Abstract
Achieving a cure for HIV infection is a global priority. There is substantial evidence supporting a central role for CD8+ T cells in the natural control of HIV, suggesting the rationale that these cells may be exploited to achieve remission or cure of this infection. In this work, we review the major challenges for achieving an HIV cure, the models of HIV remission, and the mechanisms of HIV control mediated by CD8+ T cells. In addition, we discuss strategies based on this cell population that could be used in the search for an HIV cure. Finally, we analyze the current challenges and perspectives to translate this basic knowledge toward scalable HIV cure strategies.
Keywords: HIV, Cure, Remission, CD8+ T cells, Immunotherapy
1. Introduction HIV cure: a global priority
The worldwide occurrence of HIV infection poses a significant issue for public health on a global scale, with an approximate 38.4 million individuals affected by HIV in 2021 [1]. In addition to this widespread prevalence, the efforts made by countries to address the HIV epidemic have been inadequate. There exists a delay in diagnosis and a low detection rate [2,3], and only 75% of people living with HIV received antiretroviral therapy (ART) in 2021 [1]. Numerous factors, such as adverse effects of medication, logistical challenges in accessing drugs, and the financial burden associated with lifelong treatment, contribute to suboptimal adherence to ART [4,5]. For instance, individuals receiving ART have demonstrated an increased susceptibility to cardiovascular disease, kidney problems, and bone disorders [6,7]. The clinical status before therapy initiation, virological factors (such as drug resistance mutations), sociodemographic aspects, and inadequate adherence to treatment, all contribute to an ongoing risk of developing resistance to therapy and subsequent treatment failure [8]. Additionally, the absence of a vaccine with satisfactory efficacy in preventing new infections adds to these challenges [9]. Lastly, the persistent social stigma attached to being an HIV carrier exacerbates the situation. These obstacles emphasize the urgent need for finding a cure for HIV, both in terms of public health and global research. A safe, effective, scalable, and cost-effective intervention that promotes sustained virus control in the absence of ART would provide a powerful tool for eventual epidemic control [10].
2. Why is it so difficult to find a cure for HIV?
Despite the identification of HIV in the mid-80s and important research efforts have been made to understand its pathophysiology and the mechanisms for viral control, there is no effective and scalable strategy to achieve disease remission. Numerous factors contribute to the challenge of finding a cure for HIV infection, linked to the characteristics of viral latency and intrinsic genetic variability of retroviruses. Furthermore, HIV exhibits various mechanisms of immune evasion, such as its location in multiple tissues with limited access to immune effector cells. Of note, antiretroviral drugs block infection in susceptible cells and inhibit active virus replication, but do not act on integrated proviruses in cells with latent infection [11]. The main mechanisms that determine the difficulty in achieving a cure for HIV, and the impact of the CD8+ T cell response against HIV, are described below.
2.1. HIV latency
HIV belongs to the retroviridae family, which has mechanisms to establish latent reservoirs in host cells. These viral reservoirs are characterized by cells harboring integrated viral DNA (known as provirus) that are transcriptionally silent. However, upon cellular activation, these reservoirs have the capability to generate infectious viral particles [11]. Macaque studies have demonstrated that the lentivirus reservoir is rapidly established upon viral exposure, escaping to innate and adaptive immune effector mechanisms [12]. After reservoir seeding, several mechanisms favor HIV deep latency, including epigenetic repression of HIV transcription [13], temporary absence of host transcriptional factors [14,15], and provirus integration in non-genic regions of the genome [16]. The low rate of replication in viral reservoirs determines their high stability over time. For example, previous studies have shown that proviruses with replicative capacity can persist free of selective pressure in long-lived CD4+ T cells [17]. In addition, it has been estimated that the viral reservoir has a half-life of 44 months [18], so it would take more than 70 years to naturally eradicate a reservoir consisting of only 106 cells [19]. Importantly, CD8+ T cell escape and resistance mutations [20,21], as well as resistance to type 1 interferons (IFN) and antibodies [22,23], can be found in the HIV reservoir, determining a further challenge for the design of HIV cure immunotherapies.
Intriguingly, CD8+ T cells play a dual role in the immune response against HIV. On one hand, they effectively eliminate HIV-infected cells, while on the other hand, they release soluble molecules that interact with infected CD4+ T cells. This interaction triggers a series of signaling events that lead to non-cytolytic suppression of HIV, inhibition of viral transcription, and promotion of cellular quiescence and stemness. These processes, in turn, may contribute to the establishment of viral latency [24,25]. For instance, the CD8+ T cell antiviral factor (CAF) was reported to inhibit HIV transcription by reducing the association of RNA polymerase II with the HIV promoter [26]. More recently, it has been discovered that CD8+ T cells express ligands that bind to Wnt-frizzled receptors, thereby activating the canonical Wnt/β-catenin signaling pathway. This activation leads to the suppression of HIV transcription in infected cells [27]. In addition, it was reported that this non-cytolytic CD8+ T cell-mediated suppression of HIV replication is independent of the major histocompatibility complex (MHC), and is linked to the silencing of the LTR-dependent viral transcription, and the decrease of CD4+ T cell activation and proliferation [25,28]. These mechanisms explain in part the enhancement of latency reversal mediated by several compounds when accompanied by CD8+ T cell depletion in simian immunodeficiency virus (SIV)-infected macaques [29,30], as well as the loss of ART-mediated viral suppression in SIV-infected macaques when CD8+ T cells are depleted [31].
2.2. High viral diversity
The extraordinary genetic diversity in the circulating subtypes of HIV has hampered the development of a cure [32]. There are at least three mechanisms that explain the variability of HIV: i) the high error rate of the viral reverse transcriptase [33]; ii) recombination phenomena of the strands of the viral genome [34,35]; iii) cellular factors such as APOBEC3G (apolipoprotein B mRNA editing enzyme, catalytic polypeptide-like) and RNA polymerase II, which may induce changes in the viral nucleotide sequence [36,37]. Together, these mechanisms induce a mutation rate estimated at 3.4 × 10−5 substitutions/site/replication cycle [38], leading to the generation of circulating subtypes and recombinant forms that harbor antiretroviral resistance and immune evasion mutations.
2.3. Immune evasion
In addition to the antigenic escape product of viral mutations and CD4+ T cell depletion, HIV has additional mechanisms for immune evasion. For example, it has been shown that HIV evades recognition by intracellular receptors such as Cyclic GMP-AMP synthase (cGAS), and Toll-like receptors 7, 8, and 9 inhibiting the production of type I IFN, critical for the control of viral replication [39]. Additionally, the viral protein Nef induces the downregulation of human leukocyte antigen (HLA) class I molecules, preventing recognition by CD8+ T cells [40]. On the other hand, throughout the process of HIV entry into the host cell, the virions can mask specific functional domains of the gp120 glycoprotein, thus eluding detection by antibodies [41]. Taken together, these mechanisms are associated with poor natural control of HIV replication by the host immune system.
2.4. Localization of the virus in multiple tissues with limited access to immune effector cells
Lentiviruses are localized in multiple tissues that are not easily accessible to immune cells. For example, analysis in SIV-infected macaques determined virus localization in tissues such as lymph nodes, intestine, brain, spleen, heart, kidney, lung, and liver [42]. The case of secondary lymphoid organs is relevant considering that lymphoid follicles are the main reservoirs of the virus [11], and there is poor access of cytotoxic cells to these compartments [43]. Another relevant compartment is the central nervous system, where resident macrophages and astrocytes, can be infected by HIV, persisting for a long time due to the limited access to immune effector cells [44].
2.5. Chronic immune activation and immune exhaustion
Chronic immune activation is one of the main features of HIV infection [45]. The mechanisms that determine persistent immune activation include: i) immunomodulatory functions of viral proteins and immune response against the virus; ii) reactivation of other latent infections in the individual; iii) loss of gastrointestinal mucosal integrity, followed by microbial translocation; iv) alteration in the balance of CD4+ T cells; and v) increased production of proinflammatory cytokines [46]. One consequence of chronic immune activation and antigen persistence is immune exhaustion, particularly in CD8+ T cells, which impairs the functional capacity and viral control, associated with the increased expression of inhibitory receptors such as programmed death (PD)-1 and T-cell immunoglobulin and mucin-domain containing-3 (TIM-3), metabolic dysregulation, and poor cell survival [47].
3. Models of functional cure of HIV: learning from the exceptions
The major proportion of untreated people with HIV suffers progressive disease, developing acquired immune deficiency syndrome (AIDS). However, a small proportion of individuals (<1%), known as elite controllers, maintain undetectable viremia for a long time in the absence of ART, suggesting the possibility of functional remission of the disease [48]. Viral and host-related mechanisms of resistance have been reported in HIV elite controllers [49]. The mechanisms with the major evidence are related to the adaptive immune response mediated by CD8+ T cells. Certain HLA class I alleles, such as HLA-B*27 and -B*57, are overrepresented in elite controller individuals [[50], [51], [52]], which are associated with better presentation of conserved viral peptides to CD8+ T cells [[53], [54], [55]]. Although it is not always the case, the expression of protective HLA class I alleles is associated with a greater functional capacity of CD8+ T cells [[56], [57], [58]].
HIV post-treatment controllers are the second model of a functional cure. These individuals are characterized by symptomatic acute infection that requires ART initiation. However, upon treatment interruption, these individuals can maintain low to undetectable viremia for a long time [59]. Although not well identified yet, the mechanisms of post-treatment control are related to the early ART initiation and maintenance of therapy for ∼2–3 years [60,61], which appear to limit the size and quality of the viral reservoir [60,62]. On the other hand, post-treatment controllers usually do not exhibit the classic protective HLA class I alleles and have significantly lower HIV-specific CD8+ T cell responses, in terms of their relative frequency ex vivo and HIV-suppressive capacity in vitro, compared with HIV elite controllers [60,[63], [64], [65]]. Moreover, a study also reported a poor proliferative capacity of HIV-specific CD8+ T cells in a post-treatment controller [66]. However, long-term ART initiated early in acute infection preserves functional HIV-specific CD8+ T cells with enhanced stemness, and proliferative and cytolytic potential, attributes which correlate with longer time to viral rebound after treatment interruption [67]. In line with these data, at least in a proportion of post-treatment controllers CD8+ T cells may play a critical role in sustained viral control [64]. In addition to cellular immunity, in a fraction of post-treatment controllers, the antibody response appears to play a relevant role in viral control [64,68]. Therefore, the study of post-treatment controllers may inform the design of novel immune therapies aiming at HIV functional remission.
4. Mechanisms of CD8+ T cell-mediated HIV control
CD8+ T cells are an integral part of the adaptive immune system and play a crucial role in safeguarding against intracellular pathogens and malignancies. Their function entails identifying peptides displayed by class I MHC molecules on antigen-presenting cells or target cells, resulting in the eradication of infected or transformed cells [69,70]. CD8+ T cells fulfill these biological functions through different effector mechanisms. Firstly, cytolytic mechanisms, which include the directed release of molecules such as perforin and granzymes, induce the death of target cells [[71], [72], [73]]. In addition to the cytolytic mechanisms to suppress HIV transcription previously described, CD8+ T cells also produce soluble factors that contribute to the antiviral response, including β−chemokines (CCL4, CCL5), and cytokines such as IFN-γ, tumor necrosis factor (TNF)-α and interleukin (IL)-17 [74]. In the context of HIV, β-chemokines prevent virus binding to the CCR5 co-receptor on CD4 + T cells [75]; IFN-γ induces the expression of MHC molecules and macrophage activation [76,77], while IL-17 promotes the integrity of the gastrointestinal mucosa [78].
Apart from the association observed between various HLA class I alleles and innate control of HIV, additional evidence substantiates the significant contribution of CD8+ T cells in the control of HIV infection: i) there is a temporal association between the decrease in viremia in individuals with acute infection and the appearance of HIV-specific CD8+ T cells [79,80]; ii) The persistence of HIV-specific CD8+ T cells with memory potential upon early ART initiation correlate with a lower HIV reservoir [81]; iii) HIV natural controllers and non-progressors maintain HIV-specific CD8+ T cells with enhanced functionality and cytotoxic capacity [57,58]; iv) the virus generates escape mutations to evade immune pressure from CD8+ T cells [82]. Furthermore, early macaque studies demonstrated that CD8+ T cell depletion leads to an increase in plasma viremia, and there is a decrease in viral replication upon CD8+ T cell reconstitution [83,84]. In addition, CD8+ T cell responses contribute to post-analytical treatment interruption (ATI) viral load set point in SIV-infected macaques [85]. Thus, considering the relevant anti-HIV role of CD8+ T cells, they represent an attractive option for immune therapies against this infection [86].
5. CD8+ T cell-based strategies to cure HIV infection
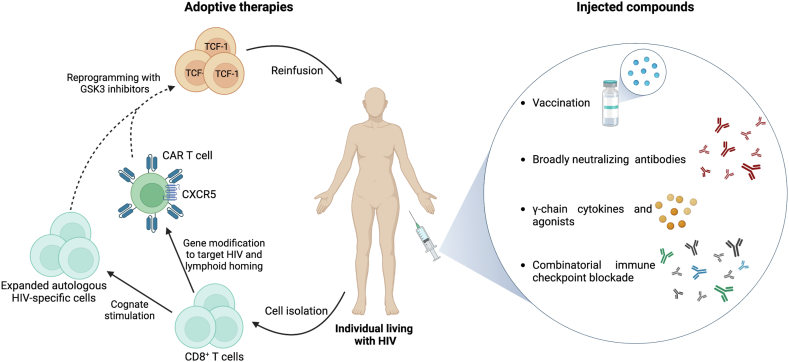
Currently, several strategies aiming at HIV cure exploit the potent antiviral potential of CD8+ T cells (Fig. 1). These include stimulating endogenous HIV-specific cell responses through vaccines and neutralizing antibodies (NAb), expansion and in vitro reprogramming of CD8+ T cells to enhance the number, functionality, and in vivo survival, and the use of cells with modified or chimeric antigen receptors (CAR). From a clinical perspective, these CD8+ T cell-based strategies could be administered to the patient in the form of injected compounds (such as vaccines, neutralizing antibodies, immunomodulators, or immune checkpoint blocking antibodies), or by adoptive cell therapy approaches (in vitro reprogrammed cells and CAR T cells). Importantly, these strategies may promote immune-mediated clearance of HIV-infected cells, but they need to be accompanied by reactivation of virus production using latency reversing agents, as well as using pro-apoptotic agents and other immunomodulatory strategies [87,88]. As such, it is unlikely that CD8+ T cell-based approaches alone will eliminate the HIV reservoir but constitute an important arm of HIV cure strategies.
Fig. 1.
CD8+T cell-based immunotherapeutic strategies for HIV cure. Various strategies seek to enhance the number and functional attributes of CD8+ T cells to favor viral control and can be divided into injected compounds and adoptive therapies. Injected compounds include i) vaccination and broadly neutralizing antibodies to promote endogenous CD8+ T cell responses, ii) γ-chain cytokines such as IL-15 and superagonists, which expand them and promote their cytotoxic capacity, and iii) combinatorial immune checkpoint blockade. For adoptive therapies, autologous CD8+ T cells could be isolated for cognate in vitro stimulation with HIV peptides to expand virus-specific cells, or for gene modification to generate CAR T cells. Chemokine receptors, such as CXCR5, could also be inserted into the genome of engineered cells to promote migration into lymphoid follicles. Expanded autologous HIV-specific cells or CAR T cells could be further reprogrammed with GSK3 inhibitors to promote TCF-1 expression and stemness, which may contribute to long-lived memory responses. Finally, in vitro-manipulated cells can be reinfused into the individual.
5.1. Stimulation of endogenous HIV-specific CD8+ T cells through vaccines and neutralizing antibodies
Vaccination has been considered throughout history a successful medical intervention for the control of infectious diseases. The objective of preventive vaccination is to induce the production of neutralizing antibodies and memory T cells, which in the context of HIV, would prevent the acquisition of the infection [89]. Another approach to vaccines is therapeutic, where the aim is to induce and/or increase adaptive immune responses that help control an already-established infection [90]. For HIV vaccines that induce CD8+ T cell responses, the outcome could potentially result in sustained viral load reduction in the absence of ART over an extended duration [91]. These types of vaccines include DNA plasmids, viral vectors, RNA-based vaccines, selected peptides based on conserved regions of HIV proteins, and dendritic cell-based vaccines [92,93]. Most of the vaccines have not passed clinical trials I and II, so the effectiveness and immunogenicity of a large proportion of them are unknown [94].
Vaccine trials in non-human primates (NHP) have shown that in animals immunized and subsequently infected with SIV, despite not inhibiting the infection, peak viremia and viral set point is reduced, largely due to the response of virus-specific CD8+ T cells [[95], [96], [97], [98]]. In humans, one of the vaccines that made the most progress in terms of clinical trials was the one based on adenovirus rAd5. This vaccine directly targeted the CD8+ T cell response, as it contained genes encoding Gag, Pol, and Nef proteins (immunodominant for CD8+ T cells), but not Env (immunodominant for humoral responses). Although this vaccine induced a strong HIV-specific CD8+ T cell response, it did not demonstrate effectiveness in protecting volunteers against acquiring the infection, nor did it reduce viral loads after infection [99,100]. These results indicate that additional strategies beyond the induction of endogenous CD8+ T cell responses are needed. For example, in a model of macaques with established SIV infection and immunized with a vaccine that promoted virus-specific CD8 + T cells, adoptive transfer of in vitro-expanded autologous CD8+ T cells helped to limit viral replication or viral rebound upon ART discontinuation [101]. Therefore, strategies that include the induction/amplification of endogenous CD8+ T cell responses through vaccines, combined with in vitro expansion and/or administration of co-adjuvants, would be more effective in achieving viral control.
The vaccine approaches employed in NHP and humans, as mentioned earlier, utilized non-persistent vectors, and aimed to generate conventional anamnestic CD8+ T cell responses characterized by a central memory, long-lived profile. These cells can expand and differentiate into effector populations after antigen re-stimulation (SIV or HIV challenge), but their expansion is delayed in relation to peak viral replication and is not sufficient to control the systemic spread of infection. Thus, vaccine-induced anamnestic CD8+ T cell responses can contain but not suppress infection in the long-term [102]. Based upon this evidence, it was hypothesized that induction of virus-specific CD8+ T cells with an effector memory profile might be a more effective approach for early and sustained SIV/HIV control. Supporting this assumption, SIV vaccines that include the persistent rhesus cytomegalovirus (RhCMV) vector elicited and maintained high frequencies of SIV-specific T cells with an effector memory-biased profile, which contributed to early and sustained viral control in >50% of animals vaccinated [103,104]. Notably, RhCMV/SIV vectors can elicit non-canonical MHC-II and MHC-E-restricted CD8+ T cells that can recognize universal epitopes termed supertopes [105,106], and are essential for RhCMV/SIV vaccine efficacy [107]. This novel mechanism of CD8+ T cell-mediated protection may exploit SIV/HIV immune-evasion adaptations, such as down-modulation of MHC-I molecules and upregulation of MHC-E.
In addition to the vaccine-mediated stimulation of CD8+ T cell responses, it was recently shown that the administration of NAb in macaques with Simian-Human Immunodeficiency Virus (SHIV) infection, or in individuals with HIV, promotes the function of virus-specific CD8+ T cells. This effect occurs probably through the modulation of T cell priming, and this vaccinal effect explains, at least in part, the sustained viral control in these individuals [[108], [109], [110]]. In this context, NAb-HIV immune complexes may activate dendritic cells to increase their capacity for conventional and crossed antigen presentation [109], but new studies are required to elucidate the mechanisms involved. Thus, immunotherapy with monoclonal antibodies offers not only viremia control mediated by virus neutralization, but also enhances the endogenous cellular response, promoting long-lived memory responses.
5.2. Cytokines and immune adjuvants
Several cytokines of the γ−common, including IL-2, IL-4, IL-7, IL-9, IL-15, and IL-21, promote the effector response of CD8+ T cells, as well as the survival of long-lived memory cells [111]. For example, IL-2 promotes the proliferation and accumulation of memory CD8+ T cells [112]; IL-7 promotes the homeostasis of naïve and memory CD8+ T cells [113,114], while IL-15 and IL-21 promote the survival of HIV-specific CD8+ T cells, the production of IFN-γ, the expression of cytotoxic molecules, and control of HIV in vitro [[115], [116], [117]]. IL-15 also promotes the metabolic plasticity of HIV-specific CD8 + T cells, a characteristic of cells derived from elite controllers [117].
Systemic administration of IL-7 or IL-21 has been explored in macaques with SIV infection. In general, the results showed low toxicity of the treatment, with induction of CD4+ and CD8+ T cell proliferation by IL-7, as well as greater expression of cytotoxic molecules induced by IL-21; however, systemic administration of these cytokines does not induce changes in viral load or viral set point [118,119]. Interestingly, comparative in vitro studies among IL-2, IL-7 and IL-15 showed that the latter cytokine is the most potent to increase Gag-specific CD8+ T cell responses [120], so it would be a good candidate for immunotherapy. Importantly, IL-15 also promotes in vitro HIV reactivation in latently infected cells [121]. However, while therapeutically-administered IL-15 is rapidly cleared from plasma in vivo, systemic administration of high doses of this cytokine can cause significant adverse effects and toxicity [122]. Therefore, this cytokine could be used as a vaccine adjuvant or as a stimulant for in vitro cell expansion and induction of effector properties. For example, immunization with HIV vaccines in vectors that also express IL-15 induced an increase in memory CD8+ T cells that persisted for several months and showed high proliferative capacity [123].
Considering that, in vivo, IL-15 effects require interaction with the IL-15 receptor alpha (IL-15Rα) [124,125], additional strategies have been devised to exploit and potentiate its function. For instance, N-803 is an IL-15 superagonist designed to increase IL-15 activity. Specifically, N-803 consists of a complex between an IL-15 mutant (containing an asparagine to aspartic acid mutation at position 72, allowing a more stable heterodimeric complex with IL-15Rα) and an IL15Rα: IgG1 Fc fusion protein. These structural properties confer to N-803 higher biological activity and longer half-life than soluble IL-15, which allow potent stimulation of NK and memory T cells [126,127]. An alternative to N-803 is the heterodimeric IL-15 (hetIL-15), a stable native complex of IL-15 and IL-15Rα. Previously, hetIL-15 has been shown to promote proliferation and effector functions of adoptively transferred tumor-specific T cells [128]. Importantly, N-803 and hetIL-15 increase cytotoxic responses and upregulate CXCR5 expression. This latter effect allows the migration of CD8+ T cells into the lymphoid follicles in SIV or SHIV-infected macaques, associated with a transient reduction in viral replication [[129], [130], [131], [132]]. In line with a role of IL-15 in promoting protective follicular cytotoxic responses, nonpathogenic SIV infection in African green monkeys was associated with enhanced NK cell migration into follicles and the presence of high levels of this cytokine presented in its membrane-bound form on follicular dendritic cells [133]. The safety and virologic impact of N-803 was recently evaluated in a phase 1 study in people living with HIV. Notably, the drug was safe and well-tolerated, with a modest reduction in the circulating inducible reservoir. In addition, N-803 treatment was associated with T cell and natural killer (NK) cell activation [134]. Larger clinical trials in ART-suppressed individuals will elucidate the effectiveness of N-803 for the promotion of follicular cytotoxic responses, latency reversal, and purge of the HIV reservoir.
In addition to cytokines of the γ-common family, other cytokines that induce a particular functional profile can also be used in vitro. An example is the induction of follicular-like CXCR5+ cells with the combination of transforming growth factor (TGF)−β and IL-23 [135], aiming at redirecting CD8+ T cells towards lymphoid follicles, an important viral replication, and reservoirs site.
Additionally, various adjuvants that promote the priming of CD8+ T cells by antigen-presenting cells through the activation of pattern-recognition receptors have been explored [136]. For example, agonist ligands of the cGAS-stimulator of interferon genes (STING) pathway have shown effectiveness as adjuvants to increase adaptive immune responses, particularly in pre-clinical trials of immunotherapy against tumors [137]. Through the induction of type I IFN, STING ligands such as cGAMP co-administered to mice together with vaccines promote the expansion and maturation of antigen-specific CD8+ T cells, conferring subsequent protection against tumors or viral infections [138]. Similar strategies could be used in the context of HIV, with the administration of vaccines along with these adjuvants, or through in vitro priming and expansion of virus-specific CD8+ T cells in the presence of cGAMP, followed by adoptive transfer.
Another activator of the innate immune response, that in turn would promote adaptive immunity, is the toll-like receptor (TLR)-7 agonist, vesatolimod, which exhibits latency reversal properties [139]. Previous studies in SIV-infected macaques demonstrated that a combination of vesatolimod with neutralizing antibodies or vaccination promotes viral control following ATI. SIV-specific T cell responses and T cell activation were the strongest correlates of virologic control [140,141]. Importantly, in a recent phase 1b clinical trial in people with HIV, vesatolimod was safe and well-tolerated and contributed to a modest delay in viral rebound upon ATI. In addition, vesatolimod induced interferon-stimulated gene expression, cytokine production, and an increase in activated and proliferating T cells [142].
5.3. Immune checkpoint blockade
Since CD8+ T cells acquire a state of functional exhaustion due to the persistent antigenic load, the inflammatory environment, and the expression of immune checkpoint receptors, a strategy to promote their function is to block certain inhibitory signals. To date, monoclonal antibodies that inhibit CTLA-4 (ipilimumab), PD-1 (pembrolizumab, nivolimumab, cemiplimab), and PD-1 ligand 1 (PD-L1; durvalumab, atezolizumab, avelumab) receptors have been approved for clinical use [143]. Anti-PD-1 antibodies have been used for therapy in a small number of people with HIV and cancer. In general, good tolerance and safety have been observed. In addition, effectiveness of immunotherapy has been observed to combat different types of cancer, as well as an increase in the CD4+ T cell count after immunotherapy, without viral rebound [144]. Other clinical trials are currently underway to evaluate the effectiveness of these immunotherapies in individuals with HIV who also have various solid cancers and lymphomas [145], as well as individuals without malignancy [146,147]. Promisingly, the use of antibodies against CTLA-4, PD-1, or PD-L1 induced an increase in the levels of cell-associated unspliced HIV RNA (indicative of latency reversal), as well as a rise in the frequency of Gag-specific CD8+ T cells that produced IFN-γ, TNF-α or CD107a in a subset of participants [147,148]. However, it should be emphasized that these results come from small clinical trials or case reports, and substantial variability is observed between studies and individuals [149]. Moreover, between 10 and 30% of patients receiving immune-checkpoint inhibitors may suffer serious immune-related adverse events [150], as has been observed in people with HIV [151]. Thus, preliminary data indicate that only a subset of people with HIV will respond to and benefit from immune checkpoint blockade, while there is a high risk of toxicities.
Of note, monotherapy with anti-PD-1 for cancer in people living with HIV is insufficient to improve virus-specific responses, probably linked to a terminal exhaustion state of HIV-specific CD8+ T cells [152]. More promising results have been obtained upon the combination of immune checkpoint blockade. As such, enhanced IL-2 and CD107a production by HIV-specific T cells was observed when cells were stimulated ex vivo with viral peptides in the presence of a combination of antibodies against Lymphocyte-activation gene 3 (LAG-3), Cytotoxic T-Lymphocyte Antigen 4 (CTLA-4), or T cell immunoreceptor with Ig and ITIM domains (TIGIT) [153]. Therefore, blocking multiple signals that inhibit effector immune mechanisms seems promising to promote more effective responses of CD8+ T cells against HIV. Moreover, a combinatory strategy that includes vaccination plus immune checkpoint blockade has shown promising results in ART-suppressed SIV-infected macaques. As such, macaques immunized with a CD40L plus TLR7 agonist–adjuvanted DNA/modified vaccinia Ankara vaccine along with anti-PD-1 therapy exhibited a higher frequency of granzyme B+ perforin+ CD8+ T cells in blood and lymph nodes, particularly within lymphoid follicles. This effect was associated with reductions in the viral reservoir, and a lower viral set point upon ATI [154]. Thus, PD-1 blockade may improve the therapeutic benefits of vaccination, and these combinatorial strategies should be further explored in HIV cure studies.
5.4. Adoptive therapies of engineered and reprogrammed CD8+ T cells
In addition to endogenous cells, CD8+ T cells whose TCR antigen receptors have been modified to recognize conserved viral peptides are another option to augment HIV-specific CD8+ T cell responses. One such example is CD8+ T cells with TCRs recognizing the SL9 epitope of the Gag protein, which exhibited potent lytic induction ability and reduced infected cells in vivo in SCID mice [155]. However, these cells can eventually recognize autoantigens, which have been associated with cardiac toxicity, limiting their clinical use [156]. Another option for CD8+ T cells with modification or redirection of their receptors are cells with chimeric receptors, which constitute a promising strategy for the treatment of HIV infection [157]. The use of these cells could overcome the limitations of endogenous CD8+ T cells, such as viral escape, HLA restriction, and immune exhaustion [158]. Previous in vitro and in vivo studies in humanized mice demonstrated that CD4-based CAR T cells containing the 4-1BB costimulatory domain efficiently control HIV replication [159]. More recently, a NAb-based CAR T cell therapy was evaluated in a small number of people living with HIV who underwent ATI. Notably, CAR T cell therapy was safe and well-tolerated, and induced a decrease of cell-associated viral RNA and intact proviruses, delaying viral rebound upon treatment interruption [160]. Another recently described platform is known as convertible CAR T cells. In this platform, an inert form of the human NKG2D extracellular domain was engineered as the ectodomain of the CAR for expression on CD8+ T cells. These CAR T cells were specifically directed to kill HIV-infected cells only in the presence of an activating bispecific antibody based on bNAb and a mutated form of the NKG2D ligand MIC/ULBP. Promisingly, convertible CAR T cells efficiently eliminate the inducible latent reservoir in cells derived from people with HIV in ex vivo assays, hence constitute a promising tool for attacking the latent HIV reservoir [161]. In addition, CAR T cells may also include the expression of receptors such as CXCR5, which directs the cells to lymphoid follicles [162]. In this regard, a recent study in SIV-infected rhesus macaques demonstrated that the adoptive transfer of CAR-T/CXCR5 cells favored the migration of these cells to lymphoid follicles, in proximity to infected cells. This cell adoptive strategy was associated with a reduction in systemic viral load upon ART interruption, without major adverse effects [163].
Parallel to cell engineering, emerging approaches to cell reprogramming by targeting metabolic or transcriptional regulators has demonstrated important therapeutic benefits in tumor and chronic infection models [[164], [165], [166]]. Previous studies have shown that stem-like memory HIV-specific CD8+ T cells expressing the transcription factor T cell factor 1 (TCF-1), endowed with potent antiviral activity and metabolic plasticity, are correlates of natural HIV and SIV control [117,[167], [168], [169]]. Based on this rationale, it was recently explored the potential of reprogramming HIV-specific CD8+ T cells from people with HIV under ART (non-controllers) to acquire properties found in HIV natural controllers. By upregulating the TCF-1 pathway with a glycogen synthase kinase 3 (GSK3) inhibitor, in vitro reprogrammed HIV-specific CD8+ T cells acquired a stem-like memory profile, with enhanced survival, polyfunctionality, metabolic plasticity, and HIV-suppressive activity [170]. These properties of such reprogrammed cells could boost the immunomodulatory potential of other therapies, such as IL-15 or CAR T cells, and these approaches could be combined to promote long-lived memory CD8+ T cells against HIV.
6. Conclusions
Although ART is efficient for the control of HIV replication, in certain cases it is an unsustainable strategy and brings negative consequences for people living with HIV, such as multi-organ disease. A cure for HIV would certainly improve the long-term health of people living with HIV, reducing community-level transmission. Viral latency, its huge diversity, multiple tissue localizations, and immune evasion mechanisms determine major barriers to an HIV cure. Since used alone CD8+ T cell-based strategies have not shown relevant clinical benefit, the combination of such strategies, along with early ART initiation in people with HIV, seems to be the way to achieve more encouraging outcomes in HIV cure studies. The study of the mechanisms for natural and post-treatment CD8+ T cell-mediated HIV control, and the transcriptional and metabolic regulators of their activity, will elucidate novel targets for the design of immune therapies.
Funding statement
This work was supported by Ministerio de Ciencia y Tecnología, Colombia(code 111577757051 to MTR) and Uniremington.
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Data availability statement
Data included in article/supp. material/referenced in article.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Federico Perdomo-Celis, Email: perdomo_federico@javeriana.edu.co.
Natalia Taborda, Email: natalia.taborda@uniremington.edu.co.
References
- 1.UNAIDS . 2022. Global HIV & AIDS Statistics — Fact Sheet; pp. 1–6.https://www.unaids.org/en/resources/fact-sheet (last accessed in june 13, 2023) [Google Scholar]
- 2.Wang H., Wolock T.M., Carter A., Nguyen G. Estimates of global, regional, and national incidence, prevalence, and mortality of HIV, 1980-2015: the Global Burden of Disease Study 2015. Lancet HIV. 2016;3:e361–e387. doi: 10.1016/S2352-3018(16)30087-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gökengin D., Oprea C., Uysal S., Begovac J. The growing HIV epidemic in Central Europe: a neglected issue? J. Virus Erad. 2016;2:156–161. doi: 10.1016/S2055-6640(20)30459-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lewin S.R., Attoye T., Bansbach C., Doehle B., Dubé K., Dybul M., SenGupta D., Jiang A., Johnston R., Lamplough R., McCune J.M., Nabel G.J., Ndung’u T., Pottage J., Ripin D., Rooney J.F., Sikazwe I., Nsubuga M., Warren M., Deeks S.G. Multi-stakeholder consensus on a target product profile for an HIV cure. Lancet HIV. 2021;8:e42–e50. doi: 10.1016/S2352-3018(20)30234-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beacroft L., Hallett T.B. The potential impact of a “curative intervention” for HIV: a modelling study. Glob. Heal. Res. Policy. 2019;4:2. doi: 10.1186/s41256-019-0107-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kelesidis T., Currier J.S. Dyslipidemia and cardiovascular risk in human immunodeficiency virus infection. Endocrinol Metab. Clin. N. Am. 2014;43:665–684. doi: 10.1016/j.ecl.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Milburn J., Jones R., Levy J.B. Renal effects of novel antiretroviral drugs. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc. - Eur. Ren. Assoc. 2017;32:434–439. doi: 10.1093/ndt/gfw064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nachega J.B., Marconi V.C., van Zyl G.U., Gardner E.M., Preiser W., Hong S.Y., Mills E.J., Gross R. HIV treatment adherence, drug resistance, virologic failure: evolving concepts. Infect. Disord.: Drug Targets. 2011;11:167–174. doi: 10.2174/187152611795589663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ndung’u T., McCune J.M., Deeks S.G. Why and where an HIV cure is needed and how it might be achieved. Nature. 2019;576:397–405. doi: 10.1038/s41586-019-1841-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deeks S.G., Archin N., Cannon P., Collins S. Research priorities for an HIV cure: international AIDS society global scientific strategy 2021. Nat. Med. 2021;27:2085–2098. doi: 10.1038/s41591-021-01590-5. [DOI] [PubMed] [Google Scholar]
- 11.Cohn L.B., Chomont N., Deeks S.G. The biology of the HIV-1 latent reservoir and implications for cure strategies. Cell Host Microbe. 2020;27:519–530. doi: 10.1016/j.chom.2020.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Whitney J.B., Hill A.L., Sanisetty S., Penaloza-MacMaster P., Liu J., Shetty M., Parenteau L., Cabral C., Shields J., Blackmore S., Smith J.Y., Brinkman A.L., Peter L.E., Mathew S.I., Smith K.M., Borducchi E.N., Rosenbloom D.I.S., Lewis M.G., Hattersley J., Li B., Hesselgesser J., Geleziunas R., Robb M.L., Kim J.H., Michael N.L., Barouch D.H. Rapid seeding of the viral reservoir prior to SIV viraemia in rhesus monkeys. Nature. 2014;512:74–77. doi: 10.1038/nature13594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Verdikt R., Hernalsteens O., Van Lint C. Epigenetic mechanisms of HIV-1 persistence. Vaccines. 2021;9 doi: 10.3390/vaccines9050514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nabel G., Baltimore D. An inducible transcription factor activates expression of human immunodeficiency virus in T cells. Nature. 1987;326:711–713. doi: 10.1038/326711a0. [DOI] [PubMed] [Google Scholar]
- 15.Williams S.A., Chen L.-F., Kwon H., Ruiz-Jarabo C.M., Verdin E., Greene W.C. NF-kappaB p50 promotes HIV latency through HDAC recruitment and repression of transcriptional initiation. EMBO J. 2006;25:139–149. doi: 10.1038/sj.emboj.7600900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Einkauf K.B., Osborn M.R., Gao C., Sun W., Sun X., Lian X., Parsons E.M., Gladkov G.T., Seiger K.W., Blackmer J.E., Jiang C., Yukl S.A., Rosenberg E.S., Yu X.G., Lichterfeld M. Parallel analysis of transcription, integration, and sequence of single HIV-1 proviruses. Cell. 2022;185:266–282.e15. doi: 10.1016/j.cell.2021.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruff C.T., Ray S.C., Kwon P., Zinn R., Pendleton A., Hutton N., Ashworth R., Gange S., Quinn T.C., Siliciano R.F., Persaud D. Persistence of wild-type virus and lack of temporal structure in the latent reservoir for human immunodeficiency virus type 1 in pediatric patients with extensive antiretroviral exposure. J. Virol. 2002;76:9481–9492. doi: 10.1128/jvi.76.18.9481-9492.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Finzi D., Blankson J., Siliciano J.D., Margolick J.B., Chadwick K., Pierson T., Smith K., Lisziewicz J., Lori F., Flexner C., Quinn T.C., Chaisson R.E., Rosenberg E., Walker B., Gange S., Gallant J., Siliciano R.F. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat. Med. 1999;5:512–517. doi: 10.1038/8394. [DOI] [PubMed] [Google Scholar]
- 19.Siliciano J.D., Kajdas J., Finzi D., Quinn T.C., Chadwick K., Margolick J.B., Kovacs C., Gange S.J., Siliciano R.F. Long-term follow-up studies confirm the stability of the latent reservoir for HIV-1 in resting CD4+T cells. Nat. Med. 2003;9:727–728. doi: 10.1038/nm880. [DOI] [PubMed] [Google Scholar]
- 20.Deng K., Pertea M., Rongvaux A., Wang L., Durand C.M., Ghiaur G., Lai J., McHugh H.L., Hao H., Zhang H., Margolick J.B., Gurer C., Murphy A.J., Valenzuela D.M., Yancopoulos G.D., Deeks S.G., Strowig T., Kumar P., Siliciano J.D., Salzberg S.L., Flavell R.A., Shan L., Siliciano R.F. Broad CTL response is required to clear latent HIV-1 due to dominance of escape mutations. Nature. 2015;517:381–385. doi: 10.1038/nature14053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang S.-H., Ren Y., Thomas A.S., Chan D., Mueller S., Ward A.R., Patel S., Bollard C.M., Cruz C.R., Karandish S., Truong R., Macedo A.B., Bosque A., Kovacs C., Benko E., Piechocka-Trocha A., Wong H., Jeng E., Nixon D.F., Ho Y.-C., Siliciano R.F., Walker B.D., Jones R.B. Latent HIV reservoirs exhibit inherent resistance to elimination by CD8+ T cells. J. Clin. Invest. 2018;128:876–889. doi: 10.1172/JCI97555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gondim M.V.P., Sherrill-Mix S., Bibollet-Ruche F., Russell R.M., Trimboli S., Smith A.G., Li Y., Liu W., Avitto A.N., DeVoto J.C., Connell J., Fenton-May A.E., Pellegrino P., Williams I., Papasavvas E., Lorenzi J.C.C., Salantes D.B., Mampe F., Monroy M.A., Cohen Y.Z., Heath S., Saag M.S., Montaner L.J., Collman R.G., Siliciano J.M., Siliciano R.F., Plenderleith L.J., Sharp P.M., Caskey M., Nussenzweig M.C., Shaw G.M., Borrow P., Bar K.J., Hahn B.H. Heightened resistance to host type 1 interferons characterizes HIV-1 at transmission and after antiretroviral therapy interruption. Sci. Transl. Med. 2021;13 doi: 10.1126/scitranslmed.abd8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bertagnolli L.N., Varriale J., Sweet S., Brockhurst J., Simonetti F.R., White J., Beg S., Lynn K., Mounzer K., Frank I., Tebas P., Bar K.J., Montaner L.J., Siliciano R.F., Siliciano J.D. Autologous IgG antibodies block outgrowth of a substantial but variable fraction of viruses in the latent reservoir for HIV-1. Proc. Natl. Acad. Sci. USA. 2020;117:32066–32077. doi: 10.1073/pnas.2020617117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mackewicz C.E., Blackbourn D.J., Levy J.A. CD8+ T cells suppress human immunodeficiency virus replication by inhibiting viral transcription. Proc. Natl. Acad. Sci. U.S.A. 1995;92:2308–2312. doi: 10.1073/pnas.92.6.2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mutascio S., Mota T., Franchitti L., Sharma A.A., Willemse A., Bergstresser S.N., Wang H., Statzu M., Tharp G.K., Weiler J., Sékaly R.-P., Bosinger S.E., Paiardini M., Silvestri G., Jones R.B., Kulpa D.A. CD8(+) T cells promote HIV latency by remodeling CD4(+) T cell metabolism to enhance their survival, quiescence, and stemness. Immunity. 2023;56:1132–1147.e6. doi: 10.1016/j.immuni.2023.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blazek D., Teque F., Mackewicz C., Peterlin M., Levy J.A. The CD8+ cell non-cytotoxic antiviral response affects RNA polymerase II-mediated human immunodeficiency virus transcription in infected CD4+ cells. J. Gen. Virol. 2016;97:220–224. doi: 10.1099/jgv.0.000326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wallace J., Narasipura S.D., Sha B.E., French A.L., Al-Harthi L. Canonical wnts mediate CD8(+) T cell noncytolytic anti-HIV-1 activity and correlate with HIV-1 clinical status. J. Immunol. 2020;205:2046–2055. doi: 10.4049/jimmunol.1801379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zanoni M., Palesch D., Pinacchio C., Statzu M., Tharp G.K., Paiardini M., Chahroudi A., Bosinger S.E., Yoon J., Cox B., Silvestri G., Kulpa D.A. Innate, non-cytolytic CD8+ T cell-mediated suppression of HIV replication by MHC-independent inhibition of virus transcription. PLoS Pathog. 2020;16 doi: 10.1371/journal.ppat.1008821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mavigner M., Liao L.E., Brooks A.D., Ke R., Mattingly C., Schoof N., McBrien J., Carnathan D., Liang S., Vanderford T.H., Paiardini M., Kulpa D., Lifson J.D., Dunham R.M., Easley K.A., Margolis D.M., Perelson A.S., Silvestri G., Chahroudi A. CD8 lymphocyte depletion enhances the latency reversal activity of the SMAC mimetic AZD5582 in ART-suppressed SIV-infected rhesus macaques. J. Virol. 2021;95 doi: 10.1128/JVI.01429-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McBrien J.B., Mavigner M., Franchitti L., Smith S.A., White E., Tharp G.K., Walum H., Busman-Sahay K., Aguilera-Sandoval C.R., Thayer W.O., Spagnuolo R.A., Kovarova M., Wahl A., Cervasi B., Margolis D.M., Vanderford T.H., Carnathan D.G., Paiardini M., Lifson J.D., Lee J.H., Safrit J.T., Bosinger S.E., Estes J.D., Derdeyn C.A., Garcia J.V., Kulpa D.A., Chahroudi A., Silvestri G. Robust and persistent reactivation of SIV and HIV by N-803 and depletion of CD8(+) cells. Nature. 2020;578:154–159. doi: 10.1038/s41586-020-1946-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cartwright E.K., Spicer L., Smith S.A., Lee D., Fast R., Paganini S., Lawson B.O., Nega M., Easley K., Schmitz J.E., Bosinger S.E., Paiardini M., Chahroudi A., Vanderford T.H., Estes J.D., Lifson J.D., Derdeyn C.A., Silvestri G. CD8+Lymphocytes are required for maintaining viral suppression in SIV-infected macaques treated with short-term antiretroviral therapy. Immunity. 2016 doi: 10.1016/j.immuni.2016.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taylor B.S., Sobieszczyk M.E., McCutchan F.E., Hammer S.M. The challenge of HIV-1 subtype diversity. N. Engl. J. Med. 2008;358:1590–1602. doi: 10.1056/NEJMra0706737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yeo J.Y., Goh G.-R., Su C.T.-T., Gan S.K.-E. The determination of HIV-1 RT mutation rate, its possible allosteric effects, and its implications on drug resistance. Viruses. 2020;12 doi: 10.3390/v12030297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rousseau C.M., Learn G.H., Bhattacharya T., Nickle D.C., Heckerman D., Chetty S., Brander C., Goulder P.J.R., Walker B.D., Kiepiela P., Korber B.T., Mullins J.I. Extensive intrasubtype recombination in South African human immunodeficiency virus type 1 subtype C infections. J. Virol. 2007;81:4492–4500. doi: 10.1128/JVI.02050-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhuang J., Jetzt A.E., Sun G., Yu H., Klarmann G., Ron Y., Preston B.D., Dougherty J.P. Human immunodeficiency virus type 1 recombination: rate, fidelity, and putative hot spots. J. Virol. 2002;76:11273–11282. doi: 10.1128/jvi.76.22.11273-11282.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Armitage A.E., Deforche K., Chang C.-H., Wee E., Kramer B., Welch J.J., Gerstoft J., Fugger L., McMichael A., Rambaut A., Iversen A.K.N. APOBEC3G-induced hypermutation of human immunodeficiency virus type-1 is typically a discrete “all or nothing” phenomenon. PLoS Genet. 2012;8 doi: 10.1371/journal.pgen.1002550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O’Neil P.K., Sun G., Yu H., Ron Y., Dougherty J.P., Preston B.D. Mutational analysis of HIV-1 long terminal repeats to explore the relative contribution of reverse transcriptase and RNA polymerase II to viral mutagenesis. J. Biol. Chem. 2002;277:38053–38061. doi: 10.1074/jbc.M204774200. [DOI] [PubMed] [Google Scholar]
- 38.Mansky L.M., Temin H.M. Lower in vivo mutation rate of human immunodeficiency virus type 1 than that predicted from the fidelity of purified reverse transcriptase. J. Virol. 1995;69:5087–5094. doi: 10.1128/JVI.69.8.5087-5094.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sandstrom T.S., Ranganath N., Angel J.B. Impairment of the type I interferon response by HIV-1: potential targets for HIV eradication. Cytokine Growth Factor Rev. 2017;37:1–16. doi: 10.1016/j.cytogfr.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 40.Blagoveshchenskaya A.D., Thomas L., Feliciangeli S.F., Hung C.H., Thomas G. HIV-1 Nef downregulates MHC-I by a PACS-1- and PI3K-regulated ARF6 endocytic pathway. Cell. 2002;111:853–866. doi: 10.1016/s0092-8674(02)01162-5. [DOI] [PubMed] [Google Scholar]
- 41.Prabakaran P., Dimitrov A.S., Fouts T.R., Dimitrov D.S. Structure and function of the HIV envelope glycoprotein as entry mediator, vaccine immunogen, and target for inhibitors. Adv. Pharmacol. 2007;55:33–97. doi: 10.1016/S1054-3589(07)55002-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Estes J.D., Kityo C., Ssali F., Swainson L., Makamdop K.N., Del Prete G.Q., Deeks S.G., Luciw P.A., Chipman J.G., Beilman G.J., Hoskuldsson T., Khoruts A., Anderson J., Deleage C., Jasurda J., Schmidt T.E., Hafertepe M., Callisto S.P., Pearson H., Reimann T., Schuster J., Schoephoerster J., Southern P., Perkey K., Shang L., Wietgrefe S.W., Fletcher C.V., Lifson J.D., Douek D.C., McCune J.M., Haase A.T., Schacker T.W. Defining total-body AIDS-virus burden with implications for curative strategies. Nat. Med. 2017 doi: 10.1038/nm.4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fukazawa Y., Lum R., Okoye A.A., Park H., Matsuda K., Bae J.Y., Hagen S.I., Shoemaker R., Deleage C., Lucero C., Morcock D., Swanson T., Legasse A.W., Axthelm M.K., Hesselgesser J., Geleziunas R., Hirsch V.M., Edlefsen P.T., Piatak M., Estes J.D., Lifson J.D., Picker L.J. B cell follicle sanctuary permits persistent productive simian immunodeficiency virus infection in elite controllers. Nat. Med. 2015 doi: 10.1038/nm.3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barton K., Winckelmann A., Palmer S. HIV-1 reservoirs during suppressive therapy. Trends Microbiol. 2016 doi: 10.1016/j.tim.2016.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Krebs S.J., Ananworanich J. Immune activation during acute HIV infection and the impact of early antiretroviral therapy. Curr. Opin. HIV AIDS. 2016 doi: 10.1097/COH.0000000000000228. [DOI] [PubMed] [Google Scholar]
- 46.Paiardini M., Müller-Trutwin M. HIV-associated chronic immune activation. Immunol. Rev. 2013;254:78–101. doi: 10.1111/imr.12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McLane L.M., Abdel-Hakeem M.S., Wherry E.J. CD8 T cell exhaustion during chronic viral infection and cancer. Annu. Rev. Immunol. 2019;37:457–495. doi: 10.1146/annurev-immunol-041015-055318. [DOI] [PubMed] [Google Scholar]
- 48.Cockerham L.R., Hatano H. Elite control of HIV: is this the right model for a functional cure? Trends Microbiol. 2015;23:71–75. doi: 10.1016/j.tim.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 49.Sáez-Cirión A., Pancino G. HIV controllers: a genetically determined or inducible phenotype? Immunol. Rev. 2013;254:281–294. doi: 10.1111/imr.12076. [DOI] [PubMed] [Google Scholar]
- 50.Kaslow R.A., Carrington M., Apple R., Park L., Muñoz A., Saah A.J., Goedert J.J., Winkler C., O’Brien S.J., Rinaldo C., Detels R., Blattner W., Phair J., Erlich H., Mann D.L. Influence of combinations of human major histocompatibility complex genes on the course of HIV-1 infection. Nat. Med. 1996;2:405–411. doi: 10.1038/nm0496-405. [DOI] [PubMed] [Google Scholar]
- 51.Migueles S.A., Sabbaghian M.S., Shupert W.L., Bettinotti M.P., Marincola F.M., Martino L., Hallahan C.W., Selig S.M., Schwartz D., Sullivan J., Connors M. HLA B*5701 is highly associated with restriction of virus replication in a subgroup of HIV-infected long term nonprogressors. Proc. Natl. Acad. Sci. U.S.A. 2000;97:2709–2714. doi: 10.1073/pnas.050567397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fellay J., V Shianna K., Ge D., Colombo S., Ledergerber B., Weale M., Zhang K., Gumbs C., Castagna A., Cossarizza A., Cozzi-Lepri A., De Luca A., Easterbrook P., Francioli P., Mallal S., Martinez-Picado J., Miro J.M., Obel N., Smith J.P., Wyniger J., Descombes P., Antonarakis S.E., Letvin N.L., McMichael A.J., Haynes B.F., Telenti A., Goldstein D.B. A whole-genome association study of major determinants for host control of HIV-1. Science. 2007;317:944–947. doi: 10.1126/science.1143767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Almeida J.R., Price D.A., Papagno L., Arkoub Z.A., Sauce D., Bornstein E., Asher T.E., Samri A., Schnuriger A., Theodorou I., Costagliola D., Rouzioux C., Agut H., Marcelin A.-G., Douek D., Autran B., Appay V. Superior control of HIV-1 replication by CD8+ T cells is reflected by their avidity, polyfunctionality, and clonal turnover. J. Exp. Med. 2007;204:2473–2485. doi: 10.1084/jem.20070784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pereyra F., Jia X., McLaren P.J., Telenti A. The major genetic determinants of HIV-1 control affect HLA class I peptide presentation. Science. 2010;330:1551–1557. doi: 10.1126/science.1195271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gaiha G.D., Rossin E.J., Urbach J., Landeros C., Collins D.R., Nwonu C., Muzhingi I., Anahtar M.N., Waring O.M., Piechocka-Trocha A., Waring M., Worrall D.P., Ghebremichael M.S., Newman R.M., Power K.A., Allen T.M., Chodosh J., Walker B.D. Structural topology defines protective CD8(+) T cell epitopes in the HIV proteome. Science. 2019;364:480–484. doi: 10.1126/science.aav5095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Migueles S.A., Laborico A.C., Shupert W.L., Sabbaghian M.S., Rabin R., Hallahan C.W., Van Baarle D., Kostense S., Miedema F., McLaughlin M., Ehler L., Metcalf J., Liu S., Connors M. HIV-specific CD8+ T cell proliferation is coupled to perforin expression and is maintained in nonprogressors. Nat. Immunol. 2002;3:1061–1068. doi: 10.1038/ni845. [DOI] [PubMed] [Google Scholar]
- 57.Betts M.R., Nason M.C., West S.M., De Rosa S.C., Migueles S.A., Abraham J., Lederman M.M., Benito J.M., Goepfert P.A., Connors M., Roederer M., Koup R.A. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood. 2006;107:4781–4789. doi: 10.1182/blood-2005-12-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Saez-Cirion A., Lacabaratz C., Lambotte O., Versmisse P., Urrutia A., Boufassa F., Barre-Sinoussi F., Delfraissy J.-F., Sinet M., Pancino G., Venet A. HIV controllers exhibit potent CD8 T cell capacity to suppress HIV infection ex vivo and peculiar cytotoxic T lymphocyte activation phenotype. Proc. Natl. Acad. Sci. USA. 2007;104:6776–6781. doi: 10.1073/pnas.0611244104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li J.Z., Blankson J.N. How elite controllers and posttreatment controllers inform our search for an HIV-1 cure. J. Clin. Invest. 2021;131 doi: 10.1172/JCI149414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sáez-Cirión A., Bacchus C., Hocqueloux L., Avettand-Fenoel V., Girault I., Lecuroux C., Potard V., Versmisse P., Melard A., Prazuck T., Descours B., Guergnon J., Viard J.-P., Boufassa F., Lambotte O., Goujard C., Meyer L., Costagliola D., Venet A., Pancino G., Autran B., Rouzioux C. Post-treatment HIV-1 controllers with a long-term virological remission after the interruption of early initiated antiretroviral therapy ANRS VISCONTI Study. PLoS Pathog. 2013;9 doi: 10.1371/journal.ppat.1003211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Namazi G., Fajnzylber J.M., Aga E., Bosch R.J., Acosta E.P., Sharaf R., Hartogensis W., Jacobson J.M., Connick E., Volberding P., Skiest D., Margolis D., Sneller M.C., Little S.J., Gianella S., Smith D.M., Kuritzkes D.R., Gulick R.M., Mellors J.W., Mehraj V., Gandhi R.T., Mitsuyasu R., Schooley R.T., Henry K., Tebas P., Deeks S.G., Chun T.-W., Collier A.C., Routy J.-P., Hecht F.M., Walker B.D., Li J.Z. The control of HIV after antiretroviral medication pause (CHAMP) study: posttreatment controllers identified from 14 clinical studies. J. Infect. Dis. 2018;218:1954–1963. doi: 10.1093/infdis/jiy479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sharaf R., Lee G.Q., Sun X., Etemad B., Aboukhater L.M., Hu Z., Brumme Z.L., Aga E., Bosch R.J., Wen Y., Namazi G., Gao C., Acosta E.P., Gandhi R.T., Jacobson J.M., Skiest D., Margolis D.M., Mitsuyasu R., Volberding P., Connick E., Kuritzkes D.R., Lederman M.M., Yu X.G., Lichterfeld M., Li J.Z. HIV-1 proviral landscapes distinguish posttreatment controllers from noncontrollers. J. Clin. Invest. 2018;128:4074–4085. doi: 10.1172/JCI120549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Frange P., Faye A., Avettand-Fenoël V., Bellaton E., Descamps D., Angin M., David A., Caillat-Zucman S., Peytavin G., Dollfus C., Le Chenadec J., Warszawski J., Rouzioux C., Sáez-Cirión A. HIV-1 virological remission lasting more than 12 years after interruption of early antiretroviral therapy in a perinatally infected teenager enrolled in the French ANRS EPF-CO10 paediatric cohort: a case report. Lancet. HIV. 2016;3:e49–e54. doi: 10.1016/S2352-3018(15)00232-5. [DOI] [PubMed] [Google Scholar]
- 64.Blazkova J., Gao F., Marichannegowda M.H., Justement J.S., Shi V., Whitehead E.J., Schneck R.F., Huiting E.D., Gittens K., Cottrell M., Benko E., Kovacs C., Lack J., Sneller M.C., Moir S., Fauci A.S., Chun T.-W. Distinct mechanisms of long-term virologic control in two HIV-infected individuals after treatment interruption of anti-retroviral therapy. Nat. Med. 2021;27:1893–1898. doi: 10.1038/s41591-021-01503-6. [DOI] [PubMed] [Google Scholar]
- 65.Etemad B., Sun X., Li Y., Melberg M., Moisi D., Gottlieb R., Ahmed H., Aga E., Bosch R.J., Acosta E.P., Yuki Y., Martin M.P., Carrington M., Gandhi R.T., Jacobson J.M., Volberding P., Connick E., Mitsuyasu R., Frank I., Saag M., Eron J.J., Skiest D., Margolis D.M., Havlir D., Schooley R.T., Lederman M.M., Yu X.G., Li J.Z. HIV post-treatment controllers have distinct immunological and virological features. Proc. Natl. Acad. Sci. USA. 2023;120 doi: 10.1073/pnas.2218960120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Uruena A., Cassetti I., Kashyap N., Deleage C., Estes J.D., Trindade C., Hammoud D.A., Burbelo P.D., Natarajan V., Dewar R., Imamichi H., Ward A.J., Poole A., Ober A., Rehm C., Jones S., Liang C.J., Chun T.-W., Nath A., Lane H.C., Smith B.R., Connors M., Migueles S.A. Prolonged posttreatment virologic control and complete seroreversion after advanced human immunodeficiency virus-1 infection. Open Forum Infect. Dis. 2021;8 doi: 10.1093/ofid/ofaa613. ofaa613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Takata H., Kakazu J.C., Mitchell J.L., Kroon E., Colby D.J., Sacdalan C., Bai H., Ehrenberg P.K., Geretz A., Buranapraditkun S., Pinyakorn S., Intasan J., Tipsuk S., Suttichom D., Prueksakaew P., Chalermchai T., Chomchey N., Phanuphak N., de Souza M., Michael N.L., Robb M.L., Haddad E.K., Crowell T.A., Vasan S., Valcour V.G., Douek D.C., Thomas R., Rolland M., Chomont N., Ananworanich J., Trautmann L., Teeratakulpisarn N., Pattanachaiwit S., Sriplienchan S., Tantivitayakul P., Kanaprach R., Ruxrungtham K., Dumrongpisutikul N., Rojnuckarin P., Chottanapund S., Poltavee K., Luekasemsuk T., Savadsuk H., Puttamsawin S., Benjapornpong K., Ratnaratorn N., Tangnaree K., Munkong C., Thaimanee R., Eamyoung P., Ubolyam S., Lerdlum S., Manasnayakorn S., Rerknimitr R., Sirivichayakul S., Wattanaboonyongcharoen P., Cowden J., Schuetz A., Akapirat S., Churikanont N., Getchalarat S., Hsu D., Turk E., Butterworth O., Milazzo M., Eller L.A., Ake J., Eller L.A., Spudich S., Fox C.L., Ratto-Kim S., DeGruttola V., Chinvarun Y., Sithinamsuwan P., Fletcher J., Shiramizu B., Schuetz A. Long-term antiretroviral therapy initiated in acute HIV infection prevents residual dysfunction of HIV-specific CD8+ T cells. EBioMedicine. 2022;84 doi: 10.1016/j.ebiom.2022.104253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Molinos-Albert L.M., Lorin V., Monceaux V., Orr S., Essat A., Dufloo J., Schwartz O., Rouzioux C., Meyer L., Hocqueloux L., Sáez-Cirión A., Mouquet H., Prazuck T., De Dieuleveult B., Bani-Sadr F., Hentzien M., Berger J.-L., Kmiec I., Pichancourt G., Nasri S., Hittinger G., Lambry V., Beauey A.-C., Pialoux G., Palacios C., Siguier M., Adda A., Foucoin J., Weiss L., Karmochkine M., Meghadecha M., Ptak M., Salmon-Ceron D., Blanche P., Piétri M.-P., Molina J.-M., Taulera O., Lascoux-Combe C., Ponscarme D., Bertaut J.D., Makhloufi D., Godinot M., Artizzu V., Yazdanpanah Y., Matheron S., Godard C., Julia Z., Bernard L., Bastides F., Bourgault O., Jacomet C., Goncalves E., Meybeck A., Huleux T., Cornavin P., Debab Y., Théron D., Miailhes P., Cotte L., Pailhes S., Ogoudjobi S., Viard J.P., Dulucq M.-J., Bodard L., Churaqui F., Guimard T., Laine L., Group A.V.S. Transient viral exposure drives functionally-coordinated humoral immune responses in HIV-1 post-treatment controllers. Nat. Commun. 2022;13:1944. doi: 10.1038/s41467-022-29511-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kägi D., Ledermann B., Bürki K., Seiler P., Odermatt B., Olsen K.J., Podack E.R., Zinkernagel R.M., Hengartner H. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature. 1994;369:31–37. doi: 10.1038/369031a0. [DOI] [PubMed] [Google Scholar]
- 70.Kägi D., Vignaux F., Ledermann B., Bürki K., Depraetere V., Nagata S., Hengartner H., Golstein P. Fas and perforin pathways as major mechanisms of T cell-mediated cytotoxicity. Science. 1994;265:528–530. doi: 10.1126/science.7518614. [DOI] [PubMed] [Google Scholar]
- 71.Dotiwala F., Mulik S., Polidoro R.B., Ansara J.A., Burleigh B.A., Walch M., Gazzinelli R.T., Lieberman J. Killer lymphocytes use granulysin, perforin and granzymes to kill intracellular parasites. Nat. Med. 2016;22:210–216. doi: 10.1038/nm.4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kuhn J.R., Poenie M. Dynamic polarization of the microtubule cytoskeleton during CTL-mediated killing. Immunity. 2002;16:111–121. doi: 10.1016/s1074-7613(02)00262-5. [DOI] [PubMed] [Google Scholar]
- 73.Makedonas G., Banerjee P.P., Pandey R., Hersperger A.R., Sanborn K.B., Hardy G.A.D., Orange J.S., Betts M.R. Rapid up-regulation and granule-independent transport of perforin to the immunological synapse define a novel mechanism of antigen-specific CD8+ T cell cytotoxic activity. J. Immunol. 2009 doi: 10.4049/jimmunol.0803945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Perdomo-Celis F., Taborda N.A., Rugeles M.T. CD8+ T-cell response to HIV infection in the era of antiretroviral therapy. Front. Immunol. 2019;10:1896. doi: 10.3389/fimmu.2019.01896. https://www.frontiersin.org/article/10.3389/fimmu.2019.01896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cocchi F., DeVico A.L., Garzino-Demo A., Arya S.K., Gallo R.C., Lusso P. Identification of RANTES, MIP-1α, and MIP-1β as the major HIV-suppressive factors produced by CD8+T cells. Science. 1995;80– doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 76.Martín-Orozco N., Isibasi A., Ortiz-Navarrete V. Macrophages present exogenous antigens by class I major histocompatibility complex molecules via a secretory pathway as a consequence of interferon-gamma activation. Immunology. 2001;103:41–48. doi: 10.1046/j.0019-2805.2001.01226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cullell-Young M., Barrachina M., López-López C., Goñalons E., Lloberas J., Soler C., Celada A. From transcription to cell surface expression, the induction of MHC class II I-Aα by interferon-γ in macrophages is regulated at different levels. Immunogenetics. 2001;53:136–144. doi: 10.1007/s002510100312. [DOI] [PubMed] [Google Scholar]
- 78.Veldhoen M. Interleukin 17 is a chief orchestrator of immunity. Nat. Immunol. 2017;18:612–621. doi: 10.1038/ni.3742. [DOI] [PubMed] [Google Scholar]
- 79.Borrow P., Lewicki H., Hahn B.H., Shaw G.M., Oldstone M.B. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J. Virol. 1994 doi: 10.1006/wmre.1996.0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Koup R.A., Safrit J.T., Cao Y., Andrews C.A., McLeod G., Borkowsky W., Farthing C., Ho D.D. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J. Virol. 1994;68(7):4650–4655. doi: 10.1128/jvi.68.7.4650-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Takata H., Buranapraditkun S., Kessing C., Fletcher J.L.K., Muir R., Tardif V., Cartwright P., Vandergeeten C., Bakeman W., Nichols C.N., Pinyakorn S., Hansasuta P., Kroon E., Chalermchai T., O’Connell R., Kim J., Phanuphak N., Robb M.L., Michael N.L., Chomont N., Haddad E.K., Ananworanich J., Trautmann L. Delayed differentiation of potent effector CD8+ T cells reducing viremia and reservoir seeding in acute HIV infection. Sci. Transl. Med. 2017 doi: 10.1126/scitranslmed.aag1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Borrow P., Lewicki H., Wei X., Horwitz M.S., Peffer N., Meyers H., Nelson J.A., Gairin J.E., Hahn B.H., Oldstone M.B.A., Shaw G.M. Antiviral pressure exerted by HIV-1-specific cytotoxic T lymphocytes (CTLs) during primary infection demonstrated by rapid selection of CTL escape virus. Nat. Med. 1997;3:205–211. doi: 10.1038/nm0297-205. [DOI] [PubMed] [Google Scholar]
- 83.Schmitz J.E., Kuroda M.J., Santra S., Sasseville V.G., Simon M.A., Lifton M.A., Racz P., Tenner-Racz K., Dalesandro M., Scallon B.J., Ghrayeb J., Forman M.A., Montefiori D.C., Peter Rieber E., Letvin N.L., Reimann K.A. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science. 1999;80– doi: 10.1126/science.283.5403.857. [DOI] [PubMed] [Google Scholar]
- 84.Jin X., Bauer D.E., Tuttleton S.E., Lewin S., Gettie A., Blanchard J., Irwin C.E., Safrit J.T., Mittler J., Weinberger L., Kostrikis L.G., Zhang L., Perelson A.S., Ho D.D., Jin B.X. Dramatic rise in plasma viremia after CD8(+) T cell depletion in simian immunodeficiency virus-infected macaques. J. Exp. Med. 1999 doi: 10.1084/jem.189.6.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Okoye A.A., Duell D.D., Fukazawa Y., Varco-Merth B., Marenco A., Behrens H., Chaunzwa M., Selseth A.N., Gilbride R.M., Shao J., Edlefsen P.T., Geleziunas R., Pinkevych M., Davenport M.P., Busman-Sahay K., Nekorchuk M., Park H., Smedley J., Axthelm M.K., Estes J.D., Hansen S.G., Keele B.F., Lifson J.D., Picker L.J. CD8+ T cells fail to limit SIV reactivation following ART withdrawal until after viral amplification. J. Clin. Invest. 2021;131 doi: 10.1172/JCI141677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Collins D.R., Gaiha G.D., Walker B.D. CD8+ T cells in HIV control, cure and prevention. Nat. Rev. Immunol. 2020 doi: 10.1038/s41577-020-0274-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kim Y., Anderson J.L., Lewin S.R. Getting the “kill” into “shock and kill”: strategies to eliminate latent HIV. Cell Host Microbe. 2018;23:14–26. doi: 10.1016/j.chom.2017.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tanaka K., Kim Y., Roche M., Lewin S.R. The role of latency reversal in HIV cure strategies. J. Med. Primatol. 2022;51:278–283. doi: 10.1111/jmp.12613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kim J., Vasan S., Kim J.H., Ake J.A. Current approaches to HIV vaccine development: a narrative review. J. Int. AIDS Soc. 2021;24(7) doi: 10.1002/jia2.25793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pantaleo G., Levy Y. Therapeutic vaccines and immunological intervention in HIV infection: a paradigm change. Curr. Opin. HIV AIDS. 2016;11:576–584. doi: 10.1097/COH.0000000000000324. [DOI] [PubMed] [Google Scholar]
- 91.Streeck H. Designing optimal HIV-vaccine T-cell responses. Curr. Opin. HIV AIDS. 2016;11 doi: 10.1097/COH.0000000000000313. https://journals.lww.com/co-hivandaids/Fulltext/2016/11000/Designing_optimal_HIV_vaccine_T_cell_responses.9.aspx [DOI] [PubMed] [Google Scholar]
- 92.Borthwick N., Ahmed T., Ondondo B., Hayes P., Rose A., Ebrahimsa U., Hayton E.-J., Black A., Bridgeman A., Rosario M., Hill A.V., Berrie E., Moyle S., Frahm N., Cox J., Colloca S., Nicosia A., Gilmour J., McMichael A.J., Dorrell L., Hanke T. Vaccine-elicited human T cells recognizing conserved protein regions inhibit HIV-1. Mol. Ther. 2014;22:464–475. doi: 10.1038/mt.2013.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.García F., Climent N., Assoumou L., Gil C., González N., Alcamí J., León A., Romeu J., Dalmau J., Martínez-Picado J., Lifson J., Autran B., Costagliola D., Clotet B., Gatell J.M., Plana M., Gallart T. A therapeutic dendritic cell-based vaccine for HIV-1 infection. J. Infect. Dis. 2011;203:473–478. doi: 10.1093/infdis/jiq077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Korber B., Fischer W. T cell-based strategies for HIV-1 vaccines. Hum. Vaccines Immunother. 2020;16:713–722. doi: 10.1080/21645515.2019.1666957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shiver J.W., Fu T.-M., Chen L., Casimiro D.R., Davies M.-E., Evans R.K., Zhang Z.-Q., Simon A.J., Trigona W.L., Dubey S.A., Huang L., Harris V.A., Long R.S., Liang X., Handt L., Schleif W.A., Zhu L., Freed D.C., V Persaud N., Guan L., Punt K.S., Tang A., Chen M., Wilson K.A., Collins K.B., Heidecker G.J., Fernandez V.R., Perry H.C., Joyce J.G., Grimm K.M., Cook J.C., Keller P.M., Kresock D.S., Mach H., Troutman R.D., Isopi L.A., Williams D.M., Xu Z., Bohannon K.E., Volkin D.B., Montefiori D.C., Miura A., Krivulka G.R., Lifton M.A., Kuroda M.J., Schmitz J.E., Letvin N.L., Caulfield M.J., Bett A.J., Youil R., Kaslow D.C., Emini E.A. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature. 2002;415:331–335. doi: 10.1038/415331a. [DOI] [PubMed] [Google Scholar]
- 96.Rosati M., von Gegerfelt A., Roth P., Alicea C., Valentin A., Robert-Guroff M., Venzon D., Montefiori D.C., Markham P., Felber B.K., Pavlakis G.N. DNA vaccines expressing different forms of simian immunodeficiency virus antigens decrease viremia upon SIVmac251 challenge. J. Virol. 2005;79:8480–8492. doi: 10.1128/JVI.79.13.8480-8492.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wilson N.A., Reed J., Napoe G.S., Piaskowski S., Szymanski A., Furlott J., Gonzalez E.J., Yant L.J., Maness N.J., May G.E., Soma T., Reynolds M.R., Rakasz E., Rudersdorf R., McDermott A.B., O’Connor D.H., Friedrich T.C., Allison D.B., Patki A., Picker L.J., Burton D.R., Lin J., Huang L., Patel D., Heindecker G., Fan J., Citron M., Horton M., Wang F., Liang X., Shiver J.W., Casimiro D.R., Watkins D.I. Vaccine-induced cellular immune responses reduce plasma viral concentrations after repeated low-dose challenge with pathogenic simian immunodeficiency virus SIVmac239. J. Virol. 2006;80:5875–5885. doi: 10.1128/JVI.00171-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Liu J., O’Brien K.L., Lynch D.M., Simmons N.L., La Porte A., Riggs A.M., Abbink P., Coffey R.T., Grandpre L.E., Seaman M.S., Landucci G., Forthal D.N., Montefiori D.C., Carville A., Mansfield K.G., Havenga M.J., Pau M.G., Goudsmit J., Barouch D.H. Immune control of an SIV challenge by a T-cell-based vaccine in rhesus monkeys. Nature. 2009;457:87–91. doi: 10.1038/nature07469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Buchbinder S.P., V Mehrotra D., Duerr A., Fitzgerald D.W., Mogg R., Li D., Gilbert P.B., Lama J.R., Marmor M., Del Rio C., McElrath M.J., Casimiro D.R., Gottesdiener K.M., Chodakewitz J.A., Corey L., Robertson M.N. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet (London, England) 2008;372:1881–1893. doi: 10.1016/S0140-6736(08)61591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.McElrath M.J., De Rosa S.C., Moodie Z., Dubey S., Kierstead L., Janes H., Defawe O.D., Carter D.K., Hural J., Akondy R., Buchbinder S.P., Robertson M.N., V Mehrotra D., Self S.G., Corey L., Shiver J.W., Casimiro D.R. HIV-1 vaccine-induced immunity in the test-of-concept Step Study: a case-cohort analysis. Lancet (London, England) 2008;372:1894–1905. doi: 10.1016/S0140-6736(08)61592-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fan J., Liang H., Ji X., Wang S., Xue J., Li D., Peng H., Qin C., Yee C., Shao Y. CTL-mediated immunotherapy can suppress SHIV rebound in ART-free macaques. Nat. Commun. 2019;10:2257. doi: 10.1038/s41467-019-09725-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Picker L.J., Hansen S.G., Lifson J.D. New paradigms for HIV/AIDS vaccine development. Annu. Rev. Med. 2012;63:95–111. doi: 10.1146/annurev-med-042010-085643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hansen S.G., Ford J.C., Lewis M.S., Ventura A.B., Hughes C.M., Coyne-Johnson L., Whizin N., Oswald K., Shoemaker R., Swanson T., Legasse A.W., Chiuchiolo M.J., Parks C.L., Axthelm M.K., Nelson J.A., Jarvis M.A., Piatak M.J., Lifson J.D., Picker L.J. Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine. Nature. 2011;473:523–527. doi: 10.1038/nature10003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hansen S.G., Piatak M.J., Ventura A.B., Hughes C.M., Gilbride R.M., Ford J.C., Oswald K., Shoemaker R., Li Y., Lewis M.S., Gilliam A.N., Xu G., Whizin N., Burwitz B.J., Planer S.L., Turner J.M., Legasse A.W., Axthelm M.K., Nelson J.A., Früh K., Sacha J.B., Estes J.D., Keele B.F., Edlefsen P.T., Lifson J.D., Picker L.J. Immune clearance of highly pathogenic SIV infection. Nature. 2013;502:100–104. doi: 10.1038/nature12519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hansen S.G., Sacha J.B., Hughes C.M., Ford J.C., Burwitz B.J., Scholz I., Gilbride R.M., Lewis M.S., Gilliam A.N., Ventura A.B., Malouli D., Xu G., Richards R., Whizin N., Reed J.S., Hammond K.B., Fischer M., Turner J.M., Legasse A.W., Axthelm M.K., Edlefsen P.T., Nelson J.A., Lifson J.D., Früh K., Picker L.J. Cytomegalovirus vectors violate CD8+ T cell epitope recognition paradigms. Science. 2013;340 doi: 10.1126/science.1237874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hansen S.G., Wu H.L., Burwitz B.J., Hughes C.M., Hammond K.B., Ventura A.B., Reed J.S., Gilbride R.M., Ainslie E., Morrow D.W., Ford J.C., Selseth A.N., Pathak R., Malouli D., Legasse A.W., Axthelm M.K., Nelson J.A., Gillespie G.M., Walters L.C., Brackenridge S., Sharpe H.R., López C.A., Früh K., Korber B.T., McMichael A.J., Gnanakaran S., Sacha J.B., Picker L.J. Broadly targeted CD8+ T cell responses restricted by major histocompatibility complex E. Science. 2016;351:714–720. doi: 10.1126/science.aac9475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hansen S.G., Hancock M.H., Malouli D., Marshall E.E., Hughes C.M., Randall K.T., Morrow D., Ford J.C., Gilbride R.M., Selseth A.N., Trethewy R.E., Bishop L.M., Oswald K., Shoemaker R., Berkemeier B., Bosche W.J., Hull M., Silipino L., Nekorchuk M., Busman-Sahay K., Estes J.D., Axthelm M.K., Smedley J., Shao D., Edlefsen P.T., Lifson J.D., Früh K., Nelson J.A., Picker L.J. Myeloid cell tropism enables MHC-E-restricted CD8(+) T cell priming and vaccine efficacy by the RhCMV/SIV vaccine. Sci. Immunol. 2022;7 doi: 10.1126/sciimmunol.abn9301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nishimura Y., Gautam R., Chun T.-W., Sadjadpour R., Foulds K.E., Shingai M., Klein F., Gazumyan A., Golijanin J., Donaldson M., Donau O.K., Plishka R.J., Buckler-White A., Seaman M.S., Lifson J.D., Koup R.A., Fauci A.S., Nussenzweig M.C., Martin M.A. Early antibody therapy can induce long-lasting immunity to SHIV. Nature. 2017;543:559–563. doi: 10.1038/nature21435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Niessl J., Baxter A.E., Mendoza P., Jankovic M., Cohen Y.Z., Butler A.L., Lu C.-L., Dubé M., Shimeliovich I., Gruell H., Klein F., Caskey M., Nussenzweig M.C., Kaufmann D.E. Combination anti-HIV-1 antibody therapy is associated with increased virus-specific T cell immunity. Nat. Med. 2020;26:222–227. doi: 10.1038/s41591-019-0747-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nishimura Y., Donau O.K., Dias J., Ferrando-Martinez S., Jesteadt E., Sadjadpour R., Gautam R., Buckler-White A., Geleziunas R., Koup R.A., Nussenzweig M.C., Martin M.A. Immunotherapy during the acute SHIV infection of macaques confers long-term suppression of viremia. J. Exp. Med. 2021;218 doi: 10.1084/jem.20201214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Leonard W.J., Lin J.-X., O’Shea J.J. The γc family of cytokines: basic biology to therapeutic ramifications. Immunity. 2019;50:832–850. doi: 10.1016/j.immuni.2019.03.028. [DOI] [PubMed] [Google Scholar]
- 112.Williams M.A., Tyznik A.J., Bevan M.J. Interleukin-2 signals during priming are required for secondary expansion of CD8+ memory T cells. Nature. 2006;441:890–893. doi: 10.1038/nature04790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Schluns K.S., Kieper W.C., Jameson S.C., Lefrançois L. Interleukin-7 mediates the homeostasis of naïve and memory CD8 T cells in vivo. Nat. Immunol. 2000;1:426–432. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- 114.Goldrath A.W., V Sivakumar P., Glaccum M., Kennedy M.K., Bevan M.J., Benoist C., Mathis D., Butz E.A. Cytokine requirements for acute and Basal homeostatic proliferation of naive and memory CD8+ T cells. J. Exp. Med. 2002;195:1515–1522. doi: 10.1084/jem.20020033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mueller Y.M., Bojczuk P.M., Halstead E.S., Kim A.H.J., Witek J., Altman J.D., Katsikis P.D. IL-15 enhances survival and function of HIV-specific CD8+ T cells. Blood. 2003;101:1024–1029. doi: 10.1182/blood-2002-07-1957. [DOI] [PubMed] [Google Scholar]
- 116.White L., Krishnan S., Strbo N., Liu H., Kolber M.A., Lichtenheld M.G., Pahwa R.N., Pahwa S. Differential effects of IL-21 and IL-15 on perforin expression, lysosomal degranulation, and proliferation in CD8 T cells of patients with human immunodeficiency virus-1 (HIV) Blood. 2007;109:3873–3880. doi: 10.1182/blood-2006-09-045278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Angin M., Volant S., Passaes C., Lecuroux C., Monceaux V., Dillies M.-A., Valle-Casuso J.C., Pancino G., Vaslin B., Le Grand R., Weiss L., Goujard C., Meyer L., Boufassa F., Müller-Trutwin M., Lambotte O., Sáez-Cirión A. Metabolic plasticity of HIV-specific CD8+ T cells is associated with enhanced antiviral potential and natural control of HIV-1 infection. Nat. Metab. 2019;1:704–716. doi: 10.1038/s42255-019-0081-4. [DOI] [PubMed] [Google Scholar]
- 118.Fry T.J., Moniuszko M., Creekmore S., Donohue S.J., Douek D.C., Giardina S., Hecht T.T., Hill B.J., Komschlies K., Tomaszewski J., Franchini G., Mackall C.L. IL-7 therapy dramatically alters peripheral T-cell homeostasis in normal and SIV-infected nonhuman primates. Blood. 2003;101:2294–2299. doi: 10.1182/blood-2002-07-2297. [DOI] [PubMed] [Google Scholar]
- 119.Pallikkuth S., Rogers K., Villinger F., Dosterii M., Vaccari M., Franchini G., Pahwa R., Pahwa S. Interleukin-21 administration to rhesus macaques chronically infected with simian immunodeficiency virus increases cytotoxic effector molecules in T cells and NK cells and enhances B cell function without increasing immune activation or viral replication. Vaccine. 2011;29:9229–9238. doi: 10.1016/j.vaccine.2011.09.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chitnis V., Pahwa R., Pahwa S. Determinants of HIV-specific CD8 T-cell responses in HIV-infected pediatric patients and enhancement of HIV-gag-specific responses with exogenous IL-15. Clin. Immunol. 2003;107:36–45. doi: 10.1016/s1521-6616(02)00051-7. [DOI] [PubMed] [Google Scholar]
- 121.Jones R.B., Mueller S., O’Connor R., Rimpel K., Sloan D.D., Karel D., Wong H.C., Jeng E.K., Thomas A.S., Whitney J.B., Lim S.-Y., Kovacs C., Benko E., Karandish S., Huang S.-H., Buzon M.J., Lichterfeld M., Irrinki A., Murry J.P., Tsai A., Yu H., Geleziunas R., Trocha A., Ostrowski M.A., Irvine D.J., Walker B.D. A subset of latency-reversing agents expose HIV-infected resting CD4+ T-cells to recognition by cytotoxic T-lymphocytes. PLoS Pathog. 2016;12 doi: 10.1371/journal.ppat.1005545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Berger C., Berger M., Hackman R.C., Gough M., Elliott C., Jensen M.C., Riddell S.R. Safety and immunologic effects of IL-15 administration in nonhuman primates. Blood. 2009;114:2417–2426. doi: 10.1182/blood-2008-12-189266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Oh S., Berzofsky J.A., Burke D.S., Waldmann T.A., Perera L.P. Coadministration of HIV vaccine vectors with vaccinia viruses expressing IL-15 but not IL-2 induces long-lasting cellular immunity. Proc. Natl. Acad. Sci. U.S.A. 2003;100:3392–3397. doi: 10.1073/pnas.0630592100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Burkett P.R., Koka R., Chien M., Chai S., Boone D.L., Ma A. Coordinate expression and trans presentation of interleukin (IL)-15Ralpha and IL-15 supports natural killer cell and memory CD8+ T cell homeostasis. J. Exp. Med. 2004;200:825–834. doi: 10.1084/jem.20041389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Schluns K.S., Klonowski K.D., Lefrançois L. Transregulation of memory CD8 T-cell proliferation by IL-15Ralpha+ bone marrow-derived cells. Blood. 2004;103:988–994. doi: 10.1182/blood-2003-08-2814. [DOI] [PubMed] [Google Scholar]
- 126.Zhu X., Marcus W.D., Xu W., Lee H., Han K., Egan J.O., Yovandich J.L., Rhode P.R., Wong H.C. Novel human interleukin-15 agonists. J. Immunol. 2009;183:3598. doi: 10.4049/jimmunol.0901244. LP – 3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Xu W., Jones M., Liu B., Zhu X., Johnson C.B., Edwards A.C., Kong L., Jeng E.K., Han K., Marcus W.D., Rubinstein M.P., Rhode P.R., Wong H.C. Efficacy and mechanism-of-action of a novel superagonist interleukin-15: interleukin-15 receptor αSu/fc fusion complex in syngeneic murine models of multiple myeloma. Cancer Res. 2013;73:3075–3086. doi: 10.1158/0008-5472.CAN-12-2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ng S.S.M., Nagy B.A., Jensen S.M., Hu X., Alicea C., Fox B.A., Felber B.K., Bergamaschi C., Pavlakis G.N. Heterodimeric IL15 treatment enhances tumor infiltration, persistence, and effector functions of adoptively transferred tumor-specific T cells in the absence of lymphodepletion. Clin. Cancer Res. an Off. J. Am. Assoc. Cancer Res. 2017;23:2817–2830. doi: 10.1158/1078-0432.CCR-16-1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ellis-Connell A.L., Balgeman A.J., Zarbock K.R., Barry G., Weiler A., Egan J.O., Jeng E.K., Friedrich T., Miller J.S., Haase A.T., Schacker T.W., Wong H.C., Rakasz E., O’Connor S.L. ALT-803 transiently reduces simian immunodeficiency virus replication in the absence of antiretroviral treatment. J. Virol. 2018;92 doi: 10.1128/JVI.01748-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Webb G.M., Li S., Mwakalundwa G., Folkvord J.M., Greene J.M., Reed J.S., Stanton J.J., Legasse A.W., Hobbs T., Martin L.D., Park B.S., Whitney J.B., Jeng E.K., Wong H.C., Nixon D.F., Jones R.B., Connick E., Skinner P.J., Sacha J.B. The human IL-15 superagonist ALT-803 directs SIV-specific CD8+ T cells into B-cell follicles. Blood Adv. 2018;2:76–84. doi: 10.1182/bloodadvances.2017012971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Webb G.M., Molden J., Busman-Sahay K., Abdulhaqq S., Wu H.L., Weber W.C., Bateman K.B., Reed J.S., Northrup M., Maier N., Tanaka S., Gao L., Davey B., Carpenter B.L., Axthelm M.K., Stanton J.J., Smedley J., Greene J.M., Safrit J.T., Estes J.D., Skinner P.J., Sacha J.B. The human IL-15 superagonist N-803 promotes migration of virus-specific CD8+ T and NK cells to B cell follicles but does not reverse latency in ART-suppressed, SHIV-infected macaques. PLoS Pathog. 2020;16 doi: 10.1371/journal.ppat.1008339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Watson D.C., Moysi E., Valentin A., Bergamaschi C., Devasundaram S., Fortis S.P., Bear J., Chertova E., Bess J.J., Sowder R., Venzon D.J., Deleage C., Estes J.D., Lifson J.D., Petrovas C., Felber B.K., Pavlakis G.N. Treatment with native heterodimeric IL-15 increases cytotoxic lymphocytes and reduces SHIV RNA in lymph nodes. PLoS Pathog. 2018;14 doi: 10.1371/journal.ppat.1006902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Huot N., Jacquelin B., Garcia-Tellez T., Rascle P., Ploquin M.J., Madec Y., Reeves R.K., Derreudre-Bosquet N., Müller-Trutwin M. Natural killer cells migrate into and control simian immunodeficiency virus replication in lymph node follicles in African green monkeys. Nat. Med. 2017;23:1277–1286. doi: 10.1038/nm.4421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Miller J.S., Davis Z.B., Helgeson E., Reilly C., Thorkelson A., Anderson J., Lima N.S., Jorstad S., Hart G.T., Lee J.H., Safrit J.T., Wong H., Cooley S., Gharu L., Chung H., Soon-Shiong P., Dobrowolski C., V Fletcher C., Karn J., Douek D.C., Schacker T.W. Safety and virologic impact of the IL-15 superagonist N-803 in people living with HIV: a phase 1 trial. Nat. Med. 2022;28:392–400. doi: 10.1038/s41591-021-01651-9. [DOI] [PubMed] [Google Scholar]
- 135.Perdomo-Celis F., Feria M.G., Taborda N.A., Rugeles M.T. Induction of follicular-like CXCR5+ CD8+ T cells by TGF-beta1/IL-23 is limited during HIV infection. Viral Immunol. 2019;32:1–11. doi: 10.1089/vim.2019.0029. [DOI] [PubMed] [Google Scholar]
- 136.Gutjahr A., Tiraby G., Perouzel E., Verrier B., Paul S. Triggering intracellular receptors for vaccine adjuvantation. Trends Immunol. 2016;37:573–587. doi: 10.1016/j.it.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 137.Corrales L., McWhirter S.M., Dubensky T.W.J., Gajewski T.F. The host STING pathway at the interface of cancer and immunity. J. Clin. Invest. 2016;126:2404–2411. doi: 10.1172/JCI86892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gutjahr A., Papagno L., Nicoli F., Kanuma T., Kuse N., Cabral-Piccin M.P., Rochereau N., Gostick E., Lioux T., Perouzel E., Price D.A., Takiguchi M., Verrier B., Yamamoto T., Paul S., Appay V. The STING ligand cGAMP potentiates the efficacy of vaccine-induced CD8+ T cells. JCI Insight. 2019;4 doi: 10.1172/jci.insight.125107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Tsai A., Irrinki A., Kaur J., Cihlar T., Kukolj G., Sloan D.D., Murry J.P. Toll-like receptor 7 agonist GS-9620 induces HIV expression and HIV-specific immunity in cells from HIV-infected individuals on suppressive antiretroviral therapy. J. Virol. 2017;91 doi: 10.1128/JVI.02166-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Borducchi E.N., Liu J., Nkolola J.P., Cadena A.M., Yu W.-H., Fischinger S., Broge T., Abbink P., Mercado N.B., Chandrashekar A., Jetton D., Peter L., McMahan K., Moseley E.T., Bekerman E., Hesselgesser J., Li W., Lewis M.G., Alter G., Geleziunas R., Barouch D.H. Antibody and TLR7 agonist delay viral rebound in SHIV-infected monkeys. Nature. 2018;563:360–364. doi: 10.1038/s41586-018-0600-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ventura J.D., Nkolola J.P., Chandrashekar A., Borducchi E.N., Liu J., Mercado N.B., Hope D.L., Giffin V.M., McMahan K., Geleziunas R., Murry J.P., Yang Y., Lewis M.G., Pau M.G., Wegmann F., Schuitemaker H., Fray E.J., Kumar M.R., Siliciano J.D., Siliciano R.F., Robb M.L., Michael N.L., Barouch D.H. Therapeutic efficacy of an Ad26/MVA vaccine with SIV gp140 protein and vesatolimod in ART-suppressed rhesus macaques. Npj Vaccines. 2022;7:53. doi: 10.1038/s41541-022-00477-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.SenGupta D., Brinson C., DeJesus E., Mills A., Shalit P., Guo S., Cai Y., Wallin J.J., Zhang L., Humeniuk R., Begley R., Geleziunas R., Mellors J., Wrin T., Jones N., Milush J., Ferre A.L., Shacklett B.L., Laird G.M., Moldt B., Vendrame E., Brainard D.M., Ramgopal M., Deeks S.G. The TLR7 agonist vesatolimod induced a modest delay in viral rebound in HIV controllers after cessation of antiretroviral therapy. Sci. Transl. Med. 2021;13 doi: 10.1126/scitranslmed.abg3071. [DOI] [PubMed] [Google Scholar]
- 143.Shiravand Y., Khodadadi F., Kashani S.M., Hosseini-Fard S.R., Hosseini S., Sadeghirad H., Ladwa R., O’Byrne K., Kulasinghe A. Immune checkpoint inhibitors in cancer therapy. Curr. Oncol. 2022;29:3044–3060. doi: 10.3390/curroncol29050247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Cook M.R., Kim C. Safety and efficacy of immune checkpoint inhibitor therapy in patients with HIV infection and advanced-stage cancer: a systematic review. JAMA Oncol. 2019;5:1049–1054. doi: 10.1001/jamaoncol.2018.6737. [DOI] [PubMed] [Google Scholar]
- 145.Rajdev L., Chiao E., Lensing S., Streicher H. AIDS malignancy consortium (AMC) 095: A phase I study of ipilimumab (IPI) and nivolumab (NIVO) in advanced HIV-associated solid tumors (ST) with expansion cohorts in HIV-associated solid tumors and classical Hodgkin lymphoma (cHL) J. Clin. Oncol. 2018;36:TPS44. doi: 10.1200/JCO.2018.36.5_suppl.TPS44. [DOI] [Google Scholar]
- 146.Colston E., Grasela D., Gardiner D., Bucy R.P., Vakkalagadda B., Korman A.J., Lowy I. An open-label, multiple ascending dose study of the anti-CTLA-4 antibody ipilimumab in viremic HIV patients. PLoS One. 2018;13 doi: 10.1371/journal.pone.0198158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Gay C.L., Bosch R.J., Ritz J., Hataye J.M., Aga E., Tressler R.L., Mason S.W., Hwang C.K., Grasela D.M., Ray N., Cyktor J.C., Coffin J.M., Acosta E.P., Koup R.A., Mellors J.W., Eron J.J. Clinical trial of the anti-PD-L1 antibody BMS-936559 in HIV-1 infected participants on suppressive antiretroviral therapy. J. Infect. Dis. 2017;215:1725–1733. doi: 10.1093/infdis/jix191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lau J.S.Y., McMahon J.H., Gubser C., Solomon A., Chiu C.Y.H., Dantanarayana A., Chea S., Tennakoon S., Zerbato J.M., Garlick J., Morcilla V., Palmer S., Lewin S.R., Rasmussen T.A. The impact of immune checkpoint therapy on the latent reservoir in HIV-infected individuals with cancer on antiretroviral therapy. AIDS. 2021;35:1631–1636. doi: 10.1097/QAD.0000000000002919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Gubser C., Chiu C., Lewin S.R., Rasmussen T.A. Immune checkpoint blockade in HIV. EBioMedicine. 2022;76 doi: 10.1016/j.ebiom.2022.103840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Martins F., Sofiya L., Sykiotis G.P., Lamine F., Maillard M., Fraga M., Shabafrouz K., Ribi C., Cairoli A., Guex-Crosier Y., Kuntzer T., Michielin O., Peters S., Coukos G., Spertini F., Thompson J.A., Obeid M. Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat. Rev. Clin. Oncol. 2019;16:563–580. doi: 10.1038/s41571-019-0218-0. [DOI] [PubMed] [Google Scholar]
- 151.Gay C.L., Bosch R.J., McKhann A., Moseley K.F., Wimbish C.L., Hendrickx S.M., Messer M., Furlong M., Campbell D.M., Jennings C., Benson C., Overton E.T., Macatangay B.J.C., Kuritzkes D.R., Miller E., Tressler R., Eron J.J., Hardy W.D. Suspected immune-related adverse events with an anti-PD-1 inhibitor in otherwise healthy people with HIV. J. Acquir. Immune Defic. Syndr. 2021;87:e234–e236. doi: 10.1097/QAI.0000000000002716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Baron M., Soulié C., Lavolé A., Assoumou L., Abbar B., Fouquet B., Rousseau A., Veyri M., Samri A., Makinson A., Choquet S., Mazières J., Brosseau S., Autran B., Costagliola D., Katlama C., Cadranel J., Marcelin A.-G., Lambotte O., Spano J.-P., Guihot A., T.F.C.T.I.I. Chiva-Investigators, T.A.C.O.S. Group Impact of anti PD-1 immunotherapy on HIV reservoir and anti-viral immune responses in people living with HIV and cancer. Cells. 2022;11 doi: 10.3390/cells11061015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Chiu C.Y., Chang J.J., Dantanarayana A.I., Solomon A., Evans V.A., Pascoe R., Gubser C., Trautman L., Fromentin R., Chomont N., McMahon J.H., Cameron P.U., Rasmussen T.A., Lewin S.R. Combination immune checkpoint blockade enhances IL-2 and CD107a production from HIV-specific T cells ex vivo in people living with HIV on antiretroviral therapy. J. Immunol. 2022;208:54–62. doi: 10.4049/jimmunol.2100367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Rahman S.A., Yagnik B., Bally A.P., Morrow K.N., Wang S., Vanderford T.H., Freeman G.J., Ahmed R., Amara R.R. PD-1 blockade and vaccination provide therapeutic benefit against SIV by inducing broad and functional CD8(+) T cells in lymphoid tissue. Sci. Immunol. 2021;6 doi: 10.1126/sciimmunol.abh3034. eabh3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Joseph A., Zheng J.H., Follenzi A., Dilorenzo T., Sango K., Hyman J., Chen K., Piechocka-Trocha A., Brander C., Hooijberg E., Vignali D.A., Walker B.D., Goldstein H. Lentiviral vectors encoding human immunodeficiency virus type 1 (HIV-1)-specific T-cell receptor genes efficiently convert peripheral blood CD8 T lymphocytes into cytotoxic T lymphocytes with potent in vitro and in vivo HIV-1-specific inhibitory activity. J. Virol. 2008;82:3078–3089. doi: 10.1128/JVI.01812-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Linette G.P., Stadtmauer E.A., V Maus M., Rapoport A.P., Levine B.L., Emery L., Litzky L., Bagg A., Carreno B.M., Cimino P.J., Binder-Scholl G.K., Smethurst D.P., Gerry A.B., Pumphrey N.J., Bennett A.D., Brewer J.E., Dukes J., Harper J., Tayton-Martin H.K., Jakobsen B.K., Hassan N.J., Kalos M., June C.H. Cardiovascular toxicity and titin cross-reactivity of affinity-enhanced T cells in myeloma and melanoma. Blood. 2013;122:863. doi: 10.1182/blood-2013-03-490565. LP – 871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Qi J., Ding C., Jiang X., Gao Y. Advances in developing CAR T-cell therapy for HIV cure. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.00361. https://www.frontiersin.org/articles/10.3389/fimmu.2020.00361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Maldini C.R., Ellis G.I., Riley J.L. CAR T cells for infection, autoimmunity and allotransplantation. Nat. Rev. Immunol. 2018;18:605–616. doi: 10.1038/s41577-018-0042-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Leibman R.S., Richardson M.W., Ellebrecht C.T., Maldini C.R., Glover J.A., Secreto A.J., Kulikovskaya I., Lacey S.F., Akkina S.R., Yi Y., Shaheen F., Wang J., Dufendach K.A., Holmes M.C., Collman R.G., Payne A.S., Riley J.L. Supraphysiologic control over HIV-1 replication mediated by CD8 T cells expressing a re-engineered CD4-based chimeric antigen receptor. PLoS Pathog. 2017;13 doi: 10.1371/journal.ppat.1006613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Liu B., Zhang W., Xia B., Jing S., Du Y., Zou F., Li R., Lu L., Chen S., Li Y., Hu Q., Lin Y., Zhang Y., He Z., Zhang X., Chen X., Peng T., Tang X., Cai W., Pan T., Li L., Zhang H. Broadly neutralizing antibody-derived CAR T cells reduce viral reservoir in individuals infected with HIV-1. J. Clin. Invest. 2021;131 doi: 10.1172/JCI150211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Herzig E., Kim K.C., Packard T.A., Vardi N., Schwarzer R., Gramatica A., Deeks S.G., Williams S.R., Landgraf K., Killeen N., Martin D.W., Weinberger L.S., Greene W.C. Attacking latent HIV with convertibleCAR-T cells, a highly adaptable killing platform. Cell. 2019;179:880–894.e10. doi: 10.1016/j.cell.2019.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Haran K.P., Hajduczki A., Pampusch M.S., Mwakalundwa G., Vargas-Inchaustegui D.A., Rakasz E.G., Connick E., Berger E.A., Skinner P.J. Simian immunodeficiency virus (SIV)-Specific chimeric antigen receptor-T cells engineered to target B cell follicles and suppress SIV replication. Front. Immunol. 2018;9:492. doi: 10.3389/fimmu.2018.00492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Pampusch M.S., Abdelaal H.M., Cartwright E.K., Molden J.S., Davey B.C., Sauve J.D., Sevcik E.N., Rendahl A.K., Rakasz E.G., Connick E., Berger E.A., Skinner P.J. CAR/CXCR5-T cell immunotherapy is safe and potentially efficacious in promoting sustained remission of SIV infection. PLoS Pathog. 2022;18 doi: 10.1371/journal.ppat.1009831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Pilipow K., Scamardella E., Puccio S., Gautam S., De Paoli F., Mazza E.M., De Simone G., Polletti S., Buccilli M., Zanon V., Di Lucia P., Iannacone M., Gattinoni L., Lugli E. Antioxidant metabolism regulates CD8+ T memory stem cell formation and antitumor immunity. JCI Insight. 2018;3 doi: 10.1172/jci.insight.122299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Guo Y., Xie Y.-Q., Gao M., Zhao Y., Franco F., Wenes M., Siddiqui I., Bevilacqua A., Wang H., Yang H., Feng B., Xie X., Sabatel C.M., Tschumi B., Chaiboonchoe A., Wang Y., Li W., Xiao W., Held W., Romero P., Ho P.-C., Tang L. Metabolic reprogramming of terminally exhausted CD8+ T cells by IL-10 enhances anti-tumor immunity. Nat. Immunol. 2021;22:746–756. doi: 10.1038/s41590-021-00940-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Verma V., Jafarzadeh N., Boi S., Kundu S., Jiang Z., Fan Y., Lopez J., Nandre R., Zeng P., Alolaqi F., Ahmad S., Gaur P., Barry S.T., Valge-Archer V.E., Smith P.D., Banchereau J., Mkrtichyan M., Youngblood B., Rodriguez P.C., Gupta S., Khleif S.N. MEK inhibition reprograms CD8+ T lymphocytes into memory stem cells with potent antitumor effects. Nat. Immunol. 2021;22:53–66. doi: 10.1038/s41590-020-00818-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Passaes C., Millet A., Madelain V., Monceaux V., David A., Versmisse P., Sylla N., Gostick E., Llewellyn-Lacey S., Price D.A., Blancher A., Dereuddre-Bosquet N., Desjardins D., Pancino G., Le Grand R., Lambotte O., Müller-Trutwin M., Rouzioux C., Guedj J., Avettand-Fenoel V., Vaslin B., Sáez-Cirión A. Optimal maturation of the SIV-specific CD8+ T cell response after primary infection is associated with natural control of SIV: ANRS SIC study. Cell Rep. 2020;32 doi: 10.1016/j.celrep.2020.108174. [DOI] [PubMed] [Google Scholar]
- 168.Sekine T., Perez-Potti A., Nguyen S., Gorin J.-B., Wu V.H., Gostick E., Llewellyn-Lacey S., Hammer Q., Falck-Jones S., Vangeti S., Yu M., Smed-Sörensen A., Gaballa A., Uhlin M., Sandberg J.K., Brander C., Nowak P., Goepfert P.A., Price D.A., Betts M.R., Buggert M. TOX is expressed by exhausted and polyfunctional human effector memory CD8+ T cells. Sci. Immunol. 2020;5 doi: 10.1126/sciimmunol.aba7918. [DOI] [PubMed] [Google Scholar]
- 169.Rutishauser R.L., Deguit C.D.T., Hiatt J., Blaeschke F., Roth T.L., Wang L., Raymond K.A., Starke C.E., Mudd J.C., Chen W., Smullin C., Matus-Nicodemos R., Hoh R., Krone M., Hecht F.M., Pilcher C.D., Martin J.N., Koup R.A., Douek D.C., Brenchley J.M., Sékaly R.-P., Pillai S.K., Marson A., Deeks S.G., McCune J.M., Hunt P.W. TCF-1 regulates HIV-specific CD8+ T cell expansion capacity. JCI Insight. 2021;6:1–16. doi: 10.1172/jci.insight.136648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Perdomo-Celis F., Passaes C., Monceaux V., Volant S., Boufassa F., de Truchis P., Marcou M., Bourdic K., Weiss L., Jung C., Bourgeois C., Goujard C., Meyer L., Müller-Trutwin M., Lambotte O., Sáez-Cirión A. Reprogramming dysfunctional CD8+ T cells to promote properties associated with natural HIV control. J. Clin. Invest. 2022;132:1–14. doi: 10.1172/JCI157549. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supp. material/referenced in article.