Abstract
Background
No data currently exist comparing the contemporary iterations of balloon‐expandable (BE) Edwards SAPIEN 3/Ultra and the self‐expanding (SE) Medtronic Evolut PRO/R34 valves. The aim of the study was the comparison of these transcatheter heart valves with emphasis on patients with small aortic annulus.
Methods and Results
In this retrospective registry, periprocedural outcomes and midterm all‐cause mortality were analyzed. A total of 1673 patients (917 SE versus 756 BE) were followed up for a median of 15 months. A total of 194 patients died (11.6%) during follow‐up. SE and BE groups showed similar survival at 1 (92.6% versus 90.6%) and 3 (80.3% versus 85.2%) years (P log‐rank=0.136).
Compared with the BE group, patients treated with the SE device had lower peak (16.3±8 mm Hg SE versus 21.9±8 mm Hg BE) and mean (8.8±5 mm Hg SE versus 11.5±5 mm Hg BE) gradients at discharge. Conversely, the BE group demonstrated lower rates of at least moderate paravalvular regurgitation postoperatively (5.6% versus 0.7% for SE and BE valves, respectively; P<0.001). In patients treated with small transcatheter heart valves (≤26 mm for SE and ≤23 mm for BE; N=284 for SE and N=260 for BE), survival was higher among patients treated with SE valves at both 1 (96.7% SE versus 92.1% BE) and 3 (91.8% SE versus 82.2% BE) years (P log‐rank=0.042). In propensity‐matched patients treated with small transcatheter heart valve, there remained a trend for higher survival among the SE group at both 1 (97% SE versus 92.3% BE) and 3 years (91.8% SE versus 78.7% BE), P log‐rank=0.096).
Conclusions
Real‐world comparison of the latest‐generation SE and BE devices demonstrated similar survival up to 3 years’ follow‐up. In patients with small transcatheter heart valves, there may be a trend for improved survival among those treated with SE valves.
Keywords: balloon expandable, paravalvular regurgitation, self‐expanding, small transcatheter heart valve, transcatheter aortic valve implantation
Subject Categories: Aortic Valve Replacement/Transcather Aortic Valve Implantation
Nonstandard Abbreviations and Acronyms
- BE
balloon expandable
- PPM
permanent pacemaker
- PVR
paravalvular regurgitation
- SE
self‐expanding
- TAVI
transcatheter aortic valve implantation
- THV
transcatheter heart valve
Clinical Perspective.
What Is New?
Contemporary generation balloon‐expandable and self‐expanding transcatheter aortic valves appear to have similar survival up to 3 years.
Balloon‐expandable valves have less paravalvular leak, and self‐expanding valves have lower transvalvular gradients, while pacemaker rates are similar.
What Are the Clinical Implications?
In the propensity‐matched cohort, there was a trend for reduced survival, albeit not statistically significant, among patients treated with small transcatheter valves in the balloon‐expandable group.
Transcatheter aortic valve implantation (TAVI) is an established treatment modality with similar efficacy to surgical treatment for patients with severe, symptomatic aortic stenosis. 1 , 2 Encouraging data from several randomized trials have further expanded the TAVI indication to intermediate and even low‐risk patients with aortic stenosis. 3 , 4 , 5
Since the introduction of TAVI, despite the variety of available technologies, 2 main platform types are used worldwide: the balloon‐expendable (BE) Edwards SAPIEN family of valves (Edwards Lifesciences Inc, Irvine, CA) and the self‐expanding (SE) Medtronic family of valves (Medtronic Inc, Minneapolis, MN). Since the first‐generation iterations of each platform (Edwards SAPIEN and Medtronic CoreValve, respectively), both platforms have evolved dramatically, resulting in improved clinical outcomes. The Edwards Sapien 3/Ultra 6 and the Evolut Pro/Pro+ are the latest iterations of each platform design available currently in Europe. 7 Both feature an external tissue skirt to minimize postprocedural paravalvular regurgitation (PVR), whereas in terms of vascular access, smaller sheath sizes (internal diameters; 14F for Evolut PRO+, 16F for Evolut PRO, 14F for 23‐ and 26‐mm Edwards S3/Ultra, and 16F for 29‐mm Edwards S3) have allowed for reduction in complications rates.
Despite being the most commonly used worldwide, head‐to‐head randomized or even confounder‐adjusted registry data comparing periprocedural and midterm outcomes in patients treated with these 2 devices are distinctly lacking. The only randomized evidence to date includes a head‐to‐head comparison of the previous generation Evolut‐R and the SAPIEN 3 device, which suggested equivalence with regard to all‐cause mortality, PVR, need for permanent pacemaker (PPM), and stroke. 8
There has been accumulating evidence that prosthesis‐patient mismatch after TAVI leads to increased hospitalization and mortality when severe. 9 This appears to hold true especially for patients with small annuli. 10 SE valves with supra‐annular design appear to have an advantage 11 in reducing transvalvular gradients in such patients. Yet, to date, no direct head‐to‐head comparisons of the new‐generation SE and BE devices have been performed in patients with small annuli, with the exception of a small registry demonstrating significantly higher gradients in BE compared with SE in day 1 and 30 after TAVI. 12
In the absence of adequately powered large randomized controlled trials, the aim of this collaborative retrospective analysis was to perform a real‐world comparison of the periprocedural and midterm outcomes between the 2 latest‐generation devices of SE valves (Evolut‐PRO/PRO+ and Evolut R 34 mm) versus the BE SAPIEN 3 and Ultra valves in all‐comers with prespecified analysis in patients treated with small transcatheter heart valves (THVs).
METHODS
Patient Characteristics: Inclusion/Exclusion Criteria: Procedure
Consecutive patients who underwent TAVI with the latest SE (Medtronic Evolut PRO, PRO+, and Evolut R 34 mm) and BE (Edwards SAPIEN 3 and Ultra) valves in 2 high‐volume centers with extensive TAVI experience (programs dating back to 2007): Athens, Greece: 3rd Department of Cardiology, University of Athens; and London, UK: Royal Brompton and Harefield Hospitals, Guy's and St Thomas' NHS Foundation Trust, from August 2017 to February 2021, were included in the ATLAS (Athens‐London‐Aortic‐Stenosis) registry and were retrospectively studied. All data required were collected by local investigators in each center, anonymized and entered into a dedicated combined TAVI database. The data that support the findings of this study are available from the corresponding author upon reasonable request. Variables collected included baseline clinical, imaging (echocardiographic, multislice computed tomography, and angiographic), and procedural characteristics, as well in‐hospital outcomes and midterm survival.
Patients treated with either the BE Edwards SAPIEN 3/Ultra valve or the SE Evolut PRO/PRO+ (23, 26, and 29 mm) and Evolut R 34 mm were included in the analysis. The SE valve patients with large anatomies (annulus perimeter >81.7 mm) were treated with the larger 34‐mm device, which was during the study period only available in the Evolut R platform (Evolut R 34 mm).
As per standard protocol, all patients with severe (valve area, <1 cm2; or aortic valve area index, <0.6 cm2/m2) symptomatic aortic valve stenosis underwent preprocedurally routine screening investigations, including transthoracic echocardiography, lung function tests, coronary angiography, and multislice computed tomography angiography. The final decision with regard to the appropriateness for TAVI, device selection, and access route was determined by the “Heart Team” (comprising cardiologists, cardiac surgeons, and anesthesiologists). Patients treated with an Edwards SAPIEN 3 or Ultra valve ≤23 mm or an Evolut PRO/PRO+ ≤26 mm were included in the “small THV” cohort. 12
Clinical End Points
The primary end point was 1‐year all‐cause mortality. Kaplan‐Meier curves were used to estimate midterm all‐cause mortality in the entire cohort and in the subpopulation of patients with small THVs. Secondary study end points were defined as per Valve Academic Research Consortium‐2 criteria, including postprocedural PVR, need for new PPM implantation, cerebrovascular accidents, and periprocedural complications, such as need for bail out valve‐in‐valve implantation, balloon postdilatation (after balloon aortic valvuloplasty), valve malpositioning (migration or embolization), and emergency conversion to full sternotomy 13 (in hospital). PVR was evaluated using discharge echocardiography and classified accordingly as none, mild, moderate, and severe. In‐hospital bleeding complications were defined using the Bleeding Academic Research Consortium classification. 14
Following consultation with our local research ethics committee, no informed consent was required as the study was part of an ongoing audit, and all data were collected retrospectively and were pseudoanonymized. Vital status was ascertained using the national Patient Demographic Service, which incorporates national death registry information as well as local notifications.
Statistical Analysis
All continuous variables were tested for normality using the Kolmogorov‐Smirnov test. Data are presented as percentages, mean±SD, or median (interquartile range). Differences in proportions were tested with the χ2 test and Fisher exact test, and differences in continuous variables were tested with a Student t test or Wilcoxon rank‐sum test for parametric and nonparametric variables, respectively. Survival was assessed using Kaplan‐Meier curves with their respective 95% CIs. Cox regression analyses were performed to adjust for confounding factors between the 2 groups. Confounding factors included in the model were those identified in univariate analysis to be significant. P<0.05 was considered statistically significant.
Propensity Matching Cohorts
The propensity scores were estimated using a nonparsimonious multivariable logistic regression model with small THV type (SE versus BE) as the dependent variable and the following variables as covariates (significantly different at baseline univariate analysis with P<0.05): age, mitral regurgitation (MR), extensive calcification of the aorta, previous balloon aortic valvuloplasty, and access site for THV delivery. Matching was performed with the use of a 1:1 matching protocol without replacement (nearest neighbor‐matching algorithm), with a caliper width equal to 0.1 of the SD of the logit of the propensity score. Standardized differences were estimated for all the baseline covariates before and after matching to assess prematch imbalance and postmatch balance and were graphically presented (histogram with overlaid kernel density estimates of standardized differences; Figures S1 and S2). Furthermore, an overall imbalance χ 2 test 9 and multivariate overall imbalance measure L1 10 were performed. In the propensity‐matched cohort, survival was assessed with the use of the Kaplan‐Meier method and compared with the use of the log‐rank test.
Statistical analysis was performed using SPSS 27 for Windows (IBM Corp, Armonk, NY).
RESULTS
Baseline Characteristics
In total, 1673 eligible patients were analyzed. Of those patients, 917 were treated with SE devices, and 756 were treated with BE devices. Baseline clinical and demographic characteristics of the 2 populations are presented in Table 1.
Table 1.
Baseline Demographic and Clinical Characteristics in the Total Cohort
| Variable | Total population (N=1673) | Evolut‐Pro/Evolut R 34 mm (N=917) | S3‐Ultra (N=756) | P value |
|---|---|---|---|---|
| General demographics | ||||
| Female sex, n (%) | 706 (42.2) | 385 (42) | 321 (42.5) | 0.827 |
| Age, y | 81.2±7.3 | 81.5±7.1 [914] | 80.9±7.5 [752] | 0.152 |
| BMI, kg/m2 | 26.8 (24–30.5) | 26.8 (24–30.5) [857] | 26.8 (24–30.5) [701] | 0.889 |
| Cardiovascular risk factors and medical history | ||||
| Diabetes, n (%) | 428 (26.4) | 228 (25.6) | 200 (27.4) | 0.382 |
| Smoking, n (%) | ||||
| Ex smoker | 727 (46.1) | 404 (46.2) | 323 (46) | 0.446 |
| Current smoker | 55 (3.5) | 35 (4) | 20 (2.8) | |
| On dialysis, n (%) | 26 (1.6) | 11 (1.3) | 15 (2) | 0.224 |
| Creatinine, mmol/L | 88 (71–112) | 87 (71–112) [857] | 88 (71–112) [714] | 0.654 |
| Previous cardiac surgery, n (%) | ||||
| Isolated CABG | 203 (12.6) | 114 (12.9) | 89 (12.2) | 0.871 |
| Valvular surgery | 64 (4) | 35 (4) | 29 (4) | |
| CABG and valve | 5 (0.3) | 2 (0.2) | 3 (0.4) | |
| Previous BAV, n (%) | 128 (7.9) | 76 (8.6) | 52 (7.1) | 0.268 |
| Previous PCI, n (%) | 422 (26.1) | 236 (26.6) | 186 (25.5) | 0.598 |
| Previous MI, n (%) | 194 (12.0) | 96 (10.8) | 98 (13.4) | 0.111 |
| PAD, n (%) | 168 (10.3) | 99 (11) | 69 (9.5) | 0.316 |
| COPD or asthma, n (%) | 333 (20.5) | 179 (20.1) | 154 (21.2) | 0.441 |
| Previous CVA, n (%) | 95 (5.8) | 48 (5.5) | 47 (6.5) | 0.135 |
| Preexisting PPM, n (%) | 114 (7.6) | 87 (9.9) | 27 (4.3) | <0.001 |
| Echocardiographic data | ||||
| LV function, n (%) | ||||
| Good (EF>50%) | 1203 (75.1) | 638 (73.5) | 565 (77.1) | |
| Moderate (EF=30%–49%) | 266 (16.6) | 168 (19.4) | 98 (13.4) | 0.002 |
| Poor (EF<30%) | 132 (8.2) | 62 (7.1) | 70 (9.5) | |
| Mean AV gradient, mm Hg | 44.1±17.8 | 44.5±18.5 [818] | 43.5±16.8 [622] | 0.113 |
| Peak AV gradient, mm Hg | 71.6±31.7 | 72.2±33.5 [822] | 70.8±29.2 [652] | 0.955 |
| Mitral regurgitation, n (%) | ||||
| None | 327 (22.7) | 160 (18.7) | 167 (28.5) | <0.001 |
| Mild | 823 (57.2) | 506 (59.3) | 317 (54.2) | |
| Moderate | 254 (17.7) | 168 (19.7) | 86 (14.7) | |
| Severe | 35 (2.4) | 20 (2.3) | 15 (2.6) | |
| Pulmonary artery pressure, mm Hg | 35 (27–45) | 34 (24–45) [571] | 37 (30–45) [269] | <0.001 |
| Bicuspid aortic valve, n (%) | 41 (2.4) | 29 (2.6) | 12 (1.6) | 0.534 |
| Coronary angiography | ||||
| Extent of epicardial CAD, n (%) | ||||
| Single vessel | 227 (14.2) | 109 (12.4) | 118 (16.5) | |
| Two vessel | 118 (7.4) | 43 (4.9) | 75 (10.5) | <0.001 |
| Three vessel | 58 (3.6) | 22 (2.5) | 36 (5) | |
| Significant LMS disease, n (%) | 21 (1.3) | 7 (0.8) | 14 (1.9) | 0.048 |
| Computed tomography data | ||||
| Annulus perimeter, mm | 78 (73–84) | 78.0 (73–84) [775] | 78.6 (73–84) [370] | 0.387 |
| Extensive calcification of the aorta, n (%)* | 33 (2.4) | 24 (3.3) | 9 (1.4) | 0.018 |
| ECG | ||||
| Bundle‐branch block, % | ||||
| None | 85.7 | 85.6 | 85.7 | 0.385 |
| LBBB | 5.2 | 4.6 | 5.2 | |
| RBBB | 3.6 | 3.7 | 3.6 | |
| Preoperative heart rhythm, n (%) | ||||
| Sinus rhythm | 1072 (65.7) | 587 (65.6) | 485 (65.8) | |
| AF/flutter | 470 (28.8) | 249 (27.8) | 221 (30) | 0.256 |
| Paced | 86 (5.3) | 56 (6.3) | 30 (4.1) | |
Data are given as mean±SD or median (interquartile range) unless otherwise indicated. Data in brackets are number of patients with available data. AF indicates atrial fibrillation; AV, aortic valve; BAV, balloon aortic valvuloplasty; BMI, body mass index; CABG, coronary artery bypass grafting; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; CVA, cerebrovascular accident; EF, ejection fraction; LBBB, left bundle‐branch block; LMS, left main stem; LV, left ventricular; MI, myocardial infarction; PAD, peripheral artery disease; PCI, percutaneous coronary intervention; PPM, permanent pacemaker; and RBBB, right bundle‐branch block.
Includes calcifications of LV outflow tract and aortic annulus.
In most variables, baseline demographics were similar between the 2 groups. Patients treated with an SE valve had higher rates of extensive aortic calcification, moderate left ventricular (LV) systolic dysfunction, and moderate MR at baseline. On the other hand, patients treated with a BE valve had more prevalent coronary artery disease (Table 1).
Procedural Characteristics
Most cases were performed via the transfemoral route (97% SE versus 95.2% BE), whereas transaxillary/subclavian (1.7% SE versus 0.7% BE) and transcarotid access (0.7% SE versus 0% BE) were used predominantly in the SE arm, and transapical (0% SE versus 3.7%BE) in the BE arm (P<0.001). With regard to valve size used, in the SE group, 5% were 23‐mm valves, 26.5% were 26‐mm valves, 41.3% were 29‐mm valves, and 27.2% were 34‐mm valves. In the BE group, 1.6% were 20‐mm valves, 33% were 23‐mm valves, 40.6% were 26‐mm valves, and 24.7% were 29‐mm valves. Aortic valve balloon predilatation was performed in 13.9% of SE cases and 13.2% of BE cases (P=0.675). Balloon postdilatation was performed more frequently in patients with SE versus BE valves (22.5% versus 7%; P<0.001).
When comparing patients with small THVs, there were 284 treated with an SE valve and 260 treated with a BE valve. In the SE group, 45 (15.8%) were treated with a 23‐mm valve, whereas the majority, 239 (84.2%), were treated with a 26‐mm valve. In the BE group, 248 (95.4%) were treated with a 23‐mm valve, whereas 12 (4.6%) were treated with a 20‐mm valve. Most of the cases, in both groups, were performed via the transfemoral route (97.8% SE versus 94.6% BE; P=0.045). Baseline demographics comparing SE and BE in this subgroup are shown in Table 2. Previous balloon aortic valvuloplasty was performed more often among BE patients. SE patients had higher rates of preprocedural moderate MR, higher calcium score, and higher rates of aortic calcification (Table 2). Aortic valve balloon predilatation was performed in 9.4% of SE cases and 10.4% of BE cases (P=0.676). Balloon postdilatation was performed more frequently in patients with SE valves (23.5% SE versus 7.3% BE; P<0.001).
Table 2.
Baseline Demographic and Clinical Characteristics in the Small Valve Cohort
| Variable | Total population (N=544) | Evolut‐Pro/Evolut R 34 mm (N=284) | S3‐Ultra (N=260) | P value |
|---|---|---|---|---|
| General demographics | ||||
| Female sex, n (%) | 440 (80.9) | 235 (82.7) | 205 (78.8) | 0.248 |
| Age, y | 81.8±7.3 | 82.4±6.4 [283] | 81±8.1 [258] | 0.029 |
| BMI, kg/m2 | 26.5 (23.4–30.2) | 26.3 (23–30.1) [268] | 26.6 (24.1– 30.7) [241] | 0.242 |
| Cardiovascular risk factors and medical history | ||||
| Diabetes, n (%) | 139 (26.2) | 75 (26.7) | 64 (25.6) | 0.529 |
| Smoking, n (%) | ||||
| Ex smoker | 186 (35.8) | 103 (37.1) | 83 (34.3) | 0.425 |
| Current smoker | 15 (2.9) | 10 (3.6) | 5 (2.1) | |
| On dialysis, n (%) | 8 (1.5) | 2 (0.7) | 6 (2.4) | 0.124 |
| Creatinine, mmol/L | 80 (65–103) | 81 (65–104) [268] | 79.5 (64.3–100) [244] | 0.735 |
| Previous cardiac surgery, n (%) | ||||
| Isolated CABG | 37 (7) | 17 (6.2) | 20 (8) | 0.882 |
| Valvular surgery | 27 (5.1) | 14 (5.1) | 13 (5.2) | |
| CABG and valve | 4 (0.8) | 2 (0.7) | 2 (0.8) | |
| Previous BAV, n (%) | 26 (4.9) | 8 (2.9) | 18 (7.2) | 0.024 |
| Previous PCI, n (%) | 121 (23.1) | 65 (23.6) | 56 (22.6) | 0.775 |
| Previous MI, n (%) | 53 (10) | 27 (9.7) | 26 (10.3) | 0.817 |
| PAD, n (%) | 39 (7.3) | 22 (7.8) | 17 (6.8) | 0.658 |
| COPD or asthma, n (%) | 111 (21) | 60 (21.5) | 51 (20.4) | 0.580 |
| Previous CVA, n (%) | 22 (4.1) | 9 (3.2) | 11 (5.2) | 0.464 |
| Echocardiographic data | ||||
| LV function, n (%) | ||||
| Good (EF>50%) | 444 (85.2) | 222 (81.9) | 222 (88.8) | 0.070 |
| Moderate (EF=30%–49%) | 61 (11.7) | 40 (14.8) | 21 (8.4) | |
| Poor (EF<30%) | 16 (3.1) | 9 (3.3) | 7 (2.8) | |
| Mean AV gradient, mm Hg | 46.9±19.3 | 46.9±19.5 [254] | 47±19.1 [218] | 0.929 |
| Peak AV gradient, mm Hg | 74.6±29.2 | 73.8±25.7 [253] | 75.5±32.6 [251] | 0.526 |
| Mitral regurgitation, n (%) | ||||
| None | 105 (21.8) | 44 (16.1) | 61 (29.3) | 0.002 |
| Mild | 282 (58.6) | 171 (62.6) | 111 (53.4) | |
| Moderate | 85 (17.7) | 55 (20.1) | 30 (14.4) | |
| Severe | 9 (1.9) | 3 (1.1) | 6 (2.9) | |
| Pulmonary artery pressure, mm Hg | 36 (29–45.8) | 36 (28–45) | 37 (30–47.5) | 0.129 |
| Bicuspid aortic valve, n (%) | 9 (1.7) | 5 (1.8) | 4 (1.7) | 0.805 |
| Coronary angiography | ||||
| Extent of epicardial CAD, n (%) | ||||
| Single vessel | 64 (12.5) | 31 (11.4) | 33 (13.7) | 0.097 |
| Two vessel | 27 (5.3) | 9 (3.3) | 18 (7.5) | |
| Three vessel | 14 (2.7) | 6 (2.2) | 8 (3.3) | |
| Significant LMS disease, n (%) | 4 (0.8) | 3 (1.1) | 1 (0.4) | 0.359 |
| Computed tomography data | ||||
| Annulus perimeter, mm | 72 (68.7–75) | 72 (68.9–75) [148] | 71 (68–75) [50] | 0.427 |
| Extensive calcification of the aorta, n (%)* | 6 (1.3) | 6 (2.4) | 0 | 0.031 |
| ECG | ||||
| Bundle‐branch block, n (%) | ||||
| None | 480 (90.6) | 244 (87.8) | 236 (93.7) | 0.112 |
| LBBB | 21 (4) | 13 (4.7) | 8 (3.2) | |
| RBBB | 13 (2.5) | 9 (3.2) | 4 (1.6) | |
| Preoperative heart rhythm, n (%) | 0.231 | |||
| Sinus rhythm | 401 (75.4) | 207 (74.2) | 194 (76.7) | |
| AF/flutter | 114 (21.4) | 59 (21.1) | 55 (21.7) | |
| Paced | 16 (3) | 12 (4.3) | 4 (1.6) | |
Data are given as mean±SD or median (interquartile range) unless otherwise indicated. Data in brackets are number of patients with available data. AF indicates atrial fibrillation; AV, aortic valve; BAV, balloon aortic valvuloplasty; BMI, body mass index; CABG, coronary artery bypass grafting; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; CVA, cerebrovascular accident; EF, ejection fraction; LBBB, left bundle‐branch block; LMS, left main stem; LVEF, left ventricular; MI, myocardial infarction; PAD, peripheral artery disease; PCI, percutaneous coronary intervention; and RBBB, right bundle‐branch block.
Includes calcifications of LV outflow tract and aortic annulus.
After propensity matching for the aforementioned variables, a total of 139 patients with SE small THVs were matched against 139 patients who had been treated with BE small THVs. The overall balance test was not statistically significant (χ 2=1.759; P=0.624). The relative multivariate imbalance (L1) was reduced after matching from 0.354 to 0.216. Standardized differences of <10% for any given covariate indicated a relatively small imbalance in the matched cohort (Figures S1 and S2). Baseline characteristics are shown in Table 3.
Table 3.
Baseline Demographic and Clinical Characteristics in the Propensity‐Matched Small THV Cohort
| Variable | Total population (N=278) | Evolut‐Pro/Evolut R 34 mm (N=139) | S3‐Ultra (N=139) | P value |
|---|---|---|---|---|
| General demographics | ||||
| Female sex, n (%) | 224 (80.6) | 114 (82) | 110 (79.1) | 0.650 |
| Age, y | 83 (78–87) | 83 (78–88) | 83 (77–87) | 0.423 |
| BMI, kg/m2 | 26.7 (23.8–30.9) | 26.6 (23–30.2) | 26.8 (24.4–32) | 0.114 |
| Cardiovascular risk factors and medical history | ||||
| Diabetes, n (%) | 78 (28.2) | 42 (30.4) | 36 (25.9) | 0.4 |
| Smoking, n (%) | ||||
| Ex smoker | 101 (37.3) | 57 (41.6) | 44 (32.8) | 0.209 |
| Current smoker | 4 (1.5) | 3 (2.2) | 1 (0.7) | |
| On dialysis, n (%) | 6 (2.2) | 1 (0.7) | 5 (3.6) | 0.214 |
| Creatinine, mmol/L | 79 (65–103) | 78 (65–103) | 80 (66–105) | 0.498 |
| Previous cardiac surgery, n (%) | ||||
| Isolated CABG | 20 (7.2) | 10 (7.2) | 10 (7.2) | 0.935 |
| Valvular surgery | 10 (3.6) | 6 (4.3) | 4 (2.9) | |
| CABG and valve | 2 (0.7) | 1 (0.7) | 1 (0.7) | |
| Previous BAV, n (%) | 0 | 0 | 0 | |
| Previous PCI, n (%) | 66 (23.7) | 31 (22.3) | 35 (25.2) | 0.573 |
| Previous MI, n (%) | 29 (10.5) | 16 (11.6) | 13 (9.4) | 0.542 |
| PAD, n (%) | 19 (6.8) | 12 (8.6) | 7 (5) | 0.235 |
| COPD or asthma, n (%) | 60 (21.7) | 31 (22.3) | 29 (21) | 0.098 |
| Previous CVA, n (%) | 12 (4.3) | 4 (2.8) | 8 (5.7) | 0.627 |
| Echocardiographic data | ||||
| LV function, n (%) | ||||
| Good (EF>50%) | 250 (89.9) | 122 (87.8) | 128 (92.1) | 0.430 |
| Moderate (EF=30%–49%) | 24 (8.6) | 15 (10.8) | 9 (6.5) | |
| Poor (EF<30%) | 4 (1.4) | 2 (1.4) | 2 (1.4) | |
| Mean AV gradient, mm Hg | 47.8±18.4 | 48.6±19.3 | 46.9±17.5 | 0.460 |
| Peak AV gradient, mm Hg | 76.4±28.4 | 75.8±30 | 76.9±26.9 | 0.770 |
| Mitral regurgitation, n (%) | 0.902 | |||
| None | 61 (21.9) | 29 (20.9) | 32 (23) | |
| Mild | 184 (66.2) | 94 (67.6) | 90 (64.7) | |
| Moderate | 33 (11.9) | 16 (11.5) | 17 (12.2) | |
| Severe | 0 | 0 | 0 | |
| Bicuspid aortic valve, n (%) | 1 (1.3) | 0 | 1 (2.3) | 1.0 |
| Coronary angiography | ||||
| Extent of epicardial CAD, n (%) | ||||
| Single vessel | 38 (14.2) | 17 (12.4) | 21 (16) | 0.688 |
| Two vessel | 9 (3.4) | 5 (3.6) | 4 (3.1) | |
| Three vessel | 8 (3.0) | 3 (2.2) | 5 (3.8) | |
| Significant LMS disease, n (%) | 2 (1.5) | 2 (1.5) | 0 | 0.498 |
| Computed tomography data | ||||
| Annulus perimeter, mm | 71 (68.2–74.9) | 71.1 (67.5–74.5) | 71 (67.3–74.8) | 0.769 |
| Extensive calcification of the aorta, %* | 0 | 0 | 0 | N/A |
| ECG | ||||
| Bundle‐branch block, n (%) | ||||
| None | 216 (93.1) | 114 (90.5) | 102 (96.2) | 0.190 |
| LBBB | 8 (3.4) | 6 (4.8) | 2 (1.9) | |
| RBBB | 4 (1.7) | 2 (1.6) | 2 (1.9) | |
| Preoperative heart rhythm, n (%) | ||||
| Sinus rhythm | 206 (74.1) | 105 (75.5) | 101 (72.7) | 0.034 |
| AF/flutter | 62 (22.3) | 25 (18) | 37 (26.6) | |
| Paced | 10 (3.6) | 9 (6.5) | 1 (0.7) | |
Data are given as median (interquartile range) unless otherwise indicated. AF indicates atrial fibrillation; AV, aortic valve; BAV, balloon aortic valvuloplasty; BMI, body mass Index; CABG, coronary artery bypass grafting; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; CVA, cerebrovascular accident; EF, ejection fraction; LBBB, left bundle‐branch block; LMS, left main stem; LVEF, left ventricular; MI, myocardial infarction; PAD, peripheral artery disease; PCI, percutaneous coronary intervention; RBBB, right bundle‐branch block; and THV, transcatheter heart valve.
Includes calcifications of LV outflow tract and aortic annulus.
Outcomes/Midterm Survival
Total Cohort
During the median follow‐up of 15 months (interquartile range, 7.3–29.9 months), 194 patients died (11.6%). The 1‐ and 3‐year Kaplan‐Meier estimated survival was similar between the 2 groups (92.6% versus 90.6% for SE and BE valves, respectively, for 1‐year survival; and 85.2% versus 80.3% for SE and BE valves, respectively, for 3‐year survival; P log‐rank=0.136; Figure 1). The crude all‐cause mortality hazard ratio (HR)(BE versus SE) was 1.25 (95% CI, 0.93–1.68) (P=0.137).
Figure 1. Midterm survival in all‐comer patients with aortic stenosis treated with contemporary self‐expanding or balloon‐expandable valves. Cum indicates cumulative.
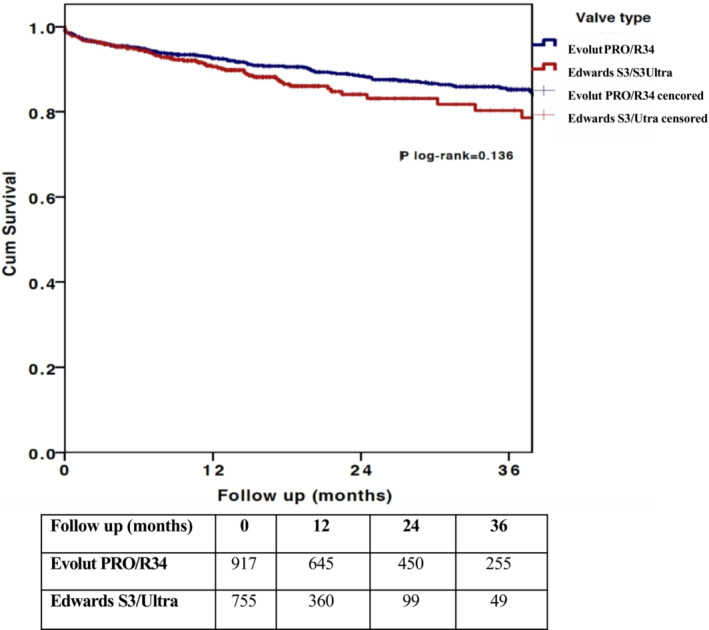
When adjusting for age, sex, baseline LV function, baseline degree of MR, epicardial coronary artery disease, and extensive calcification of the aorta, the HR remained similar in patients treated with BE versus SE valves (HR, 1.23 [95% CI, 0.8–1.9]; P=0.349).
Small THV Cohort
In patients treated with small THVs, survival was higher among the SE group at both 1 (96.7% SE versus 92.1% BE) and 3 (91.8% SE versus 82.2% BE) years (P log‐rank=0.042) (Figure 2A).
Figure 2. Midterm survival in patients with small THVs.
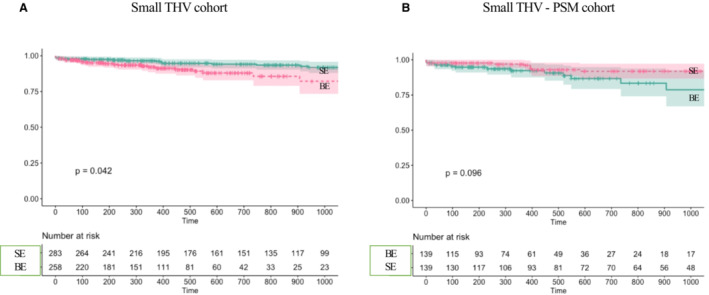
A, Increased midterm survival in the SE valve group among patients with aortic stenosis treated with small THVs. B, Midterm survival in PSM patients treated with small THVs. BE indicates balloon expandable; PSM, propensity score–matched; SE, self‐expanding; and THV, transcatheter heart valve.
Crude HR(BE versus SE) for all cause mortality was 1.88 (95% CI, 1.02–3.48; P=0.045; Table S1). When adjusting for baseline confounders (age, sex, presence of MR, calcification of aorta, and previous balloon valvuloplasty), the HR(BE versus SE) remained significant at 2.27 (95% CI, 1.1–4.68; P=0.027). When further adjusting for access route, HR(BE versus SE) was 2.2 (95% CI, 1.06–4.58; P=0.034).
When selecting transfemoral cases only in the small valve cohort, there were 273 patients treated with SE valves and 244 patients treated with BE valves. At 1 year, SE survival was 96.5%, and at 3 years, it was 91.7%, compared with 92% and 87.4%, respectively, for BE valves (P log‐rank=0.165). Although statistical significance was lost, the trend for reduced mortality remains as shown in Figure S3, despite the significantly older age of patients treated with SE valves (82.3+6.3 versus 81+8.2 years; P=0.041).
In propensity‐matched patients treated with small THVs, there remained a trend for higher survival among the SE group at both 1 (97% SE versus 92.3% BE) and 3 (91.8% SE versus 78.7% BE) years (P log‐rank=0.096) (Figure 2B).
Hemodynamic Performance
Total Cohort
With regard to hemodynamic device performance at discharge, those treated with an SE device demonstrated significantly lower peak (16.3±8.0 mm Hg for SE valves versus 21.9±8.0 mm Hg for the BE valves; P<0.001) and mean (8.8±5.0 mm Hg for SE versus 11.5±5.0 mm Hg for BE; P<0.001) gradients at discharge (Table 4).
Table 4.
Short‐Term Clinical Outcomes as Defined by Valve Academic Research Consortium‐2 Criteria and Predischarge Echocardiographic Parameters
| Variable | Total cohort | Small THV cohort | PSM small THV cohort | ||||||
| Evolut PRO/PRO+/Evolut R 34 mm (N=917) | S3/Ultra (N=756) | P value | Evolut PRO/PRO (N=284) | S3/Ultra (N=260) | P value | Evolut PRO/PRO+ (N=139) | S3/Ultra (N=139) | P value | |
| Predischarge echocardiographic parameters* | |||||||||
| Peak AV gradient, mm Hg | 16.3±8.0 [772] | 21.9±8.0 [581] | <0.001 | 18.0±10.0 [240] | 26.0±10.4 [203] | <0.001 | 18±8.3 | 25.2±8.8 | <0.001 |
| Peak AV gradient ≥20 mm Hg | 207 (26.8) | 343 (59) | <0.001 | 82 (34.2) | 151 (74.4) | <0.001 | 42 (36.8) | 77 (73.3) | <0.001 |
| Mean AV gradient, mm Hg | 8.8±5.0 [756] | 11.5±5 [513] | <0.001 | 9.7±5.5 [236] | 14.0±5.9 [181] | <0.001 | 9.7±4.6 | 13.5±5.3 | <0.001 |
| Residual PVR (moderate/severe), n (%) | 49 (5.6) | 5 (0.7) | <0.001 | 11 (4) | 3 (1.2) | <0.001 | 6 (4.4) | 3 (2.2) | <0.001 |
| Procedural complications, n (%) | |||||||||
| Valve malposition | 0.429 | ||||||||
| Valve migration | 10 (1.2) | 2 (0.3) | 0.381 | 2 (0.7) | 0 | 0.4 | 0 | 0 | |
| Valve embolization | 1 (0.1) | 1 (0.2) | 1 (0.4) | 0 | 0 | 0 | |||
| Ectopic valve deployment | 3 (0.4) | 2 (0.3) | 0 | 1 (0.5) | 0 | 1 (1) | |||
| Bail out valve in valve | 9 (1) | 5 (0.7) | 0.482 | 2 (0.7) | 3 (1.2) | 0.673 | 0 | 2 (1.5) | 0.242 |
| Tamponade | 13 (1.5) | 13 (1.8) | 0.520 | 8 (2.9) | 3 (1.2) | 0.166 | 4 (2.9) | 2 (1.5) | 0.684 |
| Conversion to full sternotomy | 6 (0.7) | 3 (0.5) | 0.619 | 4 (1.5) | 2 (1.0) | 0.710 | 2 (2) | 2 (1.5) | 1.000 |
| Periprocedural complications (during in‐hospital stay) | |||||||||
| New PPM implantation† | 114 (14.4) | 81 (13.4) | 0.58 | 29 (11.3) | 19 (9.4) | 0.503 | 10 (7.9) | 7 (6.6) | 0.698 |
| Periprocedural MI | 4 (0.4) | 4 (0.5) | 0.784 | 2 (0.7) | 1 (0.4) | 1.000 | 2 (1.4) | 1 (0.7) | 1.0 |
| Bailout PCI | 6 (0.7) | 8 (1.1) | 0.367 | 2 (0.7) | 2 (0.8) | 1.000 | 2 (1.5) | 2 (2) | 0.771 |
| CVA | 28 (3.2) | 18 (2.5) | 0.405 | 15 (5.6) | 6 (2.4) | 0.068 | 8 (6.1) | 4 (3.0) | 0.255 |
| Postprocedural renal replacement | 12 (1.4) | 6 (1.0) | 0.528 | 4 (1.5) | 2 (1.1) | 1.000 | 1 (0.7) | 2 (2.1) | 0.574 |
| Acute kidney injury (stage 3) | 27 (3.3) | 8 (2) | 0.422 | 10 (3.8) | 3 (2.3) | 0.438 | 3 (2.2) | 2 (2.4) | 0.683 |
| Periprocedural bleeding complications (life threatening+major) | 26 (3) | 9 (1.8) | 0.065 | 12 (4.4) | 3 (1.7) | 0.344 | 5 (3.7) | 3 (2.7) | 0.775 |
| Vascular major complications | 34 (3.9) | 18 (2.5) | 0.012 | 17 (6.2) | 8 (3.2) | 0.26 | 5 (3.6) | 3 (2.2) | 0.871 |
| Death at discharge | 15 (1.7) | 15 (2.1) | 0.553 | 4 (1.5) | 4 (1.6) | 1.000 | 2 (1.5) | 2 (1.5) | 1.000 |
Data are given as mean±SD unless otherwise indicated. Data in brackets are number of patients with available data. AV indicates aortic valve; CVA, cerebrovascular accident; MI, myocardial infarction; PCI, percutaneous coronary intervention; PPM, permanent pacemaker; PSM, propensity score matched; PVR, paravalvular regurgitation; and THV, transcatheter heart valve.
N=878 Evolut group and N=726 Edwards SAPIEN group in all patients, and N=234 Evolut PRO and N=190 Edwards SAPIEN group in patients with small THVs.
Excluding patients with previous PPM.
The BE group demonstrated significantly lower rates of at least moderate residual aortic regurgitation (moderate or severe PVR) postoperatively (5.6% versus 0.7% for SE and BE valves, respectively; P<0.001; Table 4). Specifically, moderate or severe PVR was seen in 4.9% of cases treated with Evolut PRO/PRO+ valves versus 7.4% for those cases treated with Evolut R 34 mm versus 0.7% in cases treated with Edwards SAPIEN 3/Ultra valves (P<0.001; Figure 3A).
Figure 3. Hemodynamic performance of SE vs BE valves.
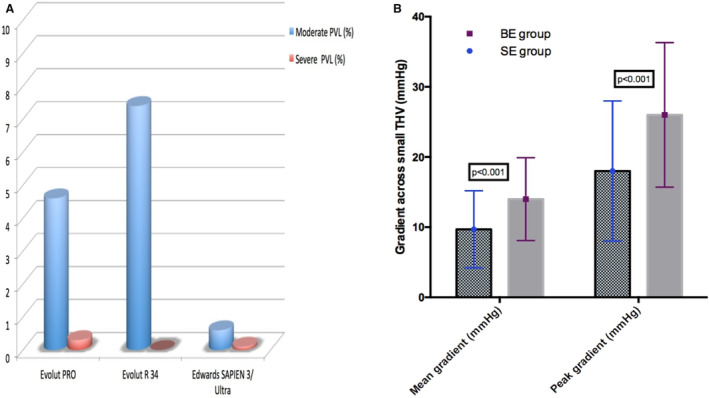
A, Higher rates of at least moderate PVL in patients with aortic stenosis treated with SE Evolut PRO or Evolut R 34‐mm valves compared with the BE Edwards SAPIEN 3 or Ultra. B, Significantly lower transcatheter gradients at discharge echocardiography among patients with aortic stenosis with small transcatheter heart valves treated with contemporary SE valves. BE indicates balloon expandable; PVL, paravalvular leak; and SE, self‐expanding.
Small THV Cohort
On echocardiography at discharge, in patients with small THVs, the SE group had significantly lower aortic valve peak (18±10 mm Hg for SE versus 26±10.4 mm Hg for BE; P<0.001) and mean gradients (9.7±5.5 for SE versus 14±5.9 mm Hg for BE; P<0.001; Table 4; Figure 3B).
The BE group demonstrated significantly lower rates of at least moderate residual aortic regurgitation (moderate or severe PVR) postoperatively (4% versus 1.2% for SE and BE valves, respectively; P<0.001; Table 4). Similar findings were found in the propensity‐matched small THV cohort (Table 4).
Periprocedural Clinical End Points
The rate of new PPM required after the device implantation in initially pacemaker‐free patients was similar for the S3/Ultra cohort compared with the SE valve group (14.4% for SE versus 13.4% for BE platform; P=0.580; Table 4). In patients with small THVs, these rates were lower (11.3% SE versus 9.4% BE; P=0.503) but still with no significant difference between the 2 groups. In the propensity‐matched small THV group, the rates remained similar (Table 4).
In the total cohort, no statistical difference was recorded between valve groups for cerebrovascular accidents (3.2% versus 2.5% for SE and BE, respectively; P=0.405) and in‐hospital mortality (1.7% versus 2.1% for SE and BE, respectively; P=0.553). No significant differences in other periprocedural complications were seen between the 2 groups in the total or the small THV cohorts (Table 4).
DISCUSSION
The current study is one of the first studies comparing, in a head‐to‐head manner, the latest generation devices of the 2 mainly used BE and SE platforms (Sapien3/Ultra and Evolut PRO/PRO+, respectively). In this multicenter study, we demonstrated that although in all‐comers midterm survival was similar between the 2 devices, in patients with smaller annuli there may be a survival advantage in those treated with SE valves. Furthermore, neither in the total nor in the small THV cohorts were there significant differences in new pacemaker, stroke, or periprocedural complications. The last generation BE platform was superior with regard to residual PVR, whereas the SE representative demonstrated lower mean and peak gradients.
Both devices represent advanced evolutions of each platform following years of research and development, incorporated in materials and design. Advanced sealing at the lower segment of both devices (alongside elongation of the sealing skirt for the Ultra device) aimed to reduce PVR rates, whereas the last generation smaller profile delivery systems ensure deliverability via the transfemoral route in the majority of the cases. In addition to technological advancements, the cumulative operators' experience and newer implantation techniques (such as cusp overlap for SE valves) have led to a reduction of major complications and new PPM implantation rates. 15
The CHOICE (Comparison of Transcatheter Heart Valves in High Risk Patients with Severe Aortic Stenosis) trial was the first trial to randomize high‐risk patients with aortic stenosis undergoing transfemoral TAVI to a BE versus an SE valve. However, the valves included were early iterations (Corevalve versus SapienXT). 16 At 5 years' follow‐up, there was no difference in the cumulative incidence of death, cardiovascular death, stroke, and hospitalization, whereas there was a trend for higher PVR in patients treated with SE valves. However, the SE valves exhibited lower Doppler gradients across the SE valves, 17 the significance of which remains to be determined in future randomized trials. The SOLVE‐TAVI (Comparison of Second‐Generation Self‐Expandable vs. Balloon‐Expandable Valves and General vs. Local Anaesthesia in Transcatheter Aortic Valve Implantation) was the second randomized trial, including 447 patients, comparing the SAPIEN 3 and Evolut‐R platforms. At 30 days’ follow‐up, Evolut‐R met the criteria for equivalence for the primary efficacy composite end point of all‐cause mortality, stroke, moderate/severe PVR, and permanent pacemaker implantation. 8
In the CENTER Collaboration study, 12 381 patients from 10 registries or trials comparing BE versus SE TAVI valves were pooled and analyzed using propensity matching. 18 Mortality at 30 days was not statistically different, whereas subanalysis of the study including the Evolut‐R device for the SE group showed comparable mortality but lower rates of strokes and new PPM implantation for the BE representative. 18 The PORTICO‐IDE (Portico Re‐Sheathable Transcatheter Aortic Valve System US Investigational Device Exemption) trial 19 was a head‐to‐head comparison of the first‐generation Portico intra‐annular SE valve versus supra‐annular SE Medtronic and BE Edwards SAPIEN valves (all iterations). This failed to demonstrate noninferiority of the Portico system compared with the other 2 valves for their primary safety end point at 30 days.
The results from the overall cohort in the current study are in line with all previously reported randomized data with regard to midterm survival (ie, no significant difference). However, we have shown that in patients with smaller anatomies, there may be a signal for survival benefit in patients treated with SE valves. This could partially be a result of increased structural valve degeneration attributable to the higher gradients observed in the BE group, 12 or attributable to the higher rates of severe prosthesis‐patient mismatch. 9 , 20 The importance of the hemodynamic differences between SE and BE prostheses on clinical outcomes and valve durability in patients with small aortic valve annuli will be assessed in great detail in the ongoing randomized SMART (Small Annuli Randomized to Evolut or SAPIEN Trial; NCT04722250). 21
Residual PVR has been shown to have a negative impact on outcomes when being more than moderate, and the BE platform was found to demonstrate lower rates of at least moderate PVR at discharge in registries, 22 , 23 but not in randomized studies. 8 One needs to take into account the presence of selection bias, given that for patients with extensive LV outflow tract calcification, extreme calcium scores, or smaller iliofemoral access, a preference is given to SE platforms with lower delivery profiles. As expected, patients with bulky leaflet and LV outflow tract calcium are also the ones more prone to PVR. 24 On the other hand, in line with previous reports, the present study confirmed a better hemodynamic performance for the latest SE valves, which is mainly attributed to its supra‐annular design. 17 , 25 Residual PVR after TAVI has emerged as an outcome of increasing importance given its link with future mortality, thus making all manufacturers aiming for low PVR rates. Contrary to 5 years’ follow‐up of CHOICE trial and SOLVE‐TAVI, which showed no difference for at least moderate PVR, the FRANCE‐TAVI nationwide registry demonstrated higher rates for previous generation SE device (15.5% versus 8.3% for SE and BE, respectively). 26 Our registry's results concur with the French data, likely reflecting the aforementioned selection bias. PVR rates of at least moderate regurgitation are significantly lower for the last‐generation SE devices compared with previous designs (5.2% versus 15.5% for our study and FRANCE‐TAVI, respectively). 25 This is largely attributable to the increased radial force and the addition of a pericardial tissue skirt at the lower part of the Evolut PRO platform, aiming for enhanced sealing. In our data, there were significantly more vascular complications and a trend toward more bleeding with the SE valve. However, this should be taken into the context of selection bias, as for patients with smaller, heavily calcified iliofemoral accesses, a preference toward SE would have been exhibited by the operators.
Interestingly, the need for new PPM implantation after TAVI in preoperatively pacemaker‐free patients was found to be similar between groups (14.4% versus 13.4% for SE and BE, respectively). Of interest, PPM rates are significantly lower for both devices compared with previous studies (23% versus 19.2% for SE and BE devices, respectively, in the SOLVE‐TAVI trial) using older valve iterations. In a recent meta‐analysis, Van Rosendael et al 27 reported rates of new PPM required after TAVI in a range of 14.7% to 26.7% for SE Medtronic Evolut‐R and 4% to 24% for BE Edwards SAPIEN 3,27 illustrating the variability of PPM rates in different registries reflecting patient confounding factors and potentially differential procedural practices and PPM implantation thresholds. The Evolut‐PRO platform has led to reduction in new PPM rates compared with its “R” predecessor, leading to statistically similar rates with the BE platform. 25 This could be attributed to the accumulating operator experience (implanting valves at optimal depth and use of the novel cusp overlap technique 15 ), the refining of pacing indications after TAVI, and possibly the reduced pressure per mm2 of tissue applied by the porcine pericardial wrap compared with the bare metallic frame of the R device. 25
With regard to cerebrovascular accidents after TAVI, the randomized SOLVE‐TAVI trial reported higher rates for the BE platform during short‐term follow‐up (4.7% versus 0.5% for BE and SE, respectively). 8 Contrary to that, 5 years’ follow‐up of the CHOICE trial showed similar stroke rates, which is in line with our short‐term results (3.4% versus 2.7% for SE and BE, respectively). 17
Study Limitations
This is a nonrandomized, retrospective study, which as such renders itself subject to selection, confounding, and time bias. Cox‐regression analysis and propensity‐matching methods aim to reduce the bias between the groups; however, unadjusted confounders cannot be tackled even with such methods. Furthermore, in our center, the BE technology was introduced at a later time, leading to shorter follow‐up times for BE valve treated patients. However, to date, it is one of the first real‐world, multicenter studies comparing outcomes between the latest‐generation devices of the 2 main representatives of SE and BE platforms. It is not always easy to randomize all‐comer patients for TAVI into different type of devices because of individual factors where a specific valve type might be favored (eg, LV outflow tract calcification).
The absence of a core laboratory may render our results susceptible to bias; however, all patients included in the analysis were scanned at the same echocardiographic department in each center, and parameters were reported in a standardized manner. Large anatomies in the SE group have been treated with the previous generation Evolut‐R 34‐mm valve, as the PRO 34 mm was not available for this group of patients until recently. 28 Such generational changes should always be accounted for when applying results of trials to current status of devices. The absence of detailed data on prosthesis‐patient mismatch and structural valve degeneration in the current cohort is another limitation that should be taken into account. However, surrogate markers, such as mean and peak gradient, indicate higher velocities across BE valves, which could potentially render themselves more prone to structural valve degeneration. Last but not least, the lack of readmission outcome data (particularly for heart failure admissions) should be acknowledged.
CONCLUSIONS
Real‐world comparison of the last‐generation BE and SE devices demonstrates similar midterm survival and major periprocedural complications rates. The BE devices exhibited lower rates of residual at least moderate PVR, at the expense, however, of significantly higher transvalvular gradients. Furthermore, in patients treated with small THVs, SE devices appear to demonstrate a trend for improved survival compared with their BE counterparts. Given, however, the retrospective nature of these findings, they should be interpreted with caution until randomized evidence comes to light.
Sources of Funding
None.
Disclosures
Dr Panoulas and Dr Vavuranakis are proctors for Medtronic. Dr Panoulas has received consultancy fees from Medtronic. The remaining authors have no disclosures to report.
Supporting information
Table S1
Figure S1–S3
Acknowledgments
We acknowledge the catheterization laboratory teams of the involved institutions who work tirelessly to deliver an excellent service to our patients with aortic stenosis.
This article was sent to John S. Ikonomidis, MD, PhD, Guest Editor, for editorial decision and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.122.028038
For Sources of Funding and Disclosures, see page 13.
References
- 1. Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J, Capodanno D, Conradi L, De Bonis M, De Paulis R, et al. 2021 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. 2022;43:561–632. doi: 10.1093/eurheartj/ehab395 [DOI] [PubMed] [Google Scholar]
- 2. Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, et al. Transcatheter aortic‐valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597–1607. doi: 10.1056/NEJMoa1008232 [DOI] [PubMed] [Google Scholar]
- 3. Leon MB, Smith CR, Mack MJ, Makkar RR, Svensson LG, Kodali SK, Thourani VH, Tuzcu EM, Miller DC, Herrmann HC, et al. Transcatheter or surgical aortic‐valve replacement in intermediate‐risk patients. N Engl J Med. 2016;374:1609–1620. doi: 10.1056/NEJMoa1514616 [DOI] [PubMed] [Google Scholar]
- 4. Vipparthy SC, Ravi V, Avula S, Kambhatla S, Mahmood M, Kabour A, Ali SS, Barzallo M, Mungee S. Meta‐analysis of transcatheter aortic valve implantation versus surgical aortic valve replacement in patients with low surgical risk. Am J Cardiol. 2020;125:459–468. doi: 10.1016/j.amjcard.2019.10.036 [DOI] [PubMed] [Google Scholar]
- 5. Thourani VH, Kodali S, Makkar RR, Herrmann HC, Williams M, Babaliaros V, Smalling R, Lim S, Malaisrie SC, Kapadia S, et al. Transcatheter aortic valve replacement versus surgical valve replacement in intermediate‐risk patients: a propensity score analysis. Lancet. 2016;387:2218–2225. doi: 10.1016/S0140-6736(16)30073-3 [DOI] [PubMed] [Google Scholar]
- 6. Binder RK, Rodes‐Cabau J, Wood DA, Webb JG. Edwards sapien 3 valve. EuroIntervention. 2012;8(suppl Q):Q83–Q87. doi: 10.4244/EIJV8SQA15 [DOI] [PubMed] [Google Scholar]
- 7. Hellhammer K, Piayda K, Afzal S, Kleinebrecht L, Makosch M, Hennig I, Quast C, Jung C, Polzin A, Westenfeld R, et al. The latest evolution of the medtronic corevalve system in the era of transcatheter aortic valve replacement: matched comparison of the evolut pro and Evolut R. JACC Cardiovasc Interv. 2018;11:2314–2322. doi: 10.1016/j.jcin.2018.07.023 [DOI] [PubMed] [Google Scholar]
- 8. Thiele H, Kurz T, Feistritzer HJ, Stachel G, Hartung P, Eitel I, Marquetand C, Nef H, Doerr O, Lauten A, et al. Comparison of newer generation self‐expandable vs. balloon‐expandable valves in transcatheter aortic valve implantation: the randomized solve‐TAVI trial. Eur Heart J. 2020;41:1890–1899. doi: 10.1093/eurheartj/ehaa036 [DOI] [PubMed] [Google Scholar]
- 9. Herrmann HC, Daneshvar SA, Fonarow GC, Stebbins A, Vemulapalli S, Desai ND, Malenka DJ, Thourani VH, Rymer J, Kosinski AS. Prosthesis‐patient mismatch in patients undergoing transcatheter aortic valve replacement: from the STS/ACC TVT registry. J Am Coll Cardiol. 2018;72:2701–2711. doi: 10.1016/j.jacc.2018.09.001 [DOI] [PubMed] [Google Scholar]
- 10. Leone PP, Regazzoli D, Pagnesi M, Sanz‐Sanchez J, Chiarito M, Cannata F, Van Mieghem NM, Barbanti M, Tamburino C, Teles R, et al. Predictors and clinical impact of prosthesis‐patient mismatch after self‐expandable TAVR in small annuli. JACC Cardiovasc Interv. 2021;14:1218–1228. doi: 10.1016/j.jcin.2021.03.060 [DOI] [PubMed] [Google Scholar]
- 11. Regazzoli D, Chiarito M, Cannata F, Pagnesi M, Miura M, Ziviello F, Picci A, Reifart J, De Marco F, Bedogni F, et al. Transcatheter self‐expandable valve implantation for aortic stenosis in small aortic annuli: the tavi‐small registry. JACC Cardiovasc Interv. 2020;13:196–206. doi: 10.1016/j.jcin.2019.08.041 [DOI] [PubMed] [Google Scholar]
- 12. Naidu S, Chen T, Fiorilli P, Li RH, Desai N, Szeto WY, Giri J, Kobayashi T, Atluri P, Herrmann HC. Measuring tavr prosthesis gradient immediately post‐procedure may underestimate its significance. JACC Cardiovasc Interv. 2022;15:120–121. doi: 10.1016/j.jcin.2021.09.012 [DOI] [PubMed] [Google Scholar]
- 13. Kappetein AP, Head SJ, Genereux P, Piazza N, van Mieghem NM, Blackstone EH, Brott TG, Cohen DJ, Cutlip DE, van Es GA, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the valve academic research consortium‐2 consensus document. J Am Coll Cardiol. 2012;60:1438–1454. doi: 10.1016/j.jacc.2012.09.001 [DOI] [PubMed] [Google Scholar]
- 14. Stortecky S, Stefanini GG, Pilgrim T, Heg D, Praz F, Luterbacher F, Piccolo R, Khattab AA, Raber L, Langhammer B, et al. Validation of the valve academic research consortium bleeding definition in patients with severe aortic stenosis undergoing transcatheter aortic valve implantation. J Am Heart Assoc. 2015;4:e002135. doi: 10.1161/JAHA.115.002135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tang GHL, Zaid S, Michev I, Ahmad H, Kaple R, Undemir C, Cohen M, Lansman SL. "Cusp‐overlap" view simplifies fluoroscopy‐guided implantation of self‐expanding valve in transcatheter aortic valve replacement. JACC Cardiovasc Interv. 2018;11:1663–1665. doi: 10.1016/j.jcin.2018.03.018 [DOI] [PubMed] [Google Scholar]
- 16. Abdel‐Wahab M, Mehilli J, Frerker C, Neumann FJ, Kurz T, Tolg R, Zachow D, Guerra E, Massberg S, Schafer U, et al. Comparison of balloon‐expandable vs self‐expandable valves in patients undergoing transcatheter aortic valve replacement: the choice randomized clinical trial. JAMA. 2014;311:1503–1514. doi: 10.1001/jama.2014.3316 [DOI] [PubMed] [Google Scholar]
- 17. Abdel‐Wahab M, Landt M, Neumann FJ, Massberg S, Frerker C, Kurz T, Kaur J, Toelg R, Sachse S, Jochheim D, et al. 5‐year outcomes after TAVR with balloon‐expandable versus self‐expanding valves: results from the CHOICE randomized clinical trial. JACC Cardiovasc Interv. 2020;13:1071–1082. doi: 10.1016/j.jcin.2019.12.026 [DOI] [PubMed] [Google Scholar]
- 18. Vlastra W, Chandrasekhar J, Munoz‐Garcia AJ, Tchetche D, de Brito FS Jr, Barbanti M, Kornowski R, Latib A, D'Onofrio A, Ribichini F, et al. Comparison of balloon‐expandable vs. self‐expandable valves in patients undergoing transfemoral transcatheter aortic valve implantation: from the center‐collaboration. Eur Heart J. 2019;40:456–465. doi: 10.1093/eurheartj/ehy805 [DOI] [PubMed] [Google Scholar]
- 19. Makkar RR, Cheng W, Waksman R, Satler LF, Chakravarty T, Groh M, Abernethy W, Russo MJ, Heimansohn D, Hermiller J, et al. Self‐expanding intra‐annular versus commercially available transcatheter heart valves in high and extreme risk patients with severe aortic stenosis (PORTICO IDE): a randomised, controlled, non‐inferiority trial. Lancet. 2020;396:669–683. doi: 10.1016/S0140-6736(20)31358-1 [DOI] [PubMed] [Google Scholar]
- 20. Flameng W, Herregods MC, Vercalsteren M, Herijgers P, Bogaerts K, Meuris B. Prosthesis‐patient mismatch predicts structural valve degeneration in bioprosthetic heart valves. Circulation. 2010;121:2123–2129. doi: 10.1161/CIRCULATIONAHA.109.901272 [DOI] [PubMed] [Google Scholar]
- 21. Herrmann HC, Abdel‐Wahab M, Attizzani GF, Batchelor W, Bleiziffer S, Verdoliva S, Chang Y, Gada H, Gillam L, Guerrero M, et al. Rationale and design of the Small Annuli Randomized to Evolut or SAPIEN Trial (SMART trial). Am Heart J. 2022;243:92–102. doi: 10.1016/j.ahj.2021.09.011 [DOI] [PubMed] [Google Scholar]
- 22. Abdel‐Wahab M, Zahn R, Horack M, Gerckens U, Schuler G, Sievert H, Eggebrecht H, Senges J, Richardt G. German transcatheter aortic valve interventions registry I. Aortic regurgitation after transcatheter aortic valve implantation: incidence and early outcome. Results from the German transcatheter aortic valve interventions registry. Heart. 2011;97:899–906. doi: 10.1136/hrt.2010.217158 [DOI] [PubMed] [Google Scholar]
- 23. Jerez‐Valero M, Urena M, Webb JG, Tamburino C, Munoz‐Garcia AJ, Cheema A, Dager AE, Serra V, Amat‐Santos IJ, Barbanti M, et al. Clinical impact of aortic regurgitation after transcatheter aortic valve replacement: insights into the degree and acuteness of presentation. JACC Cardiovasc Interv. 2014;7:1022–1032. doi: 10.1016/j.jcin.2014.04.012 [DOI] [PubMed] [Google Scholar]
- 24. Khalique OK, Hahn RT, Gada H, Nazif TM, Vahl TP, George I, Kalesan B, Forster M, Williams MB, Leon MB, et al. Quantity and location of aortic valve complex calcification predicts severity and location of paravalvular regurgitation and frequency of post‐dilation after balloon‐expandable transcatheter aortic valve replacement. JACC Cardiovasc Interv. 2014;7:885–894. doi: 10.1016/j.jcin.2014.03.007 [DOI] [PubMed] [Google Scholar]
- 25. Kalogeras K, Ruparelia N, Kabir T, Jabbour R, Naganuma T, Vavuranakis M, Nakamura S, Wang B, Sen S, Hadjiloizou N, et al. Comparison of the self‐expanding Evolut‐PRO transcatheter aortic valve to its predecessor Evolut‐R in the real world multicenter ATLAS registry. Int J Cardiol. 2020;310:120–125. doi: 10.1016/j.ijcard.2020.02.070 [DOI] [PubMed] [Google Scholar]
- 26. Van Belle E, Vincent F, Labreuche J, Auffret V, Debry N, Lefevre T, Eltchaninoff H, Manigold T, Gilard M, Verhoye JP, et al. Balloon‐expandable versus self‐expanding transcatheter aortic valve replacement: a propensity‐matched comparison from the FRANCE‐TAVI registry. Circulation. 2020;141:243–259. doi: 10.1161/CIRCULATIONAHA.119.043785 [DOI] [PubMed] [Google Scholar]
- 27. van Rosendael PJ, Delgado V, Bax JJ. Pacemaker implantation rate after transcatheter aortic valve implantation with early and new‐generation devices: a systematic review. Eur Heart J. 2018;39:2003–2013. doi: 10.1093/eurheartj/ehx785 [DOI] [PubMed] [Google Scholar]
- 28. Kalogeras K, Kabir T, Mittal T, Mirsadraee S, Skondras E, Rahman Haley S, Zuhair M, Vavuranakis M, Tousoulis D, Dalby M, et al. Real‐world comparison of the new 34 mm self‐expandable transcatheter aortic prosthesis evolut r to its 31 mm core valve predecessor. Catheter Cardiovasc Interv. 2019;93:685–691. doi: 10.1002/ccd.27862 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Figure S1–S3


