Abstract
In this review, we summarize the most recent advances in vitamin D cancer research to provide molecular clarity, as well as its translational trajectory across the cancer landscape. Vitamin D is well known for its role in regulating mineral homeostasis; however, vitamin D deficiency has also been linked to the development and progression of a number of cancer types. Recent epigenomic, transcriptomic, and proteomic studies have revealed novel vitamin D-mediated biological mechanisms that regulate cancer cell self-renewal, differentiation, proliferation, transformation, and death. Tumor microenvironmental studies have also revealed dynamic relationships between the immune system and vitamin D’s anti-neoplastic properties. These findings help to explain the large number of population-based studies that show clinicopathological correlations between circulating vitamin D levels and risk of cancer development and death. The majority of evidence suggests that low circulating vitamin D levels are associated with an increased risk of cancers, whereas supplementation alone or in combination with other chemo/immunotherapeutic drugs may improve clinical outcomes even further. These promising results still necessitate further research and development into novel approaches that target vitamin D signaling and metabolic systems to improve cancer outcomes.
Keywords: Osteosarcoma, cancer, tumor, vitamin D, vitamin D deficiency, vitamin D receptor, VDR, EMT, epithelial to mesenchymal transition, breast cancer, prostate cancer, colorectal cancer, osteoblast, metastasis, microenvironment, oxidative stress
Graphical Abstract
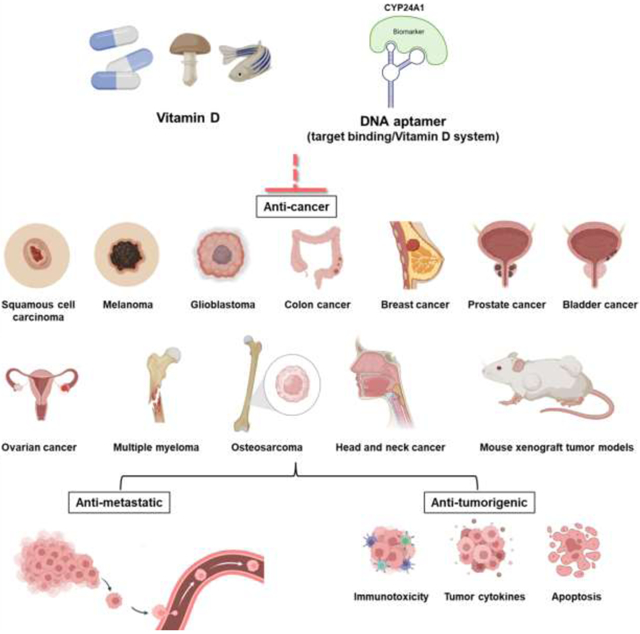
Introduction
Vitamin D deficiency and racial disparities are associated with a deluge of diseases, including cancer, resulting in an escalating burden on the healthcare system1–3. Two primary forms of vitamin D exist: vitamin D2 (ergocalciferol) and vitamin D3 (cholecalciferol)4. Vitamin D3 is generated in the skin of humans in response to UVB radiation, while vitamin D2 is derived from plant sources such as edible (UV-exposed) mushrooms in our meals, albeit with varying concentrations and diminished effectiveness5. Both forms of vitamin D are physiologically inert and must be converted by hydroxylation into 25(OH)D in the liver by vitamin D-25-hydroxylase. 25(OH)D is the primary form of vitamin D in circulation, and its measurement provides information about one’s vitamin D status in the clinical setting6. In general, the normal range for circulating 25(OH)D is 30–50 ng/mL, whereas a deficiency is defined as <20 ng/mL. 25(OH)D is hydroxylated further in the kidneys or within specialized cell types by 25(OH)D-1-OHase (CYP27B1) to create 1-alpha, 25-dihydroxyvitamin D (1,25(OH)2D or calcitriol), the physiologically active form of vitamin D4,7–11. The 1,25(OH)2D effects are mediated by the vitamin D receptor (VDR), a member of the intracellular nuclear receptor superfamily that can induce cell cycle arrest and death through post-transcriptional, post-translational and gene regulatory mechanisms7,12–22.
The recent VITAL supplementation trial demonstrated that vitamin D levels above those required for the maintenance of bone health can increase cancer patient survival, particularly among African-Americans4,23–31. According to the most recent National Health and Nutrition Examination Survey (NHANES), pigmented American Blacks, Hispanics, and Indians are 3.0, 3.0, and 2.8 times more likely to be vitamin D deficient than whites32, which may provide a plausible explanation for vitamin D’s potent impact across racial populations. Furthermore, the results of the VITAL trial have inspired additional research into understanding the mechanisms behind vitamin D’s anticancer effects33. Novel anticancer drugs and therapeutic strategies that target the metabolic and signalling networks of vitamin D and its metabolites are currently being researched34–36. For example, although immune-checkpoint blockade therapy has improved the prognosis of aggressive malignancies, many cancer patients acquire immunological resistance and do not react to these treatments. According to new studies, vitamin D-based combination therapy can overcome the immunological resistance experienced by patients on immunotherapies37–40. On the one hand, vitamin D is a well-known immune system process regulator that targets genes, regulatory transcription factors, and epigenetic modulators to promote anti-inflammatory responses in a variety of immune-related diseases, including cancer (Figure 1A)41–43. It is unknown, however, whether vitamin D has tumor-intrinsic properties that make tumors more immunogenic by influencing novel effector systems44. Given that vitamin D sensitizes cancer immunotherapy, it may interact with these putative effector systems to sensitize tumour cell death, establishing a new paradigm in vitamin D research and addressing racial disparities linked to vitamin D deficiency and increased cancer incidence. In this review, we access the biological literature from the ontological database PubMed to learn about the most recent advances in vitamin D cancer research (Figure 1B). We used NETME45, which extracted biological elements from PubMed to generate network inferring relationships between vitamin D and cancer in approximately 904 articles from 2022–2023, as shown in Table 1, forming the basis of this review.
Figure 1.
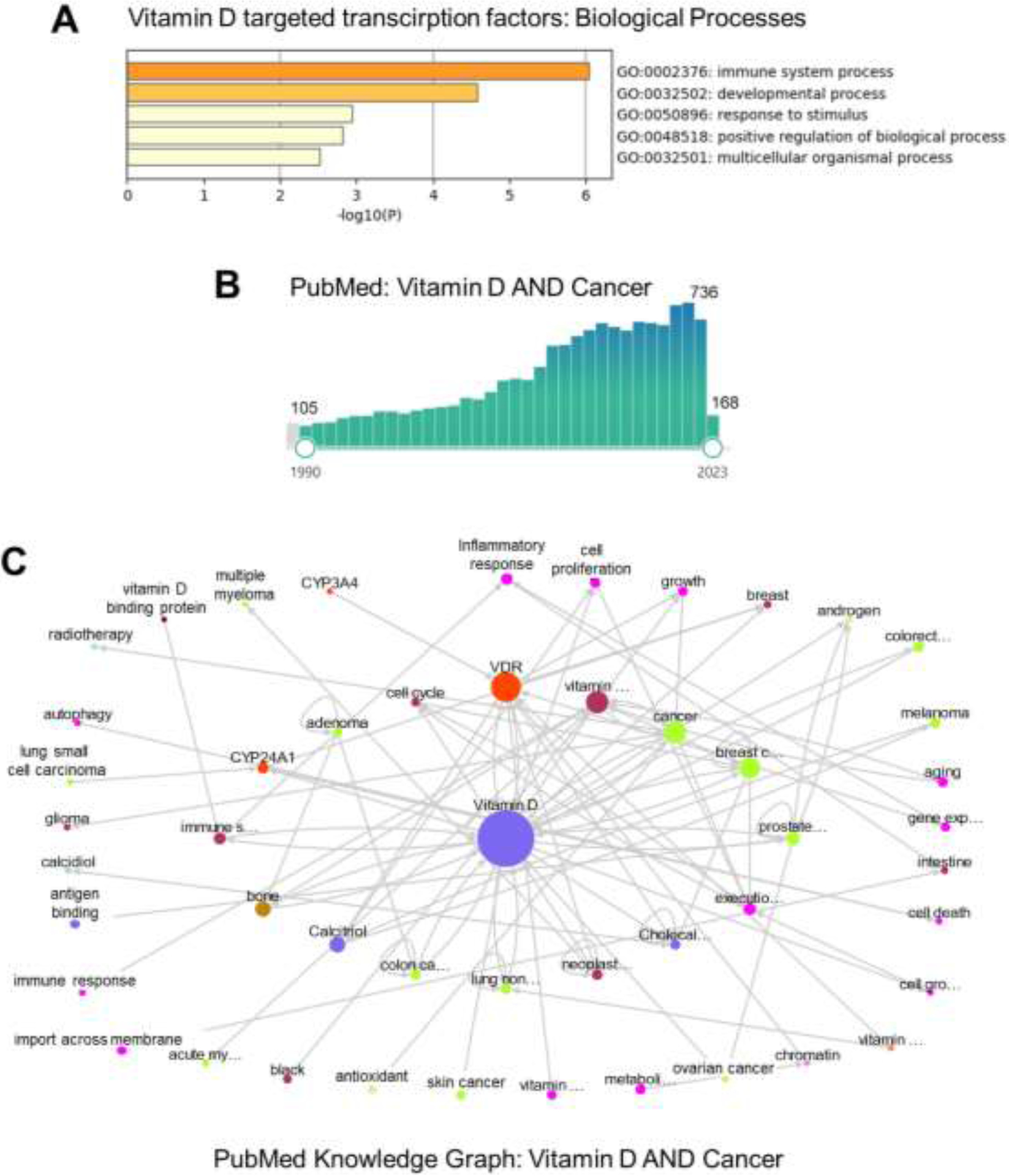
Recent advances in vitamin D cancer research.
A) Top-level Gene Ontology of Biological Processes regulated by vitamin D-targeted transcription factors: BCL6, NFE2, POU4F2, ELF4, IRF5, MAFF, MYCL, NFXL1, TFEC, MAMLD, PPARGC1B, SRA1, ZBTB46 (source Metascape.org)43.
B) A rapid increase in the number of published articles on vitamin D and cancer in PubMed between 1990 and 2023.
C) PubMed knowledge graph was used to assess vitamin D and cancer relationships in approximately 904 of the most recently accessed PubMed articles.
Table 1.
Summary of recent advancements in vitamin D cancer research from PubMed knowledge graph.
| Cancer | Vitamin D system/VDR variants | Effects | References |
|---|---|---|---|
| Breast cancer | 25(OH)D | ■ Deficiency linked to higher grade; younger/obese patients more susceptible. | 47 |
| VDR FokI T(f) variant | ■ Increased risk of developing breast cancer within North Indian women. | 48 | |
| VDR-IGF1R | ■ High VDR and IGF1R expression compounded by low 25(OH) promotes breast cancer growth. | 50 | |
| Anti-estrogens, 1,25(OH)2D, and EB1089 | ■ Activation of VDR signalling promoted ERa expression and anti-estrogen efficacy within triple-negative breast cancers. | 51,52 | |
| CYP24A1 | ■ Suppression of CYP24A1 sensitized breast cancer cells to chemotherapeutic drugs including vitamin D. | 57 | |
| Ovarian cancer | 25(OH)D | ■ Individuals with high serum 25(OH)D levels had a 37% lower risk of ovarian cancer. | 59 |
| Glioblastoma | 1,25(OH)2D, VDR agonist | ■ Induced programmed cell death and cytotoxic autophagy, and inhibited migration. | 61,62 |
| CY24A1 | ■ Acidosis induced CYP24A1 expression in glioblastoma microenvironment that impaired 1,25(OH)2D functions. | 63 | |
| Melanoma | Vitamin D3 | ■ People who regularly took vitamin D3 supplements had a lower risk of developing melanoma. | 109 |
| 25(OH)D | ■ Low serum levels associated with reduced patient survival with or without treatment with BRAF/MEK inhibitors or immunotherapy | 110,111 | |
| 1,25(OH)2D | ■ Induced expression of activated caspases as well as PTEN to promote apoptosis | 112,113 | |
| Multiple myeloma | 25(OH)D | ■ Reduced peripheral neuropathy associated with myeloma. | 65 |
| 1,25(OH)2D, bortezomib | ■ Treatment overcame bortezomib resistance in a myeloma cell line. | 67 | |
| Prostate cancer | VDR ApaI (A/C) variant | ■ Associated with increased clinical stage of prostate cancer in Egyptian men. | 70 |
| 25(OH)D, androgens | ■ Decreased Lrp2 (megalin androgen transporter) expression, correlated with African American men. | 71 | |
| 1,25(OH)2D | ■ Inhibited c-MYC and EMT gene expression; promoted unfolded protein response in prostate cancer cell lines. | 72 | |
| Head and neck squamous cell carcinomas | VDR, 1,25(OH)2D | ■ Poorly differentiated HNSCCs expressed high levels of VDR compounded by low 25(OH)D; suppressed PI3K/Akt/mTOR pathway. | 75 |
| 25(OH)D | ■ Patients with low levels on chemoradiation therapy developed skin dermatitis and mucositis. | 76 | |
| Bladder cancer | 1,25(OH)2D | ■ Improved cisplatin’s efficacy on bladder cancer cell lines. | 79 |
| Osteosarcoma | 1,25(OH)2D, calcipotriol | ■ Suppressed metastasis and tumour growth by impacting NMD, ROS and EMT pathways. | 68,77,83 |
| Colorectal cancer | Vitamin D3 | ■ Higher intake resulted in a 17% lower risk for colorectal cancer localized to the proximal colon in Norwegian women. | 85 |
| VDR, CYP3A4 | ■ Down regulated in colorectal cancer tissue suggesting impaired vitamin D metabolic pathway. | 87 | |
| rs4588*A variant of GC gene | ■ May improve tissue bioavailability of circulating vitamin D, hence protective against colorectal cancer. | 89 | |
| 1,25(OH)2D | ■ Suppressed colorectal cancer stem cells by inducing ferroptosis and downregulating SLC7A11. | 90 | |
| 25(OH)D | ■ Maintenance of adequate levels associated with lower risk of sporadic colorectal cancer. | 61 | |
| 1,25(OH)2D | ■ Activated SIRT1 in HCT 116 and HT-29 colorectal cancer cell lines via auto-deacetylation, resulting in an anti-proliferative response. | 102 | |
| VDR, bile ascites | ■ High-fat diet in mice triggered the gut microbiota to produce metabolites that suppressed inflammation and colitis-associated cancer by activating the VDR signaling pathway. | 103 | |
| VDR/p53 | ■ Both proteins interacted to induce genes that promote peroxisomal fatty acid beta-oxidation in mice as a mechanism by which vitamin D inhibits colorectal cancer. | 104 | |
| Vdr ablation | ■ Resulted in decreased Claudin-10 tight junction expression in the intestinal epithelium, leading to increased permeability, tumor number, and bacterial infiltration. | 105 | |
| Vitamin D3, neferine | ■ Synergistic anti-proliferative effects on HCT-116 colorectal cancer cells; efficacy at lower doses holds promise of reducing side effects. | 106,107 | |
| Squamous cell carcinoma | VDR FokI, Poly-A variants, 25(OH)D | ■ Variants and low 25(OH)D levels associated with disease. | 116 |
| 1,25(OH)2D | ■ Suppressed SCC utilizing both the A431 human SCC cell line and a xenograft SCC mouse mode via mTOR inhibition and activation of autophagy. | 118 | |
| Non-small cell lung cancer (lung adenocarcinoma) | CYP24Atargeting DNA aptamers | ■ Sensitized 1,25(OH)2D’s anti-proliferative effects. | 34 |
Breast cancer
Breast cancer (BC) is the leading cause of death for women worldwide, with 2.3 million new cases diagnosed each year46. Clinical studies have revealed that 25(OH)D deficiency is common in breast cancer patients, with younger and obese patients being more susceptible47. In the same study, 25(OH)D deficiency was linked to higher grade and ER negative breast cancer subtypes, implying a loss of vitamin D protective effects as well as potential extra hormonal interactions. Other studies have also clarified the role of VDR polymorphisms as potential risk factors for breast cancer in various ethnic populations, such as North Indian women in New Delhi48. The researchers not only confirmed that females with serum 25(OH)D levels in the highest quartile have a lower risk and stage of breast cancer, but they also demonstrated that women with the polymorphic T (f) allele for the VDR FokI site (genotype: CT/TT) rather than the wild C (F) allele (genotype: CC) had an increased risk of developing breast cancer. The FokI polymorphism (rs2228570) is located at the start codon in exon 2, resulting in an altered translation start site and generation of a long VDR variant of 427 amino acids with a lower efficiency of gene activation than the wild type C allele, which has been shown to promote more active anti-inflammatory responses49. This allelic combination may be the cause of the increased risk for breast cancer in north Indian women, which warrants further investigation.
Recent work investigated the relationship between the VDR and the insulin-like growth factor 1 receptor (IGF1R) in BC progression in different BC subtypes from an endocrinological and translational standpoint50. In a retrospective analysis of 48 BC patients, approximately 44% were 25(OH)D deficient, but there was strong positive VDR protein expression in 56% of cases, which was significantly associated with high IGF1R expression as well (p=0.031). These findings point to an intriguing hormone axial interaction that may affect VDR and IGF1R signalling, given that the former is anti-cancer, and the latter is cancer-promoting, and that differential levels of circulating vitamin D and IGF1 hormones may dictate BC progression. The translational implications of modulating the IGF1-vitamin D hormonal axis hold promise for the treatment of BC. Recent research also demonstrated and characterized the anti-tumoral effects of anti-estrogens, calcitriol, and EB1089 (a potent synthetic VDR agonist) in triple-negative breast cancer (TNBC) models51,52. Garcia-Becerra and colleagues demonstrated that calcitriol induced estrogen receptor alpha (ERa) expression in TNBCs, restoring antitumor responses to anti-estrogens51,52. They discovered that both VDR and retinoic X receptor (RXR) form a complex at a distinct vitamin D response element (VDRE) within the ERa gene promoter region, accompanied by a decrease in DNA methyltransferase and histone deacetylase activities. The researchers also demonstrated that EB1089 had potent anti-cancer properties in TNBC, as well as EGFR and HER2 positive breast cancer cell models and tumour-bearing mice36,53. Furthermore, because EB1089 increased ERa expression, it sensitized responses to fulvestrant (an anti-estrogen) with regard to inhibition of cell proliferation, tumour volume, and cellular metabolism in both in vitro and in vivo systems. This research suggests that treating TNBC patients with anti-estrogens and VDR agonists in combination may be a novel and efficacious therapeutic approach. Other breast cancer studies have investigated alternative methods of sensitizing chemotherapeutic responses by modulating the vitamin D catabolizing enzyme cytochrome P450 family 24 subtype A1 (CYP24A1). Numerous cancers, including breast cancer, have increased CYP24A1 expression (https://www.cancer-genetics.org)54–56, and its suppression has been shown to provide enhanced sensitivity to anti-cancer drugs, including vitamin D, with pharmacologically different modes of action in BC patients57.
Ovarian cancer
With a global incidence of 313,000 new cases per year, ovarian cancer is the most common cause of cancer death from gynecologic tumors58. Researchers recently conducted a meta-analysis of searchable databases (such as MEDLINE and the Web of Sciences) to investigate the relationships between dietary vitamin D intake and serum 25(OH)D levels and ovarian cancer relative risk (RR)59. People with high blood 25(OH)D levels had a 37% lower risk of ovarian cancer than those with low levels in 15 observational studies (pooled RR=0.63). Overall vitamin D intake, on the other hand, showed a weak inverse relationship (RR=0.92). The inverse relationship was stronger in case-control studies than in prospective studies when the studies were compared. Overall, serum 25(OH)D levels may be a better prognostic endpoint for predicting the effect on ovarian cancer.
Glioblastoma
Glioblastoma is the most common primary brain cancer, accounting for approximately 250,000 new cases each year60. In rat and human glioblastoma cell lines, 1,25(OH)2D inhibited the cell cycle, induced programmed cell death as well as cytotoxic autophagy61. Through auto-upregulation of the VDR, vitamin D also inhibited the migration and invasiveness of glioblastomas, as well as their stemness. Notably, clinically relevant anti-hypercalcemic small molecule analogues of 1,25(OH)2D initiated similar anti-cancer effects against glioblastomas in vivo, as well as synergistic effects with chemotherapeutic agents62. In addition, vitamin D supplementation studies are currently being conducted in several clinical trials of combination therapies to overcome glioma chemoresistance, but no results have been made public to date (https://clinicaltrials.gov: NCT00008086, NCT01181193). Recent research also showed that the acidic microenvironment of gliomas promotes self-renewal of stem cell-like glioma cells, with acidosis inducing CYP24A1 expression and subsequent catabolism of 1,25(OH)2D63. These findings point to the potential use of vitamin D analogues that are resistant to CYP24A1 degradation and/or inhibition of CYP24A1 in the treatment of gliomas in the future (see last section).
Multiple myeloma and peripheral neuropathy
Multiple myeloma is a rare blood cancer that affects plasma cells, with a worldwide incidence of 160,000 cases per year64. Recently, thirty-nine multiple myeloma patients were given 25(OH)D and their peripheral neuropathological effects were evaluated after six months65. In the study, 66% of patients with starting inadequate 25(OH)D levels achieved adequate 25(OH)D levels after supplementation, and peripheral neuropathy severity decreased significantly in these patients. Although the findings are promising, more cause-and-effect mechanistic studies are required to provide insights into the clinical effects of 25(OH)D supplementation. Furthermore, bortezomib is a proteasome inhibitor commonly used in the treatment of multiple myeloma, but patients frequently develop immune-mediated resistance. Because vitamin D is a major immune system regulator9,66, researchers conducted studies in the U266 multiple myeloma cell line to better understand the potential mechanisms by which vitamin D may overcome bortezomib resistance67. The authors discovered that bortezomib resistance was mediated by ATP metabolism and oxidative phosphorylation, and that vitamin D may provide adequate cellular metabolism by influencing the aforementioned resistive pathways. These findings are consistent with our recent work on the effects of vitamin D on mitochondrial energy metabolism and oxidative stress in bone cancer (see Osteosarcoma Section)68, implying that vitamin D anti-cancer pathways and mechanisms may be partially conserved across cancer types.
Prostate cancer
Prostate cancer is the most common cancer in men, with 1.4 million new cases diagnosed each year69. Recent research suggests that the ApaI (A/C) polymorphism in intron 8 of the VDR gene may be a diagnostic and prognostic marker for the stage of malignant prostate cancer in Egyptian men versus men with benign prostate hyperplasia70. Given that the ApaI (A/C) polymorphism has no effect on the encoded VDR protein, the explanation for this association is unclear, and it could represent complex epistatic and/or gene regulatory consequences. Recent work in both normal and cancerous prostates characterized the relationship between testosterone/dihydrotestosterone (DHT) levels and cellular transport mediated by the vitamin D signalling system in the African American population71. The authors discovered that prostate epithelium-specific Lrp2 (megalin transporter) loss in mice resulted in lower levels of prostate sex hormone-binding globulin bound testosterone and DHT. This association was also found in African American men, where prostatic DHT levels were high but inversely related to serum 25(OH)D status. The authors also demonstrated that 25(OH)D treatment directly decreased Lrp2, implying an anti-tumour/transformation mechanism involving androgen exclusion in a megalin-dependent manner within prostate epithelial cells. This research has clinical and therapeutic implications for prostate cancer patients, as vitamin D deficiency may influence megalin-mediated androgen transport to promote prostate cancer, whereas supplementation may protect against prostate cancer as recently described in the VITAL supplementation trial28,31. Using prostate cancer cell lines (LNCaP/22Rv1), additional mechanistic studies revealed that 1,25(OH)2D can inhibit tumour progression by negatively regulating androgenic receptor signalling, as well as c-MYC and epithelial-to-mesenchymal transition (EMT) gene expression72. The authors also demonstrated that 1,25(OH)2D induced moderate levels of unfolded protein response in prostate cancer cells via PERK/IRE1a endoplasmic reticular (ER) pathways, implying that ER-mediated apoptosis could be a potential pathological mechanism to induce cell death73.
Head and neck squamous cell carcinomas
Head and neck squamous cell carcinomas (HNSCCs) are the most common malignancies in the head and neck region, with 890,000 new cases reported each year74. HNSCCs arise from the mucosal epithelium in the oral cavity, pharynx, and larynx. Although the research on the vitamin D signalling system and HNSCCs is limited, recent studies showed that poorly differentiated HNSCCs express high VDR and Ki67 levels, whereas well-differentiated tumours do not75. Interestingly, patients with poorly differentiated HNSCCs had the lowest 25(OH)D serum levels among the patient cohorts studied, implying that the vitamin D signalling system is less activated, despite having the necessary machinery in those patients with a poor prognosis. The authors also discovered a sexual dichotomy in that female patients had higher vitamin D deficiency than males and had more poorly differentiated tumours. In the same study, adding 1,25(OH)2D to HNSCCs promoted VDR nuclear translocation, implying a therapeutically tuneable system. Furthermore, in both 2D and 3D spheroid models, co-treatment with the chemotherapeutic cisplatin significantly increased the anti-tumour effects of 1,25(OH)2D via inhibition of the PI3K/Akt/mTOR pathway. The authors proposed that when using vitamin D-based supplementation therapies for the treatment of HNSCCs, gender should be considered. Furthermore, new HNSCC data on concurrent chemoradiation therapy showed that patients with suboptimal 25(OH)D levels had more skin dermatitis and mucositis than those with optimal 25(OH)D levels76. As we observed skin polyps and dermatitis in a xenograft humanized metastatic mouse model of osteosaorcma77, these cutaneous pathologies may be associated with micrometastatic and paracrine responses of circulating cancer cells, which was suppressed by vitamin D (see Osteosarcoma section). Overall, these findings suggest that vitamin D may play a protective role in HNSCCs.
Bladder cancer
Bladder cancer is a relatively rare type of cancer that begins in the bladder lining and progresses to urothelial carcinoma, with 573,000 new cases reported globally each year78. Recent research showed that 1,25(OH)2D improved cisplatin’s efficacy on bladder cancer cell lines T24 and ECV-304 when compared to normal endothelial HUVEC cells79. The use of combination therapy increased apoptotic responses as measured by Annexin V staining and P-gp expression. This study suggests that chemotherapeutic cisplatin can be used at lower doses in conjunction with vitamin D therapy to improve efficacy, as well as reduce potential side effects in patients.
Osteosarcoma (bone cancer)
Osteosarcoma is the most common type of cancer that begins in bone-forming osteoblastic cells, with 27,000 new cases diagnosed worldwide each year25. Because of increased nonsense-mediated RNA decay (NMD), reactive oxygen species (ROS), and EMT, osteosarcomas are immune-resistant and metastatic44,68,80–82. Increased NMD in osteosarcoma has been linked to the degradation of numerous proteins that provide anti-cancer protection, including enhanced cell-cell adhesion, immunorecognition by cytotoxic T cells, and tumour suppression (e.g., p53). Although vitamin D has anti-cancer properties, its efficacy and mechanism of action against osteosarcomas are uncertain. To fill this knowledge gap, we recently investigated the effects of 1,25(OH)2D and calcipotriol, a potent non-hypercalcaemic VDR agonist, on the NMD-ROS-EMT signalling axis in in vitro and in vivo osteosarcoma cell and animal models77. We demonstrated that vitamin D inhibited osteosarcoma by reprogramming NMD and SNAI2-mediated EMT genes using epigenome and transcriptome-wide approaches, which affected outcomes such as fibrosis. To investigate the negative regulatory role of vitamin D on cell migration, we used in vitro scratch and invasion assays, as well as an in vivo lineage tracing and conditional Vdr knockout strategy, which demonstrated that vitamin D signalling, in general, directly impaired cell migration in the context of osteosarcoma and skin injury. We also demonstrated, for the first time, that calcipotriol significantly inhibited osteosarcoma spread and tumour growth in a mouse xenograft metastasis model of osteosarcoma. The paracrine effects of transmigratory human osteosarcoma cells localized to tissue compartments dictated many of the pathological features associated with the metastatic osteosarcoma model (e.g., impaired wound healing, formation of skin polyps and cysts). The anti-oxidative roles of vitamin D in osteosarcoma were central to its benefits, particularly against metastatic disease, which was largely dependent on high ROS production to promote osteosarcoma cell migration68. Our findings revealed novel osteosarcoma-inhibiting mechanisms for vitamin D and calcipotriol that could be applied to human patients, as well as potentially broader applications for the aging population by inhibiting oxidative stress83.
Colorectal cancer
Colorectal cancer develops from cells lining the colon or rectum and has a global incidence of 1.9 million new cases per year84. Recent research from the Norwegian Women and Cancer Cohort Study (95,416 participants, 1774 cases of colorectal cancer) found that patients with higher vitamin D3 intake compared to low vitamin D3 intake had a 17% lower risk of colorectal cancer localized to the proximal but not the distal or rectal colon (Hazard ratio=0.83, 95% CI, 0.68–1.02)85. These findings suggest that vitamin D may be associated with different subsites of the colon, which may be related to the known higher VDR expression in the proximal colon86. Recently, gene expression studies of vitamin D metabolic pathway genes in colorectal cancer tissue revealed that both VDR and CYP3A4 were downregulated in the disease population87. In the liver, CYP3A4 is involved in the xenobiotic transformation and degradation of many drugs, including the 4/24-hydroxylation and 25-hydroxylation of vitamin D3 and D2, respectively88. The authors hypothesized that vitamin D metabolism may be impaired in colorectal cancer development. A recent secondary analysis of a randomized clinical trial of 2,259 participants with colon adenomas revealed that the rs4588*A variant of the vitamin D-binding protein (DBP) gene, GC, may be more responsive to vitamin D3 at 1000 IU against colorectal admenoma89. Despite the fact that patients with the DBP2 isoform (encoded by the rs4588*A allele) were more likely to have lower 25(OH)D concentrations, it was hypothesized that the DBP2 isoform may improve tissue bioavailability of circulating vitamin D. More biochemical testing, however, is required to establish a clear cause-and-effect relationship.
From a mechanistic standpoint, recent research showed that 1,25(OH)2D suppressed colorectal cancer stem cells (CCSCs) by inducing ferroptosis, an iron-mediated type of programmed cell death process90. The authors demonstrated that 1,25(OH)2D (albeit at supraphysiological levels of 100nM) suppressed CCSC proliferation and the number of tumor spheroids in both in vitro and in vivo systems by generating ROS and downregulating SLC7A11 (i.e., an antiporter that promotes antioxidative cysteine uptake). TP53 and MAPK3 were also shown to be involved in the ferroptosis pathway91. Although these findings are intriguing, the supraphysiological concentrations of 1,25(OH)2D used in the studies are unlikely to be achieved in patients, let alone the potentially fatal concentrations of 1,25(OH)2D used in the in vivo studies (i.e., 30g/kg b.w.)92,93. Importantly, findings in 389 colorectal cancer patients recently revealed that serum 25(OH)D at adequate physiological levels is associated with a lower risk of sporadic colorectal cancer, emphasizing the importance of treatment doses61.
Recent research also showed that vitamin D signaling modulated anti-cancer responses in a variety of cancer types, including colorectal cancer cells, by influencing the expression and post-translational modifications of the Sirtuin (SIRT) family of proteins. Sirtuin 1 (SIRT1) is a “guardian of the genome” and a proto member of the SIRT family that acts as a NAD+-dependent histone deacetylase with dual roles in tumorigenes94. SIRT1 promotes metastasis and invasiveness in a variety of cancers, including prostate95, breast96, lung97, colon98, and melanoma99. Deacetylation of proteins involved in tumor suppression and/or DNA damage repair occurs as a result, and pharmacological inhibition of SIRT1 reduces cancer growth and proliferation100. Vitamin D downregulated both SIRT1 and 4 as part of its anti-cancer properties in osteosarcoma cells68. In contrast, SIRT1 also acts as a tumor suppressor by repressing oncogenes such as c-MYC through deacetylation101. Interestingly, recent pre-press findings using colorectal cancer cell lines HCT 116 and HT-29, showed that 1,25(OH)2D activated SIRT1 via auto-deacetylation, resulting in an anti-proliferative response, and SIRT1 pharmacological activation also recapitulated the vitamin D responses102. However, the potential negative interactions with oncogenes that govern the anti-proliferative response in colorectal cancer cells remain unknown. As a result, depending on the type and subtype of cancer, vitamin D modulation of SIRT1 can be a double-edged sword.
In new animal studies, mice on a high-fat diet produced gut microbiota metabolites that suppressed inflammation and colitis-associated cancer by activating the VDR signaling pathway103. The authors demonstrated that a high-fat diet induced secondary fecal bile ascites, which directly activated the VDR and downstream anti-inflammatory target genes in mice with colitis-associated cancer and HT29 epithelial cells. Furthermore, recent in vivo mouse studies showed that the VDR associated with the p53 tumor suppressor protein to induce genes that promote peroxisomal fatty acid beta-oxidation (FAO) as a mechanism by which vitamin D inhibited colorectal cancer104. The authors demonstrated that increased FAO caused enhanced acetylation and inhibition of Aminoimidazole-4-Carboxamide Ribonucleotide Formyl transferase/IMP Cyclohydrolase (ATIC), a catalytic enzyme in the purine biosynthetic pathway. Acetylation of ATIC via VDR/p53 was inversely related to colorectal cancer tumor growth in both mouse and human cancer samples. Interestingly, increased FAO is associated with increased ROS formation68, which may be another mechanism by which VDR/p53 limited colorectal cancer growth. Also, researchers recently discovered that ablation of the Vdr resulted in a decrease in Claudin-10 tight junction protein expression in the intestinal epithelium, leading to increased permeability, tumor number, and bacterial infiltration105. Human colorectal cancer samples exhibited similar pathogenesis, with increased tumor-invading bacteria and decreased colonic VDR and Claudin-10 mRNA and protein expression. These findings indicate that the VDR is an important host factor that could be targeted to reduce the risk of colon cancer development and progression.
Finally, there have only been a few reports on the combined effects of vitamin D and chemotherapeutic drugs on colorectal cancer. A recent study looked at the effects of cholecalciferol and neferine, a lotus seed alkaloid with anti-inflammatory, proapoptotic, and G1 arrest properties, on the growth and metastasis of the HCT-116 colorectal cancer cell line106,107. At low doses, cholecalciferol and neferine synergistically inhibited the growth of HCT-116 cells, potentially with fewer side effects if applied to patients. Furthermore, when compared to single treatment groups, both compounds together further suppressed scratch closure and colony formation capacity, as well as cell migration and invasion. Mechanistically, combined treatment reduced N-cadherin and EMT-inducer SNAI expression in HCT-116 cells.
Melanoma
Melanoma is the deadliest type of skin cancer that develops in pigment-producing melanocytes, with 325,000 new cases diagnosed each year108. Therapeutic supplemental vitamin D has also been reported to reduce cell growth in both melanoma and non-melanoma (see next section) skin cancer. A cross-sectional study of 498 adults with any type of skin cancer was recently conducted, whereby patients were stratified into groups based on self-reported use of vitamin D3 supplements to search for associations109. Logistic regression analysis revealed that among patients with a history of melanoma, the odds ratio was 0.447 (p=0.016, 95% CI, 0.231–0.862) among those who regularly used vitamin D3 supplements, indicating that the risk of melanoma was significantly lower among regular users. Additionally, a retrospective cohort study of 264 invasive melanoma patients from Barcelona University Hospital from 1998–2021 were analyzed for relationships to serum 25(OH)D levels110. The authors found that patients with lower 25(OH)D levels (<10ng/ml) were associated with worse overall survival, suggesting vitamin D deficiency could play a role in overall survival of melanoma patients. Other studies have correlated vitamin D deficit with poor clinical outcome in metastatic melanoma patients treated with BRAF/MEK inhibitors or immunotherapy as well111. Although these studies suggest that low vitamin D status is associated with increased risk and poor melanoma prognosis, and that optimizing serum 25(OH)D levels may protect against melanoma, causality still remains unknown. Recent studies in melanoma cell lines have shown that 1,25(OH)2D can induce apoptosis via modulation of caspase 3/8/9, as a potential mechanism of action112. Additional studies in melanoma cell line shown that 1,25(OH)2D can induce the expression of phosphatase and tensin homolog deleted on chromosome 10 (PTEN), a well-known tumor suppressor, as another potential mechanism of action affecting downstream effectors113.
Squamous cell carcinoma
Squamous cell carcinoma (SCC) of the skin is a common type of skin cancer that develops in the squamous cells that comprise the skin’s superficial layers, with 2.4 million new cases reported globally each year114. The functional role of polymorphic genetic variants of the VDR gene in SCC is uncertain. Recent studies examined both FokI (F and f alleles) and Poly-A (i.e., a variant microsatellite length in the 3’-untranslated region resulting in Long [L] or Short [S] variants115) VDR polymorphisms in 137 patients with a history of SCC, as well as 25(OH)D levels116. It is important to note that the Poly-A allelic variations do not affect the structural integrity of the VDR protein, but may influence mRNA degradation or translation, though this is not without uncertainty (see below)117. The researchers discovered a strong association between the FFSS or FfSS genotypes and high 25(OH)D serum levels (i.e., potentially protective) in SCC patients, whereas ffLL patients had low 25(OH)D levels (i.e., potentially susceptible). Although the Poly-A (L) allele was considered a risk allele for SCC in the study, it was previously shown to be more active in terms of VDR mRNA production, and thus potentially protective117. Nonetheless, these findings highlight the complex handling of Poly-A VDR polymorphisms that may influence cancer type and subtype risk. We also recently investigated the mechanism of 1,25(OH)2D-dependent suppression of SCC using the A431 human SCC cell line and a xenograft SCC mouse model118. We discovered that 1,25(OH)2D inhibited SCC by increasing the expression of a key inhibitor of the mTOR pathway called DNA Damage Induced Transcript 4 (DDIT4), which then activates LC3-mediated autophagy. Furthermore, 1,25(OH)2D sensitizes the anti-SCC effects of rapamycin-based pharmacological mTOR inhibition in an in vivo mouse model.
Cancer studies that showed no link with vitamin D
Although a few recent studies reported no association between respective cancers and vitamin D, most of the findings did show a positive correlation between vitamin D and cancer inhibition. Nonetheless, we include those findings here to provide a complete picture of vitamin D and cancer. The Kuopio Ischemic Heart Disease Risk Factor study (2,578 case studies) sought to establish an epidemiological link between 25(OH)D levels and lung and prostate cancer119. Overall, the authors demonstrated that serum 25(OH)D levels did not correlate with lung or prostate cancer, nor did they interact with smoking or age. Black Americans have lower circulating vitamin D levels than White people, and the response of vitamin D and calcium supplementation in Black women with cancer is unclear. With this question in mind, researchers examined data from the Women’s Health Initiative (WHI) calcium plus vitamin D (CaD) randomized clinical trial to investigate cancer incidence and cause-specific mortality among 3,325 Black women who were randomly assigned to receive calcium (1000 mg) plus vitamin D3 (400 IU) or placebo for an average of seven years120. The findings revealed no differences between the calcium/vitamin D3 and placebo groups, implying that other medical, biological, or social interventions should be considered to address health disparities among Black women with cancer. Finally, researchers examined the relationship of circulating 25(OH)D to breast cancer incidence using data from ten U.S. and seven European prospective cohorts121. The authors discovered no link between circulating 25(OH)D levels and invasive breast cancer incidence using conditional logistic regression and random-effects models.
New anti-cancer strategies that target the vitamin D signalling system
Biyani and colleagues from Kanazawa University in Japan recently demonstrated a novel method for identifying and optimizing novel DNA-derived aptamers capable of sensitizing the anti-cancer effects of 1,25(OH)2D34. In cancer patients, low vitamin D levels, as well as an increase in the enzyme vitamin D 24-hydroxylase (CYP24A1), are linked to a poor prognosis4,27–31,122–124. As a result, molecules that inhibit CYP24A1 activity could be used as antiproliferative agents in cancer treatment. Using the Systematic Evolution of Ligands by Competitive Selection (SELCOS) method, the researchers first screened a large number of DNA aptamers, which are single-stranded DNA molecules with distinct three-dimensional structures capable of competitively binding to CYP24A1 but not the related enzyme CYP27B1. After being tested for CYP24A1 inhibitory activity using HPLC to detect conversion metabolites, a 70-nucleotide DNA aptamer 7 (called Apt-7) was chosen for further investigation. The physical binding of Apt-7 to CYP24A1 was studied using electrochemical and electrophoretic methods, and the Kd was found to be in the sub-nanomolar range. Furthermore, molecular docking simulations revealed that Apt-7 inhibited CYP24A1 activity via steric inhibition of the CYP24A1 substrate binding site. The researchers then used spectral binding analysis to investigate the mode of Apt-7 binding to CYP24A1. Apt-7-containing spectra were smaller than Apt-7-free spectra, implying that Apt-7 may interfere with the enzyme active site or the enzyme-substrate complex site. Real-time high-speed atomic force microscopy was also used to characterize Apt-7 and CYP24A1 binding, and the results agreed with the molecular docking simulations. Endocytosed Apt-7 inhibited CYP24A1 activity in lung adenocarcinoma cells and sensitized 1,25(OH)2D’s anti-proliferative effects, implying that co-treatment strategies with CYP24A1-targeting DNA aptamers could be a promising vitamin D-based cancer therapy in the clinic.
Closing remarks
We have made significant progress in understanding the multiple roles that vitamin D plays in our bodies since its first description over 370 years ago in human deficiency skeletal diseases in children and adults125. This review summarizes the most recent research on vitamin D and its analogues, as well as specific manipulation of signalling and metabolic system components, with the goal of advancing their use as cancer therapeutic agents. The anti-cancer properties of vitamin D, as well as its biological presence, suggest that it may have a long-term impact on human lives as we age and succumb to injuries and disease. More research is needed to fully comprehend and appreciate vitamin D’s role in cancer biology and patient treatment.
Highlights.
The number of research articles on vitamin D cancer-related research has risen dramatically in recent years
Vitamin D continues to show positive anti-cancer effects against many types of cancer
Population studies also encourage the maintenance of adequate vitamin D levels in the hopes of avoiding cancer
Mechanistic studies show that vitamin D has intrinsic and extrinsic immune modulatory, anti-metastatic and tumorigenic effects on tumors
Funding:
T.S.L. was supported by Grant # IRG-17-183-16 from the American Cancer Society, and from the Sylvester Comprehensive Cancer Center at the Miller School of Medicine, University of Miami. EC was supported by JAX Computational Sciences and JAX Cancer Center (JAXCC) (NCI CCSG - P30CA034196) and by grant NSF 19-500, DMS 1918925/1922843.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interests: Authors have nothing to declare.
References
- 1.Gupta D, Vashi PG, Trukova K, Lis CG & Lammersfeld CA Prevalence of serum vitamin D deficiency and insufficiency in cancer: Review of the epidemiological literature. Experimental and therapeutic medicine 2, 181–193, doi: 10.3892/etm.2011.205 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bouillon R, Bischoff-Ferrari H & Willett W Vitamin D and health: perspectives from mice and man. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research 23, 974–979, doi: 10.1359/jbmr.080420 (2008). [DOI] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Wagle NS & Jemal A Cancer statistics, 2023. CA Cancer J Clin 73, 17–48, doi: 10.3322/caac.21763 (2023). [DOI] [PubMed] [Google Scholar]
- 4.Bouillon R et al. Vitamin D and human health: lessons from vitamin D receptor null mice. Endocrine reviews 29, 726–776, doi: 10.1210/er.2008-0004 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leung MF & Cheung PCK Vitamins D and D2 in Cultivated Mushrooms under Ultraviolet Irradiation and Their Bioavailability in Humans: A Mini-Review. Int J Med Mushrooms 23, 1–15, doi: 10.1615/IntJMedMushrooms.2021040390 (2021). [DOI] [PubMed] [Google Scholar]
- 6.Takeyama K et al. 25-Hydroxyvitamin D3 1alpha-hydroxylase and vitamin D synthesis. Science 277, 1827–1830, doi: 10.1126/science.277.5333.1827 (1997). [DOI] [PubMed] [Google Scholar]
- 7.Bacchetta J et al. Fibroblast growth factor 23 inhibits extrarenal synthesis of 1,25-dihydroxyvitamin D in human monocytes. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research 28, 46–55, doi: 10.1002/jbmr.1740 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bacchetta J et al. Vitamin D as a New Regulator of Iron Metabolism: Vitamin D Suppresses Hepcidin in Vitro and in Vivo. Nephrol Dial Transpl 27, 29–30 (2012). [Google Scholar]
- 9.Bacchetta J et al. Suppression of iron-regulatory hepcidin by vitamin D. Journal of the American Society of Nephrology : JASN 25, 564–572, doi: 10.1681/ASN.2013040355 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou R et al. Vitamin D and alternative splicing of RNA. J Steroid Biochem 148, 310–317, doi: 10.1016/j.jsbmb.2014.09.025 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou R et al. Concerted effects of heterogeneous nuclear ribonucleoprotein C1/C2 to control vitamin D-directed gene transcription and RNA splicing in human bone cells. Nucleic acids research 45, 606–618, doi: 10.1093/nar/gkw851 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lisse TS, Adams JS & Hewison M Identification of Novel Vitamin D Receptor Target Genes Based on Promoter Interaction with the Vitamin D Response Element Binding Protein. Endocrine reviews 31 (2010). [Google Scholar]
- 13.Lisse TS, Adams JS & Hewison M Vitamin D and microRNAs in bone. Critical reviews in eukaryotic gene expression 23, 195–214 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lagishetty V et al. 1alpha-hydroxylase and innate immune responses to 25-hydroxyvitamin D in colonic cell lines. The Journal of steroid biochemistry and molecular biology 121, 228–233, doi: 10.1016/j.jsbmb.2010.02.004 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lisse TS, Chun RF, Rieger S, Adams JS & Hewison M Vitamin D activation of functionally distinct regulatory miRNAs in primary human osteoblasts. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research 28, 1478–1488, doi: 10.1002/jbmr.1882 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lisse TS & Hewison M Vitamin D: a new player in the world of mTOR signaling. Cell cycle 10, 1888–1889 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lisse TS, Hewison M & Adams JS Hormone response element binding proteins: novel regulators of vitamin D and estrogen signaling. Steroids 76, 331–339, doi: 10.1016/j.steroids.2011.01.002 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lisse TS et al. Gene targeting by the vitamin D response element binding protein reveals a role for vitamin D in osteoblast mTOR signaling. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 25, 937–947, doi: 10.1096/fj.10-172577 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lisse TS et al. The vitamin D receptor is required for activation of cWnt and Hedgehog signaling in keratinocytes. Molecular endocrinology, me20141043, doi: 10.1210/me.2014-1043 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao H, Rieger S, Abe K, Hewison M & Lisse TS DNA Damage-Inducible Transcript 4 Is an Innate Surveillant of Hair Follicular Stress in Vitamin D Receptor Knockout Mice and a Regulator of Wound Re-Epithelialization. International journal of molecular sciences 17, doi: 10.3390/ijms17121984 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou R et al. Concerted effects of heterogeneous nuclear ribonucleoprotein C1/C2 to control vitamin D-directed gene transcription and RNA splicing in human bone cells. Nucleic acids research 45, 606–618, doi: 10.1093/nar/gkw851 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tahbazlahafi B, Paknejad M, Khaghani S, Sadegh-Nejadi S & Khalili E Vitamin D Represses the Aggressive Potential of Osteosarcoma. Endocr Metab Immune Disord Drug Targets 21, 1312–1318, doi: 10.2174/1871530320666200821155756 (2021). [DOI] [PubMed] [Google Scholar]
- 23.Mirabello L, Troisi RJ & Savage SA International osteosarcoma incidence patterns in children and adolescents, middle ages and elderly persons. International journal of cancer 125, 229–234, doi: 10.1002/ijc.24320 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ottaviani G & Jaffe N The epidemiology of osteosarcoma. Cancer Treat Res 152, 3–13, doi: 10.1007/978-1-4419-0284-9_1 (2009). [DOI] [PubMed] [Google Scholar]
- 25.Misaghi A, Goldin A, Awad M & Kulidjian AA Osteosarcoma: a comprehensive review. SICOT J 4, 12, doi: 10.1051/sicotj/2017028 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harrison DJ, Geller DS, Gill JD, Lewis VO & Gorlick R Current and future therapeutic approaches for osteosarcoma. Expert Rev Anticancer Ther 18, 39–50, doi: 10.1080/14737140.2018.1413939 (2018). [DOI] [PubMed] [Google Scholar]
- 27.Lisse TS Vitamin D Regulation of a SOD1-to-SOD2 Antioxidative Switch to Prevent Bone Cancer. Appl Sci-Basel 10, doi:ARTN2554 10.3390/app10072554 (2020). [DOI] [Google Scholar]
- 28.Manson JE et al. Vitamin D Supplements and Prevention of Cancer and Cardiovascular Disease. The New England journal of medicine 380, 33–44, doi: 10.1056/NEJMoa1809944 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Y et al. Association between vitamin D supplementation and mortality: systematic review and meta-analysis. BMJ 366, l4673, doi: 10.1136/bmj.l4673 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feldman D, Krishnan AV, Swami S, Giovannucci E & Feldman BJ The role of vitamin D in reducing cancer risk and progression. Nat Rev Cancer 14, 342–357, doi: 10.1038/nrc3691 (2014). [DOI] [PubMed] [Google Scholar]
- 31.Chandler PD et al. Effect of Vitamin D3 Supplements on Development of Advanced Cancer: A Secondary Analysis of the VITAL Randomized Clinical Trial. JAMA Netw Open 3, e2025850, doi: 10.1001/jamanetworkopen.2020.25850 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu X, Baylin A & Levy PD Vitamin D deficiency and insufficiency among US adults: prevalence, predictors and clinical implications. The British journal of nutrition 119, 928–936, doi: 10.1017/S0007114518000491 (2018). [DOI] [PubMed] [Google Scholar]
- 33.Fleet JC et al. Vitamin D Signaling Suppresses Early Prostate Carcinogenesis in TgAPT(121) Mice. Cancer Prev Res (Phila) 12, 343–356, doi: 10.1158/1940-6207.CAPR-18-0401 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Biyani M et al. Novel DNA Aptamer for CYP24A1 Inhibition with Enhanced Antiproliferative Activity in Cancer Cells. ACS Appl Mater Interfaces 14, 18064–18078, doi: 10.1021/acsami.1c22965 (2022). [DOI] [PubMed] [Google Scholar]
- 35.Martinez-Reza I et al. Calcitriol Inhibits the Proliferation of Triple-Negative Breast Cancer Cells through a Mechanism Involving the Proinflammatory Cytokines IL-1beta and TNF-alpha. J Immunol Res 2019, 6384278, doi: 10.1155/2019/6384278 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mark B Meyer C-M, Bikle Daniel D., MadhuBiyani, Campbell Moray J., Chaudhari Snehal N., SylviaChristakos, Ingles Sue A., Knuth Megan M., MinLee Seong, Lisse Thomas S., Liu Eva S., IsabellePiec, Plum Lori A., Rao Sudhaker D., Reynolds Carmen J., Thacher Tom D., White John H., Cantorna Margherita T.. Highlights from the 24th workshop on vitamin D in Austin, September 2022. The Journal of steroid biochemistry and molecular biology, doi: 10.1016/j.jsbmb.2023.106247 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang XJ et al. DNA damage-inducible transcript 4 is an innate guardian for human squamous cell carcinoma and an molecular vector for anti-carcinoma effect of 1,25(OH)(2)D-3. Experimental dermatology 28, 45–52, doi: 10.1111/exd.13815 (2019). [DOI] [PubMed] [Google Scholar]
- 38.Liu C et al. Tumor-Targeted Nanoparticles Deliver a Vitamin D-Based Drug Payload for the Treatment of EGFR Tyrosine Kinase Inhibitor-Resistant Lung Cancer. Mol Pharm 15, 3216–3226, doi: 10.1021/acs.molpharmaceut.8b00307 (2018). [DOI] [PubMed] [Google Scholar]
- 39.Verma A Combining Vitamin D with Programmed Death 1 Blockade in Head and Neck Cancer. State University of New York at Buffalo, ProQuest Dissertations Publishing, 2021. 28499420 (2021). [Google Scholar]
- 40.Verma A, Vincent-Chong VK, DeJong H, Hershberger PA & Seshadri M Impact of dietary vitamin D on initiation and progression of oral cancer. The Journal of steroid biochemistry and molecular biology 199, 105603, doi: 10.1016/j.jsbmb.2020.105603 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carlberg C Vitamin D and Its Target Genes. Nutrients 14, doi: 10.3390/nu14071354 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dimitrov V et al. Vitamin D-regulated Gene Expression Profiles: Species-specificity and Cell-specific Effects on Metabolism and Immunity. Endocrinology 162, doi: 10.1210/endocr/bqaa218 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hanel A & Carlberg C Time-Resolved Gene Expression Analysis Monitors the Regulation of Inflammatory Mediators and Attenuation of Adaptive Immune Response by Vitamin D. International journal of molecular sciences 23, doi: 10.3390/ijms23020911 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Litchfield K et al. Escape from nonsense-mediated decay associates with anti-tumor immunogenicity. Nature communications 11, 3800, doi: 10.1038/s41467-020-17526-5 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Muscolino A et al. NETME: on-the-fly knowledge network construction from biomedical literature. Appl Netw Sci 7, 1, doi: 10.1007/s41109-021-00435-x (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Arnold M et al. Current and future burden of breast cancer: Global statistics for 2020 and 2040. Breast 66, 15–23, doi: 10.1016/j.breast.2022.08.010 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rosso C, Fera N, Murugan NJ & Voutsadakis IA Vitamin D Levels in Newly Diagnosed Breast Cancer Patients according to Tumor Sub-Types. J Diet Suppl, 1–13, doi: 10.1080/19390211.2022.2144582 (2022). [DOI] [PubMed] [Google Scholar]
- 48.Chakraborty M et al. FokI polymorphism of Vitamin D receptor gene and deficiency of serum Vitamin D increases the risk of breast cancer in North Indian women. Endocrine, doi: 10.1007/s12020-023-03334-6 (2023). [DOI] [PubMed] [Google Scholar]
- 49.van Etten E et al. The vitamin D receptor gene FokI polymorphism: functional impact on the immune system. European journal of immunology 37, 395–405, doi: 10.1002/eji.200636043 (2007). [DOI] [PubMed] [Google Scholar]
- 50.Alnimer A et al. Association Between Expression of Vitamin D Receptor and Insulin-Like Growth Factor 1 Receptor Among Breast Cancer Patients. World J Oncol 14, 67–74, doi: 10.14740/wjon1550 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Santos-Martinez N et al. Calcitriol induces estrogen receptor alpha expression through direct transcriptional regulation and epigenetic modifications in estrogen receptor-negative breast cancer cells. Am J Cancer Res 11, 5951–5964 (2021). [PMC free article] [PubMed] [Google Scholar]
- 52.Segovia-Mendoza M, Garcia-Quiroz J, Diaz L & Garcia-Becerra R Combinations of Calcitriol with Anticancer Treatments for Breast Cancer: An Update. International journal of molecular sciences 22, doi: 10.3390/ijms222312741 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Segovia-Mendoza M et al. The addition of calcitriol or its synthetic analog EB1089 to lapatinib and neratinib treatment inhibits cell growth and promotes apoptosis in breast cancer cells. Am J Cancer Res 7, 1486–1500 (2017). [PMC free article] [PubMed] [Google Scholar]
- 54.Tannour-Louet M et al. Increased expression of CYP24A1 correlates with advanced stages of prostate cancer and can cause resistance to vitamin D3-based therapies. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 28, 364–372, doi: 10.1096/fj.13-236109 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mukasa H & Slade HD Structure and immunological specificity of the Streptococcus mutans group b cell wall antigen. Infection and immunity 7, 578–585, doi: 10.1128/iai.7.4.578-585.1973 (1973). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wu W et al. Drivers and suppressors of triple-negative breast cancer. Proceedings of the National Academy of Sciences of the United States of America 118, doi: 10.1073/pnas.2104162118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kamiya S et al. Suppression of the vitamin D metabolizing enzyme CYP24A1 provides increased sensitivity to chemotherapeutic drugs in breast cancer. Oncology reports 49, doi: 10.3892/or.2023.8522 (2023). [DOI] [PubMed] [Google Scholar]
- 58.Huang J et al. Worldwide Burden, Risk Factors, and Temporal Trends of Ovarian Cancer: A Global Study. Cancers (Basel) 14, doi: 10.3390/cancers14092230 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jung S, Jin S & Je Y Vitamin D Intake, Blood 25-Hydroxyvitamin D, and Risk of Ovarian Cancer: A Meta-Analysis of Observational Studies. J Womens Health (Larchmt), doi: 10.1089/jwh.2022.0432 (2023). [DOI] [PubMed] [Google Scholar]
- 60.Lin D et al. Trends in Intracranial Glioma Incidence and Mortality in the United States, 1975–2018. Front Oncol 11, 748061, doi: 10.3389/fonc.2021.748061 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ma Y et al. Adequate vitamin D level associated with reduced risk of sporadic colorectal cancer. Front Nutr 10, 1024849, doi: 10.3389/fnut.2023.1024849 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bak DH et al. Autophagy enhancement contributes to the synergistic effect of vitamin D in temozolomide-based glioblastoma chemotherapy. Experimental and therapeutic medicine 11, 2153–2162, doi: 10.3892/etm.2016.3196 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hu P et al. Acidosis enhances the self-renewal and mitochondrial respiration of stem cell-like glioma cells through CYP24A1-mediated reduction of vitamin D. Cell Death Dis 10, 25, doi: 10.1038/s41419-018-1242-1 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ludwig H, Novis Durie S, Meckl A, Hinke A & Durie B Multiple Myeloma Incidence and Mortality Around the Globe; Interrelations Between Health Access and Quality, Economic Resources, and Patient Empowerment. Oncologist 25, e1406–e1413, doi: 10.1634/theoncologist.2020-0141 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Oortgiesen BE et al. Effectiveness of a vitamin D regimen in deficient multiple myeloma patients and its effect on peripheral neuropathy. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer 31, 138, doi: 10.1007/s00520-023-07574-0 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lagishetty V et al. Vitamin D deficiency in mice impairs colonic antibacterial activity and predisposes to colitis. Endocrinology 151, 2423–2432, doi: 10.1210/en.2010-0089 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Luczkowska K, Kulig P, Baumert B & Machalinski B The Evidence That 25(OH)D3 and VK2 MK-7 Vitamins Influence the Proliferative Potential and Gene Expression Profiles of Multiple Myeloma Cells and the Development of Resistance to Bortezomib. Nutrients 14, doi: 10.3390/nu14235190 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Quigley M et al. Vitamin D Modulation of Mitochondrial Oxidative Metabolism and mTOR Enforces Stress Adaptations and Anticancer Responses. JBMR Plus 6, e10572, doi: 10.1002/jbm4.10572 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang L et al. Prostate Cancer Incidence and Mortality: Global Status and Temporal Trends in 89 Countries From 2000 to 2019. Front Public Health 10, 811044, doi: 10.3389/fpubh.2022.811044 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.El-Attar AZ et al. Vitamin D receptor polymorphism and prostate cancer prognosis. Curr Urol 16, 246–255, doi: 10.1097/CU9.0000000000000141 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Garcia J et al. Regulation of Prostate Androgens by Megalin and 25-hydroxyvitamin D Status: Mechanism for High Prostate Androgens in African American Men. Cancer Res Commun 3, 371–382, doi: 10.1158/2767-9764.CRC-22-0362 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Erzurumlu Y et al. 1,25(OH)(2) D(3) induced vitamin D receptor signaling negatively regulates endoplasmic reticulum-associated degradation (ERAD) and androgen receptor signaling in human prostate cancer cells. Cell Signal 103, 110577, doi: 10.1016/j.cellsig.2022.110577 (2023). [DOI] [PubMed] [Google Scholar]
- 73.Lisse TS et al. ER stress-mediated apoptosis in a new mouse model of osteogenesis imperfecta. PLoS genetics 4, e7, doi: 10.1371/journal.pgen.0040007 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Johnson DE et al. Head and neck squamous cell carcinoma. Nat Rev Dis Primers 6, 92, doi: 10.1038/s41572-020-00224-3 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Koll L et al. Exploiting Vitamin D Receptor and Its Ligands to Target Squamous Cell Carcinomas of the Head and Neck. International journal of molecular sciences 24, doi: 10.3390/ijms24054675 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bhanu A, Waghmare CM, Jain VS & Pawar HJ Serum 25-hydroxy vitamin-D levels in head and neck cancer chemoradiation therapy: Potential in cancer therapeutics. Indian J Cancer, doi: 10.4103/ijc.IJC_358_20 (2023). [DOI] [PubMed] [Google Scholar]
- 77.Capobianco E et al. Vitamin D inhibits osteosarcoma by reprogramming nonsense-mediated RNA decay and SNAI2-mediated epithelial-to-mesenchymal transition. bioRxiv, doi: 10.1101/2023.01.04.522778 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Saginala K et al. Epidemiology of Bladder Cancer. Med Sci (Basel) 8, doi: 10.3390/medsci8010015 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ozgen O et al. Vitamin D increases the efficacy of cisplatin on bladder cancer cell lines. Molecular biology reports 50, 697–706, doi: 10.1007/s11033-022-08044-2 (2023). [DOI] [PubMed] [Google Scholar]
- 80.Chen C et al. Immunotherapy for osteosarcoma: Fundamental mechanism, rationale, and recent breakthroughs. Cancer letters 500, 1–10, doi: 10.1016/j.canlet.2020.12.024 (2021). [DOI] [PubMed] [Google Scholar]
- 81.Wu CC et al. Immuno-genomic landscape of osteosarcoma. Nat Commun 11, 1008, doi: 10.1038/s41467-020-14646-w (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang Z, Li B, Ren Y & Ye Z T-Cell-Based Immunotherapy for Osteosarcoma: Challenges and Opportunities. Front Immunol 7, 353, doi: 10.3389/fimmu.2016.00353 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Miao D & Goltzman D Mechanisms of action of vitamin D in delaying aging and preventing disease by inhibiting oxidative stress. Vitamins and hormones 121, 293–318, doi: 10.1016/bs.vh.2022.09.004 (2023). [DOI] [PubMed] [Google Scholar]
- 84.Rawla P, Sunkara T & Barsouk A Epidemiology of colorectal cancer: incidence, mortality, survival, and risk factors. Prz Gastroenterol 14, 89–103, doi: 10.5114/pg.2018.81072 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Paulsen EM, Rylander C, Brustad M & Jensen TE Pre-diagnostic intake of vitamin D and incidence of colorectal cancer by anatomical subsites: the Norwegian Women and Cancer Cohort Study (NOWAC). The British journal of nutrition, 1–9, doi: 10.1017/S0007114523000077 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lu R, Wu S, Xia Y & Sun J The Vitamin D Receptor, Inflammatory Bowel Diseases, and Colon Cancer. Curr Colorectal Cancer Rep 8, 57–65, doi: 10.1007/s11888-011-0114-1 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sadeghi H, Hashemnia V, Nazemalhosseini-Mojarad E, Ghasemi MR & Mirfakhraie R Correlated downregulation of VDR and CYP3A4 in colorectal cancer. Molecular biology reports 50, 1385–1391, doi: 10.1007/s11033-022-08141-2 (2023). [DOI] [PubMed] [Google Scholar]
- 88.Wang Z et al. An inducible cytochrome P450 3A4-dependent vitamin D catabolic pathway. Molecular pharmacology 81, 498–509, doi: 10.1124/mol.111.076356 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gibbs DC, Barry EL, Fedirko V, Baron JA & Bostick RM Impact of Common Vitamin D-Binding Protein Isoforms on Supplemental Vitamin D3 and/or Calcium Effects on Colorectal Adenoma Recurrence Risk: A Secondary Analysis of a Randomized Clinical Trial. JAMA Oncol, doi: 10.1001/jamaoncol.2022.6924 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Guo S et al. Vitamin D Promotes Ferroptosis in Colorectal Cancer Stem Cells via SLC7A11 Downregulation. Oxid Med Cell Longev 2023, 4772134, doi: 10.1155/2023/4772134 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Guo S et al. Identification of a ferroptosis-related gene signature for prognosis prediction in colorectal cancer patients and relationship with vitamin D. The Journal of steroid biochemistry and molecular biology 227, 106234, doi: 10.1016/j.jsbmb.2022.106234 (2023). [DOI] [PubMed] [Google Scholar]
- 92.Xue Y, Karaplis AC, Hendy GN, Goltzman D & Miao D Exogenous 1,25-dihydroxyvitamin D3 exerts a skeletal anabolic effect and improves mineral ion homeostasis in mice that are homozygous for both the 1alpha-hydroxylase and parathyroid hormone null alleles. Endocrinology 147, 4801–4810, doi: 10.1210/en.2006-0403 (2006). [DOI] [PubMed] [Google Scholar]
- 93.Nagpal S, Lu J & Boehm MF Vitamin D analogs: mechanism of action and therapeutic applications. Current medicinal chemistry 8, 1661–1679, doi: 10.2174/0929867013371950 (2001). [DOI] [PubMed] [Google Scholar]
- 94.Rahman S & Islam R Mammalian Sirt1: insights on its biological functions. Cell Commun Signal 9, 11, doi: 10.1186/1478-811X-9-11 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jung-Hynes B, Nihal M, Zhong W & Ahmad N Role of sirtuin histone deacetylase SIRT1 in prostate cancer. A target for prostate cancer management via its inhibition? The Journal of biological chemistry 284, 3823–3832, doi: 10.1074/jbc.M807869200 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jin X et al. SIRT1 promotes formation of breast cancer through modulating Akt activity. J Cancer 9, 2012–2023, doi: 10.7150/jca.24275 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Han L, Liang XH, Chen LX, Bao SM & Yan ZQ SIRT1 is highly expressed in brain metastasis tissues of non-small cell lung cancer (NSCLC) and in positive regulation of NSCLC cell migration. Int J Clin Exp Pathol 6, 2357–2365 (2013). [PMC free article] [PubMed] [Google Scholar]
- 98.Lin Z & Fang D The Roles of SIRT1 in Cancer. Genes Cancer 4, 97–104, doi: 10.1177/1947601912475079 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ohanna M et al. SIRT1 promotes proliferation and inhibits the senescence-like phenotype in human melanoma cells. Oncotarget 5, 2085–2095, doi: 10.18632/oncotarget.1791 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wilking MJ, Singh C, Nihal M, Zhong W & Ahmad N SIRT1 deacetylase is overexpressed in human melanoma and its small molecule inhibition imparts anti-proliferative response via p53 activation. Archives of biochemistry and biophysics 563, 94–100, doi: 10.1016/j.abb.2014.04.001 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yuan J, Minter-Dykhouse K & Lou Z A c-Myc-SIRT1 feedback loop regulates cell growth and transformation. The Journal of cell biology 185, 203–211, doi: 10.1083/jcb.200809167 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.García-Martínez JM et al. Vitamin D induces SIRT1 activation through K610 deacetylation in colon cancer. bioRxiv, 2023.2002.2022.529558, doi: 10.1101/2023.02.22.529558 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.O’Mahony C et al. Dietary-Induced Bacterial Metabolites Reduce Inflammation and Inflammation-Associated Cancer via Vitamin D Pathway. International journal of molecular sciences 24, doi: 10.3390/ijms24031864 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhao J et al. p53 promotes peroxisomal fatty acid beta-oxidation to repress purine biosynthesis and mediate tumor suppression. Cell Death Dis 14, 87, doi: 10.1038/s41419-023-05625-2 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhang Y, Zhang J, Xia Y & Sun J Bacterial translocation and barrier dysfunction enhance colonic tumorigenesis. Neoplasia 35, 100847, doi: 10.1016/j.neo.2022.100847 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dasari S et al. Neferine, an alkaloid from lotus seed embryo targets HeLa and SiHa cervical cancer cells via pro-oxidant anticancer mechanism. Phytotherapy research : PTR 34, 2366–2384, doi: 10.1002/ptr.6687 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yang J et al. Combined effects of vitamin D and neferine on the progression and metastasis of colorectal cancer. Journal of cancer research and clinical oncology, doi: 10.1007/s00432-022-04552-7 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Saginala K, Barsouk A, Aluru JS, Rawla P & Barsouk A Epidemiology of Melanoma. Med Sci (Basel) 9, doi: 10.3390/medsci9040063 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kanasuo E, Siiskonen H, Haimakainen S, Komulainen J & Harvima IT Regular use of vitamin D supplement is associated with fewer melanoma cases compared to non-use: a cross-sectional study in 498 adult subjects at risk of skin cancers. Melanoma Res 33, 126–135, doi: 10.1097/CMR.0000000000000870 (2023). [DOI] [PubMed] [Google Scholar]
- 110.Gracia-Darder I et al. Vitamin D deficiency in melanoma patients is associated with worse overall survival: a retrospective cohort study. Melanoma Res 32, 384–387, doi: 10.1097/CMR.0000000000000842 (2022). [DOI] [PubMed] [Google Scholar]
- 111.Reichrath J et al. Low Vitamin D Status Predicts Poor Clinical Outcome in Advanced Melanoma Treated With Immune Checkpoint or BRAF/MEK Inhibitors: A Prospective Non-Interventional Side-by-Side Analysis. Front Oncol 12, 839816, doi: 10.3389/fonc.2022.839816 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sutedja EK et al. Calcitriol Inhibits Proliferation and Potentially Induces Apoptosis in B16-F10 Cells. Med Sci Monit Basic Res 28, e935139, doi: 10.12659/MSMBR.935139 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Shariev A et al. PTEN: A novel target for vitamin D in melanoma. The Journal of steroid biochemistry and molecular biology 218, 106059, doi: 10.1016/j.jsbmb.2022.106059 (2022). [DOI] [PubMed] [Google Scholar]
- 114.Zhang W et al. Global, regional and national incidence, mortality and disability-adjusted life-years of skin cancers and trend analysis from 1990 to 2019: An analysis of the Global Burden of Disease Study 2019. Cancer Med 10, 4905–4922, doi: 10.1002/cam4.4046 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Whitfield GK et al. Functionally relevant polymorphisms in the human nuclear vitamin D receptor gene. Molecular and cellular endocrinology 177, 145–159, doi: 10.1016/s0303-7207(01)00406-3 (2001). [DOI] [PubMed] [Google Scholar]
- 116.Bullock TA et al. Significant association of Poly-A and Fok1 polymorphic alleles of the vitamin D receptor with vitamin D serum levels and incidence of squamous cutaneous neoplasia. The Journal of investigative dermatology, doi: 10.1016/j.jid.2023.01.028 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Uitterlinden AG, Fang Y, Van Meurs JB, Pols HA & Van Leeuwen JP Genetics and biology of vitamin D receptor polymorphisms. Gene 338, 143–156, doi: 10.1016/j.gene.2004.05.014 (2004). [DOI] [PubMed] [Google Scholar]
- 118.Zhang X et al. DNA damage-inducible transcript 4 is an innate guardian for human squamous cell carcinoma and an molecular vector for anti-carcinoma effect of 1,25(OH)(2) D(3). Experimental dermatology 28, 45–52, doi: 10.1111/exd.13815 (2019). [DOI] [PubMed] [Google Scholar]
- 119.Voutilainen A et al. Multiplicative, additive, and interactive associations of 25-hydroxyvitamin D with lung and prostate cancer. International journal for vitamin and nutrition research. Internationale Zeitschrift fur Vitamin- und Ernahrungsforschung. Journal international de vitaminologie et de nutrition, doi: 10.1024/0300-9831/a000780 (2023). [DOI] [PubMed] [Google Scholar]
- 120.Kato I et al. Association of calcium and vitamin D supplementation with cancer incidence and cause-specific mortality in Black women: Extended follow-up of the Women’s Health Initiative calcium-vitamin D trial. Int J Cancer, doi: 10.1002/ijc.34436 (2023). [DOI] [PubMed] [Google Scholar]
- 121.Visvanathan K et al. Circulating vitamin D and breast cancer risk: an international pooling project of 17 cohorts. Eur J Epidemiol 38, 11–29, doi: 10.1007/s10654-022-00921-1 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bouillon R et al. Vitamin D and cancer. J Steroid Biochem Mol Biol 102, 156–162, doi:S0960–0760(06)00258–5 [pii] 10.1016/j.jsbmb.2006.09.014 (2006). [DOI] [PubMed] [Google Scholar]
- 123.Holick MF Vitamin D: evolutionary, physiological and health perspectives. Curr Drug Targets 12, 4–18, doi:BSP/CDT/E-Pub/00159 [pii] (2011). [DOI] [PubMed] [Google Scholar]
- 124.Holick MF Vitamin D and sunlight: strategies for cancer prevention and other health benefits. Clin J Am Soc Nephrol 3, 1548–1554, doi:CJN.01350308 [pii] 10.2215/CJN.01350308 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wheeler BJ et al. A Brief History of Nutritional Rickets. Front Endocrinol (Lausanne) 10, 795, doi: 10.3389/fendo.2019.00795 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]


