Abstract
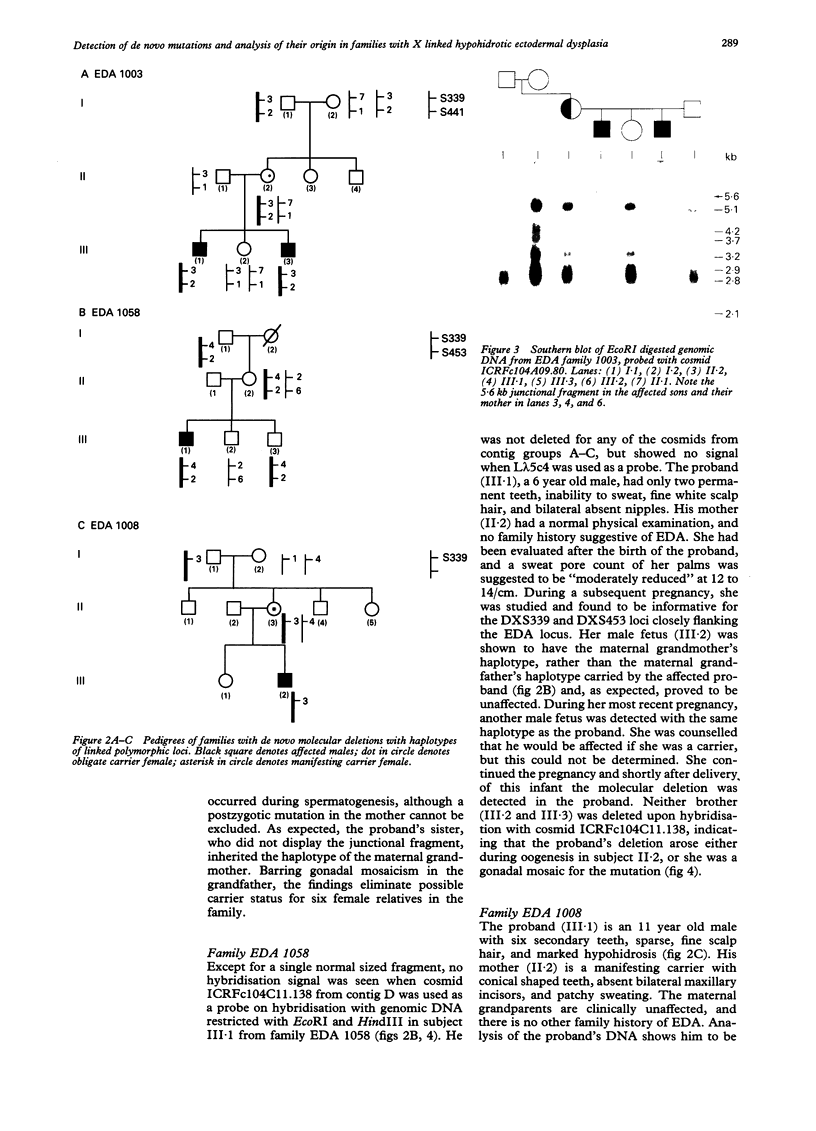
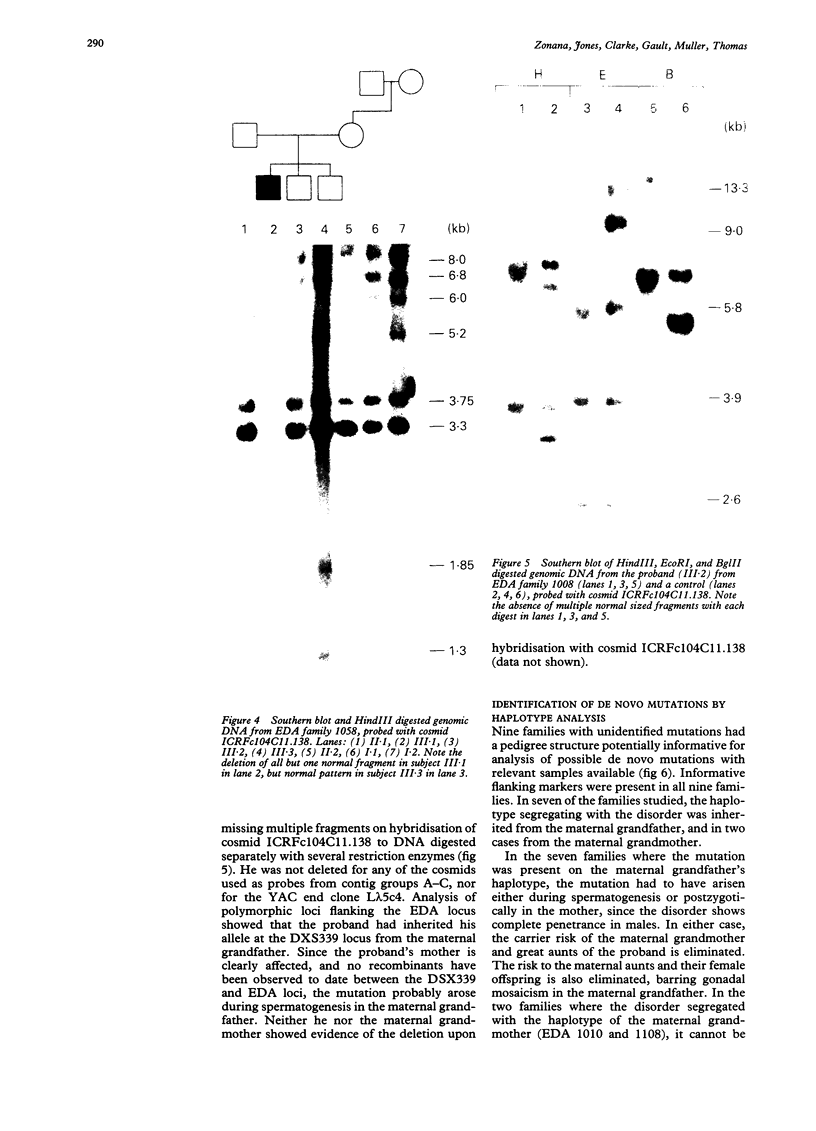
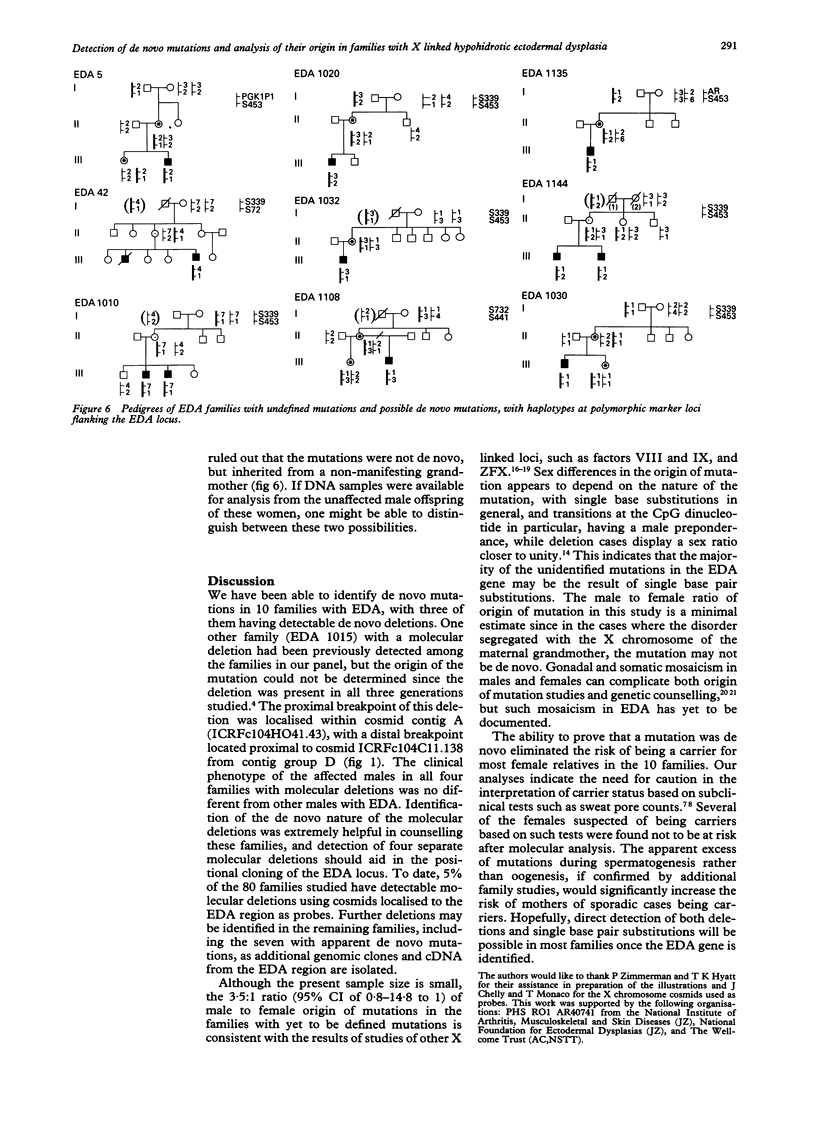
Hypohidrotic ectodermal dysplasia (EDA) has been localised to the q12-q13.1 region of the X chromosome by both physical and genetic mapping methods. Although linkage analysis using closely linked flanking markers can clarify the carrier status for many females at risk for the disorder, knowledge of the origin of the mutation in instances of possible de novo mutation is critical for accurate genetic counselling of families. Two methods have been used to confirm de novo mutation in families with EDA and to trace their origin. Direct detection of three de novo molecular deletions, one arising during oogenesis and the other two during spermatogenesis, was achieved by Southern analyses using cosmids isolated from the EDA region as probes. Seven de novo mutations arising during spermatogenesis, and two possible de novo mutations during oogenesis, were identified by an analysis of the cosegregation of the disorder with polymorphic markers closely linked to and flanking the EDA locus. The confirmation and analysis of the origin of the 10 de novo mutations greatly assisted genetic counselling in these families. The apparent 3.5:1 excess of male to female origin of mutation in families studied with unidentified types of mutation is similar to other studies of X linked disorders, and suggests that the majority of these mutations may involve single base pair substitutions.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berg D., Weingold D. H., Abson K. G., Olsen E. A. Sweating in ectodermal dysplasia syndromes. A review. Arch Dermatol. 1990 Aug;126(8):1075–1079. [PubMed] [Google Scholar]
- Clarke A., Burn J. Sweat testing to identify female carriers of X linked hypohidrotic ectodermal dysplasia. J Med Genet. 1991 May;28(5):330–333. doi: 10.1136/jmg.28.5.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke A., Phillips D. I., Brown R., Harper P. S. Clinical aspects of X-linked hypohidrotic ectodermal dysplasia. Arch Dis Child. 1987 Oct;62(10):989–996. doi: 10.1136/adc.62.10.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford P. J., Aldred M. J., Clarke A. Clinical and radiographic dental findings in X linked hypohidrotic ectodermal dysplasia. J Med Genet. 1991 Mar;28(3):181–185. doi: 10.1136/jmg.28.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards A., Civitello A., Hammond H. A., Caskey C. T. DNA typing and genetic mapping with trimeric and tetrameric tandem repeats. Am J Hum Genet. 1991 Oct;49(4):746–756. [PMC free article] [PubMed] [Google Scholar]
- Grimm T., Müller B., Müller C. R., Janka M. Theoretical considerations on germline mosaicism in Duchenne muscular dystrophy. J Med Genet. 1990 Nov;27(11):683–687. doi: 10.1136/jmg.27.11.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kere J., Grzeschik K. H., Limon J., Gremaud M., Schlessinger D., de la Chapelle A. Anhidrotic ectodermal dysplasia gene region cloned in yeast artificial chromosomes. Genomics. 1993 May;16(2):305–310. doi: 10.1006/geno.1993.1189. [DOI] [PubMed] [Google Scholar]
- Ketterling R. P., Vielhaber E., Bottema C. D., Schaid D. J., Cohen M. P., Sexauer C. L., Sommer S. S. Germ-line origins of mutation in families with hemophilia B: the sex ratio varies with the type of mutation. Am J Hum Genet. 1993 Jan;52(1):152–166. [PMC free article] [PubMed] [Google Scholar]
- Kling S., Ljung R., Sjörin E., Montandon J., Green P., Giannelli F., Nilsson I. M. Origin of mutation in sporadic cases of haemophilia-B. Eur J Haematol. 1992 Mar;48(3):142–145. doi: 10.1111/j.1600-0609.1992.tb00585.x. [DOI] [PubMed] [Google Scholar]
- Litt M., White R. L. A highly polymorphic locus in human DNA revealed by cosmid-derived probes. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6206–6210. doi: 10.1073/pnas.82.18.6206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milewicz D. M., Witz A. M., Smith A. C., Manchester D. K., Waldstein G., Byers P. H. Parental somatic and germ-line mosaicism for a multiexon deletion with unusual endpoints in a type III collagen (COL3A1) allele produces Ehlers-Danlos syndrome type IV in the heterozygous offspring. Am J Hum Genet. 1993 Jul;53(1):62–70. [PMC free article] [PubMed] [Google Scholar]
- Müller C. R., Grimm T. Estimation of the male to female ratio of mutation rates from the segregation of X-chromosomal DNA haplotypes in Duchenne muscular dystrophy families. Hum Genet. 1986 Oct;74(2):181–183. doi: 10.1007/BF00282088. [DOI] [PubMed] [Google Scholar]
- Nizetić D., Zehetner G., Monaco A. P., Gellen L., Young B. D., Lehrach H. Construction, arraying, and high-density screening of large insert libraries of human chromosomes X and 21: their potential use as reference libraries. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3233–3237. doi: 10.1073/pnas.88.8.3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ram K. T., Barker D. F., Puck J. M. Dinucleotide repeat polymorphism at the DXS441 locus. Nucleic Acids Res. 1992 Mar 25;20(6):1428–1428. doi: 10.1093/nar/20.6.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosendaal F. R., Bröcker-Vriends A. H., van Houwelingen J. C., Smit C., Varekamp I., van Dijck H., Suurmeijer T. P., Vandenbroucke J. P., Briët E. Sex ratio of the mutation frequencies in haemophilia A: estimation and meta-analysis. Hum Genet. 1990 Dec;86(2):139–146. doi: 10.1007/BF00197695. [DOI] [PubMed] [Google Scholar]
- Shimmin L. C., Chang B. H., Li W. H. Male-driven evolution of DNA sequences. Nature. 1993 Apr 22;362(6422):745–747. doi: 10.1038/362745a0. [DOI] [PubMed] [Google Scholar]
- Thomas N. S., Chelly J., Zonana J., Davies K. J., Morgan S., Gault J., Rack K. A., Buckle V. J., Brockdorff N., Clarke A. Characterisation of molecular DNA rearrangements within the Xq12-q13.1 region, in three patients with X-linked hypohidrotic ectodermal dysplasia (EDA). Hum Mol Genet. 1993 Oct;2(10):1679–1685. doi: 10.1093/hmg/2.10.1679. [DOI] [PubMed] [Google Scholar]
- Zonana J., Clarke A., Sarfarazi M., Thomas N. S., Roberts K., Marymee K., Harper P. S. X-linked hypohidrotic ectodermal dysplasia: localization within the region Xq11-21.1 by linkage analysis and implications for carrier detection and prenatal diagnosis. Am J Hum Genet. 1988 Jul;43(1):75–85. [PMC free article] [PubMed] [Google Scholar]
- Zonana J., Gault J., Davies K. J., Jones M., Browne D., Litt M., Brockdorff N., Rastan S., Clarke A., Thomas N. S. Detection of a molecular deletion at the DXS732 locus in a patient with X-linked hypohidrotic ectodermal dysplasia (EDA), with the identification of a unique junctional fragment. Am J Hum Genet. 1993 Jan;52(1):78–84. [PMC free article] [PubMed] [Google Scholar]
- Zonana J., Jones M., Browne D., Litt M., Kramer P., Becker H. W., Brockdorff N., Rastan S., Davies K. P., Clarke A. High-resolution mapping of the X-linked hypohidrotic ectodermal dysplasia (EDA) locus. Am J Hum Genet. 1992 Nov;51(5):1036–1046. [PMC free article] [PubMed] [Google Scholar]
- Zonana J., Sarfarazi M., Thomas N. S., Clarke A., Marymee K., Harper P. S. Improved definition of carrier status in X-linked hypohidrotic ectodermal dysplasia by use of restriction fragment length polymorphism-based linkage analysis. J Pediatr. 1989 Mar;114(3):392–399. doi: 10.1016/s0022-3476(89)80556-6. [DOI] [PubMed] [Google Scholar]