Abstract
Background and Purpose:
Asthma is a heterogenous disease strongly associated with inflammation that has many different causes and triggers. Current asthma treatments target symptoms such as bronchoconstriction and airway inflammation. Despite recent advances in biologic therapies, there remains a need for new classes of therapeutics with novel, upstream targets. Proteinase-activated receptor-2 (PAR2) has long been implicated in allergic airway inflammation and asthma and it remains an intriguing target for novel therapies. In this study we describe the newly developed small molecule PAR2 biased antagonist C781 in vitro and in vivo in the context of acute allergen exposure.
Experimental Approach:
A human bronchial epithelial cell line that naturally expresses PAR2 (16HBE14o- cells) was used to evaluate the ability for C781 to mediate in vitro PAR2 physiological response and downstream β-arrestin/MAPK and Gq/Ca2+ signalling. Acute Alternaria alternata sensitized and challenged mice were used to evaluate the ability for C781 to prophylactically mediate airway hyperresponsiveness, inflammation and mucus overproduction in vivo.
Key Results:
C781 was effective in reducing in vitro physiological signalling in response to ligand and proteinase activation. C781 effectively antagonized β-arrestin/MAPK signalling without significant effect on Gq/Ca2+ signalling in vitro. Given prophylactically, C781 effectively mediated airway hyperresponsiveness, airway inflammation and mucus overproduction of the small airways in an acute allergen-challenged mouse model.
Conclusions and Implications:
Our work demonstrates the first biased PAR2 antagonist for β-arrestin/MAPK signalling. C781 is efficacious as a prophylactic treatment for allergen-induced airway hyperresponsiveness and inflammation and thus, exemplifies a key pharmacophore for PAR2 that can be optimized for clinical development.
Keywords: C781, β-arrestin/MAPK signalling, Ca2+ signalling, inflammation, asthma
Graphical Abstract
INTRODUCTION:
Asthma is a multi-faceted disease with strong genetic and environmental components, presenting unique challenges for treatment development (Lambrecht & Hammad, 2015; Locksley, 2010; Martinez & Vercelli, 2013; Pavord et al., 2018). Most common treatments for asthma center around β adrenergic receptor agonists (Platts-Mills, 2015) and corticosteroids and/or inhaled steroids (Crompton, 2006; Thursby-Pelham & Kennedy, 1958). More recent medications developed at the end of the 20th century include leukotriene, IgE, IL-4, IL-5 and IL-13 modifiers (Carlsson et al., 2019; Chang, Wu, Hsu, & Hung, 2007; Katsaounou et al., 2019; Menzella, Lusuardi, Galeone, Taddei, & Zucchi, 2015; Scott & Peters-Golden, 2013; Xiong, Zhu, Wu, Fan, & Cheng, 2019). These biologics, while effective for specific asthma subtypes, frequently require multiple injections and/or infusions and come with side effects and high costs for many patients. Despite growing mechanistic understanding of asthma pathogenesis, clinical management of asthma remains relatively unchanged (Martinez & Vercelli, 2013), with current asthma guidelines relying strongly on treating symptoms rather than upstream activators that contribute to the disease (Patel & Shaw, 2015). Improvements for asthma treatment, including the development of novel drugs/drug targets are required (Pavord et al., 2018).
Proteinase-activated receptor-2 (PAR2) is a G protein-coupled receptor (GPCR) expressed in a variety of cell types throughout the body and its activation is closely associated with inflammatory diseases, including asthma (Adams et al., 2011; Knight et al., 2001; Ossovskaya & Bunnett, 2004; Ramachandran, Noorbakhsh, Defea, & Hollenberg, 2012). PAR2 is activated after proteolytic cleavage of its extracellular, amino terminus leading to activation of two distinct canonical signalling pathways: β-arrestin/MAPK and Gq/Ca2+ signalling (Adams et al., 2011; Ramachandran et al., 2012). In vivo mouse models using cockroach frass, house dust mite extracts, or potent and selective PAR2 activating peptides have all shown that PAR2 plays critical roles in allergic asthma sensitization (Arizmendi et al., 2011; Davidson et al., 2013; Page, Ledford, Zhou, Dienger, & Wills-Karp, 2010) and inflammation (Day, Zhou, Ledford, & Page, 2010; Ebeling et al., 2005; Nichols et al., 2012)}. We have shown that Alternaria alternata filtrates similarly cause proteinase-dependent asthma associated responses -- airway hyperresponsiveness (AHR), inflammation and mucus overproduction -- in acute allergen exposure mouse models (Boitano et al., 2011; Yee et al., 2018) that can be moderated with a PAR2 antagonist (Rivas et al., 2021). Complementary studies that neutralize PAR2 with antibodies were shown to reduce asthma indicators in both acute and chronic allergen exposure asthma mouse models (Asaduzzaman et al., 2018; Asaduzzaman et al., 2015). Using genetically modified mouse models we have further demonstrated that acute exposure of A. alternata induces physiologic asthma indicators (i.e., AHR, pulmonary inflammation, mucus cell hyperplasia and mucus hypersecretion after an 8 day sensitization/challenge protocol) in a manner that requires both PAR2 and β-arrestin-2 expression (Rivas et al., 2021; Yee et al., 2018). Interestingly, proteinase activated, PAR2-dependent Gq/Ca2+ signalling at the airway epithelium is associated with bronchorelaxation (Cocks et al., 1999; Henry, 2006; Nichols et al., 2012; Yee et al., 2018), a clearly beneficial outcome for asthmatics.
Noting the conflicting allergen-induced detrimental (β-arrestin/MAPK) and protective (Gq/Ca2+) effects of downstream PAR2 signalling in the airway, we sought to develop a biased antagonist of PAR2 that specifically targeted the β-arrestin/MAPK signalling arm while leaving the Gq/Ca2+ arm intact. We demonstrate here that the novel PAR2 ligand C781 reduces PAR2-dependent in vitro physiological response and selectively inhibits PAR2-associated β-arrestin/MAPK signalling while allowing Gq/Ca2+signalling to proceed unhindered in human bronchial epithelial cells. Further, in an acute A. alternata-exposure mouse model, C781 prophylactically attenuated several physiologic indicators characteristic of the acute allergic asthma response, including AHR, inflammation, and a series of inflammatory cytokines, with less pronounced impact on other indicators (e.g., mucus overproduction). These data complement our recent findings that demonstrate C781 can also prophylactically and therapeutically limit PAR2-dependent pain responses in mouse models (Kume et al., In Revision). The effectiveness of the biased PAR2 antagonist C781 in vitro and in vivo represents a promising step towards precision medicine targeting of PAR2 for asthma treatment.
METHODS
Materials:
Alternaria alternata filtrate was purchased from Stallergenes Greer Laboratories (Lenoir, NC) as a lyophilized powder, suspended at 2.5 mg/mL in Hanks Balanced Saline Solution (Life Technologies, Watham, MA) additionally buffered with 25 mM Hepes (pH 7.4, HBSS) and stored at −20°C until further use. The PAR2 agonist [2-aminothiazole-LIGR-NH2 (2at-LIGRL-NH2; 2AT) was manufactured in our laboratory as described (Boitano et al., 2015; Flynn et al., 2011). Trypsin was purchased from Sigma (Cat#T6567) and human neutrophil elastase from Worthington Biochemical (Cat# LS003703; Lakewood, NJ). C781 was developed in house and prepared as described in a supplementary file. Unless listed below, all other chemicals/components were of Molecular Biology or higher grades and purchased from Fisher Scientific (Pittsburgh, PA), ThermoFisher Scientific (Watham, MA), Sigma-Aldrich (Burlington, MA), or VWR (West Chester, PA).
Cell culture:
Naturally PAR2-expressing 16HBE14o- cells (California Pacific Lab, San Francisco; RRID:CVCL_0112) were used for all in vitro experiments. Cells were grown in media consisting of Minimum Essential Media with Earle’s salts (Life Technologies Cat# 11095-080), supplemented with 10% Fetal Bovine Serum (FBS), 1% Pen/Strep, and 1% Glutamax (MEM). Cells were grown on matrix-treated culture ware, with the matrix consisting of collagen, fibronectin, and bovine serum albumin as described (Rivas et al., 2021).
In vitro physiological response with the xCELLigence Real Time Cell Analyzer (RTCA):
RTCA is a high-capacity cell signalling detection platform that uses impedance to monitor physiologic response (Atienza et al., 2006; Xi, Yu, Wang, Xu, & Abassi, 2008). We have extensively demonstrated the ability to detect PAR2 signalling with this device (Boitano et al., 2015; Boitano et al., 2014; Flynn et al., 2013; Flynn et al., 2011; Rivas et al., 2022). Briefly, a background measurement was taken using 50 μL 2% FBS-supplemented MEM per well in an E96 plate (Agilent Technologies, Santa Clara, CA). Media was removed and cells were plated at a density of 4.5×104 cells/well suspended in 150 μL of 2% FBS-supplemented MEM and allowed to grow overnight (18 – 22 hrs) at 37°C and 5% CO2 while being monitored for Cell Index every 15 min. Upon reaching optimal confluence, recordings were switched to one min and cells were treated with 25 μL C781 (8× Final Concentration) for 5 min. The agonist treatment (25 μL, 8× dilution of 2AT, trypsin or elastase) was added and Cell Index was recorded every min for 2 hrs. For experiments using endogenous enzymes trypsin or elastase, 2% FBS MEM was replaced with FBS-free MEM to eliminate FBS interference. In these experiments, Cell Index was allowed to stabilize for 90 min prior to antagonist/agonist treatment. Agonist concentrations approximate the EC80 values (Rivas et al., 2022).
β-arrestin signalling with High Content Imaging (HCI):
β-arrestin binding was detected using probes from Montana Molecular (Bozeman, MT) and the Operetta CLS HCI (Perkin Elmer, Waltham, MA). 16HBE14o- were plated at 40,000 cells in 100μL of MEM per well in clear bottom/black sided 96 well plates and were grown overnight. On the second day of the experiment, MEM was replaced with 50 μL serum-free MEM additionally supplemented with insulin-, transferrin-, and selenium (sMEM) and grown overnight. As suggested by the manufacturer, 50 μL of BacMam vector containing the β-arrestin probe was added to each well containing 16HBE14o- cells and incubated for 6 – 8 hours. Wells were aspirated and replaced with 100 μL sMEM and grown 1 – 2 days to allow for full probe expression. Medium was replaced with fresh sMEM [175 μL (agonist experiments) or 150 μL (antagonist experiments)] and plate was transferred to the Operetta HCI (Perkin Elmer, Richmond, CA) at 37°C and 5% CO2 throughout the experiment. For agonist experiments, baseline fluorescence (Fo) was measured prior to addition of 25 μL 8× concentrations of 2AT and fluorescence (F) was measured over time. For antagonist experiments wells were first treated with 25 μL C781 (24 μM; final concentration = 3 μM) for 5 min prior to Fo recording. Experiments were conducted in quadruplicate each day and repeated on a subsequent day; F measurements obtained every 10 sec for each well. Antagonist experiments were similar with an additional pre-incubation (5 min) of C781 prior to Fo capture. Analyses were conducted using Harmony software (Perkin Elmer) and presented as F/Fo. A reduction of F/Fo was indicative of β-arrestin binding.
Mitogen Activated Protein Kinase (MAPK) signalling using In Cell Western (ICW):
ICW studies were adapted from methods described in (Flynn et al., 2011; Rivas et al., 2021). 16HBE14o- cells were plated onto matrix-coated, clear bottom, black-walled 96-well plates at a density of 2×104 cells per well. Cells were allowed to reach confluence (3 – 4 days of growth) and were subsequently serum-starved overnight in MEM supplemented with 150 μL 10 μM AEBSF and 10 μM E64. On the following day, cells were treated with 7× concentration of C781 suspended in MEM (25 μL) to a final concentration range of 100 nM - 100 μM and incubated for 15 min. This was followed by a treatment of 25 μL 2AT (8× final concentration) mixed with 1× final C781 concentration. After 5 min, cells were fixed with 4% paraformaldehyde and permeabilized with 100% methyl alcohol. Fixed cells were incubated with blocking buffer (5% goat serum, 0.3% Triton X-100) prior to incubation with primary phosphorylated MAPK antibody (p-MAPK; rabbit anti-mouse p-ERK1/2; Cat#9101 Cell Signaling Technologies, Grand Island, NY), secondary antibody (goat anti-rabbit DyLight; Cat#5151 Cell Signaling Technologies) and nuclear stain DRAQ5 (Cat#4804, Cell Signaling Technologies). Plates were imaged on an Odyssey Scanner (LI-COR, Lincoln, NE) following the manufacturer’s protocol. P-MAPK levels were normalized to DRAQ5 content to control for differences in cell number within each well.
Gq/Ca2+ signalling with digital imaging microscopy:
16HBE14o- cells were plated on matrix-coated glass coverslips and grown to confluence (3 – 5 d). Cells were loaded with 5 μM Fura-2 acetoxymethyl ester (Fura-2-AM) in HBSS for 45 min and then transferred to HBSS for at least 20 min. Cells were imaged on an Olympus 1×70 microscope hooked up to a Delta Ram monochromator (Horiba Scientific, London, ON) and an Evolve Camera (Teledyne Photometrics, Tucson, AZ) under Easy Ratio Pro software (Horiba Scientific, London ON) control. Fura-2 fluorescence (excitation at 340 and 380 nM, emission > 505 nM) was monitored at ~1 ratio/sec. Excitation experiments consisted of 20 sec in HBSS, 10 sec wash with agonist and an additional 2 min 30 sec of monitoring. Antagonist experiments consisted of 20 sec in HBSS, 10 sec wash with C781, 10 sec wash with agonist/C781 and an additional 2 min 30 sec of monitoring.
In vivo experiments:
All experiments were approved by the University of Arizona IACUC and are in accordance with ARRIVE and British Journal of Pharmacology guidelines (Kilkenny et al., 2010). Six-week-old male BALB/c mice were purchased from Jackson Laboratories (Cat #000651; Farmington, CT) and allowed to acclimate to the University of Arizona animal facility for 2 weeks. Male mice were used due to their enhanced response to allergens that allowed for statistical analysis with a reduced amount of animals. At eight weeks, mice were randomly divided into four treatment groups of six mice each and subjected to allergen sensitization and challenge using A. alternata filtrate as described (Rivas et al., 2021). Mice were first anesthetized by placing them in a chamber supplied with vaporized isoflurane and oxygen. Mice were monitored until respiration was sufficiently slowed (visible breaths ~once every 2 sec). Mice were then subjected to intranasal inoculations (50 μL) on Days 0, 3 and 7. Animals were monitored for full recovery according to IACUC standards. Inoculations included the allergen group: A. alternata alone (5 μg filtrate in 50 μL HBSS); the allergen/antagonist group: A. alternata + C781 (5 μg filtrate and 5 nmol C781 in 50 μL HBSS); and two control groups: 50 μL HBSS or 5 nmol of C781 in 50 μL HBSS. One of the mice from the Alt + C781 group died at day 4 and thus final groupings consisted of 6 mice for the HBSS, C781 and Alt groups and five mice for the Alt + C781 group. To the number of animals used, data collection (airway hyperresponsiveness, bronchoalveolar lavage, lung staining) were collected from each animal.
Airway hyperresponsiveness (AHR):
On Day 8, animals were assessed for AHR using the flexiVent (Sci Req, Montreal, QC Canada) as described (Addison, Morse, Robichaud, Daines, & Ledford, 2017; Rivas et al., 2021). Mice were anesthetized with an intraperitoneal (i.p.) injection of urethane (125 mg/mL in H2O; 16 μL/g body weight). The trachea was cannulated, and the mouse placed on the flexiVent where it was injected i.p. with pancuronium bromide (0.8 mg/mL; 10 μL/g body weight). Mice were subjected to deep inflations prior to nebulized methacholine (0, 3, 10, 30, or 100 mg/mL) administration followed by forced oscillations where 12 independent AHR data points were collected over three min. Responses were presented by the flexiVent software as total airway resistance (Rrs), total airway elastance (Ers), Newtonian resistance (Rn; resistance biased toward that in the conducting airway), tissue damping (G; loss of energy in tissue due to friction), and tissue elastance (H). Graphs of these parameters represent the area under the curve calculation related to the baseline parameter (% change from baseline) to best compare all animals across different experimental dates. One animal from the Alt group was excluded for the Rn, G and H analyses due to a lack of sufficient readings (< 4 valid points as determined by the SciReq software) to develop a curve analysis.
Inflammation and mucin analyses:
Bronchoalveolar lavage fluid (BALF) was collected immediately following the AHR procedure using up to 1.5 mL PBS supplemented with 100 μM EDTA. Lungs were then removed, placed in 4% paraformaldehyde for 48 hrs at 4°C and then switched to 70% alcohol until paraffin embedding at the University of Arizona Tissue Acquisition and Cellular/Molecular Analysis Core Facility Shared Resource. BALF fluid was centrifuged with cells resuspended into 200 μL PBS and BALF supernatant frozen at −80°C until use. Resuspended cells (< 2 × 105) were spun onto slides using a Shandon Cytospin and stained using Hema 3 stain kit (Fisher Scientific, Cat #22122911) following the manufacturer protocol. Slides were masked and differential counts were obtained from a minimum of 4 unbiased fields of view that included at least 200 cells and based on morphological criteria. Samples that did not include 200 cells were excluded. Final counts represent averages from three individuals scoring samples of five mice from each group. BALF samples (200 μL) from each treatment group were shipped to Ampersand Biosciences (Lake Clear, NY) for cytokine analysis using their Mouse MAP 4.1 multiplex immunoassay service. BALF samples that had excessive red blood cell infiltration during collection were excluded, resulting in five samples per treatment group. Samples were tested at two different concentrations to maximize sensitivity. Cytokine panel, validation and sensitivity information is available in the Supplemental Data or at https://www.ampersandbio.com/services/rodent-map-4-0-mouse-sample-testing/.
Paraffin embedded sections were cut to 5 μm thickness, de-paraffinized and stained with Hematoxylin and Eosin (H&E) for inflammatory analyses or with Periodic Acid Schiff (PAS) for mucus analyses. Slides were masked and sections were imaged using an Olympus IX70 inverted microscope with 10×, 20× or 40× air objectives. Images were captured using a MicroPublisher 6 camera and accompanying Occulus software (Qimaging, Tucson, AZ). Semi-quantitative scoring of inflammation and mucin staining was performed by histological grading. Semi-quantitative inflammatory scores ranged from 0 – 4 and were based on inflammation around the bronchis/bronchioles. A score of 0 showed well-defined bronchi/bronchioles with no cellular infiltration and intact surrounding alveoli; a score of 1 reflected noticeable bronchi/bronchiole inflammation (~20 – 25%) surrounded by intact alveoli; a score of 2 depicted moderate inflammation that mostly separated the bronchiole from adjacent alveoli; a score of 3 reflected a high level of inflammation in most bronchioles with separation from the surrounding alveoli; a score of 4 depicted most/all bronchioles demonstrating high inflammation and separation from the surrounding alveoli. Semi-quantitative evaluation of mucus production in the lung samples was performed by scoring sections on a scale of 0 – 4 on the PAS-stained sections and based on extent of staining of epithelial layer and bronchiole lumen indicative of mucus plugs. In these samples, a score of 0 represented sections free of mucus staining; a score of 1 represented diffuse mucus staining in/around the epithelial layer of the larger airways; a score of 2 indicated increased mucus staining in the larger airways but no to minimal staining in the smaller airways; a score of 3 reflected significant and abundant epithelial staining in large airways and evidence of staining throughout the small airways; a score of 4 reflected epithelial and lumenal staining of mucus in throughout the airways and presence of multiple mucus plugs. Small airways were defined as bronchi/bronchioles that lacked cartilage, contained smooth muscle and columnar or cuboidal epithelial cells. Evaluation of small airway PAS staining was based on percentage of positively stained epithelial cells where a score of 0 had minimal to no staining; a score of 1 had ~25% staining; a score of 2 had ~50% staining; a score of 3 had ~75% staining and a score of 4 had 80 – 100% staining. For both inflammation and mucus scoring, there were 6 (HBSS, C781, Alt) or 5 (Alt + C781) mice in each group. Reported scores reflect evaluation by at least 3 blinded evaluators of at least 2 lung sections with a minimum of 25 μm separation.
Data analysis and Statistics:
The manuscript complies with recommendations and requirements on experimental design proposed for the British Journal of Pharmacology (Curtis et al., 2018). Sample sizes, n, for all experiments reflect the number of independent values (either replicates for cell assays, individual mice or samples from individual mice). All data were analyzed with GraphPad Prism software (version 9.2; San Diego, CA). Only samples sizes of five or greater independent values were used to perform statistical tests. Student t tests were used for two component comparisons, One-way and two-way ANOVA were used as appropriate for multiple factored data sets. Post hoc tests in ANOVA were only executed when the p < 0.05. In all data sets, p < 0.05 was used as a cutoff for significance.
Nomenclature of Targets and Ligands:
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, and are permanently archived in the Concise Guide to PHARMACOLOGY 2021/22 (Alexander et al., 2021).
RESULTS
C781 is a proteinase-activated receptor-2 (PAR2) antagonist.
To initially characterize C781 effects on PAR2 signalling we used a high capacity in vitro physiological assay, xCELLigence Real Time Cellular analysis (RTCA). A five min pre-exposure to C781 effectively reduced 300 nM 2AT-induced signalling in a concentration-dependent manner (Figure 1A). At the highest concentration tested (10 μM) C781 reduced the 2AT-induced signalling (AUC) by 40.9% while 300 nM C781 significantly reduced the signalling by 6.3%. We next evaluated the potential for C781 to antagonize enzymatic activation of PAR2 by monitoring PAR2-dependent signalling responses of 16HBE14o- cells to both trypsin and human neutrophil elastase. Both trypsin and elastase induced concentration-dependent signalling with 16HBE14o- cells and RTCA that were dependent on PAR2 expression (Rivas et al., 2022). As with the PAR2 ligand, C781 effectively reduced 30 nM trypsin-induced in vitro physiologic signalling in a concentration dependent manner (Figure 1B, C). A 10 μM concentration of C781 reduced the trypsin-induced AUC by 89.5% whereas a 1 μM concentration reduced the AUC by 11.6%. For elastase, 10 μM C781 significantly reduced the 1 μM elastase induced RTCA signal by 70.5%, and a 3 μM concentration reduced 50.2% of the signal; however, no signal reduction was observed with 1 μM C781. These results show that C781 antagonizes PAR2 activation by peptidomimetic and proteinase-mediated receptor activation in cells naturally expressing the human receptor.
Figure 1: In vitro physiological inhibition of PAR2 activation by C781.
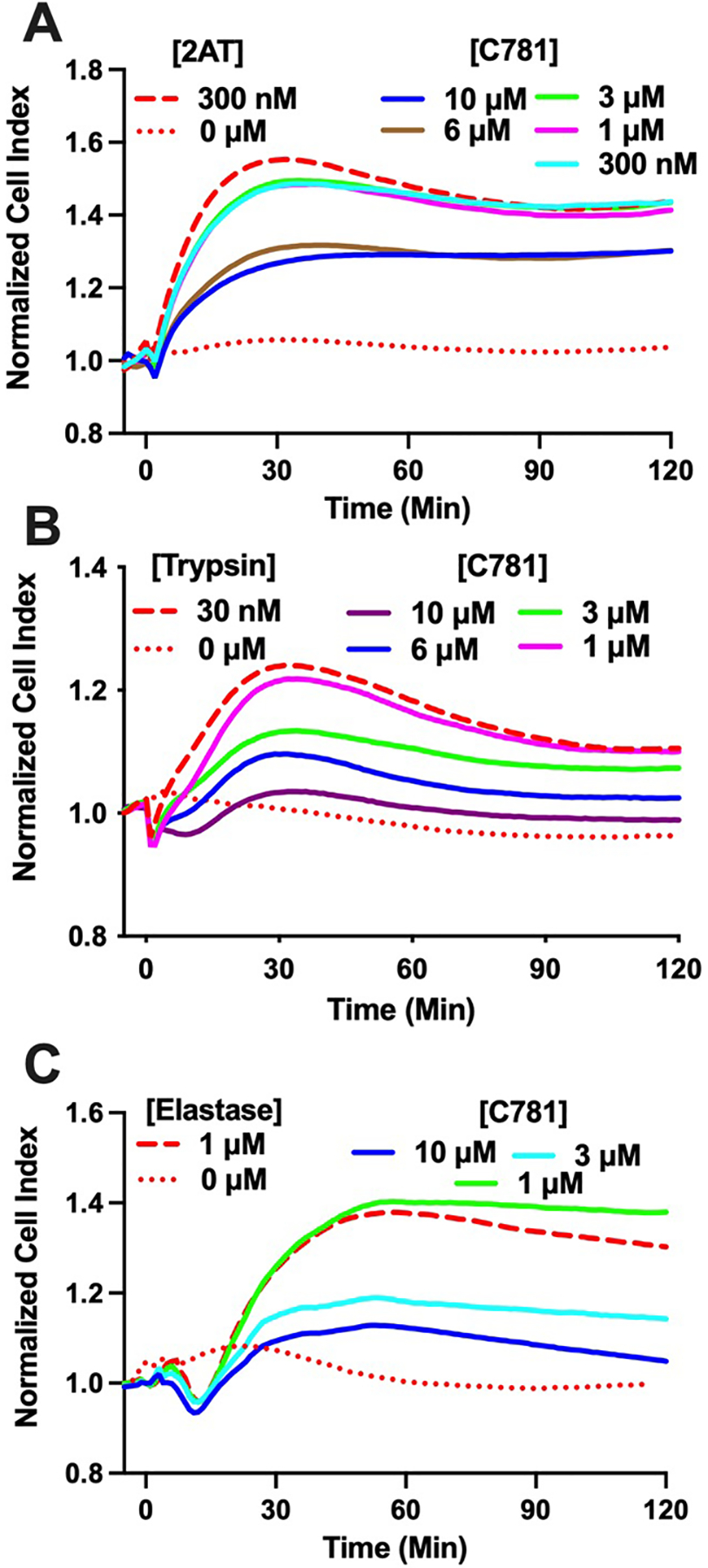
Traces show PAR2 activation and subsequent inhibition in 16HBE14o- cells by (A) 2-at-LIGRL-NH2 (2AT); (B) Trypsin; and (C) Elastase as measured by xCELLigence RTCA. Micromolar concentrations of C781 effectively reduce the in vitro physiological response to both ligand and proteinase agonists. Each trace represents the average of n = 4 (2AT, trypsin) or n = 3 (elastase) experiments from a single E-plate and are representative of multiple E-plates (2AT, E-plate n = 6; trypsin, n = 3; elastase, n = 2). Error bars are eliminated for clarity. Activating compound concentrations were EC80 and determined in (Rivas et al., 2022).
C781 is a biased-signalling antagonist for PAR2.
We further explored C781 antagonism of PAR2 using signalling-specific assays in 16HBE14o- cells and specific activation of PAR2 with 2AT. We first used a fluorescent indicator for live cell imaging of β-arrestin activity (Hoare, Tewson, Quinn, & Hughes, 2020). The probe displays reduced fluorescence upon β-arrestin binding to a compatible GPCR. Activation of PAR2 on 16HBE14o- cells by 2AT resulted in a concentration-dependent binding of β-arrestin within 30 sec that continued throughout the 150 sec experiment (Figure 2A). In a separate set of experiments following a 5 min pre-incubation with 3 μM C781, β-arrestin binding induced by 2AT in 16HBE14o- cells was severely constrained (Figure 2B). Direct comparison of maximal responses in the two experiments (Figure 2C) demonstrates the clear antagonistic effect of C781 on β-arrestin binding. To verify C781 inhibition of the β-arrestin signalling pathway, we used In Cell Westerns to evaluate C781 effects on 2AT/PAR2-induced ERK1/2 phosphorylation in 16HBE14o- cells (Flynn et al., 2011). Pre- and co-incubation with C781 effectively reduced 2AT-induced (600 nM) MAPK phosphorylation in a concentration-dependent fashion with significant inhibitory effects starting at 1 μM C781 and continuing to the highest concentration tested (100 μM; Figure 2D). In contrast to β-arrestin and MAPK signalling, C781 had no effect on the Gq/Ca2+ signalling pathway over a similar activating concentration of 2AT as measured by digital imaging microscopy (Figure 2E). Therefore, C781 antagonizes PAR2 activation with a clear bias for reducing the β-arrestin/MAPK signalling pathway and with minimal effects on the Gq/Ca2+ signalling pathway.
Figure 2: C781 reduces PAR2-dependent β-arrestin/MAPK signalling without effects on Gq/Ca2+ signalling.
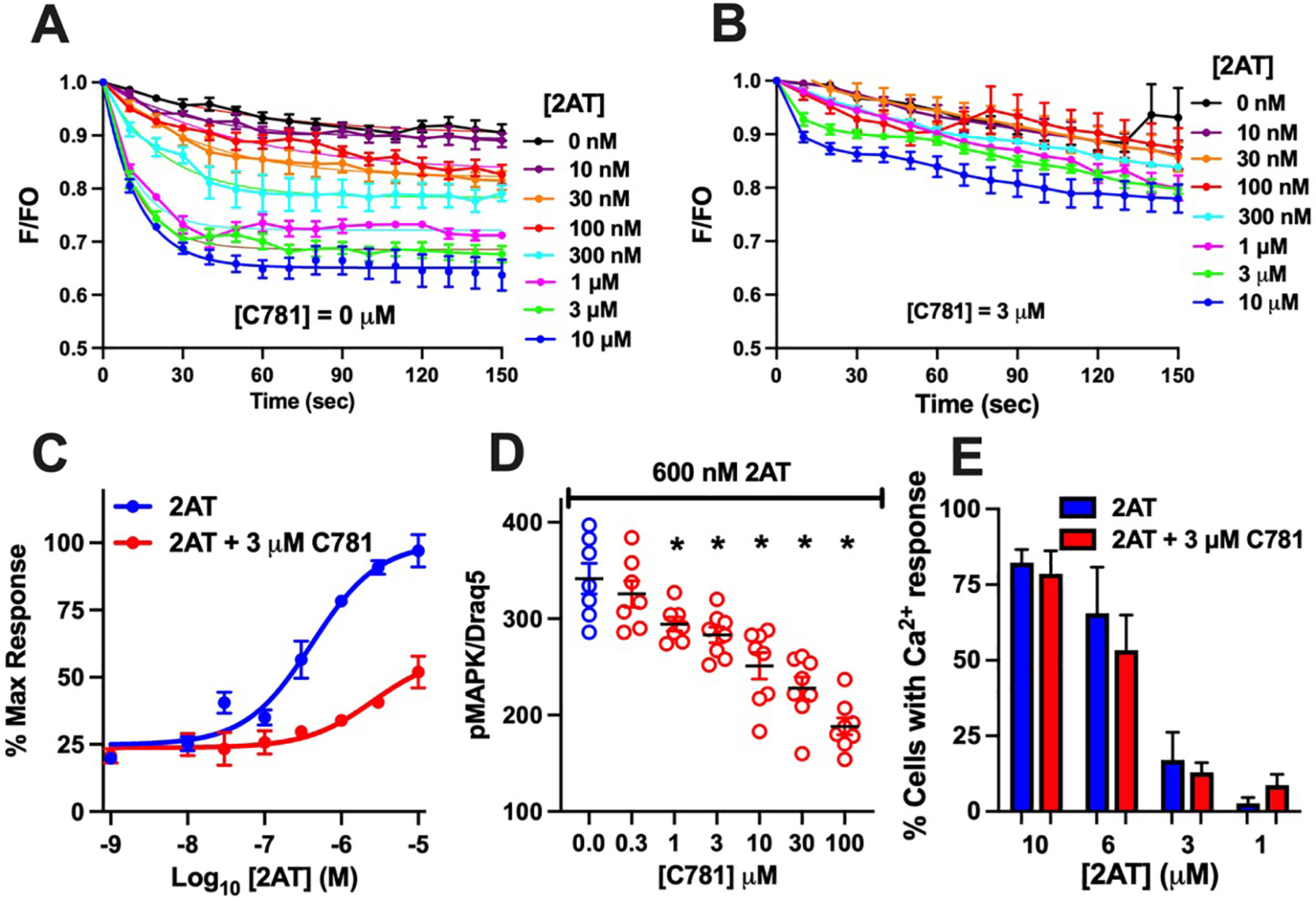
β-arrestin binding in 16HBE14o- cells was monitored in response to a concentration response of 2AT in the absence (A) or presence (B) of 3 μM C781. 2AT induced an increased binding of β-arrestin that was significantly reduced across 2AT concentrations. (C) C781 significantly reduced phosphorylation of ERK1/2 (pMAPK) in response to a full agonist dose of 2AT (600 nM) beginning at 1 μM (D). C781 (3 μM) had no effect on 2AT-induced Ca2+ signalling across a concentration range €. Data are plotted with SEM and represent n = 7, 8 for β-arrestin and pMAPK studies; 6 ≤ n ≤ 10 for Ca2+ studies. * represents significant difference from control.
C781 alters allergen-induced airway responses in a mouse model.
We have shown that the fungal allergen Alternaria alternata can induce airway hyperresponsiveness (AHR), inflammation and mucus production in an acute exposure mouse model (Rivas et al., 2021; Yee et al., 2018). Genetically modified mouse models established that these pathophysiological responses to A. alternata require both PAR2 and β-arrestin expression (Yee et al., 2018). Further, these responses could be significantly reduced or fully eliminated pharmacologically in wild type animals by the PAR2 antagonist C391, which inhibits both β-arrestin/MAPK signalling and Gq/Ca2+ signalling (Rivas et al., 2021). Here, we sought to evaluate if the in vivo responses to A. alternata could be inhibited pharmacologically with the β-arrestin/MAPK signalling-biased PAR2 antagonist C781.
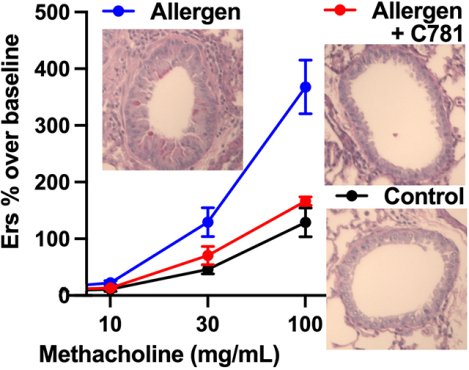
We first used the forced oscillation technique to measure AHR in response to the broncho-constrictant methacholine (MCh) in A. alternata-sensitized/challenged BALB/c mice (Addison et al., 2017; Rivas et al., 2021). A. alternata treatment resulted in a MCh dose-dependent increase in AHR in all five parameters recorded [total airway resistance (Rrs), total airway elastance (Ers), Newtonian resistance (Rn), tissue damping (G) and tissue resistance (H)] when compared to controls [HBSS or C781 alone; (Figure 3A – E)]. However, when A. alternata was administered in the presence of C781, the measured AHR parameters closely tracked with the control values throughout the experiments, with significant reduction from A. alternata-induced changes at the higher MCh doses across all measurements. The A. alternata + C781 group only differed significantly from the C781 control group in tissue damping (G) and only at 100 mg/mL MCh. The C781 group never separated from HBSS controls. These results demonstrate that PAR2 β-arrestin/MAPK biased antagonism by C781 is effective at protecting against the development of AHR in an acute, allergen sensitized/challenged murine model.
Figure 3: C781 reduces airway hyperresponsiveness in an acute A. alternata exposure model.
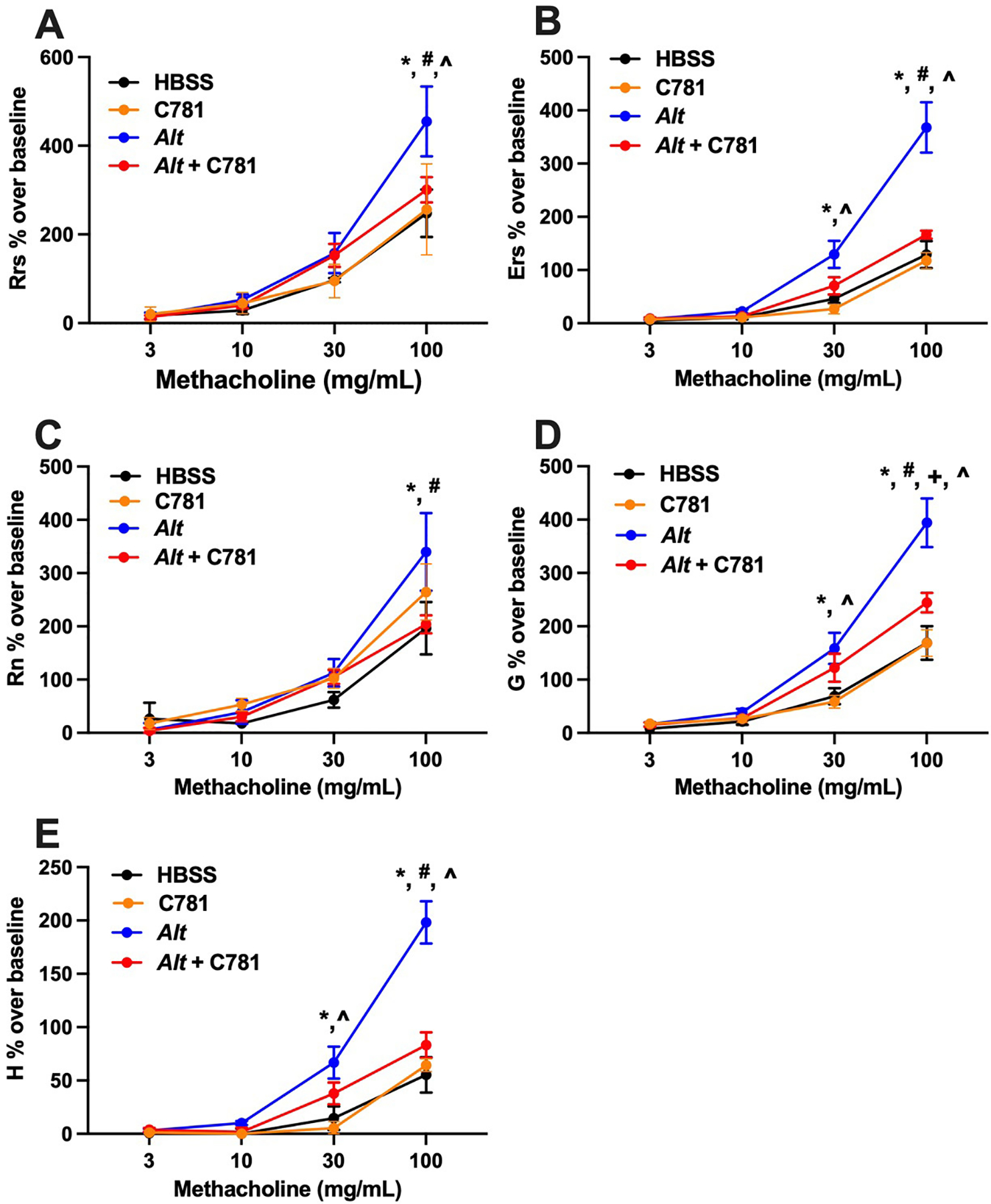
A concentration response to nebulized methacholine is shown for: (A) Total airway resistance – Rrs; (B) Total airway elastance – Ers; (C) Newtonian resistance – Rn; (D) Tissue damping – G; and (E) Tissue elastance – H. A. alternata (Alt, blue traces) significantly increased responsiveness over both controls (HBSS, black traces or C781 alone, orange traces) and A. alternata supplemented with C781 (Alt + C781, red traces) in all measures tested at the highest methacholine concentrations. * denotes significant difference between Alt and HBSS; # denotes significant difference between Alt and Alt + C781; ^ denotes significant difference between Alt and C781 control; + denotes significant difference between Alt + C781 and C781 control. For Alt (Rn, G, H) and Alt + C781 n = 5; for Alt (Rrs and Ers), HBSS and C781 controls n = 6.
We next evaluated the effect of C781 on the A. alternata-induced inflammatory response. Cellular infiltration measured in BALF-recovered samples showed that leukocytes trended higher in the A. alternata-treated samples compared to animals treated with HBSS and significantly higher than the animals treated with C781 alone. However, the animals administered C781 along with A. alternata displayed significantly less cellular infiltration compared to the A. alternata-treated group and no significant difference from the HBSS- or C781-treated controls (Figure 4A). An examination of individual cell types (Figure 4B – E) showed that A. alternata exposure caused increases in neutrophils and eosinophils that were significantly reduced by C781, whereas no significant changes in macrophage or lymphocyte cell counts were detected between any of the treatments. Histological staining of lung sections supported the BALF data, with significantly increased inflammation in the lungs from mice treated with A. alternata that was not observed when they were co-treated with C781 (Figure 4F – J). Further examination of cytokines in the BALF also supported a changed signalling environment for cell recruitment and airway remodeling. Of the 42 cytokine/secreted signalling molecules examined 24 hrs after the final challenge (Figure 5, Supplemental Figure 1), there were 11 molecules that significantly increased with A. alternata treatment (Figure 5A – K). Nine of these signalling molecules were significantly reduced by co-treatment with C781 (Eotaxin, IL-5, CCL6, GCP-2, KC/GRO, MDC, IL-10, IP-10 and TIMP-1) and two more displayed no significant difference between the A. alternata + C781 group and either control group (IL-4, and IFNγ). The cytokines increased by A. alternata treatment play key roles in eosinophil recruitment (e.g., Figure 4A – C: Eotaxin, IL-5, CCL6); neutrophil recruitment (Figure 4D, E: GCP-2, KC/GRO); T cell helper 2 (TH2) responses (Figure 4F, G: IL-4, MDC), inflammation (Figure 4H – J: IFNγ, IP-10, IL-10) and/or tissue remodeling (Figure 4K: TIMP-1). While C781 alone treatment did induce significant changes in three of the measured cytokines (Figure 4L – N: GM-CSF, IL-6, IL-1α), significant differences between A. alternata ± C781 were not observed. C781 reduced A. alternata-induced lung inflammation and cytokine signalling, maintaining an inflammatory environment consistent with the control group.
Figure 4: C781 limits acute A. alternata-induced inflammation.
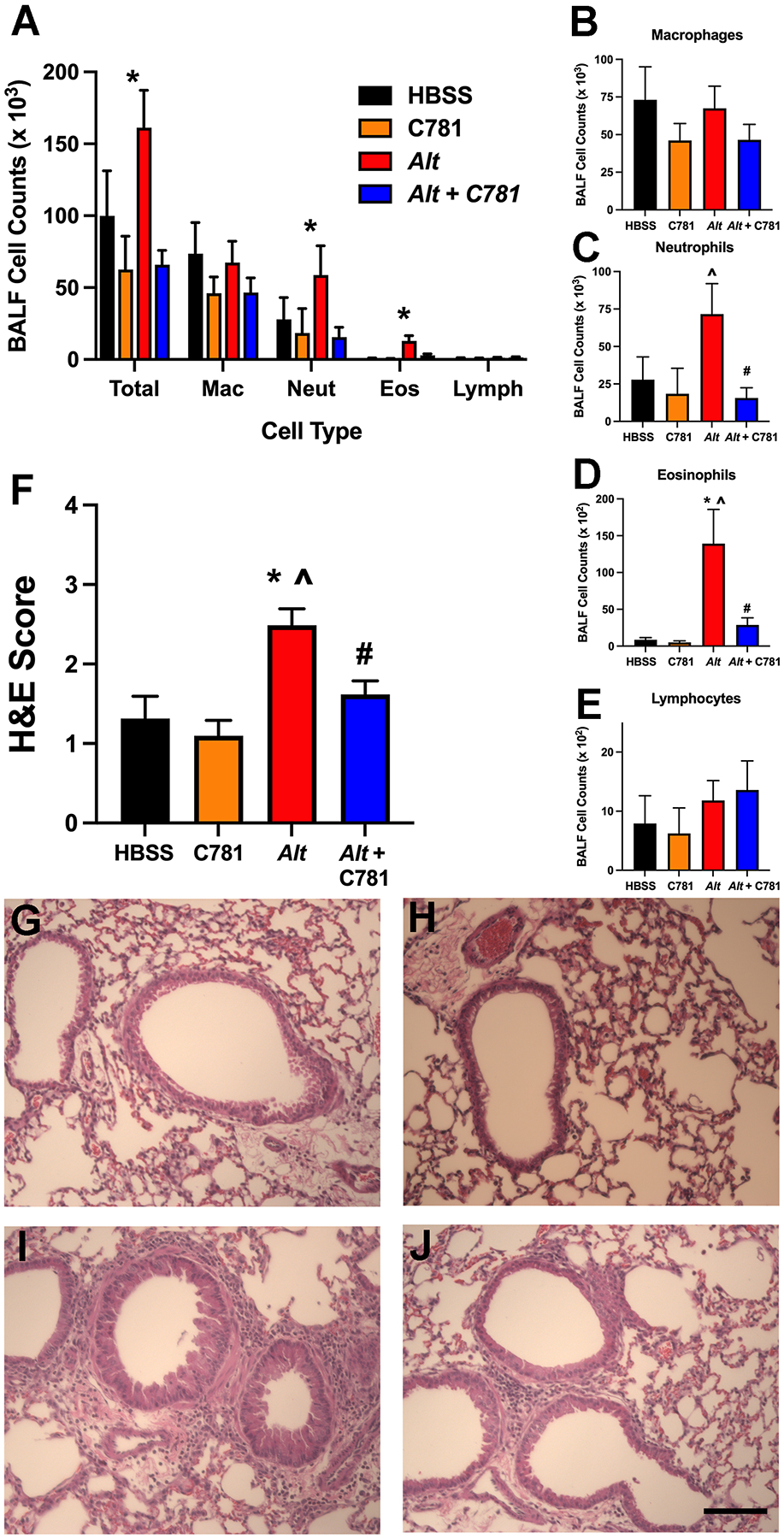
(A) A. alternata (Alt) induced a large number of leukocytes (Total Cells) recovered from the bronchoalveolar lavage (BALF) that was significantly reduced by co-treatment with C781 (Alt + C781). Individual cell graphs (B - E) demonstrate that the observed changes in cell count are driven by significant changes to neutrophils and eosinophils; these cell numbers are reduced by co-treatment with C781. (F) Inflammatory scores on lung sections stained with hematoxylin and eosin (H&E) also show significantly increased inflammation in the Alt- treated animals that was significantly reduced by co-treatment with C781. (G-J) Representative H&E staining for: (G) HBSS control; (H) C781 alone control; (I) Alt alone; and (J) Alt with C781. BALF counts were from 5 animals (HBSS); Histochemistry scores were from 6 (HBSS, C781 and Alt) and 5 (Alt + C781) animals. Bar in J denotes 100 μm. * denotes significant difference between Alt and HBSS control; # denotes significant difference between Alt and Alt + C781; ^ denotes significant difference between Alt and C781 control.
Figure 5: C781 limits acute A. alternata-induced inflammatory cytokine production.
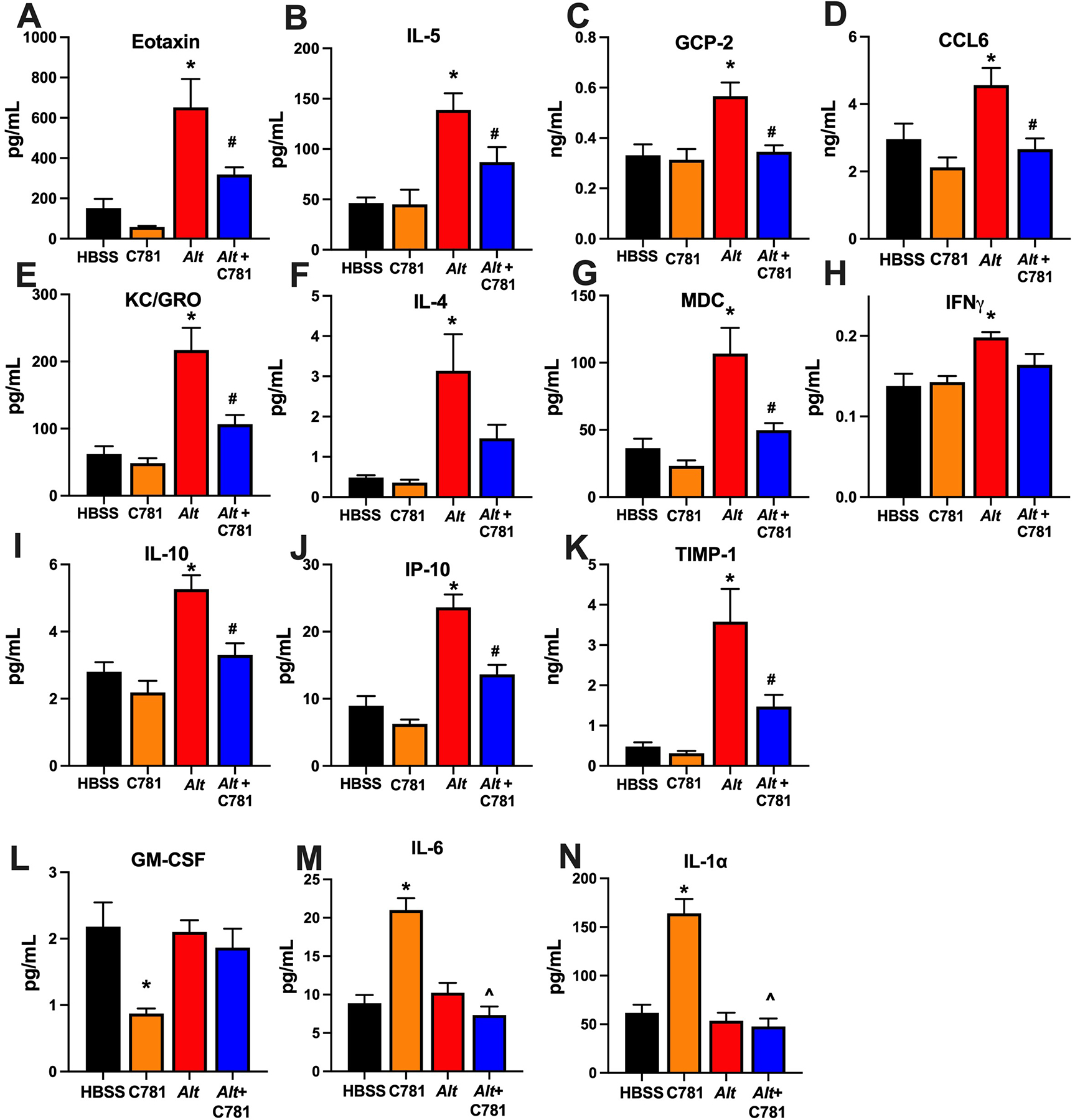
Eleven different cytokines (A - K) were significantly increased in the BALF of A. alternata-treated animals with all but two (F: IL-4, H: IFNγ) significantly reduced by C781 co-treatment. Notably both IL-4 and IFNγ in Alt + C781-treated animals were not significantly different than either control. Altered cytokines are associated with eosinophil recruitment -- (A) Eotaxin, (B) IL-5 and (C) CCL6; neutrophil recruitment -- (D) GCP-2; (E) KC/GRO; TH2 cell responses -- (F) IL-4; (G) MDC; inflammation -- (H) IFNγ, (I) IL-10, and (J) IP-10; and/or tissue remodeling -- (K) TIMP-1. Three inflammatory cytokines (L: GM-CSF, M: IL-6, N: IL1α) were significantly altered by C781 alone treatment, however, this difference was not observed after Alt exposure. Cytokine data was from 5 animals in each group. * denotes significant difference between Alt and HBSS control; # denotes significant difference between Alt and Alt + C781; ^ denotes significant difference between Alt and C781 control.
Mucus staining and mucus cell hyperplasia in the BALB/c mice were evaluated via Periodic Acid Schiff (PAS) staining. Representative images of PAS staining are shown in (Figure 6A – D). Using semi-quantitative scoring, A. alternata treatments increased PAS staining from a limited baseline score observed in the HBSS and C781 controls to a significantly higher mucus score (Figure 6E). Although the inclusion of C781 in the A. alternata treatment resulted in representative images that suggested a reduction in cellular mucus production, scoring of the whole lung sections yielded a similar score to that observed in the A. alternata treatment group (Figure 6E). A closer examination of the conducting airways that lacked cartilage but retained smooth muscle with columnar/cuboidal epithelium revealed the expected increase in mucus score of the A. alternata-treated animals as well as a significant reduction in mucus score between A. alternata alone and A. alternata + C781 (Figure 6F). Therefore, the biased PAR2 antagonist C781 limited A. alternata-induced mucin expression in collapsible airways in this acute exposure model.
Figure 6: C781 has limited effect on acute A. alternata-induced airway epithelial mucin expression.
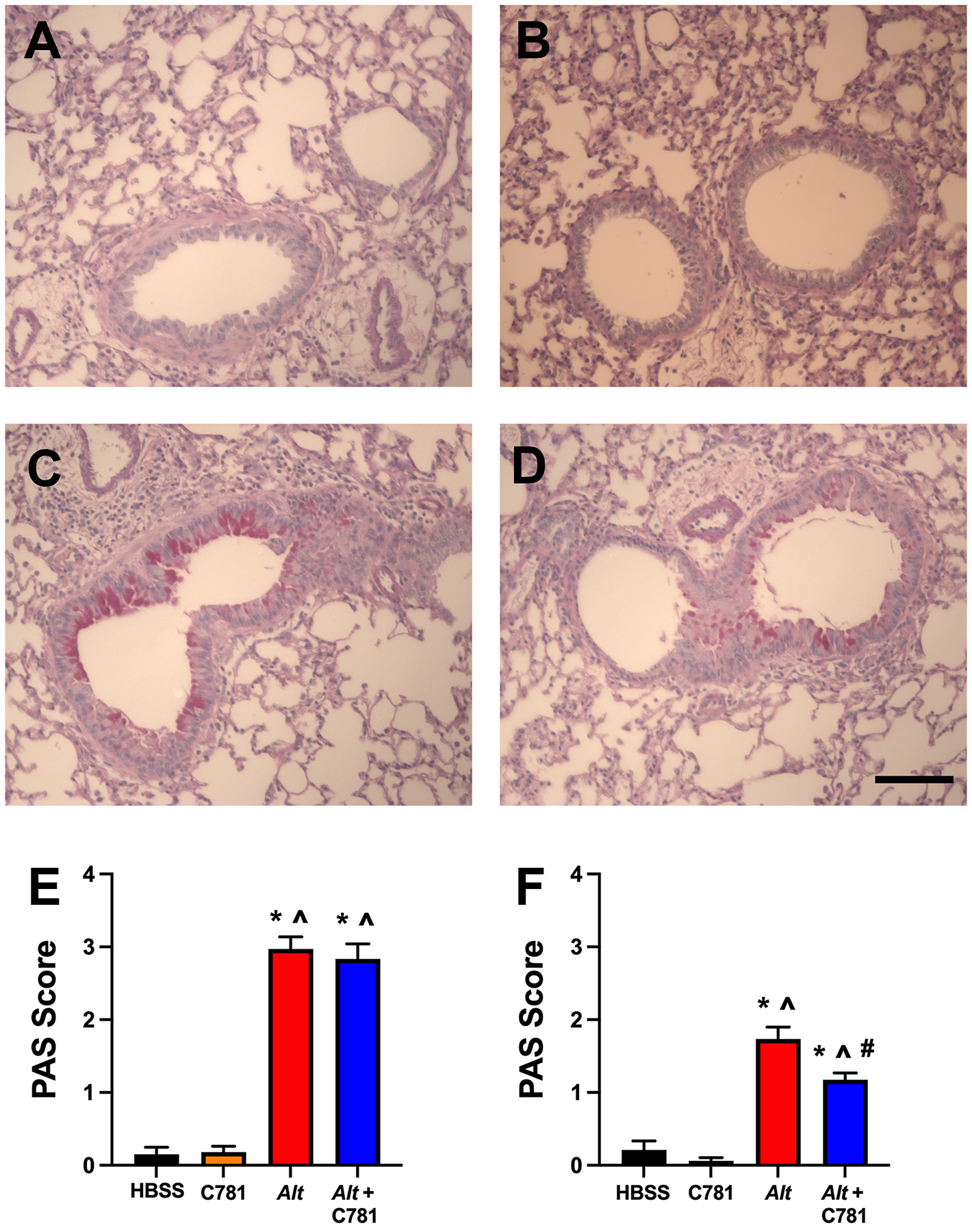
Representative histochemical stain (PAS) micrographs of lung sections showed minimal epithelial mucin staining in (A) HBSS or (B) C781 control images with an increase in staining following (C) A. alternata exposure that appeared to be reduced in (D) A. alternata + C781 exposed animals. (E) Semi-quantitative scoring of PAS-stained lung sections showed significant increases from controls in the A. alternata (Alt) and A. alternata + C781- (Alt + C781) treated animals. (E) Semi-quantitative scoring of collapsible airways in PAS-stained sections showed significant increases from controls (HBSS or C781 alone) in the A. alternata treated animals that was significantly reduced in the presence of C781. Histochemistry were from 6 (HBSS, C781 and Alt) and 5 (Alt + C781) animals. Bar in D denotes 100 μm. * denotes significant difference between Alt and HBSS control; # denotes significant difference between Alt and Alt + C781; ^ denotes significant difference between Alt and C781 control.
DISCUSSION:
We have developed C781, a biased antagonist to PAR2 that selectively blocks β-arrestin/MAPK signalling in naturally expressing PAR2 cells while leaving Gq/Ca2+-signalling downstream of the receptor in these cells intact. The effects of C781 contrast with our published PAR2 antagonist C391, which antagonizes both β-arrestin/MAPK and Gq/Ca2+ signalling, and also has partial biased Gq/Ca2+ agonist activity at high concentrations (Boitano et al., 2015). Consistent with its function as a biased antagonist of this pathway in vitro, C781 is efficacious in vivo in an acute allergen exposure animal model where it specifically reduces allergen-induced airway hyperresponsiveness (AHR), inflammation and, to a limited extent, mucus overproduction. Moreover, as shown in our companion paper, C781 is also efficacious in rodent pain models induced by ligands or proteinases that activate PAR2 in vivo (Kume et al., In Revision). These are the first demonstrations of biased β-arrestin/MAPK signalling antagonism to PAR2 in vitro and in vivo and illustrate their utility for precision medicine. A shortcoming of C781 is that it does not have favorable pharmacokinetic properties for oral or other systemic dosing routes (Kume et al., In Revision). Additional medicinal chemistry optimization will be needed to improve the pharmacokinetic profile for oral delivery. However, this may not be a requirement for further development of inhaled C781 for the treatment of respiratory conditions such as asthma.
PAR2 has emerged as a target for drug development in many inflammatory-associated diseases (Hollenberg et al., 2014; Ramachandran et al., 2012), including asthma [e.g., (Allard et al., 2014; Asaduzzaman et al., 2018; Asaduzzaman et al., 2015; Knight et al., 2001; Nichols et al., 2012; Rivas et al., 2021; Shrestha Palikhe et al., 2015; Snelgrove et al., 2014; Walker & DeFea, 2014; Yee et al., 2018)]. Activation of PAR2 in vivo via specific ligand (Nichols et al., 2012) or isolated allergen proteinase (Yee et al., 2018) is sufficient to induce detrimental physiology associated with asthma including: AHR, inflammation, and mucus overproduction. However, PAR2 activation in the airway has also been associated with bronchorelaxation (Cocks et al., 1999; Henry, 2006; Nichols et al., 2012), a clearly beneficial response for airway obstruction associated with asthma. PAR2 is known to signal through multiple pathways, including the β-arrestin/MAPK and the Gq/Ca2+ pathways, with unique physiological responses for each pathway (Walker & DeFea, 2014). β-arrestin-induced MAPK/ERK signalling, which controls the expression of numerous inflammatory factors that are involved in allergic asthma, is increased in the airway epithelium and smooth muscle cells of asthmatic patients (Liu et al., 2008). Additionally, treatment with a specific MAPK/ERK kinase inhibitor was sufficient to significantly reduced AHR and inflammatory biomarkers in a mouse allergic asthma model (Duan, Chan, Wong, Leung, & Wong, 2004). Recent work from our laboratories with PAR2 and β-arrestin-2 knock out rodent models has demonstrated the detrimental effects induced by PAR2 ligand or acute A. alternata exposure (i.e., AHR, airway inflammation and mucus overproduction) require β-arrestin signalling (Nichols et al., 2012; Yee et al., 2018). While we have shown that a full PAR2 antagonist, C391, was efficacious in preventing A. alternata-induced detrimental asthma indicators (Rivas et al., 2021), we surmised that a PAR2 antagonist specifically targeting the β-arrestin/MAPK signalling pathway would be a step forward in preventing allergen-induced lung compromise. Such a biased antagonist could prevent detrimental effects of acute allergen challenge while preserving the beneficial effects of PAR2 signalling. The reduction in AHR and both cellular and signalling components of inflammation by C781 was in line with this hypothesis. However, that C781 only offered partial protection against mucus overproduction was unexpected. While we cannot be sure why the mucus response did not follow the results in the β-arrestin-2−/− mice, several factors could be at play. First, PAR2 is expressed in a variety of cell types in the lung and it is not clear at this point which lung cells in addition to the epithelial cells that line the airways and produce mucus are susceptible to the inhalation model we used to deliver C781. We do know that the PAR2 agonist ligand 2-furoyl-LIGRL-NH2 can distribute throughout the airways, including the alveoli (Nichols et al., 2012), and the direct effects on tissue damping (G) and tissue elastance (H) shown in the AHR measurements suggest that C781 alters physiology in the lung parenchyma and thus is not restricted to the conducting airways. Second, A. alternata contains many factors independent of PAR2 that can affect the airway (e.g., via epidermal growth factor receptor) and these factors can additionally be enhanced by PAR2 Ca2+ signalling (Daines et al., 2020; Jarry et al., 2007; Nadel, 2001). It is important, however, to note that C781 was effective in reducing A. alternata-induced mucus production in collapsible airways where asthma-associated obstruction can be most damaging.
The finding that the full PAR2 antagonist C391 was able to limit mucus overproduction throughout the airways in the A. alternata sensitization/challenge model (Rivas et al., 2021) but C781 was not suggests that biased antagonism in vivo may not mimic the global reductions that can be achieved with knockout models [e.g., (Nichols et al., 2012; Yee et al., 2018)]. Consistent with this is the success in limiting inflammatory conditions associated with colitis, arthritis and pain in rodent models using the biased PAR2 Gq/Ca2+ signalling antagonist GB88 (Lieu et al., 2016; Lohman, Cotterell, Barry, et al., 2012; Lohman, Cotterell, Suen, et al., 2012; Suen et al., 2012). It will be important to fully characterize the effects of C781 and/or other biased antagonists in PAR2-mediated airway diseases to understand the therapeutic potential of biased signalling PAR2 antagonists like C781 for treatment of airway diseases in humans.
The acute allergen exposure model used herein provided rapid pulmonary responses in the absence of artificial sensitization (e.g., ovalbumin or aluminum hydroxide). The model created similar pulmonary changes to those that are frequently observed in asthmatics (AHR and inflammation) and thus, allowed for evaluation of the prophylactic potential of β-arrestin biased PAR2 antagonism. The A. alternata-induced increase in all features of AHR and subsequent control by C781 are supportive of an intervention that can include the most important physiologic symptom in asthma, obstructive breathing. In this model we also observed inflammatory cellular and cytokine responses associated with allergen-induced asthma (i.e., eosinophil recruitment and Eotaxin, IL-5 and CCL-6 expression) as well as severe asthma (i.e., neutrophil recruitment and GCP2/KC-GRO expression). While the increase in neutrophils and cytokines associated with their recruitment were somewhat unexpected, the significant reduction of both neutrophil and eosinophil recruitment by C781 are suggestive of broad control of inflammation and may help fill a gap in treatments for neutrophilia associated with severe asthma (Yamasaki, Okazaki, & Harada, 2022). We must also note that C781 did alter a trio of cytokines (GM-CSF, IL6 and IL1α) that are associated with a heightened inflammatory environment, suggesting potential off-target effects of PAR2 antagonism, possibly interreacting with endogenous protease activity under non-asthmatic conditions. While this is a concern that will require additional focus as drug development moves forward, it can also be noted that these changes were not sufficient to cause measured changes in AHR, inflammation or mucus overproduction. The acute A. alternata exposure model also has limitations. Notably, the limited exposure time precludes airway compromise prior to allergen challenge and the inflammation lacks a robustness associated with full asthma. Further studies that evaluate PAR2 antagonists in chronic exposure animal models to test efficacy in a compromised airway environment and/or evaluate therapeutic value is the logical next step. Given that PAR2 neutralizing antibodies have shown therapeutic value in a chronic asthma model (Asaduzzaman et al., 2018), and our recent findings that demonstrate C781 can be both prophylactic and therapeutic in pre-clinical models of inflammatory pain (Kume et al., In Revision), the use of chronic asthma models with C781 is an exciting next step.
To our knowledge, C781 is the first example of a biased PAR2-dependent β-arrestin/MAPK antagonist to be tested in vivo and while promising in its ability to prophylactically alter detrimental aspects of acute allergen exposure as a prophylactic, more work is needed to understand the translation from in vitro to in vivo signalling and physiologic outcome. We conclude that C781 exemplifies a new path forward for development of PAR2 antagonists for the treatment of human disease. We propose that this biased antagonist approach improves the chances for achievement of efficacy while avoiding detrimental side-effects. The demonstration of efficacy in mouse models of acute allergen challenge and proteinase-evoked pain provide proof of concept in pre-clinical models to encourage further development of biased PAR2 antagonists.
Supplementary Material
BULLET POINT SUMMARY.
What is already known:
Proteinase-activated receptor-2 (PAR2) knockout models suggest that PAR2 plays an important role in allergen-induced asthma
Full PAR2 antagonists can block the development of acute asthma indicators in animal models
What this study adds
C781 is a novel PAR2 antagonist that blocks β-arrestin/MAPK signalling without effect on Gq/Ca2+ signalling in vitro
C781 can mediate acute allergen-induced airway hyperresponsiveness and lung inflammation in an animal model
Clinical Significance
The biased PAR2 antagonist approach exemplified by C781 targets key aspects of allergen-induced asthma
C781 is an asthma drug lead that can be further developed for clinical testing
Acknowledgements:
This work was funded in part through grants from the National Institute of Health (NINDS NS098826 SB, TJP, JV, GD; NIAID AI140257 SB; HL16024, KAD, SB; ES006694, SB, pilot study; ES025494, ES. CMR is an American Physiological Society William H Townsend Porter Predoctoral Fellow. HVS is an Undergraduate Biology Research Program Student.
Declaration of transparency and scientific rigour:
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research as stated in the BJP guidelines for Design and Analysis, Immunoblotting and Immunochemistry, and Animal Experimentation, and as recommended by funding agencies, publishers and other organisations engaged with supporting research.
Footnotes
Ethics Approval Statement: Appropriate ethical approvals are inserted in the Methods Section.
Conflicts of Interest Disclosure: It is disclosed that SB, TJP, KAD, GS, and JV have patent protection for C781 and are founders of PARMedics, Inc, a company that currently holds a license to the C781 patent. These holdings did not constitute a conflict of interest.
Data Availability:
The data that support the findings of this study are available from the corresponding author upon reasonable request. Some data may not be made available because of privacy or ethical restrictions.
REFERENCES
- Adams MN, Ramachandran R, Yau MK, Suen JY, Fairlie DP, Hollenberg MD, & Hooper JD (2011). Structure, function and pathophysiology of protease activated receptors. Pharmacology & Therapeutics, 130(3), 248–282. doi: 10.1016/j.pharmthera.2011.01.003 [DOI] [PubMed] [Google Scholar]
- Addison KJ, Morse J, Robichaud A, Daines MO, & Ledford JG (2017). A Novel in vivo System to Test Bronchodilators. Journal of infectious pulmonary diseases, 3(1)doi: 10.16966/2470-3176.120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SP, Christopoulos A, Davenport AP, Kelly E, Mathie A, Peters JA, … Ye RD (2021). THE CONCISE GUIDE TO PHARMACOLOGY 2021/22: G protein-coupled receptors. British journal of pharmacology, 178 Suppl 1, S27–S156. doi: 10.1111/bph.15538 [DOI] [PubMed] [Google Scholar]
- Allard B, Bara I, Gilbert G, Carvalho G, Trian T, Ozier A, … Berger P (2014). Protease activated receptor-2 expression and function in asthmatic bronchial smooth muscle. PloS one, 9(2), e86945. doi: 10.1371/journal.pone.0086945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arizmendi NG, Abel M, Mihara K, Davidson C, Polley D, Nadeem A, … Vliagoftis H (2011). Mucosal allergic sensitization to cockroach allergens is dependent on proteinase activity and proteinase-activated receptor-2 activation. Journal of immunology, 186(5), 3164–3172. doi: 10.4049/jimmunol.0903812 [DOI] [PubMed] [Google Scholar]
- Asaduzzaman M, Davidson C, Nahirney D, Fiteih Y, Puttagunta L, & Vliagoftis H (2018). Proteinase-activated receptor-2 blockade inhibits changes seen in a chronic murine asthma model. Allergy, 73(2), 416–420. doi: 10.1111/all.13313 [DOI] [PubMed] [Google Scholar]
- Asaduzzaman M, Nadeem A, Arizmendi N, Davidson C, Nichols HL, Abel M, … Vliagoftis H (2015). Functional inhibition of PAR2 alleviates allergen-induced airway hyperresponsiveness and inflammation. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology, 45(12), 1844–1855. doi: 10.1111/cea.12628 [DOI] [PubMed] [Google Scholar]
- Atienza JM, Yu N, Kirstein SL, Xi B, Wang X, Xu X, & Abassi YA (2006). Dynamic and label-free cell-based assays using the real-time cell electronic sensing system. Assay Drug Dev Technol, 4(5), 597–607. doi: 10.1089/adt.2006.4.597 [DOI] [PubMed] [Google Scholar]
- Boitano S, Flynn AN, Sherwood CL, Schulz SM, Hoffman J, Gruzinova I, & Daines MO (2011). Alternaria alternata serine proteases induce lung inflammation and airway epithelial cell activation via PAR2. American journal of physiology. Lung cellular and molecular physiology, 300(4), L605–614. doi:ajplung.00359.2010 [pii] 10.1152/ajplung.00359.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boitano S, Hoffman J, Flynn AN, Asiedu MN, Tillu DV, Zhang Z, … Price TJ (2015). The novel PAR2 ligand C391 blocks multiple PAR2 signaling pathways in vitro and in vivo. British journal of pharmacology, 172(18), 4535–4545. doi: 10.1111/bph.13238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boitano S, Hoffman J, Tillu DV, Asiedu MN, Zhang Z, Sherwood CL, … Vagner J (2014). Development and Evaluation of Small Peptidomimetic Ligands to Protease-Activated Receptor-2 (PAR2) through the Use of Lipid Tethering. PloS one, 9(6), e99140. doi: 10.1371/journal.pone.0099140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson M, Braddock M, Li Y, Wang J, Xu W, White N, … Colice G (2019). Evaluation of Antibody Properties and Clinically Relevant Immunogenicity, Anaphylaxis, and Hypersensitivity Reactions in Two Phase III Trials of Tralokinumab in Severe, Uncontrolled Asthma. Drug Saf, 42(6), 769–784. doi: 10.1007/s40264-018-00788-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang TW, Wu PC, Hsu CL, & Hung AF (2007). Anti-IgE antibodies for the treatment of IgE-mediated allergic diseases. Advances in immunology, 93, 63–119. doi: 10.1016/S0065-2776(06)93002-8 [DOI] [PubMed] [Google Scholar]
- Cocks TM, Fong B, Chow JM, Anderson GP, Frauman AG, Goldie RG, … Moffatt JD (1999). A protective role for protease-activated receptors in the airways. Nature, 398(6723), 156–160. doi: 10.1038/18223 [DOI] [PubMed] [Google Scholar]
- Crompton G (2006). A brief history of inhaled asthma therapy over the last fifty years. Primary care respiratory journal : journal of the General Practice Airways Group, 15(6), 326–331. doi: 10.1016/j.pcrj.2006.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis MJ, Alexander S, Cirino G, Docherty JR, George CH, Giembycz MA, … Ahluwalia A (2018). Experimental design and analysis and their reporting II: updated and simplified guidance for authors and peer reviewers. British journal of pharmacology, 175(7), 987–993. doi: 10.1111/bph.14153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daines M, Zhu L, Pereira R, Zhou X, Bondy C, Pryor BM, … Chen Y (2020). Alternaria induces airway epithelial cytokine expression independent of protease-activated receptor. Respirology, 25(5), 502–510. doi: 10.1111/resp.13675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson CE, Asaduzzaman M, Arizmendi NG, Polley D, Wu Y, Gordon JR, … Vliagoftis H (2013). Proteinase-activated receptor-2 activation participates in allergic sensitization to house dust mite allergens in a murine model. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology, 43(11), 1274–1285. doi: 10.1111/cea.12185 [DOI] [PubMed] [Google Scholar]
- Day SB, Zhou P, Ledford JR, & Page K (2010). German cockroach frass proteases modulate the innate immune response via activation of protease-activated receptor-2. Journal of innate immunity, 2(5), 495–504. doi:000317195 [pii] 10.1159/000317195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan W, Chan JH, Wong CH, Leung BP, & Wong WS (2004). Anti-inflammatory effects of mitogen-activated protein kinase kinase inhibitor U0126 in an asthma mouse model. Journal of immunology, 172(11), 7053–7059. doi: 10.4049/jimmunol.172.11.7053 [DOI] [PubMed] [Google Scholar]
- Ebeling C, Forsythe P, Ng J, Gordon JR, Hollenberg M, & Vliagoftis H (2005). Proteinase-activated receptor 2 activation in the airways enhances antigen-mediated airway inflammation and airway hyperresponsiveness through different pathways. The Journal of allergy and clinical immunology, 115(3), 623–630. doi:S0091674904031203 [pii] 10.1016/j.jaci.2004.11.042 [DOI] [PubMed] [Google Scholar]
- Flynn AN, Hoffman J, Tillu DV, Sherwood CL, Zhang Z, Patek R, … Boitano S (2013). Development of highly potent protease-activated receptor 2 agonists via synthetic lipid tethering. FASEB journal : official publication of the Federation of American Societies for Experimental Biology, 27(4), 1498–1510. doi: 10.1096/fj.12-217323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn AN, Tillu DV, Asiedu MN, Hoffman J, Vagner J, Price TJ, & Boitano S (2011). The protease-activated receptor-2-specific agonists 2-aminothiazol-4-yl-LIGRL-NH2 and 6-aminonicotinyl-LIGRL-NH2 stimulate multiple signaling pathways to induce physiological responses in vitro and in vivo. The Journal of biological chemistry, 286(21), 19076–19088. doi: 10.1074/jbc.M110.185264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry PJ (2006). The protease-activated receptor2 (PAR2)-prostaglandin E2-prostanoid EP receptor axis: a potential bronchoprotective unit in the respiratory tract? European journal of pharmacology, 533(1–3), 156–170. doi:S0014–2999(05)01380–4 [pii] 10.1016/j.ejphar.2005.12.051 [DOI] [PubMed] [Google Scholar]
- Hoare SRJ, Tewson PH, Quinn AM, & Hughes TE (2020). A kinetic method for measuring agonist efficacy and ligand bias using high resolution biosensors and a kinetic data analysis framework. Scientific reports, 10(1), 1766. doi: 10.1038/s41598-020-58421-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenberg MD, Mihara K, Polley D, Suen JY, Han A, Fairlie DP, & Ramachandran R (2014). Biased signalling and proteinase-activated receptors (PARs): targeting inflammatory disease. British journal of pharmacology, 171(5), 1180–1194. doi: 10.1111/bph.12544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarry A, Dorso L, Gratio V, Forgue-Lafitte ME, Laburthe M, Laboisse CL, & Darmoul D (2007). PAR-2 activation increases human intestinal mucin secretion through EGFR transactivation. Biochem Biophys Res Commun, 364(3), 689–694. doi:S0006–291X(07)02241–3 [pii] 10.1016/j.bbrc.2007.10.073 [DOI] [PubMed] [Google Scholar]
- Katsaounou P, Buhl R, Brusselle G, Pfister P, Martinez R, Wahn U, & Bousquet J (2019). Omalizumab as alternative to chronic use of oral corticosteroids in severe asthma. Respiratory medicine, 150, 51–62. doi: 10.1016/j.rmed.2019.02.003 [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG, & Group, N. C. R. R. G. W. (2010). Animal research: reporting in vivo experiments: the ARRIVE guidelines. British journal of pharmacology, 160(7), 1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight DA, Lim S, Scaffidi AK, Roche N, Chung KF, Stewart GA, & Thompson PJ (2001). Protease-activated receptors in human airways: upregulation of PAR-2 in respiratory epithelium from patients with asthma. The Journal of allergy and clinical immunology, 108(5), 797–803. doi: 10.1067/mai.2001.119025 [DOI] [PubMed] [Google Scholar]
- Kume M, Ahmad A, Shiers S, Burton MD, DeFea KA, Boitano S, … Price TJ (In Revision). Discovery of C781, a biased antagonist at proteinase-activated receptor type-2 (PAR2) – in vivo efficacy against protease-induced pain. [DOI] [PMC free article] [PubMed]
- Lambrecht BN, & Hammad H (2015). The immunology of asthma. Nature immunology, 16(1), 45–56. doi: 10.1038/ni.3049 [DOI] [PubMed] [Google Scholar]
- Lieu T, Savage E, Zhao P, Edgington-Mitchell L, Barlow N, Bron R, … Bunnett NW (2016). Antagonism of the proinflammatory and pronociceptive actions of canonical and biased agonists of protease-activated receptor-2. British journal of pharmacology, 173(18), 2752–2765. doi: 10.1111/bph.13554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Liang Q, Balzar S, Wenzel S, Gorska M, & Alam R (2008). Cell-specific activation profile of extracellular signal-regulated kinase 1/2, Jun N-terminal kinase, and p38 mitogen-activated protein kinases in asthmatic airways. The Journal of allergy and clinical immunology, 121(4), 893–902 e892. doi: 10.1016/j.jaci.2008.02.004 [DOI] [PubMed] [Google Scholar]
- Locksley RM (2010). Asthma and allergic inflammation. Cell, 140(6), 777–783. doi: 10.1016/j.cell.2010.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohman RJ, Cotterell AJ, Barry GD, Liu L, Suen JY, Vesey DA, & Fairlie DP (2012). An antagonist of human protease activated receptor-2 attenuates PAR2 signaling, macrophage activation, mast cell degranulation, and collagen-induced arthritis in rats. FASEB journal : official publication of the Federation of American Societies for Experimental Biology, 26(7), 2877–2887. doi: 10.1096/fj.11-201004 [DOI] [PubMed] [Google Scholar]
- Lohman RJ, Cotterell AJ, Suen J, Liu L, Do AT, Vesey DA, & Fairlie DP (2012). Antagonism of protease-activated receptor 2 protects against experimental colitis. The Journal of pharmacology and experimental therapeutics, 340(2), 256–265. doi: 10.1124/jpet.111.187062 [DOI] [PubMed] [Google Scholar]
- Martinez FD, & Vercelli D (2013). Asthma. Lancet, 382(9901), 1360–1372. doi: 10.1016/S0140-6736(13)61536-6 [DOI] [PubMed] [Google Scholar]
- Menzella F, Lusuardi M, Galeone C, Taddei S, & Zucchi L (2015). Profile of anti-IL-5 mAb mepolizumab in the treatment of severe refractory asthma and hypereosinophilic diseases. J Asthma Allergy, 8, 105–114. doi: 10.2147/JAA.S40244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadel JA (2001). Role of epidermal growth factor receptor activation in regulating mucin synthesis. Respiratory research, 2(2), 85–89. doi: 10.1186/rr43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols HL, Saffeddine M, Theriot BS, Hegde A, Polley D, El-Mays T, … DeFea KA (2012). beta-Arrestin-2 mediates the proinflammatory effects of proteinase-activated receptor-2 in the airway. Proceedings of the National Academy of Sciences of the United States of America, 109(41), 16660–16665. doi: 10.1073/pnas.1208881109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossovskaya VS, & Bunnett NW (2004). Protease-activated receptors: contribution to physiology and disease. Physiol Rev, 84(2), 579–621. doi: 10.1152/physrev.00028.2003 84/2/579 [pii] [DOI] [PubMed] [Google Scholar]
- Page K, Ledford JR, Zhou P, Dienger K, & Wills-Karp M (2010). Mucosal sensitization to German cockroach involves protease-activated receptor-2. Respiratory research, 11, 62. doi:1465-9921-11-62 [pii] 10.1186/1465-9921-11-62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel M, & Shaw D (2015). A review of standard pharmacological therapy for adult asthma - Steps 1 to 5. Chronic respiratory disease, 12(2), 165–176. doi: 10.1177/1479972315573529 [DOI] [PubMed] [Google Scholar]
- Pavord ID, Beasley R, Agusti A, Anderson GP, Bel E, Brusselle G, … Bush A (2018). After asthma: redefining airways diseases. Lancet, 391(10118), 350–400. doi: 10.1016/S0140-6736(17)30879-6 [DOI] [PubMed] [Google Scholar]
- Platts-Mills TA (2015). The allergy epidemics: 1870–2010. The Journal of allergy and clinical immunology, 136(1), 3–13. doi: 10.1016/j.jaci.2015.03.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran R, Noorbakhsh F, Defea K, & Hollenberg MD (2012). Targeting proteinase-activated receptors: therapeutic potential and challenges. Nature reviews. Drug discovery, 11(1), 69–86. doi: 10.1038/nrd3615 [DOI] [PubMed] [Google Scholar]
- Rivas CM, Schiff HV, Moutul A, Khanna R, Kiela PR, Dussor G, … Boitano S (2022). Alternaria alternata-induced airway epithelial signaling and inflammatory responses via protease-activated receptor-2 expression. Biochemical and Biophysical Research Communicationsdoi: 10.1016/j.bbrc.2021.12.090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivas CM, Yee MC, Addison KJ, Lovett M, Pal K, Ledford JG, … Boitano S (2021). Novel proteinase-activated receptor-2 (PAR2) antagonist C391 inhibits Alternaria-induced human airway epithelial signaling in vitro and asthma indicators in acute exposure murine models. British journal of pharmacologydoi: 10.1111/bph.15745 [DOI] [PubMed] [Google Scholar]
- Scott JP, & Peters-Golden M (2013). Antileukotriene agents for the treatment of lung disease. American journal of respiratory and critical care medicine, 188(5), 538–544. doi: 10.1164/rccm.201301-0023PP [DOI] [PubMed] [Google Scholar]
- Shrestha Palikhe N, Nahirney D, Laratta C, Gandhi VD, Vethanayagam D, Bhutani M, … Vliagoftis H (2015). Increased Protease-Activated Receptor-2 (PAR-2) Expression on CD14++CD16+ Peripheral Blood Monocytes of Patients with Severe Asthma. PloS one, 10(12), e0144500. doi: 10.1371/journal.pone.0144500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snelgrove RJ, Gregory LG, Peiro T, Akthar S, Campbell GA, Walker SA, & Lloyd CM (2014). Alternaria-derived serine protease activity drives IL-33 mediated asthma exacerbations. The Journal of allergy and clinical immunology, 583–592. doi: 10.1016/j.jaci.2014.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suen JY, Barry GD, Lohman RJ, Halili MA, Cotterell AJ, Le GT, & Fairlie DP (2012). Modulating human proteinase activated receptor 2 with a novel antagonist (GB88) and agonist (GB110). British journal of pharmacology, 165(5), 1413–1423. doi: 10.1111/j.1476-5381.2011.01610.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thursby-Pelham DC, & Kennedy MC (1958). Prednisolone compared with cortisone in treatment of children with chronic asthma. British medical journal, 1(5065), 243–247. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/13499912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker JK, & DeFea KA (2014). Role for beta-arrestin in mediating paradoxical betaAR and PAR signaling in asthma. Current opinion in pharmacology, 16C, 142–147. doi: 10.1016/j.coph.2014.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi B, Yu N, Wang X, Xu X, & Abassi YA (2008). The application of cell-based label-free technology in drug discovery. Biotechnol J, 3(4), 484–495. doi: 10.1002/biot.200800020 [DOI] [PubMed] [Google Scholar]
- Xiong XF, Zhu M, Wu HX, Fan LL, & Cheng DY (2019). Efficacy and safety of dupilumab for the treatment of uncontrolled asthma: a meta-analysis of randomized clinical trials. Respiratory research, 20(1), 108. doi: 10.1186/s12931-019-1065-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki A, Okazaki R, & Harada T (2022). Neutrophils and Asthma. Diagnostics (Basel), 12(5)doi: 10.3390/diagnostics12051175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee MC, Nichols HL, Polley D, Saifeddine M, Pal K, Lee K, … DeFea KA (2018). Protease-activated Receptor-2 Signaling through beta-Arrestin-2 Mediates Alternaria Alkaline Serine Protease-induced Airway Inflammation. American journal of physiology. Lung cellular and molecular physiology, 315(6), L1042–L1057. doi: 10.1152/ajplung.00196.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request. Some data may not be made available because of privacy or ethical restrictions.


