Abstract
Androgenetic alopecia is the most common cause of hair loss aggravated by increased life pressure, tension, and anxiety. Although androgenetic alopecia (AGA) does not significantly effect physical health, it can have serious negative impact on the mental health and quality of life of the patient. Currently, the effect of medical treatment for AGA is not idealistic, stem cell-based regenerative medicine has shown potential for hair regrowth and follicle repair, but the long-term effect and mechanism of stem cell therapy is not quite explicit. In this review, we summarize the methods, efficacy, mechanism, and clinical progress of stem cell therapies for AGA by now, hope it will present a more comprehensive view in this topic.
Graphical Abstract
Keywords: Androgenetic Alopecia, Stem Cells, Cells Therapy, Conditioned Media
Introduction
Alopecia is a globally prevalent disease which is mainly caused by genetics, hormonal disorders, immune inflammation, malnutrition, environmental factors, mental disorders, aging, and other factors [1]. Androgenetic alopecia (AGA), the most common type of hair loss, has emerged as a medical and social issue due to its onset at a young age. It can be related to psychological problems such as depression, anxiety, and mood disorders [2]. AGA is characterized by the progressive miniaturization of hair follicles and a shortened growth period of dermal papilla cells (DPCs) leading to hair loss [3]. By the age of 50, approximately 50% of men and 45% of women are affected by AGA [4]. Although the pathogenesis of AGA remains controversial, it is generally related with the expression of dihydrotestosterone (DHT), which is converted from free testosterone by type II 5-α reductase [5]. DHT miniaturizes hair follicles by gradual hair thinning during the growth period, shortens the hair growth cycle by thinning and shallowing the original coarse and black hair, and leads to hair loss due to hair follicle atrophy and extinction [6]. DHT accumulation in androgen-sensitive hair follicles results in a higher DHT expression in balding scalp tissues compared to the non-balding scalp tissues [7, 8]. AGA has been associated with the loss of attachment between an enlarged stem cell population and the arrector pili muscle [9].
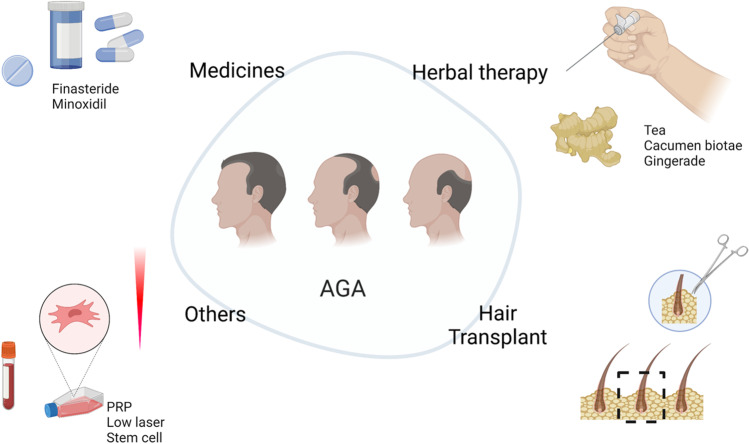
At present, the treatments promoting hair regrowth or preventing hair loss include drug treatments, surgical treatments, herbal medicines, biotherapies, and other physical treatments [10]. Though effective, these treatments are limited and have several side effects [11].The treatment of hair loss using stem cells is not only cost effective but also produces faster results with simple treatment processes Fig 1, 2 and 3. As a result, stem cell therapeutics-related research has made significant achievements [12]. Stem cell technology has long been regarded as a "regenerative medicine technology" and has been hailed as the third medical revolution after drug therapy and surgery, Stem cell therapy has emerged as a new treatment for hair loss. This review provides a systematic review of the methods, efficacy, advantages and disadvantages of stem cell therapy for androgenetic alopecia from the perspective of the etiology of hair loss.
Fig. 1.
Traditional therapies for androgenetic alopecia
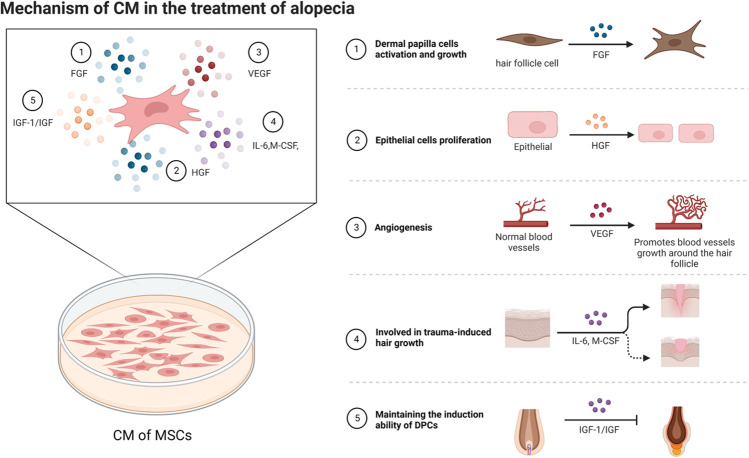
Fig. 2.
Mechanism of CM in the treatment of alopecia
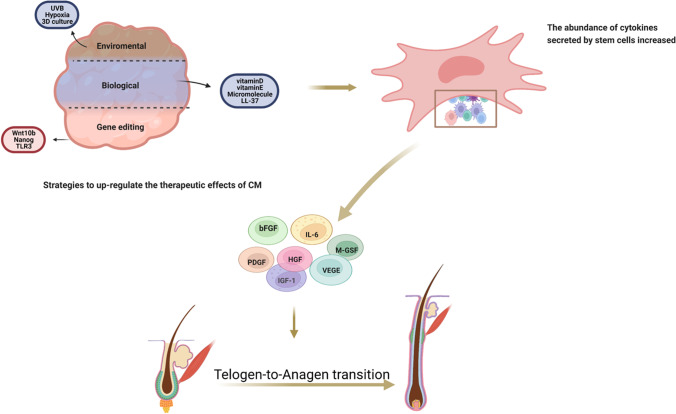
Fig. 3.
Strategies to up-regulate the therapeutic effects of CM on hair regeneration
Etiopathogenesis
Genetics
The pathogenesis of androgenetic alopecia AGA is driven by androgens, with genetic susceptibility being the main prerequisite. Data have been reported showing that 35 of 57 childhood AGA patients (22 boys and 13 girls) had a family history, and 29 of the 35 (83%) had a family history of hereditary alopecia in a first or second degree relative[13]. A study of candidate genes and genome-wide associations analysed replicate sets containing 2759 cases and 2661 controls of European descent to confirm that the analysis identified four genome-wide significant risk loci for AGA on chromosomes 2q35, 3q25.1, 5q33.3 and 12p12.1. Of these, rs7349332 obtained the strongest association signal on chr2q35, which is located in WNT10A, providing genetic evidence for the involvement of WNT signalling in AGA development[14]. It has also been demonstrated that a variant of PADI3 is present in central centrifugal scarring alopecia [15]. The study of Rui et al. identified rs6493497 and rs7176005 as significantly associated with female pattern hair loss (FPHL) [16]. In addition, the authors found that rs4646 was not associated with FPHL,While rs4646 was one of the top ranked snp in the Yip et al.'s study[17]. Another study showed that susceptibility to early-onset FHPL was associated with the AR/EDA2R locus[18]. The study of Elaine G Y Chewet al identified differentially expressed genes between balding and non-balding DPCs, revealing downregulation of vascular-related genes in balding DPCs, evidence for AR rather than EDA2R as a candidate gene at the X-chromosome AGA risk locus.It also revealed that TWIST1 and SSPN are functionally relevant AGA genes at the 7p21.1 and 12p12.1 risk loci, respectively[19].
Androgen/Androgen Receptor
Although genetic factors play an important role in androgenetic alopecia, an increasing number of studies suggest that androgen/androgen receptor is also one of the key mediators of AGA[20]. Hamilton's study of 21 pre-castration and post-castration adult males over 8 to 18 years showed no baldness after orchiectomy[21]. Castration before puberty prevents beard growth, while castration between 16 and 20 years partially prevents full beard development, and castration after 20 years has no effect on beard growth, suggesting a close association between AGA and androgens [22]. The survey found that androgens affect about 73% of men and 57% of women over the age of 80, and 58% of men over the age of 50 [23]. Androgens influence a variety of human skin functions through intracellular signalling pathways, such as sebaceous gland growth and differentiation, hair growth, epidermal barrier homeostasis and wound healing [24]. As AGA does not correlate with serum testosterone, free testosterone and bioavailable testosterone levels, its pathogenic basis may be mediated through intracellular signalling from hair follicle target cells [25]. Testosterone is a lipophilic hormone that is converted to the more potent DHT by the enzyme 5α reductase after freely penetrating the cell membrane into the cytoplasm, and is five times more potent than testosterone in binding to androgen receptors in susceptible scalp [26]. The effects of androgens such as testosterone and DHT on the skin are mainly mediated through the androgen receptor (AR), a ligand-dependent nuclear transcription factor belonging to the steroid hormone nuclear receptor superfamily [27]. Meanwhile, the androgen receptor is a polymeric complex that includes the heat shock proteins (HSP) HSP90, HSP70 and HSP56, initially located in the cytoplasm [28]. DHT is transferred to the nucleus after forming an AR-DHT dimer with an AR located in the cytoplasm of the target cell, and the AR co-activator is recruited to the AR-DHT complex, which binds to the androgen response element, leading to the transcription and eventual translation of the target gene into a protein that exerts biological activity [29]. Under the influence of androgens, the dermal papillae of the hair follicle secrete many cytokines such as TGFβ 1, IL-1α and TNFα, which induce premature termination of the early growth phase of the hair follicle [30]. Cortisone 17a-propionate (CB-03–01, also known as clascoterone, Breezula™) is a synthetic androgen receptor antagonist used to treat androgen-driven conditions, including acne vulgaris and AGA. one of the phase 2 studies (NCT02279823) compared CB-03–01 solution with 5% minoxidil solution and a control placebo for safety and efficacy in 95 male patients with AGA. After 6 months, the results showed an increase in the number of hairs in the target area in the CB-03–01 group compared to the control placebo group (12.7 vs. 2.9), but the 5% minoxidil solution was superior to both groups (18.8)[31].
Microinflammation
Although AGA is classified as a non-inflammatory, non-scarring form of hair loss, histological evidence of inflammation has long been recognised [32]. A previous series of 17 women with AGA and 5 normal controls showed a significant association between the severity of inflammatory infiltration and the degree of miniaturisation [33]. In a study with a primary cohort of women with alopecia, biopsies revealed follicular shrinkage in all specimens and perifollicular lymphocytic infiltration in most specimens (87.9% AGA, 81.6% AA), with no significant difference in prevalence [34]. In close proximity to the hair follicle, there are subdermal TREM2 + macrophages called "Trichophages", uring the resting phase, Trichophages produce the cytokine OSM (Oncostatin M) which binds to the OSM receptor on hair follicle stem cells, phosphorylating STAT5 in hair follicle stem cells (HFSCs) and inhibiting stem cell activation, leading to prolonged resting phase and delaying hair growth[35]. Jaworsky examined the hair, transitional and alopecic scalp of three men and one woman with progressive alopecia; immunohistochemical results showed that thickening of the outer follicular sheath in the transitional and alopecic areas was associated with mast cell degranulation and fibroblast activation within the fibrous sheath; control biopsies did not show follicular inflammation, while the transitional area consistently showed an infiltration of activated T cells in the lower part of the follicular funnel [36]. A previous study by John Plante et al. showed that perifollicular infiltration was present in 73% of AGA and 84% of control specimens, however, funnel and isthmus involvement was more common in AGA (P < 0.037 and P < 0.012, respectively)[34]. In a study that identified inflammation-related regulators such as activator protein 1 (AP-1) subunits (FOS, FOSB, JUN, JUNB, etc.), TLRs, PTGS, EGRs, AREG, HSPA1B were substantially upregulated in the AGA group using transcriptome analysis at different locations in the hair follicle [37]. Further studies have demonstrated the important impact of Treg cells on the maintenance of HFSC and that immune cells are essential for hair regeneration [38]. These findings highlight the link between inflammation and hair loss [39].
Others
Androgenetic alopecia is not only associated with genetics, immunity and microinflammation, but also with a number of other factors that influence the process of hair loss. Some in vitro human studies have shown increased markers of oxidative stress and increased sensitivity to oxidative stress in dermal papilla cells of balding scalp compared to non-balding scalp [40]. A study of microarray gene expression data from male patients with and without baldness on the scalp found that the expression of genes related to the oxidative stress pathway was upregulated in cultured human hair follicles and that oxidative stress has been shown to cause apoptosis and inhibit cell matrix growth [41]. Age is also strongly associated with AGA, with a decrease in HFSCs with age leading to hair loss [42]. Furthermore, it has been suggested that obesity is not only a risk factor for hair loss, but may synergize with repetitive hair cycles or aging-induced changes that significantly inhibit HFSC self-renewal [43]. Inhibition of autophagy with 3-methyladenine induces apoptosis, premature follicular regression and slows hair growth in organ culture follicles, so autophagic damage may be a potential mechanism for androgenetic alopecia [44]. A study that recruited 1,000 healthy men between the ages of 20 and 35 and developed a customised questionnaire to determine basic physical and smoking habits found that the prevalence of AGA was higher among smokers than non-smokers, and that the severity of AGA was not related to smoking intensity[45]. One questionnaire study found that age 30–40, marital status, poor sleep habits, meat consumption, junk food consumption, and heavy work were factors that influenced the development of more severe forms of AGA, another important finding of this study is that both late sleep and poor sleep quality also increase the risk of developing more severe types of AGA [46]. UV exposure also induces oxidative DNA damage and cytotoxicity in human hair follicle cells [47]. In addition, alopecia is also affected by microbiota. Hair follicles were extracted from the occipital and apical parts of alopecia patients and healthy volunteers, and 16S rRNA sequencing of the microbiome revealed that the middle hair compartment of the follicle was dominated by Burkholderia, and was less diverse; whereas bacterial diversity was higher in the lower part of the hair, with no significant differences between the occipital and apical follicles. In patients with alopecia, the lower middle compartment of the reduced hair tip contained Propionibacterium acnes, while non-reduced hair in other regions was comparable to healthy hair [48].
Current Treatment for Hair Loss
For hair loss, early prevention, diagnosis, and treatment are internationally recognized. AGA shows progressive aggravation if left untreated. Therefore, AGA treatment aims to prevent hair miniaturization, induce hair thickening, and promote regrowth [49]. To date, finasteride and minoxidil are the only two FDA-approved drugs for the treatment of AGA [50]. Finasteride affects hair growth by acting on DPCs by improving the aggregation behavior of stem cells and enhancing the expression of stem cell transcription factors Nanog and Sox-2 [51]. However, long-term use of finasteride can negatively impact sexual functions, such as decreased libido, decreased ejaculation, increased infertility, and erectile dysfunction [52]. Minoxidil 5%, a common topical drug, when applied externally can stimulate DPCs and hair follicle stromal cells. It effectively prevents miniaturization of hair follicles and promotes limited regeneration [53]. However, the side effects include aggravation of seborrheic dermatitis, irritant contact dermatitis, and allergic contact dermatitis [54]. Bimatoprost, a PGF2 analog, has some hair growth inducing effects but is less effective than minoxidil [31].
Apart from the FDA-approved drugs, there are several treatments to combat hair loss. Some herbal medicines such as Polygonum multiflorum, Astragalus membranaceus, Platycladus orientalis leaves, and plum blossom needle puncture have similar curative effects [55, 56]. Their major drawback is that they have complex components, unclear mechanisms of action, and slow affects, which often requires long-term care to be effective [57]. Hair transplantation is another alternative but instead of increasing hair thickness it redistributes hair [58]. Interestingly, the transplantation surgery is considered invasive, and scarcity of hair for redistribution can be encountered [59]. Alternatively, microneedle therapy is a minimally invasive procedure that employs multiple fine needles to create microneedles on the skin and stimulate new blood vessels, Wnt protein expression, and growth factors [60]. When combined with other drugs, microneedles can further stimulate hair growth [61, 62]. Platelet-rich plasma can also treat AGA by improving the survival of DPCs during the hair growth cycle through anti-apoptotic effects [63, 64]. Low-dose light therapy not only improves the cure rate of AGA but also improves patient satisfaction [65]. Patients who do not respond to or choose medical or surgical treatment use hair patches, wigs, or scarves. The advantages and disadvantages of various treatment methods are compared in Table 1.
Table 1.
Advantages and disadvantages of various treatments for androgenetic alopecia
| Therapy method | Therapeutic effect | Advantage | Disadvantage | Reference | |
|---|---|---|---|---|---|
| Surgical therapy | Hair Transplantation | Different degrees of shedding in the early postoperative period, no obvious effect can be seen until 6–9 months after the operation | Effective, Security | The high cost, Limited hair follicles | [66] [67] |
| Microneedle | Hair density and diameter increased | Security, well tolerated | The pain is intense, a risk of infection | [68] | |
| Drug therapy | Finasteride | Take orally 1 mg daily. Generally, hair loss decreases after 3 months | The incidence of adverse reactions was low and mild | Individual patients appear libido loss, impotence and ejaculation reduction, easy to relapse after drug withdrawal | [69] |
| Minoxidil | Hair growth is significantly increased, hair loss is reduced, 5% formula is more effective | Effective promotion of hair regeneration, high safety | Dermatitis, headache and hypertrichosis as well as poor dependence, easy to relapse after drug withdrawal | [70] | |
| Herbs therapy | Oral and Topical | Prevent and slow hair loss | High compliance, less side effects, wide spectrum of activity low price, wide range of use | Active ingredients are uncertain, plants and formulations are not standardized | [57] |
| Other therapy | Platelet plasma rich | After 6 months of subcutaneous injection, the average number and thickness of hair increased significantly | Minimally invasive, fewer safety issues and side effects | Preparation methods, frequency of treatment, and area of treatment have not been standardized | [71] |
| Laser therapy | Promote hair regeneration alleviate hair loss, hair luster significantly improved | The laser comb is convenient and easy to operate | Transient hair loss during the resting period may occur at the beginning of treatment | [72] | |
| Stem cells | Promotes the proliferation of hair follicle cells | Economy, effective | Large, more robust double-blind controlled clinical trials are lacking to further evaluate the exact mechanism, therapeutic potential and safety of stem cell-based hair treatments | [12] |
Current treatments for hair loss include drugs including finasteride and minoxidil, hair transplantation, platelet-rich plasma, low laser therapy and physical occlusion, ect.
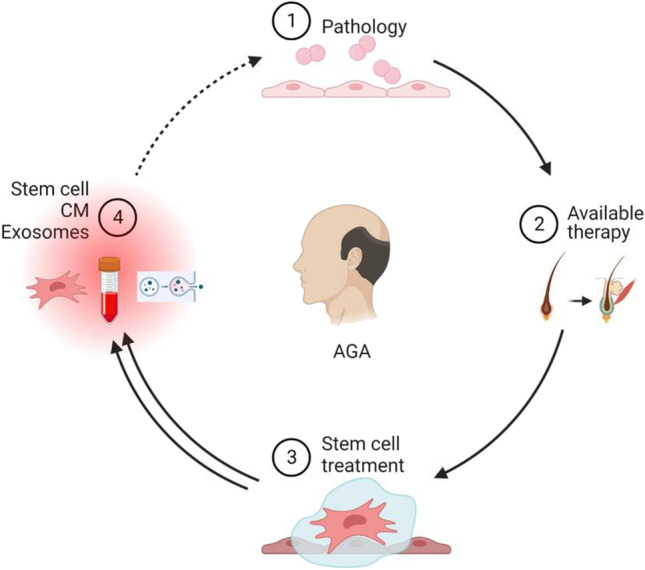
Stem Cell-based Therapies for AGA
With the development of regenerative medicine, stem cell-based therapies have received attention as potential new therapies. These therapies reactivate hair follicle stem cells, thereby promoting hair follicle growth, regeneration, and development [73, 74]. Stem cell therapy for AGA includes stem cell transplantation, stem cell-derived conditioned media, and stem cell-derived exosomes [12]. Compared to hair transplantation and drug therapy, stem cell-based biotherapy has brighter prospects. Table 2 lists some clinical trials involving stem cells for hair regeneration.
Table 2.
Clinical trials based on stem cell in androgenetic alopecia
| Stem cell source | Time | Recruitment | Method | Results | Reference |
|---|---|---|---|---|---|
| Adipose tissue | 12 weeks | 27 | Application of AAPE™ with micro-needle roller | Hair density increased from 105.4 to 122.7 hairs/cm2 (P < 0.001); Hair thickness increased from 57.5 mum to 64.0 mum (P < 0.001) | [75] |
| Hair follicles | 23 weeks | 21 | HFSCs injections 1 mL (0.2 mL·cm2) were administered to select areas of the scalp at a depth of 5 mm; Injections performed in two sessions spaced 60 days | 29% ± 5% increase in hair density for the treated area and less than a 1% increase in hair density for the placebo area | [63] |
| Adipose tissue | 16 weeks | 38 | 130-mL topical solution, apply 2 mL of this solution to the hair loss area, twice every day for 16 weeks | Hair diameter after 16 weeks was observed in IG compared with that in CG, with the total percentage change from baseline of 14.2% vs 6.3% | [76] |
| Hair follicles | 4 months | 46 | Apply one vial (5 mL) of the product on clean and dry scalp, apply every day for 5 consecutive days, stop the treatment for 2 days and then continue the application | After 2 and 4 months of treatment, the anagen rate was increased by 6.8% and 10.7%, respectively. Hair resistance to traction was decreased by 29.6% and 46.8% | [77] |
| Umbilical cord blood | 3 months | 15 | The investigational product was transported frozen to the study site, thawed just before use, and applied using a 1.5 mm derma roller by the study investigator | Patient feedback demonstrated that 92% agreed that the investigational product was effective and were highly satisfied with the treatment after 3 months. Clinical evaluation indicated that 5–10% of young patients showed improvement in the control of graying of hair | [78] |
| Umbilical cord blood | 24 weeks | 87 | Be directed to use on hair and scalp by subject her/himself at home twice a day (in the morning and evening) for 24 week | Not found |
Stem Cell Transplantation
Stem cells are a class of self-replicating, multipotential undifferentiated cells, a relatively primitive class of cells that can differentiate into cells with multiple functions under certain conditions [79]. Stem cells can repair and renew damaged organs and tissues, activate self-healing, improve immunity, and reverse aging [80]. Therefore, stem cells have been widely used in the treatment of various diseases such as diabetes, myocardial infarction, Alzheimer's disease, and Parkinson's disease [81]. Additionally, stem cells are used in plastic surgery treatments, such as stem cell hair transplant [82]. The adipose tissue, bone marrow, hair follicles, and umbilical cord are the sources of regenerative pluripotent stem cells. Adipose-derived stem cells are used in skin anti-aging treatment due to their effective re-epithelialization, easy availability, and less discomfort to the patients [83]. Adipose interstitial vascular cells (ADSVCs) are rich in stem cells and adipose tissue; hence, can be used to treat hair loss [84]. Anderi et al. reported that all 20 patients with alopecia areata after receiving ADSVC treatment showed hair regeneration within 3–6 months [85]. A randomized, double-blind, drug-controlled trial in South Korea used adipose-derived stem cell component extract (ADSC-CE) for AGA, confirming the efficacy of treatment [6]. Zhang and Ye demonstrated that the epidermal stem cell transplant can induce hair follicle regeneration. They subcutaneously injected a mixture of epidermal stem cells and DPCs into nude mice. The injection site was stained with hematoxylin and eosin (HE) to observe hair follicles and epidermal regeneration [86]. Human umbilical cord mesenchymal stem cells (hUC-MSCs), a rich source of mesenchymal stem cell, have been promoted as a cell-based treatment option for tissue repair [87]. Bak et al. transplanted umbilical cord blood mesenchymal stem (UC-MSCs) cells into the C3H/HeJ mice [88]. After 5 weeks of treatment, diffused dorsal skin darkening was observed, with no significant change in the control group. This result demonstrated that the UC-MSCs blocked apoptosis and improved the number of hair follicles. Transplantation of human hair follicle mesenchymal stem cells (hHF-MSCs) into the scalp of 27 patients treated with placebo showed an increase in the density of hair in the target area by 18.0/0.65 cm2 and 23.3 /cm2, respectively, from baseline, while in the control group it reduced by 1.1/0.65 cm2 and 0.7/cm2 (P < 0.0001), after 58 weeks.The HF-SCs improved the hair density in the treated area by 5–29% in the 11 patients with AGA [89].
AGA has been effectively treated using stem cell transplantation, however, it faces cell therapy based regulatory and safety challenges, such as tumorigenesis and infection transmission issues [90]. Another approach is to utilize stem cell-based proteins and growth factors also known as stromal cell secretion groups or conditioned media (CM). This treatment uses a mixture of stem cells derived mesenchymal growth factors, microRNAs, and polypeptides [91].
Stem Cell-derived Conditioned Media
The CM is rich in growth factors, cytokines, and beneficial proteins. In vitro and in vivo experiments that used hUC-MSCs for hair regeneration, confirmed the safety and effectiveness of CM in treating AGA [92]. CM has gained attention due to its easy collection and convenient transportation, and has broad prospects in regenerative medicine.
Applications of CM
Numerous studies and clinical trials have demonstrated the potential of CM for hair regeneration. The dermatology research team of Chung-Ang University School of Medicine in an experiment added transforming growth factor β1 (TGF-β1) protein and lithium chloride to the conditioned culture medium containing hUC-MSCs. After 24 h, the culture medium was replaced with the medium containing DPCs. After 3 days, the hUC-MCSs were collected from the conditioned culture medium. Subsequently, a 16-week clinical trial involving 30 patients with mild to moderate hair loss was conducted. The results indicated a significant increase in average hair density, hair thickness, and hair growth rate compared to the placebo group, with no adverse effects such as irritation or itching [93]. Similarly, a 3-month pilot study involving 15 volunteers using hUC-MSC-conditioned medium indicated that 86.6% of the volunteers had hair regeneration, with no side effects or adverse reactions [92]. Adipose-derived stem cell conditioning medium (ADSC-CM) too had similar effects. The results showed that after 12 weeks of ADSC-CM treatment in 27 female patients with alopecia, hair density increased from 105.4 to 122.7 roots/cm.2 (P < 0.001), and hair thickness increased from 57.5 μm to 64.0 μm (P < 0.001), with no occurrence of serious adverse reactions [75]. Based on the above results, an experiment was conducted in 40 patients for scalp regeneration. The patients were administered an intradermal injection of CM once a month for a period 6 months. The results proved that ADSC-CM not only promoted hair follicle regeneration but also promoted scalp regeneration between hair follicles [94]. Lee et al. locally applied ADSC-CM to 30 patients, who received non-ablative fractional laser treatment, and observed no adverse reactions. The experiment suggested that ADSC-CM can accelerate an increase in hair density and volume in patients with AGA after non-ablative fractal laser treatment [95]
Mechanism of CM in Treating Hair Loss
Stem cells use paracrine action of various growth factors and cytokines to perform their biological functions [96]. The CM derived from MSCs, a cell-free suspension rich in growth factors and cytokines, plays an important role in stimulating hair growth [97]. Cytokines secreted by stem cells in CM include vascular endothelial growth factor (VEGF), insulin-like growth factor (IGF), hepatocyte growth factor (HGF), platelet-derived growth factor (PDGF), bone morphogenetic proteins (BMPs), interleukin-6 (IL-6), and macrophage colony-stimulating factor (M-CSF) [98, 99]. VEGF protects CD200-rich and CD34-positive HFSCs from androgen-induced apoptosis via PI3K/Akt pathway. It reverses an increase in the Bcl-2/Bax ratio and a decrease in 5α-DHT induced increase in caspase-3 levels. To improve the hair follicle cycle, HGF promotes the growth of hair follicles by upregulating the expression of β-catenin [100], whereas IGF induces the proliferation and migration of MSCs by activating IGFR-mediated ERK1/2 signaling pathway [101]. Other experiments confirmed that PDGF and fibroblast growth factor 2 (FGF2) synergistically promote the proliferation of DPCs and enhance the expression of hair follicle-related genes to maintain their hair-inducing activity [102].BMPs maintained the DPC phenotype (the basis of HFSCs stimulation), whereas TGF-β regulated the hair-cycle signaling pathway, extracellular matrix synthesis, fibroblasts and mesenchymal stem cells proliferation, and hair follicles development [103].
Strategies to Upregulate the Regeneration Efficacy of CM
Environmental Stimulations
Byung. Park et al. demonstrated the effect of hypoxia on adipose-derived stem cells (ADSCs) stimulated hair growth. Hair regeneration induced by ADSC-CM cultured under hypoxic and normoxic conditions showed that hypoxia promoted hair growth in ADSCs. It significantly increased the secretion of insulin-like growth factor binding proteins (IGFBP-1 & IGFBP-2), M-CSF, M-CSF receptor, platelet-derived growth factor receptor-β (PDGFR-β), and VEGF [104]. ADSC-CM has a similar effect after 48 h of UVB irradiation. The passage of human skin fibroblasts, gradually decreased the relative expression of collagen I, collagen III, and elastin, whereas UVB irradiation decreased the expression of collagen I, collagen III, elastin, and TIMP-1 [105]. Moreover, after the same number of MSCs were inoculated into the 2D and 3D culture systems, the exosomes were isolated from medium supernatant and qualitative and quantitative analyses were conducted. The results showed that in 3D culture the surface markers of MSCs, and morphology and size of the 3D-Exos was unaltered. However, the total exosome production increased by 19.4 times in 3D culture compared with 2D culture. In the harvested supernatant, the level of 3D-Exos was 15.5 times higher than that of 2D-Exos. This suggested that changing the dimensions of the culture system also affects the therapeutic effect of CM [106]. Drzeniek N synthesized a 3D culture environment by encapsulating MSCs in collagen hyaluronic acid (col-HA) hydrogel. In-depth analysis of > 250 proteins in Col-HA-coated MSCs showed that the secretion spectrum of proangiogenic, neuroprotective, and immunomodulatory paracrine factors expanded.This enhanced the potential for angiogenesis [107].
Biological Factors
DPCs interact closely with the epidermal cells and play a key role in hair follicle induction and hair morphogenesis. DPCs often lose their ability to induce hair growth in in vitro monolayer culture, and it is difficult to obtain new hair follicle structures after transplantation in vivo. Therefore, small molecules such as SB431542 (SB, an inhibitor of the TGFβ/Smad pathway), CHIR99021 (CHIR, GSK3α/β inhibitor, and Wnt signal activator), and PGDF were added to the DPCs cultured with HaCaT CM. The mRNA expression of SOX2, ALP, and proteoglycans in DPCs were significantly upregulated [108]. In addition, vitamin d3 (VD3) pretreated CM significantly promoted hair growth. The experiments indicated that in preadipocytes pretreated with VD3, the VEGF level and angiogenesis rate was significantly increased in vivo than in in vitro [109]. A study on the signaling pathway indicated that ERK1/2 inhibitors reduced the production of VD3-enhanced VEGF, and the VD3 treatment increased ERK1/2 phosphorylation. Similarly, in vitamin E and selenium pretreated MSCs, the CD40 expression increased and the IL-12 expression decreased in MSC-conditioned medium. Hence, enhancing the ability of MSC to inhibit dendritic cells and enhance immune regulation [110]. LL-37, an antimicrobial peptide in the cathelicidin family, is widely found in the bone marrow, testis, neutrophils, monocytes, cervical, and vaginal squamous cells. LL-37 increased the expression of EGR1, activated MAPK, and the LL-37 pretreated ASCS-CM strongly promoted hair growth in vivo [111].
Gene Editing
Genetic modifications of stem cells can also enhance the expression of different growth factors. Wnt signaling pathway regulates hair morphogenesis and regeneration in embryos and adults. Adenovirus-mediated Wnt10b overexpression regulated the transition of hair follicles from the quiescent to the growth phase and induced hair follicle regeneration in mice [112]. Similarly, Wnt7a is associated with an increase in HFS at the wound site [113]. The generation of Wnt1A-CM by bone marrow mesenchymal stem cell (BM-MSC) accelerated the hair follicles from resting to growth stage, induced up-regulation of hair-related gene expression, and promoted the ability of DPCs to induce hair circulation and regeneration [112]. In addition, Nanog delayed senescence and maintained MSCs self-renewal ability in amniotic fluid source. Nanog expression of amniotic fluid source MSCs-CM, improved the secretion for hair regeneration and related factors, accelerated the transformation of hair follicles from the stationary to growth phase, and increased the density of HF [114]. In TLR3 agonist poly (I: C) pretreated bone marrow-derived MSCs, the antibacterial and immunomodulatory proteomic profiles of EV secretion were enhanced but the exosomes (EV) miRNA level remained unchanged [115].
Change cell culture conditions (such as 3D culture, low oxygen, ultraviolet irradiation, etc.) or with some small biological molecules training (such as vitamin D, antimicrobial peptide LL-37 etc.) or by means of genetic engineering of cells for editing strategy can increase the expression of effective components in conditioned medium quantity to increase, which affects the final pretreatment can increase the therapeutic effect of stem cells.
Stem Cell-derived Exosomes and Their Applications
EVs are small particles of 30–1000 nm diameter. They are double coated with phospholipids and contain DNA, RNA, and proteins [116]. EVs enhance tissue regeneration, participate in immune regulation, serve as a carrier for therapeutic drugs, and are a potential alternative to stem cell therapy [117], 118. The stem cell action is mainly paracrine action mediated by stem cell secretory factors [88]. EVs are important components of stem cell secretion that are abundant in body fluids and can be released into the cell medium (CCM) [119]. Derivative EVs can promote the anagen phase and delay the catagen phase. Co-culture of DPCs and HFSCs induced differentiation of HFSCs. DPC-Exos attached to HFSCs surface, regulated proliferation and differentiation of HFSCs through cell signal transduction genes, regulated fatty acid expression, and cell communication [120]. Inflammatory human pulp stem cell-derived EVs have the potential to promote angiogenesis [121]. Adipose-derived EVs reduced inflammation and promoted wound healing [122]. In addition, EVs improve aging and age-related diseases; however, the underlying mechanisms are not known. Conversely, aging also affected the generation rate and loading of mesenchymal stem cells and their EVs [123]. EVs have advantages in the treatment of hair regeneration, such as direct fusion with the target cells, biological effects, long-term storage, and transportation at -70℃. Additionally, the concentration, dosage, route, and time of medication are easy to control, with no risk of immune rejection and tumor occurrence caused by cell transplantation therapy [124]. The amount of EVs present in CM is not high, and the methods that balance efficiency and purity of EVs in CM have not been developed [125]. Additionally, EVs have the potential risk of uncontrolled genetic information, immune response, and biological distribution. Cell-derived EVs have the potential to promote angiogenesis in vitro [126]. A comparison of stem cells, CM, and EVs for hair regeneration is presented in Table 3.
Table3.
Comparison of stem cell, CM and exosomes for hair regeneration
| Therapy method | Strengths | Weaknesses |
|---|---|---|
| Stem cell transplantation |
Low immunogenicity[127] Multidirectional differentiation [128] Easy differentiation into various cell lines, and high angiogenesis potential[129] |
Oncogenic[128]Infectious transmission, strict ethical review and high cost[130] |
| CM | Low tumor risk and cost of collection[131]easy preparation[128] | Short half-life and depletion of paracrine factors may require extensive and frequent administration [131]Lack of standardization of therapy[132] |
| Exosome |
Avoid the degradation of effective active ingredients[133] Stable mass production[134] |
Lack of effective separation methods[135], standardized guidelines for mass production and potential biosafety tissues[133] |
Challenge and Frontier
Stem cell-based treatments for hair loss have become popular in recent years. Although stem cell transplantation, CM, and EV therapies have been successful in preclinical and clinical stages, each therapy has its own limitations. As the stem cell transplants are expensive and tumorigenic, CM, and EVs may be more economical and safer for treating hair loss, though both have their own limitations. Due to the cell-free nature of CM, it is safer and more immunologically compatible, but isolating compositionally consistent CM is a challenge. Similarly, using EVs for treatment has its own challenges, where based on the intended therapeutic use, it is essential to select and characterize cell sources suitable for EVs production. Another important aspect is the method and conditions of cell culture as they have an impact on EVs yield, stability, and storage. Off-the-shelf therapies need further exploration. In addition, exosome isolation and storage methods lack standardization. The characterization of therapeutic exosomes should be further validated in relevant preclinical models to assess safety/toxicology, pharmacokinetics, and pharmacodynamics. To increase the likelihood of clinical translation, standardisation of stem cell culture, collection, preservation and validation of CM and isolation of exosomes is inevitable. Although the results are promising and robust, double-blind controlled clinical trials are needed to further evaluate the exact mechanism, therapeutic potential, and safety of stem cell-based hair loss management.
Conclusion
The rapid development of regenerative medicine provides new ideas and directions for the treatment of AGA. Although, efficacy of stem cell transplantation in hair regeneration is encouraging, the biosafety of stem cells may hinder its clinical application. Owing to the secretions of stem cells, tumorigenic problems of stem cell transplantation have been eliminated. Therefore, there has been an increased interest in stem cell-conditioned media, exosomes, and related therapies. Available experimental studies have shown that both stem cells and stem cell-based non-cellular therapies can promote hair regeneration and prevent hair loss. The convenience, safety, effectiveness, ease of treatment and patient acceptance of stem cell therapy over medication and hair transplantation offer an alternative treatment option for hair loss patients.
Acknowledgements
We would like to thank Editage (www.editage.cn) for English language editing, and Biorender (www.biorender.com) for the assistance in creating figures.
Authors' Contributions
Yongcui Mao: Writing – original draft, Visualization. Pinyan Liu:Writing – review & editing, Project administration. Jiayun Wei:Data curation, Validation. Ye Xie: Data curation. Qiuxia Zheng: Datacuration. Rui Li: Data curation. Jia Yao: Conceptualization,Supervision.
Funding
This work was supported by Grant(s) from the National Natural Science Foundation of China (32160230),the Key Research Development Programs of the Provincial Science and Technology Research Projects of Gansu Province (18YF1FA109),and 1st Hospital of Lanzhou University Scientific Research Foundation(ldyyyn2019-03).
Data Availability
The datasets analyzed in this study are available from the corresponding author on reasonable request.
Declarations
Ethics Approval and Consent to Participate
Not Applicable.
Consent for Publication
All authors agree to the submission and publication of the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ho A, Shapiro J. Medical therapy for frontal fibrosing alopecia: A review and clinical approach. Journal of the American Academy of Dermatology. 2019;81(2):568–580. doi: 10.1016/j.jaad.2019.03.079. [DOI] [PubMed] [Google Scholar]
- 2.Bas Y, Seckin HY, Kalkan G, Takci Z, Citil R, Onder Y, Sahin S, Demir AK. Prevalence and types of androgenetic alopecia in north Anatolian population: A community-based study. The Journal of the Pakistan Medical Association. 2015;65(8):806–809. [PubMed] [Google Scholar]
- 3.Fu D, Huang J, Li K, Chen Y, He Y, Sun Y, Guo Y, Du L, Qu Q, Miao Y, et al. Dihydrotestosterone-induced hair regrowth inhibition by activating androgen receptor in C57BL6 mice simulates androgenetic alopecia. Biomedicine & Pharmacotherapy. 2021;137:111247. doi: 10.1016/j.biopha.2021.111247. [DOI] [PubMed] [Google Scholar]
- 4.Lee WS, Lee HJ. Characteristics of androgenetic alopecia in asian. Annals of Dermatology. 2012;24(3):243–252. doi: 10.5021/ad.2012.24.3.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Upadhyay DK, Sharma A, Sarma GS, Gupta GD, Rai VK. Mechanism of androgenic alopecia: Addressing speculations through empirical evidences. Dermatologic Therapy. 2019;32(6):e13120. doi: 10.1111/dth.13120. [DOI] [PubMed] [Google Scholar]
- 6.Tak YJ, Lee SY, Cho AR, Kim YS. A randomized, double-blind, vehicle-controlled clinical study of hair regeneration using adipose-derived stem cell constituent extract in androgenetic alopecia. STEM CELLS Translational Medicine. 2020;9(8):839–849. doi: 10.1002/sctm.19-0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.English RS. A hypothetical pathogenesis model for androgenic alopecia: Clarifying the dihydrotestosterone paradox and rate-limiting recovery factors. Medical Hypotheses. 2018;111:73–81. doi: 10.1016/j.mehy.2017.12.027. [DOI] [PubMed] [Google Scholar]
- 8.Sawaya ME, Price VH. Different levels of 5alpha-reductase type I and II, aromatase, and androgen receptor in hair follicles of women and men with androgenetic alopecia. The Journal of Investigative Dermatology. 1997;109(3):296–300. doi: 10.1111/1523-1747.ep12335779. [DOI] [PubMed] [Google Scholar]
- 9.Sinclair R, Torkamani N, Jones L. Androgenetic alopecia: new insights into the pathogenesis and mechanism of hair loss. F1000Res. 2015;4(F1000 Faculty Rev):585. doi: 10.12688/f1000research.6401.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zeeh FC, Meyer SC. Current Concepts of Pathogenesis and Treatment of Philadelphia Chromosome-Negative Myeloproliferative Neoplasms. Hämostaseologie. 2021;41(3):197–205. doi: 10.1055/a-1447-6667. [DOI] [PubMed] [Google Scholar]
- 11.Lee JY, Cho KS. Effects of 5-alpha reductase inhibitors: New insights on benefits and harms. Current Opinion in Urology. 2018;28(3):288–293. doi: 10.1097/MOU.0000000000000497. [DOI] [PubMed] [Google Scholar]
- 12.Egger, A., Tomic-Canic, M., & Tosti, A. (2020).Advances in stem cell-based therapy for hair loss. CellR4 Repair Replace Regen Reprogramn, 8, e2894. [PMC free article] [PubMed]
- 13.Gonzalez ME, Cantatore-Francis J, Orlow SJ. Androgenetic alopecia in the paediatric population: A retrospective review of 57 patients. British Journal of Dermatology. 2010;163(2):378–385. doi: 10.1111/j.1365-2133.2010.09777.x. [DOI] [PubMed] [Google Scholar]
- 14.Heilmann S, Kiefer AK, Fricker N, Drichel D, Hillmer AM, Herold C, Tung JY, Eriksson N, Redler S, Betz RC, et al. Androgenetic alopecia: Identification of four genetic risk loci and evidence for the contribution of WNT signaling to its etiology. The Journal of Investigative Dermatology. 2013;133(6):1489–1496. doi: 10.1038/jid.2013.43. [DOI] [PubMed] [Google Scholar]
- 15.Uitto J. Genetic Susceptibility to Alopecia. New England Journal of Medicine. 2019;380(9):873–876. doi: 10.1056/NEJMe1900042. [DOI] [PubMed] [Google Scholar]
- 16.Redler S, Tazi-Ahnini R, Drichel D, Birch MP, Brockschmidt FF, Dobson K, Giehl KA, Refke M, Kluck N, Kruse R, et al. Selected variants of the steroid-5-alpha-reductase isoforms SRD5A1 and SRD5A2 and the sex steroid hormone receptors ESR1, ESR2 and PGR: No association with female pattern hair loss identified. Experimental Dermatology. 2012;21(5):390–393. doi: 10.1111/j.1600-0625.2012.01469.x. [DOI] [PubMed] [Google Scholar]
- 17.Yip L, Zaloumis S, Irwin D, Severi G, Hopper J, Giles G, Harrap S, Sinclair R, Ellis J. Gene-wide association study between the aromatase gene (CYP19A1) and female pattern hair loss. British Journal of Dermatology. 2009;161(2):289–294. doi: 10.1111/j.1365-2133.2009.09186.x. [DOI] [PubMed] [Google Scholar]
- 18.Redler S, Brockschmidt FF, Tazi-Ahnini R, Drichel D, Birch MP, Dobson K, Giehl KA, Herms S, Refke M, Kluck N, et al. Investigation of the male pattern baldness major genetic susceptibility loci AR/EDA2R and 20p11 in female pattern hair loss. British Journal of Dermatology. 2012;166(6):1314–1318. doi: 10.1111/j.1365-2133.2012.10877.x. [DOI] [PubMed] [Google Scholar]
- 19.Chew EGY, Tan JHJ, Bahta AW, Ho BS, Liu X, Lim TC, Sia YY, Bigliardi PL, Heilmann S, Wan ACA, et al. Differential Expression between Human Dermal Papilla Cells from Balding and Non-Balding Scalps Reveals New Candidate Genes for Androgenetic Alopecia. The Journal of Investigative Dermatology. 2016;136(8):1559–1567. doi: 10.1016/j.jid.2016.03.032. [DOI] [PubMed] [Google Scholar]
- 20.Chen S, Xie X, Zhang G, Zhang Y. Comorbidities in Androgenetic Alopecia: A Comprehensive Review. Dermatol Ther (Heidelb) 2022;12(10):2233–2247. doi: 10.1007/s13555-022-00799-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamilton JB. Effect of castration in adolescent and young adult males upon further changes in the proportions of bare and hairy scalp. Journal of Clinical Endocrinology and Metabolism. 1960;20:1309–1318. doi: 10.1210/jcem-20-10-1309. [DOI] [PubMed] [Google Scholar]
- 22.Lai JJ, Chang P, Lai KP, Chen L, Chang C. The role of androgen and androgen receptor in skin-related disorders. Archives of Dermatological Research. 2012;304(7):499–510. doi: 10.1007/s00403-012-1265-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hamilton JB. Patterned loss of hair in man; types and incidence. Annals of the New York Academy of Sciences. 1951;53(3):708–728. doi: 10.1111/j.1749-6632.1951.tb31971.x. [DOI] [PubMed] [Google Scholar]
- 24.Inui S, Itami S. Molecular basis of androgenetic alopecia: From androgen to paracrine mediators through dermal papilla. Journal of Dermatological Science. 2011;61(1):1–6. doi: 10.1016/j.jdermsci.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 25.Faydaci G, Bilal E, Necmettin P, Fatih T, Asuman O, Ugur K. Baldness, benign prostate hyperplasia, prostate cancer and androgen levels. The Aging Male. 2008;11(4):189–192. doi: 10.1080/13685530802400995. [DOI] [PubMed] [Google Scholar]
- 26.Martinez-Jacobo L, Villarreal-Villarreal CD, Ortiz-Lopez R, Ocampo-Candiani J, Rojas-Martinez A. Genetic and molecular aspects of androgenetic alopecia. Indian Journal of Dermatology, Venereology and Leprology. 2018;84(3):263–268. doi: 10.4103/ijdvl.IJDVL_262_17. [DOI] [PubMed] [Google Scholar]
- 27.Chang C, Saltzman A, Yeh S, Young W, Keller E, Lee HJ, Wang C, Mizokami A. Androgen receptor: An overview. Critical Reviews in Eukaryotic Gene Expression. 1995;5(2):97–125. doi: 10.1615/critreveukargeneexpr.v5.i2.10. [DOI] [PubMed] [Google Scholar]
- 28.Zouboulis CC, Degitz K. Androgen action on human skin – from basic research to clinical significance. Experimental Dermatology. 2004;13(Suppl 4):5–10. doi: 10.1111/j.1600-0625.2004.00255.x. [DOI] [PubMed] [Google Scholar]
- 29.Singh SM, Gauthier S, Labrie F. Androgen receptor antagonists (antiandrogens): Structure-activity relationships. Current Medicinal Chemistry. 2000;7(2):211–247. doi: 10.2174/0929867003375371. [DOI] [PubMed] [Google Scholar]
- 30.Ceruti JM, Leiros GJ, Balana ME. Androgens and androgen receptor action in skin and hair follicles. Molecular and Cellular Endocrinology. 2018;465:122–133. doi: 10.1016/j.mce.2017.09.009. [DOI] [PubMed] [Google Scholar]
- 31.Ocampo-Garza J, Griggs J, Tosti A. New drugs under investigation for the treatment of alopecias. Expert Opinion on Investigational Drugs. 2019;28(3):275–284. doi: 10.1080/13543784.2019.1568989. [DOI] [PubMed] [Google Scholar]
- 32.Heymann WR. The inflammatory component of androgenetic alopecia. Journal of the American Academy of Dermatology. 2022;86(2):301–302. doi: 10.1016/j.jaad.2021.11.013. [DOI] [PubMed] [Google Scholar]
- 33.Ramos PM, Brianezi G, Martins AC, da Silva MG, Marques ME, Miot HA. Apoptosis in follicles of individuals with female pattern hair loss is associated with perifollicular microinflammation. International Journal of Cosmetic Science. 2016;38(6):651–654. doi: 10.1111/ics.12341. [DOI] [PubMed] [Google Scholar]
- 34.Plante J, Valdebran M, Forcucci J, Lucas O, Elston D. Perifollicular inflammation and follicular spongiosis in androgenetic alopecia. Journal of the American Academy of Dermatology. 2022;86(2):437–438. doi: 10.1016/j.jaad.2021.09.040. [DOI] [PubMed] [Google Scholar]
- 35.Dalessandri T, Kasper M. TREMendous Macrophages Inhibit Hair Growth. Cell Stem Cell. 2019;24(4):501–502. doi: 10.1016/j.stem.2019.03.014. [DOI] [PubMed] [Google Scholar]
- 36.Jaworsky C, Kligman AM, Murphy GF. Characterization of inflammatory infiltrates in male pattern alopecia: Implications for pathogenesis. British Journal of Dermatology. 1992;127(3):239–246. doi: 10.1111/j.1365-2133.1992.tb00121.x. [DOI] [PubMed] [Google Scholar]
- 37.Miao Y, Qu Q, Jiang W, Liu XM, Shi PL, Fan ZX, Du LJ, Wang GF, Liu XN, Guo ZH, et al. Identification of Functional Patterns of Androgenetic Alopecia Using Transcriptome Profiling in Distinct Locations of Hair Follicles. The Journal of Investigative Dermatology. 2018;138(4):972–975. doi: 10.1016/j.jid.2017.10.027. [DOI] [PubMed] [Google Scholar]
- 38.Maryanovich M, Frenette PS. T-Regulating Hair Follicle Stem Cells. Immunity. 2017;46(6):979–981. doi: 10.1016/j.immuni.2017.06.011. [DOI] [PubMed] [Google Scholar]
- 39.Wang L, Siegenthaler JA, Dowell RD, Yi R. Foxc1 reinforces quiescence in self-renewing hair follicle stem cells. Science. 2016;351(6273):613–617. doi: 10.1126/science.aad5440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Upton JH, Hannen RF, Bahta AW, Farjo N, Farjo B, Philpott MP. Oxidative stress-associated senescence in dermal papilla cells of men with androgenetic alopecia. The Journal of Investigative Dermatology. 2015;135(5):1244–1252. doi: 10.1038/jid.2015.28. [DOI] [PubMed] [Google Scholar]
- 41.Matsumura H, Mohri Y, Binh NT, Morinaga H, Fukuda M, Ito M, Kurata S, Hoeijmakers J, Nishimura EK. Hair follicle aging is driven by transepidermal elimination of stem cells via COL17A1 proteolysis. Science. 2016;351(6273):395. doi: 10.1126/science.aad4395. [DOI] [PubMed] [Google Scholar]
- 42.Mirmirani P. Age-related hair changes in men: Mechanisms and management of alopecia and graying. Maturitas. 2015;80(1):58–62. doi: 10.1016/j.maturitas.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 43.Morinaga H, Mohri Y, Grachtchouk M, Asakawa K, Matsumura H, Oshima M, Takayama N, Kato T, Nishimori Y, Sorimachi Y, et al. Obesity accelerates hair thinning by stem cell-centric converging mechanisms. Nature. 2021;595(7866):266–271. doi: 10.1038/s41586-021-03624-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu W, Li K, Wang G, Yang L, Qu Q, Fan Z, Sun Y, Huang J, Miao Y, Hu Z. Impairment of autophagy may be associated with follicular miniaturization in androgenetic alopecia by inducing premature catagen. Journal of Dermatology. 2021;48(3):289–300. doi: 10.1111/1346-8138.15672. [DOI] [PubMed] [Google Scholar]
- 45.Salem AS, Ibrahim HS, Abdelaziz HH, Elsaie ML. Implications of cigarette smoking on early-onset androgenetic alopecia: A cross-sectional Study. Journal of Cosmetic Dermatology. 2021;20(4):1318–1324. doi: 10.1111/jocd.13727. [DOI] [PubMed] [Google Scholar]
- 46.Yi Y, Qiu J, Jia J, Djakaya GDN, Li X, Fu J, Chen Y, Chen Q, Miao Y, Hu Z. Severity of androgenetic alopecia associated with poor sleeping habits and carnivorous eating and junk food consumption-A web-based investigation of male pattern hair loss in China. Dermatologic Therapy. 2020;33(2):e13273. doi: 10.1111/dth.13273. [DOI] [PubMed] [Google Scholar]
- 47.Gherardini J, Wegner J, Cheret J, Ghatak S, Lehmann J, Alam M, Jimenez F, Funk W, Bohm M, Botchkareva NV, et al. Transepidermal UV radiation of scalp skin ex vivo induces hair follicle damage that is alleviated by the topical treatment with caffeine. International Journal of Cosmetic Science. 2019;41(2):164–182. doi: 10.1111/ics.12521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ho BS, Ho EXP, Chu CW, Ramasamy S, Bigliardi-Qi M, de Sessions PF, Bigliardi PL. Microbiome in the hair follicle of androgenetic alopecia patients. PLoS ONE. 2019;14(5):e0216330. doi: 10.1371/journal.pone.0216330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cole JP, Cole MA, Insalaco C, Cervelli V, Gentile P. Alopecia and platelet-derived therapies. Stem Cell Investig. 2017;4:88. doi: 10.21037/sci.2017.11.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schweiger ES, Boychenko O, Bernstein RM. Update on the pathogenesis, genetics and medical treatment of patterned hair loss. Journal of Drugs in Dermatology. 2010;9(11):1412–1419. [PubMed] [Google Scholar]
- 51.Rattanachitthawat N, Pinkhien T, Opanasopit P, Ngawhirunpat T, Chanvorachote P. Finasteride Enhances Stem Cell Signals of Human Dermal Papilla Cells. In Vivo. 2019;33(4):1209–1220. doi: 10.21873/invivo.11592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Said MA, Mehta A. The Impact of 5alpha-Reductase Inhibitor Use for Male Pattern Hair Loss on Men's Health. Current Urology Reports. 2018;19(8):65. doi: 10.1007/s11934-018-0814-z. [DOI] [PubMed] [Google Scholar]
- 53.Suchonwanit P, Thammarucha S, Leerunyakul K. Minoxidil and its use in hair disorders: A review. Drug Design, Development and Therapy. 2019;13:2777–2786. doi: 10.2147/DDDT.S214907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.BinJadeed H, Almudimeegh AM, Alomran SA, Alshathry AH. A Case of Contact Allergic Dermatitis to Topical Minoxidil. Cureus. 2021;13(1):e12510. doi: 10.7759/cureus.12510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mahe YF, Cheniti A, Tacheau C, Antonelli R, Planard-Luong L, de Bernard S, Buffat L, Barbarat P, Kanoun-Copy L. Low-Level Light Therapy Downregulates Scalp Inflammatory Biomarkers in Men With Androgenetic Alopecia and Boosts Minoxidil 2% to Bring a Sustainable Hair Regrowth Activity. Lasers Surg Med. 2021;53(9):1208–1219. doi: 10.1002/lsm.23398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yuan AR, Bian Q, Gao JQ. Current advances in stem cell-based therapies for hair regeneration. European Journal of Pharmacology. 2020;881:173197. doi: 10.1016/j.ejphar.2020.173197. [DOI] [PubMed] [Google Scholar]
- 57.Zgonc Skulj A, Poljsak N, Kocevar Glavac N, Kreft S. Herbal preparations for the treatment of hair loss. Archives of Dermatological Research. 2020;312(6):395–406. doi: 10.1007/s00403-019-02003-x. [DOI] [PubMed] [Google Scholar]
- 58.Zhao J, Liu LQ, Wang YJ, Yang W, Geng WX, Wei J, Li LW, Chen FL. Treatment of alopecia by transplantation of hair follicle stem cells and dermal papilla cells encapsulated in alginate gels. Medical Hypotheses. 2008;70(5):1014–1016. doi: 10.1016/j.mehy.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 59.Ahn H, Lee SY, Jung WJ, Lee KH. Alopecia treatment using minimally manipulated human umbilical cord-derived mesenchymal stem cells: Three case reports and review of literature. World Journal of Clinical Cases. 2021;9(15):3741–3751. doi: 10.12998/wjcc.v9.i15.3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Faghihi G, Nabavinejad S, Mokhtari F, Fatemi Naeini F, Iraji F. Microneedling in androgenetic alopecia; comparing two different depths of microneedles. Journal of Cosmetic Dermatology. 2021;20(4):1241–1247. doi: 10.1111/jocd.13714. [DOI] [PubMed] [Google Scholar]
- 61.Bao L, Gong L, Guo M, Liu T, Shi A, Zong H, Xu X, Chen H, Gao X, Li Y. Randomized trial of electrodynamic microneedle combined with 5% minoxidil topical solution for the treatment of Chinese male Androgenetic alopecia. Journal of Cosmetic and Laser Therapy. 2020;22(1):1–7. doi: 10.1080/14764172.2017.1376094. [DOI] [PubMed] [Google Scholar]
- 62.Alves R, Grimalt R. Platelet-Rich Plasma and its Use for Cicatricial and Non-Cicatricial Alopecias: A Narrative Review. Dermatologic Therapy (Heidelb) 2020;10(4):623–633. doi: 10.1007/s13555-020-00408-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gentile P, Scioli MG, Bielli A, De Angelis B, De Sio C, De Fazio D, Ceccarelli G, Trivisonno A, Orlandi A, Cervelli V, et al. Platelet-Rich Plasma and Micrografts Enriched with Autologous Human Follicle Mesenchymal Stem Cells Improve Hair Re-Growth in Androgenetic Alopecia. Biomolecular Pathway Analysis and Clinical Evaluation. Biomedicines. 2019;7(2):27. doi: 10.3390/biomedicines7020027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gentile P, Garcovich S. Systematic Review of Platelet-Rich Plasma Use in Androgenetic Alopecia Compared with Minoxidil((R)), Finasteride((R)), and Adult Stem Cell-Based Therapy. International Journal of Molecular Sciences. 2020;21(8):2702. doi: 10.3390/ijms21082702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Faghihi G, Mozafarpoor S, Asilian A, Mokhtari F, Esfahani AA, Bafandeh B, Nouraei S, Nilforoushzadeh MA, Hosseini SM. The effectiveness of adding low-level light therapy to minoxidil 5% solution in the treatment of patients with androgenetic alopecia. Indian Journal of Dermatology, Venereology and Leprology. 2018;84(5):547–553. doi: 10.4103/ijdvl.IJDVL_1156_16. [DOI] [PubMed] [Google Scholar]
- 66.Stough D. Hair transplantation. JAMA. 1969;208(1):154. [PubMed] [Google Scholar]
- 67.Chen HA, Pan JY, Chiang CH, Jhang AH, Ho WT. New idea for hair transplantation to preserve more donor hair follicles. Medical Hypotheses. 2019;128:83–85. doi: 10.1016/j.mehy.2019.05.018. [DOI] [PubMed] [Google Scholar]
- 68.Yu AJ, Luo YJ, Xu XG, Bao LL, Tian T, Li ZX, Dong YX, Li YH. A pilot split-scalp study of combined fractional radiofrequency microneedling and 5% topical minoxidil in treating male pattern hair loss. Clinical and Experimental Dermatology. 2018;43(7):775–781. doi: 10.1111/ced.13551. [DOI] [PubMed] [Google Scholar]
- 69.Coskuner ER, Ozkan B, Culha MG. Sexual Problems of Men With Androgenic Alopecia Treated With 5-Alpha Reductase Inhibitors. Sexual Medicine Reviews. 2019;7(2):277–282. doi: 10.1016/j.sxmr.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 70.Lachgar S, Charveron M, Gall Y, Bonafe JL. Minoxidil upregulates the expression of vascular endothelial growth factor in human hair dermal papilla cells. British Journal of Dermatology. 1998;138(3):407–411. doi: 10.1046/j.1365-2133.1998.02115.x. [DOI] [PubMed] [Google Scholar]
- 71.Semsarzadeh N, Khetarpal S. Platelet-Rich Plasma and Stem Cells for Hair Growth: A Review of the Literature. Aesthetic Surgery Journal. 2020;40(4):NP177–NP188. doi: 10.1093/asj/sjz146. [DOI] [PubMed] [Google Scholar]
- 72.Wikramanayake TC, Villasante AC, Mauro LM, Nouri K, Schachner LA, Perez CI, Jimenez JJ. Low-level laser treatment accelerated hair regrowth in a rat model of chemotherapy-induced alopecia (CIA) Lasers in Medical Science. 2013;28(3):701–706. doi: 10.1007/s10103-012-1139-7. [DOI] [PubMed] [Google Scholar]
- 73.Ramdasi S, Tiwari SK. Human Mesenchymal Stem Cell-Derived Conditioned Media for Hair Regeneration Applications. Journal of Stem Cells. 2016;11(4):201–211. [PubMed] [Google Scholar]
- 74.Owczarczyk-Saczonek A, Krajewska-Wlodarczyk M, Kruszewska A, Banasiak L, Placek W, Maksymowicz W, Wojtkiewicz J. Therapeutic Potential of Stem Cells in Follicle Regeneration. Stem Cells Int. 2018;2018:1049641. doi: 10.1155/2018/1049641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shin H, Ryu HH, Kwon O, Park BS, Jo SJ. Clinical use of conditioned media of adipose tissue-derived stem cells in female pattern hair loss: A retrospective case series study. International Journal of Dermatology. 2015;54(6):730–735. doi: 10.1111/ijd.12650. [DOI] [PubMed] [Google Scholar]
- 76.Tak YJ, Lee SY, Cho AR, Kim YS. A randomized, double-blind, vehicle-controlled clinical study of hair regeneration using adipose-derived stem cell constituent extract in androgenetic alopecia. Stem Cells Translational Medicine. 2020;9(8):839–849. doi: 10.1002/sctm.19-0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Buonocore D, Nobile V, Michelotti A, Marzatico F. Clinical efficacy of a cosmetic treatment by Crescina((R)) human follicle stem cell on healthy males with androgenetic alopecia. Dermatologic Therapy (Heidelb) 2013;3(1):53–62. doi: 10.1007/s13555-013-0021-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mathen C, Dsouza W. In vitro and clinical evaluation of umbilical cord-derived mesenchymal stromal cell-conditioned media for hair regeneration. Journal of Cosmetic Dermatology. 2022;21(2):740–749. doi: 10.1111/jocd.14114. [DOI] [PubMed] [Google Scholar]
- 79.AlSogair S. Stem cell therapy and hair loss: Present evidence and future perspectives. Journal of Dermatology and Dermatologic Surgery. 2019;23(2):61. [Google Scholar]
- 80.Lv M, Zhang S, Jiang B, Cao S, Dong Y, Cao L, Guo S. Adipose-derived stem cells regulate metabolic homeostasis and delay aging by promoting mitophagy. The FASEB Journal. 2021;35(7):e21709. doi: 10.1096/fj.202100332R. [DOI] [PubMed] [Google Scholar]
- 81.Ebrahimi A, Ahmadi H, Pourfraidon Ghasrodashti Z, Tanide N, Shahriarirad R, Erfani A, Ranjbar K, Ashkani-Esfahani S. Therapeutic effects of stem cells in different body systems, a novel method that is yet to gain trust: A comprehensive review. Bosnian Journal of Basic Medical Sciences. 2021;21:672. doi: 10.17305/bjbms.2021.5508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jo H, Brito S, Kwak BM, Park S, Lee MG, Bin BH. Applications of Mesenchymal Stem Cells in Skin Regeneration and Rejuvenation. International Journal of Molecular Sciences. 2021;22(5):2410. doi: 10.3390/ijms22052410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Biava PM. The Use of Stem Cell Differentiation Stage Factors (SCDSFs) Taken from Zebrafish Embryos during Organogenesis and Their Role in Regulating the Gene Expression of Normal and Pathological (Stem) Cells. International Journal of Molecular Sciences . 2020;21(14):4914. doi: 10.3390/ijms21144914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bellei B, Migliano E, Tedesco M, Caputo S, Papaccio F, Lopez G, Picardo M. Adipose tissue-derived extracellular fraction characterization: Biological and clinical considerations in regenerative medicine. Stem Cell Research & Therapy. 2018;9(1):207. doi: 10.1186/s13287-018-0956-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Anderi R, Makdissy N, Azar A, Rizk F, Hamade A. Cellular therapy with human autologous adipose-derived adult cells of stromal vascular fraction for alopecia areata. Stem Cell Research & Therapy. 2018;9(1):141. doi: 10.1186/s13287-018-0889-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang M, Ye Y, Zhao P, Bai L, Li X. Preliminary studies of hair follicle regeneration by injections of epidermal stem cells and dermal papilla cells into nude mice. Cell and Tissue Banking. 2020;21(2):321–327. doi: 10.1007/s10561-020-09825-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vohra M, Sharma A, Bagga R, Arora SK. Human umbilical cord-derived mesenchymal stem cells induce tissue repair and regeneration in collagen-induced arthritis in rats. Journal of Clinical and Translational Research. 2020;6(6):203–216. [PMC free article] [PubMed] [Google Scholar]
- 88.Bak DH, Choi MJ, Kim SR, Lee BC, Kim JM, Jeon ES, Oh W, Lim ES, Park BC, Kim MJ, et al. Human umbilical cord blood mesenchymal stem cells engineered to overexpress growth factors accelerate outcomes in hair growth. The Korean Journal of Physiology & Pharmacology. 2018;22(5):555–566. doi: 10.4196/kjpp.2018.22.5.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gentile P, Scioli MG, Bielli A, Orlandi A, Cervelli V. Stem cells from human hair follicles: First mechanical isolation for immediate autologous clinical use in androgenetic alopecia and hair loss. Stem Cell Investigation . 2017;4:58. doi: 10.21037/sci.2017.06.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Luzzi S, Giotta Lucifero A, Brambilla I, Trabatti C, Mosconi M, Savasta S, Foiadelli T. The impact of stem cells in neuro-oncology: applications, evidence, limitations and challenges. Acta Biomedica. 2020;91(7-S):51–60. doi: 10.23750/abm.v91i7-S.9955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pereira T, Ivanova G, Caseiro AR, Barbosa P, Bartolo PJ, Santos JD, Luis AL, Mauricio AC. MSCs conditioned media and umbilical cord blood plasma metabolomics and composition. PLoS ONE. 2014;9(11):e113769. doi: 10.1371/journal.pone.0113769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mathen C, Dsouza W. In vitro and clinical evaluation of umbilical cord-derived mesenchymal stromal cell-conditioned media for hair regeneration. Journal of Cosmetic Dermatology. 2021;21(2):740–749. doi: 10.1111/jocd.14114. [DOI] [PubMed] [Google Scholar]
- 93.Oh HA, Kwak J, Kim BJ, Jin HJ, Park WS, Choi SJ, Oh W, Um S. Migration Inhibitory Factor in Conditioned Medium from Human Umbilical Cord Blood-Derived Mesenchymal Stromal Cells Stimulates Hair Growth. Cells. 2020;9(6):1344. doi: 10.3390/cells9061344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Narita K, Fukuoka H, Sekiyama T, Suga H, Harii K. Sequential Scalp Assessment in Hair Regeneration Therapy Using an Adipose-Derived Stem Cell-Conditioned Medium. Dermatologic Surgery. 2020;46(6):819–825. doi: 10.1097/DSS.0000000000002128. [DOI] [PubMed] [Google Scholar]
- 95.Lee YI, Kim J, Kim J, Park S, Lee JH. The Effect of Conditioned Media From Human Adipocyte-Derived Mesenchymal Stem Cells on Androgenetic Alopecia After Nonablative Fractional Laser Treatment. Dermatologic Surgery. 2020;46(12):1698–1704. doi: 10.1097/DSS.0000000000002518. [DOI] [PubMed] [Google Scholar]
- 96.Bak DH, Lee E, Choi MJ, Lee BC, Kwon TR, Kim JH, Jeon ES, Oh W, Mun SK, Park BC, et al. Protective effects of human umbilical cord bloodderived mesenchymal stem cells against dexamethasoneinduced apoptotic cell death in hair follicles. International Journal of Molecular Medicine. 2020;45(2):556–568. doi: 10.3892/ijmm.2019.4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Xiao S, Deng Y, Mo X, Liu Z, Wang D, Deng C, Wei Z. Promotion of Hair Growth by Conditioned Medium from Extracellular Matrix/Stromal Vascular Fraction Gel in C57BL/6 Mice. Stem Cells International. 2020;2020:9054514. doi: 10.1155/2020/9054514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Joseph A, Baiju I, Bhat IA, Pandey S, Bharti M, Verma M, Pratap Singh A, Ansari MM, Chandra V, Saikumar G, et al. Mesenchymal stem cell-conditioned media: A novel alternative of stem cell therapy for quality wound healing. Journal of Cellular Physiology. 2020;235(7–8):5555–5569. doi: 10.1002/jcp.29486. [DOI] [PubMed] [Google Scholar]
- 99.Ou K-L, Kuo Y-W, Wu C-Y, Huang B-H, Pai F-T, Chou H-H, Saito T, Ueno T, Cho Y-C, Huang M-S. The Potential of a Hair Follicle Mesenchymal Stem Cell-Conditioned Medium for Wound Healing and Hair Follicle Regeneration. Applied Sciences. 2020;10(8):2646. [Google Scholar]
- 100.Qi Y, Li M, Xu L, Chang Z, Shu X, Zhou L. Therapeutic role of human hepatocyte growth factor (HGF) in treating hair loss. PeerJ. 2016;4:e2624. doi: 10.7717/peerj.2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Xia Y, Chen J, Ding J, Zhang J, Chen H. IGF1- and BM-MSC-incorporating collagen-chitosan scaffolds promote wound healing and hair follicle regeneration. American Journal of Translational Research . 2020;12(10):6264–6276. [PMC free article] [PubMed] [Google Scholar]
- 102.Kiso M, Hamazaki TS, Itoh M, Kikuchi S, Nakagawa H, Okochi H. Synergistic effect of PDGF and FGF2 for cell proliferation and hair inductive activity in murine vibrissal dermal papilla in vitro. Journal of Dermatological Science. 2015;79(2):110–118. doi: 10.1016/j.jdermsci.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 103.Gentile P, Garcovich S. Systematic Review of Platelet-Rich Plasma Use in Androgenetic Alopecia Compared with Minoxidil((R)), Finasteride((R)), and Adult Stem Cell-Based Therapy. International Journal of Molecular Sciences. 2020;21(8):2702. doi: 10.3390/ijms21082702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Park BS, Kim WS, Chor JS, Kim HK, Won JH, Ohkubo F, Fukuoka H. Hair growth stimulated by conditioned medium of adipose-derived stem cells is enhanced by hypoxia: Evidence of increased growth factor secretion. Biomedical Research-Tokyo. 2010;31(1):27–34. doi: 10.2220/biomedres.31.27. [DOI] [PubMed] [Google Scholar]
- 105.Guo S, Wang T, Zhang S, Chen P, Cao Z, Lian W, Guo J, Kang Y. Adipose-derived stem cell-conditioned medium protects fibroblasts at different senescent degrees from UVB irradiation damages. Molecular and Cellular Biochemistry. 2020;463(1–2):67–78. doi: 10.1007/s11010-019-03630-8. [DOI] [PubMed] [Google Scholar]
- 106.Cao J, Wang B, Tang T, Lv L, Ding Z, Li Z, Hu R, Wei Q, Shen A, Fu Y, et al. Three-dimensional culture of MSCs produces exosomes with improved yield and enhanced therapeutic efficacy for cisplatin-induced acute kidney injury. Stem Cell Research & Therapy. 2020;11(1):206. doi: 10.1186/s13287-020-01719-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Drzeniek, N., Mazzocchi, A., Schlickeiser, S., Forsythe, S., Moll, G., Geissler S., Reinke, P., Gossen, M., Gorantla, V. S., Volk, H. D., et al. (2021). Bio-instructive hydrogel expands the paracrine potency of mesenchymal stem cells. Biofabrication, 13(4), 045002. [DOI] [PMC free article] [PubMed]
- 108.Sun D, Huang Z, Xu J, Wang Y, Chen L, Hou Y, Chi G. HaCaTconditioned medium supplemented with the small molecule inhibitors SB431542 and CHIR99021 and the growth factor PDGFAA prevents the dedifferentiation of dermal papilla cells in vitro. Molecular Medicine Reports. 2021;23(5):326. doi: 10.3892/mmr.2021.11965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jung MK, Ha S, Huh SY, Park SB, Kim S, Yang Y, Kim D, Hur DY, Jeong H, Bang SI, et al. Hair-growth stimulation by conditioned medium from vitamin D3-activated preadipocytes in C57BL/6 mice. Life Sciences. 2015;128:39–46. doi: 10.1016/j.lfs.2015.02.018. [DOI] [PubMed] [Google Scholar]
- 110.Ghasemi F, Khoshmirsafa M, Safari E, Asgari M, Alemrajabi M, Nojehdehi S, Khorrami S. Vitamin E and selenium improve mesenchymal stem cell conditioned media immunomodulatory effects. Stem Cell Investigation. 2021;8:9. doi: 10.21037/sci-2020-008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yang Y, Choi H, Seon M, Cho D, Bang SI. LL-37 stimulates the functions of adipose-derived stromal/stem cells via early growth response 1 and the MAPK pathway. Stem Cell Research & Therapy. 2016;7(1):58. doi: 10.1186/s13287-016-0313-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dong L, Hao H, Xia L, Liu J, Ti D, Tong C, Hou Q, Han Q, Zhao Y, Liu H, et al. Treatment of MSCs with Wnt1a-conditioned medium activates DP cells and promotes hair follicle regrowth. Science and Reports. 2014;4:5432. doi: 10.1038/srep05432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ito M, Yang Z, Andl T, Cui C, Kim N, Millar SE, Cotsarelis G. Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature. 2007;447(7142):316–320. doi: 10.1038/nature05766. [DOI] [PubMed] [Google Scholar]
- 114.Park J, Jun EK, Son D, Hong W, Jang J, Yun W, Yoon BS, Song G, Kim IY, You S. Overexpression of Nanog in amniotic fluid-derived mesenchymal stem cells accelerates dermal papilla cell activity and promotes hair follicle regeneration. Experimental & Molecular Medicine . 2019;51:1–5. doi: 10.1038/s12276-019-0266-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pierce LM, Kurata WE. Priming With Toll-Like Receptor 3 Agonist Poly(I:C) Enhances Content of Innate Immune Defense Proteins but Not MicroRNAs in Human Mesenchymal Stem Cell-Derived Extracellular Vesicles. Front Cell Dev Biol. 2021;9:676356. doi: 10.3389/fcell.2021.676356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tang YT, Huang YY, Zheng L, Qin SH, Xu XP, An TX, Xu Y, Wu YS, Hu XM, Ping BH, et al. Comparison of isolation methods of exosomes and exosomal RNA from cell culture medium and serum. International Journal of Molecular Medicine. 2017;40(3):834–844. doi: 10.3892/ijmm.2017.3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wiklander OPB, Brennan MA, Lotvall J, Breakefield XO, El Andaloussi S. Advances in therapeutic applications of extracellular vesicles. Science Translational Medicine . 2019;11(492):eaav8521. doi: 10.1126/scitranslmed.aav8521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ha DH, Kim HK, Lee J, Kwon HH, Park GH, Yang SH, Jung JY, Choi H, Lee JH, Sung S, et al. Mesenchymal Stem/Stromal Cell-Derived Exosomes for Immunomodulatory Therapeutics and Skin Regeneration. Cells. 2020;9(5):1157. doi: 10.3390/cells9051157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kwack MH, Seo CH, Gangadaran P, Ahn BC, Kim MK, Kim JC, Sung YK. Exosomes derived from human dermal papilla cells promote hair growth in cultured human hair follicles and augment the hair-inductive capacity of cultured dermal papilla spheres. Experimental Dermatology. 2019;28(7):854–857. doi: 10.1111/exd.13927. [DOI] [PubMed] [Google Scholar]
- 120.Yan HL, Gao Y, Ding Q, Liu J, Li Y, Jin MH, Xu H, Ma S, Wang XL, Zeng WX, et al. Exosomal Micro RNAs Derived from Dermal Papilla Cells Mediate Hair Follicle Stem Cell Proliferation and Differentiation. International Journal of Biological Sciences. 2019;15(7):1368–1382. doi: 10.7150/ijbs.33233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Huang X, Qiu W, Pan Y, Li J, Chen Z, Zhang K, Luo Y, Wu B, Xu W. Exosomes from LPS-Stimulated hDPSCs Activated the Angiogenic Potential of HUVECs In Vitro. Stem Cells International. 2021;2021:6685307. doi: 10.1155/2021/6685307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Heo JS, Kim S, Yang CE, Choi Y, Song SY, Kim HO. Human Adipose Mesenchymal Stem Cell-Derived Exosomes: A Key Player in Wound Healing. Tissue Engineering and Regenerative Medicine . 2021;18(4):537–548. doi: 10.1007/s13770-020-00316-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ahmadi M, Rezaie J. Ageing and mesenchymal stem cells derived exosomes: Molecular insight and challenges. Cell Biochemistry and Function. 2021;39(1):60–66. doi: 10.1002/cbf.3602. [DOI] [PubMed] [Google Scholar]
- 124.Di Santo R, Romano S, Mazzini A, Jovanovic S, Nocca G, Campi G, Papi M, De Spirito M, Di Giacinto F, Ciasca G. Recent Advances in the Label-Free Characterization of Exosomes for Cancer Liquid Biopsy: From Scattering and Spectroscopy to Nanoindentation and Nanodevices. Nanomaterials (Basel) 2021;11(6):1476. doi: 10.3390/nano11061476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bahrami A, Moradi Binabaj M. G AF: Exosomes: Emerging modulators of signal transduction in colorectal cancer from molecular understanding to clinical application. Biomedicine & Pharmacotherapy. 2021;141:111882. doi: 10.1016/j.biopha.2021.111882. [DOI] [PubMed] [Google Scholar]
- 126.Ajit A, Nair MD, Venugopal B. Exploring the Potential of Mesenchymal Stem Cell-Derived Exosomes for the Treatment of Alopecia. Regenerative Engineering and Translational Medicine. 2021;7:119–128. [Google Scholar]
- 127.Luzzi S, Crovace AM, Del Maestro M, Giotta Lucifero A, Elbabaa SK, Cinque B, Palumbo P, Lombardi F, Cimini A, Cifone MG, et al. The cell-based approach in neurosurgery: Ongoing trends and future perspectives. Heliyon. 2019;5(11):e02818. doi: 10.1016/j.heliyon.2019.e02818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Liang X, Lin F, Ding Y, Zhang Y, Li M, Zhou X, Meng Q, Ma X, Wei L, Fan H, et al. Conditioned medium from induced pluripotent stem cell-derived mesenchymal stem cells accelerates cutaneous wound healing through enhanced angiogenesis. Stem cell research & therapy. 2021;12(1):295. doi: 10.1186/s13287-021-02366-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ebrahimi A, Ahmadi H, Pourfraidon Ghasrodashti Z, Tanide N, Shahriarirad R, Erfani A, Ranjbar K, Ashkani-Esfahani S. Therapeutic effects of stem cells in different body systems, a novel method that is yet to gain trust: A comprehensive review. Bosnian journal of basic medical sciences. 2021;21(6):672–701. doi: 10.17305/bjbms.2021.5508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ruiz R, Rosell J, Ceccarelli G, De Sio C, De Angelis G, Pinto H, Astarita C, Graziano A. Progenitor-cell-enriched micrografts as a novel option for the management of androgenetic alopecia. Journal of cellular physiology. 2020;235(5):4587–4593. doi: 10.1002/jcp.29335. [DOI] [PubMed] [Google Scholar]
- 131.Nilforoushzadeh MA, Lotfi E, Heidari-Kharaji M, Torkamaniha E, Hanifnia AR. Autologous whole fat injection stimulates hair growth in resistant Androgenetic Alopecia: Report of nine cases. Journal of Cosmetic Dermatology. 2021;20(8):2480–2485. doi: 10.1111/jocd.13907. [DOI] [PubMed] [Google Scholar]
- 132.Raj AT, Kheur S, Bhonde R, Gupta AA, Patil S. Assessing the effect of human mesenchymal stem cell-derived conditioned media on human cancer cell lines: A systematic review. Tissue and Cell. 2021;71:101505. doi: 10.1016/j.tice.2021.101505. [DOI] [PubMed] [Google Scholar]
- 133.Wang X, Tang Y, Liu Z, Yin Y, Li Q, Liu G, Yan B. The Application Potential and Advance of Mesenchymal Stem Cell-Derived Exosomes in Myocardial Infarction. Stem Cells International . 2021;2021:5579904. doi: 10.1155/2021/5579904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Gupta S, Rawat S, Arora V, Kottarath SK, Dinda AK, Vaishnav PK, Nayak B, Mohanty S. An improvised one-step sucrose cushion ultracentrifugation method for exosome isolation from culture supernatants of mesenchymal stem cells. Stem Cell Research & Therapy. 2018;9(1):180. doi: 10.1186/s13287-018-0923-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gunawardena TNA, Rahman MT, Abdullah BJJ, Abu Kasim NH. Conditioned media derived from mesenchymal stem cell cultures: The next generation for regenerative medicine. Journal of Tissue Engineering and Regenerative Medicine. 2019;13(4):569–586. doi: 10.1002/term.2806. [DOI] [PubMed] [Google Scholar]
- 136.Tan HL, Guan XH, Hu M, Wu J, Li RZ, Wang LF, Huang HD, Yu ZP, Wang XY, Xiao YF, et al. Human amniotic mesenchymal stem cells-conditioned medium protects mice from high-fat diet-induced obesity. Stem Cell Research & Therapy. 2021;12(1):364. doi: 10.1186/s13287-021-02437-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analyzed in this study are available from the corresponding author on reasonable request.