Abstract
Schizophrenia (SCH) is a complex and severe mental disorder with high prevalence, disability, mortality and carries a heavy disease burden, the lifetime prevalence of SCH is around 0.7%–1.0%, which has a profound impact on the individual and society. In the clinical practice of SCH, key problems such as subjective diagnosis, experiential treatment, and poor overall prognosis are still challenging. In recent years, some exciting discoveries have been made in the research on objective biomarkers of SCH, mainly focusing on genetic susceptibility genes, metabolic indicators, immune indices, brain imaging, electrophysiological characteristics. This review aims to summarize the biomarkers that may be used for the prediction and diagnosis of SCH.
Keywords: accurate diagnosis, multi-omics data, objective biomarkers, personalized treatment, schizophrenia
Introduction
Schizophrenia (SCH) is a complex and severe mental disorder characterized by diverse psychopathology, the core clinical features are positive symptoms (such as hallucinations and delusions), negative symptoms (impaired motivation, reduction in spontaneous speech, and social withdrawal), and cognitive impairment (perform poorly over a wide range of cognitive functions) [1, 2]. It has a high prevalence, disability, mortality and carries a heavy disease burden, the lifetime prevalence of SCH is around 0.7%–1.0%, which has a profound impact on the individual and society [3, 4]. There are over 50% of patients with SCH having long-term psychiatric problems, which leads to chronic symptoms and disability [5]. Moreover, the unemployment rate is high at 80%–90% and the life expectancy is reduced by 10–20 years in patients with SCH [6].
Despite numerous studies on SCH have been conducted, the etiology and pathogenesis of SCH remain unknown, in which genetic and environmental factors may play a key role. Dysfunction of neurotransmission (for instance, dopaminergic, serotoninergic and glutamatergic neurotransmission) appears to contribute to the genesis of psychotic symptoms, but the evidence also points to a more widespread and variable involvement of other central nervous system and peripheral system, such as neurotrophic factors, immune system, neuroendocrine system and epigenetics.
The question that “What causes schizophrenia?” was twice included in the list of the 125 most advanced scientific questions published by Science Magazine in 2005 and 2021 separately (https://www.sciencemag.org/collections/125-questions-exploration-and-discovery). In the clinical practice of SCH, key problems such as subjective diagnosis, experiential treatment, and poor overall prognosis are still challenging [1, 2]. Due to the unknown mechanism of SCH, no effective, specific and objective biomarkers have been found, which seriously hinders the development of precise diagnosis and treatment, making it a global health, social and key scientific problem to be overcome [7]. Although many challenges remain, the past decade has occurred substantial advances in the application of genomics, epidemiology and neuroscience to SCH.
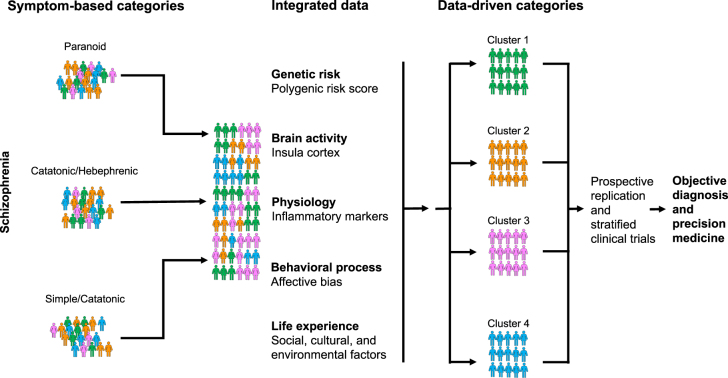
The current clinical classifications of SCH subtypes (such as paranoid, catatonic, hebephrenic, simple and undifferentiated subtypes; or defect and non-defect subtype, etc.) are mainly based on doctors’ experience and symptom description. However, as a complex disorder with highly heterogeneous clinical phenotype, such classifications of SCH are difficult to address clinical problems such as subjective diagnosis and experiential treatment. Professor Thomas Insel, former director of National Institute of National Health (NIMH), suggested that breaking down classification system based on clinical symptoms and developing a data-driven biological subtypes classification, might improve the status quo of precise diagnosis and treatment for psychiatric disorders that lack of biomarkers [8]. Utilizing the multi-dimensional longitudinal data of clinical-gene-environment-brain imaging combined with drug efficacy, it is expected to clarify the pathogenesis of SCH, discover potential biomarkers, and construct biological subtype helpful for objective and objective diagnosis and precise treatment of SCH [8, 9] (Figure 1).
Figure 1:
Data-driven biological subtype classification breaks the current clinical symptom-based classification and contributes to objective diagnosis and precision medicine (partly adapted from Insel et al. [8]).
The term “biomarker” refers to any measurable biological characteristics which can act as an indicator of disease progression, diagnosis and prognosis [10], [11], [12]. In a narrow sense, a biomarker refers to a molecular change in body tissues and fluids that can be used as a clinical indicator [13]. In a wide sense, biomarkers also refer to endophenotypes, which are associated with the illness, heritable, state independent, cosegregates within families, and is found in unaffected family members at a higher rate than expected in the general population [14]. In recent years, some exciting discoveries have been made in the research on objective biomarkers of SCH, mainly focusing on genetic susceptibility genes, biochemical indicators, brain imaging, electrophysiological characteristics (such as mismatched negative wave or eye movement trajectory, abnormal retinal function, etc.), epigenetic and transcriptional and gene environment interaction. In general, these biomarkers can be classified into three categories: diagnostic, prognostic, and theragnostic [13]. We mainly review the diagnostic biomarkers of SCH in this article, which help to identify whether a person has SCH and can ideally help differentiate SCH with other diseases.
Methods
We searched in PubMed, Embase and Cochrane Library for publications with following keywords: “schizophrenia”, “inflammation”, “neuroimmune”, “neurotransmitter”, “metabolic”, “neuroimage”, “genetic”, “epigenetic”, “transcriptome”, “single cell”, “stem cell”, “electrophysiological”, and “gut microbiota”.
Furthermore, we also acquired the information form some publicly available database, like the Psychiatric Genomics Consortium (PGC: https://www.med.unc.edu/pgc/), the Schizophrenia Spectrum Biomarkers Consortium (https://ssbcbio.org/history_and_objectives.html), Human Connectome Project (http://www.humanconnectome.org/), 1000 Functional Connectomes Project (http://fcon_1000.projects.nitrc.org/fcpClassic/FcpTable.html), OpenfMRI database (https://openfmri.org/), Database for Emotion Analysis using Physiological Signals (http://www.eecs.qmul.ac.uk/mmv/datasets/deap/), UK Biobank (https://www.ukbiobank.ac.uk/), BrainMap (http://www.brainmap.org/) and PhysioNet (https://physionet.org)
Research on neuroimmune level of SCH biomarkers
Immune system has been found to play an important role in the pathogenesis of SCH, and many evidence suggest that there is a close relationship between immune dysfunction and SCH [15]. Epidemiological studies on risk factors of SCH have found that exposure to early-life infections, including infections during prenatal life and childhood, are associated with increased risk of SCH [16], [17], [18]. Studies also suggests that SCH shares some clinical, epidemiological and genetic features with certain autoimmune diseases [19, 20]. Moreover, analyses using mendelian randomization (MR) indicated that inflammatory markers such as C-reaction protein (CRP) and cytokines have a potential causality to SCH [21, 22]. These evidence supports a putative “immunophenotype” of SCH [23], and suggests the feasibility of using neuroimmune biomarkers, which account for more than 70% of the potential biomarkers for SCH [12], in the diagnosis of SCH (Table 1).
Table 1:
Neuroimmune biomarkers in schizophrenia.
| Category | Biomarkers | Participants | Conclusions | Diagnostic aspects | Reference |
|---|---|---|---|---|---|
| Neuroimmune biomarkers | |||||
| CNS | |||||
| Cytokines | IL-6 | Meta analysis (8 studies and 1 unpublished dataset, number of participants not provided) | SCH patients showed significantly increased IL-6 levels in CSF, and IL-6 levels were higher in early stage SCH patients compared to chronic SCH patients. | Diagnostic biomarkers: the risk and stage of SCH | [28] |
| IL-8 | Meta analysis (3 studies and 1 unpublished dataset, number of participants not provided) | SCH patients showed significantly increased IL-8 levels in CSF. | Diagnostic biomarkers: the risk of SCH | [28] | |
| Peripheral | |||||
| CRP | CRP | Meta analysis (26 studies including 80,967 controls and 1,995 SCH patients) | CRP levels were moderately increased in persons with SCH regardless of the use of antipsychotics and did not change between the first episode of psychosis and with progression of SCH. CRP levels are positively related to the severity of positive symptoms and BMI, and negatively related to age. | Diagnostic biomarkers: the risk of SCH and severity of symptoms | [31] |
| Meta analysis (73 studies, including 6,112 patients) | CRP were significantly correlated with multiple domains of psychopathology | Diagnostic biomarkers: severity of symptoms | [32] | ||
| Cytokines | IFN-γ | Meta analysis (7 studies including 747 controls and 452 SCH patients) | IFN-γ levels were significantly increased in acutely ill patients with SCH, but decreased in chronically ill patients. | Diagnostic biomarkers: the risk and stage of SCH | [33] |
| IL-1β | Meta analysis (6 studies including 298 controls and 333 SCH patients) | IL-1β levels were significantly increased in both acutely and chronically ill patients with SCH, and significantly decreased following treatment. | Diagnostic biomarkers: the risk and stage of SCH | [33] | |
| Meta analysis (23 studies including 683 controls and 570 medication-naive first episode psychosis) | IL-1β levels were significantly increased in medication-naïve first episode psychosis patients | Diagnostic biomarkers: the risk of SCH | [36] | ||
| IL-1RA | Meta analysis (2 studies including 376 controls and 194 SCH patients) | IL-1RA levels were significantly increased in acutely ill patients with SCH. | Diagnostic biomarkers: the risk and stage of SCH | [33] | |
| IL-4 | Meta analysis (4 studies including 322 controls and 193 SCH patients) | IL-4 levels were significantly decreased in acutely ill patients with SCH, and significantly decreased following treatment. | Diagnostic biomarkers: the risk and stage of SCH | [33] | |
| IL-6 | Meta analysis (11 studies including 577 controls and 506 SCH patients) | IL-6 levels were significantly increased in both acutely and chronically ill patients with SCH, and significantly decreased following treatment. | Diagnostic biomarkers: the risk and stage of SCH | [33] | |
| Meta analysis (73 studies, including 6,112 patients) | Following antipsychotic treatment, changes in IL-6 levels were significantly correlated with changes in total psychopathology. | Diagnostic biomarkers: the stage of SCH and severity of symptoms | [32] | ||
| Meta analysis (23 studies including 683 controls and 570 medication-naive first episode psychosis) | IL-6 levels were significantly increased in medication-naïve first episode psychosis patients | Diagnostic biomarkers: the risk of SCH | [36] | ||
| Meta analysis (59 studies including 2,806 controls and 3,002 patients with first-episode psychosis) | IL-6 levels were significantly increased in first episode psychosis patients | [34] | |||
| IL-8 | Meta analysis (2 studies including 49 controls and 49 SCH patients) | IL-8 levels were significantly increased in acutely ill patients with SCH. | Diagnostic biomarkers: the risk and stage of SCH | [33] | |
| IL-10 | Meta analysis (4 studies including 461 controls and 357 SCH patients) | IL-10 levels were significantly increased in first-episode SCH vs. controls, but decreased in acutely ill patients with chronic SCH. | Diagnostic biomarkers: the risk and stage of SCH | [33] | |
| IL-12 | Meta analysis (3 studies including 463 controls and 258 SCH patients) | IL-12 levels were significantly increased in acutely ill patients with chronic SCH, and increased following treatment. | Diagnostic biomarkers: the risk and stage of SCH | [33] | |
| sIL-2R | Meta analysis (3 studies including 97 controls and 30 SCH patients) | sIL-2R levels were significantly increased in both acutely and chronically ill patients with SCH, and significantly increased following treatment. | Diagnostic biomarkers: the risk and stage of SCH | [33] | |
| Meta analysis (23 studies including 683 controls and 570 medication-naive first episode psychosis) | sIL-2R levels were significantly increased in medication-naïve first episode psychosis patients | Diagnostic biomarkers: the risk of SCH | [36] | ||
| TGF-β | Meta analysis (3 studies including 298 controls and 169 SCH patients) | TGF-β levels were significantly increased in acutely ill patients with SCH. | Diagnostic biomarkers: the risk and stage of SCH | [33] | |
| TNF-α | Meta analysis (9 studies including 842 controls and 587 SCH patients) | TNF-α levels were significantly increased in both acutely and chronically ill patients with SCH | Diagnostic biomarkers: the risk of SCH | [33] | |
| Meta analysis (23 studies including 683 controls and 570 medication-naive first episode psychosis) | TNF-α levels were significantly increased in medication-naïve first episode psychosis patients | Diagnostic biomarkers: the risk of SCH | [36] | ||
| Meta analysis (59 studies including 2,806 controls and 3,002 patients with first-episode psychosis) | TNF-α levels were significantly increased in first episode psychosis patients | [34] |
SCH, schizophrenia; CNS, central nervous system; CSF, cerebrospinal fluid; IL, interleukin; CRP, C-reaction protein; IFN-γ, interferon gamma; IL-1RA, interleukin-1 receptor antagonist; sIL-2R, soluble interleukin-2 receptor; TGF-β, transforming growth factor beta; TNF-α, tumor necrosis factor alpha.
Central nervous system (CNS) markers
Results of CNS neuroimmune biomarkers mainly come from studies on postmortem brains and cerebrospinal fluid (CSF). Studies on post-mortem brain tissue have shown alteration of neuroimmune-related markers in different brain regions, including prefrontal cortices, temporal cortices and the hippocampus of SCH patients [24], [25], [26]. For example, density of microglial cells, the macrophages in the brain, and their marker human leukocyte antigen-DR isotype (HLA-DR), were found to be higher in post-mortem SCH brains, in particular in those patients who committed suicide [24, 27]. In line with these findings, studies on CSF also found alternations of immune-related markers. A meta-analysis of CSF cytokines showed statistically significant increases in interleukin-6 (IL-6) and IL-8 in patients with SCH. Moreover, IL-6 levels were higher in early stage SCH patients compared to chronic SCH patients [28]. In addition, the Schizophrenia Spectrum Biomarkers Consortium (SSBC), established in 2018, have collected several CSF samples to mainly identify fluid biomarkers in schizophrenia and related disorders in a clinical research study starting in 2021. With the hypotheses of complement factor 4 (C4) associated with schizophrenia risk, measuring complement proteins in CSF have become the closest way of assessing their involvement in relevant biological processes in the brain.
Peripheral markers
As the usefulness of CNS biomarkers can be limited by their accessibility, many studies have focus on peripheral immune-related markers of SCH. Studies have suggested that peripheral immune activation may induce immune alterations in the CNS by crossing the blood–brain barrier in a subgroup of patients. Through the hypothalamic-pituitary-adrenal [29] axis, peripheral cytokines that cross the blood–brain barrier can affect brain function, precipitating changes in mood, behavior and cognition.
The majority of studies on peripheral markers have focused on C-reactive protein (CRP) and cytokine changes in serum of SCH patients. CRP is an acute-phase protein that is synthesized in the liver in response to cytokine induction, particularly IL-6 [30]. It is an attractive putative biomarker, as it can be readily assayed in most conventional laboratories in clinical settings. Meta-analyses have found an increased peripheral CRP concentrations in patients with SCH [31], with a positive correlation to the total psychopathology and negative symptoms of the disorder [32].
Cytokines are inflammatory signaling molecules that help coordinate the function of both the innate and the adaptive immune systems, and are involved with a host of physiological processes throughout the body. In a meta-analysis of first-episode psychosis (FEP) and chronic individuals with psychosis, a large number of inflammatory markers were found to be elevated in SCH, including interferon gamma (IFN-γ), IL-1β, IL-1 receptor antagonist (IL-1RA), IL-6, IL-8, IL-10, IL-12, soluble IL-2 receptor (sIL-2R), transforming growth factor beta (TGF-β), and tumor necrosis factor alpha (TNF-α), while levels of IL-4 were significantly decreased [33]. However, in a more recent meta-analysis including patients with a broader definition of FEP, only IL-6 and TNF-α were found to be significantly increased [34]. The reason for the inconsistent results may due to the use of antipsychotics, which have been shown to affect immune response [35]. In a meta-analysis of studies that only included antipsychotic-naive individuals with FEP, IL-1β, sIL-2R, IL-6, and TNF-α were found to be elevated compared to healthy controls [36]. Several of these cytokines are also found to be associated with psychopathology of SCH. In FEP, changes in IL-6 levels after antipsychotic treatment, were significantly correlated with total psychopathology of SCH [32].
Taken together, these findings suggest the potential usage of neuroimmune biomarkers in both CNS and peripheral settings as a diagnostic marker of SCH. However, as mentioned above, these neuroimmune-related markers can be affected by numerous factors including antipsychotic medication [37], and almost all neuronal disorders can demonstrate changes in inflammatory biomarkers. Neuroimmune markers with a higher specificity to the disease are yet to be established.
Research on metabolic levels of SCH biomarkers
As a heterogeneous disease, many abnormal metabolites of multiple biochemical pathways have been found to be involved in the pathophysiological mechanisms of schizophrenia. Although the specific association between these abnormal metabolites and the pathophysiological mechanism of the disease remains unclear, a large number of studies have suggested that metabolites as potential diagnostic biomarkers of SCH. Here we selectively review some of the metabolites which have a potential to be diagnostic biomarkers for SCH (Table 2).
Table 2:
Metabolic biomarkers in schizophrenia.
| Category | Biomarkers | Participants | Conclusions | Diagnostic aspects | Reference |
|---|---|---|---|---|---|
| Metabolic biomarkers | |||||
| Neurotransmitters-related metabolites | HVA | 37 SCH patients and 65 controls | In both sexes, the CSF HVA levels were significantly lower in the SCH patients, and a sex difference was observed with higher concentrations in the females. | Diagnostic biomarkers: the risk of SCH | [247] |
| 22 drug-free SCH inpatients and 33 controls | The CSF HVA levels did not differ significantly between patients and normal controls, but negatively correlated with ratings of psychosis and positive symptoms, as well as individual deficit symptoms. | Diagnostic biomarkers: the risk of SCH and severity of symptoms | [48] | ||
| MHPG | 37 SCH patients and 65 controls | In both sexes, the CSF HMPG levels were significantly higher in the schizophrenic patients. | Diagnostic biomarkers: the risk of SCH | [247] | |
| 22 drug-free SCH inpatients and 33 controls | Levels of CSF MHPG were significantly elevated in the SCH patients compared with controls, and plasma MHPG levels were positively correlated with negative symptoms. | Diagnostic biomarkers: the risk of SCH and severity of symptoms | [48] | ||
| KYNA | 16 male patients with SCH on olanzapine treatment and 29 male controls | CSF KYNA concentrations were higher in patients with SCH. | Diagnostic biomarkers: the risk of SCH | [54] | |
| 23 SCH patients and 37 controls | The CSF levels of KYNA were increased in patients compared with controls. | Diagnostic biomarkers: the risk of SCH | [55] | ||
| Glu | 20 SCH patients and 44 controls | The CSF levels of Glu were decreased in patients compared with controls. In the serum, there was no significant difference between the SCH and the controls. | Diagnostic biomarkers: the risk of SCH | [57] | |
| 32 SCH patients and 35 controls | Serum Glu concentrations were significantly increased in the male SCH patients. | Diagnostic biomarkers: the risk of SCH | [60] | ||
| 56 first-episode psychosis and 50 controls | At the first psychotic episode and 1 month after the first psychotic episode, a significant decrease (15%–25%) in plasma Glu levels was found in patients with SCH. Glu levels progressively increased towards control values during the 1-year follow up after the first episode. | Diagnostic biomarkers: the risk and stage of SCH | [62] | ||
| Gln | 216 healthy controls and 265 SCH patients | Serum Glu concentrations were significantly decreased in the male SCH patients. | Diagnostic biomarkers: the risk of SCH | [63] | |
| Gln/Glu | 32 patients with recent onset, 123 patients with chronic SCH and 128 controls | Compared with healthy controls, patients with recent onset schizophrenia showed increased Gln/Glu ratio, while patients with chronic schizophrenia showed decreased Gln/Glu ratio. | Diagnostic biomarkers: the risk and stage of SCH | [64] | |
| Fatty acids | PUFAs | 30 patients with schizophrenia or schizoaffective disorder | Reduced blood omega-3 fatty acids were associated with cognitive impairment. | Diagnostic biomarkers: the severity of symptoms | [69] |
| Not applicable | Mendelian randomization analyses indicated that long-chain omega-3 and long-chain omega-6 fatty acid concentrations were associated with a lower risk of schizophrenia. There was weak evidence that short-chain omega-3 and short-chain omega-6 fatty acids were associated with an increased risk of schizophrenia. | Diagnostic biomarkers: the risk of SCH | [70] | ||
| NASs | Cortisol | Meta analysis (26 studies including 959 individuals with first-episode psychosis and 1121 controls) | Elevated blood cortisol levels were found in individuals with first-episode psychosis. | Diagnostic biomarkers: the risk of SCH | [73] |
| Pregnenolone and pregnenolone sulfate | 22 drug-naive (first episode) SCH patients and 47 controls | In both sexes, lower levels of pregnenolone and higher levels of pregnenolone sulfate were found in patients. | Diagnostic biomarkers: the risk of SCH | [74] | |
| DHEA-S | 32 women with SCH and 32 controls | There were statistically significantly higher levels of serum DHEA-S in the study group than in the control group, and a positive correlation was determined between the negative symptoms scores and DHEA-S levels. | Diagnostic biomarkers: the risk of SCH and severity of symptoms | [78] | |
| Neurotrophins | BDNF | 88 never-medicated first-episode SCH patients and 90 healthy controls | BDNF levels were significantly lower in first-episode patients with SCH than in healthy control subjects. A significant positive correlation between BDNF levels and PANSS positive subscore was observed. Furthermore, higher BDNF levels were observed in patients with paranoid subtype of schizophrenia. | Diagnostic biomarkers: the risk of SCH and severity of symptoms | [78] |
| Meta analysis (21 studies including 2449 patients with schizophrenia-spectrum disorders) | BDNF levels were very modestly but significantly related to cognitive functioning in schizophrenia, including verbal memory, working memory, processing speed and verbal fluency performances. | Diagnostic biomarkers: severity of symptoms |
SCH, schizophrenia; HVA, homovanillic acid; CSF, cerebrospinal fluid; MHPG, 3-methoxy-4-hydroxyphenylglycol; KYNA, Kynurenic acid; Glu, glutamate; Gln, glutamine; PUFAs, polyunsaturated fatty acids; NASs, neuroactive steroids; DHEA, dehydroepiandrosterone; DHEA-S, dehydroepiandrosterone sulfate; BDNF, brain derived neurotrophic factor; PANSS, Positive and Negative Syndrome Scale.
Neurotransmitters-related metabolites
Neurotransmitter systems such as dopamine (DA), 5-hydroxytryptamine (5-HT), norepinephrine (NE), glutamate (Glu) and their related metabolites have been found to involved in the pathophysiology of SCH [38], [39], [40]. The classic dopamine hypothesis suggests that the imbalance of dopamine and the dysregulation of dopamine receptor activity in the brain is one of the causes of schizophrenia [41]. Further research suggests that 5-HT, NE and Glu systems can result in hyperactivity of the downstream mesolimbic dopamine pathway [42, 43]. Although full understandings of their interaction on the pathogenesis of SCH remains unclear, a growing body of evidence has revealed the potential diagnostic value of these biological substances to serve as biomarkers for SCH in some degree.
Homovanillic acid (HVA) is the main metabolite of dopamine, and its concentration can reflect the activity of dopamine system in the brain. Most studies have found that the level of HVA in the CSF increases in patients with SCH [44, 45], but some studies also report a reduction of HVA level in the CSF [46, 47]. 3-methoxy-4-hydroxyphenylglycol (MHPG) is a major metabolite of NE. Consistent results with an increase level in CSF and plasma have been found in SCH patients [46, 48, 49]. In addition, both HVA and MHPG were found to associate with treatment response in SCH [50, 51].
Kynurenic acid (KYNA) is the metabolite of tryptophan and plays a role as an antagonist at N-methyl-D-aspartate (NMDA) and nicotinic receptors in the brain [52]. Preclinical studies have shown an increased KYNA levels in the CSF [53], and post-mortem studies found increased KYNA in the prefrontal cortex of patients with SCH [54, 55]. KYNA was found to be positive correlated with IL-1β level in patients with SCH, and there was also positive correlation between KYNA and the severity of clinical symptoms [56].
Glutamate is a major neurotransmitter in the brain, synthesized from glucose through the tricarboxylic cycle and glutamine (Gln). Decreased Glu levels in CSF [57] and brain [58], while increased levels of Gln in the cortex [59], were found in SCH patients compared to healthy controls. However, the results for peripheral Glu and Gln levels were controversial. For example, previous studies have shown an increased level of peripheral Glu level in patients with chronic SCH [60], but not in acute SCH patients [61]. However, another study showed a lower peripheral level of Glu in FEP [62]. Decreased Gln levels were reported in the plasma of SCH patients [63]. The new idea is that circulating Glu/Glu levels are dynamic in the course of schizophrenia, with an increase of Gln/Glu ratio at the onset of SCH, and a decrease of the ratio as the disease progresses [64]. The levels of Glu, Gln and their ratio in CNS and periphery vary significantly between healthy controls and SCH patients, as well as during the progression of disease, indicating that they are potential diagnostic biomarkers of glutamatergic dysfunction in SCH [65].
Fatty acids
Fatty acids are a component of phospholipids, which are essential for lipids and cell membranes [66]. Polyunsaturated fatty acids (PUFAs) are dietary components that are crucial for the structural and functional integrity of neural cells. Deficiency of PUFAs, particularly omega-3 PUFAs, has been shown to be a risk factor for SCH [67], [68], [69]. Recent mendelian randomization analyses indicated that long-chain omega-3 and long-chain omega-6 fatty acid concentrations were associated with a lower risk of schizophrenia [70]. A recent systematic review on metabolic biomarkers of SCH have demonstrated a number of fatty acid metabolites related to SCH [71]. However, of the fatty acids reported in more than one paper, only arachidonic acid had consistent findings across all studies with significantly decreased levels reported in SCH patients compared to controls [71]. These findings suggest that reduced PUFAs, especially long-chain omega-3, long-chain omega-6 fatty acid and arachidonic acid, could be potential diagnostic biomarkers for SCH. However, investigations into the levels of PUFAs and their metabolites in CSF are yet to be conducted.
Neuroactive steroids (NASs) and steroid derivatives
Neuroactive steroids (NASs) are types of steroids that have impacts on behavioral actions, neuron excitability and neurotransmitter receptors [72]. NASs are thought to associate with pathophysiology of SCH as they can affect neurotransmitter systems. Altered circulating NASs and their related metabolites, particularly cortisol and dehydroepiandrosterone (DHEA), were found in some patients with SCH. A recent meta-analysis found that blood cortisol levels were significantly increased in individuals with FEP compared to controls with a small-to-medium effect size [73]. As for steroid derivatives, significantly decreased levels of circulating pregnenolone in drug-naive SCH patients were reported in previous study, despite the levels of its sulfate were increased [74]. The increase of pregnenolone sulfate and cortisol reflects the hyperactivity of HPA [29] axis in FEP [75]. DHEA and its sulfated metabolite, dehydroepiandrosterone sulfate (DHEA-S), are negative γ-Aminobutyric acid type A (GABAA) receptor or positive NMDA receptor modulators [76, 77]. Recent study has found an increase of serum DHEA-S levels in women SCH patients, but not serum testosterone and cortisol levels [78]. Another study has reported increased levels of cortisol/DHEA and cortisol/DHEA-S ratios as a potential biomarker of stress processing dysfunction in SCH [79]. Taken together, these findings suggest that NASs have a potential value as diagnostic markers of SCH.
Neurotrophic biomarkers
Neurotrophins are a large family of dimeric polypeptides. They are produced by nerve-innervated tissues (such as muscle) and astrocytes, and can promote the growth, differentiation and maturation of neurons in CNS and peripheral nervous systems, as well as promote survival of neurons in response to stress [80]. Abnormalities in neurotrophic molecules, particularly brain derived neurotrophic factor (BDNF), are important candidates for potential biomarkers of SCH, especially for cognitive impairment of SCH [81].
BDNF is the abundant neurotrophin factor in the body, and the most widely distributed neurotrophin in the brain. It plays an important role in neurogenesis and neuroplasticity [82]. BDNF has been associated with several psychiatric disorders, including SCH and other psychotic disorders. Valine 66 methionine (Val66Met) polymorphism of BDNF gene has been linked with brain morphology, the severity of symptoms, therapeutic response, and effectiveness, the age of onset followed by cognitive function [12]. Despite challenges to the sensitivity and accuracy of the BDNF determination, most evidence suggests that BDNF has the potential to serve as a diagnostic biomarker of SCH [83].
Evidence in both CNS and periphery has suggested that BDNF has a strong association with functional alternations in patients with SCH. Several studies report altered BDNF mRNA and protein in post-mortem brain tissue of SCH patients, with variation in levels reported across different brain regions [84], [85], [86]. A significant reduction of BDNF levels in the CSF was also found in first-episode and chronic medicated patients [87, 88]. Reduced peripheral BDNF levels were found in patients with SCH according to a meta-analysis of 41 studies [89], and found to significantly related to cognitive impairment in SCH, particularly in chronic samples [81]. Although a decrease in peripheral BDNF levels can also be observed in the acute states and then come back to normal in major depressive disorder and bipolar disorder [90], it remains a potential candidate of peripheral BDNF levels as a diagnostic biomarker for SCH.
Research on neuroimaging of SCH biomarkers
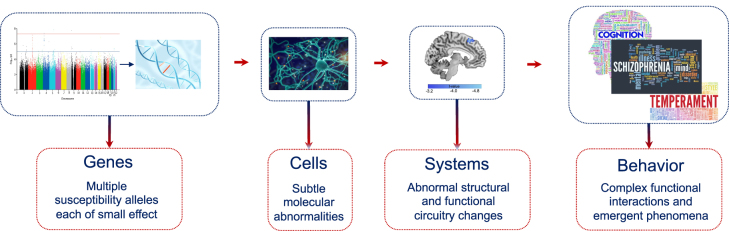
Diagnostic biomarkers are developed to index a biological process associated with objective disease signatures, which helps detect the stage of disease. With the increasing development of neuroimaging technology, neuroimaging becomes a strong candidate diagnostic biomarker for schizophrenia [91, 92]. Phenotypic variations in molecular and cellular disease targets can be captured through molecular imaging like positron emission tomography (PET) and behavior related alterations in specific brain circuits can be measured by structural and functional magnetic resonance imaging [91], [92], [93]. Furthermore, the neuroimaging biomarkers can generate the unique representation of genes, environment as well as the interaction between genes and environment (Figure 2). Here, we will selectively review mechanistically plausible diagnostic biomarker for schizophrenia in system and cell level, respectively (Table 3).
Figure 2:
Genetic and imaging biomarkers. Imaging biomarkers can be cells and systems level: Phenotypic variations in molecular and cellular disease targets captured through molecular imaging like PET, and behavior related alterations in specific brain circuits measured by structural and functional MRI. PET, positron emission tomography; MRI, magnetic resonance imaging.
Table 3:
Neuroimaging biomarkers in schizophrenia.
| Biomarkers | Methods | Participants | Conclusions | Diagnostic aspects | References |
|---|---|---|---|---|---|
| Systems level | |||||
| Brain network dysconnectivity | sMRI and dMRI | 26 chronic SCH patients and 26 controls | Chronic SCH showed reduced fractional anisotropy and fewer streamlines for both segregated and integrative frontostriatal white matter tracts | Diagnostic biomarkers: the risk of SCH | [96] |
| Task fMRI | 728 individuals including 396 controls, patients (46 SCH, 45 BP, 60 MDD), and unaffected first-degree relatives (46 SCH, 50 BP, 85 MDD) | Reduced ventral striatal-hippocampus coupling during reward processing is an endophenotype for SCH linked to positive and cognitive symptoms; ventral striatal-hippocampus coupling is familial and linked to polygenic scores for SCH | Diagnostic biomarkers: the risk of SCH | [99] | |
| Resting-state fMRI | 7 independent scanners (total 560 patients and 540 controls) | Functional striatal abnormalities (FSA) distinguished individuals with schizophrenia from healthy controls with an accuracy exceeding 80% and predicted antipsychotic treatment response. | Diagnostic and prognosis biomarkers: the risk of SCH, prognosis, and subtyping | [100] | |
| Cortical gray matter loss and thickness thinning | sMRI | 4474 SCH patients and 5098 controls | SCH showed widespread thinner cortex (Cohen’s d < −0.5) and smaller surface area (Cohen’s d < −0.25), especially in frontal and temporal lobe regions | Diagnostic biomarkers: the risk of SCH | [102] |
| sMRI | 2028 SCH patients and 2540 controls | SCH showed smaller gray matter volumes in the hippocampus, amygdala, thalamus, nucleus accumbens and total intracranial volume, as well as larger pallidum and lateral ventricle volumes | Diagnostic biomarkers: the risk of SCH | [106] | |
| sMRI | 2 cohorts (155 SCH and 79 controls; 46 SCH and 46 controls) | SCH showed significant volume deficits in the CA1 of hippocampus, with extension to other hippocampal subfields and accompanying clinical sequelae over time. | Diagnostic biomarkers: the risk of SCH | [109] | |
| Multivariate pattern recognition | Combined with sMRI and dMRI | 2 cohorts (98 patients with first-episode SCH and 106 controls; 54 patients with first-episode SCH and 48 controls) | Most prominent discriminative features in the classification (accuracy of 75.05%) included cortical thickness of left transverse temporal gyrus and right parahippocampal gyrus, the fractional anisotropy of left corticospinal tract and right external capsule. | Diagnostic biomarkers: the risk of SCH | [114] |
| Combined with sMRI, dMRI and resting-state fMRI | 2 cohorts (147 SCH patients and 147 controls; 39 SCH patients and 44 controls) | The salience network (gray matter, GM), corpus callosum (fractional anisotropy, FA), central executive and default-mode networks (fractional amplitude of low-frequency fluctuation, fALFF) were identified as modality-specific biomarkers of generalized cognition in SCH. | Diagnostic biomarkers: quantify and predict cognitive performance | [115] | |
| Auditory steady-state response | MEG, EEG | Meta analysis (20 studies including 590 controls and 606 SCH patients) | The 40-Hz ASSR spectral power and phase-locking deficits are robust in SCH, and could be useful probes for assessing circuit dysfunctions in SCH | Diagnostic biomarkers: the risk of SCH | [120] |
| EEG | 427 SCH patients and 293 controls | SCH showed significantly reduced source dipole density of gamma-band ASSR in the left superior temporal cortex, orbitofrontal cortex, and left superior frontal cortex. | Diagnostic biomarkers: the risk of SCH | [121] | |
| Excess glutamatergic neurotransmission | MRS | Meta analysis (59 studies including 1686 SCH patients and 1451 controls) | Schizophrenia is associated with elevations in glutamatergic metabolites across several brain regions. | Diagnostic biomarkers: the risk of SCH | [122] |
| MRS | Mega analysis (42 studies including 1251 SCH patients and 1197 controls) | Higher brain Glu levels may act as a biomarker of illness severity in schizophrenia. | Biomarker of valuating disease severity | [248] | |
| Cells level | |||||
| Neuroinflammation dysregulation | PET | Meta analysis (5 studies including 75 subjects with first-episode psychosis or SCH and 77 controls) | The lower levels of translocator protein (TSPO) observed in patients may correspond to altered function or lower density of brain immune cells. | Diagnostic biomarkers: the risk of SCH | [129] |
| PET | 12 studies comprising 190 SCH patients and 200 controls | SCH showed moderate elevations in TSPO tracer binding in grey matter relative to other brain tissue when using binding potential (BP) as an outcome measure, but no difference when volume of distribution (VT) is the outcome measure | Diagnostic biomarkers: the risk of SCH | [132] | |
| Dopamine hyperactivity | PET | 22 BP, 16 SCH, 22 controls | Dopamine synthesis capacity (Kicer) was significantly elevated in both BP group and SCH group, and was significantly positively correlated with positive psychotic symptom severity in the combined sample, explaining 27% of the variance in symptom severity. | Diagnostic biomarkers: the risk of SCH and symptom severity | [137] |
| PET | Meta analysis (21 studies including 269 SCH patients and 313 controls) | In individuals with SCH, dopaminergic dysfunction is greater in dorsal compared to limbic subdivisions of the striatum. | Diagnostic biomarkers: the risk of SCH | [140] | |
| Lower synaptic vesicle density | PET | 13 SCH patients and 15 controls | Synaptic vesicle density is lower across several brain regions in SCH. Frontal synaptic vesicle density correlated with psychosis symptom severity and cognitive performance on social cognition and processing speed. | The pathology of SCH | [147] |
| PET | 18 SCH patients and 18 controls | SCH exhibited lower synaptic terminal protein levels in vivo and antipsychotic drug exposure is unlikely to account for them. | The pathology of SCH | [148] |
SCH, schizophrenia; BP, bipolar disorder; MDD, major depressive disorder; MRI, magnetic resonance imaging; sMRI, structual MRI; dMRI, diffusion MRI; fMRI, functional MRI; EEG, magnetoencephalography; MEG, magnetoencephalography; MRS, magnetic resonance spectroscopy; PET, positron emission tomography.
Systems level
Brain network dysconnectivity
The dysconnectivity hypothesis is one of the well-researched pathophysiological models of psychosis. It is hypothesized that schizophrenia results from abnormal neural connectivity and the subsequent decoupling of the comprehensive thinking process in brain [94]. The abnormal structural network organization of schizophrenia mainly focused on the changes in white matter microstructure and connectivity with evidence of increased segregation and reduced integration by graph theory analysis [95], which also exhibited association with genetic risk. Reduced anatomical connectivity of white matter tracts between the dorsolateral prefrontal cortex and the associative striatum was observed in SCH, which might lead to a loss of a “normal” brain-behavior correlation in chronic patients [96]. Dopamine dysregulation in the striatum has been considered as a general feature of SCH pathology. Many studies have revealed the dysconnectivity of functional network in patients with SCH, especially for striatum. Cognitive dysmetria was found to be associated with abnormalities within the cortico-cerebellar-striatal-thalamic loop and the comparison between task-positive and task-negative cortical networks [97]. The hypoactivation in the ventral striatum, that is, deficient increases in hemodynamic responses during reward anticipation, was relevant for negative symptoms like a lack of motivation [98]. Reduced ventral striatal-hippocampus coupling during reward processing can be regarded as an endophenotype for SCH linked to positive and cognitive symptoms as well as polygenic risk [99]. A new neuroimaging biomarker was also deveolopped for schizophrenia identification, prognosis and subtyping based on striatal abonomal hyperactivity and disrupted connectivity, which was associated with dopaminergic function and the gene expression of polygenic risk [100]. Medial orbital frontal cortex (mOFC)-striatal functional connectivity was found to be reduced in SCH and correlated with the severity of negative symptoms [101]. The aberrant integration of brain networks might led by the disturbances in the excitation/inhibition balance, which in turn results in the symptoms of schizophrenia [92].
Cortical gray matter loss and thickness thinning
Cortical gray matter volume loss and cortical thickness thinning in SCH, which may be most prominent in fronto-temporal regions [92], is consistently found as a hallmark feature. The Enhancing Neuro Imaging Genetics Through Meta Analysis (ENIGMA) Consortium presented the first meta-analysis of cortical thickness and surface area abnormalities in SCH and observed widespread thinner cortex (Cohen’s d < –0.5) and smaller surface area (Cohen’s d < −0.25), especially in frontal and temporal lobe regions [102]. The overlapping pattern of cortical gray matter thickness reduction within temporal lobe and midcingulate cortex was associated with positive symptoms and aggression [103]. Positive symptom severity was also negatively related to bilateral superior temporal gyrus thickness [104]. Negative symptom severity was significantly associated with prefrontal cortical thickness in left medial orbitofrontal cortex [105]. As for gray matter volume, the prospective meta-analysis of structural brain imaging data conducted by ENIGMA found smaller volumes of SCH in the hippocampus, amygdala, thalamus, nucleus accumbens and total intracranial volume, as well as larger pallidum and lateral ventricle volumes [106]. The larger putamen and pallidum volumes were positively associated with disease duration. Greater fronto-limbic and whole brain volumes, as well as smaller ventricles was associated with better functional outcome [107]. Besides the link with disease severity across symptom dimensions [108] and overall poor clinical outcomes [107], the significant loss of gray matter volume presented at illness onset and was significantly accelerated up to middle age and plateaued thereafter [109], together with progressive illness-related loss in hippocampus [110], which suggested gray matter loss as a viable candidate biomarker not only for diagnosis but also for prognosis. Animal studies have found that the subchronic N-methyl-D-aspartate receptor (NMDAR) antagonism [111] and maternal immune activation [112] could result in gray matter reductions, indicating the abnormalities in brain maturational processes influenced by environmental factors underlie the gray matter alterations.
Multivariate pattern recognition
Recently emerging multivariate pattern recognition approaches have facilitated the search for reliable neuroimaging biomarkers [113]. Structural multiple brain features characterized by gray matter abnormalities and white matter disruptions have demonstrated with discriminative power towards pathological changes in SCH, which improved classification accuracy comparing with each modality used separately. The most prominent structural discriminative features included abnormal cortical thickness and the fractional anisotropy in specific regions [114]. Multimodal fusion has been regarded as a promising tool to discover covarying patterns of multiple imaging types impaired in brain diseases including SCH. Studies of multimodal magnetic resonance imaging (MRI) fusion have highlighted the salience network (gray matter), corpus callosum (fractional anisotropy), central executive and default-mode networks (fractional amplitude of low-frequency fluctuation) as modality-specific biomarkers of generalized cognition [115]. By leveraging multiple imaging and clinical information into one framework, a widely distributed network disruption was observed to appear in SCH patients, with synchronous changes in both functional and structural regions, especially the basal ganglia network, salience network, and the frontoparietal network [116]. These results suggested that an aggregate approach to biomarker development may be fruitful.
Auditory steady-state response
Steady-state responses (SSRs) are oscillatory responses induced by the frequency and phase of time-modulated stimulation. Using electroencephalography [117] and magnetoencephalography (MEG) to detect steady-state responses in different sensory modalities can be noninvasive. Gamma-band oscillations are assumed to establish communication between distributed neuronal ensembles [118], and their interference may be the basis of significant cognitive and perceptual changes in patients with SCH [119]. A meta-analysis revealed consistent evidence that both spectral power and phase-locking measurements decreased significantly during 40 Hz stimulation in schizophrenic patients, highlighting the obstacles generated by high-frequency oscillations and the precise temporal coordination of rhythmic activity in response to entrainment of neural circuits [120]. The gamma-band auditory steady-state response (ASSR) inter-trial coherence (ITC) in the bilateral temporal cortex was associated with positive and negative symptoms and cognitive function, the orbitofrontal cortex was associated with negative symptoms, and the left frontal epithelial layer was associated with negative symptoms and cognitive function. The results elucidated the schizophrenia-related basis of gamma-phase deficits and may identify gamma-phase deficits as an important potential biomarker of regional cortical dysfunction in schizophrenia [121].
Excess glutamatergic neurotransmission
Several lines of evidence have implicated alterations in glutamatergic neurotransmission may be fundamental to the pathophysiology of SCH. The main technique for assessing central glutamate function of human in vivo is proton magnetic resonance spectroscopy (MRS). The presence of a hyperglutamatergic state in different brain areas in patients with schizophrenia has been empirically confirmed and replicated in MRS studies, mainly including basal ganglia (Hedges’g = 0.63), thalamus (g = 0.56), basal ganglia (g = 0.39) and medial temporal lobe (g = 0.32) indicated in the meta-analysis [122]. Higher glutamate levels in medial frontal cortex and temporal lobe were also found to be associated with more severe global symptoms in SCH [123]. These finding supports the excess glutamatergic neurotransmission hypothesis in SCH. However, it is worth noting that the glutamate levels measured with MRS do not equal the amount of glutamatergic neurotransmission, but rather reflect the amount of neuronal, glial, and synaptic glutamate present in a voxel [92].
The NMDAR hypofunction model proposes that schizophrenia is related to NMDAR hypofunction on the γ-aminobutyric acid (GABAergic) interneuron, causing disinhibition of the glutamatergic pyramidal cell and leading to excess glutamate release [124], as observed through MRS studies. Preclinical data further support this target by demonstrating that experimentally induced NMDAR hypofunction results in increased firing of glutamatergic neurons in animal models and produces psychosis-like behavioral phenotypes and glutamatergic excess in healthy [125]. Furthermore, the elevated levels of NMDAR antibody may play a role in the pathogenesis of schizophrenia, leading to NMDAR dysfunction, thereby inducing symptoms of psychosis and cognitive impairment [126, 127].
Cells level
Neuroinflammation dysregulation
Neuroinflammation and abnormal immune responses are increasingly implicated in the pathophysiology of schizophrenia. Microglia are the primary immune cells of the central nervous system. Activation of microglia in response to psychosocial stress during critical developmental periods are supposed to result in aberrant neurotransmission, synaptic pruning, and structural injury of neurons and glia, which make people more vulnerable to subsequent overactivation by stressors experienced in later life [128]. To confirm the presence of a dysfunctional immune system in the brain directly, the PET and radioligands that target the glial cell marker 18 kDa translocator protein (TSPO), which is expressed in glial cells of brain including immune cells during the inflammatory response, are established to examine brain immune function in vivo. To review in vivo PET imaging studies of microglia activation, a meta-analysis with individual participant data using second-generation radioligands binding to the TSPO yielded strong evidence (effect size 0.47–0.63) of significant lower levels of TSPO in SCH compared with control subjects [129], while another subsequent meta-analysis showed no patient-control difference using the second generation TSPO radioligands and elevated brain TSPO levels using the first generation TSPO radioligand [11C]-(R)-PK11195 with a small to moderate effect size (Hedges’g=0.31) [130]. The seemingly inconclusive and even contradictory results might due to more accurate estimation of the underlying effect size by the individual participant data and low signal-to-noise ratio of [11C]-(R)-PK11195 [131]. Then a replication of meta-analysis using individual participant data adding over 200 samples confirmed the finding that SCH patients had lower TSPO levels than controls [132]. Although the lower TSPO concentrations suggested by evidence, considering the high variability of TSPO PET measurements, the discussion of increased microglia activity and pro-inflammatory activation in SCH should be kept open [133]. Additional biomarkers of neuroinflammation are needed to reflect the wide range of proinflammatory and anti-inflammatory responses that occur in the brain [131]. Furthermore, results of diffusion imaging studies reported increased extracellular free water, a proxy of inflammation, appeared more prominent in the early illness compared with chronic stages [134, 135], which might be a clue of the dynamic of biomarkers related to neuroinflammation dysregulation [92].
Dopamine hyperactivity
Neuroimaging studies investigating DA function broadly support the hypothesis of presynaptic striatal DA hyperactivity in SCH, as we partly stated above. For the cell level, striatal DA transporter (DAT) availability detected by PET and selective DAT radioligand observed higher DAT availability in midbrain, striatal, and limbic regions of in those patients with a chronic illness and long-term antipsychotic exposure. The DAT availability was involved in positive psychotic symptoms [136]. Studies have consistently demonstrated elevated presynaptic dopamine function in SCH [137]. Meta-analysis of studies using PET and single photon emission computed tomography [138] techniques showed a robust increase in striatal dopamine synthesis and release in SCH [139]. The hyperdopaminergic state associated with SCH appears greatest within the dorsal striatum. Current researches have provided consistent evidence of a striatal presynaptic hyperdopaminergic state in SCH, but little consistent evidence of altered dopamine type 2/3 (D2/3) receptor levels [137].
Lower synaptic vesicle density
SCH is hypothesized to be linked to excessive synaptic pruning within the prefrontal cortical brain circuitry [140], [141], [142]. Genetic studies have observed associations between SCH and genetic variants encoding synaptic proteins [143, 144]. Consistent changes in dendritic spines were reported by researchers as decreases in spine density and dendritic arborization in brain regions such as the primary visual cortex, the prefrontal cortex and the subiculum [145, 146]. Postmortem findings consistently reported decreased synaptic spine density in SCH. The novel PET ligand [11C] UCB-J gave the opportunity to generate a proxy measure of synaptic vesicle density in vivo. For patients with SCH, synaptic vesicle density is lower across many brain regions including frontal, occipital, parietal, and temporal cortex, as well as anterior cingulate, and hippocampus, which coincides with the findings in vivo and postmortem. Psychosis symptom severity and cognitive performance on social cognition and processing speed were found to be associated with frontal synaptic vesicle density [147]. Another study found lower level of synaptic vesicle glycoprotein 2A in the frontal and anterior cingulate cortices with large effect sizes (Cohen’s d=0.8–0.9), but not in the hippocampus [148]. Furthermore, the experiments with rats suggested that antipsychotic drug exposure was unlikely to account for the lower synaptic terminal protein levels in SCH.
Research on genetic, epigenetic and transcriptional levels of SCH biomarkers
A large number of studies have shown that SCH is caused by the joint action of genetic and environmental factors [149, 150]. Genetic factors mainly include common mutations and rare mutations. Environmental factors mainly include perinatal virus infection, childhood trauma, childhood growth environment, negative life events in adulthood and so on (Table 4).
Table 4:
Genetic biomarkers in schizophrenia.
| Biomarkers | Tools | Participants | Conclusions | Diagnostic aspects | References |
|---|---|---|---|---|---|
| Common genetic variation | |||||
| Common variants | GWAS | 3322 cases and 3587 controls | Polygenic basis to SCH involves common SNPs and explains at least a third of the total variation in liability | Diagnostic biomarkers: the risk of SCH | [153] |
| GWAS | 36,989 cases and 113,075 controls | 108 SCH-associated genetic loci were identified | Diagnostic biomarkers: the risk of SCH | [155] | |
| GWAS | 76,755 cases and 243,649 controls | Reporting common variant associations at 287 distinct genomic loci, and these associations were concentrated in genes that are expressed in excitatory and inhibitory neurons of the central nervous system | Diagnostic biomarkers: the risk of SCH | [156] | |
| Rare genetic variation | |||||
| CNV | Genome-wide analysis of CNV | 21,094 cases and 20,227 controls | Genome-wide significant evidence was obtained for eight loci, including 1q21.1, 2p16.3 (NRXN1), 3q29, 7q11.2, 15q13.3, distal 16p11.2, proximal 16p11.2 and 22q11.2. | Diagnostic biomarkers: the risk of SCH | [174] |
| DNV | NA | 4057 SCH trios | 105 genes associated with neurodevelopmental disorders were enriched for LoF DNVs, which support for these DNVs increasing liability to SCH | Diagnostic biomarkers: the risk of SCH | [173] |
| Rare coding variants | WES | 24,248 cases and 97,322 controls | Ultra-rare coding variants in 10 genes as conferring substantial risk for SCH and 32 genes at a false discovery rate of < 5%, which greatest expression in central nervous system neurons | Diagnostic biomarkers: the risk of SCH | [172] |
| PRS | |||||
| PRSice software/PGC-2 GWAS result | 628 cases and 261 controls | PRS of SCH was higher in first-admission than controls. PRS of SCH predicted more severe negative symptoms, greater illness severity, and worse cognition. PRS of SCH was the strongest predictor of diagnostic shifts from affective to non-affective psychosis. PRS of SCH predicted persistent differences in cognition and negative symptoms. PRS of SCH predicted who among those who appear to have a mood disorder with psychosis at first admission will ultimately be diagnosed with an SCH spectrum disorder. | Diagnostic and prognostic biomarkers: assist in diagnosis and differential diagnosis, evaluate disease severity and progression | [169] | |
| PRSice software/PGC-2 GWAS result | 4 cohorts (First cohort: 77 cases; second cohort: 150 cases; third cohort: 192 cases; fourth cohort: 100 cases) | Patients with higher PRS for SCH tended to have less improvement with antipsychotic drug treatment. | Theragnostic biomarkers: assist in individualized treatment strategies | [170] | |
| Methylation | |||||
| Epigenome-wide association study | 166 human fetal brain samples | The fetal brain mQTLs are enriched amongst risk loci identified in a recent large-scale GWAS of SCH | Diagnostic biomarkers: the risk of SCH | [177] | |
| Epigenome-wide association study | 469 cases and 476 controls | The observed DNA methylation aberrations in SCH patients could potentially provide a valuable resource for identifying diagnostic biomarkers and developing novel therapeutic targets to benefit SCH patients | Diagnostic biomarkers: the risk of SCH | [176] | |
| Transcriptomics | |||||
| mRNA | Cell study | NA | Bidirectional regulation of SCH-associated miR-1271-5p results in substantial remodeling of the neuronal transcriptome | The pathology of SCH | [180] |
| mRNA | iPSCs | Human brain samples | SCH-associated miR-936 upregulation in the DLPFC and can reduce glutamatergic synapses and weaken excitatory synaptic transmission, which underlie the synaptic pathology in SCH | The pathology of SCH | [183] |
| Brain expression | |||||
| TWAS | 79,845 cases and 3693 controls | 157 TWAS-significant genes were identified | Diagnostic biomarkers: the risk of SCH | [184] | |
| RNA sequencing | Single-cell sequencing | 40,000 cells | Identifying spatiotemporal loci and mapped the related loci to pyramidal cells, medium spiny neurons and interneurons in adult cortical cells, and to neural progenitors, oligodendrocyte precursors and fetal microglia | Diagnostic biomarkers: the risk of SCH | [186] |
| Gene-environment interaction | DNA methylation and childhood urbanicity | 497 healthy adults | a mediation role for ReHo, particularly increased brain activity in the superior temporal gyrus, in the urbanicity-associated speed of processing | The pathology of SCH | [191] |
SCH, schizophrenia; GWAS, genome wide association study; SNV, single nucleotide variants; CNV, copy number variants; DNV, de novo variants; WES, whole-exome sequencing; PRS, polygenic risk score; PGC, Psychiatric Genomics Consortium; mQTL, methylation quantitative trait loci; TWAS, transcriptome-wide association studies; iPSCs, induced-pluripotent stem cells; ReHo, regional homogeneity; NA, not applicable.
Heritability
Studies have shown that identical twins or both parents are SCH patients, the same disease rate is as high as 48%, and the heritability is about 80%, suggesting that SCH genetic research can help clarify its pathogenesis. The recent studies have estimated that genetic variation contributes up to 85% of the risk of developing the SCH [151, 152].
Common genetic variation
As a polygenic complex disease, the number of susceptible genes, the pathogenic risk of each gene and the pathogenic mechanism of SCH have not been clarified yet. In recent years, SCH genetics research has made important progress in European populations. For example, the genome wide association study (GWAS, can investigate the association between the clinical trait and millions of common variants simultaneously) of SCH has identified a number of susceptibility loci with significant association at genome level. In 2009, the International Schizophrenia Consortium firstly reported the loci associated with SCH at the genome-wide significant level [153]. In the subsequent decade, the Psychiatric Genomics Consortium (PGC) has identified more common genetic variants associated with SCH. The first GWAS conducted by the PGC identified seven genome-wide significant loci [154]. 108 susceptibility loci related to the risk of SCH were found in phase 2 GWAS of the SCH Working Group of PGC [155] and currently 287 genome-wide significant loci have been associated with the risk of schizophrenia in PGC phase 3 [156].
The most significant association is that across the major histocompatibility complex (MHC) locus on chromosome 6, which is known for its role in acquired immunity. The alleles of the C4 genes in the MHC region as underlying the MHC signal and the variation of it was related to increased risk for SCH [157]. The levels of C4 mRNA expression in postmortem brain from individuals with SCH were higher than matched controls, which was replicated in a transcriptomic study by the PsychEncode consortium [158]. Except for the locus in the MCH, the GWAS of SCH has also identified a number of susceptibility genes with significant association at genome level, including MIR137, CACNA1C, VRK2, TCF4, NRGN, AS3MT and so on [159], [160], [161], [162], [163], which are of positive significance to explain the pathogenesis of SCH. The above findings further highlight the key roles of dopamine D2 receptor, voltage-gated calcium channel, glutamatergic neurotransmission, B lymphocyte lineage and complement pathway related genes in the pathogenesis of SCH.
Due to the difference of population genetic structure, the results of European population research are difficult to explain the genetic risk of SCH in other population. Therefore, it is still of great significance to study the genetic mechanism of SCH in other population. For instance, in 2011, professor Weihua Yue and professor Yongyong Shi both reported multiple susceptibility genes at the level of the SCH genome in Han people at the same time in Nature Genetics, such as MHC region, MTHFR, AS3MT, VRK2, LSM1 and so on, which broke the monopoly of European genome research and clarified the mechanism of SCH neuroimmune abnormalities [159, 162]. Further summarizing the international PGC genome data, it is found that the genetic basis of SCH between East Asians and Europeans is similar. Genetic correlation analysis found that most SCH-associated variants have consistent genetic effects across East Asian and European populations, which expands the small sample study of the genetic hypothesis of cross ethnic heterogeneity of SCH [160]. There were also some GWAS studies reported in Indian [164], African American [165] and Latin American population s [166].
Application of polygenic risk score (PRS) in schizophrenia
The polygenic risk score (PRS) could detect the shared genetic aetiology among traits. First application of PRS in SCH showed that it could explain around 7.7 precent of the variance in SCH case-control status [153]. PRS is also applied to explain the clinical heterogeneity of SCH, particularly in association with response to treatment, severity of symptoms, and cognitive function. The higher PRS for SCH is related to a more chronic illness course, as well as the number and length of hospital admissions [167]. Furthermore, the PRS of SCH was associated with negative symptom and disorganized symptom dimensions [168, 169].
However, the ability of PRS to explain SCH case-control status has decreased in current GWAS cohorts [170]. Another challenge in PRS application is the different ancestral populations. PRS derived from GWAS of European explain less variance when applied to other populations than in European ancestry samples. Despite the ability of PRS to predict SCH case-control status is insufficient for diagnosis, there may be a greater promise for sampling individuals at the extreme ends of the PRS distribution. On the other hand, PRS can detect high-risk groups at a very early stage (theoretically, in the embryonic period), so that the biological characteristics of high-risk groups can be observed at an early stage before the onset of disease, excluding the impact of onset and treatment and providing a direct way to test the neurodevelopmental hypotheses about schizophrenia.
Rare genetic variation
Rare variants, with a minor allele frequency <1%, include single nucleotide variants (SNVs), altering one or a small number of bases, and insertion-deletion variants, which can vary in size from those affecting single nucleotides to those classified as copy number variants (CNVs). Currently, CNV studies are based on the same genotyping platforms, while other rare variants were studied by the whole exome sequencing (WES). However, to date, such studies in SCH are small in scale.
A WES study identified a significant association between SETD1A loss of function (LoF) variants and SCH by combining a whole-exome case-control sequencing study of 4264 schizophrenia cases, and 9343 controls [171]. Currently, the largest rare exome sequencing effort is from the Schizophrenia Exome Sequencing Meta-Analysis (SCHEMA) Consortium, which reported 10 genes (including SETD1A) reaching the genome wide significance and a further 22 reaching suggestive levels of significance [172].
De novo variants (DNVs) are variants that are present in offspring result from a new mutation event and are absent in the parents. In a recent WES study, LoF DNVs were found to be significantly enriched in LoF intolerant genes, but no gene individually achieved exome wide significance for the enrichment of LoF DNVs [173].
CNVs are either duplications or deletions, ranging from 50 base pairs to megabases in the genome. The first associated CNV for SCH was a large deletion on chromosome 22q11.2, with approximately 25% of carriers develop schizophrenia. The largest CNV study to date found eight CNVs (six deletions and two duplications) to be significantly associated with schizophrenia [174].
Epigenetic factors
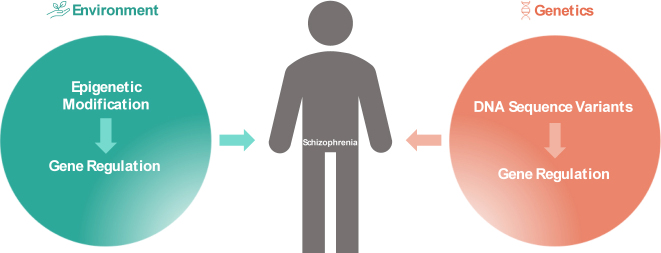
With the deepening of multi-omics research, more and more studies have begun to focus on the important role of environmental factors through epigenetic modification. Epigenetic means that DNA sequence does not change, but gene expression has a heritable change. Methylation studies reported multiple differential methylation sites related to synaptic transmission function associated with the risk of SCH (Figure 3) [175, 176]. Hannon et al. used embryonic cerebral cortex to conduct an epigenome-wide study, the results showed that differentially methylated quantitative trait loci (meQTL) (i.e. genetic polymorphisms affect methylation level) were mainly enriched at SCH genome-level susceptibility loci [177]. Adult cadaveric brain studies also found that about 60% of SCH genome-level susceptibility sites have meQTL effects, involving genes related to development and neural differentiation [178]. Considering the different developmental stages, the changes of SCH related DNA methylation mainly occur in the perinatal period, and gradually stabilize in adolescence and adulthood. It suggests that the apparent differences between SCH and healthy subjects’ brain tissues are mainly affected by embryos or postnatal period, and are relatively less affected by early adulthood experience or chronic disease [178]. The co-localization analysis of chromatin interactions in the cerebral cortex of subjects with SCH risk alleles and SCH results suggested that chromatin interactions with neurogenesis-related transcription factors were altered and involved in the dynamic regulation of SCH risk gene expression [179].
Figure 3:
Genetic and epigenetic factors on schizophrenia.
Messenger RNA and noncoding RNA
Genetic polymorphism mainly affects gene expression changes. In addition to single nucleotide polymorphism (SNP), it also includes transcription levels, such as messenger RNA and noncoding RNA (mRNA, ncRNA). Many international studies have used the cadaveric brain specimen bank to detect the specific gene mRNA transcription and expression levels, and found that the mRNA of multiple genes in patients with SCH is differentially expressed in different brain regions [180, 181]. NcRNA and mRNA have attracted more and more attention in recent years due to their rich species and extensive regulatory functions [182, 183].
Transcriptome-wide association studies (TWAS)
TWAS provides a method to predict the expression of the gene by expression quantitative trait loci (eQTLs) of genes in a specific tissue. By merging the expression data with GWAS summary statistics, the researchers can infer the causal genes related to SCH whether they are up or downregulated. A TWAS, applying summary statistics from the PGC 2 phrase GWAS, identified 157 significant genes related to SCH, and highlighted the regions responsible for gene expression regulation, which might be the potential targets [184]. A recent TWAS evaluated 5301 genes with cis-heritable expression in the dorsolateral prefrontal cortex and identified 89 genic associations [185, 186].
Gene-environment interaction affects the risk of SCH
Genetic epidemiological studies have shown that SCH is caused by a combination of genetic and environmental factors, that is, multiple pairs of micro-efficacious genes are synergistic with environmental factors [187]. A large number of studies suggest that intrauterine infection during pregnancy, perinatal complications and malnutrition, marijuana use by adolescents, and urban living environment all significantly increase the risk of neuropsychiatric disorders such as SCH [187, 188]. Compared with rural environment, childhood urban upbringing may be a risk factor for common mental disorders such as SCH. Environmental factors (perinatal viral infection, childhood abuse, urbanization, environmental pollution, etc.) interact with genetic variation, and the genetic and epigenetic mechanisms involved in the complex phenotype of SCH are the core issues in the field of SCH research.
In recent years, GWAS and the post-genome era have discovered many SCH-related genetic variation sites by integrating transcriptome, chromatin conformation and other data. These sites are mostly common variants and regulatory genetic variants located in non-coding regions. The regulatory genetic variation of schizophrenia affects the risk of disease by regulating gene expression, but its risk is generally low, and its ability to explain the phenotype is less than a quarter, which is a far from the nearly 80% heritability of schizophrenia [155, 189].
Applying the bioinformatics tools to merging the environment and genomic data, which provide some important information about the SCH. For example, Ursini G et al. combined the PRS of SCH and intra-uterine environment data and indicated that a subset of the most significant genetic variants associated with schizophrenia converge on a developmental trajectory sensitive to events that affect the placental response to stress, which may offer insights into sex biases and primary prevention [190]. The influence of genetic and environmental factors on the cognitive-emotion related brain function of the human brain has also been the focus of research in recent years. The interaction between urban and rural growth environment in childhood and genetic factors affects the cognitive functions of adult subjects, such as information processing speed and language learning ability. Differential methylation sites are located in SCH-susceptible biological pathways such as Wnt and Cadherin. The effects of childhood growth environment are influenced by the synergistic effect of regional homogeneity (ReHo)-fractional anisotropy (FA)-DNA methylation in the parietal temporal lobe and medial prefrontal cortex of the brain [191].
Single-cell studies
The TWAS provide a method to indicate the possible causal genes from GWAS summary statistics, but it cannot directly extract time-specific information nor clarify which kind of cell type is involved. To rectify this gap, the Common Mind Consortium generated transcriptomic data from dorsolateral prefrontal cortex (included 258 cases and 279 controls) and intersected with 142 GWAS associations, to demonstrate an overlap of 20 variants potentially influencing gene expression [192].
Recently, single-cell sequencing offers a new interpretation of GWAS results. As for SCH, the PsychENCODE generated single-cell RNAseq data and identified spatiotemporal loci and mapped the related loci to pyramidal cells, medium spiny neurons and interneurons in adult cortical cells, and to neural progenitors, oligodendrocyte precursors and fetal microglia [186, 193, 194].
Research on stem cell models of SCH biomarkers
The complexity of CNS presents a challenge to the research of SCH, the diversity of cell and cellular interactions also lead to the complexity of the CNS [195]. Due to lack of appropriate experimental models, the study on SCH has long been restricted to human post-mortem brain tissue or animal models, impeding the understanding of the underlying pathology of SCH. Moreover, polygenetic and psychosocial factors further complicating the understanding of mechanism. Despite these challenges, the ability of human induced pluripotent stem cells (hiPSCs) recapitulates transcriptomic changes of brain development during differentiation to neural cell types while retaining patient-specific genetic backgrounds have allowed for the in vitro study of SCH. The hiPSCs can provide insight into the cellular mechanisms underlying SCH [196], and utilization of single-cell level analyses in combination with organoid technology was proposed to discover epigenetic and/or consequent transcriptional alterations [197]-based biomarkers in diagnosing SCH [198, 199] (Table 5).
Table 5:
Cellular and molecular phenotypes revealed by iPSC studies in schizophrenia.
| iPSC derived cell types | Methods | Conclusions | Diagnostic aspects | References | |
|---|---|---|---|---|---|
| Neuron | iPSCs reprogrammed from fibroblasts and differentiated into neurons | 4 SCH patients and 4 controls | Diminished neuronal connectivity in conjunction with decreased neurite number, PSD95-protein levels and glutamate receptor expression | Diagnostic biomarkers: the risk of SCH | [202] |
| NPC | iPSCs reprogrammed from hair follicle keratinocytes and differentiated into Pax6(+)/Nestin(+) neural precursors | 3 SCH patients and 2 controls | SCH-specific defect in glutamatergic synaptic maturation; impaired mitochondrial respiration and its sensitivity to dopamine-induced inhibition | Diagnostic biomarkers: the risk of SCH | [249] |
| hiPSC derived forebrain patterned NPCs | 4 SCH patients and 6 controls | Perturbations in WNT signaling in SCH hiPSC forebrain NPCs | Diagnostic biomarkers: the risk of SCH | [207] | |
| Microglia | A high-throughput synaptosome system based on patient-derived induced microglia-like [156] cells and iPSC-derived neurons | 13 male SCH patients and 9 male controls | Increased synaptic pruning by microglia | Diagnostic biomarkers: delaying or preventing the onset of SCH in high-risk individuals | [212] |
| Astrocyte | iPSC lines generated from human dermal fibroblasts and differentiated into subtype astrocytes | 6 SCH patients and 2 controls | A significant decrease in intracellular glutamate concentrations in SCH astrocytes which was likely driven by deficient glutamate synthesis rather than reduced uptake capacity | Diagnostic biomarkers: SCH pathophysiology | [215] |
| OL | iPSCs reprogrammed from skin fibroblasts and differentiated into OLs | 6 SCH patients and 6 controls | Specific abnormalities of OL development and reduced production of late OPCs and OLs | Diagnostic biomarkers: the risk and development of SCH | [216] |
iPSC, induced pluripotent stem cell; PSD-95, postsynaptic density protein-95; NPC, neuronal progenitor cell; OL, oligodendrocyte; OPCs, oligodendrocyte progenitor cells; SCH, Schizophrenia.
The hiPSC lines of SCH were first established from Disrupted in schizophrenia 1 (DISC1) mutation-carrying chronic paranoid SCH patient in 2011 [200]. Since then, extensive research on SCH has been conducted using hiPSC models and related techniques. It is widely accepted that neural cells are primarily targets of SCH [201]. Studies have found that iPSC-derived neurons from SCH patients had impaired neuronal connectivity, decreased neurogenesis, reduced neurite outgrowth, decreased expression of synaptic proteins such as postsynaptic density protein-95 (PSD-95), and altered transcript expression [202], [203], [204]. Focusing on synaptic development of neuron, iPSC-based studies have reported SCH-specific defect in glutamatergic synaptic maturation [205, 206]. Studies on neuronal progenitor cells (NPCs) generated from SCH patients did not find any changes in the quantity of NPCs [202, 206], [207], [208]. However, several studies have demonstrated morphologic changes, abnormal organization, increased circulation, disorganized migration, delay of differentiation and reduced expression of neuronal differentiation-associated genes in iPSC-derived NPCs of SCH [205, 206, 208, 209].
Crucial role of glial cells in the brain, such as microglia and astrocytes, has also been shown to contribute to the pathology of SCH. As innate immune cells of the CNS, astrocytes and microglia interact closely with neurons and their synapses, contributing to proper synaptic connectivity and neuronal activity during development and maturation [210]. Several studies suggested that an increased inflamed state in SCH, and the GWAS of SCH further provided the evidence that the neuroinflammation played a role in SCH [155]. The involvement of microglia in aberrant synapse elimination motivated the application of anti-inflammatory drugs for SCH [211]. The iPSC-based SCH models for microglia-neuron interactions revealed increased synaptic pruning by microglia, which indicated that SCH is associated with mutations in several immune-related risk genes and an increased inflammatory state [212, 213]. As for astrocyte, an iPSC-based study found increased astrocyte differentiation in neurospheres generated from iPSC derived from SCH patients carrying a 22q11.2 microdeletion [214]. A recent iPSC-based drug study also found reduced levels of both glutamate and the NMDA receptor coagonist d-serine in subtype astrocytes derived from SCH patients, providing further evidence that NMDA receptor-mediated glutamatergic neurotransmission could play an important role in SCH pathophysiology and drug action [215]. Oligodendrocyte (OL) is another important glia cell type in CNS. iPSC-based SCH models for OLs revealed abnormal posttranslational processing, subcellular localization of mutant neuron glial antigen 2 (NG2), aberrant cellular morphology, viability and myelination potential, as well as reduced production of late oligodendrocyte progenitor cells (OPCs) and OLs in cells from subjects with SCH [216, 217]. These results directly confirm the presence of OLs dysfunction in the pathology of SCH.
Although most iPSC-based studies of SCH have some limits such as having small sample sizes, they greatly helped to elucidate the pathogenesis of SCH. By establishing in vitro homogenous neuron model of SCH, various pathogenesis of SCH can be verified, information for the early stage of the disease can be acquired, providing a valuable tool for finding early diagnostic markers of SCH.
Research on electrophysiological characteristics of SCH biomarkers
Electroencephalography can recognize the associated brain area through source analysis, it is inexpensive and noninvasive and can be useful in quantifying the sequence of various cognitive processes [218]. Due to the advantages of electroencephalography, several electroencephalography biomarkers have been extensively evaluated in psychosis. The event-related potentials (ERPs) reflect the changes in the brain’s neuroelectrophysiology during cognitive processes such as memory, expectation, arousal, intelligence, decision-making, logical reasoning, problem solving, and planning, so it is also called cognitive potentials. The main components of classical ERPs include the positive waveforms with latencies around 100 ms, 200 and 300 ms (P1, P2, P3) and the negative waveforms with latencies around 100 and 200 ms (N1, N2). ERPs still include N4 (N400), Mismatch negativity (MMN), late positive potential (LPP) and so on. MMN is a well-known electroencephalography biomarker for SCH associated with social cognition and functional outcomes [219]. It has been replicated numerous times as a mature electrophysiological biomarker [220]. The P3 component can be divided into the P3a and P3b, the P3a is associated with the orienting of attention to a novel stimulus, the P3b is associated with the motivational salience of a stimulus [221]. LPP is also closely related to attentional allocation to emotional stimuli [222]. There are a number of studies on P3 and LPP in patients with SCH, and a recent meta-analysis of the P3 and LPP in SCH showed that patients with SCH had diminished P3 and LPP amplitudes in response to positive and negative stimuli. The abnormal reduction of P3 and LPP may reflect decreased attention allocation [223]. The other abnormal changes of EPRs component were also found in the patients with SCH, such as P50 (response to two identical auditory stimuli occur 500 ms apart) related to deficits in sensory gating. Prepulse inhibition (PPI), is another commonly electroencephalogram biomarker. Abnormal activity in the gamma range (30–80 Hz) of scalp electroencephalogram gains increasing attention, patients with SCH are slower to entrain oscillatory brain activity to auditory “steady state” stimulation at 40 Hz and also show lower power in response to the stimulation overall [224] (Table 6).
Table 6:
Electrophysiological biomarkers in schizophrenia.
| Biomarkers | Tools | Participants | Conclusion | Diagnostic aspects | References |
|---|---|---|---|---|---|
| EPRs | EEG | ||||
| P3 (P3a and P3b) | Systematic review: 133 studies | Multiple EEG-indices were altered during at-risk mental state and early stages of schizophrenia | Diagnostic biomarkers: identify the risk of schizophrenia to benefit early diagnosis and intervention | [250] | |
| P50 | |||||
| MMN | 33 cases and 42 controls | Schizophrenia patients exhibited reduced MMN activity at frontocentral electrode sites | Diagnostic biomarkers: evaluate disease severity and progression | [220] | |
| LPP | 339 cases and 331 controls | Patients have slightly diminished P3 and LPP amplitudes in response to positive and negative stimuli | Diagnostic biomarkers: evaluate disease severity and progression | [223] | |
| Gamma range | 58 cases and 48 controls | the lack of age-related changes in deletion carriers indexes a potential developmental impairment in circuits underlying the maturation of neural oscillations during adolescence | Diagnostic biomarkers: identify the risk of schizophrenia | [251] | |
| Eye movement | Eye tracker | 1911 subjects | the antisaccade error rate as a putative schizophrenia spectrum marker. | Diagnostic biomarkers: identify the risk of schizophrenia | [252] |
| Retinal function | ERG and OCT | 2079 cases and 1571 controls | Retinal abnormalities may be applicable as state/trait markers in schizophrenia spectrum disorders | Diagnostic biomarkers: identify the risk of schizophrenia | [235] |
| a-waves and b-waves | |||||
| Retinal nerve fiber layer | |||||
| Macular ganglion cell layer | |||||
| Optic cup volume | |||||
| Heart rate variability | ECG | Systematic review: 34 studies | Given the association between low HRV, threat processing, emotion regulation and executive functioning, reduced vagal tone may be an endophenotype for the development of psychotic symptoms. | Diagnostic biomarkers: identify the risk of schizophrenia | [239] |
| HF | |||||
| LF |
EEG, electroencephalogram; ERPs, event-related potentials; MMN, mismatch negativity; LPP, late positive potential; ERG, electroretinogram; OCT, Optical coherence tomography; EEG, electrocardiogram; HF, high frequency; LF, low frequency.
Recently, several key non-rapid eye movement (NREM) sleep parameters of SCH have been comprehensively characterized. Marked reduction in sleep spindle density was found in SCH accompanied by altered spindle morphology, but fast and slow spindle properties were largely uncorrelated. Notably, NREM sleep metrics did not correlate with wake ERP measures, which suggested distinct aspects of SCH pathophysiology and reflected multiple distinct thalamic and thalamocortical mechanisms underlying disease abnormalities. Thus, the precise characters of sleep EEG could be regarded as the potential biomarkers for risk and SCH subtypes mapping to distinct neurophysiological deficits [225].
Corollary discharge (CD) signals are motor-related signals that exert an influence on sensory processing, which provide a simple sensorimotor basis for a subjective experience of agency by demarcating sensory experiences caused by oneself from those with external causes [226]. A disruption in the sense of agency plays a key role in many symptoms of SCH, characterized by a core disturbance in a sense of self [227, 228]. A growing amount of evidence indicates that SCH has the abnormal corollary discharge signaling associated with saccadic and smooth pursuit eye movements which impair both action plans and visual perception [229, 230]. These corollary discharge impairments may result from abnormal thalamo-cortical or cerebello-thalamo-cortical connectivity in schizophrenia [231].
The neurodegenerative changes of brain and abnormalities in neuronal fiber tracts play a role in the pathogenesis of SCH [232]. The retina is derived from the same ectoderm as the brain and is the part of the CNS, alterations of the brain can be inferred by direct observation of the retina [233]. The retinal functions as measured with the flash electroretinogram (ERG) may reflect the central dysfunctions in SCH. Two components, called the a-wave (representing the hyperpolarization of the photoreceptors) and b-wave (a positive wave, generated mainly by bipolar cells) were usually measured. Amounts of studies showed that abnormal change of a-wave and b-wave were observed in the patients with SCH, while these results were inconsistent [234]. In addition, structural changes in the retina also occur in patients with SCH, mainly characterized by thinning of the peripapillary retinal nerve fiber layer, macular ganglion cell layer-inner plexiform layer, average macular thickness, and macular volume and enlargement of the optic cup volume, which suggested that retinal abnormalities might be applicable as state/trait biomarkers in SCH [235].
Dysfunction of the autonomic nervous system has adverse effects on physical health, which reflects changes in the function of several regions of the central nervous system, and the dysfunction of autonomic nervous system was found in many mental disorders. Measurement of heart rate variability (HRV) provides a non-invasive tool for studying autonomic function. HRV can be measured as the temporal change between consecutive R waves on an electrocardiogram (time domain measurement) and the use of spectral analysis to decompose the waveform of the electrocardiogram-RR interval (heart beat interval) into frequency power bands (frequency domain measurements), the fluctuations in these intervals are thought to reflect changes in autonomic regulation of the heart [236]. Findings from studies suggested that patients with SCH reveal lower parasympathetic activity (reduced high frequency component of HRV) [237], and several HRV parameters were associated with the Positive and Negative Syndrome Scale (PANSS) scores in patients with SCH [238, 239].
Research on gut microbiota of SCH biomarkers
Gut microbiota, including Firmicutes, Bacteroidetes, Actinobacteria, Proteobacteria, Fusobacteri and Verrucomicrobia (Firmicutes and Bacteroidetes represent 90% of the gut microbiota), encodes more than three million genes, and is the essential for regulating digestion, immunity and metabolic homeostasis [240]. The brain connects with the gut via bidirectional communication involving neuroendocrine and neuro-immune pathways. Accumulating evidence indicates that the gut microbiota alteration largely associated with SCH pathogenesis. Antipsychotic could cause dysbiotic shifts in the gut microbiota, and the changes in gut microbiota composition have been found related to altered glutamate neurotransmission and cognitive impairments in SCH [241], [242], [243]. Moreover, antipsychotic could lead to the increases in adiposity and alterations in glucose homeostasis and energy balance [244].
Based on previous evidence, the gut microbiota could be applied as a mechanistic link between metabolic dysfunction and cognitive decline in SCH, as well as the programming of social behaviors and cognition, all of which are impaired in SCH. Gut microbiota alterations are also associated with the symptom of SCH. Therefore, the gut microbiota should be a potential biomarker of SCH. Furthermore, it also could be the biomarker in future psychopharmacotherapy of SCH based on the association between gut microbiota and antipsychotic treatment.
Conclusion and further direction
Biomarkers provide objective biological measures that can predict clinical outcomes leading to precision-medicine. Development and validation of biomarkers for SCH is needed for early diagnosis and treatment evaluation. The main challenge of biomarker development is that the standard disease classification in fine classification is only based on single symptoms, which largely confuses patients with different biomarkers. There is an inherent tension between the efforts to form biomarkers based on classic categorical diagnosis according to the phenotypic definitions and the currently developed biomarkers to identify more biologically homogeneous subgroups of patients.
At present, the complex pathogenesis of SCH cannot be explained from a single dimension such as genetic susceptibility genes, imaging objective biomarkers or symptom phenotypes; multi-omics and multi-dimensional data mining has become an important research strategy to explore the pathogenesis and diagnosis and treatment techniques [8, 9]. Previous literature suggests that data-driven reclassification of common mental disorders previously empirically diagnosed, combined with multi-omics research strategies such as clinical psychopathological symptomological assessment, genomewide, and brain imaging, will help clarify the clinical phenotype corresponding to a specific genotype, and then provide a more accurate evidence-based medical basis for diagnosis, classification or treatment. For example, Arnedo et al., used the PGC to evaluate the clinical symptomological phenotype of SCH patients with PANSS, and then combined with GWAS data to conduct specific genetic combination, and the phenotypic characteristics of clinical symptoms which were more finely divided and correspondingly analyzed, and it was found that data-driven analysis methods could classify SCH into eight geno-phenotype corresponding subtypes [245].
Recently, Wand et al. wrote an article in the journal Nature, and proposed a standardized analysis idea for the Polygenic Score (PGS) Catalog of complex diseases based on the Clinical Genome Resource (ClinGen) funded by the NIH in the United States [246]. Abi-Dargham et al. suggested that the most ideal strategy for the screening of objective biomarkers of mental disorders and the exploration of pathogenesis is the multi-dimensional exploration of “genetic variation-molecular effects-brain imaging features-clinical symptom phenotype” [91]. In the future, the ideal SCH precision treatment model should fully consider the multi-dimensional big data analysis based on genetic factors, personal factors, and environmental factors, such as the combination of the ClinGen complex disease working group and the PGS database (Figure 4).
Figure 4:
Recommended scheme for SCH research of objective biomarkers. SCH, Schizophrenia; PRS, polygenic risk score; SVM, Support Vector Machine; HAM-D, Hamilton Depression Rating Scale.
The ideal recommended scheme is: measurement-based care (MBC) before treatment, combined with pre-dose pharmacogenomics (PGx), and fully consider post-dose therapeutic drug monitoring (therapeutic drug monitoring, TDM).Therefore, the main research content of “exploring biomarkers and subtypes related to the diagnosis and treatment of schizophrenia based on multi-dimensional data” is to further solve the clinical diagnosis and treatment problems of SCH through multi-dimensional research strategies, and to further provide important scientific theoretical basis for clarifying the underlying pathological mechanisms of SCH.
Then, the decision to use biomarkers in clinical practice should be based on the expectation that it will have a positive impact on health; Therefore, measuring the performance of biomarkers in general terms is not enough to confirm their clinical utility. To demonstrate the clinical utility, we should firstly determine the accuracy of biomarkers, and then consider the use of biomarker information by considering the evaluation of relevant risk benefits and clinically meaningful outcome improvement when managing patients. Prospective, confirmatory multicenter studies or decision modeling methods can be used to demonstrate clinical utility.
Footnotes
Research funding: This work was supported by Academy of Medical Sciences Research Unit (2019-I2M-5-006), Chinese Institute for Brain Research at Beijing (2020-NKX-XM-12), Guizhou Province science and technology plan project ([2020]4Y064), National Natural Science Foundation of China (81825009, http://dx.doi.org/10.13039/501100001809), and PKUHSC-KCL Joint Medical Research (BMU2020KCL001).
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
Competing interests: Authors state no conflict of interest.
Ethical approval: Not applicable.
References
- 1.Owen MJ, Sawa A, Mortensen PB. Schizophrenia. Lancet. 2016;388:86–97. doi: 10.1016/s0140-6736(15)01121-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brody H. Schizophrenia. Nature. 2014;508:S1. doi: 10.1038/508s1a. [DOI] [PubMed] [Google Scholar]
- 3.GBD 2016 Disease and Injury Incidence and Prevalence Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1211–59. doi: 10.1016/S0140-6736(17)32154-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang Y, Wang Y, Wang H, Liu Z, Yu X, Yan J, et al. Prevalence of mental disorders in China: a cross-sectional epidemiological study. Lancet Psychiatr. 2019;6:211–24. doi: 10.1016/s2215-0366(18)30511-x. [DOI] [PubMed] [Google Scholar]
- 5.Kooyman I, Dean K, Harvey S, Walsh E. Outcomes of public concern in schizophrenia. Br J Psychiatr Suppl. 2007;50:s29–36. doi: 10.1192/bjp.191.50.s29. [DOI] [PubMed] [Google Scholar]
- 6.Chesney E, Goodwin GM, Fazel S. Risks of all-cause and suicide mortality in mental disorders: a meta-review. World Psychiatr. 2014;13:153–60. doi: 10.1002/wps.20128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Korth C, Fangerau H. Blood tests to diagnose schizophrenia: self-imposed limits in psychiatry. Lancet Psychiatr. 2020;7:911–4. doi: 10.1016/s2215-0366(20)30058-4. [DOI] [PubMed] [Google Scholar]
- 8.Insel TR, Cuthbert BN. Medicine. Brain disorders? Precisely. Science. 2015;348:499–500. doi: 10.1126/science.aab2358. [DOI] [PubMed] [Google Scholar]
- 9.Feczko E, Miranda-Dominguez O, Marr M, Graham AM, Nigg JT, Fair DA. The heterogeneity problem: approaches to identify psychiatric subtypes. Trends Cognit Sci. 2019;23:584–601. doi: 10.1016/j.tics.2019.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller BJ, Goldsmith DR. Inflammatory biomarkers in schizophrenia: implications for heterogeneity and neurobiology. Biomark Neurosci. 2019;1:100006. doi: 10.1016/j.bionps.2019.100006. [DOI] [Google Scholar]
- 11.Cao T, Li N, Cai H. Candidate metabolic biomarkers for schizophrenia in CNS and periphery: do any possible associations exist? Schizophr Res. 2020;226:95–110. doi: 10.1016/j.schres.2019.03.009. [DOI] [PubMed] [Google Scholar]
- 12.Patel S, Sharma D, Uniyal A, Akhilesh, Gadepalli A, Tiwari V. Recent advancements in biomarker research in schizophrenia: mapping the road from bench to bedside. Metab Brain Dis. 2022;3:1–15. doi: 10.1007/s11011-022-00926-5. [DOI] [PubMed] [Google Scholar]
- 13.Weickert CS, Weickert TW, Pillai A, Buckley PF. Biomarkers in schizophrenia: a brief conceptual consideration. Dis Markers. 2013;35:3–9. doi: 10.1155/2013/510402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gottesman I, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatr. 2003;160:636–45. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- 15.Kappelmann N, Arloth J, Georgakis MK, Czamara D, Rost N, Ligthart S, et al. Dissecting the association between inflammation, metabolic dysregulation, and specific depressive symptoms: a genetic correlation and 2-sample mendelian randomization study. JAMA Psychiatr. 2021;78:161–70. doi: 10.1001/jamapsychiatry.2020.3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khandaker GM, Zimbron J, Dalman C, Lewis G, Jones PB. Childhood infection and adult schizophrenia: a meta-analysis of population-based studies. Schizophr Res. 2012;139:161–8. doi: 10.1016/j.schres.2012.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khandaker GM, Zimbron J, Lewis G, Jones PB. Prenatal maternal infection, neurodevelopment and adult schizophrenia: a systematic review of population-based studies. Psychol Med. 2013;43:239–57. doi: 10.1017/s0033291712000736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown AS, Derkits EJ. Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. Am J Psychiatr. 2010;167:261–80. doi: 10.1176/appi.ajp.2009.09030361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benros ME, Nielsen PR, Nordentoft M, Eaton WW, Dalton SO, Mortensen PB. Autoimmune diseases and severe infections as risk factors for schizophrenia: a 30-year population-based register study. Am J Psychiatr. 2011;168:1303–10. doi: 10.1176/appi.ajp.2011.11030516. [DOI] [PubMed] [Google Scholar]
- 20.Brey RL, Holliday SL, Saklad AR, Navarrete MG, Hermosillo-Romo D, Stallworth CL, et al. Neuropsychiatric syndromes in lupus: prevalence using standardized definitions. Neurology. 2002;58:1214–20. doi: 10.1212/wnl.58.8.1214. [DOI] [PubMed] [Google Scholar]
- 21.Perry BI, Upthegrove R, Kappelmann N, Jones PB, Burgess S, Khandaker GM. Associations of immunological proteins/traits with schizophrenia, major depression and bipolar disorder: a bi-directional two-sample mendelian randomization study. Brain Behav Immun. 2021;97:176–85. doi: 10.1016/j.bbi.2021.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Williams JA, Burgess S, Suckling J, Lalousis PA, Batool F, Griffiths SL, et al. Inflammation and brain structure in Schizophrenia and other neuropsychiatric disorders: a mendelian randomization study. JAMA Psychiatr. 2022;79:498–507. doi: 10.1001/jamapsychiatry.2022.0407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller BJ, Goldsmith DR. Towards an immunophenotype of Schizophrenia: progress, potential mechanisms, and future directions. Neuropsychopharmacology. 2017;42:299–317. doi: 10.1038/npp.2016.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fillman SG, Cloonan N, Catts VS, Miller LC, Wong J, McCrossin T, et al. Increased inflammatory markers identified in the dorsolateral prefrontal cortex of individuals with schizophrenia. Mol Psychiatr. 2013;18:206–14. doi: 10.1038/mp.2012.110. [DOI] [PubMed] [Google Scholar]
- 25.Wu JQ, Wang X, Beveridge NJ, Tooney PA, Scott RJ, Carr VJ, et al. Transcriptome sequencing revealed significant alteration of cortical promoter usage and splicing in schizophrenia. PLoS One. 2012;7:e36351. doi: 10.1371/journal.pone.0036351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hwang Y, Kim J, Shin JY, Kim JI, Seo JS, Webster MJ, et al. Gene expression profiling by mRNA sequencing reveals increased expression of immune/inflammation-related genes in the hippocampus of individuals with schizophrenia. Transl Psychiatry. 2013;3:e321. doi: 10.1038/tp.2013.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Radewicz K, Garey LJ, Gentleman SM, Reynolds R. Increase in HLA-DR immunoreactive microglia in frontal and temporal cortex of chronic schizophrenics. J Neuropathol Exp Neurol. 2000;59:137–50. doi: 10.1093/jnen/59.2.137. [DOI] [PubMed] [Google Scholar]
- 28.Gallego JA, Blanco EA, Husain-Krautter S, Madeline Fagen E, Moreno-Merino P, Del Ojo-Jiménez JA, et al. Cytokines in cerebrospinal fluid of patients with schizophrenia spectrum disorders: new data and an updated meta-analysis. Schizophr Res. 2018;202:64–71. doi: 10.1016/j.schres.2018.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deshpande SB. Potentiation of serotonin-induced contractility of gastric fundus strips in lactating rats. Acta Physiol Scand. 1993;149:51–7. doi: 10.1111/j.1748-1716.1993.tb09591.x. [DOI] [PubMed] [Google Scholar]
- 30.Goldsmith DR, Crooks CL, Walker EF, Cotes RO. An update on promising biomarkers in Schizophrenia. Focus. 2018;16:153–63. doi: 10.1176/appi.focus.20170046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fernandes BS, Steiner J, Bernstein HG, Dodd S, Pasco JA, Dean OM, et al. C-reactive protein is increased in schizophrenia but is not altered by antipsychotics: meta-analysis and implications. Mol Psychiatr. 2016;21:554–64. doi: 10.1038/mp.2015.87. [DOI] [PubMed] [Google Scholar]
- 32.Miller BJ, Buckley P, Seabolt W, Mellor A, Kirkpatrick B. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatr. 2011;70:663–71. doi: 10.1016/j.biopsych.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goldsmith DR, Rapaport MH, Miller BJ. A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Mol Psychiatr. 2016;21:1696–709. doi: 10.1038/mp.2016.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fraguas D, Díaz-Caneja CM, Ayora M, Hernández-Álvarez F, Rodríguez-Quiroga A, Recio S, et al. Oxidative stress and inflammation in first-episode psychosis: a systematic review and meta-analysis. Schizophr Bull. 2019;45:742–51. doi: 10.1093/schbul/sby125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hatziagelaki E, Tsiavou A, Gerasimou C, Vavougios GD, Spathis A, Laskos E, et al. Effects of olanzapine on cytokine profile and brain-derived neurotrophic factor in drug-naive subjects with first-episode psychosis. Exp Ther Med. 2019;17:3071–6. doi: 10.3892/etm.2019.7285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Upthegrove R, Manzanares-Teson N, Barnes NM. Cytokine function in medication-naive first episode psychosis: a systematic review and meta-analysis. Schizophr Res. 2014;155:101–8. doi: 10.1016/j.schres.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 37.Lee EE, Ancoli-Israel S, Eyler LT, Tu XM, Palmer BW, Irwin MR, et al. Sleep disturbances and inflammatory biomarkers in Schizophrenia: focus on sex differences. Am J Geriatr Psychiatr. 2019;27:21–31. doi: 10.1016/j.jagp.2018.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Konradi C, Heckers S. Molecular aspects of glutamate dysregulation: implications for schizophrenia and its treatment. Pharmacol Ther. 2003;97:153–79. doi: 10.1016/s0163-7258(02)00328-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Natsubori T, Inoue H, Abe O, Takano Y, Iwashiro N, Aoki Y, et al. Reduced frontal glutamate + glutamine and N-acetylaspartate levels in patients with chronic schizophrenia but not in those at clinical high risk for psychosis or with first-episode schizophrenia. Schizophr Bull. 2014;40:1128–39. doi: 10.1093/schbul/sbt124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stone JM, Dietrich C, Edden R, Mehta MA, De Simoni S, Reed LJ, et al. Ketamine effects on brain GABA and glutamate levels with 1H-MRS: relationship to ketamine-induced psychopathology. Mol Psychiatr. 2012;17:664–5. doi: 10.1038/mp.2011.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Howes OD, Kambeitz J, Kim E, Stahl D, Slifstein M, Abi-Dargham A, et al. The nature of dopamine dysfunction in schizophrenia and what this means for treatment. Arch Gen Psychiatr. 2012;69:776–86. doi: 10.1001/archgenpsychiatry.2012.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stahl SM. Beyond the dopamine hypothesis of schizophrenia to three neural networks of psychosis: dopamine, serotonin, and glutamate. CNS Spectr. 2018;23:187–91. doi: 10.1017/s1092852918001013. [DOI] [PubMed] [Google Scholar]
- 43.Tassin JP. NE/DA interactions in prefrontal cortex and their possible roles as neuromodulators in schizophrenia. J Neural Transm Suppl. 1992;36:135–62. doi: 10.1007/978-3-7091-9211-5_7. [DOI] [PubMed] [Google Scholar]
- 44.Andreou D, Söderman E, Axelsson T, Sedvall GC, Terenius L, Agartz I, et al. Cerebrospinal fluid monoamine metabolite concentrations as intermediate phenotypes between glutamate-related genes and psychosis. Psychiatr Res. 2015;229:497–504. doi: 10.1016/j.psychres.2015.06.023. [DOI] [PubMed] [Google Scholar]
- 45.Carlborg A, Jokinen J, Nordström AL, Jönsson EG, Nordström P. Early death and CSF monoamine metabolites in schizophrenia spectrum psychosis. Nord J Psychiatr. 2011;65:101–5. doi: 10.3109/08039488.2010.503903. [DOI] [PubMed] [Google Scholar]
- 46.Bjerkenstedt L, Edman G, Hagenfeldt L, Sedvall G, Wiesel FA. Plasma amino acids in relation to cerebrospinal fluid monoamine metabolites in schizophrenic patients and healthy controls. Br J Psychiatry. 1985;147:276–82. doi: 10.1192/bjp.147.3.276. [DOI] [PubMed] [Google Scholar]
- 47.Peters JG. Dopamine, noradrenaline and serotonin spinal fluid metabolites in temporal lobe epileptic patients with schizophrenic symptomatology. Eur Neurol. 1979;18:15–8. doi: 10.1159/000115048. [DOI] [PubMed] [Google Scholar]
- 48.Pickar D, Breier A, Hsiao JK, Doran AR, Wolkowitz OM, Pato CN, et al. Cerebrospinal fluid and plasma monoamine metabolites and their relation to psychosis. Implications for regional brain dysfunction in schizophrenia. Arch Gen Psychiatr. 1990;47:641–8. doi: 10.1001/archpsyc.1990.01810190041006. [DOI] [PubMed] [Google Scholar]
- 49.Zumárraga M, Dávila R, Basterreche N, Arrue A, Goienetxea B, Zamalloa MI, et al. Catechol O-methyltransferase and monoamine oxidase A genotypes, and plasma catecholamine metabolites in bipolar and schizophrenic patients. Neurochem Int. 2010;56:774–9. doi: 10.1016/j.neuint.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 50.Yoshimura R, Nakamura J, Ueda N, Terao T. Effect of risperidone on plasma free 3-methoxy-4-hydroxyphenylglycol (pMHPG) levels in schizophrenic patients: relationship among plasma concentrations of risperidone and 9-hydroxyrisperidone, pMHPG levels, and clinical improvement. Int Clin Psychopharmacol. 2000;15:175–80. doi: 10.1097/00004850-200015030-00007. [DOI] [PubMed] [Google Scholar]
- 51.Yoshimura R, Ueda N, Shinkai K, Nakamura J. Plasma levels of homovanillic acid and the response to risperidone in first episode untreated acute schizophrenia. Int Clin Psychopharmacol. 2003;18:107–11. doi: 10.1097/00004850-200303000-00008. [DOI] [PubMed] [Google Scholar]
- 52.Stone TW. Neuropharmacology of quinolinic and kynurenic acids. Pharmacol Rev. 1993;45:309–79. [PubMed] [Google Scholar]
- 53.Pershing ML, Bortz DM, Pocivavsek A, Fredericks PJ, Jørgensen CV, Vunck SA, et al. Elevated levels of kynurenic acid during gestation produce neurochemical, morphological, and cognitive deficits in adulthood: implications for schizophrenia. Neuropharmacology. 2015;90:33–41. doi: 10.1016/j.neuropharm.2014.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Linderholm KR, Skogh E, Olsson SK, Dahl ML, Holtze M, Engberg G, et al. Increased levels of kynurenine and kynurenic acid in the CSF of patients with schizophrenia. Schizophr Bull. 2012;38:426–32. doi: 10.1093/schbul/sbq086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schwieler L, Larsson MK, Skogh E, Kegel ME, Orhan F, Abdelmoaty S, et al. Increased levels of IL-6 in the cerebrospinal fluid of patients with chronic schizophrenia--significance for activation of the kynurenine pathway. J Psychiatry Neurosci. 2015;40:126–33. doi: 10.1503/jpn.140126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Joaquim HPG, Costa AC, Gattaz WF, Talib LL. Kynurenine is correlated with IL-1β in plasma of schizophrenia patients. J Neural Transm. 2018;125:869–73. doi: 10.1007/s00702-018-1838-8. [DOI] [PubMed] [Google Scholar]
- 57.Kim JS, Kornhuber HH, Schmid-Burgk W, Holzmüller B. Low cerebrospinal fluid glutamate in schizophrenic patients and a new hypothesis on schizophrenia. Neurosci Lett. 1980;20:379–82. doi: 10.1016/0304-3940(80)90178-0. [DOI] [PubMed] [Google Scholar]
- 58.Goff DC, Freudenreich O, Evins AE. Augmentation strategies in the treatment of schizophrenia. CNS Spectr. 2001;6:907–11. doi: 10.1017/s1092852900000961. [DOI] [PubMed] [Google Scholar]
- 59.Stone JM, Morrison PD, Pilowsky LS. Glutamate and dopamine dysregulation in schizophrenia--a synthesis and selective review. J Psychopharmacol. 2007;21:440–52. doi: 10.1177/0269881106073126. [DOI] [PubMed] [Google Scholar]
- 60.Tomiya M, Fukushima T, Watanabe H, Fukami G, Fujisaki M, Iyo M, et al. Alterations in serum amino acid concentrations in male and female schizophrenic patients. Clin Chim Acta. 2007;380:186–90. doi: 10.1016/j.cca.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 61.Alfredsson G, Wiesel FA. Monoamine metabolites and amino acids in serum from schizophrenic patients before and during sulpiride treatment. Psychopharmacology (Berl) 1989;99:322–7. doi: 10.1007/bf00445551. [DOI] [PubMed] [Google Scholar]
- 62.Palomino A, González-Pinto A, Aldama A, González-Gómez C, Mosquera F, González-García G, et al. Decreased levels of plasma glutamate in patients with first-episode schizophrenia and bipolar disorder. Schizophr Res. 2007;95:174–8. doi: 10.1016/j.schres.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 63.He Y, Yu Z, Giegling I, Xie L, Hartmann AM, Prehn C, et al. Schizophrenia shows a unique metabolomics signature in plasma. Transl Psychiatry. 2012;2:e149. doi: 10.1038/tp.2012.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Madeira C, Alheira FV, Calcia MA, Silva TCS, Tannos FM, Vargas-Lopes C, et al. Blood levels of glutamate and glutamine in recent onset and chronic Schizophrenia. Front Psychiatr. 2018;9:713. doi: 10.3389/fpsyt.2018.00713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Duarte JMN, Xin L. Magnetic resonance spectroscopy in Schizophrenia: evidence for glutamatergic dysfunction and impaired energy metabolism. Neurochem Res. 2019;44:102–16. doi: 10.1007/s11064-018-2521-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tvrzicka E, Kremmyda LS, Stankova B, Zak A. Fatty acids as biocompounds: their role in human metabolism, health and disease – a review. Part 1: classification, dietary sources and biological functions. Biomed Pap Med Fac Palacky Univ Olomouc Czech Repub. 2011;155:117–30. doi: 10.5507/bp.2011.038. [DOI] [PubMed] [Google Scholar]
- 67.Janssen CI, Kiliaan AJ. Long-chain polyunsaturated fatty acids (LCPUFA) from genesis to senescence: the influence of LCPUFA on neural development, aging, and neurodegeneration. Prog Lipid Res. 2014;53:1–17. doi: 10.1016/j.plipres.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 68.Maekawa M, Owada Y, Yoshikawa T. Role of polyunsaturated fatty acids and fatty acid binding protein in the pathogenesis of schizophrenia. Curr Pharmaceut Des. 2011;17:168–75. doi: 10.2174/138161211795049615. [DOI] [PubMed] [Google Scholar]
- 69.Satogami K, Takahashi S, Yamada S, Ukai S, Shinosaki K. Omega-3 fatty acids related to cognitive impairment in patients with schizophrenia. Schizophr Res Cognit. 2017;9:8–12. doi: 10.1016/j.scog.2017.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jones HJ, Borges MC, Carnegie R, Mongan D, Rogers PJ, Lewis SJ, et al. Associations between plasma fatty acid concentrations and schizophrenia: a two-sample Mendelian randomisation study. Lancet Psychiatr. 2021;8:1062–70. doi: 10.1016/s2215-0366(21)00286-8. [DOI] [PubMed] [Google Scholar]
- 71.Davison J, O’Gorman A, Brennan L, Cotter DR. A systematic review of metabolite biomarkers of schizophrenia. Schizophr Res. 2018;195:32–50. doi: 10.1016/j.schres.2017.09.021. [DOI] [PubMed] [Google Scholar]
- 72.Tuem KB, Atey TM. Neuroactive steroids: receptor interactions and responses. Front Neurol. 2017;8:442. doi: 10.3389/fneur.2017.00442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hubbard DB, Miller BJ. Meta-analysis of blood cortisol levels in individuals with first-episode psychosis. Psychoneuroendocrinology. 2019;104:269–75. doi: 10.1016/j.psyneuen.2019.03.014. [DOI] [PubMed] [Google Scholar]
- 74.Bicikova M, Hill M, Ripova D, Mohr P, Hampl R. Determination of steroid metabolome as a possible tool for laboratory diagnosis of schizophrenia. J Steroid Biochem Mol Biol. 2013;133:77–83. doi: 10.1016/j.jsbmb.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 75.Ryan MC, Sharifi N, Condren R, Thakore JH. Evidence of basal pituitary-adrenal overactivity in first episode, drug naïve patients with schizophrenia. Psychoneuroendocrinology. 2004;29:1065–70. doi: 10.1016/j.psyneuen.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 76.Compagnone NA, Mellon SH. Dehydroepiandrosterone: a potential signalling molecule for neocortical organization during development. Proc Natl Acad Sci U S A. 1998;95:4678–83. doi: 10.1073/pnas.95.8.4678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mills DJ. The aging GABAergic system and its nutritional support. J Nutr Metab. 2021;2021:6655064. doi: 10.1155/2021/6655064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bulut SD, Bulut S, Gundogmus AG, Aydemir C. Serum DHEA-S, testosterone and cortisol levels in female patients with Schizophrenia. Endocr, Metab Immune Disord: Drug Targets. 2018;18:348–54. doi: 10.2174/1871530318666180212102128. [DOI] [PubMed] [Google Scholar]
- 79.Ritsner MS. Pregnenolone, dehydroepiandrosterone, and schizophrenia: alterations and clinical trials. CNS Neurosci Ther. 2010;16:32–44. doi: 10.1111/j.1755-5949.2009.00118.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sofroniew MV, Howe CL, Mobley WC. Nerve growth factor signaling, neuroprotection, and neural repair. Annu Rev Neurosci. 2001;24:1217–81. doi: 10.1146/annurev.neuro.24.1.1217. [DOI] [PubMed] [Google Scholar]
- 81.Bora E. Peripheral inflammatory and neurotrophic biomarkers of cognitive impairment in schizophrenia: a meta-analysis. Psychol Med. 2019;49:1971–9. doi: 10.1017/s0033291719001685. [DOI] [PubMed] [Google Scholar]
- 82.Numakawa T, Suzuki S, Kumamaru E, Adachi N, Richards M, Kunugi H. BDNF function and intracellular signaling in neurons. Histol Histopathol. 2010;25:237–58. doi: 10.14670/HH-25.237. [DOI] [PubMed] [Google Scholar]
- 83.Fernandes BS, Berk M, Turck CW, Steiner J, Gonçalves CA. Decreased peripheral brain-derived neurotrophic factor levels are a biomarker of disease activity in major psychiatric disorders: a comparative meta-analysis. Mol Psychiatr. 2014;19:750–1. doi: 10.1038/mp.2013.172. [DOI] [PubMed] [Google Scholar]
- 84.Durany N, Michel T, Zöchling R, Boissl KW, Cruz-Sánchez FF, Riederer P, et al. Brain-derived neurotrophic factor and neurotrophin 3 in schizophrenic psychoses. Schizophr Res. 2001;52:79–86. doi: 10.1016/s0920-9964(00)00084-0. [DOI] [PubMed] [Google Scholar]
- 85.Hashimoto T, Bergen SE, Nguyen QL, Xu B, Monteggia LM, Pierri JN, et al. Relationship of brain-derived neurotrophic factor and its receptor TrkB to altered inhibitory prefrontal circuitry in schizophrenia. J Neurosci. 2005;25:372–83. doi: 10.1523/jneurosci.4035-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mellios N, Huang HS, Baker SP, Galdzicka M, Ginns E, Akbarian S. Molecular determinants of dysregulated GABAergic gene expression in the prefrontal cortex of subjects with schizophrenia. Biol Psychiatr. 2009;65:1006–14. doi: 10.1016/j.biopsych.2008.11.019. [DOI] [PubMed] [Google Scholar]
- 87.Chen DC, Wang J, Wang B, Yang SC, Zhang CX, Zheng YL, et al. Decreased levels of serum brain-derived neurotrophic factor in drug-naïve first-episode schizophrenia: relationship to clinical phenotypes. Psychopharmacology (Berl) 2009;207:375–80. doi: 10.1007/s00213-009-1665-6. [DOI] [PubMed] [Google Scholar]
- 88.Tan YL, Zhou DF, Cao LY, Zou YZ, Zhang XY. Decreased BDNF in serum of patients with chronic schizophrenia on long-term treatment with antipsychotics. Neurosci Lett. 2005;382:27–32. doi: 10.1016/j.neulet.2005.02.054. [DOI] [PubMed] [Google Scholar]
- 89.Nieto RR, Carrasco A, Corral S, Castillo R, Gaspar PA, Bustamante ML, et al. BDNF as a biomarker of cognition in Schizophrenia/psychosis: an updated review. Front Psychiatr. 2021;12:662407. doi: 10.3389/fpsyt.2021.662407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lo LH, Shiea J, Huang TL. Rapid detection of alteration of serum IgG in patients with schizophrenia after risperidone treatment by matrix-assisted laser desorption ionization/time-of-flight mass spectrometry. Rapid Commun Mass Spectrom. 2016;30:2645–9. doi: 10.1002/rcm.7753. [DOI] [PubMed] [Google Scholar]
- 91.Abi-Dargham A, Horga G. The search for imaging biomarkers in psychiatric disorders. Nat Med. 2016;22:1248–55. doi: 10.1038/nm.4190. [DOI] [PubMed] [Google Scholar]
- 92.Kraguljac NV, McDonald WM, Widge AS, Rodriguez CI, Tohen M, Nemeroff CB. Neuroimaging biomarkers in Schizophrenia. Am J Psychiatr. 2021;178:509–21. doi: 10.1176/appi.ajp.2020.20030340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fuller RW, Hemrickluecke SK, Wong DT, Pearson D, Threlkeld PG, Hynes MD. Altered behavioral response to a D2 agonist, LY141865, in spontaneously hypertensive rats exhibiting biochemical and endocrine responses similar to those in normotensive rats. J Pharmacol Exp Therapeut. 1983;227:354–9. [PubMed] [Google Scholar]
- 94.van den Heuvel MP, Fornito A. Brain networks in schizophrenia. Neuropsychol Rev. 2014;24:32–48. doi: 10.1007/s11065-014-9248-7. [DOI] [PubMed] [Google Scholar]
- 95.Alloza C, Bastin ME, Cox SR, Gibson J, Duff B, Semple SI, et al. Central and non-central networks, cognition, clinical symptoms, and polygenic risk scores in schizophrenia. Hum Brain Mapp. 2017;38:5919–30. doi: 10.1002/hbm.23798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Levitt JJ, Nestor PG, Levin L, Pelavin P, Lin P, Kubicki M, et al. Reduced structural connectivity in frontostriatal white matter tracts in the associative loop in Schizophrenia. Am J Psychiatr. 2017;174:1102–11. doi: 10.1176/appi.ajp.2017.16091046. [DOI] [PubMed] [Google Scholar]
- 97.Sheffield JM, Barch DM. Cognition and resting-state functional connectivity in schizophrenia. Neurosci Biobehav Rev. 2016;61:108–20. doi: 10.1016/j.neubiorev.2015.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Radua J, Schmidt A, Borgwardt S, Heinz A, Schlagenhauf F, McGuire P, et al. Ventral striatal activation during reward processing in psychosis: a neurofunctional meta-analysis. JAMA Psychiatr. 2015;72:1243–51. doi: 10.1001/jamapsychiatry.2015.2196. [DOI] [PubMed] [Google Scholar]
- 99.Schwarz K, Moessnang C, Schweiger JI, Harneit A, Schneider M, Chen J, et al. Ventral striatal-Hippocampus coupling during reward processing as a stratification biomarker for psychotic disorders. Biol Psychiatr. 2022;91:216–25. doi: 10.1016/j.biopsych.2021.07.016. [DOI] [PubMed] [Google Scholar]
- 100.Li A, Zalesky A, Yue W, Howes O, Yan H, Liu Y, et al. A neuroimaging biomarker for striatal dysfunction in schizophrenia. Nat Med. 2020;26:558–65. doi: 10.1038/s41591-020-0793-8. [DOI] [PubMed] [Google Scholar]
- 101.Shukla DK, Chiappelli JJ, Sampath H, Kochunov P, Hare SM, Wisner K, et al. Aberrant frontostriatal connectivity in negative symptoms of Schizophrenia. Schizophr Bull. 2019;45:1051–9. doi: 10.1093/schbul/sby165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.van Erp TGM, Walton E, Hibar DP, Schmaal L, Jiang W, Glahn DC, et al. Cortical brain abnormalities in 4474 individuals with Schizophrenia and 5098 control subjects via the enhancing neuro imaging genetics through meta analysis (ENIGMA) consortium. Biol Psychiatr. 2018;84:644–54. doi: 10.1016/j.biopsych.2018.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wong TY, Radua J, Pomarol-Clotet E, Salvador R, Albajes-Eizagirre A, Solanes A, et al. An overlapping pattern of cerebral cortical thinning is associated with both positive symptoms and aggression in schizophrenia via the ENIGMA consortium. Psychol Med. 2020;50:2034–45. doi: 10.1017/s0033291719002149. [DOI] [PubMed] [Google Scholar]
- 104.Walton E, Hibar DP, van Erp TG, Potkin SG, Roiz-Santianez R, Crespo-Facorro B, et al. Positive symptoms associate with cortical thinning in the superior temporal gyrus via the ENIGMA Schizophrenia consortium. Acta Psychiatr Scand. 2017;135:439–47. doi: 10.1111/acps.12718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Walton E, Hibar DP, van Erp TGM, Potkin SG, Roiz-Santianez R, Crespo-Facorro B, et al. Prefrontal cortical thinning links to negative symptoms in schizophrenia via the ENIGMA consortium. Psychol Med. 2018;48:82–94. doi: 10.1017/s0033291717001283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.van Erp TG, Hibar DP, Rasmussen JM, Glahn DC, Pearlson GD, Andreassen OA, et al. Subcortical brain volume abnormalities in 2028 individuals with schizophrenia and 2540 healthy controls via the ENIGMA consortium. Mol Psychiatr. 2016;21:547–53. doi: 10.1038/mp.2015.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wojtalik JA, Smith MJ, Keshavan MS, Eack SM. A systematic and meta-analytic review of neural correlates of functional outcome in Schizophrenia. Schizophr Bull. 2017;43:1329–47. doi: 10.1093/schbul/sbx008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Padmanabhan JL, Tandon N, Haller CS, Mathew IT, Eack SM, Clementz BA, et al. Correlations between brain structure and symptom dimensions of psychosis in schizophrenia, schizoaffective, and psychotic bipolar I disorders. Schizophr Bull. 2015;41:154–62. doi: 10.1093/schbul/sbu075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cropley VL, Klauser P, Lenroot RK, Bruggemann J, Sundram S, Bousman C, et al. Accelerated gray and white matter deterioration with age in Schizophrenia. Am J Psychiatr. 2017;174:286–95. doi: 10.1176/appi.ajp.2016.16050610. [DOI] [PubMed] [Google Scholar]
- 110.Ho NF, Iglesias JE, Sum MY, Kuswanto CN, Sitoh YY, De Souza J, et al. Progression from selective to general involvement of hippocampal subfields in schizophrenia. Mol Psychiatr. 2017;22:142–52. doi: 10.1038/mp.2016.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Doostdar N, Kim E, Grayson B, Harte MK, Neill JC, Vernon AC. Global brain volume reductions in a sub-chronic phencyclidine animal model for schizophrenia and their relationship to recognition memory. J Psychopharmacol. 2019;33:1274–87. doi: 10.1177/0269881119844196. [DOI] [PubMed] [Google Scholar]
- 112.Crum WR, Sawiak SJ, Chege W, Cooper JD, Williams SCR, Vernon AC. Evolution of structural abnormalities in the rat brain following in utero exposure to maternal immune activation: a longitudinal in vivo MRI study. Brain Behav Immun. 2017;63:50–9. doi: 10.1016/j.bbi.2016.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kambeitz J, Kambeitz-Ilankovic L, Leucht S, Wood S, Davatzikos C, Malchow B, et al. Detecting neuroimaging biomarkers for schizophrenia: a meta-analysis of multivariate pattern recognition studies. Neuropsychopharmacology. 2015;40:1742–51. doi: 10.1038/npp.2015.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Liang S, Li Y, Zhang Z, Kong X, Wang Q, Deng W, et al. Classification of first-episode Schizophrenia using multimodal brain features: a combined structural and diffusion imaging study. Schizophr Bull. 2019;45:591–9. doi: 10.1093/schbul/sby091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sui J, Qi S, van Erp TGM, Bustillo J, Jiang R, Lin D, et al. Multimodal neuromarkers in schizophrenia via cognition-guided MRI fusion. Nat Commun. 2018;9:3028. doi: 10.1038/s41467-018-05432-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Liu S, Wang H, Song M, Lv L, Cui Y, Liu Y, et al. Linked 4-way multimodal brain differences in Schizophrenia in a large Chinese han population. Schizophr Bull. 2019;45:436–49. doi: 10.1093/schbul/sby045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Treur M, Baca E, Bobes J, Cañas F, Salvador L, Gonzalez B, et al. The cost-effectiveness of paliperidone extended release in Spain. J Med Econ. 2012;15:26–34. doi: 10.3111/13696998.2012.734884. [DOI] [PubMed] [Google Scholar]
- 118.Fries P. Rhythms for cognition: communication through coherence. Neuron. 2015;88:220–35. doi: 10.1016/j.neuron.2015.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Uhlhaas PJ, Singer W. Oscillations and neuronal dynamics in schizophrenia: the search for basic symptoms and translational opportunities. Biol Psychiatr. 2015;77:1001–9. doi: 10.1016/j.biopsych.2014.11.019. [DOI] [PubMed] [Google Scholar]
- 120.Thune H, Recasens M, Uhlhaas PJ. The 40-Hz auditory steady-state response in patients with Schizophrenia: a meta-analysis. JAMA Psychiatr. 2016;73:1145–53. doi: 10.1001/jamapsychiatry.2016.2619. [DOI] [PubMed] [Google Scholar]
- 121.Koshiyama D, Miyakoshi M, Joshi YB, Molina JL, Tanaka-Koshiyama K, Sprock J, et al. A distributed frontotemporal network underlies gamma-band synchronization impairments in schizophrenia patients. Neuropsychopharmacology. 2020;45:2198–206. doi: 10.1038/s41386-020-00806-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Merritt K, Egerton A, Kempton MJ, Taylor MJ, McGuire PK. Nature of glutamate alterations in Schizophrenia: a meta-analysis of proton magnetic resonance spectroscopy studies. JAMA Psychiatr. 2016;73:665–74. doi: 10.1001/jamapsychiatry.2016.0442. [DOI] [PubMed] [Google Scholar]
- 123.Merritt K, McGuire PK, Egerton A, Aleman A, Block W, Bloemen OJN, et al. Association of age, antipsychotic medication, and symptom severity in Schizophrenia with proton magnetic resonance spectroscopy brain glutamate level: a mega-analysis of individual participant-level data. JAMA Psychiatr. 2021;78:667–81. doi: 10.1001/jamapsychiatry.2021.0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Coyle JT. NMDA receptor and schizophrenia: a brief history. Schizophr Bull. 2012;38:920–6. doi: 10.1093/schbul/sbs076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kraguljac NV, Frolich MA, Tran S, White DM, Nichols N, Barton-McArdle A, et al. Ketamine modulates hippocampal neurochemistry and functional connectivity: a combined magnetic resonance spectroscopy and resting-state fMRI study in healthy volunteers. Mol Psychiatr. 2017;22:562–9. doi: 10.1038/mp.2016.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tong J, Huang J, Luo X, Chen S, Cui Y, An H, et al. Elevated serum anti-NMDA receptor antibody levels in first-episode patients with schizophrenia. Brain Behav Immun. 2019;81:213–9. doi: 10.1016/j.bbi.2019.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Steiner J, Walter M, Glanz W, Sarnyai Z, Bernstein HG, Vielhaber S, et al. Increased prevalence of diverse N-methyl-D-aspartate glutamate receptor antibodies in patients with an initial diagnosis of schizophrenia: specific relevance of IgG NR1a antibodies for distinction from N-methyl-D-aspartate glutamate receptor encephalitis. JAMA Psychiatr. 2013;70:271–8. doi: 10.1001/2013.jamapsychiatry.86. [DOI] [PubMed] [Google Scholar]
- 128.Howes OD, McCutcheon R. Inflammation and the neural diathesis-stress hypothesis of schizophrenia: a reconceptualization. Transl Psychiatry. 2017;7:e1024. doi: 10.1038/tp.2016.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Plaven-Sigray P, Matheson GJ, Collste K, Ashok AH, Coughlin JM, Howes OD, et al. Positron emission tomography studies of the glial cell marker translocator protein in patients with psychosis: a meta-analysis using individual participant data. Biol Psychiatr. 2018;84:433–42. doi: 10.1016/j.biopsych.2018.02.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Marques TR, Ashok AH, Pillinger T, Veronese M, Turkheimer FE, Dazzan P, et al. Neuroinflammation in schizophrenia: meta-analysis of in vivo microglial imaging studies. Psychol Med. 2019;49:2186–96. doi: 10.1017/s0033291718003057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Meyer JH, Cervenka S, Kim MJ, Kreisl WC, Henter ID, Innis RB. Neuroinflammation in psychiatric disorders: PET imaging and promising new targets. Lancet Psychiatr. 2020;7:1064–74. doi: 10.1016/s2215-0366(20)30255-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Plaven-Sigray P, Matheson GJ, Coughlin JM, Hafizi S, Laurikainen H, Ottoy J, et al. Meta-analysis of the glial marker TSPO in psychosis revisited: reconciling inconclusive findings of patient-control differences. Biol Psychiatr. 2021;89:e5–8. doi: 10.1016/j.biopsych.2020.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Plaven-Sigray P, Cervenka S. Meta-analytic studies of the glial cell marker TSPO in psychosis - a question of apples and pears? Psychol Med. 2019;49:1624–8. doi: 10.1017/s003329171800421x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lyall AE, Pasternak O, Robinson DG, Newell D, Trampush JW, Gallego JA, et al. Greater extracellular free-water in first-episode psychosis predicts better neurocognitive functioning. Mol Psychiatr. 2018;23:701–7. doi: 10.1038/mp.2017.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kraguljac NV, Anthony T, Monroe WS, Skidmore FM, Morgan CJ, White DM, et al. A longitudinal neurite and free water imaging study in patients with a schizophrenia spectrum disorder. Neuropsychopharmacology. 2019;44:1932–9. doi: 10.1038/s41386-019-0427-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Artiges E, Leroy C, Dubol M, Prat M, Pepin A, Mabondo A, et al. Striatal and extrastriatal dopamine transporter availability in Schizophrenia and its clinical correlates: a voxel-based and high-resolution PET study. Schizophr Bull. 2017;43:1134–42. doi: 10.1093/schbul/sbw192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Jauhar S, Nour MM, Veronese M, Rogdaki M, Bonoldi I, Azis M, et al. A test of the transdiagnostic dopamine hypothesis of psychosis using positron emission tomographic imaging in bipolar affective disorder and Schizophrenia. JAMA Psychiatr. 2017;74:1206–13. doi: 10.1001/jamapsychiatry.2017.2943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Shin SY, Fauman EB, Petersen AK, Krumsiek J, Santos R, Huang J, et al. An atlas of genetic influences on human blood metabolites. Nat Genet. 2014;46:543–50. doi: 10.1038/ng.2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.McCutcheon R, Beck K, Jauhar S, Howes OD. Defining the locus of dopaminergic dysfunction in Schizophrenia: a meta-analysis and test of the mesolimbic hypothesis. Schizophr Bull. 2018;44:1301–11. doi: 10.1093/schbul/sbx180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Obi-Nagata K, Temma Y, Hayashi-Takagi A. Synaptic functions and their disruption in schizophrenia: from clinical evidence to synaptic optogenetics in an animal model. Proc Jpn Acad Ser B Phys Biol Sci. 2019;95:179–97. doi: 10.2183/pjab.95.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Keshavan M, Lizano P, Prasad K. The synaptic pruning hypothesis of schizophrenia: promises and challenges. World Psychiatr. 2020;19:110–1. doi: 10.1002/wps.20725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Germann M, Brederoo SG, Sommer IEC. Abnormal synaptic pruning during adolescence underlying the development of psychotic disorders. Curr Opin Psychiatr. 2021;34:222–7. doi: 10.1097/yco.0000000000000696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Purcell SM, Moran JL, Fromer M, Ruderfer D, Solovieff N, Roussos P, et al. A polygenic burden of rare disruptive mutations in schizophrenia. Nature. 2014;506:185–90. doi: 10.1038/nature12975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Fromer M, Pocklington AJ, Kavanagh DH, Williams HJ, Dwyer S, Gormley P, et al. De novo mutations in schizophrenia implicate synaptic networks. Nature. 2014;506:179–84. doi: 10.1038/nature12929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Tendilla-Beltran H, Antonio Vazquez-Roque R, Judith Vazquez-Hernandez A, Garces-Ramirez L, Flores G. Exploring the dendritic spine pathology in a Schizophrenia-related neurodevelopmental animal model. Neuroscience. 2019;396:36–45. doi: 10.1016/j.neuroscience.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 146.Chen P, Jing H, Xiong M, Zhang Q, Lin D, Ren D, et al. Spine impairment in mice high-expressing neuregulin 1 due to LIMK1 activation. Cell Death Dis. 2021;12:403. doi: 10.1038/s41419-021-03687-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Radhakrishnan R, Skosnik PD, Ranganathan M, Naganawa M, Toyonaga T, Finnema S, et al. In vivo evidence of lower synaptic vesicle density in schizophrenia. Mol Psychiatr. 2021;26:7690–8. doi: 10.1038/s41380-021-01184-0. [DOI] [PubMed] [Google Scholar]
- 148.Onwordi EC, Halff EF, Whitehurst T, Mansur A, Cotel MC, Wells L, et al. Synaptic density marker SV2A is reduced in schizophrenia patients and unaffected by antipsychotics in rats. Nat Commun. 2020;11:246. doi: 10.1038/s41467-019-14122-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Flint J, Munafò M. Schizophrenia: genesis of a complex disease. Nature. 2014;511:412–3. doi: 10.1038/nature13645. [DOI] [PubMed] [Google Scholar]
- 150.Kahn RS. On the origins of Schizophrenia. Am J Psychiatr. 2020;177:291–7. doi: 10.1176/appi.ajp.2020.20020147. [DOI] [PubMed] [Google Scholar]
- 151.Kahn RS, Sommer IE, Murray RM, Meyer-Lindenberg A, Weinberger DR, Cannon TD, et al. Schizophrenia. Nat Rev Dis Prim. 2015;1:15067. doi: 10.1038/nrdp.2015.67. [DOI] [PubMed] [Google Scholar]
- 152.Legge SE, Santoro ML, Periyasamy S, Okewole A, Arsalan A, Kowalec K. Genetic architecture of schizophrenia: a review of major advancements. Psychol Med. 2021;51:2168–77. doi: 10.1017/s0033291720005334. [DOI] [PubMed] [Google Scholar]
- 153.Purcell SM, Wray NR, Stone JL, Visscher PM, O’Donovan MC, Sullivan PF, et al. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460:748–52. doi: 10.1038/nature08185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Schizophrenia Psychiatric Genome-Wide Association Study (GWAS) Consortium Genome-wide association study identifies five new schizophrenia loci. Nat Genet. 2011;43:969–76. doi: 10.1038/ng.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Schizophrenia Working Group of the Psychiatric Genomics Consortium Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511:421–7. doi: 10.1038/nature13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Trubetskoy V, Pardiñas AF, Qi T, Panagiotaropoulou G, Awasthi S, Bigdeli TB, et al. Mapping genomic loci implicates genes and synaptic biology in schizophrenia. Nature. 2022;604:502–8. doi: 10.1038/s41586-022-04434-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sekar A, Bialas AR, de Rivera H, Davis A, Hammond TR, Kamitaki N, et al. Schizophrenia risk from complex variation of complement component 4. Nature. 2016;530:177–83. doi: 10.1038/nature16549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Gandal MJ, Zhang P, Hadjimichael E, Walker RL, Chen C, Liu S, et al. Transcriptome-wide isoform-level dysregulation in ASD, schizophrenia, and bipolar disorder. Science. 2018;362:eaat8127. doi: 10.1126/science.aat8127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Yue WH, Wang HF, Sun LD, Tang FL, Liu ZH, Zhang HX, et al. Genome-wide association study identifies a susceptibility locus for schizophrenia in Han Chinese at 11p11.2. Nat Genet. 2011;43:1228–31. doi: 10.1038/ng.979. [DOI] [PubMed] [Google Scholar]
- 160.Lam M, Chen CY, Li Z, Martin AR, Bryois J, Ma X, et al. Comparative genetic architectures of schizophrenia in East Asian and European populations. Nat Genet. 2019;51:1670–8. doi: 10.1038/s41588-019-0512-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Yu H, Yan H, Li J, Li Z, Zhang X, Ma Y, et al. Common variants on 2p16.1, 6p22.1 and 10q24.32 are associated with schizophrenia in Han Chinese population. Mol Psychiatr. 2017;22:954–60. doi: 10.1038/mp.2016.212. [DOI] [PubMed] [Google Scholar]
- 162.Shi Y, Li Z, Xu Q, Wang T, Li T, Shen J, et al. Common variants on 8p12 and 1q24.2 confer risk of schizophrenia. Nat Genet. 2011;43:1224–7. doi: 10.1038/ng.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Li Z, Chen J, Yu H, He L, Xu Y, Zhang D, et al. Genome-wide association analysis identifies 30 new susceptibility loci for schizophrenia. Nat Genet. 2017;49:1576–83. doi: 10.1038/ng.3973. [DOI] [PubMed] [Google Scholar]
- 164.Periyasamy S, John S, Padmavati R, Rajendren P, Thirunavukkarasu P, Gratten J, et al. Association of Schizophrenia risk with disordered niacin metabolism in an Indian genome-wide association study. JAMA Psychiatr. 2019;76:1026–34. doi: 10.1001/jamapsychiatry.2019.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Fiorica PN, Wheeler HE. Transcriptome association studies of neuropsychiatric traits in African Americans implicate PRMT7 in schizophrenia. PeerJ. 2019;7:e7778. doi: 10.7717/peerj.7778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Bigdeli TB, Genovese G, Georgakopoulos P, Meyers JL, Peterson RE, Iyegbe CO, et al. Contributions of common genetic variants to risk of schizophrenia among individuals of African and Latino ancestry. Mol Psychiatr. 2020;25:2455–67. doi: 10.1038/s41380-019-0517-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Meier SM, Agerbo E, Maier R, Pedersen CB, Lang M, Grove J, et al. High loading of polygenic risk in cases with chronic schizophrenia. Mol Psychiatr. 2016;21:969–74. doi: 10.1038/mp.2015.130. [DOI] [PubMed] [Google Scholar]
- 168.Fanous AH, Zhou B, Aggen SH, Bergen SE, Amdur RL, Duan J, et al. Genome-wide association study of clinical dimensions of schizophrenia: polygenic effect on disorganized symptoms. Am J Psychiatr. 2012;169:1309–17. doi: 10.1176/appi.ajp.2012.12020218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Jonas KG, Lencz T, Li K, Malhotra AK, Perlman G, Fochtmann LJ, et al. Schizophrenia polygenic risk score and 20-year course of illness in psychotic disorders. Transl Psychiatry. 2019;9:300. doi: 10.1038/s41398-019-0612-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Zhang JP, Robinson D, Yu J, Gallego J, Fleischhacker WW, Kahn RS, et al. Schizophrenia polygenic risk score as a predictor of antipsychotic efficacy in first-episode psychosis. Am J Psychiatr. 2019;176:21–8. doi: 10.1176/appi.ajp.2018.17121363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Singh T, Kurki MI, Curtis D, Purcell SM, Crooks L, McRae J, et al. Rare loss-of-function variants in SETD1A are associated with schizophrenia and developmental disorders. Nat Neurosci. 2016;19:571–7. doi: 10.1038/nn.4267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Singh T, Poterba T, Curtis D, Akil H, Al Eissa M, Barchas JD, et al. Rare coding variants in ten genes confer substantial risk for schizophrenia. Nature. 2022;604:509–16. doi: 10.1038/s41586-022-04556-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Rees E, Han J, Morgan J, Carrera N, Escott-Price V, Pocklington AJ, et al. De novo mutations identified by exome sequencing implicate rare missense variants in SLC6A1 in schizophrenia. Nat Neurosci. 2020;23:179–84. doi: 10.1038/s41593-019-0565-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Marshall CR, Howrigan DP, Merico D, Thiruvahindrapuram B, Wu W, Greer DS, et al. Contribution of copy number variants to schizophrenia from a genome-wide study of 41, 321 subjects. Nat Genet. 2017;49:27–35. doi: 10.1038/ng.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Richetto J, Massart R, Weber-Stadlbauer U, Szyf M, Riva MA, Meyer U. Genome-wide DNA methylation changes in a mouse model of infection-mediated neurodevelopmental disorders. Biol Psychiatr. 2017;81:265–76. doi: 10.1016/j.biopsych.2016.08.010. [DOI] [PubMed] [Google Scholar]
- 176.Li M, Li Y, Qin H, Tubbs JD, Li M, Qiao C, et al. Genome-wide DNA methylation analysis of peripheral blood cells derived from patients with first-episode schizophrenia in the Chinese Han population. Mol Psychiatr. 2021;26:4475–85. doi: 10.1038/s41380-020-00968-0. [DOI] [PubMed] [Google Scholar]
- 177.Hannon E, Spiers H, Viana J, Pidsley R, Burrage J, Murphy TM, et al. Methylation QTLs in the developing brain and their enrichment in schizophrenia risk loci. Nat Neurosci. 2016;19:48–54. doi: 10.1038/nn.4182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Jaffe AE, Gao Y, Deep-Soboslay A, Tao R, Hyde TM, Weinberger DR, et al. Mapping DNA methylation across development, genotype and schizophrenia in the human frontal cortex. Nat Neurosci. 2016;19:40–7. doi: 10.1038/nn.4181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Won H, de la Torre-Ubieta L, Stein JL, Parikshak NN, Huang J, Opland CK, et al. Chromosome conformation elucidates regulatory relationships in developing human brain. Nature. 2016;538:523–7. doi: 10.1038/nature19847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kiltschewskij DJ, Geaghan MP, Cairns MJ. Characterising the transcriptional and translational impact of the Schizophrenia-associated miR-1271-5p in neuronal cells. Cells. 2020;9:1014. doi: 10.3390/cells9041014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Cai HQ, Catts VS, Webster MJ, Galletly C, Liu D, O’Donnell M, et al. Increased macrophages and changed brain endothelial cell gene expression in the frontal cortex of people with schizophrenia displaying inflammation. Mol Psychiatr. 2020;25:761–75. doi: 10.1038/s41380-018-0235-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Perkins DO, Jeffries C, Sullivan P. Expanding the ‘central dogma’: the regulatory role of nonprotein coding genes and implications for the genetic liability to schizophrenia. Mol Psychiatr. 2005;10:69–78. doi: 10.1038/sj.mp.4001577. [DOI] [PubMed] [Google Scholar]
- 183.Panja D, Li Y, Ward ME, Li Z. miR-936 is increased in Schizophrenia and inhibits neural development and AMPA receptor-mediated synaptic transmission. Schizophr Bull. 2021;47:1795–805. doi: 10.1093/schbul/sbab046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Gusev A, Mancuso N, Won H, Kousi M, Finucane HK, Reshef Y, et al. Transcriptome-wide association study of schizophrenia and chromatin activity yields mechanistic disease insights. Nat Genet. 2018;50:538–48. doi: 10.1038/s41588-018-0092-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Hall LS, Medway CW, Pain O, Pardiñas AF, Rees EG, Escott-Price V, et al. A transcriptome-wide association study implicates specific pre- and post-synaptic abnormalities in schizophrenia. Hum Mol Genet. 2020;29:159–67. doi: 10.1093/hmg/ddz253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Polioudakis D, de la Torre-Ubieta L, Langerman J, Elkins AG, Shi X, Stein JL, et al. A single-cell transcriptomic atlas of human neocortical development during mid-gestation. Neuron. 2019;103:785–801.e8. doi: 10.1016/j.neuron.2019.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.van Os J, Kenis G, Rutten BP. The environment and schizophrenia. Nature. 2010;468:203–12. doi: 10.1038/nature09563. [DOI] [PubMed] [Google Scholar]
- 188.Lederbogen F, Kirsch P, Haddad L, Streit F, Tost H, Schuch P, et al. City living and urban upbringing affect neural social stress processing in humans. Nature. 2011;474:498–501. doi: 10.1038/nature10190. [DOI] [PubMed] [Google Scholar]
- 189.Pardiñas AF, Holmans P, Pocklington AJ, Escott-Price V, Ripke S, Carrera N, et al. Common schizophrenia alleles are enriched in mutation-intolerant genes and in regions under strong background selection. Nat Genet. 2018;50:381–9. doi: 10.1038/s41588-018-0059-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Ursini G, Punzi G, Chen Q, Marenco S, Robinson JF, Porcelli A, et al. Convergence of placenta biology and genetic risk for schizophrenia. Nat Med. 2018;24:792–801. doi: 10.1038/s41591-018-0021-y. [DOI] [PubMed] [Google Scholar]
- 191.Cheng W, Luo N, Zhang Y, Zhang X, Tan H, Zhang D, et al. DNA methylation and resting brain function mediate the association between childhood Urbanicity and better speed of processing. Cerebral cortex. 2021;31:4709–18. doi: 10.1093/cercor/bhab117. (New York, NY: 1991) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Wang D, Liu S, Warrell J, Won H, Shi X, Navarro FCP, et al. Comprehensive functional genomic resource and integrative model for the human brain. Science. 2018;362:1–13. doi: 10.1126/science.aat8464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Li M, Santpere G, Imamura Kawasawa Y, Evgrafov OV, Gulden FO, Pochareddy S, et al. Integrative functional genomic analysis of human brain development and neuropsychiatric risks. Science. 2018;362:1–15. doi: 10.1126/science.aat7615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Skene NG, Bryois J, Bakken TE, Breen G, Crowley JJ, Gaspar HA, et al. Genetic identification of brain cell types underlying schizophrenia. Nat Genet. 2018;50:825–33. doi: 10.1038/s41588-018-0129-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Logan S, Arzua T, Canfield SG, Seminary ER, Sison SL, Ebert AD, et al. Studying human neurological disorders using induced pluripotent stem cells: from 2D monolayer to 3D organoid and blood brain barrier models. Compr Physiol. 2019;9:565–611. doi: 10.1002/cphy.c180025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Soldner F, Jaenisch R. Stem cells, genome editing, and the path to translational medicine. Cell. 2018;175:615–32. doi: 10.1016/j.cell.2018.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Liu W, Zahr RS, Prola J, Cetas JS, Dogan A, Fleseriu M. Clinical outcomes in patients (Male) with lactotroph adenomas that required pituitary surgery-a large single center experience. Endocr Rev. 2018;21:454–62. doi: 10.1007/s11102-018-0898-y. [DOI] [PubMed] [Google Scholar]
- 198.Lee D, Seo J, Jeong HC, Lee H, Lee SB. The perspectives of early diagnosis of Schizophrenia through the detection of epigenomics-based biomarkers in iPSC-derived neurons. Front Mol Neurosci. 2021;14:756613. doi: 10.3389/fnmol.2021.756613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Lee D, Choi YH, Seo J, Kim JK, Lee SB. Discovery of new epigenomics-based biomarkers and the early diagnosis of neurodegenerative diseases. Ageing Res Rev. 2020;61:101069. doi: 10.1016/j.arr.2020.101069. [DOI] [PubMed] [Google Scholar]
- 200.Chiang CH, Su Y, Wen Z, Yoritomo N, Ross CA, Margolis RL, et al. Integration-free induced pluripotent stem cells derived from schizophrenia patients with a DISC1 mutation. Mol Psychiatr. 2011;16:358–60. doi: 10.1038/mp.2011.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Iritani S. What happens in the brain of schizophrenia patients? an investigation from the viewpoint of neuropathology. Nagoya J Med Sci. 2013;75:11–28. [PMC free article] [PubMed] [Google Scholar]
- 202.Brennand KJ, Simone A, Jou J, Gelboin-Burkhart C, Tran N, Sangar S, et al. Modelling schizophrenia using human induced pluripotent stem cells. Nature. 2011;473:221–5. doi: 10.1038/nature09915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Grunwald LM, Stock R, Haag K, Buckenmaier S, Eberle MC, Wildgruber D, et al. Comparative characterization of human induced pluripotent stem cells (hiPSC) derived from patients with schizophrenia and autism. Transl Psychiatry. 2019;9:179. doi: 10.1038/s41398-019-0517-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Li Y, Jia X, Wu H, Xun G, Ou J, Zhang Q, et al. Genotype and phenotype correlations for SHANK3 de novo mutations in neurodevelopmental disorders. Am J Med Genet. 2018;176:2668–76. doi: 10.1002/ajmg.a.40666. [DOI] [PubMed] [Google Scholar]
- 205.Robicsek O, Karry R, Petit I, Salman-Kesner N, Müller FJ, Klein E, et al. Abnormal neuronal differentiation and mitochondrial dysfunction in hair follicle-derived induced pluripotent stem cells of schizophrenia patients. Mol Psychiatr. 2013;18:1067–76. doi: 10.1038/mp.2013.67. [DOI] [PubMed] [Google Scholar]
- 206.Wen Z, Nguyen HN, Guo Z, Lalli MA, Wang X, Su Y, et al. Synaptic dysregulation in a human iPS cell model of mental disorders. Nature. 2014;515:414–8. doi: 10.1038/nature13716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Topol A, Zhu S, Tran N, Simone A, Fang G, Brennand KJ. Altered WNT signaling in human induced pluripotent stem cell neural progenitor cells derived from four Schizophrenia patients. Biol Psychiatr. 2015;78:e29–34. doi: 10.1016/j.biopsych.2014.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Yoon KJ, Nguyen HN, Ursini G, Zhang F, Kim NS, Wen Z, et al. Modeling a genetic risk for schizophrenia in iPSCs and mice reveals neural stem cell deficits associated with adherens junctions and polarity. Cell Stem Cell. 2014;15:79–91. doi: 10.1016/j.stem.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Yu DX, Di Giorgio FP, Yao J, Marchetto MC, Brennand K, Wright R, et al. Modeling hippocampal neurogenesis using human pluripotent stem cells. Stem Cell Rep. 2014;2:295–310. doi: 10.1016/j.stemcr.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Vainchtein ID, Molofsky AV. Astrocytes and microglia: in sickness and in health. Trends Neurosci. 2020;43:144–54. doi: 10.1016/j.tins.2020.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Kim HS, Suh YH. Minocycline and neurodegenerative diseases. Behav Brain Res. 2009;196:168–79. doi: 10.1016/j.bbr.2008.09.040. [DOI] [PubMed] [Google Scholar]
- 212.Sellgren CM, Gracias J, Watmuff B, Biag JD, Thanos JM, Whittredge PB, et al. Increased synapse elimination by microglia in schizophrenia patient-derived models of synaptic pruning. Nat Neurosci. 2019;22:374–85. doi: 10.1038/s41593-018-0334-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Sellgren CM, Sheridan SD, Gracias J, Xuan D, Fu T, Perlis RH. Patient-specific models of microglia-mediated engulfment of synapses and neural progenitors. Mol Psychiatr. 2017;22:170–7. doi: 10.1038/mp.2016.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Toyoshima M, Akamatsu W, Okada Y, Ohnishi T, Balan S, Hisano Y, et al. Analysis of induced pluripotent stem cells carrying 22q11.2 deletion. Transl Psychiatry. 2016;6:e934. doi: 10.1038/tp.2016.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Akkouh IA, Hribkova H, Grabiec M, Budinska E, Szabo A, Kasparek T, et al. Derivation and molecular characterization of a morphological subpopulation of human iPSC astrocytes reveal a potential role in Schizophrenia and Clozapine response. Schizophr Bull. 2022;48:190–8. doi: 10.1093/schbul/sbab092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.McPhie DL, Nehme R, Ravichandran C, Babb SM, Ghosh SD, Staskus A, et al. Oligodendrocyte differentiation of induced pluripotent stem cells derived from subjects with schizophrenias implicate abnormalities in development. Transl Psychiatry. 2018;8:230. doi: 10.1038/s41398-018-0284-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.de Vrij FM, Bouwkamp CG, Gunhanlar N, Shpak G, Lendemeijer B, Baghdadi M, et al. Candidate CSPG4 mutations and induced pluripotent stem cell modeling implicate oligodendrocyte progenitor cell dysfunction in familial schizophrenia. Mol Psychiatr. 2019;24:757–71. doi: 10.1038/s41380-017-0004-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Banaschewski T, Brandeis D. Annotation: what electrical brain activity tells us about brain function that other techniques cannot tell us – a child psychiatric perspective. JCPP (J Child Psychol Psychiatry) 2007;48:415–35. doi: 10.1111/j.1469-7610.2006.01681.x. [DOI] [PubMed] [Google Scholar]
- 219.Shelley AM, Ward PB, Catts SV, Michie PT, Andrews S, McConaghy N. Mismatch negativity: an index of a preattentive processing deficit in schizophrenia. Biol Psychiatr. 1991;30:1059–62. doi: 10.1016/0006-3223(91)90126-7. [DOI] [PubMed] [Google Scholar]
- 220.Wynn JK, Sugar C, Horan WP, Kern R, Green MF. Mismatch negativity, social cognition, and functioning in schizophrenia patients. Biol Psychiatr. 2010;67:940–7. doi: 10.1016/j.biopsych.2009.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Polich J. Updating P300: an integrative theory of P3a and P3b. Clin Neurophysiol. 2007;118:2128–48. doi: 10.1016/j.clinph.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Hajcak G, MacNamara A, Olvet DM. Event-related potentials, emotion, and emotion regulation: an integrative review. Dev Neuropsychol. 2010;35:129–55. doi: 10.1080/87565640903526504. [DOI] [PubMed] [Google Scholar]
- 223.Castro MK, Bailey DH, Zinger JF, Martin EA. Late electrophysiological potentials and emotion in schizophrenia: a meta-analytic review. Schizophr Res. 2019;211:21–31. doi: 10.1016/j.schres.2019.07.013. [DOI] [PubMed] [Google Scholar]
- 224.Moran LV, Hong LE. High vs low frequency neural oscillations in schizophrenia. Schizophr Bull. 2011;37:659–63. doi: 10.1093/schbul/sbr056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Kozhemiako N, Wang J, Jiang C, Wang LA, Gai G, Zou K, et al. Non-rapid eye movement sleep and wake neurophysiology in schizophrenia. Elife. 2022;11:e76211. doi: 10.7554/eLife.76211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Haggard P. Sense of agency in the human brain. Nat Rev Neurosci. 2017;18:196–207. doi: 10.1038/nrn.2017.14. [DOI] [PubMed] [Google Scholar]
- 227.van der Weiden A, Prikken M, van Haren NE. Self-other integration and distinction in schizophrenia: a theoretical analysis and a review of the evidence. Neurosci Biobehav Rev. 2015;57:220–37. doi: 10.1016/j.neubiorev.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 228.Sass LA, Parnas J. Schizophrenia, consciousness, and the self. Schizophr Bull. 2003;29:427–44. doi: 10.1093/oxfordjournals.schbul.a007017. [DOI] [PubMed] [Google Scholar]
- 229.Lee J, Park S. Working memory impairments in schizophrenia: a meta-analysis. J Abnorm Psychol. 2005;114:599–611. doi: 10.1037/0021-843x.114.4.599. [DOI] [PubMed] [Google Scholar]
- 230.Phillipson OT, Harris JP. Perceptual changes in schizophrenia: a questionnaire survey. Psychol Med. 1985;15:859–66. doi: 10.1017/s0033291700005092. [DOI] [PubMed] [Google Scholar]
- 231.Sommer MA, Wurtz RH. A pathway in primate brain for internal monitoring of movements. Science. 2002;296:1480–2. doi: 10.1126/science.1069590. [DOI] [PubMed] [Google Scholar]
- 232.Kochunov P, Hong LE. Neurodevelopmental and neurodegenerative models of schizophrenia: white matter at the center stage. Schizophr Bull. 2014;40:721–8. doi: 10.1093/schbul/sbu070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.London A, Benhar I, Schwartz M. The retina as a window to the brain-from eye research to CNS disorders. Nat Rev Neurol. 2013;9:44–53. doi: 10.1038/nrneurol.2012.227. [DOI] [PubMed] [Google Scholar]
- 234.Lavoie J, Maziade M, Hébert M. The brain through the retina: the flash electroretinogram as a tool to investigate psychiatric disorders. Prog Neuro-Psychopharmacol Biol Psychiatry. 2014;48:129–34. doi: 10.1016/j.pnpbp.2013.09.020. [DOI] [PubMed] [Google Scholar]
- 235.Komatsu H, Onoguchi G, Jerotic S, Kanahara N, Kakuto Y, Ono T, et al. Retinal layers and associated clinical factors in schizophrenia spectrum disorders: a systematic review and meta-analysis. Mol Psychiatr. 2022:1–25. doi: 10.1038/s41380-022-01591-x. [DOI] [PubMed] [Google Scholar]
- 236.Stogios N, Gdanski A, Gerretsen P, Chintoh AF, Graff-Guerrero A, Rajji TK, et al. Autonomic nervous system dysfunction in schizophrenia: impact on cognitive and metabolic health. NPJ Schizophr. 2021;7:22. doi: 10.1038/s41537-021-00151-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Lazaridi M, Panagiotaropoulou G, Covanis P, Karantinos T, Aggelopoulos E, Klein C, et al. Brain-heart link in Schizophrenia: cognitive inhibitory control deficit in patients is specifically related to parasympathetic dysregulation. Schizophr Bull. 2022:1–9. doi: 10.1093/schbul/sbac033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Montaquila JM, Trachik BJ, Bedwell JS. Heart rate variability and vagal tone in schizophrenia: a review. J Psychiatr Res. 2015;69:57–66. doi: 10.1016/j.jpsychires.2015.07.025. [DOI] [PubMed] [Google Scholar]
- 239.Clamor A, Lincoln TM, Thayer JF, Koenig J. Resting vagal activity in schizophrenia: meta-analysis of heart rate variability as a potential endophenotype. Br J Psychiatry. 2016;208:9–16. doi: 10.1192/bjp.bp.114.160762. [DOI] [PubMed] [Google Scholar]
- 240.Rinninella E, Raoul P, Cintoni M, Franceschi F, Miggiano GAD, Gasbarrini A, et al. What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms. 2019;7:E14. doi: 10.3390/microorganisms7010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Kanji S, Fonseka TM, Marshe VS, Sriretnakumar V, Hahn MK, Müller DJ. The microbiome-gut-brain axis: implications for schizophrenia and antipsychotic induced weight gain. Eur Arch Psychiatr Clin Neurosci. 2018;268:3–15. doi: 10.1007/s00406-017-0820-z. [DOI] [PubMed] [Google Scholar]
- 242.Davey KJ, Cotter PD, O’Sullivan O, Crispie F, Dinan TG, Cryan JF, et al. Antipsychotics and the gut microbiome: olanzapine-induced metabolic dysfunction is attenuated by antibiotic administration in the rat. Transl Psychiatry. 2013;3:e309. doi: 10.1038/tp.2013.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Zheng P, Zeng B, Liu M, Chen J, Pan J, Han Y, et al. The gut microbiome from patients with schizophrenia modulates the glutamate-glutamine-GABA cycle and schizophrenia-relevant behaviors in mice. Sci Adv. 2019;5:eaau8317. doi: 10.1126/sciadv.aau8317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Dinan TG, Cryan JF. Schizophrenia and the microbiome: time to focus on the impact of antipsychotic treatment on the gut microbiota. World J Biol Psychiatr. 2018;19:568–70. doi: 10.1080/15622975.2018.1540793. [DOI] [PubMed] [Google Scholar]
- 245.Arnedo J, Svrakic DM, Del Val C, Romero-Zaliz R, Hernández-Cuervo H, Fanous AH, et al. Uncovering the hidden risk architecture of the schizophrenias: confirmation in three independent genome-wide association studies. Am J Psychiatr. 2015;172:139–53. doi: 10.1176/appi.ajp.2014.14040435. [DOI] [PubMed] [Google Scholar]
- 246.Wand H, Lambert SA, Tamburro C, Iacocca MA, O’Sullivan JW, Sillari C, et al. Improving reporting standards for polygenic scores in risk prediction studies. Nature. 2021;591:211–9. doi: 10.1038/s41586-021-03243-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Bjerkenstedt L, Edman G, Hagenfeldt L, Sedvall G, Wiesel F-A. Plasma amino acids in relation to cerebrospinal fluid monoamine metabolites in schizophrenic patients and healthy controls. Br J Psychiatry. 1985;147:276–82. doi: 10.1192/bjp.147.3.276. [DOI] [PubMed] [Google Scholar]
- 248.Merritt K, McGuire PK, Egerton A, Investigators HMiS , Aleman A, Bloemen O J N, Block W, et al. Association of age, antipsychotic medication, and symptom severity in Schizophrenia with proton magnetic resonance spectroscopy brain glutamate level: a mega-analysis of individual participant-level data. JAMA Psychiatr. 2021;78:667–81. doi: 10.1001/jamapsychiatry.2021.0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Robicsek O, Karry R, Petit I, Salman-Kesner N, Muller FJ, Klein E, et al. Abnormal neuronal differentiation and mitochondrial dysfunction in hair follicle-derived induced pluripotent stem cells of schizophrenia patients. Mol Psychiatr. 2013;18:1067–76. doi: 10.1038/mp.2013.67. [DOI] [PubMed] [Google Scholar]
- 250.Perrottelli A, Giordano GM, Brando F, Giuliani L, Mucci A. EEG-based measures in at-risk mental state and early stages of Schizophrenia: a systematic review. Front Psychiatr. 2021;12:653642. doi: 10.3389/fpsyt.2021.653642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Mancini V, Rochas V, Seeber M, Roehri N, Rihs TA, Ferat V, et al. Aberrant developmental patterns of gamma-band response and long-range communication disruption in youths with 22q11.2 deletion Syndrome. Am J Psychiatr. 2022;179:204–15. doi: 10.1176/appi.ajp.2021.21020190. [DOI] [PubMed] [Google Scholar]
- 252.Thomas EHX, Steffens M, Harms C, Rossell SL, Gurvich C, Ettinger U. Schizotypy, neuroticism, and saccadic eye movements: new data and meta-analysis. Psychophysiology. 2021;58:e13706. doi: 10.1111/psyp.13706. [DOI] [PubMed] [Google Scholar]