Abstract
Background
Subgroup analyses of randomized trials suggest the superiority of immune checkpoint inhibitor-based therapy over chemotherapy in patients with mismatch-repair deficient (dMMR) and/or microsatellite instability-high (MSI-high) advanced gastric or gastroesophageal junction adenocarcinoma. However, these subgroups are small and studies examining prognostic features within dMMR/MSI-high patients are lacking.
Methods
We conducted an international cohort study at tertiary cancer centers and collected baseline clinicopathologic features of patients with dMMR/MSI-high metastatic or unresectable gastric cancer treated with anti-programmed cell death protein-1 (PD-1)-based therapies. The adjusted HRs of variables significantly associated with overall survival (OS) were used to develop a prognostic score.
Results
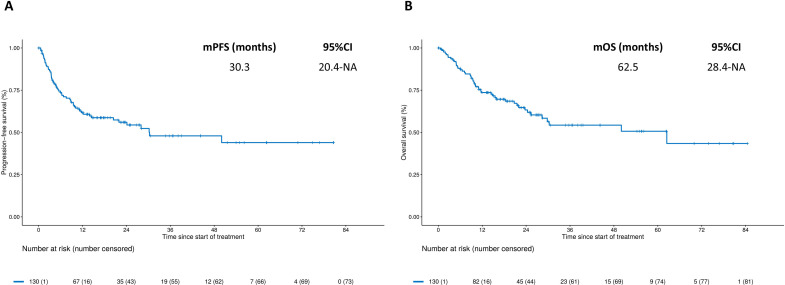
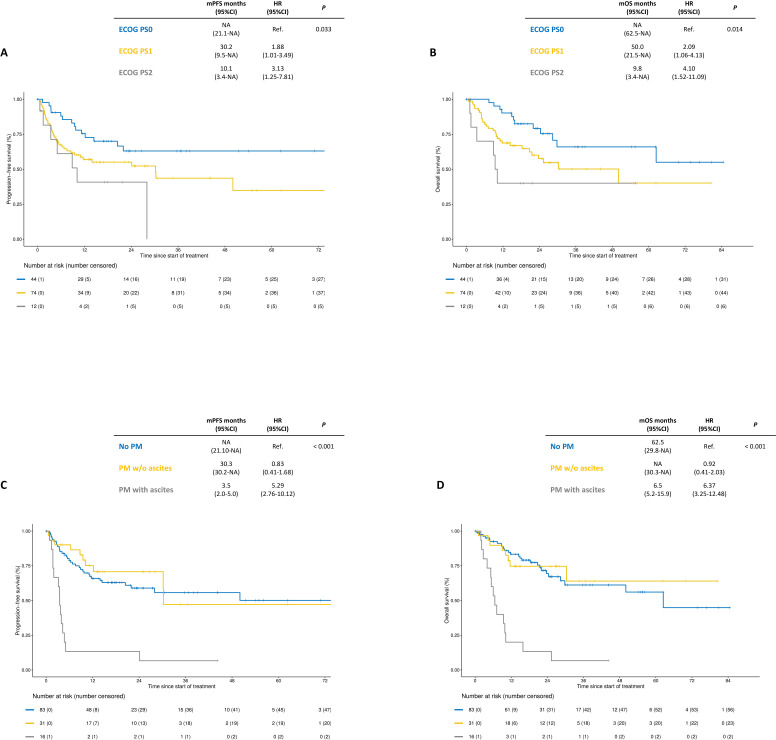
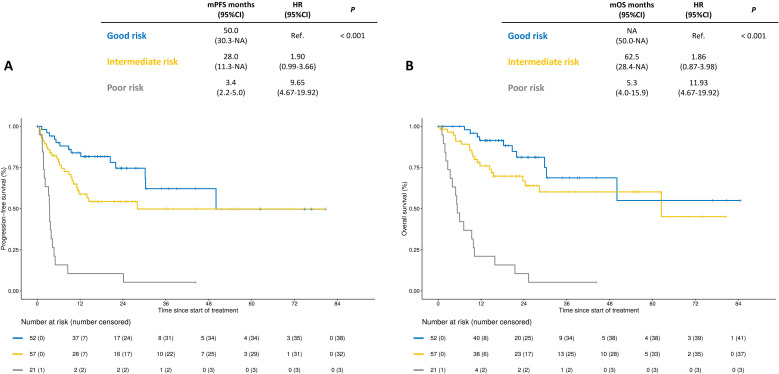
One hundred and thirty patients were included. At a median follow-up of 25.1 months, the median progression-free survival (PFS) was 30.3 months (95% CI: 20.4 to NA) and 2-year PFS rate was 56% (95% CI: 48% to 66%). Median OS was of 62.5 months (95% CI: 28.4 to NA) and 2-year OS rate was 63% (95% CI: 55% to 73%). Among the 103 Response Evaluation Criteria in Solid Tumors-evaluable patients, objective response rate was 66% and disease control rate 87% across lines of therapy. In the multivariable models, Eastern Cooperative Oncology Group Performance Status of 1 or 2, non-resected primary tumor, presence of bone metastases and malignant ascites were independently associated with poorer PFS and OS. These four clinical variables were used to build a three-category (ie, good, intermediate, and poor risk) prognostic score. Compared with patients with good risk, patients with intermediate risk score had numerically inferior PFS and OS (2-year PFS rate: 54.3% versus 74.5%, HR 1.90, 95% CI: 0.99 to 3.66; 2-year OS rate: 66.8% versus 81.2%, HR 1.86, 95% CI: 0.87 to 3.98), whereas patients with poor risk score had significantly inferior PFS and OS (2-year PFS rate: 10.6%, HR 9.65, 95% CI: 4.67 to 19.92; 2-year OS rate: 13.3%, HR 11.93, 95% CI: 5.42 to 26.23).
Conclusions
Overall outcomes with anti-PD-1-based therapies are favorable in MSI-high gastroesophageal adenocarcinomas. However, within this overall favorable subgroup a more accurate prognostication using baseline clinical characteristics might identify patients at higher risk of rapid disease progression who may deserve intensified immunotherapy combination strategies.
Keywords: immune checkpoint inhibitors, gastrointestinal neoplasms, programmed cell death 1 receptor, CTLA-4 antigen, tumor biomarkers
WHAT IS ALREADY KNOWN ON THIS TOPIC
Mismatch-repair deficient/microsatellite instability-high (dMMR/MSI-high) is rare in advanced gastric cancer (<5%). No dedicated trials with immunotherapy have been specifically conducted in this population so far; however, post hoc analyses of randomized trials confirmed dMMR/MSI-high as the strongest predictive biomarker for immunotherapy benefit.
WHAT THIS STUDY ADDS
This is the largest data set of patients with dMMR/MSI-high gastric cancer receiving immunotherapy and confirms the durable responses and efficacy of anti-programmed cell death protein-1 (PD-1)-based therapies in a real-world population. This cohort allowed us to explore for the first time the clinical prognostic biomarkers in this molecular subgroup and to build a risk score for prognostic stratification in terms of both progression-free and overall survival.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Poor Eastern Cooperative Oncology Group Performance status, non-resected primary tumor, presence of bone metastases/ascites are associated with worse outcomes on anti-PD-1-based therapies and might help to identify patients at higher risk of rapid disease progression and who could benefit from intensified anti-PD-1-based combination strategies with chemotherapy, anti-cytotoxic T-lymphocytes-associated protein 4 agents or new immune checkpoint inhibitors.
Introduction
Microsatellite instability-high (MSI-high) status is observed in about 9–10% of patients with gastric or gastroesophageal junction cancer (GC), with a decreasing prevalence to less than 5% in the advanced setting.1 2 MSI-high status is traced back to defects in the DNA mismatch repair (MMR) and accounts for specific biologic features including high tumor mutational burden (TMB) resulting from the accumulation of frameshifts and single nucleotide variants and enhanced immune response.3 Clinically, MMR deficient (dMMR)/MSI-high cancers are associated with particularly high response rates and durable benefit with immune checkpoint inhibitors (ICIs) and frontline use is supported by phase III evidence in metastatic colorectal cancer (mCRC).4–6 However, there are no prospective trials in advanced dMMR/MSI-high gastroesophageal adenocarcinomas, leading to heterogenous clinical practices and limited frontline access for patients. However, subgroup analyses from randomized clinical trials and meta-analyses showed excellent survival and long-term disease control with anti-programmed cell death protein-1 (PD-1)-based regimens compared with chemotherapy in patients with dMMR/MSI-high tumors.7–9 Therefore, dMMR/MSI-high status is currently regarded as the strongest predictor of the efficacy of ICIs.10 11 Objective response rates to anti-PD-1-based therapy are clinically meaningful and vary from 46% to 70% according to ICI treatment type (anti-cytotoxic T-lymphocytes-associated protein 4 (CTLA-4) combinations or anti-PD-1 plus or minus chemotherapy) and line of therapy.
Primary resistance is observed in up to 30% of dMMR/MSI-high patients in clinical trials but deeper understanding of features linked to resistance remains limited, partly due to very small available cohorts.12 13 Real-world data allow for exploring the impact of novel drugs in patients under-represented or excluded in clinical trials.14 To identify clinically relevant prognostic features within the overall favorable dMMR/MSI-high gastroesophageal population we assembled a large multinational cohort of patients with dMMR/MSI-high metastatic GC treated with anti-PD-1-based therapy in the real-world setting. We sought to build a prognostic risk score for identifying those patients at higher risk of early disease progression on PD-1 blockade potentially informing clinical trials evaluating intensified immunotherapy-based treatment strategies.
Patients and methods
Study population
Patients with dMMR/MSI-high locally advanced unresectable or metastatic GC treated with anti-PD-1-based therapy in any line were retrospectively retrieved from nine Academic Hospitals in European Union, USA and Asia. MMR and/or MSI status were locally assessed by means of immunohistochemistry, multiplex PCR and/or next generation sequencing as per standard institutional practices. Clinical and pathological baseline characteristics prior to ICI therapy were: age, sex, Eastern Cooperative Oncology Group (ECOG) Performance Status (PS; 1 versus 0 and 2 versus 0), primary tumor site (gastric versus gastroesophageal), histotype according to Lauren’s classification (diffuse versus intestinal and other/mixed versus intestinal), primary tumor resection (yes versus no), time-to-metastases (synchronous versus metachronous; synchronous metastatic disease was defined by diagnosis of metastases within 6 months from surgery or de novo diagnosis of metastatic or locally advanced unresectable disease), number of metastatic sites (>1 versus 1), metastatic sites, presence of malignant ascites (yes versus no), ICI treatment line (perioperative/adjuvant chemotherapy was considered as the first treatment line if disease relapse occurred within 6 months from its completion) and ICI type. Objective tumor response was assessed according to Response Evaluation Criteria in Solid Tumors (RECIST) v.1.1 in patients with measurable disease.15 Patients signed an informed consent to study participation.
Statistical analyses
Progression-free survival (PFS) was defined as the time from the beginning of the anti-PD-1-based treatment to the evidence of disease progression or death from any cause. Overall survival (OS) was defined as the time from the beginning of the anti-PD-1-based treatment to death from any cause or last follow-up. PFS and OS analyses were determined according to the Kaplan-Meier method. The Kaplan-Meier estimator and Cox proportional hazards regression were used for survival analysis using the survival, survminer, and survMisc packages (RStudio V.2022.12). Follow-up time was estimated using the reverse Kaplan-Meier method. In Cox proportional hazards regression models, all the covariates associated with PFS and OS in the univariable analyses with a p value<0.05 were included in the multivariable model. P values<0.05 were considered statistically significant. A prognostic score was built as previously reported.16 Briefly, the logs of the HR of the variables independently associated with OS obtained from the multivariable model for OS were used to derive weighting factors of a prognostic index. Coefficient estimates were ‘normalized’ by dividing by the smallest one and rounding the resulting ratios to the nearest integer value.
Results
Patient demographics
The final study population included 130 patients. Patient and disease characteristics are reported in table 1. One hundred and sixteen (89%) patients received anti-PD-1 monotherapy, whereas only 8 (6%) and 6 (5%) received combinations with chemotherapy or an anti-CTLA-4 agent, respectively. Thirty-two (25%) patients received ICIs in the first-line setting and 98 (75%) in later treatment lines.
Table 1.
Patient and disease characteristics in the overall population
| Characteristics | N (%) |
| Age (years) | |
| Median (IQR) | 68 (60–74) |
| <70 | 75 (58) |
| ≥70 | 55 (42) |
| Sex | |
| Female | 60 (46) |
| Male | 70 (54) |
| ECOG PS | |
| 0 | 44 (34) |
| 1 | 74 (57) |
| 2 | 12 (9) |
| Primary tumor site | |
| GEJ | 24 (19) |
| Gastric | 106 (81) |
| Histotype | |
| Intestinal | 51 (39) |
| Diffuse | 34 (26) |
| Mixed/other | 45 (35) |
| Primary tumor resection | |
| Yes | 62 (48) |
| No | 68 (52) |
| Time to metastases | |
| Synchronous | 99 (76) |
| Metachronous | 31 (24) |
| Metastatic sites (N) | |
| 1 | 59 (45) |
| >1 | 71 (55) |
| Liver metastases | |
| Yes | 29 (22) |
| No | 101 (78) |
| Lung metastases | |
| Yes | 18 (14) |
| No | 112 (86) |
| Lymph nodal metastases | |
| Yes | 108 (83) |
| No | 22 (17) |
| Bone metastases | |
| Yes | 4 (3) |
| No | 126 (97) |
| Peritoneal metastases | |
| No | 83 (64) |
| Yes without ascites | 31 (24) |
| Yes with ascites | 16 (12) |
| ICI treatment line | |
| 1st | 32 (25) |
| ≥2nd | 98 (75) |
| Anti-PD-1-based regimen | |
| Anti-PD-1 monotherapy | 116 (89) |
| Anti-PD-1 plus chemotherapy | 8 (6) |
| Anti-PD-1 plus anti-CTLA-4 | 6 (5) |
CTLA-4, cytotoxic T-lymphocytes-associated protein 4; ECOG PS, Eastern Cooperative Oncology Group Performance Status; GEJ, gastroesophageal junction; ICI, immune checkpoint inhibitors; PD-1, programmed cell death protein-1.
Efficacy and activity analyses
At a median follow-up of 25.1 months (IQR 15.9–44.1), 57 PFS events and 48 deaths were recorded. As shown in figure 1, the median PFS was 30.3 months (95% CI: 20.4 to NA), with a 2-year PFS estimate of 56% (95% CI: 48% to 66%); the median OS was 62.5 months (95% CI: 28.4 to NA), with a 2-year OS rate of 63% (95% CI: 55% to 73%). Kaplan-Meier curves for PFS and OS according to the specific anti-PD-1-based regimen are shown in online supplemental figure S1. Univariable and multivariable models for PFS and OS are shown in table 2. ECOG PS, resected primary tumor, presence of bone metastases and/or ascites were independently associated with both PFS and OS. Interestingly, patients with either ECOG PS 1 or PS 2 had significantly worse PFS and OS compared with those with ECOG PS 0 (figure 2A, B). The 2-year PFS rate for PS 1 and PS 2 versus PS 0 was 55.0% and 40.7% versus 63.0% (HR 1.88, 95% CI: 1.01 to 3.49 and 3.13, 95% CI: 1.25 to 7.81). The 2-year OS rate for PS 1 and PS 2 was 57.6% and 40.0% versus 79.1%, respectively (HR 2.09, 95% CI: 1.06 to 4.13 and 4.10, 95% CI: 1.52 to 11.09). Patients with peritoneal metastases and no ascites had similar PFS and OS compared with those without peritoneal metastases (2-year PFS rate: 70.7% versus 60.0%, HR 0.83, 95% CI: 0.41 to 1.68; 2-year OS rate: 74.7% versus 69.4%, HR 0.92, 95% CI: 0.41 to 2.03), whereas poorer PFS and OS were restricted to patients with peritoneal metastases and ascites (2-year PFS rate: 13.3%, HR 5.29, 95% CI: 2.76 to 10.12; 2-year OS rate: 13.3%, HR 6.37, 95% CI: 3.25 to 12.48; figure 2C, D). RECIST response data were available for 103/130 (79%) patients. We observed 23 (22%) complete responses and 45 (44%) partial responses, with 22 (17%) reported as stable disease. Therefore, the objective response rate (ORR) was 67% and the disease control rate was 87% among evaluable patients (online supplemental figure S2).
Figure 1.
Kaplan-Meier curves for progression-free survival (panel A) and overall survival (panel B) in the overall study population. mOS, median overall survival; mPFS, median progression-free survival.
Table 2.
Cox proportional hazards regression models for progression-free survival and overall survival in the study population
| Characteristics | PFS | OS | ||||||
| Univariable models | Multivariable model | Univariable models | Multivariable model | |||||
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| Sex | 0.939 | 0.965 | ||||||
| Female | Ref. | Ref. | ||||||
| Male | 0.97 (0.58 to 1.65) | 0.99 (0.56 to 1.74) | ||||||
| Age (years) | 0.584 | 0.632 | ||||||
| <70 | Ref. | Ref. | ||||||
| ≥ 70 | 1.16 (0.69 to 1.95) | 1.15 (0.65 to 2.04) | ||||||
| ECOG PS | 0.033 | 0.015 | 0.014 | 0.016 | ||||
| 0 | Ref. | Ref. | Ref. | Ref. | ||||
| 1 | 1.88 (1.01 to 3.49) | 1.25 (0.64 to 2.42) | 2.09 (1.06 to 4.13) | 1.40 (0.68 to 2.89) | ||||
| 2 | 3.13 (1.25 to 7.81) | 3.91 (1.44 to 10.65) | 4.10 (1.52 to 11.09) | 5.96 (1.90 to 18.70) | ||||
| Primary tumor site | 0.183 | 0.461 | ||||||
| Gastroesophageal junction | Ref. | Ref. | ||||||
| Gastric | 1.71 (0.77 to 3.79) | 1.35 (0.60 to 3.02) | ||||||
| Histotype | 0.886 | 0.813 | ||||||
| Intestinal | Ref. | Ref. | ||||||
| Diffuse | 0.86 (0.45 to 1.64) | 0.80 (0.39 to 1.63) | ||||||
| Mixed/other | 0.89 (0.48 to 1.64) | 0.98 (0.50 to 1.92) | ||||||
| Synchronous disease | 0.521 | 0.392 | ||||||
| No | Ref. | Ref. | ||||||
| Yes | 1.22 (0.66 to 2.28) | 1.36 (0.67 to 2.72) | ||||||
| Primary tumor resection | 0.004 | 0.011 | 0.009 | 0.033 | ||||
| No | Ref. | Ref. | Ref. | Ref. | ||||
| Yes | 0.45 (0.26 to 0.78) | 0.43 (0.22 to 0.83) | 0.44 (0.24 to 0.81) | 0.44 (0.21 to 0.94) | ||||
| Metastatic sites (N) | 0.003 | 0.060 | 0.013 | 0.443 | ||||
| 1 | Ref. | Ref. | Ref. | Ref. | ||||
| >1 | 2.26 (1.30 to 3.93) | 1.89 (0.97 to 3.65) | 2.14 (1.17 to 3.91) | 1.34 (0.63 to 2.87) | ||||
| Liver metastases | 0.180 | 0.229 | ||||||
| No | Ref. | Ref. | ||||||
| Yes | 1.50 (0.83 to 2.70) | 1.50 (0.77 to 2.89) | ||||||
| Lung metastases | 0.174 | 0.881 | ||||||
| No | Ref. | Ref. | ||||||
| Yes | 1.58 (0.82 to 3.05) | 0.94 (0.42 to 2.10) | ||||||
| Lymph nodal metastases | 0.935 | 0.547 | ||||||
| No | Ref. | Ref. | ||||||
| Yes | 1.03 (0.50 to 2.10) | 0.79 (0.37 to 1.70) | ||||||
| Bone metastases | 0.004 | 0.036 | 0.001 | 0.013 | ||||
| No | Ref. | Ref. | Ref. | Ref. | ||||
| Yes | 4.45 (1.60 to 12.52) | 3.23 (1.07 to 9.69) | 5.61 (1.98 to 15.89) | 4.70 (1.50 to 14.77) | ||||
| Peritoneal disease | <0.001 | 0.022 | <0.001 | 0.003 | ||||
| Negative | Ref. | Ref. | Ref. | Ref. | ||||
| Positive without ascites | 0.83 (0.41 to 1.68) | 0.77 (0.36 to 1.65) | 0.92 (0.41 to 2.03) | 0.97 (0.41 to 2.29) | ||||
| Positive with ascites | 5.29 (2.76 to 10.12) | 2.60 (1.18 to 5.70) | 6.37 (3.25 to 12.48) | 4.03 (1.72 to 9.44) | ||||
| ICI- treatment line | 0.908 | 0.571 | ||||||
| 1st | Ref. | Ref. | ||||||
| ≥2nd | 0.96 (0.53 to 1.77) | 0.83 (0.44 to 1.57) | ||||||
Bold marks statistically significant values and significant correlation with survivals.
ECOG PS, Eastern Cooperative Oncology Group Performance Status; ICI, immune checkpoint inhibitor; OS, overall survival; PFS, progression-free survival.
Figure 2.
Kaplan-Meier curves for progression-free survival and overall survival according to ECOG PS (panels A and B) and to the presence of peritoneal metastases with or without ascites (panels C and D). ECOG PS, Eastern Cooperative Oncology Group Performance Status; mOS, median overall survival; mPFS, median progression-free survival; PM, peritoneal metastases.
jitc-2023-007104supp002.pdf (1.4MB, pdf)
Development of the prognostic score
The prognostic score and the points assigned to each variable to calculate the individual score are reported in table 3, whereas the details on the coefficients used to build it are listed in online supplemental table S1. Patients were divided in the following three categories: 40% into the good, 44% into the intermediate and 16% into the poor risk group. Compared with patients with good risk, patients with intermediate score had numerically inferior PFS and OS (2-year PFS rate: 54.3% versus 74.5%, HR 1.90, 95% CI: 0.99 to 3.66; 2-year OS rate: 66.8% versus 81.2%, HR 1.86, 95% CI: 0.87 to 3.98), whereas patients with poor risk scoring had significantly inferior PFS and OS (2-year PFS rate: 10.6%, HR 9.65, 95% CI: 4.67 to 19.92; 2-year OS rate: 13.3%, HR 11.93, 95% CI: 5.42 to 26.23; figure 3). The ORR was lower in patients with poor risk disease (21%) compared with patients with intermediate (68%) and good risk (82%; p value<0.001).
Table 3.
Prognostic score with variables independently associated with overall survival
| Characteristics | Points assigned |
| ECOG PS0 | 0 |
| ECOG PS1 | 1 |
| ECOG PS2 | 5 |
| Resected T | 0 |
| Unresected T | 2 |
| No bone metastases | 0 |
| Bone metastases | 4 |
| No ascites | 0 |
| Ascites | 4 |
| Total points | Scoring system |
| 0–1 | Good risk |
| 2–5 | Intermediate risk |
| >5 | Poor risk |
ECOG PS, Eastern Cooperative Oncology Group Performance Status; T, primary tumor.
Figure 3.
Kaplan-Meier curves for progression-free survival (panel A) and overall survival (panel B) according to the prognostic score classified as low, intermediate or high risk. mOS, median overall survival; mPFS, median progression-free survival.
Discussion
In recent first-line trials in patients with metastatic GC, the addition of an anti-PD-1 agent to doublet chemotherapy improved the survival outcomes over chemotherapy alone, but the benefit was mostly restricted to patients with high programmed death-ligand 1 (PD-L1) expression.7 17 While the US Food and Drug Administration approved upfront nivolumab plus chemotherapy regardless of PD-L1 expression, the European Medicines Agency restricted its label to the subgroup with PD-L1 combined positive score (CPS)≥5.18 In advanced GC MMR/MSI status is the strongest independent predictor of benefit from anti-PD-1 therapies, with an OS HR of 0.34 versus chemotherapy in dMMR/MSI-high subgroup compared with 0.85 in proficient MMR or microsatellite stable one.11 In a subsequent meta-analysis, dMMR/MSI-high status outperformed PD-L1 CPS as a predictor of OS benefit.10 However, despite the tissue-agnostic approval of pembrolizumab in pretreated patients with dMMR/MSI-high solid tumors, no specific label for the upfront use of anti-PD-1-based therapy has been granted by the major Regulatory Agencies based on MMR/MSI testing alone. As a matter of fact, a non-negligible proportion of patients with dMMR/MSI-high tumors might have low CPS, even though the efficacy of ICIs is independent from PD-L1 expression in this patient population.12 19 20
Here we report the largest available cohort of dMMR/MSI-high advanced GC and provide further evidence of the effectiveness of anti-PD-1-based therapy in this patient subgroup. The activity and efficacy of ICIs in this real-world population are consistent with post hoc analyses of clinical trials, despite patient heterogeneity with 75% of our patients being previously treated with chemotherapy for metastatic disease. The high ORR and the plateau of the survival curves (with about half of patients being event-free at the 2-year time point) highlight the durable efficacy of ICIs in a real-world population including patients who are usually excluded from clinical trials, mostly because of their poor life expectancy.21 Despite overall favorable outcomes with anti-PD-1 therapies, the ORR among dMMR/MSI-high subgroup remain in the 40–60% range suggesting a sizeable proportion has some mechanism of intrinsic resistance. Small data sets have suggested that MMR heterogeneity and TMB within MSI-high disease may be able to further risk stratify patients, but overall these data are derived from small cohorts.12 22 Herein, we identified four clinical variables to inform prognosis in dMMR/MSI-high GC with statistical significance in the multivariable model: ECOG PS, resection of the primary tumor, presence of bone metastases and ascites. Worse ECOG PS—especially ECOG PS 2—was strongly associated with inferior outcomes, in line with our real-world data in patients with dMMR/MSI-high mCRC receiving ICIs.23 Similarly, the poor prognostic role of bone metastases or malignant effusions has been previously shown in dMMR/MSI-high mCRC and other tumor types.24–27 Intriguingly, an immune suppressive microenvironment has been described in bone metastases or ascites because of the upregulation of immune checkpoints (eg, T-cell immunoglobulin and mucin domain - TIM, V-domain immunoglobulin suppressor of T cell activation - VISTA), the activation of the Transforming growth factor beta (TGFβ) pathway and the increase of monocytes or M2 macrophages, potentially impairing the efficacy of PD-1 blockade.28 29 Finally, the negative prognostic role of a non-resected primary tumor may be explained by the risk of local complications and the high burden of symptoms.30 In the attempt to better predict survival in our patients’ population, we built a three-category prognostic risk score by means of the four aforementioned readily available variables. Notably, almost all patients with poor risk scoring had died within 1 year from ICIs start. Therefore, patients with adverse prognostic features may be at high risk of rapid disease progression and may benefit from intensified ICIs-based combination strategies over single-agent PD-1 blockade. In fact, dual CTLA-4/PD-1 blockade or chemo-immunotherapy may induce more rapid and deeper tumor responses compared with anti-PD-1 monotherapy and may be useful options in patients with high tumor burden. Interestingly, in the small subset of patients with dMMR/MSI-high GC who were enrolled in the CheckMate-649 trial, ipilimumab–nivolumab combination was associated with excellent ORR and OS compared with chemotherapy.7 Therefore, despite the potential clinical use of our prognostic classifier being interpreted with caution since it is not validated to drive treatment choices, intensified anti-PD-1-based treatments may be offered according to increasing risk of treatment failure (online supplemental graphical abstract). Accordingly, in our real-world data set of patients with dMMR/MSI-high mCRC, anti-CTLA-4/anti-PD-1 combination seemed to improve the survival over anti-PD-(L)−1 monotherapy especially in patients with adverse prognostic features, including poor ECOG PS related to high disease burden, high systemic inflammation indexes and malignant ascites.23 24 31 Randomized trials comparing single-agent anti-PD-(L)−1 with combinations with anti-CTLA-4 (NCT04008030; NCT04895722) or with chemotherapy and bevacizumab (NCT02997228) are still ongoing in patients with dMMR/MSI-high mCRC. These studies should be carried out also in patients with dMMR/MSI-high advanced GC, as they potentially allow to explore the efficacy of intensified treatments in key clinical subgroups of interest. Future randomized trials may allow to validate the prognostic or even the predictive role of key clinical factors or molecular biomarkers such as TMB and specific gene expression signatures.12 13
jitc-2023-007104supp001.pdf (382.8KB, pdf)
Importantly, the presence of poor prognostic features resulting in a high-risk scoring could also reflect a more advanced and aggressive disease. Therefore, considering the chance to achieve long-term benefit with ICIs, patients with dMMR/MSI-high advanced GC should be exposed to anti-PD-1-based regimens as early as possible during the disease course and before the predicted life expectancy may become too unsatisfactory. This study has several limitations. The heterogeneity of the treatment line and regimen may not fully fit the current practice of using anti-PD-1 plus chemotherapy as an upfront strategy. Second, since all patients received ICIs, the investigated clinical factors remain prognostic and their potential predictive power should be properly investigated by means of subgroup analyses of randomized clinical trials. Third, the risk score was not validated because of the lack of adequate external cohorts of patients with this rare molecular profile. Then, we acknowledge the potential importance of building a prognostic nomogram in patients with dMMR/MSI-high advanced GC. However, a high number of patients and events are needed to build complex prognostic classifiers such as nomograms; therefore, given the rarity of MSI-high in gastric cancer and the relatively limited sample size in our cohort, the development of a prognostic nomogram may lead to imprecise prognostic stratification, with clear risk of not increasing the discriminative ability as compared with our simpler prognostic tool. In conclusion, the efficacy of ICIs is confirmed in a large real-world population of patients with dMMR/MSI-high advanced GC. An improved prognostication may help to identify patients with risk of rapid disease progression and who may benefit from novel ICI-based combination strategies.
Footnotes
Twitter: @nasca_vincenzo, @klempnersam, @cowzerdarren, @FilippoPietran4
Contributors: Conception and design: GR, FP. Supervision: FP. Fundings: FP. Materials: FP, RCo, SJK, SM, JC, LF, MS, SL, TA, KS. Data collection: YA, RC, LP, VN, RCe, JC, WFB, FG, MA, PM, MS, AK, VZ, DC, VG, TA. Data analysis and interpretation: All the authors. Writer: GR, VN, FP. Responsible for the overall content: GR, FP
Funding: This study was supported by AIRC IG 23624, to FP.
Competing interests: RC has received personal fees from AstraZeneca, Bristol-Myers Squibb, Exeliom Biosciences, Enterome Bioscience, MSD Oncology, Mylan Medical, Pierre Fabre, Servier and non-financial support from Amgen, Bristol-Myers Squibb, Mylan Medical and Servier outside the submitted work. SJK reports consulting/advisory role with Astellas, Merck, BMS, Servier, Sanofi-Aventis, Novartis, Mersana, Exact Sciences, Natera, Coherus Biosciences, Daiichi-Sankyo, AstraZeneca, Pfizer, and Eli Lilly. SJK reports equity in Turning Point Therapeutics, and Nuvalent Therapeutics. SBM received consulting fees from Natera, Bicara, Novartis, Basilea, Elevation Oncology, and Daiichi Sankyo. LF reported receiving personal honoraria as invited speaker from Incyte, BMS, Lilly; research funding (to Institution) from MSD, BMS, AstraZeneca, Incyte, BeiGene, Astellas, Daiichi Sankyo, Roche; participation in advisory board for MSD, AstraZeneca, Incyte, Taiho, Servier, Daiichi Sankyo, Lilly. AK reports receiving honoraria (lecture fee) from Lilly, Bristol-Myers Squibb, Ono Pharmaceutical, Merck Pharmaceutical, Taiho Pharmaceutical, Daiichi Sankyo; receiving personal fees for advisory roles from Merck Pharmaceutical, Zymeworks; and receiving research funding from AstraZeneca, outside the submitted work. SL reports roles as consultant or advisor for Amgen, AstraZeneca, Bristol-Myers Squibb, Daiichi-Sankyo, Incyte, Lilly, Merck Serono, MSD, Servier, and reports research funding from Amgen, Astellas, AstraZeneca, Bayer, BMS Daichii Sankyo, Hutchinson, Incyte, Merck Serono, Mirati, MSD, Pfizer, Roche. She is part of speakers’ bureau of Amgen, Bristol-Myers Squibb, GlaxoSmithKline, Lilly, Merck Serono, Pierre Fabre, Roche, Servier. KS reports receiving personal fees for advisory roles from Lilly, Bristol-Myers Squibb, Takeda, Pfizer, Ono Pharmaceutical, Merck Pharmaceutical, Taiho Pharmaceutical, Astellas, Novartis, AbbVie, GlaxoSmithKline, Daiichi Sankyo, Amgen, Boehringer Ingelheim, Guardant Health Japan, and Janssen; receiving honoraria (lecture fee) from Takeda, Bristol-Myers Squibb and Janssen; and receiving research funding from Astellas, Ono Pharmaceutical, Daiichi Sankyo, Taiho Pharmaceutical, Chugai, Merck Pharmaceutical, Medi Science, Eisai and Amgen, outside the submitted work. TA reports attending advisory board meetings and receiving consulting fees from Aptitude Health, AstraZeneca, Astellas, Bristol-Myers Squibb, Gritstone Oncology, GamaMabs Pharma Sa, Gilead, GlaxoSmithKline, Merck & Co. Inc., Nordic Oncology, Pierre Fabre, Seagen, Servier and Transgène; honoraria from AstraZeneca, Bristol-Myers Squibb, GlaxoSmithKline, Merck & Co. Inc., Pierre Fabre, Roche, Sanofi Seagen and Servier; and support for meetings from Merck & Co. Inc. and Servier. FP reported receiving grants from BMS, Incyte, and AstraZeneca and personal fees from BMS, MSD, AstraZeneca, Amgen, Merck Serono, Eli Lilly & Company, Pierre Fabre, Servier, Bayer, and Organon outside the submitted work. JC received grants or contracts from Merck and Brooklyn Immunotherapeutics; consulting fees from Lilly, Merck, AstraZeneca, Foundation Medicine, Daiichi Sankyo, Amgen, Bristol-Myers Squibb, Astellas, Turning Point Therapeutics, Silverback Therapeutics, Novartis, Coherus Biosciences, Geneos, Roche, Guardant Health; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Merck, Bristol-Myers Squibb and disclose Participation on a Data Safety Monitoring Board or Advisory Board of Yiviva and Daiichi-Sankyo; disclose other financial or non-financial interests (employment) from Amgen. No other competing interests to disclose.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s).
Ethics approval
The study was approved by the Fondazione IRCCS Istituto Nazionale dei Tumori di Milano Institutional Review Board (INT 117/15) and was conducted in accordance with the ethical principles for medical research involving human subjects adopted in the Declaration of Helsinki.
References
- 1. Hause RJ, Pritchard CC, Shendure J, et al. Classification and characterization of Microsatellite instability across 18 cancer types. Nat Med 2016;22:1342–50. 10.1038/nm.4191 [DOI] [PubMed] [Google Scholar]
- 2. Pietrantonio F, Miceli R, Raimondi A, et al. Individual patient data meta-analysis of the value of Microsatellite instability as a biomarker in gastric cancer. J Clin Oncol 2019;37:3392–400. 10.1200/JCO.19.01124 [DOI] [PubMed] [Google Scholar]
- 3. Germano G, Amirouchene-Angelozzi N, Rospo G, et al. The clinical impact of the Genomic landscape of mismatch repair–deficient cancers. Cancer Discov 2018;8:1518–28. 10.1158/2159-8290.CD-18-0150 [DOI] [PubMed] [Google Scholar]
- 4. Cervantes A, Adam R, Roselló S, et al. Metastatic colorectal cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol 2023;34:10–32. 10.1016/j.annonc.2022.10.003 [DOI] [PubMed] [Google Scholar]
- 5. Diaz LA, Shiu K-K, Kim T-W, et al. Pembrolizumab versus chemotherapy for Microsatellite instability-high or mismatch repair-deficient metastatic colorectal cancer (KEYNOTE-177): final analysis of a randomised, open-label, phase 3 study. Lancet Oncol 2022;23:659–70. 10.1016/S1470-2045(22)00197-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lenz H-J, Van Cutsem E, Luisa Limon M, et al. First-line Nivolumab plus low-dose Ipilimumab for Microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer: the phase II Checkmate 142 study. J Clin Oncol 2022;40:161–70. 10.1200/JCO.21.01015 [DOI] [PubMed] [Google Scholar]
- 7. Shitara K, Ajani JA, Moehler M, et al. Nivolumab plus chemotherapy or Ipilimumab in Gastro-Oesophageal cancer. Nature 2022;603:942–8. 10.1038/s41586-022-04508-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chao J, Fuchs CS, Shitara K, et al. Assessment of Pembrolizumab therapy for the treatment of Microsatellite instability–high gastric or gastroesophageal junction cancer among patients in the KEYNOTE-059, KEYNOTE-061, and KEYNOTE-062 clinical trials. JAMA Oncol 2021;7:895. 10.1001/jamaoncol.2021.0275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Maio M, Ascierto PA, Manzyuk L, et al. Pembrolizumab in Microsatellite instability high or mismatch repair deficient cancers: updated analysis from the phase II KEYNOTE-158 study. Ann Oncol 2022;33:929–38. 10.1016/j.annonc.2022.05.519 [DOI] [PubMed] [Google Scholar]
- 10. Yoon HH, Jin Z, Kour O, et al. Association of PD-L1 expression and other variables with benefit from immune Checkpoint inhibition in advanced gastroesophageal cancer: systematic review and meta-analysis of 17 phase 3 randomized clinical trials. JAMA Oncol 2022;8:1456–65. 10.1001/jamaoncol.2022.3707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pietrantonio F, Randon G, Di Bartolomeo M, et al. Predictive role of Microsatellite instability for PD-1 blockade in patients with advanced gastric cancer: a meta-analysis of randomized clinical trials. ESMO Open 2021;6:100036. 10.1016/j.esmoop.2020.100036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kwon M, An M, Klempner SJ, et al. Determinants of response and intrinsic resistance to PD-1 blockade in Microsatellite instability–high gastric cancer. Cancer Discov 2021;11:2168–85. 10.1158/2159-8290.CD-21-0219 [DOI] [PubMed] [Google Scholar]
- 13. Chen Y, Jia K, Sun Y, et al. Predicting response to Immunotherapy in gastric cancer via multi-dimensional analyses of the tumour immune Microenvironment. Nat Commun 2022;13:4851. 10.1038/s41467-022-32570-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Penberthy LT, Rivera DR, Lund JL, et al. An overview of real-world data sources for oncology and considerations for research. CA Cancer J Clin 2022;72:287–300. 10.3322/caac.21714 [DOI] [PubMed] [Google Scholar]
- 15. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47. 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 16. Loupakis F, Intini R, Cremolini C, et al. A validated Prognostic Classifier for V600EBRAF-Mutated metastatic colorectal cancer: the 'BRAF Becool' study. Eur J Cancer 2019;118:121–30. 10.1016/j.ejca.2019.06.008 [DOI] [PubMed] [Google Scholar]
- 17. Rha SY, Wyrwicz LS, Weber PEY, et al. Vp1-2023: Pembrolizumab (Pembro) plus chemotherapy (Chemo) as first-line therapy for advanced Her2-negative gastric or gastroesophageal junction (G/GEJ) cancer: phase III KEYNOTE-859 study. Ann Oncol 2023;34:319–20. 10.1016/j.annonc.2023.01.006 [DOI] [Google Scholar]
- 18. Zhao JJ, Yap DWT, Chan YH, et al. Low programmed death-ligand 1–expressing subgroup outcomes of first-line immune Checkpoint inhibitors in gastric or Esophageal adenocarcinoma. J Clin Oncol 2022;40:392–402. 10.1200/JCO.21.01862 [DOI] [PubMed] [Google Scholar]
- 19. Mishima S, Kawazoe A, Nakamura Y, et al. Clinicopathological and molecular features of responders to Nivolumab for patients with advanced gastric cancer. J Immunother Cancer 2019;7:24. 10.1186/s40425-019-0514-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Al-Batran S-E, Lorenzen S, Thuss-Patience PC, et al. Surgical and pathological outcome, and pathological regression, in patients receiving perioperative Atezolizumab in combination with FLOT chemotherapy versus FLOT alone for Resectable Esophagogastric adenocarcinoma: interim results from DANTE, a randomized, multicenter, phase IIb trial of the FLOT-AIO German gastric cancer group and Swiss SAKK. JCO 2022;40:4003. 10.1200/JCO.2022.40.16_suppl.4003 [DOI] [Google Scholar]
- 21. Pietrantonio F, Loupakis F, Randon G, et al. Efficacy and safety of immune Checkpoint inhibitors in patients with Microsatellite Instability‐High End‐Stage cancers and poor performance status related to high disease burden. Oncologist 2020;25:803–9. 10.1634/theoncologist.2020-0014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Loupakis F, Maddalena G, Depetris I, et al. Treatment with Checkpoint inhibitors in a metastatic colorectal cancer patient with molecular and immunohistochemical heterogeneity in MSI/dMMR status. J Immunother Cancer 2019;7:297. 10.1186/s40425-019-0788-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mazzoli G, Cohen R, Lonardi S, et al. Prognostic impact of performance status on the outcomes of immune Checkpoint inhibition strategies in patients with dMMR/MSI-H metastatic colorectal cancer. Eur J Cancer 2022;172:171–81. 10.1016/j.ejca.2022.05.044 [DOI] [PubMed] [Google Scholar]
- 24. Fucà G, Cohen R, Lonardi S, et al. Ascites and resistance to immune Checkpoint inhibition in dMMR/MSI-H metastatic colorectal and gastric cancers. J Immunother Cancer 2022;10:e004001. 10.1136/jitc-2021-004001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Landi L, D’Incà F, Gelibter A, et al. Bone metastases and Immunotherapy in patients with advanced non-small-cell lung cancer. J Immunotherapy Cancer 2019;7:316. 10.1186/s40425-019-0793-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Epaillard N, Benitez JC, Gorria T, et al. Pleural effusion is a negative Prognostic factor for Immunotherapy in patients with non-small cell lung cancer (NSCLC): the Pluie study. Lung Cancer 2021;155:114–9. 10.1016/j.lungcan.2021.03.015 [DOI] [PubMed] [Google Scholar]
- 27. Murthy P, Ekeke CN, Russell KL, et al. Making cold malignant pleural effusions hot: driving novel Immunotherapies. Oncoimmunology 2019;8:e1554969. 10.1080/2162402X.2018.1554969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chow A, Schad S, Green MD, et al. Tim-4+ cavity-resident Macrophages impair anti-tumor Cd8+ T cell immunity. Cancer Cell 2021;39:973–988. 10.1016/j.ccell.2021.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang R, Song S, Harada K, et al. Multiplex profiling of peritoneal metastases from gastric adenocarcinoma identified novel targets and molecular subtypes that predict treatment response. Gut 2020;69:18–31. 10.1136/gutjnl-2018-318070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pietrantonio F, Miceli R, Rimassa L, et al. Estimating 12-week death probability in patients with refractory metastatic colorectal cancer: the colon life Nomogram. Ann Oncol 2017;28:555–61. 10.1093/annonc/mdw627 [DOI] [PubMed] [Google Scholar]
- 31. Corti F, Lonardi S, Intini R, et al. The Pan-immune-inflammation value in Microsatellite instability–high metastatic colorectal cancer patients treated with immune Checkpoint inhibitors. Eur J Cancer 2021;150:155–67. 10.1016/j.ejca.2021.03.043 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jitc-2023-007104supp002.pdf (1.4MB, pdf)
jitc-2023-007104supp001.pdf (382.8KB, pdf)
Data Availability Statement
Data are available upon reasonable request.