Abstract
The gold drugs, gold sodium thiomalate (Myocrisin), aurothioglucose (Solganal), and the orally administered auranofin (Ridaura), are utilized in modern medicine for the treatment of inflammatory arthritis including rheumatoid and juvenile arthritis; however, new gold agents have been slow to enter the clinic. Repurposing of auranofin in different disease indications such as cancer, parasitic, and microbial infections in the clinic has provided impetus for the development of new gold complexes for biomedical applications based on unique mechanistic insights differentiated from auranofin. Various chemical methods for the preparation of physiologically stable gold complexes and associated mechanisms have been explored in biomedicine such as therapeutics or chemical probes. In this Review, we discuss the chemistry of next generation gold drugs, which encompasses oxidation states, geometry, ligands, coordination, and organometallic compounds for infectious diseases, cancer, inflammation, and as tools for chemical biology via gold–protein interactions. We will focus on the development of gold agents in biomedicine within the past decade. The Review provides readers with an accessible overview of the utility, development, and mechanism of action of gold-based small molecules to establish context and basis for the thriving resurgence of gold in medicine.
Graphical Abstract

1. INTRODUCTION
Gold (Au)-containing compounds represent an attractive class of therapeutic agents and probes in chemical biology. The clinically approved Au agents for the treatment of rheumatoid arthritis and the rich history of Au in medicine, spanning several millennia, continue to ignite new research avenues toward the development of biologically relevant Au-based compounds. Major developments of Au-based therapeutic agents were reviewed in this Journal in 1999 by Frank Shaw,1 who highlighted key milestones achieved by the development of Au agents. Other significant contributions summarizing specific areas of Au-based biological reagents or mode of action have also been reported.1–11 Over the past two decades, essential aspects of the mechanism of action and new therapeutic Au compounds for different diseases have been unraveled, which will be the focus of this Chemical Review.
Au is characterized by unique chemical and physical properties that influence its reactivity and biocompatibility. Unusual relativistic effects of Au distinguish it from other transition metals including neighboring Group 11 Cu and Ag atoms.12,13 Consequently, Au possesses high ionization potential of ~2 eV due to a large 6s-orbital contraction.14,15 This direct relativistic contraction effect is orchestrated by relativistic perturbation operators that impact the regions of the nucleus and simultaneously affect the density of s-electrons within the valence shell, leading to an increase in the square of the nuclear charge (Z). Although s-contraction and stabilization factors lead to an increased first ionization potential (IP) and electron affinity (EA) for all Group 11 elements, relativistic effects substantially elevate the overall electronegativity (i.e., λIP + λEA) of Au close to that of iodine (EN = 2.2).15 Au is therefore an electronegative transition metal and often referred to as a pseudohalide. The relativistic effects described have implications on atomic, molecular, bonding, and electrochemical behavior of Au that result in its broad utility in biology and medicine. For a more focused work on the relativistic effects of Au in catalysis16 and materials, readers may refer to ref 17.
Over the course of history, dating back to ancient Egypt,18,19 the medicinal value of Au has gained enormous traction, evolved in its synthetic development, and biological utility (Figure 1).20–24 Advanced gold-containing prescriptions in Zixue dan and Zhibao dan exhibit activity to treat high body temperature and measles within the Han and Qing Dynasty of China.23
Figure 1.

Timeline of gold in medicine highlighting key steps toward the development of gold in the clinical setting.
Arnald of Villanova’s discovery of the Aurum potabile recipe to treat melancholy, although imaginary, shed light on gold therapy in the 1300s.25 Further use of this concoction continued through the 17th century, as many proclaimed alchemists fancied the use of Aurum potabile.26–28 One such medical skeptic, Paracelsus, prescribed this gold-based mixture again for the use of melancholy, as it “made one’s heart happy”.29 As the 17th century approached, many medical iconoclasts became skeptical of chemically prepared medicines and touted the use of gold for medicinal applications as dangerous. Nevertheless, gold entered the “Pharmacopeia Londinensis” drug compendia in the 17th century.30,31 Keeley’s proposition to cure alcoholism by gold therapy was not effective. Using sodium salt of gold chloride for the treatment of syphilis advanced development of gold-based therapeutics beyond alchemy in the late 19th century.32 Rational gold therapy came to light with the demonstration of antibacterial activity of gold cyanide K[Au(CN)2] by the German Robert Koch in 1895. Further, Forestier’s discovery that gold complexes exhibit antiarthritic activity brought renewed interest in gold medicine.33–36 All these scientific innovations led to the development of gold thiolate compounds that were developed along with myochrysin, allochrysin, solganal, and sanochrysin (Chart 1).1,37–42 Since then, numerous developments in synthetic strategies have been employed to establish novel gold complexes for a plethora of disease treatments.1,4,5,22,43–49
Chart 1.

Clinically Used Gold Complexes
For improved chrysotherapy (the use of gold salt for treatment of diseases) that is specific for rheumatoid arthritis (RA), Sutton and co-workers first reported the synthesis of 2,3,4,6-tetra-O-acetyl-1-thio--d-glucopyranosato-S-(triethylphosphine) gold (auranofin) in 1972.50,51 The efficacious antiarthritic properties and the oral administration of auranofin led to its approval in 1985 by the FDA.10,52,53 Despite its current use as a second-line therapy for RA, the well-established safety profile in humans makes auranofin a useful candidate for other common and rare diseases in the context of drug repurposing.54–58 Drug repurposing leverages new knowledge from unraveled molecular basis of diseases and FDA approved medication for translational therapeutic benefit.59 Auranofin is a viable drug for repurposing due to its ability to potentiate thiol-related redox homeostasis and lower inflammation. Recent clinical trials outline 14 studies that involve auranofin for the treatment of different diseases including HIV, cancer, pain syndrome, Giardia Protozoa, tuberculosis, and combination therapy for rheumatoid arthritis.10,36,50,53,60–62 These studies cover different clinical phases and across multiple continents (Figure 2). In addition to emphasizing the wide range of therapeutic benefits, auranofin offers decreased costs for discovery of novel medicine, a faster pace of drug discovery and development, and lower attrition rate. Inspired by auranofin’s success, gold-based drug discovery has garnered enormous attention with important contributions, but challenges remain. In this Review, we present recent developments of gold-based therapeutics and probes, mechanisms-of-action, and challenges that need to be addressed as well as innovative chemical strategies to circumvent these challenges toward a fuller biomedical potential.
Figure 2.

Global map of auranofin clinical trial sites.
2. SCOPE OF REVIEW AND ORGANIZATION
The high proliferation of gold-based reagents has led to important biomedical discoveries with diverse mechanisms and those yet to be unraveled. Our discussion will begin with a brief assessment of the mechanism-of-action of gold agents for disease treatment. The goal is to articulate fundamental mechanistic insights for in-depth descriptions during this Review. Au(I) complexes such as auranofin are soft and polarizable with affinity for soft nucleophilic amino acid side chains in proteins. Therefore, stable, and irreversible adducts are a result of Au(I)–protein interactions. We expand this discussion to other gold complexes that interact with proteins to form gold–protein adducts that offer structural and biophysical insights into gold–protein interactions and can lead to understanding cellular mechanisms for disease treatment. Attempts to tune gold compounds to target DNA and DNA-related processes are also expounded. This section is followed by target identification strategies to address potential biological target(s) of gold complexes. We then discuss the impact of gold agents on molecular imaging, radiodiagnostics, and radiotherapy, followed by therapeutic gold compounds that elucidates approved drugs, targeted agents, and mechanisms. We will cover Au(I) complexes and their utility as potential drugs in different diseases. Here, idiosyncratic mechanisms that result in treatment in vitro and preclinical models will be addressed. The burgeoning field of Au(III) for disease treatment will also be discussed along with potential biological targets that have been elucidated to date. Finally, we will address targeting modalities and nanodelivery approaches of biologically active gold compounds.
3. MECHANISM OF ACTION
The main mechanism of gold action has been a subject of scrutiny over many decades by scientists of multidisciplinary backgrounds. Recent advances in Cryo-electron microscopy, crystallography, bioorthogonal chemistry, affinity labeling, and chemical proteomics have led to target identification and an unbiased mode of action,63–69 which is sometimes enigmatic. The polarized character of Au(I) complexes renders them highly thio- and seleno-philic. Thus, enzymes with cysteine and selenocysteine residues within the active site are favorable targets for gold ligation. Readers may refer to other comprehensive reviews and perspectives on gold–sulfur interactions.70–73
An earlier report on the uptake of auranofin used the everted sac model of intestinal absorption. Auranofin was incubated with the everted sac, and the gold concentration in the sac after 2 h of incubation was found to be about 20% of the incubation media showing that gold passes through the intestinal wall, although this study suggests that it is the deacetylated form of auranofin that passes through the wall and not auranofin itself.74 Another study indicates that rather than through a transmucosal absorption, auranofin is absorbed via the enteric cell surface.75 The entry of auranofin in cells is by interaction with the phospholipid bilayer largely through a passive uptake profile.76 Active transport by interrogating ion channels and membrane proteins remains a possibility but unexplored. Snyder et al. proposed a ligand exchange shuttle mechanism that is different from the traditional active or passive transport for uptake of auranofin and other gold-based complexes. This model proposes that uptake is dependent on ligand selectivity for thiol groups based on their relative affinities, lipophilicity, charge, and steric factors.77 Within the cell, auranofin interacts with oxidoreductases (redox enzymes) including thioredoxin reductase (TrxR) and trypanothione reductase through substitution by cysteine or selenocysteine amino acid residues within the enzyme active site.78,79 Structural evidence by protein X-ray crystallography demonstrates that the linear geometry of auranofin allows for the displacement of the thioglucose and triethylphosphine moieties by the nucleophilic sulfhydryl groups (Figure 3).80–83 Whereas active site cysteines have been the generally accepted binding site for auranofin to confer its inhibitory activity to TrxR function, X-ray structures of Entamoeba histolytica TrxR (EhTrxR) reveal a noncatalytic Au(I) binding site at Cys286 with low affinity with no interaction with active site Cys140–Cys143 redox center.80 This is indicative of a resolute disulfide bond formation that precludes Au(I) binding even in the presence of reducing agents. Conceivably, reactivity of cysteines at the active sites of TrxR differs based on molecular weight, proximity of cysteines for disulfide formation, and the size of the catalytic motif, CXXC for EhTrxR.80 These structural insights point to mechanistic differences in the inhibition of TrxR by auranofin and other linear Au(I) complexes.
Figure 3.

Crystal structure of Au(I)–protein adduct: (a) Au(I)–EhTrxR adduct (PDB code: 4A65, gold source: AuCN), (b) Au(I)–EhTrxR adduct (PDB code: 4CBQ, gold source: auranofin).80
Given the essential role of redox homeostasis in physiological and pathophysiological conditions, modulating redox enzymes such as TrxR via the formation of stable and irreversible adducts has enormous consequences for several cellular processes and regulating intracellular reactive oxygen species (ROS).84–86 In cells that overexpress TrxR, such as parasites, cancer cells, and memory T cells, inhibition of the redox enzyme resulting in oxidative stress and eventually apoptotic cell death is therapeutically beneficial.
The discovery of relatively stable Au(III) complexes for biological application has allowed for variable ligand modification of the d8 Au(III) system, which often takes on a square planar geometry.87–92 Recent advances in omics technology, spectroscopy, and chemical biology are revolutionizing the target identification toolbox to support Au(III) mechanism of action.93,94 It has become obvious that proteins, which are the largest component of biomolecular systems in biology, are the primary target of gold-based drugs.95,96 With a few exceptions to be discussed, Au(III) complexes target proteins beyond TrxR.93,94,97–99 Au(III) complexes are relatively harder than Au(I) complexes, and ligand tuning has direct effects on biological target as well as mechanism. Passive diffusion across the plasma membrane remains the dominant transport pathway for Au(III) systems. The pathway of intracellular uptake of Au(III) complexes can be characterized more broadly under ATP-independent endocytosis and micropinocytosis processes. Active transport mechanisms of Au(III) complexes are yet to be unraveled in detail. Once in cells, Au(III) can remain intact until it reaches its biological target or can be reduced by biological nucleophiles such as glutathione (L-GSH), ascorbate to Au(I) for biological action in a prodrug format.100–102 Au(III) complexes with distinct structural scaffolds induce different mechanisms of action in cells. Au(III) porphyrins target the mitochondrial heat shock protein 60,103,104 whereas Au(III) mesoporphyrin IX target cysteine thiol containing proteins, thioredoxin, deubiquitinase, and heat shock protein 90 via an arylation of the meso carbon and sulfur atom of cysteine in a C–S bond formation (Chart 2).105 The proteasome, endoplasmic reticulum, and mitochondria are attractive targets of Au(III) complexes, providing a broad range of mechanisms of Au(III) action.89,93,100–102,106–109 The peculiar mechanistic detail will be discussed in the context of diseases within the ensuing sections of this Review.
Chart 2.

Schematic Reaction for Bioconjugation of meso-Unsubstituted Gold(III) Porphyrins with GSH under Physiological Conditions
3.1. Structural Basis for Gold–Protein Complexes
Significant progress has been achieved over the past two decades in elucidating gold–protein interactions, ranging from EXAFS, Mossbauer, NMR, and ESI-MS to X-ray crystallography data.71,73,110–117 New crystallographic information is beginning to shift our understanding of the affinity of gold for nitrogen ligands juxtaposed to the conventional sulfur and selenium ligands. Here we offer selected examples. A detailed structural analysis of gold–protein adducts was reviewed by Messori et al.118 Using X-ray crystallography, gold adducts at distinct histone sites of nucleosome core particles (NCP) using auranofin can be elucidated.119 The NCP-gold adduct reveals two-symmetry-related locations, Au1 and Au1′ along the 2-fold axis of the nucleosome and with good proximal distance from the central base. Whereas the sugar thiolate groups were substituted by the histone ligand through the histidine delta nitrogen side chains, the triethyl phosphine groups make hydrophobic interactions with surrounding H3 residues. It must be noted that the requirement for NCP-gold adduct formation is the presence of both RAPTA-T and auranofin in the NCP treatments that allows for RAPTA-T adducts to promote reactivity of the H3/H3′ H113 sites (Figure 4).119 This study adds to the knowledge of Au-histidine binding but more importantly reveals an allosteric phenomenon upon drug binding to the nucleosome acidic patch, which is a chromatin binding hotspot and may be relevant for in vivo genomic regulation and histone posttranslational modifications.119
Figure 4.

X-ray crystal structure of RAPTA-T/auranofin-nucleosome core particle (NCP). Structure reveals auranofin and RAPTA-T adduct sites. NCP is depicted on the left and zoomed adduct site displayed on the right. Gold atom (gold) bearing triethylphosphine (PEt3) bound to His113 (PDB: 5DNN, gold source: auranofin).
Metallo--lactamases (MBL) and mobilized colistin resistance (MCR) expressing Gram-negative bacteria pose a major threat to human health due their role in antibiotic resistance. To resensitize carbapenem- and colistin-resistant bacteria to antibiotics, auranofin was identified as a dual inhibitor of MBLs and MCRs.120 Enzyme activity shows that auranofin inhibits the clinically relevant New Delhi metallo--lactamase 1 (NDM-1) and MCR-1 catalysis to boost antibiotic action. Structural insights revealed that Au(I) binds NDM-1 (PDB: 6LHE) in the active site by Zn(II) displacement with two Au ions. One ion, Au282 tetrahedrally coordinates Cys208, His250, Asp124 and water molecule (w291), and Au283 tetrahedrally coordinates His122, His 120, His 189, and a water molecule (w410) (Figure 5). The Au–Au contacts possess a distance of ~3.8 Å. A remote Au ion located at the interface of two protein monomers was found to coordinate Asp223, Glu152, water molecule, and a Glu227 from a neighboring NDM-1 molecule in a distorted tetrahedral geometry.
Figure 5.

Crystal structure of the active site of Au-NDM-1 (PDB ID: 6LHE, gold source: auranofin) displaying Au ions as yellow spheres and omitting water molecules that contribute to a tetrahedral geometry. Annotated amino acid side chains within the protein active site are depicted in cyan with distinctly colored heteroatoms (N, blue; O, red; S, yellow).
Furthermore, the Au-bound MCR-1-S crystal structure (PDB: 6LI6) demonstrates Au ion displacement of Zn(II) in the catalytic site by coordinating to Glu246, Asp465, His466, and TPO285 in a distorted geometry (Figure 6). Two other Au ions coordinate to His252 or His 424 and PEt3 group or water molecule in a linear/quasi-linear geometry, respectively. Interestingly, the displacement of metal ions, e.g., Zn within the catalytic core of enzymes by Au, is a common phenomenon exhibited for enzyme inhibition.120
Figure 6.

Crystal structure of the active site of Au-MCR-1 (PDB ID: 6LI6, gold source: PEt3AuCl) displaying Au ions as yellow spheres. Annotated amino acid side chains within the protein active site is depicted in cyan with distinctly colored heteroatoms (N, blue; O, red; S, yellow). Triethylphosphine ligand is shown as green (C atoms) and orange (P atom).
Despite the incredible information obtained from X-ray crystallography to elucidate gold–protein interactions, the ability to design ligands to predict gold complex reactivity, Au compound recognition, and potential binding sites in proteins using computational aided drug design (CADD) still require dynamic evolution of transition metal parametrization in computational software as well as extensive experimentation to obtain guiding rules. Whereas this is an existential bottleneck, it presents opportunities for inorganic chemists, structural biologists, and computational chemists to work together in addressing the issue to advance the field. We predict that the modern era of artificial intelligence (AI) will facilitate the rapid identification of Au-based ligands with affinity for specific proteins.
3.2. DNA-Targeting Gold Compounds
DNA is a formidable molecular target for many drugs approved by the Food and Drug Administration (FDA), primarily for the treatment of cancer.121–126 Despite the nonspecific cytotoxic character of traditional chemotherapeutics, modern drug discovery has promoted selective agents that target DNA and associated DNA processes. Alkylating agents nondiscriminately interact with DNA and often covalently in the form of cross-links. Cisplatin, the platinum(II) antitumor drug that shares isoelectronic similarity with Au(III), was the first transition metal-based drug to be approved for the treatment of cancer. Following the serendipitous finding by Rosenberg and colleagues during an investigation of the effect of electric field on bacteria cell division,127,128 extensive work into the mechanism of cisplatin and next generation Pt(II) drugs, carboplatin and oxaliplatin, demonstrate formation of Pt-DNA cross-links as lethal complexes that lead to apoptosis.129–134 These metal agents have been remarkably transformative in the clinic against several cancers including testicular, bladder, lung, ovarian, breast, cervical, and brain tumors.61,135–137 However, toxic side effects and drug resistance are limiting concerns.138–140 Another class of agents target protein–DNA complexes with somewhat precise sequence selectivity. Agents designed to target the minor and major grooves of DNA such as polyamides and triplex forming compounds showed promise as chemotherapeutic agents. Compounds targeting secondary structures such as G-quadruplexes were particularly valuable in interrogating telomeres and transcriptional elements.2
3.2.1. Au(I) Complexes Targets Calf Thymus-DNA.
Gold(I) complexes with thiosemicarbazones ligands have been reported to interact with DNA (Chart 3). The complexes interact with calf thymus DNA (ctDNA) when incubated at different concentrations with changes in electronic transitions observed with UV–vis between 250–400 nm. The binding constant of gold complexes to ct-DNA was calculated to be within 6.26 × 104–4.42 × 106 M−1, indicating strong binding. Furthermore, competitive binding assay between the complexes at different concentration from 0–100 μM and ethidium bromide/ctDNA was used to confirm binding. Emission fluorescence shows a decrease in emission intensity and displacement of the ethidium/DNA adduct after the addition of the gold(I) complex. This is due to competition between the gold(I) derivative and ethidium bromide for binding to the DNA groove.141
Chart 3.

Chemical Structure of Au(I) Thiosemicarbazones
3.2.2. Au(III) Complexes as DNA Intercalator.
Nuclear enzymes such as the monomeric human Topoisomerase IB (TOP1) and Topoisomerase are crucial regulators of DNA topology for the orchestration of important cellular processes including DNA replication, gene transcription, and cell division.142,143 These enzymes function by inducing transient single-strand (type I) or double-strand (type II) breaks in the DNA helical structure. Despite the relatively short half-life of these enzyme–DNA complexes in vivo, they represent a viable molecular target in cancer drug discovery.144
There are two classifications of compounds targeting TOP1. First, compounds known as interfacial poisons (IFPs) interfere with enzyme–DNA complexes to prevent plausible religation of DNA. This is achieved by a noncatalytic binding of DNA–intercalating IFPs at nicked sites enzyme–DNA cleavage complexes, thereby poisoning the TOP1 enzyme. Camptothecin and indenoisoquinolines represent important examples of this class.145–148 Second, catalytic inhibitor compounds (CICs) block two crucial catalytic steps through (i) competitive inhibitor binding to Top1 or competitive binding to DNA and (ii) step 2 catalytic inhibitors (Figure 7). CICs generally convey reduced genotoxicity but are uncommon,149,150 thus raising the need for novel compounds. Au(III) compounds generally inhibit TOP1 catalytically; however, it is critical to note that inhibition of supercoiled DNA relaxation by TOP1 is not the only parameter to delineate CICs from IFPs. A novel class of pyrrole-containing Au(III) macrocycles was identified as CICs of human TOP1 and (Figure 7). The d8 complexes exist in a square planar geometry with an aromatic quinoxaline backbone that facilitate DNA intercalation with binding affinities in the low micromolar range using standard competitive displacement of DNA intercalator, ethidium bromide assay. These Au(III) macrocycles exhibit cytotoxic potency across National Cancer Institute (NCI, USA)-60 human cancer cell lines.151 The Au(III) ion plays a critical role in DNA intercalation and concomitant inhibition of TopI and TopIIa using purified DNA and enzyme.
Figure 7.

(A) Schematic representation showing the important events in the catalytic cycle of the human Topoisomerase IB (TOP1) enzyme. Detailed step by step description of the catalytic process is given in ref 151. (B) General chemical structure and derivatives of Au(III) macrocycles. Reproduced from ref 151. Copyright 2014 American Chemical Society.
3.2.2.1. Au(III) Macrocycles.
Macrocyclic Au(III) compounds containing pyrrolic fragments have demonstrated CIC and DNA intercalating properties. Studies with meso-tetraarylporphyrins (Chart 4) revealed significant interaction with duplex DNA and an intrinsic binding constant of Kb = 2.79 + 0.34 × 106 dm3 mol−1 with calf thymus DNA.152 A series of tetraarylporphyrin Au(III) complexes were prepared, bearing different substituents on the meso-aryl ring including glycosyl and methoxyphenyl groups. The complexes bind to DNA in absorption titration assays in the range 4.9 × 105–4.1 × 106 dm3 mol−1 and act as intercalators of DNA. Inhibition of Top1 by these compounds is by inducing supercoiled DNA relaxation. Additionally, in a polymerase chain reaction stop assay, Au(III) porphyrins inhibit amplification of DNA substrates with G-quadruplex structures.153
Chart 4.

Chemical Structures of DNA Interfering Substituted Au(III) Tetraphenylporphyrin
3.2.2.2. Au(III)-N-Heterocyclic Carbenes.
Another class of stable organometallic Au(III) compounds of the type, [Aun(R–ĈNĈ)n(NHC)]n+ (Chart 5) were synthesized and displayed interaction with DNA as well as in vitro and in vivo anticancer activity.154,155 The complexes interact with DNA with a binding constant of 4.5 × 105–5.3 ± 0.8 × 105 dm3 mol−1 at 298 K toward ctDNA. Further characterization reveals retardation of 123-bp DNA ladder mobility in gel-mobility-shift-assay. The complex inhibits Top1-mediated DNA relaxation at lower concentrations than the well characterized CPT. Detailed studies show that the complex is a catalytic inhibitor that inhibits the topoisomerase I cleavage reaction by preventing DNA substrate binding.
Chart 5.
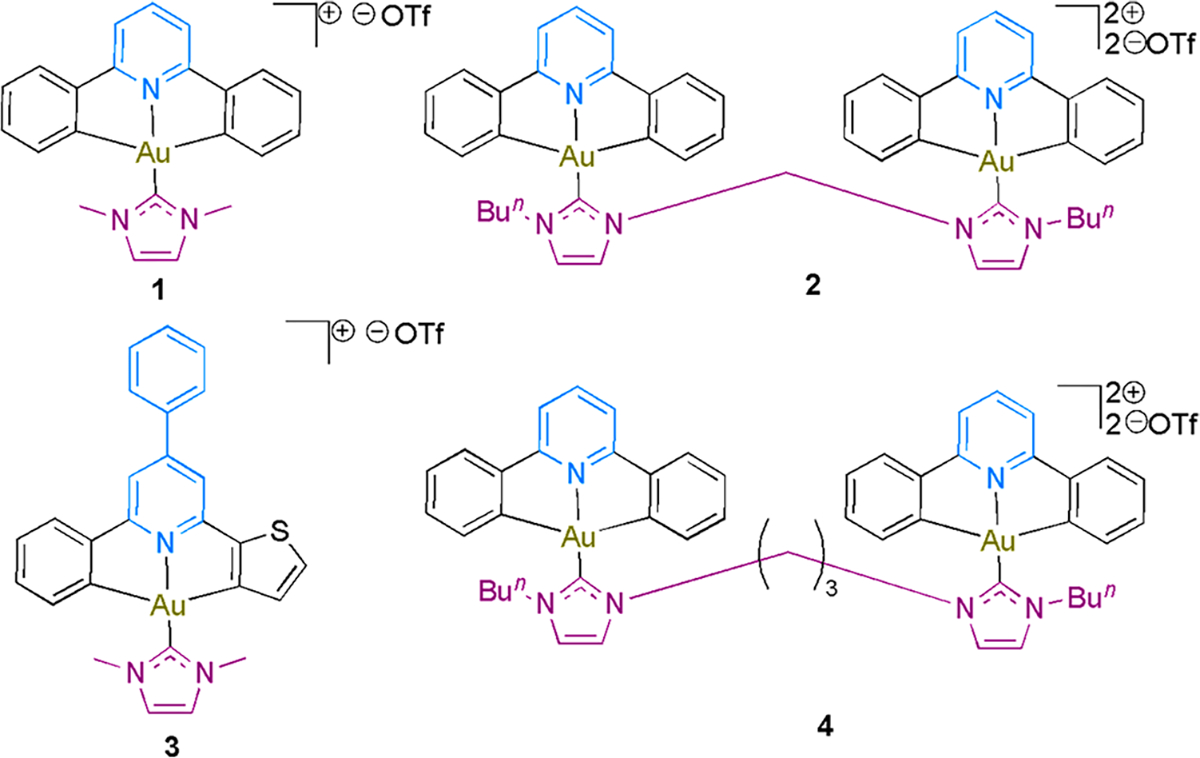
Chemical Structures of [Aun(R–ĈNĈ)n(NHC)]n+ as Inhibitors of TopI
3.2.2.3. Gold(III) Pyridyl and Isoquinolyl Complexes.
Although guiding principles for the design of gold-based agents to target DNA-associated elements appear elusive, the use of nitrogen-containing heterocycles with sufficient planarity fosters interaction with DNA and inhibition of Top1 and Top2 enzymes. The synthesis of pyridyl- and isoquinolylamido complexes of Au(III) contributes to our understanding of the affinity of gold complexes to DNA and consequent inhibition of topoisomerase (Chart 6).156 We briefly noted, with regard to the discussion above, that cationic Au(III) porphyrins revealed interaction with DNA, thus variation of multidentate amido complexes of cationic character may potentially act as cytotoxic DNA intercalators. Pyridyl or isoquinolyl ligands of the type H2Ln react with Au(III) salts to afford neutral pyridyl or isoquinolyl amido-dichloro gold(III) complexes. Perhaps the use of a base in the reaction may lead to the formation of cationic tetradentate AN2N′2 trischelates of gold toward cytotoxic complexes consistent with cationic Au(III) porphyrins. The Au(III) pyridyl- and isoquinolylamido chelates are square planar in character with the cis-Cl ions coordinated amido chelating moiety in a trans fashion. The soft, polarizable nature of gold157 facilitates covalent interaction with soft thiol or selenothiol nucleophiles compared to nitrogen nucleophiles under biological conditions. This limits DNA alkylation by gold complexes at the nucleophilic 7N-guanine of DNA. Thus, N-donor ligands promote overall gold complex stability and their planarity dictate DNA intercalation.
Chart 6.

Chemical Structures of Pyridyl and Isoquinolylamido Au(III) Complexes
3.2.2.4. Gold(III) Biscarbene Complexes.
Another class of DNA targeted gold(III) complexes consist of bis(carbene) pincer type ligand supported by a carbazole framework for gold chelation (Chart 7). The complex follows the prototypical [AuIII(CNC)Cl]+ archetype with distinct aromatic planarity and redox stability. Although these gold(III) pincer complexes form adducts with L-glutathione, their DNA binding affinity is a magnitude larger than the well-characterized DNA intercalator psoralen at a KA of 3.7(3) × 104 M−1 when ctDNA (37 °C, pH 7.4 Tris-HCl buffer) is used. It is possible that these complexes target DNA 3-way junctions, B- and Z-DNA forms. Theoretical insights show hydrophobic p-type interaction of T and A bases as well as phosphate O–Au interaction underlying B-form DNA binding and Z-DNA binding, respectively.158
Chart 7.

Chemical Structures of DNA Targeting Au(III) Pincer Complexes Supported by Carbazole Bis-carbene Ligands
4. TARGET IDENTIFICATION OF GOLD COMPLEXES
Proteins are the main targets of gold-derived bioactive compounds;159–161 however, unbiased, system-wide approaches to examine protein activity and function remain underexplored in metal-based drug discovery. New molecular biology methods combined with recent developments in sequencing and mass spectrometry-based proteomics have contributed to deciphering biological target modulation by gold complexes and associated disease phenotypes (Figure 8). In this section, we discuss recent advances in chemical biology and omics technologies that are and could revolutionize the chemical and analytical toolbox available to drive gold-based drug/probe discovery. We also shed light on the application of these analytical technologies to identify potential targets of gold complexes and mechanism of action.
Figure 8.

General schemes for affinity-based target identification and activity-based protein profiling.
Classical proteomic tools are available to profile gold complexes in cells. The pipeline involves 2-D gel electrophoresis for protein separation of lysed treated or untreated cells on an SDS-PAGE gel followed by gel staining and imaging, electrophoretic spot excision, mass spectrometry and protein identification, bioinformatic functional analysis to characterize differentially expressed genes or proteins, and validation by 2-D Western blot of selected targets and subsequent functional assays. This approach was used to characterize the potential mechanism of the organometallic Au(III) antiproliferative agent, Aubipyc, in ovarian cancer cells (A2780 and A2780 CDDP) following a 24 h incubation with the Au complex at a 10 μM concentration.162,163 Inhibition of proteins in the glycolytic pathway including GAPDH, ENO1, PKM, PGK1, ALDOC, and LDHB by Aubipyc demonstrates a promising approach for gold mechanism of action despite limitations of laborious sample preparation, high throughput, and batch-to-batch variability (Figure 9).163
Figure 9.

Classical proteomics strategy to study drug action by gel electrophoresis, mass spectrometry, bioinformatics, and validation of the organometallic Au(III), Aubipyc. Reproduced from ref 162. Copyright 2015 Royal Society of Chemistry.
Au probe-based target deconvolution is emerging as an attractive technique for target identification of bioactive Au compounds. The strategy is based on derivatization of the Au compound with affinity tags such as biotin for affinity enrichment or reactive group to facilitate immobilization to a solid support such as Sepharose beads. Combining affinity enrichment with mass spectrometry-based proteomics provides improved sensitivity, resolution, and specificity for profiling the whole cellular proteome. For details on mass spectrometry methods in drug discovery, readers may refer to recent reviews that may be applicable to gold-derived bioactive complexes.164–167 The described target identification approach is well-known as compound-centric chemoproteomics.168 Specificity can be largely enhanced by derivatizing Au compounds with reactive handles such as diazirines, aromatic azides, and benzophenones that covalently modify protein targets through photoaffinity labeling.93,104,169–172 Modular probe development using click chemistry tools can streamline synthetic efforts, whereas providing versatility with regards to tools for analyzing Au complex localization, target identification and mechanism of action.
Implementing competition-binding experiments using the parent or unmodified Au agent is a robust way to verify candidate pull down targets when using the Au probe-based target deconvolution method for Au target identification with integrated scientific rigor.
Cellular thermal shift assay (CETSA)173 can be used as a profiling tool for gold-based drug discovery when coupled with proteome-wide MS. Current use of CETSA in the context of gold-derived complexes has been limited to validating identified targets by assessing thermal protein stability changes via Western blot. This is achieved by incubating cells with a desired Au agent or vehicle, followed by cell lysis and heat across a range of temperatures. After centrifugation, the soluble fractions are subjected to gel electrophoresis and incubation with intended protein target antibodies. Direct Au complex-target engagement induces thermal stability changes to deconvolute protein targets. A major advantage with CETSA is that it does not require functionalized bioactive Au complexes, which can be difficult to develop. We posit that combining quantitative MS and CETSA in gold-based thermal proteome profiling will be a powerful analytical strategy to decipher the MoA of Au drug candidates or chemical probes. The ability to integrate 2D thermal proteome profiling will merge temperature dependent and isothermal ligand concentration-dependent studies to address prevailing false negatives associated with CETSA-MS.
The use of a clickable photoaffinity probe of a Au(III)-porphyrin complex enabled the isolation of protein binding partners, notably, heat-shock protein 60 (Hsp60). Synthesis of the probe followed tethering a hexaethylene glycol linker, clickable alkyne tag and a benzophenone photoaffinity tag to the meso-phenyl rings of the porphyrin ligand of the Au(III)-porphyrin complex (Chart 8). Photoaffinity labeling was performed by incubating cancer cells with Au(III) Probe-1 followed by UV irradiation, after which click reaction with azide-conjugated biotin was carried out. Competition experiments using the parent Au(III)-porphyrin complex showed a diminished signal of the photoaffinity-labeled protein. Subsequent click reaction using azide-conjugated Cy5 in cell lysates followed by 2D gel electrophoresis, fluorescence scanning, and MALDI-TOF/TOF MS revealed Hsp60 as the potential target. Using quantitative proteomics by stable isotope labeling by amino acids in cell culture (SILAC) and subsequent affinity isolation of biotinylated proteins and LC-MS/MS analysis using orbitrap confirmed Hsp60 identification. Additional validation of Hsp60 as the target of Au(III)-porphyrin complex was confirmed by CETSA.103
Chart 8.

Benzophenone Photoaffinity Tag Au(III)-Porphyrin Probe
Leveraging the reactivity of the meso-carbon of mesoporphyrin IX, Che and co-workers described the formation of C–S bond formation as a covalent modification strategy to target thiol containing proteins in cancer cells. The use of Au(III) mesoporphyrin IX dimethyl ester facilitates nucleophilic aromatic substitution with cysteine thiols such as thioredoxin. Other targets such as peroxiredoxin III (PRDX3) and deubiquitinase, UCHL3 were identified via thermal proteome profiling mass spectrometry and CETSA. This study highlights the value of new orthogonal approaches for target identification.174
Recently, Awuah et al. used azide–alkyne functionalization of in Au(III) complex for post-treatment click modification to enable localization and mechanism of action studies (Figure 10). Post-treatment fluorescent labeling is achieved by the initial exposure of cancer cells to alkyne functionalized P-chirogenic Au(III) followed by the biorthogonal Cu(I)-catalyzed azide–alkyne cycloaddition (CuAAC) reaction using an azide-tagged FITC fluorophore. Co-localization studies using Mito Tracker red demonstrate predominant mitochondria localization.175
Figure 10.

(a) P-chirogenic Au(III) molecule (AuPhos-19) and the alkyne functionalized probe (AuPhos-19-AP). (b) Assessment of cell viability in MDA-468 cells treated with parent molecule (AuPhos-19) versus AuPhos-19-AP. (c) Representation and result of biorthogonal Cu(I)-catalyzed azide–alkyne cycloaddition (CuAAC) reaction using an azide-tagged FITC fluorophore. Reproduced with permission from ref 175. Copyright 2022 Elsevier.
Another class of stable Au(III) complexes used as anticancer agents includes the tridentate ĈNĈ, ĈN^N, or NĈ^N carbon donor pincer or NHC ligands.176 Mechanistic studies of these complexes have largely been accelerated by the use of photoaffinity, fluorescent, and affinity labeling probes of the parent Au complexes (Chart 9) that allow for pull downs, chemoproteomics, and fluorescence imaging toward protein target engagement.176
Chart 9.

Chemical Structures of Some Au(III)-NHC Probes
The use of activity-based protein profiling (ABPP) to identify binding sites of Au complexes within the proteome is a suitable methodology to identify new druggable targets, uncover elusive targets, decipher new mechanisms, and generate broad reactive protein maps in different living species. Isotopic tandem orthogonal proteolysis-ABPP (isoTOP-ABPP) uses an amino acid residue specific (e.g., cysteine) reactive small-molecule electrophilic compound to covalently modify and enrich selective residues within the whole proteome using a chemoproteomic approach.177–179 A low-pH isoTOP-ABPP platform was developed to screen selenoprotein-targeted inhibitors in a comparative analysis with iodoacetamide electrophilic probe. Auranofin treatment of mammalian cells revealed strong sensitivity of auranofin to Sec residues of Txnrd1, Gpx4, MsrB1, and Seleno under IA-labeling conditions and analyzed by MS.179 Recently, the ligandable cysteines in Staphylococcus aureus was profiled with an organogold(III) complex using isoDTB-ABPP (isotopically labeled desthiobiotin azide-activity-based protein profiling) platform (Figure 11), which differs from the traditional ABPP by using isotopically labeled (light and heavy) desthiobiotin azide tags and is compatible with IA competition.180 The unique C–S bond via cysteine arylation mediated by [ĈN]-cyclometalated Au(III) allows for an expanded or uncovered ligandable cysteines within the proteome. Overall, 108 cysteines were modified by the [ĈN]-cyclometalated Au(III); interestingly, 59 cysteines were not liganded by previously screened organic electrophilic probes. Indicating a broader reactivity and scope of ligand ability by Au-mediated cysteine arylation.180
Figure 11.

(a) Mechanism of cystine arylation via Au(III) complex reductive elimination. (b) Workflow of isotopically labeled destiobiotin activity based protein profiling (isoDTB-ABPP). Figure reproduced from ref 180. Copyright 2022 Royal Society of Chemistry.
High-throughput screening with yeast deletions and gene knockdown systems including RNA interference (RNAi) and short-hairpin RNA (shRNA) to study drug–target interactions was pivotal in advancing chemical genetics.181–186 The discovery of CRISPR-Cas9 has emerged as a powerful tool to edit the mammalian genome with ease and is useful for biological target identification, unravel mechanism of action, and resistant pathways to chemical agents.187–189 The application of CRISPR-Cas9 screens in metallodrug target identification will be transformative. In a proof-of-concept study, Vulpe and Awuah et al. used a targeted pooled CRISPR approach known as TOXCRISPR to elucidate the targets of a chiral gold(I) anticancer agent, JHK-21 (Figure 12).190 JHK-21 largely target mitochondrial oxidative processes. In addition, ABCC1 (a gene encoding MRP1 chemical exporter) knockout sensitizes cells to JHK-21 and the loss of SPRED2, which negatively regulates the Ras-ERK pathway confers resistance to cells exposed to JHK-21.190 This work paves the way for a systematic study to identify drug targets in mammalian cells of gold-containing compounds using CRISPR-Cas9 screening.
Figure 12.

(a) Structure of JHK-21. (b) Diagram illustrating the combined CRISPR-Cas9 screening method to identify JHK-21 cellular target and mode of action. Reproduced from ref 190. Copyright 2022 American Chemical Society.
5. GOLD COMPLEXES FOR BIOMEDICAL IMAGING AND SENSING
Au complex localization in cells or whole animals reveals insights into its mechanism of action. The use of fluorescent, luminescent, and radiolabeled Au probes can be used to monitor the location of compounds in real time. Tuning the luminescence of gold complexes requires stringent conditions. Thermodynamically, the high redox potential of gold [E°(Au(III/I) = 1.41 V]191 renders it difficult to oxidize, leading to much elevated energy of radiative metal-to-ligand charge transfer (MLCT) states and low-lying HOMO levels compared to other third row transition metals such as Ir or Pt. Consequently, the photochemistry and photophysics of gold complexes are often detrimentally overwhelmed by energetically low-lying states with metal-centered (d-d) and/or ligand-to-metal charge transfer (LMCT) character that can be easily populated. Additionally, population of the excited state of structurally distorted d-d ligand field in Au(III) systems can lead to quenching via nonradiative decay processes.192 This can be circumvented by incorporating strong σ-donating ligands to elevate the energy of the ligand field state toward luminescent Au(III) complexes in solution and at room temperature beyond solid state or low temperature.193,194 In two-coordinate Au(I) complexes with filled d10 configuration, nonradiative decay can be avoided. However, examples are dominated by complexes with aurophilic interactions of metal–metal states. Two-coordinate, mononuclear Au(I) emitters with emission from MLCT states make up an attractive endeavor for biological applications.195–197 So far, efforts to develop emissive gold complexes have adopted intuitive strategies via unique mechanisms including gold–gold interactions in solution or solid state as well as in multinuclear/heteronuclear systems. Multiple strategies employed to implement Au complex imaging in cells or whole animals are discussed.
It is worth noting that the use of gold(I) alkynyls in phosphorescence has been widely explored in materials research, which is beyond the scope of this Review. The development of phosphorescent gold complexes with decreased background fluorescence in biological medium has gained traction. We refer readers to comprehensive reviews on luminescent metal-based complexes including gold.198–203,194 In this section, we focus on luminescent gold complexes used in cell imaging and sensing biomacromolecules such as DNA and proteins.
5.1. Au(I) Complexes for Luminescent Cell Imaging
Enhancing sigma donor character at the gold center, extended conjugation, gold–gold interaction, or multinuclear systems are a few strategies that facilitate single or triplet excited state transitions toward phosphorescence. The use of carbon donors such as NHCs and alkynyl ligands provides ready access to Au(I) complexes exhibiting phosphorescence in the solid state or in solution. Early demonstration of Au(I) cell imaging, made possible by the dinuclear Au(I) complex, [Au2L2]2+, bearing the bidentate cyclophane NHC ligand.204 The combination of Au–Au interaction204–206 and NHC ligand leads to a red-shifted luminescence profile that enables phosphorescence imaging in living cells and is useful for biodistribution by fluorescence microscopy. This class of Au(I) luminescent agents defines lysosomal localization in cells.
5.1.1. Au(I) Conjugated Fluorophores.
The preparation of luminescent Au(I) phosphine naphthalimide complexes enables cellular imaging, nuclei accumulation, and demonstrates antiproliferative activity, inhibition of angiogenesis in zebra fish embryo, whereas it maintains homogeneous biodistribution in zebra fish embryos by fluorescence microscopy.207 The reaction of mercaptonaphthalic anhydride with 2-(dimethylamino)ethylamine in ethanol under refluxing conditions affords the N-(N′,N′-dimethylaminoethyl)-1,8-naphthalimide-4-thiolate ligand and upon metalation with trialkyl/triphenyl phosphine Au(I) chloride analogs leads to luminescent naphthalimide gold(I) phosphine complexes.208
Expansion of luminescent naphthalimide Au(I) complexes introduced the alkynyl moiety with tunable photophysical properties and intracellular localization based on the naphthalimide substituent (Figure 13).
Figure 13.

Au(I) fluorescent alkynyl-naphthalimide complexes for cell imaging. Reproduced from ref 209. Copyright 2015 American Chemical Society.
Multinuclear Au(I) alkynyl phosphanes represent an interesting class of luminescent gold complexes both in the solid state or in solution.209 Initial efforts to synthesize water-soluble Au(I) acetylides began with the treatment of [AuCl(PR3′)], where PR3′ correspond to water-soluble phosphanes such as PTA and DAPTA with terminal alkynes in the presence of KOH in methanol to afford mononuclear phosphane Au(I) acetylides.210 In addition, dinuclear alkynyl Au(I) compounds can be prepared from bis-alkyne starting materials and employing PTA and DAPTA ligands. The use of propargyl amine leads to the formation of trinuclear Au(I) complexes under similar reaction conditions using a base and water-soluble phosphane ligands. These Au(I) alkynyl derivatives display luminescence in the solid state at room temperature with excitation maxima in the range of 356–428 and emissions between 486 and 555 nm. The photophysical character of Au(I) alkynyl phosphanes is attributed to intraligand electronic transitions, gold-centered transitions, Au–P to alkyne transitions, and often Au–Au interactions to alkyne transitions. Au-PR3′ may serve as a directing moiety to enhance the emission from the triplet states of the alkynyl luminophores.211–218 It must be noted for design purposes that the choice of phosphane ligands can influence bathochromic shift in the emission spectra of Au(I) akynyl phosphane complexes due to p–p*(C≡C) or σ(Au–P) → π*(C≡C) transitions.
Anthraquinones have been used as relevant antibiotics and antitumor agents.219,220 Functionalization of hydroxy anthraquinones with propargyl bromide generates planar, conjugated ligands to form C(sp)–Au bonds, leading to complexes that are luminescent. A key optical advantage in the context of metal complexes is the added emissive property from the anthraquinone chromophore in the visible region. Mononuclear and dinuclear Au(I) complexes bearing alkynyloxy-substituted anthraquinones can be used in cells as fluorescent imaging probes toward mechanism of action studies via cellular localization.221 Specifically, in MCF7 cells, both mono and dimetallic Au(I) anthraquinones exhibit bright fluorescence within 530–580 nm emission following a 405 nm excitation (Figure 14).
Figure 14.

Images of MCF-7 cells incubated with [L2-Au-PPh3] (100 μg/mL, 4 °C, 30 min). Excited at 405 nm, acquired 530–580 nm. Reproduced from ref 221. Copyright 2012 American Chemical Society.
To visualize the cellular distribution and intracellular targets of Au(I) therapeutics, incorporation of established fluorophores such as acridine,222,223 coumarin,224–226 and borondipyromethene (BODIPY)227 into the structural framework of Au(I) complexes makes it possible. The development of fluorescent BODIPY-Au(I) trackable probes for bioimaging over the past ten years has grown from cell imaging to whole animal imaging. We discuss in this section the modifications that have catapulted the translational application of BODIPY-Au(I) trackable probes (Chart 10). Initial work began with tracking Au complexes in live cells; this was quickly followed by research based on targeting cancer cells with sugar ligands for the glucose transporters (GLUTs) or the bombesin receptors overexpressed in cancer cells. The relatively short visible light emission of these constructs led to failed preclinical evaluation, creating opportunities to explore far-red or NIR conjugates.
Chart 10.

Chemical Structures of BODIPY Au(I) Probes
The goal to use trackable Au(I) agents in whole animals has been hamstrung by visible light emission probes. To overcome this limitation, near-infrared emitting agents for deep tissue penetration are required. Recently, Bode and Goze et al. added three NIR aza-BODIPY dinuclear Au(I) complexes to the trackable Au toolbox.228 These complexes expand the guiding principles for designing fluorescent Au(I) complexes through the incorporation of (i) NIR dyes with emission maxima >700 nm and decent fluorescence quantum yields (~25–36%) in in vitro and in vivo optical imaging; (ii) water-solubilizing groups and disruptors of solution aggregation; and (iii) dinuclear Au(I) agents for therapeutic impact. Generally, the design possesses theranostic potential in vivo. Specifically, in a CT-26 colon murine mouse model, a pronounced anticancer activity was observed when azaBDP-Au1 was administered (Figure 15).
Figure 15.

(a) Recently reported NIR aza-BODIPY dinuclear Au(I) complexes, (b) azaBDP-Au-1 localization in 4T1 cells visualized by confocal microscopy. 4T1 cells were incubated with azaBDP–Au-Cl (red) for 45 min at 5 μM, nuclei counterstain with blue, fluorescent dye (Hoesct 33342, and mitochondria labeling was done with mito-tracker green, (c) azaBDP-Au1 distribution in tumor bearing mice. (d) An intravenous injection was administered, and images were collected at the indicated times. Accumulation of the compound in the tumor area was observed as shown with arrow. Reproduced with permission from ref 228. Copyright 2021 Elsevier.
5.1.2. Au(I) Conjugated Metal Luminophores.
Improving the optical characteristics of trackable Au complexes offers opportunities for the use of luminescent metal complexes as luminophores including Ru, Re, and Ir with longer emissive lifetimes and high quantum yields of luminescence. Thus, Re(I)/Au(I),203,229–232 Ru(II)/Au(I),233 and Ir(III)/Au(I)201,234 heterometallic complexes have been synthesized as trackable probes for cell imaging. First, the use of rhenium(I) tricarbonyl [Re(CO)3] scaffold in biomedicine is attractive due to its low spin d6 electron configuration, octahedral geometry for variable coordination, kinetic inertness as a result of strong-field ligands, and photophysical properties that allow for excited triplet state transitions. Leveraging the impressive chemical properties of Re with cytotoxic Au complexes leads to theranostic agents and provides a framework to assess the mechanism of action of Au anticancer complexes. The use of polypyridyl ligands, NHC, phosphine, and alkynyl functionality enable tethering of Au(I) to Re(I) without compromising the luminescent properties of Re(I). It is important to note that there are several components to designing cell-permeable heteronuclear multimetallic Re(I)/Au(I) complexes including linker lengths and ligand types. Gimeno’s group has pioneered this field with different variations of luminescent Re(I)/Au(I) complexes for cell imaging and cancer therapy. Starting with fac-[Re(bipy)(CO)3(CF3SO3)], the displacement of the triflato ligand with Au(I) alkynylpyridine, Au(I) alkynylimidazole, or imidazole substituted Au(I) gave rise to heteronuclear Re–Au luminescent complexes.230 Strikingly, the complex localized in the nucleolus, when compared to the Re(I) species, which localized to the cytoplasm. The use of ditopic P,N-donor ligand that double as linkers lead to different Re(I)/Au(I) heteronuclear complexes of the type, fac-[Re-(bipy)(CO)3(LAuCl)]+ (Re–Au-5 and Re–Au-7) and [(fac-[Re(bipy)(CO)3(L)])2Au]3+ (Re–Au-6 and Re–Au-8) with red-shifted emission profiles up to 605 nm attributed to a triplet metal-to-ligand charge transfer transition (Chart 11).229 The quantum yield of fluorescence of these complexes is up to 12.5% in polar solvents. Fluorescence microscopy reveals a nonuniform cytoplasmic distribution as well as nuclear accumulation. These agents do not display potent antiproliferative activity with IC50s in the range of 35–76 μM when tested in A549 cells.
Chart 11.

Chemical Structures of Luminescent Re–Au Complexes
Analogs of luminescent Re(I)/Au(I) complexes bearing pyridyl N-heterocyclic carbene ligands on Re have been synthesized (Re–Au-9–11) to improve (photo)cytotoxicity in cancer cells (as low as 2.66 μM).232 The emission of these complexes is slightly blue-shifted in the range of 377–514 nm, which could be associated with a mixed MLCT from the (Re(dπ) → NHC(π*), LLCT imidazolyl/pyridyl to the NHC ligand, and ligand centered transitions. The cellular distribution of these agents reveals cytoplasm localization by fluorescence microscopy.
The incorporation of dinuclear Au(I) into Re(I)/Au(I) conjugates has the potential to enhance red-shifting to about 680 nm and maintain potent anticancer activity to 1 μM in HeLa cells.231 The design is manifested via a bis-alkynyl framework for Au-NHC metalation that is located on the N^N-bidentate ligand coordinated to the Re center. The emission profile of these complexes demonstrates a characteristic broad band between 565 and 680 nm, which can be assigned to 3MLCT transition from the dπ(Re) → π*-(diimine).235
Recently, the synthesis and antiproliferative activity of a new class of luminescent Au–Re complexes containing fused imidazo[4,5-f ]-1,10-phenanthroline core were explored (Chart 12).236 The heterobimetallic ReI/AuI and trimetallic ReI/AuI/ReI fac-[ReCl (CO)3(N^NĈAuR)]0/+ and [(fac-[ReCl (CO)3(N^NĈ)])2Au]+, where R is an iodide phenylacetylene, dodecanethiol, or 2,3,4,6-tetra-O-acetyl-1-thio--d-glucopyranose display long wavelength emission profiles in the range 641–673 nm following a 398 nm excitation. Despite the excellent photophysical profile, these complexes are not cytotoxic against cancer cells.
Chart 12.

Reaction Scheme for Synthesis of Luminescent Re–Au Complexes Bearing NHC Ligands
Second, the synthesis of luminescent heterobimetallic Au(I)–Ru(II) complexes bearing heteroditopic bipyridine-NHC ligands (Chart 13) has prospects for studying cellular distribution, uptake mechanisms, and impact on cytotoxicity against cancer cells, Leishmania infantum, and Plasmodium falciparum.233,237 The use of Ru(bipy)3 and Ru(bipy)2(dipy) as luminophores and Au(I) bearing 1-thio-β-d-glucose 2,3,4,6-tetraacetate allows for the generation of auranofin-like imaging agents to study GLUT-1 transporters and distribution in cancer cells. Tuning the gold fragment of Au(I)–Ru(II) complexes for improved stability and cytotoxicity can take advantage of strong electron donation in NHC ligands. Emission spectra of luminescent Au(I)–Ru(II) complexes are in the range of 615–630 nm with a luminescence quantum yield in water of 0.020–0.026. Whereas these photophysical properties are attractive, they are far from ideal. Challenges including longer emission wavelength, poor aqueous solubility, and bulky luminophores that obscure precise intracellular localization of desired metallodrugs exist.
Chart 13.

Chemical Structures of Luminescent Ru–Au Complexes
Third, phosphorescent iridium complexes possess excellent optical properties and have found utility in several areas of biomedical, material, energy, and catalytic research.238–241 Harnessing the remarkable photophysical properties of Ir, including high phosphorescent quantum yield, large Stokes shift, and long emission lifetimes and the cytotoxic potential of Au represent a synergistic tool for theranostics. The use of emissive cyclometalated Ir(III) complexes conjugated to Au(I) fragments does not alter the photophysical properties of Ir. The high sensitivity of cancer cells to such complexes could be attributed to the Au(I) unit. Additionally, intracellular accumulation of these luminescent conjugates via fluorescence microscopy is often characterized by lysosomal and mitochondrial localization. Access to [Ir(ppy)2(dppm)]PF6 as a precursor to Au–Ir complexes can be obtained in a single step by reacting dppm and [[Ir(ppy)2(μ-Cl)]2] in anhydrous and degassed methanol for 12 h. Metalation with Au(I) bearing ancillary ligands such as chloride, thiocytosine, and triphenylphosphine can then be carried to obtain luminescent Au(I)–Ir(III) complexes (Chart 14).201 Further, the development of Au–Ir bimetallic peptide conjugates has been explored using an enkephalin analog, Tyr-Gly-Gly-Phe-Leu, and a propargyl-substituted derivative, Tyr-Gly-Pgl-Phe-Leu, demonstrating a strong proof-of-principle agents for theranostic bimetallic peptide bioconjugates.234 Significant work is required to advance these phosphorescent heteronuclear complexes for preclinical studies.
Chart 14.

Chemical Structure of Phosphorescent Ir–Au Complexes
5.2. Bioimaging and Sensing of Au(III) Complexes
Optimized probes can be used for cellular imaging. The use of π-extended C-deprotonated [ĈNĈ] ligands readily afford organogold(III) complexes that display long-lived emissive excited states as biosensors for proteins and DNA with lifetimes and emission quantum yields of up to 282 μs (Figure 16). This fluorogenic strategy capitalizes on the low 5dx2-y2 orbital of the Au(III) metal center, which gives the overall Au(III)-NHC complex a nonemissive character in solution but upon reduction to Au(I) by biological reductants and the concomitant release of the fluorescent pincer ligand a strong emission is observed.242 Other amphiphilic Au(III) complexes that self-assemble into micelles with good biocompatibility, high in vivo permeability and retention, and in vitro phototoxicity have been developed.243
Figure 16.

(a) Synthetic scheme of Au-Avidin, (b) confocal imaging of HeLa cells treated with conjugate Au-Avidin for 4 h followed by a fluorescently tagged biotin. Reproduced from ref 242. Copyright 2015 Royal Society of Chemistry. (c) Synthesis of Au-AM self-assembled micelles. (D) Confocal microscopy images of A549 cells treated with Au-AM (33 μg/mL) (upper panel) for 4 h and without Au-AM (lower panel) under bright field or fluorescence field excitation at 405 nm. Reproduced from ref 243. Copyright 2016 Royal Society of Chemistry.
Derivatives of cationic Au(III) complexes containing highly emissive tridentate N^N^N ligands (H2N^N^N ligands, 2,6-bis(imidazol-2-yl)pyridine (H2IPI), and 2,6-bis(benzimidazol-2-yl)pyridine (H2BPB)) and supported by NHC ligands generate fluorogenic probes (Figure 17).101 These Au(III) complexes act as fluorescent thiol “switch-on” probes following reduction of Au(III) to Au(I) by thiols including glutathione. The strategy employed takes advantage of the ability of low energy Au(III) 5dx2-y2 orbital to quench intraligand emission; however, reduction activates the high emissive character of the ligands.
Figure 17.

(a) Chemical structure of Au(III)-complexes Au-IPI and Au-BPB. (b) Fluorescence images of Au-BPB derivative (left, 365 nm excitation), mitochondria-specific Mito-tracker Red stain (middle, 546 nm excitation), and the merged image (right). Reproduced from ref 101. Copyright 2013 John Wiley and Sons.
Transition metal hydrides represent an interesting class of compounds with utility in catalysis and materials.244 Following the first evidence of Au hydrides245 by Andrews et al.,246–249 these complexes were considered unstable until the first isolable Au(I) hydride bearing an NHC ancillary ligand in 2008250 and AuH stabilized by Xanthphos-phosphole ligand.250,251 Ever since, Bochmann and co-workers pioneered the development of Au(III) hydrides of the structural type [(ĈNĈ)AuH] with the hydride ligand trans to the N-donors252,253 and subsequent applications in catalytic water–gas shift reactions.252,253 Variations of this class of complexes possess emission properties with sufficient biological stability. It is possible that the Au–H bond gains susceptibility to photolability due to trans effect at which stage allows the excited state contribution to be dominated by intraligand phenyl to pyridyl transition mixed with a minor metal to ligand charge transfer transition. Further, photoinduced thiol reactivity by incubating [(ĈNĈ)AuH] (100 μM) with NAC (10 mM) in aqueous solution (H2O:20% DMF, v/v) and subsequent irradiation with 365 nm light generates ligated [Au(III)(ĈNĈ)S(NAC)] in >90% conversion in just 30 min (Figure 18).254
Figure 18.

Top. Chemical structure of [(ĈNĈ)AuH] complexes 1a–d, Bottom. (a) Emission spectrum of 1 b in dichloromethane. (b) Fluorescence microscopy image of HepG2 cells treated with 10 mm of 1 b for 1 h. (c) Bright field showing characteristics of apoptotic morphology change after irradiation. (d) Merged image. (e–h) Fluorescent images of HepG2 cells treated with 10 mm of 1b for 1 h followed by 405 nm laser irradiation at selected region (dashed box) for 2 min (e) bright field; (f) green channel; (g) red channel; (h) merged fluorescent image. Reproduced with permission from ref 254. Copyright 2020 John Wiley and Sons.
6. RADIOACTIVE AU COMPLEXES FOR RADIOTHERAPY AND BIODISTRIBUTION
Incorporation of 198Au and 199Au into the radiopharmaceutical toolbox has been largely unexplored until the past decade, largely due to synthetic complexity and stability associated with high valent Au(III) complexes. Development of radioactive 198Au and 199Au uncovers an important new class of radiopharmaceuticals for the treatment of cancer and diagnostics. 198Au and 199Au are radioactive and emitters with strong penetrating power. 198Au isotope has a days, and keV and 199Au isotope has a days, and and 208 keV. The high energy photons make these Au isotopes suitable for imaging and detection by single-photon imaging instruments. Additionally, their half-lives are optimal for production, shipping, and administration. Beyond the use of colloidal gold radionuclide 198Au colloids, which was reported by Sheppard et al. and received US approval in 1950 as an antineoplastic and liver imaging agent,255 few gold-derived monomeric radionuclide complexes have been developed.256–259 198Au and 199Au radionuclides of Au(III) bis-thiosemicarbazones bearing diversified dithiosemicarbazone ligands were synthesized and their radiochemistry characterized.257 In particular, the radionuclide with (1Z,1′Z)-N′,N″″-((2E,3E)-pentane-2,3-diylidene)bis(N-ethylcarbamohydrazonothioic acid) ligand, 198Au-TSC was synthesized with >90% radiochemical yield with good stability in phosphate-buffered saline (PBS) and mouse/human serum stability. The biodistribution of 198Au-TSC demonstrates a >50% accumulation in the bloodstream and 39% ID/g lung distribution in 4 h following administration. It must be noted that ligand systems must be carefully chosen to circumvent existing problems associated with the rapid reduction of Au(III) complexes.
Additionally, the 198Au radiolabeled Au(III) bis(pyrrolide-imine) Schiff base complex was synthesized with a high radiochemical purity of >95% and 73% yield (Chart 15).258 The high energy radiation of 198Au allowed for biodistribution of the complex in Sprague–Dawley rats using gamma capture, giving insights into blood accumulation of the hydrophilic complex and its retention in tissue as evidenced by t1/2 of 24 h in the heart and lung and excretion via the kidneys.258 Whereas these studies are of promise, the inability to use the described radiolabeled complexes to evaluate Au(III) to Au(I) reduction of drugs in animal models present limitations that require alternate radiolabeling approaches. The use of iodine (124I) radionuclide labeled Au(III) carbene complexes take advantage of the positron emission capability of 124I to monitor the speciation of Au–I-124 ex vivo and in vivo using whole body imaging by positron emission tomography (PET) and Au concentration in different organs by ICP-MS (Figure 19).259
Chart 15.

Chemical Structures of Some Radioactive Au(III) Complexes
Figure 19.

(a) Radioactivity curve of arterial blood determined by online blood sampling following the administration of Au–I-124 intravenously. (b) PET images gotten at different intervals following administration of Au–I-124 intravenously. (c) Representation of the radioactivity concentration in distinct organs at different time intervals assessed from the PET images following the administration of Au–I-124. (d) Representation of the radioactivity concentration (assessed from the PET images) and Au concentration (determined by ICP-MS) in distinct organs. Panels a–d are reproduced from ref 259. Copyright 2020 John Wiley and Sons.
7. THERAPEUTIC GOLD COMPLEXES
7.1. Approved Gold Drugs
We would like to draw readers attention to the clinical use and development of gold agents (Table 1) as a prelude to the exciting new discoveries of gold-derived compounds for therapeutic application in different disease indications. The antituberculosis effect of potassium gold cyanide by Koch spurred several therapeutic trials of cationic gold complexes across Europe in humans.260,261 The German pharmacologist Adolf Feldt introduced sodium (4-amino-2-mercaptobenzoato(2-)-O,S) aurate (Krysolgan) in 1917 for the treatment of tuberculosis and aurothioglucose (Solganol), which inhibited streptococcal infections in humans.260–263 In 1845, Fordos and Gelis synthesized sodium aurothiosulfate (Sanocrysin)264 for the first time and its preparation later refined and characterized by McCluskey and Eichelberger in 1926 as an Au(I) complex.265 Despite Mollgaard’s mischaracterization of sanocrysin as a Au(III) dimethyl complex, the chemotherapeutic investigation of sanocrysin in the context of pulmonary tuberculosis was favorable. Several independent studies by physicians and scientists from Sweden, Denmark, Germany, and France published findings of the use of cationic gold complexes in polyarthritis and rheumatoid arthritis.266 Seminal work by Jacques Forestier in 1932 that utilized sodium aurothiopropanol sulfonate (Allochrysine) proved effective against infective and rheumatoid arthritis with cases that eliminated symptoms and signs of disease progression toward clinical cure.35
Table 1.
Clinical Use of Gold Agents
| Generic Name | Trade Name | Approval Granted | Mode of Administration | Indication |
|---|---|---|---|---|
| Auranofin | Ridaura | FDA, 1985 | Oral | Rheumatoid arthritis |
| Aurothiosulfate | Sanochrysin | Abandoned 1931 due to toxicity | Intramuscular injection | Rheumatoid arthritis, tuberculosis |
| Aurothioprol | Allochrysine | Approved by EULAR, no longer recommended for use | Intramuscular injection | Rheumatoid arthritis |
| Aurothiomalate | Myochrysin | Phase 1 (USA), approved United Kingdom | Oral | PKCi, NSCLCs |
| Aurothioglucose | Solganol | Not approved | Oral | Rheumatoid arthritis |
Following the introduction of sanocrysin in 1924 by Mollgard as a chemotherapeutic agent, the first clinical use of sanocrysin in humans was fostered by Knud Secher for the treatment of pulmonary tuberculosis in Denmark.266 Secher reported that 114 patients were tubercle bacilli-symptom free and proposed a mechanism that suggests the release of toxins in air-passages after sanocrysin administration to fight the infection.266–268 Other studies around Europe contested the Mollgard–Secher theory based on unsatisfactory therapeutic effect of sanocrysin in treating tuberculosis. The lack of a formidable experimental basis of sanocrysin’s mode of action dampened enthusiasm for its use.269–272 However, several clinicians continued its use to treat tuberculosis and other indications.
Aurotioprol acid is a racemic gold(I) salt, first prepared by Lumiere and marketed by Solvay under the trade name allochrysine for the treatment of rheumatoid arthritis.36,273 This drug exists as a polymeric complex with a chiral 2-hydroxy-3-sulfidopropane-1-sulfanto ligand. It is administered via intramuscular injection and currently marketed outside the United States. In several clinical trials dating back to the 1940s allochrysine showed superior patient response to placebo as a disease modifying drug.274
Sodium aurothiomalate exists as a 50 mg injection solution with nitrogen, phenylmercuric nitrate, and water. Sanofi markets this drug under the trade name myocrisin as disease modifying agent for the management of progressive rheumatoid arthritis and juvenile chronic arthritis and administered via deep intramuscular injection. There is widespread use of myocrisine in Australia and New Zealand, where it was approved in 1969. Myocrysin was discontinued in the United Kingdom due to shortage of the Active Pharmaceutical Ingredient (API) and not due to safety concerns in June 2019.275 In The Netherlands, myocrisin was established as an alternative to aurothioglucose (Solganol) in 2001. Out of 120 patients with rheumatoid arthritis, 79% overall survival rate was recorded after 12 months. Maximum therapeutic benefit is gained in the early stages of disease. In advanced stages of the disease, where cartilage and bone damage have occurred, myocrysin is capable of management. Weekly injections of 10 mg to 50 mg of active gold until sodium aurothiomalate reaches 1 g is the general rheumatoid arthritis therapeutic dose.276
Krysolgan, also referred to as sodium (4-amino-2-mercaptobenzoato(2-)-O,S) aurate is a polymeric water-soluble complex introduced by Adolf Feldt for the treatment of tuberculosis and leprosy.277 In 1926, the use of Krysolgan in a patient with sarcoid lesions demonstrated a positive outcome including softened large nodules, flaccid skin area and disappearance of nodules following 14 injections of the drug up to 1.5 g dose.278 Application of Krysolgan in treating Lupus Erythematosus quickly emerged.279 Increasing toxicity, lack of potency, and lack of a defined mode of action, led to the discontinuation of Krysolgan as first-line therapy.280
Aurothioglucose can be viewed as a sugar derived Au(I) polymeric salt, which is often administered by intramuscular injection or intragluteally and marketed as Solganol. The American Medical Association designated Solganol for the treatment of rheumatoid arthritis, particularly for patients that have been unresponsive to conventional therapy. Although the mechanism is not fully elucidated, a general mode of action is that the gold agent accumulates in macrophages and consequently inhibits lysosomal enzymes as well as phagocytosis.276,281
Auranofin is a monomeric gold drug approved by any public health agency. It is an oral antiarthritic alkylphosphine gold(I) drug bearing a tetra-O-acetyl-1-thio-B-d-glucopyranose ligand. The search for new antiarthritic agents with improved efficacy led Sutton and the research team at Smith Kline and French laboratories, Philadelphia to synthesize a series of trialkyl-phosphine gold complexes in 1972.51 In the structure–activity relationship study that evaluated therapeutic responsiveness by the adjuvant arthritis rat model and bioavailability by measuring Au plasma levels, the triethylphosphine gold (AuPEt3) class was found to be most effective.51 Following the structural elucidation of auranofin using different spectroscopic techniques and X-ray crystallography, detailed pharmacokinetic and pharmacological profiling, and clinical trials, auranofin was approved by the US FDA in 1985 for the treatment of rheumatoid arthritis. Auranofin is marketed as Ridaura by Sebela Ireland Ltd. as a 3 mg capsule for oral administration. Recent repurposing efforts in identifying new drugs for different disease indications have positioned auranofin as an attractive drug for cancer, microbial, and viral infections beyond arthritis. We discuss the current landscape of auranofin in clinical trials around the world.
7.2. Current State of Gold Drugs in Clinical Trials
The ability for auranofin to perturb redox homeostasis by inhibiting thiol and selenocysteine rich oxidoreductases offers a broad mechanism to target for several diseases that have oxidative stress and inflammatory underpinnings such as cancer, microbial infections, and neurodegeneration.10,55,282–290 Despite the relegation of auranofin as a first line treatment for rheumatoid arthritis, there are several clinical trials that have been conducted and other ongoing trials to repurpose the gold drug to treat other diseases as summarized in Table 2.
Table 2.
Results from Clinical Trials of Auranofin in Different Disease Conditions
| Rank | Title | Status | Study Result | Conditions | Interventions | Locations | References |
|---|---|---|---|---|---|---|---|
| 1. | Oral Auranofin for Reduction of Latent Viral Reservoir in Patients with HIV Infection | Withdrawn | No Results Available | HIV | Drug: Auranofin | University of Miami - AIDS Clinical Research Unit, Miami, Florida, United States | 293 |
| 2. | Auranofin PK Following Oral Dose Administration | Completed | No Results Available | Amoebiasis | Drug: Auranofin | Quintiles Phase I Services - Overland Park, Overland Park, Kansas, United States | 294 |
| 3. | Auranofin in Treating Patients with Recurrent Epithelial Ovarian, Primary Peritoneal, or Fallopian Tube Cancer | Completed | No Results Available | Recurrent Fallopian Tube Cancer, Recurrent Ovarian Epithelial Cancer, Recurrent Primary Peritoneal Cavity Cancer | Drug: auranofin Other: laboratory biomarker analysis |
Mayo Clinic, Rochester, Minnesota, United States | 295 |
| 4. | Phase I and II Study of Auranofin in Chronic Lymphocytic Leukemia (CLL) | Completed | No Results Available | Chronic Lymphocytic Leukemia (CLL) Small Lymphocytic Lymphoma Leukemia, Prolymphocytic |
Drug: auranofin | University of Kansas Cancer Center, Westwood, Kansas, United States | 60 |
| 5. | Auranofin and Sirolimus in Treating Participants with Ovarian Cancer | Active, not recruiting | Has Results | Ovarian Serous Tumor Recurrent Ovarian Carcinoma Drug: Sirolimus | Drug: Auranofin Other: Laboratory Biomarker Analysis |
Mayo Clinic, Rochester, Minnesota, United States | 62 |
| 6 | Auranofin in Decreasing Pain in Patients with Paclitaxel-Induced Pain Syndrome | Completed | Has Results | Pain | Drug: auranofin Other: placebo Other: questionnaire administration |
Mayo Clinic, Rochester, Minnesota, United States | 296 |
| 7. | Auranofin for Giardia Protozoa | Completed | No Results Available | Amoebic Dysentery Giardiasis | Drug: Auranofin Other: Placebo |
International Center for Diarrheal Disease Research Bangladesh - Parasitology, Dhaka, Bangladesh Rajshahi Medical College Hospital, Rajshahi 6000, Bangladesh |
297 |
| 8. | Auranofin and Sirolimus in Treating Patients with Advanced Solid Tumors or Recurrent Non-Small Cell Lung Cancer | Withdrawn | No Results Available | Recurrent Nonsmall Cell Lung Cancer Unspecified Adult Solid Tumor, Protocol Specific | Drug: auranofin Drug: sirolimus Other: laboratory biomarker analysis Other: pharmacological study |
Mayo Clinic in Florida, Jacksonville, Florida, United States | 298 |
| 9. | Sirolimus and Auranofin in Treating Patients with Advanced or Recurrent Non-Small Cell Lung Cancer or Small Cell Lung Cancer | Recruiting | No Results Available | Extensive Stage Small Cell Lung Carcinoma Lung Adenocarcinoma Recurrent Non-Small Cell Lung Carcinoma Recurrent Small Cell Lung Carcinoma Squamous Cell Lung Carcinoma Stage IIIA Non-Small Cell Lung Cancer Stage IIIB Non-Small Cell Lung Cancer Stage IV Non-Small Cell Lung Cancer |
Drug: Auranofin Drug: Sirolimus Other: Laboratory Biomarker Analysis Other: Pharmacological Study |
Mayo Clinic in Arizona, Scottsdale, Arizona, United States Mayo Clinic, Jacksonville, Florida, United States | 299 |
| 10. | Multi Interventional Study Exploring HIV-1 Residual Replication: A Step Toward HIV-1 Eradication and Sterilizing Cure | Completed | No Results Available | Chronic Infection HIV | Drug: Maraviroc Drug: Dolutegravir Biological: Dendritic Cell Vaccine Drug: Auranofin Drug: Sirtuin Histone deacetylase inhibitor |
CCDI, Sao Paulo, SP, Brazil | 300 |
| 11 | TB Host Directed Therapy | Unknown status | No Results Available | Tuberculosis | Biological: Dendritic Cell Vaccine Drug: Auranofin Drug: Sirtuin Histone deacetylase inhibitor Drug: Everolimus 0.5 MG Drug: Auranofin 6 MG Drug: Vitamin D3l Drug: CC-110501 Drug: 2HRbZE/ 4HRb |
The Aurum Institute: Tembisa Clinical Research Centre, Tembisa, Gauteng, South Africa | 301 |
| 12. | A Proof-of-concept Clinical Trial Assessing the Safety of the Coordinated Undermining of Survival Paths by 9 Repurposed Drugs Combined with Metronomic Temozolomide (CUSP9v3 Treatment Protocol) for Recurrent Glioblastoma | Completed | No Results Available | Glioblastoma | Drug: Temozolomide Drug: Aprepitant Drug: Minocycline Drug: Disulfiram Drug: Celecoxib Drug: Sertraline Drug: Captopril Drug: Itraconazole Drug: Ritonavir Drug: Auranofin |
University of Ulm School of Medicine, Ulm, Baden-Wurttemberg, Germany | 302 |
| 13. | Comparative Analysis of Outcomes Among Patients Initiating Xeljanz in Combination with Oral MTX Who Withdraw MTX Versus Continue MTX | Completed | Has Results | Rheumatoid Arthritis | Pfizer, New York, New York, United States | 303 | |
| 14. | An Observational Study of MabThera/Rituxan (Rituximab) and Alternative TNF-Inhibitors in Patients with Rheumatoid Arthritis and an Inadequate Response to a Single Previous TNF-Inhibitor | Completed | Has Results | Rheumatoid Arthritis | Winnipeg, Manitoba, Canadal Saint John, New Brunswick, Canada Brampton, Ontario Canada, and 212 other locations |
304 |
Investigation into the use of auranofin as an adjunctive host directed tuberculosis therapy (TB HDT) is in phase II clinical trials. This study examines the safety and preliminary efficacy of auranofin and other drugs including everolimus, vitamin D3, and CC-11050. TB treatment is long and often prevents patient medication compliance. Additionally, TB disease burden leads to acute lung inflammation. Thus, new treatment options that shorten TB treatment and prevent permanent lung damage is of clinical need.
An observational study sponsored by Hoffman-La Roche conducted in 11 countries of 1239 enrolled participants aimed at assessing the antiarthritic biologic, rituximab, and alternative tumor necrosis factor (TNF) inhibitors in patients with rheumatoid arthritis and an inadequate response to a single previous TNF-inhibitor. The study recruited participants with previous nonbiologic disease-modifying antirheumatic drugs therapy including auranofin, aurothioglucose, allochrysine, and gold. Disease activity score-erythrocyte sedimentation rate (DAS28-ESR) is used as a measure of disease activity in rheumatoid arthritis and is calculated from the number of swollen joint count, tender joint count (TJC, 28 joints count) and ESR (millimiters per hour [mm/h]) with a higher score indicating more disease activity. This will be applied to the study over a 14-year period. In a recent study by Pfizer to examine patients initiating Xeljanz (tofitinib, Janus kinase inhibitor) for the treatment of moderate to severe active rheumatoid arthritis in combination with oral methotrexate (MTX), patient enrollment comprised those who have received Au treatment in the form of auranofin, aurothioglucose, or sodium gold thiomalate during a 1–5.2 years period before the index date. These observational models position Au drugs for potential combination therapy in the effective treatment of active rheumatoid arthritis in patients.
The use of auranofin to deplete latent viral reservoir in patients with HIV infection was supported by the hypothesis that antiretroviral therapy suppress HIV viral load and further reduction of viral load may lead to disease cure. In 2014, vaccine and gene therapy institute in collaboration with the University of Miami sponsored an interventional trial to investigate the reduction of HIV viral reservoir by oral auranofin (3–6 mg). This study was later withdrawn. Researchers in Sao Paolo launched a clinical trial toward HIV cure by studying a combination therapy involving Maraviroc and/or dolutegravir, dendritic cell vaccination, class III histone deacetylases (HDACs), surtuin-1, and auranofin to decrease the ratio of long-lived central memory/transitional memory (TTM) CD4+ T-cells.291,292 Although results are not yet available, a positive outcome will result in an efficacious combination treatment regimen for HIV sterilizing cure.
Auranofin was granted an orphan drug status for the treatment of amebiasis. Amebiasis is a parasitic disease caused by the one-celled protozoon called Entamoeba histolytica. To monitor the safety of auranofin after 7 days of daily oral administration, the National Institute of Allergy and Infectious Diseases (NIAID) completed a Phase I open label study to evaluate the pharmacokinetics of auranofin following a daily dose of 6 mg oral dose for 7 days to healthy individuals. In a separate phase IIa study, the NIAID designed a comparative study to evaluate placebo to once daily doses of 6 mg auranofin for the treatment of amebiasis or giardiasis (a diarrheal infection caused by the parasite Giardia duodenalis).
Chemotherapy remains the first line treatment for many cancers, but patients develop resistance to drug treatment and can die as a result. There is an unmet medical need to develop novel therapies for chemotherapy-resistant disease. The previous regulatory approval of auranofin established it as a reasonably safe and effective drug for rheumatoid arthritis. Thus, making auranofin attractive for the treatment of cancers. The University of Kansas Medical Center, NIH, and the Leukemia and Lymphoma Society identified auranofin as a selective inhibitor of the rare blood cancer, CLL. A phase I/II interventional trial was initiated at three sites following an IND clearance from the FDA. Due to diminished unmet need because of four promising therapies for CLL, the auranofin study was abrogated. The University of Ulm, Germany sponsored and proof of concept interventional clinical trials that combines Temozolomide with other approved drugs including auranofin for treatment of recurrent glioblastoma. The Mayo Clinic through its multiple locations in collaboration with the National Cancer Institute initiated several clinical trials to evaluate efficacy and overall tumors response rate of auranofin and sirolimus combination in treating serous and recurrent ovarian cancer. In the context of nonsmall cell lung cancer (NSCLC), phases I and II studies are currently recruiting patients to establish the maximum tolerated dose of auranofin when given in combination with sirolimus after a round of platinum-based chemotherapy as well as the potential to inhibit lung cancer growth.
7.3. Next Generation Gold Therapeutic Complexes
Ongoing research efforts to generate Au-derived compounds that are safe, efficacious, and selective have led to building unique molecular scaffolds with features akin to the FDA approved auranofin. New Au complexes have led to the discovery of novel mechanisms, potency, and precise target engagement. We describe such developments in subsequent sections of this Review. Here we discuss efforts to create novel and efficacious Au(I) and Au(III) compounds for different disease indications. Due to the broad utility of Au agents for disease treatment we have organized this section by disease indication and provided recent advances in gold-based therapeutic development for each disease category. We claim that the discussion of individual compounds is beyond the scope of this Review.
7.3.1. Antifungal Gold Complexes.
Only three types of antifungal drugs exist for treatment, namely the azoles, which in inhibit the primary fungal sterol, ergosterol; polyenes, which interact with the membranes of sterols; and 5-fluorocytosine, which is an inhibitor of macromolecular synthesis.305 The growing threat of fungal resistance to these drugs poses a major health crisis and further affirms the desperate need for new drugs with different mechanism of action. The current excitement surrounding auranofin as antimicrobial agent provides impetus for the development of Au-derived antifungal agents. Seminal work by Garneau-Tsodikova and Awuah et al. demonstrated that chiral and achiral forms of linear and square-planar Au(I) complexes (Chart 16) display broad-spectrum activity and potent antifungal effects strains of the multidrug resistant fungus, Candida auris.306 The reaction of Au(I)Cl(THT) with phosphine ligands in chloroform at room temperature give rise to dinuclear Au(I)-phosphines with a linear geometry as well as distorted tetrahedral (based on the τ4 parameter)307 counterparts, which can be separated by column chromatography to obtain highly pure complexes. Notably, the distorted tetrahedral complexes bearing chiral 1,2-bis[(2R,5R)-2,5-dimethylphospholano]benzene or 1,2-bis[(2S,5S)-2,5-dimethylphospholano]benzene display excellent antifungal activity with MIC < 1.95 μg/mL. Notably, AuFun-4 and AuFun-6 prevent biofilm formation and decrease metabolic activity of fungal biofilms.
Chart 16.

Chemical Structures of Bisphosphine-Au(I) Antifungal Complexes
Incorporating Au(III) into clinically approved antifungal azoles have been achieved via metalation of the imidazole (Chart 17).308 These Au(III) complexes display antifungal activity in multiple Candida strains including albicans, glabrata, kusei, and auris in the submicromolar range. An asexual fungus, Microsporum canis, is highly prevalent worldwide that has high relapse rates and treatment failures could benefit from more efficacious antifungal agents such as gold-based antifungals.
Chart 17.

Chemical Structures of Au(III)-Azoles
A major drawback in antifungal metallodrug discovery is the lack of target discovery and a clear mechanism of fungal inhibition. The ability to harness some of the target identification strategies expounded in the earlier sections of this Review in the context of fungus has the potential to revolutionize gold-based antifungal discovery from oral agents to topical formulations.
7.3.2. Antibacterial Gold Complexes.
The continuous rise of antibiotic resistance possesses a major health threat to society with increasing treatment challenges.309–311 Bacteria multidrug resistance mechanism against antibiotics arise through the production of enzymes that degrade antibiotics, overexpression of efflux pumps that drive antibiotics out of the bacterium, and alteration of target proteins through mutation.311,312 Most of the new set of antibacterial drugs are derivatives of existing drugs and have also shown resistance to some strains of bacteria, hence the search for more efficacious drugs. Auranofin and other gold-based complexes have been studied as potential antibacterial agents. In a report by Cassetta et al., auranofin was shown to be potent against Staphylococcus spp. in a concentration dependent manner.313 Auranofin also showed moderate bactericidal activity in Staphylococcus aureus and Pseudomonas aeruginosa biofilms.314,315 Despite auranofin’s potency against Gram-positive bacteria, its activity against Gram-negative bacteria has been suboptimal. The outer membrane of Gram-negative bacteria may create a barrier that prevents auranofin permeability. To mitigate this drawback, coadministration of auranofin with polymyxin B and E, antibiotics used for Gram-negative bacteria, has been shown as an effective way to improve auranofin activity.287
Wu et al. carried out structure activity relationship (SAR) on auranofin by modulating the thiol and phosphine ligands to create auranofin-like molecules. Forty compounds were screened for their activity against the notorious ESKAPE bacteria (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter cloacae) and noted that compounds with trimethylphosphine ligands have improved activity against Gram-negative bacteria (Table 3). Their study also revealed that the thiol ligand is necessary for the bactericidal activity of both Gram-positive and Gram-negative bacteria.316 Other reports from this lab have also shown that auranofin and its derivatives are active against Helicobacter pylori and bacteria from the Burkholderia genus.317,318
Table 3.
SAR of Auranofin and Antibacterial Activity
| A. baumannii NCTC 13420 | P. aeruginosa NCTC 13437 | S. aureus JE2 (USA300) | E. faecium ATCC 700221 | E. coli ATCC 25922 | |
|---|---|---|---|---|---|
| Aur-1 | 47 (47) | 377 (377) | 0.04 (0.09) | 0.2/0.09 (0.4) | 24 (24) |
| Aur-2 | 47 (94) | 377 (377) | 0.04 (0.09) | 0.2/0.4 (0.4) | 24 (24) |
| Aur-3 | 24 (47) | 189 (189) | 0.04/0.09 (0.09) | 0.09 (0.4) | 12 (12) |
| Aur-4 | 82 (164) | >328 | 0.01 (0.01) | 0.08 (0.2) | 21 (21) |
| Aur-5 | 33 (33) | 132 (132) | 0.008 (0.06) | 0.03/0.06 (0.1) | 17 (17) |
| Aur-6 | 66 (132) | 132 (132) | 0.03 (0.1) | 0.1 (0.3) | 33 (33) |
| Aur-7 | 12 (12) | >194 | 0.003/0.006 (0.006) | 0.05 (0.09) | 24 (24) |
| Aur-8 | 76/19 (19) | >303 | 0.04/0.009 (0.1) | 0.04/0.07 (0.3) | 152/323 (152) |
| Aur-9 | 63/16 (251) | 502 (502) | 0.06/0.1 (0.5) | 0.1 (2) | 31 (31) |
| Aur-10 | 31 (31) | 251 (251) | 0.02 (0.1) | 0.1/0.2 (0.5) | 16 (16) |
| Aur-11 | 31 (63) | 502 (502) | 0.02/0.06 (0.1) | 0.1/0.06 (0.5) | 16 (16) |
| Aur-12 | 15/29 (116) | 464 (464) | 0.02 (0.05) | 0.05/0.1 (0.5) | 7/4 (7) |
| Aur-13 | 24/48 (190) | 381 (381) | 0.02/0.05 (0.09) | 0.09 (0.4) | 24 (24) |
| Aur-14 | 24 (24) | 381 (381) | 0.09/0.04 (0.09) | 0.09/0.2 (0.4) | 12 (12) |
| Aur-15 | 19/75 (>603) | 302 (302) | 0.02 (0.6) | 0.6 (1) | 151 (151) |
| Aur-16 | 18/36 (18) | 291 (291) | 0.009 (0.6) | 0.02 (0.1) | 36 (36) |
| Aur-17 | 18(18) | 146 (146) | 0.07/0.02 (0.07) | 0.02 (0.1) | 9 (9) |
| Aur-18 | 35 (35) | 282 (282) | 0.02 (0.3) | 0.02/0.1 (0.3) | 35 (35) |
| Aur-19 | >546 | >546 | 0.0004/0.02 (0.02) | 0.06/0.1 (0.5) | >546 |
| Aur-20 | 520 (>520) | >520 | 0.02/0.1 (0.2) | 0.02 (0.2) | >520 |
| Aur-21 | 10 (82) | 41 (41) | 0.03 (0.03) | 0.2/0.3 (0.6) | 10 (10) |
| Aur-22 | 19 (76) | 305 (305) | 0.005/0.02 (0.02) | 0.1/0.3 (0.6) | 76 (76) |
| Aur-23 | 37/74 (149) | 149 (149) | 0.009 (0.1) | 0.6 (1) | 74 (74) |
| Aur-24 | 7/13 (13) | 52 (52) | 0.1/0.2 (1.6) | 0.4 (3) | 7 (7) |
| Aur-25 | 11/23 (23) | 92 (183) | 0.001/0.02 (0.2) | 0.09/0.2 (0.7) | 23 (23) |
| Aur-26 | 74/147 (147) | 74 (74) | 2 (4) | 5 (5) | >589 |
| Aur-27 | 16/129 (518) | >518 | 1/2 (4) | 4 (4) | >518 |
| Aur-28 | >438 | >438 | 2 (3) | 3 (3) | >438 |
| Aur-29 | 29/58 (233) | >467 | 1/ (4) | 2/4 (4) | >467 |
| Aur-30 | >366 | >366 | 3/1 (11) | >366 | >366 |
| Aur-31 | 6/13 (101) | 101 (101) | 0.1 (0.2) | 0.2/0.4 (0.8) | 6 (6) |
| Aur-32 | 84 (>336) | >336 | 3 (3) | 3/5 (3) | >336 |
| Aur-33 | >311 | >311 | 1/2 (2) | 5 (5) | >311 |
| Aur-34 | >281 | >281 | 1 (2) | 2/4 (4) | >281 |
| Aur-35 | >292 | >292 | 1 (2) | 2/5 (5) | >292 |
| Aur-36 | >249 | >249 | 4 (8) | 31 (62) | >249 |
| Aur-31 | 6/13 (101) | 101 (101) | 0.09 (0.2) | 0.2/0.4(0.8) | 6 (6) |
| Aur-37 | 17/4 (17) | >547 | 0.3/0.5 (1) | 0.3/0.5 (0.5) | 9 (9) |
| Aur-38 | 17/9 (17) | >547 | 0.3 (0.3) | 0.3/0.5 (0.5) | 9 (9) |
| Aur-39 | 16/8 (16) | >503 | 0.5 (0.5) | 0.2/0.5 (0.5) | 8/31 (31) |
| Aur-40 | 6/3 (23) | 23/91 (91) | 0.3 (0.7) | 0.3 (0.3) | 1/6 (11) |
Another study has also shown that auranofin inhibited, in a concentration-dependent manner, the growth of Helicobacter pylori TrxR (Gram-negative bacteria) and showed synergistic or additive effect with known H. pylori antibiotics such as amoxicillin or metronidazole and clarithromycin. Also, when the phosphane ligand on auranofin was replaced by N-heterocyclic carbene as shown in Figure 20, stability and inhibitory activity of the complexes were not altered greatly, and they show reduced in vitro toxicity.319
Figure 20.

(a) Inhibitory effect of auranofin on the activity of H. pylori TrxR. Reproduced from ref 319. Copyright 2016 Oxford University Press. (b) Combination studies of Auranofin with known H. pylori antibiotics. (c) Structures of NHC-Auranofin studied against H. pylori.
Schmidt et al. evaluated the activity of eight Au(I)-NHC complexes against ESKAPE bacteria, the bactericidal activity of the complexes Au(I)-NHC 1–8 (Chart 18) was in the lower micromolar range for Gram-positive bacteria and Gram-negative bacteria showed resistance to drug treatment, this results compared to earlier works suggest that the nature of the NHC used can be a factor in determining the activity of this class of compound and the lack of glutathione in many Gram-positive bacteria resulted in greater dependence on the thioredoxin/thioredoxin reductase system.320 Thus, sensitizing such cells to these gold compounds that inhibit TrxR. Pyrazine functionalized Au(I)-NHC complexes have also been studied as potential antibiotics. These stable neutral (Au-NHC 9) and cationic (Au-NHC 10) complexes inhibit biofilm formation, and are potent against pathogens that showed resistance to antibiotics with MIC of 2–16 μg/mL, and are nontoxic to the red blood cells with docking studies suggesting affinity for the Dap-type peptidoglycan thereby inhibiting the synthesis of cell wall.321 Another report incorporating derivatives of estrogen, ethinylestradiol (Au-NHC 12) and ethisterone (Au-NHC 13) to carbenes was shown to have lower in vitro antibacterial activity compared to the precursor carbenes in both S.aureus and E. coli strain and the complexes were nontoxic in an in vivo experiment.322
Chart 18.

Chemical Structures of Au(I)-NHC Complexes Studied for Their Antibacterial Activities
Bussing et al. also reported Au(I)-NHC complexes and their Au(III) counterpart and examined their antibacterial and thioredoxin inhibition (Chart 19). The oxidation state of the gold complexes did not affect the cytotoxicity as both classes of compounds are similar in activity with higher bactericidal activity in Gram-positive bacteria (E. faecium, methicillin-resistant S. aureus, MRSA) compared to Gram-negative bacteria (A. baumannii, E. coli, K. pneumoniae, P. aeruginosa) studied. Using an enzymatic assay, the inhibition of thioredoxin was studied in isolated E. coli TrxR. All complexes inhibited TrxR with an IC50 of 0.2–0.6 μM suggesting its mechanism of action to be inhibition of thioredoxin.323
Chart 19.

Chemical Structures of Au(I)/(III)-NHC Complexes Studied for Their Antibacterial Activities
Recent studies on Au(I) selenium NHC complexes by Chen et al. showed potent antibacterial activity on multidrug-resistant bacterial strains both in vitro and in vivo comparable to auranofin. The antibacterial mechanism of action of these Au(I) selenium complexes were related to cellular DNA degradation and irreversible inhibition of the bacterial TrxR via targeting the redox-active motif.324
Cationic Au(I) benzothiazoles (Chart 20) complexes have been shown to inhibit the spread of A. baumannii in skin and soft tissue infection (SSTI) model experiment. The inhibition of A. baumannii, a Gram-negative bacterium by Au-BTZ 1 and Au-BTZ 2 compared to neutral AuCl(PPh3) was attributed to the ability of the cationic complexes to move through the cell wall and interfere with biological processes in the cell.325
Chart 20.

Chemical Structures of Au(I) Benzothiazoles
Alkynyl gold(I) complex is another class of compound that has been studied for their bactericidal activity (Chart 21). Novel alkynyl chromone or flavone complexes bearing Au(I)PPh3 were synthesized in good yields and compared with Au(I)PPh3 against both Gram-positive and Gram-negative bacteria strains. The complexes exhibited high bactericidal activity with a MIC between 1 and 4 μg/mL for Au-chromone-1 and Au-chromone-2 comparable to Au(I) TPP in strains of Gram-positive bacteria S. aureus but were not effective in Gram-negative bacteria E. coli.326
Chart 21.

Chemical Structure of Alkynyl Au(I) Complexes and Their Antibacterial Activity
Pintus et al. synthesized Au(III) dithiolate complexes (Chart 22) and evaluated their antimicrobial activity against 10 strains of Gram-positive and Gram-negative bacteria using the agar diffusion method. The compounds were selective in their action as it inhibited growth from the 2 strains of Staphylococcus tested, this could be because of permeable cell surface structure of cocci Gram-positive bacteria compared to other strains examined. The difference in metabolism of Staphylococcus compared to Streptococcus strains may account for the disparity in sensitivity of Au(III)-dithiolate-1 to both strains. Au(III)-dithiolate-1 also interfered with biofilm formation in strains Staphylococcus at a concentration of 3.125 μg/mL.327 Fontinha and co-workers (Chart 22) further synthesized and tested 6 Au(III) bis(dithiolate) and evaluated their antibacterial and anticancer activity. Antibacterial activity of S. aureus Newman, and E. coli was determined using the microdilution method. Only Au(III)-dithiolate-2 inhibited the growth of S. aureus Newman with MIC value of 12.5 μg/mL, while other compounds had MIC values greater than 125 μg/mL indicating no inhibition. Although the MICs of these Au(III) dithiolate complexes are higher than auranofin, they provide a new structural class in expanding the library of Au(III) antibacterial agents.328
Chart 22.

Chemical Structures of Au(III)-Dithiolate Studied for Their Antibacterial Activity
To understand the mechanism of action of Au(III) complexes, Chakraborty et al. synthesized several cyclometalated Au(III) complexes. All complexes studied were inactive against E. coli and B. subtilis bacteria strains except Au(III)ĈN–Cl2 and [Au(III)ĈN^NŜ]PF6 (Chart 23) that inhibited B. subtilis colonies comparable to kanamycin, a potent antibiotic. Further experiment to decipher the mode of action of Au(III)ĈN–Cl2 on B.subtilis reveals that the compound has no effect on bacterial membrane permeability, membrane potential and there was no generation of reactive oxygen species, but a decrease in overall energy levels of the cells. RNA sequencing result shows that several metal transporters, oxidoreductases, and proteases were upregulated while genes involved in cell wall formation and ABC transporter genes were downregulated. Overall, a multimodal mechanism of action was proposed involving various cellular stress response pathways.329
Chart 23.

Chemical Structure of Cyclometalated Au(III) Complexes and Kanamycin with Their Bactericidal Activity
7.3.3. Antileishmanial Gold Complexes.
The disease burden imposed by leishmania parasites remains a major public health concern, particularly in the tropics. Current treatment approaches for visceral and cutaneous forms of leishmaniasis include miltefosine, pentamidine, paromomycin, amphotericin B, and the antimonates (urea stibamine, sodium stibogluconate, meglumine antimoniate). Whereas these agents have been employed for decades, effectiveness is a challenge in several cases, prompting resistance and requiring higher doses of drugs. New agents such as auranofin display strong leishmanicidal activity, thus uncovering an untapped chemical space for gold complexes in the treatment of leishmaniasis. The premise for the use of Au(I) complexes is derived from the established interaction of Au(I) with the thioredoxin machinery in humans that is akin to the trypanothione system in leishmania parasites for redox homeostasis.330 Establishment of auranofin as an antileishmanial agent was through a high-throughput screening campaign to identify novel antileishmanial chemotypes using a library of 2,157 bioactive compounds. Auranofin showed prominent growth inhibition of L. amazonensis promastigote and dose–response assays with EC50 comparable to the established antileishmanial drug, amphotericin B (Chart 24). In a Balb/c mouse model inoculated with 106 metacyclic L. major promastigotes, auranofin dosed 20 mg/kg/d × 10 d, or liposomal amphotericin B at 12.5 mg/kg/d × 10 d, showed comparable lesion suppression. These impressive results expanded. An expanded structural study of the gold pharmacophore against leishmaniasis provides impetus for antileishmanial drug discovery.331 For a discussion into the potential mechanisms and biological targets of leishmaniasis, we refer readers to a recent review by Abbehausen et al.332
Chart 24.

Chemical Structures and Antileishmanial Activity of Auranofin and Amphotericin B
Structure–activity relationship studies using neutral Au(I) complexes of the type Cl–Au-L, where L represent different monodentate phosphine ligands of electronic and steric variability, significant modulation of antileishamanial activity was observed.331 As shown in Table 4, water-soluble phosphines showed attenuated antileishmanial response, implicating lipophilicity as an important descriptor.
Table 4.
Antileishmanial Activity of Au(I) Complexes
| Compound | structure Cl—Au—L, where L = | prom GI EC50 (μM) | prom tox EC50 (μM) | L. amazonensis CBA EC50 (μM) |
|---|---|---|---|---|
| AuLeish-1 | P(t-Bu)2(p-(N(CH3)2)Ph) | 0.11 ± 0.02 | 10.6 ± 0.4 | 0.2 ± 0.1 |
| AuLeish-2 | P(Cy)2(t-Bu) | 0.18 ± 0.07 | NTa | 0.7 ± 0.3 |
| AuLeish-3 | P(Ph)(C5H12)2 | 0.3 ± 0.1 | 8.9 ± 0.7 | 0.23 ± 0.17 |
| AuLeish-4 | P(t-Bu)2(o-(3,5-diphenyl-1H-pyrazole)Ph) | 0.37 ± 0.04 | 6.0 ± 3.0 | 0.8 ± 0.1 |
| AuLeish-5 | P(Et)3 | 0.39 ± 0.04 | 2.7 ± 0.9 | 0.27 ± 0.08 |
| AuLeish-6 | P(Ph)2(cy) | 0.5 ± 0.1 | 8.2 ± 0.5 | 0.27 ± 0.03 |
| AuLeish-7 | P(Ph)(Et)2 | 0.6 ± 0.1 | 6.8 ± 0.5 | 0.22 ± 0.08 |
| AuLeish-8 | P(Ph)2(t-Bu) | 0.7 ± 0.1 | 11.8 ± 0.2 | 0.3 ± 0.2 |
| AuLeish-9 | P(Ph)2(i-Pr) | 0.8 ± 0.3 | 6.5 ± 1.0 | 0.17 ± 0.05 |
| AuLeish-10 | P(Ph)2(Et) | 0.8 ± 0.1 | 7.0 ± 0.5 | 0.3 ± 0.1 |
| AuLeish-11 | P(Ph)3 | 1.3 ± 0.1 | NT | 0.5 ± 0.2 |
| AuLeish-12 | P(Ph)(Me)2 | 1.4 ± 0.2 | 8.9 ± 1.1 | 0.14 ± 0.03 |
| AuLeish-13 | P(cy)2(N,N-dimethylaminobiphenyl) | 1.5 ± 0.5 | NT | 0.18 ± 0.02 |
| AuLeish-14 | P(Ph)2(Bz) | 2.1 ± 0.3 | NT | 0.6 ± 0.1 |
| AuLeish-15 | P(Ph)(CH2CH2CN)2 | 2.4 ± 0.2 | NT | 0.13 ± 0.02 |
| AuLeish-16 | P(Ph)2(4-biphenyl) | 2.5 ± 0.2 | 10.2 ± 0.4 | 0.12 ± 0.02 |
| AuLeish-17 | P(p-FPh)3 | 3.0 ± 0.3 | 13.7 ± 0.4 | 0.2 ± 0.1 |
| AuLeish-18 | P(Ph)2(2-pyridine) | 3.5 ± 0.6 | 13.6 ± 0.4 | 0.46 ± 0.02 |
| AuLeish-19 | P(p-(OCH3)Ph)3 | 3.8 ± 1.0 | 15.0 ± 0.1 | 0.2 ± 0.1 |
| AuLeish-20 | P(Ph)2(p-(N(CH3)2)Ph) | 3.9 ± 1.3 | NT | 0.4 ± 0.2 |
| AuLeish-21 | P(Ph)2(CH2CH2NCOCH2CH2Ph) | 4.2 ± 1.2 | NT | 0.16 ± 0.05 |
| AuLeish-22 | P(cy)3 | 4.4 ± 2.1 | NT | 0.5 ± 0.1 |
| AuLeish-23 | P(p-(CH3)Ph)3 | 4.6 ± 1.1 | NT | 0.5 ± 0.4 |
| AuLeish-24 | P(Ph)2(CH2CHCH2) | 5.3 ± 1.1 | NT | 0.50 ± 0.04 |
| AuLeish-25 | P(Ph)2(p-(NH2)Ph) | 5.5 ± 0.2 | >20 | 0.21 ± 0.04 |
| AuLeish-26 | P(Ph)2(CH2CH2NCOCH2Ph) | 5.6 ± 0.3 | >20 | 0.14 ± 0.06 |
| AuLeish-27 | P(2-furan)3 | 6.0 ± 0.9 | NT | 0.5 ± 0.2 |
| AuLeish-28 | P(p-ClPh)3 | 6.9 ± 1.3 | NT | 0.30 ± 0.04 |
| AuLeish-29 | P(Ph)2(p-(CO2H)Ph) | 7.6 ± 2.0 | NT | 0.40 ± 0.15 |
| AuLeish-30 | P(3,5-(CF3)2Ph)3 | 9.4 ± 0.6 | NT | 0.4 ± 0.1 |
| AuLeish-31 | P(1-naphthalene)3 | 10.5 ± 0.9 | NT | 0.9 ± 0.2 |
| AuLeish-32 | P(p-(CF3)Ph)3 | 16.8 ± 7.0 | NT | 0.3 ± 0.1 |
| AuLeish-33 | P(Ph)2(m-(SO3H)Ph) | 17.4 ± 3.5 | NT | 0.6 ± 0.2 |
| AuLeish-34 | P(Cy)2(o-Tol) | >20 | NT | 0.7 ± 0.3 |
| AuLeish-35 | P(Ph)2(m-(CO2H)Ph) | >20 | NT | 0.15 ± 0.05 |
| AuLeish-36 | P(Ph)(p-(SO3H)Ph)2 | >20 | NT | 0.3 ± 0.1 |
| AuLeish-37 | P(CH2CH2COOH)3 | >20 | NT | 0.70 ± 0.01 |
| AuLeish-38 | P(p-(SO3H)Ph)3 | >20 | NT | 0.15 ± 0.02 |
Antileishmanial activity of gold(I) compounds in L. amazonensis promastigote growth inhibition assays. Cl, chloride; prom, promastigote; GI, growth inhibition; tox, toxicity; CBA, cell-based amastigote; NT, not toxic or growth inhibitory. Data are presented as mean ± SD.
Benzimidazole-ligated Au(I) and Au(III) complexes (Chart 25) represent another class of antileishmanial agents with activity against promastigotes of L. amazonensis, L. braziliensis, and L. major.333 The condensation of o-phenylenediamine with benzaldehyde or p-anisaldehyde generated ligands in respectable yields for Au(I) or Au(III) metalation. Although the antileishmanial effects of these complexes are modest in the range of (1–54 μM), it provides a framework to expand this class of compounds.
Chart 25.

Chemical Structures of Benzimidazole Supported Au(I)/Au(III) Antileishmanial Agents
Gold-derived antileishmanial complexes bearing NHC ligands have shown encouraging results. The NHC ligands enable the formation of Au–C bonds for stabilization and demonstrates potential for diversification. Beginning with the synthesis of imidazolium salts, metalation to form carbene Au complexes could be either through transmetalation with Ag or direct metalation with Au in the presence of a base. Both monofunctionalized carbene and bis-carbene Au complexes have been achieved through the process (Chart 26).334,335 The antileishmanial activity of Au(I)-NHC complexes are in the sub micromolar range in promastigotes of L. major or L. infantum and Leishmania intracellular amastigotes. Notably, mononuclear neutral Au(I)-NHC complexes with asymmetric imidazole substitution achieve nanomolar inhibitory concentrations in L. infantum axenic amastigotes with selectivity index of >40.336 Encouraging results demonstrated the ease of functionalizing NHCs and the broad tunable characteristics imparted on biological response by NHC ligands make them attractive for exploration in leishmaniasis drug discovery. Recently Nolan et al. reported Au(III) bis-carbene complexes that act as potent inhibitors of -glucosidase and -glucuronidase and antileishmanial activity of 0.11–1.62 μM in Leishmania major promastigotes.
Chart 26.

Chemical Structures of Au(I)/Au(III)-NHC Antileishmanial Complexes
A new class of Au(I)-oxadiazole complexes (Chart 27) with antileishmanial activity was synthesized by the reaction of Au(PEt3)Cl or Au(PPh3)Cl and 5-phenyl-1,3,4-oxadiazole-2-thione ligands.337 The aromatic ring of the ligand accommodates different substituent groups, ranging from gluconolactone,338 electron withdrawing NO2, F, and Cl groups to electron donating, OCH3 groups that affect the electronic character of the complex and contribute to diversity. The antileishmanial activity did not discriminate against chemical modification.
Chart 27.

Chemical Structures of Au(I) Oxazole Complexes
In a more elaborate study with translational potential, Monte-Neto and co-workers synthesized a new class of adamantane substituted oxadiazole or thiazolidine Au(I) phosphines as antileishmanial agents.339 The complexes inhibit thioredoxin reductase in mammalian cells and trypanothione reductase in parasites, eliciting potent antileishmanial activity in the low micromolar range across multiple leishmania species via oxidative stress. In vivo efficacy demonstrates that combination of the Au agents with Miltefosine reduces lesions by up to 65% and parasitic load of up to 80% by luminescence measurements (Figure 21). Taken together, these studies set the stage for clinical development of AdT Et as a monotherapy or in combination with existing drugs for the elimination of leishmaniasis.
Figure 21.

(A) Chemical structures of adamantane Au(I)-oxazole/thiazolidinone derivatives. (B) In vivo efficacy of Au complexes in combination with Miltefosine. Reproduced from ref 339. Copyright 2020 American Chemical Society.
7.3.4. Anticancer Gold Complexes.
Several research groups have capitalized on the hopeful clinical development of auranofin for cancer therapy to develop new Au(I) and Au(III) anticancer complexes with the goal of uncovering novel mechanisms and targets, improve in vivo potency and minimize potential side effects. We describe such endeavors in subsequent sections of this Review. It is important to note that perturbations made to Au(I) and Au(III) scaffolds through ligand modification remain at their peak. Here, we discuss efforts to create novel gold complexes that are structurally distinct and have potential for targeted therapy. Given that recent reviews on gold in biology have focused on the anticancer action, we pivot this section to recent developments toward new anticancer scaffold and targeting strategies to achieve highly efficacious Au agents. Specifically, we have chosen to focus on organelle-specific targeting, tumor targeting using ligands or antibodies, and immunochemotherapy involving gold complexes.
7.3.4.1. Mitochondrial Targeting of Gold Complexes.
Mitochondria is commonly referred to as the powerhouse of the cell due to its abundant production of ATP through the redox driven oxidative phosphorylation process.340–344 In addition to being an energy hub, mitochondria are involved in anabolic and catabolic biological processes that facilitate cell signaling, differentiation, immune signaling, growth and cell death pathways.95,345–351 The mitochondria structure is defined by an outer membrane, which protects the protein-rich matrix of the organelle and the inner mitochondria membrane that is home to the electron transport chain responsible for mitochondria respiration. Intrinsic functions of the organelle include mitochondrial respiration/bioenergetics, dynamics (fusion/fission), morphology, fatty acid oxidation, and superoxide-mediated signaling to mention a few. Increasing evidence implicates mitochondria dysfunction in cancer, representing a viable target. Although many examples of gold complexes that modulate mitochondria function are known, uncovering direct targets beyond thioredoxin have been underexplored.
Early reports by Berners Price demonstrated that cationic Au(I) analogs bearing bis-phosphine or bis-carbene ligands could induce mitochondrial dysfunction through mitochondrial uncoupling activity and the disruption of thioredoxin system.95,352,353 Recent developments in omics technology have contributed to gold drug discovery in ways that uncover new mitochondrial pathways or targets. The negative inner membrane potential of the mitochondria serves as a driving force for the accumulation of lipophilic cationic structures. This phenomenon has been well studied and selective mitochondria targeting via cationic ligands has been recently reviewed.109 The Lewis acidic character of gold as earlier described coupled with the lipophilic ligands can often generate cationic Au complexes with degrees of lipophilicity >2. This structural feature makes such complexes innately attracted to the redox active mitochondria. Awuah and co-workers have exploited this feature to uncover novel mitochondria pathways impacted by rationally designed Au(I) and Au(III) complexes.
An interesting discovery of Au complexes that perturb mitochondria structure offers new tools and potential therapeutics for the treatment of diseases.336,354 Phosphine and arsine supported Au(I) complexes ligated by N^N-bidentate ligands afford unsymmetrical cationic structures in three-coordinate geometry, referred to as AuTri (Figure 22).355,356 The different Au–N bonds of the metal center to the bidentate ligand dictates the asymmetry. The rational was to rely on the labile Au–N bond for binding to macromolecules under physiological conditions. Using transmission emission tomography (TEM), the AuTri compounds induced distortions of the mitochondria structure with concomitant time-dependent depletion of mitochondria membrane proteins including, OPA1, MFN1, MFF, and TOM20 by Western blot. A global proteomics study of AuTri-9 treated in comparison to untreated breast cancer cells showed that differentially expressed proteins were largely mitochondria membrane proteins. Overall, this work highlights the potential to identify new Au complexes that target novel biological targets and further supports the report that disruption of mitochondrial structure proteins may overcome cancer drug resistance.
Figure 22.

(a) Chemical structures of three-coordinate Au(I), AuTri complexes. (b) Transmission electron microscopy of known cell death inducers, vehicle control, and AuTri-9 in MDA-MB-231. (c) Maximal cristae width. Data are representative of 10 cells chosen at random n = 10, where mitochondria were also chosen at random. (d) Immunoblots of OPA1, MFF, MFN1, and TOM20. Reproduced from ref 355. Copyright 2021 American Chemical Society.
Derivatives of Au(III) dithiocarbamate (AuDTC) have been prepared with strong proteosome inhibition and anticancer activity against breast and prostate cancer.357–360 These complexes possessed dibromido ligands and peptide ligands ligated to the Au center via a thiolate moiety. Modifications to AuDTC by incorporating ĈN-cyclometalated ligands and tuning the ancillary ligands with different dialkyl dithiocarbamate ligands generate highly potent Au(III) complexes with selective mitochondria targeting (Chart 28).361 Using transcriptomics, bioenergetics, and function mitochondrial assays the lead AuDTC complex displayed cancer cell selective inhibition of mitochondrial respiration. The impact of ligand tuning cannot be underestimated in the design of Au(III) anticancer agents, particularly in the context of mechanism of action and potency.
Chart 28.

Synthetic Scheme to Obtain a Library of Gold(III) Dithiocarbamate Complexes
A new class of organogold(III) complexes was synthesized by the reaction of ĈN-cyclometalated Au(III) complexes with bis-phosphine ligands under mild conditions, herein referred to as AuPhos (Chart 29).362 The geometry of the complexes could be square planar or square pyramidal depending on the ligand. Whereas the varying geometry is an interesting finding, more work is required to provide guiding principles and insights into the structural phenomenon. Interestingly, AuPhos modulates mitochondria activity with high potency across the NCI-60 panel and in vivo tumor inhibition in the aggressive 4T1 TNBC mouse model. Combined transcriptomics and proteomics reveal the mitochondrial electron transport chain as a potential target for the lead AuPhos-89 complex.362 A chiral form of AuPhos, using the chiral QuinoxP ligand gave rise to AuPhos-19363 which induces ATF4 activation and inhibits mitochondria respiration acutely with potent in vivo activity.175 A careful examination of the speciation of this class of compounds supports stability under physiological conditions with minor Au(I) species and concomitant reductively eliminated aryl(C)–S bond formation under reducing glutathione conditions. Expanding the chemical library of AuPhos has enormous potential for therapeutic discovery.
Chart 29.

Synthetic Scheme and SAR Depicted Library of Cyclometalated Gold(III) Phosphine Complexes
Independent studies by Ang and Awuah developed Au(III)-metformin complexes, herein 3met or auraformin (Chart 30) with significant efficacy against TNBC.364,365 Coordination of the N-donor ligands from the FDA approved metformin to the ĈN-cyclometalated Au(III) center afford square planar prodrugs with superior potency to metformin up to 6000-fold. Auraformin-1 (Chart 30) reportedly accumulates significantly in the mitochondria of MDA-MB-468 cells to efficiently impair mitochondria respiration and depolarize the mitochondria membrane. Similarly, the 3met (Auraformin-2) was reported to disrupt energy metabolism in MDA-MB-231 cells and induce ER stress and autophagy.365 In vivo efficacy of 3met (Auraformin-2) was demonstrated in athymic nude mice with orthotopic implantation of MDA-MB231 cells in the mammary fat pad. Significant tumor reduction was noted at 15 mg/kg after 3 weeks. The promising in vivo activity of this class of compounds establishes a platform for translational application in the treatment of aggressive cancer.
Chart 30.

Cyclometalated Gold(III) Complexes Ligated to Metformin and Derivatives Thereof
7.3.4.2. Gold Conjugated Cancer Targeting Ligands.
Direct interaction of anticancer agents with tumors can be greatly enriched by selective targeting of overexpressing proteins or receptors in cancer that often act as biomarkers. Gold complexes conjugated to cell adhesion molecules (e.g., integrins), epidermal growth factor receptors, G-protein coupled receptors (e.g., bombesin), hormone receptors and glucose transporters have been explored (Chart 31).366 The use of cancer targeting ligands and peptides, either linear or cyclic have been demonstrated in vitro. For example, integrins overexpressed in multiple sold tumors such as breast cancer are heterodimeric transmembrane receptors composed of an - and - subunit noncovalently associated with each other. Conjugation of RGD peptides to Au(III)ĈN–Cl2 complexes via a dithiolate moiety led to Au(III)-RGD constructs A and A′ with improved efficacy in breast cancer.367 Additionally, the conjugation of pyrazine supported pincer Au(III) complex [Au(bbfpz)(acbim)]+ (bbfpz = 2,6-bis(4-(tert-butyl)phenyl)-pyrazine; acbim = 1-methyl-3-(4-(6-aminohexyl)-carboxamido)benzylbenzimidazol-2-ylidene) to a derivative of 17-ethinylestradiol afforded Au(III)-ER conjugates, B with potent cytotoxicity and uptake in ER(+) breast cancer cells than ER(−) cells.368 Moreover, linear Au(I) complexes can be tethered to EGFR inhibitors such as erlotinib, C to improve anticancer action by ~68-fold in EGFR positive breast cancer, MDA-MB-231.369 Conjugating the human epidermal growth factor receptor (HER2) antibody, Trastuzumab or otherwise known as Herceptin to Au(PPh3)(DPTP) (DPTP = 2,5-dioxopyrrolidinyl-3-(1H-1,2,3-triazol-4-yl)propanoate) or Au-(PPh3)(MBANHS) (MBANHS = 4-mercapto-benzylmaleimido propionamide) via N-hydroxysuccinimide or maleimide groups respectively achieved constructs D and E with enhanced cytotoxicity in breast cancer cells expressing HER2 compared to the parent Au(I) complexes.370 Au(III) biotin complexes F have also been developed to target cancers that overexpress biotin receptors (BR). Selectivity of these constructs were achieved in BR(+) MCF7 compared to BR(−) HCT-116 cells.368 Despite the promising results of these targeting constructs, the lack of validation in isogenic cell lines or in vivo is a major bottleneck.
Chart 31.

Chemical Structures of Targeting Ligand Tethered Gold Agents
7.3.4.3. Immunogenic Cell Death (ICD) Induction by Gold Complexes.
Other metal complexes and several gold complexes have been shown to induce immune-potentiating effects.371,372 Gold(I) compounds not only act on tumor cells and immune cells directly, but also affect the expression of cell adhesion molecules on endothelial cells.373 Despite chemotherapy commonly increasing the risk of secondary infections via myelosuppression and lymphocytopenia, indicating that it may lead to immune suppression, an appropriate combination of cytotoxic chemotherapy and immunotherapy may exert a highly synergistic anticancer activity.373–375 Innate immunity forms the first line of defense in the human immune system. For example, NK cells are natural immune effector cells with a direct killing function that plays a key role in the clearance of tumor cells. Metal drugs have been shown to upregulate signals on cancer cells that are perceptible to the NK cell compartment, such as the NKp30 ligand B7–H6F.376 Gold compounds such as [Au(C–C-2-NC5H4)(PTA)] induce colorectal carcinoma cell death via ROS-mediated necroptosis by activating and signaling and also have been shown to exert an immunosuppressive role by inhibiting IB kinase activation and promoting cell apoptosis.377–379 Au(I) can oxidize inside phagocyte lysosomal compartments, resulting in Au(III), which plays the role of a major hapten that acts synergistically in innate immunity.380 Elie et al. investigated the antimetastatic effects of gold compounds in renal cancer cells and revealed strong inhibition of several cytokines (IL17A, IL-8, IL-6, and IL-5) by gold compounds.377–379,381 Various studies have shown that gold compounds can elicit an innate immune response, which can be ascribed to the triggering of TLR3 rather than TLR4.382 Additionally, to innate immunity, adaptive immunity presents another unique angle to approach gold-based immunotherapy. Although not thoroughly researched, a seminal study suggested that gold compounds contribute to the frequent development of adaptive immunity by directly triggering TLR3 and increasing the expression of downstream mediators.383
Immunogenic cell death is a form of cell death that can stimulate the immune response to antideath cell antigens, especially those derived from tumor cells.384 Gold compounds in combination with CRISPR/Cas9-mediated disruption of PD-L1 and mild hyperthermia induce the activation of immunogenic cell death.384,385 Additionally, gold compounds eliminate primary tumors and induce immunogenic cell death via the release of damage-associated molecular patterns (DAMPs), activation of effector cells, and induction of dendritic cell maturation. These phenomena, in a coordinated manner, eventually evoke systematic anticancer immune responses.386,387
Recently, Sessler and Arambula et al. reported Au(I) bis N-heterocyclic carbene (NHC) that induce ICD in vitro and in vivo (Figure 23).387 A potential mechanism for this phenomenon is the inhibition of thioredoxin system and promotion of ER stress to promote type II ICD as evidenced by CRT translocation and the release of ATP and HMGB1. Further, Balb/c mice were subcutaneous injected with CT26 cells pretreated with Au-ICD (5, 10, and 100 μM) and subsequently challenged with na>ve, live CT26 cells on the other flank. Strikingly, delayed or no tumors developed on the challenged in a manner consistent with the concentration of Au-ICD dosed. Demonstrably, gold compounds can induce ICD in vivo and have potential for cancer vaccine development.
Figure 23.

(a) Au(I) complex Au-ICD induces immunogenic cell death (ICD) in a CT26 colon cancer cell. (b) Depiction of in vivo experiments carried out with Au-ICD. Reproduced from ref 387. Copyright 2020 American Chemical Society.
7.3.4.4. Gold Compounds Targeting Cancer Stem Cells.
Eradication of cancer stem cells (CSCs) represents a difficult challenge in the effective treatment of cancer patients. Given the capacity of CSCs for self-renewal, differentiation and secondary tumor formation, CSCs can evade conventional chemotherapy regimen and drive tumor relapse in treated patients.388,389 Current standard of care chemotherapy agents for treatment of patients and many reported organometallic complexes are ineffective in removing CSCs.390 Hence, the need for improved treatment options effective against both bulk tumor cells as well as CSCs.
Zou et al. reported binucleargold(I) complex with mixed bridging diphosphine and bis(N-heterocyclic carbene) ligands that inhibited self-renewal ability in HeLa and U-87 MG human glioblastoma cells in vivo with 79% tumor volume inhibition in nude mice bearing HeLa xenografts.391 Roeseh et al. synthesized 6-membered phosphorus heterocycles Au(I) compounds and examined their anticancer activity in both glioblastoma cancer cells (GCC) and glioblastoma stem-like cells (GSC). The compounds were potent in GCC and demonstrated observable decrease in wound closure in glioblastoma stem-like cells.392
Suntharalingam and co-workers developed gold(I) complexes bearing nonsteroidal anti-inflammatory drugs (NSAID) to target breast cancer stem-cells (Figure 24).393 Lead complex containing indomethacin moiety showed greater inhibitory effect (80-fold) against breast CSCs than the bulk breast cancer cell population. An inquiry into the mechanism of action of the lead complex revealed cytoplasmic accumulation of the complex, inhibition of cyclooxygenase-2 (COX-2) and increased levels of intracellular ROS. In vivo efficacy for the gold(I)-indomethacin complex was demonstrated in 4T1 tumor-bearing mice; tumor was significantly reduced without affecting mice body weight. This work further demonstrates that targeting CSCs is an effective strategy for cancer treatment.393
Figure 24.

(a) Chemical structure of gold(I) complex bearing indomethacin moiety, identified as Au(I)-indo. (b) Assessment of the cell viability of HMLER-shEcad cells treated with Au(I)-indo only and in combination with z-VAD-FMK and PGE2 at 5 μM and 20 μM respectively. (c) In vivo efficacy of Au(I)-indo in 4T1 tumor bearing mice. Reproduced with permission from ref 393. Copyright 2023 Royal Society of Chemistry.
Sun et al. also reported a gold(III) meso-tetraphenylporphyrin complex that inhibit formation of spheroids from single cell suspension in U-87 glioblastoma cancer cells. The porphyrin complex demonstrated potent in vitro and in vivo toxicity in a panel of cancer cells and high physiological stability in glutathione and serum albumen. Furthermore, there was a reduction in NANOG expression (stemness marker), while deregulating 16 microRNAs linked to glioblastoma stem cell function.394
7.3.5. Antiviral Gold Complexes.
Given the timing of the review coinciding with the latter half of the pandemic, it is important to highlight the potential antiviral properties of a few gold complexes. The current outbreak of SARS-CoV-2 has resulted in an unprecedented health crisis with the number of infected well into the millions.395 The lack of an effective antiviral drug for the treatment has triggered a major surge in drug-development, specifically transition metal complexes given their success in the past. The urgent development of an effective therapeutic is an utmost priority for medicinal chemists across the globe.
Despite the long-standing history of gold complexes in medicine, it was without question that chemists would turn to gold-based complexes by either (a) repurposing old drug candidates and (b) developing new innovative scaffolds. The application of gold complexes as antiviral drugs has not been studied very intensively, although some promising results suggest a possible future use as human immunodeficiency virus (HIV) therapeutics.41,396,397 Gil-Moles et al. reported a pilot study in which select gold complexes were investigated to determine their activity against two coronavirus targets (spike protein, papain like protease, and PLpro) (Figure 25).159
Figure 25.

(a) Simple illustration of the life cycle of the SARS-CoV-2, golden bars represent gold drugs that target viral entry process and replication. Reproduced from ref 159. Copyright 2020 John Wiley and Sons. (b) Gold(I) and gold(III) benzimidazole complexes used in evaluating antiviral properties against SARS-CoV-2.
An enzymatic FRET assay was used to determine the antiviral activity of gold compounds against PLpro from SARS-CoV-1 and SARS-CoV-2. The IC50 for Au-1, Au-2, and Au-5 against PLpro from SARS-CoV-1 was determined to be within the range of 5–7 μm. This range is similar to the inhibitory concentration of Disulfiran, which was used as a reference for comparison. The gold complexes Au-3 and Au-4 showed less antiviral effect with an IC50 of 14 μm, while Auranofin had the least inhibitory effect with with IC50 of 25.5 μm as represented in Table 5.159
Table 5.
Inhibitory Values of Benzimidazole-Based Gold Complexes against Replication of Spike-ACE2
| Complex | Spike-ACE2 (IC50 μM) | PLpro SARS-CoV-1 (IC50 μm) | PLpro SARS-CoV-2 (IC50 μm) |
|---|---|---|---|
| benzimidazole | >100 | >100 | >100 |
| Chloroquine | 31.9 ± 5.4 | n.d. | n.d. |
| Disulfiram | n.d. | 6.5 ± 0.4 | 1.05 ± 0.34 |
| Auranofin | 22.2 ± 2.8 | 25.5 ± 1.2 | 0.75 ± 0.13 |
| Au-1 | 19.4 ± 5.7 | 6.3 ± 1.6 | 1.04 ± 0.02 |
| Au-2 | 20.0 ± 2.3 | 5.5 ± 0.5 | 1.44 ± 0.22 |
| Au-3 | 23.1 ± 6.8 | 14.2 ± 0.3 | >100 |
| Au-4 | 25.0 ± 4.2 | 14.1 ± 2.1 | >50 |
| Au-5 | 16.2 ± 2.4 | 6.7 ± 0.9 | 0.96 ± 0.07 |
A recent review profiled inhibition of SARS-CoV-2 by structurally diverse metal complexes including 36 gold(I)/(III) complexes.398 Inhibition of SARS-CoV-2 can occur either by the interaction of spike protein with the ACE2 receptor or by the papain-like protease PLpro. For instance, chloroauric acid showed a moderate inhibition (about 47% inhibitory activity) while the other gold compounds were poorly active or inactive.398
Also, structure–activity relationship studies reveals a preference for complexes with good leaving groups (e.g., chloride) compared to complexes with firmly coordinated ligands such as dicarbene gold complexes of the type [(NHC)2Au]+, which were inactive. Gold(III) dithiocarbamate glycoconjugates showed strong selectivity against SARS-CoV-2 PLpro.398 The gold complexes studied showed strong toxicity against Caco-2 cell line except for four complexes which were then selected and tested for SARS-CoV-2 antiviral assay, with the [Au(I)-NHC] complex showing excellent activity at micromolar range.
Auranofin has also been shown to inhibit SARS-CoV-2 replication in human cells (Huh7 cells) at a low concentration (EC50 1.4 μM)52 with about 95% reduction in the viral RNA at 48 h after infection. Treatment with auranofin showed a reduction of SARS-CoV-2-induced cytokines expression levels in human cells. These results indicate that auranofin could be potent to limit SARS-CoV-2 infection and associated lung injury due to its antiviral, anti-inflammatory and antireactive oxygen species (ROS) properties. Further in vivo study is required to establish the safety and efficacy of auranofin for the management of SARS-CoV-2 associated disease.399,400
Furthermore, highly active antiretroviral therapy (HAART) has caused decreased death rate from acquired immune deficiency syndrome due to human immunodeficiency virus.401 However, acquired drug resistance has hindered the success of current HAART, therefore the need for improved therapeutics.402–404 In addition, reports exist on gold-based inhibitors of reverse transcriptase (RT), protease (PR) and viral entry of host cells.339,405–409
Taken together, the antiviral properties of gold complexes prove to be a critical field of study for medicinal chemists to tackle as approaches to develop therapeutics remains to be confined to simply repurposing old drugs such as auranofin. Given the success auranofin has had and promising characteristics, it is up to current day medicinal chemists to explore more innovative avenues in developing new gold-based therapeutics for antiviral therapies.
7.3.6. Gold in Inflammatory Bowel Diseases.
Gold compounds (in this case auranofin) have been shown to decrease the expression of inflammatory cytokines (IL-1, IL-6 and TNF) in rheumatoid arthritis patients as well as inhibits the expression of nuclear factor kappa beta (NF-kB) which has been associated with chronic inflammatory diseases e.g., IBD.378,410–413 Given this finding, to date there have been scarce attempts at purposing gold-based complexes for IBD therapy.
In 2012, seminal work by Travnicek et al. reported the synthesis of a class of AuPPh3 complexes with anti-inflammatory activity (Chart 32).414 These complexes exhibited a strong ability to reduce the production of pro-inflammatory cytokines such as TNF-a, IL-1, and HMGB1 without effecting secretion of anti-inflammatory cytokines from LPS activated macrophages. The complexes significantly influenced the formation of edema induced by polysaccharide carrageenan in vivo. Notably, these compounds were significantly less toxic than auranofin in culture.
Chart 32.

Synthetic Scheme and Structures of Gold Complexes Investigated for Anti-inflammatory Effects in Several Cancer Cell Lines
Several Au(I) complexes bearing O-substituted 9-deazahypoxanthine derivatives (1–5; Chart 33) have been reported for their antitumor and anti-inflammatory activity. The compounds show potent anticancer activity in a panel of cancer cell lines (MCF7, HOS, A549, G361, A2780, A2780R, 22Rv1, and THP-1) with IC50s in the range of 0.6–22.8 μM. In addition, the complexes show no cross-resistance to cisplatin and are more efficacious than cisplatin in the cell lines tested. The complexes show significant selectivity for cancer cells compared to normal HEP220 cell lines. Furthermore, results from the anti-inflammatory activity of 1–5 (Chart 33) revealed that the complexes significantly decreased the production of and , attenuating the production of pro-inflammatory cytokines by blocking NF- κB signaling and inhibiting IκB degradation similar to auranofin. Also, in vivo anti-inflammatory activity of 2 (Chart 33) in a carrageenan-induced hind paw edema model reveals a pronounced antiedematous effect comparable to the FDA approved Indomethacin.415
Chart 33.

Synthetic Scheme and Depiction of Au(I) Complexes Used for Anti-inflammatory Purposes
Another recent report by Bodio et al. developed BODIPY tagged gold(I)-imidazole bimetallic complexes (as seen in section 5.1.1), which exhibited anti-inflammatory effects.227 Although these complexes were designed with anticancer therapies in mind the researchers discovered that BDP-Au7 is far less toxic: viability of PBMC is slightly superior to 60% at 10 μM. Interestingly, at 1 μM, BDP-Au7 inhibits more than 30% of the production of IL-1 without displaying any toxicity, and at 3 μM, BDP-Au7 inhibits almost all the production of IL-1 with low toxicity.
Recently, work by Wempe et al. utilized a novel gold(III) complex, termed AuPhos developed by Awuah and co-workers for the treatment of ulcerative colitis.416 Initial pharmacokinetics and biodistribution studies revealed that oral administration of AuPhos demonstrated high rates of adsorption into the small intestine and colon compared to systemic adsorption while displaying a dose-dependent uptake in IEC mitochondria. In vivo studies revealed that mice treated with AuPhos showed lower disease activity index (DAI), histology score, and FITC-dextran compared to vehicle control. Mechanistically, oral administration of AuPhos increased crypt fissioning near the mucosa while simultaneously reducing mRNA expression of pro-inflammatory cytokines.416 Further studies by the group in a piroxicam-accelerated (Px) knockout mice (an accelerated colitis model) showed that administration of AuPhos led to reduced DAI, weight loss and less crypt ablation and hyperplasia evidenced by HE sectioning.417 Mice administered with AuPhos had decreased DAI, reduction in weight loss, and resulted in less crypt ablation and hyperplasia evidenced by HE sectioning. RT-qPCR of tissue from Px-IL10 KO mice treated with AuPhos revealed significant increases in mitochondrial complex I genes (Ndufa1, Ndufa4, Ndufb6), complex IV gene (Cox5B), and stem cell markers (Lgr4, Lgr5, and Lrig1), with corresponding decreases in pro-inflammatory markers (IL-1, MCP1, and RankL).417,418 These new promising findings suggest that gold complexes can be tuned to modulate bioenergetics and metabolism to prevent inflammation-associated barrier damage when subjected to chronic colitis conditions.
8. TARGETING MODALITIES AND NANODELIVERY OF BIOACTIVE GOLD COMPLEXES
Nanobased constructs for the delivery of therapeutic agents have been clinically transformative. The ability to control the size, chemical, magnetic, and biological properties of nanocarriers and their drug cargo make nanoconstructs an excellent platform for drug delivery. Also, their enhanced bioavailability and controlled drug release profiles offer advantages for targeted delivery that minimize toxic side effects or improve efficacy.419–424 Nanodrug delivery can occur either by active or passive targeting. In active targeting, the surface of the nanocarrier is coated with ligands such as peptides and antibodies that promote recognition of specific receptors or proteins overexpressed at the target site whereas in passive targeting, the physicochemical properties of the nanocarrier such as size, shape, pH, dictate affinity, internalization and enhances permeability and retention (EPR) at target sites.423,425–427 Development of nanodelivery constructs for gold complexes have been described, employing different nanocarriers, such as liposomes, polymeric, apoferritin, albumen, collagen, and mesoporous silica materials. These have recently been reviewed by different authors,428–430 thus we refrain from giving a detailed narrative here. Nevertheless, in a review of next generation gold drugs and probes, it is imperative that we provide an overview of the significant scientific and preclinical advances made in the nanodelivery of defined gold-based complexes.
8.1. Polymeric Nanoparticles
Au(I)-loaded poly(-amino ester) micelle-like nanoparticles have been reported by Wang et al. This pH-sensitive Au(I) polymeric nanoparticle triggers cancer cell death by autophagy. Evidence for lysosomal accumulation via endocytosis and consequent pH-driven nanoparticle degradation is the likely mechanism of the Au(I) cargo (Figure 26). The released Au(I) agent subsequently inhibits TrxR activity to increase intracellular ROS, enhance oxidative stress and induce cell death.431
Figure 26.

Diagram showing dissociation of pH sensitive gold(I)-loaded poly(-amino ester)s micelle-like nanoparticles in the lysosomes and mechanism of synergistic induction of cell death. Reproduced from ref 431. Copyright 2015 American Chemical Society.
The in vivo anticancer potency of auranofin is limited by rapid ligand displacement upon interaction with serum albumin in circulation.432,433 To circumvent this limitation, Stenzel et al. developed micellar analogs of auranofin using glycopolymer-based self-assembled micelles (Figure 27). The reported analogs were cytotoxic to OVCAR-3 ovarian cancer cells (in both serum-containing media and serum-free media) and less liable to deactivation by serum proteins compared to free auranofin, possibly due to the protection offered by the micelle system. However, the micellar analogs accumulate in the lysosomes unlike free auranofin, which interacts with TrxR. This suggests that the micellar nanoconstructs may have a mechanism of action distinct from auranofin.434
Figure 27.

Formation of spherical micelles from polymeric auranofin. Reproduced from ref 434. Copyright 2015 American Chemical Society.
The triblock polymer, Pluronic F127 in combination with the amphiphilic peptide of the type (C18)2-PEG1000-G-CCK8, was used by Fregona and co-workers to form supramolecular aggregates that deliver Au(III) dithiocarbamate to enhance bioavailability. The functionalization of this aggregate system with cholecystokinin octapeptide (CCK8) act as a targeting moiety to improve tumor specificity. The resulting nanoconstruct demonstrated stability in saline solution up to 72 h and the CCK8 targeting moiety contributed to improved cytotoxicity and selectivity between A431 cells and CCK2-R-transfected A431 cells.435
Owing to the excellent physiological stability, anticancer activity, and the ability of Au(III) porphyrins (AuP) to form nanostructure, other approaches have also been utilized to deliver Au(III) porphyrin selectively to target cells. Che et al. developed Au(III) porphyrin–PEG conjugates [Au(TPP–COO–PEG5000–OCH3)]Cl and [Au(TPP–CONH– PEG5000– OCH3)]Cl that self-assemble into nanostructures.436 The conjugates feature an ester linkage that is easily hydrolyzed, leading to release of the chemotherapeutic Au(III) porphyrin [Au(TPP–COOH)]+ in vitro and in vivo. The nanostructures showed selective cytotoxicity in cancer cells ((HeLa, NCI-H460, HCT116, A2780) compared to normal cells. The lead Au(III) porphyrin–PEG conjugate [Au(TPP–COO–PEG5000–OCH3)]Cl (Au–P–P in Figure 28) significantly inhibited tumor growth in HCT116 xenografts tumor bearing mice.436 These studies highlight the potential for polymer-based self-assembled nanoparticles to facilitate the delivery of gold-derived therapeutics.
Figure 28.

(A) Structure of Au(III) porphyrin–PEG conjugate [Au(TPP–COO–PEG5000–OCH3)]Cl (Au–P–P). (B) Changes in tumor volume in HCT116 xenografts tumor bearing mice after treatment with the indicated complexes. Reproduced with permission from ref 436. Copyright 2017 Royal Society of Chemistry.
Recently, Kao and Che et al. utilized a multifunctional hydrogel and microparticle system to deliver AuP in a lung cancer xenograft.437,438 AuP was loaded into polyethylene glycol (PEG)-diacrylate (PEGdA) or an interpenetrating network system (IPN) composed of PEGdA and gelatin conjugated with PEG-cysteine (Gel-PEG-Cys). Results showed that increasing the mole ratio of PEG-400 to AuP from 636:1, 1270:1, 2540:1, 5650:1, 11,300:1, 25,400:1, to 67,800:1 led to the corresponding decrease in size of the AuP-PEG-400 constructs from 12.07 ± 1.40 μm, 5.61 ± 0.91 μm, 4.68 ± 1.28 μm, 3.37 ± 1.95 μm, 2.80 ± 0.36 μm, 1.03 ± 0.71 μm, to 0.23 ± 0.03 μm, respectively. The cumulative release profile of AuP-loaded IPN reached about 65% after 7 days following an initial burst within the first 24 h compared to the AuP-loaded PEGdA that showed about 30% release of AuP after 7 days. Cell cytotoxicity studies showed that AuP-loaded IPN exhibited significantly higher cytotoxicity in A549 and NCI-H460 lung cancer cells compared to IPN control in vitro and inhibited tumor growth in mice.437
8.2. Lipid-Based Micelles
Sterically stabilized micelles (SSM) of DSPE-PEG2000, and sterically stabilized mixed micelles (SSMM) composed of egg l--phosphatidylcholine (PC) or 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC) phospholipids (with different DSPE-PEG2000 mol ratio) as delivery systems for Au(III)-dithiocarbamate complexes have been reported. Bombesin peptide derivatives were incorporated into the micelles to improve targeting. The liposomal constructs enabled Au(III) dithiocarbamate stability, selective uptake and anticancer potential in PC-3 cells overexpressing GRP/bombesin receptors (an autocrine growth factor receptor in tumor cells).439
8.3. Apoferritin Nanoparticles
Some protein-based molecules have been used as drug delivery constructs. These macromolecules are naturally assembled protein subunits of the same protein with reduced toxicity.440 Ferritin is a blood protein for iron storage.441,442 Apoferritin can be loaded with different drugs for delivery into target cells. Merlino and co-workers developed Au(III) oxo-apoferritin complex(Apt-Auoxo) (Figure 29).443 The encapsulation of Auoxo into the ferritin core was confirmed by ICP-MS. Of note, Auoxo is capable binding histidine and cysteine side chains of proteins, which may offer insights into the mode of interaction between Auoxo and apoferritin.444,445 The Apt-Auoxo nanoparticles showed significant cytotoxicity in cancer cells compared to normal cell.443
Figure 29.

(a) Structure of the trans isomer of Auoxo3. (b) An illustration showing Auoxo3 encapsulation within apoFt (Aft) nanocage. Reproduced with permission from ref 443. Copyright 2016 Royal Society of Chemistry.
Recently, apoferritin encapsulated Au(III) thiosemicarbazones were synthesized by Zhang et al. and demonstrates high potency in glioma cancer cells with the ability to cross the blood brain barrier (Figure 30). Apoferritin-AuNPs are taken up via lysosome-mediated endocytosis in U87MG glioma cells with selective accumulation in tumors as well as promising in vivo tumor inhibition.446 Taken together, these are encouraging studies that highlight the potential of apoferritin nanoparticles for efficacious gold-based therapy.
Figure 30.

Development of the AFT-NP based Au(III) delivery system. (a) Loading of Au(III) into apoferritin. (b) Acquired SEM images of AFt nanocage and AFt-Au(III) NPs. (c) AFt and AFt-(III) NPs in glass vials. (d) Graph showing Au(III) release in vitro from the AFt-Au(III) NPs. (f) The ability of AFt-Au(III) NPs cells to target U87MG cells in vitro is assessed via ICP-MS analysis. (g) The intracellular uptake of Cy5.5-labeled AFt-Au(III) NPs by U87MG tumor cells is examined by confocal microscopy. (h) The intracellular uptake of Cy5.5-labeled AFt-Au(III) NPs by HL-7702 tumor cells is examined by confocal microscopy. Reproduced from ref 446. Copyright 2020 American Chemical Society.
8.4. Silica-Based Nanoparticles
Silica-based materials such as mesoporous silica nanocarrier (MSN) have also been used as carriers for bioactive gold compounds. Silica is considered safe by the FDA and has unique properties such as excellent encapsulation efficiency, facile large-scale production, large surface area and adjustable uniform pore size, which makes MSN a good delivery system.447–451 Che et al. reported AuP (Au-1@MSN(R)), an RGD-functionalized MSN nanoparticle carrying gold(III) porphyrin complex as cargo. The nanoparticle displayed higher anticancer activity and selectivity to normal cells compared to the free Au(III) porphyrin complex and inhibited thioredoxin reductase as a mode of apoptotic cancer cell death.452
8.5. Peptide-Based Nanoparticles
Peptides are gaining attention as promising nanosized drug delivery systems.453–455 Among several properties of peptide delivery systems, are that they undergo proteolytic degradation by proteases overexpressed by recalcitrant TNBC and renal cancer cells.456,457 Therefore, an interesting approach to increase the potency of cytotoxic gold agents is by utilizing peptide-based nanoparticle delivery systems to improve cancer cell selectivity. Recently, Contel, Ulijn, and colleagues reported the encapsulation of Au(I) N-heterocyclic carbene compounds in amphiphilic decapeptides (Figure 31). Peptide self-assembly of gold compounds, 1 or 2 and subsequent free gold precipitation and centrifugation resulted in the peptide nanostructures, which were characterized by AFM, TEM, FTIR, and zeta potential analysis. Varying encapsulation efficiency of the different peptides at 1 mM and two stock concentrations (10 μM or 500 μM) of gold compounds 1 or 2 was observed. The combination of compound 1 at 10 μM and the AD peptide yielded an encapsulation efficiency of >60%. The gold-loaded nanostructures displayed significant cytotoxicity in MDA-MB-231 and Caki-1 (renal carcinoma) cells with selectivity compared to noncancerous cells, IMR-90 (lung fibroblast). It is assumed that the proteolytic degradation of peptide filament encapsulating the drug facilitate the drug uptake by the cancer cells.458 Hence, this highlights an interesting approach to improve drug selectivity for cancer cells and consequently its cytotoxic effect.
Figure 31.

Illustration of drug-loaded peptides and structures of drugs and peptides used in this study. Reproduced from ref 458. Copyright 2022 American Chemical Society.
8.6. Noncovalent Self-Assembled Nanoparticles
Gold(III) porphyrins (AuP) display superior anticancer efficacy and with structural modifications can self-assemble to nanostructures without responsive nanocarriers. This noncovalent self-assembly strategy has been previously employed in other Sn-, Zn-, and Gd-based porphyrin systems for photocatalytic and photodynamic therapy applications.459–461 In applying this approach to gold, an Au(III) tetra-(4-pyridyl) porphyrin (AuTPyP) nanosphere (AuPNS) capable of generating intracellular ROS, and thioredoxin inhibition for synergistic chemo-photothermal therapy of tumors (Figure 32) was recently described by Bai and Shi et al.462 Full characterization of AuPNS by FTIR, XPS, and TEM support the development of spherical nanostructures with an average diameter of ~65 nm. Further functionalization of AuPNSs with cRGD produced cRGD-AuPNS, which showed improved overall pharmacokinetic behavior than free AuPNSs. Treatment of HeLa tumor-bearing mice with cRGD-AuPNS (10 mg/kg) and light irradiation (635 nm, 0.8W/cm2) for 5 min resulted in 100% tumor inhibition rate. The approach represents a new paradigm for efficacious gold-based cancer therapy.
Figure 32.

Schematic illustration of the noncovalent self-assembled Au(III) porphyrin and the heat/acid dual responsiveness of cRGD-AuPNSs for synergistic chemo-photothermal therapy of a tumor. Reproduced from ref 462. Copyright 2022 American Chemical Society.
9. CONCLUSION AND FUTURE OUTLOOK
This Review highlights research to develop next generation gold-based drugs to treat diseases and chemical probes to interrogate human physiology. As summarized throughout this Review, we articulate the rich history of gold and its relevance throughout medicinal breakthroughs and bring to prominence efforts to elucidate the mechanism of novel gold complexes. As outlined in the Review, a great deal of effort has been invested in repurposing old gold-based drugs as well as the development of novel gold complexes, notably stable Au(III) complexes, which were previously challenging to develop. To energize the scientific and medical communities, we stringed together fundamental discoveries of gold chemistry considering its applicability in basic biology such as diagnostics; radiotherapy; and preclinical studies in several disease indications as well as translational clinical trials. Subsequently, the pursuit of a more mechanistic investigation on how Au(I) and Au(III) complexes function in model systems received a boost from new omics technologies. What was a long-antiquated field in medicinal applications has now emerged as a burgeoning area of scientific rigor. As scientists have revisited the field of gold chemistry in medicine, more compound libraries have been made and have been examined to understand their true mechanism(s) of action. Furthermore, we describe novel mechanistic insights that have been published by experts all over the globe. From DNA targeting to covalent modification of proteins, and metabolic regulation just to highlight a few. Gold-derived complexes display immense potential in modulating diseases and offer new chemical tools for researchers to elucidate elusive biological processes and targets. The long history of gold in humans including FDA approved agents and current clinical trials emboldens the rationale to pursue gold drug/probe discovery. Therefore, it is critical to revisit gold-based therapeutics with a fresh sense of innovation that builds on the progress made thus far. This Review not only highlights the new classes of gold agents synthesized but further touches on the vast number of diseases in which gold has found success within the past decade. Moreover, we detail the application of gold compounds in a plethora of diseases including cancer, bacterial, leishmaniasis, microbial infections, and inflammation (e.g., RA and IBD). We posit that gold-derived agents are of therapeutic value to numerous disease indications.
Though impressive strides have been made, the stability of gold complexes remains a bottleneck toward the development of new libraries and scaffolds. This Review highlights complexes prepared by novel synthetic strategies. We must mention that detailed synthetic methodologies for the preparation of gold complexes are out of the scope of this Review. However, readers are encouraged to visit the articles cited at the end of the Review to peruse creative synthetic strategies outlined as well as a recently reviewed strategies to preparing gold anticancer complexes.463 Despite the drawbacks, new possibilities have arisen within the past decade to developing next generation gold agents beyond auranofin, the “gold standard.” Progressively, evidence of (i) the importance of gold in medicine, (ii) the success of gold in clinical trials (14 to date), (iii) the determination and creativity of scientists, and (iv) state-of-the-art technologies will propel next generation gold agents into clinical use. Leveraging the development of new gold-based libraries and high-throughput screens has the potential to accelerate first-in-class gold-based drugs/probes.
Overall, this Review highlights how fundamental discoveries of gold chemistry and mechanisms of gold action in biology have become cornerstones for researchers across the globe to unlock tool compounds and therapeutic agents that were unthinkable even 10 years ago. With the advancement of cutting-edge molecular biology tools, omic technologies, and preclinical/translational science, furthering the potential of gold-derived complexes into the clinic has never been more attainable.
ACKNOWLEDGMENTS
This work and S.G.A. was supported by grant R01CA258421-01 from the National Cancer Institute and NSF Grant No. 2203559. Figure 1 was generated using BioRender.com.
Biographies
R. Tyler Mertens was born in Madisonville, Kentucky. He received his B.S. in chemistry from Centre College. He then earned his Ph.D. in 2021 under the advisement of Prof. Samuel G. Awuah at the University of Kentucky where he developed novel gold complexes to target metabolism in cancer. In late 2021, he joined the laboratory of Prof. Roni Nowarski in the Department of Immunology at Harvard Medical School as a postdoctoral fellow where he focuses on understanding mechanisms behind metabolic regulation of inflammation during colitis.
Sailajah Gukathasan received her Ph.D. from the University of Kentucky under the supervision of Prof. Samuel G. Awuah, where her work focused on the unique niche of developing gold-based reagents for protein modifications. She is currently a postdoctoral research fellow in the lab of Prof. Eranthie Weerapana at Boston College. Her research focuses on chemical proteomics. She is originally from the idyllic city of Jaffna, Srilanka, and graduated B.Sc. with honors in chemistry and zoology from the University of Jaffna, Srilanka.
Adedamola S. Arojojoye received his B.Tech degree from Ladoke Akintola University of Technology, and MSc. degree in Chemistry from University of Lagos, Nigeria. Motivated to pursue advanced research, he proceeded to the University of Kentucky for his PhD in Chemistry. He joined Awuah Lab in Fall 2019, and his research focuses on synthetic strategies for stable organogold(I/III) complexes using structurally diverse ligands and understanding their mechanism of cytotoxic action to develop next generation therapeutics.
Chibuzor Olelewe received his BSc degree in Biochemistry from the University of Nigeria. His interest in drug discovery which lies at the interface of Chemistry and Biology motivated him to pursue a graduate degree to acquire the requisite skills needed to be successful in this field of science. As a graduate student in the Awuah lab, he is interested in understanding the mechanism of action of gold-based small molecules designed with potential as chemotherapeutics.
Samuel G. Awuah received a BSc degree in Chemistry from Kwame Nkrumah University of Science and Technology, Ghana, and a PhD from University of Oklahoma. After postdoctoral training at MIT, he accepted a position as an assistant professor of Chemistry and Pharmaceutical Sciences (joint) at the University of Kentucky. His work on gold complexes has contributed to the development of new structural scaffolds to unravel elusive targets such as mitochondrial structure and dynamics to cure diseases. He developed a new protein bioconjugation strategy known as the metal-mediated ligand affinity chemistry that utilizes transition metals such as gold in a proximity guided approach to covalently modify protein targets at their endogenous sites.
Footnotes
The authors declare the following competing financial interest(s): Samuel G. Awuah has patents pending to University of Kentucky Research Foundation.
Complete contact information is available at: https://pubs.acs.org/10.1021/acs.chemrev.2c00649
Contributor Information
R. Tyler Mertens, Department of Chemistry, University of Kentucky, Lexington, Kentucky 40506, United States.
Sailajah Gukathasan, Department of Chemistry, University of Kentucky, Lexington, Kentucky 40506, United States.
Adedamola S. Arojojoye, Department of Chemistry, University of Kentucky, Lexington, Kentucky 40506, United States
Chibuzor Olelewe, Department of Chemistry, University of Kentucky, Lexington, Kentucky 40506, United States.
Samuel G. Awuah, Department of Chemistry, University of Kentucky, Lexington, Kentucky 40506, United States Department of Pharmaceutical Sciences, College of Pharmacy, University of Kentucky, Lexington, Kentucky 40536, United States; University of Kentucky Markey Cancer Center, Lexington, Kentucky 40536, United States.
REFERENCES
- (1).Shaw CF Gold-Based Therapeutic Agents. Chem. Rev. 1999, 99, 2589–2600. [DOI] [PubMed] [Google Scholar]
- (2).Barnard PJ; Berners-Price SJ Targeting the Mitochondrial Cell Death Pathway with Gold Compounds. Coord. Chem. Rev. 2007, 251, 1889–1902. [Google Scholar]
- (3).Best SL; Sadler PJ Gold Drugs: Mechanism of Action and Toxicity. Gold Bull. 1996, 29, 87–93. [Google Scholar]
- (4).Dominelli B; Correia JDG; Kühn FE Medicinal Applications of Gold(I/III)-Based Complexes Bearing N-Heterocyclic Carbene and Phosphine Ligands. J. Organomet. Chem. 2018, 866, 153–164. [Google Scholar]
- (5).Glišić BĐ; Djuran MI Gold Complexes as Antimicrobial Agents: An Overview of Different Biological Activities in Relation to the Oxidation State of the Gold Ion and the Ligand Structure. Dalton Trans. 2014, 43, 5950–5969. [DOI] [PubMed] [Google Scholar]
- (6).Yue S; Luo M; Liu H; Wei S Recent Advances of Gold Compounds in Anticancer Immunity. Front. Chem. 2020, 8, 543–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Zou T; Lum CT; Lok C-N; Zhang J-J; Che C-M Chemical Biology of Anticancer Gold(III) and Gold(I) Complexes. Chem. Soc. Rev. 2015, 44, 8786–8801. [DOI] [PubMed] [Google Scholar]
- (8).Lu Y; Ma X; Chang X; Liang Z; Lv L; Shan M; Lu Q; Wen Z; Gust R; Liu W Recent Development of Gold(I) and Gold(III) Complexes as Therapeutic Agents for Cancer Diseases. Chem. Soc. Rev. 2022, 51, 5518–5556. [DOI] [PubMed] [Google Scholar]
- (9).Fernández-Moreira V; Herrera RP; Gimeno MC Anticancer Properties of Gold Complexes with Biologically Relevant Ligands. Pure Appl. Chem. 2019, 91, 247–269. [Google Scholar]
- (10).Roder C; Thomson MJ Auranofin: Repurposing an Old Drug for a Golden New Age. Drugs in R&D 2015, 15, 13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Balfourier A; Kolosnjaj-Tabi J; Luciani N; Carn F; Gazeau F Gold-Based Therapy: From Past to Present. Proc. Natl. Acad. Sci. U. S. A. 2020, 117, 22639–22648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Bartlett N Relativistic Effects and the Chemistry of Gold. Gold Bull. 1998, 31, 22–25. [Google Scholar]
- (13).Schwerdtfeger P; Dolg M; Schwarz WHE; Bowmaker GA; Boyd PDW Relativistic Effects in Gold Chemistry. I. Diatomic Gold Compounds. J. Chem. Phys. 1989, 91, 1762–1774. [Google Scholar]
- (14).Handschuh H; Ganteför G; Bechthold PS; Eberhardt W A Comparison of Photoelectron Spectroscopy and Two-Photon Ionization Spectroscopy: Excited States of Au2, Au3, and Au4. J. Chem. Phys. 1994, 100, 7093–7100. [Google Scholar]
- (15).Pizlo A; Jansen G; Heß BA; von Niessen W Ionization Potential and Electron Affinity of the Au Atom and the Auh Molecule by All-Electron Relativistic Configuration Interaction and Propagator Techniques. J. Chem. Phys. 1993, 98, 3945–3951. [Google Scholar]
- (16).Mertens RT; Awuah SG Gold Catalysis: Fundamentals and Recent Developments. Catalysis by Metal Complexes and Nanomaterials: Fundamentals and Applications 2019, 1317, 19–55. [Google Scholar]
- (17).Gorin DJ; Toste FD Relativistic Effects in Homogeneous Gold Catalysis. Nature 2007, 446, 395–403. [DOI] [PubMed] [Google Scholar]
- (18).Guerra MF; Calligaro T Gold Cultural Heritage Objects: A Review of Studies of Provenance and Manufacturing Technologies. Meas. Sci. Technol. 2003, 14, 1527–1537. [Google Scholar]
- (19).James TGH Gold Technology in Ancient Egypt. Gold Bull. 1972, 5, 38–42. [Google Scholar]
- (20).Higby GJ Gold in Medicine. Gold Bull. 1982, 15, 130–140. [DOI] [PubMed] [Google Scholar]
- (21).Parish RV Gold in Medicine - Chrysotherapy. Interdiscip. Sci. Rev. 1992, 17, 221–228. [Google Scholar]
- (22).Pricker SP Medical Uses of Gold Compounds: Past, Present and Future. Gold Bull. 1996, 29, 53–60. [Google Scholar]
- (23).Huaizhi Z; Yuantao N China’s Ancient Gold Drugs. Gold Bull. 2001, 34, 24–29. [Google Scholar]
- (24).Kean WF; Kean IR Clinical Pharmacology of Gold. Inflammopharmacology 2008, 16, 112–125. [DOI] [PubMed] [Google Scholar]
- (25).Pagel W Paracelsus: An Introduction to Philosophical Medicine in the Era of the Renaissance; Basel; & New York, 1958; p 399. [Google Scholar]
- (26).Multhauf R The Significance of Distillation in Renaissance Medical Chemistry. Bulletin of Historical Medicine 1956, 38, 329–346. [PubMed] [Google Scholar]
- (27).Stillman J The Story of Alchemy and Early Chemistry.; Dover Publication, 1924; p 555. [Google Scholar]
- (28).Partington J A History of Chemistry; Macmillan, 1961; p 795. [Google Scholar]
- (29).Starobinski J History of the Treatment of Melancholy from the Earliest Times to 1900; J.R. Geigy, 1972; p 100. [Google Scholar]
- (30).Zirkle C; Fulton JF; Drabkin IE; Boyer CB; Cohen IB; Strelsky K Eighty-First Critical Bibliography of the History of Science and Its Cultural Influences (to 1 January 1956). Isis 1956, 47, 247–360. [Google Scholar]
- (31).Royal College of Physicians of London and Urdang, G. Pharmacopeia Londinensis of 1618; Reproduced facsimile, 1944; p 299.
- (32).Chrestien J De La Methode Latraleptique; Ou Observations. Edinb. Med. Sur. J. 1811, 11, 239–243. [Google Scholar]
- (33).Barclay G The Keeley League. J. Ill. State Hist. Soc. 1964, 577, 344–364. [Google Scholar]
- (34).Koch R Report of Address at 10th International Medical Congress; Dutch Media Worchenschrift, Berlin, Germany, 1890. 756–757. [Google Scholar]
- (35).Forestier J The Treatment of Rheumatoid Arthritis with Gold Salts Injections. Lancet 1932, 219, 441–444. [Google Scholar]
- (36).Dequeker J; Verdickt W; Gevers G; Vanschoubroek K Longterm Experience with Oral Gold in Rheumatoid Arthritis and Psoriatic Arthritis. Clin. Rheumatol. 1984, 3, 67–74. [DOI] [PubMed] [Google Scholar]
- (37).Gaynor D; Griffith DM The Prevalence of Metal-Based Drugs as Therapeutic or Diagnostic Agents: Beyond Platinum. Dalton Trans. 2012, 41, 13239–13257. [DOI] [PubMed] [Google Scholar]
- (38).Lawrence JS Comparative Toxicity of Gold Preparations in Treatment of Rheumatoid Arthritis. Ann. Rheum. Dis. 1976, 35, 171–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Speerstra F; Reekers P; van de Putte LB; Vandenbroucke JP; Rasker JJ; de Rooij DJ Hla-Dr Antigens and Proteinuria Induced by Aurothioglucose and D-Penicillamine in Patients with Rheumatoid Arthritis. J. Rheumatol. 1983, 10, 948–953. [PubMed] [Google Scholar]
- (40).Ammon HV; Fowle SA; Cunningham JA; Komorowski RA; Loeffler RF Effects of Auranofin and Myochrysine on Intestinal Transport and Morphology in the Rat. Gut 1987, 28, 829–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Tiekink ERT Gold Compounds in Medicine: Potential Anti-Tumour Agents. Gold Bull. 2003, 36, 117–124. [Google Scholar]
- (42).Taukumova LA; Mouravjoy YV; Gribakin SG Mucocutaneous Side Effects and Continuation of Aurotherapy in Patients with Rheumatoid Arthritis. In Rheumaderm: Current Issues in Rheumatology and Dermatology, Mallia C, Uitto J, Eds.; Springer; US, 1999; pp 367–373. [DOI] [PubMed] [Google Scholar]
- (43).Kostova I Gold Coordination Complexes as Anticancer Agents. Anticancer Agents Med. Chem. 2006, 6, 19–32. [DOI] [PubMed] [Google Scholar]
- (44).Ott I; Gust R Non Platinum Metal Complexes as Anti-Cancer Drugs. Arch. Pharm. 2007, 340, 117–126. [DOI] [PubMed] [Google Scholar]
- (45).Milacic V; Fregona D; Dou QP Gold Complexes as Prospective Metal-Based Anticancer Drugs. Histol. Histopathol. 2008, 23, 101–108. [DOI] [PubMed] [Google Scholar]
- (46).Ott I On the Medicinal Chemistry of Gold Complexes as Anticancer Drugs. Coord. Chem. Rev. 2009, 253, 1670–1681. [Google Scholar]
- (47).Nobili S; Mini E; Landini I; Gabbiani C; Casini A; Messori L Gold Compounds as Anticancer Agents: Chemistry, Cellular Pharmacology, and Preclinical Studies. Med. Res. Rev 2010, 30, 550–580. [DOI] [PubMed] [Google Scholar]
- (48).Liu L-P; Hammond GB Recent Advances in the Isolation and Reactivity of Organogold Complexes. Chem. Soc. Rev. 2012, 41, 3129–3139. [DOI] [PubMed] [Google Scholar]
- (49).Mármol I; Quero J; Rodríguez-Yoldi MJ; Cerrada E Gold as a Possible Alternative to Platinum-Based Chemotherapy for Colon Cancer Treatment. Cancers (Basel) 2019, 11, 780–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Finkelstein AE; Walz DT; Batista V; Mizraji M; Roisman F; Misher A Auranofin. New Oral Gold Compound for Treatment of Rheumatoid Arthritis. Ann. Rheum. Dis. 1976, 35, 251–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Sutton BM; McGusty E; Walz DT; DiMartino MJ Oral Gold. Antiarthritic Properties of Alkylphosphinegold Coordination Complexes. J. Med. Chem. 1972, 15, 1095–1098. [DOI] [PubMed] [Google Scholar]
- (52).Rothan HA; Stone S; Natekar J; Kumari P; Arora K; Kumar M The Fda-Approved Gold Drug Auranofin Inhibits Novel Coronavirus (Sars-Cov-2) Replication and Attenuates Inflammation in Human Cells. Virology 2020, 547, 7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Capparelli EV; Bricker-Ford R; Rogers MJ; McKerrow JH; Reed SL Phase I Clinical Trial Results of Auranofin, a Novel Antiparasitic Agent. Antimicrob. Agents Chemother. 2017, 61, No. e01947–01916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Sannella AR; Casini A; Gabbiani C; Messori L; Bilia AR; Vincieri FF; Majori G; Severini C New Uses for Old Drugs. Auranofin, a Clinically Established Antiarthritic Metallodrug, Exhibits Potent Antimalarial Effects in Vitro: Mechanistic and Pharmacological Implications. FEBS Lett. 2008, 582, 844–847. [DOI] [PubMed] [Google Scholar]
- (55).Madeira JM; Renschler CJ; Mueller B; Hashioka S; Gibson DL; Klegeris A Novel Protective Properties of Auranofin: Inhibition of Human Astrocyte Cytotoxic Secretions and Direct Neuroprotection. Life Sci. 2013, 92, 1072–1080. [DOI] [PubMed] [Google Scholar]
- (56).Messori L; Marcon G Gold Complexes in the Treatment of Rheumatoid Arthritis. Met. Ions Biol. Syst. 2004, 41, 279–304. [PubMed] [Google Scholar]
- (57).Furst DE Mechanism of Action, Pharmacology, Clinical Efficacy and Side Effects of Auranofin. An Orally Administered Organic Gold Compound for the Treatment of Rheumatoid Arthritis. Pharmacotherapy 1983, 3, 284–298. [DOI] [PubMed] [Google Scholar]
- (58).Gottlieb NL Pharmacology of Auranofin: Overview and Update. Scand. J. Rheumatol. Suppl 1986, 63, 19–28. [PubMed] [Google Scholar]
- (59).Pushpakom S; Iorio F; Eyers PA; Escott KJ; Hopper S; Wells A; Doig A; Guilliams T; Latimer J; McNamee C; Norris A; Sanseau P; Cavalla D; Pirmohamed M Drug Repurposing: Progress, Challenges and Recommendations. Nat. Rev. Drug Discovery 2019, 18, 41–58. [DOI] [PubMed] [Google Scholar]
- (60).University of Kansas Medical Center The Leukemia Lymphoma Society Kansas Bioscience Authority Therapeutics for Rare Neglected Diseases. Phase I and II Study of Auranofin in Chronic Lymphocytic Leukemia (CLL), 2011. https://ClinicalTrials.gov/show/NCT01419691 (accessed 03-20-2023).
- (61).Einhorn LH Treatment of Testicular Cancer: A New and Improved Model. J. Clin. Oncol. 1990, 8, 1777–1781. [DOI] [PubMed] [Google Scholar]
- (62).Mayo Clinic; National Cancer Institute. Auranofin and Sirolimus in Treating Participants with Ovarian Cancer, 2018. https://ClinicalTrials.gov/show/NCT03456700 (accessed 03-20-2023).
- (63).Casañal A; Lohkamp B; Emsley P Current Developments in Coot for Macromolecular Model Building of Electron Cryo-Microscopy and Crystallographic Data. Protein Sci. 2020, 29, 1055–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (64).Maveyraud L; Mourey L Protein X-Ray Crystallography and Drug Discovery. Molecules 2020, 25, 1030–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (65).Renaud J-P; Chari A; Ciferri C; Liu W.-t.; Rémigy H-W; Stark H; Wiesmann C Cryo-Em in Drug Discovery: Achievements, Limitations and Prospects. Nat. Rev. Drug Discovery 2018, 17, 471–492. [DOI] [PubMed] [Google Scholar]
- (66).Nguyen SS; Prescher JA Developing Bioorthogonal Probes to Span a Spectrum of Reactivities. Nat. Rev. Chem. 2020, 4, 476–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (67).Arjmand F; Afsan Z; Sharma S; Parveen S; Yousuf I; Sartaj S; Siddique HR; Tabassum S Recent Advances in Metallodrug-Like Molecules Targeting Non-Coding Rnas in Cancer Chemotherapy. Coord. Chem. Rev. 2019, 387, 47–59. [Google Scholar]
- (68).Wenzel M; Casini A Mass Spectrometry as a Powerful Tool to Study Therapeutic Metallodrugs Speciation Mechanisms: Current Frontiers and Perspectives. Coord. Chem. Rev. 2017, 352, 432–460. [Google Scholar]
- (69).Wang H; Zhou Y; Xu X; Li H; Sun H Metalloproteomics in Conjunction with Other Omics for Uncovering the Mechanism of Action of Metallodrugs: Mechanism-Driven New Therapy Development. Curr. Opin. Chem. Biol. 2020, 55, 171–179. [DOI] [PubMed] [Google Scholar]
- (70).Giorgio A; Merlino A Gold Metalation of Proteins: Structural Studies. Coord. Chem. Rev. 2020, 407, 213175–213200. [Google Scholar]
- (71).Merlino A Recent Advances in Protein Metalation: Structural Studies. Chem. Commun. 2021, 57, 1295–1307. [DOI] [PubMed] [Google Scholar]
- (72).Bindoli A; Rigobello MP; Scutari G; Gabbiani C; Casini A; Messori L Thioredoxin Reductase: A Target for Gold Compounds Acting as Potential Anticancer Drugs. Coord. Chem. Rev. 2009, 253, 1692–1707. [Google Scholar]
- (73).Bhabak KP; Bhuyan BJ; Mugesh G Bioinorganic and Medicinal Chemistry: Aspects of Gold (I)-Protein Complexes. Dalton Trans. 2011, 40, 2099–2111. [DOI] [PubMed] [Google Scholar]
- (74).Tepperman K; Finer R; Donovan S; Elder R; Doi J; Ratliff D; Ng K Intestinal Uptake and Metabolism of Auranofin, a New Oral Gold-Based Antiarthritis Drug. Science 1984, 225, 430–432. [DOI] [PubMed] [Google Scholar]
- (75).Weisman MH; Hardison W; Walz DT Studies of the Intestinal Metabolism of Oral Gold. J. Rheumatol. 1980, 7, 633–638. [PubMed] [Google Scholar]
- (76).Suwalsky M; Gonzalez R; Villena F; Bolognin S Structural Effects of the Au (I) Drug Auranofin on Cell Membranes and Molecular Models. J. Chil. Chem. Soc. 2013, 58, 2001–2004. [Google Scholar]
- (77).Snyder RM; Mirabelli CK; Crooke ST Cellular Association, Intracellular Distribution, and Efflux of Auranofin Via Sequential Ligand Exchange Reactions. Biochem. Pharmacol. 1986, 35, 923–932. [DOI] [PubMed] [Google Scholar]
- (78).Pickering IJ; Cheng Q; Rengifo EM; Nehzati S; Dolgova NV; Kroll T; Sokaras D; George GN; Arnér ES Direct Observation of Methylmercury and Auranofin Binding to Selenocysteine in Thioredoxin Reductase. Inorg. Chem. 2020, 59, 2711–2718. [DOI] [PubMed] [Google Scholar]
- (79).Saei AA; Gullberg H; Sabatier P; Beusch CM; Johansson K; Lundgren B; Arvidsson PI; Arnér ES; Zubarev RA Comprehensive Chemical Proteomics for Target Deconvolution of the Redox Active Drug Auranofin. Redox Biol. 2020, 32, 101491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (80).Parsonage D; Sheng F; Hirata K; Debnath A; McKerrow JH; Reed SL; Abagyan R; Poole LB; Podust LM X-Ray Structures of Thioredoxin and Thioredoxin Reductase from Entamoeba Histolytica and Prevailing Hypothesis of the Mechanism of Auranofin Action. J. Struct. Biol. 2016, 194, 180–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (81).Ilari A; Baiocco P; Messori L; Fiorillo A; Boffi A; Gramiccia M; Di Muccio T; Colotti G A Gold-Containing Drug against Parasitic Polyamine Metabolism: The X-Ray Structure of Trypanothione Reductase from Leishmania Infantum in Complex with Auranofin Reveals a Dual Mechanism of Enzyme Inhibition. Amino Acids 2012, 42, 803–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (82).Angelucci F; Sayed AA; Williams DL; Boumis G; Brunori M; Dimastrogiovanni D; Miele AE; Pauly F; Bellelli A Inhibition of Schistosoma Mansoni Thioredoxin-Glutathione Reductase by Auranofin: Structural and Kinetic Aspects. J. Biol. Chem. 2009, 284, 28977–28985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (83).Angelucci F; Dimastrogiovanni D; Boumis G; Brunori M; Miele AE; Saccoccia F; Bellelli A Mapping the Catalytic Cycle of Schistosoma Mansoni Thioredoxin Glutathione Reductase by X-Ray Crystallography. J. Biol. Chem. 2010, 285, 32557–32567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (84).Giles NM; Watts AB; Giles GI; Fry FH; Littlechild JA; Jacob C Metal and Redox Modulation of Cysteine Protein Function. Chem. Biol. 2003, 10, 677–693. [DOI] [PubMed] [Google Scholar]
- (85).Augello G; Azzolina A; Rossi F; Prencipe F; Mangiatordi GF; Saviano M; Ronga L; Cervello M; Tesauro D New Insights into the Behavior of Nhc-Gold Complexes in Cancer Cells. Pharmaceutics 2023, 15, 466–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (86).Cirri D; Geri A; Massai L; Mannelli M; Gamberi T; Magherini F; Becatti M; Gabbiani C; Pratesi A; Messori L Chemical Modification of Auranofin Yields a New Family of Anticancer Drug Candidates: The Gold (I) Phosphite Analogues. Molecules 2023, 28, 1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (87).Johnson MW; DiPasquale AG; Bergman RG; Toste FD Synthesis of Stable Gold (III) Pincer Complexes with Anionic Heteroatom Donors. Organometallics 2014, 33, 4169–4172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (88).Arojojoye AS; Mertens RT; Ofori S; Parkin SR; Awuah SG Synthesis, Characterization, and Antiproliferative Activity of Novel Chiral [QuinoxP* AuCl2]+ Complexes. Molecules 2020, 25, 5735–5748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (89).da Silva Maia PI; Deflon VM; Abram U Gold (III) Complexes in Medicinal Chemistry. Future Med. Chem. 2014, 6, 1515–1536. [DOI] [PubMed] [Google Scholar]
- (90).Messori L; Abbate F; Marcon G; Orioli P; Fontani M; Mini E; Mazzei T; Carotti S; O’Connell T; Zanello P Gold (III) Complexes as Potential Antitumor Agents: Solution Chemistry and Cytotoxic Properties of Some Selected Gold (III) Compounds. J. Med. Chem. 2000, 43, 3541–3548. [DOI] [PubMed] [Google Scholar]
- (91).Garcia Santos I; Hagenbach A; Abram U Stable Gold (III) Complexes with Thiosemicarbazone Derivatives. Dalton Trans. 2004, 677–682. [DOI] [PubMed] [Google Scholar]
- (92).Gukathasan S; Parkin S; Awuah SG Cyclometalated Gold (III) Complexes Bearing Dach Ligands. Inorg. Chem. 2019, 58, 9326–9340. [DOI] [PubMed] [Google Scholar]
- (93).Bertrand B; Williams MR; Bochmann M Gold (III) Complexes for Antitumor Applications: An Overview. Eur. J. Chem. 2018, 24, 11840–11851. [DOI] [PubMed] [Google Scholar]
- (94).Messori L; Orioli P; Tempi C; Marcon G Interactions of Selected Gold (III) Complexes with Calf Thymus DNA. Biochem. Biophys. Res. Commun. 2001, 281, 352–360. [DOI] [PubMed] [Google Scholar]
- (95).Rackham O; Nichols SJ; Leedman PJ; Berners-Price SJ; Filipovska A A Gold (I) Phosphine Complex Selectively Induces Apoptosis in Breast Cancer Cells: Implications for Anticancer Therapeutics Targeted to Mitochondria. Biochem. Pharmacol. 2007, 74, 992–1002. [DOI] [PubMed] [Google Scholar]
- (96).Wedlock LE; Kilburn MR; Cliff JB; Filgueira L; Saunders M; Berners-Price SJ Visualising Gold inside Tumour Cells Following Treatment with an Antitumour Gold (I) Complex. Metallomics 2011, 3, 917–925. [DOI] [PubMed] [Google Scholar]
- (97).Abyar F; Tabrizi L New Cyclometalated Gold (III) Complex Targeting Thioredoxin Reductase: Exploring as Cytotoxic Agents and Mechanistic Insights. Biometals 2020, 33, 107–122. [DOI] [PubMed] [Google Scholar]
- (98).Quero J; Cabello S; Fuertes T; Mármol I; Laplaza R; Polo V; Gimeno MC; Rodriguez-Yoldi MJ; Cerrada E Proteasome Versus Thioredoxin Reductase Competition as Possible Biological Targets in Antitumor Mixed Thiolate-Dithiocarbamate Gold (III) Complexes. Inorg. Chem. 2018, 57, 10832–10845. [DOI] [PubMed] [Google Scholar]
- (99).Zhang J-J; Ng K-M; Lok C-N; Sun RW-Y; Che C-M Deubiquitinases as Potential Anti-Cancer Targets for Gold (III) Complexes. Chem. Commun. 2013, 49, 5153–5155. [DOI] [PubMed] [Google Scholar]
- (100).Arojojoye AS; Kim JH; Olelewe C; Parkin S; Awuah SG Chiral Gold (III) Complexes: Speciation, in Vitro, and in Vivo Anticancer Profile. Chem. Commun. 2022, 58, 10237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (101).Zou T; Lum CT; Chui SSY; Che CM Gold (III) Complexes Containing N-Heterocyclic Carbene Ligands: Thiol “Switch-on” Fluorescent Probes and Anti-Cancer Agents. Angew. Chem., Int. Ed. 2013, 52, 2930–2933. [DOI] [PubMed] [Google Scholar]
- (102).Meier SM; Gerner C; Keppler BK; Cinellu MA; Casini A Mass Spectrometry Uncovers Molecular Reactivities of Coordination and Organometallic Gold (III) Drug Candidates in Competitive Experiments That Correlate with Their Biological Effects. Inorg. Chem. 2016, 55, 4248–4259. [DOI] [PubMed] [Google Scholar]
- (103).Hu D; Liu Y; Lai YT; Tong KC; Fung YM; Lok CN; Che CM Anticancer Gold (III) Porphyrins Target Mitochondrial Chaperone Hsp60. Angew. Chem., Int. Ed. 2016, 55, 1387–1391. [DOI] [PubMed] [Google Scholar]
- (104).Tong K-C; Hu D; Wan P-K; Lok C-N; Che C-M Anticancer Gold (III) Compounds with Porphyrin or N-Heterocyclic Carbene Ligands. Front. Chem. 2020, 8, 587207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (105).Tong K-C; Lok C-N; Wan P-K; Hu D; Fung YME; Chang X-Y; Huang S; Jiang H; Che C-M An Anticancer Gold (III)-Activated Porphyrin Scaffold That Covalently Modifies Protein Cysteine Thiols. Proc. Natl. Acad. Sci. U. S. A. 2020, 117, 1321–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (106).Buac D; Schmitt S; Ventro G; Rani Kona F; Ping Dou Q Dithiocarbamate-Based Coordination Compounds as Potent Proteasome Inhibitors in Human Cancer Cells. Mini Rev. Med. Chem. 2012, 12, 1193–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (107).Dalla Via L; Nardon C; Fregona D Targeting the Ubiquitin-Proteasome Pathway with Inorganic Compounds to Fight Cancer: A Challenge for the Future. Future Med. Chem. 2012, 4, 525–543. [DOI] [PubMed] [Google Scholar]
- (108).King AP; Wilson JJ Endoplasmic Reticulum Stress: An Arising Target for Metal-Based Anticancer Agents. Chem. Soc. Rev. 2020, 49, 8113–8136. [DOI] [PubMed] [Google Scholar]
- (109).Olelewe C; Awuah SG Mitochondria as a Target of Third Row Transition Metal-Based Anticancer Complexes. Curr. Opin. Chem. Biol. 2023, 72, 102235–102244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (110).Massai L; Zoppi C; Cirri D; Pratesi A; Messori L Reactions of Medicinal Gold (III) Compounds with Proteins and Peptides Explored by Electrospray Ionization Mass Spectrometry and Complementary Biophysical Methods. Front. Chem. 2020, 8, 581648–581662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (111).Gamberi T; Pratesi A; Messori L; Massai L Proteomics as a Tool to Disclose the Cellular and Molecular Mechanisms of Selected Anticancer Gold Compounds. Coord. Chem. Rev. 2021, 438, 213905. [Google Scholar]
- (112).Messori L; Merlino A Protein Metalation by Metal-Based Drugs: X-Ray Crystallography and Mass Spectrometry Studies. Chem. Commun. 2017, 53, 11622–11633. [DOI] [PubMed] [Google Scholar]
- (113).Mazzei L; Wenzel MN; Cianci M; Palombo M; Casini A; Ciurli S Inhibition Mechanism of Urease by Au (III) Compounds Unveiled by X-Ray Diffraction Analysis. ACS Med. Chem. Lett. 2019, 10, 564–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (114).Cirri D; Bazzicalupi C; Ryde U; Bergmann J; Binacchi F; Nocentini A; Pratesi A; Gratteri P; Messori L Computationally Enhanced X-Ray Diffraction Analysis of a Gold (III) Complex Interacting with the Human Telomeric DNA G-Quadruplex. Unravelling Non-Unique Ligand Positioning. Int. J. Biol. Macromol. 2022, 211, 506–513. [DOI] [PubMed] [Google Scholar]
- (115).Elder RC; Eidsness MK Synchrotron X-Ray Studies of Metal-Based Drugs and Metabolites. Chem. Rev. 1987, 87, 1027–1046. [Google Scholar]
- (116).Hummer AA; Rompel A The Use of X-Ray Absorption and Synchrotron Based Micro-X-Ray Fluorescence Spectroscopy to Investigate Anti-Cancer Metal Compounds in Vivo and in Vitro. Metallomics 2013, 5, 597–614. [DOI] [PubMed] [Google Scholar]
- (117).Messori L; Balerna A; Ascone I; Castellano C; Gabbiani C; Casini A; Marchioni C; Jaouen G; Congiu Castellano A X-Ray Absorption Spectroscopy Studies of the Adducts Formed between Cytotoxic Gold Compounds and Two Major Serum Proteins. J. Bio. Inorg. Chem. 2011, 16, 491–499. [DOI] [PubMed] [Google Scholar]
- (118).Merlino A; Marzo T; Messori L Protein Metalation by Anticancer Metallodrugs: A Joint Esi Ms and Xrd Investigative Strategy. Eur. J. Chem. 2017, 23, 6942–6947. [DOI] [PubMed] [Google Scholar]
- (119).Adhireksan Z; Palermo G; Riedel T; Ma Z; Muhammad R; Rothlisberger U; Dyson PJ; Davey CA Allosteric Cross-Talk in Chromatin Can Mediate Drug-Drug Synergy. Nat. Commun. 2017, 8, 14860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (120).Sun H; Zhang Q; Wang R; Wang H; Wong Y-T; Wang M; Hao Q; Yan A; Kao RY-T; Ho P-L Resensitizing Carbapenem-and Colistin-Resistant Bacteria to Antibiotics Using Auranofin. Nat. Commun. 2020, 11, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (121).Mbugua SN; Njenga LW; Odhiambo RA; Wandiga SO; Onani MO Beyond DNA-Targeting in Cancer Chemotherapy. Emerging Frontiers-a Review. Curr. Top. Med. Chem. 2021, 21, 28–47. [DOI] [PubMed] [Google Scholar]
- (122).Hurley LH DNA and Its Associated Processes as Targets for Cancer Therapy. Nat. Rev. Cancer 2002, 2, 188–200. [DOI] [PubMed] [Google Scholar]
- (123).Mullard A DNA Tags Help the Hunt for Drugs. Nature 2016, 530, 367–369. [DOI] [PubMed] [Google Scholar]
- (124).Nain V; Sahi S; Verma A Cpp-Zfn: A Potential DNA-Targeting Anti-Malarial Drug. Malar. J. 2010, 9, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (125).Reinhold WC; Thomas A; Pommier Y DNA-Targeted Precision Medicine; Have We Been Caught Sleeping? Trends in cancer 2017, 3, 2–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (126).Hurley LH; Boyd FL DNA as a Target for Drug Action. Trends Pharmacol. Sci. 1988, 9, 402–407. [DOI] [PubMed] [Google Scholar]
- (127).Rosenberg B; Van Camp L; Krigas T Inhibition of Cell Division in Escherichia Coli by Electrolysis Products from a Platinum Electrode. Nature 1965, 205, 698–699. [DOI] [PubMed] [Google Scholar]
- (128).Rosenberg B; Vancamp L; Trosko JE; Mansour VH Platinum Compounds: A New Class of Potent Antitumour Agents. Nature 1969, 222, 385–386. [DOI] [PubMed] [Google Scholar]
- (129).Roberts J; Thomson A The Mechanism of Action of Antitumor Platinum Compounds. Prog. Nucleic Acid Res. Mol. Biol. 1979, 22, 71–133. [DOI] [PubMed] [Google Scholar]
- (130).Dilruba S; Kalayda GV Platinum-Based Drugs: Past, Present and Future. Cancer Chemother. Pharmacol. 2016, 77, 1103–1124. [DOI] [PubMed] [Google Scholar]
- (131).Tchounwou PB; Dasari S; Noubissi FK; Ray P; Kumar S Advances in Our Understanding of the Molecular Mechanisms of Action of Cisplatin in Cancer Therapy. J. Exp. Pharmacol. 2021, 13, 303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (132).Dasari S; Tchounwou PB Cisplatin in Cancer Therapy: Molecular Mechanisms of Action. Eur. J. Pharmacol. 2014, 740, 364–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (133).Kilic A; Barlak N; Sanli F; Aytatli A; Capik O; Karatas OF Mode of Action of Carboplatin Via Activating P53/Mir-145 Axis in Head and Neck Cancers. Laryngoscope 2020, 130, 2818–2824. [DOI] [PubMed] [Google Scholar]
- (134).Arango D; Wilson A; Shi Q; Corner G; Aranes M; Nicholas C; Lesser M; Mariadason J; Augenlicht L Molecular Mechanisms of Action and Prediction of Response to Oxaliplatin in Colorectal Cancer Cells. Br. J. Cancer 2004, 91, 1931–1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (135).de Vries G; Rosas-Plaza X; van Vugt MA; Gietema JA; de Jong S Testicular Cancer: Determinants of Cisplatin Sensitivity and Novel Therapeutic Opportunities. Cancer Treat. Rev. 2020, 88, 102054. [DOI] [PubMed] [Google Scholar]
- (136).Tandstad T; Ståhl O; Dahl O; Haugnes H; Håkansson U; Karlsdottir C; Kjellman A; Langberg C; Laurell A; Oldenburg J; et al. Treatment of Stage I Seminoma, with One Course of Adjuvant Carboplatin or Surveillance, Risk-Adapted Recommendations Implementing Patient Autonomy: A Report from the Swedish and Norwegian Testicular Cancer Group (Swenoteca). Ann. Oncol. 2016, 27, 1299–1304. [DOI] [PubMed] [Google Scholar]
- (137).Seidel C; Oechsle K; Lorch A; Dieing A; Hentrich M; Hornig M; Grünwald V; Cathomas R; Meiler J; de Wit M Efficacy and Safety of Gemcitabine, Oxaliplatin, and Paclitaxel in Cisplatin-Refractory Germ Cell Cancer in Routine Care—Registry Data from an Outcomes Research Project of the German Testicular Cancer Study Group. In Urologic Oncology: Seminars and Original Investigations, 2016; Elsevier: Vol. 34, pp 168. [DOI] [PubMed] [Google Scholar]
- (138).Oun R; Moussa YE; Wheate NJ The Side Effects of Platinum-Based Chemotherapy Drugs: A Review for Chemists. Dalton Trans. 2018, 47, 6645–6653. [DOI] [PubMed] [Google Scholar]
- (139).Um IS; Armstrong-Gordon E; Moussa YE; Gnjidic D; Wheate NJ Platinum Drugs in the Australian Cancer Chemotherapy Healthcare Setting: Is It Worthwhile for Chemists to Continue to Develop Platinums? Inorg. Chim. Acta 2019, 492, 177–181. [Google Scholar]
- (140).Rahman SA; Arifuddin S; Abdullah N Therapeutic Response and Side Effects of Chemotherapy Combination Regimen between Paclitaxel-Cisplatin and Paclitaxel-Carboplatin on Cervical Cancer Stage Iib. Gynecol Reprod Health 2019, 3, 1–5. [Google Scholar]
- (141).Almeida CM; Nascimento GP; Magalhães KG; Iglesias BA; Gatto CC Crystal Structures, DNA-Binding Ability and Influence on Cellular Viability of Gold (I) Complexes of Thiosemicarbazones. J. Coord. Chem. 2018, 71, 502–519. [Google Scholar]
- (142).Cuya SM; Bjornsti M-A; van Waardenburg RC DNA Topoisomerase-Targeting Chemotherapeutics: What’s New? Cancer Chemother. Pharmacol. 2017, 80, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (143).Pommier Y; Leo E; Zhang H; Marchand C DNA Topoisomerases and Their Poisoning by Anticancer and Antibacterial Drugs. Chem. Biol. 2010, 17, 421–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (144).Mordente A; Meucci E; EttoreMartorana G; Tavian D; Silvestrini A Topoisomerases and Anthracyclines: Recent Advances and Perspectives in Anticancer Therapy and Prevention of Cardiotoxicity. Curr. Med. Chem. 2017, 24, 1607–1626. [DOI] [PubMed] [Google Scholar]
- (145).Marzi L; Agama K; Murai J; Difilippantonio S; James A; Peer CJ; Figg WD; Beck D; Elsayed MS; Cushman M; et al. Novel Fluoroindenoisoquinoline Non-Camptothecin Topoisomerase I Inhibitorsfluoroindenoisoquinoline Top1 Inhibitors. Mol. Cancer Ther. 2018, 17, 1694–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (146).Burton JH; Mazcko C; LeBlanc A; Covey JM; Ji J; Kinders RJ; Parchment RE; Khanna C; Paoloni M; Lana S; et al. Nci Comparative Oncology Program Testing of Non-Camptothecin Indenoisoquinoline Topoisomerase I Inhibitors in Naturally Occurring Canine Lymphomanci-Cop TopII Indenoisoquinolines. Clin. Cancer Res. 2018, 24, 5830–5840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (147).Pommier Y; Cushman M; Doroshow JH Novel Clinical Indenoisoquinoline Topoisomerase I Inhibitors: A Twist around the Camptothecins. Oncotarget 2018, 9, 37286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (148).Marzi L; Szabova L; Gordon M; Weaver Ohler Z; Sharan SK; Beshiri ML; Etemadi M; Murai J; Kelly K; Pommier Y The Indenoisoquinoline Top1 Inhibitors Selectively Target Homologous Recombination-Deficient and Schlafen 11-Positive Cancer Cells and Synergize with Olaparibtop1 Inhibitors Selective for Hrd and Slfn11-Positive Cells. Clin. Cancer Res. 2019, 25, 6206–6216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (149).Matias-Barrios VM; Radaeva M; Song Y; Alperstein Z; Lee AR; Schmitt V; Lee J; Ban F; Xie N; Qi J Discovery of New Catalytic Topoisomerase II Inhibitors for Anticancer Therapeutics. Front. Oncol. 2021, 3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (150).Matias-Barrios VM; Radaeva M; Ho C-H; Lee J; Adomat H; Lallous N; Cherkasov A; Dong X Optimization of New Catalytic Topoisomerase II Inhibitors as an Anti-Cancer Therapy. Cancers (Basel) 2021, 13, 3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (151).Akerman KJ; Fagenson AM; Cyril V; Taylor M; Muller MT; Akerman MP; Munro OQ Gold(III) Macrocycles: Nucleotide-Specific Unconventional Catalytic Inhibitors of Human Topoisomerase I. J. Am. Chem. Soc. 2014, 136, 5670–5682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (152).Che C-M; Sun RW-Y; Yu W-Y; Ko C-B; Zhu N; Sun H Gold(III) Porphyrins as a New Class of Anticancer Drugs: Cytotoxicity, DNA Binding and Induction of Apoptosis in Human Cervix Epitheloid Cancer Cells. Chem. Commun. 2003, 1718–1719. [DOI] [PubMed] [Google Scholar]
- (153).Sun RWY; Li CKL; Ma DL; Yan JJ; Lok CN; Leung CH; Zhu N; Che CM Stable Anticancer Gold(III)-Porphyrin Complexes: Effects of Porphyrin Structure. Eur. J. Chem. 2010, 16, 3097–3113. [DOI] [PubMed] [Google Scholar]
- (154).Yan JJ; Chow AL-F; Leung C-H; Sun RW-Y; Ma D-L; Che C-M Cyclometalated Gold (III) Complexes with N-Heterocyclic Carbene Ligands as Topoisomerase I Poisons. Chem. Commun. 2010, 46, 3893–3895. [DOI] [PubMed] [Google Scholar]
- (155).Li CKL; Sun RWY; Kui SCF; Zhu N; Che CM Anticancer Cyclometalated [AuIIIm (C∧ N∧ C)mL]n+ Compounds: Synthesis and Cytotoxic Properties. Eur. J. Chem. 2006, 12, 5253–5266. [DOI] [PubMed] [Google Scholar]
- (156).Wilson CR; Fagenson AM; Ruangpradit W; Muller MT; Munro OQ Gold(III) Complexes of Pyridyl- and Isoquinolylamido Ligands: Structural, Spectroscopic, and Biological Studies of a New Class of Dual Topoisomerase I and II Inhibitors. Inorg. Chem. 2013, 52, 7889–7906. [DOI] [PubMed] [Google Scholar]
- (157).Geada IL; Ramezani-Dakhel H; Jamil T; Sulpizi M; Heinz H Insight into Induced Charges at Metal Surfaces and Biointerfaces Using a Polarizable Lennard-Jones Potential. Nat. Commun. 2018, 9, 716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (158).van der Westhuizen D; Slabber CA; Fernandes MA; Joubert DF; Kleinhans G; van der Westhuizen CJ; Stander A; Munro OQ; Bezuidenhout DI A Cytotoxic Bis(1,2,3-Triazol-5-Ylidene)Carbazolide Gold(III) Complex Targets DNA by Partial Intercalation. Eur. J. Chem. 2021, 27, 8295–8307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (159).Gil-Moles M; Basu U; Büssing R; Hoffmeister H; Türck S; Varchmin A; Ott I Gold Metallodrugs to Target Coronavirus Proteins: Inhibitory Effects on the Spike-Ace2 Interaction and on Plpro Protease Activity by Auranofin and Gold Organometallics. Eur. J. Chem 2020, 26, 15140–15144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (160).Gabbiani C; Messori L Protein Targets for Anticancer Gold Compounds: Mechanistic Inferences. Anti-Cancer Agents Med. Chem 2011, 11, 929–939. [DOI] [PubMed] [Google Scholar]
- (161).de Paiva REF Gold(I, III) Complexes Designed for Selective Targeting and Inhibition of Zinc Finger Proteins; Springer, 2018. [Google Scholar]
- (162).Gamberi T; Magherini F; Fiaschi T; Landini I; Massai L; Valocchia E; Bianchi L; Bini L; Gabbiani C; Nobili S; Mini E; Messori L; Modesti A Proteomic Analysis of the Cytotoxic Effects Induced by the Organogold(III) Complex Aubipyc in Cisplatin-Resistant A2780 Ovarian Cancer Cells: Further Evidence for the Glycolytic Pathway Implication. Mol. Biosyst. 2015, 11, 1653–1667. [DOI] [PubMed] [Google Scholar]
- (163).Gamberi T; Massai L; Magherini F; Landini I; Fiaschi T; Scaletti F; Gabbiani C; Bianchi L; Bini L; Nobili S; Perrone G; Mini E; Messori L; Modesti A Proteomic Analysis of A2780/S Ovarian Cancer Cell Response to the Cytotoxic Organogold(III) Compound Aubipyc. J. Proteomics 2014, 103, 103–120. [DOI] [PubMed] [Google Scholar]
- (164).Zhang X.-w.; Li Q.-h.; Dou J.-j.; et al. Mass Spectrometry-Based Metabolomics in Health and Medical Science: A Systematic Review. RSC Adv. 2020, 10, 3092–3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (165).Theiner S; Schoeberl A; Schweikert A; Keppler BK; Koellensperger G Mass Spectrometry Techniques for Imaging and Detection of Metallodrugs. Curr. Opin. Chem. Biol. 2021, 61, 123–134. [DOI] [PubMed] [Google Scholar]
- (166).Greco V; Piras C; Pieroni L; Ronci M; Putignani L; Roncada P; Urbani A Applications of Maldi-Tof Mass Spectrometry in Clinical Proteomics. Expert Rev. Proteom. 2018, 15, 683–696. [DOI] [PubMed] [Google Scholar]
- (167).Riley NM; Bertozzi CR; Pitteri SJ A Pragmatic Guide to Enrichment Strategies for Mass Spectrometry-Based Glycoproteomics. Mol. Cell. Proteomics 2021, 20, 100029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (168).Wright MH; Sieber SA Chemical Proteomics Approaches for Identifying the Cellular Targets of Natural Products. Nat. Prod. Rep. 2016, 33, 681–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (169).Mora M; Gimeno MC; Visbal R Recent Advances in Gold-Nhc Complexes with Biological Properties. Chem. Soc. Rev. 2019, 48, 447–462. [DOI] [PubMed] [Google Scholar]
- (170).Tong K-C; Hu D; Wan P-K; Lok C-N; Che C-M Anti-Cancer Gold, Platinum and Iridium Compounds with Porphyrin and/or N-Heterocyclic Carbene Ligand (S). In Adv. Inorg. Chem, Vol. 75; Elsevier, 2020; pp 87–119. [Google Scholar]
- (171).Martínez-Calvo M; Mascareñas JL Organometallic Catalysis in Biological Media and Living Settings. Coord. Chem. Rev. 2018, 359, 57–79. [Google Scholar]
- (172).Steel TR; Hartinger CG Metalloproteomics for Molecular Target Identification of Protein-Binding Anticancer Metallodrugs. Metallomics 2020, 12, 1627–1636. [DOI] [PubMed] [Google Scholar]
- (173).Sanchez TW; Ronzetti MH; Owens AE; Antony M; Voss T; Wallgren E; Talley D; Balakrishnan K; Leyes Porello SE; Rai G; Marugan JJ; Michael SG; Baljinnyam B; Southall N; Simeonov A; Henderson MJ Real-Time Cellular Thermal Shift Assay to Monitor Target Engagement. ACS Chem. Biol. 2022, 17, 2471–2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (174).Tong K-C; Lok C-N; Wan P-K; Hu D; Fung YME; Chang X-Y; Huang S; Jiang H; Che C-M An Anticancer Gold(III)-Activated Porphyrin Scaffold That Covalently Modifies Protein Cysteine Thiols. Proc. Natl. Acad. Sci. U. S. A. 2020, 117, 1321–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (175).Olelewe C; Kim JH; Ofori S; Mertens RT; Gukathasan S; Awuah SG Gold(III)-P-Chirogenic Complex Induces Mitochondrial Dysfunction in Triple-Negative Breast Cancer. iScience 2022, 25, 104340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (176).Fung SK; Zou T; Cao B; Lee P-Y; Fung YME; Hu D; Lok C-N; Che C-M Cyclometalated Gold(III) Complexes Containing N-Heterocyclic Carbene Ligands Engage Multiple Anti-Cancer Molecular Targets. Angew. Chem., Int. Ed. 2017, 129, 3950–3954. [DOI] [PubMed] [Google Scholar]
- (177).Castaldi MP; Hendricks JA; Zhang AX Design, Synthesis, and Strategic Use of Small Chemical Probes toward Identification of Novel Targets for Drug Development. Curr. Opin. Chem. Biol. 2020, 56, 91–97. [DOI] [PubMed] [Google Scholar]
- (178).Backus KM; Correia BE; Lum KM; Forli S; Horning BD; González-Páez GE; Chatterjee S; Lanning BR; Teijaro JR; Olson AJ; et al. Proteome-Wide Covalent Ligand Discovery in Native Biological Systems. Nature 2016, 534, 570–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (179).Bak DW; Gao J; Wang C; Weerapana E A Quantitative Chemoproteomic Platform to Monitor Selenocysteine Reactivity within a Complex Proteome. Cell chem. biol. 2018, 25, 1157–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (180).Schmidt C; Zollo M; Bonsignore R; Casini A; Hacker SM Competitive Profiling of Ligandable Cysteines in Staphylococcus Aureus with an Organogold Compound. Chem. Commun. 2022, 58, 5526–5529. [DOI] [PubMed] [Google Scholar]
- (181).Bassik MC; Kampmann M; Lebbink RJ; Wang S; Hein MY; Poser I; Weibezahn J; Horlbeck MA; Chen S; Mann M; et al. A Systematic Mammalian Genetic Interaction Map Reveals Pathways Underlying Ricin Susceptibility. Cell 2013, 152, 909–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (182).Lamb J; Crawford ED; Peck D; Modell JW; Blat IC; Wrobel MJ; Lerner J; Brunet J-P; Subramanian A; Ross KN; et al. The Connectivity Map: Using Gene-Expression Signatures to Connect Small Molecules, Genes, and Disease. Science 2006, 313, 1929–1935. [DOI] [PubMed] [Google Scholar]
- (183).Schenone M; Dančík V; Wagner BK; Clemons PA Target Identification and Mechanism of Action in Chemical Biology and Drug Discovery. Nat. Chem. Biol. 2013, 9, 232–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (184).Parsons AB; Lopez A; Givoni IE; Williams DE; Gray CA; Porter J; Chua G; Sopko R; Brost RL; Ho C-H; et al. Exploring the Mode-of-Action of Bioactive Compounds by Chemical-Genetic Profiling in Yeast. Cell 2006, 126, 611–625. [DOI] [PubMed] [Google Scholar]
- (185).Gregori-Puigjané E; Setola V; Hert J; Crews BA; Irwin JJ; Lounkine E; Marnett L; Roth BL; Shoichet BK Identifying Mechanism-of-Action Targets for Drugs and Probes. Proc. Natl. Acad. Sci. U. S. A. 2012, 109, 11178–11183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (186).Kampmann M; Bassik MC; Weissman JS Integrated Platform for Genome-Wide Screening and Construction of High-Density Genetic Interaction Maps in Mammalian Cells. Proc. Natl. Acad. Sci. U. S. A. 2013, 110, E2317–E2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (187).Ran Y; Liang Z; Gao C Current and Future Editing Reagent Delivery Systems for Plant Genome Editing. Sci. China Life Sci. 2017, 60, 490–505. [DOI] [PubMed] [Google Scholar]
- (188).Cong L; Zhou R; Kuo Y.-c.; Cunniff M; Zhang F Comprehensive Interrogation of Natural Tale DNA-Binding Modules and Transcriptional Repressor Domains. Nat. Commun. 2012, 3, 968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (189).Wu J; Sun L; Chen X; Du F; Shi H; Chen C; Chen ZJ Cyclic Gmp-Amp Is an Endogenous Second Messenger in Innate Immune Signaling by Cytosolic DNA. Science 2013, 339, 826–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (190).Kim JH; Ofori S; Tagmount A; Vulpe CD; Awuah SG Genome-Wide Crispr Screen Reveal Targets of Chiral Gold (I) Anticancer Compound in Mammalian Cells. ACS omega 2022, 7, 39197–39205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (191).Bratsch SG Standard Electrode Potentials and Temperature Coefficients in Water at 298.15 K. J. Phys. Chem. Ref. Data 1989, 18, 1–21. [Google Scholar]
- (192).Yam VW-W; Choi SW-K; Lai T-F; Lee W-K Syntheses, Crystal Structures and Photophysics of Organogold(III) Diimine Complexes. Dalton Trans 1993, 1001–1002. [Google Scholar]
- (193).Yam VW-W; Wong KM-C; Hung L-L; Zhu N Luminescent Gold(III) Alkynyl Complexes: Synthesis, Structural Characterization, and Luminescence Properties. Angew. Chem., Int. Ed. 2005, 44, 3107–3110. [DOI] [PubMed] [Google Scholar]
- (194).Tang M-C; Chan M-Y; Yam VW-W Molecular Design of Luminescent Gold(III) Emitters as Thermally Evaporable and Solution-Processable Organic Light-Emitting Device (OLED) Materials. Chem. Rev. 2021, 121, 7249–7279. [DOI] [PubMed] [Google Scholar]
- (195).Mihaly JJ; Stewart DJ; Grusenmeyer TA; Phillips AT; Haley JE; Zeller M; Gray TG Photophysical Properties of Organogold(I) Complexes Bearing a Benzothiazole-2,7-Fluorenyl Moiety: Selection of Ancillary Ligand Influences White Light Emission. Dalton Trans. 2019, 48, 15917–15927. [DOI] [PubMed] [Google Scholar]
- (196).Bartolomé C; Carrasco-Rando M; Coco S; Cordovilla C; Martín-Alvarez JM; Espinet P Luminescent Gold(I) Carbenes from 2-Pyridylisocyanide Complexes: Structural Consequences of Intramolecular Versus Intermolecular Hydrogen-Bonding Interactions. Inorg. Chem. 2008, 47, 1616–1624. [DOI] [PubMed] [Google Scholar]
- (197).Li T.-y.; Muthiah Ravinson DS; Haiges R; Djurovich PI; Thompson ME Enhancement of the Luminescent Efficiency in Carbene-Au(I)-Aryl Complexes by the Restriction of Renner-Teller Distortion and Bond Rotation. J. Am. Chem. Soc. 2020, 142, 6158–6172. [DOI] [PubMed] [Google Scholar]
- (198).Pujadas M; Rodríguez L Luminescent Phosphine Gold(I) Alkynyl Complexes. Highlights from 2010 to 2018. Coord. Chem. Rev. 2020, 408, 213179. [Google Scholar]
- (199).Li M; Li F; Liu S; Zhao Q Luminescent Coordination Compounds for Cell Imaging. In Fluorescent Materials for Cell Imaging, Wu F-G, Ed.; Springer; Singapore, 2020; pp 217–247. [Google Scholar]
- (200).López-de-Luzuriaga JM; Monge M; Olmos ME Luminescent Aryl-Group Eleven Metal Complexes. Dalton Trans. 2017, 46, 2046–2067. [DOI] [PubMed] [Google Scholar]
- (201).Redrado M; Benedi A; Marzo I; García-Otín AL; Fernández-Moreira V; Concepción Gimeno M Multifunctional Heterometallic IrIII-AuI Probes as Promising Anticancer and Antiangiogenic Agents. Eur. J. Chem. 2021, 27, 9885–9897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (202).Vaidya SP; Gadre S; Kamisetti RT; Patra M Challenges and Opportunities in the Development of Metal-Based Anticancer Theranostic Agents. Biosci. Rep. 2022, 42. DOI: 10.1042/BSR20212160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (203).Huang Z; Wilson JJ Therapeutic and Diagnostic Applications of Multimetallic Rhenium(I) Tricarbonyl Complexes. Eur. J. Inorg. Chem. 2021, 2021, 1312–1324. [Google Scholar]
- (204).Barnard PJ; Wedlock LE; Baker MV; Berners-Price SJ; Joyce DA; Skelton BW; Steer JH Luminescence Studies of the Intracellular Distribution of a Dinuclear Gold(I) N-Heterocyclic Carbene Complex. Angew. Chem., Int. Ed. 2006, 45, 5966–5970. [DOI] [PubMed] [Google Scholar]
- (205).Kathewad N; Kumar N; Dasgupta R; Ghosh M; Pal S; Khan S The Syntheses and Photophysical Properties of Pnp-Based Au (I) Complexes with Strong Intramolecular Au··· Au Interactions. Dalton Trans. 2019, 48, 7274–7280. [DOI] [PubMed] [Google Scholar]
- (206).Guevara-Vela JM; Hess K; Rocha-Rinza T; Pendás ÁM; Flores-Álamo M; Moreno-Alcántar G Stronger-Together: The Cooperativity of Aurophilic Interactions. Chem. Commun. 2022, 58, 1398–1401. [DOI] [PubMed] [Google Scholar]
- (207).Ott I; Qian X; Xu Y; Vlecken DHW; Marques IJ; Kubutat D; Will J; Sheldrick WS; Jesse P; Prokop A; Bagowski CP A Gold(I) Phosphine Complex Containing a Naphthalimide Ligand Functions as a Trxr Inhibiting Antiproliferative Agent and Angiogenesis Inhibitor. J. Med. Chem. 2009, 52, 763–770. [DOI] [PubMed] [Google Scholar]
- (208).Bagowski CP; You Y; Scheffler H; Vlecken DH; Schmitz DJ; Ott I Naphthalimide Gold(I) Phosphine Complexes as Anticancer Metallodrugs. Dalton Trans. 2009, 10799–10805. [DOI] [PubMed] [Google Scholar]
- (209).Langdon-Jones EE; Lloyd D; Hayes AJ; Wainwright SD; Mottram HJ; Coles SJ; Horton PN; Pope SJ Alkynyl-Naphthalimide Fluorophores: Gold Coordination Chemistry and Cellular Imaging Applications. Inorg. Chem. 2015, 54, 6606–6615. [DOI] [PubMed] [Google Scholar]
- (210).Vergara E; Cerrada E; Casini A; Zava O; Laguna M; Dyson PJ Antiproliferative Activity of Gold(I) Alkyne Complexes Containing Water-Soluble Phosphane Ligands. Organometallics 2010, 29, 2596–2603. [Google Scholar]
- (211).Che C-M; Chao H-Y; Miskowski VM; Li Y; Cheung K-K Luminescent Μ-Ethynediyl and Μ-Butadiynediyl Binuclear Gold(I) Complexes: Observation of 3(Ππ*) Emissions from Bridging Cn2- Units. J. Am. Chem. Soc. 2001, 123, 4985–4991. [DOI] [PubMed] [Google Scholar]
- (212).Yam VW-W; Kam-Wing Lo K; Man-Chung Wong K Luminescent Polynuclear Metal Acetylides. J. Organomet. Chem. 1999, 578, 3–30. [Google Scholar]
- (213).Lu W; Zhu N; Che C-M Polymorphic Forms of a Gold(I) Arylacetylide Complex with Contrasting Phosphorescent Characteristics. J. Am. Chem. Soc. 2003, 125, 16081–16088. [DOI] [PubMed] [Google Scholar]
- (214).Ferrer M; Rodrıguez L; Rossell O; Pina F; Lima JC; Bardia MF; Solans X Linear Ditopic Acetylide Gold or Mercury Complexes: Synthesis and Photophysic Studies: X-Ray Crystal Structure of Pph4[Au(C Cc5h4n)2]. J. Organomet. Chem. 2003, 678, 82–89. [Google Scholar]
- (215).Hunks WJ; MacDonald M-A; Jennings MC; Puddephatt RJ Luminescent Binuclear Gold(I) Ring Complexes. Organometallics 2000, 19, 5063–5070. [Google Scholar]
- (216).Irwin MJ; Vittal JJ; Puddephatt RJ Luminescent Gold(I) Acetylides: From Model Compounds to Polymers. Organometallics 1997, 16, 3541–3547. [Google Scholar]
- (217).Chao H-Y; Lu W; Li Y; Chan MCW; Che C-M; Cheung K-K; Zhu N Organic Triplet Emissions of Arylacetylide Moieties Harnessed through Coordination to [Au(Pcy3)]+. Effect of Molecular Structure Upon Photoluminescent Properties. J. Am. Chem. Soc. 2002, 124, 14696–14706. [DOI] [PubMed] [Google Scholar]
- (218).Vicente J; Gil-Rubio J; Barquero N; Jones PG; Bautista D Synthesis of Luminescent Alkynyl Gold Metalaligands Containing 2,2′-Bipyridine-5-Yl and 2,2′:6′,2″-Terpyridine-4-Yl Donor Groups. Organometallics 2008, 27, 646–659. [Google Scholar]
- (219).Tikhomirov AS; Shtil AA; Shchekotikhin AE Advances in the Discovery of Anthraquinone-Based Anticancer Agents. Recent Pat. Anticancer Drug Discovery 2018, 13, 159–183. [DOI] [PubMed] [Google Scholar]
- (220).Cheemalamarri C; Batchu UR; Thallamapuram NP; Katragadda SB; Reddy Shetty P A Review on Hydroxy Anthraquinones from Bacteria: Crosstalk’s of Structures and Biological Activities. Nat. Prod. Res. 2022, 1–20. [DOI] [PubMed] [Google Scholar]
- (221).Balasingham RG; Williams CF; Mottram HJ; Coogan MP; Pope SJA Gold(I) Complexes Derived from Alkynyloxy-Substituted Anthraquinones: Syntheses, Luminescence, Preliminary Cytotoxicity, and Cell Imaging Studies. Organometallics 2012, 31, 5835–5843. [Google Scholar]
- (222).Vaddamanu M; Sathyanarayana A; Masaya Y; Sugiyama S; Kazuhisa O; Velappan K; Nandeshwar M; Hisano K; Tsutsumi O; Prabusankar G Acridine N-Heterocyclic Carbene Gold (I) Compounds: Tuning from Yellow to Blue Luminescence. Chem. Asian J. 2021, 16, 521–529. [DOI] [PubMed] [Google Scholar]
- (223).Perez SA; de Haro C; Vicente C; Donaire A; Zamora A; Zajac J; Kostrhunova H; Brabec V; Bautista D; Ruiz J New Acridine Thiourea Gold (I) Anticancer Agents: Targeting the Nucleus and Inhibiting Vasculogenic Mimicry. ACS Chem. Biol. 2017, 12, 1524–1537. [DOI] [PubMed] [Google Scholar]
- (224).Arcau J; Andermark V; Aguiló E; Gandioso A; Moro A; Cetina M; Lima JC; Rissanen K; Ott I; Rodríguez L Luminescent Alkynyl-Gold (I) Coumarin Derivatives and Their Biological Activity. Dalton Trans. 2014, 43, 4426–4436. [DOI] [PubMed] [Google Scholar]
- (225).Cerrada E; Fernández-Moreira V; Gimeno MC Gold and Platinum Alkynyl Complexes for Biomedical Applications. Adv. Organomet. Chem. 2019, 71, 227–258. [Google Scholar]
- (226).Rousselle B; Massot A; Privat M; Dondaine L; Trommenschlager A; Bouyer F; Bayardon J; Ghiringhelli F; Bettaieb A; Goze C; et al. Conception and Evaluation of Fluorescent Phosphine-Gold Complexes: From Synthesis to in Vivo Investigations. ChemMedChem. 2022, 17, No. e202100773. [DOI] [PubMed] [Google Scholar]
- (227).Trommenschlager A; Chotard F; Bertrand B; Amor S; Dondaine L; Picquet M; Richard P; Bettaïeb A; Le Gendre P; Paul C; Goze C; Bodio E Gold(I)-Bodipy-Imidazole Bimetallic Complexes as New Potential Anti-Inflammatory and Anticancer Trackable Agents. Dalton Trans. 2017, 46, 8051–8056. [DOI] [PubMed] [Google Scholar]
- (228).Lescure R; Privat M; Pliquett J; Massot A; Baffroy O; Busser B; Bellaye P-S; Collin B; Denat F; Bettaïeb A; Sancey L; Paul C; Goze C; Bodio E Near-Infrared Emitting Fluorescent Homobimetallic Gold(I) Complexes Displaying Promising In vitro and In vivo Therapeutic Properties. Eur. J. Med. Chem. 2021, 220, 113483. [DOI] [PubMed] [Google Scholar]
- (229).Luengo A; Fernández-Moreira V; Marzo I; Gimeno MC Trackable Metallodrugs Combining Luminescent Re(I) and Bioactive Au(I) Fragments. Inorg. Chem. 2017, 56, 15159–15170. [DOI] [PubMed] [Google Scholar]
- (230).Fernández-Moreira V; Marzo I; Gimeno MC Luminescent Re(I) and Re(I)/Au(I) Complexes as Cooperative Partners in Cell Imaging and Cancer Therapy. Chem. Sci. 2014, 5, 4434–4446. [Google Scholar]
- (231).Luengo A; Redrado M; Marzo I; Fernández-Moreira V; Gimeno MC Luminescent Re(I)/Au(I) Species as Selective Anticancer Agents for Hela Cells. Inorg. Chem. 2020, 59, 8960–8970. [DOI] [PubMed] [Google Scholar]
- (232).Luengo A; Fernández-Moreira V; Marzo I; Gimeno MC Bioactive Heterobimetallic Re(I)/Au(I) Complexes Containing Bidentate N-Heterocyclic Carbenes. Organometallics 2018, 37, 3993–4001. [Google Scholar]
- (233).Boselli L; Carraz M; Mazères S; Paloque L; González G; Benoit-Vical F; Valentin A; Hemmert C; Gornitzka H Synthesis, Structures, and Biological Studies of Heterobimetallic Au(I)-Ru(II) Complexes Involving N-Heterocyclic Carbene-Based Multidentate Ligands. Organometallics 2015, 34, 1046–1055. [Google Scholar]
- (234).Luengo A; Marzo I; Reback M; Daubit IM; Fernández-Moreira V; Metzler-Nolte N; Gimeno MC Luminescent Bimetallic IrIII/AuI Peptide Bioconjugates as Potential Theranostic Agents. Eur. J. Chem 2020, 26, 12158–12167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (235).Kumar A; Sun S-S; Lees AJ Photophysics and Photochemistry of Organometallic Rhenium Diimine Complexes. In Photophysics of Organometallics; Lees AJ, Ed.; Springer; Berlin Heidelberg, 2010; pp 37–71. [Google Scholar]
- (236).Luengo A; Marzo I; Fernández-Moreira V; Gimeno MC Synthesis and Antiproliferative Study of Phosphorescent Multimetallic Re(I)/Au(I) Complexes Containing Fused Imidazo[4,5-F]-1,10-Phenanthroline Core. Appl. Organomet. Chem. 2022, No. e6661. [Google Scholar]
- (237).Wenzel M; de Almeida A; Bigaeva E; Kavanagh P; Picquet M; Le Gendre P; Bodio E; Casini A New Luminescent Polynuclear Metal Complexes with Anticancer Properties: Toward Structure-Activity Relationships. Inorg. Chem. 2016, 55, 2544–2557. [DOI] [PubMed] [Google Scholar]
- (238).Wilde AP; King KA; Watts RJ Resolution and Analysis of the Components in Dual Emission of Mixed-Chelate/Ortho-Metalate Complexes of Iridium(III). J. Phys. Chem. 1991, 95, 629–634. [Google Scholar]
- (239).Li T-Y; Wu J; Wu Z-G; Zheng Y-X; Zuo J-L; Pan Y Rational Design of Phosphorescent Iridium(III) Complexes for Emission Color Tunability and Their Applications in Oleds. Coord. Chem. Rev. 2018, 374, 55–92. [Google Scholar]
- (240).Lo KK-W; Zhang KY; Li SP-Y Design of Cyclometalated Iridium(III) Polypyridine Complexes as Luminescent Biological Labels and Probes. Pure Appl. Chem. 2011, 83, 823–840. [Google Scholar]
- (241).Caporale C; Massi M Cyclometalated Iridium(III) Complexes for Life Science. Coord. Chem. Rev. 2018, 363, 71–91. [Google Scholar]
- (242).Tsai JL-L; Chan AO-Y; Che C-M A Luminescent Cyclometalated Gold(III)-Avidin Conjugate with a Long-Lived Emissive Excited State That Binds to Proteins and DNA and Possesses Anti-Proliferation Capacity. Chem. Commun. 2015, 51, 8547–8550. [DOI] [PubMed] [Google Scholar]
- (243).Wang F; Lan M; To W-P; Li K; Lok C-N; Wang P; Che C-M A Macromolecular Cyclometalated Gold(III) Amphiphile Displays Long-Lived Emissive Excited State in Water: Self-Assembly and in Vitro Photo-Toxicity. Chem. Commun. 2016, 52, 13273–13276. [DOI] [PubMed] [Google Scholar]
- (244).Norton JR; Sowa J Introduction: Metal Hydrides. Chem. Rev. 2016, 116, 8315–8317. [DOI] [PubMed] [Google Scholar]
- (245).Schmidbaur H; Raubenheimer HG; Dobrzańska L The Gold-Hydrogen Bond, Au-H, and the Hydrogen Bond to Gold, Au··· H-X. Chem. Soc. Rev. 2014, 43, 345–380. [DOI] [PubMed] [Google Scholar]
- (246).Wang X; Andrews L Gold Hydrides Auh and (H2)Auh and the Auh3 Transition State Stabilized in (H2)AuH3: Infrared Spectra and Dft Calculations. J. Am. Chem. Soc. 2001, 123, 12899–12900. [DOI] [PubMed] [Google Scholar]
- (247).Wang X; Andrews L Gold Is Noble but Gold Hydride Anions Are Stable. Angew. Chem., Int. Ed. 2003, 42, 5201–5206. [DOI] [PubMed] [Google Scholar]
- (248).Andrews L; Wang X Infrared Spectra and Structures of the Stable Cuh2-, Agh2-, AuH 2-, and Auh4- Anions and the Auh2Molecule. J. Am. Chem. Soc. 2003, 125, 11751–11760. [DOI] [PubMed] [Google Scholar]
- (249).Andrews L Matrix Infrared Spectra and Density Functional Calculations of Transition Metal Hydrides and Dihydrogen Complexes. Chem. Soc. Rev. 2004, 33, 123–132. [DOI] [PubMed] [Google Scholar]
- (250).Tsui EY; Müller P; Sadighi JP Reactions of a Stable Monomeric Gold(I) Hydride Complex. Angew. Chem., Int. Ed. 2008, 47, 8937–8940. [DOI] [PubMed] [Google Scholar]
- (251).Escalle A; Mora G; Gagosz F; Mézailles N; Le Goff XF; Jean Y; Le Floch P Cationic Dimetallic Gold Hydride Complex Stabilized by a Xantphos-Phosphole Ligand: Synthesis, X-Ray Crystal Structure, and Density Functional Theory Study. Inorg. Chem. 2009, 48, 8415–8422. [DOI] [PubMed] [Google Scholar]
- (252).Rosca D-A; Smith DA; Hughes DL; Bochmann M A Thermally Stable Gold(III) Hydride: Synthesis, Reactivity, and Reductive Condensation as a Route to Gold(II) Complexes. Angew. Chem., Int. Ed. 2012, 51, 10643–10646. [DOI] [PubMed] [Google Scholar]
- (253).Pintus A; Rocchigiani L; Fernandez-Cestau J; Budzelaar PHM; Bochmann M Stereo- and Regioselective Alkyne Hydrometallation with Gold(III) Hydrides. Angew. Chem., Int. Ed. 2016, 55, 12321–12324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (254).Luo H; Cao B; Chan ASC; Sun RW-Y; Zou T Cyclometalated Gold(III)-Hydride Complexes Exhibit Visible Light-Induced Thiol Reactivity and Act as Potent Photo-Activated Anti-Cancer Agents. Angew. Chem., Int. Ed. 2020, 132, 11139–11145. [DOI] [PubMed] [Google Scholar]
- (255).Sheppard C; Goodell JP; Hahn P Colloidal Gold Containing the Radioactive Isotope Au198 in the Selective Internal Radiation Therapy of Diseases of the Lymphoid System. J. Lab. Clin. Med. 1947, 32, 1437–1441. [PubMed] [Google Scholar]
- (256).Säterborg N-E The Distribution of 198au Injected Intravenously as a Colloid and in Solution. Acta Radiol. Ther. Phys. Biol. 1973, 12, 509–528. [DOI] [PubMed] [Google Scholar]
- (257).Bottenus BN; Kan P; Jenkins T; Ballard B; Rold TL; Barnes C; Cutler C; Hoffman TJ; Green MA; Jurisson SS Gold(III) Bis-Thiosemicarbazonato Complexes: Synthesis, Characterization, Radiochemistry and X-Ray Crystal Structure Analysis. Nucl. Med. Biol. 2010, 37, 41–49. [DOI] [PubMed] [Google Scholar]
- (258).Akerman MP; Munro OQ; Mongane M; van Staden JA; Rae WID; Bester CJ; Marjanovic-Painter B; Szucs Z; Zeevaart JR Biodistribution (as Determined by the Radiolabelled Equivalent) of a Gold(III) Bis(Pyrrolide-Imine) Schiff Base Complex: A Potential Chemotherapeutic. J. Labelled Compd. Radiopharm. 2013, 56, 530–535. [DOI] [PubMed] [Google Scholar]
- (259).Guarra F; Terenzi A; Pirker C; Passannante R; Baier D; Zangrando E; Gómez-Vallejo V; Biver T; Gabbiani C; Berger W; Llop J; Salassa L 124i Radiolabeling of a AuIII-NHC Complex for in Vivo Biodistribution Studies. Angew. Chem., Int. Ed. 2020, 59, 17130–17136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (260).Koch R An Address on Bacteriological Research. Br. Med. J. 1890, 2, 380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (261).Koch R A Further Communication on a Remedy for Tuberculosis. Br. Med. J. 1890, 2, 1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (262).Feldt A The Chemotherapy of Relapsing Fever with Gold and Sulphonamlde Compounds. Klin. Wochenschr. 1941, 20. [Google Scholar]
- (263).Feldt A Zur Chemotherapie Der Tuberkulose Mit Gold. DMW-Deutsche Medizinische Wochenschrift 1913, 39, 549–551. [Google Scholar]
- (264).Fordos M-J; Gélis A Action Du Perchlorure D’or Sur L’hyposulfite De Soude; Bachelier, 1845. [Google Scholar]
- (265).McCluskey KL; Eichelberger L New Methods for the Preparation of Sodium Aurothiosulfate. J. Am. Chem. Soc. 1926, 48, 136–139. [Google Scholar]
- (266).Kayne GG The Use of Sanocrysin in the Treatment of Pulmonary Tuberculosis: (Section of Medicine). Proc. R. Soc. Med. 1935, 28, 1463–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (267).SECHER K Traitement De La Tuberculose Par La Sanocrysine. Munksgaard L, a., Ed.; Copenhagen, 1932. [Google Scholar]
- (268).Secher K; Host E; Beitr Z; Klin DT 1934, 107.
- (269).Bang O Sanocrysin Und Experimentelle Tuberkulose. Ztschr. Tuberk. 1926, 44, 298. [Google Scholar]
- (270).Benedek TG The History of Gold Therapy for Tuberculosis. J. Hist. Med. Allied Sci. 2004, 59, 50–89. [DOI] [PubMed] [Google Scholar]
- (271).Permin G Über Sanocrysinbehandlung Von Schwerer Tuberkulose Mit Kleinen Anfangsdosen. Acta Tuberc. Scand. 1925, 1, 306325. [Google Scholar]
- (272).Council TMR The Gold Treatment of Tuberculosis. Br. Med. J. 1925, 1, 735. [PMC free article] [PubMed] [Google Scholar]
- (273).Forestier J Rheumatoid Arthritis and Its Treatment by Gold Salts. Lancet 1934, 224, 646–648. [Google Scholar]
- (274).Atakhodzhaeva Z; IaA S Comparative Effectiveness of Chrysanol and Auranofin in Rheumatoid Arthritis. Ter. Arkh. 1987, 59, 30–33. [PubMed] [Google Scholar]
- (275).Miranda VM Medicinal Inorganic Chemistry: An Updated Review on the Status of Metallodrugs and Prominent Metallodrug Candidates. Rev. Inorg. Chem. 2022, 42, 29–52. [Google Scholar]
- (276).Aronson JK Meyler’s Side Effects of Drugs: The International Encyclopedia of Adverse Drug Reactions and Interactions; Elsevier, 2015. [Google Scholar]
- (277).Cummins S; Wigley J Discussion on the Therapeutic Value of Gold Compounds. Proc. R. Soc. Med. 1930, 17–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (278).Barber HW Sarcoid: Effect of Krysolgan Treatment: Gradual Absorption of Sarcoid Nodules, with Development of Prurigo (Twice Previously Shown). Proc. R. Soc. Med. 1928, 21, 671–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (279).Haldin-Davis H Four Cases of Lupus Erythematosus Treated with Gold Preparations. Proc. R. Soc. Med. 1928, 22, 89–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (280).WILE UJ; COURVILLE CJ Pityriasis-Rosea-Like Dermatitis Following Gold Therapy: Report of Two Cases. Arch. Derm. Syphilol. 1940, 42, 1105–1112. [Google Scholar]
- (281).Jessop J; Vernon-Roberts B; Harris J Effects of Gold Salts and Prednisolone on Inflammatory Cells. I. Phagocytic Activity of Macrophages and Polymorphs in Inflammatory Exudates Studied by a″ Skin-Window″ Technique in Rheumatoid and Control Patients. Ann. Rheum. Dis. 1973, 32, 294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (282).Fan C; Zheng W; Fu X; Li X; Wong Y; Chen T Enhancement of Auranofin-Induced Lung Cancer Cell Apoptosis by Selenocystine, a Natural Inhibitor of Trxr1 in Vitro and in Vivo. Cell Death Dis. 2014, 5, No. e1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (283).Li H; Hu J; Wu S; Wang L; Cao X; Zhang X; Dai B; Cao M; Shao R; Zhang R; et al. Auranofin-Mediated Inhibition of Pi3k/Akt/Mtor Axis and Anticancer Activity in Non-Small Cell Lung Cancer Cells. Oncotarget 2016, 7, 3548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (284).Marzano C; Gandin V; Folda A; Scutari G; Bindoli A; Rigobello MP Inhibition of Thioredoxin Reductase by Auranofin Induces Apoptosis in Cisplatin-Resistant Human Ovarian Cancer Cells. Free Radic. Biol. Med. 2007, 42, 872–881. [DOI] [PubMed] [Google Scholar]
- (285).Hou G-X; Liu P-P; Zhang S; Yang M; Liao J; Yang J; Hu Y; Jiang W-Q; Wen S; Huang P Elimination of Stem-Like Cancer Cell Side-Population by Auranofin through Modulation of Ros and Glycolysis. Cell Death Dis. 2018, 9, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (286).Madeira J; Gibson D; Kean W; Klegeris A The Biological Activity of Auranofin: Implications for Novel Treatment of Diseases. Inflammopharmacology 2012, 20, 297–306. [DOI] [PubMed] [Google Scholar]
- (287).Thangamani S; Mohammad H; Abushahba MF; Sobreira TJ; Hedrick VE; Paul LN; Seleem MN Antibacterial Activity and Mechanism of Action of Auranofin against Multi-Drug Resistant Bacterial Pathogens. Sci. Rep. 2016, 6, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (288).Elkashif A; Seleem MN Investigation of Auranofin and Gold-Containing Analogues Antibacterial Activity against Multidrug-Resistant Neisseria Gonorrhoeae. Sci. Rep. 2020, 10, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (289).Onodera T; Momose I; Kawada M Potential Anticancer Activity of Auranofin. Chem. Pharm. Bull. (Tokyo) 2019, 67, 186–191. [DOI] [PubMed] [Google Scholar]
- (290).Cirri D; Massai L; Giacomelli C; Trincavelli ML; Guerri A; Gabbiani C; Messori L; Pratesi A Synthesis, Chemical Characterization, and Biological Evaluation of a Novel Auranofin Derivative as an Anticancer Agent. Dalton Trans. 2022, 51, 13527–13539. [DOI] [PubMed] [Google Scholar]
- (291).Diaz RS; Shytaj IL; Giron LB; Obermaier B; della Libera E Jr; Galinskas J; Dias D; Hunter J; Janini M; Gosuen G; et al. Potential Impact of the Antirheumatic Agent Auranofin on Proviral Hiv-1 DNA in Individuals under Intensified Antiretroviral Therapy: Results from a Randomised Clinical Trial. Int. J. Antimicrob. Agents 2019, 54, 592–600. [DOI] [PubMed] [Google Scholar]
- (292).de Almeida Baptista MV; da Silva LT; Samer S; Oshiro TM; Shytaj IL; Giron LB; Pena NM; Cruz N; Gosuen GC; Ferreira PRA; et al. Immunogenicity of Personalized Dendritic-Cell Therapy in Hiv-1 Infected Individuals under Suppressive Antiretroviral Treatment: Interim Analysis from a Phase II Clinical Trial. AIDS Res. Ther. 2022, 19, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (293).Vaccine Gene Therapy Institute, Florida University of Miami. Oral Auranofin for Reduction of Latent Viral Reservoir in Patients with Hiv Infection, 2023. https://ClinicalTrials.gov/show/NCT02176135 (accessed 03-20-2023).
- (294).National Institute of Allergy Infectious Diseases. Auranofin Pk Following Oral Dose Administration, 2014. https://ClinicalTrials.gov/show/NCT02089048 (accessed 03-20-2023).
- (295).Clinic Mayo. Auranofin in Treating Patients with Recurrent Epithelial Ovarian, Primary Peritoneal, or Fallopian Tube Cancer, 2012. https://ClinicalTrials.gov/show/NCT01747798 (accessed 03-20-2023).
- (296).Mayo Clinic; National Cancer Institute. Auranofin in Decreasing Pain in Patients with Paclitaxel-Induced Pain Syndrome, 2014. https://ClinicalTrials.gov/show/NCT02063698 (accessed 03-20-2023).
- (297).National Institute of Allergy Infectious Diseases Auranofin for Giardia Protozoa, 2016. https://ClinicalTrials.gov/show/NCT02736968 (accessed 03-20-2023).
- (298).Mayo Clinic; National Cancer Institute. Auranofin and Sirolimus in Treating Patients with Advanced Solid Tumors or Recurrent Non-Small Cell Lung Cancer, 2013. https://ClinicalTrials.gov/show/NCT02126527 (accessed 03-20-2023).
- (299).Mayo Clinic; National Cancer Institute. Sirolimus and Auranofin in Treating Patients with Advanced or Recurrent Non-Small Cell Lung Cancer or Small Cell Lung Cancer, 2012. https://ClinicalTrials.gov/show/NCT01737502 (accessed 03-20-2023). [Google Scholar]
- (300).Federal University of São Paulo Fundação de Amparo à Pesquisa do Estado de São Paulo Conselho Nacional de Desenvolvimento Cientifico e Tecnológico ViiV Healthcare Multi Interventional Study Exploring Hiv-1 Residual Replication: A Step Towards Hiv-1 Eradication and Sterilizing Cure, 2015. https://ClinicalTrials.gov/show/NCT02961829 (accessed 03-20-2023).
- (301).The Aurum Institute NPC Tb Host Directed Therapy, 2016. https://ClinicalTrials.gov/show/NCT02968927 (accessed 03-20-2023).
- (302).University of Ulm Reliable Cancer Therapies Anticancer Fund, Belgium A Proof-of-Concept Clinical Trial Assessing the Safety of the Coordinated Undermining of Survival Paths by 9 Repurposed Drugs Combined with Metronomic Temozolomide (Cusp9v3 Treatment Protocol) for Recurrent Glioblastoma, 2016. https://ClinicalTrials.gov/show/NCT02770378 (accessed 03-20-2023).
- (303).Pfizer. Comparative Analysis of Outcomes among Patients Initiating Xeljanz in Combination with Oral Mtx Who Withdraw Mtx Versus Continue Mtx. (accessed 03-20-2023).
- (304).Roche Hoffmann-La. An Observational Study of Mabthera/Rituxan (Rituximab) and Alternative Tnf-Inhibitors in Patients with Rheumatoid Arthritis and an Inadequate Response to a Single Previous Tnf-Inhibitor, 2009. https://ClinicalTrials.gov/show/NCT01557348 (accessed 03-20-2023).
- (305).Ghannoum MA; Rice LB Antifungal Agents: Mode of Action, Mechanisms of Resistance, and Correlation of These Mechanisms with Bacterial Resistance. Clin. Microbiol. Rev. 1999, 12, 501–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (306).Dennis EK; Kim JH; Parkin S; Awuah SG; Garneau-Tsodikova S Distorted Gold(I)-Phosphine Complexes as Antifungal Agents. J. Med. Chem. 2020, 63, 2455–2469. [DOI] [PubMed] [Google Scholar]
- (307).Yang L; Powell DR; Houser RP Structural Variation in Copper (I) Complexes with Pyridylmethylamide Ligands: Structural Analysis with a New Four-Coordinate Geometry Index, Τ 4. Dalton Trans. 2007, 955–964. [DOI] [PubMed] [Google Scholar]
- (308).Stevanović NL; Kljun J; Aleksic I; Bogojevic SS; Milivojevic D; Veselinovic A; Turel I; Djuran MI; Nikodinovic-Runic J; Glišić BĐ Clinically Used Antifungal Azoles as Ligands for Gold(III) Complexes: The Influence of the Au(III) Ion on the Antimicrobial Activity of the Complex. Dalton Trans. 2022, 51, 5322–5334. [DOI] [PubMed] [Google Scholar]
- (309).Bassetti M; Peghin M; Vena A; Giacobbe DR Treatment of Infections Due to Mdr Gram-Negative Bacteria. Front. Med. 2019, 6, 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (310).Ratia C; Soengas RG; Soto SM Gold-Derived Molecules as New Antimicrobial Agents. Front. Microbiol. 2022, 13, 772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (311).Delcour AH Outer Membrane Permeability and Antibiotic Resistance. Biochimica et Biophysica Acta (BBA)-Proteins and Proteomics 2009, 1794, 808–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (312).Silver LL Challenges of Antibacterial Discovery. Clin. Microbiol. Rev. 2011, 24, 71–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (313).Cassetta MI; Marzo T; Fallani S; Novelli A; Messori L Drug Repositioning: Auranofin as a Prospective Antimicrobial Agent for the Treatment of Severe Staphylococcal Infections. Biometals 2014, 27, 787–791. [DOI] [PubMed] [Google Scholar]
- (314).Torres NS; Abercrombie JJ; Srinivasan A; Lopez-Ribot JL; Ramasubramanian AK; Leung KP Screening a Commercial Library of Pharmacologically Active Small Molecules against Staphylococcus Aureus Biofilms. Antimicrob. Agents Chemother. 2016, 60, 5663–5672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (315).Torres NS; Montelongo-Jauregui D; Abercrombie JJ; Srinivasan A; Lopez-Ribot JL; Ramasubramanian AK; Leung KP Antimicrobial and Antibiofilm Activity of Synergistic Combinations of a Commercially Available Small Compound Library with Colistin against Pseudomonas Aeruginosa. Front. Microbiol. 2018, 9, 2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (316).Wu B; Yang X; Yan M Synthesis and Structure-Activity Relationship Study of Antimicrobial Auranofin against Eskape Pathogens. J. Med. Chem. 2019, 62, 7751–7768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (317).Epstein TD; Wu B; Moulton KD; Yan M; Dube DH Sugar-Modified Analogs of Auranofin Are Potent Inhibitors of the Gastric Pathogen Helicobacter Pylori. ACS Infect. Dis. 2019, 5, 1682–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (318).Maydaniuk D; Wu B; Truong D; Liyanage SH; Hogan AM; Yap ZL; Yan M; Cardona ST New Auranofin Analogs with Antibacterial Properties against Burkholderia Clinical Isolates. Antibiotics 2021, 10, 1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (319).Owings JP; McNair NN; Mui YF; Gustafsson TN; Holmgren A; Contel M; Goldberg JB; Mead JR Auranofin and N-Heterocyclic Carbene Gold-Analogs Are Potent Inhibitors of the Bacteria Helicobacter Pylori. FEMS Microbiol. Lett. 2016, 363, fnw148. [DOI] [PubMed] [Google Scholar]
- (320).Schmidt C; Karge B; Misgeld R; Prokop A; Franke R; Brönstrup M; Ott I Gold (I) NHC Complexes: Antiproliferative Activity, Cellular Uptake, Inhibition of Mammalian and Bacterial Thioredoxin Reductases, and Gram-Positive Directed Antibacterial Effects. Eur. J. Chem. 2017, 23, 1869–1880. [DOI] [PubMed] [Google Scholar]
- (321).Roymahapatra G; M Mandal S; F Porto W; Samanta T; Giri S; Dinda J; L Franco O; K Chattaraj P Pyrazine Functionalized Ag (I) and Au (I)-NHC Complexes Are Potential Antibacterial Agents. Curr. Med. Chem. 2012, 19, 4184–4193. [DOI] [PubMed] [Google Scholar]
- (322).Vellé A; Maguire R; Kavanagh K; Sanz Miguel PJ; Montagner D Steroid-AuI-Nhc Complexes: Synthesis and Antibacterial Activity. ChemMedChem. 2017, 12, 841–844. [DOI] [PubMed] [Google Scholar]
- (323).Büssing R; Karge B; Lippmann P; Jones PG; Brönstrup M; Ott I Gold (I) and Gold (III) N-Heterocyclic Carbene Complexes as Antibacterial Agents and Inhibitors of Bacterial Thioredoxin Reductase. ChemMedChem. 2021, 16, 3402–3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (324).Chen X; Sun S; Huang S; Yang H; Ye Q; Lv L; Liang Y; Shan J; Xu J; Liu W; et al. Gold (I) Selenium N-Heterocyclic Carbene Complexes as Potent Antibacterial Agents against Multidrug-Resistant Gram-Negative Bacteria Via Inhibiting Thioredoxin Reductase. Redox Biol. 2023, 60, 102621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (325).Stenger-Smith J; Chakraborty I; Mascharak PK Cationic Au (I) Complexes with Aryl-Benzothiazoles and Their Antibacterial Activity. J. Inorg. Biochem. 2018, 185, 80–85. [DOI] [PubMed] [Google Scholar]
- (326).Hikisz P; Szczupak L; Koceva-Chyła A; Guspiel A; Oehninger L; Ott I; Therrien B; Solecka J; Kowalski K Anticancer and Antibacterial Activity Studies of Gold (I)-Alkynyl Chromones. Molecules 2015, 20, 19699–19718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (327).Pintus A; Aragoni MC; Cinellu MA; Maiore L; Isaia F; Lippolis V; Orru G; Tuveri E; Zucca A; Arca M [Au (Pyb-H)(Mnt)]: A Novel Gold (III) 1, 2-Dithiolene Cyclometalated Complex with Antimicrobial Activity (Pyb-H= C-Deprotonated 2-Benzylpyridine; Mnt= 1, 2-Dicyanoethene-1, 2-Dithiolate). J. Inorg. Biochem. 2017, 170, 188–194. [DOI] [PubMed] [Google Scholar]
- (328).Fontinha D; Sousa SA; Morais TS; Prudêncio M; Leitão JH; Le Gal Y; Lorcy D; Silva RA; Velho MF; Belo D; et al. Gold (III) Bis (Dithiolene) Complexes: From Molecular Conductors to Prospective Anticancer, Antimicrobial and Anti-plasmodial Agents. Metallomics 2020, 12, 974–987. [DOI] [PubMed] [Google Scholar]
- (329).Chakraborty P; Oosterhuis D; Bonsignore R; Casini A; Olinga P; Scheffers DJ An Organogold Compound as Potential Antimicrobial Agent against Drug-Resistant Bacteria: Initial Mechanistic Insights. ChemMedChem. 2021, 16, 3060–3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (330).Ilari A; Baiocco P; Messori L; Fiorillo A; Boffi A; Gramiccia M; Di Muccio T; Colotti G A Gold-Containing Drug against Parasitic Polyamine Metabolism: The X-Ray Structure of Trypanothione Reductase from Leishmania Infantum in Complex with Auranofin Reveals a Dual Mechanism of Enzyme Inhibition. Amino Acids 2012, 42, 803–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (331).Sharlow ER; Leimgruber S; Murray S; Lira A; Sciotti RJ; Hickman M; Hudson T; Leed S; Caridha D; Barrios AM; Close D; Grögl M; Lazo JS Auranofin Is an Apoptosis-Simulating Agent with in Vitro and in Vivo Anti-Leishmanial Activity. ACS Chem. Biol. 2014, 9, 663–672. [DOI] [PubMed] [Google Scholar]
- (332).Rosa LB; Aires RL; Oliveira LS; Fontes JV; Miguel DC; Abbehausen CA “Golden Age” for the Discovery of New Antileishmanial Agents: Current Status of Leishmanicidal Gold Complexes and Prospective Targets Beyond the Trypanothione System. ChemMedChem. 2021, 16, 1682–1696. [DOI] [PubMed] [Google Scholar]
- (333).Mota VZ; de Carvalho GS; da Silva AD; Costa LA; de Almeida Machado P; Coimbra ES; Ferreira CV; Shishido SM; Cuin A Gold Complexes with Benzimidazole Derivatives: Synthesis, Characterization and Biological Studies. Biometals 2014, 27, 183–194. [DOI] [PubMed] [Google Scholar]
- (334).Minori K; Rosa LB; Bonsignore R; Casini A; Miguel DC Comparing the Antileishmanial Activity of Gold(I) and Gold(III) Compounds in L. Amazonensis and L. Braziliensis in Vitro. ChemMedChem. 2020, 15, 2146–2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (335).Ouji M; Delmas SB; Álvarez ÁF; Augereau J-M; Valentin A; Hemmert C; Gornitzka H; Benoit-Vical F Design, Synthesis and Efficacy of Hybrid Triclosan-Gold Based Molecules on Artemisinin-Resistant Plasmodium Falciparum and Leishmania Infantum Parasites. ChemistrySelect 2020, 5, 619–625. [Google Scholar]
- (336).Zhang C; Bourgeade Delmas S; Fernández Álvarez A; Valentin A; Hemmert C; Gornitzka H Synthesis, Characterization, and Antileishmanial Activity of Neutral N-Heterocyclic Carbenes Gold(I) Complexes. Eur. J. Med. Chem. 2018, 143, 1635–1643. [DOI] [PubMed] [Google Scholar]
- (337).Chaves JDS; Tunes LG; de J. Franco CH; Francisco TM; Corrêa CC; Murta SMF; Monte-Neto RL; Silva H; Fontes APS; de Almeida MV Novel Gold(I) Complexes with 5-Phenyl-1,3,4-Oxadiazole-2-Thione and Phosphine as Potential Anti-cancer and Antileishmanial Agents. Eur. J. Med. Chem. 2017, 127, 727–739. [DOI] [PubMed] [Google Scholar]
- (338).Espinosa AV; Costa D. d. S.; Tunes LG; Monte-Neto R. L. d.; Grazul RM; de Almeida MV; Silva H Anticancer and Antileishmanial in Vitro Activity of Gold(I) Complexes with 1,3,4-Oxadiazole-2(3h)-Thione Ligands Derived from Δ-D-Gluconolactone. Chem. Biol. Drug Des. 2021, 97, 41–50. [DOI] [PubMed] [Google Scholar]
- (339).Tunes LG; Morato RE; Garcia A; Schmitz V; Steindel M; Corrêa-Junior JD; Dos Santos HF; Frézard F; de Almeida MV; Silva H; Moretti NS; de Barros ALB; do Monte-Neto RL Preclinical Gold Complexes as Oral Drug Candidates to Treat Leishmaniasis Are Potent Trypanothione Reductase Inhibitors. ACS Infect. Dis. 2020, 6, 1121–1139. [DOI] [PubMed] [Google Scholar]
- (340).Wallace DC Mitochondria and Cancer. Nat. Rev. Cancer 2012, 12, 685–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (341).Missiroli S; Perrone M; Genovese I; Pinton P; Giorgi C Cancer Metabolism and Mitochondria: Finding Novel Mechanisms to Fight Tumours. EBioMedicine 2020, 59, 102943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (342).Vyas S; Zaganjor E; Haigis MC Mitochondria and Cancer. Cell 2016, 166, 555–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (343).Zong W-X; Rabinowitz JD; White E Mitochondria and Cancer. Mol. Cell 2016, 61, 667–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (344).Newmeyer DD; Ferguson-Miller S Mitochondria: Releasing Power for Life and Unleashing the Machineries of Death. Cell 2003, 112, 481–490. [DOI] [PubMed] [Google Scholar]
- (345).Grasso D; Zampieri LX; Capelôa T; Van de Velde JA; Sonveaux P Mitochondria in Cancer. Cell stress 2020, 4, 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (346).Bock FJ; Tait SW Mitochondria as Multifaceted Regulators of Cell Death. Nat. Rev. Mol. Cell Biol. 2020, 21, 85–100. [DOI] [PubMed] [Google Scholar]
- (347).Chandel NS Evolution of Mitochondria as Signaling Organelles. Cell Metab. 2015, 22, 204–206. [DOI] [PubMed] [Google Scholar]
- (348).Chakrabarty RP; Chandel NS Mitochondria as Signaling Organelles Control Mammalian Stem Cell Fate. Cell Stem Cell 2021, 28, 394–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (349).Galluzzi L; Kepp O; Kroemer G Mitochondria: Master Regulators of Danger Signalling. Nat. Rev. Mol. Cell Biol. 2012, 13, 780–788. [DOI] [PubMed] [Google Scholar]
- (350).Tait SW; Green DR Mitochondria and Cell Death: Outer Membrane Permeabilization and Beyond. Nat. Rev. Mol. Cell Biol. 2010, 11, 621–632. [DOI] [PubMed] [Google Scholar]
- (351).Tait SW; Green DR Mitochondria and Cell Signalling. J. Cell Sci. 2012, 125, 807–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (352).Berners-Price SJ; Mirabelli CK; Johnson RK; Mattern MR; McCabe FL; Faucette LF; Sung C-M; Mong S-M; Sadler PJ; Crooke ST In Vivo Antitumor Activity and in Vitro Cytotoxic Properties of Bis [1, 2-Bis (Diphenylphosphino) Ethane] Gold (I) Chloride. Cancer Res. 1986, 46, 5486–5493. [PubMed] [Google Scholar]
- (353).Barnard PJ; Berners-Price SJ Targeting the Mitochondrial Cell Death Pathway with Gold Compounds. Coord. Chem. Rev. 2007, 251, 1889–1902. [Google Scholar]
- (354).Xiao Q; Liu Y; Jiang G; Liu Y; Huang Y; Liu W; Zhang Z Heteroleptic Gold (I)-BisNHC Complex with Excellent Activity in Vitro, Ex Vivo and in Vivo against Endometrial Cancer. Eur. J. Med. Chem. 2022, 236, 114302. [DOI] [PubMed] [Google Scholar]
- (355).Mertens RT; Jennings WC; Ofori S; Kim JH; Parkin S; Kwakye GF; Awuah SG Synthetic Control of Mitochondrial Dynamics: Developing Three-Coordinate Au(I) Probes for Perturbation of Mitochondria Structure and Function. JACS Au 2021, 1, 439–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (356).Mertens RT; Greif CE; Coogle JT; Berger G; Parkin S; Watson MD; Awuah SG Stable Au (I) Catalysts for Oxidant-Free Ch Functionalization with Iodoarenes. Journal of catalysis 2022, 408, 109–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (357).Zhang X; Frezza M; Milacic V; Ronconi L; Fan Y; Bi C; Fregona D; Dou QP Inhibition of Tumor Proteasome Activity by Gold-Dithiocarbamato Complexes Via Both Redox-Dependent and-Independent Processes. J. Cell. Biochem. 2010, 109, 162–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (358).Milacic V; Chen D; Ronconi L; Landis-Piwowar KR; Fregona D; Dou QP A Novel Anticancer Gold(III) Dithiocarbamate Compound Inhibits the Activity of a Purified 20s Proteasome and 26s Proteasome in Human Breast Cancer Cell Cultures and Xenografts. Cancer Res. 2006, 66, 10478–10486. [DOI] [PubMed] [Google Scholar]
- (359).Tomasello MF; Nardon C; Lanza V; Di Natale G; Pettenuzzo N; Salmaso S; Milardi D; Caliceti P; Pappalardo G; Fregona D New Comprehensive Studies of a Gold(III) Dithiocarbamate Complex with Proven Anticancer Properties: Aqueous Dissolution with Cyclodextrins, Pharmacokinetics and Upstream Inhibition of the Ubiquitin-Proteasome Pathway. Eur. J. Med. Chem. 2017, 138, 115–127. [DOI] [PubMed] [Google Scholar]
- (360).Adokoh CK Therapeutic Potential of Dithiocarbamate Supported Gold Compounds. RSC Adv. 2020, 10, 2975–2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (361).Mertens RT; Parkin S; Awuah SG Cancer Cell-Selective Modulation of Mitochondrial Respiration and Metabolism by Potent Organogold(III) Dithiocarbamates. Chem. Sci. 2020, 11, 10465–10482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (362).Kim JH; Ofori S; Parkin S; Vekaria H; Sullivan PG; Awuah SG Anticancer Gold(III)-Bisphosphine Complex Alters the Mitochondrial Electron Transport Chain to Induce in Vivo Tumor Inhibition. Chem. Sci. 2021, 12, 7467–7479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (363).Kim JH; Reeder E; Parkin S; Awuah SG Gold(I/III)-Phosphine Complexes as Potent Antiproliferative Agents. Sci. Rep. 2019, 9, 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (364).Hyun Kim J; Ofori S; Mertens RT; Parkin S; Awuah SG Water-Soluble Gold(III)-Metformin Complex Alters Mitochondrial Bioenergetics in Breast Cancer Cells. ChemMedChem. 2021, 16, 3222–3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (365).Babak MV; Chong KR; Rapta P; Zannikou M; Tang HM; Reichert L; Chang MR; Kushnarev V; Heffeter P; Meier-Menches SM; Lim ZC; Yap JY; Casini A; Balyasnikova IV; Ang WH Interfering with Metabolic Profile of Triple-Negative Breast Cancers Using Rationally Designed Metformin Prodrugs. Angew. Chem., Int. Ed. 2021, 60, 13405–13413. [DOI] [PubMed] [Google Scholar]
- (366).Machado JF; Correia JDG; Morais TS Emerging Molecular Receptors for the Specific-Target Delivery of Ruthenium and Gold Complexes into Cancer Cells. Molecules 2021, 26.3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (367).Smiłowicz D; Slootweg JC; Metzler-Nolte N Bioconjugation of Cyclometalated Gold(III) Lipoic Acid Fragments to Linear and Cyclic Breast Cancer Targeting Peptides. Mol. Pharmaceutics 2019, 16, 4572–4581. [DOI] [PubMed] [Google Scholar]
- (368).Bertrand B; O’Connell MA; Waller ZAE; Bochmann M A Gold(III) Pincer Ligand Scaffold for the Synthesis of Binuclear and Bioconjugated Complexes: Synthesis and Anticancer Potential. Chemistry (Easton) 2018, 24, 3613–3622. [DOI] [PubMed] [Google Scholar]
- (369).Ortega E; Zamora A; Basu U; Lippmann P; Rodríguez V; Janiak C; Ott I; Ruiz J An Erlotinib Gold(I) Conjugate for Combating Triple-Negative Breast Cancer. J. Inorg. Biochem. 2020, 203, 110910. [DOI] [PubMed] [Google Scholar]
- (370).Curado N; Dewaele-Le Roi G; Poty S; Lewis JS; Contel M Trastuzumab Gold-Conjugates: Synthetic Approach and in Vitro Evaluation of Anticancer Activities in Breast Cancer Cell Lines. Chem. Commun. 2019, 55, 1394–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (371).Sen S; Won M; Levine MS; Noh Y; Sedgwick AC; Kim JS; Sessler JL; Arambula JF Metal-Based Anticancer Agents as Immunogenic Cell Death Inducers: The Past, Present, and Future. Chem. Soc. Rev. 2022, 51, 1212–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (372).Sen S; Karoscik K; Maier E; Arambula JF Immunogenic Cell Death-Inducing Metal Complexes: From the Benchtop to the Clinic. Curr. Opin. Chem. Biol. 2023, 73, 102277. [DOI] [PubMed] [Google Scholar]
- (373).Eisler R Chrysotherapy: A Synoptic Review. Inflamm. Res. 2003, 52, 487–501. [DOI] [PubMed] [Google Scholar]
- (374).van der Most RG; Robinson BW; Lake RA Combining Immunotherapy with Chemotherapy to Treat Cancer. Discovery Med. 2005, 5, 265–270. [PubMed] [Google Scholar]
- (375).Gandhi L; Rodríguez-Abreu D; Gadgeel S; Esteban E; Felip E; De Angelis F; Domine M; Clingan P; Hochmair MJ; Powell SF; Cheng SY; Bischoff HG; Peled N; Grossi F; Jennens RR; Reck M; Hui R; Garon EB; Boyer M; Rubio-Viqueira B; Novello S; Kurata T; Gray JE; Vida J; Wei Z; Yang J; Raftopoulos H; Pietanza MC; Garassino MC Pembrolizumab Plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 2078–2092. [DOI] [PubMed] [Google Scholar]
- (376).Cao G; Wang J; Zheng X; Wei H; Tian Z; Sun R Tumor Therapeutics Work as Stress Inducers to Enhance Tumor Sensitivity to Natural Killer (Nk) Cell Cytolysis by up-Regulating Nkp30 Ligand B7-H6. J. Biol. Chem. 2015, 290, 29964–29973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (377).Mármol I; Virumbrales-Muñoz M; Quero J; Sánchez-de-Diego C; Fernández L; Ochoa I; Cerrada E; Yoldi MJR Alkynyl Gold(I) Complex Triggers Necroptosis Via Ros Generation in Colorectal Carcinoma Cells. J. Inorg. Biochem. 2017, 176, 123–133. [DOI] [PubMed] [Google Scholar]
- (378).Jeon KI; Byun MS; Jue DM Gold Compound Auranofin Inhibits Ikappab Kinase (Ikk) by Modifying Cys-179 of Ikkbeta Subunit. Exp. Mol. Med. 2003, 35, 61–66. [DOI] [PubMed] [Google Scholar]
- (379).Kim I; Crippen GM; Amidon GL Structure and Specificity of a Human Valacyclovir Activating Enzyme: A Homology Model of Bphl. Mol. Pharmaceutics 2004, 1, 434–446. [DOI] [PubMed] [Google Scholar]
- (380).Baeck M; Goossens A Systemic Contact Dermatitis to Corticosteroids. Allergy 2012, 67, 1580–1585. [DOI] [PubMed] [Google Scholar]
- (381).Elie BT; Pechenyy Y; Uddin F; Contel M A Heterometallic Ruthenium-Gold Complex Displays Antiproliferative, Antimigratory, and Antiangiogenic Properties and Inhibits Metastasis and Angiogenesis-Associated Proteases in Renal Cancer. J. Biol. Inorg. Chem. 2018, 23, 399–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (382).Rachmawati D; Alsalem IW; Bontkes HJ; Verstege MI; Gibbs S; von Blomberg BM; Scheper RJ; van Hoogstraten IM Innate Stimulatory Capacity of High Molecular Weight Transition Metals Au (Gold) and Hg (Mercury). Toxicol. In Vitro 2015, 29, 363–369. [DOI] [PubMed] [Google Scholar]
- (383).Martin SF; Esser PR; Weber FC; Jakob T; Freudenberg MA; Schmidt M; Goebeler M Mechanisms of Chemical-Induced Innate Immunity in Allergic Contact Dermatitis. Allergy 2011, 66, 1152–1163. [DOI] [PubMed] [Google Scholar]
- (384).Zitvogel L; Kepp O; Kroemer G Immune Parameters Affecting the Efficacy of Chemotherapeutic Regimens. Nat. Rev. Clin. Oncol. 2011, 8, 151–160. [DOI] [PubMed] [Google Scholar]
- (385).Tang H; Xu X; Chen Y; Xin H; Wan T; Li B; Pan H; Li D; Ping Y Reprogramming the Tumor Microenvironment through Second-near-Infrared-Window Photothermal Genome Editing of Pd-L1Mediated by Supramolecular Gold Nanorods for Enhanced Cancer Immunotherapy. Adv. Mater. 2021, 33, No. e2006003. [DOI] [PubMed] [Google Scholar]
- (386).Liang R; Liu L; He H; Chen Z; Han Z; Luo Z; Wu Z; Zheng M; Ma Y; Cai L Oxygen-Boosted Immunogenic Photodynamic Therapy with Gold Nanocages@Manganese Dioxide to Inhibit Tumor Growth and Metastases. Biomaterials 2018, 177, 149–160. [DOI] [PubMed] [Google Scholar]
- (387).Sen S; Hufnagel S; Maier EY; Aguilar I; Selvakumar J; DeVore JE; Lynch VM; Arumugam K; Cui Z; Sessler JL; Arambula JF Rationally Designed Redox-Active Au(I) N-Heterocyclic Carbene: An Immunogenic Cell Death Inducer. J. Am. Chem. Soc. 2020, 142, 20536–20541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (388).Reya T; Morrison SJ; Clarke MF; Weissman IL Stem Cells, Cancer, and Cancer Stem Cells. Nature 2001, 414, 105–111. [DOI] [PubMed] [Google Scholar]
- (389).Gasch C; Ffrench B; O’Leary JJ; Gallagher MF Catching Moving Targets: Cancer Stem Cell Hierarchies, Therapy-Resistance & Considerations for Clinical Intervention. Mol. Cancer 2017, 16, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (390).Zheng Q; Zhang M; Zhou F; Zhang L; Meng X The Breast Cancer Stem Cells Traits and Drug Resistance. Front. Pharmacol. 2021, 11, 599965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (391).Zou T; Lum CT; Lok C-N; To W-P; Low K-H; Che C-M A Binuclear Gold (I) Complex with Mixed Bridging Diphosphine and Bis (N-Heterocyclic Carbene) Ligands Shows Favorable Thiol Reactivity and Inhibits Tumor Growth and Angiogenesis in Vivo. Angew. Chem., Int. Ed. 2014, 126, 5920–5924. [DOI] [PubMed] [Google Scholar]
- (392).Roesch S; Fermi V; Rominger F; Herold-Mende C; Romero-Nieto C Correction: Gold (I) Complexes Based on Six-Membered Phosphorus Heterocycles as Bio-Active Molecules against Brain Cancer. Chem. Commun. 2020, 56, 15088–15088. [DOI] [PubMed] [Google Scholar]
- (393).Johnson A; Olelewe C; Kim JH; Northcote-Smith J; Mertens RT; Passeri G; Singh K; Awuah SG; Suntharalingam K The Anti-Breast Cancer Stem Cell Properties of Gold (I)-Non-Steroidal Anti-Inflammatory Drug Complexes. Chem. Sci. 2023.14557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (394).Lum CT; Wong AS-T; Lin MC; Che C-M; Sun RW-Y A Gold(III) Porphyrin Complex as an Anti-Cancer Candidate to Inhibit Growth of Cancer-Stem Cells. Chem. Commun. 2013, 49, 4364–4366. [DOI] [PubMed] [Google Scholar]
- (395).Cascella M; Rajnik M; Aleem A; Dulebohn SC; Di Napoli R Features, Evaluation, and Treatment of Coronavirus (Covid-19); StatPearls Publishing LLC., 2022. [PubMed] [Google Scholar]
- (396).Fonteh PN; Keter FK; Meyer D Hiv Therapeutic Possibilities of Gold Compounds. Biometals 2010, 23, 185–196. [DOI] [PubMed] [Google Scholar]
- (397).Bowman M-C; Ballard TE; Ackerson CJ; Feldheim DL; Margolis DM; Melander C Inhibition of Hiv Fusion with Multivalent Gold Nanoparticles. J. Am. Chem. Soc. 2008, 130, 6896–6897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (398).Fonseca C; Aureliano M Biological Activity of Gold Compounds against Viruses and Parasitosis: A Systematic Review. BioChem. 2022, 2, 145–159. [Google Scholar]
- (399).Sonzogni-Desautels K; Ndao M Will Auranofin Become a Golden New Treatment against Covid-19? Front. Immunol. 2021, 12, 683694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (400).O’Donovan SM; Imami A; Eby H; Henkel ND; Creeden JF; Asah S; Zhang X; Wu X; Alnafisah R; Taylor RT; Reigle J; Thorman A; Shamsaei B; Meller J; McCullumsmith RE Identification of Candidate Repurposable Drugs to Combat Covid-19 Using a Signature-Based Approach. Sci. Rep. 2021, 11, 4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (401).Ena J; Pasquau F Once-a-Day Highly Active Antiretroviral Therapy: A Systematic Review. Clin. Infect. Dis. 2003, 36, 1186–1190. [DOI] [PubMed] [Google Scholar]
- (402).Tam LW; Chui CK; Brumme CJ; Bangsberg DR; Montaner JS; Hogg RS; Harrigan PR The Relationship between Resistance and Adherence in Drug-Naive Individuals Initiating Haart Is Specific to Individual Drug Classes. J. Acquir. Immune Defic. Syndr. 2008, 49, 266–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (403).Burda ST; Viswanath R; Zhao J; Kinge T; Anyangwe C; Tinyami ET; Haldar B; Powell RL; Jarido V; Hewlett IK; Nyambi PN Hiv-1 Reverse Transcriptase Drug-Resistance Mutations in Chronically Infected Individuals Receiving or Naiv e to Haart in Cameroon. J. Med. Virol. 2010, 82, 187–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (404).Chirullo B; Sgarbanti R; Limongi D; Shytaj IL; Alvarez D; Das B; Boe A; DaFonseca S; Chomont N; Liotta L; Petricoin EI; Norelli S; Pelosi E; Garaci E; Savarino A; Palamara AT A Candidate Anti-Hiv Reservoir Compound, Auranofin, Exerts a Selective ’Anti-Memory’ Effect by Exploiting the Baseline Oxidative Status of Lymphocytes. Cell Death Dis. 2013, 4, No. e944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (405).Mphahlele M; Papathanasopoulos M; Cinellu MA; Coyanis M; Mosebi S; Traut T; Modise R; Coates J; Hewer R Modification of Hiv-1 Reverse Transcriptase and Integrase Activity by Gold(III) Complexes in Direct Biochemical Assays. Bioorg. Med. Chem. 2012, 20, 401–407. [DOI] [PubMed] [Google Scholar]
- (406).Milacic V; Dou QP The Tumor Proteasome as a Novel Target for Gold(III) Complexes: Implications for Breast Cancer Therapy. Coord. Chem. Rev. 2009, 253, 1649–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (407).Kirschner S; Wei Y-K; Francis D; Bergman JG Anticancer and Potential Antiviral Activity of Complex Inorganic Compounds. J. Med. Chem. 1966, 9, 369–372. [DOI] [PubMed] [Google Scholar]
- (408).Aires RL; Santos IA; Fontes JV; Bergamini FRG; Jardim ACG; Abbehausen C Triphenylphosphine Gold(I) Derivatives Promote Antiviral Effects against the Chikungunya Virus. Metallomics 2022, 14. DOI: 10.1093/mtomcs/mfac056 [DOI] [PubMed] [Google Scholar]
- (409).Anthony EJ; Bolitho EM; Bridgewater HE; Carter OWL; Donnelly JM; Imberti C; Lant EC; Lermyte F; Needham RJ; Palau M; Sadler PJ; Shi H; Wang F-X; Zhang W-Y; Zhang Z Metallodrugs Are Unique: Opportunities and Challenges of Discovery and Development. Chem. Sci. 2020, 11, 12888–12917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (410).Traber KE; Okamoto H; Kurono C; Baba M; Saliou C; Soji T; Packer L; Okamoto T Anti-Rheumatic Compound Aurothioglucose Inhibits Tumor Necrosis Factor-Alpha-Induced Hiv-1 Replication in Latently Infected Om10.1 and Ach2 Cells. Int. Immunol. 1999, 11, 143–150. [DOI] [PubMed] [Google Scholar]
- (411).Evans GF; Zuckerman SH Pharmacologic Modulation of Tnf Production by Endotoxin Stimulated Macrophages: In Vitro and in Vivo Effects of Auranofin and Other Chrysotherapeutic Compounds. Agents Actions 1989, 26, 329–334. [DOI] [PubMed] [Google Scholar]
- (412).Youn HS; Lee JY; Saitoh SI; Miyake K; Hwang DH Auranofin, as an Anti-Rheumatic Gold Compound, Suppresses Lps-Induced Homodimerization of Tlr4. Biochem. Biophys. Res. Commun. 2006, 350, 866–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (413).Jeon K-I; Jeong J-Y; Jue D-M Thiol-Reactive Metal Compounds Inhibit Nf-Κb Activation by Blocking Iκb Kinase. J. Immunol. 2000, 164, 5981–5989. [DOI] [PubMed] [Google Scholar]
- (414).Trávníček Z; Starha P; Vančo J; Silha T; Hošek J; Suchý P Jr.; Pražanová G Anti-Inflammatory Active Gold(I) Complexes Involving 6-Substituted-Purine Derivatives. J. Med. Chem. 2012, 55, 4568–4579. [DOI] [PubMed] [Google Scholar]
- (415).Vančo J; Gáliková J; Hošek J; Dvořák Z; Paráková L; Trávníček Z Gold (I) Complexes of 9-Deazahypoxanthine as Selective Antitumor and Anti-Inflammatory Agents. PLoS One 2014, 9, No. e109901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (416).Wempe L; Mohamed R; Warinner J; Goretsky T; Avdiushko M; Kim J; Abomhya A; Lee G; Awuah S; Barrett T; Kapur N Auphos, a ″First-in Class″ Oral Agent for Correcting Metabolic Dysfunction in Ibd. Gastroenterology 2022, 162, S2. [Google Scholar]
- (417).Mohamed R; Warinner J; Wempe L; Avdiushko M; Goretsky T; Kim J; Abomhya A; Lee G; Awuah S; Barrett T; Kapur N Auphos, a Novel Therapeutic That Improves Mitochondrial Function and Ameliorates Chronic Colitis. Gastroenterology 2022, 162, S2–S3. [Google Scholar]
- (418).Shashikanth N; Liu Y; Xing T; Turner J Novel Contributions of Claudin-4 to Epithelial Barrier Regulation and Colitis. Inflamm. Bowel Dis. 2022, 28, S51–S52. [Google Scholar]
- (419).Cheng Z; Li M; Dey R; Chen Y Nanomaterials for Cancer Therapy: Current Progress and Perspectives. J. Hematol. Oncol. 2021, 14, 1–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (420).Sanna V; Pala N; Sechi M Targeted Therapy Using Nanotechnology: Focus on Cancer. Int. J. Nanomedicine 2014, 9, 467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (421).Silva CO; Pinho JO; Lopes JM; Almeida AJ; Gaspar MM; Reis C Current Trends in Cancer Nanotheranostics: Metallic, Polymeric, and Lipid-Based Systems. Pharmaceutics 2019, 11, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (422).Patra JK; Das G; Fraceto LF; Campos EVR; Rodriguez-Torres M. d. P.; Acosta-Torres LS; Diaz-Torres LA; Grillo R; Swamy MK; Sharma S; et al. Nano Based Drug Delivery Systems: Recent Developments and Future Prospects. J. Nanobiotechnology 2018, 16, 1–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (423).Farinha P; Pinho JO; Matias M; Gaspar MM Nanomedicines in the Treatment of Colon Cancer: A Focus on Metallodrugs. Drug Delivery Transl. Res. 2022, 12, 49–66. [DOI] [PubMed] [Google Scholar]
- (424).Phillips MA; Gran ML; Peppas NA Targeted Nanodelivery of Drugs and Diagnostics. Nano today 2010, 5, 143–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (425).Maeda H; Wu J; Sawa T; Matsumura Y; Hori K Tumor Vascular Permeability and the Epr Effect in Macromolecular Therapeutics: A Review. J. Controlled Release 2000, 65, 271–284. [DOI] [PubMed] [Google Scholar]
- (426).Banerjee A; Pathak S; Subramanium VD; Dharanivasan G; Murugesan R; Verma RS Strategies for Targeted Drug Delivery in Treatment of Colon Cancer: Current Trends and Future Perspectives. Drug Discovery 2017, 22, 1224–1232. [DOI] [PubMed] [Google Scholar]
- (427).Attia MF; Anton N; Wallyn J; Omran Z; Vandamme TF An Overview of Active and Passive Targeting Strategies to Improve the Nanocarriers Efficiency to Tumour Sites. J. Pharm. Pharmacol. 2019, 71, 1185–1198. [DOI] [PubMed] [Google Scholar]
- (428).Moreno-Alcántar G; Picchetti P; Casini A Gold Complexes in Anticancer Therapy: From New Design Principles to Particle-Based Delivery Systems. Angew. Chem., Int. Ed. 2023. DOI: 10.1002/anie.202218000 [DOI] [PubMed] [Google Scholar]
- (429).Dantas KCF; Rosário J. d. S.; Silva-Caldeira PP Polymeric Nanosystems Applied for Metal-Based Drugs and Photosensitizers Delivery: The State of the Art and Recent Advancements. Pharmaceutics 2022, 14, 1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (430).Callari M; Aldrich-Wright JR; de Souza PL; Stenzel MH Polymers with Platinum Drugs and Other Macromolecular Metal Complexes for Cancer Treatment. Prog. Polym. Sci. 2014, 39, 1614–1643. [Google Scholar]
- (431).Lin Y-X; Gao Y-J; Wang Y; Qiao Z-Y; Fan G; Qiao S-L; Zhang R-X; Wang L; Wang H Ph-Sensitive Polymeric Nanoparticles with Gold (I) Compound Payloads Synergistically Induce Cancer Cell Death through Modulation of Autophagy. Mol. Pharmaceutics 2015, 12, 2869–2878. [DOI] [PubMed] [Google Scholar]
- (432).Ahmad S; Isab AA; Ali S; Al-Arfaj AR Perspectives in Bioinorganic Chemistry of Some Metal Based Therapeutic Agents. Polyhedron 2006, 25, 1633–1645. [Google Scholar]
- (433).Mirabelli CK; Johnson RK; Sung CM; Faucette L; Muirhead K; Crooke ST Evaluation of the in Vivo Antitumor Activity and in Vitro Cytotoxic Properties of Auranofin, a Coordinated Gold Compound, in Murine Tumor Models. Cancer Res. 1985, 45, 32–39. [PubMed] [Google Scholar]
- (434).Pearson S; Lu H; Stenzel MH Glycopolymer Self-Assemblies with Gold (I) Complexed to the Core as a Delivery System for Auranofin. Macromolecules 2015, 48, 1065–1076. [Google Scholar]
- (435).Nardon C; Boscutti G; Dalla Via L; Ringhieri P; Di Noto V; Morelli G; Accardo A; Fregona D Cck8 Peptide-Labeled Pluronic® F127 Micelles as a Targeted Vehicle of Gold-Based Anticancer Chemotherapeutics. MedChemComm 2015, 6, 155–163. [Google Scholar]
- (436).Chung CY-S; Fung S-K; Tong K-C; Wan P-K; Lok C-N; Huang Y; Chen T; Che C-M A Multi-Functional Pegylated Gold(III) Compound: Potent Anti-Cancer Properties and Self-Assembly into Nanostructures for Drug Co-Delivery. Chem. Sci. 2017, 8, 1942–1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (437).Lee P; Lok C-N; Che C-M; Kao WJ A Multifunctional Hydrogel Delivers Gold Compound and Inhibits Human Lung Cancer Xenograft. Pharm. Res. 2019, 36, 1–10. [DOI] [PubMed] [Google Scholar]
- (438).Lee P-Y; Lok C-N; Che C-M; Kao WJ Multifunctional Microparticles Incorporating Gold Compound Inhibit Human Lung Cancer Xenograft. Pharm. Res. 2020, 37, 1–10. [DOI] [PubMed] [Google Scholar]
- (439).Ringhieri P; Iannitti R; Nardon C; Palumbo R; Fregona D; Morelli G; Accardo A Target Selective Micelles for Bombesin Receptors Incorporating Au(III)-Dithiocarbamato Complexes. Int. J. Pharm. 2014, 473, 194–202. [DOI] [PubMed] [Google Scholar]
- (440).MaHam A; Tang Z; Wu H; Wang J; Lin Y Protein-Based Nanomedicine Platforms for Drug Delivery. Small 2009, 5, 1706–1721. [DOI] [PubMed] [Google Scholar]
- (441).Ma-Ham A; Wu H; Wang J; Kang X; Zhang Y; Lin Y Apoferritin-Based Nanomedicine Platform for Drug Delivery: Equilibrium Binding Study of Daunomycin with DNA. J. Mater. Chem. 2011, 21, 8700–8708. [Google Scholar]
- (442).Tosi G; Belletti D; Pederzoli F; Ruozi B Apoferritin Nanocage as Drug Reservoir: Is It a Reliable Drug Delivery System? Exp. Opin. Drug Delivery 2016, 13, 1341–1343. [DOI] [PubMed] [Google Scholar]
- (443).Ferraro G; Monti DM; Amoresano A; Pontillo N; Petruk G; Pane F; Cinellu MA; Merlino A Gold-Based Drug Encapsulation within a Ferritin Nanocage: X-Ray Structure and Biological Evaluation as a Potential Anticancer Agent of the Auoxo3-Loaded Protein. Chem. Commun. 2016, 52, 9518–9521. [DOI] [PubMed] [Google Scholar]
- (444).Messori L; Scaletti F; Massai L; Cinellu MA; Russo Krauss I; Di Martino G; Vergara A; Paduano L; Merlino A Interactions of Gold-Based Drugs with Proteins: Crystal Structure of the Adduct Formed between Ribonuclease a and a Cytotoxic Gold(III) Compound. Metallomics 2014, 6, 233–236. [DOI] [PubMed] [Google Scholar]
- (445).Russo Krauss I; Messori L; Cinellu MA; Marasco D; Sirignano R; Merlino A Interactions of Gold-Based Drugs with Proteins: The Structure and Stability of the Adduct Formed in the Reaction between Lysozyme and the Cytotoxic Gold(III) Compound Auoxo3. Dalton Trans. 2014, 43, 17483–17488. [DOI] [PubMed] [Google Scholar]
- (446).Zhang J; Zhang Z; Jiang M; Li S; Yuan H; Sun H; Yang F; Liang H Developing a Novel Gold(III) Agent to Treat Glioma Based on the Unique Properties of Apoferritin Nanoparticles: Inducing Lethal Autophagy and Apoptosis. J. Med. Chem. 2020, 63, 13695–13708. [DOI] [PubMed] [Google Scholar]
- (447).Bouchoucha M; Cote M-F; Gaudreault R; Fortin M-A; Kleitz F Size-Controlled Functionalized Mesoporous Silica Nanoparticles for Tunable Drug Release and Enhanced Anti-Tumoral Activity. Chem. Mater. 2016, 28, 4243–4258. [Google Scholar]
- (448).Farjadian F; Roointan A; Mohammadi-Samani S; Hosseini M Mesoporous Silica Nanoparticles: Synthesis, Pharmaceutical Applications, Biodistribution, and Biosafety Assessment. Chem. Eng. J. 2019, 359, 684–705. [Google Scholar]
- (449).Gao Y; Gao D; Shen J; Wang Q A Review of Mesoporous Silica Nanoparticle Delivery Systems in Chemo-Based Combination Cancer Therapies. Front. Chem. 2020, 8, 598722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (450).Croissant JG; Fatieiev Y; Almalik A; Khashab NM Mesoporous Silica and Organosilica Nanoparticles: Physical Chemistry, Biosafety, Delivery Strategies, and Biomedical Applications. Adv. Healthc. Mater 2018, 7, 1700831. [DOI] [PubMed] [Google Scholar]
- (451).Li T; Shi S; Goel S; Shen X; Xie X; Chen Z; Zhang H; Li S; Qin X; Yang H; et al. Recent Advancements in Mesoporous Silica Nanoparticles Towards Therapeutic Applications for Cancer. Acta Biomater. 2019, 89, 1–13. [DOI] [PubMed] [Google Scholar]
- (452).He L; Chen T; You Y; Hu H; Zheng W; Kwong WL; Zou T; Che CM A Cancer-Targeted Nanosystem for Delivery of Gold(III) Complexes: Enhanced Selectivity and Apoptosis-Inducing Efficacy of a Gold(III) Porphyrin Complex. Angew. Chem., Int. Ed. 2014, 126, 12740–12744. [DOI] [PubMed] [Google Scholar]
- (453).Zhang S Fabrication of Novel Biomaterials through Molecular Self-Assembly. Nat. Biotechnol. 2003, 21, 1171–1178. [DOI] [PubMed] [Google Scholar]
- (454).Cui H; Webber MJ; Stupp SI Self-Assembly of Peptide Amphiphiles: From Molecules to Nanostructures to Biomaterials. J. Pept. Sci. 2010, 94, 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (455).Lopez-Silva TL; Schneider JP From Structure to Application: Progress and Opportunities in Peptide Materials Development. Curr. Opin. Chem. Biol. 2021, 64, 131–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (456).Cancemi P; Buttacavoli M; Roz E; Feo S Expression of Alpha-Enolase (Eno1), Myc Promoter-Binding Protein-1 (Mbp-1) and Matrix Metalloproteinases (Mmp-2 and Mmp-9) Reflect the Nature and Aggressiveness of Breast Tumors. Int. J. Mol. Sci. 2019, 20, 3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (457).Sumi T; Nakatani T; Yoshida H; Hyun Y; Yasui T; Matsumoto Y; Nakagawa E; Sugimura K; Kawashima H; Ishiko O Expression of Matrix Metalloproteinases 7 and 2 in Human Renal Cell Carcinoma. Oncol. Rep. 2003, 10, 567–570. [PubMed] [Google Scholar]
- (458).Marciano Y; Del Solar V; Nayeem N; Dave D; Son J; Contel M; Ulijn RV Encapsulation of Gold-Based Anticancer Agents in Protease-Degradable Peptide Nanofilaments Enhances Their Potency. J. Am. Chem. Soc. 2022.1451234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (459).Wang Z; Medforth CJ; Shelnutt JA Self-Metallization of Photocatalytic Porphyrin Nanotubes. J. Am. Chem. Soc. 2004, 126, 16720–16721. [DOI] [PubMed] [Google Scholar]
- (460).Zhong Y; Wang J; Zhang R; Wei W; Wang H; Lü X; Bai F; Wu H; Haddad R; Fan H Morphology-Controlled Self-Assembly and Synthesis of Photocatalytic Nanocrystals. Nano Lett. 2014, 14, 7175–7179. [DOI] [PubMed] [Google Scholar]
- (461).Wang J; Wang Z; Zhong Y; Zou Y; Wang C; Wu H; Lee A; Yang W; Wang X; Liu Y; et al. Central Metal-Derived Co-Assembly of Biomimetic Gdtpp/Zntpp Porphyrin Nanocomposites for Enhanced Dual-Modal Imaging-Guided Photodynamic Therapy. Biomaterials 2020, 229, 119576. [DOI] [PubMed] [Google Scholar]
- (462).Wang X; Wang J; Wang J; Zhong Y; Han L; Yan J; Duan P; Shi B; Bai F Noncovalent Self-Assembled Smart Gold(III) Porphyrin Nanodrug for Synergistic Chemo-Photothermal Therapy. Nano Lett. 2021, 21, 3418–3425. [DOI] [PubMed] [Google Scholar]
- (463).Gukathasan S; Awuah SG Synthetic Strategies for the Preparation of Gold-Based Anticancer Agents. Encyclopedia of Inorganic and Bioinorganic Chemistry 2022, 1–32. [Google Scholar]


