Abstract
Greater intake of whole grains, compared to refined grains, is consistently associated with a reduced risk of cardiovascular disease and type 2 diabetes, both of which are associated with cognitive decline. To better understand the relationship between whole-grain intake, cognition, mood, and anxiety, a systematic review was conducted to synthesize available evidence linking whole grains to these outcomes. Four electronic databases were searched from inception to August 2021 for potentially relevant observational and interventional studies. Risk of bias (RoB) assessments were performed using the newly developed Nutrition Quality Evaluation Strengthening Tools, and the Grades of Recommendation, Assessment, Development, and Evaluation approach was used to determine the strength of evidence for each outcome. In total, 23 studies [4 randomized controlled trials (RCTs) and 19 observational studies] met the predefined eligibility criteria. Of these,12 studies included analysis of whole-grain intake and cognitive decline, 9 included mood outcomes, and 2 included both cognition and mood outcomes. The overall evidence for an association between whole-grain intake and cognition is inconclusive. With respect to mood outcomes, evidence from RCTs prospective cohort and case-control studies suggest that higher intake is linked to improved outcomes for mood and depression although the evidence is mixed for cross-sectional studies. Reporting of whole-grain intake fell short of suggested standards, and the strength of available evidence was low or very low for all outcomes. A high RoB toward studies reporting results was also noted, complicating both the interpretation of some studies and the combined evidence. Of note, few well-designed RCTs assessing the effect of whole-grain intake on measures of cognition, mood, and anxiety were identified, highlighting the need for more studies in this area. The available, although limited, evidence suggests that greater whole-grain intake is associated with better mood and anxiety-related scores and is inconclusive regarding cognitive outcomes. PROSPERO registration: CRD42021266355.
Keywords: whole grain, cereal, cognition, dementia, mood, depression, anxiety
Statement of Significance.
Evidence for whole grains reducing cognitive decline is inconclusive, although there are consistent associations between higher levels of whole-grain intake and reduced scores for mood disorders including depression and anxiety. Whole grains may be a modifiable lifestyle factor that can improve some mental health conditions, although this needs to be supported by randomized controlled trials to establish a causal relationship.
Introduction
As the proportion of older adults across many countries increases, there is an increase in the rate of cognitive diseases, with around 55 million people living with dementia worldwide. It is estimated that there are nearly 10 million new cases each year, with many more likely undiagnosed [1]. Cognitive decline covers a broad spectrum of neurological disorders related to a progressive reduction in mental capabilities with age, usually categorized as preclinical cognitive impairment, mild cognitive impairment, and late-stage cognitive impairment or dementia [2]. These disorders have severe impacts on both individual and societal health outcomes. Cognitive decline can include mental health conditions including anxiety, depression, and mood changes, as these are typically associated with the onset of mild cognitive impairment [3,4], although these conditions can act independently. Changes to the brain that lead to cognitive disease start more than a decade before symptoms of cognitive decline are clinically measurable [5,6]. To date, there are no proven clinical treatments for treating or preventing cognitive decline, emphasizing the need for lifestyle-based preventative strategies.
Cognitive decline is associated with several comorbidities including type 2 diabetes (T2D), hypertension, CVD, and obesity [[7], [8], [9]]. The coassociation of these diseases with cognitive diseases and impairment suggests there could be a protective role of increased consumption of whole-grain cereal foods, as there are consistent observational data to support that people who eat the most whole grains have a reduced risk of these diseases compared with people who eat the least whole grains [[10], [11], [12]]. Based on these findings, many countries recommend replacing at least half of refined grains with whole grains [13]. Greater intake of whole grains is associated with improved metabolic markers linked to cognitive function, including inflammation, glucose metabolism, blood pressure, and cholesterol. However, evidence from intervention studies is inconsistent or mixed [[14], [15], [16], [17], [18], [19]].
To date, there are few studies that investigated the possible association between whole-grain intake and measures of cognitive decline, usually as a secondary analysis of an overall dietary pattern study [20]. To clarify the quality of available evidence, we conducted a systematic review to synthesize all available observational and interventional studies evaluating the association between whole-grain intake and cognitive outcomes [focusing on cognitive decline and function (including dementia) and measured changes to brain structure and function] or measures of mood (including depression) and anxiety.
Methods
We followed the standard methodology outlined in the Cochrane Handbook for this systematic review [21]. We reported findings according to the PRISMA statement [22]. The study protocol was preregistered on the PROSPERO (https://www.crd.york.ac.uk/prospero) as CRD42021266355.
Literature search and study selection process
Literature searches were implemented in MEDLINE (1946 to week 3 in July 2022), Embase (1966 to 27 August 2021), Cochrane Central (1991 to 27 August 2021), and PsycINFO (1967 to 27 August 2021) databases. Searches were limited to human studies and restricted to the English language. The search strategy included specified terms for whole-grain intake and cognition outcomes (cognitive decline and mild cognitive impairment) and related disorders (including Alzheimer’s disease and dementia), brain structure, and function. Full search strategies for all databases are described in Supplemental Table 1. Secondary outcomes of anxiety, mood, and depression were included in the literature search and reviewed for inclusion. Such papers were included as part of this review if very few papers were identified to assess the relationship between whole-grain intake on cognitive outcomes. Studies in which whole grain was part of a larger dietary pattern were included if the individual food components—including whole grain—were examined in relation to cognition or anxiety, mood, and depression outcomes. These strategies did not include dietary pattern search terms (for example, Dietary Approaches to Stop Hypertension diet, Mediterranean diet). Thus, any dietary pattern papers included in this review were identified through abstracts containing search terms included in the full search strategy or additional sources (for example, reference mining of systematic reviews or hand searching). To identify randomized controlled trials (RCTs) of relevance, citation tracing was performed on included RCTs in this review. In addition, investigators conducted searches within national and international clinical trial registries. To further identify RCTs and observational studies of relevance, investigators screened several systematic reviews in related areas. Two investigators independently screened abstracts using the Rayyan software for systematic reviews [23]. Full-text articles of screened-in abstracts were independently assessed by 2 investigators using standardized forms. All rejected abstracts and full-text articles were re-examined to confirm or refute their exclusion. Disagreements were resolved by group consensus.
The study eligibility criteria are presented in Table 1. Briefly, abstracts and full-text articles were included if the study population was considered healthy or generally healthy (prediabetes or T2D populations were included as part of controlled trials); if the intervention included whole-grain foods, biomarkers of whole grain, or dietary patterns that isolate the effect or association of whole-grain foods on outcomes in the study analyses; if the comparator included no or low whole-grain foods; and if the outcome was defined as cognition (cognitive decline, cognitive function, mild cognitive impairment, dementia, Alzheimer’s disease, etc.), structural and functional changes to the brain, or conditions of mood, anxiety, and depression.
TABLE 1.
Study eligibility criteria
| Inclusion criteria | Exclusion criteria | |
|---|---|---|
| Study design | Controlled trials
|
|
| Population | Observational studies and trials
|
Exclusively unhealthy participants (unless health status is nutrient deficiency related) |
| Intervention | Whole grain
|
Dietary patterns that do not isolate the whole-grain variable (for example, whole-grain intake is not sufficiently isolated from other components in the dietary pattern). |
| Comparator | No or low whole-grain or whole-grain foods | None |
| Outcome | Cognition
|
Cerebrovascular outcomes (for example, stroke) Movement-related outcomes (for example, balance and falls) |
Abbreviation: T2D, type 2 diabetes.
Acute interventional studies are defined as 24 h after whole-grain exposure or exposure to one whole-grain–containing meal.
Generally healthy is defined as <20% of the included population has disease. Hypertension and elevated cholesterol are common conditions found in a generally healthy population. Studies were included if the prevalence of these conditions was found in <20% of the study population.
Whole-meal, brown bread, coarse bread, wheat, rye, oats, barley, corn/maize, rice, sorghum, millet, quinoa, buckwheat, amaranth, teff, etc.
Cognitive function, as assessed by the following domains: learning and memory, visuospatial and motor function, attention and concentration, language, social cognition and emotion, processing speed, and executive function.
If we did not identify enough papers with the following cognition and brain structure/function outcomes, we considered additional cognition-related outcomes such as mood, anxiety, and depression.
Data extraction
Standardized data extraction forms were created to extract population characteristics, study design details, and key findings, including observational (cross-sectional, case-control, and cohort) and interventional studies. Extracted population characteristics included age, sex, race and ethnicity, and health status. Extracted study design details included sample size (of the cohort, cases, and controls, those randomly assigned); dietary whole-grain intervention (RCT) or assessment method (observational); outcome assessment method, study cohort, or design (parallel or crossover trial); and study duration [cohort studies (follow-up duration) and RCTs (total trial duration)]. Key findings of cognition, brain structure and function, along with anxiety, mood, and depression were reported according to a symbol system, further described in the footnote of Table 2. The extracted data were separated into tables by study design to facilitate qualitative synthesis. Extractions were completed by one investigator and independently reviewed by another investigator.
TABLE 2.
Study design details, population characteristics, and key findings from randomized controlled trials assessing the effects of whole-grain intake on cognition, mood, anxiety, and depression outcomes
| Author, year | Country | N | Design | Mean age [range], y | % Male | Racial or ethnic background | Health status | Dietary whole-grain intervention | Comparator | Total trial duration | Outcome assessment method | Outcome analyzed | Key findings1 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cognition | |||||||||||||
| Kuroda, 2019 [50] | Japan | 52 | Parallel | 72.9 [NR] | 48.1 | 100% Japanese | 100% healthy | 100 g/d of ultrahigh hydrostatic pressurizing brown rice (UHHPBR) | 100 g/d of white rice | 24 mo | Cognition: HDS-R; MMSE; Frontal Assessment Battery (FAB); and the Cognitive Assessment for Dementia, iPad version (CADi). Mental health: Japanese version of Starktein’s Apathy Scale and Zung Self-Rating Depression Scale (SDS). |
Cognition: HDS-R score, MMSE score, FAB score, CADi score, FAB scores on “conceptualization,” and CADi scores on “time required” (s) Mental health: apathy score, SDS score, apathy scores |
Cognition: HDS-R score: 0; MMSE score: 0; FAB score: 0; CADi score: 0; FAB scores: ++; and CADi scores: −− Mental health: apathy score: −−; SDS score: −−; and apathy scores: −− |
| Uenobe, 20192 [30] | Japan | 31 | Crossover | 84.1 [NR] | 22.6 | 100% Japanese | 100% healthy | 3 meals/d of dewaxed brown rice | 3 meals/d of white rice | 12 mo (Period I and II: 6 mo each) | HDS-R | HDS-R score among those in group A, group B, group A + B, and the low cognitive function group3 | HDS-R score: group A: 0; group B: 0; group A + B: 0; and low cognitive function group: ++ |
| Mood, anxiety, and depression | |||||||||||||
| Esmaeilpour, 2019 [40] | Iran | 100 | Parallel | 31 [[19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45]] | 0 | NR | 100% healthy | Diet enriched with whole grains4 | Usual diet | 3 mo | Daily symptom record. Three subgroups measured, including mood symptoms (restlessness; irritability; anxiety; depression or sadness; crying; and feeling of isolation). | Mean of PMS score (mood) | Mood score: −− |
| Sakamoto, 2007 [41] | Japan | 41 | Parallel | 32.2 [NR] | 0 | 100% Japanese | 100% healthy | Pregerminated brown rice (average intake: 238 ± 35.3 g) | White rice (average intake: 270 ± 57.6 g) | 2 wk | The Profiles of Mood States (POMS): brief form Japanese version | Total mood disturbance (TMD) score POMS parameters: tension-anxiety, depression, anger-hostility, vigor, fatigue, and confusion |
TMD score: −− POMS parameters: tension-anxiety: 0; depression: −−; anger-hostility: −−; vigor: 0; fatigue: −−; confusion: 0 |
Abbreviations: HDS-R, Revised Hasegawa Dementia Scale; N, number of individuals randomly assigned; NR, not reported; PMS, premenstrual syndrome.
Key findings’ symbols: ++ significant positive effect (P ≤ 0.05); + marginally significant positive effect (0.05 < P < 0.1); 0 no effect; − marginally significant inverse effect (0.05 < P < 0.1); −− significant inverse effect (P ≤ 0.05).
This article reported both cognition and mood, anxiety, and depression outcomes.
Group A: received dewaxed brown rice for the first 6 mo then received white rice for the remaining 6 mo; group B: received white rice for the first 6 mo then received dewaxed brown rice for the remaining 6 mo; low cognitive function group had a total HDS-R score of 1 or more and <10 points (HDS-R is out of 30 points).
Replaced ≥4 servings of daily refined grains with whole grains. Given a whole-grain food list that included whole-wheat bread, brown rice, brown spaghetti, and homemade cakes and cookies using whole-wheat flour. Individuals were also given 840 g of whole-grain bread each week to replace their daily consumption of refined bread with 120 g of whole-grain bread.
Risk of bias assessment
Risk of bias (RoB) assessments were performed using the newly developed Nutrition Quality Evaluation Strengthening Tools (NUQUEST), a suite of RoB tools for nutrition RCTs, cohort, and case-control studies [24]. These tools were designed to evaluate the RoB in human nutrition studies by integrating nutrition-specific criteria into assessment domains [24]. The 4 domains assessed and rated in these 3 tools included a selection of participants (RCTs), selection of cohorts (cohort), and creation of study groups (case-control); comparability of study groups (RCTs and case-control) or cohorts (cohort); ascertainment of the exposure (case-control) or outcomes (RCTs and cohort); and nutrition-specific (all 3 tools). Each domain was rated as either good, neutral, or poor; domain ratings were reached using a prespecified point system set by the study investigators (Supplemental Table 2). An overall RoB rating was determined using the rationale described in the revised Cochrane RoB tool for randomized trials (RoB 2) guidance document [25]. For example, to fulfill the overall RoB criteria for neutral, ≥1 domain had to be rated neutral, and no domains could be rated as poor. Currently, a NUQUEST tool for cross-sectional studies does not exist. We modified the NUQUEST cohort tool to assess biases in a cross-sectional design. Details of this modification can be found in Supplemental Table 3.
Two investigators independently performed RoB assessments for each included study, with all investigators participating in reviewing the assessments. Disagreements were resolved through group discussions. RoB assessments are reported in Supplemental Table 4.
Rating of whole-grain reporting
The quality of how each study reported and estimated whole-grain intake was graded in relation to 5 criteria proposed by Ross et al. [26]. These criteria include 1) how the amount of whole grains was reported and if a clear distinction was made between whole grains (for example, “grams of whole grain as an ingredient in different foods”) and whole-grain food (for example, “grams of whole-grain–containing food”); 2) stating how whole grains had been defined; 3) differentiating between different types of cereal grain (for example, wheat, rice, maize/corn, rye, and oats); 4) distinguishing between different kinds of cereal processing or products (for example, bread, breakfast cereal, noodles, pasta, and porridge); and 5) if a biomarker of whole-grain intake had been used in the study. When criteria were partially met, a score of 0.5 for that criterion was given. Ratings for all included studies are reported in Supplemental Table 5.
Meta-analysis
Meta-analyses of RCTs were planned to combine the effects of whole-grain interventions on cognition, anxiety, mood, or depression outcomes when 2 or more RCTs reported sufficient quantitative data for the analyses. However, due to the high degree of variation in intervention duration and outcome measures coupled with a high RoB in a number of studies (Table 2), meta-analyses were deemed inappropriate. Mean differences and standardized mean differences describing the relationship between whole-grain intake and cognition, mood, and depression outcomes are reported in Supplemental Table 6.
Strength of evidence rating
The Grades of Recommendation, Assessment, Development, and Evaluation (GRADE) approach [27,28] was used to determine the strength of available evidence for each outcome. A GRADE evidence profile table was used to present synthesized data (Supplemental Table 7). All investigators rated the strength of evidence for each outcome.
Results
Study selection
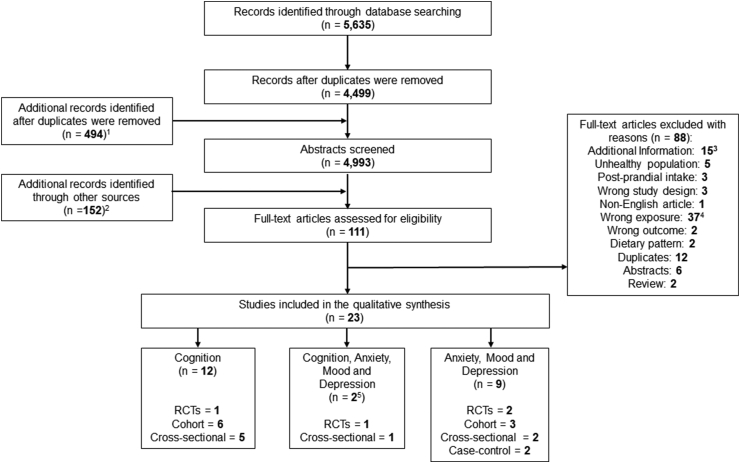
A total of 4993 eligible abstracts were identified and screened and 93 abstracts were accepted for full-text review (Figure 1). After reviewing the full-text articles, 23 papers met the eligibility criteria for inclusion. Twelve papers related to cognition [20,[29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39]], 9 to anxiety, mood, and depression [[40], [41], [42], [43], [44], [45], [46], [47], [48]], and 2 to cognition, anxiety, mood, and depression [49,50].
FIGURE 1.
PRISMA flow chart of study screening and selection process. 1Screening of the following secondary outcomes: anxiety, mood, and depression. 2Additional articles of interest were identified through reference checking of potentially eligible papers and reviews, clinical trials databases, and hand searching. 315 of the 88 articles were considered “additional information” to use as supporting information for writing the article. 4The exclusion reason “wrong exposure” includes papers that had no whole-grain exposure or no clear whole-grain exposure (for example, wheat or rice with no reference to whole grain) as well as certain dietary pattern papers that included whole-grain intake but did not isolate whole grain for separate analyses of the outcome. 5Two unique papers included both cognition and anxiety, mood, and depression outcomes. RCT, randomized controlled trial.
Several terms were used in the literature that could be interpreted as meaning or including whole grains. We accepted “nonrefined grains” and “high-fiber grains,” in which the fiber/grain ratio was defined as >7 g/100 g. Terms such as “high-fiber bread” were not included; this could imply a whole-grain food, but it could also refer to added ingredients such as added bran or purified fiber. We also accepted food descriptions that implied a whole-grain food, such as “groats.” When the term “coarse cereals” was used, a term commonly used in dietary intake research from China, we excluded publications in which major confounding existed within food groups such as legumes. Definitions of coarse cereals vary and may refer to whole grains but often include lentils and other legumes and may exclude rice and wheat [51], limiting our interpretation of “coarse cereals” as “whole grains.” Three intervention studies included brown rice that had been processed to improve palatability [ultrahigh hydrostatic pressurized brown rice (UHHPBR), dewaxed brown rice, and pregerminated brown rice]. Results from these studies were included on the basis that there was no evidence that any of the bran, germ, or endosperm were removed during processing and would, therefore, meet a whole-grain food definition [52].
Impact of whole grains on cognition
Overall, the majority of studies suggest a positive association of whole-grain consumption on measures of cognitive decline, mood, and anxiety; however, interpretation in the context of the study design is important.
Randomized trials
Cognition
Two studies examined the relationship between processed brown rice interventions compared with white rice on cognitive outcomes in relation to dementia [30,50]. Both studies found some, but not all, measures were improved with brown rice interventions. Neither study found a change to the Hasegawa Dementia Scale (HDS), although one found an improvement in HDS score in a low cognitive function subgroup associated with improved cognition [30] (Table 2, Supplemental Table 6), whereas a 2-y intervention found that daily intake of preprocessed brown rice improved changes in Frontal Assessment Battery score and Cognitive Assessment for Dementia processing time [50]. The RoB for these studies was rated as poor.
Mood, anxiety, and depression
All 3 RCTs that examined mood, depression, fatigue, and apathy found improvements in these outcomes with either processed brown rice or whole-grain wheat interventions [40,41,50] (Table 2). Notably, one study focused on mood in relation to premenstrual syndrome [40] and found that a whole-grain wheat-based intervention compared with no intervention resulted in improved measures of mood. The RoB for these 3 studies was rated as neutral [40,41] or poor [50].
Prospective cohort studies
Cognition
Of the 6 prospective cohort studies that examined whole-grain intake and cognitive decline, 3 found no associations [[32], [33], [34]] whereas 3 found a reduced risk of cognitive decline [20,31,35] (Table 3). In 1 study, the association between whole grains and improvement in cognitive score was no longer significant after full model adjustment for total EI, age at follow-up, sex, physical activity, race, diabetes, hypertension, BMI (in kg/m2) at follow-up, word reading score, education, and depressive symptoms [31]. The RoB for these 6 studies was rated as good [33,35], neutral [31,32,34] or poor [20].
TABLE 3.
Study design details, population characteristics, and key findings from cross-sectional studies describing the associations between whole-grain intake and cognition, mood, anxiety, and depression outcomes
| Author, year | Country | N | Mean age [range], y | % Male | Racial or ethnic background | Health status | Dietary whole-grain assessment method (foods) | Outcome assessment method | Outcome analyzed | Key findings1 |
|---|---|---|---|---|---|---|---|---|---|---|
| Cognition | ||||||||||
| Abbaszadeh, 20212 [49] | Iran | 181 | 20.8 [[18], [19], [20], [21], [22], [23], [24], [25]] | 0 | NR | 100% healthy | FFQ (whole-grain foods) | Cognitive Abilities Questionnaire; The Depression Anxiety Stress Scale (DASS) | Cognitive abilities and depression, anxiety, and stress3 | Cognitive abilities: 0; depression, anxiety, and stress: 0 |
| Anastasiou, 2017 [29] | Greece | 1864 | 73 [NR] | 40.6 | NR | Generally healthy4 | Greek FFQ (nonrefined cereals) | Comprehensive neuropsychological assessment: MMSE; Greek Verbal Learning Test (GVLT); Medical College of Georgia Complex Figure Test (MCG); Greek version of the Boston Diagnostic Aphasia Examination short form; Benton’s Judgment of Line Orientation; Clock Drawing Test; Trail Making Test (TMT)—part A and B; Graphical Sequence Test; and gross estimate of intellectual level. Diagnosis of dementia and its subtypes was based on DSM-IV-TR criteria5 | Dementia, cognitive status, and cognitive performance6 | Nonrefined cereal intake between those diagnosed with dementia and those without dementia: 0; cognitive status: 0; cognitive performance: ++ |
| Croll, 2018 [36] | Netherlands | 4213 | 65.7 [NR] | 43.2 | NR | Generally healthy7 | FFQ (whole-grain products) | MRI | Total brain volume, gray matter volume, white matter volume, and hippocampus volume8 | Total brain volume: ++; gray matter volume: ++; white matter volume: 0; hippocampus volume: 0 |
| Dong, 2016 [37] | China | 894 | 62.8 [[55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70], [71], [72], [73], [74], [75], [76]] | 32.8 | 100% Chinese9 | 100% healthy | FFQ (whole-grain foods) | Montreal Cognitive Assessment (MoCA) | Cognitive function (MoCA Score)10 | MoCA Score: 0 Those with mild cognitive impairment compared to controls: 011 |
| Huang, 2021 [38] | Taiwan | 1115 | 73.8 [NR] | 47.1 | NR | 100% healthy | SFFQ and 24-h dietary recall (whole-grain foods) | MMSE | Cognitive function based on degree of frailty severity (robust, prefrailty, and frailty) | Cognitive function: robust: ++; prefrailty: 0; frailty: ++ |
| Xu, 2020 [39] | China | 1262 | 72.3 [NR] | 44.5 | NR | 100% healthy | FFQ (coarse cereals) | MMSE | Mild cognitive impairment (MCI) | MCI Crude: 0; model 1: −−12; model 2: −13 |
| Mood, anxiety, and depression | ||||||||||
| Lanuza, 2021 [45] | Chile | 2031 | 65.9 [NR] | 48.2 | NR | Generally healthy14 | FFQ (whole-grain bread, whole-grain cereal, or any food product that contains whole-grain flour) | Composite International Diagnostic Interview—Short Form (CIDI-SF) | Major depressive episode (MDE)15 | MDE: 0 |
| Sadeghi, 2019 [46] | Iran | 3172 | 36.5 [NR] | 44.1 | NR | 100% healthy | DS-FFQ [wheat seed, diet bread (cooked by whole flour), and dark breads including Iranian breads of Sangak and Barbari]16 | Iranian validated version of the Hospital Anxiety and Depression Scale (HADS) | Women:
|
Women:
|
Abbreviations: DS-FFQ, dish-based FFQ; DSM-IV-TR, Diagnostic and Statistical Manual of Mental Disorders, Text Revision, Fourth Edition; IADL: Instrumental Activities of Daily Living Scale; MNA-SF: Mini Nutritional Assessment Scale-Short Form; N, number of individuals enrolled; NR, not reported; WC, waist circumference; WHR, waist-to-hip ratio.
Key findings’ symbols: ++ significant positive associations (P 0.05); + marginally significant positive associations (0.05 < P < 0.1); 0 no associations; − marginally significant inverse associations (0.05 < P < 0.1); −− significant inverse associations (P 0.05).
This article reported both cognition and mood, anxiety, and depression outcomes.
Adjusted for age, BMI, WHR, and EI.
On average, the study population was overweight (BMI: 29 kg/m2) and had a large waist circumference (WC: 100 cm).
Diagnosis of vascular dementia: clinical history of stroke, clear temporal relation between stroke and the onset of dementia and the Hachinski Ischemia Scale score. Lewy body and frontotemporal dementias were also diagnosed using specified criteria. Dementia staging was performed through a semistructured interview, using the Clinical Dementia Rating Scale, which globally assesses 6 domains of cognitive and functional performance.
Adjusted for age, sex, education, number of clinical comorbidities, and EI.
Part of the study population had hypertension (22%), hypercholesterolemia (52%), and diabetes (9%).
Adjusted for age, sex, intracranial volume, education, EI, smoking, physical activity, and BMI.
Han: 94%; Hui: 2.3%; Mengolia: 0.1%; Manchu: 1.5%; Other: 1.7%.
Adjusted for age, sex, nationality, BMI, and education level.
No differences in whole-grain intake between those diagnosed with MCI and controls.
Adjusted for age and sex.
Adjusted for age, sex, education, marital status, smoking, alcohol drinking, EI, diabetes mellitus, hypertension, physical activity, and MNA-SF and IADL scores.
Part of the study population had hypertension (72.3%), diabetes (29.6%), hypercholesterolemia (41.3%), and CVD (23.9%).
Adjusted for age, sex, region, residency area, education, tobacco status, alcohol intake, physical activity, hours of sleep, BMI, cognitive impairment, hypertension, hypercholesterolemia, diabetes, CVDs (acute MI, stroke, or peripheral artery disease), and the healthy eating score and each individual food item (as appropriate, for calculations of % of change in total effect).
Intake of whole grains was further classified into quartiles of intake for analysis.
Adjusted for age and EI (model 1); marital status, education, family size, smoking status, physical activity, home ownership, diabetes, dietary supplement use, and antipsychotic medications (model 2); intake of food groups including fruits, vegetables, red meat, fish, legumes and nuts, whole grains or refined grains, tea, and coffee (model 3); BMI (model 4); B-vitamins including thiamine, riboflavin, niacin, pantothenic acid, pyridoxine, folate, and cobalamin. All adjustments were additive across models.
A moderate consumption of whole grains (Q3) was inversely associated with anxiety in women, across all adjusted models.
Mood, anxiety, and depression
Three prospective cohort studies examined whole-grain intake and measures of depression and mental health [42–44] and found inverse associations between whole-grain intake, depression, and psychological distress (Table 3). The RoB for all 3 studies was rated as neutral.
Cross-sectional studies
Cognition
Two studies [37,49] found no association between whole-grain intake and measures of cognition (cognitive abilities, comparison between mild cognitive impairment compared with control). Four studies found a positive association between whole-grain intake and measures of cognitive performance (either based on questionnaires or total brain and gray matter volume, a marker of cognitive health) [29,36,38,39] (Table 4). The RoB for these 4 studies was rated as neutral [29,38,39] or poor [36,37,49].
TABLE 4.
Study design details, population characteristics, and key findings from prospective cohort studies describing the associations between whole-grain intake and cognition, mood, anxiety, and depression outcomes
| Author, year | Cohort | Country | N | Mean age [range], y | % Male | Racial or ethnic background (%) | Health status | Follow-up duration | Dietary whole-grain assessment method (foods) | Outcome assessment method | Outcome analyzed | Key findings1 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cognition | ||||||||||||
| Fortune, 2019 [31] | The Bogalusa Heart Study | United States | 516 | 49.7 [NR] | 37.4 | Non-Hispanic White: 72.1; Non-Hispanic Black: 27.9 | Generally healthy2 | 15 y | FFQ (whole-grain cereal, cooked oats, dark bread, kasha, couscous, and bulgur) | Wechsler Adult Intelligence Scale; Wechsler Memory Scale IV; Wide Range Achievement Test IV; Trail Making Test parts A and B | Cognitive function | Crude association,3 cognitive function: ++ Full adjustment4, cognitive function: 0 |
| Qin, 2015 [32] | China Health and Nutrition Survey (CHNS) | China | 1650 | 63.4 [NR] | 49.7 | NR | Generally healthy5 | 5 y | 24-h dietary recalls by trained interviewers (fiber-rich grains: corn grain, yellow corn flour, corn grits, and barley grain, buckwheat)6 | Modified Telephone Interview for Cognitive Status (immediate and delayed recall of a 10-word list; counting backward from 20; serial 7 subtraction and orientation) | Global Cognitive Score7 | Global cognitive score All participants: 0; age at entry <65: 0; age at entry 65: 0 |
| Samieri, 2013 [20] | NHS | United States | 16,058 | 74.3 [NR] | 0 | NR | Generally healthy8 | 6 y | FFQ (whole-grain foods) | Cognitive Battery: 1) the Telephone Interview for Cognitive Status (TICS); immediate and/or delayed recalls of 2) the East Boston Memory Test (EBMT); 3) the TICS 10-word list; 4) category fluency; and 5) digit span-backward. |
Global score Verbal memory score9,10 |
Global score: 0 Verbal memory score: 0 |
| Samieri, 2013 [33] | Women’s Health Study (WHS) | United States | 6174 | 66.0 [NR] | 0 | Non-Hispanic White: 96.0; other: ∼4.0 | Generally healthy11 | 5 y | FFQ (dark bread, brown rice, oatmeal/bran, wheat germ, crackers/wheat thins, and other grains) | Cognitive battery: immediate and delayed recalls of 1) the telephone interview of cognitive status and 2) the East Boston Memory Test | Global cognition Verbal memory12 |
Global cognition: ++ Verbal memory: 0 |
| Shakersain, 2018 [34] | Swedish National Study on Aging and Care in Kungsholmen (SNAC-K) | Sweden | 2223 | 70.6 [NR] | 39.2 | NR | Generally healthy13 | 6 y | Swedish FFQ (whole-grain breads or cereals) | MMSE | MMSE scores14 | MMSE scores: 0 |
| Wengreen, 2013 [35] | Cache County Memory Study (CCMS) | United States | 3580 | 74.1 [NR] | 43.0 | Non-Hispanic White: 90.0; other: ∼10.0 | 100% healthy | 11 y | FFQ (dark bread or pita, whole-grain cold breakfast and cooked cereal, oatmeal, popcorn, bulgur, kasha, and couscous) | Modified MMSE (3MS) | 3MS scores15 | 3MS scores: ++ |
| Mood, anxiety, and depression | ||||||||||||
| Gangwisch, 2015 [42] | Women’s Health Initiative (WHI) | United States | 69,954 | 65.7 [NR] | 0 | Non-Hispanic White: 86.6; Non-Hispanic Black: 6.2; Asian or Pacific Islander: 3.0; Hispanic: 2.7; American Indian or Alaskan Native: 1.3 | Generally healthy16 | 3 y | FFQ (whole-grain foods) | Burnam 8-item scale for depressive disorders17 | Incident depression | Incident depression Model 1: −−18 Model 2: −−19 |
| Gibson-Smith, 2020 [43] | Netherlands Study of Depression and Anxiety (NESDA) | Netherlands | 1634 | 52.0 [[18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65]] | 32.1 | NR | 100% healthy | 9 y | Dutch FFQ (nonrefined grains) | Inventory of Depressive Symptomatology—Self Report (IDS-SR); Beck Anxiety Inventory (BAI) | Severity of depression (IDS) and anxiety (BAI) scores20 | IDS scores: −− BAI scores: −− Those with current depression and anxiety disorders compared to controls: −−21 |
| Kleppang, 2021 [44] | Trøndelag Health Study (HUNT) | Norway | 2230 | NR [[23], [24], [25], [26], [27], [28], [29], [30], [31]] | 42.3 | NR | NR | 11 y | FFQ (whole-grain bread) | Conor Mental Health Index (CONOR-MHI) | Psychological distress | Psychological distress:22 Crude model: −− Adjusted model: −−23 |
Abbreviations: N, number of individuals enrolled; NR, not reported; TICS, Telephone Interview for Cognitive Status.
Key findings’ symbols: ++ significant positive associations (P ≤ 0.05); + marginally significant positive associations (0.05 < P < 0.1); 0 no associations; − marginally significant inverse associations (0.05 < P < 0.1); −− significant inverse associations (P ≤ 0.05).
Part of the study population had hypertension (75%) and diabetes (15%).
Adjusted for total caloric intake.
Adjusted for total caloric intake, sex, age at follow-up, race, physical activity, diabetes, hypertension, BMI at follow-up, word score, education, and depressive symptoms.
Part of the study population had hypertension (∼40%).
The whole-grain foods in this study were defined as “fiber-rich grain” with a fiber to carbohydrate ratio 7.4 g:100 g.
Adjusted for age, sex, region (south/north), urbanization index, education (graduated from primary/less), annual household income per capita (≥5,000 yuan/less), total EI, time, physical activity (tertile), current smoking (yes/no), BMI (linear and squared terms) and hypertension, and time interactions with each covariate.
Part of the study population had a history of MI (∼6%), hypertension (∼55%), hypercholesterolemia (∼65%), diabetes (∼10%), and depression (∼9%).
The authors in this study compared cognitive outcomes associated with whole-grain consumers (of any intake of whole grains) to nonconsumers.
Adjusted for age, education, long-term physical activity and EI, BMI, smoking, history of depression, multivitamin use, and histories of diabetes, hypertension, hypercholesterolemia, and MI.
Part of the study population had a history of hypertension (40%), hypercholesterolemia (53%), depression (6%), and diabetes (4%).
Adjusted for treatment arm, age at initial cognitive testing, Caucasian race, high education, high income, EI, physical activity, BMI, smoking, diabetes, hypertension, hypercholesterolemia, hormone use, depression, and alcohol intake.
Part of the study population had vascular disorders (∼85%), diabetes (∼31%), cancer (∼5%), and depression (∼5%).
Adjusted for total calorie intake, age, sex, education, civil status, physical activity, smoking, BMI, vitamin/mineral supplement intake, vascular disorders, diabetes, cancer, APOE ε4, and dietary components other than main exposure(s) in each model.
Adjusted for age, sex, education, BMI, frequency of moderate physical activity, multivitamin and mineral supplement use, history of drinking and smoking, and history of diabetes, heart attack, and stroke.
Part of the study population had hypertension (∼30%), diabetes (∼5%), MI (∼2%), stroke (∼1%), cancer (∼12%), and CVD (∼18%).
The Burnam scale includes 2 items from the Diagnostic Interview Schedule and 6 items from the Center for Epidemiologic Studies–Depression Scale.
Adjusted for nutrient density.
Adjusted for nutrient density, race-ethnicity, education, income, BMI, diabetes, hypertension, hormone replacement therapy, stroke, MI, Alzheimer’s disease, CVD, cancer, physical activity, stressful life events, social support, smoking, alcohol, and energy-adjusted intakes of saturated, monounsaturated, polyunsaturated, and trans FAs.
Adjusted for age, sex, education (y), partner status physical activity, and smoking status and corrected for all other food groups. Findings were significant after correction for multiple testing.
Higher nonrefined grain consumption was significantly related to a lower odds of having a current clinically diagnosed depression or anxiety disorder compared to controls.
Kleppang and authors set the reference as “daily consumption of whole-grain bread,” rather than “less than daily consumption.” Thus, the interpretation would be those who consumed whole-grain bread “less than daily” had a greater odds of psychological distress compared with those who consumed whole-grain bread daily.
Adjusted for age, sex, psychological distress in adolescence and highest education as young adults from the main effects model.
Mood, anxiety, and depression
Two studies [45,49] found no significant associations between whole-grain intake and risk of depression and anxiety. However, a possible association of a major depressive episode with whole-grain intake was observed in the Lanuza et al. [45] study (odds ratio: 0.60; 95% confidence interval: 0.27, 1.30). One study found and association between whole-grain intake and lower anxiety in women but not in men [46] (Table 4). The RoB for these studies was rated as poor.
Case-control studies
Cognition
No case-control studies examining the association between whole-grain intake and cognition were identified.
Mood, anxiety, and depression
Two case-control studies examined the relationship between whole-grain intake and mood or depression, with one study finding that whole-grain intake was greater in the control subjects compared with the cases who had a wide range of mood-related disorders, including depression [47] (Table 5). In a second study examining diet and depression, there was a higher intake of groats (cooked intact grains such as oat porridge or cooked buckwheat) in female controls compared with patients with depression. A difference in groat intake was identified between male patients and controls, but this difference was not statistically significant. No association was observed between rye bread intake in controls compared to patients with depression [53]. The RoB for these studies was rated as neutral [47] or poor [53].
TABLE 5.
Study design details, population characteristics, and key findings from case-control studies describing the associations between whole-grain intake and cognition, mood, anxiety, and depression outcomes
| Author, year | Country | Cases | Controls | Mean age [range], y | % Male | Racial or ethnic background (%) | Health status for controls | Dietary whole-grain assessment method (foods) | Outcome assessment method | Outcome analyzed | Key findings1 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mood, anxiety, and depression | |||||||||||
| Davison, 2012 [47] | Canada | 97 | 1823 | NR [[19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70]] | 46.7 | NR | Generally healthy | FFQ and 3-d food record (whole grains) | Members of the Mood Disorders Association of British Columbia (MDABC) were administered the Structured Clinical Interview for DSM-IV Axis I Disorders, Global Assessment of Functioning Scale (GAF), the Hamilton Depression Scale (Ham-D), and the Young Mania Rating Scale (YMRS) by a trained clinical interviewer | Mood disorders | Whole-grain intake was greater in controls vs. cases: ++ |
| Stefanska, 2014 [48] | Poland | 75 | 75 | 43.2 [[18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64]] | 26.7 | NR | 100% healthy | FFQ (groats and rye bread) | Identification of a 5-y, recurrent depressive disorder by a psychiatry specialist according to ICD-10 | Depression | Groats intake was greater in female controls vs. cases: ++ Groats intake between male cases and controls: 0 Rye bread intake between male and female cases and controls: 0 |
Abbreviations: DSM-IV, Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition; NR, not reported.
Key findings’ symbols: ++ significant associations (P ≤ 0.05); + marginally significant associations (0.05 < P < 0.1); 0 no associations.
Strength of evidence rating
Using the GRADE tool, the strength of evidence from the trials included in this review was rated from “insufficient” to “low” (Supplemental Table 5). This is largely due to “poor” RoB assessments for some of the studies, use of surrogate outcomes for the study question (effect on dementia risk) and end outcome (dementia), and few studies demonstrating a dose–response effect.
Whole-grain reporting quality
Overall, the rating of reporting of whole-grain intake across studies was poor, with no scores above 2 out of 5 (Supplemental Table 6). Few observational studies (3/19) reported intake as “grams whole grain” or made a distinction between grams of whole grains and grams of whole-grain food or whole-grain product. Additionally, only 2 of the 4 intervention studies included clearly stated the daily whole-grain intake during the intervention. The types of grains were usually not differentiated, except in intervention studies in which specific grain types were given (3 with processed brown rice, 1 with whole-grain wheat), and no population-based studies differentiated between different ways of preparing or processing cereals. None of the studies reviewed had applied a biomarker of whole-grain intake.
Amount of whole-grain intake in relation to positive cognitive outcomes
Seven of the 20 studies reported a measure of whole-grain intake either in grams or servings per day. In these studies, it was unclear if these referred to whole-grain foods or whole grain as an ingredient. In the intervention studies, an improvement on cognitive scores was observed with 100 g/d of UHHPBR for 24 mo [50], whereas 3 meals/d of dewaxed brown rice for 3 mo resulted in improved dementia scores in a low cognitive function subgroup (reduced dementia symptoms) [30]. Three meals/d of rice could be estimated to be between 100 to 200 g rice on a dry weight basis. In a shorter study on mood, a mean intake of 238 g/d pregerminated brown rice for 2 wk resulted in a reduction in scores of depression, anger-hostility, and fatigue [41], although it is unclear if this was on a dry or cooked basis. Due to the variation in processing methods for the intervention studies with brown rice, it is not possible to conclude if the processing had any effect on outcomes.
Of the observational studies reporting no effect on cognitive outcomes, Abbaszadeh et al. [49] reported a mean whole-grain intake of 21.7 g/d for the lowest tertile of Nordic diet adherence score and 47.6 g/d for the highest tertile, whereas Samieri et al. [33] reported the lowest quintile of 12.8 g/d compared with the highest quintile with 33.6 g/d (using 16 g/serving to convert from “servings/d”). For the observational studies reporting associations between whole-grain intake and cognitive outcomes, Sadeghi et al. [46] reported the mean of the lowest quartile of intake as 0 g/d and the highest quartile as 54 g/d for both men and women. Fortune et al. [31] reported the whole-grain intake for the lowest quintile for cognitive score at 383 g/2000 kcal and the highest quintile at 655 g/2000 kcal. Samieri et al. [20] reported whole-grain intake for the lowest and highest quintiles of a Mediterranean diet score to be 12.8 g/d and 35.2 g/d (converted from servings/d as above). Gangwisch et al. [42] reported the lowest quintile of whole-grain intake at 7.1 g/d and highest at 65 g/d. Wengreen et al. [35] and Gibson-Smith et al. [43] did not report whole-grain intake for different quartiles but did report a median population intake of 18.2 g/d (interquartile range: 9.1–35.2) and 125.5 (85.3–178.5), respectively. Both the Fortune et al. [31] and Gibson-Smith et al. [43] studies reported whole-grain intakes that are far in excess of mean whole-grain intakes reported globally [13]. Mean difference between lowest and highest quantile of whole-grain intake, excluding the studies with apparent overestimation of intake, was 23 g for studies finding no association (n = 2), and 40 g for studies finding an association with cognitive outcomes (n = 4), with an insufficient number of studies for a meaningful statistical comparison. Of the studies with outcomes on mood, anxiety, and depression, only 2 had feasible estimates of whole-grain intake [42,43], with a mean difference of 56 g/d between lowest and highest quantiles. Only one of the included observational studies differentiated between different types of grain, processing method, or food type [48], finding that women with depression had lower intake of groats compared with matched controls, but there was no difference in relation to rye bread intake in the same study.
Discussion
Summary of evidence
We aimed to conduct a systematic review and meta-analysis of the available literature on whole grains and cognition to identify if there is direct evidential support for an impact of whole grains on cognitive outcomes. We expanded our review to include mood, anxiety, and depression outcomes. Overall, of the studies meeting inclusion criteria for cognition (RCTs and observational studies), the effect of whole-grain intakes on cognitive outcomes was mixed and varied by study design. With the observational studies on cognition, the lack of a positive association in some studies [20,32,34,37,49] with cognition outcomes may reflect that these studies reported whole-grain intake as a secondary analysis of dietary pattern studies and thus estimates of whole-grain intake may have been less precise. In the majority of observational studies, a low whole-grain reporting score was applied to reported whole-grain exposures, reflected in the low rate of reporting of the amount of whole-grain intake across quantiles of cognitive scores. Of note, however, none of the studies reviewed found an adverse impact of whole grains on cognition, although there is a risk that null associations have not been reported. With respect to mood, anxiety, and depression, most studies, regardless of study design, indicated favorable changes associated with a higher level of consumption of whole grains.
The review included a wide range of populations, study designs, types of grains/grain products, and cognitive measures. There was consistent evidence that the use of brown rice processed to improve palatability and cooking time compared to a white rice control led to improvements in cognitive outcomes, although it was noted that there was some heterogeneity in the change of outcomes. For example, 2 studies [30,50] used the HDS to measure cognitive outcomes after brown rice interventions. However, only one study found a subgroup difference [30], whereas the other did not, but it did find improvements with other measures of cognition (Frontal Assessment Battery and Cognitive Assessment for Dementia) [50]. For observational studies examining cognition, there was substantial diversity in the cognition measurement tools, with very little consistency in measured outcomes between studies. Three used telephone-based tests for cognition [20,32,33], and another 5 used variants of the MMSE [29,34,35,38,39]. With the low number of studies overall, it is not possible to draw conclusions about any relationship between cognitive outcome tests used and outcomes, although out of 5 studies using the MMSE, 4 found a relationship with cognitive outcomes. Although there were a relatively large number of studies that found a positive association between whole-grain intake and better cognitive outcomes, the high degree of heterogeneity of outcome measures across studies makes it difficult to draw any firm conclusions on the effect of whole grains on cognition from the present evidence. As such, until further research is available, the evidence linking higher whole-grain intake to improvements in cognition is inconsistent.
Similarly, for mood and anxiety-related outcomes, a diverse range of tools was used to capture such outcomes, that is, mood, anxiety, and depression. All but one study found that higher intake of whole grains was associated with better scores for mood and anxiety, with the remaining study finding a nonsignificant protective association of whole grains with major depressive episodes [45]. Our review is the first to identify research on whole grains and improved these outcomes with limited bias (mostly neutral), and further RCTs examining the mood-related benefits of whole grains are warranted.
Based on the literature meeting selection criteria, there are some indications that higher whole-grain intake could be related to better cognitive function, but concerns arise as to how whole grains have been defined in these studies. The weight of the evidence suggests that higher whole-grain intake is linked to better mood and less anxiety. However, most of the available evidence is from observational studies, and in addition, people who typically eat more whole grains are also more likely to have other healthy lifestyle behaviors, further confounding any direct conclusions [54]. Moreover, most studies on cognition had a high RoB and limited information on the how they had reported whole-grain intake.
Potential mechanisms
The brain utilizes glucose as its sole energy source and has a high relative energy requirement, which in turn suggests that it may be susceptible to overall dysregulation of glucose metabolism and insulin sensitivity. Further, the brain is primarily composed of lipids, which are needed for myelin sheaths and neural conductivity, suggesting that regulation in lipid metabolism is important for neuronal maintenance. To keep the brain supplied with glucose, lipids, and other building blocks for normal function, a high degree of blood perfusion is necessary, and dysfunction in brain vasculature may play a role in cognitive decline and disease [55]. Underpinning the importance of glucose homeostasis in the brain, people with mild cognitive impairment present with altered glucose metabolism [56]. To date, there are no mechanistic studies that have considered the effect of high intakes of whole grains on cognition. However, the consistent associations between higher whole-grain intake and reduced risk of CVD and T2D [57,58] suggest that through improvements in glucose and lipid metabolism, along with hypertension risk [59], there is reduced interference or inhibition of brain maintenance functions. Studies have linked diabetes to the formation of amyloid plaques [60], one of the established risk factors for Alzheimer’s disease, and whether the wider disease prevention effects of whole grains could also reduce the rate of amyloid plaque formation warrants investigation.
Whole grains are also an important source of dietary fiber, and several micronutrients associated with reduced risk of dementia including B-vitamins and vitamin E derivatives (tocopherols and tocotrienols) [[61], [62], [63], [64]]. Dietary fiber from whole grains is structurally diverse. In very general terms, grains such as wheat, rice, and corn have proportionally more insoluble fiber, whereas oats and barley have relatively high amounts of soluble fiber (mainly β-glucan). Although soluble fibers from grains can reduce postprandial glycemia and lipidemia, there is a growing interest in the potential of whole grains to modulate the gut microbiota. Evidence for an impact of whole grains on fecal microbiota populations is mixed, with most studies finding no or limited changes to fecal microbiota relative to refined grains, possibly explained by a high degree of heterogeneity in study design and intervention foods [65,66], a reoccurring theme in whole-grain intervention studies. There is some evidence for changes to fecal metabolome composition [67], which could implicate a gut–brain axis-based mechanism. For example, rodent studies have suggested that oat β-glucan can improve recognition memory in mice through mechanisms that include altering inflammation signaling [68]. Although a mechanism related to changing gut microbiota may be unlikely, it is possible that the interaction between fermentable or nondigestible components of whole grains and gut microbiota could influence signaling between the gut and brain [69].
One underresearched mechanistic effect of whole grains is their role in reducing circulating homocysteine. Elevated homocysteine is a biomarker of several diseases, including dementia [70], and indicates when the 1-carbon metabolic cycle is perturbed. The 1-carbon metabolic cycle relies on several micronutrient intermediates and cofactors including folate, riboflavin, vitamins B6 and B12, choline, methyl donor glycine, betaine, and several amino acids. This pathway supplies methyl groups for biological functions, including DNA methylation, and interfaces with phospholipid synthesis and the endogenous antioxidant system via glutathione. Some studies have also linked differences in 1-carbon metabolism to depression [[71], [72], [73]]. Whole grains are important dietary contributors of vitamin B6 and folate. Notably, cereal foods are the major source of dietary glycine betaine [74], and studies have found that whole-grain interventions increase circulating betaine [75], whereas a wheat aleurone layer intervention reduced plasma homocysteine and increased betaine in healthy subjects [76] and other betaine-conjugated compounds [77]. Studies that have used vitamin B and folate supplementation in people with mild cognitive impairment and hyperhomocysteinemia found that a 2-y intervention reduced brain atrophy [78], similar to the higher volume of gray matter and overall brain volume in people who ate the most whole grains in the study of Croll et al. [36]. Although there is a substantial body of research linking hyperhomocysteinemia and 1-carbon metabolism to cognition-related disorders, further work is needed to establish whether whole grains could mitigate and correct dysregulation of this pathway.
Whole grains contain a wide variety of nonnutrient phytochemicals including flavonoids, phenolic acids, phenolic lipids, carotenoids, and phytosterols that may also directly or indirectly influence cognitive outcomes. Brown rice contains higher concentrations of the neurotransmitter γ-amino butyric acid [41], and together with ferulic acid, these compounds have been suggested to be responsible for the improvement of spatial learning in a β-amyloid protein-induced model of Alzheimer’s disease [79]. Long-term feeding of rice bran extract, enriched with γ-oryzanol and vitamin E derivatives, improved brain mitochondrial function in aged NMRI mice [80]. Based on studies investigating similar compounds from other plant-based foods [[81], [82], [83], [84]], these diverse phytochemicals may play a role in reducing cognitive decline. Further study on whole-grain–specific compounds and their transport across the blood–brain–barrier is required.
Of the cognitive conditions investigated in this review, it was notable that most studies investigating mood, anxiety, and whole-grain intake found that people who ate the most whole grains had reduced scores for mood disorders, including depression and anxiety. The mechanistic basis of mood disorders is complex as there can be considerable psychological confounding of biochemical mechanisms, and especially in the case of intervention studies in which the intervention is known, people may feel better if they know that they are eating a healthier diet [85]. For observational studies, there is the additional confounding of whole-grain intake being associated with other healthy lifestyle behaviors [54], although in dietary pattern studies, not all “healthy” food groups were associated with decreased risk of mood-related disorders. Inflammation and insulin resistance are among the biochemical mechanisms known to trigger or mediate stress, impaired mood, anxiety, and depression states [86]. Whole grains can impact on postprandial glucose metabolism [[87], [88], [89]], which can positively affect insulin sensitivity over the long-term and may be a mechanism in which glucose-related mood changes may be better regulated. Relationships between whole-grain intake and markers of inflammation are less clear, with only 39% of RCTs on whole grains leading to a reduction in standard biomarkers of inflammation (CRP, IL-6, and TNF) [90], although it was noted that overweight or obese populations were more likely to have significant decreases in inflammatory markers. Further, insulin resistance and inflammation are linked; insulin resistance can lead to poorly controlled signaling of the inflammation response, resulting in inflammatory cytokines crossing the circulation into the brain [91]. This unregulated inflammatory response may also be linked to the development of Alzheimer’s disease [92].
The finding that whole-grain intake was associated with greater gray matter volume [36] is interesting in the context of the requirement of particular areas of the brain to process thoughts that lead to altered mood, anxiety, and depression, such as the prefrontal cortex and amygdala [86]. Whether preservation of gray matter in particular brain structures can impact the development of cognition-related disorders warrants further investigation.
There is insufficient evidence to state any particular “dose” of whole grains is likely to lead to a protective effect, an important consideration for designing RCTs. The 3 brown rice-based studies used daily servings of 100 to 250 g, although it is unclear if all were reported on a dry or cooked weight basis. Of the observational studies that reported the amount of whole-grain intake, the reported population intakes were generally in line with what would be expected for the respective populations, irrespective of impact on cognitive outcomes, except for the studies of Fortune et al. [31] and Gibson-Smith et al. [43]. The low number of studies reporting whole-grain intake across quantiles and poor overall quality of whole-grain intake reporting lead to poor confidence in the quantitative estimates of whole-grain intake in the included studies, although the proportional differences (low compared with high whole-grain intake) should remain valid.
Limitations of the findings
The literature on whole grains and cognitive outcomes was sparse, leading to very few studies that could be directly comparable in terms of population, whole-grain exposure, and cognition measures used. This was especially the case with observational studies for cognition-related outcomes, in which 12 studies were split across 7 countries, 4 studies only recruited females, and the mean age of participants in the cross-sectional studies ranged from ages 21 to 73. Most of the observational studies only reported associations with whole-grain intake as a secondary observation or as part of investigations on total diet or dietary patterns and cognition or mood-related outcomes. In addition, although most of the observational studies found an association between greater whole-grain intake and improved cognitive or mood and depression outcomes, the observational nature of these studies indicates that no conclusion on causality can be drawn.
We expanded our search to include dietary pattern studies in which the whole-grain exposure was analyzed separately from the total diet. However, dietary pattern studies that explicitly define “whole grains” in the abstract are likely biased toward papers that report generally positive findings for whole-grain intake. Thus, our abstract screening procedures could have led to the exclusion of studies with “null” results following whole-grain intake. The problem of nonreporting of null results biasing literature reviews is known across scientific research [93].
None of the studies scored higher than 2 out of 5 for the whole-grain reporting assessment scale developed for this study, suggesting that there are considerable gaps in how whole-grain intake has been assessed and reported compared with what is recommended [26]. Most studies did not report how they defined whole grains, or whether they reported whole grains as “g whole grain/d” or other units, or if dry or wet weight for whole-grain foods was used. No studies differentiated between different types of cereal grains or different ways of processing grains (although studies on processed brown rice did describe the processing methods). Although gradually being more commonly applied in intervention and population-based studies [94], biomarkers of whole-grain intake were not included in any of the studies. Although this may not be surprising for dietary pattern studies in which whole grain was only one component of the diet under study, this highlights the difficulty comparing studies, especially across different cohorts and countries. This is particularly limiting for determining what “dose” might be required for a protective effect and if any particular cereal or processing method might be related to a positive or negative impact on cognition.
As noted in the results, we did not perform a meta-analysis on the interventional studies included in this review due to the high degree of heterogeneity in study design, duration, types of grains and processing, and cognition/mood outcome measures. There are concerns about using meta-analysis methodology, originally devised for similar clinical study designs, especially when there is considerable heterogeneity in outcome tests used and how exposure has been measured [95] as well as the resulting focus on a single metric that, if based on observational data, cannot conclusively imply causality [96]. With the wide variance in study designs and populations, and with most studies focused on dietary patterns and reporting relationships between cognition-related outcomes and whole-grain intake as a secondary analysis, meta-analysis was not appropriate for the RCTs and observational studies included. This variation in study design also suggests that it is not possible, at this point in time, to propose a suitable study duration for future studies.
Considering these limitations, and the poor overall assessments of both GRADE evidence and whole-grain intake, there is a need to improve how whole grains are assessed and reported in nutrition epidemiological research and for greater agreement on appropriate tools to assess cognition and mood, given that both the tools and how they are applied may have a major bearing on results.
Future considerations for whole grains and cognition research
The studies that have investigated whole grains and cognition come from a variety of food cultures. In the included studies, there was no explicit disentangling of the types of whole-grain foods or clarity around whether whole-grain ingredient intake or whole-grain food intake was estimated or reported. Although all grain foods require some form of processing before consumption, there has been limited examination of divergent effects of different types of grain processing (for example, fermentation for bread, boiling for rice or porridge, extrusion for breakfast cereal and pasta). Grain flour particle size impacts the glycemic response from whole-grain wheat in those with T2D [97] whereas disruption of oat grains nullifies postprandial glucose and insulin attenuation in oats [98]. Similarly, although whole grains are usually grouped together in observational trials, there is a sufficient compositional difference between different types of grain that could potentially lead to different outcomes if studied individually [99].
From the available limited research, several studies found an association between whole-grain intake and reduced risk or rate of cognitive decline. This is intriguing from the perspective of strategies to reduce the burden of cognitive disease, though there are many methodological issues around how whole-grain intake is estimated in the reviewed literature, and not all studies found an association between whole-grain intake and cognitive outcomes. As this evidence base is largely observational, no causal relationship is established, and mechanisms for how whole grains may prevent or delay cognitive decline need to be further elucidated.
Although not a direct cognition-related outcome, there is an intriguing body of evidence suggesting that whole grains are associated with better mood and anxiety scores, an aspect of health that has, like cognition, not been widely researched in connection with whole grains. This area warrants greater research attention on incorporation of whole grains into the diet given the increasing awareness of the importance of mental health to overall health. The findings of this review, although not conclusive for a role for whole grains in preventing cognitive decline, do support recommendations to increase whole-grain intake. Given the fact that cereal-based foods are a dietary staple across many cultures worldwide [100], incorporating whole-grain foods is a simple step for improving overall diet quality, and one that can have a major impact on overall health and potentially cognitive and mental health.
Overall, the current evidence for whole grains reducing risk of cognitive decline is inconclusive, against a background of considerable heterogeneity of study populations, cognitive measures, and how whole grains were defined and recorded. Almost all studies that examined whole-grain intake and mood and anxiety-related outcomes found that greater intake of whole grains was associated with reduced scores for mood and anxiety disorders. The current sparse literature on whole grains and cognition and mood are suggestive of an overall inconclusive effect of whole grains for cognition and a positive effect on mood and anxiety. This should be interpreted with caution, however, due to the generally poor–neutral RoB and low quality of evidence assessments. There are a wide range of potential mechanisms that support the possibility of whole grains being protective against cognitive decline and mood disorders, although few studies have specifically addressed this question. Given the toll of both cognitive decline and mental health, and a growing population of elderly adults at risk of cognitive decline, further research, particularly well-designed RCTs, into whether whole grains can improve measures of cognitive function, mood, and anxiety are needed.
Acknowledgments
We acknowledge Amy E LaVertu for her support in the creation of our search strategies. The authors’ responsibilities were as follows—ABR, PJ, and NM: conceived the research; ABR, MC, PJ, and NM: designed the review strategy; SPS: conducted and coordinated the systematic review; ABR, SPS, KS, MC, PJ, and NM: reviewed and evaluated abstracts and full-text articles; ABR: drafted the article; SPS, MC, PJ, and MN: made significant contributions to the article; ABR: had primary responsibility for the final content; and all authors: read and approved the final manuscript.
Funding
This research was funded by the Bell Institute of Nutrition, General Mills Inc.
Author disclosures
NM has received funding for investigator-initiated grants and for payment for serving as a consultant from General Mills Bell Institute of Health and Nutrition, speaker honorarium from Cereal Partners Worldwide, and unpaid scientific advisor on the Oldways Whole Grains Council. PJ is a member of the North America Essential Dairy and Plant-Based Advisory Board. ABR, SPS, KS, and MC report no conflicts of interest.
Disclaimer
The funding sponsor provided comments on early aspects of the study design. Interim analyses and the final data were shared with the sponsor before publication, but the final decision for all aspects of study conduct and manuscript content is that of the authors alone.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.advnut.2023.04.003.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Gauthier S., Rosa-Neto P., Morais J.A., Webster C., Gélinas I., Carver T.E., et al. World Alzheimer Report 2021: Journey through the diagnostis of dementia. Alzheimer’s Disease International. 2021 https://www.alzint.org/resource/world-alzheimer-report-2021/ [Internet] [Date cited: 22 May, 2022]. Available from: [Date cited: 22 May, 2022]. Available from: [Google Scholar]
- 2.Jack C.R., Bennett D.A., Blennow K., Carrillo M.C., Dunn B., Haeberlein S.B., et al. NIA-AA Research framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018;14(4):535–562. doi: 10.1016/j.jalz.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ismail Z., Gatchel J., Bateman D.R., Barcelos-Ferreira R., Cantillon M., Jaeger J., et al. Affective and emotional dysregulation as pre-dementia risk markers: exploring the mild behavioral impairment symptoms of depression, anxiety, irritability, and euphoria. Int. Psychogeriatr. 2018;30(2):185–196. doi: 10.1017/S1041610217001880. [DOI] [PubMed] [Google Scholar]
- 4.Ma L. Depression, anxiety, and apathy in mild cognitive impairment: current perspectives. Front. Aging Neurosci. 2020;12:9. doi: 10.3389/fnagi.2020.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elias M.F., Beiser A., Wolf P.A., Au R., White R.F., D’Agostino R.B. The preclinical phase of Alzheimer disease: a 22-year prospective study of the Framingham cohort. Arch. Neurol. 2000;57(6):808–813. doi: 10.1001/archneur.57.6.808. [DOI] [PubMed] [Google Scholar]
- 6.Dubois B., Hampel H., Feldman H.H., Scheltens P., Aisen P., Andrieu S., et al. Preclinical Alzheimer’s disease: definition, natural history, and diagnostic criteria. Alzheimers Dement. 2016;12(3):292–323. doi: 10.1016/j.jalz.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raffaitin C., Féart C., Le Goff M., Amieva H., Helmer C., Akbaraly T.N., et al. Metabolic syndrome and cognitive decline in French elders: the Three-City Study. Neurology. 2011;76(6):518–525. doi: 10.1212/WNL.0b013e31820b7656. [DOI] [PubMed] [Google Scholar]
- 8.Livingston G., Huntley J., Sommerlad A., Ames D., Ballard C., Banerjee S., et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396(10248):413–446. doi: 10.1016/S0140-6736(20)30367-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Biessels G.J., Despa F. Cognitive decline and dementia in diabetes mellitus: mechanisms and clinical implications. Nat. Rev. Endocrinol. 2018;14(10):591–604. doi: 10.1038/s41574-018-0048-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu Y., Ding M., Sampson L., Willett W.C., Manson J.E., Wang M., et al. Intake of whole grain foods and risk of type 2 diabetes: results from three prospective cohort studies. BMJ. 2020;370 doi: 10.1136/bmj.m2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tieri M., Ghelfi F., Vitale M., Vetrani C., Marventano S., Lafranconi A., et al. Whole grain consumption and human health: an umbrella review of observational studies. Int. J. Food Sci. Nutr. 2020;71(6):668–677. doi: 10.1080/09637486.2020.1715354. [DOI] [PubMed] [Google Scholar]
- 12.Reynolds A., Mann J., Cummings J., Winter N., Mete E., Te Morenga L. Carbohydrate quality and human health: a series of systematic reviews and meta-analyses. Lancet. 2019;393(10170):434–445. doi: 10.1016/S0140-6736(18)31809-9. [DOI] [PubMed] [Google Scholar]
- 13.Miller K.B. Review of whole grain and dietary fiber recommendations and intake levels in different countries. Nutr. Rev. 2020;78(Suppl 1):29–36. doi: 10.1093/nutrit/nuz052. [DOI] [PubMed] [Google Scholar]
- 14.Sanders L.M., Zhu Y., Wilcox M.L., Koecher K., Maki K.C. Whole grain intake, compared to refined grain, improves postprandial glycemia and insulinemia: a systematic review and meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2021:1–19. doi: 10.1080/10408398.2021.2017838. [DOI] [PubMed] [Google Scholar]
- 15.Guo H., Ding J., Liang J., Zhang Y. Associations of whole grain and refined grain consumption with metabolic syndrome. A meta-analysis of observational studies. Front. Nutr. 2021;8 doi: 10.3389/fnut.2021.695620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu D., Fu L., Pan D., Lu Y., Yang C., Wang Y., et al. Role of whole grain consumption in glycaemic control of diabetic patients: a systematic review and meta-analysis of randomized controlled trials. Nutrients. 2021;14(1):109. doi: 10.3390/nu14010109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghanbari-Gohari F., Mousavi S.M., Esmaillzadeh A. Consumption of whole grains and risk of type 2 diabetes: A comprehensive systematic review and dose-response meta-analysis of prospective cohort studies. Food Sci. Nutr. 2022;10(6):1950–1960. doi: 10.1002/fsn3.2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller V., Micha R., Choi E., Karageorgou D., Webb P., Mozaffarian D. Evaluation of the quality of evidence of the association of foods and nutrients with cardiovascular disease and diabetes: a systematic review. JAMA Netw. Open. 2022;5(2) doi: 10.1001/jamanetworkopen.2021.46705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rahmani S., Sadeghi O., Sadeghian M., Sadeghi N., Larijani B., Esmaillzadeh A. The effect of whole-grain intake on biomarkers of subclinical inflammation: a comprehensive meta-analysis of randomized controlled trials. Adv. Nutr. 2020;11(1):52–65. doi: 10.1093/advances/nmz063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Samieri C., Grodstein F., Rosner B.A., Kang J.H., Cook N.R., Manson J.E., et al. Mediterranean diet and cognitive function in older age. Epidemiology. 2013;24(4):490–499. doi: 10.1097/EDE.0b013e318294a065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Higgins J.P.T., Thomas J., Chandler J., Cumpston M., Li T., Page M.J., et al. John Wiley & Sons; Chichester: 2011. Cochrane Handbook for Systematic Reviews of Interventions, version 5.1.0. [Google Scholar]
- 22.Moher D., Liberati A., Tetzlaff J., Altman D.G. PRISMA Group, Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J. Clin. Epidemiol. 2009;62(10):1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 23.Ouzzani M., Hammady H., Fedorowicz Z., Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. Syst. Rev. 2016;5(1):210. doi: 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kelly S.E., Greene-Finestone L.S., Yetley E.A., Benkhedda K., Brooks S.P.J., Wells G.A., et al. NUQUEST-Nutrition Quality Evaluation Strengthening Tools: development of tools for the evaluation of risk of bias in nutrition studies. Am. J. Clin. Nutr. 2022;115(1):256–271. doi: 10.1093/ajcn/nqab335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sterne J.A.C., Savović J., Page M.J., Elbers R.G., Blencowe N.S., Boutron I., et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 26.Ross A.B., Kristensen M., Seal C.J., Jacques P., McKeown N.M. Recommendations for reporting whole-grain intake in observational and intervention studies. Am. J. Clin. Nutr. 2015;101(5):903–907. doi: 10.3945/ajcn.114.098046. [DOI] [PubMed] [Google Scholar]
- 27.Guyatt G.H., Oxman A.D., Vist G.E., Kunz R., Falck-Ytter Y., Alonso-Coello P., et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guyatt G.H., Oxman A.D., Kunz R., Vist G.E., Falck-Ytter Y., Schünemann H.J., et al. What is “quality of evidence” and why is it important to clinicians? BMJ. 2008;336(7651):995–998. doi: 10.1136/bmj.39490.551019.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anastasiou C.A., Yannakoulia M., Kosmidis M.H., Dardiotis E., Hadjigeorgiou G.M., Sakka P., et al. Mediterranean diet and cognitive health: initial results from the Hellenic Longitudinal Investigation of Ageing and Diet. PLOS ONE. 2017;12(8) doi: 10.1371/journal.pone.0182048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Uenobe M., Saika T., Waku N., Ohno M., Inagawa H. Effect of continuous dewaxed brown rice ingestion on the cognitive function of elderly individuals. J. Nutr. Sci. Vitaminol. (Tokyo). 2019;65(Suppl):S122–S124. doi: 10.3177/jnsv.65.S122. [DOI] [PubMed] [Google Scholar]
- 31.Fortune N.C., Harville E.W., Guralnik J.M., Gustat J., Chen W., Qi L., et al. Dietary intake and cognitive function: evidence from the Bogalusa Heart Study. Am. J. Clin. Nutr. 2019;109(6):1656–1663. doi: 10.1093/ajcn/nqz026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qin B., Adair L.S., Plassman B.L., Batis C., Edwards L.J., Popkin B.M., et al. Dietary patterns and cognitive decline among Chinese older adults. Epidemiology. 2015;26(5):758–768. doi: 10.1097/ede.0000000000000338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Samieri C., Okereke O.I., Devore E.E., Grodstein F. Long-term adherence to the Mediterranean diet is associated with overall cognitive status, but not cognitive decline, in women. J. Nutr. 2013;143(4):493–499. doi: 10.3945/jn.112.169896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shakersain B., Rizzuto D., Larsson S.C., Faxén-Irving G., Fratiglioni L., Xu W.L. The Nordic prudent diet reduces risk of cognitive decline in the Swedish older adults: a population-based cohort study. Nutrients. 2018;10(2):229. doi: 10.3390/nu10020229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wengreen H., Munger R.G., Cutler A., Quach A., Bowles A., Corcoran C., et al. Prospective study of Dietary Approaches to Stop Hypertension- and Mediterranean-style dietary patterns and age-related cognitive change: the Cache County Study on Memory, Health and Aging. Am. J. Clin. Nutr. 2013;98(5):1263–1271. doi: 10.3945/ajcn.112.051276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Croll P.H., Voortman T., Ikram M.A., Franco O.H., Schoufour J.D., Bos D., et al. Better diet quality relates to larger brain tissue volumes: the Rotterdam Study. Neurology. 2018;90(24):e2166–e2173. doi: 10.1212/wnl.0000000000005691. [DOI] [PubMed] [Google Scholar]
- 37.Dong L., Xiao R., Cai C., Xu Z., Wang S., Pan L., et al. Diet, lifestyle and cognitive function in old Chinese adults. Arch. Gerontol. Geriatr. 2016;63:36–42. doi: 10.1016/j.archger.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 38.Huang W.C., Huang Y.C., Lee M.S., Chang H.Y., Doong J.Y. Frailty severity and cognitive impairment associated with dietary diversity in older adults in Taiwan. Nutrients. 2021;13(2):418. doi: 10.3390/nu13020418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu R., Gao T., Cai J., Zhang H., Zhou H., Ding K., et al. Food consumption and mild cognitive impairment in Qingdao rural elderly: a cross-sectional study, Asia Pac. J. Clin. Nutr. 2020;29(4):867–875. doi: 10.6133/apjcn.202012_29(4).0023. [DOI] [PubMed] [Google Scholar]
- 40.Esmaeilpour M., Ghasemian S., Alizadeh M. Diets enriched with whole grains reduce premenstrual syndrome scores in nurses: an open-label parallel randomised controlled trial. Br. J. Nutr. 2019;121(9):992–1001. doi: 10.1017/s0007114519000333. [DOI] [PubMed] [Google Scholar]
- 41.Sakamoto S., Hayashi T., Hayashi K., Murai F., Hori M., Kimoto K., et al. Pre-germinated brown rice could enhance maternal mental health and immunity during lactation. Eur. J. Nutr. 2007;46(7):391–396. doi: 10.1007/s00394-007-0678-3. [DOI] [PubMed] [Google Scholar]
- 42.Gangwisch J.E., Hale L., Garcia L., Malaspina D., Opler M.G., Payne M.E., et al. High glycemic index diet as a risk factor for depression: analyses from the Women’s Health Initiative. Am. J. Clin. Nutr. 2015;102(2):454–463. doi: 10.3945/ajcn.114.103846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gibson-Smith D., Bot M., Brouwer I.A., Visser M., Giltay E.J., Penninx B.W.J.H. Association of food groups with depression and anxiety disorders. Eur. J. Nutr. 2020;59(2):767–778. doi: 10.1007/s00394-019-01943-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kleppang A.L., de Ridder K., Haugland S.H., Stea T.H. Physical activity, sugar-sweetened beverages, whole grain bread and insomnia among adolescents and psychological distress in adulthood: prospective data from the population-based HUNT study. Int. J. Behav. Nutr. Phys. Act. 2021;18(1):143. doi: 10.1186/s12966-021-01215-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lanuza F., Petermann-Rocha F., Celis-Morales C., Concha-Cisternas Y., Nazar G., Troncoso-Pantoja C., et al. A healthy eating score is inversely associated with depression in older adults: results from the Chilean National Health Survey 2016–2017. Public Health Nutr. 2021;25(10):1–12. doi: 10.1017/s1368980021004869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sadeghi O., Hassanzadeh-Keshteli A., Afshar H., Esmaillzadeh A., Adibi P. The association of whole and refined grains consumption with psychological disorders among Iranian adults. Eur. J. Nutr. 2019;58(1):211–225. doi: 10.1007/s00394-017-1585-x. [DOI] [PubMed] [Google Scholar]
- 47.Davison K.M., Kaplan B.J. Food intake and blood cholesterol levels of community-based adults with mood disorders. BMC Psychiatry. 2012;12(1):10. doi: 10.1186/1471-244X-12-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stefanska E., Wendołowicz A., Cwalina U., Kowzan U., Konarzewska B., Szulc A., et al. Assessment of dietary habits and nutritional status of depressive patients, depending on place of residence. Ann. Agric. Environ. Med. 2017;24(4):581–586. doi: 10.5604/12321966.1233554. [DOI] [PubMed] [Google Scholar]
- 49.Abbaszadeh A., Saharkhiz M., Khorasanchi Z., Karbasi S., Askari M., Hoseini Z.S., et al. Impact of a Nordic diet on psychological function in young students. Nutr. Health. 2021;27(1):97–104. doi: 10.1177/0260106020964981. [DOI] [PubMed] [Google Scholar]
- 50.Kuroda Y., Matsuzaki K., Wakatsuki H., Shido O., Harauma A., Moriguchi T., et al. Influence of ultra-high hydrostatic pressurizing brown rice on cognitive functions and mental health of elderly Japanese individuals: a 2-year randomized and controlled trial. J. Nutr. Sci. Vitaminol. (Tokyo) 2019;65(Suppl):S80–S87. doi: 10.3177/jnsv.65.S80. [DOI] [PubMed] [Google Scholar]
- 51.Yao Z.D., Cao Y.N., Peng L.X., Yan Z.Y., Zhao G. Coarse cereals and legume grains exert beneficial effects through their interaction with gut microbiota: a review. J. Agric. Food Chem. 2021;69(3):861–877. doi: 10.1021/acs.jafc.0c05691. [DOI] [PubMed] [Google Scholar]
- 52.Ross A.B., van der Kamp J.W., King R., Lê K.A., Mejborn H., Seal C.J., et al. Perspective: a definition for whole-grain food products-recommendations from the Healthgrain Forum. Adv. Nutr. 2017;8(4):525–531. doi: 10.3945/an.116.014001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stefańska E., Wendołowicz A., Cwalina U., Kowzan U., Konarzewska B., Szulc A., et al. Assessment of dietary habits of patients with recurrent depressive disorders. Arch. Psych. Psych. 2014;16(4):39–46. doi: 10.12740/APP/31780. [DOI] [Google Scholar]
- 54.Andersen J.L.M., Halkjær J., Rostgaard-Hansen A.L., Martinussen N., Lund A.Q., Kyrø C., et al. Intake of whole grain and associations with lifestyle and demographics: a cross-sectional study based on the Danish Diet, Cancer and Health-Next Generations cohort. Eur. J. Nutr. 2021;60(2):883–895. doi: 10.1007/s00394-020-02289-y. [DOI] [PubMed] [Google Scholar]
- 55.Sweeney M.D., Kisler K., Montagne A., Toga A.W., Zlokovic B.V. The role of brain vasculature in neurodegenerative disorders. Nat. Neurosci. 2018;21(10):1318–1331. doi: 10.1038/s41593-018-0234-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Butterfield D.A., Halliwell B. Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease. Nat. Rev. Neurosci. 2019;20(3):148–160. doi: 10.1038/s41583-019-0132-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Aune D., Keum N., Giovannucci E., Fadnes L.T., Boffetta P., Greenwood D.C., et al. Whole grain consumption and risk of cardiovascular disease, cancer, and all cause and cause specific mortality: systematic review and dose-response meta-analysis of prospective studies. BMJ. 2016;353 doi: 10.1136/bmj.i2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aune D., Norat T., Romundstad P., Vatten L.J. Whole grain and refined grain consumption and the risk of type 2 diabetes: a systematic review and dose-response meta-analysis of cohort studies. Eur. J. Epidemiol. 2013;28(11):845–858. doi: 10.1007/s10654-013-9852-5. [DOI] [PubMed] [Google Scholar]
- 59.Flint A.J., Hu F.B., Glynn R.J., Jensen M.K., Franz M., Sampson L., et al. Whole grains and incident hypertension in men. Am. J. Clin. Nutr. 2009;90(3):493–498. doi: 10.3945/ajcn.2009.27460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stanciu G.D., Bild V., Ababei D.C., Rusu R.N., Cobzaru A., Paduraru L., et al. Link between diabetes and Alzheimer’s disease due to the shared amyloid aggregation and deposition involving both neurodegenerative changes and neurovascular damages. J. Clin. Med. 2020;9(6):1713. doi: 10.3390/jcm9061713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Morris M.C. Nutritional determinants of cognitive aging and dementia. Proc. Nutr. Soc. 2012;71(1):1–13. doi: 10.1017/s0029665111003296. [DOI] [PubMed] [Google Scholar]
- 62.Yamagishi K., Maruyama K., Ikeda A., Nagao M., Noda H., Umesawa M., et al. Dietary fiber intake and risk of incident disabling dementia: the Circulatory Risk in Communities Study. Nutr. Neurosci. 2023;26(2):148–155. doi: 10.1080/1028415X.2022.2027592. [DOI] [PubMed] [Google Scholar]
- 63.Jones J.M., Korczak R., Peña R.J., Braun H.J. CIMMYT series on carbohydrates, wheat, grains, and health: impact of minerals, phytochemicals, specific grain-based foods, and dietary patterns on mild cognitive impairment, Alzheimer’s disease, and Parkinson’s disease. Cereal Foods World. 2017;62(3):104–114. doi: 10.1094/CFW-62-3-0104. [DOI] [Google Scholar]
- 64.Jones J.M., Peña R.J., Korczak R., Braun H.J. Headache, attention deficit hyperactivity disorder, and depression. CIMMYT series on carbohydrates, wheat, grains, and health: role of carbohydrates and grains in nutrition and neurological disorders. Cereal Foods World. 2017;62(4):162–171. doi: 10.1094/CFW-62-4-0162. [DOI] [Google Scholar]
- 65.Koecher K.J., McKeown N.M., Sawicki C.M., Menon R.S., Slavin J.L. Effect of whole-grain consumption on changes in fecal microbiota: a review of human intervention trials. Nutr. Rev. 2019;77(7):487–497. doi: 10.1093/nutrit/nuz008. [DOI] [PubMed] [Google Scholar]
- 66.Roager H.M., Vogt J.K., Kristensen M., Hansen L.B.S., Ibrügger S., Mærkedahl R.B., et al. Whole grain-rich diet reduces body weight and systemic low-grade inflammation without inducing major changes of the gut microbiome: a randomised cross-over trial. Gut. 2019;68(1):83–93. doi: 10.1136/gutjnl-2017-314786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ross A.B. Whole grains beyond fibre: what can metabolomics tell us about mechanisms? Proc. Nutr. Soc. 2015;74(3):320–327. doi: 10.1017/S0029665114001542. [DOI] [PubMed] [Google Scholar]
- 68.Hu M., Zhang P., Wang R., Zhou M., Pang N., Cui X., et al. Three different types of β-glucans enhance cognition: the role of the gut-brain axis. Front. Nutr. 2022;9 doi: 10.3389/fnut.2022.848930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bourassa M.W., Alim I., Bultman S.J., Ratan R.R. Butyrate, neuroepigenetics and the gut microbiome: can a high fiber diet improve brain health? Neurosci. Lett. 2016;625:56–63. doi: 10.1016/j.neulet.2016.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Whalley L.J., Duthie S.J., Collins A.R., Starr J.M., Deary I.J., Lemmon H., et al. Homocysteine, antioxidant micronutrients and late onset dementia. Eur. J. Nutr. 2014;53(1):277–285. doi: 10.1007/s00394-013-0526-6. [DOI] [PubMed] [Google Scholar]
- 71.Kurkinen K., Kärkkäinen O., Lehto S.M., Luoma I., Kraav S.L., Nieminen A.I., et al. One-carbon and energy metabolism in major depression compared to chronic depression in adolescent outpatients: a metabolomic pilot study. J. Affect. Disord. Rep. 2021;6 doi: 10.1016/j.jadr.2021.100261. [DOI] [Google Scholar]
- 72.Frankenburg F.R. The role of one-carbon metabolism in schizophrenia and depression. Harv. Rev. Psychiatry. 2007;15(4):146–160. doi: 10.1080/10673220701551136. [DOI] [PubMed] [Google Scholar]
- 73.Dayon L., Guiraud S.P., Corthésy J., Da Silva L., Migliavacca E., Tautvydaitė D., et al. One-carbon metabolism, cognitive impairment and CSF measures of Alzheimer pathology: homocysteine and beyond. Alzheimers Res. Ther. 2017;9(1):43. doi: 10.1186/s13195-017-0270-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ross A.B., Zangger A., Guiraud S.P. Cereal foods are the major source of betaine in the Western diet - analysis of betaine and free choline in cereal foods and updated assessments of betaine intake. Food Chem. 2014;145:859–865. doi: 10.1016/j.foodchem.2013.08.122. [DOI] [PubMed] [Google Scholar]
- 75.Ross A.B., Bruce S.J., Blondel-Lubrano A., Oguey-Araymon S., Beaumont M., Bourgeois A., et al. A whole-grain cereal-rich diet increases plasma betaine, and tends to decrease total and LDL-cholesterol compared with a refined-grain diet in healthy subjects. Br. J. Nutr. 2011;105(10):1492–1502. doi: 10.1017/S0007114510005209. [DOI] [PubMed] [Google Scholar]
- 76.Price R.K., Keaveney E.M., Hamill L.L., Wallace J.M.W., Ward M., Ueland P.M., et al. Consumption of wheat aleurone-rich foods increases fasting plasma betaine and modestly decreases fasting homocysteine and LDL-cholesterol in adults. J. Nutr. 2010;140(12):2153–2157. doi: 10.3945/jn.110.126961. [DOI] [PubMed] [Google Scholar]
- 77.Kärkkäinen O., Lankinen M.A., Vitale M., Jokkala J., Leppänen J., Koistinen V., et al. Diets rich in whole grains increase betainized compounds associated with glucose metabolism. Am. J. Clin. Nutr. 2018;108(5):971–979. doi: 10.1093/ajcn/nqy169. [DOI] [PubMed] [Google Scholar]
- 78.Smith A.D., Smith S.M., de Jager C.A., Whitbread P., Johnston C., Agacinski G., et al. Homocysteine-lowering by B vitamins slows the rate of accelerated brain atrophy in mild cognitive impairment: a randomized controlled trial. PLOS ONE. 2010;5(9) doi: 10.1371/journal.pone.0012244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mamiya T., Asanuma T., Kise M., Ito Y., Mizukuchi A., Aoto H., et al. Effects of pre-germinated brown rice on beta-amyloid protein-induced learning and memory deficits in mice. Biol. Pharm. Bull. 2004;27(7):1041–1045. doi: 10.1248/bpb.27.1041. [DOI] [PubMed] [Google Scholar]
- 80.Hagl S., Asseburg H., Heinrich M., Sus N., Blumrich E.M., Dringen R., et al. Effects of long-term rice bran extract supplementation on survival, cognition and brain mitochondrial function in aged NMRI mice. NeuroMolecular Med. 2016;18(3):347–363. doi: 10.1007/s12017-016-8420-z. [DOI] [PubMed] [Google Scholar]
- 81.Lefèvre-Arbogast S., Gaudout D., Bensalem J., Letenneur L., Dartigues J.F., Hejblum B.P., et al. Pattern of polyphenol intake and the long-term risk of dementia in older persons. Neurology. 2018;90(22):e1979–e1988. doi: 10.1212/wnl.0000000000005607. [DOI] [PubMed] [Google Scholar]
- 82.Ekstrand B., Scheers N., Rasmussen M.K., Young J.F., Ross A.B., Landberg R. Brain foods - the role of diet in brain performance and health. Nutr. Rev. 2021;79(6):693–708. doi: 10.1093/nutrit/nuaa091. [DOI] [PubMed] [Google Scholar]
- 83.Ngoungoure V.L.N., Schluesener J., Moundipa P.F., Schluesener H. Natural polyphenols binding to amyloid: a broad class of compounds to treat different human amyloid diseases. Mol. Nutr. Food Res. 2015;59(1):8–20. doi: 10.1002/mnfr.201400290. [DOI] [PubMed] [Google Scholar]
- 84.Pontifex M.G., Malik M.M.A.H., Connell E., Müller M., Vauzour D. Citrus polyphenols in brain health and disease: current perspectives. Front. Neurosci. 2021;15 doi: 10.3389/fnins.2021.640648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kristal A.R., Andrilla C.H., Koepsell T.D., Diehr P.H., Cheadle A. Dietary assessment instruments are susceptible to intervention-associated response set bias. J. Am. Diet. Assoc. 1998;98(1):40–43. doi: 10.1016/s0002-8223(98)00012-1. [DOI] [PubMed] [Google Scholar]
- 86.Gold P.W., Machado-Vieira R., Pavlatou M.G. Clinical and biochemical manifestations of depression: relation to the neurobiology of stress. Neural. Plast. 2015;2015 doi: 10.1155/2015/581976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Priebe M.G., Wachters-Hagedoorn R.E., Heimweg J.A., Small A., Preston T., Elzinga H., et al. An explorative study of in vivo digestive starch characteristics and postprandial glucose kinetics of wholemeal wheat bread. Eur. J. Nutr. 2008;47(8):417–423. doi: 10.1007/s00394-008-0743-6. [DOI] [PubMed] [Google Scholar]
- 88.Musa-Veloso K., Poon T., Harkness L.S., O’Shea M., Chu Y. The effects of whole-grain compared with refined wheat, rice, and rye on the postprandial blood glucose response: a systematic review and meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2018;108(4):759–774. doi: 10.1093/ajcn/nqy112. [DOI] [PubMed] [Google Scholar]
- 89.Malin S.K., Kullman E.L., Scelsi A.R., Haus J.M., Filion J., Pagadala M.R., et al. A whole-grain diet reduces peripheral insulin resistance and improves glucose kinetics in obese adults: a randomized-controlled trial. Metabolism. 2018;82:111–117. doi: 10.1016/j.metabol.2017.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Milesi G., Rangan A., Grafenauer S. Whole grain consumption and inflammatory markers: a systematic literature review of randomized control trials. Nutrients. 2022;14(2):374. doi: 10.3390/nu14020374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Spencer S.J., Korosi A., Layé S., Shukitt-Hale B., Barrientos R.M. Food for thought: how nutrition impacts cognition and emotion. npj Sci. Food. 2017;1(1):7. doi: 10.1038/s41538-017-0008-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Santiago J.A., Potashkin J.A. The impact of disease comorbidities in Alzheimer’s disease. Front. Aging Neurosci. 2021;13 doi: 10.3389/fnagi.2021.631770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Franco A., Malhotra N., Simonovits G. Publication bias in the social sciences: unlocking the file drawer. Science. 2014;345(6203):1502–1505. doi: 10.1126/science.1255484. [DOI] [PubMed] [Google Scholar]
- 94.Landberg R., Hanhineva K., Tuohy K., Garcia-Aloy M., Biskup I., Llorach R., et al. Biomarkers of cereal food intake. Genes Nutr. 2019;14(1):28. doi: 10.1186/s12263-019-0651-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shapiro S. Meta-analysis/Shmeta-analysis. Am. J. Epidemiol. 1994;140(9):771–778. doi: 10.1093/oxfordjournals.aje.a117324. [DOI] [PubMed] [Google Scholar]
- 96.Savitz D.A., Forastiere F. Do pooled estimates from meta-analyses of observational epidemiology studies contribute to causal inference? Occup. Environ. Med. 2021;78(9):621–622. doi: 10.1136/oemed-2021-107702. [DOI] [PubMed] [Google Scholar]
- 97.Åberg S., Mann J., Neumann S., Ross A.B., Reynolds A.N. Whole-grain processing and glycemic control in type 2 diabetes: a randomized crossover trial. Diabetes Care. 2020;43(8):1717–1723. doi: 10.2337/dc20-0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Musa-Veloso K., Noori D., Venditti C., Poon T., Johnson J., Harkness L.S., et al. A systematic review and meta-analysis of randomized controlled trials on the effects of oats and oat processing on postprandial blood glucose and insulin responses. J. Nutr. 2021;151(2):341–351. doi: 10.1093/jn/nxaa349. [DOI] [PubMed] [Google Scholar]
- 99.Hollænder P.L., Ross A.B., Kristensen M. Whole-grain and blood lipid changes in apparently healthy adults: a systematic review and meta-analysis of randomized controlled studies. Am. J. Clin. Nutr. 2015;102(3):556–572. doi: 10.3945/ajcn.115.109165. [DOI] [PubMed] [Google Scholar]
- 100.Awika J.M. In: Advances in Cereal Science: Implications to Food Processing and Health Promotion. Awika J.M., Piironen V., Bean S., editors. American Chemical Society; 2011. Major cereal grains production and use around the world; pp. 1–13. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.