Abstract
Invasive candidiasis (IC) is a serious infection caused by several Candida species, and the most common fungal disease in hospitals in high-income countries. Despite overall improvements in health systems and ICU care in the last few decades, as well as the development of different antifungals and microbiological techniques, mortality rates in IC have not substantially improved. The aim of this review is to summarize the main issues underlying the management of adults affected by IC, focusing on specific forms of the infection: IC developed by ICU patients, IC observed in haematological patients, breakthrough candidaemia, sanctuary site candidiasis, intra-abdominal infections and other challenging infections.
Several key challenges need to be tackled to improve the clinical management and outcomes of IC patients. These include the lack of global epidemiological data for IC, the limitations of the diagnostic tests and risk scoring tools currently available, the absence of standardized effectiveness outcomes and long-term data for IC, the timing for the initiation of antifungal therapy and the limited recommendations on the optimal step-down therapy from echinocandins to azoles or the total duration of therapy.
The availability of new compounds may overcome some of the challenges identified and increase the existing options for management of chronic Candida infections and ambulant patient treatments. However, early identification of patients that require antifungal therapy and treatment of sanctuary site infections remain a challenge and will require further innovations.
Introduction
Invasive candidiasis (IC) is a serious infection caused by several Candida species (spp.) and the most common fungal disease in hospitals in high-income countries, with a worldwide prevalence ranging from 250 000 to approximately 700 000 people per year, an incidence rate of 2–14 cases per 100 000 persons and mortality rates ranging between 40% and 55%.1–3 IC includes both candidaemia (i.e. bloodstream infections) and deep-seated tissue candidiasis, which arises from dissemination of Candida spp. to a sterile body site (e.g. abdomen, peritoneum or bone).4–6 Most IC infections are caused by five pathogens: C. albicans, Nakaseomyces glabrata (previously known as C. glabrata), C. tropicalis, C. parapsilosis and Pichia kudriavzevii (previously known as C. krusei). C. albicans is the most common species, but non-albicans species are increasing, being responsible for more than 50% of cases in some series.7 However, this trend appears limited to specific continents (e.g. Europe, mainly due to the rise of N. glabrata) and a high heterogeneity is observed between studies, overall but also in subgroups by continents.8C. auris is a novel pathogen that has emerged in 2009, triggering global outbreaks.9
Candida spp. are commensal organisms present in the gut and skin of 50%–70% of healthy individuals in low numbers due to competition within the microbiome (i.e. the gut mycobiome represents only around 0.1% of the total gut microbes10). Several factors can lead to overgrowth of Candida spp., in particular exposure to antibiotic treatment, immunosuppression and corticosteroid treatment.11 Moreover, Candida translocation from the gut into the bloodstream can be facilitated by increased permeability of the gut epithelia (for example, due to mucositis in onco-haematological patients or patients with inflammatory bowel disease),12–15 or breaches in the intestinal barrier following abdominal surgery,16 all of which significantly increase the risk of candidaemia. Additionally, the ability of Candida spp. to form biofilms on inert surfaces makes the presence of prosthetic material a risk factor for developing IC.17 Once candidaemia has developed, it can disseminate and generate deep-seated secondary infections in organs such as the lungs, liver, heart, eye, brain and bones.1
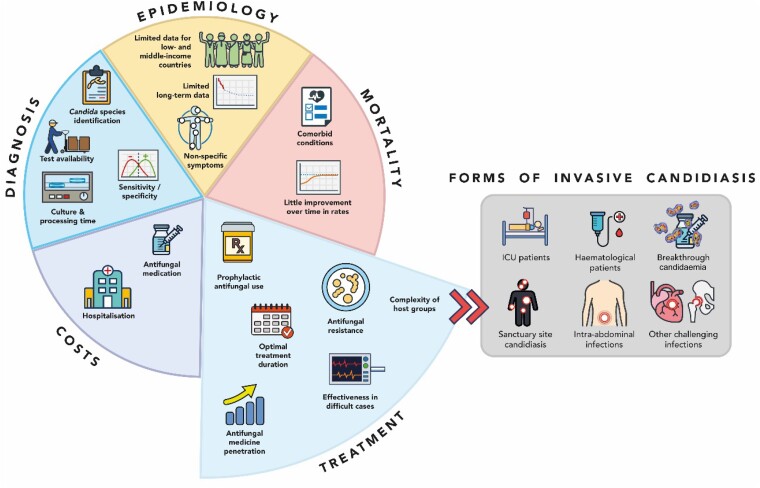
Despite overall improvements in health systems and ICU care in the last few decades, as well as the development of different antifungals and microbiological techniques, mortality rates in IC have not substantially improved.18 Several challenges hinder the clinical management of patients with IC (Figure 1). Firstly, early diagnosis of candidaemia and deep-seated candidiasis remain a challenge due to the prolonged time to positivity of blood cultures, which can take up to 5 days to become positive, and due to the low yield of culture diagnostic tests for deep-seated candidiasis (∼50%).19 Biomarkers [e.g. (1–3)-β-D-glucan (BDG), mannan (Mn)/anti-Mn antibodies] could aid earlier diagnosis; however, their role has not yet been clearly defined.19 Second, a worldwide shift to multidrug-resistant species (including C. auris, N. glabrata and P. kudriavzevii) has been observed.20 While guidelines exist to guide the choice of antifungal therapy, patients affected by IC may require a tailored approach due to heterogenous host factors and significant geographical variation in species distribution and antifungal drugs resistance rates.21–23 Moreover, the value of different treatment strategies remains to be clarified.
Figure 1.
Current clinical challenges of IC.
In addition to increasing the risk of mortality, IC is associated with significant economic burden, mainly arising from the prolonged length of hospital stay, although the economic impact of IC is difficult to measure due to comorbidities.24,25
The aim of this review is to summarize the main issues underlying the management of adults affected by IC (Figure 1), focusing on specific forms of the infection: IC developed by ICU patients, IC observed in haematological patients, breakthrough candidaemia, sanctuary site candidiasis, intra-abdominal infections and other challenging infections. The impact of such issues on the clinical and economic burden of the disease is key to understanding the unmet needs of these patients. To maximize its reliability and quality, this narrative review followed the methodological recommendations from the Scale for the Assessment of Narrative Review Articles,26 and eligible literature was identified through citation chasing of key references on IC and candidaemia and input from clinical experts (Supplementary Materials, available as Supplementary data at JAC Online).27
Global incidence, epidemiological shifts and economic burden of Candida species
Global data indicates that the incidence of IC and candidaemia is increasing, with large studies reporting an incidence rate of 3–5 per 100 000 persons in the general population, 1%–2% of all ICU admissions28 and a global annual incidence estimated to be ∼750 000 cases/year.3 Within clinical settings, almost three-quarters of the cases were reported in the ICU (60%) and cancer and transplant units (13%).3,29,30Candida spp. are the aetiology of 17% of all ICU infections in culture-positive patients.3,6,31,32 Pooled data for European countries reiterated the high ICU incidence rate, extrapolating that approximately 79 cases are diagnosed daily,32 with a cumulative incidence of IC of 7.07 episodes per 1000 ICU admissions and a crude mortality rate of 42%.33 The current incidence rate may be higher due to emerging risk groups, such as patients with severe COVID-19.32,34
Intra-abdominal candidiasis may include Candida involvement of peritoneum or intra-abdominal abscess,35,36 and is relatively common among specific high-risk groups with prevalence ranging between 5% and 30%.19 A review on the global burden of IC reported data from 29 countries worldwide, estimating a global averaged incidence rate of 1.15 cases per 100 000 for Candida peritonitis or intra-abdominal candidiasis, being associated with approximately half of the total cases of IC in ICU patients.3
Patients with haematological malignancies are also prone to developing IC, due to their compromised immune system and chemotherapy-induced mucositis, which results in the translocation of Candida into the bloodstream.6,15,37 A US prospective surveillance study of invasive fungal infections in haematopoietic stem cell transplant recipients conducted in 2001–2006 found Candida was responsible for 28% of invasive infections (mostly N. glabrata).14 A study involving 11802 Italian patients with haematological malignancies identified 175 cases of candidaemia (1.5%),13 whereas a Greek study on 27 864 candidaemia patients reported an incidence rate of 1.4 cases/1000 admissions among haematology patients (versus 0.83/1000 in non-haematology patients); candidaemia was caused predominantly by non-albicans species.12
Candida spp. distribution also differs geographically; C. albicans is the most prevalent species in most regions of the world, but in the past decade an increase in non-albicans diagnosis has been observed.3,32,38 The second most prevalent species in the USA, north-western Europe and Canada is N. glabrata, particularly among elderly patients and solid organ transplant recipients. C. parapsilosis and C. tropicalis are more common in Southern Europe, South America, India and Pakistan, while P. kudriavzevii, the least common among the five main species, is more frequent in patients with severe immunodeficiency (e.g. haematological malignancies).39,40 Other less frequent species usually present in specific hosts rather than geographically (e.g. C. dubliniensis is more common in HIV-infected patients41). It is important to note that current IC epidemiology is highly determined by antifungal selection pressure, which is influenced by both prophylaxis and treatment.42 Widespread use of antifungals has driven the shift to non-albicans and more frequently resistant Candida spp. Additionally, C. auris has emerged as a global threat causing outbreaks in all continents. C. auris,known to survive on human skin and environmental surfaces for several weeks thereby facilitating its transmission,9 is highly resistant to azole and polyene antifungals and can be resistant to some commonly used disinfectants.43,44
The collection and comparison of global data are hindered by specific challenges, including the lack of specific criteria for an incidence rate denominator.20 Moreover, there are no available data for some low- and middle-income countries due to the absence of hospital infrastructures for blood culture analyses.3 These differences between studies constrain to what extent global incidence rates can be established and comparisons across countries can be made, and point towards an underestimation of the burden of disease. Large longitudinal studies, alongside regional and local surveillance studies,45 are required to understand epidemiological trends and shifts and to collect data to guide and support antifungal therapy.46
Systematic analysis of global evidence reported that costs associated with IC are mainly driven by bed day costs, incremental hospitalization and antifungal drug expenditure.24,25 Survival and age influences costs, with both neonatal and older patients incurring higher costs, due to higher morbidity.47
Mortality rates over time and factors affecting mortality
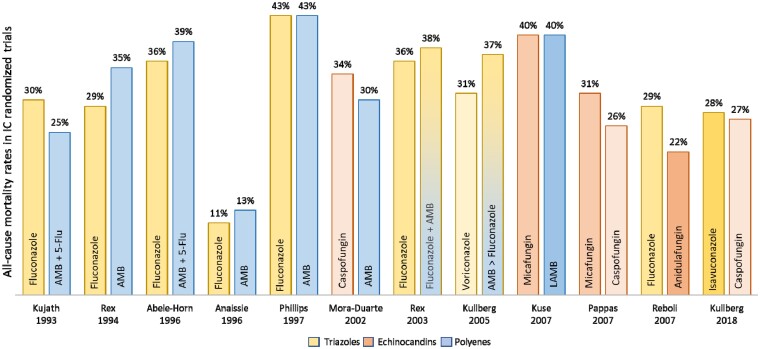
Nosocomial candidiasis has the highest rate of mortality for hospital-acquired infections, with 30-day post-diagnosis mortality estimated to range between 40% and 55%.1,2 Risk factors affecting mortality rates include older age, severity of the condition, use of immunosuppressive drugs, comorbidities, venous catheter retention and specific antifungal treatment.1,33 A large retrospective study conducted in nine European countries extrapolated the 30-day mortality rate to be 29 patients out of the 79 cases diagnosed per day.32 There have been limited changes to the mortality rate associated with IC in the past two decades (Figure 2), even though there are now several extended-spectrum triazole and echinocandin agents available for antifungal therapy, which have superior safety and potency than those antifungals agents available two decades ago.18,45,48
Figure 2.
All-cause mortality rates in IC randomized trials (based on data reported by Demir).18 The figure does not account for differences in study design, namely number of patients randomized, and only includes antifungals currently reimbursed. AMB, amphotericin B; 5-Flu, 5-fluorocytosine; LAMB, lipid formulation of amphotericin B.
Diagnostics
Culture and nonculture diagnostics
Diagnostic tests for IC should be able to accurately detect the infection and differentiate between the presence of candidaemia, deep-seated candidiasis or a combination of both.4,5,19 Identifying deep-seated candidiasis is important as patients may require longer therapy or surgical debridement.5 An early diagnosis is paramount for a timely treatment, and any delays increase the odds of mortality and healthcare associated costs.46,49
Cultures from blood and sterile sites are currently the gold standard for diagnosis of IC.50 Light microscopy using fluorescent brightener stains is also often used for the detection of Candida spp.,6 and may provide additional diagnostic benefit.51 It has been estimated that blood cultures have a sensitivity of between 63% and 83% for candidaemia in the absence of deep-seated candidiasis, and that their sensitivity is lower when deep-seated candidiasis is present,46 ranging between 21% and 71%,52 with an overall sensitivity of approximately 50%. Sensitivity can also vary according to the Candida spp., and the use of prior antifungals.22 In addition, slow turnaround times, with a median time to positivity of 2–3 days, which may be even longer for N. glabrata,53 may delay the start of the adequate antifungal therapy.22,46,49 Diagnosing IC can be further constrained by the absence of a specific clinical presentation.54
These shortcomings may be complemented by nonculture diagnostic tests for Candida, such as mannan and anti-mannan antibody detection,19,21,52 BDG detection,19C. albicans germ tube antibody (CAGTA) detection,55 PCR detection of Candida DNA56 and the T2 magnetic resonance (T2MR) Candida test.4,49 The nonculture diagnostic tests have a varying degree of sensitivity and there have been recent calls for studies to further assess the role of combination testing.19 For example, the mannan and anti-mannan assays combined were shown to achieve both sensitivity and specificity ≥80% for patients with C. albicans, N. glabrata or C. tropicalis infections.19 It has also been suggested that the combined use of biomarkers could be used as a complementary decision-support tool for the diagnosis and management of IC.19,52 For example, a combination of (1,3)-β-D-glucan, mannan and anti-mannan serum assays significantly shortened the duration of antifungal treatment in ICU patients with suspected IC, with no negative impact on outcome.57 However, nonculture diagnostic tests also have limitations (Table 1) and ideally should be used as a complement to culture tests, while also taking into account patient characteristics, including the specific host group and severity of the clinical scenario to culture tests.6,50,54,60
Table 1.
Overview of key diagnostics
| Test | Advantages | Limitations | |
|---|---|---|---|
| Microscopy | Fast turnaround46 High sensitivity when using fluorescent brightener staining6,51 |
Inability to identify species6 | |
| Blood culture | Species identification19 Susceptibility pattern |
Slow turnaround21,53 Timing of blood collection, during the course of infection19 Necessary to culture a large blood volume (40 mL) in aerobic flasks53 When Candida density is low (<1 cfu/mL), blood cultures can result in false negatives Cultures may become negative after initiating antifungal therapy5 |
|
| Sterile site cultures | Species identification Susceptibility pattern |
May require invasive procedures19 Cultures may become negative after initiating antifungal therapy5 Long incubation required for optimal performance (3 days)6 For intra-abdominal candidiasis, lack of specificity to differentiate infection from colonisation35 |
|
| Mannan antigen/anti-mannan antibodies | Early detection22,54 Useful to rule out infection22 |
Serial determinations required22 Lower utility in immunosuppred hosts52 May not distinguish between past and acute infections19 Sensitivity varies regarding Candida species (better for C. albicans, N. glabrata and C. tropicalis)54 Decreased specificity if Candida colonisation is present54 Low positive predictive value, potentially leading to antifungals overuse Limited by low serum concentrations and rapid bloodstream clearance19 Not species-specific, requiring further tests to identify the fungus46 No data on susceptibility pattern Not approved by FDA19 Not universally available |
|
| CAGTA | Fast turnaround and low cost5 Could be used to detect whether candidaemia originated in a catheter or deep organs55 |
May not distinguish between past and acute infections19 Limited by low serum concentrations and rapid bloodstream clearance19 Sensitivity varies according to Candida species (lower for C. tropicalis)5,19 Not species-specific, requiring further tests to identify the fungus46 Low positive predictive value, potentially leading to antifungals overuse No data on susceptibility pattern Not approved by FDA19 Not universally available |
|
| BDG | Early detection22 Useful to rule out infection22 |
Serial determinations required22 Lower utility in patients with haematological disease22 and immunosuppred hosts52 Sensitivity varies according to Candida species (lower for C. parapsilosis)19 May not distinguish between past and acute infections19 Not species-specific, requiring further tests to identify the fungus45 Low positive predictive value, potentially leading to antifungal overuse No data on susceptibility pattern Not universally available |
|
| Nucleic acid amplification-based methods | PCR | Early detection58 Monitoring of persistence or resolution of infection4 |
Mostly developed in-house or commercially19 Frequently performed in reference laboratories limiting the advantage of short turnaround time45 Data interpretation impaired by test heterogeneity19 Not universally available |
| T2Candida | Early detection19,59 Automated molecular diagnosis49,59 May detect candidaemia missed by cultures during empirical or pre-emptive AF therapy4 Improved performance in neutropenic patients4 |
Costs associated with the test46 Limited to some Candida species (C. albicans/C. tropicalis, N. glabrata/P. kudriavzevii, and C. parapsilosis, groupings that are based on typical antifungal susceptibility pattern)49,59 No data on susceptibility pattern59 Not universally available |
|
AF, antifungal; BDG, β-D-glucan; CAGTA, C. albicans germ tube antibody; cfu, colony forming units; FDA, Food and Drug Administration; PCR, polymerase chain reaction.
Role of candidaemia risk scores
Due to the underlying limitations of diagnostic tests, prediction rules or scoring systems have been proposed as early diagnostic tools to assess the risk of IC early in patients admitted to ICU, such as the Candida Colonization Index (CCI)61 and the Candida score.62 There are concerns that scoring systems for IC have a high negative predictive value but a low positive predictive value, which means that they may be more useful to rule out patients who do not have or will not develop IC.20,45,46,62,63 This is partially explained by the poor specificity of risk factors for developing IC, aligned with the low prevalence of IC in most clinical settings,19 which would make many patients eligible for antifungal therapy even when their real risk is low,64 thus increasing its use and consequently the risk of selecting resistant strains.65 The use of the Candida score is largely debated, and not validated for all populations. While this risk score was showed to have sensitivity and specificity for invasive candidiasis of 81% and 74%, respectively, the population tested mostly included surgical ICU patients, with only 35% of admissions for medical reasons.62 Thus, this tool may be less reliable for patients with nonsurgical reasons for ICU admission.
Other generic scoring systems that can be used to assess the risk of IC include the acute physiology and chronic health evaluation (APACHE II) score, which classifies disease severity and predict mortality in ICU patients,46,66–68 and the simplified acute physiology score (SAPS II), also a prognostic model for hospital mortality at ICU admission.69–71 However, the utility of these tests for IC is not clearly defined.
It has also been suggested that biomarkers of fungal infection, such as BDG, could be superior to candidaemia risk scores to support the decision to initiate treatment earlier.46,64,72 In one study including 95 patients with sepsis and >5 days in the ICU, a single negative BDG test at sepsis onset had a negative predictive value for candidaemia of 98.7%, and both negative and positive predictive values of such biomarkers were superior to the Candida score.72 These encouraging study results require further confirmation in high-quality studies.63 Advances in genetic polymorphisms identification in the host have shown promising results in the identification of patients with a genetic predisposition to develop IC, which would put them at higher risk and more likely to benefit from antifungal treatment.73 Using data from a prospective observational cohort study of 89 high-risk surgical ICU patients, the authors showed how one single-nucleotide polymorphism increased the susceptibility to intra-abdominal candidiasis infection.69 Albeit promising, these results need to be confirmed in larger studies.74 Finally, the use of risk scores (e.g. Candida score) could be combined with diagnostic tests with short turnaround, such as BDG detection, for a more accurate and time-efficient prediction of IC.75
Critical factors for the management of IC
Treatment
The treatment of IC has evolved in the last three decades. Generally, treatment guidelines21,76 are more relevant for patients with candidaemia, as available IC evidence mostly came from trials enrolling patients with candidaemia, with fewer trials investigating deep-seated candidiasis.21,77–82 The rarer forms of IC have seldom been studied in prospective studies; hence, treatment regimens for these forms are based on anecdotal experience and retrospective case series.
The antifungal drugs available for the treatment of IC belong to three classes: echinocandins (anidulafungin, caspofungin or micafungin), azoles (fluconazole, voriconazole, itraconazole, posaconazole, isavuconazole) and amphotericin B-based regimens.23,76 The comparative effectiveness of these agents for the treatment of IC was recently reported by a network meta-analysis, which included data from 13 trials that randomized 3528 patients to one of the three antifungal classes.18 Results showed that echinocandins were associated with best clinical outcomes (i.e. response to antifungal therapy) when compared with the other two groups of agents. Moreover, a combined analysis of clinical studies involving almost 2000 patients showed that initial therapy with an echinocandin is a significant predictor of survival.83 Overall, these agents have shown efficacy in 70%–75% of patients in randomized clinical trials.79–81,83–85 Accordingly, guidelines recommend echinocandins as a first-line treatment in most IC patients, without preference for a specific compound, mostly due to their broader spectrum of activity, higher fungicidal activity for most Candida species, low drug–drug interaction, rare acquired resistance and increased safety profile.18,76
Azoles are generally well tolerated, but they have been shown to be about 15% less effective than echinocandins on average.86 They are used instead of echinocandins as first-line therapy in some forms of deep-seated candidiasis, such as brain, intraocular and urinary tract infections, where echinocandins have lower penetration. In terms of formulations, echinocandins are approved for once-daily intravenous administration, and azoles can be administered intravenously or orally. Amphotericin B deoxycholate formulations have been associated with severe adverse events, such as nephrotoxicity and infusion-related adverse effects; hence, lipid formulations have been developed that present fewer, but still frequent, toxicities.78,87 These formulations are commonly used to treat patients who are intolerant or resistant to echinocandins and/or azoles, as well as in some deep-seated infections such as endocarditis, meningoencephalitis and endophthalmitis.76
Although the use of echinocandins as first-line therapy has increased and the major role for fluconazole in the current management of IC is for step-down therapy,7,88 fluconazole is sometimes still used as first-line therapy, as shown by a retrospective chart review of diagnostic and treatment decisions in patients with candidaemia conducted in six German hospitals.89 This contrasts with ESCMID guidelines, but is in line with the Infectious Diseases Society of America (IDSA) guidelines accepting fluconazole as alternative for those not critically ill and without prior azole exposure.21 Further deviations from international guideline recommendations include the indication, dosage, route of administration and duration, with approximately half of the prescriptions being assessed as inappropriate.90,91
Improving patient outcomes
Early initiation of antifungal therapy has been shown to reduce hospital mortality but requires starting antifungal therapy within 24 hours of taking blood cultures.92 As Candida spp. generally take longer than 24 hours to grow, the benefit from this time window is lost when relying on culture results. Most antifungal treatments are thus started empirically, when patients who are at high risk of developing IC are persistently febrile in the absence of microbiological evidence of infection.76 Recent international guidelines for the management of sepsis and septic shock suggest that empirical antifungal therapy should be preferred for adults at high risk of fungal infection.93 Empirical therapy has been associated with reduced overall mortality, although most evidence comes from uncontrolled studies.76 Importantly, a Cochrane review reported that, on the basis of 19 studies that included 2374 non-neutropenic critically ill patients, empirical antifungal treatment reduced the risk of invasive fungal infection but did not reduce all-cause mortality.94 Moreover, the broad use of empirical antifungals increases healthcare costs and is potentially linked with antifungal resistance.1,91,95,96
Prompt source control (i.e. the elimination of the focus of infection) is also key in the management of IC. This may consist of the removal of contaminated intravascular catheters, infected prosthetic devices (for example, cardiac pacemaker leads), prosthetic joints or other devices, as well as the adequate drainage of infected material (such as peritoneal fluid, pleural fluid and/or abscess material) and surgical correction of the underlying pathology (e.g. perforation or leak).97,98 Source control is important due to the ability of Candida spp. to form biofilms on implanted medical devices representing a persistent nidus of infection.99 In addition, biofilm formation is linked to the development of antifungal resistance due to decreased drug penetration and upregulation of resistance mechanisms.100,101 A recent systematic review analyzed data from 34 prospective and retrospective cohort and case-control studies, finding that a central venous catheter was associated with a significantly increased risk of developing IC (odds ratio 4.7, 95% confidence interval 2.7 to 8.1).64 Retrospective studies of adult patients diagnosed with candidaemia show how intravascular catheters were a risk factor for infection5 and how catheter removal was associated with increased odds of survival.89 Removal of indwelling intravascular catheters is therefore strongly recommended when candidaemia is present.51,76 If removal is not possible then treatment with a lipid-base amphotericin B formulation or an echinocandin is suggested,76 as these drugs have shown activity against Candida biofilms.102 Catheter lock strategies, using high antifungal concentrations locally in the catheter lumen for hours or days, have also been performed. Lipid-based amphotericin B formulations and echinocandins are again the most commonly used antifungals, showing high efficacy to decrease biofilm formation but commonly failing to eradicate the Candida infection.103 Novel strategies are being investigated including antifungal combination therapy, phototherapy, cationic peptides and even the use of plant-based therapies.104,105
Diagnosis-driven drug management
The duration of antifungal treatment is often guided by the extent of organ involvement.76 For candidaemia, the ESCMID recommends for treatment to be continued for 14 days after the last negative blood culture, whereas organ involvement may be screened with transoesophageal echocardiography and fundoscopy.76 Rapid diagnostic tests can support early discontinuation of empirical antifungal therapy, with retrospective data showing that results from T2Candida panel performed better than BDG, when combined with blood cultures, decreasing the number of days critically ill patients were on empiric echinocandin therapy.106 The decision to de-escalate treatment from intravenous echinocandins to oral fluconazole should take into account not only diagnostics but also patient’s stability, tolerance of the administration route and species susceptibility.4,76,79,89,93,107
The role of nonculture tests to guide treatment, including drug de-escalation, has also been explored. Evidence from a randomized trial of 234 critically ill non-immunocompromised patients admitted to ICU who were allocated to an echinocandin or placebo suggested that BDG monitoring could be used to decide when to de-escalate empirical therapy or when to withhold pre-emptive therapy,60 whereas T2Candida has also been shown to improve management.4,108
Therapeutic drug monitoring
Therapeutic drug monitoring (TDM) is used to measure antifungal drug levels to prevent over or underdosing. The IDSA and ESCMID recommend TDM when treating IC with voriconazole; ESCMID also advise TDM when prescribing 5-fluorocytosine and posaconazole.21,22
Whenever antifungal absorption or excretion could be hindered [i.e. mucositis, administration via nasogastric tube or gastrostomy, or in critically ill patients undergoing continuous renal replacement therapy (CRRT)], TDM should also be considered.109,110 Finally, TDM may also be helpful in those cases of treatment failure, breakthrough infections, serious toxicity or those Candida infections caused by species with high minimum inhibitory concentration (MIC).111
Drug resistance
Candida infections resistant to one or multiple types of antifungals are increasingly being diagnosed, with prior antifungal therapy found to be the main driver for resistance selection.20,112,113 The widespread use of antifungals introduces a positive selection of Candida spp. that show some intrinsic or acquired resistance to treatment, including N. glabrata and C. auris (multidrug-resistant),114,115 as well as P. kudriavzevii (azole resistant). Data gathered from population-based or multicentre studies showed that the rates of azole resistance vary considerably depending on the setting and the Candida spp., ranging from 3% to 21%.20
Acquired echinocandin resistance has also been reported, especially for N. glabrata and C. tropicalis. In the SENTRY antifungal surveillance programme, mutations in FKS gene hot spot regions were detected among echinocandin-resistant isolates, most of which were resistant to two or more echinocandins. Additionally, C. parapsilosis has an intrinsic polymorphism affecting also the FKS1 gene, leading to decreased in vitro echinocandin susceptibility.116 However, the clinical impact of such reduced susceptibility remains controversial. C. auris was initially detected in Japan in 2009 and shows some resistance to all major antifungal treatments, including multi-drug-resistant isolates, with higher resistance for fluconazole, followed by amphotericin B and echinocandins.9,117 Hospital outbreaks of C. auris with rapid spread and high mortality have been since reported in Asia (India, Pakistan), Europe (the UK, Spain, Italy), Latin America (Colombia, Venezuela, Panama) and the USA.117–119
The rise in drug-resistant IC has highlighted a need for antifungal susceptibility testing, to achieve optimal treatment and to monitor the emergence of antifungal resistance. Several tests are currently available, including broth microdilution according to the CLSI120 and the EUCAST,121 which represent gold standards for antifungal susceptibility testing. Alternative tests include disc diffusion, epsilometer tests, colorimetric broth microdilution and automated spectrophotometric systems.122 Generally, these methods can be time-consuming and/or technically complex, and the interpretation of results may be challenging.
Clinical challenges related to specific forms of IC
IC in ICU patients
Approximately 50% of episodes of IC occur in ICU, where the administration of antibiotics and immunosuppressive drugs, combined with the use of invasive procedures (e.g. the installation of central vascular catheters), total parenteral nutrition16 or intrabdominal surgery,36,38 significantly increase the risk of Candida infection.2,3,32 Length of ICU stay is consistently reported as increasing the risk of developing IC, although it is seldom possible to disentangle length of ICU stay from other confounding risk factors, as patients with longer ICU stays will have increased disease severity as well as more invasive therapies.50,64 All-cause mortality associated with candidaemia seems to be 2-fold higher for patients hospitalized in the ICU when compared with patients in other hospital settings, although these results vary for different Candida spp.123
In critically ill patients, severe comorbidities can alter the antifungal drugs’ pharmacological profile. Drug distribution can be influenced by haemodynamic alterations, while hepatic and/or renal impairment can affect drug concentration in the bloodstream, metabolism and elimination. Additionally, hypoalbuminaemia can reduce the percentage of bound antifungal, increasing activity and potentially toxicity.124
Dosing and PK/PD are also crucial during CRRT and extracorporeal membrane oxygenation (ECMO), which affect different classes of antifungals. Fluconazole is the most complex to dose in ICU during CRRT (Table 2) due to the high extracorporeal removal of fluconazole, which exceeds the normal renal clearance and necessitates a higher daily maintenance dose.125 In line with available data and as long as hepatic function remains stable, a dose of fluconazole of 500–600 mg every 12 h is recommended in critically ill patients under CRRT.126
Table 2.
Antifungal dose adaptations during CRRT
| Antifungal agent | Mechanism of action | Route of administration | Adverse effect | Elimination by CRRT | Recommended dose during CRRT |
|---|---|---|---|---|---|
| Lipid formulation of amphotericin B | Interacts with ergosterol in the fungal cell membrane | IV | Hepatic, renal and cardiovascular toxicity | Unaffected by CRRT | 5 mg/kg/d |
| Fluconazole | Interacts with 14-demethylase in the fungal cell membrane | IV or oral | Hepatic toxicity | High elimination by CRRT | 600 mg/12 h |
| Voriconazole | Reduces ergosterol synthesis | IV or oral | AKI toxicity with IV use, hepatic toxicity | Poor elimination of IV form by CRRT— No adaptations for CRRT | Loading dose: 6 mg/kg/12 h Maintenance dose: 4 mg/kg/12 h |
| Anidulafungin | Inhibits (1,3)-β-D-glucan synthetase | IV | Hepatic toxicity | Significant adsorption by CRRT adsorptive membranes | Loading dose: 200 mg/d Maintenance dose: 150 mg/d |
| Caspofungin | Interacts with 14-lanosterol demethylase in the fungal cell membrane and reduces ergosterol synthesis | IV | Severe hepatic toxicity | Unaffected by CRRT | Loading dose: 70 mg/d When BMI is >40 higher doses can be used (up to 140 mg/d) Maintenance dose: 50 mg/d (if >80 kg, maintenance with 70 mg/d is recommended) |
AKI, acute kidney injury; CRRT, continuous renal replacement therapy; d, day; IV, intravenous.
The COVID-19 pandemic led to high ICU admissions and subsequently triggered an increase in the number of secondary invasive fungal diseases, including Candida and Aspergillus,34 partially due to the high doses of corticosteroids given.127 Aggregated data showed that by September 2020 the extent of candidiasis associated with COVID-19, both superficial and invasive, ranged between 0.7% and 23.5%.128
Intra-abdominal infections
Intra-abdominal candidiasis is the most common type of deep-seated candidiasis.98 A prospective study with 176 non-neutropenic critically ill patients with severe abdominal conditions and a high prevalence of IC (18%) showed how BDG (cut-off value of 259 pg/mL) and CAGTA (positive versus negative) accurately discriminated between Candida spp. colonization and IC.129 However, currently there are no tight criteria to distinguish abdominal Candida colonization from true Candida infection, and general criteria for sepsis and septic shock are often used to differentiate between abdominal colonization and infection.130 This is an issue as most of the time sepsis and septic shock are due to causes other than true Candida infection, and thus many abdominal colonizations, especially in the post-operative period, are overtreated leading to more antifungal resistance. There is an urgent need to build an algorithm to differentiate abdominal Candida colonization from true infection.
The burden of intra-abdominal infections and its associated morbidity and mortality are higher in high-income countries, which may be partially explained by the widespread use of antibiotics and increased drug resistance.131
Clinical guidelines/expert consensus papers recommend prophylactic use of fluconazole for patients with abdominal surgery and recurrent gastrointestinal perforations or anastomotic leakages,35,76 with echinocandins or lipid formulation of amphotericin B being recommended as first-line antifungal therapy for critically ill patients or patients with previous exposure to azoles.35 However, prophylactic use of fluconazole may be associated with an increase in fluconazole-resistant species.132
The outstanding clinical challenges in intra-abdominal candidiasis thus include prophylactic use of fluconazole, need for source control and treatment selection and duration.
IC in patients with haematological malignancies
Owing to their compromised immune response, patients affected by haematological malignancies are prone to develop IC with high mortality risk, prolonged hospitalization and rising healthcare costs.15,37 This is a consequence of host defences being affected by cancer treatments, such as cytotoxic chemotherapy, ablative radiotherapy and immunosuppressive therapies. Other risk factors mentioned previously also apply to haematological cancer patients.12,37,133 Several studies have reported candidaemia-associated mortality rates in patients with haematological malignancies ranging between 29.5% and 45%.12,13,15,133
To counteract the high incidence of candidaemia and its associated mortality, the use of fluconazole prophylaxis was introduced in the early 1990s.42 Clinical guidelines recommend anti-Candida prophylaxis for patients receiving allogeneic stem cell transplantation.23 Prophylactic treatment with fluconazole has also been recommended for patients undergoing remission-induction134 or salvage-induction135 chemotherapy for acute myeloid leukaemia.
However, while prophylactic use of fluconazole succeeded in lowering the frequency of bloodstream infections caused by azole-sensitive C. albicans, it caused an increase in azole-resistant species such as N. glabrata and P. kudriavzevii, which now account for most candidaemia episodes in many cancer centres.136 Additional concerns include drug–drug interactions, tolerability and breakthrough fungal infections,134 as well as the non-specificity of signs and symptoms of IC in patients who frequently present with fever and sepsis. In a pooled analysis of 1271 patients with haematologic malignancies and patients undergoing haematopoietic stem cell transplant, echinocandins seemed to be marginally more effective than triazoles for prophylactic treatment.48
Breakthrough candidaemia
Breakthrough candidaemia is defined as candidaemia that develops during systemic antifungal therapy administered as either prophylaxis, pre-emptive, empirical or targeted therapy.21,135 It has been linked to the emergence of drug resistance and poor outcomes,137–140 and several risk factors have been identified. A retrospective study conducted in Brazil from 2011 to 2016 identified 27 breakthrough episodes from 148 candidaemia episodes, with neutropenia and mucositis being independent risk factors and non-albicans species being more frequent among these patients.141 Similarly, in a multicentre study of hospitalized adults with candidaemia, P. kudriavzevii was more frequent and fluconazole-resistance was independently associated with risk of breakthrough episodes.142 Another study identified neutropenia, use of corticosteroids and heavy antibiotic exposure (previous use of two or more antibiotics for at least 14 days) as risk factors.143 Additionally, a 3-year prospective study conducted in 567 consecutive cases of candidaemia recorded 37 cases of breakthrough candidaemia, 86% of which on fluconazole; breakthrough candidaemia was associated with gastrointestinal mucositis, graft-versus-host-disease, immunosuppression and parenteral nutrition, and non-albicans Candida were isolated in most breakthrough cases.144 Overall, breakthrough candidiasis appears to be caused by drug-resistant, non-albicans species selected by the use of antifungals and impairments in the immune response.
Sanctuary site candidiasis
CNS candidiasis
Candida CNS infections, mostly caused by C. albicans,145 are rare but severe. They can arise from haematogenous spread, mostly in neonates due to blood–brain barrier immaturity, or in the presence of ventricular drainage devices or following neurosurgical procedures.146 Increased risk in adults has been described for the immunocompromised patients145,147–149 and individuals who have a deficiency of the lectin receptor adaptor molecule CARD9.150 Moreover, Candida-caused endocarditis are associated with CNS embolic complications in 12% to 22% of cases.148,151,152
The most common manifestation of brain infection is overt meningitis, while in rarer cases chronic meningitis, brain abscesses, vasculitis with cerebral infarctions, spinal infections, ventriculitis and mycotic aneurysms can be observed.153 A recent nationwide retrospective study conducted in France and covering the period between 2005 and 2018 identified 24 adult patients with CNS candidiasis. Mortality attributed to CNS candidiasis was 42%.154
Treatment guidelines suggest the use of liposomal amphotericin B combined with flucytosine for CNS IC. Fluconazole may be used as a step-down therapy, while poor penetration of echinocandins limit their use in CNS. However, due to data scarcity, no strong recommendation is given.76 A study reported the use of amphotericin B deoxycholate combined with flucytosine for >2 weeks in a series of HIV-infected patients, with four of five patients being treated successfully.147 In two other series, 27 of 34 patients survived after similar treatments.155,156 Published data on voriconazole use in CNS candidiasis are sparse; efficacy may be limited by the variability of its concentration in the CSF.157
Urinary candidiasis
Critically ill patients and those with urinary catheters are at high risk of developing candiduria.73 In critically ill patients undergoing surgical procedures, the rates of concurrent candidaemia derived from a urinary source may reach up to 10%.158 Additional risk factors for adult candiduria include advanced age, female gender, urinary tract anatomic abnormalities, abdominal surgery, multi-morbidity, broad-spectrum antibiotics therapies and diabetes mellitus.158
If asymptomatic, then treatment is not recommended for candiduria, except in pre-operative patients who may be given flucozonale.76 If possible, the urinary catheter should be removed to clear the infection,76 and early urologic drainage procedure is also associated with improved outcomes.159 The prophylactic antifungal treatment of Candida colonization in the urinary tract is a common inappropriate use of antifungal agents.45 Different combinations of antifungal agents are available for symptomatic candiduria, including fluconazole or amphotericin B deoxycholate, with or without flucytosine; if fungus balls or casts are detected then surgical intervention is required.21,76 For the treatment of fluconazole-resistant strains, only amphotericin B and micafungin seem to reach adequate urinary concentrations, although the clinical evidence is limited.160,161
Endophthalmitis and chorioretinitis
In rare cases, candidaemia can give rise to two types of ocular infection: Candida chorioretinitis, restricted to the chorioretinal layers, and Candida endophthalmitis, usually extending into the vitreous body. The latter is associated with poor visual outcomes.162 Candidaemia-associated endophthalmitis and chorioretinitis can be treated with antifungals administered systemically or locally (i.e. via intravitreal injection). Systemic amphotericin B and echinocandins do not penetrate well in the vitreous humour, whereas fluconazole and voriconazole can reach therapeutic vitreous concentrations.163–165
A randomized multicentre trial that compared voriconazole with amphotericin B followed by fluconazole for the treatment of candidaemia reported that ocular involvement occurred in 16% of patients with candidaemia, mostly manifesting as chorioretinitis, whereas endophthalmitis was uncommon (1.6%); treatment with either voriconazole or amphotericin B followed by fluconazole was successful for ocular candidiasis in most (65%) cases.166 According to clinical guidelines, fluconazole or voriconazole are recommended as the drugs of choice for susceptible isolates, whereas liposomal amphotericin B either alone or combined with flucytosine is recommended when the susceptibility of the isolate is unknown. In the case of endophthalmitis, vitrectomy and intravitreal injection of amphotericin B are recommended in addition to systemic therapy.76
Additional challenging infection sites
Candida endocarditis
Candida species cause <2% of all infective endocarditis cases and can arise on a native valve, a prosthetic valve or in the presence of pacemaker or other implanted material. Candida endocarditis might be considered a biofilm-related infection following earlier fungal bloodstream infection. Overall, prognosis is poor with 1-year mortality >50% and substantial relapse rates.149,167,168Candida vegetations are typically larger and more friable than bacterial ones, harbouring a higher risk for embolic events, ophthalmologic complications and cutaneous lesions. Due to this high mortality, early diagnosis and initiation of antifungals is vital.167,168 Most cases are identified with transthoracic echocardiography, although transoesophageal echocardiography could help improve diagnosis. Although blood cultures are mostly positive in Candida endocarditis,169 its yield can be lower in patients receiving prior antifungals and cultures can take several days to produce results. Hence, attempts should be made to get a tissue sample for diagnosis. Moreover, nonculture methods could have an important role in early diagnosis, such as BDG detection and PCR amplification, with a sensitivity of 89% and over 92% for fungal endocarditis, respectively, according to a recent systematic review.169
A recent review of 140 cases of Candida endocarditis showed that surgery, effective antibiofilm treatment (defined in such study as antifungal treatment with liposomal amphotericin B or echinocandins) and chronic suppressive antifungal therapy were independently associated with improved prognosis.170 Accordingly, international guidelines recommend surgery whenever feasible, as well as antifungal treatment with liposomal amphotericin B, which can be combined with flucytosine,76 or high-dose echinocandins. Additionally, long-term suppressive therapy should be considered for those patients who cannot undergo valve replacement and in those cases of prosthetic valve endocarditis. Finally, risk factors for Candida endocarditis are unspecific and this entity should be considered in patients with relapsing or persistent candidaemia, especially in critically ill patients.171
Bone and joint candidiasis
These infections include osteomyelitis/spondylodiscitis, arthritis and prosthetic joint infection, commonly following haematogenous dissemination, although direct inoculation and contiguous spread are also possible. The spine is the most common site of osteomyelitis involvement.172 Although fluconazole monotherapy has been classically recommended, it is plausible that a biofilm component exists. Thus, initial ‘induction’ treatment with an echinocandin or lipid-based amphotericin, followed by long-term fluconazole therapy (6–12 months), seems advisable.21,173,174 For septic arthritis, surgery is mandatory and in cases of prosthetic joint infections, removal of the joint prosthesis is advised. If this is not possible, lifelong fluconazole therapy is commonly indicated.76 If reimplantation of the prothesis is considered, guidelines recommend administering antifungal treatment for at least 12 weeks before and 6 weeks after prothesis implantation.21 Again, biofilm-active antifungals are conceptually desirable, although their use is limited by the need for intravenous administrations. Moreover, the quality of evidence that supports these recommendations is very low, and this scenario is further complicated by the reported increase in non-albicans species with decreased azole susceptibility173,175,176 and frequent bacterial co-infection.
Candida pneumonia
While Candida species are often isolated from the respiratory tract of ICU intubated patients or patients with tracheostomies, the existence of Candida pneumonia has been largely debated.177 Indeed, the true incidence of Candida pneumonia ranges from 0.23% to 0.4%, with increased risk linked to genetic predisposition and severe immunodeficiency.7,21,177 In this context, antifungal therapy should only be considered in immunocompromised patients on mechanical ventilation with biopsy-proven candidiasis and without an alternative aetiology.177
Conclusions and perspectives
Candida is still one of the main fungal pathogens responsible for serious fungal disease, and non-albicans species as well as multidrug-resistant Candida infections are increasingly detected in clinical settings. Several key challenges need to be tackled to improve the clinical management and outcomes of IC patients. First, collecting and comparing global epidemiological data for IC is hindered by the absence of specific criteria for an incidence denominator and inconsistently collected data. Large longitudinal studies, alongside regional and local surveillance studies, are required to understand epidemiological trends and shifts and to collect data to guide and support empirical antifungal therapy. Another key challenge concerns the currently available diagnostic tests and risk scoring tools, which have a limited capacity to inform appropriate treatment and improve clinical outcomes in a very heterogeneous population of patients. Additionally, the lack of standardized effectiveness outcomes and long-term data for IC limits the ability to capture downstream consequences of treatment pathways, namely treatment failures.178 Understanding which elements of care, like survival or length of stay, are most likely to be affected by treatment, and standardizing those outcomes and their reporting across trials, would promote comparability across treatments.
The timing for the initiation of antifungal therapy is also a key aspect to improve patient outcomes, hindered by the diagnostics limitations. Empirical antifungal therapy is recommended for adults who are at high risk of fungal infection, alongside prompt source control to eliminate the focus of the infection, when possible. Finally, treatment-wise, echinocandins have shown promising efficacy and safety results18 and are recommended by guidelines as first-line treatment in most IC patients, but they carry higher costs of drug acquisition and administration compared with older-generation antifungal agents.179,180 Furthermore, available cost-effectiveness data for echinocandins in treating IC are limited, both quantitatively and qualitatively,178 and inconsistently reported. In addition, few studies are available to support recommendations on the optimal step-down therapy from echinocandins to azoles or the total duration of therapy. Strategy studies should prospectively identify optimal step-down protocols as well as explore further limitations of the total duration of antifungal treatment in selected patient groups.
Several new drugs have shown promising efficacy against IC in recent phase 2 and 3 clinical trials. Rezafungin is a novel echinocandin with an extended half-life and prolonged therapeutic drug concentrations in peripheral tissues, allowing weekly versus daily administration compared to existing echinocandins. These pharmacodynamics properties simplify treatment of outpatients requiring extended therapy and make rezafungin a viable alternative for IC prophylaxis in haematological patients. A phase 2 trial has demonstrated that the efficacy and safety of rezafungin are comparable to those of the other echinocandins,82 while recently published results of the phase 3 trial ReSTORE have shown that rezafungin is non-inferior to caspofungin in patients with candidaemia or IC regarding day-14 global cure and 30-day all-cause mortality, with no differences in adverse events occurrence.181 Ibrexafungerp is an oral glucan synthase inhibitor with broad activity against Candida spp., including azole resistant (e.g. C. auris), and it has a comparable efficacy and safety with standard of care, according to a small phase 2 trial.182,183 An ongoing salvage study suggests it could be used for drug-resistant infections as an alternative to echinocandins (NCT03059992). Fosmanogepix is a guanosine monophosphate inhibitor with a broad activity against Candida species (except against P. kudriavzevii) and can be administered orally and parenterally twice daily.184 A small phase 2 clinical trial has shown good efficacy and safety in fluconazole-resistant IC185; a similar study on C. auris-candidaemia has recently successfully met its clinical objectives (NCT04148287). ATI-2307 mitochondrial inhibitor was found to have in vitro and in vivo activity against most Candida species and has potential to be used for drug-resistant Candida infections.186 However, no comparative clinical trials have been conducted so far.
In addition to new drug development, there is an increased interest in combination therapy to tackle the rising of drug resistance and the high mortality rates in IC. A recent systematic review found that, although the effect of combination treatments varied greatly across studies depending on Candida species, drug and methodology used, some combination regimens had a synergistic effect on difficult-to-treat species or had higher efficacy than monotherapy on the prevention/reduction of biofilms and the clearance of infected tissues.187 However, these data are subject to substantial biases, and further data on combination therapy is needed.
The availability of new compounds and combination therapy overcome some of the challenges identified and increase our options for management of chronic Candida infections and ambulant patient treatments. However, early identification of patients that require antifungal therapy and treatment of sanctuary site infections remain a challenge and will require further innovations.
Supplementary Material
Acknowledgements
The authors would like to acknowledge Inga Bielicka, Sara Dickerson and Laura Sawyer for their comments on early drafts of this paper.
Contributor Information
Alex Soriano, Department of Infectious Diseases, Hospital Clinic of Barcelona, IDIBAPS, CIBERINF, University of Barcelona, Barcelona, Spain.
Patrick M Honore, CHU UCL Godinne Namur, UCL Louvain Medical School, Namur, Belgium.
Pedro Puerta-Alcalde, Department of Infectious Diseases, Hospital Clinic of Barcelona, IDIBAPS, CIBERINF, University of Barcelona, Barcelona, Spain.
Carolina Garcia-Vidal, Department of Infectious Diseases, Hospital Clinic of Barcelona, IDIBAPS, CIBERINF, University of Barcelona, Barcelona, Spain.
Anna Pagotto, Symmetron Ltd, London, UK.
Daniela C Gonçalves-Bradley, Symmetron Ltd, London, UK.
Paul E Verweij, Radboudumc-CWZ Center of Expertise for Mycology, Nijmegen, the Netherlands.
Funding
This article was sponsored by Mundipharma Research Limited.
Transparency declarations
A.S. has served as speaker and has participated in advisory boards for Pfizer, Merck Sharp & Dohme, Menarini, Shionogi, Gilead, Roche, Mundipharma and Angelini. P.M.H. has received honoraria for talks from Mundipharma, Pfizer, and Merck Sharp & Dohme; and has received grants from Pfizer. P.P.-A. has received honoraria for talks on behalf of Merck Sharp & Dohme, Lilly, ViiV Healthcare and Gilead Sciences. P.P.-A. has participated in advisory boards for Gilead Sciences. C.G.V. has served as speaker and has participated in advisory boards for Pfizer, Merck Sharp & Dohme, Menarini, Shionogi, Gilead Sciences, Roche and Janssen. D.C.G.B. and A.P. are employed by Symmetron Ltd and were commissioned by Mundipharma Research Limited to develop and write this article. PEV has received honoraria for talks from Mundipharma, F2G, Gilead Sciences and Pfizer and received research grants from F2G and Gilead Sciences. All payments were to the hospital. This article was co-developed and published based on all authors’ approval.
Supplementary data
Available as Supplementary data at JAC Online.
References
- 1. Kullberg BJ, Arendrup MC. Invasive candidiasis. N Engl J Med 2015; 373: 1445–56. 10.1056/NEJMra1315399 [DOI] [PubMed] [Google Scholar]
- 2. Logan C, Martin-Loeches I, Bicanic T. Invasive candidiasis in critical care: challenges and future directions. Intensive Care Med 2020; 46: 2001–14. 10.1007/s00134-020-06240-x [DOI] [PubMed] [Google Scholar]
- 3. Bongomin F, Gago S, Oladele ROet al. Global and multi-national prevalence of fungal diseases-estimate precision. J Fungi (Basel) 2017; 3: 57. 10.3390/jof3040057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Clancy CJ, Pappas PG, Vazquez Jet al. Detecting infections rapidly and easily for candidemia trial, part 2 (DIRECT2): a prospective, multicenter study of the T2Candida panel. Clin Infect Dis 2018; 66: 1678–86. 10.1093/cid/cix1095 [DOI] [PubMed] [Google Scholar]
- 5. Martinez-Jimenez MC, Munoz P, Guinea Jet al. Potential role of Candida albicans germ tube antibody in the diagnosis of deep-seated candidemia. Med Mycol 2014; 52: 270–5. 10.1093/mmy/myt025 [DOI] [PubMed] [Google Scholar]
- 6. Pappas PG, Lionakis MS, Arendrup MCet al. Invasive candidiasis. Nat Rev Dis Primers 2018; 4: 18026. 10.1038/nrdp.2018.26 [DOI] [PubMed] [Google Scholar]
- 7. McCarty TP, White CM, Pappas PG. Candidemia and invasive candidiasis. Infect Dis Clin North Am 2021; 35: 389–413. 10.1016/j.idc.2021.03.007 [DOI] [PubMed] [Google Scholar]
- 8. Giacobbe DR, Maraolo AE, Simeon Vet al. Changes in the relative prevalence of candidaemia due to non-albicans Candida species in adult in-patients: a systematic review, meta-analysis and meta-regression. Mycoses 2020; 63: 334–42. 10.1111/myc.13054 [DOI] [PubMed] [Google Scholar]
- 9. Du H, Bing J, Hu Tet al. Candida auris: epidemiology, biology, antifungal resistance, and virulence. PLoS Pathog 2020; 16: e1008921. 10.1371/journal.ppat.1008921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang F, Aschenbrenner D, Yoo JIet al. The gut mycobiome in health, disease, and clinical applications in association with the gut bacterial microbiome assembly. Lancet Microbe 2022; 3: e969–83. 10.1016/S2666-5247(22)00203-8 [DOI] [PubMed] [Google Scholar]
- 11. Musuuza JS, Watson L, Parmasad Vet al. Prevalence and outcomes of co-infection and superinfection with SARS-CoV-2 and other pathogens: a systematic review and meta-analysis. PLoS ONE 2021; 16: e0251170. 10.1371/journal.pone.0251170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gamaletsou MN, Walsh TJ, Zaoutis Tet al. A prospective, cohort, multicentre study of candidaemia in hospitalized adult patients with haematological malignancies. Clin Microbiol Infect 2014; 20: O50–7. 10.1111/1469-0691.12312 [DOI] [PubMed] [Google Scholar]
- 13. Pagano L, Caira M, Candoni Aet al. The epidemiology of fungal infections in patients with hematologic malignancies: the SEIFEM-2004 study. Haematologica 2006; 91: 1068–75. [PubMed] [Google Scholar]
- 14. Kontoyiannis DP, Marr KA, Park BJet al. Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001–2006: overview of the transplant-associated infection surveillance network (TRANSNET) database. Clin Infect Dis 2010; 50: 1091–100. 10.1086/651263 [DOI] [PubMed] [Google Scholar]
- 15. Sipsas NV, Lewis RE, Tarrand Jet al. Candidemia in patients with hematologic malignancies in the era of new antifungal agents (2001–2007): stable incidence but changing epidemiology of a still frequently lethal infection. Cancer 2009; 115: 4745–52. 10.1002/cncr.24507 [DOI] [PubMed] [Google Scholar]
- 16. Manolakaki D, Velmahos G, Kourkoumpetis Tet al. Candida infection and colonization among trauma patients. Virulence 2010; 1: 367–75. 10.4161/viru.1.5.12796 [DOI] [PubMed] [Google Scholar]
- 17. Mayer FL, Wilson D, Hube B. Candida albicans pathogenicity mechanisms. Virulence 2013; 4: 119–28. 10.4161/viru.22913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Demir KK, Butler-Laporte G, Del Corpo Oet al. Comparative effectiveness of amphotericin B, azoles and echinocandins in the treatment of candidemia and invasive candidiasis: a systematic review and network meta-analysis. Mycoses 2021; 64: 1098–110. 10.1111/myc.13290 [DOI] [PubMed] [Google Scholar]
- 19. Clancy CJ, Nguyen MH. Diagnosing invasive candidiasis. J Clin Microbiol 2018; 56: e01909-17. 10.1128/JCM.01909-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lamoth F, Lockhart SR, Berkow ELet al. Changes in the epidemiological landscape of invasive candidiasis. J Antimicrob Chemother 2018; 73: i4–i13. 10.1093/jac/dkx444 [DOI] [PubMed] [Google Scholar]
- 21. Pappas PG, Kauffman CA, Andes DRet al. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 2016; 62: e1–e50. 10.1093/cid/civ933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cuenca-Estrella M, Verweij PE, Arendrup MCet al. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: diagnostic procedures. Clin Microbiol Infect 2012; 18(Suppl 7):9–18. 10.1111/1469-0691.12038 [DOI] [PubMed] [Google Scholar]
- 23. Ullmann AJ, Akova M, Herbrecht Ret al. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: adults with haematological malignancies and after haematopoietic stem cell transplantation (HCT). Clin Microbiol Infect 2012; 18(Suppl 7):53–67. 10.1111/1469-0691.12041 [DOI] [PubMed] [Google Scholar]
- 24. Ismail WNAW, Jasmi N, Khan TMet al. The economic burden of candidemia and invasive candidiasis: a systematic review. Value Health Reg Issues 2020; 21: 53–8. 10.1016/j.vhri.2019.07.002 [DOI] [PubMed] [Google Scholar]
- 25. Drgona L, Khachatryan A, Stephens Jet al. Clinical and economic burden of invasive fungal diseases in Europe: focus on pre-emptive and empirical treatment of Aspergillus and Candida species. Eur J Clin Microbiol Infect Dis 2014; 33: 7–21. 10.1007/s10096-013-1944-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Baethge C, Goldbeck-Wood S, Mertens S. SANRA—a scale for the quality assessment of narrative review articles. Res Integr Peer Rev 2019; 4: 5. 10.1186/s41073-019-0064-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cooper C, Booth A, Britten Net al. A comparison of results of empirical studies of supplementary search techniques and recommendations in review methodology handbooks: a methodological review. Syst Rev 2017; 6: 234. 10.1186/s13643-017-0625-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McCarty TP, Pappas PG. Invasive candidiasis. Infect Dis Clin North Am 2016; 30: 103–24. 10.1016/j.idc.2015.10.013 [DOI] [PubMed] [Google Scholar]
- 29. Wisplinghoff H, Ebbers J, Geurtz Let al. Nosocomial bloodstream infections due to Candida spp. in the USA: species distribution, clinical features and antifungal susceptibilities. Int J Antimicrob Agents 2014; 43: 78–81. 10.1016/j.ijantimicag.2013.09.005 [DOI] [PubMed] [Google Scholar]
- 30. Marchetti O, Bille J, Fluckiger Uet al. Epidemiology of candidemia in Swiss tertiary care hospitals: secular trends, 1991–2000. Clin Infect Dis 2004; 38: 311–20. 10.1086/380637 [DOI] [PubMed] [Google Scholar]
- 31. Vincent JL, Rello J, Marshall Jet al. International study of the prevalence and outcomes of infection in intensive care units. JAMA 2009; 302: 2323–9. 10.1001/jama.2009.1754 [DOI] [PubMed] [Google Scholar]
- 32. Koehler P, Stecher M, Cornely OAet al. Morbidity and mortality of candidaemia in Europe: an epidemiologic meta-analysis. Clin Microbiol Infect 2019; 25: 1200–12. 10.1016/j.cmi.2019.04.024 [DOI] [PubMed] [Google Scholar]
- 33. Bassetti M, Giacobbe DR, Vena Aet al. Incidence and outcome of invasive candidiasis in intensive care units (ICUs) in Europe: results of the EUCANDICU project. Crit Care 2019; 23: 219. 10.1186/s13054-019-2497-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. White PL, Price JS, Backx M. Evaluation of the performance of the associates of Cape Cod STAT assay for the diagnosis of invasive fungal disease in critical-care patients with COVID-19. J Clin Microbiol 2021; 59: e0086921. 10.1128/JCM.00869-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bassetti M, Marchetti M, Chakrabarti Aet al. A research agenda on the management of intra-abdominal candidiasis: results from a consensus of multinational experts. Intensive Care Med 2013; 39: 2092–106. 10.1007/s00134-013-3109-3 [DOI] [PubMed] [Google Scholar]
- 36. Bassetti M, Righi E, Ansaldi Fet al. A multicenter multinational study of abdominal candidiasis: epidemiology, outcomes and predictors of mortality. Intensive Care Med 2015; 41: 1601–10. 10.1007/s00134-015-3866-2 [DOI] [PubMed] [Google Scholar]
- 37. Zirkel J, Klinker H, Kuhn Aet al. Epidemiology of Candida blood stream infections in patients with hematological malignancies or solid tumors. Med Mycol 2012; 50: 50–5. 10.3109/13693786.2011.587211 [DOI] [PubMed] [Google Scholar]
- 38. Klingspor L, Tortorano AM, Peman Jet al. Invasive Candida infections in surgical patients in intensive care units: a prospective, multicentre survey initiated by the European Confederation of Medical Mycology (ECMM) (2006–2008). Clin Microbiol Infect 2015; 21:87.e1–87.e10. 10.1016/j.cmi.2014.08.011 [DOI] [PubMed] [Google Scholar]
- 39. Castanheira M, Messer SA, Rhomberg PRet al. Antifungal susceptibility patterns of a global collection of fungal isolates: results of the SENTRY antifungal surveillance program (2013). Diagn Microbiol Infect Dis 2016; 85: 200–4. 10.1016/j.diagmicrobio.2016.02.009 [DOI] [PubMed] [Google Scholar]
- 40. Pfaller MA, Moet GJ, Messer SAet al. Geographic variations in species distribution and echinocandin and azole antifungal resistance rates among Candida bloodstream infection isolates: report from the SENTRY antimicrobial surveillance program (2008 to 2009). J Clin Microbiol 2011; 49: 396–9. 10.1128/JCM.01398-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sullivan D, Coleman D. Candida dubliniensis: characteristics and identification. J Clin Microbiol 1998; 36: 329–34. 10.1128/JCM.36.2.329-334.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Marr KA, Seidel K, White TCet al. Candidemia in allogeneic blood and marrow transplant recipients: evolution of risk factors after the adoption of prophylactic fluconazole. J Infect Dis 2000; 181: 309–16. 10.1086/315193 [DOI] [PubMed] [Google Scholar]
- 43. Jeffery-Smith A, Taori SK, Schelenz Set al. Candida auris: a review of the literature. Clin Microbiol Rev 2018; 31: e00029-17. 10.1128/CMR.00029-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Spivak ES, Hanson KE. Candida auris: an emerging fungal pathogen. J Clin Microbiol 2018; 56: e01588-17. 10.1128/JCM.01588-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pfaller MA, Castanheira M. Nosocomial candidiasis: antifungal stewardship and the importance of rapid diagnosis. Med Mycol 2016; 54: 1–22. [DOI] [PubMed] [Google Scholar]
- 46. Calandra T, Roberts JA, Antonelli Met al. Diagnosis and management of invasive candidiasis in the ICU: an updated approach to an old enemy. Crit Care 2016; 20: 125. 10.1186/s13054-016-1313-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Strollo S, Lionakis MS, Adjemian Jet al. Epidemiology of hospitalizations associated with invasive candidiasis, United States, 2002–2012(1). Emerg Infect Dis 2016; 23: 7–13. 10.3201/eid2301.161198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wang JF, Xue Y, Zhu XBet al. Efficacy and safety of echinocandins versus triazoles for the prophylaxis and treatment of fungal infections: a meta-analysis of RCTs. Eur J Clin Microbiol Infect Dis 2015; 34: 651–9. 10.1007/s10096-014-2287-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tang DL, Chen X, Zhu CGet al. Pooled analysis of T2 Candida for rapid diagnosis of candidiasis. BMC Infect Dis 2019; 19: 798. 10.1186/s12879-019-4419-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lagunes L, Rello J. Invasive candidiasis: from mycobiome to infection, therapy, and prevention. Eur J Clin Microbiol Infect Dis 2016; 35: 1221–6. 10.1007/s10096-016-2658-0 [DOI] [PubMed] [Google Scholar]
- 51. Schelenz S, Barnes RA, Barton RCet al. British Society for Medical Mycology best practice recommendations for the diagnosis of serious fungal diseases. Lancet Infect Dis 2015; 15: 461–74. 10.1016/S1473-3099(15)70006-X [DOI] [PubMed] [Google Scholar]
- 52. Clancy CJ, Nguyen MH. Finding the ‘missing 50%’ of invasive candidiasis: how nonculture diagnostics will improve understanding of disease spectrum and transform patient care. Clin Infect Dis 2013; 56: 1284–92. 10.1093/cid/cit006 [DOI] [PubMed] [Google Scholar]
- 53. Cobos-Trigueros N, Morata L, Torres Jet al. Usefulness of time-to-positivity in aerobic and anaerobic vials to predict the presence of Candida glabrata in patients with candidaemia. J Antimicrob Chemother 2013; 68: 2839–41. 10.1093/jac/dkt285 [DOI] [PubMed] [Google Scholar]
- 54. Mikulska M, Calandra T, Sanguinetti Met al. The use of mannan antigen and anti-mannan antibodies in the diagnosis of invasive candidiasis: recommendations from the Third European Conference on Infections in Leukemia. Crit Care 2010; 14: R222. 10.1186/cc9365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wei S, Wu T, Wu Yet al. Diagnostic accuracy of Candida albicans germ tube antibody for invasive candidiasis: systematic review and meta-analysis. Diagn Microbiol Infect Dis 2019; 93: 339–45. 10.1016/j.diagmicrobio.2018.10.017 [DOI] [PubMed] [Google Scholar]
- 56. Camp I, Spettel K, Willinger B. Molecular methods for the diagnosis of invasive candidiasis. J Fungi (Basel) 2020; 6: 101. 10.3390/jof6030101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Rouzé A, Loridant S, Poissy Jet al. Biomarker-based strategy for early discontinuation of empirical antifungal treatment in critically ill patients: a randomized controlled trial. Intensive Care Med 2017; 43:1668–77. 10.1007/s00134-017-4932-8 [DOI] [PubMed] [Google Scholar]
- 58. Avni T, Leibovici L, Paul M. PCR diagnosis of invasive candidiasis: systematic review and meta-analysis. J Clin Microbiol 2011; 49: 665–70. 10.1128/JCM.01602-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Honore PM, Redant S, Preseau Tet al. T2MR can be used as a non-culture-based test together with biomarkers to improve detection of Candida in the bloodstream and reduce time delay in treating invasive candidiasis. Expert Rev Anti Infect Ther 2022; 20:327–9. 10.1080/14787210.2021.1964954 [DOI] [PubMed] [Google Scholar]
- 60. Dupuis C, Le Bihan C, Maubon Det al. Performance of repeated measures of (1-3)-beta-D-glucan, mannan antigen, and antimannan antibodies for the diagnosis of invasive candidiasis in ICU patients: a preplanned ancillary analysis of the EMPIRICUS randomized clinical trial. Open Forum Infect Dis 2021; 8: ofab080. 10.1093/ofid/ofab080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Pittet D, Monod M, Suter PMet al. Candida colonization and subsequent infections in critically ill surgical patients. Ann Surg 1994; 220: 751–8. 10.1097/00000658-199412000-00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Leon C, Ruiz-Santana S, Saavedra Pet al. A bedside scoring system (‘Candida score’) for early antifungal treatment in nonneutropenic critically ill patients with Candida colonization. Crit Care Med 2006; 34: 730–7. 10.1097/01.CCM.0000202208.37364.7D [DOI] [PubMed] [Google Scholar]
- 63. Ostrosky-Zeichner L, Sable C, Sobel Jet al. Multicenter retrospective development and validation of a clinical prediction rule for nosocomial invasive candidiasis in the intensive care setting. Eur J Clin Microbiol Infect Dis 2007; 26: 271–6. 10.1007/s10096-007-0270-z [DOI] [PubMed] [Google Scholar]
- 64. Thomas-Ruddel D, Schlattmann P, Pletz Met al. Risk factors for invasive candida infection in critically ill patients—a systematic review and meta-analysis. Chest 2022; 161: 345–55. 10.1016/j.chest.2021.08.081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Cortegiani A, Russotto V, Raineri SMet al. Should we continue to use prediction tools to identify patients at risk of Candida spp. infection? If yes, why? Crit Care 2016; 20: 351. 10.1186/s13054-016-1521-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Knaus WA, Draper EA, Wagner DPet al. APACHE II: a severity of disease classification system. Crit Care Med 1985; 13: 818–29. 10.1097/00003246-198510000-00009 [DOI] [PubMed] [Google Scholar]
- 67. Knaus WA, Wagner DP, Draper EAet al. The APACHE III prognostic system. Risk prediction of hospital mortality for critically ill hospitalized adults. Chest 1991; 100: 1619–36. 10.1378/chest.100.6.1619 [DOI] [PubMed] [Google Scholar]
- 68. Zimmerman JE, Kramer AA, McNair DSet al. Acute Physiology and Chronic Health Evaluation (APACHE) IV: hospital mortality assessment for today's critically ill patients. Crit Care Med 2006; 34: 1297–310. 10.1097/01.CCM.0000215112.84523.F0 [DOI] [PubMed] [Google Scholar]
- 69. Moreno RP, Metnitz PG, Almeida Eet al. SAPS 3—from evaluation of the patient to evaluation of the intensive care unit. Part 2: development of a prognostic model for hospital mortality at ICU admission. Intensive Care Med 2005; 31: 1345–55. 10.1007/s00134-005-2763-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Metnitz PG, Moreno RP, Almeida Eet al. SAPS 3—from evaluation of the patient to evaluation of the intensive care unit. Part 1: objectives, methods and cohort description. Intensive Care Med 2005; 31: 1336–44. 10.1007/s00134-005-2762-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Leroy O, Bailly S, Gangneux JPet al. Systemic antifungal therapy for proven or suspected invasive candidiasis: the AmarCAND 2 study. Ann Intensive Care 2016; 6: 2. 10.1186/s13613-015-0103-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Posteraro B, De Pascale G, Tumbarello Met al. Early diagnosis of candidemia in intensive care unit patients with sepsis: a prospective comparison of (1–>3)-beta-D-glucan assay, Candida score, and colonization index. Crit Care 2011; 15: R249. 10.1186/cc10507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Wojtowicz A, Tissot F, Lamoth Fet al. Polymorphisms in tumor necrosis factor-alpha increase susceptibility to intra-abdominal Candida infection in high-risk surgical ICU patients*. Crit Care Med 2014; 42: e304–8. 10.1097/CCM.0000000000000208 [DOI] [PubMed] [Google Scholar]
- 74. Eggimann P, Pittet D. Candida colonization index and subsequent infection in critically ill surgical patients: 20 years later. Intensive Care Med 2014; 40: 1429–48. 10.1007/s00134-014-3355-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Laine ME, Flannery AH, Moody Bet al. Need for expanded Candida score for empiric antifungal use in medically critically ill patients? Crit Care 2019; 23: 242. 10.1186/s13054-019-2525-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Cornely OA, Bassetti M, Calandra Tet al. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: non-neutropenic adult patients. Clin Microbiol Infect 2012; 18(Suppl 7):19–37. 10.1111/1469-0691.12039 [DOI] [PubMed] [Google Scholar]
- 77. Mora-Duarte J, Betts R, Rotstein Cet al. Comparison of caspofungin and amphotericin B for invasive candidiasis. N Engl J Med 2002; 347: 2020–9. 10.1056/NEJMoa021585 [DOI] [PubMed] [Google Scholar]
- 78. Kuse ER, Chetchotisakd P, da Cunha CAet al. Micafungin versus liposomal amphotericin B for candidaemia and invasive candidosis: a phase III randomised double-blind trial. Lancet 2007; 369: 1519–27. 10.1016/S0140-6736(07)60605-9 [DOI] [PubMed] [Google Scholar]
- 79. Reboli AC, Shorr AF, Rotstein Cet al. Anidulafungin compared with fluconazole for treatment of candidemia and other forms of invasive candidiasis caused by Candida albicans: a multivariate analysis of factors associated with improved outcome. BMC Infect Dis 2011; 11: 261. 10.1186/1471-2334-11-261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Pappas PG, Rotstein CM, Betts RFet al. Micafungin versus caspofungin for treatment of candidemia and other forms of invasive candidiasis. Clin Infect Dis 2007; 45: 883–93. 10.1086/520980 [DOI] [PubMed] [Google Scholar]
- 81. Kullberg BJ, Viscoli C, Pappas PGet al. Isavuconazole versus caspofungin in the treatment of candidemia and other invasive Candida infections: the ACTIVE trial. Clin Infect Dis 2019; 68: 1981–9. 10.1093/cid/ciy827 [DOI] [PubMed] [Google Scholar]
- 82. Thompson GR, Soriano A, Skoutelis Aet al. Rezafungin versus caspofungin in a phase 2, randomized, double-blind study for the treatment of candidemia and invasive candidiasis: the STRIVE trial. Clin Infect Dis 2021; 73: e3647–55. 10.1093/cid/ciaa1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Andes DR, Safdar N, Baddley JWet al. Impact of treatment strategy on outcomes in patients with candidemia and other forms of invasive candidiasis: a patient-level quantitative review of randomized trials. Clin Infect Dis 2012; 54: 1110–22. 10.1093/cid/cis021 [DOI] [PubMed] [Google Scholar]
- 84. Reboli AC, Rotstein C, Pappas PGet al. Anidulafungin versus fluconazole for invasive candidiasis. N Engl J Med 2007; 356: 2472–82. 10.1056/NEJMoa066906 [DOI] [PubMed] [Google Scholar]
- 85. Kullberg BJ, Sobel JD, Ruhnke Met al. Voriconazole versus a regimen of amphotericin B followed by fluconazole for candidaemia in non-neutropenic patients: a randomised non-inferiority trial. Lancet 2005; 366: 1435–42. 10.1016/S0140-6736(05)67490-9 [DOI] [PubMed] [Google Scholar]
- 86. Pfaller MA, Andes D, Arendrup MCet al. Clinical breakpoints for voriconazole and Candida spp. revisited: review of microbiologic, molecular, pharmacodynamic, and clinical data as they pertain to the development of species-specific interpretive criteria. Diagn Microbiol Infect Dis 2011; 70: 330–43. 10.1016/j.diagmicrobio.2011.03.002 [DOI] [PubMed] [Google Scholar]
- 87. Safdar A, Ma J, Saliba Fet al. Drug-induced nephrotoxicity caused by amphotericin B lipid complex and liposomal amphotericin B: a review and meta-analysis. Medicine (Baltimore) 2010; 89: 236–44. 10.1097/MD.0b013e3181e9441b [DOI] [PubMed] [Google Scholar]
- 88. Colombo AL, Guimaraes T, Sukienik Tet al. Prognostic factors and historical trends in the epidemiology of candidemia in critically ill patients: an analysis of five multicenter studies sequentially conducted over a 9-year period. Intensive Care Med 2014; 40: 1489–98. 10.1007/s00134-014-3400-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Mellinghoff SC, Hartmann P, Cornely FBet al. Analyzing candidemia guideline adherence identifies opportunities for antifungal stewardship. Eur J Clin Microbiol Infect Dis 2018; 37: 1563–71. 10.1007/s10096-018-3285-8 [DOI] [PubMed] [Google Scholar]
- 90. Valerio M, Muñoz P, Rodriguez CGet al. Antifungal stewardship in a tertiary-care institution: a bedside intervention. Clin Microbiol Infect 2015; 21: 492.e1–e9. 10.1016/j.cmi.2015.01.013 [DOI] [PubMed] [Google Scholar]
- 91. Muñoz P, Bouza E, COMIC (Collaboration Group on Mycosis) Study Group . The current treatment landscape: the need for antifungal stewardship programmes. J Antimicrob Chemother 2016; 71: ii5–ii12. 10.1093/jac/dkw391 [DOI] [PubMed] [Google Scholar]
- 92. Ostrosky-Zeichner L, Kullberg BJ, Bow EJet al. Early treatment of candidemia in adults: a review. Med Mycol 2011; 49: 113–20. 10.3109/13693786.2010.512300 [DOI] [PubMed] [Google Scholar]
- 93. Evans L, Rhodes A, Alhazzani Wet al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med 2021; 47: 1181–247. 10.1007/s00134-021-06506-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Cortegiani A, Russotto V, Maggiore Aet al. Antifungal agents for preventing fungal infections in non-neutropenic critically ill patients. Cochrane Database Syst Rev 2016; 2016: CD004920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Bailly S, Maubon D, Fournier Pet al. Impact of antifungal prescription on relative distribution and susceptibility of Candida spp.—trends over 10 years. J Infect 2016; 72: 103–11. 10.1016/j.jinf.2015.09.041 [DOI] [PubMed] [Google Scholar]
- 96. Timsit JF, Azoulay E, Schwebel Cet al. Empirical micafungin treatment and survival without invasive fungal infection in adults with ICU-acquired sepsis, Candida colonization, and multiple organ failure: the EMPIRICUS randomized clinical trial. JAMA 2016; 316: 1555–64. 10.1001/jama.2016.14655 [DOI] [PubMed] [Google Scholar]
- 97. Kollef M, Micek S, Hampton Net al. Septic shock attributed to Candida infection: importance of empiric therapy and source control. Clin Infect Dis 2012; 54: 1739–46. 10.1093/cid/cis305 [DOI] [PubMed] [Google Scholar]
- 98. Vergidis P, Clancy CJ, Shields RKet al. Intra-abdominal candidiasis: the importance of early source control and antifungal treatment. PLoS ONE 2016; 11: e0153247. 10.1371/journal.pone.0153247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Desai JV, Mitchell AP, Andes DR. Fungal biofilms, drug resistance, and recurrent infection. Cold Spring Harb Perspect Med 2014; 4: a019729. 10.1101/cshperspect.a019729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Guinea J, Arendrup MC, Canton Ret al. Genotyping reveals high clonal diversity and widespread genotypes of Candida causing candidemia at distant geographical areas. Front Cell Infect Microbiol 2020; 10: 166. 10.3389/fcimb.2020.00166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Ramage G, Rajendran R, Sherry Let al. Fungal biofilm resistance. Int J Microbiol 2012; 2012: 528521. 10.1155/2012/528521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Davey ME, O’Toole GA. Microbial biofilms: from ecology to molecular genetics. Microbiol Mol Biol Rev 2000; 64: 847–67. 10.1128/MMBR.64.4.847-867.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Toulet D, Debarre C, Imbert C. Could liposomal amphotericin B (L-AMB) lock solutions be useful to inhibit Candida spp. biofilms on silicone biomaterials? J Antimicrob Chemother 2012; 67: 430–2. 10.1093/jac/dkr473 [DOI] [PubMed] [Google Scholar]
- 104. Cavalheiro M, Teixeira MC. Candida biofilms: threats, challenges, and promising strategies. Front Med (Lausanne) 2018; 5: 28. 10.3389/fmed.2018.00028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Cernakova L, Light C, Salehi Bet al. Novel therapies for biofilm-based Candida spp. Infections. Adv Exp Med Biol 2019; 1214: 93–123. 10.1007/5584_2019_400 [DOI] [PubMed] [Google Scholar]
- 106. Gill CM, Kenney RM, Hencken Let al. T2 Candida versus beta-D-glucan to facilitate antifungal discontinuation in the intensive care unit. Diagn Microbiol Infect Dis 2019; 95: 162–5. 10.1016/j.diagmicrobio.2019.04.016 [DOI] [PubMed] [Google Scholar]
- 107. Moreno-Garcia E, Puerta-Alcalde P, Gariup Get al. Early stepdown from echinocandin to fluconazole treatment in candidemia: a post hoc analysis of three cohort studies. Open Forum Infect Dis 2021; 8: ofab250. 10.1093/ofid/ofab250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Mylonakis E, Zacharioudakis IM, Clancy CJet al. Efficacy of T2 magnetic resonance assay in monitoring candidemia after initiation of antifungal therapy: the serial therapeutic and antifungal monitoring protocol (STAMP) trial. J Clin Microbiol 2018; 56: e01756-17. 10.1128/JCM.01756-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Gómez-López A. Antifungal therapeutic drug monitoring: focus on drugs without a clear recommendation. Clin Microbiol Infect 2020; 26: 1481–7. 10.1016/j.cmi.2020.05.037 [DOI] [PubMed] [Google Scholar]
- 110. Van Daele R, Wauters J, Lagrou Ket al. Pharmacokinetic variability and target attainment of fluconazole in critically ill patients. Microorganisms 2021; 9:2068. 10.3390/microorganisms9102068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Lewis RE, Andes DR. Managing uncertainty in antifungal dosing: antibiograms, therapeutic drug monitoring and drug-drug interactions. Curr Opin Infect Dis 2021; 34: 288–96. 10.1097/QCO.0000000000000740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Pfaller MA, Diekema DJ, Turnidge JDet al. Twenty years of the SENTRY antifungal surveillance program: results for Candida Species from 1997–2016. Open Forum Infect Dis 2019; 6: S79–94. 10.1093/ofid/ofy358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Arastehfar A, Gabaldon T, Garcia-Rubio Ret al. Drug-resistant fungi: an emerging challenge threatening our limited antifungal armamentarium. Antibiotics (Basel) 2020; 9: 877. 10.3390/antibiotics9120877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Healey KR, Perlin DS. Fungal resistance to echinocandins and the MDR phenomenon in Candida glabrata. J Fungi (Basel) 2018; 4: 105. 10.3390/jof4030105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Chow NA, Munoz JF, Gade Let al. Tracing the evolutionary history and global expansion of Candida auris using population genomic analyses. mBio 2020; 11: e03364-19. 10.1128/mBio.03364-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Pristov KE, Ghannoum MA. Resistance of Candida to azoles and echinocandins worldwide. Clin Microbiol Infect 2019; 25: 792–8. 10.1016/j.cmi.2019.03.028 [DOI] [PubMed] [Google Scholar]
- 117. Lockhart SR, Etienne KA, Vallabhaneni Set al. Simultaneous emergence of multidrug-resistant Candida auris on 3 continents confirmed by whole-genome sequencing and epidemiological analyses. Clin Infect Dis 2017; 64: 134–40. 10.1093/cid/ciw691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Chowdhary A, Sharma C, Meis JF. Candida auris: a rapidly emerging cause of hospital-acquired multidrug-resistant fungal infections globally. PLoS Pathog 2017; 13: e1006290. 10.1371/journal.ppat.1006290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Clancy CJ, Nguyen MH. Emergence of Candida auris: an international call to arms. Clin Infect Dis 2017; 64: 141–3. 10.1093/cid/ciw696 [DOI] [PubMed] [Google Scholar]
- 120. CLSI . Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts—Fourth Edition: M27. 2017. [Google Scholar]
- 121. Arendrup MC, Meletiadis J, Mouton JWet al. EUCAST definitive document E.DEF 7.3.2. Method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for yeasts , 2020. https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/AFST/Files/EUCAST_E_Def_7.3.2_Yeast_testing_definitive_revised_2020.pdf
- 122. Knabl L, Lass-Florl C. Antifungal susceptibility testing in Candida species: current methods and promising new tools for shortening the turnaround time. Expert Rev Anti Infect Ther 2020; 18: 779–87. 10.1080/14787210.2020.1760841 [DOI] [PubMed] [Google Scholar]
- 123. Kwon YJ, Won EJ, Jeong SHet al. Dynamics and predictors of mortality due to Candidemia caused by different Candida species: comparison of Intensive Care Unit-Associated Candidemia (ICUAC) and non-ICUAC. J Fungi (Basel) 2021; 7: 597. 10.3390/jof7080597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Pea F. Plasma pharmacokinetics of antimicrobial agents in critically ill patients. Curr Clin Pharmacol 2013; 8: 5–12. [PubMed] [Google Scholar]
- 125. Bouman CS, van Kan HJ, Koopmans RPet al. Discrepancies between observed and predicted continuous venovenous hemofiltration removal of antimicrobial agents in critically ill patients and the effects on dosing. Intensive Care Med 2006; 32: 2013–9. 10.1007/s00134-006-0397-x [DOI] [PubMed] [Google Scholar]
- 126. Yagasaki K, Gando S, Matsuda Net al. Pharmacokinetics and the most suitable dosing regimen of fluconazole in critically ill patients receiving continuous hemodiafiltration. Intensive Care Med 2003; 29: 1844–8. 10.1007/s00134-003-1980-z [DOI] [PubMed] [Google Scholar]
- 127. Riche CVW, Cassol R, Pasqualotto AC. Is the frequency of candidemia increasing in COVID-19 patients receiving corticosteroids. J Fungi (Basel) 2020; 6: 286. 10.3390/jof6040286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Arastehfar A, Carvalho A, Nguyen MHet al. COVID-19-associated candidiasis (CAC): an underestimated complication in the absence of immunological predispositions? J Fungi (Basel) 2020; 6: 211. 10.3390/jof6040211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Leon C, Ruiz-Santana S, Saavedra Pet al. Value of beta-D-glucan and Candida albicans germ tube antibody for discriminating between Candida colonization and invasive candidiasis in patients with severe abdominal conditions. Intensive Care Med 2012; 38: 1315–25. 10.1007/s00134-012-2616-y [DOI] [PubMed] [Google Scholar]
- 130. Delaloye J, Calandra T. Invasive candidiasis as a cause of sepsis in the critically ill patient. Virulence 2014; 5: 161–9. 10.4161/viru.26187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Poissy J, Damonti L, Bignon Aet al. Risk factors for candidemia: a prospective matched case-control study. Crit Care 2020; 24: 109. 10.1186/s13054-020-2766-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Zilberberg M, Yu HT, Chaudhari Pet al. Relationship of fluconazole prophylaxis with fungal microbiology in hospitalized intra-abdominal surgery patients: a descriptive cohort study. Crit Care 2014; 18: 590. 10.1186/s13054-014-0590-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Lortholary O, Renaudat C, Sitbon Ket al. The risk and clinical outcome of candidemia depending on underlying malignancy. Intensive Care Med 2017; 43: 652–62. 10.1007/s00134-017-4743-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Maertens JA, Girmenia C, Bruggemann RJet al. European Guidelines for primary antifungal prophylaxis in adult haematology patients: summary of the updated recommendations from the European Conference on Infections in Leukaemia. J Antimicrob Chemother 2018; 73: 3221–30. [DOI] [PubMed] [Google Scholar]
- 135. Freifeld AG, Bow EJ, Sepkowitz KAet al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis 2011; 52: 427–31. 10.1093/cid/ciq147 [DOI] [PubMed] [Google Scholar]
- 136. Kontoyiannis DP, Reddy BT, Hanna Het al. Breakthrough candidemia in patients with cancer differs from de novo candidemia in host factors and Candida species but not intensity. Infect Control Hosp Epidemiol 2002; 23: 542–5. 10.1086/502104 [DOI] [PubMed] [Google Scholar]
- 137. Girmenia C, Martino P, Cassone A. Breakthrough candidemia during antifungal treatment with fluconazole in patients with hematologic malignancies. Blood 1996; 87: 838–9. 10.1182/blood.V87.2.838.bloodjournal872838 [DOI] [PubMed] [Google Scholar]
- 138. Uzun O, Ascioglu S, Anaissie EJet al. Risk factors and predictors of outcome in patients with cancer and breakthrough candidemia. Clin Infect Dis 2001; 32: 1713–7. 10.1086/320757 [DOI] [PubMed] [Google Scholar]
- 139. Pfeiffer CD, Garcia-Effron G, Zaas AKet al. Breakthrough invasive candidiasis in patients on micafungin. J Clin Microbiol 2010; 48: 2373–80. 10.1128/JCM.02390-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Bizerra FC, Jimenez-Ortigosa C, Souza ACet al. Breakthrough candidemia due to multidrug-resistant Candida glabrata during prophylaxis with a low dose of micafungin. Antimicrob Agents Chemother 2014; 58: 2438–40. 10.1128/AAC.02189-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Breda GL, Tuon FF, Meis JFet al. Breakthrough candidemia after the introduction of broad spectrum antifungal agents: a 5-year retrospective study. Med Mycol 2018; 56: 406–15. 10.1093/mmy/myx077 [DOI] [PubMed] [Google Scholar]
- 142. Cuervo G, Garcia-Vidal C, Nucci Met al. Breakthrough candidaemia in the era of broad-spectrum antifungal therapies. Clin Microbiol Infect 2016; 22: 181–8. 10.1016/j.cmi.2015.09.029 [DOI] [PubMed] [Google Scholar]
- 143. Nucci M, Colombo AL. Risk factors for breakthrough candidemia. Eur J Clin Microbiol Infect Dis 2002; 21: 209–11. 10.1007/s10096-002-0697-1 [DOI] [PubMed] [Google Scholar]
- 144. Orasch C, Mertz D, Garbino Jet al. Fluconazole non-susceptible breakthrough candidemia after prolonged low-dose prophylaxis: a prospective FUNGINOS study. J Infect 2018; 76: 489–95. 10.1016/j.jinf.2017.12.018 [DOI] [PubMed] [Google Scholar]
- 145. Sanchez-Portocarrero J, Perez-Cecilia E, Corral Oet al. The central nervous system and infection by Candida species. Diagn Microbiol Infect Dis 2000; 37: 169–79. 10.1016/S0732-8893(00)00140-1 [DOI] [PubMed] [Google Scholar]
- 146. Nguyen MH, Yu VL. Meningitis caused by Candida species: an emerging problem in neurosurgical patients. Clin Infect Dis 1995; 21: 323–7. 10.1093/clinids/21.2.323 [DOI] [PubMed] [Google Scholar]
- 147. Casado JL, Quereda C, Oliva Jet al. Candidal meningitis in HIV-infected patients: analysis of 14 cases. Clin Infect Dis 1997; 25: 673–6. 10.1086/513746 [DOI] [PubMed] [Google Scholar]
- 148. Fennelly AM, Slenker AK, Murphy LCet al. Candida cerebral abscesses: a case report and review of the literature. Med Mycol 2013; 51: 779–84. 10.3109/13693786.2013.789566 [DOI] [PubMed] [Google Scholar]
- 149. Lefort A, Chartier L, Sendid Bet al. Diagnosis, management and outcome of Candida endocarditis. Clin Microbiol Infect 2012; 18: E99–E109. 10.1111/j.1469-0691.2012.03764.x [DOI] [PubMed] [Google Scholar]
- 150. Drummond RA, Collar AL, Swamydas Met al. CARD9-dependent neutrophil recruitment protects against fungal invasion of the central nervous system. PLoS Pathog 2015; 11: e1005293. 10.1371/journal.ppat.1005293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Baddley JW, Benjamin DK Jr, Patel Met al. Candida infective endocarditis. Eur J Clin Microbiol Infect Dis 2008; 27: 519–29. 10.1007/s10096-008-0466-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Rivoisy C, Vena A, Schaeffer Let al. Prosthetic valve Candida spp. endocarditis: new insights into long-term prognosis-the ESCAPE study. Clin Infect Dis 2018; 66: 825–32. 10.1093/cid/cix913 [DOI] [PubMed] [Google Scholar]
- 153. Schwartz S, Kontoyiannis DP, Harrison Tet al. Advances in the diagnosis and treatment of fungal infections of the CNS. Lancet Neurol 2018; 17: 362–72. 10.1016/S1474-4422(18)30030-9 [DOI] [PubMed] [Google Scholar]
- 154. Chaussade H, Cazals X, Desoubeaux Get al. Central nervous system candidiasis beyond neonates: lessons from a nationwide study. Med Mycol 2021; 59: 266–77. 10.1093/mmy/myaa051 [DOI] [PubMed] [Google Scholar]
- 155. Chen TL, Chen HP, Fung CPet al. Clinical characteristics, treatment and prognostic factors of candidal meningitis in a teaching hospital in Taiwan. Scand J Infect Dis 2004; 36: 124–30. 10.1080/00365540310017573 [DOI] [PubMed] [Google Scholar]
- 156. Smego RA J, Perfect JR, Durack DT. Combined therapy with amphotericin B and 5-fluorocytosine for Candida meningitis. Rev Infect Dis 1984; 6: 791–801. 10.1093/clinids/6.6.791 [DOI] [PubMed] [Google Scholar]
- 157. Weiler S, Fiegl D, MacFarland Ret al. Human tissue distribution of voriconazole. Antimicrob Agents Chemother 2011; 55: 925–8. 10.1128/AAC.00949-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Peman J, Ruiz-Gaitan A. Candidemia from urinary tract source: the challenge of candiduria. Hosp Pract (1995) 2018; 46: 243–5. 10.1080/21548331.2018.1538623 [DOI] [PubMed] [Google Scholar]
- 159. Cuervo G, Garcia-Vidal C, Puig-Asensio Met al. Echinocandins compared to fluconazole for candidemia of a urinary tract source: a propensity score analysis. Clin Infect Dis 2017; 64: 1374–9. 10.1093/cid/cix033 [DOI] [PubMed] [Google Scholar]
- 160. Sobel JD, Kauffman CA, McKinsey Det al. Candiduria: a randomized, double-blind study of treatment with fluconazole and placebo. The National Institute of Allergy and Infectious Diseases (NIAID) mycoses study group. Clin Infect Dis 2000; 30: 19–24. 10.1086/313580 [DOI] [PubMed] [Google Scholar]
- 161. Grau S, Luque S, Echeverria-Esnal Det al. Urinary micafungin levels are sufficient to treat urinary tract infections caused by Candida spp. Int J Antimicrob Agents 2016; 48: 212–4. 10.1016/j.ijantimicag.2016.05.010 [DOI] [PubMed] [Google Scholar]
- 162. Chhablani J. Fungal endophthalmitis. Expert Rev Anti Infect Ther 2011; 9: 1191–201. 10.1586/eri.11.139 [DOI] [PubMed] [Google Scholar]
- 163. Savani DV, Perfect JR, Cobo LMet al. Penetration of new azole compounds into the eye and efficacy in experimental Candida endophthalmitis. Antimicrob Agents Chemother 1987; 31: 6–10. 10.1128/AAC.31.1.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Barza M. Treatment options for candidal endophthalmitis [editorial comment]. Clin Infect Dis 1998; 27: 1134–6. 10.1086/514973 [DOI] [PubMed] [Google Scholar]
- 165. O’Day DM, Foulds G, Williams TEet al. Ocular uptake of fluconazole following oral administration. Arch Ophthalmol 1990; 108: 1006–8. 10.1001/archopht.1990.01070090108050 [DOI] [PubMed] [Google Scholar]
- 166. Lashof AMO, Rothova A, Sobel JDet al. Ocular manifestations of candidemia. Clin Infect Dis 2011; 53: 262–8. 10.1093/cid/cir355 [DOI] [PubMed] [Google Scholar]
- 167. Ellis ME, Al-Abdely H, Sandridge Aet al. Fungal endocarditis: evidence in the world literature, 1965–1995. Clin Infect Dis 2001; 32: 50–62. 10.1086/317550 [DOI] [PubMed] [Google Scholar]
- 168. Boland JM, Chung HH, Robberts FJet al. Fungal prosthetic valve endocarditis: Mayo Clinic experience with a clinicopathological analysis. Mycoses 2011; 54: 354–60. 10.1111/j.1439-0507.2010.01884.x [DOI] [PubMed] [Google Scholar]
- 169. Meena DS, Kumar D, Agarwal Met al. Clinical features, diagnosis and treatment outcome of fungal endocarditis: a systematic review of reported cases. Mycoses 2022; 65:294–302. 10.1111/myc.13398 [DOI] [PubMed] [Google Scholar]
- 170. Giuliano S, Guastalegname M, Russo Aet al. Candida endocarditis: systematic literature review from 1997 to 2014 and analysis of 29 cases from the Italian study of endocarditis. Expert Rev Anti Infect Ther 2017; 15: 807–18. 10.1080/14787210.2017.1372749 [DOI] [PubMed] [Google Scholar]
- 171. Foong KS, Sung A, Burnham JPet al. Risk factors predicting Candida infective endocarditis in patients with candidemia. Med Mycol 2020; 58: 593–9. 10.1093/mmy/myz104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Gamaletsou MN, Kontoyiannis DP, Sipsas NVet al. Candida osteomyelitis: analysis of 207 pediatric and adult cases (1970–2011). Clin Infect Dis 2012; 55: 1338–51. 10.1093/cid/cis660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Neofytos D, Huprikar S, Reboli Aet al. Treatment and outcomes of Candida osteomyelitis: review of 53 cases from the PATH alliance(R) registry. Eur J Clin Microbiol Infect Dis 2014; 33: 135–41. 10.1007/s10096-013-1939-0 [DOI] [PubMed] [Google Scholar]
- 174. Slenker AK, Keith SW, Horn DL. Two hundred and eleven cases of Candida osteomyelitis: 17 case reports and a review of the literature. Diagn Microbiol Infect Dis 2012; 73: 89–93. 10.1016/j.diagmicrobio.2012.02.004 [DOI] [PubMed] [Google Scholar]
- 175. Saconi ES, de Carvalho VC, de Oliveira PRDet al. Prosthetic joint infection due to Candida species: case series and review of literature. Medicine (Baltimore) 2020; 99: e19735. 10.1097/MD.0000000000019735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Lee YR, Kim HJ, Lee EJet al. Prosthetic joint infections caused by Candida species: a systematic review and a case series. Mycopathologia 2019; 184: 23–33. 10.1007/s11046-018-0286-1 [DOI] [PubMed] [Google Scholar]
- 177. Meena DS, Kumar D. Candida pneumonia: an innocent bystander or a silent killer? Med Princ Pract 2022; 31:98–102. 10.1159/000520111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Neoh CF, Slavin M, Chen SCet al. Echinocandins in the treatment of candidaemia and invasive candidiasis: clinical and economic perspectives. Int J Antimicrob Agents 2014; 43: 207–14. 10.1016/j.ijantimicag.2013.08.010 [DOI] [PubMed] [Google Scholar]
- 179. Ou HT, Lee TY, Chen YCet al. Pharmacoeconomic analysis of antifungal therapy for primary treatment of invasive candidiasis caused by Candida albicans and non-albicans Candida species. BMC Infect Dis 2017; 17: 481. 10.1186/s12879-017-2573-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Heimann SM, Cornely OA, Wisplinghoff Het al. Candidemia in the intensive care unit: analysis of direct treatment costs and clinical outcome in patients treated with echinocandins or fluconazole. Eur J Clin Microbiol Infect Dis 2015; 34: 331–8. 10.1007/s10096-014-2230-8 [DOI] [PubMed] [Google Scholar]
- 181. Thompson GR, Soriano A, Cornely OAet al. Rezafungin versus caspofungin for treatment of candidaemia and invasive candidiasis (ReSTORE): a multicentre, double-blind, double-dummy, randomised phase 3 trial. Lancet 2023; 401:49–59. 10.1016/S0140-6736(22)02324-8 [DOI] [PubMed] [Google Scholar]
- 182. Spec A, Pullman J, Thompson GRet al. MSG-10: a phase 2 study of oral ibrexafungerp (SCY-078) following initial echinocandin therapy in non-neutropenic patients with invasive candidiasis. J Antimicrob Chemother 2019; 74: 3056–62. 10.1093/jac/dkz277 [DOI] [PubMed] [Google Scholar]
- 183. Ghannoum M, Arendrup MC, Chaturvedi VPet al. Ibrexafungerp: a novel oral triterpenoid antifungal in development for the treatment of Candida auris infections. Antibiotics (Basel) 2020; 9: 539. 10.3390/antibiotics9090539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Lee A, Wang N, Carter CLet al. Therapeutic potential of fosmanogepix (APX001) for intra-abdominal candidiasis: from lesion penetration to efficacy in a mouse model. Antimicrob Agents Chemother 2021; 65: e02476-20. 10.1128/AAC.02476-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Arendrup MC, Chowdhary A, Jorgensen KMet al. Manogepix (APX001A) in vitro activity against Candida auris: head-to-head comparison of EUCAST and CLSI MICs. Antimicrob Agents Chemother 2020; 64: e00656-20. 10.1128/AAC.00656-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Wiederhold NP, Najvar LK, Jaramillo Ret al. The novel arylamidine T-2307 demonstrates in vitro and in vivo activity against Candida auris. Antimicrob Agents Chemother 2020; 64: e02198-19. 10.1128/AAC.02198-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Fioriti S, Brescini L, Pallotta Fet al. Antifungal combinations against Candida species: from bench to bedside. J Fungi (Basel) 2022; 8:1077. 10.3390/jof8101077 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.