This randomized clinical trial compares outcome data from circumferential pulmonary vein isolation treatment in patients aged 65 to 80 years with paroxysmal atrial fibrillation to explore the incremental benefit of additional substrate ablation in patients low-voltage areas.
Key Points
Question
Does additional low-voltage-area (LVA) ablation beyond circumferential pulmonary vein isolation (CPVI) reduce the recurrence of atrial arrhythmias in older patients with paroxysmal atrial fibrillation (AF) who have more atrial substrate?
Findings
In this randomized clinical trial that included 438 older patients with paroxysmal AF, additional LVA ablation was associated with a significant reduction in recurrence of atrial arrhythmias compared with CPVI alone at 23 months.
Meaning
Among older patients with paroxysmal AF, additional LVA ablation beyond CPVI might decrease the recurrence of atrial tachyarrhythmia in older patients with paroxysmal AF compared with CPVI alone.
Abstract
Importance
The overall success rate of circumferential pulmonary vein isolation (CPVI) treatment in patients with paroxysmal atrial fibrillation (AF) remains suboptimal, especially in older patients.
Objective
To explore the incremental benefit of low-voltage-area ablation after CPVI in older patients with paroxysmal AF.
Design, Setting, and Participants
This randomized clinical trial was an investigator-initiated trial to compare the efficacy of additional low-voltage-area ablation beyond CPVI vs CPVI alone in older patients with paroxysmal AF. Participants were patients aged 65 to 80 years with paroxysmal AF who were referred for catheter ablation. They were enrolled in 14 tertiary hospitals in China from April 1, 2018, to August 3, 2020, and follow-up occurred through August 15, 2021.
Interventions
Patients were randomized (1:1) to undergo CPVI plus low-voltage-area ablation or CPVI alone. Low-voltage areas were defined as areas with amplitude less than 0.5 mV in more than 3 adjacent points. If low-voltage areas existed, additional substrate ablation was performed in the CPVI plus group but not the CPVI alone group.
Main Outcomes and Measures
The primary end point of the study was freedom from atrial tachyarrhythmia as documented by electrocardiogram during a clinical visit or lasting longer than 30 seconds during Holter recordings occurring after a single ablation procedure.
Results
Among 438 patients who were randomized (mean [SD] age, 70.5 [4.4] years; 219 men [50%]), 24 (5.5%) did not complete the blanking period and were not included for efficacy analysis. After a median follow-up of 23 months, the recurrence rate of atrial tachyarrhythmia was significantly lower in the CPVI plus group (31/209 patients, 15%) compared with the CPVI alone group (49/205, 24%; hazard ratio [HR], 0.61; 95% CI, 0.38-0.95; P = .03). In subgroup analyses, among all patients with low-voltage area, CPVI plus substrate modification was associated with a 51% decreased risk of ATA recurrence compared with CPVI alone (HR, 0.49; 95% CI, 0.25-0.94; P = .03).
Conclusions and Relevance
This study found that additional low-voltage-area ablation beyond CPVI decreased the ATA recurrence in older patients with paroxysmal AF compared with CPVI alone. Our findings merit further replication by larger trials with longer follow-up.
Trial Registration
ClinicalTrials.gov Identifier: NCT03462628
Introduction
Recent evidence has shown that successful catheter ablation of atrial fibrillation (AF) can improve long-term clinical outcomes in terms of the composite end point of cardiovascular death, stroke, and hospitalization due to heart failure.1,2 For decades, research has been devoted to improve the success rate of AF ablation. So far, studies regarding additional low-voltage-area (LVA) ablation beyond circumferential pulmonary vein isolation (CPVI) have demonstrated inconsistent results.3,4,5,6,7,8,9,10,11,12 We believe that a factor accounting for the inconsistent results is the heterogeneity of patient selection in terms of the presence and extent of LVA.8,9 Therefore, defining the subset of patients with AF who may benefit from LVA ablation is an important study subject.
Our previous studies of patients with persistent AF yielded the conclusion that atrial fibrosis was the proarrhythmic substrate, which could be identified by alterations in local electrical properties and depicted as LVA on a 3-dimensional (3-D) voltage map.10,11,13,14 Of note, around 50% of the patients with persistent AF did not have LVA and needed only CPVI, while others who had LVAs received LVA ablation.10,11 This is a simplified and personalized ablation strategy for persistent AF and can avoid excessive ablation. However, compelling data on the benefit of additional LVA ablation in patients with paroxysmal AF are limited, and whether there is a patient subgroup who will benefit remains unclear. We hypothesized that older patients with paroxysmal AF who are expected to have a more proarrhythmic substrate due to fibrosis would benefit from LVA ablation, and thus their single-procedure success rate would improve. Therefore, we conducted this single-blind multicenter randomized clinical trial to compare the efficacy of CPVI plus LVA ablation vs CPVI alone in older individuals with paroxysmal AF.
Methods
Trial Design and Oversight
STABLE-SR-III was a multicenter, single-blind, randomized clinical trial comparing the effectiveness of additional LVA ablation in older patients with paroxysmal AF. All eligible patients were randomly assigned to undergo CPVI plus LVA ablation during sinus rhythm (CPVI plus group) or CPVI alone (CPVI alone group). The study protocol was approved by the institutional review boards of the First Affiliated Hospital of Nanjing Medical University and all other participating hospitals. Informed consent was obtained from all patients. The trial protocol is available in Supplement 1. This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
Patients
Fourteen hospitals in mainland China with highly experienced electrophysiologists performing AF ablation were the setting for this trial (Supplement 1). Eligible patients were aged 65 to 80 years, had 2 or more episodes of symptomatic paroxysmal AF in the 6 months before enrollment, and consented to receive radiofrequency catheter ablation. Detailed inclusion and exclusion criteria are provided in Supplement 1.
Trial Procedures
A central computerized simple randomization scheme was used. After enrollment, patients were randomly assigned (1:1) to undergo CPVI plus or CPVI alone. The patient’s procedural data were stored and reviewed by the core laboratory. Patients and the physicians who performed the follow-up were blinded to the randomization. All clinical outcomes were collected by a contracted clinical research organization (Guangzhou EnChannel Medical Information Technology).
Interventions
Periprocedural Period
All antiarrhythmic drugs were discontinued for at least 5 half-lives and amiodarone for 2 months before the procedure. An electrophysiological study was performed after overnight fasting and mild sedated state with administration of intravenous midazolam and fentanyl. A 3-D mapping system (Biosense Webster) was used to guide the mapping and ablation procedures. Ablation was performed with the use of irrigated radiofrequency energy by smart-touch contact force catheter (Biosense Webster). Ablation index–guided CPVI was performed as the initial step (power 30 to 40 W, contact force 5g to 30g) in all patients. The target ablation index value was 500 for anterior, 400 to 450 for roof, and 350 to 400 for inferior and posterior segments. Concomitant atrial flutter or tachycardias were ablated during the index procedure. If AF was still present after CPVI, sinus rhythm was restored by electrical cardioversion.
Voltage Map
After CPVI, a detailed 3-D voltage map of the left atrium was created point by point using a force-sensing ablation catheter (local contact force >5g and >150 points) during sinus rhythm in both groups. Low-voltage areas were defined as areas with amplitude less than 0.5 mV in more than 3 adjacent low-voltage points with space difference of 0.5 cm. The LVA burden was defined as the proportion of LVA over the entire left atrial surface. The details about and reasons for a contact force–adjudicated voltage map after CPVI are provided in the eMethods in Supplement 2.
Additional LVA Ablation
In the study group, additional substrate ablation after CPVI was performed if LVAs were found. The LVA ablation approach was previously described and is illustrated in eFigure 1 in Supplement 2. In the control group, no additional ablation was performed in any patient after CPVI.
Test and Validation
All PVs were rechecked after left atrial voltage mapping and LVA ablation, and re-isolation was done if the PV had reconnected. Programmed stimulation with isoproterenol infusion was performed to increase the heart rate by 50% after ablation. If non-PV triggers were observed or sustained atrial tachycardias or supraventricular tachycardias were found, further ablation was performed.
Follow-up After Ablation
All patients were treated with antiarrhythmic agents for the first 3 months if not contraindicated and were followed up in the outpatient clinic after the initial ablation; their continuation depended on atrial tachyarrhythmia (ATA) recurrence. Anticoagulation was administered for a minimum of 3 months following the procedure and at the discretion of the operators thereafter. Surface electrocardiogram (ECG) and 24-hour Holter recordings were performed at 3 and 6 months after the initial procedure, and 7-day Holter recordings were performed at 12 months. In the subsequent years, clinic visits and 24-hour Holter recordings were conducted every 6 months. Ad hoc ECGs were also performed if the patients experienced arrhythmic symptoms.
Outcomes
The primary outcome of the study was freedom from ATA by ECG documentation during clinical visit or lasting longer than 30 seconds during Holter recordings occurring after a single ablation procedure. Any ATA that occurred in the first 3 months after the index ablation (the blanking period) was not counted. The episodes of ATA were confirmed through blinded review by 2 senior electrophysiologists. In case of disagreement, a third senior electrophysiologist was consulted.
The secondary outcomes of the study included incidence of periprocedural complications and total procedural, total fluoroscopic, and total radiofrequency delivery times. Procedure-related serious adverse events were defined as disability, prolonged hospitalization duration, life-threatening complications, and death.
Statistical Analysis
Sample size was calculated based on the AF recurrence rate using contact force-sensing catheters after AF ablation in the First Affiliated Hospital of Nanjing Medical University between 2016 and 2017. The expected freedom from ATA after single procedure was 75% for CPVI alone and 85% for CPVI plus LVA ablation at the 12-month follow-up. The enrollment period was 2 years with a planned minimum follow-up period of 1 year. To test the superiority of CPVI plus LVA ablation strategy, an overall sample size of 369 patients using the log-rank test (with a randomization ratio of 1:1) was needed to achieve 90% power at a 2-sided α level of .05 to detect a hazard ratio (HR) of 0.56. Assuming a dropout rate of 15%, 434 patients would have to be enrolled (217 for each group).
The primary outcome was evaluated according to the modified intention-to-treat (mITT) principle. Freedom from ATA curves were plotted with Kaplan-Meier method and compared with the log-rank test. Cox proportion hazards regression was used to estimate HR and 95% CIs. The proportional hazard assumption was tested by including both the interaction term treatment × time and the binary treatment variable into 1 model. If the proportional hazard assumption was violated, a milestone analysis was used to estimate the treatment effect at 24 months and 36 months. Interactions by age, sex, body mass index, AF history, left atrial diameter, CHA2DS2-VASc score, and LVA were examined.
Continuous variables are expressed as mean (SD) and compared using a parametric t test or 1-way analysis of variance if normally distributed. Otherwise, continuous variables are expressed as median (IQR) and compared using a nonparametric Mann-Whitney U test or Kruskal-Wallis test. Categorical variables are expressed as number and percentage and compared using a Pearson χ2 test or Fisher exact test. A 2-sided P value less than .05 was considered statistically significant. Analyses were performed using SAS version 9.4.
Results
Patients
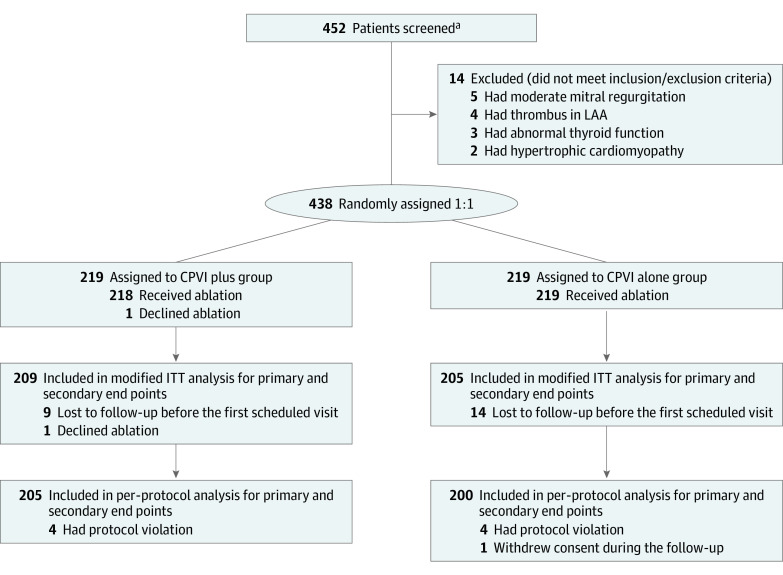
A total of 438 patients were enrolled between April 1, 2018, and August 3, 2020, and were randomly assigned to receive CPVI plus LVA ablation (n = 219) or CPVI alone (n = 219) (Figure 1). The mean (SD) age was 70.5 (4.4) years, and 219 patients (50.0%) were men. There were no differences in baseline characteristics between the 2 groups (Table 1). Because 24 patients (10 in the CPVI plus group and 14 in the CPVI alone group) dropped out before any study procedure or follow-up, 414 patients were included in the mITT analysis. The per-protocol analysis included 405 patients who completed follow-up (Figure 1). Baseline characteristics by mITT analysis and per-protocol analysis are described in eTables 1 and 2 in Supplement 2.
Figure 1. Randomization and Flow in the STABLE-SR-III Trial.
CPVI plus indicates circumferential pulmonary vein isolation plus low-voltage-area modification; CPVI alone, circumferential pulmonary vein isolation; LAA, left atrial appendage; ITT, intention to treat.
aFourteen centers were not required to provide screening logs during the recruitment phase. Thus, the number of patients for eligibility assessment is not available.
Table 1. Baseline Characteristics of Study Patients.
| Characteristic | No. (%) | |
|---|---|---|
| CPVI plus (n = 219) | CPVI alone (n = 219) | |
| Sex | ||
| Male | 111 (50.7) | 108 (49.3) |
| Female | 108 (49.3) | 111 (50.7) |
| Age, mean (SD), y | 70.2 (4.7) | 70.7 (4.1) |
| AF history, median (IQR), mo | 24.0 (6.0-48.0) | 14 (4.0-48.0) |
| BMI, mean (SD)a | 24.3 (3.5) | 24.6 (3.0) |
| <25 | 129 (62.6) | 131 (60.6) |
| ≥25 | 77 (37.4) | 85 (39.4) |
| Comorbidities | ||
| Hypertension | 130 (59.4) | 142 (64.8) |
| Diabetes | 32 (14.6) | 43 (19.6) |
| CAD | 52 (23.7) | 51 (23.3) |
| Stroke or TIA | 17 (7.8) | 21 (9.6) |
| Congestive heart failure | 2 (0.9) | 1 (0.5) |
| COPD | 4 (1.8) | 3 (1.4) |
| OSAS | 2 (0.9) | 2 (0.9) |
| CHA2DS2-VASc score, mean (SD) | 2.3 (0.8) | 2.5 (1.0) |
| 1 | 54 (24.7) | 50 (22.8) |
| 2 | 81 (37.0) | 67 (30.6) |
| 3 | 62 (28.3) | 69 (31.5) |
| >3 | 22 (10.1) | 33 (15.1) |
| LAD, mean (SD), mm | 38.8 (5.4) | 38.8 (5.4) |
| LVEF, mean (SD), % | 62.4 (5.3) | 62.4 (5.4) |
Abbreviations: AF, atrial fibrillation; BMI, body mass index; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; CPVI, circumferential pulmonary vein isolation; LAD, left atrial diameter; LVEF, left ventricular ejection fraction; OSAS, obstructive sleep apnea syndrome; TIA, transient ischemic attack.
Calculated as weight in kilograms divided by height in meters squared.
Ablation Procedure
Procedural characteristics were comparable between the 2 groups of patients who received ablation (Table 2). Successful CPVI was achieved in all patients. The mean (SD) number of points in each map was 258 (58). Low-voltage areas were identified in 88 patients (40.4%) in the CPVI plus group and 93 (42.5%) in the CPVI alone group. Overall, the prevalence of LVA was 41.4% (181/437 patients). In patients with LVA, the mean (SD) LVA burden was 7.2% (6.9%). Women had higher prevalence (50.0% vs 32.9%, P < .001) and heavier burden of LVA than men (9.1% [8.9%] vs 4.3% [4.6%]; P < .001). The prevalence of non-PV triggers and concomitant arrhythmia were not different between the 2 groups. During the period of voltage mapping and LVA ablation in the study group (lasting a mean [SD] 33.5 [19.0] minutes), the PV relapsed in 14 of 218 patients (6.4%) on the right side and 14 of 218 (6.4%) on the left side. In the control group, who received voltage mapping lasting a mean (SD) 33.6 (28.9) minutes, the PV relapsed in 11 of 219 patients (5.0%) on the right side and 13 of 219 patients (5.9%) on the left side. The reconnection rate between the 2 groups was not statistically different (P = .54), and re-isolation was performed in all patients. Procedural data by mITT and per-protocol analysis are shown in eTables 3 and 4 in Supplement 2.
Table 2. Procedural Characteristics.
| Characteristic | No. (%) | |
|---|---|---|
| CPVI plus (n = 218) | CPVI alone (n = 219) | |
| ATA at baseline | 35 (16.1) | 34 (15.5) |
| AF termination during CPVI | 69 (31.7) | 65 (29.7) |
| Cardioversion after CPVI | 13 (6.0) | 2 (1.0) |
| Non-PV triggers | 17 (7.8) | 21 (9.6) |
| Concomitant arrhythmia | 2 (0.9) | 5 (2.4) |
| CTI ablation | 24 (11.0) | 22 (10.1) |
| LVA | 88 (40.4) | 93 (42.5) |
| LVA burden 1%-10% | 67 (30.7) | 76 (34.7) |
| Area, mean (SD), cm2 | 3.9 (2.4) | 3.6 (2.6) |
| LVA burden 11%-20% | 15 (6.9) | 11 (5.0) |
| Area, mean (SD), cm2 | 13.2 (1.8) | 14.0 (3.6) |
| LVA burden >20% | 6 (2.8) | 6 (2.7) |
| Area, mean (SD), cm2 | 27.9 (9.5) | 40.6 (9.5) |
| Total procedure time, mean (SD), min | 142.5 (38.9) | 141.4 (43.6) |
| Beginning to CPVI completion | 109.1 (31.3) | 107.8 (28.3) |
| CPVI completion to end | 33.5 (19.0) | 33.6 (28.9) |
| Total fluoroscopic time, mean (SD), min | 9.3 (5.5) | 9.8 (7.1) |
| Total RF delivery time, mean (SD), min | 43.7 (18.1) | 42.0 (17.7) |
Abbreviations: AF, atrial fibrillation; ATA, atrial tachyarrhythmia; CPVI, circumferential pulmonary vein isolation; CTI, cavotricuspid isthmus; LVA, low-voltage area, PV, pulmonary vein; RF, radiofrequency.
Primary Outcome
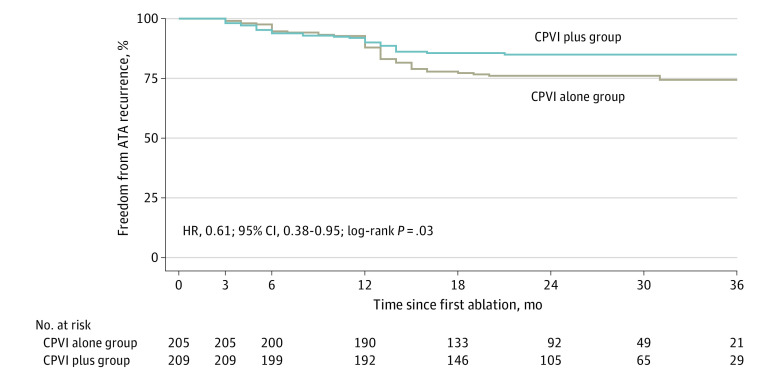
At 23 months, recurrent ATA occurred in 31 of 209 patients (15%) assigned to the CPVI plus group and 49 of 205 (24%) in the CPVI alone group. A time-to-event analysis is shown in Figure 2. The ATA recurrence rate was significantly lower in the CPVI plus group compared with the CPVI alone group (HR, 0.61; 95% CI, 0.38-0.95; P = .03). Because the proportional hazard assumption was violated, the milestone analysis was performed to further compare the difference in ATA recurrence rate between the 2 groups. The recurrence rate was 9.0% lower (95% CI, 8.9%-9.0%; P = .03) in the CPVI plus group compared with the CPVI alone group at 24 months and 10.6% lower (95% CI, 10.6%-10.7%) at 36 months (P = .01). There were 56 patients who attended unscheduled follow-up visits due to their symptoms of palpitation (31 in the CPVI plus group). Clinically detected ATA events were recorded in 10 patients in the CPVI plus group and 14 patients in the CPVI alone group (eFigure 2 in Supplement 2).
Figure 2. Kaplan-Meier Estimates of Freedom From Atrial Tachyarrhythmia (ATA) After a Single Procedure by Modified Intention-to-Treat Analysis.
There was a significant reduction in ATA recurrence for patients in the CPVI plus group compared with the CPVI alone group (P = .03). The curves began to diverge at 1 year, coinciding with the 7-day ambulatory monitoring at 12 months. CPVI plus indicates circumferential pulmonary vein isolation plus low-voltage-area modification; CPVI alone, circumferential pulmonary vein isolation; HR, hazard ratio.
Secondary Outcomes
There were no significant differences between CPVI plus and CPVI alone in mean (SD) total procedure time (142.5 [39.3] minutes vs 139.4 [42.5] minutes; P = .46), fluoroscopic time (9.2 [5.3] minutes vs 9.7 [7.1] minutes; P = .84), and radiofrequency delivery time (43.9 [18.3] minutes vs 42.2 [17.9] minutes; P = .28) (Table 2).
Subgroup Analyses
Based on their randomization assignment and the existence of LVA, all patients were categorized into 4 subgroups. Baseline and procedural characteristics by these 4 subgroups are described in eTables 5 and 6 in Supplement 2. In patients with LVA, CPVI plus substrate modification was associated with a 51% decreased risk of ATA recurrence compared with CPVI alone (HR, 0.49; 95% CI, 0.25-0.94; P = .03). In patients without LVA, there were no significant differences in ATA recurrence between the 2 ablation groups (HR, 0.77; 95% CI, 0.41-1.43; P = .40) (Figure 3A and B). In the CPVI plus group, ATA recurrence among patients with LVA was comparable with that of patients without LVA (HR, 1.06; 95% CI, 0.52-2.16; P = .88). However, in the CPVI alone group, the risk of recurrence was nonsignificantly higher in patients with LVA than those without (HR, 1.68; 95% CI, 0.96-2.95; P = .07) (Figure 3C and D). The similar results were also observed by mITT analysis in 3 subgroups based on the presence of LVA and the 2 ablation strategies: patients with LVA who received CPVI plus, patients with LVA who received CPVI alone, and all the enrolled patients without LVA of both groups (eTables 7 and 8 and eFigure 3 in Supplement 2).
Figure 3. Kaplan-Meier Curve for Freedom From Atrial Tachyarrhythmia (ATA) After a Single Procedure Among 4 Subgroups by Modified Intention-to-Treat Analysis.
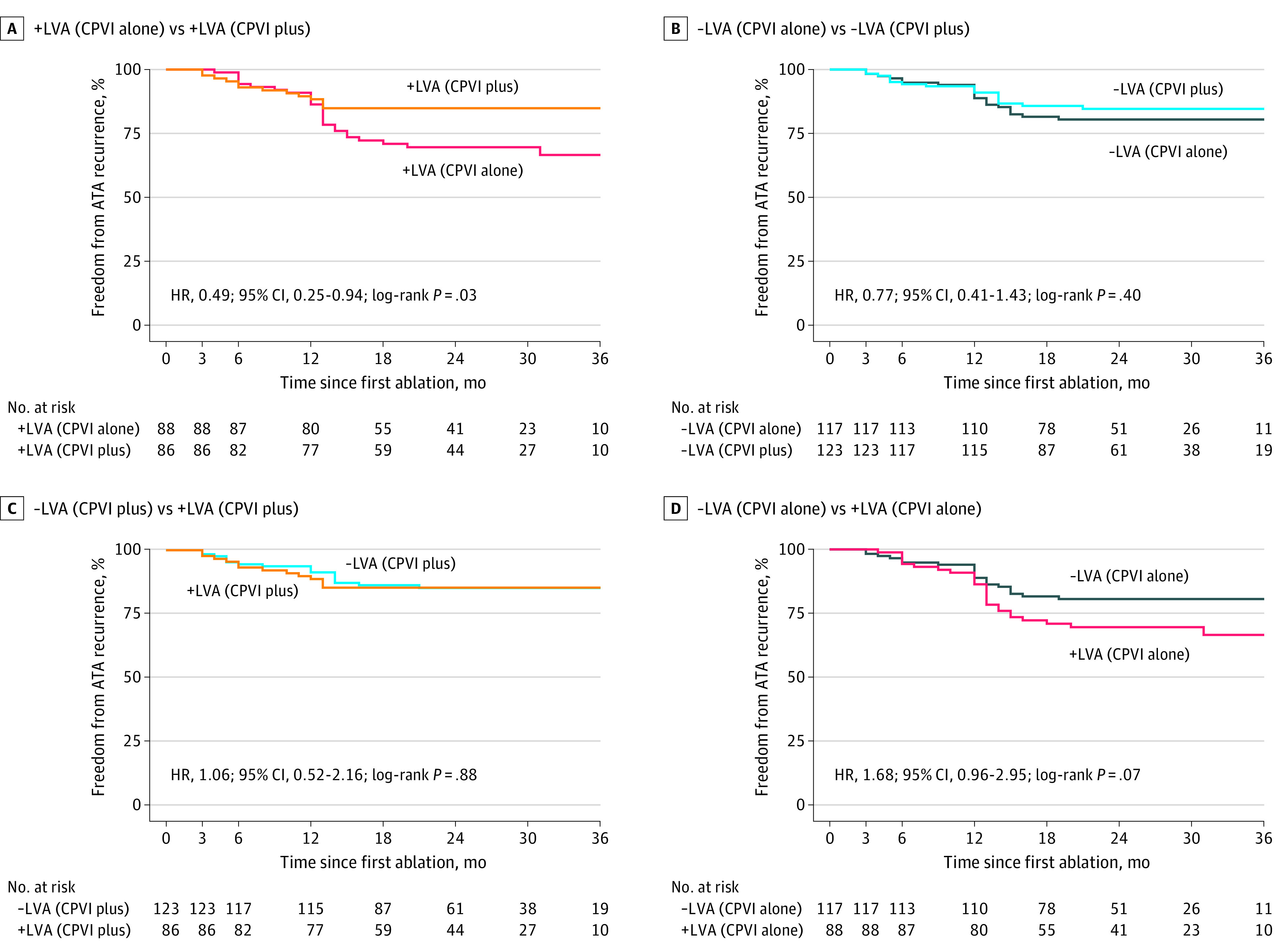
Based on randomization assignment and the existence of low-voltage areas (LVAs), all patients were divided into 4 subgroups: +LVA (CPVI plus) indicates patients with LVAs who received additional ablation beyond CPVI; +LVA (CPVI alone), patients with LVAs who received CPVI alone; −LVA (CPVI plus), patients without LVAs who received additional ablation beyond CPVI; and −LVA (CPVI alone), patients without LVAs in the CPVI alone group. Patients with LVAs who received modification in the study group had a significant reduction of ATA recurrence vs those who did not in the CPVI alone group (A). The ascertainment of recurrent ATA episodes differed at 12 months vs 3 or 6 months. CPVI plus indicates circumferential pulmonary vein isolation plus LVA modification; CPVI alone, circumferential pulmonary vein isolation; HR, hazard ratio.
Other prespecified subgroup analyses by mITT analysis are shown in eFigure 4 in Supplement 2. Of note, the effect size of LVA ablation was greater in women (HR, 0.35; 95% CI, 0.18-0.70) than in men (HR, 1.03; 95% CI, 0.55-1.93; P = .02 for interaction), while the effect was consistent in all the remaining subgroups.
Time-to-event analysis by intention to treat is shown in eFigure 5 in Supplement 2. The results of per-protocol analyses did not differ from those of mITT analyses (eTables 2, 4, 9, and 10 and eFigures 6-9 in Supplement 2).
Adverse Events
Periprocedural adverse events occurring in patients who underwent ablation are shown in eTable 11 in Supplement 2. There was no stroke or tamponade during the intraprocedural or immediate postprocedural period. No atrial-esophageal fistula occurred during follow-up. One patient in the CPVI plus group died of stroke at 25 months after the index procedure (eTable 12 in Supplement 2).
Sensitivity Analyses
Sensitivity analyses were performed to account for confounding factors that included study center, sex, and body mass index (eTable 14 in Supplement 2). After multivariable adjustment, additional LVA ablation remained significantly associated with lower recurrence rate by mITT analysis (HR, 0.57; 95% CI, 0.36-0.92; P = .02) and per-protocol analysis (HR, 0.55; 95% CI, 0.34-0.88; P = .01).
Discussion
In this randomized clinical trial, compared with CPVI alone, additional LVA ablation beyond CPVI decreased the risk of ATA recurrence by 39% in older patients with paroxysmal AF. Of note, in this population of older patients with paroxysmal AF, LVA prevalence was as high as 41.4% with an average burden of 7.2%. This benefit was consistent in subgroups with the exception of a possible greater benefit in women than in men, possibly due to the higher prevalence and burden of LVA in women.
Low-voltage areas, which indicate underlying atrial fibrosis, were a powerful predictor of AF recurrence after CPVI in both persistent and paroxysmal AF in most published studies.3,15,16,17 However, the benefit of additional LVA ablation after CPVI remains unclear. Early studies demonstrated a benefit in reducing recurrence 22% to 34% from LVA ablation.3,4,6,7 The very recent ERASE-AF trial also showed a positive result from LVA ablation in patients with persistent AF vs CPVI alone.12 In contrast, the benefit of LVA ablation was not shown in the studies of Mohanty et al9 and Masuda et al.8 Our previous STABLE-SR and STABLE-SR-II trials exclusively enrolled patients with persistent AF and showed a possible benefit from LVA ablation, but with statistical nonsignificance.10,11 Based on current data, we hypothesized that the previous inconsistent results are likely due to the heterogeneity of patient selection in terms of the extent of LVA. The STABLE-SR and STABLE-SR-II trials clearly show that CPVI alone is sufficient in patients with AF and healthy left atrium.10,11 On the other hand, the VOLCANO trial8 (which included patients with LVA >5 cm2) and the study by Mohanty et al9 (extent of LVA >60%) did not show benefit from LVA ablation. These 2 studies suggest that at the most severe end of the spectrum, patients with AF and extensive substrates may not benefit from LVA ablation. Our current trial involved older patients with paroxysmal AF who had moderate left atrial substrate, which demonstrated a favorable outcome. The recent DECAAF-II trial, which also enrolled patients with AF and heterogeneous fibrosis using magnetic resonance image (MRI)–guided fibrosis ablation, demonstrated an unfavorable result. However, the DECAAF-II trial found that patients with moderate substrate had better lesion formation than those with extensive fibrosis.18,19 This could give a supportive explanation to the findings of our trial.
Our trial demonstrated that the recurrence rate of the CPVI alone group was significantly higher than that in the CPVI plus group at a median follow-up of 23 months. However, the cumulative incidence of ATA recurrence in both groups was almost identical in the first year of follow-up and separated later. Therefore, the overall HR of 0.61 (95% CI, 0.38-0.95) was a weighted mean of the time-varying HRs, which were close to 1.0 in the first year of follow-up and decreased after 1 year. The reason for this delayed benefit might be 2-fold: first, LVA ablation might have a late benefit. This observation was supported by a recent study, which showed that preexisting LVA might contribute to a higher rate of late recurrence.20 Second, the 7-day Holter monitor was applied at 12 months or later, and more events could be detected after that point.
Prior studies have shown a positive correlation between age and atrial fibrosis using MRI, electroanatomic mapping, and pathological findings.15,21,22,23,24 Our trial, comprising patients aged 65 to 80 years, showed that the prevalence of LVA was 41.4% with the average LVA burden of 7.2%. As compared with the previously reported 10% to 15% in the entire paroxysmal AF population,3,8,15 LVA was more prevalent in this older population. The higher prevalence of LVA in older patients with paroxysmal AF may explain the suboptimal long-term outcome on the one hand,25,26 but also the improved success rate of our trial on the other hand. The other notable finding from our trial was that the LVA prevalence and burden was higher in women than in men (eTable 13 in Supplement 2). This finding is consistent with other studies.27,28 However, those studies were only observational and descriptive; no corresponding strategies targeting the LVAs were attempted.
Our trial results can have a significant clinical effect on AF ablation strategy based on its reproducibility and generalization. First, the approach was easy to conduct. Second, LVA mapping and ablation were performed during the waiting phase and did not prolong the procedure time. Whether this approach can be disseminated to other populations, such as patients with paroxysmal AF and sleep apnea, younger age, or obesity, needs to be explored further via clinical trial.
Limitations
This trial has several limitations. First, similar to other AF ablation trials, we used intermittent rhythm monitoring techniques because of practical constraints.8,9,29,30,31 In the era of wearable devices and implantable loop recorders, the intermittent monitoring protocol might miss some AF events and overestimate the success rate. Since results from different durations and frequencies of monitoring only reflect between-trial discrepancies, applying the same monitoring protocol to both ablation groups revealed the true therapeutic efficacy. Furthermore, the 7-day Holter monitor was applied at 12 months or later; more ATA events could be detected after that point. The foregoing might explain the low recurrence rate during the first year of follow-up. Second, high-density mapping, which we applied in our STABLE-SR and STABLE-SR-II trials, was not used in this study. We believe that point-by-point mapping fashion (sparsely in the healthy area, densely in areas of interest, and verified by contact force) is an acceptable and practical mapping approach (eMethods in Supplement 2).
Conclusions
In this randomized clinical trial, additional LVA ablation beyond CPVI decreased the risk of ATA recurrence in older patients with paroxysmal AF compared with CPVI alone. Our study warrants further replication to translate our findings to clinical practice.
Trial protocol
eAppendix
eMethods
eReferences
eTable 1. Baseline characteristics of study patients (modified intention to treat)
eTable 2. Baseline characteristics (per-protocol set)
eTable 3. Procedural characteristics (modified intention to treat)
eTable 4. Procedural characteristics (per-protocol set)
eTable 5. Baseline characteristics of the four subgroups (modified intention to treat)
eTable 6. Procedural characteristics of four subgroups (modified intention to treat)
eTable 7. Baseline characteristics of the study population by LVA and treatment (modified intention to treat)
eTable 8. Procedural characteristics of the study population by LVA and treatment (modified intention to treat)
eTable 9. Baseline characteristics of the study population by LVA and treatment (per-protocol set)
eTable 10. Procedural characteristics of the study population by LVA and treatment (per-protocol set)
eTable 11. Peri-procedural safety data
eTable 12. Adverse events during follow-up
eTable 13. Baseline characteristics of the study population by sex
eTable 14. Hazard ratio for primary endpoint after adjustment of study centers, sex, and BMI
eFigure 1. Sample images of LVA distribution and the corresponding ablation strategies
eFigure 2. Kaplan-Meier curve of the freedom from ATA after single procedure by specificity analysis of clinical-detected event-based finding between CPVI plus and CPVI alone groups by modified intention-to-treat analysis
eFigure 3. Kaplan-Meier curve of the freedom from ATA after single procedure among three subgroups by modified intention-to-treat analysis
eFigure 4. Primary endpoint subgroup by modified intention-to-treat analysis
eFigure 5. Kaplan-Meier estimates of the freedom from ATA after single procedure between CPVI plus and CPVI alone groups by intention-to-treat analysis
eFigure 6. Kaplan-Meier estimates of the freedom from ATA after single procedure between CPVI plus and CPVI alone groups by per-protocol analysis
eFigure 7. Kaplan-Meier curve of the freedom from ATA after single procedure among four subgroups by per-protocol analysis
eFigure 8. Kaplan-Meier curve of the freedom from ATA after single procedure among three subgroups by per-protocol analysis
eFigure 9. Primary endpoint subgroup by per-protocol analysis
Nonauthor collaborators
Data sharing statement
References
- 1.Kirchhof P, Camm AJ, Goette A, et al. ; EAST-AFNET 4 Trial Investigators . Early rhythm-control therapy in patients with atrial fibrillation. N Engl J Med. 2020;383(14):1305-1316. doi: 10.1056/NEJMoa2019422 [DOI] [PubMed] [Google Scholar]
- 2.Willems S, Borof K, Brandes A, et al. Systematic, early rhythm control strategy for atrial fibrillation in patients with or without symptoms: the EAST-AFNET 4 trial. Eur Heart J. 2022;43(12):1219-1230. doi: 10.1093/eurheartj/ehab593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rolf S, Kircher S, Arya A, et al. Tailored atrial substrate modification based on low-voltage areas in catheter ablation of atrial fibrillation. Circ Arrhythm Electrophysiol. 2014;7(5):825-833. doi: 10.1161/CIRCEP.113.001251 [DOI] [PubMed] [Google Scholar]
- 4.Kircher S, Arya A, Altmann D, et al. Individually tailored vs. standardized substrate modification during radiofrequency catheter ablation for atrial fibrillation: a randomized study. Europace. 2018;20(11):1766-1775. doi: 10.1093/europace/eux310 [DOI] [PubMed] [Google Scholar]
- 5.Schreiber D, Rieger A, Moser F, Kottkamp H. Catheter ablation of atrial fibrillation with box isolation of fibrotic areas: lessons on fibrosis distribution and extent, clinical characteristics, and their impact on long-term outcome. J Cardiovasc Electrophysiol. 2017;28(9):971-983. doi: 10.1111/jce.13278 [DOI] [PubMed] [Google Scholar]
- 6.Yamaguchi T, Tsuchiya T, Nakahara S, et al. Efficacy of left atrial voltage-based catheter ablation of persistent atrial fibrillation. J Cardiovasc Electrophysiol. 2016;27(9):1055-1063. doi: 10.1111/jce.13019 [DOI] [PubMed] [Google Scholar]
- 7.Jadidi AS, Lehrmann H, Keyl C, et al. Ablation of persistent atrial fibrillation targeting low-voltage areas with selective activation characteristics. Circ Arrhythm Electrophysiol. 2016;9(3):e002962. doi: 10.1161/CIRCEP.115.002962 [DOI] [PubMed] [Google Scholar]
- 8.Masuda M, Asai M, Iida O, et al. Additional low-voltage-area ablation in patients with paroxysmal atrial fibrillation: results of the randomized controlled VOLCANO trial. J Am Heart Assoc. 2020;9(13):e015927. doi: 10.1161/JAHA.120.015927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mohanty S, Mohanty P, Di Biase L, et al. Long-term follow-up of patients with paroxysmal atrial fibrillation and severe left atrial scarring: comparison between pulmonary vein antrum isolation only or pulmonary vein isolation combined with either scar homogenization or trigger ablation. Europace. 2017;19(11):1790-1797. doi: 10.1093/europace/euw338 [DOI] [PubMed] [Google Scholar]
- 10.Yang B, Jiang C, Lin Y, et al. ; STABLE-SR Investigators . STABLE-SR (Electrophysiological Substrate Ablation in the Left Atrium During Sinus Rhythm) for the treatment of nonparoxysmal atrial fibrillation: a prospective, multicenter randomized clinical trial. Circ Arrhythm Electrophysiol. 2017;10(11):e005405. doi: 10.1161/CIRCEP.117.005405 [DOI] [PubMed] [Google Scholar]
- 11.Yang G, Zheng L, Jiang C, et al. ; STABLE-SR-II Investigators . Circumferential pulmonary vein isolation plus low-voltage area modification in persistent atrial fibrillation: the STABLE-SR-II trial. JACC Clin Electrophysiol. 2022;8(7):882-891. doi: 10.1016/j.jacep.2022.03.012 [DOI] [PubMed] [Google Scholar]
- 12.Huo Y, Gaspar T, Schönbauer R, et al. Low-voltage myocardium-guided ablation trial of persistent atrial fibrillation. NEJM Evid. 2022;1(11):EVIDoa2200141. doi: 10.1056/EVIDoa2200141 [DOI] [PubMed] [Google Scholar]
- 13.Ju W, Li M, Wang DW, et al. Idiopathic isolated fibrotic atrial cardiomyopathy underlies unexplained scar-related atrial tachycardia in younger patients. Europace. 2018;20(10):1657-1665. doi: 10.1093/europace/eux340 [DOI] [PubMed] [Google Scholar]
- 14.Yang G, Yang B, Wei Y, et al. Catheter ablation of nonparoxysmal atrial fibrillation using electrophysiologically guided substrate modification during sinus rhythm after pulmonary vein isolation. Circ Arrhythm Electrophysiol. 2016;9(2):e003382. doi: 10.1161/CIRCEP.115.003382 [DOI] [PubMed] [Google Scholar]
- 15.Masuda M, Fujita M, Iida O, et al. Left atrial low-voltage areas predict atrial fibrillation recurrence after catheter ablation in patients with paroxysmal atrial fibrillation. Int J Cardiol. 2018;257:97-101. doi: 10.1016/j.ijcard.2017.12.089 [DOI] [PubMed] [Google Scholar]
- 16.Vlachos K, Efremidis M, Letsas KP, et al. Low-voltage areas detected by high-density electroanatomical mapping predict recurrence after ablation for paroxysmal atrial fibrillation. J Cardiovasc Electrophysiol. 2017;28(12):1393-1402. doi: 10.1111/jce.13321 [DOI] [PubMed] [Google Scholar]
- 17.Marrouche NF, Wilber D, Hindricks G, et al. Association of atrial tissue fibrosis identified by delayed enhancement MRI and atrial fibrillation catheter ablation: the DECAAF study. JAMA. 2014;311(5):498-506. doi: 10.1001/jama.2014.3 [DOI] [PubMed] [Google Scholar]
- 18.Marrouche NF, Wazni O, McGann C, et al. ; DECAAF II Investigators . Effect of MRI-guided fibrosis ablation vs conventional catheter ablation on atrial arrhythmia recurrence in patients with persistent atrial fibrillation: the DECAAF II randomized clinical trial. JAMA. 2022;327(23):2296-2305. doi: 10.1001/jama.2022.8831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.ESC 365, The Cardiology Knowledge Hub . Congress presentation: DECAAF II: efficacy of DE-MRI-guided fibrosis ablation vs. conventional catheter ablation of persistent atrial fibrillation. Accessed May 23, 2023. https://esc365.escardio.org/presentation/238817
- 20.Kanda T, Masuda M, Asai M, et al. Impact of left atrial low-voltage areas during initial ablation procedures on very late recurrence of atrial fibrillation. J Cardiovasc Electrophysiol. 2022;33(8):1697-1704. doi: 10.1111/jce.15607 [DOI] [PubMed] [Google Scholar]
- 21.Akoum N, Mahnkopf C, Kholmovski EG, Brachmann J, Marrouche NF. Age and sex differences in atrial fibrosis among patients with atrial fibrillation. Europace. 2018;20(7):1086-1092. doi: 10.1093/europace/eux260 [DOI] [PubMed] [Google Scholar]
- 22.Goette A, Juenemann G, Peters B, et al. Determinants and consequences of atrial fibrosis in patients undergoing open heart surgery. Cardiovasc Res. 2002;54(2):390-396. doi: 10.1016/S0008-6363(02)00251-1 [DOI] [PubMed] [Google Scholar]
- 23.Gramley F, Lorenzen J, Knackstedt C, et al. Age-related atrial fibrosis. Age (Dordr). 2009;31(1):27-38. doi: 10.1007/s11357-008-9077-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tuan TC, Chang SL, Tsao HM, et al. The impact of age on the electroanatomical characteristics and outcome of catheter ablation in patients with atrial fibrillation. J Cardiovasc Electrophysiol. 2010;21(9):966-972. doi: 10.1111/j.1540-8167.2010.01755.x [DOI] [PubMed] [Google Scholar]
- 25.Bahnson TD, Giczewska A, Mark DB, et al. ; CABANA Investigators . Association between age and outcomes of catheter ablation versus medical therapy for atrial fibrillation: results from the CABANA trial. Circulation. 2022;145(11):796-804. doi: 10.1161/CIRCULATIONAHA.121.055297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bunch TJ, May HT, Bair TL, et al. The impact of age on 5-year outcomes after atrial fibrillation catheter ablation. J Cardiovasc Electrophysiol. 2016;27(2):141-146. doi: 10.1111/jce.12849 [DOI] [PubMed] [Google Scholar]
- 27.Li Z, Wang Z, Yin Z, et al. Gender differences in fibrosis remodeling in patients with long-standing persistent atrial fibrillation. Oncotarget. 2017;8(32):53714-53729. doi: 10.18632/oncotarget.16342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wong GR, Nalliah CJ, Lee G, et al. Sex-related differences in atrial remodeling in patients with atrial fibrillation: relationship to ablation outcomes. Circ Arrhythm Electrophysiol. 2022;15(1):e009925. doi: 10.1161/CIRCEP.121.009925 [DOI] [PubMed] [Google Scholar]
- 29.Verma A, Jiang CY, Betts TR, et al. ; STAR AF II Investigators . Approaches to catheter ablation for persistent atrial fibrillation. N Engl J Med. 2015;372(19):1812-1822. doi: 10.1056/NEJMoa1408288 [DOI] [PubMed] [Google Scholar]
- 30.Packer DL, Mark DB, Robb RA, et al. ; CABANA Investigators . Effect of catheter ablation vs antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA. 2019;321(13):1261-1274. doi: 10.1001/jama.2019.0693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuck KH, Brugada J, Fürnkranz A, et al. ; FIRE AND ICE Investigators . Cryoballoon or radiofrequency ablation for paroxysmal atrial fibrillation. N Engl J Med. 2016;374(23):2235-2245. doi: 10.1056/NEJMoa1602014 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol
eAppendix
eMethods
eReferences
eTable 1. Baseline characteristics of study patients (modified intention to treat)
eTable 2. Baseline characteristics (per-protocol set)
eTable 3. Procedural characteristics (modified intention to treat)
eTable 4. Procedural characteristics (per-protocol set)
eTable 5. Baseline characteristics of the four subgroups (modified intention to treat)
eTable 6. Procedural characteristics of four subgroups (modified intention to treat)
eTable 7. Baseline characteristics of the study population by LVA and treatment (modified intention to treat)
eTable 8. Procedural characteristics of the study population by LVA and treatment (modified intention to treat)
eTable 9. Baseline characteristics of the study population by LVA and treatment (per-protocol set)
eTable 10. Procedural characteristics of the study population by LVA and treatment (per-protocol set)
eTable 11. Peri-procedural safety data
eTable 12. Adverse events during follow-up
eTable 13. Baseline characteristics of the study population by sex
eTable 14. Hazard ratio for primary endpoint after adjustment of study centers, sex, and BMI
eFigure 1. Sample images of LVA distribution and the corresponding ablation strategies
eFigure 2. Kaplan-Meier curve of the freedom from ATA after single procedure by specificity analysis of clinical-detected event-based finding between CPVI plus and CPVI alone groups by modified intention-to-treat analysis
eFigure 3. Kaplan-Meier curve of the freedom from ATA after single procedure among three subgroups by modified intention-to-treat analysis
eFigure 4. Primary endpoint subgroup by modified intention-to-treat analysis
eFigure 5. Kaplan-Meier estimates of the freedom from ATA after single procedure between CPVI plus and CPVI alone groups by intention-to-treat analysis
eFigure 6. Kaplan-Meier estimates of the freedom from ATA after single procedure between CPVI plus and CPVI alone groups by per-protocol analysis
eFigure 7. Kaplan-Meier curve of the freedom from ATA after single procedure among four subgroups by per-protocol analysis
eFigure 8. Kaplan-Meier curve of the freedom from ATA after single procedure among three subgroups by per-protocol analysis
eFigure 9. Primary endpoint subgroup by per-protocol analysis
Nonauthor collaborators
Data sharing statement