Abstract
‘Non-inflammatory’ pain, pain that is not associated with measures of inflammation, is common in patients with inflammatory arthritis including RA. One important cause of non-inflammatory pain is concomitant fibromyalgia. Systematic review has shown that fibromyalgia is common in inflammatory arthritis including RA affecting 1 in 5 patients and is associated with higher disease activity scores due to inflated tender joint count and patient global assessment. Consequently, many patients with RA and concomitant fibromyalgia may fail to reach treatment target and switch to alternate disease modifying drugs frequently. European Alliance of Association for Rheumatology has highlighted that concomitant fibromyalgia is an important consideration in assessing difficult-to-treat RA. The incidence and prevalence of fibromyalgia are higher in RA than the general population, raising the possibility that fibromyalgia may be ‘secondary’ to RA rather than a concomitant disease. The precise mechanisms whereby patients with RA develop fibromyalgia are unknown. In this review, we discussed fibromyalgia in RA, its clinical impact and epidemiology as well as data suggesting fibromyalgia might be ‘secondary’. Lastly, we reviewed potential pathogenic mechanisms which included inflammatory cytokines sensitizing nociceptive neurones, temporal summation, also known as windup, from chronic pain and impaired coping from poor quality sleep and mental well-being. Deciphering the exact mechanisms may lead to treatment strategies that prevent development of secondary fibromyalgia and will address a common factor associated with difficult-to-treat RA.
Keywords: RA, fibromyalgia, cytokines, central sensitisation, temporal summation, chronic pain
Rheumatology key messages.
Fibromyalgia may be secondary to rheumatoid arthritis.
Development of fibromyalgia in RA may be secondary to peripheral and/or central sensitisation of the nervous system through the effect pro-inflammatory cytokines, chronic pain stimulation or impairment of pain control from disrupted sleep and diminished mental well-being.
Introduction
Pain is one of the most common symptoms in rheumatic diseases and ranked by patients and clinicians as being the most important [1]. Pain and fatigue that are associated with physical and psychological wellbeing are unmet medical needs in RA [2]. Many patients complain of pain even in clinical and ultrasound remission or low disease activity (LDA). It leads to the concept of ‘non-inflammatory pain’ and one significant contributor is concomitant fibromyalgia [3, 4]. The primary aim of this review focuses on the impact of fibromyalgia in patients with inflammatory arthritis. Secondary aims are discussing whether fibromyalgia is secondary to inflammatory arthritis or a concomitant condition, and if so, what mechanisms are involved.
In a US survey, Radawski et al. (2019) reported that 26% of patients with RA were satisfied with treatment while 74% was unsatisfied [2]. The symptoms of RA with the greatest percentages of patients reporting moderate-to-severe impact on their life were fatigue (82%), pain (76%) and physical well-being (75%) [2]. In another study, those patients with high pain, fatigue and sleep disturbance scores as assessed by the Rheumatoid Arthritis Impact of Disease (RAID) questionnaire often failed multiple biologic DMARDs (bDMARDs) and were considered difficult-to-treat patients [5]. This paper suggested that in near-remission RA patients, the high patient global assessment score reflected both physical and psychological aspects of disease impact in RA [5]. Patients would benefit from tailored interventions such as physiotherapy, analgesia, cognitive behavioural therapy, antidepressants and education.
Is fibromyalgia a concomitant condition or secondary to inflammatory arthritis (i.e. secondary fibromyalgia)?
Fibromyalgia is common and is characterised by chronic pain, fatigue, depression, anxiety and poor non-restorative sleep [3]. Heidari et al. (2017) conducted a systematic review of 65 papers among 3 609 810 subjects from the general population and specific groups [6]. They reported the prevalence of fibromyalgia within the general population was 1.78% (95% CI: 1.65, 1.92). For patients attending a rheumatology department, the prevalence was significantly higher at 15.2% (95% CI: 13.6, 16.90) [6]. This is supported by a systematic review by Duffield et al. (2018) of concomitant fibromyalgia in patients with inflammatory arthritis, which found the prevalence was 21% in RA, 13% in ankylosing spondylitis and 18% in psoriatic arthritis [4]. Therefore, the prevalence of fibromyalgia in RA is higher than the general population, suggesting fibromyalgia in RA may not be a concomitant condition. Similarly, Collin et al. reported an annual incidence rate of fibromyalgia in the UK general population between 2001–2013 to be 32–38/100 000 patients, which is equivalent to 0.032–0.038/100 patient-year [7]. In comparison, in an inception cohort study of patients with RA followed up from the time to diagnosis, Lee et al. (2013) reported a cumulative incidence rate of 6.77 (95% CI 5.19, 8.64) per 100 person-years during the first 12 months and 3.58 (95% CI 1.86, 6.17) per 100 person-years during the period of 12–24 months with regard to the development of fibromyalgia [8]. This was the first study that noted the high incidence rate during the first 12 months of diagnosis showing that the development of chronic non-inflammatory, central pain happened early and supported the notion of ‘secondary fibromyalgia’. It also showed that objective measures such as CRP, ESR and swollen joint count did not predict development of fibromyalgia [8]. However, anti-CCP antibody positivity was the only variable inversely associated with the diagnosis of fibromyalgia [8]. Patients who use corticosteroids were significantly associated with the clinical diagnosis of fibromyalgia [8].
Impact of fibromyalgia on disease activity assessment in RA
Historically, physicians and patients’ perspectives were thought to differ with regard to assessment of disease activity in RA [9]. Physician perception is driven by joint swelling and laboratory tests while patients are concerned by symptoms such as pain. Turk et al. (2018) in an interesting study found that a third of patients with RA disagreed with their physician with regard to being in remission [9]. Patients who did not agree with their physicians showed less improvement on questions associated with emotional well-being from the RAID questionnaire, including pain and sleep [9]. This could have an impact on patient satisfaction, the relationship between physician and patient, discordance with clinical response and remission definitions [9]. They noted the importance of shared care and decision making by increasing patient involvement in their own healthcare decisions [9].
Hammer et al. (2018) reported that pain catastrophising was strongly associated with patient reported outcomes and composite measures [10]. This manifested in discrepancies between patient and physician global assessment scoring. Patients with concomitant fibromyalgia have higher Patient Global Assessment (PtGA), fatigue and anxiety [10]. PtGA in patients with RA in remission and near-remission was driven by pain, fatigue, anxiety and function rather than by indicators of active disease [10]. Furthermore, patients are likely to be concerned with regard to treatment toxicity, current pain and fears but physicians tend to be long-term goal driven with regard to joint damage and disability.
Joharatnam et al. (2015) concluded that high prevalence of fibromyalgia features, poorer mental health and higher patient-reported DAS 28 (DAS28) scores are linked in patients who live with established RA [11]. The team noted high sensitivity at non-joint points such as the anterior tibia and sternum indicating central mechanisms could contribute to pain sensitivity in RA even when inflammation might be under control [11].
A systematic review based on 19 studies concluded that fibromyalgia was significantly associated with higher disease activity score with all but one study reporting higher DAS28 in participants with comorbid fibromyalgia [3]. In 16 of these studies, statistically significant increase in the DAS in RA patients with comorbid fibromyalgia compared with those without was noted but not with clinical (swollen joint count) or laboratory (ESR, CRP) markers of disease activity. It concluded that fibromyalgia could lead to inflated disease activity measures and resulting in inappropriate escalation or stopping of treatment in the underlying index rheumatic condition [3]. This review highlights the limitations of using disease activity indices alone in assessing inflammatory activity in rheumatic patients with concomitant fibromyalgia. It is therefore important that these scores are interpreted in conjunction with knowledge of the presence of concomitant fibromyalgia to ensure optimal management and appropriate drug treatment. Indeed, the European Alliance of Associations for Rheumatology (EULAR) definition of difficult-to-treat RA suggested that concomitant non-inflammatory diseases – for example, fibromyalgia – might mimic inflammatory activity [12]. Therefore, patients with non-inflammatory conditions such as fibromyalgia may present as having difficult-to-treat RA, which is associated with a reduction in quality of life and higher clinical burden [12].
There are several studies of central pain in RA using the PainDetect Questionnaire [13–15], which is a screening tool for neuropathic pain. Neuropathic pain is defined as ‘pain caused by a lesion or disease of the somatosensory nervous system’ [16]. Although RA patients did not have lesions or disease of the somatosensory nervous system, many met the criteria for neuropathic pain by PainDetect [13–15]. Many of these RA patients with ‘neuropathic-like’ pain met the criteria for fibromyalgia, reporting tender points and worse mental and physical health when measured by the 36-item Short-Form health survey [13]. RA patients with neuropathic-like pain reflect central sensitisation, therefore non-inflammatory pain.
Mechanisms of non-inflammatory pain/fibromyalgia in inflammatory arthritis
Cytokines and non-inflammatory pain
Inflammation is characterised by chemotaxis with leucocyte that migrates from circulation into a local site. Leucocyte activation by cytokines such as TNF-α, IL-1β and IL-6 increases the prostaglandins (PG) production of eicosanoids, prostaglandins and TxA2 via the arachidonic acid pathways, which stimulates nociceptive neurons and initiates pain transmission. These cytokines can also cause persistent hyperalgesia by sensitising nociceptive sensory neurons; mainly the C and some of the Aδ-fibres for mechanical stimuli [17]. This is also known as peripheral sensitisation. Schaible reviewed the effects of proinflammatory cytokines, IL-1β, IL-6, IL-17 and TNF-α on cultured isolated sensory neurons and concluded that they may directly activate second messenger systems regulating to a change in the excitability, thus modifying ion currents, and regulate molecules involved in nociception [17]. Indeed, injecting cytokines in the normal tissue of animals will evoke pain and increase responsiveness of nociceptive sensory fibres [17]. The pro-inflammatory cytokines activate both Aδ- and C-fibres to mechanical stimulation, which can be reduced by neutralisation of these cytokines in antigen-induced arthritis. However, the effect of certain cytokines such as TNF-α are easily reversed but less so with IL-6, which suggested that different cytokines have different potency in inducing ‘sustained’ pain in RA patients.
In CIA, Inglis et al. (2007) were the first to report hyperalgesia using standard behavioural techniques [18]. It was observed that in the later stages of CIA (after 10 days from the onset of arthritis), thermal and mechanical hyperalgesia decreased due to the possible lack of pain mediators and increased level of disability [18]. They noted the critical mechanisms could be caused by astrocyte and microglia activation proven by increased activated astrocyte numbers seen in the lumbar spinal cord. They went on to show that celecoxib, a COX-2 inhibitor, had positive effects against mechanical and thermal hyperalgesia while anti-TNF therapy decreased activated astrocyte numbers, thus reducing neuropathic pain [18].
Bazzichi et al. (2007) reported that levels of plasma IL-10, IL-8 and of TNF-α were elevated in their group of patients who suffered with fibromyalgia [19]. The level of IL-6 correlated with pain [19]. Because cytokines have been reported to cross the blood-brain barrier, they may promote pain involving the sympathetic nervous system and hypothalamic-pituitary axis; as well as fatigue and depression [20]. This study suggested pro-inflammatory cytokines may be associated with the development of fibromyalgia. Raoof et al. (2018) discussed in their paper how some RA patients continue to experience pain after systemic anti-TNF-α treatment, but this may be explained by systemically administered antibodies not crossing the blood brain barrier and therefore ineffective in blocking spinal TNF-α [21]. Indeed, spinal TNF-α neutralisation is more effective in treating arthritis pain than when administered systemically [21].
Cytokine inhibition by IL-6 inhibition in RA has been associated with reduction in non-inflammatory pain. In a post-hoc analysis of clinical trial data, non-inflammatory pain was common at baseline, and the percentage of patient with non-inflammatory pain after treatment was lower after sarilumab than with adalimumab treatment [22]. Janus kinase inhibitors (JAKi) are also shown to help alleviate non-inflammatory pain. Fautrel et al. (2019) in a post-hoc analysis of a clinical trial of baricitinib (JAK1/JAK2 selective inhibitor) vs placebo or adalimumab showed that baricitinib resulted in improvements in pain, physical function, fatigue and work productivity/impairment in patients, independent of change in DAS28 [23]. A path-analysis showed that the reduction in pain by baricitinib could not be fully explained by reduction in inflammation, as measured by ESR, CRP and swollen joint count, suggesting baricitinib may have a direct effect on pain relief [23].
Simon et al. (2021) reviewed the biologic mechanism that may support these observations. It has been established that persistent afferent pain input can lead to dysregulation and synaptic changes in neurons within the central nervous system [24]. The Janus kinase (JAK)-signal transducer and activator of transcription (STAT) pathway is an intracellular signal transduction pathway whereby pro and anti-inflammatory cytokines alongside locally destructive enzymes are produced [25]. Multiple cytokine receptors, such as IL-6R, IL-1R and IL-10R and IFN-γR, are expressed on afferent nociceptors, and cytokines acting at these receptors have been implicated in pain modulation [24]. JAKis are competitive inhibitors of adenosine triphosphate, thereby prevent phosphorylation and activation of JAK preventing STAT downstream signalling [26, 27]. Simon et al. (2021) also reviewed mechanisms whereby JAK promoting nociceptive transmission in the spinal cord and descending modulatory pathways providing potential biologic mechanisms whereby JAKi may have direct effects on nocioception [24].
In addition to the effect on pain, Wittenberg et al. (2020) discussed in a systematic review suggesting that anti-IL-6 therapy reduced depression in RA [28]. Their main findings were depressive symptoms in patients with inflammatory disorders treated with an immunomodulatory drug usually causes a significant improvement, especially in the highly depressive patient subgroup [28]. However, it was unclear whether this is due to reduced disease activity, which inevitably would improve quality of life and mood [28].
Chronic pain may cause non-inflammatory pain: temporal summation/windup
Baranauskas and Nistri in 1998 described the concept of central sensitisation through ‘windup’ within the spinal cord in which repeated stimulation at constant strength of dorsal root afferents including nociceptive C fibres invoked an increase in the number of action potentials generated by interneurons and motoneurones [29]. The phenomenon was coined as ‘temporal summation’ or ‘action potential windup’ [29]. This phenomenon may occur in patients with RA especially if disease is inadequately controlled, leading to constant stimulation of nociceptive sensory neurones. Therefore, chronic pain may lead to more severe pain. If the hypothesis is correct, then disease duration and active disease will predict development of fibromyalgia [29]. A cross-sectional study showed people with RA had reduced pressure pain thresholds (PPTs) at the scapula and were associated with higher pain scores and patient-reported DAS28 components, as well as poorer mental health [30]. Many of these patients fulfilled the diagnostic criteria for fibromyalgia [30]. A statistically significant correlation was found between reduced PPTs and disease duration in a multi-variate analysis. However, Lee et al. did not find any association between disease activity and fibromyalgia in RA [31]. They postulated the possibility of a different type of pain pathway. Conventionally, it was thought that ascending nociceptive stimuli facilitate temporal summation while descending inhibitory pain pathways reduce pain including conditioned pain modulation. However, among patients who showed high inflammatory disease activity, peripheral inflammation activates the descending analgesic pain mechanisms, which could serve as an endogenous conditioning stimulus. Therefore, conditioned pain modulation impairments in certain patients may be related with elevations in disease activity measures [31]. Meanwhile, heightened conditioned pain modulation may be associated with decreases in disease activity measures in another group of RA patients. Lee et al. also suggested further longitudinal assessment of conditioned pain modulation before and after onset of inflammation would be useful in disentangling these relationships [31].
Reduced mental wellbeing and fibromyalgia in RA
Depression, anxiety and poor sleep quality are common symptoms in RA and fibromyalgia [3, 11]. They are implicated in the pathophysiology of fibromyalgia and strongly associated with chronic pain through their role in central pain processing.
Central pain processing
Over the past two decades, functional neuroimaging has advanced our knowledge and understanding of the processing of pain in the brain. Pain is not only a sensation. It is different to touch not only by the magnitude of the stimulus, but it is interpreted as being ‘unpleasant.’ In 2020, the International Association for the Study of Pain defined pain as: ‘an unpleasant sensory and emotional experience associated with, or resembling that associated with, actual or potential tissue damage’ [32]. It added that pain is always a personal experience that is influenced to varying degrees by biological, psychological and social factors. Importantly, pain and nociception are different [32]. Pain cannot be inferred solely from activity in sensory neurons. In this framework, the processing of pain in the central nervous system includes the location or source of pain, intensity, stress and emotive response, interpretation (cognition) and control (inhibition) [33]. Regions of the brain associated with processing of these different processes have been studied and identified through functional neuroimaging which include the somatosensory cortex, inferior parietal lobe, insula, putamen cingulate cortex, secondary somatosensory cortex (SII), thalamus, precuneus, amygdala, hippocampus, superior temporal gyrus, cerebellum, periaqueductal gray and ventromedial medulla [33]. In fibromyalgia, Gracely et al. (2002) first showed that comparable subjectively painful conditions resulted in activation patterns that were similar in patients and controls, although in fibromyalgia, activation occurred at lower pressure whereas similar pressures support the hypothesis that fibromyalgia is characterized by augmentation of pain processing in the brain [34].
Mental wellbeing, poor sleep quality and impaired pain control
Mental health including anxiety and depression is also strongly linked to poor sleep quality [35]. Non-refreshed sleep is a common symptom in fibromyalgia [36]. Data from polysomnography in patients with fibromyalgia found abnormal α-rhythms, reduced short-wave sleep, wakefulness during non-REM (rapid eye movement) and shortened deep sleep [37–41]. Fattinger et al. (2017) showed the importance of deep sleep mainly in restoration processes, learning and memory consolidation [42]. Initially, sleep disturbance was considered a consequence of pain. More recent evidence suggests poor sleep quality is a risk factor for developing fibromyalgia [43].
Several epidemiological studies have showed that in a healthy population, poor sleep quality was a risk factor for development of chronic widespread pain progression [44, 45]. Mork et al. (2012) reported that sleep disturbance was associated with increased risk of developing fibromyalgia [44]. McBeth et al. (2014) showed that widespread pain is common in older adults and non-restorative sleep is an independent risk factor after adjusting for psychosocial factors [45]. Sleep deprivation in healthy individuals can induce myalgia and fatigue and that interruption of slow-wave sleep (stage 4 sleep) and deprivation could cause mechanical hyperalgesia in multiple sites of the body in healthy individuals in experimental studies [46, 47]. Onen et al. (2005) showed that sleep discontinuity and disturbances would impair the diffuse noxious inhibitory controls that help with pain control and coping mechanisms [48]. Choy (2015) hypothesised that pain and sleep disturbance may have a bidirectional relationship, in which chronic pain may impair sleep and poor sleep quality leads to reduced coping with pain. A vicious circle may then lead to the development of fibromyalgia [43]. In RA, Lee et al. (2018) showed that patients with RA have psychological distress that was significantly associated with pain threshold at all sites. Importantly, they also demonstrated a good correlation between psychological distress and sleep (r = 0.65) [49]. There is a suggestion of a defect in central pain processing and distress when there are sleep issues. Nicassio et al. (1992) discussed how their analysis and data suggested that pain may aggravate sleeping difficulties in RA patients, thus contributing to depression over time [50].
Summary
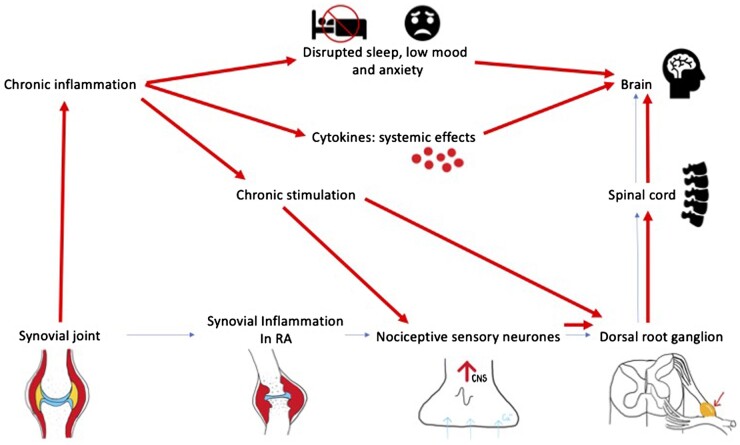
The concept of ‘secondary fibromyalgia’ in RA may be correct although definitive evidence in deciphering the precise mechanism may lead to a prevention-based treatment strategy that could reduce an area of major unmet need associated with difficult-to-treat RA. There are several potential mechanisms: inflammation causing sensitisation of nociceptive neurones, chronic stimulation of nociceptive neurons (temporary summation), impaired coping and pain inhibiting mechanisms through sleep disturbances, anxiety and depression (Fig. 1). Longitudinal studies from time of diagnosis assessing concomitant fibromyalgia, mental wellbeing, sleep quality and pain processing including quantitative sensory testing over non-inflamed sites will help to establish the temporal relationship between inflammation, mental wellbeing, pain processing and the risk of developing fibromyalgia.
Figure 1.
Potential mechanisms for the development of secondary fibromyalgia in RA. Thin arrows indicate normal nociceptive pathways caused by acute inflammation. Thick arrows indicate pathways leading to fibromyalgia
Contributor Information
Dhivya Das, Consultant Rheumatologist, Northern Care Alliance NHS Foundation Trust, University School of Medicine (Formerly with Cardiff), Cardiff, UK.
Ernest Choy, CREATE Centre, Section of Rheumatology, Division of Infection and Immunity, Cardiff University, Cardiff, UK.
Data availability
No new data were generated or analysed in this review.
Funding
No direct funding has been received for this work. The CREATE Centre was established by grant awards from Versus Arthritis and Health and Care Research Wales (20016).
Disclosure statement: E.C. has received research grants and/or served as a member of advisory boards and speaker bureaus of Abbvie, Amgen, Bio-Cancer, Biogen, Bristol Myer Squibbs, Celgene, Chugai Pharma, Eli Lilly, Fresenius Kai, Gilead, Galapagos, Inmedix, Janssen, Novartis, ObsEva, Pfizer, Regeneron, Roche, R-Pharm, Sanofi, SynAct Pharma, and UCB. D.D. has no conflict of interest.
References
- 1. Bartlett SJ, Hewlett S, Bingham CO 3rd. et al. Identifying core domains to assess flare in rheumatoid arthritis: an OMERACT international patient and provider combined Delphi consensus. Ann Rheum Dis 2012;71:1855–60. [DOI] [PubMed] [Google Scholar]
- 2. Radawski C, Genovese MC, Hauber B. et al. Patient perceptions of unmet medical need in rheumatoid arthritis: a cross-sectional survey in the USA. Rheumatol Ther 2019;6:461–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wolfe F, Smythe HA, Yunus MB. et al. The American College of Rheumatology 1990 criteria for the classification of fibromyalgia. Report of the multicenter criteria committee. Arthritis Rheum 1990;33:160–72. [DOI] [PubMed] [Google Scholar]
- 4. Duffield SJ, Miller N, Zhao S, Goodson NJ.. Concomitant fibromyalgia complicating chronic inflammatory arthritis: a systematic review and meta-analysis. Rheumatology 2018;57:1453–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ferreira RJO, Dougados M, Kirwan JR. et al. Drivers of patient global assessment in patients with rheumatoid arthritis who are close to remission: an analysis of 1588 patients. Rheumatology 2017;56:1573–8. [DOI] [PubMed] [Google Scholar]
- 6. Heidari F, Afshari M, Moosazadeh M.. Prevalence of fibromyalgia in general population and patients, a systematic review and meta-analysis. Rheumatol Int 2017;37:1527–39. [DOI] [PubMed] [Google Scholar]
- 7. Collin SM, Bakken IJ, Nazareth I, Crawley E, White PD.. Trends in the incidence of chronic fatigue syndrome and fibromyalgia in the UK, 2001-2013: a Clinical Practice Research Datalink study. J R Soc Med 2017;110:231–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lee YC, Lu B, Boire G. et al. Incidence and predictors of secondary fibromyalgia in an early arthritis cohort. Ann Rheum Dis 2013;72:949–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Turk SA, Rasch LA, van Schaardenburg D. et al. Pain, sleep and emotional well-being explain the lack of agreement between physician- and patient-perceived remission in early rheumatoid arthritis. BMC Rheumatol 2018;2:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hammer HB, Uhlig T, Kvien TK, Lampa J.. Pain catastrophizing, subjective outcomes, and inflammatory assessments including ultrasound: results from a longitudinal study of rheumatoid arthritis patients. Arthritis Care Res 2018;70:703–12. [DOI] [PubMed] [Google Scholar]
- 11. Joharatnam N, McWilliams DF, Wilson D. et al. A cross-sectional study of pain sensitivity, disease-activity assessment, mental health, and fibromyalgia status in rheumatoid arthritis. Arthritis Res Ther 2015;17:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nagy G, Roodenrijs NM, Welsing PM. et al. EULAR definition of difficult-to-treat rheumatoid arthritis. Ann Rheum Dis 2021;80:31–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Koop SM, ten Klooster PM, Vonkeman HE, Steunebrink LM, van de Laar MA.. Neuropathic-like pain features and cross-sectional associations in rheumatoid arthritis. Arthritis Res Ther 2015;17:237237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ahmed S, Magan T, Vargas M, Harrison A, Sofat N.. Use of the painDETECT tool in rheumatoid arthritis suggests neuropathic and sensitization components in pain reporting. J Pain Res 2014;7:579–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rifbjerg-Madsen S, Christensen AW, Boesen M. et al. The course of pain hypersensitivity according to painDETECT in patients with rheumatoid arthritis initiating treatment: results from the prospective FRAME-cohort study. Arthritis Res Ther 2018;20:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.International Association for the Study of Pain. https://www.iasp-pain.org/resources/terminology/#Neuropathicpain.
- 17. Schaible H-G. Nociceptive neurons detect cytokines in arthritis. Arthritis Res Ther 2014;16:470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Inglis JJ, Notley CA, Essex D. et al. Collagen-induced arthritis as a model of hyperalgesia: functional and cellular analysis of the analgesic actions of tumor necrosis factor blockade. Arthritis Rheum 2007;56:4015–23. [DOI] [PubMed] [Google Scholar]
- 19. Bazzichi L, Rossi A, Massimetti G. et al. Cytokine patterns in fibromyalgia and their correlation with clinical manifestations. Clin Exp Rheumatol 2007;25:225–30. [PubMed] [Google Scholar]
- 20. Schiepers OJG, Wichers MC, Maes M.. Cytokines and major depression. Prog Neuropsychopharmacol Biol Psychiatry 2005;29:201–17. [DOI] [PubMed] [Google Scholar]
- 21. Raoof R, Willemen HLDM, Eijkelkamp N.. Divergent roles of immune cells and their mediators in pain. Rheumatology 2018;57:429–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Choy E, Bykerk V, Lee Y. et al. SAT0102 noninflammatory pain is a frequent phenomenon in rheumatoid arthritis and responds well to treatment with sarilumab. Ann Rheum Dis 2020;79:984.1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fautrel B, Kirkham B, Pope JE. et al. Effect of baricitinib and adalimumab in reducing pain and improving function in patients with rheumatoid arthritis in low disease activity: exploratory analyses from RA-BEAM. J Clin Med 2019;8:1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Simon LS, Taylor PC, Choy EH. et al. The JAK/STAT pathway: a focus on pain in rheumatoid arthritis. Semin Arthritis Rheum 2021;51:278–84. [DOI] [PubMed] [Google Scholar]
- 25. Murray PJ. The JAK-STAT signaling pathway: input and output integration. J Immunol 2007;178:2623–9. [DOI] [PubMed] [Google Scholar]
- 26. O'Shea JJ, Schwartz DM, Villarino AV. et al. The JAK-STAT pathway: impact on human disease and therapeutic intervention. Annu Rev Med 2015;66:311–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. O'Shea JJ, Murray PJ.. Cytokine signalling modules in inflammatory responses. Immunity 2008;28:477–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wittenberg GM, Stylianou A, Zhang Y. et al. Effects of immunomodulatory drugs on depressive symptoms: a mega-analysis of randomized, placebo-controlled clinical trials in inflammatory disorders. Mol Psychiatry 2020;25:1275–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Baranauskas G, Nistri A.. Sensitization of pain pathways in the spinal cord: cellular mechanisms. Prog Neurobiol 1998;54:349–65. [DOI] [PubMed] [Google Scholar]
- 30. Pollard LC, Ibrahim F, Choy EH, Scott DL.. Pain thresholds in rheumatoid arthritis: the effect of tender point counts and disease duration. J Rheumatol 2012;39:28–31. [DOI] [PubMed] [Google Scholar]
- 31. Lee YC, Chibnik LB, Lu B. et al. The relationship between disease activity, sleep, psychiatric distress and pain sensitivity in rheumatoid arthritis: a cross-sectional study. Arthritis Res Ther 2009;11:R160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Malik NA. Revised definition of pain by “International Association for the Study of Pain”: concepts, challenges and compromises. Anaesth Pain Intens Care 2020;24:481–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Davis KD, Flor H, Greely HT. et al. Brain imaging tests for chronic pain: medical, legal and ethical issues and recommendations. Nat Rev Neurol 2017;13:624–38. [Internet] [DOI] [PubMed] [Google Scholar]
- 34. Gracely RH, Petzke F, Wolf JM, Clauw DJ.. Functional magnetic resonance imaging evidence of augmented pain processing in fibromyalgia. Arthritis Rheum 2002;46:1333–43. [DOI] [PubMed] [Google Scholar]
- 35. Oh CM, Kim HY, Na HK, Cho KH, Chu MK.. The effect of anxiety and depression on sleep quality of individuals with high risk for insomnia: a population-based study. Front Neurol 2019;10:849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bigatti SM, Hernandez AM, Cronan TA, Rand KL.. Sleep disturbances in fibromyalgia syndrome: relationship to pain and depression. Arthritis Rheum 2008;59:961–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Roizenblatt S, Moldofsky H, Benedito-Silva AA, Tufik S.. Alpha sleep characteristics in fibromyalgia. Arthritis Rheum 2001;44:222–30. [DOI] [PubMed] [Google Scholar]
- 38. Drewes AM, Nielsen KD, Taagholt SJ. et al. Sleep intensity in fibromyalgia: focus on the microstructure of the sleep process. Br J Rheumatol 1995;34:629–35. [DOI] [PubMed] [Google Scholar]
- 39. Horne JA, Shackell BS.. Alpha-like EEG activity in non-REM sleep and the fibromyalgia (fibrositis) syndrome. Electroencephalogr Clin Neurophysiol 1991;79:271–6. [DOI] [PubMed] [Google Scholar]
- 40. Branco J, Atalaia A, Paiva T.. Sleep cycles and alpha-delta sleep in fibromyalgia syndrome. J Rheumatol 1994;21:1113–7. [PubMed] [Google Scholar]
- 41. Burns JW, Crofford LJ, Chervin RD.. Sleep stage dynamics in fibromyalgia patients and controls. Sleep Med 2008;9:689–96. [DOI] [PubMed] [Google Scholar]
- 42. Fattinger S, de Beukelaar TT, Ruddy KL. et al. Deep sleep maintains learning efficiency of the human brain. Nat Commun 2017;8:15405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Choy EH. The role of sleep-in pain and fibromyalgia. Nat Rev Rheumatol 2015;11:513–20. Sep [DOI] [PubMed] [Google Scholar]
- 44. Mork P, Nilsen T.. Sleep problems and risk of fibromyalgia: longitudinal data on an adult female population in Norway. Arthritis Rheumatol 2012;64:281–4. [DOI] [PubMed] [Google Scholar]
- 45. McBeth J, Lacey RJ, Wilkie R.. Predictors of new-onset widespread pain in older adults: results from a population-based prospective cohort study in the UK. Arthritis Rheumatol 2014;66:757–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Moldofsky H, Scarisbrick P.. Induction of neurasthenic musculoskeletal pain syndrome by selective sleep stage deprivation. Psychosom Med 1976;38:35–44. [DOI] [PubMed] [Google Scholar]
- 47. Smith MT, Edwards RR, McCann UD, Haythornthwaite JA.. The effects of sleep deprivation on pain inhibition and spontaneous pain in women. Sleep 2007;30:494–505. [DOI] [PubMed] [Google Scholar]
- 48. Onen SH, Onen F, Courpron P, Dubray C.. How pain and analgesics disturb sleep. Clin J Pain 2005;21:422–31. [DOI] [PubMed] [Google Scholar]
- 49. Lee YC, Bingham CO, Edwards RR. et al. Association between pain sensitization and disease activity in patients with rheumatoid arthritis: a cross-sectional study. Arthritis Care Res 2018;70:197–204. Feb [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Nicassio PM, Wallston KA.. Longitudinal relationships among pain, sleep problems, and depression in rheumatoid arthritis. J Abnorm Psychol 1992;101:514–20. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were generated or analysed in this review.