Background:
Despite an increasing surge of exosome use throughout the aesthetic arena, a paucity of published exosome-based literature exists. Exosomes are membrane-bound extracellular vesicles derived from various cell types, exerting effects via intercellular communication and regulation of several signaling pathways. The purpose of this review was to summarize published articles elucidating mechanisms and potential applications, report available products and clinical techniques, and prompt further investigation of this emerging treatment within the plastic surgery community.
Methods:
A literature review was performed using PubMed with keywords exosomes, secretomes, extracellular vesicles, plastic surgery, skin rejuvenation, scar revision, hair growth, body contouring, and breast augmentation. Publications from 2010 to 2021 were analyzed for relevance and level of evidence. A Google search identified exosome distributors, where manufacturing/procurement details, price, efficacy, and clinical indications for use were obtained by direct contact and summarized in table format.
Results:
Exosomes are currently derived from bone marrow, placental, adipose, and umbilical cord tissue. Laboratory-based exosome studies demonstrate enhanced outcomes in skin rejuvenation, scar revision, hair restoration, and fat graft survival on the macro and micro levels. Clinical studies are limited to anecdotal results. Prices vary considerably from $60 to nearly $5000 based on company, source tissue, and exosome concentration. No exosome-based products are currently Food and Drug Administration–approved.
Conclusions:
Administered alone or as an adjunct, current reports show promise in several areas of aesthetic plastic surgery. However, ongoing investigation is warranted to further delineate concentration, application, safety profile, and overall outcome efficacy.
Takeaways
Question: What is the current evidence for exosomes in aesthetic plastic surgery, and how are these products incorporated in the clinical setting?
Findings: Literature review over 10 years showed promise in animal and cell models and in early clinical trials for skin rejuvenation, scar revision, hair restoration, and fat graft survival. Six manufacturers were identified, each supplying exosomes from different source cells. All products are advertised for topical skin rejuvenation and hair restoration. However, no products are FDA-approved.
Meaning: Exosomes show promise in several areas of nonsurgical rejuvenation; however, further studies are warranted to determine whether this modality is superior to PRP and fat grafting.
INTRODUCTION
The continued desire for aging intervention has driven the demand and rapid popularity of nonsurgical aesthetic modalities. Over the last decade, exosomes have received particular interest as a topical and injectable solution due to their described regenerative properties and potential influence on wound healing, scar modification, and hair growth.1 Although dermatology and antiaging medicine have been early adopters of this developing technology, incorporation into the field of plastic surgery is increasing, yet published studies remain limited.
Exosomes were first reported by Johnstone et al2 in 1983 while studying the maturation process of reticulocytes. These nanosized biovesicles, approximately 40-160 nm in size, were observed to be released from cellular endosomes and carried an array of proteins, nucleic acids, and lipids. Therefore, they were originally known as “cellular garbage disposals.”1 However, in the mid-1990s, exosomes were found to play a vital role in intercellular communication and immunological function.3 Exosomes are now considered an important subcategory of extracellular vesicles (ECVs) that contain a core of micro-RNA (miRNA), messenger-RNA (mRNA), transcription factors, membrane-trafficking proteins, antigen-presenting proteins, and other peptides protected within a bilayer lipid membrane.1 They express characteristics of the cell from which they are released, functioning as paracrine molecules that interact with the extracellular matrix (ECM) and adjacent cells. To date, exosomes have displayed a plethora of prospective clinical applications, including stem cell maintenance and plasticity, biomarkers, adjuncts in chemotherapy and drug delivery, and wound healing supplements that promote angiogenesis.1,3,4 However, despite their multifunctional potential, their exact mechanism of action has yet to be fully elucidated.
Given their broad regenerative potential, cell-free characteristics, and increasing use within the cosmetic arena, we sought to introduce a narrative review on exosome therapy within the plastic surgery community. Our aim was to (1) summarize the recent literature on exosome use in aesthetics, (2) describe the mechanisms and potential clinical applications, (3) report available products on the current market and techniques for procurement and preparation, and (4) initiate further discussion and investigation on this emerging topic within plastic surgery.
METHODS
A literature search was conducted through PubMed using the following key terms and their various combinations (and/or): exosomes, secretomes, ECVs, aesthetic medicine, cosmetic medicine, dermatology, plastic surgery, skin rejuvenation, skin tightening, scar revision, hair growth, body contouring, and breast augmentation, identifying a total of 62 exosome-relevant articles included in this article. Abstracts from 2010 to 2021 were analyzed for level of evidence5 and relevance to our investigation, including the following subcategories: nonsurgical skin rejuvenation, scar revision/optimization, hair restoration, and breast/body contouring, reported in table format. A Google search was conducted to identify current market exosome distributors and each contacted by phone/email for information regarding manufacturing/procurement, price, efficacy, clinical indications for use, and Food and Drug Administration (FDA) approval and summarized in table format.
HOW EXOSOMES WORK
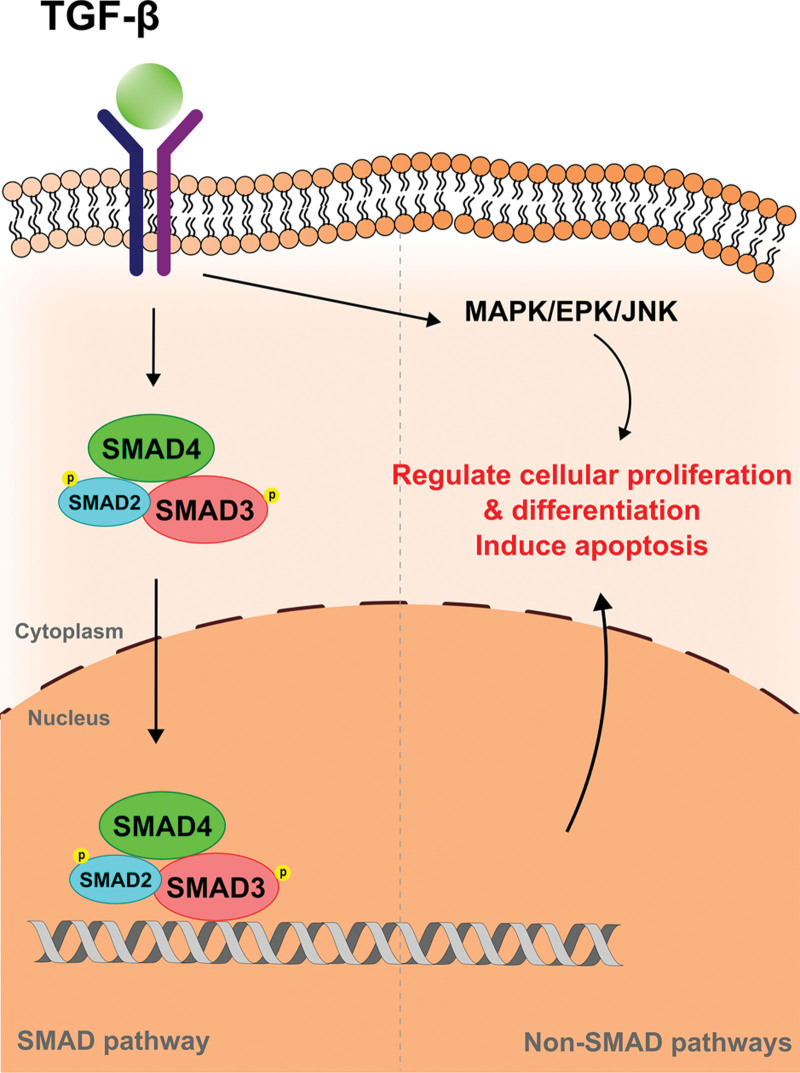
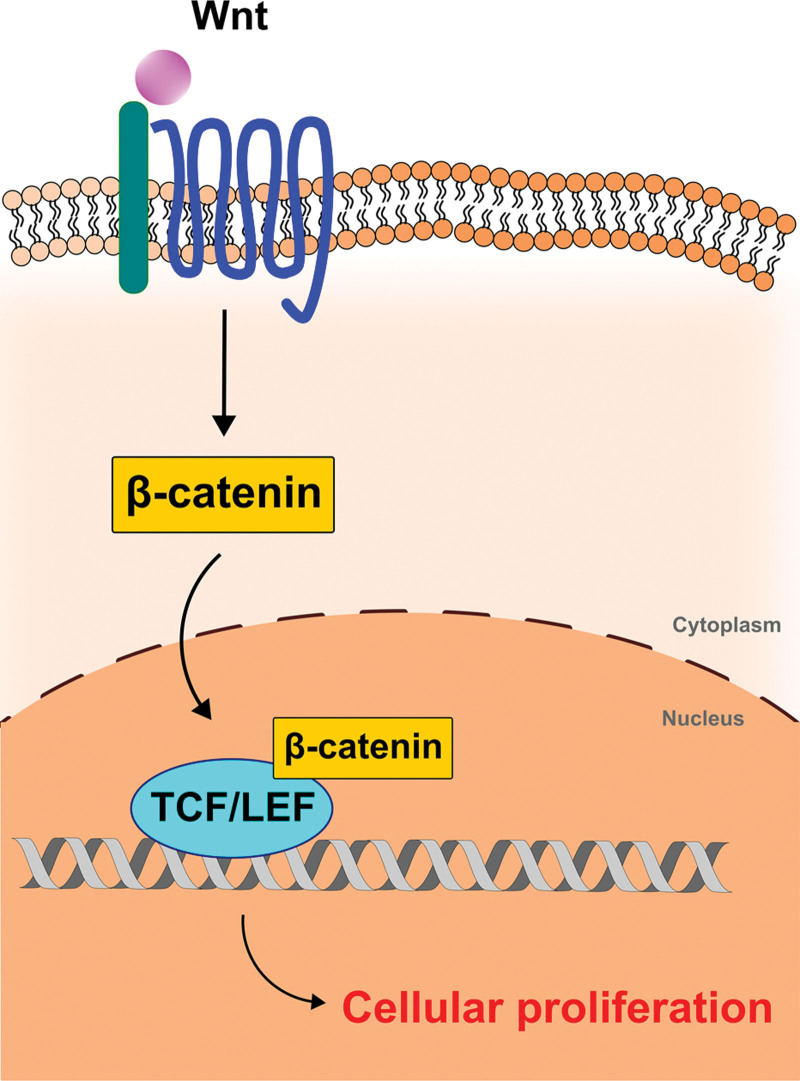
The molecular biology behind exosome signaling is daunting and not yet fully understood in the literature. Although one of the most studied exosome derivatives stems from adipose tissue, it is important to note that each derivative tissue or cell may exert specific downstream effects via its own unique mechanism. A commonly reported exosome target exerts manipulation of the TGF-β (transforming growth factor beta) superfamily, which is essential for cell differentiation, proliferation, and apoptosis (Fig. 1).6–12 TGF-β induces phosphorylation of intracellular SMAD complexes, which translocate to the nucleus and regulate gene transcription/expression. TGF-β can also activate other signaling mechanisms, such as mitogen-activated protein kinase, extracellular signal-regulated kinase, and c-Jun N-terminal kinase, and selectively modulate the cutaneous microenvironment through a single pathway. The resultant downstream effects predominantly target collagen degradation and ECM remodeling through upregulation or downregulation of matrix metalloproteinases (MMPs). Alternatively, dermal papilla-derived exosomes and keratinocyte-derived exosomes reportedly use the Wnt/β-catenin pathway (Fig. 2) potentially inhibited by adipose-derived exosomes.13–15 Known specific functions of each exosome type are discussed further throughout this review.
Fig. 1.
TGF-β pathway in exosome signaling. Adipose-derived exosomes are shown to work via both ERK/MAPK and TGF-β/SMAD pathways. TGF-β induces phosphorylation of intracellular SMAD complexes, which translocate to the nucleus. These SMAD complexes regulate gene transcription/expression. TGF-β also promotes the MAPK/EPK/JNK pathway, which can selectively augment collagen degradation and ECM remodeling downstream. Both pathways promote cell proliferation, differentiation, or apoptosis involved in scar formation. SMAD indicates decapentaplegic; MAPKs, mitogen-activated protein kinases; ERK, extracellular signal-regulated kinase; JNK, c-Jun N-terminal kinase.
Fig. 2.
Wnt/β-catenin pathway in exosome signaling. Dermal papilla-derived exosomes and keratinocyte-derived exosomes stimulate Wnt to upregulate β-catenin. β-catenin then binds TCF/LEF transcription factors in the nucleus, which promotes hair growth. TCF/LEF indicates T-cell factor/lymphoid enhancer factor; Wnt, wingless-related integration site.
APPLICATIONS IN AESTHETIC SURGERY
Nonsurgical Skin Rejuvenation
Cutaneous aging is a complex mechanism involving both intrinsic and extrinsic processes that manifest clinically as loss of epidermal and dermal thickness, deepening rhytids, pore enlargement, dyspigmentation, and diminished soft tissue elasticity.16 Skin aging is multifactorial, but a key component is senescence of vitally important cells, such as keratinocytes, fibroblasts, and melanocytes; a process now believed to be mediated impart by miRNA dysregulation.17 Consequently, structural and functional changes within the ECM occur, such as decreased organization and production of collagen, elastin, and proteoglycans–all necessary for youthful tensile strength, elasticity, and hydration of skin. Many models have been proposed as exacerbating aging, including oxidative stress, DNA damage, telomere shortening, miRNA regulation, advanced glycation end-product accumulation, genetic mutation, and inflammaging. Exosomes are thought to primarily act on oxidative stress and inflammaging pathways, impacting both ECM and collagen.17
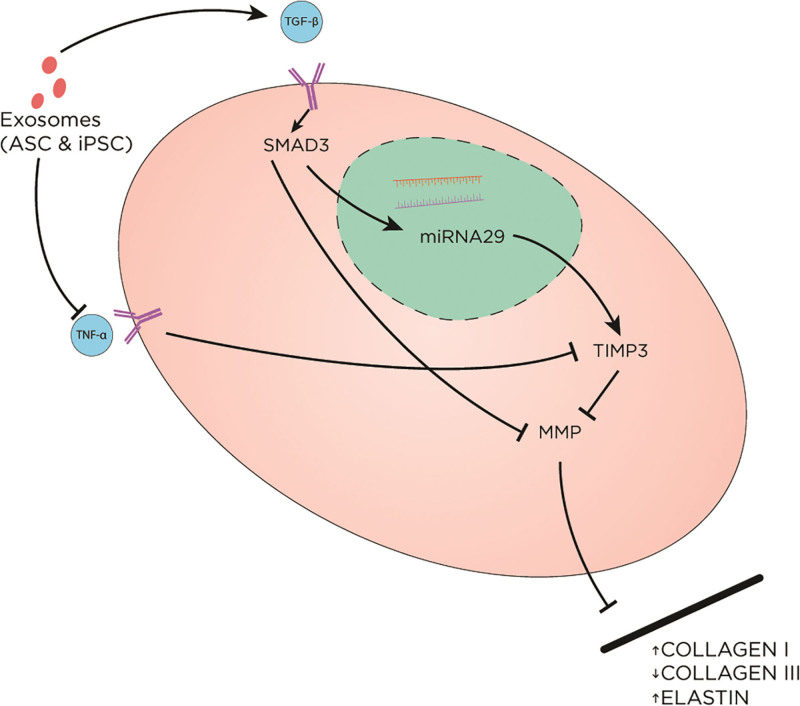
Exosome therapy raises interest by fibroblast proliferation and migration stimulation. In vitro and in vivo studies of UV-B photoaged models have shown that exosome treatment protects cells from UV-B damage by decreasing inflammatory markers, such as tumor necrosis factor alpha (TNF-α), while upregulating TGF-β and tissue inhibitor of MMP (TIMP) (Fig. 3).6,18 These mechanisms led to a reversal of fibroblast senescence with upregulation of collagen I, elastin, and fibronectin production and decreased expression of collagen III.19
Fig. 3.
The effect of exosomes on the extracellular matrix. Exosomes (derived from ASC and iPSC) upregulate TGF-β and downregulate TNF-α. TGF-β promotes SMAD pathway, leading to increased TIMP3. On the contrary, TNF-α blocks TIMP3. MMP is involved in ECM remodeling via breaking down collagen I and stimulating collagen III production. MMP is inhibited as a result of exosome treatment, leading to overall increased collagen I and elastin and decreased collagen III. ASC indicates adipose-derived stem cell-derived exosomes; iPSC, human induced pluripotent stem cell-derived exosomes; SMAD, decapentaplegic.
Recent studies evaluating topical exosomes in conjunction with nonsurgical facial treatments have displayed synergistic effects.20–22 Chernoff20 found that combining topical exosomes with facial microneedling produced greater skin quality, tone, texture, vascularity, clarity, and overall higher satisfaction among patients compared with microneedling alone. Duncan 21 added topical exosomes after facial rejuvenation procedures such as laser resurfacing, resulting in faster recovery and fewer side effects compared with laser resurfacing alone. A clinical trial conducted by Cho et al22 for hyperpigmentation treatment using adipose-derived stem cell exosomes showed a significant decrease in melanin content in the treatment group, though the decreased-melanin activity was short-lived. (See table, Supplemental Digital Content 1, which summarizes studies elucidating the proposed mechanisms of action for exosomes in skin rejuvenation and pigment regulation as well as potential applications http://links.lww.com/PRSGO/C604).
Scar Revision
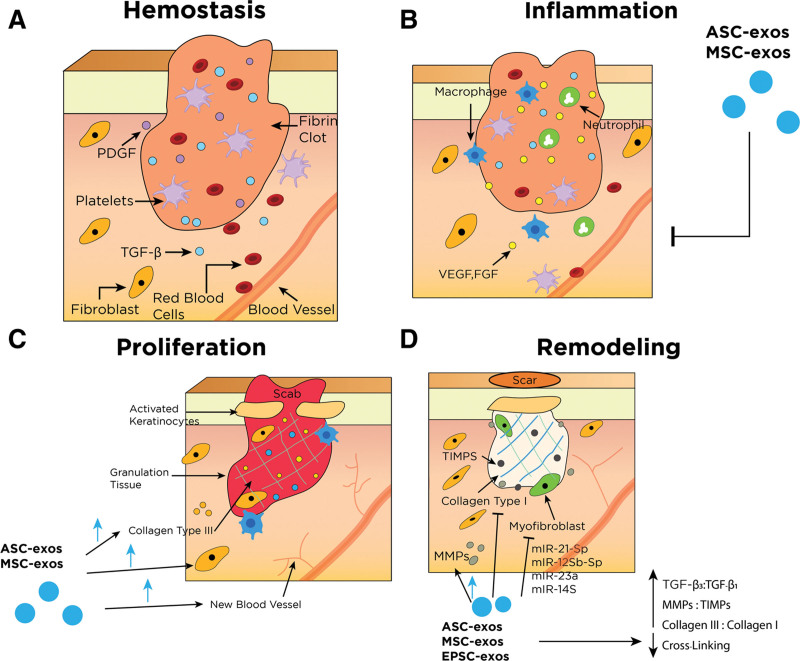
Poor, nonaesthetic scarring can present as atrophic, widened, hypertrophic, or keloid.23 Although multiple surgical and nonsurgical modalities exist, outcomes are highly variable and can be associated with a high rate of recurrence.24,25 Studies have investigated exosomes' effectiveness in reducing scar formation and promoting cutaneous regeneration through various mechanisms. (See table, Supplemental Digital Content 2, which summarizes the mechanism of exosomes in scar revision, http://links.lww.com/PRSGO/C605.) Exosomes impact several cells and signaling molecules involved in the four traditional phases of wound healing, thereby modulating the progression, length, and characteristics of the healing process (Fig. 4).9,11,12,26–32
Fig. 4.
Simplified wound healing process and its main effectors. *Other key components have been removed for simplification. Refer to table, Supplementary Digital Content 2 for more details regarding different types of exosomes and their respective effects, http://links.lww.com/PRSGO/C605. A, Hemostasis begins immediately following a disruption of epithelial integrity. This phase is characterized by the restriction of blood flow and the formation of blood clots to the injury site to control bleeding. The clot and surrounding tissue release several cytokines and growth factors that recruit inflammatory cells (chemotaxis) to the injury site and progress to the next phase. B, Inflammatory phase is characterized by localized tissue swelling via influx of inflammatory cells (neutrophils and macrophages) and transudate. Inflammatory cells are recruited to the area to degrade cellular debris and prevent infection. Macrophages play an essential role in this phase as they promote the transition to the proliferative healing phase by stimulating keratinocytes, fibroblasts, and angiogenesis. Exosomes are thought to be able to tone down the inflammatory response and prevent secondary injury in this phase. C, Proliferative phase is characterized by reepithelialization. Fibroblasts lay down a scaffold of collagen III and ECM in the wound bed and strengthen new granulation tissue. Keratinocytes migrate across the wound surface to close the defect. Exosomes increase proliferation and migration of fibroblasts, collagen III deposition, and angiogenesis. D, Remodeling phase is the final phase of wound healing and can last for years. This stage is characterized by ECM and tissue remodeling. Collagen I begins to replace collagen III and cross-linking matures, leading to flattening of scars. Myofibroblasts contract and approximate wound edges. Exosomes reduce excessive cross-linking and increased ratios of collagen III: collagen I, MMPs:TIMPs, and TGF-β3:TGF-β1. Exosomes also inhibit myofibroblast differentiation via several miRNAs’ actions. ASC-exos indicates adipose-derived exosomes; MSC-exos, mesenchymal stem cell-derived exosomes; EPSC-exos, epidermal stem cell-derived exosomes; PDGF, platelet-derived growth factor; VEGF, vascular endothelial growth factor; EGF, epidermal growth factor; FGF, fibroblast growth factor; KGF, keratinocyte growth factor.
Molecular processes involved in aesthetic scar outcomes include decreased cross-linking, minimal myofibroblasts, higher ratios of collagen III to collagen I, TGF-β3 to TGF-β1, and MMP to TIMP, which were all found to be targets of exosomes therapy.11,33 Exosomes derived from various source cell types, including epidermal stem cells, induced pluripotent stem cells, and mesenchymal stem cells (adipose tissue, bone marrow, and umbilical cord), mitigate collagen production, distribution, and the ratio of type III to type I collagen.9,26,27,30,31 By increasing the ratio of collagen III to collagen I, adipose-derived exosomes suppress properties associated with poor scarring such as excessive collagen deposition and aberrant cross-linking.29,30 Exosomes are also reported to inhibit myofibroblasts differentiation, keloid fibroblasts/hypertrophic scar fibroblasts proliferation, and migration.7,8,34
Current proposed mechanisms include blocking various components of the TGF-β/SMAD pathway (Fig. 1), resulting in overall decreased collagen production.9,11,26 Other proposed exosome-induced features include enhanced angiogenesis, reepithelialization, skin appendage regeneration, matrix deposition, cellular proliferation, and migration lending to improved scar outcomes.4,26,27,29,31,35 Exosomes have also been investigated for use in combination with scaffolding agents, such as FHE hydrogel (Pluronic F127 + oxidative hyaluronic acid + poly-ε-L-lysine), FEP dressing (Pluronic F127+ PEI + APu), and hyaluronic acid, with published results demonstrating a synergistic effect.4,35,36 Kwon et al10 published a clinical trial in 2020 assessing exosomes’ potential effects in preventing scar formation and showed a combination of adipose-derived exosomes with fractional carbon dioxide laser for facial acne scars yielded less erythema at treatment sites, shorter posttreatment downtime, fewer side effects, and overall better outcome compared with fractional carbon dioxide laser treatment alone. (See table, Supplemental Digital Content 2, which summarizes the proposed mechanisms of action for exosomes in scar revision and their potential applications, http://links.lww.com/PRSGO/C605.)
Hair Restoration
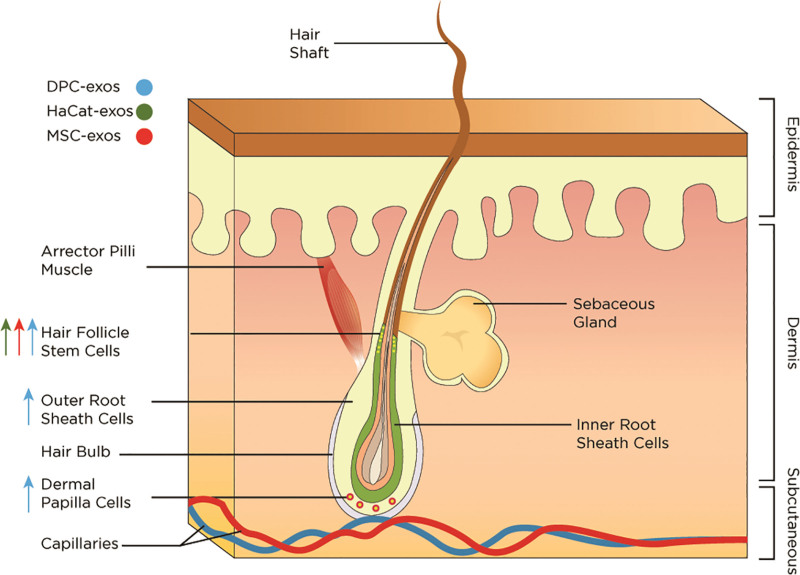
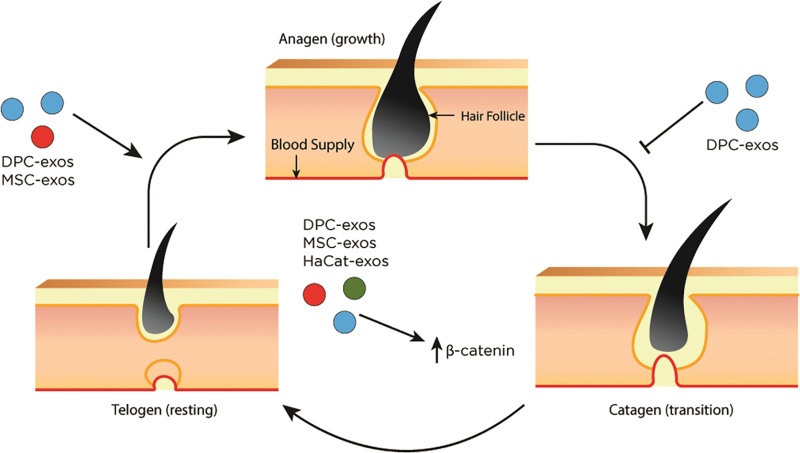
The etiology of hair loss is multifactorial. Normal hair growth occurs at the level of the hair follicle as a continuous three-phase cycle: anagen (active growth), catagen (transition and involution), and telogen (resting).37 Dermal papilla cells (DPCs), a key regulator of hair follicle development, are known to release several growth factors and communicate with epithelial cells, germ cells, and stem cells within the hair follicles.38 Hair follicle stem cells are responsible for normal hair growth and rapidly proliferate during a new hair cycle.39 Several mechanisms have been proposed regarding the exosome’s ability to replenish signals for hair growth. (See table, Supplemental Digital Content 3, which summarizes the mechanism of exosomes in hair restoration, http://links.lww.com/PRSGO/C606.) Exosomes were found to significantly induce and prolong the anagen phase, resulting in hair growth.13,14,40 Dermal papilla-derived exosomes upregulate Wnt/β-catenin signaling, a key cellular pathway involved in the regulation of hair regeneration and morphogenesis (Fig. 2).14,41,42 Additionally, dermal papilla-derived exosomes increased sonic hedgehog expression, an important player in hair follicle proliferation, as well as increased outer root sheath cell proliferation and migration.43 Interestingly, dermal papilla-derived exosomes displayed a superior effect when in spheroid form.13,40 Spheroid form of dermal papilla-derived exosomes demonstrated better capability to induce progression from telogen to anagen compared with the two-dimensional DPCs or minoxidil treatment.13 This specific form of exosomes also led to stimulated growth and viability of DPCs and outer root stem cells and facilitated elongation of hair shafts.40
Dermal papilla-derived exosomes present multiple benefits to overall hair growth. In addition to hair follicle stem cells proliferation and differentiation by way of miRNA involvement,44 longer hair shafts and hair bulges were also noted.14,40 Other source cells that have been studied for use in hair growth include mesenchymal stem cells and keratinocytes.45–47 A study conducted by Yang et al46 using microneedling, exosomes, and UK5099 (a potent inhibitor of the mitochondrial pyruvate carrier) showed increased efficiency at a lower dosage compared with subcutaneous exosome injection-alone. The effects of exosomes in hair restoration are depicted in Figures 5 and 6. (See table, Supplementary Digital Content 3, which summarizes the proposed mechanisms of action for exosomes in hair restoration and their potential applications, http://links.lww.com/PRSGO/C606.)
Fig. 5.
The effects of exosomes on hair follicles. Exosomes promote proliferation and differentiation of hair follicle stem cells, outer root sheath cells, and dermal papilla cells. DPC-exos indicates dermal papilla-derived exosomes; HaCaT-exos, keratinocyte-derived exosomes; MSC-exos, mesenchymal stem cell-derived exosomes.
Fig. 6.
The effects of exosomes on hair growth cycle. Hair follicle growth consists of a continuous three-phase cycle: anagen, catagen, and telogen. Exosomes stimulate the conversion from telogen to anagen and delay the progression to catagen, resulting in prolonged anagen. Exosomes also increase levels of β-catenin, leading to augmented hair growth. DPC-exos indicates dermal papilla-derived exosomes; HaCaT-exos, keratinocyte-derived exosomes; MSC-exos, mesenchymal stem cell-derived exosomes.
Current FDA-approved on-market hair restoration medications (ie, Minoxidil and Finasteride) can yield variable results.48,49 Minoxidil primarily exerts therapeutic function through vasodilatory and proangiogenic effects—unlikely to stimulate hair growth if DPC dormancy persists.13 In contrast, exosomes have demonstrated an ability to stimulate the growth and viability of DPCs, which may be more effective in hair loss restoration than minoxidil.40 In a clinical trial conducted by Huh and Kwon,47 exosome treatment for 12 weeks resulted in increased hair density and thickness, with no side effects reported. Although early published data appear promising, further clinical investigation is warranted in this field.
Weight Loss and Body and Breast Contouring
Obesity has been shown to affect the overall miRNA profile of an individual.50 Adipose-derived exosomes are involved in adipocyte differentiation and lipid production through the effects of miR-450a-50, and the activation of the hedgehog signaling pathway.15,51 Although these identified miRNAs have the potential to serve as targeted pathways for augmenting weight loss through exosome therapy, it is important to note that this idea is theoretical, and there is currently no additional literature or published data on this specific topic. Therefore, further research is needed to determine the feasibility of this theoretical possibility.
Autologous fat grafting (AFG) is a commonly utilized technique throughout the body with many applications, notably in postradiated breast reconstruction, cosmetic buttock augmentation, and breast augmentation. Although AFG-only breast augmentation avoids conventional prosthetic implant-related complications, a main challenge of AFG is the variable survival rate and inconsistency of grafted fat.52,53 To overcome this challenge, cell-assisted lipotransfer (CAL), the combination of adipose-derived stem cell-rich stromal vascular fraction with lipoaspirate, was developed to enhance adipogenesis and angiogenesis of the autologous fat.54 Several published studies have demonstrated the favorable results of CAL in increasing graft survival and enhancing engrafted fat volume retention given the regenerative and angiogenesis properties of adipose stem cells.54–56 As the functional molecule secreted by adipose stem cells, adipose-derived exosomes were shown to exhibit comparable effects in improving fat graft retention compared to adipose stem cells.57 Not only did adipose-derived exosomes promote survival, attenuate inflammation, and enhance neovascularization in fat grafts, Han et al. found that hypoxic preconditioning could further enhance those effects of adipose-derived exosomes.58 Additionally, in the setting of prior breast cancer, the rate of oncological recurrence was not increased in postmastectomy patients following AFG, indicating the potential use of exosomes for both cosmetic and reconstructive purposes. Although current available data suggest that adipose-derived exosomes could be a promising candidate to promote graft survival in lipotransfer, the optimal concentrations, source cells, treatment duration, and possible complications of exosome therapy are yet delineated.59
PROCUREMENT AND PREPARATION
Although various basic science and clinical studies have outlined the potential benefits of exosomes, the FDA has yet to approve exosome utilization as a topical, injectable, or intravenous treatment. Given this controversy, several exosome manufacturers have elected to advertise their products using umbrella terms such as “secretome” or “ECV.” Likewise, clinicians have been apprehensive to openly advertise this treatment modality. Despite this, an online search performed by our collaborative team found six companies that produce and supply exosome products for clinical use (Table 1).
Table 1.
Exosome Products and Manufacturers
| Name (City, State, Country) | Source Cells/Derivative | Constituents (Exosome Concentration in Billions) per Unit | Storage °C | Delivery | Thawing/Reconstitution Process | Shelf Life before/after Reconstitution or Thawing | Advertised Utilization | Approximate Surface Area for Treatment | Cost per Treatment of Face (USD) |
|---|---|---|---|---|---|---|---|---|---|
| Benev (Mission Viejo, CA, USA) | Adult adipose stem cell | 20 mL lyophilized exosome (2.5 or 5 billion) +5 mL diluent (hyaluronic acid, growth factors, coenzymes, vitamins) | Room temperature | Shipped frozen with ice pack | Reconstitute with the provided diluent or saline | 2 wk/20 min | Topical for facial rejuvenation and hair restoration | 1–2 mL per face | $68–116 |
| Direct Biologics (Austin, TX, USA) | Adult bone marrow stem cell | 1 mL exosome + 9 mL saline | −40 to −80, not in liquid nitrogen | N/A | No additional process required | 5 y/- | Topical for facial rejuvenation | 1-2 mL per face | N/A |
| Exocel Bio (San Diego, CA, USA) | Placental chorion stem cell | Exosome (5, 12, 25, 100, or 400 billion) mixed with 2–5 mL saline | -80 | Shipped on dry ice | Use as it is after thawing or can reconstitute 1:1 with saline | 6 mo–1 y/1 h | Topical for facial rejuvenation and hair restoration | 1–2 mL per face | $150–4950 |
| Kimera (Miramar, FL, USA) | Placental chorionic and amniotic stem cell | Exosome (1 or 5 billion) mixed with 1–5 mL saline | −20, not in liquid nitrogen | Shipped on dry ice | No additional process required | 1 y/48 h | Topical for facial rejuvenation, hair restoration (or scalp injection), and scar revision | 1 mL per face, and 5 mL for face, neck, and décolleté | $200–550 |
| Regan Suppliers (Scottsdale, AZ, USA) | Wharton’s Jelly stem cell | Exosome mixed with saline (1.5 billion) | −80 | Shipped on dry ice | After thawing, reconstitute 1:3 exosome to saline | 1 y/1 h | Topical and injection for facial rejuvenation and hair restoration | 1 mL per area of injection, 2 mL for hair, and 5 mL IV | $775–1075 |
| Elevai (Newport Beach, CA, USA) | Wharton’s Jelly stem cell | Exosome serum 5 mL | Room temperature | N/A | No additional process required | 1 y/- | Topical for facial rejuvenation and depigmentation | 1 mL per face | $75–149 |
| Disclose–no financial interest | |||||||||
HA, hyaluronic acid.
Some of the most notable differences among manufacturers are the source cells, methods of isolation/procurement, reconstitution requirement, and shelf life. Kimera (Miramar, Fla.), Regan Suppliers (Scottsdale, Ariz.), and Exocel Bio (San Diego, Calif.) provide aqueous exosome solutions that require frozen storage with a shelf life between 6 months to a year. Once thawed, the products can either be applied directly over the skin or mixed with saline. The unused thawed solution can be stored in a refrigerator for up to 48 hours. In contrast, Benev (Mission Viejo, Calif.) provides a freeze-dry “lyophilized” form, which requires reconstitution before use. The freeze-dry form can be stored at room temperature for up to 2 weeks before reconstitution; however, the reconstituted material must be used within 20 minutes of activation. In terms of source cells, companies listed in Table 1 derive their products from four different parental cell lines. Refer to Table 1 for more detailed information regarding source cells, exosome processing, and indicated utilization from the manufacturers. Concerning origin cell-line derivatives, potential differences in effectiveness and/or outcome based on source cells have not yet been delineated.
Future Directions
Although both interesting and promising results of exosomes are reported herein, additional studies are warranted to further investigate this topic. As with any biological therapy, one of the main challenges exosome products encounter is the stability of active ingredients.16 Therefore, additional basic science research is necessary to optimize the purification and procurement processes and mitigate the existing heterogeneity across current literature due to variable exosome source cells and purification methods. Additionally, direct comparative studies between exosomes and other modalities that may provide similar effects, such as stem cells and platelet-rich-plasma (PRP), are needed. Although the current limited evidence suggests that the cell-free characteristics of exosomes may minimize the risk of unpredictable patterns that can be associated with stem cell–based therapies, definitive evidence is currently lacking.60–62 PRP is a concentrated, autologous-derived product currently used as an adjunct for regenerative potential, and although reported PRP-based outcomes are via growth factors, the exosome itself stimulates the production of natural growth factors via mRNA activation.8,9,12,16,27,34,60 Due to laboratory-based production of exosomes, supply is nearly indefinite without human donor-related resource limitation and may contain a higher concentration of active ingredients such as growth factors, mRNAs, and cytokines when compared with PRP. The potential synergistic benefits of exosomes and PRP have yet to be fully delineated. Notably, the FDA currently recommends against more than minimally manipulated stem cell-based products, and thus, the combination of PRP and exosomes could be deemed unauthorized if it results in the alteration of the original biological characteristics.63 Additional investigation on this specific topic is required as a definitive statement on PRP and exosomes cannot yet be concluded based on the available evidence at this time. Finally, large randomized controlled trials are warranted for a better understanding of the potential impact and benefit of exosomes, overall effectiveness, associated outcomes, long-term efficacy, and safety profile.
Limitations
There are several pertinent limitations to this review that warrant discussion. First, it is important to note that this is not a systematic review and, thus, does not use strict inclusion or exclusion criteria. The majority of the studies included in this review are preclinical, reflecting a paucity of published literature on exosome application in the plastic surgery community. Published clinical reports are based on small patient cohorts and/or provide anecdotal evidence, likely due to the absence of FDA approval. Furthermore, these clinical studies provide little evidence nor elaborate on potential or observed adverse effects. Heterogeneity among these clinical studies arises from different exosome source cells and various purification processes, precluding head-to-head comparisons. Finally, there is currently no published evidence on the long-term results of exosome use.
CONCLUSIONS
Exosome utilization in medicine is receiving increasing attention and is a growing area of interest in plastic surgery. Despite several manufacturers reporting “status pending” on FDA approval on topical and intravenous infusion-based modalities, no exosome-based products are currently approved by the FDA. Further clinical studies are warranted to establish the impact, benefits, effectiveness, outcomes, and safety profile of exosomes in plastic surgery.
Supplementary Material
Footnotes
Published online 12 June 2023.
Presented at Plastic Surgery the Meeting 2022, October 27, 2022, Boston, MA.
Disclosure statements are at the end of this article, following the correspondence information.
Related Digital Media are available in the full-text version of the article on www.PRSGlobalOpen.com.
REFERENCES
- 1.Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367:eaau6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnstone RM, Bianchini A, Teng K. Reticulocyte maturation and exosome release: transferrin receptor containing exosomes shows multiple plasma membrane functions. Blood. 1989;74:1844–1851. [PubMed] [Google Scholar]
- 3.Rashed MH, Bayraktar E, Helal GK, et al. Exosomes: from garbage bins to promising therapeutic targets. Int J Mol Sci. 2017;18:E538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang M, Wang C, Chen M, et al. Efficient angiogenesis-based diabetic wound healing/skin reconstruction through bioactive antibacterial adhesive ultraviolet shielding nanodressing with exosome release. ACS Nano. 2019;13:10279–10293. [DOI] [PubMed] [Google Scholar]
- 5.Sullivan D, Chung KC, Eaves FF, et al. The level of evidence pyramid: indicating levels of evidence in plastic and reconstructive surgery articles. Plast Reconstr Surg. 2011;128:311–314. [DOI] [PubMed] [Google Scholar]
- 6.Hu S, Li Z, Cores J, et al. Needle-free injection of exosomes derived from human dermal fibroblast spheroids ameliorates skin photoaging. ACS Nano. 2019;13:11273–11282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu ZY, Zhang HJ, Zhou ZH, et al. The effect of inhibiting exosomes derived from adipose-derived stem cells via the TGF-β1/SMAD pathway on the fibrosis of keloid fibroblasts. Gland Surg. 2021;10:1046–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yuan R, Dai X, Li Y, et al. Exosomes from miR-29a-modified adipose-derived mesenchymal stem cells reduce excessive scar formation by inhibiting TGF-β2/SMAD3 signaling. Mol Med Rep. 2021;24:758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duan M, Zhang Y, Zhang H, et al. Epidermal stem cell-derived exosomes promote skin regeneration by downregulating transforming growth factor-β1 in wound healing. Stem Cell Res Ther. 2020;11:452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kwon HH, Yang SH, Lee J, et al. Combination treatment with human adipose tissue stem cell-derived exosomes and fractional CO2 laser for acne scars: a 12-week prospective, double-blind, randomized, split-face study. Acta Derm Venereol. 2020;100:adv00310adv00310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang L, Hu L, Zhou X, et al. Exosomes secreted by human adipose mesenchymal stem cells promote scarless cutaneous repair by regulating extracellular matrix remodelling. Sci Rep. 2017;7:13321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fang S, Xu C, Zhang Y, et al. Umbilical cord-derived mesenchymal stem cell-derived exosomal MicroRNAs suppress myofibroblast differentiation by inhibiting the transforming growth factor-β/SMAD2 pathway during wound healing. Stem Cells Transl Med. 2016;5:1425–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu S, Li Z, Lutz H, et al. Dermal exosomes containing miR-218-5p promote hair regeneration by regulating β-catenin signaling. Sci Adv. 2020;6:eaba1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou L, Wang H, Jing J, et al. Regulation of hair follicle development by exosomes derived from dermal papilla cells. Biochem Biophys Res Commun. 2018;500:325–332. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y, Yu M, Dai M, et al. miR-450a-5p within rat adipose tissue exosome-like vesicles promotes adipogenic differentiation by targeting WISP2. J Cell Sci. 2017;130:1158–1168. [DOI] [PubMed] [Google Scholar]
- 16.Lei X, Xu P, Cheng B. Problems and solutions for platelet-rich plasma in facial rejuvenation: a systematic review. Aesthetic Plast Surg. 2019;43:457–469. [DOI] [PubMed] [Google Scholar]
- 17.Zhang S, Duan E. Fighting against skin aging: the way from bench to bedside. Cell Transplant. 2018;27:729–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oh M, Lee J, Kim YJ, et al. Exosomes derived from human induced pluripotent stem cells ameliorate the aging of skin fibroblasts. Int J Mol Sci. 2018;19:E1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liang JX, Liao X, Li SH, et al. Antiaging properties of exosomes from adipose-derived mesenchymal stem cells in photoaged rat skin. Biomed Res Int. 2020;2020:6406395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greg C. The utilization of human placental mesenchymal stem cell derived exosomes in aging skin: an investigational pilot study. J Surg. 2021;6:1388. [Google Scholar]
- 21.Duncan DI. Combining PDO threads with exosomes for microlifting. Cosmetic Surgery. IntechOpen. 2020. https://www.intechopen.com/chapters/71782. Accessed November 15, 2022. [Google Scholar]
- 22.Cho BS, Lee J, Won Y, et al. Skin brightening efficacy of exosomes derived from human adipose tissue-derived stem/stromal cells: a prospective, split-face, randomized placebo-controlled study. Cosmetics. 2020;7:9090. [Google Scholar]
- 23.Bayat A, McGrouther DA, Ferguson MWJ. Skin scarring. BMJ. 2003;326:88–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gauglitz GG, Korting HC, Pavicic T, et al. Hypertrophic scarring and keloids: pathomechanisms and current and emerging treatment strategies. Mol Med. 2011;17:113–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lumenta DB, Siepmann E, Kamolz LP. Internet-based survey on current practice for evaluation, prevention, and treatment of scars, hypertrophic scars, and keloids. Wound Repair Regen. 2014;22:483–491. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Y, Pan Y, Liu Y, et al. Exosomes derived from human umbilical cord blood mesenchymal stem cells stimulate regenerative wound healing via transforming growth factor-β receptor inhibition. Stem Cell Res Ther. 2021;12:434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu Y, Rao SS, Wang ZX, et al. Exosomes from human umbilical cord blood accelerate cutaneous wound healing through miR-21-3p-mediated promotion of angiogenesis and fibroblast function. Theranostics. 2018;8:169–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guo S, Dipietro LA. Factors affecting wound healing. J Dent Res. 2010;89:219–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou Y, Zhao B, Zhang XL, et al. Combined topical and systemic administration with human adipose-derived mesenchymal stem cells (hADSC) and hADSC-derived exosomes markedly promoted cutaneous wound healing and regeneration. Stem Cell Res Ther. 2021;12:257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu L, Wang J, Zhou X, et al. Exosomes derived from human adipose mensenchymal stem cells accelerates cutaneous wound healing via optimizing the characteristics of fibroblasts. Sci Rep. 2016;6:32993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang J, Guan J, Niu X, et al. Exosomes released from human induced pluripotent stem cells-derived MSCs facilitate cutaneous wound healing by promoting collagen synthesis and angiogenesis. J Transl Med. 2015;13:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vu NB, Nguyen HT, Palumbo R, et al. Stem cell-derived exosomes for wound healing: current status and promising directions. Minerva Med. 2021;112:384–400. [DOI] [PubMed] [Google Scholar]
- 33.Yates CC, Hebda P, Wells A. Skin wound healing and scarring: fetal wounds and regenerative restitution. Birth Defects Res C Embryo Today. 2012;96:325–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Y, Zhang J, Shi J, et al. Exosomes derived from human adipose mesenchymal stem cells attenuate hypertrophic scar fibrosis by miR-192-5p/IL-17RA/SMAD axis. Stem Cell Res Ther. 2021;12:221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang C, Wang M, Xu T, et al. Engineering bioactive self-healing antibacterial exosomes hydrogel for promoting chronic diabetic wound healing and complete skin regeneration. Theranostics. 2019;9:65–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu K, Chen C, Zhang H, et al. Adipose stem cell-derived exosomes in combination with hyaluronic acid accelerate wound healing through enhancing re-epithelialization and vascularization. Br J Dermatol. 2019;181:854–856. [DOI] [PubMed] [Google Scholar]
- 37.Erdogen B. Anatomy and physiology of hair. Hair and Scalp Disorders. IntechOpen; 2017. https://www.intechopen.com/chapters/53880. Accessed November 15, 2022. [Google Scholar]
- 38.Kang JI, Choi YK, Koh YS, et al. Vanillic acid stimulates anagen signaling via the PI3K/Akt/ β-catenin pathway in dermal papilla cells. Biomol Ther. 2020;28:354–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Flores A, Schell J, Krall AS, et al. Lactate dehydrogenase activity drives hair follicle stem cell activation. Nat Cell Biol. 2017;19:1017–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kwack MH, Seo CH, Gangadaran P, et al. Exosomes derived from human dermal papilla cells promote hair growth in cultured human hair follicles and augment the hair-inductive capacity of cultured dermal papilla spheres. Exp Dermatol. 2019;28:854–857. [DOI] [PubMed] [Google Scholar]
- 41.Choi BY. Targeting Wnt/β-Catenin pathway for developing therapies for hair loss. Int J Mol Sci. 2020;21:E4915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Veltri A, Lang C, Lien WH. Concise review: wnt signaling pathways in skin development and epidermal stem cells. Stem Cells. 2018;36:22–35. [DOI] [PubMed] [Google Scholar]
- 43.Suzuki K, Yamaguchi Y, Villacorte M, et al. Embryonic hair follicle fate change by augmented beta-catenin through Shh and Bmp signaling. Development. 2009;136:367–372. [DOI] [PubMed] [Google Scholar]
- 44.Yan H, Gao Y, Ding Q, et al. Exosomal micro RNAs derived from dermal papilla cells mediate hair follicle stem cell proliferation and differentiation. Int J Biol Sci. 2019;15:1368–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ogawa M, Udono M, Teruya K, et al. Exosomes derived from fisetin-treated keratinocytes mediate hair growth promotion. Nutrients. 2021;13:20872087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang G, Chen Q, Wen D, et al. A therapeutic microneedle patch made from hair-derived keratin for promoting hair regrowth. ACS Nano. 2019;13:4354–4360. [DOI] [PubMed] [Google Scholar]
- 47.Huh CH, Kwon SH. Exosome for hair regeneration: from bench to bedside. J Am Acad Dermatol. 2019;81:AB62. [Google Scholar]
- 48.Mohammadi P, Youssef KK, Abbasalizadeh S, et al. Human hair reconstruction: close, but yet so far. Stem Cells Dev. 2016;25:1767–1779. [DOI] [PubMed] [Google Scholar]
- 49.Yuan AR, Bian Q, Gao JQ. Current advances in stem cell-based therapies for hair regeneration. Eur J Pharmacol. 2020;881:173197. [DOI] [PubMed] [Google Scholar]
- 50.Eirin A, Meng Y, Zhu XY, et al. The micro-RNA cargo of extracellular vesicles released by human adipose tissue-derived mesenchymal stem cells is modified by obesity. Front Cell Dev Biol. 2021;9:660851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ji Z, Cai Z, Gu S, et al. Exosomes derived from human adipose-derived stem cells inhibit lipogenesis involving hedgehog signaling pathway. Front Bioeng Biotechnol. 2021;9:734810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Salibian AA, Frey JD, Bekisz JM, et al. Fat grafting and breast augmentation: a systematic review of primary composite augmentation. Plast Reconstr Surg Glob Open. 2019;7:e2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Glass GE, Ferretti P. Adipose-derived stem cells in aesthetic surgery. Aesthet Surg J. 2019;39:423–438. [DOI] [PubMed] [Google Scholar]
- 54.Yoshimura K, Sato K, Aoi N, et al. Cell-assisted lipotransfer for cosmetic breast augmentation: supportive use of adipose-derived stem/stromal cells. Aesthetic Plast Surg. 2008;32:48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sterodimas A, de Faria J, Nicaretta B, et al. Autologous fat transplantation versus adipose-derived stem cell-enriched lipografts: a study. Aesthet Surg J. 2011;31:682–693. [DOI] [PubMed] [Google Scholar]
- 56.Kølle SFT, Duscher D, Taudorf M, et al. Ex vivo-expanded autologous adipose tissue-derived stromal cells ensure enhanced fat graft retention in breast augmentation: a randomized controlled clinical trial. Stem Cells Transl Med. 2020;9:1277–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen B, Cai J, Wei Y, et al. Exosomes are comparable to source adipose stem cells in fat graft retention with up-regulating early inflammation and angiogenesis. Plast Reconstr Surg. 2019;144:816e–827e. [DOI] [PubMed] [Google Scholar]
- 58.Han Y di, Bai Y, Yan XL, et al. Co-transplantation of exosomes derived from hypoxia-preconditioned adipose mesenchymal stem cells promotes neovascularization and graft survival in fat grafting. Biochem Biophys Res Commun. 2018;497:305–312. [DOI] [PubMed] [Google Scholar]
- 59.Groen J, Negenborn V, Twisk D, et al. Autologous fat grafting in onco-plastic breast reconstruction: a systematic review on oncological and radiological safety, complications, volume retention and patient/surgeon satisfaction. J Plast Reconstr Aesthet Surg. https://pubmed.ncbi.nlm.nih.gov/27085611/. Accessed November 15, 2022. [DOI] [PubMed] [Google Scholar]
- 60.Guo SC, Tao SC, Yin WJ, et al. Exosomes derived from platelet-rich plasma promote the re-epithelization of chronic cutaneous wounds via activation of YAP in a diabetic rat model. Theranostics. 2017;7:81–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Perocheau D, Touramanidou L, Gurung S, et al. Clinical applications for exosomes: are we there yet? Br J Pharmacol. 2021;178:2375–2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Semsarzadeh N, Andrasik W, Khetarpal S. Stem cells and exosomes in aesthetic medicine. Adv Cosmet Surg. 2021;4:59–70. [Google Scholar]
- 63.U.S. Food and Drug Administration. Regulatory considerations for human cells, tissues, and cellular and tissue-based products: minimal manipulation and homologous use. July 2020. Available at https://www.fda.gov/regulatory-information/search-fda-guidance-documents/regulatory-considerations-human-cells-tissues-and-cellular-and-tissue-based-products-minimal. Accessed November 15, 2022.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.