Abstract
Lipid mobilization through fatty acid β-oxidation is a central process essential for energy production during nutrient shortage. In yeast, this catabolic process starts in the peroxisome from where β-oxidation products enter mitochondria and fuel the tricarboxylic acid cycle. Little is known about the physical and metabolic cooperation between these organelles. Here we found that expression of fatty acid transporters and of the rate-limiting enzyme involved in β-oxidation is decreased in cells expressing a hyperactive mutant of the small GTPase Arf1, leading to an accumulation of fatty acids in lipid droplets. Consequently, mitochondria became fragmented and ATP synthesis decreased. Genetic and pharmacological depletion of fatty acids phenocopied the arf1 mutant mitochondrial phenotype. Although β-oxidation occurs in both mitochondria and peroxisomes in mammals, Arf1’s role in fatty acid metabolism is conserved. Together, our results indicate that Arf1 integrates metabolism into energy production by regulating fatty acid storage and utilization, and presumably organelle contact sites.
Subject terms: Mitochondria, Peroxisomes, Endoplasmic reticulum
Enkler et al. show that a pool of Arf1 at lipid droplets is implicated in mitochondrial ATP production control through regulation of fatty acid metabolism and acetyl-CoA transfer to mitochondria.
Main
Intracellular compartmentalization of metabolic processes involves deep and well-orchestrated inter-organelle communications to coordinate cellular functions. This requires homeostatic control of lipid, ion and metabolite transfer between organelles, and between organelles and the plasma membrane1–6. Exchanges are established through vesicular transport by means of kissing and fusing1, and membrane contact sites5,7.
Mitochondria form contacts with almost every organelle in the cell2,3,8. They establish functional interactions with peroxisomes9,10 and with lipid droplets (LDs11,12) to ensure fatty acid (FA) metabolism and ATP production. Lipids are stored in LDs in the form of triacylglycerol (TAG) and sterol esters (SE). Under nutrient shortage, FAs are released from LDs by lipolysis and metabolized by β-oxidation solely in peroxisomes in yeast, or in both peroxisomes and mitochondria in mammalian cells. Subsequently, shortened acyl-CoA (or acetylcarnitine/citrate) is transferred from peroxisomes to mitochondria by an unknown mechanism9,10, where it will fuel the tricarboxylic acid (TCA) cycle and the respiratory chain (RC) complexes for oxidative phosphorylation (OXPHOS). Hence, LDs stay in close proximity to peroxisomes and mitochondria for efficient transfer of metabolites13–17. Perturbed contact sites between these organelles and mitochondria are correlated with metabolic syndromes, liver disease and cancers, highlighting their central role in cellular homeostasis8,18,19. Nevertheless, how contact sites are organized and regulated, and which proteins are involved in metabolite transfer allowing proper lipid flux between organelles to ensure effective energy production, remains enigmatic.
Arf1 is a master regulator of vesicle formation at the Golgi20,21, and its activity is modulated by ArfGAPs (GTPase activating proteins) and ArfGEFs (guanine nucleotide exchange factors) stimulating GTP hydrolysis and GDP-to-GTP exchange, respectively. Over the past years, additional functions of Arf1 have been identified. We and others have shown that Arf1 regulates messenger RNA transport22,23, mTORC1 activity24,25, and mitochondrial dynamics and transport26–28. However, it still remains unclear how Arf1 specifically regulates mitochondrial dynamics. While we and others have observed that eliminating ARF1 in Caenorhabditis elegans or HeLa cells leads to mitochondrial hyperconnectivity26,28, mitochondria were fragmented and globular in the yeast arf1-11 mutant26,29, indicating that Arf1 might play additional roles at mitochondria.
The Arf1/COPI machinery has also been implicated in lipid metabolism by governing lipolysis, LD morphology, protein recruitment, phospholipid removal and the formation of endoplasmic reticulum (ER)–LD bridges30–36. Furthermore, Arf1 and COPI could be recruited onto peroxisomes, and Arf1 might be involved in peroxisome proliferation37–39. However, Arf1 function on peroxisomes and in FA metabolism remains elusive.
In this Article, we show that Arf1 couples FA β-oxidation to mitochondrial ATP synthesis. We demonstrate that Arf1 activity regulates expression of long-chain FA transporters Pxa1/Pxa2 and of the first and rate-limiting enzyme involved in β-oxidation, Pox1 in yeast. Arf1 modulates FA availability on LDs by promoting TAG synthesis and hydrolysis. Hyperactive Arf1 leads to an increased level of TAGs in LDs, and to reduced lipid transfer to mitochondria. This conserved mechanism is essential to sustain endomembrane homeostasis and mitochondrial ATP synthesis. Moreover, Arf1 activity drives both mitochondrial fusion and fission in yeast, thereby consolidating previous results26,28. Thus, Arf1 appears to be required for the regulation of mitochondrial dynamics and for FA metabolism and acetyl-CoA transfer to mitochondria.
Results
Arf1 regulates mitochondrial fusion and fission
To better understand the discrepancies between the hyperconnectivity of mitochondria observed in metazoans26,28 and the globular, fragmented mitochondria in the yeast temperature-sensitive arf1-11 mutant (Fig. 1a), we measured mitochondrial fission and fusion activity in the ARF1 and arf1-11 strains. For clarity, we will use the prefix ‘y’ for all yeast and ‘m’ for all mammalian genes and proteins. On the basis of growth curves and cell morphology (Fig. 1b–d), we shifted strains for 30 min to 37 °C before imaging29.
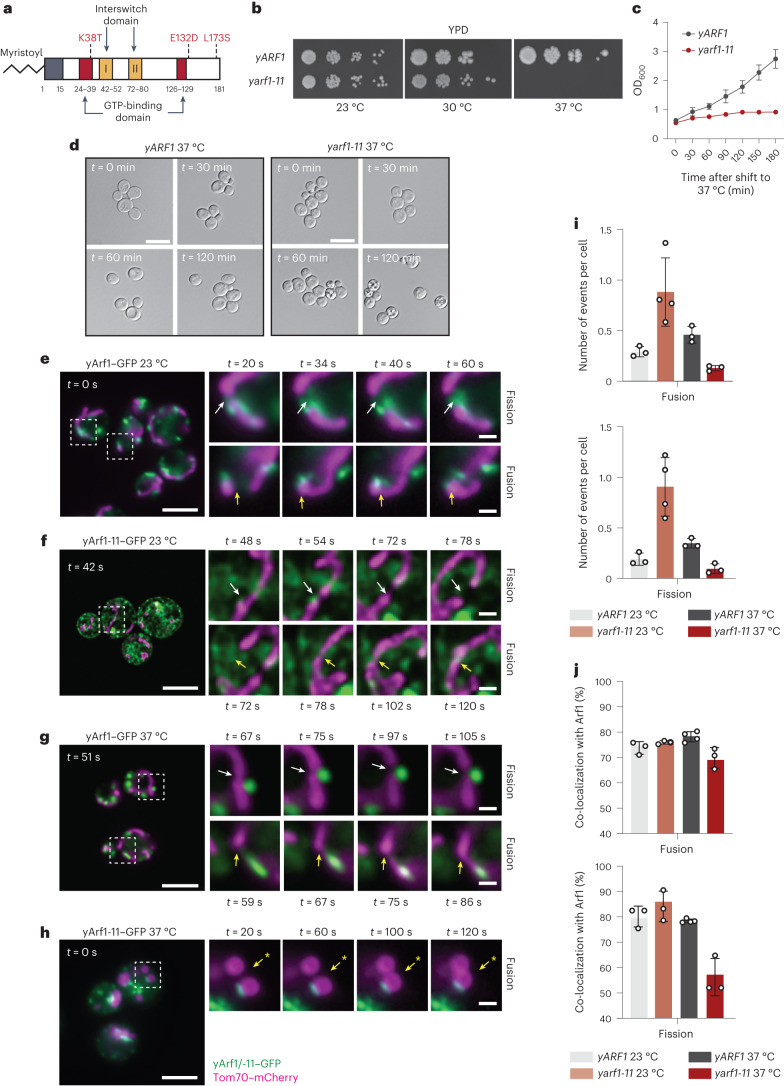
Fig. 1. yArf1 regulates mitochondria fusion and fission.
a, Schematic of the thermo-sensitive mutant Arf1-11 in yeast (Yahara et al.29). Amino acid coordinates are indicated in bold below the protein and corresponding mutated amino acids in red. b, Growth test of yARF1 and yarf1-11 strains on rich medium (YPD) and incubated at 23 °C, 30 °C and 37 °C. c, Cell viability assay of yARF1 and yarf1-11 strains performed after shifting cells to 37 °C. ODs were measured at regular timepoints. Mean and standard deviation are shown; n = 3 biological replicates. d, yARF1 and yarf1-11 strains phenotypes followed by microscopy after 0, 30, 60 and 120 min incubation time at 37 °C. Scale bar, 5 µm. e–h, Single timepoint images of movies done with strains expressing yArf1–GFP (e,g) or yArf1-11–GFP (f,h) together with the mitochondrial protein Tom70 fused to mCherry at 23 °C (e,f) or shifted to 37 °C (g,h). White arrows indicate sites of fission and yellow arrows fusion. Asterisk indicates a fusion event independent of Arf1 in h. Scale bar, 5 µm. Scale bar inlays, 2.5 µm. i,j, Measurements of mitochondrial fusion and fission events per cell (i) and the frequency of events where yArf1 is involved (j). Mean and standard deviation are shown; yARF1 23 °C = 271 cells, yARF1 37 °C = 231 cells, yarf1-11 23 °C = 379 cells and yarf1-11 37 °C = 186 cells from n = 3 biological replicates. Source numerical data are available in source data. See also Extended Data Fig. 1.
We could readily detect yArf1–GFP at mitochondrial fission and fusion sites at both 23 °C and 37 °C (Fig. 1e,g,j, Extended Data Fig. 1a,c and Supplementary Videos 1 and 2). Surprisingly, in yarf1-11 cells, the number of fission and fusion events was higher than in yARF1 cells at 23 °C (Fig. 1f,i, Extended Data Fig. 1b and Supplementary Video 3). In contrast, mitochondrial dynamics was greatly reduced at 37 °C (Fig. 1h,i, Extended Data Fig. 1d and Supplementary Video 4), even though only mildly affecting yArf1-11–GFP localization at fusion and fission sites (Fig. 1h,j and Extended Data Fig. 1d). Our data suggest that yArf1 is required for both mitochondrial fusion and fission, reconciling the findings in mammalian cells, C. elegans and yeast.
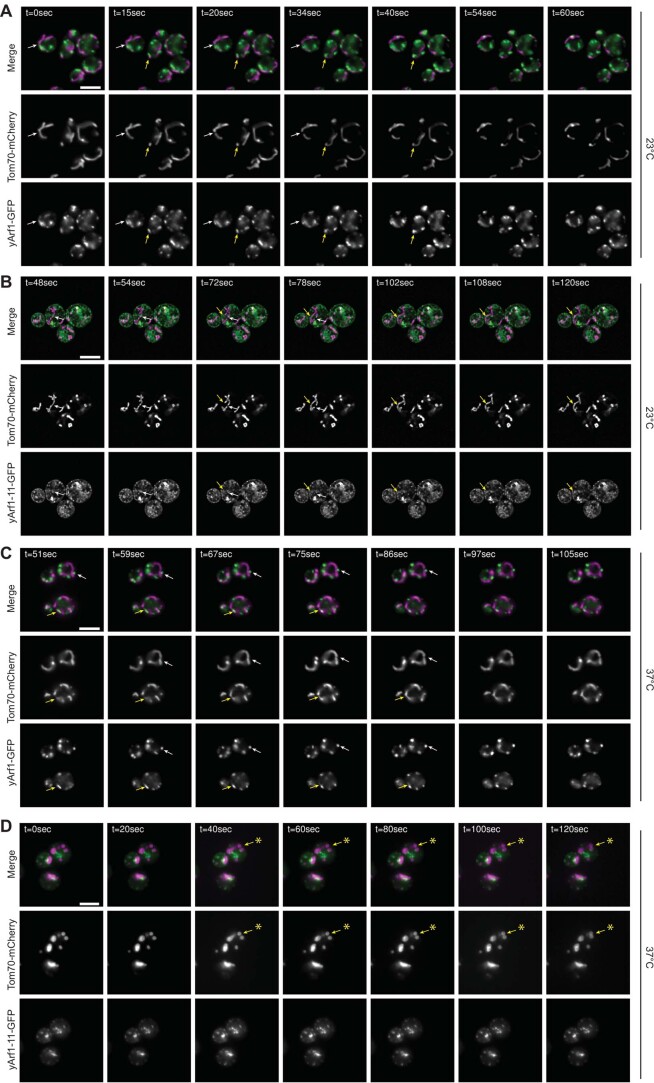
Extended Data Fig. 1. yArf1 regulates mitochondria fusion and fission.
(A–D) Single time-point images of movies done with strains expressing yArf1-GFP (A) or yArf1-11-GFP (B) and the mitochondrial protein Tom70-mCherry at 23 °C, or shifted 30 min at 37 °C for yArf1-GFP (C) and yArf1-11-GFP (D). Merge and individual GFP and mCherry channels are shown. Arrows indicate mitochondrial fusion (yellow) and fission (white). Asterisk indicates mitochondrial fusion independent of yArf1-11. Scale bars: 5 µm.
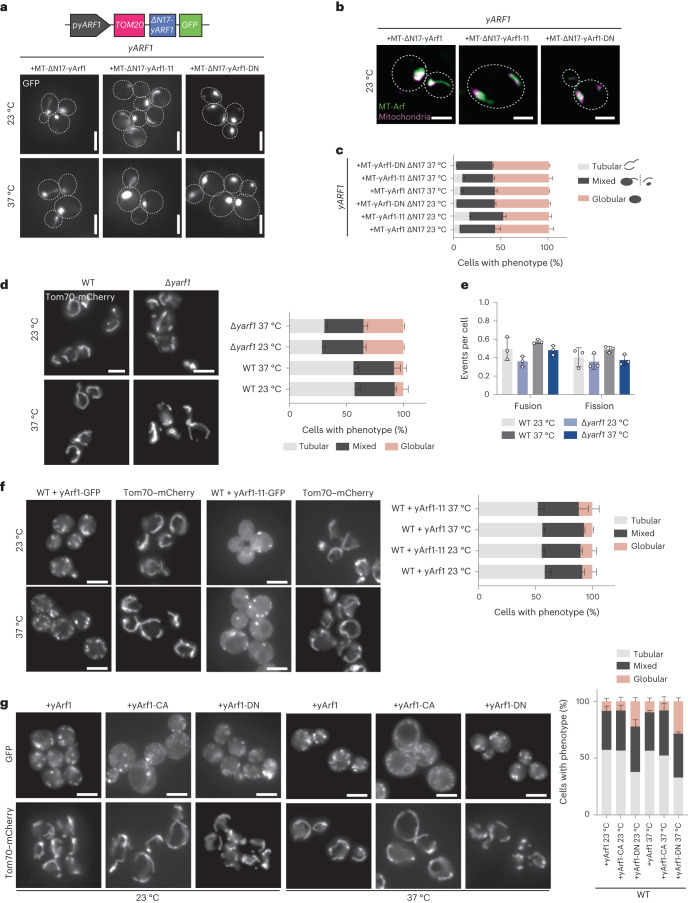
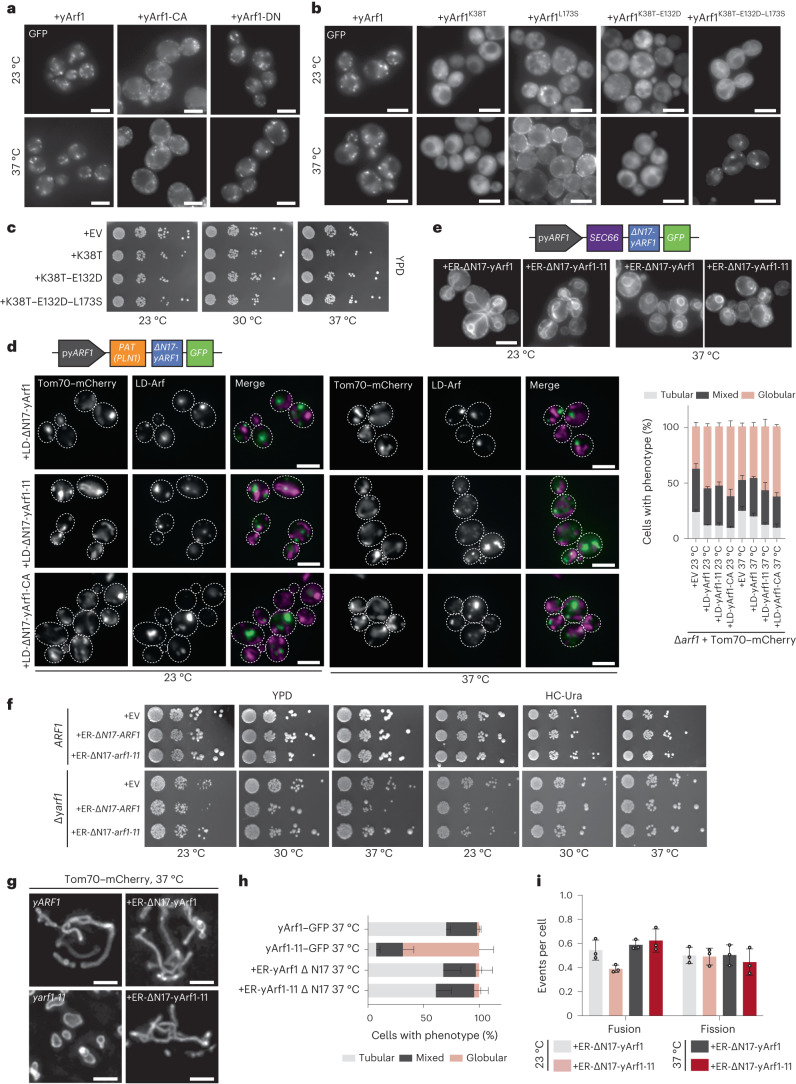
To test whether Arf1 exerts its activity directly on mitochondria, we anchored yArf1, yArf1-11 or the dominant negative version of yArf1T31N (yArf1-DN) on mitochondria via the mitochondrial translocase Tom20 (Fig. 2a,b). Irrespective of which yArf1 variant we anchored, almost no mitochondrial tubular network could be observed (Fig. 2c). Thus, the constant presence of yArf1 on mitochondria impairs mitochondrial dynamics. Deletion of yARF1, however, also impacted mitochondrial morphology, while mitochondrial dynamics were only mildly affected, presumably due to the presence of Arf2 (Fig. 2d,e and Supplementary Videos 5–8). Interestingly, yArf1-DN but not yArf1-11 or the constitutively active yArf1Q71L (yArf1-CA) had a dominant phenotype on mitochondria morphology (Fig. 2f,g), indicating that active Arf1 is necessary to maintain mitochondria morphology. Taken together, our data suggest that Arf1 cycling between GTP- and GDP-bound states might be important to sustain mitochondria homeostasis.
Fig. 2. Control of Arf1 activity is needed for mitochondria dynamics.
a, Schematic of the construct designed to anchor yArf1 on mitochondria (MT) via Tom20. yARF1 deleted in its myristoylation sequence (∆N17) was expressed from its endogenous promoter and fused to GFP on its 3′ end. Localization of MT-anchored ∆N17-yArf1–GFP, the dominant negative yArf1-DN or yArf1 bearing yArf1-11–GFP variant in yARF1 cells grown at 23 °C or shifted to 37 °C. Scale bar, 5 µm. b, High-resolution co-localization of MT-anchored ∆N17-yArf1–GFP, yArf1-DN and Arf1-11–GFP with mitochondria stained with MitoTracker Deep Red FM. A single focal plane of 0.2 µm is shown. Scale bar, 2 µm. c, Measurements of mitochondria phenotypes (tubular, mixed or globular) based on images taken in a. Mean and standard deviation are shown; ∆yarf1 + MT-Arf1 23 °C = 419 cells, ∆yarf1 + MT-Arf1-11 23 °C = 544 cells, ∆yarf1 + MT-Arf1-DN 23 °C = 606 cells, ∆yarf1 + MT-Arf1 37 °C = 509 cells, ∆yarf1 + MT-Arf1-11 37 °C = 453 cells, ∆yarf1 + MT-Arf1-DN 37 °C = 462 cells from n = 3 biological replicates. d, Mitochondria morphology were imaged in WT and ∆yarf1 strains grown at 23 °C or shifted to 37 °C using Tom70–mCherry as mitochondrial marker. Mitochondria phenotypes (tubular, mixed or globular) were measured. Mean and standard deviation are shown; WT 23 °C = 355 cells, WT 37 °C = 582 cells, ∆yarf1 23 °C = 419 cells, ∆yarf1-11 23 °C = 571 cells from n = 3 biological replicates. Scale bar, 5 µm. e, Mitochondrial fusion and fission events were measured on the basis of Supplementary Videos 5–8. Mean and standard deviation are shown; WT 23 °C = 320 cells, WT 37 °C = 279 cells, ∆yarf1 23 °C = 319 cells, ∆yarf1-11 37 °C = 321 cells from n = 3 biological replicates. f, Mitochondria morphology were imaged in WT cells expressing yArf1–GFP or yArf1-11–GFP grown at 23 °C or shifted to 37 °C using Tom70–mCherry as mitochondrial marker. For each strain the tubular, mixed and globular phenotypes were measured. Mean and standard deviation are shown. Scale bar, 5 µm. +yArf1 23 °C = 251 cells, +yArf1 37 °C = 273 cells, +yArf1-11 23 °C = 266 cells, +yArf1-11 37 °C = 273 cells from n = 3 biological replicates. g, Mitochondria morphology were imaged in WT cells expressing yArf1-, the constitutively active mutant yArf1-CA- or the dominant negative yArf1-DN–GFP grown at 23 °C or shifted to 37 °C using Tom70–mCherry as mitochondrial marker. For each strain the tubular, mixed and globular phenotypes were measured. Mean and standard deviation are shown. +yArf1 23 °C = 269 cells, +yArf1 37 °C = 275 cells, +yArf1-CA 23 °C = 268 cells, +yArf1-CA 37 °C = 287 cells, +yArf1-DN 23 °C = 243 cells, +yArf1-DN 37 °C = 411 cells from n = 3 biological replicates. Scale bar, 5 µm. Source numerical data are available in source data.
yArf1-11 is a hyperactive mutant present on the ER and LDs
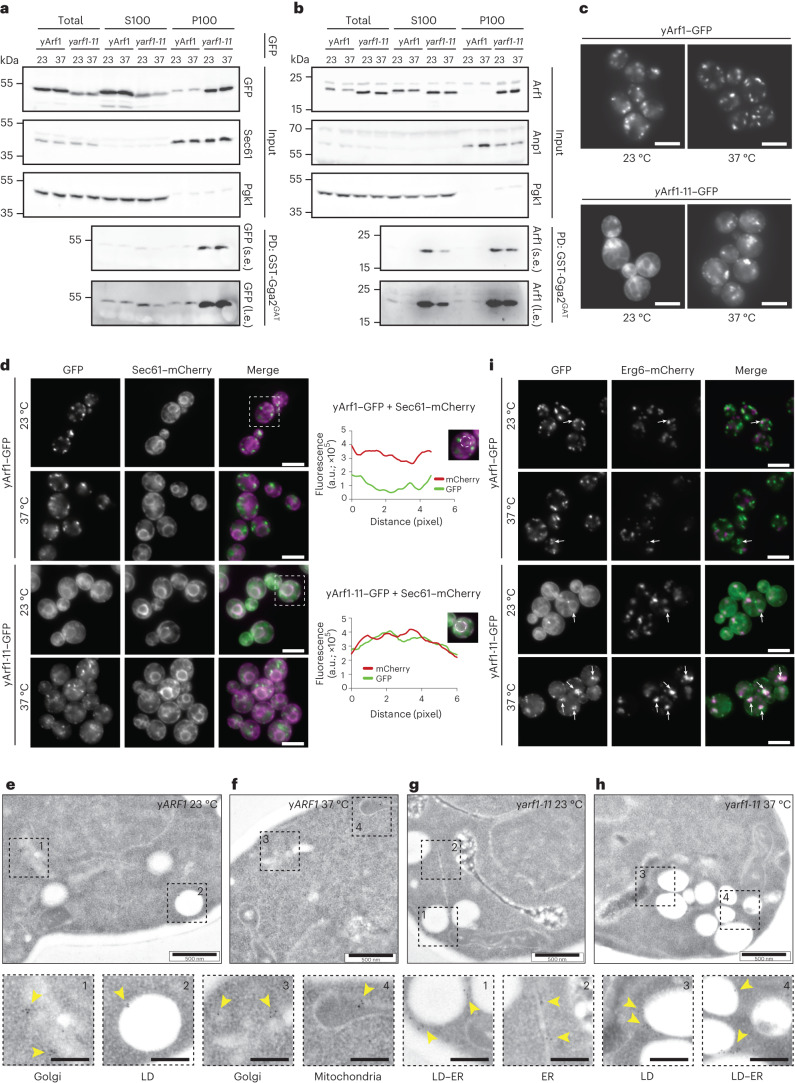
Although Arf1 activity affects mitochondrial morphology, we were puzzled by the difference in mitochondrial morphology in yeast, C. elegans and mammalian cells. A main difference between the experiments was that in yeast we used a mutant, while Arf1 was eliminated in metazoans26,28. Since two of the three mutations of yarf1-11 are located within or in close proximity to the GTP-binding domain, we asked whether GTP binding was impaired in yArf1-11. Thus, we incubated soluble (S100) and pellet (P100) yArf1-11 lysate fractions with the GAT domain of the Arf effector Gga2 (Gga2GAT), which specifically binds yArf1 in its GTP-bound form40. yArf1-11 in the P100 fraction was more efficiently retained by Gga2GAT compared with yArf1, regardless of the temperature and whether tagged or untagged Arf1 variants were used (Fig. 3a,b). At 23 °C, yArf1-11 in the S100 fraction also bound Gga2GAT (Fig. 3a,b). Our results indicate that yArf1-11 is mostly in the active conformation already at 23 °C, that GTP binding does not change upon shift to the restrictive temperature, and that hence yarf1-11 is a gain-of-function mutant. This finally explains the different observations; loss of Arf1 function yields hyperfused mitochondria, while a gain-of-function mutation results in globular mitochondria.
Fig. 3. yArf1-11 is a hyperactive mutant present on the ER and LDs.
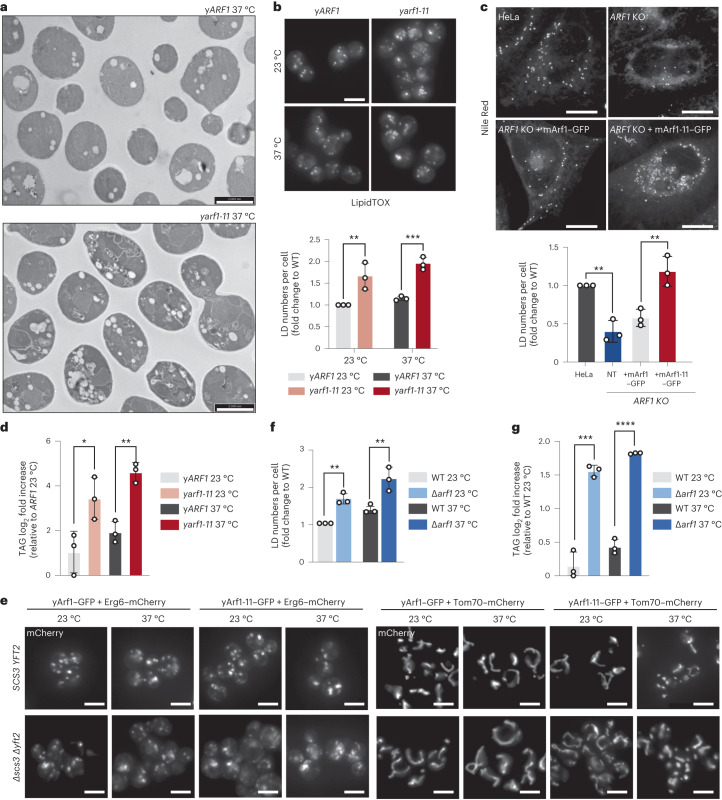
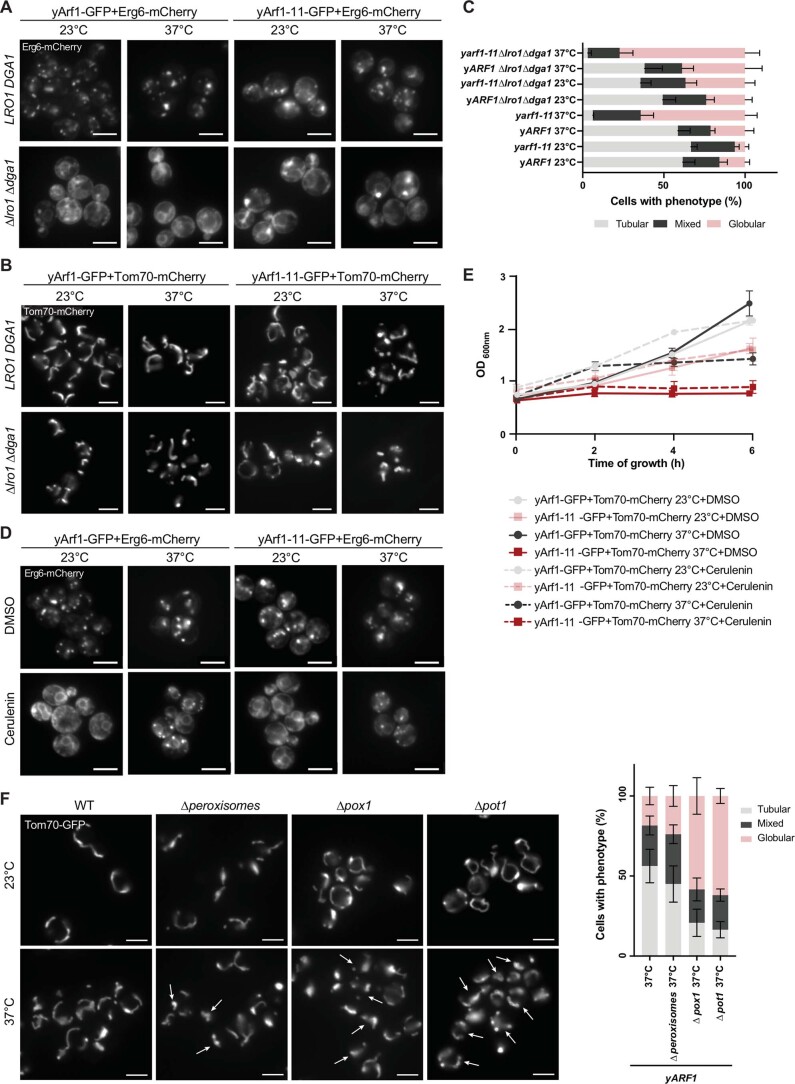
a,b, Active yArf1 pull-down and detection experiments done with strains expressing yArf1 and yArf1-11 fused to GFP (a) or endogenous untagged yArf1 and yArf1-11 (b). Protein extracts from soluble (S100) or pellet (P100) fractions from yARF1 and yarf1-11 cells grown at 23 °C or shifted to 37 °C were incubated with equal amount of purified GST-tagged GAT domain of Gga2 (Gga2GAT). Sec61 and Anp1 were used as membrane marker and Pgk1 as cytosolic marker. s.e., short exposure; l.e., long exposure; PD, pull-down. c, Localization of WT yArf1 and yArf1-11 C-terminally fused to GFP. Cells were incubated either at 23 °C or shifted at 37 °C for 30 min. Mean and standard deviation are shown. Scale bar 5 µm. d, Co-localization of yArf1–GFP and yArf1-11–GFP with the ER marker Sec61 tagged with mCherry grown at 23 °C and 37 °C. Cells highlighted by dotted squares depict GFP and mCherry co-localization. Fluorescence intensities of each channel were measured on a circle drawn around the perinuclear ER and are shown here as arbitrary units (a.u.). Scale bar, 5 µm. e–h, TEM of yARF1 (e,f) and yarf1-11 (g,h) strains grown either at 23 °C (e,g) or shifted at 37 °C (f,h) for 30 min. yArf1 and yArf1-11 localizations were detected by immunogold labeling, and dotted squares show enlargements of specific Arf1 localizations. Scale bar, 500 nm. Scale bar magnification, 200 nm. i, Co-localization of yArf1–GFP and yArf1-11–GFP with the LD marker Erg6 tagged with mCherry grown at 23 °C or shifted to 37 °C for 30 min. Arrows indicate sites of co-localization between the yArf1/yArf1-11 and LD. Scale bar, 5 µm. Unprocessed blots are available in source data. See also Extended Data Fig. 2.
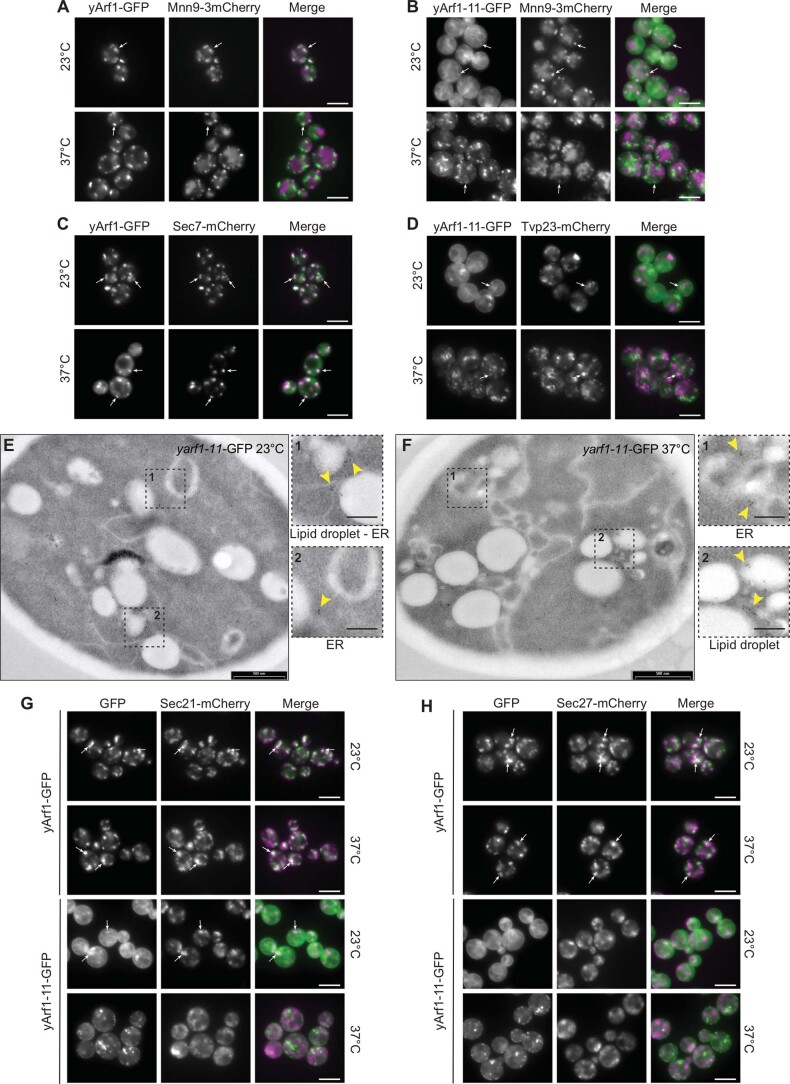
When we recorded the movies on mitochondrial dynamics, we noticed that the yArf1-11 localization pattern was different to that of yArf1. This could either be due to a difference in Golgi morphology in yarf1-11, where the bulk of yArf1 is localized, or yArf1-11 might localize to different organelles. While yArf1–GFP mainly localized to the Golgi (Fig. 3c and Extended Data Fig. 2a,c), only a minor fraction of yArf1-11–GFP was present at the Golgi (Extended Data Fig. 2b,d). Most yArf1-11–GFP was in a pattern conspicuously similar to the ER at 23 °C (Fig. 3c), which we confirmed with the ER-marker Sec61–mCherry (Fig. 3d) and by immuno-electron microscopy (EM) (Fig. 3e–h). Since yarf1-11 cells do not have a growth defect at 23 °C (Fig. 1b), Arf1-11’s ER localization does not seem to be detrimental. At 37 °C, however, yArf1-11 was massively relocated to puncta, which did not correspond to Golgi compartments (Fig. 3c and Extended Data Fig. 2b–d). Arf1 has been reported to be localized also to LDs and mitochondria30,31,33,36,41,42, which we also observed irrespective of growth temperature (Fig. 3e,f). Indeed, these yArf1-11 puncta at 37 °C corresponded to LDs (Fig. 3i and Extended Data Fig. 2e,f). Of note, the number of LDs appeared to be increased and clustered in yarf1-11 compared with wild type (WT) (Fig. 3g–i and Extended Data Fig. 2f). The presence of yArf1-11 on LDs did not prompt an increase in COPI coat components on LDs (Extended Data Fig. 2g,h), suggesting a COPI-independent function of Arf1-11 on LDs. We conclude that yArf1-11 mainly localizes to the ER at the permissive temperature and to LDs at the restrictive temperature.
Extended Data Fig. 2. yArf1-11 is a hyperactive mutant present on the ER and LDs.
(A, B) Co-localization of yArf1-GFP (A) and yArf1-11-GFP (B) with the cis-Golgi marker Mnn9-mCherry grown at 23 °C or shifted to 37 °C. Arrows indicate sites of co-localization between yArf1/yArf1-11 and Mnn9. Scale bar: 5 µm. (C, D) Co-localization of yArf1-GFP (C) and yArf1-11-GFP (D) with the trans-Golgi marker Sec7-mCherry and Tvp23-mCherry grown at 23 °C or shifted to 37 °C. Arrows indicate sites of co-localization between yArf1/yArf1-11 and the corresponding markers. (E, F) Transmission electron microscopy of the yArf1-11-GFP strain grown either at 23 °C (E) or shifted to 37 °C for 30 min (F). yArf1-11-GFP localizations were highlighted by immunogold labelling, and dotted squares shows enlargements of yArf-11 specific localizations (arrows). Scale bar: 500 nm, scale bar magnification: 200 nm. (G, H) Co-localization of yArf1-GFP and yArf1-11-GFP with the COPI markers Sec21-mCherry (G) and Sec27-mCherry (H) grown at 23 °C or shifted to 37 °C. Arrows indicate sites of co-localization between the yArf1 or yArf1-11 and COPI components. Scale bar: 5 µm.
LD-localized yArf1 induces mitochondria fragmentation
Since yArf1-11 is a gain-of-function mutant, we hypothesized that yArf1-CA might localize in a similar fashion. Indeed, yArf1-CA localized also to the ER and in smaller puncta that were quite distinct from the Golgi localization observed with yArf1 and yArf1-DN (Fig. 4a). Therefore, the active form of yArf1 can be found on the ER and most likely also on LDs.
Fig. 4. LD-localized yArf1 induces mitochondria fragmentation.
a, Localization of yArf1, constitutively active (CA) or dominant negative (DN) forms of yArf1 fused to GFP grown at 23 °C or shifted to 37 °C for 30 min. Constructs were expressed from the centromeric low copy number plasmid pGFP33. Scale bar, 5 µm. b, Localization of yArf1, or yArf1 bearing single (K38T, L173S), double (K38T–E132D) or triple (K38T–E132D–L173S) substitution yarf1-11 mutations fused to GFP in Saccharomyces cerevisiae (YPH500) grown at 23 °C or shifted to 37 °C for 30 min. Constructs were expressed from the centromeric low-copy-number plasmid pGFP33. Scale bar, 5 µm. c, Growth assay of the WT strain bearing the empty pGFP3 vector (+EV), single (K38T, L173S), double (K38T–E132D) or triple (K38T–E132D–L173S) yarf1-11 mutations fused to GFP on rich YPD plates incubated at 23 °C, 30 °C or 37 °C. d, Schematic of the construct designed to anchor yArf1 on the LD via the PAT domain of the perilipin PLN1. yARF1 deleted in its myristoylation sequence (∆N17) was expressed from its endogenous promoter and fused to GFP on its 3′ end. Localization of LD-anchored ∆N17-yArf1–GFP, the constitutively active mutant yArf1-CA, or yArf1 bearing yArf1-11–GFP variant in cells depleted of ARF1 grown at 23 °C and shifted to 37 °C. Tom70–mCherry was used as a mitochondrial marker. Mitochondria phenotypes (tubular, mixed or globular) were measured. Mean and standard deviation are shown. At 23 °C, ∆yarf1 = 406 cells, ∆yarf1 + LD-Arf1 = 477 cells, ∆yarf1 + LD-Arf1-11 = 483 cells, ∆yarf1 + LD-Arf1-CA = 403 cells; At 37 °C, ∆yarf1 = 443 cells, ∆yarf1 + LD-Arf1 = 480 cells, ∆yarf1 + LD-Arf1-11 = 523 cells, ∆yarf1 + LD-Arf1-CA = 529 cells from n = 3 biological replicates. Scale bar, 5 µm. e, Schematic of the construct designed to anchor yArf1 on the ER via Sec66. yARF1 deleted in its myristoylation sequence (∆N17) was expressed from its endogenous promoter and fused to GFP on its 3′ end. Localization of ER-anchored ∆N17-yArf1–GFP or yArf1 bearing yArf1-11–GFP variant in ∆yarf1 cells grown at 23 °C and shifted to 37 °C. Scale bar, 5 µm. f, Growth assay of the ER-anchored ∆N17-yArf1–GFP or Arf1 strains bearing yarf1-11 mutations (Arf1K38T–E132D–L173S) on rich YPD plates or synthetic medium lacking uracil (HC-Ura) incubated at 23 °C, 30 °C or 37 °C, and of the ER-anchored ∆N17-yArf1–GFP or yArf1 bearing yarf1-11 mutations (Arf1K38T–E132D–L173S) in YPH500 cells lacking yARF1 (∆yarf1) on rich YPD plates or synthetic media lacking uracil (HC -Ura) incubated at 23 °C, 30 °C or 37 °C. g, Cells expressing yArf1/11 fused to GFP, or expressing ER-∆N17-yArf1/11–GFP were grown at 37 °C for 30 min and mitochondria were imaged with Tom70–mCherry by high-resolution microscopy followed by deconvolution. A z-projection of maximum intensities is shown for each panel. Scale bar, 2 µm. h,i, Measurements of mitochondria phenotypes (tubular, mixed or globular) based on images taken in g (h), and mitochondrial fusion and fission events based on Supplementary Videos 9–12 (i). Mean and standard deviation are shown. ∆yarf1 + yArf1–GFP = 364 cells, ∆yarf1 + yArf1-11–GFP = 800 cells, ∆yarf1 + ER-yArf1–GFP = 608 cells, ∆yarf1 + ER-yArf1-11–GFP = 542 cells from n = 3 biological replicates (h); at 23 °C ∆yarf1 + ER-yArf1–GFP = 210 cells, ∆yarf1 + ER-yArf1-11–GFP = 208 cells and at 37 °C ∆yarf1 + ER-yArf1–GFP = 195 cells, ∆yarf1 + ER-yArf1-11–GFP = 190 cells from n = 3 biological replicates (i). Source numerical data are available in source data.
Next, we asked which mutation, or combination of mutations, is responsible for the localization of yArf1-11 to the ER and LDs by re-introducing yArf1-11 mutations in WT yArf1 (Supplementary Tables 1 and 2). The single mutations K38T and L173S as well as the K38T–E132D pair perturbed yArf1 localization, but failed to localize yArf1 to the ER at 23 °C (Fig. 4b). Only the reconstitution of all three mutations (K38T–E132D–L173S) caused yArf1 to be on the ER at 23 °C and on LDs at 37 °C (Fig. 4b), but none of the combinations tested elicited a dominant phenotype (Fig. 4c).
We wondered whether the localization of yArf1-11 on LDs was the cause of mitochondrial fragmentation. Therefore, we anchored yArf1 on LDs by replacing the N-terminal amphipatic helix of Arf1 with the PAT domain of the LD protein Pln1 (ref. 43) in a ∆arf1 strain. As shown above, loss of yArf1 already impacts mitochondria morphology (Fig. 4d). However, this phenotype was exacerbated irrespective of which yArf1 version was targeted to LDs (Fig. 4D), suggesting that the continuous presence of yArf1 on LDs increases the level of globular mitochondria. To corroborate our findings, we prevented yArf1 to localize on LDs by targeting yArf1 and yArf1-11 to the ER using the transmembrane domain of Sec66 (Fig. 4e). Their sequestration at the ER did not affect growth even in the absence of WT yARF1 (Fig. 4f). Under these conditions, the mitochondrial network remained tubular in cells expressing ER-anchored yArf1-11 even at 37 °C (Fig. 4g,h). Moreover, mitochondria fusion was similar between yArf1 and yArf1-11 irrespective of the temperature (Fig. 4i and Supplementary Videos 9–12). Thus, localization of yArf1-11 on the ER prevents mitochondria fragmentation at 37 °C.
Functional conservation of Arf1-11 in mammalian cells
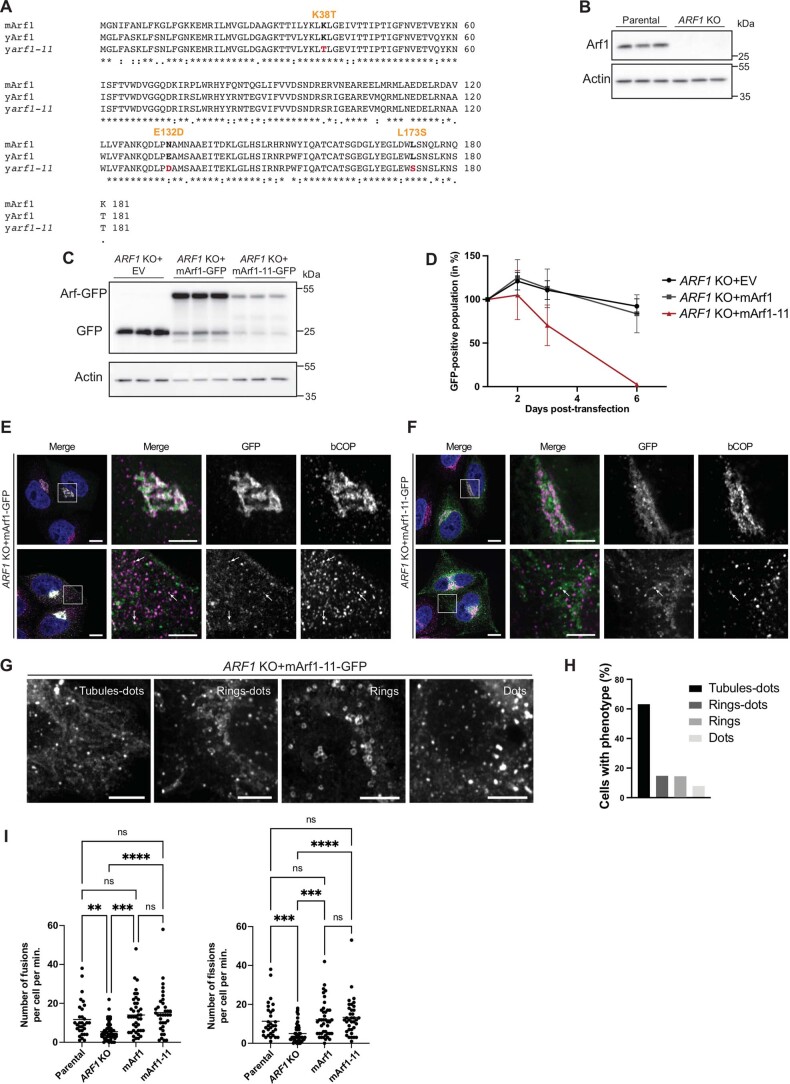
Since Arf1 also plays a role in mitochondrial dynamics in mammalian cells, we wondered whether Arf1-11 localization and function would be conserved in mammalian cells. Thus, we mutated mammalian Arf1 at the corresponding positions (mArf1-11), and C-terminally fused WT mammalian Arf1 (mArf1) and mArf1-11 to GFP (Extended Data Fig. 3a). These proteins were expressed in HeLa ARF1 knockout cells (ARF1 KO; Extended Data Fig. 3b,c)44. mArf1-11 expression in these cells led to drastic cell death after 3 days (Extended Data Fig. 3d). Thus, like in yeast, the hyperactive form of mArf1 has severe effects on cell survival.
Extended Data Fig. 3. Functional conservation of Arf1-11 in mammalian cells.
(A) Alignment of mammalian Arf1 (mArf1), yeast Arf1 (yArf1) and the yeast arf1-11 (yArf1-11) amino acids sequences. Arf1-11 mutation’s and their corresponding amino acids in mArf1 are shown. (B) Immunoblot analysis of Arf1 presence in parental HeLa cells and the CRISPR-Cas9 ARF1 KO cells. For each cell line, three independent biological replicates were analyzed on the same gel. (C) Immunoblot analysis of Arf1-GFP presence in ARF1 KO HeLa cells transfected either with an empty vector (EV), with mArf1-GFP or with mArf-11-GFP. For each cell line, three independent biological replicates were analyzed on the same gel. Actin was used as internal control. (D) Cell viability assay as percent of GFP-positive cells in the total population after transfection of EV, mArf1-GFP or mArf-11-GFP. Mean and standard deviation are shown from n = 3 biological replicates. (E, F) Co-localization of mArf1- (E) and mArf1-11-GFP (F) expressed in the ARF1 KO cell line with COPI vesicles done by immunostaining against the coatomer subunit beta (bCOP). Squares show magnification of a perinuclear and distal portion of the cell. Scale bars: 10 µm and 5 µm (inlays). Images were acquired 24 h after transfection. (G) Different localizations of mArf1-11 observed in ARF1 KO HeLa cell line expressing mArf1-11-GFP. (H) From the images in (G), four phenotypes were identified and their occurrences quantified. 380 cells were quantified. (I) Mitochondrial fusion and fission events measured in Parental cell lines, ARF1 KO, ARF1 KO expressing mArf1- or mArf1-11-GFP. Events were scored 48 h after transfection. Mean and standard deviation are shown, Parental = 31 cells, ARF1 KO = 53 cells, ARF1 KO+mArf1 = 41 cells, ARF1 KO+mArf1-11 = 38 cells from n = 3 biological replicates; Statistical analysis were done using a two-way ANOVA test with Turkey’s multiple comparison test, ****p = 0.000000000048; ***p = 0.001; **p = 0.0047. Source numerical data and unprocessed blots are available in source data. See also Supplementary data 1.
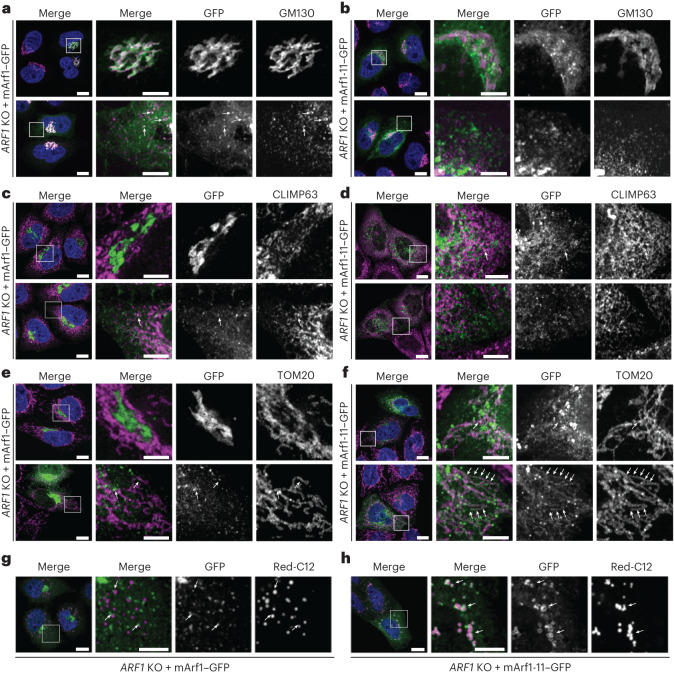
As expected, mArf1–GFP was present on the Golgi and on vesicles (Fig. 5a and Extended Data Fig. 3e). In contrast, mArf1-11 only modestly localized to the Golgi (Fig. 5b and Extended Data Fig. 3f). Instead mArf1-11 decorated tubular and large round structures (Extended Data Fig. 3g,h). The tubular structures were positive for the mitochondrial marker TOM20, but not for the ER marker CLIMP63 (Fig. 5c–f). mArf1-positive vesicles were sometimes juxtaposed to mitochondria in agreement with mArf1 function in mitochondria division or transport26–28. As expected, mArf1 knockout (KO) cells had reduced levels of mitochondria fission and fusion, which were restored to normal levels upon expression of either mArf1 or mArf1-11 (Extended Data Fig. 3i and Supplementary Videos 13–16), supporting the notion that Arf1’s functions on mitochondria are conserved from yeast to mammals. Likewise, the localization of mArf1-11 to LDs was conserved (Fig. 5g,h), encouraging us to determine the function of Arf1-11 on LDs.
Fig. 5. Functional conservation of Arf1-11 in mammalian cells.
a,b, Mammalian Arf1 (mArf1; a) or mArf1-11 (b) fused to GFP were expressed in CRISPR/Cas9-mediated ARF1 knockout HeLa cells (ARF1 KO). Co-localization with the Golgi was done by immunostaining against the marker GM130. Squares show magnification of a perinuclear and distal portion of the cell. Scale bars, 10 µm and 5 µm (inlays). c,d, Co-localization of mArf1–GFP (c) and mArf1-11–GFP (d) expressed in the ARF1 KO cell line with the ER was determined by immunostaining against the marker CLIMP63. Squares show magnification of a perinuclear and distal portion of the cell. Scale bars, 10 µm and 5 µm (inlays). e,f, Co-localization of mArf1–GFP (e) and mArf1-11–GFP (f) expressed in the ARF1 KO cell line with mitochondria was determined by immunostaining against the translocase of mitochondrial outer membrane TOM20. Squares show magnification of a perinuclear and distal portion of the cell. Scale bars, 10 µm and 5 µm (inlays). g,h, Co-localization of mArf1–GFP (g) and mArf1-11–GFP (h) expressed in the ARF1 KO cell line with LDs was determined by incubation with the fluorescent fatty-acid BODIPY Red-C12. Scale bars, 10 µm and 5 µm (inlays). Squares show magnification of distal portion of the cell. All images were acquired 24 h after transfection. See also Extended Data Fig. 3.
Hyperactive Arf1 induces TAG accumulation
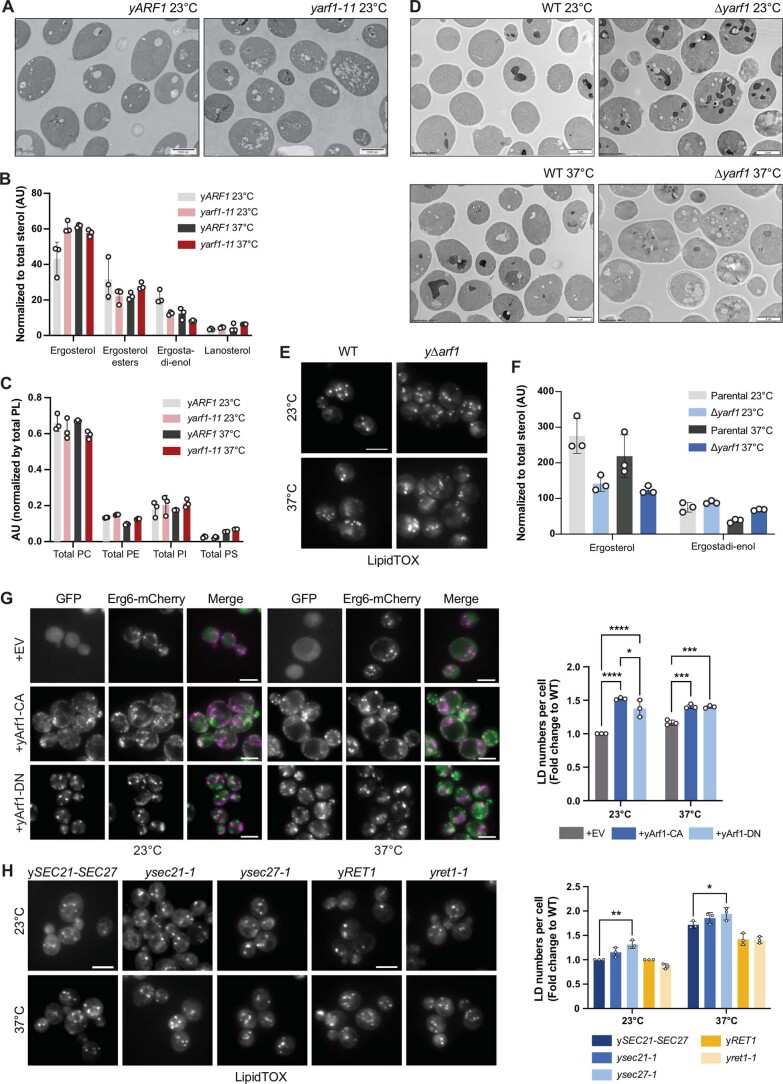
When we analysed the yeast arf1-11 mutant phenotype, we noticed in our transmission electron microscopy (TEM) pictures an increase in LD number and dilated ER (Figs. 3e–h and 6a and Extended Data Fig. 4a), which we confirmed by staining LDs with LipidTox in yeast (Fig. 6b) and with Nile Red in mammalian cells (Fig. 6c). LDs are specialized organelles primarily known for their role in energy storage in the form of neutral lipids, mainly TAG and SE. Although lipidomic analysis of yARF1 and yarf1-11 strains did not reveal any differences in the levels of SE (Extended Data Fig. 4b), we found a strong increase in TAG levels in yarf1-11 compared with yARF1 (Fig. 6d). Strikingly, these elevated TAG levels were not matched by changes in the overall phospholipid composition (Extended Data Fig. 4c).
Fig. 6. Hyperactive Arf1 induces TAG accumulation.
a, TEM of yARF1 and yarf1-11 strains grown at 37 °C for 30 min. Scale bars, 2,000 nm. b, LipidTox staining of LDs in yARF1 and yarf1-11 strains grown at 23 °C or shifted to 37 °C. Scale bar, 5 µm. Mean and standard deviation are shown; yARF1 23 °C = 408 cells, yARF1 37 °C = 408 cells, yarf1-11 23 °C = 421 cells, yarf1-11 37 °C = 422 cells from n = 3 biological replicates; two-way ANOVA using Sidak’s multiple comparison, ***P = 0.0009, **P = 0.0032. c, Nile Red staining of LDs in parental HeLa cells (control), ARF1 KO HeLa cells, and ARF1 KO HeLa cells expressing mArf1 or mArf1-11. For each cell line, the numbers of LD were quantified. Images were acquired 24 h after transfection. Mean and standard deviation are shown; HeLa control = 182 cells, ARF1 KO = 181 cells, ARF1 KO +mArf1 = 163 cells, ARF1 KO +mArf1-11 = 151 cells from n = 3 biological replicates; unpaired two-tailed t-test, **P = 0.0096. Scale bar, 5 µm. d, Measurements of TAG in the yARF1 and yarf1-11 strains grown at 23 °C or shifted to 37 °C. Mean and standard deviation are shown from n = 3 biological replicates; unpaired two-tailed t-test, *P = 0.0349; **P = 0.002. e, LDs (Erg6) and mitochondria (Tom70) morphologies imaged in the yARF1 and yarf1-11 parental strains and in strains deprived of SCS3 and YFT2 (∆scs3 ∆yft2) grown at 23 °C or shifted to 37 °C. f, LipidTox staining of LDs in WT and ∆arf1 strains grown at 23 °C or shifted to 37 °C. Mean and standard deviation are shown; WT 23 °C = 669 cells, WT 37 °C = 662, ∆arf1 23 °C = 673 cells, ∆arf1 37 °C = 659 cells from n = 3 biological replicates Two-way ANOVA using Sidak’s multiple comparison, WT versus ∆arf1 23 °C **P = 0.0032, WT versus ∆arf1 37 °C **P = 0.001. g, Measurements of TAG in the WT and ∆yarf1 strains grown at 23 °C or shifted to 37 °C. Mean and standard deviation are shown from n = 3 biological replicates; unpaired two-tailed t-test, ***P = 0.0002; ****P = 0.00000396. Source numerical data are available in source data. See also Extended Data Fig. 4.
Extended Data Fig. 4. Hyperactive Arf1 induces triacylglycerol accumulation.
(A) Transmission electron microscopy of yARF1 and yarf1-11 strains grown at 23 °C. Scale bars: 2000 nm. (B, C) Measurements of sterols (B) and phospholipids (C) in yARF1 and yarf1-11 strains grown at 23 °C or shifted to 37 °C. PC: phosphatidylcholine, PE: phosphatidylethanolamine, PI: phosphatidylinositol, PS: phosphatidylserine. Mean and standard deviation are shown from n = 3 biological replicates. (D) Transmission electron microscopy of WT and ∆yarf1 strains grown at 23 °C or shifted to 37 °C. Scale bars: 2000 nm. (E) LipidTox staining of LDs in WT and ∆yarf1 strains grown at 23 °C or shifted to 37 °C. Scale bar: 5 µm. (F) Measurements of sterols in WT and ∆yarf1 strains grown at 23 °C or shifted to 37 °C. Mean and standard deviation are shown from n = 3 biological replicates. (G) Measurements of LD numbers in WT cells expressing constitutively active yArf1-CA- or dominant negative yArf1-DN-GFP using Erg6-mCherry as marker. Mean and standard deviation are shown; WT + EV 23 °C = 303 cells, WT+yArf1-CA 23 °C = 298 cells, WT+yArf1-DN 23 °C = 289 cells, WT + EV 37 °C = 291 cells, WT+yArf1-CA 37 °C = 316 cells, WT+yArf1-DN 37 °C = 300 cells from n = 3 biological replicates. Statistical analysis were done using a two-way ANOVA test with Turkey’s multiple comparison test, +EV vs +CA 23 °C ****p = 0.000000271; +EV vs +DN 23 °C ****p = 0.00000871; ***p = 0.0006; *p = 0.022 from n = 3 biological replicates. Scale bar: 5 µm. (H) Measurements of LD numbers in COPI ts-mutants sec21-1, sec27-1, ret1-1 and their corresponding parental strains by LipidTox. Mean and standard deviation are shown; ySEC21-27 23 °C = 559 cells, ysec21-1 23 °C = 563 cells, ysec27-1 23 °C = 560 cells, ySEC21-27 37 °C = 557 cells, ysec21-1 37 °C = 527 cells, ysec27-1 37 °C = 572 cells, yRET1 23 °C = 377 cells, yret1-1 23 °C = 553, yRET1 37 °C = 427 cells, yret1-1 37 °C = 486 cells; Statistical analysis were done using a two-way ANOVA test with Turkey’s multiple comparison test, **p = 0.0034; *p = 0.0215 from n = 3 biological replicates. Scale bar: 5 µm. Source numerical data are available in source data.
We have previously shown that the ER stress response is elevated in yarf1-11 (ref. 23). Therefore, we asked whether the increase in LD biogenesis was due to ER stress. We deleted the two mammalian FIT2 homologues SCS3 and YFT2 known to connect ER stress response and LD biogenesis45, and to maintain cellular proteostasis and membrane lipid homeostasis at the ER in WT yeast (Fig. 6e)46. In these strains, LD accumulation and mitochondrial morphology remained unaffected and did not phenocopy yarf1-11. Thus, TAG accumulation and increased LD biogenesis were not a secondary effect due to ER stress in the yarf1-11 strain.
It has been previously reported that the number of LDs is increased also in a ∆arf1 mutant47, a phenotype we confirmed (Fig. 6f and Extended Data Fig. 4d,e). Similar to the yarf1-11 strain, the increase in LD number correlated with an increase in TAG levels (Fig. 6g) but not of SE (Extended Data Fig. 4f). Since both the absence of yArf1 and presence of a hyperactive yArf1 mutant induced LD formation, we asked whether Arf1 cycling between active and inactive states is needed to control LD number. LD number was significantly increased when we expressed either yArf1-CA or yArf1-DN (Extended Data Fig. 4g). This function of yArf1 on LD is independent of its role in conjunction with COPI components (Extended Data Fig. 4h). Thus, tightly controlled yArf1 activity on LD regulates TAG levels and LD number.
yArf1 regulates LD-associated functions and β-oxidation
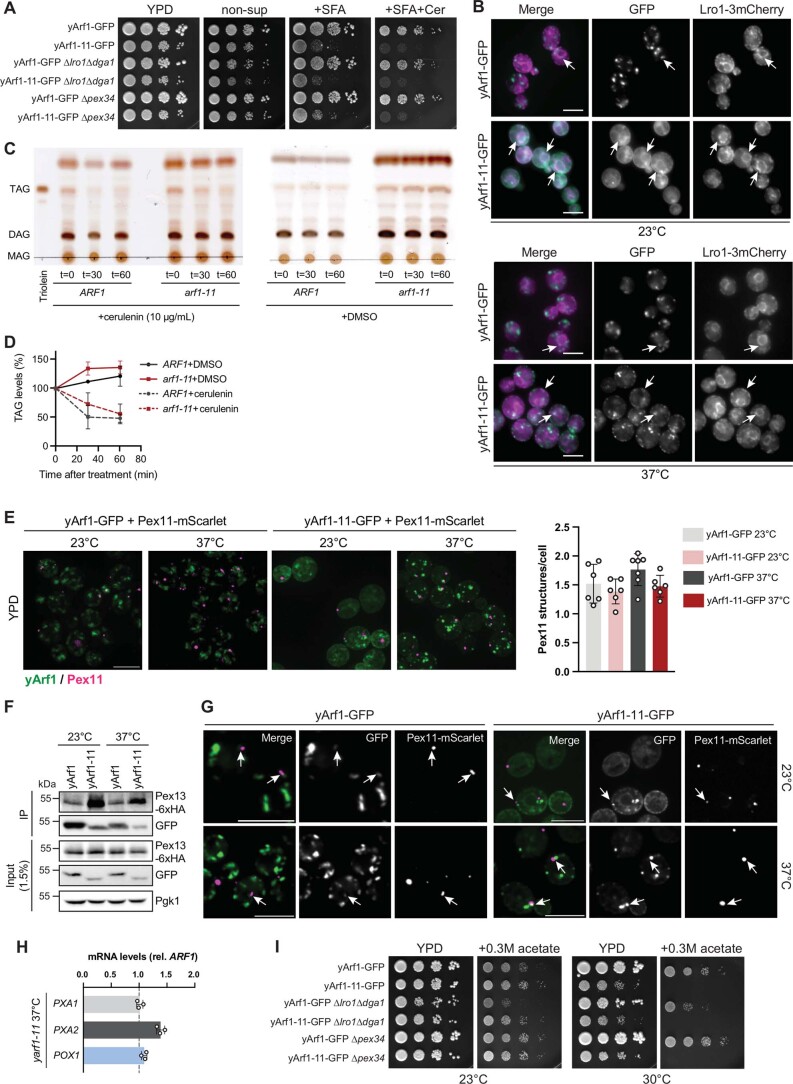
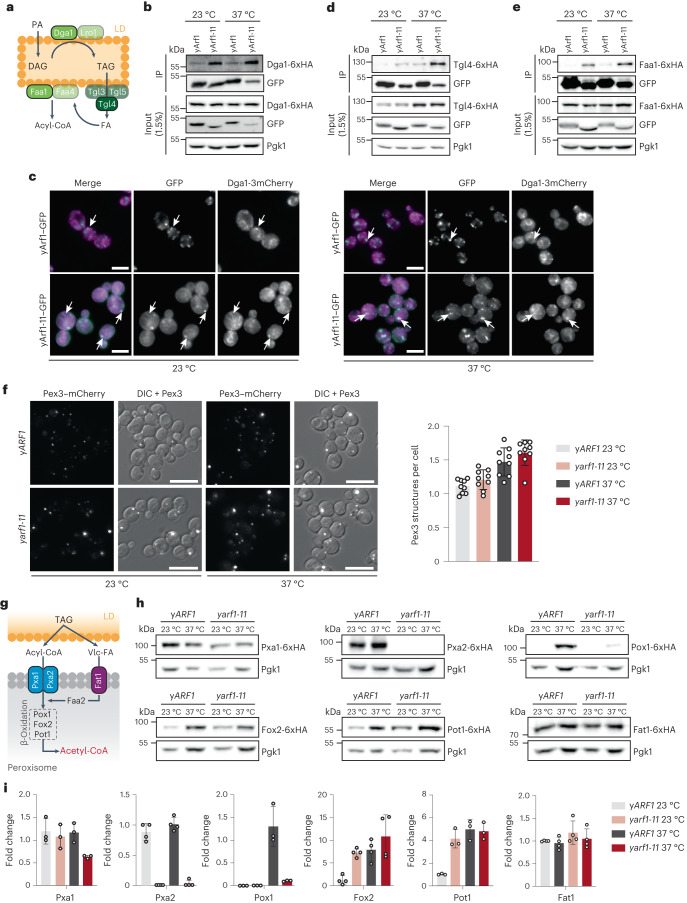
The TAG accumulation under dysregulated yArf1 activity could either be due to alteration in LD proliferation or to perturbations in FA efflux from LDs, or both. First, we tested whether yArf1-11 was involved in LD proliferation and TAG synthesis. To induce LD proliferation, we grew yARF1 and yarf1-11 cells in the presence of saturated FAs (+SFA), which resulted in a growth defect for yarf1-11 cells already at 23 °C. This phenotype was exacerbated when we blocked endogenous FA synthesis by cerulenin (+SFA+Cer) or by deleting the TAG synthases (∆lro1∆dga1) (Extended Data Fig. 5a). Moreover, Dga1 co-immunoprecipitated with both yArf1 and yArf1-11 (Fig. 7a,b). Consistently, yArf1 occasionally co-localized with or was juxtaposed to Dga1 and Lro1, while yArf1-11 co-localized with the ER pool of Dga1 and Lro1 at 23 °C and the LD pool of Dga1 at 37 °C (Fig. 7c and Extended Data Fig. 5b). These data suggest that yArf1 could positively influence TAG synthesis.
Extended Data Fig. 5. yArf1 regulates LD-associated functions and ß-oxidation.
(A) Growth test of yARF1 and yarf1-11 strains, deprived of the LRO1/DGA1 (∆lro1 ∆dga1) or of PEX34 (∆pex34), on rich media (YPD), rich media lacking saturated fatty acids (SFA) and containing cerulenin (Cer) (non-sup), containing SFA or SFA and cerulenin at 23 °C. (B) Co-localization of yArf1-GFP and yArf1-11-GFP with Lro1-3xmCherry grown at 23 °C or shifted to 37 °C for 30 min. Arrows indicate yArf1/yArf1-11 co-localizing or juxtaposed to Lro1. Scale bar: 5 µm. (C, D) In vivo TAG mobilization assay performed on yARF1 and yarf1-11 strains. Cellular TAG levels evidenced by thin-liquid chromatography extracted from cells grown in the presence of cerulenin or DMSO (C) were measured (D). Mean and standard deviation from at least n = 3 biological replicates are shown. (E) Peroxisome numbers evaluated by microscopy using Pex11-mScarlet in the yARF1- and yarf1-11-GFP strains grown at 23 °C or shifted to 37 °C. Peroxisome numbers per cell were quantified in each strain and conditions. Mean and standard deviation are shown; yArf1+Pex11-mScarlet 23 °C = 289 cells, yArf1+Pex11-mScarlet 37 °C = 431 cells, yArf1-11+Pex11-mScarlet 23 °C = 200 cells, yArf1-11+Pex11-mScarlet 37 °C = 304 cells from n = 3 biological replicates. Scale bar: 5 µm. (F) Co-immunoprecipitation of yArf1-GFP and yArf1-11-GFP with Pex13-6xHA. Strains were grown at 23 °C or shifted to 37 °C. (G) High-resolution microscopy of yARF1- and yarf1-11-GFP strains expressing Pex11-mScarlet grown at 23 °C or shifted to 37 °C. Arrows indicate Arf1 partially co-localizing with, or juxtaposed to peroxisomes. Scale bars: 5 µm. (H) mRNA levels measurement of POX1, PXA1 and PXA2 in yarf1-11 compared to yARF1 grown at 37 °C by qRT-PCR. Fold changes were measured by the 2−∆∆Ct method. Mean and standard deviation from n = 3 biological replicates are shown. (I) Growth test of yARF1, yarf1-11, ∆lro1 ∆dga1 or ∆pex34 strains, on YPD or media supplemented with 0.3 M sodium acetate at 23 °C and 30 °C. Source numerical data and unprocessed blots are available in source data.
Fig. 7. yArf1 regulates LD-associated functions and β-oxidation.
a, Schematic of TAG synthesis and breakdown on LD. Key enzymes involved in TAG synthesis (Dga1), hydrolysis (Tgl4), and FA activation (Faa1) used in our co-IP experiments are shown in green. b,d,e, Co-IP of yArf1–GFP and yArf1-11–GFP with Dga1-6xHA (b), Tgl4-6xHA (d) and Faa1-6xHA (e). Strains were grown at 23 °C or shifted to 37 °C. c, Co-localization of yArf1–GFP and yArf1-11–GFP with the diacylglycerol acyltransferase Dga1 tagged with 3xmCherry grown at 23 °C or shifted to 37 °C for 30 min. Arrows indicate sites of co-localization or juxtaposition between the yArf1/yArf1-11 and Dga1 on the ER or on LD. Scale bars, 5 µm. f, Peroxisome biogenesis followed by microscopy using the peroxisomal marker Pex3 fused to mCherry in the yARF1 and yarf1-11 strains grown at 23 °C or shifted to 37 °C (left). Quantification of peroxisomes per cell in each strain and condition (right). Mean and standard deviation are shown; yARF1 + Pex3–mCherry 23 °C = 1,496 cells, yARF1 + Pex3–mCherry 37 °C = 1,070 cells, yarf1-11 + Pex3–mCherry 23 °C = 1,025 cells, yarf1-11 + Pex3–mCherry 23 °C = 970 cells from n = 3 biological replicates. Scale bars, 5 µm. g, Schematic of TAG mobilization to synthesize acetyl-CoA by peroxisomal β-oxidation in yeast. Relevant proteins monitored in h are shown. Vlc-FA, very-long-chain FAs. h, Immunoblot analysis of all β-oxidation proteins, both acyl-CoA transporters and the Vlc-FA transporter genomically fused to 6xHA in the yARF1 and yarf1-11 strains grown at 23 °C or shifted to 37 °C. Pgk1 was used as loading control. i, Relative fold changes in protein levels from immunodetections done in h. Mean and standard deviation are shown from n = 3 biological replicates. Source numerical data and unprocessed blots are available in source data. See also Extended Data Fig. 5.
Next, we asked whether yArf1 could also be involved in FA efflux from LDs. TAGs in LD are converted into free FA by triglyceride lipases, activated by Faa1/Faa4 on LDs, and then activated free FA are imported into peroxisomes for β-oxidation (Fig. 7a). Both the triglyceride lipase Tgl4 and the acyl-CoA synthase Faa1 were co-immunoprecipitated with yArf1-11 and to a lesser extent with yArf1 (Fig. 7d,e), suggesting that yArf1 negatively regulates FA efflux from LDs. Thus, we measured TAG mobilization in yARF1 and yarf1-11 cells. Because of the yarf1-11 temperature sensitivity (Fig. 1c), we were unable to perform long kinetics. Still, we observed a slight impairment in TAG mobilization from LDs in yarf1-11 compared with yARF1 (Extended Data Fig. 5c,d), consistent with the possibility that Arf1 negatively affects TAG mobilization.
Alterations in FA efflux might also affect peroxisomes. We could, however, not detect any difference in peroxisome number (Fig. 7f and Extended Data Fig. 5e). yArf1 and mArf1 can bind to peroxisomes in vitro37,48 and Pex35 functionally interacts with yArf1 (ref. 39). Likewise, we detected interactions between yArf1 and yArf1-11 with the peroxisomal protein Pex13 (Extended Data Fig. 5f). However, neither yArf1–GFP nor yArf1-11–GFP co-localized with peroxisomes, but were rather found on juxtaposed structures (Extended Data Fig. 5g).
Next, we determined whether FA import into peroxisomes or β-oxidation was defective in yarf1-11 (Fig. 7g). The two subunits of the obligate heterodimeric FA transporter Pxa1 and Pxa2 were affected in yarf1-11. Pxa1 levels were strongly reduced at 37 °C and Pxa2 was virtually absent, both at 23 °C and 37 °C (Fig. 7h,i). Likewise, the first and rate-limiting enzyme involved in β-oxidation, the acyl-CoA oxidase Pox1, was almost undetectable at 37 °C in yarf1-11, while its levels were increased in yARF1 under the same condition. Consistent with the functional peroxisome biogenesis, not all peroxisomal proteins were affected in yarf1-11. The level of the very-long-chain FA transporter Fat1 and two other enzymes of the β-oxidation cascade, Fox2 and Pot1, were not altered in yarf1-11. The reduction of Pxa1, Pxa2 and Pox1 levels was not due to transcriptional regulation as we did not observe any decrease in the mRNA levels of all three genes in yarf1-11 at 37 °C (Extended Data Fig. 5h), indicating that the regulation of Pxa1, Pxa2 and Pox1 occurs post-transcriptionally.
The product of β-oxidation, acetyl-CoA is transferred to mitochondria for ATP production. Our data indicate that yarf1-11 might be defective in acetyl-CoA synthesis using FA as substrate. Besides FAs, acetate can also be metabolized by yeast cells to produce acetyl-CoA. In the presence of 0.3 M sodium acetate, none of the yarf1-11 mutant strains grew at the semi-permissive temperature 30 °C (Extended Data Fig. 5i). Thus, our data indicate that yarf1-11 is defective in acetyl-CoA synthesis. Taken together, our results suggest a function for Arf1 in either directly or indirectly regulating TAG synthesis and metabolism, peroxisome function and thereby FA flux into mitochondria.
Acetyl-CoA transfer loss leads to mitochondria fragmentation
We hypothesized that disruption of FA flux into mitochondria would affect mitochondria function and morphology. To test this hypothesis, we first deleted the two TAG synthases LRO1 and DGA1 and determined mitochondrial morphology. As expected, the LD marker Erg6 remained in the ER resulting from a lack of TAG and LD biogenesis (Extended Data Fig. 6a). Consistently, the proportion of cells harbouring globular mitochondria was increased (Extended Data Fig. 6b,c). Next, we investigated the impact of FA deprivation on mitochondrial morphology by treating cells with cerulenin49 (Fig. 8a–c). Cerulenin treatment efficiently reduced the levels of FAs and LDs (Extended Data Fig. 6d), and slowed down growth (Extended Data Fig. 6e). Under these conditions, the fraction of cells with globular mitochondria increased drastically (Fig. 8a,b). Moreover, abolishing β-oxidation (∆pox1 or ∆pot1) likewise resulted in globular mitochondria (Extended Data Fig. 6f). Thus, disruption of FA metabolism impairs mitochondria morphology.
Extended Data Fig. 6. Acetyl-CoA transfer loss leads to mitochondria fragmentation.
(A) Lipid droplets morphologies and Erg6 localization imaged in the yARF1 and yarf1-11 parental strains (LRO1 DGA1) or in strains deprived of LRO1 DGA1 (∆lro1∆dga1) grown at 23 °C or shifted to 37 °C. Scale bar: 5 µm. (B) Mitochondrial morphologies imaged in the parental or ∆lro1∆dga1 yARF1 and yarf1-11 strains grown at 23 °C or shifted to 37 °C. Scale bar: 5 µm. (C) Quantification of the mitochondrial phenotypes observed in (B). Mean and standard deviation are shown; yARF1 23 °C = 1213 cells, yarf1-11 23 °C = 948 cells, yARF1 ∆lro1∆dga1 23 °C = 804 cells, yarf1-11 ∆lro1∆dga1 23 °C = 1832 cells, yARF1 37 °C = 937 cells, yarf1-11 37 °C = 732 cells, yARF1 ∆lro1∆dga1 37 °C = 640 cells, yarf1-11 ∆lro1∆dga1 37 °C = 988 cells from n = 4 biological replicates. (D) Lipid droplets morphologies and Erg6 localization imaged in the yARF1 and yarf1-11 strains grown at 23 °C or shifted to 37 °C and treated with either DMSO or the fatty acid synthesis inhibitor cerulenin for 6 h. Scale bar: 5 µm. (E) Cell growth monitoring of yARF1 and yarf1-11-GFP strains treated with DMSO or with cerulenin at 23 °C and shifted to 37 °C as seen in (D). Mean and standard deviation from n = 3 biological replicates are shown. (F) Mitochondrial morphologies were imaged in the parental WT cells in which peroxisomal biogenesis was abolished (∆pex3 ∆pex19), or cells lacking enzymes of the ß-oxidation ∆pox1 or ∆pot1. All strains were grown at 23 °C or shifted to 37 °C, and Tom70-GFP was used as a mitochondrial marker. Mitochondrial phenotypes observed were quantified and mean and standard deviation are shown. yARF1 37 °C = 166 cells, yARF1 ∆peroxisomes 37 °C = 180 cells, yARF1 ∆pox1 37 °C = 574 cells, yARF1 ∆pot1 37 °C = 539 cells from n = 3 biological replicates. Scale bar: 5 µm. Source numerical data are available in source data.
Fig. 8. Acetyl-CoA transfer loss leads to mitochondria fragmentation.
a, Mitochondrial morphology imaged in the yARF1 and yarf1-11 strains grown at 23 °C or shifted to 37 °C and treated with either DMSO or the FA synthesis inhibitor cerulenin. Scale bars, 5 µm. b, Quantification of the mitochondrial phenotypes observed in a. Mean and standard deviation are shown; yARF1 + DMSO 23 °C = 751 cells, yARF1 + DMSO 37 °C = 935 cells, yARF1 + cerulenin 23 °C = 501 cells, yARF1 + cerulenin 37 °C = 654 cells, yarf1-11 + DMSO 23 °C = 616 cells, y arf1-11 + DMSO 37 °C = 740 cells, y arf1-11 + cerulenin 23 °C = 471 cells, y arf1-11 + cerulenin 37 °C = 667 cells from at least n = 3 biological replicates. c, Metabolic pathway leading to TAG synthesis. FAs are used to produce phosphatidic acid (PA), which can be further converted to diacylglycerol (DAG) and to TAG inside LDs by the Lro1 and Dga1 enzymes. The FA synthesis inhibitor cerulenin inhibits TAG synthesis. d, Schematic of the experiment done in e. Yeast cells are first grown for 30 min at 37 °C, treated with BODIPY Red-C12 for another 30 min at 37 °C, washed and imaged. e, Acetyl-CoA transfer to mitochondria monitored in the yeast ARF1 and arf1-11 strains grown at 37 °C using the fluorescent FA BODIPY Red-C12. Co-localization of GFP signal (mitochondria) over Red-C12 one was measured using Mander’s co-localization index. Mean and minimum to maximum are shown, box ranges from the first (Q1–25th percentiles) to the third quartile (Q3–75th percentiles) of the distribution; yARF1 37 °C = 115 cells, yarf1-11 37 °C = 123 cells from n = 3 biological replicates; Unpaired two-tailed t-test, ****P = 0.000000000000001. f, Schematic representation of the FA pulse-chase assay. Cells were stained with BODIPY Red-C12 for 16 h in CM, washed and chased for 9 h in nutrient-depleted medium (HBSS). Then before imaging, cells were stained for 30 min with the MitoView dye. g,h, ARF1 KO cells expressing mArf1–GFP or mArf1-11–GFP were pulsed with BODIPY Red-C12 for 16 h, incubated 1 h in CM, transferred in HBSS (0 h; g) and chased HBSS for 9 h (h). BODIPY Red-C12 was initiated 24 h after mArf1 or mArf1-11 transfection. Scale bar, 10 µm. Scale bar inlays, 2 µm. i, Relative BODIPY Red-C12 localization measured by Pearson’s co-localization index. Mean and minimum to maximum are shown, box ranges from the first (Q1–25th percentiles) to the third quartile (Q3–75th percentiles) of the distribution; ARF1 KO + mArf1 0 h = 114 cells, ARF1 KO + mArf1-11 0 h = 122 cells, ARF1 KO + mArf1 9 h = 145 cells, ARF1 KO + mArf1-11 9 h = 153 cells from n = 3 biological replicates; two-way ANOVA using Sidak’s multiple comparison test, ****P = 0.000000000000001. NS, not significant. j, Schematic of the model we propose for Arf1 role in FA metabolization and how this affects maintenance of mitochondria morphology. For more details, see Discussion. Source numerical data are available in source data. See also Extended Data Figs. 6–10.
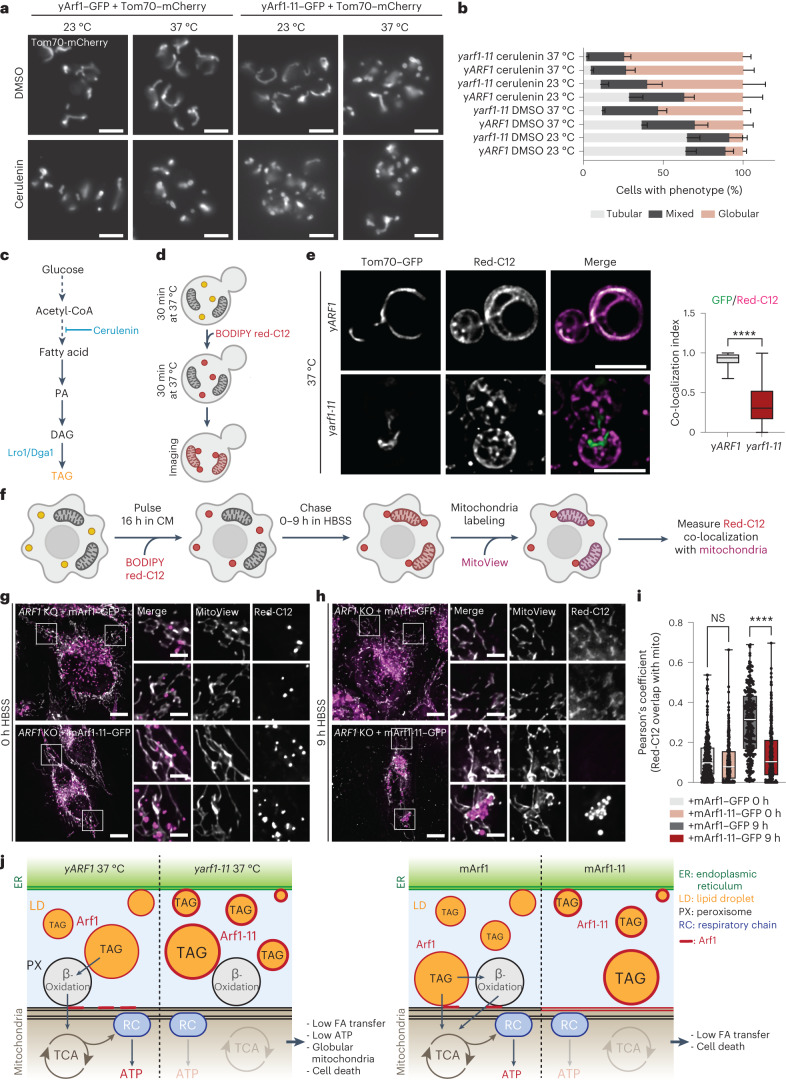
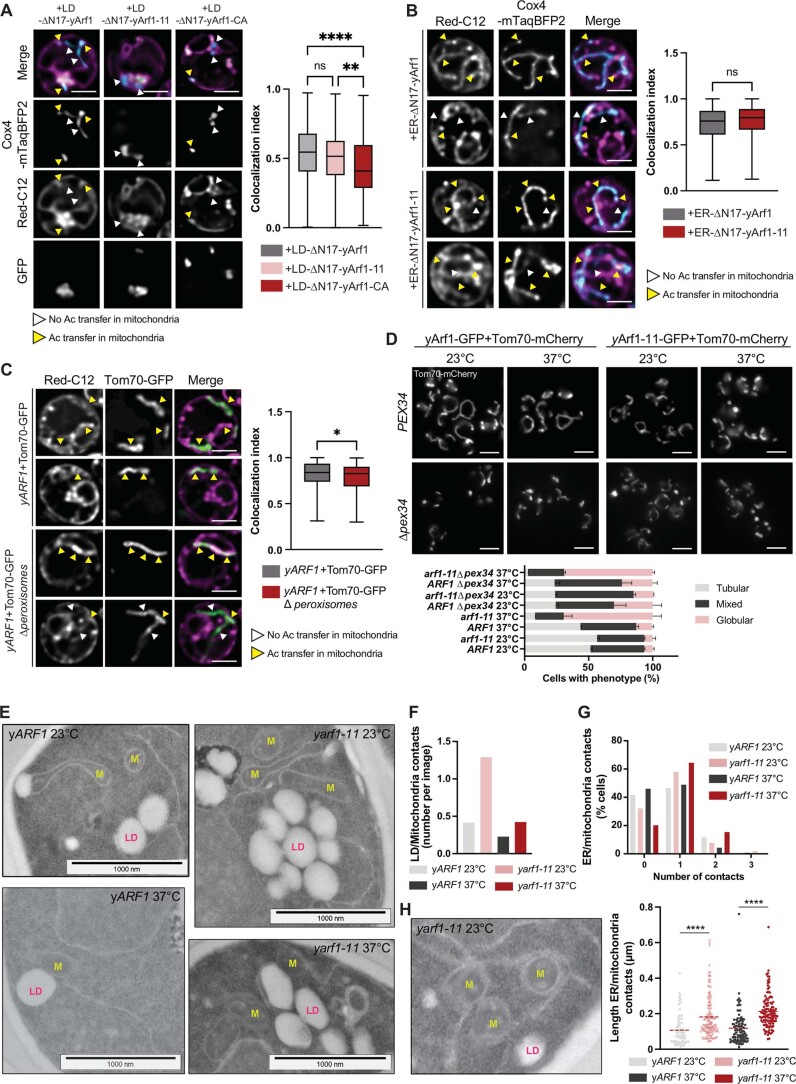
To corroborate the results above and to show that FA flux into mitochondria is impaired in yarf1-11, we followed the transport of the red-fluorescent FA derivative Bodipy C12 (Red-C12) to mitochondria (Fig. 8d). This fluorescent FA has been shown to be incorporated into LD-specific neutral lipids14,50–53, and to be metabolized by β-oxidation14,16. After 30 min, Red-C12 efficiently reached mitochondria in yARF1 (Fig. 8e), while it mainly remained in structures reminiscent of ER and LD, and was rarely transferred to mitochondria in yarf1-11 (Fig. 8e). Interestingly, Red-C12 transport into mitochondria was impaired in cells in which yArf1, yArf1-11 or yArf1-CA were locked on LDs (Extended Data Fig. 7a), but efficiently transported when yArf1-11 was anchored to the ER, consistent with the notion that Arf1 negatively regulates FA efflux from LDs (Extended Data Fig. 7b). However, abolishing peroxisome biogenesis (∆pex3∆pex19 (ref. 54)) did not affect the mitochondria phenotype (Extended Data Fig. 6f), and did not reduce the flow of Red-C12 to mitochondria (Extended Data Fig. 7c). These data suggest that loss of β-oxidation is more detrimental than the loss of peroxisomes altogether for efficient FA transfer. Taken together, our data provide evidence that Arf1 on LDs negatively regulates FA efflux from LDs and thereby contribute to the fraction of globular mitochondria.
Extended Data Fig. 7. Acetyl-CoA transfer is modulated by yArf1 localization.
(A, B) Red-C12 transfer to mitochondria in y∆arf1 + LD-anchored (A) or ER-anchored strains (B) grown at 37 °C. Cox4-mTaqBFP2 was used as mitochondrial marker. mTaqBFP2 colocalization over Red-C12 measured using Mander’s index. Two-way ANOVA test using Turkey’s multiple comparison test, ****p = 0.00000215; **p = 0.001. Mean and min to max are shown, box ranges from first (25th percentiles) to third quartile (75th percentiles); (A) LD-yArf1-GFP = 187 cells, LD-yArf1-11-GFP = 167 cells, LD-yArf1-CA-GFP = 169 cells and (B) ER-yArf1 = 224 cells, ER-yArf1-11 = 234 cells from n = 3 biological replicates. Scale bar: 2 µm. (C) Red-C12 transfer to mitochondria in yARF1 and ∆peroxisomes strains using Tom70-GFP as a mitochondrial marker. GFP colocalization over Red-C12 measured using Mander’s index. Student unpaired two-tailed t-test. *p = 0.0315. Mean and min to max are shown, box ranges from first (25th percentiles) to third quartile (75th percentiles); yARF1 = 200 cells, yARF1 ∆peroxisomes = 185 cells from n = 3 biological replicates. Scale bar: 2 µm. (D) Mitochondrial morphology in WT (PEX34) or ∆pex34 strains grown at 23 °C or shifted to 37 °C. Mean and standard deviation are shown; yARF1 23 °C = 689 cells, yARF1 37 °C = 698 cells, yarf1-11 23 °C = 743 cells, yarf1-11 37 °C = 517 cells, yARF1∆pex34 23 °C = 1740 cells, yARF1∆pex34 37 °C = 1592 cells, yarf1-11∆pex34 23 °C = 1546 cells, yarf1-11∆pex34 37 °C = 1622 cells from n = 3 biological replicates. Scale bar: 5 µm. (E) TEM of yARF1 and yarf1-11 strains grown at 37 °C for 30 min. LD: lipid droplet; M: mitochondria. Scale bars: 1000 nm. (F) LD-mitochondria contacts quantified based on TEM images (E). n = 130 cells (yARF1 23 °C), n = 136 cells (yARF1 37 °C), n = 138 cells (yarf1-11 23 °C), n = 156 cells (yarf1-11 37 °C). (G, H) ER-mitochondria contacts quantification (G) and length (H) based on (E). Image in (H) shows an example of ER-mitochondria contact. Means are shown. Unpaired two-tailed T-test, yARF1 vs yarf1-11 23 °C ****p = 0.00000638; yARF1 vs yarf1-11 37 °C ****p = 0.0000000000072. n = 101 cells (yARF1 23 °C), n = 139 cells (yARF1 37 °C), n = 155 cells (yarf1-11 23 °C), n = 104 cells (yarf1-11 37 °C). Source numerical data are available in source data.
FA transport between LDs, peroxisomes and mitochondria occurs at organellar contact sites10. Impairing peroxisome–mitochondria contacts by deletion of the PEX34 tether in yARF1 and yarf1-11 led only to a small increase of cells with globular mitochondria (Extended Data Fig. 7d), much weaker than the yarf1-11 phenotype. Additional tethers might be involved, which is also supported by previous findings10,55. Moreover, our EM data revealed an increase of ER–mitochondria contacts both in number and length, and also between LDs and mitochondria in yarf1-11 (Extended Data Fig. 7e–h). We surmise that this increase in organellar contacts might represent a compensatory mechanism. Taken together, our data indicate that β-oxidation is impaired in yarf1-11 at the restrictive temperature and hence no acetyl-CoA can be transferred to mitochondria, which ultimately leads to mitochondrial fragmentation.
OXPHOS activity and ATP synthesis are impaired in yarf1-11
Mitochondria fragmentation has been described as a general mechanism in response to various types of stress56, such as ATP synthase inhibition57, oxidative stress58, or loss of mitochondrial membrane potential (Δψm)59,60. We therefore hypothesized that the lack of metabolite transfer from peroxisomes to mitochondria leads to decreased ATP synthesis.
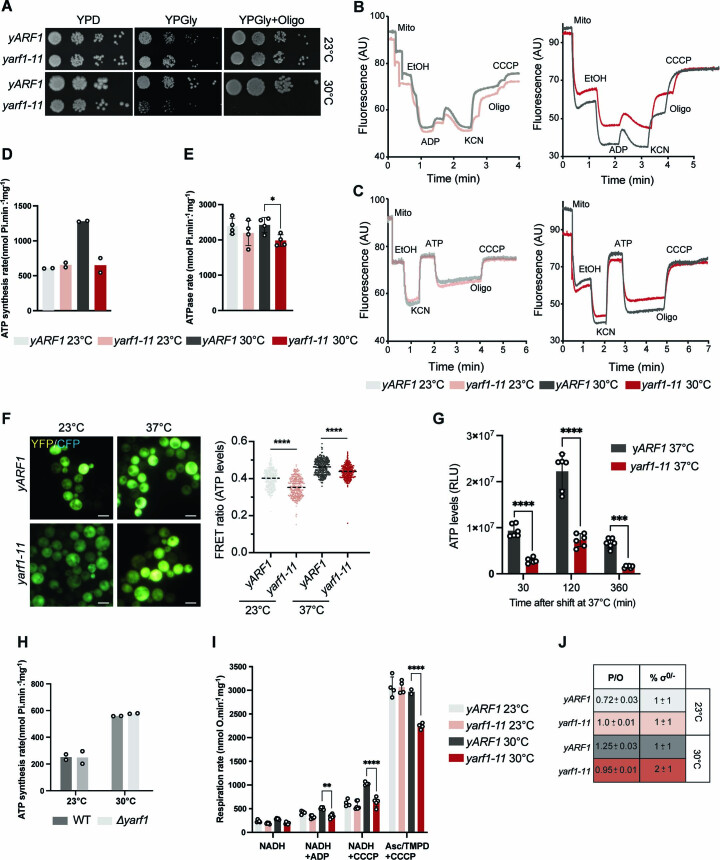
yarf1-11 did not grow on plates containing a non-fermentable carbon source (glycerol) with or without the ATP synthase inhibitor oligomycin (+Oligo), suggesting defects in RC function (Extended Data Fig. 8a). Moreover, yarf1-11 exhibited reduced Δψm (Extended Data Fig. 8b), together with impaired ATP synthesis, but not hydrolysis (Extended Data Fig. 8b,c). We confirmed these observations by directly measuring ATP synthesis and hydrolysis rates on purified mitochondria (Extended Data Fig. 8d,e), at the cellular level by using a fluorescence resonance energy transfer (FRET)-based ATP nanosensor61 (Extended Data Fig. 8f) and after 30, 120 and 360 min incubation at 37 °C (Extended Data Fig. 8g). In all cases, the outcome was a lower ATP level in yarf1-11. Conversely, ATP synthesis was not affected in ∆arf1 (Extended Data Fig. 8h), most likely due to the fact that only 40% of the mitochondria were globular in this strain (Fig. 2d). The inability of the yarf1-11 to synthesize ATP was attributed to a decrease in oxygen consumption (that is, lower respiratory rate; Extended Data Fig. 8i) and not due to uncoupled oxidative phosphorylation (P/O; Extended Data Fig. 8j). Our data indicate that the low ATP synthesis rate in yarf1-11 is a direct consequence of the low respiratory rate.
Extended Data Fig. 8. OXPHOS activity and ATP synthesis are impaired in yarf1-11.
(A) Growth test of yARF1 and yarf1-11 strain on rich media (YPD), on respiratory media (YPGly) or with oligomycine (YPGly+Oligo) at 23 °C and 30 °C. (B, C) Membrane potential measured on isolated mitochondria by Rhodamine-123 at 23 °C or 30 °C in yARF1 and yarf1-11 strains in the presence of external ADP (B) or to test ATP-driven proton pumping (C). (D, E) ATP synthesis (D) and ATPase rate (E) measured on isolated mitochondria from yARF1 and yarf1-11 strains grown at 23 °C or 30 °C. Means are shown from n = 2 biological replicates and 3 technical replicates and (E) n = 4 biological replicates. (F) Intracellular ATP levels quantified in yARF1 and yarf1-11 strains grown at 23 °C or shifted to 37 °C. FRET ratios correspond to relative ATP levels. Median and individual values are shown. Unpaired two-tailed T-test, yARF1 vs yarf1-11 23 °C ****p = 0.000000000000001; yARF1 vs yarf1-11 37 °C ****p = 0.0000000000372; yARF1 23 °C = 332 cells, yARF1 37 °C = 332 cells, yarf1-11 23 °C = 362 cells, yarf1-11 37 °C = 409 cells from n = 4 biological replicates. Scale bar: 5 µm. (G) Relative ATP levels (RLU) measured by Luciferase assay in yARF1 and yarf1-11 strains. Two-way ANOVA using Turkey’s multiple comparison test, 30 min ****p = 0.00000295; 120 min ****p = 0.000000000000028; ***p = 0.0001. Mean and standard deviation are shown from n = 6 biological replicates. (H) ATP synthesis rate measured on isolated mitochondria from WT and ∆yarf1 strains grown at 23 °C or 30 °C. Means are shown from n = 2 biological replicates and 3 technical replicates. (I) Respiration rate measured on isolated mitochondria from yARF1 and yarf1-11 strains grown at 23 °C or 30 °C. Two-way ANOVA using Sidak’s multiple comparison test. NADH + CCCP yARF1 vs yarf1-11 30 °C ****p = 0.00000000097, Asc/TMPD + CCCP yARF1 vs yarf1-11 30 °C ****p = 0.000000000014, **p = 0.0186. Median and standard deviation are shown from n = 4 biological replicates. (J) Respiration coupled to oxygen respiration (P/O) and percentage of Rho 0 and Rho-minus were measured. Source numerical data are available in source data.
Arf1 controls acetyl-CoA flux into mitochondria in mammals
We tested next whether Arf1’s role in FA metabolism was conserved in mammalian cells. We performed a Red-C12 pulse-chase assay using ARF1-KO cells expressing mArf1–GFP or mArf1-11–GFP as reported previously14. Cells were pulsed for 16 h in complete medium (CM), and chased in nutrient-deprived media (Hanks’ Balanced Salt Solution, HBSS) for 0 or 9 h (Fig. 8f). Under these conditions, FAs were present in LDs both in cells expressing mArf1 or mArf1-11 at the 0 h timepoint (Fig. 8g). While Red-C12 was efficiently transferred from LDs to mitochondria in mArf1 expressing cells after 9 h of starvation, the dye persisted in LDs in mArf1-11 cells (Fig. 8h,i). Thus, Arf1 plays an evolutionarily conserved role in acetyl-CoA transfer to mitochondria.
Taken together our results provide evidence that TAG accumulation in LDs in yeast and mammalian cells expressing the hyperactive Arf1-11 is a consequence of reduced FA metabolism and acetyl-CoA transfer into mitochondria. As a consequence, energy production is impaired, leading to mitochondrial fragmentation.
Arf1 is present at organellar contact sites
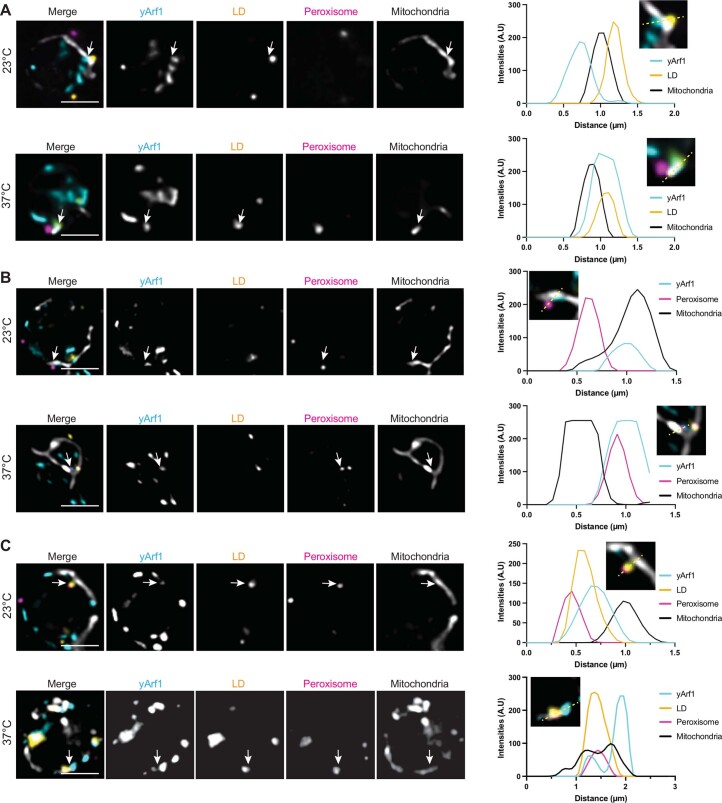
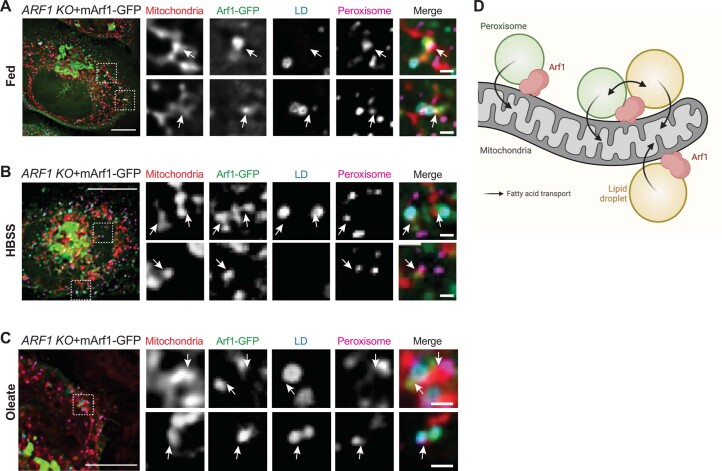
The flux of the lipid metabolites from LDs to peroxisomes and mitochondria happens through contact sites between these organelles10. Arf1 appears to regulate FA efflux from LDs, and therefore might be present at the contact sites. To test this possibility, we simultaneously labelled LDs, peroxisomes, mitochondria and yArf1 (Extended Data Fig. 9a–c). yArf1 was present at contacts between LDs and mitochondria, LDs and peroxisomes, and peroxisomes and mitochondria. More importantly, we also detected yArf1 at tripartite organellar contacts. Therefore, yArf1 is at the right location to regulate FA and acetyl-CoA flux from LDs to mitochondria. This localization was also observed in mammalian cells. mArf1 was present at mitochondria–LD, mitochondria–peroxisomes and mitochondria–LD–peroxisomes contact sites under normal (fed) conditions, nutrient starvation or in the presence of oleate (Extended Data Fig. 10a–c). Thus, Arf1 plays a conserved role in FA metabolism at organellar contact sites (Extended Data Fig. 10d).
Extended Data Fig. 9. yArf1 is present at organellar contact sites.
(A–C) High-resolution microscopy of yArf1-CFP strain expressing Erg6-YFP as LD marker, Pex3-mCherry as peroxisomal marker and MitoTracker Deep Red FM to stain for mitochondria. Cells were grown at 23 °C or shifted to 37 °C for 30 min. Localizations of LD and yArf1 (A), peroxisomes and yArf1 (B), or LD-peroxisomes and yArf1 (C) at the vicinity of mitochondria were established by following individual fluorescent intensities of each markers on a 1.5-3 µm distance. Representative images are shown and fluorescent intensities measured along the dotted lines. Single planes of 0.2 µm thickness are shown. Scale bar: 2 µm.
Extended Data Fig. 10. mArf1 is present at organellar contact sites.
(A–C) mArf1-GFP localization in ARF1 KO cells grown in complete media (A), shifted for 14 h in HBSS (B) or in the presence of oleate (C). Arrows indicate the presence of mArf1 at mitochondria-LD contact sites, mitochondria-peroxisomes contact sites or mitochondria-LD-peroxisomes contact sites. Scale bar: 10 µm, Scale bar inlays: 1 µm. (D) Schematic of Arf1 localization based on images taken in (A–C) and in Extended Data Fig. 9A–C. Created with Biorender.com.
Discussion
Here we provide evidence that Arf1 regulates mitochondria functions via two independent mechanisms. First, yArf1 is required for both fission and fusion of mitochondria, and we establish that yArf1 is present at sites on mitochondria where either fusion or fission occurs. Second, Arf1 regulates mitochondrial function by controlling the flow of FAs and metabolites from LDs to peroxisomes/mitochondria in yeast and in mammalian cells (Fig. 8j).
Previously, roles for Arf1 and the COPI coat in peroxisome biogenesis and function and on LDs have been established36–39,48,62. However, the Arf1 function we reveal here is independent of COPI. For example, coatomer mutants did not accumulate LDs. Likewise, peroxisome biogenesis appeared unaffected, but they were non-functional in yarf1-11. This functional defect is due to (1) strongly reduced acyl-CoA import into peroxisomes and (2) the almost complete absence of the acyl-CoA oxidase Pox1. We propose that FA remain stored in LDs because they cannot be metabolized in peroxisomes. This scenario is supported by the finding that deletion of Pox1 or Pxa1 in yeast leads to increased TAG levels in LDs63. Strikingly, abolishing peroxisome biosynthesis had less severe consequences than blocking β-oxidation. It is conceivable that, in the absence of peroxisomes, an alternative pathway through the ER could be activated, and that FA would flow from LDs to the ER and from there to mitochondria. This pathway would only be activated in the absence of peroxisomes and since peroxisomes, albeit non-functional, are still present in arf1-11, FA transfer is blocked entirely. In the presence of peroxisomes, neutral lipids accumulate in the ER, if transfer into LDs is abolished64–66. At any rate, as a consequence of reduced acyl-CoA import into peroxisomes and the absence of Pox1, the transfer of acetyl-CoA to mitochondria is reduced, which leads to substrate depletion for the TCA cycle and ultimately to reduced ATP production, which in turn causes mitochondrial fragmentation. We propose that the two ways by which Arf1-11 interferes with mitochondrial morphology and function drives cell death. Remarkably, the roles of Arf1 in acyl-CoA flow from LDs to mitochondria is conserved from yeast to mammals. While β-oxidation occurs in peroxisomes in yeast, it is also a mitochondrial function in mammalian cells.
mArf1 and yArf1 are found at contact sites between LDs and mitochondria, LDs and peroxisomes and at tripartite contacts, suggesting that Arf1 locally regulates organellar contacts. Moreover, the hyperactive yArf1-11 increased contacts between mitochondria, LDs and the ER. We and others have indicated that Arf1 and Arf1-11 are present on LDs36. Here we show that yArf1 co-precipitates with Dga1, which also participates in ER-LD tethering in mammalian cells67. Thus, even though co-immunoprecipitation (co-IP) does not allow us to distinguish between direct and indirect interactions, Arf1 could be involved in the regulation of tethering or TAG synthesis, or both. Likewise, Arf1 might function in LD–peroxisome tethering. The tethers between LDs and peroxisomes remain elusive. It has been speculated that proteins required for lipolysis and acyl-CoA production could act as potential tethers on LDs68. Consistent with this possibility, yArf1 co-precipitated with the triglyceride lipase Tgl4 and the acyl-CoA synthetase Faa1. In addition, yArf1-11 and mArf1-11 had a negative effect on Red-C12 transfer into peroxisomes/mitochondria and on TAG mobilization from LDs. We also detected Arf1 at contacts between LDs and mitochondria. Perlipins PLIN1 and PLIN5, MIGA2 and the mitofusin MFN2 were implicated as tethers in LD–mitochondria contacts in adipose tissue69–71. The perilipin/mitofusin tether has been proposed to be conserved between yeast and mammals72. Interestingly, in yeast arf1-11 cells the mitofusin Fzo1 formed aggregates, a phenotype that was alleviated by overexpression of Cdc48 (ref. 26).
We provide evidence that Arf1 is present at bi- and tripartite organellar contacts involving mitochondria, peroxisomes and LDs, in both yeast and mammalian cells. In yarf1-11, the contacts between mitochondria, LDs, and the ER are increased, often involving all three organelles. We cannot detect peroxisomes in our EM method. It is tempting to speculate, however, that contact sites might exist in which all four organelles come together. We envisage Arf1 to be a regulator of all these contact sites. How Arf1 regulates proteins at these contacts sites or whether Arf1 acts directly or indirectly on the TAG synthase Dga1 and the lipase Tgl4 or how it can assert its effects on peroxisomal proteins in trans remain to be established.
Strikingly, Arf1 appears to be a Jack of all trades. Historically, Arf1 has been mostly implicated in vesicle transport pathways. We have previously shown that Arf1 is also involved in mRNA transport and metabolism23. Moreover, Arf1 is also present on the ER, peroxisomes, LDs and mitochondria26–28,36,48,62. While most small GTPases have mostly rather precise set of effector molecules that they recruit, Arf1 could be much more promiscuous, since some of the processes it is involved in may not require either COPI or clathrin or its adaptors. At least, Arf1’s roles in mitochondria dynamics26 and FA efflux from LDs appear to be independent of the COPI vesicle coat. A recent study found that COPI does affect mitochondrial function and LD size in a process that is apparently not linked to TAG synthesis but rather a feedback from ROS production73. Thus, Arf1 may affect mitochondrial morphology and function in COPI-dependent and COPI-independent ways. Certainly, more studies are required to reveal the full repertoire of Arf1 function.
Methods
Strains, media and plasmids
Yeast strains were either grown in rich media composed of 1% w/v yeast extract, 1% (w/v) peptone, 40 mg l−1 adenine, 2% (w/v) glucose (YPD) or 2% glycerol (YPGly), or in synthetic complete medium (HC) composed of 0.17% (w/v) yeast nitrogen base with ammonium sulfate and without amino acids, 2% (w/v) glucose and mixtures of amino acids (MP Biomedicals) depending on the auxotrophies used for selection. Unless otherwise indicated, cells were grown at 23 °C and a subset was shifted to 37 °C for 30 min before analysis. Inhibition of FA synthesis was done by treating cells with cerulenin (10 µg ml−1; Alexis Biochemicals, Lausen, Switzerland) for 6 h, or with equal volume of dimethyl sulfoxide (DMSO) as a control. Solid media contained 2% (w/v) agar. YPD plates with SFAs contained 1% Brij58 (Fluka), 0.5 mM palmitic acid and 0.5 mM stearic acid (Sigma). Non-supplemented plates contained only YPD and 1% Brij58. YPD plates containing 0.33 M acetate pH 6 were prepared by adding 3 M sodium acetate (Ambion) to 1 litre sterilized YPD. ATP synthase activity was inhibited by adding 0.5 µg µl−1 oligomycin to YPGly plates. Cloning into the pGFP33 plasmid was performed using the Gibson assembly kit (NEB). ARF1 point mutations were generated with the Quick-change Mutagenesis kit (NEB) and KLD (Kinase, Ligase and DpnI enzymes) reactions, primers were generated with the NEBaseChanger website (http://nebasechanger.neb.com). For LD anchoring of Arf1, we subcloned the first 143 amino acids (PAT domain) of the yeast perilipin Pln1 (ref. 43).
Cell culture
ARF1 KO HeLaα cells were established, mycoplasma tested and described elsewhere44. HeLa cells were grown in high-glucose Dulbecco’s modified Eagle’s medium (Sigma-Aldrich) with 10% foetal bovine serum (FBS, Biowest), 2 mM l-glutamine, 100 U ml−1 penicillin G and 100 ng ml−1 streptomycin, 1 mM sodium pyruvate at 37 °C and 7.5% CO2. For transient cell transfections, cells were plated into six-well plates to reach 70% confluency the following day and transfected with 1 µg plasmid DNA complexed with Helix-IN transfection reagent (OZ Biosciences).
Yeast transformation
Three units of OD600 (optical density at 600 nm) of yeast cells were grown in appropriate YPD or HC media to mid-log phase. Cells were spun down and washed in 1 volume of 1× TE and 10 mM LiAc. The pellet was then resuspended in 350 µl of transformation mix (1× TE, 100 mM LiAc, 8.5% (v/v) single-stranded DNA and 70% (v/v) PEG3000), incubated with DNA (PCR product or plasmid) for 1 h at 42 °C, spun down (30 s at 10,000g at room temperature) and resuspended in 100 μl of YPD or HC media, and cells were plated onto selective media and incubated at 23 °C or 30 °C. Genomic tagging was done according to standard procedures74.
HeLa cell lines survival assay
Cells were seeded in 12-well plates at a density of 55,000 cells per well, which was confirmed by re-counting. Every 24 h for 6 consecutive days, cells from one well for each cell line were trypsinized, and resuspended in phosphate-buffered saline (PBS) complemented with 2% FCS, and GFP fluorescence from 100,000 cells per sample was measured by a Fortessa flow cytometer. After 3 days, all cell lines were trypsinized, diluted to 1:10 and transferred into fresh media.
Microscopy
Fluorescence and DIC images were acquired with an ORCA-Flash 4.0 camera (Hamamatsu) mounted on an Axio Imager.M2 fluorescence microscope with a 63× Plan-Apochromat objective (Carl Zeiss) and an HXP 120C light source with ZEN 2.6 software. Image processing was performed using OMERO.insight client, and analysed with Fiji software. Measurement of number and length of contact sites on TEM images was done with Fiji software.
High-resolution images were acquired with an ORCA-Flash 4.0 camera (Hamamatsu) mounted on a FEI-MORE microscope with a 100× U Plan-S-Apochromat objective (Olympus).
To image Pex11-mScarlet strains, high-resolution imaging was performed at 23 °C and 37 °C using an automated inverted fluorescence microscope system (Olympus) harbouring a spinning disk high-resolution module (Yokogawa CSU-W1 SoRa confocal scanner with double microlenses and 50 µm pinholes). Images of cells in 96-well plates were made using a 60× oil lens (numerical aperture 1.42) and a Hamamatsu ORCA-Flash 4.0 camera. All images were taken in a Z-stack using cellSens software. Best focal planes were deconvoluted using cellSens software, and single planes or Z-projections of maximum intensity images were processed with the Fiji software.
In HeLa cell lines, mitochondria were stained by immunofluorescence using TOM20 antibody (1:200, Santa Cruz sc-17764) as marker, GM130 antibody (1:1,000, Cell Signalling 12480S) was used as Golgi marker, β-COP (1:500, gift from the Wieland lab) as COPI vesicles marker and CLIMP63 (1:1,000, gift from the Hauri lab) as ER marker. Secondary mouse (1:500) and rabbit (1:500) Alexa-Fluor 568 (A10037 and 110042, respectively, Invitrogen) antibodies were used and mounted with Fluoromount-G mounting medium (Thermo Fischer) containing 4′,6-diamidino-2-phenylindole. Images were acquired using a LSM700 Upright confocal laser-scanning microscope with the Zen 2.6 software (Zeiss) equipped with a Plan-Apochromat 63×/1.4 oil-immersion objective lens and two photomultiplier tubes.
To image mArf1 at LD–mitochondria and peroxisomes–mitochondria contact sites in HeLa cells, Arf1 KO cells were transfected with Arf1-EGFP and mPlum-PTS1. LDs and mitochondria were stained with Lipi-Blue (Dojindo Laboratories) staining and MitoTracker Deep Red (Invitrogen) dye respectively. Cells were imaged at 37 °C with a Zeiss Axio Observer wide-field microscope with a Plan-Apochromat N 63×/1.40 oil DIC M27 objective and a Photometrics Prime 95B camera. Filters with standard specifications for GFP, TexasRed and Cy5 were used to image Lipi-Blue, Arf1-EGFP, mPlum-PTS1 and MitoTracker Deep Red dye, respectively. Z-stack images were deconvolved with Huygens Professional software using the standard deconvolution method.
Protein extraction and immunoblot analysis
For yeast cells, 10 ml of mid-log grown cultures were lysed at 4 °C in breaking buffer containing 50 mM Tris–HCl pH 8, 300 mM NaCl, 0.6% Triton X-100, 1 mM dithiothreitol (DTT) and 9 M urea, supplemented with half-volume of glass beads (0.25–0.5 mm; ROTH). Cell debris and unbroken cells were pelleted by centrifugation 3,000g for 5 min at room temperature. Equal protein concentration were loaded on 12% or 15% SDS–PAGE and transferred onto 0.45 µm nitrocellulose membranes (Amersham). Membranes were blocked with TBST (20 mM Tris, 150 mM NaCl, pH 7.6 and 0.1% Tween20) with 5% non-fat dry milk for 30 min and incubated with anti-HA primary antibody (1:5,000, Eurogentec 16B12) or anti-Pgk1 primary antibody (1:5,000, Invitrogen clone 22C5D8) overnight at 4 °C, followed by 2 h incubation with horseradish peroxidase (HRP)-conjugated secondary antibody (1:10,000; anti-mouse, Invitrogen 31430) in TBST. Chemiluminescence signals were detected using Immobilon Western HRP Substrate (Millipore) and imaged using a FusionFX (Vilber Lourmat).
Alternatively, before Gga2GAT interaction, yeast cells were resuspended in 1 ml of 0.2 M sorbitol, 25 mM KPO4 pH 7, 2 mM ethylenediaminetetraacetic acid (EDTA), 0.6% Triton X-100, 1× Halt proteases inhibitor cocktail (Thermo Scientific), transferred to Corex glass tubes filled with 500 µl glass beads (0.25–0.5 mm; ROTH) and broken 15 min by vortexing at 4 °C with 30 s intervals on ice. Unbroken cells and debris were pelleted at 3,000g for 5 min at 4 °C and supernatants (SNs) were transferred into new 1.5 ml Eppendorf tubes and pelleted at 100,000g for 30 min at 4 °C in a TLA 100-3 rotor. One millilitre of SN (S100) was saved for each sample, and the pellets (P100) were resuspended in 500 µl of Lysis buffer.
HeLa cell were lysed in 20 mM Tris–HCl pH 7.5, 150 mM NaCl, 10 mM MgCl2, 1% Triton X-100, 2 mM phenylmethylsulfonyl fluoride (PMSF) and protease inhibitors, separated by 15% SDS–PAGE and transferred to Immobilon-P polyvinylidene difluoride membranes (Millipore). Membranes were blocked with TBST (20 mM Tris, 150 mM NaCl, pH 7.6 and 0.1% Tween20) with 5% non-fat dry milk for 1 h and incubated with primary antibody in TBST with 1% milk overnight at 4 °C: anti-Arf1 (1:2,500, Abnova MAB10011), and anti-actin (1:100,000, Sigma-Aldrich MAB1501). After washing, the membranes were incubated with HRP-conjugated secondary antibody (1:10,000; anti-rabbit, Sigma-Aldrich A0545 or anti-mouse, Sigma-Aldrich A0168) in TBST with 1% milk. Chemiluminescence signals were detected using Immobilon Western HRP Substrate (Millipore) and imaged using a FusionFX (Vilber Lourmat).
Co-IP
Yeast cells were grown to mid-log phase, and 4 × 108 cells were lysed at 4 °C in IP buffer containing 25 mM Tris–HCl pH 7.5; 150 mM NaCl, 2 mM EDTA, 0.6% Triton X-100, 1 mM DTT, 1× protease inhibitor, supplemented with 100 µl glass beads (0.25–0.5 mm; ROTH). Cell debris and unbroken cells were pelleted by centrifugation 3,000g for 5 min at 4 °C. The SN was then supplemented with 25 µl anti-GFP magnetic beads (Chromotek) and incubated 1 h at 4 °C under rotation. Beads were then washed four times in IP buffer, and proteins were eluted with 2× Laemmli buffer at 95 °C for 5 min.
Arf1-Gga2 recruitment
Gga2GAT expression and purification
One litre of Escherichia coli BL21 strains harbouring the pGEX4-GST-GGA2GAT were induced with 0.5 mM isopropyl β-d-1-thiogalactopyranoside and cells were transferred from 37 °C to 30 °C for 3.5 h. Cells were then pelleted at 4,000g at room temperature for 10 min, resuspended in 20 ml ice-cold 1× PBS/5 mM EDTA buffer (2.7 mM KCl, 1.5 mM KH2PO4, 137 mM NaCl, 5.6 mM Na2HPO4, 1.4 mM NaH2PO4 and 5 mM EDTA/NaOH pH 8.0) containing 1 mM PMSF and 1× Halt protease inhibitors. The resuspended cells were lysed by sonication seven times for 10 s (50% duty) on ice, cleared at 6,000g at 4 °C for 30 min. The SN was transferred to ultracentrifuge tubes and further cleared at 100,000g for 1 h at 4 °C. To isolate GST-fusion proteins the SN was added to 500 μl glutathione Sepharose magnetic beads and incubated for 1 h at 4 °C under rotation. Glutathione Sepharose beads were spun down at 500g for 5 min and washed three times with 15 ml ice-cold 1× PBS/5 mM EDTA and twice with 1 ml 1× PBS/5 mM EDTA on a magnetic stand. Bound proteins were eluted by three consecutive treatments with 250 μl reduced glutathione buffer (20 mM reduced glutathione and 100 mM Tris–HCl pH 8.0) at 4 °C for 10 min incubation each time. SNs were dialysed in 2.5 litre dialysis buffer (10 mM HEPES/NaOH pH 7.8, 1 mM MgCl2, 1 mM DTT and 0.2 mM PMSF) with slow stirring overnight at 4 °C. The next day, samples were centrifuged for 1 min at 20,000g at 4 °C to remove precipitates. The SNs were frozen in liquid nitrogen in aliquots of 80 μg protein and stored at −80 °C.
Gga2GAT pre-loading on glutathione magnetic beads
Purified GST-GGA2GAT was loaded onto glutathione (80 µg per tube). To do so, 200 µl of resuspended glutathione magnetic beads were taken, vortexed for 10 s and washed twice in 500 and 400 µl 1× PBS, 0.5 mM EDTA on a magnetic stand. Beads were then resuspended in 200 µl lysis buffer (0.2 M sorbitol, 25 mM KPO4 pH 7, 2 mM EDTA, 0.6% Triton X-100 and 1× Halt proteases inhibitor cocktail), and pure GST-GGA2GAT was added. Binding was done for 30 min on a rotating stand at 4 °C, followed by one wash in 300 µl lysis buffer, and final resuspension in 400 µl lysis buffer.
Binding and elution
S100 and P100 fractions of yeast cells were incubated with pre-bound GGA2GAT on beads for 1 h on a rotating wheel at 4 °C. Washes were done three times in 50 µl lysis buffer, and elution was done by adding 30 µl of Laemmli 2× to the beads and 5 min incubation at 95 °C. For each gel, 10 µl per lane was used for the lysis, flow-through and elution samples.
TEM
Cells were grown to mid-log phase and fixed into the YPD media with 0.2% glutaraldehyde and 3% formaldehyde final concentration overnight at 4 °C. The next day, cells were pelleted and washed three times with 0.1 M HEPES buffer pH 7 and incubated for 30 min in 1% NaJO4 in HEPES buffer, washed three times, and free aldehydes were quenched with 50 mM NH4Cl in HEPES buffer for 30 min. After that, pellets were dehydrated through a series of methanol (50%, 70%, 90%) then infiltrated with LR Gold resin (Polysciences) according to the manufacturer’s instructions and allowed to polymerize at −10 °C under an ultraviolet lamp for 24 h. Sections of 60–70 nm were collected on carbon-coated Formvar-Ni grids. Sections were blocked with PBST (PBS + 0.05% Tween20) + 2% bovine serum albumin (BSA) for 15 min and incubated 3 h at room temperature with anti-GFP antibody (1:100, 6556 Abcam) in PBST/BSA. Sections were washed 5 × 5 min with PBS and incubated with goat anti-rabbit secondary antibody (BBI) coupled to 10 nm gold 1:100 in PBST/BSA for 2 h, washed 5 × 5 min with PBS and 3 × 2 min H2O, and stained for 10 min in 2% Ur acetate and 1 min in Pb citrate (Reynold’s solution). Sections were viewed with a Philips CM100 electron microscope.
Mitochondria fusion and fission dynamics
Yeast cells were grown to mid-log phase in YPD media, and switched to 37 °C for 30 min when indicated. Movies were acquired either with an Axio Imager.M2 (ARF1 23–37 °C and arf1-11 at 37 °C) or the FEI-MORE (arf1-11 at 23 °C), over a period of 2 min with sequential image acquisition. Images from the FEI-MORE were further deconvolved in standard mode using the Huygens Pro software. Movies were assembled with the Fiji software and single images prepared on the OMERO.insight client.
To analyse mitochondria dynamics in WT and ARF1 KO HeLa cells parental and ARF1 KO cells were seeded on a six-well plate. For rescuing the absence of Arf1 phenotype, we transfected the KO cells with 1 µg Arf1-EGFP or Arf1-11-EGFP constructs using Helix IN transfection reagent. After overnight incubation, cells were reseeded on Ibidi four-well imaging chambers and imaged the next day. Just before imaging, mitochondria were stained with 250 nM MitoTracker Deep Red for 30 min following the provider’s instructions. Staining solution was replaced with imaging medium containing OxyFluor anti-phototoxicity agent.
Cells were imaged with Olympus Spinning Disk confocal SpinSR microscope equipped with Yokogawa CSU-W1 confocal scan head using UPL Apo 100×/1.5 oil immersion objective. To avoid phototoxicity, we used 640 nm laser with 1% laser power. During aquisition, seven Z-stacks with 120 nm optical steps were taken for 2 min in every 3 s. Time-lapse movies were deconvolved with Huygens Remote Manager using Good’s Roughness Maximum Likelihood Estimation, 50 iterations, quality control criteria 0.0002. Fusion and fission events were quantified in 10.01 × 10.01-sized region of interest in each cell for 1 min using FIJI software.
Fluorescent FA pulse-chase
Yeast cells were grown to mid-log phase in HC complete medium at 23 °C, then switched to 37 °C for 30 min. Following this, 1.5 × 106 cells were incubated with 50 µM BODIPY 558/568 C12 (Invitrogen) for 30 min at 37 °C, washed twice in 1× PBS, 7.5 µM BSA and resuspended in 20 µl 1× PBS, 7.5 µM BSA supplemented with 20 µl 0.4% Trypan blue in 1× PBS. Images were taken with the FEI-MORE microscope, and deconvolved in standard mode using the Huygens Pro software.
FA pulse-chase experiments in HeLa cells were performed according to Rambold et al.14. In short, transfected cells were plated into eight-well imaging chambers (Miltenyi) and incubated for at least 6 h to let the cells adhere. Then their CM was replaced with CM containing 1 μM BODIPY 558/568 C12 (Invitrogen) and incubated overnight. Cells were washed three times with CM, incubated for 1 h in CM and then chased for 9 h in CM or in HBSS. Mitochondria were labelled with 200 nM MitoView Fix 640 (Biotinum) for 20 min before imaging. Just before imaging, CM or HBSS was replaced with imaging buffer containing 25 mM dextrose supplemented with 10% FBS or 5 mM dextrose, supplemented with 0.2% FBS, respectively.
Cells were imaged at 37 °C using an inverted Axio Observer microscope (Zeiss) with a Plan-Apochromat N 63×/1.40 oil DIC M27 objective and a Photometrics Prime 95B camera. Filters with standard specifications for GFP, dsRed and Cy5 were used to image EGFP, Bodipy 558/568 and Mitoview 640, respectively.
LD staining
Neutral lipids of mid-log grown cells in YPD media were stained with 1 µl of the lipophilic fluorophore ReadiStain Lipid Green (InVivo Biosystems) for 30 min at 23 °C and 37 °C. Cells were then washed three times in HC complete medium and prepared for microscopy.
Transfected HeLa cells were plated into eight-well imaging chambers (Ibidi, ibiTreat µ-Slide) the day before imaging to reach 50–70% confluency the following day. Just before imaging, cells were rinsed with pre-warmed PBS and replaced with imaging buffer (4.5 g l−1 dextrose, 1 mM CaCl2, 2.7 mM KCl and 0.5 mM MgCl2 in PBS supplemented with 0.2% FBS) containing 400 ng ml−1 Nile Red (Sigma) and incubated for 10 min before starting imaging. The dye was present during imaging. Confocal images were acquired at 37 °C with Olympus Fluoview FV3000 system, using an UPLSAPO 60×/1.30 objective with silicone oil, resulting in an xy pixel size of 0.1 μm. Laser intensities were at 0.5–3% for both 488 (GFP) and 561 (DsRed) wavelengths. Sampling speed was 8.0 μs per pixel with a zoom factor of 2.0. All images for corresponding experiments were processed with the same settings to ensure comparable results.
Total RNA isolation and qRT–PCR
Total RNA were extracted from 25 ml of mid-log grown cultures. Cell pellets were mixed with 300 µl AE buffer (50 mM NaOAC and 10 mM EDTA, pH 6) and 50 µl of 20% SDS. Then 300 µl of phenol–chloroform isoamyl alcohol pH 4.5 was added to the mixture, and tubes were vortexed for 15 s. Then samples were incubated at 65 °C for 5 min and flash-frozen in liquid nitrogen. Samples were centrifuged for 10 min at 20,000g at 4 °C. The upper aqueous phase was recovered and put in a new low-binding tube with 200 µl phenol–chloroform isoamyl alcohol. Tubes were again vortexed for 10 s, centrifuged for 10 min at 20,000g at 4 °C and the aqueous layer recovered. To that phase, 20 µl 3 M sodium acetate and 600 µl ethanol was added, and the mixture was chilled at −80 °C for 2 h. Samples were then centrifuged 20,000g for 30 min at 4 °C, and RNA pellets washed with 75% ethanol. Air-dried RNAs were dissolved in 50 µl water.
Four micrograms of total RNA was subjected to reverse transcription (200 ng µl−1 final RNA concentration) with the MegaScript reverse transcription mix using random (0.5 µg) and oligo-dT primers (0.5 µg) (Promega). Reactions were then incubated 5 min at 25 °C, followed by 1 h at 62 °C and 15 min at 75 °C. Using 100 ng of complementary DNA, qPCR was carried out using the GoTaq master mix. Oligonuceotides sequences are available as Supplementary Table 3.
Lipid extraction from yeast cell pellets
Lipid extraction and analysis were performed in triplicates. Frozen cell pellets (5 × 108 cells) were taken from −80 °C and 500 µl glass beads were added, then 20 µl of yeast internal standard mix and 20 µl cholesterol were added (see below), followed by 600 µl H2O, 1.5 ml methanol, then vortexed for 1 min. Then 0.75 ml chloroform was added, and the samples were vortexed at high speed for 6 min. The solution was transferred to a 13 × 100 mm screw-capped glass tube, and the beads were washed with 0.6 ml chloroform:methanol (1:2). Following vortexing, the solution was combined with the first extraction. Then 0.4 ml H2O was added and mixed by vortexing. Samples were centrifuged for 10 min at 4,000 r.p.m. (3,220g) and most of the aqueous phase was removed, followed by transfer of the organic phase to fresh 13 × 100 tubes without taking interface. Then 0.4 ml of artificial upper phase (chloroform:methanol:water (3:48:47, v/v/v)) was added and mixed by vortexing. After centrifugation for 10 min at 4,000 r.p.m. and removal of most of the aqueous phase, the organic phase was transferred to mass spectrometry (MS) vials. Then 0.3–0.4 ml was transferred to a vial insert (filled up) for analysis using the Thermo Q-Exactive Plus. The extracts in inserts were dried in the Centrivap (Labconco) and the extracts in vials under flow of N2. Internal standards used were PC31:1 (7.5 nmol per sample), PE31:1 (7.5 nmol per sample), PI31:1 (6 nmol per sample), PS31:1 (4 nmol per sample), CL56:0 (4 nmol per sample), C17Cer (1.2 nmol per sample), C8GC (2 nmol per sample) and cholesterol (20 nmol per sample).
Sterol analysis by gas chromatography–MS
Lipid extracts from approximately 40% of the total sample was dissolved in 0.3 ml chloroform:methanol (1:1), and 5 µl was loaded onto a gas chromatograph–mass spectrometer (Varian 320 MS) fitted with a fused silica capillary column (15 m × 0.32 mm inner diameter, Macherey Nagel ref. 726206.15). The gas chromatography program started at 45 °C for 4 min, then a gradient to 195 °C at 20 °C min−1, to 230 °C at 4 °C min−1, to 320 °C at 10 °C min−1, to 350 °C at 6 °C min−1 followed by a return to 45 °C at 100 °C min−1. Data were collected in the centroid mode. The different sterol species were identified by their retention times and mass spectra using the NIST database and ergosterol and cholesterol standards. Peaks were integrated and amounts calculated with correction for the yield of the internal standard, cholesterol, and using standard curves of cholesterol and ergosterol concentrations.
Lipid analysis by electrospray MS using the TSQ Vantage
Lipid analysis using nanoflow infusion (Advion Nanomate) and multiple reaction monitoring (TSQ Vantage, Thermo) was performed as described75. All runs were performed in duplicates with all transitions measured three times for a total of at least six measurements for each lipid species.
TAG analysis using the UHPLC–MS using the Q-Exactive Plus
Dried samples were resuspended by sonicating in 100 µL of liquid chromatography–MS-grade chloroform:methanol (1:1, v/v). Reversed-phase ultrahigh-performance liquid chromatography (UHPLC)–high-resolution MS analyses were performed using a Q-Exactive Plus Hybrid Quadrupole-Orbitrap mass spectrometer coupled to an UltiMate 3000UHPLC system (Thermo Fisher Scientific) equipped with an Accucore C30 column (150 × 2.1 mm, 2.6 μm) and its 20 mm guard (Thermo Fisher Scientific). Samples were kept at 8 °C in the autosampler, 10 μl was injected and eluted with a gradient starting at 10% B for 1 min, 10–70% B in 4 min, 70–100% B in 10 min, washed in 100% B for 5 min and column equilibration for an additional 3 min. Eluents were made of 5 mM ammonium acetate and 0.1% formic acid in water (solvent A) or in isopropanol/acetonitrile (2:1, v/v) (solvent B). Flow rate and column oven temperature were respectively at 350 μl min−1 and 40 °C. The mass spectrometer was operated using a heated electrospray-ionization source in positive polarity with the following settings: electrospray voltage: 3.9 kV (+); sheath gas: 51; auxiliary gas: 13; sweep gas: 3; vapourizer temperature: 431 °C; ion transfer capillary temperature: 320 °C; S-lens: 50; resolution: 140,000; m/z range: 200–1,000; automatic gain control: 1 × 106; maximum injection time: 50 ms. The following setting was used in Higher-energy C-trap dissociation fragmentation: automatic gain control: 2.5 × 105; maximum injection time: 120 ms; resolution: 35,000; (N)CE: 30. Xcaliburv.4.2 (Thermo Fisher Scientific) was used for data acquisition and processing. Major TAG species were quantified after removing background and normalization, and was presented as ratio to sample no. 1, respectively.
TAG analysis by thin liquid chromatography
ARF1 and arf1-11 strains were grown overnight at 23 °C in YPUAD media, and diluted to 0.2 optical density (OD) in the morning. When cells reached OD 0.7, cells were treated either with cerulenin (10 µg ml−1) or with the corresponding volume of DMSO. After 30 min of incubation at 23 °C, 1.5 × 108 cells were collected. Then cells were shifted to 37 °C and 1.5 × 108 cells were collected after 30 and 60 min of incubation. Lipids were extracted as described above. Dried lipid extracts were thawed and resuspended in 20 µl chlorophorm. Triolein (18:1 TG; Avanti Polar Lipids) was used as an internal marker. Samples were then spotted onto thin-liquid chromatography silica plates (aluminium sheets gel 60; Sigma-Aldrich), and neutral lipids separted by running in two successive chambers containing petroleum ether/diethyl ether (1:1, v/v) and petroleum ether/diethyl ether (49:1, v/v). For detection, plates were dipped into a solution containing MnCl2, methanol and sulfuric acid (0.63 g MnCl2.4H2O, 60 ml water, 60 ml methanol, 4 ml concentrated sulfuric acid) for 1 min and heated at 110 °C for 3 min. Plates were then scanned and analysed using the Fiji software.
ATP measurements
FRET measurement
ATP levels were measured in single cells grown to mid-log phase using a FRET-based nanosensor expressed from a cen/ars plasmid, pDR-GW AT1.03YEMK, (Addgene no. 28004). Images were acquired with the Axio Imager.M2, using dedicated CFP, YFP and FRET CFP-YFP filters. Regions of interest were measured with the Fiji software.
Biochemical assay
ATP quantitation was performed using the BacTiter-Glo Cell viability assay (Promega). Cells were resuspended in TE buffer pH 7 containing 0.7 M sorbitol and 106 cells were used for each assay. Luminescence was measured right after mixing cells with BacTiter-Glo buffer in 96× flat-white plates (Greiner) on a Tecan Infinite M1000Pro with 10 s orbital shaking (2 mm wide, 350 r.p.m.).
Mitochondrial activity measurements
Mitochondria were isolated from WT and mutant strains grown for five to six generations in 2 litres of YPGly at 23 °C or 28 °C by enzymatic method76. The cultures contained 2–5% of ρ−/ρ° cells. The values reported are averages of two biological repetitions. Respiratory, ATP synthesis activities and the variations of inner membrane potential were measured using freshly isolated, osmotically protected mitochondria buffered at pH 6.8. The oxygen consumption was measured in an oxygraph with Clarck electrode (Heito, France) at 28 °C in thermo-stabilized cuvette. Reaction mixes for assays contained 0.15 mg ml−1 of mitochondria, 4 mM NADH, 150 μM ADP, 12.5 mM ascorbate, 1.4 mM N,N,N,N,-tetramethyl-p-phenylenediamine and 4 μM carbonyl cyanide m-chlorophenyl hydrazone (CCCP). The rates of ATP synthesis were determined under the same experimental conditions in the presence of 750 µM ADP; aliquots were withdrawn from the oxygraph cuvette every 15 s and the reaction was stopped with 3.5% (w/v) perchloric acid, 12.5 mM EDTA. The ATP in samples was quantified using the Kinase-Glo Max Luminescence Kinase Assay (Promega) and a Beckman Coulter’s Paradigm Plate Reader. Variations in transmembrane potential (Δψ) were evaluated in the respiration buffer containing 0.150 mg ml−1 of mitochondria and the Rhodamine 123 (0.5 μg ml−1), with λexc of 485 nm and λem of 533 nm under constant stirring using a Cary Eclipse Fluorescence Spectrophotometer (Agilent Technologies)77. For the ATPase assays, mitochondria kept at −80 °C were thawed and the reaction was performed in absence of osmotic protection and at pH 8.4 (ref. 78).
Statistics and reproducibility
All experiments were performed at least in three independent biological replicates. Unpaired two-tailed t-test or two-way analysis of variance (ANOVA) were calculated for each experiment using GraphPad Prism9. For image analysis, pictures were taken at random places on coverslips, analysed in a blinded manner whenever possible with Omero webclient. All images are representative images from at least three independent biological experiments. For western blot, loading controls were run on a separate gel when the protein of interest had a similar molecular weight.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Online content
Any methods, additional references, Nature Portfolio reporting summaries, source data, extended data, supplementary information, acknowledgements, peer review information; details of author contributions and competing interests; and statements of data and code availability are available at 10.1038/s41556-023-01180-2.
Supplementary information
Supplementary Data 1 relative to FACS gating strategy and its legend.
Mitochondria fusion and fission monitored in yArf1–GFP expressing strain together with the mitochondrial protein Tom70 fused to mCherry at 23 °C. White arrows indicate sites of fission and yellow arrows fusion.
Mitochondria fusion and fission monitored in yArf1–GFP expressing strain together with the mitochondrial protein Tom70 fused to mCherry at 37 °C. White arrows indicate sites of fission and yellow arrows fusion.
Mitochondria fusion and fission monitored in yArf1-11–GFP expressing strain together with the mitochondrial protein Tom70 fused to mCherry at 23 °C. White arrows indicate sites of fission and yellow arrows fusion.
Mitochondria fusion and fission monitored in yArf1-11–GFP expressing strain together with the mitochondrial protein Tom70 fused to mCherry at 37 °C. White arrows indicate sites of fission and yellow arrows fusion.
Mitochondria fusion and fission monitored in the WT strain expressing the mitochondrial protein Tom70 fused to mCherry at 23 °C. White arrows indicate sites of fission and yellow arrows fusion.
Mitochondria fusion and fission monitored in the WT strain expressing the mitochondrial protein Tom70 fused to mCherry at 37 °C. White arrows indicate sites of fission and yellow arrows fusion.
Mitochondria fusion and fission monitored in the ∆yarf1 strain expressing the mitochondrial protein Tom70 fused to mCherry at 23 °C. White arrows indicate sites of fission and yellow arrows fusion.
Mitochondria fusion and fission monitored in the ∆yarf1 strain expressing the mitochondrial protein Tom70 fused to mCherry at 37 °C. White arrows indicate sites of fission and yellow arrows fusion.
Mitochondria fusion and fission monitored in ER-yArf1–GFP expressing strain together with the mitochondrial protein Tom70 fused to mCherry at 23 °C. White arrows indicate sites of fission and yellow arrows fusion.
Mitochondria fusion and fission monitored in ER-yArf1–GFP expressing strain together with the mitochondrial protein Tom70 fused to mCherry at 37 °C. White arrows indicate sites of fission and yellow arrows fusion.
Mitochondria fusion and fission monitored in ER-yArf1-11–GFP expressing strain together with the mitochondrial protein Tom70 fused to mCherry at 23 °C. White arrows indicate sites of fission and yellow arrows fusion.
Mitochondria fusion and fission monitored in ER-yArf1-11–GFP expressing strain together with the mitochondrial protein Tom70 fused to mCherry at 37 °C. White arrows indicate sites of fission and yellow arrows fusion.
Mitochondria fusion and fission monitored in parental HeLa cells using MitoTracker Deep Red. White arrows indicate sites of fission and yellow arrows fusion.
Mitochondria fusion and fission monitored in ARF1 KO HeLa cells using MitoTracker Deep Red. White arrows indicate sites of fission and yellow arrows fusion.
Mitochondria fusion and fission monitored in ARF1 KO HeLa cells expressing mArf1-GFP using MitoTracker Deep Red. White arrows indicate sites of fission and yellow arrows fusion.
Mitochondria fusion and fission monitored in ARF1 KO HeLa cells expressing mArf1-11-GFP using MitoTracker Deep Red. White arrows indicate sites of fission and yellow arrows fusion.
Supplementary Tables 1 (strains used in this study), 2 (plasmids used in this study) and 3 (list of primers used for RT–qPCR).
Acknowledgements
We thank A. Gil and H. Wechlin, S. Begum and S. Feng for excellent technical assistance with some of the experiments and F. Wieland (BZH, Heidelberg) for β-COP antibodies. This work was supported by grants of the Swiss National Science Foundation (310030B_163480 and 310030_185127) to A.S. and the University of Basel, Swiss National Science Foundation, NCCR Chemical Biology and the Leducq Foundation to H.R., Swiss National Science Foundation (31003A-182519) to M.S., and the National Science Center of Poland (2018-31-B-NZ3-01117) to R.K.
Extended data
Source data
Unprocessed western blots for all relevant figures.
Statistical source data for all relevant figures.
Author contributions
L.E. and A.S. conceived the project. L.E. performed all yeast cell biology experiments, except for the Pex11–scarlet/Arf1–GFP imaging, which was performed by R.E.A. and E.Z. V.S. and M.P. performed the experiments in mammalian cells. I.R. and H.R. did the lipidomics analyses. A.W. and R.K. carried out the biochemical analyses on yeast mitochondria. The EM experiments were executed by C.P.-B. Data were analysed by L.E., V.S., R.K., H.R., M.S. and A.S. L.E. and A.S. wrote the manuscript with input from all authors.
Peer review
Peer review information
Nature Cell Biology thanks the anonymous reviewers for their contribution to the peer review of this work. Peer reviewer reports are available.
Data availability
The authors declare that the main data supporting the findings of this study are available within the article and its supplementary information files. Source data are provided with this paper. All other data supporting the findings of this study are available from the corresponding author upon request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
is available for this paper at 10.1038/s41556-023-01180-2.
Supplementary information
The online version contains supplementary material available at 10.1038/s41556-023-01180-2.
References
- 1.Spang A. Means of intracellular communication: touching, kissing, fusing. Microb. Cell. 2021;8:87–90. doi: 10.15698/mic2021.05.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zung N, Schuldiner M. New horizons in mitochondrial contact site research. Biol. Chem. 2020;401:793–809. doi: 10.1515/hsz-2020-0133. [DOI] [PubMed] [Google Scholar]
- 3.Silva BSC, et al. Maintaining social contacts: the physiological relevance of organelle interactions. Biochim. Biophys. Acta Mol. Cell. Res. 2020;1867:118800. doi: 10.1016/j.bbamcr.2020.118800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tamura Y, Kawano S, Endo T. Organelle contact zones as sites for lipid transfer. J. Biochem. 2019;165:115–123. doi: 10.1093/jb/mvy088. [DOI] [PubMed] [Google Scholar]
- 5.Wu H, Carvalho P, Voeltz GK. Here, there, and everywhere: the importance of ER membrane contact sites. Science. 2018;361:eaan5835. doi: 10.1126/science.aan5835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spang A, Mayor S. Editorial overview: membranes and organelles: rethinking membrane structure, function and compartments. Curr. Opin. Cell Biol. 2018;53:A1–A3. doi: 10.1016/j.ceb.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 7.Stefan CJ, et al. Membrane dynamics and organelle biogenesis-lipid pipelines and vesicular carriers. BMC Biol. 2017;15:102. doi: 10.1186/s12915-017-0432-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petkovic M, O’Brien CE, Jan YN. Interorganelle communication, aging, and neurodegeneration. Genes Dev. 2021;35:449–469. doi: 10.1101/gad.346759.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen Y, et al. Peroxisomes are juxtaposed to strategic sites on mitochondria. Mol. Biosyst. 2014;10:1742–1748. doi: 10.1039/C4MB00001C. [DOI] [PubMed] [Google Scholar]
- 10.Shai N, et al. Systematic mapping of contact sites reveals tethers and a function for the peroxisome–mitochondria contact. Nat. Commun. 2018;9:1761. doi: 10.1038/s41467-018-03957-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pu J, et al. Interactomic study on interaction between lipid droplets and mitochondria. Protein Cell. 2011;2:487–496. doi: 10.1007/s13238-011-1061-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schuldiner M, Bohnert M. A different kind of love—lipid droplet contact sites. Biochim. Biophys. Acta Mol. Cell. Biol. Lipids. 2017;1862:1188–1196. doi: 10.1016/j.bbalip.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 13.Herms A, et al. AMPK activation promotes lipid droplet dispersion on detyrosinated microtubules to increase mitochondrial fatty acid oxidation. Nat. Commun. 2015;6:7176. doi: 10.1038/ncomms8176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rambold AS, Cohen S, Lippincott-Schwartz J. Fatty acid trafficking in starved cells: regulation by lipid droplet lipolysis, autophagy, and mitochondrial fusion dynamics. Dev. Cell. 2015;32:678–692. doi: 10.1016/j.devcel.2015.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cui L, Liu P. Two types of contact between lipid droplets and mitochondria. Front. Cell Dev. Biol. 2020;8:618322. doi: 10.3389/fcell.2020.618322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang CL, et al. Spastin tethers lipid droplets to peroxisomes and directs fatty acid trafficking through ESCRT-III. J. Cell Biol. 2019;218:2583–2599. doi: 10.1083/jcb.201902061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Binns D, et al. An intimate collaboration between peroxisomes and lipid bodies. J. Cell Biol. 2006;173:719–731. doi: 10.1083/jcb.200511125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim S, et al. Dysregulation of mitochondria–lysosome contacts by GBA1 dysfunction in dopaminergic neuronal models of Parkinson’s disease. Nat. Commun. 2021;12:1807. doi: 10.1038/s41467-021-22113-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dziurdzik SK, Conibear E. The Vps13 family of lipid transporters and its role at membrane contact sites. Int. J. Mol. Sci. 2021;22:2905. doi: 10.3390/ijms22062905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spang A. ARF1 regulatory factors and COPI vesicle formation. Curr. Opin. Cell Biol. 2002;14:423–427. doi: 10.1016/S0955-0674(02)00346-0. [DOI] [PubMed] [Google Scholar]
- 21.Arakel EC, et al. Dissection of GTPase-activating proteins reveals functional asymmetry in the COPI coat of budding yeast. J. Cell Sci. 2019;132:jcs232124. doi: 10.1242/jcs.232124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trautwein M, et al. Arf1p provides an unexpected link between COPI vesicles and mRNA in Saccharomyces cerevisiae. Mol. Biol. Cell. 2004;15:5021–5037. doi: 10.1091/mbc.e04-05-0411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kilchert C, et al. Defects in the secretory pathway and high Ca2+ induce multiple P-bodies. Mol. Biol. Cell. 2010;21:2624–2638. doi: 10.1091/mbc.e10-02-0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jewell JL, et al. Metabolism. Differential regulation of mTORC1 by leucine and glutamine. Science. 2015;347:194–198. doi: 10.1126/science.1259472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meng D, et al. Glutamine and asparagine activate mTORC1 independently of Rag GTPases. J. Biol. Chem. 2020;295:2890–2899. doi: 10.1074/jbc.AC119.011578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ackema KB, et al. The small GTPase Arf1 modulates mitochondrial morphology and function. EMBO J. 2014;33:2659–2675. doi: 10.15252/embj.201489039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walch L, et al. GBF1 and Arf1 interact with Miro and regulate mitochondrial positioning within cells. Sci. Rep. 2018;8:17121. doi: 10.1038/s41598-018-35190-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nagashima S, et al. Golgi-derived PI(4)P-containing vesicles drive late steps of mitochondrial division. Science. 2020;367:1366–1371. doi: 10.1126/science.aax6089. [DOI] [PubMed] [Google Scholar]
- 29.Yahara N, et al. Multiple roles of Arf1 GTPase in the yeast exocytic and endocytic pathways. Mol. Biol. Cell. 2001;12:221–238. doi: 10.1091/mbc.12.1.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakamura N, et al. ADRP is dissociated from lipid droplets by ARF1-dependent mechanism. Biochem. Biophys. Res. Commun. 2004;322:957–965. doi: 10.1016/j.bbrc.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 31.Bartz R, et al. Dynamic activity of lipid droplets: protein phosphorylation and GTP-mediated protein translocation. J. Proteome Res. 2007;6:3256–3265. doi: 10.1021/pr070158j. [DOI] [PubMed] [Google Scholar]
- 32.Beller M, et al. COPI complex is a regulator of lipid homeostasis. PLoS Biol. 2008;6:e292. doi: 10.1371/journal.pbio.0060292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soni KG, et al. Coatomer-dependent protein delivery to lipid droplets. J. Cell Sci. 2009;122:1834–1841. doi: 10.1242/jcs.045849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ellong EN, et al. Interaction between the triglyceride lipase ATGL and the Arf1 activator GBF1. PLoS ONE. 2011;6:e21889. doi: 10.1371/journal.pone.0021889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bouvet S, et al. Targeting of the Arf-GEF GBF1 to lipid droplets and Golgi membranes. J. Cell Sci. 2013;126:4794–4805. doi: 10.1242/jcs.134254. [DOI] [PubMed] [Google Scholar]
- 36.Wilfling F, et al. Arf1/COPI machinery acts directly on lipid droplets and enables their connection to the ER for protein targeting. eLife. 2014;3:e01607. doi: 10.7554/eLife.01607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lay D, et al. Binding and functions of ADP-ribosylation factor on mammalian and yeast peroxisomes. J. Biol. Chem. 2005;280:34489–34499. doi: 10.1074/jbc.M503497200. [DOI] [PubMed] [Google Scholar]
- 38.Anthonio EA, et al. Small G proteins in peroxisome biogenesis: the potential involvement of ADP-ribosylation factor 6. BMC Cell Biol. 2009;10:58. doi: 10.1186/1471-2121-10-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yofe I, et al. Pex35 is a regulator of peroxisome abundance. J. Cell Sci. 2017;130:791–804. doi: 10.1242/jcs.187914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhdankina O, et al. Yeast GGA proteins interact with GTP-bound Arf and facilitate transport through the Golgi. Yeast. 2001;18:1–18. doi: 10.1002/1097-0061(200101)18:1<1::AID-YEA644>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 41.Matto M, et al. Role for ADP ribosylation factor 1 in the regulation of hepatitis C virus replication. J. Virol. 2011;85:946–956. doi: 10.1128/JVI.00753-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ohsaki Y, et al. Inhibition of ADP-ribosylation suppresses aberrant accumulation of lipidated apolipoprotein B in the endoplasmic reticulum. FEBS Lett. 2013;587:3696–3702. doi: 10.1016/j.febslet.2013.09.036. [DOI] [PubMed] [Google Scholar]
- 43.Gao Q, et al. Pet10p is a yeast perilipin that stabilizes lipid droplets and promotes their assembly. J. Cell Biol. 2017;216:3199–3217. doi: 10.1083/jcb.201610013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pennauer M, et al. Shared and specific functions of Arfs 1–5 at the Golgi revealed by systematic knockouts. J. Cell Biol. 2022;221:e202106100. doi: 10.1083/jcb.202106100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moir RD, et al. SCS3 and YFT2 link transcription of phospholipid biosynthetic genes to ER stress and the UPR. PLoS Genet. 2012;8:e1002890. doi: 10.1371/journal.pgen.1002890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yap WS, et al. The yeast FIT2 homologs are necessary to maintain cellular proteostasis and membrane lipid homeostasis. J. Cell Sci. 2020;133:jcs248526. doi: 10.1242/jcs.248526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gaynor EC, et al. ARF is required for maintenance of yeast Golgi and endosome structure and function. Mol. Biol. Cell. 1998;9:653–670. doi: 10.1091/mbc.9.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Passreiter M, et al. Peroxisome biogenesis: involvement of ARF and coatomer. J. Cell Biol. 1998;141:373–383. doi: 10.1083/jcb.141.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Omura S. Cerulenin. Methods Enzymol. 1981;72:520–532. doi: 10.1016/S0076-6879(81)72041-X. [DOI] [PubMed] [Google Scholar]
- 50.Herms A, et al. Cell-to-cell heterogeneity in lipid droplets suggests a mechanism to reduce lipotoxicity. Curr. Biol. 2013;23:1489–1496. doi: 10.1016/j.cub.2013.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kassan A, et al. Acyl-CoA synthetase 3 promotes lipid droplet biogenesis in ER microdomains. J. Cell Biol. 2013;203:985–1001. doi: 10.1083/jcb.201305142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thumser AE, Storch J. Characterization of a BODIPY-labeled fluorescent fatty acid analogue. Binding to fatty acid-binding proteins, intracellular localization, and metabolism. Mol. Cell. Biochem. 2007;299:67–73. doi: 10.1007/s11010-005-9041-2. [DOI] [PubMed] [Google Scholar]
- 53.Wang YW, et al. Dihydronaphthalene-fused boron-dipyrromethene (BODIPY) dyes: insight into the electronic and conformational tuning modes of BODIPY fluorophores. Chemistry. 2010;16:2887–2903. doi: 10.1002/chem.200902527. [DOI] [PubMed] [Google Scholar]
- 54.Mattiazzi Usaj M, et al. Genome-wide localization study of yeast Pex11 identifies peroxisome–mitochondria interactions through the ERMES complex. J. Mol. Biol. 2015;427:2072–2087. doi: 10.1016/j.jmb.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huo Y, et al. The MFN1 and MFN2 mitofusins promote clustering between mitochondria and peroxisomes. Commun. Biol. 2022;5:423. doi: 10.1038/s42003-022-03377-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Twig G, Shirihai OS. The interplay between mitochondrial dynamics and mitophagy. Antioxid. Redox Signal. 2011;14:1939–1951. doi: 10.1089/ars.2010.3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.De Vos KJ, et al. Mitochondrial function and actin regulate dynamin-related protein 1-dependent mitochondrial fission. Curr. Biol. 2005;15:678–683. doi: 10.1016/j.cub.2005.02.064. [DOI] [PubMed] [Google Scholar]
- 58.Yu FY, et al. Citrinin induces apoptosis in HL-60 cells via activation of the mitochondrial pathway. Toxicol. Lett. 2006;161:143–151. doi: 10.1016/j.toxlet.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 59.Legros F, et al. Mitochondrial fusion in human cells is efficient, requires the inner membrane potential, and is mediated by mitofusins. Mol. Biol. Cell. 2002;13:4343–4354. doi: 10.1091/mbc.e02-06-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Miyazono Y, et al. Uncoupled mitochondria quickly shorten along their long axis to form indented spheroids, instead of rings, in a fission-independent manner. Sci. Rep. 2018;8:350. doi: 10.1038/s41598-017-18582-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bermejo C, et al. Dynamic analysis of cytosolic glucose and ATP levels in yeast using optical sensors. Biochem. J. 2010;432:399–406. doi: 10.1042/BJ20100946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Just WW, Peranen J. Small GTPases in peroxisome dynamics. Biochim. Biophys. Acta. 2016;1863:1006–1013. doi: 10.1016/j.bbamcr.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 63.Ferreira R, et al. Metabolic engineering of Saccharomyces cerevisiae for overproduction of triacylglycerols. Metab. Eng. Commun. 2018;6:22–27. doi: 10.1016/j.meteno.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Walther TC, Chung J, Farese RV., Jr. Lipid droplet biogenesis. Annu. Rev. Cell Dev. Biol. 2017;33:491–510. doi: 10.1146/annurev-cellbio-100616-060608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li D, et al. Excess diacylglycerol at the endoplasmic reticulum disrupts endomembrane homeostasis and autophagy. BMC Biol. 2020;18:107. doi: 10.1186/s12915-020-00837-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Choudhary V, et al. Architecture of lipid droplets in endoplasmic reticulum is determined by phospholipid intrinsic curvature. Curr. Biol. 2018;28:915–926 e9. doi: 10.1016/j.cub.2018.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xu N, et al. The FATP1–DGAT2 complex facilitates lipid droplet expansion at the ER–lipid droplet interface. J. Cell Biol. 2012;198:895–911. doi: 10.1083/jcb.201201139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Olzmann JA, Carvalho P. Dynamics and functions of lipid droplets. Nat. Rev. Mol. Cell Biol. 2019;20:137–155. doi: 10.1038/s41580-018-0085-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Boutant M, et al. Mfn2 is critical for brown adipose tissue thermogenic function. EMBO J. 2017;36:1543–1558. doi: 10.15252/embj.201694914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Benador IY, et al. Mitochondria bound to lipid droplets have unique bioenergetics, composition, and dynamics that support lipid droplet expansion. Cell Metab. 2018;27:869–885 e6. doi: 10.1016/j.cmet.2018.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Freyre CAC, et al. MIGA2 links mitochondria, the ER, and lipid droplets and promotes de novo lipogenesis in adipocytes. Mol. Cell. 2019;76:811–825 e14. doi: 10.1016/j.molcel.2019.09.011. [DOI] [PubMed] [Google Scholar]
- 72.Vrijsen S, et al. Inter-organellar communication in Parkinson’s and Alzheimer’s disease: looking beyond endoplasmic reticulum–mitochondria contact sites. Front. Neurosci. 2022;16:900338. doi: 10.3389/fnins.2022.900338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gasparian A, et al. Depletion of COPI in cancer cells: the role of reactive oxygen species in the induction of lipid accumulation, noncanonical lipophagy and apoptosis. Mol. Biol. Cell. 2022;33:ar135. doi: 10.1091/mbc.E21-08-0420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Janke C, et al. A versatile toolbox for PCR-based tagging of yeast genes: new fluorescent proteins, more markers and promoter substitution cassettes. Yeast. 2004;21:947–962. doi: 10.1002/yea.1142. [DOI] [PubMed] [Google Scholar]
- 75.Jimenez-Rojo N, et al. Conserved functions of ether lipids and sphingolipids in the early secretory pathway. Curr. Biol. 2020;30:3775–3787 e7. doi: 10.1016/j.cub.2020.07.059. [DOI] [PubMed] [Google Scholar]
- 76.Guerin B, Labbe P, Somlo M. Preparation of yeast mitochondria (Saccharomyces cerevisiae) with good P/O and respiratory control ratios. Methods Enzymol. 1979;55:149–159. doi: 10.1016/0076-6879(79)55021-6. [DOI] [PubMed] [Google Scholar]
- 77.Emaus RK, Grunwald R, Lemasters JJ. Rhodamine 123 as a probe of transmembrane potential in isolated rat-liver mitochondria: spectral and metabolic properties. Biochim. Biophys. Acta. 1986;850:436–448. doi: 10.1016/0005-2728(86)90112-X. [DOI] [PubMed] [Google Scholar]
- 78.Somlo M. Induction and repression of mitochondrial ATPase in yeast. Eur. J. Biochem. 1968;5:276–284. doi: 10.1111/j.1432-1033.1968.tb00368.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Data 1 relative to FACS gating strategy and its legend.
Mitochondria fusion and fission monitored in yArf1–GFP expressing strain together with the mitochondrial protein Tom70 fused to mCherry at 23 °C. White arrows indicate sites of fission and yellow arrows fusion.
Mitochondria fusion and fission monitored in yArf1–GFP expressing strain together with the mitochondrial protein Tom70 fused to mCherry at 37 °C. White arrows indicate sites of fission and yellow arrows fusion.
Mitochondria fusion and fission monitored in yArf1-11–GFP expressing strain together with the mitochondrial protein Tom70 fused to mCherry at 23 °C. White arrows indicate sites of fission and yellow arrows fusion.
Mitochondria fusion and fission monitored in yArf1-11–GFP expressing strain together with the mitochondrial protein Tom70 fused to mCherry at 37 °C. White arrows indicate sites of fission and yellow arrows fusion.
Mitochondria fusion and fission monitored in the WT strain expressing the mitochondrial protein Tom70 fused to mCherry at 23 °C. White arrows indicate sites of fission and yellow arrows fusion.
Mitochondria fusion and fission monitored in the WT strain expressing the mitochondrial protein Tom70 fused to mCherry at 37 °C. White arrows indicate sites of fission and yellow arrows fusion.
Mitochondria fusion and fission monitored in the ∆yarf1 strain expressing the mitochondrial protein Tom70 fused to mCherry at 23 °C. White arrows indicate sites of fission and yellow arrows fusion.
Mitochondria fusion and fission monitored in the ∆yarf1 strain expressing the mitochondrial protein Tom70 fused to mCherry at 37 °C. White arrows indicate sites of fission and yellow arrows fusion.
Mitochondria fusion and fission monitored in ER-yArf1–GFP expressing strain together with the mitochondrial protein Tom70 fused to mCherry at 23 °C. White arrows indicate sites of fission and yellow arrows fusion.
Mitochondria fusion and fission monitored in ER-yArf1–GFP expressing strain together with the mitochondrial protein Tom70 fused to mCherry at 37 °C. White arrows indicate sites of fission and yellow arrows fusion.
Mitochondria fusion and fission monitored in ER-yArf1-11–GFP expressing strain together with the mitochondrial protein Tom70 fused to mCherry at 23 °C. White arrows indicate sites of fission and yellow arrows fusion.
Mitochondria fusion and fission monitored in ER-yArf1-11–GFP expressing strain together with the mitochondrial protein Tom70 fused to mCherry at 37 °C. White arrows indicate sites of fission and yellow arrows fusion.
Mitochondria fusion and fission monitored in parental HeLa cells using MitoTracker Deep Red. White arrows indicate sites of fission and yellow arrows fusion.
Mitochondria fusion and fission monitored in ARF1 KO HeLa cells using MitoTracker Deep Red. White arrows indicate sites of fission and yellow arrows fusion.
Mitochondria fusion and fission monitored in ARF1 KO HeLa cells expressing mArf1-GFP using MitoTracker Deep Red. White arrows indicate sites of fission and yellow arrows fusion.
Mitochondria fusion and fission monitored in ARF1 KO HeLa cells expressing mArf1-11-GFP using MitoTracker Deep Red. White arrows indicate sites of fission and yellow arrows fusion.
Supplementary Tables 1 (strains used in this study), 2 (plasmids used in this study) and 3 (list of primers used for RT–qPCR).
Data Availability Statement
The authors declare that the main data supporting the findings of this study are available within the article and its supplementary information files. Source data are provided with this paper. All other data supporting the findings of this study are available from the corresponding author upon request.