Abstract
Approximately 50% of the individuals living with human immunodeficiency virus type 1 (HIV-1) are plagued by debilitating neurocognitive impairments (NCI) and/or affective alterations. Sizeable alterations in the composition of the gut microbiome, or dysbiosis, may underlie, at least in part, the NCI, apathy, and/or depression observed in this population. Herein, two interrelated aims will be critically addressed, including: 1) the evidence for, and functional implications of, gastrointestinal microbiome dysbiosis in HIV-1 seropositive individuals; and 2) the potential for therapeutically targeting the consequences of this dysbiosis for the treatment of HIV-1-associated NCI and affective alterations. First, gastrointestinal microbiome dysbiosis in HIV-1 seropositive individuals is characterized by decreased alpha (α) diversity, a decreased relative abundance of bacterial species belonging to the Bacteroidetes phylum, and geographic-specific alterations in Bacillota (formerly Firmicutes) spp. Fundamentally, changes in the relative abundance of Bacteroidetes and Bacillota spp. may underlie, at least in part, the deficits in γ-aminobutyric acid and serotonin neurotransmission, as well as prominent synaptodendritic dysfunction, observed in this population. Second, there is compelling evidence for the therapeutic utility of targeting synaptodendritic dysfunction as a method to enhance neurocognitive function and improve motivational dysregulation in HIV-1. Further research is needed to determine whether the therapeutics enhancing synaptic efficacy exert their effects by altering the gut microbiome. Taken together, understanding gastrointestinal microbiome dysbiosis resulting from chronic HIV-1 viral protein exposure may afford insight into the mechanisms underlying HIV-1-associated neurocognitive and/or affective alterations; mechanisms which can be subsequently targeted via novel therapeutics.
Keywords: Brain-Gut-Microbiota Axis, HIV-1-Associated Neurocognitive Disorders, Apathy, Depression, Synaptic Dysfunction, Neurotransmission
INTRODUCTION: BRAIN-GUT-MICROBIOTA AXIS
Trillions of microbes (predominantly bacteria; Qin et al., 2010, Yatsunenko et al., 2012), which outnumber human cells at a ratio of approximately 1.3:1 (Sender et al., 2016), are distributed in multiple organs throughout the human body. The gastrointestinal tract, in particular, harbors the largest microbial community (Sender et al., 2016); a bacterial community that is dominated by two phylogenetic categories, including Bacteroidetes and Bacillota (formerly Firmicutes; Hold et al., 2002; Eckburg et al., 2005; Qin et al., 2010). Nevertheless, the gut microbiome is highly diverse and individualized (Costello et al., 2009), whereby its composition is influenced by both intrinsic (e.g., Biological Sex: Mueller et al., 2006; Takagi et al., 2019; Genetics: Goodrich et al., 2014) and extrinsic (e.g., Dietary Habits: David et al., 2014; Sexual Behaviors: Noguera-Julian et al., 2016) factors. Fundamentally, the gut microbiome has a broad functional repertoire, ranging from metabolism and immunity to neurobehavioral functions (for review, Kamada et al., 2013; Sommer & Bäckhed, 2013; Sharon et al., 2016).
Resident gut microbiota dynamically communicate with the brain via multiple bidirectional pathways, which are collectively recognized as the brain-gut-microbiota axis, to influence neurobehavioral functions (for review, Cryan et al., 2019). The most direct and fastest route connecting the brain and gut is the vagus, or tenth cranial, nerve. Vagal afferent fibers, which outnumber efferent fibers at a ratio of approximately 8:2 (Agostoni et al., 1957), project from the gastrointestinal tract to the nucleus tractus solitarius via the nodose ganglion. In contrast, vagal efferent fibers exit the brain via the medulla oblongata, travel through the neck and thorax, and have branches that terminate in the stomach, small intestine, and large intestine (Berthoud et al., 1991). The functional importance of bidirectional signaling in the vagal nerve has been evidenced by both vagal nerve ablation and stimulation, whereby vagotomy induces neurocognitive impairments (NCI; Suarez et al. 2018) and affective alterations (Ghizoni et al., 2006); vagal nerve stimulation, in contrast, improves cognitive function (e.g., Clark et al., 1999; Driskill et al., 2022) and affective alterations (e.g., Rush et al., 2000). Subdiaphragmatic vagotomy of healthy mice who receive fecal microbiota transplantation from chronically stressed animals has revealed depressive-like behaviors and hippocampal neurogenesis to be dependent upon vagal afferent fibers for gut microbiome-mediated brain alterations to occur (Siopi et al., 2023). More fundamentally, the vagus nerve serves as a conduit for gut bacteria to influence both cognitive and affective function.
Sizeable alterations in the composition of the gut microbiome, or dysbiosis, may underlie, at least in part, NCI and/or affective alterations induced by infectious diseases (e.g., Human Immunodeficiency Virus Type 1 (HIV-1); Lozupone et al., 2013; Pérez-Santiago et al., 2021). Gut microbiome dysbiosis is commonly evaluated by quantifying two components of species diversity, including 1) alpha (α) diversity; and 2) beta (β) diversity. α diversity, which estimates local (within-sample) diversity, can be measured using a variety of indices (e.g., Shannon Diversity Index (Based on the Shannon and Weaver Information Theory, Shannon, 1948); Simpson’s Index: Simpson, 1949; Chao1: Chao, 1984) to reflect both the evenness and/or richness of a microbial sample. β diversity, initially formulated by Whittaker (1960, 1972), is a derived measure utilized to capture the similarity and dissimilarity between samples. Statistical methods, including Bray-Curtis (Bray & Curtis, 1957) and UniFrac (Lozupone et al., 2005), have been developed to quantify β diversity and establish the statistical significance of group differences (e.g., Permutational Multivariate ANOVA (PERMANOVA); Anderson, 2017). Furthermore, examination of the relative abundance of bacteria within a given phylum, genus, and/or species affords an opportunity to more directly interpret how group differences may mechanistically underlie NCI and/or affective alterations.
HIV-1, which afflicts approximately 38.4 million individuals worldwide (WHO, 2022), is an infectious disease that induces profound NCI (Cysique et al., 2004; Heaton et al., 2011; for review, Zenebe et al., 2022) and affective alterations (e.g., Apathy: Kamat et al., 2012; Depression: Do et al., 2014); comorbidities which necessitate the development of novel adjunctive therapeutics. Using two interrelated aims, the present review will evaluate the potential for therapeutically targeting gastrointestinal dysbiosis for the treatment of HIV-1-associated neurocognitive disorders (HAND) and apathy/depression. First, we will discuss the evidence for gastrointestinal dysbiosis in HIV-1, with a specific focus on the functional implications of these changes in HIV-1-associated neurocognitive and affective alterations. Second, the present review will examine the opportunity to mitigate NCI and/or affective alterations associated with HIV-1 by therapeutically targeting the consequences (e.g., decrease butyrate production) of gut microbiome dysbiosis.
GASTROINTESTINAL MICROBIOME DYSBIOSIS IN HIV-1
Gastrointestinal microbiome dysbiosis is established during acute HIV-1 infection (i.e., Fiebig Stage I-V), whereby it is characterized by selective deficits in α diversity, clear alterations in β diversity, and significant changes in the taxonomic composition of gut bacteria (Sortino et al., 2020). During chronic HIV-1 infection, alterations in the gut microbiome persist, despite treatment with combination antiretroviral therapy (cART, i.e., the primary treatment regimen for HIV-1 seropositive individuals).
Thus, one of the primary goals of the present review is to utilize a multi-faceted approach, including examination of α diversity, β diversity, and taxonomic composition, to characterize gut microbiome dysbiosis in adults who are chronically infected with HIV-1. Establishing how chronic HIV-1 viral protein exposure alters the gut microbiome affords an opportunity to consider the functional implications of these changes with respect to HIV-1 associated neurocognitive and/or affective alterations. Herein, 38 case-control studies (see Figure 1) comparing the gut microbiome in HIV-1 seropositive adults with chronic infection and well-matched controls were identified and evaluated.
Figure 1.
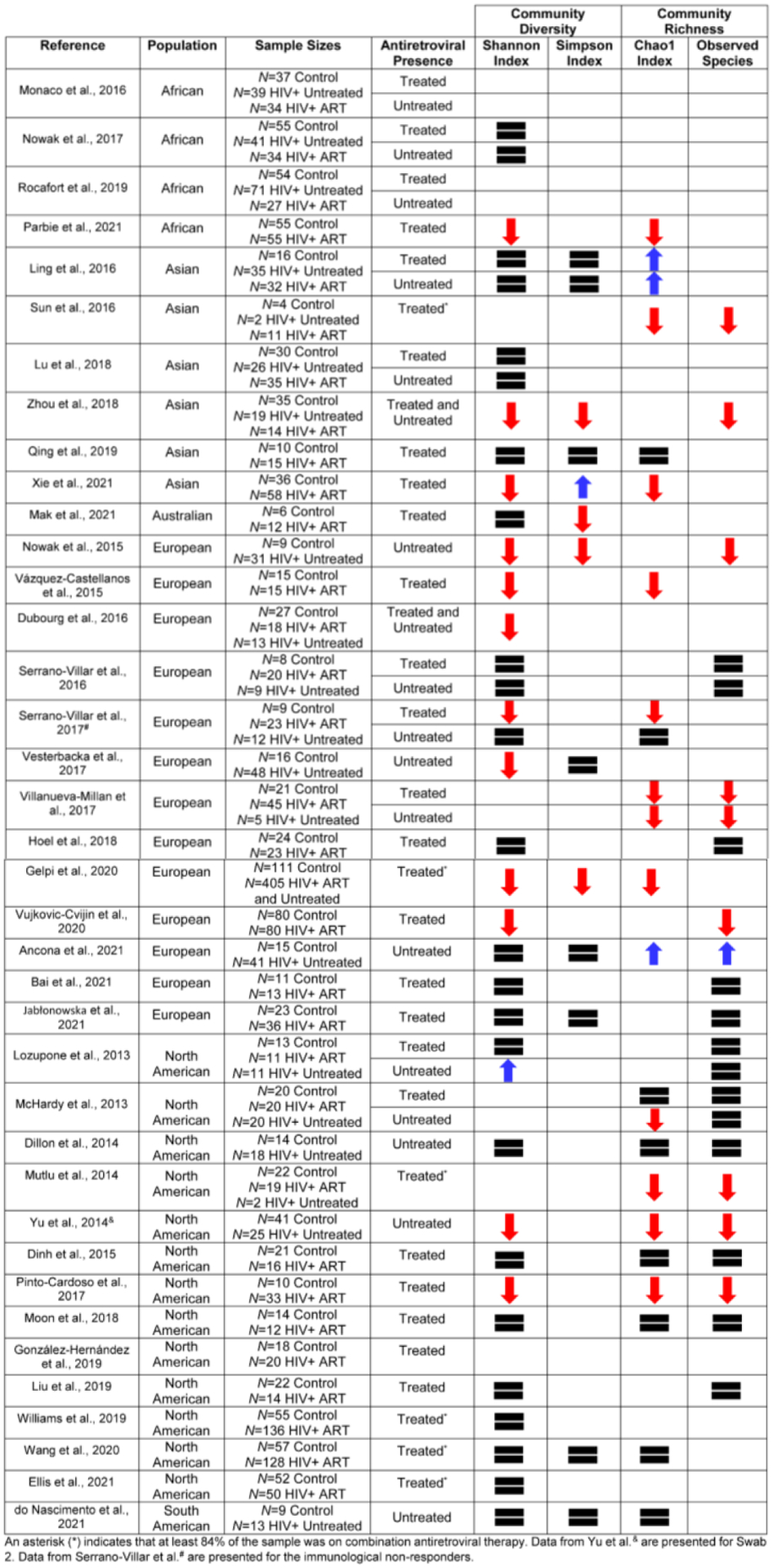
Characteristics of the 38 case-control studies comparing the gut microbiome in HIV-1 seropositive individuals with chronic infection and well-matched controls. Results from four alpha (α) diversity indices measuring community diversity (Shannon Index, Simpson Index) and community richness (Chao1 Index, Observed Species) are utilized to illustrate the significant inter-study heterogeneity observed within the field. The  indicates no statistically significant difference between HIV-1 seropositive individuals and controls, whereas the
indicates no statistically significant difference between HIV-1 seropositive individuals and controls, whereas the  and
and  illustrate significant decreases and increases, respectively, in α diversity in HIV-1 seropositive individuals relative to case-controls.
illustrate significant decreases and increases, respectively, in α diversity in HIV-1 seropositive individuals relative to case-controls.
Alpha (α) Diversity
Amongst the 36 case-control studies evaluating α diversity, the Shannon index, a measure of community diversity whose formula weighs taxa evenness more heavily than richness (Strong, 2016), was most commonly implemented (i.e., 31 of 36 studies). The Simpson index was utilized much more sparingly (i.e., 12 of 36 studies) to evaluate gastrointestinal system community diversity. With regards to measures of community richness, the Chao1 index and number of observed species were examined in a similar number of studies (i.e., 17 of 37 and 18 of 37 studies, respectively). Unsurprisingly, given similarities in α diversity indices, intra-study variability (i.e., significant and/or non-significant group differences in α diversity) was generally low.
Empirical studies provide ample evidence for a statistically significant decrease in α diversity in chronically infected HIV-1 seropositive, relative to seronegative, individuals (e.g., Mutlu et al., 2014; Vujkovic-Cvijin et al., 2020). Indeed, in empirical studies, the profound downregulation of α diversity following chronic exposure to HIV-1 viral proteins is observed across multiple geographic regions (e.g., African: Parbie et al., 2021; Asian: Zhou et al., 2018; North American: Pinto-Cardoso et al., 2017) and in both the presence (e.g., Vujkovic-Cvijin et al., 2020) and absence (e.g., Nowak et al., 2015) of cART. Meta-analytic techniques, which synthesize and analyze independent empirical studies to yield effect estimates with greater statistical power (for review, Lipsey & Wilson, 2001), have also demonstrated the association between HIV-1 serostatus and decreased α diversity in the gastrointestinal microbiome (Tuddenham et al., 2020; Zhou et al., 2020). Stratification of meta-analytic analyses by gender and/or sexual orientation revealed that decreased α diversity is specific to female and non-MSM HIV-1 seropositive individuals; no significant alterations in α diversity were observed in HIV-1 seropositive MSM (Tuddenham et al., 2020; Zhou et al., 2020). Despite the strong empirical and meta-analytic evidence for the downregulation of α diversity in chronically infected HIV-1 seropositive individuals, it is notable that statistically significant increases have also been spuriously observed (e.g., Lozupone et al., 2013; Xie et al., 2021).
Gut microbiome diversity is significantly correlated with multiple lifestyle factors and clinical markers of peripheral health and disease (Manor et al., 2020); the functional impact of α diversity alterations on cognitive and/or affective function, however, remains understudied. Stepwise regression (Canipe et al., 2021) and mediation analyses (Cai et al., 2021) have demonstrated the association between α diversity (i.e., Shannon and Simpson indices, respectively) and neurocognitive function (e.g., working memory, attention). With specific regards to HIV-1 seropositive individuals, a comparison of individuals with and without NCI revealed no statistically significant differences in α diversity (Zhang et al., 2019; Dong et al., 2021); dichotomization of individuals, however, naturally decreases variability and statistical power (Rucker et al., 2015). Regressing continuous measures of neurocognitive performance on indices of α diversity in HIV-1 seropositive individuals ought to provide increased clarity regarding the functional implications for the profound downregulation of α diversity seen in this population.
Beta Diversity
Twenty-seven of the case-control studies comparing the gut microbiome in HIV-1 seropositive individuals with chronic infection evaluated β diversity. The UniFrac (weighted or unweighted; 17 of 27 studies) and Bray-Curtis (13 of 27 studies) indices were the most commonly utilized β diversity measures. Differential clustering of samples based on HIV-1 serostatus was evaluated using both exploratory (e.g., Principal Component Analysis, Principal Coordinate Analysis) and/or inferential (e.g., PERMANOVA) statistical approaches. Statistically significant (e.g., Mutlu et al., 2014; Bai et al., 2021) differences in the overall gastrointestinal microbiota community composition between HIV-1 seropositive and case-matched controls have been reported. Identification of specific changes in the taxonomic composition of bacteria in HIV-1 seropositive individuals may afford insight into the functional implications of β diversity changes.
Relative Abundance of Bacteria
Six hierarchical taxonomic categories, including phylum, class, order, family, genus, and species, are utilized to categorize organisms based on similarities in their phylogenetic lineage and characteristics (i.e., Gram’s stain; Morphology). Within the gastrointestinal tract, the bacterial community is dominated by two phylogenetic categories, including Bacteroidetes and Bacillota (Hold et al., 2002; Eckburg et al., 2005; Qin et al., 2010); phylogenetic categories which will be the focus of the present review. However, it is notable that chronic HIV-1 viral protein exposure affects the gastrointestinal microbiome more broadly, as alterations in Pseudomonadota (formerly Proteobacteria; e.g., Dillon et al., 2014; Xie et al., 2021) have also been reported.
Bacteroidetes
Bacteria belonging to the phylum Bacteroidetes are Gram-negative rod-shaped bacteria that represent an unexpected mix of physiological types (i.e., Anaerobic: Genus, Bacteroides; Aerobic: Genus, Flavobacteria). From a taxonomic classification perspective, the phylum Bacteroidetes is subdivided into six classes (Schoch et al., 2020), whereby the majority of the gastrointestinal Bacteroidetes spp. belong to one of four families (i.e., Bacteroidaceae, Porphyromonadaceae, Prevotellaceae, or Rikenellaceae; for review, Rajilić-Stojanović & de Vos, 2014). Bacteroidetes spp. become one of the most predominant taxa in the intestinal microbiome by the end of the first year of life (Palmer et al., 2007); a taxa that, in healthy adults, is one of the most stable microbiota components across time (Rajilić-Stojanović et al., 2013). The critical role of Bacteroidetes spp. is evidenced not only by their early colonization and long-term stability, but also by the absence of Bacteroidetes spp. in a terminally ill patient (i.e., resistant tuberculosis; Dubourg et al., 2013). Indeed, as symbionts, their primary function is the degradation of proteins or polysaccharides, which is fundamental for the optimal uptake of energy from the diet. In addition, Bacteroidetes spp. interact with the immune system to activate T-cell-mediated responses (Onderdonk et al., 1982) and provide protection from pathogens (Mazmaian et al., 2008).
Given the fundamental role of gastrointestinal Bacteroidetes spp., changes in their relative abundance may underlie the development of neurocognitive and/or affective disorders associated with HIV-1. At the phylum level, a pronounced decrease in the relative abundance of Bacteroidetes has been reported in chronically infected HIV-1 seropositive, relative to seronegative, individuals (Ling et al., 2016; Sun et al., 2016; Zhou et al., 2018; Parbie et al., 2021; Xie et al., 2021). More specifically, the relative abundance of two genera, including Bacteroides (e.g., Dillon et al., 2014; Ling et al., 2016; Parbie et al., 2021) and Prevotella (e.g., Lozupone et al., 2013; Mutlu et al., 2014; Ancona et al., 2021), are significantly altered (i.e., decreased and increased, respectively) by chronic exposure to HIV-1 viral proteins. It is notable that even with cART, which mitigates CD4+ T-cells defects (Autran et al., 1997), Bacteroides spp. remain decreased in HIV-1 seropositive, relative to seronegative, individuals (Mutlu et al., 2014; Parbie et al., 2021). The potential functional implications of decreased Bacteroides and increased Prevotella with respect to neurocognitive impairments and/or apathy/depression in this population will be discussed in turn below.
Bacteroides.
Early in the course of HIV-1 infection, viral proteins productively infect CD4+ T-cells resulting in the progressive destruction of these cells (Dalgleish et al., 1984; Klatzmann et al., 1984); cell loss which may underlie, at least in part, the profound decrease in Bacteroides in chronically infected HIV-1 seropositive individuals. Bacteroides spp., including Bacteroides fragilis, Bacteroides uniformis, and Bacteroides cellulosilyticus, are capable of producing bacterial zwitterionic polysaccharides (ZPS; i.e., the presence of polysaccharide A (PSA) operons; Neff et al., 2016), whereby PSA, produced by B. fragilis, is the most well-studied. Indeed, the ZPS PSA has two fundamental characteristics, including: 1) repeating units with positively and negatively charged groups that are crucial to modulating immune responses (Tzianabos et al., 1993); and 2) reliance on CD4+ T-cell toll-like receptors for colonization (Round et al., 2011). Furthermore, the absence of PSA operons precludes the ability of B. fragilis isolates to colonize the gastrointestinal system (Round et al., 2011). The well-recognized reduction of CD4+ T-cells in chronic HIV-1 infection, therefore, supports a decrease in PSA resulting in the depletion of Bacteroides spp. in the gut microbiome of chronically infected HIV-1 seropositive individuals.
Decreased production of the inhibitory neurotransmitter γ-aminobutyric acid (GABA) is likely one of the consequences of decreased Bacteroides spp. in chronically infected HIV-1 seropositive individuals (Figure 2A). GABA can be produced by intestinal bacteria via the glutamate decarboxylase (GAD) system, which utilizes pyridoxal-5’-phosphate glutamate decarboxylase (i.e., gadA or gadB) to remove the carboxyl group from l-glutamic acid resulting in the production of GABA and carbon dioxide (Fonda, 1985). A variety of Bacteroides spp. exhibit a high prevalence of gadB orthologs (Pokusaeva et al., 2017; Strandwitz et al., 2019), express genes encoding the GAD system (Otaru et al., 2021), and functionally produce GABA (Strandwitz et al., 2019; Otaru et al., 2021). Fundamentally, the ingestion of probiotic bacteria that produce GABA (e.g., Lactobacillus rhamnosus: Lin, 2013; Bifidobacterium longum subsp. infantis: Barrett et al., 2012) has beneficial behavioral (e.g., anxiolytic) and/or neurochemical (e.g., region-dependent alterations in GABAA and GABAB receptors) effects (Bravo et al., 2011; Bercik et al., 2011; Janik et al., 2016). The therapeutic effects of both L. rhamnosus (Bravo et al., 2011) and B. longum (Bercik et al., 2011) were blocked by vagotomy supporting the involvement of vagal pathways, which express a high density of GABAB receptors (Margeta-Mitrovic et al., 1999), in the transmission of GABA from the gastrointestinal system to the brain. Additional studies are needed to evaluate the cognitive and/or affective effects of GABA produced by Bacteroides spp. on the central nervous system. Collectively, the data support a profound decrease in Bacteroides spp. in chronically infected HIV-1 seropositive individuals that is induced by the productive infection of CD4+ T-cells and leads to a dramatic reduction in GABA neurotransmission (Figure 2A).
Figure 2.
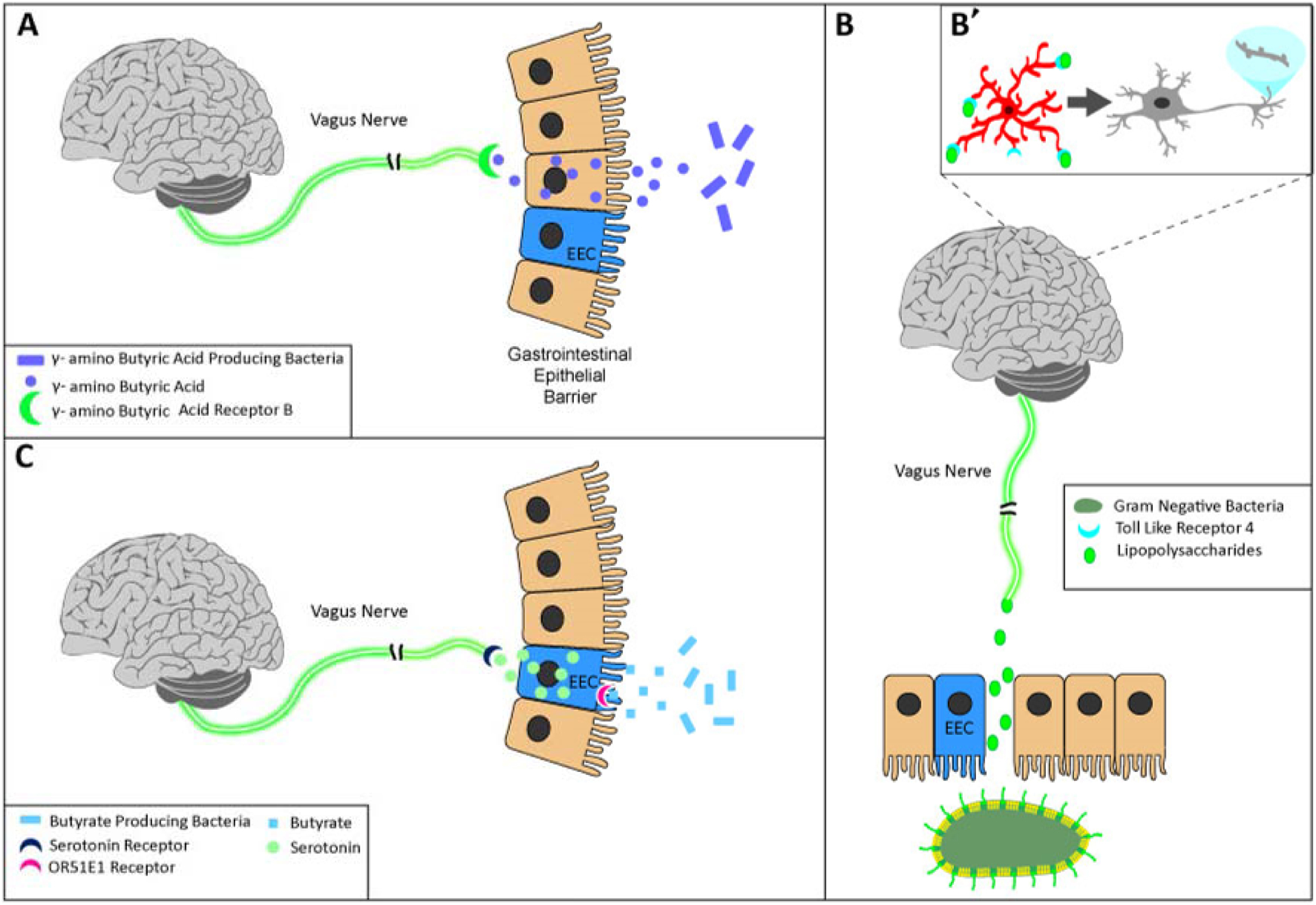
Graphical illustration of how alterations in the prevalence of Bacteroides (A), Prevotella (B), and Faecalibacterium (C) may alter brain function. (A) Decreased production of Bacteroides species in HIV-1 seropositive individuals may result in decreased production of the inhibitory neurotransmitter γ-aminobutyric acid (GABA). Bacteroides spp. produce GABA via the glutamate decarboxylase system. GABA is subsequently transported from the gastrointestinal system to the brain via vagal pathways, which express a high density of GABAB receptors. (B) A higher concentration of lipopolysaccharides (LPS) is one of the consequences of the increased prevalence of Prevotella spp. in chronically infected HIV-1 seropositive individuals. LPS can leak into circulation via the impaired gastrointestinal barrier or be transmitted to the brain via the vagus nerve. Upon entering the brain (B’), LPS binds to microglia, leading to their activation and/or dysfunction, which may subsequently underlie neurite and/or synaptic damage. (C) Faecalibacterium spp. serve as one of the main producers of butyrate, which is involved in the production and/or release of the neurotransmitter serotonin (5-HT) in a dose-dependent manner. Alterations in Faecalibacterium, as observed in HIV-1 seropositive individuals, support decreased production and/or release of 5-HT.
Prevotella.
The gastrointestinal barrier is breached by chronic HIV-1 viral protein exposure, evidenced by increased neutrophil infiltration, independent of cART (Somsouk et al., 2015), and increased circulating lipopolysaccharides (LPS; Brenchley et al., 2006; Pedro et al., 2018). Further, HIV-1 gut microbiome dysbiosis itself may compromise the integrity of the epithelial barrier, as the bacteria Akkermansia muciniphila, which serves as the “gatekeeper of the gut” (for review, Ouyang et al., 2020; Reunanen et al., 2015; Plovier et al., 2017), and Faecalibacterium spp. are decreased in HIV-1 seropositive individuals (e.g., Mutlu et al., 2014; Rocafort et al., 2019; Xie et al., 2021). Nonetheless, the impaired gut barrier induced by chronic HIV-1 infection affords a mechanism for the intestines to become permeable to harmful microbes, including Prevotella spp.
One of the key structural features of Gram-negative bacterium, including Prevotella spp., is the presence of an outer membrane that consists of phospholipids and LPS (Figure 2B; for review, Bos et al., 2007). Indeed, a positive relationship between the abundance of Prevotella spp. in the gut microbiome and LPS concentration has been observed (Leite et al., 2017); a relationship that supports a higher concentration of LPS in chronically infected HIV-1 seropositive individuals. The higher concentration of LPS can be transmitted from the gastrointestinal system to the brain either directly (Zhang et al., 2020) or indirectly (e.g., toll-like receptor 4 (TLR4; Hosoi et al., 2005)) via the vagus nerve. Further, the integrity of the blood-brain barrier (BBB) is disrupted by the constitutive expression of HIV-1 viral proteins (e.g., Petito & Cash, 1992; Chaganti et al., 2019) affording another mechanism for circulating LPS to enter the brain. Upon entering the brain, LPS binds to TLR4 receptors expressed on microglia (Bsibsi et al., 2002); the activation and/or dysfunction of microglia results in neurite and/or synaptic damage (e.g., Moraes et al., 2014; Liu et al., 2018), which may subsequently underlie cognitive and/or affective alterations. Taken together, the increased abundance of Prevotella spp. in chronically infected HIV-1 seropositive individuals likely results in a higher concentration of LPS; a concentration that induces a cascade of molecular signaling events that ultimately results in neuronal injury and/or synaptodendritic alterations.
Bacillota (Formerly Firmicutes)
When originally described by Gibbons and Murray (1978), the Bacillota phylum encompassed all Gram-positive bacteria. Under the current classification system, however, bacteria belonging to the Bacillota phylum are generally characterized by a low guanine-cytosine content in their DNA and exhibit either a round (i.e., cocci) or rod-like (i.e., bacillus) form; Gram-positive staining is no longer a unifying feature of Bacillota spp. The phylum Bacillota is subdivided into nine classes (Schoch et al., 2020), whereby the majority of the gastrointestinal Bacilloa spp. belong to one of four classes (i.e., Bacilli, Clostridia, Erysipelotrichia, and Negativicutes; Rajilić-Stojanović & de Vos, 2014). From a developmental perspective, Bacillota spp., much like bacteria belonging to the Bacteroidetes phylum, becomes preponderant in the gastrointestinal microbiome by the end of the first year of life (Palmer et al., 2007). In healthy adults, the stability of Bacillota spp. is dependent upon taxonomic order and/or genera; members of the Faecalibacterium genera, which is of particular relevance, exhibit high stability across time (Rajilić-Stojanović et al., 2013). The pivotal role of Bacillota spp. in the gastrointestinal system is evidenced by their early colonization, high prevalence, and function, whereby they are involved in the production of vitamins and short-chain fatty acids, including butyrate.
At the phylum level, the direction of changes in the relative abundance of Bacillota is dependent upon HIV-1 status and geographical region, whereby pronounced decreases are observed in HIV-1 seropositive, relative to seronegative, individuals sampled in African (Parbie et al., 2021) and North American (Dillon et al., 2014; González-Hernández et al., 2019) countries. In sharp contrast, statistically significant increases in the relative abundance of Bacillota are observed in chronically infected HIV-1 seropositive individuals in Asian countries (Ling et al., 2016; Sun et al., 2016). At the genera level, the relative abundance of Faecalibacterium is altered by chronic exposure to HIV-1 viral proteins, whereby constitutive expression of HIV-1 viral proteins induces both increases (Ling et al., 2016; Sun et al., 2016) and decreases (Mutlu et al., 2014; Nowak et al., 2015; Dubourg et al., 2016; Zhou et al., 2018; Ancona et al., 2021; Parbie et al., 2021; Xie et al., 2021) relative to seronegative controls. Notably, the relative abundance of other Bacillota genera is also altered by HIV-1 serostatus (e.g., Coprococcus: Decreased in a North American sample (Dillon et al., 2014); Lactobacillus: Increased in an Asian sample (Zhou et al., 2018)). The present review, however, will focus on the functional implications of changes in Faecalibacterium spp., as they represent approximately 5% of the total bacteria in the gastrointestinal system (Arumugam et al., 2011).
Faecalibacterium.
Bacteria belonging to the Faecalibacterium genera serve as one of the main producers of butyrate, a four-carbon short-chain fatty acid, in the gastrointestinal system (Duncan et al., 2002). Butyrate acts as a strong histone deacetylase (HDAC) inhibitor (Candido et al., 1978), whereby it favors histone acetylation (Riggs et al., 1977), and binds to G protein-coupled receptors (GPCRs; e.g., Free Fatty Acid Receptor 2 and 3, hydrocarboxylic acid receptor 2 (formerly GPR109a), for review, Priyadarshini et al., 2018). From a functional perspective, butyrate, at least at low concentrations, is critically involved in maintaining the integrity of both the gastrointestinal epithelial barrier (e.g., Mariadason et al., 1997; Peng et al., 2007) and BBB (Braniste et al., 2014); paradoxically, high concentrations of butyrate may disrupt the gastrointestinal epithelial barrier (Peng et al., 2007). Alterations in the prevalence of Faecalibacterium in chronically infected HIV-1 seropositive individuals, therefore, may be involved in the development of both gut epithelial (for review, Epple & Zeitz, 2012) and BBB (for review, Strazza et al., 2011) dysfunction in this population.
More directly, profound alterations in butyrate production induced by changes in the relative abundance of Faecalibacterium may preclude the production and/or release of the excitatory neurotransmitter serotonin (5-HT; Figure 2C). Enterochromaffin cells or neurons within the gastrointestinal system synthesize the vast majority (i.e., >90%) of the total body 5-HT, whereby 5-HT release is modulated, in part, by the interactions between butyrate and the OR51E1 GPCR (Priori et al., 2015). Fundamentally, butyrate induces 5-HT release in a dose-dependent manner (Reigstad et al., 2015). Indeed, low concentrations (i.e., 0.5 and 1 mM) of butyrate increase the transcription of tryptophan hydroxylase 1 (TPH1; i.e., the rate-limiting enzyme in 5-HT production), whereas transcription is suppressed when butyrate is absent (i.e., 0 mM) or present in high concentrations (i.e., 8mM and 16 mM; Reigstad et al., 2015). The dose-dependency of butyrate’s effects on 5-HT production/release support a convergent mechanism (i.e., Reductions in 5-HT) by which an increase or decrease in Faecalibacterium would induce functional alterations in the brain.
5-HT can be transported from the gastrointestinal system to the brain via vagal afferent fibers, which express functionally active 5-HT3 receptors (Hillsley et al., 1998; Lacolley et al., 2006) and are in close proximity to neurohormones, including 5-HT, released from enterochromaffin cells (Powley et al., 2011). An examination of the mechanism by which luminal or oral selective serotonin reuptake inhibitors (SSRIs) exert their therapeutic effects revealed increased vagal spike firing and antidepressive behavior. Beneficial behavioral effects of oral SSRIs were abolished following vagotomy (McVey Neufeld et al., 2019) supporting the vagus nerve as a communication pathway by which SSRIs exert their effects. In addition, a surge in 5-HT firing rates in the dorsal raphe nucleus, the brainstem nuclei for 5-HT, occurs after chronic stimulation of the vagus nerve (Dorr & Debonnel, 2006). Collectively, alterations in the relative abundance of Faecalibacterium induced by chronic HIV-1 viral protein exposure likely lead to profound changes in butyrate production which preclude the production and/or release of 5-HT.
Experimental Design Considerations for Microbiome Studies
Despite the compelling evidence for gastrointestinal microbiome dysbiosis in chronically infected HIV-1 seropositive individuals, it would be remiss to neglect the substantial inter-study heterogeneity (Figure 1) that has been observed. In light of the considerable inter-study heterogeneity, a brief discussion of experimental design considerations (e.g., statistical power), biological and environmental factors (e.g., age, biological sex, sexual behavior, geographic location), and the potential impact of cART underlying these discrepancies is warranted.
Statistical Power
Statistical power is defined by Cohen (1988) as “the probability [assuming the null hypothesis (H0) is false] that it will lead to the rejection of the null hypothesis”. Estimates of statistical power are influenced by three factors, including: 1) level of significance (i.e., α level); 2) effect size; and 3) sample size; increasing any of these factors yields increased statistical power. Fundamentally, the relationship between statistical power, α level, effect size, and sample size is such that one parameter can be determined as a function of the other three. For example, in an a priori power analysis, researchers can ascertain the necessary sample size based on the level of significance, effect size, and statistical power. Based on the recommendations of Cohen (1965), statistical power is conventionally established at 0.80 or greater for a medium effect size and α level of 0.05.
Inconsistencies in the defining characteristics of gastrointestinal microbiome dysbiosis in chronically infected HIV-1 seropositive individuals are likely due, in part, to low statistical power. To illustrate the risk for low statistical power in the 38 case-control studies identified in the present review, the number (i.e., sample size) of chronically infected HIV-1 seropositive individuals evaluated in each study was examined based on statistical significance in the Shannon Index (Figure 3). Indeed, studies finding statistically significant effects of HIV-1 serostatus on α diversity, measured using the Shannon Index, had a significantly greater number of HIV-1 seropositive individuals relative to studies failing to find a statistically significant effect (Main Effect of Statistical Significance: F(1,33)=6.3, p≤0.017; Three outliers, defined as being greater than 2.5 standard deviations above the mean, were removed from the analysis). More broadly, an examination of the total sample size in 28 microbiome studies that evaluated α diversity using the Chao1 index reported similar observations, whereby the distribution of sample sizes was highly skewed towards zero (Median Sample Size: 39, Mode: 8 Subjects; Kers & Saccenti, 2022).
Figure 3.
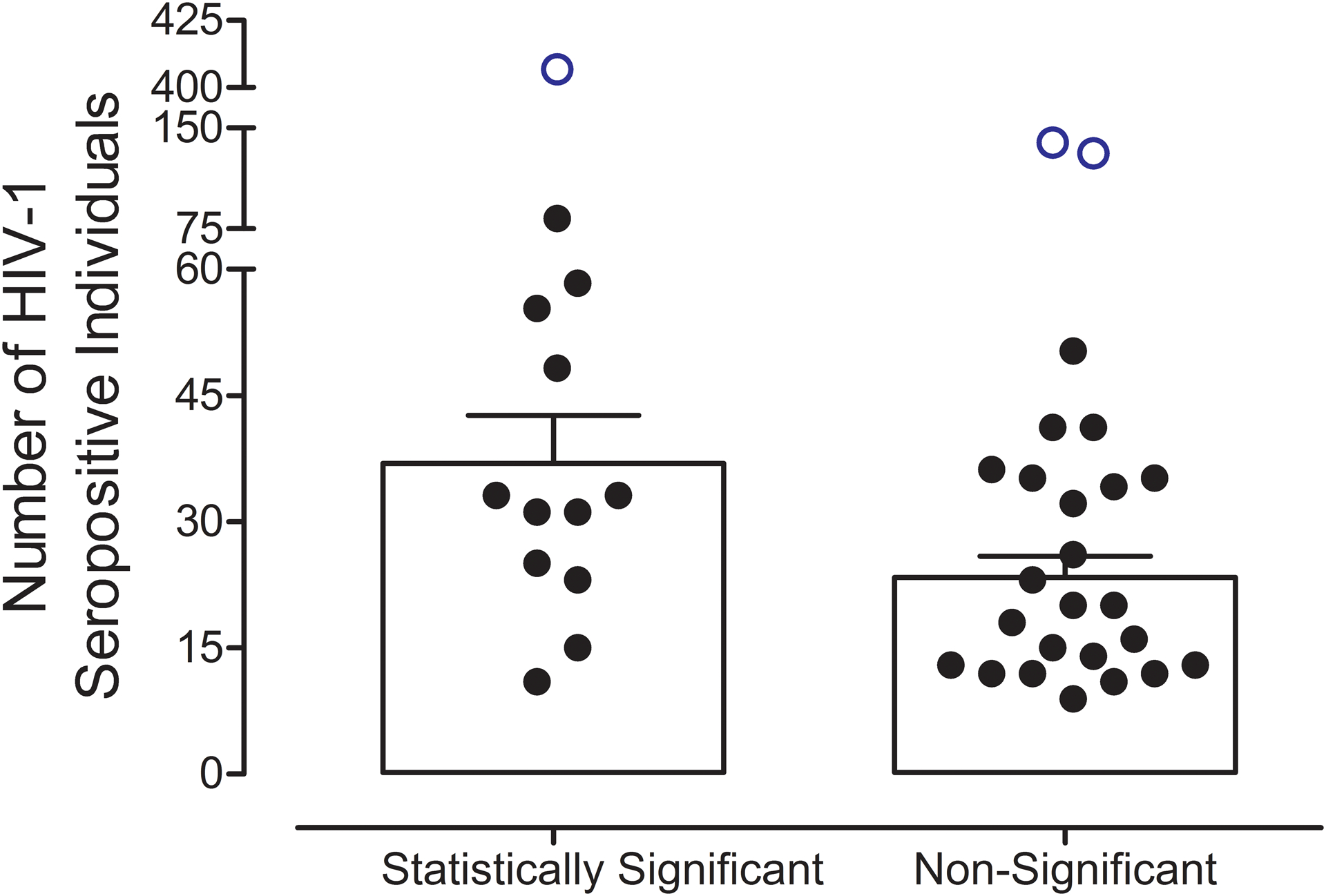
Studies finding statistically significant effects of HIV-1 serostatus on α diversity, measured using the Shannon Index, evaluated a significantly greater number of chronically infected HIV-1 seropositive individuals (Main Effect of Statistical Significance, F(1,33)=6.3, p<0.017). Three outliers, defined as being greater than 2.5 standard deviations above the mean, are indicated by the open blue circles and were not included in the analysis.
In light of these observations, it seems prudent to establish reporting practices that can enhance the biological insight gleaned from microbiome studies. First, presenting effect sizes, which highlight the practical significance of results, will aid in the interpretation of a manuscript. Indeed, effect sizes, commonly described using Cohen’s d, provide a standard metric that represents the magnitude (i.e., Small: d = 0.2; Medium: d = 0.5; Large: d ≥ 0.8) of the differences between groups. Second, effect sizes are fundamental for conducting a meta-analysis, as they are utilized as a standardized metric to represent and compare findings across studies (Lipsey & Wilson, 2001). To date, meta-analyses conducted on gut microbiota α diversity in chronically infected HIV-1 seropositive individuals have been limited by their reliance upon raw 16S rRNA gene sequence reads (Tuddenham et al., 2020; Zhou et al., 2020). Finally, published effect sizes can be utilized to estimate the sample size needed for future studies via a priori statistical power analyses.
It is acknowledged that the advanced statistical approaches implemented in the analysis of microbiome data create challenges for the calculation of both statistical power and effect size. In light of these challenges, novel approaches, including utilization of the Dirichlet-Multinomial distribution (La Rosa et al., 2012) and simulated distance matrices (Kelly et al., 2015) have been developed to estimate statistical power and sample size; a more comprehensive resource to facilitate sample size calculations for microbiome studies has also been provided by Ferdous et al. (2022). Nevertheless, continued efforts are needed to optimize methods for calculating statistical power and effect size in microbiome studies. Further, the challenges of calculating statistical power and effect size do not diminish, but rather bolster, their fundamental value as a key aspect of reporting results.
Biological and Environmental Factors
Biological and environmental factors, including age, biological sex (for review, Valeri & Endres, 2021), geography, and sexual behavior influence the diversity and composition of the gastrointestinal microbiome. The gut microbiome undergoes significant age-related development, characterized by a period of rapid change in early development (i.e., 1–3 years of age), increased stability during adulthood, and gradual modifications during aging (Yatsunenko et al., 2012; Odamaki et al., 2016). The maturation of the gastrointestinal microbiome, however, is sexually dimorphic, whereby during adulthood, biological sex is significantly associated with key components of microbial diversity (e.g., Mahnic & Rupnik, 2018) and composition (e.g., Mueller et al., 2006; Takagi et al., 2019). Similarly, both sexual behavior (i.e., MSM vs. non-MSM) and geography also influence both diversity (Sexual Behavior: Noguera-Julian et al., 2016; Zhou et al., 2020; Geography: Yatsunenko et al., 2012) and composition (Sexual Behavior: Noguera-Julian et al., 2016; Geography: He et al., 2018) of the gut microbiome. Elucidating the unique role of chronic HIV-1 viral proteins on gastrointestinal microbiome dysbiosis, therefore, requires careful consideration of both biological and environmental factors.
Unfortunately, however, biological and environmental factors are not consistently accounted for and/or reported in studies of HIV-1-associated gut microbiome dysbiosis; an experimental design failure that has the potential to confound results. For example, multiple studies include both male and female subjects, but do not appear to account for the factor of biological sex in statistical analyses (e.g., Dillon et al., 2014; Zhou et al., 2018). Further, the need to construe sexual behavior from the reported route of transmission precludes the consideration of this factor in HIV-1 seronegative individuals (e.g., Villanueva-Millán et al., 2017; Bai et al., 2021). Potentially of the greatest concern, however, is the failure to report subject characteristics of either biological and/or environmental factors that may confound the study.
Multiple approaches are readily available to mitigate these potential confounds; approaches which, when implemented, may enhance our understanding of gastrointestinal microbiome dysbiosis induced by chronic HIV-1 viral protein exposure. First, inclusion criteria for the study can be defined to only include a specific subgroup, thereby isolating the main effect of HIV-1 serostatus in these individuals (e.g., Inclusion of all Male, MSM individuals: Nowak et al., 2017). Second, the stratification of individuals (e.g., Gelpi et al., 2020; Vujkovic-Cvijin et al., 2020) and/or inclusion of interaction terms in statistical analyses (i.e., HIV-1 Serostatus × Biological Sex × Sexual Behavior) affords another practical approach to directly evaluate the influence of biological and environmental factors. The inclusion of interaction terms, however, will necessitate an increased sample size, as the sample size required to detect interactions is significantly greater than main effects (e.g., Leon & Heo, 2009). Finally, at a minimum, the implementation of stringent reporting practices, including any potential confounding subject characteristics, may improve the interpretability of findings. In addition, the evaluation of the gastrointestinal microbiome in preclinical biological systems may afford a more controlled venue with which to elucidate the specific role of chronic HIV-1 viral protein exposure on dysbiosis.
Combination Antiretroviral Therapy
cART, the primary treatment regimen for HIV-1 seropositive individuals utilizes a combination of drugs to suppress HIV-1 viral replication. HIV-1 seropositive individuals are typically prescribed a cART regimen that includes two to four drugs belonging to one of four drug classes: Integrase Strand Inhibitors (INSTI), Nucleoside Reverse Transcriptase Inhibitors (NRTI), Non-Nucleoside Reverse Transcriptase Inhibitors (NNRTI), or Protease Inhibitors (PI). The widespread implementation of cART has undoubtedly enhanced the quality of life for individuals living with HIV-1, evidenced by a dramatic increase in life expectancy (Romley et al., 2014; Teeraananchai et al., 2017) and decreased prevalence of the most severe forms of NCIs (for review, Saylor et al., 2016). Nevertheless, cART may be adversely affecting the gastrointestinal microbiome composition of HIV-1 seropositive individuals (for review, Pinto-Cardoso et al., 2018).
Drugs classified as either NRTIs or NNRTIs suppress the microbial diversity and composition of the gut microbiome in HIV-1 seropositive individuals (Imahashi et al., 2021; Ray et al., 2021; Hanttu et al., 2023), whereby treatment with zidovudine (NRTI) and/or efavirenz (NNRTI) have the most dramatic adverse effects (Shilaih et al., 2018; Ray et al., 2021). Broadly, HIV-1 seropositive individuals treated with conventional NRTI- or NNRTI-based cART exhibit a pronounced decrease in α diversity and richness across time (Imahashi et al., 2021; Ray et al., 2021) and relative to HIV-1 seronegative controls (Hanttu et al., 2023). More specifically, the NRTI zidovudine and NNRTI efavirenz show pronounced antibacterial activity. Indeed, both zidovudine and efavirenz strongly inhibit the growth of Bacteroides spp. and Prevotella spp. (Shilaih et al., 2018; Ray et al., 2021).
Other classes of drugs included in contemporary cART regimens (e.g., INSTIs, PIs) have fewer adverse effects on the gastrointestinal microbiome (Ray et al., 2021); albeit additional studies are warranted. Indeed, modifying the cART regimen by replacing the NNRTI efavirenz with the INSTI raltegravir enhances α diversity in the gastrointestinal microbiome of HIV-1 seropositive individuals (Hanttu et al., 2023). Furthermore, there are indications that treatment with a combination of NRTIs and INSTs has fewer adverse effects on gastrointestinal microbiome diversity relative to treatment with a NRTI and PI combination (Villanueva-Millán et al., 2017).
THERAPEUTICALLY TARGETING THE CONSEQUENCES OF GUT MICROBIOME DYSBIOSIS FOR NEUROCOGNITIVE AND/OR AFFECTIVE ALTERATIONS IN HIV-1
γ-Aminobutyric Acid (GABA)
A pronounced reduction in the inhibitory neurotransmitter GABA is likely one of the consequences of decreased Bacteroides spp. in chronically infected HIV-1 seropositive individuals. Indeed, a profound downregulation of GABAergic markers, including glutamate decarboxylase messenger RNA (mRNA; GAD1, GAD2), has been observed in the brains of postmortem HIV-1 seropositive individuals independent of HAND status and/or cART (Buzhdygan et al., 2016). Preclinical biological systems that express the HIV-1 transactivator of transcription (Tat) viral protein have been instrumental in defining how GABAergic neurotransmission is altered (e.g., Musante et al., 2010; Marks et al., 2016; Xu et al., 2016; Barbour et al., 2020; Xu et al., 2022). Specifically, HIV-1 Tat transgenic (Tg) mice exhibit decreased GABA exocytosis (Musante et al., 2010), dose- and sex-dependent alterations in spontaneous and miniature GABAA receptor-mediated inhibitory postsynaptic currents (sIPSC and mIPSC, respectively; Xu & Fitting, 2016; Xu et al., 2016; Xu et al., 2022), and region-specific reductions in the percentage of hippocampal somatostatin neurons (Marks et al., 2016). Related to GABAergic activity, dysregulation of glutamate metabolism is present in HIV-1 seropositive individuals. In vitro studies utilizing microglia and/or macrophages have shown that HIV-1 infection increases extracellular concentrations of glutamate in microglia and glutamine in macrophages and is correlated with elevated levels of neurotoxicity (Huang et al., 2011; Datta et al., 2016). One possible explanation may be elevated glutamine and glutaminase C levels which have been found in individuals with HIV-1-associated dementia (Huang et al., 2011). The Tat-induced decrease in GABAergic neurotransmission occurs, at least in part, via an μ-opioid receptor mechanism (Xu & Fitting, 2016) while the HIV-1 viral protein R (Vpr) is in part responsible for the dysregulation of glutamate metabolism and potentially contributes to the neuropathogenesis of HAND (Datta et al., 2016) via NMDA receptor mechanisms (Huang et al., 2011). To date, however, there is limited evidence for the direct relationship between GABAergic neurotransmission and HAND, as GABAergic mRNAs (i.e., GAD1, GAD2, GJD2) are associated with only one neurocognitive domain (i.e., verbal fluency) in HIV-1 seropositive individuals. Further, despite reaching statistical significance, GABAergic mRNAs explained merely 3.8% to 5.6% of the variance in verbal fluency in HIV-1 seropositive individuals (Buzhdygan et al., 2016). Likewise, the glutamine-glutamate cycle is indicated to be dysregulated in the presence of HIV-1 infection but has failed to reach significance in current magnetic resonance spectroscopy (MRI) studies investigating HIV-1 seropositive individuals experiencing cognitive deficits (Ernst et al., 2010).
Based on a critical review of the literature, there remains a fundamental need for additional studies elucidating the importance and/or utility of targeting GABAergic neurotransmission deficits associated with HIV-1. First, a more comprehensive evaluation of the relationship between GABAergic alterations and HIV-1-associated neurocognitive and/or affective alterations would provide clarity on whether these neurochemical changes induce functional alterations. Second, the HIV-1 genome contains nine genes and fifteen viral proteins necessitating an investigation of how GABAergic neurotransmission is altered in the presence of a more complete genome (rather than the single protein Tat). Finally, if GABAergic alterations are involved in the development of HIV-1-associated neurocognitive and/or affective alterations, evaluating novel therapeutics may be fruitful.
Even under these conditions, directly targeting the deficit in GABAergic neurotransmission observed in HIV-1 seropositive individuals may be futile for the mitigation of HIV-1-associated neurocognitive and/or affective alterations. For example, GABAergic neurotransmission can be enhanced via therapeutic treatment with benzodiazepines, which are positive allosteric modulators of GABAA receptor subtypes α1 and α2. Although benzodiazepines are beneficial for some affective alterations (e.g., anxiety, panic disorders), they have multiple adverse side effects (e.g., drowsiness, dizziness, blurry vision) and are associated with neurocognitive impairments in HIV-1 seropositive individuals (Saloner et al., 2019). Probiotic supplementation, however, may serve as an indirect way to enhance GABAergic neurotransmission via the gastrointestinal microbiome. Indeed, in HIV-1 seropositive individuals, treatment with an oral probiotic supplement that included the GABA-producing bacteria Bifidobacterium longum enhanced neurocognitive function (Ceccarelli et al., 2017a; Ceccarelli et al., 2017b); whether these beneficial effects were due to an enhancement in GABAergic neurotransmission, however, remains unknown. Taken together, the evidence for the importance and/or utility of therapeutically targeting deficits in GABAergic neurotransmission for HAND and/or apathy/depression associated with HIV-1 remains inconclusive and demands further study.
Synaptic Dysfunction and Estrogen Receptor β
The abundance of Prevotella spp., which is significantly increased in chronically infected HIV-1 seropositive individuals, is strongly associated with LPS concentration, whereby higher LPS concentrations induce microglial activation leading to neuronal and/or synaptic damage. Indeed, chronic HIV-1 viral protein exposure induces profound HIV-1-associated neuronal injury (Moore et al., 2006) and dendritic spine dysmorphology/loss (Moore et al., 2006; Gelman & Nguyen, 2010; Desplats et al., 2013; Weiss et al., 2021). Synaptodendritic alterations are also common amongst preclinical biological systems utilized to model HAND (e.g., Tat Tg Mice: Fitting et al., 2010, Fitting et al., 2013; gp120 Tg Mice: Kang et al., 2010, Speidell et al.,2020; HIV-1 Tg rat: Roscoe et al., 2014, McLaurin et al., 2019, Festa et al.,2020; chimeric HIV rat: Li et al., 2021, McLaurin et al., 2022a), whereby the generalizability (i.e., across brain regions and ages) of these deficits has been illustrated. Fundamentally, synaptic dysfunction has been implicated as a potential neural mechanism underlying HIV-1-associated neurocognitive and/or affective alterations, as it is highly correlated (i.e., r=0.648–0.827, r2=0.42–0.68) with global neuropsychological impairments in HIV-1 seropositive individuals (Moore et al., 2006) and motivational alterations in the HIV-1 Tg rat (McLaurin et al., 2022b). Therapeutically targeting the prominent neuronal injury and dendritic spine dysmorphology/loss, therefore, may mitigate HAND and/or apathy/depression associated with HIV-1.
The ovarian steroid hormone 17β-estradiol has the potential to serve as either a neuroprotective and/or neurorestorative treatment via its actions on neurons and dendritic spines, respectively. First, 17β-estradiol attenuates neuronal apoptosis induced by neurotoxic compounds, including HIV-1 (e.g., Corasaniti et al., 2005; Kendall et al., 2005; Adams et al., 2010), via an estrogen receptor β (ERβ) sensitive mechanism (Adams et al., 2010). Neuronal apoptosis, however, does not correlate with neurocognitive impairments (Adle-Biassette et al., 1999) and is rarely observed in HIV-1 seropositive individuals receiving cART. Second, 17β-estradiol promotes the growth of new dendritic spines, evidenced by increases in dendritic spine density (e.g., Khan et al. 2013; Hao et al. 2006; Tuscher et al. 2016; Wang et al., 2018); an effect resulting from the activation of the ERβ pathway (Wang et al., 2018). Further, treatment with 17β-estradiol induces a morphological shift towards more mature dendritic spines (Li et al., 2004; Hao et al., 2006). 17β-estradiol, or structurally similar compounds (i.e., phytoestrogens), may restore synaptodendritic integrity and/or function in HIV-1.
Phytoestrogens are naturally-occurring plant-derived compounds that are structurally similar to the ovarian steroid hormone 17β-estradiol (Glazier and Bowman, 2001) and exhibit a selective affinity for ERβ (e.g., Kuiper et al. 1998; Mueller et al. 2004). Daidzein (DAI), which is of particular interest, is a soy-derived phytoestrogen that is converted into the active metabolite Equol by gut microbiota (Setchell et al., 1984). Equol (7-hydroxy-3-(4-hydroxyphenyl)-chroman), whose chemical structure contains a chiral center at C-3 of the furan ring, can exist in either the R- or S- conformation, whereby S-Equol (SE) is the only enantiomer produced by humans (Setchell et al., 2005). Critically, SE penetrates the central nervous system via the blood-brain barrier (Johnson et al., 2019), supporting its potential utility to ameliorate HIV-1-associated synaptic dysfunction.
Indeed, the phytoestrogen DAI affords an efficacious therapeutic for synaptodendritic alterations resulting from HIV-1 viral protein exposure. Initial in vitro assessments demonstrated the potential utility of DAI as either a neuroprotective (i.e., via pre-treatment prior to HIV-1 Tat exposure) or neurorestorative (i.e., via induction of synaptodendritic damage by HIV-1 Tat exposure prior to treatment) therapeutic for HIV-1 associated neuronal and/or synaptic damage (Bertrand et al., 2014). Despite the promise of DAI, its clinical relevance was potentially limited by two fundamental caveats, including: 1) gastrointestinal microbiome dysbiosis, as reviewed above, is profound in chronically infected HIV-1 seropositive individuals; and 2) only 25–30% of the Western population convert DAI to SE (Rowland et al., 2000; Setchell & Cole, 2006). Subsequent in vitro studies embraced these challenges by evaluating the utility of the active metabolite SE rather than the parent compound DAI, whereby pretreatment with SE precludes interactive HIV-1 Tat and cocaine synapse loss in cortical neurons via an ERβ dependent mechanism (Bertrand et al., 2015).
In vivo studies were initiated in the HIV-1 Tg rat to evaluate whether SE can ameliorate and/or protect against HIV-1 associated neurocognitive and/or affective alterations. Treatment with SE between six to eight months of age dose-dependently mitigated deficits in a subset of HIV-1 Tg rats (i.e., 40%) in a sustained attention operant task, whereby 0.2 mg SE served as the most efficacious dose (Moran et al., 2019); a dose which was subsequently utilized to examine the generality of these effects. Indeed, the efficacious therapeutic effects of SE generalize to both HIV-1-associated neurocognitive impairments (i.e., Preattentive Processes, Sustained Attention, Selective Attention, Flexibility, and Inhibition; McLaurin et al., 2020a; McLaurin et al., 2020b) and affective alterations (i.e., motivational dysregulation; McLaurin et al., 2021), as well as across biological sex (McLaurin et al., 2020b). Further, early (i.e., Postnatal Day 28: McLaurin et al., 2020b vs. 2–3 Months of Age: McLaurin et al., 2020a vs. 6–8 Months of Age: Moran et al., 2019) initiation of SE treatment enhances its therapeutic efficacy (i.e., precluded the development of neurocognitive impairments in all HIV-1 Tg animals assessed). Fundamentally, SE treatment leads to a population shift in dendritic spine morphological parameters consistent with a more mature dendritic spine phenotype, supporting an underlying neural mechanism by which SE exerts its therapeutic effects (McLaurin et al., 2021); it remains unknown as to whether these enhancements result from mitigation of gastrointestinal microbiome dysbiosis. Collectively, the in vivo efficacy of SE for at least a subset of HIV-1 Tg animals supports its utility as either a neuroprotective and/or neurorestorative therapeutic for HIV-1 associated neurocognitive and affective alterations; a therapeutic that exerts its therapeutic effects via long-term enhancements to dendritic spines.
The clinical and translational relevance of SE cannot be understated. First, the utilization of SE, rather than its precursor DAI, fully embraces any potential heterogeneity and/or dysbiosis in the gastrointestinal microbiome. Second, the 0.2 mg dose of SE tested in the in vivo studies yields a daily amount of 0.25 to 1.0 mg/kg SE (i.e., approximately 2.5 to 10 mg in a 60 kg human), whereby the isoflavone intake of most Japanese is 30–50 mg per day (Akaza, 2012). Third, there is no evidence of any adverse peripheral effects of SE in either preclinical (Neese et al., 2014; Moran et al., 2019) or clinical (Tousen et al., 2011; Oyama et al., 2012) studies. Finally, SE is being critically evaluated in ongoing clinical trials for neurocognitive impairments associated with Alzheimer’s disease and HAND (Ausio Pharmaceuticals; NCT03101085).
Despite the evidence supporting the utility and clinical relevance of SE, it is notable that there is some, albeit not comprehensive, evidence for other therapeutics (e.g., NMDA Receptor Antagonists, Escitalopram) targeting synaptic dysfunction. NMDAR antagonists, including memantine and ifenprodil, ameliorate synaptic loss and learning deficits induced by the HIV-1 viral protein Tat supporting its potential utility as a neurorestorative therapeutic (Shin et al., 2012; Raybuck et al., 2017). To date, however, NMDAR antagonists are unable to preclude synapse loss (Shin et al., 2012) and have only been evaluated in the presence of one HIV-1 viral protein (i.e., Tat); considerations which diminish their immediate clinical relevance. With regards to the therapeutic potential of escitalopram, HIV-1 Tg animals chronically treated with escitalopram exhibited decreased apathetic and anxiolytic behaviors in conjunction with enhanced neuronal and dendritic spine morphology (Denton et al., 2021); the generality of these effects, however, has not yet been systematically evaluated. Nevertheless, cumulatively, the evidence strongly supports targeting synaptodendritic alterations as an efficacious therapeutic for HIV-1-associated neurocognitive and affective alterations.
Serotonin (5-HT)
HIV-1-induced alterations in the relative abundance of bacteria belonging to the Faecalibacterium genera may alter the production of butyrate, a four-carbon short-chain fatty acid; an effect which may ultimately preclude the production and/or release of the excitatory neurotransmitter 5-HT. Pronounced decreases in 5-HT, or its precursor tryptophan, have been reported in HIV-1 seropositive individuals since the beginning of the epidemic (Jean-Marie et al., 1998); deficits which have persisted in this population, despite treatment with cART (e.g., Keegan et al., 2016; Qi et al., 2018). The kynurenine pathway is a possible contributing factor to the alterations of tryptophan in HIV-1 seropositive individuals as reductions in the ratio of plasma kynurenine/tryptophan have been reported and are associated with the severity of cognitive impairment (Keegan et al., 2016). Further, a positive association (r=0.421) between plasma butyrate and 5-HT has been reported in chronically infected HIV-1 seropositive individuals (Ghare et al., 2022). In preclinical biological systems, constitutive expression of HIV-1 viral proteins in the HIV-1 Tg rat induces decreased serotonergic release and reuptake in the prefrontal cortex (Denton et al., 2019) and hippocampus (Denton et al., 2021). To date, only one study has systematically investigated the direct relationship between reductions in 5-HT (or tryptophan) and HIV-1-associated neurocognitive and/or affective alterations, whereby a statistically significant association between higher tryptophan and lower depression levels was observed in chronically infected HIV-1 seropositive individuals (Vadaq et al., 2022).
Pharmacological agents (e.g., SSRIs) that exert their therapeutic effects by increasing 5-HT, therefore, may be clinically relevant in this population. SSRIs, the first of which was developed and evaluated in the late 1970s (i.e., fluvoxamine; Claassen et al., 1977; Saletu et al., 1977), exhibit a selective affinity for 5-HT reuptake sites, thereby increasing extracellular 5-HT concentration. Seven SSRIs (e.g., fluvoxamine, escitalopram, sertraline) are currently approved by the United States Food and Drug Administration for the treatment of a variety of psychiatric conditions (e.g., Major Depressive Disorder; Obsessive-Compulsive Disorder; Post-Traumatic Stress Disorder; Chu & Wadhwa et al., 2022). Indeed, SSRIs are the most commonly prescribed pharmacotherapy for adults with depression (Marasine et al., 2021). Therefore, therapeutically targeting the hypo-serotonergic tone induced by chronic exposure to HIV-1 viral proteins via SSRIs may afford a readily available pharmacotherapy for HIV-1-associated affective alterations.
Indeed, given the prevalence of psychiatric comorbidities (i.e., depression, apathy; for review, Cysique & Brew, 2019) in HIV-1 seropositive individuals, the therapeutic utility of SSRIs in this population has been widely evaluated. In patients naïve to antiretroviral therapies, both open-label (e.g., Levin et al., 1990) and case-controlled studies (e.g., Elliott et al., 1998) observed moderate to strong (i.e., 50–100% of the sample responded to treatment) efficacy of SSRIs for the treatment of depression in HIV-1 seropositive individuals. The efficacy of SSRIs for depression generalizes to samples including HIV-1 seropositive individuals taking antiretroviral therapies (e.g., Ferrando et al., 1997; Rabkin et al., 1999; Currier et al., 2004; Tsai et al., 2013) and preclinical biological systems (i.e., HIV-1 Tg rat) resembling HIV-1 seropositive individuals on cART (Denton et al., 2021). The beneficial effects of SSRIs may extend beyond affective alterations in this population, as they also selectively improve neurocognitive performance (Sacktor et al., 2018) and increase adherence to antiretroviral therapies (Horberg et al., 2008).
Although SSRIs have significant therapeutic potential for HIV-1-associated affective alterations, a few considerations must also be taken into account. First, there is a minor risk for drug-drug (i.e., SSRI-cART) interactions in this population. The metabolism of nearly all SSRIs relies, at least in part, on cytochrome P450 enzymes (e.g., Obach et al., 2005; for review, Mandrioli et al., 2006); enzymes which are also responsible for metabolizing some antiretroviral medications (e.g., protease inhibitors; Chiba et al., 1996; Kumar et al., 1996). Indeed, a few HIV-1 seropositive individuals taking both fluoxetine and antiretroviral therapies known to inhibit P450 enzymes exhibited symptoms of 5-HT syndrome (DeSilva et al., 2001; Lorenzini et al., 2012), a potentially life-threatening complication resulting from the overstimulation of 5-HT (Sternbach, 1991). Second, it remains unknown as to whether SSRIs exert their therapeutic effects in HIV-1 seropositive individuals by enhancing 5-HT. Chronic escitalopram treatment, for example, likely mitigated apathetic and anxiolytic behaviors in the HIV-1 Tg rat by restoring synaptic dysfunction in the nucleus accumbens, as treatment failed to enhance hippocampal 5-HT kinetics (Denton et al., 2021).
Despite the potential utility and clinical relevance of SSRIs, it is notable that there is some, albeit not comprehensive, evidence for targeting HIV-1-induced 5-HT reductions with supplements (e.g., probiotics) and lifestyle changes (e.g., aerobic exercise). Treatment with a probiotic supplement (in addition to cART) significantly increased the serum concentration of serotonin in a small sample (n=8) of male HIV-1 seropositive individuals (Scheri et al., 2017); whether these neurochemical changes are sufficient to ameliorate neurocognitive and/or affective alterations, however, has not yet been systematically evaluated. Further, participation in an aerobic exercise and/or resistance training program significantly reduces depressive symptoms in adults living with HIV-1 (e.g., Neidig et al., 2003; Jaggers et al., 2015; Dianatinasab et al., 2018). It again, however, is unknown as to whether aerobic exercise and/or resistance training exerts its therapeutic effects by increasing 5-HT concentrations in HIV-1 seropositive individuals. Taken together, evidence supports the therapeutic utility of targeting HIV-1-induced reductions in 5-HT for affective alterations; additional research, however, is necessary to more comprehensively evaluate whether these therapeutic effects also mitigate HIV-1-associated neurocognitive impairments.
CONCLUSIONS
Gastrointestinal microbiome dysbiosis in HIV-1 seropositive individuals is characterized by decreased α diversity, a decreased relative abundance of bacterial species belonging to the Bacteroidetes phylum, and geographic-specific alterations in Bacillota spp.
Significant inter-study heterogeneity, characterized by inconsistent findings in α and β diversity, was observed in case-control studies of chronically infected (treated or untreated) HIV-1 seropositive individuals. Low statistical power and biological factors (e.g., sexual orientation, geographic location) may lead to inter-study heterogeneity, necessitating careful consideration of experimental design and reporting practices. Further, preclinical biological systems may be fundamental to elucidating the unique role of chronic HIV-1 viral protein exposure to gastrointestinal microbiome dysbiosis.
HIV-1-induced alterations in the relative abundance of Bacteroidetes and Bacillota spp. may underlie, at least in part, deficits in inhibitory (i.e., GABA) and excitatory (i.e., 5-HT) neurotransmission. In addition, the increased abundance of Gram-negative Prevotella spp. in chronically infected HIV-1 seropositive individuals likely induces a cascade of molecular signaling events that may underlie the prominent neuronal injury and/or synaptodendritic alterations commonly observed in this population.
Outstanding Questions and Future Directions
Neurocognitive and/or affective alterations in HIV-1 seropositive individuals may be mitigated by therapeutically targeting either synaptodendritic dysfunction or 5-HT reductions. Albeit, to date, the most comprehensive evidence supports early treatment with the phytoestrogen SE to ameliorate both HIV-1-associated neurocognitive and affective alterations across the factor of biological sex. Indeed, SE affords high translational relevance and promising clinical utility.
One of the largest barriers to understanding the gut-brain-microbiota axis and how the gut microbiome modulates the brain is the variability present among clinical populations. Another barrier is the amount of environmental factors (e.g., diet, exercise, medications, geographic location) that can impact the composition of the gut microbiome. Limitations in experimental control of clinical populations or even healthy control populations and the environmental factors that can impact the composition of the gut microbiome necessitate the development of preclinical biological systems. Development and use of preclinical biological systems allow for far better control of confounding variables, which in turn, would allow for a better understanding of HIV-1-associated dysbiosis and aid in developing novel adjunct therapies for HIV-1 associated neurocognitive and/or affective disorders.
ACKNOWLEDGEMENTS
This work was supported in part by grants from NIH (National Institute on Drug Abuse, R01-DA013137 to R.M.B. and C.F.M.; National Institute on Drug Abuse, K99-DA056288 to K.A.M.; National Institute on Drug Abuse, R01-DA054992 to M.S.; National Institute of Allergy and Infectious Diseases, R01-A155887 to J.L.K.; National Institute of Mental Health, R01-MH106392 to C.F.M. and R.M.B.; National Institute of Neurological Disorders and Stroke, R01-NS100624 to C.F.M. and R.M.B.) and the interdisciplinary research training program supported by the University of South Carolina Behavioral-Biomedical Interface Program (T32-GM081740).
ABBREVIATIONS
- 5-HT
Serotonin
- BBB
Blood-Brain Barrier
- cART
Combination Antiretroviral Therapy
- DAI
Daidzein
- ERβ
Estrogen Receptor β
- GABA
γ-aminobutyric Acid
- GAD
Glutamate Decarboxylase
- GPCR
G Protein-Coupled Receptors
- HAND
HIV-1-associated neurocognitive disorders
- HDAC
Histone Deacetylase
- HIV-1
Human Immunodeficiency Virus Type 1
- IPSC
Inhibitory Postsynaptic Currents
- LPS
Lipopolysaccharides
- mRNA
Messenger RNA
- NCI
Neurocognitive Impairments
- PERMANOVA
Permutational Multivariate ANOVA
- PSA
Polysaccharide A
- SE
S-Equol
- SSRI
Selective Serotonin Reuptake Inhibitors
- Tat
Transactivator of Transcription
- Tg
Transgenic
- TPH1
Tryptophan Hydroxylase 1
- ZPS
Zwitterionic Polysaccharides
- α
Alpha
- β
Beta
Footnotes
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
REFERENCES
- Adams SM, Aksenova MV, Aksenov MY, Mactutus CF, Booze RM 2010. ER-β mediated 17β-estradiol attenuation of HIV-1 Tat-induced apoptotic signaling. Synapse. 64, 829–838. 10.1002/syn.20793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adle-Biassette H, Chrétien F, Wingertsmann L, Héry C, Ereau T, Scaravilli F, Tardieu M, Gray F 1999. Neuronal apoptosis does not correlate with dementia in HIV infection but is related to microglial activation and axonal damage. Neuropathol Appl Neurobiol. 25, 123–133. 10.1046/j.1365-2990.1999.00167.x [DOI] [PubMed] [Google Scholar]
- Agostoni E, Chinnock JE, De Daly MB, Murray JG 1957. Functional and histological studies of the vagus nerve and its branches to the heart, lungs, and abdominal viscera in the cat. J Physiol. 135, 182–205. 10.1113/jphysiol.1957.sp005703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akaza H 2012. Prostate cancer chemoprevention by soy isoflavones: Role of intestinal bacteria as the “second human genome”. Cancer Sci. 103, 969–975. 10.1111/j.1349-7006.2012.02257.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ancona G, Merlini E, Tincati C, Barassi A, Calcagno A, Augello M, Bono V, Bai F, Cannizzo ES, d’Arminio Monforte A, Marchetti G 2021. Long-term suppressive cART is not sufficient to restore intestinal permeability and gut microbiota compositional changes. Front Immunol. 12, 639291. 10.3389/fimmu.2021.639291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson MJ 2017. Permutational multivariate analysis of variance (PERMANOVA), in: Balakrishnan N, Colton N, Everitt T, Piegorsch B, Ruggeri W, Teugels F, J.L. (Eds.), Wiley StatsRef: Statistics Reference Online. 10.1002/9781118445112.stat07841. [DOI] [Google Scholar]
- Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T, Mende DR, Fernandes GR, Tap J, Bruls T, Batto JM, Bertalan M, Borruel N, Casellas F, Fernandez L, Gautier L, Hansen T, Hattori M, Hayashi T, Kleerebezem M, Kurokawa K, Leclerc M, Levenez F, Manichanh C, Nielsen HB, Nielsen T, Pons N, Poulain J, Qin J, Sicheritz-Ponten T, Tims S, Torrents D, Ugarte E, Zoetendal EG, Wang J, Guarner F, Pedersen O, de Vos WM, Brunak S, Doré J, MetaHIT Constortium, Antolín M, Artiguenave F, Blottiere HM, Almeida M, Brechot C, Cara C, Chervaux C, Cultrone A, Delorme C, Denariaz G, Dervyn R, Foerstner KU, Friss C, van de Guchte M, Guedon E, Haimet F, Huber W, van Hylckama-Vlieg J, Jamet A, Juste C, Kaci G, Knol J, Lakhdari O, Layec S, Le Roux K, Maguin E, Mérieux A, Minardi RM, M’rini C, Muller J, Oozeer R, Parkhill J, Renault P, Rescigno M, Sanchez N, Sunagawa S, Torrejon A, Turner K, Vandemeulebrouck G, Varela E, Winogradsky Y, Zeller G, Weissenbach J, Ehrlich SD, Bork P 2011. Enterotypes of the human gut microbiome. Nature. 473, 174–180. 10.1038/nature09944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autran B, Carcelain G, Li TS, Blanc C, Mathez D, Tubiana R, Katlama C, Debré P, Leibowitch J 1997. Positive effects of combined antiretroviral therapy on CD4+ T cell homeostasis and function in advanced HIV disease. Science. 277, 112–116. 10.1126/science.277.5322.112. [DOI] [PubMed] [Google Scholar]
- Avci FY, Kasper DL 2010. How bacterial carbohydrates influence the adaptive immune system. Annu Rev Immunol. 28, 107–130. 10.1146/annurev-immunol-030409-101159. [DOI] [PubMed] [Google Scholar]
- Bai X, Narayanan A, Nowak P, Ray S, Neogi U, Sönnerborg A 2021. Whole-genome metagenomics analysis of the gut microbiome in HIV-1-infected individuals on antiretroviral therapy. Front Microbiol. 12: 667718. 10.3389/fmicb.2021.667718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour AJ, Hauser KF, McQuiston AR, Knapp PE 2020. HIV and opiates dysregulate K+ - Cl− cotransporter 2 (KCC2) to cause GABAergic dysfunction in primary human neurons and Tat-transgenic mice. Neurobiol Dis. 141: 104878. 10.1016/j.nbd.2020.104878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett E, Ross RP, O’Toole PW, Fitzgerald GF, Stanton C 2012. γ-aminobutyric acid production by culturable bacteria from the human intestine. J Appl Microbiol. 113, 411–417. 10.1111/j.1365-2672.2012.05344.x. [DOI] [PubMed] [Google Scholar]
- Bercik P, Park AJ, Sinclair D, Khoshdel A, Lu J, Huang X, Deng Y, Blennerhassett PA, Fahnestock M, Moine D, Berger B, Huizinga JD, Kunze W, McLean PG, Bergonzelli GE, Collins SM, Verdu EF 2011. The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut-brain communication. Neurogastroenterol Motil. 23, 1132–1139. 10.1111/j.1365-2982.2011.01796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthoud HR, Carlson NR, Powley TL 1991. Topography of efferent vagal innervation of the rat gastrointestinal tract. Am J Physiol. 260, R200–R207. 10.1152/ajpregu.1991.260.1.R200. [DOI] [PubMed] [Google Scholar]
- Bertrand SJ, Mactutus CF, Aksenova MV, Espensen-Sturges TD, Booze RM 2014. Synaptodendritic recovery following HIV Tat exposure: Neurorestoration by phytoestrogens. J Neurochem. 128, 140–151. 10.1111/jnc.12375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand SJ, Hu C, Aksenova MV, Mactutus CF, Booze RM 2015. HIV-1 Tat and cocaine mediated synaptopathy in cortical and midbrain neurons is prevented by the isoflavone Equol. Front Microbiol. 6, 894. 10.3389/fmicb.2015.00894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos MP, Robert V, Tommassen J 2007. Biogenesis of the gram-negative bacterial outer membrane. Annu Rev Microbiol. 61, 191–214. 10.1016/j.mib.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Braniste V, Al-Asmakh M, Kowal C, Anuar F, Abbaspour A, Tóth M, Korecka A, Bakocevic N, Ng LG, Kundu P, Gulyás B, Halldin C, Hultenby K, Nilsson H, Hebert H, Volpe BT, Diamond B, Pettersson S 2014. The gut microbiota influences blood-brain barrier permeability in mice. Sci Transl Med. 6, 263ra158. 10.1126/scitranslmed.3009759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, Bienenstock J, Cryan JF 2011. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci USA. 108, 16050–16055. 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray RJ, Curtis JT 1957. An ordination of the upland forest communities of southern Wisconsin. Ecol Monogr. 27, 325–349. [Google Scholar]
- Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, Kazzaz Z, Bornstein E, Lambotte O, Altmann D, Blazar BR, Rodriguez B, Teizeira-Johnson L, Landay A, Martin JN, Hecht FM, Picker LJ, Lederman MM, Deeks SG, Douek DC 2006. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 12, 1365–1371. 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- Bsibsi M, Ravid R, Gveric D, van Noort JM 2002. Broad expression of Toll-like receptors in the human central nervous system. J Neuropathol Exp Neurol. 61, 1013–1021. 10.1093/jnen/61.11.1013. [DOI] [PubMed] [Google Scholar]
- Buzhdygan T, Lisinicchia J, Patel V, Johnson K, Neugebauer V, Paessler S, Jennings K, Gelman B 2016. Neuropsychological, neurovirological and neuroimmune aspects of abnormal GABAergic transmission in HIV infection. J Neuroimmune Pharmacol. 11, 279–293. 10.1007/s11481-016-9652-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai H, Wang C, Qian Y, Zhang S, Zhang C, Zhao W, Zhang T, Zhang B, Chen J, Liu S, Zhu J, Yu Y 2021. Large-scale functional network connectivity mediate the associations of gut microbiota with sleep quality and executive functions. Hum Brain Mapp. 42, 3088–3101. 10.1002/hbm.25419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candido EP, Reeves R, Davie JR 1978. Sodium butyrate inhibits histone deacetylation in cultured cells. Cell. 14, 105–113. 10.1016/0092-8674(78)90305-7. [DOI] [PubMed] [Google Scholar]
- Canipe LG, Sioda M, Cheatham CL 2021. Diversity of the gut-microbiome related to cognitive behavioral outcomes in healthy older adults. Arch Gerontol Geriatr 96, 104454. 10.1016/j.archger.2021.104464. [DOI] [PubMed] [Google Scholar]
- Ceccarelli G, Fratino M, Selvaggi C, Giustini N, Serafino S, Schietroma I, Scheri GC, Pavone P, Passavanti G, Fegatelli DA, Mezzaroma I, Antonelli G, Vullo V, Scagnolari C, d’Ettorre G 2017a. A pilot study on the effects of probiotic supplementation on neuropsychological performance and microRNA-29a-c levels in antiretroviral-treated HIV-1-infected patients. Brain Behav. 7, e00756. 10.1002/brb3.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceccarelli G, Brenchley JM, Cavallari EN, Scheri GC, Fratino M, Pinacchio C, Schietroma I, Fard SN, Scagnolari C, Mezzaroma I, Vullo V, d’Ettorre G 2017b. Impact of hig-dose multi-strain probiotic supplementation on neurocognitive performance and central nervous system immune activation of HIV-1 infected individuals. Nutrients. 9, 1269. 10.3390/nu9111269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaganti J, Marripudi K, Staub LP, Rae CD, Gates TM, Moffat KJ, Brew BJ 2019. Imaging correlates of the blood-brain barrier disruption in HIV-associated neurocognitive disorder and therapeutic implications. AIDS. 33, 1843–1852. 10.1097/QAD.0000000000002300. [DOI] [PubMed] [Google Scholar]
- Chao A 1984. Nonparametric estimation of the number of classes in a population. Scand J Statist. 11, 265–270. [Google Scholar]
- Chiba M, Hensleigh M, Nishime JA, Balani SK, Lin JH 1996. Role of cytochrome P450 3A4 in human metabolism of MK-639, a potent human immunodeficiency virus protease inhibitor. Drug Metab Dispos. 24, 307–314. [PubMed] [Google Scholar]
- Chu A, Wadhwa R 2022. Selective serotonin reuptake inhibitors, in: StatPearls [Internet]. StatPearls Publishing, Treasure Island, FL. [PubMed] [Google Scholar]
- Claassen V, Davies JE, Hertting G, Placheta P 1977. Fluvoxamine, a specific 5-hydroxytryptamine uptake inhibitor. Br J Pharmacol. 60, 505–516. 10.1111/j.1476-5381.1977.tb07528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark KB, Naritoku DK, Smith DC, Browning RA, Jensen RA 1999. Enhanced recognition memory following vagus nerve stimulation in human subjects. Nat Neurosci. 2, 94–98. 10.1038/4600. [DOI] [PubMed] [Google Scholar]
- Cohen J 1965. Some statistical issues in psychological research, in: Wolman BB (Ed.), Handbook of Clinical Psychology, McGraw-Hill, New York, pp. 95–121. [Google Scholar]
- Cohen J 1988. Statistical Power Analysis for the Behavioral Sciences, Second Ed. Erlbaum, Hillside, NJ. [Google Scholar]
- Cohen RA, Seider TR, Navia B 2015. HIV effects on age-associated neurocognitive dysfunction: Premature cognitive aging or neurodegenerative disease? Alzheimers Res Ther. 7, 37. 10.1186/s13195-015-0123-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corasaniti MT, Amantea D, Russo R, Piccirilli S, Leta A, Corazzari M, Nappi G, Bagetta G 2005. 17beta-estradiol reduces neuronal apoptosis induced by HIV-1 gp120 in the neocortex of rat. Neurotoxicology. 26, 893–903. 10.1016/j.neuro.2005.01.019. [DOI] [PubMed] [Google Scholar]
- Costello EK, Lauber CL, Hamady M, Fierer N, Gordon JI, Knight R 2009. Bacterial community variation in human body habitats across space and time. Science. 326, 1694–1697. 10.1126/science.1177486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan JF, O’Riordan KJ, Cowan CSM, Sandhu KV, Bastiaanssen TFS, Boehme M, Codagnone MG, Cussotto S, Fulling C, Golubeva AV, Guzzetta KE, Jaggar M, Long-Smith CM, Lyte JM, Martin JA, Molinero-Perez A, Moloney G, Morelli E, Morillas E, O’Connor R, Cruz-Pereira JS, Peterson VL, Rea K, Ritz NL, Sherwin E, Spichak S, Teichman EM, van de Wouw M, Ventura-Silva AP, Wallace-Fitzsimons SE, Hyland N, Clarke G, Dinan TG 2019. The microbiota-gut-brain axis. Physiol Rev. 99, 1877–2013. 10.1152/physrev.00018.2018. [DOI] [PubMed] [Google Scholar]
- Currier MB, Molina G, Kato M 2004. Citalopram treatment of major depressive disorder in Hispanic HIV and AIDS patients: A prospective study. Psychosomatics. 45, 210–216. 10.1176/appi.psy.45.3.210. [DOI] [PubMed] [Google Scholar]
- Cysique LA, Maruff P, Brew BJ 2004. Prevalence and pattern of neuropsychological impairment in human immunodeficiency virus-infected/acquired immunodeficiency syndrome (HIV/AIDS) patients across pre- and post-highly active antiretroviral therapy eras: A combined study of two cohorts. J Neurovirol. 10, 350–357. 10.1080/13550280490521078. [DOI] [PubMed] [Google Scholar]
- Cysique LA, Brew BJ 2019. Comorbid depression and apathy in HIV-associated neurocognitive disorders in the era of chronic HIV infection. Handb Clin Neurol. 165, 71–82. 10.1016/B978-0-444-64012-3.00006-X. [DOI] [PubMed] [Google Scholar]
- Dalgleish AG, Beverley PC, Clapham PR, Crawford DH, Greaves MF, Weiss RA 1984. The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature. 312, 763–767. 10.1038/312763a0. [DOI] [PubMed] [Google Scholar]
- Datta PK, Deshmane S, Khalili K, Merali S, Gordon JC, Fecchio C, & Barrero CA (2016). Glutamate metabolism in HIV-1 infected macrophages: Role of HIV-1 Vpr. Cell Cycle, 15(17), 2288–2298. 10.1080/15384101.2016.1190054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y, Fischbach MA, Biddinger SB, Dutton RJ, Turnbaugh PJ 2014. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 505, 559–563. 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton AR, Samaranayake SA, Kirchner KN, Roscoe RF, Berger SN, Harrod SB, Mactutus CF, Hashemi P, Booze RM 2019. Selective monoaminergic and histaminergic circuit dysregulation following long-term HIV-1 protein exposure. J Neurovirol. 25, 540–550. 10.1007/s13365-019-00754-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton AR, Mactutus CF, Lateef AU, Harrod SB, Booze RM 2021. Chronic SSRI treatment reverses HIV-1 protein-mediated synaptodendritic damage. J Neurovirol. 27, 403–421. 10.1007/s13365-021-00960-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSilva KE, Le Flore DB, Marston BJ, Rimland D 2001. Serotonin syndrome in HIV-infected individuals receiving antiretroviral therapy and fluoxetine. AIDS. 15, 1281–1285. 10.1097/00002030-200107060-00010. [DOI] [PubMed] [Google Scholar]
- Desplats P, Dumaop W, Smith D, Adame A, Everall I, Letendre S, Ellis R, Cherner M, Grant I, Masliah E 2013. Molecular and pathologic insights from latent HIV-1 infection in the human brain. Neurology. 80, 1415–1423. 10.1212/WNL.0b013e31828c2e9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dianatinasab M, Fararouei M, Padehban V, Dianatinasab A, Alimohamadi Y, Beheshti S, AminiLari Z, AminiLari M 2018. The effect of a 12-week combinational exercise program on CD4 count and mental health among HIV infected women: A randomized control trial. J Exerc Sci Fit. 16, 21–25. 10.1016/j.jesf.2018.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon SM, Lee EJ, Kotter CV, Austin GL, Dong Z, Hecht DK, GIanella S, Siewe B, Smith DM, Landay AL, Robertson CE, Frank DN, Wilson CC 2014. An altered intestinal mucosal microbiome in HIV-1 infection is associated with mucosal and systemic immune activation and endotoxemia. Mucosal Immunol. 7, 983–994. 10.1038/mi.2013.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinh DM, Volpe GE, Duffalo C, Bhalchandra S, Tai AK, Kane AV, Wanke CA, Ward HD 2015. Intestinal microbiota, microbial translocation and systemic inflammation in chronic HIV infection. J Infect Dis. 211, 19–27. 10.1093/infdis/jiu409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do AN, Rosenberg ES, Sullivan PS, Beer L, Strine TW, Schulden JD, Fagan JL, Freedman MS, Skarbinski J 2014. Excess burden of depression among HIV-infected persons receiving medical care in the United States: Data from the Medical Monitoring Project and the Behavioral Risk Factors Surveillance System. PLoS One. 9, e92842. 10.1371/journal.pone.0092842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do Nascimento WM, Machiavelli A, Ferreira LGE, Silveira LC, de Azevedo SSD, Bello G, Smith DP, Mezzari MP, Petrosino JF, Duarte RTD, Zárate-Bladés CR, Pinto AR 2021. Gut microbiome profiles and associated metabolic pathways in HIV-infected treatment Naïve patients. Cells. 10, 385. 10.3390/cells10020385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong R, Lin H, Chen X, Shi R, Yuan S, Li J, Zhu B, Xu X, Shen W, Wang K, Shu XO, Ding D, He N 2021. Gut microbiota and fecal metabolites associated with neurocognitive impairment in HIV-infected population. Front Cell Infect Microbiol. 11, 723840. 10.3389/fcimb.2021.723840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorr AE, Debonnel G 2006. Effect of vagus nerve stimulation on serotonergic and noradrenergic transmission. J Pharmacol Exp Ther. 318, 890–898. 10.1124/jpet.106.104166. [DOI] [PubMed] [Google Scholar]
- Driskill CM, Childs JE, Itmer B, Rajput JS, Kroener S 2022. Acute vagus nerve stimulation facilitates short term memory and cognitive flexibility in rats. Brain Sci. 12, 1137. 10.3390/brainsci12091137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubourg G, Lagier JC, Armougom F, Robert C, Hamad I, Brouqui P, Raoult D 2013. The gut microbiota of a patient with resistant tuberculosis is more comprehensively studied by culturomics than by metagenomics. Eur J Clin Microbiol Infect Dis. 32, 637–645. 10.1007/s10096-012-1787-3. [DOI] [PubMed] [Google Scholar]
- Dubourg G, Lagier JC, Hüe S, Surenaud M, Bachar D, Robert C, Michelle C, Ravaux I, Mokhtari S, Million M, Stein A, Brouqui P, Levy Y, Raoult D 2016. Gut microbiota associated with HIV infection is significantly enriched in bacteria tolerant to oxygen. BMJ Open Gastroenterol. 3, e000080. 10.1136/bmjgast-2016-000080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan SH, Hold GL, Harmsen HJM, Stewart CS, Flint HJ 2002. Growth requirements and fermentation products of the Fusobacterium prausnitzii, and a proposal to reclassify it as Faecalibacterium prausnitzii gen. nov., comb. nov. Int J Syst Evol Microbiol. 52, 2141–2146. 10.1099/00207713-52-6-2141. [DOI] [PubMed] [Google Scholar]
- Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, Gill SR, Nelson KE, Relman DA 2005. Diversity of the human intestinal microbial flora. Science. 308, 1635–1638. 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott AJ, Uldall KK, Bergam K, Russo J, Claypoole K, Roy-Byrne PP 1998. Randomized, placebo-controlled trial of paroxetine versus imipramine in depressed HIV-positive outpatients. Am J Psychiatry. 155, 367–372. 10.1176/ajp.155.3.367. [DOI] [PubMed] [Google Scholar]
- Ellis RJ, Iudicello JE, Heaton RK, Isnard S, Lin J, Routy JP, Gianella S, Hoenigl M, Knight R 2021. Markers of gut barrier function and microbial translocation associated with lower gut microbial diversity in people with HIV. Viruses. 13, 1891. 10.3390/v13101891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epple HJ, Zeitz M 2012. HIV infection and the intestinal mucosal barrier. Ann NY Acad Sci. 1258, 19–24. 10.1111/j.1749-6632.2012.06512.x. [DOI] [PubMed] [Google Scholar]
- Ernst T, Jiang CS, Nakama H, Buchthal S, & Chang L (2010). Lower brain glutamate is associated with cognitive deficits in HIV patients: A new mechanism for HIV-associated neurocognitive disorder, Journal of Magnetic Resonance Imaging, 32(5), 1045–1053. 10.1002/jmri.22366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferdous T, Jiang L, Dinu I, Groizeleau J, Kozyrskyj AL, Greenwood CMT, Arrieta MC 2022. The rise to power of the microbiome: Power and sample size calculation for microbiome studies. Mucosal Immunol [Online Ahead of Print]. 10.1038/s41385-022-00548-1. [DOI] [PubMed] [Google Scholar]
- Ferrando SJ, Goldman JD, Charness WE 1997. Selective serotonin reuptake inhibitor treatment of depression in symptomatic HIV infection and AIDS. Improvements in affective and somatic symptoms. Gen Hosp Psychiatry. 19, 89–97. 10.1016/s0163-8343(96)00172-7. [DOI] [PubMed] [Google Scholar]
- Festa LK, Irollo E, Platt BJ, Tian Y, Floresco S, Meucci O 2020. CXCL12-induced rescue of cortical dendritic spines and cognitive flexibility. Elife. 9, e49717. 10.7554/eLife.49717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitting S, Xu R, Bull C, Buch SK, El-Hage N, Nath A, Knapp PE, Hauser KF 2010. Interactive comorbidity between opioid drug abuse and HIV-1 Tat: Chronic exposure augments spine loss and sublethal dendritic pathology in striatal neurons. Am J Pathol. 177, 1397–1410. 10.2353/ajpath.2010.090945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitting S, Ignatowska-Jankowska BM, Bull C, Skoff RP, Lictman AH, Wise LE, Fox MA, Su J, Medina AE, Krahe TE, Knapp PE, Guido W, Hauser KF 2013. Synaptic dysfunction in the hippocampus accompanies learning and memory deficits in human immunodeficiency virus type-1 Tat transgenic mice. Biol Psychiatry. 34, 443–453. 10.1016/j.biopsych.2012.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonda ML L-glutamate decarboxylase from bacteria. Methods Enzymol. 113, 11–16. 10.1016/s0076-6879(85)13005-3. [DOI] [PubMed] [Google Scholar]
- Gelman BB, Nguyen TP 2010. Synaptic proteins linked to HIV-1 infection and immunoproteasome induction: Proteomic analysis of human synaptosomes. J Neuroimmune Pharmacol. 5, 92–102. 10.1007/s11481-009-9168-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelpi M, Vestad B, Hansen SH, Holm K, Drivsholm N, Goetz A, Kirkby NS, Lindegaard B, Lebech AM, Hoel H, Michelsen AE, Ueland T, Gerstroft J, Lundgren J, Hov JR, Nielsen SD, Trøseid M 2020. Impact of human immunodeficiency virus-related gut microbiota alterations on metabolic comorbid conditions. Clin Infect Dis. 71, e359–e367. 10.1093/cid/ciz1235. [DOI] [PubMed] [Google Scholar]
- Ghare S, Singhal R, Bryant V, Gautam S, Tirumala CC, Srisailam PK, Reyes-Vega A, Ghooray D, McClain CJ, Hoffman K, Petrosino J, Bryant K, Govind V, Cohen R, Cook RL, Barve S 2022. Age-associated gut dysbiosis, marked by loss of butyrogenic potential, correlates with altered plasma tryptophan metabolites in older people living with HIV. J Acquir Immune Defic Syndr. 89, S56–S64. 10.1097/QAI.0000000000002866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghizoni DM, João LM, Neto LM, da Cunha IC, Pereira LO, Borges FRM, Battisti R, de Oliveira LG, Meneghini L, Lucinda AM, Neto JM, Paschoalini MA, Faria MS 2006. The effects of metabolic stress and vagotomy on emotional learning in an animal model of anxiety. Neurobiol Learn Mem. 86, 107–116. 10.1016/j.nlm.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Gibbons NE, Murray RGE 1978. Proposals concerning the higher taxa of bacteria. Int J Syst Bacteriol. 28, 1–6. [Google Scholar]
- Glazier MG, Bowman MA 2001. A review of the evidence for the use of phytoestrogens as a replacement for traditional estrogen replacement therapy. Arch Intern Med. 161, 1161–1172. 10.1001/archinte.161.9.1161. [DOI] [PubMed] [Google Scholar]
- González-Hernández LA, del Rocio Ruiz-Briseñ M, Sánchez-Reyes K, Alvarez-Zavala M, Vega-Magaña N, López-Iñiguez A, Díaz-Ramos JA, Martínez-Ayala P, Soria-Rodriguez RA, Ramos-Solano M, Andrade-Villanueva JF 2019. Alterations in bacterial communities, SCFA and biomarkers in an elderly HIV-positive and HIV-negative population in western Mexico. BMC Infect Dis. 19, 234. 10.1186/s12879-019-3867-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich JK, Waters JL, Poole AC, Sutter JL, Koren O, Blekhman R, Beaumont M, Van Treuren W, Knight R, Bell JT, Spector TD, Clark AG, Ley RE 2014. Human genetics shape the gut microbiome. Cell. 159, 789–799. 10.1016/j.cell.2014.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanttu AM, Pekkala S, Satokari R, Hartikainen AK, Arkkila P, Pietiläinen KH, Sutinen JP 2023. Gut microbiota alterations after switching from a protease inhibitor or effavirenze to raltegravir in a randomized, controlled study. AIDS. 37, 323–332. 10.1097/QAD.0000000000003419. [DOI] [PubMed] [Google Scholar]
- Hao J, Rapp PR, Leffler AE, Leffler SR, Janssen WGM, Lou W, McKay H, Roberts JA, Wearne SL, Hof PR, Morrison JH 2006. Estrogen alters spine number and morphology in prefrontal cortex of aged female rhesus monkeys. J Neurosci. 26, 2571–2578. 10.1523/JNEUROSCI.3440-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, Leblanc S, Corkran SH, Duarte NA, Clifford DB, Woods SP, Collier AC, Marra CM, Morgello S, Mindt MR, Taylor MJ, Marcotte TD, Atkinson JH, Wolfson T, Gelman BB, McArthur JC, Simpson DM, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I, CHARTER Groups, HNRC Group. 2011. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: Differences in rates, nature, and predictors. J Neurovirol. 17, 3–16. 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillsley K, Kirkup AJ, Grundy D 1998. Direct and indirect actions of 5-hydroxytryptamine on the discharge of mesenteric afferent fibres innervating the rat jejunum. J Physiol. 506, 551–561. 10.1111/j.1469-7793.1998.551bw.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hold GL, Pryde SE, Russell VJ, Furrie E, Flint HJ 2002. Assessment of microbial diversity in human colonic samples by 16S rDNA sequences analysis. FEMS Microbiol Ecol. 39, 33–39. 10.1111/j.1574-6941.2002.tb00904.x. [DOI] [PubMed] [Google Scholar]
- Horberg MA, Silverberg MJ, Hurley LB, Towner WJ, Klein DB, Bersoff-Matcha S, Weinberg WG, Antoniskis D, Mogyoros M, Dodge WT, Dobrinich R, Quesenberggy CP, Kovach DA 2008. Effects of depression and selective serotonin reuptake inhibitor use on adherence to highly active antiretroviral therapy and on clinical outcomes in HIV-infected patients. J Acquir Immune Defic Syndr. 47, 384–390. 10.1097/QAI.0b013e318160d53e. [DOI] [PubMed] [Google Scholar]
- Hosoi T, Okuma Y, Matsuda T, Nomura Y 2005. Novel pathway for LPS-induced afferent vagus nerve activation: Possible role of nodose ganglion. Auton Neurosci. 120,104–107. 10.1016/j.autneu.2004.11.012. [DOI] [PubMed] [Google Scholar]
- Huang Y, Zhao L, Jia B, Wu L, Li Y, Curthoys N, & Zheng JC (2011). Glutaminase Dysregulation in HIV-1-Infected Human Microglia Mediates Neurotoxicity: Relevant to HIV-1-Associated Neurocognitive Disorders. The Journal of Neuroscience, 31(42), 15195–15204. 10.1523/JNEUROSCI.2051-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imahashi M, Ode H, Kobayashi A, Nemoto M, Matsuda M, Hashiba C, Hamano A, Nakata Y, Mori M, Seko K, Nakahata M, Kogure A, Tanaka Y, Sugiura W, Yokomaku Y, Iwatani Y 2021. Impact of long-term antiretroviral therapy on gut and oral microbiotas in HIV-1-infected patients. Sci Rep. 11, 960. 10.1038/s41598-020-80247-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabłonowska E, Strzelczyk J, Piekarska A, Wójcik-Cichy K 2021. Gut microbiota diversity in HIV-infected patients on successful antiretroviral treatment is linked to sexual preferences but not CD4 nadir. Arch Immunol Ther Exp (Warsz). 69, 14. 10.1007/s00005-021-00616-7. [DOI] [PubMed] [Google Scholar]
- Jaggers JR, Hand GA, Dudgeon WD, Burgess S, Phillips KD, Durstine JL, Blair SN 2015. Aerobic and resistance training improves mood state among adults living with HIV. Int J Sports Med. 36, 175–181. 10.1055/s-0034-1385878. [DOI] [PubMed] [Google Scholar]
- Janik R, Thomason LAM, Stanisz AM, Forsythe P, Bienenstock J, Stanisz GJ 2016. Magnetic resonance spectroscopy reveals oral Lactobacillus promotion of increases in brain GABA, N-acetyl aspartate and glutamate. Neuroimage. 10.1016/j.neuroimage.2015.11.018. [DOI] [PubMed] [Google Scholar]
- Johnson SL, Kirk RD, DaSilva NA, Ma H, Seeram NP, Bertin MJ 2019. Polyphenol microbial metabolites exhibit gut and blood-brain barrier permeability and protect murine microglia against LPS-induced inflammation. Metabolites. 9, 78. 10.3390/metabo9040078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamada N, Seo SU, Chen GY, Núñez G 2013. Role of the gut microbiota in immunity and inflammatory disease. Nat Rev Immunol. 13, 321–335. 10.1038/nri3430. [DOI] [PubMed] [Google Scholar]
- Kamat R, Woods SP, Marcotte TD, Ellis RJ, Grant I, HIV Neurobehavioral Research Program (HNRP) Group. 2012. Implications of apathy for everyday functioning outcomes in persons living with HIV infection. Arch Clin Neuropsychol. 27, 520–531. 10.1093/arclin/acs055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang YJ, Digicaylioglu M, Russo R, Kaul M, Achim CL, Fletcher L, Masliah E, Lipton SA 2010. Erythropoietin plus insulin-like growth factor-I protects against neuronal damage in a muring model of human immunodeficiency virus-associated neurocognitive disorders. Ann Neurol. 68, 342–352. 10.1002/ana.22070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keegan MR, Chittiprol S, Letendre SL, Winston A, Fuchs D, Boasso A, Iudicello J, Ellis RJ, 2016. Tryptophan metabolism and its relationship with depression and cognitive impairment among HIV-infected individuals. Int J Tryptophan Res. 9, 79–88. 10.4137/IJTR.S36464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly BJ, Gross R, Bittinger K, Sherrill-Mix S, Lewis JD, Collman RG, Bushman RD, Li H 2015. Power and sample-size estimation for microbiome studies using pairwise distances and PERMANOVA. Bioinformatics. 31, 2461–2468. 10.1093/bioinformatics/btv183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendall SL, Anderson CF, Nath A, Turchan-Cholewo J, Land CL, Mactutus CF, Booze RM 2005. Gonadal steroids differentially modulate neurotoxicity of HIV and cocaine: Testosterone and ICI 182, 780 sensitive mechanism. BMC Neurosci. 6, 40. 10.1186/1471-2202-6-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kers JG, Saccenti E 2022. The power of microbiome studies Some considerations on which alpha and beta metrics to use and how to report results. Front Microbiol. 12, 796025. 10.3389/fmicb.2021.796025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan MM, Dhandapani KM, Zhang QG, Brann DW 2013. Estrogen regulation of spine density and excitatory synapses in rat prefrontal and somatosensory cerebral cortex. Steroids. 78, 614–623. 10.1016/j.steroids.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klatzmann D, Champagne E, Chamaret S, Gruest J, Guetard D, Hercend T, Gluckman JC, Montagnier L 1984. T-lymphocyte T4 molecule behaves as the receptor for human retrovirus LAV. Nature. 312, 767–768. 10.1038/312767a0. [DOI] [PubMed] [Google Scholar]
- Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, van der Burg B, Gustafsson JA 1998. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 139, 4252–4263. 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- Kumar GN, Rodrigues AD, Buko AM, Denissen JF 1996. Cytochrome P450-mediated metabolism of the HIV-1 protease inhibitor ritonavir (ABT-538) in human liver microsomes. J Pharmacol Exp Ther. 277, 423–431. [PubMed] [Google Scholar]
- Lacolley P, Owen JR, Sandock K, Lewis THJ, Bates JN, Robertson TP, Lewis SJ 2006. 5-HT activates vagal afferent cell bodies in vivo: Role of 5-HT2 and 5-HT3 receptors. Neuroscience. 143, 273–287. 10.1016/j.neuroscience.2006.07.032. [DOI] [PubMed] [Google Scholar]
- La Rosa PS, Brooks JP, Deych E, Boone EL, Edwards DJ, Wang Q, Sodergren E, Weinstock G, Shannon WD 2012. Hypothesis testing and power calculations for taxonomic-based human microbiome data. PLoS One. 7, e52078. 10.1371/journal.pone.0052078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leite AZ, de Campos Rodrigues N, Gonzaga MI, Paiolo JCC, de Souza CA, Stefanutto NAV, Omori WP, Pinheiro DG, Brisotti JL, Junior EM, Mariano VS, de Oliveira GLV 2017. Detection of increated plasma interleukin-6 levels and prevalence of Prevotella copri and Bacteroides vulgatus in the feces of type 2 diabetes patients. Front Immunol. 8, 1107. 10.3389/fimmu.2017.01107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon AC, Heo M 2009. Sample sizes required to detect interactions between two binary fixed-effects in a mixed-effects linear regression model. Comput Stat Data Anal. 53, 603–608. 10.1016/j.csda.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin S, Anderson D, Bystritsky A, Baron D 1990. A report of eight HIV-seropositive patients with major depression responding to fluoxetine. J Acquir Immune Defic Syndr (1988). 3, 1074–1077. [PubMed] [Google Scholar]
- Li C, Brake WG, Romeo RD, Dunlop JC, Gordon M, Buzescu R, Magarinos AM, Allen PB, Greengard P, Luine V, McEwen BS 2004. Estrogen alters hippocampal dendritic spine shape and enhances synaptic protein immunoreactivity and spatial memory in female mice. Proc Natl Acad Sci USA. 101, 2185–2190. 10.1073/pnas.0307313101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, McLaurin KA, Illenberger JM, Mactutus CF, Booze RM 2021. Microglial HIV-1 expression: Role in HIV-1 associated neurocognitive disorders. Viruses. 13, 924. 10.3390/v13050924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Q 2013. Submerged fermentation of Lactobacillus rhamnosus YS9 for γ-aminobutyric acid (GABA) production. Braz J Microbiol. 44, 183–187. 10.1590/S1517-83822013000100028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling Z, Jin C, Xie T, Cheng Y, Li L, Wu N 2016. Alterations in the fecal microbiota of patients with HIV-1 infection: An observational study in a Chinese population. Sci Rep. 6, 30673. 10.1038/srep30673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsey MW, Wilson BD 2001. Practical Meta-Analysis. Sage Publications, Thousand Okas, CA. [Google Scholar]
- Liu J, Johnson R, Dillon S, Kroehl M, Frank DN, Tuncil YE, Zhang X, Ir D, Robertson CE, Seifers S, Higgins J, Hamaker B, Wilson CC, Erlandson KM 2019. Among older adults, age-related changes in the stool microbiome differ by HIV-1 serostatus. EBioMedicine. 40, 583–594. 10.1016/j.ebiom.2019.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Zhang Y, Zheng X, Fang T, Yang X, Luo X, Guo A, Newell KA, Huang XF, Yu Y 2018. Galantamine improves cognition, hippocampal inflammation, and synaptic plasticity impairments induced by lipopolysaccharide in mice. J Neuroinflammation. 15, 112. 10.1186/s12974-018-1141-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzini KI, Calmy A, Ambrosioni J, Assouline B, Daali Y, Fathi M, Rebsamen M, Desmeules J, Samer CF 2012. Serotonin syndrome following drug-drug interactions and CYP2D6 and CYP2C19 genetic polymorphisms in an HIV-infected patient. AIDS. 26, 2417–2418. 10.1097/QAD.0b013e32835a11ba. [DOI] [PubMed] [Google Scholar]
- Lozupone C, Knight R 2005. UniFrac: A new phylogenetic method for comparing microbial communities. Appl Environ Microbiol. 71, 8228–8235. 10.1128/AEM.71.12.8228-8235.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozupone CA, Li M, Campbell TB, Flores SC, Linderman D, Gebert MJ, Knight R, Fontenot AP, Palmer BE 2013. Alterations in the gut microbiota associated with HIV-1 infection. Cell Host Microbe. 14, 329–339. 10.1016/j.chom.2013.08.006, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu W, Feng Y, Jing F, Han Y, Lyu N, Liu F, Li J, Song X, Xie J, Qiu Z, Zhu T, Routy B, Routy JP, Li T, Zhu B 2018. Association between gut microbiota and CD4 recovery in HIV-infected patients. Front Microbiol. 9, 1451. 10.3389/fmicb.2018.01451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak G, Zaunders JJ, Bailey M, Seddiki N, Rogers G, Leong L, Phan TG, Kelleher AD, Koelsch KK, Boyd MA, Danta M 2021. Preservation of gastrointestinal mucosal barrier function and microbiome in patients with controlled HIV infection. Front Immunol. 12:688886. 10.3389/fimmu.2021.688886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandrioli R, Forti GC, Raggi MA 2006. Fluoxetine metabolism and pharmacological interactions: The role of cytochrome p450. Curr Drug Metab. 7: 127–133. 10.2174/138920006775541561. [DOI] [PubMed] [Google Scholar]
- Manor O, Dai CL, Kornilov SA, Smith B, Price ND, Lovejoy JC, Gibbons SM, Magis AT 2020. Health and disease markers correlate with gut microbiome composition across thousands of people. Nat Commun. 11, 5206. 10.1038/s41467-020-18871-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marasine NR, Sankhi S, Lamichhane R, Marasini NR, Dangi NB 2021. Use of antidepressants among patients diagnosed with depression: A scoping review. Biomed Res Int. 2021: 6699028. 10.1155/2021/6699028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margeta-Mitrovic M, Mitrovic I, Riley RC, Jan LY, Basbaum AI 1999. Immunohistochemical localization of GABA(B) receptors in the rat central nervous system. J Comp Neurol. 405, 299–321. . [DOI] [PubMed] [Google Scholar]
- Mariadason JM, Barkla DH, Gibson PR 1997. Effect of short-chain fatty acids on paracellular permeability in Caco-2 intestinal epithelium model. Am J Physiol. 272, G705–G712. 10.1152/ajpgi.1997.272.4.G705. [DOI] [PubMed] [Google Scholar]
- Marks WD, Paris JJ, Schier CJ, Denton MD, Fitting S, McQuiston AR, Knapp PE, Hauser KF 2016. HIV-1 Tat causes cognitive deficits and selective loss of parvalbumin, somatostatin, and neuronal nitric oxide synthase expressing hippocampal CA1 interneuron subpopulations. J Neurovirol. 22, 747–762. 10.1007/s13365-016-0447-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazmanian SK, Round JL, Kasper DL 2008. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature. 453, 620–625. 10.1038/nature07008. [DOI] [PubMed] [Google Scholar]
- McHardy IH, Li X, Tong M, Ruegger P, Jacobs J, Borneman J, Anton P, Braun J 2013. HIV infection is associated with compositional and functional shifts in the rectal mucosal microbiota. Microbiome. 1, 26. 10.1186/2049-2618-1-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaurin KA, Li H, Booze RM, Mactutus CF 2019. Disruption of timing: NeuroHIV progression in the post-cART era. Sci Rep. 9, 827. 10.1038/s41598-018-36822-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaurin KA, Moran LM, Booze RM, Mactutus CF 2020a. Selective estrogen receptor β agonists: A therapeutic approach for HIV-1 associated neurocognitive disorders. J Neuroimmune Pharmacol. 15, 264–279. 10.1007/s11481-019-09900-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaurin KA, Li H, Cook AK, Booze RM, Mactutus CF 2020b. S-Equol: A neuroprotective therapeutic for chronic neurocognitive impairments in pediatric HIV. J Neurovirol. 26, 704–718. 10.1007/s13365-020-00886-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaurin KA, Bertrand SJ, Illenberger JM, Harrod SB, Mactutus CF, Booze RM 2021. S-Equol mitigates motivational deficits and dysregulation associated with HIV-1. Sci Rep. 11, 11870. 10.1038/s41598-021-91240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaurin KA, Li H, Mactutus CF, Harrod SB, Booze RM 2022a. Disrupted decision-making: EcoHIV inoculation in cocaine dependent rats. Int J Mol Sci. 23, 9100. 10.3390/ijms23169100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaurin KA, Cranston MN, Li H, Mactutus CF, Harrod SB, Booze RM 2022b. Synaptic dysfunction is associated with alterations in the initiation of goal-directed behaviors: Implications for HIV-1-associated apathy. Exp Neurol. 357, 114174. 10.1016/j.expneurol.2022.114174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVey Neufelt KA, Bienenstock J, Bharwani A, Champagne-Jorgensen K, Mao Y, West C, Liu Y, Surette MG, Kunze W, Forsythe P 2019. Oral selective serotonin reuptake inhibotors activate vagus nerve dependent gut-brain signalling. Sci Rep. 9, 14290. 10.1038/s41598-019-50807-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaco CL, Gootenberg DB, Zhao G, Handley SA, Ghebremichael MS, Lim ES, Lankowski A, Baldridge MT, Wilen CB, Flagg M, Norman JM, Keller BC, Luévano JM, Wang D, Boum Y, Martin JN, Hunt PW, Bangsberg DR, Siedner MJ, Kwon DS, Vigin HW 2016. Altered virome and bacterial microbiome in human immunodeficiency virus-associated acquire immunodeficiency syndrome. Cell Host Microbe. 19, 311–322. 10.1016/j.chom.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon JY, Zolnik CP, Wang Z, Qiu Y, Usyk M, Wang T, Kizer JR, Landay AL, Kurland IJ, Anastos K, Kaplan RC, Burk RD, Qi Q 2018. Gut microbiota and plasma metabolites associated with diabetes in women with, or at high risk for, HIV infection. EBioMedicine. 37, 392–400. 10.1016/j.ebiom.2018.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore DJ, Masliah E, Rippeth JD, Gonzalez R, Carey CL, Cherner M, Ellis RJ, Achim CL, Marcotte TD, Heaton RK, Grant I, HNRC Group. 2006. Cortical and subcortical neurodegeneration is associated with HIV neurocognitive impairment. AIDS. 20, 879–887. 10.1097/01.aids.0000218552.69834.00. [DOI] [PubMed] [Google Scholar]
- Moraes CA, Santos G, de Sampaio e Spohr TC, D’Avila JC, Lima FRS, Benjamim CF, Bozza FA, Gomes FAG 2014. Activated microglia-induced deficits in excitatory synapses through IL-1β: Implications for cognitive impairment in sepsis. Mol Neurobiol. 52, 653–663. 10.1007/s12035-014-8868-5. [DOI] [PubMed] [Google Scholar]
- Moran LM, McLaurin KA, Booze RM, Mactutus CF 2019. Neurorestoration of sustained attention in a model of HIV-1 associated neurocognitive disorders. Front Behav Neurosci. 13, 169. 10.3389/fnbeh.2019.00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller SO, Simon S, Chae K, Metzler M, Korach KS 2004. Phytoestrogens and their human metabolites show distinct agonistic and antagonistic properties on estrogen receptor alpha (ERalpha) and ERbeta in human cells. Toxicol Sci. 80, 14–25. 10.1093/toxsci/kfh147. [DOI] [PubMed] [Google Scholar]
- Musante V, Summa M, Neri E, Puliti A, Godowicz TT, Severi P, Battaglia G, Raiteri M, Pittaluga A 2010. The HIV-1 viral protein Tat increases glutamate and decreases GABA exocytosis from human and mouse neocortical nerve endings. Cereb Cortex. 20, 1974–1984. 10.1093/cercor/bhp274. [DOI] [PubMed] [Google Scholar]
- Mutlu EA, Keshavarzian A, Losurdo J, Swanson G, Siewe B, Forsyth C, French A, DeMarais P, Sun Y, Koenig L, Cox S, Engen P, Chakradeo P, Abbasi R, Gorenz A, Burns C, Landay A 2014. A compositional look at the human gastrointestinal microbiome and immune activation parameters in HIV infected subjects. PLoS Patho. 10, e1003829. 10.1371/journal.ppat.1003829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neese SL, Pisani SL, Doerge DR, Helferich WG, Sepehr E, Chittiboyina AG, Rotte SCK, Smillie TJ, Khan IA, Korol DL, Schantz SL 2014. The effects of dietary treatment with S-equol on learning and memory processes in middle-aged ovariectomized rats. Neurotoxicol Teratol. 41, 80–88. 10.1016/j.ntt.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidig JL, Smith BA, Brashers DE 2003. Aerobic exercise training for depressive symptom management in adults living with HIV infection. J Assoc Neurses AIDS Care. 14, 30–40. 10.1177/1055329002250992. [DOI] [PubMed] [Google Scholar]
- Neff CP, Rhodes ME, Arnolds KL, Collins CB, Donnelly J, Nusbacher N, Jedlicka P, Schneider JM, McCarter MD, Shaffer M, Mazmanian SK, Palmer BE, Lozupone CA 2016. Diverse intestinal bacteria contain putative zwitterionic capsular polysaccharides with anti-inflammatory properties. Cell Host Microbe. 20, 535–547. 10.1016/j.chom.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguera-Julian M, Rocafort M, Guillén Y, Rivera J, Casadellà M, Nowak P, Hildebrand F, Zeller G, Parera M, Bellido R, Rodríguez C, Carrillo J, Mothe B, Coll J, Bravo I, Estany C, Herrero C, Saz J, Sierera G, Torrela A, Navarro J, Crespo M, Brander C, Negredo E, Blanco J, Guarner F, Calle ML, Bork P, Sönnerborg A, Clotet B, Paredes R 2016. Gut microbiota linked to sexual preference and HIV infection. EBioMedicine. 5, 135–146. 10.1016/j.ebiom.2016.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak P, Troseid M, Avershina E, Barqasho B, Neogi U, Holm K, Hov JR, Noyan K, Vesterbacka J, Svärd J, Rudi K, Sönnerborg A 2015. Gut microbiota diversity predicts immune status in HIV-1 infection. AIDS. 29, 2409–2418. 10.1097/QAD.0000000000000869. [DOI] [PubMed] [Google Scholar]
- Nowak RG, Bentzen SM, Ravel J, Crowell TA, Dauda W, Ma B, Liu H, Blattner A, Baral SD, Charurat ME, TRUSTRV368 Study Group. 2017. Rectal microbiota among HIV-uninfected, untreated HIV, and treated HIV-infected in Nigeria. AIDS. 31, 857–862. 10.1097/QAD.0000000000001409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obach RS, Cox LM, Tremaine LM 2005. Sertraline is metabolized by multiple cytochrome P450 enzymes, monoamine oxidases, and glucuronyl transferases in human: An in vitro study. Drug Metab Dispos. 33, 262–270. 10.1124/dmd.104.002428. [DOI] [PubMed] [Google Scholar]
- Odamaki T, Kato K, Sugahara H, Hashikura N, Takahasi S, Xiao JZ, Abe F, Osawa R 2016. Age-related changes in gut microbiota composition from newborn to centenarian: A cross-sectional study. BMC Microbiol. 16, 90. 10.1186/s12866-016-0708-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onderdonk AB, Markham RB, Zaleznik DF, Cisneros RL, Kasper DL 1982. Evidence for T cell-dependent immunity to Bacteorides fagilis in an intraabdominal abscess model. J Clin Invest. 69, 9–16. 10.1172/jci110445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otaru N, Ye K, Mujezinovic D, Berchtold L, Constancias F, Cornejo FA, Krzystek A, de Wouters T, Braegger C, Lacroix C, Pugin B 2021. GABA production by human intestinal Bacteroides spp.: Prevalence, regulation, and role in acid stress tolerance. Front. Microbiol 12, 656895. 10.3389/fmicb.2021.656895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang J, Lin J, Isnard S, Fombuena B, Peng X, Marette A, Routy B, Messaoudene M, Chen Y, Routy JP 2020. The bacterium Akkermansia muciniphila: A sentinel for gut permeability and its relevance to HIV-related inflammation. Front Immunol. 11, 645. 10.3389/fimmu.2020.00645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyama A, Ueno T, Uchiyama S, Aihara T, Miyake A, Kondo S, Matsunaga K 2012. The effects of natural S-equol supplementation on skin aging in postmenopausal women: A pilot randomized placebo-controlled trial. Menopause. 19, 202–210. 10.1097/gme.0b013e318227427b. [DOI] [PubMed] [Google Scholar]
- Palmer C, Bik EM, DiGiulio DB, Relman DA, Brown PO 2007. Development of the human infant intestinal microbiota. PLoS Biol. 5, e177. 10.1371/journal.pbio.0050177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parbie PK, Mizutani T, Ishizaka A, Kawana-Tachikawa A, Runtuwene LR, Seki S, Abana CZY, Kushitor D, Bonney EY, Ofori SB, Uematsu S, Imoto S, Kimura Y, Kiyono H, Ishikawa K, Ampofo WK, Matano T 2021. Dysbiotic fecal microbiome in HIV-1 infected individuals in Ghana. Front Cell Infect Microbiol. 11, 646467. 10.3389/fcimb.2021.646467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedro MN, Magro DO, da Silva EUPP, Guadagnini D, Santos A, de Jesus Pedro R, Saad MJA 2018. Plasma levels of lipopolysaccharide correlate with insulin resistance in HIV patients. Diabetol Metab Syndr. 10, 5. 10.1186/s13098-018-0308-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng L, He Z, Chen W, Holzman IR, Lin J 2007. Effects of butyrate on intestinal barrier function in a Caco-2 cell monolayer model of intestinal barrier. Pediatr Res. 61, 37–41. 10.1203/01.pdr.0000250014.92242.f3. [DOI] [PubMed] [Google Scholar]
- Pérez-Santiago J, Marquine MJ, Cookson D, Giraud-Colón R, Heaton RK, Grant I, Ellis RJ, Letendre SL, Peterson SN 2021. Gut microbiota dysbiosis is associated with worse emotional states in HIV infection. J Neurovirol. 27, 228–238. 10.1007/s13365-020-00933-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petito CK, Cash KS 1992. Blood-brain barrier abnormalities in the acquired immunodeficiency syndrome: Immunohistochemical localization of serum proteins in postmortem brain. Ann Neurol. 32, 658–666. 10.1002/ana.410320509. [DOI] [PubMed] [Google Scholar]
- Pinto-Cardoso S, Lozupone C, Briceño O, Alva-Hernández S, Téllez N, Adriana A, Murakami-Ogasawara A, Reyes-Terán G 2017. Fecal bacteria communities in treated HIV infected individuals on two antiretroviral regimens. Sci Rep. 7, 43741. 10.1038/srep43741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto-Cardoso S, Klatt NR, Reyes-Terán G 2018. Impact of antiretroviral drugs on the microbiome: Unknown answers to important questions. Curr Opin HIV AIDS. 13, 53–60. 10.1097/COH.0000000000000428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plovier H, Everard A, Druart C, Depommier C, Van Hul M, Geurts L, Chilloux J, Ottman N, Duparc T, Lichtenstein L, Myridakis A, Delzenne NM, Klievink J, Bhattacharjee A, van der Ark KCH, Aalvink S, Martinez LO, Dumas ME, Maiter D, Loumaye A, Hermans MP, Thissen JP, Belzer C, de Vos WM, Cani PD 2017. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat Med. 23, 107–113. 10.1038/nm.4236. [DOI] [PubMed] [Google Scholar]
- Pokusaeva K, Johnson C, Luk B, Uribe G, Fu Y, Oezguen N, Matsunami RK, Lugo M, Major A, Mori-Akiyama Y, Hollister EB, Dann SM, Shi XZ, Engler DA, Savidge T, Versalovic J 2017. GABA-producing Bifidobacterium dentium modulates visceral sensitivity in the intestine. Neurogastroenterol Motil. 29, e12904. 10.1111/nmo.12904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powley TL, Spaulding RA, Haglof SA 2011. Vagal afferent innervation of the proximal gastrointestinal tract mucosa: Chemoreceptor and mechanoreceptor architecture. J Comp Neurol. 519, 644–660. 10.1002/cne.22541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priori D, Colombo M, Clavenzani P, Jansman AJM, Lallés JP, Trevisi P, Bosi P 2015. The olfactory receptor OR51E1 is present along the gastrointestinal tract of pigs, col-localizes with enteroendocrine cells and is modulated by intestinal microbiota. PLoS One. 10, e0129501. 10.1371/journal.pone.0129501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priyadarshini M, Kotlo KU, Dudeja PK, Layden BT 2018. Role of short chain fatty acid receptors in intestinal physiology and pathophysiology. Compr Physiol. 8, 1091–1115. 10.1002/cphy.c170050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Q, Hua S, Clish CB, Scott JM, Hanna DB, Wang T, Haberlen SA, Shah SJ, Glesby MJ, Lazar JM, Burk RD, Hodis HN, Landay AL, Post WS, Anastos K, Kaplan RC 2018. Plasma tryptophan-kynurenine metabolites are altered in human immunodeficiency virus infection and associated with progression of carotid artery atherosclerosis. Clin Infect Dis. 67, 235–242. 10.1093/cid/ciy053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, Nielsen T, Pons N, Levenez F, Yamada T, Mende DR, Li J, Xu J, Li S, Li D, Cao J, Wang B, Liang H, Zheng H, Xie Y, Tap J, Lepage P, Bertalan M, Batto JM, Hansen T, Le Paslier D, Linneberg A, Nielsen HB, Pelletier E, Renault P, Sicheritz-Ponten T, Turner K, Zhu H, Yu C, Li S, Jian M, Zhou Y, Li Y, Zhang X, Li S, Qin N, Yang H, Wang J, Brunak S, Doré J, Guarner F, Kristiansen K, Pedersen O, Parkhill J, Weissenbach J, MetaHIT Consortium, Bork P, Ehrlich SD, Wang J 2010. A human gut microbial gene catalogue established by metagenomics sequencing. Nature. 464, 59–65. 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qing Y, Xie H, Su C, Wang Y, Yu Q, Pang Q, Cui F 2019. Gut microbiome, short-chain fatty acids, and mucosa injury in young adults with human immunodeficiency virus infection. Dig Dis Sci. 64, 1830–1843. 10.1007/s10620-018-5428-2. [DOI] [PubMed] [Google Scholar]
- Rabkin JG, Wagner GJ, Rabkin R 1999. Fluoxetine treatment for depression in patients with HIV and AIDS: A randomized, placebo-controlled trial. Am J Psychiatry. 156, 101–107. 10.1176/ajp.156.1.101. [DOI] [PubMed] [Google Scholar]
- Rajilić-Stojanović M, Heilig HGHJ, Tims S, Zoetendal EG, de Vos WM 2013. Long-term monitoring of the human intestinal microbiota composition. Environ Microbiol. 15, 1146–1159. 10.1111/1462-2920.12023. [DOI] [PubMed] [Google Scholar]
- Rajilić-Stojanović M, de Vos WM 2014. The first 1000 cultured species of the human gastrointestinal microbiota. FEMS Microbiol Rev. 38, 996–1047. 10.1111/1574-6976.12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray S, Narayanan A, Giske CG, Neogi U, Sönnerborg A, Nowak P 2021. Altered gut microbiome under antiretroviral therapy: Impact of Efavirenz and Zidovudine. ACS Infect Dis. 7, 1104–1115. 10.1021/acsinfecdis.0c00536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raybuck JD, Hargus NJ, Thayer SA 2017. A GluN2B-selective NMDAR antagonist reverses synapse loss and cognitive impairment produced by the HIV-1 protein Tat. J Neurosci. 37, 7837–7847. 10.1523/JNEUROSCI.0226-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reigstad CS, Salmonson CE, Rainey JF, Szurszewski JH, Linden DR, Sonnenburg JL, Farrugia G, Kashyap PC 2015. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J. 29, 1395–1403. 10.1096/fj.14-259598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reunanen J, Kainulainen V, Huuskonen L, Ottman N, Belzer C, Huhtinen H, de Vos WM, Satokari R Akkermansia muciniphila adhere to enterocytes and strengthens the integrity of the epithelial cell layer. Appl Environ Microbiol. 81, 3655–3662. 10.1128/AEM.04050-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs MG, Whittaker RG, Neumann JR, Ingram VM 1977. n-Butyrate causes histone modification in HeLa and Friend erythroleukaemia cells. Nature. 268, 462–464. 10.1038/268462a0. [DOI] [PubMed] [Google Scholar]
- Rocafort M, Noguera-Julian M, Rivera J, Pastor L, Guillén Y, Langhorst J, Parera M, Mandomando I, Carrillo J, Urrea V, Rodríguez C, Casadellà M, Calle ML, Clotet B, Blanco J, Naniche D, Paredes R 2019. Evolution of the gut microbiome following acute HIV-1 infection. Microbiome. 7, 73. 10.1186/s40168-019-0687-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romley JA, Juday T, Solomon MD, Seekins D, Brookmeyer R, Goldman DP 2014. Early HIV treatment led to life expectancy gains valued at $80 billion for people infected in 1996–2009. Health Aff (Millwood). 33, 370–377. 10.1377/hlthaff.2013.0623. [DOI] [PubMed] [Google Scholar]
- Roscoe RF, Mactutus CF, Booze RM 2014. HIV-1 transgenic female rat: Syanptodendritic alterations of medium spiny neurons in the nucleus accumbens. J Neuroimmune Pharmacol. 9, 642–653. 10.1007/s11481-014-9555-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Round JL, Lee SM, Li J, Tran G, Jabri B, Chatila TA, Mazmanian SK 2011. The toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota. Science. 332, 974–977. 10.1126/science.1206095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland IR, Wiseman H, Sanders TA, Adlercreutz H, Bowey EA 2000. Interindividual variation in metabolism of soy isoflavones and lignans: Influence of habitual diet on equol production by the gut microflora. Nutr Cancer. 36, 27–32. 10.1207/S15327914NC3601_5. [DOI] [PubMed] [Google Scholar]
- Rucker DD, McShane BB, Preacher KJ 2015. A researcher’s guide to regression, dichotomization, and median splits of continuous variables. J Consumer Psychol. 4, 666–678. 10.1016/j.jcps.2015.04.004. [DOI] [Google Scholar]
- Rush AJ, George MS, Sackeim HA, Marangell LB, Husain MM, Giller C, Nahas Z, Haines S, Simpson RK, Goodman R 2000. Vagus nerve stimulation (VNS) for treatment-resistant depressions: A multicenter study. Biol Psychiatry. 47, 276–286. 10.1016/s0006-3223(99)00304-2. [DOI] [PubMed] [Google Scholar]
- Sacktor N, Skolasky RL, Moxley R, Wang S, Mielke MM, Munro C, Steiner J, Nath A, Haughey N, McArthur J 2018. Paroxetine and fluconazole therapy for HIV-associated neurocognitive impairment: Results from a double-blind, placebo-controlled trial. J Neurovirol. 24, 16–27. 10.1007/s13365-017-0587-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saletu B, Schjerve M, Grünberger J, Schanda H, Arnold OH 1977. Fluvoxamine-a new serotonin re-uptake inhibitor: First clinical and psychometric experiences in depressed patients. J Neural Transm. 41, 17–36. 10.1007/BF01252962. [DOI] [PubMed] [Google Scholar]
- Saloner R, Grelotti DJ, Tyree G, Sundermann EE, Ma Q, Letendre S, Heaton RK, Cherner M 2019. Benzodiazepine use is associated with an increased risk of neurocognitive impairment in people living with HIV. J Acquir Immune Defic Syndr. 82, 475–482. 10.1097/QAI.0000000000002183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saylor D, Dickens AM, Sacktor N, Haughey N, Slusher B, Pletnikov M, Mankowski JL, Brown A, Volsky DJ, McArthur JC 2016. HIV-associated neurocognitive disorder—pathogenesis and prospects for treatment. Nat Rev Neurol. 12, 234–248. 10.1038/nrneurol.2016.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheri GC, Fard SN, Schietroma I, Mastrangelo A, Pinacchio C, Giustini N, Serafino S, De Girolamo G, Cavallari EN, Statzu M, Laghi L, Vullo A, Ceccarelli G, Vullo V, d’Ettorre G 2017. Modulation of tryptophan/serotonin pathway by probiotic supplementation in human immunodeficiency virus-positive patients: Preliminary results of a new study approach. Int J Tryptophan Res. 10, 1178646917710668. 10.1177/1178646917710668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoch CL, Ciufo S, Domrachev M, Hotton CL, Kannan S, Khovanskaya R, Leipe D, Mcveigh R, O’Neill K, Robbertse B, Sharma S, Soussov V, Sullivan JP, Sun L, Turner S. Karsch-Mizrachi I. 2020. NCBI Taxonomy: A comprehensive update on curation, resources, and tools. Database (Oxford). 2020, baaa062. 10.1093/database/baaa062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sender R, Fuchs S, Milo R 2016. Revised estimates for the number of human and bacteria cells in the body. PLoS Biol. 14, e1002533. 10.1371/journal.pbio.1002533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano-Villar S, Rojo D, Martínez-Martínez M, Deusch S, Vázquez-Castellanos JF, Bargiela R, Sainz T, Vera M, Moreno S, Estrada V, Gosalbes MJ, Latorre A, Seifert J, Barbas C, Moya A, Ferrer M 2016. Gut bacteria metabolism impacts immune recovery in HIV-infected individuals. EBioMedicine. 8, 203–216. 10.1016/j.ebiom.2016.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano-Villar S, Vázquez-Castellanos JF, Vallejo A, Latorre A, Sainz T, Ferrando- Martínez S, Rojo D, Martínez-Botas J, Del Romero J, Madrid N, Leal M, Mosele JI, Motilva MJ, Barbas C, Ferrer M, Moya A, Moreno S, Gosalbes MJ, Estrada V 2017. The effects of prebiotics on microbial dysbiosis, butyrate production and immunity in HIV-infected subjects. Mucosal Immunol. 10, 1279–1293. 10.1038/mi.2016.122. [DOI] [PubMed] [Google Scholar]
- Setchell KD, Borriello SP, Hulme P, Kirk DN, Axelson M 1984. Nonsteroidal estrogens of dietary origin: Possible roles in hormone-dependent disease. Am J Clin Neutr. 40, 569–578. 10.1093/ajcn/40.3.569. [DOI] [PubMed] [Google Scholar]
- Setchell KDR, Clerici C, Lephart ED, Cole SJ, Heenan C, Castellani D, Wolfe BE, Nechemias-Zimmer L, Brown NM, Lund TD, Handa RJ, Heubi JE 2005. S-equol, a potent ligand for estrogen receptor beta, is the exclusing enantiomeric form of the soy isoflavone metabolite produced by human intestinal bacterial flora. Am J Clin Nutr. 81, 1072–1079. 10.1093/ajcn/81.5.1072. [DOI] [PubMed] [Google Scholar]
- Setchell KDR, Cole SJ, 2006. Method of defining equol-producer status and its frequency among vegetarians. J Nutr. 136, 2188–2193. 10.1093/jn/136.8.2188. [DOI] [PubMed] [Google Scholar]
- Shannon CE 1948. A mathematical theory of communication. Bell System Technical Journal. 27, 379–423. 10.1002/j.1538-7305.1948.tb01338.x [DOI] [Google Scholar]
- Sharon G, Sampson TR, Geschwind DH, Mazmanian SK 2016. The central nervous system and the gut microbiome. Cell. 167, 915–932. 10.1016/j.cell.2016.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shilaih M, Angst DC, Marzel A, Bonhoeffer S, Günthard HF, Kouyos RD 2018. Antibacterial effects of antiretrovirals, potential implications for microbiome studies in HIV. Antivir Ther. 23, 91–94. 10.3851/IMP3173. [DOI] [PubMed] [Google Scholar]
- Shin AH, Kim HJ, Thayer SA 2012. Subtype selective NMDA receptor antagonists induce recovery of synapses lost following exposure to HIV-1 Tat. Br J Pharmacol. 166, 1002–1017. 10.1111/j.1476-5381.2011.01805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson EH 1949. Measurement of diversity. Nature. 163, 688. 10.1038/163688a0 [DOI] [Google Scholar]
- Siopi E, Galerne M, Rivagorda M, Saha S, Moigneu C, Moriceau S, Bigot M, Oury F, & Lledo P-M (2023). Gut microbiota changes require vagus nerve integrity to promote depressive-like behaviors in mice. Molecular Psychiatry. 10.1038/s41380-023-02071-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DK, Kassam T, Singh B, Elliott JF 1992. Escherichia coli has two homologous glutamate decarboxylase genes that map to distinct loci. J Bacteriol. 174, 5820–5826. 10.1128/jb.174.18.5820-5826.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer F, Bäckhed F 2013. The gut microbiota—Masters of host development and physiology. Nat Rev Microbiol. 11, 227–238. 10.1038/nrmicro2974. [DOI] [PubMed] [Google Scholar]
- Somsouk M, Estes JD, Deleage C, Dunham RM, Albright R, Inadomi JM, Martin JN, Deeks SG, McCune JM, Hunt PW 2015. Gut epithelial barrier and systemic inflammation during chronic HIV infection. AIDS. 29, 43–51. 10.1097/QAD.0000000000000511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sortino O, Phanuphak N, Schuetz A, Ortiz AM, Chomchey N, Belkaid Y, Davis J, Mystakelis HA, Quiñones M, Deleage C, Ingram B, Rerknimitr R, Pinyakorn S, Rupert A, Robb ML, Ananworanich J, Brenchley J, Sereti I, RV254/SEARCH010 Study Group. 2020. Impact of acute HIV infection and early antiretroviral therapy on the human gut microbiome. Open Forum Infect Dis. 7, ofz367. 10.1093/ofid/ofz367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speidell A, Asuni GP, Wakulski R, Mocchetti I 2020. Up-regulation of the p75 neurotrophin receptor is an essential mechanism for HIV-gp120 mediated synaptic loss in the striatum. Brain Behav Immun. 89, 371–379. 10.1016/j.bbi.2020.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stackebrandt E, Murray RGE, Trüper HG 1988. Proteobacteria classis nov., a name for the phylogenetic taxon that includes the “purple bacteria and their relatives.” Int J Syst Bacteriol. 38, 321–325. 10.1099/00207713-38-3-321 [DOI] [Google Scholar]
- Sternbach H 1991. The serotonin syndrome. Am J Psychiatry. 148, 705–713. 10.1176/ajp.148.6.705. [DOI] [PubMed] [Google Scholar]
- Strandwitz P, Kim KH, Terekhova D, Liu JK, Sharma A, Levering J, McDonald D, Dietrich D, Ramadhar TR, Lekbua A, Mroue N, Liston C, Stewart EJ, Dubin MJ, Zengler K, Knight R, Gilbert JA, Clardy J, Lewis K 2019. GABA modulating bacteria of the human gut microbiota. Nat Microbiol. 4, 396–403. 10.1038/s41564-018-0307-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strazza M, Pirrone V, Wigdahl B, Nonnemacher MR 2011. Breaking down the barrier: The effects of HIV-1 on the blood-brain barrier. Brain Res. 1399, 96–115. 10.1016/j.brainres.2011.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong WL 2016. Biased richness and evenness relationships within Shannon-Wiener index values. Ecol Indic. 67, 703–713. 10.1016/j.ecolind.2016.03.043 [DOI] [Google Scholar]
- Suarez AN, Hsu TM, Liu CM, Noble EE, Cortella AM, Nakamoto EM, Hahn JD, de Lartigue G, Kanoski SE 2018. Gut vagal sensory signaling regulates hippocampus function through multi-order pathways. Nat Commun 9, 2181. 10.1038/s41467-018-04639-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Ma Y, Lin P, Tang YW, Yang L, Shen Y, Zhang R, Liu L, Cheng J, Shao J, Qi T, Tang Y, Cai R, Guan L, Luo B, Sun M, Li B, Pei Z, Lu H 2016. Fecal bacterial microbiome diversity in chronic HIV-infected patients in China. Emerg Microbes Infect. 5, e31. 10.1038/emi.2016.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teeraananchai S, Kerr SJ, Amin J, Ruxrungtham K, Law MG 2017. Life expectancy of HIV-positive people after starting combination antiretroviral therapy: A meta-analysis. HIV Med. 18, 256–266. 10.1111/hiv.12421. [DOI] [PubMed] [Google Scholar]
- Tousen Y, Ezaki J, Fujii Y, Ueno T, Nishimuta M, Ishimi Y 2011. Natural S-equol decreases bone resorption in postmenopausal, non-equol-producing Japanese women: A pilot randomized, placebo-controlled trial. Menopause. 18, 563–574. 10.1097/gme.0b013e3181f85aa7. [DOI] [PubMed] [Google Scholar]
- Tsai AC, Karasic DH, Hammer GP, Charlebois ED, Ragland K, Moss AR, Sorensen JL, Dilley JW, Bangsberg DR 2013. Directly observed antidepressant medication treatment and HIV outcomes among homeless and marginally housed HIV-positive adults: A randomized controlled trials. Am J Public Health. 103, 308–315. 10.2105/AJPH.2011.300422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuddenham SA, Koay WLA, Zhao N, White JR, Ghanem KG, Sears CL, HIV Microbiome Re-analysis Consortium. 2020. The impact of human immunodeficiency virus infection on gut microbiota α-diversity: An individual-level meta-analysis. Clin Infect Dis. 70, 615–627. 10.1093/cid/ciz258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuscher JJ, Luine V, Frankfurt M, Frick KM 2016. Estradiol-mediated spine changes in the dorsal hippocampus and medial prefrontal cortex of ovariectomized female mice depend on ERK and mTOR activation in the dorsal hippocampus. J Neurosci. 36, 1483–1489. 10.1523/JNEUROSCI.3135-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzianabos AO, Onderdonk AB, Rosner B, Cisneros RL, Kasper DL 1993. Structural features of polysaccharides that induce intra-abdominal abscesses. Science. 262, 416–419. 10.1126/science.8211161. [DOI] [PubMed] [Google Scholar]
- Vadaq N, Zhang Y, Meeder E, Van de Wijer L, Gasem MH, Joosten LA, Netea MG, de Mast Q, Matzaraki V, Schellekens A, Fu J, van der Ven AJ 2022. Microbiome-related indole and serotonin metabolites are linked to inflammation and psychiatric symptoms in people living with HIV. Int J Tryptophan Res. 15, 11786469221126888. 10.1177/11786469221126888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vázquez-Castellanos JF, Serrano-Villar S, Latorre A, Artacho A, Ferrús ML, Mdrid N, Vallejo A, Sainz T, Martínez-Botas J, Ferrando-Martínez S, Vera M, Dronda F, Leal M, Del Romero J, Moreno S, Estrada V, Gosalbes MJ, Moya A 2015. Altered metabolism of gut microbiota contributes to chronic immune activation in HIV-infected individuals. Mucosal Immunol. 8, 760–772. 10.1038/mi.2014.107. [DOI] [PubMed] [Google Scholar]
- Vesterbacka J, Rivera J, Noyan K, Parera M, Neogi U, Calle M, Paredes R, Sönnerborg A, Noguera-Julian M, Nowak P 2017. Richer gut microbiota with distinct metabolic profile in HIV infected elite controllers. Sci Rep. 7, 6269. 10.1038/s41598-017-06675-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villaneuva-Millán MJ, Péerez-Matute P, Recio-Fernández E, Rosales JML, Oteo JA 2017. Differential effects of antiretrovirals on microbial translocation and gut microbiota composition of HIV-infected patients. J Int AIDS Soc. 20, 21526. 10.7448/IAS.20.1.21526.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vujkovic-Cvijin I, Sortino O, Verheij E, Sklar J, Wit FW, Kootstra NA, Sellers B, Brenchley JM, Ananworanich J, van der Loeff MS, Belkaid Y, Reiss P, Sereti I 2020. HIV-associated gut dysbiosis is independent of sexual practice and correlates with noncommunicable diseases. Nat Commun. 11, 2448. 10.1038/s41467-020-16222-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Usyk M, Sollecito CC, Qiu Y, Williams-Nguyen J, Hua S, Gradissimo A, Wang T, Xue X, Kurland IJ, Ley K, Landay AL, Anastos K, Knight R, Kaplan RC, Burk RD, Qi Q 2020. Altered gut microbiota and host metabolite profiles in women with human immunodeficiency virus. Clin Infect Dis. 71, 2345–2353. 10.1093/cid/ciz1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Zhu J, Xu T 2018. 17β-estradiol (E2) promotes growth and stability of new dendritic spines via estrogen receptor β pathway in intact mouse cortex. Brain Res Bull. 137, 241–248. 10.1016/j.brainresbull.2017.12.011. [DOI] [PubMed] [Google Scholar]
- Weiss JJ, Calvi R, Naganawa M, Toyonaga T, Farhadian SF, Chintanaphol M, Chiarella J, Zheng MQ, Ropchan J, Huang Y, Pietrzak RH, Carson RE, Spudich S 2021. Preliminary in vivo evidence of reduced synaptic density in human immunodeficiency virus (HIV) despite antiretroviral therapy. Clin Infect Dis. 73, 1404–1411. 10.1093/cid/ciab484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner ER, Fuchs D, Hausen A, Jaeger H, Reibnegger G, Werner-Felmayer G, Dierich MP, Wachter. 1988. Tryptophan degradation in patients infected by human immunodeficiency virus. Biol Chem Hoppe Seyler. 369, 337–340. 10.1515/bchm3.1988.369.1.337. [DOI] [PubMed] [Google Scholar]
- Williams B, Weber K, Chlipala G, Evans C, Morack R, French A 2019. HIV status does not affect rectal microbiome composition, diversity, or stability over time: A Chicago Women’s Interagency HIV Study. AIDS Res Hum Retroviruses. 35, 260–266. 10.1089/AID.2018.0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittaker RH, 1960. Vegetation of the Siskiyou Mountains, Oregon and California. Ecol Monogr. 30, 279–338. 10.2307/1943563 [DOI] [Google Scholar]
- Whittaker RH 1972. Evolution and measurement of species diversity. Taxon. 21, 213–251. 10.2307/1218190. [DOI] [Google Scholar]
- Xie Y, Sun J, Wei L, Jiang H, Hu C, Yang J, Huang Y, Ruan B, Zhu B 2021. Altered gut microbiota correlate with different immune responses to HAART in HIV-infected individuals. BMC Microbiol. 21, 11. 10.1186/s12866-020-02074-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Fitting S 2016. Inhibition of GABAergic neurotransmission by HIV-1 Tat and opioid treatment in the striatum involves μ-opioid receptors. Front Neurosci. 10, 497. 10.3389/fnins.2016.00497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Hermes DJ, Mackie K, Lichtman AH, Ignatowska-Jankowska BM, Fitting S 2016. Cannabinoids occlude the HIV-1 Tat-induced decrease in GABAergic neurotransmission in prefrontal cortex slices. J Neuroimmune Pharmacol. 11, 316–331. 10.1007/s11481-016-9664-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Yadav-Samudrala BJ, Xu C, Nath B, Mistry T, Jiang W, Niphakis MJ, Cravatt BF, Mukhopadhyay S, Lichtman AH, Ignatowska-Jankowska BM, Fitting S 2022. Inhibitory neurotransmission is sex-dependently affected by Tat expression in transgenic mice and suppressed by the fatty acid amide hydrolase enzyme inhibitor PF3845 via cannabinoid type-1 receptor mechanisms. Cells. 11, 857. 10.3390/cells11050857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, Magris M, Hidalgo G, Baldassano RN, Anokhin AP, Heath AC, Warner B, Reeder J, Kuczynski J, Caporaso JG, Lozupone CA, Lauber C, Clemente JC, Knights D, Knight R, Gordon JI 2012. Human gut microbiome viewed across age and geography. Nature. 486, 222–227. 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenebe Y, Necho M, Yimam W, Akele B 2022. Worldwide occurrence of HIV-associated neurocognitive disorders and its associated factors: A systematic review and meta-analysis. Front Psychiatry. 13, 814362. 10.3389/fpsyt.2022.814362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Yang J, Ji Y, Sun M, Shen J, Sun J, Wang J, Liu L, Shen Y, Zhang R, Chen J, Lu H 2019. Gut microbiota dysbiosis is not independently associated with neurocognitive impairment in people living with HIV. Front Microbiol. 9, 3352. 10.3389/fmicb.2018.03352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Ma L, Chang L, Pu Y, Qu Y, Hashimoto K 2020. A key role of the subdiaphragmatic vagus nerve in the depression-like phenotype and abnormal composition of gut microbiota in mice after lipopolysaccharide administration. Transl Psychiatry. 10, 186. 10.1038/s41398-020-00878-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Zhang Y, Cui P, Luo L, Chen H, Liang B, Jiang J, Ning C, Tian L, Zhong X, Ye L, Liang H, Huang J 2020. Gut microbiome changes associated with HIV infection and sexual orientation. Front Cell Infect Microbiol. 10, 434. 10.3389/fcimb.2020.00434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Ou Z, Tang X, Zhou Y, Xu H, Wang X, Li K, He J, Du Y, Wang H, Chen Y, Nie Y 2018. Alterations in the gut microbiota of patients with acquired immune deficiency syndrome. J Cell Mol Med. 22, 2263–2271. 10.1111/jcmm.13508. [DOI] [PMC free article] [PubMed] [Google Scholar]


