Abstract
Background Long-term pouch surveillance outcomes for familial adenomatous polyposis (FAP) are unknown. We aimed to quantify surveillance outcomes and to determine which of selected possible predictive factors are associated with pouch dysplasia.
Methods Retrospective analysis of collected data on 249 patients was performed, analyzing potential risk factors for the development of adenomas or advanced lesions ( ≥ 10 mm/high grade dysplasia (HGD)/cancer) in the pouch body and cuff using Cox proportional hazards models. Kaplan–Meier analyses included landmark time-point analyses at 10 years after surgery to predict the future risk of advanced lesions.
Results Of 249 patients, 76 % developed at least one pouch body adenoma, with 16 % developing an advanced pouch body lesion; 18 % developed an advanced cuff lesion. Kaplan–Meier analysis showed a 10-year lag before most advanced lesions developed; cumulative incidence of 2.8 % and 6.4 % at 10 years in the pouch body and cuff, respectively. Landmark analysis suggested the presence of adenomas prior to the 10-year point was associated with subsequent development of advanced lesions in the pouch body (hazard ratio [HR] 4.8, 95 %CI 1.6–14.1; P = 0.004) and cuff (HR 6.8, 95 %CI 2.5–18.3; P < 0.001). There were two HGD and four cancer cases in the cuff and one pouch body cancer; all cases of cancer/HGD that had prior surveillance were preceded by ≥ 10-mm adenomas.
Conclusions Pouch adenoma progression is slow and most advanced lesions occur after 10 years. HGD and cancer were rare events. Pouch phenotype in the first decade is associated with the future risk of developing advanced lesions and may guide personalized surveillance beyond 10 years.
Introduction
Familial adenomatous polyposis (FAP) is caused by a constitutional pathogenic variant in the APC gene 1 . Prophylactic colectomy and ileorectal anastomosis (IRA) or restorative proctocolectomy (RPC) is advised to prevent the otherwise inevitable development of colorectal cancer 1 .
Pathogenic variants in the mutation cluster region (MCR) predict severe colorectal disease, while variants at the ends of the gene are associated with a milder phenotype. Primary RPC is recommended for patients with features predicting a high risk of rectal adenoma/cancer, including: MCR pathogenic variant; over 500 colonic or over 20 rectal adenomas 1 . Following IRA, some patients require completion restorative proctectomy (secondary RPC) owing to the development of severe rectal polyposis 2 .
RPC initially included distal rectal mucosectomy with the ileal pouch pulled through the rectal muscle tube and handsewn above the dentate line 3 , potentially leaving mucosal islands between the rectal muscle and the ileal pouch ( Fig. 1a ). A stapled anastomotic technique 4 was later adopted, preserving a rectal mucosal “cuff,” which can develop adenomas and cancer, but is accessible for surveillance ( Fig. 1b ).
Fig. 1 .
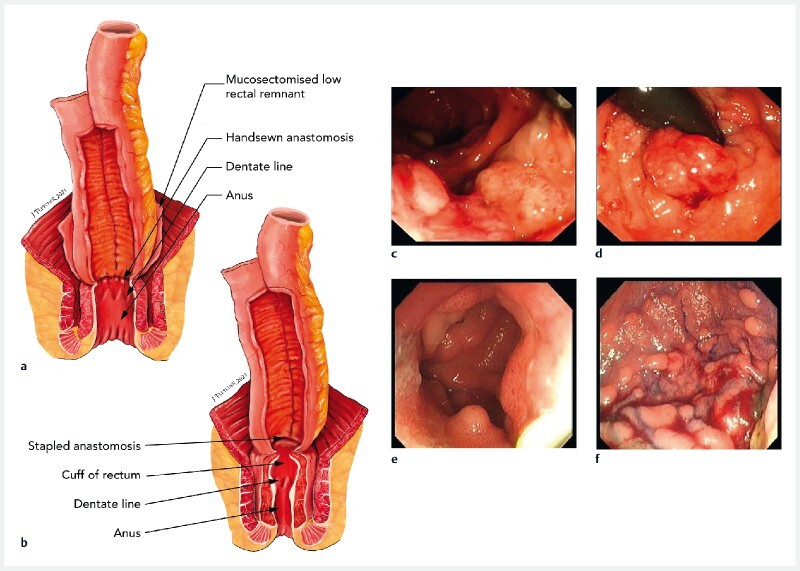
Types of anastomoses used and the adenomas that develop in pouches in familial adenomatous polyposis. a, b Illustrations of the anastomoses showing: a a handsewn anastomosis, in which there remains a hidden risk of adenoma development from residual rectal cuff mucosal islands – the ileal pouch has been advanced into a 2-cm rectal cuff following mucosectomy, and is handsewn at the dentate line, meaning islands of mucosal cells can remain behind that may develop into adenomas that cannot be visualized on direct endoscopy, and therefore require expert digital examination; b a stapled anastomosis, in which ~1–2 cm of rectal cuff remains in-situ and is proximally stapled to the ileoanal pouch with a circular stapling device, with this technique enabling direct visualization of any adenomas at pouchoscopy. c–f Endoscopic images of example pouch adenomas showing: c cancer in a cuff; d high grade dysplasia in a cuff lesion visualized on retroflexion; e adenomas in the pouch body; f diffuse polyposis within the pouch body.
Adenomas may develop in the pouch body or rectal cuff ( Fig. 1c–f ). The risk of cuff adenomas increases over time and is higher after a stapled anastomosis 5 6 7 . Cuff cancer develops in 0.5 %–3.6 % of patients 5 6 . Pouch body cancer is rare 8 9 , but adenomas are present in 7 %–15 % of patients at 5 years and 72 %–78 % at 20 years 10 11 12 . Other factors associated with pouch body adenoma development, such as genotype, sex, and intestinal phenotype, have been inconsistently reported 10 13 14 15 .
Most studies use time to development of an adenoma as the primary end point; however, it is the presence of uncontrolled pouch polyposis (hundreds of adenomas, large lesions, or high grade dysplasia [HGD]) that raises concern for cancer risk and is more clinically important. There is a need to better identify those at risk of this.
Current UK guidelines recommend annual pouchoscopy 16 , but there is a lack of data to guide surveillance intervals. Our aims were to quantify the surveillance outcomes of pouches in FAP, to better understand adenoma development, and to determine which of selected possible predictive factors are associated with pouch dysplasia.
Methods
Patient cohort
We performed a retrospective review of all patients with FAP and an ileoanal pouch on the St Mark’s Hospital Polyposis Registry database up to 2018. Inclusion criteria were a minimum of one surveillance pouchoscopy and a documented constitutional APC pathogenic variant. Genotype was categorized as: within the MCR (codon 1250–1450), 5′ of the MCR, 3′ of the MCR 2 , or a gross deletion.
Annual pouch surveillance started in 1997, performed as previously described 17 . Enhanced imaging was used at the endoscopist’s discretion. Data recorded included: patient demographics, prophylactic surgery type, endoscopic findings, intervention, and contemporaneous Spigelman stage for duodenal disease.
Follow-up outcomes
Given the rarity of cancer, presence of an “advanced lesion,” defined as an adenoma ≥ 10 mm, HGD, or cancer, was the primary outcome. Rectal cuff and pouch body findings were analyzed separately. We described risk factors for adenoma development, but focused on advanced lesions as our main clinical end point.
Statistical analysis
When analyzing neoplastic progression, data were considered right-censored if the patient had not progressed to the outcome of interest by the time of the last surveillance. Follow-up was defined as the time between pouch formation and progression or censoring. The following continuous variables were compared using Mann–Whitney U tests between those patients who did and did not progress to an advanced lesion: number of surveillance pouchoscopies; mean surveillance interval; pouch and patient age at first and last surveillance.
Statistical associations for adenoma and advanced lesion development in the pouch body and cuff were examined using univariate and multivariate Cox proportional hazards models; Kaplan–Meier curve estimations with log-rank tests were performed for potential differences in progression. Survival analyses were performed using R Studio (version 4.0.3). Pouch age was the continuous time variable. Time-independent factors included in all of the univariate Cox proportional hazards models were: sex, patient age at surgery, primary or secondary RPC, and APC pathogenic variant.
Pouch body analysis included univariate models considering Spigelman stage at time of RPC as another potential time-independent risk factor. To account for changing Spigelman stage with pouch age, univariate models were also considered for Spigelman stage as a time-varying covariate using the contemporaneous highest Spigelman stage recorded as the time-varying covariate variable (to account for therapeutic downstaging of disease).
Only 80/249 patients had a Spigelman stage recorded at pouch surgery, so we excluded this variable in the first multivariate Cox proportional hazards model for advanced lesion development. We did however investigate a second multivariate Cox proportional hazards model with the four time-independent factors and Spigelman stage included as a time-varying covariate.
Cuff analysis included univariate models with type of anastomosis as an additional time-independent risk factor. All time-independent factors were included in a multivariate model for advanced lesion development in the cuff.
Surveillance data were included until the outcome of interest or censoring at last follow-up date or pouch excision. Correction for multiple hypothesis testing was performed using the Bonferroni correction.
We hypothesized that it takes time to develop advanced lesions and that differences in the risk of developing later advanced lesions may be discernible by 10 years after pouch construction. We performed a landmark time-point analysis to determine differences in the rates of progression to advanced lesions in the pouch body and cuff, using clinical features detectable up to 10 years post-surgery 18 19 . The aim was to potentially distinguish differences in the risk factors in those who were destined to remain advanced lesion-free from those who were on a “fast-track” to developing advanced lesions in the pouch body; these risk factors were used as constant “baseline 10-year” predictors in the univariate Cox proportional hazards models. This enabled us to add adenoma presence and size at the most recent surveillance pouchoscopy prior to the 10-year mark as two additional variables in univariate Cox proportional hazards models. Spigelman stage was considered a binary variable (grouped as stages 0–2 or 3–4) owing to the small sample size. Kaplan–Meier curves were used to assess differences in the future risk of advanced lesion progression for covariates found to be statistically significant in univariate Cox proportional hazards models starting at the 10-year mark.
Secondary outcomes
We examined the maximum therapy employed for management of adenomas. We collected data on whether patients had snare polypectomy, endoscopic submucosal dissection (ESD), or surgical treatment such as examination under anesthesia (EUA) with surgical polypectomy or pouch excision.
Results
Surveillance data were available for 249 patients (131 men [53 %]) ( Table 1 ). Pouch formation was carried out at our center in 159 patients (64 %). The median age of patients at pouch construction was 29 years (range 5–66 years). The median (interquartile range [IQR]) total follow-up was 15 (8–22) years. A total of 2225 surveillance pouchoscopies were performed (median [IQR] per patient 8 4 5 6 7 8 9 10 11 12 13 14 ) over 3811 patient-years. There were 164 patients who underwent primary RPC and 85 who underwent secondary RPC; among these, significant differences were found between the mean number of surveillance examinations, mean surveillance interval, and patient age at first and last examinations ( Table 1 s , see online-only Supplementary material).
Table 1. Details of the 249 patients with familial adenomatous polyposis who underwent restorative proctocolectomy (RPC), their surgery, and surveillance examinations.
| Demographic data | |
| Sex, male, n (%) | 131 (53) |
| Patient age at time of pouch surgery, median (range) [IQR], years | 29 (5–66) [22–38] |
| Number of surveillance examinations, median (range) [IQR] | 8 (1–26) [4–14] |
| Interval between examinations, median (range) [IQR], years | 1.50 (0.14–15.93) [1.16–2.23] |
| First surveillance endoscopy post-surgery | |
| Age of pouch, median (range) [IQR], years | 3.49 (0.14–30.85) [1.39–9.91] |
| Patient age, median (range) [IQR], years | 36 (6–77) [29–46] |
| Last surveillance endoscopy | |
| Age of pouch, median (range) [IQR], years | 15.44 (0.14–37.31) [8.45–21.52] |
| Patient age, median (range) [IQR], years | 46 (10–83) [35–56] |
| Details of surgery | |
| Type of surgery, n (%) | |
|
164 (66) |
|
85 (34) |
| Configuration of pouch, n (%) | |
|
137 (55) |
|
27 (10) |
|
1 (< 1) |
|
2 (< 1) |
|
82 (33) |
| Anastomotic technique, n (%) | |
|
72 (48) |
|
79 (52) |
|
98 (–) |
| Site of APC pathogenic variant (codon), n (%) | |
|
152 (61) |
|
69 (28) |
|
20 (8) |
|
8 (3) |
IQR, interquartile range.
Time to develop first adenoma
There were 188 patients (76 %) who developed at least one pouch body adenoma; the cumulative incidence at 5, 10, 15, 20, 25, and 30 years was 19.3 %, 41.0 %, 56.6 %, 65.9 %, 73.5 %, and 74.7 %, respectively ( Fig. 1 s , part A). We did not identify significant risk factors associated with the development of a pouch body adenoma ( Table 2 s ).
A cuff adenoma developed in 179 patients (72 %); the cumulative incidence at 5, 10, 15, 20, 25, and 30 years was 26.1 %, 40.2 %, 53.0 %, 62.7 %, 67.1 %, and 70.7 %, respectively ( Fig. 1 s , part B). A stapled anastomosis was a significant risk factor on univariate analysis (hazard ratio [HR] 3.5, 95 %CI 2.3–5.2) ( Table 3 s ). No other risk factors were found to be significant after Bonferroni correction.
Of 213 patients who developed at least one adenoma (cuff or pouch body), 154 (72 %) developed adenomas at both sites; 34 (16 %) and 25 (12 %) developed adenomas uniquely in the pouch body and cuff, respectively ( Fig. 1 s , part C). Of 72 patients who developed advanced lesions, 12 (17 %) did so in both the cuff and pouch body; 27 (38 %) and 33 (46 %) developed advanced lesions uniquely in the pouch body and cuff, respectively ( Fig. 1 s , part D).
Pouch body lesions
The largest adenoma detected in the pouch body was 1–4 mm in 105 patients (42 %), 5–9 mm in 44 patients (18 %), and ≥ 10 mm in 39 patients (16 %). HGD was not identified, but there was one case of pouch body cancer ( Table 4 s ).
Advanced lesions
An advanced lesion developed in the pouch body in 39/249 patients (16 %). There was no statistically significant difference in the number of surveillance examinations between patients that did and did not progress to an advanced lesion in the pouch body ( Fig. 2a ; Table 5 s ). However, patients who developed an advanced lesion had a longer median (range) surveillance interval (1.96 years [0.66–30.44] vs. 1.46 years [0.14–16]; P = 0.006; Fig. 2b ), started surveillance later (8.54 years [0.92–30.44] vs. 2.73 years (0.14–30.85); P < 0.001), and had an older (median [IQR]) pouch at diagnosis of the advanced lesion (15.9 [12.33–22.16] vs. 14.15 [6.88–19.66] years; P = 0.03) ( Fig. 2d ; Table 5 s ). Patients were older at first surveillance in the progressors group, but the difference for patient age at last surveillance was not statistically significant ( Fig. 2e, f ).
Fig. 2.
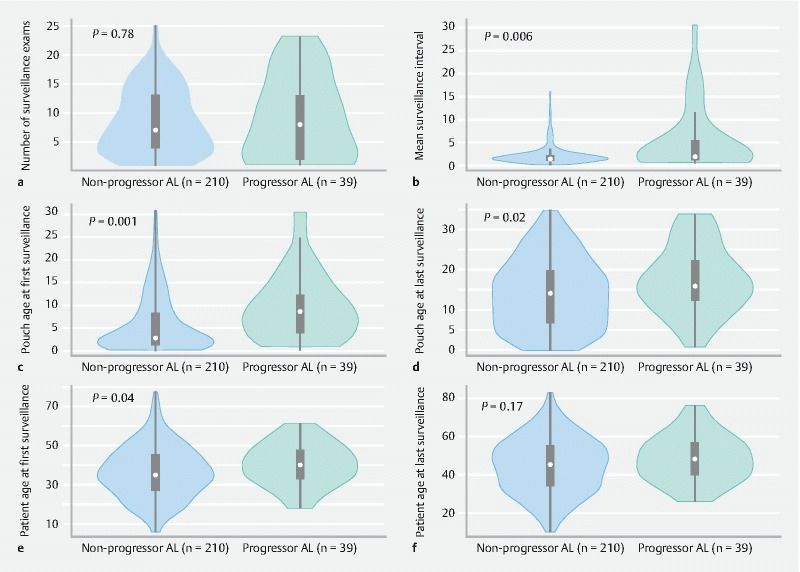
Violin plots comparing the surveillance data of patients who progressed to advanced lesions versus those who did not progress ( P values for Mann–Whitney U test), with results provided for: a number of surveillance examinations; b mean surveillance interval between examinations for each patients; c pouch age at first surveillance; d pouch age at last surveillance; e patient age at first surveillance; f patient age at last surveillance.
Adenoma counts within the pouch body increased over time ( Fig. 3a ). Advanced lesions developed in the context of both high and low adenoma counts within the pouch body as recorded at time of advanced lesion diagnosis ( Fig. 3b ).
Fig. 3.
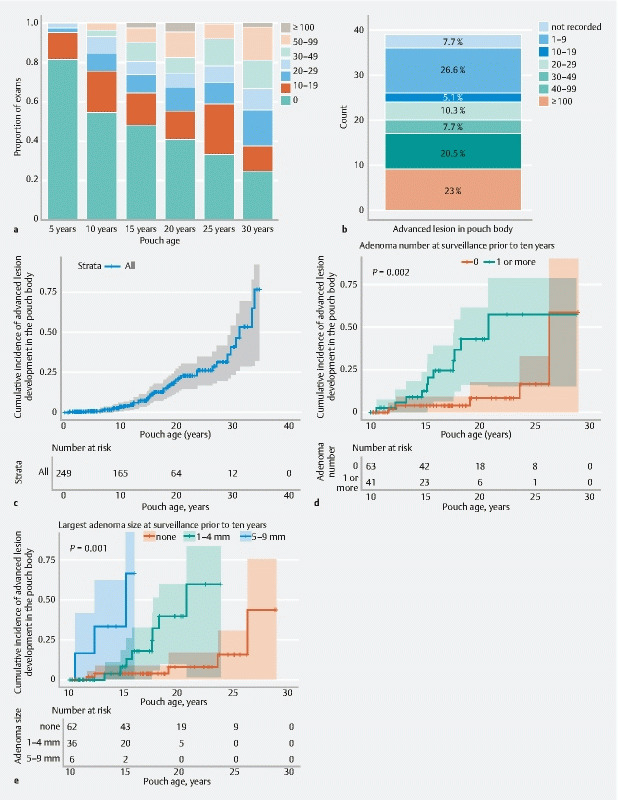
Graphs of surveillance outcomes, including advanced lesion development in the pouch body showing: a maximum adenoma count on record by 5, 10, 15, 20, 25, and 30 years; b contemporaneous adenoma count in the pouch body at the time an advanced lesion was diagnosed in the pouch body for the 39 patients with advanced lesions; c–e Kaplan–Meier curves for time to development of an advanced lesion in the pouch body: c for all patients; d (landmark at end of year 10 since surgery) stratified by presence or absence of an adenoma at the most recent surveillance prior to 10 years (of 104 patients still on study at year 10, 16 subsequently progressed to an advanced lesion; log-rank for differences in survival, P = 0.002); e (landmark at end of year 10 since surgery) stratified by largest adenoma size observed prior to 10 years (of 104 patients still on study at year 10, 16 subsequently progressed to an advanced lesion; log-rank for differences in survival, P < 0.001).
The cumulative incidence of advanced lesions at 5, 10, 15, 20, 25, and 30 years was 0.8 %, 2.8 %, 6.0 %, 10.8 %, 12.4 %, and 14.1 %, respectively ( Fig. 3c ). No baseline factors were identified as conferring increased risk of advanced lesions on univariate and multivariate Cox proportional hazard analysis.
Seven patients (2.8 %) developed advanced pouch body lesions before 10 years (median [range] pouch age at diagnosis 7 [0.9–9.7] years). Of these seven patients, two commenced annual surveillance from RPC; the remaining five started at pouch ages of 4 (n = 2), 6, 7, and 10 years (n = 1 each).
One patient, with ulcerative colitis and FAP, developed pouch body cancer 34 years after surgery. Surveillance began at our center 30 years after pouch formation, when he had approximately 300 pouch body adenomas that were up to 30 mm in size. Biopsies had shown low grade dysplasia (LGD) at the preceding surveillance pouchoscopies, before an ulcerated malignant lesion of 40–50 mm was identified.
Landmark time-point analysis for advanced lesions in the pouch body
A landmark time-point analysis was performed using 165 patients who remained at risk for the development of future advanced lesions starting at 10 years after surgery. There were 84 patients who were excluded as they had either progressed to an advanced lesion (n = 7) or been censored (n = 77) before completing 10 years of follow-up. The majority (86 % [32/37]) who progressed to an advanced lesion did so more than 10 years after RPC, indicating that the advanced lesion risk should be re-assessed for patients on surveillance at 10 years. Constant covariates (sex, age at surgery, pathogenic variant, first operation type) were considered fixed at year 10.
Considering those patients who had undergone at least one surveillance examination before 10 years and had follow-up continuing beyond 10 years (n = 104) in a univariate Cox proportional hazards models ( Table 2 ), we found that the presence of at least one pouch body adenoma (HR 4.8, 95 %CI 1.6–14.1; P = 0.004) or a larger pouch body adenoma (HR 32.4, 95 %CI 5.8–177.1; P < 0.001) ( Fig. 3 d,e ) prior to 10 years were significantly associated with an increased risk of developing advanced lesions in the subsequent years of follow-up.
Table 2. Univariate Cox proportional hazards models for progression to an advanced lesion in the pouch body and cuff, with risk factors at year 10 (landmark time-point analysis).
| Variable | Pouch body | Cuff | ||||
| Number (n = 165) | Adjusted hazard ratio (95 %CI) | P value 1 | Number (n = 158) | Adjusted hazard ratio (95 %CI) | P value 2 | |
| Female sex | 76 | 0.7 (0.4–1.4) | 0.34 | 71 | 0.7 (0.4–1.6) | 0.42 |
| Age at pouch surgery, years | 1.01 (0.98–1.05) | 0.53 | 1.0 (0.97–1.04) | 0.72 | ||
| APC pathogenic variant type | ||||||
|
48 | 1 | ref | 46 | 1 | ref |
|
99 | 1.2 (0.5–2.7) | 0.64 | 95 | 1.4 (0.5–3.5) | 0.50 |
|
12 | 3.2 (0.8–12.4) | 0.09 | 11 | 4.6 (1.3–16.7) | 0.02 |
|
6 | – | > 0.99 | 6 | 2.1 (0.4–10.5) | 0.37 |
| Type of initial pouch surgery | ||||||
|
59 | 1 | ref | 58 | 1 | ref |
|
106 | 1.09 (0.5–2.3) | 0.81 | 100 | 1.5 (0.7–3.4) | 0.33 |
| Type of anastomosis 3 | ||||||
|
60 | 1 | ref | |||
|
30 | 3.8 (1.2–11.9) | 0.02 | |||
| Adenoma number (at most recent examination where number was recorded before year 10) 4 | ||||||
|
63 | 1 | ref | 73 | 1 | ref |
|
41 | 4.8 (1.6–14.1) | 0.004 | 24 | 6.8 (2.5–18.3) | < 0.001 |
| Adenoma size (at most recent examination where size was recorded before year 10), mm 5 | ||||||
|
62 | 1 | ref | 30 | 1 | ref |
|
36 | 5.8 (1.7–20.4) | 0.005 | 12 | 3.1 (0.97–9.85) | 0.06 |
|
6 | 32.4 (5.8–177.1) | < 0.001 | 1 | – | – |
MCR, mutation cluster region; IRA, ileorectal anastomosis; RPC, restorative proctocolectomy.
Progression to an advanced lesion in the pouch body (32/165). Statistical significance in univariate analyses indicated when P < 0.01. This pragmatic approach led to the same variables being considered statistically significant as in the stricter approach requiring P < 0.008 with Bonferroni multiple testing correction for six potential variables (shown in bold).
Progression to an advanced lesion in the cuff (29/158). Statistical significance in univariate analyses indicated when P < 0.01. This pragmatic approach led to the same variables being considered statistically significant as in the stricter approach requiring P < 0.007 with Bonferroni multiple testing correction for seven potential variables (shown in bold).
Cuff group, n = 90.
Pouch body, n = 104 with 16 advanced lesions; cuff, n = 97, with 17 advanced lesions.
Pouch body, n = 104, with 16 advanced lesions; cuff, n = 43, with 13 advanced lesions.
Cuff lesions
A total of 45 patients developed an advanced lesion in the cuff: 44 (18 %) had an adenoma ≥ 10 mm, four (2 %) had cuff cancer, and one (0.6 %) had HGD.
Advanced lesions
There were 16 patients who developed an advanced lesion in the cuff before 10 years; the cumulative incidence of advanced lesions at 5, 10, 15, 20, 25, and 30 years was 2.4 %, 6.4 %, 10.8 %, 14.0 %, 16.5 %, and 17.3 %, respectively ( Fig. 4a ). Univariate Cox regression analyses ( Table 3 s ) found that patients were more likely to develop advanced lesions following a stapled anastomosis (HR 4.5, 95 %CI 2.7–11.9; P = 0.002) ( Fig. 4b ), or if they carried a 3′ APC pathogenic variant (compared to MCR), (HR 5.2, 95 %CI 1.9–14.2; P = 0.001). Only stapled anastomosis remained significant on multivariate analysis.
Fig. 4.
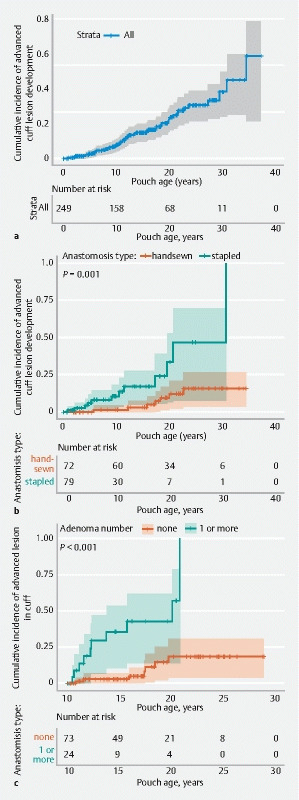
Kaplan–Meier curves for progression to an advanced lesion in the cuff: a for all patients; b stratified by anastomosis type; c (landmark at end of year 10 since surgery) stratified by presence or absence of an adenoma at the most recent surveillance prior to 10 years (of 97 patients still on study at year 10, 17 progressed to an advanced lesion on subsequent follow-up; log-rank for differences in survival, P < 0.001).
Of the 16 patients with an advanced lesion before 10 years, nine had an adenoma sized ≥ 10 mm. One patient had an adenoma sized ≥ 10 mm with subsequent HGD, a 10-mm adenoma having been observed on commencement of surveillance 6 years after pouch formation. At 8 years, the recurrence containing HGD was treated by transanal surgical excision; after four further transanal resections the pouch was excised (at pouch age 20 years) and a cuff cancer was identified on postoperative histopathology. The patient remains disease free. No patient developed cuff cancer within 10 years of the RPC.
Overall there were 44 patients with a ≥ 10-mm adenoma; three went on to develop cuff cancer (including the patient described above). The only patient who developed cuff cancer without a prior ≥ 10-mm lesion was diagnosed at first surveillance pouchoscopy after referral to our center, at pouch age 20 years.
Landmark time-point analysis for advanced lesions in the cuff
In the landmark time-point analysis at 10 years, univariate Cox regression analysis showed that the phenotype of the cuff at a surveillance examination prior to the 10-year point could predict subsequent development of an advanced lesion in the cuff. The presence of an adenoma was significantly associated with the development of an advanced lesion in the cuff (HR 6.8, 95 %CI 2.5–18.3; P < 0.001) ( Table 2 ) in the subsequent years of follow-up (Kaplan–Meier log-rank, P < 0.001) ( Fig. 4c ). Overall, however, patients who progressed to an advanced lesion in the cuff had fewer surveillance examinations compared with those who did not (median 4 [range 1–17] vs. 8 [1–21]; P = 0.002) ( Table 5 s ).
Management of advanced lesions
Most pouch body adenomas were successfully managed endoscopically. The maximum therapy used for those who developed a pouch body advanced lesion (39 patients) was: endoscopic snare polypectomy in 30 patients, pouch excision in eight patients (described below), and palliative care for one patient with pouch body cancer.
Of the 45 patients who developed advanced cuff lesions, 23 were managed endoscopically (22 snare polypectomy and one ESD) and 11 underwent EUA with surgical excision of polyps. All four patients with cuff cancer and the patient with recurrent cuff HGD were managed by pouch excision or exenterative surgery ( Table 4 s ).
Pouch excision
Pouch excision was performed for 14 patients (6 %) owing to neoplasia: nine for benign pouch body disease (five for adenoma burden in both pouch body and cuff; four for pouch body lesions only) ( Table 6 s ), and five for cuff lesions (four for cancers and one for an endoscopically unmanageable cuff lesion).
Discussion
The literature on the neoplastic outcomes of RPC in patients with FAP is inconsistent; some have authors have reported exclusively on any adenoma in either the cuff or pouch body 5 6 7 10 13 20 21 , whilst others have combined these, despite their being biologically and anatomically distinct areas 14 22 23 24 25 26 27 28 29 . No previous study has specifically considered the practical clinical end point of advanced lesion development in either the pouch body or cuff.
The risk of developing pouch adenomas in our study is consistent with the published data 6 10 12 13 22 23 24 . We observed that a pouch body adenoma developed in 65.5 % of patients by 20 years, which is similar to other published data where pouch body adenomas were observed in 45 %–78 % by 20 years 10 20 . Cuff adenoma development has generally been described according to anastomotic technique. We observed a lower rate following handsewn anastomosis compared with stapled anastomosis (72 % vs. 81 %; P = 0.001), consistent with other studies 5 6 13 21 . Although stapled anastomosis was a risk factor for advanced lesions in the cuff, two of the four cases of cuff cancer occurred after handsewn anastomosis. This emphasizes that, although the overall risk of developing a cuff adenoma is lower after mucosectomy, leaving fragments of at-risk rectal mucosa buried outside the serosa of the pouch is a disadvantage when performing surveillance. After a stapled anastomosis, the at-risk rectal mucosa can be visualized and adenomas can be resected before they develop into cancer.
We found that in the first 10 years, the vast majority of patients had no or few pouch adenomas ( Fig. 3a ). This supports a reduction in the surveillance frequency for most patients in the first 10 years. We demonstrated that the phenotype of the pouch recorded prior to finishing 10 years of surveillance could stratify the future risk of advanced lesions in both the cuff and pouch body beyond 10 years. This provides the opportunity for risk assessment at this point, to personalize clinical practice so that those at lower risk of advanced lesions can receive less intensive surveillance than those at high risk.
Studies assessing an association between duodenal and pouch adenomas have either failed to control for pouch age 10 13 or did not control for time as a confounding factor 10 11 14 22 23 24 25 26 27 28 29 , which is important because increasing age is the only consistent risk factor for duodenal disease 30 . We took into account both of these confounders and observed that the severity of duodenal disease (Spigelman stage) was not a significant risk factor for the development of adenomas or advanced lesions in the cuff or pouch body.
There is good evidence of genotype–phenotype correlation with respect to some manifestations of FAP, but no such correlation with pouch neoplasia has been consistently identified. In our study, a 3′ APC pathogenic variant appeared to be associated with cuff advanced lesions. We acknowledge the modest size of the group with a 3′ APC pathogenic variant (n = 20) and the wide confidence intervals for this statistical association. This group of patients generally has a milder colorectal phenotype and elevated risk of desmoid, so are less likely to be advised to undergo RPC. The association with advanced lesions in the cuff is surprising and we are unable to provide a plausible biological explanation.
Gastric adenomas have been reported as a risk factor for pouch adenomas 10 ; we did not explore this manifestation of FAP as only 23 patients (9 %) in our cohort had a gastric adenoma. Although an association between the colonic phenotype and subsequent pouch body and cuff adenomas has been explored previously 12 14 31 , we did not examine this as we use the colonic phenotype as a major determining factor of whether RPC is recommended, which would result in bias.
Pouch body cancer is rare and literature reviews have identified up to 23 cases 8 9 . From these publications, pouch body cancers were diagnosed between 3 and 11 years after pouch construction 14 32 33 34 35 36 37 . The true incidence is very difficult to estimate.
To our knowledge, this is the only study which has sought to identify patients at risk of developing advanced lesions in the pouch body or cuff. Given that most patients will develop pouch adenomas eventually, there is a need to identify more precisely those most at risk of developing advanced lesions. We observed that adenomas with a diameter of 10 mm preceded development of HGD or cancer; such lesions were generally not seen before 10 years.
Our cohort demonstrates that most patients develop at least one adenoma in the pouch body. All had LGD except for one case of invasive pouch body cancer that developed in a patient with both FAP and ulcerative colitis who received suboptimal surveillance and was on azathioprine for “pouchitis” (the effect of this on adenoma progression is unknown).
A current clinical challenge in patients with a pouch is to determine accurately when a pouch with many adenomas or advanced lesions should be removed to prevent cancer. Two cases of cuff cancer were diagnosed after a decision to perform pouch excision had been made owing to circumferential cuff lesions. Similarly, HGD was identified in an excised lesion after pouch excision for a circumferential adenoma.
There are limitations to our study. Despite being the largest European cohort, this is a single-center study, so some subanalyses were small. Although endoscopic practice has evolved considerably, improving adenoma detection, this leads to bias over time towards increased adenoma detection. Surveillance practice was inconsistent; pouch surgery was pioneered in 1978, but annual pouchoscopy was introduced in 1997. Some patients were referred to our center without prior surveillance and others were referred following adenoma detection.
Considering the study timespan and cohort size, procedures were performed by many endoscopists and consequently there was variability in reporting outcomes. When numerous polyps were removed, the report did not always reliably describe the exact number. We accept that the removal of adenomas alters the natural history of the development of advanced lesions.
Although a few patients may have participated in studies of chemoprevention for duodenal disease, a recent large study found sulindac to have no effect on prevention of cuff adenomas 5 . Given the use of chemoprevention is likely to have affected few patients over a short time, we have not included these data.
In conclusion, we have demonstrated that the vast majority of patients do not develop an adenoma within 5 years of pouch formation and that the development of HGD and cancer are rare events. The greatest risk appears to occur after 10 years, suggesting that surveillance in the first 10 years could be reduced for most patients, provided they have early engagement in the surveillance program. The pouch phenotype in the first decade stratified patients into high and lower risk groups for the future development of advanced lesions; risk may be assessed at 10 years to allow for a personalized surveillance strategy and the extension of surveillance intervals in the low risk group. Multicenter prospective studies are required to better assess the influence of interventions on the natural history of pouch adenomas and to validate the safety of extending the surveillance intervals beyond those in current guidelines.
Competing Interests The authors declare that they have no conflict of interest.
Contributed equally to this article
Tables 1 s–6 s, Fig. 1 s :
References
- 1.Tudyka V N, Clark S K. Surgical treatment in familial adenomatous polyposis. Ann Gastroenterol. 2012;25:201–206. [PMC free article] [PubMed] [Google Scholar]
- 2.Sinha A, Tekkis P P, Rashid S et al. Risk factors for secondary proctectomy in patients with familial adenomatous polyposis. Br J Surg. 2010;97:1710–1715. doi: 10.1002/bjs.7202. [DOI] [PubMed] [Google Scholar]
- 3.Parks A G, Nicholls R J. Proctocolectomy without ileostomy for ulcerative colitis. BMJ. 1978;2:85–88. doi: 10.1136/bmj.2.6130.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heald R J, Allen D R. Stapled ileo-anal anastomosis: a technique to avoid mucosal proctectomy in the ileal pouch operation. Br J Surg. 1986;73:571–572. doi: 10.1002/bjs.1800730719. [DOI] [PubMed] [Google Scholar]
- 5.Lee C HA, Kalady M F, Burke C A et al. Incidence and management of rectal cuff and anal transitional zone neoplasia in patients with familial adenomatous polyposis. Dis Colon Rectum. 2021;64:977–985. doi: 10.1097/DCR.0000000000001967. [DOI] [PubMed] [Google Scholar]
- 6.von Roon A C, Will O C, Man R F et al. Mucosectomy with handsewn anastomosis reduces the risk of adenoma formation in the anorectal segment after restorative proctocolectomy for familial adenomatous polyposis. Ann Surg. 2011;253:314–317. doi: 10.1097/SLA.0b013e318f3f498. [DOI] [PubMed] [Google Scholar]
- 7.Ganschow P, Treiber I, Hinz U et al. Residual rectal mucosa after stapled vs. handsewn ileal J-pouch-anal anastomosis in patients with familial adenomatous polyposis coli (FAP)--a critical issue. Langenbecks Arch Surg. 2015;400:213–219. doi: 10.1007/s00423-014-1263-x. [DOI] [PubMed] [Google Scholar]
- 8.Smith J C, Schaffer M W, Ballard B R et al. Adenocarcinomas after prophylactic surgery for familial adenomatous polyposis. J Cancer Ther. 2013;4:260–270. doi: 10.4236/jct.2013.41033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tajika M, Niwa Y, Bhatia V et al. Risk of ileal pouch neoplasms in patients with familial adenomatous polyposis. World J Gastroenterol. 2013;19:6774–6783. doi: 10.3748/wjg.v19.i40.6774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ganschow P, Trauth S, Hinz U et al. Risk factors associated with pouch adenomas in patients with familial adenomatous polyposis. Dis Colon Rectum. 2018;61:1096–1101. doi: 10.1097/DCR.0000000000001157. [DOI] [PubMed] [Google Scholar]
- 11.Parc Y R, Olschwang S, Desaint B et al. Familial adenomatous polyposis: prevalence of adenomas in the ileal pouch after restorative proctocolectomy. Ann Surg. 2001;233:360–364. doi: 10.1097/00000658-200103000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tajika M, Nakamura T, Nakahara O et al. Prevalence of adenomas and carcinomas in the ileal pouch after proctocolectomy in patients with familial adenomatous polyposis. J Gastrointest Surg. 2009;13:1266–1273. doi: 10.1007/s11605-009-0871-1. [DOI] [PubMed] [Google Scholar]
- 13.Goldstein A L, Kariv R, Klausner J M et al. Patterns of adenoma recurrence in familial adenomatous polyposis patients after ileal pouch-anal anastomosis. Dig Surg. 2015;32:421–425. doi: 10.1159/000439143. [DOI] [PubMed] [Google Scholar]
- 14.Tonelli F, Ficari F, Bargellini T et al. Ileal pouch adenomas and carcinomas after restorative proctocolectomy for familial adenomatous polyposis. Dis Colon Rectum. 2012;55:322–329. doi: 10.1097/DCR.0b013e318241e6f2. [DOI] [PubMed] [Google Scholar]
- 15.Kariv R, Rosner G, Fliss-Isakov N et al. Genotype-phenotype associations of APC mutations with pouch adenoma in patients with familial adenomatous polyposis. J Clin Gastroenterol. 2019;53:e54–e60. doi: 10.1097/MCG.0000000000000950. [DOI] [PubMed] [Google Scholar]
- 16.Monahan K J, Bradshaw N, Dolwani S et al. Guidelines for the management of hereditary colorectal cancer from the British Society of Gastroenterology (BSG)/Association of Coloproctology of Great Britain and Ireland (ACPGBI)/United Kingdom Cancer Genetics Group (UKCGG) Gut. 2020;69:411–444. doi: 10.1136/gutjnl-2019-319915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McLaughlin S D, Clark S K, Thomas-Gibson S et al. Guide to endoscopy of the ileo-anal pouch following restorative proctocolectomy with ileal pouch-anal anastomosis; indications, technique, and management of common findings. Inflamm Bowel Dis. 2009;15:1256–1263. doi: 10.1002/ibd.20874. [DOI] [PubMed] [Google Scholar]
- 18.Anderson J R, Cain K C, Gelber R D. Analysis of survival by tumor response. J Clin Oncol. 1983;1:710–719. doi: 10.1200/JCO.1983.1.11.710. [DOI] [PubMed] [Google Scholar]
- 19.Gleiss A, Oberbauer R, Heinze G. An unjustified benefit: immortal time bias in the analysis of time-dependent events. Transpl Int. 2018;31:125–130. doi: 10.1111/tri.13081. [DOI] [PubMed] [Google Scholar]
- 20.Boostrom S Y, Mathis K L, Pendlimari R et al. Risk of neoplastic change in ileal pouches in familial adenomatous polyposis. J Gastrointest Surg. 2013;17:1804–1808. doi: 10.1007/s11605-013-2319-x. [DOI] [PubMed] [Google Scholar]
- 21.Wasmuth H H, Tranø G, Myrvold H E et al. Adenoma formation and malignancy after restorative proctocolectomy with or without mucosectomy in patients with familial adenomatous polyposis. Dis Colon Rectum. 2013;56:288–294. doi: 10.1097/DCR.0b013e31827c970f. [DOI] [PubMed] [Google Scholar]
- 22.Friederich P, de Jong A E, Mathus-Vliegen L M et al. Risk of developing adenomas and carcinomas in the ileal pouch in patients with familial adenomatous polyposis. Clin Gastroenterol Hepatol. 2008;6:1237–1242. doi: 10.1016/j.cgh.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 23.Banasiewicz T, Marciniak R, Kaczmarek E et al. The prognosis of clinical course and the analysis of the frequency of the inflammation and dysplasia in the intestinal J-pouch at the patients after restorative proctocolectomy due to FAP. Int J Colorectal Dis. 2011;26:1197–1203. doi: 10.1007/s00384-011-1241-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pommaret E, Vienne A, Lefevre J H et al. Prevalence and risk factors for adenomas in the ileal pouch and the afferent loop after restorative proctocolectomy for patients with familial adenomatous polyposis. Surg Endosc. 2013;27:3816–3822. doi: 10.1007/s00464-013-2980-x. [DOI] [PubMed] [Google Scholar]
- 25.Schulz A C, Bojarski C, Buhr H J et al. Occurrence of adenomas in the pouch and small intestine of FAP patients after proctocolectomy with ileoanal pouch construction. Int J Colorectal Dis. 2008;23:437–441. doi: 10.1007/s00384-007-0422-8. [DOI] [PubMed] [Google Scholar]
- 26.Thompson-Fawcett M W, Marcus V A, Redston M et al. Adenomatous polyps develop commonly in the ileal pouch of patients with familial adenomatous polyposis. Dis Colon Rectum. 2001;44:347–353. doi: 10.1007/BF02234731. [DOI] [PubMed] [Google Scholar]
- 27.Zahid A, Kumar S, Koorey D et al. Pouch adenomas in familial adenomatous polyposis after restorative proctocolectomy. Int J Surg. 2015;13:133–136. doi: 10.1016/j.ijsu.2014.11.048. [DOI] [PubMed] [Google Scholar]
- 28.Campos F G, Perez R O, Imperiale A R et al. Surgical treatment of familial adenomatous polyposis: ileorectal anastomosis or restorative proctolectomy? Arq Gastroenterol. 2009;46:294–299. doi: 10.1590/s0004-28032009000400009. [DOI] [PubMed] [Google Scholar]
- 29.Moussata D, Nancey S, Lapalus M G et al. Frequency and severity of ileal adenomas in familial adenomatous polyposis after colectomy. Endoscopy. 2008;40:120–125. doi: 10.1055/s-2007-995363. [DOI] [PubMed] [Google Scholar]
- 30.Bulow S, Bjork J, Christensen I J et al. Duodenal adenomatosis in familial adenomatous polyposis. Gut. 2004;53:381–386. doi: 10.1136/gut.2003.027771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Remzi F H, Church J M, Bast J et al. Mucosectomy vs. stapled ileal pouch-anal anastomosis in patients with familial adenomatous polyposis: functional outcome and neoplasia control. Dis Colon Rectum. 2001;44:1590–1596. doi: 10.1007/BF02234377. [DOI] [PubMed] [Google Scholar]
- 32.Bassuini M M, Billings P J. Carcinoma in an ileoanal pouch after restorative proctocolectomy for familial adenomatous polyposis. Br J Surg. 1996;83:506. doi: 10.1002/bjs.1800830422. [DOI] [PubMed] [Google Scholar]
- 33.Palkar V M, deSouza L J, Jagannath P et al. Adenocarcinoma arising in "J" pouch after total proctocolectomy for familial polyposis coli. Indian J Cancer. 1997;34:16–19. [PubMed] [Google Scholar]
- 34.Cherki S, Glehen O, Moutardier V et al. Pouch adenocarcinoma after restorative proctocolectomy for familial adenomatous polyposis. Colorectal Dis. 2003;5:592–594. doi: 10.1046/j.1463-1318.2003.00486.x. [DOI] [PubMed] [Google Scholar]
- 35.Linehan G, Cahill R A, Kalimuthu S N et al. Adenocarcinoma arising in the ileoanal pouch after restorative proctocolectomy for familial adenomatous polyposis. Int J Colorectal Dis. 2008;23:329–330. doi: 10.1007/s00384-007-0400-1. [DOI] [PubMed] [Google Scholar]
- 36.Lee S H, Ahn B K, Chang H K et al. Adenocarcinoma in ileal pouch after proctocolectomy for familial adenomatous polyposis: report of a case. J Korean Med Sci. 2009;24:985–988. doi: 10.3346/jkms.2009.24.5.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tajika M, Nakamura T, Bhatia V et al. Ileal pouch adenocarcinoma after proctocolectomy for familial adenomatous polyposis. Int J Colorectal Dis. 2009;24:1487–1489. doi: 10.1007/s00384-009-0776-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


