Abstract
Linker and deletion mutagenesis and gene fusions were used to probe the possible domain structure of the dodecameric outer membrane secretin PulD from the pullulanase secretion pathway of Klebsiella oxytoca. Insertions of 24 amino acids close to or within strongly predicted and highly conserved amphipathic β strands in the C-terminal half of the polypeptide (the β domain) abolished sodium dodecyl sulfate (SDS)-resistant multimer formation that is characteristic of this protein, whereas insertions elsewhere generally had less dramatic effects on multimer formation. However, the β domain alone did not form SDS-resistant multimers unless part of the N-terminal region of the protein (the N domain) was produced in trans. All of the insertions except one, close to the C terminus of the protein, abolished function. The N domain alone was highly unstable and did not form SDS-resistant multimers even when the β domain was present in trans. We conclude that the β domain is a major determinant of multimer stability and that the N domain contributes to multimer formation. The entire or part of the N domain of PulD could be replaced by the corresponding region of the OutD secretin from the pectate lyase secretion pathway of Erwinia chrysanthemi without abolishing pullulanase secretion. This suggests that the N domain of PulD is not involved in substrate recognition, contrary to the role proposed for the N domain of OutD, which binds specifically to pectate lyase secreted by E. chrysanthemi (V. E. Shevchik, J. Robert-Badouy, and G. Condemine, EMBO J. 16:3007–3016, 1997).
The two-step general secretory pathway (GSP) is used by gram-negative bacteria to secrete extracellular proteins (exoproteins) and for pilus assembly (28). The second step in the GSP comprises several terminal branch pathways for exoprotein translocation from the periplasm across the outer membrane (28). The main terminal branch of the GSP, the secreton or type II secretion pathway, is present in many species of gram-negative bacteria. The Klebsiella oxytoca secreton is used exclusively for the secretion of a cell surface lipoprotein, pullulanase (PulA), and is composed of or involves up to 14 proteins: PulC, PulD, PulE, PulF, PulG, PulH, PulI, PulJ, PulK, PulL, PulM, PulN, PulO, and PulS (28). Only two of these proteins, PulD and PulS, are located exclusively in the outer membrane (4, 7, 8, 13, 14), where they form a large, tightly associated complex that has a barrel-like structure and appears to form ion-conducting channels in artificial lipid bilayers (24).
PulD and its close homologues from other secretons belong to the secretin family of proteins (12), all of which form large complexes that are poorly dissociated in sodium dodecyl sulfate (SDS) at room temperature or even at 100°C (1, 10, 13, 14, 17, 20). Secretins are apparently composed of two main domains (Fig. 1). The C-terminal half, the β domain, is predicted to contain several amphipathic transmembrane (TM) β strands that are probably embedded in the outer membrane in a manner similar to other outer membrane proteins (1). Even distantly related secretins with distinct functions share relatively high sequence similarity in this domain (12). The N-terminal half of secretins, the N domain, is predicted to face the periplasm and is conserved only in secretins from related secretion pathways. The two domains are sometimes separated by a serine-and-glycine-rich segment (12). PulD lacks this spacer sequence, and the junction between the two domains is experimentally defined as the extremity of the membrane-associated fragment that is resistant to treatment with trypsin (starting at amino acid [aa] 298 [23]) (Fig. 1). PulD has a short C-terminal domain (the S domain) that binds the pilot protein PulS, which protects PulD from proteolysis and is essential for its insertion into outer membrane (6, 13, 14) (Fig. 1). Other secretins also appear to have their own, specific pilot proteins that likewise bind to the C-terminal regions (5, 35).
FIG. 1.
Schematic representation of PulD protein showing positions of the signal peptide, the N, β, and S domains, the PulS binding site (6, 13, 14, 35, 36), the trypsin cleavage site (23), the hypothetical TM β strands (TM1 to TM13) (1), and the 24-amino-acid insertions numbered as in Table 2. Intervals of 100 aa and the position of the C terminus (aa 660) are also indicated.
The role of the N domain of secretins has been addressed in two previous studies. One of these studies provided evidence that the N domain of a phage assembly secretin (pIV of phage f1) interacts with another phage-encoded protein, the cytoplasmic membrane protein pI, to determine phage assembly/secretion specificity (6). In the other study, deletion of a 51-amino-acid segment from the N domain of Erwinia chrysanthemi OutD (residues 66 to 116) abolished exoprotein binding and secretion, whereas other deletions abolished secretion without affecting exoprotein recognition (36). Thus, OutD was proposed to bind specifically to exoproteins secreted by the Out secreton and to determine substrate specificity (36), which is in line with other studies showing that OutD protein from Erwinia carotovora, which secreted different exoproteins, cannot substitute for that of E. chrysanthemi (19).
This report describes genetic and other studies designed to test the role of each domain of PulD in multimerization and exoprotein recognition.
MATERIALS AND METHODS
Plasmids and strains.
Plasmids carrying pul and out genes used in this study are listed in Table 1. Strains used were PAP105 [Δ(lac-pro) F′ lacIq pro+ Tn10), PAP7447 [MC4100 (F′ lacIq pro+ Tn10) with pulS, pulA, and pulC-O integrated into malPp and with a large deletion in pulD (13)], and PAP7446, which is the same as PAP7447 except that pulD is wild type and pulS has a Tn5 insertion (13). Genes under lacZp control were induced with 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG), and genes under MalT control were induced with 0.4% maltose or by introduction of a malT (Con) allele. Cells were grown at 30°C in Luria-Bertani medium buffered, where appropriate, with 10% M63 medium (22) and containing antibiotics at the following concentrations: ampicillin, 200 μg/ml; tetracycline, 16 μg/ml; kanamycin, 50 μg/ml; and chloramphenicol, 25 μg/ml.
TABLE 1.
Plasmids used in this study
| Plasmida | Antibiotic resistance | Vector | Cloned gene(s) and mutations | lacZp control | Reference |
|---|---|---|---|---|---|
| pCHAP231 | Ap | pBR322 | All pul genes | − | 9 |
| pCHAP1226 | Ap | pBR322 | pCHAP231 ΔpulD pulB::kan1 | − | 27 |
| pCHAP1106 | Km | pBGS18 | malE′-′pulA | + | 25 |
| pCHAP3072 | Km | pBGS18 | pulAAB-′celZ | + | This study |
| pCHAP3671 | Ap | pUC18 | pulD | + | This study |
| pCHAP3635 | Cm | pSU18 | pulD | + | This study |
| pCHAP1349 | Cm | pSU18 | phoE′-′pulD | + | This study |
| pCHAP3608 | Cm | pSU18 | outD | + | This study |
| pCHAP1300 | Cm | pSU18 | pulA′-′pulD | + | This study |
| pCHAP585 | Ap | pUC19 | pulS | + | This study |
pCHAP3635 and pCHAP3671 carry the same pulD fragment as pCHAP362 (8). The soluble PulA encoded by pCHAP1106 lacks the fatty acylated N terminus normally found on this protein. pCHAP3072 is the same as pCHAP3044 (33) except that gene celZ encoding the E. chrysanthemi exoprotein CelZ (32) replaces blaM. The hybrid protein encoded by this gene is fully and efficiently secreted with the pullulanase secreton. pCHAP585 carries the same pulS gene fragment as pCHAP378 (7).
Mutagenesis and construction of gene fusions.
Transposon TnTAP was used as previosuly described (11) to create pulD-phoA gene fusions in pCHAP3671 (Table 1). Kanamycin-resistant clones were examined for production of stable PulD-PhoA hybrids by SDS-polyacrylamide gel electrophoresis (PAGE) and immunoblotting with antibodies against PulD and PhoA. Selected clones were then sequenced using a primer hybridizing with the 5′ end of phoA. After verification, the transposon was deleted by digesting with NotI, which cleaves sites within the inverted repeats at each end of the transposon, to leave a 24-amino-acid linker insert (11). A stop codon was introduced at the position of the NotI sites using a NotI-compatible linker. Deletions in pulD were created by ligating fragments of pulD obtained by cleaving plasmids with different NotI inserts in the gene and restriction sites flanking the gene.
The ′pulD fragment in pCHAP1349 comprises all codons starting at number 298, corresponding to the N terminus of the trypsin-resistant fragment of purified PulD in detergent micelles (23). The fragment was obtained by PCR amplification. Restriction sites introduced by the amplification primers were used to subclone the amplified DNA into plasmid pCHAP4201 (a pSU18 derivative with the 5′ end of the phoE gene in the same orientation as lacZp) to create a phoE′-′pulD gene fusion in which DNA coding for the PulD-βS fragment is fused to the PhoE signal peptide and the first three amino acids (AEF) of mature PhoE (pCHAP1349). The ′pulD fragment used to create the fatty acylated PulD, which was also obtained by PCR, comprised the complete sequence of mature PulD (9). The amplified fragment was cloned into a plasmid bearing DNA coding for the PulA signal peptide plus five amino acids starting with PulA-derived CD (pCHAP1300).
The outD gene was amplified by PCR using primers hybridizing with the 3′ end of outC and the 5′ end of outE in pCCP2236 (19) and was then subcloned using restriction sites introduced by the amplification primers. Segments of the outD and pulD genes were exchanged by using existing restriction endonuclease sites or by creating new sites at identical positions in the two genes by site-directed mutagenesis (18) in such a way that minimal sequence changes were introduced into the polypeptides. Excised fragments were exchanged between two plasmids, and recombinants were verified by restriction analysis and by immunoblotting with PulD antibodies to detect the chimera.
SDS-PAGE and immunoblotting.
Proteins were separated by SDS-PAGE in 10 or 12% acrylamide gels, were transferred onto nitrocellulose sheets, and were incubated with the antibodies indicated and then with horseradish peroxidase-coupled antirabbit immunoglobulin G (IgG). In the far-Western analysis, nitrocellulose sheets were first incubated with purified alipoPulA (pCHAP1106) then with antibodies against PulA and then with horseradish peroxidase-coupled antirabbit IgG. Immunoblots were developed by enhanced chemiluminescence (Amersham). Where indicated, PulD multimers were dissociated by phenol extraction (13) and the samples were dissolved in sample buffer (100 mM Tris [pH 8.0], 5% SDS, 12% glycerol, 1% 2-mercaptoethanol). Unless otherwise stated, all samples were heated for 5 min at 100°C before being loaded onto the gel. Metabolic labeling with radioactive palmitate was carried out as described previously (29), and labeled proteins were separated in SDS-PAGE gels that were then fixed in 10% acetic acid, soaked in Amplify (Amersham), and examined by fluorography.
Affinity chromatography, flotation gradients, and pullulanase assays.
For affinity chromatography, pullulanase was bound to amylose-agarose resin (New England Biolabs) and pCHAP3072-encoded PulAAB-CelZ, a secretable hybrid protein comprising the putative secretion signals from PulA (33) fused to the cellulase CelZ of E. chrysanthemi (30), was bound to Avicel (Serva). Membranes were solubilized in 25 mM Tris (pH 7.4) containing 2% Triton and 1 mM EDTA (approximately 25% of the total amount of PulD was solubilized), and insoluble material was removed by ultracentrifugation (120,000 × g for 1 h). The extract was then incubated with the immobilized pullulanase, which was then washed extensively with the same buffer. Bound proteins were eluted in 25 mM Tris containing 2% SDS and were examined by SDS-PAGE and immunoblotting. The presence of PulA and PulAAB-CelZ in the extracts was verified by immunoblotting with anti-PulA and anti-CelZ (a gift from F. Barras), and the presence of PulD was examined by immunoblotting with anti-PulD.
For flotation gradient analysis, outer membranes were first prepared by breaking the bacteria in a French press, removing unbroken cells by centrifugation at 3,000 × g for 5 min, and then pelleting the outer membrane by centrifugation for 1 min in a Beckman TL100 rotor at 120,000 × g. The resuspended membranes were incubated with purified pullulanase, brought to 60% (wt/wt) with sucrose, and loaded in the bottom Beckman SW55 centrifuge tube. The membranes were then overlaid with decreasing concentrations of sucrose (from 56 to 35%) in 25 mM Tris and were centrifuged at 176,000 × g for 24 h. Fractions collected from the tops of the tubes were analyzed for the presence of PulD and PulA by SDS-PAGE and immunoblotting.
Pullulanase was assayed as previously described (21), except that cells were lysed with 0.5% octylpolyoxyethylene. The level of secretion is the percentage of the total amount of pullulanase that was detectable in unlysed cells.
RESULTS
Linker insertions in pulD.
In previous studies of the pulD homologues xpsD (16) and xcpQ (1), short linker insertions often had little or no effect on secretin function. Therefore, we reasoned that larger inserts might also be tolerated and could provide information on the topology of secretin in the outer membrane and on the role of individual domains in multimerization and substrate recognition. Therefore, we used TnTAP, which creates phoA fusions and has NotI restriction sites at each end so that it can be deleted, to leave a 24-amino-acid insert containing a cleavage site for the tobacco etch virus protease (11).
Thirty eight such inserts were created at 19 positions in PulD (Fig. 1 and Table 2). The insertions were relatively well dispersed throughout the gene, though there were also some transposition hot spots (Table 2). All of the mutants contained PulD at levels that ranged from 20 to 100% of those of unaltered PulD, and degradation products were not detected when PulS was present. However, three categories of mutants could be distinguished with respect to their proportion of the total amount of PulD present as monomers and their ability to complement the ΔpulD mutation in strain PAP7447 (13) (Table 2 and Fig. 2).
TABLE 2.
Effects of 24-amino-acid insertions at different sites in PulD
| Insertion no. | Position (aa)a | Domainb | No. of examples tested | Yield and multimerizationc | % Complementationd |
|---|---|---|---|---|---|
| 0 | A | 100 | |||
| 1 | 22 | 1 | C | 0 | |
| 2 | 58 | N | 1 | C | 0 |
| 3 | 77 | N | 1 | C | 0 |
| 4 | 117 | N | 1 | C | 0 |
| 5 | 122 | N | 2 | C | 0 |
| 6 | 135 | N | 5 | C | 0 |
| 7 | 156 | N | 2 | C | 0 |
| 8 | 196 | N | 1 | C | 0 |
| 9 | 267 | N | 3 | C | 0 |
| 10 | 281 | N | 1 | D | 0 |
| 11 | 290 | N | 1 | D | 0 |
| 12 | 318 | β | 3 | D | 0 |
| 13 | 406 | β | 2 | C | 0 |
| 14 | 410 | β | 7 | C | 0 |
| 15 | 441 | β | 2 | D | 0 |
| 16 | 465 | β | 2 | D | 0 |
| 17 | 507 | β | 1 | D | 0 |
| 18 | 533 | β | 1 | D | 0 |
| 19 | 640 | S | 1 | B | 22 |
Positions are the last amino acid in the sequence of prePulD before the insertion site.
N, N domain; S, S domain.
Yields and multimerization are classified into four categories, as illustrated in Fig. 2.
Complementation tests were performed in maltose-induced cultures of PAP7447 (ΔpulD in chromosome). The results are averages of at least three independent assays.
FIG. 2.
Formation of SDS-resistant multimers and yields of representative PulD variants obtained by linker insertions in the pulD gene. Three categories of mutants were obtained: slightly reduced total yields and unaltered multimerization (B), slightly reduced yields and substantially reduced multimerization (C), and slightly reduced yields and abolished or drastically reduced multimerization (D) compared with normal PulD (A). Samples from IPTG-induced and noninduced cells of E. coli expressing pulS together with the pulD variants under lacZp control on pSU18 derivatives were examined by SDS-PAGE and immunoblotting. The same amount of total cell extract was loaded in each lane, and each panel represents the same chemiluminescence exposure time.
All of the insertions except number 19 (class B) affected, to at least some extent, the levels of SDS-resistant multimers detected. The distinction between mutants in classes C and D was based on two highly reproducible criteria (Fig. 2): normal levels of protein and reduced, but still appreciable, levels (>30%) of multimers in class C mutants compared with reduced yields (20%) and total absence of multimers in class D mutants. Since the multimers produced by class C mutants formed discrete bands that entered into the 4.5% acrylamide stacking gel and are resistant to dissociation in SDS at 100°C, they are presumed to correspond to true multimers, rather than to aggregates (Table 2 and Fig. 2; see Discussion).
Apart from insertion 19, all of the PulD insertion variants were completely inactive in complementation tests in PAP7447 (Table 2). Since all of these variants retain the complete PulS binding site at the C-terminal ends, they are probably sorted to and associate with the outer membrane, though they might not insert correctly. When production of the PulD variants was increased in these cells by IPTG induction, the bacteria grew more slowly and lysed when they were harvested and washed (not shown). This phenomenon could result from defective insertion into the outer membrane.
The only insertion that did not abolish ability to complement ΔpulD or cause lysis after IPTG induction (insertion 19, aa 640) is located within the S domain (Fig. 1, Table 2). The protein was still protected by PulS (data not shown). Therefore, its reduced ability to complement ΔpulD might be due to impaired outer membrane insertion, which requires the region around aa 640 in addition to the PulS binding site (4).
Although all of the other insertions completely abolished complementation of ΔpulD in strain PAP7447, some caused variable, partial complementation of the ΔpulD mutation carried by pCHAP1226 (data not shown). This low-level complementation (<45% secretion) might reflect partial activity that is manifested only when all other secreton components (encoded by pCHAP1226) are present at high levels.
Deletion of the PulD β and N domains and trans-induced multimerization.
The data presented above suggest that the β domain might be important for SDS-resistant multimer formation. However, these data are in contrast to the results of previous studies showing that a hybrid protein comprising the N domain (aa 1 to 99 or 1 to 395) of PulD fused to PhoA apparently produced low amounts of multimer (13). Therefore, we screened all PulD-PhoA hybrids generated by the insertion of TnTAP for the production of SDS-resistant multimers. Immunoblotting with PulD and PhoA antisera failed to reveal the presence of any multimers in any case except number 19 (aa 640), which retains all of the N and β domains. Presumably, the previously detected PulD-PhoA multimers (13) represented aggregates rather than dodecameric complexes similar to those formed by full-length PulD (24). It is also possible that the combination of a segment of the N domain of PulD and the form of PhoA used in the previous experiments could nucleate a low level of multimer production.
To obtain more information on the role of the N and β domains in multimer stability, we created truncated forms of PulD. First, we constructed a variant in which the β and S domains of PulD (from aa 298) were fused to the signal peptide and the first three amino acids of outer membrane protein PhoE (PulD-βS; pCHAP1349). Proteolysis of purified PulD dodecamers causes cleavage after residue 297 to produce a protected dodecameric PulD-βS fragment that is almost identical to PulD-βS encoded by pCHAP1349 (23). PulD-βS produced in vivo was protected from proteolysis by plasmid-encoded PulS (Fig. 3), but multimers were not detectable (data not shown). Thus, the βS alone is unable to form SDS-resistant multimers, though it presumably associates with the outer membrane through its interaction with PulS. High level (IPTG-induced) production of PulD-βS caused the bacteria to grow poorly and to lose viability at the end of exponential growth, though they did not exhibit increased sensitivity to normally nonpenetrating agents such as novobiocin or rifampicin. These observations suggest that PulD-βS causes general membrane perturbation without producing open channels in the outer membrane.
FIG. 3.
Protection of PulD-βS by PulS. Extracts from IPTG-induced cells producing PulD-βS alone (pCHAP1349) or PulD-βS plus PulS (pCHAP1349 plus pCHAP585) were examined by immunoblotting with PulD antibodies and chemiluminescence. Bands corresponding to protected and clipped PulD-βS are indicated. The band just above PulD-βS that comigrates with the 47-kDa marker protein (indicated at left) is recognized nonspecifically by the antibodies used.
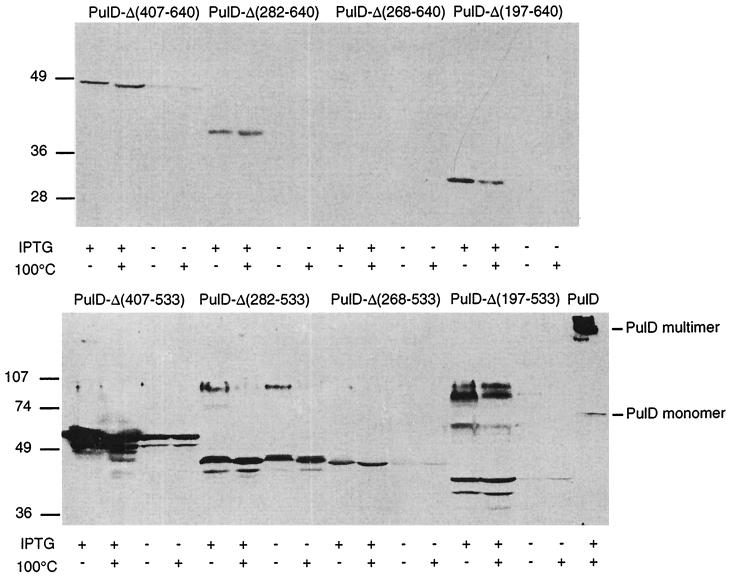
Next, a set of eight PulD variants lacking different sections of the β domain was constructed in vitro using unique NotI sites inserted at different positions within the pulD gene. The deletions spanned the region from aa 406 (insertion number 13), aa 281 (number 10), aa 267 (number 9), or aa 196 (number 8) up to aa 533 (insertion number 18) or aa 640 (number 19) (Fig. 4). Variants with deletions that terminated at aa 640 (and therefore lacking a region essential for outer membrane insertion [4]) were not detectable or were detectable only in IPTG-induced cultures irrespective of the presence of PulS, and none was present as multimers (Fig. 4). In contrast, proteins with deletions that terminated at aa 533 were detectable even without induction, and two of them (PulD-Δ282-533 and PulD-Δ197-533) were present as multimers. Unlike full-length PulD, the PulD-Δ282-533 multimers were dissociated after heating to 100°C in SDS (Fig. 4). Surprisingly, multimers were most abundant in the case of the variant with the largest deletion (PulD-Δ197-533) (Fig. 4). Furthermore, multimers produced by PulD-Δ282-533 and PulD-Δ197-533 were much closer in size than would be expected from the difference in size of the two monomeric forms (Fig. 4), suggesting that they could represent smaller multimers than the dodecamer formed by full-length PulD (24). However, since these multimers form discrete bands upon SDS-PAGE, they do not correspond to aggregates. From these results, we can conclude that the C-terminal region of the β domain and the PulS binding site (aa 534 to 640) stabilize the N domain, presumably as a result of the binding of PulS (4). Furthermore, the combined presence of aa 1 to 196 and 533 to 640 seems to permit at least some degree of multimerization of truncated PulD.
FIG. 4.
Effects of internal deletions affecting mainly the PulD β domain on the formation of SDS-resistant multimers. The proteins, resulting from deletions between linkers inserted at the indicated positions in the PulD sequence (Table 2), were produced in cells that also contained PulS. The samples at the extreme right of the lower panel were from a strain producing full-length PulD which was included as a control. The positions of monomeric and multimeric forms of PulD are indicated. The samples were from strains grown with or without IPTG to induce production of the PulD variants encoded by the lacZp-ΔpulD operon fusions on pUC18 and one of each pair of samples was heated to 100°C for 5 min before being loaded on the gel, as indicated. The positions of molecular size markers (kDa) are indicated.
To validate these observations, a stop codon-bearing linker was inserted into the NotI sites at aa 406, 281, 267, and 196 to produce PulD truncates completely or almost completely devoid of the β and S domains (called PulD-N406, PulD-N281, PulD-N267, and PulD-N196, respectively). These truncates were barely detectable, even in induced cultures (data not shown). To determine whether N domains encoded by these constructs could be stabilized by the PulD-βS in trans, the cells were transformed with pCHAP1349 (see above). Immunoblots of these cells were probed with antibodies raised against PulD or against the first 126 aa of the protein (8) to distinguish between PulD-βS and the other forms of PulD. Both antibodies recognize multimers formed from full-length PulD (data not shown). Remarkably, an increase in the levels of both the PulD-βS and the PulD-N variants (and of degradation products derived therefrom) was observed when they were produced by the same cell, indicating mutual stabilization by the two halves of the protein (an example is shown in Fig. 5). Furthermore, SDS- and heat-resistant multimers of PulD-βS were abundant in cells producing PulD-N196 (Fig. 5). This result indicates that the N domain of PulD (or part of it) can stabilize and cause at least partial multimerization of the β and S domains in trans. However, the association between the two segments is apparently disrupted by SDS, since PulD-N196 was not found in these multimers, and the multimers were smaller than would be expected if they were composed of 12 copies of the truncated monomer (Fig. 5). Surprisingly, multimers of the PulD-βS were much more abundant in cells coproducing PulD-N196 than in cells producing the longer PulD-N variants (data not shown).
FIG. 5.
Stabilization and induced multimer formation in PAP7447 cells producing PulD-βS and PulD-N196 encoded by separate plasmids, both encoded by pulD variants under lacZp control from uninduced or IPTG-induced cultures. (Note that the cells produce only low levels of chromosome-encoded PulS.) Pairs of extracts from cells producing one or other or both of the variants were subjected to SDS-PAGE and were blotted onto nitrocellulose sheets. One representative of each pair of samples was heated to 100°C for 5 min before SDS-PAGE, as indicated. Proteins were immunodetected first with antibodies against full-length PulD (bottom panels), and then the nitrocellulose sheet was stripped and reprobed with antiserum against PulD-PhoA containing only the first 126 aa of PulD, which does not react with PulD-βS (upper panels). Bands recognized nonspecifically by this antiserum (including PhoA alkaline phosphatase) are indicated by the letters NS. Other bands derived from PulD are indicated, as are the positions of molecular mass markers (kDa).
Next, we replaced the signal peptide of PulD with a lipoprotein signal peptide and the first five amino acids of a lipoprotein that includes the fatty acylated N-terminal cysteine residue and a serine at position +2 for sorting to the outer membrane (34). This protein was created to test two aspects of PulD structure and function: the possibility that added fatty acids could replace the requirement for fatty acids on PulS (14) and the ability of PulD to function when its N terminus is tethered in the lipids of the outer membrane, as is apparently the case in the related secretins XpsD (15) and BfpB (31). The PulD derivative created was fatty acylated, as shown by incorporation of radioactive palmitate, formed multimers that were the same size as those formed by normal PulD, and was protected from degradation by PulS (data not shown). However, the protein was unable to substitute for PulD. Replacement of the lipoprotein signal peptide by the classical signal peptide from the periplasmic MalE protein restored function (data not shown). Therefore, tethering of the N terminus of PulD to the outer membrane causes loss of function but not loss of multimerization. This result is of interest with respect to recent data (23) suggesting that the N terminus of PulD is sequestered within the barrel formed by the β domain (24).
PulD-OutD and OutD-PulD chimeras.
PulD cannot be replaced by OutD (14, 26), which is in line with data indicating that OutD could be a determinant of substrate specificity for the E. chrysanthemi secreton (19, 36). To analyze the proposed role of the N domains of OutD and PulD in substrate recognition (36), we used naturally existing restriction endonuclease sites or restriction sites created in pulD (pCHAP3635) and/or outD (pCHAP3608) by site-directed mutagenesis to construct genes coding for secretin chimeras. We expected that ΔpulD would be complemented only by secretin chimeras in which the N domain was derived from PulD. In fact, the data revealed the opposite to be true. Table 3 summarizes results obtained with clones that encoded secretin chimeras that had the expected size and did not show evidence of proteolysis in strains producing PulS. The following points are worthy of particular mention.
TABLE 3.
Characterization of PulD-OutD and OutD-PulD secretin chimeras
| Proteina | Plasmid | Yieldb | Predominant form at 30°Cc | % Complementationd |
|---|---|---|---|---|
| PulD (P) | pCHAP3635 | Normal | Multimer | 100 |
| OutD (O) | pCHAP3608 | Reduced | Monomer | 0 |
| P273O | pCHAP3637 | Normal | Monomer | 0 |
| O273P | pCHAP3662 | Reduced | Monomer | 55 |
| O418P | pCHAP3615 | Reduced | Monomer | 0 |
| P365O | pCHAP3643 | Normal | Monomer | 0 |
| P67O | pCHAP3638 | Reduced | Monomer | 0 |
| P158O | pCHAP3652 | Normal | Monomer | 0 |
| O158P | pCHAP3650 | Normal | Multimer | 100 |
| P67O158P | pCHAP3648 | Normal | Multimer | 100 |
| O158P273O | pCHAP3658 | Reduced | Monomer | 0 |
| P158O273P | pCHAP3665 | Normal | Multimer | 100 |
Junctions between the two proteins in the chimeras are indicate as amino acid residues in the sequence of the protein segment upstream. P, PulD; O, OutD.
Yields were estimated from immunoblots with PulD antibodies. Normal, close to yield of PulD under identical conditions; reduced, <20% of normal yield.
The multimer-monomer ratio was determined in samples that were not heated to 100°C before electrophoresis. The form of the protein that predominates under these conditions is indicated. Where monomer is indicated, the multimer was barely or totally undetectable by immunoblotting.
Complementation tests were performed in maltose-induced cultures of PAP7447 (ΔpulD in chromosome). The results are averages of at least three independent assays.
(i) PulD multimers are more resistant than those of OutD to dissociation in SDS. Secretin chimeras that exhibited PulD-like resistance to dissociation invariably included the entire β and S domains of PulD. This result underscores the critical importance of the β domain in multimer stability in SDS.
(ii) Yields and multimer stability were not affected by the presence of secreton components other than PulS (data not shown).
(iii) Only four of the constructs complemented ΔpulD in PAP7447. One of them, O273P, (see Table 3 for notations) in which almost the entire N domain is derived from OutD was only partially active in this complementation test. The other three (O158P, P67O158P, and P158O273P), including chimeras containing the OutD region from positions 66 to 116 that is part of the exoprotein binding site (36), were fully active and were the only ones that were present mainly as SDS- and heat-resistant multimers.
(iv) The gene fusions were unable to complement ΔpulC or ΔpulG mutations. Thus, complementation of ΔpulD is not due to nonspecific permeabilization of the outer membrane.
These data suggest that the N domain of OutD is not specific to the Out secreton or to exoproteins secreted by it, as might be expected from the data of Shevchik et al. (36). Instead, the β domain of PulD seems to be specifically required for the secretin chimeras to promote pullulanase secretion. Whether the partial activity of the O273P chimera and the complete absence of activity of the other chimeras lacking the PulD β domain is because of lower yields, drastically reduced multimerization (only trace amounts of heat-resistant multimer were detected for O273P and O417P), incorrect assembly into the outer membrane, or the absence of PulA recognition or interaction with another secreton component cannot be determined.
Next, we sought direct evidence of a direct interaction between pullulanase and PulD using procedures very similar to those used to demonstrate the interaction between pectate lyase and OutD (36). First, far-Western blotting experiments failed to demonstrate any interaction between multimeric or phenol-dissociated PulD renatured on nitrocellulose membranes and PulA. Second, cofractionation experiments failed to reveal any specific association of a soluble (alipo-) form of pullulanase and outer membranes containing PulD. Third, PulA did not protect PulD from degradation in the absence of PulS (which normally prevents PulD proteolysis by unidentified endogenous proteases [13]). In addition, different forms of pullulanase were immobilized on specific resins and were tested for their ability to trap PulD in detergent-solubilized extracts of outer membranes. Again, none of these assays provided any indication of a specific interaction between PulA and PulD. Therefore, the possible contribution of PulA recognition PulA by PulD to secretion specificity is questionable.
DISCUSSION
On the basis of the data presented here and comparisons with other secretins, we propose that PulD is composed of two major domains, an N-terminal (N) domain predicted to face the periplasm and a β domain. The β domain of the PulD is defined on the basis of two features. First, it is the only region of the protein predicted to contain amphipathic β strands that, by analogy with porins, are likely to be embedded in the outer membrane. Second, proteolysis of the purified protein leaves the β domain intact as a stable dodecameric complex (23). The β domain is followed by a short domain (S) to which the pilot protein PulS binds to facilitate insertion of PulD into the outer membrane (4).
The β domain of the PulD homologue XcpQ was predicted to include 13 potential amphipathic TM β strands according to an algorithm based on the known structures of outer membrane porins (1) (Fig. 1). There are unlikely to be 13 TM segments in PulD, because the N and S domains probably both face the periplasm. Ten of the 13 predicted TM segments of XcpQ are relatively well conserved in PulD and its other homologues, two (numbers 3 and 5) are moderately conserved, and one (number 4) is poorly conserved. Furthermore, two of the 13 predicted TM β strands (5 and 12) are not predicted to be in β configuration according to most commonly used structure prediction algorithms (3). No other β strands were predicted in the β domain by these algorithms. On the basis of these analyses, there appear to be 10 TM β strands in the β domain (numbers 1, 2, 3, 6, 7, 8, 9, 10, 11, and 13). Importantly, all predicted TM segments are downstream from the proteolysis cleavage site that defines the border between the N and β domains in PulD (23) and are upstream from the less-well-conserved region that corresponds to the S domain in PulD (Fig. 1). Surprisingly, the entire β domain contains only one well-conserved aromatic amino acid (F573 in PulD, on the C-terminal end of predicted TM12), whereas conserved aromatic residues, which form belts at each end of the TM barrels, are conserved in other outer membrane proteins such as porins (2). (Interestingly, F573 is absolutely conserved in all secretins, including those that are only distantly related to PulD.)
Do the consequences of the insertion of 24-amino-acid peptides in PulD conform to this prediction? Most of these insertions cause one of two quite distinct changes to PulD: increased dissociation in SDS (or reduced multimerization) (class C mutants) or complete absence of multimers and reduced total yield (class D mutants) detected by SDS-PAGE and immunoblotting (Fig. 2). The total absence of multimers in class D mutants indicates that these insertions completely destabilize the complex. Five out of 7 class D insertions (at aa 318 [number 12], aa 441 [number 15], aa 465 [number 16], aa 507 [number 17], and aa 533 [number 18]) are within or immediately adjacent to strongly predicted TM β strands (numbers 1, 6, 7, 9, and 10, respectively) (Fig. 1) and would therefore be expected to disrupt packing of the β strands. The two other insertions that abolished SDS-resistant multimer formation (numbers 10 and 11) flank a highly conserved, relatively hydrophobic sequence (282LVEVLTGIS290) that is not predicted to have a β structure by most algorithms and which is upstream of the experimentally determined N terminus of the β domain (aa 298 [23]).
All of the other insertions except number 19 belong to class C. Most of these insertions are in the N domain, but two are in the β domain (at aa 406 and 410) (Fig. 1). These insertions are in the relatively long loop between the weakly predicted TM β strands 4 and 5 and, therefore, are likely to have less dramatic effects on packing of the β strands. Taken together, these results suggest that the correct packing of the β strands is required for multimer formation or stabilization. Insertions elsewhere probably have less dramatic effects because they do not affect packing of the β strands and, presumably, destabilize multimers or reduce multimer formation for other reasons.
Although the β domain is important for multimer formation and/or stability, it is not the only region of the protein involved in multimerization because, when synthesized alone, it is exported and interacts with PulS but does not form SDS-resistant multimers. However, as will be reported elsewhere, when this domain is excised from purified PulD multimers by proteolysis, it retains its multimeric structure (23). Two further experiments reported here address the role of the N and β domains in multimerization. First, certain internal deletions in the β domain did not completely abolish multimerization if the region downstream from aa 533 (including putative TM β segments 11, 12, and 13) was retained (Fig. 4). This result suggests that the C-terminal 106 aa of the β domain might possess the ability to multimerize alone or when fused to (part of) the N domain. However, the multimers detected are considerably smaller than would be expected for a dodecameric complex (Fig. 4). Second, isolated N and β domains stabilize each other and allow multimerization of the β domain (Fig. 5). However, these multimers are also smaller than expected for a dodecamer and actually appear to increase in size when heated to 100°C (Fig. 5). In both cases, the multimers detected might represent intermediate steps in assembly of a full complex, in which case we would conclude that both the N and β domains are required for multimerization.
Studies of the OutD and PulD chimeras also contributed information on the roles of the N and β domains in multimer stability, since only those chimeras that retained the PulD β domain formed multimers that resisted heat dissociation in SDS. However, the main purpose behind the construction of these hybrids was to test the possibility that the N domain of secretins determines which substrate will be secreted. We found that a chimera in which almost all of the PulD N domain (up to aa 273) was replaced by the corresponding domain of OutD was still able to complement the ΔpulD mutation in strain PAP7447, whereas chimeras in which the βS domain was derived from OutD did not (Table 3). This result suggests that the N domain of PulD does not contain an essential binding site for pullulanase or determine secretion specificity. This is in contrast to OutD, for which there is evidence that the N domain binds specific exoproteins (36). There are several possible explanations for this striking inconsistency. First, the N domain of OutD could recognize pullulanase as well as the range of substrates normally secreted by the Out secreton of E. chrysanthemi. If this is the case, then the observed failure of E. chrysanthemi to secrete pullulanase encoded by pulA on a plasmid could be explained by a specific requirement for PulC and by the inability of OutD to replace PulD due to incompatibility between OutD and PulC. Second, specificity might be determined by the β domain of PulD. However, we did not obtain any evidence for an interaction between PulD and pullulanase by any of several different methods. Furthermore, pullulanase has no effect on the conductivity of the channels formed by purified PulD in artificial lipid bilayers (24). This leads to the third explanation, which is that PulD does not recognize PulA. If this is the case, PulD and OutD are more dissimilar than might be assumed from their highly related sequences, and the inability of OutD or the chimeras containing the β domain of OutD to replace PulD in pullulanase secretion is presumably explained by incompatibility with PulC. Since all other Pul secreton components except PulC can be replaced by the corresponding protein from the Out secreton without loss of pullulanase secretion (19, 26), we tentatively conclude that PulC, rather than PulD, participates directly in pullulanase recognition.
ACKNOWLEDGMENTS
We are grateful to Dominique Vidal-Ingigliardi for sequence analysis and structure predictions; to Olivera Francetic, Nico Nouwen, and Guillaume Vignon for providing access to unpublished data; to Nathalie Nadeau for technical assistance; to Armelle Lavenir for artwork; and to all other members of the secretion lab for their interest and encouragement. We are also grateful to Michael Ehrmann for the TnTAP system, for guidance on its use, and for providing access to unpublished data; to Alan Collmer for pCHAP2236; and to Fred Barras for providing CelZ antibodies.
This work was supported by the European Union (Training and Mobility in Research grant number FMRX-CT96-0004) and by a French Research Ministry grant in the Programme fondamentale en Microbiologie et Maladies infectieuses et parasitaires.
REFERENCES
- 1.Bitter W, Koster M, Latijnhouwers M, de Cock H, Tommassen J. Formation of oligomeric rings by XcpQ and PilQ, which are involved in protein transport across the outer membrane of Pseudomonas aeruginosa. Mol Microbiol. 1998;27:209–219. doi: 10.1046/j.1365-2958.1998.00677.x. [DOI] [PubMed] [Google Scholar]
- 2.Cowan S W, Schrimer T, Rummer G, Steiert M, Ghosh R, Pauptit R A, Jansonius J N, Rosenbush J P. Crystal structures explain functional properties of two E. coli porins. Nature. 1992;358:727–733. doi: 10.1038/358727a0. [DOI] [PubMed] [Google Scholar]
- 3.Cuff J A, Clamp M E, Siddiqui A S, Finlay M, Barton G J. JPred: a consensus secondary structure prediction server. Bioinformatics. 1998;14:892–893. doi: 10.1093/bioinformatics/14.10.892. [DOI] [PubMed] [Google Scholar]
- 4.Daefler S, Guilvout I, Hardie K R, Pugsley A P, Russel M. The C-terminal domain of the secretin PulD contains the binding site for its cognate chaperone, PulS, and confers PulS dependence on pIVf1 function. Mol Microbiol. 1997;24:465–475. doi: 10.1046/j.1365-2958.1997.3531727.x. [DOI] [PubMed] [Google Scholar]
- 5.Daefler S, Russel M. The Salmonella typhimurium InvH protein is an outer membrane lipoprotein required for the proper localization of InvG. Mol Microbiol. 1998;28:1367–1380. doi: 10.1046/j.1365-2958.1998.00908.x. [DOI] [PubMed] [Google Scholar]
- 6.Daefler S, Russel M, Model P. Module swaps between related translocator proteins pIVf1, pIVIke and PulD: identification of a specificity domain. J Mol Biol. 1997;266:978–992. doi: 10.1006/jmbi.1996.0866. [DOI] [PubMed] [Google Scholar]
- 7.d’Enfert C, Pugsley A P. Klebsiella pneumoniae pulS gene encodes an outer membrane lipoprotein required for pullulanase secretion. J Bacteriol. 1989;171:3673–3679. doi: 10.1128/jb.171.7.3673-3679.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.d’Enfert C, Reyss I, Wandersman C, Pugsley A P. Protein secretion by gram-negative bacteria: characterization of two membrane proteins required for pullulanase secretion by Escherichia coli K-12. J Biol Chem. 1989;264:17462–17468. [PubMed] [Google Scholar]
- 9.d’Enfert C, Ryter A, Pugsley A P. Cloning and expression in Escherichia coli of the Klebsiella pneumoniae genes for production, surface localization and secretion of the lipoprotein pullulanase. EMBO J. 1987;6:3531–3538. doi: 10.1002/j.1460-2075.1987.tb02679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drake S L, Sandstedt S A, Koomey M. PilP, a pilus biogenesis lipoprotein in Neisseria gonorrhoeae, affects expression of PilQ as a high-molecular-mass multimer. Mol Microbiol. 1997;23:657–668. doi: 10.1046/j.1365-2958.1997.2511618.x. [DOI] [PubMed] [Google Scholar]
- 11.Ehrmann M, Bolek P, Mondigler M, Boyd D, Lange R. TnTIN and TnTAP: mini-transposons for site-specific proteolysis in vivo. Proc Natl Acad Sci USA. 1997;94:13111–13115. doi: 10.1073/pnas.94.24.13111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Genin S, Boucher C A. A superfamily of proteins involved in different secretion pathways in gram-negative bacteria: modular structure and specificity of the N-terminal domain. Mol Gen Genet. 1994;243:112–118. doi: 10.1007/BF00283883. [DOI] [PubMed] [Google Scholar]
- 13.Hardie K R, Lory S, Pugsley A P. Insertion of an outer membrane protein in Escherichia coli requires a chaperone-like protein. EMBO J. 1996;15:978–988. [PMC free article] [PubMed] [Google Scholar]
- 14.Hardie K R, Seydel A, Guilvout I, Pugsley A P. The secretin-specific, chaperone-like protein of the general secretory pathway: separation of proteolytic protection and piloting functions. Mol Microbiol. 1996;22:967–976. doi: 10.1046/j.1365-2958.1996.01539.x. [DOI] [PubMed] [Google Scholar]
- 15.Hu N-T, Hung M-N, Liao C-T, Lin M-H. Subcellular location of XpsD, a protein required for extracellular protein secretion by Xanthomonas campestris pv. campestris. Microbiology. 1995;141:1395–1406. doi: 10.1099/13500872-141-6-1395. [DOI] [PubMed] [Google Scholar]
- 16.Hu N-T, Hung M N, Chanhan Chen D, Tsai R-T. Insertion mutagenesis of XpsD, an outer-membrane protein involved in extracellular protein secretion in Xanthomonas campestris pv. campestris. Microbiology. 1998;144:1479–1486. doi: 10.1099/00221287-144-6-1479. [DOI] [PubMed] [Google Scholar]
- 17.Koster M, Bitter W, de Cock H, Allaoui A, Cornelis G, Tommassen J. The outer membrane component, YscC, of the Yop secretion machinery of Yersinia enterocolitica forms a ring-shaped multimeric complex. Mol Microbiol. 1997;26:789–797. doi: 10.1046/j.1365-2958.1997.6141981.x. [DOI] [PubMed] [Google Scholar]
- 18.Kunkel T A, Roberts J D, Zakour R A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- 19.Lindeberg M, Salmond G P C, Collmer A. Complementation of deletion mutations in a cloned functional cluster of Erwinia chrysanthemi out genes with Erwinia carotovora out homologs reveals OutC and OutD as candidate gatekeepers of species-specific secretion of proteins via the Type II pathway. Mol Microbiol. 1996;20:175–190. doi: 10.1111/j.1365-2958.1996.tb02499.x. [DOI] [PubMed] [Google Scholar]
- 20.Linderoth N A, Model P, Russel M. Essential role of a sodium dodecyl sulfate-resistant protein IV multimer in assembly-export of filamentous phage. J Bacteriol. 1996;178:1962–1970. doi: 10.1128/jb.178.7.1962-1970.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Michaelis S, Chapon C, d’Enfert C, Pugsley A P, Schwartz M. Characterization and expression of the structural gene for pullulanase, a maltose-inducible secreted protein of Klebsiella pneumoniae. J Bacteriol. 1985;164:633–638. doi: 10.1128/jb.164.2.633-638.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 23.Nouwen, N. 1999. Unpublished data.
- 24.Nouwen N, Ranson N, Saibil H, Wolpensinger B, Engel A, Ghazi A, Pugsley A P. Secretin PulD: association with pilot protein PulS, structure and ion-conducting channel formation. Proc Natl Acad Sci USA. 1999;96:8173–8177. doi: 10.1073/pnas.96.14.8173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poquet I, Faucher D, Pugsley A P. Stable periplasmic secretion intermediate in the general secretory pathway of Escherichia coli. EMBO J. 1993;12:271–278. doi: 10.1002/j.1460-2075.1993.tb05653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Possot, O., G. Vignon, N. Bomchil, and A. P. Pugsley. Multiple interactions between pullulanase secreton components involved in stabilization and cytoplasmic membrane association of PulE. Submitted for publication. [DOI] [PMC free article] [PubMed]
- 27.Possot O M, Gérard M, Pugsley A P. Membrane association and multimerization of secreton component PulC. J Bacteriol. 1999;181:4004–4011. doi: 10.1128/jb.181.13.4004-4011.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pugsley A P. The complete general secretory pathway in gram-negative bacteria. Microbiol Rev. 1993;57:50–108. doi: 10.1128/mr.57.1.50-108.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pugsley A P, Chapon C, Schwartz M. Extracellular pullulanase of Klebsiella pneumoniae is a lipoprotein. J Bacteriol. 1986;166:1083–1088. doi: 10.1128/jb.166.3.1083-1088.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Py B, Bortoli-German I, Haiech J, Chippaux M, Barras F. Cellulase EGZ of Erwinia chrysanthemi: structural organization and importance of His98 and Glu133 residues for catalysis. Protein Eng. 1991;4:325–333. doi: 10.1093/protein/4.3.325. [DOI] [PubMed] [Google Scholar]
- 31.Ramer S W, Bieber D, Schoonik G K. BfpB, an outer membrane lipoprotein required for the biogenesis of bundle-forming pili in Enteropathogenic Escherichia coli. J Bacteriol. 1996;178:6555–6563. doi: 10.1128/jb.178.22.6555-6563.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sauvonnet N, Poquet I, Pugsley A P. Extracellular secretion of pullulanase is unaffected by minor sequence changes but is usually prevented by adding reporter proteins to its N- or C-terminal end. J Bacteriol. 1995;177:5238–5246. doi: 10.1128/jb.177.18.5238-5246.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sauvonnet N, Pugsley A P. Identification of two regions of Klebsiella oxytoca pullulanase that together are capable of promoting β-lactamase secretion by the general secretory pathway. Mol Microbiol. 1996;22:1–7. doi: 10.1111/j.1365-2958.1996.tb02650.x. [DOI] [PubMed] [Google Scholar]
- 34.Seydel, A., P. Gounon, and A. P. Pugsley. Testing the “+2 rule” for lipoprotein sorting in the Escherichia coli cell envelope with a new genetic selection. Mol. Microbiol., in press. [DOI] [PubMed]
- 35.Shevchik V E, Condemine G. Functional characterization of the Erwinia chrysanthemi OutS protein, an element of a type II secretion system. Microbiology. 1998;144:3219–3228. doi: 10.1099/00221287-144-11-3219. [DOI] [PubMed] [Google Scholar]
- 36.Shevchik V E, Robert-Badouy J, Condemine G. Specific interaction between OutD, an Erwinia chrysanthemi outer membrane protein of the general secretory pathway, and secreted proteins. EMBO J. 1997;16:3007–3016. doi: 10.1093/emboj/16.11.3007. [DOI] [PMC free article] [PubMed] [Google Scholar]