Summary
Background
Antimicrobial resistance (AMR) is an urgent global health challenge and a critical threat to modern health care. Quantifying its burden in the WHO Region of the Americas has been elusive—despite the region’s long history of resistance surveillance. This study provides comprehensive estimates of AMR burden in the Americas to assess this growing health threat.
Methods
We estimated deaths and disability-adjusted life-years (DALYs) attributable to and associated with AMR for 23 bacterial pathogens and 88 pathogen–drug combinations for countries in the WHO Region of the Americas in 2019. We obtained data from mortality registries, surveillance systems, hospital systems, systematic literature reviews, and other sources, and applied predictive statistical modelling to produce estimates of AMR burden for all countries in the Americas. Five broad components were the backbone of our approach: the number of deaths where infection had a role, the proportion of infectious deaths attributable to a given infectious syndrome, the proportion of infectious syndrome deaths attributable to a given pathogen, the percentage of pathogens resistant to an antibiotic class, and the excess risk of mortality (or duration of an infection) associated with this resistance. We then used these components to estimate the disease burden by applying two counterfactual scenarios: deaths attributable to AMR (compared to an alternative scenario where resistant infections are replaced with susceptible ones), and deaths associated with AMR (compared to an alternative scenario where resistant infections would not occur at all). We generated 95% uncertainty intervals (UIs) for final estimates as the 25th and 975th ordered values across 1000 posterior draws, and models were cross-validated for out-of-sample predictive validity.
Findings
We estimated 569,000 deaths (95% UI 406,000–771,000) associated with bacterial AMR and 141,000 deaths (99,900–196,000) attributable to bacterial AMR among the 35 countries in the WHO Region of the Americas in 2019. Lower respiratory and thorax infections, as a syndrome, were responsible for the largest fatal burden of AMR in the region, with 189,000 deaths (149,000–241,000) associated with resistance, followed by bloodstream infections (169,000 deaths [94,200–278,000]) and peritoneal/intra-abdominal infections (118,000 deaths [78,600–168,000]). The six leading pathogens (by order of number of deaths associated with resistance) were Staphylococcus aureus, Escherichia coli, Klebsiella pneumoniae, Streptococcus pneumoniae, Pseudomonas aeruginosa, and Acinetobacter baumannii. Together, these pathogens were responsible for 452,000 deaths (326,000–608,000) associated with AMR. Methicillin-resistant S. aureus predominated as the leading pathogen–drug combination in 34 countries for deaths attributable to AMR, while aminopenicillin-resistant E. coli was the leading pathogen–drug combination in 15 countries for deaths associated with AMR.
Interpretation
Given the burden across different countries, infectious syndromes, and pathogen–drug combinations, AMR represents a substantial health threat in the Americas. Countries with low access to antibiotics and basic health-care services often face the largest age-standardised mortality rates associated with and attributable to AMR in the region, implicating specific policy interventions. Evidence from this study can guide mitigation efforts that are tailored to the needs of each country in the region while informing decisions regarding funding and resource allocation. Multisectoral and joint cooperative efforts among countries will be a key to success in tackling AMR in the Americas.
Funding
Bill & Melinda Gates Foundation, Wellcome Trust, and Department of Health and Social Care using UK aid funding managed by the Fleming Fund.
Keywords: Antimicrobial resistance, AMR, Bacteria, Disease burden, Mortality, Americas
Research in context.
Evidence before this study
The burden of drug-resistant infections is growing each year. The 2016 Review on Antimicrobial Resistance estimated that in 2050 there will be 317,000 annual deaths in North America and 392,000 in South America from resistant HIV, malaria, tuberculosis, and the most burdensome bacterial pathogens. The 2013 and 2019 Antibiotic Resistance Threats Reports from the Centers for Disease Control and Prevention (CDC) estimated 23,000 and 35,000 annual deaths in the USA attributable to drug resistance, respectively. A KPMG report estimated approximately one million additional deaths in North America and Latin America arising from antimicrobial resistance (AMR), mainly attributable to third-generation cephalosporin-resistant Escherichia coli and methicillin-resistant Staphylococcus aureus. However, these studies do not report details regarding infectious syndrome, age group, or granular country-level estimates. We conducted a thorough search of articles available in PubMed, Medline, Web of Science, Scopus, and others covering exposure to resistant bacterial pathogens, seeking human-focused publications on AMR. Details on our literature review, including the search criteria used, are available in Appendix 1 (Section 2.5 pp 5–9). Despite a long history of AMR surveillance in the WHO Region of the Americas, surveillance still lacks clinical insights on patient presentation and outcome, which limits the assessment of AMR burden and its utility in informing strategies such as antimicrobial stewardship.
Added value of this study
To our knowledge, this analysis is the most comprehensive assessment of the burden of AMR in the Americas to date, providing estimates for 35 countries, 23 bacterial pathogens, and 88 pathogen–drug combinations in 2019. This study uses the major methodological innovations from the recent global burden of AMR study to quantify the burden of AMR in the Americas, with two different counterfactual scenarios as estimation endpoints (the burden directly attributable to bacterial resistance and the burden associated with bacterial resistance). This study additionally places AMR within the context of other notable causes of death by building on estimates of disease incidence, prevalence, and mortality from the Global Burden of Diseases, Injuries, and Risk Factors (GBD) Study 2019.
Implications of all the available evidence
In this study, we show that bacterial AMR is a critical public health problem in the Americas, with over half a million deaths associated with resistance. Six pathogens accounted for 79.5% of deaths (95% uncertainty interval [UI] 78.4–80.6) associated with bacterial AMR: S. aureus, E. coli, Klebsiella pneumoniae, Streptococcus pneumoniae, Pseudomonas aeruginosa, and Acinetobacter baumannii. These estimates will not only inform efforts to combat bacterial AMR by guiding prevention strategies and providing insight on specific pathogen–drug combinations but may also illuminate the optimal approach on how to collect microbiological data to improve future estimation processes and overall scientific understanding of this enormous health threat.
Introduction
Antimicrobial resistance (AMR) is one of the most serious global public health threats of our time.1 The frequently cited Review on Antimicrobial Resistance estimates that AMR could lead to the loss of ten million lives annually by 2050, in addition to significant clinical and economic consequences.2 Although these forecasts were initially challenged,3,4 the recent global burden of AMR study estimated 4.95 million deaths associated with and 1.27 million deaths attributable to resistance in bacteria in 2019.5 This positions AMR as one of the most pressing challenges to global health and modern health care, requiring collaborative global, regional, and country-specific solutions informed by data.
Surveillance of AMR in the Americas was instituted more than three decades ago. These efforts were pioneered with the surveillance of sexually transmitted infections in the late 1980s, leading to the participation in the Gonococcal Antimicrobial Stewardship Programme in 1992.6 The Regional System for Vaccines (SIREVA) was established with the leadership of the Pan-American Health Organization in 1993, and focuses on the surveillance of serotypes and antibiotic susceptibility of Streptococcus pneumoniae, Haemophilus influenzae, and Neisseria meningitidis with the aim of informing vaccine development. The Latin American Antimicrobial Resistance Surveillance Network (ReLAVRA), established in 1996 as a response to a cholera outbreak in the region, aims to support national reference laboratories and collect information on AMR.6 Most countries in the region have also provided information to the Global Antimicrobial Resistance and Use Surveillance System since 2015.
The USA also has a strong history of AMR surveillance. In 1996, the National Antimicrobial Resistance Monitoring System was founded as a collaborative programme of state and local public health departments, universities, the Centers for Disease Control and Prevention (CDC), the Food and Drug Administration, and the US Department of Agriculture.7,8 During the past decade in the USA, two national action plans for combating AMR have been released, a Presidential Advisory Council on Combating Antibiotic-Resistant Bacteria has been formed, and the CDC’s AMR Challenge has been launched to prompt global organisations into formal commitments.9 Canada has also had some notable recent developments, releasing a high-level policy document in September 2017 entitled “Tackling Antimicrobial Resistance and Antimicrobial Use: A Pan-Canadian Framework for Action,” with a primary focus on outlining strategic objectives on collaborative action against AMR.10
Notwithstanding the long history of AMR surveillance in the Americas, microbial and clinical surveillance mostly advanced as isolated endeavours until fairly recently.11 As a result, only a handful of studies present a joint analysis of routine microbial surveillance, resistance, and the clinical cases/outcomes which are fundamental to understanding the attribution of health loss due to AMR, the limitations and bias of the available data, and the pathways of action to tackle this multifaceted problem. We aim to bridge this gap and describe the impact of AMR on health loss in the Americas (with both regional and country-level estimates in 2019) as a necessary step to understand the different challenges present in this region and its component countries, as well as to inform both clinical and policy decision-making in the coming years. This manuscript was produced as part of the GBD Collaborator Network and in accordance with the GBD Protocol.12
Methods
This paper is part of a collection of studies that aim to describe a regional burden of AMR.13 The methodological approach used here extends the results of our original study to provide more granular and country-specific estimates; however, our overall methodology is the same. As such, in an effort to provide a complete description of our analytic process, parts of this methods text are taken directly from our study, “The burden of bacterial antimicrobial resistance in the WHO European region in 2019: a cross-country systematic analysis.”13
Overview and input data
This study extends the results of the global burden of AMR study5 and uses its methodological approach but provides more granular and country-specific estimates within the WHO Region of the Americas.13 More specifically, based on the global estimation of all-age and age-specific deaths and disability-adjusted life-years (DALYs) (with DALYs calculated as the sum of years of life lost due to premature mortality and years of healthy life lost due to disability) for 204 countries and territories, here we present aggregated estimates for the entire WHO Region of the Americas in 2019, as well as country-level estimates. In certain analyses, countries belonging to the WHO Region of the Americas were grouped in accordance with GBD regions (ie, high-income North America, Southern Latin America, Andean Latin America, Caribbean, Central Latin America, and Tropical Latin America). Disease burdens associated with and attributable to AMR were estimated for 12 major infectious syndromes and one residual category, 23 bacterial pathogens, and 88 pathogen-drug combinations.
Our global input data consisted of 343 million individual records or isolates covering 11,361 study-location-years obtained from surveillance systems, hospital systems, systematic literature reviews, and other sources (Appendix 1 Sections 2.1–2.7 pp 4–9). Details on data specific to the Americas are presented in the Supplemental Material (Appendix 1 Table S5 p 60). All data inputs for our models were empirical data (ie, not modelled estimates), except for a custom vaccine probe data meta-analysis which we used to estimate the fraction of pneumonia caused by Streptococcus pneumoniae. Clinical and Laboratory Standards Institute (CLSI) clinical breakpoints and methodology were used as guidance for classification of isolates into categories of susceptible or resistant.14
Our overall approach can be divided into five broad components: the number of deaths where infection was implicated, the proportion of infectious deaths attributable to a given infectious syndrome, the proportion of infectious syndrome deaths attributable to a given pathogen, the percentage of a given pathogen resistant to an antimicrobial drug of interest, and the excess risk of death or duration of an infection associated with this resistance.
We followed GATHER15 guidelines (Appendix 1 pp 67–69).
Estimation steps and burden calculation
In our approach, ten estimation steps took place within the aforementioned five broad modelling components (Appendix 1 Section 3 pp 9–38).13 In estimation steps one and two, we defined the number of deaths where infection was implicated by using the Global Burden of Disease (GBD) 2019 cause of death estimates16 to determine the number of deaths by age, sex, and location for which either the underlying cause of death was of infectious origin or the pathway to death included sepsis. We considered infectious syndromes that played a role in the pathway of sepsis deaths, some of which may not have been the underlying cause of death. In estimation steps three and four, we used multiple data sources to estimate pathogen distributions for each infectious syndrome for deaths and incident cases for each age, sex, and location. In estimation steps five through seven, we estimated the prevalence of phenotypic resistance by country for each of 88 pathogen-drug combinations (Appendix 1 Section 3.5 pp 26–33).
In estimation steps eight and nine, we estimated the relative risk of death for a resistant infection compared with that of a drug-sensitive infection for each pathogen-drug combination. Availability of input data for the above estimation steps is documented in Appendix 1 Table S5 p 60.
To generate mutually exclusive burden estimates of multi-drug resistant pathogens, we estimated a population-attributable fraction (PAF) for the resistance profiles representing each possible combination of susceptibility and resistance to the drugs analysed (Appendix 1 Section 3.5.4 pp 30–31). This method considered prevalence of resistance, excess risk, and a redistribution of burden to each antibiotic based on the respective excess risk.
In estimation step ten, we computed two counterfactual scenarios to quantify the benefit of eliminating drug-resistant infections. In a scenario where drug-resistant infections are replaced with drug-susceptible ones, we consider the excess risk of resistance, known as the “attributable to AMR” counterfactual scenario. Deaths attributable to AMR were calculated by multiplying the number of deaths for each underlying cause by the fraction of these deaths where infection was implicated, followed by multiplying the fraction of infectious deaths attributable to each infectious syndrome. This was then multiplied by the fraction of infectious syndrome deaths attributable to each pathogen, and by the PAF for each location-year and pathogen-drug combination.
Under the no-infection counterfactual scenario, infections that are resistant would not occur; this is also termed the associated with AMR scenario. Calculations here closely follow the process described for the attributable to AMR counterfactual, except the PAF is replaced with the prevalence of resistance for each location-year and pathogen-drug combination. We used a similar approach to calculate DALYs for both counterfactual scenarios (Appendix 1 Section 3.7 pp 35–38).
Modelling tools and framework
Details on our modelling approach can be found in the global burden of AMR study5 and in the Appendix of this paper (Appendix 1 Section 3 pp 9–38).13 Briefly, for estimation steps three and four we used the Bayesian meta-regression tool MR-BRT to estimate case-fatality rates (CFRs) as a function of the Healthcare Access and Quality (HAQ) Index and various bias covariates. We used multinomial estimation with partial and composite observations to incorporate heterogeneous data in the estimation of pathogen distributions for each infectious syndrome. In estimation steps five through seven, we used a two-stage spatiotemporal modelling framework to estimate the prevalence of resistance in each pathogen-drug combination (a complete list of pathogen-drug combinations can be found in Appendix Tables S3.5.1.1 and S3.5.1.2 pp 27–28).
Given the relationship between antibiotic consumption levels and AMR rates,17 we modelled antibiotic consumption at the national level to use as a covariate in the stage one models of prevalence of resistance, using an ensemble spatiotemporal Gaussian process regression model to combine antibiotic usage estimates with pharmaceutical sales data for low- and middle-income countries. In cross-country comparisons, the indicator metric “defined daily doses (DDD) per 1000 inhabitants per day” was used to report antibiotic consumption in the community and within the hospital setting by WHO Anatomical Therapeutic Chemical classification, which provided a rough estimate of the proportion of the population treated with antimicrobials on a daily basis. MR-BRT and a two-stage nested mixed effects meta-regression model were used in the estimation of both relative risk of death and excess risk of hospital stay for each pathogen-drug combination (Appendix 1 Section 3.6.2 p 33).
Uncertainty analysis
Consistent with GBD methods,16 we propagated uncertainty from each step of the analysis into the final estimates of deaths and infections attributable to and associated with drug resistance by taking the 25th and 975th of 1000 draws from the posterior distribution of each quantity of interest.13 The models were cross-validated for out-of-sample predictive validity (more details available in Appendix 1 Sections 3.2–3.6 pp 9–33).
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or the writing of the report. The first author and the corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Burden of AMR by infections and infectious syndromes
We estimated 1,327,000 million (95% uncertainty interval [UI] 993,000–1,751,000) deaths and 38,595,000 million (28,843,000–50,344,000) DALYs involving at least one of the 12 infectious syndromes in the WHO Region of the Americas in 2019 (Appendix 1 Table S8 p 66). Of these, bacterial infections, for which the burden of AMR was assessed, caused 920,000 deaths (664,000–1,242,000 million) and 23,347,000 DALYs (16,810,000–31,528,000). Bacterial lower respiratory infections, which were present as either the intermediate or underlying cause for 293,000 deaths (233,000–375,000), were the infectious syndrome responsible for the most bacterial deaths. Bloodstream infections and intra-abdominal infections were involved in 266,000 (149,000–439,000) and 181,000 bacterial deaths (120,000–259,000), respectively. Urinary tract infections were responsible for the fourth largest number of deaths caused by bacterial pathogens (80,000 [63,000–103,000]). Together, these four infectious syndromes accounted for 89.0% (85.3–91.8) of deaths related to bacterial infections in the region in 2019.
More than two of every five deaths that involved infection in the Americas in 2019 were associated with AMR (569,000 deaths [95% UI 406,000–771,000], Table 1). Of these, 141,000 deaths (99,900–196,000) were attributable to AMR. Among the 293,000 deaths involving bacterial lower respiratory infections, 189,000 deaths (149,000–241,000) were associated with and 45,700 deaths (33,700–60,900) were attributable to resistant bacteria. Of the 266,000 deaths caused by a bacterial bloodstream infection, 169,000 (94,200–278,000) were associated with and 42,600 (22,800–70,600) were attributable to AMR. Similarly, 118,000 deaths (78,600–168,000) which involved peritoneal/intra-abdominal infections were associated with AMR.5
Table 1.
Summary of deaths and DALYs by infectious syndrome for countries in the WHO Region of the Americas, 2019.
| Infectious syndrome | Associated with AMR |
Attributable to AMR |
||||||
|---|---|---|---|---|---|---|---|---|
| Deaths |
DALYs |
Deaths |
DALYs |
|||||
| Counts | Rate per 100k | Counts | Rate per 100k | Counts | Rate per 100k | Counts | Rate per 100k | |
| All | 569,000 (406,000–771,000) | 56.3 (40.2–76.3) | 14,100,000 (10,000,000–19,300,000) | 1399.1 (992.2–1910) | 141,000 (99,900–196,000) | 13.9 (9.9–19.4) | 3,480,000 (2,420,000–4,900,000) | 344.5 (239.7–485.3) |
| BSI | 169,000 (94,200–278,000) | 16.7 (9.3–27.6) | 4,680,000 (2,800,000–7,160,000) | 462.8 (277.1–709.1) | 42,600 (22,800–70,600) | 4.2 (2.3–7) | 1,170,000 (704,000–1,830,000) | 116.2 (69.7–181.5) |
| Bacterial skin infections | 18,200 (9150–34,200) | 1.8 (0.9–3.4) | 378,000 (193,000–706,000) | 37.4 (19.1–69.9) | 3840 (1760–7470) | 0.4 (0.2–0.7) | 79,600 (38,600–153,000) | 7.9 (3.8–15.1) |
| Bone and joint infections | 1910 (577–4440) | 0.2 (0.1–0.4) | 42,700 (12,600–99,700) | 4.2 (1.2–9.9) | 435 (126–1030) | 0 (0–0.1) | 9740 (2810–23,600) | 1 (0.3–2.3) |
| CNS infections | 3410 (2320–5300) | 0.3 (0.2–0.5) | 183,000 (125,000–273,000) | 18.1 (12.3–27) | 777 (515–1220) | 0.1 (0.1–0.1) | 41,500 (27,800–62,500) | 4.1 (2.7–6.2) |
| Cardiac infections | 12,000 (8260–15,600) | 1.2 (0.8–1.5) | 261,000 (189,000–338,000) | 25.8 (18.7–33.4) | 2990 (2030–3930) | 0.3 (0.2–0.4) | 64,800 (46,300–85,100) | 6.4 (4.6–8.4) |
| Diarrhoea | 556 (350–854) | 0.1 (0–0.1) | 35,500 (22,500–53,700) | 3.5 (2.2–5.3) | 126 (72–207) | 0 (0–0) | 7110 (4130–11,300) | 0.7 (0.4–1.1) |
| Gonorrhoea and chlamydia | – | – | 3600 (2110–5610) | 0.4 (0.2–0.6) | – | – | 356 (107–701) | 0 (0–0.1) |
| Intra-abdominal infections | 118,000 (78,600–168,000) | 11.7 (7.8–16.6) | 2,840,000 (1,830,000–4,140,000) | 281.4 (181.1–409.4) | 30,200 (19,700–43,300) | 3 (2–4.3) | 727,000 (463,000–1,070,000) | 72 (45.9–105.8) |
| LRI and thorax infections | 189,000 (149,000–241,000) | 18.7 (14.8–23.9) | 4,670,000 (3,620,000–6,020,000) | 462.5 (358.3–596) | 45,700 (33,700–60,900) | 4.5 (3.3–6) | 1,110,000 (823,000–1,480,000) | 110.2 (81.5–146.7) |
| Tuberculosis | 2420 (1520–3890) | 0.2 (0.2–0.4) | 87,500 (56,200–140,000) | 8.7 (5.6–13.9) | 1050 (253–2400) | 0.1 (0–0.2) | 35,000 (8290–79,800) | 3.5 (0.8–7.9) |
| Typhoid, paratyphoid, and iNTS | 68 (35–187) | 0 (0–0) | 3150 (1710–7240) | 0.3 (0.2–0.7) | 13 (3–35) | 0 (0–0) | 617 (187–1440) | 0.1 (0–0.1) |
| UTI | 53,300 (41,700–69,100) | 5.3 (4.1–6.8) | 950,000 (732,000–1,270,000) | 94 (72.4–125.8) | 12,800 (9780–17,100) | 1.3 (1–1.7) | 228,000 (170,000–318,000) | 22.6 (16.9–31.4) |
Data are estimates (95% uncertainty interval). Estimates were aggregated across drugs, accounting for the co-occurrence of resistance to multiple drugs. For gonorrhoea and chlamydia, we did not estimate the fatal burden, thus only the DALY burden is presented.
AMR = antimicrobial resistance; BSI = bloodstream infection; CNS = central nervous system; DALYs = disability-adjusted life-years; iNTS = invasive non-typhoidal Salmonella; LRI = lower respiratory infection; UTI = urinary tract infection; UI = uncertainty interval; WHO = World Health Organization.
Burden of AMR by pathogens and bacteria–drug combinations
Five bacterial pathogens each caused more than 50,000 deaths associated with AMR in the Americas in 2019 (Table 2). These were, in order of the number of associated deaths, Staphylococcus aureus (123,000 [91,300–163,000]), Escherichia coli (109,000 [78,800–149,000]), Klebsiella pneumoniae (66,100 [47,400–90,000]), Streptococcus pneumoniae (56,500 [45,800–70,200]), and Pseudomonas aeruginosa (51,000 [36,000–70,700]). Rankings of the leading pathogens with respect to burden attributable to resistance were similar, though included Acinetobacter baumannii in place of S. pneumoniae: S. aureus (30,700 [17,000–48,600]), E. coli (25,500 [17,700–36,300]), K. pneumoniae (18,100 [12,000–26,200]), A. baumannii (14,300 [7940–22,900]), and P. aeruginosa (12,500 [8040–18,700]).
Table 2.
Summary of deaths and DALYs by pathogen in the WHO Region of the Americas, 2019.
| Pathogen | Associated with AMR |
Attributable to AMR |
||||||
|---|---|---|---|---|---|---|---|---|
| Deaths |
DALYs |
Deaths |
DALYs |
|||||
| Counts | Rate per 100k | Counts | Rate per 100k | Counts | Rate per 100k | Counts | Rate per 100k | |
| All pathogens | 569,000 (406,000–771,000) | 56.3 (40.2–76.3) | 14,100,000 (10,000,000–19,300,000) | 1399.1 (992.2–1910) | 141,000 (99,900–196,000) | 13.9 (9.9–19.4) | 3,480,000 (2,420,000–4,900,000) | 344.5 (239.7–485.3) |
| Acinetobacter baumannii | 45,300 (26,400–70,800) | 4.5 (2.6–7) | 1,080,000 (642,000–1,650,000) | 106.5 (63.6–163) | 14,300 (7940–22,900) | 1.4 (0.8–2.3) | 339,000 (191,000–542,000) | 33.5 (18.9–53.6) |
| Citrobacter spp. | 2330 (1530–3370) | 0.2 (0.2–0.3) | 65,400 (42,300–96,400) | 6.5 (4.2–9.5) | 666 (385–1070) | 0.1 (0–0.1) | 18,700 (10,500–30,400) | 1.8 (1–3) |
| Enterobacter spp. | 18,500 (12,500–25,900) | 1.8 (1.2–2.6) | 504,000 (336,000–717,000) | 49.9 (33.2–71) | 4240 (2750–6110) | 0.4 (0.3–0.6) | 116,000 (74,600–167,000) | 11.4 (7.4–16.6) |
| Enterococcus faecalis | 12,000 (7460–18,100) | 1.2 (0.7–1.8) | 312,000 (202,000–450,000) | 30.9 (20–44.5) | 3410 (1730–5750) | 0.3 (0.2–0.6) | 88,300 (46,600–145,000) | 8.7 (4.6–14.3) |
| Enterococcus faecium | 33,600 (21,300–49,100) | 3.3 (2.1–4.9) | 810,000 (508,000–1,190,000) | 80.1 (50.2–118) | 10,600 (6470–16,400) | 1 (0.6–1.6) | 254,000 (151,000–399,000) | 25.1 (14.9–39.5) |
| Escherichia coli | 109,000 (78,800–149,000) | 10.8 (7.8–14.7) | 2,470,000 (1,760,000–3,390,000) | 244.8 (174–335.4) | 25,500 (17,700–36,300) | 2.5 (1.7–3.6) | 581,000 (396,000–843,000) | 57.5 (39.2–83.4) |
| Group A Streptococcus | 3520 (1930–6290) | 0.3 (0.2–0.6) | 97,100 (59,000–164,000) | 9.6 (5.8–16.2) | 337 (0– 1220) | 0 (0–0.1) | 9240 (0–30,700) | 0.9 (0–3) |
| Group B Streptococcus | 14,200 (9780–20,000) | 1.4 (1–2) | 407,000 (273,000–583,000) | 40.2 (27.1–57.7) | 1780 (0– 4190) | 0.2 (0–0.4) | 52,200 (0– 121,000) | 5.2 (0–11.9) |
| Haemophilus influenzae | 2500 (1970–3190) | 0.2 (0.2–0.3) | 75,200 (57,000–96,500) | 7.4 (5.6–9.5) | 508 (148–903) | 0.1 (0–0.1) | 15,500 (5000–27,800) | 1.5 (0.5–2.8) |
| Klebsiella pneumoniae | 66,100 (47,400–90,000) | 6.5 (4.7–8.9) | 1,770,000 (1,250,000–2,410,000) | 174.7 (124–238.6) | 18,100 (12,000–26,200) | 1.8 (1.2–2.6) | 485,000 (322,000–700,000) | 48 (31.9–69.3) |
| Morganella spp. | 447 (325–611) | 0 (0–0.1) | 7490 (5280–10,600) | 0.7 (0.5–1) | 108 (60–175) | 0 (0–0) | 1790 (984–2950) | 0.2 (0.1–0.3) |
| Mycobacterium tuberculosis | 2420 (1520–3890) | 0.2 (0.2–0.4) | 87,500 (56,200–140,000) | 8.7 (5.6–13.9) | 1050 (253–2400) | 0.1 (0–0.2) | 35,000 (8290–79,800) | 3.5 (0.8–7.9) |
| Neisseria gonorrhoeae | – | – | 3600 (2110–5610) | 0.4 (0.2–0.6) | – | – | 356 (107–701) | 0 (0–0.1) |
| Non-typhoidal Salmonella | 142 (64–277) | 0 (0–0) | 8770 (3500–19,700) | 0.9 (0.3–1.9) | 29 (3–77) | 0 (0–0) | 1510 (273–3680) | 0.1 (0–0.4) |
| Other enterococci | 10,700 (7680–14,800) | 1.1 (0.8–1.5) | 246,000 (171,000–344,000) | 24.4 (16.9–34.1) | 2440 (1140–3970) | 0.2 (0.1–0.4) | 55,300 (24,600–92,200) | 5.5 (2.4–9.1) |
| Proteus spp. | 11,400 (8080–15,500) | 1.1 (0.8–1.5) | 229,000 (160,000–315,000) | 22.7 (15.8–31.2) | 1480 (838–2340) | 0.1 (0.1–0.2) | 29,500 (16,400–47,000) | 2.9 (1.6–4.7) |
| Pseudomonas aeruginosa | 51,000 (36,000–70,700) | 5 (3.6–7) | 1,230,000 (851,000–1,730,000) | 122.1 (84.2–171.2) | 12,500 (8040–18,700) | 1.2 (0.8–1.9) | 302,000 (194,000–457,000) | 29.9 (19.2–45.2) |
| Salmonella Paratyphi | 5 (3–7) | 0 (0–0) | 208 (128–352) | 0 (0–0) | 1 (0–2) | 0 (0–0) | 41 (8–86) | 0 (0–0) |
| Salmonella Typhi | 960 (515–1670) | 0.1 (0.1–0.2) | 52,700 (28,000–91,600) | 5.2 (2.8–9.1) | 189 (40–416) | 0 (0–0) | 10,400 (2110–22,400) | 1 (0.2–2.2) |
| Serratia spp. | 3980 (2470–6140) | 0.4 (0.2–0.6) | 122,000 (76,800–183,000) | 12 (7.6–18.1) | 1040 (585–1760) | 0.1 (0.1–0.2) | 32,100 (18,400–53,200) | 3.2 (1.8–5.3) |
| Shigella spp. | 182 (73–358) | 0 (0–0) | 11,500 (4670–21,500) | 1.1 (0.5–2.1) | 37 (3–95) | 0 (0–0) | 2090 (417–4870) | 0.2 (0–0.5) |
| Staphylococcus aureus | 123,000 (91,300–163,000) | 12.2 (9–16.1) | 2,800,000 (2,000,000–3,800,000) | 277.3 (197.9–376.4) | 30,700 (17,000–48,600) | 3 (1.7–4.8) | 698,000 (376,000–1,110,000) | 69.1 (37.2–109.9) |
| Streptococcus pneumoniae | 56,500 (45,800–70,200) | 5.6 (4.5–6.9) | 1,750,000 (1,370,000–2,220,000) | 172.8 (135.8–220.2) | 11,600 (7920–15,800) | 1.1 (0.8–1.6) | 356,000 (238,000–492,000) | 35.2 (23.6–48.7) |
Data are estimates (95% uncertainty interval). Count values are rounded to three significant figures, rate values are rounded to one decimal point. Estimates were aggregated across drugs, accounting for the co-occurrence of resistance to multiple drugs.
For Neisseria gonorrhoeae, we did not estimate the fatal burden, thus only the DALY burden is presented. AMR = antimicrobial resistance; DALYs = disability-adjusted life-years; Group A Streptococcus = Streptococcus pyogenes; Group B Streptococcus = Streptococcus agalactiae; Salmonella Paratyphi = Salmonella enterica serovar Paratyphi; Salmonella Typhi = Salmonella enterica serovar Typhi; UI = uncertainty intervals; WHO = World Health Organization.
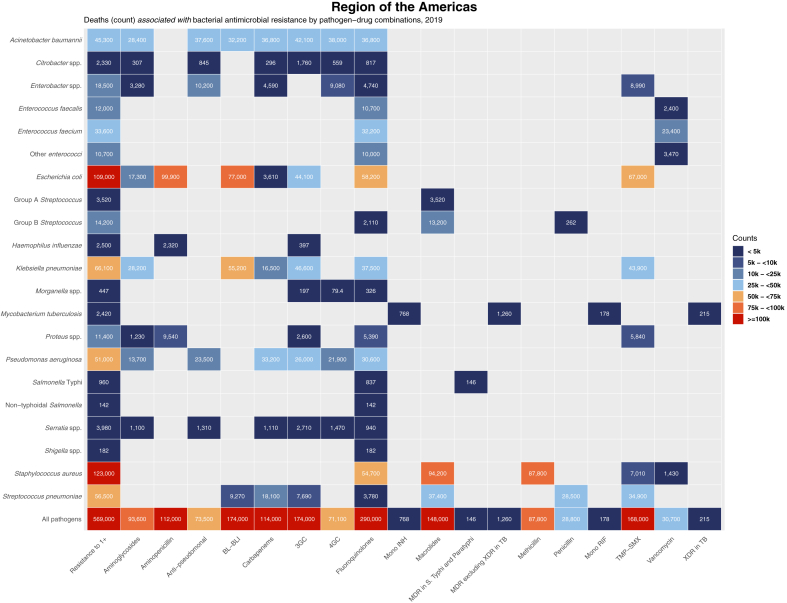
Eight pathogen–drug combinations were each associated with more than 50,000 AMR deaths in the Americas in 2019 (Fig. 1). E. coli resistant to aminopenicillin was the combination with the largest number of associated AMR deaths, 99,900 (95% UI 72,000–136,000). It was also the combination with the largest number of associated AMR deaths in 15 of the 35 countries in the region (Appendix 1 Table S1 pp 53–54). The second, third, and eighth largest combinations with the greatest associated with AMR burden were resistant strains of S. aureus: macrolide-resistant S. aureus with 94,200 deaths (70,000–126,000), methicillin-resistant S. aureus (MRSA) with 87,800 deaths (65,000–120,000), and fluoroquinolone-resistant S. aureus with 54,700 deaths (40,000–75,000). E. coli strains resistant to β-lactam with β-lactamase inhibitors (77,000 deaths [55,000–105,000]), trimethoprim-sulfamethoxazole (67,000 deaths [49,000–90,000), and fluoroquinolones (58,200 deaths [41,000–79,000]) represented the fourth, fifth, and sixth largest combinations associated with AMR fatalities. K. pneumoniae resistant to β-lactam with β-lactamase inhibitors, associated with 55,200 deaths [39,000–75,000], was the seventh leading combination and the only one not related to resistance among S. aureus or E. coli species.
Fig. 1.
Heatmap representing deaths associated with bacterial antimicrobial resistance by pathogen–drug combination in the WHO Region of the Americas, 2019. Values are rounded to three significant figures. Results for Salmonella enterica serovar Paratyphi excluded from this figure due to no appreciable AMR burden for this pathogen in the Region of the Americas (see Table 2). 3GC = third-generation cephalosporins; 4GC = fourth-generation cephalosporins; Anti-pseudomonal = anti-pseudomonal penicillins with or without β-lactamase inhibitors; BL-BLI = β-lactams with β-lactamase inhibitors; Group A Streptococcus = Streptococcus pyogenes; Group B Streptococcus = Streptococcus agalactiae; MDR = multidrug resistance; Mono INH = isoniazid mono-resistance; Mono RIF = rifampicin mono-resistance; Resistance to 1+ = resistance to one or more drugs; S Typhi = S. enterica serovar Typhi; TB = tuberculosis, TMP-SMX = trimethoprim-sulfamethoxazole; XDR = extensive drug resistance.
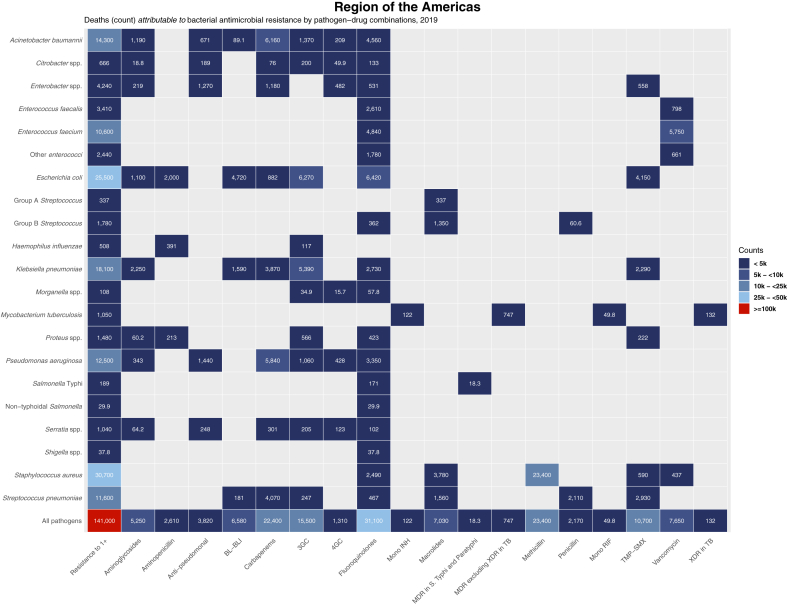
MRSA, responsible for 23,400 deaths [95% UI 10,000–39,000] attributable to resistance, was the only pathogen–drug combination with more than 10,000 attributed deaths (Fig. 2). The six combinations following MRSA in attributable mortality all had greater than 5000 deaths attributable to AMR and included five distinct pathogens, a far more diverse set than was found in the leading associated combinations. These were, ranked in decreasing order of attributable deaths: fluoroquinolone-resistant E. coli (6420 [4330–8960]), third-generation cephalosporin-resistant E. coli (6270 [2730–11,000]), carbapenem-resistant A. baumannii (6160 [3160–10,900]), carbapenem-resistant P. aeruginosa (5840 [3800–8700]), vancomycin-resistant Enterococcus faecium (5750 [3100–9500]), and third-generation cephalosporin-resistant K. pneumoniae (5390 [1900–10,200]).
Fig. 2.
Heatmap representing deaths attributable to bacterial antimicrobial resistance by pathogen-drug combination in the WHO Region of the Americas, 2019. Values are rounded to three significant figures. Results for Salmonella enterica serovar Paratyphi excluded from this figure due to no appreciable AMR burden for this pathogen in the Region of the Americas (see Table 2). 3GC = third-generation cephalosporins; 4GC = fourth-generation cephalosporins; Anti-pseudomonal = anti-pseudomonal penicillins with or without β-lactamase inhibitors; BL-BLI = β-lactams with β-lactamase inhibitors; Group A Streptococcus = Streptococcus pyogenes; Group B Streptococcus = Streptococcus agalactiae; MDR = multidrug resistance; Mono INH = isoniazid mono-resistance; Mono RIF = rifampicin mono-resistance; Resistance to 1+ = resistance to one or more drugs; S Typhi = S. enterica serovar Typhi; TB = tuberculosis; TMP-SMX = trimethoprim-sulfamethoxazole; XDR = extensive drug resistance.
Burden of AMR by location and age
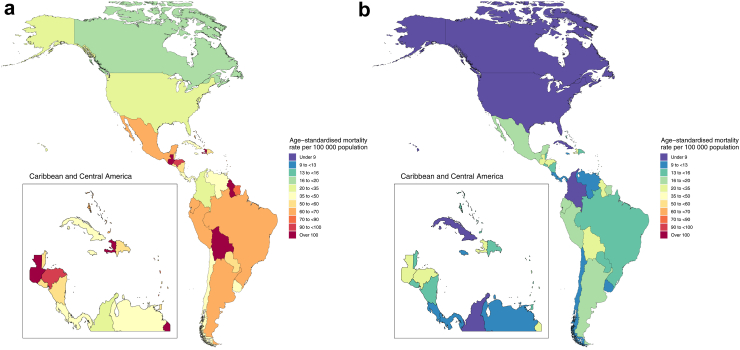
The proportion of all-cause mortality that involved infection differed widely across the countries in the Americas, as did the degree to which infectious deaths involved resistant pathogens. Infections represented the smallest share of mortality in Canada (with 13.7% of deaths involving infection [95% UI 9.9–18.6]), and the largest share of deaths in Haiti (where 33.6% of deaths were infectious in nature [27.0–40.8]) (Appendix 1 Table S6 pp 61–63). By contrast, Haiti had the lowest proportion of infectious deaths that were associated with a resistant pathogen (30.3% [27.1–33.4]), while Chile had the highest (48.2% [46.0–50.3]). Fig. 3a shows that five countries had an age-standardised mortality rate associated with AMR greater than 90 deaths per 100,000 person-years: Haiti, Bolivia, Guatemala, Guyana, and Honduras (ranked from highest to lowest). The countries with the lowest age-standardised mortality rate associated with AMR (ranked from lowest to highest) were Canada, the USA, Colombia, Cuba, Panama, Costa Rica, Chile, Venezuela, Uruguay, and Jamaica, all of which had a mortality rate lower than 50 deaths associated with AMR. Rankings of countries by age-standardised attributable mortality rate were generally similar, with the highest observed mortality in Haiti (30.0 attributable deaths per 100,000 [20.0–42.4]) and the lowest observed in Canada (4.1 attributable deaths per 100,000 [2.7–6.0]) (Fig. 3b).
Fig. 3.
Map of age-standardised mortality rates per 100,000 person-years for deaths associated with (A) and attributable to (B) antimicrobial resistance in the WHO Region of the Americas in 2019. For country-specific results presented in a heatmap, please refer to Appendix 1 Figure S4 (attributable burden) and Appendix 1 Figure S5 (associated burden).
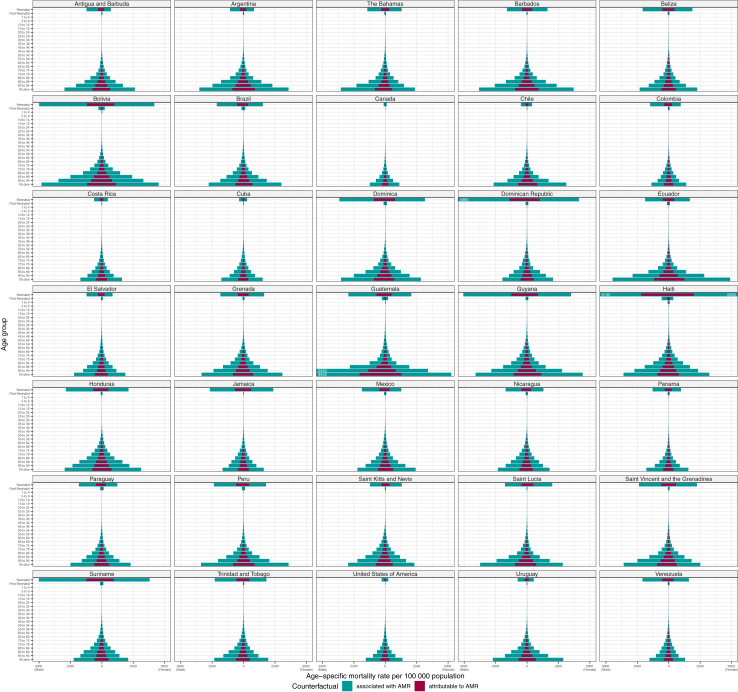
Fig. 4 shows the pattern of AMR death rates by detailed age for all the countries in the Americas. Death rates associated and attributable to AMR followed a generally consistent pattern across countries, with a spike in deaths among neonates followed by near zero rates among 1–4-year-olds that slowly increase until about age 65, after which they begin to increase more dramatically. AMR death rates among neonates were the highest observed of any age group in several countries, namely Dominica, the Dominican Republic, Guyana, Haiti, Jamaica, Suriname, and Venezuela. The rate of neonatal burden was beneath 1000 associated AMR deaths per 100,000 person-years in eight countries: Antigua and Barbuda, Argentina, Canada, Chile, Costa Rica, Cuba, the USA, and Uruguay.
Fig. 4.
Age–specific mortality rates for deaths attributable to and deaths associated with AMR per country in the WHO Region of the Americas, 2019. Rates for males are shown on the left-hand side of each plot, while rates for females are shown on the right. To improve within-country rate comparisons for countries with lower rates of burden, the X-axis is truncated at 5250. Rates higher than 5250 are represented by bars that extend to the edge of the plot, with the true rate written in white text. Results for the Region of the Americas as a whole (and the six GBD regions contained within) can be found in Appendix 1 Figure S13.
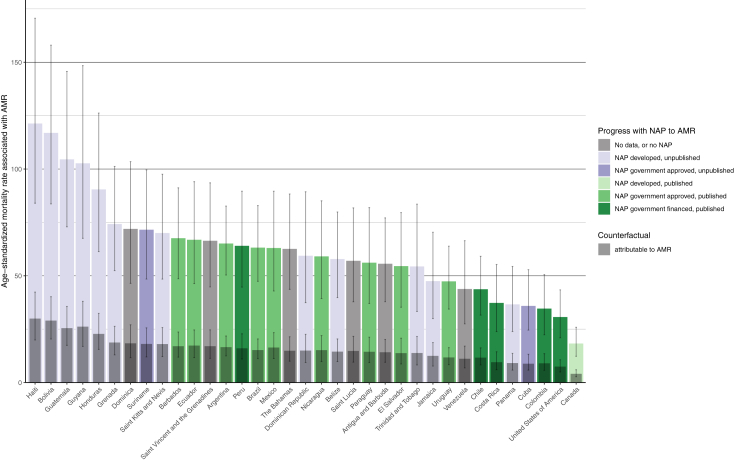
A comparison between AMR mortality rates and each country’s self-reported progress towards developing a National Action Plan (NAP) against AMR is shown in Fig. 5. The ten countries with the highest age-standardised mortality rates associated with AMR all either did not have an AMR NAP or had not published their AMR NAP. Chile, Colombia, Costa Rica, and the USA, were four of the five countries that had both published their AMR NAP and financed the plan in at least one year since 2018, and all had among the lowest AMR mortality rates.18
Fig. 5.
Age-standardised mortality rate associated with and attributable to AMR in relation to the status of NAPs for the countries in the WHO Region of the Americas. Rates per 100,000 person-years are coloured according to two dimensions NAP status: the highest self-reported government engagement with the NAP between 2018 and 2021 and whether the NAP was published online as reported in surveys or the WHO NAP website. Grey error bars indicate 95% uncertainty intervals. NAP data was acquired from the Global Database for Tracking Antimicrobial Resistance Country Self-Assessment Survey (TrACSS) responses for the years 2018–202119 and the WHO library of AMR NAPs.20 Estimates were aggregated across drugs, accounting for the co-occurrence of resistance to multiple drugs. Antigua and Barbuda, Dominica, Grenada, Haiti, Saint Kitts and Nevis and Venezuela AMR NAP progress was last reported in the 2018–2019 survey responses. AMR = antimicrobial resistance; NAP = national AMR action plan.
Discussion
To our knowledge, this is the most comprehensive study to date describing the burden of AMR in the WHO Region of the Americas for an extensive list of pathogens and pathogen–drug combinations. Our analysis reveals that 11.1% (95% UI 9.9–12.1) of the total estimated global deaths attributable to AMR and 11.5% (10.6–12.3) of the global deaths associated with AMR occurred within the Americas (a region with approximately 13% of the world’s population) in 2019, based on our published global estimates.5 In a scenario where all drug-resistant infections were replaced by no infection, 569,000 deaths in the Americas could have been prevented in 2019. Similarly, if all drug-resistant infections were replaced by drug-susceptible infections, 141,000 deaths in the region could have been prevented (Fig. 2).
We identified substantial burden caused by S. aureus and E. coli, with each responsible for more than 100,000 deaths associated with AMR in the region. These two bacteria also represent a large burden in other regions of the world,5 and are the first AMR pathogens considered part of the Sustainable Development Goals (indicator 3.d.2),21 which aims to reduce bloodstream infections related to MRSA and third-generation cephalosporin-resistant E. coli. The ranking of these two pathogens as leading AMR pathogens globally should be leveraged by health ministries and organisations to prompt calls for broad international initiatives to address their AMR risk. As the burden for S. aureus and E. coli combined represents approximately 40% of both the attributable and associated AMR burdens in the Americas, controlling the treatment, spread, and development of resistance of these pathogens will be paramount in minimising the AMR burden in the region.
With respect to S. aureus, a recent report underscored that at least 25% of S. aureus isolates carry methicillin resistance in Latin America,22 although it is likely that this percentage is higher as indicated by research showing MRSA prevalence at 48.3%,23 and a Latin America SENTRY report for the 2011–2014 time period that documented a prevalence of 44.7%.24 Accordingly, a study by Primo and colleagues25 has shown that 45.2% of excess mortality from S. aureus can be attributed to methicillin resistance in comparison to susceptible strains, driving a surge in hospitalisation rates and antimicrobial treatment costs, with 3.0 and 6.7 times higher values, respectively. Our estimates for 2019 align with and further reinforce these concerning trends, showing a median MRSA prevalence of 45% in the region, ranging from 18% in Canada to 63% in Trinidad and Tobago (Appendix 1 Figure S11 p 49).
In high-income North America, the Canadian Antimicrobial Resistance Surveillance System has documented that the rate of MRSA infections has been increasing steadily since 2012, primarily driven by the proliferation of community-acquired strains.26 Our study confirms that MRSA is the combination with the highest age-standardised mortality rate in Canada, where this pathogen–drug combination was responsible for 490 deaths (95% UI 200–870), an age-standardised mortality rate of 0.69 (0.27–1.24), and 8000 DALYs (3000–15,000) attributable to AMR. Previous studies have shown that lower prevalence of MRSA is observed in the Atlantic Provinces when compared to the national average (0.5% versus 5.6%),27 and subnational data would facilitate a refined analysis of drug consumption and resistance rates in the country.
The large burden of E. coli coincides with high burden of resistant Enterobacterales in this region more broadly, and echoes results from Karlowsky and colleagues28 that showed resistance to third-generation cephalosporins among E. coli species increased substantially in Latin America from 2015 to 2019, while K. pneumoniae resistant to third-generation cephalosporins increased substantially in Canada, Latin America, and the USA during the same time period.28,29 Carbapenem-resistant E. coli and K. pneumoniae are particularly serious health threats in Latin America and the Caribbean.29, 30, 31 In Barbados, the results of the 2017 prevalence study by Forde and colleagues on carbapenem-resistant K. pneumoniae prompted national action and changed screening recommendations to include rectal swabs for all patients hospitalised for a period longer than one month. This subsequently instigated the introduction of several AMR surveillance programmes across the English-speaking Caribbean.32 The sizable burden of K. pneumoniae we identify in Barbados, Dominica, Haiti, and Guyana aligns with the Caribbean Public Health Agency’s decision to focus on that pathogen for their Caribbean-wide pilot project to monitor AMR through phenotypic resistance assessment and whole-genome sequencing.33 Based on our results, we argue that A. baumannii and S. pneumoniae should be strongly considered as future candidates.28, 29, 30, 31, 32
It should be noted that rigid comparison of our estimates with previous publications is somewhat complicated due to our novel methodological approach; however, certain parallels can be drawn. For instance, the CDC published a 2019 report on AMR infections and deaths in the USA for 18 AMR threats by using surveillance data, and showed that more than 2.8 million AMR infections occur in the USA every year, resulting in more than 35,000 deaths.34 Our estimates for the USA indicate that 42,000 deaths (28,000–60,000) are attributable to and 173,000 deaths (120,000–243,000) associated with AMR for the 88 pathogen–drug combinations and 23 bacterial pathogens included in our study. However, the CDC analysis included resistant fungi, and these two analyses differed substantively with respect to the analysed bacterial pathogens (acknowledging the potential sources of variation and uncertainty in both sets of results). Interestingly, other than as a component of multidrug-resistant P. aeruginosa, fluoroquinolone resistance was not identified as a major threat or concern in the CDC report. By contrast, our study finds substantial burden attributable to fluoroquinolone resistance in the USA (with 9400 deaths [6200–13,900]) and fluoroquinolone-resistant E. coli is the pathogen–drug combination with the third greatest attributable burden in the country (Appendix 2 p 79). In the three years following a 2016 Food and Drug Administration warning that the risks of adverse effects of fluoroquinolones outweigh the benefits of their use in many types of infections, there was a 42% reduction in outpatient fluoroquinolone prescription fills in the USA, though prescribing patterns were enduring among some physicians, namely otolaryngologists and those 65 or older.35,36 We argue that given the historical patterns of overprescribing,37 the impact of fluoroquinolone exposure in selecting for mutations that pave the way for the development of methicillin-resistance and extended-spectrum β-lactamase production in various pathogens,38 and the high burden we identify here, there is strong justification to carefully monitor the use of and resistance to fluoroquinolones in the USA.38
Strategies to address AMR differ across countries in the Americas given the heterogeneity in wealth in the region. The region is posed in between two distinct challenges: reducing the number of infections and maintaining antibiotic efficacy. In countries where there is low socio-demographic development and deficient access to antibiotics, infection prevention and control measures and vaccination campaigns might have the largest impact on burden, as access to appropriate care once infected would be limited. For example, given low vaccination rates for S. pneumoniae in many Caribbean nations, PCV vaccination represents a promising avenue for addressing both resistant and susceptible pneumococcal disease in these countries (Appendix 1 Figure S14 p 52). Expansion of microbiological testing capacity is also vital in lower-income countries, not only to better characterise the problem of AMR in these geographies but also to inform antibiotic treatment regimens for clinicians. In countries with strong health care systems and plentiful stocks of antibiotics, conversely, opportunities for strategic antimicrobial stewardship could have the largest impact in reducing the burden from AMR. Stewardship would benefit from a surveillance monitoring network of public health officials which can provide feedback to medical teams and pharmacists regarding the current state of antibiotic use and resistance.
The fraction of all-cause mortality that involved infection and the proportion of infectious deaths that were associated with resistance are important indicators signalling the strategies that may be most beneficial for a given country. Despite having the lowest proportion of infectious deaths that were associated with resistance, Haiti has the highest age-standardised AMR attributable mortality rate due its high baseline rate of infection-related mortality, which is nearly 50% greater than that of the next highest country in the region. For countries in which infections make up a larger share of all-cause mortality—Haiti and Bolivia lead in this metric in the Americas (Appendix 1 Table S6 pp 61–63 and Appendix 1 Figure S12 p 50)—infection prevention and control could yield the greatest reductions in AMR burden. Conversely, countries in which a larger share of infectious deaths are associated with resistance, such as Chile and Mexico (Appendix 1 Table S6 pp 61–63 and Appendix 1 Figure S12 p 50), could benefit from more stringent antimicrobial stewardship and surveillance. Peru, which ranks third in the Americas for both of these metrics, is uniquely positioned to benefit from both of these strategies.
One method to institutionalise a strong surveillance monitoring network is through the implementation of national action plans (NAP). There are 14 countries in the Americas that have published their NAP online, be it developed, approved, budgeted, financed, or implemented. An additional 15 countries have developed or approved an unpublished NAP, while six countries had not yet developed a NAP or otherwise did not report their progress towards an AMR NAP.19,20 Only four countries reported sufficient capacity to collect data on AMR across all relevant sectors in 2021, and six countries reported they have weak or no policies to optimise antimicrobial use in humans.39 It is important that the systems developed are used to inform guidelines and interventions in the region. The Organisation for Economic Co-operation and Development estimated that 75% of the AMR burden could be reduced by investing US$2 per person per year across the member countries, and quantified the cost-effectiveness of policies that tackle the AMR burden on different fronts.40
The surveillance of antimicrobial use must be strengthened to monitor trends in resistance and improve future estimates. The contribution of high-income North America and Latin America and the Caribbean to the total antibiotic consumption on a global level is considerable, with 7.7% and 6.2% of global antibiotic consumption occurring in these two regions, respectively.18 In the USA, as many as 30% of antibiotic prescriptions are deemed medically unnecessary,41 and over the past two decades there has been an increasing trend in national antibiotic consumption rates for most countries in Latin America.42 Although data on antimicrobial sales for human health remain scarce in the Americas, several countries (such as Bolivia, Brazil, Costa Rica, Paraguay, and Peru) have already provided valuable data for the 2016–2018 WHO Report on Surveillance of Antibiotic Consumption.40,43 Based on our estimates, we did not find a significant correlation between overall antibiotic consumption and attributable AMR mortality rates (Appendix 3 p 2), in contrast with Europe, where such a correlation was found.13 This assessment is complicated by the juxtaposition of low-SDI countries with low antimicrobial use but high rates of infection (elevating the AMR burden, as shown in Appendix 1 Figures S9 and S10 pp 47–48) against some of the wealthiest countries in the world with established resistance monitoring regimes. Despite this heterogeneity, we do find significant correlations between the use of six (of the 13 estimated) antimicrobial classes and their corresponding attributable burden (Appendix 3). Further surveillance efforts are needed to better inform these comparisons and other research on the interaction between prescribing practices and the outbreak of resistance.
Limitations
Our study has several limitations. Notwithstanding the general issue of data scarcity, especially data points that link AMR with mortality and DALYs, there was a notable data paucity for several pathogen–drug combinations, particularly for Cuba, Paraguay, and Uruguay. Such limited data in some countries was consequential for the prevalence of resistance and relative risk modelling components of our work. Additionally, these two components were not stratified by age and sex groups nor by infectious syndromes; these methodological assumptions are explained in the Appendix of the global burden of AMR study.5 Still, our estimation process is robust as it is informed by data from all countries in the Americas. When data for a specific country are lacking, our estimates rely on regional patterns, covariates, and out-of-sample predictive validity. Even though we have accounted for various biases, we must be cognisant that selection bias may occur in passive microbial surveillance data, while potential bias and misclassification can arise when combining and standardising data from various providers (ie, different levels of care provision), as well as various domains (eg, the difference between community-acquired and health-care-associated infections). Finally, despite our use of the most recent CLSI breakpoint guidelines whenever possible,14 there are no universal laboratory standards to distinguish resistance versus susceptibility, and deferring to source laboratory interpretation for classifying the isolates in our study may have resulted in heterogeneous classification.
Conclusion
The WHO Region of the Americas has a long history of surveillance of AMR, but there are still challenges to translate this surveillance into public action. One of these challenges relates to the differentiated nature of the problem for the countries in the region. A few countries face low access to antibiotics and basic health-care services, and some also face the largest age-standardised mortality rates associated with and attributable to AMR in the region. For those, the most cost-effective solutions may lie in policies which prevent infections, especially among the age groups that are most affected by infectious diseases. However, several countries have widespread access to basic health-care services, vaccination, antenatal care, and antibiotic treatment, and may achieve the largest gains by emphasising antimicrobial stewardship. By quantifying the health loss and understanding the groups which experience the greatest burden from AMR, we aim to inform public action to address this growing global health problem.
Contributors
The corresponding author, M Naghavi, and first authors, G Robles Aguilar and L R Swetschinski, had access to and verified the data. The corresponding author, M Naghavi, confirms all authors have seen and approved the final text. Please see Appendix (pp 72–73) for more detailed information about individual author contributions to the research, divided into the following categories: managing the overall research enterprise; writing the first draft of the manuscript; primary responsibility for applying analytical methods to produce estimates; primary responsibility for seeking, cataloguing, extracting, or cleaning data; designing or coding figures and tables; providing data or critical feedback on data sources; developing methods or computational machinery; providing critical feedback on methods or results; drafting the manuscript or revising it critically for important intellectual content; and managing the estimation or publications process.
Data sharing statement
This study follows the Guidelines for Accurate and Transparent Health Estimates Reporting (GATHER). To download the data used in these analyses, please visit the Global Health Data Exchange (GHDx).
Editor note
The Lancet Group takes a neutral position with respect to territorial claims in published maps and institutional affiliations.
Declaration of interests
E Chung reports support for this work in part by the National Institutes of Health (NICHD T32HD007233 to EC). F Krapp reports grants or contracts from the Belgian Directorate of Development Cooperation (DGD) through the Framework Agreement between the Belgian DGD and the Institute of Tropical Medicine, Belgium; the Fogarty International Center of the National Institutes of Health and the University of California Global Health Institute under Award Number D43TW009343; and the Fogarty International Center and National Institute of Child Health & Human Development of the National Institutes of Health under Award Number D43 TW009763; all outside the submitted work. A Pollard reports grants or contracts paid to their institution from The Bill & Melinda Gates Foundation, Wellcome Trust, Coalition for Epidemic Preparedness Innovations (CEPI), Medical Research Council (MRC), and National Institute for Health and Care Research (NIHR); royalties or licenses from AstraZeneca in partnership with Oxford University for development of COVID-19 vaccines; consulting fees from Shionogi; and unpaid leadership or fiduciary roles as Chair of the Department of Health and Social Care’s Joint Committee on Vaccination and Immunisation, and as member of the World Health Organization (WHO) Strategic Advisory Group of Experts on Immunization (SAGE) until 2022; all outside the submitted work. K E Rudd reports support for the present manuscript from National Institutes of Health (NIH) National Heart, Lung, and Blood Institute (NHLBI) (grant T32HL007287) and NIH National Institute of General Medical Sciences (NIGMS) (grant K23GM141463); and reports consulting fees from Janssen Pharmaceuticals outside the submitted work. J Sifuentes-Osornio reports grants or contracts from Roches Pharmaceuticals (protocol number: GA42469) and Novartis Pharmaceuticals (RUXCOVID clinical trial) outside the submitted work.
Acknowledgements
Funding was provided by the Bill & Melinda Gates Foundation (OPP1176062), the Wellcome Trust (A126042), and the UK Department of Health and Social Care using UK aid funding managed by the Fleming Fund (R52354 CN001). Co-authors affiliated with this organisation provided feedback on initial maps and drafts of this manuscript. Otherwise, the funders had no role in study design, data collection, data analysis, data interpretation, writing of the final report, or decision to publish. The corresponding author had full access to all of the data in the study and had final responsibility for the decision to submit for publication.
F Krapp acknowledges support from the Belgian Directorate of Development Cooperation (DGD) through the Framework Agreement between the Belgian DGD and the Institute of Tropical Medicine, Belgium; Fogarty International Center of the National Institutes of Health and the University of California Global Health Institute under Award Number D43TW009343; and Fogarty International Center, and National Institute of Child Health and Human Development of the National Institutes of Health under Award Number D43TW009763. V Nuñez-Samudio is a member of the Sistema Nacional de Investigación (SNI), which is supported by Panamá Secretaría Nacional de Ciencia, Tecnología e Innovación (SENACYT). K E Rudd acknowledges support from National Institutes of Health (NIH) National Heart, Lung, and Blood Institute (NHLBI) (grant T32HL007287) and NIH National Institute of General Medical Sciences (NIGMS) (grant K23GM141463).
GBD 2019 Antimicrobial Resistance in the Americas Collaborators
Gisela Robles Aguilar∗, Lucien R Swetschinski∗, Nicole Davis Weaver, Kevin S Ikuta, Tomislav Mestrovic, Authia P Gray, Erin Chung, Eve E Wool, Chieh Han, Anna Gershberg Hayoon, Daniel T Araki, Ashkan Abdollahi, Ahmed Abu-Zaid, Mohammad Adnan, Ramesh Agarwal, Javad Aminian Dehkordi, Aleksandr Y Aravkin, Demelash Areda, Ahmed Y Azzam, Eitan N Berezin, Akshaya Srikanth Bhagavathula, Zulfiqar A Bhutta, Soumitra S Bhuyan, Annie J Browne, Carlos A Castañeda-Orjuela, Eeshwar K Chandrasekar, Patrick R Ching, Xiaochen Dai, Gary L Darmstadt, Fernando Pio De la Hoz, Nancy Diao, Daniel Diaz, Wendel Mombaque dos Santos, David Eyre, Coralith Garcia, Georgina Haines-Woodhouse, Mohammed Bheser Hassen, Nathaniel J Henry, Susan Hopkins, Md Mahbub Hossain, Kenneth Chukwuemeka Iregbu, Chidozie C D Iwu, Jan Adriaan Jacobs, Mark M Janko, Ronald Jones, Ibraheem M Karaye, Ibrahim A Khalil, Imteyaz A Khan, Taimoor Khan, Jagdish Khubchandani, Suwimon Khusuwan, Adnan Kisa, Giscard Wilfried Koyaweda, Fiorella Krapp, Emmanuelle A P Kumaran, Hmwe Hmwe Kyu, Stephen S Lim, Xuefeng Liu, Stephen Luby, Sandeep B Maharaj, Christopher Maronga, Miquel Martorell, Jürgen May, Barney McManigal, Ali H Mokdad, Catrin E Moore, Ebrahim Mostafavi, Efrén Murillo-Zamora, Marisa Marcia Mussi-Pinhata, Ruchi Nanavati, Hasan Nassereldine, Zuhair S Natto, Farah Naz Qamar, Virginia Nuñez-Samudio, Theresa J Ochoa, Tolulope R Ojo-Akosile, Andrew T Olagunju, Antonio Olivas-Martinez, Edgar Ortiz-Brizuela, Pradthana Ounchanum, Jose L Paredes, Venkata Suresh Patthipati, Shrikant Pawar, Marcos Pereira, Andrew Pollard, Alfredo Ponce-De-Leon, Elton Junio Sady Prates, Ibrahim Qattea, Luis Felipe Reyes, Emmanuel Roilides, Victor Daniel Rosenthal, Kristina E Rudd, Weerawut Sangchan, Samroeng Seekaew, Allen Seylani, Niloufar Shababi, Sunder Sham, Jose Sifuentes-Osornio, Harpreet Singh, Andy Stergachis, Nidanuch Tasak, Nathan Y Tat, Areerat Thaiprakong, Pascual R Valdez, Dereje Y Yada, Ismaeel Yunusa, Mikhail Sergeevich Zastrozhin, Simon I Hay, Christiane Dolecek, Benn Sartorius, Christopher J L Murray, and Mohsen Naghavi.
∗Co-first authors.
Affiliations
Nuffield Department of Medicine (G Robles Aguilar DPhil, N J Henry BS, E A P Kumaran MSc, B Sartorius PhD), Big Data Institute (A J Browne MPH, B McManigal PhD), Department of Pediatrics (Prof A Pollard FMedSci), Oxford Centre for Global Health Research (C Dolecek PhD), Centre for Tropical Medicine and Global Health (B Sartorius PhD), University of Oxford, Oxford, UK; Institute for Health Metrics and Evaluation (L R Swetschinski MSc, N Davis Weaver MPH, K S Ikuta MD, T Mestrovic PhD, A P Gray BSc, E Chung MD, E E Wool MPH, C Han BA, A Gershberg Hayoon MSc, D T Araki MPH, A Y Aravkin PhD, X Dai PhD, M Hassen BSc, H H Kyu PhD, Prof S S Lim PhD, A H Mokdad PhD, H Nassereldine MD, D Y Yada MSc, Prof S I Hay FMedSci, Prof C J L Murray DPhil, Prof M Naghavi PhD), Seattle Children's Hospital, Department of Pediatrics (E Chung MD), Department of Applied Mathematics (A Y Aravkin PhD), Department of Health Metrics Sciences, School of Medicine (A Y Aravkin PhD, X Dai PhD, H H Kyu PhD, Prof S S Lim PhD, A H Mokdad PhD, Prof A Stergachis PhD, Prof S I Hay FMedSci, B Sartorius PhD, Prof C J L Murray DPhil, Prof M Naghavi PhD), Department of Global Health (I A Khalil MD), Department of Biostatistics (A Olivas-Martinez MD), Department of Pharmacy (Prof A Stergachis PhD), University of Washington, Seattle, WA, USA; Division of Infectious Diseases (K S Ikuta MD), Veterans Affairs Greater Los Angeles, Los Angeles, LA, USA; University Centre Varazdin (T Mestrovic PhD), University North, Varazdin, Croatia; University of Texas Health Science Center, Houston, TX, USA (D T Araki MPH); Division of Cardiology (A Abdollahi MD), Department of Surgery (N Shababi MD), Johns Hopkins University, Baltimore, MD, USA; School of Medicine (A Abdollahi MD), Shiraz University of Medical Sciences, Shiraz, Iran; Department of Surgery (A Abu-Zaid MD), Alfaisal University, Riyadh, Saudi Arabia; College of Graduate Health Sciences (A Abu-Zaid MD), University of Tennessee, Memphis, TN, USA; Department of Neonatology (M Adnan MD), Indiana University Health Ball Memorial Hospital, Muncie, IN, USA; Division of Neonatology (Prof R Agarwal DM), All India Institute of Medical Sciences, New Delhi, India; Applied Science and Technology Department (J Aminian Dehkordi PhD), University of California Berkeley, Berkeley, CA, USA; Chemical Engineering Department - Biotechnology Group (J Aminian Dehkordi PhD), Tarbiat Modares University, Tehran, Iran; College of Art and Science (D Areda PhD), Ottawa University, Surprise, AZ, USA; College of Liberal Arts and Sciences (D Areda PhD), Arizona State University, Tempe, AZ, USA; Department of Neurovascular Research (A Y Azzam MD), Nested Knowledge, Inc., Saint Paul, MN, USA; Faculty of Medicine (A Y Azzam MD), October 6 University, 6th of October City, Egypt; Department of Pediatrics (Prof E N Berezin MD), University of São Paulo, São Paulo, Brazil; Department of Pediatrics (Prof E N Berezin MD), Santa Casa de São Paulo University Hospital, São Paulo, Brazil; Department of Health, Human Performance and Recreation (A S Bhagavathula PhD), University of Arkansas, Fayetteville, AR, USA; Centre for Global Child Health (Prof Z A Bhutta PhD), University of Toronto, Toronto, ON, Canada; Centre of Excellence in Women & Child Health (Prof Z A Bhutta PhD), Aga Khan University, Karachi, Pakistan; Edward J. Bloustein School of Planning and Public Policy (S S Bhuyan PhD), Department of Pediatrics (I A Khan MD), Rutgers University, New Brunswick, NJ, USA; Colombian National Health Observatory (C A Castañeda-Orjuela MD), National Institute of Health, Bogota, Colombia; Epidemiology and Public Health Evaluation Group (C A Castañeda-Orjuela MD), Department of Public Health (Prof F P De la Hoz PhD), National University of Colombia, Bogota, Colombia; Department of Anesthesiology and Perioperative Medicine (E K Chandrasekar MD), University of Rochester, Rochester, NY, USA; Division of Infectious Diseases (P R Ching MD), Washington University in St. Louis, St. Louis, MO, USA; Department of Pediatrics (Prof G L Darmstadt MD), School of Medicine (Prof S Luby MD), Stanford University, Stanford, CA, USA; Department of Environmental Health (N Diao DSc), Department of Health Policy and Oral Epidemiology (Z S Natto DrPH), Harvard University, Boston, MA, USA; Center of Complexity Sciences (Prof D Diaz PhD), National Autonomous University of Mexico, Mexico City, Mexico; Faculty of Veterinary Medicine and Zootechnics (Prof D Diaz PhD), Autonomous University of Sinaloa, Culiacán Rosales, Mexico; Responsabilidade Social (W M dos Santos PhD), Hospital Alemão Oswaldo Cruz (Oswaldo Cruz German Hospital), São Paulo, Brazil; Brazilian Centre for Evidence-based Healthcare: An Affiliate Centre of the Joanna Briggs Institute (W M dos Santos PhD), Joanna Briggs Institute, São Paulo, Brazil; NIHR Oxford Biomedical Research Centre, Oxford, UK (D Eyre DPhil); Instituto de Medicina Tropical Alexander von Humboldt (Alexander von Humboldt Institute of Tropical Medicine) (C Garcia PhD, J L Paredes MD), Alexander von Humboldt Institute of Tropical Medicine (F Krapp MD), Instituto de Medicina Tropical Alexander von Humboldt (Prof T J Ochoa MD), Universidad Peruana Cayetano Heredia (Cayetano Heredia University), Lima, Peru; Nuffield Department of Medicine (G Haines-Woodhouse MRes), Oxford University, Oxford, UK; National Data Management Center for Health (NDMC) (M Hassen BSc), Ethiopian Public Health Institute, Addis Ababa, Ethiopia; Clinical and Public Health Group (Prof S Hopkins AuD), UK Health Security Agency, London, UK; Division of Infection and Immunity (Prof S Hopkins AuD), University College London, London, UK; Social and Environmental Health Research (M Hossain MPH), Nature Study Society of Bangladesh, Khulna, Bangladesh; Department of Health Promotion and Community Health Sciences (M Hossain MPH), Texas A&M University, College Station, TX, USA; Department of Medical Microbiology (K C Iregbu MD), University of Abuja, Abuja, Nigeria; Department of Medical Microbiology (K C Iregbu MD), National Hospital, Abuja, Nigeria; School of Health Systems and Public Health (C C D Iwu MPH), University of Pretoria, Pretoria, South Africa; Department of Clinical Sciences (Prof J A Jacobs PhD), Institute of Tropical Medicine, Antwerpen, Belgium; Department of Immunology, Microbiology and Transplantation (Prof J A Jacobs PhD), Doctoral School of Biomedical Sciences (F Krapp MD), Katholieke Universiteit Leuven, Leuven, Belgium; Duke Global Health Institute (M M Janko PhD), Duke University, Durham, NC, USA; SENTRY Antimicrobial Surveillance Program (R Jones MD), JMI Laboratories, North Liberty, IA, USA; School of Health Professions and Human Services (I M Karaye MD), Hofstra University, Hempstead, NY, USA; Department of Radiation Oncology (T Khan PhD), Department of Bioengineering and Therapeutic Sciences (Prof M S Zastrozhin PhD), University of California San Francisco, San Francisco, CA, USA; Department of Public Health (Prof J Khubchandani PhD), New Mexico State University, Las Cruces, NM, USA; Department of Medicine (S Khusuwan MD), Department of Pediatrics (P Ounchanum MD), Department of Internal Medicine (S Seekaew MD), Chiang Rai Prachanukroh Hospital, Chiang Rai, Thailand (W Sangchan BS); School of Health Sciences (Prof A Kisa PhD), Kristiania University College, Oslo, Norway; Department of International Health and Sustainable Development (Prof A Kisa PhD), Tulane University, New Orleans, LA, USA; National Laboratory of Clinical Biology and Public Health, Bangui, Central African Republic (G W Koyaweda MSc); Lerner Research Institute (X Liu PhD), Cleveland Clinic, Cleveland, OH, USA; Department of Quantitative Health Science (X Liu PhD), Department of Neonatology (I Qattea MD), Case Western Reserve University, Cleveland, OH, USA; School of Pharmacy (S B Maharaj DBA), University of the West Indies, St. Augustine, Trinidad and Tobago; Fellow (S B Maharaj DBA), Planetary Health Alliance, Boston, MA, USA; Kenya Medical Research Institute Wellcome Trust Research Programme (KWTRP), Kilifi, Kenya (C Maronga MSc); Department of Nutrition and Dietetics (M Martorell PhD), University of Concepcion, Concepción, Chile; Centre for Healthy Living (M Martorell PhD), University of Concepción, Concepción, Chile; Department of Infectious Disease Epidemiology (Prof J May MD), Bernhard Nocht Institute for Tropical Medicine, Hamburg, Germany; Department of Tropical Medicine (Prof J May MD), Medical Center Hamburg-Eppendorf (UKE), Hamburg, Germany; Centre for Neonatal and Paediatric Infection (C E Moore PhD), St. George's University of London, London, UK; Department of Medicine (E Mostafavi PhD), Stanford Cardiovascular Institute (E Mostafavi PhD), Stanford University, Palo Alto, CA, USA; Clinical Epidemiology Research Unit (E Murillo-Zamora PhD), Mexican Institute of Social Security, Villa de Alvarez, Mexico; Postgraduate in Medical Sciences (E Murillo-Zamora PhD), Universidad de Colima, Colima, Mexico; Department of Pediatrics (M M Mussi-Pinhata PhD), University of São Paulo, Ribeirão Preto, Brazil; Department of Neonatology (R Nanavati MD), Seth Gordhandas Sunderdas Medical College (GSMC) and the King Edward Memorial (KEM) Hospital, Mumbai, India; Department of Dental Public Health (Z S Natto DrPH), King Abdulaziz University, Jeddah, Saudi Arabia; Department of Pediatrics and Child Health (F Naz Qamar FRCP), The Aga Khan University, Karachi, Pakistan; Unit of Microbiology and Public Health (V Nuñez-Samudio PhD), Institute of Medical Sciences, Las Tablas, Panama; Department of Public Health (V Nuñez-Samudio PhD), Ministry of Health, Herrera, Panama; School of Public Health (Prof T J Ochoa MD), University of Texas, Houston, USA; Department of Gynecology and Obstetrics (T R Ojo-Akosile MPH), Emory University, Atlanta, GA, USA; Department of Psychiatry and Behavioural Neurosciences (A T Olagunju MD), McMaster University, Hamilton, ON, Canada; Department of Psychiatry (A T Olagunju MD), University of Lagos, Lagos, Nigeria; Department of Medicine (A Olivas-Martinez MD), Instituto Nacional de Ciencias Medicas y Nutricion Salvador Zubiran (Salvador Zubiran National Institute of Medical Sciences and Nutrition), Tlalpan, Mexico; Department of Medicine (E Ortiz-Brizuela MSc), National Institute of Health and Nutrition, Mexico City, Mexico; Epidemiology Biostatistics and Occupational Health (E Ortiz-Brizuela MSc), McGill University, Montreal, QC, Canada; Department of Internal Medicine (V Patthipati MD), Advent Health, Palm Coast, FL, USA; Hospital Medicine (V Patthipati MD), Sound Physicians, Palm Coast, USA; Department of Genetics (S Pawar PhD), Yale University, New Haven, CT, USA; Institute of Collective Health (Prof M Pereira PhD), Federal University of Bahia, Salvador, Brazil; Department of Infectious Diseases (Prof A Ponce-De-Leon MD), Instituto Nacional de Ciencias Medicas y Nutricion Salvador Zubiran (Salvador Zubiran National Institute of Medical Sciences and Nutrition), Mexico City, Mexico; Department of Maternal and Child Nursing and Public Health (E J S Prates BS), Federal University of Minas Gerais, Belo Horizonte, Brazil; Unisabana Center for Translational Science (L F Reyes PhD), Universidad de La Sabana, Chia, Colombia; Critical Care Department (L F Reyes PhD), Clinica Universidad De La Sabana, Chia, Colombia; (E Roilides MD); International Nosocomial Infection Control Consortium (V D Rosenthal MD), Independent Consultant, Buenos Aires, Argentina; Department of Critical Care Medicine (K E Rudd MD), University of Pittsburgh, Pittsburgh, PA, USA; National Heart, Lung, and Blood Institute (A Seylani BS), National Institute of Health, Rockville, MD, USA; Department of Pathology, Lenox Hill Hospital (S Sham MBBS), Northwell Health, New York, NY, USA; Department of Medicine (Prof J Sifuentes-Osornio MD), National Institute of Nutrition, Tlalpan, Mexico; Department of Pulmonary and Critical Care Medicine (H Singh MD), Medical College of Wisconsin, Milwaukee, WI, USA; Chiangrai Clinical Research Unit (N Tasak BNS), Mahidol-Oxford Tropical Medicine Research Unit, Chiang Rai, Thailand; Department of Economics (N Y Tat MS), Rice University, Houston, TX, USA; Department of Research and Innovation (N Y Tat MS), Enventure Medical Innovation, Houston, TX, USA; Department of Microbiology (A Thaiprakong BSc), Mahidol-Oxford Tropical Medicine Research Unit, Bangkok, Thailand; Argentine Society of Medicine, Buenos Aires, Argentina (Prof P R Valdez MEd); Velez Sarsfield Hospital, Buenos Aires, Argentina (Prof P R Valdez MEd); Department of Clinical Pharmacy and Outcomes Sciences (I Yunusa PhD), University of South Carolina, Columbia, SC, USA; Addictology Department (Prof M S Zastrozhin PhD), Russian Medical Academy of Continuous Professional Education, Moscow, Russia; Mahidol Oxford Tropical Medicine Research Unit (C Dolecek PhD), Mahidol University, Bangkok, Thailand.
Footnotes
Corresponding author. Department of Health Metrics Sciences, Director of Subnational Burden of Disease Estimation, Institute for Health Metrics and Evaluation, School of Medicine, University of Washington, 3980 15th Ave. NE, Seattle, WA 98195, USA. E-mail address:nagham@uw.edu (M. Naghavi).
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lana.2023.100561.
Contributor Information
Antimicrobial Resistance Collaborators:
Gisela Robles Aguilar, Lucien R. Swetschinski, Nicole Davis Weaver, Kevin S. Ikuta, Tomislav Mestrovic, Authia P. Gray, Erin Chung, Eve E. Wool, Chieh Han, Anna Gershberg Hayoon, Daniel T. Araki, Ashkan Abdollahi, Ahmed Abu-Zaid, Mohammad Adnan, Ramesh Agarwal, Javad Aminian Dehkordi, Aleksandr Y. Aravkin, Demelash Areda, Ahmed Y. Azzam, Eitan N. Berezin, Akshaya Srikanth Bhagavathula, Zulfiqar A. Bhutta, Soumitra S. Bhuyan, Annie J. Browne, Carlos A. Castañeda-Orjuela, Eeshwar K. Chandrasekar, Patrick R. Ching, Xiaochen Dai, Gary L. Darmstadt, Fernando Pio De la Hoz, Nancy Diao, Daniel Diaz, Wendel Mombaque dos Santos, David Eyre, Coralith Garcia, Georgina Haines-Woodhouse, Mohammed Bheser Hassen, Nathaniel J. Henry, Susan Hopkins, Md Mahbub Hossain, Kenneth Chukwuemeka Iregbu, Chidozie C.D. Iwu, Jan Adriaan Jacobs, Mark M. Janko, Ronald Jones, Ibraheem M. Karaye, Ibrahim A. Khalil, Imteyaz A. Khan, Taimoor Khan, Jagdish Khubchandani, Suwimon Khusuwan, Adnan Kisa, Giscard Wilfried Koyaweda, Fiorella Krapp, Emmanuelle A.P. Kumaran, Hmwe Hmwe Kyu, Stephen S. Lim, Xuefeng Liu, Stephen Luby, Sandeep B. Maharaj, Christopher Maronga, Miquel Martorell, Jürgen May, Barney McManigal, Ali H. Mokdad, Catrin E. Moore, Ebrahim Mostafavi, Efrén Murillo-Zamora, Marisa Marcia Mussi-Pinhata, Ruchi Nanavati, Hasan Nassereldine, Zuhair S. Natto, Farah Naz Qamar, Virginia Nuñez-Samudio, Theresa J. Ochoa, Tolulope R. Ojo-Akosile, Andrew T. Olagunju, Antonio Olivas-Martinez, Edgar Ortiz-Brizuela, Pradthana Ounchanum, Jose L. Paredes, Venkata Suresh Patthipati, Shrikant Pawar, Marcos Pereira, Andrew Pollard, Alfredo Ponce-De-Leon, Elton Junio Sady Prates, Ibrahim Qattea, Luis Felipe Reyes, Emmanuel Roilides, Victor Daniel Rosenthal, Kristina E. Rudd, Weerawut Sangchan, Samroeng Seekaew, Allen Seylani, Niloufar Shababi, Sunder Sham, Jose Sifuentes-Osornio, Harpreet Singh, Andy Stergachis, Nidanuch Tasak, Nathan Y. Tat, Areerat Thaiprakong, Pascual R. Valdez, Dereje Y. Yada, Ismaeel Yunusa, Mikhail Sergeevich Zastrozhin, Simon I. Hay, Christiane Dolecek, Benn Sartorius, Christopher J.L. Murray, and Mohsen Naghavi
Appendix A. Supplementary data
References
- 1.WHO . 2021. Antimicrobial resistance.https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance [Google Scholar]
- 2.O’Neill J. The Review on Antimicrobial Resistance; London, UK: 2016. Tackling drug-resistant infections globally: final report and recommendations.https://apo.org.au/node/63983 [Google Scholar]
- 3.de Kraker M., Stewardson A., Harbarth S. Will 10 million people die a year due to antimicrobial resistance by 2050? PLoS Med. 2016;13 doi: 10.1371/journal.pmed.1002184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Office for Animal Health . 2016. NOAH response to final O Neill AMR review report.https://www.noah.co.uk/wp-content/uploads/2016/07/FINAL-NOAH-response-to-final-O-Neill-review-25-07-16-cle.pdf Middlesex, UK. [Google Scholar]
- 5.Antimicrobial Resistance Collaborators Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. 2022;399:629–655. doi: 10.1016/S0140-6736(21)02724-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization . World Health Organization; 2014. Antimicrobial resistance: global report on surveillance.https://apps.who.int/iris/handle/10665/112642 [Google Scholar]
- 7.Karp B.E., Tate H., Plumblee J.R., et al. National antimicrobial resistance monitoring system: two decades of advancing public health through integrated surveillance of antimicrobial resistance. Foodborne Pathog Dis. 2017;14:545–557. doi: 10.1089/fpd.2017.2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.The USA FDA . FDA; 2022. The national antimicrobial resistance monitoring system (NARMS)https://www.fda.gov/animal-veterinary/antimicrobial-resistance/national-antimicrobial-resistance-monitoring-system [Google Scholar]
- 9.Centers for Disease Control and Prevention . Cent. Dis. Control Prev.; 2022. U.S. action and events to combat antibiotic resistance. 2021.https://www.cdc.gov/drugresistance/us-activities.html [Google Scholar]
- 10.Public Health Agency of Canada . 2017. Tackling antimicrobial resistance and antimicrobial use: a pan-Canadian framework for action.https://www.canada.ca/en/health-canada/services/publications/drugs-health-products/tackling-antimicrobial-resistance-use-pan-canadian-framework-action.html [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ponce de Leon A., Merchant S., Raman G., et al. Pseudomonas infections among hospitalized adults in Latin America: a systematic review and meta-analysis. BMC Infect Dis. 2020;20:250. doi: 10.1186/s12879-020-04973-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Institute for Health Metrics and Evaluation . 2020. Protocol for the global burden of diseases, injuries, and risk factors study (GBD). Version 4.0.http://www.healthdata.org/sites/default/files/files/Projects/GBD/March2020_GBD%20Protocol_v4.pdf [Google Scholar]
- 13.European Antimicrobial Resistance Collaborators The burden of bacterial antimicrobial resistance in the WHO European region in 2019: a cross-country systematic analysis. Lancet Public Health. 2022;7:e897–e913. doi: 10.1016/S2468-2667(22)00225-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clinical and Laboratory Standards Institute . 31st ed. 2021. Performance standards for antimicrobial susceptibility testing. CLSI supplement M100.https://clsi.org/media/z2uhcbmv/m100ed31_sample.pdf Wayne, PA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stevens G.A., Alkema L., Black R.E., et al. Guidelines for accurate and transparent health estimates reporting: the GATHER statement. PLoS Med. 2016;13 doi: 10.1371/journal.pmed.1002056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.GBD 2019 Diseases and Injuries Collaborators Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet. 2020;396:1204–1222. doi: 10.1016/S0140-6736(20)30925-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Llor C., Bjerrum L. Antimicrobial resistance: risk associated with antibiotic overuse and initiatives to reduce the problem. Ther Adv Drug Saf. 2014;5:229–241. doi: 10.1177/2042098614554919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Browne A.J., Chipeta M.G., Haines-Woodhouse G., et al. Global antibiotic consumption and usage in humans, 2000-18: a spatial modelling study. Lancet Planet Health. 2021;5:e893–e904. doi: 10.1016/S2542-5196(21)00280-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.WHO Global database for tracking antimicrobial resistance (AMR) country self- assessment survey (TrACSS) https://amrcountryprogress.org/#/visualization-view
- 20.WHO Library of AMR national action plans. https://www.who.int/teams/surveillance-prevention-control-AMR/national-action-plan-monitoring-evaluation/library-of-national-action-plans
- 21.United Nations Department of Economic and Social Affairs. The sustainable development goals. Goal 3. Ensure healthy lives and promote well-being for all at all ages. https://sdgs.un.org/goals/goal3
- 22.da Silva J., Jr., Espinal M., Ramón-Pardo P. Antimicrobial resistance: time for action. Rev Panam Salud Publica. 2020;44 doi: 10.26633/RPSP.2020.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vega S., Dowzicky M.J. Antimicrobial susceptibility among Gram-positive and Gram-negative organisms collected from the Latin American region between 2004 and 2015 as part of the Tigecycline evaluation and surveillance trial. Ann Clin Microbiol Antimicrob. 2017;16:50. doi: 10.1186/s12941-017-0222-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sader H.S., Castanheira M., Farrell D.J., Flamm R.K., Mendes R.E., Jones R.N. Tigecycline antimicrobial activity tested against clinical bacteria from Latin American medical centres: results from SENTRY Antimicrobial Surveillance Program (2011-2014) Int J Antimicrob Agents. 2016;48:144–150. doi: 10.1016/j.ijantimicag.2016.04.021. [DOI] [PubMed] [Google Scholar]
- 25.Primo M.G.B., Guilarde A.O., Martelli C.M.T., Batista L.J., Turchi M.D. Healthcare-associated Staphylococcus aureus bloodstream infection: length of stay, attributable mortality, and additional direct costs. Braz J Infect Dis. 2012;16:503–509. doi: 10.1016/j.bjid.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 26.PHA of Canada . 2018. Canadian antimicrobial resistance surveillance system - update 2018: executive summary.https://www.canada.ca/en/public-health/services/publications/drugs-health-products/canadian-antimicrobial-resistance-surveillance-system-2018-report-executive-summary.html [Google Scholar]
- 27.German G.J., Frenette C., Caissy J.-A., et al. The 2018 global point prevalence survey of antimicrobial consumption and resistance in 47 Canadian hospitals: a cross-sectional survey. CMAJ Open. 2021;9:E1242–E1251. doi: 10.9778/cmajo.20200274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karlowsky J.A., Lob S.H., DeRyke C.A., et al. Prevalence of ESBL non-CRE Escherichia coli and Klebsiella pneumoniae among clinical isolates collected by the SMART global surveillance programme from 2015 to 2019. Int J Antimicrob Agents. 2022;59 doi: 10.1016/j.ijantimicag.2022.106535. [DOI] [PubMed] [Google Scholar]
- 29.Pan American Health Organization . 2020. Magnitude and trends of antimicrobial resistance in Latin America. ReLAVRA 2014, 2015, 2016. Summary report.https://www.paho.org/en/documents/magnitude-and-trends-antimicrobial-resistance-latin-america-relavra-2014-2015-2016 [Google Scholar]
- 30.Gregory C.J., Llata E., Stine N., et al. Outbreak of carbapenem-resistant Klebsiella pneumoniae in Puerto Rico associated with a novel carbapenemase variant. Infect Control Hosp Epidemiol. 2010;31:476–484. doi: 10.1086/651670. [DOI] [PubMed] [Google Scholar]
- 31.Escandón-Vargas K., Reyes S., Gutiérrez S., Villegas M.V. The epidemiology of carbapenemases in Latin America and the Caribbean. Expert Rev Anti Infect Ther. 2017;15:277–297. doi: 10.1080/14787210.2017.1268918. [DOI] [PubMed] [Google Scholar]
- 32.Forde C., Stierman B., Ramon-Pardo P., Dos Santos T., Singh N. Carbapenem-resistant Klebsiella pneumoniae in Barbados: driving change in practice at the national level. PLoS One. 2017;12 doi: 10.1371/journal.pone.0176779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heinz E., Brindle R., Morgan-McCalla A., Peters K., Thomson N.R. Caribbean multi-centre study of Klebsiella pneumoniae: whole-genome sequencing, antimicrobial resistance and virulence factors. Microb Genomics. 2019;5 doi: 10.1099/mgen.0.000266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.CDC . U.S. Department of Health and Human Services, CDC; Atlanta, GA: 2019. Antibiotic resistance threats in the United States, 2019. [Google Scholar]
- 35.Buehrle D.J., Wagener M.M., Clancy C.J. Outpatient fluoroquinolone prescription fills in the United States, 2014 to 2020: assessing the impact of Food and Drug Administration safety warnings. Antimicrob Agents Chemother. 2021;65 doi: 10.1128/AAC.00151-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Umarje S.P., Alexander C.G., Cohen A.J. Ambulatory fluoroquinolone use in the United States, 2015-2019. Open Forum Infect Dis. 2021;8 doi: 10.1093/ofid/ofab538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kabbani S., Hersh A.L., Shapiro D.J., Fleming-Dutra K.E., Pavia A.T., Hicks L.A. Opportunities to improve fluoroquinolone prescribing in the United States for adult ambulatory care visits. Clin Infect Dis. 2018;67:134–136. doi: 10.1093/cid/ciy035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fuzi M., Rodriguez Baño J., Toth A. Global evolution of pathogenic bacteria with extensive use of fluoroquinolone agents. Front Microbiol. 2020;11:271. doi: 10.3389/fmicb.2020.00271. https://www.frontiersin.org/articles/10.3389/fmicb.2020.00271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.World Health Organization . 2021. Tripartite AMR country self-assessment survey (TrACSS) 2020-2021.https://www.who.int/publications/m/item/tripartite-amr-country-self-assessment-survey-(tracss)-2020-2021 [Google Scholar]
- 40.The Organisation for Economic Co-operation and Development (OECD) Stopping antimicrobial resistance would cost just USD 2 per person a year. https://www.oecd.org/newsroom/stopping-antimicrobial-resistance-would-cost-just-usd-2-per-person-a-year.htm
- 41.Rudd K.E., Johnson S.C., Agesa K.M., et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the global burden of disease study. Lancet. 2020;395:200–211. doi: 10.1016/S0140-6736(19)32989-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Klein E.Y., Van Boeckel T.P., Martinez E.M., et al. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc Natl Acad Sci U S A. 2018;115:e3463–e3470. doi: 10.1073/pnas.1717295115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.World Health Organization . WHO; 2019. WHO report on surveillance of antibiotic consumption, 2016-2018 early implementation.https://www.who.int/publications-detail-redirect/who-report-on-surveillance-of-antibiotic-consumption [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.