SUMMARY
Clinical management of Staphylococcus aureus infections presents a challenge due to the high incidence, considerable virulence, and emergence of drug resistance mechanisms. The treatment of drug-resistant strains, such as methicillin-resistant S. aureus (MRSA), is further complicated by the development of tolerance and persistence to antimicrobial agents in clinical use. To address these challenges, membrane disruptors, that are not generally considered during drug discovery for agents against S. aureus, should be explored. The cell membrane protects S. aureus from external stresses and antimicrobial agents, but membrane-targeting antimicrobial agents are probably less likely to promote bacterial resistance. Nontypical linear cationic antimicrobial peptides (AMPs), highly modified AMPs such as daptomycin (lipopeptide), bacitracin (cyclic peptide), and gramicidin S (cyclic peptide), are currently in clinical use. Recent studies have demonstrated that AMPs and small molecules can penetrate the cell membrane of S. aureus, inhibit phospholipid biosynthesis, or block the passage of solutes between the periplasm and the exterior of the cell. In addition to their primary mechanism of action (MOA) that targets the bacterial membrane, AMPs and small molecules may also impact bacteria through secondary mechanisms such as targeting the biofilm, and downregulating virulence genes of S. aureus. In this review, we discuss the current state of research into cell membrane-targeting AMPs and small molecules and their potential mechanisms of action against drug-resistant physiological forms of S. aureus, including persister cells and biofilms.
KEYWORDS: antimicrobial peptides, biofilm, persister, small molecules, Staphylococcus aureus
INTRODUCTION
Staphylococcus aureus is a Gram-positive human pathogen carried by approximately one-third of the human population. Globally, the incidence of S. aureus cases ranges from 10 to 30 cases per 100,000 people per year (1), with hospital mortality ranging from 15% to 40% (2, 3). The death rate associated with S. aureus bacteremia is higher than those associated with AIDS, tuberculosis, and viral hepatitis combined (4). The Centers for Disease Control and Prevention (CDC) reports that methicillin-resistant S. aureus (MRSA) is a common cause of skin, wound, pneumonia, and bloodstream infections (5). Almost half of S. aureus infections acquired in hospitals in the United States are methicillin-resistant (6) and MRSA infection rates are increasing in the community (7, 8).
In this review, we discuss the concept of using AMPs and small molecules to disrupt bacterial membranes or proteins integral to membrane function in S. aureus. Furthermore, we outline how membrane targeting AMPs and small molecules are effective against the persister and biofilm forms of S. aureus.
THE NEED FOR ANTIMICROBIAL AGENTS THAT TARGET THE BACTERIAL MEMBRANE
Drug resistance, tolerance, persistence, heteroresistance and biofilm formation contribute to the treatment challenges associated with S. aureus (9). The term “resistance” refers to the ability of bacteria to grow despite antibiotic treatment, regardless of the length of time they are exposed to the antibiotics (10, 11). Antibiotic resistance is caused by various molecular mechanisms, such as modified drug targets or efflux pumps (11, 12). In comparison, when bacteria become resistant to antibiotics, a higher antibiotic concentration is required to treat a resistant strain than a susceptible strain. Generally, antibiotic resistance can be classified as either natural/intrinsic or acquired resistance. Natural/intrinsic resistance occurs when certain structures are missing or present, resulting in antibiotic ineffectiveness. Acquired resistance results from mutations of chromosomal genes or horizontal gene transfers (12).
Tolerance allows bacteria to endure a more extended period of antibiotic treatment by remaining dormant (13). During dormancy, the bacteria stay unaffected by many types of antibiotics (β-lactams and quinolones) that target the growth and functioning of the bacteria (14, 15). The development of tolerant phenotypes occurs through genetic characteristics, stochastically, or in response to environmental factors in Gram-positive and Gram-negative bacteria (12). It is critical to note that tolerance is only associated with bactericidal antibiotics, not with bacteriostatic antibiotics (16).
In addition to tolerance, persistence increases the duration of treatment for a subpopulation, even if the population is clonal (17). In simple terms, “persister” refers to a subpopulation of tolerant cells that can withstand antibiotic treatment for longer in comparison to slow-growing dying cells by entering a metabolically repressed state (nonmultiplying cells) (17, 18). Most antibacterial agents are only effective against growing cells, and persisters can survive longer without being genetically resistant by not growing (12). Importantly, most currently prescribed antibiotics are ineffective against bacterial persisters, and S. aureus persisters are tolerant to antibacterial agents, including gentamicin (protein synthesis inhibitor), ciprofloxacin (DNA replication inhibitor), vancomycin (cell wall synthesis inhibitor), and daptomycin (18–26). Generally, persister cells make up a smaller fraction of the population (less than 1%) and are killed more slowly than susceptible cells (16). An isogenic bacterial population has been shown to show an increase in persister levels when exposed to nutrient limitations, antibiotic exposure, acidic pH, high cell numbers, heat shock, and oxidative stress (16). A study on S. aureus ATCC 55585 proved that the strain was completely tolerant to fluoroquinolone and ciprofloxacin, suggesting that stationery cells may be equivalent to the persister of S. aureus (21). In studies using clinical staphylococcal isolates, persister formation was demonstrated in the presence of antibiotics like penicillin, tetracycline, clindamycin, chloramphenicol, cefoxitin, erythromycin, co-trimoxazole, gentamicin, ciprofloxacin, and moxifloxacin (27–29).
Distinct to persistence, heteroresistance occurs when isogenic subpopulations of bacteria display differing levels of susceptibility to an antibiotic, mostly at genetic levels (30). In practice, heteroresistance is defined when the highest inhibitory effect of an antibiotic is at least 8 times greater than the highest non-inhibitory concentration and is associated with the selection of more resistant subpopulations (30). Most variations in antibiotic resistance in bacteria, such as S. aureus, are caused by mutations or duplications of resistance genes and regulation factors (30). For example, in MRSA, mutations in the mecA gene and differences in penicillinase expression can cause heteroresistance to β-lactams such as methicillin (31, 32). MRSA heteroresistance may also be mediated by transcription regulators such as the Sar and the Sigma B operons (33, 34), and the SOS (save our souls) response lexA/recA gene regulators (35). The mechanism underlying the heteroresistance of MRSA to glycopeptides (vancomycin) is unclear (30) and may be associated with higher gene mutation rates of the agr gene locus (accessory gene regulator) (36). Also, VISA strains carry mutations in vraSR, walRK, and rpoB operons (37–39) and a mutation in rpoB, but not graR, is associated with heteroresistance to vancomycin in S. aureus, resulting in a thickened cell wall and reduced cell surface negative charge (40, 41).
Biofilm tolerance to drugs and other toxins is, at least in part, due to impaired antibiotic penetration (42, 43). Also, studies demonstrated that cells released from biofilms are less susceptible to antibiotics than planktonic cells (44, 45). Importantly, one of the physiological reasons for differential antibiotic susceptibility in the biofilm-dispersed cells is attributed to the retention of extracellular polymeric matrix, which may allow a varied antibiotic response compared to standard planktonic cells (46, 47). Apart from impairing the penetration of some drugs, biofilms harbor many persister cells that are often involved in the failure of treatments (48–50) (Fig. 1).
FIG 1.

S. aureus infection model of relapsing biofilms. Figure adapted based on Conlon et al. (216) and Lister et al. (217).
To improve antistaphylococcal treatment, there is a need to identify agents that overcome resistance and maintain activity against tolerant and persister cells and biofilms (20, 51, 52). The cell membrane of S. aureus is a crucial structure for cell survival and consists of the cytoplasmic plasma membrane (CM) and lipoteichoic acids (LTA) attached to a thick layer of peptidoglycan (53). Nearly one-third of proteins in the cell membrane are involved in bacterial metabolism, and damaged membranes impair homeostasis and reduce the possibility of drug resistance (54). Mehta et al. (55) hypothesized that a membrane-permeabilizing drug may also act as a chemosensitizer that enhances the activity of other antibacterial agents (55).
Consequently, the use of membrane-targeting small molecules and antimicrobial peptides (AMPs) has become a focus of antimicrobial chemotherapy (54, 56). These strategies could shorten the treatment period, reduce disease relapses, target bacterial persisters, and reduce antibiotic resistance. Antimicrobial agents that target the structure of bacterial membrane bilayers have become increasingly interesting because the membrane is essential and highly conservative (54).
MEMBRANE-ACTIVE AMPs AGAINST S. AUREUS
Antimicrobial Peptides
AMPs have evolutionarily conserved roles in the innate immune system, providing the first line of defense against microbial infection in all major phyla of plants, animals, and prokaryotes (57). Most AMPs are composed of <50 amino acids and are 40 to 60% hydrophobic and positively charged (average net charge of +3) (57) and exhibit broad-spectrum antibacterial activity against bacteria, fungi, viruses, and even cancer cells (58–60). In addition to their antimicrobial activity, AMPs may facilitate the healing of wounds, and modulate the immune system (57).
AMPs can be classified based on structure, function, or target (61). More specifically, based on their structure, AMPs are classified as α-helix, β-sheets, both α+β peptides, and non-αβ (Table 1), describing major families of AMPs primarily targeting the S. aureus membrane (Fig. 2). AMPs are also classified based on their biological functions (antibacterial, antifungal, antiviral, antiparasitic, antiprotist, anticancer, etc.). According to their potential targets, AMPs are classified as membrane-targeting or cell wall-targeting (loss of cellular integrity), or acts on intracellular functions, including ribosome, DNA, or RNA (61).
TABLE 1.
Major families of AMPs primarily targeting the S. aureus membrane
| Structural class | No. of peptides (n = 115) | Eminent examples of AMP families |
|---|---|---|
| α-helical | 48 | Dermaseptin-B2, aurein 2.5, magainin 2, melittin, buforin II, LL-37, chrysophsin-1 |
| β-sheets | 10 | Lactoferricin B, baboon theta-defensin-1, human neutrophil peptide-1, gomesin, protegrin 1, tachyplesin I, and kalata B1 |
| α + β | 6 | Human beta-defensin 1, thrombocidin-1, porcine beta-defensin 2, CXCL10, actinomycesin |
| Non-αβ | 3 | Pyrrhocoricin, penisin, and tridecaptin A1 |
FIG 2.

Examples for different types of AMPs based on their structure. (a) Aurein 2.5 (PDB ID: 6GS9); (b) Magainin 2 (PDB ID: 2MAG); (c) Melittin (PDB ID: 2MLT); (d) LL-37 (PDB ID: 2K6O); (e) Lactoferricin B (PDB ID: 1LFC); (f) Human neutrophil peptide-1 (PDB ID: 3GNY); (g) Gomesin (PDB ID: 1KFP); (h) Protegrin 1 (PDB ID: 1PG1); (i) Human β-defensin 1 (PDB ID: 1E4S); (j) Thrombocidin-1 (PDB ID: 1NAP); (k) CXCL10 (PDB ID: 1O80); (l) Actinomycesin (PDB ID: 2RU0); (m) Pyrrhocoricin (PDB ID: 5HD1); and (n) Tridecaptin A1 (PDB ID: 2N5Y). All the images were obtained from the RCSB Protein Data Bank.
Physicochemical Factors Regulating the Activity of AMPs against S. aureus Membrane
Several factors determine the action of AMPs, including the charge, hydrophobicity, conformation, length, and amphipathicity (61). The net charge in AMPs plays a critical role in determining the bacterial targets and the majority of positively charged AMPs target bacterial membranes (62). Such positively charged amino acids in AMPs interact with negatively charged phospholipids of bacterial membranes through electrostatic interactions (62). This interaction allows the hydrophobic amino acids in the AMPs to interact further with the bacterial membrane (62).
At physiological pH, the net positive charge of cationic AMPs ranges from +2 to +11 (61). In contrast, at physiological pH, anionic AMPs have a net negative charge of −1 and −8 and are usually composed of 5 to 70 amino acid residues (57). Following this electrostatic binding, the interaction between the hydrophobic amino acids in the AMPs and the hydrophobic tail of the membrane phospholipids allows the AMPs to enter deeper inside the bacterial membrane (62). Short anionic AMPs like DDDDD (pentaD) and surfactant-associated anionic peptides (SAAPs), such as those found in the pulmonary fluids of sheep and cattle, have broad-spectrum activity (63–65). However, unlike cationic AMPs that primarily target bacterial cell membranes, anionic AMPs act by constricting the ability of bacteria to absorb zinc ions (64).
After the initial electrostatic binding of the charged amino acids, the bacterial membrane permeability by the AMPs depends on the hydrophobic amino acids of the AMPs (66, 67). Hydrophobic-hydrophilic interactions between the hydrophobic amino acid of AMPs and the hydrocarbon chain of the lipids orient in various ways to form membrane deformations (66, 67) (also described below under Mechanism of Action of Membrane-Active AMPs and Small Molecules against S. aureus).
To induce effective membrane binding on different bacteria, AMPs must contain specific contents of hydrophobic amino acids (66, 67). For example, AMPs targeting Gram-positive bacteria, particularly S. aureus, requires peptides with higher hydrophobic content than AMPs selectively acting against Gram-negative bacteria (68, 69), and using an in silico approach based on database filtering technology, Mishra et al. found that AMPs specifically targeting S. aureus membranes require 61% of hydrophobicity (68). Members of our group also found that a 56 to 70% hydrophobicity is required by a short α-helical C18, and sapecin B-derived SD-8 membrane-active peptide to work against S. aureus (70, 71).
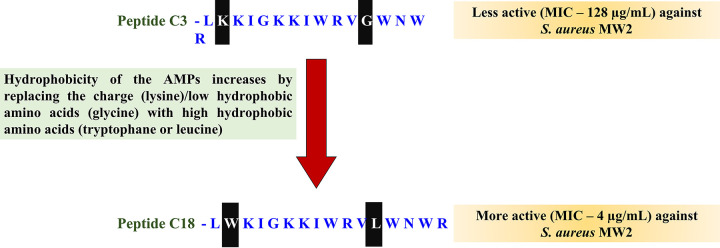
The antistaphylococcal activity of many membrane AMPs is enhanced by substituting high hydrophobic amino acid residues for low hydrophobic or charged amino acid residues (70, 72, 73) (Fig. 3). A balance of both the hydrophobic and charged amino acids is critical for maintaining the antimicrobial activity of AMPs (74). However, in AMPs with a higher number of positive charges that facilitate enough initial membrane electrostatic interactions, the additional charged amino acids can be substituted for hydrophobic amino acids to enhance antimicrobial effects. Similarly, the antistaphylococcal activity of many membrane AMPs is enhanced by substituting high hydrophobic amino acid residues for low hydrophobic or charged amino acid residues (70, 73, 74). For example, tetra-F2W peptides with arginine exchanged with tryptophan (72), and cecropin-4 peptides with glycine (70), demonstrated enhanced antimicrobial potency compared to their low hydrophobic peptide counterparts. When hydrophobic amino acids are swapped for neutral amino acids, such as tetra-F2W peptides with arginine exchanged with tryptophan and cecropin-4 peptides with glycine substituted with leucine, enhanced antimicrobial potency is demonstrated. Similarly, replacing a less hydrophobic amino acid residue with a higher hydrophobic amino acid in peptides like DFTamp1 (valine or isoleucine replaced with leucine) (68), and C18G (valine replaced with leucine) (73) also increased their antimicrobial potency against S. aureus.
FIG 3.
Replacing charged (lysine) or low hydrophobic amino acids (glycine) with high hydrophobic amino acids (tryptophane or leucine) enhances the antistaphylococcal activity of Cecropin-4-derived AMPs. The figure is adapted from Peng et al. (70).
The charged and hydrophobic amino acids in peptides determine how natural AMPs act on membranes (74). Identifying any preferential amino acid selection in natural membrane-active AMPs can guide the design of antistaphylococcal peptides. For the purposes of this review, we analyzed the amino acid composition of naturally occurring membrane-active AMPs from the Antimicrobial Peptide Database (maintained at the University of Nebraska Medical Center, Omaha) (61). The database contains about 3,425 AMPs, of which 1,658 are effective against S. aureus (57). Based on the amino acid composition of the 115 AMPs specifically targeting the S. aureus membrane (Fig. 4), lysine was preferred over arginine as a charged amino acid (lysine occurs at 11.31%, while arginine was 6.87%) and the average hydrophobicity of all peptides was 56%. In addition, a total of 307 membrane-active AMPs exhibit broad-spectrum activities, including activity against S. aureus (57). In this group of peptides, lysine was also preferred to arginine, and the average hydrophobicity of all peptides was 60%.
FIG 4.
Distribution of amino acid residues in α-helical AMPs (determined from the antimicrobial peptide database). Each amino acid is denoted with a single letter: hydrophobic (I, V, L, F, C, M, A, W, G, P), neutral (T, S, Y, N, Q), and charged residues (E, D, H, K, R). (A) Broad spectrum membrane activity. (B) S. aureus-specific membrane activity.
Interestingly, the preferential amino acid composition of membrane-active AMPs regulates peptide binding toward different lipids and the distinction in the binding affinities of these membrane-active AMPs with various membranes forms the basis of cell selectivity (75). More specifically, the membrane selectivity of an AMP results from its affinity for more anionic bacterial membranes (75). Notably, mammalian membranes contain neutral lipids such as cholesterol, which help stabilize phospholipid bilayers and AMP interactions, resulting in less cytotoxicity (57, 76).
Peptidomimetics
Peptidomimetics (or peptide mimics) are small protein chains synthesized by modifying the existing structure of a peptide (77) (Table 2). Successful peptidomimetics in clinical trials, including PMX 30063 (78) and LTX-109 (79), are hampered by complex synthesis protocols and membrane specificity (78, 80). A successful synthesis constitutes the AMP or small molecules with low toxicity and higher membrane specificity against S. aureus. The development of membrane-active drugs has been significantly enhanced by designing and optimizing cationic amphipathic mimics, which overcome these weaknesses (81). Cationic amphipathic mimics were developed and optimized to overcome weaknesses, such as stability or selectivity, to contribute significantly to the development of membrane-active agents against S. aureus (82). Most membrane-active peptide mimics contain amino groups in their molecules that form a hydrophilic area, which is crucial to their antibacterial action (83).
TABLE 2.
Membrane-active small molecules against S. aureus
| Compound name | Type of Molecules | MIC (μg/mL) | Organism | References |
|---|---|---|---|---|
| Dialkyl cationic amphiphiles | Peptide mimics | 0.5−4 | S. aureus and MRSA | 84 |
| Structural organophosphorus aminopyrimidines | Peptide mimics | 4 | MRSA | 85 |
| Polyphenylglyoxamide-based small molecular peptidomimetics | Peptide mimics | 2.9−5.6 | S. aureus | 183 |
| Honokiol/magnolol amphiphiles | Peptide mimics | 0.5−2 | MRSA clinical isolates | 113 |
| Aryl-based synthetic mimics of antimicrobial peptides (SMAMPs) | Peptide mimics | 3.13 | S. aureus | 88 |
| Hydantoin derivatives | Peptide mimics | 0.5−3.12 | MRSA | 89 |
| l-lysine-based lipidated biphenyls | Peptide mimics | 3.1 | S. aureus ATCC and MRSA | 90 |
| Dimeric lysine alkylamides | Peptide mimics | 0.75−1.5 1.5−3 3−6 |
MRSA | 81 |
| Cationic deacetyl linezolid derivatives | Peptide mimics | 2−4 | MRSA Clinical isolates | 92 |
| Acetanilide | Peptide mimics | 1 | MRSA | 91 |
| Porphyrin antibacterial agent | Repurposed drug | 4 | S. aureus SH1000 | 191 |
| NH125 (1-hexadecyl-2-methyl-3-(phenylmethyl))-1H-imidazolium iodide | Repurposed drug | 1 | MRSA-MW2 BAA-1707 | 25 |
| Veterinary drug oxyclozanide | Repurposed drug | 4 | MRSA | 96 |
| Bithionol-anthelmintic drug | Repurposed drug | 0.5−2 | MRSA-MW2 BAA-1707 and VRSA1 | 7 |
| PQ401 (diaryl urea) | Repurposed drug | 4 | MRSA and VRSA | 98 |
| α-mangostin | Natural molecules | 0.78−3.125 | S. aureus ATCC 29213 and MRSA clinical isolates | 105 |
| α-mangostin (hydrophobic scaffold of xanthone) | Natural molecules | 0.5−3 | S. aureus DM4001 clinical isolates | 106 |
| Nonpeptidic amphiphilic xanthone derivatives of α-mangostin | Natural molecules | 1.56−3.125 | MRSA clinical isolates | 108 |
| Nisin + Cinnamaldehyde | Natural molecules | 0.1875 + 0.375 | S. aureus ATCC 29213 | 103 |
| Licochalcone E Licochalcone C Licochalcone A Glabrol |
Natural molecules | 4 4 2 2 |
S. aureus ATCC 29213 and MRSA T144 | 109 |
| Balsacone C | Natural molecules | 3–11.6 | Clinical isolates of MRSA and Methicillin sensitive Staphylococcus aureus | 111 |
| Natural berberine-hybridized benzimidazole derivative 2,4-dichlorobenzyl | Natural molecules | 0.008–0.470 | S. aureus ATCC 29213 and MRSA N315 | 110 |
Several amine compounds with membrane-targeting efficacy include alkylamines, an aromatic group containing amines, and quaternary amines in the chemical composition (83). Changing the secondary amine in a series of dialkyl cationic amphiphiles bearing two identical-length lipophilic alkyl chains and one nonpeptidic amide bond improves the membrane targeting efficacy against S. aureus (84) (Fig. 5). A two-nitrogen aromatic aminopyrimidine scaffold is composed of two electron-accepting C = N groups and an electron-donating NH2 moiety. This structure interacts with biomolecules such as DNA, RNA, enzymes, and receptors with high affinity through intermolecular supramolecular interactions (85). Supramolecular interactions occur when small molecules interact noncovalently to form molecular assemblies (86). The supramolecular interaction class is characterized by its noncovalent character. Van der Waals forces, hydrogen bonds, metal-ligand coordination, and electrostatic interactions are examples of such supramolecular interactions (86).
FIG 5.
Changing the secondary amine in a series of dialkyl cationic amphiphiles by adding two identical-length lipophilic alkyl chains and one nonpeptidic amide bond to improve the membrane-targeting efficacy against S. aureus. The figure is adapted from Zhang et al. (84).
Moreover, organophosphorus functional groups may affect heterocycle reactivity, regulate key biological functions, and bind to a variety of biological targets. In organic chemistry, heterocycles are rings of atoms where one or more atoms are different from carbon. A heterocycle is often formed by combining oxygen, nitrogen, and sulfur, but it can also contain selenium, boron, silicon, arsenic, and phosphorus (87). By combining the aminopyrimidine and organophosphorus fragments with DNA-targeting membrane-active potencies, a series of novel structures known as organophosphorus aminopyrimidines were effective against S. aureus cell membrane (85). In a study by Li et al. (85), aminopyrimidine and organophosphorus structures were combined with DNA-targeting, membrane-active potencies, leading to a series of novel organophosphorus structures and aminopyrimidines which were effective against S. aureus cell membrane (85).
Small molecule structures with a central aromatic core exhibit membrane-targeting mechanisms against S. aureus (80). Considering the importance of an aromatic core in the structure of membrane-active molecules, synthetic mimics of antimicrobial peptides (SMAMPs) based on aryl groups and tuned aromatic groups were synthesized (88). Despite its complex mechanism, hydantoin still acted on the membrane and other potential targets, such as DNA and ribosomes (89). The complex mechanism of hydantoin is due to the high molecular weight (normally >1,000 Da), and structural complexity has made the synthesis tedious. Notably, compounds with optimal antibacterial activity require a balance between the hydrophobicity of the peptidomimetics with the bacterial cell membrane. For example, l-lysine-based lipidated biphenyls optimized for hydrophobicity demonstrated bactericidal properties associated with membrane-active nature (90). Also, Niu et al. (81) used a dimerization strategy to design dimeric lysine alkylamides that mimic the structure of AMPs. These AMP-mimics had well-balanced charged and hydrophobic core regions and demonstrated potent bactericidal activity and membrane disruption against MRSA, and some of these derivatives were highly selective to bacteria over mammalian cells (81).
Structure-activity relationships (SARs) indicated that an amphiphilic structure is essential for broad-spectrum activity, particularly against Gram-negative bacteria. Hence, the alkyl chain length was most important for antibacterial activity, whereas different hydrophilic amino acid residues (lysine and arginine) did not significantly influence their antibacterial activity (83). SAR analysis indicated that compounds with more positive charges are more potent against S. aureus and exhibit less toxicity toward mammalian cells (83). Wang et al. (91) used acetanilide to synthesize a new class of structurally unique para-aminobenzene sulfonyl oxadiazoles (91). Changing the structural moieties (for example, changing secondary amine, combining aminopyrimidine and organophosphorus fragments, and adding hydrophobic N-alky sulfonyl group in the existing AMPs) may increase active membrane targeting potency, reduce toxicity, and enhance selectivity against the S. aureus membrane (92, 93). The increased activity and reduced toxicity of peptidomimetics could make them superior antibacterial candidates compared to linear peptides (94).
MEMBRANE-TARGETING SMALL MOLECULES
Similar to peptidomimetics, researchers have reported small molecules that target the cell membrane of S. aureus. Some of these small molecules are chemical or structurally modified from the original structure of natural molecules or repurposed drugs to enhance the efficacy against S. aureus. Repurposing drugs for new uses have become increasingly popular (95) because clinically approved drugs have been tested for toxicity and safety, and their pharmacological properties are well understood (95). There are several membrane-active repurposed drugs tested against S. aureus (Table 2).
Other membrane-targeting small molecules, including oxyclozanide (96), NH125 (1-Hexadecyl-2-methyl-3-[phenylmethyl]-1H-imidazolium iodide) (25), bithionol (97), and PQ401 (98), showed potent bactericidal activity against MRSA. For example, NH125 kills S. aureus by inhibiting WalK, an essential histidine kinase for Gram-positive bacteria (25, 99), and has membrane-targeting efficacy against S. aureus persisters (25). Bithionol demonstrated dose-dependent membrane permeability, altered membrane fluidity and permeabilized S. aureus membrane (97). More recently, Kim et al. (98) also identified a diaryl urea compound, PQ401, which inhibits MRSA from killing the invertebrate model host Caenorhabditis elegans and causes membrane permeabilization of MRSA (98). Bithionol and retinoids showed significant synergism with gentamicin (97, 100). Most likely, synergism works better because gentamicin diffuses more easily through cell membranes of S. aureus that are damaged by retinoid or bithionol.
Notably, some of these membrane-acting agents, such as bithionol and retinoids, act additively or synergistically with established clinical antibiotics (97, 100). Most likely, synergism works better because gentamicin diffuses more easily through cell membranes of S. aureus that are damaged by retinoid or bithionol (101). The combination of antibiotics with membrane-active compounds could also reduce the required dose of both agents, thereby decreasing any potential dose related side effects (100). Moreover, combining two or more antibacterial agents increases antibacterial efficacy and delays the development of antibiotic resistance (102). The combination therapy could be dose-dependent; nisin and cinnamaldehyde in combination exhibited membrane damage against S. aureus (103), but the combination therapy also leads to toxicity in some cases (104).
Small molecules derived from natural products have also demonstrated activity against MRSA membranes (Table 2). An example is α-mangostin (xanthone derivative), a small organic compound with a molecular weight of 410.46 g/mol that resembles the antimicrobial properties of the cationic antimicrobial peptide meta-phenylene ethynylene (mPE) (105). α-mangostin could induce rapid bacterial membrane disruption and alter the proton motive force within the cell, as well as effects the structural of cytoplasmic membrane (105). It is the strong hydrophobic interaction of α-mangostin with lipid alkyl chains that is responsible for its rapid penetration into the cell (105). Isoprenyl groups conjugated to short lipid tails triggered penetration into the hydrophobic region of the membrane. Isoprenyl groups are therefore expected to reduce penetration barriers. In addition, the presence of isoprenyl groups further increased the hydrophobicity of α-mangostin, which enhanced its tendency to partition into membranes (105). In another study, Koh et al. (106) utilized the hydrophobic scaffold of xanthone derivatives to identify three components (hydrophobic xanthone core, lipophilic chains, and cationic amino acid structure) that mimicked an antimicrobial cationic peptide. Notably, even changing the structural characteristics of α-mangostin did not change the toxicity and selectivity against mammalian cells (106). Moreover, Koh et al. (107) performed an extended analysis of structure-activity relationships (SAR) of nonpeptidic xanthone derivatives to identify more compounds with lower hemolytic activity and more membrane selectivity (107). In summary, this study reports the synthesis of 46 amphiphilic xanthone derivatives categorized into cationic moieties, lipophilic chains, and triarm functionalization (108).
Combinations of membrane-targeting natural compounds such as nisin and cinnamaldehyde increased the membrane damage of S. aureus, compared to nisin alone (103). In a recent study, glabrol showed rapid killing ability by disrupting the membrane and dissipating the proton motive force of S. aureus (109). Similarly, natural molecules like berberine-hybridized benzimidazole derivative 2,4-dichlorobenzyl (110), balsacone C (dihydrochalcone extracted from Populus balsamifera) (111), 7-thiazoxime quinolones (112) and honokiol/magnolol (113) were reported to bind and alter the integrity of the S. aureus cell membrane. Notably, attaching a cationic AMP fragment to the structure of honokiol/magnolol improved the interaction with the negative charge of the bacterial cell membranes and improved the insertion of the molecule into the phospholipid bilayer membrane of S. aureus (113).
MOA OF MEMBRANE-ACTIVE AGENTS AGAINST S. AUREUS
The direct killing of bacteria by AMPs and small molecules with antimembrane activity is primarily accomplished by targeting the membrane or interfering with intercellular processes such as protein or nucleic acid synthesis, as well as other metabolic targets (62). However, several mechanisms contribute to the antimicrobial effect of AMPs and small molecules, apart from the direct killing of microbes (114). To identify and develop potential therapeutic candidates, it is essential to understand the mechanism of action (MOA) of these AMPs and small molecules (69). Even though this review focuses on the interaction between AMPs and small molecules and the bacterial membrane, it should be noted that AMPs and small molecules can also interfere with pathogen intercellular processes such as protein or nucleic acid synthesis, as well as other metabolic targets (62). Although in several membrane-active molecules, such as colistin and daptomycin, the mechanisms of actions are debatable (115, 116), understanding their MOA will help design better peptides and small molecules with antipersister and antibiofilm properties. For example, as the peptide-to-lipid ratio changes, AMPs such as BP100 can adopt multiple models of interactions (117). Peptide BP100 is an 11 residue cecropin-melittin hybrid peptide that adopts α-helical amphipathic conformation upon binding to phosphatidylglycerol containing lipid unilamellar vesicles (LUVs) (117). Circular dichroism and dynamic light scattering studies point to peptide and/or vesicle aggregation modulated by higher peptide: lipid ratio (117). Peptide BP100 releases the fluorescent entrapped dye gradually from LUVs when incubated at a low peptide-to-lipid ratio, indicating membrane perturbations (117). However, high ratios of peptide-to-lipid caused rapid loss of vesicle contents, suggesting vesicle disruptions. At high concentrations of peptides, membrane lipid clustering occurs, giving rise to peptide-lipid patches that eventually adopt a carpet-like mechanism (117). As the molecular mechanism of membrane interactions with AMPs has been thoroughly investigated, we will focus on AMP mechanisms in this section and compare them with the small molecules.
Cell Membrane as a Target for AMPs
AMPs are mostly cationic in nature and can interact effectively with the negatively charged lipoteichoic acid-rich membranes of Gram-positive bacteria (69). During this interaction, the hydrophilic domain of the peptide interacts with the charged groups in the phospholipid, resulting in the formation of an amphiphilic structure, while the hydrophobic portions of the peptide interact with the membrane phospholipid bilayer (69). In this regard, the composition of the S. aureus cell membrane makes it an ideal target for AMPs (118), as cardiolipin and phosphatidyl glycerol are both anionic lipids and cationic AMPs can effectively bind to the anionic membrane counterpart to disrupt bacterial cell membranes (118). Cardiolipin makes up 42% of the staphylococcal cell membrane, and phosphatidyl glycerol makes up the remaining 58% (118). Once the AMPs accumulate on the bacterial surface to the desired concentration, they destroy the bacterial membrane through classical and nonclassical membrane targeting. As discussed below, the classical model includes barrel-stave, toroidal pore, and carpet models (63) (Fig. 6), while the nonclassical model of membrane targeting includes the agglutination model (119), sinking raft models (120), and leaky slit models (121).
FIG 6.
Mechanism of action of small molecules and AMPs on S. aureus membrane describing barrel-stave model, toroidal pore model, and carpet model. Figure adapted based on Brogden et al. (63).
Classical models of membrane targeting.
According to the barrel stave model, AMP monomers align themselves along the membrane (122) (Fig. 6). When threshold concentrations are reached, AMP oligomers insert further along the hydrophobic core in an orientation perpendicular to the lipid bilayer (122). The interaction of AMPs with the cell membrane of Gram-positive bacteria causes transmembrane pores. Notably, the amphipathic (α-helix, β-sheet) configuration of the peptide is crucial for pore formation, in which the hydrophobic side faces the membrane lipid, and the hydrophilic side faces the inner barrel wall to form the channel lumen (63, 123). Moreover, to span the entire lipid bilayer, which is 10 nm thick, AMPs need to be at least 22 residues (α-helical) or 8 residues (β-sheet) long. These membrane pores result in the release of cytoplasmic contents, and when the penetration is severe, the bacterial membrane ruptures, killing the bacteria (124). Alamethicin (125), pardaxin (126), and protegrin (127) are examples of AMPs using the barrel stave model as MOA (Fig. 6).
In the toroidal pore model (also known as the wormhole model; Fig. 6), initial AMP adsorption is followed by inserting the AMP perpendicularly inside the lipid bilayer, which induces membrane phospholipid molecules to bend inwards (128). Peptide-peptide interactions are absent, and instead, a transitory lipid-peptide molecule is formed, called a “toroidal pore” (129). As a result, these AMPs destabilize the bacterial membrane by causing thickness changes (hydrophilic and hydrophobic arrangement of lipids is disturbed) or by aggregating and collapsing phospholipid heads that lead to increased membrane permeability. Magainin 2 (128) and melittin (130) are peptides that exhibit this MOA.
In the carpet model, AMPs are first adsorbed parallel to the lipid bilayer and accumulate until a threshold concentration is reached (131) (Fig. 6). As a result, a “carpet” is formed on the surface of the bacterial membrane, which results in the disassembly of the membrane (131). During this process, peptide-peptide interactions between the monomers create a transmembrane channel. In contrast, AMPs destroy membrane integrity by breaking them into small micelles, like aurein 1.2 (132), cecropin (70), indolicidin (133), and human cathelicidin LL-37 (134), which exhibit this MOA.
Compared to the MOA of AMPs, much less is known about the MOA of chemical compounds that target the membrane. Small molecules that cannot span the entire phospholipid membrane layer are believed to work via a carpet model. Such small molecules can first act through permeabilization mechanisms in which they induce small pores in membranes, or act through other destructive mechanisms (75, 118). Some small molecules may enter the cell, resulting in cell machinery and nutrient leaks (75, 118). Other small molecules may act through depolarization of the membrane. For the depolarization of the membrane, compounds disrupt bacterial membrane electronic gradients by forming ion-conducting pores, increasing ion permeability, or acting as ion carriers (75). The change in membrane permeability affects the proton motive force within the bacterial cell, which drives ATP synthesis and other transporters across the membrane, leading to the death of the cell or the ability of other molecules to kill it (135, 136).
Nonclassical models of membrane targeting.
The nonclassical models include agglutination, sinking rafts, and leaky raft models. Thanatin is a peptide that acts via the bacterial cell agglutination model (119). After the initial electrostatic interaction, thanatin forms a micellar complex with the peptidoglycan of Gram-positive bacteria, which induces agglutination and phagocytosis (119).
The “sinking raft” model suggests that the MOA of shorter peptides, such as mastoparsan, cannot be reconciled with an orientation perpendicular to a membrane plane (120) (Fig. 7A). According to this model, the antiparallel orientation of the diamer with its monomer forms a trimer on the membrane surface and sinks into the outer bilayer leaflet (120). During this process, the helices bend slightly, sinking deeper in the middle, so that their hydrophilic terminals remain initially in contact with water (120). A combination of relative rotation and downward movement leads to the helices moving deeper and hydrophobic residues in the trimer are in contact with lipid acyl chains (120). In addition to the formation of trimer hydrophobic residues, hydrophilic faces of helices line and form a cavity (120).
FIG 7.
(A) Sinking raft model. This model illustrates how peptide trimers are inserted and translocated across the lipid bilayer. In the cross sections of ɑ-helical peptides, the darker half-circles represent hydrophobic faces, and the lighter half-circles represent polar faces. The polar angle of δ-lysin is 180°. In step 1, the peptide forms a trimer on the phospholipid surface of the outer membrane. During step 2, the peptide sinks into the outer leaflet of the bilayer. In steps 3 and 4, a cavity is formed, which is the most unstable intermediate state. Finally, in step 5, translocation is completed symmetrically, and the trimer emerges on the inner membrane. The figure was based on the sinking raft model of δ-lysin (120, 218) (B) The “leaky slit” model is used to explain the membrane-damaging action of AMPs such as Plantaricin A (121).
Peptides that follow the “leaky slit” MOA are oriented perpendicular to the membrane, but instead of forming a circular pore, they aggregate side-by-side to form an amphipathic ribbon array (121) (Fig. 7B). In this model, the peptide hydrophobic residues face the hydrocarbon chains of the bilayer, and the hydrophilic residues are oriented toward the charged phospholipid heads. The interaction between the charged phospholipid heads of the membrane and the exposed hydrophilic amino acids from the peptides (e.g., plantaricin A) forms an aggregate, as the hydrophilic face cannot be sealed with opposing contacting bilayers (121, 137). Consequently, lipids adopt a highly positive curvature, causing the membrane to bend inwards to form a pore (121). This leaky slit arrangement of the peptide with the membranes is expected to be highly permeable to solutes and difficult for the cell to repair (121).
Other Antimicrobial Effects of Membrane-Active AMPs and Small Molecules
Membrane-targeting AMPs can also downregulate the expression of key virulence and biofilm genes in S. aureus. Several cationic AMPs, such as C18, NK-18, and LL-37, can directly bind to the DNA and AMPs are known to be regulators of gene expression (70, 138, 139). More specifically, C18 decreased the expression of fnb-A and clf-1 that control the adhesion, colonization, and invasion (70). Additionally, human β-defensin 3 inhibits biofilm formation by reducing the expression of biofilm-producing genes icaA and icaD and increasing the expression of icaR (140).
AMPs and other membrane-active molecules interact with other components of the cell envelope before encountering the plasma membrane. The bacterial cell envelope is a dynamic, multilayered structure that protects it from unpredictable and hostile conditions (53, 141). In S. aureus the bacterial cell envelope is composed of capsular polysaccharides, the cell wall, teichoic acid polymers, and the phospholipid membrane (53). The polysaccharide microcapsules produced by S. aureus and their inhibition of complement cascades can impede opsonization (142). In contrast, USA300, that is an important MRSA clone, apparently lacks the microcapsule (143). Gram-positive organisms do not have an outer membrane, but their peptidoglycan layers are thicker than Gram-negative organisms (53, 141). Generally, the peptidoglycan network is composed of up to 80 layers of alternating disaccharide N-acetylglucosamine and N-acetyl muramic acid (144). A pentapeptide chain of the sequence l-alanyl-d-glutamyl-diaminopimelyl-l-lysyl-d-alanyl-d-alanine interconnects the disaccharides and an inter bridging peptide of the sequence GGGGG links two disaccharide pentapeptide moiety (141) (Fig. 8). The glycosyltransferase MurG transfers N-acetylglucosamine residue from uridine 5′-diphospho-N-acetylglucosamine (UDP-GlcNAc) to lipid I (145). The consequent product, lipid II, is then translocated to the periplasmic side of the membrane before being incorporated into the growing peptidoglycan network by MurJ (146). Teichoic acid is an important component of the cell envelope, which plays crucial role in cell envelope physiology and pathogenesis (147–149). Other significant cell envelope components in S. aureus include capsular polysaccharides attached covalently to peptidoglycan and extracellular polysaccharides forming an amorphous outer layer (150, 151).
FIG 8.

The complex cell wall structure of S. aureus is composed of a thick peptidoglycan layer, lipoteichoic acid, surface protein, and membrane protein. Figure adapted based on Epand et al. (219).
Several membrane-active AMPs and small molecules seem to also affect peptidoglycan biosynthesis (Table 3). A peptidoglycan targeting strategy commonly used by selective small molecules and AMPs is to specifically bind to components of the peptidoglycan synthesis machinery, such as lipid II, to inhibit or destroy the cell wall (152). AMPs affecting the inhibition of cell wall synthesis are known to act in very low concentrations (153). For example, the peptide nisin inhibits peptidoglycan synthesis at low concentrations by binding to the lipid II molecule, while at higher concentrations it works by targeting the bacterial cell membranes (153). Nisin is a bacteriocin AMP classified as a type A (I) lantibiotic with several unusual amino acids (154). The FDA has approved Nisin as a generally regarded as safe (GRAS) peptide that is widely used as a food preservative (155). Human α-defensin, including the human neutrophil peptide-1, binds to lipid II to kill S. aureus (156). Macrocyclic depsipeptide, such as ramoplanin, inhibits peptidoglycan biosynthesis at the periplasmic transglycosylation step (157). Other than targeting the peptidoglycans, some linear AMPs (i.e., RWRWRW-NH2) target the respiration process in bacteria and delocalize the peripheral membrane proteins MurG and disrupts the integrity of the cell wall (158).
TABLE 3.
Examples of cell wall-targeting AMPs and small molecules with impacts on phospholipid membranes
| Compound | Action on cell wall | Cell wall references | Action on cell membrane | Cell membrane references |
|---|---|---|---|---|
| Nisin | Nisin’s A/B-ring forms a 1:1 stoichiometric cage with lipid II's pyrophosphate moiety and blocks further peptidoglycan synthesis | 220 | Membrane permeabilization of bacterial cells | 221 |
| Human α-defensin | The HNP-1 peptide bound to lipid II with high affinity (binding constant Kd-2 × 106 M-1), and reduced levels of lipid II in bacterial membranes resulted in a significant reduction in bacterial killing | 156 | In vitro channel forming ability in planar lipid bilayer membranes | 222 |
| Ramoplanin | Forms a 2:1 stoichiometric complex with lipid II that inhibits peptidoglycan biosynthesis at the periplasmic transglycosylation step | 223 | Membrane depolarization of S. aureus | 224 |
| RWRWRW-NH2 | Delocalizes essential peripheral membrane proteins involved in cell wall biosynthesis and respiration | 158 | Membrane depolarization of B. subtilis | 202 |
| CP29, Bac2A-NH2, and CP11CN | Causes laminar mesosomes-like structures arising from the septa and cell wall | 203 | Membrane depolarization and permeabilization of S. aureus | 225 |
| Lysines conjugated lipidated biphenyls (Compound 2) | Blocks peptidoglycan biosynthesis | 90 | Depolarize the cell membrane of S. aureus and leakage of intracellular K+ ions | 90 |
| 2-[1-[(2-chlorophenyl)methyl]-2-methyl-5-methylsulfanylindol-3-yl]ethanamine and 2-[1-[(3,4-dichlorophenyl)methyl]-2-methyl-5-methylsulfanylindol-3-yl]ethanamine | Interacts with lipid II substrate via pyrophosphate motif | 226 | Showed membrane depolarization and disruption activity against negatively charged lipid vesicles | 226 |
ACTIVITY AGAINST S. AUREUS BIOFILM AND PERSISTERS
AMPs and Small Molecules that Affect S. aureus Biofilms
Biofilms are targeted by multiple mechanisms like disruption of quorum sensing (QS) (152), suppression of the cellular stress response (159), downregulation of virulence/biofilm genes (70, 160), and direct disruption of the biofilm matrix (152). Biofilms can render conventional antibiotics ineffective (161). Within biofilms, polysaccharide intercellular adhesins provide a stable, hydrated matrix that binds cells together in a three-dimensional (3D) structure. The recognition of microbial surface components recognize adhesive matrix molecules determines the initial attachment of S. aureus to form biofilm on the host cell surface (162, 163). Several components play an active role in biofilm attachment, including elastin binding protein (ebpS), laminin-(eno), fibronectin-binding protein (fnbA and fnbB), fibrinogen binding protein (fib), and aggregation factor (clfA and clfB) (164). In particular, the agr system plays a significant role in the initial attachment and disassembly of biofilms by repressing adhesins and stimulating dispersal factors (165). Coordination of these factors is essential to ensure progress through the three stages of biofilm formation and the development of S. aureus chronic infections (163).
Disruption of Quorum Sensing by Membrane-Active AMPs and Small Molecules
There are limited examples of inhibition of QS by cationic AMPs and are mostly seen in Gram-negative bacteria (166). A few studies have demonstrated that membrane-active AMPs inhibit or target S. aureus QS (152). Recently, the Cec4-derived C18 peptide was found to downregulate the expression of agrA (70). Also, in a recent study, BCp12, a milk-derived AMP, is predicted to target the S. aureus cytoplasmic membrane, and also affects the QS system by downregulating agrA, agrB, and agrC (167, 168). A lack of agr increases biofilm formation because RNAIII reduces surface adhesin expression and increases capsule, toxin, and protease production (169). Surface adhesins of S. aureus are essential for the initial attachment of biofilm cells and intercellular adhesion during the biofilm maturation process (170, 171).
Suppression of the Cellular Stress Response by Membrane-Active AMPs
Under starvation conditions, bacteria produce biofilms, and their proficiency for transferring resistance genes increases and are thus more resistant to antibiotics (152, 159, 172). To coordinate the stringent response, guanosine pentaphosphate and its active moiety (ppGpp) are synthesized as small signaling nucleotides called alarmones, which bind to and change the specificity of RNA polymerase (159). A ppGpp network generally plays a role in “triggered persistence,” which occurs when specific signals, such as starvation and nutrient stress, trigger bacterial persistence (173). A loss of ppGpp will therefore not push the bacteria toward dormancy or induce persistence and would make the situation difficult for the antimicrobials to work (174, 175). RelA and SpoT are homologous proteins that control ppGpp levels, and mutants lacking both relA and spoT (or rsh) were defective in forming biofilms (159, 176). Interestingly, the S. aureus membrane-active AMP 1018 caused a complete loss of ppGpp in S. aureus (159). Upon overexpression of relA synthase, peptide IDR-1018 could no longer inhibit S. aureus biofilms, and genetic blocking of ppGpp synthesis eradicated 2-day mature biofilms (159).
Disruption of the Biofilm Matrix by Membrane-Active AMPs and Small Molecules
Several AMPs, including tryptophan-rich horine (177), DFTamp1 (68), temporins-1Ola (178), WWW motif-based peptides (72), and LL-37-derived GF-17 and 17BIPHE2 (134, 179) target the cell membrane, reduce the biomass of established S. aureus biofilms, and kill the bacterial cells within the biofilm. Mechanistically, membrane-active AMPs weaken the biofilm matrix by forming pores within lipid components (180). Some AMPs such as the Lf-KR-12 peptide (180) and esculentin-1a (181), can disrupt biofilms and alter membrane potential leading to further membrane rupture.
Nisin A and lacticin Q are bacteriocins that form stable pores on S. aureus membranes and inhibit biofilm formation (182). Similarly, membrane-active small molecules like lipidated biphenyls (90), 2,4-dichlorobenzyl derivative (110), phenylglyoxamide (183), NH125 (25), retinoids (100), and alpha mangostin (160) disrupt the mature/preformed biofilm of S. aureus (Table 4). Even though the AMPs and small molecules described in this section depolarize the S. aureus membrane and ultimately rupture it, genetic basis or proteomic evidence is still lacking.
TABLE 4.
Membrane-active small molecules against the persister and biofilm of S. aureus
| Membrane-active small molecules/AMPs | Structure of Small molecules/AMPs | S. aureus strain | Activity against S. aureus persister | Activity against S. aureus biofilm | References |
|---|---|---|---|---|---|
| nTZDpa – nonthiazolidinedione peroxisome proliferator-activated receptor gamma partial agonist |

|
MRSA-MW2 BAA-1707 | 32 μg/mL reduction ~2-log and 64 μg/mL eradicated ~5 × 107 CFU/mL MRSA persister in 2 h | 16 μg/mL eradicated ~107 CFU/mL MRSA MW2 within 2 h | 187 |
| NH125 – WalK inhibitor |

|
MRSA-MW2 BAA-1707 | 5 μg/mL eradicated 5 × 107−108 CFU/mL S. aureus persister within 4 h | 25 | |
| CD437 – synthetic retinoid |

|
MRSA-MW2 BAA-1707 | At 8–10× MIC eradicated, the persister of S. aureus within 1–4 h | 90% MRSA persister biofilm killing at 16× MIC | 100 |
| CD1530 – synthetic retinoid |

|
MRSA-MW2 BAA-1707 | At 8–10× MIC eradicated, the persister of S. aureus within 1–4 h | 100% MRSA persister biofilm killing at 32× MIC | 100 |
| α-mangostin – natural xanthone |

|
MRSA-MW2 BAA-1707 | Eradicated 99.99% of S. aureus persister within 30 min of treatment | Disrupted (96%) and inhibited (90%) the biofilm of S. aureus | 105 |
AMPs and Small Molecules against S. aureus Persisters
Hurdle et al. (54) proposed that membrane perturbation is a more profitable mechanism of action than nonmembrane-targeting antibiotics to eliminate persister cells because of the possibility of developing resistance (54). For example, membrane-active AMPs, including C10KR8d (184), horine and verine (177), DFT561d (185), SAAP-148 (186), and small molecules, including CD437 (100), and nTZDpa (187) showed least change in MICs after several serial passages against Staphylococcus aureus.
Persister cells are not actively dividing (188), and membrane disruptors can kill bacteria that are not actively dividing, unlike other antibiotics that require active division of bacteria to be effective (189). AMPs like frog-skin AMP temporin G (TG) (190), cecropin-4 derived AMPs (70), short cationic AMPs (varine and horine) (177), and SAAP-148 derived from LL-37 (186) eliminated S. aureus persisters. Interestingly, cationic AMPs show greater potential to eliminate S. aureus persisters. It is therefore likely that cationic AMPs will be effective at eliminating nondividing and antibiotic-tolerant persister S. aureus bacteria or potentiating other antibacterial agents. Several other findings support that membrane-active small molecules could kill nondividing bacterial persisters effectively (97, 187). Small molecules such as XF-73 (191), NH125 (25), lipidated biphenyls derived from l-lysine (90), nTZDpa (187), retinoids (CD437 and CD1530) (100), and bithionol (97) seem to exhibit rapid killing of persisters. A hydrophobic interaction occurs between the aromatic backbones and lipid tails of membrane-active small molecules with the hydrophilic carboxylic acid and phenolic hydroxyl moiety binding heads of S. aureus lipid bilayers (100) (Table 4). In this interaction, the lipid bilayers of the membrane are permanently damaged (100). Notably, changing the structure of nTZDpa by modifying three polar branch groups or the carboxylic acid moiety of the aryl thioether improved the efficacy against stationary-phase and persister S. aureus cells (192).
EFFICACY IN MAMMALIAN ANIMAL MODELS
The selectivity and toxicity of membrane-active AMPs and small molecules to mammalian cells are a major hurdle in moving these agents to clinical practice. Nevertheless, the local application of membrane-active drugs is already proved in animal models (Table 5). For example, mouse skin infection models were implemented in testing small molecules and AMPs like LTX-109 (79), 1% HT61 gel (193), small cationic molecule (194), and l-lysine-based lipidated biphenyls (90) against S. aureus superficial skin wound infection and dermal infections. Studies also evaluated membrane-active AMPs and small molecules in an MRSA corneal infection model (106) and the mouse thigh that mimic human deep-seated chronic infections caused by the MRSA persister model (97). Regarding systemic infection models, Mishra et al. (185) tested the in vivo toxicity and efficacy of a cationic peptide DFT561 in a neutropenic mouse model. DFT561 demonstrated 90% killing of MRSA and reduced MRSA load in the liver, spleen, and kidney of mice (185). Also, using a sepsis model, Guo et al. (113) found a significant reduction in MRSA with cationic AMP-linked honokiol/magnolol amphiphiles.
TABLE 5.
In vivo analysis of membrane-active small molecules and AMPs against S. aureus
| Membrane-active small molecules and AMPs | Effect against in vivo model | Effective concn against S. aureus | Animal model | References |
|---|---|---|---|---|
| nTZDpa | 90% survival | 16 μg/mL | C. elegans | 187 |
| PQ401 | 100% survival | 2 μg/mL | C. elegans | 98 |
| Small cationic molecule | 5.3-log MRSA biofilm reduction (>99.99%) | 40 mg/kg | Murine model of superficial skin wound infection | 194 |
| l-lysine-based lipidated biphenyls | Reduced bacterial burden (P value 0.0001) | 200 mg/kg | Murine model of skin infection | 90 |
| Xanthones derivatives | MRSA reduction by 2.56 logs (99.7%) and 3.03 logs (99.9%) | 3 mg/mL | Mouse model of corneal infection | 106 |
| Bithionol + gentamicin | 90% MRSA persister killed | 30 mg/kg of bithionol + gentamicin | Mouse thigh infections | 97 |
| Cationic peptide DFT561 | S. aureus USA300 LAC decreased by 1.8 logs (liver) and 1.4 logs (kidney) | 5 mg/kg | Neutropenic mouse model | 185 |
| AMP is based on the cationic structure of honokiol/magnolol amphiphiles | Reduction in bacterial loads in the liver, spleen, and kidney in 3 days | 5 and 10 mg/kg | Murine sepsis model | 113 |
DRAWBACKS AND CHALLENGES ASSOCIATED WITH AMPS
A critical factor that affects the binding of cationic AMPs to the bacterial membrane is the presence of salt and serum (195). The antimicrobial effect of AMPs is often reduced by a process known as the “charge shielding effect,” which involves competitive binding between cations in salts and AMPs (195). However, the charge-shielding effect can be reduced or nullified by adding more numbers of charged amino acids to the peptides (180). AMPs, such as the cecropin-derived AMP C18 (+6 net charge) and the arginine-rich decamer peptides D5 (+8 net charge) and D6 (+9 net charge) maintain high antimicrobial efficacy even in the presence of monovalent or divalent salt cations (196).
Several AMPs, including LL-37, TP4, SAAP-159, and H1α, lose their antimicrobial potency in the presence of serum (197). The interaction between LL-37 and serum albumin could reduce the ability of the peptide to bind to pathogens (186). Recently, LL-37-derived KR-12 lipopeptides interacted with serum albumin, hemoglobin, serotransferrin, and apolipoproteins in mouse serum (184). Interestingly, the d-form of KR12 lipopeptides demonstrated reduced serum binding compared to its l-form (184).
Also, AMPs such as ARVA and NATT (short synthetic AMPs discovered through a combinatorial library-based approach) (198), have poor solubility and host cell toxicity and are susceptible to proteolysis (199). Using synthetic molecular evolution, Starr et al. (199) achieved high solubility and host cell selectivity of AMPs by applying selection pressures during initial AMP screening. Similarly, by using computational simulation techniques, Pandey et al. (200) designed new nisin mutants with more solubility at physiological pH.
Moreover, linear AMPs are more susceptible to proteolysis by the host proteases (185). However, the derivatives of AMP Pep05 containing unnatural residues such as l-2,4-diaminobutanoic acid (Dab), l-2,3-diaminopropionic acid (Dap), l-homoarginine, 4-aminobutanoic acid (Aib), and l-thienylalanine, have improved stability toward trypsin, plasma proteases, and secreted bacterial proteases (201). d-analogs of DFT561 (185) and KR12 lipidated version had more enzymatic stability to proteolytic degradations (184).
BACTERIAL SYSTEM INVOLVED IN SUSCEPTIBILITY TO MEMBRANE-ACTIVE AGENTS
Several mechanisms protect bacteria from membrane-targeting antimicrobial peptides (AMPs) and small molecules (202). Among these mechanisms are regulatory systems that modify the surface charge of the bacterial cell membrane to reduce binding to cationic AMPs, breaking down AMPs with secreted exoproteases, and using AMP transporters to remove AMPs (203, 204). In the case of S. aureus, the GraRS regulatory system plays a key role in bacteria cell surface charge-mediated deactivation of membrane-targeting compounds (205). In MRSA, the expression of two key determinants of net positive surface charge (encoded by mprF and dlt) depends on the cotranscription of graR and vraG genes (205). The MprF protein helps the synthesis and movement of lysyl-PG to the outer leaflet of the bacterial membrane, increasing the net-positive surface charge (206). Similarly, the dlt operon reduces net negative surface charges in S. aureus by transferring extra d-alanine to teichoic acids (207). Other regulatory systems in S. aureus are Aps and BraRS (208–210). The Aps system is composed of a two-component histidine kinase/response regulator (ApsR, ApsS), a third protein of unknown function (ApsX) and is thought to be a widespread mechanism for sensing and regulating AMPs in bacteria (208–210). The BraRS system is quite specific to S. aureus (210). The membrane charge of S. aureus also affects the effectiveness of antibacterial agents such as moenomycin and vancomycin (211).
S. aureus cleaves membrane-active AMPs by expressing two types of proteinases: a metalloproteinase called aureolysin and a glutamyl endopeptidase called V8 protease (203, 212). Aureolysin inactivates cationic AMPs, including LL-37, by cleaving specific residues involving Arg19-Ile20, Arg23-Ile24, and Leu31-Val32, while the V8 protease hydrolyzes the Glu16-Phe17 peptide bond, making the C-terminal fragment resistant to further degradation (212). Contrary to AMPs, synthetic chemical compounds lack peptide bonds, so proteases cannot cleave them. Also, in S. aureus, the PmtA-D proteins form an ABC transporter (213). While, structurally, the Pmt transporter is composed of two membrane proteins (PmtA and C) and two ATPases (PmtB and D) (213), functionally, they are associated with exporting human-derived AMPs such as hBD3 and LL-37 (214, 215).
CONCLUSIONS
Membrane-targeting small molecules and AMPs such as colistin, daptomycin, gramicidin, and nisin indicate that membrane-active compounds have a potential role in combating S. aureus infections. However, the toxicity, serum binding, stability, and product cost of membrane-active medications remain major hurdles to the wider adoption of these agents. Studies have described the MOA of AMPs using classical and nonclassical membrane-targeting models, but to date, no clear MOA has been described for small molecules. The gap in understanding how membrane-active compounds work against S. aureus needs to be bridged to enhance drug development and application in clinical trials.
ACKNOWLEDGMENTS
This study was supported by National Institutes of Health Grant P01 AI083214 to E.M. This work was supported by P20GM121344 from the National Institute of General Medical Sciences, which funds the Center for Antimicrobial Resistance and Therapeutic Discovery to B.M.
We declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
REFERENCES
- 1.Laupland KB, Lyytikäinen O, Søgaard M, Kennedy KJ, Knudsen JD, Ostergaard C, Galbraith JC, Valiquette L, Jacobsson G, Collignon P, Schønheyder HC, International Bacteremia Surveillance Collaborative . 2013. The changing epidemiology of Staphylococcus aureus bloodstream infection: a multinational population-based surveillance study. Clin Microbiol Infect 19:465–471. doi: 10.1111/j.1469-0691.2012.03903.x. [DOI] [PubMed] [Google Scholar]
- 2.Nambiar K, Seifert H, Rieg S, Kern WV, Scarborough M, Gordon NC, Kim HB, Song KH, Tilley R, Gott H, Liao CH, Edgeworth J, Nsutebu E, López-Cortés LE, Morata L, Walker AS, Thwaites G, Llewelyn MJ, Kaasch AJ, International Staphylococcus aureus collaboration (ISAC) study group (with linked authorship to members in the Acknowledgements) and the ESCMID Study Group for Bloodstream Infections and Sepsis (ESGBIS) . 2018. Survival following Staphylococcus aureus bloodstream infection: a prospective multinational cohort study assessing the impact of place of care. J Infect 77:516–525. doi: 10.1016/j.jinf.2018.08.015. [DOI] [PubMed] [Google Scholar]
- 3.Thwaites GE, Scarborough M, Szubert A, Nsutebu E, Tilley R, Greig J, Wyllie SA, Wilson P, Auckland C, Cairns J, Ward D, Lal P, Guleri A, Jenkins N, Sutton J, Wiselka M, Armando GR, Graham C, Chadwick PR, Barlow G, Gordon NC, Young B, Meisner S, McWhinney P, Price DA, Harvey D, Nayar D, Jeyaratnam D, Planche T, Minton J, Hudson F, Hopkins S, Williams J, Török ME, Llewelyn MJ, Edgeworth JD, Walker AS, United Kingdom Clinical Infection Research Group (UKCIRG) . 2018. Adjunctive rifampicin for Staphylococcus aureus bacteraemia (ARREST): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet 391:668–678. doi: 10.1016/S0140-6736(17)32456-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Hal SJ, Jensen SO, Vaska VL, Espedido BA, Paterson DL, Gosbell IB. 2012. Predictors of mortality in Staphylococcus aureus Bacteremia. Clin Microbiol Rev 25:362–386. doi: 10.1128/CMR.05022-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.CDC MF, Form MVR. 2016. Methicillin-resistant Staphylococcus aureus (MRSA). [Google Scholar]
- 6.Weiner-Lastinger LM, Abner S, Edwards JR, Kallen AJ, Karlsson M, Magill SS, Pollock D, See I, Soe MM, Walters MS, Dudeck MA. 2020. Antimicrobial-resistant pathogens associated with adult healthcare-associated infections: summary of data reported to the National Healthcare Safety Network, 2015–2017. Infect Control Hosp Epidemiol 41:1–18. doi: 10.1017/ice.2019.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klein EY, Milkowska-Shibata M, Tseng KK, Sharland M, Gandra S, Pulcini C, Laxminarayan R. 2021. Assessment of WHO antibiotic consumption and access targets in 76 countries, 2000–15: an analysis of pharmaceutical sales data. Lancet Infect Dis 21:107–115. doi: 10.1016/S1473-3099(20)30332-7. [DOI] [PubMed] [Google Scholar]
- 8.Tickler IA, Goering RV, Mediavilla JR, Kreiswirth BN, Tenover FC, HAI Consortium . 2017. Continued expansion of USA300-like methicillin-resistant Staphylococcus aureus (MRSA) among hospitalized patients in the United States. Diagn Microbiol Infect Dis 88:342–347. doi: 10.1016/j.diagmicrobio.2017.04.016. [DOI] [PubMed] [Google Scholar]
- 9.Balaban NQ, Helaine S, Lewis K, Ackermann M, Aldridge B, Andersson DI, Brynildsen MP, Bumann D, Camilli A, Collins JJ, Dehio C, Fortune S, Ghigo J-M, Hardt W-D, Harms A, Heinemann M, Hung DT, Jenal U, Levin BR, Michiels J, Storz G, Tan M-W, Tenson T, Van Melderen L, Zinkernagel A. 2019. Definitions and guidelines for research on antibiotic persistence. Nat Rev Microbiol 17:441–448. doi: 10.1038/s41579-019-0196-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jacoby GA. 2009. AmpC beta-lactamases. Clin Microbiol Rev 22:161–182, Table of Contents. doi: 10.1128/CMR.00036-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Du D, Wang-Kan X, Neuberger A, van Veen HW, Pos KM, Piddock LJV, Luisi BF. 2018. Multidrug efflux pumps: structure, function and regulation. Nat Rev Microbiol 16:523–539. doi: 10.1038/s41579-018-0048-6. [DOI] [PubMed] [Google Scholar]
- 12.Balaban NQ, Liu J. 2019. Evolution under antibiotic treatments: interplay between antibiotic persistence, tolerance, and resistance. Persister cells and infectious disease:1–17. doi: 10.1007/978-3-030-25241-0_1. [DOI] [Google Scholar]
- 13.Meylan S, Andrews IW, Collins JJ. 2018. Targeting antibiotic tolerance, pathogen by pathogen. Cell 172:1228–1238. doi: 10.1016/j.cell.2018.01.037. [DOI] [PubMed] [Google Scholar]
- 14.Fridman O, Goldberg A, Ronin I, Shoresh N, Balaban NQ. 2014. Optimization of lag time underlies antibiotic tolerance in evolved bacterial populations. Nature 513:418–421. doi: 10.1038/nature13469. [DOI] [PubMed] [Google Scholar]
- 15.Brauner A, Fridman O, Gefen O, Balaban NQ. 2016. Distinguishing between resistance, tolerance and persistence to antibiotic treatment. Nat Rev Microbiol 14:320–330. doi: 10.1038/nrmicro.2016.34. [DOI] [PubMed] [Google Scholar]
- 16.Gefen O, Balaban NQ. 2009. The importance of being persistent: heterogeneity of bacterial populations under antibiotic stress. FEMS Microbiol Rev 33:704–717. doi: 10.1111/j.1574-6976.2008.00156.x. [DOI] [PubMed] [Google Scholar]
- 17.Fisher RA, Gollan B, Helaine S. 2017. Persistent bacterial infections and persister cells. Nat Rev Microbiol 15:453–464. doi: 10.1038/nrmicro.2017.42. [DOI] [PubMed] [Google Scholar]
- 18.Ledger EV, Mesnage S, Edwards AM. 2022. Human serum triggers antibiotic tolerance in Staphylococcus aureus. Nat Commun 13:2041. doi: 10.1038/s41467-022-29717-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meredith EM, Harven LT, Berti AD. 2022. antimicrobial efficacy against antibiotic-tolerant Staphylococcus aureus depends on the mechanism of antibiotic tolerance. Antibiotics 11:1810. doi: 10.3390/antibiotics11121810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lewis K. 2007. Persister cellules, la dormance et les maladies infectieuses. Nature 5:48–56. [Google Scholar]
- 21.Keren I, Kaldalu N, Spoering A, Wang Y, Lewis K. 2004. Persister cells and tolerance to antimicrobials. FEMS Microbiol Lett 230:13–18. doi: 10.1016/S0378-1097(03)00856-5. [DOI] [PubMed] [Google Scholar]
- 22.Allison KR, Brynildsen MP, Collins JJ. 2011. Metabolite-enabled eradication of bacterial persisters by aminoglycosides. Nature 473:216–220. doi: 10.1038/nature10069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Conlon BP, Nakayasu ES, Fleck LE, LaFleur MD, Isabella VM, Coleman K, Leonard SN, Smith RD, Adkins JN, Lewis K. 2013. Activated ClpP kills persisters and eradicates a chronic biofilm infection. Nature 503:365–370. doi: 10.1038/nature12790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim W, Conery AL, Rajamuthiah R, Fuchs BB, Ausubel FM, Mylonakis E. 2015. Identification of an antimicrobial agent effective against methicillin-resistant Staphylococcus aureus persisters using a fluorescence-based screening strategy. PLoS One 10:e0127640. doi: 10.1371/journal.pone.0127640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim W, Fricke N, Conery AL, Fuchs BB, Rajamuthiah R, Jayamani E, Vlahovska PM, Ausubel FM, Mylonakis E. 2016. NH125 kills methicillin-resistant Staphylococcus aureus persisters by lipid bilayer disruption. Future Med Chem 8:257–269. doi: 10.4155/fmc.15.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levin-Reisman I, Brauner A, Ronin I, Balaban NQ. 2019. Epistasis between antibiotic tolerance, persistence, and resistance mutations. Proc Natl Acad Sci USA 116:14734–14739. doi: 10.1073/pnas.1906169116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nguyen TK, Peyrusson F, Dodémont M, Pham NH, Nguyen HA, Tulkens PM, Van Bambeke F. 2020. The persister character of clinical isolates of Staphylococcus aureus contributes to faster evolution to resistance and higher survival in THP-1 monocytes: a study with moxifloxacin. Front Microbiol 11:587364. doi: 10.3389/fmicb.2020.587364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manandhar S, Singh A, Varma A, Pandey S, Shrivastava N. 2022. High level of persister frequency in clinical staphylococcal isolates. BMC Microbiol 22:1–11. doi: 10.1186/s12866-022-02529-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bär J, Boumasmoud M, Mairpady Shambat S, Vulin C, Huemer M, Schweizer TA, Gómez-Mejia A, Eberhard N, Achermann Y, Zingg PO, Mestres CA, Brugger SD, Schuepbach RA, Kouyos RD, Hasse B, Zinkernagel AS. 2022. Quantification of within-patient Staphylococcus aureus phenotypic heterogeneity as a proxy for the presence of persisters across clinical presentations. Clin Microbiol Infect 28:1022.e1–1022.e7. doi: 10.1016/j.cmi.2022.01.021. [DOI] [PubMed] [Google Scholar]
- 30.El-Halfawy OM, Valvano MA. 2015. Antimicrobial heteroresistance: an emerging field in need of clarity. Clin Microbiol Rev 28:191–207. doi: 10.1128/CMR.00058-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chambers HF, Hartman BJ, Tomasz A. 1985. Increased amounts of a novel penicillin-binding protein in a strain of methicillin-resistant Staphylococcus aureus exposed to nafcillin. J Clin Invest 76:325–331. doi: 10.1172/JCI111965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoshida R, Kuwahara-Arai K, Baba T, Cui L, Richardson JF, Hiramatsu K. 2003. Physiological and molecular analysis of a mecA-negative Staphylococcus aureus clinical strain that expresses heterogeneous methicillin resistance. J Antimicrob Chemother 51:247–255. doi: 10.1093/jac/dkg036. [DOI] [PubMed] [Google Scholar]
- 33.Durán SP, Kayser F, Berger-Bächi B. 1996. Impact of sar and agr on methicillin resistance in Staphylococcus aureus. FEMS Microbiol Lett 141:255–260. doi: 10.1111/j.1574-6968.1996.tb08394.x. [DOI] [PubMed] [Google Scholar]
- 34.Wu S, de Lencastre H, Tomasz A. 1996. Sigma-B, a putative operon encoding alternate sigma factor of Staphylococcus aureus RNA polymerase: molecular cloning and DNA sequencing. J Bacteriol 178:6036–6042. doi: 10.1128/jb.178.20.6036-6042.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cuirolo A, Plata K, Rosato AE. 2009. Development of homogeneous expression of resistance in methicillin-resistant Staphylococcus aureus clinical strains is functionally associated with a β-lactam-mediated SOS response. J Antimicrob Chemother 64:37–45. doi: 10.1093/jac/dkp164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harigaya Y, Ngo D, Lesse AJ, Huang V, Tsuji BT. 2011. Characterization of heterogeneous vancomycin-intermediate resistance, MIC and accessory gene regulator (agr) dysfunction among clinical bloodstream isolates of Staphyloccocus aureus. BMC Infect Dis 11:7. doi: 10.1186/1471-2334-11-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Katayama Y, Murakami-Kuroda H, Cui L, Hiramatsu K. 2009. Selection of heterogeneous vancomycin-intermediate Staphylococcus aureus by imipenem. Antimicrob Agents Chemother 53:3190–3196. doi: 10.1128/AAC.00834-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Howden BP, McEvoy CRE, Allen DL, Chua K, Gao W, Harrison PF, Bell J, Coombs G, Bennett-Wood V, Porter JL, Robins-Browne R, Davies JK, Seemann T, Stinear TP. 2011. Evolution of multidrug resistance during Staphylococcus aureus infection involves mutation of the essential two component regulator WalKR. PLoS Pathog 7:e1002359. doi: 10.1371/journal.ppat.1002359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Neoh H-m, Cui L, Yuzawa H, Takeuchi F, Matsuo M, Hiramatsu K. 2008. Mutated response regulator graR is responsible for phenotypic conversion of Staphylococcus aureus from heterogeneous vancomycin-intermediate resistance to vancomycin-intermediate resistance. Antimicrob Agents Chemother 52:45–53. doi: 10.1128/AAC.00534-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matsuo M, Hishinuma T, Katayama Y, Cui L, Kapi M, Hiramatsu K. 2011. Mutation of RNA polymerase β subunit (rpoB) promotes hVISA-to-VISA phenotypic conversion of strain Mu3. Antimicrob Agents Chemother 55:4188–4195. doi: 10.1128/AAC.00398-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cui L, Isii T, Fukuda M, Ochiai T, Neoh H-m, Camargo I, Watanabe Y, Shoji M, Hishinuma T, Hiramatsu K. 2010. An RpoB mutation confers dual heteroresistance to daptomycin and vancomycin in Staphylococcus aureus. Antimicrob Agents Chemother 54:5222–5233. doi: 10.1128/AAC.00437-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zapotoczna M, O'Neill E, O'Gara JP. 2016. Untangling the diverse and redundant mechanisms of Staphylococcus aureus biofilm formation. PLoS Pathog 12:e1005671. doi: 10.1371/journal.ppat.1005671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Høiby N, Bjarnsholt T, Givskov M, Molin S, Ciofu O. 2010. Antibiotic resistance of bacterial biofilms. Int J Antimicrob Agents 35:322–332. doi: 10.1016/j.ijantimicag.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 44.Kirker KR, Fisher ST, James GA. 2015. Potency and penetration of telavancin in staphylococcal biofilms. Int J Antimicrob Agents 46:451–455. doi: 10.1016/j.ijantimicag.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 45.França A, Carvalhais V, Vilanova M, Pier GB, Cerca N. 2016. Characterization of an in vitro fed-batch model to obtain cells released from S. epidermidis biofilms. AMB Expr 6:1–11. doi: 10.1186/s13568-016-0197-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Donlan RM. 2000. Role of biofilms in antimicrobial resistance. Asaio J 46:S47–52. doi: 10.1097/00002480-200011000-00037. [DOI] [PubMed] [Google Scholar]
- 47.Amorena B, Gracia E, Monzón M, Leiva J, Oteiza C, Pérez M, Alabart JL, Hernández-Yago J. 1999. Antibiotic susceptibility assay for Staphylococcus aureus in biofilms developed in vitro. J Antimicrob Chemother 44:43–55. doi: 10.1093/jac/44.1.43. [DOI] [PubMed] [Google Scholar]
- 48.Crabbé A, Jensen P, Bjarnsholt T, Coenye T. 2019. Antimicrobial Tolerance and Metabolic Adaptations in Microbial Biofilms. Trends Microbiol 27:850–863. doi: 10.1016/j.tim.2019.05.003. [DOI] [PubMed] [Google Scholar]
- 49.Sønderholm M, Bjarnsholt T, Alhede M, Kolpen M, Jensen P, Kühl M, Kragh KN. 2017. The Consequences of being in an infectious biofilm: microenvironmental conditions governing antibiotic tolerance. Int J Mol Sci 18:2688. doi: 10.3390/ijms18122688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Waters EM, Rowe SE, O'Gara JP, Conlon BP. 2016. Convergence of Staphylococcus aureus persister and biofilm research: can biofilms be defined as communities of adherent persister cells? PLoS Pathog 12:e1006012. doi: 10.1371/journal.ppat.1006012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Coates A, Hu Y, Bax R, Page C. 2002. The future challenges facing the development of new antimicrobial drugs. Nat Rev Drug Discov 1:895–910. doi: 10.1038/nrd940. [DOI] [PubMed] [Google Scholar]
- 52.Coates AR, Hu Y. 2008. Targeting non-multiplying organisms as a way to develop novel antimicrobials. Trends Pharmacol Sci 29:143–150. doi: 10.1016/j.tips.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 53.Silhavy TJ, Kahne D, Walker S. 2010. The bacterial cell envelope. Cold Spring Harb Perspect Biol 2:a000414. doi: 10.1101/cshperspect.a000414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hurdle JG, O'Neill AJ, Chopra I, Lee RE. 2011. Targeting bacterial membrane function: an underexploited mechanism for treating persistent infections. Nat Rev Microbiol 9:62–75. doi: 10.1038/nrmicro2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mehta D, Saini V, Aggarwal B, Khan A, Bajaj A. 2021. Unlocking the bacterial membrane as a therapeutic target for next-generation antimicrobial amphiphiles. Mol Aspects Med 81:100999. doi: 10.1016/j.mam.2021.100999. [DOI] [PubMed] [Google Scholar]
- 56.Dias C, Rauter AP. 2019. Membrane-targeting antibiotics: recent developments outside the peptide space. Future Med Chem 11:211–228. doi: 10.4155/fmc-2018-0254. [DOI] [PubMed] [Google Scholar]
- 57.Wang G, Li X, Wang Z. 2016. APD3: the antimicrobial peptide database as a tool for research and education. Nucleic Acids Res 44:D1087–93. doi: 10.1093/nar/gkv1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ballard E, Yucel R, Melchers WJG, Brown AJP, Verweij PE, Warris A. 2020. Antifungal activity of antimicrobial peptides and proteins against Aspergillus fumigatus. JoF 6:65. doi: 10.3390/jof6020065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ahmed A, Siman-Tov G, Hall G, Bhalla N, Narayanan A. 2019. Human antimicrobial peptides as therapeutics for viral infections. Viruses 11:704. doi: 10.3390/v11080704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tornesello AL, Borrelli A, Buonaguro L, Buonaguro FM, Tornesello ML. 2020. Antimicrobial peptides as anticancer agents: functional properties and biological activities. Molecules 25:2850. doi: 10.3390/molecules25122850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang G, Mishra B, Lau K, Lushnikova T, Golla R, Wang X. 2015. Antimicrobial peptides in 2014. Pharmaceuticals (Basel) 8:123–150. doi: 10.3390/ph8010123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Benfield AH, Henriques ST. 2020. Mode-of-action of antimicrobial peptides: membrane disruption vs. intracellular mechanisms. Front Med Technol 2:610997. doi: 10.3389/fmedt.2020.610997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brogden KA. 2005. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat Rev Microbiol 3:238–250. doi: 10.1038/nrmicro1098. [DOI] [PubMed] [Google Scholar]
- 64.Brogden KA, Ackermann M, McCray PB Jr., Tack BF. 2003. Antimicrobial peptides in animals and their role in host defences. Int J Antimicrob Agents 22:465–478. doi: 10.1016/s0924-8579(03)00180-8. [DOI] [PubMed] [Google Scholar]
- 65.Brogden KA, Ackermann M, Huttner KM. 1998. Detection of anionic antimicrobial peptides in ovine bronchoalveolar lavage fluid and respiratory epithelium. Infect Immun 66:5948–5954. doi: 10.1128/IAI.66.12.5948-5954.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang LJ, Gallo RL. 2016. Antimicrobial peptides. Curr Biol 26:R14–9. doi: 10.1016/j.cub.2015.11.017. [DOI] [PubMed] [Google Scholar]
- 67.Dathe M, Wieprecht T, Nikolenko H, Handel L, Maloy WL, MacDonald DL, Beyermann M, Bienert M. 1997. Hydrophobicity, hydrophobic moment and angle subtended by charged residues modulate antibacterial and haemolytic activity of amphipathic helical peptides. FEBS Lett 403:208–212. doi: 10.1016/s0014-5793(97)00055-0. [DOI] [PubMed] [Google Scholar]
- 68.Mishra B, Wang G. 2012. Ab initio design of potent anti-MRSA peptides based on database filtering technology. J Am Chem Soc 134:12426–12429. doi: 10.1021/ja305644e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mishra B, Reiling S, Zarena D, Wang G. 2017. Host defense antimicrobial peptides as antibiotics: design and application strategies. Curr Opin Chem Biol 38:87–96. doi: 10.1016/j.cbpa.2017.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Peng J, Mishra B, Khader R, Felix L, Mylonakis E. 2021. Novel Cecropin-4 Derived Peptides against Methicillin-Resistant Staphylococcus aureus. Antibiotics 10:36. doi: 10.3390/antibiotics10010036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mishra B, Srivastava VK, Chaudhry R, Somvanshi RK, Singh AK, Gill K, Somvanshi R, Patro IK, Dey S. 2010. SD-8, a novel therapeutic agent active against multidrug-resistant Gram positive cocci. Amino Acids 39:1493–1505. doi: 10.1007/s00726-010-0618-z. [DOI] [PubMed] [Google Scholar]
- 72.Zarena D, Mishra B, Lushnikova T, Wang F, Wang G. 2017. The π configuration of the WWW motif of a short trp-rich peptide is critical for targeting bacterial membranes, disrupting preformed biofilms, and killing methicillin-resistant Staphylococcus aureus. Biochemistry 56:4039–4043. doi: 10.1021/acs.biochem.7b00456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Saint Jean KD, Henderson KD, Chrom CL, Abiuso LE, Renn LM, Caputo GA. 2018. Effects of hydrophobic amino acid substitutions on antimicrobial peptide Behavior. Probiotics Antimicrob Proteins 10:408–419. doi: 10.1007/s12602-017-9345-z. [DOI] [PubMed] [Google Scholar]
- 74.Mishra B, Wang G. 2012. The importance of amino acid composition in natural AMPs: an evolutional, structural, and functional perspective. Front Immunol 3:221. doi: 10.3389/fimmu.2012.00221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Matsuzaki K. 2009. Control of cell selectivity of antimicrobial peptides. Biochim Biophys Acta 1788:1687–1692. doi: 10.1016/j.bbamem.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 76.Zasloff M. 2002. Antimicrobial peptides of multicellular organisms. Nature 415:389–395. doi: 10.1038/415389a. [DOI] [PubMed] [Google Scholar]
- 77.Marshall GR, Ballante F. 2017. Limiting assumptions in the design of peptidomimetics. Drug Dev Res 78:245–267. doi: 10.1002/ddr.21406. [DOI] [PubMed] [Google Scholar]
- 78.Fjell CD, Hiss JA, Hancock RE, Schneider G. 2011. Designing antimicrobial peptides: form follows function. Nat Rev Drug Discov 11:37–51. doi: 10.1038/nrd3591. [DOI] [PubMed] [Google Scholar]
- 79.Nilsson AC, Janson H, Wold H, Fugelli A, Andersson K, Håkangård C, Olsson P, Olsen WM. 2015. LTX-109 is a novel agent for nasal decolonization of methicillin-resistant and -sensitive Staphylococcus aureus. Antimicrob Agents Chemother 59:145–151. doi: 10.1128/AAC.03513-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ghosh C, Haldar J. 2015. Membrane-active small molecules: designs inspired by antimicrobial peptides. ChemMedChem 10:1606–1624. doi: 10.1002/cmdc.201500299. [DOI] [PubMed] [Google Scholar]
- 81.Niu Y, Wang M, Cao Y, Nimmagadda A, Hu J, Wu Y, Cai J, Ye XS. 2018. Rational design of dimeric lysine N-alkylamides as potent and broad-spectrum antibacterial agents. J Med Chem 61:2865–2874. doi: 10.1021/acs.jmedchem.7b01704. [DOI] [PubMed] [Google Scholar]
- 82.Pelay-Gimeno M, Glas A, Koch O, Grossmann TN. 2015. Structure-based design of inhibitors of protein-protein interactions: mimicking peptide binding epitopes. Angew Chem Int Ed Engl 54:8896–8927. doi: 10.1002/anie.201412070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang N, Ma S. 2019. Recent development of membrane-active molecules as antibacterial agents. Eur J Med Chem 184:111743. doi: 10.1016/j.ejmech.2019.111743. [DOI] [PubMed] [Google Scholar]
- 84.Zhang E, Bai PY, Cui DY, Chu WC, Hua YG, Liu Q, Yin HY, Zhang YJ, Qin S, Liu HM. 2018. Synthesis and bioactivities study of new antibacterial peptide mimics: the dialkyl cationic amphiphiles. Eur J Med Chem 143:1489–1509. doi: 10.1016/j.ejmech.2017.10.044. [DOI] [PubMed] [Google Scholar]
- 85.Li D, Bheemanaboina RRY, Battini N, Tangadanchu VKR, Fang XF, Zhou CH. 2018. Novel organophosphorus aminopyrimidines as unique structural DNA-targeting membrane active inhibitors towards drug-resistant methicillin-resistant Staphylococcus aureus. Medchemcomm 9:1529–1537. doi: 10.1039/c8md00301g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jayawickramarajah J, Wilson A. 2017. Volume 5. Supramolecular Medicinal Chemistry and Chemical Biology. Elsevier. doi: 10.1016/B978-0-12-409547-2.12557-0. [DOI] [Google Scholar]
- 87.Al-Mulla. 2017. A review: biological importance of heterocyclic compounds. Der Pharma Chemica 9:141–147. [Google Scholar]
- 88.Thaker HD, Som A, Ayaz F, Lui D, Pan W, Scott RW, Anguita J, Tew GN. 2012. Synthetic mimics of antimicrobial peptides with immunomodulatory responses. J Am Chem Soc 134:11088–11091. doi: 10.1021/ja303304j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Su M, Xia D, Teng P, Nimmagadda A, Zhang C, Odom T, Cao A, Hu Y, Cai J. 2017. Membrane-active hydantoin derivatives as antibiotic agents. J Med Chem 60:8456–8465. doi: 10.1021/acs.jmedchem.7b00847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ghosh C, Sarkar P, Samaddar S, Uppu D, Haldar J. 2017. l-Lysine based lipidated biphenyls as agents with anti-biofilm and anti-inflammatory properties that also inhibit intracellular bacteria. Chem Commun (Camb) (Camb) 53:8427–8430. doi: 10.1039/c7cc04206j. [DOI] [PubMed] [Google Scholar]
- 91.Wang J, Ansari MF, Zhou CH. 2021. Unique para-aminobenzenesulfonyl oxadiazoles as novel structural potential membrane active antibacterial agents towards drug-resistant methicillin resistant Staphylococcus aureus. Bioorg Med Chem Lett 41:127995. doi: 10.1016/j.bmcl.2021.127995. [DOI] [PubMed] [Google Scholar]
- 92.Bai PY, Qin SS, Chu WC, Yang Y, Cui DY, Hua YG, Yang QQ, Zhang E. 2018. Synthesis and antibacterial bioactivities of cationic deacetyl linezolid amphiphiles. Eur J Med Chem 155:925–945. doi: 10.1016/j.ejmech.2018.06.054. [DOI] [PubMed] [Google Scholar]
- 93.Konai MM, Adhikary U, Haldar J. 2017. Design and solution-phase synthesis of membrane-targeting lipopeptides with selective antibacterial activity. Chemistry 23:12853–12860. doi: 10.1002/chem.201702227. [DOI] [PubMed] [Google Scholar]
- 94.Li Petri G, Di Martino S, De Rosa M. 2022. Peptidomimetics: an overview of recent medicinal chemistry efforts toward the discovery of novel small molecule inhibitors. J Med Chem 65:7438–7475. doi: 10.1021/acs.jmedchem.2c00123. [DOI] [PubMed] [Google Scholar]
- 95.Sisignano M, Parnham MJ, Geisslinger G. 2016. Drug repurposing for the development of novel analgesics. Trends Pharmacol Sci 37:172–183. doi: 10.1016/j.tips.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 96.Rajamuthiah R, Fuchs BB, Conery AL, Kim W, Jayamani E, Kwon B, Ausubel FM, Mylonakis E. 2015. Repurposing salicylanilide anthelmintic drugs to combat drug resistant Staphylococcus aureus. PLoS One 10:e0124595. doi: 10.1371/journal.pone.0124595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kim W, Zou G, Hari TPA, Wilt IK, Zhu W, Galle N, Faizi HA, Hendricks GL, Tori K, Pan W, Huang X, Steele AD, Csatary EE, Dekarske MM, Rosen JL, Ribeiro NQ, Lee K, Port J, Fuchs BB, Vlahovska PM, Wuest WM, Gao H, Ausubel FM, Mylonakis E. 2019. A selective membrane-targeting repurposed antibiotic with activity against persistent methicillin-resistant Staphylococcus aureus. Proc Natl Acad Sci USA 116:16529–16534. doi: 10.1073/pnas.1904700116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kim W, Zou G, Pan W, Fricke N, Faizi HA, Kim SM, Khader R, Li S, Lee K, Escorba I, Vlahovska PM, Gao H, Ausubel FM, Mylonakis E. 2020. The neutrally charged diarylurea compound PQ401 kills antibiotic-resistant and antibiotic-tolerant Staphylococcus aureus. mBio 11:e01140-20. doi: 10.1128/mBio.01140-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yamamoto K, Kitayama T, Minagawa S, Watanabe T, Sawada S, Okamoto T, Utsumi R. 2001. Antibacterial agents that inhibit histidine protein kinase YycG of Bacillus subtilis. Biosci Biotechnol Biochem 65:2306–2310. doi: 10.1271/bbb.65.2306. [DOI] [PubMed] [Google Scholar]
- 100.Kim W, Zhu W, Hendricks GL, Van Tyne D, Steele AD, Keohane CE, Fricke N, Conery AL, Shen S, Pan W, Lee K, Rajamuthiah R, Fuchs BB, Vlahovska PM, Wuest WM, Gilmore MS, Gao H, Ausubel FM, Mylonakis E. 2018. A new class of synthetic retinoid antibiotics effective against bacterial persisters. Nature 556:103–107. doi: 10.1038/nature26157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Farha MA, Verschoor CP, Bowdish D, Brown ED. 2013. Collapsing the proton motive force to identify synergistic combinations against Staphylococcus aureus. Chem Biol 20:1168–1178. doi: 10.1016/j.chembiol.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 102.Gupta V, Datta P. 2019. Next-generation strategy for treating drug resistant bacteria: antibiotic hybrids. Indian J Med Res 149:97–106. doi: 10.4103/ijmr.IJMR_755_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shi C, Zhang X, Zhao X, Meng R, Liu Z, Chen X, Guo N. 2017. Synergistic interactions of nisin in combination with cinnamaldehyde against Staphylococcus aureus in pasteurized milk. Food Control 71:10–16. doi: 10.1016/j.foodcont.2016.06.020. [DOI] [Google Scholar]
- 104.Tong SYC, Davis JS. 2021. combination therapy for staphylococcus aureus bacteremia: hopes dashed again. Clin Infect Dis 72:e204–e205. doi: 10.1093/cid/ciaa994. [DOI] [PubMed] [Google Scholar]
- 105.Koh J-J, Qiu S, Zou H, Lakshminarayanan R, Li J, Zhou X, Tang C, Saraswathi P, Verma C, Tan DTH, Tan AL, Liu S, Beuerman RW. 2013. Rapid bactericidal action of alpha-mangostin against MRSA as an outcome of membrane targeting. Biochim Biophys Acta 1828:834–844. doi: 10.1016/j.bbamem.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 106.Koh J-J, Lin S, Aung TT, Lim F, Zou H, Bai Y, Li J, Lin H, Pang LM, Koh WL, Salleh SM, Lakshminarayanan R, Zhou L, Qiu S, Pervushin K, Verma C, Tan DTH, Cao D, Liu S, Beuerman RW. 2015. Amino acid modified xanthone derivatives: novel, highly promising membrane-active antimicrobials for multidrug-resistant Gram-positive bacterial infections. J Med Chem 58:739–752. doi: 10.1021/jm501285x. [DOI] [PubMed] [Google Scholar]
- 107.Koh J-J, Zou H, Mukherjee D, Lin S, Lim F, Tan JK, Tan D-Z, Stocker BL, Timmer MSM, Corkran HM, Lakshminarayanan R, Tan DTH, Cao D, Beuerman RW, Dick T, Liu S. 2016. Amphiphilic xanthones as a potent chemical entity of anti-mycobacterial agents with membrane-targeting properties. Eur J Med Chem 123:684–703. doi: 10.1016/j.ejmech.2016.07.068. [DOI] [PubMed] [Google Scholar]
- 108.Koh J-J, Zou H, Lin S, Lin H, Soh RT, Lim FH, Koh WL, Li J, Lakshminarayanan R, Verma C, Tan DTH, Cao D, Beuerman RW, Liu S. 2016. Nonpeptidic amphiphilic xanthone derivatives: structure–activity relationship and membrane-targeting properties. J Med Chem 59:171–193. doi: 10.1021/acs.jmedchem.5b01500. [DOI] [PubMed] [Google Scholar]
- 109.Wu SC, Yang ZQ, Liu F, Peng WJ, Qu SQ, Li Q, Song XB, Zhu K, Shen JZ. 2019. Antibacterial Effect and Mode of Action of Flavonoids From Licorice Against Methicillin-Resistant Staphylococcus aureus. Front Microbiol 10:2489. doi: 10.3389/fmicb.2019.02489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sun H, Ansari MF, Fang B, Zhou CH. 2021. Natural Berberine-Hybridized Benzimidazoles as Novel Unique Bactericides against Staphylococcus aureus. J Agric Food Chem 69:7831–7840. doi: 10.1021/acs.jafc.1c02545. [DOI] [PubMed] [Google Scholar]
- 111.Côté H, Pichette A, Simard F, Ouellette ME, Ripoll L, Mihoub M, Grimard D, Legault J. 2019. Balsacone C, a new antibiotic targeting bacterial cell membranes, inhibits clinical isolates of methicillin-resistant Staphylococcus aureus (MRSA) without inducing resistance. Front Microbiol 10:2341. doi: 10.3389/fmicb.2019.02341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chen JP, Battini N, Ansari MF, Zhou CH. 2021. Membrane active 7-thiazoxime quinolones as novel DNA binding agents to decrease the genes expression and exert potent anti-methicillin-resistant Staphylococcus aureus activity. Eur J Med Chem 217:113340. doi: 10.1016/j.ejmech.2021.113340. [DOI] [PubMed] [Google Scholar]
- 113.Guo Y, Hou E, Wen T, Yan X, Han M, Bai LP, Fu X, Liu J, Qin S. 2021. Development of membrane-active Honokiol/Magnolol amphiphiles as potent antibacterial agents against methicillin-resistant Staphylococcus aureus (MRSA). J Med Chem 64:12903–12916. doi: 10.1021/acs.jmedchem.1c01073. [DOI] [PubMed] [Google Scholar]
- 114.Alkatheri AH, Yap PS, Abushelaibi A, Lai KS, Cheng WH, Lim SE. 2022. Host-bacterial interactions: outcomes of antimicrobial peptide applications. Membranes 12:715. doi: 10.3390/membranes12070715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Velkov T, Roberts KD, Nation RL, Thompson PE, Li J. 2013. Pharmacology of polymyxins: new insights into an 'old' class of antibiotics. Future Microbiol 8:711–724. doi: 10.2217/fmb.13.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gray DA, Wenzel M. 2020. More than a pore: a current perspective on the in vivo mode of action of the lipopeptide antibiotic daptomycin. Antibiotics 9:17. doi: 10.3390/antibiotics9010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Manzini MC, Perez KR, Riske KA, Bozelli JC Jr, Santos TL, da Silva MA, Saraiva GK, Politi MJ, Valente AP, Almeida FC, Chaimovich H, Rodrigues MA, Bemquerer MP, Schreier S, Cuccovia IM. 2014. Peptide:lipid ratio and membrane surface charge determine the mechanism of action of the antimicrobial peptide BP100. Conformational and functional studies. Biochim Biophys Acta 1838:1985–1999. doi: 10.1016/j.bbamem.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 118.Epand RM, Epand RF. 2011. Bacterial membrane lipids in the action of antimicrobial agents. J Pept Sci 17:298–305. doi: 10.1002/psc.1319. [DOI] [PubMed] [Google Scholar]
- 119.Sinha S, Zheng L, Mu Y, Ng WJ, Bhattacharjya S. 2017. Structure and interactions of a host defense antimicrobial peptide thanatin in lipopolysaccharide micelles reveal mechanism of bacterial cell agglutination. Sci Rep 7:17795. doi: 10.1038/s41598-017-18102-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pokorny A, Birkbeck TH, Almeida PF. 2002. Mechanism and kinetics of delta-lysin interaction with phospholipid vesicles. Biochemistry 41:11044–11056. doi: 10.1021/bi020244r. [DOI] [PubMed] [Google Scholar]
- 121.Zhao H, Sood R, Jutila A, Bose S, Fimland G, Nissen-Meyer J, Kinnunen PK. 2006. Interaction of the antimicrobial peptide pheromone Plantaricin A with model membranes: implications for a novel mechanism of action. Biochim Biophys Acta 1758:1461–1474. doi: 10.1016/j.bbamem.2006.03.037. [DOI] [PubMed] [Google Scholar]
- 122.Baumann G, Mueller P. 1974. A molecular model of membrane excitability. J Supramol Struct 2:538–557. doi: 10.1002/jss.400020504. [DOI] [PubMed] [Google Scholar]
- 123.Ehrenstein G, Lecar H. 1977. Electrically gated ionic channels in lipid bilayers. Q Rev Biophys 10:1–34. doi: 10.1017/s0033583500000123. [DOI] [PubMed] [Google Scholar]
- 124.Huan Y, Kong Q, Mou H, Yi H. 2020. Antimicrobial peptides: classification, design, application and research progress in multiple fields. Front Microbiol 11:582779. doi: 10.3389/fmicb.2020.582779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.He K, Ludtke SJ, Heller WT, Huang HW. 1996. Mechanism of alamethicin insertion into lipid bilayers. Biophys J 71:2669–2679. doi: 10.1016/S0006-3495(96)79458-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Shai Y, Bach D, Yanovsky A. 1990. Channel formation properties of synthetic pardaxin and analogues. J Biol Chem 265:20202–20209. doi: 10.1016/S0021-9258(17)30490-8. [DOI] [PubMed] [Google Scholar]
- 127.Yang L, Weiss TM, Lehrer RI, Huang HW. 2000. Crystallization of antimicrobial pores in membranes: magainin and protegrin. Biophys J 79:2002–2009. doi: 10.1016/S0006-3495(00)76448-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wimley WC. 2010. Describing the mechanism of antimicrobial peptide action with the interfacial activity model. ACS Chem Biol 5:905–917. doi: 10.1021/cb1001558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kumar P, Kizhakkedathu JN, Straus SK. 2018. Antimicrobial peptides: diversity, mechanism of action and strategies to improve the activity and biocompatibility in vivo. Biomolecules 8:4. doi: 10.3390/biom8010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sengupta D, Leontiadou H, Mark AE, Marrink SJ. 2008. Toroidal pores formed by antimicrobial peptides show significant disorder. Biochim Biophys Acta 1778:2308–2317. doi: 10.1016/j.bbamem.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 131.Oren Z, Shai Y. 1998. Mode of action of linear amphipathic alpha-helical antimicrobial peptides. Biopolymers 47:451–463. doi:. [DOI] [PubMed] [Google Scholar]
- 132.Fernandez DI, Le Brun AP, Whitwell TC, Sani MA, James M, Separovic F. 2012. The antimicrobial peptide aurein 1.2 disrupts model membranes via the carpet mechanism. Phys Chem Chem Phys 14:15739–15751. doi: 10.1039/c2cp43099a. [DOI] [PubMed] [Google Scholar]
- 133.Rokitskaya TI, Kolodkin NI, Kotova EA, Antonenko YN. 2011. Indolicidin action on membrane permeability: carrier mechanism versus pore formation. Biochim Biophys Acta 1808:91–97. doi: 10.1016/j.bbamem.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 134.Wang G, Narayana JL, Mishra B, Zhang Y, Wang F, Wang C, Zarena D, Lushnikova T, Wang X. 2019. Design of antimicrobial peptides: progress made with human cathelicidin LL-37. Adv Exp Med Biol 1117:215–240. doi: 10.1007/978-981-13-3588-4_12. [DOI] [PubMed] [Google Scholar]
- 135.Te Winkel JD, Gray DA, Seistrup KH, Hamoen LW, Strahl H. 2016. Analysis of antimicrobial-triggered membrane depolarization using voltage sensitive dyes. Front Cell Dev Biol 4:29. doi: 10.3389/fcell.2016.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Benarroch JM, Asally M. 2020. The microbiologist's guide to membrane potential dynamics. Trends Microbiol 28:304–314. doi: 10.1016/j.tim.2019.12.008. [DOI] [PubMed] [Google Scholar]
- 137.Rossjohn J, Feil SC, McKinstry WJ, Tweten RK, Parker MW. 1997. Structure of a cholesterol-binding, thiol-activated cytolysin and a model of its membrane form. Cell 89:685–692. doi: 10.1016/s0092-8674(00)80251-2. [DOI] [PubMed] [Google Scholar]
- 138.Limoli DH, Rockel AB, Host KM, Jha A, Kopp BT, Hollis T, Wozniak DJ. 2014. Cationic antimicrobial peptides promote microbial mutagenesis and pathoadaptation in chronic infections. PLoS Pathog 10:e1004083. doi: 10.1371/journal.ppat.1004083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yan J, Wang K, Dang W, Chen R, Xie J, Zhang B, Song J, Wang R. 2013. Two hits are better than one: membrane-active and DNA binding-related double-action mechanism of NK-18, a novel antimicrobial peptide derived from mammalian NK-lysin. Antimicrob Agents Chemother 57:220–228. doi: 10.1128/AAC.01619-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zhu C, Tan H, Cheng T, Shen H, Shao J, Guo Y, Shi S, Zhang X. 2013. Human β-defensin 3 inhibits antibiotic-resistant Staphylococcus biofilm formation. J Surg Res 183:204–213. doi: 10.1016/j.jss.2012.11.048. [DOI] [PubMed] [Google Scholar]
- 141.Vollmer W, Blanot D, de Pedro MA. 2008. Peptidoglycan structure and architecture. FEMS Microbiol Rev 32:149–167. doi: 10.1111/j.1574-6976.2007.00094.x. [DOI] [PubMed] [Google Scholar]
- 142.Thammavongsa V, Kim HK, Missiakas D, Schneewind O. 2015. Staphylococcal manipulation of host immune responses. Nat Rev Microbiol 13:529–543. doi: 10.1038/nrmicro3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Boyle-Vavra S, Li X, Alam MT, Read TD, Sieth J, Cywes-Bentley C, Dobbins G, David MZ, Kumar N, Eells SJ, Miller LG, Boxrud DJ, Chambers HF, Lynfield R, Lee JC, Daum RS. 2015. USA300 and USA500 clonal lineages of Staphylococcus aureus do not produce a capsular polysaccharide due to conserved mutations in the cap5 locus. mBio 6:e02585-14. doi: 10.1128/mBio.02585-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Vollmer W, Höltje JV. 2004. The architecture of the murein (peptidoglycan) in gram-negative bacteria: vertical scaffold or horizontal layer(s)? J Bacteriol 186:5978–5987. doi: 10.1128/JB.186.18.5978-5987.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Mengin-Lecreulx D, Texier L, Rousseau M, van Heijenoort J. 1991. The murG gene of Escherichia coli codes for the UDP-N-acetylglucosamine: N-acetylmuramyl-(pentapeptide) pyrophosphoryl-undecaprenol N-acetylglucosamine transferase involved in the membrane steps of peptidoglycan synthesis. J Bacteriol 173:4625–4636. doi: 10.1128/jb.173.15.4625-4636.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Oluwole AO, Corey RA, Brown CM, Hernández-Rocamora VM, Stansfeld PJ, Vollmer W, Bolla JR, Robinson CV. 2022. Peptidoglycan biosynthesis is driven by lipid transfer along enzyme-substrate affinity gradients. Nat Commun 13:2278. doi: 10.1038/s41467-022-29836-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Brown S, Santa Maria JP Jr., Walker S. 2013. Wall teichoic acids of gram-positive bacteria. Annu Rev Microbiol 67:313–336. doi: 10.1146/annurev-micro-092412-155620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Percy MG, Gründling A. 2014. Lipoteichoic acid synthesis and function in gram-positive bacteria. Annu Rev Microbiol 68:81–100. doi: 10.1146/annurev-micro-091213-112949. [DOI] [PubMed] [Google Scholar]
- 149.Schneewind O, Missiakas D. 2014. Lipoteichoic acids, phosphate-containing polymers in the envelope of gram-positive bacteria. J Bacteriol 196:1133–1142. doi: 10.1128/JB.01155-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Yother J. 2011. Capsules of Streptococcus pneumoniae and other bacteria: paradigms for polysaccharide biosynthesis and regulation. Annu Rev Microbiol 65:563–581. doi: 10.1146/annurev.micro.62.081307.162944. [DOI] [PubMed] [Google Scholar]
- 151.McCarthy H, Rudkin JK, Black NS, Gallagher L, O'Neill E, O'Gara JP. 2015. Methicillin resistance and the biofilm phenotype in Staphylococcus aureus. Front Cell Infect Microbiol 5:1. doi: 10.3389/fcimb.2015.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Luo Y, Song Y. 2021. Mechanism of antimicrobial peptides: antimicrobial, anti-inflammatory and antibiofilm activities. Int J Mol Sci 22:11401. doi: 10.3390/ijms222111401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wiedemann I, Breukink E, van Kraaij C, Kuipers OP, Bierbaum G, de Kruijff B, Sahl HG. 2001. Specific binding of nisin to the peptidoglycan precursor lipid II combines pore formation and inhibition of cell wall biosynthesis for potent antibiotic activity. J Biol Chem 276:1772–1779. doi: 10.1074/jbc.M006770200. [DOI] [PubMed] [Google Scholar]
- 154.Shin JM, Gwak JW, Kamarajan P, Fenno JC, Rickard AH, Kapila YL. 2016. Biomedical applications of nisin. J Appl Microbiol 120:1449–1465. doi: 10.1111/jam.13033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Cotter PD, Hill C, Ross RP. 2005. Bacteriocins: developing innate immunity for food. Nat Rev Microbiol 3:777–788. doi: 10.1038/nrmicro1273. [DOI] [PubMed] [Google Scholar]
- 156.de Leeuw E, Li C, Zeng P, Li C, Diepeveen-de Buin M, Lu WY, Breukink E, Lu W. 2010. Functional interaction of human neutrophil peptide-1 with the cell wall precursor lipid II. FEBS Lett 584:1543–1548. doi: 10.1016/j.febslet.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Somner EA, Reynolds PE. 1990. Inhibition of peptidoglycan biosynthesis by ramoplanin. Antimicrob Agents Chemother 34:413–419. doi: 10.1128/AAC.34.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wenzel M, Chiriac AI, Otto A, Zweytick D, May C, Schumacher C, Gust R, Albada HB, Penkova M, Krämer U, Erdmann R, Metzler-Nolte N, Straus SK, Bremer E, Becher D, Brötz-Oesterhelt H, Sahl HG, Bandow JE. 2014. Small cationic antimicrobial peptides delocalize peripheral membrane proteins. Proc Natl Acad Sci USA 111:E1409–18. doi: 10.1073/pnas.1319900111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Pletzer D, Wolfmeier H, Bains M, Hancock REW. 2017. Synthetic Peptides to Target Stringent Response-Controlled Virulence in a Pseudomonas aeruginosa Murine Cutaneous Infection Model. Front Microbiol 8:1867. doi: 10.3389/fmicb.2017.01867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Felix L, Mishra B, Khader R, Ganesan N, Mylonakis E. 2022. In vitro and in vivo bactericidal and antibiofilm efficacy of alpha mangostin against staphylococcus aureus persister Cells. Front Cell Infect Microbiol 12:898794. doi: 10.3389/fcimb.2022.898794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Melander RJ, Melander C. 2019. Strategies for the Eradication of Biofilm-Based Bacterial Infections. p 499–526. Antibacterial Drug Discovery to Combat MDR: Natural Compounds, Nanotechnology and Novel Synthetic Sources. Springer. doi: 10.1007/978-981-13-9871-1_22. [DOI] [Google Scholar]
- 162.Parastan R, Kargar M, Solhjoo K, Kafilzadeh F. 2020. Staphylococcus aureus biofilms: structures, antibiotic resistance, inhibition, and vaccines. Gene Rep 20:100739. doi: 10.1016/j.genrep.2020.100739. [DOI] [Google Scholar]
- 163.Idrees M, Sawant S, Karodia N, Rahman A. 2021. Staphylococcus aureus biofilm: morphology, genetics, pathogenesis and treatment strategies. Int J Environ Res Public Health 18:7602. doi: 10.3390/ijerph18147602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Musini A, Chilumoju SP, Giri A. 2022. Biofilm Formation in Drug-Resistant Pathogen Staphylococcus aureus, p 23–46, In Microbial Biofilms. CRC Press. Boca Raton, FL. [Google Scholar]
- 165.Le KY, Otto M. 2015. Quorum-sensing regulation in staphylococci-an overview. Front Microbiol 6:1174. doi: 10.3389/fmicb.2015.01174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Overhage J, Campisano A, Bains M, Torfs EC, Rehm BH, Hancock RE. 2008. Human host defense peptide LL-37 prevents bacterial biofilm formation. Infect Immun 76:4176–4182. doi: 10.1128/IAI.00318-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Zhao Q, Shi Y, Wang X, Huang A. 2020. Characterization of a novel antimicrobial peptide from buffalo casein hydrolysate based on live bacteria adsorption. J Dairy Sci 103:11116–11128. doi: 10.3168/jds.2020-18577. [DOI] [PubMed] [Google Scholar]
- 168.Li Y, Li S, Yang K, Guo R, Zhu X, Shi Y, Huang A. 2022. Antibiofilm mechanism of a novel milk-derived antimicrobial peptide against Staphylococcus aureus by downregulating agr quorum sensing system. J Appl Microbiol 133:2198–2209. doi: 10.1111/jam.15653. [DOI] [PubMed] [Google Scholar]
- 169.Roux A, Payne SM, Gilmore MS. 2009. Microbial telesensing: probing the environment for friends, foes, and food. Cell Host Microbe 6:115–124. doi: 10.1016/j.chom.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Speziale P, Pietrocola G, Foster TJ, Geoghegan JA. 2014. Protein-based biofilm matrices in Staphylococci. Front Cell Infect Microbiol 4:171. doi: 10.3389/fcimb.2014.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Heilmann C. 2011. Adhesion mechanisms of staphylococci. Adv Exp Med Biol 715:105–123. doi: 10.1007/978-94-007-0940-9_7. [DOI] [PubMed] [Google Scholar]
- 172.Bowler P, Murphy C, Wolcott R. 2020. Biofilm exacerbates antibiotic resistance: is this a current oversight in antimicrobial stewardship? Antimicrob Resist Infect Control 9:162. doi: 10.1186/s13756-020-00830-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Pacios O, Blasco L, Bleriot I, Fernandez-Garcia L, Ambroa A, López M, Bou G, Cantón R, Garcia-Contreras R, Wood TK, Tomás M. 2020. (p)ppGpp and its role in bacterial persistence: new challenges. Antimicrob Agents Chemother 64. doi: 10.1128/AAC.01283-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Fernández-Coll L, Maciag-Dorszynska M, Tailor K, Vadia S, Levin PA, Szalewska-Palasz A, Cashel M. 2020. The absence of (p)ppGpp renders initiation of Escherichia coli chromosomal DNA synthesis independent of growth rates. mBio 11. doi: 10.1128/mBio.03223-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Mechold U, Potrykus K, Murphy H, Murakami KS, Cashel M. 2013. Differential regulation by ppGpp versus pppGpp in Escherichia coli. Nucleic Acids Res 41:6175–6189. doi: 10.1093/nar/gkt302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Jiale Z, Jian J, Xinyi T, Haoji X, Xueqin H, Xiao W. 2021. Design of a novel antimicrobial peptide 1018M targeted ppGpp to inhibit MRSA biofilm formation. AMB Express 11:49. doi: 10.1186/s13568-021-01208-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Lakshmaiah Narayana J, Mishra B, Lushnikova T, Wu Q, Chhonker YS, Zhang Y, Zarena D, Salnikov ES, Dang X, Wang F, Murphy C, Foster KW, Gorantla S, Bechinger B, Murry DJ, Wang G. 2020. Two distinct amphipathic peptide antibiotics with systemic efficacy. Proc Natl Acad Sci USA 117:19446–19454. doi: 10.1073/pnas.2005540117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Mishra B, Wang X, Lushnikova T, Zhang Y, Golla RM, Narayana JL, Wang C, McGuire TR, Wang G. 2018. Antibacterial, antifungal, anticancer activities and structural bioinformatics analysis of six naturally occurring temporins. Peptides 106:9–20. doi: 10.1016/j.peptides.2018.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Mishra B, Golla RM, Lau K, Lushnikova T, Wang G. 2016. Anti-staphylococcal biofilm effects of human cathelicidin peptides. ACS Med Chem Lett 7:117–121. doi: 10.1021/acsmedchemlett.5b00433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Ajish C, Yang S, Kumar SD, Kim EY, Min HJ, Lee CW, Shin SH, Shin SY. 2022. A novel hybrid peptide composed of LfcinB6 and KR-12-a4 with enhanced antimicrobial, anti-inflammatory and anti-biofilm activities. Sci Rep 12:4365. doi: 10.1038/s41598-022-08247-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Luca V, Stringaro A, Colone M, Pini A, Mangoni ML. 2013. Esculentin(1–21), an amphibian skin membrane-active peptide with potent activity on both planktonic and biofilm cells of the bacterial pathogen Pseudomonas aeruginosa. Cell Mol Life Sci 70:2773–2786. doi: 10.1007/s00018-013-1291-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Okuda K, Zendo T, Sugimoto S, Iwase T, Tajima A, Yamada S, Sonomoto K, Mizunoe Y. 2013. Effects of bacteriocins on methicillin-resistant Staphylococcus aureus biofilm. Antimicrob Agents Chemother 57:5572–5579. doi: 10.1128/AAC.00888-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Yu TT, Kuppusamy R, Yasir M, Hassan MM, Sara M, Ho J, Willcox MDP, Black DS, Kumar N. 2021. Polyphenylglyoxamide-based amphiphilic small molecular peptidomimetics as antibacterial agents with anti-biofilm activity. Int J Mol Sci 22:7344. doi: 10.3390/ijms22147344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Lakshmaiah Narayana J, Golla R, Mishra B, Wang X, Lushnikova T, Zhang Y, Verma A, Kumar V, Xie J, Wang G. 2021. Short and Robust Anti-Infective Lipopeptides Engineered Based on the Minimal Antimicrobial Peptide KR12 of Human LL-37. ACS Infect Dis 7:1795–1808. doi: 10.1021/acsinfecdis.1c00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Mishra B, Lakshmaiah Narayana J, Lushnikova T, Wang X, Wang G. 2019. Low cationicity is important for systemic in vivo efficacy of database-derived peptides against drug-resistant Gram-positive pathogens. Proc Natl Acad Sci USA 116:13517–13522. doi: 10.1073/pnas.1821410116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.de Breij A, Riool M, Cordfunke RA, Malanovic N, de Boer L, Koning RI, Ravensbergen E, Franken M, van der Heijde T, Boekema BK, Kwakman PHS, Kamp N, El Ghalbzouri A, Lohner K, Zaat SAJ, Drijfhout JW, Nibbering PH. 2018. The antimicrobial peptide SAAP-148 combats drug-resistant bacteria and biofilms. Sci Transl Med 10:eaan4044. doi: 10.1126/scitranslmed.aan4044. [DOI] [PubMed] [Google Scholar]
- 187.Kim W, Steele AD, Zhu W, Csatary EE, Fricke N, Dekarske MM, Jayamani E, Pan W, Kwon B, Sinitsa IF, Rosen JL, Conery AL, Fuchs BB, Vlahovska PM, Ausubel FM, Gao H, Wuest WM, Mylonakis E. 2018. Discovery and Optimization of nTZDpa as an Antibiotic Effective Against Bacterial Persisters. ACS Infect Dis 4:1540–1545. doi: 10.1021/acsinfecdis.8b00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Wood TK, Knabel SJ, Kwan BW. 2013. Bacterial persister cell formation and dormancy. Appl Environ Microbiol 79:7116–7121. doi: 10.1128/AEM.02636-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Takahashi H, Caputo GA, Kuroda K. 2021. Amphiphilic polymer therapeutics: an alternative platform in the fight against antibiotic resistant bacteria. Biomater Sci 9:2758–2767. doi: 10.1039/d0bm01865a. [DOI] [PubMed] [Google Scholar]
- 190.Casciaro B, Loffredo MR, Cappiello F, Fabiano G, Torrini L, Mangoni ML. 2020. The antimicrobial peptide temporin g: anti-biofilm, anti-persister activities, and potentiator effect of tobramycin efficacy against Staphylococcus aureus. Int J Mol Sci 21:9410. doi: 10.3390/ijms21249410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Ooi N, Miller K, Randall C, Rhys-Williams W, Love W, Chopra I. 2010. XF-70 and XF-73, novel antibacterial agents active against slow-growing and non-dividing cultures of Staphylococcus aureus including biofilms. J Antimicrob Chemother 65:72–78. doi: 10.1093/jac/dkp409. [DOI] [PubMed] [Google Scholar]
- 192.Dekarske MM, Felix LO, Ortiz CM, Csatary EE, Mylonakis E, Wuest WM. 2022. nTZDpa (non-thiazolidinedione PPARγ partial agonist) derivatives retain antimicrobial activity without improving renal toxicity. Bioorg Med Chem Lett 64:128678. doi: 10.1016/j.bmcl.2022.128678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Hu Y, Shamaei-Tousi A, Liu Y, Coates A. 2010. A new approach for the discovery of antibiotics by targeting non-multiplying bacteria: a novel topical antibiotic for staphylococcal infections. PLoS One 5:e11818. doi: 10.1371/journal.pone.0011818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Hoque J, Konai MM, Sequeira SS, Samaddar S, Haldar J. 2016. Antibacterial and antibiofilm activity of cationic small molecules with spatial positioning of hydrophobicity: an in vitro and in vivo evaluation. J Med Chem 59:10750–10762. doi: 10.1021/acs.jmedchem.6b01435. [DOI] [PubMed] [Google Scholar]
- 195.Mishra B, Felix L, Basu A, Kollala SS, Chhonker YS, Ganesan N, Murry DJ, Mylonakis E. 2022. Design and evaluation of short bovine lactoferrin-derived antimicrobial peptides against multidrug-resistant Enterococcus faecium. Antibiotics 11:1085. doi: 10.3390/antibiotics11081085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Saravanan R, Li X, Lim K, Mohanram H, Peng L, Mishra B, Basu A, Lee JM, Bhattacharjya S, Leong SS. 2014. Design of short membrane selective antimicrobial peptides containing tryptophan and arginine residues for improved activity, salt-resistance, and biocompatibility. Biotechnol Bioeng 111:37–49. doi: 10.1002/bit.25003. [DOI] [PubMed] [Google Scholar]
- 197.Tang WH, Wang SH, Wang CF, Mou Y, Lin MG, Hsiao CD, Liao YD. 2022. The lipid components of high-density lipoproteins (HDL) are essential for the binding and transportation of antimicrobial peptides in human serum. Sci Rep 12:2576. doi: 10.1038/s41598-022-06640-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Rathinakumar R, Walkenhorst WF, Wimley WC. 2009. Broad-spectrum antimicrobial peptides by rational combinatorial design and high-throughput screening: the importance of interfacial activity. J Am Chem Soc 131:7609–7617. doi: 10.1021/ja8093247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Starr CG, Ghimire J, Guha S, Hoffmann JP, Wang Y, Sun L, Landreneau BN, Kolansky ZD, Kilanowski-Doroh IM, Sammarco MC, Morici LA, Wimley WC. 2020. Synthetic molecular evolution of host cell-compatible, antimicrobial peptides effective against drug-resistant, biofilm-forming bacteria. Proc Natl Acad Sci USA 117:8437–8448. doi: 10.1073/pnas.1918427117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Pandey P, Hansmann UHE, Wang F. 2020. Altering the solubility of the antibiotic candidate nisin-a computational study. ACS Omega 5:24854–24863. doi: 10.1021/acsomega.0c03594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Lu J, Xu H, Xia J, Ma J, Xu J, Li Y, Feng J. 2020. D- and unnatural amino acid substituted antimicrobial peptides with improved proteolytic resistance and their proteolytic degradation characteristics. Front Microbiol 11:563030. doi: 10.3389/fmicb.2020.563030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Baindara P, Ghosh AK, Mandal SM. 2020. Coevolution of resistance against antimicrobial peptides. Microb Drug Resist 26:880–899. doi: 10.1089/mdr.2019.0291. [DOI] [PubMed] [Google Scholar]
- 203.Joo HS, Otto M. 2015. Mechanisms of resistance to antimicrobial peptides in staphylococci. Biochim Biophys Acta 1848:3055–3061. doi: 10.1016/j.bbamem.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Cheung AL, Bayer AS, Yeaman MR, Xiong YQ, Waring AJ, Memmi G, Donegan N, Chaili S, Yang SJ. 2014. Site-specific mutation of the sensor kinase GraS in Staphylococcus aureus alters the adaptive response to distinct cationic antimicrobial peptides. Infect Immun 82:5336–5345. doi: 10.1128/IAI.02480-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Yang SJ, Bayer AS, Mishra NN, Meehl M, Ledala N, Yeaman MR, Xiong YQ, Cheung AL. 2012. The Staphylococcus aureus two-component regulatory system, GraRS, senses and confers resistance to selected cationic antimicrobial peptides. Infect Immun 80:74–81. doi: 10.1128/IAI.05669-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Andrä J, Goldmann T, Ernst CM, Peschel A, Gutsmann T. 2011. Multiple peptide resistance factor (MprF)-mediated Resistance of Staphylococcus aureus against antimicrobial peptides coincides with a modulated peptide interaction with artificial membranes comprising lysyl-phosphatidylglycerol. J Biol Chem 286:18692–18700. doi: 10.1074/jbc.M111.226886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Peschel A, Otto M, Jack RW, Kalbacher H, Jung G, Götz F. 1999. Inactivation of the dlt operon in Staphylococcus aureus confers sensitivity to defensins, protegrins, and other antimicrobial peptides. J Biol Chem 274:8405–8410. doi: 10.1074/jbc.274.13.8405. [DOI] [PubMed] [Google Scholar]
- 208.Li M, Cha DJ, Lai Y, Villaruz AE, Sturdevant DE, Otto M. 2007. The antimicrobial peptide-sensing system aps of Staphylococcus aureus. Mol Microbiol 66:1136–1147. doi: 10.1111/j.1365-2958.2007.05986.x. [DOI] [PubMed] [Google Scholar]
- 209.Li M, Lai Y, Villaruz AE, Cha DJ, Sturdevant DE, Otto M. 2007. Gram-positive three-component antimicrobial peptide-sensing system. Proc Natl Acad Sci USA 104:9469–9474. doi: 10.1073/pnas.0702159104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Kawada-Matsuo M, Le MN, Komatsuzawa H. 2021. Antibacterial peptides resistance in Staphylococcus aureus: various mechanisms and the association with pathogenicity. Genes 12:1527. doi: 10.3390/genes12101527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Nishi H, Komatsuzawa H, Fujiwara T, McCallum N, Sugai M. 2004. Reduced content of lysyl-phosphatidylglycerol in the cytoplasmic membrane affects susceptibility to moenomycin, as well as vancomycin, gentamicin, and antimicrobial peptides, in Staphylococcus aureus. Antimicrob Agents Chemother 48:4800–4807. doi: 10.1128/AAC.48.12.4800-4807.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Sieprawska-Lupa M, Mydel P, Krawczyk K, Wójcik K, Puklo M, Lupa B, Suder P, Silberring J, Reed M, Pohl J, Shafer W, McAleese F, Foster T, Travis J, Potempa J. 2004. Degradation of human antimicrobial peptide LL-37 by Staphylococcus aureus-derived proteinases. Antimicrob Agents Chemother 48:4673–4679. doi: 10.1128/AAC.48.12.4673-4679.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Chatterjee SS, Joo HS, Duong AC, Dieringer TD, Tan VY, Song Y, Fischer ER, Cheung GY, Li M, Otto M. 2013. Essential Staphylococcus aureus toxin export system. Nat Med 19:364–367. doi: 10.1038/nm.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Cheung GYC, Fisher EL, McCausland JW, Choi J, Collins JWM, Dickey SW, Otto M. 2018. Antimicrobial peptide resistance mechanism contributes to Staphylococcus aureus Infection. J Infect Dis 217:1153–1159. doi: 10.1093/infdis/jiy024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Kawada-Matsuo M, Watanabe A, Arii K, Oogai Y, Noguchi K, Miyawaki S, Hayashi T, Komatsuzawa H. 2020. Staphylococcus aureus virulence affected by an alternative nisin a resistance mechanism. Appl Environ Microbiol 86. doi: 10.1128/AEM.02923-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Conlon BP, Rowe SE, Gandt AB, Nuxoll AS, Donegan NP, Zalis EA, Clair G, Adkins JN, Cheung AL, Lewis K. 2016. Persister formation in Staphylococcus aureus is associated with ATP depletion. Nat Microbiol 1:16051. doi: 10.1038/nmicrobiol.2016.51. [DOI] [PubMed] [Google Scholar]
- 217.Lister JL, Horswill AR. 2014. Staphylococcus aureus biofilms: recent developments in biofilm dispersal. Front Cell Infect Microbiol 4:178. doi: 10.3389/fcimb.2014.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Nayab S, Aslam MA, Rahman SU, Sindhu ZuD, Sajid S, Zafar N, Razaq M, Kanwar RAmanullah. 2022. A review of antimicrobial peptides: its function, mode of action and therapeutic potential. Int J Pept Res Ther 28:46. doi: 10.1007/s10989-021-10325-6. [DOI] [Google Scholar]
- 219.Epand RM, Walker C, Epand RF, Magarvey NA. 2016. Molecular mechanisms of membrane targeting antibiotics. Biochim Biophys Acta 1858:980–987. doi: 10.1016/j.bbamem.2015.10.018. [DOI] [PubMed] [Google Scholar]
- 220.Hasper HE, Kramer NE, Smith JL, Hillman JD, Zachariah C, Kuipers OP, de Kruijff B, Breukink E. 2006. An alternative bactericidal mechanism of action for lantibiotic peptides that target lipid II. Science 313:1636–1637. doi: 10.1126/science.1129818. [DOI] [PubMed] [Google Scholar]
- 221.Hyde AJ, Parisot J, McNichol A, Bonev BB. 2006. Nisin-induced changes in Bacillus morphology suggest a paradigm of antibiotic action. Proc Natl Acad Sci USA 103:19896–19901. doi: 10.1073/pnas.0608373104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Kagan BL, Selsted ME, Ganz T, Lehrer RI. 1990. Antimicrobial defensin peptides form voltage-dependent ion-permeable channels in planar lipid bilayer membranes. Proc Natl Acad Sci USA 87:210–214. doi: 10.1073/pnas.87.1.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Hu Y, Helm JS, Chen L, Ye XY, Walker S. 2003. Ramoplanin inhibits bacterial transglycosylases by binding as a dimer to lipid II. J Am Chem Soc 125:8736–8737. doi: 10.1021/ja035217i. [DOI] [PubMed] [Google Scholar]
- 224.Cheng M, Huang JX, Ramu S, Butler MS, Cooper MA. 2014. Ramoplanin at bactericidal concentrations induces bacterial membrane depolarization in Staphylococcus aureus. Antimicrob Agents Chemother 58:6819–6827. doi: 10.1128/AAC.00061-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Wenzel M, Schriek P, Prochnow P, Albada HB, Metzler-Nolte N, Bandow JE. 2016. Influence of lipidation on the mode of action of a small RW-rich antimicrobial peptide. Biochim Biophys Acta 1858:1004–1011. doi: 10.1016/j.bbamem.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 226.Derouaux A, Turk S, Olrichs NK, Gobec S, Breukink E, Amoroso A, Offant J, Bostock J, Mariner K, Chopra I, Vernet T, Zervosen A, Joris B, Frère JM, Nguyen-Distèche M, Terrak M. 2011. Small molecule inhibitors of peptidoglycan synthesis targeting the lipid II precursor. Biochem Pharmacol 81:1098–1105. doi: 10.1016/j.bcp.2011.02.008. [DOI] [PubMed] [Google Scholar]