Abstract
Multiple sclerosis is a multifactorial chronic inflammatory disease of the central nervous system that leads to demyelination and neuronal cell death, resulting in functional disability. Remyelination is the natural repair process of demyelination, but it is often incomplete or fails in multiple sclerosis. Available therapies reduce the inflammatory state and prevent clinical relapses. However, therapeutic approaches to increase myelin repair in humans are not yet available. The substance cytidine-5′-diphosphocholine, CDP-choline, is ubiquitously present in eukaryotic cells and plays a crucial role in the synthesis of cellular phospholipids. Regenerative properties have been shown in various animal models of diseases of the central nervous system. We have already shown that the compound CDP-choline improves myelin regeneration in two animal models of multiple sclerosis. However, the results from the animal models have not yet been studied in patients with multiple sclerosis. In this review, we summarise the beneficial effects of CDP-choline on biolipid metabolism and turnover with regard to inflammatory and regenerative processes. We also explain changes in phospholipid and sphingolipid homeostasis in multiple sclerosis and suggest a possible therapeutic link to CDP-choline.
Key Words: astrocytes, CDP-choline, cuprizone, microglia, multiple sclerosis, oligodendrocytes
Introduction
Multiple sclerosis (MS) is a chronic inflammatory disease of the central nervous system (CNS) that leads to demyelination, disruption of neuronal activity, action potential transmission, and ultimately neuronal degradation and subsequent physical disability in patients (Reich et al., 2018; Thompson et al., 2018). It is estimated that approximately 43–70% of patients with MS have cognitive impairment (Costers et al., 2021). MS is a multifactorial and complex disease with great individual heterogeneity in its course and the extent of remyelination capacity (Chung et al., 2020). In general, 85% of MS patients suffer from the remitting-relapsing form of the disease, which often progresses to a secondary progressive state. 15% of patients develop progressive MS from the beginning (Dobson and Giovannoni, 2019). Current MS therapies, mainly based on immunomodulatory effects, can successfully control the inflammatory phase in MS, but there are still no effective drugs that could restore nerve function. Therefore, a detailed understanding of the mechanisms underlying remyelination is essential. Meanwhile, the search for pharmacological agents to promote remyelination is well advanced, and numerous bioactive molecules have been identified and even successfully tested for their regenerative capacity in human studies (Gottle et al., 2019; Lubetzki et al., 2020; Cunniffe and Coles, 2021). However, many questions remain unanswered, and research is still in the early stages of successful regenerative therapy for MS.
CDP-choline is an essential intermediate and the rate-limiting step in the biosynthetic pathway (Kennedy pathway) of phosphatidylcholine (PtdCho), one of the major phospolipids in mammalian cell membranes (Weiss, 1995). Biolipids are not only essential components of biomembranes and myelin in particular but their various metabolites are intensively involved in a variety of cellular processes, including the regulation of inflammation, apoptosis, cell cycle, survival, and cell communication. Therefore, exogenous delivery of CDP-choline has numerous beneficial effects that promote cell regeneration and survival, restore biolipid balance, and reduce inflammation (Adibhatla et al., 2002; Grieb, 2014; Secades and Gareri, 2022). In addition, our previous studies in various MS animal models have shown that CDP-choline has beneficial effects on cells of oligodendrocyte lineage, particularly by increasing the proliferation of oligodendrocyte progenitor cells (OPC), leading to an enhancement of remyelination (Skripuletz et al., 2015). CDP-choline may be a new candidate for regenerative MS therapy by reducing inflammation-related disorders, promoting CNS regeneration, and restoring biolipid metabolism, which appears to be disrupted in MS (Wheeler et al., 2008; Grassi et al., 2020; Penkert et al., 2020; Alaamery et al., 2021; Pineda-Torra et al., 2021). Since CDP-choline has a favorable safety profile, its use in human trials for regenerative MS therapy is conceivable.
Search Strategy
Studies on the various topics mentioned in this narrative review were searched in PubMed at the National Institute of Health (NIH) using keywords and keyword groups for each topic (e.g., multiple sclerosis; remyelination in multiple sclerosis; astrocytes in multiple sclerosis; oligodendrocytes in multiple sclerosis; cuprizone; phospholipids; myelin; CDP-choline). The scientific articles that have been cited in this review were published in the time period from 1965 to 2023. The year of publication and/or authorship were not set as a limitation for the database search. However, special care was taken to use relatively recent articles whenever possible. In some cases, it was necessary to cite original pioneer research publications to give credit to the first authors, and therefore older citations were used.
Possible Approaches to Enhance Remyelination in Multiple Sclerosis
It is meanwhile well known that successful remyelination depends on a sufficient amount of OPC that are able to migrate, proliferate, and differentiate into myelinating oligodendrocytes, which in turn can form intact myelin sheaths around axons (Cunniffe and Coles, 2021). On the other hand, healthy neurons are needed to provide signals for myelination. The concept that remyelination protects axons from degradation and promotes restoration of axonal conduction velocity has been demonstrated in numerous animal studies and even human clinical trials (Irvine and Blakemore, 2008; Bodini et al., 2016; Mei et al., 2016). However, as we observed using the cuprizone-induced demyelination model, axons can survive demyelinated for at least some time, providing a “therapeutic window” for treatment (Gudi et al., 2017). The most damaging insults (disruption of axonal traffic) were caused by activated microglia and even injured axons could be remyelinated (Gudi et al., 2017). Several lines of evidence suggest that impairment of OPC differentiation may be a crucial factor for deficient remyelination, especially in the elderly (Shields et al., 1999; Kuhlmann et al., 2008). However, recent studies show that the assumption that promoting differentiation of resident OPC into mature oligodendrocytes leads to improved remyelination in MS may be oversimplified or not universal for the situation in the human CNS (Jakel et al., 2019; Yeung et al., 2019). Animal models are a powerful tool to study the mechanisms of de- and remyelination in detail. However, they have limitations as they usually simulate a single, local and/or completed event that has a beginning and an end. They can hardly reflect the individual, multifactorial and simultaneous processes that occur in MS. Oligodendrocytes are a heterogeneous population with different gene expression profiles in different brain regions, and this oligodendroglial heterogeneity appears to be altered in MS, suggesting different functional states of oligodendrocytes in MS lesions (Jakel et al., 2019). Moreover, the remaining mature oligodendrocytes may promote remyelination even in shadow plaques (Yeung et al., 2019). Furthermore, the number of OPC appears to be reduced in shadow plaques (Jakel et al., 2019). In animals, NG2+ OPC represent the majority of proliferating cells in the adult CNS. A two-photon imaging study in NG2-mEGFP mice revealed that NG+ OPC in cortical layers 1–3 can readily differentiate into mature oligodendrocytes without prior proliferation. However, when an OPC drives differentiation, neighboring cells proliferate and migrate into the free space (Hughes et al., 2013). Alternatively, robust remyelination might rely on coupled events of proliferation and sequential differentiation and could be promoted by a specific subclass of OPC that enter a proliferation phase in response to external signals from the environment (microglia/astrocytes). Otherwise, in our animal experiments using the cuprizone model, we would not observe a sequence of the proliferation of the existing OPC and their subsequent differentiation (Gingele et al., 2020; Gudi et al., 2021).
Remyelination can take place if the microenvironment is conducive and important molecular signals are available. Astrocytes and microglia are actively involved in developmental and regenerative myelination and support oligodendrocytes and neurons with a variety of trophic, metabolic, and conducting molecules. Astrocytes are part of the blood-brain barrier infrastructure and, together with endothelial cells, regulate the entry of vital nutrients, ions, and pathogens into the CNS. Disruptions and dysregulations of these cellular cross-talks can lead to the initiation, manifestation or/and progression of various neurological diseases (Reich et al., 2018). Recently, a significant association was found between biomarkers of aberrant astrocyte and microglial activation in the cerebrospinal fluid of MS patients and MS severity, indicating the pathogenic role of resident glial cells and their contribution to disability progression (Masvekar et al., 2019). In particular, in the secondary progressive stage of the disease, resident glial cells and the microenvironment appear to be a dominant trigger of inflammation and neurodegeneration (Liddelow et al., 2017; Masvekar et al., 2019).
Similar to other regenerative processes, remyelination becomes less efficient over time (Shields et al., 1999; Gingele et al., 2020; Lubetzki et al., 2020). Age-related changes affect not only OPC, which become increasingly less sensitive to differentiation-promoting factors, but also microglia, which produce fewer differentiation-promoting molecules and alter their inflammatory response and phagocytic activity (Angelova and Brown, 2019; Lubetzki et al., 2020). Aged astrocytes upregulate reactive genes and produce more toxic, pro-inflammatory molecules (Clarke et al., 2018). Niacin-mediated rejuvenation of macrophages/microglia, followed by upregulation of the scavenger receptor CD36, which is decreased in aged mice, was able to improve remyelination in aged mice in the lysolecithin animal model. Similar results were obtained in aged mice exposed to a juvenile systemic milieu through heterochronic parabiosis by increasing the clearance of inhibitory myelin debris by young macrophages (Ruckh et al., 2012). Therefore, a detailed understanding of the signaling pathways and key molecules that regulate oligodendrocyte lineage progression, homeostasis, and regenerative capacity is essential to identify therapeutic targets to promote remyelination. It has long been known that growth factors, particularly platelet-derived growth factor, insulin-like growth factor 1, the family of fibroblast growth factors, neurotrophins (e.g., brain-derived neurotrophic factor) and some hormones (T3, thyroid hormone, adrenocorticotropin) exert a major influence on oligodendrocyte development by modulating oligodendrocyte-related processes via Ras/Raf/ Mek/Erk- and PI3K/Akt/mTOR-dependent signaling pathways, among others (Guardiola-Diaz et al., 2012; Gottle et al., 2019; Lubetzki et al., 2020). Klotho, an anti-aging protein, can exist in three different forms: secreted, membrane bound, as a co-receptor for fibroblast growth factors and intracellular. It has been shown to act through several signaling pathways including fibroblast growth factors, FSR2-Act/Erk1/2, insulin/insulin-like growth factor 1 and Wnt by promoting oligodendrocyte maturation and myelination in vitro and accelerating remyelination in animal models of demyelination, which is a useful therapeutic target to protect the brain from age-related remodeling and promote regeneration in MS (Torbus-Paluszczak et al., 2018). Decreased levels of Klotho in the cerebrospinal fluid have been associated with progressive disability in patients with MS (Emami Aleagha et al., 2015). The G protein-coupled Smoothened (Smo) receptor and the component of the sonic hedgehog signaling pathway is closely involved in oligodendrocyte differentiation (Del Giovane and Ragnini-Wilson, 2018). Robin Franklin’s group identified the nuclear receptor, retinoid X receptor γ, as a positive regulator of endogenous oligodendrocyte progenitor cell differentiation and remyelination in aged rats and in vitro (Huang et al., 2011).
Myelination is a very plastic process that is regulated and adapted to environmental stimuli throughout life. Increasingly, epigenetic mechanisms such as histone acetylation/methylation, DNA methylation, m6A RNA modification, and ATP-dependent chromatin remodeling are reported to be closely linked to the development of oligodendroglial lineage, myelination, and remyelination. MicroRNAs (miRNAs) or long-non-coding RNAs (lncRNAs) play a critical role in oligodendrocyte development, myelination, and remyelination balancing these processes (Berry et al., 2020). Conditional deletions of the enzyme Dicer in oligodendrocytes resulted in defects in myelination, while the population of proliferating OPC increased (Berry et al., 2020). miR-219 and miR-338 showed positive effects on OPC differentiation, while miR-212 and miR-125a-3p negatively affected this process (Berry et al., 2020). Thymosin-β4, a hormone-like peptide that modulates the cellular actin-cytoskeleton and cellular migration, was shown to upregulate the microRNA miR-146a and subsequently suppress the pro-inflammatory IRAK1 and TRAF6, leading to upregulation of p38 MAPK and inhibition of phospho-c-Jun, a negative regulator of MBP promoter (Santra et al., 2014; Zhang et al., 2017). The Notch signaling pathway, the Wnt signaling pathway, LINGO-1, the muscarinic receptor, hyaluronan, chondroitin sulfate proteoglycan, and fibrinogen have also been named as negative regulators of OPC maturation (Gottle et al., 2019; Lubetzki et al., 2020), thus potential promoters of remyelination could include monoclonal antibodies that antagonize, for example, LINGO-1, a negative regulator of oligodendrocyte differentiation (opicinimab), or Nogo-A, a negative regulator of axonal growth, branching fasciculation and plasticity (ozanezumab) (Ineichen et al., 2017). The search for pharmacological agents to promote remyelination has been significantly advanced by a number of innovative screening techniques, such as high-throughput screening of OPC differentiation using pluripotent mouse epiblast stem cell-derived OPC and concentric wrapping of myelin around micropillars (Deshmukh et al., 2013; Mei et al., 2014; Najm et al., 2015). These studies revealed a number of physiologically/non-physiologically occurring small bioactive molecules with anti-muscarinic, anti-histaminic, anti-dopaminic D2/serotonin, 5HT-2, and Smo-agonist activity, such as benzatropine, atropine, oxybutynin, clemastine, quentiapine, and clobetasol, which are already used for the treatment of diseases such as Alzheimer’s or Parkinson’s disease, allergic nephritis, skin diseases such as psoriasis, schizophrenia, and urinary incontinence and therefore have a known safety profile (Cunniffe and Coles, 2021; Sapko et al., 2022). The last group of possible drugs to promote remyelination includes naturally occurring molecules such as vitamins (quercetin or AS-2P, stable form of vitamin C, vitamin D, biotin), plant flavanones (hesperidin/hesperetin), molecules with multiple cellular and organic functions that have antioxidant, anti-inflammatory, antiviral and neuroprotective properties with a favorable safety profile and are therefore traditionally used as food supplements or as cosmetics (Baradaran et al., 2018; Guo et al., 2018; Naeimi et al., 2018; Gottle et al., 2019; Gomez-Pinedo et al., 2020).
Lipid Metabolism Alterations in Multiple Sclerosis
The brain has a high lipid content. Lipids make up about 50% of the dry weight of the human brain, but this proportion varies throughout life (36–40% of dry weight in the gray matter; 49–66% in white matter) and is not equal in different species due to differences in white matter volume (Sastry, 1985; O’Brien and Sampson, 1965; Bruce et al., 2017). Lipids are an essential component of cell membranes (Figure 1 shows a simplified schematic diagram of the various membrane lipids). In general, most biomembranes are composed of approximately equal proportions of lipids and proteins, although there are fine differences in the protein/lipids content as well as in the lipid composition for the various cell compartments (Casares et al., 2019; Poitelon et al., 2020; Ardesch et al 2022). However, myelin contains a very high amount of lipids (78–81% in humans), making them one of the main targets of autoimmune attacks in MS (O’Brien and Sampson, 1965; Poitelon et al., 2020). Antibodies to membrane phospholipids, PtdCho, sphingolipids, and other lipids are frequently detected in the serum and cerebrospinal fluid of MS patients and appear to vary at different disease stages (Bakshi et al., 2016; Sadaba et al., 2020).
Figure 1.
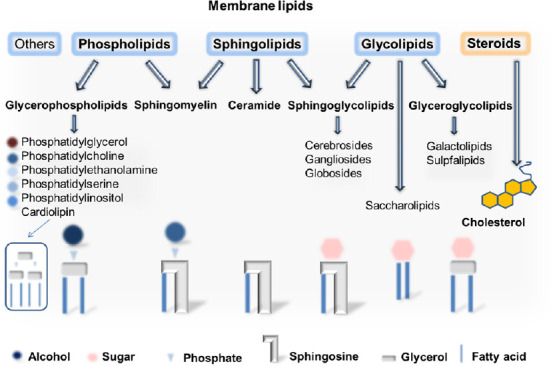
Simplified scheme of the different membrane lipids.
Adapted from Casares et al. (2019), Poitelon et al. (2020), Javaid et al. (2021), and Nelson and Cox (2021).
Lipid metabolism plays a critical role in immune cell activation, differentiation, and effector function, but also in oligodendrocyte homeostasis and, finally, in myelination and remyelination (Dimas et al., 2019; Marangon et al., 2020). The lipid-activated liver X receptors are nuclear transcription factors that play a critical role in immunity, lipids, and in particular cholesterol homeostasis, which is dysregulated in MS (Murali et al., 2020; Pineda-Torra et al., 2021). Overall, multiple studies suggest that impaired liver X receptor function may play a role in MS pathogenesis. Modulation of liver X receptors can induce reverse cholesterol transport, fatty acid, and glycosphingolipid biosynthesis, downregulation of pro-inflammatory cytokines by macrophages, stimulation of myelin gene expression by oligodendrocytes, and reduction of T-cell proliferation, differentiation into the pro-inflammatory T17 phenotype, and infiltration of T cells into the CNS (Pineda-Torra et al., 2021).
Apparently, changes in lipid profile and balance and thus aberrant lipid-mediated signaling and altered composition of lipid rafts are associated with MS (Wheeler et al., 2008; Grassi et al., 2020; Penkert et al., 2020; Alaamery et al., 2021). Recently, Ferreira et al. (2021) using liquid chromatography coupled with mass spectrometry, reported that the serum phospholipidome of MS patients is significantly different from that of healthy controls and that some phospholipids with the lowest P value, such as PtdCho (34:3), PtdCho (36:6), phosphatidylethanolamine (40:10), and PtdCho (38:1) may be suitable biomarkers for clinical applications in MS. Similarly, it was reported that the amount of PtdCho (36:1) was significantly decreased in postmortem MS brain tissue and in cuprizone-treated animals along demyelination (Trepanier et al., 2018).
Inflammation is consistently found to be directly implicated in the progression of MS regardless of the origin of the inflammatory process (exogenous, driven from peripheral immune cells or endogenous, driven from residual glial cells, or even heterogeneous) (Palumbo, 2017).
Phospholipases A2 (PLA2) is a versatile group of enzymes that cleave the fatty acids of membrane phospholipids, such as PtdCho. PLA2 is thought to play a critical role in the development and progression of MS disease (Chao et al., 2019; Trotter et al., 2019; Hoxha, 2022 Hoxha et al., 2022). Cytosolic PLA2 (cPLA2)-null mice have been shown to be resistant to experimental autoimmune encephalomyelitis (EAE) (Marusic et al., 2005). Biomembrane degradation leads to the activation of inflammatory signaling cascades associated with the generation of pro-inflammatory mediators such as arachidonic acid (AA), ceramide, sphingosine, phosphatidic acid, or prostaglandins and leukotrienes synthesized by cyclooxygenases and lipoxygenases, respectively (Palumbo, 2017). Elevated ceramide and eicosanoid levels have been detected in cerebrospinal fluid and post-mortem brain tissue from patients with MS (Dore-Duffy et al., 1991; Filippatou et al., 2021). Moreover, cyclooxygenase-2 has been shown to be expressed in actively demyelinating MS lesions, dying oligodendrocytes, and immune cells such as macrophages/microglia (Rose et al., 2004; Carlson et al., 2010). Interestingly, the 5-lipoxygenase gene has been identified as one of the major risk genes for MS (Whitney et al., 2001). Lysophosphatidylcholine, another catabolic intermediate in the PtdCho metabolic cycle is commonly used to trigger demyelination in rodent CNS and was originally thought to cause biolipid membrane disruption. Recent studies shed light on the exact mechanisms of this process (Plemel et al., 2018).
Finally, inflammation is usually accompanied by the formation of free radicals that lead to peroxidation of DNA, proteins, and lipids, resulting in cell damage or death and promoting the progression of pathological processes. Indeed, structures formed by lipid peroxidation have been observed in the cytoplasm of oligodendrocytes and some astrocytes, damaged neurons, and in dystrophic axons at sites of impaired axonal transport (Haider et al., 2011). In addition, oxidized PtdCho has been found in MS and is thought to mediate neurodegeneration in MS (Qin et al., 2007; Dong et al., 2021; Dong and Yong, 2022).
Sphingolipids, another abundant membrane lipid, are enriched in the nervous system and particularly in the myelin sheath (Vos et al., 1995; Olsen and Faergeman, 2017). There are several lines of evidence that sphingolipid metabolism is impaired in MS (Alaamery et al., 2021). Increased ceramide production and accumulation in reactive astrocytes in active MS lesions have been associated with oligodendrocyte damage and demyelination (Kim et al., 2012). Recent findings in the EAE model define a novel immunometabolic mechanism by which sphingolipid metabolism drives pro-inflammatory astrocyte activities through cPLA2-MAVS signaling with concomitant suppression of lactate formation to support neuronal metabolism (Chao et al., 2019).
Sphingosine-1-phosphate (S1P) is a catabolic product of ceramide and a pleiotropic lipid mediator involved in various cellular functions such as proliferation, migration, cytoskeletal remodeling, adhesion, and inflammation in many cell types, especially in the immune and vascular systems (Obinata and Hla, 2019). S1P gains importance in MS pathogenesis since the introduction of FTY720. FTY720 (fingolimod) is structurally analogous to S1P and functional antagonists cause the down-modulation of S1P signaling (Oo et al., 2007). Fingolimod is used for the treatment of relapsing-remitting MS. It prevents the egress of autoreactive lymphocytes from lymph nodes, thereby attenuating the infiltration of autoreactive lymphocytes and the development of inflammatory lesions (Chiba et al., 1998). In addition to immunological mechanisms, there are also FTY720-related non-immunological effects that occur in the CNS. Increased expression of S1P receptors S1P1 and S1P3 in reactive astrocytes has been found in active demyelinating and chronic MS lesions (Van Doorn et al., 2010). The suggestion that S1P receptors, particularly on astrocytes, may be involved in demyelination processes and subsequent axonal degeneration, which are essential features of the chronic progressive MS course, is gaining strength (Colombo et al., 2014; Rothhammer et al., 2017). The S1P signaling is also critical for oligodendrocyte lineage cell functionality, maintenance, and survival. Mice treated with FTY720 and CYM5422 (a selective agonist) showed a significant decrease in oligodendrocyte apoptosis and astrocyte/microglia activation, which was associated with significantly decreased demyelination in the cuprizone model of demyelination (Kim et al., 2018). Using the same animal model, a previous study by this group reported that altered sphingolipid metabolism during demyelination was restored upon active remyelination (Kim et al., 2012). Interestingly, remyelination did not occur in SphK2–/– mice (sphingosine kinase 2 (SphK2)-an enzyme for S1P synthase) after cuprizone withdrawal, although the density of progenitor cells and mature pro-myelinating oligodendrocytes increased (Song et al., 2021). The levels of pro-apoptotic sphingosine and ceramide (precursors of S1P) did not decrease in the corpus callosum of SphK2–/– mice after cuprizone was discontinued. Moreover, a significant decrease in myelin thickness with age was observed in SphK2–/– mice (Song et al., 2021), suggesting that the proper balance between ceramide and S1P might be critical for regenerative processes.
Currently, the superfamily of specialized pro-resolving lipid mediators (SPMs) is gaining increasing scientific interest. SPMs play an important role in resolving inflammation and restoring homeostasis, as well as preventing neurodegeneration by participating in cross-talk between glial cells and neurons. At the peak of inflammation, AA (ω-6) can be further converted into lipoxins, which belong to the superfamily of SPMs. In addition, affected cells utilize ω-3-eicosapentaenoic acid and ω-3-docosahexaenoic acid via different biosynthetic pathways to generate different SPMs (Tiberi and Chiurchiu, 2021).
The mechanisms underlying the regulation of bio-lipid metabolism in MS represent promising therapeutic targets and need to be further elucidated.
Beneficial Effects of CDP-Choline in Inflammatory and Degenerative Processes
In our previous study, we reported that CDP-choline improved disease progression and positively affected oligodendrocytes and myelin in EAE. Furthermore, treatment with CDP-choline effectively improved remyelination and reversed motor coordination deficits in mice after cuprizone-induced demyelination. These regenerative effects were likely achieved through an increased proliferation rate of OPC and the involvement of phospholipase C, resulting in a higher number of mature oligodendrocytes and a faster remyelination process. In our recent study, we were able to reproduce the remyelination-promoting effects of CDP-choline even when we used a 10-fold lower dose of this compound (Gudi et al., 2021).
CDP-choline (cytidine-5′-diphosphocholine, C14H26N4O11P2), a mononucleotide with a molecular weight of 488.32, is composed of ribose, cytosine, pyrophosphate, and choline and is ubiquitously present in eukaryotic cells although in minute amounts (Weiss, 1995; Secades and Gareri, 2022). CDP-choline is an essential intermediate and rate-limiting step in the biosynthetic pathway (Kennedy pathway) of PtdCho, the most abundant bilayer membrane-forming phospholipid in mammalian cells representing 32.8% of the total glycerophospholipid content of the human brain (Javaid et al., 2021). The recent results in yeast indicate the role of PtdCho in autophagosome biogenesis and autophagic flux and show that phospholipid imbalance impairs autophagosome completion (Polyansky et al., 2022). The PtdCho pool is dynamic and adapts to various intra- and extracellular changes. Lipid biosynthesis and the activity of the various enzymes relevant to the PtdCho cycle are tightly regulated throughout the cell cycle (Northwood et al., 1999). Subtle feedback mechanisms again exist between the individual components and the control of proliferation and apoptosis. Therefore, a deficiency of PtdCho leads to cell cycle arrest shortly before the S phase (Terce et al., 1994). Furthermore, PtdCho can be degraded as part of the physiological metabolic cycle by various phospholipases, including phospholipase A2. Degradation of PtdCho contributes to the formation of important lipid messengers such as diglyceride, phosphatidic acid, lyso-PtdCho and AA (Gibellini and Smith, 2010; Fagone and Jackowski, 2013). Lyso-PtdCho is in turn rapidly hydrolyzed to form free fatty acids and glycerophosphocholine (glycero-PtdCho), which provides free choline or phosphocholine. In turn, cytidilyltransferase synthesizes CDP-choline from cytidine and phosphocholine. However, under pathological conditions, the PtdCho biosynthetic cycle is disrupted/dysregulated and PLA2 activity is increased, leading to excessive production of free fatty acids and their derivate, which may exacerbate inflammation, oxidative stress, loss of membrane integrity, and subsequent cell injury and death (Adibhatla and Hatcher, 2008; Figure 2).
Figure 2.
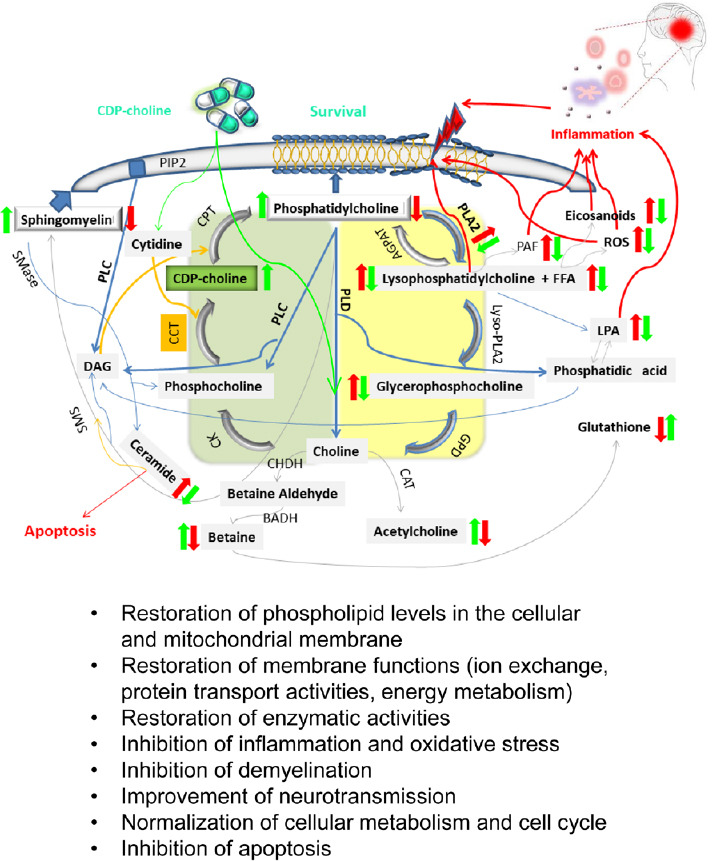
Simplified depiction of phosphatidylcholine turnover and possible positive effects of exogenously administered CDP-choline.
Simplified scheme of the de novo synthesis of phosphatidylcholine (PtdCho) (Kennedy pathway, area is highlighted in green) and its degradation (the area is colored in yellow), highlighting its importance in the production of lipid-derived signaling mediators under physiological and pathophysiological conditions (inflammation is shown in red colors, red arrows indicate an increase in expression during inflammatory processes due to endogenous or exogenous insults, blue arrows represent chemical subtraction or degradation reactions, orange arrows indicate addition reactions, and grey arrows point to some other type of chemical reaction). CDP-choline exerts protective effects by attenuating inflammation-related processes and restoring the levels of membrane lipids and thus cellular and mitochondrial membrane structures. In addition, CDP-choline serves as a source of choline, thus improving cognitive and antioxidant functions (glutathione production) and other epigenetic changes (DNA methylation). AGPAT: Acylglycerophosphate acyltransferase; BADH: betaine aldehyde dehydrogenase; CAT: choline acetyltransferase; CCT: phosphocholine cytidylyltransferase; CHDH: choline dehydrogenase; CK: choline kinase; CPT: cholinephosphotransferase; DAG: diacylglycerol; FFA: free fatty acids; GPD: glycerophosphodiesterase; LPA: lysophosphatidic acid; Lyso-PLA2: lysophospholipase A2; PAF: platelet-activating factor; PIP2: phosphatidylinositol-4,5-bisphosphat; PLA2: phospholipase A2; PLC: phospholipase C; PLD: phospholipase D; ROS: reactive oxygen species; SMase: sphingomyelinase; SMS: sphingomyeline synthase. Adapted from Adibhatla and Hatcher (2008), Fagone and Jackowski (2013), and Saito et al. (2022).
In several animal models, CDP-choline was able to restore concentrations of PtdCho and sphingomyelin, as well it attenuated AA-release, possibly by preventing activation of PLA2 (Arrigoni et al., 1987; Gimenez et al., 1999; Adibhatla and Hatcher, 2002; Secades and Gareri, 2022). Moreover, in animal models of focal brain ischemia, CDP-choline inhibited the ischemia-induced increase in glutamate levels, increased glutamate uptake, and EAAT2 glutamate transporter membrane expression in cultured rat astrocytes during oxygen and glucose deprivation, thus protected neurons from glutamate excitotoxicity under pathological conditions (Hurtado et al., 2005). Treatment with CDP-choline significantly reduced neuronal death, oxidative damage, immunoglobulin leakage, and microglial activation in the rat hippocampus after induced hypoglycemia (Kim et al., 2018a). In general, choline-containing phospholipids, such as CDP-choline or PtdCho are thought to play an important role in the maintenance and progression of neurovascular unit integrity (Roy et al., 2022).
Phosphatidylcholine is as well a major component of the phospholipid bilayer in mitochondria (Sperka-Gottlieb et al., 1988). It may be closely related to the proper composition of the mitochondrial membrane. PtdCho deficiency selectively impairs the stability of highly dynamic protein translocases such as the inner membrane TIM23 complex and the outer membrane sorting and assembly machinery (Schuler et al., 2016). Cardiolipin, another phospholipid of the inner mitochondrial membrane, is essential for cristae formation and maintenance of normal oxidative phosphorylation (Li et al., 2015). In previous studies, treatment with CDP-choline markedly restored cardiolipin levels, in gerbils after transient forebrain ischemia (Adibhatla and Hatcher, 2002). Moreover, using the H1N1 influenza A mouse model, Doolittle and colleagues showed that daily CDP-choline supplementation maintained biosynthesis of PtdCho (70% of surfactant phospholipids), which was impaired by viral infection (Woods et al., 2016) and stimulated expression of the cardiolipin remodeling enzyme tafazzin (phospholipid lysophospholipid transacylase), resulting in restoration of cardiolipin levels and subsequent stabilization of electron transport chain supercomplex assembly, mitochondrial morphology, and metabolic rescue in ATII cells from infected mice (Doolittle et al., 2022; Huckestein and Alcorn, 2022).
However, PtdCho is not only a structural component of biomembranes, CDP-choline/PtdCho cycle is fully integrated into a larger metabolic network (Fagone and Jackowski, 2013). CDP-choline serves (after its hydrolysis and dephosphorization) as one of the sources of choline, which is a precursor for the synthesis of the neurotransmitter acetylcholine (Ulus et al., 1989; Synoradzki and Grieb, 2019) and the re-synthesis of methionine via betaine aldehyde and betaine with subsequent transmethylation to homocysteine and methionine (Weiss, 1995). Exogenously administered CDP-choline affects the levels of several neurotransmitters (dopamine, serotonin, and norepinephrine) and hormones (adrenocorticotropin, somatropin) and elicits an analgesic effect (in conjunction with vasopressin) in various rodent pain models (Martinet et al., 1979; Lopez et al., 1986; Cavun and Savci, 2004; Bagdas et al., 2013). Plataras et al. (2003) have shown that CDP-choline is able to stimulate acetylcholinesterase and Na+,K+-ATPase activities in homogenates of the whole brain, but especially in the hippocampus of adult and elderly rats. This fact could explain the positive effects of CDP-choline on neuron survival and functions, thus improving memory performance, which is impaired by aging and some neuronal disorders such as MS (Costers et al., 2021). Another hydrolysis product of CDP-choline, cytidine, can be incorporated into nucleic acids.
Administration of CDP-choline resulted in improved functional nerve recovery after peripheral nerve injury (Abushukur and Knackstedt, 2022). Finally, CDP-choline increases protein levels of sirtuin-1 (SIRT-1), an enzyme involved in stress responses, protein aggregation, and inflammatory processes, and has numerous beneficial effects on age-related and neurodegenerative diseases (Lavu et al., 2008; Donmez, 2012). Overexpression of SIRT1 protein in neurons protects against EAE, and reduces neuronal loss and inflammation by activating multiple SIRT1 targets (Nimmagadda et al., 2013). Exogenously administered CDP-choline has repeatedly demonstrated significant beneficial effects on neuroprotection, reduction of infarct volume, edema, and inflammation, restoration of the blood-brain barrier and mitochondrial metabolism, and improvement of cognitive function in various preclinical models of Alzheimer’s disease, Parkinson’s disease, brain ischemia, trauma, and aging (Grieb, 2014; Grieb et al., 2021; Secades and Gareri, 2022). These findings on the efficacy of CDP-choline could be readily translated into human clinical trials (Jasielski et al., 2020; Que and Jamora, 2021; Secades, 2021). Administration of CDP-choline could improve attention in healthy adult subjects as well as cognitive function in elderly patients with memory impairment and mild vascular cognitive impairment, particularly after stroke or traumatic brain injury, while it could even inhibit disease progression in patients with degenerative dementia (Wortzel and Arciniegas, 2012; McGlade et al., 2019; Jasielski et al., 2020). Moreover, supplementation with CDP-choline provided protection of the ganglion cell complex of the retina and improves visual pathway function and parameters in glaucoma patients (Rejdak et al., 2003; Gandolfi et al., 2020). Available data from numerous clinical trials in patients after severe traumatic brain injury suggest that CDP-choline can reduce inflammation and oxidative stress, accelerate resorption of brain edema, and speed recovery, resulting in a shorter hospital stay and better outcome with a higher independence rate in patients treated with CDP-choline (Secades, 2021). However, the largest study of 1213 patients examining the efficacy of CDP-choline after traumatic brain injury, The Citicoline Brain Injury Treatment Trial (COBRIT), showed no improvement in the functional and cognitive status of patients with traumatic brain injury (Zafonte et al., 2012). The promising neuroprotective effects of CDP-choline in stroke patients were also not replicated in a large multicentre study (Davalos et al., 2012). Since stroke and brain injury have a completely different mechanism of lesion induction, the results cannot be applied to demyelinating diseases such as MS. MS lesions are accompanied by demyelination, whereas axons/neurons are not completely damaged at the onset of the disease. During the repair processes, new OPC proliferate, differentiate into mature oligodendrocytes, and form new myelin sheaths. Moreover, the results in human stroke are consistent with our animal experiments, in which we found no direct neuroprotective effects of CDP-choline (Skripuletz et al., 2015). Instead, we found beneficial effects of CDP-choline on remyelination. In general, there is a large difference between the doses used in animals and humans. In animal studies, doses of 0.5 g/kg were usually administered, which corresponds to 40 g of CDP-choline per day in an 80 kg person. However, doses of 2 g per day have been used in clinical trials. We, therefore, performed additional experiments and found that even lower doses of CDP-choline, equivalent to those used in human clinical trials, were sufficient to improve remyelination in animals (Gudi et al., 2021).
Citicoline, an international non-proprietary name for CDP-choline, is marketed in the United States and Europe as an over-the-counter dietary supplement (Commission Implementing Decision of 1 July 2014 Authorizing the Placing on the Market of Citicoline as a Novel Food Ingredient under Regulation (EC) No 258/97 of the European Parliament and of the Council). In 2013, the European Food Safety Authority (EFSA) gave a positive scientific opinion on the safety of use of citicoline, and in 2014 it was officially announced as a novel food ingredient by an implementing decision of the Commission of the European Union (EFSA, Scientific Opinion). After exogenous administration, it is rapidly metabolized to choline and cytidine (Lopez et al., 1987; Wurtman et al., 2000), which in turn readily cross the blood-brain barrier and are resynthesized by phosphocholine-cytidine transferase to CDP-choline or enter the choline and/or pyrimidine pathways. A favorable safety profile of CDP-choline has been repeatedly confirmed in preclinical animal studies and human clinical trials. The median lethal dose (LD50) of a single intravenous dose of CDP-choline is 4.6 g/kg in mice and 4.15 g/kg body weight in rats (Grau et al., 1983). When administered orally, an LD50 of 27.14 g/kg body weight for mice and 18.5 g/kg body weight for rats was determined (EFSA). The effect of chronic oral ingestion of CDP-choline was studied in dogs receiving a single daily dose of 1.5 g/kg for 6 months. No toxic effects or physiological, biochemical, neurological, or morphological abnormalities were observed (Romero et al., 1983). Overall, side effects occurred rarely and were transient. Gastrointestinal discomfort, malaise, headache, and irritability have been reported in patients and healthy volunteers participating in clinical trials (Lozano Fernandez, 1983; Cho and Kim, 2009; Secades and Gareri, 2022). According to EFSA’s scientific opinion on the safety of citicoline as a novel food ingredient, there are no safety concerns regarding the genotoxicity of CDP-choline. A bacterial mutagenicity test at citicoline concentrations up to 5000 μg/plate, a chromosomal aberration study in Chinese hamster ovary cells at doses up to 10 mM (both studies were performed in the presence or absence of metabolic activation), an in vivo micronucleus test in mice with single doses of up to 2000 mg/kg body weight of citicoline administered by intraperitoneal injection revealed no evidence of CDP-choline related genotoxicity (EFSA, Scientific Opinion).
Conclusion
In summary, numerous experimental paradigms provided comprehensive insights into the pharmacological and biochemical mode of action of CDP-choline, including protection and maintenance of biomembranes (both mitochondrial and cellular), involvement in the regulation of metabolic, enzymatic and cell cycle processes, reduction of inflammation, regulation of neurotransmitter production and uptake, and finally cell survival and thus protection and regeneration. Given the evidence to date, it is conceivable that CDP-choline could promote remyelination in MS and protect patients from progressive disability. There is an unmet clinical need for drugs that promote remyelination. Regenerative therapies to promote remyelination are currently the focus of much scientific investigation. Because CDP-choline has already been tested in human clinical trials for other diseases, the side effect profile is well known, and rapid translation of the hypothesis into a clinical trial in patients is feasible.
Footnotes
Conflicts of interest: The authors declare no conflict of interest. Outside the submitted work, the authors received honoraria for lectures, travel grants, or research grants. TS reports research support from Alnylam Pharmaceuticals, Bristol-Myers Squibb Foundation for Immuno-Oncology, Claudia von Schilling Foundation, CSL Behring, Else Kröner Fresenius Foundation, Novartis, Sanofi Genzyme, VHV Stiftung and honoraria for lectures and travel grants from Alexion, Alnylam Pharmaceuticals, Bayer Vital, Biogen, Celgene, Centogene, CSL Behring, Euroimmun, Janssen, Merck Serono, Novartis, Pfizer, Roche, Sanofi, Siemens, Sobi, Teva. RAL reports have received compensation for serving as a consultant or speaker from Biogen, Celgene/BMS, Genzyme/Sanofi, Janssen, Merck Serono, Novartis, and Roche; and have received research support from Biogen Idec and Novartis.
Data availability statement: Not applicable.
C-Editors: Zhao M, Liu WJ, Qiu Y; T-Editor: Jia Y
References
- 1.Abushukur Y, Knackstedt R. The impact of supplements on recovery after peripheral nerve injury:a review of the literature. Cureus. 2022;14:e25135. doi: 10.7759/cureus.25135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adibhatla RM, Hatcher JF. Citicoline mechanisms and clinical efficacy in cerebral ischemia. J Neurosci Res. 2002;70:133–139. doi: 10.1002/jnr.10403. [DOI] [PubMed] [Google Scholar]
- 3.Adibhatla RM, Hatcher JF. Phospholipase A(2), reactive oxygen species, and lipid peroxidation in CNS pathologies. BMB Rep. 2008;41:560–567. doi: 10.5483/bmbrep.2008.41.8.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alaamery M, Albesher N, Aljawini N, Alsuwailm M, Massadeh S, Wheeler MA, Chao CC, Quintana FJ. Role of sphingolipid metabolism in neurodegeneration. J Neurochem. 2021;158:25–35. doi: 10.1111/jnc.15044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Angelova DM, Brown DR. Microglia and the aging brain:are senescent microglia the key to neurodegeneration? J Neurochem. 2019;151:676–688. doi: 10.1111/jnc.14860. [DOI] [PubMed] [Google Scholar]
- 6.Ardesch DJ, Scholtens LH, de Lange SC, Roumazeilles L, Khrapitchev AA, Preuss TM, Rilling JK, Mars RB, van den Heuvel MP. Scaling principles of white matter connectivity in the human and nonhuman primate brain. Cereb Cortex. 2022;32:2831–2842. doi: 10.1093/cercor/bhab384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arrigoni E, Averet N, Cohadon F. Effects of CDP-choline on phospholipase A2 and cholinephosphotransferase activities following a cryogenic brain injury in the rabbit. Biochem Pharmacol. 1987;36:3697–3700. doi: 10.1016/0006-2952(87)90022-0. [DOI] [PubMed] [Google Scholar]
- 8.Bagdas D, Yucel-Ozboluk H, Orhan F, Kanat O, Isbil-Buyukcoskun N, Gurun MS. Role of central arginine vasopressin receptors in the analgesic effect of CDP-choline on acute and neuropathic pain. Neuroreport. 2013;24:941–946. doi: 10.1097/WNR.0000000000000009. [DOI] [PubMed] [Google Scholar]
- 9.Bakshi R, Yeste A, Patel B, Tauhid S, Tummala S, Rahbari R, Chu R, Regev K, Kivisakk P, Weiner HL, Quintana FJ. Serum lipid antibodies are associated with cerebral tissue damage in multiple sclerosis. Neurol Neuroimmunol Neuroinflamm. 2016;3:e200. doi: 10.1212/NXI.0000000000000200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baradaran S, Hajizadeh Moghaddam A, Ghasemi-Kasman M. Hesperetin reduces myelin damage and ameliorates glial activation in lysolecithin-induced focal demyelination model of rat optic chiasm. Life Sci. 2018;207:471–479. doi: 10.1016/j.lfs.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 11.Berry K, Wang J, Lu QR. Epigenetic regulation of oligodendrocyte myelination in developmental disorders and neurodegenerative diseases. F1000Res. 2020;9 doi: 10.12688/f1000research.20904.1. F1000 Faculty Rev 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bodini B, Veronese M, Garcia-Lorenzo D, Battaglini M, Poirion E, Chardain A, Freeman L, Louapre C, Tchikviladze M, Papeix C, Dolle F, Zalc B, Lubetzki C, Bottlaender M, Turkheimer F, Stankoff B. Dynamic imaging of individual remyelination profiles in multiple sclerosis. Ann Neurol. 2016;79:726–738. doi: 10.1002/ana.24620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bruce KD, Zsombok A, Eckel RH. Lipid processing in the brain:a key regulator of systemic metabolism. Front Endocrinol (Lausanne) 2017;8:60. doi: 10.3389/fendo.2017.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carlson NG, Rojas MA, Redd JW, Tang P, Wood B, Hill KE, Rose JW. Cyclooxygenase-2 expression in oligodendrocytes increases sensitivity to excitotoxic death. J Neuroinflammation. 2010;7:25. doi: 10.1186/1742-2094-7-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Casares D, Escriba PV, Rossello CA. Membrane lipid composition:effect on membrane and organelle structure, function and compartmentalization and therapeutic avenues. Int J Mol Sci. 2019;20:2167. doi: 10.3390/ijms20092167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cavun S, Savci V. CDP-choline increases plasma ACTH and potentiates the stimulated release of GH, TSH and LH:the cholinergic involvement. Fundam Clin Pharmacol. 2004;18:513–523. doi: 10.1111/j.1472-8206.2004.00272.x. [DOI] [PubMed] [Google Scholar]
- 17.Chao CC, Gutierrez-Vazquez C, Rothhammer V, Mayo L, Wheeler MA, Tjon EC, Zandee SEJ, Blain M, de Lima KA, Takenaka MC, Avila-Pacheco J, Hewson P, Liu L, Liliana M, Sanmarco LM, Borucki DM, Lipof GZ, Trauger SA, Clish CB, Antel JP, et al. Metabolic control of astrocyte pathogenic activity via cPLA2-MAVS. Cell. 2019;179:1483–1498. doi: 10.1016/j.cell.2019.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chiba K, Yanagawa Y, Masubuchi Y, Kataoka H, Kawaguchi T, Ohtsuki M, Hoshino Y. FTY720, a novel immunosuppressant, induces sequestration of circulating mature lymphocytes by acceleration of lymphocyte homing in rats. I. FTY720 selectively decreases the number of circulating mature lymphocytes by acceleration of lymphocyte homing. J Immunol. 1998;160:5037–5044. [PubMed] [Google Scholar]
- 19.Cho HJ, Kim YJ. Efficacy and safety of oral citicoline in acute ischemic stroke:drug surveillance study in 4,191 cases. Methods Find Exp Clin Pharmacol. 2009;31:171–176. doi: 10.1358/mf.2009.31.3.1364241. [DOI] [PubMed] [Google Scholar]
- 20.Clarke LE, Liddelow SA, Chakraborty C, Munch AE, Heiman M, Barres BA. Normal aging induces A1-like astrocyte reactivity. Proc Natl Acad Sci U S A. 2018;115:E1896–E1905. doi: 10.1073/pnas.1800165115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Colombo E, Di Dario M, Capitolo E, Chaabane L, Newcombe J, Martino G, Farina C. Fingolimod may support neuroprotection via blockade of astrocyte nitric oxide. Ann Neurol. 2014;76:325–337. doi: 10.1002/ana.24217. [DOI] [PubMed] [Google Scholar]
- 22.Commission Implementing Decision of 1 July 2014 Authorising the Placing on the Market of Citicoline as a Novel Food Ingredient under Regulation (EC) No 258/97 of the European Parliament and of the Council. [Accessed November 23, 2022]. Available at:https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32014D0423&from=IT .
- 23.Costers L, Van Schependom J, Laton J, Baijot J, Sjogard M, Wens V, De Tiege X, Goldman S, D'Haeseleer M, D'Hooghe MB, Woolrich M, Nagels G. The role of hippocampal theta oscillations in working memory impairment in multiple sclerosis. Hum Brain Mapp. 2021;42:1376–1390. doi: 10.1002/hbm.25299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cunniffe N, Coles A. Promoting remyelination in multiple sclerosis. J Neurol. 2021;268:30–44. doi: 10.1007/s00415-019-09421-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Del Giovane A, Ragnini-Wilson A. Targeting smoothened as a new frontier in the functional recovery of central nervous system demyelinating pathologies. Int J Mol Sci. 2018;19:3677. doi: 10.3390/ijms19113677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deshmukh VA, Tardif V, Lyssiotis CA, Green CC, Kerman B, Kim HJ, Padmanabhan K, Swoboda JG, Ahmad I, Kondo T, Gage FH, Theofilopoulos AN, Lawson BR, Schultz PG, Lairson LL. A regenerative approach to the treatment of multiple sclerosis. Nature. 2013;502:327–332. doi: 10.1038/nature12647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dimas P, Montani L, Pereira JA, Moreno D, Trotzmuller M, Gerber J, Semenkovich CF, Kofeler HC, Suter U. CNS myelination and remyelination depend on fatty acid synthesis by oligodendrocytes. Elife. 2019;8:e44702. doi: 10.7554/eLife.44702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dobson R, Giovannoni G. Multiple sclerosis - a review. Eur J Neurol. 2019;26:27–40. doi: 10.1111/ene.13819. [DOI] [PubMed] [Google Scholar]
- 29.Dong Y, Yong VW. Oxidized phospholipids as novel mediators of neurodegeneration. Trends Neurosci. 2022;45:419–429. doi: 10.1016/j.tins.2022.03.002. [DOI] [PubMed] [Google Scholar]
- 30.Dong Y, D'Mello C, Pinsky W, Lozinski BM, Kaushik DK, Ghorbani S, Moezzi D, Brown D, Melo FC, Zandee S, Vo T, Prat A, Whitehead SN, Yong VW. Oxidized phosphatidylcholines found in multiple sclerosis lesions mediate neurodegeneration and are neutralized by microglia. Nat Neurosci. 2021;24:489–503. doi: 10.1038/s41593-021-00801-z. [DOI] [PubMed] [Google Scholar]
- 31.Donmez G. The effects of SIRT1 on Alzheimer's disease models. Int J Alzheimers Dis. 20122012:509529. doi: 10.1155/2012/509529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Doolittle LM, Binzel K, Nolan KE, Craig K, Rosas LE, Bernier MC, Joseph LM, Woods PS, Knopp MV, Davis IC. Cytidine 5'-diphosphocholine corrects alveolar type II cell mitochondrial dysfunction in influenza-infected mice. Am J Respir Cell Mol Biol. 2022;66:682–693. doi: 10.1165/rcmb.2021-0512OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dore-Duffy P, Ho SY, Donovan C. Cerebrospinal fluid eicosanoid levels:endogenous PGD2 and LTC4 synthesis by antigen-presenting cells that migrate to the central nervous system. Neurology. 1991;41:322–324. doi: 10.1212/wnl.41.2_part_1.322. [DOI] [PubMed] [Google Scholar]
- 34.EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA) (2013) Scientific Opinion on the safety of “citicoline”as a Novel Food ingredient. EFSA J. 11:3421. [Google Scholar]
- 35.Emami Aleagha MS, Siroos B, Ahmadi M, Balood M, Palangi A, Haghighi AN, Harirchian MH. Decreased concentration of Klotho in the cerebrospinal fluid of patients with relapsing-remitting multiple sclerosis. J Neuroimmunol. 2015;281:5–8. doi: 10.1016/j.jneuroim.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 36.Fagone P, Jackowski S. Phosphatidylcholine and the CDP-choline cycle. Biochim Biophys Acta. 2013;1831:523–532. doi: 10.1016/j.bbalip.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ferreira HB, Melo T, Monteiro A, Paiva A, Domingues P, Domingues MR. Serum phospholipidomics reveals altered lipid profile and promising biomarkers in multiple sclerosis. Arch Biochem Biophys. 2021;697:108672. doi: 10.1016/j.abb.2020.108672. [DOI] [PubMed] [Google Scholar]
- 38.Filippatou AG, Moniruzzaman M, Sotirchos ES, Fitzgerald KC, Kalaitzidis G, Lambe J, Vasileiou E, Saidha S, Prince JL, Haughey N, Calabresi PA, Bhargava P. Serum ceramide levels are altered in multiple sclerosis. Mult Scler. 2021;27:1506–1519. doi: 10.1177/1352458520971816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gandolfi S, Marchini G, Caporossi A, Scuderi G, Tomasso L, Brunoro A. Cytidine 5'-diphosphocholine (citicoline):evidence for a neuroprotective role in glaucoma. Nutrients. 2020;12:793. doi: 10.3390/nu12030793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gibellini F, Smith TK. The Kennedy pathway--De novo synthesis of phosphatidylethanolamine and phosphatidylcholine. IUBMB Life. 2010;62:414–428. doi: 10.1002/iub.337. [DOI] [PubMed] [Google Scholar]
- 41.Gimenez R, Soler S, Aguilar J. Cytidine diphosphate choline administration activates brain cytidine triphosphate:phosphocholine cytidylytransferase in aged rats. Neurosci Lett. 1999;273:163–166. doi: 10.1016/s0304-3940(99)00660-6. [DOI] [PubMed] [Google Scholar]
- 42.Gingele S, Henkel F, Heckers S, Moellenkamp TM, Hummert MW, Skripuletz T, Stangel M, Gudi V. Delayed demyelination and impaired remyelination in aged mice in the cuprizone model. Cells. 2020;9:945. doi: 10.3390/cells9040945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gomez-Pinedo U, Cuevas JA, Benito-Martin MS, Moreno-Jimenez L, Esteban-Garcia N, Torre-Fuentes L, Matias-Guiu JA, Pytel V, Montero P, Matias-Guiu J. Vitamin D increases remyelination by promoting oligodendrocyte lineage differentiation. Brain Behav. 2020;10:e01498. doi: 10.1002/brb3.1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gottle P, Forster M, Weyers V, Kury P, Rejdak K, Hartung HP, Kremer D. An unmet clinical need:roads to remyelination in MS. Neurol Res Pract. 2019;1:21. doi: 10.1186/s42466-019-0026-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grassi S, Giussani P, Mauri L, Prioni S, Sonnino S, Prinetti A. Lipid rafts and neurodegeneration:structural and functional roles in physiologic aging and neurodegenerative diseases. J Lipid Res. 2020;61:636–654. doi: 10.1194/jlr.TR119000427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grau T, Romero A, Sacristan A, Ortiz JA. CDP-choline:acute toxicity study. Arzneimittelforschung. 1983;33:1033–1034. [PubMed] [Google Scholar]
- 47.Grieb P. Neuroprotective properties of citicoline:facts, doubts and unresolved issues. CNS Drugs. 2014;28:185–193. doi: 10.1007/s40263-014-0144-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grieb P, Świątkiewicz M, Kamińska A, Jünemann A, Rejdak R, Rejdak K. Citicoline:a candidate for adjunct treatment of multiple sclerosis. Pharmaceuticals (Basel) 2021;14:326. doi: 10.3390/ph14040326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guardiola-Diaz HM, Ishii A, Bansal R. Erk1/2 MAPK and mTOR signaling sequentially regulates progression through distinct stages of oligodendrocyte differentiation. Glia. 2012;60:476–486. doi: 10.1002/glia.22281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gudi V, Schafer N, Gingele S, Stangel M, Skripuletz T. Regenerative effects of CDP-choline:a dose-dependent study in the toxic cuprizone model of de- and remyelination. Pharmaceuticals (Basel) 2021;14:1156. doi: 10.3390/ph14111156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gudi V, Gai L, Herder V, Tejedor LS, Kipp M, Amor S, Suhs KW, Hansmann F, Beineke A, Baumgartner W, Stangel M, Skripuletz T. Synaptophysin is a reliable marker for axonal damage. J Neuropathol Exp Neurol. 2017;76:109–125. doi: 10.1093/jnen/nlw114. [DOI] [PubMed] [Google Scholar]
- 52.Guo YE, Suo N, Cui X, Yuan Q, Xie X. Vitamin C promotes oligodendrocytes generation and remyelination. Glia. 2018;66:1302–1316. doi: 10.1002/glia.23306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Haider L, Fischer MT, Frischer JM, Bauer J, Hoftberger R, Botond G, Esterbauer H, Binder CJ, Witztum JL, Lassmann H. Oxidative damage in multiple sclerosis lesions. Brain. 2011;134:1914–1924. doi: 10.1093/brain/awr128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hoxha M. Arachidonic acid mediators and their role in neurological disease. CNS Neurol Disord Drug Targets. 2022;21:106–107. doi: 10.2174/1871527321666220103204515. [DOI] [PubMed] [Google Scholar]
- 55.Hoxha M, Spahiu E, Prendi E, Zappacosta B. A systematic review on the role of arachidonic acid pathway in multiple sclerosis. CNS Neurol Disord Drug Targets. 2022;21:160–187. doi: 10.2174/1871527319666200825164123. [DOI] [PubMed] [Google Scholar]
- 56.Huang JK, Jarjour AA, Nait Oumesmar B, Kerninon C, Williams A, Krezel W, Kagechika H, Bauer J, Zhao C, Baron-Van Evercooren A, Chambon P, Ffrench-Constant C, Franklin RJM. Retinoid X receptor gamma signaling accelerates CNS remyelination. Nat Neurosci. 2011;14:45–53. doi: 10.1038/nn.2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huckestein BR, Alcorn JF. Improving mitochondrial function in viral infection:targeting cellular metabolism. Am J Respir Cell Mol Biol. 2022;66:598–600. doi: 10.1165/rcmb.2022-0096ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hughes EG, Kang SH, Fukaya M, Bergles DE. Oligodendrocyte progenitors balance growth with self-repulsion to achieve homeostasis in the adult brain. Nat Neurosci. 2013;16:668–676. doi: 10.1038/nn.3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hurtado O, Moro MA, Cardenas A, Sanchez V, Fernandez-Tome P, Leza JC, Lorenzo P, Secades JJ, Lozano R, Davalos A, Castillo J, Lizasoain I. Neuroprotection afforded by prior citicoline administration in experimental brain ischemia:effects on glutamate transport. Neurobiol Dis. 2005;18:336–345. doi: 10.1016/j.nbd.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 60.Ineichen BV, Kapitza S, Bleul C, Good N, Plattner PS, Seyedsadr MS, Kaiser J, Schneider MP, Zorner B, Martin R, Linnebank M, Schwab ME. Nogo-A antibodies enhance axonal repair and remyelination in neuro-inflammatory and demyelinating pathology. Acta Neuropathol. 2017;134:423–440. doi: 10.1007/s00401-017-1745-3. [DOI] [PubMed] [Google Scholar]
- 61.Irvine KA, Blakemore WF. Remyelination protects axons from demyelination-associated axon degeneration. Brain. 2008;131:1464–1477. doi: 10.1093/brain/awn080. [DOI] [PubMed] [Google Scholar]
- 62.Jakel S, Agirre E, Mendanha Falcao A, van Bruggen D, Lee KW, Knuesel I, Malhotra D, Ffrench-Constant C, Williams A, Castelo-Branco G. Altered human oligodendrocyte heterogeneity in multiple sclerosis. Nature. 2019;566:543–547. doi: 10.1038/s41586-019-0903-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jasielski P, Piedel F, Piwek M, Rocka A, Petit V, Rejdak K. Application of citicoline in neurological disorders:a systematic review. Nutrients. 2020;12:3113. doi: 10.3390/nu12103113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Javaid S, Farooq T, Rehman Z, Afzal A, Ashraf W, Rasool MF, Alqahtani F, Alsanea S, Alasmari F, Alanazi MM, Alharbi M, Imran I. Dynamics of choline-containing phospholipids in traumatic brain injury and associated comorbidities. Int J Mol Sci. 2021;22:11313. doi: 10.3390/ijms222111313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim JH, Choi BY, Kho AR, Lee SH, Jeong JH, Hong DK, Sohn M, Ryu OH, Choi MG, Suh SW. Acetylcholine precursor, citicoline (cytidine 5'-diphosphocholine), reduces hypoglycaemia-induced neuronal death in rats. J Neuroendocrinol 30. 2018a doi: 10.1111/jne.12567. doi:10.1111/jne.12567. [DOI] [PubMed] [Google Scholar]
- 66.Kim S, Steelman AJ, Zhang Y, Kinney HC, Li J. Aberrant upregulation of astroglial ceramide potentiates oligodendrocyte injury. Brain Pathol. 2012;22:41–57. doi: 10.1111/j.1750-3639.2011.00501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kim S, Bielawski J, Yang H, Kong Y, Zhou B, Li J. Functional antagonism of sphingosine-1-phosphate receptor 1 prevents cuprizone-induced demyelination. Glia. 2018b;66:654–669. doi: 10.1002/glia.23272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kuhlmann T, Miron V, Cui Q, Wegner C, Antel J, Bruck W. Differentiation block of oligodendroglial progenitor cells as a cause for remyelination failure in chronic multiple sclerosis. Brain. 2008;131:1749–1758. doi: 10.1093/brain/awn096. [DOI] [PubMed] [Google Scholar]
- 69.Lavu S, Boss O, Elliott PJ, Lambert PD. Sirtuins--novel therapeutic targets to treat age-associated diseases. Nat Rev Drug Discov. 2008;7:841–853. doi: 10.1038/nrd2665. [DOI] [PubMed] [Google Scholar]
- 70.Li XX, Tsoi B, Li YF, Kurihara H, He RR. Cardiolipin and its different properties in mitophagy and apoptosis. J Histochem Cytochem. 2015;63:301–311. doi: 10.1369/0022155415574818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, Bennett ML, Munch AE, Chung WS, Peterson TC, Wilton DK, Frouin A, Napier BA, Panicker N, Kumar M, Buckwalter MS, Rowitch DH, Dawso VL, Dawson TM, Stevens B, et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017;541:481–487. doi: 10.1038/nature21029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lopez GCI, Agut J, Von Borstel R, Wurtman RJ. Metabolism of cytidine (5?)-diphosphocholine (cdp-choline) following oral and intravenous administration to the human and the rat. Neurochem Int. 1987;11:293–297. doi: 10.1016/0197-0186(87)90049-0. [DOI] [PubMed] [Google Scholar]
- 73.Lopez I, Coviella G, Agut J, Wurtman RJ. Effect of cytidine(5')diphosphocholine (CDP-choline) on the total urinary excretion of 3-methoxy-4-hydroxyphenylglycol (MHPG) by rats and humans. J Neural Transm. 1986;66:129–134. doi: 10.1007/BF01260908. [DOI] [PubMed] [Google Scholar]
- 74.Lozano Fernandez R. Efficacy and safety of oral CDP-choline. Drug surveillance study in 2817 cases. Arzneimittelforschung. 1983;33:1073–1080. [PubMed] [Google Scholar]
- 75.Lubetzki C, Zalc B, Williams A, Stadelmann C, Stankoff B. Remyelination in multiple sclerosis:from basic science to clinical translation. Lancet Neurol. 2020;19:678–688. doi: 10.1016/S1474-4422(20)30140-X. [DOI] [PubMed] [Google Scholar]
- 76.Marangon D, Boccazzi M, Lecca D, Fumagalli M. Regulation of oligodendrocyte functions:targeting lipid metabolism and extracellular matrix for myelin repair. J Clin Med. 2020;9:470. doi: 10.3390/jcm9020470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Martinet M, Fonlupt P, Pacheco H. Effects of cytidine-5'diphosphocholine on norepinephrine, dopamine and serotonin synthesis in various regions of the rat brain. Arch Int Pharmacodyn Ther. 1979;239:52–61. [PubMed] [Google Scholar]
- 78.Marusic S, Leach MW, Pelker JW, Azoitei ML, Uozumi N, Cui J, Shen MW, DeClercq CM, Miyashiro JS, Carito BA, Thakker P, Simmons DL, Leonard JP, Shimizu T, Clark JD. Cytosolic phospholipase A2 alpha-deficient mice are resistant to experimental autoimmune encephalomyelitis. J Exp Med. 2005;202:841–851. doi: 10.1084/jem.20050665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Masvekar R, Wu T, Kosa P, Barbour C, Fossati V, Bielekova B. Cerebrospinal fluid biomarkers link toxic astrogliosis and microglial activation to multiple sclerosis severity. Mult Scler Relat Disord. 2019;28:34–43. doi: 10.1016/j.msard.2018.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mei F, Fancy SPJ, Shen YA, Niu J, Zhao C, Presley B, Miao E, Lee S, Mayoral SR, Redmond SA, Etxeberria A, Xiao L, Franklin RJM, Green A, Hauser SL, Chan JR. Micropillar arrays as a high-throughput screening platform for therapeutics in multiple sclerosis. Nat Med. 2014;20:954–960. doi: 10.1038/nm.3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mei F, Lehmann-Horn K, Shen YA, Rankin KA, Stebbins KJ, Lorrain DS, Pekarek K, A Sagan S, Xiao L, Teuscher C, von Budingen HC, Wess J, Lawrence JJ, Green AJ, Fancy SP, Zamvil SS, Chan JR. Accelerated remyelination during inflammatory demyelination prevents axonal loss and improves functional recovery. Elife. 2016;5:e18246. doi: 10.7554/eLife.18246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Murali N, Browne RW, Fellows Maxwell K, Bodziak ML, Jakimovski D, Hagemeier J, Bergsland N, Weinstock-Guttman B, Zivadinov R, Ramanathan M. Cholesterol and neurodegeneration:longitudinal changes in serum cholesterol biomarkers are associated with new lesions and gray matter atrophy in multiple sclerosis over 5 years of follow-up. Eur J Neurol. 2020;27:188–e4. doi: 10.1111/ene.14055. [DOI] [PubMed] [Google Scholar]
- 83.Naeimi R, Baradaran S, Ashrafpour M, Moghadamnia AA, Ghasemi-Kasman M. Querectin improves myelin repair of optic chiasm in lyolecithin-induced focal demyelination model. Biomed Pharmacother. 2018;101:485–493. doi: 10.1016/j.biopha.2018.02.125. [DOI] [PubMed] [Google Scholar]
- 84.Najm FJ, Madhavan M, Zaremba A, Shick E, Karl RT, Factor DC, Miller TE, Nevin ZS, Kantor C, Sargent A, Quick KL, Schlatzer DM, Tang H, Papoian R, Brimacombe KR, Shen M, Boxer MB, Jadhav A, Robinson AP, Podojil JR, et al. Drug-based modulation of endogenous stem cells promotes functional remyelination in vivo. Nature. 2015;522:216–220. doi: 10.1038/nature14335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nelson DL, Cox MM. Lehninger Principles of Biochemistry. New York: W. H. Freeman; 2021. [Google Scholar]
- 86.Nimmagadda VK, Bever CT, Vattikunta NR, Talat S, Ahmad V, Nagalla NK, Trisler D, Judge SI, Royal W, 3rd, Chandrasekaran K, Russell JW, Makar TK. Overexpression of SIRT1 protein in neurons protects against experimental autoimmune encephalomyelitis through activation of multiple SIRT1 targets. J Immunol. 2013;190:4595–4607. doi: 10.4049/jimmunol.1202584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Northwood IC, Tong AH, Crawford B, Drobnies AE, Cornell RB. Shuttling of CTP:Phosphocholine cytidylyltransferase between the nucleus and endoplasmic reticulum accompanies the wave of phosphatidylcholine synthesis during the G(0) -->G(1) transition. J Biol Chem. 1999;274:26240–26248. doi: 10.1074/jbc.274.37.26240. [DOI] [PubMed] [Google Scholar]
- 88.O'Brien JS, Sampson EL. Lipid composition of the normal human brain:gray matter, white matter, and myelin. J Lipid Res. 1965;6:537–544. [PubMed] [Google Scholar]
- 89.Obinata H, Hla T. Sphingosine 1-phosphate and inflammation. Int Immunol. 2019;31:617–625. doi: 10.1093/intimm/dxz037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Olsen ASB, Faergeman NJ. Sphingolipids:membrane microdomains in brain development, function and neurological diseases. Open Biol. 2017;7:170069. doi: 10.1098/rsob.170069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Oo ML, Thangada S, Wu MT, Liu CH, Macdonald TL, Lynch KR, Lin CY, Hla T. Immunosuppressive and anti-angiogenic sphingosine 1-phosphate receptor-1 agonists induce ubiquitinylation and proteasomal degradation of the receptor. J Biol Chem. 2007;282:9082–9089. doi: 10.1074/jbc.M610318200. [DOI] [PubMed] [Google Scholar]
- 92.Palumbo S. Pathogenesis and progression of multiple sclerosis:the role of arachidonic acid-mediated neuroinflammation. In: Zagon IS, McLaughlin PJ, editors. Multiple Sclerosis:Perspectives in Treatment and Pathogenesis. Brisbane (AU): Codon Publications; 2017. Chapter 7. [PubMed] [Google Scholar]
- 93.Penkert H, Lauber C, Gerl MJ, Klose C, Damm M, Fitzner D, Flierl-Hecht A, Kumpfel T, Kerschensteiner M, Hohlfeld R, Gerdes LA, Simons M. Plasma lipidomics of monozygotic twins discordant for multiple sclerosis. Ann Clin Transl Neurol. 2020;7:2461–2466. doi: 10.1002/acn3.51216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pineda-Torra I, Siddique S, Waddington KE, Farrell R, Jury EC. Disrupted lipid metabolism in multiple sclerosis:a role for liver X receptors? Front Endocrinol (Lausanne) 2021;12:639757. doi: 10.3389/fendo.2021.639757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Plataras C, Angelogianni P, Tsakiris S. Effect of CDP-choline on hippocampal acetylcholinesterase and Na+,K(+)-ATPase in adult and aged rats. Z Naturforsch C J Biosci. 2003;58:277–281. doi: 10.1515/znc-2003-3-423. [DOI] [PubMed] [Google Scholar]
- 96.Plemel JR, Michaels NJ, Weishaupt N, Caprariello AV, Keough MB, Rogers JA, Yukseloglu A, Lim J, Patel VV, Rawji KS, Jensen SK, Teo W, Heyne B, Whitehead SN, Stys PK, Yong VW. Mechanisms of lysophosphatidylcholine-induced demyelination:a primary lipid disrupting myelinopathy. Glia. 2018;66:327–347. doi: 10.1002/glia.23245. [DOI] [PubMed] [Google Scholar]
- 97.Poitelon Y, Kopec AM, Belin S. Myelin fat facts:an overview of lipids and fatty acid metabolism. Cells. 2020;9:812. doi: 10.3390/cells9040812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Polyansky A, Shatz O, Fraiberg M, Shimoni E, Dadosh T, Mari M, Reggiori FM, Qin C, Han X, Elazar Z. Phospholipid imbalance impairs autophagosome completion. EMBO J. 2022;41:e110771. doi: 10.15252/embj.2022110771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Qin J, Goswami R, Balabanov R, Dawson G. Oxidized phosphatidylcholine is a marker for neuroinflammation in multiple sclerosis brain. J Neurosci Res. 2007;85:977–984. doi: 10.1002/jnr.21206. [DOI] [PubMed] [Google Scholar]
- 100.Que DS, Jamora RDG. Citicoline as adjuvant therapy in Parkinson's disease:a systematic review. Clin Ther. 2021;43:e19–31. doi: 10.1016/j.clinthera.2020.11.009. [DOI] [PubMed] [Google Scholar]
- 101.Reich DS, Lucchinetti CF, Calabresi PA. Multiple sclerosis. N Engl J Med. 2018;378:169–180. doi: 10.1056/NEJMra1401483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rejdak R, Toczolowski J, Kurkowski J, Kaminski ML, Rejdak K, Stelmasiak Z, Grieb P. Oral citicoline treatment improves visual pathway function in glaucoma. Med Sci Monit. 2003;9:PI24–28. [PubMed] [Google Scholar]
- 103.Romero A, Grau T, Sacristan A, Ortiz JA. CDP-choline:6-month study on toxicity in dogs. Arzneimittelforschung. 1983;33:1038–1042. [PubMed] [Google Scholar]
- 104.Rose JW, Hill KE, Watt HE, Carlson NG. Inflammatory cell expression of cyclooxygenase-2 in the multiple sclerosis lesion. J Neuroimmunol. 2004;149:40–49. doi: 10.1016/j.jneuroim.2003.12.021. [DOI] [PubMed] [Google Scholar]
- 105.Rothhammer V, Kenison JE, Tjon E, Takenaka MC, de Lima KA, Borucki DM, Chao CC, Wilz A, Blain M, Healy L, Antel J, Quintana FJ. Sphingosine 1-phosphate receptor modulation suppresses pathogenic astrocyte activation and chronic progressive CNS inflammation. Proc Natl Acad Sci U S A. 2017;114:2012–2017. doi: 10.1073/pnas.1615413114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Roy P, Tomassoni D, Nittari G, Traini E, Amenta F. Effects of choline containing phospholipids on the neurovascular unit:A review. Front Cell Neurosci. 2022;16:988759. doi: 10.3389/fncel.2022.988759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ruckh JM, Zhao JW, Shadrach JL, van Wijngaarden P, Rao TN, Wagers AJ, Franklin RJ. Rejuvenation of regeneration in the aging central nervous system. Cell Stem Cell. 2012;10:96–103. doi: 10.1016/j.stem.2011.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sadaba MC, Rothhammer V, Munoz U, Sebal C, Escudero E, Kivisakk P, Garcia Sanchez MI, Izquierdo G, Hauser SL, Baranzini SE, Oksenberg JR, Alvarez-Lafuente R, Bakshi R, Weiner HL, Quintana FJ. Serum antibodies to phosphatidylcholine in MS. Neurol Neuroimmunol Neuroinflamm. 2020;7:e765. doi: 10.1212/NXI.0000000000000765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Saito RF, Andrade LNS, Bustos SO, Chammas R. Phosphatidylcholine-derived lipid mediators:the crosstalk between cancer cells and immune cells. Front Immunol. 2022;13:768606. doi: 10.3389/fimmu.2022.768606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Santra M, Zhang ZG, Yang J, Santra S, Chopp M, Morris DC. Thymosin beta4 up-regulation of microRNA-146a promotes oligodendrocyte differentiation and suppression of the Toll-like proinflammatory pathway. J Biol Chem. 2014;289:19508–19518. doi: 10.1074/jbc.M113.529966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sapko K, Jamroz-Wisniewska A, Rejdak K. Novel drugs in a pipeline for progressive multiple sclerosis. J Clin Med. 2022;11:3342. doi: 10.3390/jcm11123342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sastry PS. Lipids of nervous tissue:composition and metabolism. Prog Lipid Res. 1985;24:69–176. doi: 10.1016/0163-7827(85)90011-6. [DOI] [PubMed] [Google Scholar]
- 113.Schuler MH, Di Bartolomeo F, Martensson CU, Daum G, Becker T. Phosphatidylcholine affects inner membrane protein translocases of mitochondria. J Biol Chem. 2016;291:18718–18729. doi: 10.1074/jbc.M116.722694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Secades JJ. Role of citicoline in the management of traumatic brain injury. Pharmaceuticals (Basel) 2021;14:410. doi: 10.3390/ph14050410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Secades JJ, Gareri P. Citicoline:pharmacological and clinical review, 2022 update. Rev Neurol. 2022;75:S1–S89. doi: 10.33588/rn.75s05.2022311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Shields SA, Gilson JM, Blakemore WF, Franklin RJ. Remyelination occurs as extensively but more slowly in old rats compared to young rats following gliotoxin-induced CNS demyelination. Glia. 1999;28:77–83. doi: 10.1002/(sici)1098-1136(199910)28:1<77::aid-glia9>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 117.Skripuletz T, Manzel A, Gropengiesser K, Schafer N, Gudi V, Singh V, Salinas Tejedor L, Jorg S, Hammer A, Voss E, Vulinovic F, Degen D, Wolf R, Lee DH, Pul R, Moharregh-Khiabani D, Baumgartner W, Gold R, Linker RA, Stangel M. Pivotal role of choline metabolites in remyelination. Brain. 2015;138:398–413. doi: 10.1093/brain/awu358. [DOI] [PubMed] [Google Scholar]
- 118.Song H, McEwen HP, Duncan T, Lee JY, Teo JD, Don AS. Sphingosine kinase 2 is essential for remyelination following cuprizone intoxication. Glia. 2021;69:2863–2881. doi: 10.1002/glia.24074. [DOI] [PubMed] [Google Scholar]
- 119.Sperka-Gottlieb CD, Hermetter A, Paltauf F, Daum G. Lipid topology and physical properties of the outer mitochondrial membrane of the yeast, Saccharomyces cerevisiae. Biochim Biophys Acta. 1988;946:227–234. doi: 10.1016/0005-2736(88)90397-5. [DOI] [PubMed] [Google Scholar]
- 120.Synoradzki K, Grieb P. Citicoline:a superior form of choline? Nutrients. 2019;11:1569. doi: 10.3390/nu11071569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Terce F, Brun H, Vance DE. Requirement of phosphatidylcholine for normal progression through the cell cycle in C3H/10T1/2 fibroblasts. J Lipid Res. 1994;35:2130–2142. [PubMed] [Google Scholar]
- 122.Tiberi M, Chiurchiu V. Specialized pro-resolving lipid mediators and glial cells:emerging candidates for brain homeostasis and repair. Front Cell Neurosci. 2021;15:673549. doi: 10.3389/fncel.2021.673549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Torbus-Paluszczak M, Bartman W, Adamczyk-Sowa M. Klotho protein in neurodegenerative disorders. Neurol Sci. 2018;39:1677–1682. doi: 10.1007/s10072-018-3496-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Trepanier MO, Hildebrand KD, Nyamoya SD, Amor S, Bazinet RP, Kipp M. Phosphatidylcholine 36:1 concentration decreases along with demyelination in the cuprizone animal model and in post-mortem multiple sclerosis brain tissue. J Neurochem. 2018;145:504–515. doi: 10.1111/jnc.14335. [DOI] [PubMed] [Google Scholar]
- 125.Trotter A, Anstadt E, Clark RB, Nichols F, Dwivedi A, Aung K, Cervantes JL. The role of phospholipase A2 in multiple sclerosis:a systematic review and meta-analysis. Mult Scler Relat Disord. 2019;27:206–213. doi: 10.1016/j.msard.2018.10.115. [DOI] [PubMed] [Google Scholar]
- 126.Ulus IH, Wurtman RJ, Mauron C, Blusztajn JK. Choline increases acetylcholine release and protects against the stimulation-induced decrease in phosphatide levels within membranes of rat corpus striatum. Brain Res. 1989;484:217–227. doi: 10.1016/0006-8993(89)90364-8. [DOI] [PubMed] [Google Scholar]
- 127.Van Doorn R, Van Horssen J, Verzijl D, Witte M, Ronken E, Van Het Hof B, Lakeman K, Dijkstra CD, Van Der Valk P, Reijerkerk A, Alewijnse AE, Peters SL, De Vries HE. Sphingosine 1-phosphate receptor 1 and 3 are upregulated in multiple sclerosis lesions. Glia. 2010;58:1465–1476. doi: 10.1002/glia.21021. [DOI] [PubMed] [Google Scholar]
- 128.Vos JP, Giudici ML, van der Bijl P, Magni P, Marchesini S, van Golde LM, Lopes-Cardozo M. Sphingomyelin is synthesized at the plasma membrane of oligodendrocytes and by purified myelin membranes:a study with fluorescent- and radio-labelled ceramide analogues. FEBS Lett. 1995;368:393–396. doi: 10.1016/0014-5793(95)00695-6. [DOI] [PubMed] [Google Scholar]
- 129.Weiss GB. Metabolism and actions of CDP-choline as an endogenous compound and administered exogenously as citicoline. Life Sci. 1995;56:637–660. doi: 10.1016/0024-3205(94)00427-t. [DOI] [PubMed] [Google Scholar]
- 130.Wheeler D, Bandaru VV, Calabresi PA, Nath A, Haughey NJ. A defect of sphingolipid metabolism modifies the properties of normal appearing white matter in multiple sclerosis. Brain. 2008;131:3092–3102. doi: 10.1093/brain/awn190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Whitney LW, Ludwin SK, McFarland HF, Biddison WE. Microarray analysis of gene expression in multiple sclerosis and EAE identifies 5-lipoxygenase as a component of inflammatory lesions. J Neuroimmunol. 2001;121:40–48. doi: 10.1016/s0165-5728(01)00438-6. [DOI] [PubMed] [Google Scholar]
- 132.Woods PS, Doolittle LM, Rosas LE, Joseph LM, Calomeni EP, Davis IC. Lethal H1N1 influenza A virus infection alters the murine alveolar type II cell surfactant lipidome. Am J Physiol Lung Cell Mol Physiol. 2016;311:L1160–1169. doi: 10.1152/ajplung.00339.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wurtman RJ, Regan M, Ulus I, Yu L. Effect of oral CDP-choline on plasma choline and uridine levels in humans. Biochem Pharmacol. 2000;60:989–992. doi: 10.1016/s0006-2952(00)00436-6. [DOI] [PubMed] [Google Scholar]
- 134.Yeung MSY, Djelloul M, Steiner E, Bernard S, Salehpour M, Possnert G, Brundin L, Frisen J. Dynamics of oligodendrocyte generation in multiple sclerosis. Nature. 2019;566:538–542. doi: 10.1038/s41586-018-0842-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhang J, Zhang ZG, Lu M, Wang X, Shang X, Elias SB, Chopp M. MiR-146a promotes remyelination in a cuprizone model of demyelinating injury. Neuroscience. 2017;348:252–263. doi: 10.1016/j.neuroscience.2017.02.029. [DOI] [PubMed] [Google Scholar]


