Abstract
As a significant non-communicable disease, cardiovascular disease is the leading cause of death for both men and women, comprises almost twenty percent of deaths in most racial and ethnic groups, can affect greater than twenty-five million individuals worldwide over the age of twenty, and impacts global economies with far-reaching financial challenges. Multiple factors can affect the onset of cardiovascular disease that include high serum cholesterol levels, elevated blood pressure, tobacco consumption and secondhand smoke exposure, poor nutrition, physical inactivity, obesity, and concurrent diabetes mellitus. Yet, addressing any of these factors cannot completely eliminate the onset or progression of cardiovascular disorders. Novel strategies are necessary to target underlying cardiovascular disease mechanisms. The silent mating type information regulation 2 homolog 1 (Saccharomyces cerevisiae) (SIRT1), a histone deacetylase, can limit cardiovascular injury, assist with stem cell development, oversee metabolic homeostasis through nicotinamide adenine dinucleotide (NAD+) pathways, foster trophic factor protection, and control cell senescence through the modulation of telomere function. Intimately tied to SIRT1 pathways are mammalian forkhead transcription factors (FoxOs) which can modulate cardiac disease to reduce oxidative stress, repair microcirculation disturbances, and reduce atherogenesis through pathways of autophagy, apoptosis, and ferroptosis. AMP activated protein kinase (AMPK) also is critical among these pathways for the oversight of cardiac cellular metabolism, insulin sensitivity, mitochondrial function, inflammation, and the susceptibility to viral infections such as severe acute respiratory syndrome coronavirus that can impact cardiovascular disease. Yet, the relationship among these pathways is both intricate and complex and requires detailed insight to successfully translate these pathways into clinical care for cardiovascular disorders.
Keywords: AMPK, apoptosis, autophagy, FoxO, NAD+, SIRT1
The Global Implications for Cardiovascular Disease
Non-communicable diseases (NCDs) affect an increasing portion of the global population with at least fifteen million of these individuals ranging in age between thirty and sixty-nine years (Jalgaonkar et al., 2022[110]; Maiese, 2018[193], 2020[165], 2021[197]; Schell et al., 2021[276]; Speer et al., 2020[294]). In developed nations, NCDs affect at least ten percent of individuals that are less than sixty years of age. However, in low and middle-income countries, NCDs affect a greater proportion of people with at least one-third of the population affected under sixty years of age (WHO, 2011[315], 2017[314]).
In particular, cardiovascular disorders have a significant role in NCDs. NCDs are a primary cause of death that include cardiac disease, cancer, trauma, respiratory disease, stroke, Alzheimer's disease, diabetes mellitus (DM), influenza and pneumonia, kidney disease, and suicide (CDC, 2019[30] (Table 1(Tab. 1))). Cardiovascular disease is the leading cause of death for both women and men and accounts for more than forty percent of all deaths when combined with the deaths from cancer (Chen et al., 2023[36]; Huang et al., 2023[106]; Kalam et al., 2023[117]; Kostić et al., 2023[124]; Liu et al., 2023[153]; Maiese, 2015[166][191], 2017[171]; Ponzetti et al., 2023[251]; Razzaghi et al., 2023[260]; Redhwan et al., 2023[261]; Sierra-Pagan et al., 2023[290]; Yeger, 2023[328]; Zhong et al., 2023[348]). It is estimated that an individual dies every thirty-three seconds as a result of heart disease in the United States (US) alone (Heron, 2019[98]; CDC, 2023[29]). In 2021, approximately 700,000 individuals expired from cardiac disease. More than eighteen million individuals over the age of twenty experience coronary artery disease in the US and greater than 800,000 people have a myocardial infarction every year. Although ethnicity can play a role in the percentage of deaths, cardiovascular disease affects most racial and ethnic groups in at least 10 % of these populations with African Americans being affected to the highest degree at almost 23 %. In regard to financial care concerns, healthcare costs for cardiovascular disease exceed $ 555 billion US dollars and by the year 2035 they will be greater than $ 1.1 trillion US dollars.
Table 1. Highlights "Novel therapeutic strategies for cardiovascular disease".
Cardiovascular Disease and the Aging Process
Closely tied to the increasing prevalence of NCDs and cardiovascular disease are the effects of the aging process (Amidfar et al., 2023[8]; Blice-Baum et al., 2017[20]; Maiese, 2015[191], 2023[158]; Olejniczak et al., 2023[238]; Rotllan et al., 2021[266]; Sun et al., 2023[296]; Wang et al., 2021[308]). On one side of the equation for the aging process, lifespan is increasing throughout the world with the expectation of attaining at least 80 years of age (Geier and Perl, 2021[79]; Gustafsson and Ulfhake, 2021[90]; Jalgaonkar et al., 2022[110]; Maiese, 2014[162], 2021[197]; Yu et al., 2021[330]). Even in developing countries such as India and China, the elderly population is expected to increase from five to ten percent over future years (Maiese, 2015[191], 2017[175]). Across the globe, the number of individuals over the age of 65 has doubled during the previous 50 years (Hayutin, 2007[95]). Multiple factors have contributed to increased life expectancy with cardiovascular disease, except for the growing non-prescribed use of fentanyl by individuals that has resulted in a rise in mortality in this group (Wilson et al., 2020[316]). Factors that have positively promoted increased lifespan include incorporation of early diagnostic and preventive measures, rapidly identifying individuals susceptible to both acute and chronic illnesses, improved sanitation, and improved access to healthcare (Chen et al., 2023[36]; Hacioglu et al., 2021[91]; Jalgaonkar et al., 2022[110]; Jiang et al., 2023[114]; Kahmini et al., 2022[116]; Li et al., 2023[136]; Liu et al., 2022[144]; Maiese, 2018[200], 2021[160][177], 2022[205]; Odnokoz et al., 2021[234]; Patocka et al., 2021[246]; Sorrells et al., 2021[293]).
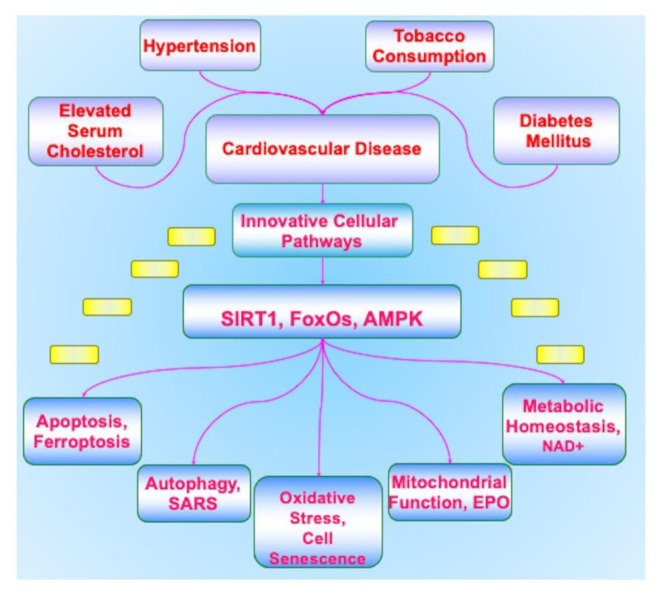
Yet, on the other side of the equation that involves aging are the underlying cellular pathways that oversee an individual's longevity (Figure 1(Fig. 1)). These cellular pathways can affect the onset and progression of cardiac disease (Blice-Baum et al., 2017[20]; Choudhery et al., 2012[51]; Du et al., 2016[64]; Maiese, 2016[185]; Okada et al., 2016[237]). Recent work has focused on the role of telomeres (TLs), complexes of deoxyribonucleic acid (DNA), that can oversee cell senescence, control stem cell renewal, cellular lifespan, cellular replication, and protection for the DNA of the genome (Alanko et al., 1996[4]; Bandara and La Thangue, 1991[12]; Chen et al., 1997[34]; Connor et al., 2001[53]; Dhakal et al., 2019[58]; Ferrara-Romeo et al., 2020[72]; Jaganjac et al., 2022[108]; Jeyaraman et al., 2022[112]; Khani et al., 2022[121]; Kita et al., 2022[122]; Li et al., 2021[137]; Mahdi et al., 1995[155]; Maiese, 2020[182][189], 2023[199]; Maiese et al., 2010[208]; O'Donnell et al., 2022[235]; Okada et al., 2016[237]; Oyefeso et al., 2021[240]; Puri et al., 2023[253]; Saxton and Pawson, 1999[275]; Sun et al., 2023[296]; Takanezawa et al., 2021[299]; Topiwala et al., 2023[304]; Wang et al., 2023[306]; Yan et al., 2021[323]; Zhao et al., 2013[345]). In patients with dilated cardiomyopathy, decreased TL length and progression of cell senescence has been observed (Barcena et al., 2023[13]) (Table 1(Tab. 1)). TLs exist at the end of chromosomes, have greater than 2000 repetitions of non-coding double-stranded DNA with the sequence 'TTAGGG”, and are completed with a guanine rich single-stranded DNA (Dhakal et al., 2019[58]; Kuan et al., 2023[126]). A number of protein complexes are associated with TLs that include telosome, shelterin, and CTC1-STN1-TEN1 (CST). These proteins control TLs activity and provide stability. The telomerase protein becomes active during cell division to maintain TL length through the addition of tandem repeat ribonucleic acid (RNA) templates. Without this process, a portion of TLs length will become lost in the amount of approximately 25-200 base pairs (Cardoso et al., 2021[26]; De Bonis et al., 2014[57]; Klionsky et al., 2021[123]; Shafi, 2016[280]). If telomerase function is also lost or the TLs become excessively short with less than 500 base pairs, cell proliferation is no longer viable and cell senescence ensues (Begum et al., 2021[16]; Cai,et al., 2021[23]; Dorvash et al., 2020[61]; Geng et al., 2021[80]; Kowalska et al., 2020[125]; Liu et al., 2020[149]; Maiese, 2014[164], 2015[194], 2016[196], 2020[189]; Rapaka et al., 2022[258]; Tabibzadeh, 2021[298]; Yu et al., 2021[330]; Zhang et al., 2020[337]; Zhou et al., 2022[349]). With the onset of cell senescence, cardiac injury can develop since reparative processes are unable to function (Blice-Baum et al., 2017[20]; Du et al., 2016[64]; Lathe and St Clair, 2023[130]; Maiese, 2014[164], 2015[170], 2016[163][198][185][196], Maiese et al., 2008[207]; Okada et al., 2016[237]; Sun et al., 2023[296]; Yamamoto et al., 2023[321]). In addition, exposure to oxidative stress can develop with the release of reactive oxygen species (ROS) during the shortening of TLs and the onset of cell senescence. Oxidative stress exposure leads to decreased cell survival and the dysfunction of mitochondrial organelles (Cardoso et al., 2021[26]; Chen et al., 2022[37]; Fields et al., 2019[75]; Gallyas et al., 2020[78]; Groen et al., 2022[86]; Lei et al., 2021[133]; Li et al., 2020[138][140]; Liu et al., 2020[149]; Maiese, 2016[198], 2020[201][189], 2021[181]; Mocayar Marón et al., 2020[226]; Odnokoz et al., 2021[234]; Oliveira et al., 2021[239]; Oyefeso et al., 2021[240]; Perluigi et al., 2021[248]; Piao et al., 2021[249]; Prasuhn and Brüggemann, 2021[252]; Raut and Khullar, 2023[259]; Tabibzadeh, 2021[298]; Xiong et al., 2022[317]; Zhang et al., 2020[337]; Zhuang et al., 2022[351]) (Figure 1(Fig. 1)). The inability to remove cells that are senescent by the immune system also may subsequently lead to tumorigenesis (Begum et al., 2021[16]; Cai et al., 2021[23]; Kowalska et al., 2020[125]; Liu et al., 2020[149]; Maiese, 2016[163][198][185], 2020[189]; Watroba and Szukiewicz, 2021[312]; Yu et al., 2021[330]; Zhang et al., 2020[337]).
Figure 1. Novel therapeutic strategies for cardiovascular disease.
Cardiovascular disease is impacted by multiple risk factors that include elevated serum cholesterol, hypertension, tobacco consumption, and concurrent disorders such as diabetes mellitus that treatment of these cannot completely avert the development of cardiovascular disease. Innovative cellular pathways that involve the silent mating type information regulation 2 homolog 1 (Saccharomyces cerevisiae) (SIRT1), mammalian forkhead transcription factors (FoxOs), and AMP activated protein kinase (AMPK) are required to address the underlying critical mechanisms for cardiovascular disease. SIRT1, FoxOs, and AMPK can oversee vital critical pathways for cardiovascular survival that involve nicotinamide adenine dinucleotide (NAD+), oxidative stress, cell senescence, mitochondrial function, trophic factor protection such as with erythropoietin (EPO), infectious agent injury such as with severe acute respiratory syndrome coronavirus (SARS-CoV-2), and the programmed cell death pathways that include apoptosis, autophagy, and ferroptosis.
Innovative Therapeutic Strategies for Cardiovascular Disease
Multiple factors can lead to the onset of cardiovascular disease. Focusing upon adults who are male or female, age sixty and over, or non-Hispanic black, or of lower income who are at high risk may reduce the onset and progression of cardiovascular disease (Fryar et al., 2012[76]; Maiese, 2008[203], 2020[178]). New imaging technology involving multiphoton microscopy and optogenetic effectors and sensors can offer improved observation of cardiac dynamics and cellular signaling (Lee et al., 2021[131]). Pulsed lasers offer the ability to possibly treat atherosclerotic plaques (Sintek et al., 2021[291]). Additional therapeutic pathways can reduce the risk of cardiovascular disorders by addressing serum cholesterol levels, elevated blood pressure, tobacco consumption and secondhand smoke exposure, poor nutrition, physical inactivity, obesity, and the presence of DM (Ahmed et al., 2020[3]; Begum et al., 2021[16]; Chong and Maiese, 2012[48]; Chong et al., 2011[49]; du Toit et al., 2022[63], 2023[62]; Januszewski et al., 2020[111]; Liu et al., 2020[147]; Maiese, 2016[185][190], 2018[200], 2019[173][174]; Maiese et al., 2008[207], 2009[209][218]; Najjar et al., 2021[230]; Quintana-Pérez et al., 2022[255]; Ran et al., 2021[257]; Raut and Khullar, 2023[259]; Rotllan et al., 2021[266]; Su et al., 2022[295]; Temiz- Resitoglu et al., 2022[302]; Wang et al., 2021[308]; Zhang et al., 2023[338]) (Figure 1(Fig. 1)). Treatment of metabolic disorders that involves DM represents a critical pathway to reduce cardiovascular disease since DM leads to the progression of cardiovascular injury. In fact, individuals with DM are twice as likely to suffer from cardiac disease or stroke when compared to individuals without DM (Hajibabaie et al., 2022[92]; Maiese, 2015[176], Maiese et al., 2009[209][214]; Pabel et al., 2021[241]; Rotllan et al., 2021[266]; Xue et al., 2019[320]). The implementation of pharmaceuticals and nutritional modification can assist with the management of DM to prevent hyperglycemic events (Arildsen et al., 2019[10]; Bayaraa et al., 2022[14]; Beegum et al., 2022[15]; Chen et al., 2021[35]; Chiareli et al., 2021[38]; Esterline et al., 2018[67]; Feng et al., 2020[71]; Gong et al., 2021[84]; Hajibabaie et al., 2022[92]; Jalgaonkar et al., 2022[110]; Jiang et al., 2023[114]; Liu et al., 2020[148]; Maiese, 2018[193], 2020[189], 2021[181]; Maiese et al., 2008[212][215]; Mocayar Marón et al., 2020[226]; Pabel et al., 2021[241]; Papachristoforou et al., 2020[244]; Rotllan et al., 2021[266]; Sakakibara et al., 2002[268]; Sanabria-de la Torre et al., 2022[272]; Tan et al., 2021[300]; Zaiou, 2020[333]; Zarneshan et al., 2020[334]; Zhou et al., 2021[350]). However, even therapies designed to effectively manage poor glycemic control are not without risks. These therapeutic regimens can affect cellular organelles and lead to decreased organ mass through processes that involve programmed cell death and autophagy (Gong et al., 2021[84]; Lee et al., 2014[132]; Li et al., 2020[135]; Mocayar Marón et al., 2020[226]) (Figure 1(Fig. 1)). With these challenges at hand, new and innovative strategies are highly warranted that can address the underlying cellular components of cardiovascular disease to include the pathways of the silent mating type information regulation 2 homolog 1 (Saccharomyces cerevisiae) (SIRT1), mammalian forkhead transcription factors (FoxOs), and AMP activated protein kinase (AMPK) (Table 1(Tab. 1)). Intimately tied to these fundamental pathways are the oversight of metabolic pathways with nicotinamide adenine dinucleotide (NAD+), cell senescence and lifespan, reactive oxygen species exposure, stem cell survival, mitochondrial injury, trophic factor protection, infectious agent injury, and programmed cell death pathways of apoptosis, autophagy, and ferroptosis.
Cardiovascular Disease, SIRT1, and Cellular Metabolism
Silent mating type information regulation 2 homolog 1 (Saccharomyces cerevisiae) (SIRT1) plays a significant role during the onset and progression of cardiovascular disease (Charles et al., 2017;[31] Cui et al., 2017[55]; Kostić et al., 2023[124]; Maiese, 2016[190], 2017[171], 2020[172][182], 2021[181][192]; Ministrini et al., 2021[225]; Piao et al., 2021[249]; Watroba and Szukiewicz, 2021[312]; Yuan et al., 2022[331]) (Table 1(Tab. 1)). A member of the sirtuin family (sirtuin 1), SIRT1 is a histone deacetylase that oversees DNA transcription by transferring acetyl groups from ε-N-acetyl lysine amino acids to the histones of DNA (Chong et al., 2012[50]; Ding et al., 2022[59]; Guimera et al., 2022[88]; Jahan et al., 2023[109]; Jalgaonkar et al., 2022[110]; Kostić et al., 2023[124]; Liu et al., 2023[153]; Maiese, 2017[171], 2020[161], 2021[197]; Sadria et al., 2022[267]; Sun et al., 2023[296]). The coenzyme ß-nicotinamide adenine dinucleotide (NAD+) is used as a substrate for SIRT1 (Chong et al., 2005[44], 2022[40]; Fangma et al., 2022[68]; Giacalone et al., 2021[82]; Jobst et al., 2023[115]; Maiese, 2015[191], 2016[198], 2020[179], 2021[181]; Maiese and Chong, 2003[206]; Maiese et al., 2009[210]; Ministrini et al., 2021[225]). Seven identified mammalian homologues of Sir2 include SIRT1 through SIRT7 (Cacabelos et al., 2019[22]; Maiese, 2016[196], 2018[200], Maiese, 2020[161]; Mori et al., 202[228]2; Wang et al., 2022[310]; Wasserfurth et al., 2021[311]; Zhang et al., 2020[337]). These histone deacetylases control metabolism, cell development and proliferation, senescence, and post-translation modifications of proteins (Begum et al., 2021[16]; Csicsar et al., 2019[54]; Kahmini et al., 2022[116]; Maiese, 2016[198], 2018[200], 2020[189], 2021[181]; Sun et al., 2023[296]; Tabibzadeh, 2021[298]; Wang et al., 2022[309]; Wasserfurth et al., 2021[311]; Yamamoto et al., 2023[321]; Yu et al., 2021[330]; Yuan et al., 2020[332]; Zhang et al., 2020[337]; Zhou et al., 2022[349]).
In the cardiovascular system, SIRT1 can increase the survival of cardiomyoblasts (Passariello et al., 2011[245]), lead to enhanced endothelial function (Charles et al., 2017[31]; Hajibabaie et al., 2022[92]; Maiese, 2016[187]; Piao et al., 2021[249]), maintain cardiac fatty acid oxidation (Kostić et al., 2023[124]), prevent cardiac DM injury (Maiese, 2015[191], 2017[171]; Xue et al., 2019[320]), reduce coronary artery disease (Maiese, 2020[161], 2021[177][192]; Yuan et al., 2022[331]) and block cellular senescence and impaired differentiation in endothelial progenitor cells (Lemarie et al., 2011[134]). SIRT1 can improve the function of aged stem cells that are senescent (Figure 1(Fig. 1)). Aged mesenchymal stem cells that are stimulated and pre-conditioned with glucose depletion demonstrate enhanced SIRT1 expression as well as trophic factor and protein kinase B (Akt) expression and can lead to increased cardiac performance (Choudhery et al., 2012[51]). Mesenchymal stem cells with SIRT1 over-expression exhibit increased blood vessel density in the area of cardiac infarcts, reduced cardiac remodeling, and improved cardiac performance in rodent models, suggesting SIRT1 as a potential target for the treatment of cardiac injury (Liu et al., 2014[150]).
Given that cellular metabolic dysfunction also can lead to cardiac injury and disability, the SIRT1 pathway is an important avenue in this respect since SIRT1 can oversee metabolic homeostasis (Chen et al., 2021[35]; Ghiasi et al., 2019[81]; Hajibabaie et al., 2022[92]; Hassanein et al., 2022[94]; Jalgaonkar et al., 2022[110]; Liu et al., 2021[146]; Maiese, 2015[180][188][191], 2016[183], 2020[161], 2021[181]; Wasserfurth et al., 2021[311]; Yang et al., 2020[324]). SIRT1 pathways are closely tied to NAD+ and the vitamin nicotinamide (Fangma et al., 2022[68]; Jobst et al., 2023[115]; Maiese, 2021[181]; Maiese et al., 2013[216]; Ministrini et al., 2021[225]; Song et al., 2019[292]; Teertam and Prakash Babu, 2021[301]; Yousafzai et al., 2021[329]; Zhang et al., 2020[337]). Nicotinamide is the amide form of the vitamin B3 (niacin) and a precursor for the coenzyme NAD+ (AlSaleh et al., 2023[6]; Fangma et al., 2022[68]; Kumar and Ou, 2023[127]; Maiese, 2020[179], 2021[181]; Rehman et al., 2022[262]; Yamamoto et al., 2023[321]; Yang et al., 2023[326]).
Nicotinamide phosphoribosyl-transferase (NAMPT) is necessary for NAD+ production and is controlled by SIRT1 and the circadian rhythm complex of CLOCK:BMAL1 (Maiese, 2021[181][192]). The NAMPT promoter uses SIRT1 to increase production of its own coenzyme (Nakahata et al., 2009[231]). Yet if NAD+ levels are diminished, altered cellular levels of nicotinamide can result in mitochondrial dysfunction, vascular disease, and cognitive loss (Oblong, 2014[232]). This is a result of cellular NAD+ pools fluctuating with circadian rhythmicity and with aging (Maiese, 2020[182]). Circadian rhythm disturbances can affect degenerative processes and metabolic dysfunction throughout the body (Amidfar et al., 2023[8]; Birnie et al., 2023[18]; Felten et al., 2023[70]; Hardeland, 2022[93]; Hsu et al., 2022[103]; Huang et al., 2023[106]; Kalam et al., 2023[117]; Klionsky et al., 2021[123]; Lathe and St Clair, 2023[130]; Luo et al., 2022[154]; Maiese, 2017[175], 2020[159][161], 2021[177]; Olejniczak et al., 2023[238]; Xu et al., 2023[319]). In relation to cellular metabolism, metformin has been reported to foster SIRT1 activity to maintain proper circadian rhythm of CLOCK and BMAL1 during obesity, since the absence of SIRT1 inhibits function of CLOCK and BMAL1 in an obese phenotype (Caton et al., 2011[25]).
In regard to nicotinamide, SIRT1 through the transfer of the acetyl residue from the acetyllysine residue of histones to the ADP-ribose moiety of NAD+ can lead to the production of nicotinamide. Yet, feedback mechanisms exist and nicotinamide can block SIRT1 activity by intercepting an ADP-ribosyl-enzyme-acetyl peptide intermediate with the regeneration of NAD+ (Jackson et al., 2003[107]). Physiological concentrations of nicotinamide can non-competitively block SIRT1 that indicates nicotinamide is a regulator of SIRT1 (Bitterman et al., 2002[19]). As a result of SIRT1 inhibition, nicotinamide at times can suppress the expression of anti-inflammatory genes (Zhang et al., 2012[339]). However, in this process, enhanced activity of SIRT1 can occur with the activation of nicotinamide phosphoribosyltransferase (NAMPT) such as during periods of glucose restriction, resulting in increased NAD+ and decreased nicotinamide, an inhibitor of SIRT1 (Fulco et al., 2008[77]).
NAD+ replenishment is believed to foster cardiac and vascular health (Rotllan et al., 2021[266]). Enhanced NAD+ levels that rely upon SIRT1 activation may reduce inflammation, metabolic instability, and cardiac injury (Maiese, 2008[203], 2015[191]; Watroba and Szukiewicz, 2021[312]) (Figure 1(Fig. 1)). Growth factors, such as erythropoietin (EPO), also may depend upon SIRT1 and NAD+ activity to offer cellular protection (Govindappa and Elfar, 2022[85]; Hu et al., 2022[104]; Liu et al., 2022[145]; Maiese, 2008[203], 2020[182]; Maiese et al., 2010[222]; Senousy et al., 2022[278]; Sergio and Rolando, 2022[279]). EPO can limit oxidative stress through pathways of NAD+ activity to preserve cellular survival in adipocytes (Wang et al., 2014[305]). EPO results in cerebral vascular protection through the subcellular trafficking of SIRT1 to the nucleus and limits mitochondrial depolarization, cytochrome c release, BCL2 associated agonist of cell death (Bad) activity, and caspase activation (Hou et al., 2011[102]). Through SIRT1 activation, EPO can increase survival of human cardiomyocytes (Cui et al., 2017[55]), control metabolic pathways (Entezari et al., 2019[66]; Fessel, 2023[73]; Montesano et al., 2019[227]; Yang et al., 2023[325]), prevent mitochondrial dysfunction (Chong et al., 2002[43], 2003[42]; Cui et al., 2017[55]; Maiese, 2016[190]; Rey et al., 2021[264]; Shang et al., 2012[284]), foster microglial survival (Shang et al., 2011[283]), and inhibit caspase activity that leads to apoptotic cell death (Shang et al., 2012[285]).
Cardiovascular Disease and Mammalian Forkhead Transcription Factors (FoxOs)
Mammalian forkhead transcription factors (FoxOs) can affect the cardiovascular system through a number of pathways that involve oxidative stress, programmed cell death, and metabolic homeostasis (Abuzenadah et al., 2018[2]; Du et al., 2016[64]; Kandula et al., 2016[118]; Klionsky et al., 2021[123]; Kostić et al., 2023[124]; Maiese et al., 2009[209][214][218], 2012[217]; Razzaghi et al., 2023[260]; Schips et al., 2011[277]) (Table 1(Tab. 1)). During oxidative stress and programmed cell death, inhibition of FoxO activity can prevent microglial cell apoptosis during oxidative stress (Czubowicz et al., 2019[56]; Guo et al., 2017[89]; Hong et al., 2012[100]; Shang et al., 2009[281]; Shi et al., 2016[288]), promote cellular protection through metabotropic glutamate receptors (Maiese, 2015[169]), and block apoptotic cell death through NAD+ precursors (AlSaleh et al., 2023[6]; Chong et al., 2022[40]; Kumar and Ou, 2023[127]; Lin et al., 2022[143]; Maiese, 2008[203], 2020[179], 2021[181]; Maiese and Chong, 2003[206]; Maiese et al., 2009[210]; Rehman et al., 2022[262]; Wang et al., 2022[309]; Ye et al., 2022[327]) (Figure 1(Fig. 1)). Metformin can prevent apoptotic cell death in ischemic myocardium through the down-regulation of FoxO3a and the inhibition of caspase activation (Elmadhun et al., 2014[65]). Once FoxO proteins become active, cytochrome c release can occur with caspase-induced apoptotic death (Hou et al., 2010[101]; Qi et al., 2013[254]; Shang et al., 2010[282]; Shi et al., 2016[288]). Similar pathways apply with inhibition of FoxO activity to preserve cardiac cell survival during DM cardiomyopathy (Kandula et al., 2016[118]; Maiese et al., 2009[218]) and cardiac ischemic-perfusion injury (Guan et al., 2016[87]; Qi et al., 2013[254]). In relation to metabolic pathways with nicotinamide, phosphorylation of FoxO3a by nicotinamide at regulatory sites that possess high affinity for Akt can block apoptotic cell injury (Chong et al., 2004[46]). Nicotinamide offers cellular protection through two mechanisms of post-translational modification of FoxO3a (Maiese et al., 2008[211], 2009[214][213]). One mechanism maintains phosphorylation of FoxO3a and inhibits caspase 3 activity (Chong et al., 2004[46]). With the second mechanism, nicotinamide can preserve the integrity of the FoxO3a protein to block FoxO3a proteolysis. If FoxO3a does not become fragmented, then the generation of “pro-apoptotic” amino-terminal (Nt) fragments that normally would result is prevented (Charvet et al., 2003[32]). Similar to the metabolic pathways of nicotinamide, EPO is dependent upon FoxOs to prevent apoptotic cell loss (Fessel, 2023[73][74]; Govindappa and Elfar, 2022[85]; Hu et al., 2022[104]; Maiese, 2016[190], 2023[158]; Maiese et al., 2005[220]; Senousy et al., 2022[278]; Sergio and Rolando, 2022[279]; Yang et al., 2023[325]). EPO leads to post-translational phosphorylation of FoxO3a (Chong et al., 2011[41]), fosters FoxO3a and 14-3-3 protein binding, and oversees the intracellular trafficking of FoxO3a (Chong and Maiese, 2007[47]; Hou et al., 2011[102]). EPO is able to reverse the acetylation of FOXO3a and FOXO1a (Mahmud et al., 2002[156]) and decrease transcriptional activity of FoxO1 (Maiese, 2021[197]; Maiese et al., 2009[209]; Zhao et al., 2015[343]).
Although FoxO proteins are expressed throughout the body, FoxOs maintain a significant role in the cardiovascular system (Abuzenadah et al., 2018[2]; Blice-Baum et al., 2017[20]; Du et al., 2016[64]; Kostić et al., 2023[124]; Maiese, 2020[165], 2021[160], 2023[158]; Margrett et al., 2022[223]). Mammalian FOXO proteins of the O class include the members FOXO1, FOXO3, FOXO4, and FOXO6 (Jalgaonkar et al., 2022[110]; Ji, Liu et al., 2022[113]; Maiese et al., 2008[211], 2009[213]; Salcher et al., 2020[269]; Salih et al., 2012[271]). The function of FoxO proteins is conserved among multiple species that include Caenorhabditis elegans, Drosophila melanogaster, and mammals. FoxO proteins are homologous to the transcription factor DAuer Formation-16 (DAF-16) in the worm Caenorhabditis elegans that can oversee metabolic insulin signaling, cell survival, cell cycle regulation, and lifespan extension (Lin et al., 1997[142]; Ogg et al., 1997[236]; Sangaletti et al., 2017[273]). FoxO proteins also affect related systems to the cardiac system that involve cerebral endothelial vascular cell survival (Hou et al., 2011[102]; Maiese et al., 2004[219]), cerebral traumatic injury (Liu et al., 2021[151]), and gluconeogenesis (Calabuig-Navarro et al., 2015[24]). FoxOs are controlled by epigenetic and post-translational protein modifications that involve phosphorylation (Maiese, 2015[169]; Peng et al., 2020[247]; Zeng et al., 2020[336]), ubiquitylation (Zeldich et al., 2014[335]), and acetylation (BinMowyna and AlFaris, 2021[17]; Ren et al., 2021[263]; Shati and El-Kott, 2021[287]). Forkhead transcription factor phosphorylation is modulated by Akt (Maiese, 2016[190], 2020[189]) such that Akt phosphorylates FoxO proteins to promote binding to 14-3-3 proteins, block nuclear translocation, and inhibit transcription of target genes that would ultimately lead to apoptosis (Maiese, 2015[169]; Sanphui et al., 2020[274]; Wang et al., 2013[307]). Akt also leads to the ubiquitination and degradation of FoxOs through the 26S proteasome. FoxOs are acetylated by histone acetyltransferases that include the CREB-binding protein (CBP), the CBP-associated factor, and p300. After FoxOs undergo acetylation, nuclear translocation of FoxOs ensues but FoxO proteins now have diminished activity. This loss of FoxO activity occurs as a result of the acetylation of lysine residues on FoxO proteins limiting the ability of FoxO proteins to bind to DNA (BinMowyna and AlFaris, 2021[17]; Farhan et al., 2017[69]; Ren et al., 2021[263]; Shati and El-Kott, 2021[287]). Interestingly, acetylation of FoxOs results in the phosphorylation of FoxOs by Akt (Matsuzaki et al., 2005[224]).
In a number of scenarios, the inhibition of FoxO activity can be beneficial for cell survival and the prevention of apoptotic pathways (BinMowyna and AlFaris, 2021[17]; Cheema et al., 2021[33]; Farhan et al., 2017[69]; Gökdoğan Edgünlü et al., 2020[83]; He et al., 2021[97]; Liu et al., 2020[149]; Maiese, 2015[169][170], 2016[168]; Sanphui et al., 2020[274]; Sharma et al., 2021[286]; Shati and El-Kott, 2021[287]; Zeng et al., 2020[336]; Zhao et al., 2023[341][346]). Yet, FoxOs can enhance survival during the activation of autophagy mediated pathways (Maiese, 2016[168], 2018[184], 2021[177]). With FoxO1 activation and the induction of autophagy, basal autophagy is increased that can reduce atherogenesis (Maiese, 2015[169]; Weikel et al., 2016[313]). Exercise induced activation of autophagy results in the down-regulation of FoxO3a and suppression of sarcopenia (Zeng et al., 2020[336]). Autophagy activation in association with modulation of FoxO signaling also results in decreased renal tubulointerstitial fibrosis (Zhao et al., 2021[344]), protection through metformin to reduce inflammation (Ali et al., 2020[5]), and reduction of cardiotoxicity during ferroptosis (He et al., 2021[97]). A limited degree of FoxO activation may be required to promote cellular survival with autophagy during cardiac injury. FoxOs through the activation of autophagy can lead to the clearance of toxic intracellular accumulations and promote neuronal survival (Saleem and Biswas, 2017[270]; Tabibzadeh, 2021[298]).
In addition to pathways of programmed cell death, oxidative stress, and cellular metabolism, FoxOs share a close relationship with SIRT1. SIRT1 can be involved with DNA transcription by transferring acetyl groups from ε-N-acetyl lysine amino acids to the histones of DNA. As a result, FoxO acetylation can be controlled by SIRT1 and other histone deacetylases (Kostić et al., 2023[124]; Li et al., 2020[139]; Maiese, 2018[186], 2021[177]; Rong et al., 2021[265]; Shati and El-Kott, 2021[287]; Yaman et al., 2020[322]). Increased SIRT1 activity can modify FoxO activity to reduce oxidative stress during cardiac ischemia (Guan et al., 2016[87]), protect against vascular cerebral injury (Hassanein et al., 2022[94]), limit DM complications (Jalgaonkar et al., 2022[110]), repair microcirculation disturbances (Rong et al., 2021[265]), improve cardiac left ventricular remodeling during renal disease (Li et al., 2020[139]), activate senescence mesenchymal stem cells through telomerase activity (Okada et al., 2016[237]), and assist with cardiac fatty acid metabolism (Kostić et al., 2023[124]). In addition, there exists an autofeedback mechanism to regulate SIRT1 activity through FoxOs. FoxOs can bind to the SIRT1 promoter region that contains a cluster of five putative core binding repeat motifs (IRS-1) and a forkhead-like consensus-binding site (FKHD-L) to modify the transcription of forkhead. FoxO proteins can oversee SIRT1 transcription and increase SIRT1 expression (Xiong et al., 2011[318]). FoxOs and SIRT1 also can function in a synergistic manner to increase cell survival and have been shown to prevent mitochondrial dysfunction during oxidative stress (Lin et al., 2015[141]).
Cardiovascular Disease and AMP-activated protein kinase (AMPK)
AMP-activated protein kinase (AMPK), a member of the mechanistic target of rapamycin (mTOR) pathway (Chong and Maiese, 2012[48]; Chong et al., 2011[49]; Hua et al., 2023[105]; Maiese, 2014[164][195], 2016[196], 2020[161][165]; Thomas et al., 2023[303]; Zhang et al., 2023[340]; Zhao et al., 2023[347]), is vital in the control of cellular metabolism and insulin sensitivity that can impact cardiovascular disease (Barcena et al., 2023[13]; Castano et al., 2014[27]; Dong et al., 2019[60]; Hua et al., 2023[105]; Maiese, 2016[196], 2017[171], 2020[165], 2023[158]; Pal et al., 2019[243]; Watroba and Szukiewicz, 2021[312]; Yang et al., 2020[324]; Zhong et al., 2023[348]) (Table 1(Tab. 1)). AMPK and mTOR also may employ metabolic pathways during the control of severe acute respiratory syndrome coronavirus (SARS-CoV-2) and coronavirus disease 2019 (COVID-19) (Abu-Eid and Ward, 2021[1]; Alves et al., 2022[7]; Bramante et al., 2023[21]; Khan, 2021[119]; Khan et al., 2021[120]; Liu et al., 2023[153]; Maiese, 2020[201][182], 2021[202], 2022[157], 2023[158]; Pinchera et al., 2022[250]; Swain et al., 2021[297]) (Figure 1(Fig. 1)). Diets with fish oil consumption increase AMPK activity and prevent endothelial progenitor cell dysfunction (Chiu et al., 2017[39]). AMPK limits insulin resistance (Liu et al., 2014[152]) and may increase lifespan (Balan et al., 2008[11]) since AMPK can be one of multiple pathways to shift to beneficial oxidative metabolism (Moroz et al., 2014[229]). Nicotinamide may limit mitochondrial stress through AMPK activation (Lai et al., 2019[128]). Metformin also has a role with AMPK. Biguanides and metformin rely upon AMPK and autophagy to maintain cellular function. Through metformin, AMPK is activated, results in autophagy induction, and protects against DM apoptotic cardiac cell loss (He et al., 2013[96]). Metformin limits lipid peroxidation in the brain and spinal cord and decreases caspase activity during toxic insults (Oda, 2017[233]). These observations of metformin to offer protection during metabolic dysfunction may be associated with the ability of autophagic pathways to limit oxidative stress (Amidfar et al., 2023[8]; Chong et al., 2005[45]; Ciesielska and Gajewska, 2023[52]; du Toit et al., 2023[62]; Maiese, 2017[204], 2018[200], 2020[189], 2023[158]; Raghuvanshi et al., 2023[256]; Raut and Khullar, 2023[259]; Zhong et al., 2023[348]). Under conditions of metabolic dysfunction, AMPK can control programmed cell death during coronary artery disease (Dong et al., 2019[60]), cholesterol efflux (An et al., 2020[9]), endothelial dysfunction during hyperglycemia (Pal et al., 2019[243]), oxidative stress (Shokri Afra et al., 2019[289]; Zhao et al., 2019[342]), and prevent mitochondrial dysfunction during ferroptosis (Zhong et al., 2023[348]). In the absence of AMPK function, cell injury, cell senescence, and mitochondrial dysfunction can ensue and lead to cardiomyopathy (Barcena et al., 2023[13]; Watroba and Szukiewicz, 2021[312]). AMPK activity limits myocardial infarct size in both non-diabetic and diabetic rat hearts following exposure to ischemia/reperfusion. This process may be controlled through the inhibition of mitochondrial permeability transition pore opening in cardiomyocytes (Paiva et al., 2011[242]).
SIRT1 is a principal pathway in overseeing the activity of AMPK that can be affected by aging, inflammation, and tumorigenesis (Guimera et al., 2022[88]; Maiese, 2016[167], 2020[182]; Sadria et al., 2022[267]; Yang et al., 2020[324]; Yu et al., 2021[330]). SIRT1 functions through the AMPK kinase, serine-threonine liver kinase B1 (LKB1). Over-expression of SIRT1 can lead to the deacetylation of LKB1 and produce the translocation of LKB1 from the nucleus to the cytoplasm to activate AMPK (Lan et al., 2008[129]). As a result, AMPK enhances SIRT1 activity but it is believed not to be through a direct mechanism. AMPK activation increases SIRT1 activity either by increasing cellular NAD+/NADH ratio, resulting in the deacetylation and modulation of the activity of downstream SIRT1 targets that include peroxisome proliferator-activated receptor-gamma coactivator-1α (PGC-1α), FoxO1, and FoxO3a (Canto and Auwerx, 2009[25]) or by up-regulating NAMPT during glucose restriction, leading to increased NAD+ and decreased activity of nicotinamide that can inhibit SIRT1 (Fulco et al., 2008[77]). The SIRT1 activator resveratrol can increase AMPK activity through SIRT1 dependent or independent mechanisms (Canto and Auwerx, 2009[25]; Herranz and Serrano, 2010[99]).
Future Perspectives
Throughout the world, cardiovascular disease is the leading cause of death for both women and men to the extent that an individual expires every thirty-three seconds as a result of heart disease in just the US alone. Healthcare costs for cardiovascular disease can be greater than $ 555 billion US dollars and are expected to exceed $ 1.1 trillion US dollars by the year 2025. Cardiovascular disease is affected by the increased lifespan of the global population that also leads to cellular pathways that mediate progressive cellular oxidative stress, the shortening of TLs, and the inception of cell senescence. The onset of cardiovascular disease has additional risks that involve age sixty and over, lower income, high serum cholesterol levels, elevated blood pressure, tobacco consumption and secondhand smoke exposure, poor nutrition, physical inactivity, obesity, and the existence of DM. Although treatment of disorders such as DM can lessen the risk of developing cardiovascular disease, cardiac disease may continue to progress and the risk of additional complications may develop, such as the loss of organ mass. These considerations for the development of novel and effective treatments for cardiovascular disease call for innovative strategies that involve SIRT1, FoxOs, and AMPK.
SIRT1, a histone deacetylase that can impact DNA transcription, can oversee metabolic homeostasis through NAD+ pathways and increase cardiomyoblast survival, improve stem cell development, assist with endothelial cell function, reduce coronary artery disease, limit cell senescence, and prevent cardiac injury during DM. SIRT1 also promotes necessary circadian rhythm pathways during obesity and treatment with metformin, mediates growth factor protection with EPO, and leads to the production of nicotinamide to maintain mitochondrial function. Yet, feedback pathways are necessary between SIRT1 and nicotinamide to effectively foster anti-inflammatory pathways.
Pathways of SIRT1 are also closely tied to FoxO activity. SIRT1 activation can oversee FoxO cardiac activity to limit oxidative stress, reduce DM complications, repair microcirculation disturbances, improve left ventricular remodeling, and activate senescence mesenchymal stem cells through telomerase activity. Feedback pathways exist for SIRT1 and FoxOs such that forkhead transcription can be altered and that FoxOs can increase SIRT1 expression. This intimate relationship is vital since under some scenarios, the increased activity of FoxOs rather than inhibition of FoxO activity can promote cell survival during cardiotoxicity and ferroptosis through the activation of autophagy pathways. With other circumstances, FoxOs and SIRT1 can function in a synergistic manner to increase cell survival and block mitochondrial dysfunction during oxidative stress.
The pathways of SIRT1 and FoxOs are also complemented by the mTOR pathway AMPK. AMPK through autophagy activation can modulate infections with COVID-19, reduce endothelial dysfunction, increase lifespan, improve insulin sensitivity, and assist nicotinamide to maintain mitochondrial function. Furthermore, AMPK can function through the application of metformin to block cardiac apoptosis during DM, reduce cardiac infarct size, and limit cellular senescence. SIRT1 also is vital in the AMPK pathway to not only oversee AMPK activity, but also for AMPK to increase SIRT1 activation to limit cardiomyopathy, cell injury, and mitochondrial dysfunction.
The cellular pathways of SIRT1, FoxOs, and AMPK offer highly novel considerations to address the onset and development of cardiovascular disease. These pathways are significant in the ability to modulate cellular oxidative stress, metabolic pathways with NAD+, cell senescence and lifespan, mitochondrial injury, trophic factor protection, infectious agent injury, and programmed cell death pathways that include apoptosis, autophagy, and ferroptosis. However, the relationship among these pathways is highly complex with the existence of multiple feedback systems that will require a continued focus on new insights to safely and effectively target these innovative mechanisms for current and future clinical translation in the treatment of cardiovascular disease.
Declaration
Acknowledgments
This research was supported by the following grants to Kenneth Maiese: American Diabetes Association, American Heart Association, NIH NIEHS, NIH NIA, NIH NINDS, and NIH ARRA.
Conflict of interest
The author declares no conflict of interest.
References
- 1.Abu-Eid R, Ward FJ. Targeting the PI3K/Akt/mTOR pathway: A therapeutic strategy in COVID-19 patients. Immunol Lett. 2021;240:1–8. doi: 10.1016/j.imlet.2021.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abuzenadah A, Al-Saedi S, Karim S, Al-Qahtani M. Role of overexpressed transcription factor FOXO1 in fatal cardiovascular septal defects in patau syndrome: molecular and therapeutic strategies. Int J Mol Sci. 2018;19(11):3547. doi: 10.3390/ijms19113547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmed A, Ahmed S, Arvidsson M, Bouzina H, Lundgren J, Rådegran G. Prolargin and matrix metalloproteinase-2 in heart failure after heart transplantation and their association with haemodynamics. ESC Heart Fail. 2020;7(1):223–234. doi: 10.1002/ehf2.12560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alanko T, Tienari J, Lehtonen E, Saksela O. FGF-2 inhibits apoptosis in human teratocarcinoma cells during differentiation on collagen substratum. Exp Cell Res. 1996;228:306–312. doi: 10.1006/excr.1996.0330. [DOI] [PubMed] [Google Scholar]
- 5.Ali T, Rahman SU, Hao Q, Li W, Liu Z, Ali Shah F, et al. Melatonin prevents neuroinflammation and relieves depression by attenuating autophagy impairment through FOXO3a regulation. J Pineal Res. 2020;69(2):e12667. doi: 10.1111/jpi.12667. [DOI] [PubMed] [Google Scholar]
- 6.AlSaleh A, Shahid M, Farid E, Bindayna K. The effect of ascorbic acid and nicotinamide on panton-valentine leukocidin cytotoxicity: an ex vivo study. Toxins (Basel) 2023;15(1):38. doi: 10.3390/toxins15010038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alves HR, Lomba GSB, Gonçalves-de-Albuquerque CF, Burth P. Irisin, exercise, and COVID-19. Front Endocrinol. 2022;13:879066. doi: 10.3389/fendo.2022.879066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amidfar M, Garcez ML, Kim YK. The shared molecular mechanisms underlying aging of the brain, major depressive disorder, and Alzheimer's disease: The role of circadian rhythm disturbances. Prog Neuropsychopharmacol Biol Psychiatry. 2023;123:110721. doi: 10.1016/j.pnpbp.2023.110721. [DOI] [PubMed] [Google Scholar]
- 9.An T, Zhang X, Li H, Dou L, Huang X, Man Y, et al. GPR120 facilitates cholesterol efflux in macrophages through activation of AMPK signaling pathway. FEBS J. 2020;287:5080–5095. doi: 10.1111/febs.15310. [DOI] [PubMed] [Google Scholar]
- 10.Arildsen L, Andersen JV, Waagepetersen HS, Nissen JBD, Sheykhzade M. Hypermetabolism and impaired endothelium-dependent vasodilation in mesenteric arteries of type 2 diabetes mellitus db/db mice. Diab Vasc Dis Res. 2019;16:539–548. doi: 10.1177/1479164119865885. [DOI] [PubMed] [Google Scholar]
- 11.Balan V, Miller GS, Kaplun L, Balan K, Chong ZZ, Li F, et al. Life span extension and neuronal cell protection by Drosophila nicotinamidase. J Biol Chem. 2008;283:27810–27819. doi: 10.1074/jbc.M804681200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bandara LR, La Thangue NB. Adenovirus E1a prevents the retinoblastoma gene product from complexing with a cellular transcription factor. Nature. 1991;351(6326):494–497. doi: 10.1038/351494a0. [DOI] [PubMed] [Google Scholar]
- 13.Barcena ML, Tonini G, Haritonow N, Breiter P, Milting H, Baczko I, et al. Sex and age differences in AMPK phosphorylation, mitochondrial homeostasis, and inflammation in hearts from inflammatory cardiomyopathy patients. Aging Cell. 2023:e13894. doi: 10.1111/acel.13894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bayaraa O, Inman CK, Thomas SA, Al Jallaf F, Alshaikh M, Idaghdour Y, et al. Hyperglycemic conditions induce rapid cell dysfunction-promoting transcriptional alterations in human aortic endothelial cells. Sci Rep. 2022;12(1):20912. doi: 10.1038/s41598-022-24999-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beegum F, P VA, George KT, K PD, Begum F, Krishnadas N, et al. Sirtuins as therapeutic targets for improving delayed wound healing in diabetes. J Drug Target. 2022;30:911–926. doi: 10.1080/1061186X.2022.2085729. [DOI] [PubMed] [Google Scholar]
- 16.Begum MK, Konja D, Singh S, Chlopicki S, Wang Y. Endothelial SIRT1 as a target for the prevention of arterial aging: promises and challenges. J Cardiovasc Pharmacol. 2021;78(Suppl 6):S63–s77. doi: 10.1097/FJC.0000000000001154. [DOI] [PubMed] [Google Scholar]
- 17.BinMowyna MN, AlFaris NA. Kaempferol suppresses acetaminophen-induced liver damage by upregulation/activation of SIRT1. Pharm Biol. 2021;59:146–156. doi: 10.1080/13880209.2021.1877734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Birnie MT, Claydon MDB, Troy O, Flynn BP, Yoshimura M, Kershaw YM, et al. Circadian regulation of hippocampal function is disrupted with corticosteroid treatment. Proc Natl Acad Sci U S A. 2023;120(15):e2211996120. doi: 10.1073/pnas.2211996120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bitterman KJ, Anderson RM, Cohen HY, Latorre-Esteves M, Sinclair DA. Inhibition of silencing and accelerated aging by nicotinamide, a putative negative regulator of yeast sir2 and human SIRT1. J Biol Chem. 2002;277:45099–45107. doi: 10.1074/jbc.M205670200. [DOI] [PubMed] [Google Scholar]
- 20.Blice-Baum AC, Zambon AC, Kaushik G, Viswanathan MC, Engler AJ, Bodmer R, et al. Modest overexpression of FOXO maintains cardiac proteostasis and ameliorates age-associated functional decline. Aging Cell. 2017;16:93–103. doi: 10.1111/acel.12543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bramante CT, Beckman KB, Mehta T, Karger AB, Odde DJ, Tignanelli CJ, et al. Metformin reduces SARS-CoV-2 in a Phase 3 Randomized Placebo Controlled Clinical Trial. medRxiv. 2023 Jun 7;Preprint:2023.06.06.23290989. doi: 10.1101/2023.06.06.23290989. [DOI] [Google Scholar]
- 22.Cacabelos R, Carril JC, Cacabelos N, Kazantsev AG, Vostrov AV, Corzo L, et al. Sirtuins in Alzheimer's Disease: SIRT2-related GenoPhenotypes and implications for PharmacoEpiGenetics. Int J Mol Sci. 2019;20(5):1249. doi: 10.3390/ijms20051249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cai J, Qi H, Yao K, Yao Y, Jing D, Liao W, et al. Non-coding RNAs steering the senescence-related progress, properties, and application of mesenchymal stem cells. Front Cell Dev Biol. 2021;9:650431. doi: 10.3389/fcell.2021.650431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Calabuig-Navarro V, Yamauchi J, Lee S, Zhang T, Liu YZ, Sadlek K, et al. FoxO6 Depletion attenuates hepatic gluconeogenesis and protects against fat-induced glucose disorder in mice. J Biol Chem. 2015;290:15581–15594. doi: 10.1074/jbc.M115.650994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Canto C, Auwerx J. Caloric restriction, SIRT1 and longevity. Trends Endocrinol Metab. 2009;20:325–331. doi: 10.1016/j.tem.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cardoso S, López IP, Piñeiro-Hermida S, Pichel JG, Moreira PI. IGF1R Deficiency modulates brain signaling pathways and disturbs mitochondria and redox homeostasis. Biomedicines. 2021;9(2):158. doi: 10.3390/biomedicines9020158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Castano D, Larequi E, Belza I, Astudillo AM, Martinez-Anso E, Balsinde J, et al. Cardiotrophin-1 eliminates hepatic steatosis in obese mice by mechanisms involving AMPK activation. J Hepatol. 2014;60:1017–1025. doi: 10.1016/j.jhep.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 28.Caton PW, Kieswich J, Yaqoob MM, Holness MJ, Sugden MC. Metformin opposes impaired AMPK and SIRT1 function and deleterious changes in core clock protein expression in white adipose tissue of genetically-obese db/db mice. Diabetes Obes Metab. 2011;13:1097–1104. doi: 10.1111/j.1463-1326.2011.01466.x. [DOI] [PubMed] [Google Scholar]
- 29.CDC, Centers for Disease Control and Prevention. National Center for Health Statistics, Mortality Data on CDC WONDER. All Ages deaths by multiple cause of death 2018–2021 (11 June 2023) on CDC WONDER Database. 2023. [June 26, 2023].
- 30.CDC, Centers for Disease Control and Prevention. CDC/National Center for Health Statistics; 2019. National Vital Statistics System. NCHS Fact Sheet, March 2019. [Google Scholar]
- 31.Charles S, Raj V, Arokiaraj J, Mala K. Caveolin1/ protein arginine methyltransferase1/sirtuin1 axis as a potential target against endothelial dysfunction. Pharmacol Res. 2017;119:1–11. doi: 10.1016/j.phrs.2017.01.022. [DOI] [PubMed] [Google Scholar]
- 32.Charvet C, Alberti I, Luciano F, Jacquel A, Bernard A, Auberger P, et al. Proteolytic regulation of Forkhead transcription factor FOXO3a by caspase-3-like proteases. Oncogene. 2003;22:4557–4568. doi: 10.1038/sj.onc.1206778. [DOI] [PubMed] [Google Scholar]
- 33.Cheema PS, Nandi D, Nag A. Exploring the therapeutic potential of forkhead box O for outfoxing COVID-19. Open Biol. 2021;11(6):210069. doi: 10.1098/rsob.210069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen Q, Benson RS, Whetton AD, Brant SR, Donowitz M, Montrose MH, et al. Role of acid/base homeostasis in the suppression of apoptosis in haemopoietic cells by v-Abl protein tyrosine kinase. J Cell Sci. 1997;110:379–387. doi: 10.1242/jcs.110.3.379. [DOI] [PubMed] [Google Scholar]
- 35.Chen Y, Huang C, Zhu SY, Zou HC, Xu CY, Chen YX. Overexpression of HOTAIR attenuates Pi-induced vascular calcification by inhibiting Wnt/β-catenin through regulating miR-126/Klotho/SIRT1 axis. Mol Cell Biochem. 2021;476:3551–3561. doi: 10.1007/s11010-021-04164-8. [DOI] [PubMed] [Google Scholar]
- 36.Chen YL, Hsieh CC, Chu PM, Chen JY, Huang YC, Chen CY. Roles of protein tyrosine phosphatases in hepatocellular carcinoma progression (Review) Oncol Rep. 2023;49(3):48. doi: 10.3892/or.2023.8485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen Z, He Y, Hu F, Li M, Yao Y. Genkwanin alleviates mitochondrial dysfunction and oxidative stress in a murine model of experimental colitis: the participation of Sirt1. Ann Clin Lab Sci. 2022;52:301–313. [PubMed] [Google Scholar]
- 38.Chiareli RA, Carvalho GA, Marques BL, Mota LS, Oliveira-Lima OC, Gomes RM, et al. The role of astrocytes in the neurorepair process. Front Cell Dev Biol. 2021;9:665795. doi: 10.3389/fcell.2021.665795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chiu SC, Chao CY, Chiang EI, Syu JN, Rodriguez RL, Tang FY. N-3 polyunsaturated fatty acids alleviate high glucose-mediated dysfunction of endothelial progenitor cells and prevent ischemic injuries both in vitro and in vivo. J Nutr Biochem. 2017;42:172–181. doi: 10.1016/j.jnutbio.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 40.Chong MC, Silva A, James PF, Wu SSX, Howitt J. Exercise increases the release of NAMPT in extracellular vesicles and alters NAD(+) activity in recipient cells. Aging Cell. 2022:e13647. doi: 10.1111/acel.13647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chong ZZ, Hou J, Shang YC, Wang S, Maiese K. EPO relies upon novel signaling of Wnt1 that requires Akt1, FoxO3a, GSK-3beta, and beta-catenin to foster vascular integrity during experimental diabetes. Curr Neurovasc Res. 2011;8:103–120. doi: 10.2174/156720211795495402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chong ZZ, Kang JQ, Maiese K. Erythropoietin fosters both intrinsic and extrinsic neuronal protection through modulation of microglia, Akt1, Bad, and caspase-mediated pathways. Br J Pharmacol. 2003;138:1107–1118. doi: 10.1038/sj.bjp.0705161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chong ZZ, Kang JQ, Maiese K. Erythropoietin is a novel vascular protectant through activation of Akt1 and mitochondrial modulation of cysteine proteases. Circulation. 2002;106:2973–2979. doi: 10.1161/01.cir.0000039103.58920.1f. [DOI] [PubMed] [Google Scholar]
- 44.Chong ZZ, Li F, Maiese K. Oxidative stress in the brain: Novel cellular targets that govern survival during neurodegenerative disease. Prog Neurobiol. 2005;75:207–246. doi: 10.1016/j.pneurobio.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 45.Chong ZZ, Li F, Maiese K. Stress in the brain: novel cellular mechanisms of injury linked to Alzheimer's disease. Brain Res Brain Res Rev. 2005;49(1):1–21. doi: 10.1016/j.brainresrev.2004.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chong ZZ, Lin SH, Maiese K. The NAD+ precursor nicotinamide governs neuronal survival during oxidative stress through protein kinase B coupled to FOXO3a and mitochondrial membrane potential. J Cereb Blood Flow Metab. 2004;24:728–743. doi: 10.1097/01.WCB.0000122746.72175.0E. [DOI] [PubMed] [Google Scholar]
- 47.Chong ZZ, Maiese K. Erythropoietin involves the phosphatidylinositol 3-kinase pathway, 14-3-3 protein and FOXO3a nuclear trafficking to preserve endothelial cell integrity. Br J Pharmacol. 2007;150:839–850. doi: 10.1038/sj.bjp.0707161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chong ZZ, Maiese K. Mammalian target of rapamycin signaling in diabetic cardiovascular disease. Cardiovasc Diabetol. 2012;11(1):45. doi: 10.1186/1475-2840-11-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chong ZZ, Shang YC, Maiese K. Cardiovascular disease and mTOR signaling. Trends Cardiovasc Med. 2011;21:151–155. doi: 10.1016/j.tcm.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chong ZZ, Shang YC, Wang S, Maiese K. SIRT1: New avenues of discovery for disorders of oxidative stress. Expert Opin Ther Targets. 2012;16:167–178. doi: 10.1517/14728222.2012.648926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Choudhery MS, Khan M, Mahmood R, Mohsin S, Akhtar S, Ali F, et al. Mesenchymal stem cells conditioned with glucose depletion augments their ability to repair-infarcted myocardium. J Cell Mol Med. 2012;16:2518–2529. doi: 10.1111/j.1582-4934.2012.01568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ciesielska K, Gajewska M. Fatty acids as potent modulators of autophagy activity in white adipose tissue. Biomolecules. 2023;13(2):255. doi: 10.3390/biom13020255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Connor CE, Burrows J, Hearps AC, Woods GM, Lowenthal RM, Ragg SJ. Cell cycle arrest of hematopoietic cell lines after treatment with ceramide is commonly associated with retinoblastoma activation. Cytometry. 2001;43:164–169. doi: 10.1002/1097-0320(20010301)43:3<164::aid-cyto1044>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 54.Csicsar A, Tarantini S, Yabluchanskiy A, Balasubramanian P, Kiss T, Farkas E, et al. Role of endothelial NAD+ deficiency in age-related vascular dysfunction. Am J Physiol Heart Circ Physiol. 2019;316:H1253–H1266. doi: 10.1152/ajpheart.00039.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cui L, Guo J, Zhang Q, Yin J, Li J, Zhou W, et al. Erythropoietin activates SIRT1 to protect human cardiomyocytes against doxorubicin-induced mitochondrial dysfunction and toxicity. Toxicol Lett. 2017;275:28–38. doi: 10.1016/j.toxlet.2017.04.018. [DOI] [PubMed] [Google Scholar]
- 56.Czubowicz K, Jesko H, Wencel P, Lukiw WJ, Strosznajder RP. The role of ceramide and sphingosine-1-phosphate in Alzheimer's Disease and other neurodegenerative disorders. Mol Neurobiol. 2019;56:5436–5455. doi: 10.1007/s12035-018-1448-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.De Bonis ML, Ortega S, Blasco MA. SIRT1 Is necessary for proficient telomere elongation and genomic stability of induced pluripotent stem cells. Stem Cell Rep. 2014;2:690–706. doi: 10.1016/j.stemcr.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dhakal S, Kushairi N, Phan CW, Adhikari B, Sabaratnam V, Macreadie I. Dietary polyphenols: a multifactorial strategy to target Alzheimer's disease. Int J Mol Sci. 2019;20(20):5090. doi: 10.3390/ijms20205090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ding MR, Qu YJ, Hu B, An HM. Signal pathways in the treatment of Alzheimer's disease with traditional Chinese medicine. Biomed Pharmacother. 2022;152:113208. doi: 10.1016/j.biopha.2022.113208. [DOI] [PubMed] [Google Scholar]
- 60.Dong Y, Chen H, Gao J, Liu Y, Li J, Wang J. Molecular machinery and interplay of apoptosis and autophagy in coronary heart disease. J Mol Cell Cardiol. 2019;136:27–41. doi: 10.1016/j.yjmcc.2019.09.001. [DOI] [PubMed] [Google Scholar]
- 61.Dorvash M, Farahmandnia M, Tavassoly I. A systems biology roadmap to decode mTOR Control system in cancer. Interdiscip Sci. 2020;12(1):1–11. doi: 10.1007/s12539-019-00347-6. [DOI] [PubMed] [Google Scholar]
- 62.du Toit WL, Kruger R, Gafane-Matemane LF, Schutte AE, Louw R, Mels CMC. Markers of arterial stiffness and urinary metabolomics in young adults with early cardiovascular risk: the African-PREDICT study. Metabolomics. 2023;19(4):28. doi: 10.1007/s11306-023-01987-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.du Toit WL, Kruger R, Gafane-Matemane LF, Schutte AE, Louw R, Mels CMC. Urinary metabolomics profiling by cardiovascular risk factors in young adults: the African Prospective study on Early Detection and Identification of Cardiovascular disease and Hypertension study. J Hypertension. 2022;40(8):1545–1555. doi: 10.1097/HJH.0000000000003182. [DOI] [PubMed] [Google Scholar]
- 64.Du WW, Yang W, Chen Y, Wu ZK, Foster FS, Yang Z, et al. Foxo3 circular RNA promotes cardiac senescence by modulating multiple factors associated with stress and senescence responses. Eur Heart J. 2016;38:1402–1412. doi: 10.1093/eurheartj/ehw001. [DOI] [PubMed] [Google Scholar]
- 65.Elmadhun NY, Sabe AA, Lassaletta AD, Chu LM, Sellke FW. Metformin mitigates apoptosis in ischemic myocardium. J Surg Res. 2014;192(1):50–58. doi: 10.1016/j.jss.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Entezari M, Flavarjani ZK, Ramezani A, Nikkhah H, Karimi S, Moghadam HF, et al. Combination of intravitreal bevacizumab and erythropoietin versus intravitreal bevacizumab alone for refractory diabetic macular edema: a randomized double-blind clinical trial. Graefes Arch Clin Exp Ophthalmol. 2019;57:2375–2380. doi: 10.1007/s00417-019-04383-2. [DOI] [PubMed] [Google Scholar]
- 67.Esterline RL, Vaag A, Oscarsson J, Vora J. Mechanisms in endocrinology: SGLT2 inhibitors;clinical benefits by restoration of normal diurnal metabolism? Eur J Endocrinol. 2018;178:R113–R125. doi: 10.1530/EJE-17-0832. [DOI] [PubMed] [Google Scholar]
- 68.Fangma Y, Wan H, Shao C, Jin L, He Y. Research progress on the role of sirtuin 1 in cerebral ischemia. Cell Mol Neurobiol. 2022;43:1769–1783. doi: 10.1007/s10571-022-01288-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Farhan M, Wang H, Gaur U, Little PJ, Xu J, Zheng W. FOXO signaling pathways as therapeutic targets in cancer. Int J Biol Sci. 2017;13:815–827. doi: 10.7150/ijbs.20052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Felten M, Dame C, Lachmann G, Spies C, Rubarth K, Balzer F, et al. Circadian rhythm disruption in critically ill patients. Acta Physiol (Oxf) 2023;238(1):e13962. doi: 10.1111/apha.13962. [DOI] [PubMed] [Google Scholar]
- 71.Feng J, Wang H, Jing Z, Wang Y, Cheng Y, Wang W, et al. Role of magnesium in Type 2 Diabetes Mellitus. Biol Trace Elem Res. 2020;196(1):74–85. doi: 10.1007/s12011-019-01922-0. [DOI] [PubMed] [Google Scholar]
- 72.Ferrara-Romeo I, Martinez P, Saraswati S, Whittemore K, Graña-Castro O, Thelma Poluha L, et al. The mTOR pathway is necessary for survival of mice with short telomeres. Nat Commun. 2020;11(1):1168. doi: 10.1038/s41467-020-14962-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fessel J. Cure of Alzheimer’s Dementia requires addressing all of the affected brain cell types. J Clin Med. 2023;12(2049):1–14. doi: 10.3390/jcm12052049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fessel J. Supplementary pharmacotherapy for the behavioral abnormalities caused by stressors in humans, focused on post-traumatic stress disorder (PTSD) J Clin Med. 2023;12(4):1680. doi: 10.3390/jcm12041680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fields CR, Bengoa-Vergniory N, Wade-Martins R. Targeting alpha-synuclein as a therapy for Parkinson's Disease. Front Mol Neurosci. 2019;12:299. doi: 10.3389/fnmol.2019.00299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fryar C, Chen T, Li X. Prevalence of uncontrolled risk factors for cardiovascular disease: United States, 1999-2010. NCHS Data Brief. 2012;(103):1–8. [PubMed] [Google Scholar]
- 77.Fulco M, Cen Y, Zhao P, Hoffman EP, McBurney MW, Sauve AA, et al. Glucose restriction inhibits skeletal myoblast differentiation by activating SIRT1 through AMPK-mediated regulation of Nampt. Dev Cell. 2008;14:661–673. doi: 10.1016/j.devcel.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gallyas F, Jr, Sumegi B, Szabo C. Role of Akt activation in PARP inhibitor resistance in cancer. Cancers. 2020;12(3):532. doi: 10.3390/cancers12030532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Geier C, Perl A. Therapeutic mTOR blockade in systemic autoimmunity: Implications for antiviral immunity and extension of lifespan. Autoimmun Rev. 2021;20(12):102984. doi: 10.1016/j.autrev.2021.102984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Geng K, Ma X, Jiang Z, Huang W, Gao C, Pu Y, et al. Innate immunity in diabetic wound healing: focus on the mastermind hidden in chronic inflammatory. Front Pharmacol. 2021;12:653940. doi: 10.3389/fphar.2021.653940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ghiasi R, Naderi R, Sheervalilou R, Alipour MR. Swimming training by affecting the pancreatic Sirtuin1 (SIRT1) and oxidative stress, improves insulin sensitivity in diabetic male rats. Hormone Mol Biol Clin Invest. 2019;40(3):/j/hmbci.2019.40.issue–/j/hmbci.2019.43/hmbci. doi: 10.1515/hmbci-2019-0011. [DOI] [PubMed] [Google Scholar]
- 82.Giacalone S, Spigariolo CB, Bortoluzzi P, Nazzaro G. Oral nicotinamide: the role in skin cancer chemoprevention. Dermatol Ther. 2021;34(3):e14892. doi: 10.1111/dth.14892. [DOI] [PubMed] [Google Scholar]
- 83.Gökdoğan Edgünlü T, Ünal Y, Karakaş Çelik S, Genç Ö, Emre U, Kutlu G. The effect of FOXO gene family variants and global DNA metylation on RRMS disease. Gene. 2020;726:144172. doi: 10.1016/j.gene.2019.144172. [DOI] [PubMed] [Google Scholar]
- 84.Gong Q, Wang H, Yu P, Qian T, Xu X. Protective or harmful: the dual roles of autophagy in diabetic retinopathy. Front Med (Lausanne) 2021;8:644121. doi: 10.3389/fmed.2021.644121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Govindappa PK, Elfar JC. Erythropoietin promotes M2 macrophage phagocytosis of Schwann cells in peripheral nerve injury. Cell Death Dis. 2022;13(3):245. doi: 10.1038/s41419-022-04671-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Groen CM, Podratz JL, Pathoulas J, Staff N, Windebank AJ. Genetic reduction of mitochondria complex i subunits is protective against cisplatin-induced neurotoxicity in drosophila. J Neurosci. 2022;42:922–937. doi: 10.1523/JNEUROSCI.1479-20.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Guan XH, Liu XH, Hong X, Zhao N, Xiao YF, Wang LF, et al. CD38 deficiency protects the heart from ischemia/reperfusion injury through activating SIRT1/FOXOs-mediated antioxidative stress pathway. Oxid Med Cell Longev. 2016;2016:7410257. doi: 10.1155/2016/7410257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Guimera AM, Clark P, Wordsworth J, Anugula S, Rasmussen LJ, Shanley DP. Systems modelling predicts chronic inflammation and genomic instability prevent effective mitochondrial regulation during biological ageing. Exp Gerontol. 2022;166:111889. doi: 10.1016/j.exger.2022.111889. [DOI] [PubMed] [Google Scholar]
- 89.Guo J, Cheng J, North BJ, Wei W. Functional analyses of major cancer-related signaling pathways in Alzheimer's disease etiology. Biochim Biophys Acta. 2017;1868:341–358. doi: 10.1016/j.bbcan.2017.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gustafsson T, Ulfhake B. Sarcopenia: what is the origin of this aging-induced disorder? Front Genet. 2021;12:688526. doi: 10.3389/fgene.2021.688526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hacioglu C, Kar F, Kanbak G. Reproductive effects of nicotinamide on testicular function and structure in old male rats: oxidative, apoptotic, hormonal, and morphological analyses. Reprod Sci. 2021;28:3352–3360. doi: 10.1007/s43032-021-00647-7. [DOI] [PubMed] [Google Scholar]
- 92.Hajibabaie F, Abedpoor N, Safavi K, Taghian F. Natural remedies medicine derived from flaxseed (secoisolariciresinol diglucoside, lignans, and α-linolenic acid) improve network targeting efficiency of diabetic heart conditions based on computational chemistry techniques and pharmacophore modeling. J Food Biochem. 2022;46:e14480. doi: 10.1111/jfbc.14480. [DOI] [PubMed] [Google Scholar]
- 93.Hardeland R. Redox biology of melatonin: discriminating between circadian and non-circadian functions. Antioxid Redox Signal. 2022;37:704–725. doi: 10.1089/ars.2021.0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hassanein EHM, Saleh FM, Ali FEM, Rashwan EK, Atwa AM, Abd El-Ghafar OAM. Neuroprotective effect of canagliflozin against cisplatin-induced cerebral cortex injury is mediated by regulation of HO-1/PPAR-γ, SIRT1/FOXO-3, JNK/AP-1, TLR4/iNOS, and Ang II/Ang 1-7 signals. Immunopharmacol Immunotoxicol. 2022;45:304–316. doi: 10.1080/08923973.2022.2143371. [DOI] [PubMed] [Google Scholar]
- 95.Hayutin A. Global demographic shifts create challenges and opportunities. PREA Quart. 2007;(Fall):46–53. [Google Scholar]
- 96.He C, Zhu H, Li H, Zou MH, Xie Z. Dissociation of Bcl-2-Beclin1 complex by activated AMPK enhances cardiac autophagy and protects against cardiomyocyte apoptosis in diabetes. Diabetes. 2013;62:1270–1281. doi: 10.2337/db12-0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.He L, Yang Y, Chen J, Zou P, Li J. Transcriptional activation of ENPP2 by FoxO4 protects cardiomyocytes from doxorubicin‑induced toxicity. Mol Med Rep. 2021;24(3):668. doi: 10.3892/mmr.2021.12307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Heron M. Deaths: Leading causes for 2017. Natl Vital Stat Rep. 2019;68(6):1–77. [PubMed] [Google Scholar]
- 99.Herranz D, Serrano M. SIRT1: recent lessons from mouse models. Nat Rev Cancer. 2010;10:819–823. doi: 10.1038/nrc2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hong YK, Lee S, Park SH, Lee JH, Han SY, Kim ST, et al. Inhibition of JNK/dFOXO pathway and caspases rescues neurological impairments in Drosophila Alzheimer's disease model. Biochem Biophys Res Commun. 2012;419:49–53. doi: 10.1016/j.bbrc.2012.01.122. [DOI] [PubMed] [Google Scholar]
- 101.Hou J, Chong ZZ, Shang YC, Maiese K. FoxO3a governs early and late apoptotic endothelial programs during elevated glucose through mitochondrial and caspase signaling. Mol Cell Endocrinol. 2010;321:194–206. doi: 10.1016/j.mce.2010.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hou J, Wang S, Shang YC, Chong ZZ, Maiese K. Erythropoietin employs cell longevity pathways of SIRT1 to foster endothelial vascular integrity during oxidant stress. Curr Neurovasc Res. 2011;8:220–235. doi: 10.2174/156720211796558069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hsu NW, Chou KC, Wang YT, Hung CL, Kuo CF, Tsai SY. Building a model for predicting metabolic syndrome using artificial intelligence based on an investigation of whole-genome sequencing. J Transl Med. 2022;20(1):190. doi: 10.1186/s12967-022-03379-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hu G, Wang T, Ma C. EPO activates PI3K-IKKα-CDK1 signaling pathway to promote the proliferation of Glial Cells under hypoxia environment. Genet Mol Biol. 2022;45(1):e20210249. doi: 10.1590/1678-4685-GMB-2021-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hua K, Li T, He Y, Guan A, Chen L, Gao Y, et al. Resistin secreted by porcine alveolar macrophages leads to endothelial cell dysfunction during Haemophilus parasuis infection. Virulence. 2023;14(1):2171636. doi: 10.1080/21505594.2023.2171636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Huang C, Zhang C, Cao Y, Li J, Bi F. Major roles of the circadian clock in cancer. Cancer Biol Med. 2023;20(1):1–24. doi: 10.20892/j.issn.2095-3941.2022.0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jackson MD, Schmidt MT, Oppenheimer NJ, Denu JM. Mechanism of nicotinamide inhibition and transglycosidation by Sir2 histone/protein deacetylases. J Biol Chem. 2003;278:50985–50998. doi: 10.1074/jbc.M306552200. [DOI] [PubMed] [Google Scholar]
- 108.Jaganjac M, Milkovic L, Zarkovic N, Zarkovic K. Oxidative stress and regeneration. Free Radic Biol Med. 2022;181:154–165. doi: 10.1016/j.freeradbiomed.2022.02.004. [DOI] [PubMed] [Google Scholar]
- 109.Jahan R, Yousaf M, Khan H, Shah SA, Khan AA, Bibi N, et al. Zinc ortho methyl carbonodithioate improved pre and post-synapse memory impairment via SIRT1/p-JNK pathway against scopolamine in adult mice. J Neuroimmune Pharmacol. 2023 Jun 1;:Epub ahead of print. doi: 10.1007/s11481-023-10067-w. [DOI] [PubMed] [Google Scholar]
- 110.Jalgaonkar MP, Parmar UM, Kulkarni YA, Oza MJ. SIRT1-FOXOs activity regulates diabetic complications. Pharmacol Res. 2022;175:106014. doi: 10.1016/j.phrs.2021.106014. [DOI] [PubMed] [Google Scholar]
- 111.Januszewski AS, Watson CJ, O'Neill V, McDonald K, Ledwidge M, Robson T, et al. FKBPL is associated with metabolic parameters and is a novel determinant of cardiovascular disease. Sci Rep. 2020;10(1):21655. doi: 10.1038/s41598-020-78676-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jeyaraman M, Muthu S, Shehabaz S, Jeyaraman N, Rajendran RL, Hong CM, et al. Current understanding of MSC-derived exosomes in the management of knee osteoarthritis. Exp Cell Res. 2022;418(2):113274. doi: 10.1016/j.yexcr.2022.113274. [DOI] [PubMed] [Google Scholar]
- 113.Ji JS, Liu L, Zeng Y, Yan LL. Effect of FOXO3 and air pollution on cognitive function: a longitudinal cohort study of older adults in China from 2000 to 2014. J Gerontol A Biol Sci Med Sci. 2022;77:1534–1541. doi: 10.1093/gerona/glac016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jiang W, Ding K, Yue R, Lei M. Therapeutic effects of icariin and icariside II on diabetes mellitus and its complications. Crit Rev Food Sci Nutr. 2023 Jan 2;Epub ahead of print:1–26. doi: 10.1080/10408398.2022.2159317. [DOI] [PubMed] [Google Scholar]
- 115.Jobst M, Kiss E, Gerner C, Marko D, Del Favero G. Activation of autophagy triggers mitochondrial loss and changes acetylation profile relevant for mechanotransduction in bladder cancer cells. Arch Toxicol. 2023;97:217–233. doi: 10.1007/s00204-022-03375-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kahmini FR, Ghaleh HD, Shahgaldi S. Sirtuins: Subtle regulators involved in convoluted mechanisms of pregnancy. Cell Physiol Biochem. 2022;56:644–662. doi: 10.33594/000000588. [DOI] [PubMed] [Google Scholar]
- 117.Kalam F, James DL, Li YR, Coleman MF, Kiesel VA, Cespedes Feliciano EM, et al. Intermittent fasting interventions to leverage metabolic and circadian mechanisms for cancer treatment and supportive care outcomes. J Natl Cancer Inst Monogr. 2023;2023(61):84–103. doi: 10.1093/jncimonographs/lgad008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kandula V, Kosuru R, Li H, Yan D, Zhu Q, Lian Q, et al. Forkhead box transcription factor 1: role in the pathogenesis of diabetic cardiomyopathy. Cardiovasc Diabetol. 2016;15(1):44. doi: 10.1186/s12933-016-0361-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Khan N. mTOR: A possible therapeutic target against SARS-CoV-2 infection. Arch Stem Cell Ther. 2021;2(1):5–7. [PMC free article] [PubMed] [Google Scholar]
- 120.Khan N, Chen X, Geiger JD. Possible therapeutic use of natural compounds against COVID-19. J Cell Signal. 2021;2(1):63–79. [PMC free article] [PubMed] [Google Scholar]
- 121.Khani F, Nafian S, Mollamohammadi S, Nemati S, Shokoohian B, Hassani SN, et al. Y Chromosome genes may play roles in the development of neural rosettes from human embryonic stem cells. Stem Cell Rev Rep. 2022;18:3008–3020. doi: 10.1007/s12015-022-10392-2. [DOI] [PubMed] [Google Scholar]
- 122.Kita A, Saito Y, Miura N, Miyajima M, Yamamoto S, Sato T, et al. Altered regulation of mesenchymal cell senescence in adipose tissue promotes pathological changes associated with diabetic wound healing. Commun Biol. 2022;5(1):310. doi: 10.1038/s42003-022-03266-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Klionsky DJ, Abdel-Aziz AK, Abdelfatah S, Abdellatif M, Abdoli A, Abel S, et al. Guidelines for the use and interpretation of assays for monitoring autophagy (4th edition) Autophagy. 2021;17(1):1–382. doi: 10.1080/15548627.2020.1797280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kostić M, Korićanac G, Tepavčević S, Stanišić J, Romić S, Ćulafić T, et al. Low-Intensity exercise affects cardiac fatty acid oxidation by increasing the nuclear content of PPARα, FOXO1, and Lipin1 in fructose-fed rats. Metab Syndr Relat Disord. 2023;21:122–131. doi: 10.1089/met.2022.0078. [DOI] [PubMed] [Google Scholar]
- 125.Kowalska M, Piekut T, Prendecki M, Sodel A, Kozubski W, Dorszewska J. Mitochondrial and nuclear DNA oxidative damage in physiological and pathological aging. DNA Cell Biol. 2020;39:1410–1420. doi: 10.1089/dna.2019.5347. [DOI] [PubMed] [Google Scholar]
- 126.Kuan XY, Fauzi NSA, Ng KY, Bakhtiar A. Exploring the causal relationship between telomere biology and Alzheimer's Disease. Mol Neurobiol. 2023;60:4169–4183. doi: 10.1007/s12035-023-03337-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kumar A, Ou Y. From bench to behavior: the role of lifestyle factors on intraocular pressure, neuroprotection, and disease progression in glaucoma. Clin Exp Ophthalmol. 2023;51:380–394. doi: 10.1111/ceo.14218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lai YF, Wang L, Liu WY. Nicotinamide pretreatment alleviates mitochondrial stress and protects hypoxic myocardial cells via AMPK pathway. Eur Rev Med Pharm Sci. 2019;23:1797–1806. doi: 10.26355/eurrev_201902_17143. [DOI] [PubMed] [Google Scholar]
- 129.Lan F, Cacicedo JM, Ruderman N, Ido Y. SIRT1 modulation of the acetylation status, cytosolic localization, and activity of LKB1. Possible role in AMP-activated protein kinase activation. J Biol Chem. 2008;283:27628–27635. doi: 10.1074/jbc.M805711200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lathe R, St Clair D. Programmed ageing: decline of stem cell renewal, immunosenescence, and Alzheimer's disease. Biol Rev Camb Philos Soc. 2023;98:1424–1458. doi: 10.1111/brv.12959. [DOI] [PubMed] [Google Scholar]
- 131.Lee FK, Lee JC, Shui B, Reining S, Jibilian M, Small DM, et al. Genetically engineered mice for combinatorial cardiovascular optobiology. eLife. 2021;10:e67858. doi: 10.7554/eLife.67858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lee JH, Lee JH, Jin M, Han SD, Chon GR, Kim IH, et al. Diet control to achieve euglycemia induces significant loss of heart and liver weight via increased autophagy compared with ad libitum diet in diabetic rats. Exp Mol Med. 2014;46:e111. doi: 10.1038/emm.2014.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lei Q, Wu T, Wu J, Hu X, Guan Y, Wang Y, et al. Roles of α‑synuclein in gastrointestinal microbiome dysbiosis‑related Parkinson's disease progression (Review) Mol Med Rep. 2021;24(4):734. doi: 10.3892/mmr.2021.12374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lemarie CA, Shbat L, Marchesi C, Angulo OJ, Deschenes ME, Blostein MD, et al. Mthfr deficiency induces endothelial progenitor cell senescence via uncoupling of eNOS and downregulation of SIRT1. Am J Physiol Heart Circ Physiol. 2011;300:H745–H753. doi: 10.1152/ajpheart.00321.2010. [DOI] [PubMed] [Google Scholar]
- 135.Li J, Lin FH, Zhu XM, Lv ZM. Impact of diabetic hyperglycaemia and insulin therapy on autophagy and impairment in rat epididymis. Andrologia. 2020;52(11):e13889. doi: 10.1111/and.13889. [DOI] [PubMed] [Google Scholar]
- 136.Li JB, Hu XY, Chen MW, Xiong CH, Zhao N, Ge YH, et al. p85S6K sustains synaptic GluA1 to ameliorate cognitive deficits in Alzheimer's disease. Transl Neurodegen. 2023;12(1):1. doi: 10.1186/s40035-022-00334-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Li M, Yang Y, Wang Z, Zong T, Fu X, Aung LHH, et al. Piwi-interacting RNAs (piRNAs) as potential biomarkers and therapeutic targets for cardiovascular diseases. Angiogenesis. 2021;24(1):19–34. doi: 10.1007/s10456-020-09750-w. [DOI] [PubMed] [Google Scholar]
- 138.Li N, Yue L, Wang J, Wan Z, Bu W. MicroRNA-24 alleviates isoflurane-induced neurotoxicity in rat hippocampus via attenuation of oxidative stress. Biochem Cell Biol. 2020;98:208–218. doi: 10.1139/bcb-2019-0188. [DOI] [PubMed] [Google Scholar]
- 139.Li P, Song X, Zhang D, Guo N, Wu C, Chen K, et al. Resveratrol improves left ventricular remodeling in chronic kidney disease via Sirt1-mediated regulation of FoxO1 activity and MnSOD expression. Biofactors. 2020;46(1):168–179. doi: 10.1002/biof.1584. [DOI] [PubMed] [Google Scholar]
- 140.Li X, Feng Y, Wang XX, Truong D, Wu YC. The critical role of SIRT1 in Parkinson's disease: mechanism and therapeutic considerations. Aging Dis. 2020;11:1608–1622. doi: 10.14336/AD.2020.0216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lin CL, Huang WN, Li HH, Huang CN, Hsieh S, Lai C, et al. Hydrogen-rich water attenuates amyloid beta-induced cytotoxicity through upregulation of Sirt1-FoxO3a by stimulation of AMP-activated protein kinase in SK-N-MC cells. Chem Biol Interact. 2015;240:12–21. doi: 10.1016/j.cbi.2015.07.013. [DOI] [PubMed] [Google Scholar]
- 142.Lin K, Dorman JB, Rodan A, Kenyon C. daf-16: An HNF-3/forkhead family member that can function to double the life-span of Caenorhabditis elegans. Science. 1997;278(5341):1319–1322. doi: 10.1126/science.278.5341.1319. [DOI] [PubMed] [Google Scholar]
- 143.Lin Y, Gong T, Ma Q, Jing M, Zheng T, Yan J, et al. Nicotinamide could reduce growth and cariogenic virulence of Streptococcus mutans. J Oral Microbiol. 2022;14(1):2056291. doi: 10.1080/20002297.2022.2056291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Liu D, Zhang M, Tian J, Gao M, Liu M, Fu X, et al. WNT1-inducible signalling pathway protein 1 stabilizes atherosclerotic plaques in apolipoprotein-E-deficient mice via the focal adhesion kinase/mitogen-activated extracellular signal-regulated kinase/extracellular signal-regulated kinase pathway. J Hypertens. 2022;40:1666–1681. doi: 10.1097/HJH.0000000000003195. [DOI] [PubMed] [Google Scholar]
- 145.Liu H, Wang C, Sun X, Zhan C, Li Z, Qiu L, et al. Silk fibroin/collagen/hydroxyapatite scaffolds obtained by 3D printing technology and loaded with recombinant human erythropoietin in the reconstruction of alveolar bone defects. ACS Biomater Sci Eng. 2022;8:5245–5256. doi: 10.1021/acsbiomaterials.2c00690. [DOI] [PubMed] [Google Scholar]
- 146.Liu L, Cao Q, Gao W, Li BY, Zeng C, Xia Z, et al. Melatonin ameliorates cerebral ischemia-reperfusion injury in diabetic mice by enhancing autophagy via the SIRT1-BMAL1 pathway. Faseb J. 2021;35(12):e22040. doi: 10.1096/fj.202002718RR. [DOI] [PubMed] [Google Scholar]
- 147.Liu L, Hu J, Yang L, Wang N, Liu Y, Wei X, et al. Association of WISP1/CCN4 with risk of overweight and gestational diabetes mellitus in chinese pregnant women. Dis Markers. 2020;2020:4934206. doi: 10.1155/2020/4934206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Liu P, Liu J, Wu Y, Xi W, Wei Y, Yuan Z, et al. Zinc supplementation protects against diabetic endothelial dysfunction via GTP cyclohydrolase 1 restoration. Biochem Biophys Res Commun. 2020;521:1049–1054. doi: 10.1016/j.bbrc.2019.11.046. [DOI] [PubMed] [Google Scholar]
- 149.Liu W, Li Y, Luo B. Current perspective on the regulation of FOXO4 and its role in disease progression. Cell Mol Life Sci. 2020;77:651–663. doi: 10.1007/s00018-019-03297-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Liu X, Chen H, Zhu W, Chen H, Hu X, Jiang Z, et al. Transplantation of SIRT1-engineered aged mesenchymal stem cells improves cardiac function in a rat myocardial infarction model. J Heart Lung Transplant. 2014;33:1083–1092. doi: 10.1016/j.healun.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 151.Liu XL, Gao CC, Qi M, Han YL, Zhou ML, Zheng LR. Expression of FOXO transcription factors in the brain following traumatic brain injury. Neurosci Lett. 2021;753:135882. doi: 10.1016/j.neulet.2021.135882. [DOI] [PubMed] [Google Scholar]
- 152.Liu Y, Palanivel R, Rai E, Park M, Gabor TV, Scheid MP, et al. Adiponectin stimulates autophagy and reduces oxidative stress to enhance insulin sensitivity during high fat diet feeding in mice. Diabetes. 2014;64(1):36–48. doi: 10.2337/db14-0267. [DOI] [PubMed] [Google Scholar]
- 153.Liu Z, Huang H, Yu Y, Jia Y, Li L, Shi X, et al. Exploring the potential mechanism of action of ursolic acid against gastric cancer and COVID-19 using network pharmacology and bioinformatics analysis. Curr Pharm Des. 2023;29:1274–1292. doi: 10.2174/1381612829666230510124716. [DOI] [PubMed] [Google Scholar]
- 154.Luo L, Li R, Wang G, Chen J, Chen L, Qin LQ, et al. Age-dependent effects of a high-fat diet combined with dietary advanced glycation end products on cognitive function and protection with voluntary exercise. Food Funct. 2022;13:4445–4458. doi: 10.1039/d1fo03241k. [DOI] [PubMed] [Google Scholar]
- 155.Mahdi T, Brizard A, Millet C, Dore P, Tanzer J, Kitzis A. In vitro p53 and/or Rb antisense oligonucleotide treatment in association with growth factors induces the proliferation of peripheral hematopoietic progenitors. J Cell Sci. 1995;108:1287–1293. doi: 10.1242/jcs.108.3.1287. [DOI] [PubMed] [Google Scholar]
- 156.Mahmud DL, M GA, Deb DK, Platanias LC, Uddin S, Wickrema A. Phosphorylation of forkhead transcription factors by erythropoietin and stem cell factor prevents acetylation and their interaction with coactivator p300 in erythroid progenitor cells. Oncogene. 2002;21:1556–1562. doi: 10.1038/sj.onc.1205230. [DOI] [PubMed] [Google Scholar]
- 157.Maiese K. Apolipoprotein-ε4 allele (APOE-ε4) as a mediator of cognitive loss and dementia in long COVID-19. Curr Neurovasc Res. 2022;19:435–439. doi: 10.2174/156720261905221227114624. [DOI] [PubMed] [Google Scholar]
- 158.Maiese K. Cellular metabolism: a fundamental component of degeneration in the nervous system. Biomolecules. 2023;13(5):816. doi: 10.3390/biom13050816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Maiese K. Circadian clock genes: targeting innate immunity for antiviral strategies against COVID-19. Curr Neurovasc Res. 2020;17:531–533. doi: 10.2174/1567202617666201203110008. [DOI] [PubMed] [Google Scholar]
- 160.Maiese K. Cognitive impairment and dementia: gaining insight through circadian clock gene pathways. Biomolecules. 2021;11(7):1–18. doi: 10.3390/biom11071002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Maiese K. Cognitive impairment with diabetes mellitus and metabolic disease: innovative insights with the mechanistic target of rapamycin and circadian clock gene pathways. Expert Rev Clin Pharmacol. 2020;13(1):23–34. doi: 10.1080/17512433.2020.1698288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Maiese K. Cutting through the Complexities of mTOR for the Treatment of Stroke. Curr Neurovasc Res. 2014;11:177–186. doi: 10.2174/1567202611666140408104831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Maiese K. Disease onset and aging in the world of circular RNAs. J Transl Sci. 2016;2:327–329. doi: 10.15761/jts.1000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Maiese K. Driving neural regeneration through the mammalian target of rapamycin. Neural Regen Res. 2014;9:1413–1417. doi: 10.4103/1673-5374.139453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Maiese K. Dysregulation of metabolic flexibility: The impact of mTOR on autophagy in neurodegenerative disease. Int Rev Neurobiol. 2020;155:1–35. doi: 10.1016/bs.irn.2020.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Maiese K. Erythropoietin and diabetes mellitus. World J Diabetes. 2015;614:1259–1273. doi: 10.4239/wjd.v6.i14.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Maiese K. Erythropoietin and mTOR: a "one-two punch" for aging-related disorders accompanied by enhanced life expectancy. Curr Neurovasc Res. 2016;13:329–340. doi: 10.2174/1567202613666160729164900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Maiese K. Forkhead transcription factors: new considerations for alzheimer’s disease and dementia. J Transl Sci. 2016;2:241–247. doi: 10.15761/JTS.1000146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Maiese K. FoxO proteins in the nervous system. Anal Cell Pathol (Amst) 2015;2015:569392. doi: 10.1155/2015/569392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Maiese K. FoxO transcription factors and regenerative pathways in Diabetes mellitus. Curr Neurovasc Res. 2015;12:404–413. doi: 10.2174/1567202612666150807112524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Maiese K. Harnessing the power of SIRT1 and non-coding RNAs in vascular disease. Curr Neurovasc Res. 2017;14:82–88. doi: 10.2174/1567202613666161129112822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Maiese K. Healing the heart with sirtuins and mammalian forkhead transcription factors. Curr Neurovasc Res. 2020;17(1):1–2. doi: 10.2174/1567202616999191209142915. [DOI] [PubMed] [Google Scholar]
- 173.Maiese K. Impacting dementia and cognitive loss with innovative strategies: mechanistic target of rapamycin, clock genes, circular non-coding ribonucleic acids, and Rho/Rock. Neural Regen Res. 2019;14:773–774. doi: 10.4103/1673-5374.249224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Maiese K. MicroRNAs for the treatment of dementia and Alzheimer's disease. Curr Neurovasc Res. 2019;16(1):1–2. doi: 10.2174/1567202616666190208094159. [DOI] [PubMed] [Google Scholar]
- 175.Maiese K. Moving to the rhythm with clock (circadian) genes, autophagy, mTOR, and SIRT1 in degenerative disease and cancer. Curr Neurovasc Res. 2017;14:299–304. doi: 10.2174/1567202614666170718092010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Maiese K. mTOR: Driving apoptosis and autophagy for neurocardiac complications of diabetes mellitus. World J Diabetes. 2015;6:217–224. doi: 10.4239/wjd.v6.i2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Maiese K. Neurodegeneration, memory loss, and dementia: the impact of biological clocks and circadian rhythm. Front Biosci (Landmark ed) 2021;26:614–627. doi: 10.52586/4971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Maiese K. New challenges and strategies for cardiac disease: autophagy, mTOR, and AMP-activated protein kinase. Curr Neurovasc Res. 2020;17:111–112. doi: 10.2174/1567202617999200207153935. [DOI] [PubMed] [Google Scholar]
- 179.Maiese K. New insights for nicotinamide: metabolic disease, autophagy, and mTOR. Front Biosci (Landmark ed) 2020;25:1925–1973. doi: 10.2741/4886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Maiese K. New insights for oxidative stress and Diabetes Mellitus. Oxid Med Cell Longev. 2015;2015:875961. doi: 10.1155/2015/875961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Maiese K. Nicotinamide as a foundation for treating neurodegenerative disease and metabolic disorders. Curr Neurovasc Res. 2021;18:134–149. doi: 10.2174/1567202617999210104220334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Maiese K. Nicotinamide: oversight of metabolic dysfunction through SIRT1, mTOR, and clock genes. Curr Neurovasc Res. 2020;17:765–783. doi: 10.2174/1567202617999201111195232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Maiese K. Novel nervous and multi-system regenerative therapeutic strategies for diabetes mellitus with mTOR. Neural Regen Res. 2016;11:372–385. doi: 10.4103/1673-5374.179032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Maiese K. Novel pathways of autophagy for the treatment of nervous system disorders. In: Ren J, Sowers JR, Zhang Y, editors. Autophagy and cardiometabolic diseases: from molecular mechanisms to translational medicine. Amsterdam: Academic Press; 2018. pp. 187–197. [Google Scholar]
- 185.Maiese K. Novel stem cell strategies with mTOR. In: Maiese K, editor. Maiese K (ed): Molecules to medicine with mTOR. Translating critical pathways into novel therapeutic strategies. Amsterdam: Academic Press; 2016. pp. 3–22. [Google Scholar]
- 186.Maiese K. Novel treatment strategies for the nervous system: circadian clock genes, non-coding RNAs, and forkhead transcription factors. Curr Neurovasc Res. 2018;15:81–91. doi: 10.2174/1567202615666180319151244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Maiese K. Picking a bone with WISP1 (CCN4): new strategies against degenerative joint disease. J Transl Sci. 2016;1(3):83–85. [PMC free article] [PubMed] [Google Scholar]
- 188.Maiese K. Programming apoptosis and autophagy with novel approaches for diabetes mellitus. Curr Neurovasc Res. 2015;12:173–188. doi: 10.2174/1567202612666150305110929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Maiese K. Prospects and perspectives for WISP1 (CCN4) in Diabetes mellitus. Curr Neurovasc Res. 2020;17:327–331. doi: 10.2174/1567202617666200327125257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Maiese K. Regeneration in the nervous system with erythropoietin. Front Biosci (Landmark ed. 2016;21:561–596. doi: 10.2741/4408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Maiese K. SIRT1 and stem cells: In the forefront with cardiovascular disease, neurodegeneration and cancer. World J Stem Cells. 2015;7:235–242. doi: 10.4252/wjsc.v7.i2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Maiese K. Sirtuins in metabolic disease: innovative therapeutic strategies with SIRT1, AMPK, mTOR, and nicotinamide. In: Maiese K, editor. Sirtuin biology in cancer and metabolic disease: cellular pathways for clinical discovery. Amsterdam: Academic Press; 2021. pp. 3–23. [Google Scholar]
- 193.Maiese K. Sirtuins: developing innovative treatments for aged-related memory loss and Alzheimer's disease. Curr Neurovasc Res. 2018;15:367–371. doi: 10.2174/1567202616666181128120003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Maiese K. Stem cell guidance through the mechanistic target of rapamycin. World J Stem Cells. 2015;7:999–1009. doi: 10.4252/wjsc.v7.i7.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Maiese K. Taking aim at Alzheimer's disease through the mammalian target of rapamycin. Ann Med. 2014;46:587–596. doi: 10.3109/07853890.2014.941921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Maiese K. Targeting molecules to medicine with mTOR, autophagy and neurodegenerative disorders. Br J Clin Pharmacol. 2016;82:1245–1266. doi: 10.1111/bcp.12804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Maiese K. Targeting the core of neurodegeneration: FoxO, mTOR, and SIRT1. Neural Regen Res. 2021;16:448–455. doi: 10.4103/1673-5374.291382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Maiese K. The bright side of reactive oxygen species: lifespan extension without cellular demise. J Transl Sci. 2016;2:185–187. doi: 10.15761/JTS.1000138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Maiese K. The implications of telomere length: advanced aging, cell senescence, MRI phenotypes, stem cells and Alzheimer's disease. Curr Neurovasc Res. 2023 May 10;:Epub ahead of print. doi: 10.2174/1567202620666230510150337. [DOI] [PubMed] [Google Scholar]
- 200.Maiese K. The mechanistic target of rapamycin (mTOR) and the silent mating-type information regulation 2 homolog 1 (SIRT1): oversight for neurodegenerative disorders. Biochem Soc Trans. 2018;46:351–360. doi: 10.1042/BST20170121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Maiese K. The mechanistic target of rapamycin (mTOR): novel considerations as an antiviral treatment. Curr Neurovasc Res. 2020;17:332–337. doi: 10.2174/1567202617666200425205122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Maiese K. The oversight of circadian clock genes for the detection, prevention, and treatment of COVID-19 infection. Curr Neurovasc Res. 2021;18:471–473. doi: 10.2174/1567202619666211223142258. [DOI] [PubMed] [Google Scholar]
- 203.Maiese K. Triple play: promoting neurovascular longevity with nicotinamide, WNT, and erythropoietin in diabetes mellitus. Biomed Pharmacother. 2008;62:218–232. doi: 10.1016/j.biopha.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Maiese K. Warming up to new possibilities with the capsaicin receptor TRPV1: mTOR, AMPK, and erythropoietin. Curr Neurovasc Res. 2017;14:184–189. doi: 10.2174/1567202614666170313105337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Maiese K. Wnt signaling and WISP1 (CCN4): critical components in neurovascular disease, blood brain barrier regulation, and cerebral hemorrhage. Curr Neurovasc Res. 2022;19:379–382. doi: 10.2174/1567202620666221019162248. [DOI] [PubMed] [Google Scholar]
- 206.Maiese K, Chong ZZ. Nicotinamide: necessary nutrient emerges as a novel cytoprotectant for the brain. Trends Pharmacol Sci. 2003;2):228–232. doi: 10.1016/S0165-6147(03)00078-6. [DOI] [PubMed] [Google Scholar]
- 207.Maiese K, Chong ZZ, Hou J, Shang YC. Erythropoietin and oxidative stress. Curr Neurovasc Res. 2008;5:125–142. doi: 10.2174/156720208784310231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Maiese K, Chong ZZ, Hou J, Shang YC. Oxidative stress: Biomarkers and novel therapeutic pathways. Exp Gerontol. 2010;45:217–234. doi: 10.1016/j.exger.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Maiese K, Chong ZZ, Hou J, Shang YC. The "O" class: crafting clinical care with FoxO transcription factors. Adv Exp Med Biol. 2009;665:242–260. doi: 10.1007/978-1-4419-1599-3_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Maiese K, Chong ZZ, Hou J, Shang YC. The vitamin nicotinamide: translating nutrition into clinical care. Molecules. 2009;14:3446–3485. doi: 10.3390/molecules14093446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Maiese K, Chong ZZ, Shang YC. OutFOXOing disease and disability: the therapeutic potential of targeting FoxO proteins. Trends Mol Med. 2008;14:219–227. doi: 10.1016/j.molmed.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Maiese K, Chong ZZ, Shang YC. Raves and risks for erythropoietin. Cytokine Growth Factor Rev. 2008;19:145–155. doi: 10.1016/j.cytogfr.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Maiese K, Chong ZZ, Shang YC, Hou J. A "FOXO" in sight: targeting Foxo proteins from conception to cancer. Med Res Rev. 2009;29:395–418. doi: 10.1002/med.20139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Maiese K, Chong ZZ, Shang YC, Hou J. FoxO proteins: cunning concepts and considerations for the cardiovascular system. Clin Sci (Lond) 2009;116:191–203. doi: 10.1042/CS20080113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Maiese K, Chong ZZ, Shang YC, Hou J. Rogue proliferation versus restorative protection: where do we draw the line for Wnt and forkhead signaling? Expert Opin Ther Targets. 2008;12:905–916. doi: 10.1517/14728222.12.7.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Maiese K, Chong ZZ, Shang YC, Wang S. Novel directions for diabetes mellitus drug discovery. Expert Opin Drug Discov. 2013;8(1):35–48. doi: 10.1517/17460441.2013.736485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Maiese K, Chong ZZ, Shang YC, Wang S. Targeting disease through novel pathways of apoptosis and autophagy. Expert Opin Ther Targets. 2012;16:1203–1214. doi: 10.1517/14728222.2012.719499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Maiese K, Hou J, Chong ZZ, Shang YC. A fork in the path: Developing therapeutic inroads with FoxO proteins. Oxid Med Cell Longev. 2009;2:119–129. doi: 10.4161/oxim.2.3.8916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Maiese K, Li F, Chong ZZ. Erythropoietin in the brain: can the promise to protect be fulfilled? Trends Pharmacol Sci. 2004;25:577–583. doi: 10.1016/j.tips.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 220.Maiese K, Li F, Chong ZZ. New avenues of exploration for erythropoietin. JAMA. 2005;293(1):90–95. doi: 10.1001/jama.293.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Maiese K, Li F, Chong ZZ, Shang YC. The Wnt signaling pathway: Aging gracefully as a protectionist? Pharmacol Ther. 2008;118(1):58–81. doi: 10.1016/j.pharmthera.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Maiese K, Shang YC, Chong ZZ, Hou J. Diabetes mellitus: channeling care through cellular discovery. Curr Neurovasc Res. 2010;7:59–64. doi: 10.2174/156720210790820217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Margrett JA, Schofield T, Martin P, Poon LW, Masaki K, Donlon TA, et al. Novel functional, health, and genetic determinants of cognitive terminal decline: Kuakini Honolulu Heart Program/Honolulu-Asia Aging Study. J Gerontol A Biol Sci Med Sci. 2022;77:1525–1533. doi: 10.1093/gerona/glab327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Matsuzaki H, Daitoku H, Hatta M, Aoyama H, Yoshimochi K, Fukamizu A. Acetylation of Foxo1 alters its DNA-binding ability and sensitivity to phosphorylation. Proc Natl Acad Sci U S A. 2005;102:11278–11283. doi: 10.1073/pnas.0502738102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Ministrini S, Puspitasari YM, Beer G, Liberale L, Montecucco F, Camici GG. Sirtuin 1 in endothelial dysfunction and cardiovascular aging. Front Physiol. 2021;12:733696. doi: 10.3389/fphys.2021.733696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Mocayar Marón FJ, Ferder L, Reiter RJ, Manucha W. Daily and seasonal mitochondrial protection: Unraveling common possible mechanisms involving vitamin D and melatonin. J Steroid Biochem Mol Biol. 2020;199:105595. doi: 10.1016/j.jsbmb.2020.105595. [DOI] [PubMed] [Google Scholar]
- 227.Montesano A, Bonfigli AR, De Luca M, Crocco P, Garagnani P, Marasco E, et al. Erythropoietin (EPO) haplotype associated with all-cause mortality in a cohort of Italian patients with Type-2 Diabetes. Sci Rep. 2019;9(1):10395. doi: 10.1038/s41598-019-46894-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Mori M, Cazzaniga G, Meneghetti F, Villa S, Gelain A. Insights on the modulation of SIRT5 activity: a challenging balance. Molecules. 2022;27(14):4449. doi: 10.3390/molecules27144449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Moroz N, Carmona JJ, Anderson E, Hart AC, Sinclair DA, Blackwell TK. Dietary restriction involves NAD -dependent mechanisms and a shift toward oxidative metabolism. Aging Cell. 2014;13:1075–1085. doi: 10.1111/acel.12273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Najjar RS, Turner CG, Wong BJ, Feresin RG. Berry-derived polyphenols in cardiovascular pathologies: mechanisms of disease and the role of diet and sex. Nutrients. 2021;13(2):387. doi: 10.3390/nu13020387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Nakahata Y, Sahar S, Astarita G, Kaluzova M, Sassone-Corsi P. Circadian control of the NAD+ salvage pathway by CLOCK-SIRT1. Science. 2009;324(5927):654–657. doi: 10.1126/science.1170803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Oblong JE. The evolving role of the NAD+/nicotinamide metabolome in skin homeostasis, cellular bioenergetics, and aging. DNA Repair (Amst) 2014;23:59–63. doi: 10.1016/j.dnarep.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 233.Oda SS. Metformin protects against experimental acrylamide neuropathy in rats. Drug Dev Res. 2017;78:349–359. doi: 10.1002/ddr.21400. [DOI] [PubMed] [Google Scholar]
- 234.Odnokoz O, Nakatsuka K, Wright C, Castellanos J, Klichko VI, Kretzschmar D, et al. Mitochondrial redox signaling is critical to the normal functioning of the neuronal system. Front Cell Dev Biol. 2021;9:613036. doi: 10.3389/fcell.2021.613036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.O'Donnell BT, Monjure TA, Al-Ghadban S, Ives CJ, L'Ecuyer MP, Rhee C, et al. Aberrant expression of COX-2 and FOXG1 in infrapatellar fat pad-derived ASCs from pre-diabetic donors. Cells. 2022;11(15):2367. doi: 10.3390/cells11152367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Ogg S, Paradis S, Gottlieb S, Patterson GI, Lee L, Tissenbaum HA, et al. The Fork head transcription factor DAF-16 transduces insulin-like metabolic and longevity signals in C. elegans. Nature. 1997;389(6654):994–999. doi: 10.1038/40194. [DOI] [PubMed] [Google Scholar]
- 237.Okada M, Kim HW, Matsu-Ura K, Wang YG, Xu M, Ashraf M. Abrogation of age-induced MicroRNA-195 rejuvenates the senescent mesenchymal stem cells by reactivating telomerase. Stem Cells. 2016;34(1):148–159. doi: 10.1002/stem.2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Olejniczak I, Pilorz V, Oster H. Circle(s) of life: the circadian clock from birth to death. Biology (Basel) 2023;12(3):383. doi: 10.3390/biology12030383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Oliveira ALL, Santos GGL, Espirito-Santo RF, Silva GSA, Evangelista AF, Silva DN, et al. Reestablishment of redox homeostasis in the nociceptive primary afferent as a mechanism of antinociception promoted by mesenchymal stem/stromal cells in oxaliplatin-induced chronic peripheral neuropathy. Stem Cells Int. 2021;2021:8815206. doi: 10.1155/2021/8815206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Oyefeso FA, Muotri AR, Wilson CG, Pecaut MJ. Brain organoids: a promising model to assess oxidative stress-induced central nervous system damage. Dev Neurobiol. 2021;8:653–670. doi: 10.1002/dneu.22828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Pabel S, Hamdani N, Luedde M, Sossalla S. SGLT2 inhibitors and their mode of action in heart failure-has the mystery been unravelled? Curr Heart Fail Rep. 2021;18:315–328. doi: 10.1007/s11897-021-00529-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Paiva MA, Rutter-Locher Z, Goncalves LM, Providencia LA, Davidson SM, Yellon DM, et al. Enhancing AMPK activation during ischemia protects the diabetic heart against reperfusion injury. Am J Physiol Heart Circ Physiol. 2011;300:H2123–H2134. doi: 10.1152/ajpheart.00707.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Pal PB, Sonowal H, Shukla K, Srivastava SK, Ramana KV. Aldose reductase regulates hyperglycemia-induced HUVEC death via SIRT1/AMPK-alpha1/mTOR pathway. J Mol Endocrinol. 2019;63:11–25. doi: 10.1530/JME-19-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Papachristoforou E, Lambadiari V, Maratou E, Makrilakis K. Association of glycemic indices (hyperglycemia, glucose variability, and hypoglycemia) with oxidative stress and diabetic complications. J Diabetes Res. 2020;2020:7489795. doi: 10.1155/2020/7489795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Passariello CL, Zini M, Nassi PA, Pignatti C, Stefanelli C. Upregulation of SIRT1 deacetylase in phenylephrine-treated cardiomyoblasts. Biochem Biophys Res Commun. 2011;407:512–516. doi: 10.1016/j.bbrc.2011.03.049. [DOI] [PubMed] [Google Scholar]
- 246.Patocka J, Kuca K, Oleksak P, Nepovimova E, Valis M, Novotny M, et al. Rapamycin: drug repurposing in SARS-CoV-2 infection. Pharmaceuticals (Basel) 2021;14(3):217. doi: 10.3390/ph14030217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Peng S, Li W, Hou N, Huang N. A review of FoxO1-regulated metabolic diseases and related drug discoveries. Cells. 2020;9(1):184. doi: 10.3390/cells9010184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Perluigi M, Di Domenico F, Barone E, Butterfield DA. mTOR in Alzheimer disease and its earlier stages: Links to oxidative damage in the progression of this dementing disorder. Free Radic Biol Med. 2021;169:382–396. doi: 10.1016/j.freeradbiomed.2021.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Piao S, Lee I, Jin SA, Kim S, Nagar H, Choi SJ, et al. SIRT1 activation attenuates the cardiac dysfunction induced by endothelial cell-specific deletion of CRIF1. Biomedicines. 2021;9(1):52. doi: 10.3390/biomedicines9010052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Pinchera B, Scotto R, Buonomo AR, Zappulo E, Stagnaro F, Gallicchio A, et al. Diabetes and COVID-19: The potential role of mTOR. Diabetes Res Clin Pract. 2022;186:109813. doi: 10.1016/j.diabres.2022.109813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Ponzetti M, Rucci N, Falone S. RNA methylation and cellular response to oxidative stress-promoting anticancer agents. Cell Cycle. 2023;22:870–905. doi: 10.1080/15384101.2023.2165632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Prasuhn J, Brüggemann N. Genotype-driven therapeutic developments in Parkinson's disease. Mol Med. 2021;27(1):42. doi: 10.1186/s10020-021-00281-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Puri D, Kelkar A, Gaurishankar B, Subramanyam D. Balance between autophagy and cell death is maintained by Polycomb-mediated regulation during stem cell differentiation. FEBS J. 2023;290:1625–1644. doi: 10.1111/febs.16656. [DOI] [PubMed] [Google Scholar]
- 254.Qi XF, Li YJ, Chen ZY, Kim SK, Lee KJ, Cai DQ. Involvement of the FoxO3a pathway in the ischemia/reperfusion injury of cardiac microvascular endothelial cells. Exp Mol Pathol. 2013;95:242–247. doi: 10.1016/j.yexmp.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 255.Quintana-Pérez JC, García-Dolores F, Valdez-Guerrero AS, Alemán-González-Duhart D, Arellano-Mendoza MG, Rojas Hernández S, et al. Modeling type 2 diabetes in rats by administering tacrolimus. Islets. 2022;14(1):114–127. doi: 10.1080/19382014.2022.2051991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Raghuvanshi DS, Chakole S, Kumar M. Relationship between vitamins and diabetes. Cureus. 2023;15(3):e36815. doi: 10.7759/cureus.36815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Ran D, Hong W, Yan W, Mengdie W. Properties and molecular mechanisms underlying geniposide-mediated therapeutic effects in chronic inflammatory diseases. J Ethnopharmacol. 2021;273:113958. doi: 10.1016/j.jep.2021.113958. [DOI] [PubMed] [Google Scholar]
- 258.Rapaka D, Bitra VR, Challa SR, Adiukwu PC. mTOR signaling as a molecular target for the alleviation of Alzheimer's disease pathogenesis. Neurochem Int. 2022;155:105311. doi: 10.1016/j.neuint.2022.105311. [DOI] [PubMed] [Google Scholar]
- 259.Raut SK, Khullar M. Oxidative stress in metabolic diseases: current scenario and therapeutic relevance. Mol Cell Biochem. 2023;478:185–196. doi: 10.1007/s11010-022-04496-z. [DOI] [PubMed] [Google Scholar]
- 260.Razzaghi A, Choobineh S, Gaeini A, Soori R. Interaction of exercise training with taurine attenuates infarct size and cardiac dysfunction via Akt-Foxo3a-caspase-8 signaling pathway. Amino Acids. 2023 May 18;:Epub ahead of print. doi: 10.1007/s00726-023-03275-4. [DOI] [PubMed] [Google Scholar]
- 261.Redhwan MAM, M GH, Samaddar S, Hard S, Yadav V, Mukherjee A, et al. Small interference (RNAi) technique: Exploring its clinical applications, benefits and limitations. Eur J Clin Invest. 2023 Jun 13;Epub ahead of print:e14039. doi: 10.1111/eci.14039. [DOI] [PubMed] [Google Scholar]
- 262.Rehman IU, Khan A, Ahmad R, Choe K, Park HY, Lee HJ, et al. Neuroprotective effects of nicotinamide against MPTP-induced Parkinson's disease in mice: impact on oxidative stress, neuroinflammation, Nrf2/HO-1 and TLR4 signaling pathways. Biomedicines. 2022;10(11):2929. doi: 10.3390/biomedicines10112929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Ren T, Chen H, Liu X, Wang Y, Fan A, Qi L, et al. ID1 inhibits foot-and-mouth disease virus replication via targeting of interferon pathways. FEBS J. 2021;288:4364–4381. doi: 10.1111/febs.15725. [DOI] [PubMed] [Google Scholar]
- 264.Rey F, Ottolenghi S, Giallongo T, Balsari A, Martinelli C, Rey R, et al. Mitochondrial metabolism as target of the neuroprotective role of erythropoietin in parkinson's disease. Antioxidants (Basel) 2021;10(1):121. doi: 10.3390/antiox10010121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Rong Y, Ren J, Song W, Xiang R, Ge Y, Lu W, et al. Resveratrol suppresses severe acute pancreatitis-induced microcirculation disturbance through targeting SIRT1-FOXO1 axis. Oxid Med Cell Longev. 2021;2021:8891544. doi: 10.1155/2021/8891544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Rotllan N, Camacho M, Tondo M, Diarte-Añazco EMG, Canyelles M, Méndez-Lara KA, et al. Therapeutic potential of emerging nAD+-increasing strategies for cardiovascular diseases. Antioxidants (Basel) 2021;10(12):1939. doi: 10.3390/antiox10121939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Sadria M, Seo D, Layton AT. The mixed blessing of AMPK signaling in Cancer treatments. BMC Cancer. 2022;22(1):105. doi: 10.1186/s12885-022-09211-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Sakakibara Y, Mitha AP, Ayoub IA, Ogilvy CS, Maynard KI. Delayed treatment with nicotinamide (vitamin B3) reduces the infarct volume following focal cerebral ischemia in spontaneously hypertensive rats, diabetic and non-diabetic Fischer 344 rats. Brain Res. 2002;931:68–73. doi: 10.1016/s0006-8993(02)02263-1. [DOI] [PubMed] [Google Scholar]
- 269.Salcher S, Spoden G, Hagenbuchner J, Fuhrer S, Kaserer T, Tollinger M, et al. A drug library screen identifies Carbenoxolone as novel FOXO inhibitor that overcomes FOXO3-mediated chemoprotection in high-stage neuroblastoma. Oncogene. 2020;39:1080–1097. doi: 10.1038/s41388-019-1044-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Saleem S, Biswas SC. Tribbles pseudokinase 3 induces both apoptosis and autophagy in amyloid-beta-induced neuronal death. J Biol Chem. 2017;292:2571–2585. doi: 10.1074/jbc.M116.744730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Salih DA, Rashid AJ, Colas D, de la Torre-Ubieta L, Zhu RP, Morgan AA, et al. FoxO6 regulates memory consolidation and synaptic function. Genes Dev. 2012;26:2780–2801. doi: 10.1101/gad.208926.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Sanabria-de la Torre R, García-Fontana C, González-Salvatierra S, Andújar-Vera F, Martínez-Heredia L, García-Fontana B, et al. The contribution of Wnt signaling to vascular complications in type 2 Diabetes mellitus. Int J Mol Sci. 2022;23(13):6995. doi: 10.3390/ijms23136995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Sangaletti R, D'Amico M, Grant J, Della-Morte D, Bianchi L. Knock-out of a mitochondrial sirtuin protects neurons from degeneration in Caenorhabditis elegans. PLoS Genet. 2017;13(8):e1006965. doi: 10.1371/journal.pgen.1006965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Sanphui P, Das AK, Biswas SC. FoxO3a requires BAF57, a subunit of chromatin remodeler SWI/SNF complex for induction of PUMA in a model of Parkinson's disease. J Neurochem. 2020;154(5):e14969. doi: 10.1111/jnc.14969. [DOI] [PubMed] [Google Scholar]
- 275.Saxton TM, Pawson T. Morphogenetic movements at gastrulation require the SH2 tyrosine phosphatase Shp2. Proc Natl Acad Sci U S A. 1999;96:3790–3795. doi: 10.1073/pnas.96.7.3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Schell M, Wardelmann K, Kleinridders A. Untangling the effect of insulin action on brain mitochondria and metabolism. J Neuroendocrinol. 2021;33(4):e12932. doi: 10.1111/jne.12932. [DOI] [PubMed] [Google Scholar]
- 277.Schips TG, Wietelmann A, Hohn K, Schimanski S, Walther P, Braun T, et al. FoxO3 induces reversible cardiac atrophy and autophagy in a transgenic mouse model. Cardiovasc Res. 2011;91:587–597. doi: 10.1093/cvr/cvr144. [DOI] [PubMed] [Google Scholar]
- 278.Senousy MA, Hanafy ME, Shehata N, Rizk SM. Erythropoietin and bacillus Calmette-Guérin vaccination mitigate 3-nitropropionic acid-induced Huntington-like disease in rats by modulating the PI3K/Akt/mTOR/P70S6K pathway and enhancing the autophagy. ACS Chem Neurosci. 2022;13:721–732. doi: 10.1021/acschemneuro.1c00523. [DOI] [PubMed] [Google Scholar]
- 279.Sergio CM, Rolando CA. Erythropoietin regulates signaling pathways associated with neuroprotective events. Exp Brain Res. 2022;240:1303–1315. doi: 10.1007/s00221-022-06331-9. [DOI] [PubMed] [Google Scholar]
- 280.Shafi O. Inverse relationship between Alzheimer's disease and cancer, and other factors contributing to Alzheimer's disease: a systematic review. BMC Neurol. 2016;16(1):236. doi: 10.1186/s12883-016-0765-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Shang YC, Chong ZZ, Hou J, Maiese K. The forkhead transcription factor FoxO3a controls microglial inflammatory activation and eventual apoptotic injury through caspase 3. Curr Neurovasc Res. 2009;6:20–31. doi: 10.2174/156720209787466064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Shang YC, Chong ZZ, Hou J, Maiese K. Wnt1, FoxO3a, and NF-kappaB oversee microglial integrity and activation during oxidant stress. Cell Signal. 2010;22:1317–1329. doi: 10.1016/j.cellsig.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Shang YC, Chong ZZ, Wang S, Maiese K. Erythropoietin and Wnt1 govern pathways of mTOR, Apaf-1, and XIAP in inflammatory microglia. Curr Neurovasc Res. 2011;8:270–285. doi: 10.2174/156720211798120990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Shang YC, Chong ZZ, Wang S, Maiese K. Prevention of beta-amyloid degeneration of microglia by erythropoietin depends on Wnt1, the PI 3-K/mTOR pathway, Bad, and Bcl-xL. Aging (Albany NY) 2012;4:187–201. doi: 10.18632/aging.100440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Shang YC, Chong ZZ, Wang S, Maiese K. WNT1 inducible signaling pathway protein 1 (WISP1) targets PRAS40 to govern beta-amyloid apoptotic injury of microglia. Curr Neurovasc Res. 2012;9:239–249. doi: 10.2174/156720212803530618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Sharma N, Shandilya A, Kumar N, Mehan S. Dysregulation of SIRT-1 signaling in multiple sclerosis and neuroimmune disorders: a systematic review of SIRTUIN activators as potential immunomodulators and their influences on other dysfunctions. Endocr Metab Immune Disord Drug Targets. 2021;21:1845–1868. doi: 10.2174/1871530321666210309112234. [DOI] [PubMed] [Google Scholar]
- 287.Shati AA, El-Kott AF. Acylated ghrelin protects against doxorubicin-induced nephropathy by activating SIRT1. Basic Clin Pharmacol Toxicol. 2021;128:805–821. doi: 10.1111/bcpt.13569. [DOI] [PubMed] [Google Scholar]
- 288.Shi C, Zhu J, Leng S, Long D, Luo X. Mitochondrial FOXO3a is involved in amyloid beta peptide-induced mitochondrial dysfunction. J Bioenerg Biomembr. 2016;48:189–196. doi: 10.1007/s10863-016-9645-0. [DOI] [PubMed] [Google Scholar]
- 289.Shokri Afra H, Zangooei M, Meshkani R, Ghahremani MH, Ilbeigi D, Khedri A, et al. Hesperetin is a potent bioactivator that activates SIRT1-AMPK signaling pathway in HepG2 cells. J Physiol Biochem. 2019;75:125–133. doi: 10.1007/s13105-019-00678-4. [DOI] [PubMed] [Google Scholar]
- 290.Sierra-Pagan JE, Dsouza N, Das S, Larson TA, Sorensen JR, Ma X, et al. FOXK1 regulates Wnt signaling to promote cardiogenesis. Cardiovasc Res. 2023;119:1728–1739. doi: 10.1093/cvr/cvad054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Sintek M, Coverstone E, Bach R, Zajarias A, Lasala J, Kurz H, et al. Excimer laser coronary angioplasty in coronary lesions: use and safety from the NCDR/CATH PCI registry. Circ Cardiovasc Interv. 2021;14(7):e010061. doi: 10.1161/CIRCINTERVENTIONS.120.010061. [DOI] [PubMed] [Google Scholar]
- 292.Song SB, Park JS, Chung GJ, Lee IH, Hwang ES. Diverse therapeutic efficacies and more diverse mechanisms of nicotinamide. Metabolomics. 2019;15(10):137. doi: 10.1007/s11306-019-1604-4. [DOI] [PubMed] [Google Scholar]
- 293.Sorrells SF, Paredes MF, Zhang Z, Kang G, Pastor-Alonso O, Biagiotti S, et al. Positive controls in adults and children support that very few, if any, new neurons are born in the adult human hippocampus. J Neurosci. 2021;41:2554–2565. doi: 10.1523/JNEUROSCI.0676-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Speer H, D'Cunha NM, Alexopoulos NI, McKune AJ, Naumovski N. Anthocyanins and human health - a focus on oxidative stress, inflammation and disease. Antioxidants (Basel) 2020;9(5):366. doi: 10.3390/antiox9050366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Su H, Wang WJ, Zheng GD, Yin ZP, Li JE, Chen LL, et al. The anti-obesity and gut microbiota modulating effects of taxifolin in C57BL/6J mice fed with high-fat diet. J Sci Food Agric. 2022;102:1598–1608. doi: 10.1002/jsfa.11496. [DOI] [PubMed] [Google Scholar]
- 296.Sun C, Bai S, Liang Y, Liu D, Liao J, Chen Y, et al. The role of Sirtuin 1 and its activators in age-related lung disease. Biomed Pharmacother. 2023;162:114573. doi: 10.1016/j.biopha.2023.114573. [DOI] [PubMed] [Google Scholar]
- 297.Swain O, Romano SK, Miryala R, Tsai J, Parikh V, Umanah GKE. SARS-CoV-2 neuronal invasion and complications: potential mechanisms and therapeutic approaches. J Neurosci. 2021;41:5338–5349. doi: 10.1523/JNEUROSCI.3188-20.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Tabibzadeh S. Signaling pathways and effectors of aging. Front Biosci (Landmark ed) 2021;26:50–96. doi: 10.2741/4889. [DOI] [PubMed] [Google Scholar]
- 299.Takanezawa Y, Tanabe S, Kato D, Ozeki R, Komoda M, Suzuki T, et al. Microglial ASD-related genes are involved in oligodendrocyte differentiation. Sci Rep. 2021;11(1):17825. doi: 10.1038/s41598-021-97257-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Tan S, Zang G, Wang Y, Sun Z, Li Y, Lu C, et al. Differences of angiogenesis factors in tumor and Diabetes mellitus. Diabetes Metab Syndr Obes. 2021;14:3375–3388. doi: 10.2147/DMSO.S315362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Teertam SK, Prakash Babu P. Differential role of SIRT1/MAPK pathway during cerebral ischemia in rats and humans. Sci Rep. 2021;11(1):6339. doi: 10.1038/s41598-021-85577-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Temiz-Resitoglu M, Guden DS, Senol SP, Vezir O, Sucu N, Kibar D, et al. Pharmacological inhibition of mammalian target of rapamycin attenuates deoxycorticosterone acetate salt-induced hypertension and related pathophysiology: regulation of oxidative stress, inflammation, and cardiovascular hypertrophy in male rats. J Cardiovasc Pharmacol. 2022;79:355–367. doi: 10.1097/FJC.0000000000001187. [DOI] [PubMed] [Google Scholar]
- 303.Thomas SD, Jha NK, Ojha S, Sadek B. mTOR signaling disruption and its association with the development of autism spectrum disorder. Molecules. 2023;28(4):1889. doi: 10.3390/molecules28041889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Topiwala A, Nichols TE, Williams LZJ, Robinson EC, Alfaro-Almagro F, Taschler B, et al. Telomere length and brain imaging phenotypes in UK Biobank. PLoS One. 2023;18(3):e0282363. doi: 10.1371/journal.pone.0282363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Wang L, Di L, Noguchi CT. AMPK is involved in mediation of erythropoietin influence on metabolic activity and reactive oxygen species production in white adipocytes. Int J Biochem Cell Biol. 2014;54:1–9. doi: 10.1016/j.biocel.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Wang R, Zhu Y, Qin LF, Xu ZG, Gao XR, Liu CB, et al. Comprehensive bibliometric analysis of stem cell research in Alzheimer's disease from 2004 to 2022. Dement Geriatr Cogn Disord. 2023;52(2):47–73. doi: 10.1159/000528886. [DOI] [PubMed] [Google Scholar]
- 307.Wang S, Chong ZZ, Shang YC, Maiese K. WISP1 neuroprotection requires FoxO3a post-translational modulation with autoregulatory control of SIRT1. Curr Neurovasc Res. 2013;10:54–60. doi: 10.2174/156720213804805945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Wang S, Schianchi F, Neumann D, Wong LY, Sun A, van Nieuwenhoven FA, et al. Specific amino acid supplementation rescues the heart from lipid overload-induced insulin resistance and contractile dysfunction by targeting the endosomal mTOR-v-ATPase axis. Mol Metab. 2021;53:101293. doi: 10.1016/j.molmet.2021.101293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Wang XY, Liu KJ, Zhang FY, Xiang B. Nicotinamide mitigates radiation injury in submandibular gland by protecting mitochondrial structure and functions. J Oral Pathol Med. 2022;51:801–809. doi: 10.1111/jop.13347. [DOI] [PubMed] [Google Scholar]
- 310.Wang Z, Wu Q, Wang H, Gao Y, Nie K, Tang Y, et al. Diosgenin protects against podocyte injury in early phase of diabetic nephropathy through regulating SIRT6. Phytomedicine. 2022;104:154276. doi: 10.1016/j.phymed.2022.154276. [DOI] [PubMed] [Google Scholar]
- 311.Wasserfurth P, Nebl J, Rühling MR, Shammas H, Bednarczyk J, Koehler K, et al. Impact of dietary modifications on plasma sirtuins 1, 3 and 5 in older overweight individuals undergoing 12-weeks of circuit training. Nutrients. 2021;13(11):3824. doi: 10.3390/nu13113824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Watroba M, Szukiewicz D. Sirtuins at the service of healthy longevity. Front Physiol. 2021;12:724506. doi: 10.3389/fphys.2021.724506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Weikel KA, Cacicedo JM, Ruderman NB, Ido Y. Knockdown of GSK3beta increases basal autophagy and AMPK signaling in nutrient-laden human aortic endothelial cells. Biosci Rep. 2016;36(5):e00382. doi: 10.1042/BSR20160174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.WHO, World Health Organization. Global action plan on the public health response to dementia 2017-2025. Geneva: WHO; 2017. [Google Scholar]
- 315.WHO, World Health Organization. Global status report on noncommunicable diseases 2010. Geneva: WHO; 2011. [Google Scholar]
- 316.Wilson N, Kariisa M, Seth P, Smith HI, Davis N. Drug and opioid-involved overdose deaths — United States, 2017–2018. MMWR Morb Mortal Wkly Rep. 2020;69:290–297. doi: 10.15585/mmwr.mm6911a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Xiong J, Bonney S, Gonçalves RV, Esposito D. Brassinosteroids control the inflammation, oxidative stress and cell migration through the control of mitochondrial function on skin regeneration. Life Sci. 2022;307:120887. doi: 10.1016/j.lfs.2022.120887. [DOI] [PubMed] [Google Scholar]
- 318.Xiong S, Salazar G, Patrushev N, Alexander RW. FoxO1 mediates an autofeedback loop regulating SIRT1 Expression. J Biol Chem. 2011;286:5289–5299. doi: 10.1074/jbc.M110.163667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Xu Y, Wang Y, Jiang Y, Liu M, Zhong W, Ge Z, et al. Relationship between cognitive dysfunction and the promoter methylation of PER1 and CRY1 in patients with cerebral small vessel disease. Front Aging Neurosci. 2023;15:1174541. doi: 10.3389/fnagi.2023.1174541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Xue P, Zhao J, Zheng A, Li L, Chen H, Tu W, et al. Chrysophanol alleviates myocardial injury in diabetic db/db mice by regulating the SIRT1/HMGB1/NF-kappaB signaling pathway. Exp Ther Med. 2019;18:4406–4412. doi: 10.3892/etm.2019.8083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Yamamoto H, Shimomura N, Oura K, Hasegawa Y. Nacre extract from pearl oyster shell prevents d-galactose-induced brain and skin aging. Mar Biotechnol (NY) 2023 Jan 11;:Epub ahead of print. doi: 10.1007/s10126-022-10192-2. [DOI] [PubMed] [Google Scholar]
- 322.Yaman D, Takmaz T, Yüksel N, Dinçer SA, Şahin F. Evaluation of silent information regulator T (SIRT) 1 and Forkhead Box O (FOXO) transcription factor 1 and 3a genes in glaucoma. Mol Biol Rep. 2020;47:9337–9344. doi: 10.1007/s11033-020-05994-3. [DOI] [PubMed] [Google Scholar]
- 323.Yan WT, Lu S, Yang YD, Ning WY, Cai Y, Hu XM, et al. Research trends, hot spots and prospects for necroptosis in the field of neuroscience. Neural Regen Res. 2021;16:1628–1637. doi: 10.4103/1673-5374.303032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Yang J, Suo H, Song J. Protective role of mitoquinone against impaired mitochondrial homeostasis in metabolic syndrome. Crit Rev Food Sci Nutr. 2020;20:1–19. doi: 10.1080/10408398.2020.1809344. [DOI] [PubMed] [Google Scholar]
- 325.Yang K, Zhang L, Chen W, Cheng J, Zhao X, Zhang Y, et al. Expression of EPO and related factors in the liver and kidney of plain and Tibetan sheep. Histol Histopathol. 2023 Jan 25;Epub ahead of print:18592. doi: 10.14670/HH-18-592. [DOI] [PubMed] [Google Scholar]
- 326.Yang W, Sun H, Yan J, Kang C, Wu J, Yang B. Enterohemorrhagic Escherichia coli senses microbiota-derived nicotinamide to increase its virulence and colonization in the large intestine. Cell Rep. 2023;42(6):112638. doi: 10.1016/j.celrep.2023.112638. [DOI] [PubMed] [Google Scholar]
- 327.Ye M, Zhao Y, Wang Y, Xie R, Tong Y, Sauer JD, et al. NAD(H)-loaded nanoparticles for efficient sepsis therapy via modulating immune and vascular homeostasis. Nat Nanotechnol. 2022;17:880–890. doi: 10.1038/s41565-022-01137-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Yeger H. CCN proteins: opportunities for clinical studies-a personal perspective. J Cell Commun Signal. 2023;17:333–352. doi: 10.1007/s12079-023-00761-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Yousafzai NA, Jin H, Ullah M, Wang X. Recent advances of SIRT1 and implications in chemotherapeutics resistance in cancer. Am J Cancer Res. 2021;11:5233–5248. [PMC free article] [PubMed] [Google Scholar]
- 330.Yu M, Zhang H, Wang B, Zhang Y, Zheng X, Shao B, et al. Key signaling pathways in aging and potential interventions for healthy aging. Cells. 2021;10(3):660. doi: 10.3390/cells10030660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Yuan L, Wang D, Wu C. Protective effect of liquiritin on coronary heart disease through regulating the proliferation of human vascular smooth muscle cells via upregulation of sirtuin1. Bioengineered. 2022;13:2840–2850. doi: 10.1080/21655979.2021.2024687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Yuan X, Liu Y, Bijonowski BM, Tsai AC, Fu Q, Logan TM, et al. NAD(+)/NADH redox alterations reconfigure metabolism and rejuvenate senescent human mesenchymal stem cells in vitro. Commun Biol. 2020;3(1):774. doi: 10.1038/s42003-020-01514-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Zaiou M. circRNAs signature as potential diagnostic and prognostic biomarker for diabetes mellitus and related cardiovascular complications. Cells. 2020;9(3):659. doi: 10.3390/cells9030659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Zarneshan SN, Fakhri S, Farzaei MH, Khan H, Saso L. Astaxanthin targets PI3K/Akt signaling pathway toward potential therapeutic applications. Food Chem Toxicol. 2020;145:111714. doi: 10.1016/j.fct.2020.111714. [DOI] [PubMed] [Google Scholar]
- 335.Zeldich E, Chen CD, Colvin TA, Bove-Fenderson EA, Liang J, Tucker Zhou TB, et al. The neuroprotective effect of Klotho is mediated via regulation of members of the redox system. J Biol Chem. 2014;289:24700–24715. doi: 10.1074/jbc.M114.567321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Zeng Z, Liang J, Wu L, Zhang H, Lv J, Chen N. Exercise-induced autophagy suppresses sarcopenia through Akt/mTOR and Akt/FoxO3a signal pathways and AMPK-mediated mitochondrial quality control. Front Physiol. 2020;11:583478. doi: 10.3389/fphys.2020.583478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Zhang GZ, Deng YJ, Xie QQ, Ren EH, Ma ZJ, He XG, et al. Sirtuins and intervertebral disc degeneration: Roles in inflammation, oxidative stress, and mitochondrial function. Clin Chim Acta. 2020;508:33–42. doi: 10.1016/j.cca.2020.04.016. [DOI] [PubMed] [Google Scholar]
- 338.Zhang WB, Huang Y, Guo XR, Zhang MQ, Yuan XS, Zu HB. DHCR24 reverses Alzheimer's disease-related pathology and cognitive impairment via increasing hippocampal cholesterol levels in 5xFAD mice. Acta Neuropathol Commun. 2023;11(1):102. doi: 10.1186/s40478-023-01593-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Zhang XM, Jing YP, Jia MY, Zhang L. Negative transcriptional regulation of inflammatory genes by group B3 vitamin nicotinamide. Mol Biol Rep. 2012;39:10367–10371. doi: 10.1007/s11033-012-1915-2. [DOI] [PubMed] [Google Scholar]
- 340.Zhang YC, Fan KY, Wang Q, Hu JX, Wang Q, Zhang HY, et al. Genetically determined levels of mTOR-dependent circulating proteins and risk of multiple sclerosis. Neurol Ther. 2023;12:751–762. doi: 10.1007/s40120-023-00455-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Zhao C, Sun G, Li Y, Kong K, Li X, Kan T, et al. Forkhead box O3 attenuates osteoarthritis by suppressing ferroptosis through inactivation of NF-κB/MAPK signaling. J Orthop Translat. 2023;39:147–162. doi: 10.1016/j.jot.2023.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Zhao D, Sun X, Lv S, Sun M, Guo H, Zhai Y, et al. Salidroside attenuates oxidized lowdensity lipoproteininduced endothelial cell injury via promotion of the AMPK/SIRT1 pathway. Int J Mol Med. 2019;43:2279–2290. doi: 10.3892/ijmm.2019.4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Zhao H, Wang R, Wu X, Liang J, Qi Z, Liu X, et al. Erythropoietin delivered via intra-arterial infusion reduces endoplasmic reticulum stress in brain microvessels of rats following cerebral ischemia and reperfusion. J Neuroimmune Pharmacol. 2015;10:153–161. doi: 10.1007/s11481-014-9571-z. [DOI] [PubMed] [Google Scholar]
- 344.Zhao HY, Li HY, Jin J, Jin JZ, Zhang LY, Xuan MY, et al. L-carnitine treatment attenuates renal tubulointerstitial fibrosis induced by unilateral ureteral obstruction. Korean J Intern Med. 2021;36(Suppl 1):S180–S195. doi: 10.3904/kjim.2019.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Zhao J, Miao K, Wang H, Ding H, Wang DW. Association between telomere length and type 2 diabetes mellitus: a meta-analysis. PLoS One. 2013;8(11):e79993. doi: 10.1371/journal.pone.0079993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Zhao T, Miao H, Song Z, Li Y, Xia N, Zhang Z, et al. Metformin alleviates the cognitive impairment induced by benzo[a]pyrene via glucolipid metabolism regulated by FTO/FoxO6 pathway in mice. Environ Sci Pollut Res Int. 2023;30:69192–69204. doi: 10.1007/s11356-023-27303-8. [DOI] [PubMed] [Google Scholar]
- 347.Zhao W, Xie C, Zhang X, Liu J, Liu J, Xia Z. Advances in the mTOR signaling pathway and its inhibitor rapamycin in epilepsy. Brain Behav. 2023;13(6):e2995. doi: 10.1002/brb3.2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Zhong S, Chen W, Wang B, Gao C, Liu X, Song Y, et al. Energy stress modulation of AMPK/FoxO3 signaling inhibits mitochondria-associated ferroptosis. Redox Biol. 2023;63:102760. doi: 10.1016/j.redox.2023.102760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Zhou J, Chen H, Wang Q, Chen S, Wang R, Wang Z, et al. Sirt1 overexpression improves senescence-associated pulmonary fibrosis induced by vitamin D deficiency through downregulating IL-11 transcription. Aging Cell. 2022;21(8):e13680. doi: 10.1111/acel.13680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Zhou Q, Tang S, Zhang X, Chen L. Targeting PRAS40: a novel therapeutic strategy for human diseases. J Drug Target. 2021;29:703–715. doi: 10.1080/1061186X.2021.1882470. [DOI] [PubMed] [Google Scholar]
- 351.Zhuang X, Ma J, Xu G, Sun Z. SHP-1 knockdown suppresses mitochondrial biogenesis and aggravates mitochondria-dependent apoptosis induced by all trans retinal through the STING/AMPK pathways. Mol Med. 2022;28(1):125. doi: 10.1186/s10020-022-00554-w. [DOI] [PMC free article] [PubMed] [Google Scholar]