Abstract
Low back pain (LBP) is a common symptom that can occur in all ages. It is the first common cause of disability globally and is associated with over 60 million disability-adjusted life-years in a single year.
Motor control exercise (MCE) has obtained increasing attention in treating LBP. However, the findings from distinct meta-analyses differed and some even reached controversial conclusions. More importantly, how MCE improves LBP-related symptoms remains unclear.
The primary aim of this study is to describe the possible improvement mechanisms of MCE on LBP from brain, biochemistry, inflammatory, and neuromuscular aspects. The secondary aim is to further conclude its effectiveness and clinical application. Further understanding of mechanisms and effectiveness could be instructive for future LBP treatments and provide more information for clinicians when making prescriptions.
MCE is effective in alleviating pain and disability among patients with acute and chronic LBP. Notably, the evidence for acute LBP is relatively low-quality and limited.
MCE might be more effective for patients with specific LBP characteristics, especially those with pre-diagnosis of impaired transversus abdominis recruitment, intermediate pain intensity, and longer MCE training duration.
MCE could remap brain representation and reverse negative brain alternation, induce exercise-induced hypoalgesia, mediate anti-inflammatory response, retain normal activation, and improve morphological deficits.
Keywords: low back pain, motor control exercise, mechanism, effect
Introduction
Low back pain (LBP) is a highly prevalent disease affecting people of all ages. LBP is the first common cause of disability globally, and it is associated with over 60 million disability-adjusted life-years in 2015 (1). It is defined as pain located between the lower rib margins and the buttock creases with or without leg pain or other neuropathic symptoms in the lower extremities (2). Considering that the specific pathology causing LBP in approximately all patients cannot be accurately diagnosed, the percentage of non-specific LBP is large (about 90%). Nearly everyone will experience an acute LBP during their lifetime. Moreover, acute LBP will normally disappear within 1 year, and some will be persistent with low-to-moderate intensity and transform into chronic LBP (CLBP). Persistent LBP may not be a simple symptom but a disease with a biopsychosocial injury model (3) because patients with CLBP generally suffer from impaired physical and mental function, low quality of life, and work incapacity (4). Thus, LBP is a large societal and economic burden, which results in direct medical costs and relatively higher indirect costs. Especially, CLBP makes up 20% of all LBP, but its costs account for 80% of the direct costs (5).
Physical therapies are recommended for patients with LBP according to several guidelines (6, 7, 8, 9). Motor control exercise (MCE) has been obtaining increasing attention in recent years (5). MCE is defined as an exercise to increase control and coordination of the spine and pelvis (10). Normally, MCE increases the weak deep trunk muscles, such as transversus abdominis and multifidus, and reduces the overactive large external trunk muscles, such as rectus abdominal and erector spinae muscles (11). Multiple systematic reviews and meta-analyses have explored the positive effectiveness of MCE on LBP patients, such as improvement in pain and disability. However, the findings from distinct meta-analyses differed due to various inclusion criteria, such as the differences in participants, interventions, comparisons, and outcomes. Meanwhile, some studies reached several controversial results, which remained to be verified (11, 12, 13). Furthermore, as LBP is a complicated disease with many potential contributors, the underlying mechanisms of how MCE improves these functions have rarely been reported.
The primary aim of this study is to describe the possible improvement mechanisms of MCE on LBP from brain, biochemistry, inflammatory, and neuromuscular aspects. The secondary aim is to further conclude its effectiveness and clinical application. Further understanding of mechanisms and effectiveness could be instructive for future LBP treatments and provide more information for clinicians when making prescriptions.
Clinical effectiveness
To better summarize the clinical effectiveness of MCE on LBP, we searched the systematic reviews and meta-analyses exploring the effect of MCE on pain and disability among patients with LBP in the past 10 years. Detailed information including population, sample size, and searching period is provided in Table 1, and the comparisons and outcomes such as standard mean difference or mean difference were concluded in Table 2. Meanwhile, the quality of evidence was assessed using Assessment of Multiple Systematic Reviews (AMSTAR), ranging from 0 to 11. The quality was determined as low, moderate, and high when the AMSTAR score was less than 4, from 4 to 7, and over 7, respectively.
Table 1.
Quality, population description, population definition and search period of studies included in the systematic reviews and meta-analyses.
| Source | Quality (AMSTAR) | Population | Population definition | Search period | |||
|---|---|---|---|---|---|---|---|
| Score | Quality | Pain type | Sample size, n | Studies, n | Pain duration | ||
| Owen et al. (5) | 11/11 | High | Chronic LBP | 5578 | 89 RCTs | ≥12 weeks | Inception to 05/2019 |
| Bernard et al. (25) | 9/11 | High | Chronic LBP | 200 | 6 RCTs | >12 weeks | Inception to 0/10/2018 |
| Byström et al. (13) | 6/11 | Moderate | Chronic LBP | 1768 | 16 RCTs | >12 weeks | Inception to 10/2011 |
| Gomes-Neto et al. (24) | 7/11 | Moderate | Chronic LBP | 895 | 11 RCTs | > 3 months + no leg pain | Inception to 11/2014 |
| Luomajoki et al. (18) | 11/11 | High | LBP | 781 | 11 RCTs | Chronic LBP: > 3 months; subacute LBP: within 3–12 weeks | Inception to 04/2017 |
| Macedo et al. (14) | 11/11 | High | Acute LBP | 197 | 3 RCTs | <6 weeks | Inception to 04/2015 |
| Niederer et al. (23) | 6/11 | Moderate | Chronic LBP | 2391 | 18 RCTs | NA | Not applicable |
| Niederer & Mueller (19) | 8/11 | High | Chronic LBP | 1081 | 10 trials | >6 weeks | Inception to 1/10/2018 |
| Saragiotto et al. (10) | 11/11 | High | Chronic LBP | 2431 | 29 RCTs | >12 weeks | Inception to 04/2015 |
| Smith et al. (21) | 7/11 | Moderate | LBP | NA | 29 RCTs | Pain or stiffness between lower rib and buttock crease (studies with specific pathology were excluded) | 10/2006 to 10/2013 |
| Wang et al. (12) | 8/11 | High | Chronic LBP | 414 | 5 RCTs | >3 months | 1970 to 10/2011 |
| Zhang et al. (22) | 8/11 | High | Chronic LBP | 1333 | 18 RCTs | >12 weeks | Inception to 08/2020 |
| Hayden et al. (17) | 10/11 | High | Chronic LBP | 20,969 | 217 RCTs | >12 weeks | Not applicable |
| Searle et al. (20) | 8/11 | High | Chronic LBP | 4462 | 45 RCTs | Pain and discomfort localized below the costal margin and above the inferior gluteal folds lasting more than 3 months | Inception to 30/10/2014 |
| Zhang et al. (72) | 7/11 | Moderate | Chronic LBP | 950 | 18 RCTs | Physician-diagnosed for more than 3 months | Inception to 01/05/2021 |
AMSTAR, Assessment of multiple sytematic reviews; RCT, randomized controlled trial; LBP, low back pain.
Table 2.
Systematic reviews and meta-analyses investigating the effect of MCE on low back pain.
| Source/intervention vs control | Outcomes | Result, MD (95% CI) | Interpretations | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Scale | Duration | Sample size | Group number | Effect | P1 | P2 | Heterogeneity | I2 | Quality of evidence | Effect size | Other | ||
| Owen et al. (5) | |||||||||||||
| Stabilization vs no treatment | |||||||||||||
| Pain | 1062 | 39 | −1.31 (−1.75 to −0.87)* | Favors stabilization | <0.001 | SUCRAs of 80% | |||||||
| Disability | 1062 | 39 | −1.13 (−1.53 to −0.74)* | Favors stabilization | <0.001 | SUCRAs of 80% | |||||||
| Mental health | 1062 | 39 | −0.78 (−1.49 to −0.07)* | Favors stabilization | 0.031 | SUCRAs of 50% | |||||||
| Bernard et al. (25) | |||||||||||||
| PFMT + exercise vs exercise | |||||||||||||
| Pain | VAS | 157 | 4 | −0.61 (−0.91 to −0.31) | Favors MCE | <0.0001 | Low | 0% | Very low | ||||
| Disability | ODI | 157 | 4 | −0.87 (−3.21 to 1.46) | Comparable | 0.46 | High | 77% | |||||
| Byström et al. (13) | |||||||||||||
| MCE vs general exercise | |||||||||||||
| Pain | 0–100 | 6–16 wk | 529 | 6 | −7.80 (−10.95 to −4.65) | Favors MCE | |||||||
| Pain | 0–100 | 4–8 mo | 523 | 3 | −6.06 (−10.94 to −1.18) | Favors MCE | |||||||
| Pain | 0–100 | 8–15 months | 632 | 4 | −3.10 (−7.03 to 0.83) | Comparable | |||||||
| Disability | 0–100 | 6–16 wk | 480 | 5 | −4.65 (−6.20 to −3.11) | Favors MCE | |||||||
| Disability | 0–100 | 4–8 mo | 523 | 3 | −4.86 (−8.59 to −1.13) | Favors MCE | |||||||
| Disability | 0–100 | 8–15 mo | 523 | 3 | −4.72 (−8.81 to −0.63) | Favors MCE | |||||||
| MCE vs spinal manual therapy | |||||||||||||
| Pain | 0–100 | 6–16 wk | 633 | 3 | −1.32 (−6.30 to 3.65) | Comparable | |||||||
| Pain | 0–100 | 4–8 mo | 586 | 2 | −2.31 (−7.85 to 3.22) | Comparable | |||||||
| Pain | 0–100 | 8–15 mo | 633 | 3 | −0.43 (−5.47 to 4.62) | Comparable | |||||||
| Disability | 0–100 | 6–16 wk | 633 | 3 | −6.12 (−11.94 to −0.30) | Favors MCE | |||||||
| Disability | 0–100 | 4–8 mo | 586 | 2 | −5.27 (−9.52 to −1.01) | Favors MCE | |||||||
| Disability | 0–100 | 8–15 mo | 633 | 3 | −5.76 (−9.21 to −2.32) | Favors MCE | |||||||
| MCE vs minimal intervention | |||||||||||||
| Pain | 0–100 | 6–16 wk | 500 | 2 | −12.48 (−19.04 to −5.93) | Favors MCE | |||||||
| Pain | 0–100 | 4–8 mo | 500 | 2 | −10.18 (−16.64 to −3.72) | Favors MCE | |||||||
| Pain | 0–100 | 8–15 mo | 500 | 2 | −13.32 (−19.75 to −6.90) | Favors MCE | |||||||
| Disability | 0–100 | 6–16 wk | 541 | 3 | −9.00 (−15.28 to −2.73) | Favors MCE | |||||||
| Disability | 0–100 | 4–8 mo | 500 | 2 | −5.62 (−10.46 to −0.77) | Favors MCE | |||||||
| Disability | 0–100 | 8–15 mo | 500 | 2 | −6.64 (−11.72 to −1.57) | Favors MCE | |||||||
| MCE vs multimodal PT | |||||||||||||
| Pain | 0–100 | 4–8 mo | 499 | 4 | −14.20 (−21.23 to −7.16) | Favors MCE | |||||||
| Disability | 0–100 | 4–8 mo | 656 | 2 | −12.98 (−19.49 to −6.47) | Favors MCE | |||||||
| Gomes-Neto et al. (24) | |||||||||||||
| STE vs general exercise | |||||||||||||
| Pain | 0–10 | 603 | 8 | −1.03 (−1.79 to −0.27) | Favors stabilization | 0.008 | <0.00001 | High | 86% | ||||
| Disability | ODI | 209 | 5 | −5.41 (−8.34 to −2.49) | Favors stabilization | 0.0003 | 0.0002 | High | 82% | ||||
| Disability | RMDQ | 310 | 2 | −0.75 (−2.26 to 0.75) | Comparable effect | 0.33 | 0.95 | Low | 0% | ||||
| STE vs manual therapy | |||||||||||||
| Pain | 0–10 | 358 | 3 | −0.38 (−0.98 to 0.22) | Comparable effect | 0.22 | 0.75 | Low | 0% | ||||
| Disability | 358 | 3 | −0.17 (−0.38 to 0.03)* | Comparable effect | 0.10 | 0.58 | Low | 0% | |||||
| Luomajoki et al. (18) | |||||||||||||
| MCE vs control intervention | |||||||||||||
| Pain | Post-treatment | 541 | 9 | −0.39 (−0.73 to −0.04)* | Favors MCE | 0.03 | 0.23 | Low | 29%, | ||||
| Pain | 12-mo FU | 366 | 5 | −0.27 (−0.62 to 0.09)* | Comparable effect | 0.14 | 0.02 | High | 75% | ||||
| Disability | Post-treatment | 642 | 11 | −0.38 (−0.68 to −0.09)* | Favors MCE | 0.01 | 0.47 | Low | 0% | Small | |||
| Disability | 12-mo FU | 425 | 6 | −0.37 (−0.69 to −0.04)* | Favors MCE | 0.03 | 0.09 | Moderate | 58% | Small | |||
| Macedo et al. (14) | |||||||||||||
| MCE vs other exercises | |||||||||||||
| Pain | 0–100 | 0–3 mo | 82 | 2 | 5.74 (−3.34 to 14.82) | Comparable effect | 0.22 | 0.93 | Low | 0% | Moderate | ||
| Pain | 0–100 | 3–12 mo | 33 | 1 | −1.20 (−18.24 to 15.84) | Comparable effect | 0.89 | Low | |||||
| Disability | 0–100 | 0–3 mo | 116 | 2 | −0.84 (−8.72 to 7.04) | Comparable effect | 0.83 | 0.28 | Low | 15% | Moderate | ||
| Disability | 0–100 | 3–12 mo | 33 | 1 | −6.70 (−22.80 to 9.40) | Comparable effect | 0.41 | Low | |||||
| Disability | 0–100 | > 12 mo | 83 | 1 | 5.70 (−1.38 to 12.78) | Comparable effect | 0.11 | Low | |||||
| MCE vs manual therapy | |||||||||||||
| Pain | 0–100 | 0–3 mo | 58 | 1 | 9.0 (−1.56 to 19.56) | Comparable effect | 0.095 | Low | |||||
| Disability | 0–100 | 0–3 mo | 85 | 1 | 4.0 (−3.38 to 11.38) | Comparable effect | 0.29 | Low | |||||
| Disability | 0–100 | >12 mo | 85 | 1 | 3.70 (−4.10 to 11.50) | Comparable effect | 0.35 | Low | |||||
| MCE + MM vs MM | |||||||||||||
| Pain | 0–100 | 0–3 mo | 41 | 1 | −9.3 (−20.41 to 1.81) | Comparable effect | 0.10 | Very low | |||||
| Disability | 0–100 | 0–3 mo | 41 | 1 | −2.4 (−4.87 to 0.07) | Comparable effect | 0.057 | Very low | |||||
| Niederer et al. (23) | |||||||||||||
| MCE vs no additional exercise | |||||||||||||
| Current pain | 3 wk | 2137 | 17 | −0.15 (−0.28 to −0.02)* | Favors MCE | 0.03 | 0.02 | Moderate | 47% | Moderate | Larger | ||
| Current pain | 6 wk | 1875 | 18 | −0.15 (−0.24 to −0.06)* | Favors MCE | 0.002 | 0.70 | Low | 0% | Moderate | Larger | ||
| Current pain | 6 mo | 1692 | 16 | −0.19 (−0.28 to −0.09)* | Favors MCE | 0.0002 | 0.55 | Low | 0% | High | Larger | ||
| Characteristic pain | 3 wk | 2137 | 17 | −0.19 (−0.35 to −0.04)* | Favors MCE | 0.01 | 0.0005 | High | 61% | Moderate | Larger | ||
| Characteristic pain | 6 wk | 1835 | 17 | −0.26 (−0.38 to −0.14)* | Favors MCE | < 0.0001 | 0.15 | Low | 27% | High | Larger | ||
| Characteristic pain | 6 mo | 1692 | 16 | −0.25 (−0.38 to −0.11)* | Favors MCE | 0.0003 | 0.07 | Moderate | 36% | Moderate | Larger | ||
| Disability | 3 wk | 2137 | 17 | −0.24 (−0.33 to −0.15)* | Favors MCE | <0.00001 | 0.44 | Low | 1% | High | Larger | ||
| Disability | 6 wk | 1835 | 17 | −0.27 (−0.40 to −0.13)* | Favors MCE | <0.0001 | 0.04 | Moderate | 41% | High | Larger | ||
| Disability | 6 mo | 1692 | 16 | −0.25 (−0.39 to −0.11)* | Favors MCE | 0.0004 | 0.04 | Moderate | 41% | Moderate | Larger | ||
| Niederer & Mueller (19) | |||||||||||||
| MCE vs control intervention | |||||||||||||
| Pain | 1210 | 13 | −0.46 (−0.78 to −0.14)* | Favors MCE | 0.004 | <0.00001 | High | 86% | Low- to-moderate | ||||
| Disability | 7311 | 12 | −0.44 (−0.80 to −0.09)* | Favors MCE | 0.01 | <0.00001 | High | 88% | Low- to-moderate | ||||
| Saragiotto et al. (10) | |||||||||||||
| MCE vs other exercises | |||||||||||||
| Pain | 0–100 | 0–3 mo | 872 | 13 | −7.53 (−10.54 to −4.52) | Favors MCE | <0.00001 | 0.05 | Moderate | 43% | Small | No clinical importance | |
| Pain | 0–100 | 3–12 mo | 588 | 6 | −2.98 (−6.96 to 0.99) | Comparable effect | 0.14 | 0.90 | Low | 0% | High | ||
| Pain | 0–100 | >12 mo | 643 | 5 | −2.69 (−6.90 to 1.53) | Comparable effect | 0.21 | 0.76 | Low | 0% | High | ||
| Disability | 0–100 | 0–3 mo | 794 | 11 | −4.82 (−6.95 to −2.68) | Favors MCE | <0.00001 | 0.03 | Moderate | 50% | Low quality | Small | No clinical importance |
| Disability | 0–100 | 3–12 mo | 588 | 6 | −2.88 (−6.92 to 1.15) | Comparable effect | 0.16 | 0.58 | Low | 0% | High | ||
| Disability | 0–100 | >12 mo | 570 | 4 | −0.71 (−4.87 to 3.45) | Comparable effect | 0.74 | 0.81 | Low | 0% | High | ||
| Mental health | 0–100 | 0–3 mo | 269 | 2 | −0.75 (−3.33 to 1.83) | Comparable effect | 0.57 | 0.62 | Low | 0% | Moderate | ||
| MCE vs manual therapy | |||||||||||||
| Pain | 0–100 | 0–3 mo | 282 | 3 | −4.36 (−9.52 to 0.81) | Comparable effect | 0.098 | 0.55 | Low | 0% | Moderate | ||
| Pain | 0–100 | 3–12 mo | 485 | 4 | −7.05 (−14.20 to 0.11) | Comparable effect | 0.054 | 0.08 | Moderate | 55% | Moderate | ||
| Pain | 0–100 | >12 mo | 406 | 4 | −3.67 (−9.28 to 1.94) | Comparable effect | 0.20 | 0.64 | Low | 0% | High | ||
| Disability | 0–100 | 0–3 mo | 282 | 3 | −2.79 (−6.60 to 1.02) | Comparable effect | 0.15 | 0.69 | Low | 0% | Moderate | ||
| Disability | 0–100 | 3–12 mo | 485 | 4 | −3.28 (−6.97 to 0.40) | Comparable effect | 0.08 | 0.41 | Low | 0% | High | ||
| Disability | 0–100 | >12 mo | 406 | 4 | −3.40 (−7.87 to 1.07) | Comparable effect | 0.14 | 0.79 | Low | 0% | High | ||
| MCE vs minimal intervention | |||||||||||||
| Pain | 0–100 | 0–3 mo | 291 | 4 | −10.01 (−15.67 to −4.35) | Favors MCE | 0.00053 | 0.19 | Moderate | 37% | Moderate | Medium | Clinically important |
| Pain | 0–100 | 3–12 mo | 348 | 4 | −12.61 (−20.53 to −4.69) | Favors MCE | 0.0018 | 0.08 | Moderate | 56% | Low | Medium | Clinically important |
| Pain | 0–100 | >12 mo | 279 | 3 | −12.97 (−18.51 to −7.42) | Favors MCE | <0.00001 | 0.95 | Low | 0%, | Moderate- | Medium | Clinically important |
| Disability | 0–100 | 0–3 mo | 332 | 5 | −8.63 (−14.78 to −2.47) | Favors MCE | 0.0060 | 0.004 | High | 74% | Very low | Small | No clinical importance |
| Disability | 0–100 | 3–12 mo | 348 | 4 | −5.47 (−9.17 to −1.77) | Favors MCE | 0.0038 | 0.25 | Low | 28% | Moderate | Small | No clinical importance |
| Disability | 0–100 | >12 mo | 279 | 3 | −5.96 (−9.81 to −2.11) | Favors MCE | 0.0024 | 0.39 | Low | 0% | Moderate | Small | No clinical importance |
| MCE vs exercise + EP agents | |||||||||||||
| Pain | 0–100 | 0–3 mo | 68 | 2 | −30.18 (−35.32 to −25.05) | Favors MCE | <0.00001 | 0.44 | Low | 0% | Low | Large | Clinically important |
| Pain | 0–100 | 3–12 mo | 179 | 2 | −19.39 (−36.83 to −1.96) | Favors MCE | 0.029 | 0.029 | High | 95% | Very low | Small | No clinical importance |
| Smith et al. (21) | |||||||||||||
| MCE vs control intervention | |||||||||||||
| Pain | 0–100 | 0–3 mo | −7.93 (−11.74 to −4.12) | Favors MCE† | High | 67% | High | No clinical importance | |||||
| Pain | 0–100 | 3–12 mo | −6.10 (−10.54 to −1.65) | Favors MCE† | Moderate | 50% | High | No clinical importance | |||||
| Pain | 0–100 | > 12 months | −6.39 (−10.14 to −2.65) | Favors MCE† | Moderate | 45% | High | No clinical importance | |||||
| Disability | 0–100 | 0–3 mo | −3.61 (−6.53 to −0.70) | Favors MCE† | High | 83% | High | No clinical importance | |||||
| Disability | 0–100 | 3–12 months | −2.31 (−5.85 to 1.23) | Comparable effect | High | 65% | |||||||
| Disability | 0–100 | > 12 mo | −3.92 (−7.25 to −0.59) | Favors MCE† | Moderate | 56% | High | No clinical importance | |||||
| Wang et al. (12) | |||||||||||||
| MCE vs general exercise | |||||||||||||
| Pain | 0–10 | 0–3 mo | 290 | 4 | −1.29 (−2.47 to −0.11) | Favors MCE | 0.03 | 0.0002 | High | 85% | |||
| Pain | 0–10 | >12 mo | 284 | 3 | −0.32 (−0.87 to 0.23) | Comparable effect | 0.25 | 0.60 | Low | 0% | |||
| Disability | ODI | 0–3 mo | 256 | 4 | −7.14 (−11.64 to 2.65) | Favors MCE | 0.002 | 0.0007 | High | 82% | |||
| Zhang et al. (22) | |||||||||||||
| MCE vs other treatments | |||||||||||||
| Pain | Post-treatment | 1489 | 20 | −0.43 (−0.66 to −0.20)* | Favors MCE | 0.0003 | <0.00001 | High | 76% | Very low | Small | ||
| Pain | 6-mo FU | 826 | 6 | −0.18 (−0.32 to −0.04)* | Favors MCE | 0.01 | 0.32 | Low | 14% | Moderate | Small | ||
| Pain | 12-mo FU | 802 | 7 | −0.08 (−0.22 to 0.06)* | Comparable effect | 0.25 | 0.69 | Low | 0% | High | |||
| Pain | 24-mo FU | 802 | 7 | −0.25 (−0.61 to 0.11)* | Comparable effect | 0.18 | 0.26 | Low | 20% | Low | |||
| Disability | Post-treatment | 1437 | 19 | −0.39 (−0.64 to −0.13)* | Favors MCE | 0.003 | <0.00001 | High | 80% | Very low | Small | ||
| Disability | 6-mo FU | 826 | 6 | −0.13 (−0.26 to 0.01)* | Comparable effect | 0.08 | 0.43 | Low | 0% | High | |||
| Disability | 12-mo FU | 820 | 6 | −0.13 (−0.27 to 0.01)* | Comparable effect | 0.07 | 0.98 | Low | 0% | High | |||
| Disability | 24-mo FU | 126 | 2 | −0.11 (−0.47 to 0.24)* | Comparable effect | 0.53 | 0.60 | Low | 0% | Very low | |||
| Hayden et al. (17) | |||||||||||||
| CS vs minimal treatment | |||||||||||||
| Pain | 0–100 | 2476 | 61 | −13.4 (−17.2 to −9.6) | Favors CS; | No clinical importance | |||||||
| Disability | 0–100 | 2320 | 56 | −6.6 (−9.0 to −4.3) | Favors CS; | No clinical importance | |||||||
| Searle et al. (20) | |||||||||||||
| STE vs other interventions | |||||||||||||
| Pain | 1343 | 12 | −0.47 (−0.77 to −0.18)* | Favors stabilization† | < 0.01 | High | 83.2% | Small | |||||
| Zhang et al. (72) | |||||||||||||
| CST vs control intervention | |||||||||||||
| Pain | 144 | 4 | −0.89 (−1.94 to 0.16)* | Comparable effect | 0.1 | High | 88% | ||||||
P1 values in bold indicate significant effect. P1 indicates difference of outcomes and P2 indicates differences in heterogeneity between intervention and control groups.
*Values are standard mean difference (95% CI); †indicates significant effect.
CS, core strengthening; CST, core strength training; EP, electrophysical; MCE, motor control exercise;MM, medical management; mo, months; ODI, Oswestry Disability Index; PFMT, Pelvic floor muscles training; PT, physical therapy; RMDQ, Roland-Morris Disability Questionnaire; STE, stabilization exercise; SUCRA, surface under cumulative ranking; VAS, visual analogue scale; wk, weeks.
For patients with acute LBP, low-to-moderate quality of evidence indicated that MCE was as effective as spinal manipulative therapy or other types of exercise, and no additional benefit of MCE combined with medical management over medical management alone was found (11, 14). Meanwhile, the safety of MCE has been demonstrated by minor or no adverse events reported in clinical trials (10, 12). Notably, the additional MCE showed less than 64% risk of 1-year recurrence than medical management alone (risk ratio 0.36, 95% CI 0.18 to 0.72, P = 0.004). This finding indicated that applying MCE in the early phase of LBP could be a potential method to prevent the transition from acute to CLBP (15). The early application of MCE is feasible possibly because MCE includes cognitive awareness and isolated activation of deep trunk muscles during the initial stages of LBP, which less irritate pain (16). Nevertheless, the quality of evidence was limited due to inconsistency and small sample sizes.
In patients with CLBP, most results indicated that MCE provided patients with reduced pain intensity and improved physical function compared with no treatment or minimal intervention at short, intermediate, and long terms (5, 10, 13, 17). The MCE can effectively relieve pain by 26 points using visual analog scale and improve disability by 14% points using Oswestry Disability Index, which was clinically significant (5). Furthermore, low-to-high quality of evidence supported the superiority of MCE over control intervention in LBP and disability improvement (12, 13, 18, 19, 20, 21, 22). In particular, the short-term effect of MCE may be more stable and significant than that in the long term (12, 18, 21, 22). Notably, although the effect size of these superiorities varied from small to large, of which some were not clinically important, the positive effects of MCE were consistent. Meanwhile, a prospective meta-analysis indicated that MCE could relieve current pain and characteristic pain intensity for patients with CLBP, and the effectiveness was sustainable for a long term based on low-to-moderate heterogeneity (23). Moreover, meta-analyses with moderate-to-high quality demonstrated that MCE was as effective as manual therapy for LBP-related symptoms, and the results were highly consistent (10, 24), whereas one study reported further effectiveness of MCE on disability (13). Only one research discovered the effectiveness of MCE on mental health compared to no treatment, which indicates its positive effect on mental health, with a surface under the cumulative ranking of 50% (5).
In the comparison between MCE and general exercise, the superiority of MCE has also been demonstrated in pain and disability reduction for patients with CLBP (10, 13, 24). A similar finding indicated that the long-term effect may be not as significant as the short-term effect (10, 13). In addition, MCE was more effective than aerobic and stretching exercises in pain relief and more helpful than stretching in disability improvement based on a network meta-analysis (17). A high-quality study focusing on the additional effect of pelvic floor muscle training (PFMT) indicated that patients undergoing exercise plus PFMT reported reduced LBP and similar dysfunction compared with those receiving single exercise (25). Considering its evident effectiveness, MCE ranks as the second and first option for treating pain and disability, respectively, with SUCRA of 80% (5). As for mental health, a moderate-quality evidence with low heterogeneity concluded that MCE was as effective as other exercises (10).
Weaken deep core muscle is one of the typical symptoms of LBP patients. A regular MCE training program has been proven to significantly improve the recruitment of transversus abdominis (26). MCE could demonstrate better pain-relieving effectiveness, especially in patients with impaired transversus abdominis activation. Therefore, a pre-diagnose through ultrasonography examining the recruitment of transversus abdominis might provide clues for clinicians to make MCE prescriptions (26). Moreover, patients with the intermediate intensity of LBP (2–2.5/11) could obtain more benefits from MCE than other patients, and the effectiveness of MCE was possibly higher for older patients than the younger ones in patients aged from 35 to 50 (23). Besides, the MCE lasting more than 8 weeks may be more important for pain reduction (25). Therefore, MCE intervention lasting for a longer duration could be recommended for patients with acute, sub-acute, or CLBP, especially those who are older or with intermediate pain intensity.
Mechanisms
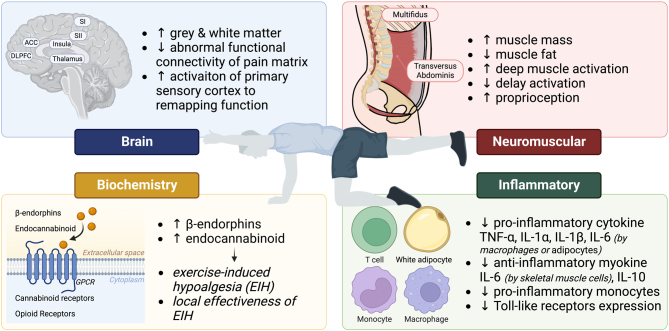
The underlying improvement mechanisms of MCE on LBP from brain, biochemistry, inflammatory, and neuromuscular aspects are illustrated in Fig. 1.
Figure 1.
The underlying improvement mechanisms of MCE on LBP from brain, biochemistry, inflammatory, and neuromuscular aspects. SI, primary somatosensory cortex; SII, secondary somatosensory cortex; DLPFC, dorsolateral prefrontal cortex; TNF-α, tumor necrosis factor alpha; IL-1α, interleukin 1α; IL-1β, interleukin 1β; IL-6, interleukin 6.
Brain
The concept ‘body map’ concluded that every part of the body is represented by a neuron network in the brain, especially in the primary somatosensory cortex (S1) (27, 28). ‘Body map’ is dynamically maintained, as it may expand or contract according to the representation extent of certain body regions. Research has found pain, movement impairment, and neglect of certain body parts could disturb and weaken the representation of S1. Meanwhile, the S1 representation extent is found to be correlated with more pain, even hyperalgesia (29). That is, pain might be a cause and consequence of ‘body map’ alternation simultaneously. To elaborate, peripheral pain in low back regions could trigger the alternation of S1 representation, which lead to further central-related pain. Additionally, pain and fear avoidance could result in the decrease of spinal movement and the neglect of low back areas, which could further weaken the representation, thereby aggravated pain. These two vicious cycles were interactive, and they might account for the transition from acute to chronic. An fMRI research indicated the regions in S1 and primary motor cortex (M1) topographically representing the core muscles were activated during MCE, indicating the remapping function (30). Therefore, MCE could re-activate deep trunk muscle, improve lumbar spine proprioception and increase sensory input which helps to retain the normal representation in corresponding brain regions, therefore breaking the vicious cycle.
In terms of brain structural alternation, the present research has revealed the differences between CLBP patients and normal people (31, 32). Decreased white matter in corpus callosum and internal capsule was found in CLBP patients. Meanwhile, less gray matter in the temporal lobe, dorsolateral prefrontal cortex (DLPFC), insula, and S1 was revealed (32, 33), most of which was related to the pain matrix (34). As a common exercise form to improve muscular strength and size, resistance training (RT) has been reported to reduce self-reported pain in LBP patients (35). Some research has found an association between skeletal muscle hypertrophy and increasing whole white matter volume, as well as the gray matter volume in the right temporal lobe (36). Moreover, RT was proven effective in alleviating white matter atrophy during age degeneration among the elderly (37, 38). Considering similar muscle contraction during MCE, the alleviating mechanism of MCE on LBP might be identical to RT.
As for brain activity, the functional connectivity in medial prefrontal cortex, cingulate cortex, insula, and S1 increased during resting state in CLBP patients compared with that in the normal population, implying the risks of brain overreaction toward innoxious stimuli (32). Meanwhile, increased activity in S1, S2, posterior cingulate cortex, and insula following mechanical pain stimuli was found, indicating an amplifying nociceptive signal within multiple brain regions (32). Increased activation of these regions evidenced an upregulated pain sensitivity pattern among CLBP patients. Exercise has been proven to alter brain activity. A 26-week RT program could decrease the functional connectivity in the anterior cingulate cortex (ACC) during the resting state (38, 39). When coping with painful stimulation, people with regular exercise habits demonstrated a significantly reduced activation in S1, insula, ACC, and DLPFC compared to those without (40). In summary, MCE might be specifically effective for LBP patients as its function in brain remapping. Furthermore, its significance in reversing brain structural maladaptation and altering brain activation mode might also benefit LBP patients, especially chronic ones.
Biochemistry
The possible mechanism of pain reduction is exercise-induced hypoalgesia (EIH) induced by MCE. EIH is the phenomenon where exercise decreases sensitivity to pain stimuli, indicating hypoalgesia during and following exercise (41). The mechanisms of EIH were not clearly revealed, but some possible ones have been proposed (42). First, exercise can increase the emission of β-endorphins that could bind with opiate receptors in peripheral and central regions. Regions with a higher level of opioid receptors were more frequently regulated compared to other regions, such as insula and descending pain inhibitory pathways (ACC, PAG) (43). Given that these regions were responsible for emotion and pain modulation, β-endorphins might be effective in alleviating pain and improving mood. Among patients with CLBP, the β-endorphin level has been proven to elevate after MCE, indicating the analgesia effect of MCE mediated by endogenous opioid system (44). Endocannabinoid (eCB), as a non-opioid substance, was also indispensable in EIH. The analgesic effect was initiated by upregulating the level of eCB, such as N-arachidonylethanolamine and 2-arachidonoylglycerol, in the circulation system, thereby activating CB1 (Type-1 cannabinoid receptors) and regulating CB1 sensitivity within pain-related brain regions and spinal cord (45, 46). The exogenous activation of CB receptors resulted in EIH. Exercises in different movement patterns, including RT, aerobic exercise, and Tai Chi, have been proven effective in modulating eCB (47, 48).
Research that discovered characteristics of EIH has reached some conclusions. For example, the EIH effect could vary based on different exercising parts. Specifically, the exercising body part generates a larger EIH effect than regions which are away from the exercising part (49). Based on this finding, MCE might be considered superior since its ‘local effectiveness’ in inducing hypoalgesia for patients with LBP. More high-quality research is needed to comprehensively explore the EIH effectiveness induced by MCE.
Inflammatory
Large proportion of research has focused on the inflammation pathogenesis of intervertebral disc degeneration, which was a main contributor to LBP (50). The events initiating inflammatory responses could be a genetic predisposition, acute trauma, or chronic overload (51). In a pathological circumstance, nucleus pulposus and annulus fibrosus cells abnormally produce pro-inflammatory molecules, including cytokines TNF-α, IL-1α, IL-1β, and IL-6 (52, 53). These molecules promote extracellular matrix degradation and recruitment of immune cells to the discal tissues (54).
The exercise-induced anti-inflammatory effects have been proposed by many researchers, which could be explained by multiple mechanisms (55, 56). Adipokines, such as TNF-α, have been mentioned previously as key factors for the development of inflammatory in LBP (57). Regular exercise could decrease fat mass through physical consumption and reduce adipokine emission. A moderate exercise program lasting for 6 months was proven to significantly reduce TNF-α levels (58). Besides, skeletal muscle, as an organ with endocrine function, could form an anti-inflammatory environment by releasing anti-inflammatory myokines (IL-6) through muscle contraction. The predominant role of IL-6 acts as an anti-inflammatory myokine by mediating the emergence of IL-1 receptor antagonist and IL-10 in circulation, thereby inhibiting the generation of inflammatory factors (59, 60, 61). In addition, exercise could decrease the expression of Toll-like receptors on macrophages, which accounted for the signaling of pro-inflammatory mediators (62). Meanwhile, decreased pro-inflammatory monocytes (CD14+ and CD16+) and increased regulatory T cells within the circulatory system were found after exercise (56).
Currently, few studies have specifically observed the specific effect of MCE on LBP from an inflammatory aspect. One research investigated the plasma concentration level changes of TNF-α and IL-6 after long-term MCE intervention among patients with LBP (63). Their results indicated TNF-α maintained whereas IL-6 increased after intervention. That is, MCE could not only prevent further generation of inflammatory factors but also contribute to an increase in anti-inflammatory factors, which inhibits the inflammatory process. Additional research is needed to verify the anti-inflammatory mechanisms of MCE.
Neuromuscular
The deep trunk muscles are attached to the thoracolumbar fascia, which can increase the stiffness of the tissue, thereby improving core stability and resisting pressure on joints (64). Normally, these muscles will be activated prior to superficial muscles to maintain core stability during daily activities. During pathological circumstances, they became dysfunctional whereas superficial ones were recruited for obtaining more spinal stability. Subsequently, patients with CLBP exhibited co-activation of agonistic and antagonistic muscles in the superficial layer (65). The emergence of this compensation mode is actually another body strategy to minimize lumbar spine instability. This abnormal activation strategy might be effective in the short term. With regard to the long term, the chronic stiffening of agonists and antagonists is identical with the mechanisms of muscle spasm, which may induce further pain related to spasticity, even neuropathic pain (66). By targeting deep trunk muscles, MCE is sufficient in adjusting incorrect activated mode by improving the strength of weak deep core muscles (67). An adequate stability provided by a strengthened core could be a signal for the brain to redefine an optimal activation pattern, instead of a co-activated and physical-demanding one.
Another feature of CLBP patients with regard to abnormal activation is the delayed response to external perturbations (68), which is a factor for injury as longer reaction time is needed for deep core muscles to be physically engaged. Soft tissues damaged by previous injury might account for the deficits in proprioceptive and nociceptive receptors, which postpones the reflex responses, thereby leading to untimely muscle activation (65). As MCE can decrease motion error and retain normal proprioception (67), repeated training on core muscles effectively stimulates muscle spindles and receptors, thereby improving sensorimotor integration.
In terms of morphology, higher intramuscular fat percentage in multifidus was found among patients with CLBP than that in the healthy population (69). Similarly, the cross-sectional area of multifidus and paraspinal muscles was decreased compared with that of normal people (70). All of the morphological alternations earlier result in impaired strength, inadequate endurance, and progressive vulnerability of deep core muscles. A lasting MCE program could result in a lower proportion of intramuscular adipose tissue (71), along with exercise-induced hypertrophy in low back muscles (67). Therefore, MCE can effectively avoid abnormal compensation, shorten reaction time, increase multi-dimensional sensory input, prevent negative morphological transformation, and reconstruct body postures in the aspect of neuromuscular.
Conclusion
This review summarized the clinical effectiveness and the mechanism of MCE in relieving LBP-related impairments. MCE is effective in alleviating pain and improving disability. Multiple mechanisms from the aspects of brain, biochemistry, inflammatory, and neuromuscular simultaneously contribute to its efficacy. MCE could remap brain representation and reverse the negative brain alternation, induce EIH to relieve pain, mediate anti-inflammatory response, retain normal activation mode, and improve morphological deficits. Although MCE has demonstrated its superiority within multiple exercise therapies for LBP, a deeper understanding is limited but indispensable. This study is instructive for future LBP treatments and provide more information for clinicians when making exercise prescriptions. Additional clinical research that specifically describes the underlying mechanism targeted MCE is needed.
ICMJE conflict of interest statement
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
The authors disclose receipt of financial support from the following for the research, authorship, and/or publication of this article: the Scientific and Technological Research Program of the Shanghai Science and Technology Committee (fund number:19080503100, 21S31902400); the Shanghai Key Lab of Human Performance (Shanghai University of Sport, fund number: 11DZ2261100); Talent Development Fund of Shanghai Municipal (2021081); Shanghai Clinical Research Center for Rehabilitation Medicine (21MC1930200); and Shanghai Youth Science and Technology Sail Project (23YF1444000).
Data availability
The data used to support the findings of this study are available on request from the corresponding author.
Author contribution statement
Y-LZ conceived the review. H-RX and Y-HZ drafted the manuscript and searched the literature to identify eligible trials. H-RX and Y-HZ extracted and analyzed data. All authors approved the final manuscript.
Acknowledgement
The authors acknowledged the use of ‘Biorender’ software to create Fig. 1 in this review.
References
- 1.GBD 2015 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 20163881545–1602. ( 10.1016/S0140-6736(1631678-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dionne CE, Dunn KM, Croft PR, Nachemson AL, Buchbinder R, Walker BF, Wyatt M, Cassidy JD, Rossignol M, Leboeuf-Yde C, et al. A consensus approach toward the standardization of back pain definitions for use in prevalence studies. Spine 20083395–103. ( 10.1097/BRS.0b013e31815e7f94) [DOI] [PubMed] [Google Scholar]
- 3.Vlaeyen JWS Maher CG Wiech K Van Zundert J Meloto CB Diatchenko L Battié MC Goossens M Koes B & Linton SJ. Low back pain. Nature Reviews. Disease Primers 20184 52. ( 10.1038/s41572-018-0052-1) [DOI] [PubMed] [Google Scholar]
- 4.Hartvigsen J, Hancock MJ, Kongsted A, Louw Q, Ferreira ML, Genevay S, Hoy D, Karppinen J, Pransky G, Sieper J, et al. What low back pain is and why we need to pay attention. Lancet 20183912356–2367. ( 10.1016/S0140-6736(1830480-X) [DOI] [PubMed] [Google Scholar]
- 5.Owen PJ Miller CT Mundell NL Verswijveren SJJM Tagliaferri SD Brisby H Bowe SJ & Belavy DL. Which specific modes of exercise training are most effective for treating low back pain? Network meta-analysis. British Journal of Sports Medicine 2020541279–1287. ( 10.1136/bjsports-2019-100886) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bernstein IA Malik Q Carville S & Ward S. Low back pain and sciatica: summary of NICE guidance. BMJ 2017356 i6748. ( 10.1136/bmj.i6748) [DOI] [PubMed] [Google Scholar]
- 7.Qaseem A, Wilt TJ, McLean RM, Forciea MA, Clinical Guidelines Committee of the American College of Physicians. Denberg TD, Barry MJ, Boyd C, Chow RD, Fitterman N, et al. Noninvasive treatments for acute, subacute, and chronic low back pain: a clinical practice guideline from the American College of Physicians. Annals of Internal Medicine 2017166514–530. ( 10.7326/M16-2367) [DOI] [PubMed] [Google Scholar]
- 8.Stochkendahl MJ, Kjaer P, Hartvigsen J, Kongsted A, Aaboe J, Andersen M, Andersen MØ, Fournier G, Højgaard B, Jensen MB, et al. National Clinical Guidelines for non-surgical treatment of patients with recent onset low back pain or lumbar radiculopathy. European Spine Journal 20182760–75. ( 10.1007/s00586-017-5099-2) [DOI] [PubMed] [Google Scholar]
- 9.Peng MS Wang R Wang YZ Chen CC Wang J Liu XC Song G Guo JB Chen PJ & Wang XQ. Efficacy of therapeutic aquatic exercise vs physical therapy modalities for patients with chronic low back pain: a randomized clinical trial. JAMA Network Open 20225 e2142069. ( 10.1001/jamanetworkopen.2021.42069) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saragiotto BT Maher CG Yamato TP Costa LOP Menezes Costa LC Ostelo RWJG & Macedo LG. Motor control exercise for chronic non-specific low-back pain. Cochrane Database of Systematic Reviews 20162016CD012004. ( 10.1002/14651858.CD012004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saragiotto BT Maher CG Yamato TP Costa LOP Costa LCM Ostelo RWJG & Macedo LG. Motor control exercise for nonspecific low back pain: a cochrane review. Spine 2016411284–1295. ( 10.1097/BRS.0000000000001645) [DOI] [PubMed] [Google Scholar]
- 12.Wang XQ, Zheng JJ, Yu ZW, Bi X, Lou SJ, Liu J, Cai B, Hua YH, Wu M, Wei ML, et al. A meta-analysis of core stability exercise versus general exercise for chronic low back pain. PLoS One 20127 e52082. ( 10.1371/journal.pone.0052082) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Byström MG Rasmussen-Barr E & Grooten WJA. Motor control exercises reduces pain and disability in chronic and recurrent low back pain: a meta-analysis. Spine (Phila Pa 1976) 201338E350–E358. ( 10.1097/BRS.0b013e31828435fb) [DOI] [PubMed] [Google Scholar]
- 14.Macedo LG Saragiotto BT Yamato TP Costa LOP Menezes Costa LC Ostelo RWJG & Maher CG. Motor control exercise for acute non-specific low back pain. Cochrane Database of Systematic Reviews 20162CD012085. ( 10.1002/14651858.CD012085) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Navalgund A Buford JA Briggs MS & Givens DL. Trunk muscle reflex amplitudes increased in patients with subacute, recurrent LBP treated with a 10-week stabilization exercise program. Motor Control 2013171–17. ( 10.1123/mcj.17.1.1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Unsgaard-Tøndel M Fladmark AM Salvesen Ø & Vasseljen O. Motor control exercises, sling exercises, and general exercises for patients with chronic low back pain: a randomized controlled trial with 1-year follow-up. Physical Therapy 2010901426–1440. ( 10.2522/ptj.20090421) [DOI] [PubMed] [Google Scholar]
- 17.Hayden JA Ellis J Ogilvie R Stewart SA Bagg MK Stanojevic S Yamato TP & Saragiotto BT. Some types of exercise are more effective than others in people with chronic low back pain: a network meta-analysis. Journal of Physiotherapy 202167252–262. ( 10.1016/j.jphys.2021.09.004) [DOI] [PubMed] [Google Scholar]
- 18.Luomajoki HA Bonet Beltran MB Careddu S & Bauer CM. Effectiveness of movement control exercise on patients with non-specific low back pain and movement control impairment: a systematic review and meta-analysis. Musculoskeletal Science and Practice 2018361–11. ( 10.1016/j.msksp.2018.03.008) [DOI] [PubMed] [Google Scholar]
- 19.Niederer D & Mueller J. Sustainability effects of motor control stabilisation exercises on pain and function in chronic nonspecific low back pain patients: a systematic review with meta-analysis and meta-regression. PLoS One 202015 e0227423. ( 10.1371/journal.pone.0227423) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Searle A Spink M Ho A & Chuter V. Exercise interventions for the treatment of chronic low back pain: a systematic review and meta-analysis of randomised controlled trials. Clinical Rehabilitation 2015291155–1167. ( 10.1177/0269215515570379) [DOI] [PubMed] [Google Scholar]
- 21.Smith BE Littlewood C & May S. An update of stabilisation exercises for low back pain: a systematic review with meta-analysis. BMC Musculoskeletal Disorders 201415 416. ( 10.1186/1471-2474-15-416) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang C Li Y Zhong Y Feng C Zhang Z & Wang C. Effectiveness of motor control exercise on non-specific chronic low back pain, disability and core muscle morphological characteristics: a meta-analysis of randomized controlled trials. European Journal of Physical and Rehabilitation Medicine 202157793–806. ( 10.23736/S1973-9087.21.06555-2) [DOI] [PubMed] [Google Scholar]
- 23.Niederer D, Engel T, Vogt L, Arampatzis A, Banzer W, Beck H, Moreno Catalá M, Brenner-Fliesser M, Güthoff C, Haag T, et al. Motor control stabilisation exercise for patients with non-specific low back pain: a prospective meta-analysis with multilevel meta-regressions on intervention effects. Journal of Clinical Medicine 20209. ( 10.3390/jcm9093058) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gomes-Neto M Lopes JM Conceição CS Araujo A Brasileiro A Sousa C Carvalho VO & Arcanjo FL. Stabilization exercise compared to general exercises or manual therapy for the management of low back pain: a systematic review and meta-analysis. Physical Therapy in Sport 201723136–142. ( 10.1016/j.ptsp.2016.08.004) [DOI] [PubMed] [Google Scholar]
- 25.Bernard S Gentilcore-Saulnier E Massé-Alarie H & Moffet H. Is adding pelvic floor muscle training to an exercise intervention more effective at improving pain in patients with non-specific low back pain? A systematic review of randomized controlled trials. Physiotherapy 202111015–25. ( 10.1016/j.physio.2020.02.005) [DOI] [PubMed] [Google Scholar]
- 26.Ferreira PH Ferreira ML Maher CG Refshauge K Herbert RD & Hodges PW. Changes in recruitment of transversus abdominis correlate with disability in people with chronic low back pain. British Journal of Sports Medicine 2010441166–1172. ( 10.1136/bjsm.2009.061515) [DOI] [PubMed] [Google Scholar]
- 27.Adriaan Louw P Kevin Farrell P Lauren Wettach PTD Justine Uhl PTD Katherine Majkowski PTD & Marcus Welding PTD. Immediate effects of sensory discrimination for chronic low back pain: a case series. New Zealand and Journal of Physiotherapy 201543 58. ( 10.15619/NZJP/43.2.06) [DOI] [Google Scholar]
- 28.Stavrinou ML Della Penna S Pizzella V Torquati K Cianflone F Franciotti R Bezerianos A Romani GL & Rossini PM. Temporal dynamics of plastic changes in human primary somatosensory cortex after finger webbing. Cerebral Cortex 2007172134–2142. ( 10.1093/cercor/bhl120) [DOI] [PubMed] [Google Scholar]
- 29.Marinus J Moseley GL Birklein F Baron R Maihöfner C Kingery WS & van Hilten JJ. Clinical features and pathophysiology of complex regional pain syndrome. Lancet. Neurology 201110637–648. ( 10.1016/S1474-4422(1170106-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim DH Lee JJ & You SJH. Best core stabilization exercise to facilitate subcortical neuroplasticity: a functional MRI neuroimaging study. Technology and Health Care 201826401–407. ( 10.3233/THC-171051) [DOI] [PubMed] [Google Scholar]
- 31.Mansour AR Baliki MN Huang L Torbey S Herrmann KM Schnitzer TJ & Apkarian VA. Brain white matter structural properties predict transition to chronic pain. Pain 20131542160–2168. ( 10.1016/j.pain.2013.06.044) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kregel J Meeus M Malfliet A Dolphens M Danneels L Nijs J & Cagnie B. Structural and functional brain abnormalities in chronic low back pain: a systematic review. Seminars in Arthritis and Rheumatism 201545229–237. ( 10.1016/j.semarthrit.2015.05.002) [DOI] [PubMed] [Google Scholar]
- 33.Baliki MN Schnitzer TJ Bauer WR & Apkarian AV. Brain morphological signatures for chronic pain. PLoS One 20116 e26010. ( 10.1371/journal.pone.0026010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davis KD & Moayedi M. Central mechanisms of pain revealed through functional and structural MRI. Journal of Neuroimmune Pharmacology 20138518–534. ( 10.1007/s11481-012-9386-8) [DOI] [PubMed] [Google Scholar]
- 35.Kristensen J & Franklyn-Miller A. Resistance training in musculoskeletal rehabilitation: a systematic review. British Journal of Sports Medicine 201246719–726. ( 10.1136/bjsm.2010.079376) [DOI] [PubMed] [Google Scholar]
- 36.Kilgour AHM Todd OM & Starr JM. A systematic review of the evidence that brain structure is related to muscle structure and their relationship to brain and muscle function in humans over the lifecourse. BMC Geriatrics 201414 85. ( 10.1186/1471-2318-14-85) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Best JR Chiu BK Liang Hsu C Nagamatsu LS & Liu-Ambrose T. Long-term effects of resistance exercise training on cognition and brain volume in older women: results from a randomized controlled trial. Journal of the International Neuropsychological Society 201521745–756. ( 10.1017/S1355617715000673) [DOI] [PubMed] [Google Scholar]
- 38.Herold F Törpel A Schega L & Müller NG. Functional and/or structural brain changes in response to resistance exercises and resistance training lead to cognitive improvements - a systematic review. European Review of Aging and Physical Activity 201916 10. ( 10.1186/s11556-019-0217-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suo C, Singh MF, Gates N, Wen W, Sachdev P, Brodaty H, Saigal N, Wilson GC, Meiklejohn J, Singh N, et al. Therapeutically relevant structural and functional mechanisms triggered by physical and cognitive exercise. Molecular Psychiatry 2016211633–1642. ( 10.1038/mp.2016.19) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Geisler M Ritter A Herbsleb M Bär KJ & Weiss T. Neural mechanisms of pain processing differ between endurance athletes and nonathletes: a functional connectivity magnetic resonance imaging study. Human Brain Mapping 2021425927–5942. ( 10.1002/hbm.25659) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koltyn KF. Exercise-induced hypoalgesia and intensity of exercise. Sports Medicine 200232477–487. ( 10.2165/00007256-200232080-00001) [DOI] [PubMed] [Google Scholar]
- 42.Wu B Zhou L Chen C Wang J Hu LI & Wang X. Effects of exercise-induced hypoalgesia and its neural mechanisms. Medicine and Science in Sports and Exercise 202254220–231. ( 10.1249/MSS.0000000000002781) [DOI] [PubMed] [Google Scholar]
- 43.Scheef L, Jankowski J, Daamen M, Weyer G, Klingenberg M, Renner J, Mueckter S, Schürmann B, Musshoff F, Wagner M, et al. An fMRI study on the acute effects of exercise on pain processing in trained athletes. Pain 20121531702–1714. ( 10.1016/j.pain.2012.05.008) [DOI] [PubMed] [Google Scholar]
- 44.Paungmali A Joseph LH Punturee K Sitilertpisan P Pirunsan U & Uthaikhup S. Immediate effects of core stabilization exercise on β-endorphin and cortisol levels among patients with chronic nonspecific low back pain: a randomized crossover design. Journal of Manipulative and Physiological Therapeutics 201841181–188. ( 10.1016/j.jmpt.2018.01.002) [DOI] [PubMed] [Google Scholar]
- 45.Tantimonaco M Ceci R Sabatini S Catani MV Rossi A Gasperi V & Maccarrone M. Physical activity and the endocannabinoid system: an overview. Cellular and Molecular Life Sciences 2014712681–2698. ( 10.1007/s00018-014-1575-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koltyn KF Brellenthin AG Cook DB Sehgal N & Hillard C. Mechanisms of exercise-induced hypoalgesia. Journal of Pain 2014151294–1304. ( 10.1016/j.jpain.2014.09.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Watkins BA. Endocannabinoids, exercise, pain, and a path to health with aging. Molecular Aspects of Medicine 20186468–78. ( 10.1016/j.mam.2018.10.001) [DOI] [PubMed] [Google Scholar]
- 48.Schönke M Martinez-Tellez B & Rensen PC. Role of the endocannabinoid system in the regulation of the skeletal muscle response to exercise. Current Opinion in Pharmacology 20205252–60. ( 10.1016/j.coph.2020.05.003) [DOI] [PubMed] [Google Scholar]
- 49.Vaegter HB Handberg G & Graven-Nielsen T. Similarities between exercise-induced hypoalgesia and conditioned pain modulation in humans. Pain 2014155158–167. ( 10.1016/j.pain.2013.09.023) [DOI] [PubMed] [Google Scholar]
- 50.Chou D Samartzis D Bellabarba C Patel A Luk KDK Kisser JMS & Skelly AC. Degenerative magnetic resonance imaging changes in patients with chronic low back pain: a systematic review. Spine 201136(21)(Supplement) S43–S53. ( 10.1097/BRS.0b013e31822ef700) [DOI] [PubMed] [Google Scholar]
- 51.Johnson ZI Schoepflin ZR Choi H Shapiro IM & Risbud MV. Disc in flames: roles of TNF-α and IL-1β in intervertebral disc degeneration. European Cells and Materials 201530104–116. ( 10.22203/ecm.v030a08) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Séguin CA Pilliar RM Roughley PJ & Kandel RA. Tumor necrosis factor-alpha modulates matrix production and catabolism in nucleus pulposus tissue. Spine (Phila Pa 1976) 2005301940–1948. ( 10.1097/01.brs.0000176188.40263.f9) [DOI] [PubMed] [Google Scholar]
- 53.Le Maitre CL Hoyland JA & Freemont AJ. Catabolic cytokine expression in degenerate and herniated human intervertebral discs: il-1beta and TNFalpha expression profile. Arthritis Research and Therapy 20079 R77. ( 10.1186/ar2275) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Risbud MV & Shapiro IM. Role of cytokines in intervertebral disc degeneration: pain and disc content. Nature Reviews. Rheumatology 20141044–56. ( 10.1038/nrrheum.2013.160) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pedersen BK. Exercise-induced myokines and their role in chronic diseases. Brain, Behavior, and Immunity 201125811–816. ( 10.1016/j.bbi.2011.02.010) [DOI] [PubMed] [Google Scholar]
- 56.Gleeson M. Anti-inflammatory effects of exercise. Obesity, Inflammation and Cancer. Springer; 2013. pp. 401–424. ( ) [Google Scholar]
- 57.Ouchi N Parker JL Lugus JJ & Walsh K. Adipokines in inflammation and metabolic disease. Nature Reviews. Immunology 20111185–97. ( 10.1038/nri2921) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Santos RVT Viana VAR Boscolo RA Marques VG Santana MG Lira FS Tufik S & de Mello MT. Moderate exercise training modulates cytokine profile and sleep in elderly people. Cytokine 201260731–735. ( 10.1016/j.cyto.2012.07.028) [DOI] [PubMed] [Google Scholar]
- 59.Opal SM & DePalo VA. Anti-inflammatory cytokines. Chest 20001171162–1172. ( 10.1378/chest.117.4.1162) [DOI] [PubMed] [Google Scholar]
- 60.Saavedra Ramírez PG Vásquez Duque GM & González Naranjo LA. Interleucina-6:¿ amiga o enemiga? Bases para comprender su utilidad como objetivo terapéutico. Iatreia 201124157–166. ( 10.17533/udea.iatreia.9600) [DOI] [Google Scholar]
- 61.Petersen AMW & Pedersen BK. The anti-inflammatory effect of exercise. Journal of Applied Physiology 2005981154–1162. ( 10.1152/japplphysiol.00164.2004) [DOI] [PubMed] [Google Scholar]
- 62.Gleeson M McFarlin B & Flynn M. Exercise and toll-like receptors. Exercise Immunology Review 20061234–53. [PubMed] [Google Scholar]
- 63.Minobes-Molina E Nogués MR Giralt M Casajuana C de Souza DLB Jerez-Roig J & Romeu M. Effectiveness of specific stabilization exercise compared with traditional trunk exercise in women with non-specific low back pain: a pilot randomized controlled trial. PeerJ 20208 e10304. ( 10.7717/peerj.10304) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gordon R & Bloxham S. A systematic review of the effects of exercise and physical activity on non-specific chronic low back pain. Healthcare (Basel) 20164. ( 10.3390/healthcare4020022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Radebold A Cholewicki J Panjabi MM & Patel TC. Muscle response pattern to sudden trunk loading in healthy individuals and in patients with chronic low back pain. Spine 200025947–954. ( 10.1097/00007632-200004150-00009) [DOI] [PubMed] [Google Scholar]
- 66.Costigan M Scholz J & Woolf CJ. Neuropathic pain: a maladaptive response of the nervous system to damage. Annual Review of Neuroscience 2009321–32. ( 10.1146/annurev.neuro.051508.135531) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hlaing SS Puntumetakul R Khine EE & Boucaut R. Effects of core stabilization exercise and strengthening exercise on proprioception, balance, muscle thickness and pain related outcomes in patients with subacute nonspecific low back pain: a randomized controlled trial. BMC Musculoskeletal Disorders 202122 998. ( 10.1186/s12891-021-04858-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hodges PW & Richardson CA. Delayed postural contraction of transversus abdominis in low back pain associated with movement of the lower limb. Journal of Spinal Disorders 19981146–56. ( 10.1097/00002517-199802000-00008) [DOI] [PubMed] [Google Scholar]
- 69.Mengiardi B Schmid MR Boos N Pfirrmann CWA Brunner F Elfering A & Hodler J. Fat content of lumbar paraspinal muscles in patients with chronic low back pain and in asymptomatic volunteers: quantification with MR spectroscopy. Radiology 2006240786–792. ( 10.1148/radiol.2403050820) [DOI] [PubMed] [Google Scholar]
- 70.Fortin M & Macedo LG. Multifidus and paraspinal muscle group cross-sectional areas of patients with low back pain and control patients: a systematic review with a focus on blinding. Physical Therapy 201393873–888. ( 10.2522/ptj.20120457) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hebert JJ Le Cara EC Koppenhaver SL Hoffman MD Marcus RL Dempsey AR & Albert WJ. Predictors of clinical success with stabilization exercise are associated with lower levels of lumbar multifidus intramuscular adipose tissue in patients with low back pain. Disability and Rehabilitation 202042679–684. ( 10.1080/09638288.2018.1506510) [DOI] [PubMed] [Google Scholar]
- 72.Zhang SK Yang Y Gu ML Mao SJ & Zhou WS.. Effects of Low Back Pain Exercises on Pain Symptoms and Activities of Daily Living: A Systematic Review and Meta-Analysis. Perceptual and Motor Skills 2022 129 63-89. ( 10.1177/00315125211059407) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available on request from the corresponding author.



 This work is licensed under a
This work is licensed under a