Abstract
Importance
The Cochrane review (2016) on kangaroo mother care (KMC) demonstrated a significant reduction in the risk of mortality in low birth weight infants. New evidence from large multi-centre randomised trials has been available since its publication.
Objective
Our systematic review compared the effects of KMC vs conventional care and early (ie, within 24 hours of birth) vs late initiation of KMC on critical outcomes such as neonatal mortality.
Methods
Eight electronic databases, including PubMed®, Embase, and Cochrane CENTRAL, from inception until March 2022, were searched. All randomised trials comparing KMC vs conventional care or early vs late initiation of KMC in low birth weight or preterm infants were included.
Data extraction and synthesis
The review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and was registered with PROSPERO.
Main outcomes and measures
The primary outcome was mortality during birth hospitalization or 28 days of life. Other outcomes included severe infection, hypothermia, exclusive breastfeeding rates, and neurodevelopmental impairment. Results were pooled using fixed-effect and random-effects meta-analyses in RevMan 5.4 and Stata 15.1 (StataCorp, College Station, TX).
Results
In total, 31 trials with 15 559 infants were included in the review; 27 studies compared KMC with conventional care, while four compared early vs late initiation of KMC. Compared with conventional care, KMC reduces the risks of mortality (relative risk (RR) 0.68; 95% confidence interval (CI) 0.53 to 0.86; 11 trials, 10 505 infants; high certainty evidence) during birth hospitalisation or 28 days of age and probably reduces severe infection until the latest follow-up (RR 0.85, 95% CI 0.79 to 0.92; nine trials; moderate certainty evidence). On subgroup analysis, the reduction in mortality was noted irrespective of gestational age or weight at enrolment, time of initiation, and place of initiation of KMC (hospital or community); the mortality benefits were greater when the daily duration of KMC was at least 8 hours per day than with shorter-duration KMC. Studies comparing early vs late-initiated KMC demonstrated a reduction in neonatal mortality (RR 0.77, 95% CI 0.66 to 0.91; three trials, 3693 infants; high certainty evidence) and a probable decrease in clinical sepsis until 28-days (RR 0.85, 95% CI 0.76 to 0.96; two trials; low certainty evidence) following early initiation of KMC.
Conclusions and relevance
The review provides updated evidence on the effects of KMC on mortality and other critical outcomes in preterm and low birth weight infants. The findings suggest that KMC should preferably be initiated within 24 hours of birth and provided for at least 8 hours daily.
Keywords: public health, systematic review
WHAT IS ALREADY KNOWN ON THIS TOPIC
Kangaroo mother care (KMC) is a simple and cost-effective intervention that decreases neonatal mortality and the risk of infection in low birth weight infants.
The WHO recommends the initiation of KMC among low birth weight infants after clinical stabilisation.
WHAT THIS STUDY ADDS
Compared with conventional care, KMC initiated either in the hospital or at home reduces mortality during birth hospitalisation or 28 days of age and probably reduces severe infection until the latest follow-up among preterm and low birth weight infants.
KMC provided for at least 8 hours a day probably results in greater benefits than a shorter duration of KMC.
KMC initiated within 24 hours of birth reduces neonatal mortality and may reduce clinical sepsis until 28 days compared with later initiation.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
The results of this updated review will likely influence health providers to initiate KMC in all low birth weight and preterm infants managed in health facilities and at home. Efforts might be undertaken to initiate KMC within 24 hours of birth and to provide it for at least 8 hours a day.
Introduction
Prematurity (gestational age <37 weeks) and low birth weight (defined as <2500 g) are important causes of neonatal and infant mortality and long-term neurodevelopmental disability.1 Low- and middle-income countries (LMIC) have the highest burden of preterm and low birth weight infants. Kangaroo mother care (KMC) is a simple and cost-effective intervention that has been shown to reduce neonatal mortality and the risk of infection in low birth weight infants.2 The Cochrane review on KMC, published in 2016, included 21 studies involving 3042 infants and demonstrated a significant reduction in the risks of mortality and severe infection in low birth weight infants.3
New evidence from large multi-country and community-based randomised trials became available after the publication of the Cochrane review.4 5 A few of these trials examined the effect of early KMC, that is, KMC initiated within the first 24 hours of delivery.5 6 The timing of initiation of KMC is critical because KMC is usually commenced after the infant is stabilised. The WHO guidelines also recommend the initiation of KMC after clinical stabilisation. However, stabilisation of preterm/low birth weight neonates may take anything from hours to days, depending on the gestation, birth weight, and general condition at birth. The median age at initiation of KMC in the facility-based studies included in the Cochrane review varied from 3 to 24 days. KMC initiated after 3 days of life would not naturally reduce the risk of deaths occurring in the first 3 days, which account for about 62% of total neonatal deaths.7 The efficacy and safety of early initiation of KMC – within 24 hours of life – are unknown.
This systematic review aimed to compare the effects of (a) KMC with conventional care and (b) early initiation, that is, KMC within 24 hours of age, with late initiation of KMC on neonatal and infant mortality and severe morbidities among low birth weight and preterm infants. This review would provide critical evidence for policymakers and other stakeholders and may help to formulate clinical practice guidelines.
Methods
Inclusion and exclusion criteria
Our review included individually-randomised and cluster-randomised trials that compared KMC with conventional care or early initiation (ie, in the first 24 hours after birth) of KMC with late-initiated KMC among low birth weight and preterm infants, irrespective of the duration of KMC, infant stability at enrolment, study setting, and breastfeeding patterns. Trials reported as only abstracts were included if sufficient information on study methods was available to assess the eligibility and the risk of bias. We excluded quasi-randomised and crossover trials, studies evaluating KMC among term infants or those with birthweight >2500 g, and studies assessing KMC on only physiological parameters, pain scores, maternal mental health, infant colic, or during neonatal transport or as a part of a package of interventions.
Search strategy
We systematically reviewed the relevant publications by searching the electronic databases of MEDLINE (1966 to March 2022) via PubMed® and OVID, Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library, Issue 1 to March 2022), EMBASE (1988 to March 2022), CINAHL (1981 to March 2022), and the databases PsycINFO, AMED, EMCARE, BNI from inception until March 2022. We used the search terms “kangaroo care,” “kangaroo mother care,” “skin-to-skin care,” and “neonates or infants” in the search strategy. The search was initially conducted until March 2021 (for the presentation of review findings to the WHO Guideline Development Group of the guidelines on the care of low birth weight infants); the search was then updated to March 2022. The search strategy, search results, and the definitions used in the review are provided in online supplemental file 1. We also searched the databases of clinical trials and reference lists of retrieved articles for eligible studies.
bmjgh-2022-010728supp001.pdf (343.5KB, pdf)
Outcomes
The primary outcome was mortality during birth hospitalisation or by day 28 of life. Other outcomes were mortality by 6–12 months of age, severe infections, infant growth, neurodevelopment, hypothermia, length of hospital stay, readmission to hospital, and exclusive breastfeeding at discharge and at one and 6 months of age.
Data extraction
The two review authors (SS and MJS) extracted data using a standardised and pre-tested data abstraction form. The data included study characteristics, sample size, details of KMC initiation, duration, breastfeeding, time of hospital discharge, study setting (hospital or community), outcomes including neonatal mortality, hypothermia, sepsis, rates of exclusive breastfeeding, and weight gain. Discrepancies, if any, were resolved by mutual discussion between the reviewers.
Quality assessment and statistical analysis
The review authors independently evaluated the quality of studies using Cochrane’s Risk of Bias-1 tool, extracted data, and synthesised the effect estimates – relative risks (RR) or mean difference (MD) – using RevMan version 5.4 (The Cochrane Collaboration, 2020) or Stata 15.1 (StataCorp, College Station, TX, USA). The RR and 95% confidence intervals (CI) were calculated based on the extracted frequencies and denominators. Results were pooled using fixed-effect meta-analyses using the Mantel-Haenszel method. The heterogeneity of the pooled studies was assessed using the test of homogeneity of study-specific effect sizes and the I2 statistic, in addition to visual confirmation from forest plots. If substantial heterogeneity was detected, the reasons for heterogeneity were explored. If there was no critical clinical or methodological heterogeneity among the studies, we pooled their results using the random-effects model. We evaluated the likelihood of potential publication bias using funnel plots.
We used the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach8 to assess the quality of evidence for critical outcomes such as mortality at discharge, severe infection/sepsis at the latest follow-up, weight gain, exclusive breastfeeding, and neurodevelopmental outcomes. Evidence from randomised controlled trials was considered high quality; still, it could be downgraded by one or two levels for serious and very serious limitations, respectively, based on the risk of bias, imprecision, inconsistency, indirectness of study results, and publication bias. The review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and was registered in PROSPERO (CRD42021240336).
Planned subgroup analyses
For the comparison of KMC vs conventional care, we performed subgroup analyses according to different gestational and birth weight categories and by median duration KMC in hours (<8 hours, 8–16 hours, and >16 hours); time of initiation of KMC – early (≤24 hours of life) vs late initiation; stable vs unstable neonates; health facility vs community settings; and countries (high income vs LMIC settings).
Patient and public involvement
The study is a systematic review of the existing literature on the efficacy of KMC in preterm and low birth weight infants. No subjects were enrolled in the review. Therefore, parents, parent advisors, or the public were not involved in developing the research question and outcome measures.
Role of the funding source
The WHO, Geneva, funded the review. The WHO staff helped finalise the protocol and the manuscript; they had no role in the literature search, data extraction, or data analysis. The corresponding author had the final responsibility for the decision to submit for publication.
Results
Of the 3458 records identified from the database and bibliographic searches, 314–6 9–35 studies enrolling 15 559 infants were included in the review (figure 1); 25 studies were conducted in LMIC (two from multiple countries5 14 while seven were conducted in high-income countries12 20 24 26 29 30 34 (Appendix). Twenty-seven studies compared KMC with conventional care, while four compared early with late initiation of KMC.5 6 24 25 KMC was initiated in the health facility in 29 studies and at home (community) in two trials.4 11 While the sample sizes of earlier hospital-based studies ranged from 28 to 777, the most recent facility-based study – WHO iKMC study5 – had a sample size of 3211. Of the two community-based studies, one trial had enrolled around 8400 infants.4 Only six studies included infants with birthweight <1500 g.12 13 19 28 30 34 Figure 2 depicts the risk of bias in the included studies in specific domains. Many studies had an unclear or high risk of selection bias (due to a lack of information on allocation concealment) and detection bias (because the outcomes assessors were not masked to the intervention group).
Figure 1.
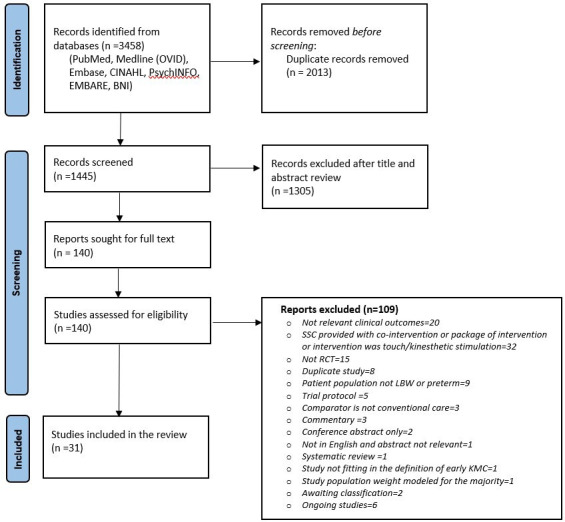
Flow chart of search results (adapted from PRISMA 2009 flow diagram).
Figure 2.
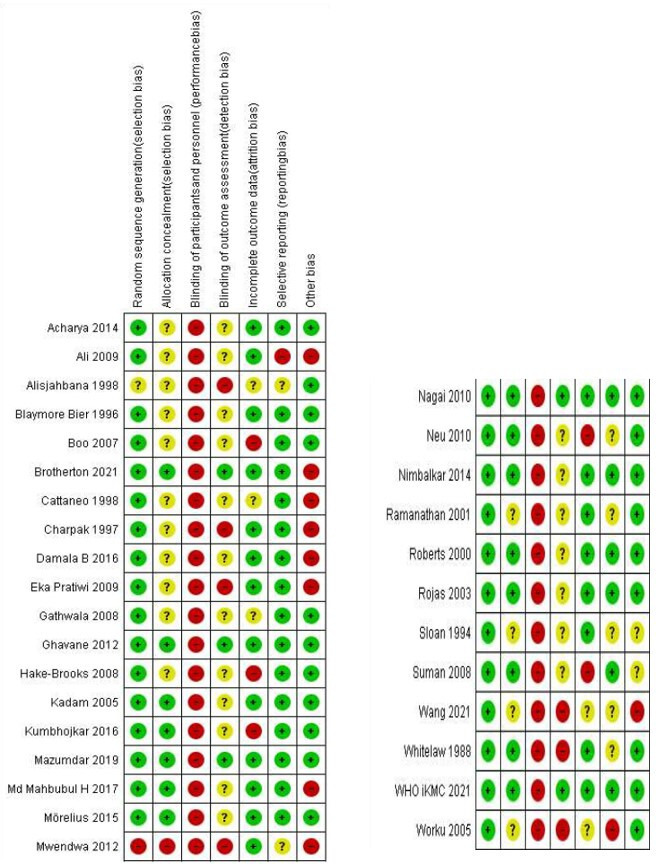
Risk of bias in included studies. Green circle indicates low-risk, red indicates high-risk and yellow, unclear-risk of bias.
KMC versus conventional newborn care
The comparison included 27 studies that enrolled 11 956 infants. The characteristics of included studies are provided in table 1. All but one study enrolled infants after stabilisation (variably defined in different studies as cardiorespiratory stability, off oxygen or any form of respiratory support, or off intravenous fluids). KMC was started within 24 hours after birth in two studies, between 1 and 7 days in 10 studies, and after 7 days in 12 studies (3 studies did not report the time of initiation). The duration of KMC was <8 hours in 9 studies, 8–16 hours in 9 studies, and >16 hours in 4 studies (5 studies did not report the duration).
Table 1.
KMC vs conventional newborn care – characteristics of included studies
| S. no | Author, Year | Country | Stabilisation status | Intervention group | Control group | Age at initiation of KMC (days) |
KMC duration (hours per day*) |
Last follow-up | Schema for follow-up |
| 1. | Ali et al 10 | India | Stable | KMC in facility | Conventional warmer or cots | 4.7 | 6.3±1.5 (range 4 to 12) | 6 months corrected age | Weekly until 40 weeks’ postmenstrual age, fortnightly until 3 months corrected age, and monthly until 6 months corrected age |
| 2. | Alisjahbana et al 11 | Indonesia | Stable | KMC in community | Conventional home care | – | – | 4 weeks after discharge | Weekly for 4 weeks after discharge |
| 3. | Archarya et al 9 | Nepal | Stable | KMC in facility | Conventional warmer or cots | – | 6 | In-hospital | Not reported |
| 4. | Bier et al 12 | USA | Stable | KMC in facility | Conventional clothed | 29 | – | 6 months after hospital discharge | At 1, 3, and 6 months after hospital discharge |
| 5. | Boo et al 13 | Malaysia | Stable | KMC in facility | Conventional NICU | 25 | 1 | In-hospital | Not reported |
| 6. | Cattaneo et a 14 | Ethiopia | Stable | KMC in facility | Conventional open cribs, incubator or warmer | 10 | 20 | 30 days postnatal age | Four times: at 3, 10, 20, and 30 days, and as usually scheduled at each hospital afterwards |
| 7. | Charpak et al 15 | Columbia | Stable | KMC in facility | Conventional incubator | 4 | 24 | 1 year and 20 years for a subset of enrolled subjected | At least once a week until 40 weeks’postmenstrual age; then monthly up to 3 months' corrected age, every 6 weeks until at least 6 months' corrected age, and every third month until 12 months' corrected age |
| 8. | Bhavana et al 16 | India | Stable | KMC in facility | Conventional warmer or cots | – | 13–14 | Until 2.5 kg weight | After discharge, babies were followed-up twice a week for the first week and weekly until babies reached 2.5 kg |
| 9. | Pratiwi et al 17 | Indonesia | Stable | KMC in facility | Conventional warmer or cots | 1 | 10.0±1.8 | In-hospital | – |
| 10. | Gathwala et al 18 | India | Stable | KMC in facility | Conventional warmer or cots | 1.7 | 10.2±1.5 | 3 months of age | Weekly until 3 months of age |
| 11. | Ghavane et al 19 | India | Stable | KMC in facility | Conventional warmer or cots | 14 | 8 | 40 weeks postmenstrual age | Weekly until 40 weeks' postmenstrual age |
| 12. | Hake-Brooks et al 20 | USA | Stable | KMC in facility | Conventional warmer or cots | 1 | – | Follow-up done telephonically at 6 weeks and 3 months and by an interview in the clinic at 6, 12, and 18 months | |
| 13. | Kadam et al 21 | India | Stable | KMC in facility | Conventional warmer or cots | 3.2 | 9.8±3.7 | In-hospital | – |
| 14. | Kumbhojkar et al 22 | India | Stable | KMC in facility | Conventional warmer or cots | 3 | 11.5 | 40 weeks’ PMA or weight of 2500 g | Weekly until 40 weeks' postmenstrual age in preterm infants, or until a weight of 2500 g was reached |
| 15. | Mazumder et al 4 | India | Stable | KMC in community | Conventional home care | 32.7 hours | 11.5 vs 0.2 | 28 days postnatal age and 1 year follow-up | Mothers and infants in the intervention group were visited at home (days 1–3, 5, 7, 10, 14, 21, and 28) to support kangaroo mother care |
| 16. | Md Mahbubul et al
23 |
Bangladesh | Stable | KMC in facility | Conventional warmer or cots | 1.8 | – | In-hospital | Outcome measures ended before hospital discharge |
| 17. | Mwendwa 2012 |
Kenya | Stable | KMC in facility | Conventional warmer or cots | 10 | 8 | In-hospital | None. Only in-hospital outcomes reported |
| 18. | Neu and Robinson26 | USA | Stable | KMC in facility | Conventional | 15 | 1 | 6 months of age | Twice a week for 2 weeks, followed by weekly visits for 6 months |
| 19. | Nimbalkar et al 27 | India | Stable | KMC in facility | Conventional | Immediate | 17.0±0.3 | In-hospital | – |
| 20. | Ramanathan et al 282001 | India | Stable | KMC in facility | Conventional warmer or cots | 11.8 | 4 | In-hospital | – |
| 21. | Roberts et al 29 | Australia | Stable | KMC in facility | Conventional clothed | 31 | 1.6±0.9 | 6 months of age | At 6 weeks after discharge or at 3 months of age, whichever was later, and at 6 months |
| 22. | Rojas et al 30 | USA | Stable | KMC in facility | Conventional incubator | 19 | 1.3±0.7 | In-hospital | – |
| 23. | Sloan et al 31 | Ecuador | Stable | KMC in facility | Conventional incubator or crib | 13 | – | 6 months of age | At 1, 1.5, 2, 3, 4, 5, and 6 months of age |
| 24. | Suman et al 32 | India | Stable | KMC in facility | Conventional warmer or cots | 3.7 | 13.5 | 40 weeks post menstrual age or until weight of 2500 g | Weekly until 40 weeks' postmenstrual age in preterm infants, or until a weight of 2500 g in term SGA infants |
| 25. | Wang et al 33 | China | Stable | KMC in facility | Conventional | 2.5 | 6 months corrected age | Follow-up appointments in outpatient setting at 40 weeks, 3 months, and 6 months CA | |
| 26. | Whitelaw et al 34 | UK | Stable | KMC in facility | Conventional incubator or crib | 16 | 0.6 (0 to 1.5) |
12 months of age | At 6, 9, and 12 months of age |
| 27. | Worku et al 35 | Ethiopia | Unstable | KMC in facility | Conventional incubator or crib | 10 | Continuous | Until hospital discharge | – |
*mean (± SD) duration of KMC in KMC group
CA, corrected age; KMC, Kangaroo mother care; NICU, give details; SGA, small for gesational age.
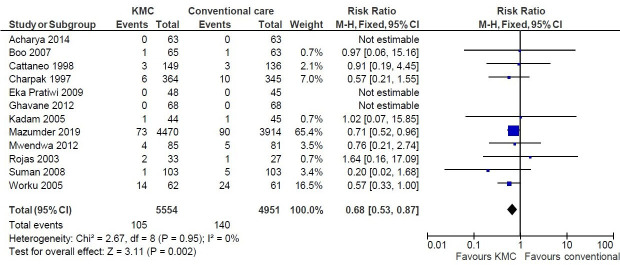
Pooled analysis revealed a 32% reduction in mortality during birth hospitalisation or by 28 days after birth or 40 weeks of postmenstrual age (risk ratio (RR) 0.68; 95% CI (CI) 0.53 to 0.86; I2=0%; 12 studies; 10 505 infants; fixed-effect model; high certainty evidence; figure 3). The funnel plot did not show any evidence of a potential publication bias (online supplemental efigure 1). The benefits of KMC in the primary outcome of mortality during birth hospitalisation or by 28 days of age were observed in all subgroup analyses: gestational age category (≤34 weeks vs. >34 weeks), weight at birth/enrolment (≤2000 g vs. >2000 g), setting (health facility vs. community) and time of initiation of KMC (within 24 hours after birth vs later); the benefits were greater when the daily duration of KMC was at least 8 hours per day than with shorter duration (online supplemental efigure 2). Pooled analysis of 4 studies that had reported mortality by 6 months of age showed a 25% reduction in mortality (RR 0.75; 95% CI 0.62 to 0.92; fixed-effect model; high certainty of evidence).
Figure 3.
Kangaroo mother care (KMC) vs. conventional care –Risk ratio of mortality during birth hospitalisation or 28 days of life.
bmjgh-2022-010728supp002.pdf (335.9KB, pdf)
KMC probably results in a 15% reduction in severe infection/sepsis at the latest follow-up (RR 0.85, 95% CI 0.79 to 0.92; 9 trials, 9847 infants; moderate certainty evidence) and 68% reduction in the risk of hypothermia (RR 0.32, 95% CI 0.19 to 0.53; 11 trials, 1169 infants; moderate-certainty evidence). Infants in the KMC arm had a higher gain in anthropometric parameters, namely weight gain per day and length and head circumference gain per week (table 2). The exclusive breastfeeding rates were higher at discharge/28 days of life (RR 1.48, 95% CI 1.44 to 1.52; 9 trials, 9983 infants, very low certainty evidence), but the evidence was uncertain; also, there was no difference in breastfeeding rates at 1–3 months of age. KMC may result in little to no difference in the Griffith Quotients or the risk of cerebral palsy at 12 months of corrected age36 or IQ scores at 20 years of age.
Table 2.
KMC vs conventional newborn care: key outcomes
| Outcome and subgroup | Studies | N | Pooled relative risk (95% CI) |
| Mortality during birth hospitalisation or by 28 days of age or 40 weeks’ PMA | 12 | 10 505 | 0.68 (0.53 to 0.87) |
| Health facilities | 11 | 2121 | 0.62 (0.41 to 0.94) |
| Community settings | 1 | 8384 | 0.71 (0.52 to 0.96) |
| Mortality 6 months follow-up | 4 | 8031 | 0.75 [(.62 to 0.92) |
| Health facilities | 3 | 1047 | 0.74 (0.44 to 1.23) |
| Community settings | 1 | 6984 | 0.76 (0.61 to 0.95) |
| Severe infection*/sepsis at latest follow-up | 9 | 9847 | 0.85 (0.79, 0.92) |
| Health facilities | 8 | 1463 | 0.50 (0.36, 0.69) |
| Community settings* | 1 | 8384 | 0.89 (0.82, 0.97) |
| Hypothermia by discharge or by 40–41 weeks’ PMA or 28 days follow-up | 11 | 1169 | 0.32 (0.19, 0.53) |
| Exclusive breastfeeding at discharge or at 28 days of age | 9 | 9983 | 1.48 (1.44, 1.52) |
| Health facilities | 8 | 1599 | 1.18 (1.10, 1.27) |
| Community settings | 1 | 8384 | 1.54 (1.49, 1.59) |
| Exclusive breastfeeding at 1 to 3 months' follow-up | 7 | 8139 | 1.39 (0.99, 1.97) |
| Weight gain at latest follow-up (g/d) | 11 | 1198 | MD 4.08 (2.30, 5.86) |
| Length gain at latest follow-up (cm/week) | 3 | 377 | MD 0.21 (0.03, 0.38) |
| Head circumference gain at latest follow-up (cm/week) | 5 | 652 | MD 0.18 (0.09, 0.27) |
| Cerebral palsy at 12 months' corrected age | 1 | 588 | 0.65 (0.21, 2.02) |
| Severe disability at 20 years | 1 | 264 | 0.34 (0.09, 1.24) |
| Neurodevelopmental outcomes at 12 months of age using BSID-III | |||
| Cognitive score | 1 | 516 | MD 0.21 (-1.84, 2.26) |
| Language score | 1 | 516 | MD −0.91 (-2.46, 0.64) |
| Motor score | 1 | 516 | MD −0.85 (-2.65, 0.95) |
*In community settings, the diagnosis of sepsis or severe infection was based on the WHO definition of possible serious bacterial infection.
BSID-III, Bayley Scales of Infant Development-III; MD, mean difference; PMA, postmenstrual age.
Early-initiated versus late-initiated KMC
The evidence was derived from 4 trials that enrolled 3603 infants. One study was done in a high-come country (Sweden), 2 studies were done in low-income countries (Madagascar and The Gambia), and 1 study was multi-country conducted in LMICs (Ghana, India, Malawi, Nigeria, and Tanzania). All trials were conducted in health facilities. Infant stability at enrolment, duration of KMC achieved, and time of initiation of KMC in the included studies are provided in table 3. In two studies (Mörelius et al 24 and WHO iKMC)5 KMC was initiated in the delivery room. Brotherton et al 6 enrolled moderately unstable infants in the early KMC arm and stable infants after >24 hour of admission in the control arm. Nagai et al began KMC within 24 hours of birth in the early arm and after 24 hours in the late arm.
Table 3.
Early vs late-initiated KMC – characteristics of included studies
| S. no | Author, Year | Inclusion criteria | Exclusion criteria | Intervention: early KMC as planned/ as achieved |
Control: late KMC as planned/as achieved |
| 1 | WHO iKMC 2021 | All infants with birth weight of 1.0 to 1.799 kg, regardless of gestation, type of delivery, or singleton or twin status (irrespective of clinical stability). | Infants who were unable to breathe spontaneously by 1 hour or who had a major congenital malformation |
Immediately after birth; Median initiation time of 1.3 hours after birth |
KMC began after the neonate recovered from preterm birth complications and was at least 24 hours old; Median initiation time 53.6 hours after birth |
| 2 | Brotherton 2021 | Birth weight <2000 g and age 1–24 hours | Stable and severely unstable neonates were excluded. Triplets, major congenital malformations, severe jaundice, seizures, and lack of study bed were the other exclusion criteria | KMC initiated <24 hours after admission; Median initiation time 13.6 hours |
KMC once stable at >24 hours after admission; Median initiation time 104.5 hours |
| 3 | Mörelius 201524 | Vaginally born singleton preterm infants (32–35 weeks’ gestation) | Infants with congenital malformations and severely unstable infants | Continuous skin-to-skin contact, beginning in the delivery room; Median initiation time not provided |
KMC began in the NICU; On day 2, both groups were practicing KMC |
| 4 | Nagai 201025 | Birth weight <2500 g, age <24 hours, no serious malformations, and relatively stable clinical condition | Apnea and intravenous infusion | KMC begun soon as possible, within 24 hours post-birth; Median initiation time 19 hours (IQR 13.00–23.00) |
KMC began after complete stabilisation (generally after 24 hours post-birth) Median initiation time 28.5 hours (IQR 25–40) |
KMC, Kangaroo mother care; NICU, neonatal intensive care unit.
Early-initiated KMC showed a reduction in the risks of mortality by 28 days of age (RR 0.78, 95% CI 0.66 to 0.92; 3 trials, 3533 infants, high certainty evidence; online supplemental efigure 3) and hypothermia by discharge or at 28 days (RR 0.74, 95% CI 0.61 to 0.90; high certainty evidence). It probably reduces the risk of clinical sepsis until 28-day follow-up (RR 0.85, 95% CI 0.76 to 0.96; table 4; low certainty evidence) and improves exclusive breastfeeding at discharge (RR 1.1.2, 95% CI 1.10 to 1.19; moderate certainty evidence). There was also a decrease in the length of hospital stay (table 4).
Table 4.
Early vs late-initiated KMC – critical outcomes
| Outcome | Studies | Number of participants | Pooled relative risk (95% CI) |
| Mortality by 28 days of life | 3 | 3533 | 0.78 (0.66 to 0.92) |
| Mortality at 6 months of age | 1 | 72 | 1.0 (0.15 to 6.72) |
| Sepsis until 28 days | 2 | 3415 | 0.85 (0.76 to 0.96) |
| Exclusive breastfeeding at discharge | 3 | 3464 | 1.12 (1.07 to 1.16) |
| Exclusive breastfeeding at 28 days of age | 3 | 2841 | 1.01 (0.98 to 1.04) |
| Hypothermia at discharge or by 28 days | 3 | 3553 | 0.74 (0.61 to 0.90) |
| Weight gain at 28-day follow-up (g/d) | 1 | 204 | MD −2.20 (−5.26 to 0.86) |
| Nosocomial sepsis | |||
| Clinical sepsis | 2 | 3415 | 0.85 (0.75 to 0.95) |
| Culture-positive sepsis | 1 | 279 | 1.53 (0.44 to 5.31) |
| Re-admission to hospital at 4 weeks of age | 1 | 73 | 1.95 (0.18 to 20.5) |
| Length of hospital stay (days) | 3 | 3498 | −0.30 (−0.31 to −0.29) |
MD, mean difference.
On subgroup analysis, there was evidence of a reduction in 28-day mortality for infants with GA ≤34 weeks and BW ≤2000, but there was little data for infants >34 weeks and weighing >2000 g at birth. The mortality reduced with a duration of KMC of at least >16 hours per day, with little data for daily KMC duration of <8 hours or 8–16 hours per day.
Quality of the evidence
For the comparison of KMC vs conventional newborn care, the certainty of the evidence was assessed as high for neonatal mortality and moderate for sepsis/severe infection and hypothermia (table 5). For early vs late-initiated KMC, the certainty of the evidence was high for neonatal mortality and hypothermia, moderate for exclusive breastfeeding at discharge, and low for nosocomial clinical sepsis (table 6). A few outcomes, such as weight gain, breastfeeding, and length of hospital stay, showed a high degree of heterogeneity, partly due to clinical and methodological heterogeneity among the studies (varied definitions of hypothermia and time points of assessment; different methods of breastfeeding assessment, etc.).
Table 5.
Summary of findings – KMC vs conventional newborn care
| Summary of findings table 1. Kangaroo mother care compared with conventional newborn care in preterm or low birth weight infants | |||||
|
Patient or population
: preterm or low birth weight infants
Setting : Hospital or community/home Intervention : Kangaroo mother care Comparison : Conventional newborn care | |||||
| Outcomes |
№ of participants
(studies) Follow-up |
Certainty of the evidence
(GRADE) |
Relative effect
(95% CI) |
Anticipated absolute effects | |
| Risk with conventional neonatal care | Risk difference with Kangaroo mother care | ||||
| Mortality during birth hospitalisation or 28 days of age or 40 weeks’ PMA | 10 505 (12 RCTs) |
⊕⊕⊕⊕
HIGH* |
RR 0.68
(0.53 to 0.87) |
28 per 1000 | nine fewer per 1000 (from 13 fewer to four fewer) |
| Severe infection or sepsis until latest follow-up |
9847
(9 RCTs) |
⊕⊕⊕Ο
MODERATE† |
RR 0.85
(0.79 to 0.92) |
215 per 1000 | 32 fewer per 1000 (45 fewer to 17 fewer) |
| Hypothermia by discharge or 40 weeks’ PMA or 28 days after birth |
1169
(11 RCTs) |
⊕⊕⊕Ο
MODERATEठ|
RR 0.32
(0.19 to 0.53) |
257 per 1000 |
175 fewer per 1000
(from 208 fewer to 121 fewer) |
| Weight gain at latest follow-up (g/d) |
1198
(11 RCTs) |
⊕⊕ΟΟ
LOW§¶ |
– | Mean weight gain at latest follow-up was 17 grams/day | MD 4.08 g/day higher (2.3 higher to 5.86 higher) |
| Exclusive breastfeeding at discharge or at 40 to 41 weeks' PMA or at 28 days of age |
9983
(9 RCTs) |
⊕ΟΟΟ
VERY LOW §** |
RR 1.48
(1.44 to 1.52) |
546 per 1000 |
262 more per 1000
(from 240 more to 284 more) |
| Neurodevelopmental outcome at 12 months' using BSID-III (stable LBW infants) |
516
(1 RCT) |
⊕⊕ΟΟ
LOW††‡‡§§ |
Post-hoc equivalence testing using two one-sided tests of equivalence (TOST) demonstrated that composite scores for cognitive, language, and motor domains at 12 months among the study arms were statistically equivalent | ||
|
GRADE Working Group grades of evidence
High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
*All 12 studies were at risk of performance bias because the participants/parents/clinical team were not masked to intervention. In all except Mazumder’s study (weightage 65.4%), the outcome assessors were also not masked to the intervention. However, mortality being a ‘hard’ outcome, we did not downgrade for either performance or outcome assessment bias. Six studies, Acharya, Boo, Cattaneo, Charpak, Eka Prawiti, and Worku, contributing to 26.3% weightage in the pooled analysis, are at unclear risk of allocation concealment. Four studies - Boo, Cattaneo, Suman, and Worku - were also at risk of attrition bias due to incomplete outcome data. But they together account for only 22.7% weightage in the pooled analysis. The risk of bias was therefore not downgraded to ‘serious’ risk. One study Mwendwa 2012 was at high risk of bias for random sequence generation and allocation concealment. It contributed to 3.5% weightage. The total number of neonates enrolled is quite large (~10 500) – therefore, the evidence was not downgraded for imprecision.
†All studies were at moderate or severe risk of bias as participants and outcome assessors were not masked to intervention and outcomes. Only in Mazumder et al the assessors were masked to the intervention. Though culture-positive sepsis is a ‘hard’ outcome, the largest study Mazumder 2019 that accounted for 91% of weightage defined sepsis based on WHO PSBI signs and not on culture positivity; five studies (4.7% weightage; Ali 2009, Eka Prawiti 2009, Kadam 2005, Kumbhojkar 2016, Suman 2008) did not define sepsis in their studies; another study (Rojas 2008; weightage 9.2%) defined it as both clinical and culture-positive sepsis, and only Boo 2007 defined it as culture-positive sepsis. Therefore, the risk of bias was downgraded to ‘serious’ risk. Allocation concealment was unclear in four studies (Charpak 1997, Ali 2009 Boo, 2007 Eka Pratiwi 2009) that together contribute to 4.8% weightage.
‡All studies were at high risk of outcome ascertainment bias as participants and outcome assessors were not masked to intervention and outcomes. However, weight gain is considered a ‘hard’ outcome. Therefore, we did not downgrade for the risk of bias. Seven studies (Acharya, Ali, Bier, Boo, Cattaneo, Gathwala, and Ramanathan accounting for 64% weightage) were at risk of allocation concealment bias. Therefore, the evidence was downgraded for ‘serious’ risk of bias.
§Substantial heterogeneity >50%.
¶All studies were at high risk of outcome ascertainment bias as participants and outcome assessors were not masked to intervention and outcomes. However, weight gain is considered a ‘hard’ outcome. Therefore, we did not downgrade for the risk of bias. Seven studies (Acharya, Ali, Bier, Boo, Cattaneo, Gathwala, and Ramanathan accounting for 64% weightage) were at risk of allocation concealment bias. Therefore, the evidence was downgraded for ‘serious’ risk of bias.
**All studies were at high risk of outcome ascertainment bias because the participants and outcome assessors were not masked to the intervention and the outcome was not a ‘hard’ outcome. Allocation concealment was unclear in six studies that accounted for 82% of weightage.
††95% CI overlap no effect (ie, CI includes RR of 1.0).
‡‡One study Charpak 1997 with moderate risk of bias (unclear allocation concealment; lack of blinding of participants/parents/clinical team and outcome assessors). The follow-up rate at 12–18 months was 80%. The characteristics of infants of KMC and conventional groups who completed follow-up were similar.
§§Single study.
MD, Mean difference; PMA, Postmenstrual age; RR, Risk ratio.
Table 6.
Summary of findings – early initiated KMC vs late-initiated KMC in preterm or low-birth weight infants
| Summary of findings table 2. Early initiated KMC compared with late initiated KMC in preterm or low birth weight infants | |||||
|
Patient or population: preterm or low birth weight infants
Setting: Hospital or community/home Intervention: Early initiated KMC (within 24 hours after birth) Comparison: late initiated KMC (more than 24 hours after birth) | |||||
| Outcomes |
№ of participants
(studies) Follow-up |
Certainty of the evidence
(GRADE) |
Relative effect
(95% CI) |
Anticipated absolute effects | |
| Risk with late initiated KMC | Risk difference with early initiated KMC | ||||
| Mortality by 28 days of age | 3693 (3 RCTs) | ⊕⊕⊕⊕ HIGH* |
RR 0.77 (0.66 to 0.91) |
156 per 1000 | 36 fewer per 1000 (53 fewer to 14 fewer) |
| Sepsis until 28 days | 3694 (2 RCTs) |
⊕⊕ΟΟ
LOW†‡ |
RR 0.85 (0.76 to 0.96) |
249 per 1000 | 37 fewer per 1000 (from 60 fewer to 10 fewer) |
| Exclusive breastfeeding - At discharge | 3464 (3 RCTs) | ⊕⊕⊕Ο MODERATE‡§ |
RR 1.12 (1.07 to 1.16) |
688 per 1000 | 83 more per 1000 (from 48 more to 110 more) |
| Exclusive breastfeeding at 28 days of age | 2841 (3 RCTs) | ⊕⊕⊕Ο MODERATE‡§¶ |
RR 1.01 (0.98 to 1.04) |
855 per 1000 | nine more per 1000 (from 17 fewer to 34 more) |
| Hypothermia at discharge or by 28 days | 3713 (4 RCTs) | ⊕⊕⊕⊕ HIGH** |
RR 0.74 (0.61 to 0.90) |
109 per 1000 | 28 fewer per 1000 (from 42 fewer to 11 fewer) |
| Weight gain at 28 day follow-up (g/d) | 204 (1 RCTs) |
⊕⊕ΟΟ
LOW††‡‡ |
– | Mean weight gain at 28 day follow-up was 12.5 g/day | MD 2.2 g/day lower (5.26 lower to 0.86 higher) |
*Though parents and the clinical team were not masked to the intervention, mortality was considered a 'hard' outcome, so the evidence was not downgraded.
†In both studies, the participants and clinicians were not masked to the intervention. Both diagnosed sepsis based on WHO’s PSBI definition and not by culture positivity. Though the outcome assessment was done by an independent team who was unaware of group allocation in the WHO iKMC study (accounting for 95% of weightage), the risk of performance bias by the clinical team and researchers in a subjective outcome like clinical sepsis or PSBI cannot be ruled out.
‡Significant heterogeneity >50%.
§In three studies, participants and the clinical team were masked. Assessment of exclusive or any breastfeeding is prone to bias. However, the outcome assessment in the WHO iKMC study, which contributed to the maximum weightage in the pooled analysis, was done by an independent team not involved in the intervention. The risk of performance bias – by the clinical team or researchers – in breastfeeding outcomes was considered low; hence, the evidence was not downgraded.
¶95% CI overlap no effect (ie, CI includes RR of 1.0), but they also exclude important benefits as well as important harm; so not downgraded.
**All three studies were at low risk of bias. Although parents and clinical team were not masked to the intervention, measurement of temperature is less prone to outcome assessment bias. Hence not downgraded.
††A single study that was prematurely terminated at 75% enrolment. We did not downgrade for lack of masking of caregivers or outcome assessors because weight measurement is an objective outcome.
‡‡95% CI overlaps no effect (ie, CI includes RR of 1.0).
MD, Mean difference; RR, Risk ratio.
Discussion
The systematic review showed that KMC reduces mortality during birth hospitalisation or by 28 days of age and probably reduces severe infection at the latest follow-up in preterm and low birth weight infants in health facilities and at home. KMC may result in a slight increment in growth parameters (weight and length) and exclusive breastfeeding rates at discharge. KMC may result in little to no difference in neurodevelopmental outcomes at 12 months compared with conventional care. Compared with delayed initiation (>24 hours) of KMC, early-initiated KMC (<24 hours) results in a 33% reduction in mortality by 28 days and a slight reduction in clinical sepsis by 28 days.
Three recent systematic reviews examined the effect of KMC compared with conventional care on infant clinical outcomes.3 37 38 The Cochrane review in 2016 found 21 studies enrolling 3042 low birth weight infants.3 Our systematic review used a similar search strategy and inclusion criteria and included studies until 2022. We found 10 newer studies that provided data on 12 517 additional infants with similar gestation and birth weight range. The Cochrane review reported a similar decrease in mortality at discharge or 40 weeks of postmenstrual age (RR 0.60, 95% CI 0.39 to 0.92; 8 trials, 1736 infants) and similar effects on infection, hypothermia, and anthropometry. However, the certainty of the evidence was graded as moderate to very low in the Cochrane review. The addition of information from 12,000-odd infants has improved the precision and certainty of the evidence of the critical outcomes in the current review. In 2020, a systematic review of 416 preterm neonates reported that KMC significantly reduced apneic events in preterm neonates.38 Another review in 2019 concluded that KMC had a significant positive impact on growth and breastfeeding rates in very low birth weight (VLBW) neonates.37
We investigated the effect of mean duration KMC in hours and prespecified three categories (<8 hours, 8–16 hours, and >16 hours). The effects on mortality were comparable in the >16 hour and 8–16 hour groups, but there was insufficient data in the <8 hours group. The Cochrane review (2016) explored the effects of the duration of KMC in three different categories; <2 hours and 6–15 hours, and >20 hours per day, and found benefits only when KMC was done for 20 hours or more. We found beneficial effects of KMC in prespecified subgroups of ≤2.0 kg and >2.0 kg and infants with gestational age ≤34 and >34 weeks at birth. The two community-based studies that enrolled infants at home also showed significant benefits on mortality. We found no additional trials – other than the study by Worku et al included in the Cochrane review – that compared KMC with conventional care in unstable infants.
Only one systematic review – the Cochrane review published in 2016 – has evaluated the effects of early vs late initiation of KMC in low birth weight infants. It also used a cut-off of 24 hours to define early initiation but found only one study of 73 relatively stable low birth weight infants.25 Our review included three additional studies that recruited 3530 preterm/low birth weight infants and found significant beneficial effects with early initiation of KMC.5 6 24
The results of our review have substantial implications for policymaking, particularly in LMIC. First, KMC should be provided to all low birth weight and preterm infants irrespective of the settings – both health facilities and at home. Second, given the probable dose-effect response, KMC should preferably be practiced for at least 8 hours a day for optimal benefits. Third, KMC should be initiated within the first 24 hours of life. Indeed, our findings have helped to make recommendations on KMC in the new WHO guidelines on the care of preterm and low birth weight neonates.39
The strengths of the current review include a comprehensive and systematic search of the literature with updated evidence to March 2022. Compared with the existing Cochrane reviews on KMC, our review identified additional studies that had enrolled almost 13 000 low birth weight infants, which resulted in high precision of estimates and improved the certainty of the evidence. The review also had some limitations. The included studies were not blinded, although outcome assessors were blinded in many studies. However, the risk of bias in the included studies was generally low, and the certainty of the evidence for the primary outcomes was moderate to high. Very low birth weight, extremely preterm neonates, and severely unstable neonates were often excluded from studies. More evidence is needed before extrapolating the study results in these high-risk groups.
To conclude, our findings support the practice of KMC for preterm and low birth weight infants as soon as possible after birth and for at least 8 hours a day. Future research should focus on overcoming barriers and facilitators to large-scale implementation of KMC in facility and community settings. Data on long-term neurodevelopmental outcomes are also needed.
Acknowledgments
We acknowledge the support and guidance provided by Dr. Rajiv Bahl, Dr. Karen Edmond, and Dr. Shuchita Gupta from the WHO, Geneva, in finalising the protocol and interpreting the results.
Footnotes
Handling editor: Seema Biswas
Contributors: Both authors, MJS and SS, contributed equally to protocol development, literature search, data extraction and analysis and interpretation. SS drafted the manuscript with inputs from MJS. Both authors reviewed and approved the final manuscript. MJS acts as the guarantor of the paper.
Funding: The World Health Organization. Grant number- not applicable.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. All data relevant to the study are included in the article or uploaded as supplementary information. Data are available upon reasonable request from the corresponding author.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Not applicable.
References
- 1. Milner KM, Neal EFG, Roberts G, et al. Long-Term neurodevelopmental outcome in high-risk newborns in resource-limited settings: a systematic review of the literature. Paediatr Int Child Health 2015;35:227–42. 10.1179/2046905515Y.0000000043 [DOI] [PubMed] [Google Scholar]
- 2. Boundy EO, Dastjerdi R, Spiegelman D, et al. Kangaroo mother care and neonatal outcomes: a meta-analysis. Pediatrics 2016;137:e20152238. 10.1542/peds.2015-2238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Conde-Agudelo A, Díaz-Rossello JL. Kangaroo mother care to reduce morbidity and mortality in low birthweight infants. Cochrane Database Syst Rev 2016;2016:CD002771. 10.1002/14651858.CD002771.pub4 [DOI] [PubMed] [Google Scholar]
- 4. Mazumder S, Taneja S, Dube B, et al. Effect of community-initiated kangaroo mother care on survival of infants with low birthweight: a randomised controlled trial. Lancet 2019;394:1724–36. 10.1016/S0140-6736(19)32223-8 [DOI] [PubMed] [Google Scholar]
- 5. WHO Immediate KMC Study Group, Arya S, Naburi H, et al. Immediate "kangaroo mother care'' and survival of infants with low birth weight. N Engl J Med 2021;384:2028–38. 10.1056/NEJMoa2026486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brotherton H, Gai A, Kebbeh B, et al. Impact of early kangaroo mother care versus standard care on survival of mild-moderately unstable neonates < 2000 Grams: a randomised controlled trial. EClinicalMedicine 2021;39:101050. 10.1016/j.eclinm.2021.101050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sankar MJ, Natarajan CK, Das RR, et al. When do newborns die? A systematic review of timing of overall and cause-specific neonatal deaths in developing countries. J Perinatol 2016;36:S1–11. 10.1038/jp.2016.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Balshem H, Helfand M, Schünemann HJ, et al. Grade guidelines: 3. rating the quality of evidence. J Clin Epidemiol 2011;64:401–6. 10.1016/j.jclinepi.2010.07.015 [DOI] [PubMed] [Google Scholar]
- 9. Acharya N, Singh RR, Bhatta NK, et al. Randomized control trial of kangaroo mother care in low birth weight babies at a tertiary level Hospital. J Nepal Paedtr Soc 2014;34:18–23. 10.3126/jnps.v34i1.8960 [DOI] [Google Scholar]
- 10. Ali SM, Sharma J, Sharma R, et al. Kangaroo mother care as compared to conventional care for low birth weight babies. Dicle Medical Journal 2009;36:155–60. [Google Scholar]
- 11. Alisjahbana A, Usman A, Lrawaty S, et al. Prevention of hypothermia of low birth infants using the kangaroo method. PI 1998;38:205. 10.14238/pi38.9-10.1998.205-14 [DOI] [Google Scholar]
- 12. Bier JA, Ferguson AE, Morales Y, et al. Comparison of skin-to-skin contact with standard contact in low-birth-weight infants who are breast-fed. Arch Pediatr Adolesc Med 1996;150:1265–9. 10.1001/archpedi.1996.02170370043006 [DOI] [PubMed] [Google Scholar]
- 13. Boo NY, Jamli FM. Short duration of skin-to-skin contact: effects on growth and breastfeeding. J Paediatr Child Health 2007;43:831–6. 10.1111/j.1440-1754.2007.01198.x [DOI] [PubMed] [Google Scholar]
- 14. Cattaneo A, Davanzo R, Worku B, et al. Kangaroo mother care for low birthweight infants: a randomized controlled trial in different settings. Acta Paediatr 1998;87:976–85. 10.1111/j.1651-2227.1998.tb01769.x [DOI] [PubMed] [Google Scholar]
- 15. Charpak N, Ruiz-Peláez JG, Figueroa de CZ, et al. Kangaroo mother versus traditional care for newborn infants. Pediatrics 1997;100:682–8. 10.1542/peds.100.4.682 [DOI] [PubMed] [Google Scholar]
- 16. Bhavana DrD, Lakshmi DBV, Sruthi DT, et al. Effect of kangaroo mother care in the management of low birth weight babies one year randomized controlled trial at NRI Hospital. Int J Pediatr Res 2016;3:546–52. 10.17511/ijpr.2016.i08.01 [DOI] [Google Scholar]
- 17. Pratiwi I, Soetjiningsih S, Kardana IM. Effect of kangaroo method on the risk of hypothermia and duration of birth weight regain in low birth weight infants: a randomized controlled trial. PI 2009;49:253. 10.14238/pi49.5.2009.253-8 [DOI] [Google Scholar]
- 18. Gathwala G, Singh B, Singh J. Effect of kangaroo mother care on physical growth, breastfeeding and its acceptability. Trop Doct 2010;40:199–202. 10.1258/td.2010.090513 [DOI] [PubMed] [Google Scholar]
- 19. Ghavane S, Murki S, Subramanian S, et al. Kangaroo mother care in kangaroo ward for improving the growth and breastfeeding outcomes when reaching term gestational age in very low birth weight infants. Acta Paediatr 2012;101:e545–9. 10.1111/apa.12023 [DOI] [PubMed] [Google Scholar]
- 20. Hake-Brooks SJ, Anderson GC. Kangaroo care and breastfeeding of mother-preterm infant dyads 0-18 months: a randomized, controlled trial. Neonatal Netw 2008;27:151–9. 10.1891/0730-0832.27.3.151 [DOI] [PubMed] [Google Scholar]
- 21. Kadam S, Binoy S, Kanbur W, et al. Feasibility of kangaroo mother care in Mumbai. Indian J Pediatr 2005;72:35–8. 10.1007/BF02760578 [DOI] [PubMed] [Google Scholar]
- 22. Kumbhojkar S, Mokase Y, Sarawade S. Kangaroo Mother Care (KMC): an alternative to conventional method of care for low birth weight babies. Int J Health Sci Res 2016;6:36. [Google Scholar]
- 23. Md Mahbubul H, Nishat J, Md Maksudur R. Effectiveness of KMC on success of breast feeding in preterm low birth weight neonate. Acad J Ped Neonatol 2017;3:555617. 10.19080/AJPN.2017.03.555617 [DOI] [Google Scholar]
- 24. Mörelius E, Örtenstrand A, Theodorsson E, et al. A randomised trial of continuous skin-to-skin contact after preterm birth and the effects on salivary cortisol, parental stress, depression, and breastfeeding. Early Hum Dev 2015;91:63–70. 10.1016/j.earlhumdev.2014.12.005 [DOI] [PubMed] [Google Scholar]
- 25. Nagai S, Andrianarimanana D, Rabesandratana N, et al. Earlier versus later continuous Kangaroo Mother Care (KMC) for stable low-birth-weight infants: a randomized controlled trial. Acta Paediatr 2010;99:827–35. 10.1111/j.1651-2227.2009.01676.x [DOI] [PubMed] [Google Scholar]
- 26. Neu M, Robinson J. Maternal holding of preterm infants during the early weeks after birth and dyad interaction at six months. J Obstet Gynecol Neonatal Nurs 2010;39:401–14. 10.1111/j.1552-6909.2010.01152.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nimbalkar SM, Patel VK, Patel DV, et al. Effect of early skin-to-skin contact following normal delivery on incidence of hypothermia in neonates more than 1800 G: randomized control trial. J Perinatol 2014;34:364–8. 10.1038/jp.2014.15 [DOI] [PubMed] [Google Scholar]
- 28. Ramanathan K, Paul VK, Deorari AK, et al. Kangaroo mother care in very low birth weight infants. Indian J Pediatr 2001;68:1019–23. 10.1007/BF02722345 [DOI] [PubMed] [Google Scholar]
- 29. Roberts KL, Paynter C, McEwan B. A comparison of kangaroo mother care and conventional cuddling care. Neonatal Netw 2000;19:31–5. 10.1891/0730-0832.19.4.31 [DOI] [PubMed] [Google Scholar]
- 30. Rojas MA, Kaplan M, Quevedo M, et al. Somatic growth of preterm infants during skin-to-skin care versus traditional holding: a randomized, controlled trial. J Dev Behav Pediatr 2003;24:163–8. 10.1097/00004703-200306000-00006 [DOI] [PubMed] [Google Scholar]
- 31. Sloan NL, Camacho LW, Rojas EP, et al. Kangaroo mother method: randomised controlled trial of an alternative method of care for stabilised low-birthweight infants. Maternidad Isidro Ayora Study Team. Lancet 1994;344:782–5. 10.1016/s0140-6736(94)92341-8 [DOI] [PubMed] [Google Scholar]
- 32. Suman RPN, Udani R, Nanavati R. Kangaroo mother care for low birth weight infants: a randomized controlled trial. Indian Pediatr 2008;45:17–23. [PubMed] [Google Scholar]
- 33. Wang Y, Zhao T, Zhang Y, et al. Positive effects of kangaroo mother care on long-term breastfeeding rates, growth, and neurodevelopment in preterm infants. Breastfeed Med 2021;16:282–91. 10.1089/bfm.2020.0358 [DOI] [PubMed] [Google Scholar]
- 34. Whitelaw A, Heisterkamp G, Sleath K, et al. Skin to skin contact for very low birthweight infants and their mothers. Arch Dis Child 1988;63:1377–81. 10.1136/adc.63.11.1377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Worku B, Kassie A. Kangaroo mother care: a randomized controlled trial on effectiveness of early kangaroo mother care for the low birthweight infants in Addis ababa, Ethiopia. J Trop Pediatr 2005;51:93–7. 10.1093/tropej/fmh085 [DOI] [PubMed] [Google Scholar]
- 36. Charpak N, Ruiz-Pelaez JG, Figueroa de C Z, et al. A randomized, controlled trial of kangaroo mother care: results of follow-up at 1 year of corrected age. Pediatrics 2001;108:1072–9. 10.1542/peds.108.5.1072 [DOI] [PubMed] [Google Scholar]
- 37. Sharma D, Farahbakhsh N, Sharma S, et al. Role of kangaroo mother care in growth and breast feeding rates in very low birth weight (VLBW) neonates: a systematic review. J Matern Fetal Neonatal Med 2019;32:129–42. 10.1080/14767058.2017.1304535 [DOI] [PubMed] [Google Scholar]
- 38. Montealegre-Pomar A, Bohorquez A, Charpak N. Systematic review and meta-analysis suggest that kangaroo position protects against apnoea of prematurity. Acta Paediatr 2020;109:1310–6. 10.1111/apa.15161 [DOI] [PubMed] [Google Scholar]
- 39. WHO . WHO recommendations for care of the preterm or low birthweight infant. Geneva: World Health Organization, 2022. Available: https://apps.who.int/iris/bitstream/handle/10665/363697/9789240058262-eng.pdf [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjgh-2022-010728supp001.pdf (343.5KB, pdf)
bmjgh-2022-010728supp002.pdf (335.9KB, pdf)
Data Availability Statement
Data are available upon reasonable request. All data relevant to the study are included in the article or uploaded as supplementary information. Data are available upon reasonable request from the corresponding author.