Summary
Background
Innovative GLP-1 receptor agonist (GLP-1RA)-based treatment strategies—such as tirzepatide, GLP-1RA plus basal insulin fixed-ratio combinations [FRC], GLP-1RA plus sodium glucose cotransporter-2 inhibitors [SGLT-2i] combinations, and high-dose GLP-1RA—have been listed among the most efficacious options for type 2 diabetes management. However, differences in their glucometabolic effects have not been assessed in dedicated head-to-head trials. In the absence of such trials, we aimed to provide a useful comparison among these treatment strategies to guide clinical practice.
Methods
In this network meta-analysis, we searched PubMed, MEDLINE, and Web of Science (from database inception to June 24, 2023) for randomised controlled studies, published in English, that enrolled individuals with type 2 diabetes treated with tirzepatide, iGlarLixi, iDegLira, GLP-1RA plus SGLT-2i combination, or high-dose GLP-1RA (dulaglutide 3 mg and 4.5 mg, semaglutide 2 mg) compared with placebo or active comparators. Eligible studies reported change from baseline in HbA1c as an outcome, which was the primary outcome of this analysis. Secondary outcomes were changes in fasting and post-prandial glucose, bodyweight, LDL-cholesterol, blood pressure and risk of hypoglycaemia. We assessed risk of bias through the Cochrane Collaboration's tool (RoB2 tool), publication bias through visual inspection of funnel plots and Egger's test, and heterogeneity by comparing the magnitude of the common between-study variance (τ2) for each outcome with empirical distributions of heterogeneity variances. This network meta-analysis was registered in PROSPERO (CRD42022329878).
Findings
40 trials were included. Tirzepatide 15 mg ranked first in terms of HbA1c reduction compared to other GLP-1RA-based strategies, even those including insulin (vs. iDegLira MD −0.40%, 95% CI [−0.66; −0.14], low certainty; vs. iGlarLixi MD −0.48%, 95% CI [−0.75; −0.21], low certainty), without increasing the risk of hypoglycaemia (vs. iDegLira OR 0.35, 95% CI [0.16; 0.79], high certainty; vs. iGlarLixi OR 0.31, 95% CI [0.20; 0.48], high certainty). Tirzepatide 15 mg was also the most efficacious on weight lowering, even compared to high-dose GLP-1RA (eg, semaglutide 2 mg MD −6.56 kg, 95% CI [−7.38; −5.73], low certainty) and GLP-1RA plus SGLT-2i combination (MD −4.61 kg, 95% CI [−5.29; −3.93], low certainty). Risk of bias and publication bias were generally low throughout studies, while high levels of heterogeneity were detected for most outcomes.
Interpretation
Aiming to support clinicians in tailoring treatment to patients’ needs, we suggest that a hierarchy among treatment strategies be devised considering the best options for type 2 diabetes. Tirzepatide, followed by GLP-1RA plus basal insulin FRC and GLP-1RA plus SGLT-2i combination, was associated with greater benefit on HbA1c than high-dose GLP-1RA.
Funding
Fondazione per la Ricerca Biomedica “Saverio e Isabella Cianciola” and Next Generation EU, in the context of the National Recovery and Resilience Plan, Investment PE8—Project Age-It: Ageing Well in an Ageing Society.
Keywords: Tirzepatide, GLP1-RA, SGLT-2i, Fixed-ratio combination, Network meta-analysis
Research in context.
Evidence before this study
We repeatedly searched PubMed until June 24, 2023, without date or study duration restrictions. We did not find any systematic review that compared new GLP-1 receptor agonist (GLP-1RA)-based therapeutic strategies (ie, tirzepatide, GLP-1RA plus basal insulin fixed-ratio combinations [FRC], GLP-1RA plus sodium glucose cotransporter-2 inhibitors [SGLT-2i] combination and high-dose GLP-1RA). Despite these treatment strategies being regarded as among the most efficacious options for diabetes management, the available literature to date suggests that there are differences in glucose lowering potential between them. In the absence of head-to-head comparisons in the form of direct trials, we aimed to conduct a network meta-analysis to provide a useful comparison among these highly efficacious treatment strategies to guide clinical practice.
Added value of this study
The present analysis found that tirzepatide 15 mg yielded greater HbA1c and bodyweight lowering efficacy compared to other GLP-1RA-based treatments, without increasing the risk of serious adverse events and hypoglycaemia with respect to placebo, nor of gastrointestinal side effects compared to GLP-1RA plus SGLT-2i combination and high-dose GLP-1RA. Moreover, GLP-1RA plus basal insulin FRC and tirzepatide 15 mg and 10 mg ranked higher in terms of fasting plasma glucose (FPG) lowering, while iGlarLixi appeared as the most efficacious in post-prandial glucose (PPG) lowering, followed by GLP-1RA plus SGLT-2i combination and tirzepatide 15 mg. Our analysis hinted that the effects of GLP-1RA plus basal insulin FRC might match that of tirzepatide 15 mg in patients with baseline diabetes duration above 10 years, suggesting that tirzepatide could be preferentially considered for the management of early stages of type 2 diabetes, to exploit its unparalleled weight lowering effect that could be beneficial for restoring insulin secretion and action, whereas GLP-1RA plus basal insulin FRC could be considered as an equally efficacious option in patients with longer disease duration. No significant differences among these GLP-1RA-based treatments were detected in terms of LDL cholesterol (LDL-c) and systolic blood pressure (SBP) lowering.
Implications of all the available evidence
To our knowledge, this is the first time that these highly efficacious treatment strategies of great relevance for clinical practice have been compared with a rigorous methodology. Our results are consistent with the mechanism of action of investigated treatments and previous attempts of indirect comparisons. The results of this analysis highlight the need for further studies, preferentially randomised clinical trials, to confirm the differences herein described, specifically investigating whether GLP-1RA-based treatments could differ in their effects on HbA1c, FPG and PPG considering patients' characteristics such as diabetes duration and using homogeneous assessment methods. Meanwhile, aiming to support clinicians in tailoring treatment to patients’ needs, we suggest a hierarchy among treatment strategies considered as the best options for type 2 diabetes management.
Introduction
The prevalence of type 2 diabetes is increasing worldwide,1 while the proportion of patients achieving glycaemic control is declining,2 posing a significant medical, social and economic threat. In the last decade, glucagon-like peptide-1 receptor agonists (GLP-1RA) have become available for the treatment of type 2 diabetes, with clear evidence of improved glucose control, weight loss, and cardiorenal protection.3,4 However, despite a glucose-lowering potential similar to insulin, treatment with GLP-1RA allows to achieve a target HbA1c <7% in only 40–80% of patients, while no more than 25% lose 10% of bodyweight (BW).5 Aiming to fill in the gaps of traditional GLP-1RA therapy, new GLP-1RA-based treatment strategies have been recently developed to improve clinical outcomes and/or simplify treatment regimens, including high-dose GLP-1RA, combination of GLP-1RA with sodium glucose cotransporter-2 inhibitors (SGLT-2i), fixed-ratio combinations (FRC) of GLP-1RA with basal insulin, and dual incretin receptor agonists. Predictably, high-dose GLP-1RA have proven to be more efficacious than standard dose GLP-1RA in achieving both glycaemic (mean HbA1c reduction −2.2 to −1.87%) and weight targets (mean BW reduction −6.9 to −5.0 kg).5, 6, 7 The combination of GLP-1RA and SGLT-2i has been associated with a greater reduction in HbA1c (up to −1.32%), BW (up to −0.93 kg) and blood pressure compared to each drug alone due to their complementary mechanisms of action.8,9 Also, GLP-1RA and basal insulin FRC allowed to achieve greater glycaemic control (mean HbA1c reduction −1.5 to −1.89%), exploiting the distinct effect of their components on fasting and prandial glucose, respectively, while reducing the risk of adverse events such as hypoglycaemia, weight gain and gastrointestinal disturbances and increasing adherence.10 Lastly, tirzepatide, a dual GLP-1 and glucose-dependent insulinotropic polypeptide (GIP) receptor agonist, has been recently introduced as a highly efficacious anti-diabetes agent, with unprecedented glucose (mean HbA1c reduction up to −2.59%) and weight lowering (mean BW reduction up to −12.9 kg) effects.11 A deeper understanding of the multifactorial pathogenesis of type 2 diabetes12 supports the combination of therapeutic agents to simultaneously address multiple mechanisms promoting hyperglycaemia. This approach could likely be beneficial in the management of diabetes cardio-metabolic complication,13,14 even though further evidence is needed.15
The American Diabetes Association (ADA) recommendations encouraged a prompt intervention to achieve tailored glycaemic targets, listing these new GLP-1RA-based treatments (tirzepatide, GLP-1RA plus SGLT-2i, GLP-1RA plus basal insulin FRC, high-dose GLP-1RA) among the strategies with greatest glucose-lowering potential.16 Tirzepatide and semaglutide are also the most effective drugs for weight management.16 Existing literature hints at differences among these treatment options,5,9,10 however, in the absence of head-to-head trials, it is unclear whether they should be regarded as equally efficacious in obtaining glucometabolic and weight control. This network meta-analysis aims to compare for the first time the effects of tirzepatide, GLP-1RA plus basal insulin FRC, GLP-1RA plus SGLT-2i combination and high-dose GLP-1RA on HbA1c and other glucose outcomes, as well as on weight, blood pressure and lipids, in randomised controlled trials (RCT) in people with type 2 diabetes, in order to help physicians navigating within the multiple highly efficacious GLP-1RA-based treatment options currently available for clinical use.
Methods
The protocol for this network meta-analysis was registered in PROSPERO (CRD42022329878).
Data sources
We searched PubMed, including MEDLINE, and Web of Science from inception to June 24th, 2023 (search strings in Supplementary files). Corresponding Authors were contacted in case of missing data for any of the outcomes of interest.
Ethics approval
Analyses were performed on data extracted from published papers. Patient consent for publication was not required.
Study selection
We included RCT with a follow-up duration ranging from a minimum of 12 weeks to a maximum of 78 weeks, published in English, enrolling individuals with type 2 diabetes treated with tirzepatide or GLP-1RA plus basal insulin FRC (iGlarLixi, iDegLira) or GLP-1RA plus SGLT-2i combination or high-dose GLP-1RA (dulaglutide 3 mg and 4.5 mg, semaglutide 2 mg) compared to placebo or active comparators reporting change from baseline in HbA1c. Of note, in the Asian population, specifically tailored doses and dose-ratios of GLP-1RA and GLP-1RA plus basal insulin FRC were included.
With the exception of high-dose GLP-1RA, which were considered individually as dulaglutide 3 mg and 4.5 mg and semaglutide 2 mg, all compounds belonging to the GLP-1RA and SGLT-2i drug classes were considered altogether, GLP-1RA plus SGLT-2i combination therapy was considered as a group, and only RCT evaluating the simultaneous initiation of these anti-diabetes agents were included, excluding trials evaluating add-on schemes; in case of trial arms with different doses of GLP-1RA/SGLT2i in combination treatment, we selected the arm with the higher doses of each drug.
Extension studies were excluded. Animal studies, trials conducted in non-diabetic individuals or people with type 1 diabetes, prediabetes, gestational diabetes were excluded.
The main outcome of interest was the mean difference in HbA1c change from baseline. If available, the mean difference in change from baseline for fasting blood glucose (FPG), post-prandial glucose (PPG), BW, systolic (SBP) and diastolic blood pressure (DBP), LDL cholesterol (LDL-c) levels and prevalence of hypoglycaemic events were also assessed as secondary outcomes. The definition of a hypoglycaemic event was heterogeneous across included studies; in the present analysis we considered the prevalence of hypoglycaemic events defined as blood glucose levels <70 mg/dL.
Data extraction
Study data were extracted independently by three reviewers (IC, LDG and SDM). Conflicts were settled by debate, with the aid of another reviewer (FG). For RCT evaluating two doses of the same compound belonging to the class of SGLT-2i, the higher dose was considered. Data from intention to treat analyses were selected; if unavailable, per protocol analyses were considered. Mean change from baseline in HbA1c was extracted from each RCT as primary outcome. Mean changes from baseline in FPB, PPG, BW, SBP, DBP, LDL-c and prevalence of hypoglycaemic events were also collected. Change from baseline was considered at end of study. If standard deviation (SD) was missing for a specific outcome, it was calculated from the standard error (SE), by multiplying SE by the square root of the sample size, or from the 95% confidence interval (CI), by dividing the length of the CI by 3.92, and then multiplying by the square root of the sample size. When none of them was described, the largest SD among the other studies was reported. If hypoglycaemia was expressed as Person-Years, the prevalence was calculated as (Person-Years ∗ study duration [years]/number of patients).
Risk of bias assessment
Risk of bias was assessed independently by two reviewers (IC, LDG) through the Cochrane Collaboration's tool (RoB2 tool) evaluating the following domains: randomisation process; deviations from intended intervention; missing outcome data; measurement of the outcome; selection of the reported result; overall bias. Each domain was deemed at low, with some concerns or high risk of bias.
Statistical analysis
Pairwise meta-analyses were conducted for direct comparisons. The transitivity assumption that a network meta-analysis approach could be appropriate was assessed by comparing the distribution of potential effect modifiers across treatment comparisons (year of publication of the study, sample size, study duration, ethnicity, sex, BMI, duration of diabetes, age, baseline HbA1c). Differences in BMI, duration of diabetes and year of publication were found across trials, hence we planned to conduct subgroup analyses for BMI and duration of diabetes, while year of publication was not taken into account due to statistically but not clinically relevant differences. We subsequently performed frequentist random effects network meta-analysis,17 calculating mean differences (MDs) and 95% confidence intervals (CIs) for change in HbA1c, FPG, PPG, BW, SBP, DBP and LDL-c, and odds ratios (ORs) and 95% CIs for the risk of hypoglycaemic events. We assessed heterogeneity by comparing the magnitude of the common between-study variance (τ2) for each outcome with empirical distributions of heterogeneity variances.18 We evaluated local consistency in networks by comparing direct with indirect evidence19 and global consistency with the design-by-treatment interaction model.20 Japan and worldwide approved iDegLira and iGlarLixi doses were analysed altogether. We also conducted subgroup analyses according to patients’ mean baseline BMI < or ≥30 kg/m2, background basal insulin treatment (with or without basal insulin as background therapy), ethnicity (RCT conducted in exclusively Asian population or not), and diabetes duration < or ≥ 10 years. For all outcomes, we performed sensitivity analyses restricted to trials at a low risk of bias or excluding trials for which SDs were imputed. An exploratory analysis of adverse events (serious adverse events, any adverse event, cholelithiasis, pancreatitis, nausea, vomiting, diarrhoea, constipation) was performed. All analyses were performed using RStudio 2023.03.1 Build 446 (MacOS, Apple Silicon version), R 4.3.0 (2023-04-21) and R packages meta21 version 6.2.1 and netmeta22 version 2.8-2. We assessed confidence in network meta-analysis estimates using the CINeMA (Confidence In Network Meta-Analysis) framework and online application.23
Role of the funding source
The funders had no role in study design, data collection, data analyses, interpretation, or writing, but did provide funding for publication.
Results
Study characteristics
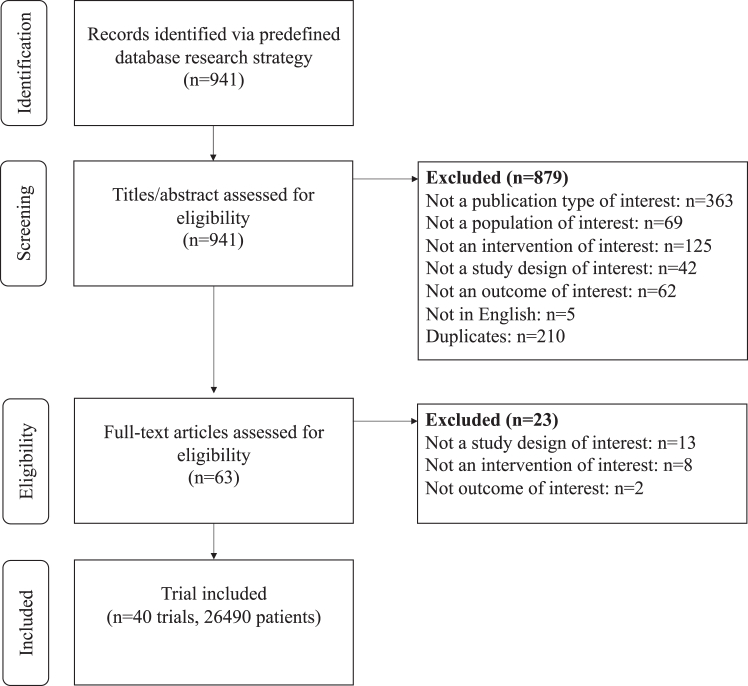
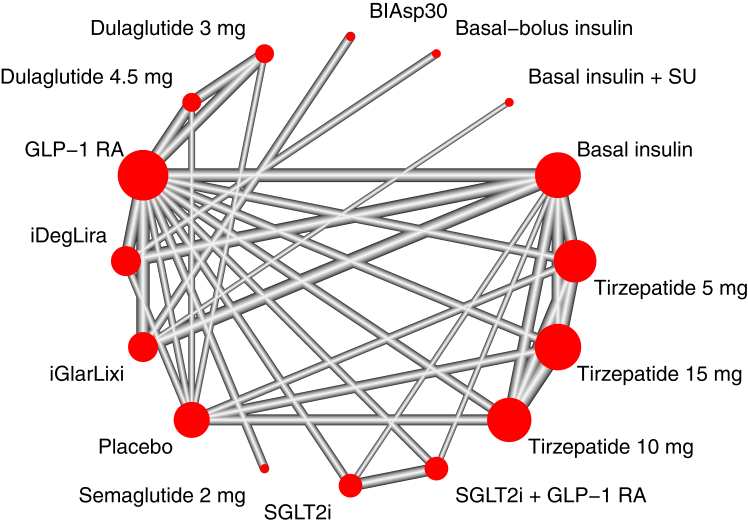
A total of 40 trials, enrolling 26,490 patients, were included in the systematic review and network meta-analysis (Fig. 1). Eleven studies evaluated the efficacy and safety of tirzepatide, four studies GLP-1RA plus SGLT-2i combination therapy, three studies high-dose GLP-1RA (two dulaglutide 3 and 4.5 mg; one semaglutide 2 mg), and twenty-one trials GLP-1RA plus basal insulin FRC (ten iDegLira; eleven iGlarLixi). The networks of trials used in the meta-analysis for evaluating HbA1c, FPG, PPG, BW, LDL-c, SBP, DBP, and hypoglycaemia are shown in Fig. 2 and Supplementary Appendix S11, S16, S21, S26, S31, S36 and S40. The characteristics of studies and patients' baseline features are presented in Supplementary Appendix S2. The median follow-up length of included studies was 26 (interquartile range [IQR] 26–40) weeks. Most studies (n = 22) had a duration of 26 weeks or less with a median of 26 (IQR 24–26) weeks, whereas the remaining 18 studies had a median duration of 46 (IQR 34–52) weeks. All but one trials were funded by the pharmaceutical industry. Across all trials, there was a median (IQR) percentage of Asians and female individuals of 28% (3.2%–100%) and 45.3% (37.6–49.6%) respectively; the median (IQR) baseline age was 57.1 (55.7–58.8) years. The mean (SD) diabetes duration was 9.4 (2.5) years. The median (IQR) HbA1c was 8.3% (8.1–8.5%). Median (IQR) BMI was 31.6 (27.9–32.9) kg/m2. In 14 out of 40 trials, patients were overweight (mean baseline BMI ≥25 kg/m2 and <30 kg/m2), while 26 out of 40 trials enrolled obese patients (mean baseline BMI ≥30 kg/m2). No RCT reported a mean BMI <25 kg/m2. A substantial amount of heterogeneity was detected for HbA1c, FPG, PPG, BW; moderate heterogeneity was found for LDL-c, SBP, DBP and for risk of hypoglycaemia. Global inconsistency at design-by-treatment interaction model was high for all outcomes, however global inconsistency was generally low, except for BW (Supplementary Appendix S3). Overall risk of bias for the main outcome was deemed low for 30 trials and of some concern for 10 trials (Supplementary Appendix S4). Comparison-adjusted funnel plots did not suggest the presence of publication bias for BW, LDL-c, SBP, DBP and hypoglycaemia (Supplementary Appendix S5). Evidence certainty was generally low for each of the main comparisons and is summarised in dedicated Tables for each outcome but DBP in the Supplementary Appendix (S8, S13, S18, S23, S28, S33 and S40.5).
Fig. 1.
PRISMA flowchart for study selection.
Fig. 2.
Meta-analysis networks for change in HbA1c level. Each circle indicates a treatment node, and its size is proportional to the number of trials evaluating each treatment. Lines connecting two nodes represent direct comparisons between two treatments; the thickness of the lines is proportional to the number of trials directly comparing the 2 connected treatments. BIAsp30, biphasic insulin aspart 30/70; GLP-1RA, glucagon-like peptide-1 receptor agonists; SGLT-2i, sodium glucose cotransporter-2 inhibitors; SU, sulfonylurea.
HbA1c
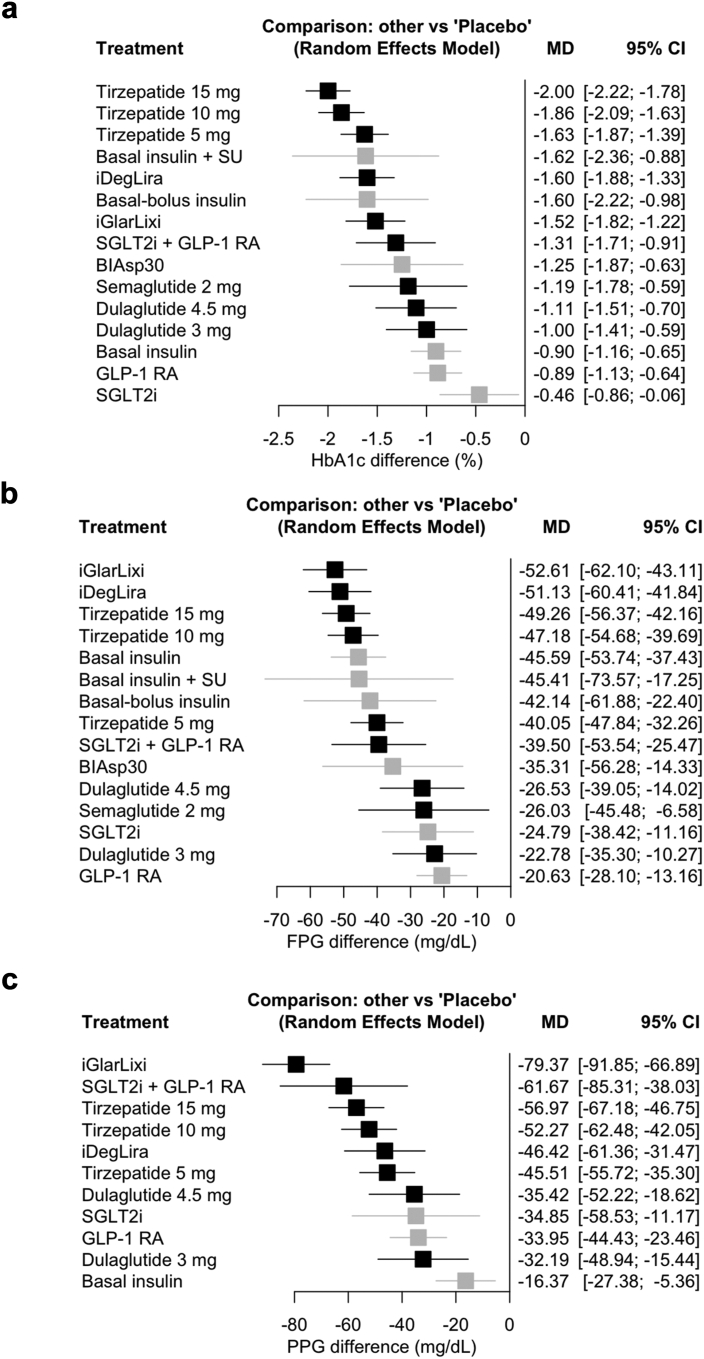
A total of 40 studies (26,490 patients) were included in the main analysis evaluating the change from baseline in HbA1c. Pairwise meta-analysis results are presented in Supplementary Appendix S6.1. Network meta-analysis results are presented in Fig. 3a, Table 1 and Supplementary Appendix S7. All innovative GLP-1RA-based treatment strategies significantly reduced HbA1c compared to placebo. Tirzepatide 15 mg ranked first in terms of HbA1c lowering efficacy (6 studies, 1320 patients, MD −2.00%, 95% CI [−2.22; −1.78], high certainty). The HbA1c lowering efficacy of tirzepatide 15 mg and 10 mg was comparable (10 studies, 4553 patients, MD −0.14, 95% CI [−0.31; 0.04], moderate certainty), while tirzepatide 15 mg was superior to tirzepatide 5 mg (9 studies, 3941 patients, MD −0.37%, 95% CI [−0.56; −0.18], moderate certainty), iDegLira (MD −0.40%, 95% CI [−0.66; −0.14], low certainty), iGlarLixi (MD −0.48%, 95% CI [−0.75; −0.21], low certainty), GLP-1RA plus SGLT-2i combination (MD −0.69%, 95% CI [−1.07; −0.30], low certainty), and high-dose GLP-1RA (vs. semaglutide 2 mg MD −0.81%, 95% CI [−1.40; −0.23], low certainty; vs. dulaglutide 4.5 mg MD −0.89%, 95% CI [−1.31; −0.48], low certainty; vs. dulaglutide 3 mg MD −1.00, 95% CI [−1.41; −0.59], low certainty), as shown in Table 1. Tirzepatide 10 mg was superior to all other treatments except tirzepatide 15 mg and iDegLira. The efficacy of tirzepatide 5 mg, GLP-1RA plus SGLT-2i combination, semaglutide 2 mg, iDegLira and iGlarLixi was comparable, while dulaglutide 4.5 mg and 3 mg was less effective compared to tirzepatide and GLP-1RA plus basal insulin FRC but still comparable to the rest of the above treatments in reducing HbA1c. All the latest GLP-1RA-based treatment strategies but high-dose GLP-1RA were more efficacious than basal insulin, while no significant difference was found with basal bolus insulin therapy.
Fig. 3.
Network meta-analysis results for change from baseline in a. HbA1c, b. fasting plasma glucose (FPG), and c. post-prandial glucose (PPG) compared with placebo. Treatments are presented according to their effect estimate compared with placebo. Effect sizes are presented as mean difference (MD) and 95% confidence intervals (CI). New GLP-1RA-based treatments are highlighted in black, other treatments in grey. SGLT-2i and GLP-1RA compounds and doses in included studies are described in Supplementary Appendix S2. BIAsp30, biphasic insulin aspart 30/70; GLP-1 RA, glucagon-like peptide-1 receptor agonists; SGLT-2i, sodium glucose cotransporter-2 inhibitors; SU, sulfonylurea.
Table 1.
Change from baseline in HbA1c for all treatments in the main analysis.
 |
The lower half presents network meta-analysis results, while the upper half presents pairwise meta-analysis results.
Main treatments are reported in efficacy ranking order.
Treatment estimates are expressed as mean difference and 95% confidence intervals of the column-defining treatment compared with the row-defining treatment for change from baseline in HbA1c.
Mean differences lower than 0 favor the column-defining treatment for network meta-analysis and the row-defining treatment for pairwise meta-analysis.
Significant results are in bold.
GLP-1RA, glucagon-like peptide-1 receptor agonists; SGLT2i, sodium glucose cotransporter-2 inhibitors.
FPG and PPG
Change in FPG was reported in 38 studies (26,000 patients) while change in PPG was reported in 21 studies (16,392 patients). Results of pairwise meta-analyses for both outcomes are presented in Supplementary Appendix S6.2 and S6.3. Network meta-analysis results are presented in Fig. 3b and c and Supplementary Appendix S12 and S17. All innovative GLP-1RA-based treatment strategies significantly reduced FPG and PPG compared to placebo. IGlarLixi ranked as the most efficacious treatment in reducing FPG (MD −52.61 mg/dL, 95% CI [−62.10; −43.11], moderate certainty) and especially PPG (MD −79.37 mg/dL, 95% CI [−91.85; −66.89], moderate certainty) (Fig. 3b and c). GLP-1RA plus basal insulin FRC and tirzepatide 15 mg and 10 mg had comparable effects on FPG and were superior to tirzepatide 5 mg and high-dose GLP-1RA. No significant difference was found among GLP-1RA plus SGLT-2i combination and GLP-1RA plus basal insulin FRC and all doses of tirzepatide, whereas tirzepatide 5 mg and GLP-1RA plus SGLT-2i combination were superior to most high-dose GLP-1RA (Supplementary Appendix S12). The effect of iGlarLixi and GLP-1RA plus SGLT-2i combination on PPG lowering was similar (MD −17.70 mg/dL, 95% CI [−40.92; 5.51], low certainty). IGlarLixi was superior to all other treatment strategies on PPG reduction, while no significant differences were detected between GLP-1RA plus SGLT-2i combination, all doses of tirzepatide and iDegLira. GLP-1RA plus SGLT-2i combination and tirzepatide 15 mg and 10 mg were more efficacious than high-dose GLP-1RA (dulaglutide 4.5 mg and 3 mg), while the effects of tirzepatide 5 mg and high-dose GLP-1RA were similar (Supplementary Appendix S17).
Bodyweight
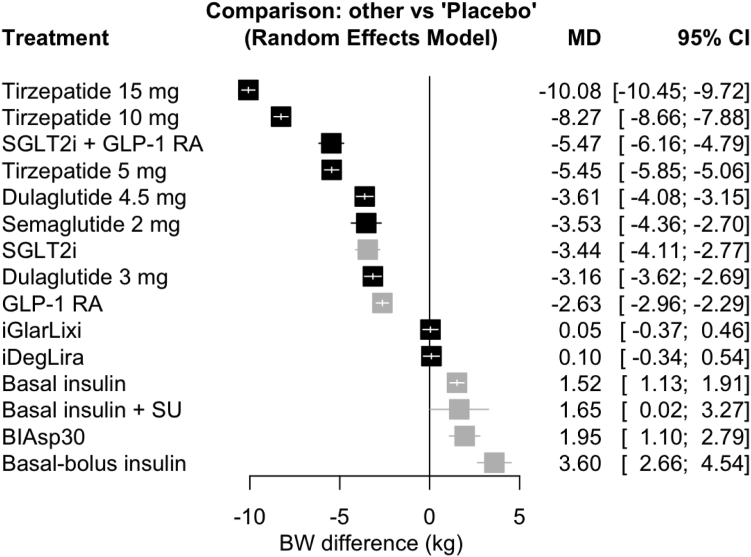
A total of 40 studies (26,490 patients) were included in the main analysis for change in BW. Pairwise meta-analysis results are presented in Supplementary Appendix S6.4, while network meta-analysis results are presented in Fig. 4 and Supplementary Appendix S22. All doses of tirzepatide, GLP-1RA plus SGLT-2i combination and high-dose GLP-1RA significantly reduced BW compared to placebo, while the two GLP-1RA plus basal insulin FRC were neutral. Tirzepatide 15 mg ranked first in terms of BW lowering (6 studies, 1320 patients, MD −10.08 kg, 95% CI [−10.45; −9.72], high certainty) and was significantly more efficacious than all other treatment strategies (Supplementary Appendix S22). Tirzepatide 5 mg and GLP-1RA plus SGLT-2i combination were equally efficacious (MD −0.02 kg, 95% CI [−0.71; 0.67], low certainty) and superior to high-dose GLP-1RA, which in turn were superior to either GLP-1RA plus basal insulin FRC.
Fig. 4.
Network meta-analysis results for change from baseline in bodyweight (BW) compared with placebo. Treatments are presented according to their effect estimate compared with placebo. Effect sizes are presented as mean difference (MD) and 95% confidence intervals (CI). New GLP-1RA-based treatments are highlighted in black, other treatments in grey. BIAsp30, biphasic insulin aspart 30/70; GLP-1 RA, glucagon-like peptide-1 receptor agonists; SGLT-2i, sodium glucose cotransporter-2 inhibitors; SU, sulfonylurea.
Cardiovascular (CV) risk factors (LDL-c, SBP, DBP)
A total of 13 studies (9448 patients), 18 studies (12,711 patients) and 16 studies (12,237 patients) were included in the main analyses for change in LDL-c, SBP and DBP, respectively. Pairwise meta-analyses and network meta-analysis results are presented in Supplementary Appendix. IDegLira and all doses of tirzepatide equally ranked first in terms of LDL-c lowering compared to placebo, while the effect of GLP-1RA plus SGLT-2i combination and high-dose GLP-1RA on this parameter was neutral. However, no significant difference among new GLP-1RA-based treatment strategies on LDL-c was detected (Supplementary Appendix S27.2).
GLP-1RA plus SGLT-2i combination ranked first in SBP lowering compared to placebo, followed by all doses of tirzepatide; the effect of iDegLira and high-dose GLP-1RA was neutral. No significant difference among new GLP-1RA-based treatment strategies was detected, except for the superiority of GLP-1RA plus SGLT-2i combination and tirzepatide 15 mg on iDegLira and GLP-1RA plus SGLT-2i combination on semaglutide 2 mg and dulaglutide 3 mg (Supplementary Appendix S32.2). In regard to DBP reduction, all doses of tirzepatide were superior to placebo, while other treatment strategies were neutral. The effect of all doses of tirzepatide on DBP was superior to that of iDegLira; other new GLP-1RA-based treatment streategies were comparable to each other in reducing DBP (Supplementary Appendix S37.1).
Adverse events
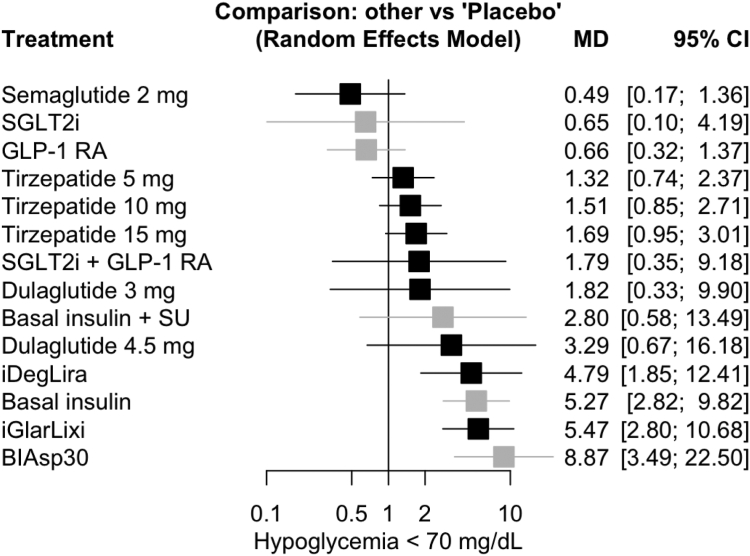
A total of 25 studies (15,503 patients) reported change in hypoglycaemia. Pairwise meta-analysis results are presented in Supplementary Appendix S6.8, and network meta-analysis results are presented in Fig. 5 and Supplementary Appendix S40.4. Innovative GLP-1RA-based treatment strategies had a neutral effect on the risk of hypoglycaemia compared to placebo, except for iGlarLixi and iDegLira, which were associated with an increased risk of hypoglycaemia (OR 5.47, 95% CI [2.8; 10.68], high certainty; OR 4.79, 95% CI [1.85; 12.41], high certainty). No significant difference was found between semaglutide 2 mg, dulaglutide 3 mg and GLP-1RA plus SGLT-2i combination, while semaglutide 2 mg could be associated with a reduction in the risk of hypoglycaemia compared to dulaglutide 4.5 mg (OR 0.15, 95% CI [0.03; 0.77], moderate certainty) and tirzepatide 5 mg (OR 0.37, 95% CI [0.15; 0.91], low certainty), 10 mg (OR 0.32, 95% CI [0.13; 0.79], low certainty) and 15 mg (OR 0.29, 95% CI [0.12; 0.71], high certainty) (Supplementary Appendix S40.4). No significant difference in serious adverse events was found in patients on GLP-1RA-based treatment strategies compared to placebo (Supplementary Appendix S40.2). A similar rate of any adverse events occurred in GLP-1RA-based treatment strategies, with tirzepatide, high-dose GLP-1RA and GLP-1RA plus SGLT-2i combination displaying a similar risk of common gastrointestinal side effects, while treatment with GLP-1RA plus basal insulin FRC, particularly iDegLira, exhibited a lower risk of nausea, vomiting, diarrhoea and constipation (Supplementary Appendix S40). Due to the exiguous number of events detected throughout included studies, any comparison regarding pancreatitis and cholelithiasis was unfeasible.
Fig. 5.
Network meta-analysis results for hypoglycaemia compared with placebo. Treatments are presented according to their effect estimate compared with placebo. Effect sizes are presented as odds ratio (OR) and 95% confidence intervals (CI). New GLP-1RA-based treatments are highlighted in black, other treatments in grey. BIAsp30, biphasic insulin aspart 30/70; GLP-1 RA, glucagon-like peptide-1 receptor agonists; SGLT-2i, sodium glucose cotransporter-2 inhibitors; SU, sulfonylurea.
Subgroup and sensitivity analyses
Sensitivity analyses including only trials at low risk of bias or excluding trials with imputed SD yielded similar results to those of the main analysis for all prespecified outcomes (Supplementary Appendix S10, S15, S20, S25, S30, S35, S39) except for risk of hypoglycaemia (Supplementary Appendix S40.7), according to which all innovative GLP-1RA-based treatment strategies yielded a neutral effect on the risk of hypoglycaemia compared to placebo, with the exception of a plausible increased risk of hypoglycaemia with iGlarLixi.
The ranking in HbA1c lowering yielded by the main analysis was mostly confirmed, regardless of baseline BMI and patients’ ethnicity (Supplementary Appendix S9). However, in patients with a diabetes duration greater than 10 years, all doses of tirzepatide were numerically outranked by iDegLira and iGlarLixi, yet no significant differences among treatments were detected (Supplementary Appendix S9). The subgroup analysis for FPG lowering according to BMI showed that in trials with baseline BMI <30 kg/m2, tirzepatide outranked iGlarLixi in efficacy, probably due to the lower doses of basal insulin, and consequently GLP-1RA, required in these individuals. The same pattern emerged in studies conducted in an exclusively Asian population, with a lower mean baseline BMI (Supplementary Appendix S14).
The ranking of the main analysis for the effect on PPG, BW, CV risk factors and risk of hypoglycaemia was mostly confirmed, regardless of baseline BMI, diabetes duration and whether or not trials were conducted in an exclusively Asian population (Supplementary Appendix S19, S24, S29, S34, S38, S40.6).
Discussion
Tirzepatide, GLP-1RA plus basal insulin FRC, GLP-1RA plus SGLT-2i combination and high-dose GLP-1RA are all innovative GLP-1RA-based therapeutic strategies listed among those with the highest glucose-lowering efficacy according to various RCT and the current ADA/EASD recommendations. Our network meta-analysis found that tirzepatide 15 mg yielded greater HbA1c and BW lowering efficacy compared to other GLP-1RA-based treatments, without increasing the risk of serious adverse events and hypoglycaemia and with a similar rate of gastrointestinal side effects to high-dose GLP-1RA and GLP-1RA plus SGLT-2i combination. Moreover, GLP-1RA plus basal insulin FRC and tirzepatide 15 mg and 10 mg were superior to the other investigated treatments in terms of FPG lowering, while iGlarLixi appeared as the most efficacious in PPG lowering, followed by GLP-1RA plus SGLT-2i combination and tirzepatide 15 mg. No significant differences among these GLP-1RA-based treatments were detected in terms of LDL-c and SBP lowering.
The present network meta-analysis provides results that are largely coherent with existing literature. Indeed, RCT conducted so far demonstrated a dose-dependent and unprecedented benefit in HbA1c lowering with tirzepatide compared not only to placebo but also to basal insulin and semaglutide, attaining normoglycaemia in approximately half of enrolled patients.5,11 Also, the paramount role of tirzepatide as the most efficacious treatment strategy for weight loss regardless of baseline BMI is coherent with its outstanding performance in RCT conducted in patients with type 2 diabetes24, 25, 26, 27, 28 and non-diabetic obese individuals.29 The mechanism underlying the therapeutic efficacy of tirzepatide is yet to be fully elucidated. Heise et al. recently found that tirzepatide improved peripheral insulin sensitivity, enhanced insulin secretion and reduced post-meal glucagon levels compared to placebo and semaglutide 1 mg.30 However, these experiments did not address the relative contribution of weight loss and GIP receptor agonism to the superiority of tirzepatide over the GLP-1RA. The role of GIP receptor agonism remains an unresolved issue in the light of the resistance to GIP effects that was shown in patients with type 2 diabetes.31 Indeed, sustained >10% weight loss has been associated with a greater likelihood of achieving normoglycaemia,32 especially in patients with shorter disease duration.33
Our analysis showed that GLP-1RA plus basal insulin FRC numerically outranked tirzepatide 15 mg in patients with baseline diabetes duration above 10 years, in agreement with previous findings indicating that longer disease duration may be associated with the need for insulin replacement.34,35 Awaiting evidence from dedicated ongoing trials (eg, NCT05433584), these findings suggest that tirzepatide could be preferentially considered for the management of early stages of type 2 diabetes, to exploit its unparalleled weight-lowering effect that could be beneficial for restoring insulin secretory function, whereas GLP-1RA plus basal insulin FRC could be considered as an equally efficacious option in patients with longer disease duration.
Despite figuring as the best choice for HbA1c lowering, tirzepatide was outranked by iGlarLixi as the best option for PPG lowering. The short-acting GLP-1RA lixisenatide is traditionally regarded as particularly beneficial in reducing PPG excursions, mainly due to its persisting inhibition of gastric emptying, complementing the typical GLP-1RA-mediated modulation of insulin and glucagon secretion.36 GLP-1RA plus SGLT-2i combination also exhibited a strong PPG reduction, comparable to the effect of tirzepatide (Supplementary Appendix S17). SGLT-2i induce both FPG and PPG lowering by augmenting renal glucose excretion, and the combination with GLP-1RA probably enhances their effect on glucose excursions by counteracting the SGLT-2i-mediated increase in glucagon levels and related glucoregulatory effects in a sub-additive fashion.37 However, it should be noted that PPG measurement was heterogeneous among trials, being evaluated following a standard meal test or as a mean of post-prandial values from SMBG profiles; also, the certainty of evidence for this outcome appears to be generally low to very low, except for comparisons involving iGlarLixi.
The few significant differences among GLP-1RA-based treatment strategies on CV risk factors should be interpreted with caution given that not all included trials reported data on these outcomes and none of them was powered to appreciate differences between treatments. However, certainty of evidence for change from baseline in LDL-c was mostly moderate. Existing literature attributed a modest benefit to tirzepatide38,39 and GLP-1RA plus basal insulin FRC40,41 on BP and LDL-c, while GLP-1RA plus SGLT-2i combination was beneficial only on BP with a neutral effect on LDL-c,42,43 with yet hypothetical mechanisms. The broader CV protection of the above agents has not been as extensively investigated as that of GLP-1RA and SGLT-2i. A prespecified meta-analysis of seven RCTs from the SURPASS program showed that treatment with all doses of tirzepatide for up to 104 weeks did not increase the risk of major CV events and actually showed a trend for reduction with respect to comparators.44 No CV outcomes RCTs are planned for GLP-1RA plus SGLT-2i combination and GLP-1RA plus basal insulin FRC. Real-world evidence highlighted that GLP-1RA plus SGLT-2i combination was associated with a reduced risk of major adverse cardiovascular and cerebrovascular events in patients with type 2 diabetes in primary prevention compared to other regimens.13 Data on the CV safety of GLP-1RA plus basal insulin FRC as a class are lacking, while a retrospective study directly comparing iGlarLixi and iDegLira showed a lower risk of myocardial infarction, stroke and heart failure in patients using iGlarLixi.45
The results of our study can be easily translated to clinical practice, as we chose to compare treatment strategies featuring among the glucose-lowering options with greatest efficacy currently available for everyday use using a rigorous methodology. Our results are consistent with previous attempts of indirect comparisons, such as the analyses comparing tirzepatide and semaglutide 2 mg46 and iDegLira and iGlarLixi.47 Given the possibility of a weakened effect for some of the investigated treatments over time,48 only studies with a limited follow-up duration of up to 1 year following end of titration were included. Also, when investigating GLP-1RA plus SGLT-2i combination, we included only trials evaluating initial combination rather than add-on schemes to avoid biases related to patients non-responsive to either one of the two drugs; indeed, studies involving add-on strategies include only patients not reaching glycaemic targets with one of the investigated compounds.
However, our study had some limitations. Since the investigated treatment strategies have all been implemented in recent years, a relatively low number of studies was included in this analysis, probably accounting for the high levels of heterogeneity and global inconsistency for the main outcomes and consequently a generally low level of certainty. Specifically, a very low number of studies investigated high-dose GLP-1RA and GLP-1RA plus SGLT-2i combinations. However, to our knowledge, the present analysis is timely as there are no currently ongoing trials aiming to compare these GLP-1RA-based therapeutic strategies. Moreover, included studies investigating GLP-1RA plus SGLT-2i combination were not conducted with dulaglutide, subcutaneous semaglutide and oral semaglutide, which represent the most efficacious GLP-1RA now available, possibly leading to underestimate the effects of this treatment strategy. However, relevant results achieved with this treatment strategy were described in SUSTAIN-9, investigating the effect of semaglutide 1 mg in addition to SGLT-2i monotherapy, with 56.1% of patients reaching HbA1c <6.5%, a result roughly inferior to what was observed with all doses of tirzepatide across the SURPASS program.49
Further trials are awaited to confirm the differences described in the present network meta-analysis, specifically investigating whether GLP-1RA-based treatments could differ in their effects on HbA1c, FPG and PPG considering patients’ characteristics, such as diabetes duration and using homogeneous assessment methods. The mechanisms underlying the glycaemic and weight-lowering efficacy of tirzepatide are yet to be fully elucidated, particularly the role of GIP on glucose excursions and weight loss. The mechanisms of the benefit of the GLP-1RA plus SGLT-2i combination on blood pressure are also still unclear, and the effect on hard CV endpoints should be addressed in comparison to other GLP-1RA-based treatment strategies.
The exploitation of different mechanisms of action in the dual GIP/GLP-1 agonist tirzepatide, GLP-1RA plus basal insulin FRC and GLP-1RA plus SGLT-2i combination was associated with a generally greater glucometabolic benefit compared to high-dose GLP-1RA, likely due to tackling of multiple pathophysiological defects of type 2 diabetes. Aiming to support clinicians in tailoring treatment to patients’ needs, the results of this analysis suggest a hierarchy among treatment strategies considered as the best options for type 2 diabetes management.
Contributors
IC, AC and FG contributed to the study conception and design. IC, LDG, SDM and AC designed the statistical plan, performed the statistical search, collected the data, and performed the analysis. SCP and PN supervised the statistical analysis and the assessment of the certainty of evidence. IC, LDG and SDM gave the major contribution in writing the manuscript. LDG created the figures. IC, LDG, SDM, AC, LL, SP, PN, AN, GFMS and FG revised the article and contributed to the discussion. FG supervised the project and finalised the manuscript. All authors read and approved the final manuscript. FG, IC, LDG, SDM accessed and verified the underlying data.
Data sharing statement
All data relevant to the study are included in the article or uploaded as supplementary information. Statistical code and data set: Available on reasonable request from Professor F. Giorgino (e-mail, francesco.giorgino@uniba.it).
Declaration of interests
AC reports the following: AstraZeneca, Eli Lilly, Novo Nordisk, Roche Diagnostics, Sanofi Aventis (honoraria). AN reports the following: AstraZeneca, Novo Nordisk, and Sanofi Aventis (honoraria). FG reports the following: Eli Lilly, Roche Diabetes Care (grants); Eli Lilly, Novo Nordisk (consulting fees); AstraZeneca, Boehringer-Ingelheim, Eli Lilly, Lifescan, Merck Sharp & Dohme, Medtronic, Novo Nordisk, Roche Diabetes Care, Sanofi Aventis; Eli Lilly, Sanofi Aventis (support for attending meetings/travel); AstraZeneca, Boehringer-Ingelheim, Eli Lilly, Lifescan, Merck Sharp & Dohme, Medimmune, Medtronic, Novo Nordisk, Roche Diabetes Care, Sanofi Aventis (participation on Advisory Boards); EASD/EFSD, Società Italiana di Endocrinologia (SIE), Fo.Ri.SIE (unpaid leadership); AstraZeneca, Eli Lilly, Novo Nordisk, Sanofi Aventis (support for medical writing and statistical analysis). GFMS: no competing interests. IC reports the following: Eli Lilly, Novo Nordisk (honoraria); Eli Lilly, Novo Nordisk (support for attending meeting or travels); LDG reports the following: Eli Lilly, MOVI SpA, Roche Diabetes Care (honoraria). LL reports the following: Abbott, AstraZeneca, Boehringer-Ingelheim, Eli Lilly, Merck Sharp & Dohme, Medtronic, Menarini, MOVI SpA, Mundipharma, Novo Nordisk, Roche Diabetes Care, Sanofi Aventis, Terumo (honoraria); Abbott, AstraZeneca, Boeringher-Ingelheim, Eli Lilly Italia, Medtronic, MOVI SpA, Novo Nordisk, Roche Diabetes Care, Sanofi Aventis, Terumo (participation on Advisory Boards). PN: no competing interests. SDM reports the following: Ascensia, MOVI SpA, Roche Diabetes Care (honoraria); Ascensia, MOVI SpA, Roche Diabetes Care (participation on Advisory Boards). SCP: no competing interests. SP reports the following: AstraZeneca, Eli Lilly, Novo Nordisk, Sanofi Aventis (honoraria).
Acknowledgements
We acknowledge co-funding from the Fondazione per la Ricerca Biomedica “Saverio e Isabella Cianciola” and from Next Generation EU, in the context of the National Recovery and Resilience Plan, Investment PE8—Project Age-It: “Ageing Well in an Ageing Society”. This resource was co-financed by the Next Generation EU [DM 1557 11.10.2022]. The views and opinions expressed are only those of the authors and do not necessarily reflect those of the European Union or the European Commission; neither the European Union nor the European Commission can be held responsible for them.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2023.102181.
Appendix A. Supplementary data
References
- 1.Saeedi P., Petersohn I., Salpea P., et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the international diabetes federation diabetes atlas, 9th edition. Diabetes Res Clin Pract. 2019;157 doi: 10.1016/j.diabres.2019.107843. [DOI] [PubMed] [Google Scholar]
- 2.Fang M., Wang D., Coresh J., Selvin E. Trends in diabetes treatment and control in U.S. Adults, 1999-2018. N Engl J Med. 2021;384:2219–2228. doi: 10.1056/NEJMsa2032271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nauck M.A., Quast D.R., Wefers J., Meier J.J. GLP-1 receptor agonists in the treatment of type 2 diabetes–state-of-the-art. Mol Metab. 2021;46 doi: 10.1016/j.molmet.2020.101102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ussher J.R., Drucker D.J. Glucagon-like peptide 1 receptor agonists: cardiovascular benefits and mechanisms of action. Nat Rev Cardiol. 2023;20:463–474. doi: 10.1038/s41569-023-00849-3. [DOI] [PubMed] [Google Scholar]
- 5.De Block C.E.M., Dirinck E., Verhaegen A., Van Gaal L.F. Efficacy and safety of high-dose glucagon-like peptide-1, glucagon-like peptide-1/glucose-dependent insulinotropic peptide, and glucagon-like peptide-1/glucagon receptor agonists in type 2 diabetes. Diabetes Obes Metab. 2022;24:788–805. doi: 10.1111/dom.14640. [DOI] [PubMed] [Google Scholar]
- 6.Frias J.P., Bonora E., Nevarez Ruiz L., et al. Efficacy and safety of dulaglutide 3.0 mg and 4.5 mg versus dulaglutide 1.5 mg in metformin-treated patients with type 2 diabetes in a randomized controlled trial (AWARD-11) Diabetes Care. 2021;44:765–773. doi: 10.2337/dc20-1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frías J.P., Auerbach P., Bajaj H.S., et al. Efficacy and safety of once-weekly semaglutide 2.0 mg versus 1.0 mg in patients with type 2 diabetes (SUSTAIN FORTE): a double-blind, randomised, phase 3B trial. Lancet Diabetes Endocrinol. 2021;9:563–574. doi: 10.1016/S2213-8587(21)00174-1. [DOI] [PubMed] [Google Scholar]
- 8.Singh A.K., Singh R. Metabolic and cardiovascular benefits with combination therapy of SGLT-2 inhibitors and GLP-1 receptor agonists in type 2 diabetes. World J Cardiol. 2022;14:329–342. doi: 10.4330/wjc.v14.i6.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gourdy P., Darmon P., Dievart F., Halimi J.-M., Guerci B. Combining glucagon-like peptide-1 receptor agonists (GLP-1 RAs) and sodium-glucose cotransporter-2 inhibitors (SGLT2is) in patients with type 2 diabetes mellitus (T2DM) Cardiovasc Diabetol. 2023;22:79. doi: 10.1186/s12933-023-01798-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giorgino F., Caruso I., Napoli R. Titratable fixed-ratio combination of insulin glargine plus lixisenatide: a simplified approach to glycemic control in type 2 diabetes mellitus. Diabetes Res Clin Pract. 2020;170 doi: 10.1016/j.diabres.2020.108478. [DOI] [PubMed] [Google Scholar]
- 11.Karagiannis T., Avgerinos I., Liakos A., et al. Management of type 2 diabetes with the dual GIP/GLP-1 receptor agonist tirzepatide: a systematic review and meta-analysis. Diabetologia. 2022;65:1251–1261. doi: 10.1007/s00125-022-05715-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Defronzo R.A. Banting Lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes. 2009;58:773–795. doi: 10.2337/db09-9028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wright A.K., Carr M.J., Kontopantelis E., et al. Primary prevention of cardiovascular and heart failure events with SGLT2 inhibitors, GLP-1 receptor agonists, and their combination in type 2 diabetes. Diabetes Care. 2022;45:909–918. doi: 10.2337/dc21-1113. [DOI] [PubMed] [Google Scholar]
- 14.Gastaldelli A., Cusi K., Fernandez Landò L., et al. Effect of tirzepatide versus insulin degludec on liver fat content and abdominal adipose tissue in people with type 2 diabetes (SURPASS-3 MRI): a substudy of the randomised, open-label, parallel-group, phase 3 SURPASS-3 trial. Lancet Diabetes Endocrinol. 2022;10(6):393–406. doi: 10.1016/S2213-8587(22)00070-5. [DOI] [PubMed] [Google Scholar]
- 15.Muzurović E.M., Volcansek S., Tomsic K.Z., et al. Glucagon-like peptide-1 receptor agonists and dual glucose-dependent insulinotropic polypeptide/glucagon-like peptide-1 receptor agonists in the treatment of obesity/metabolic syndrome, prediabetes/diabetes and non-Alcoholic fatty liver disease-current evidence. J Cardiovasc Pharmacol Ther. 2022;27 doi: 10.1177/10742484221146371. [DOI] [PubMed] [Google Scholar]
- 16.ElSayed N.A., Aleppo G., Aroda V.R., et al. 9. Pharmacologic Approaches to glycemic treatment: standards of Care in diabetes-2023. Diabetes Care. 2023;46:S140–S157. doi: 10.2337/dc23-S009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shim S.R., Kim S.-J., Lee J., Rücker G. Network meta-analysis: application and practice using R software. Epidemiol Health. 2019;41 doi: 10.4178/epih.e2019013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rhodes K.M., Turner R.M., Higgins J.P.T. Predictive distributions were developed for the extent of heterogeneity in meta-analyses of continuous outcome data. J Clin Epidemiol. 2015;68:52–60. doi: 10.1016/j.jclinepi.2014.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dias S., Welton N.J., Caldwell D.M., Ades A.E. Checking consistency in mixed treatment comparison meta-analysis. Stat Med. 2010;29:932–944. doi: 10.1002/sim.3767. [DOI] [PubMed] [Google Scholar]
- 20.Higgins J.P.T., Jackson D., Barrett J.K., Lu G., Ades A.E., White I.R. Consistency and inconsistency in network meta-analysis: concepts and models for multi-arm studies. Res Synth Methods. 2012;3:98–110. doi: 10.1002/jrsm.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schwarzer G. Others. meta: an R package for meta-analysis. R News. 2007;7:40–45. [Google Scholar]
- 22.Rücker G., Krahn U., König J., Efthimiou O., Schwarzer G. 2020. netmeta: network meta-analysis using frequentist methods. R package version 1.2-1. [Google Scholar]
- 23.Nikolakopoulou A., Higgins J.P.T., Papakonstantinou T., et al. CINeMA: an approach for assessing confidence in the results of a network meta-analysis. PLoS Med. 2020;17 doi: 10.1371/journal.pmed.1003082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosenstock J., Wysham C., Frías J.P., et al. Efficacy and safety of a novel dual GIP and GLP-1 receptor agonist tirzepatide in patients with type 2 diabetes (SURPASS-1): a double-blind, randomised, phase 3 trial. Lancet. 2021;398:143–155. doi: 10.1016/S0140-6736(21)01324-6. [DOI] [PubMed] [Google Scholar]
- 25.Frías J.P., Davies M.J., Rosenstock J., et al. Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes. N Engl J Med. 2021;385:503–515. doi: 10.1056/NEJMoa2107519. [DOI] [PubMed] [Google Scholar]
- 26.Ludvik B., Giorgino F., Jódar E., et al. Once-weekly tirzepatide versus once-daily insulin degludec as add-on to metformin with or without SGLT2 inhibitors in patients with type 2 diabetes (SURPASS-3): a randomised, open-label, parallel-group, phase 3 trial. Lancet. 2021;398:583–598. doi: 10.1016/S0140-6736(21)01443-4. [DOI] [PubMed] [Google Scholar]
- 27.Del Prato S., Kahn S.E., Pavo I., et al. Tirzepatide versus insulin glargine in type 2 diabetes and increased cardiovascular risk (SURPASS-4): a randomised, open-label, parallel-group, multicentre, phase 3 trial. Lancet. 2021;398:1811–1824. doi: 10.1016/S0140-6736(21)02188-7. [DOI] [PubMed] [Google Scholar]
- 28.Dahl D., Onishi Y., Norwood P., et al. Effect of subcutaneous tirzepatide vs placebo added to titrated insulin glargine on glycemic control in patients with type 2 diabetes: the SURPASS-5 randomized clinical trial. JAMA. 2022;327:534–545. doi: 10.1001/jama.2022.0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jastreboff A.M., Aronne L.J., Ahmad N.N., et al. Tirzepatide once weekly for the treatment of obesity. N Engl J Med. 2022;387:205–216. doi: 10.1056/NEJMoa2206038. [DOI] [PubMed] [Google Scholar]
- 30.Heise T., Mari A., DeVries J.H., et al. Effects of subcutaneous tirzepatide versus placebo or semaglutide on pancreatic islet function and insulin sensitivity in adults with type 2 diabetes: a multicentre, randomised, double-blind, parallel-arm, phase 1 clinical trial. Lancet Diabetes Endocrinol. 2022;10:418–429. doi: 10.1016/S2213-8587(22)00085-7. [DOI] [PubMed] [Google Scholar]
- 31.Nauck M.A., Quast D.R., Wefers J., Pfeiffer A.F.H. The evolving story of incretins (GIP and GLP-1) in metabolic and cardiovascular disease: a pathophysiological update. Diabetes Obes Metab. 2021;23(Suppl 3):5–29. doi: 10.1111/dom.14496. [DOI] [PubMed] [Google Scholar]
- 32.ElSayed N.A., Aleppo G., Aroda V.R., et al. 8. Obesity and weight management for the prevention and treatment of type 2 diabetes: standards of care in diabetes-2023. Diabetes Care. 2023;46:S128–S139. doi: 10.2337/dc23-S008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taylor R., Al-Mrabeh A., Sattar N. Understanding the mechanisms of reversal of type 2 diabetes. Lancet Diabetes Endocrinol. 2019;7:726–736. doi: 10.1016/S2213-8587(19)30076-2. [DOI] [PubMed] [Google Scholar]
- 34.Gentile S., Strollo F., Viazzi F., et al. Five-year predictors of insulin initiation in people with type 2 diabetes under real-life conditions. J Diabetes Res. 2018;2018 doi: 10.1155/2018/7153087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Donner T., Muñoz M. Update on insulin therapy for type 2 diabetes. J Clin Endocrinol Metab. 2012;97:1405–1413. doi: 10.1210/jc.2011-2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miñambres I., Pérez A. Is there a justification for classifying GLP-1 receptor agonists as basal and prandial? Diabetol Metab Syndr. 2017;9:6. doi: 10.1186/s13098-017-0204-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Baar M.J.B., van Ruiten C.C., Muskiet M.H.A., van Bloemendaal L., IJzerman R.G., van Raalte D.H. SGLT2 inhibitors in combination therapy: from mechanisms to clinical considerations in type 2 diabetes management. Diabetes Care. 2018;41:1543–1556. doi: 10.2337/dc18-0588. [DOI] [PubMed] [Google Scholar]
- 38.Wilson J.M., Nikooienejad A., Robins D.A., et al. The dual glucose-dependent insulinotropic peptide and glucagon-like peptide-1 receptor agonist, tirzepatide, improves lipoprotein biomarkers associated with insulin resistance and cardiovascular risk in patients with type 2 diabetes. Diabetes Obes Metab. 2020;22:2451–2459. doi: 10.1111/dom.14174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nauck M.A., Mirna A.E.A., Quast D.R. Meta-analysis of head-to-head clinical trials comparing incretin-based glucose-lowering medications and basal insulin: an update including recently developed glucagon-like peptide-1 (GLP-1) receptor agonists and the glucose-dependent insulinotropic polypeptide/GLP-1 receptor co-agonist tirzepatide. Diabetes Obes Metab. 2023;25:1361–1371. doi: 10.1111/dom.14988. [DOI] [PubMed] [Google Scholar]
- 40.Giorgino F., Shaunik A., Liu M., Saremi A. Achievement of glycaemic control is associated with improvements in lipid profile with iGlarLixi versus iGlar: a post hoc analysis of the LixiLan-L trial. Diabetes Obes Metab. 2019;21:2712–2717. doi: 10.1111/dom.13857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vilsbøll T., Blevins T.C., Jodar E., et al. Fixed-ratio combination of insulin degludec and liraglutide (IDegLira) improves cardiovascular risk markers in patients with type 2 diabetes uncontrolled on basal insulin. Diabetes Obes Metab. 2019;21:1506–1512. doi: 10.1111/dom.13675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Szekeres Z., Toth K., Szabados E. The effects of SGLT2 inhibitors on lipid metabolism. Metabolites. 2021;11 doi: 10.3390/metabo11020087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Ruiten C.C., Smits M.M., Kok M.D., et al. Mechanisms underlying the blood pressure lowering effects of dapagliflozin, exenatide, and their combination in people with type 2 diabetes: a secondary analysis of a randomized trial. Cardiovasc Diabetol. 2022;21:63. doi: 10.1186/s12933-022-01492-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sattar N., McGuire D.K., Pavo I., et al. Tirzepatide cardiovascular event risk assessment: a pre-specified meta-analysis. Nat Med. 2022;28:591–598. doi: 10.1038/s41591-022-01707-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cowart K., Gonzalez R., Carris N.W. Cardiovascular and microvascular outcomes with iGlarLixi versus iDegLira: a real-world, population-based cohort study. Diabetes Obes Metab. 2022;24:348–353. doi: 10.1111/dom.14579. [DOI] [PubMed] [Google Scholar]
- 46.Vadher K., Patel H., Mody R., et al. Efficacy of tirzepatide 5, 10 and 15 mg versus semaglutide 2 mg in patients with type 2 diabetes: an adjusted indirect treatment comparison. Diabetes Obes Metab. 2022;24:1861–1868. doi: 10.1111/dom.14775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Home P.D., Aroda V.R., Blonde L., et al. Efficacy and safety of iGlarLixi versus IDegLira in adults with type 2 diabetes inadequately controlled by glucagon-like peptide-1 receptor agonists: a systematic literature review and indirect treatment comparison. Diabetes Obes Metab. 2020;22:2170–2178. doi: 10.1111/dom.14136. [DOI] [PubMed] [Google Scholar]
- 48.Li C., Luo J., Jiang M., Wang K. The efficacy and safety of the combination therapy with GLP-1 receptor agonists and SGLT-2 inhibitors in type 2 diabetes mellitus: a systematic review and meta-analysis. Front Pharmacol. 2022;13 doi: 10.3389/fphar.2022.838277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zinman B., Bhosekar V., Busch R., et al. Semaglutide once weekly as add-on to SGLT-2 inhibitor therapy in type 2 diabetes (SUSTAIN 9): a randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 2019;7:356–367. doi: 10.1016/S2213-8587(19)30066-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.