Abstract
Background
Vitamin B12 involves several physiological functions, and malabsorption is reported with medication use.
Objectives
Studies have reported an inverse association between the use of metformin or acid-lowering agents (ALAs), such as proton pump inhibitors, histamine 2 receptor antagonists, and blood vitamin B12 concentration, because of malabsorption. The concomitant use of these medications is underreported. We sought to examine these associations in a cohort of Boston-area Puerto Rican adults.
Methods
This analysis was conducted within the Boston Puerto Rican Health Study (BPRHS), an ongoing longitudinal cohort that enrolled 1499 Puerto Rican adults aged 45–75 y at baseline. Our study comprised 1428, 1155, and 782 participants at baseline, wave2 (2.2 y from baseline), and wave3 (6.2 y from baseline), respectively. Covariate-adjusted linear and logistic regression was used to examine the association between baseline medication use and vitamin B12 concentration or deficiency (vitamin B12 <148 pmol/L or methylmalonic acid >271 nmol/L), and long-term medication use (continuous use for ∼6.2 y) and wave3 vitamin B12 concentration and deficiency. Sensitivity analyses were done to examine these associations in vitamin B12 supplement users.
Results
At baseline, we observed an association between metformin use (β = –0.069; P = 0.03) and concomitant ALA and metformin use (β = –0.112; P = 0.02) and vitamin B12 concentration, but not a deficiency. We did not observe associations between ALA, proton pump inhibitors, or histamine 2 receptor antagonists, individually, with vitamin B12 concentration or deficiency.
Conclusions
These results suggest an inverse relationship between metformin, concomitant ALA, metformin use, and serum vitamin B12 concentration.
Keywords: vitamin B12, acid-lowering agents (ALA), proton pump inhibitors (PPI), histamine 2 receptor antagonists (H2RA), metformin
Introduction
Vitamin B12 (cobalamin) is an essential micronutrient available through animal-derived dietary sources and vitamin B12-fortified foods and supplements [1,2]. The recommended dietary allowance for vitamin B12 intake for adults is 2.4 μg/d [1,3]. Vitamin B12 is an important cofactor for enzymes involved in DNA and neurotransmitter synthesis, and vitamin B12 deficiency is implicated in megaloblastic anemia, axonal demyelination, neuropathy, and neurocognitive disorders [1,2,4]. Risk factors for vitamin B12 deficiency include aging, poor diet quality, vegan or vegetarian dietary patterns without vitamin B12-supplementation, gastrointestinal (GI) disorders, infections, gut-microbiome changes, GI surgery, medication use, pernicious anemia, autoimmune atrophic gastritis, and destruction of gastric cells and subsequent lack of intrinsic factor (IF) [1,3,5]. The use of medications, such as proton pump inhibitors (PPI), histamine 2 receptor antagonists (H2RA), and metformin, especially long-term use, has been associated with low vitamin B12 concentration and deficiency in several studies [2,3,[6], [7], [8]]. However, only a few studies have examined the impact of polypharmacy with long-term use of these medications, particularly among populations at risk of vitamin B12 deficiency, including Latinos.
PPIs and H2RAs are FDA-approved acid-lowering agents (ALAs) used to treat acid-related GI disorders. However, there is an increasing off-label utilization, with evidence suggesting 25–70% use without appropriate indication [9]. Long-term ALA use may lead to vitamin B12 malabsorption because of decreased gastric acid secretion in the stomach [10] because vitamin B12 must be released from dietary protein by gastric enzymes that require HCl, followed by complexing with IF and internalization in ileal cells by receptor-mediated endocytosis [1,2]. Metformin, an FDA-approved drug, is widely used to treat type 2 diabetes (T2D), insulin resistance, and polycystic ovary syndrome [9,11] but has been implicated in vitamin B12 malabsorption and deficiency [9,[11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22]]. Reported mechanisms include parietal cell dysfunction, decreased IF release, antagonism of vitamin B12-IF endocytic receptors on ileal cells, changes in gut motility, and bile acid secretion leading to small intestinal bacterial overgrowth, which preferentially bind vitamin B12-IF complex, leading to decreased vitamin B12 availability [11]. This is reported to result in metformin-induced cobalamin deficiency, causing a mixed phenotype of T2D and metformin-induced cobalamin deficiency-related neuropathy among T2D patients [11]. A recent review notes insufficient guidelines for vitamin B12 screening among medication users and suggests that more data is needed on factors contributing to deficiency among metformin users [11].
Further, a 40% prevalence of gastroesophageal reflux disease is estimated among those with T2D [17]. This could potentially lead to the concomitant use of acid-lowering medications and metformin [17]. Although no adverse interaction between these drugs is reported [23], there could be underlying comorbidities and increased malabsorption because of the additive effects [2,17,24], and this may increase risk of vitamin B12 deficiency because of gastric acid and ileal cell receptor dysfunction [2,24]. Only a few studies have reported on concomitant medication use and vitamin B12 deficiency [12,25,26], and among these, the analytical methods have differed widely, leading to divergent findings. For example, among a cohort of Asian participants (mean age 71 y), Ting et al. [12] showed an association between metformin use and vitamin B12 deficiency but no additional risk of deficiency with concomitant PPI or H2RA use. Chappell et al. [25] examined PPI and metformin dual therapy (participant mean age 53 y) and, in univariate analyses, observed no mean differences in vitamin B12 concentration compared with without concomitant PPI and metformin use. In contrast, Long et al. [26] reported an elevated incidence of vitamin B12 deficiency (defined as vitamin B12 concentration <300 pg/mL) among concomitant medication users, compared to either medication alone among participants of mean age 65 y.
The Massachusetts Hispanic Elders Study reported low vitamin B12 intake and status among Hispanic adults of Caribbean origin ≥60 y of age [27]. Latinos, and especially Puerto Ricans, have unique dietary patterns [28] as well high burden of medication use and polypharmacy [29]. Given the high prevalence of diabetes among the Hispanic population [30], the increasing use of acid-lowering medications in a cohort of Boston-area Puerto Ricans [29], and the lack of studies examining these associations in the minority population such as Latinos/Hispanics [25], we sought to examine the associations between use of these medications and vitamin B12 (cobalamin) deficiency in a high-risk Latino population, with a particular focus on concomitant and long-term use.
Methods
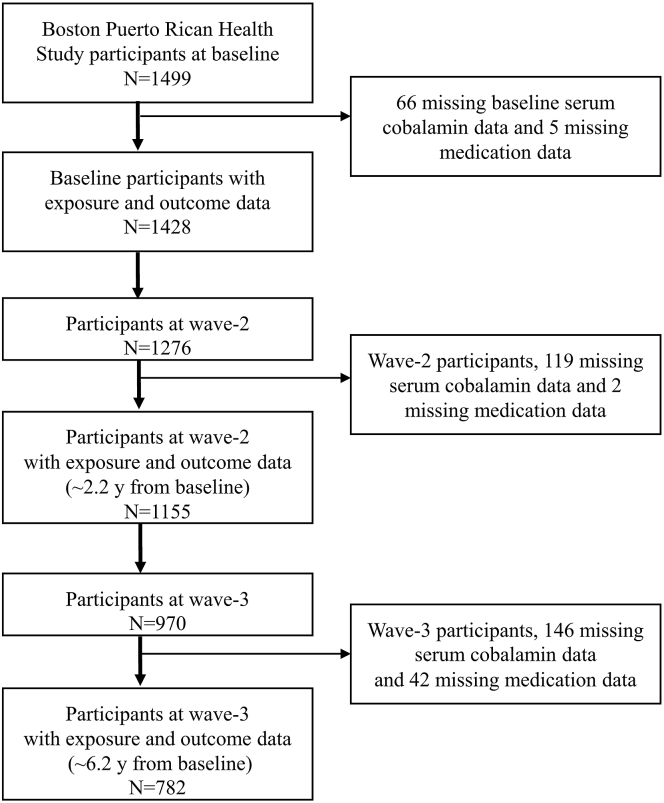
The Boston Puerto Rican Health Study (BPRHS) is an ongoing longitudinal cohort that enrolled 1499 self-identified Puerto Rican adults aged 45–75 y at baseline [31]. In the BPHRS, 1428, 1155, and 782 participants had complete data on serum vitamin B12 concentration and medication use (PPI, H2RA, and metformin use) at baseline, wave2 (mean 2.2 ± 0.61 y from baseline) and wave3 (mean 6.2 ± 0.98 y from baseline), respectively (Figure 1). Study protocols were approved by the institutional review boards at Tufts Medical Center and the University of Massachusetts Lowell.
FIGURE 1.
Participant flow chart.
Assessment of outcomes: Serum vitamin B12 concentration and vitamin B12 deficiency
Blood vitamin B12 concentration was determined by radio-assay using Immunoassay kits from Siemens Medical Solutions Diagnostics for use on the IMMULITE 1000 [31]. MMA was extracted from serum and derivatized to form a volatile compound, as described by Rasmussen [32]. This derivative was analyzed by GC-MS and quantified based on the amount of a deuterated form of MMA (D3) added to the samples prior to extraction. Analysis was done on a Hewlett-Packard 5791A (Hewlett-Packard) and dried on a Techne’s Dri-block (Bibby Scientific) [31]. Continuous serum vitamin B12 concentration was the primary outcome. The supporting outcome of vitamin B12 deficiency was defined as serum vitamin B12 <148 pmol/L or serum MMA >271 nmol/L, and these cut-offs were based on previous reports [3,31,33]. MMA was measured only among 394 participants with plasma vitamin B12 concentration <350 pg/mL.
Assessment of exposure: Medication use
Medication data from the BPHRS baseline, wave2, and wave3 were used for analyses. Prescription and over-the-counter medication use was ascertained by careful examination of medicine containers, and use was classified dichotomously (use/no use). Having reported medication use at baseline, participants were asked to self-report on medication use during subsequent waves, and the same methods of assessments were used in waves 2 and 3 [31]. PPIs reported in the BPRHS include omeprazole, esomeprazole, lansoprazole, pantoprazole and rabeprazole. H2RAs reported include Cimetidine, Famotidine, Nizatidine, and Ranitidine. The exposure measure of ALA was defined as using either a PPI or H2RA. Metformin (Glucophage) use was self-reported as use compared with no use.
Concomitant medication use was defined as the use of ALA (i.e., PPI or H2RA) and metformin. Long-term medication use was defined as a response of “yes” to the use of that at baseline, wave2, and wave3, i.e., ∼6.2 y of continuous use. In our analyses, we considered individual and concomitant medication use both at baseline and long-term use (∼6.2 y of continuous use).
Assessment of covariates
All analyses were adjusted for covariates known or hypothesized to be associated with vitamin B12 concentration or deficiency from our previous analyses or reported in the literature. Multivariable models were adjusted for age, sex, alcohol frequency, smoking, BMI (in kg/m2), serum creatinine, multivitamin, and ACE inhibitor. Additionally, individual ALA, PPI, H2RA, and metformin exposures were adjusted for metformin or H2RA and metformin or PPI and metformin or PPI and H2RA, respectively. Baseline cross-sectional analyses were adjusted for covariates from baseline, and wave3 cross-sectional analyses were adjusted for corresponding covariates measured at wave3.
Medications, including ACE inhibitor and multivitamin supplement use, were assessed via examination of containers [31]. At each time point, participants self-reported health conditions, information on health insurance, and self-rated health status[31]. Smoking and alcohol use frequency, history, and type were assessed. Alcohol use was categorized as nondrinker (none within the past year), moderate, or heavy drinker. Smoking status was categorized as nonsmoker (lifetime use of <100 cigarettes), past or current smoker. Physical activity score was based on a modified questionnaire, as described previously [31], and computed as the sum of hours spent on activities over 24-h and multiplied by weights for the rate of oxygen consumption associated with each activity. BMI was computed as weight (kg) divided by height (m) squared. BMI of 25–29.9 was classified as overweight, 30–39.9 as obese class I and II, and ≥40 as extremely obese. Cholesterol was analyzed from EDTA plasma with an enzymatic endpoint reaction [31]. CRP was measured in serum using the IMMULITE 1000 High Sensitive CRP kit (Seimens Medical Solutions Diagnostics) [31]. Kidney dysfunction (serum creatinine concentration) is reported to be associated with the concentration of vitamin B12 biomarkers, such as MMA [17]. Serum creatinine was measured with a colorimetric, kinetic reaction on the Olympus AU400e with Olympus Creatinine Reagents (OSR6178) (Olympus America Inc.) [31]. Medications, including ACE inhibitor and multivitamin supplement use, were ascertained by careful assessment via examination of containers [31]. Diabetes was defined as fasting plasma glucose ≥126 mg/dL or the use of diabetes medication [31].
Statistical analyses
Analyses were performed using R version 4.1.0 (R Foundation for Statistical Computing). We report on prevalence estimates at baseline, wave2, and wave3. Descriptive statistics for medication users compared with nonusers were evaluated using the Wilcox test for univariate median differences for continuous variables and Fisher’s exact test or t-tests for univariate differences for categorical variables.
For cross-sectional analyses at baseline, linear regression models, adjusted for baseline covariates, were used to examine the association between medication use and log-transformed serum vitamin B12 concentration. To examine the association between medication use and dichotomously defined vitamin B12 deficiency (vitamin B12 <148 pmol/L or MMA >271 nmol/L), we used logistic regression, adjusted for the same covariates as above. Analogously, for the cross-sectional analyses at wave3, linear regression models were adjusted for wave3 covariates to examine the association between long-term medication use (continuous use for ∼6.2 y) and log-transformed wave3 serum vitamin B12 concentration. Covariate-adjusted multivariable logistic regression models were used to examine associations between long-term use and wave3 vitamin B12 deficiency.
We performed sensitivity analyses to examine if the associations at baseline were consistent when restricted to participants with low serum vitamin B12 (vitamin B12 <148 pmol/L) based on cut-offs reported previously [3,31,33]. We did not perform these analyses for long-term medication use because of the low number of participants in the subgroups. A second set of sensitivity analyses were done to examine these associations among participants taking vitamin B12 supplements (yes/no) both at baseline and wave3. The use of vitamin B12 supplements was assessed using a semi-quantitative FFQ that asked about 1) vitamin B12 supplementation and 2) the use of B-complex vitamins [28]. Participants who replied “yes” to either were labeled users, and those who replied “no” to both were labeled as nonusers.
Results
Prevalence estimates
Baseline
Among a total of 1499 BPRHS baseline participants, 1428, 1155, and 782 had complete outcomes (serum vitamin B12 concentration) and exposure data (PPI, H2RA, and metformin use) at baseline, wave2 (mean 2.2 y from baseline) and wave3 (mean 6.2 y from baseline), respectively (Figure 1). Among these 1428 baseline participants, there were 361 (25.3%) and 112 (7.8%) self-reported PPI and H2RA use, respectively (Supplementary Figure 1). There were 18 (1.3%) concomitant users of PPI and H2RA. 308 (21.6%) participants self-reported metformin users (Supplementary Figure 1). There were 120 (8.4%) concomitant users of ALA and metformin at baseline.
Among the 1428 baseline participants, 128 (9.0%) were vitamin B12 deficient, 41 (2.9%) had vitamin B12 <148 pmol, and the mean baseline serum vitamin B12 concentration was 401 pmol/L (±208 pmol/L). Four hundred forty-nine (31.4%) used a supplement containing vitamin B12 (446 vitamin B12 supplements and 3 vitamin B complex supplement users).
Wave2 (∼2.2 y after baseline)
Thousand hundred fifty-five participants had complete exposure and outcome data at wave2 (Figure 1). Among them, 347 (30%), 97 (8.4%), and 300 (25.9%) were PPI, H2RA, and metformin users, respectively (Supplementary Figure 1). Between baseline and wave2, 248 (21.5%) participants used ALA (211 PPI and 45 H2RA), with 17 (1.5%) concomitant users of PPI and H2RA, of whom 4 utilized these medications continuously and concomitantly for 2 y. There were 212 (18.4%) metformin users with continuous use from baseline to wave2 and 60 (5.2%) concomitant and continuous users of ALA and metformin from baseline to wave2. Among 1155 wave2 participants, 97 (8.4%) were vitamin B12 deficient.
Wave3 (∼6.2 y after baseline)
Seven hundred eighty-two participants had complete exposure and outcome data at wave3 (Figure 1). Among these, 288 (36.8%), 53 (6.8%), and 245 (31.3%) were PPI, H2RA, and metformin users, respectively (Supplementary Figure 1). Hundred twenty-four (15.8%) participants reported ALA use continuously from baseline through wave3 (112 PPI and 12 H2RA). There were no long-term concomitant users of PPI and H2RA. There were 118 (15.1%) long-term metformin users and 23 (2.9%) concomitant and long-term ALA and metformin users. Among 782 wave3 participants, 67 (8.6%) were vitamin B12 deficient. Four hundred twenty-one (53.8%) utilized a supplement containing vitamin B12.
We observed increasing utilization of PPI from baseline (25.3% users) to the wave3 (36.8% users), with a similar upward trend for metformin users from baseline (21.6%) to the wave3 (31.3%) (Supplementary Figure 1). We did not observe a similar trend with concomitant use of ALA and metformin (8.4% at baseline to 2.9% at wave3).
Baseline demographics
At baseline, ALA users had higher median age (median 58 y compared with 55 y; P < 0.01), were more likely to be postmenopausal females without estrogen use (65.3% compared with 53.2%; P < 0.01), to have low education (53.8% compared with 43.2%; P < 0.01), low physical activity (P < 0.01), overweight or obese BMI (P < 0.01), high utilization of metformin (P < 0.01) and multivitamin (P = 0.05) and high inflammation, as assessed by CRP concentration (P = 0.01), compared to non-ALA users, and a trend toward T2D (P = 0.07) (Table 1). Metformin users had higher median age (median 59 y compared with 56 y; P < 0.01), were more likely to be postmenopausal females without estrogen use (64.3% compared with 55.1%; P < 0.01), to have low education (53.6% compared with 44.8%; P < 0.01), low physical activity (P < 0.01), overweight or obese BMI (P < 0.01), and high PPI (P = 0.01) and ACE inhibitor use (P < 0.01), compared to non-metformin users (Table 2). As expected, metformin users had higher blood glucose concentrations compared to nonusers (P < 0.01) and with 100% prevalence of T2D (Table 2).
TABLE 1.
Baseline demographics based on proton pump inhibitors use in the Boston Puerto Rican Health Study
| Medication n (%) | ALA (PPI or H2RA) use |
No ALA 973 (68.1) | P value1 | ||
|---|---|---|---|---|---|
| PPI user 361 (25.3) | H2A user 112 (7.84) | ALA user 455 (31.9) | |||
| Age (y), median (min, max) | 59 (45, 75) | 57 (45, 72) | 58 (45, 75) | 55 (45, 75) | <0.012 |
| Male n (%) | 83 (22.9) | 22 (19.6) | 100 (21.9) | 321 (32.9) | <0.013 |
| Premenopausal with estrogen use n (%) | 48 (13.3) | 14 (12.5) | 58 (12.7) | 134 (13.8) | |
| Postmenopausal without estrogen use n (%) | 230 (63.7) | 76 (67.9) | 297 (65.3) | 518 (53.2) | |
| Education <8th grade n (%) | 191 (52.9) | 65 (58.0) | 245 (53.8) | 420 (43.2) | <0.013 |
| Education ≥8th grade n (%) | 169 (46.8) | 47 (41.9) | 209 (45.9) | 551 (56.6) | |
| Never smoker n (%) | 163 (45.2) | 47 (42.0) | 203 (44.7) | 440 (45.3) | 0.863 |
| Past and current smoke n (%) | 197 (54.6) | 65 (58.0) | 251 (55.3) | 531 (54.7) | |
| Alcohol intake never n (%) | 235 (65.1) | 67 (59.8) | 289 (63.5) | 494 (50.1) | <0.013 |
| Alcohol intake moderate n (%) | 104 (28.8) | 34 (30.4) | 134 (29.5) | 383 (39.4) | |
| Alcohol intake heavy n (%) | 18 (5.0) | 9 (8.0) | 26 (5.7) | 89 (9.2) | |
| Physical activity median (min, max) | 29.2 (24.3, 53.1) | 29.7 (25.4, 44.1) | 29.4 (24.3, 53.1) | 30.8 (24.8, 62.6) | <0.012 |
| BMI <25 n (%) | 40 (11.1) | 13 (11.6) | 48 (10.5) | 149 (15.3) | <0.013 |
| BMI ≥25 and <30 n (%) | 92 (25.5) | 30 (26.8) | 116 (25.5) | 309 (31.8) | |
| BMI ≥30 n (%) | 229 (63.4) | 69 (61.6) | 291 (63.9) | 515 (52.9) | |
| Hypertension n (%) | 268 (74.2) | 88 (78.6) | 343 (75.4) | 618 (63.5) | <0.014 |
| Stroke n (%) | 24 (6.65) | 4 (3.57) | 27 (5.93) | 27 (2.77) | <0.014 |
| Diabetes n (%) | 154 (42.7) | 51 (45.5) | 194 (42.6) | 365 (37.5) | 0.074 |
| Gastrointestinal disorders n (%) | 228 (63.3) | 61 (54.5) | 278 (61.1) | 175 (18.0) | <0.014 |
| Creatinine (mg/dL) median (min, max) | 0.8 (0.4, 4.7) | 0.8 (0.5, 3.3) | 0.8 (0.4, 4.7) | 0.8 (0.4, 7.9) | 0.472 |
| CRP (mg/L) median (min, max) | 4.1 (0.1, 56.6) | 3.9 (0, 69.8) | 4.1 (0, 69.8) | 3.4 (0, 127) | 0.012 |
| Cholesterol (mg/dL) median (min, max) | 184 (89, 375) | 175 (94, 320) | 182 (89, 375) | 182 (87, 355) | 0.352 |
| Glucose (mg/dL) median (min, max) | 104 (64, 476) | 107.5 (64, 255) | 105 (64, 476) | 102 (47, 587) | 0.332 |
| Metformin n (%) | 96 (26.6) | 30 (26.8) | 120 (26.4) | 188 (19.3) | 0.0034 |
| Multivitamin n (%) | 128 (35.5) | 34 (30.36) | 157 (34.5) | 266 (27.3) | 0.0514 |
| ACE inhibitor n (%) | 27 (7.48%) | 5 (4.46%) | 31 (6.81%) | 45 (4.62%) | 0.114 |
| Plasma folate (ng/mL) | 40.3 (9.8, 155) | 41.3 (7.3, 131) | 40.3 (7.3, 155) | 38.8 (5.9, 199) | 0.082 |
| Plasma homocysteine (μmol/L) | 8.1 (3.5, 69.4) | 8.4 (4.1, 55) | 8.1 (3.5, 69.4) | 8.1 (3.9, 56) | 0.992 |
ALA, acid-lowering agent; H2RA, histamine 2 receptor antagonists; min, max, minimum, maximum; PPI, proton pump inhibitor.
ALA user compared with never user.
Wilcox test.
Fisher's exact test.
χ2 test.
TABLE 2.
Baseline demographics based on metformin use in the Boston Puerto Rican Health Study
| n (%) | Metformin user 308 (21.6) | No metformin 1120 (78.4) | P value1 |
|---|---|---|---|
| Age (y), median (min, max) | 59 (45, 75) | 56 (45, 75) | <0.012 |
| Male n (%) | 84 (27.3) | 337 (30.1) | <0.013 |
| Premenopausal with estrogen use n (%) | 26 (8.44) | 166 (14.8) | |
| Postmenopausal without estrogen use n (%) | 198 (64.3) | 617 (55.1) | |
| Education <8th grade n (%) | 165 (53.6) | 500 (44.8) | <0.013 |
| Education ≥8th grade n (%) | 143 (46.4) | 617 (55.2) | |
| Never smoker n (%) | 140 (45.5) | 503 (45.0) | 0.893 |
| Past and current smoke n (%) | 168 (54.6) | 614 (54.9) | |
| Alcohol intake never n (%) | 183 (60.4) | 600 (53.9) | <0.013 |
| Alcohol intake moderate n (%) | 108 (35.6) | 409 (36.8) | |
| Alcohol intake heavy n (%) | 12 (3.96) | 103 (9.3) | |
| Physical activity median (min, max) | 29.9 (25.2, 54.2) | 30.5 (24.3, 62.6) | <0.012 |
| BMI <25 n (%) | 28 (9.1) | 169 (15.1) | <0.013 |
| BMI ≥25 and <30 n (%) | 72 (23.4) | 353 (31.5) | |
| BMI ≥30 n (%) | 208 (67.5) | 598 (53.4) | |
| Hypertension n (%) | 266 (86.6) | 695 (62.6) | <0.014 |
| Stroke n (%) | 13 (4.25) | 41 (3.66) | 0.764 |
| Diabetes n (%) | 308 (100) | 251 (22.4) | <0.014 |
| Gastrointestinal disorders n (%) | 103 (33.7) | 350 (31.3) | 0.484 |
| Creatinine (mg/dL) median (min, max) | 0.8 (0.4, 2.0) | 0.8 (0.4, 7.9) | 0.092 |
| CRP median (mg/L) (min, max) | 3.8 (0,127) | 3.5 (0, 67.8) | 0.292 |
| Cholesterol (mg/dL) median (min, max) | 165 (89, 355) | 187 (87, 375) | <0.012 |
| Glucose (mg/dL) median (min, max) | 136.5 (47, 587) | 99 (51, 519) | <0.012 |
| Proton pump inhibitor n (%) | 96 (31.2) | 265 (23.7) | <0.014 |
| Histamine 2 receptor antagonist n (%) | 30 (9.74) | 82 (7.32) | 0.194 |
| Multivitamin n (%) | 64 (20.8) | 215 (19.2) | 0.574 |
| ACE inhibitor 5n (%) | 36 (11.7) | 40 (3.57) | <0.014 |
| Plasma folate (ng/mL) | 41.3 (14.1, 109) | 38.5 (5.9, 199) | <0.012 |
| Plasma homocysteine (μmol/L) | 8.2 (3.5, 27.8) | 8.1 (3.9, 69.4) | 0.592 |
min, max, minimum, maximum.
Metformin user compared with never user.
Wilcox test.
Fisher's exact test.
χ2 test.
Angiotensin-converting enzyme (ACE)
Although there were no significant differences in median homocysteine or folate concentrations among ALA users compared with nonusers (Table 1), we observed higher folate concentrations among metformin users compared with nonusers (P < 0.01) (Table 2).
Cross-sectional associations between baseline medication use and baseline vitamin B12 concentration and deficiency
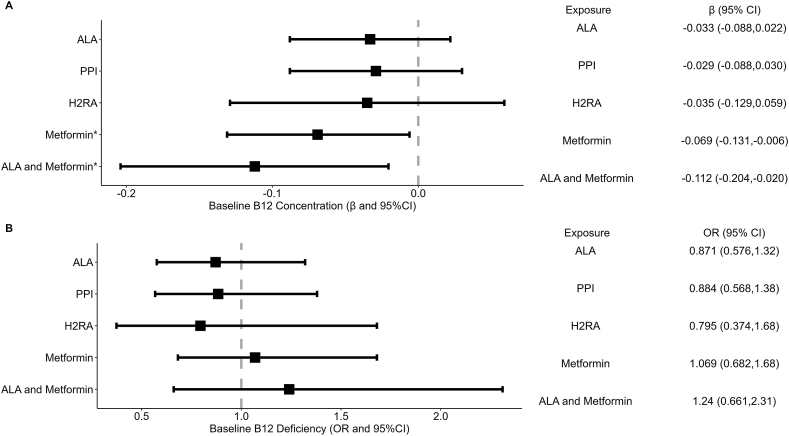
In analyses adjusted for baseline covariates, as described above, we did not observe associations between ALA, PPI, or H2RA use and log serum vitamin B12 (all P ≥ 0.24) (Figure 2A) or vitamin B12 deficiency (all P ≥ 0.51) (Figure 2B). We observed an inverse association between metformin use and log serum vitamin B12 concentration (β = –0.069; P = 0.03) (Figure 2A) but not vitamin B12 deficiency (OR = 1.069; P = 0.77) (Figure 2B), and an inverse association between concomitant ALA and metformin use and log serum vitamin B12 concentration (β = –0.112; P = 0.02) (Figure 2A) but not vitamin B12 deficiency (OR = 1.24; P = 0.51) (Figure 2B).
FIGURE 2.
(A) Association between medication use and serum vitamin B12 concentration among baseline BPHRS participants. (B) Association between medication use and vitamin B12 deficiency among baseline BPHRS participants. Thousand four hundred thirteen participants with the complete outcome and exposure data after adjusting for relevant covariates. Exposure ALA adjusted for age, sex, alcohol frequency, smoking, BMI level, multivitamin, ACE inhibitor, metformin use, and serum creatinine. Exposure PPI adjusted for age, sex, alcohol frequency, smoking, BMI level, multivitamin, ACE inhibitor, H2RA and metformin use, and serum creatinine. Exposure H2RA adjusted for age, sex, alcohol frequency, smoking, BMI level, multivitamin, ACE inhibitor, PPIs and metformin use, and serum creatinine. Exposure ALA and metformin adjusted for age, sex, alcohol frequency, smoking, BMI level, multivitamin, ACE inhibitor use, PPI use, H2RA use, and serum creatinine. ∗P ≤ 0.05. ALA, acid-lowering agent; BPHRS, Boston Puerto Rican Health H2RA, histamine 2 receptor antagonists; PPI, proton pump inhibitor.
Cross-sectional associations between long-term medication use (continuous use ∼6.2 y) and wave3 vitamin B12 concentration and deficiency
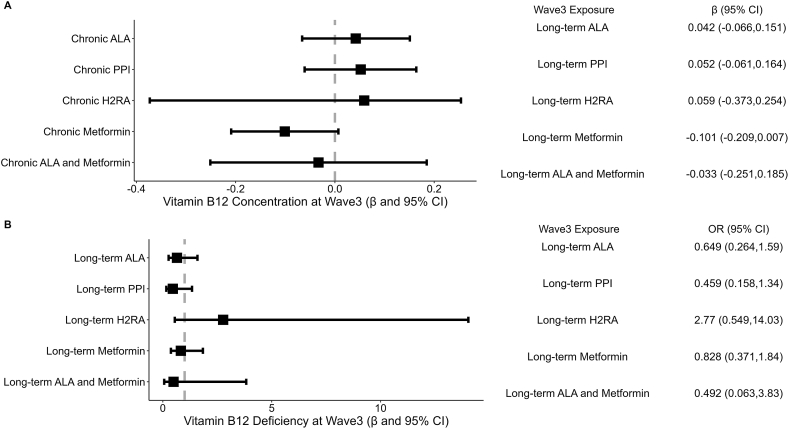
In analyses adjusted for relevant wave3 covariates, we did not observe an association between long-term ALA, PPI, H2RA, or concomitant use (all P ≥ 0.37), but we observed a trend toward a negative association between long-term metformin use and wave3 log serum vitamin B12 concentration (β = –0.101; P = 0.07) (Figure 3A). We did not observe an association between long-term ALA, PPI, H2RA, metformin, or concomitant use and wave3 vitamin B12 deficiency (all P ≥ 0.15) (Figure 3B).
FIGURE 3.
(A) Association between long-term medication use (continuous use for ∼6.2 y) and wave3 vitamin B12 concentration among wave3 BPHRS participants. (B) Association between long-term medication use and wave3 vitamin B12 deficiency among wave3 BPHRS participants. Seven hundred eighteen participants with complete wave3 outcome and wave3 exposure data after adjusting for wave3 covariates. Exposure long-term ALA (∼6.2 y continuous use) adjusted for wave3 covariates: age, sex, alcohol frequency, smoking, BMI level, multivitamin, ACE inhibitor, metformin use, and serum creatinine. Exposure long-term PPI (∼6.2 y continuous use) adjusted for wave3 covariates: age, sex, alcohol frequency, smoking, BMI level, multivitamin, ACE inhibitor, H2RA and metformin use, and serum creatinine. Exposure long-term H2RA (∼6.2 y continuous use) adjusted for wave3 covariates: age, sex, alcohol frequency, smoking, BMI level, multivitamin, ACE inhibitor, PPI and metformin use, and serum creatinine. Exposure metformin (∼6.2 y continuous use) adjusted for wave3 covariates: age, sex, alcohol frequency, smoking, BMI level, multivitamin, ACE inhibitor use, PPI use, H2RA use, and serum creatinine. ALA, acid-lowering agent; BPHRS, Boston Puerto Rican Health Study; H2RA, histamine 2 receptor antagonists; PPI, proton pump inhibitor.
Sensitivity analyses
Among baseline participants with low serum vitamin B12 concentration (<148 pmol/L; not accounting for serum MMA), after adjusting for relevant covariates, we did not observe associations between ALA, metformin, or concomitant use and vitamin B12 concentration (all P ≥ 0.3). However, our power may have been limited because of the small numbers: 41 baseline participants had vitamin B12 concentration <148 pmol/L, among these, 12 used metformin, 13 used ALA (9 PPI and 4 H2RA users), and 7 were concomitant users.
In sensitivity analyses among vitamin B12 supplement users at baseline, after adjusting for relevant covariates, we did not observe cross-sectional associations between ALA, PPI, and H2RA and log serum vitamin B12 concentration or deficiency (all P ≥ 0.39). Results were similar for long-term medication use, with no associations between long-term ALA, PPI, H2RA, or concomitant use (all P ≥ 0.37) and vitamin B12 outcomes. However, in cross-sectional analyses among vitamin B12 supplement users, we continued to observe an inverse association between metformin (β = –0.125; P = 0.03) and concomitant ALA and metformin use (β = –0.1802; P = 0.03) and log serum vitamin B12 concentration, although this did not extend to deficiency (P = 0.57) (Supplementary Table 1). Sensitivity analyses focused on long-term medication use among users of wave3 vitamin B12 supplements did not observe an association between long-term ALA, H2RA, metformin, and concomitant use and wave3 log serum vitamin B12 concentration or deficiency (all P ≥ 0.15) (Supplementary Table 1).
Discussion
Although there is evidence suggesting an association between ALA (PPI or H2RA) and metformin use and vitamin B12 status [2,3,6,9], only a few studies have examined concomitant use, with divergent results [12,25,26]. Concomitant use of these medications is hypothesized to increase the severity of vitamin B12 deficiency because of the additive effects [2,24]. In a cohort of Boston-area Puerto Rican adults ≥45 y of age, we examined the association between individual and concomitant use of PPI, H2RA, and metformin and vitamin B12 status. In fully adjusted models, we observed an inverse association between metformin use and the use of concomitant PPI or H2RA and metformin and serum vitamin B12 concentration. Our results are supported by a study that also reported an association between concomitant use of these medications and low vitamin B12 status [26]. Further, these associations persisted in concomitant medication users with vitamin B12 supplementation in baseline cross-sectional analysis. The additive effects of these medications by a combination of mechanisms may influence the absorption of vitamin B12 in the gastric and iliac regions [6,24]. Underlying comorbidities associated with polypharmacy may also explain these associations. The clinical implication of vitamin B12 status is relevant to those with T2D and comorbid gastroesophageal reflux disease[11].
In previous studies, long-term medication use was significantly associated with vitamin B12 status compared to shorter duration of use [34,35]. Therefore, we examined associations between long-term medication use (∼6.2 y of continuous use) and vitamin B12 status. However, we did not observe an association between long-term PPI or H2RA use and serum vitamin B12 concentration or deficiency. Our results agree with Elzen et al. [36], who also did not observe an association between serum vitamin B12 and PPI use (>3 y use) among community-dwelling participants, compared to controls (partners of study participants to account for the influence of dietary habits). Another case-controlled retrospective observational study reported no association between PPI use, PPI dose or duration of use, and serum vitamin B12 among participants ≥65 y of age [37]. Further, H2RA monotherapy is not widely reported in the literature, likely because of use as needed [6], and we see decreasing use of H2RA from baseline to wave3 in our cohort. Therefore, we do not report on the associations for long-term H2RA use because of the low number of participants in this group. Overall, there were fewer participants at wave3 compared to baseline, and our results indicate that more participants are needed to observe these associations.
Our results on metformin agree with a previous study [20] that reported an association between metformin use and blood vitamin B12 concentration but not vitamin B12 deficiency (that included MMA measures). It is possible that, although metformin use may reduce the concentration of circulating serum vitamin B12, suggesting marginal deficiency, this does not extend to clinical deficiency in most individuals. Blood vitamin B12 concentration does not reflect total body stores because of the efficient utilization of vitamin B12 by enterohepatic recycling [3,38], which may prevent the manifestation of clinical deficiency for several years. At baseline, the inverse association between metformin use and serum vitamin B12 concentration persisted despite vitamin B12 supplementation, likely because vitamin B12 supplement use does not influence mechanisms associated with metformin and vitamin B12 absorption, such as ileal cell receptor-mediated dysfunction. In our study, although long-term metformin use showed a trend toward association with wave3 serum vitamin B12 concentration, this did not reach significance. There were fewer participants at wave3 than baseline, and a larger sample size may be needed for a better estimate.
In this study, we observed a detrimental impact of metformin on vitamin B12. Vitamin B12 has been protectively associated with brain health, including in our cohort[39]. There is a robust association between T2D and risk of Alzheimer’s disease (AD) and, on the one hand, metformin improves insulin resistance and glycemic which is protective against AD and neuropathy [22,40], and metformin may also reduce dementia risk by suppressing AD-related genes [41]. On the contrary, worse cognitive decline among T2D patients using metformin than those not using metformin is reported [42]. It is hypothesized that the effect of metformin on cognitive performance may be mediated by alterations in vitamin B12 concentration[42]. Metformin-associated vitamin B12 deficiency may also be associated with neuropathy [22]. This paradoxical role of metformin is further highlighted in other neurodegenerative disorders, such as Parkinson’s disease. Metformin, as a disease-modifying therapy for Parkinson’s disease, was recently suggested because of its role in enhancing autophagic and mitochondrial functions and decreasing reactive oxygen species and protein aggregation [43]. However, this study also mentioned risk for vitamin B12 deficiency with long-term metformin use [43].
There is a high degree of heterogeneity in prior work on PPI, H2RA, and metformin [38], which may be attributed to differences in study design, variation in population characteristics, age of study participants, dose and duration of medication use, definitions and cut-off values for vitamin B12 deficiency [3], or covariates adjusted in analyses. For example, although Valuck et al. [44]and Cotter et al. [37] examine these associations in a university-based geriatric setting and use the outcome of serum vitamin B12 concentration <130 pg/mL and <150 pmol/L, Lam et al. [35] utilized data from Kaiser Permanente Northern California integrated healthcare system and reported vitamin B12 deficiency based on the ICD-9 codes, respectively. In this study, we add to these data by examining the concomitant use of medications in a minority population with a high risk of vitamin B12 deficiency, utilizing a rigorous, MMA-supplemented definition of vitamin B12 deficiency. Further, vitamin B12 status may be influenced by vitamin B12 supplements, and, to our knowledge, only a few prior studies have included this important modifying factor [45]. Therefore, in sensitivity analyses, we examined these associations among vitamin B12 supplement users. In addition, alcohol use is a risk factor for vitamin B12 depletion [35], and only a few prior studies have adjusted for alcohol intake [45]. Among studies that have adjusted for alcohol intake, Porter et al. [45] reported a significant association between PPI use and vitamin B12 status; they also observed a significantly high intake of alcohol among PPI users compared to nonusers in their cohort. In our cohort, PPI users had significantly low alcohol intake compared to nonusers, which may partly explain the lack of association. However, although metformin users also had low alcohol intake compared to non-metformin users, there was a significant association between metformin use and vitamin B12 concentration at baseline. These differences may be because of the differential mechanisms of these drugs on vitamin B12 absorption.
Strengths of our study include rich exposure, outcome, and covariate data from baseline through wave3 (∼6.2 y of follow-up) in the BPHRS, which allowed us to examine the association between long-term medication use and vitamin B12 status. The comprehensive set of measurements at all waves of data collection allowed us to adjust for relevant variables reported to influence vitamin B12 status. Information on vitamin B12 supplementation in the BPRHS allowed us to examine these associations among users of vitamin B12 supplements. A key strength of our study is the assessment of vitamin B12 outcomes utilizing both circulating and functional vitamin B12 biomarkers for serum concentration and deficiency [2,3,33]. Limitations of our study include self-reported comorbidities, medication, and supplement use. We also did not have information on pernicious anemia, GI surgery, and other risk factors that would increase risk of vitamin B12 deficiency. We did not have information on the dose of the drug; therefore, dose-response characteristics could not be evaluated. We also did not have a prebaseline duration of medication use. It is reported that CYP2C19 catalyzes the metabolism of PPI, and polymorphism of this enzyme is associated with vitamin B12 deficiency [17], and we did not have information on this. Finally, all metformin users in our cohort had T2D; we could not distinguish between the contribution of metformin compared with T2D to the participant’s vitamin B12 status.
In conclusion, we observe an association between metformin use and vitamin B12 concentration, which persists despite vitamin B12 supplementation. Mainland Puerto Ricans are a growing subset of the population in the United States, with reported low vitamin B12 status, with >8% showing deficiency [27,39]. Our results suggest that further research is needed to understand the mechanisms involved in medication use and vitamin B12 deficiency, especially in the context of polypharmacy.
Funding
This study was supported by NIH grants P50 HL105185, P01 AG023394, and R01 AG055948.
Author contributions
The authors’ responsibilities were as follows– DD, KLT, and NP: contributed to the design; DD: conducted the data analyses and drafted the manuscript; NP, JSL, TMS, and KLT: provided analytical feedback and revised the manuscript; KLT: is the principal investigator of the Boston Puerto Rican Health Study and all authors: read and approved the final manuscript.
Author disclosures
The authors report no conflicts of interest.
Data availability
Data described in the manuscript, code book, and analytic code will be made available upon request, pending approval by the authors.
Acknowledgments
We thank Esther Jennings, Liam Fouhy, and Xiyuan Zhang for their assistance with data management.
Footnotes
This article was presented in part at Nutrition 2022 by DD, JSL, TMS, KLT, and NP. "Acid-Lowering Agents and Trajectory of Vitamin B-12 Deficiency in the Boston Puerto Rican Health Study." Current Developments in Nutrition 6, no. Supplement_1 (2022): 1178-1178, July 2022. https://nutrition.org/nutrition-2022-registration/.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.tjnut.2023.05.031.
Contributor Information
Deepika Dinesh, Email: deepika_dinesh@uml.edu.
Natalia Palacios, Email: natalia_palacios@uml.edu.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Watanabe F. Vitamin B12 sources and bioavailability. Exp. Biol. Med. (Maywood). 2007;232(10):1266–1274. doi: 10.3181/0703-MR-67. [DOI] [PubMed] [Google Scholar]
- 2.Miller J.W. Proton pump inhibitors, H2-receptor antagonists, metformin, and vitamin B-12 deficiency: clinical implications. Adv. Nutr. 2018;9(4):511S–518S. doi: 10.1093/advances/nmy023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Green R., Allen L.H., Bjørke-Monsen A.-L., Brito A., Guéant J.-L., Miller J.W., et al. Vitamin B12 deficiency. Nat. Rev. Dis. Primers. 2017;3(1):1–20. [Google Scholar]
- 4.Riggs K.M., Spiro A., 3rd, Tucker K., Rush D. Relations of vitamin B-12, vitamin B-6, folate, and homocysteine to cognitive performance in the Normative Aging Study. Am. J. Clin. Nutr. 1996;63(3):306–314. doi: 10.1093/ajcn/63.3.306. [DOI] [PubMed] [Google Scholar]
- 5.Andrès E., Loukili N.H., Noel E., Kaltenbach G., Abdelgheni M.B., Perrin A.E., et al. Vitamin B12 (cobalamin) deficiency in elderly patients. CMAJ. 2004;171(3):251–259. doi: 10.1503/cmaj.1031155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Linder L., Tamboue C., Clements J.N. Drug-induced vitamin B12 deficiency: a focus on proton pump inhibitors and histamine-2 antagonists. J. Pharm. Pract. 2017;30(6):639–642. doi: 10.1177/0897190016663092. [DOI] [PubMed] [Google Scholar]
- 7.Liu Q., Li S., Quan H., Li J. Vitamin B12 status in metformin treated patients: systematic review. PLoS One. 2014;9(6) doi: 10.1371/journal.pone.0100379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tomkin G.H., Hadden D.R., Weaver J.A., Montgomery D.A. Vitamin-B12 status of patients on long-term metformin therapy. Br. Med. J. 1971;2(5763):685–687. doi: 10.1136/bmj.2.5763.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jaynes M., Kumar A.B. The risks of long-term use of proton pump inhibitors: a critical review. Ther. Adv. Drug. Saf. 2019;10 doi: 10.1177/2042098618809927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li M., Luo Z., Yu S., Tang Z. Proton pump inhibitor use and risk of dementia: systematic review and meta-analysis. Medicine. 2019;98(7) doi: 10.1097/MD.0000000000014422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Infante M., Leoni M., Caprio M., Fabbri A. Long-term metformin therapy and vitamin B12 deficiency: an association to bear in mind. World J. Diabetes. 2021;12(7):916–931. doi: 10.4239/wjd.v12.i7.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ting R.Z.-W., Szeto C.C., Chan M.H.-M., Ma K.K., Chow K.M. Risk factors of vitamin B12 deficiency in patients receiving metformin. Arch. Intern. Med. 2006;166(18):1975–1979. doi: 10.1001/archinte.166.18.1975. [DOI] [PubMed] [Google Scholar]
- 13.Reinstatler L., Qi Y.P., Williamson R.S., Garn J.V., Oakley G.P., Jr. Association of biochemical B₁₂ deficiency with metformin therapy and vitamin B₁₂ supplements: the National Health and Nutrition Examination Survey, 1999-2006. Diabetes Care. 2012;35(2):327–333. doi: 10.2337/dc11-1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sato Y., Ouchi K., Funase Y., Yamauchi K., Aizawa T. Relationship between metformin use, vitamin B12 deficiency, hyperhomocysteinemia and vascular complications in patients with type 2 diabetes. Endocr. J. 2013;60(12):1275–1280. doi: 10.1507/endocrj.ej13-0332. [DOI] [PubMed] [Google Scholar]
- 15.Ko S.H., Ko S.H., Ahn Y.B., Song K.H., Han K.D., Park Y.M., et al. Association of vitamin B12 deficiency and metformin use in patients with type 2 diabetes. J. Korean Med. Sci. 2014;29(7):965–972. doi: 10.3346/jkms.2014.29.7.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wong C.W., Leung C.S., Leung C.P., Cheng J.N. Association of metformin use with vitamin B12 deficiency in the institutionalized elderly. Arch. Gerontol. Geriatr. 2018;79:57–62. doi: 10.1016/j.archger.2018.07.019. [DOI] [PubMed] [Google Scholar]
- 17.Wakeman M., Archer D.T. Metformin and micronutrient status in type 2 diabetes: does polypharmacy involving acid-suppressing medications affect vitamin B12 levels? Diabetes Metab. Syndr. Obes. 2020;13:2093–2108. doi: 10.2147/DMSO.S237454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martin D., Thaker J., Shreve M., Lamerato L., Budzynska K.J.B.N. Assessment of vitamin B12 deficiency and B12 screening trends for patients on metformin: a retrospective cohort case review. BMJ Nutr. Prev. Health. 2021;4(1):30–35. doi: 10.1136/bmjnph-2020-000193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kancherla V., Garn J.V., Zakai N.A., Williamson R.S., Cashion W.T., Odewole O., et al. Multivitamin use and serum vitamin B12 concentrations in older-adult metformin users in REGARDS, 2003-2007. PLoS One. 2016;11(8) doi: 10.1371/journal.pone.0160802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Obeid R. Metformin causing vitamin B12 deficiency: a guilty verdict without sufficient evidence. Diabetes Care. 2014;37(2):e22–e23. doi: 10.2337/dc13-2278. [DOI] [PubMed] [Google Scholar]
- 21.Ahmed M.A. Metformin and vitamin B12 deficiency: where do we stand? J. Pharm. Pharm. Sci. 2016;19(3):382–398. doi: 10.18433/J3PK7P. [DOI] [PubMed] [Google Scholar]
- 22.Out M., Kooy A., Lehert P., Schalkwijk C.A., Stehouwer C.D.A. Long-term treatment with metformin in type 2 diabetes and methylmalonic acid: post hoc analysis of a randomized controlled 4.3 year trial. J. Diabetes Complications. 2018;32(2):171–178. doi: 10.1016/j.jdiacomp.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 23.Flory J., Haynes K., Leonard C.E., Hennessy S. Proton pump inhibitors do not impair the effectiveness of metformin in patients with diabetes. Br. J. Clin. Pharmacol. 2015;79(2):330–336. doi: 10.1111/bcp.12506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zdilla M.J. Metformin with either histamine H2-receptor antagonists or proton pump inhibitors: A polypharmacy recipe for neuropathy via vitamin B12 depletion. Clin. Diabetes. 2015;33(2):90–95. doi: 10.2337/diaclin.33.2.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chappell L., Brown S.A., Wensel T.M. Evaluation of vitamin B12 monitoring in patients on concomitant metformin and proton pump inhibitors. Innov. Pharm. 2020;11(4) doi: 10.24926/iip.v11i4.3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Long A.N., Atwell C.L., Yoo W., Solomon S.S. Vitamin B12 deficiency associated with concomitant metformin and proton pump inhibitor use. Diabetes Care. 2012;35(12):e84–e. doi: 10.2337/dc12-0980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kwan L.L., Bermudez O.I., Tucker K.L. Low vitamin B-12 intake and status are more prevalent in Hispanic older adults of Caribbean origin than in neighborhood-matched non-Hispanic whites. J. Nutr. 2002;132(7):2059–2064. doi: 10.1093/jn/132.7.2059. [DOI] [PubMed] [Google Scholar]
- 28.Tucker K.L., Bianchi L.A., Maras J., Bermudez O.I. Adaptation of a food frequency questionnaire to assess diets of Puerto Rican and non-Hispanic adults. Am. J. Epidemiol. 1998;148(5):507–518. doi: 10.1093/oxfordjournals.aje.a009676. [DOI] [PubMed] [Google Scholar]
- 29.Dinesh D., Lee J.S., Scott T.M., Tucker K.L., Palacios N. Proton pump inhibitor use and cognitive function in the Boston Puerto Rican health study. J. Gerontol. A. Biol. Sci. Med. Sci. 2022 doi: 10.1093/gerona/glac231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tucker K.L., Bermudez O.I., Castaneda C. Type 2 diabetes is prevalent and poorly controlled among Hispanic elders of Caribbean origin. Am. J. Public Health. 2000;90(8):1288. doi: 10.2105/ajph.90.8.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tucker K.L., Mattei J., Noel S.E., Collado B.M., Mendez J., Nelson J., et al. The Boston Puerto Rican Health Study, a longitudinal cohort study on health disparities in Puerto Rican adults: challenges and opportunities. BMC Public Health. 2010;10(1):107. doi: 10.1186/1471-2458-10-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rasmussen K. Solid-phase sample extraction for rapid determination of methylmalonic acid in serum and urine by a stable-isotope-dilution method. Clin. Chem. 1989;35(2):260–264. [PubMed] [Google Scholar]
- 33.Yetley E.A., Pfeiffer C.M., Phinney K.W., Bailey R.L., Blackmore S., Bock J.L., et al. Biomarkers of vitamin B-12 status in NHANES: a roundtable summary. Am. J. Clin. Nutr. 2011;94(1):313S–321S. doi: 10.3945/ajcn.111.013243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jung S.B., Nagaraja V., Kapur A., Eslick G.D. Association between vitamin B 12 deficiency and long-term use of acid-lowering agents: a systematic review and meta-analysis. Intern. Med. J. 2015;45(4):409–416. doi: 10.1111/imj.12697. [DOI] [PubMed] [Google Scholar]
- 35.Lam J.R., Schneider J.L., Zhao W., Corley D.A. Proton pump inhibitor and histamine 2 receptor antagonist use and vitamin B12 deficiency. JAMA. 2013;310(22):2435–2442. doi: 10.1001/jama.2013.280490. [DOI] [PubMed] [Google Scholar]
- 36.Den Elzen W.P., Groeneveld Y., De Ruijter W., Souverijn J.H., Le Cessie S., Assendelft W.J., et al. Long-term use of proton pump inhibitors and vitamin B12 status in elderly individuals, Aliment. Pharmacol. Ther. 2008;27(6):491–497. doi: 10.1111/j.1365-2036.2008.03601.x. 2008. [DOI] [PubMed] [Google Scholar]
- 37.Cotter P.E., O’Keeffe S.T. Use of proton pump inhibitors is not associated with vitamin B12 deficiency and in older hospital patients: a case control study. Eur. Geriatr. Med. 2011;2(4):253–255. [Google Scholar]
- 38.Niafar M., Hai F., Porhomayon J., Nader N.D. The role of metformin on vitamin B12 deficiency: a meta-analysis review. Intern. Emerg. Med. 2015;10:93–102. doi: 10.1007/s11739-014-1157-5. [DOI] [PubMed] [Google Scholar]
- 39.Boumenna T., Scott T.M., Lee J.-S., Palacios N., Tucker K.L. Folate, vitamin B-12, and cognitive function in the Boston Puerto Rican Health Study. Am. J. Clin. Nutr. 2021;113(1):179–186. doi: 10.1093/ajcn/nqaa293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liao W., Xu J., Li B., Ruan Y., Li T., Liu J. Deciphering the Roles of Metformin in Alzheimer's Disease: A Snapshot. Front Pharmacol. 2022;12:4123. doi: 10.3389/fphar.2021.728315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Charpignon M.-L., Vakulenko-Lagun B., Zheng B., Magdamo C., Su B., Evans K., et al. Causal inference in medical records and complementary systems pharmacology for metformin drug repurposing towards dementia. Nat Commun. 2022;13(1):1–17. doi: 10.1038/s41467-022-35157-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moore E.M., Mander A.G., Ames D., Kotowicz M.A., Carne R.P., Brodaty H., et al. Increased risk of cognitive impairment in patients with diabetes is associated with metformin. Diabetes Care. 2013;36(10):2981–2987. doi: 10.2337/dc13-0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Agostini F., Masato A., Bubacco L., Bisaglia M. Metformin repurposing for Parkinson disease therapy: opportunities and challenges. Int. J. Mol. Sci. 2021;23(1):398. doi: 10.3390/ijms23010398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Valuck R.J., Ruscin J.M. A case-control study on adverse effects: H2 blocker or proton pump inhibitor use and risk of vitamin B12 deficiency in older adults. J. Clin. Epidemiol. 2004;57(4):422–428. doi: 10.1016/j.jclinepi.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 45.Porter K.M., Hoey L., Hughes C.F., Ward M., Clements M., Strain J., et al. Associations of atrophic gastritis and proton-pump inhibitor drug use with vitamin B-12 status, and the impact of fortified foods, in older adults. Am. J. Clin. Nutr. 2021;114(4):1286–1294. doi: 10.1093/ajcn/nqab193. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the manuscript, code book, and analytic code will be made available upon request, pending approval by the authors.