Abstract
The phenotype of schizophrenia, regardless of etiology, represents the most studied psychotic disorder with respect to neurobiology and distinct phases of illness. The early phase of illness represents a unique opportunity to provide effective and individualized interventions that can alter illness trajectories. Developmental age and illness stage, including temporal variation in neurobiology, can be targeted to develop phase-specific clinical assessment, biomarkers, and interventions. We review an earlier model whereby an initial glutamate signaling deficit progresses through different phases of allostatic adaptation, moving from potentially reversible functional abnormalities associated with early psychosis and working memory dysfunction, and ending with difficult-to-reverse structural changes after chronic illness. We integrate this model with evidence of dopaminergic abnormalities, including cortical D1 dysfunction, which develop during adolescence. We discuss how this model and a focus on a potential critical window of intervention in the early stages of schizophrenia impact the approach to research design and clinical care. This impact includes stage-specific considerations for symptom assessment as well as genetic, cognitive, and neurophysiological biomarkers. We examine how phase-specific biomarkers of illness phase and brain development can be incorporated into current strategies for large-scale research and clinical programs implementing coordinated specialty care. We highlight working memory and D1 dysfunction as early treatment targets that can substantially affect functional outcome.
Neurodevelopment of Psychotic Disorders
In the late 20th century, phenomenological descriptions of young persons characterized behavioral, motoric, and cognitive alterations as antecedents of later development of psychosis (1, 2, 3). While nonspecific, findings offered indirect evidence of altered neurodevelopment in those at risk for psychosis, in line with a neurodevelopmental model of schizophrenia (SZ) (4, 5, 6). Psychosis, as a syndrome, is characterized by symptoms including hallucinations, delusions, and changes in behavior and communication; these can be present in different disorders. However, the SZ phenotype, as a disorder, is viewed as the representative psychotic disorder. We acknowledge that the diagnosis of SZ is based on expression of a common phenotypic pathway arising from various possible genetic and environmental interactions. The recent National Institute of Mental Health Research Domain Criteria emphasis on dimensional biological and cognitive processes that lead to mental health and illness does not result in diagnostic classifications, which in the past have posed limitations in elucidating neurobiology associated with psychiatric symptoms. Indeed, there have been ongoing considerations regarding alternatives to the SZ construct that may lead to new nomenclature and novel phenotypic classifications (7). To accommodate the evolving shift away from viewing SZ as a distinct disorder with unitary causality, we will refer to the phenotype of SZ in this article. This phenotype of SZ has provided the opportunity to study genetics and environment, biomarkers, and phases of illness in an enriched sample. Family studies led to the discovery of heritable markers of brain processing associated with the SZ phenotype, for example, smooth pursuit eye movement dysfunction (8,9), sensory gating deficits (10), and certain cognitive deficits, such as working memory (WM) (11,12), associated with familial vulnerability for psychosis. Potential risk genes, such as single nucleotide polymorphisms, copy number variants, and de novo mutations, were associated with later development of psychosis (13). A recently updated large genome-wide association study (14) in SZ phenotype cases and controls identified 270 risk loci. The role of environmental factors in psychosis risk is also critical, including numerous nonspecific influences during prenatal, perinatal, early childhood, and adolescent development (15). In the diathesis–stress model, genetic risk interacts with environmental influences to result in altered brain development. According to this concept, transition to psychosis and subsequent chronic illness relates to neurodevelopment mediated by common and/or rare gene alterations in interaction with various adverse environmental influences at any time during brain development, starting in utero up to close to onset of illness (16). Thus, early environmental influences (e.g., infections, nutritional deficiency, and neglect during intrauterine to early childhood period) may exert influences that can alter neurodevelopment directly, or indirectly through gene expression. Later events (e.g., drugs, brain injury) more proximal to the onset of psychosis may disrupt dopaminergic homeostasis. This model is neither unitary nor discrete, and a person may undergo exposure to multiple risk factors. This developmental perspective promotes understanding of how distinct phases in the pathophysiology of the phenotype of SZ deviate from neurotypical brain development spanning the prenatal period through adulthood.
Early Phase of Illness
Alterations in behavior across various dimensions, lasting from weeks to years, prior to emergence of psychosis have been described. Nonspecific at-risk symptoms in childhood and adolescence may include anxiety, depression, reduced emotional experience, cognitive difficulties, and social withdrawal (17). Specific syndromes identifying psychosis prodrome are embodied in clinical high-risk (CHR) (18) and ultra high-risk (UHR) (19,20) constructs. CHR/UHR classifications are primarily based on acute or increasing subthreshold positive symptoms, including unusual thought content, perceptual anomalies, and disordered thinking/speech, with relatively intact reality testing. In individuals identified as experiencing attenuated psychosis syndrome (APS), transition to threshold psychosis occurs in an estimated 20% to 35% within 2 to 3 years (21). Young persons who do not transition may experience persistent symptoms, in some cases consistent with schizotypy or another nonpsychotic disorder, or may experience symptom resolution. Innovative transdiagnostic clinical staging models developed in recent years aim to provide conceptual frameworks for characterizing risk, development, and progression of a broad array of youth mental health outcomes (22, 23, 24, 25, 26). Risk calculator (27, 28, 29, 30, 31, 32) and machine learning (33,34) approaches, which incorporate an array of risk factors, have been developed to predict progression to first-episode psychosis (FEP). Thus, the FEP outcome is inherently probabilistic rather than determined (24), as delineated in a growing literature supporting the construct of transdiagnostic pluripotential risk (35).
The emergence of FEP is often associated with changes in mood, anxiety, cognition, and substance use problems resulting in uncertain/inconsistent endorsement of target symptoms, phenomenological heterogeneity, and diagnostic uncertainty. Yet as the clinical course evolves over time, eventual clinical diagnosis in FEP becomes evident and remains relatively stable for SZ (36). In the United States, about 100,000 young people experience FEP annually, with a lifetime prevalence of 3%. About one-third of those with FEP develop the phenotype of SZ; the remainder are diagnosed with a range of nonaffective, affective, and substance-induced psychoses. Traditionally, SZ has been viewed as an illness with limited recovery rates, high disability-adjusted life years, and a 20% reduction in average life expectancy, which underscores the need for more effective interventions.
Importance of Early Intervention
Intervention programs in young persons at CHR have been implemented in numerous countries and aim to attenuate or possibly prevent progression to psychosis, mostly through nonspecific treatments and education (37). In FEP, early intervention can preserve or improve functional abilities (38, 39, 40) and potentially alter neurobiological progression of untreated illness. Educational and social developments proceed rapidly during adolescence and young adulthood, and even brief disruption can produce enduring consequences for attaining important maturational milestones. These, in turn, lead to challenges for young persons and their supports, most commonly their family. Consequently, individuals may experience emotional distress, social alienation, and stigma (41). It has been postulated, although with inconsistent empirical support, that the duration of untreated psychosis (DUP) appears to exert adverse or toxic effects on brain functioning (42). DUP has been associated with poorer outcome (43), even years later (44), leading the World Health Organization to recommend constraining DUP to <3 months. Long mean DUP of several years in the representative North American FEP trial (45) illustrates how far the field is from attaining the goal of early intervention, particularly in community settings in the United States. This critical delay is due to multiple contributors, including limited mental health literacy in the general public, absence of universal screening and mobile detection strategies, limited access to dedicated care, and societal stigma. In addition, lack of significant improvement within a few months to years after onset is associated with limited long-term recovery (46,47). These harsh findings highlight the pressing need to invest in effective prevention and early intervention approaches to reduce rates of outcome and disability that have not changed significantly in many years despite novel antipsychotics and other treatment modalities.
Typical FEP treatment in general psychiatric settings makes little differentiation between illness phases, and young persons with FEP may receive similar treatment as individuals with chronic illness. Consequently, individuals with FEP are particularly sensitive to both therapeutic and side effects of medications (48). They can easily experience excessive exposure to antipsychotics and a failure to implement recovery-oriented interventions. For young patients, traditional intervention approaches in settings serving more chronically ill persons can contribute to resistance to illness acceptance and proposed treatment, demoralization, and enforced stigma of serious mental illness, leading to poor outcome.
FEP-specific interventions in dedicated settings were developed in Australia and Europe in the past 3 decades and have been more widely implemented in the United States since 2015 through support of the Substance Abuse and Mental Health Services Administration. FEP-specific interventions are provided by a dedicated team of coordinated specialty care (CSC), including case management, psychotherapy, medication management, supported employment and education, and family support and education. A recent meta-analysis (49) of 10 randomized controlled trials in FEP provides empirical support for improved outcomes in individuals engaged in 6 to 24 months of early intervention services, compared with treatment as usual. However, 10-year outcome data from the OPUS trial did not support sustained clinical and functional improvements in individuals with CSC compared with usual care, highlighting the need for additional longer-term outcome studies (50). CSC programs in the United States remain scarce in many regions (51) and are thus inaccessible to many individuals experiencing FEP.
Neurodevelopmental Mechanisms
Prior Phase-Specific Model of SZ Pathophysiology
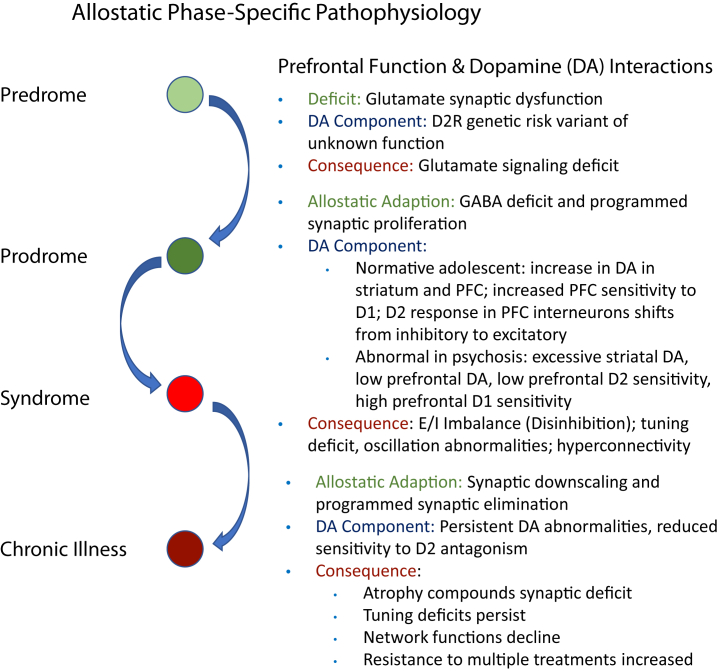
Individuals with FEP are more sensitive to standard D2 receptor (D2R)–blocking antipsychotics compared with those with chronic illness (52,53). Similar early-stage sensitivity was recently noted with respect to novel glutamatergic interventions (54). Thus, treatment response appears greater in early stages, suggesting that pathophysiology of illness differs according to illness stage. Krystal and Anticevic (55) previously outlined a model of stage-specific pathophysiology and its dynamic evolution over time. In this model, the earliest illness stage (predrome) is marked by minimally symptomatic glutamatergic hypofunction. Allostatic adaptations to this initial excitatory deficit, particularly reduced GABA (gamma-aminobutyric acidergic) signaling, lead to cortical disinhibition/overexcitation during the prodromal phase. This, in turn, causes abnormalities in neural tuning, oscillations, and functional hyperconnectivity, which produce frank psychotic symptoms, and cognitive impairment including WM dysfunction. Further adaptations to hyperexcitation and hyperconnectivity, such as accelerated synaptic pruning, cause atrophy and worsening neural circuit dysfunction over time, producing worsening psychosis and potentially irreversible structural changes. One limitation of this exciting model is that it addressed dopaminergic systems only in passing. Here, we expand this model (Figure 1) to incorporate literature on developmental and phase-specific changes in dopaminergic function, with a particular focus on dopamine D1Rs in the prefrontal cortex (PFC).
Figure 1.
Developmental schema for neurobiological progression of schizophrenia, adapted from Krystal and Anticevic (55) by incorporating dopaminergic components. D2R, D2 receptor; DA, dopamine; E:I, excitatory-to-inhibitory balance; GABA, gamma-aminobutyric acid; PFC, prefrontal cortex.
Normal and Abnormal Development, Focusing on PFC Dopamine D1 System
The Krystal and Anticevic (55) model coheres with earlier suggestions that SZ pathophysiology reflects abnormal early neurodevelopmental vulnerability, with frank illness only manifesting in the context of further adolescent brain development (56,57). Adolescence is marked by profound normative changes in dopaminergic innervation, receptor levels, and signaling, and behavioral responses to dopaminergic agents (e.g., stimulants) peak during adolescence (56, 57, 58, 59). This contributes to broader maturation of the PFC, which involves synaptic pruning and myelination continuing well into the third decade in humans (60,61). Indeed, adolescence has been proposed as a neurodevelopmental critical window of heightened plasticity for PFC and its functions, including WM (58).
Dopaminergic midbrain neurons continue to arborize into the nucleus accumbens as well as into the PFC across adolescence. While these changes affect the entire PFC, dopaminergic innervation is particularly increased during adolescence in cortical layer 3 of the dorsolateral PFC, which is strongly implicated both in WM and SZ phenotype pathophysiology (59,62,63). Human postmortem data indicate that D1R levels increase from infancy through adulthood, while D2R levels decrease (64). Response to dopamine receptor stimulation in the PFC also changes dramatically during adolescence. Dopamine effects on PFC neurons are complex, with a mix of excitatory and inhibitory impacts varying across cell types, subcellular components, and developmental stages. D1R signaling at low levels directly increases PFC pyramidal neuronal firing by enhancing NMDA response, while at higher dopamine levels, the D1R has a net inhibitory effect on pyramidal cells via modulation of dendritic potassium channels (63,65) as well as through excitation of interneurons, particularly parvalbumin-positive fast-spiking interneurons (FSNs) (63,66,67). D1R responsivity in PFC neurons generally increases across adolescence (68, 69, 70). D1R and D2R signaling have a complex interaction in PFC, with some similar and some opposing effects (69,71). This interaction varies according to basal dopamine levels, which can shift the direction of D1R effects, contributing to inhibitory tuning (63). The interaction also varies dramatically across adolescence, as D2R effects change direction from inhibitory to excitatory on FSNs during this period (thereby increasing cortical inhibition) (56). FSNs, implicated in WM and in SZ pathophysiology, show progressive increases in synaptogenesis throughout primate development, including adolescence, and are also particularly sensitive to dopamine modulation compared with other interneurons (62,65). Dopamine also directly accelerates the development of FSNs and their synapses (72). Dopaminergic maturation in the PFC thus interacts with the maturing GABAergic system, contributing to increased inhibition and reduced excitatory-to-inhibitory balance across adolescence (62,69). Developmental dopamine dysregulation can be impacted by abnormalities of perineuronal nets, extracellular matrix structures that increase across adolescence and protect parvalbumin interneurons from damage while also reducing their plasticity and more broadly contributing to closure of developmental critical windows (73,74).
In addition to indirect effects via GABAergic interneurons, dopamine's direct effects on glutamatergic neurons also change across adolescence. In particular, the excitatory component of D1R signaling on PFC pyramidal neurons increases across adolescence, partly through alterations in NMDA receptor function (56,69,75,76).
While we focus on the PFC here, PFC dopaminergic systems also interact with striatal dopamine systems and related limbic circuitry (57,77). Activity of the striatal dopamine system peaks during adolescence (78,79), and striatal hyperdopaminergia is strongly associated with psychosis (80). In contrast, PFC dopamine is abnormally low in SZ (77,81). Dopamine systems in the PFC and accumbens can have reciprocal or antagonistic effects (82,83), and this interaction changes dynamically across adolescence (84, 85, 86). Striatal dopamine is already elevated in the prodromal phase, increasing further with progression to frank psychosis (87). Beyond direct corticostriatal interactions, the hippocampus and amygdala also participate in circuits linking to the PFC as well as the ventral striatum, and are likely to be important in neurodevelopmental risks for psychosis. Early developmental injury to the hippocampus recapitulates features of SZ in animal models in part through dysregulation of the PFC, and disruption of the PFC may, in turn, cause dysregulated hippocampal and amygdala function, particularly in the context of elevated stress (57). Disturbance of PFC maturation may thus contribute to progression of the striatal dopamine and limbic dysfunction component of pathophysiology, and vice versa.
Adolescent neurodevelopmental vulnerabilities can be exacerbated by adverse environments that produce anxiety and stress. In animal models, nondopaminergic agents including GABAergic modulators used clinically as anti-anxiety medications, as well as metabotropic glutamate modulators, demonstrate the ability to prevent development of hyperdopaminergic states (57,88). Such mechanisms may have important interactions with environmental risk and resilience factors—environmental enrichment also blocks development of hyperdopaminergia (89), and adverse environmental effects on psychosis risk are likely mediated at least in part by the increase in anxiety and stress they cause (90, 91, 92).
Updated Model Incorporating Dopamine Systems
While many details remain unknown, the above literature points to the need to incorporate dopaminergic systems into the prior model of allostatic phase-specific pathophysiology. Dopaminergic maturation in adolescence and early adulthood represents a key illness phase vulnerability point in SZ pathophysiology, and PFC dopamine abnormalities contribute to a disinhibited and hyperconnected PFC associated with WM impairment (57,93). In models of SZ, the PFC dopamine system appears to be shifted toward greater D1R sensitivity and reduced D2R sensitivity. Reduced D2R sensitivity directly impacts interneuron activity, leading to pyramidal disinhibition. This disinhibition likely drives the PFC hyperglutamatergia and hyperconnectivity found using magnetic resonance imaging (MRI) in early-stage but not later-stage illness, perhaps owing to progressive synaptic loss (94, 95, 96); D1R agonism reduces PFC pyramidal cell hyperconnectivity in primates (97). The impact of increased D1R sensitivity may depend on basal dopamine levels, as D1R is more excitatory for pyramidal cells when dopamine levels are low but is more inhibitory when dopamine levels are high; the weight of the evidence indicates that dopamine levels are low in the PFC in SZ. These dopamine effects are likely to be greatest during adolescence and young adulthood, although dopamine abnormalities may also impact earlier stages of pathophysiology.
Application of the Developmental Model to Illness Phase and Potential Interventions
This updated model integrates glutamatergic and dopaminergic abnormalities into a dynamic model of pathophysiology that can guide biomarker development and therapeutic development specific to illness phase and brain development. An implication is that dopamine modulators other than D2R-blocking antipsychotics should be considered in early illness and for symptoms outside the positive symptom domain. The combination of abnormally low dopamine, increased D1R sensitivity, and reduced D2R sensitivity points to potential benefits of a D1R agonist, which in the low-dopamine regime would be expected to bring pyramidal neuronal firing into a more optimal range, improving inhibitory tuning and reducing hyperconnectivity. Given the inverted-U effects of D1R on PFC physiology and WM (63,77), a partial D1R agonist would be expected to have a similar effect but with reduced risk of overshooting the optimal range, compared with a full D1R agonist. Challenges of effective PFC D1R stimulation and selection of a novel partial D1R agonist as a candidate to improve WM have been described (77).
Another critical implication is that biomarker and treatment studies should stratify according to illness phase, and further work is needed to identify appropriate definitions of illness and brain development phases. Initially, specific age or DUP cutoffs may be selected, but eventually, biomarker-based staging may specify an individual's neurodevelopmental phase and illness phase, providing more accurate stratification. The critical window hypothesis suggests that adolescence and early adulthood represent a period in which treatments work differentially and that aberrant plasticity developed may get cemented once this window closes. This highlights the urgency of implementing treatments specifically effective during this critical window to prevent illness progression or even reverse it. A more speculative implication of the critical window concept is that treatments that reopen this window of neuroplasticity (for example, histone deacetylases) could potentially reinstate sensitivity even during the chronic phase of illness (92). Interventions to prolong neuroplasticity could be combined with other treatments to normalize circuit functioning.
Advances in phase-specific treatment will depend critically on biomarker development. These could include measures that more precisely capture an individual's developmental stage and illness phase and/or different components of the pathophysiological model and its behavioral sequelae, including prefrontal dopamine levels, D1R sensitivity, prefrontal tuning, and WM. These considerations have driven the design of the TRANSCENDS (Translational Neuroscience Computational Evaluation of a D1R Partial Agonist for Schizophrenia) study including D1R intervention and a WM biomarker (77).
Unique Challenges and Opportunities in Early Psychosis Assessment and Treatment
Stage-Specific Screening, Assessment, and Ttreatment
Warning signs of early psychosis (EP) may arise in nonclinical settings (e.g., school or work) or clinical settings without proper recognition, and the condition often goes unaddressed, contributing to DUP. Interventions for CHR/UHR persons include different, mostly nonpharmacological, modalities and may protect against transition to psychosis, which occurs in a minority of persons. Reasons for nontransition remain to be better elucidated (98) and may include protective behavioral, environmental, and genetic factors. Young persons with FEP commonly endorse changes in mood, anxiety, and cognition, and may use substances, contributing to phenomenological heterogeneity and delayed identification of psychosis. The clinician needs to be comfortable with diagnostic uncertainty in FEP (99,100). Initial diagnoses of affective psychosis (i.e., major depressive or bipolar disorder) may change to nonaffective psychotic disorders and (less commonly) vice versa. Over time, considering clinical course, resolution of symptoms, relapse, or chronic illness, the clinical diagnosis becomes evident and remains relatively stable for SZ (36). Conveying diagnostic fluidity to the person with EP and their family members protects them from confusion and potential stigma associated with premature diagnosis and allows treatment to remain symptom focused.
Over the past 20 years, the psychiatric field has moved toward implementation of CSC in persons with EP. Here, a small interactive team of providers offers multiple treatment components in settings dedicated to young persons. Longitudinal assessment of psychosis and nonpsychosis symptom domains represent an important component of CSC and allows for more accurate eventual diagnosis. Stage-specific assessments of core psychosis symptoms include brief screeners (101, 102, 103) and structured assessments (18,104) for CHR and FEP (105,106). Other important EP assessments include mood, anxiety, cognition, substance use, and developmental disorders (e.g., attention-deficit/hyperactivity disorder, autism spectrum). Assessment of functional (role/social) capacity informs an individualized treatment plan across CSC components, minimizing disruption of the expected developmental trajectory in this vital developmental period. For FEP, the Early Psychosis Intervention Network Core Assessment Battery (107) was specifically developed, incorporating validated and widely used instruments.
Increasingly, psychiatry has moved away from placing undue emphasis on a single intervention in FEP (i.e., antipsychotic monotherapy), instead embracing combination treatments of mostly pharmacological interventions with different behavioral modalities. CSC effectiveness and its longer-term effects beyond CSC (108) may result from synchronized delivery of multiple treatment components by a small team, albeit early benefits may not be sustained (50). In the United States, FEP services are modeled after the RAISE (Recovery After an Initial Schizophrenia Episode) trial (45) and longstanding EP services in other countries. Many young persons with EP make their own treatment decisions but remain dependent on logistical, financial, and emotional support from their families. Including families in formulating an individualized treatment plan can increase implementation of services and potentially improve outcomes. While we do not want to overstate the benefits of CSC, the dedicated setting and treatment team are best positioned to adopt new approaches.
Potential Role of Biomarkers in EP Assessment
For many years, the field has explored potential biomarkers associated with psychosis. Biomarkers can improve our understanding of neuropathological mechanisms involved in development of psychosis and assist in illness staging by contrasting the clinical phenotype against static or illness-phase dynamic biological markers, resulting in improved diagnosis and prediction of clinical course. Biomarkers could potentially be targeted for individualized treatment or serve as endpoints in treatment studies. Candidates for biomarkers are derived from findings in genetics, electrophysiology, neuroimaging, neurocognition, inflammation, and neuroendocrinology (109). For example, in 40 individuals with CHR (110), conversion to psychosis was predicted with high accuracy by combining demographic and clinical data with blood biomarkers and quantitative electroencephalography (EEG). Similarly, a baseline panel of 15 serum analytes modulating immune system function, hypothalamic-pituitary function, and oxidative stress differentiated CHR persons who developed psychosis from unaffected individuals with high predictive validity (111). While promising, larger-scale and adequately powered studies are necessary to explore the role of biomarkers in psychosis detection and how they can inform treatment course.
Cognition represents a strong predictor of functional outcome and quality of life in SZ (112). The illness is associated with dysfunction in multiple domains, including attention, WM, semantic memory, executive functioning, and social cognition. Cognitive difficulties may present in the predromal phase, can worsen during prodomal and FEP phases, and respond poorly to existing interventions. Even in EP patients whose positive psychotic symptoms are effectively treated, cognitive difficulties (113), particularly in WM, significantly affect independent daily functioning and scholastic and occupational advancement. PFC-dependent WM impairment (77) presents an endophenotypic marker of psychosis, given its occurrence irrespective of clinical acuity (114, 115, 116) and in unaffected first-degree family members (117,118). In fact, WM may differentiate between those who do versus do not transition to psychosis (119). Thus, WM represents a highly suitable candidate as a biomarker and target of novel interventions in EP. The recently established Accelerating Medicines Partnership-Schizophrenia represents a ground-breaking new phase in EP research (120). The Accelerating Medicines Partnership–Schizophrenia is a global partnership among the National Institutes of Health, the U.S. Food and Drug Administration, and multiple public and private organizations. The Accelerating Medicines Partnership–Schizophrenia focuses specifically on CHR to identify promising biological targets and biomarkers and build an infrastructure for testing CHR-specific treatments in over 2000 youths. Over 2 years, participants will undergo clinical and biomarker assessments, including psychopathology, cognition, genetics, behavior, language analysis, and brain structure and function, to evaluate whether biomarkers can predict individual clinical trajectories. The Early Psychosis Intervention Network (121), through eight regional hubs including over 100 FEP clinics in 17 states, represents a similar but more intervention-oriented initiative to develop improved CSC models.
Implementing Biomarkers Into CSC for EP and Potential Novel Compounds
Large-scale networks focused on phenotyping EP provide a promising platform to investigate specific and individualized treatments, based on both conceptual models of neurodevelopmental staging and empirical findings provided by biomarkers. During this stage of neurodevelopment and early illness, a time-limited window of opportunity exists to change the curve from poor outcome and prognosis commonly associated with more chronic illness. This assertion is supported by neuroplasticity during brain development in adolescence and early adulthood, which diminishes with aging and illness chronicity. Higher rates of treatment response and remission in EP (52) support temporary potential reversibility of neurochemical brain changes associated with psychosis.
Recent biomarker studies in EP, including neuroanatomy (122,123), neurochemistry (124,125), EEG (126), peripheral inflammatory markers (126, 127, 128), and oxidative stress (129), were found to relate to conversion to psychosis (123), antipsychotic response (124,127), and functional outcome (122,128, 129, 130). Current advances of in-depth phenotyping in EP combining demographic, clinical, and stage-specific biomarker findings offer the exciting potential for earlier detection and more targeted treatment options improving clinical and functional outcome. Potential biomarkers in EP settings include digital phenotyping, serum analytes, genetic markers, cognitive functioning, EEG, and MRI. Digital phenotyping in behavioral science (131) is rapidly expanding and has been implemented in persons with psychosis (132), in particular actigraphy (133) and ecological momentary assessment (134), to monitor mood and activity. Serum analytes can include markers of stress, inflammation, and oxidative stress (135). Molecular genetic studies (13,136) have identified variations in neurotrophic, serotonin, cell adhesion, and sodium-channel systems and their association with neurocognition and social cognition in SZ. Last, commercial pharmacogenetic testing is rapidly expanding and provides information about medication metabolism to tailor medication choices to individuals (137).
Cognitive testing, EEG, and MRI can monitor possible biomarkers but are more labor intensive and costly, requiring access to suitable equipment. Cognitive batteries exist in paper and computerized format that can be administered online. The MATRICS Consensus Cognitive Battery (138) has been widely implemented in SZ. The PhenX Toolkit, a Web-based catalogue of high-priority assessment measures (139) for close to 1000 conditions, also includes neurocognition (140). Promising biomarker paradigms in EEG include mismatch negativity (141), steady-state responses (142), and resting EEG (143). Structural brain MRI can reveal minor developmental abnormalities or, rarely, clinically significant abnormalities; multivariate patterns of subtle structural changes can help predict psychosis transition risk and functional outcomes in CHR (33). Potential utilization of biomarkers, either alone or in combination, in identifying illness, monitoring progression, and governing treatment choices remains a promising concept.
An area of particular promise is the incorporation of biomarkers into interventional studies. Past studies have shown that antipsychotics do not prevent psychosis (144), but modulation of the dopaminergic system remains a focus of psychosis intervention. For example, as outlined above, selective enhancement of prefrontal D1R signaling may enhance prefrontal cortical activity and downregulate mesolimbic hyperactivity associated with psychosis symptoms. Over the past 20 years, multiple efforts have attempted to augment cortical glutamate functioning based on the NMDA hypothesis in SZ (145). While exploration of NMDA receptor hypofunctioning has informed our understanding of SZ pathophysiology, modulation of NMDA activity has produced variable success using glycine, D-serine, and cycloserine (146) or allosteric metabotropic glutamate receptor (147) agonists. Other potential agents exist for addressing cortical excitatory-to-inhibitory balance imbalance proposed by the model put forth by Krystal and Anticevic (55) and warrant further investigation. These include GABA agonists, N-acetylcysteine, α7 nicotinic acetylcholine receptor agonists, and D-amino acid oxidase inhibitors. Potential candidate interventions via nondopaminergic mechanisms include fatty acids, modulation of serotoninergic or cannabinoid systems, and immunomodulators (e.g., nonsteroidal anti-inflammatory medications, antioxidants, nutrients, vitamins, and anti-inflammatory herbal products). Beyond pharmacological approaches, cognitive and neuromodulatory interventions, either alone or in combination, also hold promise and may benefit from incorporation of phase-specific biomarkers.
Conclusions
Development and dissemination of standardized CSC in EP has been a fairly recent development. CSC care, providing an individualized multimodal approach, represents our best option to retain patients in treatment and can be further enhanced through implementation of biomarkers. Early years of illness represent a critical and narrow window for potential recovery or significant clinical improvement. CSC programs also offer ideal settings to explore novel interventions in EP. In concert with biomarkers, employing pharmacological or behavioral interventions in EP before progression to more chronic or relapsing illness offers the opportunity to stabilize or reverse the proposed dynamically developing cortical dysfunction. WM functions mediated by prefrontal physiology hold particular promise as an early biomarker with a strong relationship to outcome (77). Based on our conceptual model, WM provides a promising target of specific interventions in early illness, before PFC maturation is completed, and this impairment becomes more difficult to modulate. Rapidly developing knowledge regarding potential mechanistic biomarkers, linked with new large-scale efforts toward standardized assessment in CHR/UHR and EP, positions the field to discover developmentally informed phase-specific interventions that are both standardized and personalized. These efforts should ultimately encompass a broad array of options for prevention and treatment, including medications, neuromodulation, psychotherapy, and cognitive remediation, to improve long-term outcomes.
Acknowledgments and Disclosures
This work was supported by the National Institute of Mental Health (Grant No. U01MH121766 [to AA]).
We are grateful for the contributions of the TRANSCENDS Group.
AA-D has served as a consultant to Sunovion, Otsuka, Merck, and Neurocrine; and owns stock options in Systems 1 Bio and in Terran Biosciences. AA has served on the Technology Advisory Board for RBNC Therapeutics and is co-founder of Manifest Sciences. CF has served as a consultant for RBNC Therapeutics. RRG has performed consulting work within the last 3 years for IMS Expert Services, Noble Insights, and Fowler White Burnett; and has received royalties from Wipf and Stock Books and Routledge/Taylor & Francis. JTK has received consulting payments within the last 24 months from Alphasights, Charles River Associates, Medscape, Putnam, techspert.io, Third Bridge, MEDACorp, Parexel, GroupH, Simon Kucher, ECRI Institute, ExpertConnect, Parexel, Schlesinger Group, CelloHealth, Acsel Health, Strafluence, Guidepoint, L.E.K., and System Analytic; has served on the MedinCell Psychiatry and Karuna Mechanism of Action Advisory Boards; has conducted clinical research supported by the National Institute of Mental Health, Sunovion, Roche, Alkermes, Cerevance, Corcept, Takeda, Taisho, Lundbeck, Boehringer Ingelheim, NeuroRX, and Teva within the last 24 months; was a co-investigator on a study that receives Lumateperone and reimbursement for safety testing for an investigator-initiated research from Intra-Cellular Therapies Inc; and owns a small number of shares of common stock from GSK. JHK has received consulting payments from AstraZeneca Pharmaceuticals, Biogen, Biomedisyn Corporation, Bionomics, Boehringer Ingelheim International, COMPASS Pathways, Concert Pharmaceuticals, Epiodyne, EpiVario, Heptares Therapeutics, Janssen Research and Development, Otsuka America Pharmaceutical, Perception Neuroscience Holdings, Spring Care, Sunovion Pharmaceuticals, Takeda Industries, and Taisho Pharmaceutical; has served on advisory boards for Bioasis Technologies, Biohaven Pharmaceuticals, BioXcel Therapeutics, BlackThorn Therapeutics, Cadent Therapeutics, Cerevel Therapeutics, EpiVario, Eisai, Lohocla Research Corporation, Novartis Pharmaceuticals Corporation, and PsychoGenics; is a co-sponsor of a patent for the intranasal administration of ketamine for the treatment of depression and for the treatment of suicide risk that was licensed by Janssen Pharmaceuticals; has a patent related to the use of riluzole to treat anxiety disorders that was licensed by Biohaven Pharmaceuticals; has stock or stock options in Biohaven Pharmaceuticals, Blackthorn Therapeutics, Luc Therapeutics, Cadent Pharmaceuticals, Terran Biosciences, Spring Healthcare, and Sage Pharmaceuticals; has served on the Board of Directors of Inheris Pharmaceuticals; and receives compensation for serving as editor of the journal Biological Psychiatry. JAL neither accepts nor receives any personal financial remuneration for consulting, speaking, or research activities from any pharmaceutical, biotechnology, or medical device companies; has received support administered through Columbia University and the Research Foundation for Mental Hygiene in the form of funding and medication supplies for investigator-initiated research from Denovo, Taisho, Sunovion, and Genentech, and for company-sponsored phase 2, 3, and 4 studies from Alkermes, Allergan, and Boehringer Ingelheim, but none of this research support contributes to his institutional compensation; has served as a consultant to or member of the advisory board of Intracellular Therapies, Pear Therapeutics, and Gilgamesh Therapeutics, for which he receives no remuneration; has served as a paid consultant for Signant, a clinical research services organization; and holds a patent from Repligen that neither has nor currently yields any royalties. JDM has served on the Technology Advisory Board for RBNC Therapeutics and is co-founder of Manifest Sciences. ZT has served as a consultant for RBNC Therapeutics. DLG is an employee and shareholder of Cerevel Therapeutics (which owns rights to CVL-562 and has other D1 receptor agonists in development). All other authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
TRANSCENDS Group authors: Yale School of Medicine: Deepak D'Souza, Vinod Srihari, Ralitza Gueorguieva, Prashant Patel, Kimberlee Forselius-Bielen, Jing Lu, Audrey Butler, Geena Fram, Yvette Afriyie-Agyemang, Alexandria Selloni, Laura Cadavid, Sandra Gomez-Luna, Aarti Gupta, Rajiv Radhakrishnan, Ali Rashid, Ryan Aker, Philisha Abrahim, Anahita Bassir Nia, Toral Surti; Columbia University, College of Physicians and Surgeons/New York State Psychiatric Institute: Lawrence S. Kegeles, Marlene Carlson, Terry Goldberg, James Gangwisch, Erinne Benedict, Preetika Govil, Stephanie Brazis, Megan Mayer, Nathalie de la Garrigue; Stony Brook University Renaissance School of Medicine: Natalka Fallon, Topaz Baumvoll, Sameera Abeykoon, Greg Perlman, Kelly Bobchin; University of Pennsylvania, Perelman School of Medicine: Mark Elliott, Lyndsay Schmidt, Sage Rush, Allison Port, Zac Heffernan, Nina Laney, Jenna Kantor, Thomas Hohing.
Contributor Information
Christian G. Kohler, Email: kohler@pennmedicine.upenn.edu.
TRANSCENDS Group:
Deepak D'Souza, Vinod Srihari, Ralitza Gueorguieva, Prashant Patel, Kimberlee Forselius-Bielen, Jing Lu, Audrey Butler, Geena Fram, Yvette Afriyie-Agyemang, Alexandria Selloni, Laura Cadavid, Sandra Gomez-Luna, Aarti Gupta, Rajiv Radhakrishnan, Ali Rashid, Ryan Aker, Philisha Abrahim, Anahita Bassir Nia, Toral Surti, Lawrence S. Kegeles, Marlene Carlson, Terry Goldberg, James Gangwisch, Erinne Benedict, Preetika Govil, Stephanie Brazis, Megan Mayer, Nathalie de la Garrigue, Natalka Fallon, Topaz Baumvoll, Sameera Abeykoon, Greg Perlman, Kelly Bobchin, Mark Elliott, Lyndsay Schmidt, Sage Rush, Allison Port, Zac Heffernan, Nina Laney, Jenna Kantor, and Thomas Hohing
References
- 1.Fish B. Infant predictors of the longitudinal course of schizophrenic development. Schizophr Bull. 1987;13:395–409. doi: 10.1093/schbul/13.3.395. [DOI] [PubMed] [Google Scholar]
- 2.Walker E.F., Savoie T., Davis D. Neuromotor precursors of schizophrenia. Schizophr Bull. 1994;20:441–451. doi: 10.1093/schbul/20.3.441. [DOI] [PubMed] [Google Scholar]
- 3.Cannon T.D., Rosso I.M., Bearden C.E., Sanchez L.E., Hadley T. A prospective cohort study of neurodevelopmental processes in the genesis and epigenesis of schizophrenia. Dev Psychopathol. 1999;11:467–485. doi: 10.1017/s0954579499002163. [DOI] [PubMed] [Google Scholar]
- 4.Johnstone E.C., Crow T.J., Frith C.D., Husband J., Kreel L. Cerebral ventricular size and cognitive impairment in chronic schizophrenia. Lancet. 1976;2:924–926. doi: 10.1016/s0140-6736(76)90890-4. [DOI] [PubMed] [Google Scholar]
- 5.Lewis S.W., Murray R.M. Obstetric complications, neurodevelopmental deviance, and risk of schizophrenia. J Psychiatr Res. 1987;21:413–421. doi: 10.1016/0022-3956(87)90088-4. [DOI] [PubMed] [Google Scholar]
- 6.Weinberger D.R. Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry. 1987;44:660–669. doi: 10.1001/archpsyc.1987.01800190080012. [DOI] [PubMed] [Google Scholar]
- 7.Tandon R., Keshavan M., Nasrallah H. Reinventing schizophrenia. Updating the construct. Schizophr Res. 2022;242:1–3. doi: 10.1016/j.schres.2022.02.024. [DOI] [PubMed] [Google Scholar]
- 8.Holzman P.S., Proctor L.R., Levy D.L., Yasillo N.J., Meltzer H.Y., Hurt S.W. Eye-tracking dysfunctions in schizophrenic patients and their relatives. Arch Gen Psychiatry. 1974;31:143–151. doi: 10.1001/archpsyc.1974.01760140005001. [DOI] [PubMed] [Google Scholar]
- 9.Iacono W.G. Young psychophysiologist award address, 1982. Psychophysiology and genetics: A key to psychopathology research. Psychophysiology. 1983;20:371–383. doi: 10.1111/j.1469-8986.1983.tb00916.x. [DOI] [PubMed] [Google Scholar]
- 10.Siegel C., Waldo M., Mizner G., Adler L.E., Freedman R. Deficits in sensory gating in schizophrenic patients and their relatives. Evidence obtained with auditory evoked responses. Arch Gen Psychiatry. 1984;41:607–612. doi: 10.1001/archpsyc.1984.01790170081009. [DOI] [PubMed] [Google Scholar]
- 11.Lee J., Park S. Working memory impairments in schizophrenia: A meta-analysis. J Abnorm Psychol. 2005;114:599–611. doi: 10.1037/0021-843X.114.4.599. [DOI] [PubMed] [Google Scholar]
- 12.Gur R.E., Calkins M.E., Gur R.C., Horan W.P., Nuechterlein K.H., Seidman L.J., et al. The Consortium on the Genetics of Schizophrenia: Neurocognitive endophenotypes. Schizophr Bull. 2007;33:49–68. doi: 10.1093/schbul/sbl055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Avramopoulos D. Recent advances in the genetics of schizophrenia. Mol Neuropsychiatry. 2018;4:35–51. doi: 10.1159/000488679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schizophrenia Working Group of the Psychiatric Genomics Consortium. Ripke S., Walters J.T., O’Donovan M.C. Mapping genomic loci prioritises genes and implicates synaptic biology in schizophrenia. medRxiv. 2020 doi: 10.1101/2020.09.12.20192922. [DOI] [Google Scholar]
- 15.Fusar-Poli P., Tantardini M., De Simone S., Ramella-Cravaro V., Oliver D., Kingdon J., et al. Deconstructing vulnerability for psychosis: Meta-analysis of environmental risk factors for psychosis in subjects at ultra high-risk. Eur Psychiatry. 2017;40:65–75. doi: 10.1016/j.eurpsy.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 16.Wahbeh M.H., Avramopoulos D. Gene-environment interactions in schizophrenia: A literature review. Genes (Basel) 2021;12:1850. doi: 10.3390/genes12121850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taylor J.H., Calkins M.E., Gur R.E. Markers of psychosis risk in the general population. Biol Psychiatry. 2020;88:337–348. doi: 10.1016/j.biopsych.2020.02.002. [DOI] [PubMed] [Google Scholar]
- 18.McGlashan T., Walsh B.C., Woods S.W. Oxford University Press; New York: 2010. The Psychosis-Risk Syndrome: Handbook for Diagnosis and Follow-Up. [Google Scholar]
- 19.McGorry P.D., Mei C. Ultra-high-risk paradigm: Lessons learnt and new directions. Evid Based Ment Health. 2018;21:131–133. doi: 10.1136/ebmental-2018-300061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Phillips L.J., Yung A.R., McGorry P.D. Identification of young people at risk of psychosis: Validation of Personal Assessment and Crisis Evaluation Clinic intake criteria. Aust N Z J Psychiatry. 2000;34:S164–S169. doi: 10.1080/000486700239. [DOI] [PubMed] [Google Scholar]
- 21.Fusar-Poli P., Bonoldi I., Yung A.R., Borgwardt S., Kempton M.J., Valmaggia L., et al. Predicting psychosis: Meta-analysis of transition outcomes in individuals at high clinical risk. Arch Gen Psychiatry. 2012;69:220–229. doi: 10.1001/archgenpsychiatry.2011.1472. [DOI] [PubMed] [Google Scholar]
- 22.McGorry P.D., Hickie I.B., Yung A.R., Pantelis C., Jackson H.J. Clinical staging of psychiatric disorders: A heuristic framework for choosing earlier, safer and more effective interventions. Aust N Z J Psychiatry. 2006;40:616–622. doi: 10.1080/j.1440-1614.2006.01860.x. [DOI] [PubMed] [Google Scholar]
- 23.McGorry P., Nelson B. Why we need a transdiagnostic staging approach to emerging psychopathology, early diagnosis, and treatment. JAMA Psychiatry. 2016;73:191–192. doi: 10.1001/jamapsychiatry.2015.2868. [DOI] [PubMed] [Google Scholar]
- 24.Shah J.L., Scott J., McGorry P.D., Cross S.P.M., Keshavan M.S., Nelson B., et al. Transdiagnostic clinical staging in youth mental health: A first international consensus statement. World Psychiatry. 2020;19:233–242. doi: 10.1002/wps.20745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scott J., Leboyer M., Hickie I., Berk M., Kapczinski F., Frank E., et al. Clinical staging in psychiatry: A cross-cutting model of diagnosis with heuristic and practical value. Br J Psychiatry. 2013;202:243–245. doi: 10.1192/bjp.bp.112.110858. [DOI] [PubMed] [Google Scholar]
- 26.Gupta T., Mittal V.A. Advances in clinical staging, early intervention, and the prevention of psychosis. F1000Res. 2019;8:F1000. doi: 10.12688/f1000research.20346.1. Faculty Rev-2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cannon T.D., Yu C., Addington J., Bearden C.E., Cadenhead K.S., Cornblatt B.A., et al. An individualized risk calculator for research in prodromal psychosis. Am J Psychiatry. 2016;173:980–988. doi: 10.1176/appi.ajp.2016.15070890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fusar-Poli P., Rutigliano G., Stahl D., Davies C., Bonoldi I., Reilly T., et al. Development and validation of a clinically based risk calculator for the transdiagnostic prediction of psychosis. JAMA Psychiatry. 2017;74:493–500. doi: 10.1001/jamapsychiatry.2017.0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Osborne K.J., Mittal V.A. External validation and extension of the NAPLS-2 and SIPS-RC personalized risk calculators in an independent clinical high-risk sample. Psychiatry Res. 2019;279:9–14. doi: 10.1016/j.psychres.2019.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang T., Xu L., Li H., Woodberry K.A., Kline E.R., Jiang J., et al. Calculating individualized risk components using a mobile app-based risk calculator for clinical high risk of psychosis: Findings from ShangHai At Risk for Psychosis (SHARP) program. Psychol Med. 2021;51:653–660. doi: 10.1017/S003329171900360X. [DOI] [PubMed] [Google Scholar]
- 31.Zhang T., Xu L., Tang Y., Li H., Tang X., Cui H., et al. Prediction of psychosis in prodrome: Development and validation of a simple, personalized risk calculator. Psychol Med. 2019;49:1990–1998. doi: 10.1017/S0033291718002738. [DOI] [PubMed] [Google Scholar]
- 32.Moore T.M., Calkins M.E., Rosen A.F.G., Butler E.R., Ruparel K., Fusar-Poli P., et al. Development of a probability calculator for psychosis risk in children, adolescents, and young adults. Psychol Med. 2022;52:3159–3167. doi: 10.1017/S0033291720005231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koutsouleris N., Kambeitz-Ilankovic L., Ruhrmann S., Rosen M., Ruef A., Dwyer D.B., et al. Prediction models of functional outcomes for individuals in the clinical high-risk state for psychosis or with recent-onset depression: A multimodal, multisite machine learning analysis. JAMA Psychiatry. 2018;75:1156–1172. doi: 10.1001/jamapsychiatry.2018.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tognin S., van Hell H.H., Merritt K., Winter-van Rossum I., Bossong M.G., Kempton M.J., et al. Towards precision medicine in psychosis: Benefits and challenges of multimodal multicenter studies-PSYSCAN: Translating neuroimaging findings from research into clinical practice. Schizophr Bull. 2020;46:432–441. doi: 10.1093/schbul/sbz067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hartmann J.A., McGorry P.D., Destree L., Amminger G.P., Chanen A.M., Davey C.G., et al. Pluripotential risk and clinical staging: Theoretical considerations and preliminary data from a transdiagnostic risk identification approach. Front Psychiatry. 2020;11 doi: 10.3389/fpsyt.2020.553578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bromet E.J., Kotov R., Fochtmann L.J., Carlson G.A., Tanenberg-Karant M., Ruggero C., et al. Diagnostic shifts during the decade following first admission for psychosis. Am J Psychiatry. 2011;168:1186–1194. doi: 10.1176/appi.ajp.2011.11010048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Catalan A., Salazar de Pablo G., Vaquerizo Serrano J., Mosillo P., Baldwin H., Fernandez-Rivas A., et al. Annual Research Review: Prevention of psychosis in adolescents - systematic review and meta-analysis of advances in detection, prognosis and intervention. J Child Psychol Psychiatry. 2021;62:657–673. doi: 10.1111/jcpp.13322. [DOI] [PubMed] [Google Scholar]
- 38.Westfall M.B.E., Kohler C.G., Hurford I., Abegunde C., Agosti D., Brinen A., et al. Pennsylvania coordinated specialty care programs for first-episode psychosis: 6- and 12-month outcomes. Early Interv Psychiatry. 2021;15:1395–1408. doi: 10.1111/eip.13084. [DOI] [PubMed] [Google Scholar]
- 39.Nossel I., Wall M.M., Scodes J., Marino L.A., Zilkha S., Bello I., et al. Results of a coordinated specialty care program for early psychosis and predictors of outcomes. Psychiatr Serv. 2018;69:863–870. doi: 10.1176/appi.ps.201700436. [DOI] [PubMed] [Google Scholar]
- 40.Oluwoye O., Reneau H., Stokes B., Daughtry R., Venuto E., Sunbury T., et al. Preliminary evaluation of Washington State's Early Intervention Program for First-Episode Psychosis. Psychiatr Serv. 2020;71:228–235. doi: 10.1176/appi.ps.201900199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baba Y., Nemoto T., Tsujino N., Yamaguchi T., Katagiri N., Mizuno M. Stigma toward psychosis and its formulation process: Prejudice and discrimination against early stages of schizophrenia. Compr Psychiatry. 2017;73:181–186. doi: 10.1016/j.comppsych.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 42.Rund B.R. Does active psychosis cause neurobiological pathology? A critical review of the neurotoxicity hypothesis. Psychol Med. 2014;44:1577–1590. doi: 10.1017/S0033291713002341. [DOI] [PubMed] [Google Scholar]
- 43.Perkins D.O., Gu H., Boteva K., Lieberman J.A. Relationship between duration of untreated psychosis and outcome in first-episode schizophrenia: A critical review and meta-analysis. Am J Psychiatry. 2005;162:1785–1804. doi: 10.1176/appi.ajp.162.10.1785. [DOI] [PubMed] [Google Scholar]
- 44.Jabar L.S.A., Sorensen H.J., Nordentoft M., Hjorthoj C., Albert N. Associations between duration of untreated psychosis and domains of positive and negative symptoms persist after 10 years of follow-up: A secondary analysis from the OPUS trial. Schizophr Res. 2021;228:575–580. doi: 10.1016/j.schres.2020.11.027. [DOI] [PubMed] [Google Scholar]
- 45.Kane J.M., Robinson D.G., Schooler N.R., Mueser K.T., Penn D.L., Rosenheck R.A., et al. Comprehensive versus usual community care for first-episode psychosis: 2-Year outcomes from the NIMH RAISE Early Treatment Program. Am J Psychiatry. 2016;173:362–372. doi: 10.1176/appi.ajp.2015.15050632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dazzan P., Lappin J.M., Heslin M., Donoghue K., Lomas B., Reininghaus U., et al. Symptom remission at 12-weeks strongly predicts long-term recovery from the first episode of psychosis. Psychol Med. 2020;50:1452–1462. doi: 10.1017/S0033291719001399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lally J., Ajnakina O., Stubbs B., Cullinane M., Murphy K.C., Gaughran F., et al. Remission and recovery from first-episode psychosis in adults: Systematic review and meta-analysis of long-term outcome studies. Br J Psychiatry. 2017;211:350–358. doi: 10.1192/bjp.bp.117.201475. [DOI] [PubMed] [Google Scholar]
- 48.Whale R., Harris M., Kavanagh G., Wickramasinghe V., Jones C.I., Marwaha S., et al. Effectiveness of antipsychotics used in first-episode psychosis: A naturalistic cohort study. BJPsych Open. 2016;2:323–329. doi: 10.1192/bjpo.bp.116.002766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Correll C.U., Galling B., Pawar A., Krivko A., Bonetto C., Ruggeri M., et al. Comparison of early intervention services vs treatment as usual for early-phase psychosis: A systematic review, meta-analysis, and meta-regression. JAMA Psychiatry. 2018;75:555–565. doi: 10.1001/jamapsychiatry.2018.0623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Secher R.G., Hjorthoj C.R., Austin S.F., Thorup A., Jeppesen P., Mors O., et al. Ten-year follow-up of the OPUS specialized early intervention trial for patients with a first episode of psychosis. Schizophr Bull. 2015;41:617–626. doi: 10.1093/schbul/sbu155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Prodrome and Early Psychosis Program Network, P-RaEPPN Early Psychosis Program Directory. med.stanford.edu/peppnet/interactivedirectory.html Available at:
- 52.Carbon M., Correll C.U. Clinical predictors of therapeutic response to antipsychotics in schizophrenia. Dialogues Clin Neurosci. 2014;16:505–524. doi: 10.31887/DCNS.2014.16.4/mcarbon. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McGorry P.D., Cocks J., Power P., Burnett P., Harrigan S., Lambert T. Very low-dose risperidone in first-episode psychosis: A safe and effective way to initiate treatment. Schizophr Res Treatment. 2011. 2011 doi: 10.1155/2011/631690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kinon B.J., Millen B.A., Zhang L., McKinzie D.L. Exploratory analysis for a targeted patient population responsive to the metabotropic glutamate 2/3 receptor agonist pomaglumetad methionil in schizophrenia. Biol Psychiatry. 2015;78:754–762. doi: 10.1016/j.biopsych.2015.03.016. [DOI] [PubMed] [Google Scholar]
- 55.Krystal J.H., Anticevic A. Toward illness phase-specific pharmacotherapy for schizophrenia. Biol Psychiatry. 2015;78:738–740. doi: 10.1016/j.biopsych.2015.08.017. [DOI] [PubMed] [Google Scholar]
- 56.O'Donnell P. Cortical disinhibition in the neonatal ventral hippocampal lesion model of schizophrenia: New vistas on possible therapeutic approaches. Pharmacol Ther. 2012;133:19–25. doi: 10.1016/j.pharmthera.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 57.Sonnenschein S.F., Gomes F.V., Grace A.A. Dysregulation of midbrain dopamine system and the pathophysiology of schizophrenia. Front Psychiatry. 2020;11:613. doi: 10.3389/fpsyt.2020.00613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Larsen B., Luna B. Adolescence as a neurobiological critical period for the development of higher-order cognition. Neurosci Biobehav Rev. 2018;94:179–195. doi: 10.1016/j.neubiorev.2018.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rosenberg D.R., Lewis D.A. Postnatal maturation of the dopaminergic innervation of monkey prefrontal and motor cortices: A tyrosine hydroxylase immunohistochemical analysis. J Comp Neurol. 1995;358:383–400. doi: 10.1002/cne.903580306. [DOI] [PubMed] [Google Scholar]
- 60.Patel P.K., Leathem L.D., Currin D.L., Karlsgodt K.H. Adolescent neurodevelopment and vulnerability to psychosis. Biol Psychiatry. 2021;89:184–193. doi: 10.1016/j.biopsych.2020.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gogtay N., Giedd J.N., Lusk L., Hayashi K.M., Greenstein D., Vaituzis A.C., et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lewis D.A., Hashimoto T., Volk D.W. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- 63.Arnsten A.F., Wang M., Paspalas C.D. Dopamine's actions in primate prefrontal cortex: Challenges for treating cognitive disorders. Pharmacol Rev. 2015;67:681–696. doi: 10.1124/pr.115.010512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rothmond D.A., Weickert C.S., Webster M.J. Developmental changes in human dopamine neurotransmission: Cortical receptors and terminators. BMC Neurosci. 2012;13:18. doi: 10.1186/1471-2202-13-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Trantham-Davidson H., Kroner S., Seamans J.K. Dopamine modulation of prefrontal cortex interneurons occurs independently of DARPP-32. Cereb Cortex. 2008;18:951–958. doi: 10.1093/cercor/bhm133. [DOI] [PubMed] [Google Scholar]
- 66.Kroner S., Krimer L.S., Lewis D.A., Barrionuevo G. Dopamine increases inhibition in the monkey dorsolateral prefrontal cortex through cell type-specific modulation of interneurons. Cereb Cortex. 2007;17:1020–1032. doi: 10.1093/cercor/bhl012. [DOI] [PubMed] [Google Scholar]
- 67.Gorelova N., Seamans J.K., Yang C.R. Mechanisms of dopamine activation of fast-spiking interneurons that exert inhibition in rat prefrontal cortex. J Neurophysiol. 2002;88:3150–3166. doi: 10.1152/jn.00335.2002. [DOI] [PubMed] [Google Scholar]
- 68.Heng L.J., Markham J.A., Hu X.T., Tseng K.Y. Concurrent upregulation of postsynaptic L-type Ca(2+) channel function and protein kinase A signaling is required for the periadolescent facilitation of Ca(2+) plateau potentials and dopamine D1 receptor modulation in the prefrontal cortex. Neuropharmacology. 2011;60:953–962. doi: 10.1016/j.neuropharm.2011.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kang S., Cox C.L., Gulley J.M. High frequency stimulation-induced plasticity in the prelimbic cortex of rats emerges during adolescent development and is associated with an increase in dopamine receptor function. Neuropharmacology. 2018;141:158–166. doi: 10.1016/j.neuropharm.2018.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Diaz Heijtz R., Scott L., Forssberg H. Alteration of dopamine D1 receptor-mediated motor inhibition and stimulation during development in rats is associated with distinct patterns of c-fos mRNA expression in the frontal-striatal circuitry. Eur J Neurosci. 2004;19:945–956. doi: 10.1111/j.0953-816x.2004.03154.x. [DOI] [PubMed] [Google Scholar]
- 71.Choi S.J., Mukai J., Kvajo M., Xu B., Diamantopoulou A., Pitychoutis P.M., et al. A schizophrenia-related deletion leads to KCNQ2-dependent abnormal dopaminergic modulation of prefrontal cortical interneuron activity. Cereb Cortex. 2018;28:2175–2191. doi: 10.1093/cercor/bhx123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Porter L.L., Rizzo E., Hornung J.P. Dopamine affects parvalbumin expression during cortical development in vitro. J Neurosci. 1999;19:8990–9003. doi: 10.1523/JNEUROSCI.19-20-08990.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhu X., Cabungcal J.H., Cuenod M., Uliana D.L., Do K.Q., Grace A.A. Thalamic reticular nucleus impairments and abnormal prefrontal control of dopamine system in a developmental model of schizophrenia: Prevention by N-acetylcysteine. Mol Psychiatry. 2021;26:7679–7689. doi: 10.1038/s41380-021-01198-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Woodward E.M., Coutellier L. Age- and sex-specific effects of stress on parvalbumin interneurons in preclinical models: Relevance to sex differences in clinical neuropsychiatric and neurodevelopmental disorders. Neurosci Biobehav Rev. 2021;131:1228–1242. doi: 10.1016/j.neubiorev.2021.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Flores-Barrera E., Thomases D.R., Heng L.J., Cass D.K., Caballero A., Tseng K.Y. Late adolescent expression of GluN2B transmission in the prefrontal cortex is input-specific and requires postsynaptic protein kinase A and D1 dopamine receptor signaling. Biol Psychiatry. 2014;75:508–516. doi: 10.1016/j.biopsych.2013.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tseng K.Y., O'Donnell P. Post-pubertal emergence of prefrontal cortical up states induced by D1-NMDA co-activation. Cereb Cortex. 2005;15:49–57. doi: 10.1093/cercor/bhh107. [DOI] [PubMed] [Google Scholar]
- 77.Abi-Dargham A., Javitch J.A., Slifstein M., Anticevic A., Calkins M.E., Cho Y.T., et al. Dopamine D1R receptor stimulation as a mechanistic pro-cognitive target for schizophrenia. Schizophr Bull. 2022;48:199–210. doi: 10.1093/schbul/sbab095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ernst M., Luciana M. Neuroimaging of the dopamine/reward system in adolescent drug use. CNS Spectr. 2015;20:427–441. doi: 10.1017/S1092852915000395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Satterthwaite T.D., Ruparel K., Loughead J., Elliott M.A., Gerraty R.T., Calkins M.E., et al. Being right is its own reward: Load and performance related ventral striatum activation to correct responses during a working memory task in youth. Neuroimage. 2012;61:723–729. doi: 10.1016/j.neuroimage.2012.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.McCutcheon R.A., Abi-Dargham A., Howes O.D. Schizophrenia, dopamine and the striatum: From biology to symptoms. Trends Neurosci. 2019;42:205–220. doi: 10.1016/j.tins.2018.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Slifstein M., van de Giessen E., Van Snellenberg J., Thompson J.L., Narendran R., Gil R., et al. Deficits in prefrontal cortical and extrastriatal dopamine release in schizophrenia: A positron emission tomographic functional magnetic resonance imaging study. JAMA Psychiatry. 2015;72:316–324. doi: 10.1001/jamapsychiatry.2014.2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fusar-Poli P., Howes O.D., Allen P., Broome M., Valli I., Asselin M.C., et al. Abnormal prefrontal activation directly related to pre-synaptic striatal dopamine dysfunction in people at clinical high risk for psychosis. Mol Psychiatry. 2011;16:67–75. doi: 10.1038/mp.2009.108. [DOI] [PubMed] [Google Scholar]
- 83.Meyer-Lindenberg A., Miletich R.S., Kohn P.D., Esposito G., Carson R.E., Quarantelli M., et al. Reduced prefrontal activity predicts exaggerated striatal dopaminergic function in schizophrenia. Nat Neurosci. 2002;5:267–271. doi: 10.1038/nn804. [DOI] [PubMed] [Google Scholar]
- 84.Benoit-Marand M., O'Donnell P. D2 dopamine modulation of corticoaccumbens synaptic responses changes during adolescence. Eur J Neurosci. 2008;27:1364–1372. doi: 10.1111/j.1460-9568.2008.06107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Huppe-Gourgues F., O'Donnell P. Periadolescent changes of D(2) -AMPA interactions in the rat nucleus accumbens. Synapse. 2012;66:1–8. doi: 10.1002/syn.20976. [DOI] [PubMed] [Google Scholar]
- 86.Brenhouse H.C., Lukkes J.L., Andersen S.L. Early life adversity alters the developmental profiles of addiction-related prefrontal cortex circuitry. Brain Sci. 2013;3:143–158. doi: 10.3390/brainsci3010143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Howes O., Bose S., Turkheimer F., Valli I., Egerton A., Stahl D., et al. Progressive increase in striatal dopamine synthesis capacity as patients develop psychosis: A PET study. Mol Psychiatry. 2011;16:885–886. doi: 10.1038/mp.2011.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sonnenschein S.F., Grace A.A. Peripubertal mGluR2/3 agonist treatment prevents hippocampal dysfunction and dopamine system hyperactivity in adulthood in MAM model of schizophrenia. Schizophr Bull. 2021;47:1806–1814. doi: 10.1093/schbul/sbab047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhu X., Grace A.A. Prepubertal environmental enrichment prevents dopamine dysregulation and hippocampal hyperactivity in MAM schizophrenia model rats. Biol Psychiatry. 2021;89:298–307. doi: 10.1016/j.biopsych.2020.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Corcoran C., Walker E., Huot R., Mittal V., Tessner K., Kestler L., et al. The stress cascade and schizophrenia: Etiology and onset. Schizophr Bull. 2003;29:671–692. doi: 10.1093/oxfordjournals.schbul.a007038. [DOI] [PubMed] [Google Scholar]
- 91.Howes O.D., McCutcheon R., Owen M.J., Murray R.M. The role of genes, stress, and dopamine in the development of schizophrenia. Biol Psychiatry. 2017;81:9–20. doi: 10.1016/j.biopsych.2016.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gomes F.V., Zhu X., Grace A.A. The pathophysiological impact of stress on the dopamine system is dependent on the state of the critical period of vulnerability. Mol Psychiatry. 2020;25:3278–3291. doi: 10.1038/s41380-019-0514-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.O'Donnell P. Adolescent onset of cortical disinhibition in schizophrenia: Insights from animal models. Schizophr Bull. 2011;37:484–492. doi: 10.1093/schbul/sbr028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Anticevic A., Corlett P.R., Cole M.W., Savic A., Gancsos M., Tang Y., et al. N-methyl-D-aspartate receptor antagonist effects on prefrontal cortical connectivity better model early than chronic schizophrenia. Biol Psychiatry. 2015;77:569–580. doi: 10.1016/j.biopsych.2014.07.022. [DOI] [PubMed] [Google Scholar]
- 95.Anticevic A., Hu X., Xiao Y., Hu J., Li F., Bi F., et al. Early-course unmedicated schizophrenia patients exhibit elevated prefrontal connectivity associated with longitudinal change. J Neurosci. 2015;35:267–286. doi: 10.1523/JNEUROSCI.2310-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Merritt K., Egerton A., Kempton M.J., Taylor M.J., McGuire P.K. Nature of glutamate alterations in schizophrenia: A meta-analysis of proton magnetic resonance spectroscopy studies. JAMA Psychiatry. 2016;73:665–674. doi: 10.1001/jamapsychiatry.2016.0442. [DOI] [PubMed] [Google Scholar]
- 97.Castner S.A., Williams G.V. Tuning the engine of cognition: A focus on NMDA/D1 receptor interactions in prefrontal cortex. Brain Cogn. 2007;63:94–122. doi: 10.1016/j.bandc.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 98.Addington J., Stowkowy J., Liu L., Cadenhead K.S., Cannon T.D., Cornblatt B.A., et al. Clinical and functional characteristics of youth at clinical high-risk for psychosis who do not transition to psychosis. Psychol Med. 2019;49:1670–1677. doi: 10.1017/S0033291718002258. [DOI] [PubMed] [Google Scholar]
- 99.Cadenhead K.S., Mirzakhanian H. A case of attenuated psychosis syndrome: A broad differential diagnosis requires broad-spectrum treatment. Am J Psychiatry. 2016;173:321–329. doi: 10.1176/appi.ajp.2015.15060789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Woods S.W., Bearden C.E., Sabb F.W., Stone W.S., Torous J., Cornblatt B.A., et al. Counterpoint. Early intervention for psychosis risk syndromes: Minimizing risk and maximizing benefit. Schizophr Res. 2021;227:10–17. doi: 10.1016/j.schres.2020.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Loewy R.L., Pearson R., Vinogradov S., Bearden C.E., Cannon T.D. Psychosis risk screening with the Prodromal Questionnaire--brief version (PQ-B) Schizophr Res. 2011;129:42–46. doi: 10.1016/j.schres.2011.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Miller T.J., Cicchetti D., Markovich P.J., McGlashan T.H., Woods S.W. The SIPS screen: A brief self-report screen to detect the schizophrenia prodrome. Schizophr Res. 2004;70:78. [Google Scholar]
- 103.Kobayashi H., Nemoto T., Koshikawa H., Osono Y., Yamazawa R., Murakami M., et al. A self-reported instrument for prodromal symptoms of psychosis: Testing the clinical validity of the PRIME Screen-Revised (PS-R) in a Japanese population. Schizophr Res. 2008;106:356–362. doi: 10.1016/j.schres.2008.08.018. [DOI] [PubMed] [Google Scholar]
- 104.Yung A.R., Yuen H.P., McGorry P.D., Phillips L.J., Kelly D., Dell'Olio M., et al. Mapping the onset of psychosis: The Comprehensive Assessment of At-Risk Mental States. Aust N Z J Psychiatry. 2005;39:964–971. doi: 10.1080/j.1440-1614.2005.01714.x. [DOI] [PubMed] [Google Scholar]
- 105.First M.B., Williams J.B.W., Karg R.S., Spitzer R.L. American Psychiatric Association; Arlington, VA: 2015. Structured Clinical Interview for DSM-5, Research Version (SCID-5 for DSM-5, Research Version; SCID-5_RV) [Google Scholar]
- 106.Sheehan D.V., Lecrubier Y., Sheehan K.H., Amorim P., Janavs J., Weiller E., et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59 Suppl 20:22-33, quiz 34-57. [PubMed] [Google Scholar]
- 107.Early Psychosis Intervention Network Core Assessment Battery (CAB) https://nationalepinet.org/core-assessment-battery-cab/ Available at:
- 108.Posselt C.M., Albert N., Nordentoft M., Hjorthoj C. The Danish OPUS early intervention services for first-episode psychosis: A phase 4 prospective cohort study with comparison of randomized trial and real-world data. Am J Psychiatry. 2021;178:941–951. doi: 10.1176/appi.ajp.2021.20111596. [DOI] [PubMed] [Google Scholar]
- 109.Mirzakhanian H., Singh F., Cadenhead K.S. Biomarkers in psychosis: An approach to early identification and individualized treatment. Biomark Med. 2014;8:51–57. doi: 10.2217/bmm.13.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Clark S.R., Baune B.T., Schubert K.O., Lavoie S., Smesny S., Rice S.M., et al. Prediction of transition from ultra-high risk to first-episode psychosis using a probabilistic model combining history, clinical assessment and fatty-acid biomarkers. Transl Psychiatry. 2016;6:e897. doi: 10.1038/tp.2016.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Perkins D.O., Jeffries C.D., Addington J., Bearden C.E., Cadenhead K.S., Cannon T.D., et al. Towards a psychosis risk blood diagnostic for persons experiencing high-risk symptoms: Preliminary results from the NAPLS project. Schizophr Bull. 2015;41:419–428. doi: 10.1093/schbul/sbu099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Green M.F. What are the functional consequences of neurocognitive deficits in schizophrenia? American Journal of Psychiatry. 1996;153:321–330. doi: 10.1176/ajp.153.3.321. [DOI] [PubMed] [Google Scholar]
- 113.Bora E., Murray R.M. Meta-analysis of cognitive deficits in ultra-high risk to psychosis and first-episode psychosis: Do the cognitive deficits progress over, or after, the onset of psychosis? Schizophr Bull. 2014;40:744–755. doi: 10.1093/schbul/sbt085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fatouros-Bergman H., Cervenka S., Flyckt L., Edman G., Farde L. Meta-analysis of cognitive performance in drug-naive patients with schizophrenia. Schizophr Res. 2014;158:156–162. doi: 10.1016/j.schres.2014.06.034. [DOI] [PubMed] [Google Scholar]
- 115.Choi J.S., Park J.Y., Jung M.H., Jang J.H., Kang D.H., Jung W.H., et al. Phase-specific brain change of spatial working memory processing in genetic and ultra-high risk groups of schizophrenia. Schizophr Bull. 2012;38:1189–1199. doi: 10.1093/schbul/sbr038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wood S.J., Pantelis C., Proffitt T., Phillips L.J., Stuart G.W., Buchanan J.A., et al. Spatial working memory ability is a marker of risk-for-psychosis. Psychol Med. 2003;33:1239–1247. doi: 10.1017/s0033291703008067. [DOI] [PubMed] [Google Scholar]
- 117.Bora E. A comparative meta-analysis of neurocognition in first-degree relatives of patients with schizophrenia and bipolar disorder. Eur Psychiatry. 2017;45:121–128. doi: 10.1016/j.eurpsy.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 118.Snitz B.E., MacDonald A.W., 3rd, Carter C.S. Cognitive deficits in unaffected first-degree relatives of schizophrenia patients: A meta-analytic review of putative endophenotypes. Schizophr Bull. 2006;32:179–194. doi: 10.1093/schbul/sbi048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.De Herdt A., Wampers M., Vancampfort D., De Hert M., Vanhees L., Demunter H., et al. Neurocognition in clinical high risk young adults who did or did not convert to a first schizophrenic psychosis: A meta-analysis. Schizophr Res. 2013;149:48–55. doi: 10.1016/j.schres.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 120.National Institutes of Health Acclerated Medicines Partnership. Schizophrenia. https://www.nih.gov/research-training/accelerating-medicines-partnership-amp/schizophrenia Available at:
- 121.Early Psychosis Intervention Network. https://nationalepinet.org Available at:
- 122.Kambeitz-Ilankovic L., Meisenzahl E.M., Cabral C., von Saldern S., Kambeitz J., Falkai P., et al. Prediction of outcome in the psychosis prodrome using neuroanatomical pattern classification. Schizophr Res. 2016;173:159–165. doi: 10.1016/j.schres.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 123.Collin G., Nieto-Castanon A., Shenton M.E., Pasternak O., Kelly S., Keshavan M.S., et al. Brain functional connectivity data enhance prediction of clinical outcome in youth at risk for psychosis. Neuroimage Clin. 2020;26 doi: 10.1016/j.nicl.2019.102108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Egerton A., Murphy A., Donocik J., Anton A., Barker G.J., Collier T., et al. Dopamine and glutamate in antipsychotic-responsive compared with antipsychotic-nonresponsive psychosis: A multicenter positron emission tomography and magnetic resonance spectroscopy study (STRATA) Schizophr Bull. 2021;47:505–516. doi: 10.1093/schbul/sbaa128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Reyes-Madrigal F., Guma E., Leon-Ortiz P., Gomez-Cruz G., Mora-Duran R., Graff-Guerrero A., et al. Striatal glutamate, subcortical structure and clinical response to first-line treatment in first-episode psychosis patients. Prog Neuropsychopharmacol Biol Psychiatry. 2022;113 doi: 10.1016/j.pnpbp.2021.110473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Naatanen R., Todd J., Schall U. Mismatch negativity (MMN) as biomarker predicting psychosis in clinically at-risk individuals. Biol Psychol. 2016;116:36–40. doi: 10.1016/j.biopsycho.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 127.Enache D., Nikkheslat N., Fathalla D., Morgan B.P., Lewis S., Drake R., et al. Peripheral immune markers and antipsychotic non-response in psychosis. Schizophr Res. 2021;230:1–8. doi: 10.1016/j.schres.2020.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kose M., Pariante C.M., Dazzan P., Mondelli V. The role of peripheral inflammation in clinical outcome and brain imaging abnormalities in psychosis: A systematic review. Front Psychiatry. 2021;12 doi: 10.3389/fpsyt.2021.612471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Fournier M., Scolamiero M., Gholam-Rezaee M.M., Cleusix M., Jenni R., Ferrari C., et al. Topology predicts long-term functional outcome in early psychosis. Mol Psychiatry. 2021;26:5335–5346. doi: 10.1038/s41380-020-0826-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wenneberg C., Glenthoj B.Y., Glenthoj L.B., Fagerlund B., Krakauer K., Kristensen T.D., et al. Baseline measures of cerebral glutamate and GABA levels in individuals at ultrahigh risk for psychosis: Implications for clinical outcome after 12 months. Eur Psychiatry. 2020;63:e83. doi: 10.1192/j.eurpsy.2020.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Insel T.R. Digital phenotyping: Technology for a new science of behavior. JAMA. 2017;318:1215–1216. doi: 10.1001/jama.2017.11295. [DOI] [PubMed] [Google Scholar]
- 132.Benoit J., Onyeaka H., Keshavan M., Torous J. Systematic review of digital phenotyping and machine learning in psychosis spectrum illnesses. Harv Rev Psychiatry. 2020;28:296–304. doi: 10.1097/HRP.0000000000000268. [DOI] [PubMed] [Google Scholar]
- 133.Wee Z.Y., Yong S.W.L., Chew Q.H., Guan C., Lee T.S., Sim K. Actigraphy studies and clinical and biobehavioural correlates in schizophrenia: A systematic review. J Neural Transm (Vienna) 2019;126:531–558. doi: 10.1007/s00702-019-01993-2. [DOI] [PubMed] [Google Scholar]
- 134.Mote J., Fulford D. Ecological momentary assessment of everyday social experiences of people with schizophrenia: A systematic review. Schizophr Res. 2020;216:56–68. doi: 10.1016/j.schres.2019.10.021. [DOI] [PubMed] [Google Scholar]
- 135.Barron H., Hafizi S., Andreazza A.C., Mizrahi R. Neuroinflammation and oxidative stress in psychosis and psychosis risk. Int J Mol Sci. 2017;18:651. doi: 10.3390/ijms18030651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zai G., Robbins T.W., Sahakian B.J., Kennedy J.L. A review of molecular genetic studies of neurocognitive deficits in schizophrenia. Neurosci Biobehav Rev. 2017;72:50–67. doi: 10.1016/j.neubiorev.2016.10.024. [DOI] [PubMed] [Google Scholar]
- 137.van Schaik R.H.N., Muller D.J., Serretti A., Ingelman-Sundberg M. Pharmacogenetics in psychiatry: An update on clinical usability. Front Pharmacol. 2020;11 doi: 10.3389/fphar.2020.575540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Nuechterlein K.H., Green M.F., Kern R.S., Baade L.E., Barch D.M., Cohen J.D., et al. The MATRICS Consensus Cognitive Battery, part 1: Test selection, reliability, and validity. Am J Psychiatry. 2008;165:203–213. doi: 10.1176/appi.ajp.2007.07010042. [DOI] [PubMed] [Google Scholar]
- 139.Hamilton C.M., Strader L.C., Pratt J.G., Maiese D., Hendershot T., Kwok R.K., et al. The PhenX Toolkit: Get the most from your measures. Am J Epidemiol. 2011;174:253–260. doi: 10.1093/aje/kwr193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gur R.C., Richard J., Hughett P., Calkins M.E., Macy L., Bilker W.B., et al. A cognitive neuroscience-based computerized battery for efficient measurement of individual differences: Standardization and initial construct validation. J Neurosci Methods. 2010;187:254–262. doi: 10.1016/j.jneumeth.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Light G.A., Swerdlow N.R. Future clinical uses of neurophysiological biomarkers to predict and monitor treatment response for schizophrenia. Ann N Y Acad Sci. 2015;1344:105–119. doi: 10.1111/nyas.12730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.O'Donnell B.F., Vohs J.L., Krishnan G.P., Rass O., Hetrick W.P., Morzorati S.L. The auditory steady-state response (ASSR): A translational biomarker for schizophrenia. Suppl Clin Neurophysiol. 2013;62:101–112. doi: 10.1016/b978-0-7020-5307-8.00006-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Murphy M., Ongur D. EEG microstate sequences suggest abnormally chaotic brain dynamics in psychosis. Neuropsychopharmacology. 2021;46:223–224. doi: 10.1038/s41386-020-00800-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zhang T., Xu L., Tang X., Wei Y., Hu Q., Hu Y., et al. Real-world effectiveness of antipsychotic treatment in psychosis prevention in a 3-year cohort of 517 individuals at clinical high risk from the SHARP (ShangHai At Risk for Psychosis) Aust N Z J Psychiatry. 2020;54:696–706. doi: 10.1177/0004867420917449. [DOI] [PubMed] [Google Scholar]
- 145.Olney J.W., Newcomer J.W., Farber N.B. NMDA receptor hypofunction model of schizophrenia. J Psychiatr Res. 1999;33:523–533. doi: 10.1016/s0022-3956(99)00029-1. [DOI] [PubMed] [Google Scholar]
- 146.Lieberman J.A., Small S.A., Girgis R.R. Early detection and preventive intervention in schizophrenia: From fantasy to reality. Am J Psychiatry. 2019;176:794–810. doi: 10.1176/appi.ajp.2019.19080865. [DOI] [PubMed] [Google Scholar]
- 147.Stansley B.J., Conn P.J. The therapeutic potential of metabotropic glutamate receptor modulation for schizophrenia. Curr Opin Pharmacol. 2018;38:31–36. doi: 10.1016/j.coph.2018.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]