Abstract
In this study, we investigated in vitro the potential of Trichoderma harzianum to produce bioactive secondary metabolites that can be used as alternatives to synthetic compounds. The study focused on analyzing two extracts of T. harzianum using ethyl acetate and n-butanol solvents with different polarities. The extracts were examined using phytochemical analysis to determine the content of polyphenols, flavonoids, tannins, and alkaloids. Thin-layer chromatography (TLC) and Gas chromatography-mass spectroscopy (GC-MS) analysis were used to profile volatile organic metabolites (VOCs) present in the extracts. Furthermore, the extracts were tested for their antifungal ability using the poison food technique. For measuring antioxidant activity, the 1,1-diphenyl-2-picryl-hydrazyl (DPPH) test was used. Trichoderma harzianum was shown to have a significantly high content of tannins and alkaloids, with a noticeable difference between the two extracts. GC-MS analysis identified 33 potential compounds with numerous benefits that could be used in agriculture and the medicinal industry. Moreover, strong antifungal activity was identified against Sclerotinia sclerotiorum by 94.44%, Alternaria sp. by 77.04%, and Fusarium solani by 51.48; similarly, the IC50 of antioxidant activity was estimated for ethyl acetate extract by 71.47% and n-butanol extract by 56.01%. This leads to the conclusion that Trichoderma harzianum VOCs play a significant role as an antifungal and antioxidant agent when taking into account the advantageous bioactive chemicals noted in the extracts. However, to our knowledge, this is the first study in Algeria presenting detailed phytochemical analysis and GC-MS profiling of Trichoderma harzianum for two extracts, ethyl acetate and n-butanol.
Keywords: Trichoderma harzianum, bioactive metabolite, antifungal, natural products, antioxidant
1. Introduction
Endophytic fungi are an integral part of a plant’s mycobiome. They frequently appear in the intercellular space of their plant hosts; however, they do not appear to cause any disease symptoms [1]. Endophytes are species of microorganisms that are underutilized for the discovery of novel chemicals since they coexist asymptomatically with their hosts. They produce an array of metabolites and have the ability to produce substances that are separated from, and only produced by, higher plants [2,3]. When agrochemicals are applied poorly or excessively, phytopathogens become resistant and less susceptible [4]. Endophytes are biocontrol agents that can be utilized to control plant diseases and advance sustainable agriculture [5]. Trichoderma species have been the subject of extensive research and usage in biological control against phytopathogenic fungi due to their strong ability to produce significant amounts of enzymes and metabolites [6,7]. Trichoderma is a filamentous fungus with a wide range of uses in industry, agriculture, and the environment [8]. It has the benefit of producing a variety of bioactive metabolites and potential drug leads. Trichoderma is frequently used in agriculture as biofungicides and bioremediation agents because they protect the host plant throughout its entire life cycle and can therefore act as biocontrol agents [4,9,10]. Bio-efficient substances are an excellent source of potential novel therapies [11]. The demand for novel therapeutic and therapeutically beneficial chemicals is expected to continue to increase in order to face the challenges posed by rising antibiotic resistance [12]. The secondary metabolites have not been thoroughly or methodically assembled. To date, nearly 200 Trichoderma sp. compounds have been identified as terpenoids, polyketides, peptides, alkaloids, and lactones [7]. Furthermore, because Trichoderma species have a natural resistance to many agricultural agents, such as fungicides, they are integrated into pest management strategies [13].
In this field, few studies were interested in examining the potential antagonistic effects of Trichoderma hazianum in inhibiting the growth of the plant and pathogenic fungi [14], as the primary goal of these investigations was to protect the plant against pathogenic microorganisms, in particular the antifungal effects against Fusarium graminarium and Asper-gillus terreus [15]. Some species of this fungus have the ability to clean up contaminated environments. Trichoderma harzianum is one of the various methods for decreasing the detrimental effects of heavy metals on plants [16]. Sesquiterpenes and diterpenes isolated from Trichoderma species have been shown to exhibit anti-microbial, anti-microalgae, anti-cancer, and phytotoxic properties [17].
A wide class of carbon-based substances known as fungal volatile organic compounds (VOCs) has low molecular weights, low polarity, low boiling temperatures, and high vapor pressure [18]. These substances are frequently lipophilic and include alcohols, benzenoids, aldehydes, alkenes, acids, esters, ketones, thiols, and their derivatives, among other chemical classes [19,20].
Aside from having few adverse effects and promising therapeutic applications, bio-efficient natural compounds are a promising source of new antioxidants and antibacterial agents [11]. This study aims to investigate the bio-efficiency of the secondary metabolites of T. harzianum by using phytochemical analysis, TLC, and GC-MS. Furthermore, this study focuses on identifying the compounds’ capacity for antioxidant and antifungal activities as well as any potential advantages and applications.
2. Results
T. harzianum was grown and dried, and two extracts were prepared using 79.75 g of the fine powder: ethyl acetate, yielding 1.81% of the extract and n-butanol, yielding 1.17%.
2.1. Phytochemical Analysis
Phytochemical analysis was carried out on T. harzianum ethyl acetate and n-butanol extracts; a noticeable difference in the contents is shown in Table 1. A high tannin content of 1584.16 mg TAE (Tannic acid equivalent)/g DE for ethyl extract and 2192.5 mg TAE/g DE for n-butanol extract, followed by flavonoids of 266.18 mg QE (Quercetin equivalent)/g DE and 203.62 mg QE/g DE for ethyl extract and n-butanol extract, respectively. Phenolic content was estimated to be 70.54 mg GAE (Gallic acid equivalent)/g DE for ethyl acetate extract and 40.12 mg GAE/g DE for n-butanol extract. The alkaloid content is considered to be the lowest in our analysis, as we noted 0.83 mg NE (Nicotine equivalent)/g DE for ethyl acetate and 0.77 mg NE/g DE for n-butanol extract.
Table 1.
Phytochemical analysis of T. harzianum extracts.
| Ethyl Acetate Extract | n-butanol Extract | Curve Equation | R2 | |
|---|---|---|---|---|
| Total phenolics (mg GAE/g DE) |
70.54 ± 5.92 | 40.12 ± 1.21 | ABS = 0.009x + 0.194 | 0.999 |
| Total flavonoids (mg QE/g DE) |
266.18 ± 15.11 | 203.62 ± 4.28 | ABS = 0.001x + 0.031 | 0.996 |
| Total alkaloids (mg NE/g DE) |
0.83 ± 0.11 | 0.77 ± 0.10 | ABS = 0.0441x + 0.1002 | 0.999 |
| Total tannins (mg TAE/g DE) |
1584.16 ± 407.22 | 2192.5 ± 50 | ABS = 4 × 10−5x + 0.039 | 0.995 |
GAE: Gallic acide equivalent. QE: Quercetin equivalent. NE: Nicotine equivalent. TAE: Tannic acid equivalent. DE: Dry extract.
ANOVA analysis results show that the amount of secondary metabolites in ethyl acetate extract showed significant differences between groups F (40.028) and p < 0.0001. Multiple comparisons using a Tukey test indicated that there is a significant difference for the polyphenol, while the other variables (alkaloid, tannin, and flavonoid) do not show a significant difference between the subsets. The n-butanol showed significant differences between the groups F (340.98) and p < 0.0001. Multiple comparisons using Tukey’s HSD test indicate that there is also a significant difference between the groups for the variable sub-homogeneous sets. In this case, there are three subsets (1, 2, and 3) for which comparisons were made. The groups show significant variations in terms of this variable (polyphenol alkaloid, tannin, and flavonoid).
2.2. Thin Layer Chromatography Analysis (TLC)
Thin-layer chromatography was performed for T. harzianum ethyl acetate and n-butanol extracts. Several patterns of composition were determined based on the presence or absence of discriminant spots in the first visual inspection of the fungal extract.
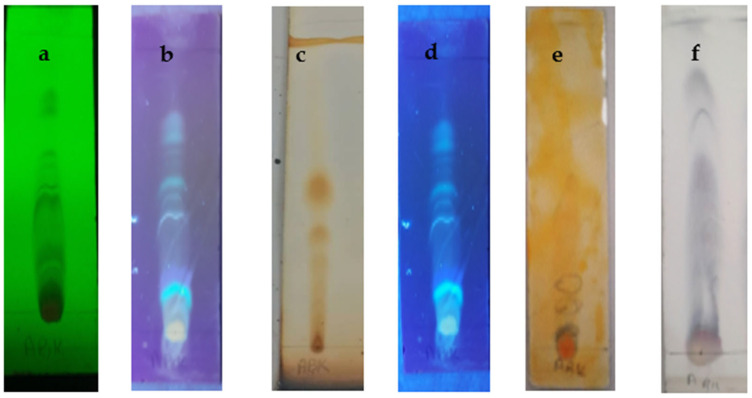
TLC profiles of ethyl acetate extract and the results of spraying with Vanillin/Sulfuric acid, Aluminum AlCl3, Iodine, and the Dragendroff test (UV 245 and 365 nm) are illustrated in Figure 1. Nine fractions were identified (F1-F9) by the Vanillin/Sulfuric test (Figure 1f), characterized by an Rf: 0.05 and 0.08 with orange color and 0.13, 0.2, 0.33, 0.36, 0.41, 0.5, and 0.7 with blue color, indicating a wide color range that shows the presence of different compounds (carbonyl compounds). Eight fractions were identified (F1–F8) by the flavonoid type aluminum test and are characterized by an Rf of 0.05 with an orange color and an Rf of 0.08, 0.13, 0.2, 0.41, 0.5, 0.62, and 0.7 with a similar blue color under UV (365) (Figure 1b). After the Dragendroff test in the system was used (Figure 1e), we noticed the presence of three fractions, including an Rf of 0.05 and 0.2 blue and an Rf of 0.13 yellow, which indicates the presence of alkaloids.
Figure 1.
Thin layer chromatography (TLC) of n-butanol of T. harzianum. (a): Under UV (254); (b): Under UV (365); (c): Iodine reagent; (d): Aluminum AlCl3; (e): Dragendroff reagent; (f) Vanillin/Sulfuric acid reagent.
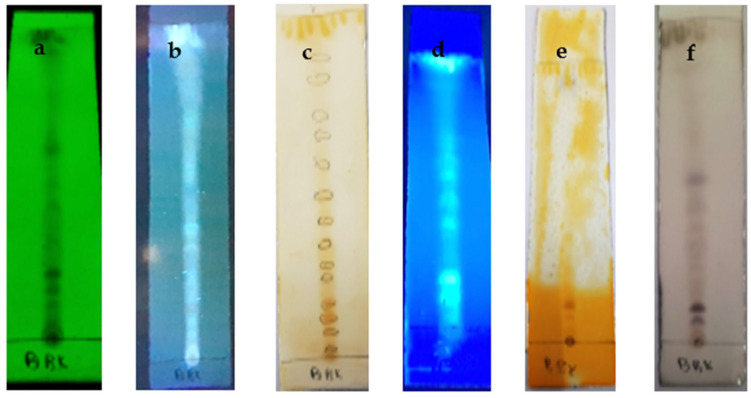
According to the results of the TLC plates of n-butanol extract, we noted the Vanillin/Sulfuric reagent (Figure 2f) presents 11 spots characterized by an Rf of 0.6, 0.11, 0.23, 0.4, 0.5, and 0.83 with a similar color blue. An Rf of 0.15, 0.26, or 0.57 is a yellow color. An Rf between 0.33 and 0.63 is a green color, denoting the existence of several compounds (carbonyl compounds, ketones, and phenols). The aluminum reagent presents 8 spots (Figure 2d) characterized by an Rf of 0.11, 0.4 orange color, an Rf of 0.26, and 0.34 blue color, and an Rf of 0.46, 0.5, 0.67, and 0.72 yellow color. This indicates the presence of flavonoids. The Dragendroff reagent (Figure 2e) presents three spots with an Rf of 0.09 and 0.77 in blue and an Rf of 0.069 in green.
Figure 2.
Thin layer chromatography (TLC) of n-butanol of T. harzianum. (a): Under UV (254); (b): Under UV (365); (c): Iodine reagent; (d): Aluminum AlCl3; (e): Dragendroff reagent; (f): Vanillin/Sulfuric acid reagent.
2.3. GC-MS Analysis
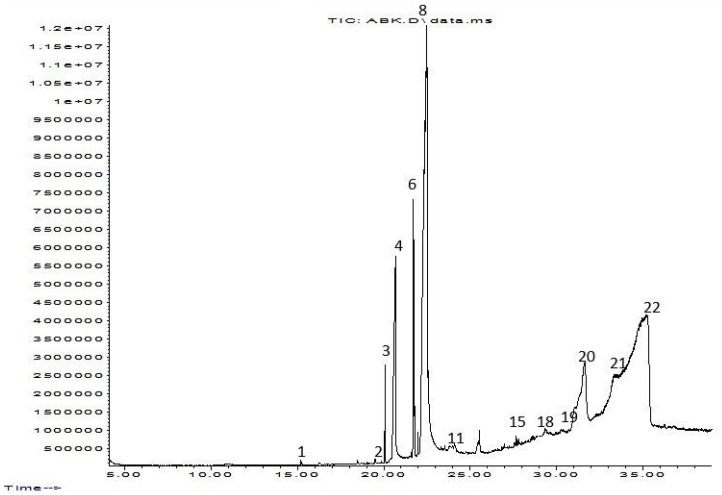
T. harzianum extracts by ethyl acetate and n-butanol were subjected to GC-MS examination. The investigations revealed an array of biomolecules (Table 2 and Table 3), and a GC-MS chromatogram of ethyl acetate extract showed 27 peaks, as shown in Figure 3. The primary components were s-Triazine trichloride (33.24%), Linoleic acid (26.95%), Ethylene sulfate (11.66%), 5-(Dimethylamino)-3,4-dihydro-4-isopropyl-4-methyl-2H-imidazol-2- one (9.90%), and Palmitinic acid (5.86%).
Table 2.
Chemical composition of ethyl acetate extract of T. harzianum by GC-MS.
| N° | RT | Compound | Structure | Molecular Formula | MW g/mol |
Peak Area % | Compound Nature |
|---|---|---|---|---|---|---|---|
| 1 | 15.201 | Massoialactone |
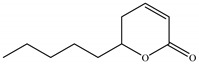
|
C10H16O2 | 168.23 | 0.05 | Pyranone |
| 2 | 19.511 | Pentadecanoic acid |
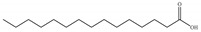
|
C15H30O2 | 242.39 | 0.06 | Fatty Acid |
| 3 | 20.071 | Palmitic acid, methyl ester |
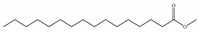
|
C17H34O2 | 270.45 | 0.59 | Fatty Acid |
| 4 | 20.654 | Palmitinic acid |
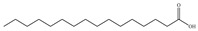
|
C16H32O2 | 256.4241 | 5.86 | Fatty Acid |
| 5 | 21.282 | Capric acid |
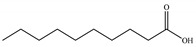
|
C10H20O2 | 172.26 | 0.14 | Fatty Acid |
| 6 | 21.717 | Methyl linolelaidate |

|
C19H34O2 | 294.48 | 3.23 | Fatty Acid |
| 7 | 21.974 | Methyl stearate |
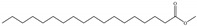
|
C19H38 O2 | 298.503 | 0.33 | Fatty Acid |
| 8 | 22.477 | Linoleic acid |

|
C18H32 O2 | 280.45 | 26.95 | Fatty Acid |
| 9 | 23.523 | 9,17-Octadecadienal, (Z) |

|
C18H32O | 264.44 | 0.73 | Aldehyde |
| 10 | 23.523 | 14-Methyl-8-hexadecyn-1-ol |
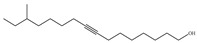
|
C17H32O | 252.44 | 0.46 | Fatty Alcohol |
| 11 | 24.089 | Ethyl linoleate |

|
C20H36O | 308.49 | 0.71 | Fatty Acid |
| 12 | 26.975 | (16S)-12,16-epoxy-6.beta.-hydroxy-17(15-16)-abeo-abieta-8,12-diene- 3,11,14-trione |
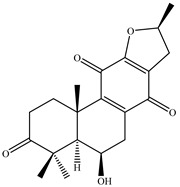
|
C20H24O5 | 344.41 | 0.20 | Ketone |
| 13 | 27.558 | Ethyl 2-(2-chloroacetamido)-3,3,3-trifluoro-2-(2-fluoroanilino) propionate |
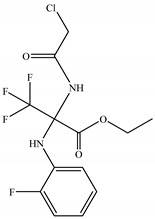
|
C13H13ClF4N2O3 | 356.70 | 1.58 | Ester |
| 14 | 27.667 | Allyl 2-Nitrophenylpyruvate Oxime |
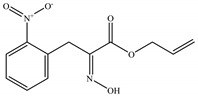
|
C12H12N2O5 | 264.24 | 0.16 | Ester |
| 15 | 27.804 | 3-Méthyl mercaptopropanal |
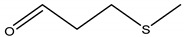
|
C4H8OS | 104.17 | 0.23 | Aldehyde |
| 16 | 28.610 | N(2)-[bis(hexafluoromethyl)methylene] oxamoyl hydrazide | / | / | / | 1.03 | Hydrazide |
| 17 | 28.833 | 1-Monolinoleoylglycerol trimethylsilyl ether |
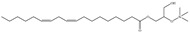
|
C24H46O4Si | 426.71 | 0.80 | Ester |
| 18 | 29.330 | 2-Methoxy-3-methylpyrazine |
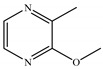
|
C6H8N2O | 124.14 | 0.98 | Pyrazine |
| 19 | 30.273 | 1-Monolinoleoylglycerol trimethylsilyl ether |
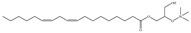
|
C24H46O4Si | 426.71 | 1.12 | Ester |
| 20 | 31.627 | 5-(Dimethylamino)-3,4-dihydro-4-isopropyl-4-methyl-2H-imidazol-2- one |
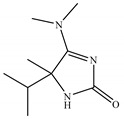
|
C9H17N3O | 183.26 | 9.90 | Imidazole |
| 21 | 33.348 | Ethylene sulfate |
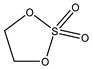
|
C2H4O4S | 124.11 | 11.66 | / |
| 22 | 35.200 | s-Triazine trichloride |
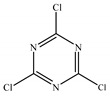
|
C3Cl3N3 | 184.40 | 33.24 | Triazine |
Table 3.
Chemical composition of n-butanol extract of T. harzianum by GC-MS.
| N° | RT | Compound | Structure | Molecular Formula | MW g/mol |
Peak Area % | Compound Nature |
|---|---|---|---|---|---|---|---|
| 1 | 20.52 | Palmitic acid |
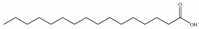
|
C16H32O2 | 256.42 | 2.72 | Fatty Acid |
| 2 | 21.70 | Ethyl linoleate |

|
C20H36O2 | 308.51 | 0.44 | Fatty Acid |
| 3 | 22.19 | Linoleic acid |
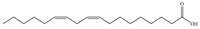
|
C20H36O2 | 308.50 | 14.05 | Fatty Acid |
| 4 | 1 | (Z)-11-Hexadecenal |
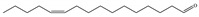
|
C16H30O | 238.40 | 1.54 | Aldehyde |
| 5 | 23.62 | Biperiden |
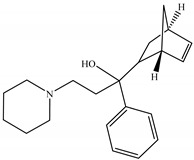
|
C21H29NO | 311.46 | 0.54 | Alcohol |
| 6 | 27.25 | Erucylamide |

|
C22H43NO | 337.58 | 1.43 | Fatty amide |
| 7 | 27.45 | 6-nitro-1H-indazole-4-carboxylic acid |
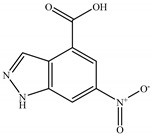
|
C8H5N3O4 | 207.15 | 2.26 | Fatty Acid |
| 8 | 28.007 | Monolinolein TMS |
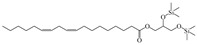
|
C27H54O4Si2 | 498.88 | 1.47 | Ester |
| 9 | 29.33 | Ethyl 2-(2-chloroacetamido)-3,3,3-trifluoro-2-(3-fluoroanilino)propionate |
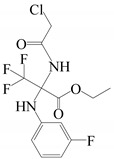
|
C13H13ClF4N2O3 | 356.70 | 1.13 | Ester |
| 10 | 31.91 | Glyceryl 1-oleate, diacetate |
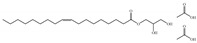
|
C25H48O8 | 476.65 | 5.23 | Fatty Acid |
Figure 3.
Chromatography–mass spectrometry (GC-MS) separation chromatograms for ethyl acetate extract of T. harzianum.
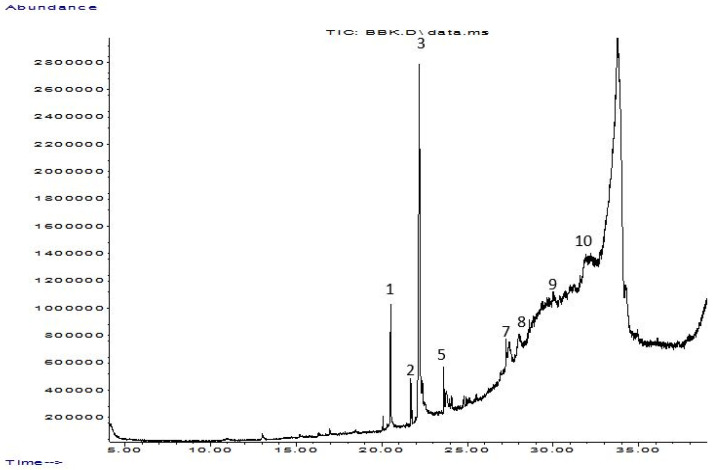
Regarding the n-butanol extract, it presented 10 peaks as shown in the chromatogram (Figure 4). The main constituents were Ethyl linoleate 14.05%, Glyceryl 1-oleate, diacetate 5.23%, Palmitic acid 2.72%,1H-Indazole-5-carboxylic acid, 6-nitro- 2.26%, and (Z)-11-Hexadecenal 1.54%.
Figure 4.
Chromatography–mass spectrometry (GC-MS) separation chromatograms for n-butanol extract of T. harzianum.
2.4. Antioxidant Activity
DPPH radical scavenging assays were used to evaluate the antioxidant activity of T. harzianum ethyl acetate and n-butanol extracts in different concentrations. Our results (Table 4) demonstrate that the IC50 obtained was higher than that of ascorbic acid (0.265 mg/mL), where we noted the IC50 and the inhibition of ethyl acetate extract were 7.147 mg/mL, which was higher than the n-butanol extract with an IC50 of 5.415 mg/mL and 56.01%.
Table 4.
Inhibition percentage IC50 of ethyl acetate and n-butanol mL extracts of T. harzianum.
| DPPH Assays | IC50 mg/mL |
|---|---|
| Ethyl acetate extract | 7.147 ± 0.181 |
| n-butanolextarct | 5.415 ± 0.238 |
| Ascorbic acid | 0.265 ± 0.007 |
±: standard deviation.
2.5. Antifungal Activity
The results of the antifungal activity of T. harzianum in ethyl acetate and n-butanol extracts are presented in Table 5. Both extracts were shown to have inhibitory activity on all tested pathogens in comparison with the control. In particular, we noted a strong activity of n-butanol extract against Sclerotinia sclerotiorum at 93.70%; however, we noted 58% against Alternaria sp., and ethyl acetate extract was more effective against Alternaria sp. with 77.04% and 51.48% against Fusarium solani.
Table 5.
Inhibitory activity of T. harzianum extracts against pathogenic fungi.
| Extracts | Concentration | Pathogenic Fungi % | ||
|---|---|---|---|---|
| Alternaria sp. | Fusarium solani | Sclerotinia sclerotiorum | ||
| Ethyl acetate extract | 100% | 77.04 ± 0.83 (a) | 51.48 ± 1.09 (b) | ND |
| 50% | 71.48 ± 3.12 (a) | 9.63 ± 0.39 (c) | ND | |
| 25% | 44.07 ± 1.7 (b) | 1.85 ± 0.17 (c) | ND | |
| n-butanol extract | 100% | 58 ± 10.13 (a) | ND | 93.70 ± 0.02 (a) |
| 50% | 64 ± 5.05 (b) | ND | 94.44 ± 0.01 (a) | |
| 25% | 68 ± 12.72 (b) | ND | 61.48 ± 2.38 (b) | |
| Fungicide (Fosetyl-Alumium 310 g/L) |
100% | 55 ± 0.80 | 35 ± 0.9 | 60 ± 1.41 |
| 50% | 15 ± 0.25 | 31 ± 0.88 | 42 ± 1.55 | |
| 25% | 0 | 0 | 0 | |
ND; not determined. The letters a, b, and c indicate the significant differences between the groups, according to Tukey’s multiple comparison test with a level of significance of 0.05. Means with the same letter are not significantly different.
Based on the results presented in Table 5, the results of the normality test of ethyl acetate against Alternaria sp. and Fusarium solani show an abnormal distribution, as the p-values for all tests are less than 0.05. The Dunn test for independent samples proved that the null hypothesis was rejected. We have the difference between the two groups as indicated by the results reported in Sig. ajus., and add to that the differences between an average of 100% and 25%.
For the statistical analysis of n-butanol extract against Alternaria sp., the data follow the normal distribution. Based on Tukey’s HSD test (p < 0.05), it can be concluded that there are significant differences in the means of the groups.
The results suggest that the data for Sclerotinia sclerotiorum are not normally distributed. Based on the Dunn test with p-values less than 0.05, the null hypothesis is rejected. We have the difference between the two groups as indicated by the results reported in Sig. ajus., and add to that the differences between an average of 100% and the control.
3. Discussion
Trichoderma species have a major impact on the production of secondary metabolites, which offer specific benefits in processes including competition, symbiosis, metal transport, growth differentiation, signaling, and mycoparasitic activity [21]. The present study investigated the potentiality of T. harzianum secondary metabolites and phytochemical analysis such as polyphenols, flavonoids, alkaloids, and tannins for ethyl acetate and n-butanol extracts of varying polarities. Da Silva et al. [22] noted polyphenols for T. longibrachiatum in an ethyl acetate extract of 103.62 mg g−1 and in an n-butanol extract of 140.07 mg g−1 in their study on flavonoids in ethyl acetate (105.07 mg g−1) and n-butanol (162.81 mg g−1). Our findings from this investigation on secondary metabolites were higher than those reported by [23], where he noted 3.85 ± 0.04 mg g−1 on polyphenol and 36.54 ± 3.17 mg g−1 on flavonoids, whereas the total phenolic compounds and total flavonoids content of T. harzianum were estimated to be 12.18 and 6.33 mg QE/100 mL, respectively. According to [24], Trichoderma metabolites have displayed beneficial effects on plants, increasing plant growth and development and inducing defense responses to abiotic stresses and pathogens [25]. The presence of flavonoids and phenolic compounds in the extract is associated with the growth of Trichoderma sp. [26]. In response to Trichoderma species, phenolic compound accumulation has been linked to biochemical defense against plant diseases. Furthermore, the increased synthesis of phenols and flavonoids has a direct effect on antioxidant activity by acting as free radical scavengers and contributing to cell wall formation, which defends plants from instances of biotic stress [27,28,29]. Flavonoids serve as endogenous regulators of auxin movements and mediate developmental regulation [30,31]. Moreover, polyphenolic compounds have been recognized to possess pharmacological properties such as antioxidative, hepatoprotective, antibacterial, anti-inflammatory, anticancer, and potential antiviral properties [32]. Polyphenolic substances known as tannins and alkaloids have astringent, diuretic, anti-inflammatory, antiseptic, antioxidant, and hemostatic characteristics. The treatment of gastric and duodenal cancers is another application for them [33,34]. The presence of alkaloids and tannins was confirmed by [35] for Trichoderma sp.; however, [36] noted the presence of tannins and the lack of alkaloids for T. harzianum. Ref. [37] reported that T. harzianum and T. viren both contain only alkaloid compounds. Ecologically, the accumulation of alkaloids is an important chemical defensive strategy used by plants to adapt to environmental stresses such as endophytes, pathogens, and herbivores [38,39].
Thin-layer chromatography was performed for ethyl acetate and n-butanol extracts of T. harzianum. The sequential extraction of the extract obtained revealed 1.81% and 1.17% extraction percentages, respectively. The presence of several bioactive biomolecules was detected using thin-layer chromatography in T. harzianum. Spraying with different reagents showed a wide range of colors that denote the presence of various compounds (carbonylated compositions). The TLC profile suggests interesting chemical compositions that have bioactive compounds related to abiotic activities present in the ethyl acetate and n-butanol extracts.
In the present study, T. harzianum extracts were subjected to Chromatography-mass spectrometry (GC-MS), showing a profile of secondary metabolites that covers all the numerous substances that a fungus may make on a certain substrate, including antibiotics and other outwardly directed substances [40]. 22 compounds were identified in ethyl acetate extracts and 11 in n-butanol extracts. The major compounds are fatty acid, ester, aldehyde, hydrazide, pyrazine, imidazole, triazine, and fatty amide. A few of the compounds found were similar between the two extracts.
Researchers have found that a large number of fatty acids have antimicrobial activities and the potential for medicine in nutritional therapy [41]. Some fatty acids have the potential for antituberculosis therapy [42]. Linolenic acid, linoleic acid, and oleic acid possess substantial activity against R. solani. In recent work [13], the antibacterial activity of linolenic acid was demonstrated, while [43] showed that linoleic acid and oleic acid possessed insecticidal activity against the fourth-instar Aedes aegypti larvae. Recently, linoleic acid and glycerol monolinolate have been reported to have sporogenic activities, enhancing the asexual spores of Alternaria tomato [44] and Sclerotinia fructicola [45], respectively. Thus, linoleic acid and its congeners may have important regulatory roles in sexual as well as asexual reproductive processes in fungi [46]. Microorganism extracts are considered another alternative to traditional fungicides and pesticides as they produce bioagents that are effective against bacteria and fungi. Ref. [1] mentioned that T. harzianum can be used as a biopesticide against different insect pests, and [47] reported that the compounds Hexadecenoic acid and 7,10-Octadecadienoic acid can be characterized as pesticides, nematicides, and insecticides. The efficacy of pentadecanal and pentadecanoic acids as anti-biofilm agents has been recently reported against different bacterial strains, and [48] reported that Glyceryl 1-oleate and diacetate have antifungal and antimicrobial activity.
An ethyl acetate extract of T. harzianum showed the presence of the compound massoia lactone, characterized as a new type of biosurfactant that can be produced by Aureobasidiumpullulans [49], Cyberlindnera samutprakarnensis [50], and Candida sp. [51]. Some biosurfactants are also biologically active compounds with antifungal, antitumor, and anticancer proliferative activities [52]. Massoia lactone has strong anti-fungal activity and many modes of action and may be a good option for development as an efficient and environmentally friendly bio-fungicide [53].
Pyrazines are aromatic heterocyclic nitrogen-containing compounds that are important flavoring agents in many raw and roasted food products. Most alkyl pyrazines found in food result from the condensation of aldehydes. Pyrazines are also found to be produced by a wide variety of insects and play a role as pheromones [54]. For instance, some fungi imitate flowers to draw in insects that act as vectors for the spread of fungi [55]. Due to the aromatic properties of pyrazines, they have many uses in the flavor and fragrance industries [56], and 2-methoxy-3-butylpyrazine can be used as an ingredient in various perfumes [57].
Searching for strains capable of producing active ester hydrolases such as ethylene glycol against PET films is an important step in worldwide recycling [58]. Producers such as Trichoderma [59], Aspergillus [60], Penicillium [61], Alternaria [62], and Fusarium [52] are widely used in biotechnological applications and organic chemistry. Their use as a model for plastic biodegradation and chemical analyses has been reported in many studies [63,64].
Modern heterocyclic chemistry relies on the synthesis, reactions, and biological characteristics of substituted imidazoles. Compounds possessing an imidazole ring system have been found to exhibit a number of pharmacological properties, such as anticancer [65], carboxypeptidase [66], anti-aging [67], anti-fungal [68], anti-bacterial [69], anti-diabetic [70], and anti-malarial [71].
Given its broad spectrum of biological applications, s-triazine has attracted a considerable amount of attention from chemists due to its rich source of pharmacological activities, including antibacterial [72,73,74], antimalarial [73], antiprotozoal [75], antifungal [76], anticancer [77], antimycobacterial [78], and antiviral [79].
Regarding the activity shown by the compounds, it is known that secondary metabolites possessing aldehyde groups, especially unsaturated aldehydes, are bioactive and were found to be inhibitory to seed germination [80,81], pollen germination [82], pathogenic fungi [83], and bacteria [84].
In addition, hydrazide derivatives are available in numerous bioactive atoms and show a wide variety of biological activities. Hydrazide has been demonstrated to possess antibacterial [85], anticancer [86,87], antitubercular [88,89], anti-inflammatory [90], and antifungal activity [91].
Strategies can be developed to use these fungi for the exploitation of bioactive compounds. In addition, the use of endophytes as potential factories for the production of secondary metabolites might revolutionize agricultural, pharmaceutical, and biotechnological research in the near future [92].
An n-butanol extract of T. harzianum showed the presence of (Z)-11-Hexadecenal. Ref. [93] mentioned that (Z)-11-hexadecenal, a major component of M. separata sex pheromone, was found to attract early-instar larvae of M. separata, and this could aid in the development of olfaction-based methods for controlling M. separata crop damage in the larval stage. The use of (Z)-11-Hexadecenal pheromone is expected to reduce the use of chemical pesticides in C. perspectalis [94]. Numerous studies have mentioned biperiden as an anticholinergic drug [95].
Erucylamide is a known compound with recognized antimicrobial activity [96]. The antimicrobial effect of (Z)-docos-13-enamide, one of the most abundant constituents detected in the studied fractions known as erucamide, has been studied by [97], who detected the formation of hydrogen bridges between erucamide and amino acid residues of tubulin and glucosamine-6-P synthase, which could explain their anthelmintic and antibacterial actions.
The compounds without traceable or known biological activity may be novel ones that should be further investigated to reveal their functions. The varying biological activities of the bioactive compounds may account for the treatment of health disorders such as high blood pressure, diabetes, asthma, fever, and cancer [98].
In the present study, the ethyl acetate and n-butanol extracts of T. harzianum were evaluated for antifungal activity against three phytopathogenic fungi: Sclerotinia sclerotiorum, Alternaria sp., and Fusarium solani. Similar to our results, Trichoerma species have been used with efficacy to treat plant diseases brought on by Fusarium [99], Alternaria, and Sclerotium [100]. The potency of metabolites derived from Trichoderma species as antifungal agents against plant diseases was reported by Živković et al. [101]. For example, they inhibited Fusarium solani (74.4%), Alternaria solani (70.0%), Pythium aphanidermatum (67.7%), and Macrophomina phaseolina (50.0%). The bioactivity of the sample secondary metabolites from T. harzianum shows that all four Trichoderma species significantly inhibited the mycelial growth of the four pathogens, Sclerotinia sclerotiorum [102]. Strains of Trichoderma such as T. harzianum, T. hamatum, T. asperellum, and T. atroviride are applied for the control of phytopathogens and also as plant growth promoters in agriculture and inhibit mycelia growth as well [103]. The metabolite of T. harzianum produced in agar culture inhibited the growth of all 3 pathogenic fungi tested in vitro (Table 1). Trichoderma species are known to produce a number of antibiotics, such as trichodermin, trichodermol, harzianum A, and harzianolide [104]. Our study demonstrated the involvement of volatile metabolites in the inhibition of pathogenic fungi. The secondary metabolites produced by the fungal strains have broken down into various classes of antifungal compounds and contribute to antifungal activity, as shown in the GC-MS analysis of the two extracts. Our results implicated that the content of phenols and flavonoids, as well as the productivity of these aforementioned compounds by T. harzianum, could be responsible for the anti-fungal activity, modulators of pathogenicity, and activators of plant defense [26].
The antioxidant activity of ethyl acetate and n-butanol in T. harzianum was studied. Correspondingly, the antioxidant activity of T. longibrachiatum in the ethyl acetate extract was estimated to be 3.77 mg g−1 and in the butanoic extract, 304.18 mg g−1 [22]. However, [105] noted an antioxidant activity of 54.61% for the ethyl acetate extract of Trichoderma sp., whereas [51] noted 72.72% for T. longibrachiatum and 53.43% for T. subviride. The extracts obtained from T. harzianum exhibit strong antioxidant activity [106]. The antioxidant activity of the stable radical DPPH demonstrates the ability of molecules from the fungal extracts to scavenge these radicals. The high activity can be linked to the presence of numerous secondary metabolites, as shown in the phytochemical and GC-MS analyses [107,108,109]. Secondary metabolite analysis provides information for developing new pharmacological agents that can act as antioxidants. These active compounds can be used as sources of natural antioxidants and replace extraction from actual plants. The majority of antioxidants used today are produced industrially, although they are responsible for liver damage and carcinogenesis [110]. Contrarily, antioxidants of natural origin, such as those created by endophytes, have not been proven dangerous, particularly because of the rich diversity of life and the evolution of biochemistry [111].
4. Material and Methods
4.1. Chemical and Fungi Material
All chemical material and endophytic fungi were provided by the Biopesticides Laboratory, INRAA, Touggourt. The chemicals used are n-butanol, ethyl acetate, Folin-Ciocalteu reagent, sodium carbonate, sodium nitrite, aluminum chloride, hydrochloric acid, vanillin, phosphate buffer, BCG solution, chloroform, Hexane, acetic acid, Dragendroff, dimethyl sulfoxide (DMSO), 1, 1-diphenyl-2-picryl-hydrazyl (DPPH), and methanol.
The endophytic fungi evaluated in this study are T. harzianum, which was isolated from soil collected from the southeast of Algeria in Sidi Mehdi (33°4′18.27″ N 6°5′43.14″ E). Three pathogenic fungi, Sclerotinia sclerotiorum and Alternaria sp., were isolated from Solanum tuberosum l and collected from Ouadsouf (33°22′4.12″ N 6°51′5.91″ E), and Fusarium solani was isolated from Solanum lycopersicum collected from Touggourt (33°06′00″ N 6° 04′00″ E).
4.2. Extaction Method
The extraction of secondary metabolites from T. harzianum was done using the modified method of [112]. After the cultivation of fungus under fermentation conditions in a liquid medium of PDB for 14 days, the mycelia were separated from the broth through vacuum filtration. After drying, the mycelia were placed in Soxhlet, n-butanol, and ethyl acetate extraction was performed for 2 h, then the extract was evaporated by a rotary evaporator and stored until use.
4.3. Phytochemical Screening
Phytochemical analyses of polyphenols, flavonoids, alkaloids, and tannins were performed for ethyl acetate and n-butanol extracts of T. harzianum. All tests were done in triplicate [113]. All analyses were performed in the Biopesticides Laboratory, INRAA, Touggourt.
4.3.1. Quantification of Polyphenol Content
The Folin—Ciocalteu reagent was used to detect total polyphenols spectrophotometrically using the colorimetric technique [114]. This evaluation is based on a measurement of the total number of hydroxyl groups present in the extract. In a nutshell, 200 µL of each extract, 800 µL of a 7.5% sodium carbonate solution, and a combination of 1 mL of reactive Folin-Ciocalteu that had been diluted 10 times were added to glass hemolyze tubes. For 30 min, the tubes were swirled and held. The absorption was focused at 765 nm. In parallel, a series of etaloning tests using various concentrations of acetic acid (0 to 1000 g/mL) were carried out under the same operating conditions [115].
4.3.2. Quantification of Flavonoids Content
A method for measuring flavonoids was used based on the aluminum chloride and oxygen atmospheres found on carbons 4 and 5 of the flavonoids coming together to form a very stable combination [116]. The procedure utilized, with a few modifications, is based on those explained by Zhishen et al. [117] and Kim et al. [118]. In a glass hemolysis tube, 120 μL of NaNO2 at 5% was mixed with 400 μL of extract, etalon, or distillate water for reference. 120 µL of 10% AlCl3 was added after 5 min, and the mixture was thoroughly mixed. After 6 min, 800 μL of NaOH at 1 M was added to the solution. The absorbance was measured immediately at 510 nm against the reference. A quercetin methyl ester solution was prepared. The etaloning curve can be tracked using various solutions derived from the mother solution that range in concentration from 0 to 1000 g/mL.
4.3.3. Quantification of Tannis Content
We combined the HCl approach with the vanillin method. Tanins’ capacity to transform into red anthocyanidols by interaction with vanillin explains how this approach depends on the reactivity of vanillin with the terminal grouping of the TCs’ flavonoids and the generation of red complexes [119,120]. The vanillin technique published by [121] was used to determine the concentration of condensed tannins. 1500 μL of the vanillin/methanol solution at 1% were added with 50 μL of each extract, and the mixture was stirred well. Hydrochloric acid (HCl) at a concentration of 4% was then added in a volume of 750 μL. The completed combination was allowed to rest for 20 min at room temperature. The absorbance was measured at 550 nm. Different concentrations ranging from 0 to 1000 g/mL prepared from the catalytic mother solution will enable the tracing of the etaloning curve.
4.3.4. Quantification of Alkaloids Content
Accurately measured aliquots (0.4, 0.6, 0.8, 1, and 1.2 mL) of fungi extract were transferred to different separatory funnels. Then 5 mL of pH 4.7 phosphate buffer and 5 mL of bromocresol green were taken, and the mixture was shaken with extracts of 1, 2, 3, and 4 mL of chloroform. The extracts were then collected in a 10 mL volumetric flask and then diluted to adjust the solution with chloroform. The absorbance of the complex in chloroform was measured at 470 nm in a UV-spectrophotometer (SHIMADZU UV-1800, Kyoto, Japan) against the blank prepared as above but without a standard [122].
4.4. Thin Layer Chromatography
Apart from gas chromatography, thin-layer chromatography (TLC) is the most efficient technique for separating and identifying fungus composition. When a fraction is investigated by TLC, it is possible that a fraction that seemed homogenous by gas chromatography really included many components [123].
Both ethyl acetate and n-butanol extracts were examined by TLC using the solvent systems: for the ethyl acetate extract, Chloroform/Hexane/acetic acid (8:2:0.1) and for the n-butanol extract, n-butanol/water/acetic acid (4:2:0.1) on silica gel G plates (20 × 10 cm). One set of duplicated TLC plates served as the reference chromatogram. Spots and bands were visualized by UV irradiation (254 and 365 nm) and by spraying with the following reagents: Vanillin/Sulfuric acid, Aluminum AlCl3, and Dragendroff [124]. They are characterized by a retention factor (Rf).
4.5. Gas Chromatography-Mass Spectroscopy GC-MS
Separation of hydrocarbons and other volatile compounds from ethyl acetate and n-butanol extracts of T. harzianum was determined with a GC (C.R.A.P.C., Bou-Ismail, Algeria). Chromatograph: Hewlett-Packard Agilent 6890 plus Mass spectrometer: Hewlett Packard Agilent, CA, USA. GC–MS analyses were done with an ionization energy of 70 eV.
The putative identification of volatile metabolites was performed by three different chromatographic runs using three different capillary columns with different stationary phases. The putative identification of volatile metabolites was performed in capillary columns with different stationary phases. With a non-polar column (HP-5MS) of 30 m, 0.25 mm, and 0.25 µm, the oven program had an initial temperature of 60 °C for 5 min, 10 °C/min up to 300 °C, and isotherm for 10 min; injector temperature was kept at 250 °C (splitless), and detector temperature was at 280 °C.
4.6. Antifungal Activity
The Poison Food Technique method was used to assess the antifungal activity. The activity of the two extracts was evaluated against three pathogens: Sclerotinia sclerotiorum, Alternaria sp., and Fusarium solani. Each extract was reconstituted (4 mg/mL) in dimethyl sulfoxide (DMSO) in 20 mL PDA, and a 6 mm pathogen disc was put in the center of each Petri plate. The plates were then incubated at 27 °C until the control plate reached the edges. The diameters of the inhibition zones were measured in centimeters. The activity was performed in triplicate, and all treated plates were compared with the controls to calculate the inhibition percentage of growth of T. harzianum [125].
The minimal inhibitory concentration (MIC) was defined as the lowest concentration, determined by performing a series of dilutions of 100%, 50%, and 25%. The lowest concentrations showing a clear zone of inhibition were taken as the MIC [126].
Mycelia growth was monitored by measuring colony diameter in centimeters. The inhibition percentage of mycelia growth is calculated by the following formula [127]:
| Inhibition percentage I% = (C1 − C2)/C1 × 100 |
C1: Diametrical growth of the control.
C2: Diametrical growth of the fungus in the presence of a precise concentration (C) of the extract.
4.7. Antioxidant Activity
The free radical scavenging activities were assessed for ethyl acetate and n-butanol extracts of T. harzianum, and the extracts were measured using 1,1-diphenyl-2-picryl-hydrazyl (DPPH). Serial dilutions were made to check the IC50. An extract concentration of 0.1 mg/mL−1 of endophytic crude extract dissolved in methanol (75 μL) was mixed with 250 μL of a methanolic solution containing 1,1-diphenyl-2-picrylhydrazyl (DPPH, Sigma, St. Louis, MO, USA). radicals obtained from a fresh DPPH solution that was prepared by mixing 24 mg of DPPH in 100 mL of methanol and storing it at 20 °C before use. After aggressively shaking the mixture and letting it stand in the dark for 30 min, the absorbance was measured at 517 nm. Ascorbic acid was used as the standard antioxidant. All readings were taken in triplicate [128]. The following formula was used to determine the capacity to scavenge the DPPH radical equation:
| DPPH scavenging (%) = (A0 − A1)/A0 × 100 |
A0: Absorance of the control reaction.
A1: Absorance in the presence of the sample.
4.8. Statistical Analysis
In a statistical descriptive study of the inhibition rate of fungi, the key characteristics of a dataset that includes measurements of the inhibition rate of fungi are summarized and described [22]. The software IBM SPSS Statistics (Statistical Package for the Social Sciences) v.24 for Windows was used to perform parametric (ANOVA followed by Tukey’s HSD test) and non-parametric (Kruskal–Wallis followed by Dunn test) tests [129,130,131]. All the series were first checked for normality (Kolmogorov–Smirnov test) and equality of variance (Bartlett test) to decide which tests were more appropriate.
5. Conclusions
T. harzianum biosynthesizes biopotent products and has varied nutritional, industrial, and medical applications. A gas chromatography-mass spectroscopy examination demonstrates the presence of 33 compounds in total. The present study shows a variety of bioactive compounds in T. harzianum extracts that provide beneficial effects. Furthermore, it possesses the distinctive ability to produce bioactive secondary metabolites for human use as proficient therapeutic agents against various diseases. Ethyl acetate and n-butanol extracts demonstrated different levels of polyphenols, flavonoids, and alkaloids. The antifungal inhibitory effect of ethyl acetate and n-butanol extracts obtained in this study against pathogenic fungi might be a result of antifungal antibiotics present in secondary metabolites. T. harzianum possesses the advantage of large-scale production of diverse bioactive metabolites and potential drug leads, which is not always possible in plants. They are widely used in agriculture as biofungicides and bioremediation agents.
Acknowledgments
The authors extend their appreciation to the Princess Nourah bint Abdulrahman University Researchers Supporting Project Number (PNURSP2023R47), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Author Contributions
Writing—original draft, W.L. and I.B.; validation and writing—original draft, M.M.B. and H.B. (Hamdi Bendif); literature investigation, H.K. (Hafida Khelafi) and H.B. (Hakim Bachir); review and editing, A.L. and D.M.; funding acquisition, review, and editing, T.S.A. and N.A.; literature investigation and formal analysis, H.H. and H.K. (Hanane Khelil); statistical analyses, N.B.; conceptualization, review, and editing, F.B.; conceptualization, supervision, review, and editing, A.D. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All the data in the article are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Sample Availability
Samples of all the compounds are available from the authors.
Funding Statement
The authors extend their appreciation to the Princess Nourah bint Abdulrahman University Researchers Supporting Project Number (PNURSP2023R47), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Ahmed A.M., El-Katatny M.H. Entomopathogenic Fungi as Biopesticides against the Egyptian Cotton Leaf Worm, Spodoptera littoralis: Between Biocontrol-Promise and Immune-Limitation. J. Egypt. Soc. Toxicol. 2007;37:39–51. [Google Scholar]
- 2.Schulz B., Boyle C., Draeger S., Römmert A.-K., Krohn K. Endophytic Fungi: A Source of Novel Biologically Active Secondary Metabolites. Mycol. Res. 2002;106:996–1004. doi: 10.1017/S0953756202006342. [DOI] [Google Scholar]
- 3.Nicoletti R., Fiorentino A. Plant Bioactive Metabolites and Drugs Produced by Endophytic Fungi of Spermatophyta. Agriculture. 2015;5:918–970. doi: 10.3390/agriculture5040918. [DOI] [Google Scholar]
- 4.Segaran G., Sathiavelu M. Fungal Endophytes: A Potent Biocontrol Agent and a Bioactive Metabolites Reservoir. Biocatal. Agric. Biotechnol. 2019;21:101284. doi: 10.1016/j.bcab.2019.101284. [DOI] [Google Scholar]
- 5.Peters L.P., Prado L.S., Silva F.I., Souza F.S., Carvalho C.M. Selection of Endophytes as Antagonists of Colletotrichum gloeosporioides in Açaí Palm. Biol. Control. 2020;150:104350. doi: 10.1016/j.biocontrol.2020.104350. [DOI] [Google Scholar]
- 6.Guo R., Li G., Zhang Z., Peng X. Structures and Biological Activities of Secondary Metabolites from Trichoderma harzianum. Mar. Drug. 2022;20:701. doi: 10.3390/md20110701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khan R.A.A., Najeeb S., Hussain S., Xie B., Li Y. Bioactive Secondary Metabolites from Trichoderma spp. against Phytopathogenic Fungi. Microorganisms. 2020;8:817. doi: 10.3390/microorganisms8060817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mukherjee P.K., Horwitz B.A., Singh U.S., Mala Mukherjee M.M., Schmoll M. Trichoderma: Biology and Applications. CABI; Botany, Australia: 2013. Trichoderma in Agriculture, Industry and Medicine: An Overview; pp. 1–9. [Google Scholar]
- 9.Adeleke B.S., Babalola O.O. Pharmacological Potential of Fungal Endophytes Associated with Medicinal Plants: A Review. J. Fungi. 2021;7:147. doi: 10.3390/jof7020147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sheeba H., Ali M.S., Anuradha V. Bioactive Compounds and Antimicrobial Activity of Fungal Crude Extract from Medicinal Plants. J. Pharm. Sci. Res. 2019;11:1826–1833. [Google Scholar]
- 11.Farag R., Swaby S. Antimicrobial Effects of Wasp (Vespa orientalis) Venom. Egypt. Pharm. J. 2018;17:218 [Google Scholar]
- 12.Moshikur R.M., Chowdhury M.R., Fujisawa H., Wakabayashi R., Moniruzzaman M., Goto M. Design and Characterization of Fatty Acid-Based Amino Acid Ester as a New “Green” Hydrophobic Ionic Liquid for Drug Delivery. ACS Sustain. Chem. Eng. 2020;8:13660–13671. doi: 10.1021/acssuschemeng.0c03419. [DOI] [Google Scholar]
- 13.Lee S., Yap M., Behringer G., Hung R., Bennett J.W. Volatile Organic Compounds Emitted by Trichoderma Species Mediate Plant Growth. Fungal Biol. Biotechnol. 2016;3:7. doi: 10.1186/s40694-016-0025-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abo-Elyousr K.A., Abdel-Hafez S.I., Abdel-Rahim I.R. Isolation of Trichoderma and Evaluation of Their Antagonistic Potential against Alternaria porri. J. Phytopathol. 2014;162:567–574. doi: 10.1111/jph.12228. [DOI] [Google Scholar]
- 15.Leelavathi M.S., Vani L., Reena P. Antimicrobial Activity of Trichoderma harzianum against Bacteria and Fungi. Int. J. Curr. Microbiol. Appl. Sci. 2014;3:96–103. [Google Scholar]
- 16.Ahmadi-Nouraldinvand F., Afrouz M., Elias S.G., Eslamian S. Green Synthesis of Copper Nanoparticles Extracted from Guar Seedling under Cu Heavy-Metal Stress by Trichoderma harzianum and Their Bio-Efficacy Evaluation against Staphylococcus Aureus and Escherichia Coli. Environ. Earth Sci. 2022;81:54. doi: 10.1007/s12665-022-10184-4. [DOI] [Google Scholar]
- 17.Zhang J.-L., Tang W.-L., Huang Q.-R., Li Y.-Z., Wei M.-L., Jiang L.-L., Liu C., Yu X., Zhu H.-W., Chen G.-Z. Trichoderma: A Treasure House of Structurally Diverse Secondary Metabolites with Medicinal Importance. Front. Microbiol. 2021;12:723828. doi: 10.3389/fmicb.2021.723828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herrmann A. The Chemistry and Biology of Volatiles. John Wiley & Sons; Hoboken, NJ, USA: 2010. Profragrances and Properfumes; pp. 333–362. [Google Scholar]
- 19.Hung R., Lee S., Bennett J.W. Fungal Volatile Organic Compounds and Their Role in Ecosystems. Appl. Microbiol. Biotechnol. 2015;99:3395–3405. doi: 10.1007/s00253-015-6494-4. [DOI] [PubMed] [Google Scholar]
- 20.Lemfack M.C., Gohlke B.-O., Toguem S.M.T., Preissner S., Piechulla B., Preissner R. MVOC 2.0: A Database of Microbial Volatiles. Nucleic Acids Res. 2018;46:D1261–D1265. doi: 10.1093/nar/gkx1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manganiello G., Sacco A., Ercolano M.R., Vinale F., Lanzuise S., Pascale A., Napolitano M., Lombardi N., Lorito M., Woo S.L. Modulation of Tomato Response to Rhizoctonia Solani by Trichoderma Harzianum and Its Secondary Metabolite Harzianic Acid. Front. Microbiol. 2018;9:1966. doi: 10.3389/fmicb.2018.01966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Da Silva M.H.R., Cueva-Yesquén L.G., Júnior S.B., Garcia V.L., Sartoratto A., de Angelis D.d.F., de Angelis D.A. Endophytic Fungi from Passiflora incarnata: An Antioxidant Compound Source. Arch. Microbiol. 2020;202:2779–2789. doi: 10.1007/s00203-020-02001-y. [DOI] [PubMed] [Google Scholar]
- 23.Yan Y., Mao Q., Wang Y., Zhao J., Fu Y., Yang Z., Peng X., Zhang M., Bai B., Liu A. Trichoderma Harzianum Induces Resistance to Root-Knot Nematodes by Increasing Secondary Metabolite Synthesis and Defense-Related Enzyme Activity in Solanum lycopersicum L. Biol. Control. 2021;158:104609. doi: 10.1016/j.biocontrol.2021.104609. [DOI] [Google Scholar]
- 24.Ghoniem A.A., Abd El-Hai K.M., El-Khateeb A.Y., Eldadamony N.M., Mahmoud S.F., Elsayed A. Enhancing the Potentiality of Trichoderma Harzianum against Pythium Pathogen of Beans Using Chamomile (Matricaria chamomilla L.) Flower Extract. Molecules. 2021;26:1178. doi: 10.3390/molecules26041178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cardoza R.-E., Hermosa M.-R., Vizcaíno J.-A., Sanz L., Monte E., Gutiérrez S. Microorganism for Industrial Enzymes and Biocontrol. Research Signpost; Thiruvananthapuram, India: 2005. Secondary Metabolites Produced by Trichoderma and Their Importance in the Biocontrol Process; pp. 1–22. [Google Scholar]
- 26.Surekha C.H., Neelapu N.R.R., Kamala G., Prasad B.S., Ganesh P.S. Efficacy of Trichoderma viride to Induce Disease Resistance and Antioxidant Responses in Legume Vigna Mungo Infested by Fusarium oxysporum and Alternaria alternata. Int. J. Agric. Sci. Res. 2013;3:285–294. [Google Scholar]
- 27.Surekha C.H., Neelapu N.R.R., Prasad B.S., Ganesh P.S. Induction of Defense Enzymes and Phenolic Content by Trichoderma viride in Vigna Mungo Infested with Fusarium oxysporum and Alternaria alternata. Int. J. Agric. Sci. Res. 2014;4:31–40. [Google Scholar]
- 28.Ahanger M.A., Agarwal R.M., Tomar N.S., Shrivastava M. Potassium Induces Positive Changes in Nitrogen Metabolism and Antioxidant System of Oat (Avena sativa L. cultivar Kent) J. Plant Interact. 2015;10:211–223. doi: 10.1080/17429145.2015.1056260. [DOI] [Google Scholar]
- 29.Hashem A., Abd_Allah E.F., Alqarawi A.A., Egamberdieva D. Bioremediation of Adverse Impact of Cadmium Toxicity on Cassia italica Mill by Arbuscular Mycorrhizal Fungi. Saudi J. Biol. Sci. 2016;23:39–47. doi: 10.1016/j.sjbs.2015.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brunetti C., Di Ferdinando M., Fini A., Pollastri S., Tattini M. Flavonoids as Antioxidants and Developmental Regulators: Relative Significance in Plants and Humans. Int. J. Mol. Sci. 2013;14:3540–3555. doi: 10.3390/ijms14023540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mona S.A., Hashem A., Abd_Allah E.F., Alqarawi A.A., Soliman D.W.K., Wirth S., Egamberdieva D. Increased Resistance of Drought by Trichoderma harzianum Fungal Treatment Correlates with Increased Secondary Metabolites and Proline Content. J. Integr. Agric. 2017;16:1751–1757. doi: 10.1016/S2095-3119(17)61695-2. [DOI] [Google Scholar]
- 32.Kumar G., Kumar K. Design of an Evolutionary Approach for Intrusion Detection. Sci. World J. 2013;2013:962185. doi: 10.1155/2013/962185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dolara P., Luceri C., De Filippo C., Femia A.P., Giovannelli L., Caderni G., Cecchini C., Silvi S., Orpianesi C., Cresci A. Red Wine Polyphenols Influence Carcinogenesis, Intestinal Microflora, Oxidative Damage and Gene Expression Profiles of Colonic Mucosa in F344 Rats. Mutat. Res./Fundam. Mol. Mech. Mutagen. 2005;591:237–246. doi: 10.1016/j.mrfmmm.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 34.Saxena M.S.J., Nema R., Sigh D., Gupta A. Phytochemsitry of Medical Plants. J. Pharm. Phytochem. 2013;1:168–182. [Google Scholar]
- 35.Omomowo I.O., Fadiji A.E., Omomowo O.I. Antifungal Evaluation and Phytochemical Profile of Trichoderma harzianum and Glomus versiforme Secondary Metabolites on Cowpea Pathogens. Asian J. Microbiol. Biotechnol. Environ. Sci. 2020;22:265–272. [Google Scholar]
- 36.Ladoh-Yemeda C.F., Nyegue M.A., Ngene J.P., Benelesse G.E., Lenta B., Wansi J.D., Mpondo E.M., Dibong S.D. Identification and Phytochemical Screening of Endophytic Fungi from Stems of Phragmanthera capitata (Sprengel) S. Balle (Loranthaceae) J. Appl. Biosci. 2015;90:8355–8360. doi: 10.4314/jab.v90i1.7. [DOI] [Google Scholar]
- 37.Sriwati R., Chamzurn T., Soesanto L., Munazhirah M. Field Application of Trichoderma Suspension to Control Cacao Pod Rot (Phytophthora palmivora) AGRIVITA J. Agric. Sci. 2019;41:175–182. doi: 10.17503/agrivita.v41i1.2146. [DOI] [Google Scholar]
- 38.Joosten L., van Veen J.A. Defensive Properties of Pyrrolizidine Alkaloids against Microorganisms. Phytochem. Rev. 2011;10:127–136. doi: 10.1007/s11101-010-9204-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matsuura H.N., Fett-Neto A.G. Plant Alkaloids: Main Features, Toxicity, and Mechanisms of Action. Plant Toxins. 2015;2:1–15. [Google Scholar]
- 40.Frisvad J.C., Andersen B., Thrane U. The Use of Secondary Metabolite Profiling in Chemotaxonomy of Filamentous Fungi. Mycol. Res. 2008;112:231–240. doi: 10.1016/j.mycres.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 41.Kar B., Özköse E., Ekinci M.S. The Comparisons of Fatty Acid Composition in Some Anaerobic Gut Fungi Neocallimastix, Orpinomyces, Piromyces, and Caecomyces. An. Acad. Bras. Ciências. 2021;93:e20200896. doi: 10.1590/0001-3765202120200896. [DOI] [PubMed] [Google Scholar]
- 42.Morbidoni H.R., Vilchèze C., Kremer L., Bittman R., Sacchettini J.C., Jacobs W.R., Jr. Dual Inhibition of Mycobacterial Fatty Acid Biosynthesis and Degradation by 2-Alkynoic Acids. Chem. Biol. 2006;13:297–307. doi: 10.1016/j.chembiol.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 43.Ramsewak R.S., Nair M.G., Murugesan S., Mattson W.J., Zasada J. Insecticidal Fatty Acids and Triglycerides from Dirca palustris. J. Agric. Food Chem. 2001;49:5852–5856. doi: 10.1021/jf010806y. [DOI] [PubMed] [Google Scholar]
- 44.Hyeon S.B. Chemical Studies on the Factors Controlling Sporulation of Fungi. Chem. Regul. Plants. 1976;42:1431–1433. [Google Scholar]
- 45.Katayama M., Marumo S. R (–)-Glycerol Monolinolate, a Minor Sporogenic Substance of Sclerotinia fructicola. Agric. Biol. Chem. 1978;42:1431–1433. doi: 10.1271/bbb1961.42.1431. [DOI] [Google Scholar]
- 46.Nukina M., Sassa T., Ikeda M., Takahashi K., Toyota S. Linoleic Acid Enhances Perithecial Production in Neurospora crassa. Agric. Biol. Chem. 1981;45:2371–2373. doi: 10.1271/bbb1961.45.2371. [DOI] [Google Scholar]
- 47.Abdullah R.R. Insecticidal Activity of Secondary Metabolites of Locally Isolated Fungal Strains against Some Cotton Insect Pests. J. Plant Prot. Pathol. 2019;10:647–653. doi: 10.21608/jppp.2019.79456. [DOI] [Google Scholar]
- 48.Egbung G.E., Anosike C., Utu-Baku A.B., Ogar I., Nna V.U. Phytochemical Evaluation and GC-MS Analysis of Hyptis verticillata Cultivated in Calabar Cross River State, Nigeria. Int. J. Biol. Chem. Sci. 2017;11:2548–2559. doi: 10.4314/ijbcs.v11i5.47. [DOI] [Google Scholar]
- 49.Luepongpattana S., Thaniyavarn J., Morikawa M. Production of Massoia Lactone by Aureobasidium pullulans YTP6-14 Isolated from the Gulf of Thailand and Its Fragrant Biosurfactant Properties. J. Appl. Microbiol. 2017;123:1488–1497. doi: 10.1111/jam.13598. [DOI] [PubMed] [Google Scholar]
- 50.Poomtien J., Thaniyavarn J., Pinphanichakarn P., Jindamorakot S., Morikawa M. Production and Characterization of a Biosurfactant from Cyberlindnera samutprakarnensis JP52T. Biosci. Biotechnol. Biochem. 2013;77:2362–2370. doi: 10.1271/bbb.130434. [DOI] [PubMed] [Google Scholar]
- 51.Kim H.-S., Jeon J.-W., Kim B.-H., Ahn C.-Y., Oh H.-M., Yoon B.-D. Extracellular Production of a Glycolipid Biosurfactant, Mannosylerythritol Lipid, by Candida sp. SY16 Using Fed-Batch Fermentation. Appl. Microbiol. Biotechnol. 2006;70:391–396. doi: 10.1007/s00253-005-0092-9. [DOI] [PubMed] [Google Scholar]
- 52.Rodrigues C., Cassini S.T.A., s Antunes P.W.P., Pinotti L.M., de Pinho Keller R., Gonçalves R.F. Lipase-Producing Fungi for Potential Wastewater Treatment and Bioenergy Production. Afr. J. Biotechnol. 2016;15:759–767. [Google Scholar]
- 53.Wang Z., Zhang M., Chi Z., Liu G.-L., Chi Z.-M. Liamocin Overproduction by the Mutants of Aureobasidium melanogenum 9–1 for Effectively Killing Spores of the Pathogenic Fungi from Diseased Human Skin by Massoia Lactone. World J. Microbiol. Biotechnol. 2022;38:107. doi: 10.1007/s11274-022-03290-9. [DOI] [PubMed] [Google Scholar]
- 54.Mahomed Ali A.B. Master’s Thesis. University of Pretoria; Pretoria, South Africa: 2010. Production of Pyrazine Flavours by Mycelial Fungi. [Google Scholar]
- 55.Ngugi H.K., Scherm H. Biology of Flower-Infecting Fungi. Annu. Rev. Phytopathol. 2006;44:261–282. doi: 10.1146/annurev.phyto.44.070505.143405. [DOI] [PubMed] [Google Scholar]
- 56.Endrédi H., Billes F., Keresztury G. Revised Assignment of the Vibrational Spectra of Methylpyrazines Based on Scaled DFT Force Fields. J. Mol. Struct. THEOCHEM. 2004;677:211–225. doi: 10.1016/j.theochem.2004.01.031. [DOI] [Google Scholar]
- 57.Bramwell A.F., Burrell J.W.K., Riezebos G. Characterisation of Pyrazines in Galbanum Oil. Tetrahedron Lett. 1969;10:3215–3216. doi: 10.1016/S0040-4039(01)88391-X. [DOI] [Google Scholar]
- 58.Malafatti-Picca L., de Barros Chaves M.R., de Castro A.M., Valoni É., de Oliveira V.M., Marsaioli A.J., de Franceschi de Angelis D., Attili-Angelis D. Hydrocarbon-Associated Substrates Reveal Promising Fungi for Poly (Ethylene Terephthalate) (PET) Depolymerization. Braz. J. Microbiol. 2019;50:633–648. doi: 10.1007/s42770-019-00093-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kashmiri M.A., Adnan A., Butt B.W. Production, Purification and Partial Characterization of Lipase from Trichoderma viride. Afr. J. Biotechnol. 2006;5:878–882. [Google Scholar]
- 60.Contesini F.J., Calzado F., Valdo J. Fungal Metabolites. Springer; Berlin/Heidelberg, Germany: 2017. (Reference Series in Phytochemistry). [Google Scholar]
- 61.Schneider W.D.H., Gonçalves T.A., Uchima C.A., Couger M.B., Prade R., Squina F.M., Dillon A.J.P., Camassola M. Penicillium Echinulatum Secretome Analysis Reveals the Fungi Potential for Degradation of Lignocellulosic Biomass. Biotechnol. Biofuels. 2016;9:66. doi: 10.1186/s13068-016-0476-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Iftikhar T., Abdullah R., Iqtedar M., Kaleem A., Aftab M., Niaz M., Sidra B.T., Majeed H. Production of Lipases by Alternaria Sp.(Mbl 2810) through Optimization of Environmental Conditions Using Submerged Fermentation Technique. Int. J. Biosci. 2015;6655:178–186. [Google Scholar]
- 63.Sharma R., Chisti Y., Dan Banerjee U.C. Production, Purification, Characterization and Applicatiosn of Lipases. Biotechnol. Adv. 2001;19:627–662. doi: 10.1016/S0734-9750(01)00086-6. [DOI] [PubMed] [Google Scholar]
- 64.Zafar U., Houlden A., Robson G.D. Fungal Communities Associated with the Biodegradation of Polyester Polyurethane Buried under Compost at Different Temperatures. Appl. Environ. Microbiol. 2013;79:7313–7324. doi: 10.1128/AEM.02536-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Congiu C., Cocco M.T., Onnis V. Design, Synthesis, and In Vitro Antitumor Activity of New 1, 4-Diarylimidazole-2-Ones and Their 2-Thione Analogues. Bioorganic Med. Chem. Lett. 2008;18:989–993. doi: 10.1016/j.bmcl.2007.12.023. [DOI] [PubMed] [Google Scholar]
- 66.Han M.S., Kim D.H. Effect of Zinc Ion on the Inhibition of Carboxypeptidase A by Imidazole-Bearing Substrate Analogues. Bioorganic Med. Chem. Lett. 2001;11:1425–1427. doi: 10.1016/S0960-894X(01)00226-8. [DOI] [PubMed] [Google Scholar]
- 67.Govindhara Sujatha P., Tamilselvan Pazhanisamy V. Synthesis, Spectral Characterization, Computational Studies and Antimicrobial Activities of Imidazole Derivatives. DJ J. Eng. Chem. Fuel. 2016;1:60–72. doi: 10.18831/djchem.org/2016041006. [DOI] [Google Scholar]
- 68.Emami S., Foroumadi A., Falahati M., Lotfali E., Rajabalian S., Ebrahimi S.-A., Farahyar S., Shafiee A. 2-Hydroxyphenacyl Azoles and Related Azolium Derivatives as Antifungal Agents. Bioorganic Med. Chem. Lett. 2008;18:141–146. doi: 10.1016/j.bmcl.2007.10.111. [DOI] [PubMed] [Google Scholar]
- 69.Sharma D., Narasimhan B., Kumar P., Judge V., Narang R., De Clercq E., Balzarini J. Synthesis, Antimicrobial and Antiviral Evaluation of Substituted Imidazole Derivatives. Eur. J. Med. Chem. 2009;44:2347–2353. doi: 10.1016/j.ejmech.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 70.Crane L., Anastassiadou M., El Hage S., Stigliani J.L., Baziard-Mouysset G., Payard M., Leger J.M., Bizot-Espiard J.-G., Ktorza A., Caignard D.-H. Design and Synthesis of Novel Imidazoline Derivatives with Potent Antihyperglycemic Activity in a Rat Model of Type 2 Diabetes. Bioorganic Med. Chem. 2006;14:7419–7433. doi: 10.1016/j.bmc.2006.07.026. [DOI] [PubMed] [Google Scholar]
- 71.Narasimhan B., Sharma D., Kumar P. Biological Importance of Imidazole Nucleus in the New Millennium. Med. Chem. Res. 2011;20:1119–1140. doi: 10.1007/s00044-010-9472-5. [DOI] [Google Scholar]
- 72.Bhushan Singh R., Das N., Jana S., Das A. Synthesis and In Vitro Antibacterial Screening of Some New 2, 4, 6-Trisubstituted-1, 3, 5-Triazine Derivatives. Lett. Drug Des. Discov. 2012;9:316–321. doi: 10.2174/157018012799129936. [DOI] [Google Scholar]
- 73.Gahtori P., Ghosh S.K., Parida P., Prakash A., Gogoi K., Bhat H.R., Singh U.P. Antimalarial Evaluation and Docking Studies of Hybrid Phenylthiazolyl-1,3,5-Triazine Derivatives: A Novel and Potential Antifolate Lead for Pf-DHFR-TS Inhibition. Exp. Parasitol. 2012;130:292–299. doi: 10.1016/j.exppara.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 74.Kumar Ghosh S., Saha A., Hazarika B., Pratap Singh U., Raj Bhat H., Gahtori P. Design, Facile Synthesis, Antibacterial Activity and Structure-Activity Relationship of Novel Di-and Tri-Substituted 1, 3, 5-Triazines. Lett. Drug Des. Discov. 2012;9:329–335. doi: 10.2174/157018012799129846. [DOI] [Google Scholar]
- 75.Baliani A., Bueno G.J., Stewart M.L., Yardley V., Brun R., Barrett M.P., Gilbert I.H. Design and Synthesis of a Series of Melamine-Based Nitroheterocycles with Activity against Trypanosomatid Parasites. J. Med. Chem. 2005;48:5570–5579. doi: 10.1021/jm050177+. [DOI] [PubMed] [Google Scholar]
- 76.Singh U.P., Bhat H.R., Gahtori P. Antifungal Activity, SAR and Physicochemical Correlation of Some Thiazole-1, 3, 5-Triazine Derivatives. J. Mycol. Médicale. 2012;22:134–141. doi: 10.1016/j.mycmed.2011.12.073. [DOI] [PubMed] [Google Scholar]
- 77.Menicagli R., Samaritani S., Signore G., Vaglini F., Dalla Via L. In Vitro Cytotoxic Activities of 2-Alkyl-4, 6-Diheteroalkyl-1, 3, 5-Triazines: New Molecules in Anticancer Research. J. Med. Chem. 2004;47:4649–4652. doi: 10.1021/jm0495374. [DOI] [PubMed] [Google Scholar]
- 78.Patel R.V., Kumari P., Rajani D.P., Chikhalia K.H. Synthesis, Characterization and Pharmacological Activities of 2-[4-Cyano-(3-Trifluoromethyl) Phenyl Amino)]-4-(4-Quinoline/Coumarin-4-Yloxy)-6-(Fluoropiperazinyl)-s-Triazines. J. Fluor. Chem. 2011;132:617–627. doi: 10.1016/j.jfluchem.2011.06.021. [DOI] [Google Scholar]
- 79.Chen X., Zhan P., Liu X., Cheng Z., Meng C., Shao S., Pannecouque C., Clercq E.D., Liu X. Design, Synthesis, Anti-HIV Evaluation and Molecular Modeling of Piperidine-Linked Amino-Triazine Derivatives as Potent Non-Nucleoside Reverse Transcriptase Inhibitors. Bioorganic Med. Chem. 2012;20:3856–3864. doi: 10.1016/j.bmc.2012.04.030. [DOI] [PubMed] [Google Scholar]
- 80.Bradow J.M. Relationships between Chemical Structure and Inhibitory Activity of C 6 through C 9 Volatiles Emitted by Plant Residues. J. Chem. Ecol. 1991;17:2193–2212. doi: 10.1007/BF00988001. [DOI] [PubMed] [Google Scholar]
- 81.Gardner H.W., Dornbos D.L., Jr., Desjardins A.E. Hexanal, Trans-2-Hexenal, and Trans-2-Nonenal Inhibit Soybean, Glycine Max, Seed Germination. J. Agric. Food Chem. 1990;38:1316–1320. doi: 10.1021/jf00096a005. [DOI] [Google Scholar]
- 82.Hamilton-Kemp T.R., Loughrin J.H., Archbold D.D., Andersen R.A., Hildebrand D.F. Inhibition of Pollen Germination by Volatile Compounds Including 2-Hexenal and 3-Hexenal. J. Agric. Food Chem. 1991;39:952–956. doi: 10.1021/jf00005a031. [DOI] [Google Scholar]
- 83.Hamilton-Kemp T.R., McCracken C.T., Loughrin J.H., Andersen R.A., Hildebrand D.F. Effects of Some Natural Volatile Compounds on the Pathogenic Fungi Alternaria alternata and Botrytis cinerea. J. Chem. Ecol. 1992;18:1083–1091. doi: 10.1007/BF00980064. [DOI] [PubMed] [Google Scholar]
- 84.Deng W., Hamilton-Kemp T.R., Nielsen M.T., Andersen R.A., Collins G.B., Hildebrand D.F. Effects of Six-Carbon Aldehydes and Alcohols on Bacterial Proliferation. J. Agric. Food Chem. 1993;41:506–510. doi: 10.1021/jf00027a030. [DOI] [Google Scholar]
- 85.Küçükgüzel S.G., Mazi A., Sahin F., Öztürk S., Stables J. Synthesis and Biological Activities of Diflunisal Hydrazide–Hydrazones. Eur. J. Med. Chem. 2003;38:1005–1013. doi: 10.1016/j.ejmech.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 86.Mohareb R.M., EL-Sharkawy K.A., Al Farouk F.O. Synthesis, Cytotoxicity against Cancer and Normal Cell Lines of Novel Hydrazide–Hydrazone Derivatives Bearing 5H-Chromen-5-One. Med. Chem. Res. 2019;28:1885–1900. doi: 10.1007/s00044-019-02421-6. [DOI] [Google Scholar]
- 87.Terzioglu N., Gürsoy A. Synthesis and Anticancer Evaluation of Some New Hydrazone Derivatives of 2, 6-Dimethylimidazo [2, 1-b][1, 3, 4] Thiadiazole-5-Carbohydrazide. Eur. J. Med. Chem. 2003;38:781–786. doi: 10.1016/S0223-5234(03)00138-7. [DOI] [PubMed] [Google Scholar]
- 88.Joshi S.D., More Y., Vagdevi H.M., Vaidya V.P., Gadaginamath G.S., Kulkarni V.H. Synthesis of New 4-(2, 5-Dimethylpyrrol-1-Yl)/4-Pyrrol-1-Yl Benzoic Acid Hydrazide Analogs and Some Derived Oxadiazole, Triazole and Pyrrole Ring Systems: A Novel Class of Potential Antibacterial, Antifungal and Antitubercular Agents. Med. Chem. Res. 2013;22:1073–1089. doi: 10.1007/s00044-012-0112-0. [DOI] [PubMed] [Google Scholar]
- 89.Özdemir A., Turan-Zitouni G., Kaplancikli Z.A., Tunali Y. Synthesis and Biological Activities of New Hydrazide Derivatives. J. Enzym. Inhib. Med. Chem. 2009;24:825–831. doi: 10.1080/14756360802399712. [DOI] [PubMed] [Google Scholar]
- 90.Küçükgüzel Ş.G., Küçükgüzel I., Tatar E., Rollas S., Şahin F., Güllüce M., De Clercq E., Kabasakal L. Synthesis of Some Novel Heterocyclic Compounds Derived from Diflunisal Hydrazide as Potential Anti-Infective and Anti-Inflammatory Agents. Eur. J. Med. Chem. 2007;42:893–901. doi: 10.1016/j.ejmech.2006.12.038. [DOI] [PubMed] [Google Scholar]
- 91.Backes G.L., Neumann D.M., Jursic B.S. Synthesis and Antifungal Activity of Substituted Salicylaldehyde Hydrazones, Hydrazides and Sulfohydrazides. Bioorganic Med. Chem. 2014;22:4629–4636. doi: 10.1016/j.bmc.2014.07.022. [DOI] [PubMed] [Google Scholar]
- 92.Suresh A.K., Pelletier D.A., Doktycz M.J. Relating Nanomaterial Properties and Microbial Toxicity. Nanoscale. 2013;5:463–474. doi: 10.1039/C2NR32447D. [DOI] [PubMed] [Google Scholar]
- 93.Tian Z., Li R., Cheng S., Zhou T., Liu J. The Mythimna Separata General Odorant Binding Protein 2 (MsepGOBP2) Is Involved in the Larval Detection of the Sex Pheromone (Z)-11-hexadecenal. Pest Manag. Sci. 2023;79:2005–2016. doi: 10.1002/ps.7373. [DOI] [PubMed] [Google Scholar]
- 94.Mohammadpour K., Moezipour M., Avand-Faghih A. Efficacy of Different Pheromone Trap Design in Monitoring of the Box Tree Moth, Cydalima Perspectalis in the Northern Forests of Iran. Acta Phytopathol. Entomol. Hung. 2022;57:43–48. [Google Scholar]
- 95.Hu T.-M., Wu C.-L. Fluconazole-induced Delirium in an Older Patient with Schizophrenia. Psychogeriatrics. 2022;22:588. doi: 10.1111/psyg.12844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gómez O.C. Ph.D. Thesis. The Federal University of Alfenas; Alfenas, Brazil: 2022. Identificação de Metabólitos Secundários Com Potencial Antimicrobiano Produzidos Pelo Fungo Endofítico Lasiodiplodia Sp. Isolado Do Ipê Rosa (Handroanthus Impetiginosus) [Google Scholar]
- 97.Adnan M., Nazim Uddin Chy M., Mostafa Kamal A.T.M., Azad M., Paul A., Uddin S., Barlow J., Faruque M., Park C., Cho D. Investigation of the Biological Activities and Characterization of Bioactive Constituents of Ophiorrhiza Rugosa Var. Prostrata (D.Don) & Mondal Leaves through In Vivo, In Vitro, and In Silico Approaches. Molecules. 2019;24:1367. doi: 10.3390/molecules24071367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Agoreyo B.O., Oseghale E.I. Effect of post-harvest storage at ambient temperature on nutritional constituents, in vitro antioxidant activity and gc-ms profile of king tuber mushroom (pleurotus tuber-regium) Eur. J. Food Sci. Technol. 2019;7:24–46. [Google Scholar]
- 99.John R.P., Tyagi R.D., Prévost D., Brar S.K., Pouleur S., Surampalli R.Y. Mycoparasitic Trichoderma Viride as a Biocontrol Agent against Fusarium Oxysporum f. Sp. Adzuki and Pythium Arrhenomanes and as a Growth Promoter of Soybean. Crop Prot. 2010;29:1452–1459. doi: 10.1016/j.cropro.2010.08.004. [DOI] [Google Scholar]
- 100.Arzanlou M., Khodaei S., Narmani A., Babai-Ahari A., Azar A.M. Inhibitory Effect of Trichoderma Isolates on Growth of Alternaria Alternata, the Causal Agent of Leaf Spot Disease on Sunflower, under Laboratory Conditions. Arch. Phytopathol. Plant Prot. 2014;47:1592–1599. doi: 10.1080/03235408.2013.853453. [DOI] [Google Scholar]
- 101.Živković S., Stojanović S., Ivanović Ž., Gavrilović V., Popović T., Balaž J. Screening of Antagonistic Activity of Microorganisms against Colletotrichum acutatum and Colletotrichum gloeosporioides. Arch. Biol. Sci. 2010;62:611–623. doi: 10.2298/ABS1003611Z. [DOI] [Google Scholar]
- 102.Rajani P., Rajasekaran C., Vasanthakumari M.M., Olsson S.B., Ravikanth G., Shaanker R.U. Inhibition of Plant Pathogenic Fungi by Endophytic Trichoderma Spp. through Mycoparasitism and Volatile Organic Compounds. Microbiol. Res. 2021;242:126595. doi: 10.1016/j.micres.2020.126595. [DOI] [PubMed] [Google Scholar]
- 103.Meena M., Swapnil P., Zehra A., Dubey M.K., Upadhyay R.S. Antagonistic Assessment of Trichoderma Spp. by Producing Volatile and Non-Volatile Compounds against Different Fungal Pathogens. Arch. Phytopathol. Plant Prot. 2017;50:629–648. doi: 10.1080/03235408.2017.1357360. [DOI] [Google Scholar]
- 104.Küçük Ç., Kivanç M. In Vitro Antifungal Activity of Strains of Trichoderma Harzianum. Turk. J. Biol. 2004;28:111–115. [Google Scholar]
- 105.Narendran R., Kathiresan K. Antimicrobial Activity of Crude Extracts from Mangrove-Derived Trichoderma Species against Human and Fish Pathogens. Biocatal. Agric. Biotechnol. 2016;6:189–194. doi: 10.1016/j.bcab.2016.03.003. [DOI] [Google Scholar]
- 106.Ameen O.M., Garuba T., Zubair M.F., Baker M.T., Arowolo B.Z., Yakubu A.O. Structural And Phytochemical Characterization of Bioactive Components of the Endophytic Fungi (Trichoderma Harzianum) Extracts. J. Appl. Sci. Environ. Manag. 2022;26:689–693. doi: 10.4314/jasem.v26i4.18. [DOI] [Google Scholar]
- 107.Vitti A., Pellegrini E., Nali C., Lovelli S., Sofo A., Valerio M., Scopa A., Nuzzaci M. Trichoderma Harzianum T-22 Induces Systemic Resistance in Tomato Infected by Cucumber Mosaic Virus. Front. Plant Sci. 2016;7:1520. doi: 10.3389/fpls.2016.01520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lombardi N., Caira S., Troise A.D., Scaloni A., Vitaglione P., Vinale F., Marra R., Salzano A.M., Lorito M., Woo S.L. Trichoderma Applications on Strawberry Plants Modulate the Physiological Processes Positively Affecting Fruit Production and Quality. Front. Microbiol. 2020;11:1364. doi: 10.3389/fmicb.2020.01364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Saravanakumar K., Park S., Sathiyaseelan A., Mariadoss A.V.A., Park S., Kim S.-J., Wang M.-H. Isolation of Polysaccharides from Trichoderma Harzianum with Antioxidant, Anticancer, and Enzyme Inhibition Properties. Antioxidants. 2021;10:1372. doi: 10.3390/antiox10091372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yuan J.-F., Zhang Z.-Q., Fan Z.-C., Yang J.-X. Antioxidant Effects and Cytotoxicity of Three Purified Polysaccharides from Ligusticum Chuanxiong Hort. Carbohydr. Polym. 2008;74:822–827. [Google Scholar]
- 111.Fernandes M.d.R.V., Silva T.A.C., Pfenning L.H., da Costa-Neto C.M., Heinrich T.A., de Alencar S.M., de Lima M.A., Ikegaki M. Biological Activities of the Fermentation Extract of the Endophytic Fungus Alternaria Alternata Isolated from Coffea arabica L. Braz. J. Pharm. Sci. 2009;45:677–685. doi: 10.1590/S1984-82502009000400010. [DOI] [Google Scholar]
- 112.Anwar J., Iqbal Z. Effect of Growth Conditions on Antibacterial Activity of Trichoderma harzianum against Selected Pathogenic Bacteria. Sarhad J. Agric. 2017;33:501–510. [Google Scholar]
- 113.Ali-Rachedi F., Meraghni S., Touaibia N., Mesbah S. Analyse quantitative des composés phénoliques d’une endémique algérienne Scabiosa Atropurpurea sub. Maritima L. Bull. Société R. Sci. Liège. 2018;87:13–21. doi: 10.25518/0037-9565.7398. [DOI] [Google Scholar]
- 114.Singleton V.L., Orthofer R., Lamuela-Raventós R.M. Methods in Enzymology. Volume 299. Elsevier; Amsterdam, The Netherlands: 1999. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent; pp. 152–178. [Google Scholar]
- 115.Singleton V.L., Rossi J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965;16:144–158. doi: 10.5344/ajev.1965.16.3.144. [DOI] [Google Scholar]
- 116.Lagnika L. Etude Phytochimique et Activité Biologique de Substances Naturelles Isolées de Plantes Béninoises. Université Louis Pasteur Starsbourg; Strasbourg, France: 2005. [Google Scholar]
- 117.Zhishen J., Mengcheng T., Jianming W. The Determination of Flavonoid Contents in Mulberry and Their Scavenging Effects on Superoxide Radicals. Food Chem. 1999;64:555–559. doi: 10.1016/S0308-8146(98)00102-2. [DOI] [Google Scholar]
- 118.Kim D.-O., Chun O.K., Kim Y.J., Moon H.-Y., Lee C.Y. Quantification of Polyphenolics and Their Antioxidant Capacity in Fresh Plums. J. Agric. Food Chem. 2003;51:6509–6515. doi: 10.1021/jf0343074. [DOI] [PubMed] [Google Scholar]
- 119.Schofield P., Mbugua D.M., Pell A.N. Analysis of Condensed Tannins: A Review. Anim. Feed Sci. Technol. 2001;91:21–40. doi: 10.1016/S0377-8401(01)00228-0. [DOI] [Google Scholar]
- 120.Sun B., Ricardo-da-Silva J.M., Spranger I. Critical Factors of Vanillin Assay for Catechins and Proanthocyanidins. J. Agric. Food Chem. 1998;46:4267–4274. doi: 10.1021/jf980366j. [DOI] [Google Scholar]
- 121.Julkunen-Tiitto R. Phenolic Constituents in the Leaves of Northern Willows: Methods for the Analysis of Certain Phenolics. J. Agric. Food Chem. 1985;33:213–217. doi: 10.1021/jf00062a013. [DOI] [Google Scholar]
- 122.Ajanal M., Gundkalle M.B., Nayak S.U. Estimation of Total Alkaloid in Chitrakadivati by UV-Spectrophotometer. Anc. Sci. Life. 2012;31:198. doi: 10.4103/0257-7941.107361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hollo J., Kurucz E., Torky A.A., Biacs P. Application de la CCM à l’étude des huiles volatiles. Plant Food Hum. Nutr. 1968;16:51–62. doi: 10.1007/BF01103863. [DOI] [Google Scholar]
- 124.Nakamura C.V., Ueda-Nakamura T., Bando E., Melo A.F.N., Cortez D.A.G., Dias Filho B.P. Antibacterial Activity of Ocimum Gratissimum L. Essential Oil. Memórias Inst. Oswaldo Cruz. 1999;94:675–678. doi: 10.1590/S0074-02761999000500022. [DOI] [PubMed] [Google Scholar]
- 125.Apeti G.K., Marra D., Semihinva A., Komlan B., Koffi A. Evaluation De L’activité Antifongique De Ficus Platyphylla Del.(Moraceae) Eur. Sci. J. 2013;9:252–260. [Google Scholar]
- 126.El Mansouri K., Moutaj R. Ph.D. Thesis. Universite Cadi Ayyad; Marrakesh, Morocco: 2013. Recherche et Évaluation de l’activité Antifongique Des Extraits de Plantes Médicinales. [Google Scholar]
- 127.De Albuquerque C.C., Camara T.R., Mariano R.d.L.R., Willadino L., Marcelino Júnior C., Ulisses C. Antimicrobial Action of the Essential Oil of Lippia Gracilis Schauer. Braz. Arch. Biol. Technol. 2006;49:527–535. doi: 10.1590/S1516-89132006000500001. [DOI] [Google Scholar]
- 128.Rahmawati N., Isfandito A.R., Astuti D.I., Aditiawati P. Endophytic Fungi from Surian (Toona Sinensis Roem) and Antioxidant Potency from Its Culture. Asian J. Plant Sci. 2016;15:8. doi: 10.3923/ajps.2016.8.15. [DOI] [Google Scholar]
- 129.Potshangbam M., Devi S.I., Sahoo D., Strobel G.A. Functional Characterization of Endophytic Fungal Community Associated with Oryza sativa L. and Zea mays L. Front. Microbiol. 2017;8:325. doi: 10.3389/fmicb.2017.00325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Maxwell S.E., Delaney H.D., Kelley K. Designing Experiments and Analyzing Data: A Model Comparison Perspective. Routledge; Abingdon, UK: 2017. [Google Scholar]
- 131.Rangkadilok N., Tongchusak S., Boonhok R., Chaiyaroj S.C., Junyaprasert V.B., Buajeeb W., Akanimanee J., Raksasuk T., Suddhasthira T., Satayavivad J. In Vitro Antifungal Activities of Longan (Dimocarpus Longan Lour.) Seed Extract. Fitoterapia. 2012;83:545–553. doi: 10.1016/j.fitote.2011.12.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data in the article are available from the corresponding author upon reasonable request.