Abstract
Background
Emerging research has shown racial and ethnic variations in the magnitude of association between the apolipoprotein ε4 (APOE ε4) allele and the risk of developing Alzheimer’s disease and related dementias (ADRD). Studies researching this association among Hispanic groups within and outside of the U.S. have produced inconsistent results.
Objective
To examine the association between the APOE ε4 allele and the risk of developing ADRD in global Hispanic populations from different ethnic regions of origin.
Methods
PubMed, Embase, Scopus, and PsycInfo were searched for studies relating to Hispanic/Latin American origin, APOE ε4, and ADRD. Odds ratios (OR) of ADRD risk for individuals with APOE ε4 vs. those without APOE ε4 were extracted and calculated using random effects analysis.
Results
20 eligible studies represented Caribbean Hispanic, Mexican, South American, Spanish, and Cuban groups. Overall, APOE ε4 was significantly associated with increased risk of ADRD (Odds Ratio [OR] 3.80, 95% CI: 2.38-6.07). The association was only significant in the South American (OR: 4.61, 95% CI: 2.74 – 7.75) subgroup.
Conclusion
There was an association between APOE ε4 and increased ADRD risk for the South American subgroup. The strength of this association varied across Hispanic subgroups. Data is limited with more studies especially needed for adjusted analysis on Spanish, Central American, Cuban Hispanic, and Caribbean Hispanic groups.. Results suggest additional environmental or genetic risk factors are associated with ethnic variations.
Keywords: Alzheimer disease, dementia, hispanic or latino, apolipoprotein E4
INTRODUCTION
As a leading cause of morbidity and mortality, 12.7 million adults in America are projected to be affected by Alzheimer’s disease and related dementias (ADRD) 2050 [1]. Apolipoprotein E (ApoE) ε4 is the strongest known genetic risk factor for ADRD, but has primarily been examined for populations with European ancestry [1]. Emerging research has shown racial and ethnic variations in the magnitude of association between the APOE ε4 allele and ADRD risk [1-3]. Studies researching this association among Hispanic groups within and outside of the U.S. have produced inconsistent results. For example, among a Caribbean Hispanic population in New York City, there is a weaker association between APOE ε4 presence and ADRD risk with sporadic Alzheimer’s disease (AD), compared to a stronger association with late onset cases with a strong family history of AD [4]. Additionally, APOE ε4 has a lower prevalence and weaker association with ADRD risk amongst Mexican Hispanic populations as compared to non-Hispanic White populations [5,6].
These differences in the associations between APOE ε4 and ADRD have been hypothesized to be related to the heterogeneity of Hispanic genetic ancestry [5,6]. Genetic ancestry of Hispanic groups varies amongst Amerindian, European, and African depending on country of origin [5]. As differences in the association between APOE ε4 and ADRD have been established depending on African or Amerindian ancestry, there is likely a difference in the associations between APOE ε4 and ADRD based on regions of origin across Hispanic populations [5,7]. However, there remain inconsistencies, as one recent study found African admixture had no effect on the association between APOE ε4 and dementia when comparing Non-Hispanic White, African American, Caribbean Hispanic, and Hispanic American groups [8].
Studies have also suggested an association between social and environmental lifestyle factors and ADRD risk [9,10]. The Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER) [11], showed an effect of lifestyle interventions on cognitive function of Finnish older adults at risk of dementia, leading to an adapted global approach led by the Alzheimer’s Association (WW-FINGERS) [12]. Of existing studies that address the relationship between APOE ε4 and ADRD among Hispanic populations, most focus on specific populations in South America. There are limited studies in this area on other Hispanic groups such as Mexican Hispanic groups. Hispanic populations are heterogenous in their geographic locations and genetic ancestry, and the population of older Hispanic individuals continues to grow rapidly [13]. Thus, a comprehensive assessment of the associations between APOE ε4 and ADRD by Hispanic subgroup is needed.
To address this gap, this meta-analysis aimed to evaluate the variation in the association between APOE ε4 and ADRD risk amongst Hispanic populations by region of origin. We also explored the association between APOE ε4 and mild cognitive impairment (MCI), as MCI can be viewed as an early stage of the disease continuum for ADRD [1]. A better understanding of the association between APOE ε4 and ADRD in different Hispanic subgroups will help to improve understanding of the underlying pathology and ultimately to reduce disparities in ADRD.
METHODS
This meta-analysis is registered in PROSPERO (CRD42021221598) and was conducted according to PRISMA 2020 guidelines [14]. A comprehensive search strategy consisted of search terms related to Hispanic and Latin American origin, ADRD, cognitive decline, and ApoE (Supplementary Table 1). PubMed, Embase, Scopus, and PsycInfo were chosen as the primary databases for this meta-analysis and reference lists of the included studies were hand-searched to identify additional eligible studies. Studies were also manually searched for from publications of well-known population-based ADRD studies such as the Dementia 10/66 [15]. The final search was completed on February 15th, 2022 to capture any studies that were published before that date.
The following inclusion criteria was used to identify eligible studies. Studies should (1) focus on Hispanic and Latino populations of any origin, i.e. Spanish, Caribbean Hispanic, Latin American, Mexican, Cuban, Puerto Rican, self-identified Hispanic, (2) address the presence of APOE ε4 allele as a genetic risk factor with a focus on dementia, ADRD, cognitive function, and/or mild cognitive impairment as the study outcomes, (3) specify the regional ethnic origin of the Hispanic individuals, (4) be designed as population-based studies, (5) involve an adult population (18+), (6) be published in English, and (7) be dataset based original studies.
Studies were excluded if the study (1) did not involve Hispanic ethnic groups, (2) did not specify the ethnic region of Hispanic origin (i.e. Cuban Hispanic, Mexican Hispanic, etc.), (3) did not include data on APOE ε4, (4) did not specify allele/genotype frequency for each ethnic subgroup, (5) study participants were recruited through familial relation or analyses were conducted at the genomic level rather than at an individual level, (6) outcomes did not include dementia, ADRD, cognitive function, and/or MCI, (7) non-dataset based original studies (e.g. editorial, trial design paper, case reviews), systematic reviews/meta-analysis, (8) thesis and other non-peer reviewed studies (9) non-English study.
A dual review approach was implemented throughout the screening and data extraction [16]. Three reviewers independently screened articles and resolved any resulting conflicts. When multiple articles reported data from the same parent study/dataset, only one study with the largest sample size, the most specific breakdown of APOE and a focus on ADRD outcomes was included. The following information was extracted independently from each included study: study design, country setting, region of origin of study population, sample size, age, education, sex, frequency of APOE genotypes, frequency of population with at least one copy of APOE ε4, study outcomes, and outcome measures. Two reviewers independently conducted risk of bias assessments using the Newcastle-Ottawa scale presented in Table 1 [17]. Discrepancies in scores were resolved between the two reviewers.
Table 1.
Newcastle Ottawa Scale Risk of Bias Assessment
| Study | Selection | Comparability | Outcome or Exposure | Total |
|---|---|---|---|---|
| Case-Control Studies | ||||
| Alavez-Rubio 2020 [33] | *** | ** | ** | 7 |
| Arboleda 2001 [24] | ** | ** | * | 5 |
| Bartrés-Faz 2001 [34] | **** | * | 5 | |
| Campos 2013 [5] | *** | ** | * | 6 |
| Jacquier 2001 [21] | *** | ** | * | 6 |
| Cristiá-Lara 2017 [35] | **** | ** | ** | 8 |
| Forero 2006 [22] | *** | ** | * | 6 |
| Mateo 2002 [37] | **** | * | 5 | |
| Moreno 2017 [23] | **** | ** | ** | 8 |
| Sevush 2000 [29] | **** | * | * | 6 |
| Teruel 2011 [36] | **** | ** | ** | 8 |
| Villalpando-Berumen 2008 [31] | **** | ** | * | 7 |
| Marca-Ysabel 2021 [25] | **** | ** | * | 7 |
| Aguilar-Navarro 2021 [32] | *** | ** | * | 6 |
| Cohort Studies | ||||
| Granot-Hershkovitz 2020 [6] | *** | ** | * | 6 |
| Allegri 2018 [27] | **** | ** | ** | 8 |
| Molero 2001 [26] | **** | ** | * | 7 |
| Villarreal 2016 [38] | **** | ** | ** | 8 |
| O'Bryant 2013 [28] | **** | ** | ** | 8 |
| Tosto 2015 [30] | *** | ** | ** | 7 |
Newcastle-Ottawa-Scale scores for the risk of bias assessment of included studies.
Case-Control: Selection (max 4 stars): (1) Is the case definition adequate? (2) Representativeness of the cases (3) Selection of Controls (4) Definition of Controls Comparability (max 2 stars): (1) Comparability of cases and controls on the basis of the design or analysis- A. study controls for the most important factor (*) B. study controls for any additional factor (*) Exposure (max 3 stars): (1) Ascertainment of exposure (2) Same method of ascertainment for cases and controls (3) Non-response rate
Cohort: Selection (max 4 stars): (1) Representativeness of the exposed cohort (2) Selection of the non-exposed cohort (3) Ascertainment of exposure (4) Demonstration that outcome of interest was not present at start of study Comparability (max 2 stars): (1) Comparability of cohorts on the basis of the design or analysis- A. study controls for the most important factor (*) B. study controls for any additional factor (*) Outcome (max 3 stars): (1) Assessment of outcome (2) Was follow-up >5 years (3) Adequacy of follow up of cohorts
Meta-analysis was conducted in Stata 16 SE. Odd Ratios (ORs) and 95% Confidence Interval (CI) were extracted and/or calculated for developing ADRD or mild cognitive impairment respectively for individuals with the APOE ε4 allele (ε4+) vs. individuals without the APOE ε4 allele (ε4-) by Hispanic ethnicity region. Adjusted ORs were extracted if available and meta-analysis was conducted to calculate the pooled adjusted risk for developing ADRD. The pooled adjusted estimate for developing MCI was unable to be obtained due to small sample size. The DerSimonian-Laird random effect model was used due to high heterogeneity across studies for each region [18-20]. Q value and I2 were used to assess the heterogeneity among the included studies.
RESULTS
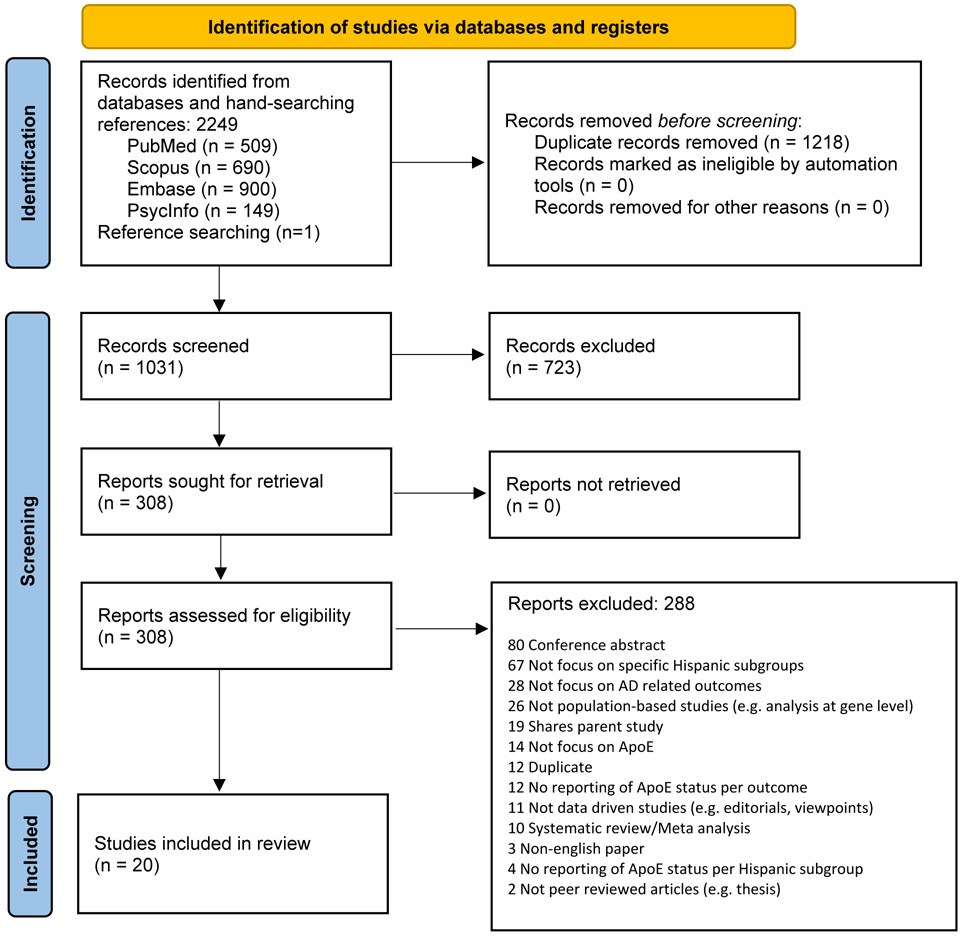
There were 2248 articles identified in the initial database search and one study was identified from reference lists. Of the 2249 articles, 308 were screened for full-text eligibility. A total of 20 articles were included in this meta-analysis (Figure 1). Among 20 selected studies, a total of 16,178 participants were included, with 6259 cases of ADRD, 829 cases of MCI, and 9090 cognitively normal controls.
Figure 1.
PRISMA Flow Diagram
From: Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. doi: 10.1136/bmj.n71
PRISMA Flow Diagram of eligible article selection from initial database searches in PubMed, Embase, Scopus, and PsycInfo and reference lists.
Six Hispanic ethnic regions were represented: South American (n=8), Mexican Hispanic (n=6), Cuban Hispanic (n=4), Caribbean Hispanic (Puerto Rico and the Dominican Republic, n=2), Spanish (n=2), and Central American (Panama and “Central American”, n=2). One study included data on Caribbean, South American, Central American, and Cuban regions [6]. All other studies focused on one region of origin. Within the South American region, four studies focused on a Colombian population [21-24], there was one study focused on each of the Peruvian [25], Venezuelan [26], and Argentinian [27] populations, and one study did not specify a country of focus [6]. Five studies were conducted with Hispanic groups living in the US [5,6,28-30], four in Colombia [21-24], three in Mexico [31-33], two each in Cuba and Spain [34-37] and one each in Argentina, Panama, Peru, and Venezuela [25-27,38].
Ten of the included studies are based on data from one or more large parent studies that have produced multiple publications. The represented parent studies include: Hispanic Community Health Study/Study of Latinos (HCHS/SOL) [6], Study on Aging and Dementia in Mexico (SADEM) [33], Argentinian Alzheimer’s Disease Neuroimaging Initiative (ADNI) [27], Playa Dementia and Alzheimer Study (EDAP) [35], Project FRONTIER (Facing Rural Obstacles to Health Now Through Intervention, Education & Research) [28], the Texas Alzheimer’s Research & Care Consortium (TARCC) [28], The Maracaibo Aging Study [26], Panama Aging Research Initiative (PARI) [38], 10/66 Cuba [36], the Washington Heights and Inwood Columbia Aging Project (WHICAP) [30], Northern Manhattan Study (NOMAS) [30], and Prevalence Survey of Dementia in the Mexico City Elderly Population (ESEC) [31].
Tables 2a, 2b, and 2c present the study characteristics and demographic information for each study by ADRD, MCI, and cognitively normal outcomes, respectively. Sample sizes varied and ranged from 18 to 2063 among cognitively normal groups, 24 to 148 among MCI groups, and ranged from 28 to 2451 among the groups with ADRD. Eighteen out of 20 studies reported age data. The pooled mean age was 67.72 for cognitively normal cases, 67.91 for individuals with MCI, and 76.46 for individuals with ADRD. Sixteen out of 20 studies reported data on sex. Education was reported in 13 out of the 20 studies. Three studies reported education as a categorical variable [6,23,36] and 10 studies reported education in years of education. Among these 10 studies, the pooled average years of education was 9.45 for MCI (n=7), 7.64 for cognitively normal groups (n=7), and 5.93 for ADRD (n=8). Seven studies did not report data on education [5,21,25,29,30,37].
Table 2a.
Study Characteristics and Demographic Data of ADRD outcomes
| Ethnic Region |
Author , Year |
Study Design |
Setting | Specific Ethnicity |
Outcome | N | Age (SD) |
female (%) |
Education (SD) |
|---|---|---|---|---|---|---|---|---|---|
| Caribbean Hispanic | Tosto, 2015 [30] | Cohort | USA | Caribbean Hispanic | LOAD | 2451 | 78.6 (8) | 1640 (66.9) | |
| Central American | Villarreal, 2016 [38] | Cohort | Panama | Panamian Hispanic | ADRD | 31 | 82.1 (8.8) | 24 (77.4) | 6.9 (3.6) |
| Cuban | Cristiá-Lara, 2017[35] | Cross-sectional | Cuba | Cuban | ADRD | 58 | 76.9 (6.8) | 41 (70.7) | 6.6 (3.6) |
| Cuban | Sevush, 2000 [29] | Case-control | USA | Cuban | ADRD | 80 | |||
| Cuban | Teruel, 2011 [36] | Case-control | Cuba | Cuban | Dementia | 2247 | 75 (7)* | 1654 (65.6) | none - 58, some - 511, primary - 778, secondary - 564, tertiary - 412 |
| Mexican Hispanic | Campos, 2013 [5] | Cross-sectional | US | Mexican Hispanic | ADRD | 28 | |||
| Mexican | Villalpando-Berumen, 2008 [31] | Case-control | Mexico | Mexican Mestizo | AD | 49 | 77.9 (7.1) | 34 (69.3) | 4.1 (4.5) |
| Mexican Hispanic | O'Bryant, 2013 [28] | Cohort | US | Mexican American | ADRD | 35 | 73.6 (9.1) | 19 (55) | 5.9 (4.5) |
| Mexican Hispanic | Alavez-Rubio, 2020 [33] | Case-control | Mexico | Mestizo Mexican | ADRD | 196 | 77 | 128 (65.31) | 5.71 (5.49) |
| South American | Moreno, 2017 [23] | Case-control | Colombia | Colombian | ADRD | 280 | 75.5 (7.23) | 213 (76.1) | 0-4 years: 19, 5 years: 157, 6-11 years: 69, 12-23 years: 30 |
| South American | Arboleda, 2001 [24] | Case-control | Colombia | Caucasian Mestizo | ADRD | 61 | 67.6 (9.1) | 45 (73.77) | |
| South American | Jacquier, 2001 [21] | Case-control | Colombia | Colombian | ADRD | 83 | 73.3 (9.5) | ||
| South American | Marca-Ysabel, 2021 [25] | Case-control | Peru | Peruvian | AD | 79 | 72.3 (8.4) | 53 (67) | |
| South American | Forero, 2006 [22] | Case-control | Colombia | Colombian | ADRD | 106 | 73.3 (8.8) | 75 (71) | 7.1 (4.2) |
| South American | Allegri, 2018 [27] | Cohort | Argentina | Argentinian | ADRD | 43 | 72.3 (14.4) | 22 (51) | 13.7 (4.2) |
| South American | Molero, 2001 [26] | Cohort | Venezuela | Venezuelan | AD | 121 | 78.1 (9) | 101 (83.5) | 2.7 (3.4) |
| Spanish | Mateo, 2002 [37] | Case-control | Spain | Spanish | AD | 311 | 75.3 (8.9) | 208 (67) |
LOAD: late-onset Alzheimer’s disease
study did not specify this variable by outcome, number refers to the overall study participants
Table 2b.
Study Characteristics and Demographic Data of MCI Outcomes
| Ethnic Region |
Author , Year |
Study Design |
Setting | Specific Ethnicity |
Outcome | N | Age (SD) |
female (%) |
Education (SD) |
|---|---|---|---|---|---|---|---|---|---|
| Caribbean Hispanic | Granot-Hershkovitz, 2020 [6] | Cohort | US | Dominican | MCI | 47 | 66.7 (8.99) | 13 (27.7) | <12 yrs: 26 (55.3); 12 yrs: 6 (12.8); >12 years: 15 (31.9) |
| Caribbean Hispanic | Granot-Hershkovitz, 2020 [6] | Cohort | US | Puerto Rican | MCI | 87 | 64.38 (8.01) | 40 (46) | <12 yrs: 47 (54); 12 yrs: 13 (14.9); >12 yrs: 27 (31) |
| Central American | Granot-Hershkovitz, 2020 [6] | Cohort | US | Central American | MCI | 46 | 63.91 (8.46) | 17 (37) | <12 yrs: 23 (50); 12 yrs: 9 (19.6); >12 yrs: 14 (30.4) |
| Central American | Villarreal, 2016 [38] | Cohort | Panama | Panamian Hispanic | MCI | 43 | 80.2 (7.8) | 27 (62.8) | 6.7 (3.5) |
| Cuban | Cristiá-Lara, 2017 [35] | Cross-sectional | Cuba | Cuban | MCI | 52 | 73.2 (4.9) | 36 (69.2) | 6.9 (4) |
| Cuban | Granot-Hershkovitz, 2020 [6] | Cohort | US | Cuban | MCI | 85 | 65.04 (8.2) | 46 (36.5) | <12 yrs: 28 (32.9); 12 yrs: 22 (25.9); >12 yrs: 35 (41.2) |
| Mexican | Aguilar-Navarro, 2021 [32] | Case-control | Mexico | Mexican | naMCI | 50 | 74.2 (7.2) | 31 (62) | 12.6 (5.6) |
| Mexican | Aguilar-Navarro, 2021 [32] | Case-control | Mexico | Mexican | aMCI | 24 | 78.2 (7.4) | 15 (62.5) | 9.6 (5.9) |
| Mexican Hispanic | Granot-Hershkovitz, 2020 [6] | Cohort | US | Mexican | MCI | 148 | 64.58 (8.01) | 50 (33.8) | <12 yrs: 86 (58.1); 12 yrs: 27 (18.2); >12 yrs: 35 (23.6) |
| Mexican Hispanic | O'Bryant, 2013 [28] | Cohort | US | Mexican American | MCI | 67 | 61.9 (12.3) | 42 (62) | 6.6 (4.2) |
| South American | Allegri, 2018 [27] | Cohort | Argentina | Argentinian | MCI | 89 | 72.8 (8.4) | 44 (49) | 14.2 (4.2) |
| South American | Granot-Hershkovitz, 2020 [6] | Cohort | US | South American | MCI | 28 | 64.21 (7.99) | 9 (32.1) | <12 yrs: 5 (17.9); 12 yrs: 6 (21.4); >12 yrs: 17 (60.7) |
| Spanish | Bartrés-Faz, 2001 [34] | Case-control | Spain | Spanish | MCI | 63 | 67.7 (10.23) | 7.2 (3.78) |
aMCI: amnestic mild cognitive impairment; naMCI: non-amnestic mild cognitive impairment; specific ethnicity: the specific ethnicity of the study population as specified by included studies
study did not specify this variable by outcome, number refers to the overall study participants
Table 2c.
Study Characteristics and Demographic Data of Cognitively Normal Outcomes
| Ethnic Region |
Author , Year |
Study Design |
Setting | Specific Ethnicity |
Outcome | N | Age (SD) |
female (%) |
Education (SD) |
|---|---|---|---|---|---|---|---|---|---|
| Caribbean Hispanic | Granot-Hershkovitz, 2020 [6] | Cohort | US | Puerto Rican | cognitively normal | 491 | 62.07 (6.94) | 199 (40.5) | <12 yrs: 197 (40.1); 12 yrs: 118 (24); >12 yrs: 176 (35.8) |
| Caribbean Hispanic | Tosto, 2015 [30] | Cohort | USA | Caribbean Hispanic | cognitively normal | 2063 | 73.5 (8) | 1376 (66.7) | |
| Caribbean Hispanic | Granot-Hershkovitz, 2020 [6] | Cohort | US | Dominican | cognitively normal | 284 | 60.62 (7.05) | 81 (28.5) | <12 yrs: 126 (44.4); 12 yrs: 59 (20.8); >12 yrs: 99 (34.9) |
| Central American | Villarreal, 2016 [38] | Cohort | Panama | Panamian Hispanic | cognitively normal | 185 | 76.7 (6.9) | 93 (50.5) | 8.5 (4) |
| Central American | Granot-Hershkovitz, 2020 [6] | Cohort | US | Central American | cognitively normal | 304 | 60.88 (6.07) | 101 (33.22) | <12 yrs: 118 (38.8); 12 yrs: 63 (20.7); >12 yrs: 123 (40.5) |
| Cuban | Granot-Hershkovitz, 2020 [6] | Cohort | US | Cuban | cognitively normal | 638 | 61.69 (7.3) | 284 (44.5) | <12yrs: 153 (24); 12 yrs: 169 (26.5); >12 yrs: 316 (49.5) |
| Cuban | Cristiá-Lara, 2017 [35] | Cross-sectional | Cuba | Cuban | cognitively normal | 33 | 71.1 (5) | 21 (63.6) | 10.6 (4.5) |
| Cuban | Sevush, 2000 [29] | Case-control | USA | Cuban | cognitively normal | 21 | |||
| Cuban | Teruel, 2011 [36] | Case-control | Cuba | Cuban | cognitively normal | 273 | 75 (7)* | 1654 (65.6) | none - 58, some - 511, primary - 778, secondary - 564, tertiary - 412 |
| Mexican Hispanic | O'Bryant, 2013 [28] | Cohort | US | Mexican American | cognitively normal | 337 | 58.7 (9.9) | 239 (71) | 8.1 (4.2) |
| Mexican | Alavez-Rubio, 2020 [33] | Case-control | Mexico | Mestizo Mexican | cognitively normal | 204 | 70 | 130 (63.73) | 6.55 (5.09) |
| Mexican | Villalpando-Berumen, 2008 [31] | Case-control | Mexico | Mexican Mestizo | cognitively normal | 141 | 72.1 (6.3) | 101 (71.6) | 4.7 (4.4) |
| Mexican | Aguilar-Navarro, 2021 [32] | Case-control | Mexico | Mexican | cognitively normal | 63 | 71.3 (6.2) | 53 (84.1) | 13.7 (3.7) |
| Mexican Hispanic | Granot-Hershkovitz, 2020 [6] | Cohort | US | Mexican | cognitively normal | 971 | 60.94 (6.89) | 356 (36.7) | <12 yrs: 475 (48.9); 12 yrs: 200 (20.6); >12 yrs: 296 (30.5) |
| Mexican Hispanic | Campos, 2013 [5] | Cross-sectional | US | Mexican Hispanic | cognitively normal | 28 | |||
| South American | Granot-Hershkovitz, 2020 [6] | Cohort | US | South American | cognitively normal | 225 | 61.7 (6.95) | 93 (41.3) | <12 yrs: 52 (23.1); 12 yrs: 45 (20); >12 yrs: 128 (56.9) |
| South American | Arboleda, 2001[24] | Case-control | Colombia | Caucasian Mestizo | cognitively normal | 61 | 72.4 (8.7) | 45 (73.77) | |
| South American | Jacquier, 2001 [21] | Case-control | Colombia | Colombian | cognitively normal | 44 | 65.8 (7.2) | ||
| South American | Allegri, 2018 [27] | Cohort | Argentina | Argentinian | cognitively normal | 18 | 69.2 (5.8) | 8 (44) | 14.8 (2.9) |
| South American | Forero, 2006 [22] | Case-control | Colombia | Colombian | cognitively normal | 97 | 72.2 (8.7) | ||
| South American | Moreno, 2017 [23] | Case-control | Colombia | Colombian | cognitively normal | 357 | 71.04 (7.08) | 264 (73.9) | 0-4 years: 29, 5 years: 228, 6-11 years: 60, 12-23 years: 22 |
| South American | Molero, 2001[26] | Cohort | Venezuela | Venezuelan | cognitively normal | 1665 | 66.5 (8.4) | 1109 (66.6) | 6 (4.1) |
| South American | Marca-Ysabel, 2021 [25] | Case-control | Peru | Peruvian | cognitively normal | 128 | 75 (6.6) | 75.65 (59.1) | |
| Spanish | Mateo, 2002 [37] | Case-control | Spain | Spanish | cognitively normal | 346 | 80.4 (7.6) | 242 (70) | |
| Spanish | Bartrés-Faz, 2001 [34] | Case-control | Spain | Spanish | cognitively normal | 124 | 65 (13.2) |
specific ethnicity: the specific ethnicity of the study population as specified by included studies
study did not specify this variable by outcome, number refers to the overall study participants
The reports on frequency/proportion of APOE genotypes (ε2/ε2, ε2/ε3, ε2/ε4, ε3/ε3, ε3/ ε4, ε4/ε4) and alleles (ε2, ε3, ε4) varied. Only six studies reported data on the frequency and/or proportion of all possible genotypes and alleles [5,21,24,26,34,36]. Five studies reported on all genotypic frequencies and no allelic frequencies [23,29,32,33,38], and two studies reported on some genotypes or some alleles but not all [25,38], and seven studies only reported on ε4 data [6,22,27,28,30,35,37]. As the data on the frequency of APOE ε4 carriers (ε4+) and APOE ε4 non-carriers (ε4-) were available or could be calculated for each study, this meta-analysis focuses on assessing the relationship between the presence of APOE ε4 and the risks of developing MCI and/or ADRD. Frequencies of ε4- and ε4+ individuals for ADRD and MCI outcomes and controls are reported by region of origin in Table 3.
Table 3.
Frequency of ApoE e4 by Ethnic Region of Origin
| Ethnic Region of Origin | ||||||
|---|---|---|---|---|---|---|
| Caribbean Hispanic |
Central American |
Cuban | Mexican | South American |
Spanish | |
| e4+ (AD) | 958 | 16 | 144 | 68 | 324 | 170 |
| e4− (AD) | 1492 | 12 | 268 | 240 | 412 | 141 |
| Total (AD) | 2450 | 28 | 412 | 308 | 736 | 311 |
| e4+p (AD) | 39.10 | 57.14 | 34.95 | 22.08 | 44.02 | 54.66 |
| e4+ (MCI) | 38 | 28 | 36 | 72 | 52 | 15 |
| e4− (MCI) | 96 | 48 | 101 | 217 | 65 | 48 |
| Total (MCI) | 134 | 76 | 137 | 289 | 117 | 63 |
| e4+p (MCI) | 28.36 | 36.84 | 26.28 | 24.91 | 44.44 | 23.81 |
| e4+ (controls) | 731 | 72 | 472 | 297 | 513 | 62 |
| e4− (controls) | 2097 | 309 | 2467 | 1384 | 2075 | 284 |
| Total (controls) | 2828 | 381 | 2939 | 1681 | 2588 | 346 |
| e4+p (controls) | 25.85 | 18.90 | 16.06 | 17.67 | 19.82 | 17.92 |
e4+ : carriers of ApoE e4 allele; e4− : non-carriers of ApoE e4 allele; e4+p: percentage of ApoE e4 carriers
Most studies used the National Institute for Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA) criteria to assess ADRD outcomes (n=11) [21,24-26,29-31,33,35,37,38]. Other criteria used included the Diagnostic and Statistical Manual of Mental Disorders (DMS-IV-R) criteria for dementia (n=7) [22,23,27,31,33,35,36], National Institute on Aging-Alzheimer’s Association (n=2) [27,28], the National Institute of Neurological Disorders and Stroke Association Internationale Pour la Recherche et Enseignement en Neurosciences (NINDS-AIREN) (n=1) [33], 10/66 dementia diagnosis algorithm (n=1) [36], and unspecified “standardized criteria” (n=1) [5].
To classify MCI, four studies used criteria established by Peterson and Morris [27,28,32,34] and one study used National Institute on Aging-Alzheimer’s Association criteria [6].
APOE ε4 and ADRD risk
Based on unadjusted estimates, there was a statistically significant association with APOE ε4 presence and risk of developing ADRD for Caribbean Hispanic (OR: 1.88, 95% CI: 1.66 - 2.14), Central American (OR: 5.08, 95% CI: 2.01 −12.87), Cuban (OR: 3.69, 95% CI: 1.86 – 7.33), and South American (OR: 3.51, 95% CI: 2.26 – 5.45) groups (Supplementary Figure 1). The association between APOE ε4 and ADRD was not statistically significant for the Mexican group. There was only one included study in each of the Caribbean Hispanic [30], Central American [38], and Spanish groups [37], thus the presented estimate of the APOE ε4/ADRD association for these groups is not robust.
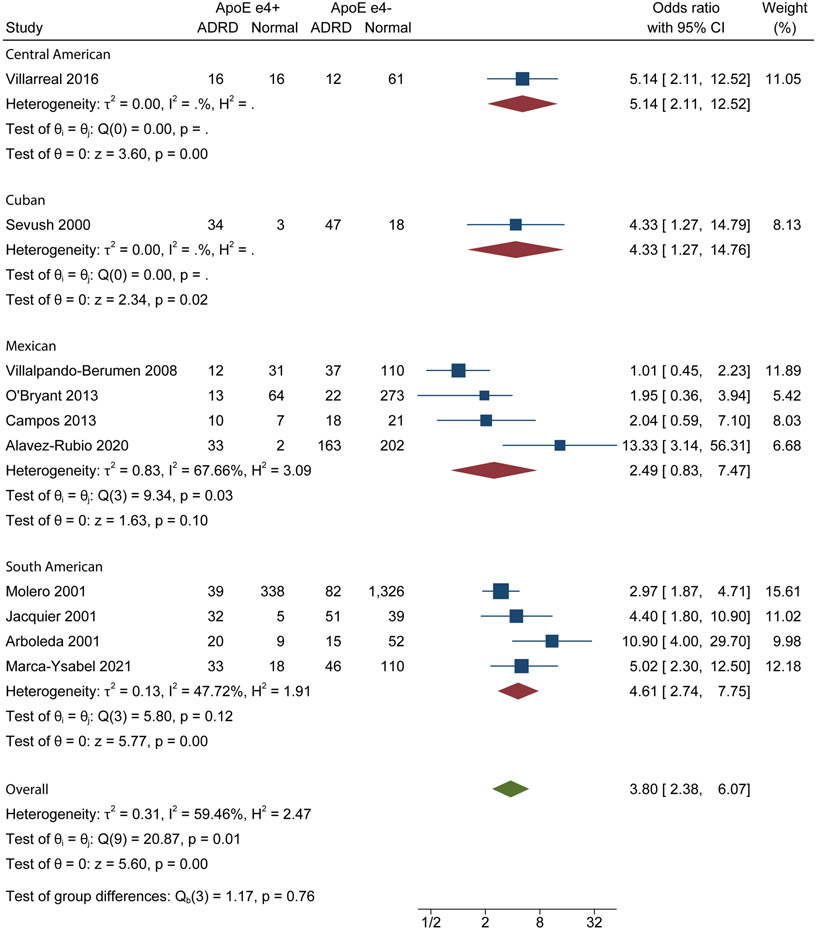
Twelve studies reported adjusted OR values with common adjustments being age, sex, and education [5,21,24-26,28,29,31-33,35,38]. Results from pooled estimates based on adjusted ORs showed that there was a statistically significant association between APOE ε4 and ADRD for the South American (OR: 4.61, 95% CI: 2.74 – 7.75) group (Figure 2). There was not a statistically significant association between APOE ε4 and ADRD for the Mexican group. Adjusted estimates were not presented for the Central American, Cuban Hispanic, Caribbean Hispanic and Spanish groups due to a lack of studies with adjusted data.
Figure 2.
ADRD Adjusted Forest Plot
Random-effects REML model
Forest plot of adjusted analysis of ADRD association with APOE ε4. APOE ε4+: individuals with the APOE ε4 allele; APOE ε4−: +: individuals without the APOE ε4 allele
Heterogeneity from adjusted analysis was not calculated for Central American and Cuban Hispanic groups, since these groups were represented by one study each. Heterogeneity was moderate for the South American group (I2=47.72%) and substantial for the Mexican Hispanic group (I2=67.66%).
In meta-regression for unadjusted analysis, higher mean age and more females included did not significantly increase the effect among studies focused on South American, Mexican, or Cuban Hispanic groups. For the adjusted analysis, higher mean age and more females included did not significantly increase the effect among studies focused on South American or Mexican Hispanic groups.
APOE ε4 and MCI risk
Supplementary Figure 2 presents the pooled estimates on unadjusted ORs for MCI. There was a statistically significant association with APOE ε4 presence and MCI only for the Central American (OR: 2.45, 95% CI: 1.14 – 5.22) group. Each ethnic group contained only two studies, except for Mexican (n=3) and Spanish (n=1) groups. As only one study reported adjusted ORs for each Hispanic subgroup, the pooled estimates on adjusted ORs were unable to be obtained. Heterogeneity was generally low across regions of origin, with South American, Mexican and Caribbean Hispanic groups having a I2 value of 0%. The Cuban (I2=82.17%) and Central American (I2=44.15%) groups had high and moderate heterogeneity, respectively.
Publication Bias
Funnel plots for unadjusted and adjusted ORs for AD and unadjusted ORs for MCI were generated to investigate the possibility of publication bias for each region of origin (Supplementary figures 3-5). Distribution around the mean effect size varied among regions of origin. In the adjusted plot, the Mexican plot had one point outside of the 95% CI, and the South American plot had one point on the edge of the 95% CI line. In the unadjusted plots, the Mexican plot had one outlier outside of the 95% CI, the South American plot showed three points on the border of the 95% CI, and the Cuban plot did not have any point outside of the 95% CI.
Study Quality
A risk of bias assessment was conducted using the Newcastle-Ottawa Scale (Table 1) [17]. Out of a maximum score of nine, 11 studies were rated high quality (7-9), nine studies were of fair quality (4-6), and none were of poor quality (0-3). The quality was poorest among studies focusing on Spanish populations. There were only two included Spanish studies, both with a score of 5.
DISCUSSION
This meta-analysis evaluated the variation of the association between APOE ε4 presence and ADRD risk amongst Hispanic groups from six different ethnic regions of origin. There was a significant association between APOE ε4 presence and ADRD risk for South American groups based on adjusted analysis. In unadjusted analysis, APOE ε4 was significantly associated with ADRD risk for Caribbean Hispanic, Central American, Cuban, and South American groups. There was not a statistically significant association between APOE ε4 and ADRD risk for the Mexican group in both adjusted and unadjusted analyses. Only the Central American group showed a significant association between APOE ε4 presence and MCI risk.
The variation in the association of APOE ε4 and ADRD risk has been supported by studies evaluating the influence of genetic ancestry on the association of APOE ε4 with ADRD as well as MCI [6,39,40].
In this meta-analysis, the South American countries included were Colombia, Argentina, Venezuela, and Peru. APOE ε4 presence and ADRD risk varied between among included studies of different South American countries, as well as studies within the same countries. This contrast may be attributed to differences in the ethnic makeup of the South American group, a very large and diverse region. One study focused on Caucasian Mestizos in Columbia found a strong association between APOE ε4 and ADRD risk [24]. Contrastingly, a different Colombian based study found no association in APOE ε4 presence and ADRD risk in men, but a significant association for women [21]. Similarly, one study conducted in Venezuela only found a significant association between APOE ε4 and AD risk for women [26]. Therefore, future studies are needed that cover a wide array of countries in South America to further reveal the association between APOE ε4 and AD. In this meta-analysis, while the heterogeneity of Hispanic populations is acknowledged by assessing the association between APOE ε4 and ADRD by region, the heterogeneity among studies within each region remains high. Heterogeneity indicated by high I2 values points to large variation in sample characteristics within regions, not only across regions. This variation in the strength of APOE ε4 as a genetic risk factor for ADRD, suggests that in certain ethnic subgroups, there may be additional important ADRD risk factors other than APOE ε4, such as environmental exposures, stressful life events, nativity status and immigration history, or how well a population is assimilated in their country.
We were not able to further test the impact of nativity for two reasons: (1) limited number of studies were conducted in the U.S., (2) and among those few U.S. based studies, lack of comprehensive data to differentiate first vs. second (or more) generation of immigration. Future research should consider comparing the associations in three groups (e.g. Mexicans, Mexican immigrants in the U.S., and Mexican Americans). Assessing outcomes by nativity status and immigration history are important areas for future research.
Although some studies adjusted for potential other risk factors, such as education, sex, and age, limited studies adjusted for lifestyle and comorbidities [23,28,33]. The diagnoses of ADRD and MCI have evolved over time with the advances in disease biomarkers. Thus, more recent diagnoses of either condition are much more robust than diagnoses based on older criteria [41]. Because the contemporary diagnosis standards rest on biological evidence of disease, less diagnostic variability is observed in more recent studies. This consistency would improve comparison across different cultures and allow greater precision in inferences around the role of different regions of origin in the relationship of genetic factors to ADRD risk. Additionally, ADRD risk among Hispanic populations residing in the US may be different than Hispanics residing in their home countries due to changes in environment and lifestyle [42]. Future studies are needed to assess the possibility of environmental and lifestyle risk factors, by focusing on one group with similar environmental exposures while accounting for other risk factors as has been done in studies of ADRD risk in other sociocultural groups [43].
The variation in APOE ε4 and ADRD risk may also be due to additional genetic or biochemical factors associated with ADRD in Hispanic subgroups. Genetic factors other than APOE have been found to be associated with ADRD for other ethnic groups. ATP-binding cassette transporter protein (ABCA7) was found to double the risk of AD among African American individuals [44]. Mutations in genes coding for presenilin 1 (PSEN1), presenilin 2 (PSEN2), and amyloid precursor protein (APP) have been found to be risk factors for early-onset familial ADRD (EOAD) [45]. It is possible that among the regions of origin with weaker association between APOE ε4 and ADRD, there is a stronger association of ADRD with these genetic factors and/or higher prevalence of EOAD as compared to late-onset ADRD, which shows a higher association with APOE ε4 [45].
Genetic ancestry may play a role in the association between APOE ε4 presence and ADRD risk among Hispanic populations [6]. It has been found that Hispanic groups with higher proportions of European ancestry have a stronger association between APOE ε4 and ADRD as compared to those with African or Amerindian ancestry [6]. Additionally, Amerindian ancestry may have a protective effect against the effect of APOE ε4 on ADRD development [6]. Still, a recent study comparing Non-Hispanic White, African American, Caribbean Hispanic (Cuba, Puerto Rico, Dominican Republic, Venezuela), and Hispanic American individuals found ancestry had no effect on association between APOE ε4 and ADRD risk [8]. In this meta-analysis, only three of the four studies focused on Cuban Hispanic groups included information on genetic ancestry. Samples from the three studies had similar genetic ancestry makeup despite moderate heterogeneity across studies. Therefore, more research is needed to further assess the role of genetic ancestry in the association between APOE ε4 and ADRD risk.
There is a need for increased studies assessing APOE ε4 prevalence and ADRD association among Central American and Spanish populations as well as a need for greater representation of heterogenous Hispanic groups amongst studies of ADRD risk factors. Only one included study focused on ADRD within a Spanish group and one study focused on a Central American group in Panama. There may be a need for more studies conducted in Central American countries other than Panama. There were limited studies focused on Central American and Cuban Hispanic groups with more rigorous design that allowed us to account for factors associated with ADRD risk. Study quality was poorest among studies focusing on Spanish populations. There were only two included Spanish studies, both with a score of 5 out of 9. This may indicate a need for more higher-quality studies focused on Spanish populations.
Three non-English studies were excluded from this meta-analysis. Two of the non-English studies were based on Mexican populations [46,47]. The third study was the Spanish translation of an English study that is included in this meta-analysis [27]. Thus, it is unlikely that this meta-analysis is missing significant data from studies published in Spanish. Future studies should consider incorporating data from non-English articles if applicable.
Another future research direction is a more comprehensive investigation of the association of APOE ε4 with MCI using data from studies that apply the new biological definitions of MCI due to AD [41]. The diagnosis of MCI is unstable when based solely on the clinical features of the disease, with many diagnosed with MCI reverting to cognitively normal [1]. Knowing the association between APOE ε4 and MCI due to AD may help identify individuals with a higher risk of progressing to dementia and identify factors that might mitigate disease progression. Among the 20 included studies, only seven assessed the association between APOE ε4 and MCI. Therefore, additional studies with diverse Hispanic groups are needed to investigate the association of APOE ε4 and/or other risk factors with MCI.
The APOE ε4 allele is significantly associated with increased ADRD risk for the South American group but not for other Hispanic subgroups. This variation suggests that there are environmental factors and/or genetic factors outside of APOE ε4 that contribute to the risk of ADRD amongst diverse Hispanic groups. A better understanding of ethnic variations in both genetic and environmental ADRD risk factors may help to reduce the globally disproportionate burden of ADRD among Hispanic populations.
Supplementary Material
ACKNOWLEDGEMENTS
Thank you to Dr. Nina Tang Sherwood (Duke University Biology Department) for her early review of this project in the thesis stage. This work was presented at the Alzheimer’s Association APOE and Immunity Conference in October 2021.
FUNDING
HX received partial funding for this research from the National Institute on Minority Health and Health Disparities (U54MD012530).
Footnotes
CONFLICT OF INTEREST
The authors have no conflicts of interest to report.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no datasets were generated or analyzed during this study.
REFERENCES
- [1].Alzheimer's Association (2022) Alzheimer’s disease facts and figures. Alzheimers Dement 18, 700–789. [DOI] [PubMed] [Google Scholar]
- [2].Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, Myers RH, Pericak-Vance MA, Risch N, van Duijn CM (1997) Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA 278, 1349–1356. [PubMed] [Google Scholar]
- [3].Tang MX, Stern Y, Marder K, Bell K, Gurland B, Lantigua R, Andrews H, Feng L, Tycko B, Mayeux R (1998) The APOE-ϵ4 Allele and the Risk of Alzheimer Disease Among African Americans, Whites, and Hispanics. JAMA 279, 751–755. [DOI] [PubMed] [Google Scholar]
- [4].Romas SN, Santana V, Williamson J, Ciappa A, Lee JH, Rondon HZ, Estevez P, Lantigua R, Medrano M, Torres M, Stern Y, Tycko B, Mayeux R (2002) Familial Alzheimer Disease Among Caribbean Hispanics: A Reexamination of Its Association With APOE. Arch Neurol 59, 87–91. [DOI] [PubMed] [Google Scholar]
- [5].Campos M, Edland SD, Peavy GM (2013) An Exploratory Study of APOE-ε4 Genotype and Risk of Alzheimer’s Disease in Mexican Hispanics. J Am Geriatr Soc 61, 1038–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Granot-Hershkovitz E, Tarraf W, Kurniansyah N, Daviglus M, Isasi CR, Kaplan R, Lamar M, Perreira KM, Wassertheil-Smoller S, Stickel A, Thyagarajan B, Zeng D, Fornage M, DeCarli CS, González HM, Sofer T (2020) APOE alleles’ association with cognitive function differs across Hispanic/Latino groups and genetic ancestry in the study of Latinos-investigation of neurocognitive aging (HCHS/SOL). Alzheimers Dement 17, 466–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Barnes LL, Bennett DA (2015) Cognitive resilience in APOE*ε4 carriers—is race important? Nat Rev Neurol 11, 190–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Llibre-Guerra JJ, Li J, Qian Y, Llibre-Rodriguez JJ, Jiménez-Velázquez IZ, Acosta D, Salas A, Llibre-Guerra JC, Valvuerdi A, Harrati A, Weiss J, Liu MM, Dow WH (2022) Apolipoprotein E (APOE) genotype, dementia, and memory performance among Caribbean Hispanic versus US populations. Alzheimers Dement 19, 602–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Norton S, Matthews FE, Barnes DE, Yaffe K, Brayne C (2014) Potential for primary prevention of Alzheimer’s disease: an analysis of population-based data. Lancet Neurol 13, 788–794. [DOI] [PubMed] [Google Scholar]
- [10].Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, Brayne C, Burns A, Cohen-Mansfield J, Cooper C, Costafreda SG, Dias A, Fox N, Gitlin LN, Howard R, Kales HC, Kivimäki M, Larson EB, Ogunniyi A, Orgeta V, Ritchie K, Rockwood K, Sampson EL, Samus Q, Schneider LS, Selbaek G, Teri L, Mukadam, N (2020) Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. The Lancet 396, 413–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ngandu T, Lehtisalo J, Solomon A, Levälahti E, Ahtiluoto S, Antikainen R, Bäckman L, Hänninen T, Jula A, Laatikainen T, Lindström J, Mangialasche F, Paajanen T, Pajala S, Peltonen M, Rauramaa R, Stigsdotter-Neely A, Strandberg T, Tuomilehto J, Soininen H, Kivipelto M (2015) A 2-year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. The Lancet. 385, 2255–2263. [DOI] [PubMed] [Google Scholar]
- [12].Kivipelto M, Mangialasche F, Snyder HM, Allegri R, Andrieu S, Arai H, Baker L, Belleville S, Brodaty H, Brucki SM, Calandri I, Caramelli P, Chen C, Chertkow H, Chew E, Choi SH, Chowdhary N, Crivelli L, Torre R, Du Y, Dua T, Espeland M, Feldman HH, Hartmanis M, Hartmann T, Heffernan M, Henry CJ, Hong CH, Hakånsson K, Iwatsubo T, Jeong JH, Jimenez-Maggiora G, Koo EH, Launer LJ, Lehtisalo J, Lopera F, Martínez-Lage P, Martins R, Middleton L, Molinuevo JL, Montero-Odasso M, Moon SY, Morales-Pérez K, Nitrini R, Nygaard HB, Park YK, Peltonen M, Qiu C, Quiroz YT, Raman R, Rao N, Ravindranath V, Rosenberg A, Sakurai T, Salinas RM, Scheltens P, Sevlever G, Soininen H, Sosa AL, Suemoto CK, Tainta-Cuezva M, Velilla L, Wang Y, Whitmer R, Xu X, Bain LJ, Solomon A, Ngandu T, Carrillo MC (2020) World-Wide FINGERS Network: A global approach to risk reduction and prevention of dementia. Alzheimers Dement. 16, 1078–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].The United States Census Bureau, 65 and Older Population Grows Rapidly as Baby Boomers Age. https://www.census.gov/newsroom/press-releases/2020/65-older-population-grows.html, Accessed February 9, 2021.
- [14].Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 372, 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].10/66 Dementia Research Group, https://1066.alzint.org/, Accessed May 14, 2022.
- [16].Stoll CRT, Izadi S, Fowler S, Green P, Suls J, Colditz GA (2019) The value of a second reviewer for study selection in systematic reviews. Res Synth Methods. 10, 539–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ottawa Hospital Research Institute, http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp, Accessed April 16, 2021.
- [18].Pang S, Li J, Zhang Y, Chen J (2018) Meta-Analysis of the Relationship between the APOE Gene and the Onset of Parkinson’s Disease Dementia. Park Dis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Higgins JPT, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med. 21, 1539–1558. [DOI] [PubMed] [Google Scholar]
- [20].Dersimonian R (1996) Meta-Analysis in the Design and Monitoring of Clinical Trials. Stat Med. 15, 1237–1248. [DOI] [PubMed] [Google Scholar]
- [21].Jacquier M, Arango D, Villareal E, Torres O, Serrano ML, Cruts M, Montañes P, Cano C, Rodriguez MN, Serneels S, Van Broeckhoven C (2001) APOE epsilon4 and Alzheimer’s disease: positive association in a Colombian clinical series and review of the Latin-American studies. Arq Neuropsiquiatr. 59, 11–17. [DOI] [PubMed] [Google Scholar]
- [22].Forero DA, Arboleda G, Yunis JJ, Pardo R, Arboleda H (2006) Association Study of Polymorphisms in LRP1, Tau and 5-HTT Genes and Alzheimer’s Disease in a Sample of Colombian Patients. J Neural Transm (Vienna), 113, 1253–1262 [DOI] [PubMed] [Google Scholar]
- [23].Moreno DJ, Pino S, Ríos Á, Lopera F, Ostos H, Via M, Bedoya G (2017) Genetic Ancestry and Susceptibility to Late-Onset Alzheimer Disease (LOAD) in the Admixed Colombian Population. Alzheimer Dis Assoc Disord. 31, 225–231. [DOI] [PubMed] [Google Scholar]
- [24].Arboleda GH, Yunis JJ, Pardo R, Gómez CM, Hedmont D, Arango G, Arboleda H (2001) Apolipoprotein E genotyping in a sample of Colombian patients with Alzheimer’s disease. Neurosci Lett. 305, 135–138. [DOI] [PubMed] [Google Scholar]
- [25].Marca-Ysabel MV, Rajabli F, Cornejo-Olivas M, Whitehead PG, Hofmann NK, Illanes Manrique MZ, Veliz Otani DM, Milla Neyra AK, Castro Suarez S, Meza Vega M, Adams LD, Mena PR, Rosario I, Cuccaro ML, Vance JM, Beecham GW, Custodio N, Montesinos R, Mazzetti Soler PE, Pericak-Vance MA (2021) Dissecting the role of Amerindian genetic ancestry and the ApoE ε4 allele on Alzheimer disease in an admixed Peruvian population. Neurobiol Aging. 101, 11–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Molero AE, Pino-Ramírez G, Maestre GE (2001) Modulation by age and gender of risk for Alzheimer’s disease and vascular dementia associated with the apolipoprotein E-epsilon4 allele in Latin Americans: findings from the Maracaibo Aging Study. Neurosci Lett. 307, 5–8. [DOI] [PubMed] [Google Scholar]
- [27].Allegri RF, Chrem Mendez P, Russo MJ, Cohen G, Calandri I, Campos J, Nahas F, Surace E, Vazquez S, Sevlever G (2021) Biomarkers of Alzheimer’s disease in mild cognitive impairment: Experience in a memory clinic from Latin America. Neurol Barc Spain. 36, 201–208. [DOI] [PubMed] [Google Scholar]
- [28].O’Bryant SE, Johnson L, Balldin V, Edwards M, Barber R, Williams B, Devous M, Cushings B, Knebl J, Hall J (2013) Characterization of Mexican Americans with Mild Cognitive Impairment and Alzheimer’s Disease. J Alzheimers Dis JAD. 33, 373–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Sevush S, Peruyera G, Crawford F, Mullan M (2000) Apolipoprotein-E epsilon 4 allele frequency and conferred risk for Cuban Americans with Alzheimer’s disease. Am J Geriatr Psychiatry Off J Am Assoc Geriatr Psychiatry. 8, 254–256. [PubMed] [Google Scholar]
- [30].Tosto G, Fu H, Vardarajan BN, Lee JH, Cheng R, Reyes-Dumeyer D, Lantigua R, Medrano M, Jimenez-Velazquez IZ, Elkind MS, Wright CB, Sacco RL, Pericak-Vance M, Farrer L, Rogaeva E, St George-Hyslop P, Reitz C, Mayeux R (2015) F-box/LRR-repeat protein 7 is genetically associated with Alzheimer’s disease. Ann Clin Transl Neurol. 2, 810–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Villalpando-Berumen JM, Mejia-Arango S, Aguilar-Salinas CA, Ordonez-Sanchez ML, Gutierrez-Robledo LM (2008) Apolipoprotein E ε4, Alzheimer’s Disease, and Cognitive Performance in Elderly Mexican Mestizos. J Am Geriatr Soc. 56, 677–682. [DOI] [PubMed] [Google Scholar]
- [32].Aguilar-Navarro SG, Gonzalez-Aparicio II, Avila-Funes JA, Juárez-Cedillo T, Tusié-Luna T, Mimenza-Alvarado AJ (2021) Association between ApoE ε4 Carrier Status and Cardiovascular Risk Factors on Mild Cognitive Impairment among Mexican Older Adults. Brain Sci. 11, 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Alavez-Rubio JS, Martínez-Rodríguez N, Escobedo-de-la-Peña J, Garrido-Acosta O, Juárez-Cedillo T (2021) Relationship Between Genetic Variants of ACAT1 and APOE with the Susceptibility to Dementia (SADEM Study). Mol Neurobiol. 58, 905–912. [DOI] [PubMed] [Google Scholar]
- [34].Bartrés-Faz D, Junqué C, López-Alomar A, Valveny N, Moral P, Casamayor R, Salido A, Bel C, Clemente IC (2001) Neuropsychological and genetic differences between age-associated memory impairment and mild cognitive impairment entities. J Am Geriatr Soc. 49, 985–990. [DOI] [PubMed] [Google Scholar]
- [35].Cristiá-Lara L, Sosa-Pérez S, Urrutia-Amble N, Garrudo-Guirado A, Posada-García A, Galán-García L, Verde-Corvo L, Rodríguez-Morera M, Díaz-Casañas MD, Bobes-León MA, Rodríguez-Tanty C, Sablón-Carrazana M, Manrique-Suárez V (2017) Association of Antineuronal Antibody Levels with Cognitive Impairment in Older Cuban Adults. MEDICC Rev. 19, 32–39. [DOI] [PubMed] [Google Scholar]
- [36].Teruel BM, Rodríguez JJL, McKeigue P, Mesa T TC, Fuentes E, Cepero A AV, Hernandez MA, Copeland JRM JRM, Ferri CP, Prince MJ (2011) Interactions between genetic admixture, ethnic identity, APOE genotype and dementia prevalence in an admixed Cuban sample; a cross-sectional population survey and nested case-control study. BMC Med Genet. 12, 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Mateo I, Sánchez-Guerra M, Combarros O, Llorca J, Infante J, González-García J, del Molino JP, Berciano J (2002) Lack of association between cathepsin D genetic polymorphism and Alzheimer disease in a Spanish sample. Am J Med Genet. 114, 31–33. [DOI] [PubMed] [Google Scholar]
- [38].Villarreal AE, Grajales S, O’Bryant SE, Edwards M, López L, Montalván A, Britton GB, Panama Aging Research Initiative (PARI) (2016) Characterization of Alzheimer’s Disease and Mild Cognitive Impairment in Older Adults in Panama. J Alzheimers Dis. 54, 897–901. [DOI] [PubMed] [Google Scholar]
- [39].Alzheimer’s Association (2020) 2020 Alzheimer’s Disease Facts and Figures. Alzheimers Dement. 16, 391+. [DOI] [PubMed] [Google Scholar]
- [40].González HM, Tarraf W, Schneiderman N, Fornage M, Vásquez PM, Zeng D, Youngblood M, Gallo LC, Daviglus ML, Lipton RB, Kaplan R, Ramos AR, Lamar M, Thomas S, Chai A, DeCarli C (2019) Prevalence and correlates of mild cognitive impairment among diverse Hispanics/Latinos: Study of Latinos-Investigation of Neurocognitive Aging results. Alzheimers Dement J Alzheimers Assoc. 15, 1507–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Jack CR Jr., Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, Holtzman DM, Jagust W, Jessen F, Karlawish J, Liu E, Molinuevo JL, Montine T, Phelps C, Rankin KP, Rowe CC, Scheltens P, Siemers E, Snyder HM, Sperling R, Contributors (2018) NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 14, 535–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Xu H, Zhang Y, Wu B (2017) Association between migration and cognitive status among middle-aged and older adults: a systematic review. BMC Geriatr. 17, 184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Hendrie HC, Ogunniyi A, Hall KS, Baiyewu O, Unverzagt FW, Gureje O, Gao S, Evans RM, Ogunseyinde AO, Adeyinka AO, Musick B, Hui SL (2001) Incidence of Dementia and Alzheimer Disease in 2 Communities: Yoruba Residing in Ibadan, Nigeria, and African Americans Residing in Indianapolis, Indiana. JAMA. 285, 739–747. [DOI] [PubMed] [Google Scholar]
- [44].Reitz C, Jun G, Naj A, Rajbhandary R, Vardarajan BN, Wang LS, Valladares O, Lin CF, Larson EB, Graff-Radford NR, Evans D, De Jager PL, Crane PK, Buxbaum JD, Murrell JR, Raj T, Ertekin-Taner N, Logue M, Baldwin CT, Green RC, Barnes LL, Cantwell LB, Fallin MD, Go RCP, Griffith P, Obisesan TO, Manly JJ, Lunetta KL, Kamboh MI, Lopez OL, Bennett DA, Hendrie H, Hall KS, Goate AM, Byrd GS, Kukull WA, Foroud TM, Haines JL, Farrer LA, Pericak-Vance MA, Schellenberg GD, Mayeux R, Alzheimer Disease Genetics Consortium (2013) Variants in the ATP-Binding Cassette Transporter (ABCA7), Apolipoprotein E ϵ4, and the Risk of Late-Onset Alzheimer Disease in African Americans. JAMA. 309, 1483–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Liu CC, Kanekiyo T, Xu H, Bu G (2013) Apolipoprotein E and Alzheimer disease: risk, mechanisms, and therapy. Nat Rev Neurol. 9, 106–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Genis-Mendoza AD, Martínez-Magaña JJ, Bojórquez C, Téllez-Martínez JA, Jiménez-Genchi J, Roche A, Bojorge A, Chávez M, Castañeda C, Guzmán R, Zapata L, Aguilar-Méndez D, Lanzagorta N, Rebolledo I, Castro-Chavira S, Fernández T, Orozco L, Nicolini H, Martínez-Hernández AG (2018) Programa de detección del alelo APOE-E4 en adultos mayores mexicanos con deterioro cognitivo. Gac Med Mex. 154, 555–560. [DOI] [PubMed] [Google Scholar]
- [47].Suástegui Román RA, Yescas Gómez P, Guerrero Camacho JL, Ochoa Morales A, Granados J, Jara Prado A, López-Caro OA, Alonso Vilatela ME (2002) Frequency of apolipoprotein E in a Nahua population. Rev Investig Clin Organo Hosp Enfermedades Nutr. 54, 415–421. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during this study.