ABSTRACT
Exposure to environmental pollutants is linked to increased risk of cardiovascular disease. Beyond the extensive evidence for particulate air pollution, accumulating evidence supports that exposure to nonessential metals such as lead, cadmium, and arsenic is a significant contributor to cardiovascular disease worldwide. Humans are exposed to metals through air, water, soil, and food and extensive industrial and public use. Contaminant metals interfere with critical intracellular reactions and functions leading to oxidative stress and chronic inflammation that result in endothelial dysfunction, hypertension, epigenetic dysregulation, dyslipidemia, and changes in myocardial excitation and contractile function. Lead, cadmium, and arsenic have been linked to subclinical atherosclerosis, coronary artery stenosis, and calcification as well as to increased risk of ischemic heart disease and stroke, left ventricular hypertrophy and heart failure, and peripheral artery disease. Epidemiological studies show that exposure to lead, cadmium, or arsenic is associated with cardiovascular death mostly attributable to ischemic heart disease. Public health measures reducing metal exposure are associated with reductions in cardiovascular disease death. Populations of color and low socioeconomic means are more commonly exposed to metals and therefore at greater risk of metal‐induced cardiovascular disease. Together with strengthening public health measures to prevent metal exposures, development of more sensitive and selective measurement modalities, clinical monitoring of metal exposures, and the development of metal chelation therapies could further diminish the burden of cardiovascular disease attributable to metal exposure.
Keywords: AHA Scientific Statements, arsenic, cadmium, cardiac risk factors, coronary disease, heavy metals, lead, myocardial infarction
Cardiovascular disease (CVD) is the predominant cause of death worldwide, leading to at least 18 million lives lost per year worldwide. Decades of study have identified modifiable risk factors for atherosclerosis, including tobacco smoke, dyslipidemia, hypertension, diabetes, obesity, and a sedentary lifestyle. However, the known risk factors do not present a complete risk profile. In this American Heart Association scientific statement we review evidence linking chronic exposure to low and low‐moderate levels of 3 contaminant metals (lead, cadmium, and arsenic) to coronary and peripheral artery atherosclerosis, including stroke, highlighting the clinical and public health implications for health care professionals, researchers, and the public. 1 In the statement, we exclude the acute consequences of high‐level exposures, such as industrial, accidental, or suicidal, which affect a few thousand individuals annually in the United States. Exposure to low‐level environmental metal contamination, a risk factor for atherosclerotic vascular disease, is nearly ubiquitous, and its manifestations occur over many years. Understanding the effect of low‐level metal contamination is thus critical for developing a complete picture of the drivers of atherosclerotic vascular disease.
METHODS
A writing group of experts on environmental health, metals, toxicology, epidemiology, atherosclerosis, cardiology, clinical trials, and public health convened and developed an outline covering the scientific goals of the statement. Literature search engines were used to identify relevant articles. For clinical cardiovascular outcomes, we updated an extant systematic review using the same strategy. 1 The writing group gave greatest weight to prospective epidemiologic studies and systematic reviews. 1 , 2 , 3 , 4
PERSPECTIVE ON CONTAMINANT METALS AND CARDIOVASCULAR RISK
The sum of traditional risk factors and biological mechanisms incompletely defines the totality of atherosclerotic risk. 5 In recent years, exposure to environmental pollutants, such as inhaled particulate matter and exposure to toxic metals, has been firmly linked to the progression of CVD and the incidence of cardiovascular events. 6 These links and the efforts to understand and act on them have been termed environmental cardiology. 7 , 8 Although the European Society of Cardiology and the American Heart Association have considered air pollution as a cardiovascular risk factor, to date, medical societies have not uniformly focused on vascular toxicity from contaminant metals.
The cornerstone concept of environmental cardiology 8 is that exposure to pollutants, including toxic metals, and other environmental conditions constitutes an important, modifiable component of CVD risk. Metal exposure occurs largely within the context of social and personal activities, such as the use of tobacco products, contaminated groundwater, or industrial fertilizers, and the addition of metals to widely used commercial products and public projects, including paint, gasoline, electronics, water pipes, and some foods. In this statement, we highlight near‐universal exposure to toxic metals and how sociodemographic factors modify metal exposure and the resulting cardiovascular manifestations as well. Finally, we describe potential opportunities for interventions at the organizational, public health, pharmacologic, individual practitioner, and patient levels that might guide avoidance or mitigation of toxic metal exposure as part of routine prevention and treatment of CVD.
SOURCES OF EXPOSURE AND PHARMACOKINETICS OF SELECTED METALS
Humans are exposed to a wide range of metals through air, water, soil, and food (Figure 1). In this statement, we prioritize 3 environmentally ubiquitous metals: lead, cadmium, and arsenic. These metals have no essential biological function and affect most populations on a global scale, and robust evidence links them to cardiovascular toxicity (Table 1).
Lead was one of the first metals mined and smelted by humans. It is a divalent cation widespread in the biosphere because of its use in consumer products. 9 , 10 In the 20th century, there was a steep increase in lead production attributable to its extensive industrial and public use, including gasoline and paint. Lead persists in the environment, and legacy uses such as in water pipes contribute to lead exposure today. Manufacture of lead products continues, for example, in wheel weights and acid‐lead batteries. Consequently, lead production has continued to grow, from 8 million tons globally in 2006 to 12 million tons in 2018. Currently, the general population is exposed to lead from a variety of sources including old paint, tobacco products (conventional cigarettes and e‐cigarettes), secondhand smoke, acid‐lead batteries, contaminated foods, water pipes and conduits for drinks and drinking water, herbal remedies and spices, toys, cosmetics, electronics, and emissions from industrial facilities, incinerators, and old heating systems.
Cadmium was discovered in the 19th century as an impurity of zinc ores. 9 , 10 In the second half of the 20th century, its use expanded in nickel‐cadmium batteries, pigments, plastic stabilizers, ceramics and glassware, construction, and other products. Industrially produced fertilizers use phosphate rock naturally rich in cadmium, contaminating root vegetables and green leafy plants (including tobacco). These plants bioconcentrate cadmium from the soil, as this element resembles the essential element zinc.
Absorption of lead and cadmium. Lead and cadmium are divalent cations and both can be efficiently absorbed through the respiratory and gastrointestinal tracts. 9 , 10 The extent of absorption defines what is known as the internal dose—the amount of a substance that is taken up by the body—and is determined by genetic and nongenetic factors. Both metals gain intracellular access via transporters of essential metals. The absorption of lead and cadmium, especially through the gastrointestinal tract, depends on systemic homeostasis of essential metals and on differential bioavailability across different dietary sources. Absorption of lead and cadmium through the respiratory tract, however, is almost complete, as metal‐contaminated air easily reaches and crosses the alveolar‐capillary membrane. The principal reservoir for lead is bone, and for cadmium, kidneys, liver, and other visceral organs. Excretion of these metals has half‐lives of decades.
Arsenic. Inorganic arsenic is a potent toxic and carcinogenic metalloid (intermediate properties between metals and nonmetals) found in water, soil, food (rice), and air. 9 , 10 Known as a poison for centuries, it was widely used in medicine before the introduction of antibiotics. The main source of exposure to inorganic arsenic, globally, is contaminated groundwater, affecting private wells and community water systems. Millions of people in Asia, the Americas including the United States, and also parts of Europe, Africa, and Oceania are exposed to arsenic levels above the current World Health Organization and US standards (eg, Environmental Protection Agency standard for drinking water is 10 μg/L, and for the states of New Jersey and New Hampshire it is 5 μg/L). Inorganic arsenic in water is completely absorbed through the gastrointestinal tract using water channels (aquaporins). After exposure, inorganic arsenic is methylated into mono‐ and dimethyl arsenic compounds, metabolites that are excreted in the urine together with inorganic arsenic. Arsenic metabolism is genetically determined. Women, in general, are more efficient arsenic methylators than men. Arsenic is rapidly excreted from the body (in contrast to lead and cadmium), and removal of the exposure source quickly reduces arsenic body burden.
Other metals and metal mixtures. Studies have reported that exposure to excessive levels of copper, cobalt, tungsten, titanium, and antimony have adverse cardiovascular effects. 9 , 10 Consequently, there is growing interest in evaluating the cardiovascular effects of metals, alone and as a mixture. 11 , 12
Figure 1. Sources of exposure, pharmacokinetics, and cardiovascular outcomes of environmental metals lead (Pb), cadmium (Cd), and arsenic (As).
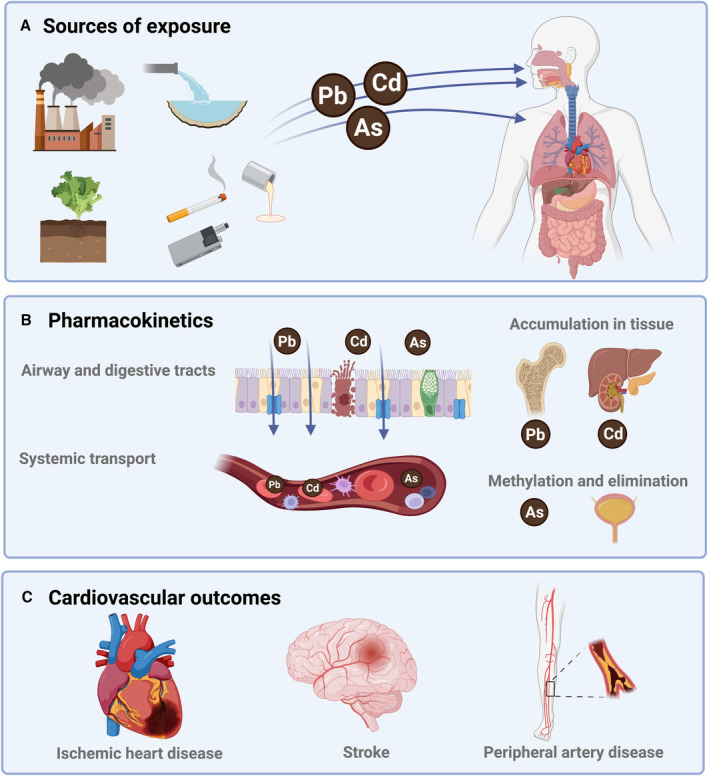
Figure created with Biorender.com.
Table 1.
Main Sources of Exposure, Pharmacokinetics, and Public Health and Regulatory Guidelines of Established Cardiotoxic Metals Relevant for General Populations
| Lead | Cadmium | Arsenic | |
|---|---|---|---|
| Sources of exposure |
Old paint Soil Water Smoking Drinks (wine) Ammunition Air (incinerators, combustion) Aviation fuels |
Smoking Food (organ meats, shellfish, root vegetables, green leafy vegetables) Air (incinerators, combustion, tires) |
Water Rice Drinks (apple juice, wine) Air and dust |
| Absorption |
Respiratory tract (100%) Gastrointestinal tract (<50%) Dermal contact (not relevant, except pica in children) |
Respiratory tract (100%) Gastrointestinal tract (<50%) Dermal contact (not relevant) |
Gastrointestinal tract (100% in water, <50% in food) Respiratory tract (<50%) Dermal contact (<5%) |
| Biotransformation | No | No | Methylation (via 1 carbon metabolism) |
| Accumulation |
Cortical bone (half‐life >30 y) Trabecular bone (half‐life 5–10 y) |
Liver, kidney, other soft tissues (half‐life >30 y) | No |
| Elimination | Minimal | Minimal | Urine |
| Established biomarkers |
Blood (half‐life 1 mo, reflects both external and bone levels) Bone (noninvasively through K‐shell XRF) |
Blood (half‐life 1 mo, reflects both external and soft‐tissue levels) Urine (half‐life of decades) |
Urine (half‐life in 3 phases, ranging from 1–4 d to 1 mo)* Toenail (past exposures ~6 mo ago) |
| Guidelines |
CDC blood reference value for children and pregnancy: 3.5 μg/dL OSHA occupational standard: 40 μg/dL ACOEM recommendation for regular blood testing: 10 μg/dL |
OSHA action urinary level: 3 μg/g creatinine OSHA action blood level: 5 μg/L |
Biological Exposure Index for the sum of inorganic and methylated arsenic in urine: 35 μg/g creatinine* EPA standard in drinking water is 10 μg/L |
ACOEM indicates American College of Occupational and Environmental Medicine; CDC, Centers for Disease Control and Prevention; EPA, Environmental Protection Agency; OSHA, Occupational and Safety Health Administration; and XRF, x‐ray fluorescence.
When assessing arsenic in the urine, it is important to either avoid seafood intake for at least 7 days (if only total arsenic is measured) or to analyze arsenic species including arsenobetaine (which is a specific biomarker of arsenicals in seafood). Arsenobetaine and other organic arsenic species in seafood are generally nontoxic.
BIOLOGICAL MECHANISMS
Overview
Experimental studies in vivo and in vitro indicate that exposure to contaminant metals alters key biological pathways with shared roles on the regulation of cardiac and vascular functions, 4 including impaired vascular endothelial function, 13 chronic inflammation, 14 hypertension, kidney toxicity (affecting primarily the proximal tubule), 15 oxidative stress, 16 lipid metabolism, 17 myocardial electric perturbations, 18 cardiotoxic effects, 19 and epigenetic effects 20 , 21 , 22 (Figure 2). A common mechanism for several metals to cause such disparate effects involves replacement of essential divalent cations. 9 , 10 Lead replaces calcium in tissues and metabolic pathways where calcium is involved, interfering with critical intracellular reactions and functions. Cadmium replaces zinc (again a divalent cation) in numerous enzymes and metalloproteins, accumulating in liver, kidney, and other soft tissues, and rendering those enzymes and proteins dysfunctional. Other essential divalent cations, notably copper, may be substituted as well. Unique toxic effects of each metal may be present as well, without necessarily invoking the replacement of essential metals. With regards to exposure levels and health effects, this statement focuses not on metal poisoning, as is seen with high level industrial exposures, but rather on low levels that are reached daily in our communities. Many mechanisms discussed are dose dependent, but data from in vitro and in vivo studies using model organisms are difficult to extrapolate to human exposures. When possible, we have highlighted evidence at lower doses. With the evidence available, none of the 3 metals discussed exhibit a clear threshold effect, below which one would expect no detectable cardiovascular damage when compared with a population without exposure.
Figure 2. Pharmacokinetics and relevant mechanisms for the subclinical and clinical effects of lead (Pb), cadmium (Cd), and arsenic (As). Figure created with Biorender.com.
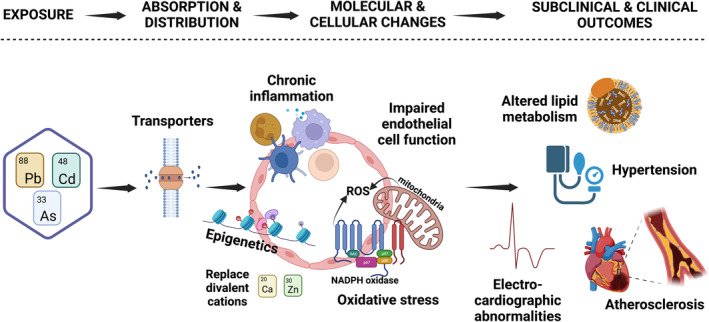
This is not a complete figure on mechanisms. For example, evidence on metals and hyperglycemia and thrombosis also exists. Ca indicates calcium; ROS, reactive oxygen species; and Zn, zinc.
Endothelial Injury
Exposure to metals induces endothelial injury. Cadmium and arsenic enhance endothelial cell expression of adhesion molecules, altering signaling, increasing permeability, and inducing oxidative stress and inflammation, all proatherosclerotic stimuli. 14 , 23 Lead and arsenic are associated with increased levels of soluble adhesion molecules in blood. 14 , 24 These changes are associated with changes in vascular function in vitro and in vivo, including altered contractility, disruption of localized blood flow, arterial stiffness measured by pulse wave velocity, and resultant hypertension. Airborne, metal‐containing fine particles (particulate matter ≤2.5 microns) can induce endothelial injury by impacting endothelial progenitor cell mobilization from bone marrow to peripheral blood and inhibiting signaling events triggered by vascular endothelial growth factor–receptor stimulation. 25
Inflammatory Mediators
While likely dependent on dose and cellular target, lead, cadmium, and arsenic exposures in vitro, in rodents, and in humans correlate with increased release of proinflammatory cytokines and inflammatory mediators, such as cyclooxygenase‐2, lipoxygenases, prostaglandins, and acute‐phase proteins, such as C‐reactive protein. 14 , 26 , 27
Oxidative Stress
Increased oxidative stress via production of reactive oxygen species has been proposed as a principal mechanism for the detrimental health effects of metals. 4 Lead and cadmium compete with copper and zinc, essential elements that play a fundamental role on cellular transport and redox balance maintenance. 9 , 10 Divalent toxic metals bind to sulfhydryl groups, which counteract the antioxidant properties of glutathione, metallothionein and copper/zinc superoxide dismutase, 28 , 29 as well as promote mitochondrial and endoplasmic reticulum stresses. 30 , 31 Increased levels of reactive oxygen species can increase oxidized lipids/lipoproteins, promoting atherosclerotic plaque formation.
Lipid Metabolism
Chronic exposure to environmental metals can affect lipid metabolism systemically and at the cellular level. Lead and cadmium levels in the body have been associated with differential circulating lipid profiles. 17 , 32 , 33 Arsenic alters cellular lipid homeostasis, such that macrophages retain lipids resulting in foam cell formation and increased atherosclerotic plaque. 34
Heart Rhythm and the ECG
Chronic arsenic exposure demonstrates unique cardiovascular toxicity by interfering with intracellular calcium accumulation in myocardial tissues via reduced surface expression of the cardiac potassium channel human ether‐à‐go‐go–related gene resulting in increased risk of QT prolongation and torsade de pointes. 35 Although the effect of inorganic arsenic in QT prolongation is a side effect of arsenic trioxide in the treatment of acute promyelocytic leukemia, an association between arsenic and QT prolongation has also been found at low‐chronic exposure levels. 36 , 37 Effects of lead and cadmium on another ECG parameter reflecting autonomic balance are reviewed in section Heart Rate Variability and Electrocardiographic Abnormalities.
Epigenomic Effects
Lead, cadmium, and arsenic have epigenomic effects, including effects on DNA methylation and histone modifications, influencing gene expression and downstream transcription. 20 , 21 , 22 They also show multiple gene–environment interactions. 38 The epigenetic modifications induced by lead and cadmium may be related to their replacement of zinc, which is required for the activity of DNA methyltransferases, histone acetyltransferases, histone deacetylases, and histone demethylases, although other mechanisms might be present. 39 Persistent cardiac epigenetic effects of lead exposure have been documented in animal models. 40 An epigenetic biomarker of lead levels in blood and bone has been developed using blood DNA methylation arrays. 41 These epigenetic biomarkers of lead internal dose in blood and bone have been associated with incident CVD in an independent population. 42 Arsenic is associated with blood DNA methylation‐based biomarkers that explain in part the association of arsenic with CVD. 22 These epigenomic and molecular effects of metals provide opportunities for the overlap of environmental and precision cardiology, informing clinical decisions, for instance, through the assessment of metal exposure biomarker levels directly, or indirectly through molecular signatures of metal exposure and internal dose.
EPIDEMIOLOGIC EVIDENCE
Most of the clinical evidence supporting this statement consists of epidemiological data strengthened by the mechanistic evidence presented in the prior section. The epidemiologic evidence has been curated to give the most weight to prospective cohort studies, especially for clinical end points. We focus largely on studies conducted in general populations, with levels of metals exposure ranging from low to moderate. The organization of the section has preclinical disease first, and then newly diagnosed cardiovascular events including nonfatal and fatal. We end with a discussion related to potential confounding.
Preclinical Atherosclerosis
Lead, cadmium, and arsenic are associated with preclinical measures of atherosclerosis. Higher blood lead levels are associated with the presence of carotid plaque in the Malmö Diet and Cancer Study cardiovascular cohort and in individuals with diabetes from 7 communities in China, as well as with moderate‐to‐severe coronary artery stenosis in Korean adults ongoing elective coronary computed angiography screening. 43 , 44 , 45 Higher blood cadmium levels are associated with carotid intima‐media thickness levels among Austrian women, the prevalence of atherosclerotic plaque in the Swedish Malmö Diet and Cancer study cardiovascular cohort, and the prevalence of coronary artery calcium (Agatston score >0) in the population‐based SCAPIS (Swedish Cardiopulmonary Bioimage Study). 46 In the Aragon Workers Health Study, a study of mostly male workers in a car manufacturing plant in Spain (levels of metal exposure relevant to general populations), urinary cadmium was positively associated with atherosclerosis in the carotid, femoral, and coronary vascular beds, and urinary arsenic was positively associated with carotid atherosclerotic plaque. 47 Higher urinary arsenic levels were associated with carotid intima‐media thickness levels in adults from Bangladesh 48 and with carotid intima‐media thickness and carotid plaque in the Strong Heart Study, a population‐based study in American Indian communities in the Southwest and the Great Plains. 49 The association of metals with preclinical atherosclerosis provides key information reconstructing the natural history of the disease from early atherosclerosis to the development of clinical symptoms and disease.
Heart Rate Variability and Electrocardiographic Abnormalities
Lead is associated with decreased heart rate variability, a marker of poor cardiovascular health that could be related to the neurotoxic effects of lead on the autonomic nervous system (in general populations and even in children). 3 , 50 , 51 In elderly men in the Normative Aging Study in Boston, tibia lead levels were associated with autonomic dysfunction overall, and the association was stronger among participants with metabolic syndrome. 51 Cadmium has also been associated with electrocardiographic abnormalities and with decreased heart rate variability in several studies. 52 , 53 General populations exposed to low‐level arsenic in drinking water including the Strong Heart Study and the Normative Aging Study found longer QT intervals in the presence of arsenic exposure. In a study in Poland, arsenic contamination in air pollution was associated with out‐of‐hospital cardiac arrest and sudden death. 54
Ischemic Heart Disease and Stroke
A systematic review published in 2018 in the British Medical Journal (37 studies, 348 259 nonoverlapping participants) reported that exposures to lead, cadmium, and inorganic arsenic contribute to clinical cardiovascular outcomes in general populations with a clear dose–response (Figure 3). 1 The pooled relative risk for ischemic heart disease (coronary heart disease) comparing the top versus bottom third of baseline level of each metal was 1.85 (95% CI, 1.27–2.69) for lead, 1.28 (95% CI, 0.98–1.71) for cadmium, and 1.23 (95% CI, 1.04–1.45) for arsenic; for stroke they were 1.63 (95% CI, 1.14–2.34) for lead, 1.72 (95% CI, 1.29–2.28) for cadmium, and 1.15 (95% CI, 0.92–1.43) for arsenic. Although most studies for lead exposure in this systematic review measured lead in blood, the Normative Aging Study measured lead in both blood and bone (in tibia and patella), using K‐shell x‐ray fluorescence, and correlated results with cardiovascular outcomes. 55 , 56 The adjusted hazard ratio (HR) for a 1‐log‐unit increase of blood, patella, and tibia lead was 1.45 (95% CI, 1.01–2.06), 2.64 (95% CI, 1.09–6.37), and 1.84 (95% CI, 0.57–5.90), respectively, suggesting that bone lead might better reflect the association of long‐term lead exposure compared with blood lead. 55
Figure 3. Pooled relative risks of lead, cadmium, and arsenic exposure and incident cardiovascular outcomes in a systematic review.
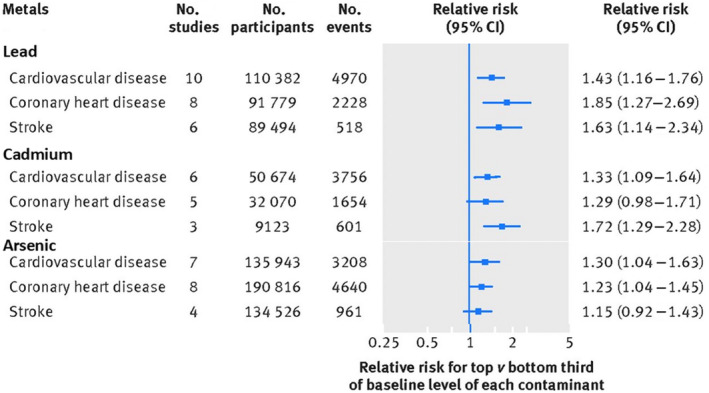
Adapted with permission from Chowdhury et al. 1 © Copyright 2018 BMJ Publishing Group Ltd.
Since the British Medical Journal systematic review in 2018, additional studies have been published. In the Hortega Study 57 , a general population from Spain, urinary cadmium was associated with incident CVD. In several studies from China, cadmium and arsenic were associated with increased incidence of ischemic heart disease, ischemic stroke, and overall stroke. 58 , 59 , 60 In the REGARDS (Reasons for Geographic and Racial Differences in Stroke) study, a case‐cohort study across the Southern United States, urinary cadmium as well as monomethylarsonate, a metabolite of inorganic arsenic, were positively associated with incident ischemic stroke. 58 , 61 The association between urinary cadmium and ischemic stroke in REGARDS remained positive but not significant in never smokers. Toxic metals other than lead, cadmium, and arsenic have also been associated with stroke. 62
Heart Failure and Measures of Cardiac Geometry and Function
The number of studies evaluating the association of metals with heart failure and left ventricular function is relatively small compared with other cardiovascular outcomes. Blood lead has been associated with left ventricular hypertrophy in several studies. 3 In young adults from the Strong Heart Study, urinary arsenic was associated with an increase in left ventricular wall thickness and left ventricular hypertrophy. 63 Higher urinary cadmium was positively associated with heart failure incidence in the Strong Heart Study (HR per interquintile range, 1.39 [95% CI, 1.01–1.94]); the Hortega Study (HR per interquintile range, 3.95 [95% CI, 1.44–10.9]); and nonsmokers of the Danish Diet, Cancer and Health cohort, in which the association of cadmium with heart failure appeared stronger in men (HR, 1.5 [95% CI, 1.2–1.9]). 64 These findings suggest that metals might contribute to cardiac remodeling and ultimately result in heart failure.
Peripheral Artery Disease
There is robust evidence supporting the association between contaminant metals and peripheral artery disease (PAD). In a 2021 statement, the American Heart Association recognized contaminant metals as nonconventional risk factors in PAD. 65 The National Health and Nutrition Examination Survey (NHANES) 1999 to 2000 revealed blood lead and cadmium levels to be 13.8% and 16.1% higher, respectively, in participants with PAD compared with those without PAD. 66 The Strong Heart Study in American Indian adults showed a prospective association between urine cadmium and PAD (ankle‐brachial index <0.9 or >1.4), independent from smoking, 67 and urine arsenic was associated with noncompressible PAD (ankle‐brachial index >1.4). 67 In Swedish women, cadmium was associated with incident PAD (ankle‐brachial index ≤0.9). 68 An environment‐wide association study in NHANES 1999 to 2004 evaluating hundreds of self‐reported and measured data points found urine cadmium as 1 of only 4 markers independently associated with PAD. 69 A small study suggested an association of PAD severity with increasing levels of urine cadmium in patients with established ischemic heart disease: urine cadmium levels were lower in patients with ischemic heart disease but not PAD, greater in patients with ischemic heart disease and PAD, and highest in patients with ischemic heart disease and critical limb‐threatening ischemia, the most severe stage of PAD. 70
Death
Extensive epidemiologic research shows that lead, cadmium, and arsenic are associated with premature death, attributable in large part to increased CVD risk. In the analysis of global burden conducted by the World Health Organization, the impact of lead exposure on cardiovascular death is estimated through the established effects of lead exposure increasing blood pressure and the subsequent effect of high blood pressure on CVDs. The risk however, as reviewed above, extends well beyond the effects on blood pressure and its complications.
In the United States, lead exposure, as measured in blood, was first associated with cardiovascular death in NHANES II (1976–1980), and confirmed in all subsequent NHANES studies, even at current markedly lower lead exposure levels than in the leaded gasoline era (Table S1). In NHANES 1999 to 2012, with follow‐up through 2015, the HR for CVD death comparing the 75th versus 25th percentile (2.49 versus 1.10 μg/dL) of blood lead levels was 1.45 (95% CI, 1.21–1.74). 5 In NHANES III (1988–1994), participants followed through 2011 (n=14 289), the annual excess deaths in the United States attributable to blood lead levels in the 90th versus 10th percentile (1.0–6.7 μg/dL) was estimated to be 412 000 total deaths, including 256 000 CVD deaths, of which 185 000 were attributable to ischemic heart disease deaths. 71 Data from men in Boston, women from 4 US cities, and men occupationally exposed to lead also supported positive associations with increased CVD death (Table S1). In men from Boston in the Normative Aging Study, the association of patella and tibia lead with CVD death and with ischemic heart disease death was also stronger compared with the corresponding association with blood lead. 56
The first study of an association between cadmium exposure and death reported higher death rates from atherosclerotic heart disease with higher atmospheric levels of cadmium in 28 US cities. 72 In NHANES III (1988–1994), urinary cadmium was associated with CVD death, although only among men. 73 In NHANES 1999 to 2004 (average follow‐up, 4.8 years), both blood and urine cadmium were associated with CVD death and ischemic heart disease death overall and in men and women. 74 In the Strong Heart Study, the HR comparing the 80th versus 20th percentile of urinary cadmium levels (1.62 and 0.55 μg/g creatinine) was 1.43 (95% CI, 1.21–1.70) for CVD death and 1.34 (95% CI, 1.10–1.63) for ischemic heart disease death. These studies were included in the British Medical Journal systematic review published in 2018. 1 Multiple studies have been published since then, including new studies in the United States using NHANES 1999 to 2012 data, and studies in Europe, China, and Australia, generally with consistent findings including fatal and nonfatal events (Table S2).
Studies in Taiwan, Chile, and Bangladesh have consistently shown that arsenic levels in drinking water >50 μg/L are associated with increased all‐cause and cardiovascular death. 75 , 76 Arsenic exposure has also been related to cardiovascular death in populations exposed to levels <50 μg/L in drinking water including rural communities in the United States (Strong Heart Study, San Luis Valley Diabetes Study in Colorado and New Hampshire), and in regions of Italy and Denmark (Table S3). In a dose–response meta‐analysis, a 2‐fold increase in arsenic levels in drinking water (eg, 20 versus 10 μg/L), the pooled relative risk was 1.07 (95% CI, 1.01–1.14) for CVD death and 1.16 (95% CI, 1.07–1.26) for ischemic heart disease death. 75 In NHANES 2003 to 2014, the HR for CVD death comparing 75th versus 25th percentile of urinary arsenic not derived from seafood (6.5 versus 2.30 μg/L) was 1.20 (95% CI, 0.83–1.74). 77 Although the number of deaths was small and the study lacked power, the point estimates are consistent with the previous meta‐analyses and supports cardiovascular harm even at low levels of arsenic exposure.
Reduced Cardiovascular Death Attributable to Public Health Measures
The reduction in death attributable to reduced lead and cadmium exposure in the United States suggests that public health measures have been partially successful. Using the difference in coefficients approach, Ruiz‐Hernandez et al 78 estimated that of 230.7 CVD deaths/100 000 person‐years avoided in the United States comparing NHANES 1999 to 2004 to NHANES III (1988–1994), 22.5% (52 deaths per 100 000 person‐years) were attributed to reductions in blood lead levels, and 8.4% (19.4 deaths per 100 000 person‐years) were attributed to reductions in blood cadmium levels observed from 1988 to 1994 and 1999 to 2004. However, work is still to be done, as increased cardiovascular risk remains easily detectable at the currently lower lead and cadmium exposure levels. Equivalent efforts for the impact of lowering arsenic exposure in the United States and other populations are not available.
Potential Sources of Bias in Epidemiological Studies
Despite the largely consistent associations across diverse populations, confounding remains a potential concern. Lead, cadmium, and arsenic are more prevalent in communities of color, which, in the United States, generally relates to lower socioeconomic status and exposure to psychosocial stressors including systemic racism (see Social Determinants of Metal Exposure: Inequalities and Environmental Justice). Cigarette smoking, a major risk factor for atherosclerotic CVD, also constitutes an important source of both cadmium and lead. Multivariable analyses have shown that after adjustment for tobacco smoking, including cotinine as an objective measure of smoking dose 66 and pack‐years as a measure of cumulative smoking, 67 lead and cadmium carry independent, significant risks. In Danish never smokers, urinary cadmium was consistently associated with an increased risk of acute myocardial infarction and heart failure but not with stroke (Table S2). In the REGARDS study of arsenic, sourcing drinking water from contaminated groundwater could be confounded by other water contaminants or community characteristics, as this is most common in rural areas. However, analyses in homogenous populations by sociodemographic and other environmental characteristics suggest that arsenic risk is independent of socioeconomic status. 75 Finally, measurement error, which is likely nondifferential and related to the challenges to measure cumulative metal exposures, could underestimate the actual associations.
SOCIAL DETERMINANTS OF METAL EXPOSURE: INEQUALITIES AND ENVIRONMENTAL JUSTICE
Exposure to environmental pollutants, including metals constitutes a part of the totality of environmental exposures that determine cardiovascular disease risk. To evaluate and understand the cardiovascular effects of metals, it is essential to consider their effects within the wider range of natural and social environmental conditions as well as local and personal environments that collectively determine the risk of exposure and its biological response. In the United States, race and ethnicity are highly correlated with residential location. 79 , 80 People of underrepresented races and ethnicities may encounter greater exposure to metals in drinking water and air because of proximity to environmental hazards (eg, major roadways, industrial sources, hazard waste sites, older homes), poor enforcement of environmental regulations (eg, drinking water), and inadequate response to community complaints. 81 , 82 , 83 , 84 , 85
Several studies have analyzed metal biomarker data from NHANES to identify differences by race and ethnicity. 86 , 87 , 88 , 89 , 90 Metal concentrations were found to be generally higher in Black, Hispanic, and Asian participants in comparison with White participants, as well as in foreign‐born versus US‐born participants, particularly for cadmium and lead. There are also differences in metal exposure at the neighborhood level by race and ethnicity. Lead contamination in drinking water is disproportionately higher in neighborhoods populated predominantly by people of underrepresented races and ethnicities. 91 , 92 American Indian and Hispanic communities are exposed to higher arsenic in drinking water compared with other groups. 93 , 94 US urban areas with a higher population of people of underrepresented races and ethnicities also have higher soil concentrations of lead, cadmium, and arsenic. 84 Globally, lower‐income communities in Asia, Africa, and the Americas are disproportionately exposed to lead, cadmium, and arsenic through contaminated air, water, and soil.
Despite overall declines in CVD morbidity and death in the United States in recent decades, populations defined by race and ethnicity continue to experience striking disparities in CVD. Because metals can be monitored and regulated, understanding the influence of metal exposure on these differences may suggest strategies for reducing health disparities.
MONITORING METAL EXPOSURE
The measurement of metals through environmental monitoring and biomonitoring is a critical prerequisite for public health and clinical interventions. So far, however, no regulation, public health recommendation, or clinical guideline has been developed to specifically address whether there is a need for universal or population‐specific monitoring in the context of CVD prevention.
Environmental Regulations
The Environmental Protection Agency regulates lead in ambient air as one of the criteria pollutants of the Clean Air Act, the limits of which remain unchanged since 2008 (Table S4). The Lead and Copper rule establishes that if >10% of customer tap‐water samples in a system exceed 15 μg/L, corrosion must be controlled. Implementation of this rule often fails. In Flint, Michigan; Newark, New Jersey; and other US cities and towns, lead concentration in the water supply has exceeded federal standards.
In 2021, the Environmental Protection Agency updated clearance levels for lead in dust from old housing to protect children. Exceeding limits in air, water, dust, and soil remains common, affecting numerous populations.
Cadmium and arsenic are regulated in drinking water through the Environmental Protection Agency Safe Drinking Water Act. Both are also listed as hazardous air pollutants by the Clean Air Act, but no limits have been set. For cadmium, there is an oral minimal risk level estimated through food, based on kidney outcomes.
The Occupational Safety and Health Administration limits occupational exposures, but the limits for the 3 metals are relatively high. The National Institute for Occupational Safety and Health and other agencies have additional advisory environmental and biomonitoring guidelines at lower levels, but those are not enforceable, leaving many workers unprotected.
Despite governmental regulations, however, problems with metal exposure persist. In NHANES 2011 to 2016, for example, ≈385 775 children aged 1 to 11 years had blood lead levels ≥5 μg/dL. 95
Individual Biomonitoring
The blood lead reference value established by the Centers for Disease Control and Prevention is the only biomonitoring guideline for public health and clinical action of any of the metals reviewed in this statement. Set to identify children with high levels of lead exposure, it was lowered from 5 to 3.5 μg/dL in 2022. The goal is to allow parents, doctors, public health officials, and communities to act earlier to reduce lead exposures. The Centers for Disease Control and Prevention/National Institute for Occupational Safety and Health have established 5 μg/dL as the reference level of lead exposure for adults. Although pediatricians routinely monitor blood lead levels in children in many states, no equivalent monitoring for lead or any other metal is established for adult patients. Potentially, it might be more relevant to measure bone lead, which is a better marker of cumulative lead exposure and more strongly associated with CVD in studies with those measures available. 55 , 56 Although measuring bone lead is challenging and results in radiation exposure, surrogate blood‐based epigenetic markers of bone lead provide some promise. 42
A common question is whether measuring metals in blood, urine, or bone can help identify and protect people at risk of developing CVD. Lead, cadmium, and arsenic biomarkers are well established in research (Table 2), and some are used by public health (blood lead) and occupational agencies (blood lead, blood and urine cadmium, urine arsenic) for surveillance purposes. Whether this is an effective strategy to identify and protect people at risk of CVD is unknown at present but may become a recognized strategy in the future. 5
Table 2.
Possible Biomarkers for Metal Assessment as Part of Clinical Practice for Cardiovascular Disease Protection
| Metal | Specimen (half‐life) | Method | Additional information | Possible reference value for adults |
|---|---|---|---|---|
| Lead |
Blood (30–100 d)* Bone (decades) Postchelation urine (decades) † |
ICPMS K‐shell XRF ICPMS |
Blood is the common marker Postchelation urine is an established measure of total body burden |
3.5 μg/dL (similar to children) … … |
| Cadmium |
Blood (30–100 d)* Urine (decades) Postchelation urine (unknown) † |
ICPMS ICPMS ICPMS |
Smokers have markedly high levels | 1.0 μg/L both blood and urine‡ (based on NHANES) |
| Arsenic |
Urine (1–30 d) Toenail (weeks of exposure 6 prior mo) |
ICPMS ICPMS or nuclear activation analysis |
Avoid seafood for 7 d before sample Measurement error is large |
5 μg/L (based on water standards) ‡ … |
ICPMS indicates inductively coupled plasma mass spectrometry; NHANES, National Health and Nutrition Examination Survey; and XRF, x‐ray fluorescence.
Reflects both exogenous and endogenous exposure from bone and other tissues.
Chelating agents for lead are intravenous (EDTA) or oral (dimercaptosuccinic acid [succimer]); the chelatable urine lead is considered a marker of lead body burden. Intravenous EDTA also chelates cadmium, however, whether postchelation urine cadmium reflects total cadmium body burden is not established.
First morning urine void (for spot urine samples, report per gram of creatinine). For cadmium, this limit is around 3 times the geometric mean in urine in NHANES (similar for blood). For arsenic, the measure of total arsenic requires no seafood in the preceding 7 days or using arsenic speciation (sum inorganic and methylated species). The possible guideline is proposed on the basis of the drinking water standard in New Jersey and New Hampshire and that the ratio in water and urine is 1.
INTERVENTIONS
Public Health Interventions
Public health measures through legislation and the mitigation and control of sources of exposures are essential to minimize metal contamination of air, water, food, and soil and to protect the population as a whole. For example, urgent interventions are needed to protect community water systems from lead contamination in urban settings and from arsenic contamination in rural community water systems. There is also a need to protect private well users, who in many instances are unaware of their metal exposures. Protection of food quality such as rice (cadmium and arsenic) would also serve to reduce public exposure.
Tobacco control measures remain critical to prevent lead and cadmium exposure, and new assessments and development of product standards could help reduce metal exposure. Under the Family Smoking Prevention and Tobacco Control Act of 2009, the US Food and Drug Administration has the authority to regulate tobacco products, and it could set product standards for preventing or minimizing metal exposure from both combustible and noncombustible tobacco products, including e‐cigarettes. However, to date, no such product standards have been set by the US Food and Drug Administration.
The most toxic air pollution components are the fine particles, which can rapidly pass into the circulation. Some fine particles (eg, those originating from coal fire power plants, harbors, incinerators, or heating systems) can be particularly rich in metals including lead, cadmium, and arsenic. Particles rich in metals are particularly toxic to the cardiovascular system. 96 , 97 Additional measures for preventing metal exposure thus include controlling emissions from industrial sites, incinerators, and heating systems, and cleaning the air from traffic pollution and other forms of combustion. Individual protection from inhaled metal components in fine particles include portable air cleaners, masks, and air filtering in indoor spaces. Whether trials can be designed to demonstrate efficacy of any single or combined strategy and whether any result would be scalable has yet to be defined. 98 Despite these efforts, metal exposure likely remains pervasive and additional strategies are needed to limit exposure to metals, including those derived from past exposures.
Medical Interventions
The basic and epidemiologic studies reviewed above convincingly indicate that even at low levels, lead, cadmium, and arsenic are linked to vascular damage and atherosclerotic disease. Yet pharmacotherapy to counteract these vascular effects does not exist. Whether removal of contaminant metals from an individual patient may reduce cardiovascular risk remains an important objective of environmental cardiology today.
Chelating agents with high affinity for toxic metals, particularly the edetates (EDTA and its salts), and dimercaptosuccinic acid (succimer) remove contaminant metals, especially lead and cadmium, from the human body. 99 A systematic review of all edetate clinical trials suggested a signal of benefit, with optimal clinical effect in patients with PAD and diabetes. 100 , 101 Among the trials reviewed, the 10‐year, National Institutes of Health–funded TACT (Trial to Assess Chelation Therapy) merits attention. TACT enrolled 1708 patients following myocardial infarction and targeted 40 infusions of EDTA‐based chelation versus placebo for each participant. At a median 5‐year follow‐up, there was an 18% (P=0.035) relative risk reduction of combined cardiovascular events, mostly on the basis of a 41% (P=0.0002) event reduction in 633 participants with diabetes. 102 , 103 Unfortunately, metal levels were not measured. A small case series suggested benefit and amputation prevention in patients with no‐option critical limb‐threatening ischemia. 104 TACT2, another National Institutes of Health–funded trial that should finish in 2023, aims to replicate TACT, and it also includes a metal biomarker component. Thus, there are lines of research in progress, addressing the potential benefits of modifying these novel risk factors at the individual level.
GAPS IN KNOWLEDGE
Identification of a pervasive family of risk factors attributable to a series of bioactive metal contaminants that affect the general population currently leads to more questions than solutions. The ideal measurement technique to identify contaminating metals in a wide range of matrices with high precision and sensitivity remain a critical impediment in widespread evaluation of the contribution of metals to the global burden of CVD. Indeed, even limits of exposure and the target level of risk factors remains unidentified. The National Institutes of Health–funded TACT2 105 will compare lead and cadmium levels over time following repeated chelation with EDTA in 1000 participants and may inform the laboratory science of clinically useful metal assays for the prevention of CVD.
Therapeutic intervention at the individual patient level could be considered in the future. For the general population with low‐level exposures, research can investigate the potential mitigation of the health effects of metals by healthy diet/lifestyle and nutritional supplementation such as folate and N‐acetyl cysteine. For instance, randomized clinical trials have shown that arsenic excretion can be accelerated and internal dose reduced using folate supplementation. 106 N‐acetyl cysteine, a source of sulfidryl groups with antioxidant properties has shown to counteract lead and cadmium toxicity in experimental models. 107 , 108 The clinical benefit from these nutritional and supplementation interventions from randomized trials or prospective cohort studies is scant. Treatments with inexpensive, powerful, and safe chelating agents that increase the excretion of lead and cadmium have been identified. These drugs, however, are not Food and Drug Administration approved for the prevention of CVD. The only drug tested in an National Institutes of Health–funded trial requires intravenous infusion, so even if positive results should be replicated, it will be difficult to scale. In addition to the confirmatory study TACT2, a pilot study for limb events (TACT3a) is also in progress. Still, a future direction of research should include how individuals who were exposed can be protected with therapies other than chelation.
CONCLUSIONS
Extensive literature, reviewed in this statement, suggests that exposure to metals constitutes a significant risk factor for CVD, including ischemic heart disease, stroke, and PAD. There is also convincing evidence that higher plasma or urinary levels of metals such as arsenic, lead, and cadmium are associated with subclinical CVD, hypertension, dyslipidemia, and electrical and functional disturbances in the myocardium. The totality of evidence supports the notion that environmental metal exposure increases the risk of premature cardiovascular death by contributing to CVD progression, severity, and clinical outcomes. The biological plausibility is strengthened by the results of mechanistic studies showing that contaminant metals may replace biologically essential metals 105 , 109 bound to critical proteins and that such protein dysfunction contributes to tissue oxidative stress as well as local and systemic inflammation. The light of this evidence and ongoing trials may enable wide recognition of metal exposure as a significant and possibly preventable CVD risk factor. Greater recognition of the contribution of metals to CVD would also spur basic scientists focused on drug development to develop new pharmacological interventions to remove metals or attenuate their effects. Political and public health leaders could set goals and regulations and implement programs to prevent and treat water, air, soil, and food pollution incorporating cost–benefit analyses that estimate the cardiovascular benefits of preventing metal exposure. This multipronged approach to environmental cardiology, if implemented, may provide a reduced‐pollution environment for all, not just for the privileged, leading to a proportional reduction in cardiovascular events.
Disclosures
Writing Group Disclosures
| Writing group member | Employment | Research grant | Other research support | Speakers’ bureau/honoraria | Expert witness | Ownership interest | Consultant/advisory board | Other |
|---|---|---|---|---|---|---|---|---|
| Gervasio A. Lamas | Columbia University, Mount Sinai Medical Center | NIH grants managed by Mount Sinai† | None | None | None | None | None | Columbia University Irving Medical Center (Professor)† |
| Ana Navas‐Acien | Columbia University | NIH (several NIH grants managed by Columbia University)† | None | None | None | None | None | Columbia University (professor)† |
| Aruni Bhatnagar | University of Louisville | None | None | None | None | None | None | None |
| Miranda R. Jones | Johns Hopkins Bloomberg School of Public Health | None | None | None | None | None | None | None |
| Koren K. Mann | McGill University (Canada) | None | None | None | None | None | None | None |
| Khurram Nasir | Houston Methodist | None | None | None | None | None | None | None |
| Maria Tellez‐Plaza | Instituto de Salud Carlos III, Spain | Intramural funds for research in health sciences, Instituto de Salud Carlos III, Spain, managed by Instituto de Salud Carlos III* | None | None | None | None | None | Universidad Autonoma de Madrid (Adjunct Associate Professor)* |
| Francisco Ujueta | Mount Sinai Medical Center | None | None | None | None | None | None | None |
This table represents the relationships of writing group members that may be perceived as actual or reasonably perceived conflicts of interest as reported on the Disclosure Questionnaire, which all members of the writing group are required to complete and submit. A relationship is considered to be “significant” if (a) the person receives $5000 or more during any 12‐month period, or 5% or more of the person's gross income; or (b) the person owns 5% or more of the voting stock or share of the entity, or owns $5000 or more of the fair market value of the entity. A relationship is considered to be “modest” if it is less than “significant” under the preceding definition.
*Modest.
†Significant.
Reviewer Disclosures
| Reviewer | Employment | Research grant | Other research support | Speakers’ bureau/honoraria | Expert witness | Ownership interest | Consultant/advisory board | Other |
|---|---|---|---|---|---|---|---|---|
| Jordi Bañeras | Hospital Universitari Vall Hebron (Spain) | None | None | None | None | None | None | None |
| Shohreh F. Farzan | Keck School of Medicine of the University of Southern California | None | None | None | None | None | None | None |
| Jaymie Meliker | Stony Brook University | NIH† | None | None | None | None | None | None |
| Jonathan D. Newman | New York University Grossman School of Medicine | None | NIH (Have received salary support as co‐director of the Clinical Coordiating Center and co‐site PI for the TACT2 trial)* | None | None | None | None | None |
| Sung Kyun Park | University of Michigan School of Public Health | None | None | None | None | None | None | None |
This table represents the relationships of reviewers that may be perceived as actual or reasonably perceived conflicts of interest as reported on the Disclosure Questionnaire, which all reviewers are required to complete and submit. A relationship is considered to be “significant” if (a) the person receives $5000 or more during any 12‐month period, or 5% or more of the person's gross income; or (b) the person owns 5% or more of the voting stock or share of the entity, or owns $5000 or more of the fair market value of the entity. A relationship is considered to be “modest” if it is less than “significant” under the preceding definition.
*Modest.
†Significant.
Supporting information
Appendix S1
The American Heart Association makes every effort to avoid any actual or potential conflicts of interest that may arise as a result of an outside relationship or a personal, professional, or business interest of a member of the writing panel. Specifically, all members of the writing group are required to complete and submit a Disclosure Questionnaire showing all such relationships that might be perceived as real or potential conflicts of interest.
This statement was approved by the American Heart Association Science Advisory and Coordinating Committee on January 26, 2023, and the American Heart Association Executive Committee on February 25, 2023. A copy of the document is available at https://professional.heart.org/statements by using either “Search for Guidelines & Statements” or the “Browse by Topic” area.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.123.029852
The opinions and views expressed in this statement are those of the authors and do not necessarily represent the official position of the Instituto de Salud Carlos III (Spain).
The American Heart Association requests that this document be cited as follows: Lamas GA, Bhatnagar A, Jones MR, Mann KK, Nasir K, Tellez‐Plaza M, Ujueta F, Navas‐Acien A; on behalf of the American Heart Association Council on Epidemiology and Prevention; Council on Cardiovascular and Stroke Nursing; Council on Lifestyle and Cardiometabolic Health; Council on Peripheral Vascular Disease; and Council on the Kidney in Cardiovascular Disease on behalf of the American Heart Association. Contaminant metals as cardiovascular risk factors: a scientific statement from the American Heart Association. J Am Heart Assoc. 2023;12:e028489. doi: 10.1161/JAHA.123.029852
The expert peer review of AHA‐commissioned documents (eg, scientific statements, clinical practice guidelines, systematic reviews) is conducted by the AHA Office of Science Operations. For more on AHA statements and guidelines development, visit https://professional.heart.org/statements. Select the “Guidelines & Statements” drop‐down menu, then click “Publication Development.”
References
- 1. Chowdhury R, Ramond A, O'Keeffe LM, Shahzad S, Kunutsor SK, Muka T, Gregson J, Willeit P, Warnakula S, Khan H, et al. Environmental toxic metal contaminants and risk of cardiovascular disease: systematic review and meta‐analysis. BMJ. 2018;362:k3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tellez‐Plaza M, Jones MR, Dominguez‐Lucas A, Guallar E, Navas‐Acien A. Cadmium exposure and clinical cardiovascular disease: a systematic review. Curr Atheroscler Rep. 2013;15:356. doi: 10.1007/s11883-013-0356-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Navas‐Acien A, Guallar E, Silbergeld EK, Rothenberg SJ. Lead exposure and cardiovascular disease—a systematic review. Environ Health Perspect. 2007;115:472–482. doi: 10.1289/ehp.9785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lind L, Araujo JA, Barchowsky A, Belcher S, Berridge BR, Chiamvimonvat N, Chiu WA, Cogliano VJ, Elmore S, Farraj AK, et al. Key characteristics of cardiovascular toxicants. Environ Health Perspect. 2021;129:95001. doi: 10.1289/EHP9321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang X, Mukherjee B, Park SK. Does information on blood heavy metals improve cardiovascular mortality prediction? J Am Heart Assoc. 2019;8:e013571. doi: 10.1161/JAHA.119.013571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bhatnagar A. Cardiovascular effects of particulate air pollution. Annu Rev Med. 2022;73:393–406. doi: 10.1146/annurev-med-042220-011549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bañeras J, Iglesies‐Grau J, Téllez‐Plaza M, Arrarte V, Báez‐Ferrer N, Benito B, Ruiz RC, Cecconi A, Domínguez‐Rodríguez A, Rodríguez‐Sinovas A, et al. Environment and cardiovascular health: causes, consequences and opportunities in prevention and treatment. Rev Esp Cardiol (Engl Ed). 2022;75:1050–1058. doi: 10.1016/j.rec.2022.05.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bhatnagar A. Environmental cardiology: studying mechanistic links between pollution and heart disease. Circ Res. 2006;99:692–705. doi: 10.1161/01.RES.0000243586.99701.cf [DOI] [PubMed] [Google Scholar]
- 9. Navas‐Acien A, Tellez‐Plaza M. Metals and health: science and practice. In: Boulton ML, Wallace RB, eds. Maxcy‐Rosenau‐Last Public Health & Preventive Medicine. 16th ed. McGraw Hill; 2022. [Google Scholar]
- 10. Nordberg GF, Fowler BA, Nordberg M, Friberg LT. Handbook on the Toxicology of Metals. Elsevier; 2007. [Google Scholar]
- 11. Domingo‐Relloso A, Grau‐Perez M, Briongos‐Figuero L, Gomez‐Ariza JL, Garcia‐Barrera T, Dueñas‐Laita A, Bobb JF, Chaves FJ, Kioumourtzoglou MA, Navas‐Acien A, et al. The association of urine metals and metal mixtures with cardiovascular incidence in an adult population from Spain: the Hortega follow‐up study. Int J Epidemiol. 2019;48:1839–1849. doi: 10.1093/ije/dyz061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yim G, Wang Y, Howe CG, Romano ME. Exposure to metal mixtures in association with cardiovascular risk factors and outcomes: a scoping review. Toxics. 2022;10:10. doi: 10.3390/toxics10030116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Singh P, O'Toole TE, Conklin DJ, Hill BG, Haberzettl P. Endothelial progenitor cells as critical mediators of environmental air pollution‐induced cardiovascular toxicity. Am J Physiol Heart Circ Physiol. 2021;320:H1440–H1455. doi: 10.1152/ajpheart.00804.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. States JC, Srivastava S, Chen Y, Barchowsky A. Arsenic and cardiovascular disease. Toxicol Sci. 2009;107:312–323. doi: 10.1093/toxsci/kfn236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Marques M, Millás I, Jiménez A, García‐Colis E, Rodriguez‐Feo JA, Velasco S, Barrientos A, Casado S, López‐Farré A. Alteration of the soluble guanylate cyclase system in the vascular wall of lead‐induced hypertension in rats. J Am Soc Nephrol. 2001;12:2594–2600. doi: 10.1681/ASN.V12122594 [DOI] [PubMed] [Google Scholar]
- 16. Solenkova NV, Newman JD, Berger JS, Thurston G, Hochman JS, Lamas GA. Metal pollutants and cardiovascular disease: mechanisms and consequences of exposure. Am Heart J. 2014;168:812–822. doi: 10.1016/j.ahj.2014.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhu X, Fan Y, Sheng J, Gu L, Tao Q, Huang R, Liu K, Yang L, Chen G, Cao H, et al. Association between blood heavy metal concentrations and dyslipidemia in the elderly. Biol Trace Elem Res. 2021;199:1280–1290. doi: 10.1007/s12011-020-02270-0 [DOI] [PubMed] [Google Scholar]
- 18. Eum KD, Nie LH, Schwartz J, Vokonas PS, Sparrow D, Hu H, Weisskopf MG. Prospective cohort study of lead exposure and electrocardiographic conduction disturbances in the Department of Veterans Affairs normative aging study. Environ Health Perspect. 2011;119:940–944. doi: 10.1289/ehp.1003279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shen J, Wang X, Zhou D, Li T, Tang L, Gong T, Su J, Liang P. Modelling cadmium‐induced cardiotoxicity using human pluripotent stem cell‐derived cardiomyocytes. J Cell Mol Med. 2018;22:4221–4235. doi: 10.1111/jcmm.13702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Riffo‐Campos AL, Fuentes‐Trillo A, Tang WY, Soriano Z, De Marco G, Rentero‐Garrido P, Adam‐Felici V, Lendinez‐Tortajada V, Francesconi K, Goessler W, et al. In silico epigenetics of metal exposure and subclinical atherosclerosis in middle aged men: pilot results from the Aragon Workers Health Study. Philos Trans R Soc Lond B Biol Sci. 2018;373(1748). doi: 10.1098/rstb.2017.0084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sanchez OF, Lee J, Yu King Hing N, Kim SE, Freeman JL, Yuan C. Lead (Pb) exposure reduces global DNA methylation level by non‐competitive inhibition and alteration of dnmt expression. Metallomics. 2017;9:149–160. doi: 10.1039/C6MT00198J [DOI] [PubMed] [Google Scholar]
- 22. Domingo‐Relloso A, Makhani K, Riffo‐Campos AL, Tellez‐Plaza M, Klein KO, Subedi P, Zhao J, Moon KA, Bozack AK, Haack K, et al. Arsenic exposure, blood DNA methylation, and cardiovascular disease. Circ Res. 2022;131:e51–e69. doi: 10.1161/CIRCRESAHA.122.320991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Messner B, Knoflach M, Seubert A, Ritsch A, Pfaller K, Henderson B, Shen YH, Zeller I, Willeit J, Laufer G, et al. Cadmium is a novel and independent risk factor for early atherosclerosis mechanisms and in vivo relevance. Arterioscler Thromb Vasc Biol. 2009;29:1392–1398. doi: 10.1161/ATVBAHA.109.190082 [DOI] [PubMed] [Google Scholar]
- 24. Camaj PR, Graziano JH, Preteni E, Popovac D, LoIacono N, Balac O, Factor‐Litvak P. Long‐term effects of environmental Lead exposure on blood pressure and plasma soluble cell adhesion molecules in young adults: a follow‐up study of a prospective cohort in Kosovo. J Environ Public Health. 2018;2018:3180487–3180410. doi: 10.1155/2018/3180487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Haberzettl P, Lee J, Duggineni D, McCracken J, Bolanowski D, O'Toole TE, Bhatnagar A, Conklin DJ. Exposure to ambient air fine particulate matter prevents VEGF‐induced mobilization of endothelial progenitor cells from the bone marrow. Environ Health Perspect. 2012;120:848–856. doi: 10.1289/ehp.1104206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Colacino JA, Arthur AE, Ferguson KK, Rozek LS. Dietary antioxidant and anti‐inflammatory intake modifies the effect of cadmium exposure on markers of systemic inflammation and oxidative stress. Environ Res. 2014;131:6–12. doi: 10.1016/j.envres.2014.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ma S, Zhang J, Xu C, da M, Xu Y, Chen Y, Mo X. Increased serum levels of cadmium are associated with an elevated risk of cardiovascular disease in adults. Environ Sci Pollut Res Int. 2022;29:1836–1844. doi: 10.1007/s11356-021-15732-2 [DOI] [PubMed] [Google Scholar]
- 28. Bridges CC, Zalups RK. Molecular and ionic mimicry and the transport of toxic metals. Toxicol Appl Pharmacol. 2005;204:274–308. doi: 10.1016/j.taap.2004.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Klaassen CD, Liu J. Metallothionein transgenic and knock‐out mouse models in the study of cadmium toxicity. J Toxicol Sci. 1998;23(Suppl 2):97–102. doi: 10.2131/jts.23.SupplementII_97 [DOI] [PubMed] [Google Scholar]
- 30. Dabravolski SA, Sadykhov NK, Kartuesov AG, Borisov EE, Sukhorukov VN, Orekhov AN. Interplay between Zn(2+) homeostasis and mitochondrial functions in cardiovascular diseases and heart ageing. Int J Mol Sci. 2022;23:6890. doi: 10.3390/ijms23136890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zachariah M, Maamoun H, Milano L, Rayman MP, Meira LB, Agouni A. Endoplasmic reticulum stress and oxidative stress drive endothelial dysfunction induced by high selenium. J Cell Physiol. 2021;236:4348–4359. doi: 10.1002/jcp.30175 [DOI] [PubMed] [Google Scholar]
- 32. Kim DW, Ock J, Moon KW, Park CH. Association between heavy metal exposure and dyslipidemia among Korean adults: from the Korean National Environmental Health Survey, 2015–2017. Int J Environ Res Public Health. 2022;19:3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhou Z, Lu YH, Pi HF, Gao P, Li M, Zhang L, Pei LP, Mei X, Liu L, Zhao Q, et al. Cadmium exposure is associated with the prevalence of dyslipidemia. Cell Physiol Biochem. 2016;40:633–643. doi: 10.1159/000452576 [DOI] [PubMed] [Google Scholar]
- 34. Lemaire M, Lemarié CA, Flores Molina M, Guilbert C, Lehoux S, Mann KK. Genetic deletion of LXRα prevents arsenic‐enhanced atherosclerosis, but not arsenic‐altered plaque composition. Toxicol Sci. 2014;142:477–488. doi: 10.1093/toxsci/kfu197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ficker E, Kuryshev YA, Dennis AT, Obejero‐Paz C, Wang L, Hawryluk P, Wible BA, Brown AM. Mechanisms of arsenic‐induced prolongation of cardiac repolarization. Mol Pharmacol. 2004;66:33–44. doi: 10.1124/mol.66.1.33 [DOI] [PubMed] [Google Scholar]
- 36. Moon KA, Zhang Y, Guallar E, Francesconi KA, Goessler W, Umans JG, Best LG, Howard BV, Devereux RB, Okin PM, et al. Association of low‐moderate urine arsenic and QT interval: cross‐sectional and longitudinal evidence from the strong heart study. Environ Pollut. 2018;240:894–902. doi: 10.1016/j.envpol.2018.04.129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mordukhovich I, Wright RO, Amarasiriwardena C, Baja E, Baccarelli A, Suh H, Sparrow D, Vokonas P, Schwartz J. Association between low‐level environmental arsenic exposure and QT interval duration in a general population study. Am J Epidemiol. 2009;170:739–746. doi: 10.1093/aje/kwp191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Grau‐Perez M, Pichler G, Galan‐Chilet I, Briongos‐Figuero LS, Rentero‐Garrido P, Lopez‐Izquierdo R, Navas‐Acien A, Weaver V, García‐Barrera T, Gomez‐Ariza JL, et al. Urine cadmium levels and albuminuria in a general population from Spain: a gene‐environment interaction analysis. Environ Int. 2017;106:27–36. doi: 10.1016/j.envint.2017.05.008 [DOI] [PubMed] [Google Scholar]
- 39. Brito S, Lee MG, Bin BH, Lee JS. Zinc and its transporters in epigenetics. Mol Cells. 2020;43:323–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Svoboda LK, Wang K, Jones TR, Colacino JA, Sartor MA, Dolinoy DC. Sex‐specific alterations in cardiac DNA methylation in adult mice by perinatal lead exposure. Int J Environ Res Public Health. 2021;18. doi: 10.3390/ijerph18020577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Colicino E, Just A, Kioumourtzoglou MA, Vokonas P, Cardenas A, Sparrow D, Weisskopf M, Nie LH, Hu H, Schwartz JD, et al. Blood DNA methylation biomarkers of cumulative lead exposure in adults. J Expo Sci Envir Epidemiol. 2021;31:108–116. doi: 10.1038/s41370-019-0183-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lieberman‐Cribbin W, Domingo‐Relloso A, Navas‐Acien A, Cole S, Haack K, Umans J, Tellez‐Plaza M, Colicino E, Baccarelli AA, Gao X, et al. Epigenetic biomarkers of lead exposure and cardiovascular disease: prospective evidence in the strong heart study. J Am Heart Assoc. 2022;11:e026934. doi: 10.1161/JAHA.122.026934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Harari F, Barregard L, Östling G, Sallsten G, Hedblad B, Forsgard N, Borné Y, Fagerberg B, Engström G. Blood lead levels and risk of atherosclerosis in the carotid artery: results from a Swedish cohort. Environ Health Perspect. 2019;127:127002. doi: 10.1289/EHP5057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kim S, Kang W, Cho S, Lim DY, Yoo Y, Park RJ, Lee BC, Moon JD, Park WJ. Associations between blood Lead Levels and coronary artery stenosis measured using coronary computed tomography angiography. Environ Health Perspect. 2021;129:27006. doi: 10.1289/EHP7351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wan H, Chen S, Cai Y, Chen Y, Wang Y, Zhang W, Chen C, Wang N, Guo Y, Lu Y. Lead exposure and its association with cardiovascular disease and diabetic kidney disease in middle‐aged and elderly diabetic patients. Int J Hyg Environ Health. 2021;231:113663. doi: 10.1016/j.ijheh.2020.113663 [DOI] [PubMed] [Google Scholar]
- 46. Barregard L, Sallsten G, Harari F, Andersson EM, Forsgard N, Hjelmgren O, Angerås O, Fagman E, Persson M, Lundh T, et al. Cadmium exposure and coronary artery atherosclerosis: a cross‐sectional population‐based study of Swedish middle‐aged adults. Environ Health Perspect. 2021;129:67007. doi: 10.1289/EHP8523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Grau‐Perez M, Caballero‐Mateos MJ, Domingo‐Relloso A, Navas‐Acien A, Gomez‐Ariza JL, Garcia‐Barrera T, Leon‐Latre M, Soriano‐Gil Z, Jarauta E, Cenarro A, et al. Toxic metals and subclinical atherosclerosis in carotid, femoral, and coronary vascular territories: the Aragon Workers Health Study. Arterioscler Thromb Vasc Biol. 2022;42:87–99. doi: 10.1161/ATVBAHA.121.316358 [DOI] [PubMed] [Google Scholar]
- 48. Chen Y, Hakim ME, Parvez F, Islam T, Rahman AM, Ahsan H. Arsenic exposure from drinking‐water and carotid artery intima‐medial thickness in healthy young adults in Bangladesh. J Health Popul Nutr. 2006;24:253–257. [PubMed] [Google Scholar]
- 49. Mateen FJ, Grau‐Perez M, Pollak JS, Moon KA, Howard BV, Umans JG, Best LG, Francesconi KA, Goessler W, Crainiceanu C, et al. Chronic arsenic exposure and risk of carotid artery disease: the strong heart study. Environ Res. 2017;157:127–134. doi: 10.1016/j.envres.2017.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yu YL, Thijs L, Yu CG, Yang WY, Melgarejo JD, Wei DM, Wei FF, Nawrot TS, Verhamme P, Roels HA, et al. Two‐year responses of heart rate and heart rate variability to first occupational lead exposure. Hypertension. 2021;77:1775–1786. doi: 10.1161/HYPERTENSIONAHA.120.16545 [DOI] [PubMed] [Google Scholar]
- 51. Park SK, Schwartz J, Weisskopf M, Sparrow D, Vokonas PS, Wright RO, Coull B, Nie H, Hu H. Low‐level lead exposure, metabolic syndrome, and heart rate variability: the VA normative aging study. Environ Health Perspect. 2006;114:1718–1724. doi: 10.1289/ehp.8992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Feng W, He X, Chen M, Deng S, Qiu G, Li X, Liu C, Li J, Deng Q, Huang S, et al. Urinary metals and heart rate variability: a cross‐sectional study of urban adults in Wuhan, China. Environ Health Perspect. 2015;123:217–222. doi: 10.1289/ehp.1307563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Thapa S, Delhey L, Jin J, Abouelenein S, Morad W, Delongchamp R, Faramawi MF. Association between urinary cadmium and QRS|T angle among adults in the United States. J Occup Environ Med. 2018;60:e412–e415. [DOI] [PubMed] [Google Scholar]
- 54. Sielski J, Kaziród‐Wolski K, Jóźwiak MA, Jóźwiak M. The influence of air pollution by PM2.5, PM10 and associated heavy metals on the parameters of out‐of‐hospital cardiac arrest. Sci Total Environ. 2021;788:147541. doi: 10.1016/j.scitotenv.2021.147541 [DOI] [PubMed] [Google Scholar]
- 55. Jain NB, Potula V, Schwartz J, Vokonas PS, Sparrow D, Wright RO, Nie H, Hu H. Lead levels and ischemic heart disease in a prospective study of middle‐aged and elderly men: the VA normative aging study. Environ Health Perspect. 2007;115:871–875. doi: 10.1289/ehp.9629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Weisskopf MG, Jain N, Nie H, Sparrow D, Vokonas P, Schwartz J, Hu H. A prospective study of bone lead concentration and death from all causes, cardiovascular diseases, and cancer in the department of veterans affairs normative aging study. Circulation. 2009;120:1056–1064. doi: 10.1161/CIRCULATIONAHA.108.827121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Domingo‐Relloso A, Grau‐Perez M, Briongos‐Figuero L, Gomez‐Ariza JL, Garcia‐Barrera T, Dueñas‐Laita A, Bobb JF, Chaves FJ, Kioumourtzoglou M‐A, Navas‐Acien A, et al. The association of urine metals and metal mixtures with cardiovascular incidence in an adult population from Spain: the Hortega follow‐up study. Int J Epidemiol. 2019;48:1839–1849. doi: 10.1093/ije/dyz061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Chen C, Xun P, Tsinovoi C, McClure LA, Brockman J, MacDonald L, Cushman M, Cai J, Kamendulis L, Mackey J, et al. Urinary cadmium concentration and the risk of ischemic stroke. Neurology. 2018;91:e382–e391. doi: 10.1212/WNL.0000000000005856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Yuan Y, Xiao Y, Feng W, Liu Y, Yu Y, Zhou L, Qiu G, Wang H, Liu B, Liu K, et al. Plasma metal concentrations and incident coronary heart disease in Chinese adults: the Dongfeng‐Tongji cohort. Environ Health Perspect. 2017;125:107007. doi: 10.1289/EHP1521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wen Y, Huang S, Zhang Y, Zhang H, Zhou L, Li D, Xie C, Lv Z, Guo Y, Ke Y, et al. Associations of multiple plasma metals with the risk of ischemic stroke: a case‐control study. Environ Int. 2019;125:125–134. doi: 10.1016/j.envint.2018.12.037 [DOI] [PubMed] [Google Scholar]
- 61. Tsinovoi CL, Xun P, McClure LA, Carioni VM, Brockman JD, Cai J, Guallar E, Cushman M, Unverzagt FW, Howard VJ, et al. Arsenic exposure in relation to ischemic stroke: the reasons for geographic and racial differences in stroke study. Stroke. 2018;49:19–26. doi: 10.1161/STROKEAHA.117.018891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Xiao Y, Yuan Y, Liu Y, Yu Y, Jia N, Zhou L, Wang H, Huang S, Zhang Y, Yang H, et al. Circulating multiple metals and incident stroke in Chinese adults. Stroke. 2019;50:1661–1668. doi: 10.1161/STROKEAHA.119.025060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Pichler G, Grau‐Perez M, Tellez‐Plaza M, Umans J, Best L, Cole S, Goessler W, Francesconi K, Newman J, Redon J, et al. Association of arsenic exposure with cardiac geometry and left ventricular function in young adults. Circ Cardiovasc Imaging. 2019;12:e009018. doi: 10.1161/CIRCIMAGING.119.009018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Sears CG, Eliot M, Raaschou‐Nielsen O, Poulsen AH, Harrington JM, Howe CJ, James KA, Roswall N, Overvad K, Tjønneland A, et al. Urinary cadmium and incident heart failure: a case‐cohort analysis among never‐smokers in Denmark. Epidemiology. 2022;33:185–192. doi: 10.1097/EDE.0000000000001446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Criqui MH, Matsushita K, Aboyans V, Hess CN, Hicks CW, Kwan TW, McDermott MM, Misra S, Ujueta F. Lower extremity peripheral artery disease: contemporary epidemiology, management gaps, and future directions: a scientific statement from the American Heart Association. Circulation. 2021;144:e171–e191. doi: 10.1161/CIR.0000000000001005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Navas‐Acien A, Selvin E, Sharrett AR, Calderon‐Aranda E, Silbergeld E, Guallar E. Lead, cadmium, smoking, and increased risk of peripheral arterial disease. Circulation. 2004;109:3196–3201. doi: 10.1161/01.CIR.0000130848.18636.B2 [DOI] [PubMed] [Google Scholar]
- 67. Tellez‐Plaza M, Guallar E, Fabsitz RR, Howard BV, Umans JG, Francesconi KA, Goessler W, Devereux RB, Navas‐Acien A. Cadmium exposure and incident peripheral arterial disease. Circ Cardiovasc Qual Outcomes. 2013;6:626–633. doi: 10.1161/CIRCOUTCOMES.112.000663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Fagerberg B, Bergström G, Borén J, Barregard L. Cadmium exposure, intercellular adhesion molecule‐1 and peripheral artery disease: a cohort and an experimental study. BMJ Open. 2013;3:e002489. doi: 10.1136/bmjopen-2012-002489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Zhuang X, Ni A, Liao L, Guo Y, Dai W, Jiang Y, Zhou H, Hu X, du Z, Wang X, et al. Environment‐wide association study to identify novel factors associated with peripheral arterial disease: evidence from the National Health and nutrition examination survey (1999‐2004). Atherosclerosis. 2018;269:172–177. doi: 10.1016/j.atherosclerosis.2018.01.006 [DOI] [PubMed] [Google Scholar]
- 70. Ujueta F, Arenas IA, Diaz D, Yates T, Beasley R, Navas‐Acien A, Lamas GA. Cadmium level and severity of peripheral artery disease in patients with coronary artery disease. Eur J Prev Cardiol. 2019;26:1456–1458. doi: 10.1177/2047487318796585 [DOI] [PubMed] [Google Scholar]
- 71. Lanphear BP, Rauch S, Auinger P, Allen RW, Hornung RW. Low‐level lead exposure and mortality in US adults: a population‐based cohort study. Lancet Public Health. 2018;3:e177–e184. doi: 10.1016/S2468-2667(18)30025-2 [DOI] [PubMed] [Google Scholar]
- 72. Carroll RE. The relationship of cadmium in the air to cardiovascular disease death rates. JAMA. 1966;198:267–269. doi: 10.1001/jama.1966.03110160095029 [DOI] [PubMed] [Google Scholar]
- 73. Menke A, Muntner P, Silbergeld EK, Platz EA, Guallar E. Cadmium levels in urine and mortality among U.S. adults. Environ Health Perspect. 2009;117:190–196. doi: 10.1289/ehp.11236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Tellez‐Plaza M, Navas‐Acien A, Menke A, Crainiceanu CM, Pastor‐Barriuso R, Guallar E. Cadmium exposure and all‐cause and cardiovascular mortality in the U.S. general population. Environ Health Perspect. 2012;120:1017–1022. doi: 10.1289/ehp.1104352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Moon KA, Oberoi S, Barchowsky A, Chen Y, Guallar E, Nachman KE, Rahman M, Sohel N, D'Ippoliti D, Wade TJ, et al. A dose‐response meta‐analysis of chronic arsenic exposure and incident cardiovascular disease. Int J Epidemiol. 2017;46:1924–1939. doi: 10.1093/ije/dyx202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Argos M, Kalra T, Rathouz PJ, Chen Y, Pierce B, Parvez F, Islam T, Ahmed A, Rakibuz‐Zaman M, Hasan R, et al. Arsenic exposure from drinking water, and all‐cause and chronic‐disease mortalities in Bangladesh (HEALS): a prospective cohort study. Lancet. 2010;376:252–258. doi: 10.1016/S0140-6736(10)60481-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Nigra AE, Moon KA, Jones MR, Sanchez TR, Navas‐Acien A. Urinary arsenic and heart disease mortality in NHANES 2003‐2014. Environ Res. 2021;200:111387. doi: 10.1016/j.envres.2021.111387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Ruiz‐Hernandez A, Navas‐Acien A, Pastor‐Barriuso R, Crainiceanu CM, Redon J, Guallar E, Tellez‐Plaza M. Declining exposures to lead and cadmium contribute to explaining the reduction of cardiovascular mortality in the US population, 1988–2004. Int J Epidemiol. 2017;46:1903–1912. doi: 10.1093/ije/dyx176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Williams DR, Collins C. Racial residential segregation: a fundamental cause of racial disparities in health. Public Health Rep. 2001;116:404–416. doi: 10.1016/S0033-3549(04)50068-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Williams DR. Race and health: basic questions, emerging directions. Ann Epidemiol. 1997;7:322–333. doi: 10.1016/S1047-2797(97)00051-3 [DOI] [PubMed] [Google Scholar]
- 81. Gee GC, Payne‐Sturges DC. Environmental health disparities: a framework integrating psychosocial and environmental concepts. Environ Health Perspect. 2004;112:1645–1653. doi: 10.1289/ehp.7074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Bullard RD, Wright BH. Environmental justice for all: community perspectives on health and research needs. Toxicol Ind Health. 1993;9:821–841. doi: 10.1177/074823379300900508 [DOI] [PubMed] [Google Scholar]
- 83. Maantay J. Zoning, equity, and public health. Am J Public Health. 2001;91:1033–1041. doi: 10.2105/AJPH.91.7.1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Jones DH, Yu X, Guo Q, Duan X, Jia C. Racial disparities in the heavy metal contamination of urban soil in the southeastern United States. Int J Environ Res Public Health. 2022;19. doi: 10.3390/ijerph19031105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Morello‐Frosch R, Lopez R. The riskscape and the color line: examining the role of segregation in environmental health disparities. Environ Res. 2006;102:181–196. doi: 10.1016/j.envres.2006.05.007 [DOI] [PubMed] [Google Scholar]
- 86. Awata H, Linder S, Mitchell LE, Delclos GL. Biomarker levels of toxic metals among Asian populations in the United States: NHANES 2011‐2012. Environ Health Perspect. 2017;125:306–313. doi: 10.1289/EHP27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Jones MR, Tellez‐Plaza M, Vaidya D, Grau‐Perez M, Post WS, Kaufman JD, Guallar E, Francesconi KA, Goessler W, Nachman KE, et al. Ethnic, geographic and dietary differences in arsenic exposure in the multi‐ethnic study of atherosclerosis (MESA). J Expo Sci Environ Epidemiol. 2019;29:310–322. doi: 10.1038/s41370-018-0042-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Mijal RS, Holzman CB. Blood cadmium levels in women of childbearing age vary by race/ethnicity. Environ Res. 2010;110:505–512. doi: 10.1016/j.envres.2010.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Teye SO, Yanosky JD, Cuffee Y, Weng X, Luquis R, Farace E, Wang L. Exploring persistent racial/ethnic disparities in lead exposure among American children aged 1‐5 years: results from NHANES 1999–2016. Int Arch Occup Environ Health. 2021;94:723–730. doi: 10.1007/s00420-020-01616-4 [DOI] [PubMed] [Google Scholar]
- 90. Wen X, Li T, Xu X. Cadmium exposure in US adults, research based on the National Health and nutrition examination survey from 1988 to 2018. Environ Sci Pollut Res Int. 2022;29:22293–22305. doi: 10.1007/s11356-021-17484-5 [DOI] [PubMed] [Google Scholar]
- 91. Levin R, Zilli Vieira CL, Rosenbaum MH, Bischoff K, Mordarski DC, Brown MJ. The urban lead (Pb) burden in humans, animals and the natural environment. Environ Res. 2021;193:110377. doi: 10.1016/j.envres.2020.110377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Gostin LO. Lead in the water: a tale of social and environmental injustice. JAMA. 2016;315:2053–2054. doi: 10.1001/jama.2016.5581 [DOI] [PubMed] [Google Scholar]
- 93. Sobel M, Sanchez TR, Zacher T, Mailloux B, Powers M, Yracheta J, Harvey D, Best LG, Bear AB, Hasan K, et al. Spatial relationship between well water arsenic and uranium in Northern Plains native lands. Environ Pollut. 2021;287:117655. doi: 10.1016/j.envpol.2021.117655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Nigra AE, Chen Q, Chillrud SN, Wang L, Harvey D, Mailloux B, Factor‐Litvak P, Navas‐Acien A. Inequalities in public water arsenic concentrations in counties and community water systems across the United States, 2006–2011. Environ Health Perspect. 2020;128:127001. doi: 10.1289/EHP7313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Egan KB, Cornwell CR, Courtney JG, Ettinger AS, Blood Lead Levels in U.S . Children ages 1–11 years, 1976–2016. Environ Health Perspect. 2021;129:37003. doi: 10.1289/EHP7932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Rahmatinia T, Kermani M, Farzadkia M, Nicknam MH, Soleimanifar N, Mohebbi B, Jafari AJ, Shahsavani A, Fanaei F. Potential cytotoxicity of PM2.5‐bound PAHs and toxic metals collected from areas with different traffic densities on human lung epithelial cells (A549). J Environ Health Sci Eng. 2021;19:1701–1712. doi: 10.1007/s40201-021-00724-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Thurston GD, Chen LC, Campen M. Particle toxicity's role in air pollution. Science. 2022;375:506. doi: 10.1126/science.abn4481 [DOI] [PubMed] [Google Scholar]
- 98. Newman JD, Bhatt DL, Rajagopalan S, Balmes JR, Brauer M, Breysse PN, Brown AGM, Carnethon MR, Cascio WE, Collman GW, et al. Cardiopulmonary impact of particulate air pollution in high‐risk populations: JACC state‐of‐the‐art review. J Am Coll Cardiol. 2020;76:2878–2894. doi: 10.1016/j.jacc.2020.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Arenas IA, Navas‐Acien A, Ergui I, Lamas GA. Enhanced vasculotoxic metal excretion in post‐myocardial infarction patients following a single edetate disodium‐based infusion. Environ Res. 2017;158:443–449. doi: 10.1016/j.envres.2017.06.039 [DOI] [PubMed] [Google Scholar]
- 100. Lamas GA, Goertz C, Boineau R, Mark DB, Rozema T, Nahin RL, Drisko JA, Lee KL. Design of the Trial to assess chelation therapy (TACT). Am Heart J. 2012;163:7–12. doi: 10.1016/j.ahj.2011.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Ravalli F, Vela Parada X, Ujueta F, Pinotti R, Anstrom KJ, Lamas GA, Navas‐Acien A. Chelation therapy in patients with cardiovascular disease: a systematic review. J Am Heart Assoc. 2022;11:e024648. doi: 10.1161/JAHA.121.024648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Lamas GA, Goertz C, Boineau R, Mark DB, Rozema T, Nahin RL, Lindblad L, Lewis EF, Drisko J, Lee KL, et al. Effect of disodium EDTA chelation regimen on cardiovascular events in patients with previous myocardial infarction: the TACT randomized trial. JAMA. 2013;309:1241–1250. doi: 10.1001/jama.2013.2107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Escolar E, Lamas GA, Mark DB, Boineau R, Goertz C, Rosenberg Y, Nahin RL, Ouyang P, Rozema T, Magaziner A, et al. The effect of an EDTA‐based chelation regimen on patients with diabetes mellitus and prior myocardial infarction in the trial to assess chelation therapy (TACT). Circ Cardiovasc Qual Outcomes. 2014;7:15–24. doi: 10.1161/CIRCOUTCOMES.113.000663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Arenas I, Ujueta F, Diaz D, Yates T, Olivieri B, Beasley R, Lamas G. Limb preservation using Edetate disodium‐based chelation in patients with diabetes and critical limb ischemia: an open‐label pilot study. Cureus. 2019;11:e6477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Lamas GA, Anstrom KJ, Navas‐Acien A, Boineau R, Kim H, Rosenberg Y, Stylianou M, Jones TLZ, Joubert BR, Santella RM, et al. The trial to assess chelation therapy 2 (TACT2): rationale and design. Am Heart J. 2022;252:1–11. doi: 10.1016/j.ahj.2022.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Bozack AK, Hall MN, Liu X, Ilievski V, Lomax‐Luu AM, Parvez F, Siddique AB, Shahriar H, Uddin MN, Islam T, et al. Folic acid supplementation enhances arsenic methylation: results from a folic acid and creatine supplementation randomized controlled trial in Bangladesh. Am J Clin Nutr. 2019;109:380–391. doi: 10.1093/ajcn/nqy148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Jaafarzadeh M, Mahjoob Khaligh R, Mohsenifar Z, Shabani A, Rezvani Gilkalaei MM, Rajabi Keleshteri S, Beigi Harchegani A. Protecting effects of N‐acetyl cysteine supplementation against lead and cadmium‐induced brain toxicity in rat models. Biol Trace Elem Res. 2022;200:4395–4403. doi: 10.1007/s12011-021-03034-0 [DOI] [PubMed] [Google Scholar]
- 108. Siddarth M, Chawla D, Raizada A, Wadhwa N, Banerjee BD, Sikka M. Lead‐induced DNA damage and cell apoptosis in human renal proximal tubular epithelial cell: attenuation via N‐acetyl cysteine and tannic acid. J Biochem Mol Toxicol. 2018;32:e22038. doi: 10.1002/jbt.22038 [DOI] [PubMed] [Google Scholar]
- 109. Check L, Marteel‐Parrish A. The fate and behavior of persistent, bioaccumulative, and toxic (PBT) chemicals: examining lead (Pb) as a PBT metal. Rev Environ Health. 2013;28:85–96. doi: 10.1515/reveh-2013-0005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1


