Abstract
Obesity plays a crucial role in the development of non-alcoholic fatty liver disease (NAFLD). However, the underlying mechanism for the pathogenesis of obesity-associated NAFLD remains largely obscure. Although the “multiple hit” theory provides a more accurate explanation of NAFLD pathogenesis, it still cannot fully explain precisely how obesity causes NAFLD. The liver is the key integrator of the body's energy needs, receiving input from multiple metabolically active organs. Thus, recent studies have advocated the “multiple crosstalk” hypothesis, highlighting that obesity-related hepatic steatosis may be the result of dysregulated “crosstalk” among multiple extra-hepatic organs and the liver in obesity. A wide variety of circulating endocrine hormones work together to orchestrate this “crosstalk”. Of note, with deepening understanding of the endocrine system, the perception of hormones has gradually risen from the narrow sense (i.e. traditional hormones) to the broad sense of hormones as organokines and exosomes. In this review, we focus on the perspective of organic endocrine hormones (organokines) and molecular endocrine hormones (exosomes), summarizing systematically how the two types of new hormones mediate the dialogue between extra-hepatic organs and liver in the pathogenesis of obesity-related NAFLD.
Keywords: Exosomes, Hormones, Non-alcoholic fatty liver disease (NAFLD), Obesity, Organokines
Introduction
Non-alcoholic fatty liver disease (NAFLD) is the most common liver disease worldwide. The estimated global prevalence of NAFLD is 25.24%, with the highest prevalence in South America (31%) and the Middle East (32%), and the lowest in Africa (14%).1 In China, the prevalence of NAFLD in adults was estimated to be 20.1–29.2%, and has increased over time.2 However, the treatment status of NAFLD/non-alcoholic steatohepatitis (NASH) remains unsatisfactory, there are no FDA-approved NASH medications currently.3 The ongoing medical therapies for NAFLD/NASH that can be concluded as the following categories: anti-diabetic agents, such as glucagon-like peptide-1 receptor (GLP-1R) agonist, dipeptidyl peptidase 4 (DPP4) inhibitor, thiazolidinedione and sodium-glucose cotransporter 2 (SGLT2) inhibitors; new agents targeting intermediary metabolism in NAFLD, such as farnesoid X receptor (FXR) agonists, peroxisome proliferator-activated receptor (PPAR) agonists, thyroid hormone receptor (THR) agonists, inhibitors of de novo lipogenesis (DNL) and fibroblast growth factors 19 and 21 (FGF19 and FGF21); vitamin E and drugs to regulate gut microbiota, et al.3,4 Though, several drugs are in advanced stages of development for NAFLD/NASH, multiple failures have occurred due to disease heterogeneity, variable placebo response, low efficacy, and in some cases, over-interpretation of phase II results. Therefore, new therapeutic targets remain imminent.
Obesity is the most common and well-described risk factor for NAFLD.5 As it is estimated that 70–80% of individuals with obesity have hepatic steatosis and 15–30% have NASH.6 NAFLD can be detected by ultrasound in approximately 65% of patients with overweight, compared to only 25% of normal individuals.7 Although NAFLD is strongly associated with obesity, the etiopathogenesis is still in the process of being defined. Obesity-induced overnutrition and insulin resistance (IR) are the main risk factors for the occurrence and development of NAFLD.8 However, the underlying mechanism for the development and progression of obesity-associated NAFLD is complex and multifactorial. Different theories have been proposed. The most widely regarded theory is the so-called “two-hit hypothesis”.9 This theory proposes that steatosis is the first hit for a non-alcoholic fatty liver (NAFL), and a second hit, such as reactive oxygen species (ROS) and endoplasmic reticulum (ER) stress, are needed for the progression to NASH and advanced fibrosis. The “two-hit” pathophysiological theory has subsequently been challenged, hepatic steatosis is considered to represent an epiphenomenon of several distinct injurious mechanisms, rather than a true “first hit”.10 For this reason, a “multiple parallel hits” hypothesis, involving a myriad of factors, offers a more acceptable delineation of the pathogenesis of NAFLD.11 However, these hypotheses are focused on the end-stage pathogenesis of obesity-associated NAFLD, the contributing factors that trigger these pathological mechanisms have rarely been summarized in the literature, particularly as related to the state of obesity.
The liver is a key integrator of the body's energy needs and metabolic balance, receiving multiple afferent metabolic signals from metabolically active organs. Accordingly, attention has recently been focused on the “multiple crosstalk” hypothesis, which advocates that obesity-related NAFLD may be the result of dysregulated “crosstalk” between multiple extra-hepatic organs and the liver, in the setting of obesity.12 The “crosstalk” is generally considered to be mediated by metabolic signals in an endocrine manner.13 Apart from metabolites (such as lipids, glucose and lipopolysaccharides [LPS], etc.), hormones are recognized to be the mainstay of endocrine metabolic signals. Of note, with deepening understanding of the endocrine system, the perception of hormones has gradually expanded from the narrow sense of traditional hormones to the broad sense of hormones as organokines and components in exosomes.14, 15, 16 In this review, we propose a potential pathological link between obesity and NAFLD from a macro-endocrine to a micro-endocrine perspective, summarizing the current understanding of how the new sense of hormones (organokines and exosomes) mediate the inter-organ and inter-cell crosstalk in the pathogenesis of obesity-associated NAFLD.
Organokines in obesity-associated NAFLD
Recently, evidence has emerged that adipose tissue, skeletal muscle, gut, and bone also function as endocrine organs that each secrete a complex array of cytokines, called organokines.17 Similar to traditional hormones, organokines exert autocrine, paracrine, or endocrine effects for the maintenance of inter-organ crosstalk and energy homeostasis.18,19 In the state of obesity, the balance of organokines is disrupted, accompanied by increased secretion of harmful organokines or decreased secretion of beneficial organokines targeting the liver (Supplemental table). This is proposed to be a new mechanism contributing to obesity-linked NAFLD. In this section, we outline the recent updates on how metabolic organokines (adipokines, myokines, gut cytokines and osteokines) influence the pathogenesis of obesity-associated NAFLD in an endocrine manner.
Adipokines
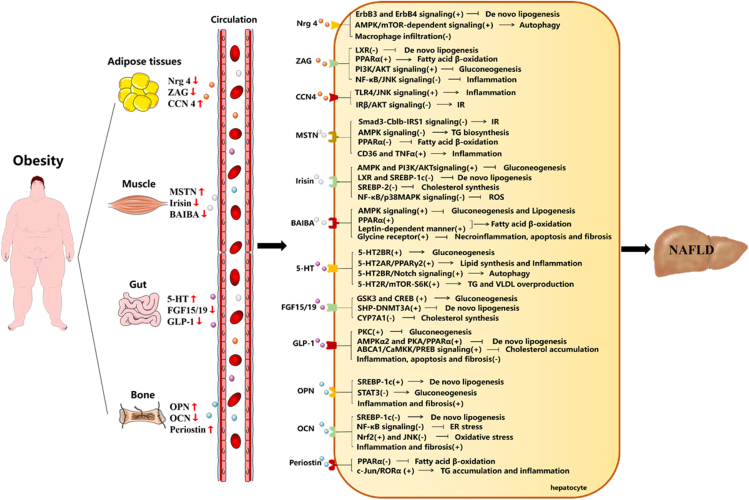
The current multiple crosstalk hypothesis has thrown the dialogue between adipose tissue and the liver into sharp focus.20 Adipose tissue produces circulating factors, known as adipokines, which exert several effects on hepatocytes, promoting the onset of NAFLD and its progression to NASH in subjects with obesity.21 Data of the association between NAFLD and some well-documented adipokines (e.g., adiponectin, leptin, and tumor necrosis factor alpha [TNF-α]) or inconclusive adipokines (e.g., IL-6, resistin, visfatin) are summarized in a chart (Supplementary table). Below, we describe in detail the role and mechanisms of three newly identified and better recognized adipokines (Nrg4, ZAG and CCN4) in obesity-linked fatty liver (Fig. 1).
Figure 1.
Potential roles and mechanisms of organokines in obesity-associated NAFLD. In the state of obesity, the balance of organokines (adipokines, myokines, gut cytokines, and osteokines) is disrupted, accompanied by increased secretion of harmful organokines or decreased secretion of beneficial organokines. The organokines target their hepatic receptor respectively and regulate hepatic metabolism in different pathways. ↑ increase, ↓ decrease, (+) activate, (−) inactivate, → promote,  restrain.
restrain.
Nrg4
Neuregulin 4 (Nrg4), a newly identified adipokine, is hypothesized to play a crucial role in metabolism.20 It is highly enriched in brown adipose tissue (BAT), and rarely expressed in skeletal muscle, heart, liver, and brain.22 Both serum and adipose tissue Nrg4 expression are reduced in rodents and humans with obesity.23 Consistently, serum Nrg4 level is significantly lower in the NAFLD group with obesity.24 Moreover, Nrg4−/− mice fed a high-fat diet (HFD) exhibit a significant increase in body weight as well as an exacerbation of hepatic steatosis.23 In contrast, transgenic expression of Nrg4 in adipose tissue alleviated diet-induced NAFLD.25 To determine the target tissue of Nrg4, researchers perform binding assays on sections of BAT, heart, muscle, liver and spleen, and found that Nrg4 binding is restricted to the liver, emphasizing that Nrg4 appears to be involved in crosstalk between BAT and the liver.23,26 Mechanistically, based on the available literature, BAT-derived Nrg4 counteracts obesity-linked NAFLD at least in three ways. First, it activates erb-b2 receptor tyrosine kinase 3/4 (ErbB3/4) signaling in hepatocytes and negatively regulates DNL mediated by liver x nuclear receptor (LXR) and sterol regulatory element binding protein-1c (SREBP-1c) in an endocrine manner.23,27 Second, it alleviates hepatic steatosis by enhancing autophagy via the amp-activated protein kinase (AMPK)/mammalian target of rapamycin (mTOR)-dependent signaling pathway.28 Third, it reduces macrophage infiltration in the liver and lowers expression of inflammatory factors, such as F4/80, Cd68, Cd11, and Mcp1.25 Thus, obesity-induced reduction of circulating BAT-derived Nrg4 may be one of the main causes of disturbed hepatic metabolic homeostasis.
ZAG
Recently, it has been proposed that the zinc-alpha2-glycoprotein (ZAG) could be a new lipolytic adipose tissue-derived candidate involved in the pathogenesis of obesity.29 Numerous data have shown that the serum concentration of ZAG is significantly lower in subjects and mice with obesity.30,31 Uniformly, ZAG expression is also significantly lower in the adipose tissue of patients with obesity, as well as HFD-induced obesity and genetic leptin-deficient obese (ob/ob) mice.32,33 Very recently, work from our group have uncovered a novel role of ZAG in regulating hepatic steatosis, IR, and inflammation in the obesity-related fatty liver.33,34 More specifically, ZAG ameliorates hepatic intracellular lipid accumulation via upregulating adiponectin and lipolytic genes, while downregulating lipogenic genes. ZAG restores HFD- or palmitic acid-induced impaired hepatic insulin receptor substrate (IRS)/AKT signaling, thereby exerting a protective effect against obesity-related IR. ZAG also inhibits the nuclear factor kappa-B (NF-κB)/jun n-terminal kinase (JNK) signaling, resulting in suppression of obesity-related inflammation in hepatocytes. Moreover, our current unpublished study showed that adipose tissue-specific ZAG-knockout mice display significantly reduced serum ZAG levels and exhibit more severe hepatic steatosis than their littermate control mice when challenged with a HFD. This indicates that ZAG is an protective endocrine factor that mediates fat and liver dialogue under obesity.
CCN4
Cellular communication network factor 4 (CCN4), a member of the CCN family of secreted, extracellular matrix-associated signaling proteins, was recently validated as a novel adipokine involved in pathophysiology of obesity and its related metabolic diseases.35,36 Since CCN4 is a matricellular protein, it may be sequestered in the extracellular matrix, however, CCN4 is found in the circulation.37 In humans, circulating CCN4 levels are higher in men with obesity than in normal-weight men.38,39 Moreover, CCN4 expression in visceral adipose tissue is 1.9-fold higher in men with obesity than in normal-weight men. Likewise, feeding mice a HFD also increases the expression of CCN4 in adipose tissue.36 Furthermore, knockdown of CCN4 in HFD-fed mice significantly attenuates hepatic lipid accumulation, inflammation, and IR.40 Mechanistically, the increases in circulating adipose tissue-derived CCN4 during obesity directly impacts the liver through two main pathways.38,40 First, it contributes to hepatic steatosis and inflammation through a toll like receptor 4 (TLR4)-activated inflammation/JNK signaling pathway. Secondly, it impairs insulin action on the phosphorylation of insulin receptor β (IRβ), Akt, and glycogen synthase kinase 3 beta (GSK3β), demonstrating an deteriorative role of CCN4 in obesity-associated NAFLD hepatic steatosis.
Myokines
Recent advances have shown the existence of direct muscle‒liver crosstalk in response to obesity,41 indicating that muscle-derived myokines may play a key role in energy homeostasis and in the pathogenesis of obesity-linked NAFLD (Supplementary table). Here, we summarize three candidate myokines (myostatin, irisin, BAIBA), and describe how they contribute to the evolution of fatty liver in the state of obesity (Fig. 1).
Myostatin
Myostatin (MSTN) is the first secreted muscle factor that fulfils the criteria of a myokine.42 Beyond its traditional role, MSTN has been reported to be secreted into the circulation in an endocrine manner, facilitating biological crosstalk among organs via activin-type II B receptors.43 Several lines of evidence have demonstrated that obesity is associated with increased MSTN levels. Clinically, circulating MSTN levels are increased in individuals with obesity.44 In animal study, MSTN levels are also elevated in plasma and peripheral tissues from ob/ob and HFD-obese mice.45 Conversely, exercise and weight loss reduce muscle and circulating MSTN levels in human subjects.46 Evidence suggests that increased circulating MSTN levels are observed in patients with liver disease and are associated with worse survival in patients with liver cirrhosis.47 Moreover, a constitutive MSTN loss-of-function mutation attenuates hepatic steatosis in mice fed an HFD.48 Even though the details of the mechanism by which MSTN affects liver metabolism remains largely unknown, some possible molecular signaling pathways have recently been proposed. MSTN induces IR by degrading the IRS1 protein via cbl proto-oncogene B (Cblb), an E3 ligase, in a Smad3-dependent manner.45 MSTN elevates hepatocellular lipid levels by suppressing AMPK signaling.49,50 MSTN reduces fatty acid β-oxidation by diminishing hepatic PPARα signaling.49 MSTN promotes hepatic inflammation via upregulating CD36 and TNF-α.48 Thus, it is plausible that an obesity-induced increase in plasma MSTN may contribute to development of obesity-related NAFLD.
Irisin
Initially identified as a myokine, muscle-derived irisin represents ca. 72% of the total circulating levels of the protein.51 Several studies have emphasized that obesity is closely related to a disorder of serum irisin.52,53 As irisin plays a protective role in obesity, it might be expect that levels of irisin would be lower in populations with obesity. However, most, but not all, clinical studies have described a positive association between circulating levels of irisin and BMI.54,55 To comprehend this somewhat contradictory clinical picture, a plausible explanation for this phenomenon is “irisin resistance” similar to that observed for insulin.53 In this regard, the hyper-irisinemia seen in obesity might be a compensatory mechanism to maximize energy usage and glucose homeostasis to combat irisin resistance.52,53 In the event of decompensation, compensatory high secretion of irisin in simple obesity switches to low secretion, further triggering obesity-related metabolic syndrome.56 In line with this, clinical studies have indicated that serum irisin levels are reduced in patients with obesity-related NAFLD.57 A study with 125I-labeled irisin showed high radioactivity in the mouse liver, implying that the liver is a target for irisin.57 In connection with this concept, recent studies have shown several candidate signaling pathways of irisin in hepatocytes. Irisin improves glucose homeostasis by activating the phosphoinositide-3-kinase (PI3K)-AKT and AMPK pathways.52,56 Irisin prevents palmitic acid-induced lipid accumulation by inhibiting hepatic lipogenic regulators (LXR and SREBP-1c).56 Irisin reduces cholesterol content by inhibiting SREBP-2.56 It downregulats inflammatory markers via NF-κB and p38 mitogen activated protein kinase (p38 MAPK) pathways and ameliorates hepatic oxidative stress in a protein arginine methyltransferase 3 (PRMT3) dependent manner.52,58 Hence, obesity-induced decompensated reduction of irisin levels is an important factor mediating disorder of muscle‒liver dialogue, leading to obesity-related fatty liver.
BAIBA
β-Aminoisobutyric acid (BAIBA), a newly identified myokine, is a muscle-derived signal factor, acting particularly on metabolic organs (such as the liver) to improve the metabolic profile.59 In humans, plasma BAIBA concentrations are reduced in obesity and are inversely associated with metabolic risk factors.60 Inversely, exercise training intervention increases plasma BAIBA concentration.61 Moreover, accumulating experimental evidence suggests that BAIBA treatment improves HFD-induced body adiposity and hepatic steatosis.62,63 It has been clarified that BAIBA can prevent body fatness through stimulating browning of white adipose tissue.62 However, the mechanisms by which BAIBA favors improvement of hepatic steatosis are more comprehensive and complicated, as follows. BAIBA improves hepatic IR by decreasing gluconeogenesis via both the IRS1/AKT and AMPK pathways.63 BAIBA down-regulates expression of ACC and SREBP-1c, reduces apoB-containing lipoprotein production by activation of AMPK in hepatocytes.63 BAIBA drives an increase in hepatic fatty acid β-oxidation through a conserved PPARα and leptin-dependent mechanism.62,63 BAIBA reduces hepatic necroinflammation, apoptosis, and fibrosis, possibly through activation of the glycine receptor in ob/ob mice.62,63 Accordingly, considering that BAIBA plays such an protective role in liver metabolism, obesity-induced reduction in muscle-derived BAIBA levels should be taken into account in the pathogenesis of obesity-related fatty liver.
Gut cytokines
In the recent years, research on the biology of the gut–liver axis has assisted in understanding the basic biology of obesity-associated fatty liver.64 Obesity impairs the gut barrier, which results in the translocation of disordered gut-derived hormones into the circulation and distant metabolic organs, particularly the liver (Supplementary table). Here, we focus on three key gut cytokines [5-hydroxytryptamine (5-HT), FGF15/19 and GLP-1] that are involved in the dialogue between obesity and fatty liver (Fig. 1).
5-HT
Emerging evidence suggests an important role for peripheral 5-HT as a factor that enhances nutrient absorption and storage.65 Peripheral 5-HT is synthesized by enterochromaffin (EC) cells scattered throughout the gastrointestinal tract. It can enter the bloodstream and act as an endocrine factor to promote a dialogue between gut and multiple metabolic organs, particularly white adipose tissue and the liver.66 Recent findings support a positive correlation between obesity and peripheral 5-HT levels. For example, elevated serum 5-HT levels are observed both in individuals with obesity and HFD mice.67,68 Moreover, obesity also increases EC cell numbers and disrupts the circadian rhythm of circulating 5-HT.69 Of interest, researchers have also highlighted the involvement of 5-HT in the development of obesity-related NAFLD, as evidence suggests that serum 5-HT levels are elevated in both NAFLD patients and rats with obesity.70 In terms of the mechanism, a previous study has demonstrated an indirect effect of 5-HT on promoting fatty liver by inhibiting BAT thermogenesis in mice with obesity.71 Besides the indirect manner, recent studies have implicated a direct role for gut-derived 5-HT in the pathogenesis of hepatic steatosis: locally produced 5-HT can directly travel to and affect the liver via the hepatic portal vein in HFD mice.72 The direct action of 5-HT on the liver is dependent on its receptors (5-HT2AR and 5-HT2BR).72,73 5-HT promotes liver gluconeogenesis and inhibits glucose uptake via activation of 5-HT2BR.74 5-HT promotes lipid synthesis and an inflammatory response through the 5-HT2AR/PPARγ signaling in hepatocytes.70 It induces autophagy-mediated hepatic steatosis via 5-HT2BR/Notch signaling.73 It stimulates overproduction of hepatic TG and very low-density lipoprotein (VLDL) by acting 5-HT2R/mTOR-S6K pathway.74 5-HT also activates 5-HT2R in hepatic stellate cells to trigger liver fibrosis and steatohepatitis.75 Hence, on the basis of the available evidence, we conclude that 5-HT may be one of the deteriorative gut-to-liver signals mediating obesity-associated NAFLD.
FGF15/19
FGF19 (and its mouse ortholog FGF15) mainly controls bile acid (BA) metabolism in various target organs (liver, adipose tissue, and brain).76 Recently, interest has focused on the role of FGF15/19 in obesity. FGF15/FGF19 overexpression confers resistance to diet-induced obesity (DIO) and alleviates genetic obesity in ob/ob mice.77 Conversely, FGF15−/− mice show increased weight gain and exacerbated systemic adiposity when fed a HFD.78 Basal circulating FGF19 levels are significantly lower in patients with obesity than in non-obese controls.79 Moreover, circulating FGF19 levels in patients with obesity are increased after bariatric surgery-induced weight loss.80 Recently, growing evidence suggests that changes in circulating levels of FGF19 contribute to the pathogenesis of obesity-related NAFLD. Most reports have indicated decreased fasting FGF19 levels in patients with obesity-related NAFLD as compared to controls.81 Nevertheless, some studies have reported that patients with NAFLD present basal FGF19 levels similar to healthy subjects, but their hepatic response to FGF19 is impaired.82 Furthermore, in the context of obesity-induced NAFLD, transgenic expression of FGF19 or its pharmacological administration to obese mice resulted in increased insulin sensitivity and reduced hepatosteatosis.78 In terms of the potential underlying mechanism, the direct signaling pathways of FGF15/19 triggered by its FGFR4-β-klotho receptor complex in the hepatocyte can be summarized as follows. FGF15/19 stimulates glycogen synthesis, but reduces glucogenesis by inactivating GSK3 and camp responsive element binding protein (CREB).83,84 It represses hepatic lipogenesis via inhibiting SREBP-1c‒stearoyl-coa desaturase 1 (SCD1) and activating SHP‒dna methyltransferase 3 alpha (DNMT3A) axis.83,85 FGF15/19 also suppresses the synthesis of bile acid by inhibiting cytochrome p450 family 7 subfamily a member 1 (CYP7A1).83 Taken together, obesity-associated reduction of gut-derived FGF19 should certainly be taken into account in the pathogenesis of obesity-associated NAFLD.
GLP-1
Recently, reports have emphasized that GLP-1 is an interesting target for the treatment of obesity.86 Although increased and unchanged GLP-1 responses have also been reported,87,88 it has generally been accepted that there is an attenuated postprandial GLP-1 response in subjects with obesity. A large study population of 1462 Danish adults demonstrated that individuals with obesity have an up to 20% reduced GLP-1 response to oral glucose as compared with normal weight individuals.89 In addition, the attenuation of the postprandial GLP-1 response in obesity is somewhat improved by jejunoileal bypass surgery and dietary-induced weight reduction.90 Of note, the lower GLP-1 response to a meal in individuals with obesity was likely to be a consequence, rather than a cause. Obesity-induced increased concentrations of non-esterified fatty acid (NEFA) and glucose are thought to be inhibitors of GLP-1 release.91 Moreover, the obesity-induced delay in gastric emptying also leads to impaired secretion of GLP-1.92 Congruously, GLP-1 responses are also blunted in patients with obesity-induced NAFLD.93 Importantly, growing evidence indicates a direct protective effect of GLP-1 on the liver, which can be summarized as follows. GLP-1 exerts an insulin-sensitizing action via PKC and PPARγ signaling.94,95 GLP-1 also decreases hepatic DNL by activating AMPKα2 and PKA-dependent increased of PPARα.95,96 GLP-1 reduces hepatic cholesterol accumulation via the ATP binding cassette subfamily a member 1 (ABCA1)/calcium/calmodulin dependent protein kinase kinase (CaMKK)/prolactin regulatory element binding (PREB) pathway.97 GLP-1 alleviates hepatic ER stress by enhancing the SIRT1/heat shock transcription factor 1 (HSF1)/HSPs pathway.98 GLP-1 mitigates hepatic inflammation by inducing liver-specific anti-inflammatory M2 macrophages polarization via signal transducer and activator of transcription 3 (STAT3) activation.95,99 GLP-1 deactivates hepatic stellate cells by decreasing expression of fibrosis-associated proteins, and therefore reduces liver fibrosis and cirrhosis.95,99 These effects seem to be GLP-1R-mediated, since the treatment of GLP-1R-deficient mice with GLP-1 analogues has no effect on hepatic lipid concentration. Whether GLP-1R is expressed on hepatocytes is controversial. It has been suggested that GLP-1 can even signal through a neural circuit originating in the hepatic-portal area.100 All in all, as a direct protective effect on the liver, obesity-induced reduced GLP-1 responses are crucial factors contributing to obesity-associated NAFLD.
Osteokines
Bone-derived secreted factors, called osteokines, comprise an important endocrine system that is finely tuned to other organs (particularly adipose tissues, muscle, and liver) to ensure homeostatic balance and health.101 The evidence obtained over the past few decades have shown that at least three hormones or osteokines from bone cells have endocrine functions and promote hepatic metabolic disorders in response to obesity (Supplementary table). Here, we highlight three well-documented osteokines (osteopontin, osteocalcin, and periostin) and describe their contribution to the evolvement of obesity-associated NAFLD (Fig. 1).
Osteopontin
Besides its function as a pivotal molecule regulating bone mineralization, osteopontin (OPN) has recently come to light as a key component in the development of obesity and metabolic disorders.102 Elevated plasma levels of OPN have been detected in humans and mice with obesity.103,104 Moreover, diet-induced weight loss could decrease plasma OPN levels.104 It has been proposed that elevated circulating and local OPN concentrations in individuals with obesity are a key risk factor for obesity-related NAFLD,105 an effect that is mainly attributed to OPN-induced systemic metabolic inflammation.106,107 Additionally, genetic OPN deficiency and antibody-mediated neutralization improve obesity-associated hepatic TG synthesis and inflammation in murine models.108,109 More specifically, the direct mechanisms connecting OPN to obesity-associated NAFLD can be concluded as follows. OPN facilitates hepatic steatosis by up-regulating PPARγ and down-regulating PPARα and PGC1α expression.108 It promotes hepatic IR by inactivating STAT3, further increasing the gluconeogenetic enzymes phosphoenolpyruvate carboxykinase (PEPCK) and glucose-6-phosphatase (G6P) expression.102 OPN accelerates hepatic inflammation by augmenting hepatic macrophage infiltration.109 OPN exacerbates hepatic fibrosis via activation of the PI3K/pAkt/NF-κB pathway in hepatic stellate cells.110 Hence, it is reasonable to assume that OPN acts as one of the key drivers in the development of obesity-related NAFLD by mediating the dialogue between bone and the liver.
Osteocalcin
Osteocalcin (OCN) is a bone-derived protein, classically considered as a clinical marker of bone turnover. It has recently arisen as a circulating hormone involved in the regulation of energy metabolism.111 OCN-deficient mice exhibit increased fat mass and glucose intolerance.112 In contrast, OCN treatment in HFD-fed mice results in improved glucose tolerance and less visceral fat deposition.113 Several clinical studies have shown that individuals with obesity present lower serum levels of OCN than normal-weight individuals.114,115 Of note, recent studies have indicated that circulating OCN is negatively associated with NAFLD,116 consistent with the situation in obesity. Fernández-Real et al pointed out that OCN may play a role in the development of obesity-associated fatty liver disease.117 Moreover, previous animal studies also showed that OCN treatment prevents obesity-induced hepatic TG accumulation and liver injury in mice.114,115 We summarize the possible mechanisms connecting OCN to obesity-associated NAFLD as follows. OCN reduces HFD-induced hepatic lipogenesis in a SREBP-1c-dependent manner.118 It attenuates ER stress and rescues impaired insulin sensitivity via the NF-κB signaling.119 OCN also protects HFD mice from oxidative stress by activating the hepatic nuclear factor 2 (Nrf2) and inhibiting the JNK pathway.120 Moreover, it robustly reduces expression of pro-inflammatory and pro-fibrotic genes (Cd68, Mcp1, Spp1, and Col1a2) in the liver.121 Therefore, it is feasible that the reduced circulatory levels of the bone-derived hormone OCN observed in patients with obesity could be a potential link between the presence of obesity and obesity-associated NAFLD.
Periostin
Periostin is a secreted cell-adhesion protein that was initially identified in periosteal osteocytes and osteoblasts. It functions as a homophilic adhesion molecule during bone formation.122 Beyond bone, periostin has been linked to several primarily metabolic inflammatory diseases, including obesity, type 2 diabetes, and atherosclerosis.123 More recently, increasing evidence has suggested that periostin is involved in the development of obesity-linked hepatosteatosis.124 Epidemiological studies have noted that serum periostin concentrations are increased in human with obesity, and have speculated that an elevated serum periostin level is associated with an increased risk of NAFLD among overweight and obese individuals.125,126 Consistently, preclinical study also revealed that obese HFD-fed and ob/ob mice have higher circulating periostin levels.127 To clarify the precise role of periostin in the pathogenesis of obesity-induced hepatosteatosis further, both gain- and loss-of-function mouse models were used in the research. Overexpression of periostin in the liver of wild-type mice promoted hepatic steatosis, while knockdown of the periostin gene or administration of a periostin-neutralizing antibody in obese mice reduced hepatic and serum TG and improved steatosis.127 Furthermore, periostin-knockout mice fed a methionine choline deficient (MCD) diet, a model known to develop NASH, had a markedly lower degree of hepatic steatosis, inflammation, and fibrosis than wild-type mice fed an MCD diet.128 Two possible mechanistic pathways were illuminated by the studies: First, periostin reduces hepatic fatty acid oxidation via downregulation of PPARα. Secondly, periostin suppresses rar related orphan receptor α (RORα) transcriptional activity, which finally results in hepatic TG accumulation and inflammation. Together, periostin is expected to be a promising extracellular diagnostic biomarker and mediator of obesity-linked NAFLD.127,128
Exosomes in obesity-associated NAFLD
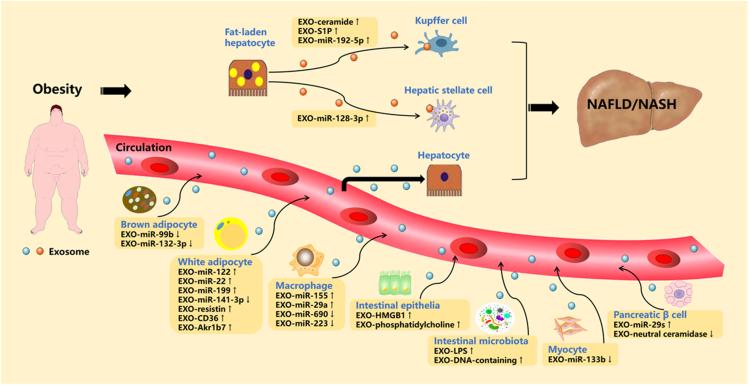
Extracellular vesicles (EVs, including exosomes), a new class of microscopic endocrine hormone, mediate long-distance cell-to-cell communication at the molecular level. Exosomes are circulating, cell-derived nanoparticles containing proteins and nucleic acids. An emerging property of exosomes is their ability to target and modify specific cells.16 In the context of obesity, exosomes may serve as mediators of NAFLD. This section aims to improve understanding of exosome-mediated crosstalk of various cells (adipocytes, macrophages, intestinal epithelia, etc.) with hepatocytes or hepatic macrophages/stellate cells in the pathogenesis of obesity-associated NAFLD (Fig. 2).
Figure 2.
Potential roles of exosomes in obesity-associated NAFLD/NASH. In the state of obesity, adipocytes, macrophages, intestinal epithelia, microbiota, myocytes and pancreatic β cells derived exosomal miRNAs or proteins mediate obesity-associated fatty liver by talking to hepatocytes in an endocrine manner. ↑ increase, ↓ decrease.
Adipocyte-derived exosomes
microRNA-containing exosomes
Recent years have witnessed a growing interest in exosomal miRNA-mediated crosstalk among metabolic organs. Interestingly, the recent work of Thomou and co-authors showed that the majority of circulating exosomal miRNAs are derived from adipose tissue and can travel to the liver, resulting in functional changes in hepatocytes.129 Of note, recent findings have suggested that obesity changes the profile of circulating exosomal miRNAs in humans and mice, thus disturbing the normal dialogue between adipose tissue and the liver, which may lead to the progression of obesity-related fatty liver.130 To date, four types of white adipocyte-derived exosomal miRNAs (EXO-miR-122, EXO-miR-22, EXO-miR-199, and EXO-miR-141–3p) have been confirmed to be involved in obesity-linked fatty liver. These miRNAs have been reported to undergo significant changes in circulating levels in obesity.131, 132, 133, 134 In the state of obesity, elevated EXO-miR-122 could inhibit hepatic fatty acid oxidation via the LKB1/AMPK/Sirt1 pathway.135 Elevated EXO-miR-22 contributes to development of steatosis by simultaneously reducing hepatic FGF21.136 Elevated EXO-miR-199 promotes hepatic lipid accumulation by down-regulating hepatic macrophage stimulating 1 (MST1) expression.133 Reduced EXO-miR-141–3p significantly inhibits hepatic insulin sensitivity and glucose uptake.134 In addition to white adipocyte-derived exosomal miRNAs, brown adipocyte-derived exosomal miRNAs have also been confirmed to mediate obesity-induced fatty liver. Obesity alters the composition of brown adipocyte-derived exosomal miRNAs. Among them, exosomal miR-99 b and miR-132–3p were identified to regulate hepatic glucose tolerance and lipogenesis by targeting FGF21 and sterol regulatory element binding transcription factor 1 (Srebf1) in mouse liver, respectively.129,137
Protein-containing exosomes
Interestingly, recent findings have pointed out that adipocyte-derived exosomal proteins can also be endocytosed by hepatocytes and take part in the development of obesity-related NAFLD. For example, adipocyte-derived exosomal resistin is responsible for the communication from adipose tissue to the liver, leading to hepatic ER stress by inhibiting AMPKα signaling, which finally contributes to the development of obesity-linked hepatic steatosis.138 Furthermore, obesity-associated inactivation of AMPKα1 in white adipose tissue contributes to increased adipocyte-derived exosomal CD36 secretion, mediating HFD-induced hepatic lipid accumulation and inflammation.139 Recently, Gu et al indicated that obesity-induced adipose tissue ER stress promotes release of adipocyte-derived exosomal Aldo-keto-reductase 1b7 (Akr1b7), leading to hepatic steatosis, inflammation, and fibrosis.140
Macrophages derived exosomes
In addition to anti-inflammatory and pro-inflammatory cytokines, adipose tissue macrophages (ATMs) can also secrete miRNA-containing exosomes into the circulation. Importantly, ATM-derived exosomes containing increased levels of “harmful” miRNAs and decreased levels of “protective” miRNAs, in obese mice, can be transported to the liver and where they regulate hepatic gene expression. For instance, M1-polarized macrophage-derived exosomal miR-155 and miR-29a are increased in obesity and impair hepatic glucose output by targeting PPAR-γ and PPAR-δ, respectively.141,142 However, miR-690, an exosome-derived miRNA from M2-polarized macrophages, is decreased in obesity. This “protective” miRNA improves hepatic insulin sensitivity by targeting Nadk in obese mice.143 More recently, macrophage-derived exosomal miR-223 has been identified as an anti-fibrotic factor that hinders liver fibrosis in HFD-fed mice by targeting tafazzin (TAZ), a well-known factor that promotes NASH-fibrosis.144
Intestinal epithelial and microbial-derived exosomes
Emerging evidence has indicated that gut-derived exosomes are also enriched in the circulation in individuals with obesity, and could be targeted to the liver, triggering obesity-associated hepatic lipid deposition and inflammation. One such example is exosomal high mobility group box 1 (HMGB1), the levels of which are elevated in both intestinal epithelia and circulation of mice with obesity as compared with lean mice.145 HMGB1 is a proinflammatory cytokine, which serves as a damage-associated molecular pattern molecule (DAMP) that mediates TLR4 activation. HMGB1 is released via the exosomes from the intestine due to HFD-induced gut dysbiosis and then exerts its harmful action on the liver. A previous study also revealed that a HFD markedly changes the lipid profile of intestinal epithelial exosomes from predominantly phosphatidylethanolamine (PE) to phosphatidylcholine (PC), which results in inhibition of the hepatic insulin response via binding of PC to the aryl hydrocarbon receptor expressed in hepatocytes.146 Moreover, recent studies have underlined the pathogenic effects of obesity-associated microbial EVs on the development of NAFLD.147 For example, a recent study has shown that patients with intestinal barrier dysfunction demonstrate increased systemic levels of LPS-positive bacterial EVs. As bacterial EVs can reach and accumulate in the liver, hepatocytes/Kupffer cells carry LPS from EVs into the cytosol by TLR4‒toll like receptor adaptor molecule 1 (TRIF) signaling, mediating inflammation and other pathological processes related to NAFLD.148 Furthermore, a recent study revealed that microbiota-derived bacterial DNA-containing EVs can pass through the disrupted intestinal barrier, subsequently exacerbating obesity-linked hepatic inflammation and IR via the cGAS/STING pathway.149
Myocyte-derived exosomes
No direct evidence yet exists that muscle-derived exosomal miRNAs facilitate communication with the liver in the state of obesity. Only one study has reported that exercise triggers the release of exosomes by the trained muscle, carrying the miR-133 b signature that induces forkhead box o1 (FoxO1) expression changes in the liver, finally contributing to increased insulin sensitivity.150 Since exercise can combat obesity and enhance the secretion of muscle-derived EXO-miR-133 b, obesity may impair the secretion of EXO-miR-133 b. Hence, decreased EXO-miR-133 b may further interfere with the normal dialogue between the muscle and the liver, contributing to obesity-related hepatic IR. This assumption should be addressed in future studies.
Pancreatic β cell-derived exosomes
It has recently been verified that pancreatic islets release not only conventional hormones but also exosomal miRNAs/proteins to mediate the dialogue between the pancreas and insulin-responsive organs, including the liver, adipose tissue, and skeletal muscle. Of note, pancreas‒liver crosstalk mediated by exosomes has been confirmed to be involved in obesity-associated fatty liver disease. For example, one study has indicated that, in response to chronic obesity-associated high levels of free fatty acids, pancreatic β cells secrete exosomal miR-29s to target hepatocytes and promote hepatic IR and steatosis.151 Furthermore, an in vitro experiment noted that neutral ceramidase-enriched exosomes from INS-1 cells rescue palmitic acid-induced hepatic IR and ROS production.152
Hepatocyte-derived exosomes
Activation of hepatic stellate cells (HSCs) and Kupffer cells play critical roles in the development of NASH. Evidence has suggested that exosomes have a critical function in paracrine actions between lipotoxic hepatocytes and macrophages in the liver, exacerbating obesity-associated NASH. For instance, palmitic acid-induced, hepatocyte-derived EVs are enriched in both C16:0 ceramide and its metabolite sphingosine-1-phosphate (S1P), which are effectors of macrophage infiltration into the liver microenvironment during hepatic lipotoxicity.153,154 Moreover, Liu et al reported that lipotoxic injury-induced release of hepatocyte-derived exosomal miR-192–5p plays a critical role in the activation of M1 macrophages and hepatic inflammation by modulating Rictor/Akt/FoxO1 signaling.155 Additionally, there is cross-talk between fat-laden hepatocytes and hepatic stellate cells via exosomes, which plays a key role in liver fibrosis during NAFLD. It has been reported that overloading of hepatocytes with toxic lipids results in the release of exosomal miR-128–3p, which can be efficiently internalized by HSCs and induce a profibrogenic phenotype by suppressing PPARγ expression.156
Conclusions
Previously, the mechanisms underlying NAFLD have been summarized by investigators mainly focusing on the end-stage pathogenesis, such as hepatic lipidosis, IR, ER stress, ROS, mitochondrial autophagy, ferroptosis and pyroptosis. However, the contributing factors that trigger these pathological mechanisms have rarely been summarized in the literature, particularly as related to the state of obesity. This review provides a comprehensive view of how obesity drives the development and progression of fatty liver from an endocrine perspective. We focused on the perspective of organic endocrine hormones (organokines) and molecular endocrine hormones (exosomes), to improve understanding of how they are altered in the circulation, whether they are in direct dialogue with the liver, and how they influence the mechanisms of metabolic disturbances in hepatocytes during obesity.
The discovery and elucidation of novel endocrine regulatory cytokines is critical for the early diagnosis and treatment of NAFLD. The current identification of novel cytokines is based on genomics, proteomics, transcriptomics, metabolomics and lipidomics, and even on the combination of part or all these omics.157 Here, we summarized a series of new candidate hormones that may mediate obesity-associated fatty liver, the preclinical mechanisms of some of which have been intensively studied, only a few have been developed for their clinical value. For example, among the series of hormones we summarized, only organokines (adiponectin, leptin, resistin, visfatin, RBP4, FABP4, FGF21) and miRNAs (miRNA-34a, miRNA-122 and miRNA-192) were considered as promising clinical indicators for the diagnosis of NAFLD and NASH.157,158 In addition, only FGF19, FGF21 and GLP-1 are currently beyond phase II for the treatment of NAFLD/NASH.4 Hence, gaps and limitations in current knowledge are highlighted together with the need to further decipher the full array of the summarized hormones in their actions in actual clinical application.
Of note, the hormone network is complex, in addition to acting directly on the liver, the hormones could also indirectly interact with other hormones or organs to regulate liver metabolism. For example, upregulation of hormones such as irisin, IL-15, FGF21, ZAG and OCN has been shown in preclinical studies to stimulate the release of the hepatoprotective hormone adiponectin.159 Besides, hormones such as ZAG, Nrg4, FGF21, GLP-1, IL-6, BAIBA, irisin and OCN may indirectly improve NAFLD by promoting anorexigenic hormone brain-derived neurotrophic factor (BDNF) synthesis in the central nervous system (CNS) or promoting browning of WAT.159,160 Moreover, the dialogue between organokines and exosomes also plays an important role in the development of NAFLD. For instance, adiponectin was found to enhance exosomes biogenesis and secretion, leading to a decrease in cellular ceramides. While excess ceramide is known to cause IR and fatty liver phenotype.161 In addition, obesity-related circulating exosomes could reduce FGF21 levels and impair hepatic insulin signaling pathways.129 It is well known that exercise and diet restriction are the most effective ways to amplify the synergistic effects of metabolically beneficial hormonal network. Hence, regardless of the progress that has been, or will be, made in diagnostic tests and drug treatments, weight reduction through exercise and diet restriction remains crucial for the prevention and treatment of NAFLD/NASH, as obesity is the main driver of this common liver disease and its associated metabolic comorbidities.3
Conflict of interests
Authors declare no conflict of interests.
Footnotes
Peer review under responsibility of Chongqing Medical University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.gendis.2021.12.013.
Contributor Information
Jiang-Hua Liu, Email: jianghua990@126.com.
Xin-Hua Xiao, Email: xinhua0102@163.com.
Funding
This work was supported by The National Natural Science Foundation of China (No. 82070873, 82000813) and Major Special Projects of Hunan Provincial Health and Family Planning Commission (No. A2017011).
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Younossi Z.M., Koenig A.B., Abdelatif D., et al. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 2.Zhou F., Zhou J., Wang W., et al. Unexpected rapid increase in the burden of NAFLD in China from 2008 to 2018: a systematic review and meta-analysis. Hepatology. 2019;70(4):1119–1133. doi: 10.1002/hep.30702. [DOI] [PubMed] [Google Scholar]
- 3.Powell E.E., Wong V.W., Rinella M. Non-alcoholic fatty liver disease. Lancet. 2021;397(10290):2212–2224. doi: 10.1016/S0140-6736(20)32511-3. [DOI] [PubMed] [Google Scholar]
- 4.Ferguson D., Finck B.N. Emerging therapeutic approaches for the treatment of NAFLD and type 2 diabetes mellitus. Nat Rev Endocrinol. 2021;17(8):484–495. doi: 10.1038/s41574-021-00507-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harris R., Card T.R., Delahooke T., et al. Obesity is the most common risk factor for chronic liver disease: results from a risk stratification pathway using transient elastography. Am J Gastroenterol. 2019;114(11):1744–1752. doi: 10.14309/ajg.0000000000000357. [DOI] [PubMed] [Google Scholar]
- 6.Lall C.G., Aisen A.M., Bansal N., et al. Nonalcoholic fatty liver disease. AJR Am J Roentgenol. 2008;190(4):993–1002. doi: 10.2214/AJR.07.2052. [DOI] [PubMed] [Google Scholar]
- 7.Arab J.P., Arrese M., Trauner M. Recent insights into the pathogenesis of nonalcoholic fatty liver disease. Annu Rev Pathol. 2018;13:321–350. doi: 10.1146/annurev-pathol-020117-043617. [DOI] [PubMed] [Google Scholar]
- 8.Roberts E.A. Pediatric nonalcoholic fatty liver disease (NAFLD): a "growing" problem? J Hepatol. 2007;46(6):1133–1142. doi: 10.1016/j.jhep.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 9.Day C.P., James O.F. Steatohepatitis: a tale of two "hits. Gastroenterology. 1998;114(4):842–845. doi: 10.1016/s0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- 10.Bulankina A.V., Deggerich A., Wenzel D., et al. TIP47 functions in the biogenesis of lipid droplets. J Cell Biol. 2009;185(4):641–655. doi: 10.1083/jcb.200812042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tilg H., Moschen A.R. Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology. 2010;52(5):1836–1846. doi: 10.1002/hep.24001. [DOI] [PubMed] [Google Scholar]
- 12.Zhang X., Ji X., Wang Q., et al. New insight into inter-organ crosstalk contributing to the pathogenesis of non-alcoholic fatty liver disease (NAFLD) Protein Cell. 2018;9(2):164–177. doi: 10.1007/s13238-017-0436-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nobili V., Svegliati-Baroni G., Alisi A., et al. 360-degree overview of paediatric NAFLD: recent insights. J Hepatol. 2013;58(6):1218–1229. doi: 10.1016/j.jhep.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 14.Marino L., Jornayvaz F.R. Endocrine causes of nonalcoholic fatty liver disease. World J Gastroenterol. 2015;21(39):11053–11076. doi: 10.3748/wjg.v21.i39.11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oh K.J., Lee D.S., Kim W.K., et al. Metabolic adaptation in obesity and type II diabetes: myokines, adipokines and hepatokines. Int J Mol Sci. 2016;18(1):8. doi: 10.3390/ijms18010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang-Doran I., Zhang C.Y., Vidal-Puig A. Extracellular vesicles: novel mediators of cell communication in metabolic disease. Trends Endocrinol Metabol. 2017;28(1):3–18. doi: 10.1016/j.tem.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 17.Montgomery M.K., De Nardo W., Watt M.J. Impact of lipotoxicity on tissue “CrossTalk” and metabolic regulation. Physiology. 2019;34(2):134–149. doi: 10.1152/physiol.00037.2018. [DOI] [PubMed] [Google Scholar]
- 18.de Oliveira Dos Santos A.R., de Oliveira Zanuso B., Miola V.F.B., et al. Adipokines, myokines, and hepatokines: crosstalk and metabolic repercussions. Int J Mol Sci. 2021;22(5):2639. doi: 10.3390/ijms22052639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chung H.S., Choi K.M. Organokinesindisease. Adv Clin Chem. 2020;94:261–321. doi: 10.1016/bs.acc.2019.07.012. [DOI] [PubMed] [Google Scholar]
- 20.Azzu V., Vacca M., Virtue S., et al. Adipose tissue-liver cross talk in the control of whole-body metabolism: implications in nonalcoholic fatty liver disease. Gastroenterology. 2020;158(7):1899–1912. doi: 10.1053/j.gastro.2019.12.054. [DOI] [PubMed] [Google Scholar]
- 21.Ouchi N., Parker J.L., Lugus J.J. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11(2):85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pfeifer A. NRG4: an endocrine link between brown adipose tissue and liver. Cell Metabol. 2015;21(1):13–14. doi: 10.1016/j.cmet.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 23.Wang G.X., Zhao X.Y., Meng Z.X., et al. The brown fat-enriched secreted factor Nrg4 preserves metabolic homeostasis through attenuation of hepatic lipogenesis. Nat Med. 2014;20(12):1436–1443. doi: 10.1038/nm.3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dai Y.N., Zhu J.Z., Fang Z.Y., et al. A case-control study: association between serum neuregulin 4 level and non-alcoholic fatty liver disease. Metabolism. 2015;64(12):1667–1673. doi: 10.1016/j.metabol.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 25.Ma Y., Gao M., Liu D. Preventing high fat diet-induced obesity and improving insulin sensitivity through neuregulin 4 gene transfer. Sci Rep. 2016;6:26242. doi: 10.1038/srep26242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blüher M. Neuregulin 4: a "hotline" between Brown fat and liver. Obesity. 2019;27(10):1555–1557. doi: 10.1002/oby.22595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang W., Zhang Y., Yang C., et al. Transplantation of neuregulin 4-overexpressing adipose-derived mesenchymal stem cells ameliorates insulin resistance by attenuating hepatic steatosis. Exp Biol Med. 2019;244(7):565–578. doi: 10.1177/1535370219839643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu B., Mei W., Jiao T., et al. Neuregulin 4 alleviates hepatic steatosis via activating AMPK/mTOR-mediated autophagy in aged mice fed a high fat diet. Eur J Pharmacol. 2020;884:173350. doi: 10.1016/j.ejphar.2020.173350. [DOI] [PubMed] [Google Scholar]
- 29.Gong F.Y., Deng J.Y., Zhu H.J., et al. Fatty acid synthase and hormone-sensitive lipase expression in liver are involved in zinc-alpha2-glycoprotein-induced body fat loss in obese mice. Chin Med Sci J. 2010;25(3):169–175. doi: 10.1016/s1001-9294(10)60043-0. [DOI] [PubMed] [Google Scholar]
- 30.Selva D.M., Lecube A., Hernández C., et al. Lower zinc-alpha2-glycoprotein production by adipose tissue and liver in obese patients unrelated to insulin resistance. J Clin Endocrinol Metab. 2009;94(11):4499–4507. doi: 10.1210/jc.2009-0758. [DOI] [PubMed] [Google Scholar]
- 31.Liu M., Zhu H., Dai Y., et al. Zinc-α2-Glycoprotein is associated with obesity in Chinese people and HFD-induced obese mice. Front Physiol. 2018;9:62. doi: 10.3389/fphys.2018.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mracek T., Gao D., Tzanavari T., et al. Downregulation of zinc-{alpha}2-glycoprotein in adipose tissue and liver of obese ob/ob mice and by tumour necrosis factor-alpha in adipocytes. J Endocrinol. 2010;204(2):165–172. doi: 10.1677/JOE-09-0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xiao X.H., Wang Y.D., Qi X.Y., et al. Zinc alpha2 glycoprotein protects against obesity-induced hepatic steatosis. Int J Obes. 2018;42(8):1418–1430. doi: 10.1038/s41366-018-0151-9. [DOI] [PubMed] [Google Scholar]
- 34.Xiao X., Li H., Qi X., et al. Zinc alpha2 glycoprotein alleviates palmitic acid-induced intracellular lipid accumulation in hepatocytes. Mol Cell Endocrinol. 2017;439:155–164. doi: 10.1016/j.mce.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 35.Klimontov V.V., Bulumbaeva D.M., Fazullina O.N., et al. Circulating Wnt1-inducible signaling pathway protein-1 (WISP-1/CCN4) is a novel biomarker of adiposity in subjects with type 2 diabetes. J Cell Commun Signal. 2020;14(1):101–109. doi: 10.1007/s12079-019-00536-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murahovschi V., Pivovarova O., Ilkavets I., et al. WISP1 is a novel adipokine linked to inflammation in obesity. Diabetes. 2015;64(3):856–866. doi: 10.2337/db14-0444. [DOI] [PubMed] [Google Scholar]
- 37.Stephens S., Palmer J., Konstantinova I., et al. A functional analysis of Wnt inducible signalling pathway protein -1 (WISP-1/CCN4) J Cell Commun Signal. 2015;9(1):63–72. doi: 10.1007/s12079-015-0267-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hörbelt T., Tacke C., Markova M., et al. The novel adipokine WISP1 associates with insulin resistance and impairs insulin action in human myotubes and mouse hepatocytes. Diabetologia. 2018;61(9):2054–2065. doi: 10.1007/s00125-018-4636-9. [DOI] [PubMed] [Google Scholar]
- 39.Tacke C., Aleksandrova K., Rehfeldt M., et al. Assessment of circulating Wnt1 inducible signalling pathway protein 1 (WISP-1)/CCN4 as a novel biomarker of obesity. J Cell Commun Signal. 2018;12(3):539–548. doi: 10.1007/s12079-017-0427-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jung T.W., Kang C., Goh J., et al. WISP1 promotes non-alcoholic fatty liver disease and skeletal muscle insulin resistance via TLR4/JNK signaling. J Cell Physiol. 2018;233(8):6077–6087. doi: 10.1002/jcp.26449. [DOI] [PubMed] [Google Scholar]
- 41.Severinsen M.C.K., Pedersen B.K. Muscle-organ crosstalk: the emerging roles of myokines. Endocr Rev. 2020;41(4):594–609. doi: 10.1210/endrev/bnaa016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Argilés J.M., Orpí M., Busquets S., López-Soriano F.J. Myostatin: more than just a regulator of muscle mass. Drug Discov Today. 2012;17(13–14):702–709. doi: 10.1016/j.drudis.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 43.Han H.Q., Zhou X., Mitch W.E., et al. Myostatin/activin pathway antagonism: molecular basis and therapeutic potential. Int J Biochem Cell Biol. 2013;45(10):2333–2347. doi: 10.1016/j.biocel.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 44.Hittel D.S., Berggren J.R., Shearer J., et al. Increased secretion and expression of myostatin in skeletal muscle from extremely obese women. Diabetes. 2009;58(1):30–38. doi: 10.2337/db08-0943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bonala S., Lokireddy S., McFarlane C., et al. Myostatin induces insulin resistance via Casitas B-lineage lymphoma b (Cblb)-mediated degradation of insulin receptor substrate 1 (IRS1) protein in response to high calorie diet intake. J Biol Chem. 2014;289(11):7654–7670. doi: 10.1074/jbc.M113.529925. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 46.Hittel D.S., Axelson M., Sarna N., et al. Myostatin decreases with aerobic exercise and associates with insulin resistance. Med Sci Sports Exerc. 2010;42(11):2023–2029. doi: 10.1249/MSS.0b013e3181e0b9a8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nishikawa H., Enomoto H., Ishii A., et al. Elevated serum myostatin level is associated with worse survival in patients with liver cirrhosis. J Cachexia Sarcopenia Muscle. 2017;8(6):915–925. doi: 10.1002/jcsm.12212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wilkes J.J., Lloyd D.J., Gekakis N. Loss-of-function mutation in myostatin reduces tumor necrosis factor alpha production and protects liver against obesity-induced insulin resistance. Diabetes. 2009;58(5):1133–1143. doi: 10.2337/db08-0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu X.H., Pan J.P., Bauman W.A., et al. AdipoRon prevents myostatin-induced upregulation of fatty acid synthesis and downregulation of insulin activity in a mouse hepatocyte line. Phys Rep. 2019;7(13):e14152. doi: 10.14814/phy2.14152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zarfeshani A., Ngo S., Sheppard A.M. Leucine alters hepatic glucose/lipid homeostasis via the myostatin-AMP-activated protein kinase pathway - potential implications for nonalcoholic fatty liver disease. Clin Epigenet. 2014;6(1):27. doi: 10.1186/1868-7083-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boström P., Wu J., Jedrychowski M.P., et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481(7382):463–468. doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Perakakis N., Triantafyllou G.A., Fernández-Real J.M., et al. Physiology and role of irisin in glucose homeostasis. Nat Rev Endocrinol. 2017;13(6):324–337. doi: 10.1038/nrendo.2016.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gamas L., Matafome P., Seiça R. Irisin and myonectin regulation in the insulin resistant muscle: implications to adipose tissue: muscle crosstalk. J Diabetes Res. 2015;2015:359159. doi: 10.1155/2015/359159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kurdiova T., Balaz M., Vician M., et al. Effects of obesity, diabetes and exercise on Fndc5 gene expression and irisin release in human skeletal muscle and adipose tissue: in vivo and in vitro studies. J Physiol. 2014;592(5):1091–1107. doi: 10.1113/jphysiol.2013.264655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stengel A., Hofmann T., Goebel-Stengel M., et al. Circulating levels of irisin in patients with anorexia nervosa and different stages of obesity--correlation with body mass index. Peptides. 2013;39:125–130. doi: 10.1016/j.peptides.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 56.Polyzos S.A., Anastasilakis A.D., Efstathiadou Z.A., et al. Irisin in metabolic diseases. Endocrine. 2018;59(2):260–274. doi: 10.1007/s12020-017-1476-1. [DOI] [PubMed] [Google Scholar]
- 57.Hu J., Ke Y., Wu F., et al. Circulating irisin levels in patients with nonalcoholic fatty liver disease: a systematic review and meta-analysis. Gastroenterol Res Pract. 2020;2020:8818191. doi: 10.1155/2020/8818191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Park M.J., Kim D.I., Choi J.H., et al. New role of irisin in hepatocytes: the protective effect of hepatic steatosis in vitro. Cell Signal. 2015;27(9):1831–1839. doi: 10.1016/j.cellsig.2015.04.010. [DOI] [PubMed] [Google Scholar]
- 59.Roberts L.D., Boström P., O'Sullivan J.F., et al. β-Aminoisobutyric acid induces browning of white fat and hepatic β-oxidation and is inversely correlated with cardiometabolic risk factors. Cell Metabol. 2014;19(1):96–108. doi: 10.1016/j.cmet.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rietman A., Stanley T.L., Clish C., et al. Associations between plasma branched-chain amino acids, β-aminoisobutyric acid and body composition. J Nutr Sci. 2016;5:e6. doi: 10.1017/jns.2015.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Short K.R., Chadwick J.Q., Teague A.M., et al. Effect of obesity and exercise training on plasma amino acids and amino metabolites in American Indian adolescents. J Clin Endocrinol Metab. 2019;104(8):3249–3261. doi: 10.1210/jc.2018-02698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Begriche K., Massart J., Abbey-Toby A., et al. Beta-aminoisobutyric acid prevents diet-induced obesity in mice with partial leptin deficiency. Obesity. 2008;16(9):2053–2067. doi: 10.1038/oby.2008.337. [DOI] [PubMed] [Google Scholar]
- 63.Tanianskii D.A., Jarzebska N., Birkenfeld A.L., et al. Beta-Aminoisobutyric acid as a novel regulator of carbohydrate and lipid metabolism. Nutrients. 2019;11(3):524. doi: 10.3390/nu11030524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Konrad D., Wueest S. The gut-adipose-liver axis in the metabolic syndrome. Physiology. 2014;29(5):304–313. doi: 10.1152/physiol.00014.2014. [DOI] [PubMed] [Google Scholar]
- 65.Yabut J.M., Crane J.D., Green A.E., et al. Emerging roles for serotonin in regulating metabolism: new implications for an ancient molecule. Endocr Rev. 2019;40(4):1092–1107. doi: 10.1210/er.2018-00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jones L.A., Sun E.W., Martin A.M., et al. The ever-changing roles of serotonin. Int J Biochem Cell Biol. 2020;125:105776. doi: 10.1016/j.biocel.2020.105776. [DOI] [PubMed] [Google Scholar]
- 67.Young R.L., Lumsden A.L., Martin A.M., et al. Augmented capacity for peripheral serotonin release in human obesity. Int J Obes. 2018;42(11):1880–1889. doi: 10.1038/s41366-018-0047-8. [DOI] [PubMed] [Google Scholar]
- 68.Kim H.J., Kim J.H., Noh S., et al. Metabolomic analysis of livers and serum from high-fat diet induced obese mice. J Proteome Res. 2011;10(2):722–731. doi: 10.1021/pr100892r. [DOI] [PubMed] [Google Scholar]
- 69.Kwon O., Yu J.H., Jeong E., et al. Meal-related oscillations in the serum serotonin levels in healthy young men. Clin Endocrinol. 2018;88(4):549–555. doi: 10.1111/cen.13545. [DOI] [PubMed] [Google Scholar]
- 70.Wang L., Fan X., Han J., et al. Gut-derived serotonin contributes to the progression of non-alcoholic steatohepatitis via the liver htr2a/PPARγ2 pathway. Front Pharmacol. 2020;11:553. doi: 10.3389/fphar.2020.00553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Crane J.D., Palanivel R., Mottillo E.P., et al. Inhibiting peripheral serotonin synthesis reduces obesity and metabolic dysfunction by promoting brown adipose tissue thermogenesis. Nat Med. 2015;21(2):166–172. doi: 10.1038/nm.3766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Choi W., Namkung J., Hwang I., et al. Serotonin signals through a gut-liver axis to regulate hepatic steatosis. Nat Commun. 2018;9(1):4824. doi: 10.1038/s41467-018-07287-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Niture S., Gyamfi M.A., Kedir H., et al. Serotonin induced hepatic steatosis is associated with modulation of autophagy and notch signaling pathway. Cell Commun Signal. 2018;16(1):78. doi: 10.1186/s12964-018-0282-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li X., Guo K., Li T., et al. 5-HT2 receptor mediates high-fat diet-induced hepatic steatosis and very low density lipoprotein overproduction in rats. Obes Res Clin Pract. 2018;12(Suppl 2):16–28. doi: 10.1016/j.orcp.2016.03.015. [DOI] [PubMed] [Google Scholar]
- 75.Kim D.C., Jun D.W., Kwon Y.I., et al. 5-HT2A receptor antagonists inhibit hepatic stellate cell activation and facilitate apoptosis. Liver Int. 2013;33(4):535–543. doi: 10.1111/liv.12110. [DOI] [PubMed] [Google Scholar]
- 76.Somm E., Jornayvaz F.R. Fibroblast growth factor 15/19: from basic functions to therapeutic perspectives. Endocr Rev. 2018;39(6):960–989. doi: 10.1210/er.2018-00134. [DOI] [PubMed] [Google Scholar]
- 77.Fu L., John L.M., Adams S.H., et al. Fibroblast growth factor 19 increases metabolic rate and reverses dietary and leptin-deficient diabetes. Endocrinology. 2004;145(6):2594–2603. doi: 10.1210/en.2003-1671. [DOI] [PubMed] [Google Scholar]
- 78.Alvarez-Sola G., Uriarte I., Latasa M.U., et al. Fibroblast growth factor 15/19 (FGF15/19) protects from diet-induced hepatic steatosis: development of an FGF19-based chimeric molecule to promote fatty liver regeneration. Gut. 2017;66(10):1818–1828. doi: 10.1136/gutjnl-2016-312975. [DOI] [PubMed] [Google Scholar]
- 79.So S.S.Y., Yeung C.H.C., Schooling C.M., et al. Targeting bile acid metabolism in obesity reduction: a systematic review and meta-analysis. Obes Rev. 2020;21(7):e13017. doi: 10.1111/obr.13017. [DOI] [PubMed] [Google Scholar]
- 80.Gallego-Escuredo J.M., Gómez-Ambrosi J., Catalan V., et al. Opposite alterations in FGF21 and FGF19 levels and disturbed expression of the receptor machinery for endocrine FGFs in obese patients. Int J Obes. 2015;39(1):121–129. doi: 10.1038/ijo.2014.76. [DOI] [PubMed] [Google Scholar]
- 81.Jiao N., Baker S.S., Chapa-Rodriguez A., et al. Suppressed hepatic bile acid signalling despite elevated production of primary and secondary bile acids in NAFLD. Gut. 2018;67(10):1881–1891. doi: 10.1136/gutjnl-2017-314307. [DOI] [PubMed] [Google Scholar]
- 82.Schreuder T.C., Marsman H.A., Lenicek M., et al. The hepatic response to FGF19 is impaired in patients with nonalcoholic fatty liver disease and insulin resistance. Am J Physiol Gastrointest Liver Physiol. 2010;298(3):G440–G445. doi: 10.1152/ajpgi.00322.2009. [DOI] [PubMed] [Google Scholar]
- 83.Degirolamo C., Sabbà C., Moschetta A. Therapeutic potential of the endocrine fibroblast growth factors FGF19, FGF21 and FGF23. Nat Rev Drug Discov. 2016;15(1):51–69. doi: 10.1038/nrd.2015.9. [DOI] [PubMed] [Google Scholar]
- 84.Potthoff M.J., Boney-Montoya J., Choi M., et al. FGF15/19 regulates hepatic glucose metabolism by inhibiting the CREB-PGC-1α pathway. Cell Metabol. 2011;13(6):729–738. doi: 10.1016/j.cmet.2011.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kim Y.C., Seok S., Zhang Y., et al. Intestinal FGF15/19 physiologically repress hepatic lipogenesis in the late fed-state by activating SHP and DNMT3A. Nat Commun. 2020;11(1):5969. doi: 10.1038/s41467-020-19803-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hira T., Pinyo J., Hara H. What is GLP-1 really doing in obesity? Trends Endocrinol Metabol. 2020;31(2):71–80. doi: 10.1016/j.tem.2019.09.003. [DOI] [PubMed] [Google Scholar]
- 87.Tricò D., Baldi S., Tulipani A., et al. Mechanisms through which a small protein and lipid preload improves glucose tolerance. Diabetologia. 2015;58(11):2503–2512. doi: 10.1007/s00125-015-3710-9. [DOI] [PubMed] [Google Scholar]
- 88.Smushkin G., Sathananthan A., Man C.D., et al. Defects in GLP-1 response to an oral challenge do not play a significant role in the pathogenesis of prediabetes. J Clin Endocrinol Metab. 2012;97(2):589–598. doi: 10.1210/jc.2011-2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Færch K., Torekov S.S., Vistisen D., et al. GLP-1 response to oral glucose is reduced in prediabetes, screen-detected type 2 diabetes, and obesity and influenced by sex: the ADDITION-PRO study. Diabetes. 2015;64(7):2513–2525. doi: 10.2337/db14-1751. [DOI] [PubMed] [Google Scholar]
- 90.Verdich C., Toubro S., Buemann B., et al. The role of postprandial releases of insulin and incretin hormones in meal-induced satiety--effect of obesity and weight reduction. Int J Obes Relat Metab Disord. 2001;25(8):1206–1214. doi: 10.1038/sj.ijo.0801655. [DOI] [PubMed] [Google Scholar]
- 91.Ranganath L.R., Beety J.M., Morgan L.M., et al. Attenuated GLP-1 secretion in obesity: cause or consequence? Gut. 1996;38(6):916–919. doi: 10.1136/gut.38.6.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Meyer-Gerspach A.C., Wölnerhanssen B., Beglinger B., et al. Gastric and intestinal satiation in obese and normal weight healthy people. Physiol Behav. 2014;129:265–271. doi: 10.1016/j.physbeh.2014.02.043. [DOI] [PubMed] [Google Scholar]
- 93.Matikainen N., Bogl L.H., Hakkarainen A., et al. GLP-1 responses are heritable and blunted in acquired obesity with high liver fat and insulin resistance. Diabetes Care. 2014;37(1):242–251. doi: 10.2337/dc13-1283. [DOI] [PubMed] [Google Scholar]
- 94.Gupta N.A., Mells J., Dunham R.M., et al. Glucagon-like peptide-1 receptor is present on human hepatocytes and has a direct role in decreasing hepatic steatosis in vitro by modulating elements of the insulin signaling pathway. Hepatology. 2010;51(5):1584–1592. doi: 10.1002/hep.23569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bifari F., Manfrini R., Dei Cas M., et al. Multiple target tissue effects of GLP-1 analogues on non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH) Pharmacol Res. 2018;137:219–229. doi: 10.1016/j.phrs.2018.09.025. [DOI] [PubMed] [Google Scholar]
- 96.Ben-Shlomo S., Zvibel I., Shnell M., et al. Glucagon-like peptide-1 reduces hepatic lipogenesis via activation of AMP-activated protein kinase. J Hepatol. 2011;54(6):1214–1223. doi: 10.1016/j.jhep.2010.09.032. [DOI] [PubMed] [Google Scholar]
- 97.Lyu J., Imachi H., Fukunaga K., et al. Role of ATP-binding cassette transporter A1 in suppressing lipid accumulation by glucagon-like peptide-1 agonist in hepatocytes. Mol Metabol. 2020;34:16–26. doi: 10.1016/j.molmet.2019.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zheng X., Xu F., Liang H., et al. SIRT1/HSF1/HSP pathway is essential for exenatide-alleviated, lipid-induced hepatic endoplasmic reticulum stress. Hepatology. 2017;66(3):809–824. doi: 10.1002/hep.29238. [DOI] [PubMed] [Google Scholar]
- 99.Somm E., Montandon S.A., Loizides-Mangold U., et al. The GLP-1R agonist liraglutide limits hepatic lipotoxicity and inflammatory response in mice fed a methionine-choline deficient diet. Transl Res. 2021;227:75–88. doi: 10.1016/j.trsl.2020.07.008. [DOI] [PubMed] [Google Scholar]
- 100.Nakabayashi H., Nishizawa M., Nakagawa A., et al. Vagal hepatopancreatic reflex effect evoked by intraportal appearance of tGLP-1. Am J Physiol. 1996;271(5Pt 1):E808–E813. doi: 10.1152/ajpendo.1996.271.5.E808. [DOI] [PubMed] [Google Scholar]
- 101.Han Y., You X., Xing W., et al. Paracrine and endocrine actions of bone-the functions of secretory proteins from osteoblasts, osteocytes, and osteoclasts. Bone Res. 2018;6:16. doi: 10.1038/s41413-018-0019-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kahles F., Findeisen H.M., Bruemmer D. Osteopontin: a novel regulator at the cross roads of inflammation, obesity and diabetes. Mol Metabol. 2014;3(4):384–393. doi: 10.1016/j.molmet.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nomiyama T., Perez-Tilve D., Ogawa D., et al. Osteopontin mediates obesity-induced adipose tissue macrophage infiltration and insulin resistance in mice. J Clin Invest. 2007;117(10):2877–2888. doi: 10.1172/JCI31986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gómez-Ambrosi J., Catalán V., Ramírez B., et al. Plasma osteopontin levels and expression in adipose tissue are increased in obesity. J Clin Endocrinol Metab. 2007;92(9):3719–3727. doi: 10.1210/jc.2007-0349. [DOI] [PubMed] [Google Scholar]
- 105.Bruha R., Vitek L., Smid V. Osteopontin - a potential biomarker of advanced liver disease. Ann Hepatol. 2020;19(4):344–352. doi: 10.1016/j.aohep.2020.01.001. [DOI] [PubMed] [Google Scholar]
- 106.Wang C., He M., Peng J., et al. Increased plasma osteopontin levels are associated with nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus. Cytokine. 2020;125:154837. doi: 10.1016/j.cyto.2019.154837. [DOI] [PubMed] [Google Scholar]
- 107.Bertola A., Deveaux V., Bonnafous S., et al. Elevated expression of osteopontin may be related to adipose tissue macrophage accumulation and liver steatosis in morbid obesity. Diabetes. 2009;58(1):125–133. doi: 10.2337/db08-0400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kiefer F.W., Neschen S., Pfau B., et al. Osteopontin deficiency protects against obesity-induced hepatic steatosis and attenuates glucose production in mice. Diabetologia. 2011;54(8):2132–2142. doi: 10.1007/s00125-011-2170-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lancha A., Rodríguez A., Catalán V., et al. Osteopontin deletion prevents the development of obesity and hepatic steatosis via impaired adipose tissue matrix remodeling and reduced inflammation and fibrosis in adipose tissue and liver in mice. PLoS One. 2014;9(5):e98398. doi: 10.1371/journal.pone.0098398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Song Z., Chen W., Athavale D., et al. Osteopontin takes center stage in chronic liver disease. Hepatology. 2021;73(4):1594–1608. doi: 10.1002/hep.31582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yilmaz Y. Review article: non-alcoholic fatty liver disease and osteoporosis-clinical and molecular crosstalk. Aliment Pharmacol Ther. 2012;36(4):345–352. doi: 10.1111/j.1365-2036.2012.05196.x. [DOI] [PubMed] [Google Scholar]
- 112.Lee N.K., Sowa H., Hinoi E., et al. Endocrine regulation of energy metabolism by the skeleton. Cell. 2007;130(3):456–469. doi: 10.1016/j.cell.2007.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ferron M., Hinoi E., Karsenty G., Ducy P. Osteocalcin differentially regulates beta cell and adipocyte gene expression and affects the development of metabolic diseases in wild-type mice. Proc Natl Acad Sci U S A. 2008;105(13):5266–5270. doi: 10.1073/pnas.0711119105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kord-Varkaneh H., Djafarian K., Khorshidi M., Shab-Bidar S. Association between serum osteocalcin and body mass index: a systematic review and meta-analysis. Endocrine. 2017;58(1):24–32. doi: 10.1007/s12020-017-1384-4. [DOI] [PubMed] [Google Scholar]
- 115.Liu X., Liu Y., Mathers J., et al. Osteocalcin and measures of adiposity: a systematic review and meta-analysis of observational studies. Arch Osteoporos. 2020;15(1):145. doi: 10.1007/s11657-020-00812-6. [DOI] [PubMed] [Google Scholar]
- 116.Amin S., El Amrousy D., Elrifaey S., Gamal R., Hodeib H. Serum osteocalcin levels in children with nonalcoholic fatty liver disease. J Pediatr Gastroenterol Nutr. 2018;66(1):117–121. doi: 10.1097/MPG.0000000000001768. [DOI] [PubMed] [Google Scholar]
- 117.Fernández-Real J.M., Ortega F., Gómez-Ambrosi J., Salvador J., Frühbeck G., Ricart W. Circulating osteocalcin concentrations are associated with parameters of liver fat infiltration and increase in parallel to decreased liver enzymes after weight loss. Osteoporos Int. 2010;21(12):2101–2107. doi: 10.1007/s00198-010-1174-9. [DOI] [PubMed] [Google Scholar]
- 118.Xia M., Rong S., Zhu X., et al. Osteocalcin and non-alcoholic fatty liver disease: lessons from two population-based cohorts and animal models. J Bone Miner Res. 2021;36(4):712–728. doi: 10.1002/jbmr.4227. [DOI] [PubMed] [Google Scholar]
- 119.Zhou B., Li H., Xu L., et al. Osteocalcin reverses endoplasmic reticulum stress and improves impaired insulin sensitivity secondary to diet-induced obesity through nuclear factor-κB signaling pathway. Endocrinology. 2013;154(3):1055–1068. doi: 10.1210/en.2012-2144. [DOI] [PubMed] [Google Scholar]
- 120.Du J., Zhang M., Lu J., et al. Osteocalcin improves nonalcoholic fatty liver disease in mice through activation of Nrf2 and inhibition of JNK. Endocrine. 2016;53(3):701–709. doi: 10.1007/s12020-016-0926-5. [DOI] [PubMed] [Google Scholar]
- 121.Gupte A.A., Sabek O.M., Fraga D., et al. Osteocalcin protects against nonalcoholic steatohepatitis in a mouse model of metabolic syndrome. Endocrinology. 2014;155(12):4697–4705. doi: 10.1210/en.2014-1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Merle B., Garnero P. The multiple facets of periostin in bone metabolism. Osteoporos Int. 2012;23(4):1199–1212. doi: 10.1007/s00198-011-1892-7. [DOI] [PubMed] [Google Scholar]
- 123.Liu A.Y., Zheng H., Ouyang G. Periostin, a multifunctional matricellular protein in inflammatory and tumor microenvironments. Matrix Biol. 2014;37:150–156. doi: 10.1016/j.matbio.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 124.Wu T., Wu S., Ouyang G. Periostin: a new extracellular regulator of obesity-induced hepatosteatosis. Cell Metabol. 2014;20(4):562–564. doi: 10.1016/j.cmet.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 125.Polyzos S.A., Kountouras J., Anastasilakis A.D., et al. Circulating periostin in patients with nonalcoholic fatty liver disease. Endocrine. 2017;56(2):438–441. doi: 10.1007/s12020-016-1144-x. [DOI] [PubMed] [Google Scholar]
- 126.Yang Z., Zhang H., Niu Y., et al. Circulating periostin in relation to insulin resistance and nonalcoholic fatty liver disease among overweight and obese subjects. Sci Rep. 2016;6:37886. doi: 10.1038/srep37886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lu Y., Liu X., Jiao Y., et al. Periostin promotes liver steatosis and hypertriglyceridemia through downregulation of PPARα. J Clin Invest. 2014;124(8):3501–3513. doi: 10.1172/JCI74438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Li Y., Wu S., Xiong S., et al. Deficiency of periostin protects mice against methionine-choline-deficient diet-induced non-alcoholic steatohepatitis. J Hepatol. 2015;62(2):495–497. doi: 10.1016/j.jhep.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 129.Thomou T., Mori M.A., Dreyfuss J.M., et al. Adipose-derived circulating miRNAs regulate gene expression in other tissues. Nature. 2017;542(7642):450–455. doi: 10.1038/nature21365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Dorairaj V., Sulaiman S.A., Abu N., et al. Extracellular vesicles in the development of the non-alcoholic fatty liver disease: an update. Biomolecules. 2020;10(11):1494. doi: 10.3390/biom10111494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Baranova A., Maltseva D., Tonevitsky A. Adipose may actively delay progression of NAFLD by releasing tumor-suppressing, anti-fibrotic miR-122 into circulation. Obes Rev. 2019;20(1):108–118. doi: 10.1111/obr.12765. [DOI] [PubMed] [Google Scholar]
- 132.de Mendonça M., Rocha K.C., de Sousa É, et al. Aerobic exercise training regulates serum extracellular vesicle miRNAs linked to obesity to promote their beneficial effects in mice. Am J Physiol Endocrinol Metab. 2020;319(3):E579–E591. doi: 10.1152/ajpendo.00172.2020. [DOI] [PubMed] [Google Scholar]
- 133.Li Y., Luan Y., Li J., et al. Exosomal miR-199a-5p promotes hepatic lipid accumulation by modulating MST1 expression and fatty acid metabolism. Hepatol Int. 2020;14(6):1057–1074. doi: 10.1007/s12072-020-10096-0. [DOI] [PubMed] [Google Scholar]
- 134.Dang S.Y., Leng Y., Wang Z.X., et al. Exosomal transfer of obesity adipose tissue for decreased miR-141-3p mediate insulin resistance of hepatocytes. Int J Biol Sci. 2019;15(2):351–368. doi: 10.7150/ijbs.28522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Long J.K., Dai W., Zheng Y.W., et al. miR-122 promotes hepatic lipogenesis via inhibiting the LKB1/AMPK pathway by targeting Sirt1 in non-alcoholic fatty liver disease. Mol Med. 2019;25(1):26. doi: 10.1186/s10020-019-0085-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hu Y., Liu H.X., Jena P.K., et al. miR-22 inhibition reduces hepatic steatosis via FGF21 and FGFR1 induction. JHEP Rep. 2020;2(2):100093. doi: 10.1016/j.jhepr.2020.100093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kariba Y., Yoshizawa T., Sato Y., et al. Brown adipocyte-derived exosomal miR-132-3p suppress hepatic Srebf1 expression and thereby attenuate expression of lipogenic genes. Biochem Biophys Res Commun. 2020;530(3):500–507. doi: 10.1016/j.bbrc.2020.05.090. [DOI] [PubMed] [Google Scholar]
- 138.Rong B., Feng R., Liu C., et al. Reduced delivery of epididymal adipocyte-derived exosomal resistin is essential for melatonin ameliorating hepatic steatosis in mice. J Pineal Res. 2019;66(4):e12561. doi: 10.1111/jpi.12561. [DOI] [PubMed] [Google Scholar]
- 139.Yan C., Tian X., Li J., et al. A high-fat diet attenuates AMPK α1 in adipocytes to induce exosome shedding and nonalcoholic fatty liver development in vivo. Diabetes. 2021;70(2):577–588. doi: 10.2337/db20-0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gu H., Yang K., Shen Z., et al. ER stress-induced adipocytes secrete-aldo-keto reductase 1B7-containing exosomes that cause nonalcoholic steatohepatitis in mice. Free Radic Biol Med. 2021;163:220–233. doi: 10.1016/j.freeradbiomed.2020.12.011. [DOI] [PubMed] [Google Scholar]
- 141.Ying W., Riopel M., Bandyopadhyay G., et al. Adipose tissue macrophage-derived exosomal miRNAs can modulate in vivo and in vitro insulin sensitivity. Cell. 2017;171(2):372–384. doi: 10.1016/j.cell.2017.08.035. e12. [DOI] [PubMed] [Google Scholar]
- 142.Liu T., Sun Y.C., Cheng P., et al. Adipose tissue macrophage-derived exosomal miR-29a regulates obesity-associated insulin resistance. Biochem Biophys Res Commun. 2019;515(2):352–358. doi: 10.1016/j.bbrc.2019.05.113. [DOI] [PubMed] [Google Scholar]
- 143.Ying W., Gao H., Dos Reis F.C.G., et al. MiR-690, an exosomal-derived miRNA from M2-polarized macrophages, improves insulin sensitivity in obese mice. Cell Metabol. 2021;33(4):781–790. doi: 10.1016/j.cmet.2020.12.019. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hou X., Yin S., Ren R., et al. Myeloid-cell-specific IL-6 signaling promotes MicroRNA-223-enriched exosome production to attenuate NAFLD-associated fibrosis. Hepatology. 2021;74(1):116–132. doi: 10.1002/hep.31658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Chen Y., Sun H., Bai Y., et al. Gut dysbiosis-derived exosomes trigger hepatic steatosis by transiting HMGB1 from intestinal to liver in mice. Biochem Biophys Res Commun. 2019;509(3):767–772. doi: 10.1016/j.bbrc.2018.12.180. [DOI] [PubMed] [Google Scholar]
- 146.Kumar A., Sundaram K., Mu J., et al. High-fat diet-induced upregulation of exosomal phosphatidylcholine contributes to insulin resistance. Nat Commun. 2021;12(1):213. doi: 10.1038/s41467-020-20500-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ji Y., Yin Y., Li Z., et al. Gut microbiota-derived components and metabolites in the progression of non-alcoholic fatty liver disease (NAFLD) Nutrients. 2019;11(8):1712. doi: 10.3390/nu11081712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Tulkens J., Vergauwen G., Van Deun J., et al. Increased levels of systemic LPS-positive bacterial extracellular vesicles in patients with intestinal barrier dysfunction. Gut. 2020;69(1):191–193. doi: 10.1136/gutjnl-2018-317726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Luo Z., Ji Y., Gao H., et al. CRIg+ macrophages prevent gut microbial DNA-containing extracellular vesicle-induced tissue inflammation and insulin resistance. Gastroenterology. 2021;160(3):863–874. doi: 10.1053/j.gastro.2020.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Castaño C., Mirasierra M., Vallejo M., et al. Delivery of muscle-derived exosomal miRNAs induced by HIIT improves insulin sensitivity through down-regulation of hepatic FoxO1 in mice. Proc Natl Acad Sci U S A. 2020;117(48):30335–30343. doi: 10.1073/pnas.2016112117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Li J., Zhang Y., Ye Y., et al. Pancreatic β cells control glucose homeostasis via the secretion of exosomal miR-29 family. J Extracell Vesicles. 2021;10(3):e12055. doi: 10.1002/jev2.12055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zhu Q., Zhu R., Jin J. Neutral ceramidase-enriched exosomes prevent palmitic acid-induced insulin resistance in H4IIEC3 hepatocytes. FEBS Open Bio. 2016;6(11):1078–1084. doi: 10.1002/2211-5463.12125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kakazu E., Mauer A.S., Yin M., et al. Hepatocytes release ceramide-enriched pro-inflammatory extracellular vesicles in an IRE1α-dependent manner. J Lipid Res. 2016;57(2):233–245. doi: 10.1194/jlr.M063412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Liao C.Y., Song M.J., Gao Y., et al. Hepatocyte-derived lipotoxic extracellular vesicle sphingosine 1-phosphate induces macrophage chemotaxis. Front Immunol. 2018;9:2980. doi: 10.3389/fimmu.2018.02980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Liu X.L., Pan Q., Cao H.X., et al. Lipotoxic hepatocyte-derived exosomal MicroRNA 192-5p activates macrophages through rictor/akt/forkhead box transcription factor O1 signaling in nonalcoholic fatty liver disease. Hepatology. 2020;72(2):454–469. doi: 10.1002/hep.31050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Povero D., Panera N., Eguchi A., et al. Lipid-induced hepatocyte-derived extracellular vesicles regulate hepatic stellate cell via microRNAs targeting PPAR-γ. Cell Mol Gastroenterol Hepatol. 2015;1(6):646–663. doi: 10.1016/j.jcmgh.2015.07.007. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wong V.W., Adams L.A., de Lédinghen V., et al. Noninvasive biomarkers in NAFLD and NASH - current progress and future promise. Nat Rev Gastroenterol Hepatol. 2018;15(8):461–478. doi: 10.1038/s41575-018-0014-9. [DOI] [PubMed] [Google Scholar]
- 158.Liu C.H., Ampuero J., Gil-Gómez A., et al. miRNAs in patients with non alcoholic fatty liver disease: a systematic review and meta-analysis. J Hepatol. 2018;69(6):1335–1348. doi: 10.1016/j.jhep.2018.08.008. [DOI] [PubMed] [Google Scholar]
- 159.Gonzalez-Gil A.M., Elizondo-Montemayor L. The role of exercise in the interplay between myokines, hepatokines, osteokines, adipokines, and modulation of inflammation for energy substrate redistribution and fat mass loss: a review. Nutrients. 2020 Jun;12(6):1899. doi: 10.3390/nu12061899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Pedersen B.K. Physical activity and muscle-brain crosstalk. Nat Rev Endocrinol. 2019;15(7):383–392. doi: 10.1038/s41574-019-0174-x. [DOI] [PubMed] [Google Scholar]
- 161.Kita S., Maeda N., Shimomura I. Interorgan communication by exosomes, adipose tissue, and adiponectin in metabolic syndrome. J Clin Invest. 2019;129(10):4041–4049. doi: 10.1172/JCI129193. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.