Abstract
Study Objectives
To investigate the frequency and characteristics of large muscle group movements (LMMs) during sleep in healthy adults.
Methods
LMMs were scored following the International Restless Legs Syndrome Study Group criteria in 100 healthy participants aged 19–77 years. A LMM was defined as a temporally overlapping increase in EMG activity and/or the occurrence of movement artifacts in at least two channels. LMM indices and durations in total sleep time (TST), NREM and REM sleep, and association with arousals, awakenings, and/or respiratory events were calculated. Correlations of LMMs indices and durations with sleep architecture, respiratory and motor events, and subjective sleep quality were investigated.
Results
Median LMMs index in TST was 6.8/h (interquartile range (IQR), 4.5–10.8/h), median mean duration 12.4 s (IQR 10.7–14.4 s). Mean LMMs duration was longer in NREM (median 12.7 s, IQR 11.1–15.2 s) versus REM sleep (median 10.3 s, IQR 8.0–13.5s), p < 0.001. LMMs associated with awakening increased with age (p = 0.029). LMMs indices in TST were higher in men than women (p = 0.018). LMMs indices correlated positively with N1 sleep percentage (ρ = 0.49, p < 0.001), arousal index (ρ = 0.40, p = 0.002), sleep stages shift index (ρ = 0.43, p < 0.001, apnea index (ρ = 0.36, p = 0.017), and video-visible movements indices (ρ = 0.45, p < 0.001), and negatively with N3 sleep (ρ = −0.38, p= 0.004) percentage.
Conclusions
This is the first study providing normative data on LMMs frequency in healthy adults. LMMs are a ubiquitous phenomenon often associated with other events. Correlation with arousals and respiratory events suggests a potential clinical significance of LMMs in adults that awaits further investigation.
Keywords: LMM, restlessness, movements during sleep, sleep-related motor disorders, healthy sleep
Graphical abstract
Graphical Abstract.
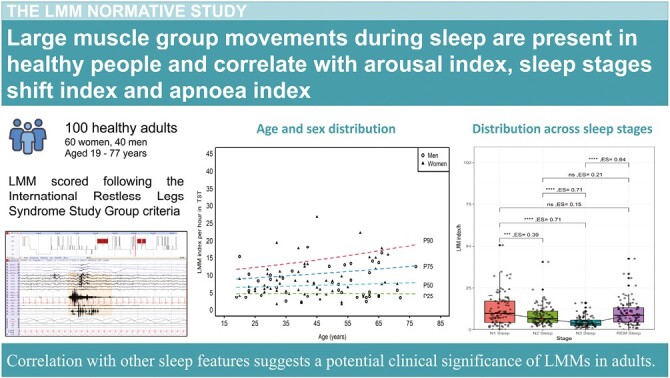
Statement of Significance.
Large body movements during sleep have been suggested to have a clinical significance. Criteria to score large muscle group movements during sleep have been recently developed. Our study applies these criteria for the first time to 100 healthy adults, to provide normative values (including age and sex distribution, occurrence in the different sleep stages, correlation to arousals or awakenings, respiratory events, and other motor events). We found a correlation between large muscle group movement indices and features indicative of sleep fragmentation, suggesting that they could represent a marker of sleep quality. This work represents a necessary step for future studies assessing the clinical significance of large muscle group movements in adults, and their potential relevance in different sleep disorders.
Introduction
The current American Academy of Sleep Medicine sleep scoring Manual (AASM, Version 2.6) contains rules to define specific movements during sleep. These include periodic leg movements (LM), alternating leg muscle activation, hypnagogic foot tremor, excessive fragmentary myoclonus, bruxism, and REM sleep without atonia [1]. However, other motor events not defined in the AASM manual can occur during sleep (e.g. neck myoclonus, non-periodic LM), and may have clinical implications [2, 3]. Most minor movements during sleep are well-defined [4] and have been systematically investigated in healthy adults. Large movements during sleep have been visually assessed based on PSG-synchronized video in sleep disorders (e.g. NREM parasomnias [5], REM sleep behavior disorder [6–8], narcolepsy [9, 10]) and healthy adults [11]. However, no particular focus was set on identification of large movements during sleep using PSG signals alone.
The Rechtschaffen and Kales scoring manual considered “movement time” as a distinct stage [12]. This stage was abolished with the release of the AASM Manual in 2007, mainly due to the lack of a clear definition [13, 14], which contributed to a gap in the scoring of this type of motor event. Recently, a task force of the International Restless Legs Syndrome Study Group (IRLSSG) developed criteria to detect and score large muscle group movements (LMMs) during sleep [15]. These criteria rely on standard polysomnographic signals without video-synchronized input and represent the first expert consensus to quantitatively assess large movements during sleep in a standardized way [15]. However, these post hoc scoring criteria have not been applied in clinical studies on adults until now, and normative values, which would be of interest for differentiating between normal and pathological movements in adults, are not available yet.
The clinical relevance of large body movements has been highlighted in children, particularly with the recent identification of a sleep-related movement disorder called restless sleep disorder (RSD) [16]. The PSG hallmark of RSD is the presence of large body movements documented during sleep, which are linked to disturbed sleep architecture, increased sympathetic activation during sleep, and higher occurrence of NREM parasomnias, emphasizing their clinical importance [17–19]. Moreover, large body movements were shown to be increased in children with Gilles de la Tourette syndrome and with attention deficit hyperactivity disorder compared to healthy children [20, 21].
While restlessness during sleep is a frequent patient complaint [22–25], no attempts have been made yet to objectively measure restlessness during sleep in adults. Additionally, the relationship between large body movements during sleep and other sleep variables and clinical disorders in healthy adults has not been thoroughly investigated so far, likely due to the absence of homogenous scoring rules.
The aim of this study was to investigate for the first time the frequency and characteristics of LMMs according to the recently published IRLSSG criteria in a large cohort of healthy adults and to establish normative values. To characterize LMMs, we additionally aimed to examine their correlation with other sleep features including sleep architecture, respiratory parameters, other motor activities during sleep, visible movements, anxiety and depression measures, and self-reported sleep quality.
Methods
Data used in this study were acquired as part of a large project on motor activity during sleep in healthy people [4]. Normative values for motor events during healthy sleep assessed with surface EMG and video analysis have been reported elsewhere [4, 11]. For the present study, we scored LMMs in this previously reported cohort of 100 healthy people.
Cohort
One-hundred healthy participants between 19 and 77 years of age were previously recruited from a random sample representative of the population of Tyrol/Austria. The major inclusion criterion was that participating participants perceived their sleep as healthy, and that there was no suspicion of sleep disorders after a two-step screening process (i.e. structured telephone interview and interview with a sleep expert). Detailed inclusion and exclusion criteria are reported elsewhere [26]. Exclusion criteria included the presence of any relevant disease, pregnancy, regular alcohol consumption, and a history suggestive of any sleep disorder. Details are published elsewhere [4]. The study was approved by the local ethical committee of the Medical University of Innsbruck. All participants were granted written informed consent prior to study participation.
Polysomnography
All participants underwent 8-hour v-PSG, conducted according to international standards [27]. V-PSG consisted of vertical and horizontal electrooculography (EOG), EEG (F3, F4, C3, C4, O1, O2, M1, and M2 electrodes), cardiorespiratory recording (single channel electrocardiography [ECG], recording of nasal airflow [thermocouple], nasal pressure cannula, tracheal microphone, thoracic and abdominal respiratory movements [piezo], and transcutaneous oxygen saturation), EMG of the mental, submental, both flexor digitorum superficialis and both anterior tibialis muscles (AT), and digital videography. Sleep stages were scored according to the 2007 American Academy of Sleep Medicine (AASM) criteria [27]. The awakening index was defined as the number of changes from any sleep stage to wake divided by total sleep time (TST) in hours.
LMM scoring
LMMs were scored according to the IRLSSG criteria [15]. Briefly summarized, a LMM was defined as temporally overlapping increase in EMG activity (twice the baseline signal) and/or the presence of movement artifacts in any combinations of the recording channels. A LMM was scored when it was preceded by at least 10 seconds of sleep, and when its duration was ≥3 but ≤45 seconds. LMM onset was identified as the first detectable increase in any channel involved and ended with the return to baseline signal for ≥1 second. Technical artifacts, parasomnias, seizures, and movements that met the criteria for any other scored movement following the AASM manual were not scored as LMM. The following channels were included for LMM scoring: six EEG signals, four EOGs, one ECG, EMG of the chin, bilateral AT, and bilateral flexor digitorum superficialis muscles.
LMMs indices (LMMs number/hour) and mean duration in TST, NREM, and REM sleep, as well as for each NREM sleep stage were calculated. Indices were calculated for LMMs occurring alone, as well as for those associated with arousals, awakenings, either arousals or awakenings, or respiratory events. In addition, the association of LMMs with more than one event type (i.e. arousal, awakening, or respiratory event) was recorded. A LMM and a respiratory event or arousal were considered associated with each other when they occurred simultaneously, overlapped, or when there were less than 0.5 seconds between the end of one event and the onset of the other event, regardless of which is first.
LMMs were scored by a board-certified sleep disorders specialist (AI) and reviewed by a board-certificated neurologist and sleep expert (AS), who was part of the IRLSSG task force developing the LMMs scoring criteria.
Correlation to other sleep features
LMMs indices and durations were investigated for correlations with sleep architecture, i.e. TST, sleep efficiency, sleep latency, REM sleep latency, time and percentage of the different sleep stages, wake after sleep onset, arousal index, and sleep stage transitions index in TST. Sleep stage transitions index was defined as the number of transitions between wake, N1, N2, N3, and REM sleep per hour in TST.
As exploratory investigations, correlations of LMMs with the following features were investigated:
a) Breathing parameters: apnea–hypopnea index (AHI), apnea index, hypopnea index, and the mean oxygen saturation during the night.
b) LM: scored according to the 2006 World Association of Sleep Medicine criteria [28]. For isolated LM and periodic leg movements during sleep (PLMS), correlations were investigated for indices in TST, NREM (as a whole and for each NREM sleep stage), and REM sleep. For PLMS, correlations were additionally investigated for periodicity indices in the different sleep stages, number of sequences, PLMS duration, PLMS mean interval duration, and PLMS associated with arousals.
c) Other motor events during sleep: high-frequency leg movements, fragmentary myoclonus, and neck myoclonus, scored as previously described [4].
d) REM sleep without atonia (RWA): quantification was performed manually according to the SINBAR (Sleep Innsbruck Barcelona) criteria [4, 29].
e) Visible movements: scored based on video analyses and classified according to type of movement and topographical distribution, as described elsewhere [11].
f) Subjective daytime sleepiness, sleep quality, chronotype, and anxiety and depression measures: with the exception of chronotype, these measures were based on questionnaires, i.e. Epworth Sleepiness Scale, Pittsburgh sleep quality index, and the hospital anxiety and depression scale (HADS-A/D). Chronotype was classified as “morning type,” “evening type” or “indifferent type” based on clinical history.
g) Lifestyle factors: alcohol (intake per week) and smoking status.
Statistics
Statistical analyses were performed with R version 4.1.2 and IBM SPSS V.26 (SPSS, Inc., Chicago, IL, USA) for Windows. Data were tested for normal distribution using the Shapiro–Wilks test. Depending on distribution, data are reported as median (interquartile range, IQR) or mean (standard deviation, SD). The Mann–Whitney test was used to compare LMMs indices between men and women. Tests for paired data were used to compare LMMs features in NREM and REM sleep, depending on data distribution either parametric (paired t-test) or nonparametric (Wilcoxon signed-rank test in case of two groups, Kruskal–Wallis test in case of more than two groups). The effect size (ES) was calculated as Z statistic divided by the square root of the sample size. Additionally, we performed a post hoc power analysis to consider the effect of age on the LMM index.
Participants were grouped into age groups: ≤30 years, 31–40 years, 41–50 years, 51–60 years, and > 60 years. Percentiles (90th and 95th) for the LMMs indices and durations for the total cohort and each age group were calculated.
A linear regression analysis was performed to assess sex- and age-dependent changes in LMMs indices. In the linear regression analysis, age was added as a continuous independent variable and non-normal variables were naturally log-transformed. Here R squared value was considered as the ES. To estimate percentiles for individual LMM duration instead of mean night duration, a gamma distribution with 500 bins was fitted using the pooled LMM durations from all participants.
For the correlation analyses, correlation coefficients were calculated using either Pearson r or Spearman rho, depending on data distribution. In addition, the point-biserial correlation coefficient was used to compare the correlation between categorical and continuous data. Finally, reference centile curves were built using the Lambda-Mu-Sigma method derived from a generalized additive model for location, scale, and shape (GAMLSS) model [30].
Two-tailed P-values < 0.05 were considered statistically significant. Adjustment for multiple comparisons was made using the Benjamini–Hochberg procedure. The interpretation values for the ES are as follows: 0.10 − < 0.3 (small effect), 0.30 − < 0.5 (moderate effect), and ≥0.5 (large effect) [31].
Results
Demographics and general sleep parameters
A total of 100 healthy participants (60 women and 40 men) participated in this study. The median (interquartile range [IQR]) age at the time of V-PSG was 43 (19–77) years. The median AHI index was 1.7/h (0.0–25.0/h), the median arousal index was 13.4/h (2.6–41.1/h) and the median awakening index was 4.1/h (2.9–5.6/h). Detailed sleep data are published elsewhere [4].
LMMs in REM and NREM sleep
Table 1 provides characteristics of LMMs indices and duration in TST, NREM, and REM sleep, as well as indices and durations of LMMs associated with awakenings, arousals, and/or respiratory events. The median LMMs index in TST was 6.8 (4.5–10.8)/h, with a median mean duration of 12.4 (10.7–14.4) s. The mean LMMs duration was longer in NREM compared to REM sleep (10.3 vs. 12.7 s, p < 0.001). Overall, the longest LMMs were those associated with awakenings (18.3 ± 3.8) s, and the shortest were the isolated ones (6.8 [5.4–8.4]) s. The majority of LMMs (83.4%) occurred either with arousals or awakenings, with (10.7%) or without (72.7%) respiratory events, whereas isolated LMMs constituted 14.7% of the total.
Table 1.
LMMs Indices in TST, NREM, and REM Sleep
| LMM indices (per hour), median [IQR] Mean LMM duration (seconds), median [IQR] |
TST | NREM | REM | P-values NREM vs. REM; unadjusted adjusted | ES | |
|---|---|---|---|---|---|---|
| Total LMM index | 6.8 (4.5–10.8) | 6.2 (4.3–10.1) | 8.4 (4.4–12.9) | 0.028* | 0.363 | 0.22* |
| Mean duration | 12.3 (10.7–14.4) | 12.7 (11.1–15.2) | 10.4 (8.3–13.2) | <0.001*** | <0.001*** | 0.50** |
| LMM not associated to any event | 0.7 (0.3–1.6) | 0.6 (0.2–1.6) | 0.7 (0–1.8) | 0.390 | 0.850 | 0.07 |
| Mean duration | 6.8 (5.4–8.4) | 6.8 (5.6–8.6) | 5.8 (4.6–7.7) | 0.005* | 0.087 | 0.38** |
| LMM associated with arousals or awakenings | 5.6 (4.1–9.2) | 5.5 (3.7–8.6) | 6.6 (3.9–10.7) | 0.067 | 0.629 | 0.18* |
| Mean duration | 13.3 (11.8–15.2) | 14.0 (11.7–15.8) | 11.6 (8.5–14.4) | <0.001*** | <0.001*** | 0.47** |
| LMM associated with arousals or awakenings, without respiratory events | 5.0 (3.5–8.1) | 5.0 (3.3–8.1) | 5.3 (2.6–8.6) | 0.850 | 0.850 | 0.02 |
| Mean duration | 13.4 (11.8–15.6) | 13.8 (12.0–16.2) | 11.4 (8.5–15.2) | <0.001*** | 0.002** | 0.41** |
| LMM associated with arousals | 3.2 (2.0–5.4) | 3.0 (1.7–5.2) | 3.7 (2.1–7.8) | 0.004** | 0.074 | 0.28* |
| Mean duration | 9.3 (8.4–10.9) | 10.0 (2.2) | 8.2 (6.6–10.9) | <0.001*** | 0.006** | 0.38** |
| LMM associated with arousals, without respiratory events | 2.9 (1.6–4.6) | 2.7 (1.5–4.7) | 2.7 (1.4–5.8) | 0.084 | 0.629 | 0.17* |
| Mean duration | 9.2 (8.1–10.7) | 9.6 (8.2–11.1) | 8.1 (6.5–10.9) | <0.001*** | 0.011* | 0.38** |
| LMM associated with awakenings | 2.4 (−1.9–3.1) | 2.4 (1.7–3.2) | 2.0 (0.9–3.5) | 0.023** | 0.328 | 0.23* |
| Mean duration, mean (SD) | 18.3 (3.8) | 18.5 (4.1) | 16.6 (11.7–20.7) | 0.081 | 0.629 | 0.19* |
| LMM associated with awakenings, without respiratory events | 2.2 (1.8–3.0) | 2.3 (1.6–3.2) | 1.7 (0.7–3.1) | 0.002** | 0.040* | 0.31** |
| Mean duration, mean (SD) | 18.3 (3.8) | 18.5 (4.0) | 16.7 (11.5–20.7) | 0.090 | 0.629 | 0.19* |
| LMM associated with respiratory events | 0.4 (0.1–1.3) | 0.3 (0–0.9) | 0 (0–1.9) | 0.040** | 0.485 | 0.15* |
| Mean duration | 10.8 (8.6–14.0) | 11.3 (9.2–15.2) | 9.6 (3.8) | 0.004** | 0.073 | 0.45** |
| LMM associated with respiratory events, without arousals or awakenings | 0 (0–0.1) | 0 (0–0.1) | 0 (0–0) | 0.169 | 0.850 | 0.29* |
| Mean duration, mean (SD) | 8.1 (3.4) | 8.0 (3.3) | 8.3 (3.5) | 0.705 | 0.835 | 0.18* |
| LMM associated with respiratory events and arousals | 0.3 (0–0.7) | 0.2 (0–0.6) | 0 (0–1.4) | 0.010** | 0.154 | 0.21* |
| Mean duration | 10.7 (8.6–13.7) | 11.3 (8.6–14.8) | 8.5 (5.9–11.8) | 0.064 | 0.629 | 0.31** |
| LMM associated with respiratory events and awakenings | 0 (0–0.1) | 0 (0–0.1) | 0 (0–0) | 0.296 | 0.850 | 0.05 |
| Mean duration, mean (SD) | 17.6 (6.9) | 18.1 (7.7) | 16.7 (9.2) | 0.623 | 0.835 | 0.18* |
The P-value is the represent the significance tested between REM and NREM sleep. Bold* represent significant difference (p < 0.05) or ES > 0.3. One star means p < 0.05 or ES > 0.1, 2 stars means p < 0.01 or ES > 0.3, 3 stars < 0.001or ES > 0.5.
IQR, Interquartile range; LMM, Large muscle group movements; NREM, Non-rapid eye movements; REM, Rapid eye movement.
Figure 1 and Table 2 show LMMs indices, durations, and their percentiles in the different sleep stages. LMMs indices in NREM sleep decreased from N1 to N2 to N3 sleep, with LMMs durations showing an opposite trend. No significant difference between REM and N1 sleep stages in either index or duration was present. LMMs index in N3 sleep was significantly lower compared to all other sleep stages (p < 0.001) and LMMs mean duration was significantly longer in N3 sleep compared to all other sleep stages (p < 0.001). When looking into LMMs distribution throughout the night, mean LMMs indices were significantly higher during the first and the last hour of the night. Time of the night was a significant predictor of LMM index in a quadratic regression model (p = 0.029) but not a linear regression (p = 0.300).
Figure 1.
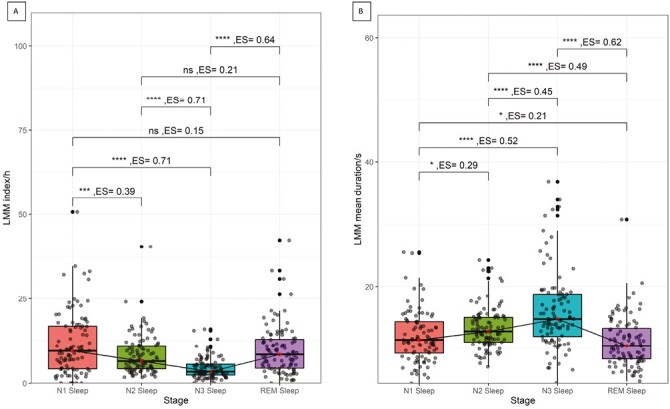
Distribution of LMMs indices across the different sleep stages. Box and whiskers plot representing LMM indices (A) and mean durations (B) in the different sleep stages. The red dot represents the median, and the whiskers indicate the interquartile range. A line to visualize the trend of change across the sleep stages connects medians. One star means significance < 0.05, 2 stars < 0.01, 3 stars < 0.001, ns means not significant. P-values were corrected for multiple comparisons with the Benjamini–Hochberg procedure. LMM, large muscle group movements; REM, Rapid eye movement.
Table 2.
Percentiles of the LMMs Indices and Durations Across the Different Sleep Stages
| Sleep stage | 25th percentile | 50th percentile | 75th percentile | 90th percentile | 95th percentile |
|---|---|---|---|---|---|
| LMM index (per h) | |||||
| N1 | 4.2 | 9.5 | 16.8 | 24.0 | 32.2 |
| N2 | 4.2 | 6.4 | 10.8 | 15.8 | 17.5 |
| N3 | 2.4 | 3.4 | 5.5 | 8.3 | 11.1 |
| REM | 4.4 | 8.4 | 12.9 | 18.5 | 21.4 |
| Mean LMM duration (seconds) | |||||
| N1 | 9.2 | 11.4 | 14.3 | 16.6 | 19.8 |
| N2 | 11.0 | 12.7 | 15.0 | 15.8 | 17.9 |
| N3 | 11.9 | 14.7 | 18.7 | 25.1 | 31.4 |
| REM | 8.3 | 10.4 | 13.4 | 16.2 | 17.1 |
LMM, Large muscle group movements; REM, Rapid eye movement.
Normative values, age, and gender distribution
Ninetieth and 95th percentiles of LMM indices and durations in TST, NREM, and REM sleep are reported in Table 3. LMMs indices in women and men are reported in Table 4. Sex- and age-related distribution of LMMs indices and durations in TST, NREM, and REM sleep, and in the different sleep stages is shown in Figure 2. The 90th and 95th percentiles of LMMs indices and durations in the different age groups are shown in Table 5. LMMs indices and duration tended to increase with age. This increase was however not significant in a linear regression model adjusting for sex (Table 5). The LMMs increase with age was significant only for LMMs associated with awakenings and those associated with respiratory events (Table 5). The median time with LMMs was 9.5 (6.4–13.1) minutes, and did not change significantly with age (p = 0.673). Given the achieved ES and sample size, post hoc power analysis showed an achieved power of 0.70 and 0.90 for the effect of age on the total LMM index, and LMM index associated with awakening, respectively.
Table 3.
Ninetieth and 95th Percentiles of LMMs Indices and Durations in TST, NREM and REM Sleep
| LMM index (per hour) and corresponding mean LMM duration (seconds) | TST | NREM | REM | |||
|---|---|---|---|---|---|---|
| Percentile | 90th | 95th | 90th | 95th | 90th | 95th |
| Total LMM index | 15.0 | 17.6 | 15.7 | 18.1 | 17.9 | 21.4 |
| Mean duration | 16.5 | 17.6 | 17.6 | 18.4 | 16.2 | 16.9 |
| LMM not associated to any event | 2.8 | 3.2 | 2.9 | 3.2 | 3.7 | 5.0 |
| Mean duration | 10.2 | 12.0 | 10.2 | 12.6 | 9.7 | 11.4 |
| LMM associated with arousals or awakenings | 12.1 | 14.1 | 13.2 | 14.7 | 15.3 | 19.1 |
| Mean duration | 18.0 | 20.2 | 18.9 | 20.6 | 17.0 | 19.5 |
| LMM associated with arousals or awakenings, without respiratory events | 10.6 | 11.9 | 11.1 | 12.6 | 11.5 | 13.4 |
| Mean duration | 18.9 | 20.7 | 19.1 | 20.7 | 18.1 | 20.7 |
| LMM associated with arousals | 8.2 | 8.9 | 8.4 | 9.9 | 11.2 | 14.3 |
| Mean duration | 12.8 | 13.8 | 13.1 | 14.4 | 13.1 | 14.8 |
| LMM associated with arousals, without respiratory events | 6.2 | 7.2 | 6.4 | 7.3 | 8.8 | 10.0 |
| Mean duration | 12.9 | 14.2 | 13.2 | 14.5 | 13.0 | 14.9 |
| LMM associated with awakenings | 4.4 | 5.8 | 4.6 | 6.1 | 4.9 | 5.6 |
| Mean duration, mean (SD) | 23.5 | 24.4 | 23.9 | 25.4 | 25.7 | 29.2 |
| LMM associated with awakenings, without respiratory events | 4.1 | 5.3 | 4.4 | 5.9 | 4.5 | 5.4 |
| Mean duration, mean (SD) | 23.5 | 24.2 | 23.9 | 24.6 | 25.8 | 29.8 |
| LMM associated with respiratory events | 3.2 | 4.2 | 2.7 | 4.4 | 4.6 | 7.2 |
| Mean duration | 18.9 | 20.8 | 20.8 | 24.2 | 14.7 | 15.7 |
| LMM associated with respiratory events, without arousals or awakenings | 0.5 | 0.6 | 0.5 | 0.7 | 0.1 | 1.1 |
| Mean duration, mean (SD) | 12.6 | 13.7 | 11.8 | 13.4 | 12.9 | 13.2 |
| LMM associated with respiratory events and arousals | 2.3 | 3.3 | 2.0 | 3.4 | 3.5 | 5.0 |
| Mean duration | 19.1 | 21.2 | 20.2 | 22.7 | 14.3 | 17.6 |
| LMM associated with respiratory events and awakenings | 0.5 | 0.6 | 0.5 | 0.6 | 0.9 | 1.2 |
| Mean duration, mean (SD) | 25.2 | 31.3 | 29.9 | 31.7 | 28.7 | 33.9 |
LMM, Large muscle group movements; NREM, Non-rapid eye movements; REM, Rapid eye movement.
Table 4.
Median and Interquartile Range of LMMs Indices in Women and Men.
| LMM index (per hour) and corresponding mean LMM duration (seconds), median [IQR] | Women | Men | P-value | ES |
|---|---|---|---|---|
| Total LMM index | 5.8(4.4–14.3) | 8.4(6.0–11.8) | 0.017* | 0.24* |
| Mean duration | 12.4(10.7–1.2) | 12.3(11–14.7) | 0.936 | 0.01 |
| LMM not associated to any event | 0.7(0.3–8.8) | 0.9(0.4–1.9) | 0.080 | 0.18 |
| Mean duration | 6.8(5.3–8.1) | 6.8(5.7–7.7) | 0.965 | 0.01 |
| LMM associated with arousals or awakenings | 5.2(3.7–15.2) | 6.7(4.6–10.2) | 0.053 | 0.19 |
| Mean duration | 13.1(11.6–7.3) | 13.7(12.2–15.5) | 0.473 | 0.07 |
| LMM associated with arousals or awakenings, without respiratory events | 4.8(3.5–15.4) | 5.6(4.0–8.5) | 0.127 | 0.15 |
| Mean duration | 12.9(11.8–4.7) | 14.6(12.1–16.1) | 0.209 | 0.13 |
| LMM associated with arousals | 2.9(1.7–11.2) | 4.0(2.6–6.2) | 0.077 | 0.18 |
| Mean duration | 9.4(8.2–4.1) | 9.4(8.6–10.4) | 0.942 | 0.01 |
| LMM associated with arousals, without respiratory events | 2.6(1.5–10.9) | 3.0(1.9–5.1) | 0.304 | 0.10 |
| Mean duration | 9.3(8.2–3) | 9.3(8.1–10.1) | 0.880 | 0.02 |
| LMM associated with awakenings | 2.3(1.9–20.1) | 2.6(2–3.2) | 0.209 | 0.13 |
| Mean duration, mean (SD) | 17.5(14.6–2.9) | 19.5(16.9–22.5) | 0.026* | 0.22* |
| LMM associated with awakenings, without respiratory events | 2.1(1.8–19.6) | 2.5(1.9–3.2) | 0.137 | 0.15 |
| Mean duration, mean (SD) | 17.5(14.5–0.8) | 19.4(16.9–22.7) | 0.024* | 0.23* |
| LMM associated with respiratory events | 0.3(0–16.3) | 0.8(0.4–1.8) | 0.001** | 0.34** |
| Mean duration | 11.2(8.7–0) | 10.7(8.8–13.1) | 0.447 | 0.09 |
| LMM associated with respiratory events, without arousals or awakenings | 0(0–11.0) | 0.1(0–0.3) | 0.005** | 0.28* |
| Mean duration, mean (SD) | 8.2(6.3–0.5) | 7.7(5.4–10.4) | 0.575 | 0.10 |
| LMM associated with respiratory events and arousals | 0.2(0–16.5) | 0.7(0.3–1.3) | 0.001** | 0.35** |
| Mean duration | 10.8(7.6–0.1) | 10.7(8.8–12.4) | 0.707 | 0.04 |
| LMM associated with respiratory events and awakenings | 0(0–19.5) | 0(0–0.2) | 0.494 | 0.07 |
| Mean duration, mean (SD) | 18(14.4–1) | 18.6(12–21.8) | 0.731 | 0.06 |
Bold* represent significant difference (p < 0.05) or ES > 0.3. One star means p < 0.05 or ES > 0.1, 2 stars means p < 0.01 or ES > 0.3, 3 stars < 0.001or ES > 0.5.
IQR, Interquartile range; LMM, Large muscle group movements; NREM, Non-rapid eye movements; REM, Rapid eye movement.
Figure 2.
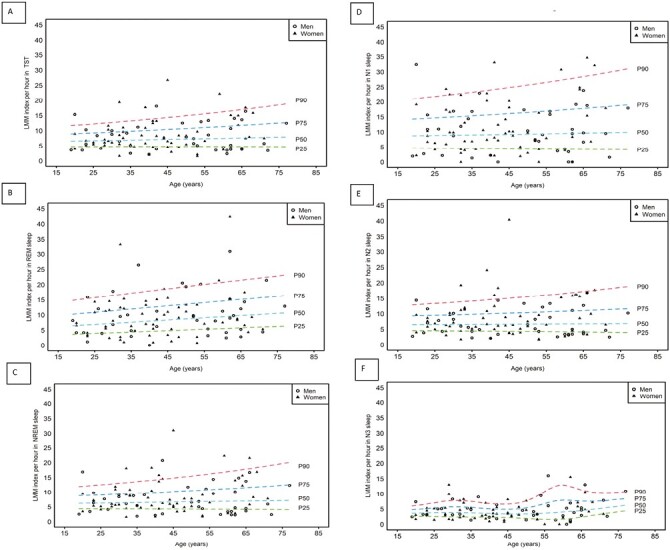
Sex- and age-related distribution of LMM in TST (A), NREM (B), REM (C) sleep, N1 sleep (D), N2 sleep (E), and N3 sleep (F). The panel shows the modeled percentiles (25th, 50th, 75th, and 90th) for the LMM indices in relation to change in age. Individuals are presented as either empty circles (men) or full triangles (women). LMM, Large muscle group movements; NREM, Non-rapid eye movements; P25, 25th percentile; P50, 50th percentile; P75, 75th percentile; P90, 90th percentile; REM, Rapid eye movement.
Table 5.
Ninetieth and 95th Percentiles of Different LMMs Indices and Durations in the Different Age Groups
| LMM index per hour and corresponding mean LMM duration in seconds | Total | ≤30 y | 31–40 y | 41–50 y | 51–60 y | >60 y | P-value | R2 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Percentile | 90th | 95th | 90th | 95th | 90th | 95th | 90th | 95th | 90th | 95th | 90th | 95th | ||
| Total LMM index | 15.0 | 17.6 | 10.3 | 11.5 | 11.8 | 17.7 | 13.2 | 18.1 | 13.2 | 16.4 | 15.8 | 16.3 | 0.220 | 0.06 |
| Mean duration | 16.5 | 17.6 | 15.3 | 15.7 | 20.4 | 23.0 | 14.5 | 14.7 | 18.1 | 19.1 | 16.6 | 16.9 | 0.887 | 0.00 |
| LMM not associated to any event | 2.8 | 3.2 | 2.6 | 3.0 | 2.7 | 3.2 | 2.0 | 2.9 | 2.0 | 2.3 | 3.0 | 4.0 | 0.422 | 0.03 |
| Mean duration | 10.2 | 12.0 | 9.6 | 10.8 | 13.9 | 17.4 | 11.4 | 11.8 | 9.0 | 9.4 | 9.6 | 10.0 | 0.752 | 0.00 |
| LMM associated with arousals or awakenings | 12.1 | 14.1 | 8.9 | 9.4 | 10.4 | 14.2 | 12.7 | 14.1 | 10.9 | 13.8 | 12.6 | 13.8 | 0.135 | 0.05 |
| Mean duration | 18.0 | 20.2 | 16.7 | 18.3 | 20.4 | 23.0 | 15.0 | 15.3 | 19.5 | 21.4 | 17.1 | 17.7 | 0.477 | 0.01 |
| LMM associated with arousals or awakenings, without respiratory events | 10.6 | 11.9 | 8.6 | 9.0 | 9.4 | 10.5 | 11.0 | 13.4 | 9.8 | 12.2 | 11.2 | 11.4 | 0.610 | 0.02 |
| Mean duration | 18.9 | 20.7 | 16.9 | 18.4 | 20.9 | 23.0 | 15.1 | 15.6 | 19.5 | 21.5 | 18.0 | 21.0 | 0.584 | 0.02 |
| LMM associated with arousals | 8.2 | 8.9 | 5.1 | 6.1 | 7.3 | 8.4 | 8.9 | 9.1 | 7.8 | 11.1 | 8.1 | 8.3 | 0.930 | 0.02 |
| Mean duration | 12.8 | 13.8 | 13.0 | 13.4 | 11.9 | 12.1 | 10.3 | 13.3 | 11.3 | 12.8 | 14.9 | 15.3 | 0.602 | 0.00 |
| LMM associated with arousals, without respiratory events | 6.2 | 7.2 | 4.9 | 5.8 | 6.4 | 6.7 | 7.2 | 7.6 | 5.6 | 8.2 | 6.2 | 7.1 | 0.571 | 0.01 |
| Mean duration | 12.9 | 14.2 | 12.9 | 13.4 | 12.2 | 13.0 | 10.3 | 13.3 | 11.7 | 13.1 | 14.0 | 14.7 | 0.667 | 0.00 |
| LMM associated with awakenings | 4.4 | 5.8 | 3.2 | 4.6 | 3.5 | 4.6 | 3.6 | 4.1 | 3.6 | 4.2 | 5.8 | 7.1 | 0.006** | 0.10 |
| Mean duration | 23.5 | 24.4 | 23.3 | 24.1 | 24.5 | 25.9 | 20.7 | 22.0 | 23.6 | 24.6 | 22.2 | 23.5 | 0.023* | 0.10 |
| LMM associated with awakenings, without respiratory events | 4.1 | 5.3 | 3.2 | 4.6 | 3.3 | 4.3 | 3.5 | 4.1 | 3.3 | 3.9 | 5.5 | 7.1 | 0.098 | 0.06 |
| Mean duration | 23.5 | 24.2 | 23.3 | 24.0 | 24.6 | 26.7 | 20.2 | 22.0 | 23.6 | 24.9 | 22.2 | 23.5 | 0.029* | 0.10 |
| LMM associated with respiratory events | 3.2 | 4.2 | 0.9 | 1.6 | 2.8 | 3.2 | 3.4 | 4.1 | 3.3 | 5.2 | 5.0 | 5.5 | 0.014* | 0.08 |
| Mean duration | 18.9 | 20.8 | 16.6 | 18.8 | 12.5 | 12.8 | 19.7 | 20.8 | 18.8 | 19.1 | 21.0 | 22.6 | 0.638 | 0.01 |
| LMM associated with respiratory events, without arousals or awakenings | 0.5 | 0.6 | 0.2 | 0.3 | 0.5 | 0.5 | 0.4 | 0.5 | 0.3 | 0.4 | 1.1 | 1.3 | 0.838 | 0.09 |
| Mean duration | 12.6 | 13.7 | 9.8 | 10.6 | 11.7 | 12.3 | 15.8 | 16.5 | 11.5 | 11.9 | 11.9 | 12.7 | 0.531 | 0.02 |
| LMM associated with respiratory events and arousals | 2.3 | 3.3 | 0.9 | 0.9 | 2.0 | 2.3 | 2.4 | 3.5 | 2.9 | 4.2 | 2.9 | 3.3 | 0.157 | 0.05 |
| Mean duration | 19.1 | 21.2 | 18.5 | 24.1 | 12.7 | 13.0 | 19.2 | 19.7 | 13.2 | 16.1 | 21.1 | 23.0 | 0.558 | 0.01 |
| LMM associated with respiratory events and awakenings | 0.5 | 0.6 | 0.1 | 0.1 | 0.3 | 0.6 | 0.4 | 0.4 | 0.4 | 0.7 | 1.0 | 1.2 | 0.118 | 0.06 |
| Mean duration | 25.2 | 31.3 | 10.3 | 11.5 | 19.2 | 19.3 | 23.7 | 24.2 | 20.7 | 21.3 | 25.3 | 28.5 | 0.118 | 0.06 |
P values are adjusted for sex.
Bold* represent significant difference (p < 0.05) or ES > 0.3. One star means p < 0.05 or ES > 0.1, 2 stars means p < 0.01 or ES > 0.3, 3 stars < 0.001or ES > 0.5
LMM, large muscle group movements.
Gamma distribution of the pooled LMMs durations in TST, NREM, and REM sleep is shown in Supplementary Figure S1.
Correlation analysis
Figure 3 shows the correlation of LMMs indices and durations with sleep architecture. LMMs indices correlated positively with N1 sleep percentage, arousal index, sleep stage shifts index in TST and apnea index, and negatively with N3 sleep percentages.
Figure 3.
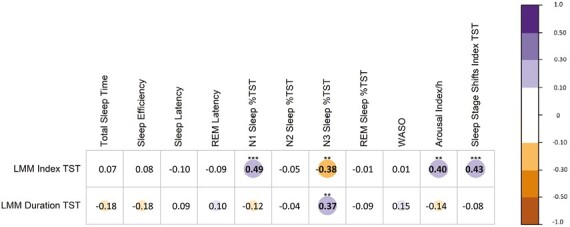
Correlation between LMMs indices and sleep architecture features. Correlogram representing the correlation between the LMM indices/durations and the sleep architecture- The purple color represents a positive correlation, red represents a negative one. Correlations more than 0.1 are represented with a circle/color as follows: 0.1–0.3 as small, <0.3–0.5 as medium, and > 0.5 as large (Cohen, J. (1988). Statistical Power Analysis for the Behavioral Sciences (second ed.). Erlbaum, Hillsdale, NJ). The number inside the circle shows the correlation coefficient, the P-value is marked above the correlation coefficient, one-star means < 0.05, 2 stars < 0.01, 3 stars < 0.001. All P-values are corrected with the Benjamin–Hochberg procedure. LMM, large muscle group movements; REM, rapid eye movements; WASO, wake after sleep onset.
The correlations of LMMs indices and durations with motor events in sleep, visible video movements, subjective sleep, anxiety, and depression measures, as well as chronotype and lifestyle factors, are shown in Supplementary Figure S2. LMMs indices were positively correlated with HADS-A and HADS-D scores, isolated LM in TST and NREM sleep, and visible movements.
Discussion
This study assessed LMMs frequency during sleep in healthy adults and investigated the relationship between LMMs and other sleep features, providing normative values. In this cohort of 100 healthy people, 90% had a LMMs index between 1.5–15.0/h in TST, indicating that LMMs are a ubiquitous physiological motor phenomenon.
In 90% of participants, the mean LMM duration during sleep was 6.9–16.5 seconds, with significantly longer LMM durations in NREM sleep compared to REM sleep. Durations were inversely correlated with the LMM indices. Most LMM were either associated with arousals or awakenings, while isolated LMM and those associated with respiratory events occurred less frequently.
LMM indices and durations did not change significantly with age, except for those associated with awakenings which increased across the lifespan. A sex difference in LMM was present in our cohort, with higher LMM indices in men compared to women.
LMM in the different sleep stages
In our cohort, LMM indices were highest during N1 sleep, and decreased from N1 to N2 to N3 sleep. Of note, indices in REM sleep were similar to those in N1 sleep. Furthermore, LMM duration showed an inverse correlation with indices, i.e. the higher the index, the shorter the duration. This is in line with historical results, which reported a similar pattern of the relationship between body movements rate and sleep stages (W > N1 > REM > N2 > N3 [S3 + S4]), despite the different scoring methodologies [32]. Of note, the change in LMM duration and frequency was sleep stage dependent.
Movements during sleep are likely regulated by stage-dependent mechanisms, due to the different regulation mechanisms of muscle tone in different sleep stages. While the decrease of LMM indices from lighter to deeper NREM sleep stages is expected, the higher emergence of LMM in REM sleep is not. However, a possible explanation is that due to the physiological atonia during REM sleep, LMM are fragmented into smaller movements during this sleep stage. This hypothesis is supported by the significantly shorter LMM durations in REM versus NREM sleep.
The high frequency of LMM in N1 can be related to the AASM scoring rules for sleep stages, as an epoch with a major body movement is scored the same as the epoch that follows it, which frequently is N1 sleep or wake [27]. We did not find any correlation between LMM indices in REM sleep and RWA indices, underlying that applying the scoring criteria different kinds of motor events are identified. The lack of correlation may also relate to the application of the 3-second SINBAR scoring rules for RWA, which exclude arousals (and thus LMM associated with arousals or awakenings) from RWA scoring.
LMM across the lifespan
In children, a LMM index > 5/h was reported to have high accuracy in distinguishing people with RSD from those with RLS and healthy controls [25]. However, in that study large body movements were scored based on video PSG [25]. Here we showed that healthy adults have an LMM index of < 15/h. This might indicate a trend towards an increasing frequency of body movements during sleep from childhood (not infancy) to adulthood.
Notably, LMM indices were stable throughout adulthood in healthy participants. Nevertheless, those associated with awakenings increased with age. Previous studies similarly showed that spontaneous awakenings preceded by body movements are more common in elderly than in young people [33, 34] In contrast to our results, a small sample study found a decrease in body movements with age in healthy sleepers. In that study, a different scoring methodology was applied and movement detection was derived from EOG and EEG artifacts only [33], which may have missed some movements, e.g. LM, which were shown to increase with age [35].
Based on our findings, LMM duration in healthy adults seems to be longer than in children [25], and similar to values previously reported in adults [36] assessed using video PSG and analyzed post hoc [15]. Age did not influence mean LMM duration in our cohort of healthy adults. The post hoc power analysis of the effect of aging on LMMs suggested that our study had adequate statistical power to detect the effect of age on the total LMM index and a strong statistical power to detect the effect on the LMM index associated with awakening. However, in order to achieve a power of at least 0.80, and thus further minimize a type II error for our ES, at least 125 patients would have been required.
LMMs associated with respiratory events significantly increased with age. Of note, the AHI was significantly higher in older individuals [37], which could also account for some of the age-related LMMs differences.
Sex differences in LMM
The total LMM index was higher in men compared to women. A higher index of visible movements in men compared to women was also reported in the same cohort [11]. Recent actigraphy data align with this finding, recording fewer movements in women during sleep [38]. This could partially explain the better objective sleep quality reported in women [39–41].
Notably, LMM index associated with respiratory events was also higher in men. Although our cohort included only healthy sleepers, this may indicate a link between some LMM and respiratory events. This observation deserves further evaluation in future studies assessing sex differences in LMM, and LMM features in people with sleep-related breathing disorder.
LMM correlation with objective sleep features
LMM were strongly correlated with multiple sleep architecture features. LMM indices positively correlated with N1 sleep percentage, arousal index, and sleep stage shifts index in TST, suggesting a link with sleep fragmentation even in healthy individuals. Adding to that, LMM indices negatively correlated with N3 sleep percentages. In line with our findings, one study showed that large movements during sleep delay transition to slow wave sleep in adults [39]. Furthermore, a recent study found that children with RSD had an increased NREM instability [17]. According to the literature, N3 sleep putatively contributes to next-day cognitive and physical performance, subjective sleep quality, and long-term cardiovascular health [42]. Future studies should investigate if these features are altered in people with high LMM indices.
LMM indices correlated positively with the apnea index. This suggests a potential link between LMM and respiratory events in adults, which deserves further investigation to clarify the clinical relevance of LMM in people with sleep-related breathing disorder. Alternatively, LMM associated with respiratory events may relate to movements at the end of apneas [43].
LMM correlation with anxiety and depression
In our cohort of healthy people, LMM indices were positively correlated with HADS-A and HADS-D scores. Recent studies in children with RSD reported daytime dysfunction as measured by an overall decreased pediatric quality of life inventory and decreased psychosocial and physical functioning sub-scores compared to children with PLMD or RLS [44]. Considering previous findings in children, our data may suggest a link between LMM and anxiety or depression in adults.
LMM correlation to other motor events and visible movements
As expected, LMMs did not correlate with PLMS (according to the scoring criteria, PLMS are not considered LMMs [15]). However, LMMs correlated with isolated LMs in TST and NREM sleep. As it was previously shown that both periodic and non-periodic LM are preceded by EEG activation and accompanied by autonomic changes [45], our findings may indicate a link between LMMs and autonomic changes. Thus, LMMs deserve further investigation to assess their clinical relevance.
LMMs correlated to visible movements scored as described previously [11], in particular with bilateral, multifocal, and elementary movements. The correlation was present for all body parts, except the upper limbs. Higher indices of visible movements and lack of correlation between duration of LMMs and visible movements are likely explained by the different methodologies and approaches in the two studies. When performing the video analysis, voluntary movements associated with arousals were not considered, and all other visible movements during sleep were identified independently from their duration. Most of them were brisk (median duration 1.1 seconds, IQR 0.8–1.5), whereas a minimum duration of three seconds is required for LMM.
Strengths and limitations of the present study
Major strengths of the current study were the inclusion of healthy people and the comprehensive analysis of correlation with sleep and sleep-related features, including sleep architecture, respiratory events, all motor events for which criteria are available, and visible movements. Furthermore, all LMM parameters and their associations with other events were reported, including not only recommended but also optional parameters suggested in the original publication by the IRLSSG taskforce on LMM.
Potential limitations include skewed sex distribution and the lack of ethnic diversity in our cohort. Additionally, only a single night was used. Thus, inter-night variability of LMM could not be assessed.
Conclusions
This study demonstrated that LMM during sleep are frequent in healthy people. LMMs were more frequent but shorter in REM compared to NREM sleep, with the lowest rates in N3 sleep. A sex difference was present, with higher LMM indices in men compared to women. Despite a stable LMM prevalence among different age groups, LMMs leading to awakening increased with age. LMM indices correlated with features indicative of sleep fragmentation, thus they could represent a candidate marker of sleep quality and/or restlessness.
Future studies should assess whether an index > 15/h could be linked to anxiety and depression, as well as impaired quality of life, and explore LMMs in different adult patients’ populations, e.g. in people with sleep-related breathing disorder and insomnia. We provide first LMM normative values, which represent a prerequisite for investigating the clinical relevance of LMMs in adults.
Supplementary Material
Contributor Information
Abubaker Ibrahim, Department of Neurology, Medical University of Innsbruck, Innsbruck, Austria.
Raffaele Ferri, Sleep Research Centre, Department of Neurology IC, Oasi Research Institute - IRCCS, Troina, Italy.
Matteo Cesari, Department of Neurology, Medical University of Innsbruck, Innsbruck, Austria.
Birgit Frauscher, Analytical Neurophysiology Lab, Montreal Neurological Institute and Hospital, McGill University, Montreal, QC, Canada.
Anna Heidbreder, Department of Neurology, Medical University of Innsbruck, Innsbruck, Austria.
Melanie Bergmann, Department of Neurology, Medical University of Innsbruck, Innsbruck, Austria.
Birgit Högl, Department of Neurology, Medical University of Innsbruck, Innsbruck, Austria.
Ambra Stefani, Department of Neurology, Medical University of Innsbruck, Innsbruck, Austria.
Disclosure Statement
None Declared.
References
- 1. Berry RB, Quan SF, Abreu AR, et al. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Westchester: American Academy of Sleep Medicine; 2020. [Google Scholar]
- 2. Stefani A, Hogl B.. Diagnostic criteria, differential diagnosis, and treatment of minor motor activity and less well-known movement disorders of sleep. Curr Treat Options Neurol. 2019;21(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ferri R, Rundo F, Silvani A, et al. Short-interval leg movements during sleep entail greater cardiac activation than periodic leg movements during sleep in restless legs syndrome patients. J Sleep Res. 2017;26(5):602–605. doi: 10.1111/jsr.12529 [DOI] [PubMed] [Google Scholar]
- 4. Frauscher B, Gabelia D, Mitterling T, et al. Motor events during healthy sleep: a quantitative polysomnographic study. Sleep. 2014;37(4):763–773, 773A–773B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zucconi M, Oldani A, Ferini-Strambi L, Bizzozero D, Smirne S.. Nocturnal paroxysmal arousals with motor behaviors during sleep: frontal lobe epilepsy or parasomnia? J Clin Neurophysiol. 1997;14(6):513–522. doi: 10.1097/00004691-199711000-00008 [DOI] [PubMed] [Google Scholar]
- 6. Frauscher B, Gschliesser V, Brandauer E, et al. Video analysis of motor events in REM sleep behavior disorder. Mov Disord. 2007;22(10):1464–1470. doi: 10.1002/mds.21561 [DOI] [PubMed] [Google Scholar]
- 7. Manni R, Terzaghi M, Glorioso M.. Motor-behavioral episodes in REM sleep behavior disorder and phasic events during REM sleep. Sleep. 2009;32(2):241–245. doi: 10.1093/sleep/32.2.241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Frauscher B, Gschliesser V, Brandauer E, Ulmer H, Poewe W, Hogl B.. The relation between abnormal behaviors and REM sleep microstructure in patients with REM sleep behavior disorder. Sleep Med. 2009;10(2):174–181. doi: 10.1016/j.sleep.2008.01.003 [DOI] [PubMed] [Google Scholar]
- 9. Frauscher B, Gschliesser V, Brandauer E, et al. Motor disturbances during non-REM and REM sleep in narcolepsy-cataplexy: a video-polysomnographic analysis. J Sleep Res. 2011;20(4):514–521. doi: 10.1111/j.1365-2869.2011.00906.x [DOI] [PubMed] [Google Scholar]
- 10. Franceschini C, Ferri R, Pizza F, et al. Motor events during REM sleep in patients with narcolepsy-cataplexy: a video-polysomnographic pilot study. Sleep Med. 2011;12(suppl 2):S59–S63. doi: 10.1016/j.sleep.2011.10.013 [DOI] [PubMed] [Google Scholar]
- 11. Stefani A, Gabelia D, Mitterling T, Poewe W, Hogl B, Frauscher B.. A prospective video-polysomnographic analysis of movements during physiological sleep in 100 healthy sleepers. Sleep. 2015;38(9):1479–1487. doi: 10.5665/sleep.4994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rechtschaffen A, Kales A.. A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. Los Angeles: Brain Information Service/Brain Research Institute; 1968. [Google Scholar]
- 13. Moser D, Anderer P, Gruber G, et al. Sleep classification according to AASM and Rechtschaffen & Kales: effects on sleep scoring parameters. Sleep. 2009;32(2):139–149. doi: 10.1093/sleep/32.2.139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Iber C, Ancoli-Israel C, Chesson A, Quan SF; For the American Academy of Sleep Medicine. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 15. Ferri R, DelRosso LM, Provini F, Stefani A, Walters AS, Picchietti DL.. Scoring of large muscle group movements during sleep: an International Restless Legs Syndrome Study Group position statement. Sleep. 2021;44(9). doi: 10.1093/sleep/zsab092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. DelRosso LM, Ferri R, Allen RP, et al.; International Restless Legs Syndrome Study Group (IRLSSG). Consensus diagnostic criteria for a newly defined pediatric sleep disorder: restless sleep disorder (RSD). Sleep Med. 2020;75:335–340. doi: 10.1016/j.sleep.2020.08.011 [DOI] [PubMed] [Google Scholar]
- 17. DelRosso LM, Hartmann S, Baumert M, Bruni O, Ruth C, Ferri R.. Non-REM sleep instability in children with restless sleep disorder. Sleep Med. 2020;75:276–281. doi: 10.1016/j.sleep.2020.07.033 [DOI] [PubMed] [Google Scholar]
- 18. DelRosso LM, Bruni O, Ferri R.. Heart rate variability during sleep in children and adolescents with restless sleep disorder: a comparison with restless legs syndrome and normal controls. J Clin Sleep Med. 2020;16(11):1883–1890. doi: 10.5664/jcsm.8718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Senel GB, Kizilkilic EK, Karadeniz D.. Restless sleep disorder in children with NREM parasomnias. Sleep. 2021;44(7). doi: 10.1093/sleep/zsab049 [DOI] [PubMed] [Google Scholar]
- 20. Hashimoto T, Endo S, Fukuda K, et al. Increased body movements during sleep in Gilles de la Tourette syndrome. Brain Dev. 1981;3(1):31–35. doi: 10.1016/s0387-7604(81)80003-4 [DOI] [PubMed] [Google Scholar]
- 21. Nakatani M, Okada S, Shimizu S, et al. Body movement analysis during sleep for children with ADHD using video image processing. Annu Int Conf IEEE Eng Med Biol Soc. 2013;2013:6389–6392. doi: 10.1109/EMBC.2013.6611016 [DOI] [PubMed] [Google Scholar]
- 22. Zou C, Sun H, Lu C, Chen W, Guo VY.. Nighttime sleep duration, restlessness and risk of multimorbidity - A longitudinal study among middle-aged and older adults in China. Arch Gerontol Geriatr. 2022;99:104580. doi: 10.1016/j.archger.2021.104580 [DOI] [PubMed] [Google Scholar]
- 23. DelRosso LM, Picchietti DL, Spruyt K, et al.; International Restless Legs Syndrome Study Group (IRLSSG). Restless sleep in children: a systematic review. Sleep Med Rev. 2021;56:101406. doi: 10.1016/j.smrv.2020.101406 [DOI] [PubMed] [Google Scholar]
- 24. Gjerstad MD, Tysnes OB, Larsen JP.. Increased risk of leg motor restlessness but not RLS in early Parkinson disease. Neurology. 2011;77(22):1941–1946. doi: 10.1212/WNL.0b013e31823a0cc8 [DOI] [PubMed] [Google Scholar]
- 25. DelRosso LM, Jackson CV, Trotter K, Bruni O, Ferri R.. Video-polysomnographic characterization of sleep movements in children with restless sleep disorder. Sleep. 2019;42(4). doi: 10.1093/sleep/zsy269 [DOI] [PubMed] [Google Scholar]
- 26. Frauscher B, Mitterling T, Bode A, et al. A prospective questionnaire study in 100 healthy sleepers: non-bothersome forms of recognizable sleep disorders are still present. J Clin Sleep Med. 2014;10(6):623–629. doi: 10.5664/jcsm.3784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Iber C, Ancoli-Israel C, Chesson A, Quan SF.. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 28. Zucconi M, Ferri R, Allen R, et al.; International Restless Legs Syndrome Study Group (IRLSSG). The official World Association of Sleep Medicine (WASM) standards for recording and scoring periodic leg movements in sleep (PLMS) and wakefulness (PLMW) developed in collaboration with a task force from the International Restless Legs Syndrome Study Group (IRLSSG). Sleep Med. 2006;7(2):175–183. doi: 10.1016/j.sleep.2006.01.001 [DOI] [PubMed] [Google Scholar]
- 29. Frauscher B, Iranzo A, Gaig C, et al.; SINBAR (Sleep Innsbruck Barcelona) Group. Normative EMG values during REM sleep for the diagnosis of REM sleep behavior disorder. Sleep. 2012;35(6):835–847. doi: 10.5665/sleep.1886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cole TJ. The Lms method for constructing normalized growth standards. Eur J Clin Nutr. 1990;44(1):45–60. [PubMed] [Google Scholar]
- 31. Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd edn. Hillsdale, NJ: Erlbaum; 1988. [Google Scholar]
- 32. Wilde-Frenz J, Schulz H.. Rate and distribution of body movements during sleep in humans. Percept Mot Skills. 1983;56(1):275–283. doi: 10.2466/pms.1983.56.1.275 [DOI] [PubMed] [Google Scholar]
- 33. Gori S, Ficca G, Giganti F, Di Nasso I, Murri L, Salzarulo P.. Body movements during night sleep in healthy elderly subjects and their relationships with sleep stages. Brain Res Bull. 2004;63(5):393–397. doi: 10.1016/j.brainresbull.2003.12.012 [DOI] [PubMed] [Google Scholar]
- 34. Kronholm E, Alanen E, Hyyppa MT.. Nocturnal motor activity in a community sample. Sleep. 1993;16(6):565–571. doi: 10.1093/sleep/16.6.565 [DOI] [PubMed] [Google Scholar]
- 35. Pennestri MH, Whittom S, Adam B, Petit D, Carrier J, Montplaisir J.. PLMS and PLMW in healthy subjects as a function of age: prevalence and interval distribution. Sleep. 2006;29(9):1183–1187. doi: 10.1093/sleep/29.9.1183 [DOI] [PubMed] [Google Scholar]
- 36. Loddo G, Baldassarri L, Zenesini C, et al. Seizures with paroxysmal arousals in sleep-related hypermotor epilepsy (SHE): dissecting epilepsy from NREM parasomnias. Epilepsia. 2020;61(10):2194–2202. doi: 10.1111/epi.16659 [DOI] [PubMed] [Google Scholar]
- 37. Mitterling T, Hogl B, Schonwald SV, et al. Sleep and respiration in 100 healthy caucasian sleepers--a polysomnographic study according to American Academy of Sleep Medicine Standards. Sleep. 2015;38(6):867–875. doi: 10.5665/sleep.4730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kovacova K, Stebelova K.. Sleep characteristics according to gender and age measured by wrist actigraphy. Int J Environ Res Public Health. 2021;18(24):13213. doi: 10.3390/ijerph182413213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Redline S, Kirchner HL, Quan SF, Gottlieb DJ, Kapur V, Newman A.. The effects of age, sex, ethnicity, and sleep-disordered breathing on sleep architecture. Arch Intern Med. 2004;164(4):406–418. doi: 10.1001/archinte.164.4.406 [DOI] [PubMed] [Google Scholar]
- 40. Roehrs T, Kapke A, Roth T, Breslau N.. Sex differences in the polysomnographic sleep of young adults: a community-based study. Sleep Med. 2006;7(1):49–53. doi: 10.1016/j.sleep.2005.05.008 [DOI] [PubMed] [Google Scholar]
- 41. Bixler EO, Papaliaga MN, Vgontzas AN, et al. Women sleep objectively better than men and the sleep of young women is more resilient to external stressors: effects of age and menopause. J Sleep Res. 2009;18(2):221–228. doi: 10.1111/j.1365-2869.2008.00713.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. McCarter SJ, Hagen PT, St Louis EK, et al. Physiological markers of sleep quality: a scoping review. Sleep Med Rev. 2022;64:101657. doi: 10.1016/j.smrv.2022.101657 [DOI] [PubMed] [Google Scholar]
- 43. Iranzo A, Santamaria J.. Severe obstructive sleep apnea/hypopnea mimicking REM sleep behavior disorder. Sleep. 2005;28(2):203–206. doi: 10.1093/sleep/28.2.203 [DOI] [PubMed] [Google Scholar]
- 44. Liu WK, Dye TJ, Horn P, Patterson C, Garner D, Simakajornboon N.. Large body movements on video polysomnography are associated with daytime dysfunction in children with restless sleep disorder. Sleep. 2022;45(4). doi: 10.1093/sleep/zsac005 [DOI] [PubMed] [Google Scholar]
- 45. Ferri R, Zucconi M, Rundo F, Spruyt K, Manconi M, Ferini-Strambi L.. Heart rate and spectral EEG changes accompanying periodic and non-periodic leg movements during sleep. Clin Neurophysiol. 2007;118(2):438–448. doi: 10.1016/j.clinph.2006.10.007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


