Abstract
With rates of obesity and dyslipidemia rising among young adults, this meta-analysis aimed to compare the effects of high-intensity interval training (HIIT) versus moderate-intensity continuous training (MICT) and sedentary controls (CON) on low-density lipoprotein (LDL), high-density lipoprotein (HDL), triglyceride (TG), and total cholesterol (TC) in nondiabetic overweight and obese young adults to determine if HIIT or MICT is more efficacious in improving dyslipidemia. Studies included in the analysis had to be randomized controlled trials or quasi-experimental studies, comparing the effects of HIIT versus MICT or CON on at least three variables of interest: LDL, HDL, TG, and TC, in nondiabetic adults, with body mass indexes (BMIs) above 25, and average ages between 18–30. The quality of the studies was evaluated using the Physiotherapy Evidence Database (PEDro) scale. Eight studies fulfilled the selection criteria, with a mean PEDro quality score of 5.8. Compared to CON, HIIT significantly decreased the concentrations of LDL (−12.14 mg/dL, p = < 0.00001) and TC (−9.27 mg/dL, p = 0.003), without significantly affecting HDL or TG. Compared to MICT, HIIT significantly decreased the concentrations of LDL (−6.23 mg/dL, p = 0.05) and TC (−7.85 mg/dL, p = 0.02), without significantly affecting HDL or TG concentrations. HIIT is superior to MICT and CON in improving the concentrations of LDL and TC in our target population. As early management of dyslipidemia improves long-term health, we recommend clinicians consider HIIT training protocols for their nondiabetic overweight and obese young adult patients.
Keywords: Longevity, increased healthspan, lipid fraction, cardiovascular disease, atherosclerosis, steady state training, sprint interval training
INTRODUCTION
Obesity is on the rise in the United States, specifically among 18–25-year-olds, with a study showing that between 1976–1980 the prevalence of obesity was 6.2%, and by 2017–2018 it had increased to 32.7% in this age group (13). The National Health and Nutrition Examination Survey (NHANES) III (1988–1994) survey showed the prevalence of obesity in adults greater than twenty years old to be 22.9% in all age groups (59) versus NHANES survey 2017–2020 which showed the prevalence increased to 41.9% (11). Examination of the association between poor health outcomes and weight gain in early middle adulthood found that there were increased rates of type 2 diabetes, cardiovascular disease, obesity-related cancer, and mortality compared to the same-age cohort who did not gain weight (70). Additionally, obesity has been associated with dyslipidemia with increases in triglycerides (TG) and low-density lipoprotein (LDL) and decreases in high-density lipoprotein (HDL) (30).
Dyslipidemia is an abnormal blood lipid profile resulting from increased cholesterol, LDL, TG, and decreased HDL (40) and is associated with atherosclerotic cardiovascular disease (37), ischemic stroke (17), and metabolic syndrome (MetS) (1). With the increasing rates of obesity in this age group and the subsequent increase in the risk of dyslipidemia, effective prevention strategies must be established to manage lipid profiles in obese young adults.
Aerobic exercise has been shown to improve lipid profiles by decreasing LDL and total cholesterol (TC), while increasing HDL (18). However, it has been observed that these favorable changes in lipid profiles were only seen following high-intensity aerobic exercise when compared to moderate-intensity exercise (39). It is important to note, however, that this single study was conducted on individuals older than our target age group. Additionally, the differences in lipid profiles between low and high-intensity aerobic exercise could be a function differing rates of adaptations in lipid profiles between the two exercise modalities. It has been shown that exercise intensity has a significant positive correlation with levels of growth hormone, epinephrine, and fat oxidation. Growth hormone and epinephrine have a lipolytic effect, and with the additional increase in fat oxidation, this could positively affect lipid levels in individuals (4,43,50). With high-intensity interval training (HIIT) having a greater exercise intensity than moderate-intensity continuous training (MICT), it is possible that greater elevations in growth hormone, epinephrine, and subsequent fat oxidation with HIIT would affect lipid profiles differently than MICT. Thus, while varying forms of aerobic exercise may improve patients’ lipid profiles, it is important to understand which forms of aerobic exercise work best for specific age groups and metabolic profiles.
HIIT is an exercise composed of alternating short to long stretches of high-intensity exercise, which are spaced by recovery periods of varying lengths (9, 63). Olympians have utilized HIIT in the past to increase performance (7). More recently, HIIT has been popularized among nonathletes to improve cardiovascular health (34), weight loss (64), and insulin sensitivity (46). MICT is a more traditional form of exercise (especially among non-athletes) that involves steady and continuous effort, without rest intervals, such as cycling or jogging. Previous reviews of the literature aimed at comparing the effect of HIIT vs. MICT have suggested that HIIT was not more effective than MICT at improving blood lipid profiles (28, 65). However, one of these did not perform a meta-analysis (28), and the other did not analyze a standardized patient population (65). Exercise protocols to improve health conditions should be supported by data and research specific to each patient population (38). This specificity will allow for the best outcomes in patients with dyslipidemia, as the training recommendations can be tailored to their unique physiology. To our knowledge, this is the first meta-analysis aimed at examining the effects of HIIT vs. MICT on blood lipid profiles in a homogenous population of non-diabetic obese young adults between 18–30 years old. We hope that through this analysis, this specific population will have a better understanding of protocols that may improve their dyslipidemia.
Thus, this study aims to conduct a meta-analysis to compare the effects of HIIT versus MICT and sedentary controls (CON) on HDL, LDL, TC, and TG in nondiabetic overweight and obese young adults. We hypothesize that HIIT will lead to greater improvements in these measures of dyslipidemia than MICT in this population.
METHODS
To achieve the aim of this paper, this analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (36), and its results are presented accordingly.
Participants
Studies included in this meta-analysis had to meet the following criteria: a) Studies had to be randomized controlled trials, controlled trials, or quasi-experimental studies. b) Studies had to measure the effects on humans of a HIIT intervention versus MICT or sedentary controls on at least three of the four metrics of interest: LDL, HDL, TG, and TC. The specific form of HIIT was not an inclusion or exclusion criterion. The study only had to have HIIT as the intervention form of exercise. c) Studies had to have more than ten total participants to allow for adequate sample size in the meta-analysis. d) Participants had to be categorized as overweight or obese through average body mass index (BMI) values of > 25 or > 30, respectively, as these are the categorizations of obesity set by the World Health Organization (WHO) (66). e) Participants’ average age had to be between 18 – 30 years old. f) Studies had to be published before December 1st, 2021. g) Studies had to be written in English. h) Studies had to be freely available on PubMed. i) Studies had to present data in tables with mean and standard deviation clearly reported. This was to ensure that included studies provided numerical values for mean and standard deviation (or standard error) both pre- and post-intervention which are required for use in the meta-analysis. Studies that did not meet the eligibility criteria were excluded from the meta-analysis.
Protocol
A systematic search process was utilized to identify potential studies for analysis. Author C.M. performed a thorough search of English-language results in PubMed from inception to December 2021. The search terms were: “The effects of HIIT on (blood pressure OR BMI OR body composition OR body fat mass OR bodyweight OR LDL OR HDL OR Triglycerides OR lean body mass OR VO2max).” The search was limited to publications listed in PubMed that were freely available in their entirety, as we lacked the institutional resources allowing us to access non-freely available articles. The search was limited to publications dated before December 1st, 2021. The abstract and title were screened to remove papers that did not meet the selection criteria by author C.M.. A full review of the papers was performed by author C.M., to identify studies that measured the metrics of interest (LDL, HDL, TC, and TG) and conducted the trials on the population of interest (nondiabetic overweight and obese young adults). During the systematic search and selection of papers for inclusion in the meta-analysis, if it was questionable if a study met the strict selection criteria, it was not included in the meta-analysis.
We extracted data from the included studies, specifically: author, year of publication, number of subjects, biological sex, age, initial BMI, exercise type, frequency and duration of the training, training protocols, and mean and standard deviation of PRE and POST concentrations of LDL, HDL, TC, and TG. If not reported in mg/dL, concentration measures were standardized to mg/dL through unit conversion. For TC, HDL, and LDL the units were converted using 1 mmol/L = 38.67 mg/dL (45). For TG the units were converted as 1 mmol/L = 88.57 mg/dL (45).
The quality of the studies was determined using the Physiotherapy Evidence Database Scale (PEDro) (41, 61). The PEDro scale evaluates each study along ten metrics indicative of methodological quality. The score for each study is summed, with scores ranging from 6 to 10 indicating high quality, 4–5 indicating acceptable quality, and a score lower than 3 showing low quality (60). Studies with a PEDro scale of 4 and higher were included in the meta-analysis to ensure every study was of acceptable or high methodological quality. The Cochrane Collaboration tool was utilized to determine the risk of bias in the randomized trials, with higher values (on a scale of 1–6) indicating a lower risk of bias (20). Author C.M. assessed the quality of the studies and the risk of bias using the PEDro and Cochrane scales as described above.
Statistical Analysis
The statistical analyses were performed using the Revman 5.4.1 software (The Nordic Cochrane Centre, Copenhagen, Denmark). The tests for heterogeneity and overall effect were performed by Revman 5.4.1 as well. The properties used in the Revman statistical analysis were as follows: 1) Continuous data, 2) Random-effects inverse-variance analysis model and statistical method, and 3) 95% study and total confidence interval. This statistical analysis model was used to allow for different effect sizes, as found across the studies included in our meta-analysis (8).
Revman 5.4.1 bases its statistical analysis on the mean difference (MD) and standard deviation (SD) of the MD, as well as the sample size for each study. For studies where the MD and SD of the MD were not reported by the study authors, the MD and SD of the MD were calculated as follows (65):
(1) MD = Meanpost-intervention − Meanpre-intervention
(2) SD = [ (SDpre-treatment)2 + (SDpost-treatment)2 − (2r * SDpre-treatment * SDpost-treatment) ]½
A correlation coefficient of (r) = 0.5 was used, which is considered a conservative estimate (15). For studies that provided their data in Standard Error (SE), the following equation was used to calculate SD:
(3) SE = SD / (N)1/2
Studies were grouped in the meta-analysis according to the lipid fractions they analyzed and the training protocols they utilized. The data from each study were pooled for analysis. P-values for the overall effect were calculated, with statistical significance indicated by p ≤ 0.05 and strong statistical significance indicated by p ≤ 0.001 (48). The heterogeneity of the studies was quantified using the I2 test, in which values ranged from 100% (indicative of complete heterogeneity) to 0% (indicative of complete homogeneity) (21).
RESULTS
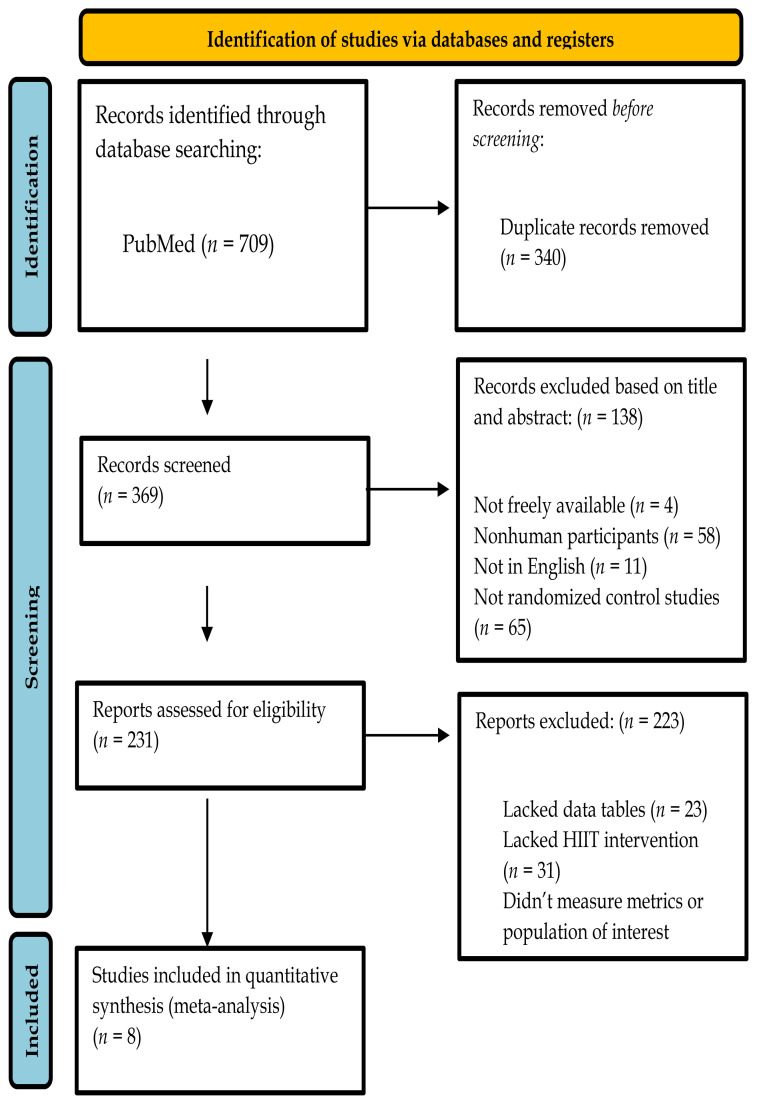
The combined searches generated a total of 709 articles. From the total, 340 papers were identified as duplicates and removed before abstract and title review. After reviewing the title and abstract of the 369 papers, 138 papers were removed. Four articles were not freely available on PubMed, 58 articles utilized nonhuman participants, 11 were not published in English, and 65 were not the desired type of study outlined in the “Study Selection and Eligibility Criteria” section, with the most common non-compliant type being review articles. A total of 231 papers underwent a complete analysis, and 223 were removed. Of the 223, 23 were removed for not presenting data in data tables, 31 lacked HIIT interventions, and 169 did not measure the metrics of interest or the desired population, as described above. The remaining eight studies were used in the meta-analysis (6, 14, 16, 29, 31, 49, 57, 68). A flow chart of the study selection can be found in Figure 1.
Figure 1.
Flow chart for the selection of studies in the meta-analysis.
Characteristics of Included Studies
The characteristics of the studies utilized in this meta-analysis are presented in Table 1. The selected studies were published between 2015 and 2021. The total sample size was 286 participants, with 118 in HIIT treatment groups, 88 in MICT treatment groups, and 80 as sedentary controls. Four studies contained female participants (6, 31, 49, 68), and four included male participants (14, 16, 29, 57), with no studies including both males and females. The majority of included studies did not detail the ethnicity of the participants used in the study, so ethnicity was not considered in the meta-analysis. The duration of the trials varied from 3 (14, 16, 29) weeks to 26 (6) weeks, with the frequency ranging from 3 (6, 14, 16, 29, 68) to 5 (14) sessions per week. Cycle ergometer exercise was used for five of the studies (14, 31, 49, 57, 68), outdoor running was used in one (29), treadmill running was used in one (16), and cardio equipment of choice was used in two of the studies (6). The study by Benham et al. (6) includes the measurement of the participants following HIIT protocols of two different lengths, and each protocol is considered separately in the analysis.
Table 1.
Characteristics of the studies included in the meta-analysis
| Study (A-Z) | Study Design | Participants n, gender | Average Age (Y) | Initial BMI | Exercise Type, HIIT/MICT/SED Protocol | Sessions per Week | Duration (Weeks) |
|---|---|---|---|---|---|---|---|
| Benham et al., 2021 a. (4) | RCT | 42 - Female | 29.2 | 31.4 | Cardio equipment of choice: HIIT 10x 30s @ 90% HRR w/90s low-intensity active rest. MICT: 40mins @ 50–60% HRR | 3 | 13 |
| Benham et al., 2021 b. (4) | RCT | 38 - Female | 29.2 | 31.4 | Cardio equipment of choice: HIIT 10x 30s @ 90% HRR w/90s low-intensity active rest. MICT: 40mins @ 50–60% HRR | 3 | 26 |
| Fisher et al., 2015 (10) | RCT | 38 - Male | 20 | 29.5 | Ergocycle: HIIT: 4x 30s @ 85% Max-AP w/4 min active rest @ 15% Max-AP. MICT: 45–60 min @ 55–65% VO2peak | 3 - HIIT; 5 - MICT | 3 |
| Gerosa-Neto et al., 2019 (12) | RCT | 26 - Male | 29.6 | 35.1 | Treadmill: HIIT: 10x 1 min @ 100% MAV w/1 min passive rest. MICT: 30 min @ 65% MAV | 3 | 3 |
| Khammassi et al., 2018 (24) | RCT | 20 - Male | 18–21 | 29.2 | Running: HIIT: 5–9x 30s @ 100% MAV w/30s active rest @ 50% MAV. Sedentary Control | 3 | 3 |
| Kong et al., 2016 (26) | RCT | 26 - Female | 21 | 25.7 | Ergocycle: HIIT: 60x 8s @ 100% VO2peak, MICT:40 min @ 60% of VO2 peak | 4 | 5 |
| Soltani et al., 2020 (41) | QET | 30 - Female | 18–25 | 29.2 | Ergocycle + Resistance Training: HIIT+RT: 4x (45s 75–90%, 15s transition, 45s 50–70% 1RM, 15s transition, 45s 75–90%, 15s transition, 45s 50–70 1RM, 3min active rest. Sedentary Control | 4 | 10 |
| Tucker et al., 2021 (46) | RCT | 28 - Male | 29 | 29.8 | Ergocycle: HIIT: 8–11x 1 min @ 90–95% HRmax. MICT: 30–45 min @ 50% VO2 max, Sedentary Control | 4 | 4 |
| Zapata-Lamana et al., 2018 (56) | RCT | 48 - Female | 21.7 | 32.4 | Ergocycle: HIIT: 4 × (4 × 60s @ 90% VO2 peak with 120s active recovery between sets) MICT: 45–50 min at 95% of pVT1 | 3 | 12 |
RCT - Randomized Control Trial; QET - Quasi-Experimental Trial; HRR - Heart Rate Reserve; Max-AP - Maximum Anaerobic Power; MAV - Maximum Aerobic Volume;
Measure of Study Quality
The PEDro quality scale resulted in a mean score of 5.8 for the included papers. The scores ranged from 4 to 8, with higher scores indicative of better quality. Eight studies stated the eligibility criteria, and all studies randomly allocated participants to treatment groups and had groups matched at baseline. No studies performed blinding of the subjects, while three performed blinding to the consultants who measured key outcomes. All groups reported between-group statistical analysis results and provided point estimates for effect size. The PEDro Scale for each study and scoring criteria are provided in Table 2 (41, 47).
Table 2.
The results of the PEDro Scale for included studies, with the criteria listed below.
| Study | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | Score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Benham et al., 2021 a. (4) | + | + | + | + | − | − | + | + | + | + | + | 8 |
| Benham et al., 2021 b. (4) | + | + | + | + | − | − | + | + | + | + | + | 8 |
| Fisher et al., 2015 (10) | + | + | + | + | − | − | − | − | + | + | + | 6 |
| Gerosa-Neto et al., 2019 (12) | + | + | − | + | − | − | − | − | − | + | + | 4 |
| Khammassi et al., 2018 (24) | − | + | − | + | − | − | − | − | − | + | + | 4 |
| Kong et al., 2016 (26) | + | + | − | + | − | − | − | − | − | + | + | 4 |
| Soltani et al., 2020 (41) | + | + | + | + | − | − | + | + | − | + | + | 7 |
| Tucker et al., 2021 (46) | + | + | − | + | − | − | + | + | − | + | + | 6 |
| Zapata-Lamana et al., 2018 (56) | + | + | − | + | − | − | − | − | + | + | + | 5 |
The numbers of the columns correspond to the following items of the PEDro scale:
- Eligibility criteria were specified (not included in the score).
- Subjects were randomly allocated to groups.
- Allocations were concealed.
- The groups were similar at baseline regarding the most important prognostic indicator.
- There was blinding of all subjects.
- There was blinding of all therapists who administered the therapy.
- There was blinding of all consultants who measured at least one key outcome.
- Measures of at least one key outcome were obtained from more than 85% of the subjects initially allocated to groups.
- All subjects for whom outcome measures were available received the treatment or control as allocated or, where this was not the case, data for at least one key outcome was analyzed by intention to treat.
- The results of between-group statistical comparisons are reported for at least one key outcome.
- The study provides both point measurements and measures of variability for at least one key outcome.
The Cochrane Scale was used to assess the risk of bias within the studies included in the meta-analysis. All studies had low levels of risk in the categories of random sequence generation, incomplete outcome data, and selective reporting. Four papers indicated low bias in allocation concealment. All papers indicated a high risk regarding the participant and personnel blinding. Four papers indicated low risk in outcome assessment blinding. On average, each study scored 4.375 out of 6, with higher scores indicating a lower risk of bias. The entire risk of bias assessment can be observed in Table 3 (20, 47).
Table 3.
The results of the Cochrane Scale for included studies, with the criteria listed below.
| Study | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| Benham et al., 2021 a. (4) | + | + | − | + | + | + |
| Benham et al., 2021 b. (4) | + | + | − | + | + | + |
| Fisher et al., 2015 (10) | + | + | − | − | + | + |
| Gerosa-Neto et al., 2019 (12) | + | − | − | − | + | + |
| Khammassi et al., 2018 (24) | + | − | − | − | + | + |
| Kong et al., 2016 (26) | + | − | − | − | + | + |
| Soltani et al., 2020 (41) | + | + | − | + | + | + |
| Tucker et al., 2021 (46) | + | − | − | + | + | + |
| Zapata-Lamana et al., 2018 (56) | + | − | − | − | + | + |
Risk of bias assessment of the included studies. (+) indicates a low risk of bias, (?) indicates an unclear risk of bias, and (−) indicates a high risk of bias:
- Random sequence generation (selection bias).
- Allocation concealment (selection bias).
- Blinding (participants and personnel (performance bias).
- Blinding (outcome assessment) (detection bias).
- Incomplete outcome data (attrition bias).
- Selective reporting (reporting bias).
Blood Lipid Fractions
The full results of the data analysis for blood lipid fractions are included in Figures 2 through 9. Each figure contains the mean, standard deviation, sample size, weighting, and 95% Confidence Interval (CI) for each study, as well as the overall forest plot, heterogeneity, and statistical significance tests. The x-axis labels in the forest plot of “Favours HIIT, “Favours CON,” and “Favours MICT” indicate whether the results of the individual study or overall meta-analysis found HIIT, CON, or MICT to have a larger effect on the analyzed lipid fraction. Thus, if the diamond on the forest plot is predominantly on the “Favours HIIT” compared to the “Favours CON” side, it indicates that HIIT decreased the concentration of the lipid fraction to a larger degree, compared to CON.
Figure 2.
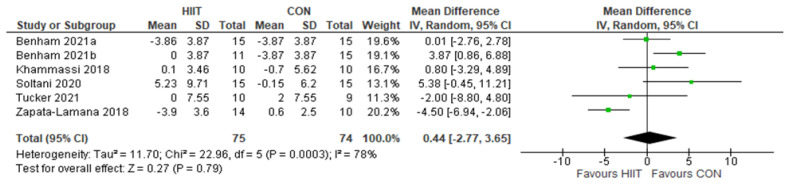
HDL: HIIT v. CON: High-density lipoprotein-cholesterol. Mean (Mean Difference = MeanHIIT - MeanCON) and SD values are expressed as mg/dL. Total indicates the sample size. HIIT, high-intensity interval training. CON, control group.
Figure 3.
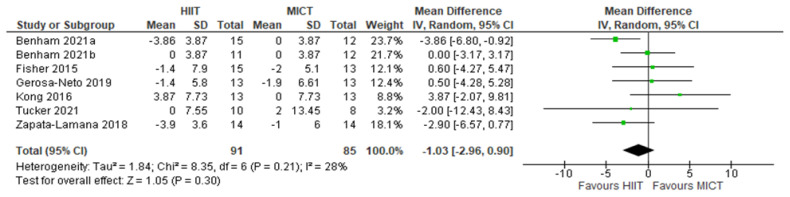
HDL: HIIT v. MICT: High-density lipoprotein-cholesterol. Mean (Mean Difference = MeanHIIT - MeanMICT) and SD values are expressed as mg/dL. Total indicates the sample size. HIIT, high-intensity interval training. MICT, moderate-intensity continuous training.
Figure 4.
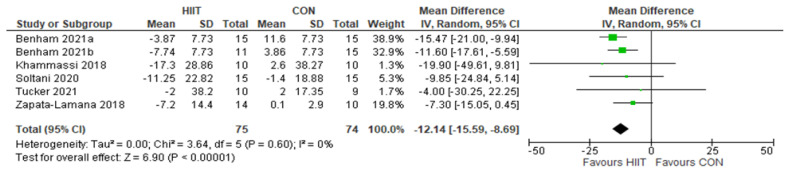
LDL: HIIT v. CON: Low-density lipoprotein-cholesterol. Mean (Mean Difference = MeanHIIT - MeanCON) and SD values are expressed as mg/dL. Total indicates the sample size. HIIT, high-intensity interval training. CON, control group.
Figure 5.
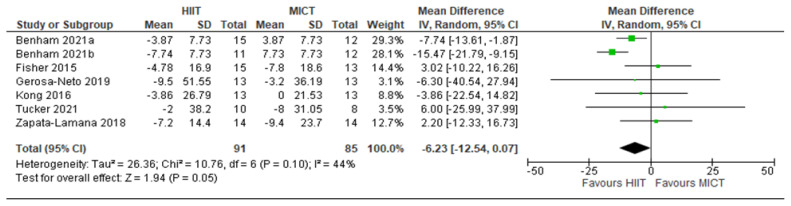
LDL: HIIT v. MICT: Low-density lipoprotein-cholesterol. Mean (Mean Difference = MeanHIIT - MeanMICT) and SD values are expressed as mg/dL. Total indicates the sample size. HIIT, high-intensity interval training. MICT, moderate-intensity continuous training.
Figure 6.
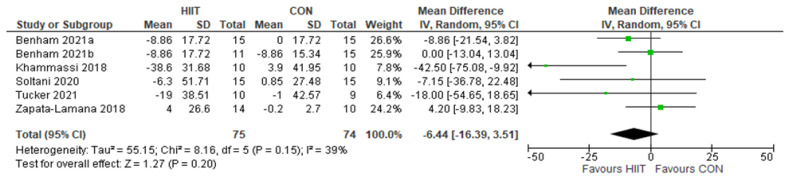
TG: HIIT v. CON: Triglycerides. Mean (Mean Difference = MeanHIIT - MeanCON) and SD values are expressed as mg/dL. Total indicates the sample size. HIIT, high-intensity interval training. CON, control group.
Figure 7.
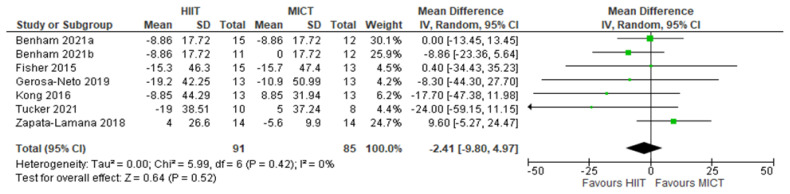
TG: HIIT v. MICT: Triglycerides. Mean (Mean Difference = MeanHIIT - MeanMICT) and SD values are expressed as mg/dL. Total indicates the sample size. HIIT, high-intensity interval training. MICT, moderateintensity continuous training.
Figure 8.
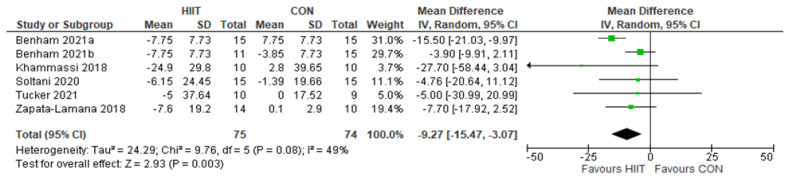
TC: HIIT v. CON: Total cholesterol. Mean (Mean Difference = MeanHIIT - MeanCON) and SD values are expressed as mg/dL. Total indicates the sample size. HIIT, high-intensity interval training. CON, control group.
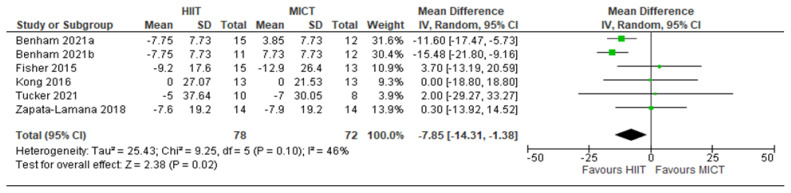
Figure 9.
TC: HIIT v. MICT: Total Cholesterol. Mean (Mean Difference = MeanHIIT - MeanMICT) and SD values are expressed as mg/dL. Total indicates the sample size. HIIT, high-intensity interval training. MICT, moderateintensity continuous training.
High-density lipoprotein cholesterol
Five studies analyzed the effects of HIIT compared to CON on HDL concentrations with a total of 149 participants (75 HIIT, 74 CON). HIIT did not have a significant impact on HDL concentration compared to sedentary controls with a MD (HIIT-CON) of 0.44 mg/dL (p = 0.79, I2 = 78%, 95% CI of −2.77 to 3.65 mg/dL); see Figure 2. Six studies analyzed the effects of HIIT compared to MICT on HDL concentrations with a total of 176 participants (91 HIIT, 85 MICT). HIIT did not have a significant impact on HDL compared to MICT with a MD (HIIT-MICT) of − 1.03 mg/dL (p = 0.30, I2 = 28%, 95% CI of −2.96 to 0.90 mg/dL); see Figure 3.
Low-density lipoprotein cholesterol
Five studies analyzed the effects of HIIT compared to CON on LDL concentrations with a total of 149 participants (75 HIIT, 74 CON). HIIT had a strongly significant impact on lowering LDL compared to sedentary controls with a MD (HIIT-CON) of −12.14 mg/dL (p < 0.00001, I2 = 0%, 95% CI of −15.59 to −8.69 mg/dL); see Figure 4. Six studies analyzed the effects of HIIT compared to MICT on LDL concentrations with a total of 176 participants (91 HIIT, 85 MICT). HIIT had a significant impact on lowering LDL compared to MICT with a MD (HIIT-MICT) of −6.23 mg/dL (p = 0.05, I2 = 44%, 95% CI of −12.54 to 0.07 mg/dL); see Figure 5.
Triglycerides
Five studies analyzed the effects of HIIT compared to CON on TG concentrations with a total of 149 participants (75 HIIT, 74 CON). HIIT did not have a significant impact on TG concentration compared to sedentary controls with a MD (HIIT-CON) of −6.44 mg/dL (p = 0.20, I2 = 39%, 95% CI of −16.39 to 3.51 mg/dL); see Figure 6. Six studies analyzed the effects of HIIT compared to MICT on TG concentrations with a total of 176 participants (91 HIIT, 85 MICT). HIIT did not have a significant impact on TG compared to MICT with a MD (HIIT-MICT) of −2.41 mg/dL (p = 0.52, I2 = 0%, 95% CI of −9.80 to 4.97 mg/dL); see Figure 7.
Total Cholesterol
Five studies analyzed the effects of HIIT compared to CON on TC concentrations with a total of 149 participants (75 HIIT, 74 CON). HIIT had a significant impact on decreasing TC compared to sedentary controls with a MD (HIIT-CON) of −9.27 mg/dL (p = 0.003, I2 = 49%, 95% CI of - 15.47 to −3.07 mg/dL); see Figure 8. Five studies analyzed the effects of HIIT compared to MICT on TC concentrations with a total of 150 participants (78 HIIT, 72 MICT). HIIT had a significant impact on decreasing TC compared to MICT with a MD (HIIT-MICT) of −7.85 mg/dL (p = 0.02, I2 = 46%, 95% CI of −14.31 to −1.38 mg/dL); see Figure 9.
DISCUSSION
Meta-analysis
This meta-analysis aimed to compare the effectiveness of HIIT versus MICT and sedentary controls in modulating the concentrations of HDL, LDL, TG, and TC in nondiabetic overweight and obese young adults. To the best of the authors’ knowledge, this analysis is the first study to directly compare HIIT vs MICT and CON on lipid fraction concentrations in nondiabetic overweight and obese young adults. This analysis demonstrates that HIIT has a statistically significant advantage over MICT and CON in decreasing the concentrations of LDL and TC, with there being no statistically significant effect on the concentrations of HDL or TG between the intervention and control groups.
Lipid Fractions
High-density lipoprotein cholesterol
HIIT did not improve HDL concentrations compared to MICT and CON. Our finding is similar to the results of other studies, such as recent systematic reviews (28,33,56) and meta-analyses (25,26). However, these studies did not look solely at the results of HIIT on lipid fraction concentrations. Instead, they were broader in their analysis and included studies that measured the change in lipid concentration following various exercise interventions, such as HIIT, MICT, resistance training (RT), HIIT + RT, and MICT + RT vs. sedentary controls. Our results differ from other studies that found HIIT to increase HDL concentrations slightly but used different populations, consisting of participants with BMI values ranging from healthy to obese (65), MetS participants (47,65), and obese adults of all ages (27). However, the studies that found HIIT to induce positive changes in HDL, measured a small effect of about a 3% increase in HDL values, which may not be of clinical significance. Regardless, with a larger sample size, our analysis would have been more sensitive to the effects of HIIT, and we may have found HIIT to induce a small positive change on HDL levels, as other studies have.
Previous studies have analyzed the implications of abnormal HDL concentrations in young adults on the quality of cardiovascular health in later adult life. For example, a study of 4860 participants indicated that low HDL levels in young adults do not independently contribute to cardiovascular disease (CVD) or coronary heart disease (CHD) events as they age (42). This result is consistent with another study that failed to find a correlation between abnormal HDL levels in young adults with the prevalence of myocardial infarctions in later life (62). Consequently, HDL may not be a critical factor in CVD risk among overweight and obese young adults (35). Therefore, while HIIT may not significantly alter HDL concentrations in overweight and obese young adults, there is limited data to suggest that positively affecting HDL concentrations would have any impact on the long-term cardiovascular health of the studied population. Further research is needed to fully determine the implication of HDL concentrations on long-term cardiovascular health and the clinical role exercise modalities such as HIIT may have on modulating HDL levels.
Low-density lipoprotein cholesterol
Our analysis demonstrates that HIIT decreases LDL concentration in our target population to a larger degree than MICT and CON. Our result agrees with recent meta-analyses that showed various aerobic and resistance exercise has a positive impact on LDL levels, compared to an absence of exercise, in the general population (25, 26). Additionally, our result is consistent with a meta-analysis that demonstrated HIIT was superior to MICT in positively altering LDL concentrations in overweight and obese adults of all ages (53), as opposed to examining solely young adults as we have here. Our result indicating HIIT has a larger effect on LDL than MICT is consistent with the literature, which suggests that the higher intensity associated with HIIT induces a larger release of growth hormone compared to MICT (43). This increased release of growth hormone after HIIT, compared to MICT, likely increases fat oxidation following completion of HIIT exercise (4). This increased lipid oxidation mediated by growth hormone release may partly explain why HIIT induces larger decreases in LDL compared to MICT. Our result disagrees with other meta-analyses (27, 67) that compared HIIT vs. CON and reported no significant changes in LDL following HIIT protocols. However, these studies utilized heterogeneous populations with regard to age and BMI. Our results may have differed from these studies, as they used healthy populations with LDL concentrations lower than obese populations (30). As such, these studies may be less sensitive to the effects of HIIT, as a portion of their populations are within normal levels and consequently have less room to decrease compared to those with elevated LDL levels, as in our study (18). Collectively, these results may indicate that HIIT is particularly effective for lowering LDL in our focus population.
It has been demonstrated that elevated LDL levels in young adults is associated with an increased prevalence of CVD events, such as coronary artery calcification (32), regardless of LDL levels in later adult life and the acquisition of other health risk factors (42). Specifically, if young adults have LDL concentrations that exceed 100 mg/dL, the risk of developing CHD was 64% higher than those with LDL concentrations lower than 100 mg/dL (69). Additionally, for every decrease of 40 mg/dL of LDL in young adults, there is a corresponding 20% decrease in coronary mortality in later life (5). Based on our 95% CI results, this correlation indicates that nondiabetic overweight and obese young adults may experience a 4.3% to 7.8% decrease in later life coronary mortality if they follow HIIT protocols compared to their sedentary peers. As such, clinicians could consider promoting HIIT protocols to their nondiabetic overweight and obese young adult patients, as engaging in HIIT exercise may lead to improvements in long-term cardiovascular health.
Triglycerides
HIIT did not appear to have a meaningful impact on TG compared to MICT or CON. Our result is similar to other studies that showed HIIT did not have a significant impact on TG concentrations among their study population (28, 53, 65, 67). Our result differs from other studies that found aerobic exercise (including but not limited to walking, jogging, cycling, and swimming in a large range of weekly frequencies, durations, and intensities as measured by %VO2max), decreased TG concentrations in adults of various ages, and BMI values (25, 26, 27).
However, the inclusion of participants with a wide range of BMI values may explain the difference in outcome between these studies and our own. It has been shown that healthy and trained individuals may oxidize TG at rates 17 times higher than obese and untrained individuals, when exercising at 85% of VO2max (3). This difference in lipid metabolism may be why studies including healthy participants saw decreases in TG, while our study containing untrained, overweight, and obese individuals did not reveal significant decreases in TG concentration following similar HIIT protocols. It is plausible that longer duration training protocols that result in significant changes in fitness (i.e., subjects become more ‘trained’) may more favorably affect TG concentrations in our target population.
There have been few studies investigating the effects of elevated TG levels in young adults on CVD prevalence in later life. However, it has been found that obesity is correlated to elevated TG levels (30). Additionally, TG is an independent risk factor for developing CVD, suggesting that elevated TG levels, especially in overweight and obese young adults, increases the risk of developing CVD in later life (22). Furthermore, it has been determined that individuals that have TG concentrations that place them in the middle tertile have a 50% increased risk of ischemic heart disease compared to those in the lowest tertile, with those in the highest tertile experiencing a 120% increased risk compared to those in the lowest tertile, even after adjusting for smoking, diabetes, physical activity, BMI, smoking and alcohol intake (24). Thus, despite the lack of direct evidence, lowering TG levels in nondiabetic overweight and obese young adults may decrease the risk of CVD in later life. While the benefits of exercise are well-documented and we encourage participation in exercise, our data suggests that HIIT may not be an effective clinical solution to modulating TG concentrations in our studied population. Other management strategies that are proven effective in managing TG should be considered, for example pharmaceutical alternatives such as Fibrates have been shown to improve TG concentrations in young adults (19).
Total Cholesterol
We found that HIIT led to a larger decrease in TC concentration in our population of interest compared to MICT and CON. Our result is consistent with other studies that did not differentiate between MICT and HIIT but found aerobic exercise to improve TC concentration in a participant population ranging from young to old adults with BMI values ranging from healthy to obese (25, 26, 27). Our results differ from a meta-analysis that found HIIT did not significantly impact TC compared to CON, in its nonhomogenous population with regards to age or BMI (28). However, that meta-analysis (28) drew its data from studies that primarily used participants whose baseline TC levels were within clinically healthy ranges, thus the opportunity for improvement in TC concentration may have been limited. Studies have found that MICT and HIIT induce similar changes in mitochondrial enzymes responsible for lipid oxidation rates, such as 3-hydroxyacyl CoA dehydrogenase (β-HAD) (10), in addition to similar increases in other factors associated with lipid metabolism such as the plasma membrane fatty acid binding protein (FABPpm) (55). This might suggest that the change in TC concentration might be similar between MICT and HIIT in some instances, in contrast to the results of our meta-analysis. Additional research on the mechanisms associated with the impact of exercise intensity on lipid concentrations are warranted to fully elucidate how HIIT and MICT might differentially impact TC concentration, particularly in our target population.
While the mechanism may not be clear, our result showing that HIIT positively affects TC is clinically relevant, as the literature suggests that young adults who decrease their TC levels may reduce the likelihood of developing CVD compared to their peers that maintain elevated TC levels (23). Specifically, for every 29.1 mg/dL decrease in TC, there is a corresponding decrease in ischemic heart disease of 2–4% for those in the middle tertile of TC concentration, and 6–9% for those in the highest tertile (23). Additionally, there is growing support for the “Cumulative Damage Hypothesis,” which suggests that the atherosclerotic changes induced by elevated TC concentrations begin at a young age (58). Specifically, 17% of teenagers 13 to 19 years old and 37% of young adults 20 to 29 years old possess atherosclerotic lesions, when categorizing lesions with the conservative threshold of 0.3mm (58). While these lesions are clinically benign (51), a higher prevalence of atherosclerotic lesions in children and young adults results in higher rates of damaging plaque build-up in later life (52). Cumulatively, the indication that HIIT decreases TC levels, combined with the correlation between decreasing TC levels benefiting and improving the cardiovascular health of young adults later in life, indicates that clinicians could consider implementing HIIT protocols for their nondiabetic overweight and obese young adult populations, to improve their long-term cardiovascular health.
Risk of Bias and Quality Assessment
After completing the PEDro and Cochrane scales, we conclude that the included studies were of acceptable quality and had a low risk of bias. The studies used in this meta-analysis had an average PEDro score of 5.8 which demonstrates the quality of the studies on the whole were acceptable (4–5) or high quality (6–10). For the Cochrane scale of risk of bias, the studies utilized in this analysis had an average value of 4.4 on a scale of 1–6, with higher values indicating a lower risk of bias. This indicates the studies included in our analysis were in the top tertile for low risk of bias. With the acknowledgment that none of the studies were free of risk bias, the PEDro and Cochrane analysis leads us to conclude that the risk of bias and quality of the included studies makes them appropriate to be included in the meta-analysis and does not limit the interpretation of our results.
Strengths and Limitations
To the authors’ knowledge, this quantitative meta-analysis is the first to compare the effects of HIIT vs. MICT and CON on HDL, LDL, TC, and TG in specifically nondiabetic overweight and obese young adults.
A limitation of this meta-analysis was that while a thorough search was conducted, it is possible that some studies were missed. Specifically, studies containing quality data published in languages other than English and not freely available on PubMed may not have been included in the analysis.
This meta-analysis drew its data from a relatively small number of studies that varied in training protocols. Specifically, the studies varied in the metrics of exercise intensity, interval length, number of intervals, the ratio of intensity vs. rest, utilization of passive or active rest, session frequency, study duration, and the mode of exercise. Additionally, the duration of the studies was relatively short. While the average length of the studies was 8.78 weeks, five of the included nine data sets had durations of less than six weeks. If the studies utilized longer training durations, further understanding of the impact of training on the lipid fractions may have been elucidated.
When the studies used in the current analysis did not provide the MD and the SD of the MD, the values had to be calculated as described previously in the “Statistical Analysis” section. Additionally, not all studies published results in the same units: mg/dL or mmol/L. Data conversion to mg/dL for use in the meta-analyses may have introduced uncertainty as the statistical analyses were based on extrapolated and converted data. The culmination of these limitations may have impacted the results of this quantitative meta-analysis.
CONCLUSION
Rates of both obesity and dyslipidemia among young adults have increased in recent decades due to a multitude of factors, including but not limited to decreased physical activity, decreased participation in sports, increased screen time, and increased daily calorie intake, according to a 2016 report from the American Heart Association (37). When considered in conjunction with data indicating obesity and dyslipidemia are correlated to increased risk of CVD, as described in the discussion, it is important that methods are explored to help manage and limit the adverse effects of these conditions with the goal of improving the long-term health of overweight and obese young adults. HIIT exercise shows potential to be an effective non-pharmaceutical intervention in the management of these health issues. The results of our meta-analysis show that HIIT was more effective than MICT and CON in decreasing the concentration of LDL and TC in our target population of non-diabetic, overweight, and obese young adults. However, HIIT did not have a significant impact on the concentrations of HDL or TG compared to MICT and CON. As improving the concentrations of LDL and TC in early adulthood has been demonstrated to potentially decrease the prevalence of CVD and CHD in later life, clinicians might consider prescribing HIIT training as a component of their treatment plan, as HIIT interventions may play a role in improving long-term cardiovascular health. However, more research is required before HIIT can be definitively recommended as a method to improve lipid concentrations in all patient populations.
Exercise is only one part of a complete regimen to improve health. To experience the largest modification in lipid fractions, one should incorporate exercise into a healthy lifestyle focusing on eating a healthy diet (44), getting quality sleep (2), and avoiding cigarettes (54) and alcohol (12). Thus, while HIIT may have a significant impact on one’s lipid concentrations, it is just one of many factors that lead to a healthy life free from disease and chronic illness.
ACKNOWLEDGEMENTS
The authors would like to thank all the referenced researchers, as well as all the researchers whose work was read, but not included in the study. The authors would also like to thank Dr. Brook Swanson from Gonzaga University for their help with the initial shaping of the project that became this study.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- 1.Alberti KGMM, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120(16):1640–5. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 2.Araghi MH, Thomas GN, Taheri S. The potential impact of sleep duration on lipid biomarkers of cardiovascular disease. Clin Lipidol. 2012;7(4):443–53. [Google Scholar]
- 3.Aslankeser Z, Balcı ŞS. Re-examination of the contribution of substrates to energy expenditure during high-intensity intermittent exercise in endurance athletes. PeerJ. 2017;5:e3769. doi: 10.7717/peerj.3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bahr R, Høstmark AT, Newsholme EA, Grønnerød O, Sejersted OM. Effect of exercise on recovery changes in plasma levels of FFA, glycerol, glucose and catecholamines. Acta Physiol Scand. 1991;143(1):105–15. doi: 10.1111/j.1748-1716.1991.tb09205.x. [DOI] [PubMed] [Google Scholar]
- 5.Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, et al. Efficacy and safety of cholesterol-lowering treatment: Prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet Lond Engl. 2005;366(9493):1267–78. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 6.Benham JL, Booth JE, Corenblum B, Doucette S, Friedenreich CM, Rabi DM, et al. Exercise training and reproductive outcomes in women with polycystic ovary syndrome: A pilot randomized controlled trial. Clin Endocrinol (Oxf) 2021;95(2):332–43. doi: 10.1111/cen.14452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Billat LV. Interval training for performance: a scientific and empirical practice. Special recommendations for middle- and long-distance running. Part I: Aerobic interval training. Sports Med Auckl NZ. 2001;31(1):13–31. doi: 10.2165/00007256-200131010-00002. [DOI] [PubMed] [Google Scholar]
- 8.Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods. 2010;1(2):97–111. doi: 10.1002/jrsm.12. [DOI] [PubMed] [Google Scholar]
- 9.Buchheit M, Laursen PB. High-intensity interval training, solutions to the programming puzzle: Part I: Cardiopulmonary emphasis. Sports Med Auckl NZ. 2013;43(5):313–38. doi: 10.1007/s40279-013-0029-x. [DOI] [PubMed] [Google Scholar]
- 10.Burgomaster KA, Howarth KR, Phillips SM, Rakobowchuk M, MacDonald MJ, McGee SL, et al. Similar metabolic adaptations during exercise after low volume sprint interval and traditional endurance training in humans. J Physiol. 2008;586(Pt 1):151–60. doi: 10.1113/jphysiol.2007.142109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Center for Disease Control. NHANES 2017-March 2020 Pre-pandemic. n.d. Available at: https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/default.aspx?Cycle=2017-2020.
- 12.Crouse JR, Grundy SM. Effects of alcohol on plasma lipoproteins and cholesterol and triglyceride metabolism in man. J Lipid Res. 1984;25(5):486–96. [PubMed] [Google Scholar]
- 13.Ellison-Barnes A, Johnson S, Gudzune K. Trends in obesity prevalence among adults aged 18 through 25 years, 1976–2018. JAMA. 2021;326(20):2073–4. doi: 10.1001/jama.2021.16685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fisher G, Brown AW, Bohan Brown MM, Alcorn A, Noles C, Winwood L, et al. High intensity interval- vs moderate intensity-training for improving cardiometabolic health in overweight or obese males: A randomized controlled trial. PloS One. 2015;10(10):e0138853. doi: 10.1371/journal.pone.0138853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fu R, Vandermeer BW, Shamliyan TA, O’Neil ME, Yazdi F, Fox SH, et al. Methods Guide for Effectiveness and Comparative Effectiveness Reviews. Rockville (MD): Agency for Healthcare Research and Quality (US); 2008. Handling continuous outcomes in quantitative synthesis [Internet] [cited 2022 Sep 15] [PubMed] [Google Scholar]
- 16.Gerosa-Neto J, Panissa VLG, Monteiro PA, Inoue DS, Ribeiro JPJ, Figueiredo C, et al. High- or moderate-intensity training promotes change in cardiorespiratory fitness, but not visceral fat, in obese men: A randomised trial of equal energy expenditure exercise. Respir Physiol Neurobiol. 2019;266:150–5. doi: 10.1016/j.resp.2019.05.009. [DOI] [PubMed] [Google Scholar]
- 17.Goldstein LB, Adams R, Becker K, Furberg CD, Gorelick PB, Hademenos G, et al. Primary prevention of ischemic stroke: A statement for healthcare professionals from the Stroke Council of the American Heart Association. Stroke. 2001;32(1):280–99. doi: 10.1161/01.str.32.1.280. [DOI] [PubMed] [Google Scholar]
- 18.Greene NP, Martin SE, Crouse SF. Acute exercise and training alter blood lipid and lipoprotein profiles differently in overweight and obese men and women. Obes Silver Spring Md. 2012;20(8):1618–27. doi: 10.1038/oby.2012.65. [DOI] [PubMed] [Google Scholar]
- 19.Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the management of blood cholesterol: A report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation. 2019;139(25):e1082–143. doi: 10.1161/CIR.0000000000000625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hokanson JE, Austin MA. Plasma triglyceride level is a risk factor for cardiovascular disease independent of high-density lipoprotein cholesterol level: A meta-analysis of population-based prospective studies. J Cardiovasc Risk. 1996;3(2):213–9. [PubMed] [Google Scholar]
- 23.Jeong S-M, Choi S, Kim K, Kim SM, Lee G, Park SY, et al. Effect of change in total cholesterol levels on cardiovascular disease among young adults. J Am Heart Assoc. 2018;7(12):e008819. doi: 10.1161/JAHA.118.008819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jeppesen J, Hein HO, Suadicani P, Gyntelberg F. Triglyceride concentration and ischemic heart disease: An eight-year follow-up in the Copenhagen Male Study. Circulation. 1998;97(11):1029–36. doi: 10.1161/01.cir.97.11.1029. [DOI] [PubMed] [Google Scholar]
- 25.Kelley GA, Kelley KS. Aerobic exercise and lipids and lipoproteins in men: A meta-analysis of randomized controlled trials. J Mens Health Gend. 2006;3(1):61–70. doi: 10.1016/j.jmhg.2005.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kelley GA, Kelley KS, Tran ZV. Aerobic exercise and lipids and lipoproteins in women: A meta-analysis of randomized controlled trials. J Womens Health. 2002;2004;13(10):1148–64. doi: 10.1089/jwh.2004.13.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kelley GA, Kelley KS, Vu Tran Z. Aerobic exercise, lipids and lipoproteins in overweight and obese adults: A meta-analysis of randomized controlled trials. Int J Obes. 2005;2005;29(8):881–93. doi: 10.1038/sj.ijo.0802959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kessler HS, Sisson SB, Short KR. The potential for high-intensity interval training to reduce cardiometabolic disease risk. Sports Med Auckl NZ. 2012;42(6):489–509. doi: 10.2165/11630910-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 29.Khammassi M, Ouerghi N, Hadj-Taieb S, Feki M, Thivel D, Bouassida A. Impact of a 12-week high-intensity interval training without caloric restriction on body composition and lipid profile in sedentary healthy overweight/obese youth. J Exerc Rehabil. 2018;14(1):118–25. doi: 10.12965/jer.1835124.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klop B, Elte JWF, Cabezas MC. Dyslipidemia in obesity: Mechanisms and potential targets. Nutrients. 2013;5(4):1218–40. doi: 10.3390/nu5041218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kong Z, Fan X, Sun S, Song L, Shi Q, Nie J. Comparison of high-intensity interval training and moderate-tovigorous continuous training for cardiometabolic health and exercise enjoyment in obese young women: A randomized controlled trial. PloS One. 2016;11(7):e0158589. doi: 10.1371/journal.pone.0158589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Loria CM, Liu K, Lewis CE, Hulley SB, Sidney S, Schreiner PJ, et al. Early adult risk factor levels and subsequent coronary artery calcification: The CARDIA Study. J Am Coll Cardiol. 2007;49(20):2013–20. doi: 10.1016/j.jacc.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 33.Mann S, Beedie C, Jimenez A. Differential effects of aerobic exercise, resistance training, and combined exercise modalities on cholesterol and the lipid profile: Review, synthesis and recommendations. Sports Med Auckl NZ. 2014;44(2):211–21. doi: 10.1007/s40279-013-0110-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martin-Smith R, Cox A, Buchan DS, Baker JS, Grace F, Sculthorpe N. High intensity interval training (HIIT) improves cardiorespiratory fitness (CRF) in healthy, overweight and obese adolescents: A systematic review and meta-analysis of controlled studies. Int J Environ Res Public Health. 2020;17(8):E2955. doi: 10.3390/ijerph17082955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.März W, Kleber ME, Scharnagl H, Speer T, Zewinger S, Ritsch A, et al. HDL cholesterol: Reappraisal of its clinical relevance. Clin Res Cardiol Off J Ger Card Soc. 2017;106(9):663–75. doi: 10.1007/s00392-017-1106-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann Intern Med. 2009;151(4):264–9. W64. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 37.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Executive summary: Heart disease and stroke statistics--2016 update: A report from the American Heart Association. Circulation. 2016;133(4):447–54. doi: 10.1161/CIR.0000000000000366. [DOI] [PubMed] [Google Scholar]
- 38.Müller P, Rehfeld K, Schmicker M, Müller N. P52. Future directions for physical exercise as personalized medicine. Clin Neurophysiol. 2018;129(8):e88. [Google Scholar]
- 39.O’Donovan G, Owen A, Bird SR, Kearney EM, Nevill AM, Jones DW, et al. Changes in cardiorespiratory fitness and coronary heart disease risk factors following 24 wk of moderate- or high-intensity exercise of equal energy cost. J Appl Physiol. 1985;2005;98(5):1619–25. doi: 10.1152/japplphysiol.01310.2004. [DOI] [PubMed] [Google Scholar]
- 40.Pappan N, Rehman A. StatPearls. Treasure Island (FL): StatPearls Publishing; 2022. Dyslipidemia [Internet] [cited 2022 Sep 15] [Google Scholar]
- 41.PEDro. PEDro scale [Internet] 1999. Available at: https://www.pedro.org.au/wpcontent/uploads/PEDro_scale.pdf.
- 42.Pletcher MJ, Vittinghoff E, Thanataveerat A, Bibbins-Domingo K, Moran AE. Young adult exposure to cardiovascular risk factors and risk of events later in life: The Framingham Offspring study. PloS One. 2016;11(5):e0154288. doi: 10.1371/journal.pone.0154288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pritzlaff CJ, Wideman L, Blumer J, Jensen M, Abbott RD, Gaesser GA, et al. Catecholamine release, growth hormone secretion, and energy expenditure during exercise vs. recovery in men. J Appl Physiol. 1985;2000;89(3):937–46. doi: 10.1152/jappl.2000.89.3.937. [DOI] [PubMed] [Google Scholar]
- 44.Iggman D, Rosqvist F, Larsson A, Arnlöv J, Beckman L, Rudling M, et al. Role of dietary fats in modulating cardiometabolic risk during moderate weight gain: a randomized double-blind overfeeding trial (LIPOGAIN study) J Am Heart Assoc. 2014;3(5):e001095. doi: 10.1161/JAHA.114.001095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rugge B, Balshem H, Sehgal R, Relevo R, Gorman P, Helfand M. Screening and treatment of subclinical hypothyroidism or hyperthyroidism. Rockville, MD: Agency for Healthcare Research and Quality (US); 2011. [PubMed] [Google Scholar]
- 46.Ryan BJ, Schleh MW, Ahn C, Ludzki AC, Gillen JB, Varshney P, et al. Moderate-intensity exercise and high-intensity interval training affect insulin sensitivity similarly in obese adults. J Clin Endocrinol Metab. 2020;105(8):dgaa345. doi: 10.1210/clinem/dgaa345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Serrablo-Torrejon I, Lopez-Valenciano A, Ayuso M, Horton E, Mayo X, Medina-Gomez G, et al. High intensity interval training exercise-induced physiological changes and their potential influence on metabolic syndrome clinical biomarkers: a meta-analysis. BMC Endocr Disord. 2020;20(1):167. doi: 10.1186/s12902-020-00640-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Singh P. P value, statistical significance, and clinical significance. J Clin Prev Cardiol. 2013;2(4):202–4. [Google Scholar]
- 49.Soltani N, Marandi SM, Kazemi M, Esmaeil N. Meta-inflammatory state and insulin resistance can improve after 10 weeks of combined all-extremity high-intensity interval training in sedentary overweight/obese females: A quasi-experimental study. J Diabetes Metab Disord. 2020;19(2):717–26. doi: 10.1007/s40200-020-00550-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Soria M, Ansón M, Lou-Bonafonte JM, Andrés-Otero MJ, Puente JJ, Escanero J. Fat oxidation rate as a function of plasma lipid and hormone response in endurance athletes. J Strength Cond Res. 2020;34(1):104–13. doi: 10.1519/JSC.0000000000003034. [DOI] [PubMed] [Google Scholar]
- 51.Steinberg D. Earlier intervention in the management of hypercholesterolemia: What are we waiting for? J Am Coll Cardiol. 2010;56(8):627–9. doi: 10.1016/j.jacc.2009.12.057. [DOI] [PubMed] [Google Scholar]
- 52.Strong JP, Malcom GT, McMahan CA, Tracy RE, Newman WP, Herderick EE, et al. Prevalence and extent of atherosclerosis in adolescents and young adults: Implications for prevention from the Pathobiological Determinants of Atherosclerosis in Youth study. JAMA. 1999;281(8):727–35. doi: 10.1001/jama.281.8.727. [DOI] [PubMed] [Google Scholar]
- 53.Su L, Fu J, Sun S, Zhao G, Cheng W, Dou C, et al. Effects of HIIT and MICT on cardiovascular risk factors in adults with overweight and/or obesity: A meta-analysis. PloS One. 2019;14(1):e0210644. doi: 10.1371/journal.pone.0210644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Szkup M, Jurczak A, Karakiewicz B, Kotwas A, Kopeć J, Grochans E. Influence of cigarette smoking on hormone and lipid metabolism in women in late reproductive stage. Clin Interv Aging. 2018;13:109–15. doi: 10.2147/CIA.S140487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Talanian JL, Galloway SDR, Heigenhauser GJF, Bonen A, Spriet LL. Two weeks of high-intensity aerobic interval training increases the capacity for fat oxidation during exercise in women. J Appl Physiol. 1985;2007;102(4):1439–47. doi: 10.1152/japplphysiol.01098.2006. [DOI] [PubMed] [Google Scholar]
- 56.Tambalis K, Panagiotakos DB, Kavouras SA, Sidossis LS. Responses of blood lipids to aerobic, resistance, and combined aerobic with resistance exercise training: A systematic review of current evidence. Angiology. 2009;60(5):614–32. doi: 10.1177/0003319708324927. [DOI] [PubMed] [Google Scholar]
- 57.Tucker WJ, Jarrett CL, D’Lugos AC, Angadi SS, Gaesser GA. Effects of indulgent food snacking, with and without exercise training, on body weight, fat mass, and cardiometabolic risk markers in overweight and obese men. Physiol Rep. 2021;9(22):e15118. doi: 10.14814/phy2.15118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tuzcu EM, Kapadia SR, Tutar E, Ziada KM, Hobbs RE, McCarthy PM, et al. High prevalence of coronary atherosclerosis in asymptomatic teenagers and young adults: evidence from intravascular ultrasound. Circulation. 2001;103(22):2705–10. doi: 10.1161/01.cir.103.22.2705. [DOI] [PubMed] [Google Scholar]
- 59.University of California. Plan and operation of the third National Health and Nutrition Examination Survey 1988–94. Series 1: programs and collection procedures. U.S. Department of Health and Human Services, Public Health Service Centers for Disease Control and Prevention, National Center for Health Statistics; 1994. [Google Scholar]
- 60.Valkenet K, van de Port IGL, Dronkers JJ, de Vries WR, Lindeman E, Backx FJG. The effects of preoperative exercise therapy on postoperative outcome: A systematic review. Clin Rehabil. 2011;25(2):99–111. doi: 10.1177/0269215510380830. [DOI] [PubMed] [Google Scholar]
- 61.Verhagen AP, de Vet HC, de Bie RA, Kessels AG, Boers M, Bouter LM, et al. The Delphi list: A criteria list for quality assessment of randomized clinical trials for conducting systematic reviews developed by Delphi consensus. J Clin Epidemiol. 1998;51(12):1235–41. doi: 10.1016/s0895-4356(98)00131-0. [DOI] [PubMed] [Google Scholar]
- 62.Voight BF, Peloso GM, Orho-Melander M, Frikke-Schmidt R, Barbalic M, Jensen MK, et al. Plasma HDL cholesterol and risk of myocardial infarction: A mendelian randomisation study. Lancet Lond Engl. 2012;380(9841):572–80. doi: 10.1016/S0140-6736(12)60312-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Weston KS, Wisløff U, Coombes JS. High-intensity interval training in patients with lifestyle-induced cardiometabolic disease: A systematic review and meta-analysis. Br J Sports Med. 2014;48(16):1227–34. doi: 10.1136/bjsports-2013-092576. [DOI] [PubMed] [Google Scholar]
- 64.Wewege M, van den Berg R, Ward RE, Keech A. The effects of high-intensity interval training vs. moderate-intensity continuous training on body composition in overweight and obese adults: A systematic review and meta-analysis. Obes Rev Off J Int Assoc Study Obes. 2017;18(6):635–46. doi: 10.1111/obr.12532. [DOI] [PubMed] [Google Scholar]
- 65.Wood G, Murrell A, van der Touw T, Smart N. HIIT is not superior to MICT in altering blood lipids: A systematic review and meta-analysis. BMJ Open Sport Exerc Med. 2019;5(1):e000647. doi: 10.1136/bmjsem-2019-000647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.World Health Organization. A healthy lifestyle – WHO recommendations. 2010. Available at: https://www.who.int/europe/news-room/fact-sheets/item/a-healthy-lifestyle---who-recommendations.
- 67.Xie B, Yan X, Cai X, Li J. Effects of high-intensity interval training on aerobic capacity in cardiac patients: A systematic review with meta-analysis. BioMed Res Int. 2017;2017;5420840 doi: 10.1155/2017/5420840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zapata-Lamana R, Henríquez-Olguín C, Burgos C, Meneses-Valdés R, Cigarroa I, Soto C, et al. Effects of polarized training on cardiometabolic risk factors in young overweight and obese women: a randomized-controlled trial. Front Physiol. 2018;9:1287. doi: 10.3389/fphys.2018.01287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang Y, Vittinghoff E, Pletcher MJ, Allen NB, Zeki Al Hazzouri A, Yaffe K, et al. Associations of blood pressure and cholesterol levels during young adulthood with later cardiovascular events. J Am Coll Cardiol. 2019;74(3):330–41. doi: 10.1016/j.jacc.2019.03.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zheng Y, Manson JE, Yuan C, Liang MH, Grodstein F, Stampfer MJ, et al. Associations of weight gain from early to middle adulthood with major health outcomes later in life. JAMA. 2017;318(3):255–69. doi: 10.1001/jama.2017.7092. [DOI] [PMC free article] [PubMed] [Google Scholar]