ABSTRACT
Antimicrobial resistance (AMR) poses a substantial risk to public health. In low-income and middle-income (LMICs) nations, the impact of AMR is significantly more severe. The absence of data from low-income countries (LMICs) causes this topic to be frequently overlooked. Additionally, the COVID-19 pandemic could make the AMR issue even worse. Earlier guidelines recommended antibiotic use in patients with COVID-19, even in those without bacterial coinfection. This study aims to investigate the proportion of antibiotic prescriptions in LMICs among patients with and without coronavirus disease-2019 (COVID-19), the proportion of inappropriate antibiotics, and multi-antibiotic prescribing. We followed the Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA). We retrieved data through online databases, including PubMed, Scopus, and ScienceDirect. Amongst COVID-19 patients, the meta-analytic estimate of antibiotic prescription was 0.80 (95% CI: 0.72–0.88), whereas antibiotic use among patients with non-COVID-19 infections was 0.54 (95% CI: 0.49–0.58). Half of those prescribed antibiotics (0.52, 95% CI: 0.32–0.72) are inappropriate prescriptions. In addition, we found that one-third of antibiotics prescriptions consisted of more than one antibiotic (0.32, 95% CI: 0.21–0.43). In conclusion, antibiotics are highly prescribed across LMICs, and their use is increased in patients with COVID-19. Amongst those prescriptions, inappropriate and multiple use was not uncommon. This study has several limitations, as it included two studies in an ambulatory setting, and some of the studies included in the analysis were conducted on a small scale. Nevertheless, our findings suggest that urgent action to improve prescribing practices is essential.
KEYWORDS: Antibiotic prescription, COVID-19, low-income countries, middle-income countries, antimicrobial resistance
Introduction
Antibiotic or antimicrobial resistance (AMR) poses a significant public health threat [1]. AMR has been estimated as the cause of 700,000 death globally. By 2050, the number was predicted to swell to 10 million, much higher than cancer (8.2 million) [2]. The growth of AMR rates also leads to increased morbidity and economic burden amongst patients, thus, reducing the patient quality of life. Globally, in 2019 there were 192,000 and 47,900 disability-adjusted life years (DALYs) associated and attributed with AMR, respectively [2].
The risk for AMR is attributed to several factors. Those include the high incidence of infectious diseases, low awareness of antibiotics and their risk of resistance, and limited laboratory resources that favor the use of antibiotics empirically [3]. Inappropriate use and prescribing of more than one antibiotic is also reported as a growing concern as a cause of AMR [4].
AMR impacts all countries. Nonetheless, the burden is disproportionately higher in LMICs compared to High-Income Countries (HICs) [5]. AMR is predicted to account for 80% of deaths in LMICs [6]. Partly, the high burden of AMR in LMICs is caused by the high rate of communicable diseases are seen in these countries. LMICs may also have the fewest resources and little information on the prevalence of AMR, thus compounding the AMR problem [3].
A considerable increase of 114% in antibiotics consumption was observed in LMICs between 2000–2015 [7]. It could mean mortality from infectious diseases is decreasing if these antibiotics are used appropriately. However, in LMICs, the driver for inappropriate prescribing of antibiotics is prevalent, leading to frequent antibiotic misuse [8]. Those drivers include a lack of understanding among antibiotic prescribers, insufficient education and supervision given to healthcare professionals, absence of diagnostic tools, and financial incentives for suppliers and prescribers [8].
Approximately 83% of the world’s population lives in LMICs [9,10]. With its dense population, the immense burden of infectious disease, limited laboratory resources, and common misuse of antibiotics, LMICs serve as an important reservoir for the occurrence and spread of AMR [6]. Special attention must be addressed to the LMICs to halt the spread of AMR. However, the fact that AMR is a critical problem in LMICs is often neglected due to a research gap. In these countries, there is a scarcity of studies about AMR and antibiotic use, and the majority of research focuses more on HICs [2].
The COVID-19 pandemic may further complicate the AMR problem in LMICs. Various antibiotic agents were mentioned in earlier guidelines to treat COVID-19 infection, even in those without bacterial coinfection [11]. The demand of antibiotics is also increasing during the COVID-19 pandemic [12,13].
The increased use of antibiotics during this pandemic could be attributed to an early study from Wuhan, China, that reported half of COVID-19 mortality is associated with a secondary bacterial infection [14]. However, newer research suggested that empirical antibiotic in COVID-19 is unnecessary and bacterial coinfection were only found in 6.9% and 8.1% of COVID-19 patients and critically ill COVID-19 patients, respectively [15].
We conducted a systematic review to assess the proportion of antibiotic prescriptions in LMICs amongst patients with COVID-19 and without COVID-19, inappropriate prescription of antibiotics, and the proportion of prescribing more than one antibiotic in LMICs.
Methods
We Followed Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA). The protocol for the systematic review has been registered in the International Prospective Register of Systematic Reviews (PROSPERO) as CRD42022306232.
Eligibility criteria, search strategy, and study selection
We searched online databases, including PubMed, Scopus, and ScienceDirect. The search was limited to literature published between 2012–2022 and to that written in English. The search addressed the following query ((Antibiotic) OR (antimicrobial) OR (antibacterial) AND (((trend) OR (prescription])) OR (use])). Studies will be included if they describe antibiotics prescription in healthcare settings in the LMICs and reported by the number of patients or by the total prescription. Studies were to be excluded if they: (1) done in special populations in which antibiotics use was mainly prophylactic (for example, people with neutropenic fever, rheumatic heart disease, patients who have undergone prophylactic antibiotics including surgical prophylaxis and HIV/AIDS); (2) reported veterinary use of antibiotics; (3) done in the HMICs. The income-based classification of countries into low, lower-middle, upper-middle, or high-income follows the World Bank categorization at the year the study started.
Data extraction and outcomes of interest
We incorporated search results into EndNote 20 software (Clarivate Analytics) and removed the duplicates. Two authors (YAAS and MSU) worked independently on the screening according to the inclusion and exclusion criteria. Any disagreement was resolved by the discussion with the third reviewer (AP). We used a form incorporated into an Excel spreadsheet (Microsoft Corporation) to extract information from all eligible publications.
Eligible studies were reviewed, and the following data were extracted: (1) first author of the study; (2) country in which the study was conducted; (3) diagnosis (reported as COVID-19 and non-COVID-19); (4) data collection method; (5) type of denominator; (6) total of study’s participants; (7) number of antibiotic prescriptions; (8) most common antibiotics.
The primary outcome was the proportion of antibiotic prescriptions in LMICs amongst patients with COVID-19 and without COVID-19. In addition, secondary outcomes were inappropriate prescription of antibiotics and the proportion of prescribing more than one antibiotic. The effect measure will be reported as a proportion of antibiotic prescriptions.
Bias assessment
We use the tool Hoy et al [8]. developed to assess publication bias. Two authors (YAAS and MSU) evaluated each study to ensure that the included study carries a minimal risk of bias. Any disagreements will be resolved by a third author (AP). We categorized a summary of the overall bias of individual studies into low, medium, and high risk, according to Hoy et al [16].
Data synthesis and statistical analysis
Data were divided into two subgroups according to the diagnosis of the patients as follows: (1) COVID-19 and (2) without COVID-19. To assess the between-study heterogeneity, we performed an I2 statistic. We conducted Knapp – Hartung adjustment to investigate the sources of heterogeneity. In addition, a leave-one-out sensitivity analysis was performed to know whether the included studies changed the overall result. The proportions of antibiotic prescriptions were then pooled using a random-effects meta-analysis. A Forest plot was provided to illustrate the summary. Statistical analysis was conducted in Stata version 17 (Stata Corp).
Results
Study selection and characteristics
Our search yielded 8,392 unique studies. After screening the abstracts, we reviewed 152 full texts, of which 69 were included in the systematic review (Figure 1). Nine studies were conducted in low-income countries, 29 in lower-middle-income countries, 28 in upper-middle-income countries, and 1 study was done in 8 countries (low- and lower-middle-income countries). Amongst the included studies, 42 reported the use of antibiotics with patients as the denominator. Twenty-seven studies reported the number of prescriptions as the denominator. In addition, ten studies were done on COVID-19 patients and 59 studies on non-COVID-19 patients. Table 1 summarizes the characteristics of the included studies.
Figure 1.

PRISMA flow diagram of the study.
Table 1.
Summary of the individual studies.
| Study Author | Country | Income | Health Care Level | Diagnosis | Denominator | Total | Antibiotic | Effect Size (95% CI) |
|---|---|---|---|---|---|---|---|---|
| Bajis (2014) [17] | Afghanistan | Low | secondary | Non-COVID | Prescription | 9678 | 5417 | 0.56 (0.55–0.57) |
| Baltzell (2019) [18] | Malawi | Low | primary | Non-COVID | Patient | 9924 | 5464 | 0.55 (0.54–0.56) |
| Bonniface (2021) [19] | Uganda | Low | secondary and tertiary | Non-COVID | Prescription | 45160 | 10402 | 0.23 (0.23–0.23) |
| Bunduki (2021) [20] | Malawi | Low | tertiary | Non-COVID | Patient | 105 | 29 | 0.28 (0.19–0.37) |
| Chaw (2018) [21] | Gambia | Low | tertiary | Non-COVID | Patient | 917 | 496 | 0.54 (0.51–0.57) |
| Mukonzo (2013) [22] | Uganda | Low | primary, secondary, tertiary | Non-COVID | Prescription | 23935 | 9691 | 0.40 (0.40–0.41) |
| Worku (2018) [23] | Ethiopia | Low | primary | Non-COVID | Patient | 900 | 504 | 0.56 (0.53–0.59) |
| Yebyo (2016) [24] | Ethiopia | Low | primary | Non-COVID | Patient | 414 | 363 | 0.88 (0.84–0.91) |
| Yousif (2016) [25] | Sudan | Low | primary | Non-COVID | Prescription | 220 | 120 | 0.55 (0.48–0.61) |
| Fink (2019) [26] | 8 countries | Low and lower-middle | primary | Non-COVID | Patient | 22519 | 14120 | 0.63 (0.62–0.63) |
| Abdulah (2019) [27] | Indonesia | Lower-middle | primary | Non-COVID | Prescription | 10118 | 2373 | 0.23 (0.23–0.24) |
| Abubakar (2020) [28] | Nigeria | Lower-middle | secondary | Non-COVID | Patient | 321 | 156 | 0.49 (0.43–0.54) |
| Adisa (2015) [29] | Nigeria | Lower-middle | primary | Non-COVID | Prescription | 400 | 220 | 0.55 (0.50–0.60) |
| Ahiabu (2015) [30] | Ghana | Lower-middle | primary | Non-COVID | Prescription | 1600 | 306 | 0.19 (0.17–0.21) |
| Ahmadi (2017) [31] | Iran | Lower-middle | primary | Non-COVID | Prescription | 352399 | 183600 | 0.52 (0.52–0.52) |
| Ahmed (2018) [32] | Bangladesh | Lower-middle | tertiary | Non-COVID | Patient | 3570 | 1395 | 0.39 (0.37–0.41) |
| Alkaff (2019) [33] | Indonesia | Lower-middle | primary | Non-COVID | Patient | 203 | 100 | 0.49 (0.42–0.56) |
| Andrajati (2016) [34] | Indonesia | Lower-middle | primary | Non-COVID | Prescription | 4287 | 1209 | 0.28 (0.27–0.30) |
| Ankrah (2021) [35] | Ghana | Lower-middle | tertiary | Non-COVID | Patient | 988 | 343 | 0.35 (0.32–0.38) |
| Ansar (2017) [36] | Pakistan | Lower-middle | tertiary | Non-COVID | Prescription | 400 | 137 | 0.34 (0.29–0.39) |
| Atif (2017) [37] | Pakistan | Lower-middle | tertiary | Non-COVID | Prescription | 1000 | 823 | 0.82 (0.80–0.85) |
| Bediako-Bowan (2019) [38] | Ghana | Lower-middle | secondary and tertiary | Non-COVID | Patient | 540 | 261 | 0.48 (0.44–0.53) |
| Boone (2020) [39] | Bangladesh | Lower-middle | secondary and tertiary | Non-COVID | Patient | 448 | 329 | 0.73 (0.69–0.78) |
| Chaudhary (2014) [40] | India | Lower-middle | secondary | Non-COVID | Prescription | 100 | 45 | 0.45 (0.35–0.55) |
| Chem (2018) [41] | Cameroon | Lower-middle | primary | Non-COVID | Prescription | 30096 | 11035 | 0.37 (0.36–0.37) |
| Dat (2022) [42] | Viet Nam | Lower-middle | secondary and tertiary | Non-COVID | Patient | 1747 | 1112 | 0.64 (0.61–0.66) |
| Dodoo (2021) [43] | Ghana | Lower-middle | tertiary | Non-COVID | Patient | 300 | 182 | 0.61 (0.55–0.66) |
| Fahimzad (2016) [44] | Iran | Lower-middle | secondary and tertiary | Non-COVID | Patient | 858 | 523 | 0.61 (0.58–0.64) |
| Gandra (2018) [45] | India | Lower-middle | secondary and tertiary | Non-COVID | Patient | 403 | 208 | 0.52 (0.47–0.57) |
| Jose (2016) [46] | India | Lower-middle | primary | Non-COVID | Patient | 552 | 404 | 0.73 (0.69–0.77) |
| Mekuria (2019) [47] | Kenya | Lower-middle | primary | Non-COVID | Prescription | 85484 | 21870 | 0.26 (0.25–0.26) |
| Mustafa (2022) [48] | Pakistan | Lower-middle | secondary | COVID-19 | Patient | 444 | 377 | 0.85 (0.81–0.88) |
| Ndhlovu (2015) [49] | Zambia | Lower-middle | primary and secondary | Non-COVID | Patient | 872 | 470 | 0.54 (0.51–0.57) |
| Nepal (2020) [50] | Nepal | Lower-middle | primary and secondary | Non-COVID | Patient | 6860 | 3064 | 0.45 (0.43–0.46) |
| Raza (2014) [51] | Pakistan | Lower-middle | primary and secondary | Non-COVID | Prescription | 1097 | 627 | 0.57 (0.54–0.60) |
| Safaeian (2015) [52] | Iran | Lower-middle | primary | Non-COVID | Prescription | 7439709 | 3794252 | 0.51 (0.51–0.51) |
| Sarwar (2018) [53] | Pakistan | Lower-middle | primary | Non-COVID | Prescription | 7200 | 5869 | 0.82 (0.81–0.82) |
| Saweri (2017) [54] | Papua New Guinea | Lower-middle | primary | Non-COVID | Prescription | 6008 | 4370 | 0.73 (0.72–0.74) |
| Suranadi (2022) [55] | Indonesia | Lower-middle | tertiary | COVID-19 | Patient | 410 | 342 | 0.83 (0.80–0.87) |
| Thobari (2019) [56] | Indonesia | Lower-middle | primary and secondary | Non-COVID | Patient | 1621 | 551 | 0.34 (0.32–0.36) |
| Ababneh (2017) [57] | Jordan | Upper-middle | primary | Non-COVID | Patient | 5829 | 4575 | 0.78 (0.77–0.80) |
| Ababneh (2021) [58] | Jordan | Upper-middle | tertiary | Non-COVID | Patient | 683 | 144 | 0.21 (0.18–0.24) |
| Aksoy (2021) [59] | Turkey | Upper-middle | primary | Non-COVID | Prescription | 1054261396 | 318941829 | 0.30 (0.30–0.30) |
| Alkhaldi (2021) [60] | Jordan | Upper-middle | ambulatory | Non-COVID | Prescription | 73701 | 20133 | 0.27 (0.27–0.28) |
| Al-Shatnawi (2021) [61] | Jordan | Upper-middle | tertiary | Non-COVID | Prescription | 20494 | 15883 | 0.78 (00.77–0.78) |
| Bozic (2015) [62] | Serbia | Upper-middle | primary | Non-COVID | Patient | 1353714 | 728285 | 0.54 (0.54–0.54) |
| Cao (2020) [63] | China | Upper-middle | secondary and tertiary | COVID-19 | Patient | 199 | 189 | 0.95 (0.92–0.98) |
| Chautrakarn (2020) [64] | Thailand | Upper-middle | tertiary | Non-COVID | Patient | 644 | 279 | 0.43 (0.39–0.47) |
| Chen (2020) [65] | China | Upper-middle | secondary | COVID-19 | Patient | 274 | 249 | 0.91 (0.88–0.94) |
| Choez (2018) [66] | Ecuador | Upper-middle | ambulatory | Non-COVID | Patient | 1393 | 523 | 0.38 (0.35–0.40) |
| Ergül (2018) [67] | Turkey | Upper-middle | tertiary | Non-COVID | Patient | 113 | 80 | 0.71 (0.62–0.80) |
| Gasson (2018) [68] | South Africa | Upper-middle | primary | Non-COVID | Patient | 654 | 449 | 0.69 (0.65–0.72) |
| Greer (2018) [69] | Thailand | Upper-middle | primary | Non-COVID | Patient | 83661 | 81691 | 0.98 (0.98–0.98) |
| Guan (2020) [70] | China | Upper-middle | secondary and tertiary | COVID-19 | Patient | 1099 | 637 | 0.58 (0.55–0.61) |
| He (2020) [71] | China | Upper-middle | secondary | COVID-19 | Patient | 65 | 49 | 0.75 (0.64–0.86) |
| Kalkan (2021) [72] | Turkey | Upper-middle | tertiary | Non-COVID | Patient | 927 | 748 | 0.81 (0.78–0.83) |
| Lima (2017) [73] | Brazil | Upper-middle | primary | Non-COVID | Patient | 399 | 71 | 0.18 (0.14–0.22) |
| Liu (2019) [74] | China | Upper-middle | primary | Non-COVID | Prescription | 428475 | 189719 | 0.44 (0.44–0.44) |
| Mashalla (2017) [75] | Botswana | Upper-middle | primary | Non-COVID | Prescription | 550 | 235 | 0.43 (0.39–0.47) |
| Rahman (2016) [76] | Malaysia | Upper-middle | primary | Non-COVID | Patient | 27587 | 5810 | 0.21 (0.21–0.22) |
| Sencan (2022) [77] | Turkey | Upper-middle | secondary and tertiary | COVID-19 | Patient | 1500 | 1118 | 0.75 (0.72–0.77) |
| Sun (2015) [78] | China | Upper-middle | primary and secondary | Non-COVID | Prescription | 8400 | 979 | 0.12 (0.11–0.12) |
| Wang (2014) [79] | China | Upper-middle | primary | Non-COVID | Prescription | 10199 | 6105 | 0.60 (0.59–0.61) |
| Wang (2020) [80] | China | Upper-middle | tertiary | COVID-19 | Patient | 138 | 89 | 0.64 (0.56–0.73) |
| Wang (2020b) [81] | China | Upper-middle | tertiary | COVID-19 | Patient | 107 | 85 | 0.79 (0.71–0.87) |
| Yang (2020) [82] | China | Upper-middle | secondary | COVID-19 | Patient | 52 | 49 | 0.94 (0.87–1.02) |
| Yin (2019) [83] | China | Upper-middle | primary | Non-COVID | Prescription | 14526 | 5851 | 0.40 (0.39–0.41) |
| Zhan (2019) [84] | China | Upper-middle | primary and secondary | Non-COVID | Prescription | 2470 | 1313 | 0.53 (0.51–0.55) |
| Zhang (2017) [85] | China | Upper-middle | primary and secondary | Non-COVID | Patient | 9340 | 3425 | 0.37 (0.36–0.38) |
Risk oF bias assessment
The results of the methodological quality of the individual studies are presented in Appendix 1. Figure 2 shows the summary of the quality assessment. The majority of the studies were classified as having a low risk of bias (n = 41, 59.42%). Seventeen studies (24.64%) were judged as moderate risk, whilst 11 studies (15.94%) were at high risk of bias. The most significant issue of bias was the representativeness of the national population; most of the studies only selected a few healthcare facilities within one or two provinces.
Figure 2.
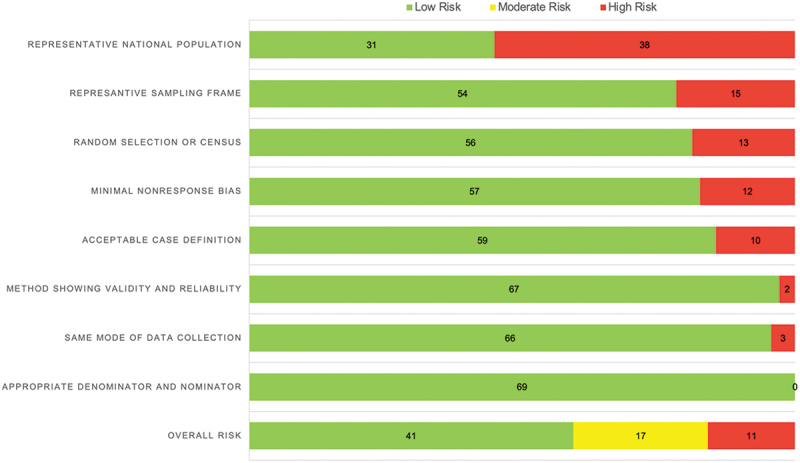
Risk of bias assessment.
Antibiotic prescription prevalence
Ten studies (n = 4,288) reported the prevalence of antibiotic prescriptions among COVID-19 patients. All studies were conducted on hospitalized patients. The reported antibiotic prescriptions in COVID-19 patients ranged from 0.58 (95% CI: 0.55–0.61) to 0.95 (95% CI: 0.91–0.98), with the meta-analytic estimate was 0.80 (95% CI: 0.720–0.88; P>Q 0.000; tau2: 0.017; I2: 97.85%).
Fifty-nine studies (n = 1,064,378,108) reported antibiotic use amongst patients with non-COVID-19 infection. The use of antibiotics in non-COVID-19 patients ranged from 0.117 (95% CI: 0.110–0.123) to 0.977 (95% CI: 0.975–0.978), and the meta-analytic estimate was 0.540 (95% CI: 0.493–0.588; P>Q 0.000; tau2: 0.041; I2: 100.00). Overall, the pooled estimate was 0.540 (95% CI: 0.493–0.588). Two studies were done in an ambulatory setting, in which Alkhaldi et al. reported the prevalence of antibiotic prescription to be 0.27 (95% CI: 0.27–0.28) and the prevalence of 0.38 (95% CI: 0.35–0.40) was reported by Choez et al. Figure 3 shows a forest plot that summarizes the meta-analysis.
Figure 3.
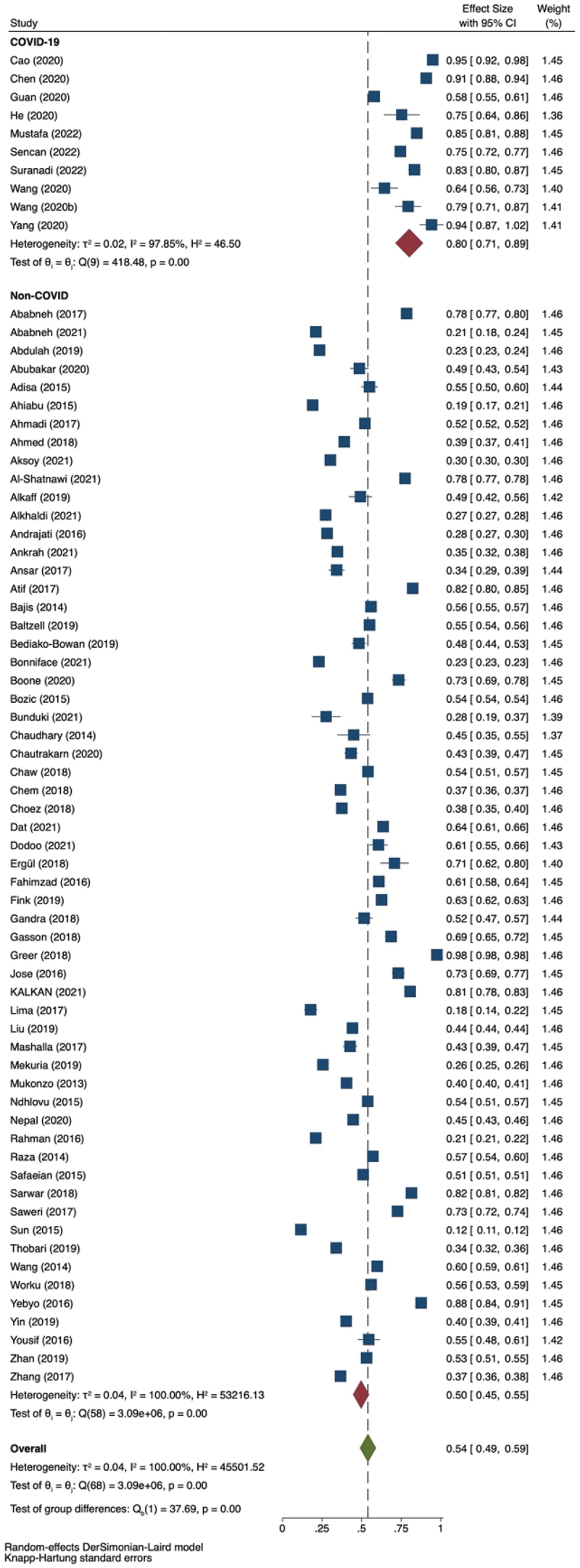
Forest plot for the prevalence of antibiotics prescription.
Prevalence of inappropriate antibiotic prescription
If available, we retrieved the proportion of inappropriate antibiotic prescriptions within individual studies. Nine studies reported inappropriate antibiotic prescriptions. Inappropriate prescriptions are defined by included studies as unnecessary use of antibiotics when several criteria are met, such as antibiotic is not indicated, unnecessary use of multiple antibiotics, irrational drug frequency, or inappropriate antibiotic dose. Table 2 showed the characteristics of the study reporting inappropriate antibiotic prescription. The lowest proportion of inappropriate antibiotic prescriptions was 0.16 (95% CI: 0.15–0.17), and the highest was 0.90 (95% CI: 0.88–0.93). Figure 4 showed a pooled effect estimate of 0.52 (95% CI: 0.32–0.72). The study reported the highest inappropriate prescription (0.90, 95% CI: 0.88–0.93) was a study done in an ambulatory setting by Choez et al.
Table 2.
Characteristics of the study reporting inappropriate antibiotic prescription.
| Study Author | Country | Income | Health care level | Diagnosis | COVID-19 status | Denominator | Effect Size (95% CI) |
|---|---|---|---|---|---|---|---|
| Ababneh (2017) [57] | Jordan | UMIC | primary | acute respiratory infection | Non-COVID | Patient | 0.59 (0.57–0.60) |
| Alkhaldi (2021) [60] | Jordan | UMIC | secondary and tertiary | respiratory tract infection | Non-COVID | Prescription | 0.30 (0.29–0.31) |
| Choez (2018) [66] | Ecuador | UMIC | ambulatory | upper respiratory tract infection | Non-COVID | Patient | 0.90 (0.88–0.93) |
| Ergül (2018) [67] | Turkey | UMIC | tertiary | any | Non-COVID | Patient | 0.34 (0.23–0.45) |
| Gasson (2018) [68] | South Africa | UMIC | primary | any | Non-COVID | Patient | 0.68 (0.64–0.72) |
| Sarwar (2018) [53] | Pakistan | LMIC | primary | any | Non-COVID | Prescription | 0.16 (0.15–0.17) |
| Sun (2015) [78] | China | UMIC | primary and secondary | upper respiratory tract infection, common cold | Non-COVID | Prescription | 0.89 (0.87–0.91) |
| Wang (2014) [79] | China | UMIC | primary | any | Non-COVID | Prescription | 0.47 (0.45–0.48) |
| Zhan (2019) [84] | China | UMIC | primary and secondary | any | Non-COVID | Prescription | 0.37 (0.34–0.39) |
Figure 4.
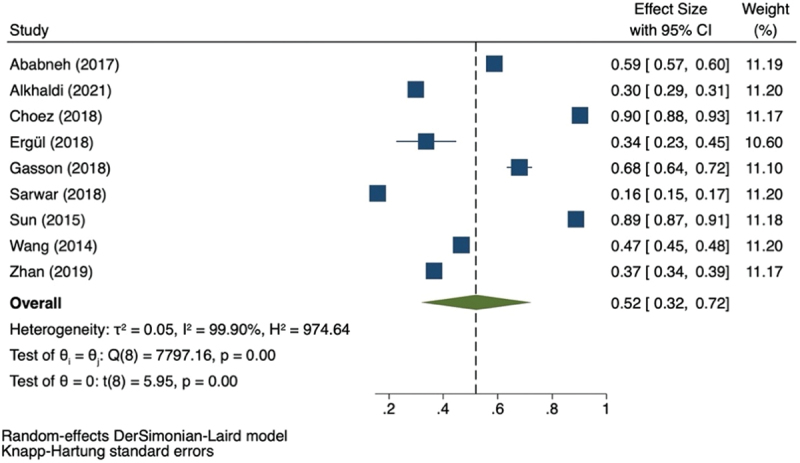
Forest plot for the inappropriate antibiotics prescription.
Prevalence of multiple antibiotic prescription
Twenty-one studies reported the use of more than one antibiotic (Table 3). The prevalence of multiple antibiotic prescriptions ranged from 0.02 (95% CI: 0.01–0.02) to 0.82 (95% CI: 0.77–0.86) (Figure 5). The study that reported the highest use of multiple antibiotics was done in tertiary and secondary settings. The most commonly combined antibiotics reported in the study were β-lactam with β-lactamase inhibitor and nitroimidazole [38]. Another study reported cefotaxime/ceftriaxone with metronidazole as the most common combination [37]. One study also observed redundant antibiotic treatments such as dual anaerobic and dual beta-lactam [28].
Table 3.
Prevalence of multiple antibiotic prescriptions.
| Study Author | Country | Income | Health care level | Diagnosis | COVID-19 Status | Denominator | Effect Size (95% CI) |
|---|---|---|---|---|---|---|---|
| Ababneh (2017) [57] | Jordan | UMIC | primary | acute respiratory infection | Non-COVID | Patient | 0.02 (0.01–0.02) |
| Abubakar (2020) [28] | Nigeria | LMIC | secondary | any | Non-COVID | Patient | 0.45 (0.37–0.53) |
| Ankrah (2021) [35] | Ghana | LMIC | tertiary | any | Non-COVID | Patient | 0.77 (0.72–0.81) |
| Ansar (2017) [36] | Pakistan | LMIC | tertiary | any | Non-COVID | Prescription | 0.20 (0.13–0.27) |
| Atif (2017) [37] | Pakistan | LMIC | tertiary | any | Non-COVID | Prescription | 0.35 (0.32–0.38) |
| Bediako-Bowan (2019) [38] | Ghana | LMIC | secondary and tertiary | any | Non-COVID | Patient | 0.82 (0.77–0.86) |
| Bunduki (2021) [20] | Malawi | LIC | secondary | hospital-acquired infection | Non-COVID | Patient | 0.55 (0.36–0.74) |
| Chautrakarn (2020) [64] | Thailand | UMIC | tertiary | any | Non-COVID | Patient | 0.59 (0.53–0.65) |
| Chem (2018) [41] | Cameroon | LMIC | primary | any | Non-COVID | Prescription | 0.05 (0.04–0.05) |
| Dat (2021) [42] | Viet Nam | LMIC | secondary | any | Non-COVID | Patient | 0.42 (0.39–0.45) |
| Gandra (2018) [45] | India | LMIC | secondary and tertiary | any | Non-COVID | Patient | 0.60 (0.53–0.67) |
| Liu (2019) [74] | China | UMIC | primary | any | Non-COVID | Prescription | 0.21 (0.21–0.21) |
| Mashalla (2017) [75] | Botswana | UMIC | primary | Non-COVID | Prescription | 0.19 (0.14–0.24) |
|
| Ndhlovu (2015) [49] | Zambia | LMIC | primary and secondary | suspected malaria | Non-COVID | Patient | 0.05 (0.03–0.07) |
| Nepal (2020) [50] | Nepal | LMIC | primary and secondary | any | Non-COVID | Patient | 0,09 (0.08–0.10) |
| Rahman (2016) [76] | Malaysia | UMIC | primary | any | Non-COVID | Patient | 0.03 (0.03–0.04) |
| Sencan (2022) [77] | Turkey | UMIC | secondary and tertiary | COVID-19 | COVID-19 | Patient | 0.37 (0.34–0.40) |
| Suranadi (2022) [55] | Indonesia | LMIC | tertiary | COVID-19 | COVID-19 | Patient | 0.46 (0.40–0.51) |
| Thobari (2019) [56] | Indonesia | LMIC | primary and secondary | any | Non-COVID | Patient | 0.12 (0.09–0.15) |
| Yin (2019) [83] | China | UMIC | primary | any | Non-COVID | Prescription | 0.18 (0.18–0.19) |
| Zhang (2017) [85] | China | UMIC | primary and secondary | upper respiratory tract infection | Non-COVID | Patient | 0.40 (0.39–0.42) |
Figure 5.
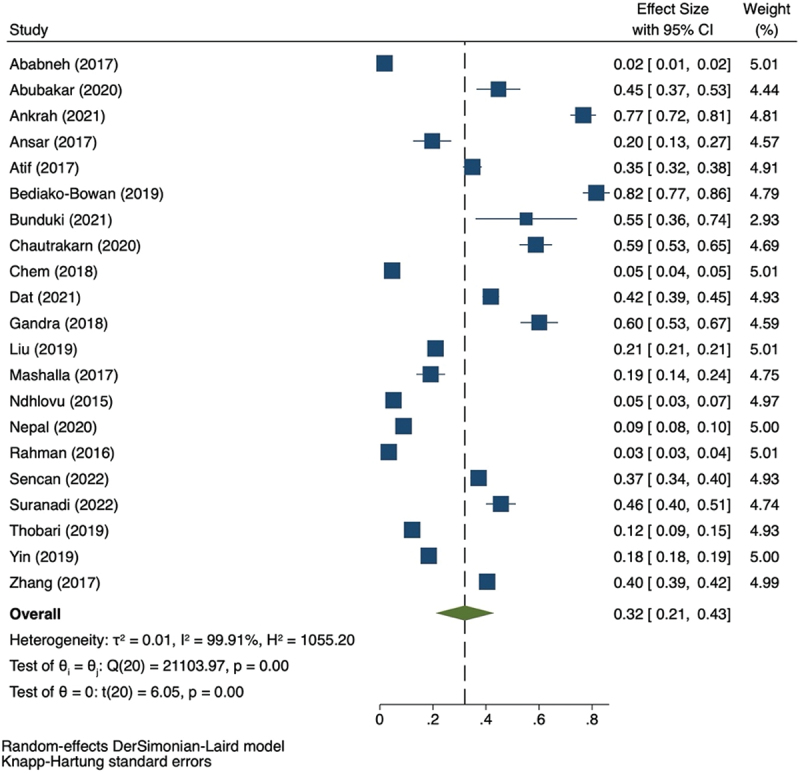
Forest plot for the prevalence of multiple antibiotics prescription.
Discussion
This systematic review summarizes data from 69 studies describing antibiotic prescriptions. Our meta-analysis suggests that antibiotics prescriptions in low-income and middle-income countries were 0.54 (95% CI: 0.49–0.59) of the total patient prescriptions. This means that for every 100 patients or prescriptions, 54 would receive antibiotics, reflecting a high antibiotic use in the LMICs.
COVID-19 pandemics complicate the antibiotic overprescription problem. Our finding showed that in COVID-19 patients, the antibiotic prescription was as high as 0.80 (95%CI: 0.71–0.89) compared to the non-COVID-19 patients with a prevalence value of 0.50 (95%CI: 0.45–0.55). Those numbers are much higher than the World Health Organization indicator, which recommends that antibiotic prescriptions should be lower than 30% of total prescriptions [86]. However, studies on patients with COVID-19 only include those who were hospitalized. This circumstance may cause an overestimate or even underestimate of the actual condition because outpatients were not included in the studies.
One study done in China reported antibiotics usage amongst hospitalized COVID-19 patients with a nosocomial infection [71]. Interestingly, the proportion of antibiotic prescriptions was lower compared to several other studies [48,55,63,65,81,82]. The study that reported the highest prevalence of antibiotic prevalence was a trial of lopinavir-ritonavir in severe COVID-19. It was reported that antibiotic was part of the standard care, alongside supportive therapy.
The high infectious disease burden in the LMICs could be the reason for prevalent antibiotic use. As mentioned before, the prevalent use of antibiotics could be good in LMICs, as it means that access to antibiotics has improved and could decrease the mortality rate of infectious diseases [87]. However, our findings showed a potential concern about the misuse of antibiotics. Half of those prescribed antibiotics (0.52, 95% CI: 0.32–0.72) received an inappropriate antibiotic prescription. Inappropriate antibiotic prescriptions are one of the causes of antimicrobial resistance apart from overuse of antibiotics and the use of multiple antibiotics [4].
The highest circumstances in which antibiotics were inappropriately prescribed were ambulatory settings [66]. Meanwhile, the study reporting the lowest prevalence of inappropriate antibiotic prescriptions was done in primary health care [53]. Upper respiratory tract infection appeared to be a common diagnosis in both the low or high rate of inappropriate antibiotic prescription. None of the studies reporting inappropriate antibiotic prescriptions were done in COVID-19 patients.
Multiple antibiotic prescriptions were also prevalent based on our findings. We found that one-third of antibiotics prescriptions consisted of more than one antibiotic (0.32, 95% CI: 0.21–0.43). In contrast, World Health Organization recommends that antibiotic prescriptions be lower than two medicines [86]. Multiple antibiotics, if misused, also lead to antibiotic resistance [4].
This study showed a very high level of heterogeneity (I2 were above 50%). However, our meta-analysis’s pooled 95% CI were not showing wide intervals (0.49–0.59; 0.32–0.72; 0.21–0.43; for antibiotic prescription prevalence, inappropriate antibiotic prescription, and multiple antibiotic prescriptions, respectively). Thus, indicating that a similar systematic review study would yield a similar proportion of effect size.
In conclusion, antibiotics prescriptions accounted for half of the drug prescriptions in LMICs, and their use is increased amongst patients with COVID-19. Inappropriate antibiotics prescription accounted for half of the total antibiotic use. In addition, multiple antibiotic use is also common practice in LMICs. The burden of antibiotic use in LMICs corresponds to the decrease in mortality from infectious diseases. However, there is an extent to relying upon antibiotics as their inappropriate and overuse are associated with AMR. Mainly, physicians should use antibiotics appropriately and minimize multiple uses when not indicated.
Our study is not without a limitation. We included studies with a high risk of bias in the analysis, and one research conducted in an ambulatory setting was included in this study. Furthermore, many of the studies were conducted on a small scale. Hence, there is a need for a larger-scale study to accurately capture the prevalence of antibiotic prescription in a country, specifically LMICs. Ideally, the study should also observe the appropriateness of the treatment and the antibiotic agent of choice. Nevertheless, our findings suggest that urgent action to improve prescribing practices is essential.
Funding Statement
The author(s) reported there is no funding associated with the work featured in this article.
Disclosure statement
No potential conflict of interest was reported by the authors.
Availability of data and material
All data used in this manuscript can be found in the online versions of the studies that were accessed. Our own data synthesis of these manuscripts is available from the author upon a reasonable request.
References
- [1].Ventola CL. The antibiotic resistance crisis: part 1: causes and threats. P T Peer-Rev J Formul Manag. 2015;40:277–283. [PMC free article] [PubMed] [Google Scholar]
- [2].Murray CJ, Ikuta KS, Sharara F. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet Lond Engl. 2022;399:629–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Sartelli M, C Hardcastle T, Catena F, et al. Antibiotic use in low and middle-income countries and the challenges of antimicrobial resistance in surgery. Antibiotics. 2020;9:497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Tomas A, Aljadeeah S. The overlooked issue of outpatient combination antibiotic prescribing in low- and middle-income countries: an example from syria. Antibiotics. 2022;11:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Pokharel S, Raut S, Adhikari B. Tackling antimicrobial resistance in low-income and middle-income countries. BMJ Glob Health. 2019;4:e002104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Sulis G, Sayood S, Gandra S. Antimicrobial resistance in low- and middle-income countries: current status and future directions. Expert Rev Anti Infect Ther. 2022;20:147–160. [DOI] [PubMed] [Google Scholar]
- [7].Klein EY, Van Boeckel TP, Martinez EM, et al. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc Natl Acad Sci USA. 2018;115:E3463–3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wu S, Tannous E, Haldane V, et al. Barriers and facilitators of implementing interventions to improve appropriate antibiotic use in low- and middle-income countries: a systematic review based on the consolidated framework for implementation research. Implement Sci. 2022;17:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].World Bank . Low & middle income | Data. 2022. [cited 2022 Dec 9]; Available from: https://data.worldbank.org/country/XO
- [10].World Bank . Population, total | Data. 2022. [cited 2022 Dec 9]; Available from: https://data.worldbank.org/indicator/SP.POP.TOTL
- [11].Adebisi YA, Jimoh ND, Ogunkola IO, et al. The use of antibiotics in COVID-19 management: a rapid review of national treatment guidelines in 10 African countries. Trop Med Health. 2021;49:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Dutta S, Kaur RJ, Bhardwaj P, et al. Demand of COVID-19 medicines without prescription among community pharmacies in Jodhpur, India: findings and implications. J Fam Med Prim Care. 2022;11:503–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Jirjees F, Ahmed M, Sayyar S, et al. Self-medication with antibiotics during COVID-19 in the Eastern Mediterranean region countries: a review. Antibiotics. 2022;11:733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet Lond Engl. 2020;395:1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Langford BJ, So M, Raybardhan S, et al. Bacterial coinfection and secondary infection in patients with COVID-19: a living rapid review and meta-analysis. Clin Microbiol Infect. 2020;26:1622–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hoy D, Brooks P, Woolf A, et al. Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. J Clin Epidemiol. 2012;65:934–939. [DOI] [PubMed] [Google Scholar]
- [17].Bajis S, Bernardo RS, Esmati S, et al. Antibiotic use in a district hospital in Kabul, Afghanistan: are we overprescribing? Public Health Action. 2014;4(4):259–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Baltzell K, Kortz T, Scarr E, et al. “Not all fevers are malaria”: a mixed methods study of non-malarial fever management in rural southern Malawi. Rural Remote Health. 2019. DOI: 10.22605/RRH4818 [DOI] [PubMed] [Google Scholar]
- [19].Bonniface M, Nambatya W, Rajab K. An evaluation of antibiotic prescribing practices in a rural refugee settlement district in Uganda. Antibiotics. 2021;10:172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Bunduki GK, Feasey N, Henrion MYR, et al. Healthcare-associated infections and antimicrobial use in surgical wards of a large urban central hospital in Blantyre, Malawi: a point prevalence survey. Infect Prev Pract. 2021;3:100163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Chaw PS, Schlinkmann KM, Raupach-Rosin H, et al. Antibiotic use on paediatric inpatients in a teaching hospital in the Gambia, a retrospective study. Antimicrob Resist Infect Control. 2018;7:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Jackson M, Namuwenge, Okure G, et al. Over-the-counter suboptimal dispensing of antibiotics in Uganda. J Multidiscip Healthc. 2013;303. DOI: 10.2147/JMDH.S49075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Worku F, Tewahido D. Retrospective assessment of antibiotics prescribing at public primary healthcare facilities in addis Ababa, Ethiopia. Interdiscip Perspect Infect Dis. 2018;1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Yebyo H, Medhanyie AA, Spigt M, et al. C-reactive protein point-of-care testing and antibiotic prescribing for acute respiratory tract infections in rural primary health centres of North Ethiopia: a cross-sectional study. NPJ Prim Care Respir Med. 2016;26:15076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Yousif BME, Supakankunti S. General practitioners’ prescribing patterns at primary healthcare centers in national health insurance, Gezira, Sudan. Drugs Real World Outcomes. 2016;3:327–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Fink G, D’Acremont V, Leslie HH, et al. Antibiotic exposure among children younger than 5 years in low-income and middle-income countries: a cross-sectional study of nationally representative facility-based and household-based surveys. Lancet Infect Dis. 2020;20:179–187. [DOI] [PubMed] [Google Scholar]
- [27].Abdulah R, Insani WN, Putri NE, et al. Pattern of medication use in geriatric patients at primary healthcare facilities in Karawang, Indonesia. Drug Healthc Patient Saf. 2019;11:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Abubakar U. Antibiotic use among hospitalized patients in northern Nigeria: a multicenter point-prevalence survey. BMC Infect Dis. 2020;20:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Adisa R, Orherhe OM, Fakeye TO. Evaluation of antibiotic prescriptions and use in under-five children in Ibadan, SouthWestern Nigeria. Afr Health Sci. 2018;18:1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ahiabu M-A, Tersbøl BP, Biritwum R, et al. A retrospective audit of antibiotic prescriptions in primary healthcare facilities in Eastern Region, Ghana. Health Policy Plan. 2016;31:250–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Ahmadi F, Zarei E. Prescribing patterns of rural family physicians: a study in Kermanshah Province, Iran. BMC Public Health. 2017;17:908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Ahmed S, Korpe P, Ahmed T, et al. Burden and risk factors of antimicrobial use in children less than 5 years of age with diarrheal illness in rural Bangladesh. Am J Trop Med Hyg. 2018;98:1571–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Alkaff RN, Kamigaki T, Saito M, et al. Use of antibiotics for common illnesses among children aged under 5 years in a rural community in Indonesia: a cross-sectional study. Trop Med Health. 2019;47:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Andrajati R, Tilaqza A, Supardi S. Factors related to rational antibiotic prescriptions in community health centers in Depok city, Indonesia. J Infect Public Health. 2017;10:41–48. [DOI] [PubMed] [Google Scholar]
- [35].Ankrah D, Owusu H, Aggor A, et al. Point prevalence survey of antimicrobial utilization in ghana’s premier hospital: implications for antimicrobial stewardship. Antibiotics. 2021;10:1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Ansar A, Iftikhar S, Mir WA, et al. Original article drug prescription pattern in a tertiary care hospital in Pakistan. Med Forum. 2017;28:4. [Google Scholar]
- [37].Atif M, Azeem M, Saqib A, et al. Investigation of antimicrobial use at a tertiary care hospital in Southern Punjab, Pakistan using WHO methodology. Antimicrob Resist Infect Control. 2017;6:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Bediako-Bowan AAA, Owusu E, Labi A-K, et al. Antibiotic use in surgical units of selected hospitals in Ghana: a multi-centre point prevalence survey. BMC Public Health. 2019;19:797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Boone K, Morris SK, Doshi S, et al. Antimicrobial prescribing during infant hospital admissions in a birth Cohort in Dhaka. Bangladesh J Trop Pediatr. 2021;67:fmaa093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Chaudhary P, Bahl A, Kumar A. Trends of prescribing and utilization of antibiotics in the paediatric outpatient population of a secondary care hospital in Gurgaon, India. Indian J Med Spec. 2014;5:93–96. [Google Scholar]
- [41].Chem ED, Anong DN, J-FKT A. Prescribing patterns and associated factors of antibiotic prescription in primary health care facilities of Kumbo East and Kumbo West health districts, North West Cameroon. PLoS ONE. 2018;13:e0193353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Dat VQ, Dat TT, Hieu VQ, et al. Antibiotic use for empirical therapy in the critical care units in primary and secondary hospitals in Vietnam: a multicenter cross-sectional study. Lancet Reg Health - West Pac. 2022;18:100306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Dodoo CC, Orman E, Alalbila T, et al. Antimicrobial prescription pattern in ho teaching hospital, Ghana: seasonaL determination using a point prevalence survey. Antibiotics. 2021;10:199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Fahimzad A, Eydian Z, Karimi A, et al. Surveillance of antibiotic consumption point prevalence survey 2014: antimicrobial prescribing in pediatrics wards of 16 Iranian hospitals Arch Iran Med. 2016;19(3):204–209. [PubMed] [Google Scholar]
- [45].Gandra S, Alvarez-Uria G, Murki S, et al. Point prevalence surveys of antimicrobial use among eight neonatal intensive care units in India: 2016. Int J Infect Dis. 2018;71:20–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Jose J. Paediatric prescription analysis in a primary health care institution. J Clin Diagn Res. 2016. DOI: 10.7860/JCDR/2016/22350.8797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Mekuria LA, de Wit TF, Spieker N, et al. Analyzing data from the digital healthcare exchange platform for surveillance of antibiotic prescriptions in primary care in urban Kenya: a mixed-methods study. PLoS ONE. 2019;14:e0222651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Mustafa ZU, Kow CS, Salman M, et al. Pattern of medication utilization in hospitalized patients with COVID-19 in three district headquarters hospitals in the Punjab province of Pakistan. Explor Res Clin Soc Pharm. 2022;5:100101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Ndhlovu M, Nkhama E, Miller JM, et al. Antibiotic prescribing practices for patients with fever in the transition from presumptive treatment of malaria to ‘confirm and treat’ in Zambia: a cross-sectional study. Trop Med Int Health. 2015;20:1696–1706. [DOI] [PubMed] [Google Scholar]
- [50].Nepal A, Hendrie D, Robinson S, et al. Analysis of patterns of antibiotic prescribing in public health facilities in Nepal. J Infect Dev Ctries. 2020;14:18–27. [DOI] [PubMed] [Google Scholar]
- [51].Raza UA, Khursheed, T, Irfan, M. et al. Prescription patterns of general practitioners in Peshawar, Pakistan. Pak J Med Sci. 2014;30:462–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Safaeian L, Salami S, Pakmehr F, et al. Seasonality and physician-related factors associated with antibiotic prescribing: a cross-sectional study in Isfahan, Iran. Int J Prev Med. 2015;6:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Sarwar MR, Saqib A, Iftikhar S, et al. Antimicrobial use by WHO methodology at primary health care centers: a cross sectional study in Punjab, Pakistan. BMC Infect Dis. 2018;18:492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Saweri OPM, Hetzel MW, Mueller I, et al. The treatment of non-malarial febrile illness in Papua New Guinea: findings from cross sectional and longitudinal studies of health worker practice. BMC Health Serv Res. 2017;17:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Suranadi IW, IMAK S, Fatmawati NND, et al. A retrospective analysis of the bacterial infections, antibiotic use, and mortality predictors of COVID-19 patients. Int J Gen Med. 2022;15:3591–3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].At Thobari J, Satria CD, Ridora Y, et al. Antimicrobial use in an Indonesian community cohort 0-18 months of age. PLoS ONE. 2019;14:e0219097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Ababneh MA, Al-Azzam SI, Ababneh R, et al. Antibiotic prescribing for acute respiratory infections in children in Jordan. Int Health. 2017;9:124–130. [DOI] [PubMed] [Google Scholar]
- [58].Ababneh MA, Jaber M, Rababa’h A, et al. Prevalence of antimicrobial use in a tertiary academic hospital: a venue for antimicrobial stewardship programs. Expert Rev Anti Infect Ther. 2021;19:1047–1051. [DOI] [PubMed] [Google Scholar]
- [59].Aksoy M, Isli F, Kadi E, et al. Evaluation of more than one billion outpatient prescriptions and eight‐year trend showing a remarkable reduction in antibiotic prescription in Turkey: a success model of governmental interventions at national level. Pharmacoepidemiol Drug Saf. 2021;30:1242–1249. [DOI] [PubMed] [Google Scholar]
- [60].Alkhaldi SM, Yaseen NA, Bataineh EA, et al. Patterns of antibiotic prescribing and appropriateness for respiratory tract infections in a teaching hospital in Jordan. Int J Clin Pract. 2021;75. DOI: 10.1111/ijcp.14113. [DOI] [PubMed] [Google Scholar]
- [61].Al‐shatnawi SF, Al‐hosban SY, Altawalbeh SM, et al. Antibiotic prescribing patterns for childhood infections in ambulatory settings in Jordan. Int J Clin Pract. 2021;75. DOI: 10.1111/ijcp.14740. [DOI] [PubMed] [Google Scholar]
- [62].Bozic B, Bajcetic M. Use of antibiotics in paediatric primary care settings in Serbia. Arch Dis Child. 2015;100:966–969. [DOI] [PubMed] [Google Scholar]
- [63].Cao B, Wang Y, Wen D, et al. A trial of lopinavir–ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382:1787–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Chautrakarn S, Anugulruengkitt S, Puthanakit T, et al. Antimicrobial prescription patterns in a tertiary‐care pediatric unit in Thailand. Pediatr Int. 2020;62:683–687. [DOI] [PubMed] [Google Scholar]
- [65].Chen T, Wu D, Chen H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;m1091. DOI: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Sánchez Choez X, Armijos Acurio ML, Jimbo Sotomayor RE. Appropriateness and adequacy of antibiotic prescription for upper respiratory tract infections in ambulatory health care centers in Ecuador. BMC Pharmacol Toxicol. 2018;19:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Ergül AB, İ G, Çelik T, et al. Assessment of inappropriate antibiotic use in pediatric patients: point-prevalence study. Turk Arch Pediatr Pediatri Arş. 2018;53:17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Gasson J, Blockman M, Willems B. Antibiotic prescribing practice and adherence to guidelines in primary care in the Cape town metro District, South Africa. S Afr Med J. 2018;108:304. [DOI] [PubMed] [Google Scholar]
- [69].Greer RC, Intralawan D, Mukaka M, et al. Retrospective review of the management of acute infections and the indications for antibiotic prescription in primary care in northern Thailand. BMJ Open. 2018;8:e022250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Guan W, Ni Z, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].He Y, Li W, Wang Z, et al. Nosocomial infection among patients with COVID-19: a retrospective data analysis of 918 cases from a single center in Wuhan, China. Infect Control Hosp Epidemiol. 2020;41:982–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Kalkan İA, Çinar G, PehliVanli A, et al. Pattern of systemic antibiotic use and potential drug interactions: evaluations through a point prevalence study in Ankara University Hospitals. Turk J Med Sci. 2021;51(2):523–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Lima MG, Dutra KR, Martins UCM. Prescribing indicators in primary health care in Belo Horizonte, Brazil: associated factors. Int J Clin Pharm. 2017;39:913–918. [DOI] [PubMed] [Google Scholar]
- [74].Liu C, Liu C, Wang D, et al. Intrinsic and external determinants of antibiotic prescribing: a multi-level path analysis of primary care prescriptions in Hubei, China. Antimicrob Resist Infect Control. 2019;8:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Mashalla Y, Setlhare V, Massele A, et al. Assessment of prescribing practices at the primary healthcare facilities in Botswana with an emphasis on antibiotics: findings and implications. Int J Clin Pract. 2017;71:e13042. [DOI] [PubMed] [Google Scholar]
- [76].Ab Rahman N, Teng CL, Sivasampu S. Antibiotic prescribing in public and private practice: a cross-sectional study in primary care clinics in Malaysia. BMC Infect Dis. 2016;16:208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Şencan İ, Çağ Y, Karabay O, et al. Antibiotic use and influencing factors among hospitalized patients with COVID-19: a multicenter point-prevalence study from Turkey. Balk Med J. 2022;39:209–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Sun Q, Dyar OJ, Zhao L, et al. Overuse of antibiotics for the common cold – attitudes and behaviors among doctors in rural areas of Shandong Province, China. BMC Pharmacol Toxicol. 2015;16:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Wang J, Wang P, Wang X, et al. Use and prescription of antibiotics in primary health care settings in China. JAMA Intern Med. 2014;174:1914. [DOI] [PubMed] [Google Scholar]
- [80].Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323:1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Wang D, Yin Y, Hu C, et al. Clinical course and outcome of 107 patients infected with the novel coronavirus, SARS-CoV-2, discharged from two hospitals in Wuhan, China. crit care. 2020;24:188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Yin J, Dyar OJ, Yang P, et al. Pattern of antibiotic prescribing and factors associated with it in eight village clinics in rural Shandong Province, China: a descriptive study. Trans R Soc Trop Med Hyg. 2019;113:714–721. [DOI] [PubMed] [Google Scholar]
- [84].Zhan Q, Wang YL, Chen X. Evaluation of antibacterial use in outpatients of township and community primary medical institutions in a district of Sichuan Province, China. J Glob Antimicrob Resist. 2019;19:201–206. [DOI] [PubMed] [Google Scholar]
- [85].Zhang Z, Hu Y, Zou G, et al. Antibiotic prescribing for upper respiratory infections among children in rural China: a cross-sectional study of outpatient prescriptions. Glob Health Action. 2017;10:1287334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].World Health Organization . Using indicators to measure country pharmaceutical situations: fact book on WHO level I and level II monitoring indicators. Geneva: World Health Organization; 2006. [Google Scholar]
- [87].Laxminarayan R, Matsoso P, Pant S, et al. Access to effective antimicrobials: a worldwide challenge. Lancet Lond Engl. 2016;387:168–175. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data used in this manuscript can be found in the online versions of the studies that were accessed. Our own data synthesis of these manuscripts is available from the author upon a reasonable request.


