Abstract
As the demand for clinically effective fluoride-free oral care products for consumers increases, it is important to document which types of toothpastes have been shown in clinical studies to be effective in improving oral health. In this review, we included different indications, i.e., caries prevention, improving periodontal health, reducing dentin hypersensitivity, protecting against dental erosion, and safely improving tooth whitening in defining what constitutes improvement in oral health. While there are several professional and consumer fluoride-containing formulations fortified with calcium-phosphate-based ingredients, this review focuses on fluoride-free toothpastes containing biomimetic calcium-phosphate-based molecules as the primary active ingredients. Several databases were searched, and only clinical trials in human subjects were included; in vitro and animal studies were excluded. There were 62 oral health clinical trials on biomimetic hydroxyapatite (HAP), 57 on casein phosphopeptide-amorphous calcium phosphate (CPP-ACP), 26 on calcium sodium phosphosilicate (CSPS, or so called Bioglass), and 2 on β-tricalcium phosphate (β-TCP). HAP formulations were tested the most in clinical trials for benefits in preventing caries, dentin hypersensitivity, improving periodontal health, and tooth whitening. Based on the current clinical evidence to date, fluoride-free HAP toothpaste formulations are the most versatile of the calcium phosphate active ingredients in toothpastes for improving oral health.
Keywords: fluoride-free, toothpaste, calcium phosphate, hydroxyapatite (HAP), casein phosphoprotein-amorphous calcium phosphate (CPP-ACP), beta-tricalcium phosphate (β-TCP), calcium sodium phosphosilicate (CSPS; Bioglass), caries, periodontitis, gingivitis, erosion, whitening
1. Introduction
Even in the 21st century, poor oral health remains a major human affliction burdening health care systems in countries all over the world. Dental decay (caries) is still the most common affliction of children and very common in adults [1]. Periodontal disease today is the main reason for tooth loss throughout industrialized countries [2]. However, these human afflictions are preventable with improved diets, healthy nutrition, and especially with improved oral hygiene using toothpastes with active ingredients designed to prevent these common health issues [3]. Furthermore, as teeth are expected to last for a lifetime in ageing populations, dental tissues need to be protected from dental erosion. Some oral care products help protect teeth from mineral loss improving the longevity of the dentition [4]. In addition, people today want whiter and healthier looking teeth. Adults value the cosmetic appearance of their teeth; a whiter dentition improves confidence, improves social acceptance and even employment prospects [5]. Therefore, there is a need to develop active ingredients for toothpastes designed to help with one or more of the preventive roles in home oral care.
Fluoride has been the active ingredient most used in toothpastes throughout the world for the prevention of dental caries for a long time. That fluoride toothpaste reduces dental decay has been documented with many placebo-controlled clinical trials [6]. In order to improve fluoride toothpaste formulations to also help prevent gingivitis and lower the risk of periodontal disease, additional ingredients are added. These include pyrophosphates to help reduce calculus formation [7], bicarbonate for dental plaque removal [8], as well as antibacterial agents such as stannous salts [9], zinc salts [10], and chlorhexidine at low concentrations [11]. Natural ingredients such as herbs and plant-based antimicrobials have also been tested mostly in non-fluoride toothpastes [12].
Fluoridated toothpastes pose safety issues for children under age 6 since there is risk of dental fluorosis from fluoride ingestion [13]. Children under age 3 swallow a significant amount of toothpaste even if they are able to rinse and spit [14]. Because of the risk of fluoride ingestion, dentists in the US and Canada are advised to recommend families with children under the age of 3 year to use a pea-sized amount of fluoridated toothpaste [15,16]. In Europe, children up to age 2 should use a rice-size smear, and those aged 2 to 6 years, a pea size amount [17]. However, children, but also their parents when applying toothpaste for their children, still tend to use more toothpaste, and the majority of those ages ≤ 3 years use it 2 times a day or more often [18]. There is no direct evidence that these smaller amounts of toothpaste can prevent cavities [19]. One study showed that the pea-size amount is less effective in cleaning teeth compared to larger toothpaste amounts [20]. Recent concerns about fluoride’s potential neurotoxicity on developing brains [21,22] have also spurred on research to find alternatives to fluoride as an active ingredient in toothpastes. There is now a concerted effort to find effective non-fluoride anti-caries agents. However, because there is also the need to improve general oral health by also reducing the risk of gingivitis, reducing dentin sensitivity, preventing dental mineral loss, and improving on the appearance of teeth, the active ingredient needs to be very versatile and provide more than one benefit. One ingredient, hydroxyapatite (HAP), has been tested clinically as a general multifunctional useful active ingredient [23].
The most promising candidate active ingredients in toothpastes for achieving all these goals in the future are the calcium-phosphate-based molecules [24]. There is a wide range of these inorganic molecules and the most researched ingredients in this class that have already been tested in toothpastes are amorphous calcium derivatives (casein phosphoprotein-amorphous calcium phosphate, or CPP-ACP), hydroxyapatite, calcium sodium phosphosilicate (CSPS, Novamin, Bioglass), and beta-tricalcium phosphate (β-TCP). A recent review on randomized clinical trials comparing calcium-phosphate-based ingredients was published [25], but the authors omitted clinical evidence from in situ trials, where active ingredients are applied to human enamel slabs imbedded in appliances worn by volunteer subjects. Additionally, the authors did not examine the clinical evidence for hydroxyapatite’s usefulness in controlling caries, even though it has been shown to clinically produce calcium phosphate ions required for remineralization and there have been clinical trials published to show reversal of carious lesions [26].
This review was conducted to examine the clinical evidence published for fluoride-free calcium-phosphate-based toothpastes in order to compare them for determining which one might be a versatile, overall effective toothpaste formulation in promoting good overall oral health.
2. Materials and Methods
A PICO framework was used to guide the search. The following question was posed: “Do fluoride-free toothpastes containing calcium-phosphate-based active ingredients help to improve oral health”? The target populations (P) were humans of all ages. The intervention (I) was using one of the following calcium-phosphate-based active ingredients in a human subject clinical trial, including in situ trials using human enamel imbedded in intra-oral appliances worn by human subjects: amorphous calcium derivatives (casein phosphoprotein-amorphous calcium phosphate, or CPP-ACP), hydroxyapatite (HAP), calcium sodium phosphosilicate, (CSPS, Bioglass) and beta-tricalcium phosphate (β-TCP). The controls (C) were untreated teeth or placebo toothpastes, or positive control toothpastes, and the outcome (O) was one of the following: lowered caries or reduction in white spot lesions, reduced dentin hypersensitivity, protection against dental erosion, improvement of gingival or periodontal health, and/or improved appearance of teeth. The literature was searched using the University of Toronto databases PubMed (Medline), Scopus, and Web of Science, as well as Google Scholar, from inception to 1 June 2023. For the active ingredients, the search terms were “hydroxyapatite”, or “nano-hydroxyapatite”; “casein phosphopeptide-amorphous calcium phosphate” or “CPP-ACP”, or “amorphous calcium phosphate” or “ACP”; “calcium sodium phosphosilicate” or “CSPS” or “bioglass” or “novamin”; “beta-tricalcium phosphate” or “β-TCP” or “tricalcium phosphate” or “TCP”. For the vehicle, the search terms were “toothpaste” or “dentifrice”. For the experimental conditions, the search terms were “in vivo”; ”in situ”; “clinical trial”. For the remineralization outcomes, the search terms were “caries” or “white spot lesion” or “WSL”; “remineralization”; “erosion”. For the dentin hypersensitivity outcomes, they were “sensitivity” or “hypersensitivity”. For the gingival health outcomes, the search terms were “gingivitis” or “gingival” or “periodontal” or “periodontitis”. For the tooth whitening outcomes, the search words were “whiten(s)” or “whitening”.
Inclusion and exclusion criteria: The selection of studies was based on the need to focus on only clinical trials that produced direct clinical evidence for the outcomes directly related to oral health improvement. Animal and in vitro studies were excluded, even those that provide support for the mechanisms of how the active ingredients provide benefits since the evidence needs to be gathered from clinical trials in human subjects. In situ studies were included if the enamel slabs imbedded in appliances worn by volunteer subjects were derived from human (not bovine) enamel. In vivo effects on Streptococcus mutans and intra-oral mineral release studies were excluded. All reviews, abstracts, and book chapters were excluded. There were no language restrictions.
Microsoft Excel spreadsheets of the publications were produced by manually downloading the particulars of each publication of interest (authors, title, journal, abstract, key words) or converting “cvs” files generated by the databases, such as Scopus. The studies were ordered alphabetically, and duplicates were manually removed. Even though the collection of papers was obtained systematically, qualitative syntheses (risk of bias) and quantitative syntheses (meta-analysis) were not carried out. Qualitative (risk of bias) and quantitative (meta-analyses) have been conducted elsewhere on hydroxyapatite-containing oral care products [5,26,27], so the aim of this review was to systematically document the studies published for other fluoride-free calcium phosphate toothpastes, in comparison to the current literature on hydroxyapatite toothpastes, in order to determine the volume and extent of this evidence. Qualitative and quantitative meta-analysis of that literature was not the focus of this review.
3. Results
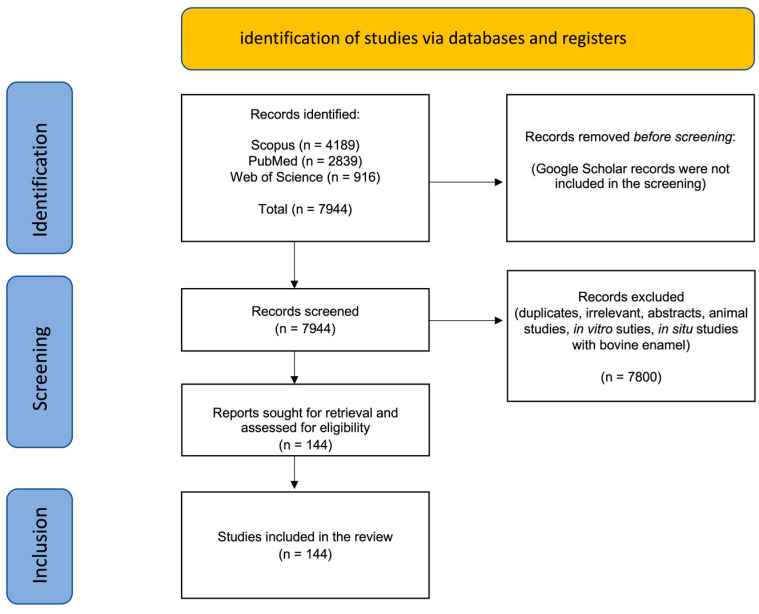
The results of the search are shown in Figure 1.
Figure 1.
Summary of the search results showing numbers of publications from each database identified and the total records included after screening and exclusion of records.
A total of 144 clinical trials and in situ clinical studies resulted after applying the exclusion and inclusion criteria. The majority (>80%) of the clinical studies were conducted on HAP- and CPP-ACP-containing toothpastes. Clinical studies on CSPS were mostly on dentin hypersensitivity (DH), and there were only two clinical trials found testing fluoride-free TCP toothpaste. With so many search term combinations, the Google Scholar search yielded an imprecise and excessively large number of titles which, after rapid screening, contained many citations, duplicates, and irrelevant publications. The focus was, therefore, on the titles retrieved in the PubMed, Scopus, and Web of Science databases. Both Scopus and Web of Science permitted “search within results” where subsets of publications were obtained from the large list of publications found using the starting primary search word (e.g., “hydroxyapatite”).
Table S1 shows the distribution of the clinical studies found using the main databases as a result of the various combinations of search terms. The publications that were retrieved in full and carefully read for each of the calcium-phosphate-based toothpaste active ingredients are summarized in Table 1, Table 2, Table 3 and Table 4. Some studies were cited more than once in the tables because they examined more than one aspect of improving oral health in the same study.
Table 1.
(a) Hydroxyapatite (HAP) clinical trials on reducing caries or white spot lesions (WSL) or preventing erosion, listed chronologically. (b) HAP studies in situ using human enamel to measure remineralization or erosion resistance. (c) HAP clinical trials on reducing dentin hypersensitivity listed chronologically. (d) Hydroxyapatite (HAP) clinical trials on improvement of gingival health listed chronologically. (e) Hydroxyapatite (HAP) clinical trials on improving tooth appearance listed chronologically.
| (a) | ||||||
| Study First Author | Year |
Test
(HAP Used) |
Controls |
Trial Subjects,
Duration |
Outcome | Does HAP Reduce Dental Caries? |
| Kani [28] | 1989 | Apato 5% HAP | Kirara (HAP and F-free) | 181 children, -3 years |
Significant reduction in DMFT in the HAP group | yes |
| Lelli [29] | 2014 | Biorepair (ZnCO3/n-HAP) |
Sensodyne Pronamel | Extracted premolars after 8 weeks treatment | Zinc-carbonated HAP showed better repair of damaged enamel than fluoride toothpaste | yes |
| Makeeva [30] | 2016 | Apadent Total Care | No control | 30 subjects -3 mo. |
The long-term use of HAP toothpaste increases caries resistance of enamel | yes (but no control) |
| Schlagenhauf [31] | 2019 | Karex (10% HAP) | 1400 ppm fluoride toothpaste (amine fluoride + SnF2) |
150 subjects -6 mo. |
Works as well as fluoride paste in reducing caries (ICDAS) progression | yes |
| Bossù [32] | 2019 | Biorepair (ZnCO3/n-HAP) |
1. Ordinary toothpaste 2. 500 ppm fluoride toothpaste 3. 1400 ppm toothpaste |
40 extracted primary teeth after 15 days trial | Zinc-carbonated HAP showed better remineralization properties than fluoride toothpaste | yes |
| Badiee [33] | 2020 | 6.7% HAP toothpaste | Fluoride toothpaste | 50 post-orthodontic subjects | HAP toothpaste outperformed in fluoride toothpaste in caries reduction | yes |
| Grocholewicz [34] | 2020 | ApaCare Repair (10% HAP gel) |
(1) ozone (2) not treatment |
92 subjects -2 years |
HAP gel provided significant reversal of caries | yes |
| Paszinska [35] | 2021 | Kinder Karex (10% HAP) |
Elmex Kinder Zahnpasta (500 ppm F−) |
77 children -1 year |
HAP and fluoride toothpaste were equivalent in slowing caries progression (ICDAS | yes |
| Verma [36] | 2021 | Apagard Premio Toothpaste (10% HAP) |
Amflor toothpaste (amine fluoride) | 30 orthodontic subjects -15 days |
The HAP toothpaste was superior to fluoride toothpaste in restoring the enamel surface post-orthodontic bonding | yes |
| Butera [37] | 2021 | MicroRepair (ZnCO3/n-HAP) | Sensodyne Repair and Protect | 20 subjects with orthodontic buttons -30 days |
More deposition of Phosphate and Calcium on the composite resin in the HAP group | likely |
| Butera [38] | 2022 | Biorepair Total Protective Repair | Same toothpaste + ZnCO3/n-HAP mouthwash | 40 rugby players -90 days |
Erosion index improved in both test and control | likely |
| Paszynska [39] | 2023 | 10% HAP | NaF toothpaste (1450 ppm fluoride) | 171 adults -18 months |
HAP toothpaste was equivalent to fluoride toothpaste in preventing new caries lesions as measured by DMFS and DIAGNOcam | yes |
| (b) | ||||||
| Study First Author | Year |
Test
(HAP Used) |
Controls |
Trial Subjects,
Duration |
Outcome | Does HAP Remineralize Human Enamel? |
| Najibfarb [40] | 2011 | Apagard (5% HAP or 10% HAP) |
Crest fluoride toothpaste | 30 subjects -28 days per phase |
10% hydroxyapatite tooth-paste caused remineralization comparable to a fluoride den-tifrice, inhibiting in situ caries development as effectively as fluoride toothpaste | yes |
| Amaechi [41] | 2019 | Karex (10% HAP) | Elmex (Amine fluoride toothpaste, 500 ppm F−) |
32 subjects wearing appliances with imbedded human enamel blocks -2 mo. (with crossover) |
The HAP toothpaste works as well as the fluoride toothpaste in remineralizing enamel | yes |
| Amaechi [42] | 2021 | Apagard Deep Care (5% nHAP) along with Apagard M-plus (5% nHAP) | Placebo along with Apagard M-Plus (5% n-HAP) |
32 subjects wearing appliances with imbedded human enamel blocks -2 mo. (with crossover) |
5% HAP toothpaste remineralized enamel and the added 5% HAP lotion improved the remineralization | yes |
| Amaechi [43] | 2022 | Bioniq Repair-Zahncreme (20% HAP) | Colgate Komplett 8 Zahnpaste (1450 ppm F−) |
15 subjects wearing appliances with imbedded human enamel blocks -1 mo. (with crossover) |
HAP toothpaste achieved significantly higher remineralization of MIH lesions than the fluoride toothpaste | yes |
| (c) | ||||||
| Study First Author | Year |
Test
(HAP Product Used) |
Controls |
Trial Subjects,
Duration |
Outcome |
Does HAP Toothpaste
Desensitize Teeth? |
| Hüttemann [44] | 1987 | 17% HAP A: with 6 µm particles B: with 2 µm particles |
B: 17% salt, C: 0.125% benzocaine, D: placebo, E: 9% HAP. 8% salt, 0.125% benzocaine, F: 17% HAP, 6% SrCl2, G: 17% HAP, 5% SrCl2, 1% amine fluoride | 140 adults -2 weeks |
HAP reduced DH over controls | yes |
| Barone [45] | 1991 | 15% HAP paste | no treatment control | 40 adults -24 weeks |
reduced DH in the HAP groupbased on before and after measurements | maybe |
| Park [46] | 2005 | HAP toothpaste | no treatment control | 44 adults -8 weeks |
the HAP toothpaste reduced DH | maybe |
| Kim [47] | 2008 | Diome Plus PRTC (10% HAP) toothpaste |
Strontium chloride toothpaste (Sensodyne GSK) | 100 adults -4 weeks |
HAP toothpaste worked as well as strontium toothpaste to lower DH | yes |
| Kang [48] | 2009 | Diome Plus PRTC (10% HAP) toothpaste |
Fluoride toothpaste (2080 Korea) Strontium chloride toothpaste (Sensodyne GSK) |
150 adults -4 weeks |
HAP toothpaste reduced DH | yes |
| Kim [49] | 2009 | Diome Plus PRTC (10% HAP) toothpaste |
Strontium chloride toothpaste (Sensodyne GSK) | 55 adults -8 weeks |
HAP toothpaste worked as well as strontium toothpaste to lower DH | yes |
| Orsini [50] | 2010 | Biorepair Plus (30% Zn-carbonate HAP) |
Sensodyne Pronamel | 75 adults -8 weeks |
Zn-Carbonated HAP toothpaste reduced DH | yes |
| Shetty [51] | 2010 | A: HAP in dry sol powder B: HAP liquid |
C: placebo D: no treatment |
45 adults -8 weeks |
the HAP toothpaste reduced DH more that the controls | yes |
| Browning [52] | 2012 | Renamel nHAP toothpaste | placebo | 42 adults -2 weeks |
HAP toothpaste reduced DH | yes |
| Orsini [53] | 2013 | Biorepair Plus (30% Zn-carbonate HAP) |
Colgate Sensitive (8% arginine, MFP at 1450 ppm fluoride) Sensodyne Rapid Relief (8% strontium acetate, NaF at 1044 ppm fluoride) |
90 adults -3 days |
all three toothpastes reduced DH equally | yes |
| Jena [54] | 2015 | NanoXIM (15% HAP) |
Vantej (5% Novamin) Sensitive Pro-Relief |
45 adults -4 weeks |
HAP toothpaste was more effective than 5% Novamin toothpaste | yes |
| Pinojj [55] | 2014 | HAP toothpaste (SHY NM) | CSPS toothpaste (SHY) CPP-ACP |
80 teeth (adult subjects) -3 months |
the HAP and CSPS toothpastes worked better to reduce DH than the CPP-ACP paste | yes |
| Reddy [56] | 2014 | Acclaim (15% HAP) |
Colgate ProArgin | 30 adults 3 days |
both toothpastes (HAP and arginine) reduced DH | yes |
| Vano [57] | 2014 | Prevdent (15% HAP) |
Colgate Cavity Protection (1500 ppm fluoride in MFP) Placebo |
105 adults -4 weeks |
the HAP toothpaste worked better than the fluoride toothpaste to reduce DH | yes |
| VJ Narmantha [58] | 2014 | Acclaim (1% HAP) |
Sensodent-K (5% KNO3) Propolis |
45 adults -4 weeks |
HAP toothpaste and Propolis toothpaste both reduced DH | yes |
| Amin [59] | 2015 | Acclaim (15% HAP) |
none | 30 adults -6 mo. |
HAP toothpaste reduced DH | maybe |
| Gopinath [60] | 2015 | Acclaim (10% nHAP) |
Shy-NM (5% CSPS) | 36 adults -4 weeks |
both HAP and CSPS toothpastes lowered DH | yes |
| Lee [61] | 2015 | Denti-guard Sensitive (20% Carbonated HAP, 8% silica) |
Sensodyne (10% CaCO3, 10% Strontium chloride) Laser treatment |
82 adults -4 weeks |
HAP toothpaste worked as well as strontium chloride toothpaste and professional laser treatment | yes |
| Vano [62] | 2015 | Prevdent (2% HAP in 6% hydrogen peroxide toothpaste) |
6% hydrogen peroxide toothpaste control | 60 subjects -2 weeks |
the HAP added to peroxide toothpaste reduced DH | yes |
| Makeeva [30] | 2016 | Apadent Total Care (10% HAP) |
No treatment control | 30 adults -3 months |
HAP toothpaste reduced DH | maybe |
| Anand [63] | 2017 | 1% nHAP toothpaste | Pro-Argin sensitivity fluoride toothpaste | 60 adults -4 weeks |
both nHAP and Pro-Argin reduce DH | yes |
| Makeeva [64] | 2018 | Innova paste (6% Nano-HAP) + Liquid Enamel (1% Nano-HAP liquid) |
No treatment control | 40 adults -2 weeks |
The combination of HAP toothpaste and HAP mouthwash reduced DH | maybe |
| Amaechi [65] | 2018 | Apadent Pro dental cream (20% HAP) | 20% silica cream | 52 adults -8 weeks |
HAP-group showed reduced DH compared to silica | yes |
| Vano [66] | 2018 | Cavex Bite & White ExSense (2% nHAP toothpaste) |
Colgate Cavity Protection Gel placebo |
105 adults -4 weeks |
HAP toothpaste reduced DH more than the placebo | yes |
| Al Asmari [67] | 2019 | Biorepair (20% Zn-carbonate hydroxyapatite) |
no treatment control | 72 adults -8 weeks |
reduced DH | maybe |
| Kondyurova [68] | 2019 | SPLAT Sensitive Ultra (0.5% nHAP) | 0.1% nHAP (Splat Professional Sensitive White) | 60 adults -4 weeks |
both concentrations of HAP reduced DH | yes |
| Alancar [69] | 2020 | nHAP toothpaste (± laser) | Placebo + laser Placebo + simulated laser |
32 adults -1 mo. |
HAP toothpaste reduced DH over control | yes |
| Ding [70] | 2020 | Dentiguard Sensitive (20% nanocarbonate-apatite) |
placebo | 45 adults -6 weeks |
HAP toothpaste reduced DH relative to control | yes |
| Alharith [71] | 2021 | Nano XIM toothpaste (15% HAP) |
Placebo Fluorophat (5% NaF) |
63 adults -1 week |
HAP toothpaste reduced DH better than fluoride | yes |
| Amaechi [72] | 2021 | 10% and 15% nHAP toothpaste | 10% HAP + 5% KNO3 Na-MFP (1400 ppm F-) + CPSC |
104 adults -8 weeks |
10% HAP ± KNO3 reduced DH and 15% HAP worked better than 10% HAP | yes |
| Ehlers [73] | 2021 | Kinder Karex (10% HAP) |
Elmex Junior (amine fluoride at 1400 ppm fluoride) | 21 children -8 weeks |
HAP toothpaste worked as well as fluoride toothpaste in lowering DH | yes |
| Polyakova [74] | 2022 | 20% HAP toothpaste | Zn-magnesium HAP n-FAP toothpaste |
30 adults -1 month |
the Zn-Magnesium HAP toothpaste worked better that the 20% HAP and n-FAP toothpaste | yes |
| Vlasova [75] | 2022 | GARDA SILK (HAP toothpaste with Polyol Germanium Complex) | fluoride toothpaste no toothpaste control |
120 adults -2 weeks |
HAP toothpaste with PGC reduced DH better than conventional fluoride toothpaste (no HAP-free PGC supplemented placebo used) | maybe |
| Butera [76] | 2022 | Biorepair (30% Zn-HAP) | -no treatment control | 25 MIH patients -9 mo. |
Zn-HAP showed desensitization of MIH teeth | yes |
| (d) | ||||||
| Study First Author | Year |
Test
(HAP Product Used) |
Controls |
Trial Subjects,
Duration |
Outcome |
Does HAP Toothpaste
Improve Gingival Health? |
| Harks [77] | 2016 | Zn-HAP | previously used toothpaste | 46 adults -4 weeks |
subjective improvement of oral health in both groups (HAP and antibacterial toothpaste) | maybe |
| Doroshina [78] | 2019 | Zn-carbonate HAP (CHA) | CSPS toothpaste Herbal toothpaste |
25 adults | CHA did not perform as well as the CSPS and herbal toothpastes in reducing gingival health measurements (bleeding on probing) | maybe |
| Monterubbianesi [79] | 2020 | Sensitive Ultra Splat | Biorepair Gum Protection Curaprox Enzycal Zero no paste brushing control |
80 adults -14 days |
all pastes improved gingival health | yes |
| Steinert [23] | 2020 | 20% Zn-HAP | amine fluoride/stannous fluoride toothpaste | 46 subjects -3 months |
pocket depth, bleeding on probing improved with both toothpastes | yes |
| Brauner [80] | 2022 | the test toothpaste in combination of the mouthwash improved gingival health | not shown directly | |||
| Andrea [81] | 2022 | Biorepair Peribioma | regular toothpaste (no preference) | 50 adults -2 months |
The HAP toothpaste improved gingival health parameters | yes |
| (e) | ||||||
| Study First Author | Year |
Test
(HAP Product Used) |
Controls |
Trial Subjects,
Duration |
Outcome |
Does HAP Toothpaste
Whiten or Improve the Appearance of Teeth? |
| Niwa [82] | 2001 | 3% HAP toothpaste 15% HAP toothpaste |
placebo toothpaste | 12 adults -1 month |
whitening of teeth was achieved (without polishing) | yes |
| Raoufi [83] | 2010 | 0.1% HAP toothpaste | calcium peroxide toothpaste placebo toothpaste |
150 adults -3 months |
unable to demonstrate tooth whitening (HAP concentration was too low) | no, concentration was too low |
| Woo [84] | 2014 | 0.25% HAP toothpaste | 0.075% Hydrogen peroxide placebo toothpaste |
85 adults -3 months |
hydrogen peroxide whitening teeth more than HAP toothpaste | maybe, even at a very low concentration |
| Bommer [85] | 2018 | self-assembling peptide matrix and HAP | no treatment control | 40 adults -1 month |
whitening based on diffuse reflection in vitro was seen in vivo | yes |
| Steinert [86] | 2020 | HAP gel | 25 adults -1 month |
subjective whitening of teeth was achieved by HAP | yes | |
| Steinert [23] | 2020 | 20% Zn-HAP | 46 subjects -1 month |
patients reported smoother, whiter teeth when using HAP | yes | |
WSL: white spot lesion; HAP: hydroxyapatite; nHAP: nano-hydroxyapatite; F: fluoride; ICDAS: international caries detection and assessment system; DH: dentin hypersensitivity.
Table 2.
(a) Casein phosphopeptide-amorphous calcium phosphate (CPP-ACP) clinical trials on reducing caries or white spot lesions (WSL) or preventing erosion, listed chronologically. (b) Casein phosphopeptide-amorphous calcium phosphate (CPP-ACP) studies in situ using human enamel to measure remineralization or erosion resistance, listed chronologically. (c) Casein phosphopeptide-amorphous calcium phosphate (CPP-ACP) clinical trials on reducing dentin hypersensitivity listed chronologically. (d) Casein phosphopeptide-amorphous calcium phosphate (CPP-ACP) clinical trials on improvement of gingival health. (e) Casein phosphopeptide-amorphous calcium phosphate (CPP-ACP) clinical trials on improving tooth appearance.
| (a) | ||||||
| Study First Author | Year |
Test
(CPP-ACP Used) |
Controls |
Trial Subjects,
Duration |
Outcome |
Does F-Free CPP-ACP Clinically
Reverse WSLs? |
| Andersson [87] | 2007 | Topacal 1st 3 mo. |
0.05% NaF rinse + brushing with F toothpaste | 26 adolescents -12 mo. |
CPP-ACP = 63% complete visual loss of WSL compared to 25% with F | yes |
| Bailey [88] | 2009 | CPP-ACP in addition to regular F toothpaste use | Placebo in addition to regular F toothpaste use | 45 teens -3 mo. |
CPP-ACP = 31% more regression of ICDAS II scores than placebo | yes |
| Rao [89] | 2009 | 2% CPP | (1) 0.76% Na MFP (2) placebo paste |
150 children -24 mo |
Both CPP and MFP significantly but equally reduced caries increment compared to placebo | yes |
| Uysal [90] | 2010 | Tooth Mousse | (1) Fluoridin N5 (2) placebo |
21 orthodontic volunteers donated 60 teeth after 2 mo. trial |
Both test groups successfully inhibited caries better than controls | yes |
| Bröchner [91] | 2011 | Tooth Mousse in addition to regular F toothpaste use | Pearl Powder gel in addition to regular F toothpaste use | 30 subjects, -12 mo. |
Reduction in QLF for WSL for both test and control | yes |
| Akin [92] | 2012 | Tooth Mousse | (1) brush (2) 0.025% F rinse |
80 subjects, -6 mo. |
CPP-ACP = 58% reduction in WSL area (1) 45%, (2) 48% (3) micro-abrasion 97% |
yes |
| Sitthisettapong [93] | 2012 | 10% CPP-ACP in addition to regular F toothpaste use | Regular toothbrushing with a F paste | 296 preschoolers -12 mo. |
ICDAS scores were no difference between test and control | no |
| Wang [94] | 2012 | Tooth Mousse | 1100 ppm F paste | 20 orthodontic patients -6 mo. |
CPP-ACP significantly reduced WSL as measured by enamel decalcification index- the F paste control did not | yes |
| Krithikadatta [95] | 2013 | 10% CPP-ACP | (1) MI Paste plus 0.2% NaF (2) 0.5% NaF mouth rinse |
45 subjects -1 mo. |
Both CPP-ACP groups had fewer WSL (visual) with decrease in DIAGNOdent readings compared to the control | yes |
| Plonka [96] | 2013 | 10% CPP-ACP along with 0.304% F paste | 0.12% Chlorhexidine (CHX) along with 0.304% F paste | 622 children -24 mo. |
No significant caries increment benefit over fluoride for CPP-ACP or CHX | no |
| Aykut- Yetkiner [97] |
2014 | Tooth Mousse | F toothpaste | 60 children -3 mo. |
CPP-ACP provided a slight remineralization effect as measured by DIAGNOdent compared to F paste | yes |
| Yazıcıoğlu [98] | 2014 | Tooth Mousse | (1) no treatment (2) ozone (3) APF gel (4) Clearfil Protect Bond |
125 approximal lesions -18 mo |
All groups arrested approximal lesions compared to the non-treatment group | yes |
| Zhang [99] | 2014 | CPP-ACP | Fluoride varnish (Duraphat) | 112 head cancer patients -12 mo |
CPP-ACP reduced radiation caries more than FV | yes |
| Llena [100] | 2015 | CPP-ACP | (1) CPP-ACFP (2) fluoride varnish (FV) monthly |
786 WSLs in children 3 mo. |
CPP-ACP reduced DIAGNOdent and ICDASII scores, but CPP-ACFP and FV were superior | yes |
| Memarpour [101] | 2015 | Tooth Mousse | (1) OHI, dietary counseling (2) (1) + fluoride varnish (FV) (3) no treatment |
140 children -12 mo. |
CPP-ACP reduced the size of WSL and produced smaller increases of dmft scores compared to counselling and FV | yes |
| Sitthisettapong [102] | 2015 | 10% CPP-ACP in addition to regular F toothpaste use | Regular toothbrushing with a F paste | 103 children -12 mo. |
There was significant reduction in QFL but no significant difference between test and control | no |
| Sim [103] | 2019 | CPP-ACP along with 0.4% SnF2 + 0.32% NaF paste | Placebo | 24 head and neck cancer patients -3 mo. |
The test subjects had a 51% reduction in caries as measured by ICDASII | yes |
| Esenlik [104] | 2016 | Tooth Mousse | No other treatment | 57 patients -12 mo. |
CPP-ACP significantly reduced WSLs | yes |
| Güçlü [105] | 2016 | CPP-ACP | (1) 5% NaF varnish (FV) (2) FV + CPP-ACP (3) no treatment |
21 children -3 mo. |
Control FV< CPP-ACP or CPP-ACP + FV in laser fluorescent and visual assessment of WSLs | yes |
| Munjal [106] | 2016 | Tooth Mousse | No treatment, no orthodontics | 679 WSLs (20 treatment group children) -3 mo. |
CPP-ACP significantly reduced the WSLs compared to controls according to computerized image analysis | yes |
| Singh [107] | 2016 | 10% CPP-ACP in addition to regular F toothpaste use | (1) Fluoride varnish in addition to regular toothpaste use (2)regular fluoride toothpaste use |
45 subjects post orthodontics -6 mo. |
Both FV and CPP-ACP were more effective than F-toothpaste in reducing WSLs (visual, DIAGNOdent readings) | yes |
| Karabekiroğlu [108] | 2017 | 10% CPP-ACP | F toothpaste | 41 subjects -36 mo. |
CPP-ACP was not better than regular F paste in reducing WSLs as measured by DIAGNOdent, Gorelik index, ICDAS II | no |
| Mendes [109] | 2018 | CPP-ACP | (1) CPP-ACP + fluoride (2) F gel (3) placebo paste |
36 children -3 mo. |
All treatments produced decreased DIAGNOdent readings, with the best result obtained with CPP-ACP + F | yes |
| Wang [110] | 2018 | Tooth Mousse in addition to regular F toothpaste use | (1) F paste + 0.01% F mouth rinse (2) F paste only |
21 orthodontic patients -6 mo. |
WSL areas were reduced in all groups and the CPP-ACP had the greatest effect | yes |
| Bobu [111] | 2019 | 10% CPP-ACP | (1) CPP-ACFP (2) 2–10% CPP-ACP + 0.2% NaF paste (3) 0.05% NaF mouth rinse (4) control |
80 subjects -3 mo. |
All treatment groups significantly lowered DIAGNOdent readings and visual appearance of early caries lesions | yes |
| Tingyun [112] | 2019 | MI Paste | (1) F-free placebo (2) OHOLV toothpaste |
15 orthodontic patients donated 60 premolars after 10-day treatment | CPP-ACP and OHOLV produced higher calcium and phosphate levels in demineralized enamel | yes |
| Al-Batayneh [113] | 2020 | Tooth Mousse | (1) 500 ppm F toothpaste (2) 1 + Tooth Mousse |
114 children -6 mo. |
CPP-ACP = fluoride paste in reducing QFL, WSL area -CPP-ACP not a booster for F paste) |
yes |
| Bangi [114] | 2020 | Tooth Mousse | (1) Colgate Strong toothpaste (2) Colgate Phos-Flur mouthwash (3) SHY-NM (CSPS glass paste) |
80 subjects, -6 mo. |
All significantly reduced WSL decalcification index, but CPP-ACP outperformed the others | yes |
| Perić [115] | 2020 | CPP-ACP | (1) CPP-ACFP (2) 0.05% NaF mouth rinse |
30 Sjögren’s patients -6 mo |
Reduction in WSL in all groups, but no significant difference in DMFS | yes |
| Ashour [116] | 2021 | Tooth Mousse in addition to regular F toothpaste use | (1) Tooth Mousse Plus + F toothpaste (2) F toothpaste only |
51 subjects -6 mo. |
All treatment groups provided slight remineralization as judged by Vistacam scores | yes |
| Juárez-López [117] | 2021 | CPP-ACP in addition to regular F toothpaste use | (1) Chewing gum with CPP-ACP (2) F toothpaste only |
90 children -3 mo. |
CPP-ACP in chewing gum was more effective than CPP-ACP cream in decreasing fluorescence | yes |
| El-Sherif [118] | 2022 | CPP-ACP | Pearl powder | 57 subjects -3 mo. |
CPP-APP and pearl powder both reduced WSL areas and improved their color | yes |
| Hamdi [119] | 2022 | CPP-ACP | (1) SDF-KI (2) tricalcium silicate (TCS) |
45 patients -24 mo. |
Both CPP-ACP and TSC reduced DIAGNOdent readings. SDF-KI significantly remineralized early carious lesions | yes |
| Olgen [120] | 2022 | CPP-ACP | (1)CPP-ACFP (2) fluoride varnish (FV) |
49 children with MIH -24 mo. |
All treatments significantly reduced DIAGNOdent and ICDAS scores with no significant difference between them | yes |
| Salah [121] | 2022 | CPP-ACP | (1) BiominF (2) Novamin |
60 orthodontic subjects -6 mo. |
ICDASII scores, WSL areas and DIAGNOdent scores were reduced by all treatments-BiominF was best | yes |
| Simon [122] | 2022 | Tooth Mousse | ICON resin infiltration | 60 children -12 mo. |
Both treatments reduced WSL areas using ICDASII scores, digitized photos | yes |
| (b) | ||||||
| Study First Author | Year |
Test
(CPP-ACP Product Used) |
Controls | Study Design | Outcome |
Does F-Free CPP-ACP
Remin- eralize Human Enamel? |
| Srinivasan [123] | 2010 | CPP-ACP | (1) CPP-ACFP (2) saliva placebo |
5 volunteers wearing human enamel slabs imbedded in appliances | CPP-ACFP remineralized the enamel slabs better than CPP-ACP and both were substantially better than saliva | yes |
| Shen [124] | 2011 | Tooth Mousse (TM) | (1) 1000 ppm F paste (2) Clinpro with 950 ppm F (3) 5000 ppm F paste (4) Tooth Mousse + 900 ppm F (TMP) (4) placebo |
Volunteers wearing human enamel slabs in appliances | TMP was better than TM and both were better at remineralization than ClinproF or 5000 ppm F paste as measured by transverse microradiography | yes |
| Perić [125] | 2015 | CPP-ACP | (1) CPP-ACFP (2) 0.05% NaF mouth rinse |
30 Sjögren’s patients -enamel slabs on appliances 1 mo. |
Both CPP-ACP agents reduced enamel defects better than NaF mouthrinse | yes |
| Garry [126] | 2017 | Tooth Mousse (along with F toothpaste) | F toothpaste control | 12 patients wearing fixed orthodontic appliances | CPP-ACP significantly improved remineralization as measured by transverse microradiography | yes |
| Zawaideh [127] | 2017 | Tooth Mousse | (1) Pronamel (2) no treatment |
20 subjects wearing appliances with human enamel slabs from permanent and primary teeth | CPP-ACP and fluoride protected against dental erosion as measured by surface microhardness | yes |
| Yu [128] | 2018 | Tooth Mousse | Water control | 12 volunteers wearing human enamel slabs in appliances | CPP-ACP reduced erosion as measured by microhardness | yes |
| de Oliveira [129] | 2020 | Mi Paste | (1) MI Paste Plus (2) 1000 ppm fluoride toothpaste (3) placebo toothpaste |
10 participants -four 10-day experiments |
Remineralizing agents (MP, MPP, and DF) were able to inhibit demineralization of human enamel subjected to high cariogenic challenge in situ. | yes |
| de Oliveira [130] | 2022 | MI Paste | (1) MI Paste Plus (2) 1000 ppm fluoride toothpaste (3) placebo toothpaste |
10 participants -four 10-day experiments |
CPP-ACP and fluoride both prevent demineralization as measured by microhardness | yes |
| Kumar [131] | 2022 | CPP-ACP | Fluoride varnish (FV) | 30 subjects wearing ortho appliances with human MIH enamel slabs -6 month |
CPP-ACP = FV in remineralizing MIH enamel | yes |
| (c) | ||||||
| Study First Author | Year |
Test
(CPP-ACP Product Used) |
Controls | Study Design | Outcome |
Does F-Free CPP-ACP
reduce tooth sensitivity? |
| Borgess [132] | 2012 | CPP-ACP | No sensitivity treatment | 3 patients -some teeth were treated with 20% carbamide peroxide with CPP-ACP |
CPP-ACP reduce sensitivity compared to no treatment (pilot study) | yes |
| Özgül [133] | 2013 | MI Paste | (1) CPP-ACFP (2) CPP-ACFP + ozone (3) fluoride varnish (Bifluorid) (4) FV + ozone (5) CPP-ACP + ozone |
42 MIH patients -3 mo. |
Ozone prolonged the desensitization effect of CPP-ACP and FV, but not CPP-ACFP -all 3 effectively reduced tooth sensitivity |
yes |
| Maghaireh [134] | 2014 | 10% CPP-ACP | (1) 2% NaF gel (2) placebo gel |
51 patients after bleaching -14 days |
CPP-ACP can lower sensitivity post bleaching as well as F | yes |
| Mahesuti [135] | 2014 | MI Paste | (1) UltraEZ (KNO3) (2) UltraEZ placebo (3) MI Paste placebo |
102 subjects -2 mo. |
MI Paste has sustained pain relief compared to KNO3 | yes |
| Zhang [136] | 2014 | CPP-ACP | Fluoride varnish (Duraphat) | 112 head and neck cancer patients -12 mo |
CPP-ACP reduced post radiation tooth sensitivity more than FV | yes |
| Konekeri [137] | 2015 | CPP-ACP | (2) KNO3 treatment | 48 patients -6 weeks |
CPP-ACP was better at reducing tooth sensitivity than KNO3 | yes |
| Nanjundasetty [138] | 2016 | Tooth Mousse | (1) Sensodyne KF (2) placebo |
69 fluorosis patients -10 min. after each bleaching session (2) -7 days |
MI Paste and Sensodyne equally reduced tooth sensitivity compared to the placebo | yes |
| Tarique [139] | 2017 | CPP-ACP | (1) 5% NaF varnish (2) 5% KNO3 |
36 patients after bleaching -10 day for 3 mo. |
CPP-ACP effectively reduced tooth sensitivity more than the other two test groups | yes |
| Pasini [140] | 2018 | CPP-ACP | F paste | 40 MIH patients -3 mo. |
CPP-ACP reduced tooth sensitivity compared to the F paste control | yes |
| Yassin [141] | 2019 | CPP-ACP | Placebo paste | 24 patients -custom tray application 30 min/day, 7 days after bleaching |
CPP-ACP effectively reduced tooth sensitivity compared to the placebo paste | yes |
| Adil [142] | 2021 | CPP-ACP | (1) KO3 + Na MFP (2) placebo gel |
2011 patients -12 hr. for 3 days after bleaching |
CPP-ACP and F effectively reduced tooth sensitivity | yes |
| Gümüştaş [143] | 2022 | CPP-ACP | (1) HAP (2) NaF gel |
64 subjects -4 min application before bleaching |
HAP and F treatments reduced sensitivity, CPP-ACP did not | no |
| (d) | ||||||
| Study First Author | Year |
Test
(CPP-ACP Product Used) |
Controls |
Trial Subjects,
Duration |
Outcome |
Does CPP-ACP Toothpaste
Improve Gingival Health? |
| Perić [115] | 2020 | CPP-ACP toothpaste | CPP-ACPP (with 0.5% NaF) toothpaste 0.5% NaF toothpaste |
30 Sjögren’s patients -4 weeks |
no significant improvement in gingival health but improvement in dry mouth symptoms | not shown |
| (e) | ||||||
| No studies were found. | ||||||
CPP-ACP: 10% casein phosphoprotein-amorphous calcium phosphate (MI Paste, Tooth Mousse); CPP-ACFP: 10% casein phosphoprotein-amorphous calcium phosphate with added fluoride to 900 ppm (MI Paste Plus, Tooth Mousse Plus); F: fluoride; FV: fluoride varnish; WSL: white spot lesion; QFL: quantitative fluorescent light; ICDAS II: international caries detection and assessment system (modified from ICDAS I).
Table 3.
(a) Calcium sodium phosphosilicate (CSPS) clinical trials on reducing caries or white spot lesions (WSL) or preventing erosion, listed chronologically. (b) Calcium sodium phosphosilicate (CSPS) in situ clinical trials on reducing caries on preventing erosion. (c) Calcium sodium phosphosilicate (CSPS) clinical trials on reducing dentin hypersensitivity listed chronologically. (d) Calcium sodium phosphosilicate (CSPS) clinical trials on improvement of gingival health.
| (a) | ||||||
| Study First Author | Year |
Test
(CSPS Used) |
Controls |
Trial Subjects,
Duration |
Outcome | Does CSPS Toothpaste Reduce Dental Caries? |
| Salah [121] | 2022 | Biomin slurry and toothpaste | BiominF slurry and toothpaste CPP-ACP toothpaste |
60 orthodontic patients -4 weeks |
All three reduced WSL, with BiominF performing the best | yes |
| Tiwari [144] | 2023 | NovaMin toothpaste | probiotic toothpaste fluoride toothpaste |
93 orthodontic patients -6 months |
All three toothpastes reduced WSLs (the probiotic toothpaste group had the least WSLs) | yes |
| (b) | ||||||
| No studies were found. | ||||||
| (c) | ||||||
| Study First Author | Year |
Test
(CSPS Used) |
Controls |
Trial Subjects,
Duration |
Outcome | Does CSPS Toothpaste Desensitise Teeth |
| Du [145] | 2008 | NovaMin Toothpaste (2.5% and 7.5% CSPS) | placebo toothpaste Strontium chloride toothpaste |
71 adults -6 weeks |
NovaMin reduced SDH better than placebo and strontium chloride toothpaste | yes |
| Litkowski [146] | 2010 | NovaMin Toothpaste (2.5% and 7.5% CSPS) | placebo toothpaste | 66 adults -8 weeks |
NovaMin reduced SDH better than placebo | yes |
| Narongdej [147] | 2010 | NovaMin powder and toothpaste | placebo powder + NovaMin toothpaste placebo powder + fluoride/KNO3 toothpaste |
60 adults -4 weeks |
NovaMin powder and toothpaste reduced DH better than the Potassium nitrate/fluoride toothpaste | yes |
| Pradeep [148] | 2010 | NovaMin toothpaste SHY-NM (5% CSPS) | placebo potassium nitrate toothpaste |
110 adults -6 weeks |
NovaMin reduced DH better than the placebo and potassium nitrate toothpastes | yes |
| Salian [149] | 2010 | NovaMin (5% CSPS) | 5% potassium nitrate toothpaste placebo toothpaste |
30 adults -4 weeks |
NovaMin reduced DH better than the placebo and potassium nitrate toothpastes | yes |
| Sharma [150] | 2010 | NovaMin (7.5% CSPS) | 5% potassium nitrate toothpaste 0.4% Stannous fluoride toothpaste |
120 subjects -12 weeks |
All three reduced DH but NovaMin worked better than the others at early time points | yes |
| West [151] | 2011 | NovaMin (5% CSPS) | 8% arginine toothpaste water control placebo toothpaste |
volunteers wore appliances with dentin slices -4 days |
NovaMin showed better dentin occlusion and retention than the arginine toothpaste | yes |
| Pradeep [152] | 2012 | Novamin SHY (5% CSPS) | 5% potassium nitrate toothpaste 3.88% amine fluoride toothpaste placebo toothpaste |
149 adults -6 weeks |
The Novamin toothpaste showed better results than the others in lowering DH | yes |
| Rajesh [153] | 2012 | Novamin SHY (5% CSPS) | Pepsodent toothpaste | 30 adults -8 weeks |
NovaMin reduced DH better than the placebo toothpaste | yes |
| Surve [154] | 2012 | CSPS toothpaste | potassium nitrate toothpaste | 20 adults -8 weeks |
both reduced DH | yes |
| Acharya [155] | 2013 | CSPS toothpaste | potassium nitrate toothpaste | 20 adults -8 weeks |
both reduced DH but the CSPS toothpaste worked better early in the in the trial | yes |
| Jena [54] | 2015 | Vantej (NovaMin 5% CSPS) | Colgate Sensitive Pro-Relief -8% arginine with fluoride) nanoXIM (15% HAP) |
45 adults -4 weeks |
all three reduced DH, but nHAP toothpaste performed the best | yes |
| Pintado-Palomino [156] | 2015 | Bioglass 45S5 | 7.5% Biosilicate toothpaste Sensodyne toothpaste Odontis RX Sensi Block toothpaste Desesibilize Nano P (HAP toothpaste |
140 adults -2 weeks |
Toothpaste containing Bioactive glass reduced tooth sensitivity caused by vital bleaching | yes |
| Samuel [157] | 2015 | NovaMin toothpaste | ProArgin toothpaste Gluma Desensitizer |
147 adults -1 month |
ProArgin toothpaste and Gluma sealer reduced DH from a single application compared to NovaMin | yes |
| Majji [158] | 2016 | NovaMin (5% CSPS) | 5% potassium nitrate toothpaste 10% strontium chloride herbal toothpaste |
160 adults -2 months |
the CSPS toothpaste showed better reduction in DH than the others | yes |
| Sufi [159] | 2016 | 5% CSPS | placebo CSPS fluoride toothpaste |
134 adults -8 weeks |
small and inconsistent outcomes | no |
| Sufi [160] | 2016 | 5% CSPS | placebo CSPS fluoride toothpaste |
134 adults -8 weeks |
CSPS paste reduced DH similar to placebo | no |
| Athurulu [161] | 2017 | 5% CSPS | 5% potassium nitrate toothpaste 3.85% Amine fluoride toothpaste Placebo toothpaste |
68 adults -12 weeks |
CSPS toothpaste was found to be more effective in reducing DH as the others | yes |
| Hall [162] | 2017 | 5% CSPS | 8% arginine/calcium carbonate toothpaste regular fluoride toothpaste |
133 adults -11 weeks |
CSPS and arginine toothpastes performed equally in reducing DH | yes |
| Fu [163] | 2019 | 2.5% CSPS toothpaste | 8% arginine toothpaste placebo toothpaste |
147 adults -8 weeks |
the CSPS and qarginine toothpastes both equally reduced DH more than the control | yes |
| Alsherbiney [164] | 2020 | CSPS toothpaste | Zn-carbonate nHAP toothpaste | 42 adults -appliances worn with dentin slices |
both toothpastes occluded dentin tubules but the HAP toothpaste provided immediate occlusion of dentin tubules | |
| Bhowmik [165] | 2021 | NovaMin toothpaste SHY-NM (7.5% CSPS) | Elgydium (fluorinol) toothpaste | 30 adults -4 weeks |
CSPS toothpaste reduced DH | yes |
| Ongphichetmetha [166] | 2022 | 5% CSPS | 8% arginine | 45 adults -2 weeks |
SCPS and arginine toothpaste reduce DH | yes |
| (d) | ||||||
| Study First Author | Year |
Test
(CSPS Used) |
Controls |
Trial Subjects,
Duration |
Outcome | Does CSPS Toothpaste Improve Gingival Health? |
| Monterubbianesi [79] | 2020 | CSPS toothpaste | HAP toothpaste herbal toothpaste |
25 adults -2 weeks |
CSPS toothpaste supported gingival health as well as the herbal toothpaste and better than the HAP toothpaste | yes |
Table 4.
(a) Tricalcium phosphate (TCP) clinical trials on reducing caries or white spot lesions (WSL) or preventing erosion. (b) Tricalcium phosphate (TCP) in situ clinical trials on reducing caries on preventing erosion. (c) Tricalcium phosphate (TCP) clinical trials on reducing dentin hypersensitivity. (d) Tricalcium phosphate (TCP) clinical trials on improvement of gingival health. (e) TCP clinical trials on improving tooth appearance.
| (a) | ||||||
| Study First Author | Year |
Test
(TCP Used) |
Controls |
Trial Subjects,
Duration |
Outcome | Does TCP Toothpaste Reduce Dental Caries? |
| Detsomboonrat [167] | 2016 | Pureen | 1000 ppm fluoride toothpaste 500 ppm fluoride toothpaste |
131 mother-child dyads -1 year |
caries were reduced by the TCP toothpaste as well as the fluoride toothpastes | yes |
| (b) | ||||||
| No studies were found. | ||||||
| (c) | ||||||
| Study First Author | Year |
Test
(CSPS Product Used) |
Controls |
Trial Subjects,
Duration |
Outcome |
Does TCP Toothpaste
Desensitize Teeth? |
| Jang [168] | 2023 | Vussen S (190% TCP) | Sensodyne Pleasia (fluoride free) |
53 adults -4 weeks |
TCP toothpaste effectively reduces DH better than placebo | yes |
| (d) | ||||||
| No studies were found. | ||||||
| (e) | ||||||
| No studies were found. | ||||||
3.1. Hydroxyapatite (HAP)
The authors of this review have previously published systematic reviews of the clinical evidence that HAP reduces dental caries [26], reduces dentin hypsersenstivity [27], and improves tooth color [5]. That literature has been updated in this review to include the most recent publications. A total of 62 clinical trials were found where HAP toothpaste was shown to reduce caries, remineralize enamel and protect against erosion, reduce dentin hypesensitivity, improve tooth color, and support gingival health (Table 1).
3.2. Casein Phosphopeptide-Amorphous Calcium Phosphate (CPP-ACP)
A total of 57 clinical trials were found on CPP-ACP toothpaste showing that this form of calcium-phosphate-based toothpaste reverses white spot lesions, protects against dental erosion and reduces dentin hypersensitivity (Table 2). Only one study was found where CPP-ACP toothpaste was tested to improve gingival health. Several studies were found to show that CPP-ACP reduced dentin hypersensitivity in studies measuring the effectiveness of professional peroxide bleaching products and that the CPP-ACP did not interfere with the whitening process, but none were found where the active ingredient CPP-ACP was tested on its own in a toothpaste for whitening teeth.
3.3. Calcium Sodium Phosphosilicate (CSPS, Novamin, Biomin, Bioglass)
There have been several studies on fluoride toothpastes fortified with Novamin (CSPS), but those were not summarized in this review since the focus was on fluoride-free toothpastes. Recently, two studies examined CSPS as an active ingredient in fluoride-free toothpastes for controlling caries or white spot lesions [122,145]. There were 23 clinical studies found showing that CSPS was also capable of reducing dentin hypersensitivity. One study was found where CSPS as an isolated active ingredient was able to control gingival health. No studies were found where CSPS toothpastes were tested to improve the color of teeth. These studies are summarized in Table 3.
3.4. Beta-Tricalcium Phosphate (β-TCP)
The clinical literature on tricalcium phosphate toothpaste in improving oral health was very limited. While there were a number of in vitro studies and studies conducted on fluoride toothpaste with added TCP (called ‘functionalized’ TCP), only one clinical trial was found where a fluoride-free TCP toothpaste was tested in a clinical trial for reducing caries, and one clinical trial examined how fluorid-free TCP in toothpaste affected dentin hypersensitivity (Table 4).
4. Discussion
This systematic review was conducted to compare the clinical evidence that has been published on the calcium-phosphate-containing toothpastes designed to improve oral health. We were interested in comparing the calcium-phosphate-based active ingredients without fluoride. Many fluoride toothpaste formulations contain calcium phosphate additives in an attempt to improve the remineralization and protection of tooth enamel, but recent studies have shown that some ingredients, such as hydroxyapatite, perform as well if not better than fluoridated toothpaste [24,26,27]. Dental fluorosis has been an increasing concern, particularly in those countries that continue to fluoridate their drinking water supplies [169]. In addition, there are concerns that prenatal and even postnatal exposure to fluoride is linked to interference with brain function during early development and growth [170]. For these reasons, it is worthwhile to seek alternatives to fluoridated toothpaste.
The fluoride-free, calcium-phosphate-containing toothpaste formulations tested in the studies summarized in this review show great promise in that they have been shown in clinical trials to prevent dental decay, reverse white spot lesions, remineralize tooth enamel, protecting it from erosion, desensitize hypersensitive root surfaces and even improve gingival health, all while whitening and brightening the dentition.
There were 62 clinical studies found where HAP was the active ingredient and almost an equal number of clinical studies conducted on CPP-ACP. The vast majority of them used fluoride-toothpaste as positive controls. No study was conducted to compare HAP vs. CPP-ACP in a head-to-head clinical trial. Toothpastes containing CPP-ACP, which contains casein peptides, cannot be used in patients who are allergic to milk proteins. Neither can that toothpaste be given a ‘vegan’ designation. Calcium phosphate ingredients, if accidentally swallowed, are considered safe since they dissociate in the stomach into their constituent inorganic components (calcium and phosphate ions), which are not only harmless but actually contribute to needed dietary sources [171].
One other fluoride-free calcium-phosphate active ingredient that should have been considered but not included in the search was calcium glycerophosphate (CaGP), an active ingredient mentioned in the review by Enax et al. [172] on the remineralization strategies of molar incisor hypocalcification. While this ingredient is used mainly to fortify fluoride toothpaste, it has only been tested in three clinical trials as an active ingredient without fluoride [173,174,175]. In those recent trials, it has been shown to be effective on its own and should really be counted as the fifth active ingredient for fluoride-free calcium-phosphate-containing toothpaste with the potential to reverse white spot lesions.
5. Future Directions
While the clinical evidence to date on the effectiveness of biomimetic fluoride-free calcium-phosphate ingredients in oral care products is already quite extensive and based on dozens of clinical trials, the development of new strategies and products for the prevention and control of oral diseases and maintaining good oral health should continue. Randomized clinical trials (RCTs) where calcium-phosphate-based toothpaste formulations are tested in head-to-head experiments have not been conducted. These would be useful in order to determine which active ingredients most meet the needs of the average consumer in improving overall oral health. Additional clinical trials are required using subjects in susceptible populations and in all age groups.
6. Conclusions
Because of the concern by families of the lasting negative effects of fluoride ingestion with the use of fluoridated toothpaste, there is increased interest by researchers in preventive dentistry to clinically test fluoride-free toothpastes for the potential to be effective in improving oral health. While there is extensive clinical evidence that the biomimetic approach of using hydroxyapatite, casein phopshopeptide-amorphous calcium phosphate, or calcium sodium phosphosilicate has proven successful, additional clinical studies would help identify the most effective active ingredients so that dentists can tailor targeted preventive regimens best suited for patients’ needs. Based on the current clinical evidence to date, fluoride-free hydroxyapatite seems to be an all-round, versatile, and effective agent for improving oral health, in comparison to the other calcium phosphate active ingredients in toothpastes tested clinically.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biomimetics8040331/s1, Table S1: Search results using designated search terms.
Author Contributions
Conceptualization, H.L., F.M. and J.E.; literature search, H.L., F.M. and J.E.; qualitative synthesis and writing, H.L.; review and editing, F.M. and J.E.; supervision, final editing, corresponding author, H.L. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data used in this review was published data in the studies referenced. Online information was referenced and accessed as shown in the reference list. No new data were created.
Conflicts of Interest
J.E. and F.M. are senior scientists and employees of Dr. Kurt Wolff GmbH & Co. KG in Germany.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Meyer F., Enax J. Early childhood caries: Epidemiology, aetiology, and prevention. Int. J. Dent. 2018;2018:1415873. doi: 10.1155/2018/1415873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kwon T., Lamster I.B., Levin L. Current Concepts in the Management of Periodontitis. Int. Dent. J. 2021;71:462–476. doi: 10.1111/idj.12630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fraihat N., Madae’en S., Bencze Z., Herczeg A., Varga O. Clinical Effectiveness and Cost-Effectiveness of Oral-Health Promotion in Dental Caries Prevention among Children: Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health. 2019;16:2668. doi: 10.3390/ijerph16152668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zanatta R.F., Caneppele T.M.F., Scaramucci T., El Dib R., Maia L.C., Ferreira D.M.T.P., Borges A.B. Protective effect of fluorides on erosion and erosion/abrasion in enamel: A systematic review and meta-analysis of randomized in situ trials. Arch. Oral Biol. 2020;120:104945. doi: 10.1016/j.archoralbio.2020.104945. [DOI] [PubMed] [Google Scholar]
- 5.Limeback H., Meyer F., Enax J. Tooth Whitening with Hydroxyapatite: A Systematic Review. Dent. J. 2023;11:50. doi: 10.3390/dj11020050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walsh T., Worthington H.V., Glenny A.M., Marinho V.C., Jeroncic A. Fluoride toothpastes of different concentrations for preventing dental caries. Cochrane Database Syst. Rev. 2019;3:CD007868. doi: 10.1002/14651858.CD007868.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hong I., Lee H.G., Keum H.L., Kim M.J., Jung U.W., Kim K., Kim S.Y., Park T., Kim H.J., Kim J.J., et al. Clinical and Microbiological Efficacy of Pyrophosphate Containing Toothpaste: A Double-Blinded Placebo-Controlled Randomized Clinical Trial. Microorganisms. 2020;8:1806. doi: 10.3390/microorganisms8111806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taschieri S., Tumedei M., Francetti L., Corbella S., Del Fabbro M. Efficacy of 67% sodium bicarbonate toothpaste for plaque control and gingivitis control. A systematic review and meta-analysis. J. Evid. Based Dent. Pract. 2022;22:101709. doi: 10.1016/j.jebdp.2022.101709. [DOI] [PubMed] [Google Scholar]
- 9.Lorenz K., Hoffmann T., Heumann C., Noack B. Effect of toothpaste containing amine fluoride and stannous chloride on the reduction of dental plaque and gingival inflammation. A randomized controlled 12-week home-use study. Int. J. Dent. Hyg. 2019;17:237–243. doi: 10.1111/idh.12392. [DOI] [PubMed] [Google Scholar]
- 10.Prasad K.V., Therathil S.G., Agnihotri A., Sreenivasan P.K., Mateo L.R., Cummins D. The Effects of Two New Dual Zinc plus Arginine Dentifrices in Reducing Oral Bacteria in Multiple Locations in the Mouth: 12-Hour Whole Mouth Antibacterial Protection for Whole Mouth Health. J. Clin. Dent. 2018;29:A25–A32. [PubMed] [Google Scholar]
- 11.Yates R., Jenkins S., Newcombe R., Wade W., Moran J., Addy M. A 6-month home usage trial of a 1% chlorhexidine toothpaste (1). Effects on plaque, gingivitis, calculus and toothstaining. J. Clin. Periodontol. 1993;20:130–138. doi: 10.1111/j.1600-051X.1993.tb00327.x. [DOI] [PubMed] [Google Scholar]
- 12.Rajendiran M., Trivedi H.M., Chen D., Gajendrareddy P., Chen L. Recent Development of Active Ingredients in Mouthwashes and Toothpastes for Periodontal Diseases. Molecules. 2021;26:2001. doi: 10.3390/molecules26072001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Santos A.P., Oliveira B.H., Nadanovsky P. Effects of low and standard fluoride toothpastes on caries and fluorosis: Systematic review and meta-analysis. Caries Res. 2013;47:382–390. doi: 10.1159/000348492. [DOI] [PubMed] [Google Scholar]
- 14.van Loveren C., Ketley C.E., Cochran J., Duckworth R.M., O’Mullane D.M. Fluoride ingestion from toothpaste: Fluoride recovered from the toothbrush, the expectorate and the after-brush rinses. Commun. Dent. Oral. Epid. 2004;32((Suppl. S1)):54–61. doi: 10.1111/j.1600-0528.2004.00140.x. [DOI] [PubMed] [Google Scholar]
- 15.American Dental Association Council on Scientific Affairs Fluoride Toothpaste Use for Young Children. [(accessed on 4 May 2023)];J. Am. Dent. Assoc. 2014 145:190–191. doi: 10.14219/jada.2013.47. Available online: https://jada.ada.org/article/S0002-8177(14)60226-9/pdf. [DOI] [PubMed] [Google Scholar]
- 16.Canadian Dental Association CDA Position on Fluoride. Feb, 2021. [(accessed on 4 May 2023)]. Available online: https://www.cda-adc.ca/_files/position_statements/fluoride.pdf.
- 17.Toumba K.J., Twetman S., Splieth C., van Loveren C., Lygidakis N.A. Guidelines on the use of fluoride for caries prevention in children: And updated EAPD policy document. Eur. Arch. Paed. Dent. 2019;20:507–516. doi: 10.1007/s40368-019-00464-2. [DOI] [PubMed] [Google Scholar]
- 18.Thornton-Evans G., Junger M.L., Lin M., Wei L., Espinosa L., Beltran-Aguilar E. Use of toothpaste and toothbrushing among children and adolescents—United States, 2013–2016. MMWR Morb. Mortal. Wkly. Rep. 2019;68:87–90. doi: 10.15585/mmwr.mm6804a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Creeth J., Bosma M.L., Govier K. How much is a ‘pea-sized amount’? A study of dentifrice dosing by parents in three countries. Int. Dent. J. 2013;63((Suppl. S2)):25–30. doi: 10.1111/idj.12076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sarembe S., Ufer C., Kiesow A., Limeback H., Meyer F., Fuhrmann I., Enax J. Influence of the Amount of Toothpaste on Cleaning Efficacy: An In Vitro Study. Eur. J. Dent. 2022;17:497–503. doi: 10.1055/s-0042-1747953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Till C., Green R. Controversy: The evolving science of fluoride: When new evidence doesn’t conform with existing beliefs. Pediatr. Res. 2020;90:1093–1095. doi: 10.1038/s41390-020-0973-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National Toxicology Program NTP Board of Scientific Counselors Working Group Report on the Draft State of the Science Monograph and the Draft Meta-Analysis Manuscript on Fluoride. [(accessed on 4 May 2023)];2023 Available online: https://ntp.niehs.nih.gov/sites/default/files/2023-04/wgrptBSC20230400.pdf.
- 23.Steinert S., Zwanzig K., Doenges H., Kuchenbecker J., Meyer F., Enax J. Daily application of a toothpaste with biomimetic hydroxyapatite and its subjective impact on dentin hypersensitivity, tooth smoothness, tooth whitening, gum bleeding, and feeling of freshness. Biomimetics. 2020;5:17. doi: 10.3390/biomimetics5020017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meyer F., Amaechi B.T., Fabritius H.-O., Enax J. Overview of Calcium Phosphates in Biomimetic Oral Care. Open Dent. J. 2018;12:406–423. doi: 10.2174/1874210601812010406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singal K., Sharda S., Gupta A., Malik V.S., Singh M., Chauhan A., Agarwal A., Pradhan P., Singh M. Effectiveness-of Calcium Phosphate derivative agents on the prevention and remineralization of caries among children- A systematic review & meta-analysis of randomized controlled trials. J. Evid. Based Dent. Pract. 2022;22:101746. doi: 10.1016/j.jebdp.2022.101746. [DOI] [PubMed] [Google Scholar]
- 26.Limeback H., Enax J., Meyer F. Biomimetic hydroxyapatite and caries prevention: A systematic review and meta-analysis. Can. J. Dent. Hyg. 2021;55:148–159. [PMC free article] [PubMed] [Google Scholar]
- 27.Limeback H., Enax J., Meyer F. Clinical Evidence of Biomimetic Hydroxyapatite in Oral Care Products for Reducing Dentin Hypersensitivity: An Updated Systematic Review and Meta-Analysis. Biomimetics. 2023;8:23. doi: 10.3390/biomimetics8010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kani K., Kani M., Isozaki A., Shintani H., Ohashi T., Tokumoto T. Effect of apatite-containing dentifrices on dental caries in school children. J. Dent. Health. 1989;19:104–109. doi: 10.5834/jdh.39.104. [DOI] [Google Scholar]
- 29.Lelli M., Putignano A., Marchetti M., Foltran I., Mangani F., Procaccini M., Roveri N., Orsini G. Remineralization and repair of enamel surface by biomimetic Zn-carbonate hydroxyapatite containing toothpaste: A comparative in vivo study. Front. Physiol. 2014;5:333. doi: 10.3389/fphys.2014.00333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Makeeva I.M., Polyakova M.A., Avdeenko O.E., Paramonov Y.O., Kondrati’ev S.A., Pilyagina A.A. Evaluation of the effectiveness of long-term use of Apadent Total Care toothpaste containing medical nano-hydroxyapatite. Stomatologiia. 2016;95:34–36. doi: 10.17116/stomat201695434-36. [DOI] [PubMed] [Google Scholar]
- 31.Schlagenhauf U., Kunzelmann K.H., Hannig C., May T.W., Hösl H., Gratza M., Viergutz G., Nazet M., Schamberger S., Proff P. Impact of a non-fluoridated microcrystalline hydroxyapatite dentifrice on enamel caries progression in highly caries-susceptible orthodontic patients: A randomized, controlled 6-month trial. J. Investig. Clin. Dent. 2019;10:e12399. doi: 10.1111/jicd.12399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bossù M., Saccucci M., Salucci A., Giorgio G.D., Bruni E., Uccelletti D., Sarto M.S., Familiari G., Relucenti M., Polimeni A. Enamel remineralization and repair results of biomimetic hydroxyapatite toothpaste on deciduous teeth: An effective option to fluoride toothpaste. J. Nanobiotech. 2019;17:17. doi: 10.1186/s12951-019-0454-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Badiee M., Jafari N., Fatemi S., Ameli N., Kasraei S., Ebadifar A. Comparison of the effects of toothpastes containing nanohydroxyapatite and fluoride on white spot lesions in orthodontic patients: A randomized clinical trial. Dent. Res. J. 2020;17:354–359. [PMC free article] [PubMed] [Google Scholar]
- 34.Grocholewicz K., Matkowska-Cichocka G., Makowiecki P., Droździk A., Ey-Chmielewska H., Dziewulska A., Tomaski M., Trybek G., Janiszewska-Olszowska J. Effect of nano-hydroxyapatite and ozone on approximal initial caries: A randomized clinical trial. Sci. Rep. 2020;10:11192. doi: 10.1038/s41598-020-67885-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paszynska E., Pawinska M., Gawriolek M., Kaminska I., Otulakowska-Skrzynska J., Marczuk-Kolada G., Rzatowski S., Sokolowskaq K., Olszewska A., Schlagenhauf U., et al. Impact of a toothpaste with microcrystalline hydroxyapatite on the occurrence of early childhood caries: A 1-year randomized clinical trial. Sci. Rep. 2021;11:2650. doi: 10.1038/s41598-021-81112-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Verma P., Pandian S.M. Bionic effects of nano hydroxyapatite dentifrice on demineralised surface of enamel post orthodontic debonding: In-vivo split mouth study. Prog. Orthod. 2021;22:39. doi: 10.1186/s40510-021-00381-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Butera A., Pascadopoli M., Gallo S., Lelli M., Tarterini F., Giglia F., Scribante A. SEM/EDS Evaluation of the Mineral Deposition on a Polymeric Composite Resin of a Toothpaste Containing Biomimetic Zn-Carbonate Hydroxyapatite (microRepair®) in Oral Environment: A Randomized Clinical Trial. Polymers. 2021;13:2740. doi: 10.3390/polym13162740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Butera A., Gallo S., Pascadopoli M., Montasser M.A., Abd El Latief M.H., Modica G.G., Scribante A. Home Oral Care with Biomimetic Hydroxyapatite vs. Conventional Fluoridated Toothpaste for the Remineralization and Desensitizing of White Spot Lesions: Randomized Clinical Trial. Int. J. Environ. Res. Public Health. 2022;19:8676. doi: 10.3390/ijerph19148676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paszynska E., Pawinska M., Enax J., Meyer F., Schulze zur Wiesche E., May T.W., Amaechi B.T., Limeback H., Hernik A., Otulakowska-Skrzynska J., et al. Caries-preventing effect of a hydroxyapatite-toothpaste in adults: A 18 months double-blinded randomized clinical trial. Front. Public Health. 2023;11:1199728. doi: 10.3389/fpubh.2023.1199728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Najibfard K., Ramalingam K., Chedjieu I., Amaechi B.T. Remineralization of early caries by a nano-hydroxyapatite dentifrice. J. Clin. Dent. 2011;22:139–143. [PubMed] [Google Scholar]
- 41.Amaechi B.T., AbdulAzees P.A., Alshareif D.O., Shehata M.A., Lima P.P.d.C.S., Abdollahi A., Kalkhorani P.S., Evans V. Comparative efficacy of a hydroxyapatite and a fluoride toothpaste for prevention and remineralization of dental caries in children. BDJ Open. 2019;5:18. doi: 10.1038/s41405-019-0026-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Amaechi B.T., Alshareif D.O., AbdulAzees P.A., Shehata M.A., Lima P.P., Abdollahi A., Kalkhorani P.S., Evans V., Bagheri A., Okoye L.O. Anti-caries evaluation of a nano-hydroxyapatite dental lotion for use after toothbrushing: An in situ study. J. Dent. 2021;115:103863. doi: 10.1016/j.jdent.2021.103863. [DOI] [PubMed] [Google Scholar]
- 43.Amaechi B.T., Farah R., Liu J.A., Phillips T.S., Perozo B.I., Kataoka Y., Meyer F., Enax J. Remineralization of molar incisor hypomineralization (MIH) with a hydroxyapatite toothpaste: An in-situ study. BDJ Open. 2022;8:33. doi: 10.1038/s41405-022-00126-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hüttemann R.W., Dönges H. Investigations for treating hypersensitive necks of teeth with hydroxyapatite. Dtsch. Zahnärztl Z. 1987;42:486–488. [PubMed] [Google Scholar]
- 45.Barone M., Malpassi M. Clinical trial of a 15% supermicronized hydroxyapatite gel for dentin hypersensitivity. G. Ital. Endod. 1991;5:43–47. [PubMed] [Google Scholar]
- 46.Park J.J., Park J.B., Kwon Y.H., Herr Y., Chung J.H. The effects of microcrystalline hydroxyapatite containing toothpaste in the control of tooth hypersensitivity. J. Korean Acad. Periodontol. 2005;35:577–590. doi: 10.5051/jkape.2005.35.3.577. [DOI] [Google Scholar]
- 47.Kim M.S., Chae G.J., Choi S.H., Chai J.K., Kim C.K., Cho K.S. Effect of hydroxyapatite containing dentifrice on teeth hypersensitivity after periodontal therapy. J. Korean Acad. Periodontol. 2008;38:1–6. doi: 10.5051/jkape.2008.38.1.1. [DOI] [Google Scholar]
- 48.Kang S.J., Kwon Y.H., Park J.B., Herr Y., Chung J.H. The effects of hydroxyapatite toothpaste on tooth hypersensitivity. J. Korean Acad. Periodontol. 2009;39:9–16. doi: 10.5051/jkape.2009.39.1.9. [DOI] [Google Scholar]
- 49.Kim S.H., Park J.B., Lee C.W., Koo K.T., Kim T.I., Seol Y.J., Lee Y.M., Ku Y., Chung C.P., Rhyu I.C. The clinical effects of a hydroxyapatite containing toothpaste for dentine hypersensitivity. J. Korean Acad. Periodontol. 2009;39:87–94. doi: 10.5051/jkape.2009.39.1.87. [DOI] [Google Scholar]
- 50.Orsini G., Procaccini M., Manzoli L., Giuliodori F., Lorenzini A., Putignano A. A double-blind randomized-controlled trial comparing the desensitizing efficacy of a new dentifrice containing carbonate/hydroxyapatite nanocrystals and a sodium fluoride/potassium nitrate dentifrice. J. Clin. Periodontol. 2010;37:510–517. doi: 10.1111/j.1600-051X.2010.01558.x. [DOI] [PubMed] [Google Scholar]
- 51.Shetty S., Kohad R., Yeltiwar R. Hydroxyapatite as an in-office agent for tooth hypersensitivity: A clinical and scanning electron microscopic study. J. Periodontol. 2010;81:1781–1789. doi: 10.1902/jop.2010.100172. [DOI] [PubMed] [Google Scholar]
- 52.Browning W.D., Cho S.D., Deschepper E.J. Effect of a nano-hydroxyapatite paste on bleaching-related tooth sensitivity. J. Esthet. Restor. Dent. 2012;24:268–276. doi: 10.1111/j.1708-8240.2011.00437.x. [DOI] [PubMed] [Google Scholar]
- 53.Orsini G., Procaccini M., Manzoli L., Sparabombe S., Tiriduzzi P., Bambini F., Putignano A. A 3-day randomized clinical trial to investigate the desensitizing properties of three dentifrices. J. Periodontol. 2013;84:e65–e73. doi: 10.1902/jop.2013.120697. [DOI] [PubMed] [Google Scholar]
- 54.Jena A., Shashirekha G. Comparison of efficacy of three different desensitizing agents for in-office relief of dentin hypersensitivity: A 4 weeks clinical study. J. Conserv. Dent. 2015;18:389–393. doi: 10.4103/0972-0707.164052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pinojj A., Shetty A., Shetty D., Shetty S. A comparison of clinical efficacy of dentifrices containing calcium sodium phosphosilicate, nanoparticle hydroxyapatite and a dentifrice containing casein phosphopeptide amorphous calcium phosphate on dentinal hypersensitivity: A comparative triple blind randomized study. Adv. Hum. Biol. 2014;4:57–64. [Google Scholar]
- 56.Reddy S., Prasad M.G.S., Prasad S., Bhowmik N., Ashwini N., Sravya L., Singh S. The effect of pro-argin technology vs nano technology using commercially available dentifrice: A comparative study. Int. J. Appl. Dent. Sci. 2014;1:26–30. [Google Scholar]
- 57.Vano M., Derchi G., Barone A., Covani U. Effectiveness of nano-hydroxyapatite toothpaste in reducing dentin hypersensitivity: A double-blind randomized controlled trial. Quintessence Int. 2014;45:703–710. doi: 10.3290/j.qi.a32240. [DOI] [PubMed] [Google Scholar]
- 58.Narmatha V.J., Thakur S. An in-vivo comparative study of the efficacy of Propolis, nano-hydroxyapatite and potassium nitrate-containing desensitizing agents. RRJDS. 2014;2:113–118. [Google Scholar]
- 59.Amin M., Mehta R., Duseja S., Desai K. Evaluation of the efficacy of commercially available nano hydroxypatite paste (Aclaim) as a desensitizing agent. Adv. Human Biol. 2015;5:34–38. [Google Scholar]
- 60.Gopinath N.M., John J., Nagappan N., Prabhu S., Kumar E.S. Evaluation of dentifrice containing nano-hydroxyapatite for dentinal hypersensitivity: A randomized controlled trial. J. Int. Oral Health. 2015;7:118–122. [PMC free article] [PubMed] [Google Scholar]
- 61.Lee S.-Y., Jung H.-I., Jung B.-Y., Cho Y.-S., Kwon H.-K., Kim B.-I. Desensitizing efficacy of nano-carbonate apatite dentifrice and Er,Cr:YSGG laser: A randomized clinical trial. Photomed. Laser Surg. 2015;33:9–14. doi: 10.1089/pho.2014.3787. [DOI] [PubMed] [Google Scholar]
- 62.Vano M., Derchi G., Barone A., Genovesi A., Covani U. Tooth bleaching with hydrogen peroxide and nano-hydroxyapatite: A 9-month follow-up randomized clinical trial. Int. J. Dent. Hyg. 2015;13:301–307. doi: 10.1111/idh.12123. [DOI] [PubMed] [Google Scholar]
- 63.Anand S., Rejula F., Sam J.V.G., Christaline R., Nair M.G., Dinakaran S. Comparative evaluation of effect of nano-hydroxyapatite and 8% arginine containing toothpastes in managing dentin hypersensitivity: Double blind randomized clinical trial. Acta Medica. 2017;60:114–119. doi: 10.14712/18059694.2018.3. [DOI] [PubMed] [Google Scholar]
- 64.Makeeva I.M., Polyakova M.A., Doroshina V.Y., Sokhova I.A., Arakelyan M.G., Makeeva M.K. Efficiency of paste and suspension with nano-hydroxyapatite on the sensitivity of teeth with gingival recession. Stomatologiia. 2018;97:23–27. doi: 10.17116/stomat20189704123. [DOI] [PubMed] [Google Scholar]
- 65.Amaechi B.T., Lemke K.C., Saha S., Gelfond J. Clinical efficacy in relieving dentin hypersensitivity of nanohydroxyapatite-containing cream: A randomized controlled trial. Open Dent. J. 2018;12:572–585. doi: 10.2174/1874210601812010572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vano M., Derchi G., Barone A., Pinna R., Usai P., Covani U. Reducing dentine hypersensitivity with nano-hydroxyapatite toothpaste: A double-blind randomized controlled trial. Clin. Oral Investig. 2018;22:313–320. doi: 10.1007/s00784-017-2113-3. [DOI] [PubMed] [Google Scholar]
- 67.Al Asmari D., Khan M.K. Evaluate efficacy of desensitizing toothpaste containing zinc-carbonate hydroxyapatite nanocrystals: Non-comparative eight-week clinical study. J. Int. Soc. Prev. Community Dent. 2019;9:566–570. doi: 10.4103/jispcd.JISPCD_261_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kondyurova E.V., Lisevtsova J.V., Eliseykina E.V., Vilikotskiy A.E., Zakirova S.A. Clinical evaluation of a dentifrice containing Nhap for the reduction of dentin hypersensitivity. Int. J. Oral Dent. Health. 2019;5:104. doi: 10.23937/2469-5734/1510104. [DOI] [Google Scholar]
- 69.Alencar C.D., Ortiz M.I., Silva F.A., Alves E.B., Araújo J.L., Silva C.M. Effect of nanohydroxyapatite associated with photobiomodulation in the control of dentin hypersensitivity: A randomized, double-blind, placebo-controlled clinical trial. Am. J. Dent. 2020;33:138–144. [PubMed] [Google Scholar]
- 70.Ding P.H., Dai A., Hu H.J., Huang J.P., Liu J.M., Chen L.L. Efficacy of nano-carbonate apatite dentifrice in relief from dentine hypersensitivity following non-surgical periodontal therapy: A randomized controlled trial. BMC Oral Health. 2020;20:170. doi: 10.1186/s12903-020-01157-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Alharith D.N., Al-Omari M., Almnea R., Basri R., Alshehri A.H., Al-Nufiee A.A. Clinical efficacy of single application of plain nano-hydroxyapatite paste in reducing dentine hypersensitivity—A randomized clinical trial. Saudi Endod. J. 2021;11:24–30. [Google Scholar]
- 72.Amaechi B.T., Lemke K.C., Saha S., Luong M.N., Gelfond J. Clinical efficacy of nanohydroxyapatite-containing toothpaste at relieving dentin hypersensitivity: An 8 weeks randomized control trial. BDJ Open. 2021;7:23. doi: 10.1038/s41405-021-00080-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ehlers V., Reuter A.K., Kehl E.B., Enax J., Meyer F., Schlecht J., Schmidtmann I., Deschner J. Efficacy of a toothpaste based on microcrystalline hydroxyapatite on children with hypersensitivity caused by MIH: A randomised controlled trial. Oral Health Prev. Dent. 2021;19:647–658. doi: 10.3290/j.ohpd.b2403649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Polyakova M., Sokhova I., Doroshina V., Arakelyan M., Novozhilova N., Babina K. The effect of toothpastes containing hydroxyapatite, fluoroapatite, and Zn-Mg-hydroxyapatite nanocrystals on dentin hypersensitivity: A randomized clinical trial. J. Int. Soc. Prev. Commun. Dent. 2022;12:252. doi: 10.4103/jispcd.JISPCD_333_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vlasova N., Samusenkov V., Novikova I., Nikolenko D., Nikolashvili N., Gor I., Danilina A. Clinical efficacy of hydroxyapatite toothpaste containing Polyol Germanium Complex (PGC) with threonine in the treatment of dentine hypersensitivity. Saudi Dent. J. 2022;34:310–314. doi: 10.1016/j.sdentj.2022.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Butera A., Pascadopoli M., Pellegrini M., Trapani B., Gallo S., Radu M., Scribante A. Biomimetic hydroxyapatite paste for molar-incisor hypomineralization: A randomized clinical trial. Oral Dis. 2022:preprint. doi: 10.1111/odi.14388. [DOI] [PubMed] [Google Scholar]
- 77.Harks I., Jockel-Schneider Y., Schlagenhauf U., May T.W., Gravemeier M., Prior K., Petersilka G., Ehmke B. Impact of the Daily Use of a Microcrystal Hydroxyapatite Dentifrice on De Novo Plaque Formation and Clinical/Microbiological Parameters of Periodontal Health. A Randomized Trial. PLoS ONE. 2016;11:e0160142. doi: 10.1371/journal.pone.0160142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Doroshina V.Y., Sokhova I.A., Polyakova M.A., Margaryan E.G. Comparative evaluation of the effectiveness of oral care products in inflammatory disease of the oral cavity, accompanied by teeth hyperesthesia. New Am. Med. J. 2019;13:34–40. [Google Scholar]
- 79.Monterubbianesi R., Sparabombe S., Tosco V., Profili F., Mascitti M., Hosein A., Putignano A., Orsini G. Can Desensitizing Toothpastes Also Have an Effect on Gingival Inflammation? A Double-Blind, Three-Treatment Crossover Clinical Trial. Int. J. Environ. Res. Public Health. 2020;17:8927. doi: 10.3390/ijerph17238927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Brauner E., Di Cosola M., Ambrosino M., Cazzolla A.P., Dioguardi M., Nocini R., Topi S., Mancini A., Maggiore M.E., Scacco S., et al. Efficacy of bioactivated anticalculus toothpaste on oral health: A single-blind, parallel-group clinical study. Minerva Dent. Oral Sci. 2022;71:31–38. doi: 10.23736/S2724-6329.21.04606-4. [DOI] [PubMed] [Google Scholar]
- 81.Andrea B., Lanteri V., Martina B., Fabio D.F., Eleonora F., Annamaria G. Proactive therapy in maintenance of periodontal orthodontic patient. Int. J. Clin. Dent. 2022;15:39–47. [Google Scholar]
- 82.Niwa M., Sato T., Li W., Aoki H., Aoki H., Daisaku T. Polishing and whitening properties of toothpaste containing hydroxyapatite. J. Mater. Sci. Mater. Med. 2001;12:277–281. doi: 10.1023/A:1008927502523. [DOI] [PubMed] [Google Scholar]
- 83.Raoufi S., Birkhed D. Effect of whitening toothpastes on tooth staining using two different colour-measuring devices-a 12-week clinical trial. Int. Dent. J. 2010;60:419–423. [PubMed] [Google Scholar]
- 84.Woo G.-J., Kim E.-K., Jeong S.-H., Song K.-B., Goo H.-J., Jeon E.-S., Choi Y.-H. Comparison of the whitening effect of toothpastes containing 0.25% hydroxyapatite and 0.75% hydrogen peroxide. J. Korean Acad. Oral Health. 2014;38:3–9. doi: 10.11149/jkaoh.2014.38.1.3. [DOI] [Google Scholar]
- 85.Bommer C., Flessa H.P., Xu X., Kunzelmann K.H. Hydroxyapatite and self-assembling peptide matrix for non-oxidizing tooth whitening. J. Clin. Dent. 2018;29:57–63. [PubMed] [Google Scholar]
- 86.Steinert S., Kuchenbecker J., Meyer F., Simader B., Zwanzig K., Enax J. Whitening effects of a novel oral care gel with biomimetic hydroxyapatite: A 4-week observational pilot study. Biomimetics. 2020;5:65. doi: 10.3390/biomimetics5040065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Andersson A., Sköld-Larsson K., Hallgren A., Petersson L.G., Twetman S. Effect of a dental cream containing amorphous cream phosphate complexes on white spot lesion regression assessed by laser fluorescence. Oral Health Prev. Dent. 2007;5:229–233. [PubMed] [Google Scholar]
- 88.Bailey D.L., Adams G.G., Tsao C.E., Hyslop A., Escobar K., Manton D.J., Reynolds E.C., Morgan M.V. Regression of post-orthodontic lesions by a remineralizing cream. J. Dent. Res. 2009;88:1148–1153. doi: 10.1177/0022034509347168. [DOI] [PubMed] [Google Scholar]
- 89.Rao S.K., Bhat G.S., Aradhya S., Devi A., Bhat M. Study of the efficacy of toothpaste containing casein phosphopeptide in the prevention of dental caries: A randomized controlled trial in 12- to 15-year-old high caries risk children in Bangalore, India. Caries Res. 2009;43:430–435. doi: 10.1159/000252976. [DOI] [PubMed] [Google Scholar]
- 90.Uysal T., Amasyali M., Koyuturk A.E., Ozcan S. Effects of different topical agents on enamel demineralization around orthodontic brackets: An in vivo and in vitro study. Aust. Dent. J. 2010;55:268–274. doi: 10.1111/j.1834-7819.2010.01233.x. [DOI] [PubMed] [Google Scholar]
- 91.Bröchner A., Christensen C., Kristensen B., Tranæus S., Karlsson L., Sonnesen L., Twetman S. Treatment of post-orthodontic white spot lesions with casein phosphopeptide-stabilised amorphous calcium phosphate. Clin. Oral Investig. 2011;15:369–373. doi: 10.1007/s00784-010-0401-2. [DOI] [PubMed] [Google Scholar]
- 92.Akin M., Basciftci F.A. Can white spot lesions be treated effectively? Angle Orthod. 2012;82:770–775. doi: 10.2319/090711.578.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sitthisettapong T., Phantumvanit P., Huebner C., Derouen T. Effect of CPP-ACP paste on dental caries in primary teeth: A randomized trial. J. Dent. Res. 2012;91:847–852. doi: 10.1177/0022034512454296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang J.X., Yan Y., Wang X.J. Clinical evaluation of remineralization potential of casein phosphopeptide amorphous calcium phosphate nanocomplexes for enamel decalcification in orthodontics. Chin. Med. J. 2012;125:4018–4021. [PubMed] [Google Scholar]
- 95.Krithikadatta J., Fredrick C., Abarajithan M., Kandaswamy D. Remineralisation of occlusal white spot lesion with a combination of 10% CPP-ACP and 0.2% sodium fluoride evaluated using Diagnodent: A pilot study. Oral Health Prev. Dent. 2013;11:191–196. doi: 10.3290/j.ohpd.a29736. [DOI] [PubMed] [Google Scholar]
- 96.Plonka K.A., Pukallus M.L., Holcombe T.F., Barnett A.G., Walsh L.J., Seow W.K. Randomized controlled trial: A randomized controlled clinical trial comparing a remineralizing paste with an antibacterial gel to prevent early childhood caries. Pediatr. Dent. 2013;35:8–12. [PubMed] [Google Scholar]
- 97.Aykut-Yetkiner A., Kara N., Ateş M., Ersin N., Ertuğrul F. Does casein phosphopeptide amorphous calcium phosphate provide remineralization on white spot lesions and inhibition of Streptococcus mutans? J. Clin. Pediatr. Dent. 2014;38:302–306. doi: 10.17796/jcpd.38.4.b4q401v6m4818215. [DOI] [PubMed] [Google Scholar]
- 98.Yazıcıoğlu O., Ulukapi H. The investigation of non-invasive techniques for treating early approximal carious lesions: An in vivo study. Int. Dent. J. 2014;64:1–11. doi: 10.1111/idj.12056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhang Q., Zou J., Yang R., Zhou X. Remineralization effects of casein phosphopeptide-amorphous calcium phosphate crème on artificial early enamel lesions of primary teeth. Int. J. Paediatr. Dent. 2011;21:374–381. doi: 10.1111/j.1365-263X.2011.01135.x. [DOI] [PubMed] [Google Scholar]
- 100.Llena C., Leyda A.M., Forner L. CPP-ACP and CPP-ACFP versus fluoride varnish in remineralisation of early caries lesions. A prospective study. Eur. J. Paediatr. Dent. 2015;16:181–186. [PubMed] [Google Scholar]
- 101.Memarpour M., Fakhraei E., Dadaein S., Vossoughi M. Efficacy of fluoride varnish and casein phosphopeptide-amorphous calcium phosphate for remineralization of primary teeth: A randomized clinical trial. Med. Princ. Pract. 2015;24:231–237. doi: 10.1159/000379750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sitthisettapong T., Doi T., Nishida Y., Kambara M., Phantumvanit P. Effect of CPP-ACP Paste on Enamel Carious Lesion of Primary Upper Anterior Teeth Assessed by Quantitative Light-Induced Fluorescence: A One-Year Clinical Trial. Caries Res. 2015;49:434–441. doi: 10.1159/000434728. [DOI] [PubMed] [Google Scholar]
- 103.Sim C.P.C., Wee J., Xu Y., Cheung Y.-B., Soong Y.-L., Manton D.J. Anti-caries effect of CPP-ACP in irradiated nasopharyngeal carcinoma patients. Clin. Oral Investig. 2015;19:1005–1011. doi: 10.1007/s00784-014-1318-y. [DOI] [PubMed] [Google Scholar]
- 104.Esenlik E., Uzer Çelik E., Bolat E. Efficacy of a casein phosphopeptide amorphous calcium phosphate (CPP-ACP) paste in preventing white spot lesions in patients with fixed orthodontic appliances: A prospective clinical trial. Eur. J. Paediatr. Dent. 2016;17:274–280. [PubMed] [Google Scholar]
- 105.Güçlü Z.A., Alaçam A., Coleman N.J. A 12-Week Assessment of the Treatment of White Spot Lesions with CPP-ACP Paste and/or Fluoride Varnish. Biomed. Res. Int. 2016;2016:8357621. doi: 10.1155/2016/8357621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Munjal D., Garg S., Dhindsa A., Sidhu G.K., Sethi H.S. Assessment of White Spot Lesions and In-Vivo Evaluation of the Effect of CPP-ACP on White Spot Lesions in Permanent Molars of Children. J. Clin. Diagn. Res. 2016;10:ZC149-54. doi: 10.7860/JCDR/2016/19458.7896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Singh S., Singh S.P., Goyal A., Utreja A.K., Jena A.K. Effects of various remineralizing agents on the outcome of post-orthodontic white spot lesions (WSLs): A clinical trial. Prog. Orthod. 2016;17:25. doi: 10.1186/s40510-016-0138-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Karabekiroğlu S., Ünlü N., Küçükyilmaz E., Şener S., Botsali M.S., Malkoç S. Treatment of post-orthodontic white spot lesions with CPP-ACP paste: A three year follow up study. Dent. Mater. J. 2017;36:791–797. doi: 10.4012/dmj.2016-228. [DOI] [PubMed] [Google Scholar]
- 109.Mendes A.C., Restrepo M., Bussaneli D., Zuanon A.C. Use of Casein Amorphous Calcium Phosphate (CPP-ACP) on White-spot Lesions: Randomised Clinical Trial. Oral Health Prev. Dent. 2018;16:27–31. doi: 10.3290/j.ohpd.a39749. [DOI] [PubMed] [Google Scholar]
- 110.Wang Y.H., Liu F., Liu H.N., Wang Q.X., Xing W.Z., Li Z.C. Impact assessment on enamel remineralization after orthodontic treatment with casein phosphopeptide calcium phosphate complex. Shanghai Kou Qiang Yi Xue. 2018;27:382–385. [PubMed] [Google Scholar]
- 111.Bobu L., Murariu A., Topor G., Beznea A., Vasluianu R. Comparative evaluation of casein phosphopeptide—Amorphous calcium phosphate and fluoride in managing early caries lesions. Rev. Chim. 2019;70:3746–3749. doi: 10.37358/RC.19.10.7638. [DOI] [Google Scholar]
- 112.Tingyun W., Detang W., Youjia Z., Qiong R., Aimin W., Shangqun H., Xiaofang Z. Effects of different types of fluoride-free toothpaste on the remineralization of enamel after acid erosion: An in vivo study. Chin. J. Tiss. Eng. Res. 2019;23:2842–2846. [Google Scholar]
- 113.Al-Batayneh O.B., Bani Hmood E.I., Al-Khateeb S.N. Assessment of the effects of a fluoride dentifrice and GC Tooth Mousse on early caries lesions in primary anterior teeth using quantitative light-induced fluorescence: A randomised clinical trial. Eur. Arch. Paediatr. Dent. 2020;21:85–93. doi: 10.1007/s40368-019-00451-7. [DOI] [PubMed] [Google Scholar]
- 114.Bangi S.L., Konda P., Talapaneni A.K., Fatima A., Hussain A. Evaluation of three commercially available materials in reducing the white spot lesions during fixed orthodontic treatment: A prospective randomized controlled trial. J. Ind. Orthod. Soc. 2020;54:100–105. [Google Scholar]
- 115.Perić T., Marković D., Tomić-Spirić V., Petrović B., Perić-Popadić A., Marković E. Clinical efficacy of casein phosphopeptide-amorphous calcium phosphate and casein phosphopeptide-amorphous calcium fluoride phosphate and their influence on the quality of life in patients with Sjögren’s syndrome. Srp. Arh. Celok. Lek. 2020;148:528–534. doi: 10.2298/SARH191222041P. [DOI] [Google Scholar]
- 116.Ashour D.G., Farid M.R., Mosallam R.S., Abouauf E.A. Effect of CPP-ACP pastes with/without fluoride on white spot lesion progression, salivary pH, and fluoride release in high caries risk patients: A randomized clinical trial. J. Int. Oral Health. 2021;13:336–343. [Google Scholar]
- 117.Juárez-López M.L.A., Gómez-Rivas Y.C., Murrieta-Pruneda F. Casein-phosphopeptide-amorphous-calcium-phosphate plus brushing with fluoride toothpaste for remineralization of early caries. Acta Pediátrica México. 2021;42:272–279. [Google Scholar]
- 118.El-Sherif S.A., Shaalan O.O., Hamza N.K., El Baz M.A. Remineralization Potential of Pearl Powder Compared to Casein Phosphopeptide Amorphous Calcium Phosphate on Enamel White Spot Lesions (Randomized Clinical Trial) J. Pharm. Negat. Results. 2022;13:1662–1671. [Google Scholar]
- 119.Hamdi K., Hamama H.H., Motawea A., Fawzy A., Mahmoud S.H. Long-term evaluation of early-enamel lesions treated with novel experimental tricalcium silicate paste: A 2-year randomized clinical trial. J. Esthet. Restor. Dent. 2022;34:1113–1121. doi: 10.1111/jerd.12941. [DOI] [PubMed] [Google Scholar]
- 120.Olgen I.C., Sonmez H., Bezgin T. Effects of different remineralization agents on MIH defects: A randomized clinical study. Clin. Oral. Investig. 2022;26:3227–3238. doi: 10.1007/s00784-021-04305-9. [DOI] [PubMed] [Google Scholar]
- 121.Salah R., Afifi R.R., Kehela H.A., Aly N.M., Rashwan M. Efficacy of a novel bioactive glass in the treatment of enamel white spot lesions: A randomized controlled trial. J. Evid. Based Dent. Pract. 2022;22:101725. doi: 10.1016/j.jebdp.2022.101725. [DOI] [PubMed] [Google Scholar]
- 122.Simon L.S., Dash J.K., U D., Philip S., Sarangi S. Management of Post Orthodontic White Spot Lesions Using Resin Infiltration and CPP-ACP Materials- A Clinical Study. J. Clin. Paeditr. Dent. 2022;46:70–74. doi: 10.17796/1053-4625-46.1.12. [DOI] [PubMed] [Google Scholar]
- 123.Srinivasan N., Kavitha M., Loganathan S.C. Comparison of the remineralization potential of CPP-ACP and CPP-ACP with 900 ppm fluoride on eroded human enamel: An in situ study. Arch. Oral. Biol. 2010;55:541–544. doi: 10.1016/j.archoralbio.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 124.Shen P., Walker G.D., Yuan Y., Reynolds C., Stanton D.P., Fernando J.R., Reynolds E.C. Importance of bioavailable calcium in fluoride dentifrices for enamel remineralization. J. Dent. 2018;78:59–64. doi: 10.1016/j.jdent.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 125.Perić T., Markovic D., Petrovic B., Radojevic V., Todorovic T., Radicevic B.A., Heinemann R.J., Susic G., Popadic A.P., Spiric V.T. Efficacy of pastes containing CPP-ACP and CPP-ACFP in patients with Sjögren’s syndrome. Clin. Oral Investig. 2015;19:2153–2165. doi: 10.1007/s00784-015-1444-1. [DOI] [PubMed] [Google Scholar]
- 126.Garry A.P., Flannigan N.L., Cooper L., Komarov G., Burnside G., Higham S.M. A randomised controlled trial to investigate the remineralising potential of Tooth Mousse™ in orthodontic patients. J. Orthod. 2017;44:147–156. doi: 10.1080/14653125.2017.1341729. [DOI] [PubMed] [Google Scholar]
- 127.Zawaideh F.I., Owais A.I., Mushtaha S. Effect of CPP-ACP or a potassium nitrate sodium fluoride dentifrice on enamel erosion prevention. J. Clin. Paediatr. Dent. 2017;41:135–140. doi: 10.17796/1053-4628-41.2.135. [DOI] [PubMed] [Google Scholar]
- 128.Yu H., Jiang N.-W., Ye X.-Y., Zheng H.-Y., Attin T., Cheng H. In situ effect of Tooth Mousse containing CPP-ACP on human enamel subjected to in vivo acid attacks. J. Dent. 2018;76:40–45. doi: 10.1016/j.jdent.2018.05.021. [DOI] [PubMed] [Google Scholar]
- 129.de Oliveira P.R.A., Barboza C.M., Barreto L.S.C., Tostes M.A. Effect of CPP-ACP on remineralization of artificial caries-like lesion: An in situ study. Braz. Oral Res. 2020;34:e061. doi: 10.1590/1807-3107bor-2020.vol34.0061. [DOI] [PubMed] [Google Scholar]
- 130.de Oliveira P.R.A., Barreto L.S.D.C., Tostes M.A. Effectiveness of CPP-ACP and fluoride products in tooth remineralization. Int. J. Dent. Hyg. 2022;20:635–642. doi: 10.1111/idh.12542. [DOI] [PubMed] [Google Scholar]
- 131.Kumar A., Goyal A., Gauba K., Kapur A., Singh S.K., Mehta S.K. An evaluation of remineralised MIH using CPP-ACP and fluoride varnish: An in-situ and in-vitro study. Eur. Arch. Paediatr. Dent. 2022;23:79–97. doi: 10.1007/s40368-021-00630-5. [DOI] [PubMed] [Google Scholar]
- 132.Borges B.C., Borges J.S., de Melo C.D., Pinheiro I.V., Santos A.J., Braz R., Montes M.A. Efficacy of a novel at-home bleaching technique with carbamide peroxides modified by CPP-ACP and its effect on the microhardness of bleached enamel. Oper. Dent. 2011;36:521–528. doi: 10.2341/11-013-L. [DOI] [PubMed] [Google Scholar]
- 133.Özgül B.M., Saat S., Sönmez H., Oz F.T. Clinical evaluation of desensitizing treatment for incisor teeth affected by molar-incisor hypomineralization. J. Clin. Pediatr. Dent. 2013;38:101–105. doi: 10.17796/jcpd.38.2.92mx26l6n482j682. [DOI] [PubMed] [Google Scholar]
- 134.Maghaireh G.A., Alzraikat H., Guidoum A. Assessment of the effect of casein phosphopeptide-amorphous calcium phosphate on postoperative sensitivity associated with in-office vital tooth whitening. Oper. Dent. 2014;39:239–247. doi: 10.2341/12-527-C. [DOI] [PubMed] [Google Scholar]
- 135.Mahesuti A., Duan Y.L., Wang G., Cheng X.R., Matis B.A. Short-term Efficacy of Agents Containing KNO3 or CPP-ACP in Treatment of Dentin Hypersensitivity. Chin. J. Dent. Res. 2014;17:43–47. [PubMed] [Google Scholar]
- 136.Zhang D., Zhang X., Peng H., Sun H. Casein phosphopeptide-amorphous calcium phosphate nanocomplexes as a preventive agent for radiation caries and dental sensitivity in irradiated head and neck cancer patients. Chin. J. Clin. Oncol. 2014;41:1293–1296. [Google Scholar]
- 137.Konekeri V., Bennadi D., Manjunath M., Kshetrimayum N., Siluvai S., Reddy C.V.K. A clinical study to assess the effectiveness of CPP-ACP (casein phosphopeptide-amorphous calcium phosphate) versus potassium-nitrate (KNO3) on cervical dentine hypersensitivity. J. Young Pharm. 2015;7:217–224. doi: 10.5530/jyp.2015.3.12. [DOI] [Google Scholar]
- 138.Nanjundasetty J.K., Ashrafulla M. Efficacy of desensitizing agents on postoperative sensitivity following an in-office vital tooth bleaching: A randomized controlled clinical trial. J. Conserv. Dent. 2016;19:207–211. doi: 10.4103/0972-0707.181927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Tarique N., Awan R., Saleh M.I. Vital tooth bleaching and management of post operative sensitivity: A clinical trial evaluating the efficacy of different desensitizing materials. Pak. J. Med. Health Sci. 2017;11:1564–1567. [Google Scholar]
- 140.Pasini M., Giuca M.R., Scatena M., Gatto R., Caruso S. Molar incisor hypomineralization treatment with casein phosphopeptide and amorphous calcium phosphate in children. Minerva Stomatol. 2018;67:20–25. doi: 10.23736/S0026-4970.17.04086-9. [DOI] [PubMed] [Google Scholar]
- 141.Yassin O., Milly H. Effect of CPP-ACP on efficacy and postoperative sensitivity associated with at-home vital tooth bleaching using 20% carbamide peroxide. Clin. Oral Investig. 2019;23:1555–1559. doi: 10.1007/s00784-018-2574-z. [DOI] [PubMed] [Google Scholar]
- 142.Adil H.M., Jouhar R., Ahmed M.A., Basha S., Ahmed N., Abbasi M.S., Maqsood A., Nagarajappa A.K., Alam M.K. Comparison of casein phosphopeptide with potassium nitrate and sodium monofluorophosphate desensitizing efficacy after in-office vital bleaching—A randomized trial. Appl. Sci. 2021;11:9291. doi: 10.3390/app11199291. [DOI] [Google Scholar]
- 143.Gümüştaş B., Dikmen B. Effectiveness of remineralization agents on the prevention of dental bleaching induced sensitivity: A randomized clinical trial. Int. J. Dent. Hyg. 2021;20:650–657. doi: 10.1111/idh.12524. [DOI] [PubMed] [Google Scholar]
- 144.Tiwari A., Jain R.K. Comparative evaluation of white spot lesions incidence between NovaMin, Probiotic and fluoride containing dentifrices during orthodontic treatment using laser fluorescence- a prospective randomized controlled trial. Clin. Investig. Orthog. 2023;82:75–82. doi: 10.1080/27705781.2023.2190950. [DOI] [Google Scholar]
- 145.Du M.Q., Bian Z., Jiang H., Greenspan D.C., Burwell A.K., Zhong J., Tai B.J. Clinical evaluation of a dentifrice containing calcium sodium phosphosilicate (NovaMin) for the treatment of dentin hypersensitivity. Am. J. Dent. 2008;21:210–214. [PubMed] [Google Scholar]
- 146.Litkowski L., Greenspan D.C. A clinical study of the effect of calcium sodium phosphosilicate on dentin hypersensitivity-Proof of principle. J. Clin. Dent. 2010;21:77–81. [PubMed] [Google Scholar]
- 147.Narongdej T., Sakoolnamarka R., Boonroung T. The effectiveness of a calcium sodium phosphosilicate desensitizer in reducing cervical dentin hypersensitivity: A pilot study. J. Am. Dent. Assoc. 2010;141:995–999. doi: 10.14219/jada.archive.2010.0313. [DOI] [PubMed] [Google Scholar]
- 148.Pradeep A.R., Sharma A. Comparison of clinical efficacy of a dentifrice containing calcium sodium phosphosilicate to a dentifrice containing potassium nitrate and to a placebo on dentinal hypersensitivity: A randomized clinical trial. J. Periodontol. 2010;81:995–999. doi: 10.1902/jop.2010.100056. [DOI] [PubMed] [Google Scholar]
- 149.Salian S., Thakur S., Kulkarni S., Latorre G. A randomized controlled clinical study evaluating the efficacy of two desensitizing dentifrices. J. Clin. Dent. 2010;21:82–87. [PubMed] [Google Scholar]
- 150.Sharma N., Roy S., Kakar A., Greenspan D.C., Scott R. A clinical study comparing oral formulations containing 7.5% calcium sodium phosphosilicate (Novamin®), 5% potassium nitrate, and 0.4% stannous fluoride for the management of dentin hypersensitivity. J. Clin. Dent. 2010;21:88–92. [PubMed] [Google Scholar]
- 151.West N.X., Macdonald E.L., Jones S.B., Claydon N.C.A., Hughes N., Jeffery P. Randomized in situ clinical study comparing the ability of two new desensitizing toothpaste technologies to occlude patent dentin tubules. J. Clin. Dent. 2011;22:82–89. [PubMed] [Google Scholar]
- 152.Pradeep A.R., Agarwal E., Naik S.B., Bajaj P., Kalra N. Comparison of efficacy of three commercially available dentrifices on dentinal hypersensitivity: A randomized clinical trial. Aust. Dent. J. 2012;57:429–434. doi: 10.1111/j.1834-7819.2012.01726.x. [DOI] [PubMed] [Google Scholar]
- 153.Rajesh K.S., Hedge S., Kumar A., Shetty D.G. Evaluation of the efficacy of a 5% calcium sodium phosphosilicate (Novamin®) containing dentifrice for the relief of dentinal hypersensitivity: A clinical study. Ind. J. Dent. Res. 2012;23:363–367. doi: 10.4103/0970-9290.102228. [DOI] [PubMed] [Google Scholar]
- 154.Surve S.M., Acharya A.B., Shetty A., Thakur S.L. Efficacy of calcium sodium phosphosilicate in managing dentinal hypersensitivity. Gen. Dent. 2012;60:e308–e311. [PubMed] [Google Scholar]
- 155.Acharya A.B., Surve S.M., Thakur S.L. A clinical study of the effect of calcium sodium phosphosilicate on dentin hypersensitivity. J. Clin. Exp. Dent. 2013;5:e18–e22. doi: 10.4317/jced.50955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Pintado-Palomino K., Peitl Filho O., Zanotto E.D., Tirapelli C. A clinical, randomized, controlled study on the use of desensitizing agents during tooth bleaching. J. Dent. 2015;43:1099–1105. doi: 10.1016/j.jdent.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 157.Samuel S.R., Khatri S.G., Acharya S., Patil S.T. Evaluation of instant desensitization after a single topical application over 30 days: A randomized trial. Aust. Dent. J. 2015;60:336–342. doi: 10.1111/adj.12341. [DOI] [PubMed] [Google Scholar]
- 158.Majji P., Murthy K.R. Clinical efficacy of four interventions in the reduction of dentinal hypersensitivity: A 2-month study. Ind. J. Dent. Res. 2016;27:477–482. doi: 10.4103/0970-9290.195618. [DOI] [PubMed] [Google Scholar]
- 159.Sufi F., Hall C., Mason S., Shaw D., Kennedy L., Gallob J.T. Efficacy of an experimental toothpaste containing 5% calcium sodium phosphosilicate in the relief of dentin hypersensitivity: An 8-week randomized study (Study 1) Am. J. Dent. 2016;29:93–100. [PubMed] [Google Scholar]
- 160.Sufi F., Hall C., Mason S., Shaw D., Milleman J., Milleman K. Efficacy of an experimental toothpaste containing 5% calcium sodium phosphosilicate in the relief of dentin hypersensitivity: An 8-week randomized study (Study 2) Am. J. Dent. 2016;29:101–109. [PubMed] [Google Scholar]
- 161.Athuluru D., Reddy C., Sudhir K., Kumar K., Gomasani S., Nagarakanti S. Evaluation and comparison of efficacy of three desensitizing dentifrices on dentinal hypersensitivity and salivary biochemical characteristics: A randomized controlled trial. Dent. Res. J. 2017;14:150–157. [PMC free article] [PubMed] [Google Scholar]
- 162.Hall C., Mason S., Cooke J. Exploratory randomised controlled clinical study to evaluate the comparative efficacy of two occluding toothpastes—A 5% calcium sodium phosphosilicate toothpaste and an 8% arginine/calcium carbonate toothpaste—for the longer-term relief of dentine hypersensitivity. J. Dent. 2017;60:36–43. doi: 10.1016/j.jdent.2017.02.009. [DOI] [PubMed] [Google Scholar]
- 163.Fu Y., Sufi F., Wang N., Young S., Feng X. An exploratory randomised study to evaluate the efficacy of an experimental occlusion-based dentifrice in the relief of dentin hypersensitivity. Oral Health Prev. Dent. 2019;17:107–115. doi: 10.3290/j.ohpd.a42372. [DOI] [PubMed] [Google Scholar]
- 164.Alsherbiney H., El-Deeb H., Alsherbiney A., Abdou A., Mobarak E., Hamza O. Effect of calcium sodium phosphosilicate-containing compared to nanohydroxyapatite-containing toothpastes on dentinal tubule occlusion: A randomized clinical in situ study. J. Int. Oral Health. 2020;12:305–312. [Google Scholar]
- 165.Bhowmik E., Pawar Chandrashekhar D., Sharma Hareesha M. Comparative evaluation of fluorinol and calcium sodium phosphosilicate-containing toothpastes in the treatment of dentin hypersensitivity. Int. J. Dent. Hyg. 2021;19:421–428. doi: 10.1111/idh.12495. [DOI] [PubMed] [Google Scholar]
- 166.Ongphichetmetha N., Lertpimonchai A., Champaiboon C. Bioactive glass and arginine dentifrices immediately relieved dentine hypersensitivity following non-surgical periodontal therapy: A randomized controlled trial. J. Periodontol. 2022;93:246–255. doi: 10.1002/JPER.21-0091. [DOI] [PubMed] [Google Scholar]
- 167.Detsomboonrat P., Trairatvorakul C., Pisarnturakit P.P. Similar 1-year caries increment after use of fluoride or non-fluoride toothpaste in infants and toddlers. Fluoride. 2016;49:313–326. [Google Scholar]
- 168.Jang J.-H., Oh S., Kim H.-J., Kim D.-S. A randomized clinical trial for comparing the efficacy of desensitizing toothpastes on the relief of dentin hypersensitivity. Nat. Sci. Rep. 2023;13:5171. doi: 10.1038/s41598-023-31616-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Neurath C., Limeback H., Osmunson B., Connett M., Kanter V., Wells C.R. Dental Fluorosis Trends in US Oral Health Surveys: 1986 to 2012. JDR Clin. Trans. Res. 2019;4:298–308. doi: 10.1177/2380084419830957. [DOI] [PubMed] [Google Scholar]
- 170.Farmus L., Till C., Green R., Hornung R., Martinez Mier E.A., Ayotte P., Muckle G., Lanphear B.P., Flora D.B. Critical windows of fluoride neurotoxicity in Canadian children. Environ. Res. 2021;200:111315. doi: 10.1016/j.envres.2021.111315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Enax J., Meyer F., Schulze Zur Wiesche E., Epple M. On the Application of Calcium Phosphate Micro- and Nanoparticles as Food Additive. Nanomaterials. 2022;12:4075. doi: 10.3390/nano12224075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Enax J., Amaechi B.T., Farah R., Liu J.A., Schulze zur Wiesche E., Meyer F. Remineralization Strategies for Teeth with Molar Incisor Hypomineralization (MIH): A Literature Review. Dent. J. 2023;11:80. doi: 10.3390/dj11030080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Sezer B., Kargul B. Effect of Remineralization Agents on Molar-Incisor Hypomineralization-Affected Incisors: A Randomized Controlled Clinical Trial. J. Clin. Pediatr. Dent. 2022;46:192–198. doi: 10.17796/1053-4625-46.3.4. [DOI] [PubMed] [Google Scholar]
- 174.Sezer B., Tuğcu N., Calışkan C., Baş Durmus B., Kupets T., Bekiroğlu N., Kargül B., Bourgeois D. Effect of casein phosphopeptide amorphous calcium fluoride phosphate and calcium glycerophosphate on incisors with molar-incisor hypomineralization: A cross-over, randomized clinical trial. Bio-Med. Mater. Eng. 2022;33:325–335. doi: 10.3233/BME-211371. [DOI] [PubMed] [Google Scholar]
- 175.Yilmaz M.A., Yildiz P.K., Gokkaya B., Bilsel S.O., Kargul B. The effect of a novel toothpaste in children with white spot lesions. J. Pak. Med. Assoc. 2022;72:2170–2174. doi: 10.47391/JPMA.2409. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used in this review was published data in the studies referenced. Online information was referenced and accessed as shown in the reference list. No new data were created.