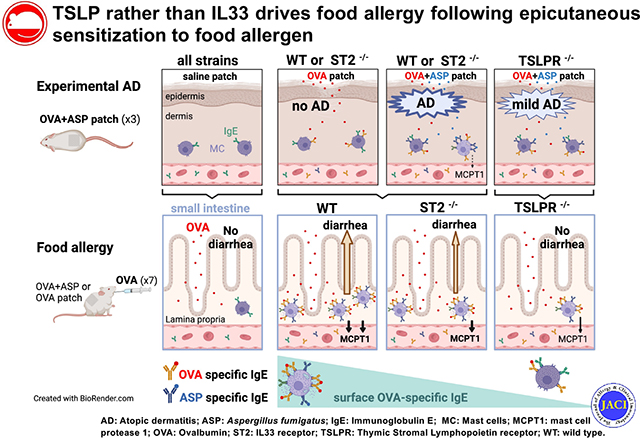
Abstract
Background:
A major route of sensitization to food allergen is through an impaired skin barrier. IL-33 and thymic stromal lymphopoietin (TSLP) have both been implicated in epicutaneous sensitization and food allergy, albeit in different murine models.
Objective:
We assessed the respective contributions of TSLP and IL-33 to the development of atopic dermatitis (AD) and subsequent food allergy in TSLP and IL-33 receptor (ST2)-deficient mice using an AD model that does not require tape stripping.
Method:
TSLP receptor (TSLPR)−/−, ST2−/−, and BALB/cJ control mice were exposed to 3 weekly epicutaneous skin patches of one of saline, ovalbumin (OVA), or a combination of OVA and Aspergillus fumigatus (ASP), followed by repeated intragastric OVA challenges and development of food allergy.
Results:
ASP and/or OVA patched, but not OVA-alone patched, BALB/cJ mice developed an AD-like skin phenotype. However, epicutaneous OVA sensitization occurred in OVA patched mice and was decreased in ST2−/− mice, resulting in lower intestinal mast cell degranulation and accumulation, as well as OVA-induced diarrhea occurrences on intragastric OVA challenges. In TSLPR−/− mice, intestinal mast cell accumulation was abrogated, and no diarrhea was observed. AD was significantly milder in OVA + ASP patched TSLPR−/− mice compared to wild type and ST2−/− mice. Accordingly, intestinal mast cell accumulation and degranulation were impaired in OVA + ASP patched TSLPR−/− mice compared to wild type and ST2−/− mice, protecting TSLPR−/− mice from developing allergic diarrhea.
Conclusion:
Epicutaneous sensitization to food allergen and development of food allergy can occur without skin inflammation and is partly mediated by TSLP, suggesting that prophylactic targeting of TSLP may be useful in mitigating the development of AD and food allergy early in life in at-risk infants.
Keywords: Epicutaneous sensitization, atopic dermatitis, food allergy, allergic diarrhea, TSLP, IL-33, ST2, IgE, mast cell
Graphical Abstract
INTRODUCTION
It is increasingly recognized that one of the major routes of sensitization to food allergens is through an impaired skin barrier.1,2 Accordingly, associations between altered skin barrier and/or atopic dermatitis (AD) and food allergy have been reported.1,2 Food allergy is dependent on accumulation of intestinal mast cells and allergen-specific IgE-mediated mast cell degranulation.3,4 Mechanistically, IL-33 and thymic stromal lymphopoietin (TSLP) have both been proposed to mediate sensitization to food allergen, accumulation of intestinal mast cells, and development of food allergy, albeit in different animal models.5 To promote food allergen recognition by the cutaneous immune system, murine epidermal skin barrier is either weakened naturally by a gene defect (eg, Flg mutation) or after alterations of the epidermal barrier through chemical or proteolytic degradation and/or by mechanical injury (scratching, tape stripping). In a food allergy model using neonatal mice heterozygous for the Flg and Tmem79 mutations, epicutaneous sensitization to peanut in the first weeks of life required tape stripping and preexposure to a proteolytic aeroallergen, Alterneria alternata, and was abrogated by blocking the IL-33 receptor ST2.6 Repeatedly tape stripping the shaved back of BALB/c mice induces IL-33 and, to a lesser extent, TSLP.7 However, the associated 2-fold increase in intestinal mucosal mast cells was abrogated in IL-33 receptor–deficient mice but not in TSLP receptor (TSLPR)-deficient mice, demonstrating that there is a role for IL-33 rather than TSLP in intestinal mucosal mast cell accumulation.7 In contrast, in a model of inducible TSLP specifically in keratinocytes, overexpression of TSLP in the skin during intradermal ovalbumin (OVA) injections promoted acute allergic diarrhea on oral OVA gavage.8 Further, when AD-like skin inflammation was induced by application of vitamin D (calcipotriol, MC903) and OVA, elevated skin levels of TSLP were associated with increased OVA-specific IgE as well as intestinal mast cell accumulation and degranulation on intragastric OVA challenges in wild type (WT) mice but not in TSLPR-deficient mice.9
To assess the respective contribution of TSLP and IL-33 to the development of food allergy after epicutaneous sensitization, we used TSLPR-deficient mice, mice lacking the receptor for IL-33 (ST2), and BALB/cJ control mice, and subjected their shaved back skin to epicutaneous exposures to a common food allergen (OVA) in combination with a proteolytic Aspergillus fumigatus (ASP) extract known to disrupt the skin barrier. We have previously shown that mice exposed to 3 repeated weekly patches of ASP extract developed an AD-like skin phenotype, characterized by mixed TH2/TH17 skin inflammation.10 After assessing skin barrier integrity and sensitization to OVA, these mice were subjected to repeated intragastric OVA challenges, and the development of food allergy was determined by monitoring diarrhea and intestinal mast cell accumulation and degranulation. Detailed methods are available in the Methods section in this article’s Online Repository at www.jacionline.org.
RESULTS AND DISCUSSION
In order to promote epicutaneous OVA sensitization, which is thought to require tape stripping and/or preexposing skin to a proteolytic allergen, we chose to patch mice with a combination of OVA and ASP (250 μL of OVA with or without ASP at 1 mg/mL each) (Fig 1, A). Consistent with our prior published findings,10 skin exposure to ASP resulted in skin excoriations, epidermal thickening, and an impaired epidermal barrier, as indicated by elevated transepidermal water loss (TEWL) a day after patch removal (Fig 1, B and C). ASP-specific IgE levels were elevated in mice with ASP-induced eczema (Fig 1, D). Surprisingly, OVA-specific IgE levels were not only strongly increased in OVA + ASP patched mice but also in OVA patched mice (Fig 1, E), which displayed no skin excoriations or elevated TEWL (Fig 1, B and C). It is possible that minor skin wounding around the patched area may allow for OVA sensitization, but this is not reflected by TEWL. Thus, in our model, epicutaneous sensitization to food allergen does not require AD-like skin lesions—similar to what is observed in some children, who develop food sensitization in the absence of AD.11 This underappreciated finding further highlights the importance of noninflamed skin as a significant route for IgE sensitization to food allergen.
FIG 1.
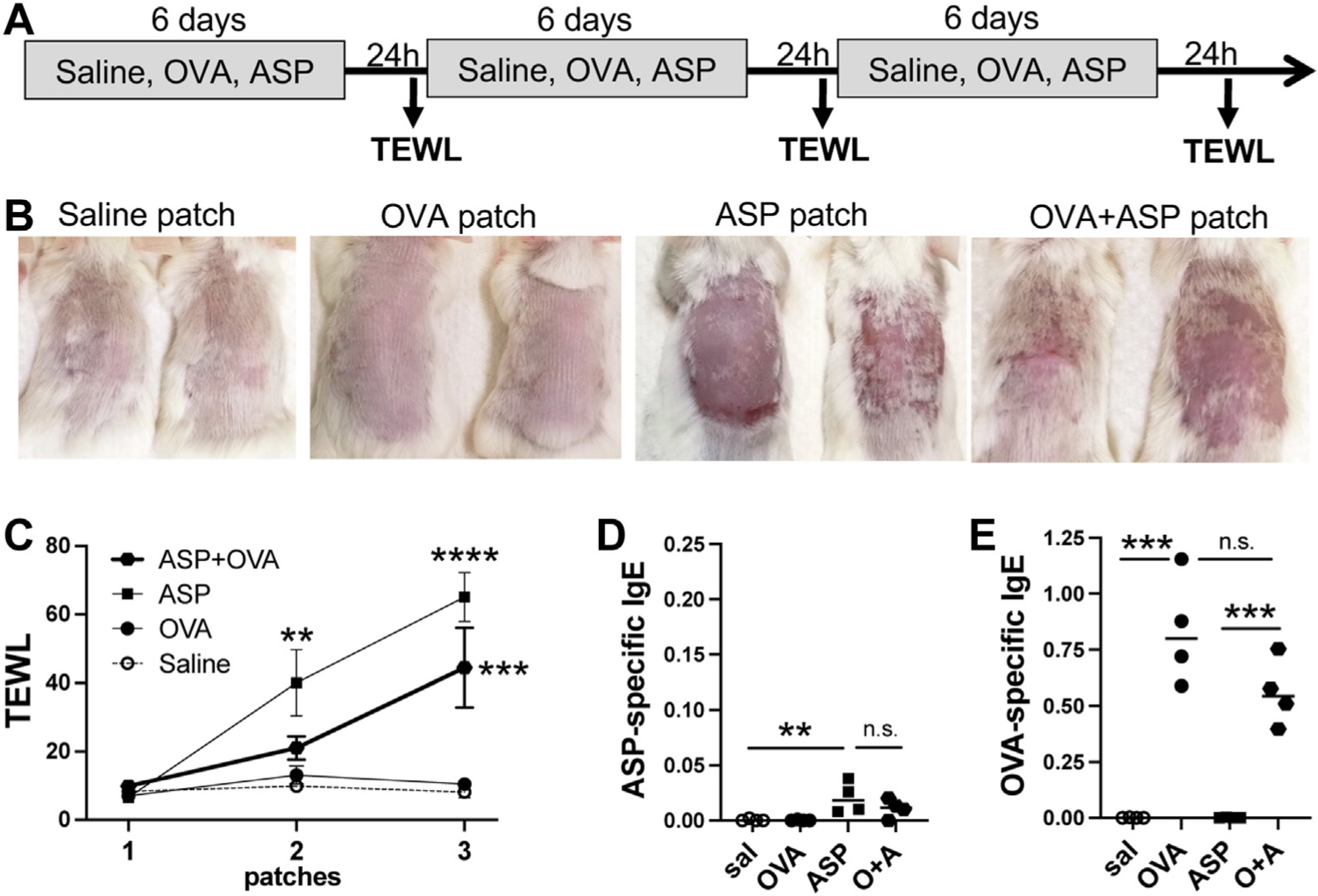
Epicutaneous sensitization to OVA does not require AD-like skin lesions. (A) Protocol: WT BALB/cJ mice were exposed to 3 weekly patches of one of saline, OVA, ASP, or OVA + ASP (1 mg/mL each). (B) Pictures were taken 24 hours after removal of third patch. (C) TEWL was measured 24 hours after patch removal (n = 4 mice per group; significance assessed by 2-way ANOVA). Plasma levels of (D) ASP-specific IgE and (E) OVA-specific IgE (significance assessed by 1-way ANOVA; **P < .01, ***P < .001, ****P < .0001, ns = not significant).
We therefore investigated the respective contribution of TSLP and IL-33 to epicutaneous food sensitization and the development of food allergy by exposing IL-33 and TSLPR-deficient mice and BALB/cJ control mice to 3 weekly skin patches of either saline or OVA, before repeated oral OVA challenges and monitoring mice for allergic diarrhea as previously described (Fig 2, A).3 After the third patch, the OVA patched WT mice did not develop skin lesions or elevated TEWL, but OVA-specific IgE levels were elevated. In contrast, OVA-specific IgE levels were lower in ST2−/− mice and notably lower in TSLPR−/− mice after the third patch (Fig 2, B). When we assessed mast cell degranulation after the first intragastric OVA challenge, MCPT1 levels were elevated in WT mice, reduced in ST2−/− mice, and further reduced in TSLPR−/− mice (Fig 2, C). After repeated OVA challenges, most WT mice developed allergic diarrhea, whereas only a third of ST2−/− mice and no TSLPR−/− mice developed allergic diarrhea (Fig 2, D). Accordingly, blood MCPT1 levels and jejunum mucosal mast cell counts were elevated in WT mice, lower in ST2−/−, and very low in TSLPR−/− mice (Fig 2, E–G). This was confirmed at the mRNA level for MCPT1+ mucosal mast cells and MCPT7+ connective tissue mast cells (Fig 2, H). Regardless of their genotype, mice with diarrhea were characterized by a large increase in the number of mucosal mast cells and elevated MCPT1 blood levels (Fig 2, E–H). In contrast, TSLPR−/− mice were protected from developing allergic diarrhea despite being sensitized (Fig 2, B and D), likely as a result of a near absence of intestinal mast cell accumulation (Fig 2, F–H).
FIG 2.
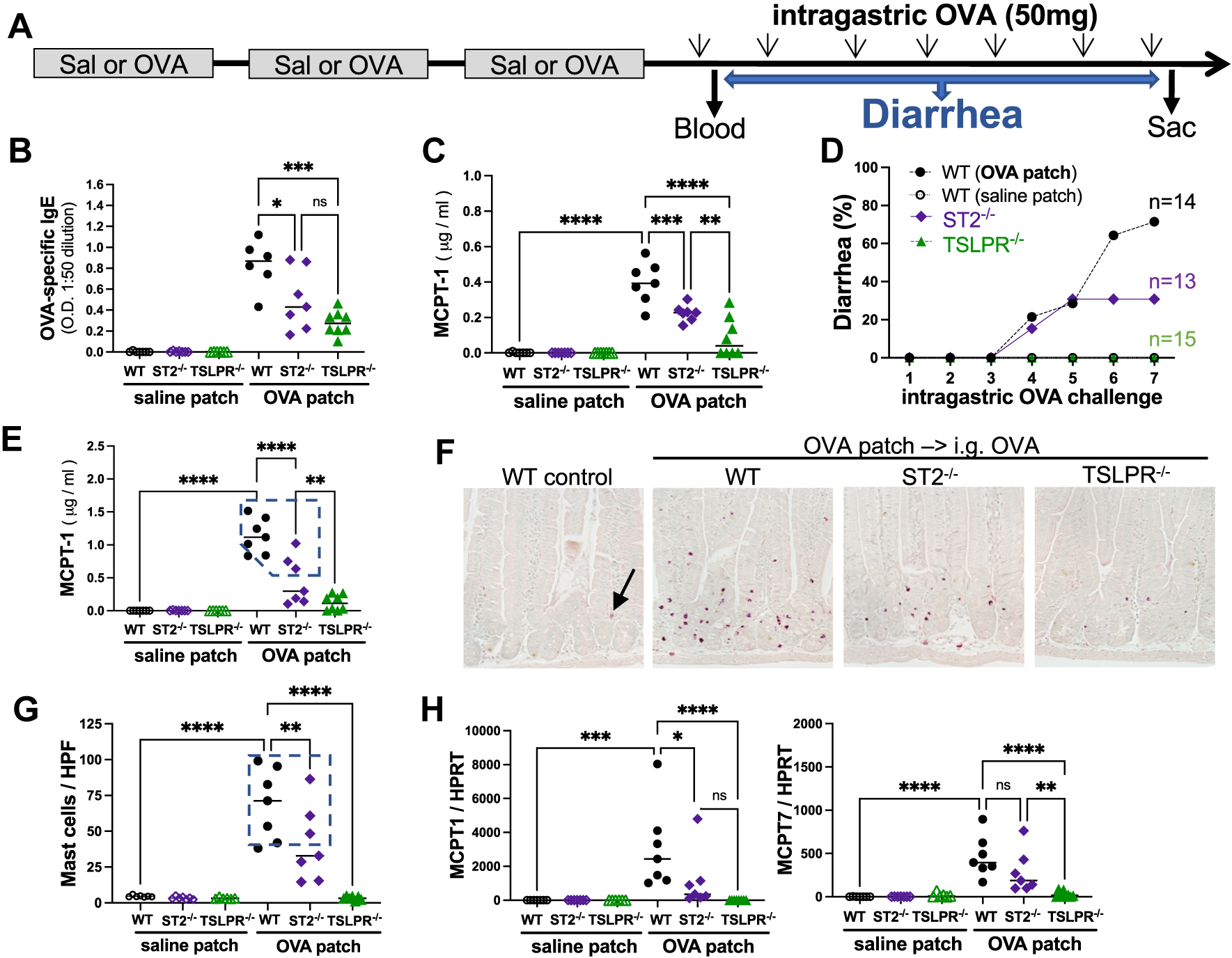
OVA-induced food allergy is milder in ST2-deficient mice and abrogated in TSLPR-deficient mice. (A) Protocol. Blood levels of (B) OVA-specific IgE and (C) MCPT1 were assessed 2 hours after first intragastric OVA challenge. (D) After each intragastric OVA challenge, diarrhea occurrences were monitored. (E) MCPT1 blood levels were assessed a day after last OVA challenge. (F) Representative images of CEA-stained jejunum mast cells (red), (G) counted in at least 15 HPF (original magnification ×20). (H) Relative mRNA levels of MCPT1 and MCPT7 in upper jejunum. Significance was assessed by 1-way ANOVA (*P < .05, **P < .01, ***P < .001, ****P < .0001, ns = not significant). CEA, Chloro-acetate esterase mast cell stain; HPF, high-power field.
Because AD is recognized as a major risk factor for developing a food allergy, we next assessed the respective importance of TSLP and IL-33 in Aspergillus-induced eczema and in sensitization to food allergen via inflamed skin (Fig 3, A). In OVA + ASP patched mice, TEWL measurements were not significantly lower in ST2-deficient mice compared to WT mice. In contrast, TSLPR−/− mice had a milder skin phenotype, as evidenced by decreased TEWL (Fig 3, B and C). Skin histology demonstrated that epidermal thickening was increased in OVA + ASP patched WT and ST2−/− mice, and to a lesser degree in TSLPR−/− mice (Fig 3, D). OVA-specific IgE levels were significantly decreased in TSLPR−/− mice compared to WT and ST2−/− mice (Fig 3, E). The recruitment of eosinophils was significantly impaired in OVA + ASP patched TSLPR−/− mice (Fig 3, F), suggesting that TSLP, rather than IL-33, is driving Aspergillus-induced skin inflammation in our model. Indeed, TSLP, in addition to promoting TH2 responses, has also been demonstrated to directly act on skin sensory neurons and induce itching, thereby exacerbating allergen-induced pruritus.12
FIG 3.
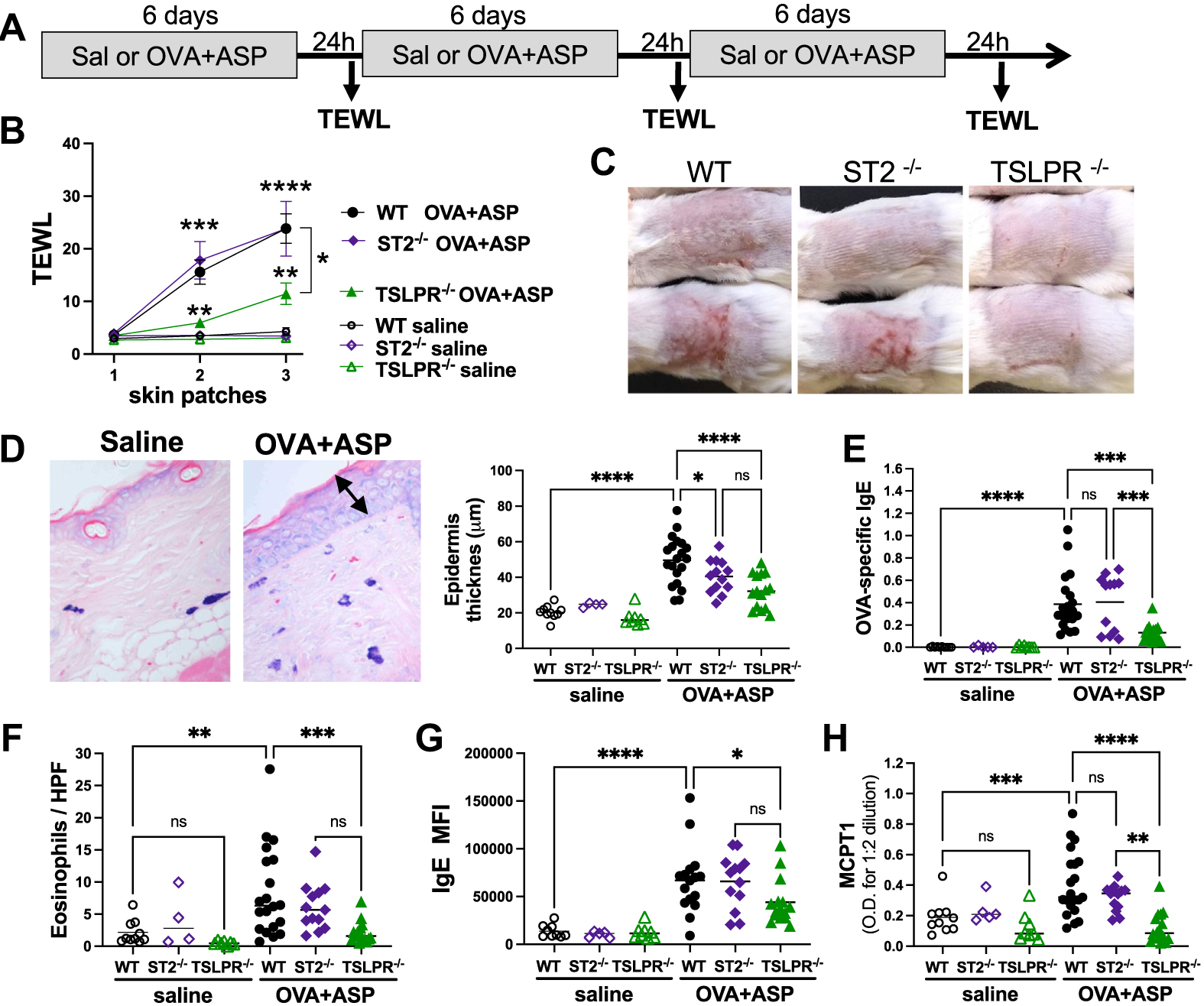
AD-like skin phenotype is alleviated in TSLPR-deficient mice. (A) Protocol. (B) TEWL was measured 24 hours after patch removal (n = 5–20 mice per group from 2 separate experiments; significance assessed by 2-way ANOVA). (C) Representative pictures taken 24 hours after removal of third patch. (D) Representative skin section of toluidine blue–stained mast cells. Epidermal thickness was measured by Image Pro Plus 4.1. (E) OVA-specific IgE blood levels. (F) Number of dermal eosinophils per HPF (original magnification ×40). (G) Amount of surface IgE on mast cells was determined by MFI on lineage-negative cKit+IgE+ skin cells. (H) Blood levels of MCPT1 after third patch. Significance was assessed by 1-way ANOVA (*P < .05, **P < .01, ***P < .001, ****P < .0001, ns = not significant). HPF, High-power field; MFI, mean fluorescent intensity.
Although skin mast cell numbers only modestly increased in OVA + ASP patched mice (data not shown),11 cKit+ IgE+ skin mast cells from OVA + ASP patched mice had higher levels of IgE detected on their surface compared to saline patched mice, as demonstrated by increased IgE mean fluorescent intensity (Fig 3, G). The observed lower peripheral blood levels of OVA-specific IgE in TSLPR−/− mice were associated with lower detection of IgE on skin mast cells from TSLPR−/− mice (Figs 3, E and G). Because histology suggested that some mast cells had degranulated (Fig 3, D), we measured the levels of MCPT1 in the blood after removal of the third patch. A mild but significant increase in MCPT1 levels was observed in WT and ST2−/−, but not in TSLPR−/−, OVA + ASP patched mice compared to controls (Fig 3, H).
It has been previously shown that OVA patched mice have an increased accumulation of mast cells in their small intestine in an AD model requiring tape stripping.7,13 In our model, when cells were isolated from the small intestine and IgE+cKit+ mast cells were identified by flow cytometry, no increase in overall intestinal mast cell frequency was observed in OVA + ASP patched mice (Fig 4, A). Interestingly, slightly lower mast cell numbers were observed in TSLPR−/− mice (Fig 4, A). Importantly, like skin mast cells (Fig 3, G), intestinal mast cells from OVA + ASP patched mice had increased IgE on their surface compared to saline patched mice (Fig 4, A). Slightly lower levels of surface-bound IgE were observed on intestinal mast cells from TSLPR−/− mice compared to WT and ST2−/− mice (Fig 4, A), suggesting that TSLPR−/− mast cells may be less susceptible to degranulate on OVA challenge. In a separate set of experiments, we gave OVA + ASP patched mice a single intragastric OVA challenge, resulting in a large release of MCPT1 into the blood of OVA + ASP patched WT and ST2−/− mice, but significantly lower MCPT1 levels were observed in TSLPR−/− mice (Fig 4, B), suggesting that the attenuated mast cell degranulation observed in TSLPR−/− might prevent the development of food allergy.
FIG 4.
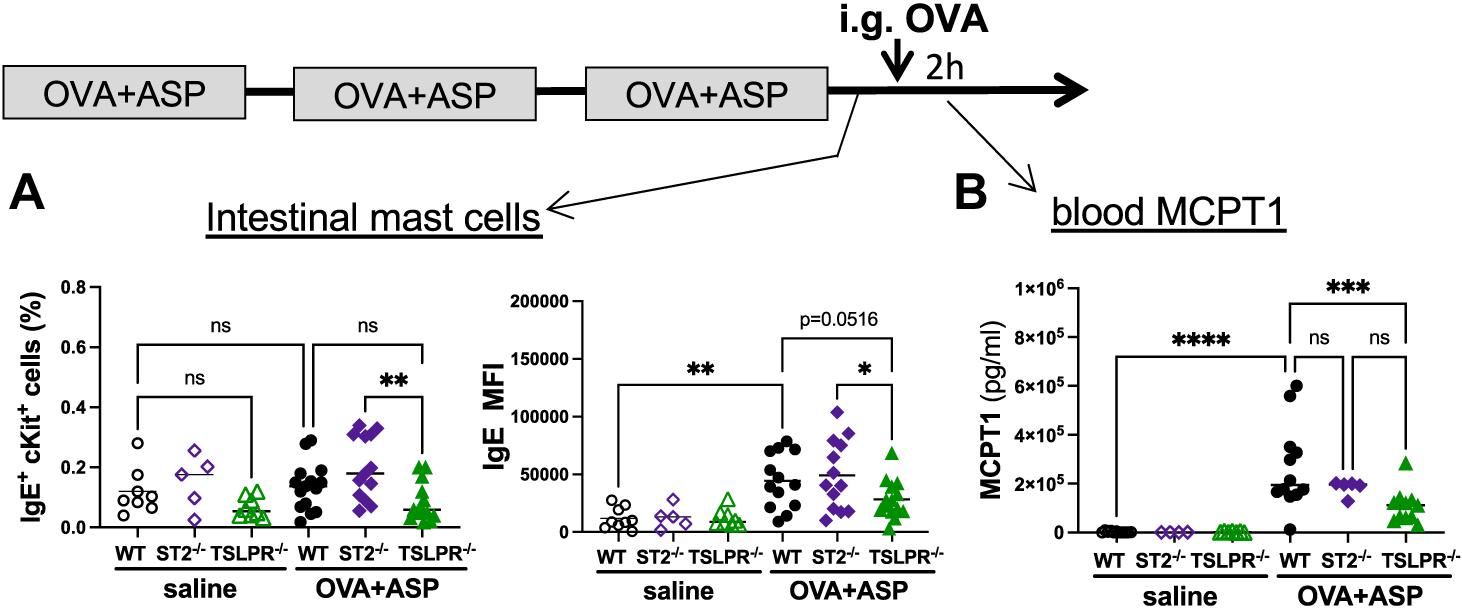
OVA-induced intestinal mast cell degranulation is impaired in TSLPR-deficient mice. (A) Frequency of lineage negative cKit+IgE+ mast cells isolated from small intestine; IgE presence on surface of mast cells was determined by MFI. (B) MCPT1 blood levels 2 hours after intragastric OVA challenge. Significance was assessed by 1-way ANOVA (*P < .05, **P < .01, ***P < .001, ****P < .0001, ns = not significant). MFI, Mean fluorescent intensity.
We have previously demonstrated that repeated OVA challenges of intraperitoneally sensitized mice promote allergic diarrhea, which is critically dependent on IgE and mast cells.3 Thus, we repeatedly challenged saline, OVA + ASP patched WT, ST2−/−, and TSLPR−/− mice with OVA and monitored them for diarrhea after each challenge (Fig 5, A). In contrast to ST2−/− mice, which were only partially resistant to developing diarrhea compared to WT mice, TSLPR−/− mice were completely protected from developing OVA-induced diarrhea (Fig 5, B). Diarrhea was associated with elevated blood MCPT1 levels (Fig 5, C) and was only observed in OVA + ASP patched WT and ST2−/− mice with the largest increase in lamina propria mucosal mast cell numbers (Fig 5, D). Thus, a certain threshold of degranulating mucosal mast cells must be reached to generate acute diarrhea, as previously suggested.4,13 Notably, there was no significant increase in mucosal mast cells in TSLPR−/− mice (Fig 5, D), and accordingly very little MCPT1 release (Fig 5, C).
FIG 5.
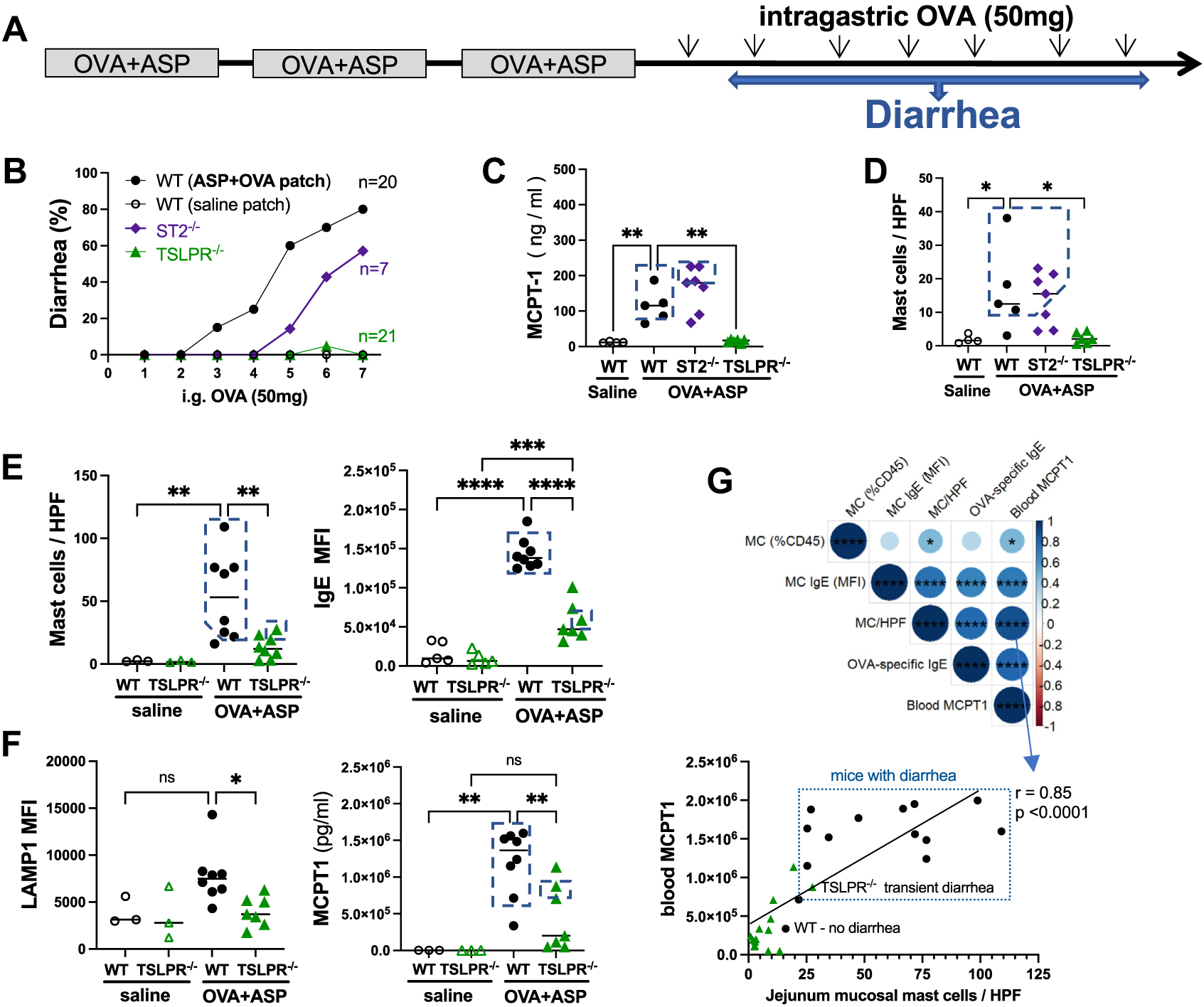
OVA-induced allergic diarrhea is abrogated in TSLPR-deficient mice. (A) Protocol. (B) Percentage of diarrhea occurrences in OVA + ASP patched mice was compiled from 3 separate experiments. (C) Blood levels of MCPT1 in OVA + ASP patched mice were assessed 1 day after last intragastric OVA challenge. (D and E) CEA-stained jejunum mucosal mast cells were counted in at least 15 HPFs (original magnification 320). Among lin−cKit+IgE+ mast cells, amount of (E) surface IgE and (F) surface LAMP1 was determined by MFI. Blood MCPT1 levels after last intragastric OVA challenge. (G) Spearman correlation matrix among parameters in mice. Dotted-line boxes denote mice with diarrhea. CEA, Chloro-acetate esterase mast cell stain; HPF, high-power field; MFI, mean fluorescent intensity.
Next, we focused on isolated intestinal mast cells from WT and TSLPR−/− mice and assessed the relative amounts of surface IgE (Fig 5, E). A substantial increase in surface IgE was observed on mast cells from OVA + ASP patched WT mice, but this was significantly attenuated in TSLPR−/− mice (Fig 5, E). This was associated with slightly increased surface expression of CD107a (LAMP1), a marker of degranulation, in WT but not TSLPR−/− mice (Fig 5, F). Accordingly, blood levels of MCPT1 were elevated in OVA + ASP patched WT mice and significantly attenuated in TSLPR−/− mice (Fig 5, F). Furthermore, intestinal mucosal mast cell numbers and MCPT1 blood levels were highly correlated; elevated levels of both were only observed in mice with diarrhea (Fig 5, G). Of note, the only TSLPR−/− mouse with mild, transient diarrhea had elevated intestinal mast cell numbers and high MCPT1 blood levels. Hence, accumulation of mucosal mast cells with high IgE surface expression during repeated food allergen exposures leads to elevated release of mast cell proteases and, after reaching a certain threshold, diarrhea. Taken together, these results point to a central role of TSLP in mediating the development of food allergen–induced allergic diarrhea.
We propose a model in which early exposure of noninflamed skin to allergen has TSLP rather than IL-33 playing the dominant role in promoting allergen-specific IgE generation, and subsequent intestinal mast cell accumulation and degranulation as well as symptoms of food allergy (diarrhea) on oral exposure. Furthermore, TSLP rather IL-33 contributes to ASP-induced skin inflammation in a model mimicking mixed TH2/TH17 eczema in young children.14 However, when cutaneous exposure to food allergen occurs during a more chronic phase of AD, after cycles of itching and scratching (modeled in mice by tape stripping), large amounts of IL-33 are released into the dermis and bloodstream, activating intestinal group 2 innate lymphoid cells, which release TH2 cytokines, which drive the expansion of intestinal mast cells and thus promote food allergy.7,13 In our model, no changes in intestinal group 2 innate lymphoid cell numbers were observed (data not shown). The contribution of other cell types, notably to the generation of IgE, which is critical to mast cell–mediated food allergy, has not been addressed in the present study; nor has the potential impact that large intragastric OVA doses containing lipopolysaccharide could have on the intestinal microbiome. A strength of our study is the finding that epicutaneous sensitization to food allergen can occur in the absence of AD-like skin inflammation.
In conclusion, these results suggest that prophylactic targeting of TSLP, possibly in combination with emollients, could mitigate the development of food sensitization early in life, especially in at-risk children (skin barrier defect, atopic parents). Although blocking TSLP in recently sensitized mice has been shown to prevent food-induced anaphylaxis, suppression of established food allergy requires targeting multiple pro-TH2 cytokines (IL-33, IL-25, and TSLP), as demonstrated in a murine model of food allergy.15
METHODS
Mice
WT BALB/cJ mice were purchased from The Jackson Laboratory (Bar Harbor, Maine) and bred in house alongside ST2−/− and TSLPR−/− mice in a pathogen-free facility. ST2−/−mice were the kind gift of Andrew McKenzie (Cambridge University, Cambridge, United Kingdom); and TSLPR-deficient mice were obtained from Steven Ziegler (Benaroya Research Institute, Seattle, Wash). The mice were maintained and handled under Institutional Animal Care and Use Committee–approved procedures (Cincinnati Children’s Hospital Medical Center) and the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, National Research Council).
Epicutaneous allergen exposure
Mice were anesthetized with isoflurane (IsoFlo; Abbott Laboratories, Chicago, Ill) and their backs shaved with an electric razor 1 or 2 days before first allergen exposure. A total of 250 μL of sterile saline solution, OVA (albumin from chicken egg white, OVA grade V, Sigma-Aldrich, St Louis, Mo), ASP (XPM110D3A4; Greer, Lenoir, NC), or both at a concentration of 1 mg/mL was applied to a 2 × 2 cm patch of sterile gauze. The patch was secured with TegaDerm (3M, Maplewood, Minn), and the mouse was wrapped with a Band-Aid and waterproof tape. After 6 days, the patch was removed; 24 hours later, a new patch was applied, for a total of 3 patches over a 3-week period, as shown in Fig 1, A. Endotoxin levels in OVA and ASP were assessed using the QCL-1000 LAL assay (Lonza, Basel, Switzerland): 1 mg/mL of OVA grade V contains about 250 EU/mL of endotoxin (50 EU/mL per patch), and 1 mg/mL of ASP contains between 0.04 EU/mL (lot 357280) and 0.14 EU/mL (lot 194776).
Measurement of TEWL
TEWL was measured by using a DermaLab USB module (Cortex Technology, Hadsund, Denmark). TEWL was assessed over a 1-minute period by placing the probe against the skin surface in the center of an area exposed to the patch. An average of 2 readings per mouse was used, and TEWL measurements were recorded as grams per square meter per hour.
Allergen-specific antibody levels
Allergen-specific IgG1 and IgE plasma levels were measured by ELISA. Briefly, plates were coated with either OVA or ASP (100 μg/mL) overnight at 4°C. Blocking was done with 1% BSA in PBS, and all washes were performed with 0.05% Tween 20 in PBS. Plasma samples were serially diluted to determine the optimal dilution for IgG1 and IgE. After 2 hours of incubation, the biotinylated secondary antibodies, respectively biotin rat anti-mouse IgG1 (A85–1; Pharmingen; BD Biosciences, San Jose, Calif) and biotin rat antimouse IgE (R35–118; Pharmingen, BD Biosciences), were added for 1 hour, followed by washing and incubation with streptavidin–horseradish peroxidase (DY998, R&D Systems, Minneapolis, Minn). Samples were developed by adding tetramethylbenzidine substrate (BD Biosciences). MCPT1 plasma levels were measured according to the manufacturer’s instructions (Invitrogen; Thermo Fisher Scientific, Waltham, Mass). All reactions were stopped with 2N H2SO4, and absorbance was read at 450 nm.
Histology
Skin stripes were collected from the patched area, and sections 2 to 3 cm long of upper jejunum were collected from each mouse and fixed in 10% formalin before being processed according to standard histologic techniques. Paraffin-embedded tissues were cut into 5 μm sections and stained with either hematoxylin and eosin to assess skin thickness and identify eosinophils, or with Leder stain to identify mast cells.11 Epidermal thickness was quantified by morphometric software (Image Pro Plus 4.1; Media Cybernetics, Silver Spring, Md). To stain intestinal mucosal mast cells, we mixed 50 μL of New Fuchsin (1 g resuspended in 25 mL of HCl 2N) with 50 μL sodium nitrate 4%. After the hexazotized solution turned yellow, we added 900 μL of Naphthol AS-D chloroacetate (100 mg resuspended in 50 mL of N-N-dimethylformamide and stored at −20°C). A total of 500 μL was taken, and to this we added 10 mL of filtered phosphate buffer, as follows: 13 mL of monobasic sodium phosphate (0.2 mol) + 87 mL of dibasic sodium phosphate (0.2 mol) + 100 mL of H2O, adjusted to pH 7.6. Freshly prepared solution was added to tissue sections for 10 minutes. Staining was stopped by washing slides in H2O before lightly counterstaining with methyl green or with diluted 1:10 hematoxylin. Chloroacetate esterase–stained mucosal mast cells were counted and expressed as an average over 15 to 20 high-power fields at ×20 magnification.
RNA isolation and real-time quantitative PCR
Total RNAwas isolated from each mouse’s homogenized skin or intestine using RiboZol according to the manufacturer’s instructions (VWR, Radnor, Pa), then DNase treated and purified using the RNeasy MinElute Cleanup kit (Qiagen, Valencia, Calif), before being reverse transcribed with SuperScript IV First-Strand Synthesis System (Invitrogen; Thermo Fisher Scientific). Generated cDNA was diluted 1:15 before being amplified using LightCycler FastStart DNA Master SYBR Green I and the following primers: mMCPT1 forward 5′-TGGCACTTCTCTTGCCTTCTG-3′ and reverse 5′-TCAGAACCTCTGTCCGTGATG-3′, mMCPT7 forward 5′-AGGCTGGGGTAACATCGACA-3′ and reverse 5′-AATGGGAACTTGCACCTCCTT, mHPRT forward 5′-TGCCGAGGATTTGGAAAAAG-3′ and reverse 5′-CCCCCCTTGAGCACACAG-3′. Quantitative real-time PCR was performed on a LightCycler 96 device (Roche, Basel, Switzerland), and mast cell protease gene expression was normalized to the housekeeping gene HPRT.
Skin and intestinal cell isolations
A 1 × 2 cm skin section below the patched area was subcutaneously injected twice with 50 μL of digestion media, cut out, and placed in 1 mL of digestion media (RPMI 1640 containing penicillin/streptomycin, and 0.25 mg/mL Liberase DL and 0.2 mg/mL DNaseI from Roche) and incubated at 37°C. After 30 minutes, skin fat was manually scraped off with a scalpel, and skin sheets were cut into 2 or 3 pieces, then digested for another 90 minutes at 37°C. Digested skin sheets were homogenized with the Medicon/Medimachine tissue homogenizer system according to the manufacturer’s instructions (BD Biosciences). Small intestines were flushed with PBS, cut longitudinally after removal of Peyer patches, and incubated in HBSS with 5 mmol EDTA at 4°C for 5 minutes before vortexing to remove epithelial cells. After 3 repetitions, the remaining tissues were rinsed in HBSS before being minced and digested for 30 minutes at 37°C in a solution of RPMI 1640 + 1% FBS containing 2.4 mg/mL collagenase A (Roche) and 0.2 mg/mL DNaseI (Roche). The remaining tissue fragments were disrupted by aspiration through an 18-gauge needle, and tissue debris was removed by passage through a 70 μm filter. Intestinal cells resuspended in 44% Percoll were then carefully loaded above 67% Percoll before centrifugation (20 minutes at 1800 rpm). Lamina propria cells were collected from the interface between 44% and 67% Percoll into 8 mL of RPMI 1640 + 10% FBS, centrifuged 5 minutes at 1300 rpm, and resuspended in magnetic cell sorting buffer.
Flow cytometry
For jejunum cells, 5 × 105 to 106 cells were transferred to a 96-well plate with V-shaped wells on ice, centrifuged, and resuspended in PBS containing TruStain FcX (anti-mouse CD16/32; BioLegend, San Diego, Calif). Cells were stained with the antibodies listed in Table E1, and mast cells were identified as lineage-negative (B220, CD3, CD4, CD8, CD11b, CD11c, Gr1) IgEhighcKit+ cells (Fig E1). All cells were labeled with Live/Dead Fixable Zombie UV Dye according to the manufacturer’s instructions (Invitrogen). Data from flow cytometry experiments were acquired on a BD LSR Fortessa device using BD FACSDiva software (BD Biosciences), and all data were analyzed by FlowJo v10.8 software (Treestar, Ashland, Ore).
Statistical analysis
Statistical significance was assessed by either 1-way ANOVA followed by Sidak multiple comparisons tests between relevant groups, or 2-way ANOVA followed by the Tukey multiple comparisons tests. Relationships between variables were assessed by Spearman coefficient analysis. Statistical analyses were performed by GraphPad Prism v9.4 software (GraphPad Software, La Jolla, Calif) and R v4.1.0 (R Project; www.r-project.org). P < .05 was considered significant.
Supplementary Material
Key messages.
In an AD murine model that does not rely on repeated tape stripping, TSLP rather than IL-33 mediates Aspergillus-induced eczema.
Epicutaneous sensitization to food allergen does not require AD-like skin lesions.
Presence of IgE on intestinal mast cells is lower in OVA sensitized TSLPR−/− mice compared to ST2−/− and WT mice, resulting in impaired mast cell degranulation.
Acknowledgments
Supported by National Institutes of Health grants 2U19AI70235 and R01AI127392.
Abbreviations used
- AD
Atopic dermatitis
- ASP
Aspergillus fumigatus extract
- MCPT1
Mast cell protease 1
- OVA
Ovalbumin
- ST2
IL-33 receptor
- TEWL
Transepidermal water loss
- TSLP
Thymic stromal lymphopoietin
- TSLPR
TSLP receptor
- WT
Wild type
Footnotes
Disclosure of potential conflict of interest: The authors declare that they have no relevant conflicts of interest.
TSLPR-deficient mice were obtained from Steven Ziegler (Benaroya Research Institute, Seattle, Wash) and ST2−/− mice from Andrew McKenzie (Cambridge University, Cambridge, United Kingdom). We acknowledge the assistance of the Research Flow Cytometry Core in the Division of Rheumatology at Cincinnati Children’s Hospital Medical Center. We thank Heidi Rider for editorial assistance and Paige Bolcas, Brittany Grashel, and David Ohayon for technical assistance.
REFERENCES
- 1.Brough HA, Nadeau KC, Sindher SB, Alkotob SS, Chan S, Bahnson HT, et al. Epicutaneous sensitization in the development of food allergy: what is the evidence and how can this be prevented? Allergy 2020;75:2185–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tham EH, Rajakulendran M, Lee BW, van Bever HPS. Epicutaneous sensitization to food allergens in atopic dermatitis: what do we know? Pediatr Allergy Immunol 2020;31:7–18. [DOI] [PubMed] [Google Scholar]
- 3.Brandt EB, Strait RT, Hershko D, Wang Q, Muntel EE, Scribner TA, et al. Mast cells are required for experimental oral allergen-induced diarrhea. J Clin Invest 2003;112:1666–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahrens R, Osterfeld H, Wu D, Chen CY, Arumugam M, Groschwitz K, et al. Intestinal mast cell levels control severity of oral antigen-induced anaphylaxis in mice. Am J Pathol 2012;180:1535–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Han H, Roan F, Ziegler SF. The atopic march: current insights into skin barrier dysfunction and epithelial cell–derived cytokines. Immunol Rev 2017;278:116–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walker MT, Green JE, Ferrie RP, Queener AM, Kaplan MH, Cook-Mills JM. Mechanism for initiation of food allergy: dependence on skin barrier mutations and environmental allergen costimulation. J Allergy Clin Immunol 2018;141:1711–25.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leyva-Castillo JM, Galand C, Kam C, Burton O, Gurish M, Musser MA, et al. Mechanical skin injury promotes food anaphylaxis by driving intestinal mast cell expansion. Immunity 2019;50:1262–75.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Han H, Thelen TD, Comeau MR, Ziegler SF. Thymic stromal lymphopoietin-mediated epicutaneous inflammation promotes acute diarrhea and anaphylaxis. J Clin Invest 2014;124:5442–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Noti M, Kim BS, Siracusa MC, Rak GD, Kubo M, Moghaddam AE, et al. Exposure to food allergens through inflamed skin promotes intestinal food allergy through the thymic stromal lymphopoietin–basophil axis. J Allergy Clin Immunol 2014;133:1390–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brandt EB, Gibson AM, Bass S, Rydyznski C, Khurana Hershey GK. Exacerbation of allergen-induced eczema in TLR4- and TRIF-deficient mice. J Immunol 2013;191:3519–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berdyshev E, Goleva E, Bronova I, Bronoff AS, Hoffman BC, Ramirez-Gama MA, et al. Unique skin abnormality in patients with peanut allergy but no atopic dermatitis. J Allergy Clin Immunol 2021;147:361–7.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilson SR, Thé L, Batia LM, Beattie K, Katibah GE, McClain SP, et al. The epithelial cell–derived atopic dermatitis cytokine TSLP activates neurons to induce itch. Cell 2013;155:285–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galand C, Leyva-Castillo JM, Yoon J, Han A, Lee MS, McKenzie ANJ, et al. IL-33 promotes food anaphylaxis in epicutaneously sensitized mice by targeting mast cells. J Allergy Clin Immunol 2016;138:1356–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Esaki H, Brunner PM, Renert-Yuval Y, Czarnowicki T, Huynh T, Tran G, et al. Early-onset pediatric atopic dermatitis is TH2 but also TH17 polarized in skin. J Allergy Clin Immunol 2016;138:1639–51. [DOI] [PubMed] [Google Scholar]
- 15.Khodoun Mv, Tomar S, Tocker JE, Wang YH, Finkelman FD. Prevention of food allergy development and suppression of established food allergy by neutralization of thymic stromal lymphopoietin, IL-25, and IL-33. J Allergy Clin Immunol 2018;141:171–9.e1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


