INTRODUCTION
Male infertility affects 15% of couples, with azoospermia diagnosed among 1% of the general male population and 10–15% of males presenting with infertility. Use of well-defined diagnostic tools enable clinicians to reliably classify the etiology of azoospermia as pre-testicular, testicular, or post-testicular causes. Shared decision-making is recommended in navigating potential therapeutic options for couples wishing to pursue fertility. The purpose of this guideline is to equip the Canadian clinician with a diagnostic and management approach for males presenting with azoospermia. To achieve this, we provide a narrative overview and highlight three priority clinical questions, which were identified though a needs assessment among CUA members. For these priority topics, we performed a critical evaluation of the literature using EtD GRADE framework to arrive at transparent, evidence-based recommendations.
SUMMARY OF RECOMMENDATIONS
The committee identified three specific clinical circumstances in the management of NOA by which to apply the GRADE EtD framework for a transparent and systematic approach to make well-informed healthcare choices and recommendations. See section on “Management of azoospermia” for detailed explanation of each recommendation.
Among males with NOA, does cryopreservation of surgically retrieved sperm lower IVF-ICSI live birth rates in comparison to fresh sperm and oocytes?
■ RECOMMENDATION 1
We suggest cryopreservation of surgically retrieved sperm for most couples with NOA and subsequent IVF-ICSI (Conditional recommendation, very low certainty of evidence).
Among males with testicular failure NOA and a varicocele, does varicocele repair prior to surgical sperm retrieval and IVF-ICSI improve live birth rates compared to observation?
■ RECOMMENDATION 2
We suggest observation of varicoceles for most couples with testicular failure NOA and a varicocele considering surgical sperm retrieval and IVF-ICSI as compared to pre-treatment with varicocelectomy (Conditional recommendation, very low certainty of evidence).
Among males with testicular failure NOA, does neoadjuvant hormonal therapy improve IVF-ICSI live birth rates compared to conservative management?
■ RECOMMENDATION 3
We do not suggest neoadjuvant hormone therapies for males with testicular failure NOA for the purpose of improving IVF-ICSI live birth rates (Conditional recommendation, very low certainty of evidence).
METHODOLOGY
The guideline can be considered in two parts — one in which the panel attempted to assess priority areas of controversy in a systematic way using GRADE EtD framework, and the second as a narrative summary of background information for learners that is essential to understanding the topic area.
The work in this guideline was carried out by a panel of 17 subspecialty urologists. This panel was selected to represent a broad geographic representation of Canadian male reproductive experts. At the onset, each panel member contributed to the scope, audience, and topics of interest to cover in the guideline. In parallel, 10 community urologists were contacted across the country to perform a needs assessment for the content and topics they would like discussed in the guideline (see Appendix 1; available at cuaj.ca). Virtual meetings were then conducted among all members of the guideline committee to further define scope, topics for discussion, and topics to evaluate through EtD GRADE methodology.1,2
The expert panel assessed available literature and existing guidelines from the AUA3 and previous CUA4 guidelines and incorporated expert opinion from the 17 members of the panel, with input from 10 community urologists, to arrive at a list of 26 general topics felt to be important to include in the guideline. The expert panel reviewed the 26 topics and determined, through expert opinion and literature review, that there were eight controversial topics where a more in-depth assessment using the GRADE EtD framework might provide a more informed review and benefit the urological and reproductive communities. Given the time-intensive nature of using the GRADE EtD framework and available resources, the panel decided that only three high-priority, controversial questions could practically be addressed in this guideline. The selection of the three topics to study using the GRADE EtD framework was then decided by a consensus of the expert panel. The panel recognizes that there may be additional important questions that merit systematic evaluation and can be addressed in subsequent iterations of this guideline. The selected topics are listed below.
Among males with NOA, does cryopreservation of surgically retrieved sperm lower IVF-ICSI live birth rates in comparison to fresh sperm and oocytes?
Among males with NOA and a varicocele, does varicocele repair prior to surgical sperm retrieval and IVF-ICSI improve live birth rates compared to observation?
Among males with hypergonadotropic-hypogonadism (testis failure) NOA, does neoadjuvant hormonal therapy improve IVF-ICSI live birth rates compared to conservative management?
The narrative component of this guideline provides an overview of the background, evaluation and standard management of males with azoospermia for a general Canadian urologist. This section is largely based on clinical principles and expert opinion, which is listed throughout for clarity but should not be mistaken for a systematic assessment of evidence. Other goals derived from the panel’s discussions and needs assessment were to define clinical scenarios requiring referral to a fertility subspecialist to support the general Canadian urologist and provide optimal patient care.
About GRADE
The GRADE approach facilitates assigning certainty of evidence for each outcome. Using this methodology, the level of certainty was categorized as very low, low, moderate, or high.2,5 The EtD framework facilitated a systematic approach to make clinical recommendations based on a balance of critical components that underly clinical decision-making. These include desirable effects, undesirable effects, balance of these effects, certainty in estimates of effect, cost effectiveness, equity, resources required, patients’ values and preferences, feasibility, and acceptability.5 The summary of findings and EtD framework tables were generated using the GRADE pro GTD application and are available in the Appendix at cuaj.ca.6,7
Since the GRADE and EtD framework were used to answer three questions in the management of NOA, live birth rates, clinical pregnancy rates, and sperm retrieval rates were considered as outcomes when available. The effect estimates for these three questions were derived from the most recent and comprehensive existing meta-analyses and systematic reviews, by reproducing some stages of the review to ensure accuracy of the effect sizes. These stages included overall appraisal of the systematic reviews and meta-analyses, review and evaluation of additional literature to locate missing studies, evaluating risk of bias from the original studies, then re-performing the meta-analyses again.
Shared decision-making between the clinician and patient is key to interpreting recommendations from the GRADE framework, where patient values and preferences are central. Recommendations are categorized by direction, for or against, and by strength, strong or conditional. A strong recommendation implies that the panel believes that a significant majority of patients would be in alignment with the recommendation when aware of the available evidence. A conditional recommendation indicates that the panel believes most patients would agree with the recommendation, but a significant proportion would not. Thus, for a minority of patients, it may be appropriate not to proceed with the conditional recommendation. Therefore, for conditional recommendations, it is critical for the clinician and patient to engage in shared decision-making considering available evidence, as well as patient values and preferences to arrive at the “best” course of action.
INCIDENCE & DEFINITION OF AZOOSPERMIA
Male infertility affects 15% of couples globally, with male factors contributing to 50% of cases.8–10 The most severe form of infertility is termed azoospermia and is defined by the complete absence of sperm after microscopic evaluation of two separate semen samples after centrifugation at 3000 g for 15 minutes.11,12 Azoospermia is found in 1% of the general population and 10–15% of males presenting for fertility evaluation.13
CLASSIFICATION & ETIOLOGIES OF AZOOSPERMIA
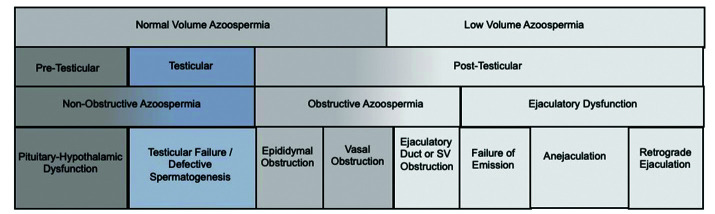
Azoospermia may be classified through several approaches (Figure 1). Anatomical classification includes division by pre-testicular, testicular, and post-testicular aetiologies.
Figure 1.
Classifications of azoospermia. SV: seminal vesicle.
Pre-testicular azoospermia accounts for 2% of males with azoospermia.4,14–16 It is defined as a failure of sperm production (NOA) due to a hypothalamic or pituitary abnormality, presenting as hypo-gonadotropic-hypo-gonadism (HH). This may be due to congenital abnormalities (i.e., normosmic idiopathic HH; or anosmic HH, i.e., Kallman syndrome) or may be acquired (i.e., pituitary tumor, pituitary or hypothalamic trauma, or through exogenous testosterone or hormone therapies).17
Testicular failure azoospermia (NOA) accounts for 49–93% of males with azoospermia.4,14–16 It is defined as a failure of sperm production due to testicular failure, presenting as hyper-gonadotropic-hypo-gonadism. This may be due to congenital abnormalities (i.e., Klinefelter Syndrome, Y-chromosome microdeletions) or may be acquired (i.e., chemotherapy, radiation therapy, etc.). Histopathological classification is often used to subcharacterize testis failure NOA. This includes hypospermatogenesis, where rare spermatozoa are identified; maturation arrest, where germ cell differentiation stops at any immature stage; or Sertoli cell-only syndrome. where no germ cells are identified on histology.
-
Post-testicular azoospermia accounts for 7–51% of cases with azoospermia.4,14–16 It is due to either obstruction of the male reproductive tract, that may be acquired or congenital, such as absence of vas deferens, or an ejaculatory abnormality in the presence of otherwise normal spermatogenesis.
○ Obstruction may occur at the level of the epididymis, vas deferens, or ejaculatory duct. Epididymal obstruction may be acquired following epididymitis, idiopathic, or secondary to blockage associated with vasectomy or incidentally with scrotal surgery in up to 10–15% of cases. Vas deferens obstruction may be acquired during vasectomy, inguinal hernia repair or pelvic surgeries. Congenital obstruction of the epididymis or vas deferens may be associated with mutations of the CFTR gene. Ejaculatory duct obstruction may be due to Mullerian or ejaculatory duct cysts or secondary to urethral instrumentation.
○ Ejaculatory abnormalities may include retrograde ejaculation, anejaculation, or failure of emission. Retrograde ejaculation may be associated with benign prostatic hyperplasia, prostate-ablating surgeries (i.e., transurethral resection of prostate), or medications altering the bladder neck (i.e., alpha-blockers). Anejaculation is most often associated with SSRIs but may also be associated with hyperprolactinemia, low testosterone, or psychogenic causes. Failure of emission can occur from similar causes as retrograde ejaculation but is often associated with neurologic dysfunction, as seen in spinal cord injury and pelvic nerve injuries (i.e., retroperitoneal lymph node dissection).18
EVALUATION OF THE MALE WITH AZOOSPERMIA
Investigation of the male with azoospermia through a systematic and algorithmic approach will result in accurate diagnosis in most circumstances, as demonstrated in Figures 2 and 3. Some investigations may be initiated by general practitioners and general urologists, while final diagnosis and management is best offered through a male infertility expert connected to appropriate clinical facilities with a full breadth of clinical and surgical expertise.
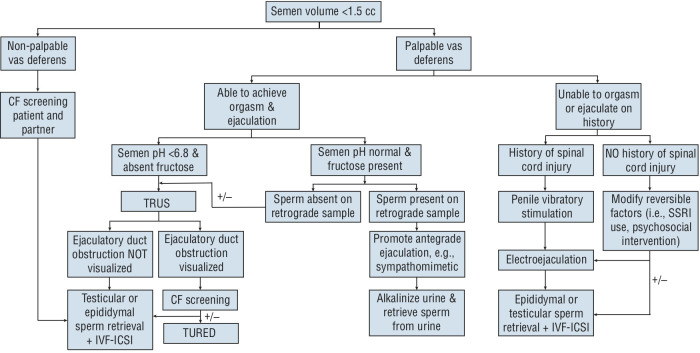
Figure 2.
Diagnostic and management flow chart for the evaluation of males with low-volume azoospermia. Note: Counselling for sperm donation, adoption, or child-free living are important options in each clinical scenario. Following low-volume azoospermia workflow is with assumption that follicle-stimulating hormone (FSH) is normal. If FSH is abnormal, consider following normal volume azoospermia workflow. If fructose or pH is not available on semen analyses, consider performing a post-ejaculate urine evaluation. CF: cystic fibrosis; IVF-ICSI: in vitro fertilization intracytoplasmic sperm injection; TRUS: transrectal ultrasound; TURED: transurethral resection of ejaculatory duct.
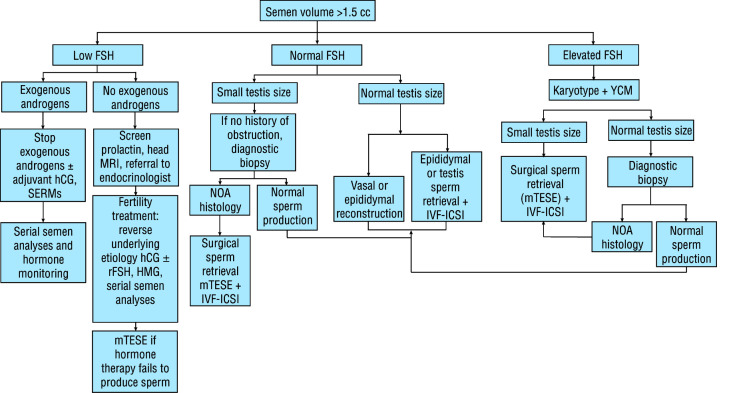
Figure 3.
Diagnostic and management flow chart for the evaluation of normal volume azoospermia in males. Note: Counselling for sperm donation, adoption, or child-free living are important options in each clinical scenario. FSH: follicle stimulating hormone; hCG: human chorionic gonadotropin; HMG: human menopausal hormone; IVF-ICSI: in vitro fertilization intracytoplasmic sperm injection; MRI: magnetic resonance imaging; mTESE: microdissection testicular sperm extraction; NOA: non-obstructive azoospermia; rFSH: recombinant follicle stimulating hormone; SERM: selective estrogen receptor modulator; YCM: Y chromosome microdeletion.
Standard of care
Males found to have azoospermia on semen analysis, should undergo a second semen analysis with microscopic evaluation of the centrifuged pellet. In addition to a thorough history and physical exam, patients should undergo serum FSH, morning TT testing and LH. (Note: LH is not required if TT is normal; however, if TT low, then LH, estradiol, and prolactin testing are indicated). These patients should undergo further evaluation by a male reproductive expert for specialized testing, as well as medical and surgical management. Their partner should also undergo a complete female fertility evaluation with a reproductive endocrinology/infertility specialist (Clinical principle).
History
The aim of the infertility history is to identify underlying etiologies and potentially modifiable factors to enhance chances of fertility. It is also important to understand and tailor management to the couple’s fertility goals. Table 1 summarizes areas of importance when taking an infertility history, in addition to open-ended questions (Expert opinion).
Table 1.
History for infertility evaluation
| Medical history | Areas of focus |
|---|---|
| History of presenting illness (HPI) |
|
| Past medical history |
|
| Past surgical history |
|
| Medications |
|
| Social history |
|
| Family history |
|
Physical examination
Physical exam is extremely important in helping distinguish the etiology of azoospermia. Key features on physical exam are summarized in Table 2. An emphasis of importance is placed on testis size, epididymal fullness, and presence of vas deferens (Expert opinion).
Table 2.
Key physical exam features for an infertility evaluation
| Location of examination | Areas of focus |
|---|---|
| General |
|
| Scrotum |
|
| Abdomen |
|
| Chest |
|
Standard investigations (based on expert opinion)
Mandatory: These include two semen analyses, serum morning TT, and serum FSH:
Semen analyses should be centrifugated at 3000 g for 15 minutes with microscopic evaluation of the pellet for rare sperm.11,12 Semen volume is critical in guiding further azoospermia evaluation.
Serum morning TT, LH, and FSH help differentiate pre-testicular, testicular, and post-testicular etiologies of azoospermia. Undetectably low FSH and low TT suggest pre-testicular azoospermia. Normal FSH and TT suggest post-testicular azoospermia. Elevated FSH with normal or low TT suggest azoospermia associated with testicular failure.
Optional tests include LH, prolactin, and estradiol if TT is low. Scrotal ultrasound is not routinely ordered, but may be considered if physical examination is difficult or testicular mass is suspected (Expert opinion).
Specialized investigations (based on expert opinion)
Karyotype is routinely ordered for all males with azoospermia with suspected testicular failure. While numerous abnormalities have been associated with azoospermia, Klinefelter Syndrome, defined as the presence of at least one additional X chromosome (i.e., 47, XXY), is the most common genetic abnormality in males with azoospermia and is identified in 10.8% of males with azoospermia due to testicular failure.19 Other chromosomal abnormalities may include, but are not limited to, inversion, translocations, and other sex chromosome abnormalities.19,20 Couples with karyotypic abnormalities may benefit from referral for genetic counselling.
Y chromosome microdeletion (YCM) is routinely ordered for all males with azoospermia with suspected testicular failure. This test evaluates for DNA deletions of the azoospermia factor region on the Y chromosome. Commonly evaluated YCMs include the AZFa, AZFb, and AZFc deletion, and are found in 7.5% of azoospermic males.21 Complete deletions of AZFa and AZFb regions have not been associated with successful sperm retrieval, and thus sperm retrieval should not be attempted. Thirty three percent of men with complete AZFc deletions have been found to have sperm present in the ejaculate, while 47% of men with azoospermia with complete AZFc deletions have successful surgical sperm retrieval.22 Couples with YCMs may benefit from referral for genetic counselling.
Cystic fibrosis transmembrane conductance regulatory gene (CFTR) is indicated for males with absent vas deferens (unilateral or bilateral), idiopathic epididymal obstruction, or absent/hypoplastic seminal vesicles on TRUS. Numerous mutations have been described and commonly used assays report on frequently identified mutations. CFTR pathogenic variants are identified in 80–97% of men with congenital bilateral absence of vas deferens.23,24 When CFTR mutations are identified, the female partner should also be evaluated for CFTR mutations, and the couple referred for genetic counselling to discuss the risk of cystic fibrosis in the offspring.
Post-ejaculate urine analysis is indicated in low-volume azoospermia (<1.5 mL) to evaluate for presence and quantity of sperm in the urine reflective of retrograde ejaculation.
Transrectal ultrasound is indicated in low-volume azoospermia (<1.5 mL), when semen pH is <6.8, fructose is negative, and retrograde urine analysis is negative. CFTR testing is indicated in cases with absent or hypoplastic seminal vesicles and abdominal vas deferens, as the male will have 80% risk of carrying a CFTR mutation.25 Enlarged seminal vesicles (>1.5 cm wide) typically reflect ejaculatory duct obstruction, which may be accompanied by a cystic structure in the prostate.26
Testis biopsy is not routinely performed in the evaluation of azoospermia. Testis biopsy is reserved for male reproductive experts and can be used to confirm a presumptive diagnosis of obstructive azoospermia, typically in situations where history, physical exam findings, and investigations (serum FSH) point to a post-testicular etiology of azoospermia but are unclear. If testis biopsy is indicated, unilateral biopsy should be performed if symmetrical testis volume. If asymmetrical testis volumes, the larger of the two testes should be biopsied.
Abdominal ultrasound is indicated in the presence of unilateral or bilateral non-palpable vas deferens. The purpose is to evaluate for embryologic mesonephric (Wolffian) duct anomalies (i.e., renal agenesis, malformation, or malposition).
DNA sequencing. While reports of single gene defects have been reported in the literature,27,28 routine whole genome sequencing, whole exome sequencing, or gene arrays are not currently indicated for routine clinical testing.
Biomarkers for NOA have been investigated for decades. Anti-Mullerian hormone (AMH) and inhibin B have been investigated but do not meaningfully predict chances of sperm retrieval for males with testis failure NOA.29–33 Recent reports of AMH:testosterone appear intriguing; however, additional data is required prior to application to widespread clinical practice.
In summary, history, physical, and investigations will help the clinician delineate the underlying classification and cause of azoospermia. Azoospermia evaluative algorithms are divided into low-volume azoospermia (Figure 2) and normal-volume azoospermia (Figure 3).
MANAGEMENT OF AZOOSPERMIA
Couples should be counselled that their fertility options may include child-free living, adoption, donor sperm, or attempts to have children genetically related to the male. The following management approaches are directed toward having children genetically related to the male. Figures 2 and 3 summarize the diagnostic and treatment algorithms for males with azoospermia. Management of males with azoospermia should be performed by urologists with expertise in male reproduction with access to advanced fertility centre services.
Pre-testicular azoospermia
The treating clinician should reverse any identified causes of pre-testicular azoospermia. Advanced hormonal manipulation should be done under the supervision of a fertility specialist comfortable with prescribing the medications and monitoring these patients (Expert opinion).
Congenital pre-testicular azoospermia
Congenital pre-testicular azoospermia is most commonly managed with gonadotropic therapy. While several different medications and combinations may be used, in most instances, hCG is routinely used, with 1000–2500 IU injected subcutaneously two to three times per week. Addition of recombinant FSH, 75–150 IU three times per week, or human menopausal gonadotropins (hMG), 75–150 IU three times per week, may be considered after 3–6 months if no sperm is present in the ejaculate. Alternative options may include pulsatile gonadotropin-releasing hormone pump. Spermatogenesis may be achieved in 80% by six months34 and overall up to 90% of males with gonadotropin therapy. Surgical sperm retrieval using a technique such as mTESE is only considered after an adequate trial of gonadotropin therapy by a reproductive expert34–37 (Expert opinion).
Acquired pre-testicular azoospermia
Exogenous testosterone, steroids, or hormone therapy should be stopped when safe or augmented with gonadotropins if not otherwise possible. The patient may be followed for natural recovery of the hypothalamic-pituitary-gonadal (HPG) axis by following serum LH, FSH, TT ± estradiol and semen analyses every 1–3 months. Clinicians may consider use of off-label gonadotropin therapy (i.e., hCG), selective estrogen receptor modulators (SERM s) (i.e., clomiphene), or aromatase inhibitors (AIs) (i.e., anastrazole or letrozole) to augment HPG recovery. If HPG recovery is not possible, the clinician should use gonadotropin therapy, as discussed above for congenital pre-testicular azoospermia. Elevated prolactin should be repeated, and if it remains elevated, patients should have expert endocrine evaluation, such as referral to an endocrinologist, when the treating reproductive urologist does not have the expertise for further evaluation and management (Expert opinion).
Testicular failure azoospermia (NOA)
Fertility options for couples where the male has testicular failure NOA includes attempted surgical sperm retrieval combined with IVF-ICSI, donor sperm, adoption, or child-free living. Sperm retrieval techniques described have included TESE,38 fine needle aspiration mapping,39 and mTESE.40 mTESE is the most accepted technique among male reproductive experts. While no head-to-head controlled randomized trials exist comparing surgical techniques, among studies controlled for histopathology, mTESE is 1.3 times more likely to retrieve sperm than conventional TESE. This corresponds to a NNT to treat of eight, and drops to a NNT of five in cases of Sertoli cell-only, where sperm retrieval is 2.3 times more likely with mTESE.41
mTESE leverages the ability to take advantage of intratesticular heterogeneity of spermatogenesis. It requires use of an operating microscope to visualize the seminiferous tubules within the testis, allowing the surgeon to select the healthiest appearing tubules potentially containing sites of active spermatogenesis. This tissue is further dissociated and analyzed using a bench microscope to find rare sperm in 52% of males.42
Sperm used with IVF-ICSI leads to oocyte fertilization in approximately 57% of attempts and results in clinical pregnancy and live birth rates in up to 39% and 24% of patients that undergo embryo transfers, respectively. 43 No apparent differences exist in congenital malformations for offspring of fathers with NOA compared to OA controls undergoing IVF-ICSI.44 mTESE procedures may be offered to all males with testicular failure NOA, but should only be undertaken in a center with expertise in mTESE and where an IVF-ICSI laboratory has expertise in processing the tissue. Males with NOA should also be made aware of options for sperm donation, adoption, and child-free living (Expert opinion).
Unique considerations
In males with complete AZFa or AZFb deletions, sperm have not been previously identified during retrieval and the utility of retrieval by any technique is negligible.
While controversy exists among timing of attempted surgical sperm retrieval in males with Klinefelter syndrome, the committee recommends performing surgical sperm retrieval for males at the time of desired fertility or post-pubertally prior to initiating exogenous testosterone therapy when they and their families have been adequately counselled and wish to attempt cryopreservation45 (Expert opinion).
GRADE ETD RECOMMENDATIONS
The panel identified three specific clinical circumstances in the management of NOA by which to apply the GRADE EtD framework for a transparent and systematic approach to make well-informed healthcare choices and recommendations. These recommendations are summarized at the beginning of this guideline and should be used as guidance and not definitive rules, as shared decision-making is an essential element for personalized patient management.
Among males with NOA, does cryopreservation of surgically retrieved sperm lower IVF-ICSI live birth rates in comparison to fresh sperm and oocytes?
Based upon the available evidence, the panel conditionally recommends cryopreserving microsurgically retrieved sperm in males with NOA to use for a subsequently staged cycle of IVF-ICSI. This recommendation should be considered as a general guiding principal, where certain patient and couple-centered factors may influence the approach of performing fresh mTESE sperm retrievals concomitant with fresh/frozen oocytes for IVF-ICSI vs. staged mTESE sperm retrieval with cryopreservation and subsequent IVF-ICSI cycle. Of particular note, the current healthcare coverage in Quebec favors the use of fresh sperm retrieval and IVF-ICSI due to substantial cost savings for the couple compared to a cryopreservation protocol. For couples looking for the highest chance of live birth irrespective of all other considerations, then a fresh sperm retrieval and IVF-ICSI cycle may be preferred. Appendix 2 (available at cuaj.ca) transparently summarizes the panel’s summary of judgements related to components underlying clinical decision-making for this question such as: desirable effects, undesirable effects, balance of these effects, certainty in estimates of effect, cost effectiveness, equity, resources required, patients’ values and preferences, feasibility, and acceptability.5
The panel reviewed 24 studies46–69 from Amer and Fakhry’s 2021 systematic review,70 and subsequent studies published since this review, where an appropriate comparator arm was available. The panel acknowledges that the evidence has significant limitations, as only seven of 23 cryopreservation studies reported an intention-to-treat-like methodology, where patients were included if sperm were found during surgical sperm retrieval, thereby capturing potential unusable sperm after cryopreservation; the remainder of the studies potentially underestimate the negative impact of cryopreservation on primary and secondary outcomes by only reporting IVF-ICSI outcomes with useable sperm in fresh or cryopreserved conditions.
We calculate a 92.2% rate of finding useable sperm for IVF-ICSI after cryopreservation and thawing of NOA-derived testicular sperm among all studies reporting this data, meaning that 7.8% of couples may not have useable sperm following thawing of a cryopreserved sample. In this context, the meta-analysis results for the primary outcome — live birth rate — demonstrated that cases using cryopreserved sperm had a RR of 0.77 (95% CI 0.67–0.89) compared to those cases using fresh sperm; similarly, we performed a sensitivity analysis including only the seven studies using an intention-to-treat-like methodologically demonstrating that using cryopreserved sperm had a RR of 0.73 (95% CI 0.60–0.88). Collectively, both the primary meta-analysis and sensitivity analyses favor the use of fresh sperm over cryopreserved sperm in patients undergoing surgical sperm retrieval and IVF-ICSI (see Appendices 3, 4A, 4B, 5A, 5B for summary of literature, effect size and summary of findings; available at cuaj.ca).
While performing coordinated fresh sperm retrieval and oocyte retrieval for IVF-ICSI appears to have an advantage for overall live birth rates, there are notable potential disadvantages in this approach compared to sperm retrieval and cryopreservation prior to oocyte retrieval and IVF-ICSI, such as increased cost,71 harms to the female partner when committing to an IVF cycle if backup donor sperm is not desired, and limitations to accessing such logistics at many centers. Therefore, the panel suggests that most couples would overall benefit from cryopreservation of surgically retrieved mTESE sperm with subsequent IVF-ICSI; however, it is also acceptable to perform coordinated fresh sperm retrieval and oocyte retrieval to meet the needs of individual couples, such as couples desiring the highest chance of live birth rate irrespective of all other considerations, couples willing to use back up donor sperm, or couples living in Quebec.
Among males with NOA and a varicocele, does varicocele repair prior to surgical sperm retrieval and IVF-ICSI improve live birth rates compared to observation?
Based upon the available evidence, the panel conditionally recommends observing varicoceles prior to surgical sperm retrieval in patients with NOA. This recommendation should be considered as a general guiding principal, where certain patient and couple-centered factors may influence the approach of performing varicocele repairs prior to attempted sperm retrieval. Couples with younger female age may be amenable to an attempted varicocele repair, where the delay in attempted sperm retrieval and IVF-ICSI doesn’t change the statistical IVF-ICSI outcomes. Appendix 6 (available at cuaj.ca) transparently summarizes the panel’s summary of judgements related to components underlying clinical decision-making for this question, such as desirable effects, undesirable effects, balance of these effects, certainty in estimates of effect, cost effectiveness, equity, resources required, patients’ values and preferences, feasibility, and acceptability.5
The panel reviewed three studies for sperm retrieval, 72–74 one study for clinical pregnancy and live birth rates74 derived from several recent meta-analyses and systematic reviews,75–77 and numerous studies evaluating varicocele repair complications and recurrences.78–85 The studies by Haydardedeoglu et al86 and Kizilkan et al87 were excluded, which deviated from previously published meta-analyses, due to the comparator group not having a varicocele; thus, preventing the comparison of a treatment effect of a varicocele repair. Based on the primary outcome of live birth rates, males that underwent varicocele repair had a RR of 1.43 (95% CI 0.34–6.11) compared to males that had a varicocele and did not undergo repair.
Secondary outcomes of clinical pregnancy rate demonstrated that males undergoing varicocele repair had a RR of 1.75 (95% CI 0.42–7.27) compared to males with a varicocele who did not undergo repair, and sperm retrieval rate demonstrated that males undergoing a varicocele repair had a RR of 1.19 (95% CI 0.82–1.73) compared to males with a varicocele that did not undergo a VR (see Appendices 7, 8, 9, 10 for summary of literature, effect size and summary of findings; available at cuaj.ca) With a significant paucity of data, further studies with appropriate comparator groups are needed to further evaluate the role of VRs in this group.
Among males with testicular failure NOA, does neoadjuvant hormonal therapy improve IVF-ICSI live birth rates compared to conservative management?
Based on the available evidence, the panel conditionally recommends proceeding directly to microsurgical sperm retrieval in the context of neoadjuvant hormone therapy for the sole purpose of improving fertility outcomes, such as live birth rate. This recommendation should be considered as a general guiding principal, where certain patient and couple-centered factors may influence the approach of neoadjuvant hormone therapy prior to attempted sperm retrieval. Patients that are hypogonadal and symptomatic may still benefit from adjuvant therapies, such as SERMs and aromatase inhibitors; and gonadotropins, such as hCG. Appendix 11 (available at cuaj.ca) transparently summarizes the panel’s summary of judgements related to the components underlying clinical decision-making for this question, such as desirable effects, undesirable effects, balance of these effects, certainty in estimates of effect, cost effectiveness, equity, resources required, patients’ values and preferences, feasibility, and acceptability.5
The panel reviewed studies derived from the recent meta-analysis by Tharakan et al.88 These included three studies reporting on clinical pregnancy rates and live birth outcomes89–91 and 12 studies evaluating sperm retrieval rates.89–99 It should be prefaced that the treatment regimens were extremely heterogeneous; sensitivity analyses for specific regimens are included in Appendices 13, 14, 15 (available at cuaj.ca). Similarly, the patient populations were highly heterogeneous, including some patients with Klinefelter syndrome, others with idiopathic NOA, primary mTESE s, and some salvage mTESE s. Based on the primary outcome, the RR of live birth rate for males treated with neoadjuvant hormone therapy was 0.75 (95% CI 0.52–1.08) relative to males who did not. The secondary outcomes of clinical pregnancy rate demonstrated that males treated with neoadjuvant hormone therapy had a RR of 0.94 (95% CI 0.57–1.53) compared to males with no treatment, and surgical sperm retrieval rates demonstrated a RR of 1.40 (95% CI 1.01–1.93) in males treated with neoadjuvant hormone therapy relative to males that did not (See Appendices 12, 13, 14, 15 for summary of literature, effect size and summary of findings; available at cuaj.ca). Therefore, it is emphasized that subpopulations may have potential benefit as additional, higher-quality data is generated and considered; however, at the present time, the patient populations and neoadjuvant hormonal strategies remain poorly understood to support generalized use of neoadjuvant hormones in all males with NOA.
Post-testicular azoospermia (based on expert opinion)
Management of post-testicular azoospermia may be achieved through appropriate surgical reconstruction/correction, medical therapies (ejaculatory failure), or through sperm retrieval and ART.
Obstructive azoospermia
Epididymal obstruction may be successfully reconstructed micro-surgically to facilitate motile sperm to be transported into the semen in 48–84% of cases,100 and facilitate a natural pregnancy in approximately 20–50% of cases.101 Various surgical techniques have been described for VEs, with longitudinal intussuscepted vasoepididymostomy being broadly cited with respectable success rates,100,102 although microsurgical technique of the surgeon’s preference is appropriate. An operating microscope should be used in all epididymal reconstruction procedures. Patients should be offered the opportunity to cryopreserve sperm at the time of the procedure, since a non-trivial number of patients undergoing epididymal reconstruction are not ultimately successful in establishing motile sperm in the ejaculate. Patients may also be offered the opportunity to cryopreserve sperm following successful epididymal reconstruction where sperm is present in the semen postoperatively, in case they acquire a late failure of the anastomosis.
Vasal obstruction encountered in the scrotum, inguinal canal, or intra-abdominally may be successfully reconstructed micro-surgically to facilitate motile sperm to be transported into the semen to accommodate a natural pregnancy. Numerous techniques exist with adequate reported success. Multilayer anastomoses with an operating microscope are recommended. Patency rates have been reported to vary from 70–99.5%,101,103 with the highest reported rates using multilayer, microdot techniques.104
Vasectomy reversals may be performed by surgeons who possess the clinical and microsurgical expertise, operating microscope availability, equipment, and environment necessary to perform either a vasovasostomy or VE at the time of reconstruction. Vasectomy reversals should not be routinely offered if the ability to adequately perform a VE is not within the skillset of the surgeon, as an estimated 13–60% of vasectomy reversal cases require at least unilateral VE,105–108 including up to 20% within obstructive intervals of 0–3 years,106 and no available preoperative definitive diagnostic test can determine the presence of epididymal obstruction. To determine which type of reconstruction is necessary — vasal or epididymal — intra-vasal fluid should be evaluated microscopically at the time of the reconstruction. Patients seeking vasectomy reversals should be made aware of sperm retrieval and IVF-ICSI as another viable fertility option for couples desiring biologic children, or the ability to concomitantly perform sperm retrieval at the time of vasectomy reversal.
Ejaculatory duct obstruction (EDO) may be successfully treated through TURED. This procedure is successful in improving semen parameters among 59% of patients with complete EDO and 94% with partial EDO, resulting in spontaneous pregnancy rates of 12.5–31%. TURED is best performed with intraoperative, real-time TRUS guidance to precisely unroof the ejaculatory duct cyst. Preoperative counselling on potential surgical complications and the risks of restenosis postoperatively is important.109
Ejaculatory failure
Failure of emission may be overcome through EEJ in nearly all men to successfully obtain sperm.
Retrograde ejaculation may be managed through use of oral sympathomimetics, such as pseudoephedrine 60–120 mg prior to ejaculation. In refractory cases, a retrograde urine sample may be collected for subsequent ART. Urine alkalinization using NaHCO3 should be performed prior to retrograde sample collection to optimize sperm quality.
Anejaculation may be managed through modifying any reversible factors (i.e., SSRI discontinuation) or through psycho-sexual education and counselling when appropriate. Limited data exists for off-label medication use, such as cabergoline.110 In refractory cases, sperm may be retrieved through EEJ or directly from the testicle or epididymis.
In cases where spinal cord injuries are present, penile vibratory stimulation may be performed with success in eliciting antegrade ejaculation among 86% of males whose level of injury is T10 or cranial, and in 17% of males whose level of injury is T10 or caudal.111 In refractory cases, EEJ may be considered, which elicits ejaculation among 97% of males.111 It is important to monitor and be prepared to treat males with spinal cord injuries receiving penile vibratory stimulation or EEJ for autonomic dysreflexia.
Sperm retrieval for post-testicular azoospermia
Sperm may be retrieved from the epididymis percutaneously (PESA) or microsurgically (MESA). Sperm may also be retrieved directly from the testicle percutaneously (TESA) or through a small incision (TESE). Sperm retrieved through these techniques require pairing with IVF-ICSI to achieve pregnancy. PESA, TESE, or TESA may be performed in an outpatient setting. While MESA may result in higher quantity of sperm retrieved, it requires an operating microscope typically associated with sedation or a general anesthetic and is more costly. Common practice among Canadian practitioners is to offer PESA, TESA, or TESE to limit costs, with acceptable rates of success; however, each of the aforementioned methods of sperm retrieval in this population are appropriate.112
FUTURE PRIORITIES OF RESEARCH
Azoospermia remains an important area of male reproduction that has been vastly underserved with respect to research. The committee has identified several clinical, translational, and basic science areas of research as future priorities.
Clinical
Nearly all clinical data arises from retrospective case series and, in rare circumstances, prospective series or clinical trials. Prospective, multicentered, randomized clinical trials would elevate the level of evidence in the evaluation and management of azoospermia. Among men with NOA, the impact of varicocele repair on sperm retrieval, clinical pregnancy, and live birth rate has yet to be definitively determined. Use of neoadjuvant medical therapy, including SERM s, gonadotropins, aromatase inhibitors, and other strategies for males with testicular failure NOA is of interest and requires further evaluation. Further, value-based research on patients with NOA and their partners is necessary to better understand patient perspectives on treatment pathways and their multidimensional impact.
Translational
Identification of reliable biomarkers and diagnostic testing to predict sperm retrieval in males with NOA is of interest. Technologies to identify rare sperm for patients with NOA would be of value to the male reproductive field. Medical or regenerative approaches to stimulating sperm production in patients with NOA is a challenging problem.
Basic science
Understanding the mechanisms of NOA remains an elusive problem for scientists and clinicians alike. Further, well-characterized studies evaluating the pathogenesis and mechanistic genomic and molecular biological events contributing to NOA would be of paramount value to the field of reproductive medicine. Such knowledge could lead to effective interventions to restore spermatogenesis, especially for men with maturation arrest and identifiable but potentially treatable abnormalities affecting sperm development. Similarly, development of regenerative strategies to promote spermatogenesis and spermiogenesis in vivo or in vitro would be valuable for future therapeutic intervention.
Supplementary Information
ACRONYMS
- ART
advanced reproductive therapies
- AUA
American Urological Association
- CFTR
cystic fibrosis transmembrane regulatory
- CI
confidence interval
- CUA
Canadian Urological Association
- EDO
ejaculatory duct obstruction
- EEJ
electroejaculation
- EtD
evidence-to-decision
- FSH
follicle-stimulating hormone
- hCG
human chorionic gonadotropin therapy
- ICSI
intracytoplasmic sperm injection
- IVF
in vitro fertilization
- LH
luteinizing hormone
- MESA
microsurgical epididymal sperm aspiration
- mTESE
microdissection testicular extraction of sperm
- NNT
number needed to treat
- NOA
non-obstructive azoospermia
- OA
obstructive azoospermia
- PESA
percutaneous epididymal sperm aspiration
- RR
risk ratio
- SERM
selective estrogen receptor modulators
- SSRI
selective serotonin reuptake inhibitors
- TESA
testicular sperm aspiration
- TRUS
transrectal ultrasound
- TT
total testosterone
- TURED
transurethral resection of ejaculatory duct
- VE
vasoepididymostomy
- YCM
Y chromosome microdeletion
Footnotes
Appendices available at cuaj.ca
COMPETING INTERESTS: Dr. Flannigan has been an advisory board member for Acerus; has received speaker honoraria from Boston Scientific and Paladin; has equity in Teumo Health Technologies Inc.; and received product from Theralogix for clinical trial evaluating impact of antioxidant supplements on sperm DNA fragmentation. Dr. Patel has been a consultant for Boston Scientific and Nestle Health; and has received honoraria from Paladin. Dr. Mak has served in advisory boards for Astellas, Bayer, Ferring, Janssen, Sanofi, and TerSera; and has been a presenter for AbbVie, Amgen, Astellas, Duchesnay, Ferring, Janssen, Sanofi, and TerSera. Dr. Fischer owns shares in J&J. Dr. Chow has participated in a speaker training session for Astellas. Dr. Wu participated in an advisory board for TerSera (Zoladex); and has give talks supported by Astellas and Pfizer. The remaining authors do not report any competing personal or financial interests related to this work.
Contributor Information
Peter N. Schlegel, Urology, Weill Cornell Medicine, New York, NY, United States
Matthew Roberts, The Ottawa Hospital, Uniersity of Otttawa, Ottawa, ON, Canada.
REFERENCES
- 1.Guyatt GH, Oxman AD, Vist GE, et al. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–6. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alonso-Coello P, Schunemann HJ, Moberg J, et al. GRADE Evidence to Decision (EtD) frameworks: A systematic and transparent approach to making well-informed healthcare choices. 1: Introduction. BMJ. 2016;353:i2016. doi: 10.1136/bmj.i2016. [DOI] [PubMed] [Google Scholar]
- 3.Schlegel PN, Sigman M, Collura B, et al. Diagnosis and treatment of infertility in men: AUA/ASRM guideline part I. Fertil Steril. 2021;115:54–61. doi: 10.1016/j.fertnstert.2020.11.015. [DOI] [PubMed] [Google Scholar]
- 4.Jarvi K, Lo K, Grober E, et al. The workup and management of azoospermic males. Can Urol Assoc J. 2015;9:229–35. doi: 10.5489/cuaj.3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balshem H, Helfand M, Schunemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64:401–6. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 6.Guyatt GH, Oxman AD, Santesso N, et al. GRADE guidelines: 12. Preparing summary of findings tables-binary outcomes. J Clin Epidemiol. 2013;66:158–72. doi: 10.1016/j.jclinepi.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 7.Guyatt GH, Thorlund K, Oxman AD, et al. GRADE guidelines: 13. Preparing summary of findings tables and evidence profiles-continuous outcomes. J Clin Epidemiol. 2013;66:173–83. doi: 10.1016/j.jclinepi.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 8.Thoma ME, McLain AC, Louis JF, et al. Prevalence of infertility in the United States as estimated by the current duration approach and a traditional constructed approach. Fertil Steril. 2013;99:1324–31. doi: 10.1016/j.fertnstert.2012.11.037. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Kretser DM. Male infertility. Lancet. 1997;349:787–90. doi: 10.1016/S0140-6736(96)08341-9. [DOI] [PubMed] [Google Scholar]
- 10.Thonneau P, Marchand S, Tallec A, et al. Incidence and main causes of infertility in a resident population (1,850,000) of three French regions (1988–1989) Hum Reprod. 1991;6:811–6. doi: 10.1093/oxfordjournals.humrep.a137433. [DOI] [PubMed] [Google Scholar]
- 11.Male Infertility Best Practice Policy Committee of the American Urological A, Practice Committee of the American Society for Reproductive M. Report on optimal evaluation of the infertile male. Fertil Steril. 2006;86:S202–9. doi: 10.1016/j.fertnstert.2006.08.029. [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization Manual: WHO laboratory manual for the examination and processing of human semen July27202135–6.Available at: https://www.who.int/publications/i/item/9789240030787.
- 13.Stephen EH, Chandra A. Declining estimates of infertility in the United States: 1982–2002. Fertil Steril. 2006;86:516–23. doi: 10.1016/j.fertnstert.2006.02.129. [DOI] [PubMed] [Google Scholar]
- 14.Fedder J, Cruger D, Oestergaard B, et al. Etiology of azoospermia in 100 consecutive non-vasectomized men. Fertil Steril. 2004;82:1463–5. doi: 10.1016/j.fertnstert.2004.06.035. [DOI] [PubMed] [Google Scholar]
- 15.Matsumiya K, Namiki M, Takahara S, et al. Clinical study of azoospermia. Int J Androl. 1994;17:140–2. doi: 10.1111/j.1365-2605.1994.tb01233.x. [DOI] [PubMed] [Google Scholar]
- 16.Jarow JP, Espeland MA, Lipshultz LI. Evaluation of the azoospermic patient. J Urol. 1989;142:62–5. doi: 10.1016/S0022-5347(17)38662-7. [DOI] [PubMed] [Google Scholar]
- 17.Fraietta R, Zylberstejn DS, Esteves SC. Hypogonadotropic hypogonadism revisited. Clinics (Sao Paulo) 2013;68(Suppl1):81–8. doi: 10.6061/clinics/2013(Sup01)09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Otani T. Clinical review of ejaculatory dysfunction. Reprod Med Biol. 2019;18:331–43. doi: 10.1002/rmb2.12289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Assche E, Bonduelle M, Tournaye H, et al. Cytogenetics of infertile men. Hum Reprod. 1996;11(Suppl4):1–24. doi: 10.1093/humrep/11.suppl_4.1. discussion 25–6. [DOI] [PubMed] [Google Scholar]
- 20.Flannigan R, Schlegel PN. Genetic diagnostics of male infertility in clinical practice. Best Pract Res Clin Obstet Gynaecol. 2017;44:26–37. doi: 10.1016/j.bpobgyn.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 21.Colaco S, Modi D. Genetics of the human Y chromosome and its association with male infertility. Reprod Biol Endocrinol. 2018;16:14. doi: 10.1186/s12958-018-0330-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yuen W, Golin AP, Flannigan R, et al. Histology and sperm retrieval among men with Y chromosome microdeletions. Transl Androl Urol. 2021;10:1442–56. doi: 10.21037/tau.2020.03.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Casals T, Bassas L, Ruiz-Romero J, et al. Extensive analysis of 40 infertile patients with congenital absence of the vas deferens: In 50% of cases only one CFTR allele could be detected. Hum Genet. 1995;95:205–11. doi: 10.1007/BF00209403. [DOI] [PubMed] [Google Scholar]
- 24.Chillon M, Casals T, Mercier B, et al. Mutations in the cystic fibrosis gene in patients with congenital absence of the vas deferens. N Engl J Med. 1995;332:1475–80. doi: 10.1056/NEJM199506013322204. [DOI] [PubMed] [Google Scholar]
- 25.Jarvi K, McCallum S, Zielenski J, et al. Heterogeneity of reproductive tract abnormalities in men with absence of the vas deferens: Role of cystic fibrosis transmembrane conductance regulator gene mutations. Fertil Steril. 1998;70:724–8. doi: 10.1016/S0015-0282(98)00247-7. [DOI] [PubMed] [Google Scholar]
- 26.Jhaveri KS, Mazrani W, Chawla TP, et al. The role of cross-sectional imaging in male infertility: A pictorial review. Can Assoc Radiol J. 2010;61:144–55. doi: 10.1016/j.carj.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 27.Kherraf ZE, Cazin C, Bouker A, et al. Whole-exome sequencing improves the diagnosis and care of men with non-obstructive azoospermia. Am J Hum Genet. 2022;109:508–17. doi: 10.1016/j.ajhg.2022.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nagirnaja L, Morup N, Nielsen JE, et al. Variant PNLDC1, defective piRNA processing, and azoospermia. N Engl J Med. 2021;385:707–19. doi: 10.1056/NEJMoa2028973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alfano M, Ventimiglia E, Locatelli I, et al. Anti-Mullerian hormone-to-testosterone ratio is predictive of positive sperm retrieval in men with idiopathic non-obstructive azoospermia. Sci Rep. 2017;7:17638. doi: 10.1038/s41598-017-17420-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ballesca JL, Balasch J, Calafell JM, et al. Serum inhibin B determination is predictive of successful testicular sperm extraction in men with non-obstructive azoospermia. Hum Reprod. 2000;15:1734–8. doi: 10.1093/humrep/15.8.1734. [DOI] [PubMed] [Google Scholar]
- 31.Vernaeve V, Tournaye H, Schiettecatte J, et al. Serum inhibin B cannot predict testicular sperm retrieval in patients with non-obstructive azoospermia. Hum Reprod. 2002;17:971–6. doi: 10.1093/humrep/17.4.971. [DOI] [PubMed] [Google Scholar]
- 32.Zarezadeh R, Fattahi A, Nikanfar S, et al. Hormonal markers as non-invasive predictors of sperm retrieval in non-obstructive azoospermia. J Assist Reprod Genet. 2021;38:2049–59. doi: 10.1007/s10815-021-02176-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fenichel P, Rey R, Poggioli S, et al. Anti-Mullerian hormone as a seminal marker for spermatogenesis in non-obstructive azoospermia. Hum Reprod. 1999;14:2020–4. doi: 10.1093/humrep/14.8.2020. [DOI] [PubMed] [Google Scholar]
- 34.Kumar R. Medical management of non-obstructive azoospermia. Clinics (Sao Paulo) 2013;68(Suppl 1):75–9. doi: 10.6061/clinics/2013(Sup01)08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zitzmann M, Nieschlag E. Hormone substitution in male hypogonadism. Mol Cell Endocrinol. 2000;161:73–88. doi: 10.1016/S0303-7207(99)00227-0. [DOI] [PubMed] [Google Scholar]
- 36.Han TS, Bouloux PM. What is the optimal therapy for young males with hypogonadotropic hypogonadism? Clin Endocrinol (Oxf) 2010;72:731–7. doi: 10.1111/j.1365-2265.2009.03746.x. [DOI] [PubMed] [Google Scholar]
- 37.Flannigan R, Bach PV, Schlegel PN. Microdissection testicular sperm extraction. Transl Androl Urol. 2017;6:745–52. doi: 10.21037/tau.2017.07.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Devroey P, Liu J, Nagy Z, et al. Pregnancies after testicular sperm extraction and intracytoplasmic sperm injection in non-obstructive azoospermia. Hum Reprod. 1995;10:1457–60. doi: 10.1093/HUMREP/10.6.1457. [DOI] [PubMed] [Google Scholar]
- 39.Turek PJ, Cha I, Ljung BM. Systematic fine-needle aspiration of the testis: Correlation to biopsy and results of organ “mapping” for mature sperm in azoospermic men. Urology. 1997;49:743–8. doi: 10.1016/S0090-4295(97)00154-4. [DOI] [PubMed] [Google Scholar]
- 40.Schlegel PN, Li PS. Microdissection TESE: Sperm retrieval in non-obstructive azoospermia. Hum Reprod Update. 1998;4:439. doi: 10.1093/humupd/4.4.439. [DOI] [PubMed] [Google Scholar]
- 41.Esteves SC, Ramasamy R, Colpi GM, et al. Sperm retrieval rates by micro-TESE vs. conventional TESE in men with non-obstructive azoospermia – the assumption of independence in effect sizes might lead to misleading conclusions. Hum Reprod Update. 2020;26:603–5. doi: 10.1093/humupd/dmaa006. [DOI] [PubMed] [Google Scholar]
- 42.Bernie AM, Mata DA, Ramasamy R, et al. Comparison of microdissection testicular sperm extraction, conventional testicular sperm extraction, and testicular sperm aspiration for non-obstructive azoospermia: A systematic review and meta-analysis. Fertil Steril. 2015;104:1099–103e1. doi: 10.1016/j.fertnstert.2015.07.1136. [DOI] [PubMed] [Google Scholar]
- 43.Achermann APP, Pereira TA, Esteves SC. Microdissection testicular sperm extraction (micro-TESE) in men with infertility due to nonobstructive azoospermia: Summary of current literature. Int Urol Nephrol. 2021 Nov;53(11):2193–2210. doi: 10.1007/s11255-021-02979-4. [DOI] [PubMed] [Google Scholar]
- 44.Esteves SC, Agarwal A. Reproductive outcomes, including neonatal data, following sperm injection in men with obstructive and nonobstructive azoospermia: Case series and systematic review. Clinics (Sao Paulo) 2013;68(Suppl1):141–50. doi: 10.6061/clinics/2013(Sup01)16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Corona G, Pizzocaro A, Lanfranco F, et al. Sperm recovery and ICSI outcomes in Klinefelter syndrome: A systematic review and meta-analysis. Hum Reprod Update. 2017;23:265–75. doi: 10.1093/humupd/dmx008. [DOI] [PubMed] [Google Scholar]
- 46.Friedler S, Raziel A, Soffer Y, et al. Intracytoplasmic injection of fresh and cryopreserved testicular spermatozoa in patients with nonobstructive azoospermia--a comparative study. Fertil Steril. 1997;68:892–7. doi: 10.1016/S0015-0282(97)00358-0. [DOI] [PubMed] [Google Scholar]
- 47.Ben-Yosef D, Yogev L, Hauser R, et al. Testicular sperm retrieval and cryopreservation prior to initiating ovarian stimulation as the first line approach in patients with nonobstructive azoospermia. Hum Reprod. 1999;14:1794–801. doi: 10.1093/humrep/14.7.1794. [DOI] [PubMed] [Google Scholar]
- 48.Habermann H, Seo R, Cieslak J, et al. In vitro fertilization outcomes after intracytoplasmic sperm injection with fresh or frozen-thawed testicular spermatozoa. Fertil Steril. 2000;73:955–60. doi: 10.1016/S0015-0282(00)00416-7. [DOI] [PubMed] [Google Scholar]
- 49.Friedler S, Raziel A, Strassburger D, et al. Factors influencing the outcome of ICSI in patients with obstructive and non-obstructive azoospermia: A comparative study. Hum Reprod. 2002;17:3114–21. doi: 10.1093/humrep/17.12.3114. [DOI] [PubMed] [Google Scholar]
- 50.Sousa M, Cremades N, Silva J, et al. Predictive value of testicular histology in secretory azoospermic subgroups and clinical outcome after microinjection of fresh and frozen-thawed sperm and spermatids. Hum Reprod. 2002;17:1800–10. doi: 10.1093/humrep/17.7.1800. [DOI] [PubMed] [Google Scholar]
- 51.Wu B, Wong D, Lu S, et al. Optimal use of fresh and frozen-thawed testicular sperm for intracytoplasmic sperm injection in azoospermic patients. J Assist Reprod Genet. 2005;22:389–94. doi: 10.1007/s10815-005-7481-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hauser R, Yogev L, Amit A, et al. Severe hypospermatogenesis in cases of nonobstructive azoospermia: Should we use fresh or frozen testicular spermatozoa? J Androl. 2005;26:772–8. doi: 10.2164/jandrol.05044. [DOI] [PubMed] [Google Scholar]
- 53.Konc J, Kanyo K, Cseh S. The effect of condition/state of testicular spermatozoa injected to the outcome of TESE-ICSI-ET cycles. Eur J Obstet Gynecol Reprod Biol. 2008;141:39–43. doi: 10.1016/j.ejogrb.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 54.Akarsu C, Caglar G, Vicdan K, et al. Pregnancies achieved by testicular sperm recovery in male hypogonadotrophic hypogonadism with persistent azoospermia. Reprod Biomed Online. 2009;18:455–9. doi: 10.1016/S1472-6483(10)60119-8. [DOI] [PubMed] [Google Scholar]
- 55.Karacan M, Alwaeely F, Erkan S, et al. Outcome of intracytoplasmic sperm injection cycles with fresh testicular spermatozoa obtained on the day of or the day before oocyte collection and with cryopreserved testicular sperm in patients with azoospermia. Fertil Steril. 2013;100:975–80. doi: 10.1016/j.fertnstert.2013.06.031. [DOI] [PubMed] [Google Scholar]
- 56.Abdel Raheem A, Rushwan N, Garaffa G, et al. Factors influencing intracytoplasmic sperm injection (ICSI) outcome in men with azoospermia. BJU Int. 2013;112:258–64. doi: 10.1111/j.1464-410X.2012.11714.x. [DOI] [PubMed] [Google Scholar]
- 57.Tavukcuoglu S, Al-Azawi T, Al-Hasani S, et al. Using fresh and frozen testicular sperm samples in couples undergoing ICSI-microTESE treatment. J Reprod Infertil. 2013;14:79–84. [PMC free article] [PubMed] [Google Scholar]
- 58.Madureira C, Cunha M, Sousa M, et al. Treatment by testicular sperm extraction and intracytoplasmic sperm injection of 65 azoospermic patients with non-mosaic Klinefelter syndrome with birth of 17 healthy children. Andrology. 2014;2:623–31. doi: 10.1111/j.2047-2927.2014.00231.x. [DOI] [PubMed] [Google Scholar]
- 59.Park YS, Lee SH, Lim CK, et al. Effect of testicular spermatozoa on embryo quality and pregnancy in patients with non-obstructive azoospermia. Syst Biol Reprod Med. 2015;61:300–6. doi: 10.3109/19396368.2015.1056885. [DOI] [PubMed] [Google Scholar]
- 60.Schachter-Safrai N, Karavani G, Levitas E, et al. Does cryopreservation of sperm affect fertilization in non-obstructive azoospermia or cryptozoospermia? Fertil Steril. 2017;107:1148–52. doi: 10.1016/j.fertnstert.2017.03.009. [DOI] [PubMed] [Google Scholar]
- 61.Okuyama N, Obata R, Oka N, et al. Long-term clinical outcomes of testicular sperm extraction and intracytoplasmic sperm injection for infertile men. Reprod Med Biol. 2018;17:82–8. doi: 10.1002/rmb2.12073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Falah K. Intracytoplasmic sperm injection with fresh versus cryopreserved testicular sperm in azoospermic patients. Middle East Fertil Soc J. 2020;24:2352. doi: 10.1186/s43043-019-0010-1. [DOI] [Google Scholar]
- 63.Zhang HL, Mao JM, Liu DF, et al. Clinical outcomes of microdissection testicular sperm extraction-intracytoplasmic sperm injection with fresh or cryopreserved sperm in patients with nonobstructive azoospermia. Asian J Androl. 2021;23:211–4. doi: 10.4103/aja.aja_38_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Barros P, Cunha M, Barros A, et al. Clinical outcomes of 77 TESE treatment cycles in non-mosaic Klinefelter syndrome patients. JBRA Assist Reprod. 2022;26:412–21. doi: 10.5935/1518-0557.20210081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kalsi J, Thum MY, Muneer A, et al. Analysis of the outcome of intracytoplasmic sperm injection using fresh or frozen sperm. BJU Int. 2011;107:1124–8. doi: 10.1111/j.1464-410X.2010.09545.x. [DOI] [PubMed] [Google Scholar]
- 66.Kavoussi PK, West BT, Chen SH, et al. A comprehensive assessment of predictors of fertility outcomes in men with non-obstructive azoospermia undergoing microdissection testicular sperm extraction. Reprod Biol Endocrinol. 2020;18:90. doi: 10.1186/s12958-020-00646-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Verheyen G, Vernaeve V, Van Landuyt L, et al. Should diagnostic testicular sperm retrieval followed by cryopreservation for later ICSI be the procedure of choice for all patients with non-obstructive azoospermia? Hum Reprod. 2004;19:2822–30. doi: 10.1093/humrep/deh490. [DOI] [PubMed] [Google Scholar]
- 68.Zhang Z, Jing J, Luo L, et al. ICSI outcomes of fresh or cryopreserved spermatozoa from micro-TESE in patients with non-obstructive azoospermia: CONSORT. Medicine (Baltimore) 2021;100:e25021. doi: 10.1097/MD.0000000000025021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yapeng Wang DL, Ren Xiulian, et al. Fresh gametes might get better clinical results than cryopreserved sperm or oocytes for non-obstructive azoospermia patients underwent micro-TESE. Clin Experiment Obstet Gynecol. 2022;49:128–33. doi: 10.31083/j.ceog4906128. [DOI] [Google Scholar]
- 70.Amer M, Fakhry E. Fresh vs. frozen testicular sperm for assisted reproductive technology in patients with non-obstructive azoospermia: A systematic review. Arab J Urol. 2021;19:247–54. doi: 10.1080/2090598X.2021.1932303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wong J, Sparanese S, Witherspoon L, et al. Patient and practitioner expectations for treatment of non-obstructive azoospermia. Can Urol Assoc J. 2023;17:64–8. doi: 10.5489/cuaj.8027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zampieri N, Bosaro L, Costantini C, et al. Relationship between testicular sperm extraction and varicocelectomy in patients with varicocele and nonobstructive azoospermia. Urology. 2013;82:74–7. doi: 10.1016/j.urology.2013.03.037. [DOI] [PubMed] [Google Scholar]
- 73.Schlegel PN, Kaufmann J. Role of varicocelectomy in men with non-obstructive azoospermia. Fertil Steril. 2004;81:1585–8. doi: 10.1016/j.fertnstert.2003.10.036. [DOI] [PubMed] [Google Scholar]
- 74.Inci K, Hascicek M, Kara O, et al. Sperm retrieval and intracytoplasmic sperm injection in men with non-obstructive azoospermia and treated and untreated varicocele. J Urol. 2009;182:1500–5. doi: 10.1016/j.juro.2009.06.028. [DOI] [PubMed] [Google Scholar]
- 75.Kirby EW, Wiener LE, Rajanahally S, et al. Undergoing varicocele repair before assisted reproduction improves pregnancy rate and live birth rate in azoospermic and oligospermic men with a varicocele: A systematic review and meta-analysis. Fertil Steril. 2016;106:1338–43. doi: 10.1016/j.fertnstert.2016.07.1093. [DOI] [PubMed] [Google Scholar]
- 76.Esteves SC, Miyaoka R, Roque M, et al. Outcome of varicocele repair in men with nonobstructive azoospermia: Systematic review and meta-analysis. Asian J Androl. 2016;18:246–53. doi: 10.4103/1008-682X.169562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jensen S, Ko EY. Varicocele treatment in non-obstructive azoospermia: A systematic review. Arab J Urol. 2021;19:221–6. doi: 10.1080/2090598X.2021.1956838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ghanem H, Anis T, El-Nashar A, et al. Subinguinal microvaricocelectomy vs. retroperitoneal varicocelectomy: Comparative study of complications and surgical outcome. Urology. 2004;64:1005–9. doi: 10.1016/j.urology.2004.06.060. [DOI] [PubMed] [Google Scholar]
- 79.Jungwirth A, Gogus C, Hauser G, et al. Clinical outcome of microsurgical subinguinal varicocelectomy in infertile men. Andrologia. 2001;33:71–4. doi: 10.1046/j.1439-0272.2001.00407.x. [DOI] [PubMed] [Google Scholar]
- 80.Kumar R, Gupta NP. Subinguinal microsurgical varicocelectomy: Evaluation of the results. Urol Int. 2003;71:368–72. doi: 10.1159/000074087. [DOI] [PubMed] [Google Scholar]
- 81.Watanabe M, Nagai A, Kusumi N, et al. Minimal invasiveness and effectivity of subinguinal microscopic varicocelectomy: a comparative study with retroperitoneal high and laparoscopic approaches. Int J Urol. 2005;12:892–8. doi: 10.1111/j.1442-2042.2005.01142.x. [DOI] [PubMed] [Google Scholar]
- 82.Yavetz H, Levy R, Papo J, et al. Efficacy of varicocele embolization vs. ligation of the left internal spermatic vein for improvement of sperm quality. Int J Androl. 1992;15:338–44. doi: 10.1111/j.1365-2605.1992.tb01133.x. [DOI] [PubMed] [Google Scholar]
- 83.Shlansky-Goldberg RD, VanArsdalen KN, Rutter CM, et al. Percutaneous varicocele embolization vs. surgical ligation for the treatment of infertility: Changes in seminal parameters and pregnancy outcomes. J Vasc Interv Radiol. 1997;8:759–67. doi: 10.1016/S1051-0443(97)70657-2. [DOI] [PubMed] [Google Scholar]
- 84.Al-Kandari AM, Shabaan H, Ibrahim HM, et al. Comparison of outcomes of different varicocelectomy techniques: Oinguinal, laparoscopic, and subinguinal microscopic varicocelectomy: A randomized clinical trial. Urology. 2007;69:417–20. doi: 10.1016/j.urology.2007.01.057. [DOI] [PubMed] [Google Scholar]
- 85.Orhan I, Onur R, Semercioz A, et al. Comparison of two different microsurgical methods in the treatment of varicocele. Arch Androl. 2005;51:213–20. doi: 10.1080/01485010590919648. [DOI] [PubMed] [Google Scholar]
- 86.Haydardedeoglu B, Turunc T, Kilicdag EB, et al. The effect of prior varicocelectomy in patients with nonobstructive azoospermia on intracytoplasmic sperm injection outcomes: A retrospective pilot study. Urology. 2010;75:83–6. doi: 10.1016/j.urology.2009.09.023. [DOI] [PubMed] [Google Scholar]
- 87.Kizilkan Y, Toksoz S, Turunc T, et al. Parameters predicting sperm retrieval rates during microscopic testicular sperm extraction in nonobstructive azoospermia. Andrologia. 2019;51:e13441. doi: 10.1111/and.13441. [DOI] [PubMed] [Google Scholar]
- 88.Tharakan T, Corona G, Foran D, et al. Does hormonal therapy improve sperm retrieval rates in men with non-obstructive azoospermia: A systematic review and meta-analysis. Hum Reprod Update. 2022;28:609–28. doi: 10.1093/humupd/dmac016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Guo F, Fang A, Fan Y, et al. Role of treatment with human chorionic gonadotropin and clinical parameters on testicular sperm recovery with microdissection testicular sperm extraction and intracytoplasmic sperm injection outcomes in 184 Klinefelter syndrome patients. Fertil Steril. 2020;114:997–1005. doi: 10.1016/j.fertnstert.2020.05.043. [DOI] [PubMed] [Google Scholar]
- 90.Reifsnyder JE, Ramasamy R, Husseini J, et al. Role of optimizing testosterone before microdissection testicular sperm extraction in men with non-obstructive azoospermia. J Urol. 2012;188:532–6. doi: 10.1016/j.juro.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 91.Umit Gul TT. The effect of human chorionic gonadotropin treatment before testicular sperm extraction in non-obstructive azoospermia. J Clin Analyt Med. 2016;7:55–9. [Google Scholar]
- 92.Arunan Sujenthiran JT, Broussil P, Homa S, et al. Hormone stimulation prior to micro-TESE improves success rates in Klinefelter syndrome patients. J Urol. 2010;201:e679. doi: 10.1097/01.JU.0000556297.34238.f0. [DOI] [Google Scholar]
- 93.Hussein A, Ozgok Y, Ross L, et al. Optimization of spermatogenesis-regulating hormones in patients with non-obstructive azoospermia and its impact on sperm retrieval: A multicenter study. BJU Int. 2013;111:E110–4. doi: 10.1111/j.1464-410X.2012.11485.x. [DOI] [PubMed] [Google Scholar]
- 94.Majzoub A, Arafa M, Al Said S, et al. Outcome of testicular sperm extraction in non-mosaic Klinefelter syndrome patients: What is the best approach? Andrologia. 2016;48:171–6. doi: 10.1111/and.12428. [DOI] [PubMed] [Google Scholar]
- 95.Amer MK, Ahmed HEH, Gamal El Din SF, et al. Evaluation of neoadjuvant gonadotropin administration with downregulation by testosterone prior to second time microsurgical testicular sperm extraction: A prospective case-control study. Urologia. 2020;87:185–190. doi: 10.1177/0391560320913401. [DOI] [PubMed] [Google Scholar]
- 96.Cocci A, Cito G, Russo GI, et al. Effectiveness of highly purified urofollitropin treatment in patients with idiopathic azoospermia before testicular sperm extraction. Urologia. 2018;85:19–21. doi: 10.5301/uj.5000253. [DOI] [PubMed] [Google Scholar]
- 97.Aydos K, Unlu C, Demirel LC, et al. The effect of pure FSH administration in nonobstructive azoospermic men on testicular sperm retrieval. Eur J Obstet Gynecol Reprod Biol. 2003;108:54–8. doi: 10.1016/S0301-2115(02)00412-8. [DOI] [PubMed] [Google Scholar]
- 98.Shashwati Sen TEC, Su Ling Y, Zhang X, et al. Effect of recombinant hCG pretreatment on surgical sperm retrieval rates in patients with non-obstructive azoospermia — an audit of our practice. PCRS Abstracts. 2020;113:e22. doi: 10.1016/j.fertnstert.2020.02.048. [DOI] [Google Scholar]
- 99.Shiraishi K, Ohmi C, Shimabukuro T, et al. Human chorionic gonadotrophin treatment prior to microdissection testicular sperm extraction in non-obstructive azoospermia. Hum Reprod. 2012;27:331–9. doi: 10.1093/humrep/der404. [DOI] [PubMed] [Google Scholar]
- 100.Farber NJ, Flannigan R, Li P, et al. The kinetics of sperm return and late failure following vasovasostomy or vasoepididymostomy: A systematic review. J Urol. 2019;201:241–50. doi: 10.1016/j.juro.2018.07.092. [DOI] [PubMed] [Google Scholar]
- 101.Wosnitzer MS, Goldstein M. Obstructive azoospermia. Urol Clin North Am. 2014;41:83–95. doi: 10.1016/j.ucl.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 102.Chan PT, Li PS, Goldstein M. Microsurgical vasoepididymostomy: A prospective randomized study of 3 intussusception techniques in rats. J Urol. 2003;169:1924–9. doi: 10.1097/01.ju.0000059360.97108.c4. [DOI] [PubMed] [Google Scholar]
- 103.Crosnoe LE, Kim ED, Perkins AR, et al. Angled vas cutter for vasovasostomy: Technique and results. Fertil Steril. 2014;101:636–9e2. doi: 10.1016/j.fertnstert.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 104.Goldstein M, Li PS, Matthews GJ. Microsurgical vasovasostomy: the microdot technique of precision suture placement. J Urol. 1998;159:188–90. doi: 10.1016/S0022-5347(01)64053-9. [DOI] [PubMed] [Google Scholar]
- 105.Silber SJ, Grotjan HE. Microscopic vasectomy reversal 30 years later: A summary of 4010 cases by the same surgeon. J Androl. 2004;25:845–59. doi: 10.1002/j.1939-4640.2004.tb03150.x. [DOI] [PubMed] [Google Scholar]
- 106.Mui P, Perkins A, Burrows PJ, et al. The need for epididymovasostomy at vasectomy reversal plateaus in older vasectomies: A study of 1229 cases. Andrology. 2014;2:25–9. doi: 10.1111/j.2047-2927.2013.00143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Parekattil SJ, Kuang W, Agarwal A, et al. Model to predict if a vasoepididymostomy will be required for vasectomy reversal. J Urol. 2005;173:1681–4. doi: 10.1097/01.ju.0000154608.08496.f2. [DOI] [PubMed] [Google Scholar]
- 108.Grober ED, Tobe S. Microscopic evaluation of the vasal fluid for sperm at the time of vasectomy reversal: Do we really need to check? Can Urol Assoc J. 2021;15:E397–9. doi: 10.5489/cuaj.6980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Avellino GJ, Lipshultz LI, Sigman M, et al. Transurethral resection of the ejaculatory ducts: Etiology of obstruction and surgical treatment options. Fertil Steril. 2019;111:427–43. doi: 10.1016/j.fertnstert.2019.01.001. [DOI] [PubMed] [Google Scholar]
- 110.Hollander AB, Pastuszak AW, Hsieh TC, et al. Cabergoline in the treatment of male orgasmic disorder — a retrospective pilot analysis. Sex Med. 2016;4:e28–33. doi: 10.1016/j.esxm.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Brackett NL, Ibrahim E, Iremashvili V, et al. Treatment for ejaculatory dysfunction in men with spinal cord injury: An 18-year single center experience. J Urol. 2010;183:2304–8. doi: 10.1016/j.juro.2010.02.018. [DOI] [PubMed] [Google Scholar]
- 112.Esteves SC, Miyaoka R, Agarwal A. Sperm retrieval techniques for assisted reproduction. Int Braz J Urol. 2011;37:570–83. doi: 10.1590/S1677-55382011000500002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.