Summary
Background
Melatonin prescriptions for children and adolescents have increased substantially during the last decade. Existing clinical recommendations focus on melatonin as a treatment for insomnia related to neurodevelopmental disorders. To help guide clinical decision-making, we aimed to construct a recommendation on the use of melatonin in children and adolescents aged 5–20 years with idiopathic chronic insomnia.
Methods
A systematic search for guidelines, systematic reviews and randomised controlled trials (RCT) were performed in Medline, Embase, Cochrane Library, PsycInfo, Cinahl, Guidelines International Network, Trip Database, Canadian Agency for Drugs and Technologies in Health, American Academy of Sleep Medicine, European Sleep Research Society and Scandinavian Health Authorities databases. A search for adverse events in otherwise healthy children and adolescents was also performed. The latest search for guidelines, systematic reviews, and adverse events was performed on March 18, 2023. The latest search for RCTs was performed on to February 6, 2023. The language was restricted to English, Danish, Norwegian, and Swedish. Eligible participants were children and adolescents (5–20 years of age) with idiopathic chronic insomnia, in whom sleep hygiene practices have been inadequate and melatonin was tested. There were no restrictions on dosage, duration of treatment, time of consumption, or release formula. Primary outcomes were quality of sleep, daytime functioning and serious adverse events. Secondary outcomes included total sleep time, sleep latency, awakenings, drowsiness, quality of life, all-cause dropouts, and non-serious adverse events. Outcomes were assessed at different time points to assess short-term and long-term effects. Meta-analysis was performed using inverse variance random-effects model and risk of bias was assessed using Cochrane risk of bias tool. If possible, funnel plots would be constructed to investigate publication bias. Heterogeneity was calculated via I2 statistics. A multidisciplinary guideline panel formulated the recommendation according to Grading of Recommendations Assessment, Development and Evaluation (GRADE). The certainty of evidence was considered either high, moderate, low or very low depending on the extent of risk of bias, inconsistency, imprecision, indirectness, or publication bias. The evidence-to-decision framework was subsequently used to discuss the feasibility and acceptance of the constructed recommendation alongside the impact on resources and equity. The protocol is registered with the Danish Health Authority.
Findings
We included eight RCTs with 419 children and adolescents with idiopathic chronic insomnia. Melatonin led to a moderate increase in total sleep time by 30.33 min (95% confidence interval (CI) 18.96–41.70, 4 studies, I2 = 0%) and a moderate reduction in sleep latency by 18.03 min (95% CI −26.61 to −9.44, 3 studies, I2 = 0%), both as assessed by sleep diary. No other beneficial effects were found. None of the studies provided information on serious adverse events, yet the number of participants experiencing non-serious adverse events was increased (Relative risk 3.44, 95% CI 1.25–9.42, 4 studies, I2 = 0%). Funnel plots were not constructed due to the low number of studies. The certainty of evidence was very low on the quality of sleep and low for daytime functioning.
Interpretation
Evidence of very low certainty shows that benefits are limited and unwanted events are likely when melatonin is used to treat otherwise healthy children and adolescents with chronic insomnia. Melatonin should never be the first choice of treatment for this particular population, yet carefully monitored short-term use may be considered if sleep hygiene practices and non-pharmacological interventions have proven inadequate, and only if daytime function is compromised.
Funding
The Danish Health Authority and the Parker Institute, Bispebjerg and Frederiksberg Hospital supported by the Oak Foundation.
Keywords: Children and adolescents, Idiopathic, Chronic insomnia, Melatonin, Evidence-based recommendation
Research in context.
Evidence before this study
The number of young people using prescribed melatonin has increased substantially in the past decade. Previous recommendations focus on melatonin as a treatment for insomnia related to neurodevelopmental disorders, whereas no recommendations exist for children and adolescents with insomnia of unknown cause. We aimed to address this gap in recommendations.
Added value of this study
To the best of our knowledge, this is the first evidence-based clinical recommendation on this topic in children and adolescents with idiopathic chronic insomnia. Evidence of very low certainty shows that the efficacy of melatonin, in otherwise healthy children and adolescents aged 5–20 years, is restricted to a moderate improvement in sleep continuity parameters, without having any impact on more personal-perceived outcomes, such as quality of sleep and daytime functioning. There is an increased risk of non-serious adverse events. Our recommendation, outlined below, were constructed by a multidisciplinary guideline panel in accordance with Grading of Recommendations Assessment, Development and Evaluation (GRADE).
Implications of all the available evidence
Based on our findings, we recommend that healthcare providers aim for rational use of melatonin in otherwise healthy children and adolescents, in which it is only administered in special cases and for a short period of time. Prior to initiating such off-label use, it is essential that the limited benefits, the likelihood of unwanted events, and what may constitute realistic magnitudes of effect are discussed with the child/adolescent. Further studies are needed to determine the long-term consequences of melatonin treatment in healthy, young individuals with greater certainty.
Introduction
Insomnia is common and found in conjunction with neurodevelopmental and psychiatric disorders, yet also reported in up to 25% of otherwise healthy children and adolescents.1,2 Chronic insomnia disorder refers to repeated difficulties with either initiating—or maintaining sleep, or issues with the duration–or quality of sleep, despite optimal circumstances for achieving sleep. The issues with sleep are present at least three times a week, for at least three months and are related to a reduction in daytime functioning such as fatigue, impaired attention, mood—and cognitive disturbances, daytime sleepiness and reduced motivation.3,4 Inadequate sleep may have widespread consequences for the child or young individual. Apart from a potential negative impact school/work performance, inadequate sleep has also been linked to symptoms of depression, growth restriction, diabetes, and obesity.5, 6, 7, 8, 9, 10, 11, 12 Thus, sufficient intervention is needed to break a potential negative trajectory.
Within the last decade, melatonin has gained popularity as a sleep aid and is now considered one of the most commonly prescribed drugs to treat insomnia in children and adolescents.1,13, 14, 15 Melatonin is an endogenous hormone that promotes sleep.16, 17, 18, 19 Registry data from Denmark show a substantial increase in the number of young people using prescribed melatonin from 2011 to 2021, as users between the age of 0–17 years more than tripled, whereas a sevenfold increase was seen in users between 18 and 24 years.20 This substantial increase was also seen from 2012 to 2018 in Norway and Sweden.21,22 During the same time period (from 2012 to 2021) the number of annual paediatric melatonin ingestions reported to the United States poison control centres increased by 530%, which was considered a likely result of a surge in the use of melatonin for both adults and children in the United States.23 In Australia, the prescription of melatonin increased by just over 600% from 2011 to 2018 in children and adolescents below 19 years of age.24 The regulation of synthetic melatonin variates considerably across countries. In some European countries, melatonin is obtained by prescription, whereas it can be bought as a dietary supplement in the United States.25 In Europe, melatonin is indicated for a narrow set of paediatric patients who have difficulties sleeping, including those with autism spectrum disorder, and attention-deficit/hyperactive disorder (ADHD).26,27 For children and adolescents with chronic insomnia of unknown cause, the use of melatonin is off label. Thus, for this population, it is the prescribing doctor who, based on the evidence at hand, bears the responsibility for each individual decision leading to off-label use. As such, several recommendations exist for the use of melatonin in children and adolescents who present with chronic insomnia due to underlying neurodevelopmental disorders such as autism spectrum disorder, and ADHD.28, 29, 30 There are currently no recommendations that may guide clinical decision-making specifically when it comes to prescribing melatonin to children and adolescents with chronic insomnia that is not due to any sleep -, medical -, or mental disorder or the use of medication/substances.
Our objective was to develop a recommendation on the use of melatonin for children and adolescents (age 5–20 years), who despite optimisation of sleep hygiene practices, continue to display deficits in daytime functioning due to idiopathic chronic insomnia.
Methods
Methodology and work process
This study is a part of national clinical recommendations published by the Danish Health Authority in 2022.3 The work follows the Population, Intervention, Comparison and Outcome (PICO) framework,31 GRADE,32 the Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA)33, 34, 35 and guidelines of the Cochrane Collaboration.36 A prespecified protocol in Danish was registered and approved by management from the Department of Evidence Based Medicine at the Danish Health Authority on December 21, 2021 and is publicly available on the Danish Health Authority website at https://www.sst.dk/da/Udgivelser/2022/NKA_-Behandling-med-melatonin-ved-soevnforstyrrelser-hos-boern-og-unge. The PRISMA checklist as well as description of the guidelines panel and work process is found in the Supplementary.
PICO question
The population included children and adolescents (5–20 years of age) with idiopathic chronic insomnia, where sleep hygiene practices have been inadequate. Chronic insomnia refers to the diagnosis of chronic insomnia disorder as defined by the International Classification of Sleep Disorders, version 3 (ICSD-3). The term “idiopathic” was chosen in the context of this review, to reflect that the observed sleep disturbances are not due to any sleep -, medical -, or mental disorder or the use of medication/substances. The diagnosis of chronic insomnia disorder found in the ICD-3 applies to all patients presenting with insomnia symptoms, irrespective of whether or not these may be linked to an underlying medical—or mental disorder.4 However, from a clinical perspective, we have in this review chosen to solely focus on the patient group presenting with idiopathic insomnia, as the referral and treatment of these patients tend to differ from those presenting with underlying disorders. Whereas the former may be referred to a general practitioner, the latter is often treated for insomnia symptoms in specialised medical facilities due to the complexity of potentially conflicting symptoms and concomitant medications. Thus, studies on participants with primary psychiatric, or somatic disorders were excluded. Sleep hygiene refers to the counselling of the parents/young individual on what type of behaviour and routines facilitate adequate sleep, such as establishing fixed bedtime and routines, limiting the use of electronics close to bedtime and avoiding excessive daytime sleeping. The intervention was melatonin, with no restrictions on dosage, duration, time of consumption or release formula. The comparison was no treatment (or placebo) or non-pharmacological interventions such as, but not restricted to, physical activity, cognitive behavioural therapy, or weighted blankets. Primary outcomes were quality of sleep, daytime functioning and serious adverse events. Secondary outcomes included total sleep time, sleep latency, awakenings, drowsiness, quality of life, dropouts, and non-serious adverse events. Outcomes were assessed at different timeframes to assess both the impact on short- and long-term treatments (overview of outcomes provided in Supplementary). A thorough assessment of long-term adverse events (pubertal development and bone health) will be published separately.37 We did not a priori make any requirements as to how outcomes were to be assessed, as this would depend on the data available in the current literature.
Search for literature and study selection
A systematic search was performed in four individual steps for 1) guidelines and health technology assessments, published within the last 12 years 2) systematic reviews and metanalysis, published within the last 7 years 3) randomised controlled trials (RCT), with no date restriction and 4) a restricted search for adverse events, with no date restriction. Search for guidelines, systematic reviews and the restricted search for adverse events was performed up to March 2023 (last search date March 18). The search for RCTs was performed up to February 2023 (last search date February 6). The restricted time frame for guidelines and systematic reviews was chosen to ensure that any identified evidence synthesis would include the latest primary studies and thus would still be considered relevant. No restriction was put on the search for randomised studies to ensure that all relevant primary studies were identified. Searches were performed in Medline, Embase, Cochrane Library, PsycInfo, Cinahl, Guidelines International Network, Trip Database, Canadian Agency for Drugs and Technologies in Health, American Academy of Sleep Medicine, European Sleep Research Society and the Scandinavian Health Authorities databases. Search words included medical subject headings and free-text search words. Language was restricted to English and Scandinavian languages. There were no restrictions on publication status. The reference lists of included studies were searched for additional relevant studies. The guideline panel was consulted to ensure that no known literature was missing. The search strategies are found in the Supplementary.
Identified studies were imported to RefWorks and duplicates were removed. Remaining studies were imported to Covidence,38 and final selection was based on the described PICO criteria. One reviewer (HEC) assessed title and abstracts. Full text assessment was done by two independent reviewers (HEC and HKA). Reviewers were not blinded during study selection. Any disagreements were solved through discussion.
Data extraction and risk of bias
The Covidence software was used for data extraction using a predefined template,38 which included: study design, funding, diagnosis, age, dosage, length of treatment and outcomes. For cross-over trials, data of the first period were prioritised, in an attempt to diminish potential carry-over effects.36 If case clock times were provided, these were transformed into minutes. Risk of bias was assessed using the Cochrane risk of bias tool (version 1).39 If possible, funnel plots would be constructed to investigate publication bias.40 Two reviewers (HEC and HKA) performed data extraction and risk of bias assessment independently. Discrepancies were resolved through discussion. The authors of the included studies were not contacted for further information.
Summary measures and statistical analysis
We constructed a table providing an overview of the reported outcomes across the included studies (outcome matrix available upon request). Forest plots were performed in RevMan 5, version 5.3 (Cochrane collaboration), using inverse variance random-effects models.41 For dichotomous outcomes, relative risk and 95% confidence interval (CI) were calculated. For continuous outcomes, either a mean difference (MD) or standardised mean difference (SMD) alongside a 95% CI was calculated. Heterogeneity was calculated via I2 statistics, with I2 > 50% considered to be substantial.42 Wherever possible, post hoc subgroup analysis were performed investigating the impact of dosage (below/above 5 mg), age (below/above 12 years) and type of release formular applied (fast release/sustained release). Sleep continuity parameters (total sleep time and sleep latency) measured by both actigraphy and sleep diaries, were assessed separately. In case a significant result was identified, further assessment of baseline values was made to assist in interpreting the clinical relevance. Baseline values were extracted from the studies when available and the range of values was presented. Sensitivity analysis were not performed due to the low number of studies.
Certainty of evidence and creation of recommendation
The certainty of evidence was considered either high, moderate, low or very low depending on the extent of risk of bias, inconsistency, imprecision, indirectness or publication bias.32 The overall certainty of evidence relied on the lowest level of evidence of the primary outcomes. The final recommendation was based on a weighted assessment of benefits and harms, overall certainty of evidence and patient values and preferences, which led to either a strong or conditional recommendation, for or against an intervention. The guideline panel discussed the feasibility and acceptance of the constructed recommendation alongside the impact on resources and equity in accordance with the evidence-to-decision framework (EtD).43 Factors relevant to healthcare decision-makers and patient preferences were based on experiences and clinical judgment of the multidisciplinary guideline panel, with the potential for adjustments following a public hearing.
Role of funding source
The work was funded by the Danish Health Authority. The Parker Institute, Bispebjerg and Frederiksberg Hospital, is supported by a core grant from the Oak Foundation. The Danish Health Authority was involved in all steps of this study, including the study design, data extraction and analysis, results interpretation and development of the final recommendation.
Results
Literature search and study selection
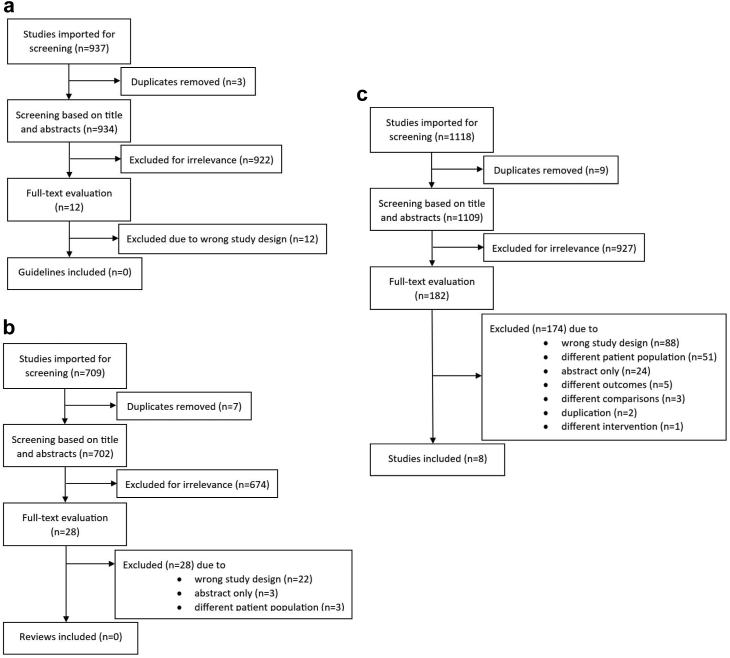
No relevant guidelines or health technology assessments were identified (Fig. 1a). The search for systematic reviews resulted in 28 reviews that underwent full-text evaluation. Of these 28 reviews, 14 reviews came close to our research question,25,44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56 yet these were excluded following a thorough read-through (Fig. 1b). A search for primary studies resulted in 1109 studies, leading to the final inclusion of eight RCTs57, 58, 59, 60, 61, 62, 63, 64; (Fig. 1c). The reference lists of the identified systematic reviews and the separate search for RCTs investigating adverse events did not contribute with any further studies. PRISMA flowcharts for all searches performed and an overview of excluded primary studies are included in the Supplementary.
Fig. 1.
PRISMA flowcharts for the screening of (a) guidelines, (b) systematic reviews and (c) randomised controlled studies. Initial and updated search combined.
Description of included studies
The eight RCTs included a total of 419 participants, aged 6–19 years, with idiopathic chronic sleep onset insomnia. All studies investigated the effects of melatonin in comparison to placebo.57, 58, 59, 60, 61, 62, 63, 64 No studies were identified in which melatonin was compared to no-pharmacological interventions such as physical activity, cognitive behavioural therapy or weighted blankets. Five studies explicitly mentioned that sleep hygiene measures had been tested before the initiation of the trial.58, 59, 60, 61, 62 In one study, information on sleep hygiene was provided at the start of the study.63 The dosages of melatonin ranged from 1 to 10 mg. Fast-releasing melatonin was used in five studies.58, 59, 60,62,63 Three studies did not explicitly state which release-formula had been used.57,61,64 The time of ingestion mainly took place 1.5–2 h before bedtime. The length of treatment varied from 1 to 5 weeks and outcomes were mainly assessed after the end of treatment.
Study characteristics are seen in Table 1.
Table 1.
Characteristics of the eight included randomised controlled trials evaluating the effect of melatonin in children and adolescents with idiopathic chronic insomnia.
| Author, year, country, trial registration | Demographics sex (male/female) age (provided as range or mean, SD) | Design and funding | Intervention (s) | Comparison | Duration | Outcomes of interest, measurement |
|---|---|---|---|---|---|---|
| Eckerberg, 2012, Sweden57 No protocol identified |
n = 21, participants with sleep-onset difficulties Sex: 10/11 Overall age, years: 14–19 Melatonin group (n = 21) Sex: 10/11 Age, years: 14–19 Control (placebo) group (n = 21) Sex: 10/11 Age, years: 14–19 |
RCT, crossover trial Funding: NATURAL PHARMA International, Stockholm Sweden provided the melatonin and placebo capsules. |
Melatonin, 1 mg fast release capsules (5-methoxy-N-acetyltryptamine) | Placebo–capsules | 5 weeks |
Total sleep time: Sleep diary Sleepiness and fatigue during daytime: Karolinska Sleepiness Scale |
| Smits, 2001, the Netherlands58 No protocol identified |
n = 40, participants with idiopathic chronic sleep-onset insomnia Sex: 27/11 Overall age, years: 6–12 Melatonin group (n = 20) Sex: 11/8 Age, years: 6–12 Control (placebo) group (n = 20) Sex: 16/3 Age, years: 6–12 |
RCT, single center parallel group, two arms. Funding: The Jan Dekker and dr. Ludgardine Bouwman Foundation and the Dutch Society for Sleep-Wake Research. |
Melatonin, 5 mg fast release capsules. Administration at 18:00 |
Placebo—capsules similar to the ones in intervention group | 4 weeks |
Total sleep time: Actigraph (Gähwiler Electronics, Hombrechtikon, Switzerland) Sleep onset latency: Actigraph Dropouts: Number of participants |
| Smits, 2003, the Netherlands59 No protocol identified |
n = 62, participants with idiopathic chronic sleep-onset insomnia Sex: 49/13 Overall age, years: 6–12 Melatonin group (n = 27) Sex: 20/7 Age, years: 9.2 (2.1) Control (placebo) group (n = 35) Sex: 29/6 Age, years: 10.1 (1.7) |
RCT, single center, parallel group, two arms. Funding: The Jan Dekker and dr. Ludgardine Bouwman Foundation. |
Melatonin 5 mg fast release capsules (Duchefa Farma BV, Harlem, the Netherlands). Administration at 19:00 |
Placebo—capsules similar to the ones in intervention group | 4 weeks |
Daytime functioning: Funktional status II tool Total sleep time: Sleep log/Actigraph Sleep onset latency: Sleep log/Actigraph Dropouts: Number of participants |
| Van der Heijden, 2005, the Netherlands60 No protocol identified |
n = 110, participants with idiopathic chronic sleep onset insomnia Sex: 76/24 Overall age, years: 6–12 Melatonin group (n = 55) Sex: 31/15 Age, years: 6–9 and 10–13 Control (placebo) group (n = 55) Sex: 45/9 Age, years: 6–9 and 10–13 |
RCT, single center, parallel group, two arms. Funding: The Dr. Ludgardine Bouwman Foundation. |
Melatonin 5 mg fast release capsules (Duchefa Farma BV, Harlem, the Netherlands). | Placebo–capsules similar to the ones in intervention group | 4 weeks | Individual patient data of two previously published randomised, placebo-controlled, double blind, clinical trials, using similar methodology (Smits et al., 2001, 2003), were combined. Total sleep time: Actigraph Sleep onset latency: Actigraph |
| Van Geijlswijk, 2010, the Netherlands61 Title registration: International Standard Randomized Controlled Trial Number Register (ISRCTN20033346). |
n = 72, participants with chronic sleep onset insomnia Sex: 30/42 Overall age, years: 6–12 Melatonin groups (n = 53) Sex: 24/29 Age, years: 6–12 Control (placebo) group (n = 17) Sex: 6/11 Age, years: 6–12 |
RCT, single center, parallel group, four arms. Funding: None mentioned |
Melatonin 0.05 or 0.1 or 0.15 mg/kg (supplied by Pharma Nord, Denmark) in the appropriate calculated dosage and microcrystalline cellulose. Administered between 17:30 and 19:30. |
Placebo containing only microcrystalline cellulose (Bufa, Haarlem, The Netherlands) | 2 weeks |
Sleep onset: Actigraph Sleep onset latency: Actigraph Wake-up time: Actigraph Total sleep time: Actigraph |
| Van Geijlswijk, 2011, the Netherlands62 Trial registration: International Standard Randomized Controlled Trial Number Register (ISRCTN20033346). |
n = 59, participants with chronic sleep onset insomnia No sex data available Subgroup a. Age, years: <13 years Subgroup b. Age, years: >13 years Melatonin group (n = 59) |
Follow up from the RCT study Van Geijlswijk, 2010 Funding: None mentioned |
Long term use of melatonin, mean dose 2.69 mg (min 0.3 mg, max 10 mg) | 6 months |
Dropouts: Number of participants. Quality of sleep: Children sleep health questionnaire Effects of prolonged use of Melatonin—Puberty development: Tanner score (Tanner Stages standard deviation scores could be determined for 16 boys and 30 girls) |
|
| Van Maanen, 2017, the Netherlands63 No protocol identified |
n = 54, participants with idiopathic chronic sleep onset insomnia Sex: 33/21. Overall age, years: 7–12 Melatonin group (n = 26) Sex: 17/9 Age, years: 10.01 (1.47) Control (placebo) group (n = 28) Sex: 16/12 Age, years: 10.04 (1.63) |
RCT, parallel group, two arms. A third arm received light (30 children) Funding: Pharma Nord sponsored the melatonin and placebo tablets for the study |
Melatonin tablets (3 mg. fast release, Pharma Nord) Administration at 19:00. |
Placebo–tablets, similar to the ones in intervention group | 3–4 weeks |
Quality of sleep: Sleep efficacy (%)/AW4 actiwatches (Cambridge Neurotechnology Ltd, Cambridge, UK)–Actigraph Total sleep time: Actigraph Sleep onset latency: Actigraph Wake after sleep onset: Actigraph |
| Jalilolghadr 2022, Iran64 Trial registration: Iranian Registry of Clinical Trials. IRCT 2015111225008N1. |
n = 60, healthy participants with insomnia No sex data available Overall age, years: 7–12 Melatonin group (n = 30) Mean age 9.79 (2.02) Control (placebo) (n = 30) Mean age 9.38 (1.05) |
RCT, single center, parallel group, two arms Funding: None |
Melatonin tablets (Weber Nature company) containing 3 mg Administration at 19:00 |
Placebo tablets | 4 weeks | Daytime functioning: children's sleep habits questionnaire, daily performance subscale |
SD: Standard deviation, RCT: Randomised controlled trial, mg: milligram, kg: kilogram, min: minimum, max: maximum.
Risk of bias in the included studies
Of the eight included RCTs, the domains considered to represent the highest level of concern (e.g. blinding, incomplete and selective outcome reporting) were unclear in five studies.58,60,61,63,64 The risk of selection bias, as indicated by sequence generation and allocation concealment, was unclear in six of the studies.57, 58, 59, 60,62,64 Risk of bias assessment is found in the Supplementary. It was not possible to construct funnel plots due to the low number of studies.
Estimated effects
Primary outcomes
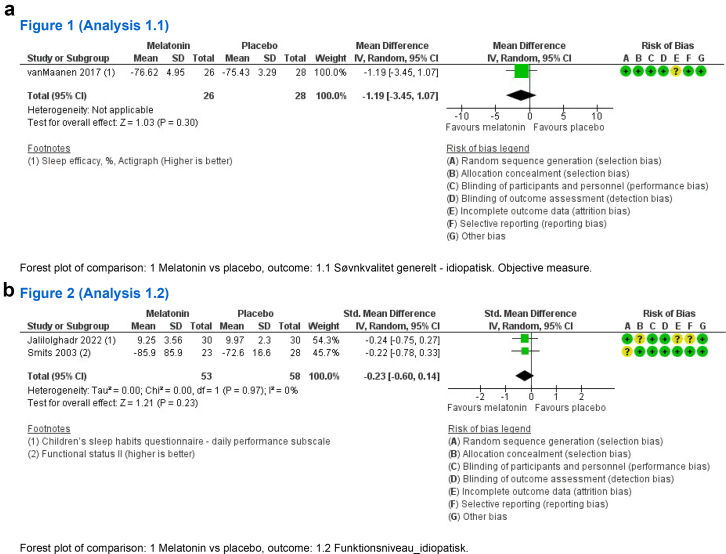
We found no statistical effect on the quality of sleep reported as sleep efficiency (%) measured by actigraphy (MD −1.19, 95% CI −3.45 to 1.07, 1 study, very low certainty) (see Fig. 2a). No statistical effect was found on daytime functioning (SMD −0.23, CI 95% −0.60 to 0.14, 2 studies, low certainty) when measured using parent-rated questionnaires (children sleep habit questionnaire and functional status II) (see Fig. 2b and Table 2). None of the studies provided information on serious adverse events. All forest plots are provided in the Supplementary.
Fig. 2.
Findings of the primary outcome (a) quality of sleep and (b) daytime functioning. None of the studies provided information on serious adverse events. SD: Standard deviation, CI: Confidence interval.
Table 2.
Estimated effects and certainty of evidence for each outcome in accordance with the GRADE approach.
| Outcome | Results | Effect estimates |
Certainty of evidence | Conclusion | |
|---|---|---|---|---|---|
| No melatonin | Melatonin | ||||
| All-cause dropout | Relative risk: 2.48 (CI 95% 0.39–15.54) Based on data from 215 patients in 3 studiesa |
19 per 1.000 | 47 per 1.000 |
Moderate Due to serious imprecisionb |
Melatonin probably has no or little effect on all-cause dropout |
| Difference: 28 more per 1.000 (CI 95% 11 fewer–272 more) | |||||
| Quality of sleep | Measured by: Sleep efficacy (%), actigraphy Higher is better Based on data from 54 patients in 1 studc |
Difference: MD 1.19 lower (CI 95% 3.45 lower–1.07 higher) |
Very low Due to very serious imprecisiond |
Melatonin may have no or little effect on quality of sleep | |
| Daytime functioning | Measured by: Functional status II (total score); Children's sleep habits questionnaire (daily performance) (parent-rated). Scale: Lower is better Based on data from 111 patients in 2 studiese |
Difference: SMD 0.23 lower (CI 95% 0.60 lower–0.14 higher) |
Low Due to serious imprecisionf |
Melatonin may have no or little effect on daytime functioning | |
| Total sleep time | Measured by: Total time spent sleeping in minutes, assessed by sleep diary (parent—and self-reported) Scale: Higher is better Based on data from 184 patients in 4 studiesg |
Difference: MD 30.33 higher (CI 95% 18.96 higher–41.70 higher) | High | Melatonin leads to a moderate increase in the total sleep time | |
| Sleep latency | Measured by: Time taken to fall asleep in minutes, assessed by sleep diary (parent-reported) Scale: Lower is better Based on data from 142 patients in 3 studiesh |
Difference: MD 18.03 lower (CI 95% 26.61 lower–9.44 lower) | High | Melatonin leads to a moderate decrease in sleep latency | |
| Sleepiness | Measured by: Karolinska sleepiness scale (self-rated) Scale: Range 1–9. Lower is better Based on data from 42 patients in 1 studyi |
Difference: MD 0.57 lower (CI 95% 0.66 lower–0.48 lower) |
Low Due to serious imprecisionj |
Melatonin may have no or little effect on sleepiness/drowsiness | |
| Wakening after sleep onset | Measured by: Wakening after sleep onset in minutes assessed by actigraph. Scale: Lower is better Based on data from 54 patients in 1 studyc |
Difference: MD 18.49 higher (CI 95% 5.87 higher–31.11 higher) |
Very low Due to very serious imprecisionk |
Melatonin may lead to a moderate increase in wakening after sleep onset | |
| Non-serious adverse events | Relative risiko: 3.44 (CI 95% 1.25–9.42) Based on data from 212 patients in 4 studiesl |
32 per 1.000 | 125 per 1.000 |
Moderate Due to serious imprecisionm |
Melatonin probably leads to an increase in non-serious adverse events |
| Difference: 93 more per 1.000 (CI 95% 8 more–275 more) | |||||
CI: Confidence interval, MD: Mean difference, SMD: Standardised mean difference.
Smits 2001, Smits 2003, vanGeijlswijk 2010.
Serious imprecision: wide confidence interval.
VanMaanen 2017.
Very serious imprecision: data based on one study; few patients; wide confidence interval.
Smits 2003, Jalilolghadr 2022.
Serious imprecision: few patients; wide confidence interval.
Smits 2001, vanMaanen 2017, Smits 2003, Eckerberg 2012.
Smits 2001, Smits 2003, vanMaanen 2017.
Eckerberg 2012.
Serious imprecision: data based on one study; few patients.
Serious imprecision: data based on one study; few patients; wide confidence interval.
Smits 2001, Smits 2003, vanGeijlswijk 2010, Eckerberg 2012.
Serious imprecision: wide confidence interval.
Secondary outcomes
Total sleep time increased by 30.33 min (95% CI 18.96–41.70, 4 studies, high certainty) when assessed by sleep diaries (parent—and self-reported) (see Table 2). This was considered a moderate clinical effect compared to estimates reported at baseline (ranging between 7 and 9.5 h). Further tests for subgroup differences showed no statistical effect for age (p = 0.86). No statistical effect was found on total sleep time when assessed by actigraphy (2.40 min, 95% CI −115.84 to 120.64, 1 study, very low certainty). All forest plots are found in the Supplementary.
Sleep latency was reduced by 18.03 min (95% CI −26.61 to −9.44, 3 studies, high certainty) when assessed using a sleep diary (parent-reported), and by 27.04 min (95% CI −38.07 to −16.01, 1 study, very low certainty) when assessed using actigraphy (see Table 2). Both estimates were considered a moderate clinical effect compared to estimates at baseline (ranging between 51 and 60 min). We found a statistical, yet no clinical effect on sleepiness measured by the Karolinska sleepiness scale (self-rated) (MD −0.57, CI 95% −0.66 to −0.48, 1 study, low certainty) (see Table 2). A potential increase in wakefulness after sleep onset (WASO) was found by actigraphy (MD 18.49, CI 95% 5.87–31.11, 1 study, very low certainty). Melatonin showed no statistical impact on all-cause dropouts (RR 2.48, CI 95% 0.39–15.54, 3 studies, moderate certainty) (see Table 2). Melatonin increased the number of participants experiencing non-serious adverse events (RR 3.44, 95% CI 1.25–9.42, 4 studies, moderate certainty) (see Table 2). Forest plots and an overview of reported non-serious adverse events are found in the Supplementary.
None of the studies reported on quality of sleep or daytime functioning at 3–6 months or quality of life. Due to a lack of data, it was not possible to perform subgroup analysis investigating the effect of dosage or release-form.
Certainty of evidence
The certainty of evidence was very low on quality of sleep and low for daytime functioning. None of the studies provided any information on serious adverse events. Thus, the overall certainty of evidence was very low (see Table 3).
Table 3.
Evidence to decision framework (EtD).
 |
The table displays to parameters taken into account in the development of the recommendation. These include the benefits and harms, certainty of evidence, patient values and preferences, resources, equity, acceptability and feasibility.
Evidence-based recommendation
Emphasis was put on evidence showing a moderate reduction in sleep latency and moderate increase in the total sleep time as assessed by sleep diary, yet without melatonin having an impact on quality of sleep and daytime functioning. This was weighed against an increased risk of adverse events, yet with no indication of them being serious of nature, and the uncertain long-term consequences.37 The overall certainty of evidence was very low (see Table 3). The guideline panel believes that melatonin should never be the first choice of treatment for insomnia in children and adolescents. However, due to the potential negative impact of chronic insomnia, carefully monitored short-term treatment may be tried in those who continue to display deficits in daytime functioning. For these individuals and their families, the guideline panel believes that there is a general interest in trying melatonin as a treatment option.
Therefore, a conditional recommendation was provided for the use of melatonin to treat idiopathic chronic insomnia in children and adolescents (5–20 years), who continue to display deficits in daytime functioning, despite the optimisation of sleep hygiene practices. If sleep hygiene practices have proven inadequate, the guideline panel believes that non-pharmacological interventions should also be considered prior to initiating treatment with melatonin. In accordance, some effects of cognitive behavioural therapy (CBT), as well as other psychological treatments, have been found for treating insomnia in children and adolescents.65, 66, 67, 68, 69 The working group recommends that treatment with melatonin should be as short as possible and always be re-assessed after 14 days, and again after three months. If there is little or no effect, as assessed by sleep diary on parameters such as total sleep time and sleep latency, use of melatonin should be discontinued.
The implementation of this recommendation may be affected by the accessibility of non-pharmacological interventions and counselling on sleep hygiene measures. Equity and resources may be affected by the price of the melatonin product being prescribed. The guideline panel believes that the child/adolescents and parents consider melatonin to be an acceptable treatment for chronic insomnia. This has also been found by others.70 Nevertheless, the use of melatonin for idiopathic chronic insomnia is off-label, which the child/adolescents and family needs to be made aware of prior to initiating treatment (see Table 3).
Discussion
We recommend that melatonin may be used for a short period of time to treat idiopathic chronic insomnia in children and adolescents (5–20 years), yet with the prerequisite that sleep-hygiene practices and non-pharmacological interventions, such as cognitive behavioural therapy and other measures, have proven inadequate, and only in cases where daytime functioning is compromised. To our knowledge, this is the first clinical, evidence-based recommendation on the topic.
Our body of evidence consists of eight RCTs investigating the effect of melatonin in 419 participants, aged 6–19 years, diagnosed with idiopathic chronic sleep onset insomnia. For these individuals, melatonin led to a moderate increase in total sleep time with 30 min and a moderate decrease in sleep latency with approximately 18 min, in both cases when assessed using sleep diaries. These results are however based on few studies with small sample sizes and rely on estimates provided by the parents or the participants themselves, and thus the data bears a considerable level of uncertainty. One study with few patients (n = 54) reported that melatonin may increase wakening after sleep onset, however further studies on this matter are needed. We found no other clinical–or statistical effect of melatonin on the remaining outcomes. Thus, it seems that the effect of melatonin is restricted to a moderate improvement in sleep continuity parameters, without this translating into a positive effect on personal perceived outcomes such as quality of sleep and daytime functioning. If treatment with melatonin is considered, these limited benefits, including what may be considered realistic magnitudes of effect, are important when consulting the parents and young individuals. Due to the lack of data, it was not possible to investigate the impact of dosage, timing of administration or release formula. The decision on these variables continues to rely on expert-opinions and consensus reports, until further studies have been conducted.28 None of the included studies provided information on whether any serious adverse events were found when melatonin was used in children and adolescents with idiopathic chronic insomnia. Yet, others have shown that melatonin, in general, is not associated with serious adverse events when assessed across a wider population.37 Treatment with melatonin however increased the number of participants experiencing non-serious adverse events, which included a range of unwanted events such as headache, nausea, red eyes, drowsiness, change in mood and cognition and gastrointestinal problems. The certainty of evidence for non-serious adverse events was downgraded to moderate due to a wide confidence interval, indicating some level of uncertainty regarding the extent to which melatonin leads to non-serious adverse events. The current evidence on safety has several limitations. Only half of the identified studies reported on adverse events, and of these, the systematic assessment of adverse events in all cases relied on spontaneous reporting either by the parents or the participant. None of the studies provided an extensive list of all the adverse events that occurred during the trial period. Despite an increase in unwanted events, melatonin did not impact all-cause dropouts, indicating that treatment is tolerable. We were able to identify 14 existing systematic reviews that came close to our subject, yet without them being a complete match. Nevertheless, there was a considerable overlap of the included primary studies across the existing reviews, and thus not surprisingly, reviews tended to report on the same effects, as also found here. To go beyond what has already been identified and published, there is a need for new, larger randomised studies, which apart from evaluating efficacy beyond 4–5 weeks of treatment also should include a systematic safety assessment. The guideline panel agrees that optimisation of sleep hygiene practices constitutes the first choice of treatment for children and adolescents with idiopathic chronic insomnia, alongside trying non-pharmacological interventions. The feasibility of implementing our recommendation may, however, be affected by especially the access to counselling in sleep hygiene practices and non-pharmacological measures. Here, it is worth mentioning, that only five out of eight studies had tested sleep hygiene practices prior to initiating treatment with melatonin, despite it being simple and straightforward to perform. The importance of optimising bed-time behaviour and routines to improve sleep in young individuals should not be underestimated. A recent meta-analysis showed that interventions simply focusing on earlier bedtimes, may increase sleep duration by 47 min in healthy children.71 The equity may also be affected by our recommendation, due to the price of the melatonin products available for prescription. The issues of high costs may favour the use of self-bought products, which has been reported in Sweden.72 The use of melatonin products not prescribed by a physician should be discouraged, as studies have found that the content may vary significantly from what is being advertised, with some products having serotonin content at potential clinically important levels.73,74
Overall, melatonin has a moderate effect on sleep continuity parameters, without having an impact on more personal-perceived outcomes. The use of melatonin should never be first choice of treatment for otherwise healthy children and adolescents with chronic insomnia. However, given the potential negative impact of continuous sleep problems, melatonin may be administered in special cases and only for a short period of time. The limited benefits of melatonin, the likelihood of non-serious adverse events and what may constitute realistic magnitudes of effect has to be discussed prior to initiating treatment.
The strengths of the current review include transparent methods adhering to PRISMA guidelines, Cochrane collaboration and the GRADE approach. The work is based on a pre-specified protocol, formulated by a multidisciplinary guideline panel and pre-registered at the Danish Health Authority. The search is based on a systematic and comprehensive search strategy. Selection of studies, data extraction and quality assessment have been done independently by duplicate reviewers. Limitations include restriction to English and Scandinavian languages. This was done to avoid any misinterpretation of results published in another foreign language. It is not known whether there may be additional relevant studies published in other languages. Reviewers were not being blinded during selection of studies, as this was not possible with the current software and since evaluation of the full text was mandated to assess the eligibility of the study for inclusion. Authors of the included studies were not contacted for further information and grey literature was not considered for inclusion. Assessment of title and abstract was only done by one reviewer. Amendments to the pre-registered protocol were inclusion of studies in which sleep hygiene had not been tested prior to initiation of the trial. We were not able to evaluate the frequency of adverse events as initially planned, due to limitations in the reporting of data. Due to lack of data, we were not able to evaluate the pre-specified outcomes following 3–6 months of treatment. Daytime functioning was only assessed using parent-rated questionnaires and not by a clinician as initially pre-specified. The age range in our pre-specified protocol was 5–20 years, whereas the included studies assessed children and adolescents aged 6–19 years. We do however believe that the current evidence is still representative of our pre-specified age range, and thus no amendments in regards to this were made. Our cut-off values used in the subgroup analysis investigating dosage effects (below/above 5 mg) were chosen post-hoc. As such, these cut-off values are based on what data was available in the identified literature, and thus do not per se reflect any clinical, scientific or theoretical reason of choice.
Contributors
The authors confirm contribution to the paper as follows: study design: all authors, data collection: HEC, HKA, MNH; analysis of results: HEC, HKA, MNH; interpretation of results: all authors; construction of final recommendation: all authors; draft of manuscript: HEC, with input from HKA, MNH. All authors reviewed and approved the final version of the manuscript. HEC, HKA and MNH accessed and verified the underlying data.
Data sharing statement
Data and all other relevant materials are publicly available either at the Danish Health Authority website (www.sst.dk) or upon request to the corresponding author.
Declaration of interests
LB is a member of the Danish medication reimbursement committee. AV has previously received honoraria for lectures at AGB pharma, Takeda & Medice and holds stocks at Novo Nordisk. All other authors declare no competing interests. Statements of conflicts of interests can be found for all members of the guideline panel, the external reviewer of the national clinical guideline, the reference–and project group at the Danish Health Authority website (www.sst.dk).
Acknowledgments
We would like to thank the guideline panel, reference group and the secretary of the “National clinical recommendation for the use of melatonin in children and adolescents with insomnia” published by the Danish Health Authority. The Parker Institute, Bispebjerg and Frederiksberg Hospital, is supported by a core grant from the Oak Foundation Oak Foundation (OCAY-18-774-OFIL).
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2023.102048.
Appendix A. Supplementary data
References
- 1.Owens J.A., Rosen C.L., Mindell J.A. Medication use in the treatment of pediatric insomnia: results of a survey of community-based pediatricians. Pediatrics. 2003;111(5 Pt 1):e628. doi: 10.1542/peds.111.5.e628. [DOI] [PubMed] [Google Scholar]
- 2.Mindell J.A., Emslie G., Blumer J., et al. Pharmacologic management of insomnia in children and adolescents: consensus statement. Pediatrics. 2006;117(6):e1223. doi: 10.1542/peds.2005-1693. [DOI] [PubMed] [Google Scholar]
- 3.The Danish Health Authority . 2022. National Clinical Recommendation for the use of melatonin to treat sleep problems in children and adolescents.https://files.magicapp.org/guideline/29d92ec7-7da4-4d84-974d-d26f397bd02d/published_guideline_5982-1_0.pdf [Google Scholar]
- 4.Sateia M.J. International classification of sleep disorders-third edition highlights and modifications. Chest. 2014;146(5):1387–1394. doi: 10.1378/chest.14-0970. [DOI] [PubMed] [Google Scholar]
- 5.Copinschi G., Leproult R., Spiegel K. The important role of sleep in metabolism. Front Horm Res. 2014;42:59–72. doi: 10.1159/000358858. [DOI] [PubMed] [Google Scholar]
- 6.Chorney D.B., Detweiler M.F., Morris T.L., Kuhn B.R. The interplay of sleep disturbance, anxiety, and depression in children. J Pediatr Psychol. 2008;33(4):339–348. doi: 10.1093/jpepsy/jsm105. [DOI] [PubMed] [Google Scholar]
- 7.Gregory A.M., Rijsdijk F.V., Dahl R.E., McGuffin P., Eley T.C. Associations between sleep problems, anxiety, and depression in twins at 8 years of age. Pediatrics. 2006;118(3):1124–1132. doi: 10.1542/peds.2005-3118. [DOI] [PubMed] [Google Scholar]
- 8.Matthews K.A., Dahl R.E., Owens J.F., Lee L., Hall M. Sleep duration and insulin resistance in healthy black and white adolescents. Sleep. 2012;35(10):1353–1358. doi: 10.5665/sleep.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fatima Y., Doi S.A.R., Mamun A.A. Sleep quality and obesity in young subjects: a meta-analysis. Obes Rev. 2016;17(11):1154–1166. doi: 10.1111/obr.12444. [DOI] [PubMed] [Google Scholar]
- 10.Wing Y.K., Li S.X., Li A.M., Zhang J., Kong A.P.S. The effect of weekend and holiday sleep compensation on childhood overweight and obesity. Pediatrics. 2009;124(5):e994. doi: 10.1542/peds.2008-3602. [DOI] [PubMed] [Google Scholar]
- 11.Casazza K., Hanks L.J., Fernandez J.R. Shorter sleep may be a risk factor for impaired bone mass accrual in childhood. J Clin Densitom. 2011;14(4):453. doi: 10.1016/j.jocd.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Combs D., Goodwin J.L., Quan S.F., Morgan W.J., Shetty S., Parthasarathy S. Insomnia, health-related quality of life and health outcomes in children: a seven year longitudinal cohort. Sci Rep. 2016;6 doi: 10.1038/srep27921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Owens J.A. Pharmacotherapy of pediatric insomnia. J Am Acad Child Adolesc Psychiatry. 2009;48(2):99–107. doi: 10.1097/CHI.0b013e3181930639. [DOI] [PubMed] [Google Scholar]
- 14.Owens J.A., Rosen C.L., Mindell J.A., Kirchner H.L. Use of pharmacotherapy for insomnia in child psychiatry practice: a national survey. Sleep Med. 2010;11(7):692–700. doi: 10.1016/j.sleep.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 15.Hartz I., Furu K., Bratlid T., Handal M., Skurtveit S. Hypnotic drug use among 0-17 year olds during 2004-2011: a nationwide prescription database study. Scand J Public Health. 2012;40(8):704–711. doi: 10.1177/1403494812464446. [DOI] [PubMed] [Google Scholar]
- 16.Buscemi N., Vandermeer B., Hooton N., et al. Efficacy and safety of exogenous melatonin for secondary sleep disorders and sleep disorders accompanying sleep restriction: meta-analysis. BMJ. 2006;332(7538):385–388. doi: 10.1136/bmj.38731.532766.F6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scheer F.A.J.L., Wright K.P., Kronauer R.E., Czeisler C.A. Plasticity of the intrinsic period of the human circadian timing system. PLoS One. 2007;2(8) doi: 10.1371/journal.pone.0000721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Checa-Ros A., Muñoz-Hoyos A., Molina-Carballo A., et al. Analysis of different melatonin secretion patterns in children with sleep disorders: melatonin secretion patterns in children. J Child Neurol. 2017;32(12):1000–1008. doi: 10.1177/0883073817726680. [DOI] [PubMed] [Google Scholar]
- 19.Salti R., Galluzzi F., Bindi G., et al. Nocturnal melatonin patterns in children. J Clin Endocrinol Metab. 2000;85(6):2137–2144. doi: 10.1210/jcem.85.6.6656. [DOI] [PubMed] [Google Scholar]
- 20.The Danish Health Data Authority. Medstat.dk; 2015. Accessed February 9, 2023.
- 21.Wesselhoeft R., Rasmussen L., Jensen P.B., et al. Use of hypnotic drugs among children, adolescents, and young adults in Scandinavia. Acta Psychiatr Scand. 2021;144(2):100–112. doi: 10.1111/acps.13329. [DOI] [PubMed] [Google Scholar]
- 22.Bliddal M., Kildegaard H., Rasmussen L., et al. Melatonin use among children, adolescents, and young adults: a Danish nationwide drug utilization study. Eur Child Adolesc Psychiatry. 2022:1–9. doi: 10.1007/s00787-022-02035-1. [DOI] [PubMed] [Google Scholar]
- 23.Centers for Disease control and Prevention Pediatric melatonin ingestions — United States, 2012–2021. https://www.cdc.gov/mmwr/volumes/71/wr/mm7122a1.htm?s_cid=mm7122a1_w Available from:
- 24.Klau J., Bernardo C.D.O., Gonzalez-Chica D.A., Raven M., Jureidini J. Trends in prescription of psychotropic medications to children and adolescents in Australian primary care from 2011 to 2018. Aust N Z J Psychiatry. 2022;56(11):1477–1490. doi: 10.1177/00048674211067720. [DOI] [PubMed] [Google Scholar]
- 25.Skrzelowski M., Brookhaus A., Shea L.A., Berlau D.J. Melatonin use in pediatrics: evaluating the discrepancy in evidence based on country and regulations regarding production. J Pediatr Pharmacol Ther. 2021;26(1):4–20. doi: 10.5863/1551-6776-26.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.European Medicines Agency Slenyto (melatonin) www.ema.europa.eu/contact Available from:
- 27.Danish Medicines Agency Melatonin AGB. http://produktresume.dk/AppBuilder/search?utf8=&;&id=&type=&q=Melatonin+AGB&button=Søg Available from:
- 28.Bruni O., Alonso-Alconada D., Besag F., et al. Current role of melatonin in pediatric neurology: clinical recommendations. Eur J Paediatr Neurol. 2015;19(2):122–133. doi: 10.1016/j.ejpn.2014.12.007. https://pubmed.ncbi.nlm.nih.gov/25553845/ [cited 2022 Oct 11] Available from: [DOI] [PubMed] [Google Scholar]
- 29.Danish Health Authority . 2021. National clinical guideline on the treatment of children and adolescence with Autism.https://app.magicapp.org/#/guideline/3992 Available at: [Google Scholar]
- 30.Danish Health Authority . 2021. National clinical guideline on the treatment of children and adolescence with ADHD.https://app.magicapp.org/#/guideline/4512 Available at: [Google Scholar]
- 31.Guyatt G.H., Oxman A.D., Kunz R., et al. GRADE guidelines: 2. Framing the question and deciding on important outcomes. J Clin Epidemiol. 2011;64(4):395–400. doi: 10.1016/j.jclinepi.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 32.Guyatt G.H., Oxman A.D., Vist G.E., et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shamseer L., Moher D., Clarke M., et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;350 doi: 10.1136/bmj.g7647. [DOI] [PubMed] [Google Scholar]
- 34.Page M.J., McKenzie J.E., Bossuyt P.M., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. 2021;10(1) doi: 10.1186/s13643-021-01626-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moher D., Shamseer L., Clarke M., et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):148–160. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cumpston M., Li T., Page M.J., et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. 2019;10 doi: 10.1002/14651858.ED000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Händel M.N., Andersen H.K., Ussing A., et al. The short-term and long-term adverse consequences of melatonin treatment in children and adolescents: a systematic review and GRADE assessment. eClinicalMedicine. 2023 doi: 10.1016/j.eclinm.2023.102083. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Covidence Systematic Review Software: Veritas Health Innovation: Melbourne–Better Systematic Review Management. 2015. covidence.org. Accessed February 9, 2023.
- 39.Higgins J.P.T., Altman D.G., Gøtzsche P.C., et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343(7829) doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sterne J.A.C., Sutton A.J., Ioannidis J.P.A., et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343(7818) doi: 10.1136/bmj.d4002. [DOI] [PubMed] [Google Scholar]
- 41.DerSimonian R., Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 42.Higgins J.P.T., Thompson S.G. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 43.Moberg J., Oxman A.D., Rosenbaum S., et al. The GRADE Evidence to Decision (EtD) framework for health system and public health decisions. Health Res policy Syst. 2018;16(1):45. doi: 10.1186/s12961-018-0320-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abdelgadir I.S., Gordon M.A., Akobeng A.K. Melatonin for the management of sleep problems in children with neurodevelopmental disorders: a systematic review and meta-analysis. Arch Dis Child. 2018;103(12):1155–1162. doi: 10.1136/archdischild-2017-314181. [DOI] [PubMed] [Google Scholar]
- 45.Beresford B., McDaid C., Parker A., et al. Pharmacological and non-pharmacological interventions for non-respiratory sleep disturbance in children with neurodisabilities: a systematic review. Health Technol Assess. 2018;22(60):1–117. doi: 10.3310/hta22600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Besag F.M.C., Vasey M.J., Lao K.S.J., Wong I.C.K. Adverse events associated with melatonin for the treatment of primary or secondary sleep disorders: a systematic review. CNS Drugs. 2019;33(12):1167–1186. doi: 10.1007/s40263-019-00680-w. [DOI] [PubMed] [Google Scholar]
- 47.Bruni O., Angriman M., Calisti F., et al. Practitioner Review: treatment of chronic insomnia in children and adolescents with neurodevelopmental disabilities. J Child Psychol Psychiatry. 2018;59(5):489–508. doi: 10.1111/jcpp.12812. [DOI] [PubMed] [Google Scholar]
- 48.Bueno A.P.R., Savi F.M., Alves I.A., Bandeira V.A.C. Regulatory aspects and evidences of melatonin use for sleep disorders and insomnia: an integrative review. Arq Neuropsiquiatr. 2021;79(8):732–742. doi: 10.1590/0004-282X-ANP-2020-0379. [DOI] [PubMed] [Google Scholar]
- 49.Hollway J.A., Aman M.G. Pharmacological treatment of sleep disturbance in developmental disabilities: a review of the literature. Res Dev Disabil. 2011;32(3):939–962. doi: 10.1016/j.ridd.2010.12.035. [DOI] [PubMed] [Google Scholar]
- 50.McDonagh M.S., Holmes R., Hsu F. Pharmacologic treatments for sleep disorders in children: a systematic review. J Child Neurol. 2019;34(5):237–247. doi: 10.1177/0883073818821030. [DOI] [PubMed] [Google Scholar]
- 51.Parker A., Beresford B., Dawson V., et al. Oral melatonin for non-respiratory sleep disturbance in children with neurodisabilities: systematic review and meta-analyses. Dev Med Child Neurol. 2019;61(8):880–890. doi: 10.1111/dmcn.14157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wei S., Smits M.G., Tang X., et al. Efficacy and safety of melatonin for sleep onset insomnia in children and adolescents: a meta-analysis of randomized controlled trials. Sleep Med. 2020;68:1–8. doi: 10.1016/j.sleep.2019.02.017. [DOI] [PubMed] [Google Scholar]
- 53.Choi K., Lee Y.J., Park S., Je N.K., Suh H.S. Efficacy of melatonin for chronic insomnia: systematic reviews and meta-analyses. Sleep Med Rev. 2022;66 doi: 10.1016/j.smrv.2022.101692. [DOI] [PubMed] [Google Scholar]
- 54.Iwamoto B.K., Decker K.M., Byars K.C., Van Dyk T.R. Impact of exogenous melatonin on sleep and daytime functioning in healthy, typically developing adolescents. Curr Sleep Med Rep. 2022;8(4):62–73. [Google Scholar]
- 55.Kennaway D.J. What do we really know about the safety and efficacy of melatonin for sleep disorders? Curr Med Res Opin. 2022;38(2):211–227. doi: 10.1080/03007995.2021.2000714. [DOI] [PubMed] [Google Scholar]
- 56.Salanitro M., Wrigley T., Ghabra H., et al. Efficacy on sleep parameters and tolerability of melatonin in individuals with sleep or mental disorders: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2022;139 doi: 10.1016/j.neubiorev.2022.104723. [DOI] [PubMed] [Google Scholar]
- 57.Eckerberg B., Lowden A., Nagai R., Åkerstedt T. Melatonin treatment effects on adolescent students' sleep timing and sleepiness in a placebo-controlled crossover study. Chronobiol Int. 2012;29(9):1239–1248. doi: 10.3109/07420528.2012.719962. [DOI] [PubMed] [Google Scholar]
- 58.Smits M.G., Nagtegaal E.E., van der Heijden J., Coenen A.M.L., Kerkhof G.A. Melatonin for chronic sleep onset insomnia in children: a randomized placebo-controlled trial. J Child Neurol. 2001;16(2):86–92. doi: 10.1177/088307380101600204. [DOI] [PubMed] [Google Scholar]
- 59.Smits M.G., Van Stel H.F., Van Der Heijden K., Meijer A.M., Coenen A.M.L., Kerkhof G.A. Melatonin improves health status and sleep in children with idiopathic chronic sleep-onset insomnia: a randomized placebo-controlled trial. J Am Acad Child Adolesc Psychiatry. 2003;42(11):1286–1293. doi: 10.1097/01.chi.0000085756.71002.86. [DOI] [PubMed] [Google Scholar]
- 60.Van Der Heijden K.B., Smits M.G., Van Someren E.J.W., Boudewijn Gunning W. Prediction of melatonin efficacy by pretreatment dim light melatonin onset in children with idiopathic chronic sleep onset insomnia. J Sleep Res. 2005;14(2):187–194. doi: 10.1111/j.1365-2869.2005.00451.x. [DOI] [PubMed] [Google Scholar]
- 61.Van Geijlswijk I.M., Van Der Heijden K.B., Egberts A.C.G., Korzilius H.P.L.M., Smits M.G. Dose finding of melatonin for chronic idiopathic childhood sleep onset insomnia: an RCT. Psychopharmacology (Berl) 2010;212(3):379–391. doi: 10.1007/s00213-010-1962-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Van Geijlswijk I.M., Mol R.H., Egberts T.C.G., Smits M.G. Evaluation of sleep, puberty and mental health in children with long-term melatonin treatment for chronic idiopathic childhood sleep onset insomnia. Psychopharmacology (Berl) 2011;216(1):111–120. doi: 10.1007/s00213-011-2202-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Van Maanen A., Meijer A.M., Smits M.G., Van Der Heijden K.B., Oort F.J. Effects of melatonin and bright light treatment in childhood chronic sleep onset insomnia with late melatonin onset: a randomized controlled study. Sleep. 2017;40(2):zsw038. doi: 10.1093/sleep/zsw038. [DOI] [PubMed] [Google Scholar]
- 64.Jalilolghadr S., Roozmehr S., Yazdi Z., Soltanabadi M. The effect of treatment with melatonin on primary school aged children with difficulty in initiation and maintenance of sleep. Turk J Pediatr. 2022;64(6):993. doi: 10.24953/turkjped.2018.1381. [DOI] [PubMed] [Google Scholar]
- 65.Tsai H.J., Yang A.C., Zhu J.D., Hsu Y.Y., Hsu T.F., Tsai S.J. Effectiveness of digital cognitive behavioral therapy for insomnia in young people: preliminary findings from systematic review and meta-analysis. J Pers Med. 2022;12(3):481. doi: 10.3390/jpm12030481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dӧnmez Ş., Abdullaev K. Efficacy of cognitive behavioral therapy in children and adolescents with insomnia: a systematic review and meta-analysis. Braz J Med Biol Res. 2018;51(6) doi: 10.1590/1414-431X20187070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Taylor D.J., Roane B.M. Treatment of insomnia in adults and children: a practice-friendly review of research. J Clin Psychol. 2010;66(11):1137–1147. doi: 10.1002/jclp.20733. [DOI] [PubMed] [Google Scholar]
- 68.Bourchtein E., Langberg J.M., Eadeh H.M. A review of pediatric nonpharmacological sleep interventions: effects on sleep, secondary outcomes, and populations with co-occurring mental health conditions. Behav Ther. 2020;51(1):27–41. doi: 10.1016/j.beth.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 69.Hill C. Practitioner review: effective treatment of behavioural insomnia in children. J Child Psychol Psychiatry. 2011;52(7):731–740. doi: 10.1111/j.1469-7610.2011.02396.x. [DOI] [PubMed] [Google Scholar]
- 70.Waldron A.Y., Spark M.J., Dennis C.M. The use of melatonin by children: parents' perspectives. J Clin Sleep Med. 2016;12(10):1395–1401. doi: 10.5664/jcsm.6198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Magee L., Goldsmith L.P., Chaudhry U.A.R., et al. Nonpharmacological interventions to lengthen sleep duration in healthy children: a systematic review and meta-analysis. JAMA Pediatr. 2022;176:1084. doi: 10.1001/jamapediatrics.2022.3172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Støre S.J. Swedish Internet forum users' views and experiences of melatonin treatments for troubled sleep. Sleep Health. 2022;8(2):225–229. doi: 10.1016/j.sleh.2021.12.005. [DOI] [PubMed] [Google Scholar]
- 73.Erland L.A.E., Saxena P.K. Melatonin natural health products and supplements: presence of serotonin and significant variability of melatonin content. J Clin Sleep Med. 2017;13(2):275–281. doi: 10.5664/jcsm.6462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Williamson B.L., Tomlinson A.J., Mishra P.K., Gleich G.J., Naylor S. Structural characterization of contaminants found in commercial preparations of melatonin: similarities to case-related compounds from L-tryptophan associated with eosinophilia-myalgia syndrome. Chem Res Toxicol. 1998;11(3):234–240. doi: 10.1021/tx970202h. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.