Abstract
Background and Objectives
Increasing evidence indicates that a subset of cognitively normal individuals has subtle cognitive impairment at baseline. We sought to identify them using the Stages of Objective Memory Impairment (SOMI) system. Symptomatic cognitive impairment was operationalized by a Clinical Dementia Rating (CDR) ≥0.5. We hypothesized that incident impairment would be higher for participants with subtle retrieval impairment (SOMI-1), higher still for those with moderate retrieval impairment (SOMI-2), and highest for those with storage impairment (SOMI-3/4) after adjusting for demographics and APOE ε4 status. A secondary objective was to determine whether including biomarkers of β-amyloid, tau pathology, and neurodegeneration in the models affect prediction. We hypothesized that even after adjusting for in vivo biomarkers, SOMI would remain a significant predictor of time to incident symptomatic cognitive impairment.
Methods
Among 969 cognitively normal participants, defined by a CDR = 0, from the Knight Alzheimer Disease Research Center, SOMI stage was determined from their baseline Free and Cued Selective Reminding Test scores, 555 had CSF and structural MRI measures and comprised the biomarker subgroup, and 144 of them were amyloid positive. Cox proportional hazards models tested associations of SOMI stages at baseline and biomarkers with time to incident cognitive impairment defined as the transition to CDR ≥0.5.
Results
Among all participants, the mean age was 69.35 years, 59.6% were female, and mean follow-up was 6.36 years. Participants in SOMI-1–4 had elevated hazard ratios for the transition from normal to impaired cognition in comparison with those who were SOMI-0 (no memory impairment). Individuals in SOMI-1 (mildly impaired retrieval) and SOMI-2 (moderately impaired retrieval) were at nearly double the risk of clinical progression compared with persons with no memory problems. When memory storage impairment emerges (SOMI-3/4), the hazard ratio for clinical progression increased approximately 3 times. SOMI stage remained an independent predictor of incident cognitive impairment after adjusting for all biomarkers.
Discussion
SOMI predicts the transition from normal cognition to incident symptomatic cognitive impairment (CDR ≥0.5). The results support the use of SOMI to identify those cognitively normal participants most likely to develop incident cognitive impairment who can then be referred for biomarker screening.
Early detection is important for prevention of Alzheimer disease (AD) and related dementias.1 In the most common approach to secondary prevention trials, ostensibly cognitively normal participants are enrolled based on positive biomarkers of AD.2 These studies have largely aimed to demonstrate the effectiveness of amyloid- or tau-targeted therapies in biomarker-positive but cognitively asymptomatic individuals. This approach is best exemplified by the AHEAD 3-453 and Anti-Amyloid in Asymptomatic Alzheimer's (A4) studies.4
There is increasing evidence that a subset of cognitively normal individuals has subtle cognitive impairment at baseline.5-7 Not all cognitively normal, biomarker-positive individuals clinically progress to cognitive impairment over the typical duration of a clinical trial, say 18–36 months.8,9 Because the objective of treatment in many clinical trials is to slow the rate of cognitive decline, inclusion of individuals unlikely to decline, even if randomized to placebo, will attenuate power to identify treatment effects in trials.10 Using sensitive neuropsychological tests to detect subtle cognitive impairment in biomarker-positive individuals categorized as cognitively normal (Clinical Dementia Rating [CDR] = 0) may improve our ability to identify a group of individuals who are more likely to decline.
The Stages of Objective Memory Impairment (SOMI) system, based on Free and Cued Selective Reminding Test (FCSRT) performance, provides an approach to identifying subtle cognitive impairment in this group.11,12 The FCSRT provides measures of memory retrieval (Free Recall; FR) and memory storage (Total Recall; TR). FR scores predict incident mild cognitive impairment (MCI) and incident dementia, particularly dementia due to AD,13-17 and often outperforms other cognitive tests.18,19 Furthermore, FR appears to be the component of the Preclinical Alzheimer's Cognitive Composite most sensitive to changes in pathologic hallmarks of AD.20,21 Impaired TR indicates prevalent dementia.22,23
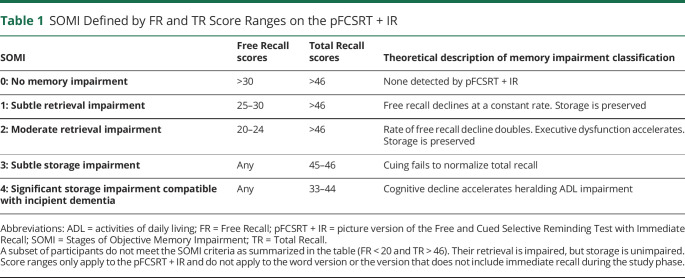
The SOMI system was based on the extensive literature mapping FR and TR scores to clinical outcomes in longitudinal aging cohorts and to anti- and post-mortem markers of AD pathology.11 Table 1 shows the SOMI cutoffs for classifying individuals into 1 of 5 stages based on FR and TR scores from the picture version of the FCSRT with Immediate Recall (pFCSRT + IR). In SOMI-0, FR and TR are normal. The next 2 SOMI stages (SOMI-1 and -2) reflect increasing retrieval difficulty, shown by declining FR in the context of intact TR beginning 7–8 years before clinical dementia.24,25 In the next 2 SOMI stages (SOMI-3 and -4), cuing fails to recover all items missed in FR, indicating a memory storage deficit occurring about 1–3 years before clinical dementia.23
Table 1.
SOMI Defined by FR and TR Score Ranges on the pFCSRT + IR
When the SOMI system was applied to the baseline FCSRT scores of 142 cases who developed AD in the Einstein Aging Study, the temporal trajectory of FR and TR decline mirrored the proposed SOMI stages.11 Cases with intact memory at baseline (SOMI-0) developed clinical dementia 7 years later, with subtle retrieval impairment (SOMI-1) 5 years later and moderate retrieval impairment (SOMI-2) 4 years later. With the addition of storage impairment (SOMI-3 and -4) dementia developed sooner, 2½ years. When the SOMI system was applied to the baseline FCSRT scores from the A4 study, 20% of the cognitively normal participants were SOMI-2 or higher.12 SOMI's advantage over individual FR and TR scores is because it permits the measurement of retrieval impairment separately from memory storage impairment. Because these processes breakdown at different points in the predementia phase,21,26 the ability to distinguish between them provides an estimate of the participant's location along the AD continuum.
In this study, we used data from the Knight Alzheimer Disease Research Center (ADRC) to assess SOMI's usefulness in predicting incident cognitive impairment among those who had a Clinical Dementia Rating (CDR)27 of 0 at baseline. Symptomatic cognitive impairment was operationalized by a CDR ≥0.5; CDR 0.5 comprises both mild cognitive impairment and very mild dementia. We hypothesized that incident cognitive impairment would be higher for participants with subtle retrieval impairment (SOMI-1), higher still for those with moderate retrieval impairment (SOMI-2), and highest for those with storage impairment (SOMI-3/4) after adjusting for demographics and APOE ε4 status. A secondary objective was to determine whether including biomarkers of β-amyloid (Aβ), tau pathology, and neurodegeneration in the models affected prediction. We hypothesized that even after adjusting for in vivo biomarkers, SOMI would remain a significant predictor of time to incident cognitive impairment. Finally, because secondary prevention trials in AD often enroll only amyloid-positive participants,28 we performed a sensitivity analysis in the amyloid-positive subsample.
Methods
Participants
Participants were enrolled in longitudinal studies of aging and AD from the Knight ADRC at Washington University in St. Louis, MO. Details about recruitment procedures have been reported previously.29 Briefly, Knight ADRC participants are community dwelling, and most reside in the St. Louis, MO, area. Participants were required to be in good general health, have no serious illnesses (e.g., end-stage renal disease requiring dialysis) that would preclude participation, nor medical contraindications to either CSF or MRI studies.
Study protocols were approved by the Human Research Protection Office at Washington University School of Medicine, and all participants provided written informed consent at the time of enrollment. The current study was also approved by the Institutional Review Board at Albert Einstein College of Medicine.
Clinical Assessment
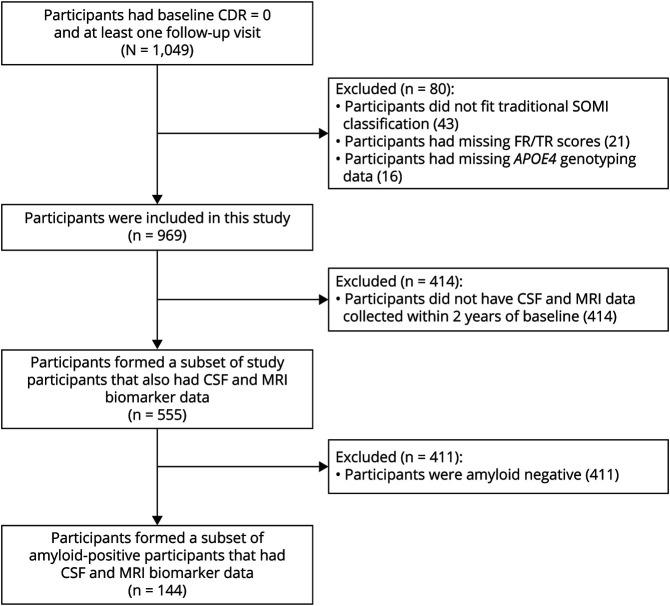
All participants in Knight ADRC studies have annual clinical and neuropsychological assessments that include the FCSRT. Participants are assessed by experienced clinicians, who assign CDR scores to individuals based on neurologic examinations, interviews with each participant, and separate interviews with an informant who knows the participant well.27 A total of 969 participants who were cognitively normal at baseline (CDR = 0), had FCSRT scores, and at least 1 wave of follow-up were included in this study. A subset of participants (N = 555) had CSF collection and volumetric MRI measurement within 2 years of baseline clinical assessment. Participants who were amyloid-positive (N = 144) provided the final subset. A flowchart of study participants is shown in Figure 1.
Figure 1. Flowchart of Study Participants.
CDR Clinical Dementia Rating; FR = Free Recall; SOMI = Stages of Objective Memory Impairment; TR = Total Recall.
The Free and Cued Selective Reminding Test
Unlike other episodic memory tests, the FCSRT controls the conditions of learning.30,31 Participants are asked to identify pictured items in response to category cues that are used in the test phase to prompt recall of items not retrieved in FR. This controlled learning procedure induces semantic processing and coordinates encoding and retrieval for maximum recall. Participants whose performance cannot be remediated by these procedures have a genuine memory deficit due to impairment of specific memory processes (storage or retrieval mechanisms) and not an apparent memory deficit due to use of inefficient strategies or impairment of other cognitive processes that occur in normal aging.31 Participants who have genuine memory deficits progress to MCI or clinical dementia.16,23,32,33
The pFCSRT + IR 331 was administered as part of the neuropsychological assessment. Pictures were presented 4 at a time for identification and then removed, and immediate cued recall was tested to ensure successful encoding and practice at retrieval before the test phase. Following the study phase, there were 3 test trials of 16 pictures for a maximum score of 48. Scores include FR (range 0–48) and TR (range 0–48). Participants were stratified into different SOMI stages using the score ranges of FR and TR shown in Table 1. Forty-two individuals (4%), whose retrieval was impaired but storage was unimpaired, could not be classified by the SOMI system and were excluded from the analysis. To increase statistical power, given the small number of participants in SOMI-3 and -4 stages, SOMI-3 and -4 were merged into a single group (SOMI-3/4).
CSF Biomarkers
CSF sampling procedures have been described previously.34 In brief, lumbar puncture was performed by experienced neurologists at 8 am after an overnight fast. CSF levels of Aβ40, Aβ42, phosphorylated tau 181 (p-Tau181), and total tau (t-Tau) were measured using ELISA (INNOTEST; Fujirebio [formerly Innogenetics], Tokyo, Japan). CSF levels of the Aβ42/Aβ40 ratio (indicative of amyloid deposition) are reduced in AD, whereas biomarkers of tauopathy (p-Tau181) and neurodegeneration (t-Tau) are elevated.35 Amyloid positivity, used to determine the sensitivity analysis subsample, was defined as Aβ42/Aβ40 ratio <0.0673.36
Imaging Biomarkers
Volumetric MRI
FreeSurfer 5.3 (freesurfer.net) was used for automated volumetric segmentation to identify the hippocampus. Hippocampal volume (HV) summed across hemispheres and adjusted for differences in intracranial volume with a regression approach.37
APOE ε4 Genotyping
DNA samples were genotyped using either Illumina 610 or Omniexpress chips following procedures previously described.38
Statistical Analysis
Statistical analysis of characteristic data was performed using MATLAB (version 2021a) where α = 0.05 indicated statistical significance, and all tests were 2 tailed. Sample characteristics among SOMI groups and the entire cohort were examined using analysis of variance for continuous variables and χ2 tests for categorical variables.
Prediction of Incident CDR ≥0.5
Cox proportional hazards models were used to examine the association of SOMI stage and incident cognitive impairment defined by the transition of CDR from 0 at baseline to CDR ≥0.5 during follow-up. Data from study participants who did not experience a change in CDR over the course of follow-up were right censored at the date of the last clinical assessment. SOMI stage was treated as a categorical variable with SOMI-0 as the reference stage. Age, sex, years of education, and APOE ε4 status were included in all models. Initially, a model was developed on the whole sample to assess the association between SOMI stage and incident CDR ≥05 (model 1). Data from the subset of participants who had both CSF data and structural MRI were used to develop models 2–8. A partial likelihood ratio test between nested models assessed model improvement as biomarkers were added. The Maddala-Magee index 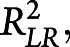 a measure of explained variation derived as an R2 measure,39 was calculated. Kaplan-Meier survival curves graphically illustrate survival probability. Because secondary prevention trials often seek to recruit individuals that are amyloid positive, we additionally performed a sensitivity analysis in the subsample of amyloid-positive individuals.
a measure of explained variation derived as an R2 measure,39 was calculated. Kaplan-Meier survival curves graphically illustrate survival probability. Because secondary prevention trials often seek to recruit individuals that are amyloid positive, we additionally performed a sensitivity analysis in the subsample of amyloid-positive individuals.
Data Availability
The data used for the purpose of this study were available from the Knight ADRC for eligible researchers on reasonable request. We used the Strengthening the Reporting of Observational Studies in Epidemiology cohort checklist when writing our report.40
Results
Sample Characteristics
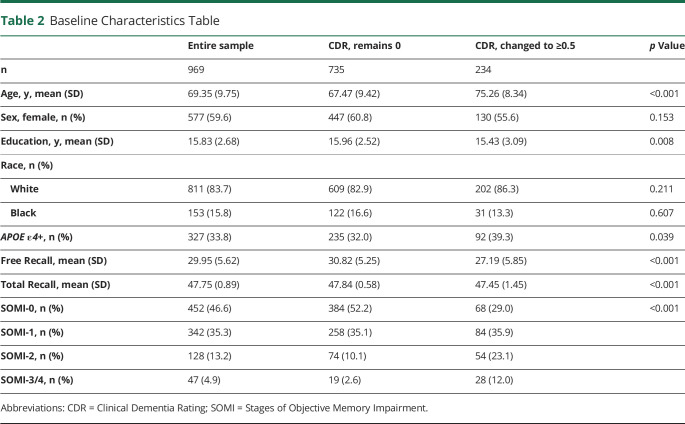
Table 2 provides a summary of the baseline characteristics of the entire cohort and a summary stratified by change in CDR status. Data from 969 participants, ranging in age from 40.5 to 100.2 at baseline (mean ± SD 69.35 ± 9.75 years), were used. Participants had 15.84 (SD 2.68) years of education, 59.6% were women, 83.7% were White and 15.8% were Black, and 33.8% were APOE ε4 positive. Participants were followed for up to 10 years with a mean follow-up period of 6.36 ± 3.17 years. The median follow-up time for the whole sample, defined by the median time to censoring, was 7.25 years. During follow-up, 234 (24.1%) participants had incident cognitive impairment (progressors), and 735 (75.9%) remained cognitively normal. The median follow-up time for progressors, defined by median time to event, was 4.83 years. Participants who remained cognitively normal during follow-up in comparison with those who progressed to CDR ≥0.5 were on average younger (67.5 vs 75.3, p < 0.001), had higher education levels (15.9 vs 15.4, p = 0.001), and had a lower frequency of APOE ε4 positivity (32.0% vs 39.3%, p = 0.039). They also had higher FR scores (30.8 vs 27.2, p < 0.001) and TR scores (47.8 vs 47.4, p < 0.001).
Table 2.
Baseline Characteristics Table
eTable 1 (links.lww.com/WNL/C732) summarizes characteristics for the subset who had CSF and structural MRI data. eTable 2 compares the characteristics of participants with and without biomarker information. Participants with biomarker data, compared with those without, were found to be younger (66.8 vs 72.7 years of age, p < 0.001), had more years of education (16.1 vs 15.5, p = 0.001), and had higher FR scores (30.8 vs 28.8, p < 0.001). In addition, participants with biomarkers had a higher proportion of SOMI-0 (53.0% vs 38.2%, p < 0.001). eTable 3 summarizes characteristics for the subsample of amyloid-positive individuals used in the sensitivity analysis. eTable 4 shows which variables were included in each model.
SOMI Prediction of Incident Cognitive Impairment (CDR ≥0.5)
Using data from the whole sample and demographics and APOE ε4 status as covariates, the Cox proportional hazards model indicated that SOMI-2 (hazard ratio [HR] 2.07, 95% CI 1.32–3.01, p < 0.001) and SOMI-3/4 (HR 3.11, 95% CI 1.94–4.97, p < 0.001) were significantly associated with incident cognitive impairment using SOMI-0 as the reference (Table 3). Given the wide age range of our sample (40–100 years), we stratified the sample by age (<70 and ≥70) and reran the analysis (eTable 5, links.lww.com/WNL/C732). Although we lose power after stratification, results largely remain the same. The Kaplan-Meier curves representing the association between SOMI stages and time to incident CDR ≥0.5 for the 969 participants (Figure 2) show that cognitive impairment–free survival declined in an orderly fashion as SOMI stage increased.
Table 3.
Cox Proportional Hazard Model Predicting Time to the Development of Incident Cognitive Impairment (CDR ≥0.5) for the Entire Sample (N = 969)
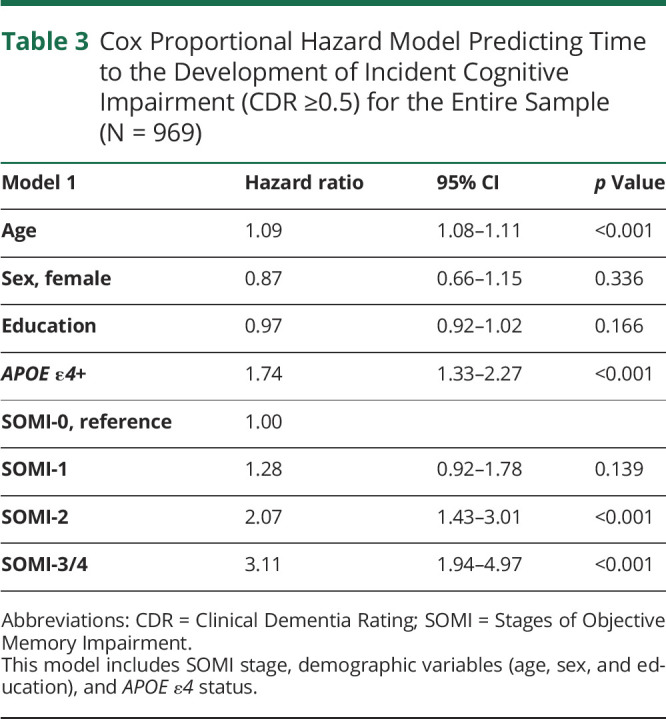
Figure 2. Kaplan-Meier Survival Curves for SOMI Stages.
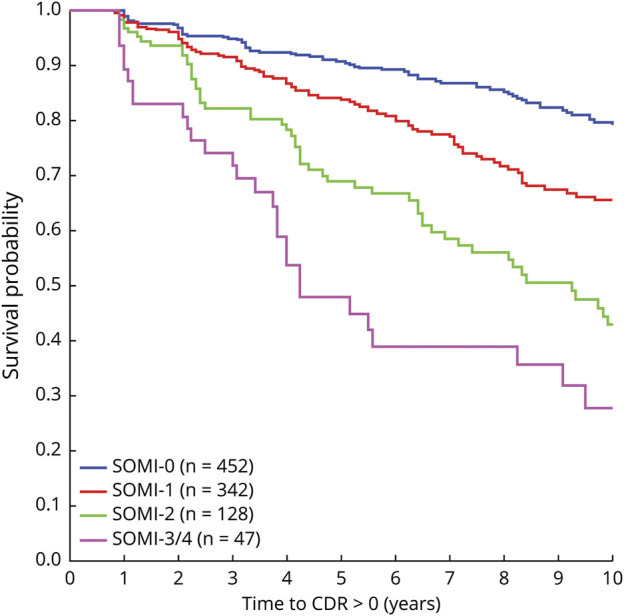
Time to first change in CDR from 0 to ≥0.5 for participants based on their initial SOMI stage. CDR Clinical Dementia Rating; SOMI = Stages of Objective Memory Impairment.
SOMI and CSF Biomarker Prediction of Incident Cognitive Impairment (CDR ≥0.5)
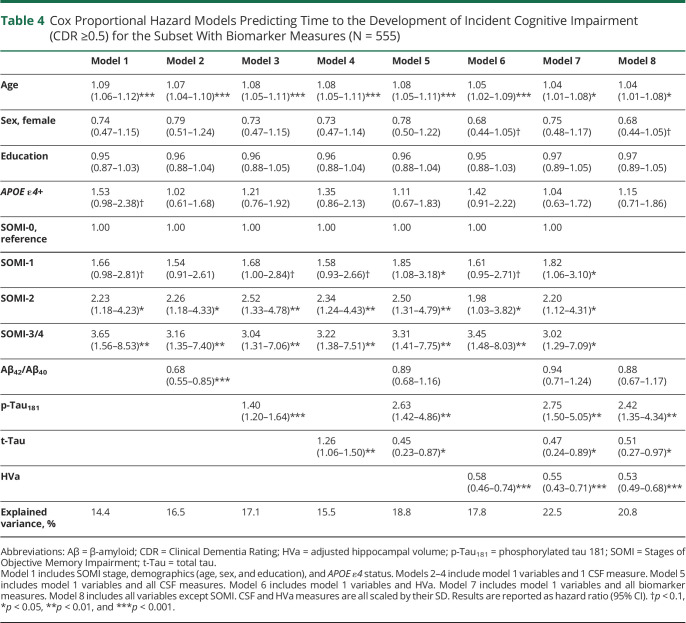
Model 1 used only demographics, APOE ε4 status, and SOMI, whereas models 2–5 additionally used the CSF information of each participant (Table 4). Model 1 indicated that SOMI-1 (HR 1.66, 95% CI 0.98–2.81, p = 0.057) was marginally and SOMI-2 (HR 2.23, 95% CI 1.18–4.23, p = 0.014) and SOMI-3/4 (HR 3.65, 95% CI 1.56–8.53, p = 0.003) were significantly associated with time to CDR ≥0.5. When Aβ42/Aβ40, p-Tau181, and t-Tau were individually added to model 1, SOMI-2 (p < 0.050), SOMI-3/4 (p < 0.010), and each CSF measure (p < 0.010) were significantly associated with incident CDR ≥0.5 (models 2–4). When all CSF measures were included (model 5), SOMI-1 (HR 1.85, 95% CI 1.08–3.18, p = 0.025), SOMI-2 (HR 2.50, 95% CI 1.31–4.79, p = 0.006), and SOMI-3/4 (HR 3.31, 95% CI 1.41–7.75, p = 0.006) were associated with incident cognitive impairment.
Table 4.
Cox Proportional Hazard Models Predicting Time to the Development of Incident Cognitive Impairment (CDR ≥0.5) for the Subset With Biomarker Measures (N = 555)
Using the metric of explained variance, the percent of explained variance by the SOMI stages, demographics, and APOE ε4 (model 1) was 14.4%. Models 2–4 increased the explained variance relative to model 1 (Table 4). The partial likelihood ratio test showed that the addition of Aβ42/Aβ40 or p-Tau181 to model 1 improved model fit (p < 0.001 for both) as did the addition of t-Tau (p = 0.013). Model 5, with all CSF measures, had an explained variance of 18.8%, significantly improving model fit (p < 0.001).
SOMI, CSF Biomarkers, and HV Prediction of CDR ≥0.5
When HVa was added to model 1, SOMI-2 (HR 1.98, 95% CI 1.03–3.82, p = 0.040) and SOMI-3/4 (HR 3.45, 95% CI 1.48–8.03, p = 0.004) were significantly associated with time to CDR ≥0.5 as was HVa (HR 0.58, 95% CI 0.46–0.74, p < 0.001) (model 6). In model 7 that included all biomarkers, the following were significant: SOMI-1 (HR 1.82, 95% CI 1.06–3.10, p = 0.029), SOMI-2 (HR 2.20, 95% CI 1.12–4.31, p = 0.021), SOMI-3/4 (HR 3.02, 95% CI 1.29–7.09, p = 0.011), p-Tau181 (HR 2.75, 95% CI 1.50–5.05, p = 0.001), t-Tau (HR 0.47, 95% CI 0.24–0.89, p = 0.021), and HVa (HR 0.55, 95% CI 0.43–0.71, p < 0.001). When SOMI was excluded, biomarker HRs were unchanged (model 8): p-Tau181 (HR 2.42, 95% CI 1.35–4.34, p = 0.003), t-Tau (HR 0.51, 95% CI 0.27–0.97, p = 0.039), and HVa (HR 0.53, 95% CI 0.49–0.68, p < 0.001). A summary of the Cox proportional hazards results for these models can be found in Table 4.
In the models with HVa, model 6 had an explained variance of 17.8%, model 7 had 22.5%, and model 8 had 20.8%. The partial likelihood ratio test showed that models 6 and 7 were significantly improved by the inclusion of additional biomarkers (p < 0.001 for all). Model 7 was significantly improved over model 8 by including SOMI (p = 0.003).
Sensitivity Analysis Using the Amyloid-Positive Subsample
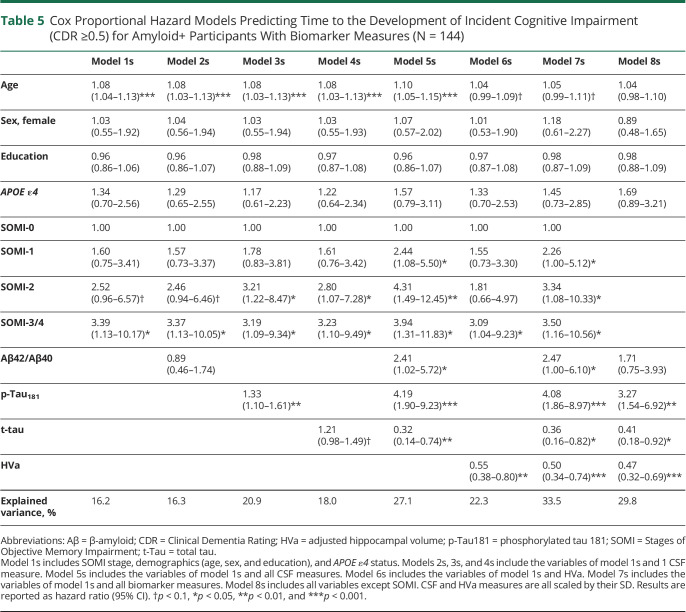
Models 1s–7s were developed using the subsample (N = 144) of amyloid-positive individuals with CSF and MRI data (Table 5). In model 1s, only SOMI-3/4 (HR 3.39, 95% CI 1.13–10.17, p = 0.029) was significantly associated with time to CDR ≥0.5. The same was true in model 2s, SOMI-3/4 (HR 3.37, 95% CI 1.13–10.05, p = 0.030) while Aβ42/Aβ40 was not significantly associated with time to CDR ≥0.5. Models 3s–7s had similar risk profiles as models 3–7. When all CSF measures were included (model 5s), SOMI-1 (HR 1.85, 95% CI 1.08–3.18, p = 0.025), SOMI-2 (HR 2.50, 95% CI 1.31–4.79, p = 0.006), and SOMI-3/4 (HR 3.31, 95% CI 1.41–7.75, p = 0.006) were associated with incident cognitive impairment as were all the biomarkers. In model 7s, with all CSF measures and HVa added, SOMI-1 (HR 2.26, 95% CI 1.00–5.12, p = 0.050), SOMI-2 (HR 3.34, 95% CI 1.08–10.33, p = 0.037), SOMI-3/4 (HR 3.50, 95% CI 1.16–10.56, p = 0.026), all CSF measures (p < 0.050 for all), and HVa (p < 0.001) were significantly associated with time to CDR ≥0.5. Without SOMI (model 8s), significant predictors were p-Tau181 (HR 3.27, 95% CI 1.54–6.92, p = 0.002), t-Tau (HR 0.41, 95% CI 0.18–0.92, p = 0.031), and HVa (HR 0.47, 95% CI 0.32–0.69, p < 0.001).
Table 5.
Cox Proportional Hazard Models Predicting Time to the Development of Incident Cognitive Impairment (CDR ≥0.5) for Amyloid+ Participants With Biomarker Measures (N = 144)
The percent of explained variance by the SOMI stages, demographics, and APOE ε4 (model 1s) was 16.2% (Table 5). Adding Aβ42/Aβ40 did not improve it. Explained variance with all CSF measures was 27.1% (model 5s), 22.3% with only HVa (6s), 33.5% with all biomarkers (7s), and 29.8% without SOMI (8s).
Neither Aβ42/Aβ40 (p = 0.736) nor T-tau (p = 0.086) improved model 1s (p = 0.736) while the addition of p-Tau181 did (p = 0.005). Inclusion of all CSF measures compared with 1 or fewer measures significantly improved the models (p < 0.010 for all). In addition, the inclusion of HVa to model 1s improved the model (p = 0.002), and the inclusion of all CSF measures and HVa improved all previous models (p < 0.001 for all). Model 7s was significantly improved over model 8s by including SOMI (p = 0.019).
Contrasting SOMI With Other Neurocognitive Tests
Finally, to evaluate whether commonly used neuropsychological tests, specifically the Mini-Mental State Examination (MMSE), Animal Fluency Test, and Trail Making Test Part B (TMT-B) predict incident cognitive impairment, we repeated the analyses in the entire sample, excluding SOMI. None of these tests were significant predictors (eTables 6–8, links.lww.com/WNL/C732).
Discussion
We used the SOMI system, based on the FCSRT, to identify subtle cognitive impairment in ostensibly cognitively normal participants and showed that they are at increased risk of incident cognitive impairment (CDR ≥0.5). Participants with retrieval deficits or memory storage deficits were at increased risk of developing cognitive impairment. Results from our first set of analyses indicate that SOMI-2 and SOMI-3/4 are both associated with higher likelihood of CDR ≥0.5 onset. SOMI remained a significant predictor of time to CDR ≥0.5 when measures of Aβ and tau pathology and HV were included in the models. In the presence of SOMI and all other biomarkers as predictors, only p-Tau181, t-Tau, and HV were significantly associated with incident cognitive impairment. Overall, our primary hypotheses were largely confirmed. Participants with moderate retrieval deficits SOMI-2 were twice as likely to progress to incident cognitive impairment than those whose memory was intact (SOMI-0) while participants with memory storage deficits (SOMI-3, 4) were 3 times as likely to progress. In the subsamples with biomarkers and amyloid-positive status, SOMI-1 participants were also at increased risk of incident cognitive impairment. Although SOMI predicted incident symptomatic cognitive impairment, MMSE, Animal Fluency, and TMT-B did not.
Current results indicate that 18.1% of the cognitively normal participants in the Knight ADRC cohort have memory storage or retrieval impairment. In our prior studies using data from several longitudinal cohorts, we demonstrated that SOMI staging can identify the subset of cognitively normal participants who have impairments in memory retrieval or storage, including the A4 study (20%),12 the Harvard Aging Brain Study (HABS: 15%),41 and the Baltimore Longitudinal Study of Aging (16%).42 Another advantage of SOMI is that it identifies the participants with intact memory who are unlikely to develop incident cognitive impairment during 18 months to 5 years of follow-up in clinical trials.
This is another demonstration that cognitively normal people at higher SOMI stages are at increased risk for incident symptomatic cognitive impairment. Our results indicate that individuals in SOMI-1 and SOMI-2 stages are at nearly double the risk of clinical progression compared with persons with no memory problems. This HR increases to approximately 3 times for individuals in SOMI-3/4 stage when memory storage impairments emerge. These risk ratios are in line with our previous findings that showed a first acceleration of FR decline among SOMI-1 individuals approximately 7 years before onset of clinical dementia.24,25 In another study, we showed that individuals, who have moderately impaired retrieval but intact memory storage (SOMI-2), were at increased risk of progression to dementia over 5 years (HR 58.7, 95% CI 7.5–458).43
In this study, for models that included SOMI and CSF biomarkers or HV, SOMI-2 and SOMI-3/4 stages consistently remained associated with incident cognitive impairment despite the known shared variance between SOMI staging and AD biomarkers in cognitively normal adults.12,41 In the HABS cohort, SOMI-3/4 stage had smaller HVs and higher entorhinal and inferior temporal tau PET deposition than SOMI-0 or SOMI-1 stages.41 In the A4 trial, higher SOMI stage was associated with higher global amyloid standardized uptake value ratio level and smaller medial temporal lobe structures.12
Our results also indicate that the presence of APOE ε4 was associated with onset of cognitive impairment only in models that did not include other AD biomarkers. This is likely due to the shared variance between APOE ε4 status and AD biomarkers and that APOE ε4 influence on cognition is mediated in part through amyloid or tau biomarkers.44 In the model that included all biomarkers, Aβ failed to retain significance in the presence of tau pathology and neurodegeneration. This is consistent with the widely accepted view that the severity of cognitive impairment correlates best with the burden of neocortical neurofibrillary tangles.45
Based on the pathophysiologic processes of AD,46 the presence of AD biomarkers such as CSF amyloid and tau and neurodegeneration based on structural MRI precede onset of cognitive impairment (MCI or dementia stage). The observed order in this cascade (delayed onset of symptomatic cognitive impairment relative to biomarkers) might be partially explained by the lack of sensitivity of the cognitive tests used to identify the impairment. Subtle cognitive impairment already exists when AD pathology starts accumulating in the brain at detectable thresholds.5,6,12,41,47,48 Subthreshold levels of Aβ PET imaging were associated with a decline in FR scores in the A4 study.20 FR was most sensitive to Aβ-related decreases in average cognitive scores, outperforming all tests including logical memory.19 Current results largely support this hypothesis.
Although our goal was predicting the transition from cognitive normality to impairment in a cognitively normal group with mixed amyloid status, we hypothesized that SOMI would be as effective in the amyloid+ subgroups that are typically enrolled in secondary prevention trials. The SOMI risk profile in the amyloid-positive subgroup tended to be higher than in the biomarker subgroup, especially for the SOMI-1 and -2 despite the much smaller sample size. This is consistent with the decline in FR among cognitively normal amyloid-positive participants in secondary prevention trials at 3 and 5 years of follow-up.21 The results support the use of SOMI to identify those cognitively normal participants most likely to develop incident symptomatic cognitive impairment who can then be referred for biomarker screening. SOMI can also be used to exclude participants with normal memory who are unlikely to decline during the trial period.
The word version of the FCSRT with IR is used in several European longitudinal studies.14,17,19 It should be possible to develop a SOMI system using the word version of FCSRT if it includes IR; however, the score ranges will likely be slightly lower than the picture version reflecting the picture superiority effect.49 Importantly, in the word version without IR, scores are much lower than scores when IR is part of the study phase.50 This is because IR confirms and enhances correct initial encoding and provides retrieval practice before the test phase.31 Without IR, storage and retention are less robust and may not be as distinguishable.
The primary strength of this study was that we were able to evaluate the predictive validity of the SOMI system among persons who were cognitively normal (CDR = 0) at baseline, using data from a single site that collected CSF and MRI biomarkers. However, there were some limitations. We used a convenience sample of older adults, 95% of whom were well-educated Caucasian adults who were willing to be followed longitudinally and were able to tolerate MRI and lumbar punctures, which reduces the generalizability of the findings. Considering the small sample size (4.9%) of SOMI-3 and SOMI-4, the results should not be overinterpreted. It is noteworthy, however, that 5% of the ostensibly cognitively normal seniors had cognitive impairment consistent with incipient dementia at baseline. The amount of time during which data were collected varied over the course of Knight cohorts such that more information was available for some data (e.g., CDR and FCSRT) than for others (e.g., MRIs). Greater statistical power and stability of findings is yielded for measures with higher sample size. Here, we focused mainly on the CSF biomarkers for measurement of amyloid and tau pathology and a single structural region (hippocampus), which by nature lack spatial information about the underlying pathology. Extending analysis to include measures of amyloid, tau, and neurodegeneration from different regions of the brain may provide insight into the mechanistic understanding of disease expression and progression. Finally, because the majority of the studied sample were White, subgroup analysis in minority groups was not a possibility. Larger studies with more diverse samples are required to confirm the generalizability of our findings.
In conclusion, we showed the SOMI stage predicts the transition from cognitive normality (CDR = 0) to incident symptomatic cognitive impairment (CDR ≥0.5). Predictive validity persists in models that include ATN biomarkers. The SOMI system may find application in future AD clinical trials and as a tool for identifying high-risk patients in clinical practice.
Glossary
- Aβ
β-amyloid
- A4
Anti-Amyloid in Asymptomatic Alzheimer's
- AD
Alzheimer disease
- ADRC
Alzheimer Disease Research Center
- CDR
Clinical Dementia Rating
- FCSRT
Free and Cued Selective Reminding Test
- FR
Free Recall
- HABS
Harvard Aging Brain Study
- HR
hazard ratio
- HV
hippocampal volume
- IR
Immediate Recall
- MCI
mild cognitive impairment
- MMSE
Mini-Mental State Examination
- pFCSRT
picture version of the FCSRT
- p-Tau181
phosphorylated tau 181
- SOMI
Stages of Objective Memory Impairment
- TR
Total Recall
- TMT-B
Trail Making test Part B
- t-Tau
total tau
Appendix. Authors

Study Funding
The authors of this study were supported in part by grants from the NIH (NIA K23 AG063993, A.E.; 2PO1 AG003949, R.B.L); the Alzheimer's Association (2019-AACSF-641329; A.E.); Cure Alzheimer Fund (A.E. and R.B.L.), and the Leonard and Sylvia Marx Foundation (R.B.L.). J.C.M. is funded by NIH grant numbers P30 AG066444, P01AG003991, P01AG026276, U19 AG032438, and U19 AG024904. R.B.L. also receives research support from the following sources unrelated to this manuscript: 5U10 NS077308 (PI), R21 AG056920 (Investigator), 1RF1 AG057531 (Site PI), RF1 AG054548 (Investigator), 1RO1 AG048642 (Investigator), R56 AG057548 (Investigator), U01062370 (Investigator), RO1 AG060933 (Investigator), RO1 AG062622 (Investigator), 1UG3FD006795 (mPI), and 1U24NS113847 (Investigator).
Disclosure
E. Grober receives a small royalty for commercial use of the Free and Cued Selective Reminding Test with Immediate Recall (FCSRT + IR). The test is available at no cost to researchers and clinicians. The Albert Einstein College of Medicines holds the copyright for the test. Inquiries should be addressed to Dr. Nilam Sinha in the Office of Biotechnology and Business Development (Nilam.Sinha@einsteinmed.org). She is a paid consultant for Genentech-Roche. K.K. Petersen reports no disclosures relevant to the manuscript. R.B. Lipton serves as a consultant and advisory board member or has received honoraria for his headache work from AbbVie (Allergan), the American Academy of Neurology, the American Headache Society, Amgen, Biohaven, Eli Lilly, GlaxoSmithKline, Grifols, Lundbeck (Alder), Merck, Pfizer, Teva, Vector, and Vedanta. He receives royalties from Wolff's Headache 7th and 8th Edition, Oxford Press University, 2009, Wiley and Informa. J. Hassenstab, J.C. Morris, B. Gordon, and A.Ezzati report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43(11):2412-2414. [DOI] [PubMed] [Google Scholar]
- 2.Donohue MC, Sperling RA, Salmon DP, et al. The preclinical Alzheimer cognitive composite: measuring amyloid-related decline. JAMA Neurol. 2014;71(8):961-970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aisen PS, Zhou J, Irizarry MC, et al. AHEAD 3‐45 study design: a global study to evaluate the efficacy and safety of treatment with BAN2401 for 216 weeks in preclinical Alzheimer's disease with intermediate amyloid (A3 trial) and elevated amyloid (A45 trial) Clinical trial design and implementation. Alzheimers Dement. 2020;16(S9):e044511. [Google Scholar]
- 4.Sperling RA, Rentz DM, Johnson KA, et al. The A4 study: stopping AD before symptoms begin? Sci Transl Med. 2014;6(228):228fs213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomas KR, Bangen KJ, Edmonds EC, et al. Objective subtle cognitive decline and plasma phosphorylated tau181: early markers of Alzheimer's disease-related declines. Alzheimers Dement (Amst). 2021;13(1):e12238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shen X-N, Kuo K, Yang Y-X, et al. Subtle cognitive impairment as a marker of Alzheimer's pathologies and clinical progression in cognitively normal individuals. Alzheimers Dement (Amst). 2021;13(1):e12198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duke Han S, Nguyen CP, Stricker NH, Nation DA. Detectable neuropsychological differences in early preclinical Alzheimer's disease: a meta-analysis. Neuropsychol Rev. 2017;27(4):305-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baker JE, Lim YY, Pietrzak RH, et al. Cognitive impairment and decline in cognitively normal older adults with high amyloid-β: a meta-analysis. Alzheimers Dement (Amst). 2016;6(1):108-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roe CM, Ances BM, Head D, et al. Incident cognitive impairment: longitudinal changes in molecular, structural and cognitive biomarkers. Brain. 2018;141(11):3233-3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ezzati A, Davatzikos C, Wolk DA, Hall CB, Habeck C, Lipton RB. Application of predictive models in boosting power of Alzheimer's disease clinical trials: a post hoc analysis of phase 3 solanezumab trials. Alzheimers Dement (NY). 2022;8(1):e12223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grober E, Veroff AE, Lipton RB. Temporal unfolding of declining episodic memory on the Free and Cued Selective Reminding Test in the predementia phase of Alzheimer's disease: implications for clinical trials. Alzheimers Dement (Amst). 2018;10(1):161-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grober E, Lipton RB, Sperling RA, et al. Associations of stages of objective memory impairment (SOMI) with amyloid PET and structural MRI: the A4 study. Neurology. 2022;98(13):e1327-e1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Katz MJ, Lipton RB, Hall CB, et al. Age-specific and sex-specific prevalence and incidence of mild cognitive impairment, dementia, and Alzheimer dementia in blacks and whites: a report from the Einstein Aging Study. Alzheimer Dis Assoc Disord. 2012;26(4):335-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Auriacombe SM, Helmer CMDP, Amieva HP, Berr CP, Dubois BM, Dartigues JFMDP. Validity of the free and cued selective reminding test in predicting dementia: the 3C study. Neurology. 2010;74(22):1760-1767. [DOI] [PubMed] [Google Scholar]
- 15.Di Stefano F, Epelbaum S, Coley N, et al. Prediction of Alzheimer's disease dementia: data from the GuidAge prevention trial. J Alzheimers Dis. 2015;48(3):793-804. [DOI] [PubMed] [Google Scholar]
- 16.Lemos R, Maroco J, Simoes MR, Santiago B, Tomas J, Santana I. The free and cued selective reminding test for predicting progression to Alzheimer's disease in patients with mild cognitive impairment: a prospective longitudinal study. J Neuropsychol. 2017;11(1):40-55. [DOI] [PubMed] [Google Scholar]
- 17.Teichmann M, Epelbaum S, Samri D, et al. Free and Cued Selective Reminding Test: accuracy for the differential diagnosis of Alzheimer's and neurodegenerative diseases: a large-scale biomarker-characterized monocenter cohort study (ClinAD). Alzheimers Dement. 2017;13(8):913-923. [DOI] [PubMed] [Google Scholar]
- 18.Derby CA, Burns LC, Wang C, et al. Screening for predementia AD: time-dependent operating characteristics of episodic memory tests. Neurology. 2013;80(14):1307-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mura T, Coley N, Amieva H, et al. Cognitive decline as an outcome and marker of progression toward dementia, in early preventive trials. Alzheimers Dement. 2022;18(4):676-687. [DOI] [PubMed] [Google Scholar]
- 20.Insel PS, Donohue MC, Sperling R, Hansson O, Mattsson-Carlgren N. The A4 study: beta-amyloid and cognition in 4432 cognitively unimpaired adults. Ann Clin Transl Neurol. 2020;7(5):776-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mormino EC, Papp KV, Rentz DM, et al. Early and late change on the preclinical Alzheimer's cognitive composite in clinically normal older individuals with elevated beta-amyloid. Alzheimers Dement. 2017;13(9):1004-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grober E, Buschke H, Crystal H, Bang S, Dresner R. Screening for dementia by memory testing. Neurology. 1988;38(6):900-903. [DOI] [PubMed] [Google Scholar]
- 23.Grober E, Sanders AE, Hall C, Lipton RB. Free and cued selective reminding identifies very mild dementia in primary care. Alzheimer Dis Assoc Disord. 2010;24(3):284-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grober E, Hall CB, Lipton RB, Zonderman AB, Resnick SM, Kawas C. Memory impairment, executive dysfunction, and intellectual decline in preclinical Alzheimer's disease. J Int Neuropsychol Soc. 2008;14(2):266-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grober E, An Y, Lipton RB, Kawas C, Resnick SM. Timing of onset and rate of decline in learning and retention in the pre-dementia phase of Alzheimer's disease. J Int Neuropsychol Soc. 2019;25(7):699-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Papp K, Rentz D, Mormino EC, et al. Cued memory decline in biomarker defined preclinical Alzheimer's disease. Neurology. 2017;88(15):1431-1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morris JC. The clinical dementia rating (CDR): current version and. Young. 1991;41:1588-1592. [DOI] [PubMed] [Google Scholar]
- 28.Kryscio RJ. Secondary prevention trials in Alzheimer disease: the challenge of identifying a meaningful end point. JAMA Neurol. 2014;71(8):947-949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hassenstab J, Chasse R, Grabow P, et al. Certified normal: Alzheimer's disease biomarkers and normative estimates of cognitive functioning. Neurobiol Aging. 2016;43:23-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buschke H. Cued recall in amnesia. J Clin Neuropsychol. 1984;6(4), 433-440. [DOI] [PubMed] [Google Scholar]
- 31.Grober E, Buschke H. Genuine memory deficits in dementia. Dev Neuropsychol. 1987;3(1):13-36. [Google Scholar]
- 32.Grober E, Kawas C. Learning and retention in preclinical and early Alzheimer's disease. Psychol Aging. 1997;12(1):183-188. [DOI] [PubMed] [Google Scholar]
- 33.Schindler SE, Jasielec MS, Weng H, et al. Neuropsychological measures that detect early impairment and decline in preclinical Alzheimer disease. Neurobiol Aging. 2017;56:25-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roe CM, Fagan AM, Grant EA, et al. Amyloid imaging and CSF biomarkers in predicting cognitive impairment up to 7.5 years later. Neurology. 2013;80(19):1784-1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Molinuevo JL, Ayton S, Batrla R, et al. Current state of Alzheimer's fluid biomarkers. Acta Neuropathol. 2018;136(6):821-853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Volluz KE, Schindler SE, Henson RL, et al. Correspondence of CSF biomarkers measured by Lumipulse assays with amyloid PET. Alzheimer Dement. 2021;17(S5):e051085. [Google Scholar]
- 37.Mormino EC, Betensky RA, Hedden T, et al. Synergistic effect of β-amyloid and neurodegeneration on cognitive decline in clinically normal individuals. JAMA Neurol. 2014;71(11):1379-1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cruchaga C, Kauwe JS, Nowotny P, et al. Cerebrospinal fluid APOE levels: an endophenotype for genetic studies for Alzheimer's disease. Hum Mol Genet. 2012;21(20):4558-4571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Magee L. R2 measures based on Wald and likelihood ratio joint significance tests. Am Statistician. 1990;44(3):250-253. [Google Scholar]
- 40.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg. 2014;12(12):1495-1499. [DOI] [PubMed] [Google Scholar]
- 41.Grober E, Papp KV, Rentz DM, et al. Neuroimaging correlates of Stages of Objective Memory Impairment (SOMI) system. Alzheimers Dement (Amst). 2021;13(1):e12224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grober E, Wang C, Kitner-Triolo M, Lipton RB, Kawas C, Resnick SM. Prognostic value of learning and retention measures from the free and cued selective reminding test to identify incident mild cognitive impairment. J Int Neuropsychol Soc. 2022;28(3):292-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grober E, Lipton RB, Hall C, Crystal H. Memory impairment on free and cued selective reminding predicts dementia. Neurology. 2000;54(4):827-832. [DOI] [PubMed] [Google Scholar]
- 44.Petersen KK, Lipton RB, Grober E, Davatzikos C, Sperling RA, Ezzati A. Predicting amyloid positivity in cognitively unimpaired older adults: a machine learning approach using the A4 data. Neurology. 2022;98(24):e2425-e2435. doi: 10.1212/WNL.0000000000200553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nelson PT, Alafuzoff I, Bigio EH, et al. Correlation of Alzheimer disease neuropathologic changes with cognitive status: a review of the literature. J Neuropathol Exp Neurol. 2012;71(5):362-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jack CR, Knopman DS, Jagust WJ, et al. Tracking pathophysiological processes in Alzheimer's disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12(2):207-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Edmonds EC, Delano-Wood L, Galasko DR, Salmon DP, Bondi MW. Subtle cognitive decline and biomarker staging in preclinical Alzheimer's disease. J Alzheimers Dis. 2015;47(1):231-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bondi MW, Edmonds EC, Jak AJ, et al. Neuropsychological criteria for mild cognitive impairment improves diagnostic precision, biomarker associations, and progression rates. J Alzheimers Dis. 2014;42(1):275-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Paivio A. Dual coding theory: retrospect and current status. Can J Psychol. 1991;45(3):255-287. [Google Scholar]
- 50.Pena-Casanova J, Gramunt-Fombuena N, Quinones-Ubeda S, et al. Spanish multicenter normative studies (NEURONORMA project): norms for the Rey-Osterrieth complex figure (copy and memory), and free and cued selective reminding test. Arch Clin Neuropsychol. 2009;24(4):371-393. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used for the purpose of this study were available from the Knight ADRC for eligible researchers on reasonable request. We used the Strengthening the Reporting of Observational Studies in Epidemiology cohort checklist when writing our report.40