Abstract
The drug efflux transporters ABCB1 and ABCG2 at the blood-brain barrier limit the delivery of drugs into the brain. Strategies to overcome ABCB1/ABCG2 have been largely unsuccessful, which poses a tremendous clinical problem to successfully treat central nervous system (CNS) diseases. Understanding basic transporter biology, including intracellular regulation mechanisms that control these transporters, is critical to solving this clinical problem.
In this comprehensive review, we summarize current knowledge on signaling pathways that regulate ABCB1/ABCG2 at the blood-brain barrier. In Section I, we give a historical overview on blood-brain barrier research and introduce the role that ABCB1 and ABCG2 play in this context. In Section II, we summarize the most important strategies that have been tested to overcome the ABCB1/ABCG2 efflux system at the blood-brain barrier. In Section III, the main component of this review, we provide detailed information on the signaling pathways that have been identified to control ABCB1/ABCG2 at the blood-brain barrier and their potential clinical relevance. This is followed by Section IV, where we explain the clinical implications of ABCB1/ABCG2 regulation in the context of CNS disease. Lastly, in Section V, we conclude by highlighting examples of how transporter regulation could be targeted for therapeutic purposes in the clinic.
Significance Statement
The ABCB1/ABCG2 drug efflux system at the blood-brain barrier poses a significant problem to successful drug delivery to the brain. The article reviews signaling pathways that regulate blood-brain barrier ABCB1/ABCG2 and could potentially be targeted for therapeutic purposes.
I. Introduction
A. The Blood-Brain Barrier
1. History of Blood-Brain Barrier Knowledge
The discovery of the blood-brain barrier in 1885 by German microbiologist Paul Ehrlich was a serendipitous event that arose from Ehrlich’s work to determine the oxygen demand of the body (Ehrlich, 1885). In his experiments, Ehrlich injected rabbits intravenously with the “vital dyes” alizarin blue and indophenol blue and observed that all organs were stained by the dyes except for the brain. Several years later, in 1909, Ehrlich’s student Edwin Goldmann repeated the original studies in mice and rats to determine the organ distribution of the vital dye trypan blue (Goldmann, 1909). After intravenous injection, Goldmann observed that all peripheral organs were stained, albeit at a different staining intensity, while the cerebrospinal fluid (CSF) and all other parts of the central nervous system (CNS) remained unstained (Goldmann, 1909). Goldmann inferred such differences in dye distribution to be due to differences in secretion and architecture of the respective organs and concluded that a “physiological barrier membrane” (Physiologische Grenzmembran) separated the blood from the CNS. To test his hypothesis, Goldmann injected trypan blue into the mouse cranium and observed that the brain parenchyma and spinal cord were stained whereas the peripheral organs were not—the opposite effect of intravenous dye injection (Goldmann, 1913). This finding supported Goldmann’s hypothesis and provided further evidence for the existence of a barrier between the peripheral blood circulation and the CNS.
Today, Ehrlich’s and Goldmann’s experiments are considered the dawn of blood-brain barrier research. However, the term “blood-brain barrier” was not introduced until 1921 when Russian physiologist Lina Stern referred to it as barrière hémato-encéphalique (Stern and Gautier, 1921). Stern coined this term based on a series of experiments in which she injected guinea pigs, rabbits, cats, and dogs with a variety of substances, including bromides, strychnine, or bile salts, and then analyzed blood, CSF, and urine using colorimetric assays. After intravenous injection, Stern detected these substances only in blood and urine, whereas after intraventricular injection, she detected them only in the CSF. These results convinced Stern that the blood-brain barrier was not an anatomic but a functional structure that protects the CNS, prevents the uptake of toxic substances, and maintains normal physiologic conditions in the brain (Stern and Gautier, 1921).
In the years following these fundamental discoveries, a heated scientific controversy erupted over the nomenclature, location, and physiologic function of the blood-brain barrier. After much discussion, Hugo Spatz hypothesized in the early 1920s that barrier function must reside in the brain’s capillary endothelial cells (Spatz, 1934). Danish Nobel Laureate in Physiology or Medicine August Krogh argued that the blood-brain barrier could not be completely impermeable as Spatz had suggested years earlier, since Krogh’s own studies showed that nutrients and ions reached the brain parenchyma (Krogh, 1946). Definitive proof of blood-brain barrier location and function was provided by the seminal work of Reese and Karnovsky (1967) and Brightman and Reese (1969). These researchers intravenously injected mice, chicken, and goldfish with the enzyme horseradish peroxidase. Using electron microscopy, they showed in fixed brain slices that horseradish peroxidase remained confined in the lumen of brain microvessels due to brain endothelial tight junctions that prevented paracellular diffusion of the enzyme into the brain (Reese and Karnovsky, 1967; Brightman and Reese, 1969). These findings unequivocally demonstrated that the tight junctions that had been identified between brain capillary endothelial cells several years earlier by Muir and Peters (1962) restrict paracellular diffusion of solutes across the blood-brain barrier (Reese and Karnovsky, 1967; Brightman and Reese, 1969).
2. The Neurovascular Unit
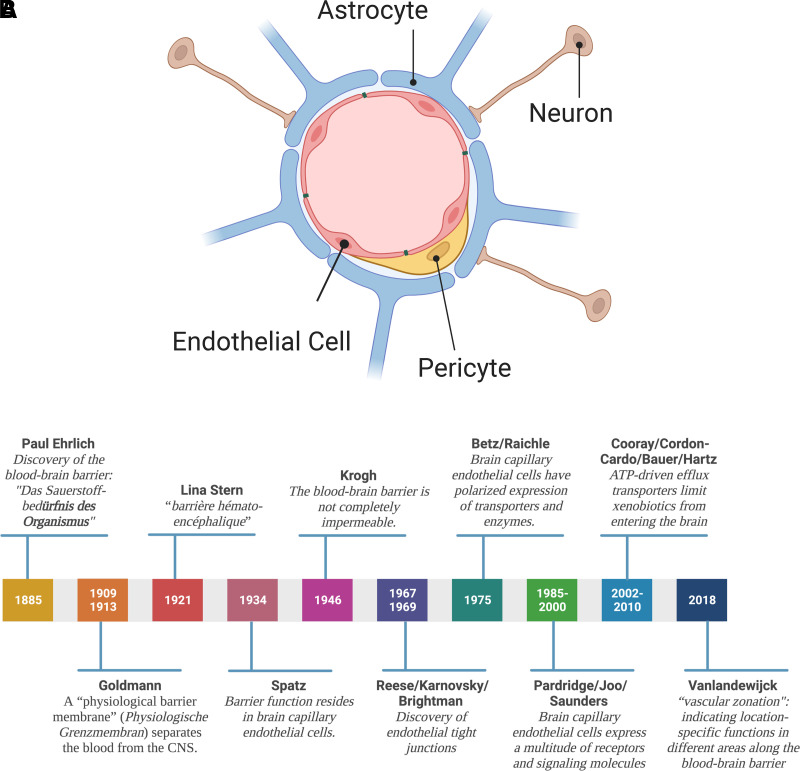
In the early 2000s, a new concept arose: Barrier function is not solely based on endothelial cell properties but rather relies on the anatomic and functional interaction of endothelial cells with pericytes, astrocytes, and neurons. Together, these cells form an anatomically complex and functionally highly regulated and dynamic multicell structure referred to as the “neurovascular unit” (Fig. 1; Stroke Progress Review Group, 2002).
Fig. 1.
(A) The neurovascular unit. The neurovascular unit consists of endothelial cells surrounded by a basement membrane, astrocytes, pericytes, and neurons. This four-cell structure also known as the “neurovascular unit” is responsible for the regulation of blood-brain barrier function. (B) History of the blood-brain barrier. Timeline of fundamental discoveries made in the blood-brain barrier field. Created with BioRender.com.
In the neurovascular unit, brain capillary endothelial cells form the first layer of barrier function. Characteristics of brain capillary endothelial cells that contribute to barrier function include high expression levels of tight junction proteins, lack of fenestration, low pinocytic activity, and a large number of mitochondria that provide ATP to support a high energy demand (Reese and Karnovsky, 1967; Brightman and Reese, 1969; Oldendorf and Brown, 1975; Oldendorf et al., 1977; Betz and Goldstein, 1978; Betz et al., 1980; Yoshida et al., 1988). Brain capillary endothelial cells also have polarized expression of transporters and enzymes. Alkaline phosphatase and other enzymes localize to the luminal membrane facing the blood, while Na+/K+-ATPase and the Na+-dependent small amino acid carrier are located in the abluminal membrane, facing the brain. This polar protein expression is the prerequisite for directional transport across the blood-brain barrier and has been described for glucose and several amino acids, including leucine and isoleucine (Betz et al., 1975; Raichle et al., 1975). Moreover, protein expression also changes along the vascular continuum of the blood-brain barrier, a phenomenon recently described as “vascular zonation,” indicating location-specific functions in different areas along the vasculature of the blood-brain barrier (Vanlandewijck et al., 2018). Brain capillary endothelial cells are surrounded by a basement membrane that consists of collagens and laminins, as well as proteins involved in extracellular matrix and basement membrane reorganization, e.g., matrix metalloproteinases (Bonnans et al., 2014; Joutel et al., 2016). The basement membrane provides structure and support for endothelial cells and is involved in signal transduction between brain capillary endothelial cells and brain parenchymal cells (Hynes, 2009; Baeten and Akassoglou, 2011; Nehra et al., 2022). Embedded within the basement membrane are pericytes (multifunctional mural cells), the second cell type of the neurovascular unit, that cover the abluminal brain capillary surface (Cuevas et al., 1984; Armulik et al., 2005, 2010; Winkler et al., 2011; Sweeney et al., 2016). Depending on the brain region, pericytes cover 20% to 99% of the abluminal surface of brain capillaries (Mathiisen et al., 2010; Hartmann et al., 2015; Herndon et al., 2017; Berthiaume et al., 2018). Astrocytes, the third cell type of the neurovascular unit, have end feet that sit on top of the basement membrane and cover approximately 60% of the abluminal surface area of brain capillaries (Wolff, 1963; Mathiisen et al., 2010; Korogod et al., 2015). The fourth and last cell type at the neurovascular unit is neurons. Neurons interact with the neurovascular unit either through astrocytic connections or through direct interaction of interneurons with endothelial cells (Niwa et al., 2000; Gotoh et al., 2001).
Together, the neurovascular unit, consisting of endothelial cells, pericytes, astrocytes, and neurons, maintains brain homeostasis, protects the CNS from neurotoxic compounds, and is responsible for communication between the periphery and the CNS.
a. Communication at the neurovascular unit
The neurovascular unit represents a critical blood-brain interface that ensures regulated bidirectional communication between the periphery and the CNS (Stern and Gautier, 1921; Terrando et al., 2011; Marchi et al., 2013; Chen et al., 2020) and is a highly regulated anatomic structure that senses and responds to information flowing from the periphery to the brain and vice versa (Wyss-Coray and Rogers, 2012; Chen et al., 2020).
To enable communication, cells of the neurovascular unit are highly specialized and equipped with a myriad of signaling molecules. For example, brain capillary endothelial cells express a multitude of receptors and signaling molecules (Stefanovich, 1979; Karnushina et al., 1980; Joo, 1985, 1993; Pardridge et al., 1985). Among these proteins are signaling molecules like cAMP (Stefanovich, 1979; Karnushina et al., 1980), insulin receptor (Frank and Pardridge, 1981; Pardridge et al., 1985), hormone receptors (Edvinsson and Owman, 1975), as well as receptor tyrosine kinases like platelet-derived growth factor receptor (Smits et al., 1989). Additionally, enzymes involved in the synthesis and degradation of signaling molecules like cyclooxygenase (Baba et al., 1985) and phosphodiesterases (Stefanovich, 1979) are also present at the blood-brain barrier (Joo, 1985; 1993; Saunders et al., 2014). These signaling molecules enable endothelial cells to communicate between the periphery and the brain.
Pericytes are in direct, basolateral contact with brain capillary endothelial cells, which allows for direct communication between the two cell types through gap junctions (Cuevas et al., 1984; Armulik et al., 2005, 2010; Winkler et al., 2011; Sweeney et al., 2016). Pericytes play a vital role in blood-brain barrier development and maintenance of barrier function. In this regard, loss of pericyte function results in abnormal capillary development and increased capillary permeability (Hellström et al., 2001; Armulik et al., 2010).
Astrocytes cover a large area of the basolateral side of brain capillary endothelial cells and, therefore, are in an ideal position to communicate with brain capillary endothelial cells and regulate barrier function. Signaling from astrocytes to brain capillary endothelial cells is essential for development of tight junctions and localization of transporters and other endothelial proteins. One such regulatory pathway involves the signaling peptide sonic hedgehog that is released by astrocytes. After secretion, sonic hedgehog binds to the Patch-1 receptor on brain capillary endothelial cells inducing downstream activation of the transcription factor GLI family zinc finger 1 (Alvarez et al., 2011, 2013). Activation of GLI family zinc finger 1, in turn, increases the expression of the tight junction proteins claudin 5 and occludin, decreasing blood-brain barrier permeability (Alvarez et al., 2011). Astrocytes are uniquely located between endothelial cells and neurons and enable communication between those two cell types (Niwa et al., 2000; Gotoh et al., 2001). Astrocyte-endothelial cell communication is referred to as neurovascular coupling, indicating the close interaction of neuronal activity and cerebral blood flow. Cerebral blood flow is selectively increased in areas with high neuronal activity to compensate for higher energy consumption (Cox et al., 1993; Chaigneau et al., 2003). Additionally, interneurons regulate cerebral blood flow by releasing vasoactive molecules, such as prostaglandins or nitric oxide (NO) (Niwa et al., 2000; Gotoh et al., 2001; Iadecola, 2017). Together, cells of the neurovascular unit work together to ensure effective communication among themselves as well as the periphery and the brain.
3. Barrier Function
Barrier function is pivotal for protecting and ensuring nutrient supply to the brain. The blood-brain barrier achieves this through tightly regulated interplay among enzymes, transporters, and structural proteins that cooperate through four different mechanisms. First, tight junction proteins form a physical barrier by sealing off paracellular pathways, which prevents passive diffusion of hydrophilic endo- and xenobiotics (Reese and Karnovsky, 1967; Brightman and Reese, 1969; Saunders et al., 2014). Second, metabolic enzymes expressed in endothelial cells form a metabolic barrier by degrading, and thereby deactivating, CNS-active drugs before they can reach their targets (Ghersi-Egea et al., 1994; Dauchy et al., 2009; Saunders et al., 2017). Third, influx transporters facilitate the uptake of specific nutrients like glucose and amino acids (Oldendorf, 1971; Daneman et al., 2010; Saunders et al., 2017). These transporters belong to the solute carrier superfamily and are either facilitative, secondary, or tertiary active transporters (Deng et al., 2014; Morris et al., 2017; Yan et al., 2019b). Fourth, ATP-driven efflux transporters export metabolic waste and limit xenobiotics, including a myriad of therapeutic drugs, from entering the brain (Cordon-Cardo et al., 1989; Cooray et al., 2002; Hartz et al., 2005, 2009, 2010b; Bauer et al., 2006; Hartz and Bauer, 2010; Miller, 2010; Saunders et al., 2017). Notably, influx and efflux transporters make up approximately 15% of blood-brain barrier–specific proteins indicating a high relevance for proper barrier function (Li et al., 2001; Shusta et al., 2002; Pardridge, 2007; Kamiie et al., 2008; Uchida et al., 2011; Uchida et al., 2014).
B. ATP-Binding Cassette Efflux Transporters
Efflux transporters belong to the ATP-binding cassette (ABC) transporter superfamily of primary active transporters and are organized based on their gene structure, amino acid sequence, and phylogenetic analyses into seven subfamilies (ABCA–ABCG; Sarkadi et al., 2006; Robey et al., 2018). ABC transporter structure and function are conserved across the different subfamilies as well as across multiple species, including fungi, bacteria, protozoa, insects, fish, and mammals (Klokouzas et al., 2003; Kovalchuk and Driessen, 2010; Gebhard, 2012; Luckenbach et al., 2014; Kowalski et al., 2020). The human genome contains 48 different ABC transporters (Vasiliou et al., 2009; Morris et al., 2017; Robey et al., 2018); 19 of these transporters are expressed in the CNS, most of them at the blood-brain barrier (Hartz and Bauer, 2011). Common structural features of ABC transporters include two transmembrane domains (TMD) and two nucleotide-binding domains (NBD); (Loo et al., 2002). The TMDs form the substrate binding pocket and facilitate substrate movement across the blood-brain barrier and other membranes, while the NBDs hydrolyze ATP to provide the energy for active substrate movement against a concentration gradient (Loo et al., 2002). The NBD structure is highly conserved across the seven ABC subfamilies and across species. Common motifs, including the Walker A (G-x(4)-GK-[TS]) and B ([RK]-x(3)-G-x(3)-LhhhD) motifs, are preserved throughout all ABC transporters (Hyde et al., 1990; Dean et al., 2001). The TMD sequences on the other hand are highly variable, which allows a broad, diverse substrate spectrum that includes lipophilic drugs, hydrophilic metabolites, glucuronides, and sulfate conjugates (Dean et al., 2001; de Vries et al., 2007; Kruh et al., 2007). The two most prominent efflux transporters at the blood-brain barrier, P-glycoprotein (P-glycoprotein nucleotide-binding domains; ABCB1) and breast cancer resistance protein (BCRP; ABCG2), are the main topic of this review and are discussed in detail later.
1. History of ABCB1
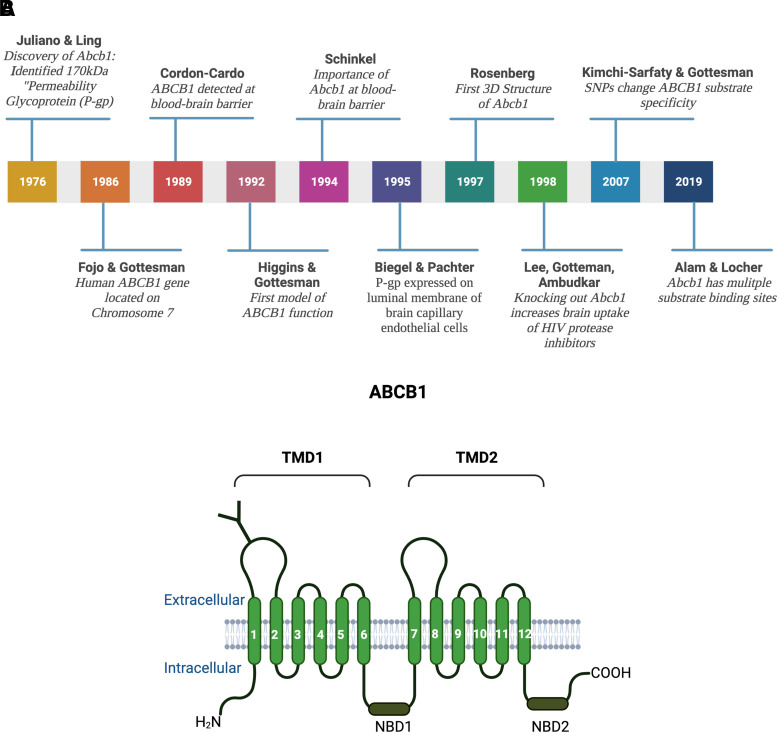
The development of mustard gas derivatives, antimetabolites, and antibiotics as anticancer drugs in the 1940s and 1950s significantly improved the survival of cancer patients (Goodman et al., 1946; Hitchings and Elion, 1954; Pinkel, 1959; DeVita and Chu, 2008). Success in treatment, however, also revealed that patients could be resistant to anticancer drugs (Law, 1952; Niero et al., 2014). Work analyzing this drug resistance in bacterial and mammalian cells eventually led to the discovery of ABCB1 and other ABC transporters (Fig. 2).
Fig. 2.
(A) History of ABCB1. From the discovery of the “permeability glycoprotein” by Juliano and Ling in 1976 to structural insights into substrate and inhibitor discrimination by human ABCB1 revealed by Alam and Locher in 2019. (B) ABCB1 structure. ABCB1 consists of two transmembrane domains TMD1 and TMD2, each of which has six transmembrane spanning α-helices and a nucleotide binding domain (NBD1 and NBD2). ABCB1 is N-glycosylated at the first extracellular loop. Created with BioRender.com.
In 1970, June Biedler postulated that resistance to actinomycin D and other anticancer drugs in Chinese hamster ovary cells was caused by changes in cell permeability (Biedler and Riehm, 1970). Biedler’s experiments showed that multidrug resistance was established through stable chromosome changes, possibly in membrane-related genes (Biedler and Riehm, 1970). Later, Juliano and Ling determined that the expression of a 170 kDa glycoprotein in the membrane of drug-resistant Chinese hamster ovary cells correlated with the level of drug resistance (Juliano and Ling, 1976). Since the protein was not expressed in wild-type cells and drug resistance corresponded with changes in drug permeation, Juliano and Ling postulated that this new glycoprotein changed the permeability of the cell membrane and, therefore, named it “permeability glycoprotein” or P-glycoprotein [old nomenclature: PGY1, MDR1, CLCS; current: ABCB1 (human protein), Abcb1a/Abcb1b (rodent proteins), Abcb1a/Abcb1b (rodent genes), ABCB1 (human gene) (Juliano and Ling, 1976)]. Homolog genes and proteins were later detected in bacteria as well as in mice and humans, where the gene is located on chromosome 7 (chromosome 5 in mice; Chen et al., 1986; Fojo et al., 1986; Gros et al., 1986; Callen et al., 1987).
In human samples, ABCB1 localizes to the apical surface of epithelial and endothelial cells of excretory and barrier organs and tissues such as liver, kidney, intestine, colon, and placenta, suggesting a role in secretion, elimination, and protection (Thiebaut et al., 1987; Croop et al., 1989). The role of ABCB1 in protecting critical organs was further demonstrated when it was detected at the human blood-brain barrier in 1989 (Cordon-Cardo et al., 1989). At the blood-brain barrier, ABCB1 is expressed in the luminal membrane of brain capillary endothelial cells (Biegel et al., 1995). Even though ABCB1 had been identified at the blood-brain barrier, its role and significance were initially obscure. Based on tissue distribution and expression in drug-resistant cancer cells, the leading hypothesis was that ABCB1 was involved in the active excretion of toxic xenobiotics and metabolites from the brain and other excretory tissues. To test the physiologic role of Abcb1, Schinkel et al. developed an Abcb1a (originally referred to as mdr1a) knockout mouse (Schinkel et al., 1994). Shortly after establishing this unique knockout mouse, unexpected circumstances led to a serendipitous finding. Due to a mite infestation of Schinkel’s mouse colonies, all animals—wild-type and Abcb1a knockout—were treated with ivermectin, a standard veterinary anthelmintic drug. After treatment, all Abcb1a knockout mice, presented with paralytic symptoms and died from neurotoxicity; however, none of the wild-type mice died. Toxicological testing showed that Abcb1a knockout mice had 90-fold higher ivermectin brain levels compared with wild-type or heterozygous littermates, which correlated with a 100-fold increase in sensitivity to ivermectin-induced neurotoxicity (Schinkel et al., 1994). Today, Abcb1 deficiency is well recognized in dogs and cats and is routinely screened for in pets to prevent ivermectin-induced toxicity (Roulet et al., 2003; Mealey et al., 2023). Based on their observations in mice, Schinkel and coworkers concluded that blood-brain barrier Abcb1a was important for protecting the brain and creating a pharmacological sanctuary. This was further corroborated by results from Kim et al. showing that knocking out Abcb1a increased oral absorption and brain uptake of human immunodeficiency virus (HIV) protease inhibitors (Kim et al., 1998a,b; Lee et al., 1998). Combined, these findings indicated that Abcb1a acts as a double-edged sword at the blood-brain barrier: on the one hand, Abcb1a-mediated efflux is vital for protecting the brain; on the other hand, Abcb1a prevents uptake of potentially CNS-active drugs, significantly limiting their CNS efficacy.
At that time in the 1990s, initial models were proposed to explain ABCB1-mediated transport function. The original model hypothesized a central pore that facilitates active substrate expulsion through the apical plasma membrane (Borst and Schinkel, 1997). However, the first 3D structure for ABCB1 proposed by Rosenberg and colleagues showed that ABCB1 is closed toward the cytoplasm side, contradicting the pore model (Rosenberg et al., 1997). A second model attempting to explain ABCB1 transport function, referred to as the “hydrophobic vacuum cleaner model,” postulates that a substrate moves laterally through the membrane until ABCB1 removes it through a flipping process, indicating that ABCB1 acts as a flippase (Higgins and Gottesman, 1992; Gottesman and Pastan, 1993). Additional data from experiments to elucidate ABCB1 structure showed a central, polyspecific substrate binding chamber that is accessible from the cytoplasm as well as the lipid membrane, suggesting that ABCB1 efflux function is most likely based on a combination of both models (Rosenberg et al., 2005; Aller et al., 2009). However, understanding ABCB1 function is further complicated by several synonymous single nucleotide polymorphisms (SNPs) that are inconsequential for ABCB1 protein structure but affect function and substrate binding (Kimchi-Sarfaty et al., 2007; Fung and Gottesman, 2009; Dickens et al., 2013; Hattori et al., 2018). Recently, Alam et al. found multiple substrate binding pockets in the ABCB1 molecule and concluded that the pocket a compound binds to determines if this compound is an ABCB1 substrate or inhibitor (Alam et al., 2019). In addition, Dastvan et al. (2019) demonstrated that ABCB1 substrate binding decreases the activation energy for ATP hydrolysis and showed that ATP hydrolysis must occur before or simultaneously to substrate translocation (Dastvan et al., 2019). To this date, exactly how ABCB1 functions remains unclear, and more research is necessary to fully elucidate the mechanism of ABCB1-mediated efflux transport.
2. History of ABCG2
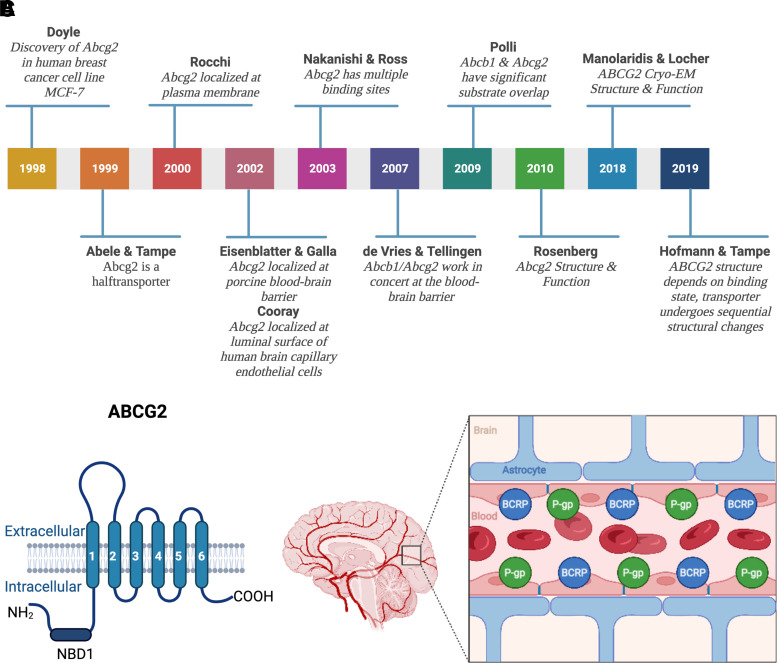
In 1992, Nakagawa et al. discovered that exposing MCF-7 human breast cancer cells to the ABCB1 inhibitor verapamil did not reverse mitoxantrone resistance (Nakagawa et al., 1992). Moreover, daunorubicin and rhodamine 123 efflux from MCF-7 cells was not affected by the ABCB1 inhibitor cyclosporin A but was reversed by depleting ATP (Lee et al., 1997). These data indicated the existence of another active, ATP-driven efflux transporter in cancer cells. In 1998, Doyle et al. (Doyle et al., 1998) compared gene expression in parental versus doxorubicin-resistant MCF-7 cells and revealed a differentially expressed messenger RNA (mRNA) that coded for a new ABC transporter: breast cancer resistance protein [old nomenclature: EST157481, MXR, BCRP, ABCP, CD338; current: ABCG2 (human protein), Abcg2 (rodent protein), Abcg2 (rodent gene), ABCG2 (human gene; Fig. 3A; Doyle et al., 1998)].
Fig. 3.
(A) History of ABCG2. From the discovery of the “breast cancer resistance protein” ABCG2 in 1998 to its cryo-EM structure and function. (B) ABCG2 structure. ABCG2 consists of one transmembrane domain that has six transmembrane spanning α-helices and one nucleotide binding domain (NBD1). ABCG2 is a half transporter that needs to homodimerize to fully function. (C) ABCB1 and ABCG2 at the blood-brain barrier. ABCB1 and ABCG2 are both located at the luminal membrane of endothelial cells comprising the blood-brain barrier. They act as a “first line of defense” by limiting xenobiotics including a large number of therapeutic drugs from entering into the brain. Created with BioRender.com.
Physiologically, ABCG2 is expressed in barrier organs and tissues including the blood-brain barrier, where ABCG2 localizes to the luminal plasma membrane of endothelial cells and facilitates directional efflux across the blood-brain barrier from brain to blood (Cooray et al., 2002; Eisenblätter and Galla, 2002; Zhang et al., 2003). However, in contrast to other ABC transporters, ABCG2 codes for only one transmembrane domain with one nucleotide binding site, resulting in a protein of approximately 70 kDa, which is half the size of other ABC transporters. Therefore, ABCG2 is a so-called half transporter that needs to homodimerize to fully function (Fig. 3B; Abele and Tampe, 1999; Rocchi et al., 2000; Kage et al., 2002).
Structural studies, homology modeling, and transport studies with ABCG2 mutants identified multiple substrate binding sites that confirmed an overlapping substrate spectrum with ABCB1 (Nakanishi et al., 2003; Clark et al., 2006; Xu et al., 2007; Rosenberg et al., 2010). The exact mechanism of transport function, however, was unknown until Manolaridis et al. (2018) recently constructed cryo-electron microscopy structures of ABCG2 in substrate- and ATP-bound pre- and post-translocation states. These different conformations revealed that substrates bind to a central, hydrophobic binding pocket that faces the cytoplasm. Upon ATP binding and hydrolysis, a conformational shift collapses the substrate binding pocket, which opens an external cavity and pushes the substrate across the membrane and out of the cell (Manolaridis et al., 2018). Hofmann et al. (2019) confirmed these findings and found two distinct cryo-electron microscopy structures of an ABCG2 bacterial homolog. Based on these structures, the authors determined that transporter conformation depends on substrate- and ATP-binding state and suggested sequential conformation changes during the transport process. In addition to exogenous drug transport, ABCG2 has also been implicated in the transport of endogenous metabolites including estrogens, steroids, and folates (Imai et al., 2003; Suzuki et al., 2003; Ifergan et al., 2004, 2005).
Like ABCB1, several SNPs in ABCG2 have been identified in patients (Zamber et al., 2003; Furukawa et al., 2009; Delord et al., 2013). For example, Allegra et al. (2018) recently demonstrated that the SNP 1194 + 928 rs13120400 T>C (position 89033527), an intronic variant of ABCG2, is associated with decreased brain uptake of ceftriaxone in patients.
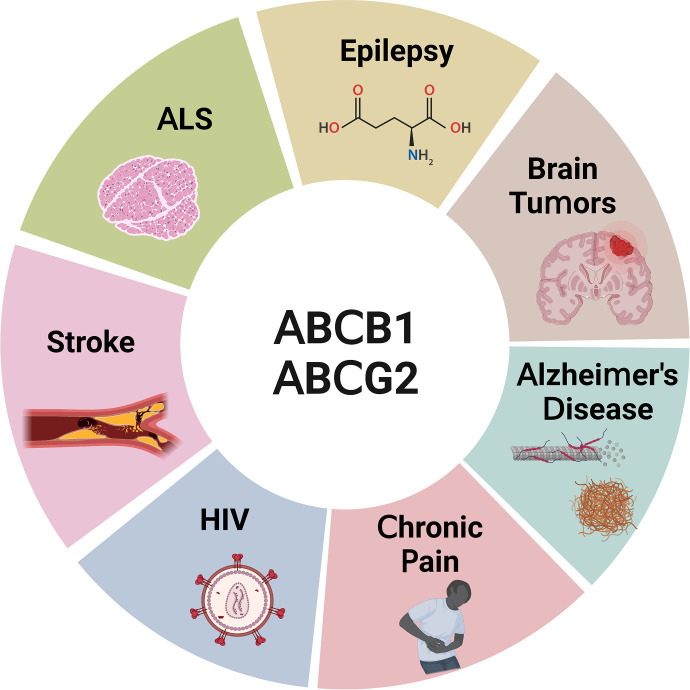
Together, at the blood-brain barrier, ABCB1 and ABCG2 restrict brain uptake of substrate drugs and decrease their efficacy, representing a formidable obstacle to the successful therapy of many CNS diseases (Fig. 3C). Thus, understanding ABCB1 and ABCG2 substrate specificities can make the difference between therapeutic failure or success.
3. ABCB1/ABCG2 Substrates
ABCB1 and ABCG2 were first described as mediators of anticancer drug resistance in cancer cells. While ABCB1 was implicated in resistance against daunorubicin, ABCG2 was found to contribute to resistance against mitoxantrone, doxorubicin, and daunorubicin, indicating important roles for both transporters in multidrug resistance (Biedler and Riehm, 1970; Juliano and Ling, 1976; Doyle et al., 1998). de Vries et al. confirmed these findings and showed that Abcb1a/Abcb1b and Abcg2 have overlapping substrate spectra and work together in concert in restricting topotecan brain uptake (de Vries et al., 2007).
Over the past decades, many anticancer drugs have been identified as substrates of either ABCB1, ABCG2, or in many cases both transporters (de Vries et al., 2007; Agarwal and Elmquist, 2012; Traxl et al., 2019). ABCB1/ABCG2 restrict the brain uptake of anticancer drugs and significantly limit their efficacy in the treatment of primary and metastatic brain tumors (Marchetti et al., 2008; Agarwal et al., 2011; de Vries et al., 2012; Taskar et al., 2012; Laramy et al., 2017; de Gooijer et al., 2018a; Sorf et al., 2018). Substrates of ABCB1/ABCG2 are not restricted to a specific class of anticancer drugs but span the entire spectrum of chemotherapeutic compounds. ABCB1/ABCG2 substrates include antibiotics, such as daunorubicin (Juliano and Ling, 1976), alkylating agents like temozolomide (de Gooijer et al., 2018b), microtubule inhibitors including paclitaxel (Kemper et al., 2003, 2004), topoisomerase inhibitors (Marchetti et al., 2008), cell cycle disruptors such as ribociclib (Sorf et al., 2018), and tyrosine kinase inhibitors like lapatinib or sorafenib (Polli et al., 2008, 2009; Agarwal et al., 2011). While many anticancer drugs show promising effects against different brain cancer cell lines in vitro, their efficacy in vivo and in clinical trials has been marginal at best, in large part due to ABCB1/ABCG2-mediated efflux at the blood-brain barrier. Since the seminal work by de Vries et al. in 2007, the overlap in ABCB1 and ABCG2 substrate spectra was expanded from anticancer drugs to include a multitude of other drug classes. ABCB1 significantly restricts brain uptake of some antiseizure drugs and limits their efficacy in the treatment of epilepsy (Cox et al., 2001; van Vliet et al., 2006; Tang et al., 2017). Other drugs that are ABCB1/ABCG2 substrates include HIV protease inhibitors (Kim et al., 1998a,b; Lee et al., 1998), the dopamine hydroxylase inhibitor etamicastat (Bicker et al., 2018), riluzole, one of the few Food and Drug Administration (FDA)-approved drugs for amyotrophic lateral sclerosis (ALS) therapy (Jablonski et al., 2014), and a myriad of drugs including opioids (Letrent et al., 1999; Dagenais et al., 2004; Bauer et al., 2006; Sharma and Ali, 2006; Hassan et al., 2007; Yousif et al., 2008, 2012; Chaves et al., 2016; Schaefer et al., 2018). For example, since oxycodone, morphine, and methadone are weak Abcb1a substrates, they can cross the blood-brain barrier, resulting in substantial brain uptake and CNS activity (Gibbs et al., 2018). On the other hand, active efflux of opioids at the blood-brain barrier has been exploited to develop peripherally active opioids for the treatment of diarrhea. Take loperamide as an example, which is a good Abcb1a/b substrate and therefore does not easily enter the brain (Watari et al., 2019). Loperamide has a four times higher Abcb1a-mediated transport rate compared with methadone, which significantly restricts loperamide brain uptake (Gibbs et al., 2018).
Taken together, ABCB1 and ABCG2 have largely overlapping substrate spectra that comprise a wide range of compounds including anticancer drugs, antiseizure drugs, HIV protease inhibitors, opioids, and a large number of other therapeutically used drugs. The consequence of this overlap in substrates is that both transporters compensate for each other. In other words, drugs directed to the brain have to overcome not one but two transporters—the ABCB1/ABCG2 drug efflux system.
II. Overcoming The ABCB1/ABCG2 Drug Efflux System
The blood-brain barrier is a challenge in the treatment of many CNS diseases. Over the decades, multiple strategies to overcome the blood-brain barrier have been developed with the goal of improving drug therapy for CNS disorders. These strategies can largely be divided into transporter-independent and transporter-dependent strategies.
Transporter-independent strategies to overcome the barrier include blood-brain barrier disruption with focused ultrasound (Hynynen et al., 2001; Burgess et al., 2011; Mainprize et al., 2019), hyperosmotic solutions (Neuwelt et al., 1986; Doolittle et al., 2000; Angelov et al., 2009; Chakraborty et al., 2016; Lesniak et al., 2019), transport vehicles that target receptor-mediated transcytosis (Pardridge, 2001; Kariolis et al., 2020; Ullman et al., 2020), direct drug delivery via intraparenchymal infusion, waver implantation (Valtonen et al., 1997) or convection-enhanced delivery (Laske et al., 1997; Lidar et al., 2004), intranasal delivery (Thorne et al., 1995; Frey, 1997, 2001), or the use of liposomes and nanoparticles (Huwyler et al., 1996; Ulbrich et al., 2009; Fan et al., 2018). Currently, only intraarterial injection of hyperosmotic mannitol and implantable drug wavers are FDA-approved therapeutics (National Cancer Institute, 2022). Other transporter-independent strategies exist (Doolittle et al., 2000; Hynynen et al., 2001; Westphal et al., 2003; Duntze et al., 2013; Mainprize et al., 2019; Kariolis et al., 2020; Ullman et al., 2020).
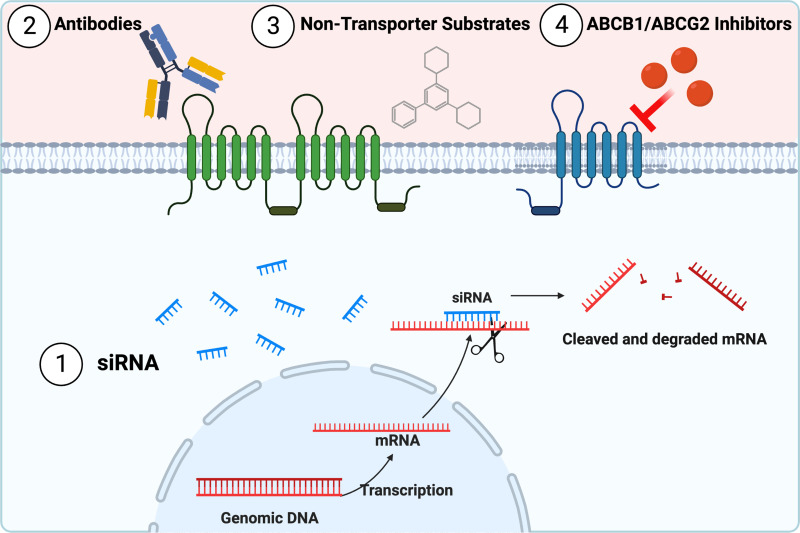
In the following section, we discuss advantages and disadvantages of the main transporter-dependent strategies. Transporter-dependent strategies (Fig. 4) focus on inhibiting and overcoming ABCB1- and ABCG2-mediated drug efflux using small interfering (siRNA), antibodies, nontransporter, or transporter inhibitors.
Fig. 4.
Transporter-dependent strategies to overcome ABCB1 and ABCG2 drug efflux. Transporter-dependent strategies focus on inhibiting and overcoming ABCB1- and ABCG2-mediated drug efflux by using (1) siRNA, (2) antibodies, (3) nontransporter substrates, or (4) transporter inhibitors. Created with BioRender.com.
A. Small Interfering RNA
In vitro, siRNA reduce ABCB1/ABCG2 mRNA and ABCB1/ABCG2 protein expression in drug-resistant cancer cells such as U87 glioblastoma cells or hepatocellular carcinoma cells (Fisher et al., 2007; Zhao et al., 2008; Li et al., 2012). ABCB1/ABCG2 knockdown also decreases transporter function, increases the accumulation of rhodamine 123, and enhances the cytotoxicity of doxorubicin (Rittierodt et al., 2004; Fisher et al., 2007; Zhao et al., 2008; Li et al., 2012). However, synthetic siRNAs have not yet been tested in vivo.
While superficially similar to siRNA, microRNAs (miRNA) have several specific differences. Both are endogenous, small noncoding RNAs that act as a recognition sequence to permit the RNA-induced silencing complex to bind target mRNAs. However, siRNAs silence genes by cleaving mRNA before translation, while miRNAs function to silence the translation apparatus. In addition, siRNA targeting relies on (near) 100% complementarity, whereas miRNA binding requirements are less stringent. The stem-loop structures that give rise to miRNAs are also shorter than the long double-stranded RNA that gives rise to siRNA (Mack, 2007; Qureshi et al., 2014).
miRNAs target the 3′-untranslated region (3′-UTR) of an mRNA. Binding of the 3′-UTR prevents the assembly of the translational complex and decreases the expression of the target protein (Ambros, 2004; Bartel, 2004). However, in some instances, miRNAs target other regions of an mRNA, including the 5′-UTR and the protein-coding sequence, and miRNA activity upregulates translation of some targets (Long et al., 2019) Several groups have identified differentially expressed miRNAs in tumor cells and at the blood-tumor barrier in samples from glioblastoma patients. For example, expression of miR-145 is decreased in tumor samples from glioblastoma patients. Transfecting U87 glioblastoma cells with synthetic miR-145 decreases both ABCB1 and ABCG2 protein levels, which in return increases sunitinib cytotoxicity (Liu et al., 2015). Another miRNA, miR-4539, altered expression of ABCB1 in T98G glioblastoma cells and aligned strongly with the 3′-UTR mRNA sequence of ABCB1. Cotreatment of cells with doxorubicin and miR-4539 increased toxicity by at least 40%, depending on miRNA dose (Medarova et al., 2020).
While these in vitro data seem promising, only a few in vivo studies have been conducted in animal disease models. Li et al. showed that miR-378 increases the treatment response in orthotopic glioblastoma mouse models in vivo (Li et al., 2018). Deng et al. found that miR-146a-5p expression is lower in the brains of rats after status epilepticus compared with control rats (Deng et al., 2019). Downregulation of miR146a-5p increased Abcb1 protein levels at the blood-brain barrier of rats with status epilepticus. Additionally, injecting miR-146a-5p into the hippocampus of rats with status epilepticus decreased Abcb1 mRNA and protein expression (Deng et al., 2019). However, these authors did not evaluate treatment response. To fully evaluate the impact of siRNA and miRNA approaches on drug brain delivery and efficacy, further studies in animal models are necessary.
B. Antibodies
Several anti-ABCB1 antibodies have been tested both in vitro and in vivo. The antibodies MRK16 and MRK17 inhibited Abcb1-mediated efflux in vitro and in animal tumor models and increased doxorubicin efficacy (Broxterman et al., 1988; Tsuruo et al., 1989; Mechetner and Roninson, 1992). MRK16 also increased efficacy of other anticancer drugs in ABCB1-overexpressing cells but had no effect on the parent cells (Hamada and Tsuruo, 1986; Pearson et al., 1991). Other antibodies, such as MRK17 and UIC2, had cytotoxic effects themselves, possibly through antibody-dependent cytotoxicity (Hamada and Tsuruo, 1986; Mechetner and Roninson, 1992). While these initial studies were promising, this strategy has not been further developed since the mid-1990s.
C. Nontransporter Substrates
Another approach has been to develop compounds that are pharmacologically active in the CNS but not substrates for blood-brain barrier efflux transporters. Among these nontransporter substrates are the epidermal growth factor receptor (EGFR) inhibitors buparlisib and avitinib (Heffron et al., 2016a,b; Sio et al., 2014; Wu et al., 2017; de Gooijer et al., 2018c; Wang et al., 2018). However, both drugs are associated with significant adverse effects (Borson-Chazot et al., 2018; Di Leo et al., 2018). The PI3K/Akt/mTOR inhibitor GDC-0084 had promising brain distribution and efficacy in preclinical models (Heffron, 2016; Salphati et al., 2016) but was ineffective in a recent phase 1 clinical trial in patients with recurrent glioblastoma (Wen et al., 2020). Thus, developing CNS drugs that are neither an ABCB1 nor an ABCG2 substrate is challenging.
D. ABCB1/ABCG2 Inhibitors
In the past decades, most research efforts in the drug efflux transporter field have been spent on developing inhibitors for ABCB1, ABCG2, or dual inhibitors for both transporters. Tsuruo et al. were the first to discover that the calcium channel blocker verapamil overcomes ABCB1-mediated resistance against vinca alkaloids (Tsuruo et al., 1981; Broxterman et al., 1988). However, due to its primary effect on the cardiovascular system, verapamil is associated with cardiovascular toxicity (Pennock et al., 1991). Similarly, cyclosporin A, another promising first-generation ABCB1 inhibitor, is associated with immunosuppression, nephrotoxicity, and hemodynamic adverse events (Mechetner and Roninson, 1992; Tsuji et al., 1993; Desrayaud et al., 1997). While both verapamil and cyclosporin A enhanced brain delivery of several drugs in animal brain cancer and epilepsy models (Tatsuta et al., 1992; Chikhale et al., 1995; Drion et al., 1996; Cox et al., 2001), responses were small due to their low ABCB1 binding affinity and competitive transporter inhibition that was easily overcome (Cisternino et al., 2001; Kemper et al., 2003; Thomas and Coley, 2003). Today, first-generation ABCB1 inhibitors are used as positron emission tomography tracers to test the efficacy of newly developed transporter inhibitors in humans (Hendrikse et al., 1999; Bankstahl et al., 2008; Bauer et al., 2017).
Second-generation ABCB1 inhibitors, like valspodar (PSC833), were developed with increased potency and reduced off-target effects and toxicity (Boesch et al., 1991; Friche et al., 1992; List, 1996; Tidefelt et al., 2000; Thomas and Coley, 2003). Data from in vivo studies in mice show that valspodar increased brain levels of several Abcb1 substrates without affecting their plasma pharmacokinetics (Drion et al., 1996; Desrayaud et al., 1997; Mayer et al., 1997; Cisternino et al., 2001; Kemper et al., 2003; Hubensack et al., 2008). Fellner and colleagues (2002) demonstrated that valspodar given in combination with paclitaxel reduced tumor volume by 90% in a mouse glioblastoma model and concluded that Abcb1 inhibition would potentially allow anticancer drugs to reach a tumor in the brain (Fellner et al., 2002). However, second-generation ABCB1 inhibitors inhibit several other ABC transporters due to their low selectivity and are highly bound to plasma proteins (Simon et al., 1998; Thomas and Coley, 2003). Moreover, many second-generation ABCB1 inhibitors are metabolized by CYP 450 enzymes, resulting in drug-drug interactions (Wandel et al., 1999; O’Byrne et al., 2001; Kemper et al., 2003).
Third-generation ABCB1 inhibitors such as tariquidar (XR9576) and elacridar (GF120918) are highly specific and lack CYP 450 enzyme interactions (Thomas and Coley, 2003). These inhibitors induce long-lasting, dose-dependent Abcb1 inhibition without causing adverse effects in mice (Stewart et al., 2000; Abraham et al., 2001, 2009; Cisternino et al., 2001; Ferry et al., 2001; Thomas et al., 2001; Dorner et al., 2009). Both elacridar and tariquidar increase the delivery of drugs into the brain, including anticancer drugs, opioids, and HIV protease inhibitors (Letrent et al., 1999; Edwards et al., 2002; Kemper et al., 2003; Walker et al., 2004; Pusztai et al., 2005; Choo et al., 2006; van Vliet et al., 2006; Fox and Bates, 2007; Bankstahl et al., 2008; Hubensack et al., 2008; Kurnik et al., 2008; Chen et al., 2009; Lagas et al., 2009, 2010; Agarwal et al., 2011; Hendrikx et al., 2014; Traxl et al., 2015; Mittapalli et al., 2016). Tariquidar and elacridar inhibit the ATPase activity of human ABCB1 and mouse Abcb1 and were initially thought to not interact with the substrate binding site (Martin et al., 1999; Cisternino et al., 2001; Mistry et al., 2001; Dorner et al., 2009). However, tariquidar has recently been shown to be both an ABCB1 and ABCG2 inhibitor and substrate (Kannan et al., 2011). Drawbacks of third-generation ABCB1 inhibitors include that they are poorly soluble and have highly variable pharmacokinetics depending on the route of administration (Ward and Azzarano, 2004; Sane et al., 2012; Matzneller et al., 2018). Today, third-generation ABCB1 inhibitors are also used in positron emission tomography imaging to determine brain uptake in the preclinical and clinical setting (Wagner et al., 2009; Bauer et al., 2010; Bauer et al., 2019; Traxl et al., 2019).
Current research efforts are focused on developing extended-release formulations to overcome solubility and pharmacokinetic issues associated with transporter inhibitors (Matzneller et al., 2018). The development of ABCB1/ABCG2 inhibitors is further complicated by species differences in transporter expression and activity levels. These differences become apparent when comparing rodents with humans but also when comparing humans with other higher species, including monkeys or dogs (Roulet et al., 2003; Haritova et al., 2008; Kamiie et al., 2008; Ito et al., 2011; Uchida et al., 2011). Thus, the “ideal” transporter inhibitor is yet to be found.
In summary, none of the described transporter-dependent strategies to overcome the blood-brain barrier in general or ABCB1/ABCG2 specifically were clinically successful, mostly due to low efficacy, high toxicity, and frequent adverse events, especially in combination with standard of care treatment. Therefore, new strategies to overcome ABCB1/ABCG2-mediated efflux at the blood-brain barrier and to improve the treatment of CNS disorders need to be pursued. One such strategy is to pharmacologically target molecules that directly or indirectly regulate the expression and activity of ABCB1 and ABCG2 at the blood-brain barrier (Hartz and Bauer, 2010). In the following section we summarize current knowledge of ABCB1 and ABCG2 regulation and discuss target molecules that could be used to modulate blood-brain barrier transporter expression and activity.
III. Regulation of ABCB1/ABCG2 at the Blood-Brain Barrier
Three main pathways regulate ABCB1 and ABCG2 at the blood-brain barrier: (1) nuclear receptors, (2) inflammatory and oxidative stress signaling, and (3) receptor tyrosine kinase/growth factor signaling. In the following sections we describe these pathways in detail and highlight their clinical significance where appropriate. We also summarize other signaling mechanisms involved in ABCB1/ABCG2 regulation and briefly describe their clinical relevance.
A. Nuclear Receptors
Nuclear receptors are critical transcription factors. By binding directly to DNA and inducing or inhibiting the transcription of target genes, nuclear receptors regulate important cellular functions in development, homeostasis, and metabolism (Evans, 1988; Olefsky, 2001). Ligands of nuclear receptors are classified as hormones, vitamins, or xenobiotic endocrine disruptors (Overington et al., 2006). After activation by their respective ligands, nuclear receptors form homo- or heterodimers with heat shock protein or retinoid X receptor (RXR) that bind to specific response elements in the promotor regions of their target genes (Klinge et al., 1997; Linja et al., 2004; Amoutzias et al., 2007). For ABCB1 and ABCG2, the response elements for several nuclear receptors are located in their respective proximal promotor (Nakanishi and Ross, 2012). Therefore, activation of nuclear receptors regulates the transcription of ABCB1 and ABCG2 changing transporter expression and activity at the blood-brain barrier but also in other barrier organs, such as placenta, testes, intestine, liver, and kidney (Rigalli et al., 2019b). With a few exceptions, this process involves transcription and translation and is, therefore, relatively slow (Miller and Cannon, 2014).
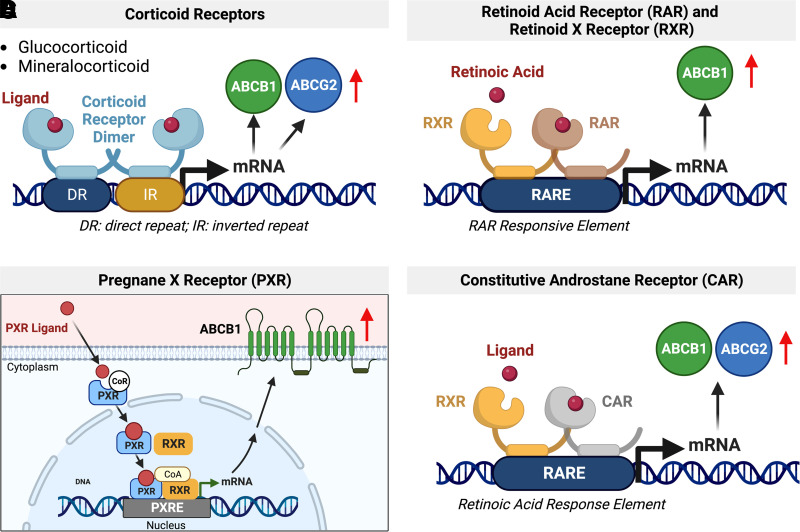
1. Corticoid Receptors
In 1992, Loffreda et al. were the first to detect nuclear receptors at the blood-brain barrier. (Loffreda et al., 1992). These researchers found mineralocorticoid receptor (MR) and glucocorticoid receptor (GR) mRNA in isolated rat brain capillaries. Stimulating mineralocorticoid and GR with dexamethasone increases Abcb1 and Abcg2 expression in vitro in primary rat brain capillary endothelial cells and in vivo at the mouse blood-brain barrier (Narang et al., 2008; Petropoulos et al., 2010; Chan et al., 2013; Miller, 2015; Yasuda et al., 2015; Chaves et al., 2017). This effect was dose-dependent and reversible and could be inhibited with GR antagonists (Narang et al., 2008).
In general, corticoid receptors are activated by endogenous hormones such as glucocorticoids as well as exogenous xenobiotics (Fig. 5A). Upon activation, the receptor translocates from the cytoplasm to the nucleus, where it binds to the response element of its target genes, resulting in transcription (Miller, 2010). Corticoid signaling is critical during blood-brain barrier development. Activation of maternal GR during development induces early Abcb1 expression in brain capillaries isolated from Guinea pig fetuses at different developmental stages (Iqbal et al., 2016).
Fig. 5.
Regulation of ABCB1 and ABCG2 via corticoid receptors RAR/RXR, PXR, and CAR. (A) Upon ligand binding, the corticoid receptor dimer binds to the direct repeat and inverted repeat region of the target gene to increase ABCB1 and ABCG2 mRNA expression levels. (B) Upon ligand binding, RAR and RXR form a heterodimer that binds and activates the RAR response element (RARE), which increases ABCB1 expression. (C) A PXR ligand binds to inactivated PXR in the cytoplasm. Ligand binding then triggers conformational change of PXR during which the corepressor dissociates. Activated PXR translocates into the nucleus and heterodimerizes with retinoic X receptor α (RXRα). The complex PXR-RXRα together with its coactivators binds to the xenobiotic response element in the promotor region on ABCB1. This results in increased transcription of the gene and protein expression. (D) CAR forms a heterodimer with RXR that binds to RARE, which leads to an increase in ABCB1 and ABCG2 levels. Created with BioRender.com.
Glucocorticoids are often used to prevent edema in patients with brain tumors. However, glucocorticoid activation of GR upregulates ABCB1/ABCG2 at the blood-brain barrier. Increased efflux transporter expression and activity then further restricts brain uptake of anticancer drugs, limiting their efficacy in the treatment of brain tumors (Petropoulos et al., 2010).
2. Retinoid Acid Receptor and Retinoid X Receptor
In 1997, El Hafny et al. showed that retinoic acid increases Abcb1 expression and ABCB1 activity in a rat brain capillary endothelial cell line in a concentration-dependent manner. Retinoic acid binds to retinoid acid receptor (RAR) and induces the formation of heterodimers with RXR (Fig. 5B). The RAR-RXR heterodimer activates the retinoic acid response element in the Abcb1 promotor resulting in transporter upregulation (El Hafny et al., 1997). A similar process involves several other RAR ligands (Xu et al., 2005; Sarkadi et al., 2006; Chan et al., 2013).
3. Pregnane X Receptor
In 1998, Kliewer and colleagues discovered pregnane X receptor (PXR) (Kliewer et al., 1998). PXR functions as a xenobiotic sensor, and its activation increases levels of proteins involved in detoxification and xenobiotic clearance (Kliewer et al., 1998). Upon activation, PXR forms heterodimers with RXR (Bauer et al., 2005) or other orphan nuclear receptors (Xu et al., 2005) and binds to its response element in the promotor region of its target genes (Fig. 5C; Song et al., 2004; Miller et al., 2008). In 2001, Synold et al. (2001) showed that PXR regulates ABCB1 protein levels. We found that PXR is expressed in isolated rat brain capillaries and first reported that PXR activation upregulates rodent Abcb1 protein levels and transport activity at the blood-brain barrier (Bauer et al., 2004, 2006; Hartz and Bauer, 2010). Other groups later confirmed our findings and also showed that PXR activation increases Abcb1 and Abcg2 protein levels and activity at the blood-brain barrier in rodents (Chan et al., 2011; Yasuda et al., 2015; Chaves et al., 2017).
Many drugs that are ABCB1 and ABCG2 substrates increase their own efflux at the blood-brain barrier through PXR-mediated upregulation. For example, antiretroviral drugs, antiseizure drugs, and several other drugs, including rifampicin and hyperforin, are PXR agonists. These drugs induce ABCB1/ABCG2 expression at the blood-brain barrier by activating PXR and, thus, restrict their own brain uptake and efficacy (Chan et al., 2011; Potschka, 2012; Chan et al., 2013).
4. Constitutive Androstane Receptor
Upon activation, constitutive androstane receptor (CAR) forms heterodimers with RXR, which bind to the retinoic acid response element in the promotor sequence of target genes (Xu et al., 2005; Sarkadi et al., 2006; Fig. 5D). Both Abcb1 and Abcg2 are among those target genes. In this regard, xenobiotics and drugs, such as phenobarbital, increase the expression of both Abcb1 and Abcg2 and their accompanying proteins’ activity in isolated brain capillaries from mice and rats (Wang et al., 2010; Yasuda et al., 2015) This also occurs in hCMEC/D3 cells, a human brain microvascular endothelial cell line (Chan et al., 2011). ABCB1 mRNA and ABCB1 protein levels increased after exposing hCMEC/D3 cells to the CAR ligand 6-(4-chlorophenyl)imidazo[2,1-b][1,3]thiazole-5-carbaldehyde O-(3,4-dichlorobenzyl)oxime. This upregulation was inhibited by coexposing the cells to 6-(4-chlorophenyl)imidazo[2,1-b][1,3]thiazole-5-carbaldehyde O-(3,4-dichlorobenzyl)oxime and the CAR inhibitor meclizine (Chan et al., 2011). In a follow-up study, Chan et al. demonstrated that the antiretroviral drugs abacavir, efavirenz, and nevirapine are CAR ligands and upregulate ABCB1 in hCMEC/D3 cells (Chan et al., 2013).
Acetaminophen is a common over-the-counter pain and fever-relieving agent. High doses of acetaminophen activate CAR, which increases Abcb1 mRNA levels and accompanying protein activity in isolated rat brain capillaries (Slosky et al., 2013). In addition, five FDA-approved drugs were identified that facilitate CAR transport into the nucleus, including the antihypertensive drug telmisartan (Lynch et al., 2013). These drugs could potentially affect transporters at the blood-brain barrier.
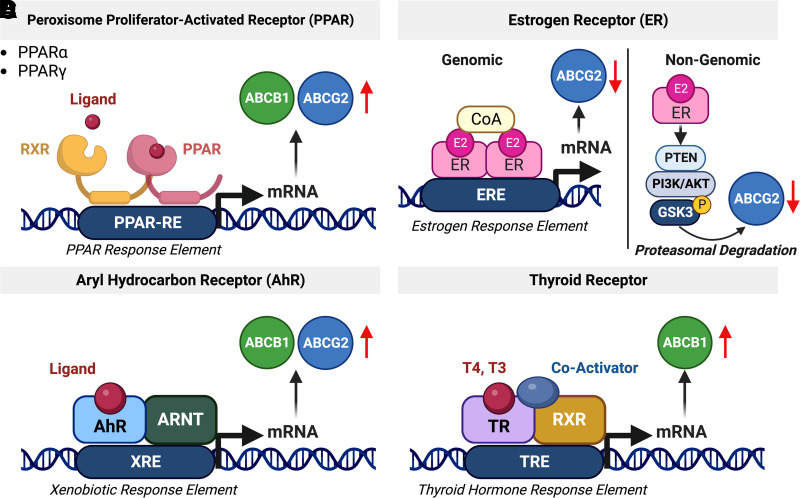
5. Peroxisome Proliferator-Activated Receptor
Three types of peroxisome proliferator-activated receptors (PPARα, PPARβ, PPARγ) have been identified, but only PPARα and PPARγ are involved in ABC transporter regulation (Xu et al., 2005). Clofibrate, linoleic acid, and other PPARα agonists increase expression levels of Abcb1 and Abcg2 mRNAs and accompanying proteins as well as transporter activity in isolated mouse brain capillaries and in hCMEC/D3 cells (Hoque et al., 2012; Chan et al., 2013; Hoque et al., 2015; More et al., 2017). After heterodimerizing with RXR, the PPARγ/RXR complex binds to the PPAR response element upstream of the Abcg2 promotor, which induces drug resistance in cancer cells (Nakanishi and Ross, 2012; Fig. 6A). PPARγ also regulates ABCB1 and ABCG2 in human glioblastoma cell lines in vitro (Szatmari et al., 2006; Han et al., 2015). Cannon et al. (2020) showed that ammonium 2,3,3,3-tetrafluoro-2-(heptafluoropropoxy) propanoate, a chemical precursor used in the production of Teflon, rapidly inhibits Abcb1 transport activity in isolated rat brain capillaries and that this inhibition is dependent on PPARγ activity.
Fig. 6.
Regulation of ABCB1 and ABCG2 via the nuclear receptors PPAR, ER, AhR, and thyroid hormone receptor. (A) PPAR forms a heterodimer with RXR that binds to and activates the PPAR response element, which leads to increased ABCB1 and ABCG2 levels. (B) Genomic regulation of ABCG2 is driven by the estrogen receptor that binds to the estrogen response element in the ABCG2 promotor region. In addition, ABCG2 is also regulated via rapid, nongenomic ER signaling involving PTEN/PI3K/Akt/GSK3. (C) AhR translocates into the nucleus and dimerizes with the aryl hydrocarbon receptor nuclear translocator resulting in the regulation of its target genes, including ABCB1 and ABCG2. (D) The thyroid receptor forms a complex with RXR and coactivators. This complex binds to the thyroid hormone response element and activates transcription of ABCB1. Created with BioRender.com.
Fibrates, a class of drugs used treat hypercholesterolemia, are PPARα agonists. Clofibrate upregulates Abcb1/Abcg2 mRNA and associated protein levels as well as efflux transporter activity in isolated rat brain capillaries and in hCMEC/D3 cells in vitro (Hoque et al., 2015; More et al., 2017). The thiazolidinediones are a class PPARγ agonists approved for treatment of type II diabetes and include pioglitazone, rosiglitazone, and lobeglitazone. Of these, pioglitazone increases docosahexaenoic acid trafficking into the brain (Low et al., 2020), crosses the blood-brain barrier, and reduces tumor growth in a human xenograft model (Grommes et al., 2013). Rosiglitazone, on the other hand, appears to reinforce the integrity of the blood-brain barrier (Sivandzade and Cucullo, 2019; Zhao et al., 2019).
6. Estrogen Receptor
Estrogen receptors (ERs) are hormone-activated nuclear receptors (ERα and ERβ) or G-protein coupled membrane receptors (GPR30, ER-X, and Gq-mER). Estrogen binding to these receptors triggers either a rapid (minutes) response through nongenomic pathways or a slow response (hours-days) through genomic signaling pathways (Fig. 6B).
In 2002, Imai et al. (2002) showed that 17β-estradiol enhances the cytotoxicity of several anticancer drugs in vitro by decreasing expression of ABCG2 in human leukemia cells (Imai et al., 2002). The estrogen response element was detected in the ABCG2 promotor region (Ee et al., 2004). In addition to the genomic regulation of ABCG2, Imai et al. (2005) also discovered that 17β-estradiol activation of ERα increases topotecan cytotoxicity via a nongenomic pathway through posttranscriptional regulation of ABCG2 in human breast cancer cells (Imai et al., 2005). In 2010, we showed that estrogen signaling regulates Abcg2 at the blood-brain barrier (Hartz et al., 2010a,b). We found that 17β-estradiol (E2) decreased Abcg2 activity within minutes and this effect did not involve transcription, translation, or proteasomal degradation, indicating a nongenomic mechanism (Hartz et al., 2010b). Experiments with ERα and ERβ knockout mice showed that rapid loss of Abcg2 activity was due to E2 signaling through both receptors. In a follow-up study we demonstrated that 6-hour E2 exposure of isolated brain capillaries resulted in a loss of Abcg2 activity that was accompanied by reduced Abcg2 protein expression levels. Altogether, we found that the signaling process responsible for these effects in isolated rat brain capillaries involved E2 signaling through ERβ, which inhibits the PTEN/PI3K/Akt/GSK3 pathway leading to Abcg2 proteasomal degradation (Hartz et al., 2010b). Thus, E2 acting through either ER can signal an initial loss of Abcg2 transport activity, but only signaling through ERβ mediates reduced ABCG2 protein expression and activity levels.
Another estrogenic compound, the synthetic xenoestrogen bisphenol A, is a common component of plastic products that also activates ERs. Bisphenol A decreased Abcg2 protein and activity levels in isolated rat brain capillaries via an ERα-dependent genomic pathway (Nickel and Mahringer, 2014). Specifically, upon bisphenol A-mediated activation, ERα binds to the estrogen response element in the Abcg2 promotor where it acts as a negative regulator resulting in a slow decrease in Abcg2 expression and activity levels in isolated mouse brain capillaries (Zhang et al., 2010; Shin et al., 2018). Phytoestrogens from soybeans also induce ABCG2 expression and protein activity through a genomic signaling pathway in breast cancer cell lines (Rigalli et al., 2019a,b). However, this particular pathway has not yet been identified at the blood-brain barrier. A similar ERβ-dependent, nongenomic pathway for ABCB1 that is activated by androstanes also exists (Zuloaga et al., 2012). Further information on ER-dependent, nongenomic ABCB1 and ABCG2 regulation is in Section C.3.
7. Aryl Hydrocarbon Receptor
The aryl hydrocarbon receptor (AhR) does not belong to the family of 48 known human nuclear receptors but is a member of the basic Helix-Loop-Helix-Period/ARNT/Single-minded family of dimerizing transcription factors. Similar to xenobiotic-sensing nuclear receptors, after binding and activation by aromatic aryl hydrocarbons, from which its name derives, AhR translocates into the nucleus and dimerizes with the aryl hydrocarbon receptor nuclear translocator (Xu et al., 2005; Fig. 6C) resulting in the regulation of its target genes, including transporters. AhR is highly expressed in hCMEC/D3 cells (Dauchy et al., 2008, 2009) and increases Abcb1 and Abcg2 mRNA expression levels and activity levels of the respective proteins in several tissues, including the blood-brain barrier of mice and rats (Klaassen and Slitt, 2005; Campos et al., 2012; Nakanishi and Ross, 2012; Chan et al., 2013; Le Vee et al., 2015; Chaves et al., 2017). AhR inhibition with ethanol decreases Abcb1 and Abcg2 mRNA expression and associated protein levels at the rat blood-brain barrier (Hammad et al., 2019), but other AhR signaling in brain endothelial cells is unknown.
8. Thyroid Receptors
Thyroid hormone signaling regulates processes including growth, development, and metabolism. The main thyroid hormones are thyroxin (T4) and 3,3,3′-triiodo-L-thyronine, which enter the brain by crossing the blood-brain barrier. The role of thyroid hormones in the regulation of ABCB1 and ABCG2 at the blood-brain barrier, however, is not well investigated and limited to few studies. In this regard, Saljé et al. (2012) treated rats with T4 (9 μg/kg for 9 days) and showed upregulation of Abcb1 protein expression in brain and liver tissue (Fig. 6D). Kassem and colleagues (2007) found that ABCB1 regulates T4 levels in the CSF by facilitating T4 transport between the choroid plexus, the brain, and the CSF. However, thyroid regulation of blood-brain barrier ABCB1 and ABCG2 remains largely unexplored.
9. Other Nuclear Receptors
Several other nuclear receptors such as Farnesoid X receptor (FXR), liver X receptor (LXR), and vitamin D receptor (VDR) have been implicated in transporter regulation at the blood-brain barrier. Thus far, however, they have not been studied in detail, and little is known about their role in transporter regulation at the blood-brain barrier. For example, the FXR ligand chenodeoxycholic acid upregulates the efflux transporter Abcc2 in isolated rat brain capillaries, indicating that FXR could be involved in the regulation of its target genes at the blood-brain barrier (Bauer et al., 2008a). Further, the VDR regulates protein expression levels of both ABCB1 and ABCG2 (Sarkadi et al., 2006; Chan et al., 2013; Chaves et al., 2017). In contrast, the LXR regulates Abca1 mRNA levels in an immortalized rat brain capillary endothelial cell line (TR-BBB13) but has no effect on Abcg2 mRNA levels (Akanuma et al., 2008). More studies are needed to understand the role FXR, LXR, and VDR play in transporter regulation at the blood-brain barrier.
B. Inflammatory and Oxidative Stress Signaling
1. Inflammation
The brain is not immune-privileged, as originally anticipated, and immune cells do cross the blood-brain barrier and enter the brain (Engelhardt et al., 2017). The barrier itself contributes to inflammation, and brain capillary endothelial cells respond to inflammatory stimuli and release cytokines. Neuroinflammation is common among all CNS diseases including epilepsy (Choi and Koh, 2008; Alyu and Dikmen, 2017; Rana and Musto, 2018), brain tumors (Jiang et al., 2017; Couto et al., 2019), and Alzheimer’s disease (Akiyama et al., 2000; Wyss-Coray and Rogers, 2012; Mosher and Wyss-Coray, 2014; Lai et al., 2017). Neuroinflammatory signaling is driven by cytokines and oxidative stress, both of which are implicated in the regulation of blood-brain barrier transporters through activating different signaling pathways. In the following sections, we discuss four key regulators of ABC transporters at the blood-brain barrier: (1) nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), (2) tumor necrosis factor α (TNFα), (3) prostaglandins, and (4) cytokines.
a. Nuclear factor kappa-light-chain-enhancer of activated B cells
NF-κB is a rapidly acting, primary transcription factor that is constitutively expressed in the cytoplasm of all cells (Jacobs and Harrison, 1998). In its inactive state, NF-κB is bound to the inhibitor of κB, which prevents translocation into the nucleus (Jacobs and Harrison, 1998). Upon activation by infectious and inflammatory stimuli or through cell surface receptors, inhibitor of κB is ubiquitinated and degraded, which releases NF-κB, allowing translocation into the nucleus. There, NF-κB binds to the promoters of its target genes and stimulates transcription (Deptala et al., 1998; Gilmore, 2006). Among NF-κB target genes are proinflammatory cytokines as well as markers of cell survival and proliferation (Renard et al., 1997; Basu et al., 1998; Deptala et al., 1998; Chandel et al., 2000; Qin et al., 2005; Fitzgerald et al., 2007).
In 2005, Dixit et al. showed that interferon γ stimulates ABCB1 expression and ABCB1 activity in human intestinal cells in vitro via NO synthase and NF-κB (Dixit et al., 2005). Pan et al. discovered a similar pathway showing that lipopolysaccharide-induced inflammation increases Abcb1 mRNA expression levels at the blood-brain barrier of wild-type but not NF-κB knockout mice, indicating an important role for NF-κB in Abcb1 regulation (Pan et al., 2010). Since then, several groups have shown that NF-κB activation by inflammatory stimuli or cellular stress increases the expression of Abcb1 and associated protein activity at the rodent blood-brain barrier in vivo and in vitro (Bauer et al., 2007; Pan et al., 2010; Ronaldson et al., 2010; Zhang et al., 2014). In contrast, stimuli that inhibit NF-κB signaling decrease Abcb1 and Abcg2 mRNA expression and associated protein activity levels at the blood-brain barrier. For example, in cultured rat microvessel endothelial cells in vitro, insulin inhibits NF-κB through the insulin receptor, which decreases both Abcb1 and Abcg2 mRNA expression and protein activity levels (Liu et al., 2009; Liu et al., 2011). Additionally, in vivo experiments in diabetic rats showed the opposite effect: increased ABC transporter expression and activity at the blood-brain barrier due to decreased insulin plasma levels (Maeng et al., 2007). Thus, NF-κB is a key transcription factor that regulates both Abcb1 and Abcg2 at the blood-brain barrier.
Multiple drug candidates for repurposing to regulate NF-κB include clemastine, topotecan, bortezomib, and dexamethasone (Roberti et al., 2022) and are known to affect ABC transporters (Hartz et al., 2016). Other drugs, like methamphetamine, weaken the blood-brain barrier by inhibiting NF-κB (Coelho-Santos et al., 2015), and another drug of abuse, mephedrone, activates NF-κB and increases blood-brain barrier permeability (Buzhdygan et al., 2021). At this point, it is unclear if these compounds affect ABC transporters.
b. Wnt/β-catenin signaling
Wnt/β-catenin signaling is part of several inflammatory signaling cascades. During canonical Wnt signaling, β-catenin is degraded by a so-called “destruction complex” formed by GSK3β, APC, and axin. Upon activation, Wnt binds to the Frizzled receptor, which recruits axin and inhibits GSK3Β. Consequently, the destruction complex cannot assemble, and β-catenin accumulates in the cytosol. After translocation into the nucleus, β-catenin acts as transcription factor and induces transcription of genes involved in cell proliferation and survival (Atlasi et al., 2014). At the blood-brain barrier, β-catenin regulates the transcription of Abcb1 and Abcg2. β-catenin leads to increased transporter expression and activity levels at the blood-brain barrier in vitro in hCMEC/D3 cells and in mice and rats in vivo (Lim et al., 2008, 2009; Harati et al., 2013; Paolinelli et al., 2013; Strazielle and Ghersi-Egea, 2015; Laksitorini et al., 2019).
c. Tumor necrosis factor alpha
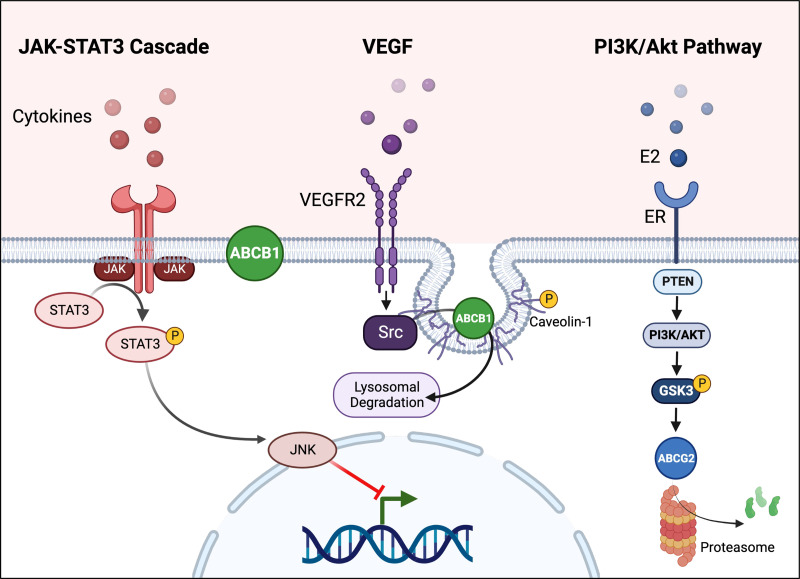
TNFα is commonly involved in CNS inflammation (Probert et al., 1997; Fresegna et al., 2020; Raffaele et al., 2020). In 1992, Sharief and Thompson described increased TNFα levels in the cerebrospinal fluid from patients with multiple sclerosis that correlated with blood-brain barrier dysfunction (Sharief and Thompson, 1992). Maternal infections in guinea pigs led to TNFα release, which decreased Abcb1 function at the blood-brain barrier of the fetus, consequently rendering the fetal brain vulnerable to potentially teratogenic compounds (Iqbal et al., 2012, 2016). At later stages of development, TNFα signaling decreases Abcg2 activity at the rat blood-brain barrier (Harati et al., 2012). In adulthood, TNFα has differential effects on Abcb1 and Abcg2 expression levels and associated protein and activity levels, depending on exposure time and concentration. For example, we showed that acute short-term exposure of isolated rat brain capillaries to nanomolar concentrations of TNFα activated the TNF receptor 1, which activated endothelin converting enzyme (Fig. 7; Hartz et al., 2006). Endothelin converting enzyme activation, in turn, leads to the production of endothelin 1, which signals through the endothelin receptor B to activate the inducible nitric oxide synthase. NO stimulates protein kinase Cβ1 (PKCβ1) and sphingosine release from the brain capillary membrane (Pilorget et al., 2007; Rigor et al., 2010). Sphingosine is phosphorylated by sphingosine kinase and binds to the sphingosine-1-phosphate receptor decreasing Abcb1 and Abcg2 mRNA levels and associated protein activity levels at the blood-brain barrier in vitro and in vivo (Hartz et al., 2004, 2006; Evseenko et al., 2007; Pilorget et al., 2007; von Wedel-Parlow et al., 2009; Hawkins et al., 2010; Heemskerk et al., 2010; Cannon et al., 2012; Harati et al., 2013). In addition, the PKCβ1 activator 12-deoxyphorbol-13-phenylacetate-20-acetate significantly increases brain uptake of the ABCB1 substrate [3H]-verapamil in rats, indicating that downregulating Abcb1 expression and protein activity enhances brain drug delivery (Rigor et al., 2010).
Fig. 7.
Inflammatory and oxidative stress signaling. (A) NFkB, a primary transcription factor, is activated by infectious and inflammatory stimuli. NF-κB binds to the promoters of its target genes and stimulates transcription. Both ABCB1 and ABCG2 are regulated via NFkB signaling at the blood-brain barrier. (B) Upon activation, Wnt binds to the Frizzled receptor, which recruits axin and inhibits GSK3Β. Consequently, the destruction complex cannot assemble, and β-catenin accumulates in the cytosol. After translocation into the nucleus, β-catenin acts as transcription factor and induces transcription of both ABCB1 and ABCG2. (C) In isolated brain capillaries, TNFα signals through TNF receptor 1 activating the endothelin converting enzyme, which, in turn, leads to the production of endothelin 1, which signals through the endothelin receptor B to activate the inducible nitric oxide synthase. NO stimulates protein kinase C, which leads to the activation of NF-κB, which upregulates ABCB1 protein expression and transport activity. (D) Seizure-induced glutamate release activates NMDAR- cytosolic phospholipase A2-COX-2 signaling that leads to the generation of prostaglandin E2 (PGE2) by the microsomal prostaglandin synthase. PGE2 activates the prostaglandin EP1 receptor, which via NF-κB activation ultimately leads to increased ABC transporter expression and activity levels at the blood-brain barrier. Created with BioRender.com.
Long-term exposure (6 hours) of isolated rat brain capillaries to TNFα leads to endothelin 1 release, which in turn activates both endothelin receptor A receptors and endothelin receptor B receptors, stimulating NO release and activation of PKCβ2. This signaling pathways results in downstream activation of NF-κB, which translocates to the nucleus and induces transcription and translation of Abcb1 at the blood-brain barrier (Rigor et al., 2010; Bauer et al., 2007; Mayati et al., 2017).
d. Prostaglandins
In 1995, Tishler et al. analyzed resected brain tissue from patients with medically intractable (refractory or drug-resistant) epilepsy and found increased ABCB1 mRNA levels (Tishler et al., 1995). This led to the transporter hypothesis of refractory epilepsy, which states that ABCB1 overexpression at the blood-brain barrier in epilepsy restricts antiseizure brain drug uptake, thus, leading to antiseizure drug resistance (Tang et al., 2017). Much research has been done to understand the role of blood-brain barrier ABC transporters in epilepsy and lead to the partial unraveling of signaling pathways that control these transporters after seizures.
Release of glutamate in the CNS of patients with epilepsy is linked to seizure activity and subsequent CNS damage (Ronne-Engstrom et al., 1992). Extracellular glutamate upregulates Abcb1 mRNA and associated protein levels in rat brain capillary endothelial cells in vitro and proposed that glutamate activates the N-methyl-D-aspartate receptor (NMDAR) and triggers a signaling cascade that increases Abcb1 expression at the blood-brain barrier (Zhu and Liu, 2004). At the same time, efflux of anticonvulsive drugs by ABC transporters at the blood-brain barrier was considered as one of the main causes of refractory epilepsy (van Vliet et al., 2005).
Since then, we and others have identified several signaling steps through which seizures upregulate ABC transporters at the blood-brain barrier. Specifically, seizure-induced glutamate release activates the NMDAR in brain capillaries (Hartz et al., 2019; Mohamed et al., 2019). NMDAR activation stimulates cytosolic phospholipase A2 to cleave arachidonic acid from triglycerides in the cell membrane (Hartz et al., 2019). Arachidonic acid is converted by cyclooxygenase 2 to prostaglandin H2, which is then converted to prostaglandin E2 by microsomal prostaglandin synthase 1, a process first described in isolated rat brain capillaries (Baba et al., 1985; Bauer et al., 2008b; Zibell et al., 2009; Schlichtiger et al., 2010; van Vliet et al., 2010; Soldner et al., 2019). Prostaglandin E2 activates the prostaglandin EP1 receptor in brain capillary endothelial cells and via NF-κB activation ultimately leads to increased ABC transporter expression and activity levels (Pekcec et al., 2009; Soldner et al., 2019). Targeting signaling steps in this pathway has the potential to prevent ABCB1 upregulation at the blood-brain barrier and thus overcome drug resistance in patients with epilepsy (Bauer et al., 2008b; Pekcec et al., 2009; Zibell et al., 2009; Schlichtiger et al., 2010; van Vliet et al., 2010; Hartz et al., 2019; Mohamed et al., 2019; Soldner et al., 2019). This pathway is active at the blood-brain barrier of patients with epilepsy and ALS (Avemary et al., 2013; Mohamed et al., 2019). In addition to epileptic seizures, morphine withdrawal also activates this pathway and upregulates Abcb1 expression and associated protein activity levels at the rat blood-brain barrier (Yousif et al., 2012; Chaves et al., 2017). Other cell membrane lipids like ceramide 1-phosphate and other sphingolipids also stimulate this pathway and increase Abcb1 activity at the blood-brain barrier (Mesev et al., 2017). Taken together, prostaglandin signaling is a key pathway that regulates ABCB1 and ABCG2 and associated proteins at the blood-brain barrier.
Multiple drugs operate through modifying prostaglandin levels or activity. These include bimatoprost (glaucoma treatment), carboprost (induce uterine contractions), dinoprost (cervical dilation during labor), misoprostol (abortifacient, gastric ulcer treatment), and latanoprost (glaucoma treatment). The antibiotic cefmetazole can inhibit prostaglandin transport out of the brain across the blood-brain barrier (Akanuma et al., 2011). However, focused research on modifying prostaglandin activity to regulate blood-brain barrier activity is currently lacking.
e. Other cytokines
Several other cytokines are involved in transporter regulation at the blood-brain barrier but have not been studied extensively. For example, interleukin (IL) 1β decreases expression and activity levels of both Abcb1 and Abcg2 and associated proteins (Evseenko et al., 2007; Ronaldson et al., 2008; Robey et al., 2009; Ashraf et al., 2011). Another example includes members of the IL-6 family, including leukemia inhibitory factor and ciliary neurotrophic factor that stimulate NF-κB signaling and increase Abcb1 activity at the blood-brain barrier (Monville et al., 2002; Evseenko et al., 2007; Ashraf et al., 2011). ABCG2 expression levels, on the other hand, are decreased by IL-6 (Poller et al., 2010). While the underlying mechanisms of how cytokines affect transporters are not well understood, some data indicate that cytokine signaling alters caveolae in the brain capillary endothelium, which moves transporters from intracellular storage vesicles into the luminal membrane (Tome et al., 2016).
2. Oxidative Stress
Oxidative stress occurs in many CNS disorders (Qosa et al., 2016a; Singh et al., 2019; Mendiola et al., 2020). Reactive oxygen species (ROS) generated during oxidative stress damage cells lead to cytokine release, which in turn affects cellular processes including transport (Mendiola et al., 2020). In 2002, Felix et al. first demonstrated that hypoxia-induced ROS increase Abcb1 mRNA and protein expression levels in rat brain capillary endothelial cells in vitro (Felix and Barrand, 2002; Neuhaus et al., 2014). Following this initial discovery, other groups also unraveled signaling pathways through which oxidative stress increases Abcb1 expression and associated protein activity levels at the blood-brain barrier. For example, ROS stimulate the extracellular signal-regulated kinase (ERK) signaling cascade that includes protein kinase C, c-Jun, and Akt (Bauer et al., 2005; Miller et al., 2008). The key relay in this pathway is NF-κB that, once activated, increases Abcb1 protein levels (Bauer et al., 2005; Miller et al., 2008; Qosa et al., 2016a; Grewal et al., 2017). In contrast, ROS-mediated ERK1 and ERK2 stimulation in mouse brain capillary endothelial cells in vitro induces Abcg2 downregulation (Neuhaus et al., 2014; Grewal et al., 2017). ROS also oxidizes Kelch-like ECH-associated protein 1, which releases nuclear factor E2-related factor 2 (Nrf2) allowing its translocation into the nucleus. There, Nrf2 binds to the antioxidant response element in the promoter region of its target genes resulting in increased transcription. These target genes code for detoxification enzymes, antioxidant proteins, proteins involved in xenobiotic metabolism, and efflux transporters including Abcb1 (Klaassen and Slitt, 2005; Maher et al., 2005; Copple, 2010, 2012; Aleksunes and Klaassen, 2012). Nrf2 also activates p53 and stimulates the p38 mitogen-activated protein kinase (MAPK) cascade that activates NF-κB, which increases Abcb1 expression and associated protein levels in isolated rat brain capillaries (Wang et al., 2014; Grewal et al., 2017). On the other hand, oxidative stress activates pathways that decrease ABCB1 expression and associated protein levels at the blood-brain barrier. For example, oxidative stress activates Abl and Src kinases that phosphorylate caveolin-1, which triggers internalization of both caveolin-1 and colocalized ABCB1 reducing ABCB1 expression and protein activity levels (Hoshi et al., 2019).
If ROS affects transport, one might expect antioxidants like N-acetylcysteine to reverse this. Indeed, exposing cultured rat capillary endothelial cells or isolated rat brain capillaries to N-acetylcysteine reversed the ROS effects on the expression and activity of Abcb1 and Abcg2 and associated proteins (Zhu and Liu, 2004; Li et al., 2016; Zhou et al., 2019), which might provide a future clinical avenue.
3. Clinical Relevance of Inflammatory and Oxidative Stress Signaling
Our research shows that, in epilepsy, seizure-induced glutamate release activates a prostaglandin-dependent signaling pathway leading to Abcb1 and Abcg2 upregulation at the blood-brain barrier (Bauer et al., 2008b; Pekcec et al., 2009; Zibell et al., 2009; Hartz et al., 2017). In addition, proinflammatory cytokines are increased in the blood and brain of patients with epilepsy indicating neuroinflammation. Cytokines that are upregulated in the brain of patients with epilepsy include IL1α, IL1β, IL6, and TNFα, all of which regulate blood-brain barrier ABCB1 and ABCG2 (Arend et al., 2018; de Vries et al., 2016; Gao et al., 2016; Mercado-Gomez et al., 2018; Kothur et al., 2019). Moreover, our and other data indicate that inflammation contributes to elevated protein levels and functional activity of ABCB1/Abcb1 and ABCG2/Abcg2 and is also involved in epileptogenesis (Hartz et al., 2004; Bauer et al., 2007, 2008a; Alyu and Dikmen, 2017; Rana and Musto, 2018). Neuroinflammation exacerbates seizures and increased expression levels of drug efflux transporters in the brain endothelium could hinder antiseizure drugs from entering the brain (Cox et al., 2001; van Vliet et al., 2006). Uncontrolled seizures in patients cause more neuroinflammation driving a vicious cycle of disease progression and drug resistance. In addition to epilepsy, neuroinflammation is part of many other CNS disorders. In glioblastoma, for example, tumor cells stimulate microglia to release proinflammatory cytokines such as IL1β, IL6, TNFα, and prostaglandins (Maruno et al., 1997; Matsuo et al., 2001; Samaras et al., 2007; Schwartzbaum et al., 2007, 2017; Wang et al., 2009; Jiang et al., 2017; Couto et al., 2019; Gao et al., 2019; Ham et al., 2019). In this context, microglia help form a proinflammatory tumor microenvironment that is conducive to gliomagenesis and tumor growth and progression including tumor migration and invasion (Maruno et al., 1997; Matsuo et al., 2001; Samaras et al., 2007; Wang et al., 2009; Desmarais et al., 2015; Lepore et al., 2018). In addition, cytokines transcriptionally upregulate ABCB1/ABCG2 and enhance translation of their associated proteins at the blood-brain barrier, which restricts anticancer drug delivery into the brain. Blocking cytokine signaling prevented tumor growth and invasion, which improved survival in glioblastoma animal models (Desmarais et al., 2015; Kast et al., 2017; Lamano et al., 2019). Thus, such treatment approaches could help prevent or reverse ABCB1/ABCG2 overexpression and potentially improve drug delivery and efficacy in patients.
Brain levels of proinflammatory cytokines are also increased in neurodegenerative diseases, including Alzheimer’s disease (Akiyama et al., 2000; Cai et al., 2014). Amyloid β (Aβ), a neurotoxic peptide and one hallmark of Alzheimer’s disease, activates microglia, which in turn generate and release IL1, IL6, TNFα, and prostaglandins into the brain parenchyma. These inflammatory mediators, in turn, activate NF-κB signaling, which leads to more inflammation and even higher Aβ levels in Alzheimer’s disease patients, ultimately leading to neuronal death (Bauer et al., 1991; Buxbaum et al., 1992; Strauss et al., 1992; Mackenzie et al., 1995; Pasinetti and Aisen, 1998; Ringheim et al., 1998; Ho et al., 1999; Kitamura et al., 1999; Meda et al., 1999; Tarkowski et al., 1999; Yates et al., 2000; Combs et al., 2001; Haas et al., 2002; McGeer and McGeer, 2003; Sochocka et al., 2013; Cai et al., 2014; Mosher and Wyss-Coray, 2014; Heppner et al., 2015; Bhattacharya et al., 2020).
While levels of proinflammatory mediators are increased, ABCB1/Abcb1 and ABCG2/Abcg2 expression and associated protein activity levels are decreased in animal models of Alzheimer’s disease as well as in patients with Alzheimer’s disease (Vogelgesang et al., 2002, 2004; Hartz et al., 2010c, 2012, 2018; Wijesuriya et al., 2010; Jeynes and Provias, 2011b; van Assema et al., 2012b; Mehta et al., 2013; Carrano et al., 2014; Chiu et al., 2015; Wang et al., 2016; Bauer et al., 2017; Kannan et al., 2017; Shubbar and Penny, 2018; Al-Majdoub et al., 2019). At this point it is unclear if neuroinflammation contributes to the loss of ABCB1/ABCG2 proteins at the blood-brain barrier in Alzheimer’s disease.
Research and development of anti-inflammatory strategies for neurologic disorders that involve the blood-brain barrier are currently in progress. Currently, 14 clinical trials are reported by clinicaltrials.gov as recruiting, enrolling, completed, or in planning. However, none of these trials address blood-brain barrier transporters.
C. Receptor Tyrosine Kinases and Growth Factor Signaling
Growth factors, cytokines, and hormones activate receptor tyrosine kinases (RTK) that are critical in survival and apoptosis (Robinson et al., 2000). RTKs have a hydrophobic transmembrane domain that connects the extracellular N terminus with the ligand binding domain and the intracellular C terminus containing the catalytic kinase domain (Hubbard, 1999; Zwick et al., 2001). Ligands, like growth factors, cytokines, and hormones, bind to the ligand binding domain and induce receptor dimerization and rapid activation of the kinase domain. Autophosphorylation of the receptor allows signal transfer through the cell membrane. The phosphorylated receptor interacts with adaptor proteins that act as linkers to downstream kinases, such as Src or phospholipase C. These kinases further activate a network of redundant pathways with feedback loops, crosstalk, and compensatory mechanisms (Zwick et al., 2001; Lemmon and Schlessinger, 2010). Mutations in RTKs or downstream signaling partners are implicated in the development and progression of cancer (Zwick et al., 2001). RTK signaling cascades regulate proliferation and modify protein expression and activity (Lemmon and Schlessinger, 2010). Several growth factors and their respective RTKs regulate ABCB1 and ABCG2 at the blood-brain barrier, including epidermal growth factor (EGF), platelet-derived growth factor (Smits et al., 1989; Bleau et al., 2009b), transforming growth factor beta (Dohgu et al., 2004; Baello et al., 2014), and vascular endothelial growth factor (VEGF). In general, activation of RTKs and downstream signaling cascades increases expression levels of blood-brain barrier ABCB1 and ABCG2 but also of other transporters such as Oatp1a1 (Ronaldson et al., 2011). In contrast, inhibition of this signaling decreases transporter expression levels.
The FDA has approved several RTK inhibitors for anticancer use, including axitinib, cabozantinib, lenvatinib, nintedanib, and others (Hou et al., 2021). Effects of such drugs on the blood-brain barrier have not been characterized, and RTK agonists are even less investigated.
1. Janus Kinase and Signal Transducer and Activator of Transcription 3 Cascade
The Janus kinase and signal transducer and activator of transcription 3 (JAK-STAT3) cascade is commonly activated by cytokines (Fig. 8). Downregulation and inhibition of JAK1, STAT3, or phosphorylated STAT3 downregulate ABCB1 at the blood-brain barrier (Jagadeeshan et al., 2017). Similar effects occur after p38 MAPK inhibition. Specifically, inhibiting p38 MAPK in epileptic rats decreased Abcb1 expression and associated protein activity levels, which correlated with increased brain drug uptake (Shao et al., 2016). The signaling molecules JNK, ERK, and c-Jun are also involved in this cascade, and activation of the c-Jun NH2 terminal kinase deactivates c-Jun and reduces Abcb1 mRNA expression levels (Zhou et al., 2006; Ronaldson et al., 2008). However, activating ERK and c-Jun with EGF via EGFR induces Abcb1 and Abcg2 expression at the blood-brain barrier (Bauer et al., 2005; Evseenko et al., 2007; Nakanishi and Ross, 2012; Munoz et al., 2014).
Fig. 8.
Regulation of ABCB1 by JAK-STAT3. Cytokines activate the JAK-STAT3 cascade, which leads to phosphorylation of STAT3, which leads to activation of the c-Jun NH2 terminal kinase that in turn deactivates c-Jun and reduces Abcb1 mRNA expression levels. Regulation by VEGF. VEGF signals through VEGFR2 to activate the nonreceptor tyrosine kinase Src. Activation of Src then induces phosphorylation of caveolin-1, which is followed by Abcb1 internalization and lysosomal degradation of the transporter. Regulation by the PI3K/Akt pathway. E2 signaling through ERβ inhibits the PTEN/PI3K/Akt/GSK3 pathway, which in turn leads to proteasomal degradation of Abcg2. Created with BioRender.com.
Currently approved JAK inhibitors include tofacitinib and ruxolitinib that are used for the treatment of rheumatoid arthritis and autoimmune diseases (Taylor, 2019). Other indications include ulcerative colitis, myelofibrosis, and atopic dermatitis (Hu et al., 2021). However, targeting the JAK-STAT3 signaling cascade for blood-brain barrier manipulation is little explored.
2. Vascular Endothelial Growth Factor
VEGF activates its receptors VEGFR2 and Flk-1 that in turn activate the nonreceptor tyrosine kinase Src (Fig. 8; Agarwal et al., 2011). Src activation induces phosphorylation of caveolin-1 followed by Abcb1 internalization and degradation (Hawkins et al., 2010; Miller and Cannon, 2014; Qosa et al., 2015). Phosphorylated caveolin-1 colocalizes with Abcb1 in the plasma membrane and initiates Abcb1 endocytosis and vesicular trafficking to the endosome or lysosome. There, Abcb1 is either recycled and trafficked back to the plasma membrane or, as in most cases, undergoes lysosomal degradation (Tome et al., 2015).
EGFR is highly expressed in some cancers, and EGFR inhibitors are used to block cancer cell growth. In glioblastoma patients, currently available EGFR inhibitors do not provide a survival benefit but could be used to overcome blood-brain barrier ABCB1/ABCG2-mediated drug resistance via transporter internalization/degradation (de Gooijer et al., 2018b; Kim et al., 2019b). In particular, EGFR inhibitors with improved brain partitioning, like buparlisib, might be useful to increase drug delivery into the brain and thus be beneficial in the treatment of glioblastoma patients (de Gooijer et al., 2018b).
3. PI3K/Akt/mTOR Pathway
In cancer, including gliomas, the PI3K/Akt/mTOR signaling pathway is dysregulated due to the loss of the tumor suppressor PTEN or constitutively active PI3K and Akt mutants. PTEN loss and PI3K/Akt mutations induce proliferation and prevent apoptosis of cancer cells and are associated with migration, invasion, and resistance to both radiation and chemotherapy (Balsara et al., 2004; Chen et al., 2005; Mellinghoff et al., 2005; Tang et al., 2006; Jiang et al., 2007; Cancer Genome Atlas Research Network, 2008; Verhaak et al., 2010; Song et al., 2012; Zhang et al., 2015; Wang et al., 2017). PI3K/Akt/mTOR also regulates transporters and in several cancers PI3K/Akt/mTOR signaling leads to transporter-mediated drug resistance. For example, constitutive overactivity of the PI3K/Akt pathway induces ABCB1 and ABCG2 expression and their associated proteins’ translocation to the plasma membrane of brain microvasculature endothelial cells, which increases drug resistance of brain tumors (Takada et al., 2005; Bleau et al., 2009b; Agarwal et al., 2011; Huang et al., 2013; Huang et al., 2014b). In this regard, we showed that estradiol activation of ERβ activates PTEN, leading to PI3K/Akt inactivation followed by GSK3 phosphorylation. Phosphorylated GSK3 then induces ABCG2 internalization and proteasomal degradation (Fig. 8; Hartz et al., 2010a,b). Similarly, PI3K inhibition with LY294002 stimulates ABCG2 transporter internalization and degradation (Mogi et al., 2003; Takada et al., 2005; Bleau et al., 2009a).
Until recently, the cause of transporter upregulation in cancer was unknown. Matsumoto et al. (1991) found ABCB1 overexpression in glioblastoma samples from patients after initial treatment and concluded that glioblastoma acquires ABCB1-mediated resistance during treatment (Matsumoto et al., 1991). However, transporter overexpression could also be due to pathway dysregulation. In support of this model, RTK signaling components, including downstream kinases like PI3K, are mutated in 95% of glioblastoma patients (Schlessinger, 2000; Brennan et al., 2013; Eskilsson et al., 2018). Overactive PI3K signaling drives tumor progression and upregulates ABCB1 and ABCG2 expression and associated protein activity in tumors and at the blood-brain barrier, contributing to drug resistance (Schlessinger, 2000; Shinojima et al., 2003; Brennan et al., 2013; Eskilsson et al., 2018). Specifically, activation of the PI3K/Akt signaling cascade through increased phosphorylation of PI3K and Akt, PI3K/Akt overexpression or loss of PTEN, significantly increases ABCB1 and ABCG2 expression and associated protein activity at the blood-brain barrier (Brennan et al., 2013; Huang et al., 2013, 2014a). Thus, inhibiting PI3K, mTOR, or upstream RTKs holds the potential to attenuate transporter upregulation (Huang et al., 2013; de Gooijer et al., 2018c).
In tumor cells and at the blood-brain barrier, ABCB1 and ABCG2 restrict the uptake of anticancer drugs, and transporter expression levels were thought to correlate with the extent of multidrug resistance (Marchetti et al., 2008; Agarwal et al., 2010, 2011; de Vries et al., 2012; Laramy et al., 2017; de Gooijer et al., 2018c). This transporter-centric view on multidrug resistance in cancer, however, is controversial.
D. Other Pathways
Several other pathways that regulate blood-brain barrier ABCB1/ABCG2 have been identified in recent years and are summarized in the following sections.
1. Adenosine
Adenosine receptor A2 and adenylate cyclase, both key components of the adenosine signaling pathway, exist at the blood-brain barrier (Fig. 9A; Kalaria and Harik, 1986). In 2016, Kim and Bynoe (2016) demonstrated that activation of the adenosine receptor A2A in hCMEC/D3 cells in vitro decreases ABCB1 and ABCG2 expression and associated protein activity through membrane metalloprotease 9-mediated cleavage followed by ubiquitination and proteasomal degradation (Kim and Bynoe, 2016). A2A activation decreases the expression of tight junction proteins, which increases paracellular permeability (Kim and Bynoe, 2015). The adenosine receptor A2B has a low affinity for adenosine and to be activated requires high adenosine concentrations that are usually associated with pathologic conditions, such as brain tumors (Fredholm et al., 2001; Hasko et al., 2009; Aherne et al., 2011). Jackson et al. (2016, 2018) demonstrated that A2B inhibition disrupts blood-brain barrier function and improves brain drug uptake in rats (Jackson et al., 2016, 2018). Later studies showed that A2B signaling through protein kinase A and phospholipase C increases ABCB1 protein levels in endothelial cells, thereby contributing to drug resistance (Hasko et al., 2009; Aherne et al., 2011; Yan et al., 2019a).
Fig. 9.
Other signaling pathways. Diagram showing several other signaling pathways identified to regulate ABCB1 and/or ABCG2 at the blood-brain barrier: (A) adenosine, (B) circadian rhythm, (C) epigenic changes, and (D) P53. Created with BioRender.com.
Adenosine levels are increased in various pathologic conditions including brain cancer (Hasko et al., 2009; Aherne et al., 2011; Jackson et al., 2016; Yan et al., 2019a). Several preclinical studies suggest that increased adenosine levels could drive tumor invasion and drug resistance in glioblastoma models (Yan et al., 2019a,b; Zavala-Tecuapetla et al., 2020). Preclinical studies also suggest that adenosine inhibition improves drug brain uptake and efficacy in animal glioblastoma and epilepsy models. In a 2017 pilot study with patients with cardiovascular disease, A2A inhibition increased brain levels of the imaging reagent (99m)Tc-sestamibi (Jackson et al., 2017). However, A2A inhibition did not translate into increased temozolomide levels in a phase 1 clinical trial in glioblastoma patients (Jackson et al., 2018). At this point the therapeutic value of adenosine receptor inhibition to regulate blood-brain barrier transporters with the goal of improving brain drug levels needs further evaluation.
2. Circadian Rhythm
The circadian rhythm regulates multiple physiologic processes, including metabolism and transport, which affects drug delivery and elimination and,f thus, efficacy (Fig. 9B; Erol et al., 2001; Okyar et al., 2012; Filipski et al., 2014; Kervezee et al., 2014; Savolainen et al., 2016). At the molecular level, the circadian rhythm is controlled by a complex system of transcription factors including the main circadian regulator CLOCK, its heterodimer BMAL1, PAR domain basic leucine zipper proteins, Period, and E4BP4, a transcriptional activator protein that follows an opposing oscillating cycle and acts as a negative regulator during the sleep phase (Gekakis et al., 1998; Murakami et al., 2008). These proteins regulate a number of processes including the circadian oscillations of metabolizing enzymes and drug transporters.
In this regard, Zhang et al. (2018) first reported that xenobiotic efflux at the blood-brain barrier in fruit flies (Drosophila melanogaster) underlies circadian rhythm regulation. Specifically, they studied Mdr65, which is a pesticide-resistance protein with homology to ABCB1, along with Mdr49. Mdr65 activity is regulated through opposing variation of intracellular Mg2+ and Ca2+ concentrations without changing transporter expression. Importantly, increased Mdr65-mediated efflux during the active period of the fruit fly decreased brain accumulation of xenobiotics (Zhang et al., 2018). The oscillating changes in efflux are repressed in Period knockout flies, further supporting circadian rhythm regulation of efflux transporter activity. Furthermore, Abcb1 activity at the blood-brain barrier of mice and ABCB1 activity in hCMEMC/D3 cells in vitro also underly circadian rhythm regulation (Zhang et al., 2021). Of particular note is that circadian oscillation of ABCB1 intestinal expression in primates (Macaca fascicularis) altered the pharmacokinetics of its substrates, suggesting a similar principle operating in the brain of higher mammal (Iwasaki et al., 2015).
In this regard, in the 1970s researchers discovered that mice with leukemia responded better to treatment when anticancer drug therapy was adjusted to the circadian rhythm (Haus et al., 1972). Thus, a dosing schedule following the circadian rhythm has the potential to improve brain uptake and efficacy of CNS therapeutics. Several studies show that brain uptake of the Abcb1 substrate quinidine is increased during the resting phase of mice compared with the active phase, when Abcb1 activity is increased (Kervezee et al., 2014; Savolainen et al., 2016). Human patients had higher serum drug levels and better seizure control when phenytoin was dosed at night compared with the morning (Yegnanarayan et al., 2006). These findings suggest that decreased ABCB1 activity during the resting phase—daytime in rodents, nighttime in patients—is sufficient to improve drug brain uptake and efficacy while decreasing side effects (Yegnanarayan et al., 2006; Filipski et al., 2014).
3. Epigenetic Markers
Epigenetic markers include methylation and hydroxymethylation of target gene DNA and methylation, acetylation, phosphorylation, ubiquitylation, and sumoylation of associated histones that alter gene transcriptional activity without changing DNA sequence (Fig. 9C; Liberman et al., 2019). Regarding ABCB1, promotor (de)methylation is an important regulator of transporter expression (Scotto, 2003; Sarkadi et al., 2006; Nakanishi and Ross, 2012). ABCB1 promotor methylation increases the association of chromatin with deacetylated histones and methyl-CpG-binding protein 2, which represses transcription and, in turn, reduces ABCB1 mRNA and protein expression levels (El-Osta et al., 2002). Promotor demethylation triggers the release of methyl-CpG-binding protein 2 and causes chromatin relaxation, which enables ABCB1 gene transcription (El-Osta et al., 2002). While histone acetylation does not activate the ABCB1 promotor, it induces ABCB1 transcription and causes transporter overexpression (El-Osta et al., 2002; Nakanishi and Ross, 2012). Several groups have investigated histone acetyltransferase and histone deacetylase (HDAC) and their effects on ABCB1 expression. HDAC inhibition specifically increases acetylation of histone H4, which regulates the expression of Rab GTPases that stimulate vesicular trafficking of ABCB1 protein to the membrane (Noack et al., 2016). HDAC inhibition and histone acetyltransferase activation combined activate the ABCB1 promotor (Jin and Scotto, 1998; Sarkadi et al., 2006; You et al., 2019). You et al. (2019) showed that HDAC inhibitors increase ABCB1 mRNA and protein levels in cultured hCMEC/D3 cells, accompanied by increased AhR binding to the ABCB1 promotor, indicating a coregulatory mechanism.
Another epigenetic pathway involves melatonin, a hormone that controls the sleep-wake cycle and methylates the ABCG2 promotor, which ultimately leads to decreased ABCG2 protein levels in brain tumor stem cells. Since melatonin is a DNA methyltransferase substrate, methyltransferase inhibitors prevent ABCG2 downregulation (Martin et al., 2013). Recently, Jumnongprakhon et al. (2017) identified an alternative melatonin signaling pathway that regulates Abcb1 in primary rat brain microvascular endothelial cells. They showed that melatonin reverses methamphetamine-induced reduction in Abcb1 mRNA expression and associated protein levels at the blood-brain barrier by preventing internalization, ubiquitination, and proteasomal degradation (Jumnongprakhon et al., 2017). Since melatonin is not an Abcb1 substrate, competitive inhibition is unlikely to contribute to these effects (Tran et al., 2009).
Gene amplification, alternative promotors, and multiple transcription start sites are important ABCB1 regulators in the context of bacterial antibiotic resistance and cancer multidrug resistance; their role at the blood-brain barrier is unknown (Nakanishi and Ross, 2012).
Epigenetic changes are common in cancer cells, where they drive tumor progression and drug resistance (Hegi et al., 2005; Dong and Cui, 2019). In this regard, anticancer drugs, specifically DNA alkylating agents, increase DNA methylation, which causes double-strand breaks and leads to apoptosis. While this is the main mechanism of action of DNA alkylating agents, drugs from this category also change expression levels of other genes, including ABCB1 and ABCG2 (Munoz et al., 2015). This might explain why temozolomide and carmustine, two clinically used alkylating agents for the treatment of glioblastoma, decrease their own brain distribution and efficacy in glioblastoma patients (Valtonen et al., 1997; Stupp et al., 2005). However, opposing effects of alkylating agents have been described in glioblastoma cells (Riganti et al., 2013).
4. p53
p53 (gene: TP53) is one of the best-studied tumor suppressor proteins. Mutations and loss of p53 in different cancer types upregulate transcription and translation of ABCB1 (Fig. 9D; Bush and Li, 2002; Marroni et al., 2003; Sarkadi et al., 2006). Wild-type p53 decreases Ras/Raf signaling and phospholipase C activity, which increases ABCB1 expression levels (Chin et al., 1992; Bush and Li, 2002; Scotto, 2003). At the blood-brain barrier, DNA damage induces ataxia-telangiectasia-mutated kinase, which, in turn, activates p53, p38, and NF-κB, leading to ABCB1 overexpression (Bush and Li, 2002; Bleau et al., 2009b; Miller, 2015). Interestingly, the TP53 promoter is activated by intracellular Aβ, an ABCB1 substrate (Ohyagi et al., 2005).
TP53, which encodes p53, is the most mutated gene in cancer, including 30% of all brain tumors (Cancer Genome Atlas Research Network, 2008; Brennan et al., 2013; Duffy et al., 2017). TP53 mutations result in p53 loss-of-function, which dysregulates cell cycle progression, proliferation, and tumor growth.
IV. Overall Clinical Implication
Approximately 1 billion people worldwide and 100 million people in the United States suffer from CNS diseases, accounting for 6.3% of total global disease burden [World Health Organization (WHO), 2006; Gooch et al., 2017]. Many CNS diseases are difficult to treat, and the economic and social impact of treatment failure is significant (WHO, 2006; Gooch et al., 2017). Consequently, new therapeutic strategies are urgently needed. Two main obstacles exist to successful treatment of CNS disorders. First, the efflux transporters ABCB1 and ABCG2 at the blood-brain barrier prevent access of drugs to the brain and thus significantly interfere with the treatment of CNS diseases (Schinkel et al., 1994; Kim et al., 1998b; de Vries et al., 2007). Second, changes in the blood-brain barrier contribute to disease pathology that further restricts drug uptake into the brain, adding another layer of intricacy to the successful treatment of CNS diseases. Next we discuss some of the distinct changes that occur at the blood-brain barrier in patients with CNS disease or in animal disease models and highlight how those changes affect the progression and treatment of the respective disease (Fig. 10).
Fig. 10.
Clinical implications. Overview of diseases where ABCB1 and/or ABCG2 are changed and affect the progression and treatment of the respective disease. Created with BioRender.com.
A. Epilepsy
The WHO estimates that about 46 million people worldwide suffer from epilepsy, a disease characterized by recurring, unprovoked seizures that also impair cognition and sleep (Beghi, 2020). Approximately one-third of epilepsy patients do not respond to pharmacological treatment with antiseizure drugs and thus suffer from refractory, drug-resistant epilepsy and uncontrolled seizures (Kwan and Brodie, 2000). One major contributor to this drug resistance is ABCB1 and potentially other drug efflux transporters including ABCG2 at the blood-brain barrier, which prevent the delivery of antiseizure drugs into the brain (Cox et al., 2001; van Vliet et al., 2006; Tang et al., 2017). The significance ABCB1 plays in refractory epilepsy is further amplified by the fact that ABCB1 is upregulated at the blood-brain barrier of patients with refractory epilepsy (Tishler et al., 1995; Dombrowski et al., 2001). Abcb1 is overexpressed specifically in epileptogenic brain regions of chronic epileptic rats (van Vliet et al., 2006). In a case report, Iannetti et al. demonstrated that the ABCB1 inhibitor verapamil increased the efficacy of antiseizure drugs in a boy with epilepsy (Iannetti et al., 2005). However, the use of ABCB1 inhibitors is currently not a viable clinical strategy due to severe adverse effects.
B. Brain Tumors
Brain tumors account for 2% of all cancer cases worldwide but disproportionately contribute to cancer morbidity and mortality (Kleihues et al., 2014). In the United States, approximately 77,000 patients are newly diagnosed with a brain tumor every year, which makes brain tumors the most common and deadliest cancer in children and the sixth most common cancer in adults (Ostrom et al., 2019). Treatment options are limited and mostly involve surgical resection and radiotherapy (Stupp et al., 2005). Chemotherapy of primary and secondary brain tumors is often unsuccessful, in part because ABCB1 and ABCG2 at the blood-brain barrier restrict access to a wide range of anticancer drugs to the brain and impair their efficacy (de Vries et al., 2012; Taskar et al., 2012; de Gooijer et al., 2018d; Sorf et al., 2018). The situation is especially dire for patients with glioblastoma multiforme, the most common malignant primary brain tumor (Ostrom et al., 2018). Median survival after diagnosis is 15 to 23 months, and fewer than 7% of glioblastoma patients survive 5 years or longer (Ostrom et al., 2018, 2019). While many anticancer drugs are promising in vitro, their efficacy in vivo and in clinical trials has been marginal at best, due to ABCB1- and ABCG2-mediated efflux at the blood-brain barrier. Recently, Kim et al. demonstrated and visualized this “ABC challenge” in an elegant study (Kim et al., 2018, 2019a). In brief, Kim and coworkers showed that the MDM2 inhibitor SAR405838 significantly decreased the viability of patient-derived glioblastoma cell lines (Kim et al., 2018). In addition, SAR405838 prevented growth of glioblastoma cells injected into the flanks of immunocompromised mice (Kim et al., 2018). In contrast, when the tumor cells were injected into the brain, the drug did not reach the brain and, therefore, had no beneficial therapeutic effect on survival in the orthotopic xenograft model (Kim et al., 2018). The data further showed that SAR405838 is both an ABCB1 and ABCG2 substrate (Kim et al., 2019a). Even though SAR405838 was effective in vitro and in flank models, ABCB1- and ABCG2-mediated efflux at the blood-brain barrier prevented drug entry into the brain, rendering the compound ineffective (Kim et al., 2019a).
Diffuse intrinsic pontine glioma (DIPG) is another example of a CNS tumor. DIPG is the most common brain tumor in children (Ostrom et al., 2019). DIPGs reside in the brain stem and, therefore, cannot be surgically removed or biopsied (Ostrom et al., 2019). Consequently, to date, little information is available on the molecular composition of DIPGs, hindering the development of targeted therapies. Data from recent studies with animal DIPG models indicate that blood-brain barrier ABC transporters contribute to the low efficacy of chemotherapy (Veringa et al., 2013; Becher and Wechsler-Reya, 2014; Chung et al., 2014; Duchatel et al., 2019; Chaves et al., 2020). Similar data have also been obtained for secondary, metastatic brain tumors of lung and breast cancer as well as melanoma. Several research groups have also shown increased uptake of fluorescent dextrans into brain metastases exists, suggesting a disrupted blood-brain/tumor barrier (Palmieri et al., 2009; Lockman et al., 2010; Taskar et al., 2012; Mittapalli et al., 2016; Osswald et al., 2016; Terrell-Hall et al., 2017; Mohammad et al., 2018). However, de Gooijer et al. (2021) and other groups have demonstrated that “ATP-binding cassette transporters restrict drug delivery and efficacy against brain tumors even when blood-brain barrier integrity is lost,” suggesting that ABCB1 and ABCG2 overcome barrier leakage (Thomas et al., 2009; Adkins et al., 2013; Dudek et al., 2013; Li et al., 2013; Ballard et al., 2016; Vaidhyanathan et al., 2016; Yang et al., 2016; Gampa et al., 2018, 2019; Ippen et al., 2019).
In addition to the blood-brain barrier, ABCB1 is also upregulated in tumor tissue samples from brain tumor patients. One underlying mechanism for ABCB1 upregulation in cancer cells is the amplification of the ABCB1-containing 7q21 chromosomal region, which confers multidrug resistance (Genovese et al., 2017). Thus, ABCB1 copy number plays an important role in cancer-associated drug resistance. In glioblastoma, ABCB1 protein expression levels, measured with Western blotting and immunohistochemistry, were increased in patient samples from the second resection compared with those from the first resection (Matsumoto et al., 1991). ABCB1 expression increased in astrocyte-derived glioma cells compared with healthy human astrocytes (Spiegl-Kreinecker et al., 2002; Marroni et al., 2003). The increase in ABCB1 expression in glioblastoma patient samples may be mainly due to increased expression at the blood-tumor barrier, and transporter expression levels in tumor cells seem to play a minor role in drug resistance (Tanaka et al., 1994; Tews et al., 2000; Veringa et al., 2013).
C. Alzheimer’s Disease
Alzheimer’s disease is the leading cause of dementia worldwide. In the USA, an estimated 6.5 million people over the age of 65 suffer from the disease (Alzheimer's Association, 2022). One hallmark of Alzheimer’s disease is the accumulation and aggregation of Aβ in the brain (Murphy and LeVine, 2010; Serrano-Pozo et al., 2011). The mechanism underlying brain Aβ accumulation is not fully understood, but data from recent studies suggest that loss of ABC transporters at the blood-brain barrier impairs Aβ clearance from the brain, contributing to an imbalance between Aβ production and clearance.
In 2001, Lam et al. (2001) were the first to show that ABCB1 transports Aβ in vitro (Lam et al., 2001; Kuhnke et al., 2007; Hartz et al., 2010c; Callaghan et al., 2020). Abcb1 cooperates with low-density lipoprotein receptor-related protein-1 (LRP1) located in the abluminal membrane of the brain capillary endothelium where it shuttles Aβ from the brain into brain capillary endothelial cells (Shibata et al., 2000; Deane et al., 2004; Storck et al., 2018). Additional data indicate that ABCB1 then clears Aβ from the endothelial cell into the blood (Cirrito et al., 2005; Hartz et al., 2010c; Mawuenyega et al., 2010; Hartz et al., 2018; Krohn et al., 2018).
In 2002, Vogelgesang and colleagues (2002) showed in brain tissue samples from nondemented elderly patients (n = 243; 50–91 years) that blood-brain barrier ABCB1 expression and associated protein levels are decreased in areas with high Aβ load (Vogelgesang et al., 2002). Such a reduction in transporter levels also occurs in animal models of Alzheimer’s disease and in Alzheimer’s disease patients (Vogelgesang et al., 2004; Wijesuriya et al., 2010; Jeynes and Provias, 2011a; van Assema et al., 2012a; Mehta et al., 2013; Carrano et al., 2014; Chiu et al., 2015; Wang et al., 2016; Bauer et al., 2017; Kannan et al., 2017; Shubbar and Penny, 2018; Al-Majdoub et al., 2019). ABCB1 loss is particularly pronounced in areas directly surrounding Aβ plaques (Jeynes and Provias, 2011a; Chiu et al., 2015). Our group has shown that exposing isolated capillaries from mice and rats to nanomolar concentrations of human Aβ 40 decreases Abcb1 protein expression and activity levels (Hartz et al., 2010c, 2016). This effect is abolished by inhibiting ubiquitination, transporter internalization, and proteasomal degradation (Akkaya et al., 2015; Hartz et al., 2016, 2018; Ding et al., 2021). In addition to posttranslational modifications, other pathways including RAGE and NF-κB also regulate Abcb1 expression and associated protein levels at the blood-brain barrier in response to Aβ exposure (Park et al., 2014). While the role ABCB1 plays in Aβ clearance from the brain is well established, potential involvement of ABCG2 is less clear. In brain slices from Alzheimer’s disease patients capillaries surrounding Aβ plaques have decreased ABCG2 protein levels compared with cognitive normal controls (Carrano et al., 2014). However, other groups reported increased ABCG2 protein levels at the blood-brain barrier of Alzheimer’s disease patients (Xiong et al., 2009) or found no changes at all (Wijesuriya et al., 2010). Thus, currently it is unclear if ABCG2 is involved in Aβ clearance from the brain (Pahnke et al., 2008; Hartz et al., 2010c; Krohn et al., 2011). The involvement of other ABC transporters such as ABCA1 or ABCC1 in Alzheimer’s disease has been reviewed in detail by Wolf et al. (2012).
D. Chronic Pain
Chronic pain is one of the leading causes of disability and significantly impairs patients’ ability to participate in daily activities (Gooch et al., 2017; Vlaeyen et al., 2018). Opioids (natural and synthetic) are commonly used to treat chronic pain and are also, coincidentally, ABCB1 substrates (Letrent et al., 1999; Dagenais et al., 2004; Bauer et al., 2006; Sharma and Ali, 2006; Hassan et al., 2007; Yousif et al., 2008, 2012; Chaves et al., 2016; Schaefer et al., 2018). For example, oxycodone, morphine, and methadone are weak ABCB1 substrates and therefore cross the blood-brain barrier and exert activity on the CNS (Gibbs et al., 2018). Some centrally active opioids such as oxycodone, morphine, or fentanyl, induce Abcb1 expression, diminishing their own brain uptake and efficacy (Letrent et al., 1999; Dagenais et al., 2004; Sharma and Ali, 2006; Hassan et al., 2007; Yousif et al., 2008, 2012; Chaves et al., 2016; Schaefer et al., 2018). Other opioids such as loperamide and naldemedine are more active Abcb1 substrates and thus only have low brain uptake, which minimizes their central action and side effects (Watari et al., 2019). Loperamide has a four times higher Abcb1-mediated transport rate than methadone, which significantly restricts its brain uptake and makes loperamide a safe and effective drug for diarrhea treatment without CNS side effects (Gibbs et al., 2018). Of potential note is that λ-carrageenan-induced inflammatory pain increased brain uptake and antinociception of codeine (Hau et al., 2004). This suggests that inflammatory pain may be an important consideration in therapeutic drug dosing, potential adverse effects, and neurotoxicity.
E. Human Immunodeficiency Virus
Major breakthroughs in human immunodeficiency virus (HIV) treatment have transformed this originally deadly disease into a manageable chronic condition. The development of several classes of antiretroviral drugs with good safety and efficacy profiles significantly improved and prolonged the lives of HIV patients (Kwon et al., 2020). However, one obstacle remains: ABCB1 at the blood-brain barrier restricts HIV protease inhibitor uptake into the brain, thereby creating a sanctuary where the virus can persist and replicate (Kim et al., 1998a,b; Lee et al., 1998; Edwards et al., 2002). Virus replication in the brain causes decline in motor and cognitive function and can lead to dementia (Edwards et al., 2002). Further, antiretroviral drugs are a double-edged sword: They are effective against HIV in the periphery but increase ABCB1 expression at the blood-brain barrier through activation of nuclear receptors such as PXR or CAR, thereby hindering drugs from reaching the brain (Chan et al., 2013). In a recent study, McRae et al. provided evidence that the HIV protein Tat1 increased Abcb1 expression and Abcb1 activity levels at the blood-brain barrier in mouse models, further restricting brain uptake of antiretroviral drugs (McRae et al., 2019).
F. Stroke
Stroke is one of the deadliest neurologic diseases causing annually more than 3 million deaths worldwide (Katan and Luft, 2018). Only 20% of patients who survive a stroke regain complete independence, making stroke the CNS disease responsible for the highest rate of long-term disability (WHO, 2006). In patients who survive, stroke is known to induce neuroinflammation and blood-brain barrier damage, mainly by disrupting tight junctions (Yang et al., 2019). Further, Dazert et al. showed in a rat ischemic stroke model that middle cerebral artery occlusion increases the expression of several ABC transporters, including Abcb1 and Abcg2, in the infarct region, potentially limiting brain uptake of neuroprotective drugs (Dazert et al., 2006). A follow-up study by DeMars et al. (2017) confirmed that Abcb1 protein levels are significantly increased after ischemic stroke in the brain capillary endothelium in vivo (DeMars et al., 2017). The presence of blood-brain barrier ABC transporters decreased drug concentrations in the ischemic brain by up to an order of magnitude, thus reducing neuroprotective drug efficacy (Spudich et al., 2006; Kilic et al., 2008; ElAli and Hermann, 2010). Inhibiting Abcb1 or silencing Abcb1 with siRNA reduced levels of inflammatory cytokines, matrix metalloproteinases (membrane metalloprotease-2 and -9), and adhesion molecules (ICAM-1 and VCAM-1), which resulted in reduced infarct volume (Huang et al., 2022). These findings suggest that Abcb1 impairs brain drug entry of therapeutic drugs and may also contribute to barrier dysfunction in ischemic stroke and could potentially be a therapeutic target. More research is needed in this area to clarify the role of ABC transporters during stroke.
G. Amyotrophic Lateral Sclerosis
ALS is a neurodegenerative disease leading to neuronal damage and loss of voluntary muscle movement. To this day, there is no cure, and only limited treatment options are available. Riluzole is one of two FDA-approved drugs for ALS therapy and is also an ABCB1 substrate, restricting riluzole brain uptake and efficacy (Jablonski et al., 2014). A preclinical study in a mouse ALS model showed that the Abcb1 inhibitor elacridar increases riluzole brain uptake and improves drug efficacy (Jablonski et al., 2014). Drug brain uptake is further restricted due to Abcb1 upregulation in brain capillary endothelial cells and in astrocytes of mice with a SOD1 mutation, a commonly used animal ALS model (Jablonski et al., 2012; Qosa et al., 2016b; Chan et al., 2017). ABCB1 expression increases in human pluripotent stem cell-derived brain endothelial cells that were cocultured with astrocytes isolated from ALS patient samples (Mohamed et al., 2019).
V. Concluding Remark
CNS disorders make up approximately 50% of the total health burden in the United States, significantly contributing to mortality and disability (Gooch et al., 2017). Moreover, CNS disorders are often difficult to treat (Gooch et al., 2017), which is in part because many CNS active drugs are substrates of ABCB1 and ABCG2 at the blood-brain barrier. We now know that ABCB1 and ABCG2, and possibly other ABC transporters, work together in restricting brain drug uptake, rendering CNS pharmacotherapy extremely difficult. Over the past decades, numerous therapeutic approaches have been tested to overcome blood-brain barrier efflux transporters and to improve treatment outcomes in patients with CNS disorders. In this regard, we have worked to unravel signaling pathways that regulate transporters (Hartz and Bauer, 2010). Part of this research involves identifying target molecules that can be manipulated to control ABC transporter expression and/or activity. For example, this approach could be used to increase transporter expression and/or activity to protect the brain while treating peripheral diseases when CNS effects are not desired (Bauer et al., 2004, 2007). Consider the chemotherapy of peripheral cancers, where one prominent side effect is the development of “chemo brain,” a form of drug-induced dementia (Joshi et al., 2010; Ren et al., 2019; Stefancin et al., 2020). In this case, shielding the brain from anticancer drugs by upregulating ABC transporters could be beneficial to prevent chemo brain, which would improve patients’ overall health and quality of life.
On the other hand, targeting transporter regulation has the potential to open the barrier for a short “window in time” and allow drug uptake when needed while protecting the brain in between treatments (Hartz and Bauer, 2010). Selectively turning off ABC transporters to increase brain uptake of CNS therapeutics could be an important tool to improve the treatment of various CNS disorders (Hartz and Bauer, 2010). Since reducing ABC transporter activity could exacerbate conditions such as Alzheimer’s disease, including prodromal stages, where ABCB1 activity is critical for clearance of Aβ from the brain, chronic downregulation of ABC transporters could bear risks. This further supports the idea of time-limited ABCB1/ABCG2 reduction to maximize drug delivery and therapeutic outcomes, while minimizing risk that could stem from chronic transporter suppression. Several of the pathways described here could be targeted with existing FDA-approved drugs with the potential of regulating ABCB1/ABCG2 expression and associated proteins’ activity at the blood-brain barrier (Table 1). For example, ER-mediated decrease of ABCB1/ABCG2 expression and associated proteins activity levels at the blood-brain barrier could potentially be accomplished with ethinyl estradiol or could be blocked with fulvestrant (Imai et al., 2002, 2005; Hartz et al., 2010a,b; Zuloaga et al., 2012). Inflammation-mediated changes in ABCB1/ABCG2 could be blocked with the anti-TNFα antibodies adalimumab or infliximab or with the cyclooxygenase 2 inhibitor celecoxib to prevent ABCB1 and ABCG2 upregulation (Hartz et al., 2004, 2006; Bauer et al., 2007; Zibell et al., 2009). Another option is the use of tyrosine kinase inhibitors such as lapatinib (EGFR), erlotinib (EGFR), sunitinib (VEGFR), or bevacizumab (VEGFR), to decrease ABCB1/ABCG2 expression/activity levels at the blood-brain barrier (Evseenko et al., 2007; Hawkins et al., 2010; Tome et al., 2015). While this approach seems promising, more research is needed to evaluate the effect of increased or decreased ABC transporter expression and activity at the blood-brain barrier on drug brain levels, efficacy, and overall disease progression.
TABLE 1.
Examples of FDA-approved drugs that could downregulate ABCB1/ABCG2 and provide a window-in-time for brain drug delivery
| Target | Drug Name | Drug Action | Predicted Outcome |
|---|---|---|---|
| COX-2 | Fenoprofen | Inhibitor | ABCB1 ↓ |
| COX-2 | Acetic salicylic acid | Inhibitor | ABCB1 ↓ |
| COX-2 | Diclofenac | Inhibitor | ABCB1 ↓ |
| COX-2 | Celecoxib | Inhibitor | ABCB1 ↓ |
| COX-2 | Bromfenac | Inhibitor | ABCB1 ↓ |
| COX-2 | Etoricoxib | Inhibitor | ABCB1 ↓ |
| COX-2 | Firocoxib | Inhibitor | ABCB1 ↓ |
| COX-2 | Flurbiprofen | Inhibitor | ABCB1 ↓ |
| COX-2 | Ibuprofen | Inhibitor | ABCB1 ↓ |
| COX-2 | Indomethacin | Inhibitor | ABCB1 ↓ |
| COX-2 | Ketorolac | Inhibitor | ABCB1 ↓ |
| COX-2 | Meloxicam | Inhibitor | ABCB1 ↓ |
| COX-2 | Nabumetone | Inhibitor | ABCB1 ↓ |
| COX-2 | Naproxen | Inhibitor | ABCB1 ↓ |
| COX-2 | Oxaprozin | Inhibitor | ABCB1 ↓ |
| COX-2 | Parecoxib | Inhibitor | ABCB1 ↓ |
| COX-2 | Piroxicam | Inhibitor | ABCB1 ↓ |
| COX-2 | Tenoxicam | Inhibitor | ABCB1 ↓ |
| EGFR | Cetuximab | EGFR neutralizing antibody | ABCB1 ↓; ABCG2 ↓ |
| EGFR | Erlotinib | Inhibitor | ABCB1 ↓; ABCG2 ↓ |
| EGFR | Lapatinib | Inhibitor | ABCB1 ↓; ABCG2 ↓ |
| EGFR | Mobocertinib | Inhibitor | ABCB1 ↓; ABCG2 ↓ |
| EGFR | Necitumumab | EGFR neutralizing antibody | ABCB1 ↓; ABCG2 ↓ |
| EGFR | Osimertinib | Inhibitor | ABCB1 ↓; ABCG2 ↓ |
| EGFR | Panitumumab | EGFR neutralizing antibody | ABCB1 ↓; ABCG2 ↓ |
| EGFR | Gefitinib | Inhibitor | ABCB1 ↓; ABCG2 ↓ |
| ER | Estradiol | Agonist | ABCB1 ↓; ABCG2 ↓ |
| ER | Estramustine | Agonist | ABCB1 ↓; ABCG2 ↓ |
| ER | Ethinyl estradiol | Agonist | ABCB1 ↓; ABCG2 ↓ |
| ER | Norethisterone | Agonist | ABCB1 ↓; ABCG2 ↓ |
| ER | Norethynodrel | Agonist | ABCB1 ↓; ABCG2 ↓ |
| GR | Mifepristone | Antagonist | ABCB1 ↓; ABCG2 ↓ |
| GR | Ulipristal | Antagonist | ABCB1 ↓; ABCG2 ↓ |
| MR | Fineronone | Antagonist | ABCB1 ↓; ABCG2 ↓ |
| NMDAR | Esketamine | Antagonist | ABCB1 ↓ |
| NMDAR | Memantine | Antagonist | ABCB1 ↓ |
| mTOR | Everolimus | Inhibitor | ABCB1 ↓; ABCG2 ↓ |
| mTOR | Sirolimus | Inhibitor | ABCB1 ↓; ABCG2 ↓ |
| mTOR | Temsirolimus | Inhibitor | ABCB1 ↓; ABCG2 ↓ |
| PI3K | Alpelisib | Inhibitor | ABCB1 ↓; ABCG2 ↓ |
| PI3K | Copanlisib | Inhibitor | ABCB1 ↓; ABCG2 ↓ |
| PI3K | Duvelisib | Inhibitor | ABCB1 ↓; ABCG2 ↓ |
| TNFα | Adalimumab | TNFα neutralizing antibody | ABCB1 ↓ |
| TNFα | Certolizumab | TNFα neutralizing antibody | ABCB1 ↓ |
| TNFα | Etanercept | TNFα inhibitor | ABCB1 ↓ |
| TNFα | Golimumab | TNFα neutralizing antibody | ABCB1 ↓ |
| TNFα | Infliximab | TNFα neutralizing antibody | ABCB1 ↓ |
Materials and FDA-approval status were determined based on the FDALabel database in September 2021 (https://www.fda.gov/science-research/bioinformatics-tools/fdalabel-full-text-search-drug-product-labeling), specifically Section 12.1, “Mechanism of Action.”
Acknowledgments
The authors thank the members of the Bauer and Hartz laboratories, especially Bryan Maloney, for proofreading and suggesting improvements to this manuscript.
Abbreviations
- 3′-UTR
3′-untranslated region
- Aβ
amyloid β
- ABC
ATP-binding cassette
- AhR
aryl hydrocarbon receptor
- ALS
amyotrophic lateral sclerosis
- CAR
constitutive androstane receptor
- CNS
central nervous system
- CSF
cerebral spinal fluid
- E2
17β-estradiol
- DIPG
diffuse intrinsic pontine glioma
- EGF
epidermal growth factor
- EGFR
epidermal growth factor receptor
- ER
estrogen receptor
- ERK
extracellular signal-regulated kinase
- FDA
Food and Drug Administration
- FXR
Farnesoid X receptor
- GR
glucocorticoid receptor
- HDAC
histone acetyltransferase and histone deacetylase
- HIV
human immunodeficiency virus
- IL
interleukin
- JAK-STAT3
Janus kinase and signal transducer and activator of transcription 3
- LXR
liver X receptor
- MAPK
p38 mitogen-activated protein kinase
- miRNA
micro RNA
- mRNA
messenger RNA
- NBD
nucleotide binding domains
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- NMDAR
N-methyl-D-aspartate receptor
- NO
nitric oxide
- Nrf2
nuclear factor E2-related factor 2
- PPAR
peroxisome proliferator-activated receptor
- PXR
pregnane X receptor
- RAR
retinoid acid receptor
- ROS
reactive oxygen species
- RTK
receptor tyrosine kinase
- RXR
retinoid X receptor
- siRNA
small interfering RNA
- SNP
single nucleotide polymorphisms
- T4
thyroxin
- TMD
transmembrane domain
- TNFα
tumor necrosis factor α
- VDR
vitamin D receptor
- VEGF
vascular endothelial growth factor
- WHO
World Health Organization
Authorship Contributions
Wrote or contributed to the writing of the manuscript: Schulz, Hartz, Bauer.
Footnotes
This project was supported by National Institutes of Health National Institute of Neurologic Disorders and Stroke [Grants R01-NS107548 and 2R01-NS079507] (to B.B.) and National Institute on Aging [Grants 2R01-AG039621 and R01-AG075583] (to A.M.S.H.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke, National Institute on Aging, or the National Institutes of Health. Additional funding was provided by the University of Kentucky College of Pharmacy (Pharmaceutical Sciences Excellence in Graduate Achievement Fellowship) and by a PhRMA Foundation Pre-Doctoral Fellowship Pharmacology/Toxicology (to J.A.S.).
The authors declare no conflicts of interest.
Primary laboratory of origin: Björn Bauer.
This publication is the basis for the Introduction section of the doctoral dissertation by J.A.S. that can be found online at: https://uknowledge.uky.edu/pharmacy_etds/124/
References
- Abele R, Tampé R (1999) Function of the transport complex TAP in cellular immune recognition. Biochim Biophys Acta 1461:405–419. [DOI] [PubMed] [Google Scholar]
- Abraham J, Edgerly M, Wilson R, Chen C, Rutt A, Bakke S, Robey R, Dwyer A, Goldspiel B, Balis F, et al. (2009) A phase I study of the P-glycoprotein antagonist tariquidar in combination with vinorelbine. Clin Cancer Res 15:3574–3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham J, Edgerly M, Wilson R, Medina W, Hermonstine L, Rutt A, Bakke S, Robey R, Grem J, Bates S, et al. (2001) A study of the novel P-glycoprotein (P-gp) antagonist, XR9576 in combination with vinorelbine. Clin Cancer Res 7:3739S–3740S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adkins CE, Mittapalli RK, Manda VK, Nounou MI, Mohammad AS, Terrell TB, Bohn KA, Yasemin C, Grothe TR, Lockman JA, et al. (2013) P-glycoprotein mediated efflux limits substrate and drug uptake in a preclinical brain metastases of breast cancer model. Front Pharmacol 4:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal S, Elmquist WF (2012) Insight into the cooperation of P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2) at the blood-brain barrier: a case study examining sorafenib efflux clearance. Mol Pharm 9:678–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal S, Sane R, Gallardo JL, Ohlfest JR, Elmquist WF (2010) Distribution of gefitinib to the brain is limited by P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2)-mediated active efflux. J Pharmacol Exp Ther 334:147–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal S, Sane R, Ohlfest JR, Elmquist WF (2011) The role of the breast cancer resistance protein (ABCG2) in the distribution of sorafenib to the brain. J Pharmacol Exp Ther 336:223–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aherne CM, Kewley EM, Eltzschig HK (2011) The resurgence of A2B adenosine receptor signaling. Biochim Biophys Acta 1808:1329–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akanuma S, Hori S, Ohtsuki S, Fujiyoshi M, Terasaki T (2008) Expression of nuclear receptor mRNA and liver X receptor-mediated regulation of ABC transporter A1 at rat blood-brain barrier. Neurochem Int 52:669–674. [DOI] [PubMed] [Google Scholar]
- Akanuma S, Uchida Y, Ohtsuki S, Tachikawa M, Terasaki T, Hosoya K (2011) Attenuation of prostaglandin E2 elimination across the mouse blood-brain barrier in lipopolysaccharide-induced inflammation and additive inhibitory effect of cefmetazole. Fluids Barriers CNS 8:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL, et al. (2000) Inflammation and Alzheimer’s disease. Neurobiol Aging 21:383–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akkaya BG, Zolnerciks JK, Ritchie TK, Bauer B, Hartz AMS, Sullivan JA, Linton KJ (2015) The multidrug resistance pump ABCB1 is a substrate for the ubiquitin ligase NEDD4-1. Mol Membr Biol 32:39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Majdoub ZM, Al Feteisi H, Achour B, Warwood S, Neuhoff S, Rostami-Hodjegan A, Barber J (2019) Proteomic quantification of human blood-brain barrier SLC and ABC transporters in healthy individuals and dementia patients. Mol Pharm 16:1220–1233. [DOI] [PubMed] [Google Scholar]
- Alam C, Aufreiter S, Georgiou CJ, Hoque MT, Finnell RH, O’Connor DL, Goldman ID, Bendayan R (2019) Upregulation of reduced folate carrier by vitamin D enhances brain folate uptake in mice lacking folate receptor alpha. Proc Natl Acad Sci USA 116:17531–17540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleksunes LM, Klaassen CD (2012) Coordinated regulation of hepatic phase I and II drug-metabolizing genes and transporters using AhR-, CAR-, PXR-, PPARα-, and Nrf2-null mice. Drug Metab Dispos 40:1366–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allegra S, Cardellino CS, Fatiguso G, Cusato J, De Nicolò A, Avataneo V, Bonora S, D’Avolio A, Di Perri G, Calcagno A (2018) Effect of ABCC2 and ABCG2 gene polymorphisms and CSF-to-serum albumin ratio on ceftriaxone plasma and cerebrospinal fluid concentrations. J Clin Pharmacol 58:1550–1556. [DOI] [PubMed] [Google Scholar]
- Aller SG, Yu J, Ward A, Weng Y, Chittaboina S, Zhuo R, Harrell PM, Trinh YT, Zhang Q, Urbatsch IL, et al. (2009) Structure of P-glycoprotein reveals a molecular basis for poly-specific drug binding. Science 323:1718–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez JI, Dodelet-Devillers A, Kebir H, Ifergan I, Fabre PJ, Terouz S, Sabbagh M, Wosik K, Bourbonnière L, Bernard M, et al. (2011) The Hedgehog pathway promotes blood-brain barrier integrity and CNS immune quiescence. Science 334:1727–1731. [DOI] [PubMed] [Google Scholar]
- Alvarez JI, Katayama T, Prat A (2013) Glial influence on the blood brain barrier. Glia 61:1939–1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alyu F, Dikmen M (2017) Inflammatory aspects of epileptogenesis: contribution of molecular inflammatory mechanisms. Acta Neuropsychiatr 29:1–16. [DOI] [PubMed] [Google Scholar]
- Alzheimer’s Association (2022) Alheimers and Dementia—Facts and Figures, Alzheimer's Association, Chicago, IL. [Google Scholar]
- Ambros V (2004) The functions of animal microRNAs. Nature 431:350–355. [DOI] [PubMed] [Google Scholar]
- Amoutzias GD, Pichler EE, Mian N, De Graaf D, Imsiridou A, Robinson-Rechavi M, Bornberg-Bauer E, Robertson DL, Oliver SG (2007) A protein interaction atlas for the nuclear receptors: properties and quality of a hub-based dimerisation network. BMC Syst Biol 1:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelov L, Doolittle ND, Kraemer DF, Siegal T, Barnett GH, Peereboom DM, Stevens G, McGregor J, Jahnke K, Lacy CA, et al. (2009) Blood-brain barrier disruption and intra-arterial methotrexate-based therapy for newly diagnosed primary CNS lymphoma: a multi-institutional experience. J Clin Oncol 27:3503–3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arend J, Kegler A, Caprara ALF, Almeida C, Gabbi P, Pascotini ET, de Freitas LAV, Miraglia C, Bertazzo TL, Palma R, et al. (2018) Depressive, inflammatory, and metabolic factors associated with cognitive impairment in patients with epilepsy. Epilepsy Behav 86:49–57. [DOI] [PubMed] [Google Scholar]
- Armulik A, Abramsson A, Betsholtz C (2005) Endothelial/pericyte interactions. Circ Res 97:512–523. [DOI] [PubMed] [Google Scholar]
- Armulik A, Genové G, Mäe M, Nisancioglu MH, Wallgard E, Niaudet C, He L, Norlin J, Lindblom P, Strittmatter K, et al. (2010) Pericytes regulate the blood-brain barrier. Nature 468:557–561. [DOI] [PubMed] [Google Scholar]
- Ashraf T, Ronaldson PT, Persidsky Y, Bendayan R (2011) Regulation of P-glycoprotein by human immunodeficiency virus-1 in primary cultures of human fetal astrocytes. J Neurosci Res 89:1773–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atlasi Y, Looijenga L, Fodde R (2014) Cancer stem cells, pluripotency, and cellular heterogeneity: a WNTer perspective. Curr Top Dev Biol 107:373–404. [DOI] [PubMed] [Google Scholar]
- Avemary J, Salvamoser JD, Peraud A, Rémi J, Noachtar S, Fricker G, Potschka H (2013) Dynamic regulation of P-glycoprotein in human brain capillaries. Mol Pharm 10:3333–3341. [DOI] [PubMed] [Google Scholar]
- Baba A, Kimoto M, Tatsuno T, Inoue T, Iwata H (1985) Membrane-bound lipoxygenase of rat cerebral microvessels. Biochem Biophys Res Commun 127:283–288. [DOI] [PubMed] [Google Scholar]
- Baello S, Iqbal M, Bloise E, Javam M, Gibb W, Matthews SG (2014) TGF-β1 regulation of multidrug resistance P-glycoprotein in the developing male blood-brain barrier. Endocrinology 155:475–484. [DOI] [PubMed] [Google Scholar]
- Baeten KM, Akassoglou K (2011) Extracellular matrix and matrix receptors in blood-brain barrier formation and stroke. Dev Neurobiol 71:1018–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard P, Yates JW, Yang Z, Kim DW, Yang JC, Cantarini M, Pickup K, Jordan A, Hickey M, Grist M, et al. (2016) Preclinical comparison of osimertinib with other EGFR-TKIs in EGFR-mutant NSCLC brain metastases models, and early evidence of clinical brain metastases activity. Clin Cancer Res 22:5130–5140. [DOI] [PubMed] [Google Scholar]
- Balsara BR, Pei J, Mitsuuchi Y, Page R, Klein-Szanto A, Wang H, Unger M, Testa JR (2004) Frequent activation of AKT in non-small cell lung carcinomas and preneoplastic bronchial lesions. Carcinogenesis 25:2053–2059. [DOI] [PubMed] [Google Scholar]
- Bankstahl JP, Kuntner C, Abrahim A, Karch R, Stanek J, Wanek T, Wadsak W, Kletter K, Müller M, Löscher W, et al. (2008) Tariquidar-induced P-glycoprotein inhibition at the rat blood-brain barrier studied with (R)-11C-verapamil and PET. J Nucl Med 49:1328–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116:281–297. [DOI] [PubMed] [Google Scholar]
- Basu S, Rosenzweig KR, Youmell M, Price BD (1998) The DNA-dependent protein kinase participates in the activation of NF kappa B following DNA damage. Biochem Biophys Res Commun 247:79–83. [DOI] [PubMed] [Google Scholar]
- Bauer B, Hartz AM, Fricker G, Miller DS (2004) Pregnane X receptor up-regulation of P-glycoprotein expression and transport function at the blood-brain barrier. Mol Pharmacol 66:413–419. [DOI] [PubMed] [Google Scholar]
- Bauer B, Hartz AM, Lucking JR, Yang X, Pollack GM, Miller DS (2008a) Coordinated nuclear receptor regulation of the efflux transporter, Mrp2, and the phase-II metabolizing enzyme, GSTpi, at the blood-brain barrier. J Cereb Blood Flow Metab 28:1222–1234. [DOI] [PubMed] [Google Scholar]
- Bauer B, Hartz AM, Miller DS (2007) Tumor necrosis factor alpha and endothelin-1 increase P-glycoprotein expression and transport activity at the blood-brain barrier. Mol Pharmacol 71:667–675. [DOI] [PubMed] [Google Scholar]
- Bauer B, Hartz AM, Pekcec A, Toellner K, Miller DS, Potschka H (2008b) Seizure-induced up-regulation of P-glycoprotein at the blood-brain barrier through glutamate and cyclooxygenase-2 signaling. Mol Pharmacol 73:1444–1453. [DOI] [PubMed] [Google Scholar]
- Bauer B, Yang X, Hartz AM, Olson ER, Zhao R, Kalvass JC, Pollack GM, Miller DS (2006) In vivo activation of human pregnane X receptor tightens the blood-brain barrier to methadone through P-glycoprotein up-regulation. Mol Pharmacol 70:1212–1219. [DOI] [PubMed] [Google Scholar]
- Bauer B, Hartz AM, Fricker G, Miller DS (2005) Modulation of p-glycoprotein transport function at the blood-brain barrier. Exp Biol Med (Maywood) 230:118–127. [DOI] [PubMed] [Google Scholar]
- Bauer F, Kuntner C, Bankstahl JP, Wanek T, Bankstahl M, Stanek J, Mairinger S, Dörner B, Löscher W, Müller M, et al. (2010) Synthesis and in vivo evaluation of [11C]tariquidar, a positron emission tomography radiotracer based on a third-generation P-glycoprotein inhibitor. Bioorg Med Chem 18:5489–5497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer J, Strauss S, Schreiter-Gasser U, Ganter U, Schlegel P, Witt I, Yolk B, Berger M (1991) Interleukin-6 and alpha-2-macroglobulin indicate an acute-phase state in Alzheimer’s disease cortices. FEBS Lett 285:111–114. [DOI] [PubMed] [Google Scholar]
- Bauer M, Karch R, Wulkersdorfer B, Philippe C, Nics L, Klebermass EM, Weber M, Poschner S, Haslacher H, Jäger W, et al. (2019) A proof-of-concept study to inhibit ABCG2- and ABCB1-mediated efflux transport at the human blood-brain barrier. J Nucl Med 60:486–491. [DOI] [PubMed] [Google Scholar]
- Bauer M, Wulkersdorfer B, Karch R, Philippe C, Jäger W, Stanek J, Wadsak W, Hacker M, Zeitlinger M, Langer O (2017) Effect of P-glycoprotein inhibition at the blood-brain barrier on brain distribution of (R)-[11 C]verapamil in elderly vs. young subjects. Br J Clin Pharmacol 83:1991–1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becher OJ, Wechsler-Reya RJ (2014) Cancer. For pediatric glioma, leave no histone unturned. Science 346:1458–1459. [DOI] [PubMed] [Google Scholar]
- Beghi E (2020) The epidemiology of epilepsy. Neuroepidemiology 54:185–191. [DOI] [PubMed] [Google Scholar]
- Berthiaume AA, Grant RI, McDowell KP, Underly RG, Hartmann DA, Levy M, Bhat NR, Shih AY (2018) Dynamic remodeling of pericytes in vivo maintains capillary coverage in the adult mouse brain. Cell Rep 22:8–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz AL, Firth JA, Goldstein GW (1980) Polarity of the blood-brain barrier: distribution of enzymes between the luminal and antiluminal membranes of brain capillary endothelial cells. Brain Res 192:17–28. [DOI] [PubMed] [Google Scholar]
- Betz AL, Gilboe DD, Drewes LR (1975) Kinetics of unidirectional leucine transport into brain: effects of isoleucine, valine, and anoxia. Am J Physiol 228:895–900. [DOI] [PubMed] [Google Scholar]
- Betz AL, Goldstein GW (1978) Polarity of the blood-brain barrier: neutral amino acid transport into isolated brain capillaries. Science 202:225–227. [DOI] [PubMed] [Google Scholar]
- Bhattacharya A, Kaushik DK, Lozinski BM, Yong VW (2020) Beyond barrier functions: roles of pericytes in homeostasis and regulation of neuroinflammation. J Neurosci Res 98:2390–2405. [DOI] [PubMed] [Google Scholar]
- Bicker J, Alves G, Fortuna A, Soares-da-Silva P, Falcão A (2018) In vitro assessment of the interactions of dopamine β-hydroxylase inhibitors with human P-glycoprotein and breast cancer resistance protein. Eur J Pharm Sci 117:35–40. [DOI] [PubMed] [Google Scholar]
- Biedler JL, Riehm H (1970) Cellular resistance to actinomycin D in Chinese hamster cells in vitro: cross-resistance, radioautographic, and cytogenetic studies. Cancer Res 30:1174–1184. [PubMed] [Google Scholar]
- Biegel D, Spencer DD, Pachter JS (1995) Isolation and culture of human brain microvessel endothelial cells for the study of blood-brain barrier properties in vitro. Brain Res 692:183–189. [DOI] [PubMed] [Google Scholar]
- Bleau A-M, Huse JT, Holland EC (2009a) The ABCG2 resistance network of glioblastoma. Cell Cycle 8:2936–2944. [PubMed] [Google Scholar]
- Bleau AM, Hambardzumyan D, Ozawa T, Fomchenko EI, Huse JT, Brennan CW, Holland EC (2009b) PTEN/PI3K/Akt pathway regulates the side population phenotype and ABCG2 activity in glioma tumor stem-like cells. Cell Stem Cell 4:226–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boesch D, Gavériaux C, Jachez B, Pourtier-Manzanedo A, Bollinger P, Loor F (1991) In vivo circumvention of P-glycoprotein-mediated multidrug resistance of tumor cells with SDZ PSC 833. Cancer Res 51:4226–4233. [PubMed] [Google Scholar]
- Bonnans C, Chou J, Werb Z (2014) Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol 15:786–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borson-Chazot F, Dantony E, Illouz F, Lopez J, Niccoli P, Wassermann J, Do Cao C, Leboulleux S, Klein M, Tabarin A, et al. (2018) Effect of buparlisib, a pan-class I PI3K inhibitor, in refractory follicular and poorly differentiated thyroid cancer. Thyroid 28:1174–1179. [DOI] [PubMed] [Google Scholar]
- Borst P, Schinkel AH (1997) Genetic dissection of the function of mammalian P-glycoproteins. Trends Genet 13:217–222. [DOI] [PubMed] [Google Scholar]
- Brennan CW, Verhaak RG, McKenna A, Campos B, Noushmehr H, Salama SR, Zheng S, Chakravarty D, Sanborn JZ, Berman SH, et al. ; TCGA Research Network (2013) The somatic genomic landscape of glioblastoma. Cell 155:462–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brightman MW, Reese TS (1969) Junctions between intimately apposed cell membranes in the vertebrate brain. J Cell Biol 40:648–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broxterman HJ, Kuiper CM, Schuurhuis GJ, Tsuruo T, Pinedo HM, Lankelma J (1988) Increase of daunorubicin and vincristine accumulation in multidrug resistant human ovarian carcinoma cells by a monoclonal antibody reacting with P-glycoprotein. Biochem Pharmacol 37:2389–2393. [DOI] [PubMed] [Google Scholar]
- Burgess A, Ayala-Grosso CA, Ganguly M, Jordão JF, Aubert I, Hynynen K (2011) Targeted delivery of neural stem cells to the brain using MRI-guided focused ultrasound to disrupt the blood-brain barrier. PLoS One 6:e27877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush JA, Li G (2002) Cancer chemoresistance: the relationship between p53 and multidrug transporters. Int J Cancer 98:323–330. [DOI] [PubMed] [Google Scholar]
- Buxbaum JD, Oishi M, Chen HI, Pinkas-Kramarski R, Jaffe EA, Gandy SE, Greengard P (1992) Cholinergic agonists and interleukin 1 regulate processing and secretion of the Alzheimer beta/A4 amyloid protein precursor. Proc Natl Acad Sci USA 89:10075–10078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzhdygan TP, Rodrigues CR, McGary HM, Khan JA, Andrews AM, Rawls SM, Ramirez SH (2021) The psychoactive drug of abuse mephedrone differentially disrupts blood-brain barrier properties. J Neuroinflammation 18:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Z, Hussain MD, Yan LJ (2014) Microglia, neuroinflammation, and beta-amyloid protein in Alzheimer’s disease. Int J Neurosci 124:307–321. [DOI] [PubMed] [Google Scholar]
- Callaghan R, Gelissen IC, George AM, Hartz AMS (2020) Mamma mia, P-glycoprotein binds again. FEBS Lett 594:4076–4084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callen DF, Baker E, Simmers RN, Seshadri R, Roninson IB (1987) Localization of the human multiple drug resistance gene, MDR1, to 7q21.1. Hum Genet 77:142–144. [DOI] [PubMed] [Google Scholar]
- Campos CR, Schröter C, Wang X, Miller DS (2012) ABC transporter function and regulation at the blood-spinal cord barrier. J Cereb Blood Flow Metab 32:1559–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancer Genome Atlas Research Network (2008) Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature 455:1061–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon RE, Peart JC, Hawkins BT, Campos CR, Miller DS (2012) Targeting blood-brain barrier sphingolipid signaling reduces basal P-glycoprotein activity and improves drug delivery to the brain. Proc Natl Acad Sci USA 109:15930–15935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon RE, Richards AC, Trexler AW, Juberg CT, Sinha B, Knudsen GA, Birnbaum LS (2020) Effect of GenX on P-glycoprotein, breast cancer resistance protein, and multidrug resistance-associated protein 2 at the blood-brain barrier. Environ Health Perspect 128:37002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrano A, Snkhchyan H, Kooij G, van der Pol S, van Horssen J, Veerhuis R, Hoozemans J, Rozemuller A, de Vries HE (2014) ATP-binding cassette transporters P-glycoprotein and breast cancer related protein are reduced in capillary cerebral amyloid angiopathy. Neurobiol Aging 35:565–575. [DOI] [PubMed] [Google Scholar]
- Chaigneau E, Oheim M, Audinat E, Charpak S (2003) Two-photon imaging of capillary blood flow in olfactory bulb glomeruli. Proc Natl Acad Sci USA 100:13081–13086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty S, Filippi CG, Wong T, Ray A, Fralin S, Tsiouris AJ, Praminick B, Demopoulos A, McCrea HJ, Bodhinayake I, et al. (2016) Superselective intraarterial cerebral infusion of cetuximab after osmotic blood/brain barrier disruption for recurrent malignant glioma: phase I study. J Neurooncol 128:405–415. [DOI] [PubMed] [Google Scholar]
- Chan GN, Hoque MT, Bendayan R (2013) Role of nuclear receptors in the regulation of drug transporters in the brain. Trends Pharmacol Sci 34:361–372. [DOI] [PubMed] [Google Scholar]
- Chan GN, Hoque MT, Cummins CL, Bendayan R (2011) Regulation of P-glycoprotein by orphan nuclear receptors in human brain microvessel endothelial cells. J Neurochem 118:163–175. [DOI] [PubMed] [Google Scholar]
- Chan GNY, Evans RA, Banks DB, Mesev EV, Miller DS, Cannon RE (2017) Selective induction of P-glycoprotein at the CNS barriers during symptomatic stage of an ALS animal model. Neurosci Lett 639:103–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandel NS, Trzyna WC, McClintock DS, Schumacker PT (2000) Role of oxidants in NF-kappa B activation and TNF-alpha gene transcription induced by hypoxia and endotoxin. J Immunol 165:1013–1021. [DOI] [PubMed] [Google Scholar]
- Chaves C, Declèves X, Taghi M, Menet MC, Lacombe J, Varlet P, Olaciregui NG, Carcaboso AM, Cisternino S (2020) Characterization of the blood-brain barrier integrity and the brain transport of SN-38 in an orthotopic xenograft rat model of diffuse intrinsic pontine glioma. Pharmaceutics 12:399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaves C, Gómez-Zepeda D, Auvity S, Menet MC, Crété D, Labat L, Remião F, Cisternino S, Declèves X (2016) Effect of subchronic intravenous morphine infusion and naloxone-precipitated morphine withdrawal on P-gp and Bcrp at the rat blood-brain barrier. J Pharm Sci 105:350–358. [DOI] [PubMed] [Google Scholar]
- Chaves C, Remiao F, Cisternino S, Decleves X (2017) Opioids and the blood-brain barrier: a dynamic interaction with consequences on drug disposition in brain. Curr Neuropharmacol 15:1156–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CJ, Chin JE, Ueda K, Clark DP, Pastan I, Gottesman MM, Roninson IB (1986) Internal duplication and homology with bacterial transport proteins in the mdr1 (P-glycoprotein) gene from multidrug-resistant human cells. Cell 47:381–389. [DOI] [PubMed] [Google Scholar]
- Chen MB, Yang AC, Yousef H, Lee D, Chen W, Schaum N, Lehallier B, Quake SR, Wyss-Coray T (2020) Brain endothelial cells are exquisite sensors of age-related circulatory cues. Cell Rep 30:4418–4432.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Agarwal S, Shaik NM, Chen C, Yang Z, Elmquist WF (2009) P-glycoprotein and breast cancer resistance protein influence brain distribution of dasatinib. J Pharmacol Exp Ther 330:956–963. [DOI] [PubMed] [Google Scholar]
- Chen Z, Trotman LC, Shaffer D, Lin HK, Dotan ZA, Niki M, Koutcher JA, Scher HI, Ludwig T, Gerald W, et al. (2005) Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis. Nature 436:725–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikhale EG, Burton PS, Borchardt RT (1995) The effect of verapamil on the transport of peptides across the blood-brain barrier in rats: kinetic evidence for an apically polarized efflux mechanism. J Pharmacol Exp Ther 273:298–303. [PubMed] [Google Scholar]
- Chin K-V, Ueda K, Pastan I, Gottesman MM (1992) Modulation of activity of the promoter of the human MDR1 gene by Ras and p53. Science 255:459–462. [DOI] [PubMed] [Google Scholar]
- Chiu C, Miller MC, Monahan R, Osgood DP, Stopa EG, Silverberg GD (2015) P-glycoprotein expression and amyloid accumulation in human aging and Alzheimer’s disease: preliminary observations. Neurobiol Aging 36:2475–2482. [DOI] [PubMed] [Google Scholar]
- Choi J, Koh S (2008) Role of brain inflammation in epileptogenesis. Yonsei Med J 49:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choo EF, Kurnik D, Muszkat M, Ohkubo T, Shay SD, Higginbotham JN, Glaeser H, Kim RB, Wood AJ, Wilkinson GR (2006) Differential in vivo sensitivity to inhibition of P-glycoprotein located in lymphocytes, testes, and the blood-brain barrier. J Pharmacol Exp Ther 317:1012–1018. [DOI] [PubMed] [Google Scholar]
- Chung AH, Mittapalli RK, Elmquist WF, Becher OJ (2014) ABCG2 and ABCB1 promote brainstem gliomagenesis and limit the efficacy of Dasatinib In A Diffuse Intrinsic Pontine Glioma (DIPG) Mouse Model. Neuro-oncol 16:46–47. [Google Scholar]
- Cirrito JR, Deane R, Fagan AM, Spinner ML, Parsadanian M, Finn MB, Jiang H, Prior JL, Sagare A, Bales KR, et al. (2005) P-glycoprotein deficiency at the blood-brain barrier increases amyloid-beta deposition in an Alzheimer disease mouse model. J Clin Invest 115:3285–3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisternino S, Rousselle C, Dagenais C, Scherrmann JM (2001) Screening of multidrug-resistance sensitive drugs by in situ brain perfusion in P-glycoprotein-deficient mice. Pharm Res 18:183–190. [DOI] [PubMed] [Google Scholar]
- Clark R, Kerr ID, Callaghan R (2006) Multiple drugbinding sites on the R482G isoform of the ABCG2 transporter. Br J Pharmacol 149:506–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho-Santos V, Leitão RA, Cardoso FL, Palmela I, Rito M, Barbosa M, Brito MA, Fontes-Ribeiro CA, Silva AP (2015) The TNF-α/NF-κB signaling pathway has a key role in methamphetamine-induced blood-brain barrier dysfunction. J Cereb Blood Flow Metab 35:1260–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combs CK, Karlo JC, Kao SC, Landreth GE (2001) Beta-amyloid stimulation of microglia and monocytes results in TNFalpha-dependent expression of inducible nitric oxide synthase and neuronal apoptosis. J Neurosci 21:1179–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooray HC, Blackmore CG, Maskell L, Barrand MA (2002) Localisation of breast cancer resistance protein in microvessel endothelium of human brain. Neuroreport 13:2059–2063. [DOI] [PubMed] [Google Scholar]
- Copple IM (2012) The Keap1-Nrf2 cell defense pathway—a promising therapeutic target? Adv Pharmacol 63:43–79. [DOI] [PubMed] [Google Scholar]
- Copple IM, Goldring CE, Kitteringham NR, Park BK (2010) The keap1-nrf2 cellular defense pathway: mechanisms of regulation and role in protection against drug-induced toxicity. Handb Exp Pharmacol 196:233–266. [DOI] [PubMed] [Google Scholar]
- Cordon-Cardo C, O’Brien JP, Casals D, Rittman-Grauer L, Biedler JL, Melamed MR, Bertino JR (1989) Multidrug-resistance gene (P-glycoprotein) is expressed by endothelial cells at blood-brain barrier sites. Proc Natl Acad Sci USA 86:695–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couto M, Coelho-Santos V, Santos L, Fontes-Ribeiro C, Silva AP, Gomes CMF (2019) The interplay between glioblastoma and microglia cells leads to endothelial cell monolayer dysfunction via the interleukin-6-induced JAK2/STAT3 pathway. J Cell Physiol 234:19750–19760. [DOI] [PubMed] [Google Scholar]
- Cox DS, Scott KR, Gao H, Raje S, Eddington ND (2001) Influence of multidrug resistance (MDR) proteins at the blood-brain barrier on the transport and brain distribution of enaminone anticonvulsants. J Pharm Sci 90:1540–1552. [DOI] [PubMed] [Google Scholar]
- Cox SB, Woolsey TA, Rovainen CM (1993) Localized dynamic changes in cortical blood flow with whisker stimulation corresponds to matched vascular and neuronal architecture of rat barrels. J Cereb Blood Flow Metab 13:899–913. [DOI] [PubMed] [Google Scholar]
- Croop JM, Raymond M, Haber D, Devault A, Arceci RJ, Gros P, Housman DE (1989) The three mouse multidrug resistance (mdr) genes are expressed in a tissue-specific manner in normal mouse tissues. Mol Cell Biol 9:1346–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuevas P, Gutierrez-Diaz JA, Reimers D, Dujovny M, Diaz FG, Ausman JI (1984) Pericyte endothelial gap junctions in human cerebral capillaries. Anat Embryol (Berl) 170:155–159. [DOI] [PubMed] [Google Scholar]
- Dagenais C, Graff CL, Pollack GM (2004) Variable modulation of opioid brain uptake by P-glycoprotein in mice. Biochem Pharmacol 67:269–276. [DOI] [PubMed] [Google Scholar]
- Daneman R, Zhou L, Agalliu D, Cahoy JD, Kaushal A, Barres BA (2010) The mouse blood-brain barrier transcriptome: a new resource for understanding the development and function of brain endothelial cells. PLoS One 5:e13741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dastvan R, Mishra S, Peskova YB, Nakamoto RK, Mchaourab HS (2019) Mechanism of allosteric modulation of P-glycoprotein by transport substrates and inhibitors. Science 364:689–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauchy S, Dutheil F, Weaver RJ, Chassoux F, Daumas-Duport C, Couraud PO, Scherrmann JM, De Waziers I, Declèves X (2008) ABC transporters, cytochromes P450 and their main transcription factors: expression at the human blood-brain barrier. J Neurochem 107:1518–1528. [DOI] [PubMed] [Google Scholar]
- Dauchy S, Miller F, Couraud PO, Weaver RJ, Weksler B, Romero IA, Scherrmann JM, De Waziers I, Declèves X (2009) Expression and transcriptional regulation of ABC transporters and cytochromes P450 in hCMEC/D3 human cerebral microvascular endothelial cells. Biochem Pharmacol 77:897–909. [DOI] [PubMed] [Google Scholar]
- Dazert P, Suofu Y, Grube M, Popa-Wagner A, Kroemer HK, Jedlitschky G, Kessler C (2006) Differential regulation of transport proteins in the periinfarct region following reversible middle cerebral artery occlusion in rats. Neuroscience 142:1071–1079. [DOI] [PubMed] [Google Scholar]
- de Gooijer MC, Buil LCM, Çitirikkaya CH, Hermans J, Beijnen JH, van Tellingen O (2018a) ABCB1 attenuates the brain penetration of the PARP inhibitor AZD2461. Mol Pharm 15:5236–5243. [DOI] [PubMed] [Google Scholar]
- de Gooijer MC, de Vries NA, Buckle T, Buil LCM, Beijnen JH, Boogerd W, van Tellingen O (2018b) Improved brain penetration and antitumor efficacy of temozolomide by inhibition of ABCB1 and ABCG2. Neoplasia 20:710–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Gooijer MC, Kemper EM, Buil LCM, Çitirikkaya CH, Buckle T, Beijnen JH, van Tellingen O (2021) ATP-binding cassette transporters restrict drug delivery and efficacy against brain tumors even when blood-brain barrier integrity is lost. Cell Rep Med 2:100184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Gooijer MC, Zhang P, Buil LCM, Çitirikkaya CH, Thota N, Beijnen JH, van Tellingen O (2018c) Buparlisib is a brain penetrable pan-PI3K inhibitor. Sci Rep 8:10784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Gooijer MC, Zhang P, Weijer R, Buil LCM, Beijnen JH, van Tellingen O (2018d) The impact of P-glycoprotein and breast cancer resistance protein on the brain pharmacokinetics and pharmacodynamics of a panel of MEK inhibitors. Int J Cancer 142:381–391. [DOI] [PubMed] [Google Scholar]
- de Vries EE, van den Munckhof B, Braun KP, van Royen-Kerkhof A, de Jager W, Jansen FE (2016) Inflammatory mediators in human epilepsy: a systematic review and meta-analysis. Neurosci Biobehav Rev 63:177–190. [DOI] [PubMed] [Google Scholar]
- de Vries NA, Buckle T, Zhao J, Beijnen JH, Schellens JH, van Tellingen O (2012) Restricted brain penetration of the tyrosine kinase inhibitor erlotinib due to the drug transporters P-gp and BCRP. Invest New Drugs 30:443–449. [DOI] [PubMed] [Google Scholar]
- de Vries NA, Zhao J, Kroon E, Buckle T, Beijnen JH, van Tellingen O (2007) P-glycoprotein and breast cancer resistance protein: two dominant transporters working together in limiting the brain penetration of topotecan. Clin Cancer Res 13:6440–6449. [DOI] [PubMed] [Google Scholar]
- Dean M, Rzhetsky A, Allikmets R (2001) The human ATP-binding cassette (ABC) transporter superfamily. Genome Res 11:1156–1166. [DOI] [PubMed] [Google Scholar]
- Deane R, Wu Z, Sagare A, Davis J, Du Yan S, Hamm K, Xu F, Parisi M, LaRue B, Hu HW, et al. (2004) LRP/amyloid β-peptide interaction mediates differential brain efflux of Abeta isoforms. Neuron 43:333–344. [DOI] [PubMed] [Google Scholar]
- Delord M, Rousselot P, Cayuela JM, Sigaux F, Guilhot J, Preudhomme C, Guilhot F, Loiseau P, Raffoux E, Geromin D, et al. (2013) High imatinib dose overcomes insufficient response associated with ABCG2 haplotype in chronic myelogenous leukemia patients. Oncotarget 4:1582–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMars KM, Yang C, Hawkins KE, McCrea AO, Siwarski DM, Candelario-Jalil E (2017) Spatiotemporal changes in P-glycoprotein levels in brain and peripheral tissues following ischemic stroke in rats. J Exp Neurosci 11:1179069517701741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng D, Xu C, Sun P, Wu J, Yan C, Hu M, Yan N (2014) Crystal structure of the human glucose transporter GLUT1. Nature 510:121–125. [DOI] [PubMed] [Google Scholar]
- Deng X, Shao Y, Xie Y, Feng Y, Wu M, Wang M, Chen Y (2019) MicroRNA-146a-5p downregulates the expression of P-glycoprotein in rats with lithium-pilocarpine-induced status epilepticus. Biol Pharm Bull 42:744–750. [DOI] [PubMed] [Google Scholar]
- Deptala A, Bedner E, Gorczyca W, Darzynkiewicz Z (1998) Activation of nuclear factor kappa B (NF-kappaB) assayed by laser scanning cytometry (LSC). Cytometry 33:376–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmarais G, Charest G, Fortin D, Bujold R, Mathieu D, Paquette B (2015) Cyclooxygenase-2 inhibitor prevents radiation-enhanced infiltration of F98 glioma cells in brain of Fischer rat. Int J Radiat Biol 91:624–633. [DOI] [PubMed] [Google Scholar]
- Desrayaud S, Guntz P, Scherrmann JM, Lemaire M (1997) Effect of the P-glycoprotein inhibitor, SDZ PSC 833, on the blood and brain pharmacokinetics of colchicine. Life Sci 61:153–163. [DOI] [PubMed] [Google Scholar]
- DeVita VT Jr, Chu E (2008) A history of cancer chemotherapy. Cancer Res 68:8643–8653. [DOI] [PubMed] [Google Scholar]
- Di Leo A, Johnston S, Lee KS, Ciruelos E, Lønning PE, Janni W, O’Regan R, Mouret-Reynier MA, Kalev D, Egle D, et al. (2018) Buparlisib plus fulvestrant in postmenopausal women with hormone-receptor-positive, HER2-negative, advanced breast cancer progressing on or after mTOR inhibition (BELLE-3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 19:87–100. [DOI] [PubMed] [Google Scholar]
- Dickens D, Owen A, Alfirevic A, Pirmohamed M (2013) ABCB1 single nucleotide polymorphisms (1236C>T, 2677G>T, and 3435C>T) do not affect transport activity of human P-glycoprotein. Pharmacogenet Genomics 23:314–323. [DOI] [PubMed] [Google Scholar]
- Ding Y, Zhong Y, Baldeshwiler A, Abner EL, Bauer B, Hartz AMS (2021) Protecting P-glycoprotein at the blood-brain barrier from degradation in an Alzheimer’s disease mouse model. Fluids Barriers CNS 18:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixit SG, Zingarelli B, Buckley DJ, Buckley AR, Pauletti GM (2005) Nitric oxide mediates increased P-glycoprotein activity in interferon-gamma-stimulated human intestinal cells. Am J Physiol Gastrointest Liver Physiol 288:G533–G540. [DOI] [PubMed] [Google Scholar]
- Dohgu S, Yamauchi A, Takata F, Naito M, Tsuruo T, Higuchi S, Sawada Y, Kataoka Y (2004) Transforming growth factor-beta1 upregulates the tight junction and P-glycoprotein of brain microvascular endothelial cells. Cell Mol Neurobiol 24:491–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombrowski SM, Desai SY, Marroni M, Cucullo L, Goodrich K, Bingaman W, Mayberg MR, Bengez L, Janigro D (2001) Overexpression of multiple drug resistance genes in endothelial cells from patients with refractory epilepsy. Epilepsia 42:1501–1506. [DOI] [PubMed] [Google Scholar]
- Dong Z, Cui H (2019) Epigenetic modulation of metabolism in glioblastoma. Semin Cancer Biol 57:45–51. [DOI] [PubMed] [Google Scholar]
- Doolittle ND, Miner ME, Hall WA, Siegal T, Jerome E, Osztie E, McAllister LD, Bubalo JS, Kraemer DF, Fortin D, et al. (2000) Safety and efficacy of a multicenter study using intraarterial chemotherapy in conjunction with osmotic opening of the blood-brain barrier for the treatment of patients with malignant brain tumors. Cancer 88:637–647. [DOI] [PubMed] [Google Scholar]
- Dörner B, Kuntner C, Bankstahl JP, Bankstahl M, Stanek J, Wanek T, Stundner G, Mairinger S, Löscher W, Müller M, et al. (2009) Synthesis and small-animal positron emission tomography evaluation of [11C]-elacridar as a radiotracer to assess the distribution of P-glycoprotein at the blood-brain barrier. J Med Chem 52:6073–6082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle LA, Yang W, Abruzzo LV, Krogmann T, Gao Y, Rishi AK, Ross DD (1998) A multidrug resistance transporter from human MCF-7 breast cancer cells. Proc Natl Acad Sci USA 95:15665–15670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drion N, Lemaire M, Lefauconnier J-M, Scherrmann JM (1996) Role of P-glycoprotein in the blood-brain transport of colchicine and vinblastine. J Neurochem 67:1688–1693. [DOI] [PubMed] [Google Scholar]
- Duchatel R, Jackson E, Patabendige A, Cain J, Tsoli M, Monje M, Alvaro F, Ziegler D, Dun M (2019) Targeting PI3K using the blood brain barrier penetrable inhibitor, GDC-0084, for the treatment of diffuse intrinsic pontine glioma (DIPG). Neuro-oncol 21:ii68. [Google Scholar]
- Dudek AZ, Raza A, Chi M, Singhal M, Oberoi R, Mittapalli RK, Agarwal S, Elmquist WF (2013) Brain metastases from renal cell carcinoma in the era of tyrosine kinase inhibitors. Clin Genitourin Cancer 11:155–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy MJ, Synnott NC, Crown J (2017) Mutant p53 as a target for cancer treatment. Eur J Cancer 83:258–265. [DOI] [PubMed] [Google Scholar]
- Duntze J, Litré CF, Eap C, Théret E, Debreuve A, Jovenin N, Lechapt-Zalcman E, Metellus P, Colin P, Guillamo JS, et al. (2013) Implanted carmustine wafers followed by concomitant radiochemotherapy to treat newly diagnosed malignant gliomas: prospective, observational, multicenter study on 92 cases. Ann Surg Oncol 20:2065–2072. [DOI] [PubMed] [Google Scholar]
- Edvinsson L, Owman C (1975) A pharmacologic comparison of histamine receptors in isolated extracranial and intracranial arteries in vitro. Neurology 25:271–276. [DOI] [PubMed] [Google Scholar]
- Edwards JE, Brouwer KR, McNamara PJ (2002) GF120918, a P-glycoprotein modulator, increases the concentration of unbound amprenavir in the central nervous system in rats. Antimicrob Agents Chemother 46:2284–2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ee PL, Kamalakaran S, Tonetti D, He X, Ross DD, Beck WT (2004) Identification of a novel estrogen response element in the breast cancer resistance protein (ABCG2) gene. Cancer Res 64:1247–1251. [DOI] [PubMed] [Google Scholar]
- Ehrlich P (1885) Das Sauerstoff-Bedürfniss des Organismus, A.Hirschwald, Berlin, Germany. [Google Scholar]
- Eisenblätter T, Galla H-J (2002) A new multidrug resistance protein at the blood-brain barrier. Biochem Biophys Res Commun 293:1273–1278. [DOI] [PubMed] [Google Scholar]
- El-Osta A, Kantharidis P, Zalcberg JR, Wolffe AP (2002) Precipitous release of methyl-CpG binding protein 2 and histone deacetylase 1 from the methylated human multidrug resistance gene (MDR1) on activation. Mol Cell Biol 22:1844–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Hafny B, Chappey O, Piciotti M, Debray M, Boval B, Roux F (1997) Modulation of P-glycoprotein activity by glial factors and retinoic acid in an immortalized rat brain microvessel endothelial cell line. Neurosci Lett 236:107–111. [DOI] [PubMed] [Google Scholar]
- ElAli A, Hermann DM (2010) Apolipoprotein E controls ATP-binding cassette transporters in the ischemic brain. Sci Signal 3:ra72. [DOI] [PubMed] [Google Scholar]
- Engelhardt B, Vajkoczy P, Weller RO (2017) The movers and shapers in immune privilege of the CNS. Nat Immunol 18:123–131. [DOI] [PubMed] [Google Scholar]
- Erol K, Kiliç FS, Batu OS, Yildirim E (2001) Morning-evening administration time differences in digoxin kinetics in healthy young subjects. Chronobiol Int 18:841–849. [DOI] [PubMed] [Google Scholar]
- Eskilsson E, Røsland GV, Solecki G, Wang Q, Harter PN, Graziani G, Verhaak RGW, Winkler F, Bjerkvig R, Miletic H (2018) EGFR heterogeneity and implications for therapeutic intervention in glioblastoma. Neuro-oncol 20:743–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans RM (1988) The steroid and thyroid hormone receptor superfamily. Science 240:889–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evseenko DA, Paxton JW, Keelan JA (2007) Independent regulation of apical and basolateral drug transporter expression and function in placental trophoblasts by cytokines, steroids, and growth factors. Drug Metab Dispos 35:595–601. [DOI] [PubMed] [Google Scholar]
- Fan K, Jia X, Zhou M, Wang K, Conde J, He J, Tian J, Yan X (2018) Ferritin nanocarrier traverses the blood brain barrier and kills glioma. ACS Nano 12:4105–4115. [DOI] [PubMed] [Google Scholar]
- Felix RA, Barrand MA (2002) P-glycoprotein expression in rat brain endothelial cells: evidence for regulation by transient oxidative stress. J Neurochem 80:64–72. [DOI] [PubMed] [Google Scholar]
- Fellner S, Bauer B, Miller DS, Schaffrik M, Fankhänel M, Spruss T, Bernhardt G, Graeff C, Färber L, Gschaidmeier H, et al. (2002) Transport of paclitaxel (Taxol) across the blood-brain barrier in vitro and in vivo. J Clin Invest 110:1309–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferry D, Price L, Atsmon J(2001) A phase IIa pharmacokinetic and pharmacodynamic study of the P-glycoprotein inhibitor, XR9576 in patients treated with doxorubicin chemotherapy. Proc Annu Meet Am Soc Clin Oncol 42:950. [Google Scholar]
- Filipski E, Berland E, Ozturk N, Guettier C, van der Horst GT, Lévi F, Okyar A (2014) Optimization of irinotecan chronotherapy with P-glycoprotein inhibition. Toxicol Appl Pharmacol 274:471–479. [DOI] [PubMed] [Google Scholar]
- Fisher M, Abramov M, Van Aerschot A, Xu D, Juliano RL, Herdewijn P (2007) Inhibition of MDR1 expression with altritol-modified siRNAs. Nucleic Acids Res 35:1064–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald DC, Meade KG, McEvoy AN, Lillis L, Murphy EP, MacHugh DE, Baird AW (2007) Tumour necrosis factor-alpha (TNF-alpha) increases nuclear factor kappaB (NFkappaB) activity in and interleukin-8 (IL-8) release from bovine mammary epithelial cells. Vet Immunol Immunopathol 116:59–68. [DOI] [PubMed] [Google Scholar]
- Fojo A, Lebo R, Shimizu N, Chin JE, Roninson IB, Merlino GT, Gottesman MM, Pastan I (1986) Localization of multidrug resistance-associated DNA sequences to human chromosome 7. Somat Cell Mol Genet 12:415–420. [DOI] [PubMed] [Google Scholar]
- Fox E, Bates SE (2007) Tariquidar (XR9576): a P-glycoprotein drug efflux pump inhibitor. Expert Rev Anticancer Ther 7:447–459. [DOI] [PubMed] [Google Scholar]
- Frank HJ, Pardridge WM (1981) A direct in vitro demonstration of insulin binding to isolated brain microvessels. Diabetes 30:757–761. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, Irenius E, Kull B, Schulte G (2001) Comparison of the potency of adenosine as an agonist at human adenosine receptors expressed in Chinese hamster ovary cells. Biochem Pharmacol 61:443–448. [DOI] [PubMed] [Google Scholar]
- Fresegna D, Bullitta S, Musella A, Rizzo FR, De Vito F, Guadalupi L, Caioli S, Balletta S, Sanna K, Dolcetti E, et al. (2020) Re-examining the role of TNF in MS pathogenesis and therapy. Cells 9:2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey, 2nd WH; Ramsey Foundation; (1997) Method for administering neurologic agents to the brain. U.S. patent 5,624,898. [Google Scholar]
- Frey, 2nd WH; Chiron Corporation; (2001) Method for administering insulin to the brain. U.S. patent 6313093B1. [Google Scholar]
- Friche E, Jensen PB, Nissen NI (1992) Comparison of cyclosporin A and SDZ PSC833 as multidrug-resistance modulators in a daunorubicin-resistant Ehrlich ascites tumor. Cancer Chemother Pharmacol 30:235–237. [DOI] [PubMed] [Google Scholar]
- Fung KL, Gottesman MM (2009) A synonymous polymorphism in a common MDR1 (ABCB1) haplotype shapes protein function. Biochim Biophys Acta 1794:860–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa T, Wakabayashi K, Tamura A, Nakagawa H, Morishima Y, Osawa Y, Ishikawa T (2009) Major SNP (Q141K) variant of human ABC transporter ABCG2 undergoes lysosomal and proteasomal degradations. Pharm Res 26:469–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gampa G, Kim M, Cook-Rostie N, Laramy JK, Sarkaria JN, Paradiso L, DePalatis L, Elmquist WF (2018) Brain distribution of a novel MEK inhibitor E6201: implications in the treatment of melanoma brain metastases. Drug Metab Dispos 46:658–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gampa G, Kim M, Mohammad AS, Parrish KE, Mladek AC, Sarkaria JN, Elmquist WF (2019) Brain distribution and active efflux of three panRAF inhibitors: considerations in the treatment of melanoma brain metastases. J Pharmacol Exp Ther 368:446–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Lau EYY, Wan JH, Lau CS, Mok MY (2016) Systemic lupus erythematosus patients with past neuropsychiatric involvement are associated with worse cognitive impairment: a longitudinal study. Lupus 25:637–644. [DOI] [PubMed] [Google Scholar]
- Gao Y, Zhang E, Liu B, Zhou K, He S, Feng L, Wu G, Cao M, Wu H, Cui Y, et al. (2019) Integrated analysis identified core signal pathways and hypoxic characteristics of human glioblastoma. J Cell Mol Med 23:6228–6237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebhard S (2012) ABC transporters of antimicrobial peptides in Firmicutes bacteria—phylogeny, function and regulation. Mol Microbiol 86:1295–1317. [DOI] [PubMed] [Google Scholar]
- Gekakis N, Staknis D, Nguyen HB, Davis FC, Wilsbacher LD, King DP, Takahashi JS, Weitz CJ (1998) Role of the CLOCK protein in the mammalian circadian mechanism. Science 280:1564–1569. [DOI] [PubMed] [Google Scholar]
- Genovese I, Ilari A, Assaraf YG, Fazi F, Colotti G (2017) Not only P-glycoprotein: amplification of the ABCB1-containing chromosome region 7q21 confers multidrug resistance upon cancer cells by coordinated overexpression of an assortment of resistance-related proteins. Drug Resist Updat 32:23–46. [DOI] [PubMed] [Google Scholar]
- Ghersi-Egea JF, Leninger-Muller B, Suleman G, Siest G, Minn A (1994) Localization of drug-metabolizing enzyme activities to blood-brain interfaces and circumventricular organs. J Neurochem 62:1089–1096. [DOI] [PubMed] [Google Scholar]
- Gibbs ME, Wilt LA, Ledwitch KV, Roberts AG (2018) A conformationally gated model of methadone and loperamide transport by P-glycoprotein. J Pharm Sci 107:1937–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore TD (2006) Introduction to NF-kappaB: players, pathways, perspectives. Oncogene 25:6680–6684. [DOI] [PubMed] [Google Scholar]
- Goldmann EE (1909) Die äussere und innere sekretion des gesunden und kranken organismus im lichte der “vitalen Färbung.” Beiträg Klinische Chirurgie 64:192–265. [Google Scholar]
- Goldmann EE (1913) Vitalfärbung am zentralnervensyatem. Beitrag zur physio-pathologie des plexus chorioideus und der Hirnhäute. Abh preuss. Akad Wiss Phys-Math Kl 1:1–60. [Google Scholar]
- Gooch CL, Pracht E, Borenstein AR (2017) The burden of neurological disease in the United States: a summary report and call to action. Ann Neurol 81:479–484. [DOI] [PubMed] [Google Scholar]
- Goodman LS, Wintrobe MM, Sameshek W, Goodman MJ, Gilman AG, McLennan MT (1946) Use of methyl-bid(beta-chloroethyl)amine hydrochloride and Tris(beta-chloroethyl)amine hydrochloride for Hodgkin’s disease, lymphosarcoma, leukemia and certain allied and miscellaneous disorders. JAMA 132:126–132. [DOI] [PubMed] [Google Scholar]
- Gotoh J, Kuang TY, Nakao Y, Cohen DM, Melzer P, Itoh Y, Pak H, Pettigrew K, Sokoloff L (2001) Regional differences in mechanisms of cerebral circulatory response to neuronal activation. Am J Physiol Heart Circ Physiol 280:H821–H829. [DOI] [PubMed] [Google Scholar]
- Gottesman MM, Pastan I (1993) Biochemistry of multidrug resistance mediated by the multidrug transporter. Annu Rev Biochem 62:385–427. [DOI] [PubMed] [Google Scholar]
- Grewal GK, Kukal S, Kanojia N, Saso L, Kukreti S, Kukreti R (2017) Effect of oxidative stress on ABC transporters: contribution to epilepsy pharmacoresistance. Molecules 22:365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grommes C, Karlo JC, Caprariello A, Blankenship D, Dechant A, Landreth GE (2013) The PPARγ agonist pioglitazone crosses the blood-brain barrier and reduces tumor growth in a human xenograft model. Cancer Chemother Pharmacol 71:929–936. [DOI] [PubMed] [Google Scholar]
- Gros P, Croop J, Housman D (1986) Mammalian multidrug resistance gene: complete cDNA sequence indicates strong homology to bacterial transport proteins. Cell 47:371–380. [DOI] [PubMed] [Google Scholar]
- Haas J, Storch-Hagenlocher B, Biessmann A, Wildemann B (2002) Inducible nitric oxide synthase and argininosuccinate synthetase: co-induction in brain tissue of patients with Alzheimer’s dementia and following stimulation with beta-amyloid 1-42 in vitro. Neurosci Lett 322:121–125. [DOI] [PubMed] [Google Scholar]
- Ham SW, Jeon HY, Jin X, Kim EJ, Kim JK, Shin YJ, Lee Y, Kim SH, Lee SY, Seo S, et al. (2019) TP53 gain-of-function mutation promotes inflammation in glioblastoma. Cell Death Differ 26:409–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada H, Tsuruo T (1986) Functional role for the 170- to 180-kDa glycoprotein specific to drug-resistant tumor cells as revealed by monoclonal antibodies. Proc Natl Acad Sci USA 83:7785–7789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammad AM, Alasmari F, Sari Y, Scott Hall F, Tiwari AK (2019) Alcohol and cocaine exposure modulates ABCB1 and ABCG2 transporters in male alcohol-preferring rats. Mol Neurobiol 56:1921–1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S, Lv X, Wang Y, Gong H, Zhang C, Tong A, Zhang B, Yao H (2015) Effect and mechanism of peroxisome proliferator-activated receptor-γ on the drug resistance of the U-87 MG/CDDP human malignant glioma cell line. Mol Med Rep 12:2239–2246. [DOI] [PubMed] [Google Scholar]
- Harati R, Benech H, Villégier AS, Mabondzo A (2013) P-glycoprotein, breast cancer resistance protein, organic anion transporter 3, and transporting peptide 1a4 during blood-brain barrier maturation: involvement of Wnt/β-catenin and endothelin-1 signaling. Mol Pharm 10:1566–1580. [DOI] [PubMed] [Google Scholar]
- Harati R, Villégier AS, Banks WA, Mabondzo A (2012) Susceptibility of juvenile and adult blood-brain barrier to endothelin-1: regulation of P-glycoprotein and breast cancer resistance protein expression and transport activity. J Neuroinflammation 9:273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haritova AM, Schrickx J, Lashev LD, Fink-Gremmels J (2008) Expression of MDR1, MRP2 and BCRP mRNA in tissues of turkeys. J Vet Pharmacol Ther 31:378–385. [DOI] [PubMed] [Google Scholar]
- Hartmann DA, Underly RG, Grant RI, Watson AN, Lindner V, Shih AY (2015) Pericyte structure and distribution in the cerebral cortex revealed by high-resolution imaging of transgenic mice. Neurophotonics 2:041402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartz AM, Bauer B (2011) ABC transporters in the CNS—an inventory. Curr Pharm Biotechnol 12:656–673. [DOI] [PubMed] [Google Scholar]
- Hartz ABB, Baehr C, Miller D, Fricker G (2005) Drug delivery across the blood-brain barrier. Curr Nanosci 1:203–209. [Google Scholar]
- Hartz AM, Bauer B (2010) Regulation of ABC transporters at the blood-brain barrier: new targets for CNS therapy. Mol Interv 10:293–304. [DOI] [PubMed] [Google Scholar]
- Hartz AM, Bauer B, Fricker G, Miller DS (2004) Rapid regulation of P-glycoprotein at the blood-brain barrier by endothelin-1. Mol Pharmacol 66:387–394. [DOI] [PubMed] [Google Scholar]
- Hartz AM, Bauer B, Fricker G, Miller DS (2006) Rapid modulation of P-glycoprotein-mediated transport at the blood-brain barrier by tumor necrosis factor-alpha and lipopolysaccharide. Mol Pharmacol 69:462–470. [DOI] [PubMed] [Google Scholar]
- Hartz AM, Bauer B, Soldner EL, Wolf A, Boy S, Backhaus R, Mihaljevic I, Bogdahn U, Klünemann HH, Schuierer G, et al. (2012) Amyloid-β contributes to blood-brain barrier leakage in transgenic human amyloid precursor protein mice and in humans with cerebral amyloid angiopathy. Stroke 43:514–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartz AM, Madole EK, Miller DS, Bauer B (2010a) Estrogen receptor beta signaling through phosphatase and tensin homolog/phosphoinositide 3-kinase/Akt/glycogen synthase kinase 3 down-regulates blood-brain barrier breast cancer resistance protein. J Pharmacol Exp Ther 334:467–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartz AM, Mahringer A, Miller DS, Bauer B (2010b) 17-β-Estradiol: a powerful modulator of blood-brain barrier BCRP activity. J Cereb Blood Flow Metab 30:1742–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartz AM, Miller DS, Bauer B (2010c) Restoring blood-brain barrier P-glycoprotein reduces brain amyloid-beta in a mouse model of Alzheimer’s disease. Mol Pharmacol 77:715–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartz AM, Notenboom S, Bauer B (2009) Signaling to P-glycoprotein—a new therapeutic target to treat drug-resistant epilepsy? Drug News Perspect 22:393–397. [DOI] [PubMed] [Google Scholar]
- Hartz AM, Pekcec A, Soldner EL, Zhong Y, Schlichtiger J, Bauer B (2017) P-gp protein expression and transport activity in rodent seizure models and human epilepsy. Mol Pharm 14:999–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartz AMS, Rempe RG, Soldner ELB, Pekcec A, Schlichtiger J, Kryscio R, Bauer B (2019) Cytosolic phospholipase A2 is a key regulator of blood-brain barrier function in epilepsy. FASEB J 33:14281–14295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartz AM, Zhong Y, Wolf A, LeVine H 3rd, Miller DS, Bauer B (2016) Aβ40 reduces P-glycoprotein at the blood-brain barrier through the ubiquitin-proteasome pathway. J Neurosci 36:1930–1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartz AMS, Zhong Y, Shen AN, Abner EL, Bauer B (2018) Preventing P-gp ubiquitination lowers Aβ brain levels in an Alzheimer’s disease mouse model. Front Aging Neurosci 10:186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haskó G, Csóka B, Németh ZH, Vizi ES, Pacher P (2009) A(2B) adenosine receptors in immunity and inflammation. Trends Immunol 30:263–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan HE, Myers AL, Lee IJ, Coop A, Eddington ND (2007) Oxycodone induces overexpression of P-glycoprotein (ABCB1) and affects paclitaxel’s tissue distribution in Sprague Dawley rats. J Pharm Sci 96:2494–2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori S, Suda A, Kishida I, Miyauchi M, Shiraishi Y, Fujibayashi M, Tsujita N, Ishii C, Ishii N, Moritani T, et al. (2018) Effects of ABCB1 gene polymorphisms on autonomic nervous system activity during atypical antipsychotic treatment in schizophrenia. BMC Psychiatry 18:231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hau VS, Huber JD, Campos CR, Davis RT, Davis TP (2004) Effect of lambda-carrageenan-induced inflammatory pain on brain uptake of codeine and antinociception. Brain Res 1018:257–264. [DOI] [PubMed] [Google Scholar]
- Haus E, Halberg F, Pauly JE, Cardoso S, Kühl JF, Sothern RB, Shiotsuka RN, Hwang DS (1972) Increased tolerance of leukemic mice to arabinosyl cytosine with schedule adjusted to circadian system. Science 177:80–82. [DOI] [PubMed] [Google Scholar]
- Hawkins BT, Rigor RR, Miller DS (2010) Rapid loss of blood-brain barrier P-glycoprotein activity through transporter internalization demonstrated using a novel in situ proteolysis protection assay. J Cereb Blood Flow Metab 30:1593–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heemskerk S, Peters JG, Louisse J, Sagar S, Russel FG, Masereeuw R (2010) Regulation of P-glycoprotein in renal proximal tubule epithelial cells by LPS and TNF-alpha. J Biomed Biotechnol 2010:525180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffron TP (2016) Small molecule kinase inhibitors for the treatment of brain cancer. J Med Chem 59:10030–10066. [DOI] [PubMed] [Google Scholar]
- Heffron TP, McClory A, Stumpf A (2016a) The discovery and process chemistry development of GDC-0084, a brain penetrating inhibitor of PI3K and mTOR, in Comprehensive Accounts of Pharmaceutical Research and Development: from Discovery to Late Stage Process Development, Vol. 1 (AbdelMagid AF, Pesti J, Vaidyanathan R, eds) pp 147–173, American Chemical Society, Washington, DC. [Google Scholar]
- Heffron TP, Ndubaku CO, Salphati L, Alicke B, Cheong J, Drobnick J, Edgar K, Gould SE, Lee LB, Lesnick JD, et al. (2016b) Discovery of clinical development candidate GDC-0084, a brain penetrant inhibitor of PI3K and mTOR. ACS Med Chem Lett 7:351–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, Kros JM, Hainfellner JA, Mason W, Mariani L, et al. (2005) MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med 352:997–1003. [DOI] [PubMed] [Google Scholar]
- Hellström M, Gerhardt H, Kalén M, Li X, Eriksson U, Wolburg H, Betsholtz C (2001) Lack of pericytes leads to endothelial hyperplasia and abnormal vascular morphogenesis. J Cell Biol 153:543–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrikse NH, de Vries EG, Eriks-Fluks L, van der Graaf WT, Hospers GA, Willemsen AT, Vaalburg W, Franssen EJ (1999) A new in vivo method to study P-glycoprotein transport in tumors and the blood-brain barrier. Cancer Res 59:2411–2416. [PubMed] [Google Scholar]
- Hendrikx JJ, Lagas JS, Wagenaar E, Rosing H, Schellens JH, Beijnen JH, Schinkel AH (2014) Oral co-administration of elacridar and ritonavir enhances plasma levels of oral paclitaxel and docetaxel without affecting relative brain accumulation. Br J Cancer 110:2669–2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heppner FL, Ransohoff RM, Becher B (2015) Immune attack: the role of inflammation in Alzheimer disease. Nat Rev Neurosci 16:358–372. [DOI] [PubMed] [Google Scholar]
- Herndon JM, Tome ME, Davis TP(2017) Development and maintenance of the blood–brain barrier, in Primer on Cerebrovascular Diseases (Caplan LR ed) pp 51–56, Academic Press, New York, NY. [Google Scholar]
- Higgins CF, Gottesman MM (1992) Is the multidrug transporter a flippase? Trends Biochem Sci 17:18–21. [DOI] [PubMed] [Google Scholar]
- Hitchings GH, Elion GB (1954) The chemistry and biochemistry of purine analogs. Ann N Y Acad Sci 60:195–199. [DOI] [PubMed] [Google Scholar]
- Ho L, Pieroni C, Winger D, Purohit DP, Aisen PS, Pasinetti GM (1999) Regional distribution of cyclooxygenase-2 in the hippocampal formation in Alzheimer’s disease. J Neurosci Res 57:295–303. [DOI] [PubMed] [Google Scholar]
- Hofmann S, Januliene D, Mehdipour AR, Thomas C, Stefan E, Brüchert S, Kuhn BT, Geertsma ER, Hummer G, Tampé R, et al. (2019) Conformation space of a heterodimeric ABC exporter under turnover conditions. Nature 571:580–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoque MT, Robillard KR, Bendayan R (2012) Regulation of breast cancer resistant protein by peroxisome proliferator-activated receptor α in human brain microvessel endothelial cells. Mol Pharmacol 81:598–609. [DOI] [PubMed] [Google Scholar]
- Hoque MT, Shah A, More V, Miller DS, Bendayan R (2015) In vivo and ex vivo regulation of breast cancer resistant protein (Bcrp) by peroxisome proliferator-activated receptor alpha (Pparα) at the blood-brain barrier. J Neurochem 135:1113–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi Y, Uchida Y, Tachikawa M, Ohtsuki S, Couraud PO, Suzuki T, Terasaki T (2019) Oxidative stress-induced activation of Abl and Src kinases rapidly induces P-glycoprotein internalization via phosphorylation of caveolin-1 on tyrosine-14, decreasing cortisol efflux at the blood-brain barrier. J Cereb Blood Flow Metab 40:420–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou W, Ding M, Li X, Zhou X, Zhu Q, Varela-Ramirez A, Yi C (2021) Comparative evaluation of cardiovascular risks among nine FDA-approved VEGFR-TKIs in patients with solid tumors: a Bayesian network analysis of randomized controlled trials. J Cancer Res Clin Oncol 147:2407–2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Li J, Fu M, Zhao X, Wang W (2021) The JAK/STAT signaling pathway: from bench to clinic. Signal Transduct Target Ther 6:402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang FF, Wu DS, Zhang L, Yu YH, Yuan XY, Li WJ, Chen XP, Zhao XL, Chen FP, Zeng H (2013) Inactivation of PTEN increases ABCG2 expression and the side population through the PI3K/Akt pathway in adult acute leukemia. Cancer Lett 336:96–105. [DOI] [PubMed] [Google Scholar]
- Huang FF, Zhang L, Wu DS, Yuan XY, Yu YH, Zhao XL, Chen FP, Zeng H (2014a) PTEN regulates BCRP/ABCG2 and the side population through the PI3K/Akt pathway in chronic myeloid leukemia. PLoS One 9:e88298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Yang Y, Yang J, Li X (2014b) Regenerating gene family member 4 promotes growth and migration of gastric cancer through protein kinase B pathway. Int J Clin Exp Med 7:3037–3044. [PMC free article] [PubMed] [Google Scholar]
- Huang L, Chen Y, Liu R, Li B, Fei X, Li X, Liu G, Li Y, Xu B, Fang W (2022) P-glycoprotein aggravates blood brain barrier dysfunction in experimental ischemic stroke by inhibiting endothelial autophagy. Aging Dis 13:1546–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard SR (1999) Structural analysis of receptor tyrosine kinases. Prog Biophys Mol Biol 71:343–358. [DOI] [PubMed] [Google Scholar]
- Hubensack M, Müller C, Höcherl P, Fellner S, Spruss T, Bernhardt G, Buschauer A (2008) Effect of the ABCB1 modulators elacridar and tariquidar on the distribution of paclitaxel in nude mice. J Cancer Res Clin Oncol 134:597–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huwyler J, Wu D, Pardridge WM (1996) Brain drug delivery of small molecules using immunoliposomes. Proc Natl Acad Sci USA 93:14164–14169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde SC, Emsley P, Hartshorn MJ, Mimmack MM, Gileadi U, Pearce SR, Gallagher MP, Gill DR, Hubbard RE, Higgins CF (1990) Structural model of ATP-binding proteins associated with cystic fibrosis, multidrug resistance and bacterial transport. Nature 346:362–365. [DOI] [PubMed] [Google Scholar]
- Hynes RO (2009) The extracellular matrix: not just pretty fibrils. Science 326:1216–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynynen K, McDannold N, Vykhodtseva N, Jolesz FA (2001) Noninvasive MR imaging-guided focal opening of the blood-brain barrier in rabbits. Radiology 220:640–646. [DOI] [PubMed] [Google Scholar]
- Iadecola C (2017) The neurovascular unit coming of age: a journey through Neurovascular coupling in health and disease. Neuron 96:17–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannetti P, Spalice A, Parisi P (2005) Calcium-channel blocker verapamil administration in prolonged and refractory status epilepticus. Epilepsia 46:967–969. [DOI] [PubMed] [Google Scholar]
- Ifergan I, Jansen G, Assaraf YG (2005) Cytoplasmic confinement of breast cancer resistance protein (BCRP/ABCG2) as a novel mechanism of adaptation to short-term folate deprivation. Mol Pharmacol 67:1349–1359. [DOI] [PubMed] [Google Scholar]
- Ifergan I, Shafran A, Jansen G, Hooijberg JH, Scheffer GL, Assaraf YG (2004) Folate deprivation results in the loss of breast cancer resistance protein (BCRP/ABCG2) expression. A role for BCRP in cellular folate homeostasis. J Biol Chem 279:25527–25534. [DOI] [PubMed] [Google Scholar]
- Imai Y, Asada S, Tsukahara S, Ishikawa E, Tsuruo T, Sugimoto Y (2003) Breast cancer resistance protein exports sulfated estrogens but not free estrogens. Mol Pharmacol 64:610–618. [DOI] [PubMed] [Google Scholar]
- Imai Y, Ishikawa E, Asada S, Sugimoto Y (2005) Estrogen-mediated post transcriptional down-regulation of breast cancer resistance protein/ABCG2. Cancer Res 65:596–604. [PubMed] [Google Scholar]
- Imai Y, Tsukahara S, Ishikawa E, Tsuruo T, Sugimoto Y (2002) Estrone and 17β-estradiol reverse breast cancer resistance protein-mediated multidrug resistance. Jpn J Cancer Res 93:231–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ippen FM, Grosch JK, Subramanian M, Kuter BM, Liederer BM, Plise EG, Mora JL, Nayyar N, Schmidt SP, Giobbie-Hurder A, et al. (2019) Targeting the PI3K/Akt/mTOR pathway with the pan-Akt inhibitor GDC-0068 in PIK3CA-mutant breast cancer brain metastases. Neuro-oncol 21:1401–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal M, Baello S, Javam M, Audette MC, Gibb W, Matthews SG (2016) Regulation of multidrug resistance p-glycoprotein in the developing blood-brain barrier: interplay between glucocorticoids and cytokines. J Neuroendocrinol 28:12360. [DOI] [PubMed] [Google Scholar]
- Iqbal M, Ho HL, Petropoulos S, Moisiadis VG, Gibb W, Matthews SG (2012) Pro-inflammatory cytokine regulation of P-glycoprotein in the developing blood-brain barrier. PLoS One 7:e43022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K, Uchida Y, Ohtsuki S, Aizawa S, Kawakami H, Katsukura Y, Kamiie J, Terasaki T (2011) Quantitative membrane protein expression at the blood-brain barrier of adult and younger cynomolgus monkeys. J Pharm Sci 100:3939–3950. [DOI] [PubMed] [Google Scholar]
- Iwasaki M, Koyanagi S, Suzuki N, Katamune C, Matsunaga N, Watanabe N, Takahashi M, Izumi T, Ohdo S (2015) Circadian modulation in the intestinal absorption of P-glycoprotein substrates in monkeys. Mol Pharmacol 88:29–37. [DOI] [PubMed] [Google Scholar]
- Jablonski MR, Jacob DA, Campos C, Miller DS, Maragakis NJ, Pasinelli P, Trotti D (2012) Selective increase of two ABC drug efflux transporters at the blood-spinal cord barrier suggests induced pharmacoresistance in ALS. Neurobiol Dis 47:194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablonski MR, Markandaiah SS, Jacob D, Meng NJ, Li K, Gennaro V, Lepore AC, Trotti D, Pasinelli P (2014) Inhibiting drug efflux transporters improves efficacy of ALS therapeutics. Ann Clin Transl Neurol 1:996–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson S, Anders NM, Mangraviti A, Wanjiku TM, Sankey EW, Liu A, Brem H, Tyler B, Rudek MA, Grossman SA (2016) The effect of regadenoson-induced transient disruption of the blood-brain barrier on temozolomide delivery to normal rat brain. J Neurooncol 126:433–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson S, George RT, Lodge MA, Piotrowski A, Wahl RL, Gujar SK, Grossman SA (2017) The effect of regadenoson on the integrity of the human blood-brain barrier, a pilot study. J Neurooncol 132:513–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson S, Weingart J, Nduom EK, Harfi TT, George RT, McAreavey D, Ye X, Anders NM, Peer C, Figg WD, et al. (2018) The effect of an adenosine A2A agonist on intra-tumoral concentrations of temozolomide in patients with recurrent glioblastoma. Fluids Barriers CNS 15:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs MD, Harrison SC (1998) Structure of an IkappaBalpha/NF-kappaB complex. Cell 95:749–758. [DOI] [PubMed] [Google Scholar]
- Jagadeeshan S, David D, Jisha S, Manjula S, Asha Nair S (2017) Solanum nigrum unripe fruit fraction attenuates adriamycin resistance by down-regulating multi-drug resistance protein (Mdr)-1 through Jak-STAT pathway. BMC Complement Altern Med 17:370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeynes B, Provias J (2011a) The case for blood-brain barrier dysfunction in the pathogenesis of Alzheimer’s disease. J Neurosci Res 89:22–28. [DOI] [PubMed] [Google Scholar]
- Jeynes B, Provias J (2011b) An investigation into the role of P-glycoprotein in Alzheimer’s disease lesion pathogenesis. Neurosci Lett 487:389–393. [DOI] [PubMed] [Google Scholar]
- Jiang J, Qiu J, Li Q, Shi Z (2017) Prostaglandin E2 signaling: alternative target for glioblastoma? Trends Cancer 3:75–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Z, Pore N, Cerniglia GJ, Mick R, Georgescu MM, Bernhard EJ, Hahn SM, Gupta AK, Maity A (2007) Phosphatase and tensin homologue deficiency in glioblastoma confers resistance to radiation and temozolomide that is reversed by the protease inhibitor nelfinavir. Cancer Res 67:4467–4473. [DOI] [PubMed] [Google Scholar]
- Jin S, Scotto KW (1998) Transcriptional regulation of the MDR1 gene by histone acetyltransferase and deacetylase is mediated by NF-Y. Mol Cell Biol 18:4377–4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joó F (1985) The blood-brain barrier in vitro: ten years of research on microvessels isolated from the brain. Neurochem Int 7:1–25. [DOI] [PubMed] [Google Scholar]
- Joó F (1993) The blood-brain barrier in vitro: the second decade. Neurochem Int 23:499–521. [DOI] [PubMed] [Google Scholar]
- Joshi G, Aluise CD, Cole MP, Sultana R, Pierce WM, Vore M, St Clair DK, Butterfield DA (2010) Alterations in brain antioxidant enzymes and redox proteomic identification of oxidized brain proteins induced by the anti-cancer drug adriamycin: implications for oxidative stress-mediated chemobrain. Neuroscience 166:796–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joutel A, Haddad I, Ratelade J, Nelson MT (2016) Perturbations of the cerebrovascular matrisome: a convergent mechanism in small vessel disease of the brain? J Cereb Blood Flow Metab 36:143–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano RL, Ling V (1976) A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochim Biophys Acta 455:152–162. [DOI] [PubMed] [Google Scholar]
- Jumnongprakhon P, Sivasinprasasn S, Govitrapong P, Tocharus C, Tocharus J (2017) Activation of melatonin receptor (MT1/2) promotes P-gp transporter in methamphetamine-induced toxicity on primary rat brain microvascular endothelial cells. Toxicol In Vitro 41:42–48. [DOI] [PubMed] [Google Scholar]
- Kage K, Tsukahara S, Sugiyama T, Asada S, Ishikawa E, Tsuruo T, Sugimoto Y (2002) Dominant-negative inhibition of breast cancer resistance protein as drug efflux pump through the inhibition of S-S dependent homodimerization. Int J Cancer 97:626–630. [DOI] [PubMed] [Google Scholar]
- Kalaria RN, Harik SI (1986) Adenosine receptors of cerebral microvessels and choroid plexus. J Cereb Blood Flow Metab 6:463–470. [DOI] [PubMed] [Google Scholar]
- Kamiie J, Ohtsuki S, Iwase R, Ohmine K, Katsukura Y, Yanai K, Sekine Y, Uchida Y, Ito S, Terasaki T (2008) Quantitative atlas of membrane transporter proteins: development and application of a highly sensitive simultaneous LC/MS/MS method combined with novel in-silico peptide selection criteria. Pharm Res 25:1469–1483. [DOI] [PubMed] [Google Scholar]
- Kannan P, Brimacombe KR, Kreisl WC, Liow JS, Zoghbi SS, Telu S, Zhang Y, Pike VW, Halldin C, Gottesman MM, et al. (2011) Lysosomal trapping of a radiolabeled substrate of P-glycoprotein as a mechanism for signal amplification in PET. Proc Natl Acad Sci USA 108:2593–2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannan P, Schain M, Kretzschmar WW, Weidner L, Mitsios N, Gulyás B, Blom H, Gottesman MM, Innis RB, Hall MD, et al. (2017) An automated method measures variability in P-glycoprotein and ABCG2 densities across brain regions and brain matter. J Cereb Blood Flow Metab 37:2062–2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kariolis MS, Wells RC, Getz JA, Kwan W, Mahon CS, Tong R, Kim DJ, Srivastava A, Bedard C, Henne KR, et al. (2020) Brain delivery of therapeutic proteins using an Fc fragment blood-brain barrier transport vehicle in mice and monkeys. Sci Transl Med 12:eaay1359. [DOI] [PubMed] [Google Scholar]
- Karnushina I, Tóth I, Dux E, Joó F (1980) Presence of the guanylate cyclase in brain capillaries: histochemical and biochemical evidence. Brain Res 189:588–592. [DOI] [PubMed] [Google Scholar]
- Kassem NA, Deane R, Segal MB, Chen R, Preston JE (2007) Thyroxine (T4) transfer from CSF to choroid plexus and ventricular brain regions in rabbit: contributory role of P-glycoprotein and organic anion transporting polypeptides. Brain Res 1181:44–50. [DOI] [PubMed] [Google Scholar]
- Kast RE, Hill QA, Wion D, Mellstedt H, Focosi D, Karpel-Massler G, Heiland T, Halatsch ME (2017) Glioblastoma-synthesized G-CSF and GM-CSF contribute to growth and immunosuppression: potential therapeutic benefit from dapsone, fenofibrate, and ribavirin. Tumour Biol 39:1010428317699797. [DOI] [PubMed] [Google Scholar]
- Katan M, Luft A (2018) Global burden of stroke. Semin Neurol 38:208–211. [DOI] [PubMed] [Google Scholar]
- Kemper EM, Cleypool C, Boogerd W, Beijnen JH, van Tellingen O (2004) The influence of the P-glycoprotein inhibitor zosuquidar trihydrochloride (LY335979) on the brain penetration of paclitaxel in mice. Cancer Chemother Pharmacol 53:173–178. [DOI] [PubMed] [Google Scholar]
- Kemper EM, van Zandbergen AE, Cleypool C, Mos HA, Boogerd W, Beijnen JH, van Tellingen O (2003) Increased penetration of paclitaxel into the brain by inhibition of P-Glycoprotein. Clin Cancer Res 9:2849–2855. [PubMed] [Google Scholar]
- Kervezee L, Hartman R, van den Berg DJ, Shimizu S, Emoto-Yamamoto Y, Meijer JH, de Lange EC (2014) Diurnal variation in P-glycoprotein-mediated transport and cerebrospinal fluid turnover in the brain. AAPS J 16:1029–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilic E, Spudich A, Kilic U, Rentsch KM, Vig R, Matter CM, Wunderli-Allenspach H, Fritschy JM, Bassetti CL, Hermann DM (2008) ABCC1: a gateway for pharmacological compounds to the ischaemic brain. Brain 131:2679–2689. [DOI] [PubMed] [Google Scholar]
- Kim AE, Dintaman JM, Waddell DS, Silverman JA (1998a) Saquinavir, an HIV protease inhibitor, is transported by P-glycoprotein. J Pharmacol Exp Ther 286:1439–1445. [PubMed] [Google Scholar]
- Kim DG, Bynoe MS (2015) A2A adenosine receptor regulates the human blood-brain barrier permeability. Mol Neurobiol 52:664–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DG, Bynoe MS (2016) A2A adenosine receptor modulates drug efflux transporter P-glycoprotein at the blood-brain barrier. J Clin Invest 126:1717–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Laramy JK, Gampa G, Parrish KE, Brundage R, Sarkaria JN, Elmquist WF (2019a) Brain distributional kinetics of a novel MDM2 inhibitor SAR405838: implications for use in brain tumor therapy. Drug Metab Dispos 47:1403–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Laramy JK, Mohammad AS, Talele S, Fisher J, Sarkaria JN, Elmquist WF (2019b) Brain distribution of a panel of epidermal growth factor receptor inhibitors using cassette dosing in wild-type and Abcb1/Abcg2-deficient mice. Drug Metab Dispos 47:393–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Ma DJ, Calligaris D, Zhang S, Feathers RW, Vaubel RA, Meaux I, Mladek AC, Parrish KE, Jin F, et al. (2018) Efficacy of the MDM2 inhibitor SAR405838 in glioblastoma is limited by poor distribution across the blood-brain barrier. Mol Cancer Ther 17:1893–1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim RB, Fromm MF, Wandel C, Leake B, Wood AJ, Roden DM, Wilkinson GR (1998b) The drug transporter P-glycoprotein limits oral absorption and brain entry of HIV-1 protease inhibitors. J Clin Invest 101:289–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimchi-Sarfaty C, Oh JM, Kim IW, Sauna ZE, Calcagno AM, Ambudkar SV, Gottesman MM (2007) A “silent” polymorphism in the MDR1 gene changes substrate specificity. Science 315:525–528. [DOI] [PubMed] [Google Scholar]
- Kitamura Y, Shimohama S, Koike H, Kakimura Ji, Matsuoka Y, Nomura Y, Gebicke-Haerter PJ, Taniguchi T (1999) Increased expression of cyclooxygenases and peroxisome proliferator-activated receptor-gamma in Alzheimer’s disease brains. Biochem Biophys Res Commun 254:582–586. [DOI] [PubMed] [Google Scholar]
- Klaassen CD, Slitt AL (2005) Regulation of hepatic transporters by xenobiotic receptors. Curr Drug Metab 6:309–328. [DOI] [PubMed] [Google Scholar]
- Kleihues P, Barnholtz-Sloan J, Ohgaki H (2014) Tumors of the nervous system, in World Cancer Report 2014 (Cavenee WK, Paulus W, eds) World Health Organization, Geneva, Switzerland. [Google Scholar]
- Kliewer SA, Moore JT, Wade L, Staudinger JL, Watson MA, Jones SA, McKee DD, Oliver BB, Willson TM, Zetterström RH, et al. (1998) An orphan nuclear receptor activated by pregnanes defines a novel steroid signaling pathway. Cell 92:73–82. [DOI] [PubMed] [Google Scholar]
- Klinge CM, Bodenner DL, Desai D, Niles RM, Traish AM (1997) Binding of type II nuclear receptors and estrogen receptor to full and half-site estrogen response elements in vitro. Nucleic Acids Res 25:1903–1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klokouzas A, Shahi S, Hladky SB, Barrand MA, van Veen HW (2003) ABC transporters and drug resistance in parasitic protozoa. Int J Antimicrob Agents 22:301–317. [DOI] [PubMed] [Google Scholar]
- Korogod N, Petersen CC, Knott GW (2015) Ultrastructural analysis of adult mouse neocortex comparing aldehyde perfusion with cryo fixation. eLife 4:o5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kothur K, Bandodkar S, Wienholt L, Chu S, Pope A, Gill D, Dale RC (2019) Etiology is the key determinant of neuroinflammation in epilepsy: elevation of cerebrospinal fluid cytokines and chemokines in febrile infection-related epilepsy syndrome and febrile status epilepticus. Epilepsia 60:1678–1688. [DOI] [PubMed] [Google Scholar]
- Kovalchuk A, Driessen AJ (2010) Phylogenetic analysis of fungal ABC transporters. BMC Genomics 11:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalski P, Baum M, Körten M, Donath A, Dobler S (2020) ABCB transporters in a leaf beetle respond to sequestered plant toxins. Proc Biol Sci 287:20201311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogh A (1946) The active and passive exchanges of inorganic ions through the surfaces of living cells and through living membranes generally. Proc R Soc Med 133:140–200. [DOI] [PubMed] [Google Scholar]
- Krohn M, Lange C, Hofrichter J, Scheffler K, Stenzel J, Steffen J, Schumacher T, Brüning T, Plath AS, Alfen F, et al. (2011) Cerebral amyloid-β proteostasis is regulated by the membrane transport protein ABCC1 in mice. J Clin Invest 121:3924–3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krohn M, Wanek T, Menet MC, Noack A, Declèves X, Langer O, Löscher W, Pahnke J (2018) Humanization of the blood-brain barrier transporter ABCB1 in mice disrupts genomic locus—lessons from three unsuccessful approaches. Eur J Microbiol Immunol (Bp) 8:78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruh GD, Guo Y, Hopper-Borge E, Belinsky MG, Chen ZS (2007) ABCC10, ABCC11, and ABCC12. Pflugers Arch 453:675–684. [DOI] [PubMed] [Google Scholar]
- Kuhnke D, Jedlitschky G, Grube M, Krohn M, Jucker M, Mosyagin I, Cascorbi I, Walker LC, Kroemer HK, Warzok RW, et al. (2007) MDR1-P-glycoprotein (ABCB1) mediates transport of Alzheimer’s amyloid-beta peptides—implications for the mechanisms of Abeta clearance at the blood-brain barrier. Brain Pathol 17:347–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurnik D, Sofowora GG, Donahue JP, Nair UB, Wilkinson GR, Wood AJ, Muszkat M (2008) Tariquidar, a selective P-glycoprotein inhibitor, does not potentiate loperamide’s opioid brain effects in humans despite full inhibition of lymphocyte P-glycoprotein. Anesthesiology 109:1092–1099. [DOI] [PubMed] [Google Scholar]
- Kwan P, Brodie MJ (2000) Early identification of refractory epilepsy. N Engl J Med 342:314–319. [DOI] [PubMed] [Google Scholar]
- Kwon KJ, Timmons AE, Sengupta S, Simonetti FR, Zhang H, Hoh R, Deeks SG, Siliciano JD, Siliciano RF (2020) Different human resting memory CD4+ T cell subsets show similar low inducibility of latent HIV-1 proviruses. Sci Transl Med 12:eaax6795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagas JS, van Waterschoot RA, Sparidans RW, Wagenaar E, Beijnen JH, Schinkel AH (2010) Breast cancer resistance protein and P-glycoprotein limit sorafenib brain accumulation. Mol Cancer Ther 9:319–326. [DOI] [PubMed] [Google Scholar]
- Lagas JS, van Waterschoot RA, van Tilburg VA, Hillebrand MJ, Lankheet N, Rosing H, Beijnen JH, Schinkel AH (2009) Brain accumulation of dasatinib is restricted by P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2) and can be enhanced by elacridar treatment. Clin Cancer Res 15:2344–2351. [DOI] [PubMed] [Google Scholar]
- Lai KSP, Liu CS, Rau A, Lanctôt KL, Köhler CA, Pakosh M, Carvalho AF, Herrmann N (2017) Peripheral inflammatory markers in Alzheimer’s disease: a systematic review and meta-analysis of 175 studies. J Neurol Neurosurg Psychiatry 88:876–882. [DOI] [PubMed] [Google Scholar]
- Laksitorini MD, Yathindranath V, Xiong W, Hombach-Klonisch S, Miller DW (2019) Modulation of Wnt/β-catenin signaling promotes blood-brain barrier phenotype in cultured brain endothelial cells. Sci Rep 9:19718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam FC, Liu R, Lu P, Shapiro AB, Renoir JM, Sharom FJ, Reiner PB (2001) Beta-amyloid efflux mediated by p-glycoprotein. J Neurochem 76:1121–1128. [DOI] [PubMed] [Google Scholar]
- Lamano JB, Lamano JB, Li YD, DiDomenico JD, Choy W, Veliceasa D, Oyon DE, Fakurnejad S, Ampie L, Kesavabhotla K, et al. (2019) Glioblastoma-derived IL6 induces immunosuppressive peripheral myeloid cell PD-L1 and promotes tumor growth. Clin Cancer Res 25:3643–3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laramy JK, Kim M, Gupta SK, Parrish KE, Zhang S, Bakken KK, Carlson BL, Mladek AC, Ma DJ, Sarkaria JN, et al. (2017) Heterogeneous binding and central nervous system distribution of the multitargeted kinase inhibitor ponatinib restrict orthotopic efficacy in a patient-derived xenograft model of glioblastoma. J Pharmacol Exp Ther 363:136–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laske DW, Youle RJ, Oldfield EH (1997) Tumor regression with regional distribution of the targeted toxin TF-CRM107 in patients with malignant brain tumors. Nat Med 3:1362–1368. [DOI] [PubMed] [Google Scholar]
- Law LW (1952) Origin of the resistance of leukaemic cells to folic acid antagonists. Nature 169:628–629. [DOI] [PubMed] [Google Scholar]
- Le Vee M, Jouan E, Stieger B, Lecureur V, Fardel O (2015) Regulation of human hepatic drug transporter activity and expression by diesel exhaust particle extract. PLoS One 10:e0121232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CG, Gottesman MM, Cardarelli CO, Ramachandra M, Jeang KT, Ambudkar SV, Pastan I, Dey S (1998) HIV-1 protease inhibitors are substrates for the MDR1 multidrug transporter. Biochemistry 37:3594–3601. [DOI] [PubMed] [Google Scholar]
- Lee JS, Scala S, Matsumoto Y, Dickstein B, Robey R, Zhan Z, Altenberg G, Bates SE (1997) Reduced drug accumulation and multidrug resistance in human breast cancer cells without associated P-glycoprotein or MRP overexpression. J Cell Biochem 65:513–526. [PubMed] [Google Scholar]
- Lemmon MA, Schlessinger J (2010) Cell signaling by receptor tyrosine kinases. Cell 141:1117–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepore F, D’Alessandro G, Antonangeli F, Santoro A, Esposito V, Limatola C, Trettel F (2018) CXCL16/CXCR6 axis drives microglia/macrophages phenotype in physiological conditions and plays a crucial role in glioma. Front Immunol 9:2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesniak WG, Chu C, Jablonska A, Behnam Azad B, Zwaenepoel O, Zawadzki M, Lisok A, Pomper MG, Walczak P, Gettemans J, et al. (2019) PET imaging of distinct brain uptake of a nanobody and similarly-sized PAMAM dendrimers after intra-arterial administration. Eur J Nucl Med Mol Imaging 46:1940–1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letrent SP, Pollack GM, Brouwer KR, Brouwer KL (1999) Effects of a potent and specific P-glycoprotein inhibitor on the blood-brain barrier distribution and antinociceptive effect of morphine in the rat. Drug Metab Dispos 27:827–834. [PubMed] [Google Scholar]
- Li H, Zhou S, Li T, Liu Z, Wu J, Zeng G, Liu C, Gong J (2012) Suppression of BCRP expression and restoration of sensitivity to chemotherapy in multidrug-resistant HCC cell line HEPG2/ADM by RNA interference. Hepatogastroenterology 59:2238–2242. [DOI] [PubMed] [Google Scholar]
- Li JY, Boado RJ, Pardridge WM (2001) Blood-brain barrier genomics. J Cereb Blood Flow Metab 21:61–68. [DOI] [PubMed] [Google Scholar]
- Li L, Agarwal S, Elmquist WF (2013) Brain efflux index to investigate the influence of active efflux on brain distribution of pemetrexed and methotrexate. Drug Metab Dispos 41:659–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Liu Y, Yang W, Han X, Li S, Liu H, Gerweck LE, Fukumura D, Loeffler JS, Yang BB, et al. (2018) MicroRNA-378 enhances radiation response in ectopic and orthotopic implantation models of glioblastoma. J Neurooncol 136:63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Zhang H, Assaraf YG, Zhao K, Xu X, Xie J, Yang DH, Chen ZS (2016) Overcoming ABC transporter-mediated multidrug resistance: molecular mechanisms and novel therapeutic drug strategies. Drug Resist Updat 27:14–29. [DOI] [PubMed] [Google Scholar]
- Liberman N, Wang SY, Greer EL (2019) Transgenerational epigenetic inheritance: from phenomena to molecular mechanisms. Curr Opin Neurobiol 59:189–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidar Z, Mardor Y, Jonas T, Pfeffer R, Faibel M, Nass D, Hadani M, Ram Z (2004) Convection-enhanced delivery of paclitaxel for the treatment of recurrent malignant glioma: a phase I/II clinical study. J Neurosurg 100:472–479. [DOI] [PubMed] [Google Scholar]
- Lim JC, Kania KD, Wijesuriya H, Chawla S, Sethi JK, Pulaski L, Romero IA, Couraud PO, Weksler BB, Hladky SB, et al. (2008) Activation of beta-catenin signalling by GSK-3 inhibition increases p-glycoprotein expression in brain endothelial cells. J Neurochem 106:1855–1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim JC, Mickute Z, Zaman M, Hopkins S, Wijesuriya H, Steckler T, Moechars D, Van Leuven F, Sarnyai Z, Hladky SB, et al. (2009) Decreased expression of multidrug efflux transporters in the brains of GSK-3beta transgenic mice. Brain Res 1276:1–10. [DOI] [PubMed] [Google Scholar]
- Linja MJ, Porkka KP, Kang Z, Savinainen KJ, Jänne OA, Tammela TL, Vessella RL, Palvimo JJ, Visakorpi T (2004) Expression of androgen receptor coregulators in prostate cancer. Clin Cancer Res 10:1032–1040. [DOI] [PubMed] [Google Scholar]
- List AF (1996) The role of multidrug resistance and its pharmacological modulation in acute myeloid leukemia. Leukemia 10(Suppl 1):S36–S38. [PubMed] [Google Scholar]
- Liu H, Liu Z, Jiang B, Huo L, Liu J, Lu J (2015) Synthetic miR-145 mimic enhances the cytotoxic effect of the antiangiogenic drug sunitinib in glioblastoma. Cell Biochem Biophys 72:551–557. [DOI] [PubMed] [Google Scholar]
- Liu H, Yang H, Wang D, Liu Y, Liu X, Li Y, Xie L, Wang G (2009) Insulin regulates P-glycoprotein in rat brain microvessel endothelial cells via an insulin receptor-mediated PKC/NF-kappaB pathway but not a PI3K/Akt pathway. Eur J Pharmacol 602:277–282. [DOI] [PubMed] [Google Scholar]
- Liu X, Jing XY, Jin S, Li Y, Liu L, Yu YL, Liu XD, Xie L (2011) Insulin suppresses the expression and function of breast cancer resistance protein in primary cultures of rat brain microvessel endothelial cells. Pharmacol Rep 63:487–493. [DOI] [PubMed] [Google Scholar]
- Lockman PR, Mittapalli RK, Taskar KS, Rudraraju V, Gril B, Bohn KA, Adkins CE, Roberts A, Thorsheim HR, Gaasch JA, et al. (2010) Heterogeneous blood-tumor barrier permeability determines drug efficacy in experimental brain metastases of breast cancer. Clin Cancer Res 16:5664–5678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loffreda N, Eldin P, Auzou G, Frelin C, Claire M (1992) Corticosteroid receptors in cells derived from rat brain microvessels: mRNA identification and aldosterone binding. Am J Physiol 262:C156–C163. [DOI] [PubMed] [Google Scholar]
- Long JM, Maloney B, Rogers JT, Lahiri DK (2019) Novel upregulation of amyloid-β precursor protein (APP) by microRNA-346 via targeting of APP mRNA 5′-untranslated region: implications in Alzheimer’s disease. Mol Psychiatry 24:345–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo TW, Bartlett MC, Clarke DM (2002) The “LSGGQ” motif in each nucleotide-binding domain of human P-glycoprotein is adjacent to the opposing walker A sequence. J Biol Chem 277:41303–41306. [DOI] [PubMed] [Google Scholar]
- Low YL, Jin L, Morris ER, Pan Y, Nicolazzo JA (2020) Pioglitazone increases blood-brain barrier expression of fatty acid-binding protein 5 and docosahexaenoic acid trafficking into the brain. Mol Pharm 17:873–884. [DOI] [PubMed] [Google Scholar]
- Luckenbach T, Fischer S, Sturm A (2014) Current advances on ABC drug transporters in fish. Comp Biochem Physiol C Toxicol Pharmacol 165:28–52. [DOI] [PubMed] [Google Scholar]
- Lynch C, Pan Y, Li L, Ferguson SS, Xia M, Swaan PW, Wang H (2013) Identification of novel activators of constitutive androstane receptor from FDA-approved drugs by integrated computational and biological approaches. Pharm Res 30:489–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack GS (2007) MicroRNA gets down to business. Nat Biotechnol 25:631–638. [DOI] [PubMed] [Google Scholar]
- Mackenzie IR, Hao C, Munoz DG (1995) Role of microglia in senile plaque formation. Neurobiol Aging 16:797–804. [DOI] [PubMed] [Google Scholar]
- Maeng HJ, Kim MH, Jin HE, Shin SM, Tsuruo T, Kim SG, Kim DD, Shim CK, Chung SJ (2007) Functional induction of P-glycoprotein in the blood-brain barrier of streptozotocin-induced diabetic rats: evidence for the involvement of nuclear factor-kappaB, a nitrosative stress-sensitive transcription factor, in the regulation. Drug Metab Dispos 35:1996–2005. [DOI] [PubMed] [Google Scholar]
- Maher JM, Cheng X, Slitt AL, Dieter MZ, Klaassen CD (2005) Induction of the multidrug resistance-associated protein family of transporters by chemical activators of receptor-mediated pathways in mouse liver. Drug Metab Dispos 33:956–962. [DOI] [PubMed] [Google Scholar]
- Mainprize T, Lipsman N, Huang Y, Meng Y, Bethune A, Ironside S, Heyn C, Alkins R, Trudeau M, Sahgal A, et al. (2019) Blood-brain barrier opening in primary brain tumors with non-invasive MR-guided focused ultrasound: a clinical safety and feasibility study. Sci Rep 9:321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manolaridis I, Jackson SM, Taylor NMI, Kowal J, Stahlberg H, Locher KP (2018) Cryo-EM structures of a human ABCG2 mutant trapped in ATP-bound and substrate-bound states. Nature 563:426–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetti S, de Vries NA, Buckle T, Bolijn MJ, van Eijndhoven MA, Beijnen JH, Mazzanti R, van Tellingen O, Schellens JH (2008) Effect of the ATP-binding cassette drug transporters ABCB1, ABCG2, and ABCC2 on erlotinib hydrochloride (Tarceva) disposition in in vitro and in vivo pharmacokinetic studies employing Bcrp1-/-/Mdr1a/1b-/- (triple-knockout) and wild-type mice. Mol Cancer Ther 7:2280–2287. [DOI] [PubMed] [Google Scholar]
- Marchi N, Bazarian JJ, Puvenna V, Janigro M, Ghosh C, Zhong J, Zhu T, Blackman E, Stewart D, Ellis J, et al. (2013) Consequences of repeated blood-brain barrier disruption in football players. PLoS One 8:e56805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marroni M, Agrawal ML, Kight K, Hallene KL, Hossain M, Cucullo L, Signorelli K, Namura S, Bingaman W, Janigro D (2003) Relationship between expression of multiple drug resistance proteins and p53 tumor suppressor gene proteins in human brain astrocytes. Neuroscience 121:605–617. [DOI] [PubMed] [Google Scholar]
- Martin C, Berridge G, Mistry P, Higgins C, Charlton P, Callaghan R (1999) The molecular interaction of the high affinity reversal agent XR9576 with P-glycoprotein. Br J Pharmacol 128:403–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín V, Sanchez-Sanchez AM, Herrera F, Gomez-Manzano C, Fueyo J, Alvarez-Vega MA, Antolín I, Rodriguez C (2013) Melatonin-induced methylation of the ABCG2/BCRP promoter as a novel mechanism to overcome multidrug resistance in brain tumour stem cells. Br J Cancer 108:2005–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruno M, Kovach JS, Kelly PJ, Yanagihara T (1997) Distribution of endogenous tumour necrosis factor alpha in gliomas. J Clin Pathol 50:559–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathiisen TM, Lehre KP, Danbolt NC, Ottersen OP (2010) The perivascular astroglial sheath provides a complete covering of the brain microvessels: an electron microscopic 3D reconstruction. Glia 58:1094–1103. [DOI] [PubMed] [Google Scholar]
- Matsumoto T, Tani E, Kaba K, Shindo H, Miyaji K (1991) Expression of P-glycoprotein in human glioma cell lines and surgical glioma specimens. J Neurosurg 74:460–466. [DOI] [PubMed] [Google Scholar]
- Matsuo M, Yonemitsu N, Zaitsu M, Ishii K, Hamasaki Y, Fukuyama K, Tabuchi K, Miyazaki S (2001) Expression of prostaglandin H synthase-2 in human brain tumors. Acta Neuropathol 102:181–187. [DOI] [PubMed] [Google Scholar]
- Matzneller P, Kussmann M, Eberl S, Maier-Salamon A, Jäger W, Bauer M, Langer O, Zeitlinger M, Poeppl W (2018) Pharmacokinetics of the P-gp inhibitor tariquidar in rats after intravenous, oral, and intraperitoneal administration. Eur J Drug Metab Pharmacokinet 43:599–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mawuenyega KG, Sigurdson W, Ovod V, Munsell L, Kasten T, Morris JC, Yarasheski KE, Bateman RJ (2010) Decreased clearance of CNS β-amyloid in Alzheimer’s disease. Science 330:1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayati A, Moreau A, Le Vée M, Stieger B, Denizot C, Parmentier Y, Fardel O (2017) Protein kinases C-mediated regulations of drug transporter activity, localization and expression. Int J Mol Sci 18:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer U, Wagenaar E, Dorobek B, Beijnen JH, Borst P, Schinkel AH (1997) Full blockade of intestinal P-glycoprotein and extensive inhibition of blood-brain barrier P-glycoprotein by oral treatment of mice with PSC833. J Clin Invest 100:2430–2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeer EG, McGeer PL (2003) Inflammatory processes in Alzheimer’s disease. Prog Neuropsychopharmacol Biol Psychiatry 27:741–749. [DOI] [PubMed] [Google Scholar]
- McRae M, Jones AM, Paris JJ, Kim WK, Knapp PE, Kashuba AMD, Hauser KF, Leibrand CR (2019) Opiates and HIV-1 perturb the cellular/regional biodistribution of antiretrovirals in the brain by disrupting the blood-brain barrier and efflux transporter function. J Neuroimmune Pharmacol 14:334. [Google Scholar]
- Mealey KL, Owens JG, Freeman E (2023) Canine and feline P-glycoprotein deficiency: what we know and where we need to go. J Vet Pharmacol Ther 46:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechetner EB, Roninson IB (1992) Efficient inhibition of P-glycoprotein-mediated multidrug resistance with a monoclonal antibody. Proc Natl Acad Sci USA 89:5824–5828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meda L, Baron P, Prat E, Scarpini E, Scarlato G, Cassatella MA, Rossi F (1999) Proinflammatory profile of cytokine production by human monocytes and murine microglia stimulated with beta-amyloid[25-35]. J Neuroimmunol 93:45–52. [DOI] [PubMed] [Google Scholar]
- Medarova Z, Pantazopoulos P, Yoo B (2020) Screening of potential miRNA therapeutics for the prevention of multi-drug resistance in cancer cells. Sci Rep 10:1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta DC, Short JL, Nicolazzo JA (2013) Altered brain uptake of therapeutics in a triple transgenic mouse model of Alzheimer’s disease. Pharm Res 30:2868–2879. [DOI] [PubMed] [Google Scholar]
- Mellinghoff IK, Wang MY, Vivanco I, Haas-Kogan DA, Zhu S, Dia EQ, Lu KV, Yoshimoto K, Huang JH, Chute DJ, et al. (2005) Molecular determinants of the response of glioblastomas to EGFR kinase inhibitors. N Engl J Med 353:2012–2024. [DOI] [PubMed] [Google Scholar]
- Mendiola AS, Ryu JK, Bardehle S, Meyer-Franke A, Ang KK, Wilson C, Baeten KM, Hanspers K, Merlini M, Thomas S, et al. (2020) Transcriptional profiling and therapeutic targeting of oxidative stress in neuroinflammation. Nat Immunol 21:513–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercado-Gómez OF, Córdova-Dávalos L, García-Betanzo D, Rocha L, Alonso-Vanegas MA, Cienfuegos J, Guevara-Guzmán R (2018) Overexpression of inflammatory-related and nitric oxide synthase genes in olfactory bulbs from frontal lobe epilepsy patients. Epilepsy Res 148:37–43. [DOI] [PubMed] [Google Scholar]
- Mesev EV, Miller DS, Cannon RE (2017) Ceramide 1-phosphate increases P-glycoprotein transport activity at the blood-brain barrier via prostaglandin E2 signaling. Mol Pharmacol 91:373–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DS (2010) Regulation of P-glycoprotein and other ABC drug transporters at the blood-brain barrier. Trends Pharmacol Sci 31:246–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DS (2015) Regulation of ABC transporters blood-brain barrier: the good, the bad, and the ugly, in ABC Transporters and Cancer (Schuetz JD, Ishikawa T, eds) pp 43–70, Elsevier/ Academic Press, San Diego, CA. [DOI] [PubMed] [Google Scholar]
- Miller DS, Bauer B, Hartz AM (2008) Modulation of P-glycoprotein at the blood-brain barrier: opportunities to improve central nervous system pharmacotherapy. Pharmacol Rev 60:196–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DS, Cannon RE (2014) Signaling pathways that regulate basal ABC transporter activity at the blood- brain barrier. Curr Pharm Des 20:1463–1471. [DOI] [PubMed] [Google Scholar]
- Mistry P, Stewart AJ, Dangerfield W, Okiji S, Liddle C, Bootle D, Plumb JA, Templeton D, Charlton P (2001) In vitro and in vivo reversal of P-glycoprotein-mediated multidrug resistance by a novel potent modulator, XR9576. Cancer Res 61:749–758. [PubMed] [Google Scholar]
- Mittapalli RK, Chung AH, Parrish KE, Crabtree D, Halvorson KG, Hu G, Elmquist WF, Becher OJ (2016) ABCG2 and ABCB1 limit the efficacy of dasatinib in a PDGF-B-driven brainstem glioma model. Mol Cancer Ther 15:819–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogi M, Yang J, Lambert JF, Colvin GA, Shiojima I, Skurk C, Summer R, Fine A, Quesenberry PJ, Walsh K (2003) Akt signaling regulates side population cell phenotype via Bcrp1 translocation. J Biol Chem 278:39068–39075. [DOI] [PubMed] [Google Scholar]
- Mohamed LA, Markandaiah SS, Bonanno S, Pasinelli P, Trotti D (2019) Excess glutamate secreted from astrocytes drives upregulation of P-glycoprotein in endothelial cells in amyotrophic lateral sclerosis. Exp Neurol 316:27–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammad AS, Adkins CE, Shah N, Aljammal R, Griffith JIG, Tallman RM, Jarrell KL, Lockman PR (2018) Permeability changes and effect of chemotherapy in brain adjacent to tumor in an experimental model of metastatic brain tumor from breast cancer. BMC Cancer 18:1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monville C, Fages C, Feyens AM, D’Hondt V, Guillet C, Vernallis A, Gascan H, Peschanski M (2002) Astroglial expression of the P-glycoprotein is controlled by intracellular CNTF. BMC Cell Biol 3:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- More VR, Campos CR, Evans RA, Oliver KD, Chan GNY, Miller DS, Cannon RE (2017) PPAR-α, a lipid-sensing transcription factor, regulates blood-brain barrier efflux transporter expression. J Cereb Blood Flow Metab 37:1199–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris ME, Rodriguez-Cruz V, Felmlee MA (2017) SLC and ABC transporters: expression, localization, and species differences at the blood-brain and the blood-cerebrospinal fluid barriers. AAPS J 19:1317–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosher KI, Wyss-Coray T (2014) Microglial dysfunction in brain aging and Alzheimer’s disease. Biochem Pharmacol 88:594–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir AR, Peters A (1962) Quintuple-layered membrane junctions at terminal bars between endothelial cells. J Cell Biol 12:443–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz JL, Rodriguez-Cruz V, Greco SJ, Nagula V, Scotto KW, Rameshwar P (2014) Temozolomide induces the production of epidermal growth factor to regulate MDR1 expression in glioblastoma cells. Mol Cancer Ther 13:2399–2411. [DOI] [PubMed] [Google Scholar]
- Munoz JL, Walker ND, Scotto KW, Rameshwar P (2015) Temozolomide competes for P-glycoprotein and contributes to chemoresistance in glioblastoma cells. Cancer Lett 367:69–75. [DOI] [PubMed] [Google Scholar]
- Murakami Y, Higashi Y, Matsunaga N, Koyanagi S, Ohdo S (2008) Circadian clock-controlled intestinal expression of the multidrug-resistance gene mdr1a in mice. Gastroenterology 135:1636–1644 e1633. [DOI] [PubMed] [Google Scholar]
- Murphy MP, LeVine H 3rd (2010) Alzheimer’s disease and the amyloid-beta peptide. J Alzheimers Dis 19:311–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa M, Schneider E, Dixon KH, Horton J, Kelley K, Morrow C, Cowan KH (1992) Reduced intracellular drug accumulation in the absence of P-glycoprotein (mdr1) overexpression in mitoxantrone-resistant human MCF-7 breast cancer cells. Cancer Res 52:6175–6181. [PubMed] [Google Scholar]
- Nakanishi T, Doyle LA, Hassel B, Wei Y, Bauer KS, Wu S, Pumplin DW, Fang HB, Ross DD (2003) Functional characterization of human breast cancer resistance protein (BCRP, ABCG2) expressed in the oocytes of Xenopus laevis. Mol Pharmacol 64:1452–1462. [DOI] [PubMed] [Google Scholar]
- Nakanishi T, Ross DD (2012) Breast cancer resistance protein (BCRP/ABCG2): its role in multidrug resistance and regulation of its gene expression. Chin J Cancer 31:73–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narang VS, Fraga C, Kumar N, Shen J, Throm S, Stewart CF, Waters CM (2008) Dexamethasone increases expression and activity of multidrug resistance transporters at the rat blood-brain barrier. Am J Physiol Cell Physiol 295:C440–C450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Cancer Institute. (2022) Drugs approved for brain tumors. https://www.cancer.gov/about-cancer/treatment/drugs/brain
- Nehra G, Bauer B, Hartz AMS (2022) Blood-brain barrier leakage in Alzheimer’s disease: From discovery to clinical relevance. Pharmacol Ther 234:108119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhaus W, Gaiser F, Mahringer A, Franz J, Riethmüller C, Förster C (2014) The pivotal role of astrocytes in an in vitro stroke model of the blood-brain barrier. Front Cell Neurosci 8:352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuwelt EA, Howieson J, Frenkel EP, Specht HD, Weigel R, Buchan CG, Hill SA (1986) Therapeutic efficacy of multiagent chemotherapy with drug delivery enhancement by blood-brain barrier modification in glioblastoma. Neurosurgery 19:573–582. [DOI] [PubMed] [Google Scholar]
- Nickel S, Mahringer A (2014) The xenoestrogens ethinylestradiol and bisphenol A regulate BCRP at the blood-brain barrier of rats. Xenobiotica 44:1046–1054. [DOI] [PubMed] [Google Scholar]
- Niero EL, Rocha-Sales B, Lauand C, Cortez BA, de Souza MM, Rezende-Teixeira P, Urabayashi MS, Martens AA, Neves JH, Machado-Santelli GM (2014) The multiple facets of drug resistance: one history, different approaches. J Exp Clin Cancer Res 33:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa K, Araki E, Morham SG, Ross ME, Iadecola C (2000) Cyclooxygenase-2 contributes to functional hyperemia in whisker-barrel cortex. J Neurosci 20:763–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noack A, Noack S, Buettner M, Naim HY, Löscher W (2016) Intercellular transfer of P-glycoprotein in human blood-brain barrier endothelial cells is increased by histone deacetylase inhibitors. Sci Rep 6:29253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Byrne K, Steward W, Leonard R, Wysowskyj H, Dugan M, Pfister C, Moss D (2001) A phase 1 study of escalating doses of docetaxel when administered with valspodar (PSC833). Clin Cancer Res 7:3739S–3740S. [Google Scholar]
- Ohyagi Y, Asahara H, Chui DH, Tsuruta Y, Sakae N, Miyoshi K, Yamada T, Kikuchi H, Taniwaki T, Murai H, et al. (2005) Intracellular Abeta42 activates p53 promoter: a pathway to neurodegeneration in Alzheimer’s disease. FASEB J 19:255–257. [DOI] [PubMed] [Google Scholar]
- Okyar A, Dressler C, Hanafy A, Baktir G, Lemmer B, Spahn-Langguth H (2012) Circadian variations in exsorptive transport: in situ intestinal perfusion data and in vivo relevance. Chronobiol Int 29:443–453. [DOI] [PubMed] [Google Scholar]
- Oldendorf WH (1971) Brain uptake of radiolabeled amino acids, amines, and hexoses after arterial injection. Am J Physiol 221:1629–1639. [DOI] [PubMed] [Google Scholar]
- Oldendorf WH, Brown WJ (1975) Greater number of capillary endothelial cell mitochondria in brain than in muscle. Proc Soc Exp Biol Med 149:736–738. [DOI] [PubMed] [Google Scholar]
- Oldendorf WH, Cornford ME, Brown WJ (1977) The large apparent work of the blood-brain barrier: the mitochondria1 content of capillary endothelial cells in brain and other tissues of the rat. Ann Neurol 1:403–417. [DOI] [PubMed] [Google Scholar]
- Olefsky JM (2001) Nuclear receptor minireview series. J Biol Chem 276:36863–36864. [DOI] [PubMed] [Google Scholar]
- Osswald M, Blaes J, Liao Y, Solecki G, Gömmel M, Berghoff AS, Salphati L, Wallin JJ, Phillips HS, Wick W, et al. (2016) Impact of blood-brain barrier integrity on tumor growth and therapy response in brain metastases. Clin Cancer Res 22:6078–6087. [DOI] [PubMed] [Google Scholar]
- Ostrom QT, Cioffi G, Gittleman H, Patil N, Waite K, Kruchko C, Barnholtz-Sloan JS (2019) CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2012-2016. Neuro-oncol 21(Suppl 5):v1–v100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrom QT, Gittleman H, Truitt G, Boscia A, Kruchko C, Barnholtz-Sloan JS (2018) CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2011-2015. Neuro-oncol 20(Suppl 4):iv1–iv86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overington JP, Al-Lazikani B, Hopkins AL (2006) How many drug targets are there? Nat Rev Drug Discov 5:993–996. [DOI] [PubMed] [Google Scholar]
- Pahnke J, Wolkenhauer O, Krohn M, Walker LC (2008) Clinico-pathologic function of cerebral ABC transporters—implications for the pathogenesis of Alzheimer’s disease. Curr Alzheimer Res 5:396–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmieri D, Lockman PR, Thomas FC, Hua E, Herring J, Hargrave E, Johnson M, Flores N, Qian Y, Vega-Valle E, et al. (2009) Vorinostat inhibits brain metastatic colonization in a model of triple-negative breast cancer and induces DNA double-strand breaks. Clin Cancer Res 15:6148–6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan W, Yu C, Hsuchou H, Kastin AJ (2010) The role of cerebral vascular NFkappaB in LPS-induced inflammation: differential regulation of efflux transporter and transporting cytokine receptors. Cell Physiol Biochem 25:623–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolinelli R, Corada M, Ferrarini L, Devraj K, Artus C, Czupalla CJ, Rudini N, Maddaluno L, Papa E, Engelhardt B, et al. (2013) Wnt activation of immortalized brain endothelial cells as a tool for generating a standardized model of the blood brain barrier in vitro. PLoS One 8:e70233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardridge WM (2001) Brain drug targeting and gene technologies. Jpn J Pharmacol 87:97–103. [DOI] [PubMed] [Google Scholar]
- Pardridge WM (2007) Blood-brain barrier genomics. Stroke 38(2, Suppl):686–690. [DOI] [PubMed] [Google Scholar]
- Pardridge WM, Eisenberg J, Yang J (1985) Human blood-brain barrier insulin receptor. J Neurochem 44:1771–1778. [DOI] [PubMed] [Google Scholar]
- Park R, Kook SY, Park JC, Mook-Jung I (2014) Aβ1-42 reduces P-glycoprotein in the blood-brain barrier through RAGE-NF-κB signaling. Cell Death Dis 5:e1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasinetti GM, Aisen PS (1998) Cyclooxygenase-2 expression is increased in frontal cortex of Alzheimer’s disease brain. Neuroscience 87:319–324. [DOI] [PubMed] [Google Scholar]
- Pearson JW, Fogler WE, Volker K, Usui N, Goldenberg SK, Gruys E, Riggs CW, Komschlies K, Wiltrout RH, Tsuruo T, et al. (1991) Reversal of drug resistance in a human colon cancer xenograft expressing MDR1 complementary DNA by in vivo administration of MRK-16 monoclonal antibody. J Natl Cancer Inst 83:1386–1391. [DOI] [PubMed] [Google Scholar]
- Pekcec A, Unkrüer B, Schlichtiger J, Soerensen J, Hartz AM, Bauer B, van Vliet EA, Gorter JA, Potschka H (2009) Targeting prostaglandin E2 EP1 receptors prevents seizure-associated P-glycoprotein up-regulation. J Pharmacol Exp Ther 330:939–947. [DOI] [PubMed] [Google Scholar]
- Pennock GD, Dalton WS, Roeske WR, Appleton CP, Mosley K, Plezia P, Miller TP, Salmon SE (1991) Systemic toxic effects associated with high-dose verapamil infusion and chemotherapy administration. J Natl Cancer Inst 83:105–110. [DOI] [PubMed] [Google Scholar]
- Petropoulos S, Gibb W, Matthews SG (2010) Developmental expression of multidrug resistance phosphoglycoprotein (P-gp) in the mouse fetal brain and glucocorticoid regulation. Brain Res 1357:9–18. [DOI] [PubMed] [Google Scholar]
- Pilorget A, Demeule M, Barakat S, Marvaldi J, Luis J, Béliveau R (2007) Modulation of P-glycoprotein function by sphingosine kinase-1 in brain endothelial cells. J Neurochem 100:1203–1210. [DOI] [PubMed] [Google Scholar]
- Pinkel D (1959) Actinomycin D in childhood cancer; a preliminary report. Pediatrics 23:342–347. [PubMed] [Google Scholar]
- Poller B, Drewe J, Krähenbühl S, Huwyler J, Gutmann H (2010) Regulation of BCRP (ABCG2) and P-glycoprotein (ABCB1) by cytokines in a model of the human blood-brain barrier. Cell Mol Neurobiol 30:63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polli JW, Humphreys JE, Harmon KA, Castellino S, O’Mara MJ, Olson KL, John-Williams LS, Koch KM, Serabjit-Singh CJ (2008) The role of efflux and uptake transporters in [N-{3-chloro-4-[(3-fluorobenzyl)oxy]phenyl}-6-[5-({[2-(methylsulfonyl)ethyl]amino} methyl)-2-furyl]-4-quinazolinamine (GW572016, lapatinib) disposition and drug interactions. Drug Metab Dispos 36:695–701. [DOI] [PubMed] [Google Scholar]
- Polli JW, Olson KL, Chism JP, John-Williams LS, Yeager RL, Woodard SM, Otto V, Castellino S, Demby VE (2009) An unexpected synergist role of P-glycoprotein and breast cancer resistance protein on the central nervous system penetration of the tyrosine kinase inhibitor lapatinib (N-{3-chloro-4-[(3-fluorobenzyl)oxy]phenyl}-6-[5-({[2-(methylsulfonyl)ethyl]amino} methyl)-2-furyl]-4-quinazolinamine; GW572016). Drug Metab Dispos 37:439–442. [DOI] [PubMed] [Google Scholar]
- Potschka H (2012) Role of CNS efflux drug transporters in antiepileptic drug delivery: overcoming CNS efflux drug transport. Adv Drug Deliv Rev 64:943–952. [DOI] [PubMed] [Google Scholar]
- Probert L, Akassoglou K, Kassiotis G, Pasparakis M, Alexopoulou L, Kollias G (1997) TNF-alpha transgenic and knockout models of CNS inflammation and degeneration. J Neuroimmunol 72:137–141. [DOI] [PubMed] [Google Scholar]
- Pusztai L, Wagner P, Ibrahim N, Rivera E, Theriault R, Booser D, Symmans FW, Wong F, Blumenschein G, Fleming DR, et al. (2005) Phase II study of tariquidar, a selective P-glycoprotein inhibitor, in patients with chemotherapy-resistant, advanced breast carcinoma. Cancer 104:682–691. [DOI] [PubMed] [Google Scholar]
- Saljé K, Lederer K, Oswald S, Dazert E, Warzok R, Siegmund W (2012) Effects of rifampicin, dexamethasone, St. John’s wort and thyroxine on maternal and foetal expression of Abcb1 and organ distribution of talinolol in pregnant rats. Basic Clin Pharmacol Toxicol 111:99–105. [DOI] [PubMed] [Google Scholar]
- Qin H, Wilson CA, Lee SJ, Zhao X, Benveniste EN (2005) LPS induces CD40 gene expression through the activation of NF-kappaB and STAT-1alpha in macrophages and microglia. Blood 106:3114–3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qosa H, Lichter J, Sarlo M, Markandaiah SS, McAvoy K, Richard JP, Jablonski MR, Maragakis NJ, Pasinelli P, Trotti D (2016a) Astrocytes drive upregulation of the multidrug resistance transporter ABCB1 (P-glycoprotein) in endothelial cells of the blood-brain barrier in mutant superoxide dismutase 1-linked amyotrophic lateral sclerosis. Glia 64:1298–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qosa H, Miller DS, Pasinelli P, Trotti D (2015) Regulation of ABC efflux transporters at blood-brain barrier in health and neurological disorders. Brain Res 1628(Pt B):298–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qosa H, Mohamed LA, Alqahtani S, Abuasal BS, Hill RA, Kaddoumi A (2016b) Transporters as drug targets in neurological diseases. Clin Pharmacol Ther 100:441–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi A, Thakur N, Monga I, Thakur A, Kumar M (2014) VIRmiRNA: a comprehensive resource for experimentally validated viral miRNAs and their targets. Database (Oxford) 2014:bau103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffaele S, Lombardi M, Verderio C, Fumagalli M (2020) TNF production and release from microglia via extracellular vesicles: impact on brain functions. Cells 9:2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, Larson KB, Phelps ME, Grubb RL Jr, welch MJ, Ter-Pogossian MM (1975) In vivo measurement of brain glucose transport and metabolism employing glucose- -11C. Am J Physiol 228:1936–1948. [DOI] [PubMed] [Google Scholar]
- Rana A, Musto AE (2018) The role of inflammation in the development of epilepsy. J Neuroinflammation 15:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese TS, Karnovsky MJ (1967) Fine structural localization of a blood-brain barrier to exogenous peroxidase. J Cell Biol 34:207–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren X, Boriero D, Chaiswing L, Bondada S, St Clair DK, Butterfield DA (2019) Plausible biochemical mechanisms of chemotherapy-induced cognitive impairment (“chemobrain”), a condition that significantly impairs the quality of life of many cancer survivors. Biochim Biophys Acta Mol Basis Dis 1865:1088–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renard P, Zachary MD, Bougelet C, Mirault ME, Haegeman G, Remacle J, Raes M (1997) Effects of antioxidant enzyme modulations on interleukin-1-induced nuclear factor kappa B activation. Biochem Pharmacol 53:149–160. [DOI] [PubMed] [Google Scholar]
- Rigalli JP, Scholz PN, Tocchetti GN, Ruiz ML, Weiss J (2019a) The phytoestrogens daidzein and equol inhibit the drug transporter BCRP/ABCG2 in breast cancer cells: potential chemosensitizing effect. Eur J Nutr 58:139–150. [DOI] [PubMed] [Google Scholar]
- Rigalli JP, Tocchetti GN, Weiss J (2019b) Modulation of ABC transporters by nuclear receptors: physiological, pathological and pharmacological aspects. Curr Med Chem 26:1079–1112. [DOI] [PubMed] [Google Scholar]
- Riganti C, Salaroglio IC, Caldera V, Campia I, Kopecka J, Mellai M, Annovazzi L, Bosia A, Ghigo D, Schiffer D (2013) Temozolomide downregulates P-glycoprotein expression in glioblastoma stem cells by interfering with the Wnt3a/glycogen synthase-3 kinase/β-catenin pathway. Neuro-oncol 15:1502–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigor RR, Hawkins BT, Miller DS (2010) Activation of PKC isoform beta(I) at the blood-brain barrier rapidly decreases P-glycoprotein activity and enhances drug delivery to the brain. J Cereb Blood Flow Metab 30:1373–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringheim GE, Szczepanik AM, Petko W, Burgher KL, Zhu SZ, Chao CC (1998) Enhancement of beta-amyloid precursor protein transcription and expression by the soluble interleukin-6 receptor/interleukin-6 complex. Brain Res Mol Brain Res 55:35–44. [DOI] [PubMed] [Google Scholar]
- Rittierodt M, Tschernig T, Harada K (2004) Modulation of multidrug-resistance-associated P-glycoprotein in human U-87 MG and HUV-ECC cells with antisense oligodeoxynucleotides to MDR1 mRNA. Pathobiology 71:123–128. [DOI] [PubMed] [Google Scholar]
- Roberti A, Chaffey LE, Greaves DR (2022) NF-κB signaling and inflammation-drug repurposing to treat inflammatory disorders? Biology (Basel) 11:372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robey RW, Pluchino KM, Hall MD, Fojo AT, Bates SE, Gottesman MM (2018) Revisiting the role of ABC transporters in multidrug-resistant cancer. Nat Rev Cancer 18:452–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robey RW, To KK, Polgar O, Dohse M, Fetsch P, Dean M, Bates SE (2009) ABCG2: a perspective. Adv Drug Deliv Rev 61:3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DR, Wu YM, Lin SF (2000) The protein tyrosine kinase family of the human genome. Oncogene 19:5548–5557. [DOI] [PubMed] [Google Scholar]
- Rocchi E, Khodjakov A, Volk EL, Yang CH, Litman T, Bates SE, Schneider E (2000) The product of the ABC half-transporter gene ABCG2 (BCRP/MXR/ABCP) is expressed in the plasma membrane. Biochem Biophys Res Commun 271:42–46. [DOI] [PubMed] [Google Scholar]
- Ronaldson PT, Ashraf T, Bendayan R (2010) Regulation of multidrug resistance protein 1 by tumor necrosis factor alpha in cultured glial cells: involvement of nuclear factor-kappaB and c-Jun N-terminal kinase signaling pathways. Mol Pharmacol 77:644–659. [DOI] [PubMed] [Google Scholar]
- Ronaldson PT, Finch JD, Demarco KM, Quigley CE, Davis TP (2011) Inflammatory pain signals an increase in functional expression of organic anion transporting polypeptide 1a4 at the blood-brain barrier. J Pharmacol Exp Ther 336:827–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronaldson PT, Persidsky Y, Bendayan R (2008) Regulation of ABC membrane transporters in glial cells: relevance to the pharmacotherapy of brain HIV-1 infection. Glia 56:1711–1735. [DOI] [PubMed] [Google Scholar]
- Ronne-Engström E, Hillered L, Flink R, Spännare B, Ungerstedt U, Carlson H (1992) Intracerebral microdialysis of extracellular amino acids in the human epileptic focus. J Cereb Blood Flow Metab 12:873–876. [DOI] [PubMed] [Google Scholar]
- Rosenberg MF, Bikadi Z, Chan J, Liu X, Ni Z, Cai X, Ford RC, Mao Q (2010) The human breast cancer resistance protein (BCRP/ABCG2) shows conformational changes with mitoxantrone. Structure 18:482–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg MF, Callaghan R, Ford RC, Higgins CF (1997) Structure of the multidrug resistance P-glycoprotein to 2.5 nm resolution determined by electron microscopy and image analysis. J Biol Chem 272:10685–10694. [DOI] [PubMed] [Google Scholar]
- Rosenberg MF, Callaghan R, Modok S, Higgins CF, Ford RC (2005) Three-dimensional structure of P-glycoprotein: the transmembrane regions adopt an asymmetric configuration in the nucleotide-bound state. J Biol Chem 280:2857–2862. [DOI] [PubMed] [Google Scholar]
- Roulet A, Puel O, Gesta S, Lepage JF, Drag M, Soll M, Alvinerie M, Pineau T (2003) MDR1-deficient genotype in Collie dogs hypersensitive to the P-glycoprotein substrate ivermectin. Eur J Pharmacol 460:85–91. [DOI] [PubMed] [Google Scholar]
- Salphati L, Alicke B, Heffron TP, Shahidi-Latham S, Nishimura M, Cao T, Carano RA, Cheong J, Greve J, Koeppen H, et al. (2016) Brain distribution and efficacy of the brain penetrant PI3K inhibitor GDC-0084 in orthotopic mouse models of human glioblastoma. Drug Metab Dispos 44:1881–1889. [DOI] [PubMed] [Google Scholar]
- Samaras V, Piperi C, Korkolopoulou P, Zisakis A, Levidou G, Themistocleous MS, Boviatsis EI, Sakas DE, Lea RW, Kalofoutis A, et al. (2007) Application of the ELISPOT method for comparative analysis of interleukin (IL)-6 and IL-10 secretion in peripheral blood of patients with astroglial tumors. Mol Cell Biochem 304:343–351. [DOI] [PubMed] [Google Scholar]
- Sane R, Agarwal S, Elmquist WF (2012) Brain distribution and bioavailability of elacridar after different routes of administration in the mouse. Drug Metab Dispos 40:1612–1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkadi B, Homolya L, Szakács G, Váradi A (2006) Human multidrug resistance ABCB and ABCG transporters: participation in a chemoimmunity defense system. Physiol Rev 86:1179–1236. [DOI] [PubMed] [Google Scholar]
- Saunders NR, Dreifuss JJ, Dziegielewska KM, Johansson PA, Habgood MD, Møllgård K, Bauer HC (2014) The rights and wrongs of blood-brain barrier permeability studies: a walk through 100 years of history. Front Neurosci 8:404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders NR, Dziegielewska KM, Møllgård K, Habgood MD(2017) General introduction to barrier mechanisms in the central nervous system, in The Blood Brain Barrier and Inflammation (Lyck R, Enzmann G, eds) pp 1–22. Springer, New York, NY. [Google Scholar]
- Savolainen H, Meerlo P, Elsinga PH, Windhorst AD, Dierckx RA, Colabufo NA, van Waarde A, Luurtsema G (2016) P-glycoprotein function in the rodent brain displays a daily rhythm, a quantitative in vivo PET study. AAPS J 18:1524–1531. [DOI] [PubMed] [Google Scholar]
- Schaefer CP, Arkwright NB, Jacobs LM, Jarvis CK, Hunn KC, Largent-Milnes TM, Tome ME, Davis TP (2018) Chronic morphine exposure potentiates p-glycoprotein trafficking from nuclear reservoirs in cortical rat brain microvessels. PLoS One 13:e0192340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schinkel AH, Smit JJM, van Tellingen O, Beijnen JH, Wagenaar E, van Deemter L, Mol CAAM, van der Valk MA, Robanus-Maandag EC, te Riele HPJ, et al. (1994) Disruption of the mouse mdr1a P-glycoprotein gene leads to a deficiency in the blood-brain barrier and to increased sensitivity to drugs. Cell 77:491–502. [DOI] [PubMed] [Google Scholar]
- Schlessinger J (2000) Cell signaling by receptor tyrosine kinases. Cell 103:211–225. [DOI] [PubMed] [Google Scholar]
- Schlichtiger J, Pekcec A, Bartmann H, Winter P, Fuest C, Soerensen J, Potschka H (2010) Celecoxib treatment restores pharmacosensitivity in a rat model of pharmacoresistant epilepsy. Br J Pharmacol 160:1062–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzbaum J, Wang M, Root E, Pietrzak M, Rempala GA, Huang RP, Johannesen TB, Grimsrud TK (2017) A nested case-control study of 277 prediagnostic serum cytokines and glioma. PLoS One 12:e0178705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzbaum JA, Ahlbom A, Lönn S, Malmer B, Wigertz A, Auvinen A, Brookes AJ, Collatz Christensen H, Henriksson R, Johansen C, et al. (2007) An international case-control study of interleukin-4Ralpha, interleukin-13, and cyclooxygenase-2 polymorphisms and glioblastoma risk. Cancer Epidemiol Biomarkers Prev 16:2448–2454. [DOI] [PubMed] [Google Scholar]
- Scotto KW (2003) Transcriptional regulation of ABC drug transporters. Oncogene 22:7496–7511. [DOI] [PubMed] [Google Scholar]
- Serrano-Pozo A, Frosch MP, Masliah E, Hyman BT (2011) Neuropathological alterations in Alzheimer disease. Cold Spring Harb Perspect Med 1:a006189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Y, Wang C, Hong Z, Chen Y (2016) Inhibition of p38 mitogen-activated protein kinase signaling reduces multidrug transporter activity and anti-epileptic drug resistance in refractory epileptic rats. J Neurochem 136:1096–1105. [DOI] [PubMed] [Google Scholar]
- Sharief MK, Thompson EJ (1992) In vivo relationship of tumor necrosis factor-alpha to blood-brain barrier damage in patients with active multiple sclerosis. J Neuroimmunol 38:27–33. [DOI] [PubMed] [Google Scholar]
- Sharma HS, Ali SF (2006) Alterations in blood-brain barrier function by morphine and methamphetamine. Ann N Y Acad Sci 1074:198–224. [DOI] [PubMed] [Google Scholar]
- Shibata M, Yamada S, Kumar SR, Calero M, Bading J, Frangione B, Holtzman DM, Miller CA, Strickland DK, Ghiso J, et al. (2000) Clearance of Alzheimer’s amyloid-ss(1-40) peptide from brain by LDL receptor-related protein-1 at the blood-brain barrier. J Clin Invest 106:1489–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin JA, Jeong SI, Kim HW, Jang G, Ryu DR, Ahn YH, Choi JH, Choi YH, Park EM (2018) Repression of adenosine triphosphate-binding cassette transporter ABCG2 by estrogen increases intracellular glutathione in brain endothelial cells following ischemic reperfusion injury. Neurobiol Aging 66:138–148. [DOI] [PubMed] [Google Scholar]
- Shinojima N, Tada K, Shiraishi S, Kamiryo T, Kochi M, Nakamura H, Makino K, Saya H, Hirano H, Kuratsu J, et al. (2003) Prognostic value of epidermal growth factor receptor in patients with glioblastoma multiforme. Cancer Res 63:6962–6970. [PubMed] [Google Scholar]
- Shubbar MH, Penny JI (2018) Effect of amyloid beta on ATP-binding cassette transporter expression and activity in porcine brain microvascular endothelial cells. Biochim Biophys Acta, Gen Subj 1862:2314–2322. [DOI] [PubMed] [Google Scholar]
- Shusta EV, Boado RJ, Mathern GW, Pardridge WM (2002) Vascular genomics of the human brain. J Cereb Blood Flow Metab 22:245–252. [DOI] [PubMed] [Google Scholar]
- Simon N, Dailly E, Combes O, Malaurie E, Lemaire M, Tillement JP, Urien S (1998) Role of lipoproteins in the plasma binding of SDZ PSC 833, a novel multidrug resistance-reversing cyclosporin. Br J Clin Pharmacol 45:173–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A, Kukreti R, Saso L, Kukreti S (2019) Oxidative stress: a key modulator in neurodegenerative diseases. Molecules 24:1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sio TT, Oberoi RK, Grams MP, Furutani KM, Gupta SK, Wilson ZC, Pokorny JL, Bakken KK, Schroeder MA, Carlson BL, et al. (2014) The impact of hemi-brain irradiation on accumulation of PI3K/mTOR inhibitors with limited (GDC-0980) and robust (GNE-317) blood-brain barrier penetration. Int J Radiat Oncol Biol Phys 90:S197. [Google Scholar]
- Sivandzade F, Cucullo L (2019) Assessing the protective effect of rosiglitazone against electronic cigarette/tobacco smoke-induced blood-brain barrier impairment. BMC Neurosci 20:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slosky LM, Thompson BJ, Sanchez-Covarrubias L, Zhang Y, Laracuente ML, Vanderah TW, Ronaldson PT, Davis TP (2013) Acetaminophen modulates P-glycoprotein functional expression at the blood-brain barrier by a constitutive androstane receptor-dependent mechanism. Mol Pharmacol 84:774–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits A, Hermansson M, Nistér M, Karnushina I, Heldin CH, Westermark B, Funa K (1989) Rat brain capillary endothelial cells express functional PDGF B-type receptors. Growth Factors 2:1–8. [DOI] [PubMed] [Google Scholar]
- Sochocka M, Koutsouraki ES, Gasiorowski K, Leszek J (2013) Vascular oxidative stress and mitochondrial failure in the pathobiology of Alzheimer’s disease: a new approach to therapy. CNS Neurol Disord Drug Targets 12:870–881. [DOI] [PubMed] [Google Scholar]
- Soldner EL, Hartz AM, Akanuma S, Pekcec A, Doods H, Kryscio R, Hosoya K, Bauer B(2019) Inhibition of human microsomal PGE2 synthase-1 reduces seizure-induced increases of P-glycoprotein expression and activity at the blood-brain barrier. FASEB J 33:13966–13981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song MS, Salmena L, Pandolfi PP (2012) The functions and regulation of the PTEN tumour suppressor. Nat Rev Mol Cell Biol 13:283–296. [DOI] [PubMed] [Google Scholar]
- Song X, Xie M, Zhang H, Li Y, Sachdeva K, Yan B (2004) The pregnane X receptor binds to response elements in a genomic context-dependent manner, and PXR activator rifampicin selectively alters the binding among target genes. Drug Metab Dispos 32:35–42. [DOI] [PubMed] [Google Scholar]
- Sorf A, Hofman J, Kučera R, Staud F, Ceckova M (2018) Ribociclib shows potential for pharmacokinetic drug-drug interactions being a substrate of ABCB1 and potent inhibitor of ABCB1, ABCG2 and CYP450 isoforms in vitro. Biochem Pharmacol 154:10–17. [DOI] [PubMed] [Google Scholar]
- Spatz H (1934) Die Bedeutung der vitalen Färbung für die Lehre vom Stoffaustausch zwischen dem Zentralnervensystem und dem übrigen Körper. Arch Psychiatr Nervenkr 101:267–358. [Google Scholar]
- Spiegl-Kreinecker S, Buchroithner J, Elbling L, Steiner E, Wurm G, Bodenteich A, Fischer J, Micksche M, Berger W (2002) Expression and functional activity of the ABC-transporter proteins P-glycoprotein and multidrug-resistance protein 1 in human brain tumor cells and astrocytes. J Neurooncol 57:27–36. [DOI] [PubMed] [Google Scholar]
- Stroke Progress Review Group (2002) Report of the Stroke Progress Review Group, National Institutes of Health, Bethesda, MD. [Google Scholar]
- Spudich A, Kilic E, Xing H, Kilic U, Rentsch KM, Wunderli-Allenspach H, Bassetti CL, Hermann DM (2006) Inhibition of multidrug resistance transporter-1 facilitates neuroprotective therapies after focal cerebral ischemia. Nat Neurosci 9:487–488. [DOI] [PubMed] [Google Scholar]
- Stefancin P, Cahaney C, Parker RI, Preston T, Coulehan K, Hogan L, Duong TQ (2020) Neural correlates of working memory function in pediatric cancer survivors treated with chemotherapy: an fMRI study. NMR Biomed 33:e4296. [DOI] [PubMed] [Google Scholar]
- Stefanovich V (1979) Cyclic 3′,5′-adenosine monophosphate phosphodiesterase (cAMP PDE) and cyclic 3′,5′-guanosine monophosphate phosphodiesterase (cGMP PDE) in microvessels isolated from bovine cortex. Neurochem Res 4:681–687. [DOI] [PubMed] [Google Scholar]
- Stern L, Gautier R (1921) Recherches sur le liquide céphalo-rachidien. 1. Les rapports entre le liquide céphalo-rachidien et la circulation sanguine. Arch Int Physiol 17:138–192. [Google Scholar]
- Stewart A, Steiner J, Mellows G, Laguda B, Norris D, Bevan P (2000) Phase I trial of XR9576 in healthy volunteers demonstrates modulation of P-glycoprotein in CD56+ lymphocytes after oral and intravenous administration. Clin Cancer Res 6:4186–4191. [PubMed] [Google Scholar]
- Storck SE, Hartz AMS, Bernard J, Wolf A, Kachlmeier A, Mahringer A, Weggen S, Pahnke J, Pietrzik CU (2018) The concerted amyloid-beta clearance of LRP1 and ABCB1/P-gp across the blood-brain barrier is linked by PICALM. Brain Behav Immun 73:21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss S, Bauer J, Ganter U, Jonas U, Berger M, Volk B (1992) Detection of interleukin-6 and alpha 2-macroglobulin immunoreactivity in cortex and hippocampus of Alzheimer’s disease patients. Lab Invest 66:223–230. [PubMed] [Google Scholar]
- Strazielle N, Ghersi-Egea JF (2015) Efflux transporters in blood-brain interfaces of the developing brain. Front Neurosci 9:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, et al. ; European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987–996. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Suzuki H, Sugimoto Y, Sugiyama Y (2003) ABCG2 transports sulfated conjugates of steroids and xenobiotics. J Biol Chem 278:22644–22649. [DOI] [PubMed] [Google Scholar]
- Sweeney MD, Ayyadurai S, Zlokovic BV (2016) Pericytes of the neurovascular unit: key functions and signaling pathways. Nat Neurosci 19:771–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Synold TW, Dussault I, Forman BM (2001) The orphan nuclear receptor SXR coordinately regulates drug metabolism and efflux. Nat Med 7:584–590. [DOI] [PubMed] [Google Scholar]
- Szatmári T, Lumniczky K, Désaknai S, Trajcevski S, Hídvégi EJ, Hamada H, Sáfrány G (2006) Detailed characterization of the mouse glioma 261 tumor model for experimental glioblastoma therapy. Cancer Sci 97:546–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada T, Suzuki H, Gotoh Y, Sugiyama Y (2005) Regulation of the cell surface expression of human BCRP/ABCG2 by the phosphorylation state of Akt in polarized cells. Drug Metab Dispos 33:905–909. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Abe Y, Tsugu A, Takamiya Y, Akatsuka A, Tsuruo T, Yamazaki H, Ueyama Y, Sato O, Tamaoki N, et al. (1994) Ultrastructural localization of P-glycoprotein on capillary endothelial cells in human gliomas. Virchows Arch 425:133–138. [DOI] [PubMed] [Google Scholar]
- Tang F, Hartz AMS, Bauer B (2017) Drug-resistant epilepsy: multiple hypotheses, few answers. Front Neurol 8:301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang JM, He QY, Guo RX, Chang XJ (2006) Phosphorylated Akt overexpression and loss of PTEN expression in non-small cell lung cancer confers poor prognosis. Lung Cancer 51:181–191. [DOI] [PubMed] [Google Scholar]
- Tarkowski E, Blennow K, Wallin A, Tarkowski A (1999) Intracerebral production of tumor necrosis factor-alpha, a local neuroprotective agent, in Alzheimer disease and vascular dementia. J Clin Immunol 19:223–230. [DOI] [PubMed] [Google Scholar]
- Taskar KS, Rudraraju V, Mittapalli RK, Samala R, Thorsheim HR, Lockman J, Gril B, Hua E, Palmieri D, Polli JW, et al. (2012) Lapatinib distribution in HER2 overexpressing experimental brain metastases of breast cancer. Pharm Res 29:770–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsuta T, Naito M, Oh-hara T, Sugawara I, Tsuruo T (1992) Functional involvement of P-glycoprotein in blood-brain barrier. J Biol Chem 267:20383–20391. [PubMed] [Google Scholar]
- Taylor PC (2019) Clinical efficacy of launched JAK inhibitors in rheumatoid arthritis. Rheumatology (Oxford) 58(Suppl 1):i17–i26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrando N, Eriksson LI, Ryu JK, Yang T, Monaco C, Feldmann M, Jonsson Fagerlund M, Charo IF, Akassoglou K, Maze M (2011) Resolving postoperative neuroinflammation and cognitive decline. Ann Neurol 70:986–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrell-Hall TB, Nounou MI, El-Amrawy F, Griffith JIG, Lockman PR (2017) Trastuzumab distribution in an in-vivo and in-vitro model of brain metastases of breast cancer. Oncotarget 8:83734–83744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tews DS, Nissen A, Külgen C, Gaumann AKA (2000) Drug resistance-associated factors in primary and secondary glioblastomas and their precursor tumors. J Neurooncol 50:227–237. [DOI] [PubMed] [Google Scholar]
- Thiebaut F, Tsuruo T, Hamada H, Gottesman MM, Pastan I, Willingham MC (1987) Cellular localization of the multidrug-resistance gene product P-glycoprotein in normal human tissues. Proc Natl Acad Sci USA 84:7735–7738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas FC, Taskar K, Rudraraju V, Goda S, Thorsheim HR, Gaasch JA, Mittapalli RK, Palmieri D, Steeg PS, Lockman PR, et al. (2009) Uptake of ANG1005, a novel paclitaxel derivative, through the blood-brain barrier into brain and experimental brain metastases of breast cancer. Pharm Res 26:2486–2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas H, Coley HM (2003) Overcoming multidrug resistance in cancer: an update on the clinical strategy of inhibiting p-glycoprotein. Cancer Contr 10:159–165. [DOI] [PubMed] [Google Scholar]
- Thomas H, Steiner JA, Mould GP(2001) A phase IIa pharmacokinetic study of the P-glycoprotein inhibitor, XR9576, in combination with paclitaxel in patients with ovarian cancer. Proc Annu Meet Am Soc Clin Oncol 20:288. [Google Scholar]
- Thorne RG, Emory CR, Ala TA, Frey WH 2nd (1995) Quantitative analysis of the olfactory pathway for drug delivery to the brain. Brain Res 692:278–282. [DOI] [PubMed] [Google Scholar]
- Tidefelt U, Liliemark J, Gruber A, Liliemark E, Sundman-Engberg B, Juliusson G, Stenke L, Elmhorn-Rosenborg A, Möllgård L, Lehman S, et al. (2000) P-glycoprotein inhibitor valspodar (PSC 833) increases the intracellular concentrations of daunorubicin in vivo in patients with P-glycoprotein-positive acute myeloid leukemia. J Clin Oncol 18:1837–1844. [DOI] [PubMed] [Google Scholar]
- Tishler DM, Weinberg KI, Hinton DR, Barbaro N, Annett GM, Raffel C (1995) MDR1 gene expression in brain of patients with medically intractable epilepsy. Epilepsia 36:1–6. [DOI] [PubMed] [Google Scholar]
- Tome ME, Herndon JM, Schaefer CP, Jacobs LM, Zhang Y, Jarvis CK, Davis TP (2016) P-glycoprotein traffics from the nucleus to the plasma membrane in rat brain endothelium during inflammatory pain. J Cereb Blood Flow Metab 36:1913–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tome ME, Schaefer CP, Jacobs LM, Zhang Y, Herndon JM, Matty FO, Davis TP (2015) Identification of P-glycoprotein co-fractionating proteins and specific binding partners in rat brain microvessels. J Neurochem 134:200–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran HT, Tran PH, Lee BJ (2009) New findings on melatonin absorption and alterations by pharmaceutical excipients using the Ussing chamber technique with mounted rat gastrointestinal segments. Int J Pharm 378:9–16. [DOI] [PubMed] [Google Scholar]
- Traxl A, Mairinger S, Filip T, Sauberer M, Stanek J, Poschner S, Jäger W, Zoufal V, Novarino G, Tournier N, et al. (2019) Inhibition of ABCB1 and ABCG2 at the mouse blood-brain barrier with marketed drugs to improve brain delivery of the model ABCB1/ABCG2 substrate [11C]erlotinib. Mol Pharm 16:1282–1293. [DOI] [PubMed] [Google Scholar]
- Traxl A, Wanek T, Mairinger S, Stanek J, Filip T, Sauberer M, Müller M, Kuntner C, Langer O (2015) Breast cancer resistance protein and P-glycoprotein influence in vivo disposition of 11C-erlotinib. J Nucl Med 56:1930–1936. [DOI] [PubMed] [Google Scholar]
- Tsuji A, Tamai I, Sakata A, Tenda Y, Terasaki T (1993) Restricted transport of cyclosporin A across the blood-brain barrier by a multidrug transporter, P-glycoprotein. Biochem Pharmacol 46:1096–1099. [DOI] [PubMed] [Google Scholar]
- Tsuruo T, Hamada H, Sato S, Heike Y (1989) Inhibition of multidrug-resistant human tumor growth in athymic mice by anti-P-glycoprotein monoclonal antibodies. Jpn J Cancer Res 80:627–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuruo T, Iida H, Tsukagoshi S, Sakurai Y (1981) Overcoming of vincristine resistance in P388 leukemia in vivo and in vitro through enhanced cytotoxicity of vincristine and vinblastine by verapamil. Cancer Res 41:1967–1972. [PubMed] [Google Scholar]
- Uchida Y, Ohtsuki S, Katsukura Y, Ikeda C, Suzuki T, Kamiie J, Terasaki T (2011) Quantitative targeted absolute proteomics of human blood-brain barrier transporters and receptors. J Neurochem 117:333–345. [DOI] [PubMed] [Google Scholar]
- Uchida Y, Wakayama K, Ohtsuki S, Chiba M, Ohe T, Ishii Y, Terasaki T (2014) Blood-brain barrier pharmacoproteomics-based reconstruction of the in vivo brain distribution of P-glycoprotein substrates in cynomolgus monkeys. J Pharmacol Exp Ther 350:578–588. [DOI] [PubMed] [Google Scholar]
- Ulbrich K, Hekmatara T, Herbert E, Kreuter J (2009) Transferrin- and transferrin-receptor-antibody-modified nanoparticles enable drug delivery across the blood-brain barrier (BBB). Eur J Pharm Biopharm 71:251–256. [DOI] [PubMed] [Google Scholar]
- Ullman JC, Arguello A, Getz JA, Bhalla A, Mahon CS, Wang J, Giese T, Bedard C, Kim DJ, Blumenfeld JR, et al. (2020) Brain delivery and activity of a lysosomal enzyme using a blood-brain barrier transport vehicle in mice. Sci Transl Med 12:eaay1163. [DOI] [PubMed] [Google Scholar]
- Vaidhyanathan S, Wilken-Resman B, Ma DJ, Parrish KE, Mittapalli RK, Carlson BL, Sarkaria JN, Elmquist WF (2016) Factors influencing the central nervous system distribution of a novel phosphoinositide 3-kinase/mammalian target of rapamycin inhibitor GSK2126458: implications for overcoming resistance with combination therapy for melanoma brain metastases. J Pharmacol Exp Ther 356:251–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valtonen S, Timonen U, Toivanen P, Kalimo H, Kivipelto L, Heiskanen O, Unsgaard G, Kuurne T (1997) Interstitial chemotherapy with carmustine-loaded polymers for high-grade gliomas: a randomized double-blind study. Neurosurgery 41:44–48, discussion 48–49. [DOI] [PubMed] [Google Scholar]
- van Assema DM, Lubberink M, Bauer M, van der Flier WM, Schuit RC, Windhorst AD, Comans EF, Hoetjes NJ, Tolboom N, Langer O, et al. (2012a) Blood-brain barrier P-glycoprotein function in Alzheimer’s disease. Brain 135:181–189. [DOI] [PubMed] [Google Scholar]
- van Assema DME, Lubberink M, Boellaard R, Schuit RC, Windhorst AD, Scheltens P, Lammertsma AA, van Berckel BNM (2012b) P-glycoprotein function at the blood-brain barrier: effects of age and gender. Mol Imaging Biol 14:771–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Vliet EA, Redeker S, Aronica E, Edelbroek PM, Gorter JA (2005) Expression of multidrug transporters MRP1, MRP2, and BCRP shortly after status epilepticus, during the latent period, and in chronic epileptic rats. Epilepsia 46:1569–1580. [DOI] [PubMed] [Google Scholar]
- van Vliet EA, van Schaik R, Edelbroek PM, Redeker S, Aronica E, Wadman WJ, Marchi N, Vezzani A, Gorter JA (2006) Inhibition of the multidrug transporter P-glycoprotein improves seizure control in phenytoin-treated chronic epileptic rats. Epilepsia 47:672–680. [DOI] [PubMed] [Google Scholar]
- van Vliet EA, Zibell G, Pekcec A, Schlichtiger J, Edelbroek PM, Holtman L, Aronica E, Gorter JA, Potschka H (2010) COX-2 inhibition controls P-glycoprotein expression and promotes brain delivery of phenytoin in chronic epileptic rats. Neuropharmacology 58:404–412. [DOI] [PubMed] [Google Scholar]
- Vanlandewijck M, He L, Mäe MA, Andrae J, Ando K, Del Gaudio F, Nahar K, Lebouvier T, Laviña B, Gouveia L, et al. (2018) A molecular atlas of cell types and zonation in the brain vasculature. Nature 554:475–480. [DOI] [PubMed] [Google Scholar]
- Vasiliou V, Vasiliou K, Nebert DW (2009) Human ATP-binding cassette (ABC) transporter family. Hum Genomics 3:281–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhaak RG, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD, Miller CR, Ding L, Golub T, Mesirov JP, et al. ; Cancer Genome Atlas Research Network (2010) Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 17:98–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veringa SJ, Biesmans D, van Vuurden DG, Jansen MH, Wedekind LE, Horsman I, Wesseling P, Vandertop WP, Noske DP, Kaspers GJ, et al. (2013) In vitro drug response and efflux transporters associated with drug resistance in pediatric high grade glioma and diffuse intrinsic pontine glioma. PLoS One 8:e61512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlaeyen JWS, Maher CG, Wiech K, Van Zundert J, Meloto CB, Diatchenko L, Battié MC, Goossens M, Koes B, Linton SJ (2018) Low back pain. Nat Rev Dis Primers 4:52. [DOI] [PubMed] [Google Scholar]
- Vogelgesang S, Cascorbi I, Schroeder E, Pahnke J, Kroemer HK, Siegmund W, Kunert-Keil C, Walker LC, Warzok RW (2002) Deposition of Alzheimer’s beta-amyloid is inversely correlated with P-glycoprotein expression in the brains of elderly non-demented humans. Pharmacogenetics 12:535–541. [DOI] [PubMed] [Google Scholar]
- Vogelgesang S, Warzok RW, Cascorbi I, Kunert-Keil C, Schroeder E, Kroemer HK, Siegmund W, Walker LC, Pahnke J (2004) The role of P-glycoprotein in cerebral amyloid angiopathy; implications for the early pathogenesis of Alzheimer’s disease. Curr Alzheimer Res 1:121–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Wedel-Parlow M, Wölte P, Galla HJ (2009) Regulation of major efflux transporters under inflammatory conditions at the blood-brain barrier in vitro. J Neurochem 111:111–118. [DOI] [PubMed] [Google Scholar]
- Wagner CC, Bauer M, Karch R, Feurstein T, Kopp S, Chiba P, Kletter K, Löscher W, Müller M, Zeitlinger M, et al. (2009) A pilot study to assess the efficacy of tariquidar to inhibit P-glycoprotein at the human blood-brain barrier with (R)-11C-verapamil and PET. J Nucl Med 50:1954–1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J, Martin C, Callaghan R (2004) Inhibition of P-glycoprotein function by XR9576 in a solid tumour model can restore anticancer drug efficacy. Eur J Cancer 40:594–605. [DOI] [PubMed] [Google Scholar]
- Wandel C, Kim RB, Kajiji S, Guengerich P, Wilkinson GR, Wood AJ (1999) P-glycoprotein and cytochrome P-450 3A inhibition: dissociation of inhibitory potencies. Cancer Res 59:3944–3948. [PubMed] [Google Scholar]
- Wang H, Zhang L, Hu P, Zheng X, Si X, Zhang X, Wang M (2018) Penetration of the blood-brain barrier by avitinib and its control of intra/extra-cranial disease in non-small cell lung cancer harboring the T790M mutation. Lung Cancer 122:1–6. [DOI] [PubMed] [Google Scholar]
- Wang M, Wu H, Li S, Xu Z, Li X, Yang Y, Li B, Li Y, Guo J, Chen H (2017) SYNJ2BP promotes the degradation of PTEN through the lysosome-pathway and enhances breast tumor metastasis via PI3K/AKT/SNAI1 signaling. Oncotarget 8:89692–89706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Chen HL, Liu L, Sheps JA, Phillips MJ, Ling V (2009) Compensatory role of P-glycoproteins in knockout mice lacking the bile salt export pump. Hepatology 50:948–956. [DOI] [PubMed] [Google Scholar]
- Wang X, Sykes DB, Miller DS (2010) Constitutive androstane receptor-mediated up-regulation of ATP-driven xenobiotic efflux transporters at the blood-brain barrier. Mol Pharmacol 78:376–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Campos CR, Peart JC, Smith LK, Boni JL, Cannon RE, Miller DS (2014) Nrf2 upregulates ATP binding cassette transporter expression and activity at the blood-brain and blood-spinal cord barriers. J Neurosci 34:8585–8593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward KW, Azzarano LM (2004) Preclinical pharmacokinetic properties of the P-glycoprotein inhibitor GF120918A (HCl salt of GF120918, 9,10-dihydro-5-methoxy-9-oxo-N-[4-[2-(1,2,3,4-tetrahydro-6,7-dimethoxy-2-isoquinolinyl)ethyl]phenyl]-4-acridine-carboxamide) in the mouse, rat, dog, and monkey. J Pharmacol Exp Ther 310:703–709. [DOI] [PubMed] [Google Scholar]
- Watari R, Matsuda A, Ohnishi S, Hasegawa H (2019) Minimal contribution of P-gp on the low brain distribution of naldemedine, a peripherally acting μ-opioid receptor antagonist. Drug Metab Pharmacokinet 34:126–133. [DOI] [PubMed] [Google Scholar]
- Wang W, Bodles-Brakhop AM, Barger SW (2016) A role for P-glycoprotein in clearance of alzheimer amyloid β -peptide from the brain. Curr Alzheimer Res 13:615–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen PY, Cloughesy TF, Olivero AG, Morrissey KM, Wilson TR, Lu X, Mueller LU, Coimbra AF, Ellingson BM, Gerstner E, et al. (2020) First-in-human phase I study to evaluate the brain-penetrant PI3K/mTOR inhibitor GDC-0084 in patients with progressive or recurrent high-grade glioma. Clin Cancer Res 26:1820–1828. [DOI] [PubMed] [Google Scholar]
- Westphal M, Hilt DC, Bortey E, Delavault P, Olivares R, Warnke PC, Whittle IR, Jääskeläinen J, Ram Z (2003) A phase 3 trial of local chemotherapy with biodegradable carmustine (BCNU) wafers (Gliadel wafers) in patients with primary malignant glioma. Neuro-oncol 5:79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijesuriya HC, Bullock JY, Faull RL, Hladky SB, Barrand MA (2010) ABC efflux transporters in brain vasculature of Alzheimer’s subjects. Brain Res 1358:228–238. [DOI] [PubMed] [Google Scholar]
- Winkler EA, Bell RD, Zlokovic BV (2011) Central nervous system pericytes in health and disease. Nat Neurosci 14:1398–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf A, Bauer B, Hartz AM (2012) ABC transporters and the Alzheimer’s disease enigma. Front Psychiatry 3:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff J (1963) Beitraege zur Ultrastruktur der Kapillaren in der normalen Grosshirnrinde. Zeitschrift fuer Zellforschung 60:409–431. [Google Scholar]
- World Health Organization (2006) Neurological Disorders: Public Health Challenges, WHO Press, Geneva, Switzerland. [Google Scholar]
- Wu CP, Hsiao SH, Murakami M, Lu YJ, Li YQ, Huang YH, Hung TH, Ambudkar SV, Wu YS (2017) Alpha-mangostin reverses multidrug resistance by attenuating the function of the multidrug resistance-linked ABCG2 transporter. Mol Pharm 14:2805–2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyss-Coray T, Rogers J (2012) Inflammation in Alzheimer disease—a brief review of the basic science and clinical literature. Cold Spring Harb Perspect Med 2:a006346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong H, Callaghan D, Jones A, Bai J, Rasquinha I, Smith C, Pei K, Walker D, Lue L-F, Stanimirovic D, et al. (2009) ABCG2 is upregulated in Alzheimer’s brain with cerebral amyloid angiopathy and may act as a gatekeeper at the blood-brain barrier for Abeta(1-40) peptides. J Neurosci 29:5463–5475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Li CY, Kong AN (2005) Induction of phase I, II and III drug metabolism/transport by xenobiotics. Arch Pharm Res 28:249–268. [DOI] [PubMed] [Google Scholar]
- Xu J, Peng H, Chen Q, Liu Y, Dong Z, Zhang JT (2007) Oligomerization domain of the multidrug resistance-associated transporter ABCG2 and its dominant inhibitory activity. Cancer Res 67:4373–4381. [DOI] [PubMed] [Google Scholar]
- Yan A, Joachims ML, Thompson LF, Miller AD, Canoll PD, Bynoe MS (2019a) CD73 Promotes glioblastoma pathogenesis and enhances its chemoresistance via A2B adenosine receptor signaling. J Neurosci 39:4387–4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan R, Zhao X, Lei J, Zhou Q (2019b) Structure of the human LAT1-4F2hc heteromeric amino acid transporter complex. Nature 568:127–130. [DOI] [PubMed] [Google Scholar]
- Yang Q, Langston JC, Tang Y, Kiani MF, Kilpatrick LE (2019) The role of tyrosine phosphorylation of protein kinase C delta in infection and inflammation. Int J Mol Sci 20:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Guo Q, Wang Y, Chen K, Zhang L, Cheng Z, Xu Y, Yin X, Bai Y, Rabbie S, et al. (2016) AZD3759, a BBB-penetrating EGFR inhibitor for the treatment of EGFR mutant NSCLC with CNS metastases. Sci Transl Med 8:368ra172. [DOI] [PubMed] [Google Scholar]
- Yasuda K, Cline C, Lin YS, Scheib R, Ganguly S, Thirumaran RK, Chaudhry A, Kim RB, Schuetz EG (2015) In vivo imaging of human MDR1 transcription in the brain and spine of MDR1-luciferase reporter mice. Drug Metab Dispos 43:1646–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates SL, Burgess LH, Kocsis-Angle J, Antal JM, Dority MD, Embury PB, Piotrkowski AM, Brunden KR (2000) Amyloid beta and amylin fibrils induce increases in proinflammatory cytokine and chemokine production by THP-1 cells and murine microglia. J Neurochem 74:1017–1025. [DOI] [PubMed] [Google Scholar]
- Yegnanarayan R, Mahesh SD, Sangle S (2006) Chronotherapeutic dose schedule of phenytoin and carbamazepine in epileptic patients. Chronobiol Int 23:1035–1046. [DOI] [PubMed] [Google Scholar]
- Yoshida Y, Yamada M, Wakabayashi K, Ikuta F (1988) Endothelial fenestrae in the rat fetal cerebrum. Brain Res Dev Brain Res 44:211–219. [DOI] [PubMed] [Google Scholar]
- You D, Wen X, Gorczyca L, Morris A, Richardson JR, Aleksunes LM (2019) Increased MDR1 transporter expression in human brain endothelial cells through enhanced histone acetylation and activation of Aryl hydrocarbon receptor signaling. Mol Neurobiol 56:6986–7002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousif S, Chaves C, Potin S, Margaill I, Scherrmann JM, Declèves X (2012) Induction of P-glycoprotein and Bcrp at the rat blood-brain barrier following a subchronic morphine treatment is mediated through NMDA/COX-2 activation. J Neurochem 123:491–503. [DOI] [PubMed] [Google Scholar]
- Yousif S, Saubaméa B, Cisternino S, Marie-Claire C, Dauchy S, Scherrmann JM, Declèves X (2008) Effect of chronic exposure to morphine on the rat blood-brain barrier: focus on the P-glycoprotein. J Neurochem 107:647–657. [DOI] [PubMed] [Google Scholar]
- Zamber CP, Lamba JK, Yasuda K, Farnum J, Thummel K, Schuetz JD, Schuetz EG (2003) Natural allelic variants of breast cancer resistance protein (BCRP) and their relationship to BCRP expression in human intestine. Pharmacogenetics 13:19–28. [DOI] [PubMed] [Google Scholar]
- Zavala-Tecuapetla C, Orozco-Suarez S, Manjarrez J, Cuellar-Herrera M, Vega-Garcia A, Buzoianu-Anguiano V (2020) Activation of adenosine receptors modulates the efflux transporters in brain capillaries and restores the anticonvulsant effect of carbamazepine in carbamazepine resistant rats developed by window-pentylenetetrazole kindling. Brain Res 1726:146516. [DOI] [PubMed] [Google Scholar]
- Zhang J, Zhang M, Sun B, Li Y, Xu P, Liu C, Liu L, Liu X (2014) Hyperammonemia enhances the function and expression of P-glycoprotein and Mrp2 at the blood-brain barrier through NF-κB. J Neurochem 131:791–802. [DOI] [PubMed] [Google Scholar]
- Zhang L, Zhang S, Yao J, Lowery FJ, Zhang Q, Huang WC, Li P, Li M, Wang X, Zhang C, et al. (2015) Microenvironment-induced PTEN loss by exosomal microRNA primes brain metastasis outgrowth. Nature 527:100–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SL, Lahens NF, Yue Z, Arnold DM, Pakstis PP, Schwarz JE, Sehgal A (2021) A circadian clock regulates efflux by the blood-brain barrier in mice and human cells. Nat Commun 12:617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SL, Yue Z, Arnold DM, Artiushin G, Sehgal A (2018) A circadian clock in the blood-brain barrier regulates xenobiotic efflux. Cell 173:130–139.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Mojsilovic-Petrovic J, Andrade MF, Zhang H, Ball M, Stanimirovic DB (2003) The expression and functional characterization of ABCG2 in brain endothelial cells and vessels. FASEB J 17:2085–2087. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Wang H, Wei L, Li G, Yu J, Gao Y, Gao P, Zhang X, Wei F, Yin D, et al. (2010) Transcriptional modulation of BCRP gene to reverse multidrug resistance by toremifene in breast adenocarcinoma cells. Breast Cancer Res Treat 123:679–689. [DOI] [PubMed] [Google Scholar]
- Zhao P, Zhang Y, Sun M, He Y (2008) Reversion of multidrug resistance in human glioma by RNA interference. Neurol Res 30:562–566. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Wei X, Song J, Zhang M, Huang T, Qin J (2019) Peroxisome proliferator-activated receptor γ agonist rosiglitazone protects blood-brain barrier integrity following diffuse axonal injury by decreasing the levels of inflammatory mediators through a caveolin-1-dependent pathway. Inflammation 42:841–856. [DOI] [PubMed] [Google Scholar]
- Zhou J, Liu M, Aneja R, Chandra R, Lage H, Joshi HC (2006) Reversal of P-glycoprotein-mediated multidrug resistance in cancer cells by the c-Jun NH2-terminal kinase. Cancer Res 66:445–452. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Zhou J, Li P, Xie Q, Sun B, Li Y, Chen Y, Zhao K, Yang T, Zhu L, et al. (2019) Increase in P-glycoprotein levels in the blood-brain barrier of partial portal vein ligation /chronic hyperammonemia rats is medicated by ammonia/reactive oxygen species/ERK1/2 activation: in vitro and in vivo studies. Eur J Pharmacol 846:119–127. [DOI] [PubMed] [Google Scholar]
- Zhu HJ, Liu GQ (2004) Glutamate up-regulates P-glycoprotein expression in rat brain microvessel endothelial cells by an NMDA receptor-mediated mechanism. Life Sci 75:1313–1322. [DOI] [PubMed] [Google Scholar]
- Zibell G, Unkrüer B, Pekcec A, Hartz AMS, Bauer B, Miller DS, Potschka H (2009) Prevention of seizure-induced up-regulation of endothelial P-glycoprotein by COX-2 inhibition. Neuropharmacology 56:849–855. [DOI] [PubMed] [Google Scholar]
- Zuloaga KL, Swift SN, Gonzales RJ, Wu TJ, Handa RJ (2012) The androgen metabolite, 5α-androstane-3β,17β-diol, decreases cytokine-induced cyclooxygenase-2, vascular cell adhesion molecule-1 expression, and P-glycoprotein expression in male human brain microvascular endothelial cells. Endocrinology 153:5949–5960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwick E, Bange J, Ullrich A (2001) Receptor tyrosine kinase signalling as a target for cancer intervention strategies. Endocr Relat Cancer 8:161–173. [DOI] [PubMed] [Google Scholar]