Abstract
Since the beginning of the coronavirus disease 19 (COVID-19) pandemic, efforts in defining risk factors and associations between the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), clinical, and molecular features have initiated. After three years of pandemic, it became evident that men have higher risk of adverse outcomes. Such evidence provided the impetus for defining the biological fundaments of such a gender disparity. Our objective was to analyze the most recent literature with the aim of defining the relationship between COVID-19 and fertility, in particular, we assessed the interplay between SARS-CoV-2 and testosterone in a systematic review of literature from December 2019 (first evidence of a novel coronavirus in the Hubei province) until March 2022. As a fundamental basis for understanding, articles pertaining preclinical aspects explaining the gender disparity (n=9) were included. The main review categories analyzed the risk of being infected with SARS-CoV-2 according to testosterone levels (n=5), the impact of serum testosterone on outcomes of COVID-19 (n=23), and the impact SARS-CoV-2 on testosterone levels after infection (n=19). Preclinical studies mainly evaluated the relation between angiotensin-converting enzyme 2 (ACE2) and its androgen-mediated regulation, articles exploring the risk of COVID-19 according to testosterone levels were few. Although most publications evaluating the effect of COVID-19 on fertility found low testosterone levels after the infection, follow-up was short, with some also suggesting no alterations during recovery. More conclusive findings were observed in men with low testosterone levels, that were generally at higher risk of experiencing worse outcomes (i.e., admission to intensive care units, longer hospitalization, and death). Interestingly, an inverse relationship was observed in women, where higher levels of testosterone were associated to worse outcomes. Our finding may provide meaningful insights to better patient counselling and individualization of care pathways in men with testosterone levels suggesting hypogonadism.
Keywords: COVID-19, COVID-19 pandemic, Fertility, SARS-CoV-2, Testosterone
INTRODUCTION
The rapidly spreading new severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) led the World Health Organization (WHO) to declare a global coronavirus disease 19 (COVID-19) pandemic in March 2020 [1]. By June 2022, more than 500 million cases, with more than 6 million deaths have been documented (https://covid19.who.int/). The clinical course of COVID-19 may be characterized by mild symptoms, up to an acute respiratory distress syndrome (ARDS) leading to multiorgan failure (MOF) and death [2]. Moreover, long- and short-term sequelae of COVID-19 have been observed, and represent a significant proportion in the burden of morbidity and mortality for patients recovering from SARS-CoV-2 infection [3]. Early evidence from patients affected by COVID-19 has shown a higher incidence and mortality in elderly patients, or those with a high burden of comorbidities, such as hypertension, diabetes mellitus, pulmonary, and cardiovascular disease [3,4,5,6]. More recently, a concomitant diagnosis of cancer has been established as a risk factor for poorer outcomes in patients with COVID-19 [7]. Although sex-disaggregated data have been not readily available for all countries, a gender disparity in COVID-19 severity was evident, as it has been previously observed with other coronaviruses [8,9]. It is now established that, although a disparity in prevalence is not evident, men run a three-fold increased risk of adverse COVID-19 outcomes compared to women, particularly in terms of intensive care unit (ICU) admission and mortality [10,11]. Since then, efforts have been made in exploring potential factors explaining this observation. Such gender disparity might in fact arise from a combination of sociodemographic characteristics, lifestyle habits, as well as hormonal and immunological differences [12]. It has been observed, for example, that women mount a stronger T-cell response to SARS-CoV-2 compared to men [13]. The SARS-CoV-2 spike proteins mediate entry in human cells, following priming by the transmembrane protease serine 2 (TMPRSS2) and subsequent binding to the angiotensin-converting enzyme 2 (ACE2) receptors [14,15,16]. ACE2 is expressed primarily in the lungs, however, among other tissues, it is expressed in the testes in Leydig and Sertoli cells, and spermatogonia [17,18]. Evidence from studies in prostate cancer have shown how androgen metabolism induces the expression of TMPRSS2, and its androgen-mediated fusion with the erythroblast transformation-specific related gene (ERG) oncogene, resulting in prostate cancer development [19,20]. Hypothesis have been formulated including serum testosterone as a potential player in the mechanism explaining gender differences in COVID-19 susceptibility [15]. For example, some postulated an interplay between testosterone and the development of MOF during COVID-19 [21]. Notably, taken these considerations together, SARS-CoV-2 infection might also determine an effect on male gonadal axis resulting in impaired testosterone metabolism and hypogonadism. With this regard, there is yet conflicting evidence as to whether SARS-CoV-2 might be found in the semen from recovered males [22]. We aimed at assessing the most recent medical literature to explore the complex interplay between COVID-19 and serum testosterone. A better insight into such a complex topic is of paramount importance in the definition of personalized follow-up for patients recovering from COVID-19, or whether men with hypogonadism shall be considered at higher risk of experiencing adverse outcomes when infected with SARS-CoV-2 [23,24,25].
MATERIALS AND METHODS
We performed a systematic review following the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines [26]. An EMBASE, Scopus, and PubMed research to retrieve English-only articles was carried out through April 2022, in a time frame spanning from December 2019 to March 2022, assessing the relationship between COVID-19 and testosterone. The search terms included (coronavirus AND testosterone) OR (COVID-19 AND testosterone) OR (SARS-CoV-2 AND testosterone) OR (coronavirus AND fertility) OR (COVID-19 AND fertility) OR (SARS-CoV-2 AND fertility). Duplicated papers, reviews, systematic reviews, meta-analyses, editorials, commentaries, case reports and congress abstracts were excluded. Moreover, further relevant articles were selected from manuscript bibliographies to avoid omission of relevant articles, this task was performed by a co-author (SB). We divided our primary outcomes in three main categories and a fourth secondary category. We aimed at differentiating studies with testosterone concentrations measured prior to SARS-CoV-2 exposure from those where testosterone was assessed in confirmed COVID-19 cases. The first group would reflect the hypothalamic–pituitary–gonadal (HPG) axis function before infection, whereas in the second, the HPG axis may be suppressed due to the acute phase of the infection. In the latter scenario, testosterone levels would be lower with more severe infections, which may correlate with poorer outcomes [27]. Those categories consisted of the risk of SARS-CoV-2 infection and outcomes according to premorbid testosterone levels, the impact of serum testosterone on outcomes of COVID-19, and the impact SARS-CoV-2 on testosterone levels after infection. A fourth category pertaining evidence in the preclinical setting was included since a massive effort in defining molecular determinants of outcomes of COVID-19 treatment has been made since the beginning of the pandemic [28]. Of note, when analyzed studies evaluated outcomes pertaining two or more categories, they were separately analyzed percategory. Initial screening was independently carried by two co-authors (RL and FB). Full text of reports of potential relevance were reviewed and disagreements were resolved by consultation with a third co-author (PC). Studies were considered eligible if evaluated the interplay of COVID-19 and serum testosterone, we selected articles specifically evaluating such relationship in both sexes. Retrospective and prospective studies, as well as randomized trials and studies based on registry data were included. Since our aim was to explore more domains, given the high heterogeneity of the included articles, we did not perform analyses on interventions and comparators. No restriction on participants was applied, nor a protocol-defined outcome was assessed. In consideration of the actuality of the topic, considering most studies had a short, non-standardized follow-up, and no high-level evidence exists in term of the relationship between COVID-19 and fertility, our review protocol did not include a meta-analysis. Risk of bias (RoB) for non-randomized comparative studies was independently assessed by two reviewers (RL and FB) using the Risk Of Bias In Non-randomized Studies - of Exposure (ROBINS-E) tool, and were visualized with the Risk-of-bias VISualization (robvis) tool [29,30]. The overall RoB level was judged as “low,” “raising some concerns/intermediate”, “high”, and “very high”, or “no information” whether the relative domain could not be assessed. Since we relied on studies pertaining the preclinical aspects of our analysis, a separate assessment for those studies was performed with the same tool.
RESULTS
1. Evidence synthesis
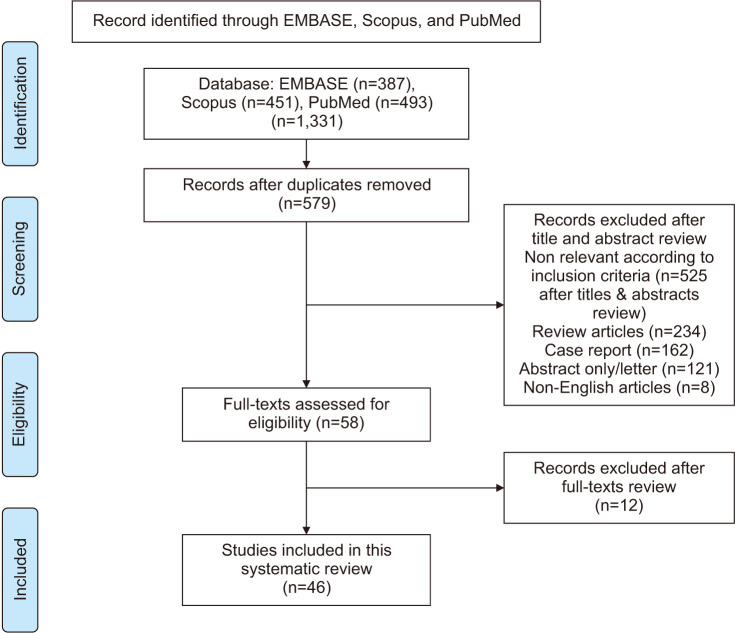
The study selection process is outlined in the PRISMA flowchart as shown in Fig. 1, after removal of duplicates, a total of 579 abstracts were assessed for eligibility, yielding to 58 full texts, of which further 12 were excluded as non-relevant. We included 46 studies exploring the relationship between COVID-19 and testosterone, critically, 9 of these evaluated preclinical aspects of our interest.
Fig. 1. PRISMA flowchart of paper selection process and inclusion. PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-analyses.
2. Risk of bias assessment
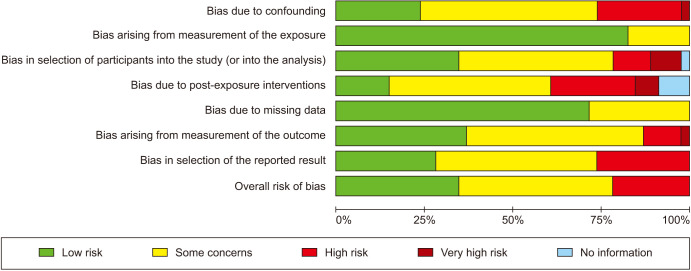
This study had a non-negligible RoB, as it was determined raising some concerns in 20 (43.5%) studies, and high in 10 (21.7%), as summarized in Fig. 2. Of note, bias arising from measurement of the exposure, and bias due to missing data were low in >50% of included studies. A detailed breakdown of studies and domains is reported in Supplement Fig. 1. When assessing RoB in studies in the preclinical setting, it raised some concerns in 6 (66.7%), and was high in 1 (11.1%), as shown in Supplement Fig. 2 and 3.
Fig. 2. Risk Of Bias In Non-randomized Studies - of Exposure (ROBINS-E) visualized with the robvis tool for studies included in the systematic review.
3. Preclinical models of the interplay between COVID-19 and testosterone
Since the beginning of the pandemic, the link between COVID-19 and testosterone has been explored in preclinical studies, following the observation of an androgen-mediated susceptibility to SARS-CoV-2 infection [14,15]. We identified 9 publications, as shown in Table 1. In a recent paper, Ghosh et al [31] investigated the impact of COVID-19 on semen proteome (1,099 proteins and 8,503 peptides) following complete clinical recovery of the patients. It was observed that despite clinical remission, the modification of the semen proteome in response to COVID-19 disrupts male reproductive function. In COVID-19-recovered males, 21 proteins were downregulated and 27 were upregulated, out of 48 differentially expressed genes between the healthy and COVID-19-recovered groups. According to bioinformatic analysis, restricted to proteins with ≤1% false discovery rate, pathways involved in reproductive functions were downregulated in COVID-19-recovered patients, including sperm-oocyte recognition, testosterone response, cell motility regulation, adhesion regulation, extracellular matrix adhesion, and endopeptidase activity. Furthermore, considerable downregulation of two proteins linked to male fertility, semenogelin 1 and prosaposin was found [31]. However, the interplay between testosterone and COVID-19 appears bidirectional. In fact, shorter polyQ alleles in the androgen receptor (AR) provided protection against severe outcomes. When endocrine feedback fails to overcome AR signaling faults by boosting testosterone levels during infection, the polyQ tract takes over as the vital step for clinical outcomes [32]. Vishvakarma et al [33] studied the interaction of sex hormones (testosterone and progesterone) with SARS-CoV-2 main protease (Mpro) for uncovering potential therapeutic targets against COVID-19. It was discovered that testosterone binds strongly to Mpro determining its inhibition. Molecular dynamics simulations at various temperatures were used to further investigate the binding. The creation of complexes between Mpro and testosterone/progesterone was better at 27°C compared to higher temperatures. These results show that testosterone has the potential to inhibit the Mpro of COVID-19 [33]. Similar results were obtained in different animal studies. In a recent study, a mouse model was used to investigate the expression of ACE2 and the potential risk of infection in the testis. Testis had higher ACE2 RNA expression than the other organs and SARS-CoV-2 was also found in Leydig cells. In the animal model, green fluorescence was only found in the Leydig cells after intratesticular injection of the pseudovirus SARS-CoV-2 [34]. The same results were obtained when infecting hamsters with a model for mild human SARS-CoV-2. Up to one month after infection, viral RNA was found in testes samples without any histopathological alterations [35]. Another paper involving the hamster model was published by Li et al [36]. An intranasal SARS-CoV-2 administration resulted in an immediate drop in sperm count, serum testosterone, and inhibin B, as well as the lower testicular size and weight, and serum sex hormone levels. Acute histological damage was observed at four days post infection, including inflammation, hemorrhage, and necrosis, as well as seminiferous tubule degradation and disruption of normal spermatogenesis. Sertoli and Leydig cells showed signs of degeneration and necrosis and these effects lasted up to 120 days post infection and appeared more severe with higher virus inoculum [36]. Testosterone’s role in the risk of thromboembolism in COVID-19 patients has also been investigated in the preclinical setting. In a sample of 1186 people infected with SARS-CoV-2, homozygosity of the cell adhesion molecule P-selectin gene (SELP) rs6127, previously linked to thrombotic risk, was found to be linked to the severity in a male subcohort of 513 individuals. Since testosterone suppresses the SELP gene, an increased odds ratio (OR) was observed in men after their 50s (OR, 2.42) [37]. COVID-19’s age-related outcome could also be influenced by a synergy between vitamin D and sex hormones. In older males, a substantial positive association between testosterone and 25(OH)D was observed, indicating that sex hormones play a role in maintaining 25(OH)D levels [38]. On the base of these interactions between testosterone and COVID-19, a clinical trial was started to evaluate the effect of androgen deprivation therapy (ADT) on SARS-CoV-2 infection, but it was terminated early as it was observed that men treated with had longer hospitalization, and no clear benefit of ADT was determined [39].
Table 1. Summary of publications pertaining the preclinical and molecular aspects of the interaction between testosterone and COVID-19.
| Study | Year | Country | n | Sexa | Method | Summary of relevant findings |
|---|---|---|---|---|---|---|
| Baldassarri et al, 2021 [32] | 2020 | Italy | 1,178 | M/F | Comparison of poly-amino acids variability from WES data in pts with severe versus mild COVID-19. | Higher AR activity associated to milder COVID-19. |
| Zhang et al, 2021 [34] | 2020 | China | 40 | M | Bioinformatic analysis on public databases. Mouse model. Males recovered from COVID-19. |
ACE2 expression higher in testis compared with other organs. In mouse model, green fluorescence only detected in Leydig cells after SARS-CoV-2 injection. |
| Peruzzu et al, 2021 [38] | 2020 | Italy | 160 | M/F | Pts (male and female) with COVID-19. | Potential interplay between vitamin D and sex hormones contributing to the age-related outcome of COVID-19. |
| Campos et al, 2021 [35] | 2020 | USA | - | - | Syrian golden hamsters. | Testicular damage observed in severe COVID-19 could be determined from SARS-CoV-2 infection of testicular cells. |
| Fallerini et al, 2021 [37] | 2021 | Italy | 1,186 | M/F | Patients infected by SARS-CoV-2 with different severity, stratified by sex and adjusted by age. | These results provide a rationale for the repurposing of antibodies against P-selectin as adjuvant therapy in rs6127 male homozygotes. |
| Vishvakarma et al, 2022 [33] | 2021 | India | - | - | No human participants nor animals. | Testosterone showed better binding affinity with Mpro and thus, more inhibition of the main protease. |
| Welén et al, 2022 [39] | 2020–2021 | Sweden | 7,936 | M/F | 42 hospitalized COVID-19 patients before safety evaluation. Population-based retrospective study of 7,894 SARS-CoV-2-positive prostate cancer patients. |
No support in favor of enzalutamide or preventive effects of bicalutamide or ADT in COVID-19. |
| Li et al, 2022 [36] | 2021 | China | - | - | Male Syrian hamsters infected with SARS-CoV-2 wild-type strain HK-13, and Delta and Omicron variants. | SARS-CoV-2 causes acute testicular damage and chronic asymmetric testicular atrophy with hormonal changes. |
| Ghosh et al, 2022 [31] | 2022 | India | 30 | M | COVID-19 recovered individuals and healthy individuals as controls. | Findings on dysregulated proteins and pathways associated with the reproductive process during COVID-19. |
ACE2: angiotensin-converting enzyme 2, ADT: androgen deprivation therapy, AR: androgen receptor, COVID-19: coronavirus disease 19, F: female, M: male, Mpro: SARS-CoV-2 main protease, SARS-CoV-2: severe acute respiratory syndrome coronavirus 2, WES: whole exome sequencing.
aReferred to studies in humans.
4. The impact of premorbid serum testosterone on the risk, and outcomes of COVID-19
Although SARS-CoV-2 infection appears evenly distributed between sexes, males show greater COVID-19 severity and fatality [10,11]. To date, limited data is available regarding how testosterone levels before SARS-CoV-2 infection influences the risk of infection itself, or its severity. We were able to find four publications exploring this relationship, as shown in Table 2. Yeap et al [40] investigating data from 159,964 men reported that a higher total testosterone (TT) level was linked to a higher risk of COVID-19-related death, but no association was found between testosterone and risk of COVID-19. Karkin and Alma [41] found higher testosterone concentration associated to need of ICU admission in a series of men with erectile dysfunction who were subsequently exposed to SARS-CoV-2. Androgens may upregulate the mucosa-specific serine protease TMPRSS2, which plays a vital role in spike protein priming, and facilitates SARS-CoV-2 entrance, as initially observed by Hoffmann et al [14]. Higher circulating testosterone concentrations may enhance the entry of SARS-CoV-2, leading to more severe infection. AR signaling may modulate ACE2 expression in tissues including the lung, providing clues on potential pathways by which high serum testosterone concentrations may enhance viral entry. Similar results were observed by Lee et al [42] in a cohort of 246,087 male patients with COVID-19. Among them, 3,057 were exposed to ADT and 36,096 were diagnosed with cancer and did not receive ADT. In these patients, ADT was linked to a lower incidence and severity of COVID-19 rising the idea that testosterone and AR signaling may lead to a severe COVID-19 phenotype in men and enhance the risk of SARS-CoV-2 infection [42]. The relationship between testosterone and COVID-19 was explored also in the context of Gender-Affirming Hormone Therapy. In comparison to patients with female to male (FtM) gender dysphoria (GD) who received estrogen and anti-androgen medication, the chance of developing COVID-19 increased 3.46 times in people with FtM GD who had received testosterone therapy. Furthermore, when compared to those in which COVID-19 was not diagnosed, those with FtM GD who contracted COVID-19 had a longer course of testosterone therapy [43]. Although Koç et al [44], as well as Gul et al [45] measured testosterone before and after COVID-19 infection, no correlation with infection severity was performed.
Table 2. Summary of publications pertaining the relationship between premorbid serum testosterone, risk of COVID-19, and its clinical outcomes.
| Study | Year | Country | n | Sexa | Population | Summary of relevant findings |
|---|---|---|---|---|---|---|
| Yeap et al, 2022 [40] | 2022 | UK | 159,964 | M | Community-dwelling males aged 40–69 years. | Middle-aged and older men with high premorbid total testosterone and SHBG are at greater risk of COVID-19-related mortality. |
| Karkin and Alma, 2021 [41] | 2021 | Turkey | 70 | M | Males complaining ED after COVID-19. | High testosterone associated to increased ICU admission. |
| Lee et al, 2022 [42] | 2022 | USA | 246,087 | M | Males with COVID-19 and healthy controls. | ADT linked to lower COVID-19 incidence and severity. |
| Durcan et al, 2022 [43] | 2021 | Turkey | 238 | M/F | Individuals with FtM or MtF GD. | Risk of contracting COVID-19 increased 3.46 times in pts with FtM GD in comparison to pts with MtF GD. |
ADT: androgen deprivation therapy, COVID-19: coronavirus disease 19, ED: erectile dysfunction, F: female, FtM: female to male, GD: gender dysphoria, ICU: intensive care unit, M: male, MtF: male to female, SHBG: sex hormone binding globulin.
aReferred to studies in humans.
5. The impact of serum testosterone on clinical outcomes of COVID-19
Serum TT has been identified as a determinant of prognosis for COVID-19 infection across 23 studies. Table 3 summarizes findings pertaining this relationship. The majority of included studies (n=16) focused on the negative association between TT and admission to higher intensity care (i.e., ICU admission, need of extra oxygen care), inflammatory parameters (C-reactive protein [CRP], interleukin-6 [IL6], and D-Dimer, among the most relevant ones), as well as with COVID-19 related mortality [24,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60]. In a recent paper by Wong et al, machine learning algorithms were used to predict the risk of developing severe, or fatal COVID-19 in a large population study. Different risk factors were proposed such as age, central obesity, impaired renal function, multiple comorbidities, and testosterone, the latter emerging as a negative prognostic factor [61]. Such relationship was demonstrated also in case-control studies and prospective series, as outlined by Cinislioglu et al [54], who observed lower testosterone levels in men with COVID-19, compared to healthy controls in a study including 450 individuals [51,54,55,58]. In terms of COVID-19 severity, it was observed that not only testosterone correlated with disease severity itself, but lower testosterone levels predicted worsening to more severe forms after being diagnosed with a milder stage, as observed by Dhindsa et al [53]. In terms of mortality, one study [52] found no association between lower testosterone and COVID-19-related mortality, while those using multivariable models observed that lower testosterone was an independent predictor of mortality, as observed by Vena et al [58] and Beltrame et al [59]. Along with testosterone, higher luteinizing hormone (LH), a lower testosterone to LH ratio, as well as high estradiol to testosterone ratio were observed in men with COVID-19 and was associated to an inflammatory status [48,56,62]. Interestingly, two studies found an inverse trend when evaluating women with COVID-19, in particular, Schroeder at al [62] found in a retrospective series that high levels of estradiol and low levels of TT were associated to worse prognosis in men, but not in women admitted at the ICU due to COVID-19 infection. Di Stasi et al [63] found a positive correlation between serum TT and inflammatory markers, as well as with COVID-19 disease severity and longer stay in the respiratory ICU in 17 women admitted for COVID-19. On the contrary, four studies had discordant findings compared to others. Two demonstrated that higher testosterone levels correlated with worse outcomes, and two others found no association between TT and clinical outcomes [23,41,64,65]. Salciccia et al [64] found higher testosterone levels associated to longer time to negative PCR in men with COVID-19 infection. Karkin and Alma [41] found higher testosterone to be associated to the need of ICU care in 70 men complaining ED after COVID-19 infection. Xu et al [23] found no association between COVID-19 infection severity and testosterone in 39 recovering men, as well as no association between testosterone and laboratory parameters. Apaydin et al [65] found no significant difference in baseline testosterone between mild and severe COVID-19 cases, while only free testosterone levels were correlated with inflammatory parameters and ICU admission.
Table 3. Summary of publications pertaining the relationship between testosterone and outcomes after COVID-19.
| Study | Year | Country | n | Sexa | Population | Summary of relevant findings |
|---|---|---|---|---|---|---|
| Ilias et al, 2022 [60] | 2021 | Greece | 94 | M | Males with COVID-19 and age-matched non-COVID-19 hospitalized males. | Testosterone is low in men with COVID-19 and is associated to adverse outcomes. |
| Wong et al, 2021 [61] | 2020 | UK | 7,846 | M/F | Infected individuals and healthy controls. | Lower testosterone, among other covariates, may predispose to worse COVID-19 outcomes. |
| Rastrelli et al, 2021 [46] | 2020 | Italy | 31 | M | Males with SARS-CoV-2 pneumonia. | Low total al free-testosterone predicted poor prognosis. |
| Lanser et al, 2021 [47] | 2020 | Austria | 377 | M/F | Hospitalized men and women with SARS-CoV-2 infection. | Hospitalized men with COVID-19 had low testosterone levels linked to immune activation and an increased risk for ICU admission or death. |
| Ma et al, 2021 [48] | 2021 | China | 404 | M | Reproductive-age males with COVID-19 infection and age-matched controls. | Lower testosterone levels linked to inflammatory parameters. |
| Çayan et al, 2020 [49] | 2020 | Turkey | 221 | M | Hospitalized males with SARS-CoV-2. | Low testosterone levels at baseline correlate with the risk for ICU admission and mortality. |
| Salciccia et al, 2020 [50] | 2020 | Italy | 29 | M | Males admitted for COVID-19. | Testosterone levels lower in the ARDS group and associated with worse clinical outcomes. |
| Xu et al, 2021 [23] | 2021 | China | 39 | M | COVID-19 patients. | No associations between testosterone and disease duration or severity. |
| Kadihasanoglu et al, 2021 [51] | 2021 | Turkey | 262 | M | Group 1: Men with COVID-19 (n=89). Group 2: Cases with non–COVID-19 pneumonia (n=30). Group 3: Age-matched controls (n=143). |
COVID-19 associated with low testosterone and high prolactin and LH. |
| Salonia et al, 2021 [24] | 2020 | Italy | 567 | M | Symptomatic men with COVID-19 compared to a cohort of healthy men. | Testosterone levels associated with COVID-19 severity, ICU admission and mortality. |
| Salciccia et al, 2021 [64] | 2020 | Italy | 25 | M | Pts hospitalized for COVID-19. | Higher testosterone levels in males with COVID-19 associated with shorter durations of positive COVID-19 testing. |
| Camici et al, 2021 [52] | 2021 | Italy | 48 | M | Males with COVID-19. | Pts with severe COVID-19 had lower testosterone compared to pts with milder symptoms. |
| Dhindsa et al, 2021 [53] | 2020 | USA | 152 | M/F | Patients (males and females) with COVID-19. | Lower testosterone during hospitalization associated with increased severity and inflammation in men. |
| Cinislioglu et al, 2022 [54] | 2020–2021 | Turkey | 450 | M | Males with COVID-19 compared to healthy controls. | Testosterone in pts with COVID-19 lower compared to controls. |
| Schroeder et al, 2021 [62] | 2021 | Germany | 181 | M | Male and female COVID-19 pts. Non-COVID-19 ICU pts. Non-COVID-19 patients with comorbidities. |
High estradiol levels in critically ill male COVID-19 pts compared to all control cohorts. Testosterone reduced in critically ill male COVID-19 patients compared to controls. |
| van Zeggeren et al, 2021 [55] | 2020 | Netherlands | 40 | M/F | Patients (males and females) with COVID-19. | Low SHBG levels were associated with mortality rate in patients with COVID-19, and low total and free testosterone were associated with mortality in men. |
| Infante et al, 2021 [56] | 2020 | Italy | 59 | M | Symptomatic males with SARS-CoV-2 infection. | Low testosterone associated with inflammation and mortality. |
| Di Stasi et al, 2022 [63] | 2021 | Italy | 17 | F | Women affected by SARS-CoV-2 pneumonia and recovered in the RICU. | High testosterone associated with inflammation. |
| Zheng et al, 2022 [57] | 2021 | China | 61 | M | Males with COVID-19. | Testosterone is an independent risk factor for disease severity and inflammation. |
| Vena et al, 2022 [58] | 2020–2021 | Italy | 221 | M | Adult males hospitalized for COVID-19. | Testosterone maintained significant associations with respiratory insufficiency and mortality. |
| Apaydin et al, 2022 [65] | 2020 | Turkey | 81 | M | Pts with COVID-19. | Low testosterone correlated with inflammatory parameters and need of ICU admission. |
| Beltrame et al, 2022 [59] | 2020 | Italy | 138 | M/F | Pts (males and females) hospitalized for COVID-19. | Higher testosterone protects against unfavorable outcomes, higher estradiol is associated with a higher probability of death regardless of sex. |
| Rambhatla et al, 2021 [80] | 2020 | USA | 95 | M | Males with COVID-19 on TRT, propensity score matched to males diagnosed with COVID-19 and not on TRT. | TRT not associated with a worse clinical outcome in males diagnosed with COVID-19. |
ARDS: acute respiratory distress syndrome, COVID-19: coronavirus disease 19, F: female, ICU: intensive care unit, LH: luteinizing hormone, M: male, RICU: respiratory ICU, SARS-CoV-2: severe acute respiratory syndrome coronavirus 2, SHBG: sex hormone binding globulin, TRT: testosterone replacement therapy.
aReferred to studies in humans.
6. Short- and long-term effects of COVID-19 on serum testosterone
It has been observed that SARS-CoV-2 might alter post-infection testosterone levels across 19 studies summarized in Table 4. In general, testosterone levels appear to remain low immediately after infection and rise during the recovery phase [24,34,41,44,49,51,57,65,66,67,68,69,70]. However, results are mixed and non-conclusive towards a certain trend, in that nearly half of analyzed studies found no significant TT alterations in the recovery phase, including those evaluating severe vs. mild COVID-19 [22,23,71,72,73,74]. Moreover, follow-up was short in the majority of analyzed studies. Among studies with a long follow-up, it was observed that testosterone tended to raise during the recovery phase, while no definitive evidence towards a stronger trend in younger patients was observed [24,57,65,66]. Salonia et al [24,66] observed that COVID-19 was an independent predictor of lower testosterone, and testosterone levels suggestive of hypogonadism, this trend tended to resolve at a 7-month follow-up, with however 55% of patients with testosterone levels still suggesting hypogonadism. Zheng et al [57] observed that testosterone tended to raise in men younger than 50 years-old admitted to ICU and those managed in lower-intensity areas, while in older men admitted to ICU, serum TT showed a steady, lower trend, compared to those not admitted to the ICU. Such relationship was observed in case-control studies, both when comparing COVID-19 versus healthy controls, as well as when comparing patients with COVID-19 pneumonia and those with non-COVID-19 pneumonia [51,68,69]. In particular, Kadihasanoglu et al [51] prospectively evaluated 262 men, and found lower testosterone levels in men with COVID-related respiratory infection, compared to matched controls with non-COVID respiratory infection and age matched healthy controls. A high burden of comorbidities, as well as obesity, were correlated to higher odds of lower testosterone during the recovery phase [66,67]. On the other hand, six studies did not found alterations in testosterone during recovery compared to baseline [22,23,71,72,73,74]. Those included a study evaluating short- versus long-lasting COVID-19 (i.e., >50 days of viral shedding), as well as one case-control study [23,72]. Interestingly, Gul et al [45] demonstrated that treatment with favipiravir and hydroxychloroquine did not impact semen parameters and hormonal levels in a prospective series of 29 men recovering from COVID-19 with available baseline semen and hormonal parameters.
Table 4. Summary of publications pertaining the impact of SARS-CoV-2 on testosterone levels.
| Study | Year | Country | n | Sexa | Population | Summary of relevant findings |
|---|---|---|---|---|---|---|
| Çayan et al, 2020 [49] | 2020 | Turkey | 221 | M | Males hospitalized for COVID-19. | Reduced testosterone from baseline in 24 men after COVID-19 |
| Ruan et al, 2021 [22] | 2020 | China | 74 | M | Males recovered from COVID-19. | Hormonal profiles remained normal during recovery. |
| Xu et al, 2021 [23] | 2020 | China | 39 | M | Pts with COVID-19. | In males with COVID-19, most sex-related hormones remain within the normal reference ranges after recovery from COVID-19. No significant associations observed between testosterone and disease duration or severity. |
| Okçelik, 2021 [71] | 2020 | Turkey | 44 | M | Pts recovered from COVID-19. | Testosterone decreased during acute COVID-19. |
| Temiz et al, 2021 [72] | 2020 | Turkey | 65 | M | 55 pts with COVID-19 and 10 healthy controls. | Sperm morphology and serum sex hormone levels were significantly different between groups. Patients before treatment had significantly lower serum FSH, LH and testosterone levels than controls. |
| Kadihasanoglu et al, 2021 [51] | 2020 | Turkey | 262 | M | Group 1: Men with COVID-19 (n=89). Group 2: Cases with non–COVID-19 pneumonia (n=30). Group 3: Age-matched controls (n=143). |
COVID-19 associated with low testosterone and high prolactin and LH. |
| Moreno-Perez et al, 2022 [67] | 2020 | Spain | 143 | M | Adult surviving patients of COVID-19. | High prevalence of low serum testosterone or Sertoli cell dysfunction, spermatogenesis disruption, 8–12 weeks after disease onset. |
| Koç and Keseroğlu, 2021 [44] | 2020 | Turkey | 21 | M | Infertile men with semen analyses before and after COVID-19. | COVID-19 worsens semen parameters. Testosterone declined after COVID-19 diagnosis. |
| Salonia et al, 2022 [66] | 2020 | Italy | 121 | M | Males recovering from COVID-19. | Testosterone increased over time after COVID-19, however, more than 50% still had testosterone levels suggestive for a condition of hypogonadism at 7-mo follow-up. |
| Gul et al, 2021 [45] | 2019–2021 | Turkey | 49 | M | Recovered COVID-19 patients who had semen analysis prior to the pandemic. | Treatment with favipiravir and hydroxychloroquine did not affect spermatogenesis and serum androgen levels in the long-term period. |
| Scroppo et al, 2021 [73] | 2020–2021 | Italy | 15 | M | Males with COVID-19. | No alteration in the inflammatory indices, nor gonadotropins and testosterone levels. |
| Saad et al, 2022 [68] | 2021 | Egypt | 197 | M | Males with COVID-19. | Significant difference in testosterone between cases and controls |
| Zheng et al, 2022 [57] | 2021 | China | 61 | M | Males with COVID-19. | Testosterone levels in the second week after onset is valuable for evaluating disease severity. |
| Huang et al, 2022 [74] | 2020 | Singapore | 63 | M | Males with COVID-19. | ACE2 and TMPRSS2 detected in in semen, potentially correlated to changes in male hormone profiles and age. |
| Karkin and Alma, 2021 [41] | 2020 | Turkey | 70 | M | Males complaining ED after COVID-19. | Testosterone after COVID-19 in different compared to the period before COVID-19. |
| Enikeev et al, 2022 [70] | 2021 | Russia | 88 | M | Males with COVID-19 and healthy controls. | COVID-19 and its management affect male hormones and sperm quality during the disease. |
| Gonen et al, 2022 [69] | 2021 | Turkey | 77 | M/F | Patients hospitalized for COVID-19 and healthy controls. | Pts with COVID-19 had lower baseline testosterone levels, and higher cortisol and prolactin levels when compared with healthy volunteers. |
| Apaydin et al, 2022 [65] | 2020 | Turkey | 81 | M | Pts with COVID-19. | Nearly half of recovered pts had low testosterone levels at a 6-mo follow-up. |
| Zhang et al, 2021 [34] | 2020 | China | 40 | M | Males recovered from COVID-19. | In the clinical follow-up, serum total testosterone level was statistically lower in patients who had recovered from COVID-19 compared with healthy men. |
ACE2: angiotensin-converting enzyme 2, COVID-19: coronavirus disease 19, ED: erectile dysfunction, F: female, FSH: follicle-stimulating hormone, LH: luteinizing hormone, M: male, SARS-CoV-2: severe acute respiratory syndrome coronavirus 2, TMPRSS2: transmembrane protease, serine 2.
aReferred to studies in humans.
DISCUSSION
In this systematic review we examined the interplay between testosterone and SARS-CoV-2, highlighting the complexity of such a relationship. Given the emergent situation, a vast number of publications was available. While the majority of studies focused either on testosterone levels measured in the early phases of the infection, and its impact on outcomes of COVID-19, as well as the impact of SARS-CoV-2 itself on the male gonadal axis after infection, only a minority explored the effect of testosterone concentrations on the risk of infection. We also considered publications exploring preclinical and molecular evidence even though of limited number, a novel approach that provide meaningful insights on measurable clinical characteristics.
In a recent meta-analysis of 35 studies, an evidence of impaired testosterone levels and sperm parameters shortly after recovering from COVID-19 was observed. The Authors however pointed out that a longer follow-up is warranted to assess long-term effects of SARS-CoV-2 on the male gonadal axis [75]. Testosterone levels suggesting secondary hypogonadism might be the consequence of the adaptive response of human organism to a variety of conditions such as infection, downregulating testosterone-dependent functions like reproduction. SARS-CoV-2 related inflammation might therefore disrupt the HPG axis, resulting in low testosterone levels associated to a more severe clinical course. Such a response is observed in a variety of chronic systemic conditions such as cardiovascular diseases, obesity, osteoporosis, HIV infection, renal failure, and obstructive pulmonary diseases [76,77]. Low testosterone levels suggesting hypogonadism have been observed at a 7-month follow-up after COVID-19 recovery by Salonia et al [66]. Moreover, Yamamoto et al [78] observed that up to 50% of males recovering from COVID-19 fulfilled criteria for late-onset hypogonadism in a small prospective series. On this premises, testosterone replacement therapy (TRT) might be appealing in the context of hypogonadal men diagnosed with COVID-19 [79]. Critically, neither a harm nor a benefit of TRT on adverse COVID-19 outcomes has been observed yet [80,81]. For these reasons, TRT cannot be recommended in men diagnosed with COVID-19 unless high quality evidence from randomised controlled trials, proving benefit and excluding harms, is available [27,82].
On the other hand, evidence suggest that lower levels of testosterone are associated to an inflammatory state in men with COVID-19, in particular, higher levels of CRP, IL-6 and other pro-inflammatory cytokines have been observed in men with low testosterone, moreover, an association with worse outcomes in these patients has been observed [47,48,56,57,63,65]. Montopoli et al [83] observed that men with prostate cancer on ADT had a lower incidence of COVID-19 compared to those with prostate cancer not on ADT in a large population-based study of men with COVID-19 in Italy. However, other reports did not confirm such observation [84]. Based on these premises, the phase II COVIDENZA trial was started with the aim of testing whether the administration of enzalutamide was able to reduce COVID-19 severity. This trial was however discontinued since no benefit was observed, moreover, in vitro and large-scale epidemiological evidence showed no effect of enzalutamide on lung cells in term of susceptibility to SARS-CoV-2 infection, nor an effect of bicalutamide treatment in men with prostate cancer in terms of risk of COVID-19 [39].
While the majority of analyzed studies have evaluated outcomes in men, it appears that women have a somewhat inverse relationship between testosterone and COVID-19, although publications are scarce. This was observed by Schroeder et al [62], and Di Stasi et al [63], the former observing no relationship between testosterone and COVID-19 outcomes in women, the latter observing an inverse relationship (i.e., positive correlation) between testosterone and inflammatory parameters in women with COVID-19. The gender differences in terms of COVID-19 outcomes may be explained by the fact that in women adrenocorticotropic hormone (ACTH) stimulation on the adrenal gland might result in a prevalent increase in androgens of adrenal origin. However, also the protective role of female hormones (i.e., estrogens and progesterone) through the downregulation of the expression of ACE-2 may underline the observation that male sex is considered a risk factor for severe COVID-19 [85] Interestingly, Subramanian et al found higher risk of SARS-CoV-2 infection in women with polycystic ovarian syndrome (PCOS) compared to matched controls, in a large, UK-based population study, supporting the evidence of an inflammatory status predisposing to SARS-CoV-2 infection, possibly related to higher cytokines levels in women with PCOS [86,87]. Evaluating the relationship between sex hormones, female sex, and SARS-CoV-2 was not the primary objective of this systematic review, further research is therefore warranted to explore this interplay.
Various preclinical studies investigated the interplay between COVID-19 and testosterone. It was found that SARS-CoV-2 can infect the testis and may impact hormone production. At the same time, it was noted how testosterone also plays a role in the biological mechanisms underlying the infection, inhibiting the docking of the SARS-CoV-2 to host cells. Regarding the role of testosterone in the risk of infection, literature is limited and heterogeneous. Some studies have associated higher testosterone with increased risk of infection or poorer outcomes, whereas other studies associated lower testosterone with poorer outcomes from infection. Some studies suggest that testicular damage may be a consequence of COVID-19, but others have found no evidence of long-term detrimental effects.
Systematic reviews like ours are prone to intrinsic limitations, the main being undoubtedly the non-negligible risk of bias, the natural consequence of the actuality of the topic, arising from studies with a follow-up too short to assess long-term effects of the relationship between SARS-CoV-2 and fertility [66]. Moreover, embracing the task of reviewing broad fields of interest reduces the possibility of meta-analyses, which have been recently published evaluating short-term outcomes [75]. Including findings from the preclinical field, and those where testosterone levels were available prior to exposure represent the strength of our approach, that, even characterized by structural limitations, has a strong support arising both from the animal and bioinformatics model [34,35,36,40,42,69].
CONCLUSIONS
The complex relationship between COVID-19 and male reproductive function extends beyond the interplay between testosterone and SARS-CoV-2. Acknowledging the potential androgen-related biological mechanisms of SARS-CoV-2 susceptibility may offer meaningful insights for patient counselling and health policy making. In general, since the majority of analyzed studies were limited in a three-year time span, resulting in significant risk of bias due to postexposure intervention, longer follow-up is warranted to assess long-term effects of COVID-19 on testosterone levels and fertility, and to draw solid conclusions.
Footnotes
Conflict of Interest: The authors have nothing to disclose.
Funding: None.
- Conceptualization: PC, RL, FB.
- Data curation: RL, FB, SB, PC.
- Formal analysis: RL, FB, SB, PC.
- Funding acquisition: none.
- Investigation: RL, FB, SB, FIS, EZ, GA, FM, AS, GC, PC, FD.
- Methodology: RL, FB, SB, FIS, EZ, GA, FM, AS, GC, PC, FD.
- Project administration: RL, FB, SB, PC.
- Resources: RL, FB, SB, PC.
- Software: RL, FB.
- Supervision: PC, FD.
- Validation: RL, FB, SB, FIS, EZ, GA, FM, AS, GC, PC, FD.
- Visualization: RL, FB.
- Writing – original draft: RL, FB, SB, PC.
- Writing – review & editing: FIS, EZ, GA, FM, AS, GC PC, FD.
Supplementary Materials
Supplementary materials can be found via https://doi.org/10.5534/wjmh.220143.
Risk Of Bias In Non-randomized Studies - of Exposure (ROBINS-E) assessment for all studies included in the systematic review.
Risk Of Bias In Non-randomized Studies - of Exposure (ROBINS-E) assessment for studies in the preclinical setting included in the systematic review.
Risk Of Bias In Non-randomized Studies - of Exposure (ROBINS-E) visualized with the robvis tool for studies in the preclinical setting included in the systematic review.
References
- 1.Carvalho T, Krammer F, Iwasaki A. The first 12 months of COVID-19: a timeline of immunological insights. Nat Rev Immunol. 2021;21:245–256. doi: 10.1038/s41577-021-00522-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324:782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 3.Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:934–943. doi: 10.1001/jamainternmed.2020.0994. Erratum in: JAMA Intern Med 2020;180:1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. Erratum in: Lancet 2020;395:1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Onder G, Rezza G, Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020;323:1775–1776. doi: 10.1001/jama.2020.4683. Erratum in: JAMA 2020;323:1619. [DOI] [PubMed] [Google Scholar]
- 6.de Bruin S, Bos LD, van Roon MA, Tuip-de Boer AM, Schuurman AR, Koel-Simmelinck MJA, et al. Amsterdam UMC COVID-19 Biobank Investigators. Clinical features and prognostic factors in Covid-19: a prospective cohort study. EBioMedicine. 2021;67:103378. doi: 10.1016/j.ebiom.2021.103378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khoury E, Nevitt S, Madsen WR, Turtle L, Davies G, Palmieri C. Differences in outcomes and factors associated with mortality among patients with SARS-CoV-2 infection and cancer compared with those without cancer: a systematic review and meta-analysis. JAMA Netw Open. 2022;5:e2210880. doi: 10.1001/jamanetworkopen.2022.10880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gebhard C, Regitz-Zagrosek V, Neuhauser HK, Morgan R, Klein SL. Impact of sex and gender on COVID-19 outcomes in Europe. Biol Sex Differ. 2020;11:29. doi: 10.1186/s13293-020-00304-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karlberg J, Chong DS, Lai WY. Do men have a higher case fatality rate of severe acute respiratory syndrome than women do? Am J Epidemiol. 2004;159:229–231. doi: 10.1093/aje/kwh056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Porcheddu R, Serra C, Kelvin D, Kelvin N, Rubino S. Similarity in case fatality rates (CFR) of COVID-19/SARS-COV-2 in Italy and China. J Infect Dev Ctries. 2020;14:125–128. doi: 10.3855/jidc.12600. [DOI] [PubMed] [Google Scholar]
- 11.Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, et al. COVID-19 Lombardy ICU Network. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA. 2020;323:1574–1581. doi: 10.1001/jama.2020.5394. Erratum in: JAMA 2021;325:2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mohamed MS, Moulin TC, Schiöth HB. Sex differences in COVID-19: the role of androgens in disease severity and progression. Endocrine. 2021;71:3–8. doi: 10.1007/s12020-020-02536-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takahashi T, Ellingson MK, Wong P, Israelow B, Lucas C, Klein J, et al. Reply to: A finding of sex similarities rather than differences in COVID-19 outcomes. Nature. 2021;597:E10–E11. doi: 10.1038/s41586-021-03645-6. [DOI] [PubMed] [Google Scholar]
- 14.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Samuel RM, Majd H, Richter MN, Ghazizadeh Z, Zekavat SM, Navickas A, et al. Androgen signaling regulates SARS-CoV-2 receptor levels and is associated with severe COVID-19 symptoms in men. Cell Stem Cell. 2020;27:876–889.e12. doi: 10.1016/j.stem.2020.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mjaess G, Karam A, Aoun F, Albisinni S, Roumeguère T. COVID-19 and the male susceptibility: the role of ACE2, TMPRSS2 and the androgen receptor. Prog Urol. 2020;30:484–487. doi: 10.1016/j.purol.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li MY, Li L, Zhang Y, Wang XS. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect Dis Poverty. 2020;9:45. doi: 10.1186/s40249-020-00662-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Douglas GC, O'Bryan MK, Hedger MP, Lee DK, Yarski MA, Smith AI, et al. The novel angiotensin-converting enzyme (ACE) homolog, ACE2, is selectively expressed by adult Leydig cells of the testis. Endocrinology. 2004;145:4703–4711. doi: 10.1210/en.2004-0443. [DOI] [PubMed] [Google Scholar]
- 19.Tomlins SA, Rhodes DR, Perner S, Dhanasekaran SM, Mehra R, Sun XW, et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science. 2005;310:644–648. doi: 10.1126/science.1117679. [DOI] [PubMed] [Google Scholar]
- 20.Lin B, Ferguson C, White JT, Wang S, Vessella R, True LD, et al. Prostate-localized and androgen-regulated expression of the membrane-bound serine protease TMPRSS2. Cancer Res. 1999;59:4180–4184. [PubMed] [Google Scholar]
- 21.Pozzilli P, Lenzi A. Commentary: Testosterone, a key hormone in the context of COVID-19 pandemic. Metabolism. 2020;108:154252. doi: 10.1016/j.metabol.2020.154252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruan Y, Hu B, Liu Z, Liu K, Jiang H, Li H, et al. No detection of SARS-CoV-2 from urine, expressed prostatic secretions, and semen in 74 recovered COVID-19 male patients: a perspective and urogenital evaluation. Andrology. 2021;9:99–106. doi: 10.1111/andr.12939. [DOI] [PubMed] [Google Scholar]
- 23.Xu H, Wang Z, Feng C, Yu W, Chen Y, Zeng X, et al. Effects of SARS-CoV-2 infection on male sex-related hormones in recovering patients. Andrology. 2021;9:107–114. doi: 10.1111/andr.12942. [DOI] [PubMed] [Google Scholar]
- 24.Salonia A, Pontillo M, Capogrosso P, Gregori S, Tassara M, Boeri L, et al. Severely low testosterone in males with COVID-19: a case-control study. Andrology. 2021;9:1043–1052. doi: 10.1111/andr.12993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giagulli VA, Guastamacchia E, Magrone T, Jirillo E, Lisco G, De Pergola G, et al. Worse progression of COVID-19 in men: is testosterone a key factor? Andrology. 2021;9:53–64. doi: 10.1111/andr.12836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salonia A, Bettocchi C, Carvalho J, Corona G, Jones TH, Kadioglu A, et al. EAU guidelines on sexual and reproductive health [Internet] Arnhem: EAU; c2022. [cited 2022 Jul 01]. Available from: https://uroweb.org/guidelines/sexual-and-reproductive-health/summary-of-changes . [Google Scholar]
- 28.Muñoz-Fontela C, Dowling WE, Funnell SGP, Gsell PS, Riveros-Balta AX, Albrecht RA, et al. Animal models for COVID-19. Nature. 2020;586:509–515. doi: 10.1038/s41586-020-2787-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Higgins J, Morgan R, Rooney A, Taylor K, Thayer K, Silva R . Risk of bias in non-randomized studies - of exposure (ROBINS-E). Launch version, 1 [Internet] Bristol: ROBINS-E Development Group; c2022. [cited 2022 Sep 20]. Available from: https://www.riskofbias.info/welcome/robins-e-tool . [Google Scholar]
- 30.McGuinness LA, Higgins JPT. Risk-of-bias VISualization (robvis): an R package and Shiny web app for visualizing risk-of-bias assessments. Res Synth Methods. 2021;12:55–61. doi: 10.1002/jrsm.1411. [DOI] [PubMed] [Google Scholar]
- 31.Ghosh S, Parikh S, Nissa MU, Acharjee A, Singh A, Patwa D, et al. Semen proteomics of COVID-19 convalescent men reveals disruption of key biological pathways relevant to male reproductive function. ACS Omega. 2022;7:8601–8612. doi: 10.1021/acsomega.1c06551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baldassarri M, Picchiotti N, Fava F, Fallerini C, Benetti E, Daga S, et al. Shorter androgen receptor polyQ alleles protect against life-threatening COVID-19 disease in European males. EBioMedicine. 2021;65:103246. doi: 10.1016/j.ebiom.2021.103246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vishvakarma VK, Pal S, Singh P, Bahadur I. Interactions between main protease of SARS-CoV-2 and testosterone or progesterone using computational approach. J Mol Struct. 2022;1251:131965. doi: 10.1016/j.molstruc.2021.131965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang J, Wu Y, Li S, Wang X, Wang R, Wang X. Bioinformatic and mouse model reveal the potential high vulnerability of Leydig cells on SARS-CoV-2. Ann Transl Med. 2021;9:678. doi: 10.21037/atm-21-936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Campos RK, Camargos VN, Azar SR, Haines CA, Eyzaguirre EJ, Rossi SL. SARS-CoV-2 infects hamster testes. Microorganisms. 2021;9:1318. doi: 10.3390/microorganisms9061318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li C, Ye Z, Zhang AJX, Chan JFW, Song W, Liu F, et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection by intranasal or intratesticular route induces testicular damage. Clin Infect Dis. 2022;75:e974–e990. doi: 10.1093/cid/ciac142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fallerini C, Daga S, Benetti E, Picchiotti N, Zguro K, Catapano F, et al. SELP Asp603Asn and severe thrombosis in COVID-19 males. J Hematol Oncol. 2021;14:123. doi: 10.1186/s13045-021-01136-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peruzzu D, Pagano MT, Pierdominici M, Ruggieri A, Antinori A, D'Offizi G, et al. INMI-ISS COVID-19 Team. Synergy between vitamin D and sex hormones in respiratory functionality of patients affected by COVID-19. Front Pharmacol. 2021;12:683529. doi: 10.3389/fphar.2021.683529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Welén K, Rosendal E, Gisslén M, Lenman A, Freyhult E, Fonseca-Rodríguez O, et al. A phase 2 trial of the effect of antiandrogen therapy on COVID-19 outcome: no evidence of benefit, supported by epidemiology and in vitro data. Eur Urol. 2022;81:285–293. doi: 10.1016/j.eururo.2021.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yeap BB, Marriott RJ, Manning L, Dwivedi G, Hankey GJ, Wu FCW, et al. Higher premorbid serum testosterone predicts COVID-19-related mortality risk in men. Eur J Endocrinol. 2022;187:159–170. doi: 10.1530/EJE-22-0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Karkin K, Alma E. Erectile dysfunction and testosterone levels prior to COVID-19 disease: what is the relationship? Arch Ital Urol Androl. 2021;93:460–464. doi: 10.4081/aiua.2021.4.460. [DOI] [PubMed] [Google Scholar]
- 42.Lee KM, Heberer K, Gao A, Becker DJ, Loeb S, Makarov DV, et al. A population-level analysis of the protective effects of androgen deprivation therapy against COVID-19 disease incidence and severity. Front Med (Lausanne) 2022;9:774773. doi: 10.3389/fmed.2022.774773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Durcan E, Turan S, Bircan BE, Yaylamaz S, Demirel O, Demir AN, et al. TransCOVID: does gender-affirming hormone therapy play a role in contracting COVID-19? J Sex Marital Ther. 2022;48:415–426. doi: 10.1080/0092623X.2021.2000535. [DOI] [PubMed] [Google Scholar]
- 44.Koç E, Keseroğlu BB. Does COVID-19 worsen the semen parameters? Early results of a tertiary healthcare center. Urol Int. 2021;105:743–748. doi: 10.1159/000517276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gul A, Zengin S, Dundar G, Ozturk M. Do SARS-CoV-2 infection (COVID-19) and the medications administered for its treatment impair testicular functions? Urol Int. 2021;105:944–948. doi: 10.1159/000517925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rastrelli G, Di Stasi V, Inglese F, Beccaria M, Garuti M, Di Costanzo D, et al. Low testosterone levels predict clinical adverse outcomes in SARS-CoV-2 pneumonia patients. Andrology. 2021;9:88–98. doi: 10.1111/andr.12821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lanser L, Burkert FR, Thommes L, Egger A, Hoermann G, Kaser S, et al. Testosterone deficiency is a risk factor for severe COVID-19. Front Endocrinol (Lausanne) 2021;12:694083. doi: 10.3389/fendo.2021.694083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ma L, Xie W, Li D, Shi L, Ye G, Mao Y, et al. Evaluation of sex-related hormones and semen characteristics in reproductive-aged male COVID-19 patients. J Med Virol. 2021;93:456–462. doi: 10.1002/jmv.26259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Çayan S, Uğuz M, Saylam B, Akbay E. Effect of serum total testosterone and its relationship with other laboratory parameters on the prognosis of coronavirus disease 2019 (COVID-19) in SARS-CoV-2 infected male patients: a cohort study. Aging Male. 2020;23:1493–1503. doi: 10.1080/13685538.2020.1807930. [DOI] [PubMed] [Google Scholar]
- 50.Salciccia S, Del Giudice F, Gentile V, Mastroianni CM, Pasculli P, Di Lascio G, et al. Interplay between male testosterone levels and the risk for subsequent invasive respiratory assistance among COVID-19 patients at hospital admission. Endocrine. 2020;70:206–210. doi: 10.1007/s12020-020-02515-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kadihasanoglu M, Aktas S, Yardimci E, Aral H, Kadioglu A. SARS-CoV-2 pneumonia affects male reproductive hormone levels: a prospective, cohort study. J Sex Med. 2021;18:256–264. doi: 10.1016/j.jsxm.2020.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Camici M, Zuppi P, Lorenzini P, Scarnecchia L, Pinnetti C, Cicalini S, et al. ReCoVeRi Study Group. Role of testosterone in SARS-CoV-2 infection: a key pathogenic factor and a biomarker for severe pneumonia. Int J Infect Dis. 2021;108:244–251. doi: 10.1016/j.ijid.2021.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dhindsa S, Zhang N, McPhaul MJ, Wu Z, Ghoshal AK, Erlich EC, et al. Association of circulating sex hormones with inflammation and disease severity in patients with COVID-19. JAMA Netw Open. 2021;4:e2111398. doi: 10.1001/jamanetworkopen.2021.11398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cinislioglu AE, Cinislioglu N, Demirdogen SO, Sam E, Akkas F, Altay MS, et al. The relationship of serum testosterone levels with the clinical course and prognosis of COVID-19 disease in male patients: a prospective study. Andrology. 2022;10:24–33. doi: 10.1111/andr.13081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.van Zeggeren IE, Boelen A, van de Beek D, Heijboer AC, Vlaar APJ, Brouwer MC Amsterdam UMC COVID-19 Biobank. Sex steroid hormones are associated with mortality in COVID-19 patients: level of sex hormones in severe COVID-19. Medicine (Baltimore) 2021;100:e27072. doi: 10.1097/MD.0000000000027072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Infante M, Pieri M, Lupisella S, D'Amore L, Bernardini S, Fabbri A, et al. Low testosterone levels and high estradiol to testosterone ratio are associated with hyperinflammatory state and mortality in hospitalized men with COVID-19. Eur Rev Med Pharmacol Sci. 2021;25:5889–5903. doi: 10.26355/eurrev_202110_26865. [DOI] [PubMed] [Google Scholar]
- 57.Zheng S, Zou Q, Zhang D, Yu F, Bao J, Lou B, et al. Serum level of testosterone predicts disease severity of male COVID-19 patients and is related to T-cell immune modulation by transcriptome analysis. Clin Chim Acta. 2022;524:132–138. doi: 10.1016/j.cca.2021.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vena W, Pizzocaro A, Maida G, Amer M, Voza A, Di Pasquale A, et al. Humanitas COVID19 Task Force. Low testosterone predicts hypoxemic respiratory insufficiency and mortality in patients with COVID-19 disease: another piece in the COVID puzzle. J Endocrinol Invest. 2022;45:753–762. doi: 10.1007/s40618-021-01700-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Beltrame A, Salguero P, Rossi E, Conesa A, Moro L, Bettini LR, et al. Association between sex hormone levels and clinical outcomes in patients with COVID-19 admitted to hospital: an observational, retrospective, cohort study. Front Immunol. 2022;13:834851. doi: 10.3389/fimmu.2022.834851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ilias I, Pratikaki M, Diamantopoulos A, Jahaj E, Mourelatos P, Athanasiou N, et al. Testosterone, free, bioavailable and total, in patients with COVID-19. Minerva Endocrinol (Torino) 2022;47:111–116. doi: 10.23736/S2724-6507.21.03610-1. [DOI] [PubMed] [Google Scholar]
- 61.Wong KC, Xiang Y, Yin L, So HC. Uncovering clinical risk factors and predicting severe COVID-19 cases using UK Biobank data: machine learning approach. JMIR Public Health Surveill. 2021;7:e29544. doi: 10.2196/29544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schroeder M, Schaumburg B, Mueller Z, Parplys A, Jarczak D, Roedl K, et al. High estradiol and low testosterone levels are associated with critical illness in male but not in female COVID-19 patients: a retrospective cohort study. Emerg Microbes Infect. 2021;10:1807–1818. doi: 10.1080/22221751.2021.1969869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Di Stasi V, Rastrelli G, Inglese F, Beccaria M, Garuti M, Di Costanzo D, et al. Higher testosterone is associated with increased inflammatory markers in women with SARS-CoV-2 pneumonia: preliminary results from an observational study. J Endocrinol Invest. 2022;45:639–648. doi: 10.1007/s40618-021-01682-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Salciccia S, Eisenberg ML, Maggi M, Lai S, Mastroianni CM, Pasculli P, et al. Modeling the contribution of male testosterone levels to the duration of positive COVID testing among hospitalized male COVID-19 patients. Diagnostics (Basel) 2021;11:581. doi: 10.3390/diagnostics11040581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Apaydin T, Sahin B, Dashdamirova S, Dincer Yazan C, Elbasan O, Ilgin C, et al. The association of free testosterone levels with coronavirus disease 2019. Andrology. 2022;10:1038–1046. doi: 10.1111/andr.13152. [DOI] [PubMed] [Google Scholar]
- 66.Salonia A, Pontillo M, Capogrosso P, Gregori S, Carenzi C, Ferrara AM, et al. Testosterone in males with COVID-19: a 7-month cohort study. Andrology. 2022;10:34–41. doi: 10.1111/andr.13097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Moreno-Perez O, Merino E, Alfayate R, Torregrosa ME, Andres M, Leon-Ramirez JM, et al. COVID19-ALC Research Group. Male pituitary-gonadal axis dysfunction in post-acute COVID-19 syndrome-prevalence and associated factors: a Mediterranean case series. Clin Endocrinol (Oxf) 2022;96:353–362. doi: 10.1111/cen.14537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Saad HM, GamalEl Din SF, Elbokl OM, Adel A. Predictive factors of erectile dysfunction in Egyptian individuals after contracting COVID-19: a prospective case-control study. Andrologia. 2022;54:e14308. doi: 10.1111/and.14308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gonen MS, De Bellis A, Durcan E, Bellastella G, Cirillo P, Scappaticcio L, et al. Assessment of neuroendocrine changes and hypothalamo-pituitary autoimmunity in patients with COVID-19. Horm Metab Res. 2022;54:153–161. doi: 10.1055/a-1764-1260. [DOI] [PubMed] [Google Scholar]
- 70.Enikeev D, Taratkin M, Morozov A, Petov V, Korolev D, Shpikina A, et al. Prospective two-arm study of the testicular function in patients with COVID-19. Andrology. 2022;10:1047–1056. doi: 10.1111/andr.13159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Okçelik S. COVID-19 pneumonia causes lower testosterone levels. Andrologia. 2021;53:e13909. doi: 10.1111/and.13909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Temiz MZ, Dincer MM, Hacibey I, Yazar RO, Celik C, Kucuk SH, et al. Investigation of SARS-CoV-2 in semen samples and the effects of COVID-19 on male sexual health by using semen analysis and serum male hormone profile: a cross-sectional, pilot study. Andrologia. 2021;53:e13912. doi: 10.1111/and.13912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Scroppo FI, Costantini E, Zucchi A, Illiano E, Trama F, Brancorsini S, et al. COVID-19 disease in clinical setting: impact on gonadal function, transmission risk, and sperm quality in young males. J Basic Clin Physiol Pharmacol. 2021;33:97–102. doi: 10.1515/jbcpp-2021-0227. [DOI] [PubMed] [Google Scholar]
- 74.Huang Z, Do DV, Beh D, Lee CK, Yan B, Foo R, et al. Effects of acute severe acute respiratory syndrome coronavirus 2 infection on male hormone profile, ACE2 and TMPRSS2 expression, and potential for transmission of severe acute respiratory syndrome coronavirus 2 in semen of Asian men. F S Sci. 2022;3:29–34. doi: 10.1016/j.xfss.2021.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Corona G, Vena W, Pizzocaro A, Pallotti F, Paoli D, Rastrelli G, et al. Andrological effects of SARS-Cov-2 infection: a systematic review and meta-analysis. J Endocrinol Invest. 2022 doi: 10.1007/s40618-022-01801-x. [Epub] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Corona G, Vignozzi L, Sforza A, Maggi M. Risks and benefits of late onset hypogonadism treatment: an expert opinion. World J Mens Health. 2013;31:103–125. doi: 10.5534/wjmh.2013.31.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kalyani RR, Gavini S, Dobs AS. Male hypogonadism in systemic disease. Endocrinol Metab Clin North Am. 2007;36:333–348. doi: 10.1016/j.ecl.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 78.Yamamoto Y, Otsuka Y, Sunada N, Tokumasu K, Nakano Y, Honda H, et al. Detection of male hypogonadism in patients with post COVID-19 condition. J Clin Med. 2022;11:1955. doi: 10.3390/jcm11071955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Punjani N, Flannigan R. Androgens and COVID-19: exploring the role of testosterone replacement therapy. Int J Impot Res. 2022 doi: 10.1038/s41443-021-00524-6. [Epub] [DOI] [PubMed] [Google Scholar]
- 80.Rambhatla A, Bronkema CJ, Corsi N, Keeley J, Sood A, Affas Z, et al. COVID-19 infection in men on testosterone replacement therapy. J Sex Med. 2021;18:215–218. doi: 10.1016/j.jsxm.2020.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Baillargeon J, Kuo YF, Westra J, Lopez DS, Urban RJ, Williams SB, et al. Association of testosterone therapy with disease progression in older males with COVID-19. Andrology. 2022;10:1057–1066. doi: 10.1111/andr.13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mulhall JP, Trost LW, Brannigan RE, Kurtz EG, Redmon JB, Chiles KA, et al. Evaluation and management of testosterone deficiency: AUA guideline. J Urol. 2018;200:423–432. doi: 10.1016/j.juro.2018.03.115. [DOI] [PubMed] [Google Scholar]
- 83.Montopoli M, Zumerle S, Vettor R, Rugge M, Zorzi M, Catapano CV, et al. Androgen-deprivation therapies for prostate cancer and risk of infection by SARS-CoV-2: a population-based study (N = 4532) Ann Oncol. 2020;31:1040–1045. doi: 10.1016/j.annonc.2020.04.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Koskinen M, Carpen O, Honkanen V, Seppänen MRJ, Miettinen PJ, Tuominen JA, et al. Androgen deprivation and SARS-CoV-2 in men with prostate cancer. Ann Oncol. 2020;31:1417–1418. doi: 10.1016/j.annonc.2020.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Taslem Mourosi J, Anwar S, Hosen MJ. The sex and gender dimensions of COVID-19: a narrative review of the potential underlying factors. Infect Genet Evol. 2022;103:105338. doi: 10.1016/j.meegid.2022.105338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Subramanian A, Anand A, Adderley NJ, Okoth K, Toulis KA, Gokhale K, et al. Increased COVID-19 infections in women with polycystic ovary syndrome: a population-based study. Eur J Endocrinol. 2021;184:637–645. doi: 10.1530/EJE-20-1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Puder JJ, Varga S, Kraenzlin M, De Geyter C, Keller U, Müller B. Central fat excess in polycystic ovary syndrome: relation to low-grade inflammation and insulin resistance. J Clin Endocrinol Metab. 2005;90:6014–6021. doi: 10.1210/jc.2005-1002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Risk Of Bias In Non-randomized Studies - of Exposure (ROBINS-E) assessment for all studies included in the systematic review.
Risk Of Bias In Non-randomized Studies - of Exposure (ROBINS-E) assessment for studies in the preclinical setting included in the systematic review.
Risk Of Bias In Non-randomized Studies - of Exposure (ROBINS-E) visualized with the robvis tool for studies in the preclinical setting included in the systematic review.