Abstract
Besides plants and animals, the Fungi kingdom describes several species characterized by various forms and applications. They can be found in all habitats and play an essential role in the excellent functioning of the ecosystem, for example, as decomposers of plant material for the cycling of carbon and nutrients or as symbionts of plants. Furthermore, fungi have been used in many sectors for centuries, from producing food, beverages, and medications. Recently, they have gained significant recognition for protecting the environment, agriculture, and several industrial applications. The current article intends to review the beneficial roles of fungi used for a vast range of applications, such as the production of several enzymes and pigments, applications regarding food and pharmaceutical industries, the environment, and research domains, as well as the negative impacts of fungi (secondary metabolites production, etiological agents of diseases in plants, animals, and humans, as well as deteriogenic agents).
Keywords: biodeterioration, filamentous fungi, fungal biotechnology, natural products, secondary metabolites
1. Introduction
The fungal kingdom comprises a plethora of eukaryotic species that proliferate in diverse environments; fungi also have essential roles as components of the microbiota, where they act as symbionts, endophytes, parasites, or saprotrophs [1,2]. Studies aiming to characterize the microbiota of diverse species across kingdoms have revealed an unexpected double nature of the fungi in the microbiome: they colonize higher eukaryotes from plants to humans [3]. In the meantime, as with all other eukaryotes, fungi host their microbiota, consisting of microbial communities that adhere to the hyphal surface, develop among the pseudotissues produced by hyphal aggregation, or colonize the fungal cytoplasm. Fungi, generally, can be microscopic to macroscopic, and include unicellular organisms such as yeasts and multicellular organisms such as filamentous fungi. Filamentous fungi grow long 2–10 μm thin filaments (hyphae) into intricate network structures (mycelium) that are observable to the naked eye and can grow to the centimeter to meter scale [4,5].
The filamentous growth mode and the secretion capacity of proteins and primary and secondary metabolites facilitate fungal proliferation in nature. Industry uses these properties to produce proteins, small molecule compounds, and, recently, mycelium materials. These bio-based products could be used as thermal and acoustic insulation and packaging [6]. Pure fungal materials are the result of complete substrate degradation or are obtained by removing the fungal skin from the surface of a substrate. The properties of the mycelium depend on the substrate, the type of fungus, and growth conditions [7].
Fungi could contribute to the aspiration to develop more sustainable manufacturing to protect the environment, being an optimal candidate to produce several products such as textiles and myco-leather, biofuels, building materials, wastewater treatment, and sustainable meat substitutes [8,9]. Besides industrial and food applications, fungi are also used in medicine for the production of a lot of compounds, e.g., antibiotics (such as penicillin), or a compound named cyclosporine, produced by Tolypocladium inflatum, as well as lovastatin, a drug for lowering the cholesterol from blood produced from Aspergillus terreus [10,11].
In agriculture, fungi play a significant role, including plant growth and protection. For example, mycorrhizal fungi establish a mutualistic association with plant roots, improving the plant’s nutrient uptake by increasing the surface area of the root system. This relationship helps the plant to access nutrients, such as phosphorus and nitrogen, that are not readily available in the soil [12,13,14]. Another essential agricultural fungal species is the endophytic fungi that colonize plant tissue. The complex interaction between endophytic fungi and plant tissue involves modulating the plant’s defense mechanism in terms of inhibiting phytopathogens and stimulating the growth of the plants even under biotic and abiotic stress conditions [15]. However, in a specific situation, some fungal species exert a less beneficial action on plant health, causing various plant diseases by colonizing roots, leaves, and tissue [16].
Regarding the environmental issues related to pollution and toxic wastes, fungi are quickly surfacing as essential protagonists, involved in practices such as the bioremediation of pharmaceutical compounds, agricultural wastes, or degradation of various pollutants.
Although fungi are widely spread in the environment and co-exist with many organisms, in certain circumstances, fungi, like any other microorganisms, can harm their host. Fungi are characterized by high resilience in stressful conditions and a remarkable ability to adapt to different environments. Infectious fungi can spread through the air and water and be transmitted by different carriers such as animals, humans, or plants [17,18]. Regarding the interaction of fungi with plant organisms, more than 80% of the plants from our planet are symbiotic with fungi. However, some of the fungal strains can sometimes enter inside plants through damaged leaves and stomata, thus turning themselves into plant pathogens with a high impact on plant health.
This review aims to characterize the beneficial roles of fungi used for a wide range of applications, such as the production of several enzymes (cellulase, proteinases, amylases, invertase, pectinase, chitinases, lipases, and, respectively, lignocellulolytic enzymes, citric acid, gluconic, itaconic, lactic, fumaric, malic, succinic, and oxalic acids); pigments (such as polyketide pigments, carotenoids, or melanin); applications regarding food (e.g., worldwide farmed mushrooms species such as Agaricus bisporus, Pleurotus ostreatus, Flammulina, and Lentinula shiitake, or the usage of filamentous fungi strains to produce different fermented foods such as cheese, bread, and beer, or the molds to manufacture fermented sausages, or alcoholic beverages production such as wine and beer); and in pharmaceutical industries, the environment (hydrocarbon degrading fungi, bioremediation, biofuels production, mycofiltration), and research domains (as research model organisms). Furthermore, this review summarize the negative impacts of fungi secondary metabolites production (B1, B2, G1, and G2 aflatoxins; A, B, and C ochratoxins; A, B, C, and P fumonisins; more than 200 trichothecenes; zearalenone and its derivatives; patulin), etiological agents of diseases in plants, animals and humans (e.g., Alternaria and Cladosporium genus comprise phytopathogenic species or can are responsible for human or animal infectious diseases), as well as deteriogenic agents of different substrates such as stone, wooden, paintings, textiles, parchments, paper and paper-based materials or heritage buildings.
In terms of novelty, this review brings together comprehensive data regarding the beneficial roles of fungi used for a wide range of applications (e.g., production of several enzymes, medicine, agriculture, industry, environmental safety, and research) and the damaging effects of fungi, acting as etiological agents of diseases in plants, animals, and humans. In addition, in this paper, we present the deterioration action of fungi on cultural heritage objects. Therefore, the rationale for choosing this subject is the impact of fungi on human health and environmental safety. People suffering from opportunistic and primary invasive fungal infections urgently need resources and research efforts to bring them new diagnostics and treatments regardless of commercial potential. In agriculture, the presented applications have the potential to improve crop yield, reduce the use of synthetic fertilizers and pesticides, avoid the use of toxic compounds, and promote sustainable agriculture practices. Thus, further attention must be paid to uncovering the biomolecules from fungi for agriculture and pharmaceutical applications through studying metagenomics, genomics, and proteomics.
2. Significance of Fungi in Different Sectors
2.1. Beneficial Roles
Fungi are the kingdom of those organisms whose species can populate practically all ecosystems. They are found as free-living and symbiotic unicellular or multicellular organisms and exist under varied morphologies [19]. They exist in almost all environmental types, from soil to water, and are best known for their essential roles in ecology as decomposers and symbionts. Fungi have also been used for centuries in several practices in the food and medicine fields. Recently, fungi have emerged as a valuable resource in modern biotechnology, with numerous applications across different sectors and as a sustainable candidate.
Fungi appear in various sizes, starting with microfungi such as molds and yeasts and progressing to macromyctes such as mushrooms or truffles. The macro-sized fungi are most often used for human consumption as supplements or food; on the other hand, the micro-sized fungi, including species such as Aspergillus, Penicillium, and Saccharomyces, are used for synthesizing enzymes and metabolites. According to these described abilities, fungi are considered one of the cornerstones of modern biotechnology [20].
Fungi indubitably dominate the biotechnology sphere; therefore, it is expected that their utilization is going to grow exponentially hereafter. They play a crucial role in various industrial processes, including manufacturing enzymes, pigments, vitamins, and so on [21]. Moreover, they are used to manufacture different types of food pigments such as benzoquinone (Penicillium europium), anthraquinones (Paecilomyces farinosus), melanin (Aspergillus spp.), and β-carotene (Blackeslea trispora) [8,9].
Fungi could contribute to the aspiration to develop more sustainable manufacturing to protect the environment, being an optimal candidate to produce several products such as textiles and myco-leather, biofuels, building materials, wastewater treatment, and sustainable meat substitutes [4,22,23].
Before we identifed the different fungi species and understood the definition of fungi, they had been used to produce various food in different parts of the world, such as fermented food, bread, wine, and cheese [20]. Today, a significant part of worldwide cuisine is represented by products made with fungi, usually products that result after fermentation.
2.1.1. Medicine/Health
Natural products (NPs) produced by fungi are responsible for several effects, such as antimicrobial, immunosuppressive, anticancer, antidiabetic, immunomodulatory, and anti-inflammatory effects, many of which have been developed as treatments and have potential therapeutic applications for human diseases.
NPs such as non-ribosomal peptides (i.e., penicillin produced by Penicillium rubens; cephalosporin C by Acremonium chrysogenum, pneumocandins by Glarea lozoyensis and Pezicula (Cryptosporiopsis), or ribosomal peptides (amatoxins, piperazines), polyketides, lipopeptides, lipodepsipeptides and secondary metabolites produced by fungi mediate antimicrobial resistance and virulence and act in competition against other microorganisms [24].
Regarding antibiotic production by filamentous fungi, it has been proved that they initiated the golden era of natural antibiotics in the 20th century, as a consequence of extensive antibiotic use, especially in hospital settings, with the appearance of antimicrobial resistance phenomenon in the 21st century, especially to ESKAPE pathogens (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species) [25].
In Table 1, we list the NPs known as antimicrobial agents produced by different filamentous fungi strains or macromycetes.
Table 1.
Antimicrobial agents produced by filamentous fungi.
| Antimicrobial Agents | Producer | Active Against | Mode of Action | References |
|---|---|---|---|---|
| Non-ribosomal peptides | ||||
| Mycophenolic acid | Penicillium brevicompactum | several species | antibacterial, antifungal, antiviral, antitumor, antipsoriasis and immunosuppressive, anti-angiogenic activities | [26] |
| Penicillin | Penicillium notatum | Staphylococcus aureus | [27] | |
| Penicillin G | P. rubens | Streptococcus, Staphylococcus, Enterococcus, Clostrodium and Treponema spp. | inhibit the peptidoglycan synthesis | [28,29] |
| Cephalosporin C | A. chrysogenum | broad spectrum antibiotic | [2] | |
| Ribosomal peptides | ||||
| Amatoxin Phallotoxin |
Amanita spp. | anticancer drugs | RNA polymerase II inhibitors | [30] |
| Ustilotoxin | Ustilaginoidea virens | cytotoxicity against different anticancer cell lines | anti-mitotic activity | [31] |
| Depsipeptides Beauvericin (A–H) Beauveriolide |
Fusarium, Alternaria, Calonectria, Cochliobolus, Cordyceps cardinalis, Ophiocordyceps communis Cordyceps militaris |
antimicrobial and insecticidal activity anti-aging activity against S. cerevisiae |
[32,33,34,35] | |
| Piperazines | ||||
| Roquefortine C | Penicillium roqueforti | acute toxicity in mice and dogs | [36,37] | |
| Gliotoxin | Aspergillus fumigatus | antifungal activity against Candida albicans and Aspergillus spp. | inhibit the activation of lymphocyte B and T | [38,39] |
| Polyketides | ||||
| Griseofulvin | Penicillium griseofulvum | dermatophytes fungal infections in humans and animals;non-fungal inflammatory diseases; cardiovascular, antitumor and antiviral applications; |
inhibit fungal cell mitosis and nuclear acid synthesis | [40] |
| Patulin | Aspergillus clavatus | mycotoxin, fungistatic activity against Rhizoctonia solani, S. cerevisiae, Didymella bryoniae, Botrytis cinerea, Fusarium oxysporum, clerotium rolfsii, Pithium ultinum | destabilization of of the plasma membrane integrity, blockage in rRNA, tRNA, and mRNA synthesis | [41,42] |
| Strobilurins | Strobilurus tenacellus, Oudemansiella mucida | antifungal activity | inhibit the transfer of electrons between complexes II and III of the electron transport chain in the mitochondria, resulting in impaired cell respiration and ATP synthesis | [43] |
| Uredinorubellin derivatives | Torrubiella spp. | antibacterial activity against S. aureus strains | [34] | |
| Rubellins antraquinones | Ramularia collo-cygni | antiproliferative, cytotoxic, aggregation inhibitory and antimicrobial activity against B. subtilis, S. aureus, S. aureus MRSA, Enterococcus faecalis clinical and reference strains | phytotoxic activity | [44] |
| Viriditoxin | Penicillium radicum | antimicrobial activity against S. aureus MRSA | inhibiting FtsZ, the bacterial tubulin | [45] |
| Lindgomycin | Lindgomycetaceae family | antimicrobial (against Gram-positive and C. albicans strains) and antiviral activity | [24] | |
| Lipopeptides | ||||
| Echinocandin B | Aspergilllus nidulans | inhibiting β (1,3)-glucan synthase | [46] | |
| Pneumocandin B0 | Glarea lozoyensis | antifungal activity against C. albicans and Pneumocystis carinii | inhibiting β (1,3)-glucan synthase | |
| Caspofungin | G. lozoyensis | blocking cell wall biosynthesis by inhibiting β (1,3)-D-glucan synthase |
[47] | |
| Micafungin | Coleophoma empetri | |||
| Anidulafungin | A. nidulans | |||
| Mulundocandin | Aspergillus sydowii |
Aspergillus niger, C. albicans, Candida non-albicans |
[48] | |
| Anidulafungin | A. nidulans |
Candida parapsilosis, Candida guilliermondii Aspergillus spp. Fusarium spp. |
||
| Rezafungin | Candida spp., Aspergillus spp., Pneumocystis murina | [48,49] | ||
| Cryptocandin | Cryptosporiopsis quercina | antifungal activity against Tricophyton rubrum, Sclerotinia sclerotiorum, Botrytis cinerea | [50,51] | |
| Lipodepsipeptide | ||||
| Aureobasidin A | Aureobasidium pullulans | fungicidal activity against Candida spp., C. neoformans, Blastomyces dermatitidis, and Histoplasma capsulatum | noncompetitive inhibition of the inositol phosphorylceramide synthase | [52,53] |
| Nucleosidic peptide | ||||
| Arthrichitin FR-90403 | Arthrinium phaeospermum and Kernia spp. | C. albicans | chitin synthase inhibitors | [54] |
| Other peptides | ||||
| Aspergillomarasmine | Aspergillus versicolor | Gram-negative rods | inhibit the NDM-1 and VIM-2 metallo-β-lactamases | [55] |
| Cyclosporin A | Tolypocladium nivenum | immunosuppressive, anti- coronaviruses activity | [56] | |
| Peptaibols | Trichoderma reesei | antimicrobial activity against Alternaria alternata, Phoma cucurbitaceum, Fusarium spp., A. fumigatus | [57] | |
| Plectasin | Pseudoplectania nigrella | Streptococcus pneumoniae | [58] | |
| Leucinostatin A | Purpureocillium lilacinum | antifungal activity against Candida spp. (including C. albicans, Candida krusei, Candida tropicalis, and C. guilliermondii); antitrypanosomal and antitumoral activities |
[59,60,61] | |
| Terpene derivated metabolites | ||||
| Enfumafungin Ergokonin |
Hormonema spp. Trichoderma spp. |
antimicrobial activity against Bacillus subtilis, Cryptococcus neoformans, C. albicans, A. fumigatus | glucan synthesis inhibitors | [62,63] |
| Antifungal metabolites | ||||
| Parnafungin | Fusarium lavarum | inhibits inhibit mRNA polyadenylation in Candida albicans and pathogenic fungi | [64,65] | |
| Other pharmaceutical agents | ||||
| Lovastatin | Aspergillus terreus | hypercholesterolemia treatment | [2] | |
| Mevastatin | Penicillium citrinum | [66] | ||
| Pravastatin | Penicillium chrysogenum | [67] | ||
| Other bioactive compounds | ||||
| Clavatol |
A. clavatus, Aspergillus clavatonanicus |
fungistatic activiy against C. albicans, A. niger, F. oxysporum, Rhizoctonia solani, Pythium ultimum, Didymella bryoniae, B. cinerea | [68] | |
| Pyranonigrins A, B, C, D, E, S | A. niger | [69] | ||
| Pyranonigrins A and F | Penicillium brocae | Antimicrobial activity against Gram-positive and Gram-negative strains | [25] | |
2.1.2. Fungi in Agriculture
Fungi can also act as biological control agents against plant pathogens. Trichoderma, for instance, demonstrated antagonistic effects against a wide range of plant-pathogenic fungi [13,70,71,72,73]. Trichoderma species have been used intensively in different biotechnological fields. Even so, they represent an outstanding contribution to agriculture because they show an excellent potential to defend against disease crops and attenuate the unfavorable conditions that can affect plant growth and stimulate plant growth [74,75,76,77,78]. These fungi are involved in biocontrol applications, versus fungi that can be plant-pathogenic, oomycetes, or nematodes [79]. Several fungi are used to control insect pests; for instance, species such as Beauveria bassiana attacks corn borer, Verticillium lecanii is known to control whitefly and aphids, and Metarhizium anisopliae are used against scarab larvae [34,80,81]. The action of the fungi is to infect the body surface, which leads to the attachment of the fungus to the integuments of the affected insect, where it will develop and continue to proliferate until the fungus entirely covers the insect [82]. Plant-parasitic nematodes depict another threat to the wellness of plants. Using fungi can be a sustainable strategy to avoid the intensive use of chemical nematicides. The leading group of filamentous fungi that were studied for the biocontrol of nematode pests is known as Trichoderma. The mechanisms involved in damaging nematodes can be found in the antibiosis (production of secondary metabolites), enzymes, or space competition [83].
The presented applications have the potential to improve crop yield, reduce the use of synthetic fertilizers and pesticides, avoid the use of toxic compounds, and promote sustainable agriculture practices. Therefore, fungi have an essential contribution to the agriculture sector, and they must be exploited [84,85,86].
2.1.3. Industry
Fungi play an essential role in the food industry. They have been used since ancient times for various purposes, such as fermentation, production of enzymes, and as a source of food.
For centuries, humans learned to select and collect several macroscopic fungi known as mushrooms. They can be wild-harvested or commercially raised, and they are rich in protein; also, they can develop on inexpensive substrates, sometimes even agro-industrial wastes [87]. The most frequently farmed species worldwide are A. bisporus, P. ostreatus, Flammulina, and Lentinula edodes, used in salads, soups, and other recipes. Many additional mushrooms are collected from the wild for personal use or sale, amongst which are king boletes, milk mushrooms, morels, chanterelles, truffles, black trumpets, and porcini mushrooms. They frequently appear in upscale cuisine [88].
Filamentous fungi have long been used to produce fermented foods such as cheese, bread, and beer. Soy sauce, miso, tempeh, mold-cheeses, and alcoholic beverages, including beer, wine, and spirits, are all products of traditional fungi and yeast fermentation techniques. Using fungi in these industries has significantly improved the final products’ quality, taste, and shelf life. One example of the use of filamentous fungi is cheese production. The first evidence of various cheese types dates from 897 for Gorgonzola, 1070 for Roquefort, and 1791 for Camembert [89]. Mold-ripening is the central aspect for an increased quality of the cheese for a reason during this process, as the flavor and consistency are improved. P. roqueforti makes blue cheese (Roquefort, Gorgonzola, Danish blue, etc.). It produces blue-green veins and gives the cheese its distinctive flavor after inoculation and growth [90]. On the other hand, Penicillium camemberti is inoculated on the surface of the cheese, and it changes the consistency—a soft texture—more than the flavor. These types of cheese are Camembert and Brie [91].
Molds have been used to manufacture fermented sausages since the Greek and Roman empires, where fermented and air-dried sausage had widespread popularity among peasants due to its long-term stability at room temperature as a replacement for refrigerators nowadays. Various types of meat, including cow, goat, horse, lamb, pork, and chicken, are used to make salami [92]. Penicillium nalgiovense is most frequently used as a starting culture when curing fermented meat products; also, the species is recognized as safe to use [93].
The potential use of fungi is great because, currently, farming is conducted at higher rates. There is a need to find other alternatives to avoid soil erosion speeding up and environmental pollution; therefore, the alternative is microorganism cultivation aiming at the production of edible biomass [94]. Quorn™ is a company with fungi-based food products producing dried fungal biomass from Fusarium venenatum. The product is a mycoprotein, low-fat, low-calorie, cholesterol-free food that is highly popular in Anglo-Saxon nations [95]. Mycoprotein is known to help against body weight issues because it gives a feeling of satiety. Therefore, an investigation of the safety of F. venenatum mycelia for human eating was conducted between 1970 and 1980 following the isolation and subsequent suitability testing of F. venenatum (at that time, still known as F. graminearum). The potential for the fungus to create mycotoxins was a significant worry, as many Fusarium species are notorious for producing these toxins due to their role as phytopathogens [77,96].
For the production of Japanese traditional goods, such as seasonings or alcoholic drinks (soy sauce, miso, soju), Aspergillus oryzae is the predominant species used in fermentation processes. Starting in China between 3000 and 2000 years ago, koji became a popular item in Japan with the purpose of a starter for secondary fermentation [97]. Applying A. oryzae spores to heated rice produces koji. The resulting mixture is combined with soybeans or other steamed rice, water, and yeasts to ferment. Besides A. oryzae, other filamentous fungi are used to make koji, such as Aspergillus sojae, Aspergillus kawachii, and Aspergillus awamori [98].
Manufacturing alcoholic beverages such as wine and beer is another common usage of the fungal species in the food industry. For example, Botrytis cinerea, a plant-pathogenic fungi, is exploited in southern France and other locations to increase the sugar level in grapes before harvesting, producing “noble rot,” a sweet and premium wine [99]. For centuries, mankind has used Saccharomyces yeasts to make beer, bread, and wine. Since high quantities of ethanol harm most other bacteria, yeasts have historically been utilized as efficient methods to protect food and beverages’ nutritional value and security. The most well-known among all the beneficial yeasts is S. cerevisiae. It can be utilized to make wine, beer, and bread. Additionally, kefir is created by the symbiotic relationship between bacteria and Saccharomyces yeasts [100].
One of the numerous beneficial features of fungi is the synthesis of fungal enzymes and organic acids. Cellulase (Penicillium funiculosum, Trichoderma viride), α-amylases and invertase (A. niger, A. oryzae), proteinases (A. oryzae), and citric acid (A. niger) can be listed among them.
Several fungal species have been used regarding food processes since the beginning of agriculture. Fungal biodiversity is undoubtedly an important provider of resources for food as well as other higher value uses.
In the present, society confronts many manufactured challenges, among which high pollution levels or the lack of nutritional resources to support population growth occupy a central position [101,102]. The transition to a zero-carbon sustainable bioeconomy is the only direction that offers humankind the possibility to address these challenges, and it involves the transformation of a linear economy into a sustainable circular economy. Microorganisms are critical players in the circular economy since they harbor many intrinsic characteristics that recommend them for biobased industrial applications [23,102]. Among microbial strains with industrial potential and a high impact on the circular bio-economy, fungal strains have the unique metabolic ability to convert many organic materials (including wastes) into various by-products relevant to different industrial applications [23].
Metabolites Produced by Fungi with Industrial Applications
Different chemical compounds with industrial applications, such as organic acids, enzymes, flavors, vitamins, and colorants, might be obtained more cost-efficiently using fermentation processes based on fungal strains [103].
Organic acids are used in the food industry mainly as acidulants, flavoring agents, or preservatives, but their biotechnological potential is not limited only to the food industry. Citric acid is a weak organic acid highly demanded worldwide, especially in the food and pharmaceutical industries. The bioproduction of citric acid comprises the traditional process of industrial-scale fermentation. Among the most common organic acids used in industry is citric acid, which has a variety of uses, and the global market for 2025 is estimated at USD 3.6 billion [104]. Besides the use in food and beverage industries, the mentioned organic acid is utilized in pharmaceutical, cosmetic, and detergent industries [104]. Karl William Scheele is recognized for the isolation of citric acid for the first time from lemon juice in 1784. James Currie discovered in 1917 that A. niger could create citric acid from sugar using the surface fermentation method, which later served as the foundation for Aspergillus’s application in industrial production. Because of its higher production yield, A. niger is better than other microorganisms for the industrial synthesis of citric acid. It provides excellent yields, can ferment a variety of inexpensive basic materials, and is simple to handle [105].
In the food and beverage industry, it is used as a preservative or as a flavors and aromas enhancer, for preventing the deterioration of frozen food products or for the development of non-toxic plastic films for foodstuffs protection, and also as an emulsifying agent for ice cream and cheese-based food products [106]. In the pharmaceutical industry, as an antioxidant, citric acid is used to preserve vitamins, as a pH buffer, and in association with other chemicals such as iron (as iron citrate tables) or for the development of citrate-based biomaterials with potential use for regenerative engineering [107,108]. Other possible applications of citric acid are for the chemical industry, mainly for producing conditioners and laundry detergents or as chelating agents in cleaning solutions used for removing limescale [106,109,110]. Every year more than 6 million tons of citric acid are used for beverages, food, detergents, cosmetics, and pharmaceutical production. High quantities of citric acid are assured using the fungal submerged fermentation of sucrose and molasses or synthetically from acetone or glycerol. Numerous Aspergillus strains belonging to A. niger, A. awamori, Aspergillus foetidus, Aspergillus wentii, Aspergillus aculeatus, Aspergillus carbonarius species or T. viride, Penicillium restrictum, and Mucor piriformis are currently evaluated as being good producers of citric acid [103,105,111]. However, among them, A. niger is considered most suitable for industrial production due to its high ability to assimilate and ferment many cheap agro-industrial derived materials, and thus it is a cost-effective technology [105]. Among cheap agro-industrial wastes used for citric acid production using A. niger, it is worth mentioning pineapple peels [112]; apple processing wastes [113]; banana peels [114]; cocoa pod and coffee husk processing wastes [115,116] or sweet potato starch hydrolyzate [117]. Gluconic acid is another highly valuable organic acid for the food industry, which can be obtained using fungal fermentation [103]. Gluconic acid is a mild organic acid frequently used to pickle foods, prevent milkstone in the dairy production industry, or for clean cans used in these circumstances. Gluconic acid derivatives such as D-glucono-δ-lactone are important as leavening agents for preleavened products, for reducing fat absorption in doughnuts, for the coagulation of soybean proteins in tofu manufacture, and for improving the heat stability of milk [118,119]. Gluconic acid salts (sodium gluconate, calcium gluconate, ferric gluconate) have a broadened industrial potential from metallurgy where they can act as alkaline derusting agents and anticorrosive agents to use as additives to cement [120,121]. Calcium and iron salts of gluconic acid have important biomedical potential, being used for calcium therapy for osteoarthritis [122] and the treatment of anaemia [123], and they also can be used in animal feed [124] or in agriculture as foliar feed formulations [118]. Annually, more than 60 thousand tons of gluconic acid and its derivatives are produced worldwide through various chemical, electrochemical, or fermentation processes. By far, fermentation is considered the most efficient and dominant technique that can be used to obtain gluconic acid. Among the microbial strains involved in gluconic acid production through fermentation, A. niger, Penicillium funiculosum, Penicillium variable, Penicillium amagasakiens, and members of Glicladium, Scopulariopsis, Gonatobotrys, and Endomycopsis genera are well known [118]. A. niger is considered a main fungal species that can produce gluconic acid at an industrial level. Members of this species produce all the enzymes involved in converting carbohydrates, such as glucose, into gluconic acid. Since industrial production requires the reduction of costs, a strategy presented in the scientific literature was to replace the substrate represented by glucose with cheaper raw materials such as breadfruit hydrolysate [125], grape must [126], waste office paper hydrolysate [127], whey [128], dry dilute acid pre-treated corn stover [129], corn starch [130], sugarcane molasses [131], or banana must [119].
Apart from citric and gluconic acids, fungi are also used for obtaining other organic acids such as itaconic, lactic, fumaric, malic, succinic, and oxalic acids, but to a lesser extent [103]. Itaconic acid has been commercially available since the mid-twentieth century, significant for the industrial production of adhesives, detergents, and shampoo formulations. More than that, its vinyl esters are relevant for producing plastics, elastomers, and coatings with light colors for carpets and book covers [132]. For the biomedical field, itaconic acid is used for ophthalmic, dental and drug delivery fields [133,134]. A. terreus and Ustilago maydis species are considered model organisms for producing itaconic acid. They are also characterized by outstanding tolerance to acidic pH values. However, apart from them, other fungal species such as Ustilago vetiveriae, Ustilago xerochloae, or Aspergillus niveus were described as being able to produce this organic acid. As an alternative growth substrate for the production of itaconic acid using fungi, it is worth mentioning enzymatically digested wood chips [135], corn stover hydrolysate [136], pre-treated rice husk [137], sweet potatoes, wheat flour, corn starch [138] beech wood [139,140], and glycerol [141]. Lactic acid is widely used in the food industry as a preservative (preventing the proliferation of spoilage microorganisms) and for the production of yogurt and cheese (involved in decreasing pH and casein aggregation) [142]. Apart from the food industry, lactic acid is a precursor for propylene glycol and acrylic polymers that can be used to develop biodegradable packaging and labeling materials [143,144] and also for biomedical prosthetic devices or sutures [145]. Although it can be obtained through chemical synthesis, the main advantage of fermentation processes for obtaining lactic acid for the food industry is that selecting the right microbial strain can yield a pure form of L(+)-lactic acid, which is preferred since it is not harmful to humans [146]. Usually, lactic acid bacteria are preferred for lactic acid production, but since this group of microorganisms exhibits special nutritional requirements, fungal strains represent a cheaper yet productive alternative. Rhizopus spp. represents the most critical fungal genera that can be used for lactic acid production. Members of this genus have the advantage of producing only L-lactic acid isomers, thus reducing the cost associated with the purification of the fermentation products. Although glucose is preferred as a carbon source for producing lactic acid using Rhizopus strains, other substrates such as raw starch from potatoes, cassava, wheat, corn, and rice wastes [147,148,149,150] or lignocellulose wastes [151,152,153] can contribute to cost reduction [146]. Malic and fumaric acids are other valuable organic acids that can be obtained using fungal strains. Their industrial applications vary from the manufacture of chemical products such as resins, biodegradable polymers, lubricating oils, inks, plasticizers, or lacquers to the production of food and pharmaceutical additives (acidulants, flavor enhancers, precursors for malic or aspartic acid) or drugs (including those with antimicrobial properties, antioxidant, and anticarcinogenic effects) [154]. Fungi species such as Rhizopus arrhizus, Rhizopus oryzae, Mucor spp., Cunninghamella spp., or Aspergillus spp. are presented as good producers of fumaric acid, both through aerobic and anaerobic fermentation, while for malic acid good results are reported for A. oryzae, A. niger and Aureobasidium pullulans [155,156,157]. Good yields of fumaric acid production were obtained using different substrates for fungal biomass accumulation, of which it is worth mentioning: apple industry waste biomass [158] or different food wastes disposed by restaurant, kitchens, and cafeterias [155,159,160].
Filamentous fungi are also used for producing enzymes at large scales. Their versatile metabolism assures obtaining large quantities of amylases, proteases, pectic enzymes, galactosidases, lipases, chitinases, or lignocellulolytic enzymes.
Amylases with biotechnological importance are extracellular enzymes involved in starch degradation. These enzymes represent approximately 25% of the world enzyme market, relevant to many industrial processes such as those in the food, fermentation, textile, paper, and pharmaceutical industries (Table 2). Three types of amylases are produced using microbial strains: α-amylases, β-amylases, and γ-amylases. α-amylases or endo-1,4-α-D-glucan glucohydrolase (EC 3.2.1.1) catalyze the hydrolysis of random 1,4-α-D-glycosidic bonds between glucose units from short linear amylase chains [161]. Unlike these, the β-amylases or β-1,4-glucan maltohydrolase (EC 3.2.1.2) are responsible for the hydrolysis of the second 1,4-α-D-glycosidic bond from the non-reducing end of the starch molecule, thus producing disaccharides such as maltose.
On the other hand, γ-amylases and glucan 1,4-α-glucosidase (EC 3.2.1.3) are usually highly stable enzymes in acidic conditions, and these enzymes are mainly responsible for the cleavage of 1,4-α or 1,6-α-D-glycosidic bonds on the external glucose residues of amylose or amylopectin from the non-reducing end, thus producing only glucose [162]. The industrial production of amylases usually involves submerged fermentation, but recently solid-state fermentation has received greater interest due to its superior productivity, reduced energy requirement, and simpler fermentation media. In addition, many studies have reported the optimal production conditions of fungal amylases in terms of the cultivation conditions (pH, presence of different inhibitors, temperature a.s.o) and the substrate used to obtain the biomass (Table 2).
Proteases constitute a large group of enzymes responsible for hydrolysis peptide bonds. In general terms, according to the position of the cleaved peptide bond, proteases can be divided into two major groups: endopeptidase and exopeptidase. Fungal strains can produce both types of protease, thus having great importance for their production. Fungal proteases can be obtained using both submerged fermentation and solid-state fermentation. Both options seem more advantageous in fungi than other protease-producing organisms (microbial or not) [103,163]. Among the protease-producing fungi, thermophilic fungi such as Thermoascus aurantiacus [164] or Thermomyces lanuginosus [165] are of great interest since they possess the ability to secrete thermostable proteases that act in the temperature range 60–85 °C.
Pectinase, in general terms, refers to a group of enzymes that catalyzes pectic substance depolymerization (pectin hydrolases and lyases) and de-esterification (pectin esterases). Pectinase represents approximately 10% of the overall production of enzymes, and its utilization is highly valuable for the food industry, but not only. Microbial pectinases, in general, are relevant for the natural carbon cycle involved in the decomposition of dead plant material. However, for the producing microorganism itself, these enzymes represent a tool in the phytopathologic process and plant-microbe symbiosis [166]. Either way, pectinase can be successfully used in the industry for various applications (Table 2).
According to their substrate specificity, the galactosidases are glycoside hydrolases classified as α-galactosidases or β-galactosidases. α-Galactosidases (EC 3.2.1.22) catalyze the removal of α-linked terminal non-reducing galactose residues from small oligosaccharides. This enzyme is also responsible for the cleavage of α-1,6 linkage between galactose and glucose in melibiose. α-Galactosidases have proven helpful in the food and feed industry, mainly increasing the sucrose yield by eliminating raffinose [167]. β-Galactosidase (EC. 3.2.1.23), also known as lactase, is responsible for the hydrolysis of D-galactosyl residues from polymers. Fungal β-galactosidases are highly stable to acidic pH, thus being an excellent instrument for whey reintegration into the economic circuit [168,169,170].
Chitinases (EC. 3.2.1.14) are enzymes responsible for chitin—the second-most-abundant polymer found in nature—degradation. Fungi are the most important group of microorganisms able to produce and secrete chitinase. Fungal chitinases are the only enzymes that can efficiently degrade chitin by hydrolyzing chitin to form chito-oligosaccharides with a minimum chain length of two carbon atoms [171]. Apart from their involvement in fungal morphogenesis, cell division, mycoparasitism, and autolysis, from a biotechnological point of view, chitinases are essential for the functional reintegration of chitin trapped in the biomass in the economic circuit [172].
Lipases (EC. 3.1.1.3), or triacylglycerol hydrolases, catalyze glycerol and fatty acids hydrolysis. It was also noticed that their processes, including the extraction and purification of lipases from fungi, are comparatively more accessible and cheaper than other sources of lipases. Fungal lipases have applications not only in the hydrolysis of fats and oils (triglycerides) but are also involved in synthetic reactions such as esterification, acidolysis, alcoholysis, interesterification, and aminolysis. Although some fungal species produce intracellular lipases, most can secrete this enzyme outside the cell. Major genera of filamentous fungi capable of producing lipases are Rhizopus, Aspergillus, Penicillium, Mucor, and Geotrichum, and the lipases produced have special biotechnological applications that are intensively studied both from a functional and genetic point of view [173,174].
Lignocellulolytic enzymes are involved in lignocellulose degradation, and this group of enzymes includes ligninases, hemicellulases, and cellulases. Ligninases are responsible for deleting lignin into more minor compounds that microorganisms can assimilate. In general, ligninases can be divided into laccase or phenol oxidase and peroxidases or lignin peroxidases. Laccases (EC 1.10.3.2), or p-diphenol: dioxygen oxidoreductases, are the enzymes responsible for the attack of the phenolic subunits of lignin leading to Cα-Cβ cleavage and aryl-alkyl cleavage [175]. Lignin peroxidase (EC. 1.11.1.14), or diaryl propane oxygenase, is a heme-containing enzyme that catalyzes lignin’s hydrogen peroxide-dependent oxidative degradation. These enzymes belong to the oxidase group and are mainly used for reducing environmental pollution. Ligninases are widely found in nature and produced by various plants, fungal species, or bacteria. Among fungal species, white rot fungi are the best ligninases producers, and their biotechnological and industrial potential has been intensely studied in recent decades [176]. Cellulases are a group of enzymes that contain endoglucanase (EC 3.2.1.4), exoglucanase (EC 3.2.1.91), and β-D-glucoside glucanhydrolase (EC 3.2.1.21). Among the microorganisms, fungi are the principal cellulose decomposers, responsible for about 80% of the cellulose breakdown on earth. In industry, fungal cellulases are usually preferred, being much easier to be obtained in large quantities. Fungal species, including T. reesei, Rasamsonia emersonii, Aspergillus spp., and Penicillium spp. produce extracellular cellulases during their growth in aerobic conditions, and thus are promising candidates for various industrial applications [177,178].
Table 2.
The most common filamentous fungi enzyme producers and their applications in industry.
| Enzymes | Fungal Species | Non-Conventional Growth Substrates | Applications | References | ||
|---|---|---|---|---|---|---|
| Amylases |
A. niger A. oryzae A. fumigatus Aspergillus flavus; A. awamori; A. kawachii; Penicillium brunneum; Penicillium expansum; P. roqueforti; P. camemberti; Helminthosporium oxysporum; Penicillium frequestans P. chrysogenum Penicillium fellutanum |
Coconut oil cake; groundnut oil cake; sesame oil cake; olive oil cake; wheat bran; corncob leaf; rye straw; wheat straw; banana waste; residues obtained from rice husking; cassava peels; yam peels; pomegranate peel; molasses |
|
[161,162,179,180,181,182,183,184,185,186] | ||
| Proteases |
A. flavus; Aspergillus ochraceus Conidiobolus coronatus; Rhizomucor miehei; Endothia parasitica; Mucor circinelloides; Mucor pusillus; P. camemberti; P. citrinum; Penicillium griseoroseum; Penicillium restrictum; P. roqueforti; A. flavus; A. oryzae; A. niger; R. oryzae; T. reesei; Trichoderma harzianum |
Wheat and rice bran, soybean meal; oil seed cake |
|
[49,163,187,188,189,190,191,192,193,194,195] | ||
| Pectinase |
A. niger
A. flavus A. sojae A. terreus Alternaria citri Claviceps purpurea Fusarium moniliforme Botrytis cinerea A. kawakii Thermoascus aurantiacus Acrophialophora nainiana Aspergillus japonicus |
Wheat bran; rice husk and bran; papaya peel; mango peel; sugarcane bagasse; sunflower head; grape and strawberry pomace |
|
[166,196,197,198,199,200,201,202,203,204] | ||
| Galactosidases | α-Galactosidases |
Mortierella vinaceae
Tricholoma matsutake A. niger A. oryzae A. fumigatus |
Soybean meal and wheat bran red gram plant waste; soy flour |
|
[101,102] | |
| β-galactosidase |
A. niger
A oryzae A. flavus Aspergillus uvarum P. brevicompactum F. oxysporum |
Lemon peel, pineapple peel, musk melon peel, banana peel, musambi peel, pomegranate peel, orange peel; soybean residue, okara, soymilk; wheat straw, rice straw, and peanut pod |
|
[168,205,206,207,208,209] | ||
| Chitinases |
Thermomyces lanuginosus; T. viride; T. harzianum; A. nidulans, A. fumigatus, P. chrysogenum |
Wheat bran; rice bran; chitin flakes; waste products obtained from crabs, shrimps and prawn |
|
[171,210,211,212,213,214] | ||
| Lipases |
Mucor circinelloides
Penicillium aurantiogriseum Rhizopus rhizopodiformis Rhizomucor pusillus Rhizopus oligosporus P. restrictum Penicillium simplicissimum Aspergillus carneus Penicillium verrucossum P. chrysogenum A. awamori A. terreus Fusarium solani |
Soya bean oil; olive oil cake; babassu oil cake Almond meal; mustard oil cake, sunflower oil; soybean bran; rice bran oil; olive mill wastewater |
|
[215,216,217,218,219,220,221,222,223,224] | ||
| Lignocellulolytic enzymes | Cellulase |
A. niger
T. reesei Aspergillus heteromorphus A. fumigatus R. oryzae |
Wheat straw and bran; maize straw; banana peel Coir waste; grass; sugarcane bagasse; corn cob residue |
|
[225,226,227,228,229,230] | |
| Ligninanses | Laccases |
Aspergillus niveus
Rhizoctonia solani B. cinerea Myceliophthora thermophila Pycnoporus cinnabarinus Trametes villosa Coriolopsis gallica Coprinopsis cinerea |
Wheat bran, rice husk, mango peel, orange peel, groundnut husk and saw dusk |
|
[175,231,232,233,234,235,236,237,238] | |
| Peroxidases |
Phanerochaete chrysosporium; A. sclerotiorum Cladosporium cladosporioides M. racemosus Neurospora discreta |
Cocopeat, sugarcane bagasse |
|
[239,240,241] | ||
Pigments: Filamentous fungi are also known for their ability to produce natural pigments with a high potential of replacing artificial synthetic dyes. Even today, synthetic colorants are widely used in foodstuff, cosmetics, pharmaceutical, and textile manufacturing, but some are hazardous to human health. In this context, there is a strong interest in replacing these colorants with their natural alternative [242]. Fungal pigments are an excellent alternative to synthetic dyes, which are easy to obtain and less expensive [243]. Many fungal species of Aspergillus, Fusarium, Penicillium, and Trichoderma genera produce large amounts of pigments during their growth. Fungal pigments are classified into polyketides, polyketide-derivates, carotenoids, and melanins [242]. Polyketide pigments have a polyketide chain with four or eight C2 units, and in this group are included secondary metabolites such as anthraquinone, hydroxyanthraquinones, naphtoquinone, and azaphilone. These compounds are responsible for a wide range of colors from yellow to red and even blue shades [244] and are successfully used for textile dyeing and as antibacterial agents. Polyketide pigments-producing fungi mainly belong to Fusarium sporotrichioides, Penicillium spp., Aspergillus ustus, and Monascus purpureus, and their potential utilization in the benefit of humankind varies from developing anti-aging, anti-acne, and skin-whitening agents to anticancer drugs [245]. Some species of fungi are used in the production of pigments, such as Monascus species, which produce red, orange, and yellow pigments used as natural colorants in the food, cosmetic, and pharmaceutical industries, as well as the dyeing, textile, and printing domains [246]. Carotenoids are tetraterpenoid pigments comprising xanthophylls and carotenes. In this class of natural pigments, more than 750 chemical compounds are included; among them, most important is β-carotene, a vitamin A precursor and a vital antioxidant agent. It is used as an orange-red pigment in the food industry and is mainly produced by Blakeslea trispora strains [103]. Other carotenoid-producing fungi belong to Aschersonia aleyrodis; Aspergillus giganteus; B. trispora; F. fujikuroi; M. circinelloides; B. trispora; Sclerotinia sclerotiorum; Fusarium sporotrichioides; Phycomyces blakesleeanus; Neurospora crassa; Puccinia distincta and Allomyces arbusculus [247]. At the industrial level, B. trispora is widely known, with members of this species being used to produce β-carotene [245]. More than that, fungal carotenoids can be obtained in a cost-efficient manner using alternative growth substrates such as waste cooking oil [248], deproteinized hydrolyzed cheese waste [249], or oat flakes/spent malt grain [250]. Melanins are dark brown or black pigments widely found in animals, plants, and microorganisms. From an industrial point of view, fungal melanins have gained significant interest in the last decades, being eco-friendly and biodegradable. According to their chemical structure and precursors involved in their biosynthesis, fungal melanins are classified into five main groups: eumelanin, 1,8-dihydroxynaphtalene melanins, pyomelanin, pheomelanin, and glutaminylhydroxybenzene melanin [251]. Being extremely diverse from a chemical point of view, fungal melanins positively impact biomedicine; the dyeing industry; the food industry for developing new packaging materials; for cosmetic industry; and environmental protection a.s.o [252].
2.1.4. Fungi and the Environment
Currently, concerns about environmental safety are emerging since there are issues relating to pollution and toxic wastes. Therefore, there is a need to develop new strategies to sustain the environment’s health. Fungi are an integral part of the environment and play essential roles in many ecosystems. Besides the impact on nutrient cycling, decomposition, and soil health, fungi exhibit great potential for developing strategies that enhance environmental protection. For instance, in the fight against pollution, climate change, and various other issues, fungi are quickly surfacing as essential protagonists, involved in practices such as the bioremediation of pharmaceutical compounds, agricultural wastes, or degradation of various pollutants.
Mycoremediation is mediated by two mechanism types: enzymatic (fungal secreted enzymes) and non-enzymatic (adsorption of toxic compounds inside the cell wall, biosurfactants production) [253]. For example, filamentous fungi belonging to the Trichoderma, Penicillium, and Aspergillus genera are able, through absorption mechanisms, to absorb heavy metals such as copper and cobalt [254].
A study by Asemoloye et al. established that two fungal strains belonging to Mucor irregularis and A. oryzae, isolated from oil-contaminated places, could be used to clean up the soil after hydrocarbon contamination. Moreover, the two fungi displayed a remarkable capacity to degrade hydrocarbons [255].
Filamentous fungi are highly efficient in the process of decolorization. There is evidence that filamentous fungi produce the enzymes laccase and manganese peroxidase to achieve this. By converting complex synthetic dye molecules into non-colored, safer, and environmentally secure structures, fungal laccases were widely used for bioremediation [253]. It has been demonstrated that microorganisms exploit agricultural waste, specifically cellulose—the most renewable source of biomass in the biosphere—to produce valuable goods, such as sugars, cheap energy resources, and enzymes. Waste products from industry and agriculture are some of the things that pollute the environment [228]. Their transformation into beneficial products might lessen the issues they create. These wastes, including grains, leaves, corn cobs, and other materials, are underutilized.
As mentioned in the previous section, fungi can secrete cellulase, enzymes responsible for breaking down the cellulose in agricultural wastes into simple glucose molecules. Cellulolytic fungi, including Chaetomium, Fusarium, Myrothecium, and Trichoderma, produce celluloses through cellulolysis [256].
Another way to save the environment is using renewable energy from living organisms, known as biofuels. Biodiesel production was greatly enhanced by cultivating filamentous oleaginous fungi with lignocellulosic biomass, such as Mortierella isabellina and Aspergillus terreus [257]. A second way to produce biofuels is to develop bio-ethanol. Biomass of crops from grains and corn, rich in sugar and starch, is the base of bio-ethanol production. Filamentous fungi can convert sugars to ethanol. The pre-treatment of the biomass is realized with the aid of fungi and lignin-degrading enzymes (pectinases, xylanases, mannanases). Pre-treatment methods can improve enzyme cellulose availability during enzymatic hydrolysis, which converts sugars into fermented ethanol [258].
Utilizing biofuels is an excellent alternative to diminish the use of petroleum oil, which leads to reducing carbon dioxide emissions, air pollution, and a safe environment.
The additional use of fungi to enhance environmental wellness is described by a new strategy named mycofiltration. Mycofiltration, or the process of treating contaminated water by passing it through a network of fungal mycelium, is one way that fungi are used in mycoremediation [259]. For example, a preliminary study by Taylor and their team proved the use of basidiomycete Stropharia rugoso-annulata as an adjuvant to improve synthetic stormwater Escherichia coli removal through wood chips [260].
2.1.5. Research
In order to facilitate the study of particular biological phenomena, yeasts and filamentous fungi are used as research model organisms. Studies on these fungi provide relevant biological insights into other organisms, such as genetics, cell biology, meiosis, and pathogenesis. Yeasts and filamentous fungi are fascinating lower eukaryotes involved in understanding cellular processes. Their advantages are that they are easy to grow on inexpensive media and have easy access to molecular and classical genetics. The fact that fungi are more closely related to animals than plants underscores these organisms’ value as convenient models of human cells [261]. Fungi provide an excellent model for understanding the structure and function of chromatin in both actively transcribed regions (euchromatin) and transcriptionally silent regions (heterochromatin). Saccharomyces and Aspergillus are among the most prevalent fungi preferred by geneticists and molecular developmental biologists, but the first species used was Neurospora. Learning about epigenetic phenomena in other systems without the filamentous fungus N. crassa would have been challenging or impossible. S. cerevisiae, for instance, does not have the same characteristics in Neurospora, including DNA methylation and unique RNA interferences that act in mitotic and meiotic cells. Moreover, it contains an (RNAi)-based silencing system [262].
The initial usage of fungi for nanotechnology applications dates back to the early 2000s, making fungal nanotechnology a relatively new field of study. For example, palladium is a precious metal frequently used in catalysis, and in 2002, researchers at the University of California, Riverside, reported synthesizing palladium nanoparticles using a fungus called N. crassa. It has now been proven for the first time that fungi can operate as biological factories to create nanoparticles with distinct features. Since then, fungal nanotechnology has proliferated and has a wide range of potential uses, including the production of antibacterial agents, biosensors, and drug delivery systems. In addition, researchers are investigating methods to create various nanoparticles with varying shapes, sizes, and surface qualities utilizing several fungal species, including Aspergillus, Fusarium, and Trichoderma [263]. Apart from S. cerevisiae, many other yeast species, such as Kluyveromyces (K. marxianus and K. lactis); Pichia (Pichia pastoris renamed Komagataella phaffii, Pichia anomala renamed Wickerhamomyces anomalus); Hansenula polymorpha (renamed Ogataea polymorpha) and Yarrowia lipolytica were described as suitable research models, especially for biotechnological studies. Members of K. marxianus species are Crabtree-negative, can metabolize a broad spectrum of low-cost feedstocks such as whey and dairy industry wastes, and presents an exceptional ability to grow at elevated temperatures, thus being helpful in their use as a versatile host for a wide range of applications in the food, feed and pharmaceutical industries [264,265]. Y. lipolytica is considered a real industrial workhorse—the most extensively studied non-conventional yeast. Members of this species are strictly aerobic and are used to produce a variety of industrially important metabolites such as lipids, biosurfactants, and enzymes. Additionally, Y. lipolytica was used as a research model for dimorphism studies in yeasts and as a popular system for expressing heterologous proteins [266,267]. K. phaffii was recognized as an important host for the industrial production of heterologous proteins due to its possibility to run high-density fermentation associated with high secretory efficiency and its specific eukaryotic post-translational modifications [268].
2.2. Damaging Effects
2.2.1. Etiological Agents of Diseases in Plants, Animals and Humans
The pathogenic potential of microorganisms, in general, is described as their ability to invade the host and produce toxic compounds that affect the well-functioning host. Although fungi are widely spread in the environment and co-exist with many organisms, in certain circumstances, fungi, like any other microorganisms, can harm their host. Fungi are characterized by high resilience in stressful conditions and a unique ability to adapt to different environments. Infectious fungi can spread through the air and water and be transmitted by different carriers such as animals, humans, or plants [18]. Regarding the interaction of fungi with plant organisms, more than 80% of the plants from our planet are symbiotic with fungi. However, some of the fungal strains can sometimes enter inside plants through damaged leaves and stomata, thus turning themselves into plant pathogens with a high impact on plant health. Plant fungal pathogens can be classified into biotrophs, hemibiotrophs, and necrotrophs, according to the mechanism involved in pathogenicity and the time required for complete host damage [269].
Necrotrophic fungi infect many hosts and can produce and secrete large hydrolytic enzymes that degrade plant cell walls. Thus, their pathogenic effect is rapid, causing the host’s death [270].
Both biotrophic and hemibiotrophic fungi require living plant tissue for their development, so their negative impact is much slower than necrotrophic fungi. Biotrophic fungi interact with the living host via special haustoria hyphae and can secrete specific molecules that suppress the plant’s immune system. The invasion of the plant organisms is assured by appressoria, which is involved in the attachment of the fungus to the substrate, allowing the cell wall penetration using mechanical force followed by affecting the plant’s normal metabolism by taking its nutrients [271]. Hemibiotrophic fungi combine both biotrophic and necrotrophic invasion mechanisms. First, these fungal pathogens invade the plant organism through mechanisms similar to those described for biotrophic fungi, followed by a necrotrophic phase that ends with the death of the affected plant [269,271]. Although tremendous progress has been made in recent decades to prevent and combat the fungal contamination of crops, devastating crop yield losses are still a significant problem. Therefore, many fungal species were described as phytopathogens associated with significant economic losses. Members of the Botrytis genus include pathogens of monocotyledons and dicotyledonous plants. Among the 22 species of Botrytis, B. cinerea is considered the most damaging, being able to infect numerous hosts [76,267]. The other 21 species have a narrow host range, especially infecting monocotyledonous plants. Gray mold is the most common disease caused by B. cinerea, affecting the mature or senescent tissue of the dicotyledonous host. Usually, the contamination occurs on the field, but severe damage is caused during storage in an improper condition of crops [272,273,274]. B. cinerea routes of infection vary according to the plant species and the environmental conditions. In general, B. cinerea conidia attach to the plant surface and germinate. Later it forms germ tubs that differentiate simple appressoria and infection cushions, structures involved in host penetration. Many models for the establishment of B. cinerea were presented. However, in general, after host invasion, the fungi produce specific molecules to suppress the death of host cells, which allows the fungi to accumulate enough biomass. After that, the fungus replaces autophagy-suppressing molecules with proteins that promote the plant’s ability to secrete enzymes involved in apoptosis, thus leading to the plant tissue’s death [275]. Cladosporium fulvum is responsible for tomato leaf mold, a common disease affecting production. The disease is associated with the exposure of tomato plants to high temperatures and humid environments, which promotes the growth of the pathogenic fungus. Usually, leaves are the main organs infected, and after contamination on the foliar surface appears, irregular chlorotic spots and the leaves edge becomes curly and wilted [276]. C. fulvum is a biotrophic fungus belonging to the Dothidiomycete class. Its infectious cycle starts with the germination of the conidia and the development of hypha, which enters the host through open stomata. Within two weeks, C. fulvum produces many conidiophores that block the stomata and cause leaf necrosis [273]. The Magnaporthe grisea species complex includes many fungal species responsible for causing disease in grass and sedge crops such as rice, wheat, barley, maize, oats, and finger millet. Magnaporthe oryzae occupies a central position among members of this group since it is a hemibiotrophic ascomycete known as the etiological agent of rice blast disease. This fungus mainly affects the rice plant’s aerial parts, leading to leaf, collar rot, and node blasts [277]. When invading the host, M. oryzae conidia develop melanized appressorium penetrating the rice cell wall through mechanical pressure. After that, the primary hyphae spread through plant cells and form a complex structure responsible for the secretion of effectors that suppress the plant’s defense responses. In time, the fungus changes its metabolism, secreting other toxins responsible for inducing the death of the plant tissue [273]. Another fungal species considered phytopathogenic is Ustilago maydis. Members of this species are also biotrophic plant pathogens responsible for causing corn smut disease. In this case, rapid proliferation is triggered after invading the host, which is associated with the development of tumors. Although this species has fewer damaging crops, U. maydis is considered a prime model organism for smut fungi characterized by a biphasic life cycle. The triggering of the pathogenic character is associated with the formation of diploids due to the mating of haploid cells that have contaminated the host plant [278]. Mycosphaerella graminicola (Septoria tritici) is responsible for septoria tritici blotch (STB), the most important foliar disease of wheat, which is associated with necrotic lesions on leaves and stems [279]. Members of this species are well characterized regarding their pathogenic character. This pathogen is spread by wind, and its propagation is ensured by both sexual ascospores and asexual pycnidiospores [280].
As mentioned before, the pathogenicity of a microorganism is strongly influenced by the affected host. Therefore, when an organism is analyzed as a potential host for microbial infections, it must be taken into account that in terms of intracellular organization, there are some similarities between plant and animal cells (organelles with similar or standard functions, cytoskeleton elements a.s.o), but the differences between them are that they are significantly more numerous when discussing the cell wall or the cell membrane. More than that, in the case of multicellular organisms, the plant cellular immune systems are entirely different from those of animals. In these circumstances, plant and animal pathogens have a divergent evolution that allows them to adapt to the conditions encountered in the infected organism [245].
Despite their divergent evolutionary pathways, there are some pieces of evidence that phytopathogenic fungi can cause infections in humans or animals. A good example is supported by another Alternaria species, Alternaria alternata, which is a phytopathogenic fungi [281]. In addition, Alternaria infectoria, a fungal species responsible for causing severe blossom blight [282], might also be involved in causing phaehyphomycosis after renal transplant [283]. Other examples are the members of the Cladosporium genus, which were isolated both from infected plant and animal hosts [18,284,285,286].
Among the numerous amounts of different types of fungi, only a tiny percentage of these species are responsible for human infections or diseases. However, these pathogens cause various infections, starting from affecting internal organs to superficial infections of the skin and mucosal surfaces, allergies, and mycoses, and are more likely to arise in immunocompromised people with weakened immune systems [287,288]. Since the human body temperature is a significant barrier to fungal development, only a few fungi are potentially harmful organisms. Therefore, they are frequently seen outside the human body, such as ringworm or athlete’s foot [289].
The most common fungal infection is represented by fungal nail infection. The principal fungi responsible are fungi from the genera Trichophyton and Microsporum. This infection enters the nail bed and diffuses into the margins on the sides of the upper side of the nail, causing the nail to develop a white surface, discoloration, and thickening. In addition, this infection causes the skin around it to become scaly. Chlorazol Black E is used in microscopic and staining procedures to identify the onchomycosis. Chemical remedies or surgery are both options for treating nail infections. Terbinafine and itraconazole are just a few medications used to treat onchomycosis. However, they all have some adverse side effects. The typical infection is athlete’s foot, mostly in wet areas [290].
Cryptococcus neoformans, a fungal pathogen, can cause fatal fungal pneumonia and meningitis among immunocompromised patients. Naturally, it lives on waste and soil polluted with chicken, pigeon, and bat excrement. It penetrates the brain, extrapulmonary tissues, and the lungs. These infections most frequently result in infections of the lungs, skin, prostate, central nervous system, and eyes. If left untreated, it produces deadly cryptococcal meningoencephalitis. Antifungals such as flucytosine and intraventricular miconazole are used during treatment [288].
A. fumigatus is the fungus that causes aspergillosis. It is a saprophytic fungus that makes asexual spores from vegetative mycelium found in soil. The inhalation of A. fumigatus conidia results in lung infection. These strains can lead to invasive fungal infection in people with deficient immune systems. Patients with compromised immune systems can develop chronic pulmonary aspergillosis, one of the most prevalent invasive fungal diseases. Galactomannan antigen may be used to diagnose it. In addition, antifungal medications are used to treat aspergillosis. To treat aspergillosis, ergosterol, components of the fungal membrane, and 1,3 glucan are among the potential targets for antifungal compounds [288,291].
A healthy part of the human microbiome is C. albicans. C. albicans, however, transforms into a pathogen when the harmonious relationship between the organism and the host cells is perturbed. It then overgrows on skin and mucosal surfaces, invades host tissue, spreads to circulation, and colonizes solid organs. Patients with this illness have excruciating pain and agitation, especially immunocompromised patients. Oropharyngeal and esophageal candidiasis are both conditions, and the agent that causes them is C. albicans [292].
R. oryzae is a fungus that causes a medical condition known as mucormycosis. It is a member of the Mucorales order. Based on where the illness occurs, mucormycosis can be classified into five different groups: gastrointestinal, cutaneous, pulmonary, disseminated, and miscellaneous. Patients with neutropenia and dysfunctional phagocytes (caused by acidosis and hyperglycemia) are more likely to contract the illness. Mucormycosis etiology is associated with higher patient serum iron, such as cryptococcosis. Therefore, a quick and accurate diagnosis is crucial for illness therapy. Unfortunately, no PCR-based or serological assays are available for quick diagnosis. Therefore, treatment includes quick detection and surgical excision of the diseased tissue. Treatment options include quick diagnosis, surgical removal of the infected tissue to stop further invasion, and the use of antifungal medications such as amphotericin B deoxycholate and its lipid derivatives, azoles such as itraconazole, voriconazole, posaconazole, and ravuconazole, investigational triazoles, and echinocandins such as caspofungin [288,293].
2.2.2. Mycotoxin Production
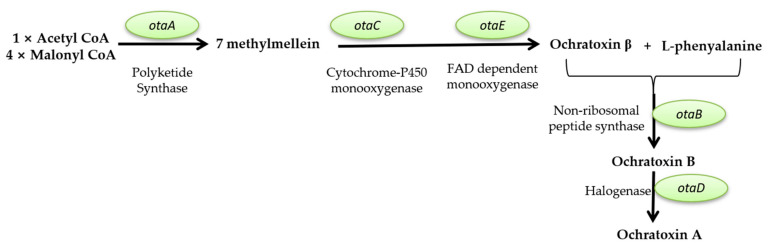
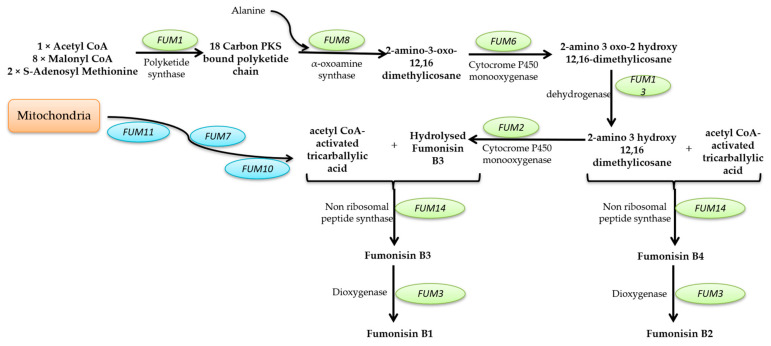
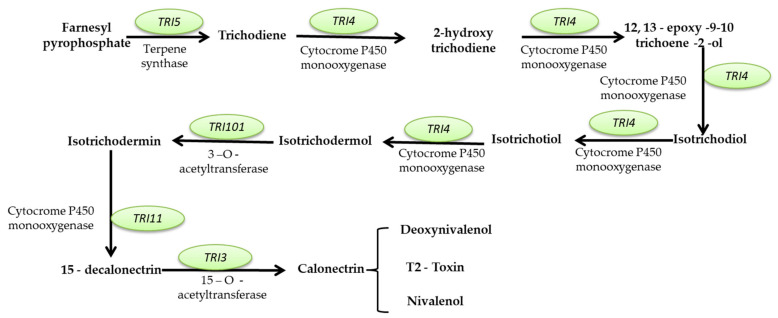
Mycotoxins are toxic secondary metabolites produced by numerous fungal species with a high negative impact on the health of humans and livestock, which can persist in food commodities after harvesting or processing [294]. Although in recent decades tremendous advances in understanding the biochemistry, genetics, and regulation of mycotoxin biosynthesis have been made, mycotoxin contamination of food products remains a problem far from being solved. These secondary metabolites are produced by toxigenic fungi belonging to Aspergillus, Fusarium, Talaromyces, and Penicillium genera. In food security and safety, their involvement in reducing the quality and quantity of food commodities requires intensive research [295]. Today, more than 300 mycotoxins of fungal origin are known, and their chemical structure varies from simple molecules with four carbon atoms to more complex ones. Although mycotoxins are secondary metabolites of different fungal strains, they do not intervene in fungal growth but rather act as a defensive mechanism against other organisms and as a strategy to maintain the oxidative status of the fungal cell [296]. In general, there are six types of mycotoxins considered most dangerous for human health: aflatoxins, ochratoxins, trichothecene, patulin, fumonisins, and zearalenone [297,298,299].
Mycotoxins Biosynthetic Pathways—Mechanisms and Genetic Background
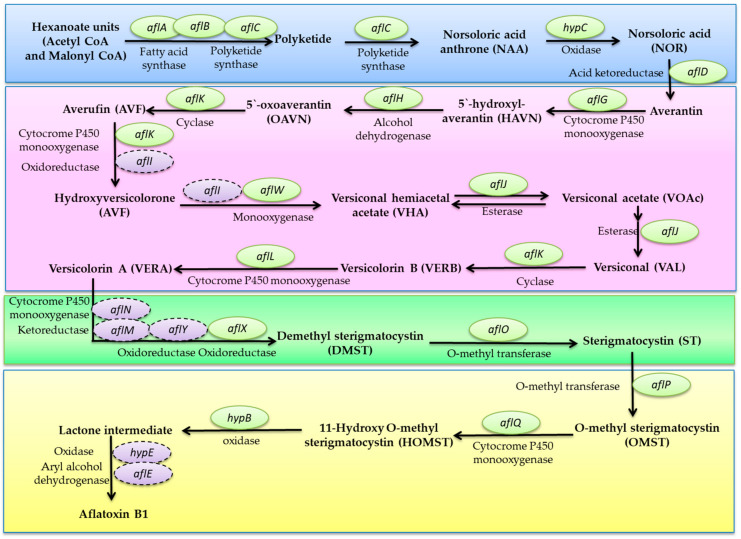
Aflatoxins are produced mainly by A. flavus and A. parasiticus strains, found in soil, decaying vegetation, or grains, and less frequently by Aspergillus bombycis, A. ochraceus, Aspergillus nomius, and Aspergillus pseudotamari species. Depending on their fluorescence underneath UV light and relative chromatographic mobility, four main types of aflatoxins (B1, B2, G1, and G2) were described [297], but based on their toxicity, B1 aflatoxin is considered the most genotoxic [292,293]. Aflatoxin B1 (AFB1) biosynthesis is a complex process that involves at least 27 enzymatic reactions. Based on the intermediates formed during the entire cascade, the biosynthesis pathway of AFB1 can be divided into four main stages. The first stage is the conversion of acetate into norsolorinic acid. The cascade reaction starts with forming hexanoate units from acetyl-CoA and malonyl-CoA and their transformation into norsolorinic acid (NOR). The first reaction is catalyzed by two fatty acid synthases encoded by aflA (fas-2) and aflB (fas-1) genes. After that, the acetate derivatives are subjected to chain elongation catalyzed by a polyketide synthase encoded by the aflC (pksA) gene [300,301,302,303]. The resulting norsolorinic acid anthrone (NAA) is oxidized to norsolorinic acid (NOR) by the anthrone oxidase HypC encoded by the hypC (hypB1) gene [304] (Figure 1, blue color). The second stage of AFB1 synthesis is converting norsolorinic acid into versicolorin A through a series of 10 enzymatic reactions. First, the norsolorinic acid is transformed into averantin (AVN) by a ketoreductase encoded by the aflD (nor-1) gene. The averantin is hydroxylated in a reaction catalyzed by a P-450 monooxygenase encoded by the aflG (avnA) gene to form 5’-hydroxyaverantin (HAVN), which is subsequently converted to 5’-oxoaverantin (OAVN) through a reaction catalyzed by an alcohol dehydrogenase encoded by aflH (adhA) gene. A cyclase encoded by aflK (vbs) catalyzes the reaction of transforming 5’-oxoaverantin (OAVN) in averufin (AVF), which is further transformed to versiconal hemiacetal acetate (VHA) through two successive reactions catalyzed by monooxygenases (a P450 monooxygenase encoded by aflV (cypX) and a cytosolic monooxygenase encoded by aflW (moxY)), the intermediate compound being hydroxyversicolorone (HVN). In this process, an enzyme encoded by the aflI (avfA) gene also intervenes, which might be responsible for the reaction needed for the ring-closure step in the formation of hydroxyversicolorone (HVN) [301,303,305]. Versiconal hemiacetal acetate (VHA) is further transformed into versiconal (VAL) by an esterase encoded by the aflJ (estA) gene, and versiconal (VAL) is transformed into VERB through a reaction catalyzed by the cyclase encoded by aflK (vbs) [306]. Versicolorin B (VERB) is the critical branch point leading to forming AFB1/AFG1 or AFB2/AFG2. VERB contains both a tetrahydrobisfuran ring (such as AFB2/AFG2) and a dihydrobisfuran ring (such as AFB1/AFG1), so the conversion of versicolorin B (VERB) to versicolorin A (VERA) requires the desaturation of the bisfuran ring under the action of a P450 monooxygenase encoded by aflL (verb), whose activity is highly dependent of the cultural conditions [303,307] (Figure 1, pink color). The third stage of AFB1 biosynthesis is the conversion of versicolorin A into sterigmatoxystin through a series of three successive reactions catalyzed by different enzymes encoded by aflM (ver-1), aflN (verA), aflY (hypA), aflX (ordB) and aflO (omtB) genes. The intermediate compounds formed are not yet fully understood, but the final step of this stage is the conversion of demethylsterigmatoxystin (DMST) into sterigmatocystin (ST) through a reaction catalyzed by an O-methyltransferase [301,308,309] (Figure 1, green color). The last stage of AFB1 production is the conversion of sterigamtocystin into aflatoxin B2. The conversion of sterigamatocystin into O–methylsterigmatocystin (OMST) is catalyzed by an O-methyltransferase encoded by the aflP (omtA) gene, whose expression is highly influenced by the growth conditions [310,311]. The A-ring of O–methylsterigmatocystin (OMST) is oxidized, and an intermediate named 11-hydroxy-O-methylsterigmatocystin (HOMST) is formed. The reaction is catalyzed by a P450 monooxygenase encoded by the aflQ (ordA) gene. Moreover, 11-hydroxy-O-methylsterigmatocystin (HOMST) is further oxidized to a lactone intermediate by an oxidase encoded by hypB (hypB2), whose expression is also strongly influenced by the culturing conditions, and the final steps of aflatoxin B1 biosynthesis are catalyzed by different enzymes most probably encoded by hypE (aflLa) and aflE (norA) genes (Figure 1, yellow color) [301,312,313].
Many genes involved in aflatoxin production are organized as a cluster from chromosome 3 in the case of A. flavus. A similar cluster was also described for A. parasiticus, the second-most-critical fungal species that can produce large quantities of aflatoxins. Between the two of them, the homology of the clustered genes is 90–99%, and in terms of functionality, the main difference is that A. flavus strains are mainly producers of B types of aflatoxins while A. parasiticus strains produce both B and G types. Although many questions regarding the biosynthetic pathway of AFB1 have found their answer, many other problems remained unsolved in recent years. Apart from the genes that encode enzymes directly involved in AFB1 biosynthesis for other genes, such as aflT and hypD (aflNa), the function was not fully elucidated [301,314]. In the case of A. flavus, the aflatoxin pathway is regulated by a transcriptional factor encoded by the aflR gene. The Cys6Zn2 transcriptional factor binds to at least 17 genes from the aflatoxin genes cluster and acts as a positive regulator enhancing their transcription and, thus, aflatoxins production up to 50 times. Similar transcriptional factors were also characterized for A. parasiticus and A. nidulans strains [301,315]. Another possible transcriptional factor in the aflatoxin biosynthesis pathway is a protein encoded by the aflS gene. This factor might influence the expression of aflC, aflD, aflM, and aflP genes, but its exact mechanisms of action are not yet fully elucidated [301,305,316].
Figure 1.
Aspergillus spp. aflatoxin B1 biosynthesis pathway and the genes involved (according to [305,312,314,317,318] (green circles with full border- genes with proven function; purple circles with dashed edges- putative genes).
Ochratoxins are polyketide-derived secondary metabolites classified into three main types: A, B, and C. Chemically, ochratoxin A (OTA) contains a dihydrocoumarin moiety coupled to L-β-phenylalanine and is considered to be the most toxic [319]. Ochratoxin B (OTB) is the non-chlorinated form of ochratoxin A, while ochratoxin C (OTC) is an ethyl ester form of ochratoxin A [320]. OTA is produced mainly by Aspergillus (A. ochraceus, Aspergillus carbonarius, A. niger, Aspergillus alliaceus, Aspergillus sclerotiorum, Aspergillus sulphureus, Aspergillus albertensis, Aspergillus auricomus, A. fumigatus, A. versicolor, Aspergillus pseudoelegans, Aspergillus roseoglobulosus, Aspergillus westerdijkiae, Aspergillus welwitshiae and A. wentii) [319,321,322] and Penicillium (Penicillium verrucosum, Penicillium nordicum, P. expansum and less frequently P. chrysogenum) [319,323,324] fungal strains, and it is found in many types of food commodities such as cereals, dried fruits, nuts, and oilseeds. OTA biosynthetic pathway starts with 7-methylmellein production from acetyl-CoA and malonyl-CoA through a reaction catalyzed by the OtaA enzyme (encoded by PKS gene-otaA) which is a polyketide synthase. Then, 7-methylmellein is further oxidized to β-ochratoxin; the reaction is catalyzed by a cytochrome P450 monooxygenase named OtaC (encoded by otaC). Subsequently, β-ochratoxin is combined with L-β-phenylalanine by NRPS enzyme (otaB), resulting in an amide bond and OTB. The next step involves the chlorination of OTB by a halogenase named OtaD (otaD), thus resulting in the final compound OTA (Figure 2).
The genes involved in OTA biosynthesis, in the case of A. ochraceus, A. carbonarius, A. niger, A. steynii, and P. nordicum, are grouped as a cluster formed by five genes, of which four are responsible for encoding enzymes (otaA, otaB, otaC, otaD). One is responsible for the production of a transcription factor—otaR1. Additionally, there are two other genes relevant to the OTA biosynthetic pathway otaE, which encodes a flavin-adenine dinucleotide-dependent oxidoreductase and a second transcriptional factor (encoded by otaR2). Thus, the OTA biosynthetic pathway regulation is ensured by the otaR1 transcriptional factor, which controls the expression of otaA, B, C, and D genes, and the otaR2 factor responsible for modulating the expression of otaA, B, and D [318,325,326].
Figure 2.
Ochratoxin A biosynthesis pathway and the genes involved (according to [318,319,325,326,327]) (green circles with full border- genes with proven function).
Fumonisins are polyketides consisting of a 19- or 20-carbon backbone with hydroxyl, methyl, and tricarballylic acid moieties, produced mainly by Fusarium verticillioides, Fusarium proliferatum, and Fusarium nygamai fungi that contaminate maize and maize-derived products [297,328]. These mycotoxins are classified into four major groups: A, B, C, and P, but only B (fumonisins B1, B2, B3, and B4) and A (fumonisins A1 and A2) groups are considered extremely important for human health; most frequently they are encountered in the human food chain [329]. Among group B fumonisins, fumonisin B1 is the most abundant in contaminated foods, representing up to 80% of the total fumonisin percentage [330]. The precursors for fumonisins biosynthesis are acetyl-CoA, malonyl-CoA, and S-adenosyl methionine. One molecule of acetyl-CoA, eight molecules of malonyl-CoA, and two molecules of S-adenosyl methionine are used to obtain 18-carbon PKS-bound polyketide chain compounds through a reaction catalyzed by polyketide synthase (PKS) encoded by FUM1. The α-oxoamine synthase encoded by FUM8 catalyzes the reactions of the decarboxylation of the amino acid and acyl-CoA thioester, and two carbon atoms of alanine are incorporated into the intermediate, forming a 22-carbon compound (2-amino-3-oxo-12,16-dimethyleicosane). An intermediate is formed through a reaction of hydroxylation at C14 and C15 catalyzed by a decarboxylase encoded by the FUM6 gene. This intermediate is further reduced to 2-amino-3-hydroxy-12, 16-dimethylicosane by a reaction catalyzed by a short-chain dehydrogenase encoded by FUM13 [331]. Subsequently, another hydroxylation at C10 occurs under the action of an enzyme encoded by the FUM2 gene resulting in hydrolyzed B3 fumonisin. The final stage of fumonisins production involves another precursor represented by an acetyl CoA-activated tricarballylic acid. The presence of acetyl CoA-activated tricarballylic acid is assured by the action of other enzymes and transporters encoded by FUM7, FUM10, and FUM11 [318]. FB3 and FB4 are formed by the esterification of acetyl CoA-activated tricarballylic acid at the C14 and C15 carbon atoms of hydrolyzed fumonisin B3 or 2-amino-3-hydroxy-12,16-dimethylicosane through reactions catalyzed by enzymes encoded by FUM14 [332]. The hydroxylation of FB3 and FB4 under the action of the FUM3 encoded enzyme leads to the formation of FB1 and FB2 [318,328,330] (Figure 3). From a genetic point of view, many genes related to fumonisins production were identified in the F. verticillioides genome. However, a cluster formed by 17 co-regulated genes is essential among them. The cluster contains both enzyme-encoding genes such as FUM1, FUM3, FUM6, FUM8, FUM 10, and FUM13 [318], transporters such as FUM11 [332], and DNA-binding transcription factors such as FUM21 [333]. More than that, in the same cluster are included genes that might be responsible for fungal self-protection during fumonisins production, such as FUM17 and FUM18 [334,335].
Figure 3.
General aspects regarding fumonisins biosynthesis pathway and the genes involved (according to [318,328,330,336]) (green circles—genes with proven function; blue circles—gene-encoding enzymes involved in acetyl CoA tricarballylic acid transportation or processing FUM11-tricarboxyl transporter; FUM7-alcohol dehydrogenase; FUM10-Acyl CoA synthase).
Trichothecenes represent the most diverse class of mycotoxins produced mainly by Fusarium species but also by Myrothecium and Stachybotrys strains. This group of mycotoxins contains more than 200 toxins, with molecular weights ranging from 200 to 500 Da. These compounds share a sesquiterpenoid structure. The presence or absence of macrocyclic esters or ester ether bridges between C-4 and C-15 and double bonds between C-9 and C10 with different side-chain substitutions ensures the differences between them. Many trichothecenes also contain 12,13-epoxyalkylene groups responsible for their cytotoxicity [337,338]. Trichothecenes are divided into four groups: type A containing T-2, HT-2, neosolaniol (ENNS) and diacetoxyscirpenol (DAS); type B that includes deoxynivalenol (DON) and its derivatives (3-acetyl deoxynivalenol and 15-acetyl deoxynivalenol), nivalenol (NIV) and Fusarenon-X; type C whose main representative compound is crotocin; and type D that includes roridin A, errucarin A and satratoxin H [337,339]. In the case of Fusarium species, the biosynthesis of trichothecenes starts with the cyclization of farnesyl pyrophosphate, resulting in trichodiene. The reaction is catalyzed by Tri5 synthase, encoded by the TRI5 gene. Then, trichodiene undergoes a reaction of oxygenation catalyzed by a cytochrome P450 monooxygenase, encoded by TRI4. The oxygenation consists in adding four oxygen atoms at C-2, C-3, C-11, and C-12 positions, resulting in isotrichotriol. Through non-enzymatic isomerization and cyclization, isotrichotriol is transformed into isotrichodermol. The last compound is converted into isotrichodermin through a reaction catalyzed by an acetyltransferase encoded by TRI101. Then, a second hydroxyl group is added to C-15 by an enzyme encoded by TRI11, resulting in 15-decalonectrin. This compound is acetylated in the presence of the TRI3 encoded enzyme, thus forming calonectrin [318,330,338,339] (Figure 4). The calonectrin compound can be considered an intermediate compound necessary for the biosynthesis of several types of trichothecenes, namely T-2 toxin, deoxynivalenol, and nivalenol.
Figure 4.
General aspects regarding trichothecenes biosynthesis pathway and the genes involved (according to [318,330,338,339]) (green circles—genes with proven function).
Zearalenone (ZEN), previously known as F-2 toxin, is a resorcyclic acid lactone produced by several Fusarium species such as Fusarium graminearum (Gibberella zeae), Fusarium culmorum, Fusarium crookwellense (F. cerealis), Fusarium semitectum and Fusarium equiseti. Zearalenone and its derivatives (α-zearalenol (α-ZEL); β-zearalenol (β-ZEL); zearalanone-(ZAN); α-zearalanol (α-ZAL) and β-zearalanol (β-ZAL)) usually contaminate corn, barley, wheat, beer, and soybean [337]. Chemically, α-ZEL and β-ZEL differ from ZEN and ZAN by replacing the C6’ keto group with a hydroxyl group. The main difference between ZAL and ZEN and ZEN derivatives is ensured by the lack of a C-1’-C-2’ double bond [336,337,340]. In the case of F. gramniareum, four genes were described as being involved in ZEN biosynthesis: PKS4, PKS13, ZEB1, and ZEB2. Except for ZEB2, which encodes a transcriptional factor, the other three encode enzymes involved in biosynthesis. The first step of ZEN biosynthesis is condensing acetyl-CoA and malonyl-CoA molecules to form a hexaketide starter unit. The reaction is catalyzed by a polyketide synthase encoded by PKS4 [341]. Then, the hexaketide undergoes transacylation and chain extension by adding three extra malonyl-CoA molecules. The reactions are catalyzed by a polyketide synthase encoded by the PKS13 gene. After a spontaneous aromatization of the ZEN backbone, a compound with a macrolide ring structure and lactone bond named zearalenol is formed. This compound is further converted into zearalenone through a reaction catalyzed by an isoamyl alcohol oxidase, encoded by the ZEB1 gene (Figure 5) [337,340].
Figure 5.
General aspects regarding zearalenone biosynthesis pathway and the genes involved (according to [318,340,341,342]) (green circles—genes with proven function).
Patulin is a polyketide lactone frequently encountered in fruits and fruit products. Although initially investigated as a possible antimicrobial agent, today, patulin is considered a dangerous compound that affects human health when ingested [343]. Patulin is produced by many fungal strains belonging to Penicillium genera (Penicillium carneum, Penicillium clavigerum, Penicillium concentricum, Penicillium coprobium, Penicillium dipodomyicola, P. expansum, Penicillium glandicola, Penicillium gladioli, P. griseofulvum, Penicillium marinum, Penicillium paneum, Penicillium sclerotigenum, Penicillium vulpinum); Aspergillus genera (A. clavatus, A. giganteus and Aspergillus longivesica); Paecilomyces saturnus; and Byssochlamys fulva [344,345]. The biosynthetic pathway of patulin presents ten chemical reactions starting from one molecule of acetyl-CoA and three molecules of malonyl-CoA, which are condensed through a reaction catalyzed by a multifunctional enzyme-6-methylsalicyclic acid synthase (6MSAS) encoded by PatK gene to form 6-methylsalicylic acid (6MSA). The resulting compound is further transformed into m-cresol by 6MSA decarboxylase, and the methyl group of m-cresol is oxidized to form an aldehyde. After a hydroxylation reaction, it forms gentisyaldehyde. Gentisyaldehyde is converted into phyllostine through a reaction catalyzed by an isoamyl alcohol oxidase (PatO), and the last compound is converted into neopatulin by neopatulin synthase (PatF). The last step might involve the conversion of E-ascladiol into patulin via a ring-closing reaction mediated by a glucose-methanol-choline oxidoreductase encoded by PatE, which is similar to versicolorin B synthase, described in the AFB1 biosynthetic pathway. Although in recent years, many studies have made much progress in elucidating the patulin biosynthesis pathway, certain steps remain open to question. An example is represented by the reactions necessary for converting gentisylalcohol into gentisylaldehyde (Figure 6) [345,346,347]. More than that, the genes involved in patulin biosynthesis seem highly conserved. For A. clavatus, a cluster of 15 genes responsible for producing this toxin was described. In the same cluster are gene-encoding enzymes such as PatK, PatG, PatI, PatO, PatD, PatF, or PatE, specific regulatory factors, and transporters [345,348]. A similar cluster was described for Penicillium species, with the mention that, in their case, the order of the genes in the cluster is quite different. Despite that, the biosynthetic pathway is not influenced [346].
Figure 6.
General aspects regarding patulin biosynthesis pathway and the genes involved (according to [345,346,349]) (green circles with full border- genes with proven function; purple circles with dashed edges- putative genes).
Health Implications of Mycotoxins Exposure
In 1962, when more than 100,000 turkeys from a poultry farm in London died after being fed with contaminated Brazilian groundnut meal, the “second mycotoxixology era” began. Later, it was proven that the incident was caused by secondary metabolites, today known as aflatoxins, produced by A. flavus. These fungal species contaminated the biomass used as feed on the farm [350]. More than 300 types of mycotoxins were described from this point forward, and many studies reported their negative impact on animal and human health. Only a few of the total number of mycotoxins described to date pose severe risks to public health, and most represent a real concern when they enter the human food supply chain (Table 3). Mycotoxins are classified according to their toxic activity into five main groups: Group 1, in which human carcinogens are included (e.g., aflatoxins); Group 2A, with probably carcinogenic mycotoxins for which no representative mycotoxins were described; Group 2B, with possible carcinogens (ochratoxins and fumonisins); Group 3, in which mycotoxins that are not yet considered human carcinogens (trichothecenes; zearalenones) are included [351]; and Group 4, with mycotoxins that are probably not carcinogenic to humans [352]. Aflatoxin B1 is considered to have the highest carcinogenicity agent among mycotoxins since it can penetrate the cell membrane due to its liposoluble character. After entering the cell, it is rapidly metabolized to a highly reactive and unstable compound: aflatoxin-8,9- epoxide, which binds to DNA or proteins. The mutagenic effect of aflatoxin B1 consists of GC to TA transversions, directly affecting the function of the P53 gene, which encodes a tumor suppressor protein that inhibits the development of tumors [353,354]. In the case of ochratoxin A, the second dangerous mycotoxin, its carcinogenic effect is mainly based on the fact that this compound disturbs cellular physiology by disrupting the phenylalanine metabolism or by inhibiting the mitochondrial ATP production [353]. Although included in Group 2B of mycotoxins, fumonisins disrupt the sphingolipid metabolism and inhibit protein and DNA synthesis [353,355]. Mycotoxins present a real concern to public health because these compounds are widely spread into the world’s food supply [296]. Mycotoxigenic fungi are frequently encountered in all agricultural regions. Because of that, more than 20% of crops harvested worldwide are contaminated with mycotoxins (even those from the field) every year, followed by an increase in their concentration during storage [296,349].
Table 3.
| Mycotoxins | Source | Effect on Human/Animal Health | References | |
|---|---|---|---|---|
| Aflatoxins | B1 B2 G1 G2 |
Maize, peanuts, copra, corn, coffee beans, rice, sorghum, soybean |
|
[353,356,357,358] |
| Ochratoxins | A | barley, wheat, coffee beans, citrus, grape, beer, fruits, soybean, cereals; dried fruits; breast milk of exposed mothers; smoked and salted dried fish; cheese |
|
[359,360,361,362,363] |
| Fumonisins | Maize; rice, wheat, sorghum; barley, oats |
|
[337,364,365,366] | |
| Zearalenone | Maize; wheat; barley; oats; grains; animal feed |
|
[367,368,369,370,371] | |
| Patulin | Fruits; fruit juices, cheese, wheat |
|
[372,373,374] | |
| Trichothecenes | Deoxynivalenol | Maize; wheat; barley; oats; grains; animal feed |
|
[337,375,376,377,378] |
| Nivalenol |
|
[337,379,380] | ||
| T-2 toxin and HT-2 toxin | Maize, oat, barley, wheat, rice, soybean |
|
[337,381,382,383,384,385] | |
2.2.3. Deteriogenic Agents
Cultural heritage, known as a connection between the past and the present, requires specific safeguarding efforts because it brings together both tangible cultural heritage [significant elements that support the history and culture of a country, i.e., monuments (of different substrate types), books, art mills, paintings, photographs, parchments, mosques, sanctuaries, monasteries, churches, chapels, bridges of wooden, stone, museums] and intangible cultural heritage (traditional music, folklore, language, traditional practices, customs, customs, crafts, crafts, etc.) [386].
The biodeterioration of cultural heritage objects, involving aesthetic, architectural, and economic damages, is a big challenge for conserving cultural heritage materials and community safety. The available methods for preventing biodeterioration are most often effective when removing deteriogenic microbial biofilms but not when preventing the recontamination of heritage objects. In certain situations, the repeated use of current decontamination methods leads to complex alterations of heritage objects. The biodeterioration of heritage objects is currently a significant problem worldwide. Modern conservation strategies underline the need to know and understand the structure and function of complex microbial communities, which represents one of the leading causes of the biodegradation of the objects included in the cultural heritage. To achieve adequate conservation actions, it is necessary to identify community species of microorganisms and their activity on a substrate. Attempts by researchers to elucidate biogeochemical mechanisms of the biodeterioration process were perfected by introducing next-generation techniques and whole-genome sequencing. Then, the first step is represented by identifying the microorganisms responsible for the process that means fungi are resistant to drying out, and they benefit from spore diffusion as a transport mechanism. Fungi also produce a wide range of organic acids and provide mineral nutrients to other microorganisms, especially phototrophs [387].
The Most Frequently Encountered Cultural Heritage Objects and Their Deterioration by Fungi
Stone Objects
Stones are the primary structural components of ancient structures, starting from numerous types of buildings, monuments, statues, tombs, and weather conditions that have influenced their quality. Anthropogenic and natural forces mostly cause stone modification and decay because it is an extreme environment for biotic factors. The enounced forces cause various physical, chemical, and biological degradation. The lack of nutrients in their composition, water, and intense microclimate oscillation makes the stone objects an unfavorable surface for microorganism colonization. Although, if the conditions are convenient, cyanobacteria, algae, and lichens are considered the primary colonizers, whereas hemolithotrophic and hemoorganotrophic bacteria and fungi have been described as the secondary colonizers [388,389].
Fungi may be the most significant deteriogenic species on exposed building stones due to their great erosiveness [86,390]. Fungi may enter the stone depending on the material’s physical characteristics. Black fungi are the principal culprits behind the process known as bio-pitting, which results in fissures up to 2 cm in diameter and depth in stone. Although it has been seen on old glass, bio-pitting is most common on marble and limestone [391]. The most widespread stone-degrading fungi genera are presented in Table 4.
Stone may become damaged by fungi through chemical and physical processes. Chemical mechanisms include the release of acidic metabolites and pigments and the oxidation of cations that produce minerals. In contrast, physical mechanisms include hyphal penetration of the rock surface, which results in its fragmentation. Fungi are thought to be the most powerful creatures in nature that break down rocks and minerals, even though numerous microorganisms can produce acids. The creation of numerous secondary mycogenic minerals is caused by synthesizing various acidic metabolites, which cause the mineral substrate to dissolve through biocorrosion [388].
Wooden Objects
The majority of cultural heritage objects were manufactured using prime material wood. In accordance with UNESCO, wooden cultural heritage items can be divided into three categories: moveable, such as furniture, frames, sculptures, and musical instruments; immovable, for example, buildings such as windmills, monasteries, churches, temples, and bridges; and underwater, such as shipwrecks, foundation piles, wooden cargo or contents that were partially or entirely underwater, periodically or continuously, for at least 100 years [392].
Almost each wooden cultural heritage object is constantly undergoing chemical changes, and an expected action that may appear is degradation over time. Depending on the wood species that may be particularly robust or the exposure circumstances that may be moderate or non-aggressive, the degradation of wood items could be quite slow [393]. However, many of these objects are deteriorating and vanishing due to neglect and unintentional mistreatment. Because of the agents of deterioration, the wood species, and the climatic conditions, the level of wooden item degradation ranges from a nearly everyday look to being disintegrated and significantly altered. Among the factors of deterioration, we can name biotic factors such as the action of fungi, bacteria, or insects, and abiotic factors, such as humidity, temperature, and weathering [86,392].
Although all types of microorganisms can contribute to the destruction of wood cultural items, fungi have the most ability to degrade this particular category. The mechanisms of biodeterioration can imply the growth of fungus on the surface or between internal components, the generation of extracellular enzymes, and the structural modification of fundamental biopolymers, which finally leads to alterations of the item evident to the naked eye [388].
Most of the time, filamentous fungi can be recognized easily due to their macroscopic aspects of culture. They have fast growth on the surface of the substrates, where the conidia develop rapidly. Due to the specific color of the conidia, wood colonized by a multitude of mold species will present a multicolored image on its surface, such as black, given by the presence of A. niger, or green, given by Trichoderma spp., Penicillium spp. The species of Trichoderma spp. occur most frequently on beech roots, and beech stems that are stored are frequently colonized by Bispora antennata, causing black spots. Molds develop on fresh cuttings after felling trees and usually on those improperly stored and undergoing not-tolerable conditions. The hyphae penetrate the wood only a few millimeters and develop on the parenchyma cells. Moldy wood loses all its value. Using it for decorative purposes is impossible; it is not suitable because color spots cannot be removed mechanically but can only be hidden by paint. Infected wood is not suitable to be used for hygienic reasons; for example, it is not suitable to be used for packaging [394]. Untreated, the molds that appear on the surface of the wood are dangerous for its durability over time. Fungi will degrade the material, changing its composition and losing its original characteristics.
Three types of fungi alterations that destroy wood are known as white rot, brown rot, and soft rot. The most frequently encountered genera are included in Table 4.
Wood degradation can be evaluated by X-rays and computed tomography. This is a non-destructive alternative that allows the determination of the material’s structural state. This is crucial for cultural preservation because it enables an accurate definition of the state of deterioration, which helps develop a conservation strategy to stop the loss of heritage assets. For example, Alfieri et al. analyzed the wood alteration of locomotive wood slats by a white rot fungus, Phellinus chaquensis. They used X-rays and computed tomography to observe the area filled by mycelium and basidiomata and later processed the images. Therefore, they could observe the area affected by wood decay fungi [395].
Paintings
Oil painting on canvas has become one of the most significant art expressions, resulting in remarkable works with significant historical and cultural worth. In addition, oil paintings on canvas may offer the most remarkable diversity of microhabitats and nutrients utilized by a wide range of microbial species compared to other artworks [86,396,397].
The chemical composition of canvas paintings is diverse and includes organic and inorganic elements. Textile fibers are frequently used as the foundation material in oil canvas paintings. Glues, gelatin, casein, egg yolk, flour, rubber, oil, or resin are frequently used in the ground, pictorial, and protective layers [396]. These substances serve as nutritive substrates for different microorganisms that can colonize canvas paintings and are thus vulnerable to deterioration. The pigments often contain inorganic chemicals, frequently heavy metal compounds, which may have harmful effects and prevent the growth of some fungi and bacteria [398].
Another substrate that can be deteriorated by fungal activity is represented by wall paintings. As with the oil paintings, the wall paintings are also a substrate rich in nutrients (egg whites, milk, oils, casein, etc.) that suffice as a microhabitat for fungal growth. The most frequently reported fungi on canvas oil and wall paintings are included in Table 4.
Textiles
Textile biodeterioration is a prevalent issue affecting various materials with various chemical compositions broken down by various microorganisms in various environmental settings. Of course, the deterioration method differs for each type of product, and the care of several circumstances, such as usage, storage, cleaning, and exposure to climatic conditions, can prevent it. However, as it offers a mechanism to eliminate waste textiles in the environment, the microbiological deterioration of textiles is not necessarily bad. Therefore, when considering final usage and disposal, adding chemical protectants to textiles is only sometimes required. Due to the organic composition of most textile products, microorganisms deteriorate quickly because they are a source of nutrients. Therefore, synthetic fibers are less susceptible to deteriogens than fibers made from natural source materials. Weaves and other textile-based products show signs of biodeterioration as exterior changes, most frequently discoloration and related disagreeable odors. The chemical alterations brought on by the development of microorganisms cause diminished weave strength and eventually result in a partial or complete material breakdown [399]. The most widespread deteriogenic fungi are included in Table 4.
Paper and Paper-Based Materials
Paper-based materials are typically stored in libraries, archives, and museums. These include books, essays, antique maps, photos, and more. In addition, paper is the world’s most significant material for recording and preserving historical events, primarily created through the mechanical and chemical processing of cellulose fibers derived from wood. Cellulolysis, the enzymatic hydrolysis of cellulose polymer into glucose units, is a process by which fungal microorganisms may break down cellulose fibers. In this regard, isolating the fungus capable of producing cellulolytic enzymes from paper, mainly from ancient books or papers stored in libraries, archives, and museum depots, is common practice. Members of the genera Chaetomium, Penicillium, Aspergillus, Eurotium, Trichoderma, and so on are among the most common genera responsible for paper deterioration (Table 4) [86].
Parchments
Because ancient parchment-based writings were a crucial tool for human communication, they are of immeasurable historical importance to our society. Before the Middle Ages, they were the primary writing materials, but since the invention of paper, parchment has mainly been employed for noble reasons [400].
Collagen, the primary constituent of treated animal skin, is used to make parchment. Numerous microorganisms can degrade collagen, which they use as a source of energy and carbon. In parchment deterioration and that of other archival materials, fungi play a crucial role. As a result of this biodeterioration, parchment begins to lose some of its original qualities. For example, it becomes distorted, and there is a chance that white films, fading lettering, and spots may appear. The biodeterioration of parchment is frequently caused by the action of fungi that have extracellular enzymes that enable them to metabolize protein, generating various spots of different colors (brown, black, or reddish). In addition to this chemical activity, fungal hyphae can break down the parchment’s fiber structures and cause mechanical harm to the document. Table 4 includes the most common fungal deteriogenes genera that can colonize and degrade parchments.
Table 4.
Deterioration of different cultural heritage substrate types by fungi.
| Deteriogenic Agents/Group | Genus | Substrate | Alteration Types | References |
|---|---|---|---|---|
| Ascomycota Phyllum | Acrodictys spp. | stone artifacts | [401] | |
| Dothideomycetes class | Aureobasidium spp. | black patina, black spots, biofilm formation, discolorations, stone erosion, and disintegration | [402,403,404] | |
| Capnobotryella spp. | black spots, crater shaped lesions, superficial deposit, and biofilm formation | [403,405] | ||
| Coniosporium spp. | black patina, black spots, pitting, exfoliation, superficial deposit, and biofilm formation | [403,406] | ||
| Phoma spp. | black spots, black patinas, exfoliation, pitting, superficial deposit, biofilm formation | [404,407,408] | ||
| Alternaria spp. | black spots, black patina, biofilm formation, black crusts | |||
| Cladosporium spp. | black spots, patinas, pitting, biofilm formation, erosion, discoloration, disintegration | |||
| Epicoccum spp. | black spots, black patinas, superficial deposit, biofilm formation with salt efflorescence | |||
| Eurotiomycetes class | Exophiala spp. | black patina, black spots, detachment of discolorations, visible damage | [389,406] | |
| Knuffia spp. | stone | black spots, patinas, pitting, discolorations, visible damage | [389,405,406,409] | |
| Lithophyla spp. | black patina, black spots | [245,402] | ||
| Dothideomycetes class |
Chaetomium spp., Aureobasidium spp., Epicoccum spp., Cladosporium spp. |
wall paintings | brown discolorations, degrade protein binders of the painted layer, which results in the lifting and separation of the painted layer from the support |
[86,410,411] |
| Eurotiomycetes class |
Penicillium spp., Aspergillus spp. |
primary fresco deteriogens, damage of wall paintings due to its intensely sporulation degree |
[86,412] | |
| Zygomycetes class |
Mucor spp., Rhizopus spp., Actinomucor spp. |
surface contaminants | [413,414] | |
| Basidiomycetes class | Coprinus spp. | contamination | [413] | |
| Dothideomycetes and Eurotiomycetes classes |
A. alternata, A. flavus, A. niger, A. versicolor, Aureobasidium pullulans, Chaetomium globosum, C. cladosporoides, Eurotium chevalieri, and P. chrysogenum | canvas oil paintings | the detachment of the paint layer from the support, the loss of material due to the excretion of metabolites, esthetic changes of materials, biofilm formation, chromatic alteration of the painted surfaces and detachment of the support | [89,393,410] |
| Zygomycetes class |
Cunninghamella spp., Mucor spp., Rhizopus spp. Phycomyces spp. |
dust deposits | [398] | |
| Basidiomycetes class | Puccinia spp. | contaminants | [415] | |
| Basidiomycetes class | Bjerkandera spp., Donkioporia spp., Fomes spp., Irpex spp., Phanerochaete spp., Pholiota spp., Pleurotus spp., Trametes spp. | wooden | white rot fungi complete depolymerization and degradation of lignin, cellulose, and hemicellulose components |
[86,416] |
| Eurotiomycetes class Sordariomycetes class |
Aspergillus spp., Fusarium spp., | [86] | ||
| Basidiomycetes class |
Antrodia spp., Coniophora spp., Coriolellus spp., Gloeophyllum spp., Paxillus spp., Poria spp., Postia spp., Serpula (Merulius) lacrymans |
brown rot fungi cellulose and hemicellulose decomposition and lignin degradation is restricted to methoxyl group demethylation |
[86,417] | |
| Dothideomycetes class | Alternaria spp., Stemphylium spp., | soft rot fungi cellulose and hemicellulose decomposition and lignin degradation is restricted to methoxyl group demethylation, leading to discoloration and cracking pattern | [86,392,417] | |
| Sordariomycetes class |
Chaetomium spp., Daldinia spp., Humicola spp., Xylaria spp. |
|||
| Eurotiomycetes Sordariomycetes and Dothideomycetes classes |
Penicillium spp., Aspergillus spp., Eurotium spp., Myxotrichum spp. Trichoderma spp., Chaetomium spp., Acremonium spp., Paecilomyces spp., Stachybotrys spp., Myrothecium spp., Cladosporium spp., Bipolaris spp., Aureobasidium spp., Alternaria spp., Epicoccum spp. |
paper and paper-based materials | pigments and organic acid production, brown to red spots (foxing) | [86,418,419,420,421,422] |
| Basidiomycetes classTritirachiomycetes classes |
Bjerkandera spp., Tritirachium spp. |
|||
| Zygomycetes class | Rhizopus arrhyzus | |||
| Eurotiomycetes and Sordariomycetes classes |
Aspergillus spp., Penicillium spp., Microsporum spp., Trichophyton spp., Chaetomium spp., Fusarium spp., |
textiles | wool fibers degradation | [86,423] |
| Zygomycetes class | Rhizopus spp. | wool fibers degradation | [423] | |
| Eurotiomycetes class | Chaetomium globosum | silk deterioration, causing cracks and gaps in fibroin fibers | [424] | |
| Dothideomycetes and Eurotiomycetes classes |
Alternaria spp., Aureobasidium spp., Cladosporium spp., Epicoccum spp., Penicillium spp. |
parchment | [86,425,426] | |
| Ascomycota phyllum | Diploospora rosea | the detachment of large parts of the artwork’s preparative layer and the overlying illumination. | [427] |
3. Conclusions
Fungi have been used as a cell factory to produce enzymes and small molecule compounds for almost a century. The biomass produced during these production processes has generally been considered a waste stream. This inconvenience may change in the future since fungal biomass is now being explored as the basis of sustainable biomaterials. In agriculture, the presented applications have the potential to improve crop yield, reduce the use of synthetic fertilizers and pesticides, avoid the use of toxic compounds, and promote sustainable agriculture practices. Therefore, further attention must be paid to uncovering the biomolecules from fungi for agriculture and pharmaceutical applications through studying metagenomics, genomics, and proteomics.
Humans are endowed by evolution with robust defenses against invasive fungal diseases, successfully treating many of them. However, we are still vulnerable to invasive fungal infections. People suffering from opportunistic and primary invasive fungal infections urgently need resources and research efforts to bring them new diagnostics and treatments regardless of commercial potential. Enormous work over the past three decades has opened vast new views in fungal biology; we can expand upon them to fulfill the promises of modern medical advances.
Author Contributions
I.G.-B. and T.E.Ș. conceived, revised, and corrected the manuscript; I.G.-B., V.M.C., A.Ș.D. and C.O.V. drafted chapters 1 and 2; V.M.C. and I.G.-B. drafted the tables and figures. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. The funding had no role in the study design, data collection, analysis, the decision to publish, or the preparation of the manuscript.
Funding Statement
This study was supported by the Romanian Executive Agency for Higher Education, Research, Development, and Innovation (https://uefiscdi.gov.ro/) research projects PN-III-P2-2.1-PED-2021-2526 (736 PED/2022) and by the Ministry of Research, Innovation, and Digitalization through Program 1—Development of the national R&D system, Subprogram 1.2—Institutional performance—Financing projects for excellence in RDI research projects C1.2.PFE-CDI.2021-587, contract no. 41PFE/30.12.2021 and CNFIS-FDI-0609/2023.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Bonfante P., Venice F., Lanfranco L. The mycobiota: Fungi take their place between plants and bacteria. Curr. Opin. Microbiol. 2019;49:18–25. doi: 10.1016/j.mib.2019.08.004. [DOI] [PubMed] [Google Scholar]
- 2.Wösten H.A.B. Filamentous fungi for the production of enzymes, chemicals and materials. Curr. Opin. Biotechnol. 2019;59:65–70. doi: 10.1016/j.copbio.2019.02.010. [DOI] [PubMed] [Google Scholar]
- 3.Nash A.K., Auchtung T.A., Wong M.C. The gut mycobiome of the human microbiome project healthy cohort. Microbiome. 2017;5:153. doi: 10.1186/s40168-017-0373-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hüttner S., Johansson A., Gonçalves Teixeira P., Achterberg P., Nair R.B. Recent advances in the intellectual property landscape of filamentous fungi. Fungal Biol. Biotechnol. 2020;7:16. doi: 10.1186/s40694-020-00106-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mei Y.Z., Zhu Y.L., Huang P.W., Yang Q., Dai C.C. Strategies for gene disruption and expression in filamentous fungi. Appl. Microbiol. Biotechnol. 2019;103:6041–6059. doi: 10.1007/s00253-019-09953-2. [DOI] [PubMed] [Google Scholar]
- 6.Jones M.P., Huynh T., Dekiwadia C.D., Daver F., John S. Mycelium composites: A review of engineering characteristics and growth kinetics. J. Bionanosci. 2017;11:241–257. doi: 10.1166/jbns.2017.1440. [DOI] [Google Scholar]
- 7.Appels F.V.W., Dijksterhuis J., Lukasiewicz C.E. Hydrophobin gene deletion and environmental growth conditions impact mechanical properties of mycelium by affecting the density of the material. Sci. Rep. 2018;8:4703. doi: 10.1038/s41598-018-23171-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pombeiro-Sponchiado S.R., Sousa G.S., Andrade J.C.R., Lisboa H.F., Gonçalves R.C.R. Melanin. IntechOpen; London, UK: 2017. Production of melanin pigment by fungi and its biotechnological applications. [Google Scholar]
- 9.Poorniammal R., Prabhu S., Dufossé L., Kannan J. Safety evaluation of fungal pigments for food applications. J. Fungi. 2021;7:692. doi: 10.3390/jof7090692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iram W., Anjum T. Production enhancement of cyclosporin “A” by Aspergillus terreus through mutation. Afr. J. Biotechnol. 2015;11:1736–1743. doi: 10.5897/AJB10.1330. [DOI] [Google Scholar]
- 11.Karwehl S., Stadler M. Exploitation of fungal biodiversity for discovery of novel antibiotics. In: Stadler M., Dersch P., editors. How to Overcome the Antibiotic Crisis. Current Topics in Microbiology and Immunology. Volume 398. Springer; Berlin/Heidelberg, Germany: 2016. pp. 303–338. [DOI] [PubMed] [Google Scholar]
- 12.Smith S.E., Read D. The symbionts forming arbuscular mycorrhizas. In: Smith S.E., Read D., editors. Mycorrhizal Symbiosis. Academic Press; Cambridge, MA, USA: 2008. pp. 13–41. [Google Scholar]
- 13.Şesan T.E., Oancea F. Trichoderma viride Pers.—Experimental model for biological and biotechnological investigations of mycromyceta with importance in obtaining plant protection bioproducts. J. Plant Dev. 2010;17:49–62. [Google Scholar]
- 14.Şesan T.E., Oancea F., Toma C., Matei G.-M., Matei S.M., Chira F., Chira D., Fodor E., Mocan C., Ene M.A., et al. Approaches to the study of mycorrhizas in Romania. Ed. Univ. Buc. 2010;51:75–85. doi: 10.1007/s13199-010-0093-z. [DOI] [Google Scholar]
- 15.Galindo-Solís J.M., Fernández F.J. Endophytic fungal terpenoids: Natural role and bioactivities. Microorganisms. 2022;10:339. doi: 10.3390/microorganisms10020339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaur C., Mishra Y., Mishra V., Saraogi G.K., Tambuwala M.M. Recent advancement and biomedical applications of fungal metabolites. In: Singh J., Gehlot P., editors. New and Future Developments in Microbial Biotechnology and Bioengineering. Elsevier; Amsterdam, The Netherlands: 2021. pp. 47–67. [Google Scholar]
- 17.Fodor E., Şesan T.E. Fitopatogeni în Ecosistemele Forestiere [Phytopathogens in Forest Ecosystems] Editura Universitatii din Bucyresti; Bucharest, Romania: 2014. p. 648. [Google Scholar]
- 18.Kim J.-S., Yoon S.-J., Park Y.-J., Kim S.-Y., Ryu C.-M. Crossing the kingdom border: Human diseases caused by plant pathogens. Environ. Microbiol. 2020;22:2485–2495. doi: 10.1111/1462-2920.15028. [DOI] [PubMed] [Google Scholar]
- 19.Stajich J.E. Fungal genomes and insights into the evolution of the kingdom. Microbiol. Spectr. 2017;5:1–15. doi: 10.1128/microbiolspec.FUNK-0055-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mukherjee D., Singh S., Kumar M., Kumar V., Datta S., Dhanjal D.S. Fungal biotechnology: Role and aspects. In: Gehlot P., Singh J., editors. Fungi and their Role in Sustainable Development: Current Perspectives. Springer; Berlin/Heidelberg, Germany: 2018. pp. 91–103. [Google Scholar]
- 21.Adrio J.L., Demain A.L. Fungal biotechnology. Int. Microbiol. 2003;6:191–199. doi: 10.1007/s10123-003-0133-0. [DOI] [PubMed] [Google Scholar]
- 22.Cerimi K., Akkaya K.C., Pohl C., Schmidt B., Neubauer P. Fungi as Source for new bio-based materials: A patent review. Fungal Biol. Biotechnol. 2019;6:17. doi: 10.1186/s40694-019-0080-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meyer V., Basenko E.Y., Benz J.P., Braus G.H., Caddick M.X., Csukai M., de Vries R.P., Endy D., Frisvad J.C., Gunde-Cimerman N., et al. Growing a circular economy with fungal biotechnology: A white paper. Fungal Biol. Biotechnol. 2020;7:5. doi: 10.1186/s40694-020-00095-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jakubczyk D., Dussart F. Selected fungal natural products with antimicrobial properties. Molecules. 2020;25:911. doi: 10.3390/molecules25040911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rani A., Saini K.C., Bast F., Varjani S., Mehariya S., Bhatia S.K., Sharma N., Funk C. A Review on microbial products and their perspective application as antimicrobial agents. Biomolecules. 2021;11:1860. doi: 10.3390/biom11121860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sebastian L., Madhusudana S.N., Ravi V., Desai A. Mycophenolic acid inhibits replication of japanese encephalitis virus. Chemotherapy. 2011;57:56–61. doi: 10.1159/000321483. [DOI] [PubMed] [Google Scholar]
- 27.Demain A., Martens E. Production of valuable compounds by molds and yeasts. J. Antibiot. 2017;70:347–360. doi: 10.1038/ja.2016.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller E.L. The penicillins: A review and update. J. Midwifery Womens Health. 2002;47:426–434. doi: 10.1016/S1526-9523(02)00330-6. [DOI] [PubMed] [Google Scholar]
- 29.Tipper D.J. Mode of action of beta-lactam antibiotics. Pharmacol. Ther. 1985;27:1–35. doi: 10.1016/0163-7258(85)90062-2. [DOI] [PubMed] [Google Scholar]
- 30.Moldenhauer G., Salnikov A.V., Lüttgau S., Herr I., Anderl J., Faulstich H. Therapeutic potential of amanitin-conjugated anti-epithelial cell adhesion molecule monoclonal antibody against pancreatic carcinoma. J. Natl. Cancer Inst. 2012;104:622–634. doi: 10.1093/jnci/djs140. [DOI] [PubMed] [Google Scholar]
- 31.Koiso Y., Li Y., Iwasaki S., Hanaka K., Kobayashi T., Sonoda R., Fujita Y., Yaegashi H., Sato Z. Ustiloxins, antimitotic cyclic peptides from false smut balls on rice panicles caused by Ustilaginoidea virens. J. Antibiot. 1994;47:765–773. doi: 10.7164/antibiotics.47.765. [DOI] [PubMed] [Google Scholar]
- 32.Anke H., Laatsch H. Cyclic peptides and depsipeptides from Fungi. In: Esser K., editor. Physiology and Genetics. The Mycota (A Comprehensive Treatise on Fungi as Experimental Systems for Basic and Applied Research) Volume 15. Springer; Berlin/Heidelberg, Germany: 2009. pp. 331–335. [Google Scholar]
- 33.Umeyama A., Takahashi K., Grudniewska A., Shimizu M., Hayashi S., Kato M., Okamoto Y., Suenaga M., Ban S., Kumada T., et al. In vitro antitrypanosomal activity of the cyclodepsipeptides, cardinalisamides A-C, from the insect pathogenic fungus Cordyceps cardinalis NBRC 103832. J. Antibiot. 2014;67:163–166. doi: 10.1038/ja.2013.93. [DOI] [PubMed] [Google Scholar]
- 34.Haritakun R., Sappan M., Suvannakad R., Tasanathai K., Isaka M. An antimycobacterial cyclodepsipeptide from the entomopathogenic fungus Ophiocordyceps communis BCC 16475. J. Nat. Prod. 2010;73:75–78. doi: 10.1021/np900520b. [DOI] [PubMed] [Google Scholar]
- 35.Nakaya S., Mizuno S., Ishigami H., Yamakawa Y., Kawagishi H., Ushimaru T. New rapid screening method for anti-aging compounds using budding yeast and identification of beauveriolide I as a potent active compound. Biosci. Biotechnol. Biochem. 2012;76:1226–1228. doi: 10.1271/bbb.110872. [DOI] [PubMed] [Google Scholar]
- 36.Arnold D., Scott P., McGuire P., Harwig J., Nera E. Acute toxicity studies on roquefortine and pr toxin, metabolites of the Penicillium roqueforti in the mouse. Food Cosmet. Toxicol. 1978;16:369–371. doi: 10.1016/S0015-6264(78)80009-1. [DOI] [PubMed] [Google Scholar]
- 37.Eriksen G.S., Jäderlund K.H., Moldes-Anaya A., Schönheit J., Bernhoft A., Jæger G., Rundberget T., Skaar I. Poisoning of dogs with tremorgenic Penicillium toxins. Med. Mycol. 2010;48:188–196. doi: 10.3109/13693780903225821. [DOI] [PubMed] [Google Scholar]
- 38.Pahl B.H.L., Kraub B., Schulze-osthoff K., Decker T., Traenckner E.B., Myersfl C., Parksfl T., Warring P., Miihlbacher I.I.A., Czernilofiky A. The immunosuppressive fungal metabolite gliotoxin specifically inhibits transcription factor NF-KB. J. Exp. Med. 1996;183:1829–1840. doi: 10.1084/jem.183.4.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coleman J.J., Ghosh S., Okoli I., Mylonakis E. Antifungal activity of microbial secondary metabolites. PLoS ONE. 2011;6:e25321. doi: 10.1371/journal.pone.0025321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aris P., Wei Y., Mohamadzadeh M., Xia X. Griseofulvin: An updated overview of old and current knowledge. Molecules. 2022;18:7034. doi: 10.3390/molecules27207034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Riley R.T., Showker J.L. The mechanism of patulin’s cytotoxicity and the antioxidant activity of indole tetramic acids. Toxicol. Appl. Pharm. 1991;109:108–126. doi: 10.1016/0041-008X(91)90195-K. [DOI] [PubMed] [Google Scholar]
- 42.Sumbu Z.L., Thomart P., Bechet J. Action of patulin on yeast. Appl. Environ. Microbiol. 1983;45:110–115. doi: 10.1128/aem.45.1.110-115.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Feng Y., Huang Y., Zhan H., Bhatt P., Chen S. Species specificity and mechanism of action of strobilurins. Dechema Monogr. 1993;129:27–38. [Google Scholar]
- 44.Miethbauer S., Gaube F., Mollmann U., Dahse H.M., Schimidtke M., Gareis M., Pickhardt M., Liebermann B. Antimicrobial, antiproliferative, cytotoxic, and tau inhibitory activity of rubellins and caeruleoramularin produced by the phytopathogenic fungus Ramularia collo-cygni. Planta Med. 2009;75:1523–1525. doi: 10.1055/s-0029-1185835. [DOI] [PubMed] [Google Scholar]
- 45.Schueffler A., Anke T. Fungal natural products in research and development. Nat. Prod. Rep. 2014;31:1425–1448. doi: 10.1039/C4NP00060A. [DOI] [PubMed] [Google Scholar]
- 46.Denning D. Echinocandins: A new class of antifungal. J. Antimicrob. Chemother. 2002;49:889–891. doi: 10.1093/jac/dkf045. [DOI] [PubMed] [Google Scholar]
- 47.Houšť J., Spížek J., Havlíček V. Antifungal drugs. Metabolites. 2020;10:106. doi: 10.3390/metabo10030106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bouz G., Doležal M. Advances in antifungal drug development: An up-to-date mini review. Pharmaceuticals. 2021;14:1312. doi: 10.3390/ph14121312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.El-Khonezy M.I., Elgammal E.W., Ahmed E.F., Abd-Elaziz A.M. Detergent stable thiol-dependant alkaline protease produced from the endophytic fungus Aspergillus ochraceus BT21: Purification and kinetics. Biocatal. Agric. Biotechnol. 2021;35:102046. doi: 10.1016/j.bcab.2021.102046. [DOI] [Google Scholar]
- 50.Strobel G., Daisy B. Bioprospecting for microbial endophytes and their natural products. Microbiol. Mol. Biol. Rev. 2003;67:491–502. doi: 10.1128/MMBR.67.4.491-502.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Strobel G.A., Miller R.V., Martinez-Miller C., Condron M.M., Teplow D.B., Hess W.M. Cryptocandin, a potent antimycotic from the endophytic fungus Cryptosporiopsis cf. quercina. Microbiology. 1999;145:1919–1926. doi: 10.1099/13500872-145-8-1919. [DOI] [PubMed] [Google Scholar]
- 52.Endo M., Takesako K., Kato I., Yamaguchi H. Fungicidal action of aureobasidin A, a cyclic depsipeptide antifungal antibiotic, against Saccharomyces cerevisiae. Antimicrob. Agents Chemother. 1997;41:672–676. doi: 10.1128/AAC.41.3.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tan H.W., Tay S.T. The inhibitory effects of aureobasidin A on Candida planktonic and biofilm cells. Mycoses. 2013;56:150–156. doi: 10.1111/j.1439-0507.2012.02225.x. [DOI] [PubMed] [Google Scholar]
- 54.Vijayakumar E.K.S., Roy K., Chatterjee S., Deshmukh S.K., Ganguli B.N., Fehlhaber H.W., Kogler H. Arthrichitin. A new cell wall active metabolite from Arthrinium phaeospermum. J. Org. Chem. 1996;61:6591–6593. doi: 10.1021/jo960769n. [DOI] [PubMed] [Google Scholar]
- 55.King A.M. Aspergillomarasmine A overcomes metallo-β-lactamase antibiotic resistance. Nature. 2014;510:503–506. doi: 10.1038/nature13445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Borel J.F., Feurer C., Gabler H.U., Stahelin H. Biological effects of cyclosporine A: A new anti- lymphocytic agent. Agents Action. 1976;6:468–475. doi: 10.1007/BF01973261. [DOI] [PubMed] [Google Scholar]
- 57.Marik T., Tyagi C., Balázs D., Urbán P., Szepesi Á., Bakacsy L., Endre G., Rakk D., Szekeres A., Andersson M.A. Structural diversity and bioactivities of peptaibol compounds from the Longibrachiatum clade of the filamentous fungal genus Trichoderma. Front. Microbiol. 2019;10:1434. doi: 10.3389/fmicb.2019.01434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mygind P.H., Fischer R.L., Schnorr K.M., Hansen M.T., Sönksen C.P., Ludvigsen S., Raventós D., Buskov S., Christensen B., De Maria L., et al. Plectasin is a peptide antibiotic with therapeutic potential from a saprophytic fungus. Nature. 2005;437:975–980. doi: 10.1038/nature04051. [DOI] [PubMed] [Google Scholar]
- 59.Shima A., Fukushima K., Arai T., Terada H. Dual Inhibitory effects of the peptide antibiotics leucinostatins on oxidative phosphorylation in mitochondria. Cell Struct. Funct. 1990;15:53–58. doi: 10.1247/csf.15.53. [DOI] [PubMed] [Google Scholar]
- 60.Iwatsuki M., Kinoshita Y., Niitsuma M., Hashida J., Mori M., Ishiyama A., Namatame M., Nishihara-Tsukashima A., Nonaka K., Masuma R., et al. Antitrypanosomal peptaibiotics, trichosporins B-VIIa and B-VIIb, produced by Trichoderma polysporum FKI-4452. J. Antibiot. 2010;63:331–333. doi: 10.1038/ja.2010.41. [DOI] [PubMed] [Google Scholar]
- 61.Shiomi K., Yamada H., Omura S. In vitro and in vivo antitrypanosomal activities of three peptide antibiotics: Leucinostatin A and B, alamethicin I and tsushimycin. J. Antibiot. 2009;62:303–308. doi: 10.1038/ja.2009.32. [DOI] [PubMed] [Google Scholar]
- 62.Pelaez F., Cabello A., Platas G., Díez M.T., Del Val A.G., Basilio A., Martán I., Vicente F., Bills G.F., Giacobbe R.A. The discovery of enfumafungin, a novel antifungal compound produced by an endophytic Hormonema species biological activity and taxonomy of the producing organisms. Syst. Appl. Microbiol. 2000;23:333–343. doi: 10.1016/S0723-2020(00)80062-4. [DOI] [PubMed] [Google Scholar]
- 63.Vicente M.F., Cabello A., Platas G., Basilio A., Díez M.T., Dreikorn S., Giacobbe R.A., Onishi J.C., Meinz M., Kurtz M.B. Antimicrobial activity of ergokonin A from Trichoderma longibrachiatum. J. Appl. Microbiol. 2001;91:806–813. doi: 10.1046/j.1365-2672.2001.01447.x. [DOI] [PubMed] [Google Scholar]
- 64.Harvey A.L., Edrada-Ebel R., Quinn R.J. The re-emergence of natural products for drug discovery in the genomics era. Nat. Rev. Drug Discov. 2015;14:111–129. doi: 10.1038/nrd4510. [DOI] [PubMed] [Google Scholar]
- 65.Bills G.F., Platas G., Overy D.P., Collado J., Fillola A., Jiménez M.R., Martín J., Val A.G., Francisca Vicente J.R.T., Peláez F., et al. Discovery of the parnafungins, antifungal metabolites that inhibit MRNA polyadenylation, from the Fusarium larvarum complex and other hypocrealean fungi. Mycologia. 2009;101:449–472. doi: 10.3852/08-163. [DOI] [PubMed] [Google Scholar]
- 66.Manzoni M., Rollini M. Biosynthesis and biotechnological production of statins by filamentous fungi and application of these cholesterol-lowering drugs. Appl. Microbiol. Biotechnol. 2002;58:555–564. doi: 10.1007/s00253-002-0932-9. [DOI] [PubMed] [Google Scholar]
- 67.McLean K.J., Hans M., Meijrink B., Scheppingen W.B., Vollebregt A., Tee K.L., Laan J.-M., Leys D., Munro A.W., Berg M.A. Single-step fermentative production of the cholesterol lowering drug pravastatin via reprogramming of Penicillium chrysogenum. Proc. Natl. Acad. Sci. USA. 2015;112:2847–2852. doi: 10.1073/pnas.1419028112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang C.-L., Zheng B.-Q., Lao J.-P., Mao L.-J., Chen S.-Y., Kubicek C.P., Lin F.-C. Clavatol and patulin formation as the antagonistic principle of Aspergillus clavatonanicus, an endophytic fungus of Taxus mairei. Appl. Microbiol. Biotechnol. 2008;78:833–840. doi: 10.1007/s00253-008-1371-z. [DOI] [PubMed] [Google Scholar]
- 69.Riko R., Nakamura H., Shindo K. Studies on pyranonigrins–isolation of pyranonigrin E and biosynthetic studies on pyranonigrin A. J. Antibiot. 2014;67:179–181. doi: 10.1038/ja.2013.91. [DOI] [PubMed] [Google Scholar]
- 70.Şesan T.E. Studiul biologic al speciilor de ciuperci antagoniste faţă de unii patogeni cu produc micoze la plante [Biological study of fungi species antagonistic towards some phytopathogens] ICEBiol. 1986;1:89. [Google Scholar]
- 71.Şesan T.E. Trichoderma spp. Applications in Agriculture and Horticulture. Editura Universitatii din Bucuresti; Bucharest, Romania: 2017. p. 380. [Google Scholar]
- 72.Şesan T.E. Sustainable management of gray mold (Botrytis spp.) of horticultural crops. Adv. Plant Dis. Manag. Res. Signpost. 2003;37:121–152. [Google Scholar]
- 73.Hermosa R., Rubio B., Cardoza R., Nicolas C., Monte E., Gutierrez S. The Contribution of Trichoderma to balancing the costs of plant growth and defense. Int. Microbiol. 2013;16:69–80. doi: 10.2436/20.1501.01.181. [DOI] [PubMed] [Google Scholar]
- 74.Lorito M., Woo S.L., Harman G.E., Monte E. Translational research on Trichoderma: From omics to the field. Annu. Rev. Phytopathol. 2010;48:395–418. doi: 10.1146/annurev-phyto-073009-114314. [DOI] [PubMed] [Google Scholar]
- 75.Lahlali R., Ezrari S., Radouane N., Kenfaoui J., Esmaeel Q., El Hamss H., Belabess Z., Barka E.A. Biological control of plant pathogens: A global perspective. Microorganisms. 2022;10:596. doi: 10.3390/microorganisms10030596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Şesan T.E. Ciuperci cu importanţă practică în combaterea biologică a micozelor plantelor [Microfungi with practical importance in biocontrol of plant mycoses] Red Prop. Tehnol. Agric. 1986;1:56. [Google Scholar]
- 77.Şesan T.E., Enache E., Iacomi B.M., Oprea M., Oancea F., Iacomi C. In vitro antifungal activity of some plant extracts against Fusarium oxysporum in the blackcurrant crop (Ribes Nigrum L.) Acta Sci. Pol. Hortorum. Cultus. 2017;16:163–172. doi: 10.24326/asphc.2017.6.15. [DOI] [Google Scholar]
- 78.Şesan T.E., Oancea A.O., Ştefan L., Mănoiu V.S., Ghiurea M., Răuţ I., Constantinescu-Aruxandei D., Toma A., Savin S., Bira A.F., et al. Effect of foliar treatment with a Trichoderma plant biostimulant consortium on Passiflora caerulea L. yield and quality. Microorganisms. 2020;8:123. doi: 10.3390/microorganisms8010123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Monte E. Understanding Trichoderma: Between biotechnology and microbial ecology. Int. Microbiol. 2001;4:1–4. doi: 10.1007/s101230100001. [DOI] [PubMed] [Google Scholar]
- 80.Feng M., Zhang Y., Coates B.S., Du Q., Gao Y., Li L., Yuan H., Sun W., Chang X., Zhou S., et al. Assessment of Beauveria bassiana for the biological control of corn borer, Ostrinia furnacalis, in sweet maize by irrigation application. BioControl. 2023;68:49–60. doi: 10.1007/s10526-022-10175-1. [DOI] [Google Scholar]
- 81.Javed K., Javed H., Mukhtar T., Qiu D. Efficacy of Beauveria Bassiana and Verticillium Lecanii for the management of whitefly and aphid. Pak. J. Agric. Sci. 2019;56:669–674. [Google Scholar]
- 82.Yuvaraj M., Ramasamy M. Biostimulants in Plant Sciences. IntechOpen; London, UK: 2020. Chapter 7 Role of Fungi in agriculture. [DOI] [Google Scholar]
- 83.Poveda J., Abril-Urias P., Escobar C. Biological control of plant-parasitic nematodes by filamentous fungi inducers of resistance: Trichoderma, mycorrhizal and endophytic fungi. Front. Microbiol. 2020;11:992. doi: 10.3389/fmicb.2020.00992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Șesan T.E., Crișan A. Cercetări de biologie asupra ciupercii Coniothyrium minitans Campbell—Specie hiperparazită nou semnalată în România [Researches on the biology of C. minitans Campbell—Fungal hyperparasitic species newly recorded in Romania] St. Cerc. Biol. Biol. Veget. 1988;40:71–77. [Google Scholar]
- 85.Şesan T.E., Csép N. Investigations on Coniothyrium minitans and Trichoderma spp. to control diseases of industrial crops caused by Sclerotinia sclerotiorum. IOBC Wprs Bull. 1995;18:26–33. [Google Scholar]
- 86.Şesan T.E., Tănase C. Fungi cu Importanţă în Agricultură, Medicină şi Patrimoniu [Fungi with Importance in Agriculture, Medicine and Patrimony. Editura Universitatii din Bucuresti; Bucharest, Romania: 2009. p. 305. [Google Scholar]
- 87.Chang S.T., Hayes W.A.P. Biology and Cultivation Edible Mushrooms. Academic Press; London, UK: 1978. [Google Scholar]
- 88.Amara A.A., El-Baky N.A. Fungi as a source of edible proteins and animal feed. J. Fungi. 2023;9:73. doi: 10.3390/jof9010073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Scott R. Cheesemaking Practice. 2nd ed. Elsevier Applied Science Publishers; London, UK: 1986. [Google Scholar]
- 90.Ropars J., Didiot E., Vega R.C., Bennetot B., Coton M., Poirier E., Coton E., Snirc A., Le Prieur S., Giraud T. Domestication of the emblematic white cheese-making fungus Penicillium camemberti and its diversification into two varieties. Curr. Biol. 2020;30:4441–4453. doi: 10.1016/j.cub.2020.08.082. [DOI] [PubMed] [Google Scholar]
- 91.Ropars J., Cruaud C., Lacoste S., Dupont J. A taxonomic and ecological overview of cheese fungi. Int. J. Food Microbiol. 2012;155:199–210. doi: 10.1016/j.ijfoodmicro.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 92.Pederson C.S. Microbiology of Food Fermentations. AVI Publishing Co. Inc.; Westport, CN, USA: 1971. [Google Scholar]
- 93.Laranjo M., Elias M., Fraqueza M.J. The use of starter cultures in traditional meat products. J. Food Qual. 2017;2017:9546026. doi: 10.1155/2017/9546026. [DOI] [Google Scholar]
- 94.Whittaker J., Johnson R., Finnigan T., Avery S., Dyer P. The biotechnology of Quorn mycoprotein: Past, present and future challenges. In: Nevalainen H., editor. Grand Challenges in Fungal Biotechnology. Springer; Berlin/Heidelberg, Germany: 2020. pp. 59–79. Grand Challenges in Biology and Biotechnology. [Google Scholar]
- 95.Wiebe M.G. QuornTM myco-protein—Overview of a successful fungal product. Mycologist. 2004;18:17–20. doi: 10.1017/S0269915X04001089. [DOI] [Google Scholar]
- 96.Nelson P.E., Desjardins A.E., Plattner R.D. Fumonisins, mycotoxins produced by Fusarium species: Biology, chemistry and significance. Annu. Rev. Phytopathol. 1993;31:233–252. doi: 10.1146/annurev.py.31.090193.001313. [DOI] [PubMed] [Google Scholar]
- 97.Machida M., Yamada O., Gomi K. Genomics of Aspergillus oryzae: Learning from the history of koji mold and exploration of its future. DNA Res. 2018;15:173–183. doi: 10.1093/dnares/dsn020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kitamoto K. Molecular biology of the koji molds. In: Laskin A.I., Bennett J.W., Gadd G.M., editors. Advances in Applied Microbiology. Academic Press; Cambridge, MA, USA: 2002. pp. 129–153. [DOI] [PubMed] [Google Scholar]
- 99.Fournier E., Gladieux P., Giraud T. The ‘Dr Jekyll and Mr Hyde Fungus’: Noble rot versus gray mold symptoms of Botrytis cinerea on grapes. Evol. Appl. 2013;6:960–969. doi: 10.1111/eva.12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dupont J., Dequin S., Giraud T., Le Tacon F., Marsit S., Ropars J., Richard F., Selosse M.A. Fungi as a source of food. Microbiol. Spectr. 2017;5:1063–1085. doi: 10.1128/microbiolspec.FUNK-0030-2016. [DOI] [PubMed] [Google Scholar]
- 101.Antranikian G., Streit W.R. Microorganisms harbor keys to a circular bioeconomy making them useful tools in fighting plastic pollution and rising CO2 levels. Extremophiles. 2022;26:10. doi: 10.1007/s00792-022-01261-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.O’Connor K.E. Microbiology challenges and opportunities in the circular economy. Microbiol. Read. 2021;167:001026. doi: 10.1099/mic.0.001026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Copetti M.V. Fungi as industrial producers of food ingredients. Curr. Opin. Food Sci. 2019;25:52–56. doi: 10.1016/j.cofs.2019.02.006. [DOI] [Google Scholar]
- 104.Mores S., Souza Vandenberghe L., Irineudo Magalhães Júnior A., Carvalho J.C., Mello A.F., Pandey A., Soccol C.R. Citric acid bioproduction and downstream processing: Status, opportunities, and challenges. Bioresour. Technol. 2021;320:124426. doi: 10.1016/j.biortech.2020.124426. [DOI] [PubMed] [Google Scholar]
- 105.Show P.L., Oladele K.O., Siew Q.Y., Aziz Zakry F.A., Lan J.C.-W., Ling T.C. Overview of citric acid production from Aspergillus niger. Front. Life Sci. 2015;8:271–283. doi: 10.1080/21553769.2015.1033653. [DOI] [Google Scholar]
- 106.Behera B.C. Citric acid from Aspergillus niger: A comprehensive overview. Crit. Rev. Microbiol. 2020;46:727–749. doi: 10.1080/1040841X.2020.1828815. [DOI] [PubMed] [Google Scholar]
- 107.Ma C., Gerhard E., Lu D., Yang J. Citrate chemistry and biology for biomaterials design. Biomaterials. 2018;178:383–400. doi: 10.1016/j.biomaterials.2018.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tran R.T., Yang J., Ameer G.A. Citrate-based biomaterials and their applications in regenerative engineering. Annu. Rev. Mater. Res. 2015;45:277–310. doi: 10.1146/annurev-matsci-070214-020815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ciriminna R., Meneguzzo F., Delisi R., Pagliaro M. Citric acid: Emerging applications of key biotechnology industrial product. Chem. Cent. J. 2017;11:22. doi: 10.1186/s13065-017-0251-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nangare S., Vispute Y., Tade R., Dugam S., Patil P. Pharmaceutical applications of citric acid. Future J. Pharm. Sci. 2021;7:54. doi: 10.1186/s43094-021-00203-9. [DOI] [Google Scholar]
- 111.Berovic M., Legisa M. Citric acid production. In: El-Gewely M.R., editor. Biotechnology Annual Review. Volume 13. Elsevier; Amsterdam, The Netherlands: 2007. pp. 303–343. [DOI] [PubMed] [Google Scholar]
- 112.Kareem S.O., Akpan I., Alebiowu O.O. Production of citric acid by Aspergillus niger using pineapple waste. Malays. J. Microbiol. 2010;6:161–165. doi: 10.21161/mjm.19009. [DOI] [Google Scholar]
- 113.Dienye B.N., Ahaotu I., Agwa O.K., Odu N.N. Citric acid production potential of Aspergillus niger using Chrysophyllum albidum peel. Adv. Biosci. Biotechnol. 2018;9:190–203. doi: 10.4236/abb.2018.94013. [DOI] [Google Scholar]
- 114.Kareem S., Rahman R. Utilization of banana peels for citric acid production by Aspergillus niger. Agric. Biol. J. North Am. 2013;4:384–387. doi: 10.5251/abjna.2013.4.4.384.387. [DOI] [Google Scholar]
- 115.De Oliveira P.Z., de Souza Vandenberghe L.P., Rodrigues C., de Melo Pereira G.V., Soccol C.R. Exploring cocoa pod husks as a potential substrate for citric acid production by solid-state fermentation using Aspergillus niger mutant strain. Process. Biochem. 2022;113:107–112. doi: 10.1016/j.procbio.2021.12.020. [DOI] [Google Scholar]
- 116.Ramachandra Y.L., Narayanamurthy G., Jois S., Chavan A., Satwadi P.R. Production of citric acid in basal coffee husk medium by Aspergillus niger under solid state fermentation. Adv. Biol. Res. 2013;7:234–240. [Google Scholar]
- 117.Betiku E., Adesina O.A. Statistical approach to the optimization of citric acid production using filamentous fungus Aspergillus niger grown on sweet potato starch hydrolyzate. Biomass Bioenergy. 2013;55:350–354. doi: 10.1016/j.biombioe.2013.02.034. [DOI] [Google Scholar]
- 118.Ramachandran S., Fontanille P., Pandey A., Larroche C. Gluconic acid: Properties, applications and microbial production. Food Technol. Biotechnol. 2006;44:185–195. [Google Scholar]
- 119.Sharma A.A., Gomashe D.A., Bawane H. Gluconic acid production by Aspergillus niger from banana must. J. Innov. Sci. 2015;2:2394. [Google Scholar]
- 120.Elhousni L., Galai M., ElKamraoui F.Z., Dkhireche N., Touhami M.E., Chebabe D., Sfaira M., Zarrouk A. Study of sodium gluconate and cetyltrimethyl ammonium bromide as inhibitor for copper in moroccan industrial cooling water systems. J. Mater. Environ. Sci. 2016;7:2513–2525. [Google Scholar]
- 121.Rabie A.I., El-Din H.A. Sodium gluconate as a new environmentally friendly iron controlling agent for HP/HT acidizing treatments; Proceedings of the SPE Middle East Oil & Gas Show and Conference; Manama, Bahrain. 8 March 2015; Richardson, TX, USA: OnePetro; 2015. [Google Scholar]
- 122.García-Padilla S., Duarte-Vázquez M.A., Gonzalez-Romero K.E., del Caamaño M.C., Rosado J.L. Effectiveness of intra-articular injections of sodium bicarbonate and calcium gluconate in the treatment of osteoarthritis of the knee: A randomized double-blind clinical trial. BMC Musculoskelet. Disord. 2015;16:114. doi: 10.1186/s12891-015-0568-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Miller H.J., Hu J., Valentine J.K., Gable P.S. Efficacy and tolerability of intravenous ferric gluconate in the treatment of iron deficiency anemia in patients without kidney disease. Arch. Intern. Med. 2007;167:1327–1328. doi: 10.1001/archinte.167.12.1327. [DOI] [PubMed] [Google Scholar]
- 124.Silva R.F., Álvarez M.E., Ríos D.L., López C., Carmona J.U., Rezende C.M. Evaluation of the effect of calcium gluconate and bovine thrombin on the temporal release of transforming growth factor beta 1 and platelet-derived growth factor isoform BB from feline platelet concentrates. BMC Vet. Res. 2012;8:212. doi: 10.1186/1746-6148-8-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ajala E.O., Ajala M.A., Ogunniyi D.S., Sunmonu M.O. Kinetics of gluconic acid production and cell growth in a batch bioreactor by Aspergillus niger using breadfruit hydrolysate. J. Food Process. Eng. 2017;40:e12461. doi: 10.1111/jfpe.12461. [DOI] [Google Scholar]
- 126.Singh O.V., Singh R.P. Bioconversion of grape must into modulated gluconic acid production by Aspergillus niger ORS-4·410. J. Appl. Microbiol. 2006;100:1114–1122. doi: 10.1111/j.1365-2672.2006.02870.x. [DOI] [PubMed] [Google Scholar]
- 127.Ikeda Y., Park E.Y., Okuda N. Bioconversion of waste office paper to gluconic acid in a turbine blade reactor by the filamentous fungus Aspergillus niger. Bioresour. Technol. 2006;97:1030–1035. doi: 10.1016/j.biortech.2005.04.040. [DOI] [PubMed] [Google Scholar]
- 128.Mukhopadhyay R., Chatterjee S., Chatterjee B.P., Banerjee P.C., Guha A.K. Production of gluconic acid from whey by free and immobilized Aspergillus niger. Int. Dairy J. 2005;15:299–303. doi: 10.1016/j.idairyj.2004.07.010. [DOI] [Google Scholar]
- 129.Zhang H., Zhang J., Bao J. High titer gluconic acid fermentation by Aspergillus niger from dry dilute acid pretreated corn stover without detoxification. Bioresour. Technol. 2016;203:211–219. doi: 10.1016/j.biortech.2015.12.042. [DOI] [PubMed] [Google Scholar]
- 130.Katsuya Tooyama T.M. Simultaneous saccharification of corn starch in gluconic acid production by Aspergillus niger immobilized on nonwoven fabric in a pressurized reactor. Microb. Biochem. Technol. 2013;5:1000106. doi: 10.4172/1948-5948.1000106. [DOI] [Google Scholar]
- 131.Sharma A., Vivekanand V., Singh R.P. Solid-state fermentation for gluconic acid production from sugarcane molasses by Aspergillus niger ARNU-4 employing tea waste as the novel solid support. Bioresour. Technol. 2008;99:3444–3450. doi: 10.1016/j.biortech.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 132.Huang J., Huang L., Lin J., Xu Z., Cen P. Organic chemicals from bioprocesses in China. Adv. Biochem. Eng. Biotechnol. 2010;122:43–71. doi: 10.1007/10_2010_75. [DOI] [PubMed] [Google Scholar]
- 133.Hajian H., Yusoff W.M.W. Itaconic acid production by microorganisms: A review. Curr. Res. J. Biol. Sci. 2015;7:37–42. doi: 10.19026/crjbs.7.5205. [DOI] [Google Scholar]
- 134.Teleky B.-E., Vodnar D.C. Recent advances in biotechnological itaconic acid production, and application for a sustainable approach. Polymers. 2021;13:3574. doi: 10.3390/polym13203574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bellasio M., Mattanovich D., Sauer M., Marx H. Organic acids from lignocellulose: Candida lignohabitans as a new microbial cell factory. J. Ind. Microbiol. Biotechnol. 2015;42:681–691. doi: 10.1007/s10295-015-1590-0. [DOI] [PubMed] [Google Scholar]
- 136.Liu Y., Liu G., Zhang J., Balan V., Bao J. Itaconic acid fermentation using activated charcoal-treated corn stover hydrolysate and process evaluation based on aspen plus model. Biomass Conv. Bioref. 2020;10:463–470. doi: 10.1007/s13399-019-00423-3. [DOI] [Google Scholar]
- 137.Pedroso G.B., Montipó S., Mario D.A.N., Alves S.H., Martins A.F. Building block itaconic acid from left-over biomass. Biomass Conv. Bioref. 2017;7:23–35. doi: 10.1007/s13399-016-0210-1. [DOI] [Google Scholar]
- 138.Gnanasekaran R., Dhandapani B., Gopinath K.P., Iyyappan J. Synthesis of itaconic acid from agricultural waste using novel Aspergillus niveus. Prep. Biochem. Biotechnol. 2018;48:605–609. doi: 10.1080/10826068.2018.1476884. [DOI] [PubMed] [Google Scholar]
- 139.Regestein L., Klement T., Grande P., Kreyenschulte D., Heyman B., Maßmann T., Eggert A., Sengpiel R., Wang Y., Wierckx N., et al. From beech wood to itaconic acid: Case study on biorefinery process integration. Biotechnol. Biofuels. 2018;11:279. doi: 10.1186/s13068-018-1273-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Tippkötter N., Duwe A.-M., Wiesen S., Sieker T., Ulber R. Enzymatic hydrolysis of beech wood lignocellulose at high solid contents and its utilization as substrate for the production of biobutanol and dicarboxylic acids. Bioresour. Technol. 2014;167:447–455. doi: 10.1016/j.biortech.2014.06.052. [DOI] [PubMed] [Google Scholar]
- 141.Reddy C.S.K., Singh R.P. Enhanced Production of itaconic acid from corn starch and market refuse fruits by genetically manipulated Aspergillus terreus SKR10. Bioresour. Technol. 2002;85:69–71. doi: 10.1016/S0960-8524(02)00075-5. [DOI] [PubMed] [Google Scholar]
- 142.Castillo Martinez F.A., Balciunas E.M., Salgado J.M., Domínguez González J.M., Converti A., Oliveira R.P. de S. Lactic acid properties, applications and production: A review. Trends Food Sci. Technol. 2013;30:70–83. doi: 10.1016/j.tifs.2012.11.007. [DOI] [Google Scholar]
- 143.Aung S.P.S., Shein H.H.H., Aye K.N., Nwe N. Environment-friendly biopolymers for food packaging: Starch, protein, and poly-lactic acid (PLA) In: Ahmed S., editor. Bio-Based Materials for Food Packaging: Green and Sustainable Advanced Packaging Materials. Springer; Singapore: 2018. pp. 173–195. [Google Scholar]
- 144.Tawakkal I.S.M.A., Cran M.J., Miltz J., Bigger S.W. A review of Poly(Lactic Acid)-based materials for antimicrobial packaging. J. Food Sci. 2014;79:R1477–R1490. doi: 10.1111/1750-3841.12534. [DOI] [PubMed] [Google Scholar]
- 145.Blasi P. Poly(Lactic Acid)/Poly(Lactic-Co-Glycolic Acid)-Based Microparticles: An Overview. J. Pharm. Investig. 2019;49:337–346. doi: 10.1007/s40005-019-00453-z. [DOI] [Google Scholar]
- 146.Zhang Z.Y., Jin B., Kelly J.M. Production of lactic acid from renewable materials by Rhizopus fungi. Biochem. Eng. J. 2007;35:251–263. doi: 10.1016/j.bej.2007.01.028. [DOI] [Google Scholar]
- 147.Huang L.P., Jin B., Lant P., Zhou J. Biotechnological production of lactic acid integrated with potato wastewater treatment by Rhizopus arrhizus. J. Chem. Technol. Biotechnol. 2003;78:899–906. doi: 10.1002/jctb.877. [DOI] [Google Scholar]
- 148.Kumar R., Shivakumar S. Production of L-lactic acid from starch and food waste by amylolytic Rhizopus oryzae MTCC 8784. Int. J. ChemTech Res. 2014;6:527–537. [Google Scholar]
- 149.Ruengruglikit C., Hang Y.D. L(+)-lactic acid production from corncobs by Rhizopus oryzae NRRL-395. LWT Food Sci. Technol. 2003;36:573–575. doi: 10.1016/S0023-6438(03)00062-8. [DOI] [Google Scholar]
- 150.Shahri S.Z., Vahabzadeh F., Mogharei A. Lactic acid production by loofah-immobilized Rhizopus oryzae through one-step fermentation process using starch substrate. Bioprocess Biosyst. Eng. 2020;43:333–345. doi: 10.1007/s00449-019-02231-5. [DOI] [PubMed] [Google Scholar]
- 151.Chen X., Wang X., Xue Y., Zhang T.-A., Li Y., Hu J., Tsang Y.F., Zhang H., Gao M.-T. Influence of rice straw-derived dissolved organic matter on lactic acid fermentation by Rhizopus oryzae. J. Biosci. Bioeng. 2018;125:703–709. doi: 10.1016/j.jbiosc.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 152.Oonkhanond B., Jonglertjunya W., Srimarut N., Bunpachart P., Tantinukul S., Nasongkla N., Sakdaronnarong C. Lactic acid production from sugarcane bagasse by an integrated system of lignocellulose fractionation, saccharification, fermentation, and ex-situ nanofiltration. J. Environ. Chem. Eng. 2017;5:2533–2541. doi: 10.1016/j.jece.2017.05.004. [DOI] [Google Scholar]
- 153.Vially G., Marchal R., Guilbert N. L(+) Lactate Production from Carbohydrates and Lignocellulosic Materials by Rhizopus Oryzae UMIP 4.77. World J. Microbiol. Biotechnol. 2010;26:607–614. doi: 10.1007/s11274-009-0210-4. [DOI] [Google Scholar]
- 154.Ilica R.-A., Kloetzer L., Galaction A.-I., Caşcaval D. Fumaric Acid: Production and Separation. Biotechnol. Lett. 2019;41:47–57. doi: 10.1007/s10529-018-2628-y. [DOI] [PubMed] [Google Scholar]
- 155.Dörsam S., Fesseler J., Gorte O., Hahn T., Zibek S., Syldatk C., Ochsenreither K. Sustainable Carbon Sources for Microbial Organic Acid Production with Filamentous Fungi. Biotechnol. Biofuels. 2017;10:242. doi: 10.1186/s13068-017-0930-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Iyyappan J., Baskar G., Bharathiraja B., Saravanathamizhan R. Malic Acid Production from Biodiesel Derived Crude Glycerol Using Morphologically Controlled Aspergillus niger in Batch Fermentation. Bioresour. Technol. 2018;269:393–399. doi: 10.1016/j.biortech.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 157.Zou X., Zhou Y., Yang S.-T. Production of Polymalic Acid and Malic Acid by Aureobasidium pullulans Fermentation and Acid Hydrolysis. Biotechnol. Bioeng. 2013;110:2105–2113. doi: 10.1002/bit.24876. [DOI] [PubMed] [Google Scholar]
- 158.Das R.K., Brar S.K., Verma M. A fermentative approach towards optimizing directed biosynthesis of fumaric acid by Rhizopus oryzae 1526 utilizing apple industry waste biomass. Fungal Biol. 2015;119:1279–1290. doi: 10.1016/j.funbio.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 159.Fan T., Liu X., Zhao R., Zhang Y., Liu H., Wang Z., Wang F., Nie K., Deng L. Hydrolysis of food waste by hot water extraction and subsequent Rhizopus fermentation to fumaric acid. J. Environ. Manag. 2020;270:110954. doi: 10.1016/j.jenvman.2020.110954. [DOI] [PubMed] [Google Scholar]
- 160.Liu H., Ma J., Wang M., Wang W., Deng L., Nie K., Yue X., Wang F., Tan T. Food waste fermentation to fumaric acid by Rhizopus arrhizus RH7-13. Appl. Biochem. Biotechnol. 2016;180:1524–1533. doi: 10.1007/s12010-016-2184-7. [DOI] [PubMed] [Google Scholar]
- 161.De Souza P.M. Application of microbial -amylase in industry—A review. Braz. J. Microbiol. 2010;41:850–861. doi: 10.1590/S1517-83822010000400004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Saranraj P., Stella D. Fungal Amylase—A Review. Int. J. Microbiol. Res. 2013;4:203–211. [Google Scholar]
- 163.De Souza P.M., Bittencourt M.L.d.A., Caprara C.C., de Freitas M., de Almeida R.P.C., Silveira D., Fonseca Y.M., Ferreira Filho E.X., Pessoa Junior A., Magalhães P.O. A biotechnology perspective of fungal proteases. Braz. J. Microbiol. 2015;46:337–346. doi: 10.1590/S1517-838246220140359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Merheb C.W., Cabral H., Gomes E., Da-Silva R. Partial characterization of protease from a thermophilic fungus, Thermoascus aurantiacus, and its hydrolytic activity on bovine casein. Food Chem. 2007;104:127–131. doi: 10.1016/j.foodchem.2006.11.010. [DOI] [Google Scholar]
- 165.Ghareib M., Fawzi E.M., Aldossary N.A. Thermostable alkaline protease from thermomyces lanuginosus: Optimization, purification and characterization. Ann. Microbiol. 2014;64:859–867. doi: 10.1007/s13213-013-0725-7. [DOI] [Google Scholar]
- 166.Pedrolli D.B., Monteiro A.C., Gomes E., Carmona E.C. Pectin and pectinases: Production, characterization and industrial application of microbial pectinolytic enzymes. Open Biotechnol. J. 2009;3:9–18. doi: 10.2174/1874070700903010009. [DOI] [Google Scholar]
- 167.Katrolia P., Jia H., Yan Q., Song S., Jiang Z., Xu H. Characterization of a protease-resistant α-Galactosidase from the thermophilic fungus Rhizomucor miehei and its application in removal of raffinose family oligosaccharides. Bioresour. Technol. 2012;110:578–586. doi: 10.1016/j.biortech.2012.01.144. [DOI] [PubMed] [Google Scholar]
- 168.Balu V., Muthusamy P., Gopalakrishnan V.K. Screening and optimization of β-galactosidase from fungal strains by using agro residues. World J. Pharm. Pharm. Sci. 2014;3:1809–1821. [Google Scholar]
- 169.Saqib S., Akram A., Halim S.A., Tassaduq R. Sources of β-galactosidase and its applications in food industry. 3 Biotech. 2017;7:79. doi: 10.1007/s13205-017-0645-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Singh R.V., Sambyal K. β-galactosidase as an industrial enzyme: Production and potential. Chem. Pap. 2023;77:11–31. doi: 10.1007/s11696-022-02507-3. [DOI] [Google Scholar]
- 171.Hartl L., Zach S., Seidl-Seiboth V. Fungal chitinases: Diversity, mechanistic properties and biotechnological potential. Appl. Microbiol. Biotechnol. 2012;93:533–543. doi: 10.1007/s00253-011-3723-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.El-Gendi H., Saleh A.K., Badierah R., Redwan E.M., El-Maradny Y.A., El-Fakharany E.M. A comprehensive insight into fungal enzymes: Structure, classification, and their role in mankind’s challenges. J. Fungi. 2022;8:23. doi: 10.3390/jof8010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Mehta A., Bodh U., Gupta R. Fungal lipases: A review. J. Biotech Res. 2017;8:58–77. [Google Scholar]
- 174.Shu Z.-Y., Yan Y.-J., Yang J.-K., Xu L. Aspergillus niger lipase: Gene cloning, over-expression in Escherichia coli and in vitro refolding. Biotechnol. Lett. 2007;29:1875–1879. doi: 10.1007/s10529-007-9470-y. [DOI] [PubMed] [Google Scholar]
- 175.Viswanath B., Rajesh B., Janardhan A., Kumar A.P., Narasimha G. Fungal laccases and their applications in bioremediation. Enzym. Res. 2014;2014:163242. doi: 10.1155/2014/163242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Senthivelan T., Kanagaraj J., Panda R.C. Recent trends in fungal laccase for various industrial applications: An eco-friendly approach—A review. Biotechnol. Bioprocess Eng. 2016;21:19–38. doi: 10.1007/s12257-015-0278-7. [DOI] [Google Scholar]
- 177.Sajith S., Priji P., Sreedevi S., Benjamin S. An overview on fungal cellulases with an industrial perspective. J. Nutr. Food Sci. 2016;6:461. [Google Scholar]
- 178.Payne C.M., Knott B.C., Mayes H.B., Hansson H., Himmel M.E., Sandgren M., Ståhlberg J., Beckham G.T. Fungal cellulases. Chem. Rev. 2015;115:1308–1448. doi: 10.1021/cr500351c. [DOI] [PubMed] [Google Scholar]
- 179.Adeniran A.H., Abiose S.H. Amylolytic potentiality of fungi isolated from some nigerian agricultural wastes. Afr. J. Biotechnol. 2009;8:59910. [Google Scholar]
- 180.Anto H., Trivedi U.B., Patel K.C. Glucoamylase production by solid-state fermentation using rice flake manufacturing waste products as substrate. Bioresour. Technol. 2006;97:1161–1166. doi: 10.1016/j.biortech.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 181.Baeyens J., Kang Q., Appels L., Dewil R., Lv Y., Tan T. Challenges and opportunities in improving the production of bio-ethanol. Prog. Energy Combust. Sci. 2015;47:60–88. doi: 10.1016/j.pecs.2014.10.003. [DOI] [Google Scholar]
- 182.Farooq M.A., Ali S., Hassan A., Tahir H.M., Mumtaz S., Mumtaz S. Biosynthesis and industrial applications of α-amylase: A review. Arch. Microbiol. 2021;203:1281–1292. doi: 10.1007/s00203-020-02128-y. [DOI] [PubMed] [Google Scholar]
- 183.Ghorai S., Banik S.P., Verma D., Chowdhury S., Mukherjee S., Khowala S. Fungal biotechnology in food and feed processing. Food Res. Int. 2009;42:577–587. doi: 10.1016/j.foodres.2009.02.019. [DOI] [Google Scholar]
- 184.Rana N., Walia A., Gaur A. α-amylases from microbial sources and its potential applications in various industries. Natl. Acad. Sci. Lett. 2013;36:9–17. doi: 10.1007/s40009-012-0104-0. [DOI] [Google Scholar]
- 185.Sakthi S.S., Kanchana D., Saranraj P., Usharani G. Evaluation of amylase activity of the amylolytic fungi Aspergillus niger using cassava as substrate. Int. J. Appl. Microbiol. Sci. 2012;1:24–34. [Google Scholar]
- 186.Singh S., Singh S., Bali V., Sharma L., Mangla J. Production of fungal amylases using cheap, readily available agriresidues, for potential application in textile industry. BioMed Res. Int. 2014;2014:e215748. doi: 10.1155/2014/215748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Bah C.S.F., Bekhit A.E.-D.A., McConnell M.A., Carne A. Generation of bioactive peptide hydrolysates from cattle plasma using plant and fungal proteases. Food Chem. 2016;213:98–107. doi: 10.1016/j.foodchem.2016.06.065. [DOI] [PubMed] [Google Scholar]
- 188.Brandelli A., Daroit D.J., Riffel A. Biochemical features of microbial keratinases and their production and applications. Appl. Microbiol. Biotechnol. 2010;85:1735–1750. doi: 10.1007/s00253-009-2398-5. [DOI] [PubMed] [Google Scholar]
- 189.Chutmanop J., Chuichulcherm S., Chisti Y., Srinophakun P. Protease production by Aspergillus oryzae in solid-state fermentation using agroindustrial substrates. J. Chem. Technol. Biotechnol. 2008;83:1012–1018. doi: 10.1002/jctb.1907. [DOI] [Google Scholar]
- 190.Ha M., Bekhit A.E.-D., Carne A., Hopkins D.L. Comparison of the proteolytic activities of new commercially available bacterial and fungal proteases toward meat proteins. J. Food Sci. 2013;78:C170–C177. doi: 10.1111/1750-3841.12027. [DOI] [PubMed] [Google Scholar]
- 191.Kranthi V.S., Rao D.M., Jaganmohan P. Production of protease by Aspergillus flavus through solid state fermentation using different oil seed cakes. Int. J. Microbiol. Res. 2012;3:12–15. [Google Scholar]
- 192.Kumar S., Sharma N.S., Saharan M.R., Singh R. Extracellular acid protease from Rhizopus oryzae: Purification and characterization. Process. Biochem. 2005;40:1701–1705. doi: 10.1016/j.procbio.2004.06.047. [DOI] [Google Scholar]
- 193.Novelli P.K., Barros M.M., Fleuri L.F. Novel inexpensive fungi proteases: Production by solid state fermentation and characterization. Food Chem. 2016;198:119–124. doi: 10.1016/j.foodchem.2015.11.089. [DOI] [PubMed] [Google Scholar]
- 194.Savitha S., Sadhasivam S., Swaminathan K., Lin F.H. Fungal protease: Production, purification and compatibility with laundry detergents and their wash performance. J. Taiwan Inst. Chem. Eng. 2011;42:298–304. doi: 10.1016/j.jtice.2010.05.012. [DOI] [Google Scholar]
- 195.Zanutto-Elgui M.R., Vieira J.C.S., do Prado D.Z., Buzalaf M.A.R., Padilha P.d.M., Elgui de Oliveira D., Fleuri L.F. Production of milk peptides with antimicrobial and antioxidant properties through fungal proteases. Food Chem. 2019;278:823–831. doi: 10.1016/j.foodchem.2018.11.119. [DOI] [PubMed] [Google Scholar]
- 196.Anand G., Yadav S., Yadav D. Production, purification and biochemical characterization of an exo-polygalacturonase from Aspergillus niger MTCC 478 suitable for clarification of orange juice. 3 Biotech. 2017;7:122. doi: 10.1007/s13205-017-0760-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Demir H., Tarı C. Valorization of wheat bran for the production of polygalacturonase in SSF of Aspergillus sojae. Ind. Crops Prod. 2014;54:302–309. doi: 10.1016/j.indcrop.2014.01.025. [DOI] [Google Scholar]
- 198.Nikolić M.V., Mojovic L. Hydrolysis of apple pectin by the coordinated activity of pectic enzymes. Food Chem. 2007;101:1580–1584. doi: 10.1016/j.foodchem.2005.12.053. [DOI] [Google Scholar]
- 199.Patidar M.K., Nighojkar S., Kumar A., Nighojkar A. Pectinolytic enzymes-solid state fermentation, assay methods and applications in fruit juice industries: A review. 3 Biotech. 2018;8:199. doi: 10.1007/s13205-018-1220-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Sharma N., Rathore M., Sharma M. Microbial pectinase: Sources, characterization and applications. Rev. Environ. Sci. Biotechnol. 2013;12:45–60. doi: 10.1007/s11157-012-9276-9. [DOI] [Google Scholar]
- 201.Ortiz G.E., Ponce-Mora M.C., Noseda D.G., Cazabat G., Saravalli C., López M.C., Gil G.P., Blasco M., Albertó E.O. Pectinase production by Aspergillus giganteus in solid-state fermentation: Optimization, scale-up, biochemical characterization and its application in olive-oil extraction. J. Ind. Microbiol. Biotechnol. 2017;44:197–211. doi: 10.1007/s10295-016-1873-0. [DOI] [PubMed] [Google Scholar]
- 202.Tai E.-S., Hsieh P.-C., Sheu S.-C. Effect of polygalacturonase and feruloyl esterase from Aspergillus tubingensis on demucilage and quality of coffee beans. Process. Biochem. 2014;49:1274–1280. doi: 10.1016/j.procbio.2014.05.001. [DOI] [Google Scholar]
- 203.Barman S., Sit N., Badwaik L.S., Deka S.C. Pectinase Production by Aspergillus niger using banana (Musa balbisiana) peel as substrate and its effect on clarification of banana juice. J. Food Sci. Technol. 2015;52:3579–3589. doi: 10.1007/s13197-014-1413-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Sethi B.K., Nanda P.K., Sahoo S. Enhanced production of pectinase by Aspergillus terreus NCFT 4269.10 Using Banana Peels as Substrate. 3 Biotech. 2016;6:36. doi: 10.1007/s13205-015-0353-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Geng X., Tian G., Zhao Y., Zhao L., Wang H., Ng T.B. A fungal α-galactosidase from Tricholoma matsutake with broad substrate specificity and good hydrolytic activity on raffinose family oligosaccharides. Molecules. 2015;20:13550–13562. doi: 10.3390/molecules200813550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Bhatia S., Singh A., Batra N., Singh J. Microbial production and biotechnological applications of α-galactosidase. Int. J. Biol. Macromol. 2020;150:1294–1313. doi: 10.1016/j.ijbiomac.2019.10.140. [DOI] [PubMed] [Google Scholar]
- 207.Martarello R.D., Cunha L., Cardoso S.L., de Freitas M.M., Silveira D., Fonseca-Bazzo Y.M., Homem-de-Mello M., Filho E.X.F., Magalhães P.O. Optimization and partial purification of beta-galactosidase production by Aspergillus niger isolated from brazilian soils using soybean residue. AMB Express. 2019;9:81. doi: 10.1186/s13568-019-0805-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Kazemi S., Khayati G., Faezi-Ghasemi M. β-galactosidase production by Aspergillus niger ATCC 9142 using inexpensive substrates in solid-state fermentation: Optimization by orthogonal arrays design. Iran. Biomed. J. 2016;20:287–294. doi: 10.22045/ibj.2016.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Silvério S.C., Macedo E.A., Teixeira J.A., Rodrigues L.R. New β-galactosidase producers with potential for prebiotic synthesis. Bioresour. Technol. 2018;250:131–139. doi: 10.1016/j.biortech.2017.11.045. [DOI] [PubMed] [Google Scholar]
- 210.Dario Rafael O.-H., Luis Fernándo Z.-G., Abraham P.-T., Pedro Alberto V.-L., Guadalupe G.-S., Pablo P.J. Production of chitosan-oligosaccharides by the chitin-hydrolytic system of Trichoderma harzianum and their antimicrobial and anticancer effects. Carbohydr. Res. 2019;486:107836. doi: 10.1016/j.carres.2019.107836. [DOI] [PubMed] [Google Scholar]
- 211.Abu-Tahon M.A., Isaac G.S. Anticancer and antifungal efficiencies of purified chitinase produced from Trichoderma viride under submerged fermentation. J. Gen. Appl. Microbiol. 2020;66:32–40. doi: 10.2323/jgam.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 212.Zhang M., Puri A.K., Govender A., Wang Z., Singh S., Permaul K. The multi-chitinolytic enzyme system of the compost-dwelling thermophilic fungus Thermomyces lanuginosus. Process. Biochem. 2015;50:237–244. doi: 10.1016/j.procbio.2014.11.008. [DOI] [Google Scholar]
- 213.Nampoothiri K.M., Baiju T.V., Sandhya C., Sabu A., Szakacs G., Pandey A. Process optimization for antifungal chitinase production by Trichoderma harzianum. Process. Biochem. 2004;39:1583–1590. doi: 10.1016/S0032-9592(03)00282-6. [DOI] [Google Scholar]
- 214.Sudhakar P., Nagarajan P. Production of chitinase by solid state fermentation from rice bran. Int. J. Environ. Sci. Dev. 2010;1:435–440. doi: 10.7763/IJESD.2010.V1.84. [DOI] [Google Scholar]
- 215.Marchut-Mikolajczyk O., Kwapisz E., Wieczorek D., Antczak T. Biodegradation of diesel oil hydrocarbons enhanced with Mucor circinelloides enzyme preparation. Int. Biodeterior. Biodegrad. 2015;104:142–148. doi: 10.1016/j.ibiod.2015.05.008. [DOI] [Google Scholar]
- 216.Wang X., Lu D., Jönsson L.J., Hong F. Preparation of a PET-hydrolyzing lipase from Aspergillus oryzae by the addition of Bis(2-hydroxyethyl) terephthalate to the culture medium and enzymatic modification of PET fabrics. Eng. Life Sci. 2008;8:268–276. doi: 10.1002/elsc.200700058. [DOI] [Google Scholar]
- 217.Romdhane I.B.-B., Fendri A., Gargouri Y., Gargouri A., Belghith H. A novel thermoactive and alkaline lipase from Talaromyces thermophilus fungus for use in laundry detergents. Biochem. Eng. J. 2010;53:112–120. doi: 10.1016/j.bej.2010.10.002. [DOI] [Google Scholar]
- 218.Colla L.M., Primaz A.L., Benedetti S., Loss R.A., de Lima M., Reinehr C.O., Bertolin T.E., Costa J.A.V. Surface response methodology for the optimization of lipase production under submerged fermentation by filamentous fungi. Braz. J. Microbiol. 2016;47:461–467. doi: 10.1016/j.bjm.2016.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Aguieiras E.C.G., Cavalcanti-Oliveira E.D., Freire D.M.G. Current status and new developments of biodiesel production using fungal lipases. Fuel. 2015;159:52–67. doi: 10.1016/j.fuel.2015.06.064. [DOI] [Google Scholar]
- 220.Lima V.M.G., Krieger N., Sarquis M.I.M., Mitchell D.A., Ramos L.P., Fontana J.D. Effect of nitrogen and carbon sources on lipase production by Penicillium aurantiogriseum. Food Technol. Biotechnol. 2003;41:105–110. [Google Scholar]
- 221.Gombert A.K., Pinto A.L., Castilho L.R., Freire D.M.G. Lipase production by Penicillium restrictum in solid-state fermentation using babassu oil cake as substrate. Process. Biochem. 1999;35:85–90. doi: 10.1016/S0032-9592(99)00036-9. [DOI] [Google Scholar]
- 222.Gutarra M.L.E., Godoy M.G., Castilho L.R., Freire D.M.G. Inoculum strategies for Penicillium simplicissimum lipase production by solid-state fermentation using a residue from the babassu oil industry. J. Chem. Technol. Biotechnol. 2007;82:313–318. doi: 10.1002/jctb.1674. [DOI] [Google Scholar]
- 223.Ruela H.S., Sutili F.K., Leal I.C.R., Carvalho N.M.F., Miranda L.S.M., de Souza R.O.M.A. Lipase-catalyzed synthesis of secondary glucose esters under continuous flow conditions. Eur. J. Lipid Sci. Technol. 2013;115:464–467. doi: 10.1002/ejlt.201200321. [DOI] [Google Scholar]
- 224.Sethi B.K., Nanda P.K., Sahoo S. Characterization of biotechnologically relevant extracellular lipase produced by Aspergillus Terreus NCFT 4269.10. Braz. J. Microbiol. 2016;47:143–149. doi: 10.1016/j.bjm.2015.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Wang H., Kaur G., Pensupa N., Uisan K., Du C., Yang X., Lin C.S.K. Textile waste valorization using submerged filamentous fungal fermentation. Process. Saf. Environ. Prot. 2018;118:143–151. doi: 10.1016/j.psep.2018.06.038. [DOI] [Google Scholar]
- 226.Hu Y., Du C., Leu S.-Y., Jing H., Li X., Lin C.S.K. Valorisation of textile waste by fungal solid state fermentation: An example of circular waste-based biorefinery. Resour. Conserv. Recycl. 2018;129:27–35. doi: 10.1016/j.resconrec.2017.09.024. [DOI] [Google Scholar]
- 227.Chen Y., Wan J., Zhang X., Ma Y., Wang Y. Effect of beating on recycled properties of unbleached eucalyptus cellulose fiber. Carbohydr. Polym. 2012;87:730–736. doi: 10.1016/j.carbpol.2011.08.051. [DOI] [PubMed] [Google Scholar]
- 228.Kuhad R.C., Gupta R., Singh A. Microbial cellulases and their industrial applications. Enzym. Res. 2011;2011:280696. doi: 10.4061/2011/280696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Mrudula S., Murugammal R. Production of cellulase by Aspergillus niger under submerged and solid state fermentation using coir waste as a substrate. Braz. J. Microbiol. 2011;42:1119–1127. doi: 10.1590/S1517-83822011000300033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Yasmin S., Mattoo R.L., Nehvi F.A. Isolation, characterization and molecular weight determination of cellulase from Trichoderma viride. Afr. J. Biotechnol. 2013;12:4503–4511. doi: 10.5897/AJB2013.12275. [DOI] [Google Scholar]
- 231.Garcia L.F., Lacerda M.F.A.R., Thomaz D.V., de Souza Golveia J.C., das Pereira M.G.C., de Souza Gil E., Schimidt F., Santiago M.F. Optimization of laccase-alginate-chitosan-based matrix toward 17 α-ethinylestradiol removal. Prep. Biochem. Biotechnol. 2019;49:375–383. doi: 10.1080/10826068.2019.1573195. [DOI] [PubMed] [Google Scholar]
- 232.Garcia-Ubasart J., Esteban A., Vila C., Roncero M.B., Colom J.F., Vidal T. Enzymatic treatments of pulp using laccase and hydrophobic compounds. Bioresour. Technol. 2011;102:2799–2803. doi: 10.1016/j.biortech.2010.10.020. [DOI] [PubMed] [Google Scholar]
- 233.Vantamuri A.B., Kaliwal B.B. Purification and characterization of laccase from Marasmius species BBKAV79 and effective decolorization of selected textile dyes. 3 Biotech. 2016;6:189. doi: 10.1007/s13205-016-0504-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Xu L., Sun K., Wang F., Zhao L., Hu J., Ma H., Ding Z. Laccase production by Trametes versicolor in solid-state fermentation using tea residues as substrate and its application in dye decolorization. J. Environ. Manag. 2020;270:110904. doi: 10.1016/j.jenvman.2020.110904. [DOI] [PubMed] [Google Scholar]
- 235.Renzetti S., Courtin C.M., Delcour J.A., Arendt E.K. Oxidative and proteolytic enzyme preparations as promising improvers for oat bread formulations: Rheological, biochemical and microstructural background. Food Chem. 2010;119:1465–1473. doi: 10.1016/j.foodchem.2009.09.028. [DOI] [Google Scholar]
- 236.De Peixoto-Nogueira S.C., Betini J.H.A., Michelin M., de Carvalho C.C., Lucca A.L., Vici A.C., de Jorge J.A.M. Laccase production by Aspergillus niveus on SSF using wheat bran as alternative carbon source and its synergistic effect on pulp biobleaching using a mix of laccase/xylanase from the same microorganism. J. Biochem. Technol. 2015;6:929–937. [Google Scholar]
- 237.Omeje K.O., Nnolim N.E., Ezema B.O., Ozioko J.N., Eze S.O.O. Synthetic dyes decolorization potential of agroindustrial waste-derived thermo-active laccase from Aspergillus species. Biocatal. Agric. Biotechnol. 2020;29:101800. doi: 10.1016/j.bcab.2020.101800. [DOI] [Google Scholar]
- 238.Singh Arora D., Kumar Sharma R. Ligninolytic fungal laccases and their biotechnological applications. Appl. Biochem. Biotechnol. 2010;160:1760–1788. doi: 10.1007/s12010-009-8676-y. [DOI] [PubMed] [Google Scholar]
- 239.Falade A.O., Nwodo U.U., Iweriebor B.C., Green E., Mabinya L.V., Okoh A.I. Lignin peroxidase functionalities and prospective applications. MicrobiologyOpen. 2017;6:e00394. doi: 10.1002/mbo3.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Bonugli-Santos R.C., Durrant L.R., Da Silva M., Sette L.D. Production of laccase, manganese peroxidase and lignin peroxidase by brazilian marine-derived fungi. Enzym. Microb. Technol. 2010;46:32–37. doi: 10.1016/j.enzmictec.2009.07.014. [DOI] [Google Scholar]
- 241.Pamidipati S., Ahmed A. Degradation of lignin in agricultural residues by locally isolated fungus Neurospora discreta. Appl. Biochem. Biotechnol. 2017;181:1561–1572. doi: 10.1007/s12010-016-2302-6. [DOI] [PubMed] [Google Scholar]
- 242.Akilandeswari P., Pradeep B.V. Exploration of industrially important pigments from soil fungi. Appl. Microbiol. Biotechnol. 2016;100:1631–1643. doi: 10.1007/s00253-015-7231-8. [DOI] [PubMed] [Google Scholar]
- 243.Lopes F.C., Tichota D.M., Pereira J.Q., Segalin J., de Oliveira Rios A., Brandelli A. Pigment production by filamentous fungi on agro-industrial byproducts: An eco-friendly alternative. Appl. Biochem. Biotechnol. 2013;171:616–625. doi: 10.1007/s12010-013-0392-y. [DOI] [PubMed] [Google Scholar]
- 244.Räisänen R. Fungal colorants in applications—Focus on Cortinarius Species. Color. Technol. 2019;135:22–31. doi: 10.1111/cote.12376. [DOI] [Google Scholar]
- 245.Meruvu H., dos Santos J.C. Colors of life: A review on fungal pigments. Crit. Rev. Biotechnol. 2021;41:1153–1177. doi: 10.1080/07388551.2021.1901647. [DOI] [PubMed] [Google Scholar]
- 246.Chen W., Chen R., Liu Q., He Y., He K., Ding X., Kang L., Guo X., Xie N., Zhou Y., et al. Orange, red, yellow: Biosynthesis of azaphilone pigments in Monascus fungi. Chem. Sci. 2017;8:4917–4925. doi: 10.1039/C7SC00475C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Rapoport A., Guzhova I., Bernetti L., Buzzini P., Kieliszek M., Kot A.M. Carotenoids and some other pigments from fungi and yeasts. Metabolites. 2021;11:92. doi: 10.3390/metabo11020092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Nanou K., Roukas T. Waste cooking oil: A new substrate for carotene production by Blakeslea trispora in submerged fermentation. Bioresour. Technol. 2016;203:198–203. doi: 10.1016/j.biortech.2015.12.053. [DOI] [PubMed] [Google Scholar]
- 249.Roukas T., Varzakakou M., Kotzekidou P. From cheese whey to carotenes by Blakeslea trispora in a bubble column reactor. Appl. Biochem. Biotechnol. 2015;175:182–193. doi: 10.1007/s12010-014-1260-0. [DOI] [PubMed] [Google Scholar]
- 250.Klempová T., Slaný O., Šišmiš M., Marcinčák S., Čertík M. Dual production of polyunsaturated fatty acids and beta-carotene with Mucor wosnessenskii by the process of solid-state fermentation using agro-industrial waste. J. Biotechnol. 2020;311:1–11. doi: 10.1016/j.jbiotec.2020.02.006. [DOI] [PubMed] [Google Scholar]
- 251.Bayram S. A comparative characterization study between fungal and bacterial eumelanin pigments. Indian J. Microbiol. 2022;62:393–400. doi: 10.1007/s12088-022-01012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Liu R., Meng X., Mo C., Wei X., Ma A. Melanin of Fungi: From Classification to Application. World J. Microbiol. Biotechnol. 2022;38:228. doi: 10.1007/s11274-022-03415-0. [DOI] [PubMed] [Google Scholar]
- 253.Ghosh S., Rusyn I., Dmytruk O.V., Onyeaka H., Gryzenhout M., Gafforov Y. Filamentous fungi for sustainable remediation of pharmaceutical compounds, heavy metal and oil hydrocarbons. Front. Bioeng. Biotechnol. 2023;11:1106973. doi: 10.3389/fbioe.2023.1106973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Dusengemungu L., Kasali G., Gwanama C., Ouma K.O. Recent advances in biosorption of copper and Cobalt by filamentous fungi. Front. Microbiol. 2020;11:582016. doi: 10.3389/fmicb.2020.582016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Asemoloye M.D., Tosi S., Daccò C., Wang X., Xu S., Marchisio M.A., Gao W., Jonathan S.G., Pecoraro L. Hydrocarbon degradation and enzyme activities of Aspergillus oryzae and Mucor irregularis isolated from nigerian crude oil-polluted sites. Microorganisms. 2020;8:1912. doi: 10.3390/microorganisms8121912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Milala M., Shugaba A., Gidado A., Ene A.C., Wafar J.A. Studies on the use of agricultural wastes for cellulase enzyme production by Aspegillus niger. Res. J. Agric. Biol. Sci. 2005;1:325–328. [Google Scholar]
- 257.Zheng W., Zheng Q., Xue Y., Hu J., Gao M.T. Influence of rice straw polyphenols on cellulase production by Trichoderma Reesei. J. Biosci. Bioeng. 2017;123:731–738. doi: 10.1016/j.jbiosc.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 258.Vasić K., Knez Ž., Leitgeb M. Bioethanol production by enzymatic hydrolysis from different lignocellulosic sources. Molecules. 2021;26:753. doi: 10.3390/molecules26030753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Mnkandla M., Otomo P. Effectiveness of mycofiltration for removal of contaminants from water: A systematic review protocol. Environ. Evid. 2021;10:17. doi: 10.1186/s13750-021-00232-0. [DOI] [Google Scholar]
- 260.Taylor A., Flatt A., Beutel M.W., Wolff M., Brownson K., Stamets P. Removal of Escherichia coli from synthetic stormwater using mycofiltration. Ecol. Eng. 2015;78:79–86. doi: 10.1016/j.ecoleng.2014.05.016. [DOI] [Google Scholar]
- 261.Van Der Aa Kühle A., Jespersen L. The taxonomic position of Saccharomyces boulardii as evaluated by sequence analysis of the D1/D2 domain of 26S RDNA, the ITS1-5.8S RDNA-ITS2 region and the mitochondrial cytochrome-c oxidase II gene. Syst. Appl. Microbiol. 2003;26:564–571. doi: 10.1078/072320203770865873. [DOI] [PubMed] [Google Scholar]
- 262.Aramayo R., Selker E.U. Neurospora crassa, a model system for epigenetics research. Cold Spring Harb. Perspect. Biol. 2013;5:a017921. doi: 10.1101/cshperspect.a017921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Abd-Elsalam K.A. Special issue: Fungal nanotechnology. J. Fungi. 2021;7:583. doi: 10.3390/jof7080583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Lane M.M., Morrissey J.P. Kluyveromyces marxianus: A yeast emerging from its sister’s shadow. Fungal Biol. Rev. 2010;24:17–26. doi: 10.1016/j.fbr.2010.01.001. [DOI] [Google Scholar]
- 265.Bilal M., Ji L., Xu Y., Xu S., Lin Y., Iqbal H.M.N., Cheng H. Bioprospecting Kluyveromyces marxianus as a robust host for industrial biotechnology. Front. Bioeng. Biotechnol. 2022;10:851768. doi: 10.3389/fbioe.2022.851768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Coelho M.A.Z., Amaral P.F.F., Belo I. Yarrowia lipolytica: An Industrial Workhorse. Formatex Research Center; Badajoz, Spain: 2010. [Google Scholar]
- 267.Park Y.-K., Ledesma-Amaro R. What makes Yarrowia lipolytica well suited for industry? Trends Biotechnol. 2023;41:242–254. doi: 10.1016/j.tibtech.2022.07.006. [DOI] [PubMed] [Google Scholar]
- 268.Barone G.D., Emmerstorfer-Augustin A., Biundo A., Pisano I., Coccetti P., Mapelli V., Camattari A. Industrial production of proteins with Pichia pastoris (Komagataella phaffii) Biomolecules. 2023;13:441. doi: 10.3390/biom13030441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Nazarov P.A., Baleev D.N., Ivanova M.I., Sokolova L.M., Karakozova M.V. Infectious plant diseases: Etiology, current status, problems and prospects in plant protection. Acta Nat. 2020;12:46–59. doi: 10.32607/actanaturae.11026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Wang X., Jiang N., Liu J., Liu W., Wang G.-L. The role of effectors and host immunity in plant-necrotrophic fungal interactions. Virulence. 2014;5:722–732. doi: 10.4161/viru.29798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Zeilinger S., Gupta V.K., Dahms T.E.S., Silva R.N., Singh H.B., Upadhyay R.S., Gomes E.V., Tsui C.K.-M., Nayak S.C. Friends or foes? emerging insights from fungal interactions with plants. FEMS Microbiol. Rev. 2016;40:182–207. doi: 10.1093/femsre/fuv045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Şesan T.E., Enache E., Iacomi B.M., Oprea M., Oancea F., Iacomi C. Antifungal activity of some plant extracts against Botrytis cinerea Pers. in the blackcurrant crop (Ribes nigrum L.) Acta Sci. Pol. Hortorum Cultus. 2015;14:29–43. [Google Scholar]
- 273.Doehlemann G., Ökmen B., Zhu W., Sharon A. Plant pathogenic fungi. Microbiol. Spectr. 2017;5:703–726. doi: 10.1128/microbiolspec.FUNK-0023-2016. [DOI] [PubMed] [Google Scholar]
- 274.Hassan E.A., Mostafa Y.S., Alamri S., Hashem M., Nafady N.A. Biosafe management of Botrytis grey mold of strawberry fruit by novel bioagents. Plants. 2021;10:2737. doi: 10.3390/plants10122737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Bi K., Liang Y., Mengiste T., Sharon A. Killing softly: A roadmap of Botrytis cinerea pathogenicity. Trends Plant. Sci. 2023;28:211–222. doi: 10.1016/j.tplants.2022.08.024. [DOI] [PubMed] [Google Scholar]
- 276.Zhao T., Pei T., Jiang J., Yang H., Zhang H., Li J., Xu X. Understanding the mechanisms of resistance to tomato leaf mold: A review. Hortic. Plant. J. 2022;8:667–675. doi: 10.1016/j.hpj.2022.04.008. [DOI] [Google Scholar]
- 277.Skamnioti P., Gurr S.J. Against the grain: Safeguarding rice from rice blast disease. Trends Biotechnol. 2009;27:141–150. doi: 10.1016/j.tibtech.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 278.Doehlemann G., Wahl R., Vranes M., de Vries R.P., Kämper J., Kahmann R. Establishment of compatibility in the Ustilago maydis/maize pathosystem. J. Plant. Physiol. 2008;165:29–40. doi: 10.1016/j.jplph.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 279.Ponomarenko A., Goodwin S.B., Kema G.H.J. Septoria Tritici Blotch (STB) Wheat. APSnet; St. Paul, MN, USA: 2011. Septoria tritici blotch (STB) of wheat. [Google Scholar]
- 280.Orton E.S., Deller S., Brown J.K.M. Mycosphaerella graminicola: From genomics to disease control. Mol. Plant Pathol. 2011;12:413–424. doi: 10.1111/j.1364-3703.2010.00688.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Șesan T.E., Enache E., Iacomi B.M., Oprea M., Oancea F., Iacomi C. Antifungal activity of some plant extracts against Alternaria alternata (Fr.) Keissl. in the blackcurrant crop (Ribes nigrum L.) Acta Sci. Pol. Hortorum. Cultus. 2016;15:57–68. [Google Scholar]
- 282.Pryor B.M., Michailides T.J. Morphological, pathogenic, and molecular characterization of Alternaria isolates associated with Alternaria late blight of pistachio. Phytopathology. 2002;92:406–416. doi: 10.1094/PHYTO.2002.92.4.406. [DOI] [PubMed] [Google Scholar]
- 283.Halaby T., Boots H., Vermeulen A., van der Ven A., Beguin H., van Hooff H., Jacobs J. Phaeohyphomycosis caused by Alternaria infectoria in a renal transplant recipient. J. Clin. Microbiol. 2001;39:1952–1955. doi: 10.1128/JCM.39.5.1952-1955.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Sandoval-Denis M., Sutton D.A., Martin-Vicente A., Cano-Lira J.F., Wiederhold N., Guarro J., Gené J. Cladosporium species recovered from clinical samples in the United States. J. Clin. Microbiol. 2015;53:2990–3000. doi: 10.1128/JCM.01482-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Wang W.-Y., Luo H.-B., Hu J.-Q., Hong H.-H. Pulmonary Cladosporium infection coexisting with subcutaneous Corynespora cassiicola infection in a patient: A case report. World J. Clin. Cases. 2022;10:3490–3495. doi: 10.12998/wjcc.v10.i11.3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Solomon P.S., Oliver R.P. The nitrogen content of the tomato leaf apoplast increases during infection by Cladosporium fulvum. Planta. 2001;213:241–249. doi: 10.1007/s004250000500. [DOI] [PubMed] [Google Scholar]
- 287.Kim J.-Y. Human fungal pathogens: Why should we learn? J. Microbiol. 2016;54:145–148. doi: 10.1007/s12275-016-0647-8. [DOI] [PubMed] [Google Scholar]
- 288.Ijaz N., Tanveer W., Qureshi A. Review on common fungal diseases of humans. Pak. Res. J. Biol. Sci. 2021;1:14–22. [Google Scholar]
- 289.Robert V.A., Casadevall A. Vertebrate endothermy restricts most fungi as potential pathogens. J. Infect. Dis. 2009;200:1623–1626. doi: 10.1086/644642. [DOI] [PubMed] [Google Scholar]
- 290.Berker D. Fungal nail disease. N. Engl. J. Med. 2009;60:2108–2116. doi: 10.1056/NEJMcp0804878. [DOI] [PubMed] [Google Scholar]
- 291.Brakhage A. Systemic fungal infections caused by Aspergillus species: Epidemiology, infection process and virulence determinants. Curr. Drug Targets. 2005;6:875–886. doi: 10.2174/138945005774912717. [DOI] [PubMed] [Google Scholar]
- 292.Sherman R.G., Prusinski L., Ravenel M.C., Joralmon R.A. Oral candidiasis. Quintessence Int. 2002;33:455–459. [PubMed] [Google Scholar]
- 293.Ibrahim A.S., Edwards J.E., Jr., Bryant R., Spellberg B. Economic burden of mucormycosis in the United States: Can a vaccine be cost-effective? Med. Mycol. 2009;47:592–600. doi: 10.1080/13693780802326001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Dey D.K., Kang J.I., Bajpai V.K., Kim K., Lee H., Sonwal S., Simal-Gandara J., Xiao J., Ali S., Huh Y.S., et al. Mycotoxins in food and feed: Toxicity, preventive challenges, and advanced detection techniques for associated diseases. Crit. Rev. Food Sci. Nutr. 2022;62:1–22. doi: 10.1080/10408398.2022.2059650. [DOI] [PubMed] [Google Scholar]
- 295.Balendres M.A.O., Karlovsky P., Cumagun C.J.R. Mycotoxigenic fungi and mycotoxins in agricultural crop commodities in the Philippines: A review. Foods. 2019;8:249. doi: 10.3390/foods8070249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Daou R., Joubrane K., Maroun R.G., Khabbaz L.R., Ismail A., Khoury A.E., Daou R., Joubrane K., Maroun R.G., Khabbaz L.R., et al. Mycotoxins: Factors influencing production and control strategies. AIMS Agric. Food. 2021;6:416–447. doi: 10.3934/agrfood.2021025. [DOI] [Google Scholar]
- 297.Elkenany R.M., Awad A. Types of mycotoxins and different approaches used for their detection in foodstuffs. Mansoura Vet. Med. J. 2021;22:25–32. doi: 10.21608/mvmj.2021.161191. [DOI] [Google Scholar]
- 298.Tola M., Kebede B. Occurrence, importance and control of mycotoxins: A review. Cogent Food Agric. 2016;2:1191103. doi: 10.1080/23311932.2016.1191103. [DOI] [Google Scholar]
- 299.Tabuc C., Marin D., Guerre P., Sesan T., Bailly J.D. Molds and mycotoxin content of cereals in southeastern Romania. J. Food Prot. 2009;72:662–665. doi: 10.4315/0362-028X-72.3.662. [DOI] [PubMed] [Google Scholar]
- 300.Liao J., He Z., Xia Y., Lei Y., Liao B. A review on biosynthesis and genetic regulation of aflatoxin production by major Aspergillus fungi. Oil Crop. Sci. 2020;5:166–173. doi: 10.1016/j.ocsci.2020.11.001. [DOI] [Google Scholar]
- 301.Caceres I., Al Khoury A., El Khoury R., Lorber S., Oswald P.I., El Khoury A., Atoui A., Puel O., Bailly J.-D. Aflatoxin biosynthesis and genetic regulation: A review. Toxins. 2020;12:150. doi: 10.3390/toxins12030150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Roze L.V., Hong S.-Y., Linz J.E. Aflatoxin biosynthesis: Current frontiers. Annu. Rev. Food Sci. Technol. 2013;4:293–311. doi: 10.1146/annurev-food-083012-123702. [DOI] [PubMed] [Google Scholar]
- 303.Yu J. Current understanding on aflatoxin biosynthesis and future perspective in reducing aflatoxin contamination. Toxins. 2012;4:1024–1057. doi: 10.3390/toxins4111024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Ehrlich K.C., Li P., Scharfenstein L., Chang P.-K. HypC, the anthrone oxidase involved in aflatoxin biosynthesis. Appl. Environ. Microbiol. 2010;76:3374–3377. doi: 10.1128/AEM.02495-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Khan R., Ghazali F.M., Mahyudin N.A., Samsudin N.I.P. Aflatoxin biosynthesis, genetic regulation, toxicity, and control strategies: A review. J. Fungi. 2021;7:606. doi: 10.3390/jof7080606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Sakuno E., Wen Y., Hatabayashi H., Arai H., Aoki C., Yabe K., Nakajima H. Aspergillus parasiticus cyclase catalyzes two dehydration steps in aflatoxin biosynthesis. Appl. Environ. Microbiol. 2005;71:2999–3006. doi: 10.1128/AEM.71.6.2999-3006.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Yabe K., Nakajima H. Enzyme reactions and genes in aflatoxin biosynthesis. Appl. Microbiol. Biotechnol. 2004;64:745–755. doi: 10.1007/s00253-004-1566-x. [DOI] [PubMed] [Google Scholar]
- 308.Henry K.M., Townsend C.A. Ordering the reductive and cytochrome P450 oxidative steps in demethylsterigmatocystin formation yields general insights into the biosynthesis of aflatoxin and related fungal metabolites. J. Am. Chem. Soc. 2005;127:3724–3733. doi: 10.1021/ja0455188. [DOI] [PubMed] [Google Scholar]
- 309.Yu J., Woloshuk C.P., Bhatnagar D., Cleveland T.E. Cloning and characterization of AvfA and OmtB genes involved in aflatoxin biosynthesis in three Aspergillus species. Gene. 2000;248:157–167. doi: 10.1016/S0378-1119(00)00126-8. [DOI] [PubMed] [Google Scholar]
- 310.Li T., Zhang Z., Wang Y., Li Y., Zhu J., Hu R., Yang Y., Liu M. Quantitative proteomic analysis for high- and low-aflatoxin-yield Aspergillus flavus strains isolated from natural environments. Front. Microbiol. 2021;12:741875. doi: 10.3389/fmicb.2021.741875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Scherm B., Palomba M., Serra D., Marcello A., Migheli Q. Detection of transcripts of the aflatoxin genes AflD, AflO, and AflP by reverse transcription-polymerase chain reaction allows differentiation of aflatoxin-producing and non-producing isolates of Aspergillus flavus and Aspergillus Parasit. Int. J. Food Microbiol. 2005;98:201–210. doi: 10.1016/j.ijfoodmicro.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 312.Ehrlich K.C. Predicted roles of the uncharacterized clustered genes in aflatoxin biosynthesis. Toxins. 2009;1:37–58. doi: 10.3390/toxins1010037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Zeng H., Hatabayashi H., Nakagawa H., Cai J., Suzuki R., Sakuno E., Tanaka T., Ito Y., Ehrlich K.C., Nakajima H., et al. Conversion of 11-hydroxy-O-methylsterigmatocystin to aflatoxin G1 in Aspergillus parasiticus. Appl. Microbiol. Biotechnol. 2011;90:635–650. doi: 10.1007/s00253-010-2999-z. [DOI] [PubMed] [Google Scholar]
- 314.Caceres I., El Khoury R., Bailly S., Oswald I.P., Puel O., Bailly J.-D. Piperine inhibits aflatoxin B1 production in Aspergillus flavus by modulating fungal oxidative stress response. Fungal Genet. Biol. 2017;107:77–85. doi: 10.1016/j.fgb.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 315.Flaherty J.E., Payne G.A. Overexpression of AflR Leads to upregulation of pathway gene transcription and increased aflatoxin production in Aspergillus flavus. Appl. Environ. Microbiol. 1997;63:3995–4000. doi: 10.1128/aem.63.10.3995-4000.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Chang P.-K. The Aspergillus parasiticus protein AFLJ interacts with the aflatoxin pathway-specific regulator AFLR. Mol. Genet. Genomics. 2003;268:711–719. doi: 10.1007/s00438-003-0809-3. [DOI] [PubMed] [Google Scholar]
- 317.Bhatnagar D., Cary J.W., Ehrlich K., Yu J., Cleveland T.E. Understanding the genetics of regulation of aflatoxin production and Aspergillus flavus development. Mycopathologia. 2006;162:155–166. doi: 10.1007/s11046-006-0050-9. [DOI] [PubMed] [Google Scholar]
- 318.Kolawole O., Meneely J., Petchkongkaew A., Elliott C. A Review of mycotoxin biosynthetic pathways: Associated genes and their expressions under the influence of climatic factors. Fungal Biol. Rev. 2021;37:8–26. doi: 10.1016/j.fbr.2021.04.003. [DOI] [Google Scholar]
- 319.Wang Y., Wang L., Liu F., Wang Q., Selvaraj J.N., Xing F., Zhao Y., Liu Y. Ochratoxin A producing fungi, biosynthetic pathway and regulatory mechanisms. Toxins. 2016;8:83. doi: 10.3390/toxins8030083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Adeyeye S.A.O. Fungal mycotoxins in foods: A review. Cogent Food Agric. 2016;2:1213127. doi: 10.1080/23311932.2016.1213127. [DOI] [Google Scholar]
- 321.Davolos D., Pietrangeli B. A molecular and bioinformatic study on the ochratoxin A (OTA)-producing Aspergillus affinis (section Circumdati) Mycotoxin Res. 2014;30:113–122. doi: 10.1007/s12550-014-0195-1. [DOI] [PubMed] [Google Scholar]
- 322.Gil-Serna J., Patiño B., Cortes L., Gonzalez-Jaen M.T., Vazquez C. Aspergillus steynii and Aspergillus westerdijkiae as potential risk of OTA contamination in food products in warm climates. Food Microbiol. 2015;46:168–175. doi: 10.1016/j.fm.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 323.Chen A.J., Tang D., Zhou Y.Q., Sun B.D., Li X.J., Wang L.Z., Gao W.W. Identification of ochratoxin A producing fungi associated with fresh and dry liquorice. PLoS ONE. 2013;8:e78285. doi: 10.1371/journal.pone.0078285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Schmidt-Heydt M., Graf E., Stoll D., Geisen R. The biosynthesis of Ochratoxin A by Penicillium as one mechanism for adaptation to NaCl rich foods. Food Microbiol. 2012;29:233–241. doi: 10.1016/j.fm.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 325.Gallo A., Ferrara M., Perrone G. Recent advances on the molecular aspects of ochratoxin A biosynthesis. Curr. Opin. Food Sci. 2017;17:49–56. doi: 10.1016/j.cofs.2017.09.011. [DOI] [Google Scholar]
- 326.Wang Y., Wang L., Wu F., Liu F., Wang Q., Zhang X., Selvaraj J.N., Zhao Y., Xing F., Yin W.-B., et al. A Consensus ochratoxin A biosynthetic pathway: Insights from the genome sequence of Aspergillus ochraceus and a comparative genomic analysis. Appl. Environ. Microbiol. 2018;84:e01009-18. doi: 10.1128/AEM.01009-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Ferrara M., Gallo A., Perrone G., Magistà D., Baker S.E. Comparative genomic analysis of ochratoxin A biosynthetic cluster in producing fungi: New evidence of a cyclase gene involvement. Front. Microbiol. 2020;11:e01009-18. doi: 10.3389/fmicb.2020.581309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Visentin I., Montis V., Döll K., Alabouvette C., Tamietti G., Karlovsky P., Cardinale F. Transcription of genes in the biosynthetic pathway for fumonisin mycotoxins is epigenetically and differentially regulated in the fungal maize pathogen Fusarium verticillioides. Eukaryot. Cell. 2012;11:252–259. doi: 10.1128/EC.05159-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Ahangarkani F., Rouhi S., Gholamour Azizi I. A review on incidence and toxicity of fumonisins. Toxin Rev. 2014;33:95–100. doi: 10.3109/15569543.2013.871563. [DOI] [Google Scholar]
- 330.Alexander N.J., Proctor R.H., McCormick S.P. Genes, gene clusters, and biosynthesis of trichothecenes and fumonisins in Fusarium. Toxin Rev. 2009;28:198–215. doi: 10.1080/15569540903092142. [DOI] [Google Scholar]
- 331.Yi H., Bojja R.S., Fu J., Du L. Direct evidence for the function of FUM13 in 3-ketoreduction of mycotoxin fumonisins in Fusarium verticillioides. J. Agric. Food Chem. 2005;53:5456–5460. doi: 10.1021/jf050062e. [DOI] [PubMed] [Google Scholar]
- 332.Lia Y., Lou L., Cerny R.L., Butchko R.A.E., Proctor R.H., Shen Y., Du L. Tricarballylic ester formation during biosynthesis of fumonisin mycotoxins in Fusarium verticillioides. Mycology. 2013;4:179–186. doi: 10.1080/21501203.2013.874540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Brown D.W., Butchko R.A.E., Busman M., Proctor R.H. The Fusarium verticillioides FUM gene cluster encodes a Zn(II)2Cys6 protein that affects FUM gene expression and fumonisin production. Eukaryot. Cell. 2007;6:1210–1218. doi: 10.1128/EC.00400-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Janevska S., Ferling I., Jojić K., Rautschek J., Hoefgen S., Proctor R.H., Hillmann F., Valiante V. Self-protection against the sphingolipid biosynthesis inhibitor fumonisin B1 is conferred by a FUM cluster-encoded ceramide synthase. mBio. 2020;11:e00455-20. doi: 10.1128/mBio.00455-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Sun L., Chen X., Gao J., Zhao Y., Liu L., Hou Y., Wang L., Huang S. Effects of disruption of five FUM genes on fumonisin biosynthesis and pathogenicity in Fusarium proliferatum. Toxins. 2019;11:327. doi: 10.3390/toxins11060327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Huffman J., Gerber R., Du L. Recent advancements in the biosynthetic mechanisms for polyketide-derived mycotoxins. Biopolymers. 2010;93:764–776. doi: 10.1002/bip.21483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Ji F., He D., Olaniran A.O., Mokoena M.P., Xu J., Shi J. Occurrence, toxicity, production and detection of Fusarium mycotoxin: A review. Food Prod. Process. Nutr. 2019;1:6. doi: 10.1186/s43014-019-0007-2. [DOI] [Google Scholar]
- 338.McCormick S.P., Stanley A.M., Stover N.A., Alexander N.J. Trichothecenes: From simple to complex mycotoxins. Toxins. 2011;3:802–814. doi: 10.3390/toxins3070802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Chen Y., Kistler H.C., Ma Z. Fusarium graminearum trichothecene mycotoxins: Biosynthesis, regulation, and management. Annu. Rev. Phytopathol. 2019;57:15–39. doi: 10.1146/annurev-phyto-082718-100318. [DOI] [PubMed] [Google Scholar]
- 340.Nahle S., El Khoury A., Atoui A. Current status on the molecular biology of zearalenone: Its biosynthesis and molecular detection of zearalenone producing Fusarium species. Eur. J. Plant. Pathol. 2021;159:247–258. doi: 10.1007/s10658-020-02173-9. [DOI] [Google Scholar]
- 341.Lysøe E., Klemsdal S.S., Bone K.R., Frandsen R.J.N., Johansen T., Thrane U., Giese H. The PKS4 gene of Fusarium graminearum is essential for zearalenone production. Appl. Environ. Microbiol. 2006;72:3924–3932. doi: 10.1128/AEM.00963-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Borzekowski A., Drewitz T., Keller J., Pfeifer D., Kunte H.-J., Koch M., Rohn S., Maul R. Biosynthesis and characterization of zearalenone-14-Sulfate, zearalenone-14-glucoside and zearalenone-16-glucoside using common fungal strains. Toxins. 2018;10:104. doi: 10.3390/toxins10030104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Vidal A., Ouhibi S., Ghali R., Hedhili A., De Saeger S., De Boevre M. The mycotoxin patulin: An updated short review on occurrence, toxicity and analytical challenges. Food Chem. Toxicol. 2019;129:249–256. doi: 10.1016/j.fct.2019.04.048. [DOI] [PubMed] [Google Scholar]
- 344.Frisvad J.C., Smedsgaard J., Larsen T.O., Samson R.A. Mycotoxins, drugs and other extrolites produced by species in Penicillium subgenus Penicillium. Stud. Mycol. 2004;49:e41. [Google Scholar]
- 345.Puel O., Galtier P., Oswald I.P. Biosynthesis and toxicological effects of patulin. Toxins. 2010;2:613–631. doi: 10.3390/toxins2040613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Li B., Chen Y., Zhang Z., Qin G., Chen T., Tian S. Molecular basis and regulation of pathogenicity and patulin biosynthesis in Penicillium expansum. Compr. Rev. Food Sci. Food Saf. 2020;19:3416–3438. doi: 10.1111/1541-4337.12612. [DOI] [PubMed] [Google Scholar]
- 347.Saleh I., Goktepe I. The characteristics, occurrence, and toxicological effects of patulin. Food Chem. Toxicol. 2019;129:301–311. doi: 10.1016/j.fct.2019.04.036. [DOI] [PubMed] [Google Scholar]
- 348.Artigot M.P., Loiseau N., Laffitte J., Mas-Reguieg L., Tadrist S., Oswald I.P., Puel O. Molecular cloning and functional characterization of two CYP619 cytochrome P450s involved in biosynthesis of patulin in Aspergillus clavatus. Microbiology. 2009;155:1738–1747. doi: 10.1099/mic.0.024836-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Li B., Chen Y., Zong Y., Shang Y., Zhang Z., Xu X., Wang X., Long M., Tian S. Dissection of patulin biosynthesis, spatial control and regulation mechanism in Penicillium expansum. Environ. Microbiol. 2019;21:1124–1139. doi: 10.1111/1462-2920.14542. [DOI] [PubMed] [Google Scholar]
- 350.Pickova D., Ostry V., Toman J., Malir F. Aflatoxins: History, significant milestones, recent data on their toxicity and ways to mitigation. Toxins. 2021;13:399. doi: 10.3390/toxins13060399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Omotayo O.P., Omotayo A.O., Mwanza M., Babalola O.O. Prevalence of mycotoxins and their consequences on human health. Toxicol. Res. 2019;35:1–7. doi: 10.5487/TR.2019.35.1.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Claeys L., Romano C., De Ruyck K., Wilson H., Fervers B., Korenjak M., Zavadil J., Gunter M.J., De Saeger S., De Boevre M., et al. Mycotoxin exposure and human cancer risk: A systematic review of epidemiological studies. Compr. Rev. Food Sci. Food Saf. 2020;19:1449–1464. doi: 10.1111/1541-4337.12567. [DOI] [PubMed] [Google Scholar]
- 353.Ahmed Adam M.A., Tabana Y.M., Musa K.B., Sandai D.A. Effects of different mycotoxins on humans, cell genome and their involvement in cancer (review) Oncol. Rep. 2017;37:1321–1336. doi: 10.3892/or.2017.5424. [DOI] [PubMed] [Google Scholar]
- 354.Moradi S., Azari H., Jafari Anarkooli I., Qasemi-Panahi B., Elhami S., Forouharmehr A. Effect of aflatoxin B1 on BRCA1 and BRCA2 genes expression under in vitro cultured cell line of normal human mammary epithelial cells (HMEC) J. Police Med. 2015;3:211–220. doi: 10.30505/3.4.211. [DOI] [Google Scholar]
- 355.Kouadio J.H., Mobio T.A., Baudrimont I., Moukha S., Dano S.D., Creppy E.E. Comparative study of cytotoxicity and oxidative stress induced by deoxynivalenol, zearalenone or fumonisin B1 in human intestinal cell line Caco-2. Toxicology. 2005;213:56–65. doi: 10.1016/j.tox.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 356.Mohsenzadeh M.S., Hedayati N., Riahi-Zanjani B., Karimi G. Immunosuppression following dietary aflatoxin B1 exposure: A review of the existing evidence. Toxin Rev. 2016;35:121–127. doi: 10.1080/15569543.2016.1209523. [DOI] [Google Scholar]
- 357.Qian G., Tang L., Guo X., Wang F., Massey M.E., Su J., Guo T.L., Williams J.H., Phillips T.D., Wang J.-S. Aflatoxin B1 modulates the expression of phenotypic markers and cytokines by splenic lymphocytes of male F344 Rats. J. Appl. Toxicol. 2014;34:241–249. doi: 10.1002/jat.2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Valtchev I., Koynarski T., Sotirov L., Nikolov Y., Petkov P. Effect of aflatoxin B1 on moulard duck’s natural immunity. Pak. Vet. J. 2015;35:67–70. [Google Scholar]
- 359.Bui-Klimke T.R., Wu F. Ochratoxin A and human health risk: A review of the evidence. Crit. Rev. Food Sci. Nutr. 2015;55:1860–1869. doi: 10.1080/10408398.2012.724480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Limonciel A., Jennings P. A review of the evidence that ochratoxin A is an Nrf2 inhibitor: Implications for nephrotoxicity and renal carcinogenicity. Toxins. 2014;6:371–379. doi: 10.3390/toxins6010371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Malir F., Ostry V., Pfohl-Leszkowicz A., Malir J., Toman J. Ochratoxin A: 50 years of research. Toxins. 2016;8:191. doi: 10.3390/toxins8070191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Paradells S., Rocamonde B., Llinares C., Herranz-Pérez V., Jimenez M., Garcia-Verdugo J.M., Zipancic I., Soria J.M., Garcia-Esparza M.A. Neurotoxic effects of ochratoxin A on the subventricular zone of adult mouse brain. J. Appl. Toxicol. 2015;35:737–751. doi: 10.1002/jat.3061. [DOI] [PubMed] [Google Scholar]
- 363.Park S., Lim W., You S., Song G. Ochratoxin A exerts neurotoxicity in human astrocytes through mitochondria-dependent apoptosis and intracellular calcium overload. Toxicol. Lett. 2019;313:42–49. doi: 10.1016/j.toxlet.2019.05.021. [DOI] [PubMed] [Google Scholar]
- 364.Stockmann-Juvala H., Savolainen K. A review of the toxic effects and mechanisms of action of fumonisin B1. Hum. Exp. Toxicol. 2008;27:799–809. doi: 10.1177/0960327108099525. [DOI] [PubMed] [Google Scholar]
- 365.Summerell B.A., Leslie J.F. Fifty years of Fusarium: How could nine species have ever been enough? Fungal Divers. 2011;50:135–144. doi: 10.1007/s13225-011-0132-y. [DOI] [Google Scholar]
- 366.Voss K.A., Riley R.T. Fumonisin toxicity and mechanism of action: Overview and current perspectives. Food Saf. 2013;1:2013006. doi: 10.14252/foodsafetyfscj.2013006. [DOI] [Google Scholar]
- 367.Ahamed S., Foster J.S., Bukovsky A., Wimalasena J. Signal Transduction through the Ras/Erk pathway is essential for the mycoestrogen zearalenone-induced cell-cycle progression in MCF-7 Cells. Mol. Carcinog. 2001;30:88–98. doi: 10.1002/1098-2744(200102)30:2<88::AID-MC1017>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 368.Koraichi F., Videmann B., Mazallon M., Benahmed M., Prouillac C., Lecoeur S. Zearalenone exposure modulates the expression of ABC transporters and nuclear receptors in pregnant rats and fetal liver. Toxicol. Lett. 2012;211:246–256. doi: 10.1016/j.toxlet.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 369.Pistol G.C., Braicu C., Motiu M., Gras M.A., Marin D.E., Stancu M., Calin L., Israel-Roming F., Berindan-Neagoe I., Taranu I. Zearalenone mycotoxin affects immune mediators, MAPK signalling molecules, nuclear receptors and genome-wide gene expression in pig spleen. PLoS ONE. 2015;10:e0127503. doi: 10.1371/journal.pone.0127503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Zhang G.-L., Feng Y.-L., Song J.-L., Zhou X.-S. Zearalenone: A mycotoxin with different toxic effect in domestic and laboratory animals’ granulosa cells. Front. Genet. 2018;9:667. doi: 10.3389/fgene.2018.00667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Zhang Y., Jia Z., Yin S., Shan A., Gao R., Qu Z., Liu M., Nie S. Toxic effects of maternal zearalenone exposure on uterine capacity and fetal development in gestation rats. Reprod. Sci. 2014;21:743–753. doi: 10.1177/1933719113512533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Koçkaya E., Selmanoğlu G., Ozsoy N., Gül N. Evaluation of patulin toxicity in the thymus of growing male rats. Arch. Ind. Hyg. Toxicol. 2009;60:411–418. doi: 10.2478/10004-1254-60-2009-1973. [DOI] [PubMed] [Google Scholar]
- 373.Lupescu A., Jilani K., Zbidah M., Lang F. Patulin-induced suicidal erythrocyte death. Cell. Physiol. Biochem. 2013;32:291–299. doi: 10.1159/000354437. [DOI] [PubMed] [Google Scholar]
- 374.Selmanoğlu G. Evaluation of the reproductive toxicity of patulin in growing male rats. Food Chem. Toxicol. 2006;44:2019–2024. doi: 10.1016/j.fct.2006.06.022. [DOI] [PubMed] [Google Scholar]
- 375.Berthiller F., Krska R., Domig K.J., Kneifel W., Juge N., Schuhmacher R., Adam G. Hydrolytic fate of deoxynivalenol-3-glucoside during digestion. Toxicol. Lett. 2011;206:264–267. doi: 10.1016/j.toxlet.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Brown R., Priest E., Naglik J.R., Richardson J.P. Fungal toxins and host immune responses. Front. Microbiol. 2021;12:643639. doi: 10.3389/fmicb.2021.643639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Fang H., Zhi Y., Yu Z., Lynch R.A., Jia X. The embryonic toxicity evaluation of deoxynivalenol (DON) by murine embryonic stem cell test and human embryonic stem cell test models. Food Control. 2018;86:234–240. doi: 10.1016/j.foodcont.2017.10.018. [DOI] [Google Scholar]
- 378.Islam Z., Gray J.S., Pestka J.J. P38 Mitogen-activated protein kinase mediates IL-8 induction by the ribotoxin deoxynivalenol in human monocytes. Toxicol. Appl. Pharmacol. 2006;213:235–244. doi: 10.1016/j.taap.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 379.Gerez J.R., Pinton P., Callu P., Grosjean F., Oswald I.P., Bracarense A.P.F.L. Deoxynivalenol alone or in combination with nivalenol and zearalenone induce systemic histological changes in pigs. Exp. Toxicol. Pathol. 2015;67:89–98. doi: 10.1016/j.etp.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 380.Nagashima H., Nakagawa H. Differences in the toxicities of trichothecene mycotoxins, deoxynivalenol and nivalenol, in cultured cells. Jpn. Agric. Res. Q. JARQ. 2014;48:393–397. doi: 10.6090/jarq.48.393. [DOI] [Google Scholar]
- 381.Fang H., Wu Y., Guo J., Rong J., Ma L., Zhao Z., Zuo D., Peng S. T-2 toxin induces apoptosis in differentiated murine embryonic stem cells through reactive oxygen species-mediated mitochondrial pathway. Apoptosis. 2012;17:895–907. doi: 10.1007/s10495-012-0724-3. [DOI] [PubMed] [Google Scholar]
- 382.Janik E., Niemcewicz M., Podogrocki M., Ceremuga M., Stela M., Bijak M. T-2 Toxin—The most toxic trichothecene mycotoxin: Metabolism, toxicity, and decontamination strategies. Molecules. 2021;26:6868. doi: 10.3390/molecules26226868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Li Y., Wang Z., Beier R.C., Shen J., De Smet D., De Saeger S., Zhang S. T-2 Toxin, a trichothecene mycotoxin: Review of toxicity, metabolism, and analytical methods. J. Agric. Food Chem. 2011;59:3441–3453. doi: 10.1021/jf200767q. [DOI] [PubMed] [Google Scholar]
- 384.Madheswaran R., Balachandran C., Murali Manohar B. Influence of dietary culture material containing aflatoxin and T2 toxin on certain serum biochemical constituents in japanese quail. Mycopathologia. 2004;158:337–341. doi: 10.1007/s11046-005-8399-8. [DOI] [PubMed] [Google Scholar]
- 385.Weidner M., Lenczyk M., Schwerdt G., Gekle M., Humpf H.-U. Neurotoxic potential and cellular uptake of T-2 toxin in human astrocytes in primary culture. Chem. Res. Toxicol. 2013;26:347–355. doi: 10.1021/tx3004664. [DOI] [PubMed] [Google Scholar]
- 386.Indrie L., Oana D., Ilies M., Ilies D.C., Lincu A., Ilies A., Baias S., Herman G.V., Onet A., Costea M., et al. Indoor air quality of museums and conservation of textiles art works. Case study: Salacea Museum House, Romania. Ind. Text. 2019;70:88–93. doi: 10.35530/IT.070.01.1608. [DOI] [Google Scholar]
- 387.Zhang G.X., Gong C.J., Gu J.G., Katayama Y., Someya T., Gu J.D. Biochemical reactions and mechanisms involved in the biodeterioration of stone world cultural heritage under the tropical climate conditions. Int. Biodeterior. Biodegrad. 2019;143:104723. doi: 10.1016/j.ibiod.2019.104723. [DOI] [Google Scholar]
- 388.Savković Ž., Stupar M., Unković N., Knežević A., Vukojević J., Ljaljević Grbić M. Biodegradation. IntechOpen; London, UK: 2022. Fungal deterioration of cultural heritage objects. [DOI] [Google Scholar]
- 389.Owczarek-Kościelniak M., Krzewicka B., Piątek J., Kołodziejczyk Ł.M., Kapusta P. Is there a link between the biological colonization of the gravestone and its deterioration? Int. Biodeterior. Biodegrad. 2020;148:104879. doi: 10.1016/j.ibiod.2019.104879. [DOI] [Google Scholar]
- 390.Scheerer S., Ortega-Morales O.C.G. Microbial deterioration of stone monuments—An updated overview. Adv. Appl. Microbiol. 2009;66:97–139. doi: 10.1016/S0065-2164(08)00805-8. [DOI] [PubMed] [Google Scholar]
- 391.Piñar G., Garcia-Valles M., Gimeno-Torrente D., Fernandez-Turiel J.L., Ettenauer J., Sterflinger K. Microscopic, chemical, and molecular-biological investigation of the decayed medieval stained window glasses of two catalonian churches. Int. Biodeterior. Biodegrad. 2013;84:388–400. doi: 10.1016/j.ibiod.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Kim Y.S., Singh A.P. Wood as cultural heritage material and its deterioration by biotic and abiotic agents. In: Kim Y.S., Funada R., Singh A.P., editors. Secondary Xylem Biology: Origins, Functions, and Applications. Academic Press; London, UK: 2016. pp. 233–257. Chapter 12. [DOI] [Google Scholar]
- 393.Koestler R.J., Koestler V.R., Charola A.E., Nieto-Fernandez F.E. Art, Biology, and Conservation: Biodeterioration of Works of Art. The Metropolitan Museum of Art; New York, NY, USA: 2010. [Google Scholar]
- 394.Schmidt O. Wood and Tree Fungi. Springer; Berlin/Heidelberg, Germany: 2006. Chapter 6 Wood Discoloration; p. 120. [Google Scholar]
- 395.Alfieri P.V., Correa M.V. Fungi observation in deteriorated wooden heritage: Analysis using different imaging techniques. Rev. Argent. Microbiol. 2017;49:120–122. doi: 10.1016/j.ram.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 396.Poyatos F., Morales F., Nicholson A.W., Giordano A. Physiology of biodeterioration on canvas paintings. J. Cell Physiol. 2018;233:2741–2751. doi: 10.1002/jcp.26088. [DOI] [PubMed] [Google Scholar]
- 397.Caselli E., Pancaldi S., Baldisserotto C., Petrucci F., Impallaria A., Volpe L., D’Accolti M., Soffritti I., Coccagna M., Sassu G., et al. Characterization of Biodegradation in a 17th century easel painting and potential for a biological approach. PLoS ONE. 2018;13:e0207630. doi: 10.1371/journal.pone.0207630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Santos A., Cerrada A., García S., Andrés M.S., Abrusci C., Marquina D. Application of molecular techniques to the elucidation of the microbial community structure of antique paintings. Microb. Ecol. 2009;58:692–702. doi: 10.1007/s00248-009-9564-2. [DOI] [PubMed] [Google Scholar]
- 399.Szostak-Kotowa J. Biodeterioration of textiles. Int. Biodeterior. Biodegrad. 2004;53:165–170. doi: 10.1016/S0964-8305(03)00090-8. [DOI] [Google Scholar]
- 400.Pinzari F., Colaizzi P., Maggi O., Persiani A.M., Schütz R., Rabin I. Fungal bioleaching of mineral components in a twentieth-century illuminated parchment. Anal. Bioanal. Chem. 2012;402:1541–1550. doi: 10.1007/s00216-011-5263-1. [DOI] [PubMed] [Google Scholar]
- 401.Pinna D., Salvadori O. Plant Biology for Cultural Heritage. The Getty Conservation Institute; Los Angeles, CA, USA: 2008. Stone and Related Materials; pp. 128–144. [Google Scholar]
- 402.Isola D., Zucconi L., Onofri S., Caneva G., Hoog G.S., Selbmann L. Extremotolerant rock inhabiting black fungi from italian monumental sites. Fungal Divers. 2016;76:75–96. [Google Scholar]
- 403.Sterflinger K. Fungi: Their role in deterioration of cultural heritage. Fungal Biol. Rev. 2010;24:47–55. doi: 10.1016/J.FBR.2010.03.003. [DOI] [Google Scholar]
- 404.Sazanova K.V., Zelenskaya M.S., Vlasov A.D., Bobir S.Y., Yakkonen K.L., Vlasov D.Y. Microorganisms in superficial deposits on the stone monuments in Saint Petersburg. Microorganisms. 2022;10:316. doi: 10.3390/microorganisms10020316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Sert H.B., Sümbül H., Sterflinger K. Microcolonial fungi from antique marbles in Perge/Side/Termessos (Antalya/Turkey) Antonie Van Leeuwenhoek J. Microb. 2007;91:217–227. doi: 10.1007/s10482-006-9111-9. [DOI] [PubMed] [Google Scholar]
- 406.Sterflinger K., Prillinger H. Molecular taxonomy and biodiversity of rock fungal communities in an urban environment (Vienna, Austria) Antonie Van Leeuwenhoek J. Microb. 2001;80:275–286. doi: 10.1023/A:1013060308809. [DOI] [PubMed] [Google Scholar]
- 407.Ricca M., Urzì C.E., Rovella N., Sardella A., Bonazza A., Ruffolo S.A., Leo F., Randazzo L., Arcudi A., Russa M.F. Multidisciplinary approach to characterize archaeological materials and status of conservation of the roman thermae of Reggio Calabria site (Calabria, South Italy) Appl. Sci. 2020;10:5106. doi: 10.3390/app10155106. [DOI] [Google Scholar]
- 408.Ruffolo S.A., Leo F., Ricca M., Arcudi A., Silvestri C., Bruno L., Urzì C., Russa M.F. Medium-term in situ experiment by using organic biocides and titanium dioxide for the mitigation of microbial colonization on stone surfaces. Int. Biodeterior. Biodegrad. 2017;123:17–26. [Google Scholar]
- 409.Sterflinger K., Baere R., Hoog G.S., Wachter R., Krumbein W.E., Haase G. Coniosporium perforans and C. apollinis, two new rock-inhabiting fungi isolated from marble in the sanctuary of delos (Cyclades, Greece) Antonie Van Leeuwenhoek J. Microb. 1997;72:349–363. doi: 10.1023/A:1000570429688. [DOI] [PubMed] [Google Scholar]
- 410.Ruga L., Orlandi F., Romano B., Fornaciari M. The assessment of fungal bioaerosols in the crypt of St. Peter in Perugia (Italy) Int. Biodeterior. Biodegrad. 2015;98:121–130. [Google Scholar]
- 411.Pangallo D., Bučková M., Kraková L., Puškárová A., Šaková N., Grivalský T., Chovanová K., Zemánková M. Biodeterioration of epoxy resin: A microbial survey through culture-independent and culture-dependent approaches. Environ. Microbiol. 2014;17:462–479. doi: 10.1111/1462-2920.12523. [DOI] [PubMed] [Google Scholar]
- 412.Nevalainen A., Täubel M., Hyvärinen A. Indoor fungi: Companions and contaminants. Indoor Air. 2015;25:125–156. doi: 10.1111/ina.12182. [DOI] [PubMed] [Google Scholar]
- 413.Ciferri O. Microbial degradation of paintings. Appl. Environ. Microbiol. 1999;65:879–885. doi: 10.1128/AEM.65.3.879-885.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Ma W., Wu F., Tian T., He D., Zhang Q., Gu J.-D., Duan Y., Ma D., Wang W., Feng H. Fungal diversity and its contribution to the biodeterioration of mural paintings in two 1700-year-old tombs of China. Int. Biodeterior. Biodegrad. 2020;152:104972. [Google Scholar]
- 415.López-Miras M.D.M., Martín-Sánchez I., Yebra-Rodríguez Á., Romero-Noguera J., Bolívar-Galiano F., Ettenauer J., Sterflinger K., Piñar G. Contribution of the microbial communities detected on an oil painting on canvas to its biodeterioration. PLoS ONE. 2013;8:e80198. doi: 10.1371/JOURNAL.PONE.0080198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Tiano P. Biodegradation of cultural heritage: Decay mechanisms and control methods; Proceedings of the 9th ARIADNE Workshop “Historic Material and Their Diagnostic” ARCCHIP; Prague, Czech Republic. 22–28 April 2002. [Google Scholar]
- 417.Pyzik A., Ciuchcinski K., Dziurzynski M., Dziewit L. The bad and the good-microorganisms in cultural heritage environments—An update on biodeterioration and biotreatment approaches. Materials. 2021;14:177. doi: 10.3390/ma14010177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Arai H. Foxing caused by fungi: Twenty-five years of study. Int. Biodeterior. Biodegrad. 2000;46:181–188. doi: 10.1016/S0964-8305(00)00063-9. [DOI] [Google Scholar]
- 419.Corte A.M., Ferroni A., Salvo V.S. Isolation of fungal species from test samples and maps damaged by foxing, and correlation between these species and the environment. Int. Biodeterior. Biodegrad. 2003;51:167–173. doi: 10.1016/S0964-8305(02)00137-3. [DOI] [Google Scholar]
- 420.Koul B., Upadhyay H. Fungi-Mediated Biodeterioration of Household Materials, Libraries, Cultural Heritage and Its Control. In: Gehlot P., Singh J., editors. Fungi and Their Role in Sustainable Development: Current Perspectives. Springer; Singapore: 2018. pp. 597–615. [Google Scholar]
- 421.Nitiu D.S., Mallo A.C., Saparrat M.C.N. Fungal melanins that deteriorate paper cultural heritage: An overview. Mycologia. 2020;112:859–870. doi: 10.1080/00275514.2020.1788846. [DOI] [PubMed] [Google Scholar]
- 422.Pinheiro A.C., Sequeira S.O., Macedo M.F. Fungi in archives, libraries, and museums: A review on paper conservation and human health. Crit. Rev. Microbiol. 2019;45:686–700. doi: 10.1080/1040841X.2019.1690420. [DOI] [PubMed] [Google Scholar]
- 423.Kavkler K., Demšar A. Impact of fungi on contemporary and accelerated aged wool fibres. Polym. Degrad. Stab. 2012;97:786–792. doi: 10.1016/j.polymdegradstab.2012.02.002. [DOI] [Google Scholar]
- 424.Ljaljević Grbić M., Unković N., Stupar M., Vukojević J., Nedeljković T. Implementation of ATP biolumenescence method in the study of the fungal deterioration of textile artefacts. Fibres Text. East. Eur. 2014;22:132–136. [Google Scholar]
- 425.Troiano F., Polo A., Villa F., Cappitelli F. Assessing the microbiological risk to stored sixteenth century parchment manuscripts: A holistic approach based on molecular and environmental studies. Biofouling. 2014;30:299–311. doi: 10.1080/08927014.2013.871539. [DOI] [PubMed] [Google Scholar]
- 426.Paiva de Carvalho H., Mesquita N., Trovão J., Peixoto da Silva J., Rosa B., Martins R., Bandeira A.M.L., Portugal A. Diversity of fungal species in ancient parchments collections of the archive of the University of Coimbra. Int. Biodeterior. Biodegrad. 2016;108:57–66. doi: 10.1016/j.ibiod.2015.12.001. [DOI] [Google Scholar]
- 427.Tanney J.B., Nguyen H.D.T., Pinzari F., Seifert K.A. A century later: Rediscovery, culturing and phylogenetic analysis of Diploöspora rosea, a rare onygenalean hyphomycete. Antonie van Leeuwenhoek. 2015;108:1023–1035. doi: 10.1007/s10482-015-0555-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.