Abstract
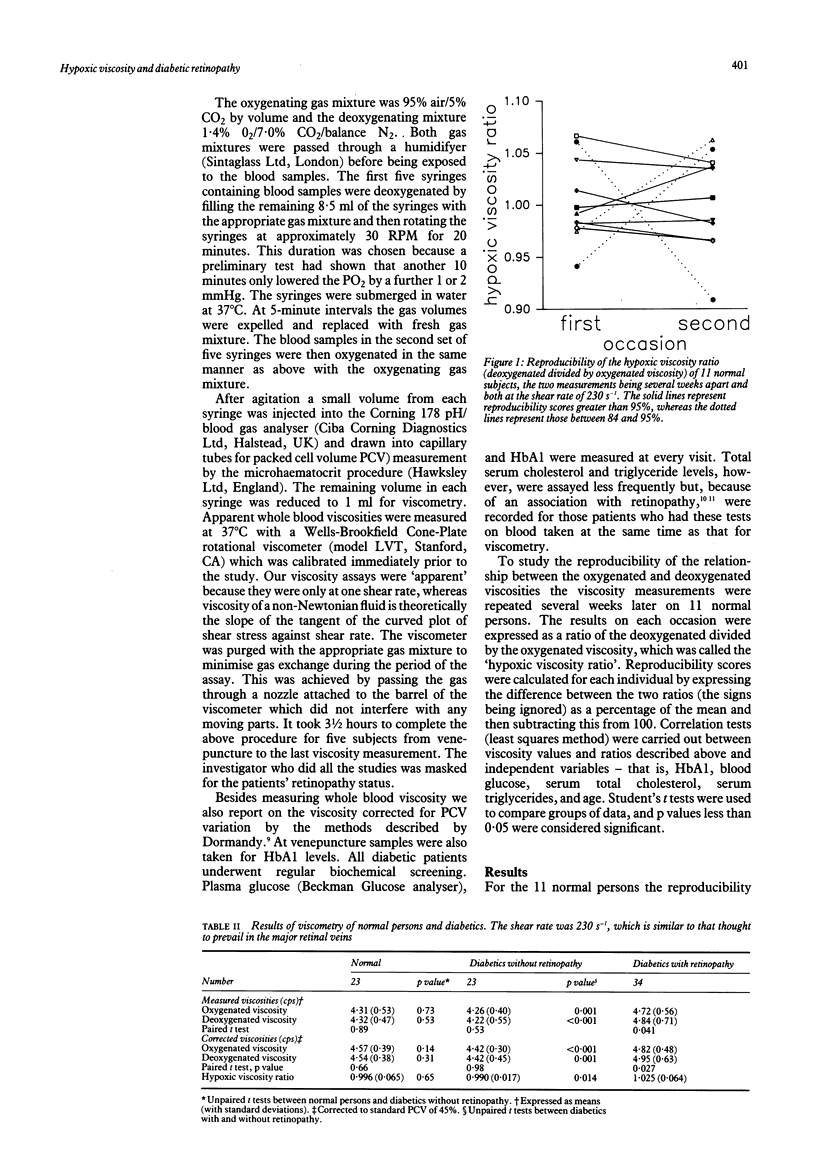
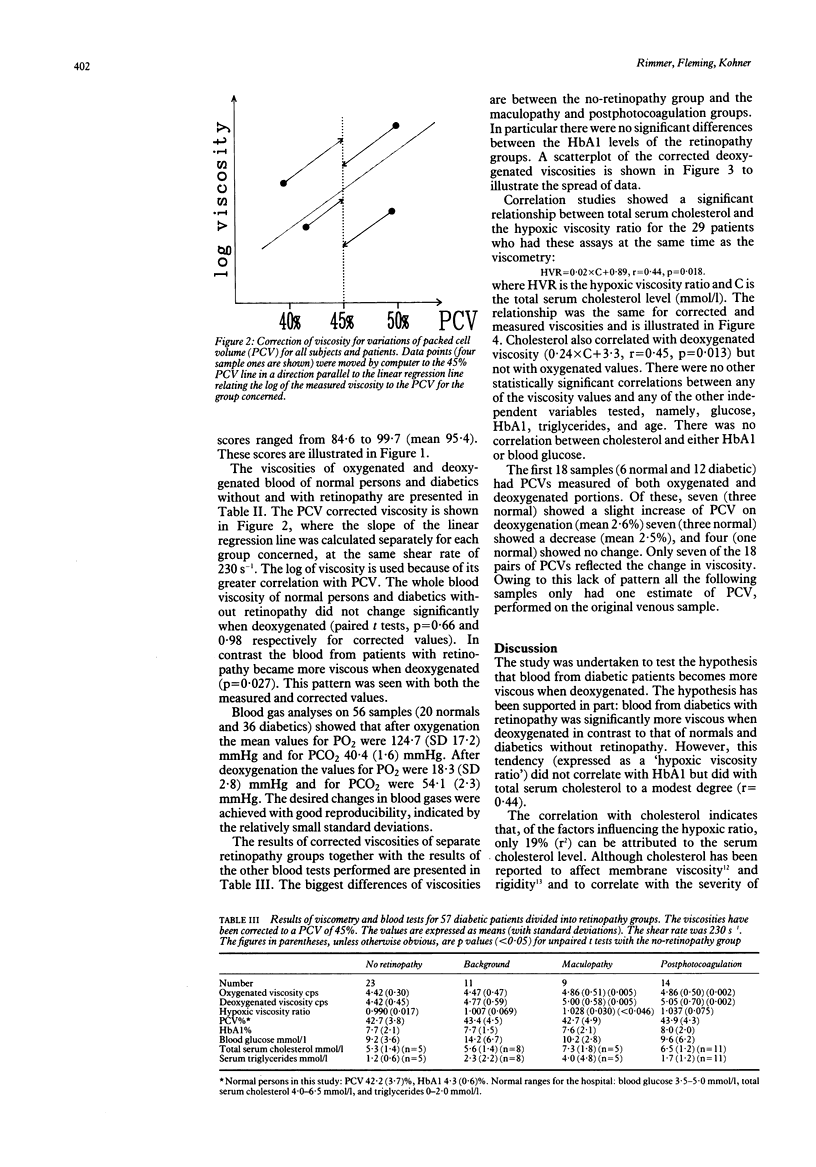
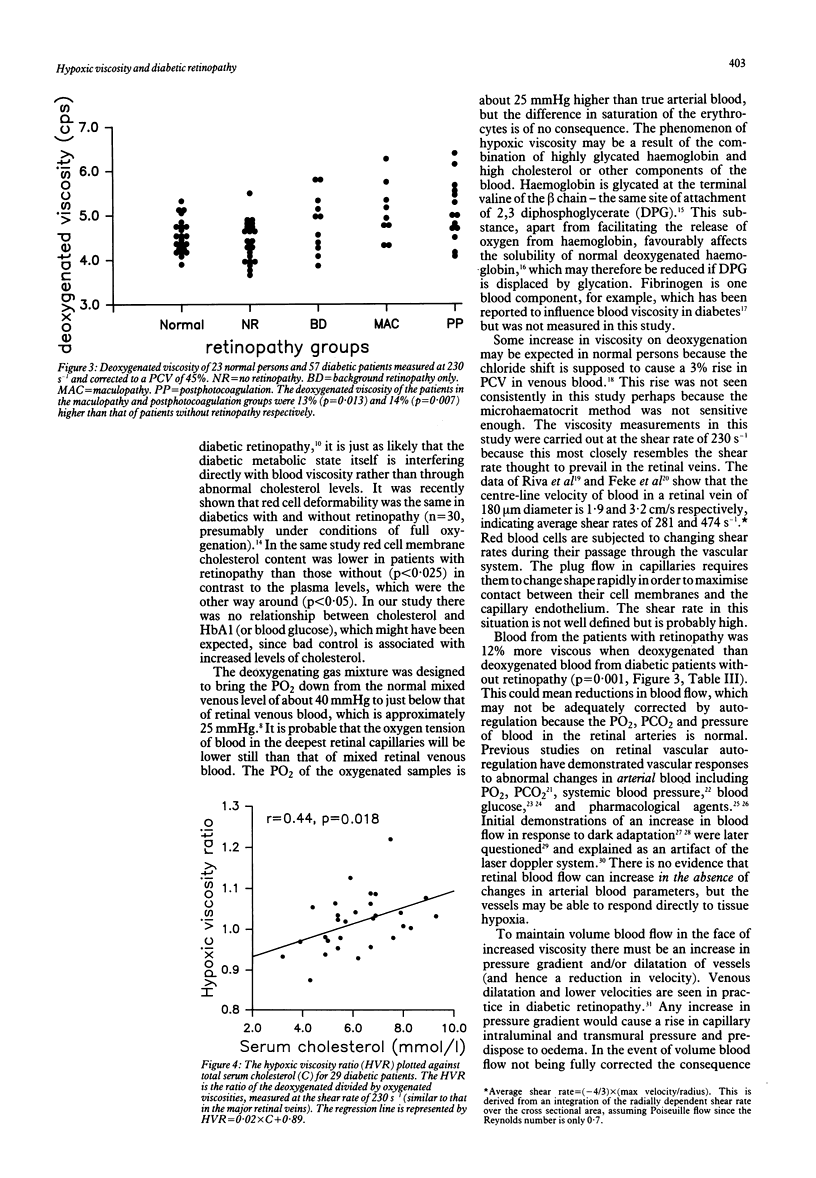
Diabetic and sickle retinopathy have features in common--for example, venous dilatation, microaneurysms, and capillary closure preceding neovascularisation. Bearing in mind that haemoglobin in poorly controlled diabetes is abnormal and that extremely low oxygen tensions (known to cause sickling) exist in the healthy cat retina, we wished to explore the possibility that diabetic blood, like that of sickle cell disease, may become more viscous when deoxygenated. To do this we measured whole blood viscosity, under oxygenated and deoxygenated conditions, of 23 normal persons, 23 diabetic patients without retinopathy, and 34 diabetic patients with retinopathy. The shear rate used was 230 s-1, which is similar to that thought to prevail in the major retinal veins. The viscosity of blood from normal persons, corrected for packed cell volume, did not change significantly on deoxygenation: mean 4.54 (SD 0.38) cps, versus, 4.57 (0.39) paired t test, p = 0.66. Similarly the blood from diabetics without retinopathy showed no change: 4.42 (0.45) versus 4.42 (0.30), p = 0.98; whereas the blood from patients with retinopathy changed from 4.82 (0.48) to 4.95 (0.63), p = 0.027. The hypoxic viscosity ratio (deoxygenated divided by oxygenated viscosity) correlated with total serum cholesterol (r = 0.44, p = 0.018) but not with HbA1, serum glucose, triglycerides, or age. A disproportionate increase in venous viscosity relative to arterial viscosity would lead to increased intraluminal and transmural pressure and therefore exacerbate leakage across capillary walls.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSON B., Jr, SALTZMAN H. A. RETINAL OXYGEN UTILIZATION MEASURED BY HYPERBARIC BLACKOUT. Arch Ophthalmol. 1964 Dec;72:792–795. doi: 10.1001/archopht.1964.00970020794009. [DOI] [PubMed] [Google Scholar]
- Alder V. A., Cringle S. J., Constable I. J. The retinal oxygen profile in cats. Invest Ophthalmol Vis Sci. 1983 Jan;24(1):30–36. [PubMed] [Google Scholar]
- Barnes A. J., Locke P., Scudder P. R., Dormandy T. L., Dormandy J. A., Slack J. Is hyperviscosity a treatable component of diabetic microcirculatory disease? Lancet. 1977 Oct 15;2(8042):789–791. doi: 10.1016/s0140-6736(77)90724-3. [DOI] [PubMed] [Google Scholar]
- Bunn H. F., Gabbay K. H., Gallop P. M. The glycosylation of hemoglobin: relevance to diabetes mellitus. Science. 1978 Apr 7;200(4337):21–27. doi: 10.1126/science.635569. [DOI] [PubMed] [Google Scholar]
- Condon P. I., Serjeant G. R. Ocular findings in homozygous sickle cell anemia in Jamaica. Am J Ophthalmol. 1972 Apr;73(4):533–543. doi: 10.1016/0002-9394(72)90005-0. [DOI] [PubMed] [Google Scholar]
- Dornan T. L., Carter R. D., Bron A. J., Turner R. C., Mann J. I. Low density lipoprotein cholesterol: an association with the severity of diabetic retinopathy. Diabetologia. 1982 Mar;22(3):167–170. doi: 10.1007/BF00283746. [DOI] [PubMed] [Google Scholar]
- Fallon T. J., Sleightholm M. A., Merrick C., Chahal P., Kohner E. M. The effect of acute hyperglycemia on flow velocity in the macular capillaries. Invest Ophthalmol Vis Sci. 1987 Jun;28(6):1027–1030. [PubMed] [Google Scholar]
- Feinstein M. B., Fernandez S. M., Sha'afi R. I. Fluidity of natural membranes and phosphatidylserine and ganglioside dispersions. Effect of local anesthetics, cholesterol and protein. Biochim Biophys Acta. 1975 Dec 16;413(3):354–370. doi: 10.1016/0005-2736(75)90121-2. [DOI] [PubMed] [Google Scholar]
- Feke G. T., Tagawa H., Deupree D. M., Goger D. G., Sebag J., Weiter J. J. Blood flow in the normal human retina. Invest Ophthalmol Vis Sci. 1989 Jan;30(1):58–65. [PubMed] [Google Scholar]
- Feke G. T., Zuckerman R., Green G. J., Weiter J. J. Response of human retinal blood flow to light and dark. Invest Ophthalmol Vis Sci. 1983 Jan;24(1):136–141. [PubMed] [Google Scholar]
- Ffytche T. J., Bulpitt C. J., Archer D., Kohner E. M., Dollery C. T. Effects of papaverine on the retinal microcirculation. Br J Ophthalmol. 1973 Dec;57(12):910–920. doi: 10.1136/bjo.57.12.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg M. F. Classification and pathogenesis of proliferative sickle retinopathy. Am J Ophthalmol. 1971 Mar;71(3):649–665. doi: 10.1016/0002-9394(71)90429-6. [DOI] [PubMed] [Google Scholar]
- Grunwald J. E., Riva C. E., Sinclair S. H., Brucker A. J., Petrig B. L. Laser Doppler velocimetry study of retinal circulation in diabetes mellitus. Arch Ophthalmol. 1986 Jul;104(7):991–996. doi: 10.1001/archopht.1986.01050190049038. [DOI] [PubMed] [Google Scholar]
- Hill D. W., Houseman J. Retinal blood flow in the cat following periods of light and darkness. Exp Eye Res. 1985 Aug;41(2):219–225. doi: 10.1016/0014-4835(85)90027-2. [DOI] [PubMed] [Google Scholar]
- Linsenmeier R. A. Effects of light and darkness on oxygen distribution and consumption in the cat retina. J Gen Physiol. 1986 Oct;88(4):521–542. doi: 10.1085/jgp.88.4.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan R., Mohan V., Susheela L., Ramachandran A., Viswanathan M. Increased LDL cholesterol in non-insulin-dependent diabetics with maculopathy. Acta Diabetol Lat. 1984 Jan-Mar;21(1):85–89. [PubMed] [Google Scholar]
- Pierluissi J., Campbell J. Metasomatotrophic diabetes and its induction: basal insulin secretion and insulin release responses to glucose, glucagon, arginine and meals. Diabetologia. 1980 Mar;18(3):223–228. doi: 10.1007/BF00251920. [DOI] [PubMed] [Google Scholar]
- Riva C. E., Grunwald J. E., Petrig B. L. Reactivity of the human retinal circulation to darkness: a laser Doppler velocimetry study. Invest Ophthalmol Vis Sci. 1983 Jun;24(6):737–740. [PubMed] [Google Scholar]
- Riva C. E., Grunwald J. E., Sinclair S. H., Petrig B. L. Blood velocity and volumetric flow rate in human retinal vessels. Invest Ophthalmol Vis Sci. 1985 Aug;26(8):1124–1132. [PubMed] [Google Scholar]
- Robinson F., Riva C. E., Grunwald J. E., Petrig B. L., Sinclair S. H. Retinal blood flow autoregulation in response to an acute increase in blood pressure. Invest Ophthalmol Vis Sci. 1986 May;27(5):722–726. [PubMed] [Google Scholar]
- Sebag J., Delori F. C., Feke G. T., Weiter J. J. Effects of optic atrophy on retinal blood flow and oxygen saturation in humans. Arch Ophthalmol. 1989 Feb;107(2):222–226. doi: 10.1001/archopht.1989.01070010228027. [DOI] [PubMed] [Google Scholar]
- Yan H. Y., Chiou G. C. Effects of L-timolol, D-timolol, haloperidol and domperidone on rabbit retinal blood flow measured with laser Doppler method. Ophthalmic Res. 1987;19(1):45–48. doi: 10.1159/000265470. [DOI] [PubMed] [Google Scholar]
- Zamorano A. F., Arnalich F., Ferro-Sánchez A., Grande C., Lahoz C., Pallardo L. F. Deformabilidad eritrocitaria y lípidos de la membrana eritrocitaria en la diabetes mellitus. Med Clin (Barc) 1987 Nov 21;89(17):717–720. [PubMed] [Google Scholar]


