Abstract
Background
Chronic low back pain (cLBP) is a complex with a heterogenous clinical presentation. A better understanding of the factors that contribute to cLBP is needed for accurate diagnosis, optimal treatment, and identification of mechanistic targets for new therapies. The Back Pain Consortium (BACPAC) Research Program provides a unique opportunity in this regard, as it will generate large clinical datasets, including a diverse set of harmonized measurements. The Theoretical Model Working Group was established to guide BACPAC research and to organize new knowledge within a mechanistic framework. This article summarizes the initial work of the Theoretical Model Working Group. It includes a three-stage integration of expert opinion and an umbrella literature review of factors that affect cLBP severity and chronicity.
Methods
During Stage 1, experts from across BACPAC established a taxonomy for risk and prognostic factors (RPFs) and preliminary graphical depictions. During Stage 2, a separate team conducted a literature review according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines to establish working definitions, associated data elements, and overall strength of evidence for identified RPFs. These were subsequently integrated with expert opinion during Stage 3.
Results
The majority (∼80%) of RPFs had little strength-of-evidence confidence, whereas seven factors had substantial confidence for either a positive association with cLBP (pain-related anxiety, serum C-reactive protein, diabetes, and anticipatory/compensatory postural adjustments) or no association with cLBP (serum interleukin 1-beta / interleukin 6, transversus muscle morphology/activity, and quantitative sensory testing).
Conclusion
This theoretical perspective will evolve over time as BACPAC investigators link empirical results to theory, challenge current ideas of the biopsychosocial model, and use a systems approach to develop tools and algorithms that disentangle the dynamic interactions among cLBP factors.
Keywords: Chronic Pain, Low Back Pain, Measurement, Research, Spine, Theoretical Model
Introduction
Chronic low back pain (cLBP) is characterized by three primary dimensions: sensory–discriminative, affective–motivational, and cognitive–evaluative [1]. cLBP conditions have many potential causes and trajectories—a situation that is further complicated by the hypothesis that “pain” can become separated from an original nociceptive source (i.e., central sensitization) [2]. Patient-specific pathophysiological mechanisms are complex and difficult to untangle. The biopsychosocial model (BPSM) has become the dominant framework for explaining the diversity of potential risk factors, prognostic factors, protective factors, and comorbidities that influence and affect cLBP experiences and their presentation [3, 4]. However, although the BPSM is intended to encourage viewing the patient as a continuum of mind, body, and environment [5], some note that the BPSM is too vague to be testable and is difficult to implement in clinical practice [6]. Paradoxically, it can encourage compartmental, reductionist thinking, making it hard to operationalize in a holistic manner [7, 8].
A primary goal of the Back Pain Consortium (BACPAC) is to promote evidence-based understanding of the dynamic interactions among the physiological, psychological, and environmental factors that contribute to cLBP conditions. That knowledge can then be used to develop tools and algorithms that facilitate therapeutic decision-making processes, thereby providing guidance about for whom, when, and how to intervene. Deep phenotyping of patients with cLBP is intended to support the achievement of that BACPAC goal.
Multiple disciplines are involved with cLBP research and clinical care. Diverse perspectives lead to inconsistent language, varying conceptual frameworks, and reliance on siloed literature during the development of theories about links between risk factors and outcomes. To address this dilemma, BACPAC established a Theoretical Model Working Group (TMWG) with a charge to develop and incrementally improve Theoretical Model Schemas (TMS), defined as conceptual representations, mental models, or patterns of knowledge on cLBP that are intended to facilitate interpretation and understanding of new information. Within BACPAC, TMS can support achieving four broad goals: 1) Integrate observations from diverse BACPAC research activities, 2) facilitate transdisciplinary discussions and collaboration, 3) organize theories on which clinical hypotheses can be based, and 4) develop executable models supporting the three preceding activities. Specific TMS are expected to develop into coherent, mechanism-oriented rationales for developing and prioritizing improved clinical measures.
Although clinical algorithms rooted in basic science are expected to provide more robust predictions, TMS can also serve as communication tools for data scientists who will be conducting large-scale, multimodal analyses but might be inexpert at interpreting biological, biomechanical, and biobehavioral evidence. Grounding those analyses to TMS could help reduce the chance of focusing on interesting but spurious clinical correlations.
Developing, challenging, and iteratively improving TMS are requisite for 1) bringing together observations and facts from separate investigations; 2) summarizing and linking findings into accessible, coherent, useful structures; 3) improving explanations of cLBP phenomena—both the “what” and “why” of their occurrence; and 4) providing relational maps to improve insight into dynamic, multidimensional causal influences. TMS are expected to strike a balance between interpreting data-driven discoveries and developing applications of medical knowledge to better meet the needs of patients with cLBP [5]. We expect that TMS will undergo continual refinement in response to increased use across BACPAC and the broader spine research community.
Methods
We used a three-stage approach in developing the TMS. During Stage 1, TMWG subject-matter experts from across BACPAC reviewed prior BPSM representations, established a taxonomy for risk and prognostic factors (RPFs), and reached consensus on initial depictions. During Stage 2, an umbrella literature review was performed to identify meta-analyses and systematic reviews that describe risk factors (those that discriminate between individuals with cLBP and individuals without cLBP) or prognostic factors (those that discriminate between individuals who did and individuals who did not respond to a particular cLBP treatment). Finally, during Stage 3, working domain definitions and associated TMS were finalized on the basis of the umbrella review results.
Schemas
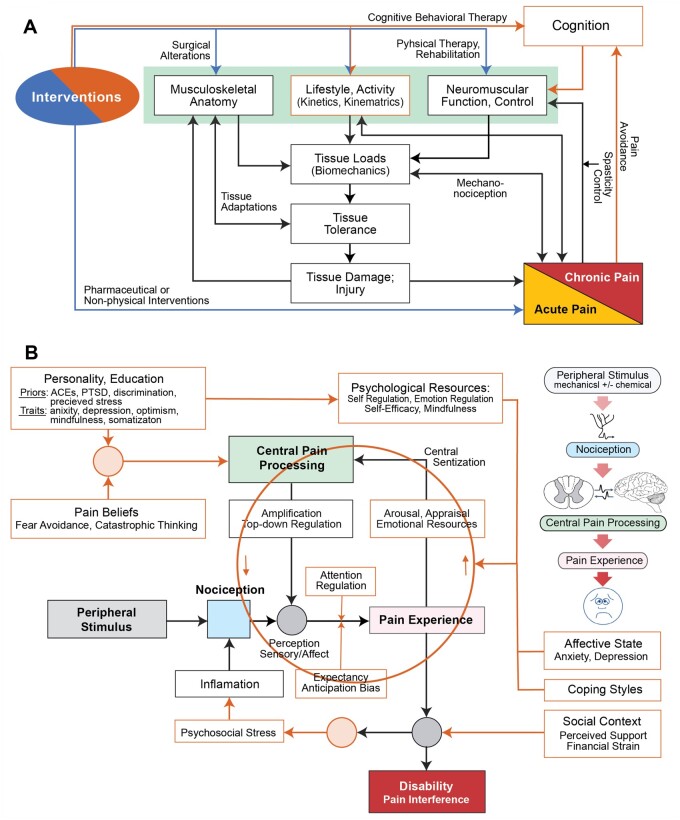
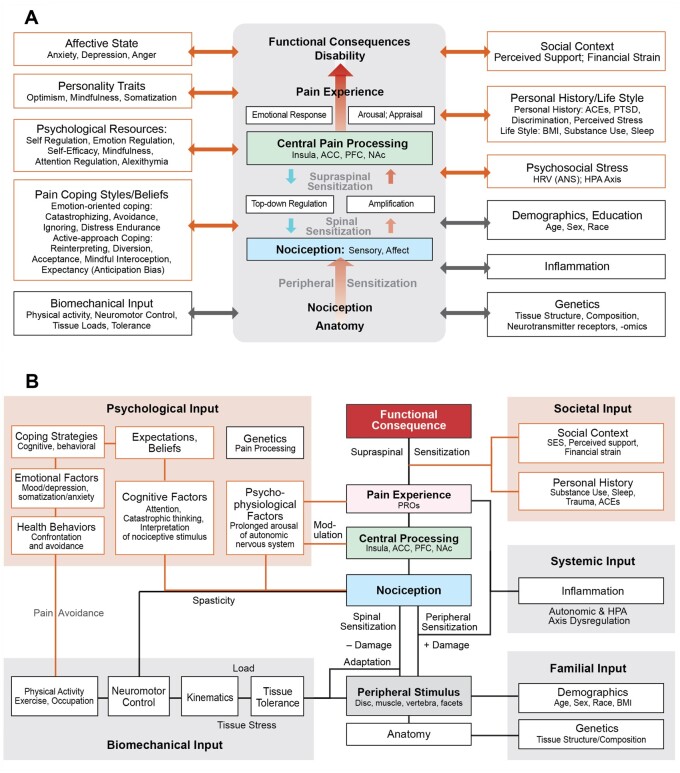
During a series of biweekly TMWG meetings between December 2019 and June 2020, discussions included, for example, development of predictive algorithms, issues associated with planning BACPAC clinical trials, possible explanations for cLBP phenomena and their trajectories, strategies to manage potential treatment options, novel outcome measures, and BPSM characteristics at various degrees of granularity. Discussions were often centered on ad hoc and working schemas. Several early working schemas included focused “sub-models” (Figure 1) that were factored into coarse-grain overviews (Figure 2 and (Figure 3). Broader-ranging discussions were frequently predicated on schemas representing the conceptual models provided by one or more group members.
Figure 1.
Two exemplary working sub-models developed to support focused TMWG discussions. (A) This scheme illustrates features and activities contributing to loads generated in the spine during daily-living activities. Tissue stresses triggering nociception can exceed tissue tolerances, cause damage, and thus facilitate the development of neuropathic pain. Concurrent feedback phenomena can affect neuromuscular control and function. (B) This scheme illustrates how social, biobehavioral, psychological, and patient-specific features and phenomena can influence central pain processing, pain experience, and ultimately disability. The circle highlights features and phenomena that can contribute dynamic bidirectional (“top-down,” “bottom-up”) influences. Abbreviations: ACE = agreeableness, conscientiousness, extraversion; PTSD = post-traumatic stress disorder.
Figure 2.
Course-grained theoretical schemes. (A) A scheme used to support intervention-focused discussions emphasizing psychological and social perspectives. The idealized central scheme depicts the canonical sequence of features and phenomena characterizing cLBP experiences and their functional consequences. On both sides are categories of factors that can influence the central process and vice versa. (B) This scheme supports ongoing TMWG discussions of phenotypes, interventions, explanatory theories, machine learning, etc. Factors in A are reorganized into five broad domain clusters. The scheme specifies plausible, bidirectional interactions between factors within clusters and between those factors and central features. A peripheral stimulus source is included between anatomy and nociception. ACE = agreeableness, conscientiousness, extraversion; ACC = anterior cingulate cortex; ANS = autonomic nervous system; BMI = body mass index; HRV = heart rate variability; HPA = hypothalamic-pituitary-adrenal axis; NAc = nucleus accumbens; PTSD = post-traumatic stress disorder; PFC = prefrontal cortex; PRO = patient-reported outcome; SES = socioeconomic status.
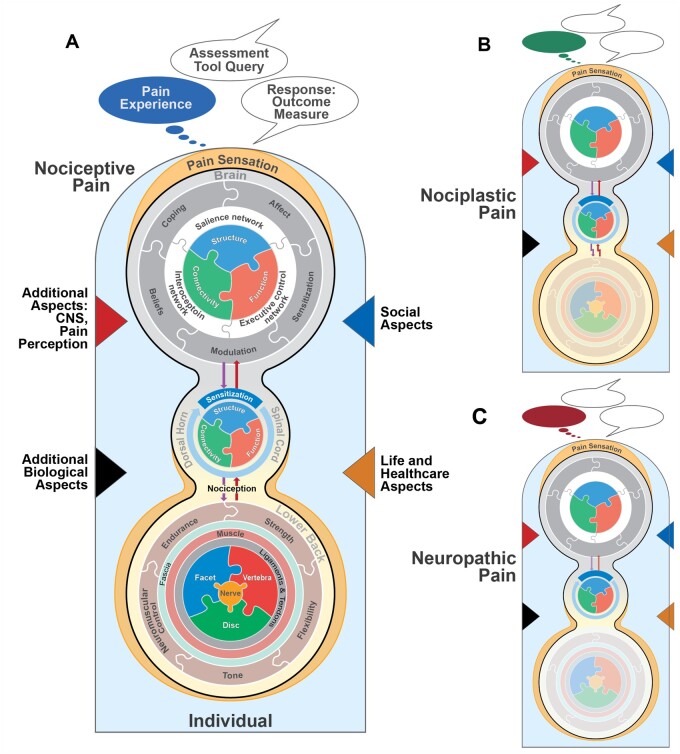
Figure 3.
Illustrations highlighting selected aspects contributing to the complexity of an individual’s cLBP condition when current pain is one of three types. Temporal changes are not illustrated. (A) Nociceptive pain: The three concentric groupings depict dynamically networked (entangled) features within brain, spine, and lower back. All other features are grouped into one of four categories, represented by colored triangles—two internal (left: green and red) and two external (right: blue and brown). (B) Nociplastic pain emerges from altered nociception despite no clear evidence of actual or threatened tissue damage. (C) Neuropathic pain can be caused by an abnormality or disease of the somatosensory nervous system. The top speech balloon depicts the same clinical assessment question being posed. Each subjective response is based on the individual’s current or recalled pain experience, illustrated by the different-colored thought balloons. All three responses can be identical even though an individual’s experience is unique and dependent on pain type. An individual’s current cLBP condition might (or might not) involve a combination of pain types.
Umbrella Literature Review
A subset of TMWG members performed a “systematic review of systematic reviews,” or umbrella review, in which the articles analyzed were either systematic reviews of cLBP RPFs or related publication types (e.g., meta-analyses, scoping reviews). The review was conducted and is reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The protocol for this umbrella review was registered with the Open Science Framework (Center of Open Science; https://osf.io/8ajvf) [9].
Search Strategy
We searched for systematic reviews and meta-analyses of studies that investigate factors associated with trajectories of measures of a patient’s cLBP condition with or without a specific treatment intervention. PubMed, EMBASE, and Web of Science Core searches were limited to January 2005 to December 2020. The keywords and Medical Subject Headings were selected to capture diverse but relevant systematic reviews and meta-analyses (Supplementary Data 1). Only full-text reviews published in English were retained for evaluation.
Two coauthors (AChau and EW) performed electronic searches and removed duplicates (using Endnote X9 [Clarivate, Chandler, AZ, USA]). Next, a team of 13 coauthors determined eligibility on the basis of title and abstract content. Two or more coauthors screened each article. Results were tracked in Rayyan (https://www.rayyan.ai). When relevance consensus was not reached, a full-text screening was undertaken. Each remaining article was checked against the eligibility criteria on a full-text basis. Disagreement was resolved by a third coauthor. An additional Web of Science cited reference search was conducted in October to November of 2021 to retrieve more recent, relevant articles that cited reviews from our original pool of articles. To ensure completeness, the references of the original pool were searched for relevant articles, as well.
Eligibility Criteria
Reviews were eligible if they 1) included a separate analyzable population of adult patients with cLBP (defined as pain lasting >3 months); 2) measured the association between biopsychosocial RPFs and treatment choice, prognosis, or treatment outcome; and 3) were published in English with a readily available full text. Reviews were excluded if the cited studies 1) focused on pediatric-only populations; 2) were limited to either acute or subacute low back pain exclusively; or 3) included a mixed population of subjects with pain without a separable cLBP subgroup analysis. Reviews were also excluded if the article type was an umbrella review or an intervention article, and conference annals, protocols, and supplements were excluded.
Data Extraction
Review evaluators extracted the following data: the number of studies reviewed; the number of subjects with cLBP across the included studies; the factors assessed; the study authors’ assessment of the strength of evidence (SOE) for an association between factors and clinical outcomes (as reported through odds ratios, relative risk, and confidence intervals); and the study authors’ description of relevant mechanisms.
Risk-of-Bias Assessment
Methodological quality was assessed with a modified version of the Assessing the Methodological Quality of Systematic Reviews (AMSTAR) 2 quality-assessment tool [10]. It is designed to work with both interventional and non-interventional systematic reviews. It consists of 16 items, each evaluated as “yes,” “no,” or “partially” on the basis of the completion of specific criteria for each item. To enable application to the final set of reviews, minor modifications were made to the AMSTAR 2 template (Supplementary Data 2). Overall confidence in the results of each review was determined primarily from the number of critical AMSTAR 2 domains that were satisfied, with higher or lower ratings justified by satisfaction of noncritical domains. The seven critical AMSTAR 2 domains are 1) protocol registered before commencement of the review; 2) adequacy of the literature search; 3) justification for the exclusion of individual studies; 4) inclusion of the risk of bias from individual studies in the review; 5) appropriateness of the meta-analytical methods; 6) consideration of risk of bias in the interpretation of the results of the review; and 7) assessment of the presence and likely impact of publication bias. Summary assessments were used to rank the quality of each review as high, moderate, low, or critically low.
Data Synthesis and SOE Assessment
The overall SOE for each RPF was assessed with a modified version of the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach [11]. For each review article, the study authors’ assessment of the SOE that associated studied factors and outcomes was documented. The GRADE quality rating was modified to distinguish four SOE ranks that were affiliated to the study authors’ language: association with high/moderate evidence; association with low/weak evidence; inconclusive evidence for any association; or high/moderate evidence of no association. (Table 1). Finally, a combination of the AMSTAR 2 assessment of overall study quality and the study authors’ assessment of the SOE was used to establish an overall rating of confidence that a given factor associates with a cLBP outcome (summary SOE) (Table 2).
Table 1.
Mapping between study authors’ SOE language, the modified GRADE SOE definition, and the present summary authors’ SOE definition
| SOE Definition Used in the Present Summary |
||||||
|---|---|---|---|---|---|---|
| Association (High, Moderate) | Association (Weak, Low) | Inconclusive | No Association (High, Moderate) | |||
| Modified GRADE definition | Further research is very unlikely to change our confidence in the estimated effect. | Further research is likely to have an important impact on our confidence in the estimate of effect and could change the estimate. | Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. | Further research is unlikely to change our confidence in the estimated effect. | ||
| High | Moderate | Weak | Low | |||
| Language examples from the articles |
|
|
|
|
|
|
Table 2.
Integration of AMSTAR rating and study authors’ SOE assessment into overall SOE rating
| Study Authors’ SOE and Strength of Association |
|||||
|---|---|---|---|---|---|
| AMSTAR | High/Moderate Evidence for Association | Weak/Low Evidence for Association | Inconclusive Evidence for Association | High/Moderate Evidence for No Association | |
| High | No or one noncritical weakness: The systematic review provides an accurate and comprehensive summary of the results of the available studies that address the question of interest. | Substantial confidence | Substantial confidence | Little | Substantial confidence |
| Moderate | More than one noncritical weakness: The systematic review has more than one weakness but no critical flaws. It might provide an accurate summary of the results of the available studies that were included in the review. | Substantial confidence | Some | Little | Substantial confidence |
| Low | One critical flaw with or without noncritical weaknesses: The review has a critical flaw and might not provide an accurate and comprehensive summary of the available studies that address the question of interest. | Some | Little | Little | Some |
| Critically Low | More than one critical flaw with or without noncritical weaknesses: The review has more than one critical flaw and should not be relied on to provide an accurate and comprehensive summary of the available studies. | Little | Little | Little | Little |
 Little confidence that the prognostic factor does or does not associate with cLBP.
Little confidence that the prognostic factor does or does not associate with cLBP.
 Some confidence that the prognostic factor does or does not associate with cLBP.
Some confidence that the prognostic factor does or does not associate with cLBP.
 Substantial confidence that the prognostic factor associates with cLBP.
Substantial confidence that the prognostic factor associates with cLBP.
 Substantial confidence that the prognostic factor does not associate with cLBP.
Substantial confidence that the prognostic factor does not associate with cLBP.
Definitions of RPF domains (provided below) and the associated data elements (ADEs) for each were adjusted on the basis of the information and language extracted from the selected reviews. When available, we documented the study authors’ hypotheses or theories with regard to potential mechanisms underlying the associations between RPFs and cLBP outcomes or measures of patients’ cLBP experiences.
Results
Schemas
The TMWG adopted a hierarchy of established medical terms, detailed in the section “Cluster and Domain Descriptions,” to describe components and features of working TMS. Because group discussions often centered around plausible causal influences and potentially mitigating treatment intervention ideas, discussants tended to identify corresponding schema features as either inputs or outputs, where the latter map to outcome measures. For a given output, biological, psychological, or socio-environmental sources of inputs or influences (potential RPFs) were identified by BACPAC investigators with corresponding expertise (Figure 2A and 2B provide examples). For convenience, we referred to each source as a primary domain. To better align with BPSM concepts, we grouped related primary domains into six broad domain clusters, described below (Figure 2B). The domains are also intended to align with data elements collected during BACPAC clinical studies. The first five clusters are based on Figure 2B domains. The sixth is based on the central schema in Figure 2A and 2B, which depict how noxious and “threat” signals from nociceptors are processed and modulated by peripheral and central factors and ultimately determine the pain experience and associated functional consequences [12]. ADEs for the primary domains were initially identified by TMWG members and subsequently refined during the umbrella review (Table 3).
Table 3.
Affiliation of data elements and model domains
| ADEs | |
|---|---|
| Cluster 1—Psychological Attributes | |
| PAIN BELIEFS | |
|
|
| Fear avoidance |
|
| Catastrophizing |
|
| Pain beliefs |
|
| Patient expectations | CEQ—Credibility/Expectancy Questionnaire |
| COPING SKILLS | |
| Coping strategies / reaction to pain |
|
| Emotional support system / psychosocial aspects |
|
| Stress | PSS—Perceived Stress Scale |
| Coping | PSEQ—Pain Self-Efficacy Questionnaire (assesses confidence in continuing activities in spite of pain; self-efficacy, pain-related coping strategies) |
| Acceptance |
|
| AFFECTIVE STATE | |
| Depression |
|
| Anxiety |
|
| Pain somatization | SSI—Symptom Severity Index |
| Positive affect |
|
| PERSONALITY TRAITS |
|
| PSYCHOLOGICAL RESOURCES | |
| Negative psychological state |
|
| Mindfulness | FFMQ—Five-Facet Mindfulness Questionnaire |
| Cluster 2—Biomechanical Attributes | |
| PHYSICAL ACTIVITY | |
| Physical functioning / activities of daily living |
|
| Real-world measurements |
|
| SENSORIMOTOR CONTROL |
|
| LOAD, OCCUPATION |
|
| TISSUE TOLERANCE |
|
| Cluster 3—Societal Attributes | |
| SOCIODEMOGRAPHICS | |
| Sociodemographic factors |
|
| Emotional and social support systems |
|
| OCCUPATION | |
| Work characteristics and work-related attitudes |
|
| Fatigue | MFI—Multidimensional Fatigue Inventory (measures five dimensions: general, physical, motivation, activity, mental) |
| PERSONAL/MEDICAL HISTORY |
|
| Cluster 4—Systemic Attributes | |
| INFLAMMATION |
|
| Cluster 5—Familial Attributes | |
| GENETICS | Biosample analysis |
| DEMOGRAPHICS | Demographic questionnaires |
| Cluster 6—Central Schema Attributes | |
| ANATOMY | MRI—magnetic resonance imaging X-ray |
| PERIPHERAL STIMULUS |
|
| NOCICEPTION AND NEUROPATHIC PAIN |
|
| CENTRAL PAIN PROCESSING |
|
| PAIN PERCEPTION | |
| Aspects of pain |
|
| PAIN EXPERIENCE | |
| Pain intensity |
|
| Pain experience |
|
| Cognitive fatigue |
|
| Pain location/duration |
|
| FUNCTIONAL CONSEQUENCE | |
| Disability |
|
| Functional capacity tests |
|
| Pain interference |
|
| Physical functioning |
|
“Load,” “tissue stress,” and “adaptation” were included in working TMS variants to represent hypothesized phenomena that link specific domains and could be capable of changing the behaviors of the linked domains. However, because they are not directly measurable in patients, they are neither risk nor prognostic factors. Nevertheless, we included them below to encourage explanatory and mechanistic thinking and to support hypothesis generation with regard to how these phenomena might interact and alter networked causal influences.
Umbrella Literature Review
Study Characteristics
Our initial literature search yielded 2,234 studies for title and abstract screening. This first round of screening excluded 1,836 studies, leaving 398 studies for full-text review. During full-text review, an additional 196 studies were identified in their cited literature and via Web of Science identified articles that cited the reviewed studies. During full-text screening, 549 studies were excluded, leaving 45 studies that were compatible with our search criteria.
Across the 45 studies, the study authors individually presented conclusions on SOE for 89 RPF–outcome associations (Supplementary Data 3). After data on similar RPFs were combined to adjust for overlap, conclusions were drawn on a total of 52 different RPF–outcome associations (Table 4). Of the study authors’ SOE assessments for the 52 RPFs, 39.3% were classified as high/moderate association; 24.7% and 14.6% were classified as low/weak association and no association, respectively; and 21.3% were classified as inconclusive.
Table 4.
Overall assessment of RPF SOE organized by domain clusters
| Cluster and Domain | Factors | ADEs | Outcome Measure(s) | Overall Assessment | Article |
|---|---|---|---|---|---|
| Cluster 1—Psychological Attributes | |||||
| Pain beliefs | Patient expectations | Pain intensity, disability, quality of life |

|
Mohamed (2020) [44] | |
| Fear-avoidance beliefs | ALBPSQ, FABQ, FAB, TSK, PCS, SES, PCL, CSQ, BBQ, PSES | Work status, functional limitation, ODI, RMDQ, CPG, RTW, transition from acute to chronic pain as measured by functional disability / pain intensity / RTW, FNA, ENA, QOL, RTW, GCPS, Pain Intensity, LBP-related disability, TSK, FABQ-P, FABQ-W, VAS, SF-36, ODI, MVK pain and disability, lifting low-capacity tests (PILE, IWS-FCE, WEST2-Work Capacity Evaluation, Work Well FCE), static lifting |
|
||
| Pain-related anxiety | Disability |

|
Martinez-Calderon (2019) [13] | ||
| Affective state | Depression | SCL-90, ALBPSQ, HKF-R 10, CES-D, BDI | Work status, functional limitation, pain, lifting capacity tests (PILE, IWS-FCE, WEST2-Work Capacity Evaluation, Work Well FCE), disability (e.g., activity limitations, participation restriction), overall health status (e.g., health-related quality of life), opioid use |
|
|
| Coping skills | Coping strategies / reaction to pain | ALBPSQ, CPCI, HFK-R 10, INTERMED, PPS | Work status, pain, functional limitation |

|
Melloh (2009) [44] |
| Catastrophizing | Disability |

|
Wertli (2014) [51] | ||
| Specific self-efficacy | SES | Lifting capacity tests (PILE, IWS-FCE, WEST2-Work Capacity Evaluation, Work Well FCE), carrying |

|
van Abbema (2011) [47] | |
| Psychological resources | Negative psychological states | ALBPSQ, HFK-R, INTERMED, PPS | Work status, pain, functional limitation |

|
Melloh (2009) [44] |
| Cluster 2—Biomechanical Attributes | |||||
| Physical activity | Physical functioning / activities of daily living | ALBPSQ, CPCI, PPS | Work status, pain, functional limitation |

|
Melloh (2009) [44] |
| Physical activity | Accelerometry, PARS, 7-day physical activity recall questionnaire, Baecke physical activity questionnaire | Quebec Back Pain Disability scale, RMDQ, ODI, Von Korff Disability Questionnaire, pain intensity, disability, any measure of recovery |

|
||
| Sensorimotor control | Anticipatory postural adjustments | APAs | Classification as having cLBP |

|
Knox (2018) [16] |
| Compensatory postural adjustments | CPAs | Classification as having cLBP |

|
Knox (2018) [16] | |
| Proprioception | JRE, TTDPM, AJRS, PJrS, passive lumbar flexion, passive lumbar extension | Pain-related disability, VAS, RMDQ, ODI |

|
Ghamkhar (2019) [54] Lin (2019) [55] |
|
| Lumbar repositioning error; absolute error, repositioning error, constant error, variable error | Classification as having cLBP |

|
Rausch Osthoff (2015) [56] | ||
| Trunk mobility, strength, endurance | VAS, Quebec Questionnaire, Dallas Pain Questionnaire, RMDQ, JOA score, PDI, ODI |

|
Steiger (2012) [57] | ||
| Load | |||||
| Tissue tolerance | |||||
| Cluster 3—Societal Attributes | |||||
| Sociodemographics | Emotional and social support systems | ALBPSQ, CPCI, VDPQ | Work status, pain, functional limitation |

|
Melloh (2009) [44] |
| Personal/medical history | Vitamin D Deficiency | cLBP after surgery |

|
Zadro (2017) [58] | |
| Sleep quality, insomnia severity, sleep quantity | NRS, VAS, MPQ-SF, SF-36, JOABPEQ, self-reported questionnaire, NPRS, PRI (MPQ), PPI (MPQ), QST, AIS, ODQ, ISI, PROMIS Sleep, PSQI, 4-item Jenkins Sleep Questionnaire, self-reported questionnaire, Actiwatch, ESS |

|
Van Looveren (2021) [59] | ||
| Effect of functional shoes (unstable shoes and orthopedic shoes) on LBP | MILLION questionnaire, VAS, NRS, RMDQ, EMG, lumbar spine ROM, JOABPEQ, ODI, QSLS, QOL, EMG, balance |

|
Kong (2020) [60] | ||
| Effect of functional insoles (custom-made orthotics), foot orthoses and foot-supporting insoles on LBP | MILLION questionnaire, VAS, NRS, RMDQ, EMG, lumbar spine ROM, JOABPEQ, ODI, QSLS, QOL, EMG, balance |

|
Kong (2020) [60] | ||
| Using pain medication | VAS, pain rating scale, LBPRS-P, NRS, Borg CR-10, RMDQ, Aberdeen, LBPRS-F, ODI, PSFS |

|
Hayden (2020) [61] | ||
| Medical aspects | HKF-R 10, VDPQ, INTERMED | Work status, pain |

|
Melloh (2009) [44] | |
| Prevalence of PTSD | Diagnosis of cLBP |

|
Fishbain (2017) [62] | ||
| Occupation | Work characteristics and work attitudes | ALBPSQ, VDPQ | Work status, functional limitation |

|
Melloh (2009) [44] |
| Fewer physical demands at work | VAS, pain rating scale, LBPRS-P, NRS, Borg CR-10, RMDQ, Aberdeen, LBPRS-F, ODI, PSFS |

|
Hayden (2020) [61] | ||
| Cluster 4—Systemic Attributes | |||||
| Inflammation | TNF-alpha | Presence of cLBP and/or patient-oriented outcomes |

|
Morris (2020) [14] | |
| CRP | Presence of cLBP and/or patient-oriented outcomes |

|
Morris (2020) [14] | ||
| IL-6, IL-1b | Presence of cLBP and/or patient-oriented outcomes |

|
Morris (2020) [14] | ||
| Type 1 or type 2 diabetes | Self-reported back pain |

|
Pozzobon (2019) [15] | ||
| Physical comorbidities | Lower body mass index | VAS, pain rating scale, LBPRS-P, NRS, Borg CR-10, RMDQ, Aberdeen, LBPRS-F, ODI, PSFS |

|
Hayden (2020) [61] | |
| Cluster 5—Familial Attributes | |||||
| Genetics | Genetic variant. rs71321981, NFIB gene | Presence of sciatica |

|
Lemmela (2016) [63] | |
| Genetic variant, Sox5, CCDC26/GSDMC, DCC | Self-reported cLBP |

|
Suri (2018) [64] | ||
| Demographics | Age | Lifting capacity tests (PILE, IWS-FCE, WEST2-Work Capacity Evaluation, Work Well FCE), carrying |

|
van Abbema (2011) [47] | |
| Gender | Lifting capacity tests (PILE, IWS-FCE, WEST2-Work Capacity Evaluation, Work Well FCE), carrying |

|
van Abbema (2011) [47] | ||
| Cluster 6—Central Schema Attributes | |||||
| Central pain processing | Brain anatomy, gray and white matter and volume, functional brain changes | Bilateral mPFC and left anterior insula from MRI, functional brain imaging | cLBP, cLBP vs control by VAS, NRS, SF-MPQ |
|
|
| QST | Pressure pain threshold, heat pain threshold, pain tolerance, temporal summation | VAS, NRS, ODI, LBP status at follow-up, including: pain intensity, functional status or disability, work status, health-related quality of life, global perceived effect/recovery |

|
||
| Pain perception | Aspects of pain | ALBPSQ, HKF-R10, INTERMED, PPS, VDPQ | Work status, pain, functional limitation |

|
Melloh (2009) [44] |
| QST | Pressure pain threshold, heat pain threshold, pain tolerance, temporal summation | VAS, NRS, ODI |

|
Hubscher (2013) [69] | |
| Pain experience | Pain intensity | MPQ, VAS | Lifting capacity tests (PILE, IWS-FCE, WEST2-Work Capacity Evaluation, Work Well FCE), carrying |

|
van Abbema (2011) [47] |
| Pain duration | Lifting capacity tests (PILE, IWS-FCE, WEST 2-Work Capacity Evaluation, Work Well FCE), pain proportion (% of patients with LBP at 1, 3, 6, and 12 months), physical activity, sick leave, pain intensity, fear-avoidance beliefs, disabling LBP, functional status |

|
|||
| Neuropathic pain | Neuropathic pain | LANSS, PDQ, DN4 |

|
Fishbain (2014) [71] | |
| Functional consequence | Self-reported disability | QTFC, RMDQ | Lifting capacity tests (PILE, IWS-FCE, WEST2-Work Capacity Evaluation, Work Well FCE), carrying |

|
van Abbema (2011) [47] |
| OMPSQ, SBT | Transition from acute to chronic pain as measured by functional disability; pain intensity; return-to-work status |

|
Pauli (2019) [72] | ||
| Peripheral stimulus | Bacterial infection in discs | Symptomatic, degenerative disc disease |

|
Ganko (2015) [73] | |
| Disc protrusion, nerve root impingement, disc degeneration, HIZ | MRI | Presence of cLBP and/or patient-oriented outcomes |

|
Endean (2011) [74] | |
| Modic changes | Presence of back pain, LBP intensity, activity limitation |

|
Herlin (2018) [75] | ||
| Facet joint osteoarthritis | ODI, NRS |

|
Baroncini (2021) [76] | ||
| Anatomy | Morphology or activity of TA and MF | RMDQ, VAS, ODI, NPRS |

|
Wong (2014) [17] | |
| Lumbar multifidi (LM) | US, CT, MRI | Pain VAS, BPS, RMDQ, MPQ, FRIQ, ODI, SF-36, surface electromyographic, motor control exercises, LM cross-sectional area, thickness, fat area |

|
||
| Composite Measures | |||||
| Composite measures, predictive models | Composite of risk factors (psychological, biomechanical, familial attributes) | ODI, RMDQ |

|
Hilfiker (2007) [37] | |
| Composite measures of physical and psychosocial domains | SBT, OMPSQ | SBT, OMPSQ | RMDQ, QALYs, PCS, TSK, HADS, QOL, VAS, BBQ, DRAM, MSPQ |

|
Meyer (2018) [39] |
| Composite measure of psychosocial yellow flags | OMPQ | Self-reported global recovery, long-term pain, disability, sick leave |

|
Hockings (2008) [38] | |
Aberdeen = Chinese Aberdeen Scale; AIS = Athens Insomnia Scale; BDI = Beck Depression Inventory; Borg Cr-10 = Borg Category-Rating Scale; BPS = Back Performance Scale; CCDC26/GSDMC = Long non-coding RNA; CT = Computed Tomography; DCC = gene Deleted in Colorectal Carcinoma; DRAM = Distress and Risk Assessment Method; ENA = emotional non-adjustment; ESS = Epworth Sleepiness Scale; FAB = Fear avoidance beliefs; FCE = Functional Capacity Evaluation; FNA = functional non-adjustment; FRIQ = Functional Rating Index Questionnaire; ISI = Insomnia Severity Index; IWS-FCE = Isernhagen Work Systems Functional Capacity Evaluation; JOABPEQ = Japanese Orthopedic Association Back Pain Evaluation Questionnaire; MVK = Modified von Korff; NFIB = Nuclear Factor 1 B-type; ODQ = Oswestry Disability Questionnaire; PCL = Pain Cognition List; PDI = Pain Disability Index; PILE = Progressive isoinertial lifting evaluation; PPI = Present Pain Index; PRI = Pain Rating Index; PSES = Pain Self-efficacy Beliefs questionnaire; PSFS = Patient specific functional scale; PSQI = Pittsburgh Sleep Quality Index; QALYs = Quality-Adjusted Life Years; QOL = quality of life; QSLS = Quantitative Score of Lumbago Symptom; ROM = Range of Motion; RTW = Return to work; SES = Self Efficacy Scale; Sox5 = SRY-Box Transcription Factor 5; TNF-alpha = Tissue Necrosis Factor alpha; US = ultrasound.
The majority of the conclusions on RPFs reported were in the Psychological Attributes category (n = 27), followed by the Central Schema Attributes category (n = 24), the Societal Attributes category (n = 13), the Biomechanical Attributes category (n = 10), the Familial Attributes category (n = 6), and the Systemic Attributes category (n = 5) (Table 4). Linked to each RPF was one or more ADE(s), which were assessed during the reviewed studies.
When combined with AMSTAR 2 assessment of the overall study quality, 9.0% of factors were classified as having Substantial Confidence, 19.1% were classified as having Some Confidence, and 71.9% were classified as having Little Confidence. Substantial confidence for an association was noted for pain-related anxiety (with disability) [13], C-reactive protein (CRP) (with self-reported cLBP) [14], diabetes (with self-reported cLBP) [15], and anticipatory and compensatory postural adjustments (with self-reported cLBP) [16]. Substantial confidence for a lack of association was noted for interleukin (IL)-6 and IL-1b (with the presence of chronic nonspecific low back pain) [14], transverse abdominis muscle morphology and activity after conservative treatments (with changes in cLBP clinical outcomes) [17], and quantitative sensory testing (QST) (with low back pain outcomes) [18].
The main reasons that study authors downgraded quality included heterogeneity of methods, outcome measures, and populations (subject diversity, low back pain duration, comorbidities, insufficient demographic data); small sample sizes; and inadequate investigator blinding and bias assessment.
Cluster and Domain Descriptions
Information extracted from the cited reviews was used to modify and augment domain language used during TMWG discussions. Several RPFs could be assigned to multiple domains. For organizational simplicity, we assigned each to one domain. In addition, note that outcome measures can be influenced by multiple RPFs, which makes it impracticable to study them in isolation. Furthermore, the same subjective or objective ADE can be affiliated with RPFs listed under multiple domains.
Psychological Attributes—Cluster 1
During the past several decades, numerous studies have shown the influence of psychological parameters on the development and trajectory of cLBP conditions [19]. Psychological parameters are defined as constructs that capture patient beliefs based on prior experiences and future expectations, affect states and traits, attitudes, personality traits, behaviors, coping styles and resources, attention styles towards pain, self-efficacy, and others. The following interconnected constructs—often studied in isolation—provide a crude categorization of factors that have found repeated entry into studies of RPFs and possible cLBP mechanisms.
Pain Beliefs
Pain beliefs and associated behaviors have been associated with cLBP activity interference, frequency of pain behavior, pain severity, and depression severity. The most notable beliefs are fear of movement and catastrophizing (maladaptive cognition). The patients’ capability to function (physically, psychologically, and socially) is influenced by their perceived self-efficacy or the belief that they can perform, their perceived ability to endure or reduce pain, their confidence that the pain will improve, and their coping skills.
Nine studies reported on expectations (one RPF with little SOE confidence [20]), fear avoidance (10 RPFs with little to some SOE confidence [13, 21–27]), and pain-related anxiety (one RPF with substantial SOE confidence [13]), as assessed by questionnaire, in relation to pain severity, disability, lifting capacity, or transition from acute to chronic pain. Study authors discussed how negative beliefs about pain can lead to catastrophizing and fear avoidance, which can result in further distress and reinforcement of a deleterious cycle.
Affective State
Affect has components of valence (positive vs negative) and arousal (perceived intensity). Negative affect could constitute a long-term, trait-like vulnerability, has been found to be a risk factor for greater pain severity, and is associated with emotions such as anger, sadness, irritability, state anxiety, and fear. In contrast, positive affective states (e.g., joy, hope, love, enthusiasm) are associated with better pain outcomes in longitudinal and experimental studies. Affect balance style is a measure of the relative levels of positive and negative trait affect within an individual. Having a depressive style (low positive affect relative to negative affect) has been associated with greater pain severity or perception of pain without any relation to nociception or bodily symptoms (somatization). Personality trait components can influence certain affective states that can, in turn, influence reported pain sensitivity.
Three studies summarized data on measures of affective state [22, 25, 28]. These studies drew eight depression-related conclusions (with little to some SOE confidence) in relation to work status, opioid use, disability, lifting capacity, pain severity, and overall health status. The study authors discussed how depression is a common comorbidity of cLBP conditions and contributes via mechanisms included in the biopsychosocial and fear-avoidance models.
Coping Skills
Coping with pain is an important element in pain perception and responses. The coping theory by Lazarus and Folkman has become widely applied in behavioral medicine [29]. Coping is the behavioral response to pain aimed at generating and maintaining psychological well-being despite living with a serious condition. Coping styles can be emotion-oriented coping (such as catastrophizing and avoidance; ignoring and distress endurance) or active-approach coping (such as reinterpretation, diversion, acceptance, and mindful interoception). The behavioral active efforts refer to measures taken to reduce pain, and the cognitive ones are aimed at reinterpreting pain or distraction. The avoidance-of-activity element in the fear-avoidance model can be viewed as a maladaptive coping style and catastrophizing as an emotion-oriented coping style.
Three studies described research on coping skills that include coping strategies (two RPFs with little SOE confidence [22]), catastrophizing (one RPF with little SOE confidence [30]), and self-efficacy (two RPFs with little to some SOE confidence [25]) in relation to disability, functional limitation, lifting capacity, work status, and transition from acute low back pain to cLBP. Similar to pain beliefs, the study authors discussed how fear-avoidance and reduced activity can lead to worsening physical conditioning.
Psychological Resources
Coping style is dependent on an individual’s psychological resources and can be limited by numerous potentially modifiable traits. These have been operationalized as distinct but often overlapping psychological constructs associated with resilience or vulnerability to pain. These have shown strong influences on the bothersomeness of pain, such as pain self-efficacy, resilience, the capacity to self-regulate, the capacity to regulate emotions, psychological flexibility, mindfulness, and interoceptive awareness.
One study reported data on measures of negative psychological states (two RPFs with little SOE confidence [22]) in relation to work status, pain, and functional limitations.
Personality Traits
Personality influences how people constitute the meanings and implications of pain, as well as their adoption of different types of pain-related beliefs and coping strategies. Cognitive, emotional, and behavioral patterns influence pain perception. Personality traits represent a pattern of thinking that can influence the processing and interpretation of one’s pain experience. Features linked to chronic pain sufferers include higher harm avoidance (e.g., being fearful, pessimistic, or sensitive to criticism; requiring high levels of reassurance), and lower self-directedness (manifested by difficulty in defining and setting meaningful goals, low motivation, and problems with adaptive coping). Trait neuroticism is known to increase vulnerability to the development of depression, anxiety, kinesiophobia, and pain catastrophizing. Extroversion has been associated with more pain complaints. Trait anxiety is a personality characteristic of constant high arousal with a sense of tension, worry, or apprehension relative to something adverse that might happen in the future. Positive traits include optimism, agreeableness, openness, emotional flexibility, and conscientiousness, which help with maintenance of a social support network and acceptance of the situation and add to coping resources (see above Psychological Resources).
None of the cited studies reported solely on personality traits.
Biomechanical Attributes—Cluster 2
The main functions of the spine are biomechanical: to protect the spinal cord, to support upper body loads, and to facilitate trunk mobility [31]. These functions are enabled by a complex integration of passive tissues (vertebrae, discs, facet joints, ligaments) and active tissues (muscles) plus the neuromuscular control system [32]. Disruption of one or more of these components from injury, overuse, or aging can lead to functional pathology that includes painful stress concentrations within innervated tissues [33].
Physical Activity
Physical activity imposes fluctuating loads and movements on the spine. Literature has demonstrated a dose-dependent influence of loading, where both sedentary and strenuous activities are thought to be detrimental. Physical activity categories can include occupational activity, recreational activity, and sports-related activity. The World Health Organization defines physical activity as “any bodily movement produced by skeletal muscle that results in a substantial increase over the resting energy expenditure.” Whereas disability focuses on what people cannot do or what they are told they should not do, the concept of physical activity focuses on what people are able to do or actually do in daily living.
Three studies reported data on physical functioning (two RPFs with little SOE confidence [22]) and activity (two RPFs with little to some SOE confidence [34, 35]), as assessed by questionnaire or accelerometry, in relation to work status, disability, and pain severity. Potential mechanisms discussed include the fear-avoidance or avoidance-endurance models, with the hypothesis that patients avoid activities because of fear of reinjury that, in turn, leads to deconditioning and further disability.
Sensorimotor Control
The spinal column is biomechanically stabilized by three subsystems: 1) a passive subsystem that includes bone, cartilage, ligaments, and intervertebral discs; 2) an active subsystem that includes the paraspinal muscles; and 3) the neural control subsystem. These subsystems are often conceptualized separately, but they are functionally interdependent. Motor control and function include muscle recruitment, strength, and endurance. An important feedback component of neuromotor control is proprioception, which refers to afferent information arising from internal peripheral areas that contribute to postural control, joint stability, and several conscious sensations. Movement and control disorders presumably lead to a proprioceptive deficit because of stress on local muscle spindles and joint receptors in the painful area resulting from stress to a joint caused by an individual’s maladaptive movement. Subsequently, abnormal joint and tissue loading during daily activities and postures can affect local proprioceptors and maintain this vicious cycle. Changes in muscle activity have been linked to spinal pain (muscle-tension or pain-spasm-pain model) or restriction of spinal motion (pain adaptation).
Five studies reported data related to sensorimotor control, including anticipatory postural adjustments (one RPF with substantial SOE confidence [16]), compensatory postural adjustments (one RPF with substantial SOE confidence [16]), proprioception (two RPFs with little SOE confidence [36, 37]), lumbar repositioning error (one RPF with little SOE confidence [38]), and trunk strength and mobility (one RPF with little SOE confidence [39]), in relation to cLBP classification (yes/no) and pain severity or disability as assessed by questionnaire. The study authors hypothesized that movement control disorders cause spine tissue overloading with increased risk for nociceptive activation.
Load
Muscle and gravity loads cause the lumbar spine to be one of the most highly stressed structures in the body. Loading induces a combination of compression, bending, torsion, and shear that vary from level to level. Spinal loads induce stresses within the various sub-tissues, which can be anabolic (stimulate remodeling via cell-mediated processes) or catabolic (induce damage and inflammation). Obesity can contribute to increased biomechanical loading of spinal joints.
None of the cited studies reported on load.
Tissue Tolerance
Spinal loading can become problematic when the magnitude and duration exceed the tolerance of the active and passive stabilizers. Cumulative trauma can lead to the accumulation of structural damage when applied stresses exceed tissue strength (material properties) and tissue repair capability (biological activity). Tissue inflammation, neoinnervation, and pain can result. The biochemical milieu of diabetes (hyperglycemia and dyslipidemia) can facilitate tissue damage mainly because of detrimental effects on blood vessels, leading to reduced muscle blood flow and increased likelihood of disc degeneration.
None of the cited studies reported on tissue tolerance.
Societal Attributes—Cluster 3
The chronic pain experience can be influenced by social context: the quality of an individual’s social relationships [40] and society’s (cultural, family, therapeutic) responses to the individual’s pain [41].
Sociodemographics
Several social factors or determinants influence the cLBP experience, such as socioeconomic status as a function of education, income, and occupation; access to care; race; and gender. We define race as a social construct levied upon individuals and groups by society, and we define gender as how a person aligns with socially constructed roles, behaviors, and expressions. A patient’s experience of health and illness is framed by not only their social and cultural context, but also the social, cultural, and historical context of those delivering care. Pain is perceived and experienced differently according to ethno-culture, with its health beliefs and expectations. Characteristics of the social environment include social support and invalidating, stigmatizing, or discriminating responses from others. Coping strategies can include religious or spiritual practices, like meditation and prayer, that are incorporated as a cognitive process of self-management. These can vary across cultures. The effect of socioeconomic factors is (only) partly mediated by stress (perceived: self-report; objective: hypothalamic-pituitary-adrenal axis and autonomic nervous system measures) and coping styles with stressors.
One study reported data related to emotional and social support systems (two RPFs with little SOE confidence [22]) in relation to work status, pain, and functional limitation.
Personal/Medical History
One’s personal history spans many facets of life, including employment status, occupation, marital status, and insurance status. It also includes social history, such as alcohol, tobacco, and substance use, in addition to personal preferences, expectations, and habits, such as sleep schedule, clothing choices, and diet. One’s medical history of isolated and chronic illnesses is important, as well. All of these entities likely contribute to how pain is experienced in each individual.
Six studies reported data on personal history, including vitamin D deficiency (one RPF with little SOE confidence [42]), sleep quality and insomnia (one RPF with some SOE confidence [43]), functional shoes/insoles (two RPFs with little SOE confidence [44]), pain medication (one RPF with little SOE confidence [45]), medical aspects (two RPFs with little SOE confidence [22]), and post-traumatic stress disorder (one RPF with little SOE confidence [46]), in relation to cLBP after surgery, pain, sleep, disability, work status, quality of life, and biomechanical assessments, including electromyography and balance.
Occupation
Occupational factors and work characteristics, such as employment status, job satisfaction, work attitudes, and social support at the workplace can be associated with cLBP. Physical fatigue is exhibited as exhaustion, tiredness, or symptoms of aches and pains.
Two studies reported data on how work status and functional limitation affect work characteristics and attitudes (two RPFs with little SOE confidence [22]) and on how reduced physical work demands could be treatment effect modifiers (one RPF with little SOE confidence [45]).
Systemic Attributes—Cluster 4
One’s pain experience can be significantly influenced by systemic factors and comorbidities, which include nutritional status, metabolic diseases, immunologic conditions, endocrine disorders, and sleep disorders [47]. These conditions can lead to detrimental crosstalk among inflammatory, clinical, physical, and psychosocial factors [48].
Inflammation
Chronic inflammation is associated with the increased production of cytokines and activation of proinflammatory pathways that ultimately could contribute to low back pain. This chronic inflammation can be at both the tissue and systemic levels. Pathological processes associated with facet and sacroiliac joint osteoarthritis, vertebral Modic changes, and degeneration of intervertebral discs and spinal ligaments can trigger inflammatory back pain. Obesity has been associated with metabolic, low-grade systemic inflammation. Tissue-level biomarkers can include IL-1b, IL-6, and tumor necrosis factor alpha. Increased cytokine production leads to modulation of other signaling molecules and increased production of downstream mediators, such as substance P, prostaglandin, nitrous oxide, and matrix metalloproteinases.
Two studies reported data on tumor necrosis factor alpha (one RPF with little SOE confidence [14]), CRP (one RPF with substantial SOE confidence [14]), IL-6 or IL-1b (one RPF with substantial non-association SOE confidence [14]), and diabetes (one RPF with substantial SOE confidence [15]) in relation to cLBP by self-report. Study authors discussed the potential links between systemic inflammation and central sensitization.
Physical Comorbidities
Physical comorbidities include body mass index, osteoarthritis, and degenerative joint disease. These contribute to one’s pain experience, biomechanics, and the overall stress for which the body must compensate. A direct association has been observed with obesity (body mass index) and age (fifth and sixth decades).
One study reported data associating lower body mass index (one RPF with little SOE confidence [45]) with improved outcomes from exercise interventions.
Perceived Stress
Autonomic dysregulation and hypothalamic-pituitary-adrenal axis dysregulation have a systemic effect. They not only produce physiological symptoms but also alter factors on a microbiological level.
None of the cited studies reported on perceived stress.
Familial Attributes—Cluster 5
Genetic and environmental conditions shared in families can contribute to the development and maintenance of chronic pain [49, 50]. The cellular mechanisms that link inflammation, peripheral sensitization, and pain can be modified by complex interactions between the genome and environment [51].
Genetics
Heredity can play a significant role in defining an individual’s back pain risk. Genetic association has been reported for structural factors, such as disc degeneration, disc herniation, disc height loss, osteophytes, lumbar stenosis, and Modic changes [52, 53]. Additionally, some evidence associates genetic variations with expression of structural matrix proteins, as well as pain perception and pain sensitivity [54, 55]. Personality traits, the tendency to experience certain emotional states, and pain response all have heritable components [56].
Two included studies discussed evidence for genetic mechanisms and described genetic variants that increase susceptibility to sciatica (one RPF with little SOE confidence [57]) and self-reported cLBP (one RPF with little SOE confidence [58]).
Demographics
Epidemiological factors that associate with cLBP include age, sex (chromosomal assignment at birth), ethnicity (which is inherited through family and culture and is often oversimplified as race), and inheritance (which is the main way that wealth or the lack thereof is transferred between generations). In cLBP, we see a higher prevalence in women than in men, higher prevalence in Whites and Blacks than in Hispanics, and higher odds with low income and lower level of education.
One study considered how gender (two RPFs with little to some SOE confidence [25]) and age (two RPFs with little to some SOE confidence) [25] are associated with lifting and carrying capacity tests.
Central Schema Attributes—Cluster 6
Many factors determine how pain signals from the periphery are transformed into physiological, cognitive, affective, and behavioral responses that can ultimately disable a patient [12]. These bidirectional factors include the strength of the peripheral nociceptive stimulation, peripheral or central inflammation, central pain modulation networks, and psychological factors [59] (Figure 2).
Central Pain Processing
Sensory disturbances, such as pain sensitivity, are frequent features of chronic pain. Increased pain sensitivity in the primary pain area is considered a sign of peripheral sensitization, whereas pain sensitivity in areas anatomically remote from the primary pain area reflects a more central phenomenon. People with heightened pain sensitivity report higher levels of pain and disability due to dysregulation of the central or peripheral nervous systems via cortical reorganization and neuronal sensitization/hyperexcitability. These are characterized by generalized hypersensitivity to both noxious and non-noxious stimuli (e.g., hyperalgesia, allodynia). Functional magnetic resonance imaging studies have demonstrated evidence of central pain amplification and altered nociceptive processing (nociplastic pain state), where a clear peripheral tissue injury might be completely absent. Quantitative measures of gray matter volume by magnetic resonance imaging in patients with cLBP have demonstrated decreases in regions predominantly related to pain perception and integration. The assessment of sensory function with QST can provide evidence of pain hypersensitivity.
Six studies reported data related to central pain processing, including brain anatomy and functional changes (four RPFs with little to some SOE confidence [60–63]) and QST (two RPFs with little SOE confidence to substantial non-association RPF confidence [18, 64]), in relation to cLBP classification (yes/no), pain severity, and disability, as assessed by questionnaire. The study authors discussed how maladaptive neuroplasticity can occur with chronic pain, which can associate with disrupted emotional and cognitive functioning that disrupts the complex interaction between peripheral input and central processing. Central sensitization can result.
Pain Perception
Patient’s perception of pain is differentiated into the sensorial and affective dimensions, which follow separate neural pathways. How attention is directed to pain can vary greatly and can be avoidant, evaluative or immediate sensory, accepting, and nonjudgmental. Pain perception is interoception, the process by which the nervous system senses, interprets, and integrates bodily clues and signals, modulated by top-down and bottom-up neural activity. Increased attentional focus on physical sensations has commonly been associated with anxiety, hypervigilance, somatization, and hypochondriasis (maladaptive interoceptive awareness) but can be mindful and adaptive.
Two studies reported data on pain perception, including QST (one RPF with little SOE confidence [64]) and aspects of pain (one RPF with little SOE confidence [22]), in relation to work status, functional limitation, and pain assessment tools.
Pain Experience
Various instruments have been developed to evaluate the two key dimensions of the pain experience: pain intensity (how much a person hurts) and pain affect (how much a person suffers). Other domains include pain frequency and pain location. A key element of a pain experience is perception bias based on prior experience and expectancy. Cognitive processing of pain information modifies the pain experience. Cognitive evaluation of pain involves the appraisal of the pain sensation (as described in the coping theory) and the emotional reaction to the pain sensation. Threat appraisal contributes to pain chronicity. Cognitive fatigue results in concentration and memory problems or difficulty making decisions.
Two studies reported data on pain experience, including pain intensity (two RPFs with little to some SOE confidence [25]) and pain duration (three RPFs with little to some SOE confidence [25, 65]), in association with lifting and carrying capacity tests, physical activity, sick leave, fear-avoidance behavior, disability, and functional status.
Nociception and Neuropathic Pain
Nociception is the neurochemical process by which specific nociceptors convey pain signals through peripheral neural pathways to the central nervous system. Nociceptive pain arises from the actual or pending damage to non-neural spine tissues due to activation of nociceptors. Activation is typically due to a combination of mechanical and chemical sensitization (peripheral sensitization). Neuropathic pain is due to clinical problems with the involved nerves, such as nerve damage from compression, inflammation, or degeneration.
One study showed that neuropathic pain (one RPF with little SOE confidence [66]) is present in cLBP and postulated that signs and symptoms of pseudoradiculopathy and radiculopathy reflect a disease continuum as opposed to difference disease entities. We did not identify any studies that reported on nociception.
Functional Consequence
Pain often results in functional alterations and limitations. This can impact a person’s independence, which many consider a foundational pillar of existence. Functional consequences secondary to pain impact one’s overall quality of life.
Two studies reported data on self-reported disability (two RPFs with little to some SOE confidence [25]) and self-reported composite measures of disability and pain beliefs (Orebro Musculoskeletal Pain Screening Questionnaire and STarT Back Screening Tool; one RPF with little SOE confidence [23]) in relation to lifting/carrying capacity tests, pain intensity, return-to-work status, and the transition from acute to chronic pain as measured by functional disability.
Peripheral Stimulus
Structural abnormalities of several different anatomic structures can be responsible for lumbar spine pain. Symptoms are typically associated with pathological alterations in the intervertebral disc, facet joints, sacroiliac joints, vertebral endplates, vertebral bodies, or the paraspinal muscles, although the reverse—that those pathological alterations regularly predict pain—is not necessarily true. Painful pathological features typically include matrix damage, neovascularization, neoinnervation, and inflammation. Some of these features can be detectable with advanced imaging techniques.
Four studies described data for peripheral tissue dysfunction, including discs (two RPFs with little SOE confidence [67, 68]), vertebra (one RPF with little SOE confidence [69]), and facets (one RPF with little SOE confidence [70]), in relation to cLBP classification, pain severity, and disability, as assessed by questionnaire. Local inflammatory changes and loading of sensitized nociceptors are potential mechanisms.
Anatomy
Anatomy refers to the shape and size of lumbosacral and brain structures. The shape and size of spinal structures (vertebrae, facets, intervertebral discs, sacroiliac joints, muscles, ligaments, and pelvis) influence the capacity of the spine to support loads and maintain the biomechanical stability of the spine. Also important are the brain areas involved in nociceptive processing (prefrontal cortex, sensorimotor areas, insula, basal ganglia, thalamus, and brainstem). Abnormalities of these spine and brain structures can increase risk.
Three studies compared transverse abdominis morphology or activity with clinical outcomes (one RFA with substantial non-association SOE confidence [17]) and lumbar multifidi size and ultrasound characteristics with fat area, pain, motor control exercises, and electromyography findings (two RPFs with some SOE confidence [71, 72]).
Composite Factors
Three studies reported on the SOE for aggregate of measures that incorporate multiple domains [73–75]. Consequently, these were organized separately.
Discussion
A primary TMS use case is to organize and focus thinking across the consortium as novel clinical data are generated and predictive models are developed and tested. Thousands of patients with cLBP are being enrolled in BACPAC clinical studies. Each patient undergoes a battery of quantitative tests and completes diverse questionnaires. Study results will provide uniquely rich datasets for the application of supervised learning methods. Algorithm development and training will benefit from the complementary experiences of BACPAC scientists. For the latter, TMS will support our abilities to think clearly about the cLBP condition from multiple perspectives, leverage prior research, and promote application of mechanistic principles (where appropriate) to help minimize the risk of misclassification that might occur with purely data-driven techniques. In particular, affiliating ADEs with TMS domains will help identify gaps, guide analyses meant to uncover relationships between factors, and narrow the list of canonical measurements to those that have demonstrable prognostic and treatment-allocating utility. For these reasons, we expect that the BACPAC studies will overcome prior roadblocks to operationalize the BPSM perspective in both research and clinical care.
The umbrella review was used to assess the relative SOE for cLBP RPFs (Table 4) and to refine our theoretical model domain definitions and ADEs (Table 3). Our SOE assessment combined conclusions from reviewed study authors and our assessment of the reviewed studies’ quality with AMSTAR criteria. Our results indicate a dominant interest by the research community in psychological factors, which had the greatest number of citations included in our review. There was a clear underrepresentation of research on societal and systemic factors. It was also notable that the studies were mostly one-dimensional relative to their consideration of BPSM domains. When both the study authors’ assessment and our AMSTAR rating were factored, the majority (71.9%) of factors were found to have little confidence in their overall SOE. Just seven factors were identified as having substantial confidence in either a positive association or no association with cLBP (positive association: pain-related anxiety, serum CRP, diabetes, and anticipatory and compensatory postural adjustments; no association: serum IL-1b / IL-6, transversus muscle morphology/activity, and QST). These results underscore the need for more comprehensive studies with larger sample sizes and harmonized methodologies, which is a particular emphasis of BACPAC.
The BPSM is a useful construct for thinking holistically about a patient’s pain experience from mechanistic, philosophic, clinical, and practical perspectives [5]. However, it lacks features that are necessary to support hypothesis-testing and guide implementation [6]. Challenges to operationalizing the BPSM include vague semantics (the trichotomization of the person in pain with arbitrary boundaries between domains) [8]; absent representation of the dynamic integration of domains, particularly the psychological and the biophysical [76], and changes over time [6]; and no specification of variables among domains and related algorithms [6]. Recently, some authors have advocated for more complete representation of the patient’s dynamic and reciprocal relationship with their environment [77], particularly the growing digital world [6]. We expect that integration of TMS into machine learning approaches will help overcome challenges in achieving BACPAC’s goals for establishing algorithms that customize cLBP care. In particular, the TMS can serve to add context and representational diversity of data used in artificial intelligence computation (e.g., coverage of TMS domains, as well as patient demographics) [78]. In addition to helping assure that the right canonical data elements are included, data element combination consistent with the mechanistic underpinnings could help identify prototypical elements (e.g., features that discriminate patient sub-phenotypes) that will improve computational model training and generalizability, while bridging explainability and interpretability gaps that might, in the future, stymie interactions among clinicians, their patients, and the broader health system.
A limitation is that this work is a qualitative literature synthesis of limited scope. We focus on only a narrow slice of the low back pain literature—systematic reviews in which the authors have specific statements on cLBP RPFs. There were many other studies that focused more broadly on chronic pain or spine pain and included cLBP cohorts but did not perform cLBP sub-analyses. Also, for practical reasons, we did not review the broader primary literature of preclinical and clinical studies. Consequently, the included studies were mainly systematic reviews focused on appraising RPFs from previously reported cross-sectional or longitudinal clinical cohorts, rather than studies designed to rigorously identify cLBP causal mechanisms. To address these weaknesses moving forward, we are using more contemporary techniques to develop information systems to mine the rapidly growing cLBP literature to facilitate formulating new hypotheses and drawing new inferences. For example, knowledge graphs are extensible models that can facilitate understanding complex systems where there is a need to integrate unstructured and semistructured data coming from heterogeneous sources. The supporting metadata and enabling methods also facilitate identifying and mitigating sources of biases. We expect that knowledge graph pipelines and related technology will become valuable resources to cLBP stakeholder communities. Together with TMS schema, knowledge documentation and exploration technology will help cLBP experts and stakeholders bridge syntactic (vocabulary), semantic (data interpretations), and pragmatic (prior knowledge) boundaries [79].
Despite the above limitations, this umbrella review serves as a first step in preparing TMS to serve as backbones for future hypothesis generation, experimentation, and design of cLBP clinical trials. As such, TMS will naturally evolve as new knowledge is generated and cLBP definitions are revised and supplemented over time. Within BACPAC, TMS are currently being used to anchor legacy and novel biomarkers to networks of cLBP factors and to establish connections between diagnosis and intervention. The domains represented serve as conceptual representations of tangible measurements that we can use to phenotype cLBP and identify dominant pathways active within individuals—and thereby provide realistic guidance for gathering the “right” biopsychosocial data that feed into validated predictive algorithms to support improved patient care. The provided domain and sub-domain descriptions are intended to support the generation of standardized terminology that is needed to systematically operationalize BPSM fundamentals to help explain the most likely biological processes contributing to an individual’s cLBP and to aid in communicating these among providers, payers, and patients.
Supplementary Material
Contributor Information
Anthony Chau, Department of Orthopaedic Surgery, University of California San Francisco, San Francisco, California, USA.
Sharis Steib, Department of Physical Medicine and Rehabilitation, University of Michigan, Ann Arbor, Michigan, USA.
Evans Whitaker, Department of Orthopaedic Surgery, University of California San Francisco, San Francisco, California, USA.
David Kohns, Department of Physical Medicine and Rehabilitation, University of Michigan, Ann Arbor, Michigan, USA.
Alexander Quinter, Department of Medicine, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, USA.
Anita Craig, Department of Physical Medicine and Rehabilitation, University of Michigan, Ann Arbor, Michigan, USA.
Anthony Chiodo, Department of Physical Medicine and Rehabilitation, University of Michigan, Ann Arbor, Michigan, USA.
SriKrishan Chandran, Department of Physical Medicine and Rehabilitation, University of Michigan, Ann Arbor, Michigan, USA.
Ann Laidlaw, Department of Physical Medicine and Rehabilitation, University of Michigan, Ann Arbor, Michigan, USA.
Zachary Schott, Department of Physical Medicine and Rehabilitation, University of Michigan, Ann Arbor, Michigan, USA.
Nathan Farlow, Department of Physical Medicine and Rehabilitation, University of Michigan, Ann Arbor, Michigan, USA.
John Yarjanian, Department of Physical Medicine and Rehabilitation, University of Michigan, Ann Arbor, Michigan, USA.
Ashley Omwanghe, Department of Orthopaedic Surgery, University of California San Francisco, San Francisco, California, USA.
Ronald Wasserman, Department of Anesthesiology, University of Michigan, Ann Arbor, Michigan, USA.
Conor O’Neill, Department of Orthopaedic Surgery, University of California San Francisco, San Francisco, California, USA.
Dan Clauw, Department of Internal Medicine, University of Michigan, Ann Arbor, Michigan, USA.
Anton Bowden, Department of Mechanical Engineering, Brigham Young University, Provo, Utah, USA.
William Marras, Department of Integrated Systems Engineering, Ohio State University, Columbus, Ohio, USA.
Tim Carey, Department of Medicine, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, USA.
Wolf Mehling, Department of Family and Community Medicine, University of California San Francisco, San Francisco, California, USA.
C Anthony Hunt, Department of Bioengineering and Therapeutic Sciences, University of California at San Francisco, San Francisco, California, USA.
Jeffrey Lotz, Department of Orthopaedic Surgery, University of California San Francisco, San Francisco, California, USA.
Supplementary Data
Supplementary Data may be found online at http://painmedicine.oxfordjournals.org.
Funding
This article was developed as part of the National Institutes of Health (NIH) Back Pain Consortium (BACPAC) Research Program that was funded as part of the NIH Helping to End Addiction Long-term Initiative (HEAL Initiative) (U19AR076737).
Conflicts of interest: Anthony Chiu, Sharis Steib, Evans Whitaer, David Kohns, Alexader Quinter, Anita Craig, Anthony Chiodo, SriKrishan Chandran, Ann Laidlaw, Zachary Schott, Nathan Farlow, John Yarjanian, Ashley Omwanghe, Ronald Wasserman, Anton Bowden, William Marras, Tim Carey, Wolf Mehling, Anthony Hunt declare no conflict of interest for this work. Jeffrey Lotz has royalties/licenses with Aclarion, royalties/licenses/consulting fees with Relievant MedSystems, and stock/stock options with Relievant MedSystems, Aclarion, and Bioniks, and is the Secretary of the International Society for the Study of the Lumbar Spine ISSLS (no payments). Dan Clauw performs consulting and receives consulting fees from Pfizer, Lilly, Tonix, Virias, IMC, Lundbeck, Teva, Aptinyx, and Sammumed. He also received payments for an expert testimony against opioid manufacturers in Oklahoma and Florida. Conor O'Neill has stock/stock options with and Aclarion.
Authorship has been granted only to those individuals who have contributed substantively to the work presented in this article.
Study registration: Open Science Framework (Center of Open Science; https://osf.io/8ajvf).
Supplement sponsorship
This article appears as part of the supplement entitled “Back Pain Consortium (BACPAC) Research Program” supported by the National Institutes of Health through the NIH HEAL Initiative under award number AR076730-01.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or its NIH HEAL Initiative.
References
- 1. Corns J. Recent work on pain. Anal Rev 2018;78(4):737–53. [Google Scholar]
- 2. Maher C, Underwood M, Buchbinder R.. Non-specific low back pain. Lancet 2017;389(10070):736–47. [DOI] [PubMed] [Google Scholar]
- 3. Hartvigsen J, Hancock MJ, Kongsted A, et al. ; Lancet Low Back Pain Series Working Group. What low back pain is and why we need to pay attention. Lancet 2018;391(10137):2356–67. [DOI] [PubMed] [Google Scholar]
- 4. Edwards RR, Dworkin RH, Sullivan MD, Turk DC, Wasan AD.. The role of psychosocial processes in the development and maintenance of chronic pain. J Pain 2016;17(Suppl 9):T70–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Borrell-Carrio F, Suchman AL, Epstein RM.. The biopsychosocial model 25 years later: Principles, practice, and scientific inquiry. Ann Fam Med 2004;2(6):576–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Card AJ. The biopsychosociotechnical model: A systems-based framework for human-centered health improvement. Health Syst 2022;1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Daluiso-King G, Hebron C.. Is the biopsychosocial model in musculoskeletal physiotherapy adequate? An evolutionary concept analysis. Physiother Theory Pract 2022;38(3):373–89. [DOI] [PubMed] [Google Scholar]
- 8. Cormack B, Stilwell P, Coninx S, Gibson J.. The biopsychosocial model is lost in translation: From misrepresentation to an enactive modernization. Physiother Theory Pract 2022;1–16. [DOI] [PubMed] [Google Scholar]
- 9. Parums DV. Editorial: Review articles, systematic reviews, meta-analysis, and the updated Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guidelines. Med Sci Monit 2021;27:e934475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bojcic R, Todoric M, Puljak L.. Adopting AMSTAR 2 critical appraisal tool for systematic reviews: Speed of the tool uptake and barriers for its adoption. BMC Med Res Methodol 2022;22(1):104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Guyatt GH, Oxman AD, Vist GE, et al. ; GRADE Working Group. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Garland EL. Pain processing in the human nervous system: A selective review of nociceptive and biobehavioral pathways. Prim Care 2012;39(3):561–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Martinez-Calderon J, Flores-Cortes M, Morales-Asencio JM, Luque-Suarez A.. Pain-related fear, pain intensity and function in individuals with chronic musculoskeletal pain: A systematic review and meta-analysis. J Pain 2019;20(12):1394–415. [DOI] [PubMed] [Google Scholar]
- 14. Morris P, Ali K, Merritt M, Pelletier J, Macedo LG.. A systematic review of the role of inflammatory biomarkers in acute, subacute and chronic non-specific low back pain. BMC Musculoskelet Disord 2020;21(1):142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pozzobon D, Ferreira PH, Dario AB, et al. Is there an association between diabetes and neck and back pain? A systematic review with meta-analyses. PLoS One 2019;14(2):e0212030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Knox MF, Chipchase LS, Schabrun SM, Romero RJ, Marshall PWM.. Anticipatory and compensatory postural adjustments in people with low back pain: A systematic review and meta-analysis. Spine J 2018;18(10):1934–49. [DOI] [PubMed] [Google Scholar]
- 17. Wong AY, Parent EC, Funabashi M, Kawchuk GN.. Do changes in transversus abdominis and lumbar multifidus during conservative treatment explain changes in clinical outcomes related to nonspecific low back pain? A systematic review. J Pain 2014;15(4):377.e1–35. [DOI] [PubMed] [Google Scholar]
- 18. Marcuzzi A, Dean CM, Wrigley PJ, Chakiath RJ, Hush JM.. Prognostic value of quantitative sensory testing in low back pain: A systematic review of the literature. J Pain Res 2016;9:599–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pincus T, McCracken LM.. Psychological factors and treatment opportunities in low back pain. Best Pract Res Clin Rheumatol 2013;27(5):625–35. [DOI] [PubMed] [Google Scholar]
- 20. Mohamed Mohamed WJ, Joseph L, Canby G, et al. Are patient expectations associated with treatment outcomes in individuals with chronic low back pain? A systematic review of randomised controlled trials. Int J Clin Pract 2020;74(11):e13680. [DOI] [PubMed] [Google Scholar]
- 21. Alamam DM, Leaver A, Alsobayel HI, et al. Low back pain–related disability is associated with pain-related beliefs across divergent non–English-speaking populations: Systematic review and meta-analysis. Pain Med 2021;22(12):2974–89. [DOI] [PubMed] [Google Scholar]
- 22. Melloh M, Elfering A, Egli Presland C, et al. Identification of prognostic factors for chronicity in patients with low back pain: A review of screening instruments. Int Orthop 2009;33(2):301–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pauli J, Starkweather A, Robins JL.. Screening tools to predict the development of chronic low back pain: An integrative review of the literature. Pain Med 2019;20(9):1651–77. [DOI] [PubMed] [Google Scholar]
- 24. Pincus T, Vogel S, Burton AK, Santos R, Field AP.. Fear avoidance and prognosis in back pain: A systematic review and synthesis of current evidence. Arthritis Rheum 2006;54(12):3999–4010. [DOI] [PubMed] [Google Scholar]
- 25. van Abbema R, Lakke SE, Reneman MF, et al. Factors associated with functional capacity test results in patients with non-specific chronic low back pain: A systematic review. J Occup Rehabil 2011;21(4):455–73. [DOI] [PubMed] [Google Scholar]
- 26. Wertli MM, Rasmussen-Barr E, Held U, et al. Fear-avoidance beliefs—a moderator of treatment efficacy in patients with low back pain: A systematic review. Spine J 2014;14(11):2658–78. [DOI] [PubMed] [Google Scholar]
- 27. Wertli MM, Rasmussen-Barr E, Weiser S, Bachmann LM, Brunner F.. The role of fear avoidance beliefs as a prognostic factor for outcome in patients with nonspecific low back pain: A systematic review. Spine J 2014;14(5):816–36 e4. [DOI] [PubMed] [Google Scholar]
- 28. Wong JJ, Tricco AC, Cote P, Rosella LC.. The association between depressive symptoms or depression and health outcomes in adults with low back pain with or without radiculopathy: Protocol of a systematic review. Syst Rev 2019;8(1):267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Boothby JL, Thorn BE, Stroud MW, Jensen MP.. Coping with Pain. Psychosocial Factors in Pain: Critical Perspectives. New York, NY: The Guilford Press; 1999:343–59. [Google Scholar]
- 30. Wertli MM, Eugster R, Held U, et al. Catastrophizing—a prognostic factor for outcome in patients with low back pain: A systematic review. Spine J 2014;14(11):2639–57. [DOI] [PubMed] [Google Scholar]
- 31. Ferguson SJ, Steffen T.. Biomechanics of the aging spine. Eur Spine J 2003;12(Suppl 2):S97–S103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Panjabi MM. Clinical spinal instability and low back pain. J Electromyogr Kinesiol 2003;13(4):371–9. [DOI] [PubMed] [Google Scholar]
- 33. Adams MA. Biomechanics of back pain. Acupunct Med 2004;22(4):178–88. [DOI] [PubMed] [Google Scholar]
- 34. Lin CC, McAuley JH, Macedo L, et al. Relationship between physical activity and disability in low back pain: A systematic review and meta-analysis. Pain 2011;152(3):607–13. [DOI] [PubMed] [Google Scholar]
- 35. Oliveira CB, Pinheiro MB, Teixeira RJ, et al. Physical activity as a prognostic factor of pain intensity and disability in patients with low back pain: A systematic review. Eur J Pain 2019;23(7):1251–63. [DOI] [PubMed] [Google Scholar]
- 36. Ghamkhar L, Kahlaee AH.. Pain and pain-related disability associated with proprioceptive impairment in chronic low back pain patients: A systematic review. J Manipulative Physiol Ther 2019;42(3):210–7. [DOI] [PubMed] [Google Scholar]
- 37. Lin J, Halaki M, Rajan P, Leaver A.. Relationship between proprioception and pain and disability in people with non-specific low back pain: A systematic review with meta-analysis. Spine (Phila Pa 1976 )2019;44(10):E606–17. [DOI] [PubMed] [Google Scholar]
- 38. Rausch Osthoff AK, Ernst MJ, Rast FM, et al. Measuring lumbar reposition accuracy in patients with unspecific low back pain: Systematic review and meta-analysis. Spine (Phila Pa 1976) 2015;40(2):E97–111. [DOI] [PubMed] [Google Scholar]
- 39. Steiger F, Wirth B, de Bruin ED, Mannion AF.. Is a positive clinical outcome after exercise therapy for chronic non-specific low back pain contingent upon a corresponding improvement in the targeted aspect(s) of performance? A systematic review. Eur Spine J 2012;21(4):575–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Philpot LM, Schumann ME, Ebbert JO.. Social relationship quality among patients with chronic pain: A population-based sample. J Patient Exp 2020;7(3):316–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. McCracken LM. Social context and acceptance of chronic pain: The role of solicitous and punishing responses. Pain 2005;113(1-2):155–9. [DOI] [PubMed] [Google Scholar]
- 42. Zadro J, Shirley D, Ferreira M, et al. Mapping the association between vitamin D and low back pain: A systematic review and meta-analysis of observational studies. Pain Physician 2017;20(7):611–40. [PubMed] [Google Scholar]
- 43. Van Looveren E, Bilterys T, Munneke W, et al. The association between sleep and chronic spinal pain: A systematic review from the last decade. J Clin Med 2021;10(17):1–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kong L, Zhou X, Huang Q, et al. The effects of shoes and insoles for low back pain: A systematic review and meta-analysis of randomized controlled trials. Res Sports Med 2020;28(4):572–87. [DOI] [PubMed] [Google Scholar]
- 45. Hayden JA, Wilson MN, Stewart S, et al. ; Chronic Low Back Pain IPDM-AG. Exercise treatment effect modifiers in persistent low back pain: An individual participant data meta-analysis of 3514 participants from 27 randomised controlled trials. Br J Sports Med 2020;54(21):1277–8. [DOI] [PubMed] [Google Scholar]
- 46. Fishbain DA, Pulikal A, Lewis JE, Gao J.. Chronic pain types differ in their reported prevalence of post-traumatic stress disorder (PTSD) and there is consistent evidence that chronic pain is associated with PTSD: An evidence-based structured systematic review. Pain Med 2017;18(4):711–35. [DOI] [PubMed] [Google Scholar]
- 47. Canli K, Billens A, Van Oosterwijck J, Meeus M, De Meulemeester K.. Systemic cytokine level differences in patients with chronic musculoskeletal spinal pain compared to healthy controls and its association with pain severity: A systematic review. Pain Med 2022;23(12):1947–64. [DOI] [PubMed] [Google Scholar]
- 48. Paley CA, Johnson MI.. Physical activity to reduce systemic inflammation associated with chronic pain and obesity: A narrative review. Clin J Pain 2016;32(4):365–70. [DOI] [PubMed] [Google Scholar]
- 49. James SK. Chronic postsurgical pain: Is there a possible genetic link? Br J Pain 2017;11(4):178–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kerr JI, Burri A.. Genetic and epigenetic epidemiology of chronic widespread pain. J Pain Res 2017;10:2021–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Buchheit T, Van de Ven T, Shaw A.. Epigenetics and the transition from acute to chronic pain. Pain Med 2012;13(11):1474–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Dario AB, Ferreira ML, Refshauge KM, et al. The relationship between obesity, low back pain, and lumbar disc degeneration when genetics and the environment are considered: A systematic review of twin studies. Spine J 2015;15(5):1106–17. [DOI] [PubMed] [Google Scholar]
- 53. Vigeland MD, Flam ST, Vigeland MD, et al. ; AIM Study Group. Correlation between gene expression and MRI STIR signals in patients with chronic low back pain and Modic changes indicates immune involvement. Sci Rep 2022;12(1):215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tegeder I, Lotsch J.. Current evidence for a modulation of low back pain by human genetic variants. J Cell Mol Med 2009;13(8B):1605–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Bjorland S, Moen A, Schistad E, Gjerstad J, Roe C.. Genes associated with persistent lumbar radicular pain: A systematic review. BMC Musculoskelet Disord 2016;17(1):500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Freidin M, Kraatari M, Skarp S, et al. Genome-wide meta-analysis identifies genetic locus on chromosome 9 associated with Modic changes. J Med Genet 2019;56(7):420–6. [DOI] [PubMed] [Google Scholar]
- 57. Lemmela S, Solovieva S, Shiri R, et al. Genome-wide meta-analysis of sciatica in Finnish population. PLoS One 2016;11(10):e0163877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Suri P, Palmer MR, Tsepilov YA, et al. Genome-wide meta-analysis of 158,000 individuals of European ancestry identifies three loci associated with chronic back pain. PLoS Genet 2018;14(9):e1007601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Li W, Gong Y, Liu J, et al. Peripheral and central pathological mechanisms of chronic low back pain: A narrative review. J Pain Res 2021;14:1483–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kregel J, Meeus M, Malfliet A, et al. Structural and functional brain abnormalities in chronic low back pain: A systematic review. Semin Arthritis Rheum 2015;45(2):229–37. [DOI] [PubMed] [Google Scholar]
- 61. Ng SK, Urquhart DM, Fitzgerald PB, et al. The relationship between structural and functional brain changes and altered emotion and cognition in chronic low back pain brain changes: A systematic review of MRI and fMRI studies. Clin J Pain 2018;34(3):237–61. [DOI] [PubMed] [Google Scholar]
- 62. Schouppe S, Van Oosterwijck S, Danneels L, Van Damme S, Van Oosterwijck J.. Are functional brain alterations present in low back pain? A systematic review of EEG studies. J Pain 2020;21(1-2):25–43. [DOI] [PubMed] [Google Scholar]
- 63. Yuan C, Shi H, Pan P, et al. Gray matter abnormalities associated with chronic back pain: A Meta-analysis of voxel-based morphometric studies. Clin J Pain 2017;33(11):983–90. [DOI] [PubMed] [Google Scholar]
- 64. Hubscher M, Moloney N, Leaver A, et al. Relationship between quantitative sensory testing and pain or disability in people with spinal pain-a systematic review and meta-analysis. Pain 2013;154(9):1497–504. [DOI] [PubMed] [Google Scholar]
- 65. Itz CJ, Geurts JW, van Kleef M, Nelemans P.. Clinical course of non-specific low back pain: A systematic review of prospective cohort studies set in primary care. Eur J Pain 2013;17(1):5–15. [DOI] [PubMed] [Google Scholar]
- 66. Fishbain DA, Cole B, Lewis JE, Gao J.. What is the evidence that neuropathic pain is present in chronic low back pain and soft tissue syndromes? An evidence-based structured review. Pain Med 2014;15(1):4–15. [DOI] [PubMed] [Google Scholar]
- 67. Ganko R, Rao PJ, Phan K, Mobbs RJ.. Can bacterial infection by low virulent organisms be a plausible cause for symptomatic disc degeneration? A systematic review. Spine (Phila Pa 1976) 2015;40(10):E587–92. [DOI] [PubMed] [Google Scholar]
- 68. Endean A, Palmer KT, Coggon D.. Potential of magnetic resonance imaging findings to refine case definition for mechanical low back pain in epidemiological studies: A systematic review. Spine (Phila Pa 1976 )2011;36(2):160–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Herlin C, Kjaer P, Espeland A, et al. Modic changes: Their associations with low back pain and activity limitation: A systematic literature review and meta-analysis. PLoS One 2018;13(8):e0200677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Baroncini A, Maffulli N, Eschweiler J, et al. Management of facet joints osteoarthritis associated with chronic low back pain: A systematic review. Surgeon 2021;19(6):e512–e18. [DOI] [PubMed] [Google Scholar]
- 71. Pinto SM, Boghra SB, Macedo LG, et al. Does motor control exercise restore normal morphology of lumbar multifidus muscle in people with low back pain? A systematic review. J Pain Res 2021;14:2543–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Rummens S, Robben E, De Groef A, et al. Factors associated with the ultrasound characteristics of the lumbar multifidus: A systematic review. PM R 2020;12(1):82–100. [DOI] [PubMed] [Google Scholar]
- 73. Hilfiker R, Bachmann LM, Heitz CA, et al. Value of predictive instruments to determine persisting restriction of function in patients with subacute non-specific low back pain. Systematic review. Eur Spine J 2007;16(11):1755–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Hockings RL, McAuley JH, Maher CG.. A systematic review of the predictive ability of the Orebro Musculoskeletal Pain Questionnaire. Spine (Phila Pa 1976) 2008;33(15):E494–500. 2008 [DOI] [PubMed] [Google Scholar]
- 75. Meyer C, Denis CM, Berquin AD.. Secondary prevention of chronic musculoskeletal pain: A systematic review of clinical trials. Ann Phys Rehabil Med 2018;61(5):323–38. [DOI] [PubMed] [Google Scholar]
- 76. Keefe FJ, France CR.. Pain: Biopsychosocial mechanisms and management. Curr Dir Psychol Sci 1999;8(5):137–41. [Google Scholar]
- 77. Coninx S, Stilwell P.. Pain and the field of affordances: An enactive approach to acute and chronic pain. Synthese 2021;199(3-4):7835–63. [Google Scholar]
- 78. Ameen S, Wong M-C, Yee K-C, Turner P.. AI and clinical decision making: the limitations and risks of computational reductionism in bowel cancer screening. Appl Sci 2022;12(7):3341. [Google Scholar]
- 79. Broniatowski DA, Magee CL.. The emergence and collapse of knowledge boundaries. IEEE Trans Eng Manag 2017;64(3):337–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.