Abstract
Background
Patients with advanced esophageal cancer carry poor prognoses; limited data exist to guide second-line therapy in the metastatic setting. Paclitaxel has been used yet is associated with limited efficacy. There is preclinical evidence of synergy between paclitaxel and cixutumumab, a monoclonal antibody targeting insulin-like growth factor-1 receptor. We conducted a randomized phase II trial of paclitaxel (arm A) versus paclitaxel plus cixutumumab (arm B) in the second-line for patients with metastatic esophageal or gastroesophageal junction (GEJ) cancers.
Methods
The primary endpoint was progression-free survival (PFS); 87 patients (43 in arm A, 44 in arm B) were treated.
Results
Median PFS was 2.6 months in arm A [90% CL 1.8-3.5] and 2.3 months in arm B [90% 2.0-3.5], P = .86. Stable disease was observed in 29 (33%) patients. Objective response rates for Arms A and B were 12% [90% CI, 5-23%] and 14% [90% CI, 6-25%]. Median overall survival was 6.7 months [90% CL 4.9-9.5] in arm A and 7.2 months [90% CL 4.9-8.1] in arm B, P = 56.
Conclusion
The addition of cixutumumab to paclitaxel in second-line therapy of metastatic esophageal/GEJ cancer was well tolerated but did not improve clinical outcomes relative to standard of care (ClinicalTrials.gov Identifier: NCT01142388).
Keywords: esophageal, gastroesophageal junction, insulin-like growth factor-1 receptor, xixutumumab
Paclitaxel has been used for second-line treatment of esophageal cancer, with limited efficacy. Considering the preclinical evidence of synergy between paclitaxel and cixutumumab, this randomized phase II trial of paclitaxel (arm A) versus paclitaxel plus cixutumumab (arm B) was conducted.
Lessons Learned.
The addition of insulin-like growth factor-1 receptor monoclonal antibody cixutumumab to paclitaxel was well-tolerated as second-line therapy in patients with metastatic esophageal or gastroesophageal junction (GEJ) carcinomas.
The primary endpoint was not met, as this combination of agents did not significantly improve progression-free survival.
Since the time of study completion, changes in the treatment landscape of esophageal and gastroesophageal junction cancer have highlighted the need for histology and biomarker-directed therapy.
Discussion
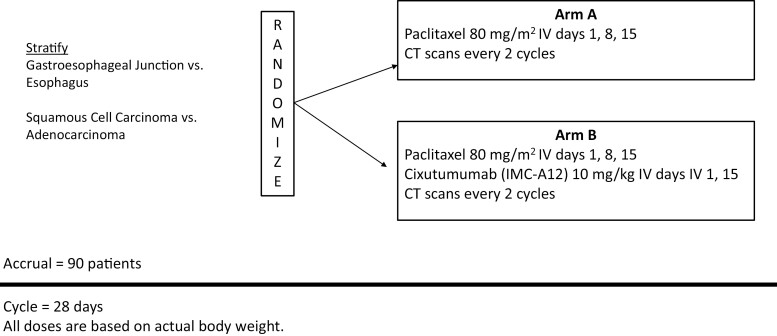
This randomized, multicenter, phase II trial evaluated paclitaxel (arm A) versus paclitaxel plus cixutumumab (arm B) as second-line therapy in patients with metastatic esophageal or gastroesophageal junction (GEJ) cancers (Fig. 1). The study was conducted from September 2010 to October 2012 and analyzed in 2014. Each regimen was well tolerated, with >Grade 3 toxicities observed in 53% [90% CI, 38-66%] of arm A patients and 52% [90% CI, 39-65%] of arm B patients. There was no improvement in clinical outcomes. The primary endpoint of improved progression-free survival (PFS) was not met. Meaningful differences were not detected in secondary endpoints, including overall survival (OS) and overall response rate (ORR).
Figure 1.
Study schema.
Taxanes have served as a cornerstone therapy for patients with platin-refractory metastatic esophageal and GEJ malignancies; however, treatment resistance is inevitable. As such, enhancing therapeutic potential of a single agent taxane is an appealing area of exploration. The insulin-like growth factor-1 receptor (IGF-1R) was of interest as such a therapeutic target, based on preclinical evidence for its role in treatment resistance in esophageal and GEJ tumors. In addition to the negative trial we report here, other studies of IGF-1R inhibition in gastrointestinal malignancies have also been negative. While IGF-1R inhibition is not currently advancing in studies in esophagogastric cancer, a number of other targets have now been validated in the clinic. Specifically, anti-angiogenic therapies directed against the VEGF receptor family have demonstrated meaningful clinical anti-tumor activity. Ramucirumab and paclitaxel gained FDA approval November 5, 2014 for the second-line treatment of unselected metastatic GEJ adenocarcinoma. Data from this trial were analyzed just prior to this regulatory licensure, based on data updates through July 15, 2014. It has also become clear that histology profoundly impacts treatment response in esophageal cancers. In squamous cell subtypes, immunotherapy has emerged as a second-line option, which was not standard of care at the time of enrollment on this trial. In adenocarcinomas, the development of biomarker-directed agents has created additional options for patients with tumors that overexpress certain protein markers (eg, Her2, FGFR2). Going forward, expanding the treatment landscape for refractory metastatic esophageal cancer will require special attention to histology and improved patient selection approaches.
| Trial Information | |
|---|---|
| Disease | Esophageal or GEJ cancer |
| Stage of disease/treatment | Stage IV |
| Prior therapy | One line of prior systemic therapy |
| Type of study | Phase II randomized |
| Primary endpoint | Median PFS |
| Secondary endpoints | Toxicity, median OS, ORR |
| Investigator’s analysis | Level of activity did not meet planned end point |
| Additional details of endpoints or study design | Patients were randomized 1:1 to arm A (paclitaxel) or arm B (paclitaxel plus cixutumumab). |
| Drug Information | Arm A | Arm B |
|---|---|---|
| Generic/working name | Paclitaxel | Paclitaxel plus cixutumumab |
| Company name drug type | Taxol | Taxol, IMC-A12 |
| Drug class | Taxane | Taxane, monoclonal antibody against IGF-1R |
| Dose | 80 mg/m2 | 80 mg/m2, 10 mg/kg |
| Route | IV | IV, IV |
| Schedule of administration | Days 1, 8, 15 of every 28 day cycle | Days 1, 8, 15 of every 28 day cycle; days 1, 15 of every 28 day cycle |
| Patient Characteristics: Overall Study Population | |
|---|---|
| Number of patients, male | 68 |
| Number of patients, female | 19 |
| Stage | IV (87) |
| Age: median (range) | 62 (40-89) years |
| Number of prior systemic therapies: median (range) | 1 |
| Performance status: ECOG | 0: 34 |
| 1: 49 | |
| 2: 4 | |
| 3: 0 | |
| 4: 0 | |
| Cancer types or histologic subtypes | Adenocarcinoma, 71; adenosquamous carcinoma, 2; squamous cell carcinoma, 14 |
| Primary Assessment Method: Median Progression-Free Survival, Arm A | |
|---|---|
| Number of patients enrolled | 94 |
| Number of patients evaluable for toxicity | 87 (43 in Arm A, 44 in Arm B) |
| Number of patients evaluated for efficacy | 84 |
| Evaluation method | RECIST 1.1 |
| Response assessment, CR | 0 (0%) |
| Response assessment, PR | 5 (11.6%) |
| Response assessment, SD | 14 (32.6%) |
| Response assessment, PD | 13 (30.2) |
| Median duration assessments, PFS | 2.6 months (95% CI, 1.8-3.5) |
| Median duration assessments, OS | 6.7 months (95% CI, 4.9-9.5) |
| Median duration of treatment | 2.0 cycles |
| Primary Assessment Method: Median Progression-Free Survival, Arm B | |
|---|---|
| Number of patients evaluable for toxicity | 87 (43 in Arm A, 44 in Arm B) |
| Evaluation method | RECIST 1.1 |
| Response assessment, CR | 1 (2.3%) |
| Response assessment, PR | 5 (11.4%) |
| Response assessment, SD | 15 (34.1%) |
| Response assessment, PD | 18 (40.9) |
| Median duration assessments, PFS | 2.3 months (95% CI, 2.0-3.5) |
| Median duration assessments, OS | 7.2 months (95% CI, 4.9-8.1) |
| Median duration of treatment | 2.0 cycles |
Assessment, Analysis, and Discussion
| Completion | Study completed |
| Investigator’s assessment | Level of activity did not meet planned end point |
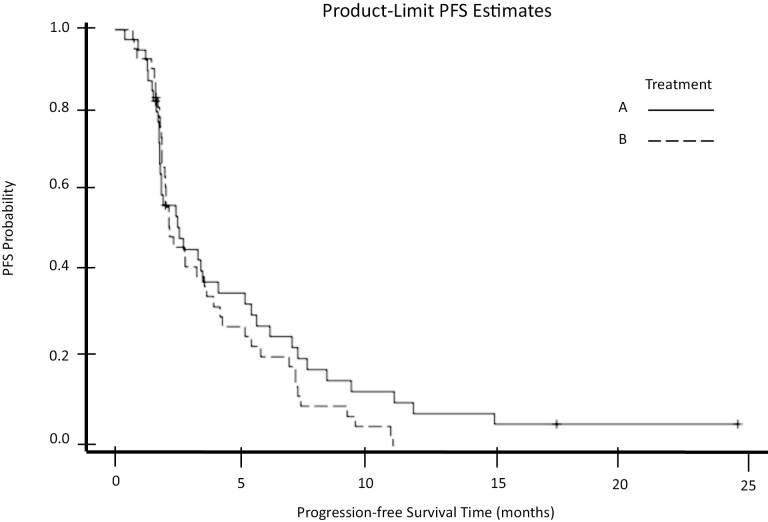
Our trial began enrollment on September 21, 2010 and closed to accrual on October 15, 2012. The data presented here represents updates through July 15, 2014. At the time when this study was conducted, IGF1-R was being investigated as a potential therapeutic target in various gastrointestinal malignancies. This randomized phase II trial assessed paclitaxel (arm A) and paclitaxel plus IGF1-R inhibitor cixutumumab (arm B) in the second-line setting for patients with esophageal or GEJ cancer. A total of 94 patients enrolled, with 7 patients deemed ineligible after enrollment. All 87 eligible patients were included in efficacy analyses, and the 84 patients who started treatment were included in safety analyses. Of the total study population, 40 patients had tumors of the esophagus, and 47 had GEJ tumors. By histology, there were 71 patients with adenocarcinoma, 2 with adenosquamous carcinoma, and 14 with squamous cell carcinoma. The majority of patients were male (78.2%) non-Hispanic White (94.3%) and possessed an ECOG PS of 1 (56.3%). The GEJ was the most common primary site represented (54%). Patients completed a median of 2.0 cycles of therapy in each arm. Toxicity rates were similar between arms (Table 1). Grade ≥3 toxicity rates were 53% in arm A [90% CI, 38-66%] and 52% [90% CI 39,-65%] in arm B; these were predominantly hematologic toxicities. Notably, 2 patients experienced grade 5 toxicities classified as treatment-related adverse events: one in arm A defined as death not otherwise specified, and one in arm B defined as death due to respiratory failure. Per intention-to-treat analysis, median mPFS for arm s A and B was 2.6 (90% CI, 1.8-3.5) and 2.3 (90% CI, 2.0-3.5) months, respectively (P = 0.86), and thus the primary endpoint was not met (Fig. 2). The median (mOS) for arms A and B were 6.7 (90% CI, 4.9-9.5) and 7.2 (90% CI, 4.9-8.1) months, respectively (P = .56). There were five partial responses in arm A, and five partial response and one complete response in arm B. Overall response rates were 11.6% in arm A and 13.7% in arm B. Despite the study treatment being relatively well-tolerated, we did not find meaningful clinical benefit from adding cixutumumab to paclitaxel in this patient population.
Table 1.
Toxicity by arm (frequency)
| Toxicity type | Treatment arm | |||
|---|---|---|---|---|
| A (n = 40) | B (n = 44) | |||
| Grade | Grade | |||
| 1,2 | ≥3a | 1,2 | ≥3a | |
| Hematologic | ||||
| Anemia | 28 | 4 | 27 | 4 |
| White blood cell count decreased | 11 | 2 | 15 | 6 |
| Lymphocyte count decreased | 13 | 8 | 12 | 8 |
| Neutrophil count decreasedb | 8 | 3 | 10 | 8 |
| Platelet count decreased | 6 | — | 10 | 1 |
| Constitutional | ||||
| Fatigue | 26 | 3 | 27 | 1 |
| Pruritis | 3 | — | 5 | — |
| Weight loss | 8 | — | 8 | 1 |
| Myalgia | 1 | 1 | 5 | — |
| Dizziness | 2 | — | 5 | — |
| Anorexia | 7 | — | 12 | — |
| Gastrointestinal | ||||
| Constipation | 4 | — | 2 | — |
| Diarrhea | 8 | — | 10 | — |
| Mucositis | 2 | — | 3 | 2 |
| Nausea | 8 | 1 | 13 | 1 |
| Vomiting | 6 | — | 8 | 2 |
| Alanine aminotransferase increased | 5 | — | 4 | — |
| Alkaline phosphatase increased | 5 | — | 10 | — |
| Aspartate aminotransferase increased | 5 | — | 8 | — |
| Dysgeusia | 1 | — | 5 | — |
| Electrolyte abnormality | ||||
| Hypocalcemia | — | — | 5 | — |
| Hypokalemia | 3 | 1 | 3 | — |
| Hypomagnesemia | 5 | — | 6 | — |
| Hyponatremia | 3 | — | 8 | 1 |
| Hypophosphatemia | 2 | 2 | 2 | 1 |
| Endocrine | ||||
| Glucose intolerance | 3 | — | 4 | 1 |
| Hyperglycemia | 9 | 2 | 15 | 5 |
| Dermatologic | ||||
| Acneiform rash | 1 | — | 5 | — |
| Maculopapular rash | 2 | 1 | 8 | — |
| Other | ||||
| Peripheral sensory neuropathy | 13 | 1 | 15 | 1 |
| Edema of limbs | 4 | — | — | — |
| Alopecia | 13 | — | 12 | — |
| Visual flashing lights | — | — | 7 | — |
aOne treatment-related Grade 5 event was observed in each arm - death not otherwise specified in arm A, and death due to respiratory failure in arm B.
bNo neutropenic fever was observed.
Figure 2.
Progression-free survival (PFS) by arm.
When this trial was designed, preclinical data suggested that IGF-1R may serve as a therapeutic target in esophageal cancer, with in vitro tumor models demonstrating overexpression of IGF-1R.1,2 In numerous tumor types, including gastric cancer, IGF-1R overexpression has been associated with poor prognosis and chemoresistance.3–5 Monoclonal antibodies against IGF-1R have since been studied in multiple gastrointestinal malignancies without evidence of clinical activity. In a phase II trial of cixutumumab with or without cetuximab in patients with cetuximab or panitumumab-refractory metastatic colorectal cancer, neither monotherapy nor combination therapy improved overall response rate meaningfully for patients.6 Istiratumab, a monoclonal antibody targeting both IGF-1R Her3, was evaluated with and without gemcitabine and nab-paclitaxel in patients with metastatic pancreatic adenocarcinoma in the randomized phase II CARRIE trial. This study also failed to find a clinical benefit, with no meaningful difference in PFS between the trial arms.7 Studies investigating the resistance mechanisms to IGF-1R inhibition have suggested downstream receptor tyrosine kinase (RTK) activation as a compensatory response. It has been proposed that antibody targeting of IGF-1R can bias the receptor to association with arrestin-1 and, thus, actually promote ERK1/2 signaling.8 In theory, giving combination therapy to target downstream RTKs could augment the poor response rates seen to IGF-1R inhibitor monotherapy. However, in clinical trials of multikinase inhibitors targeting IGF-1R and downstream RTKs, it does not appear that there were meaningful improvement in objective response over other RTKs that do not target IGF-1R.9,10
In the time, since this study was completed, the treatment landscape for esophageal and GEJ cancers has evolved. Histology has emerged as a key consideration in formulating a treatment plan, as management of esophageal adenocarcinoma is now distinct from squamous cell carcinoma. Our study predominantly consisted of patients with adenocarcinoma as is observed mostly in Western patient populations. The preclinical data, however, suggest greater expression of IGF-1R in esophageal squamous cell carcinoma, suggesting that perhaps this might have been a more ideal patient population to test the agent.11 While taxanes remain an acceptable second-line treatment option for patients with refractory metastatic esophageal cancer, immunotherapy, and biomarker- selected agents are now available as well.12 In patients with adenocarcinoma, Her2, and FGFR2b are potentially actionable targets. For instance, based on the phase 2 trial DESTINY-Gastric02, patients with Her2-overexpressing GEJ cancers can now receive trastuzumab deruxtecan in the second-line setting.13 Anti-VEGF targeting combination therapy has also emerged as standard of care therapy for GEJ adenocarcinoma based on the RAINBOW trial, a phase III randomized controlled trial of ramucirumab plus paclitaxel versus paclitaxel monotherapy.14 In patients with squamous cell esophageal cancers, immunotherapy is now widely used in the second-line setting, with nivolumab approved regardless of irrespective of tumor PD-L1 status and pembrolizumab approved in patients with tumor PD-L1 expression levels of CPS of ≥10.15,16 Despite these advances, the prognosis of patients with metastatic esophageal and GEJ cancer remains poor; there remains a need to continue to look for novel targets and tolerable therapeutics for these targets.
Acknowledgments
This study was coordinated by the ECOG-ACRIN Cancer Research Group (Peter J. O’Dwyer, MD and Mitchell D. Schnall, MD, PhD, Group Co-Chairs). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Contributor Information
Shannon Stockton, Department of Medicine, Vanderbilt University Medical Center, Nashville, TN, USA.
Paul Catalano, Dana-Farber Cancer Institute, ECOG-ACRIN Biostatistics Center, Boston, MA, USA.
Steven J Cohen, Jefferson Health System/Abington Memorial Hospital, Abington, PA, USA.
Barbara A Burtness, Yale University, New Haven, CT, USA.
Edith P Mitchell, Thomas Jefferson University, Philadelphia, PA, USA.
Efrat Dotan, Fox Chase Cancer Center, Philadelphia, PA, USA.
Sam J Lubner, University of Wisconsin, Madison, WI, USA.
Pankaj Kumar, Illinois CancerCare, Peoria, IL, USA.
Mary F Mulcahy, Northwestern University, Evanston, IL, USA.
George A Fisher, Jr., Stanford Cancer Center, Stanford University, Palo Alto, CA, USA
Theodore L Crandall, University of Pittsburgh, Pittsburgh, PA, USA.
Al Benson, Northwestern University, Evanston, IL, USA.
Funding
This study was supported by the National Cancer Institute of the National Institutes of Health under award numbers: U10CA180820, U10CA180794, UG1CA180799, UG1CA180826, UG1CA180844, UG1CA189830, U G1CA233270, UG1CA233320, and UG1CA233341.
Conflict of Interest
Sam J. Lubner reported consulting for Elephas Bio and research support from AstraZeneca, Iincyte, and BMS. Al Benson reported consulting or advisory role for Bristol-Myers Squibb DMC, Novartis DMC, Pfizer, Therabionic, Mirati Therapeutics, GSK, Tempus, Boehringer-Ingelheim, Astrellas DMC, Mirati, and AM Immunotech and research funding from ITM, Elevar Therapeutics INC, Merk Sharp and Dohme LLC, ST Pharm CO Ltd, The Nathan Cummings Foundation, and Cardiff Oncology. The other authors indicated no financial relationships.
Data Availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Zhang T, Shen H, Dong W, et al. Antitumor effects and molecular mechanisms of figitumumab, a humanized monoclonal antibody to IGF-1 receptor, in esophageal carcinoma. Sci Rep. 2014;4:6855. 10.1038/srep06855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Imsumran A, Adachi Y, Yamamoto H, et al. Insulin-like growth factor-I receptor as a marker for prognosis and a therapeutic target in human esophageal squamous cell carcinoma. Carcinogenesis. 2007;28(5):947-956. 10.1093/carcin/bgl247. [DOI] [PubMed] [Google Scholar]
- 3. Ge J, Chen Z, Wu S, et al. Expression levels of insulin-like growth factor-1 and multidrug resistance-associated protein-1 indicate poor prognosis in patients with gastric cancer. Digestion. 2009;80(3):148-158. 10.1159/000226089. [DOI] [PubMed] [Google Scholar]
- 4. Hardman RA, Kari FW, Barrett JC.. Insulin-like growth factor 1 (IGF-1) alters drug sensitivity of HBL100 human breast cancer cells by inhibition of apoptosis induced by diverse anticancer drugs. Cancer Res. 1997;57(13):2687-2693. [PubMed] [Google Scholar]
- 5. Sun HZ, Wu SF, Tu ZH.. Blockage of IGF-1R signaling sensitizes urinary bladder cancer cells to mitomycin-mediated cytotoxicity. Cell Res. 2001;11(2):107-115. 10.1038/sj.cr.7290075. [DOI] [PubMed] [Google Scholar]
- 6. Reidy DL, Vakiani E, Fakih MG, et al. Randomized, phase II study of the insulin-like growth factor-1 receptor inhibitor IMC-A12, with or without cetuximab, in patients with cetuximab- or panitumumab-refractory metastatic colorectal cancer. J Clin Oncol. 2010;28(27):4240-4246. 10.1200/JCO.2010.30.4154. Epub 2010 Aug 16. PMID: 20713879; PMCID: PMC3296668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kundranda M, Gracian AC, Zafar SF, et al. Randomized, double-blind, placebo-controlled phase II study of istiratumab (MM-141) plus nab-paclitaxel and gemcitabine versus nab- paclitaxel and gemcitabine in front-line metastatic pancreatic cancer (CARRIE). Ann Oncol. 2020;31(1):79-87. 10.1016/j.annonc.2019.09.004. Erratum in: Ann Oncol. 2020 Aug;31(8):1094. PMID: 31912800. [DOI] [PubMed] [Google Scholar]
- 8. Zheng H, Shen H, Oprea I, et al. β-Arrestin-biased agonism as the central mechanism of action for insulin-like growth factor 1 receptor-targeting antibodies in Ewing’s sarcoma. Proc Natl Acad Sci USA. 2012;109(50):20620-20625. 10.1073/pnas.1216348110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Xing P, Zhao Q, Zhang L, et al. Conteltinib (CT-707) in patients with advanced ALK-positive non-small cell lung cancer: a multicenter, open-label, first-in-human phase 1 study. BMC Med. 2022;20(1):453. 10.1186/s12916-022-02646-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kuenzi BM, Remsing Rix LL, Stewart PA, et al. Polypharmacology- based ceritinib repurposing using integrated functional proteomics. Nat Chem Biol. 2017;13(12):1222-1231. 10.1038/nchembio.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Doyle SL, Donohoe CL, Finn SP, et al. IGF-1 and its receptor in esophageal cancer: association with adenocarcinoma and visceral obesity. Am J Gastroenterol. 2012;107(2):196-204. 10.1038/ajg.2011.417. Epub 2011 Dec 6. PMID: 22146489 [DOI] [PubMed] [Google Scholar]
- 12. National Comprehensive Cancer Network. Esophageal Cancer (Version 5.2022). Accessed December 19, 2022.https://www.nccn.org/professionals/physician_gls/pdf/esophageal.pdf
- 13. Ku G, di Bartolomeo M, Smyth E, et al. Updated analysis of DESTINY-Gastric02: a phase II single-arm trial of trastuzumab deruxtecan (T-DXd) in western patients (Pts) with HER2-postitive (HER2+) unresectable/metastatic gastric/gastroesophageal junction (GEJ) cancer who progressed on or after trastuzumab-containing regimen. Presented at: European Society for Medical Oncology Congress 2022; September 9-13, 2022; Paris, France.
- 14. Wilke H, Muro K, Van Cutsem E, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. 2014;15(11):1224-1235. 10.1016/S1470-2045(14)70420-6. Epub 2014 Sep 17. PMID: 25240821. [DOI] [PubMed] [Google Scholar]
- 15. Kato K, Cho BC, Takahashi M, et al. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20(11):1506-1517. 10.1016/S1470-2045(19)30626-6. Epub 2019 Sep 30. Erratum in: Lancet Oncol. 2019 Nov;20(11):e613. PMID: 31582355. [DOI] [PubMed] [Google Scholar]
- 16. Sun JM, Shen L, Shah MA, et al. . Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomised, placebo- controlled, phase 3 study. Lancet. 2021;398(10302):759-771. 10.1016/S0140-6736(21)01234-4. Erratum in: Lancet. 2021 Nov 20;398(10314):1874. PMID: 34454674. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.