Abstract
Morris water maze (MWM) test is widely used to evaluate the learning and memory deficits in rodents. Image processing and pattern recognition can be used to analyse videos and recognize automatically the tracking in MWM. There are several commercial and free access software that allows analyzing the behavioral tasks although they also have limitations such as automation, cost, user intervention among other things. The aim of this paper was to develop a new image processing technique to automatically analyse the track of the rat in the MWM, which we called RatsTrack. The MWM test was performed with an animal model for Alzheimer, and the videos were recorded to measure the distance, time, and speed. The segmentation method based on the projection of the video frames was made for pool identification, eliminating the rat, while conserving the shape of the pool. Then, the Hough transformation was used to recognize the position and radius of the pool. Finally, the frame in which the rat is released into the pool was established automatically using mathematical morphology techniques and added as a plugin on free access ImageJ software. The new image processing technique, RatsTrack, successfully detected and located the pool and rat without user intervention, significantly decreasing operational time and providing results for distance, time, speed, and acceleration parameters of the MWM test. Alzheimer's rats compared with the control group presented significant data measured with the RatsTrack. RatsTrack is a plugin of ImageJ software and will be made freely available for public use.
Keywords: Image processing, Pattern recognition, Morris water maze, ImageJ, Tracking, Memory
1. Introduction
A large number of diseases of the brain and nervous system cause memory deficits, many of which are evaluated by behavioural tests [1]. One such approach is the Morris water maze test (MWM), which is used to asses spatial memory in rodents. A rat is placed in a circular pool where the animal learns to swim towards a platform to escape from the water based on spatial information [2]. The rodent's path to the platform is recorded with a video camera and processed by a computer software to finally analyse the parameters of the rat's trajectory [3]. This test is essential for studies that play an important role in molecular, pathological and pharmacological research in memory impairment [4].
There are different software programs for the analysis of behavioural tests in rodents, some commercial such as Ethovision (Noldus), Top Scan (Clever Sys Inc.), Smart Video Tracking Software (PanLab) and VideoTrack Rodent Behavior (ViewPoint), and others free of charge such as Use Tracker (Tracker), Anymaze (Stoelting Co.) and MouBeAT [5]. However, most require user intervention to locate and draw the pool and platform contour, mark the quadrants into which the pool is divided and even the shape of the rat. In addition, it is required to identify when the rat is released.
Also, the presence of the experimenter's hand, while releasing the rodent affects the results, as some programs confuse the hand with the animal. In addition, some programs are based on MATLAB, which increases the cost, requires significant time to obtain results and limits the number of videos that can be analyzed. Therefore, it is necessary to develop a low-cost, fast, and easy-to-use software application that can be used for the analysis of MWM tests, obtaining reproducible results and requiring minimal user intervention.
Thus, this paper introduces a new technique, RatsTrack, for the evaluation of the rat's path in the MWM test, where the location of the pool and the rat, its time of release and arrival at the platform is performed automatically, greatly reducing the time spent in processing the videos, improving the accuracy and reproducibility of the results. RatsTrack, a plugin for the ImageJ program [6], was developed and employed to obtain very accurate parameters in an animal model for Alzheimer in the MWM, demonstrating its ability to determine the test duration, as well as the trajectory and speed of the rats during the experiment.
This method is an improvement of a previously published work [7] which now yields better results. In addition, it now includes a tool that allows a large set of videos to be evaluated automatically, saving the results so the user can review them later. The results were compared with those obtained with Ethovision showing the reliability of the proposed method.
2. Materials and methods
2.1. Animals
Twenty 3-month-old male Sprague-Dawley rats, weighing approximately 250 g at baseline, under a 12/12 h light/dark cycle were used for the development and evaluation of RatsTrack under a 12/12 h light/dark cycle. The sample size was based on previous pilot studies in which rats were evaluated and the behavioural tasks were reproducible and meaningful. Food and water were provided ad libitum throughout the experiment. In addition, rats were monitored daily by animal care staff for clinical, behavioural, and health observation. The animals were randomly assigned to both the control and the experimental groups, using a non-repeating random number table from 1 to 20; the principal investigator was aware of the group assignments at the different stages of the experiment. All selected animals were included in the study and in the final analysis. Potential confounding variables were minimised by placing the cages at the same level, the order of the evaluations was always the same and they were always performed at the same time, between 09:00 and 13:00. Animal handling and experimental procedures were approved by the ethical guidelines of the Universidad Peruana Cayetano Heredia System (CIEA-102069) [8]. Rats were obtained from the bioterium of the Research and Development Laboratory, Universidad Peruana Cayetano Heredia, Lima, Peru. They were kept under temperature (25 ± 2 °C) and humidity (60%) controlled conditions. The rats' behaviour was monitored daily by animal care staff to ensure that the animals were safe and healthy [8]. Factors considered in deciding whether to euthanise before the planned end of the experiment included weight loss; severe pain that could not be controlled; tumour growth; coughing, wheezing or severe nasal discharge; nervous signs such as tremors, convulsions and paresis; incapacitation and other behavioural signs of distress, but not adverse events were observed. No animal survived this experiment, as all were euthanised to preserve their brains for future pathological experiments.
2.2. Alzheimer rat model
The Aβ-injected group consisted of ten rats. Amyloid β1–42 (A9810, Sigma-Aldrich®) was diluted in Dimethyl Sulphoxide (DMSO) at a concentration of 10 μg/μl and stored at −20C. The working solutions were diluted in phosphate buffered saline (PBS) at the final concentration of 0.5 and incubated at 37 °C for 7 days prior to injection into the rat brain to allow fibrillar formation [9]. Rats were anesthetised with an intraperitoneal injection of 10% chloral hydrate in distilled water (400 mg/kg) and placed on a stereotaxic apparatus (KOPF ® 900, David Kopf Instruments) where their heads were shaved and disinfected using chlorhexidine 0.5%. Then, a 2.5 cm longitudinal incision was made in the midline of the scalp between the eyes to the back of the ears [10]. Peptide was subsequently injected with a Hamilton® microsyringe into the hippocampus (ML, ±2.6 mm; AP, - 3.0 mm, DV, - 3.0 mm relative to the bregma) [11], three μl of Aβ1–42 were infused into each brain hemisphere at concentrations of 0.5 μg/μl, while ten rats from the control group were injected with saline solution at the same volume in the same area. The solution was injected slowly over 6 min, and the microsyringe was held at the injection site for a further 6 min to facilitate diffusion of the Aβ.
2.3. Morris water maze test (MWM)
The MWM test was performed 14 days after the injection to evaluate spatial memory in rats [3]. The maze was built with a circular black plastic pool, 126 cm in diameter and 75 cm high, filled with water at a temperature of 21–22 °C to a depth of 35 cm. A black pool was chosen because the rats were white, which increases their visibility and detection using RatsTrack, the image processing technique presented in this work. The pool was also placed against a light background to facilitate its automatic detection using RatsTrack. The pool was divided into four quadrants: Northeast (NE), Northwest (NW), Southeast (SE), Southwest (SW), which were labelled based on arbitrary compass directions along imaginary lines for the quantification of study variables. The maze contained a movable transparent plastic platform, 10 cm in diameter and 33 cm high, placed in a quadrant 10 cm away from the pool wall. Four different white geometric shapes, one per quadrant, were placed on the inside wall of the pool to guides the rats. The rats' swimming trajectories were recorded with a webcam placed above the pool so that their entire outer circular contour was within the video frame, allowing for automatic detection. A dim light was placed over the pool so as not to affect the rats' behaviour and to avoid water reflections interfering with the rat detection.
The reference memory protocol used consisted of six sessions, one session per day. The first day was the familiarisation session, from the second to the fifth day was the acquisition session, and the sixth day was the memorisation session. In the familiarisation, four trials were performed the day 1; while in the acquisition, eight trials were performed from day 2 to day 5 with the platform hidden, the objective was to evaluate the spatial learning of animals by placing rats in all 4 quadrants of the pool and evaluating their velocities to reach the platform. In the memorisation on the day 6, the rat was placed in the pool without the platform and only one trial per rat was performed, the objective of this trial is to record the reference memory of rats by monitoring the swimming trajectories of rats placed into the pool without any platform. In each trial, the rat was removed from the cage and placed in the water maze at one of four randomly determined locations with its head facing the pool wall and the interval between tests was 5 min. In all experiments, the initial position of the rat in the maze was chosen pseudo-randomly by placing the rodent in any of the quadrants, so that by the end of every fourth session the animal had already started the run in all four quadrants in any order, while the position of the platform was always the same [8].
A video of each trial was recorded. Each video was analyzed from the time the rat was released into the pool until it climbed onto the platform. If the rat did not climb within 90 s the trial was over. In the familiarisation tests, where there was no platform, the experiment was ended after 90 s. The rat was placed in the pool wearing a glove of a very different colour to the black pool bottom, to allow automatic detection of the moment when the rat was released. The results of the behavioural tests were expressed as the mean ± SEM and were assessed with Levene's test for homogeneity, Shapiro Wilk's normality test and the non-parametric Mann-Whitney test to evaluate the difference between the groups, with statistical significance p < 0.05. Stata 13.0 software was used to analyse the data and GraphPrism 8.0 software to produce graphs.
2.4. Video acquisition
For this research, a total of 117 videos were recorded, ranging from 2 to 114 s in duration, of the corresponding number of animals. The acquisition protocol used was as follows: a black pool was placed in orden to contrast with the white colour of the rat. The pool was fully located within the video frame. The lighting was dim but sufficiently intense to obtain good contrast between the pool background and the animal, while avoiding light reflections. The videos were acquired with a Logitech HD Pro C920 webcam in mp4 format with a resolution of 854 × 480 pixels and 30 frames per second, and in 3gp format with a resolution of 352 × 288 pixels and 24 frames per second. The videos were then converted to AVI format for further processing. For the development of the application a Hewlett-Packard computer model 14-r022la Intel(R) Core(TM), I5–4210U CPU at 1.70 GHz 2.40 GHz with 8 GB RAM, Intel(R) HD Graphics Family, running on Windows 10 Pro platform was used. The method was implemented in Java as a plugin for the freely available software ImageJ. The software and some videos will be available for free use after this paper publication on the authors' ResearchGate website.
2.5. Image processing
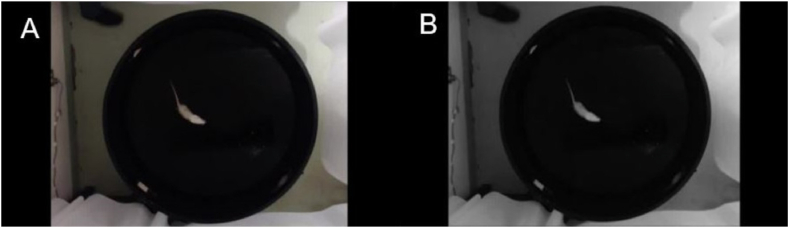
As shown in Fig. 1A, the videos are dimly light, so each video frame is characterised by low contrast. Each starts before the operator introduces the rat into the pool and releases it. As can be seen, colour does not provide relevant information. Therefore and to gain computational efficiency, the videos were converted to grey levels, as illustrated in Fig. 1b, by simply averaging the values of the colour channels in each video frame, i.e.,
| (1) |
where r, g, and b represent the red, green, and blue components of the pixel at position (x,y) and q the grey value obtained after conversion.
Fig. 1.
Video of the Morris water maze (MWM) test. (A) Original color frame. (B) Grayscale converted frame. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
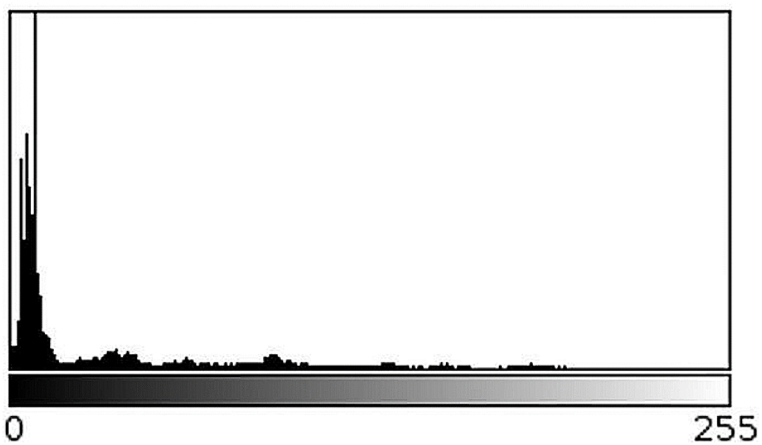
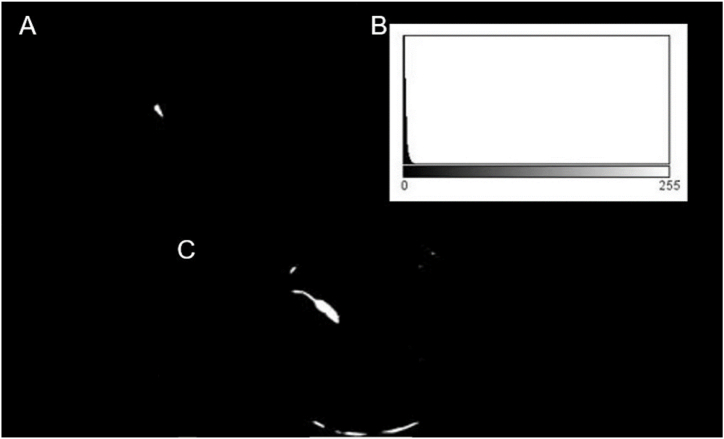
To find the pool location, each video can be interpreted as a stack of images where each frame is stacked one on top of the other, where the x, y axes correspond to the position within each frame and the z-axis to the depth, given by each of the frames in the video. From the stack, a minimum image projection is made by mapping the pixels onto the z-axis, corresponding to the minimum pixel intensity for each x,y position. As shown in Fig. 2A, the resulting image is quite dark because the minimum intensity value is obtained at each pixel, where the pool intensity is very close to zero. As can be observed, some other areas of the background are also dark, but this drawback does not affect the developed method. However, the pool can be placed on a completely white background to improve its delimitation. To separate the pool from the background, an automatic threshing technique is used by Forero and Langevin [12] to isolate the head from the background in magnetic resonance images. This method consists of using the grey level corresponding to the first local minimum of the histogram as threshold, as illustrated in Fig. 3. The resulting image, shown in Fig. 2B, is eroded and the contour of the pool, illustrated in Fig. 2C, is obtained by subtracting the eroded image from the binarised one.
Fig. 2.
Process followed to obtain the contour of the pool. (A) Projection of minimum intensity of the pixels through the stack. (B) Binary image. (C) Contour of the pool, obtained by mathematical morphology.
Fig. 3.
Typical histogram of a 3D stack projection. Histogram of the image in Fig. 2A.
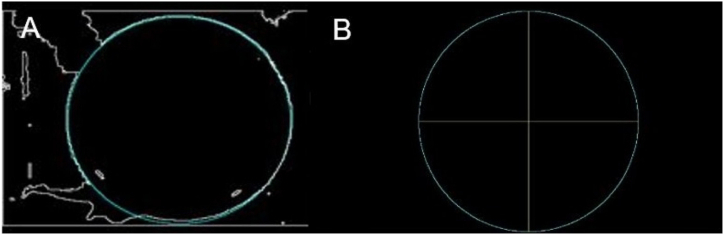
To find the circumference formed by the edge of the pool, the Hough transform is used. This is a slow process, as the possible circumferences passing through each white pixel of the image are evaluated. Since the approximate diameter of the pool is known, the search for the circumference is confined to a close range, thus making this step much more efficient. Fig. 4A illustrates the obtained circumference superimposed on the binary image in Fig. 2C. Once the circumference is found, it is divided into quadrants following the protocol established by the MWM test for the evaluation of the rat's movement, as shown in Fig. 4B.
Fig. 4.
Circumference of the pool obtained using the Hough transform. (A) Circumference superimposed on the edge of the pool. (B) Subdivision into quadrants, in yellow, for the evaluation of the Morris test. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
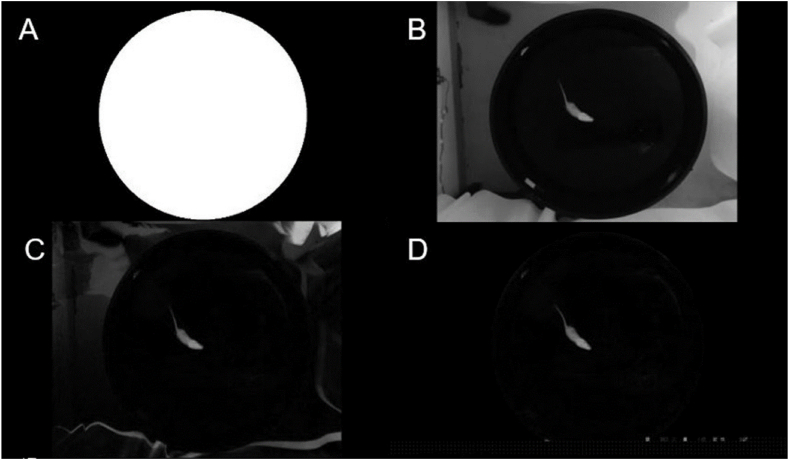
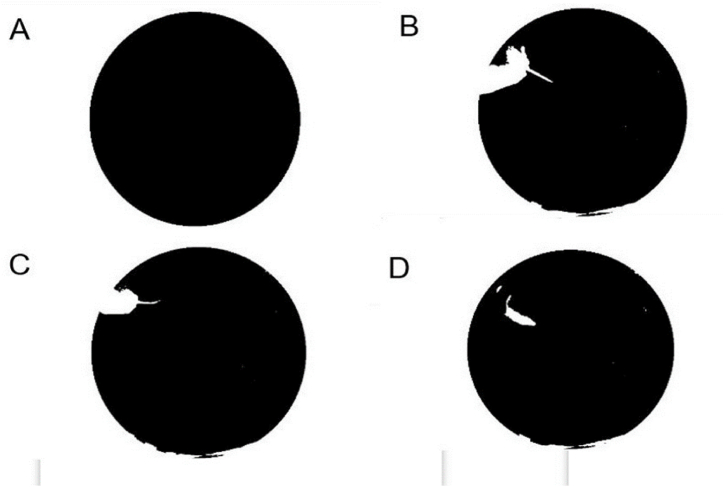
Once the pool has been located, the search for the rat is initiated, preceded by the elimination of the geometric shapes that appear in white within the pool. This is achieved by filling the circumference formed by the edge of the pool, as depicted in Fig. 5A. Subsequently, the grayscale video (Fig. 5B) is subtracted from the image of minimum intensity obtained by projection on the z-axis of the video (Fig. 2A), resulting in the image presented in Fig. 5C. As the animal is confined to the pool, the search is restricted to the interior of the pool. Thus, the filled circle in Fig. 5A is utilized as a mask to superimpose on the grey level video, thus retaining solely the area inside the pool, as illustrated in Fig. 5D. As the rat is characterised by its white colour and is located on a black background, binarisation is used to separate its silhouette from the background. Since the size of the rat is very small compared to the background popular binarisation techniques, such as Otsu's or maximum entropy, do not provide an adequate threshold and frequently yield erroneous results, as illustrated in Fig. 6A, where a brightness in the image is identified as part of the object of interest. However, as a typical histogram such as Fig. 6B shows, there is a clear separation between the two modes. A statistical study of twenty videos then showed that a threshold of twenty correctly separates the rat from the background, as shown in Fig. 6C.
Fig. 5.
Process followed to detect objects in the pool. (A) Mask used to detect the rat in the pool. (B) Grayscale frame. (C) Subtract minimum intensity from the video in grey levels. (D) Objects detected inside the mask.
Fig. 6.
Process followed to detect the rat in the pool. (A) Example of incorrect binarisation obtained by the Otsu threshold method. (B) Typical histogram of the result obtained after using a circular mask to remove the information outside the pool by means of a circular mask. (C) The silhouette of the rat obtained by the threshold value found statistically.
Once the image was binarised, besides the rat, smaller noise was found in the background. To eliminate it, the objects are labelled, and only the largest one within the circumference, corresponding to the rodent, is retained. At this stage, the frame in which the animal was released is also detected. Simply, the largest object in the whole image is found and removed. This object corresponds to the space outside the pool (Fig. 7A) and includes the operator's arm and the rat when the animal has not yet been released, as illustrated in Fig. 7B and C, since the grey level of both the operator's arm and the rat is well above the threshold chosen to binarise the image. As soon as it is released, the rat appears as a new object in the pool, which is thus detected.
Fig. 7.
Detection of the exact moment when the rat is released. (A) Mask used to detect the rat inside the pool. (B) Silhouette of the user holding the rat. (C) Releasing time. The rat can be seen appearing as a new object inside the pool. (D) Silhouette of the rat inside the pool.
The animal is tracked by finding the centre of the rat silhouette in each frame. Thus, the trajectory of the rodent is obtained by connecting the centres of the silhouettes through the video, as illustrated in Fig. 8A.
Fig. 8.
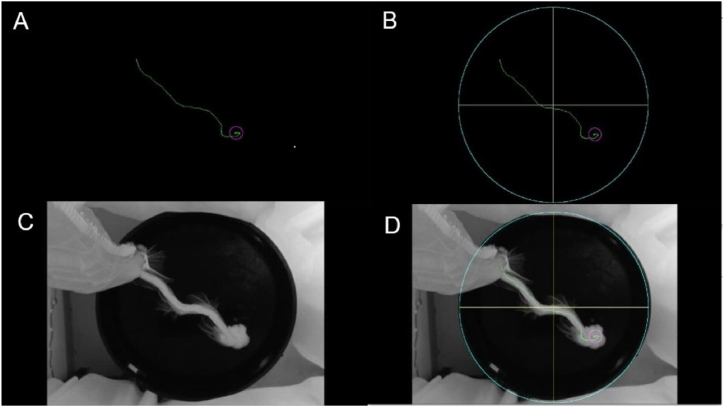
Trajectory of a rat. (A) Trajectory obtained. (B) The trajectory shown by RatsTrack. (C) Actual path. (D) Superposition of Figures B and C.
Finally, the trajectory is superimposed on the previously obtained circumference to graphically show the movement of the rat in the pool, as shown in Fig. 8A. If a platform inside the pool is included in the experiment, the arrival of the rat to the platform is detected by measuring its movement from one frame to the next. If it is close to zero, for a certain number of frames, empirically set at seven, the rat is assumed to have arrived at the platform and a purple circle is drawn centred on that position, as shown in Fig. 8B. To verify whether the trajectory obtained with the developed method corresponds to the actual one, a maximum intensity projection of the video was obtained. In this way, the actual displacement of the rat over time can be seen in the projection, as illustrated in Fig. 8C. Fig. 8D shows the overlap between the actual path and the one obtained by RatsTrack, allowing the coincidence between both to be seen. If a platform is not placed in the pool, the software will not search for it and, consequently, a purple circle will not be placed at the end of the trajectory, as illustrated in Fig. 9A. Fig. 9B displays the actual trajectory of the rat, and Fig. 9C shows the superposition of Fig. 9A and B, enabling observation of the obtained result coinciding with the real trajectory of the rat.
Fig. 9.
Trajectory of a rat in the pool without a platform. (A) Results obtained with RatsTrack. (B) Actual trajectory. (C) Superposition of Figures A and B.
The data obtained is used to calculate the information of interest, such as total distance travelled, distance travelled per quadrant, total time, time per quadrant, average speed, and average acceleration, which is delivered by RatsTrack in a table, to be further processed with statistical software. If several videos, acquired with the same protocol, are to be processed, RatsTrack allows them to be processed in a continuous way without user intervention, saving the resulting videos, the path image, and the table containing the data obtained in a folder for further analysis and review.
Although the algorithm was initially designed for the analysis of white rats, it can be customised to track black rats swimming in a white pool, provided that the established acquisition protocol is followed. Specifically, the pool must be positioned against a black background and must be entirely captured within the video frame. In such cases, the negative of the video is used as input to RatsTrack. Additionally, the plugin can be fine-tuned to analyse other rodent species, including mice, by adjusting the size parameters of the target animal.
3. Results
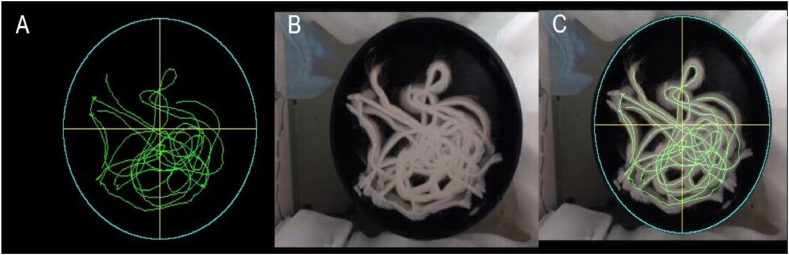
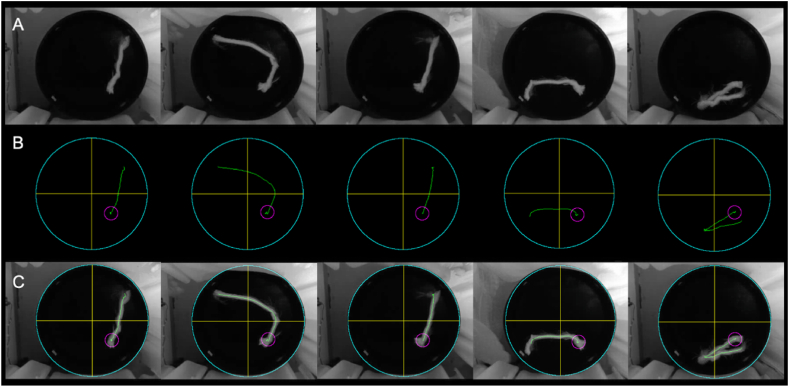
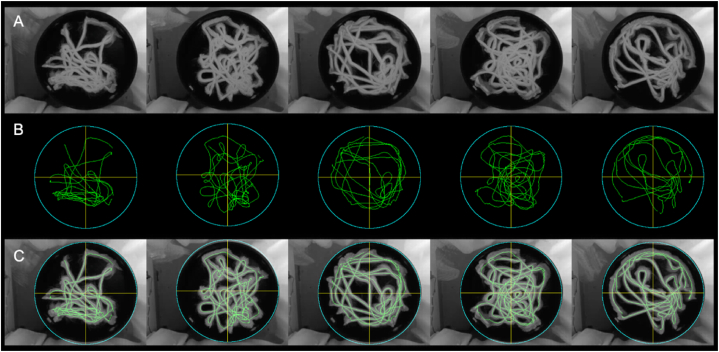
The 117 test videos underwent evaluation using RatsTrack in order to determine the rat's path. The obtained result was deemed valid for all videos. Fig. 10, Fig. 11 illustrate the outcomes generated by RatsTrack for a total of 10 videos, 5 with the platform and 5 without. The graphical representations display the maximum intensity projection of each video (Fig. 10, Fig. 11A), the rat's path as determined by our method (Fig. 10, Fig. 11B), and the superimposition of the maximum intensity projection and the trajectory generated by RatsTrack (Fig. 10, Fig. 11C). These figures clearly demonstrate the congruity between both images.
Fig. 10.
Rats trajectory using the MWM protocol with platform. (A) Actual path obtained by maximum intensity projection on the z-axis of the video. (B) Rat trajectory obtained with RatsTrack. (C) Overlapping of Images A and B.
Fig. 11.
Rats trajectory using the MWM protocol without platform. (A) Actual path obtained by maximum intensity projection on the z-axis of the video. (B) Rat trajectory obtained with RatsTrack. (C) Overlapping of Images A and B.
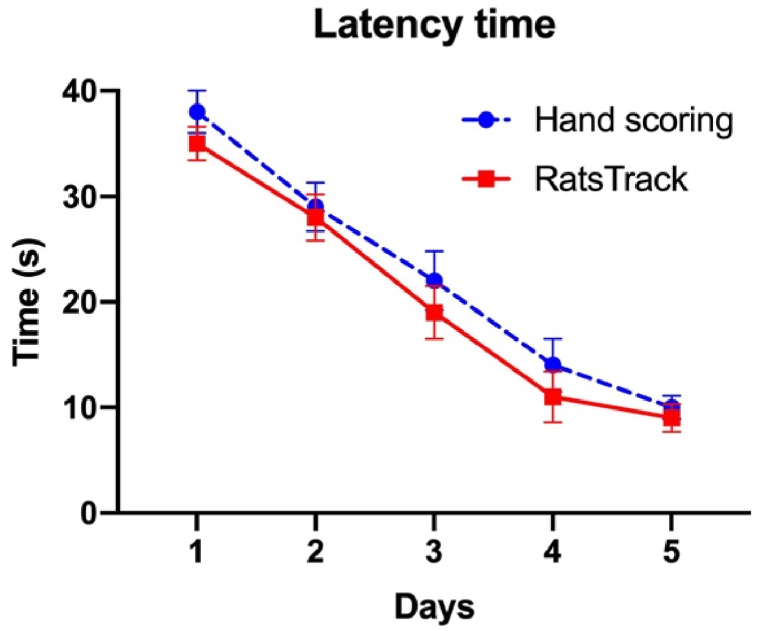
To validate the results produced with the developed software, six videos taken from MWM tests on ten rats were taken, averaged, and compared with those obtained by hand. Fig. 12 shows the average time values obtained. However, the manual scoring evaluation could not get the time per quadrant, distance, distance per quadrant, acceleration, and speed due to the complexity of the animal's trajectory and lead to confusion and bias of the results. In contrast, RatsTrack was able to obtain these variables, which were subsequently used to evaluate the animal model of Alzheimer's disease using the MWM test.
Fig. 12.
Average latency time found per session in ten rats using the technique developed and handmade for the Morris water maze (MWM) test. Latency time was recorded during the first five days of training. The results are expressed as mean ± SEM. N = 10 per group. *P < 0,05.
The results obtained by manual scoring and using RatsTrack in ten 1-min videos of a rat in the MWM test are presented in Table 1.
Table 1.
Average time consumed in determining the path taken by a rat in ten 1-min videos in the Morris water maze (MWM) test.
| Method | Mean ± SD (min) |
|---|---|
| Hand scoring | 105 ± 6.5 |
| RatsTrack | 3 ± 1.7 |
Unlike other software, such as Ethovision and MouBeat, RatsTrack allows to automatically obtain the path of the rat in each test, without user intervention. It is a user-friendly and easy-to-use tool, developed for ImageJ software, so it can be used on any platform. In addition, another plugin, LotsRatsTrack, has been developed, which allows the application to be run with a large number of videos acquired using the same protocol, placed in a folder, so that the program processes one after another without user intervention and saves the results in another folder, allowing subsequent analysis of the results obtained.
The quantitative variables returned by RatsTrack are distance, time, time per quadrant, distance per quadrant, acceleration and velocity. No issues have been experienced with the program as long as the established protocol for video acquisition is followed. However, failure to adhere to the protocol, such as in cases where there is poor lighting, low contrast between the video background, or the pool is not entirely located within the video frame, may cause the technique to fail.
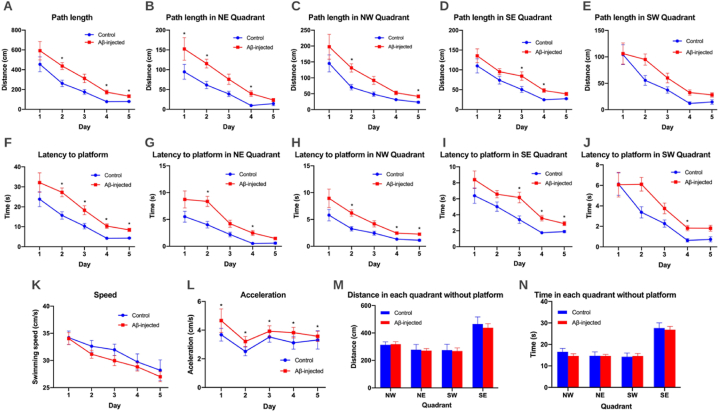
Once the technique was implemented and validated, RatsTrack was used in a case study to evaluate its usefulness. Thus, it was employed to evaluate the Morris water maze (MWM) test in an animal model using rats with Alzheimer's disease and control rats, finding significant differences in the spatial memory of both groups, as can be seen in Fig. 13. Observing the results obtained during the five days of training, differences were found in each variable: distance travelled, distances covered in each quadrant, total time, time spent in each quadrant, speed and acceleration. The Morris Water Maze results between the control and AB-injected groups showed a large difference when comparing path length, the Αβ-injected group showed a greater path length than the control group (Day 2, p = 0.02, Day 4, p = 0.01, Day 5 p = 0.04) and this was evident in each quadrant of the pool (Fig. 13A–E). A significant difference was also observed when comparing latency to the platform, with latency being greater in the Αβ-injected group than the control group (Day 2, p = 0.01, Day 3, p = 0.01, Day 4, p = 0.00, Day 5 p = 0.00) and this was also evident in each of the quadrants of the pool (Fig. 13F–J). On the sixth day of evaluation (Fig. 13 M, N), memory was evaluated without using a platform in the pool, finding that the control group spent more time and had a longer run in the SE quadrant, where the platform was located on the previous days, compared to the Aβ injected group. These results show a decrease in memory scores in the Aβ-injected group compared to the control one, indicating that the Aβ-injected group showed memory impairment in line with other animal models of Alzheimer's disease described in the literature.
Fig. 13.
RatsTrack in the spatial memory evaluation of rats using the Morris water maze test. The graphs show the results obtained in control and Αβ-injected rats, represented as mean values (±SEM). Graphs (A–L) show the results obtained during the first five days of training with the platform in the SE quadrant. (A) Total distance travelled. (B) Distance travelled in the NE quadrant. (C) Distance travelled in the NW quadrant. (D) Distance travelled in the SE quadrant. (E) Distance travelled in the SW quadrant. (F) Total travel time. (G) Time spent in NE quadrant. (H) Time spent in NW quadrant. (I) Time spent in SE quadrant. (J) Time spent in SW quadrant. (K) Speed. (L) Acceleration. Graphs (M − N) show the results obtained on the sixth evaluation day without the platform. (M) Total distance travelled in each quadrant. (N) Total time spent in each quadrant. N = 10 Control, N = 10 Αβ-injected (0.5 μg/μl). *p < 0.05.
4. Conclusions
In this work, a new automatic technique for detecting and tracking of a rat's path in a swimming pool for the evaluation of the MWM test was introduced. The results show that the technique works in all cases, reducing analysis time and user intervention. The method is highly reliable and accurate, allowing a whole group of videos to be processed without user intervention, automatically detecting the position of the pool, the instant at which the rodent is released, its displacement and its arrival on the platform, allowing the cognitive and motor level of the rodent to be assessed.
The developed algorithm uses the Hough transform to locate the pool and the centre of the rat's silhouette to estimate its position in each image. The path followed by the rodent is then processed to obtain the data required for the analysis of its behaviour, such as distance travelled, time duration, acceleration, and velocity. This data is used to compare the behaviour of the rats and to evaluate drugs in development for the treatment of different diseases. Based on the method described here, the RatsTrack plugin was implemented, whose results were compared with those obtained manually and with other available program, showing the superiority of RatsTrack in terms of efficiency and execution time. In addition, the projection of the maximum intensity through the video was used to visualise the rat's path, which, when superimposed with the one obtained by RatsTrack, allowed to confirm the close approximation between the path obtained and the actual one. By automatically detecting the rodent, its time of release, its arrival on the platform and the pool position, the plugin developed allowed faster and more reproducible results to be obtained than those achieved with other applications.
It is recommended to establish an acquisition protocol for the videos, always using the same characteristics of pool size and location, video files, and if possible, acquiring them with the same camera to give greater validity to the comparative results of the rats' behaviour. Since the program developed so far is the only fully automatic for the analysis of the MWM test, it also allows the analysis of multiple videos continuously without user intervention, storing the results for later review and use, requiring only that the videos have been acquired with the same protocol. Although this work was carried out with white rats on a black background pool, it can be used with other types of rodents, adjusting the protocol according to the type of video and size of the animal.
The results obtained were validated, allowing verification of the quality of the proposed method for comparing rats with Alzheimer's disease and a control group. Other automatic applications for the analysis of rodent behavioural tests can be performed in the same way.
Author contribution statement
Manuel G. Forero: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Natalia C. Hernández and Cristian M. Morera: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Luis A. Aguilar: Contributed reagents, materials, analysis tools or data; Wrote the paper.
Ruth Aquino: Performed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Laura E. Baquedano: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Data availability statement
Data will be made available on request.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Luis Aguilar reports article publishing charges was provided by University of Lima.
References
- 1.Dumont M. Behavioral phenotyping of mouse models of neurodegeneration. Methods Mol. Biol. 2011;793:229–237. doi: 10.1007/978-1-61779-328-8_15. [DOI] [PubMed] [Google Scholar]
- 2.Vorhees C.V., Williams M.T. Assessing spatial learning and memory in rodents. ILAR J. 2014;55:310–332. doi: 10.1093/ilar/ilu013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wenk G.L. 2004. Assessment of Spatial Memory Using the Radial Arm Maze and Morris Water Maze. Current Protocols in Neuroscience. (Chapter 8), Unit 8 5A. [DOI] [PubMed] [Google Scholar]
- 4.Moreno-Treviño M.G., Castillo-López J., Meester I. Moving away from amyloid Beta to move on in Alzheimer research. Front. Aging Neurosci. 2015;7:2. doi: 10.3389/fnagi.2015.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bello-Arroyo E., Roque H., Marcos A., Orihuel J., Higuera-Matas A., Desco M., Caiolfa V.R., Ambrosio E., Lara-Pezzi E., Gómez-Gaviro M.V. MouBeAT: a new and open toolbox for guided analysis of behavioral tests in mice. Front. Behav. Neurosci. 2018;12:201. doi: 10.3389/fnbeh.2018.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rasband, Rasband, W.S., ImageJ, U. S. National institutes of health, Bethesda, Maryland, USA ImageJ, U. S. National Institutes of Health, Bethesda, Maryland, USA. https://imagej.nih.gov/ij/, 1997-2018.
- 7.Forero M.G., Hernández N.C., Morera C.M., Baquedano L.E., Aguilar L.A. Proc. SPIE 11137, Applications of Digital Image Processing XLII. 2019. New automatic method for tracking rats in a pool for medication studies; p. 111372B. [DOI] [Google Scholar]
- 8.Aquino R., de Concini V., Dhenain M., Lam S., Gosset D., Baquedano L., Forero M.G., Menuet A., Baril P., Pichon C. Intrahippocampal inoculation of aβ1–42 peptide in rat as a model of alzheimer's disease identified MicroRNA-146a-5p as blood marker with anti-inflammatory function in astrocyte cells. Cells. 2023;12(5):694. doi: 10.3390/cells12050694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borbély E., Horváth J., Furdan S., Bozsó Z., Penke B., Fülöp L. Simultaneous changes of spatial memory and spine density after intrahippocampal administration of fibrillar aβ1-42 to the rat brain. BioMed Res. Int. 2014;2014 doi: 10.1155/2014/345305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu Y., Xu J., Xu J., Cheng J., Jiao D., Zhou C., Dai Y., Chen Q. Lower serum levels of miR-29c-3p and miR-19b-3p as biomarkers for Alzheimer's disease. Tohoku J. Exp. Med. 2017;242:129–136. doi: 10.1620/tjem.242.129. [DOI] [PubMed] [Google Scholar]
- 11.Paxinos G., Watson C. Academic Press; 1998. The Rat Brain in Stereotaxic Coordinates. [DOI] [PubMed] [Google Scholar]
- 12.Forero M.G., Langevin F. Proc. 17th Conf. IEEE-EMBS'95. Montreal. Canada. 1995. Electrical brain mapping on the real surface of the scalp obtained by IRM. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.