Abstract
Spinal cord injury is characterized by different aetiologies, complex pathogenesis, and diverse pathological changes. Current treatments are not ideal, and prognosis is generally poor. After spinal cord injury, neurons die due to various forms of cell death. Among them, ferroptosis causes dysfunction after spinal cord injury, and no existing traditional treatments have been indicated to block its occurrence. Meanwhile, emerging therapies using mesenchymal stem cells, extracellular vesicles, and transcranial magnetic stimulation therapy are promising for reversing spinal cord neuronal ferroptosis after spinal cord injury. However, no definitive studies have demonstrated the effectiveness of these approaches. This review summarizes the existing research on the mechanisms of ferroptosis; ferroptosis after spinal cord injury; treatment of spinal cord injury with mesenchymal stem cells, extracellular vesicles, and transcranial magnetic stimulation; and treatment of ferroptosis using mesenchymal stem cells, extracellular vesicles, and transcranial magnetic stimulation. Inhibiting ferroptosis can promote the reversal of neurological dysfunction after spinal cord injury. In addition, mesenchymal stem cells, extracellular vesicles, and transcranial magnetic stimulation can reverse adverse outcomes of spinal cord injury and regulate ferroptosis-related factors. Thus, it can be inferred that mesenchymal stem cells, extracellular vesicles, and transcranial magnetic stimulation have the potential to inhibit ferroptosis after spinal cord injury. This review serves as a reference for future research to confirm these conclusions.
Key Words: exosomes, extracellular vesicles, ferroptosis, iron overload, lipid peroxidation, mesenchymal stem cells, miRNAs, spinal cord injury, stem cells, transcranial magnetic stimulation
Introduction
Spinal cord injury (SCI) is a common neurological injury seen in clinical practice, with various associated pathological changes (Fan et al., 2018) often manifested as physical motor, sensory, and even autonomic dysfunction in patients; SCI is difficult to cure and has poor prognosis. SCI is most common after traumatic events (Silvestro et al., 2020) and mostly occurs in young men, with the prevalence increasing annually (Flack et al., 2022).
Given the complex pathological mechanisms, difficult recovery, and poor treatment effects associated with SCI, the medical community has worked to clarify its mechanisms and is constantly exploring effective treatment methods (Flack et al., 2022). After SCI, neurons undergo various forms of cell death, and previous studies have confirmed that apoptosis is the main damaging process, while autophagy is the main protective process. As a newly discovered method of programmed cell death, ferroptosis is also considered to be closely related to post-SCI dysfunction (Feng et al., 2021). Furthermore, emerging therapeutic approaches, especially the use of mesenchymal stem cells (MSCs), extracellular vesicles (EVs), and transcranial magnetic stimulation (TMS) therapy, appear to block neuronal death after SCI and even significantly improve SCI patient prognosis (Silvestro et al., 2020). Therefore, MSCs, EVs and TMS therapy could theoretically improve prognosis by inhibiting ferroptosis after SCI (Figure 1). However, to date, directly relevant research is still lacking. This review summarizes studies related to ferroptosis after SCI; MSCs, EVs, and TMS treatment; as well as interactions between these treatments and ferroptosis to provide support for further research.
Figure 1.
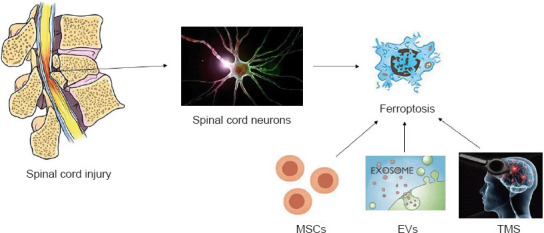
MSCs, EVs, and TMS inhibit ferroptosis after spinal cord injury.
MSCs, EVs, and TMS reverse ferroptosis after spinal cord injury, reduce spinal cord neuron death, and improve neurological function. EVs: Extracellular vesicles; MSCs: mesenchymal stem cells; TMS: transcranial magnetic stimulation.
Search Strategy
The references and studies cited in this narrative review were searched and filtered as shown in Figure 2.
Figure 2.

Search strategy.
EVs: Extracellular vesicles; MSCs: mesenchymal stem cells; SCI: spinal cord injury; TMS: transcranial magnetic stimulation.
Aetiology Staging and Pathological Characteristics of Spinal Cord Injury
The aetiology of SCI is diverse (Flack et al., 2022) with intricate mechanisms, and SCI is mainly divided into two categories: traumatic and nontraumatic (Silvestro et al., 2020). The former is relatively common, and the mechanisms include contusions and continuous compression, impact-induced compression, and tearing, transection, and tensile separation (Alizadeh et al., 2019). The latter is mostly attributed to congenital dysplasia, degenerative nervous system diseases, tumors, infections, and metabolic disorders (Venkatesh et al., 2019).
The pathogenesis of SCI can be divided into primary and secondary injury stages. The former often determines the severity and prognosis of the disease (Anjum et al., 2020), and the latter determines the recovery time (Jiang et al., 2022). The primary and secondary injury stages can be further divided into the early stage (within 2 hours), acute stage (2–48 hours), subacute stage (48 hours–2 weeks), intermediate stage (2 weeks–6 months), and chronic stage (> 6 months) (Fiani et al., 2021a). After the occurrence of SCI, the body’s homeostasis is disrupted, and a series of pathological changes occur, including neuroinflammation, scarring, tissue hematoma, tissue edema, neuronal cell death, and various ion disorders (Fan et al., 2018). The pathological changes in different stages are also different (Table 1).
Table 1.
Stages and pathological changes in spinal cord injury
| Stage | Phase | Duration | Characteristics | Treatment |
|---|---|---|---|---|
| Primary injury stage | Immediate phase | 2 h | Direct injury: nerve cell death, vascular injury, and disruption of the blood-spinal cord barrier | Neuroprotective therapy |
| Acute phase | 2–48 h | Bleeding, edema, inflammation, internal environment disturbances: the formation of free radicals, oxidative reaction and ion disturbances, ischaemia and hypoperfusion, various forms of nerve cell death, nerve demyelination | Inhibit inflammation, maintain perfusion, and correct disturbances | |
| Secondary injury stage | Subacute phase | 48 h–2 wk | Scar formation, syringomyelia formation, blood-spinal cord barrier repair | Cell therapy |
| Intermediate phase | 2 wk–6 mon | Scar maturation, syringomyelia, axonal regeneration and reconnection failure | Inhibit scar formation | |
| Chronic phase | More than 6 mon | Scar maturation, syringomyelia, axonal regeneration and reconnection failure | Rehabilitation treatment |
In addition, after the occurrence of SCI, the damaged nerve tissue, the most important of which are nerves, will also spontaneously repair and regenerate. This process mainly includes three stages: neuroinflammation and cell death, cell proliferation and tissue regeneration, and tissue remodelling (Anjum et al., 2020).
Ferroptosis and Spinal Cord Injury
Damage to the nervous system after SCI is extensive and involves damage at the tissue, cellular, and molecular levels (Fan et al., 2018). Various forms of cell death, including apoptosis, necroptosis, pyroptosis, autophagy, and ferroptosis, are also seen, with different mechanisms and cellular morphological changes (Table 2).
Table 2.
Various cell death pathways and characteristics in spinal cord injury
| Characteristics | Core characteristic | Others | |
|---|---|---|---|
| Necrosis | Organelle swelling, plasma membrane rupture, DNA fragmentation, enzyme participation, and ATP consumption | – | Nonprocedural, unregulated |
| Apoptosis | Apoptotic body formation, nuclear rupture, cellular pyknosis, and plasma membrane blebbing | Caspase | Active cell death |
| Necroptosis | Swelling and rupture of cells and organelles, rupture after plasma membrane perforation | RIP1/RIP3/MLKL mediated necrosis | Passive destruction of cells, alternative means of apoptosis |
| Pyroptosis | Bubble (pyrosome) formation, relatively low degree of cell swelling, inflammasome formation, and inflammatory response | Gasdermin D/E | Inflammation related process |
| Autophagy | The formation of autophagic vesicles, autophagosomes and autolysosomes | Lysosome | Maintain homeostasis |
| Ferroptosis | Iron metabolism disorder, reduced mitochondrial volume, thickened membranes, and reduced cristae, nuclei are not significantly altered | Iron input; glutathione peroxidase 4 inactivation | Iron dependence |
Snapshot of ferroptosis
In 2003, a new iron-related cell death mode was discovered and officially named ferroptosis in 2012 (Dixon et al., 2012). As a type of novel iron-dependent, non-apoptotic programmed cell death, ferroptosis is characterized by disturbances in iron metabolism and the accumulation of lipid reactive oxygen species (ROS).
Cells undergoing ferroptosis exhibit a series of unique changes in cell morphology, with a characteristic round shape similar to that of cells before dying of necrosis, but with no characteristic plasma membrane rupture or swelling of the cytoplasm or organelles, as are seen in necrosis. Furthermore, when ferroptosis occurs, mitochondria are reduced, the cell membrane thicken, and cristae are reduced (Li et al., 2020a), while the nucleus of the cell maintains structural integrity. Ultimately, the cell membrane ruptures after lipid peroxidation and cell lysis. Thus, characteristic changes in mitochondria can verify the occurrence of ferroptosis in cells. In addition, mitochondria can generate a large amount of ROS; studies have shown that mitochondria may be involved in the occurrence and development of ferroptosis (Hirschhorn and Stockwell, 2019).
Mechanisms and pathways of ferroptosis
The specific mechanisms of ferroptosis are not completely clear (Li et al., 2020a), with iron overload, oxidative system activation, and antioxidant inhibition considered important. In addition, studies attribute ferroptosis to the long-term accumulation of previously unrepaired damage (Stockwell et al., 2017). It is now known that the key steps in ferroptosis include intracellular iron input and the inactivation of glutathione peroxidase 4 (GPX4) caused by glutathione deficiency (Proneth and Conrad, 2019), while lipid oxidation, ferritin decomposition, and other reactions are significantly enhanced (Shen et al., 2020). Under physiological conditions, antioxidant enzymes, represented by GPX4, limit the accumulation of oxidative lipids and inhibit ferroptosis. In pathological states, antioxidant enzymes are downregulated, leading to the occurrence of lipid peroxidation and iron overload, which mediate the occurrence of ferroptosis. Recent studies have demonstrated (Riegman et al., 2020) that the essence of ferroptosis is cell rupture mediated by plasma membrane pores, which spreads through cell populations with a wavy appearance not observed in other forms of cell death, leading to widespread tissue damage. Recent studies have shown that autophagy may trigger ferroptosis after SCI; this is called ferritin phagocytosis. The degradation of ferritin by lysosomes in the process of autophagy recycle intracellular redox-active iron. Therefore, autophagy can increase levels of free iron by degrading iron storage proteins such as ferritin, thereby leading to ferroptosis; studies have boldly hypothesized that active lysosomes are an important cause of ferroptosis (Hirschhorn and Stockwell, 2019).
Ferroptosis is regulated by multiple genes, promoting and antagonizing it in several ways (Lei et al., 2022). Elevated concentrations of free iron is an important factor leading to ferroptosis. After ferroptosis, with the consumption of free iron and most of the iron in the body existing in bound form, the body’s resistance to ferroptosis will gradually increase. However, as described above, reactions such as autophagy convert iron in ferritin to free iron, thereby lowering the threshold for ferroptosis (Stockwell et al., 2020). Lipid peroxidation also plays an important role in ferroptosis and is necessary for ferroptosis to occur. Most lipids in which peroxidation occurs have polyunsaturated acyl tails (PL-PUFAs), and PL-PUFAs undergo peroxidation after binding to iron-dependent enzymes and labile iron. In a sense, the occurrence of ferroptosis depends in part on the concentration and location of PL-PUFAs in the phospholipid bilayer. Regardless, the regulatory and control pathways to eliminate lipid peroxidation mainly include four parallel pathways (Figure 3): (1) the GPX4-glutathione (GSH) system (cytoplasm and mitochondria); (2) the ferroptosis inhibitory protein 1 (FSP1)-ubiquinol (CoQH2) system (plasma membrane); (3) the dihydroorotate dehydrogenase (DHODH)-CoQH2 system (mitochondria); and (4) the guanosine triphosphate cyclohydrolase 1 (GCH1)-tetrahydrobiopterin (BH4) system (potentially cytoplasmic, unclear) (Lei et al., 2022).
Figure 3.

Key regulators of ferroptosis.
Four parallel pathways suppress lipid peroxidation: the GPX4-GSH system (cytoplasm and mitochondria); the FSP1-CoQH2 system (plasma membrane); the DHODH-CoQH2 system (mitochondria); and the GCH1-BH4 system (potentially cytoplasmic, unclear). BH4: Tetrahydrobiopterin; CoQ: ubiquinone; CoQH2: ubiquinol; DHODH: dihydroorotate dehydrogenase; FSP1: ferroptosis inhibitory protein 1; GCH1: guanosine triphosphate cyclohydrolase 1; GPX4: glutathione peroxidase 4; GSH: glutathione; GTP: guanosine triphosphate.
Ferroptosis after SCI
Iron is necessary for the maintenance of normal neurological function. Iron overload has been shown to aggravate nerve damage, which enhances the production of hydroxyl radicals, and high expression of ferritin in tissues has also been shown to be positively correlated with the severity of SCI (Stockwell et al., 2020). The balance between ROS induced by iron overload and the antioxidant system is also a key factor in ferroptosis, and an imbalance leads to lipid peroxidation. In addition, the occurrence of lipid peroxidation leads to mitochondrial damage (Park et al., 2021) and even cell rupture (Zhang et al., 2021) in spinal cord neurons, accelerating SCI progression. Although the exact mechanisms of cellular damage are still unclear, its role in ferroptosis has been confirmed (Li et al., 2022). There are also pathological changes in the spinal cord, such as hemorrhage, cell fragmentation, accumulation of ROS, and increased glutamate excitotoxicity, and all of these changes can theoretically be used as factors to induce ferroptosis (Hu et al., 2021).
Once we observe the occurrence of iron overload, lipid peroxidation, lipid ROS accumulation, and characteristic mitochondrial changes and the nucleus has not undergone significant morphological changes, it can often be inferred that cells have undergone ferroptosis. In studies based on rat models, iron-induced lipid ROS accumulation in the motor cortex of rats with SCI was detected (Feng et al., 2021). Yao et al. (2019) also observed characteristic changes in ferroptotic mitochondria in SCI. A previous study has also detected a significant increase in iron deposition in the motor cortex of SCI rats, while activated microglia secreted large amounts of nitric oxide (NO), inducing iron overload in motor neurons (Feng et al., 2021). All of the above findings confirmed the existence of ferroptosis after SCI.
The types of nerve cells involved in ferroptosis are mainly neurons and oligodendrocytes (Zhang et al., 2020b). More importantly, ferroptosis of neurons reduces the number of neurons in the motor cortex, which eventually manifests as structural atrophy, accompanied by the death of the corresponding axons, which is considered to be one of the reasons for the difficulty in functional recovery in patients with SCI (Feng et al., 2021). Finally, preliminary studies have demonstrated that ruptured cells after ferroptosis release inflammatory damage-associated molecular patterns that ultimately lead to further tissue damage (Proneth and Conrad, 2019). In conclusion, ferroptosis is considered to play a vital role in the damage caused by secondary injury in SCI, but related research has only been carried out for a short period of time and is still in its infancy, and further research is still needed.
Inhibiting ferroptosis as a therapy for SCI
As mentioned above, once iron overload, ROS aggregation, lipid peroxidation, or characteristic changes in mitochondria are observed in cells, ferroptosis can occur. Research has confirmed the occurrence of ferroptosis after SCI, so ferroptosis can be regarded as an important mechanism in the death of spinal cord neurons. Studies have also confirmed that the inhibition of ferroptosis after SCI is expected to improve prognosis (Chen et al., 2020). Lipid peroxidation and iron overload are two important causes of ferroptosis after SCI, and they can theoretically be used as therapeutic targets.
Inhibiting lipid peroxidation
GPX4 inhibits oxidative reactions under physiological conditions and was the first ferroptosis inhibitor discovered (Stockwell et al., 2020); decreased GPX4 levels after SCI is one of the causes of ferroptosis. Initially, GPX4 was considered indispensable in the inhibition of ferroptosis. However, subsequent studies revealed that FSP1 can also prevent ferroptosis in the absence of GPX4 as a potential intervention (Liang et al., 2020). Liproxstatin-1 (Lipro-1) can not only inhibit mitochondrial lipid peroxidation but also restore the expression levels of GSH, GPX4, and FSP1. Lipoxin A4 can also play a role in preventing lipid oxidation (Wei et al., 2021). NRF2 is a stress-inducible transcription factor, and its related signalling pathway plays a role in mediating lipid peroxidation and ferroptosis (Dodson et al., 2019). Therefore, NRF2 can be used as a target to interfere with ferroptosis after SCI. For example, zinc inhibits ferroptosis by degrading lipid peroxides through the NRF2/GPX4 signalling pathway (Ge et al., 2021). Prokineticin-2 is an important secreted protein that inhibits lipid peroxidation by inhibiting lipid peroxidative substrate synthesis (Bao et al., 2021). EGCG, the main catechin in green tea, also has antioxidant effects, and studies have demonstrated its role in inhibiting ferroptosis after SCI (Wang et al., 2020).
Inhibiting iron overload
Iron overload after SCI triggers neuronal ferroptosis to inhibit functional recovery (Feng et al., 2021). Ferroportin 1 is the only mammalian nonheme iron exporter identified thus far, and its upregulation can prevent iron overload (Yang et al., 2021). Deferoxamine, an iron chelator, also prevents iron overload (Yao et al., 2019) and acts directly on neurons (Zhang et al., 2020a). Dynasore, a dynamin protein inhibitor, inhibits lipid peroxidation by inhibiting iron uptake and mitochondrial respiration and reduces ferroptosis through combined action (Clemente et al., 2020).
Mesenchymal Stem Cells and Spinal Cord Injury
MSCs and other cells
Stem cells are divided into several types, including MSCs, embryonic stem cells, neural stem cells, and pluripotent stem cells. There are also other types of cells with the ability to differentiate, including Schwann cells and olfactory ensheathing cells. These different cell types show different physicochemical properties (Shao et al., 2019; Table 3).
Table 3.
Cells and their effects on SCI
| Definition | Effects on SCI | Advantages | Limitations | |
|---|---|---|---|---|
| Mesenchymal stem cells | Originate from multipotent progenitor cells of the mesoderm | Differentiate into neurons and glial cells, paracrine activity | Rapid proliferation and strong differentiation, easy access, few side effects, no ethical concerns | Heterogeneous, efficacy uncertain |
| Embryonic stem cells | Highly undifferentiated cells isolated from early embryos or primitive gonads | Differentiate into neurons and glial cells, remyelination | Long-term research, adequate research, multidirectional differentiation ability | Ethical issues, immune rejection, tumor risk |
| Neural stem cells | The lateral ventricle of the brain, the dentate gyrus of the hippocampus, and the central canal of the spinal cord | Differentiate into neurons and glial cells, produce neurotrophic factors, reduce inflammation | Can remain dormant for a long time, directly obtained from the foetal spinal cord | Regional heterogeneity, timing, route, and dose of treatment, appropriate sources |
| Pluripotent stem cells | Artificial induction transforms primary cells into a pluripotent state through genetic reprogramming | Ability to self-renew and differentiate into most nerve cells, provide nutritional support, promote regeneration | No ethical issues, multidirectional differentiation, wide range of sources, no immunosuppression required | Induces tumors, genetic alterations, expensive |
| Schwann cells | Glial cells of the peripheral nervous system | Provide a structural scaffold for regeneration, guide axonal growth, promote axonal and myelin regrowth | Autografts are available, well-studied, proven efficacy | Difficulty in isolation, culture and purification; cause inflammation, scaring, tissue damage |
| Olfactory ensheathing cells | Unique glial cells located within the olfactory mucosa and olfactory bulb | Phagocytosis of harmful substances, protect nerves, exhibit anti-inflammatory activity, nourish nerves, provide regeneration promoting substrates | Strong cellular plasticity, widespread distribution, easy access | Poor posttransplant survival, lack of reliable identification, and poor purification methods |
SCI: Spinal cord injury.
MSCs have shown a series of advantages (Cofano et al., 2019). MSCs possess immunomodulatory capabilities and the ability to autonomously migrate to lesions (Cofano et al., 2019), which is called the “homing” feature. According to various sources, MSCs can be divided into bone marrow (BM), umbilical cord, adipose and other types of MSCs (Table 4). The biological properties of different types of MSCs are also heterogeneous (Costa et al., 2021).
Table 4.
MSCs and their effect on SCI
| Invasive procedure | Passage continuity | Proliferation | Neural differentiation capacity | Anti-inflammatory effect on SCI | Glial scar reduction | Immuno-modulatory activity | Secretome | Related research | |
|---|---|---|---|---|---|---|---|---|---|
| Bone marrow MSCs | +++ | + | + | ++ | ++ | ++ | +++ | Mainly angiogenic factors | +++ |
| Umbilical cord MSCs | – | +++ | +++ | +++ | +++ | ++ | ++ | Mainly neurotrophic factors | ++ |
| Adipose MSCs | + | ++ | ++ | + | ++ | ++ | ++ | Mainly angiogenic factors | + |
* –: None; +: mild degree; ++: moderate degree; +++: severe degree. MSCs: Mesenchymal stem cells; SCI: spinal cord injury.
MSC therapy for SCI
Neuroprotective treatment methods including therapeutic hypothermia, hormones, and decompression surgery are mainly used in clinical practice (Fiani et al., 2021b), but the effects of the abovementioned treatments are not satisfactory, with debates ongoing regarding their dose, time, efficacy, and safety (Hu et al., 2020).
Given the potential of stem cells to differentiate into nerve cells, people are optimistic regarding the use of stem cell transplantation to reverse dysfunction after SCI. The earliest research on stem cell transplantation was performed approximately 50 years ago (Flack et al., 2022). In recent years, with the development of animal model research and clinical trials for SCI, the results seem to be as hoped (Silvestro et al., 2020).
The use of stem cells, especially MSCs, in the treatment of SCI is not limited to direct differentiation into neurons (Figure 4) but also plays a role in inhibiting neuroinflammation and scarring, stimulating angiogenesis, and providing nutritional support (Silvestro et al., 2020). Specifically, MSCs can inhibit immune rejection by inhibiting the proliferation of T cells and B cells and, at the same time, provide nutritional support by secreting growth factors related to nerve regeneration and angiogenesis to damaged tissue sites. MSCs inhibit gliosis and reduce scarring after SCI (Liau et al., 2020). Finally, the antioxidant properties of MSCs can stimulate cells to produce antioxidant enzymes and reduce tissue damage (Liau et al., 2020). Because of the extensive role of MSCs in the treatment of SCI, experiments based on animal models have emerged, and exciting conclusions have been drawn. The success of MSCs in animal models of SCI has inspired extensive clinical trials. According to query results on ClinicalTrials.gov, a total of 87 clinical trials on the treatment of SCI using MSCs have been carried out before the writing of this article. A total of 22 treatment strategies have been assessed, and the results show that MSCs can improve dysfunction after SCI to varying degrees (Liau et al., 2020; Silvestro et al., 2020). The success rate and accuracy of MSC transplantation has also improved significantly with the application of auxiliary means, including magnetic resonance imaging (Ali et al., 2020), protein modification (Lee et al., 2020a), gene remodelling (Ocansey et al., 2020), biological scaffolds (Cofano et al., 2019), growth factors (Ahuja et al., 2020), and degradable bioscaffolds (Wang et al., 2019).
Figure 4.

Different sources of MSCs and neural differentiation pathways.
Bone marrow (BM)/umbilical cord (UC)/adipose (AD)-derived mesenchymal stem cells (MSCs) transform into neural progenitor cells (NPCs)/neural stem cells (NSCs) and ultimately differentiate into neurons/oligodendrocytes/astrocytes.
Limitations of MSC therapy for SCI
Although MSC transplantation has various advantages, some researchers have noted concerns (Lukomska et al., 2019). First, the heterogeneity of MSCs should be considered. In addition, potential side effects (Lukomska et al., 2019; Liu et al., 2020) are also prohibitive. The issues of MSCs cell administration routes, dosage, and frequency all need to be resolved before large-scale application (Liau et al., 2020). Finally, its efficacy is not entirely convincing. Existing completed clinical studies have used small sample sizes and had low completion rates, and none of them have demonstrated improved outcomes for all patients.
Extracellular Vesicles and Spinal Cord Injury
EVs and exosomes
EVs are vesicles discovered in sheep reticulocytes (Wang et al., 2019) and secreted by cells to perform various cellular functions. Almost all cells can secrete EVs. EVs can be divided into exosomes (30–100 nm), retrovirus-like particles (90–100 nm), microvesicles (50–2000 nm), and apoptotic bodies (500–4000 nm) (Akers et al., 2013). Exosomes, which were named in 1987, are a subset of EVs (Kalluri and LeBleu, 2020). Because exosomes are the main functional component of EVs, people sometimes use them instead of EVs. However, in this review, we mainly discuss EVs. EVs contain active components such as DNA, RNA, proteins, and phospholipids, the most important and most studied of which are microRNAs (miRNAs). EVs can play a variety of roles, including maintaining homeostasis, exerting cellular functions, facilitating intercellular communication, and transferring substances.
As mentioned above, nearly all cells can secrete EVs, and MSCs have also been shown to have auto/paracrine activity (Baez-Jurado et al., 2019) that is stronger than that seen in other cells. Most of the EVs produced by MSCs are nanosized, round vesicles composed of a phospholipid bilayer containing biologically active substances. Similar to MSCs, EVs are also heterogeneous (Costa et al., 2021). Studies have demonstrated that MSCs act in SCI through EVs rather than through direct differentiation (Gazdic et al., 2018; Lim et al., 2019).
EV therapy for SCI
In contrast to the various disadvantages of MSCs, EVs have the advantages of safety, controllability, and easy transportation and storage and have been applied in the treatment of various diseases (Lelek and Zuba-Surma, 2020). EVs can play a role in neuroprotection (Nakazaki et al., 2021), promoting nerve tissue regeneration (Wang et al., 2021; Zhou et al., 2021), reducing scarring (Jiang et al., 2020; Chen et al., 2021), and inhibiting oxidative stress and angiogenesis (Peng et al., 2021) in SCI.
In addition, EVs from MSCs can also reduce oxidative stress and restore the integrity of the blood-brain barrier (Guy and Offen, 2020; Figure 5). Moreover, EVs from MSCs have been observed to inhibit various forms of cell death including neuronal apoptosis (Flack et al., 2022) and ferroptosis (Bao et al., 2020). In addition to playing a direct therapeutic role, EVs can also be used as drug carriers (Guo et al., 2021) with the advantages of small size, strong penetrability, and the ability to avoid being cleared by the body’s immune system. EVs exert the abovementioned effects not only by inhibiting or even eliminating related pathological reactions but also by activating the regeneration signalling pathways of nerves and blood vessels (Guy and Offen, 2020).
Figure 5.

The functions of EVs in the treatment of spinal cord injury.
Besides direct differentiation into neurons, EVs play a variety of roles in spinal cord injury, including neuroprotection, promoting nerve tissue regeneration, reducing scarring, inhibiting oxidative stress and angiogenesis, and reducing inflammation and immunomodulation. EVs: Extracellular vesicles.
The future development of SCI treatment lies in combination therapies (Flack et al., 2022). When EVs are combined with degradable bioscaffolds, combination therapy exhibits significantly better effects than single therapy and can reduce complications or promote the migration of nerve repair-related nutrients to lesions (Wang et al., 2019).
Limitations of EV therapy for SCI
Although the current research on EV treatment has made great breakthroughs, it also faces many challenges (Liu et al., 2021a). First, similarly to MSCs, there is heterogeneity in EVs. Second, the methods for the separation of EVs lack a unified standard, and the purity of EVs obtained by different methods varies. In addition, it also includes various problems, such as insufficient production capacity and a short half-life. In response to the above problems, improvements were proposed, such as the use of nanocapsules (Lee et al., 2020b), an aqueous two-phase system (Kırbaş et al., 2019), hydrogel encapsulation (Mol et al., 2019), and labelling and assay methods (Ghafouri-Fard et al., 2021).
Transcranial Magnetic Stimulation and Spinal Cord Injury
Snapshot of TMS
TMS is a novel brain stimulation technique with the advantages of being non-invasive and well-tolerated (Rawji et al., 2020), and it can be used to monitor and modulate nervous system function and intervene in cognitive and behavioural functions (Ferrarelli and Phillips, 2021). As the only non-invasive means of eliciting action potentials in cortical neurons via suprathreshold stimulation, TMS can accurately and reproducibly be localized to brain surface regions to specifically activate targeted brain sites (de Lara et al., 2021). TMS not only can be used to assess human motor corticospinal tract function (Ferrarelli and Phillips, 2021) but also has been widely used in the evaluation and treatment of various neurological diseases (Rawji et al., 2020).
TMS therapy for SCI
TMS can be used to assess neurological function after SCI. TMS is a means of stimulating neuronal activity by attaching a coil tightly to the skull to generate a pulsed magnetic field in selected cortical regions. Therefore, the excitability and inhibition of the corticospinal tract can be indirectly reflected by measuring evoked potentials in the muscles corresponding to the stimulated cortical regions (Benedetti et al., 2022).
TMS can also improve neurological dysfunction after SCI. Experiments based on rat models compared the motor and sensory functions of the two groups of SCI rats receiving repetitive transcranial magnetic stimulation (rTMS) stimulation within 10 minutes and 2 weeks after SCI, respectively; both groups showed significant improvement, and the recovery of the former group was significantly better than that of the latter (Krishnan et al., 2019). Subsequent clinical studies using different frequencies of rTMS stimulation combined with exercise therapy have demonstrated that rTMS at 20–25 Hz can improve dysfunction in patients with SCI (Wincek et al., 2021). TMS combined with spinal cord stimulation has been proven to increase corticospinal excitability and functional motor output (Al’joboori et al., 2021). Notably, based on the current trend of combined treatments for SCI, a method using TMS combined with olfactory ensheathing cell transplantation for the treatment of SCI was developed (Delarue et al., 2021).
Limitations of TMS therapy for SCI
Although TMS is generally a safe, dependable, and easy-to-implement treatment, the application of TMS to assess and improve neurological dysfunction after SCI also has limitations. The reliability of TMS in the assessment of neurological indicators in SCI is affected by multiple factors such as the “muscle grade” of the muscles tested, the patients’ baseline motor function, and the course of SCI (Baker et al., 2016). In addition, the efficacy of TMS in improving neurological dysfunction after SCI is also affected by factors such as stimulation intensity, frequency, and location (Tazoe and Perez, 2015), and published clinical studies have included too few cases (Gunduz et al., 2017).
Mesenchymal Stem Cells, Extracellular Vesicles and Transcranial Magnetic Stimulation for Ferroptosis
As mentioned above, ferroptosis plays an important role in the secondary injury phase of SCI, and inhibition of ferroptosis has also been shown to benefit functional recovery after SCI. Studies have shown that treatment with MSCs and EVs, and especially with EVs, can inhibit ferroptosis. Unfortunately, related studies on the treatment of ferroptosis using MSCs and EVs have mainly been performed in the context of cancer treatment (Wu et al., 2021). There are only a few studies on the treatment of neurological diseases that can provide a partial basis for the treatment of ferroptosis using MSCs and EVs, and there are almost no studies directly on ferroptosis after SCI. Furthermore, the field of TMS inhibition of ferroptosis is even less well studied.
MSC therapy for ferroptosis
Only a few studies have been conducted on stem cell interventions in ferroptosis. After repetitive mild traumatic brain injury, abnormal iron metabolism, lipid peroxidation, and decreased GPX4 levels are seen. MSC treatment can effectively reverse these changes and improve cognitive impairments caused by repetitive mild traumatic brain injury (Wang et al., 2022). Xu et al. (2021) demonstrated that intravenous infusion of MSCs in a male domestic pig model of cardiopulmonary resuscitation after cardiac arrest could significantly reduce the incidence of ferroptosis, thereby reducing the functional impairments caused by the resuscitation of brain nerve tissue. Unfortunately, the specific mechanisms by which MSCs inhibit ferroptosis in these experiments remain unclear.
As mentioned above, MSCs have anti-inflammatory, antioxidant, and immune response inhibition effects. These are the causes of ferroptosis, and breakthroughs may be found when investigating these aspects. Animal model experiments have confirmed the antioxidant properties of MSCs (Stavely and Nurgali, 2020). The mechanisms include activating antioxidant pathways, scavenging free radicals, inhibiting ROS to regulate the immune system, altering cellular or mitochondrial bioenergetics, and regenerating mitochondria in damaged cells. Follow-up studies have shown that MSC activity is related to the body’s antioxidant capacity (Kubben et al., 2016). A decrease in MSC activity in the body will lead to premature ageing, and the restoration of its activity can effectively reverse this, such as by activating NRF2.
EV therapy for ferroptosis
EVs, which are the most important MSC components that play a therapeutic role, have also been observed to inhibit ferroptosis. A recent study in mouse SCI models demonstrated that EVs from MSCs could inhibit ferroptosis in neurons through the lncGm36569/miR-5627-5p/FSP1 axis and inhibit the production of ROS and Fe2+ (Shao et al., 2022). Unfortunately, other related studies were in diseases other than SCI. Studies based on a mouse model of acute myocardial infarction (Song et al., 2021) showed that EVs derived from human umbilical cord -MSCs indirectly inhibited ferroptosis by inhibiting the expression of divalent metal transporter 1 to reduce myocardial injury after acute myocardial infarction. Both MSCs, and EVs secreted by other stem/progenitor cells inhibit ferroptosis. EVs secreted by endothelial progenitor cells not only inhibit ferroptosis in vascular endothelial cells after atherosclerosis (Li et al., 2021a) but also prevent steroid-induced osteoporosis by inhibiting the ferroptosis pathway in osteoblasts (Lu et al., 2019).
Few studies on exosome therapies for neurological disease-related ferroptosis have focused on miRNAs. miRNAs are a series of single-chain, non-coding molecules with small size and light weight (Munir et al., 2020). Moreover, miRNAs control mRNA and protein expression (Hill and Tran, 2021). Because miRNAs can remain stable in EVs without degradation, they are able to pass easily through biofluids. miRNAs can remain fully functional in receptors, and they are the most important and the most studied components of EVs (Liu et al., 2019; Munir et al., 2020); there have been a few studies confirming their inhibitory effects on ferroptosis. Cancer-associated fibroblasts suppress ferroptosis in cancer cells by targeting arachidonic acid lipoxygenase 15 and blocking lipid ROS accumulation via miR-522 in secreted exosomes (Zhang et al., 2020a). Li et al. (2020b) confirmed that endothelial progenitor cell-EVs overexpressing miR-137 could inhibit ferroptosis through the miR-137-COX2/PGE2 signalling pathway, thereby protecting neurons after stroke. Bao et al. (2020) studied a mouse model of intracerebral hemorrhage and concluded that the downregulation of miR124 could indirectly upregulate ferroportin 1 levels, inhibit ferroptosis, and ultimately exert neuroprotective effects. Additionally, Yi and Tang (2021) analysed an intracerebral hemorrhage mouse model and observed ferroptosis inhibition and reduced neuronal injury after the infusion of miR-19b-3p-modified EVs from adipose-MSCs. As a key factor that regulates ferroptosis, miR-212-5p overexpression can directly target and negatively regulate prostaglandin endoperoxidase 2 to inhibit ferroptosis (Xiao et al., 2019). After traumatic brain injury, the level of miR-212-5p in brain cells is downregulated, and restoring its level via EVs can effectively protect brain nerve tissue. Finally, Li et al. (2021b) observed that ferroptosis caused by the upregulation of miR-335 and downregulation of ferritin heavy chain 1 was one of the pathogenic factors of Parkinson’s disease, and the latter is negatively affected by the former.
However, an alternative approach is to focus on inhibiting key factors of ferroptosis or activating antioxidant reactions. EVs from MSCs can reduce ROS production and reduce mitochondrial damage by stabilizing NRF2 levels (Liu et al., 2021b). EVs from MSCs can also stabilize the levels of SLC7A11 (Lin et al., 2022) and GPX4 (Tan et al., 2022). MSCs-EVs are rich in miRNAs with antioxidant capacity, which can exert extraordinary antioxidant effects, effectively reducing ROS production and lipid/protein oxidation while increasing the activity of glutathione peroxidase, catalase, and superoxide dismutase (Luo et al,. 2021).
TMS therapy for ferroptosis
Compared with the few studies on the inhibition of ferroptosis by MSCs or EVs, research on TMS inhibition of ferroptosis is lacking at the time of writing this review. Various forms of cell death occur in neurons after SCI, and existing studies have shown that TMS treatment has inhibitory effects on both apoptosis (Sasso et al., 2016) and pyroptosis (Luo et al., 2022). Whether TMS can inhibit ferroptosis by activating the ferroptosis defence pathway or inhibiting iron overload and lipid peroxidation is still unknown, and further studies are needed to confirm this hypothesis. However, if we also change our perspective, starting from the regulatory factors related to ferroptosis, there may be some advantages of TMS therapy. For example, studies have shown that TMS can activate and upregulate the level of NRF2 and further play an antioxidant role (Tasset et al., 2013). TMS can also regulate the levels of antioxidant factors including GSH (Medina-Fernandez et al., 2018a). In addition, TMS itself can also be used as an antioxidant treatment (Medina-Fernández et al., 2018b). The conclusions of the above study undoubtedly greatly increase the possibility of ferroptosis inhibition by TMS and encourage the development of follow-up studies.
In summary, theoretically using MSCs, EVs, or TMS to intervene in ferroptosis in the nervous system after various neurological diseases is expected to improve patient prognosis and has relatively broad research prospects (Figure 6). However, in the field of SCI, relevant experiments are urgently needed for verification.
Figure 6.

MSCs, EVs, and TMS inhibit ferroptosis after spinal cord injury: potential mechanisms.
During spinal cord injury, spinal cord neurons die after ferroptosis. Death of neurons will lead to neurological dysfunction. MSCs, EVs, and TMS could inhibit ferroptosis by reactivating the defence pathways of ferroptosis and/or by inhibiting key factors of ferroptosis to recover neurological function. BH4: Tetrahydrobiopterin; CoQH2: ubiquinol; DHODH: dihydroorotate dehydrogenase; EVs: extracellular vesicles; FSP1: ferroptosis inhibitory protein 1; GCH1: guanosine triphosphate cyclohydrolase 1; GPX4: glutathione peroxidase 4; GSH: glutathione; MSCs: mesenchymal stem cells; TMS: transcranial magnetic stimulation.
Conclusions and Future Perspectives
SCI is a devastating neurological disease that has no cure and often develops into chronic SCI. After decades of research, the pathogenesis and pathological changes in SCI have been more deeply understood on a clinical level, and many new treatments have been proposed. Neuronal death after SCI, especially programmed cell death such as ferroptosis, is gradually being accepted as an important factor that causes dysfunction after SCI. Emerging treatment strategies such as MSC, EV, and TMS therapy also provide hope for SCI treatment. Different forms of cell death, such as ferroptosis, are presumed to play an important role in the pathogenesis of SCI, and MSC, EV, and TMS therapy are expected to improve the prognosis of SCI by inhibiting neuronal death. However, the relevant mechanisms are not fully understood, and convincing research results are still lacking. We believe that further research will provide important progress toward a cure for SCI.
Additional file: Open peer review reports 1 (88.6KB, pdf) and 2 (97.4KB, pdf) .
Footnotes
Author contributions: QFS conceived and wrote the manuscript; QC and YSW polished the manuscript; LXZ revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Conflicts of interest: The authors declare no conflict of interest.
Data availability statement: The data are available from the corresponding author on reasonable request.
Open peer reviewers: Renata Ciccarelli, University of Chieti-Pescara, Italy; Chen Chen, Indiana University School of Medicine, USA.
P-Reviewers: Ciccarelli R, Chen C; C-Editor: Zhao M; S-Editor: Li CH; L-Editors: Li CH, Song LP; T-Editor: Jia Y
References
- 1.Ahuja CS, Mothe A, Khazaei M, Badhiwala JH, Gilbert EA, van der Kooy D, Morshead CM, Tator C, Fehlings MG. The leading edge:Emerging neuroprotective and neuro-regenerative cell-based therapies for spinal cord injury. Stem Cells Transl Med. 2020;9:1509–1530. doi: 10.1002/sctm.19-0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akers JC, Gonda D, Kim R, Carter BS, Chen CC. Biogenesis of extracellular vesicles (EV):exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. J Neurooncol. 2013;113:1–11. doi: 10.1007/s11060-013-1084-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ali AAA, Shahror RA, Chen KY. Efficient labeling of mesenchymal stem cells for high sensitivity long-term mri monitoring in live mice brains. Int J Nanomedicine. 2020;15:97–114. doi: 10.2147/IJN.S211205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alizadeh A, Dyck SM, Karimi Abdolrezaee S. Traumatic spinal cord injury:an overview of pathophysiology, models and acute injury mechanisms. Front Neurol. 2019;10:282. doi: 10.3389/fneur.2019.00282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al'joboori Y, Hannah R, Lenham F, Borgas P, Kremers CJP, Bunday KL, Rothwell J, Duffell LD. The immediate and short-term effects of transcutaneous spinal cord stimulation and peripheral nerve stimulation on corticospinal excitability. Front Neurosci. 2021;15:749042. doi: 10.3389/fnins.2021.749042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anjum A, Yazid MD, Daud MF, Idris J, Ng AMH, Naicker AS, Ismail OHR, Kumar RKA, Lokanathan Y. Spinal cord injury:pathophysiology, multimolecular interactions, and underlying recovery mechanisms. Int J Mol Sci. 2020;21:7533. doi: 10.3390/ijms21207533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baez-Jurado E, Hidalgo-Lanussa O, Barrera-Bailón B, Sahebkar A, Ashraf GM, Echeverria V, Barreto GE. Secretome of mesenchymal stem cells and its potential protective effects on brain pathologies. Mol Neurobiol. 2019;56:6902–6927. doi: 10.1007/s12035-019-1570-x. [DOI] [PubMed] [Google Scholar]
- 8.Baker KAP, Janini DP, Frost FS, Chabra P, Varnerin N, Cunningham DA, Sankarasubramanian V, Plow EB. Reliability of TMS metrics in patients with chronic incomplete spinal cord injury. Spinal Cord. 2016;54:980–990. doi: 10.1038/sc.2016.47. [DOI] [PubMed] [Google Scholar]
- 9.Bao WD, Zhou XT, Zhou LT, Wang FD, Yin XP, Lu YM, Zhu LQ, Liu D. Targeting miR-124/Ferroportin signaling ameliorated neuronal cell death through inhibiting apoptosis and ferroptosis in aged intracerebral hemorrhage murine model. Aging Cell. 2020;19:e13235. doi: 10.1111/acel.13235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bao ZY, Liu YL, Chen BL, Miao Z, Tu YM, Li C, Chao HL, Ye YF, Xu XP, Sun GC, Zhao PZ, Liu N, Liu Y, Wang XM, Lam SM, Kagan VE, Bayır H, Ji J. Prokineticin-2 prevented neuronal cell deaths in model of traumatic brain injury. Nat Commun. 2021;12:4220. doi: 10.1038/s41467-021-24469-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benedetti B, Weidenhammer A, Reisinger M, Couillard-Despres S. Spinal cord injury and loss of cortical inhibition. Int J Mol Sci. 2022;23:5622. doi: 10.3390/ijms23105622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen YX, Liu SX, Li JJ, Li Z, Quan J, Liu XZ, Tang YB, Liu B. The latest view on the mechanism of ferroptosis and its research progress in spinal cord injury. Oxid Med Cell Longev. 2020;2020:6375938. doi: 10.1155/2020/6375938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen YY, Tian ZM, He L, Liu C, Wang NX, Rong LM, Liu B. Exosomes derived from miR-26a-modified MSCs promote axonal regeneration via the PTEN/AKT/mTOR pathway following spinal cord injury. Stem Cell Res Ther. 2021;12:224. doi: 10.1186/s13287-021-02282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clemente LP, Rabenau M, Tang S, Stanka J, Cors E, Stroh J, Culmsee C, von Karstedt S. Dynasore blocks ferroptosis through combined modulation of iron uptake and inhibition of mitochondrial respiration. Cells. 2020;9:2259. doi: 10.3390/cells9102259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cofano F, Boido M, Monticelli M, Zenga F, Ducati A, Vercelli A, Garbossa D. Mesenchymal stem cells for spinal cord injury:current options, limitations, and future of cell therapy. Int J Mol Sci. 2019;20:2698. doi: 10.3390/ijms20112698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Costa LA, Eiro N, Fraile M, Gonzalez LO, Saá J, Garcia-Portabella P, Vega B, Schneider J, Vizoso FJ. Functional heterogeneity of mesenchymal stem cells from natural niches to culture conditions:implications for further clinical uses. Cell Mol Life Sci. 2021;78:447–467. doi: 10.1007/s00018-020-03600-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Lara LIN, Daneshzand M, Mascarenas A, Paulson D, Pratt K, Okada Y, Raij T, Makarov SN, Nummenmaa A. A 3-axis coil design for multichannel TMS arrays. Neuroimage. 2021;224:117355. doi: 10.1016/j.neuroimage.2020.117355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Delarue Q, Robac A, Massardier R, Marie JP, Guérout N. Comparison of the effects of two therapeutic strategies based on olfactory ensheathing cell transplantation and repetitive magnetic stimulation after spinal cord injury in female mice. J Neurosci Res. 2021;99:1835–1849. doi: 10.1002/jnr.24836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS, Morrison 3rd B, Stockwell BR. Ferroptosis:an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dodson M, Castro Portuguez R, Zhang DD. NRF2 plays a critical role in mitigating lipid peroxidation and ferroptosis. Redox Biol. 2019;23:101107. doi: 10.1016/j.redox.2019.101107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fan B, Wei Z, Yao X, Shi G, Cheng X, Zhou X, Zhou H, Ning G, Kong X, Feng S. Microenvironment imbalance of spinal cord injury. Cell Transplant. 2018;27:853–866. doi: 10.1177/0963689718755778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feng Z, Min LX, Chen H, Deng WW, Tan ML, Liu HL, Hou JM. Iron overload in the motor cortex induces neuronal ferroptosis following spinal cord injury. Redox Bio. 2021;43:101984. doi: 10.1016/j.redox.2021.101984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferrarelli F, Phillips ML. Examining and modulating neural circuits in psychiatric disorders with transcranial magnetic stimulation and electroencephalography:present practices and future developments. Am J Psychiatry. 2021;178:400–413. doi: 10.1176/appi.ajp.2020.20071050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fiani B, Kondilis A, Soula M, Tao A, Alvi MA. Novel methods of necroptosis inhibition for spinal cord injury using translational research to limit secondary injury and enhance endogenous repair and regeneration. Neurospine. 2021a;18:261–270. doi: 10.14245/ns.2040722.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fiani B, Arshad MA, Shaikh ES, Baig A, Farooqui M, Ayub MA, Zafar A, Quadri SA. Current updates on various treatment approaches in the early management of acute spinal cord injury. Rev Neurosci. 2021b;32:513–530. doi: 10.1515/revneuro-2020-0148. [DOI] [PubMed] [Google Scholar]
- 26.Flack JA, Sharma KD, Xie JY. Delving into the recent advancements of spinal cord injury treatment:a review of recent progress. Neural Regen Res. 2022;17:283–291. doi: 10.4103/1673-5374.317961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gazdic M, Volarevic V, Harrell CR, Fellabaum C, Jovicic N, Arsenijevic N, Stojkovic M. Stem cells therapy for spinal cord injury. Int J Mol Sci. 2018;19:1039. doi: 10.3390/ijms19041039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ge MH, Tian H, Mao L, Li DY, Lin JQ, Hu HS, Huang SC, Zhang CJ, Mei XF. Zinc attenuates ferroptosis and promotes functional recovery in contusion spinal cord injury by activating Nrf2/GPX4 defense pathway. CNS Neurosci Ther. 2021;27:1023–1040. doi: 10.1111/cns.13657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ghafouri-Fard S, Niazi V, Hussen BM, Omrani MD, Taheri M, Basiri A. The emerging role of exosomes in the treatment of human disorders with a special focus on mesenchymal stem cells-derived exosomes. Front Cell Dev Biol. 2021;9:653296. doi: 10.3389/fcell.2021.653296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gunduz A, Rothwell J, Vidal J, Kumru H. Non-invasive brain stimulation to promote motor and functional recovery following spinal cord injury. Neural Regen Res. 2017;12:1933–1938. doi: 10.4103/1673-5374.221143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guo SW, Redenski I, Levenberg S. Spinal cord repair:from cells and tissue engineering to extracellular vesicles. Cells. 2021;10:1872. doi: 10.3390/cells10081872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guy R, Offen D. Promising opportunities for treating neurodegenerative diseases with mesenchymal stem cell-derived exosomes. Biomolecules. 2020;10:1320. doi: 10.3390/biom10091320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hill M, Tran N. miRNA interplay:mechanisms and consequences in cancer. Dis Model Mech. 2021;14:dmm047662. doi: 10.1242/dmm.047662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hirschhorn T, Stockwell BR. The development of the concept of ferroptosis. Free Radic Biol Med. 2019;133:130–143. doi: 10.1016/j.freeradbiomed.2018.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hu XL, Chen HW, Xu H, Wu YS, Wu CY, Jia C, Li Y, Sheng SR, Xu C, Xu HZ, Ni WF, Zhou KL. Role of pyroptosis in traumatic brain and spinal cord injuries. Int J Biol Sci. 2020;16:2042–2050. doi: 10.7150/ijbs.45467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hu XL, Xu Y, Xu H, Jin CQ, Zhang HJ, Su HH, Li Y, Zhou KL, Ni WF. Progress in understanding ferroptosis and its targeting for therapeutic benefits in traumatic brain and spinal cord injuries. Front Cell Dev Biol. 2021;9:705786. doi: 10.3389/fcell.2021.705786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiang DD, Gong FY, Ge XH, Lv CT, Huang CY, Feng S, Zhou Z, Rong YL, Wang JX, Ji CY, Chen J, Zhao WE, Fan J, Liu W, Cai WH. Neuron-derived exosomes-transmitted miR-124-3p protect traumatically injured spinal cord by suppressing the activation of neurotoxic microglia and astrocytes. J Nanobiotechnology. 2020;18:105. doi: 10.1186/s12951-020-00665-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jiang YZ, Guo JB, Tang XW, Wang XH, Hao DJ, Yang H. The immunological roles of olfactory ensheathing cells in the treatment of spinal cord injury. Front Immunol. 2022;13:881162. doi: 10.3389/fimmu.2022.881162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367:eaau6977. doi: 10.1126/science.aau6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kırbaş OK, Bozkurt BT, Asutay AB, Mat B, Ozdemir B, Öztürkoğlu D, Ölmez H, İşlek Z, Şahin F, Taşlı PN. Optimized isolation of extracellular vesicles from various organic sources using aqueous two-phase system. Sci Rep. 2019;9:19159. doi: 10.1038/s41598-019-55477-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krishnan VS, Shin SS, Belegu V, Celnik P, Reimers M, Smith KR, Pelled G. Multimodal evaluation of TMS - induced somatosensory plasticity and behavioral recovery in rats with contusion spinal cord injury. Front Neurosci. 2019;13:387. doi: 10.3389/fnins.2019.00387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kubben N, Zhang WQ, Wang LX, Voss TC, Yang JP, Qu J, Liu GH, Misteli T. Repression of the antioxidant NRF2 pathway in premature aging. Cell. 2016;165:1361–1374. doi: 10.1016/j.cell.2016.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee J, Cha H, Park TH, Park JH. Enhanced osteogenic differentiation of human mesenchymal stem cells by direct delivery of Cbfβprotein. Biotechnol Bioeng. 2020a;117:2897–2910. doi: 10.1002/bit.27453. [DOI] [PubMed] [Google Scholar]
- 44.Lee JR, Kyung JW, Kumar H, Kwon SP, Song SY, Han IB, Kim BS. Targeted delivery of mesenchymal stem cell-derived nanovesicles for spinal cord injury treatment. Int J Mol Sci. 2020b;21:4185. doi: 10.3390/ijms21114185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lei G, Zhuang L, Gan BY. Targeting ferroptosis as a vulnerability in cancer. Nat Rev Cancer. 2022;22:381–396. doi: 10.1038/s41568-022-00459-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lelek J, Zuba-Surma EK. Perspectives for future use of extracellular vesicles from umbilical cord- and adipose tissue-derived mesenchymal stem/stromal cells in regenerative therapies—synthetic review. Int J Mol Sci. 2020;21:799. doi: 10.3390/ijms21030799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li F, Wang HF, Chen H, Guo JN, Dang XQ, Ru Y, Wang HY. Mechanism of ferroptosis and its role in spinal cord injury. Front Neurol. 2022;13:926780. doi: 10.3389/fneur.2022.926780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li J, Cao F, Yin HL, Huang ZJ, Lin ZT, Mao N, Sun B, Wang G. Ferroptosis:past, present and future. Cell Death Dis. 2020a;11:88. doi: 10.1038/s41419-020-2298-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li L, Wang HN, Zhang J, Chen X, Zhang ZW, Li Q. Effect of endothelial progenitor cell-derived extracellular vesicles on endothelial cell ferroptosis and atherosclerotic vascular endothelial injury. Cell Death Discov. 2021a;7:235. doi: 10.1038/s41420-021-00610-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li XR, Si WW, Li Z, Tian Y, Liu XL, Ye SY, Huang ZF, Ji YC, Zhao CP, Hao XQ, Chen DF, Zhu ML. miR-335 promotes ferroptosis by targeting ferritin heavy chain 1 in in vivo and in vitro models of Parkinson's disease. Int J Mol Med. 2021b;47:61. doi: 10.3892/ijmm.2021.4894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li YC, Wang JJ, Chen SZ, Wu P, Xu SC, Wang CL, Shi HZ, Bihl J. miR-137 boosts the neuroprotective effect of endothelial progenitor cell-derived exosomes in oxyhemoglobin-treated SHSY5Y cells partially via COX2/PGE2 pathway. Stem Cell Res Ther. 2020b;11:330. doi: 10.1186/s13287-020-01836-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liang DG, Deng L, Jiang XJ. A new checkpoint against ferroptosis. Cell Res. 2020;30:3–4. doi: 10.1038/s41422-019-0258-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liau LL, Looi QH, Chia WC, Subramaniam T, Ng MH, Law JX. Treatment of spinal cord injury with mesenchymal stem cells. Cell Biosci. 2020;10:112. doi: 10.1186/s13578-020-00475-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lim WL, Liau LL, Ng MH, Chowdhury SR, Law JX. Current progress in tendon and ligament tissue engineering. Tissue Eng Regen Med. 2019;16:549–571. doi: 10.1007/s13770-019-00196-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lin FY, Chen WY, Zhou JH, Zhu JQ, Yao QG, Feng B, Feng XD, Shi XW, Pan QL, Yu J, Li LJ, Cao HC. Mesenchymal stem cells protect against ferroptosis via exosome-mediated stabilization of SLC7A11 in acute liver injury. Cell Death Dis. 2022;13:271. doi: 10.1038/s41419-022-04708-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu AR, Zhang XW, He HL, Zhou L, Naito Y, Sugita S, Lee JW. Therapeutic potential of mesenchymal stem/stromal cell-derived secretome and vesicles for lung injury and disease. Expert Opin Biol Ther. 2020;20:125–140. doi: 10.1080/14712598.2020.1689954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu T, Zhang Q, Zhang JK, Li C, Miao YR, Lei Q, Li QB, Guo AY. EV miRNA:a database of miRNA profiling in extracellular vesicles. Nucleic Acids Res. 2019;47:D89–D93. doi: 10.1093/nar/gky985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu W, Tang PY, Wang JX, Ye W, Ge XH, Rong YL, Ji CY, Wang ZH, Bai JL, Fan J, Yin GY, Cai WH. Extracellular vesicles derived from melatonin-preconditioned mesenchymal stem cells containing USP29 repair traumatic spinal cord injury by stabilizing NRF2. J Pineal Res. 2021b;71:e12769. doi: 10.1111/jpi.12769. [DOI] [PubMed] [Google Scholar]
- 59.Liu WZ, Ma ZJ, Li JR, Kang XW. Mesenchymal stem cell-derived exosomes:therapeutic opportunities and challenges for spinal cord injury. Stem Cell Res Ther. 2021a;12:102. doi: 10.1186/s13287-021-02153-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lu JS, Yang JZ, Zheng YS, Chen XY, Fang SY. Extracellular vesicles from endothelial progenitor cells prevent steroid-induced osteoporosis by suppressing the ferroptotic pathway in mouse osteoblasts based on bioinformatics evidence. Sci Rep. 2019;9:16130. doi: 10.1038/s41598-019-52513-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lukomska B, Stanaszek L, Zuba-Surma E, Legosz P, Sarzynska S, Drela K. Challenges and controversies in human mesenchymal stem cell therapy. Stem Cells Int. 2019;2019:9628536. doi: 10.1155/2019/9628536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Luo L, Liu MX, Fan YH, Zhang JJ, Liu L, Li Y, Zhang QQ, Xie HY, Jiang CY, Wu JF, Xiao X, Wu Y. Intermittent theta-burst stimulation improves motor function by inhibiting neuronal pyroptosis and regulating microglial polarization via TLR4/NFκB/NLRP3 signaling pathway in cerebral ischemic mice. J Neuroinflammation. 2022;19:141. doi: 10.1186/s12974-022-02501-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Luo Q, Xian PP, Wang T, Wu SX, Sun TN, Wang WT, Wang B, Yang H, Yang YP, Wang H, Liu WP, Long QF. Antioxidant activity of mesenchymal stem cell-derived extracellular vesicles restores hippocampal neurons following seizure damage. Theranostics. 2021;11:5986–6005. doi: 10.7150/thno.58632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Medina-Fernandez FJ, Escribano BM, Luque E, Caballero-Villarraso J, Gomez-Chaparro JL, Feijoo M, Garcia-Maceira FI, Pascual-Leone A, Drucker-Colin R, Tunez I. Comparative of transcranial magnetic stimulation and other treatments in experimental autoimmune encephalomyelitis. Brain Res Bull. 2018a;137:140–145. doi: 10.1016/j.brainresbull.2017.11.018. [DOI] [PubMed] [Google Scholar]
- 65.Medina-Fernández FJ, Escribano BM, Padilla-Del-Campo C, Drucker-Colín R, Pascual-Leone A, Túnez I. Transcranial magnetic stimulation as an antioxidant. Free Radic Res. 2018b;52:381–389. doi: 10.1080/10715762.2018.1434313. [DOI] [PubMed] [Google Scholar]
- 66.Mol EA, Lei ZY, Roefs MT, Bakker MH, Goumans MJ, Doevendans PA, Dankers PYW, Vader P, Sluijter JPG. Injectable supramolecular ureidopyrimidinone hydrogels provide sustained release of extracellular vesicle therapeutics. Adv Healthc Mater. 2019;8:e1900847. doi: 10.1002/adhm.201900847. [DOI] [PubMed] [Google Scholar]
- 67.Munir J, Yoon JK, Ryu S. Therapeutic miRNA-enriched extracellular vesicles:current approaches and future prospects. Cells. 2020;9:2271. doi: 10.3390/cells9102271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nakazaki M, Morita T, Lankford KL, Askenase PW, Kocsis JD. Small extracellular vesicles released by infused mesenchymal stromal cells target M2 macrophages and promote TGF-βupregulation, microvascular stabilization and functional recovery in a rodent model of severe spinal cord injury. J Extracell Vesicles. 2021;10:e12137. doi: 10.1002/jev2.12137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ocansey DKW, Pei B, Yan YM, Qian H, Zhang X, Xu WR, Mao F. Improved therapeutics of modified mesenchymal stem cells:an update. J Transl Med. 2020;18:42. doi: 10.1186/s12967-020-02234-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Park MW, Cha HW, Kim J, Kim JH, Yang H, Yoon S, Boonpraman N, Yi SS, Yoo ID, Moon JS. NOX4 promotes ferroptosis of astrocytes by oxidative stress-induced lipid peroxidation via the impairment of mitochondrial metabolism in Alzheimer's diseases. Redox Biol. 2021;41:101947. doi: 10.1016/j.redox.2021.101947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Peng W, Wan LY, Luo ZX, Xie Y, Liu YD, Huang TM, Lu HB, Hu JZ. Microglia-derived exosomes improve spinal cord functional recovery after injury via inhibiting oxidative stress and promoting the survival and function of endothelia cells. Oxid Med Cell Longev. 2021;2021:1695087. doi: 10.1155/2021/1695087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Proneth B, Conrad M. Ferroptosis and necroinflammation, a yet poorly explored link. Cell Death Differ. 2019;26:14–24. doi: 10.1038/s41418-018-0173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rawji V, Latorre A, Sharma N, Rothwell JC, Rocchi L. On the use of TMS to Investigate the pathophysiology of neurodegenerative diseases. Front Neurol. 2020;11:584664. doi: 10.3389/fneur.2020.584664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Riegman M, Sagie L, Galed C, Levin T, Steinberg N, Dixon SJ, Wiesner U, Bradbury MS, Niethammer P, Zaritsky A, Overholtzer M. Ferroptosis occurs through an osmotic mechanism and propagates independently of cell rupture. Nat Cell Biol. 2020;22:1042–1048. doi: 10.1038/s41556-020-0565-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sasso V, Bisicchia E, Latini L, Ghiglieri V, Cacace F, Carola V, Molinari M, Viscomi MT. Repetitive transcranial magnetic stimulation reduces remote apoptotic cell death and inflammation after focal brain injury. J Neuroinflammation. 2016;13:150. doi: 10.1186/s12974-016-0616-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shao AW, Tu S, Lu JN, Zhang JM. Crosstalk between stem cell and spinal cord injury:pathophysiology and treatment strategies. Stem Cell Res Ther. 2019;10:238. doi: 10.1186/s13287-019-1357-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shao CL, Chen Y, Yang TY, Zhao HB, Li DZ. Mesenchymal stem cell derived exosomes suppress neuronal cell ferroptosis via lncGm36569/miR-5627-5p/FSP1 axis in acute spinal cord injury. Stem Cell Rev Rep. 2022;18:1127–1142. doi: 10.1007/s12015-022-10327-x. [DOI] [PubMed] [Google Scholar]
- 78.Shen LS, Lin DF, Li XY, Wu HJ, Lenahan C, Pan YB, Xu WL, Chen YD, Shao AW, Zhang JM. Ferroptosis in acute central nervous system injuries:the future direction? Front Cell Dev Biol. 2020;8:594. doi: 10.3389/fcell.2020.00594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Silvestro S, Bramanti P, Trubiani O, Mazzon E. Stem cells therapy for spinal cord injury:an overview of clinical trials. Int J Mol Sci. 2020;21:659. doi: 10.3390/ijms21020659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Song YF, Wang BC, Zhu XL, Hu JL, Sun JJ, Xuan JZ, Ge ZW. Human umbilical cord blood–derived MSCs exosome attenuate myocardial injury by inhibiting ferroptosis in acute myocardial infarction mice. Cell Biol Toxicol. 2021;37:51–64. doi: 10.1007/s10565-020-09530-8. [DOI] [PubMed] [Google Scholar]
- 81.Stavely R, Nurgali K. The emerging antioxidant paradigm of mesenchymal stem cell therapy. Stem Cells Transl Med. 2020;9:985–1006. doi: 10.1002/sctm.19-0446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Stockwell BR, Angeli JPF, Bayir H, Bush AI, Conrad M, Dixon SJ, Fulda S, Gascón S, Hatzios SK, Kagan VE, Noel K, Jiang XJ, Linkermann A, Murphy ME, Overholtzer M, Oyagi A, Pagnussat GC, Park J, Ran QT, Rosenfeld CS, et al. Ferroptosis:a regulated cell death nexus linking metabolism, redox biology, and disease. Cell. 2017;71:273–285. doi: 10.1016/j.cell.2017.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Stockwell BR, Jiang XJ, Gu W. Emerging mechanisms and disease relevance of ferroptosis. Trends Cell Biol. 2020;30:478–490. doi: 10.1016/j.tcb.2020.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tan YW, Huang Y, Mei R, Mao F, Yang DK, Liu JW, Xu WR, Qian H, Yan YM. HucMSC-derived exosomes delivered BECN1 induces ferroptosis of hepatic stellate cells via regulating the xCT/GPX4 axis. Cell Death Dis. 2022;13:319. doi: 10.1038/s41419-022-04764-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tasset I, Pérez-Herrera A, Medina FJ, Arias-Carrión O, Drucker-Colín R, Túnez I. Extremely low-frequency electromagnetic fields activate the antioxidant pathway Nrf2 in a Huntington's disease-like rat model. Brain Stimul. 2013;6:84–86. doi: 10.1016/j.brs.2012.03.015. [DOI] [PubMed] [Google Scholar]
- 86.Tazoe T, Perez MA. Effects of repetitive transcranial magnetic stimulation on recovery of function after spinal cord injury. Arch Phys Med Rehabil. 2015;96:S145–155. doi: 10.1016/j.apmr.2014.07.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Venkatesh K, Ghosh SK, Mullick M, Manivasagam G, Sen D. Spinal cord injury:pathophysiology, treatment strategies, associated challenges, and future implications. Cell Tissue Res. 2019;377:125–151. doi: 10.1007/s00441-019-03039-1. [DOI] [PubMed] [Google Scholar]
- 88.Wang D, Zhang SS, Ge XT, Yin ZY, Li MM, Guo MT, Hu TP, Han ZL, Kong XD, Li D, Zhao J, Wang L, Liu Q, Chen FL, Lei P. Mesenchymal stromal cell treatment attenuates repetitive mild traumatic brain injury-induced persistent cognitive deficits via suppressing ferroptosis. J Neuroinflammation. 2022;19:185. doi: 10.1186/s12974-022-02550-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang JJ, Chen Y, Chen L, Duan YZ, Kuang XJ, Peng Z, Li CH, Li YH, Xiao Y, Jin H, Tan QD, Zhang SF, Zhu BP, Tang YJ. EGCG modulates PKD1 and ferroptosis to promote recovery in ST rats. Transl Neurosci. 2020;11:173–181. doi: 10.1515/tnsci-2020-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang XZ, Botchway BOA, Zhang Y, Yuan JY, Liu XH. Combinational treatment of bioscaffolds and extracellular vesicles in spinal cord injury. Front Mol Neurosci. 2019;12:81. doi: 10.3389/fnmol.2019.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang Y, Lai XW, Wu DP, Liu B, Wang NX, Rong LM. Umbilical mesenchymal stem cell-derived exosomes facilitate spinal cord functional recovery through the miR-199a-3p/145-5pmediated NGF/TrkA signaling pathway in rats. Stem Cell Res Ther. 2021;12:117. doi: 10.1186/s13287-021-02148-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wei N, Lu T, Yang LB, Dong YH, Liu XT. Lipoxin A4 protects primary spinal cord neurons from Erastin-induced ferroptosis by activating the Akt/Nrf2/HO-1 signaling pathway. FEBS Open Bio. 2021;11:2118–2126. doi: 10.1002/2211-5463.13203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wincek A, Huber J, Leszczyńska K, Fortuna W, Okurowski S, Chmielak K, Tabakow P. The long-term effect of treatment using the transcranial magnetic stimulation rTMS in patients after incomplete cervical or thoracic spinal cord injury. J Clin Med. 2021;10:2975. doi: 10.3390/jcm10132975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wu S, Li TY, Liu WW, Huang YY. Ferroptosis and cancer:complex relationship and potential application of exosomes. Front Cell Dev Biol. 2021;9:733751. doi: 10.3389/fcell.2021.733751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Xiao X, Jiang YJ, Liang WB, Wang YY, Cao SQ, Yan H, Gao LB, Zhang L. miR-212-5p attenuates ferroptotic neuronal death after traumatic brain injury by targeting Ptgs2. Mol Brain. 2019;12:78. doi: 10.1186/s13041-019-0501-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Xu JF, Zhang MH, Liu F, Shi L, Jiang XK, Chen C, Wang JG, Diao MY, Khan ZU, Zhang M. Mesenchymal stem cells alleviate post-resuscitation cardiac and cerebral injuries by inhibiting cell pyroptosis and ferroptosis in a swine model of cardiac arrest. Front Pharmacol. 2021;12:793829. doi: 10.3389/fphar.2021.793829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yang MX, Lu ZJ, Li FL, Shi F, Zhan FB, Zhao LJ, Li YN, Li J, Lin L, Qin ZD. Escherichia coli induced ferroptosis in red blood cells of grass carp (Ctenopharyngodon idella) Fish Shellfish Immunol. 2021;112:159–167. doi: 10.1016/j.fsi.2020.09.036. [DOI] [PubMed] [Google Scholar]
- 98.Yao X, Zhang Y, Hao J, Duan HQ, Zhao CX, Sun C, Li B, Fan BY, Wang X, Li WX, Fu XH, Hu Y, Liu C, Kong XH, Feng SQ. Deferoxamine promotes recovery of traumatic spinal cord injury by inhibiting ferroptosis. Neural Regen Res. 2019;14:532–541. doi: 10.4103/1673-5374.245480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yi X, Tang XQ. Exosomes From miR-19b-3p-modified ADSCs inhibit ferroptosis in intracerebral hemorrhage mice. Front Cell Dev Biol. 2021;9:661317. doi: 10.3389/fcell.2021.661317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhang HY, Deng T, Liu R, Ning T, Yang HO, Liu DY, Zhang QM, Lin D, Ge SH, Bai M, Wang XY, Zhang L, Li HL, Yang YC, Ji Z, Wang HL, Ying GG, Ba Y. CAF secreted miR-522 suppresses ferroptosis and promotes acquired chemoresistance in gastric cancer. Mol Cancer. 2020a;19:43. doi: 10.1186/s12943-020-01168-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang Y, Fan BY, Pang YL, Shen WY, Wang X, Zhao CX, Li WX, Liu C, Kong XH, Ning GZ, Feng SQ, Yao X. Neuroprotective effect of deferoxamine on erastininduced ferroptosis in primary cortical neurons. Neural Regen Res. 2020b;15:1539–1545. doi: 10.4103/1673-5374.274344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhang Y, Wu JL, Jiang L, Lu CK, Huang ZW, Liu B. Prospects for the role of ferroptosis in fluorosis. Front Physiol. 2021;12:773055. doi: 10.3389/fphys.2021.773055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhou WS, Silva M, Feng C, Zhao SM, Liu LL, Li S, Zhong JM, Zheng WH. Exosomes derived from human placental mesenchymal stem cells enhanced the recovery of spinal cord injury by activating endogenous neurogenesis. Stem Cell Res Ther. 2021;12:174. doi: 10.1186/s13287-021-02248-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


