Abstract
Background
We evaluated the clinical relevance of achieving histologic endoscopic mucosal improvement (HEMI) and the more stringent target of histologic endoscopic mucosal remission (HEMR) in the phase 3 maintenance trial of upadacitinib for moderately to severely active ulcerative colitis.
Methods
Clinical and patient-reported outcomes were assessed in patients with clinical response after 8- or 16-week upadacitinib induction who received 52-week upadacitinib maintenance treatment. Cross-sectional and predictive analyses evaluated the relationship between HEMR or HEMI at Week 8/16 and Week 52, respectively, and outcomes at Week 52. Adjusted odds ratios (aOR) were derived from logistic regressions for patients achieving HEMR or HEMI without HEMR versus those not achieving HEMI.
Results
Cross-sectional analyses showed that patients with HEMR had greater odds of achieving all clinical and patient-reported outcomes at Week 52 than those not achieving HEMI. In predictive analyses, patients with HEMR at Week 8/16 had significantly greater odds of achieving clinical remission (aOR = 3.6, p = 0.001) and endoscopic remission (aOR = 3.9, p < 0.001) at Week 52 than patients not achieving HEMI and HEMR. For patients achieving HEMI without HEMR, these odds were lower: clinical remission (aOR = 3.2, p < 0.001) and endoscopic remission (aOR = 2.4, p = 0.010). The odds of achieving clinically meaningful improvements in most patient-reported outcomes were directionally similar between HEMI and HEMR, but not statistically different to patients not achieving HEMI. No hospitalizations or surgeries were observed in patients with HEMR at Week 52.
Conclusions
Achievement of HEMR or HEMI is clinically relevant with HEMR being associated with greater likelihood of improvement in long-term clinical and patient-reported outcomes. https://www.clinicaltrials.gov NCT02819635.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00535-023-02013-7.
Keywords: Histologic endoscopic mucosal improvement, Histologic endoscopic mucosal remission, Inflammatory bowel disease
Introduction
Treatment of ulcerative colitis (UC), a chronic, progressive, debilitating inflammatory disease of the gastrointestinal tract [1] has evolved over time. Today, disease activity and therapeutic benefits can be evaluated by clinical symptoms, endoscopy, patient-reported outcomes, biomarkers, and histologic methods. The Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE-II) consensus guidelines recommend a treat-to-target approach in UC [2] to improve outcomes and reduce the risk of end-organ damage. Per STRIDE-II recommendations, short-term treatment targets include symptomatic relief along with normalization of serum and fecal inflammatory markers, while long-term treatment targets include clinical remission, endoscopic mucosal healing, and restoration of quality of life, as well as decreased rates of flares, dysplasia, and colectomy [2]. Histologic remission was suggested as an adjunct to endoscopic remission to achieve a deeper level of healing [2]. The STRIDE authors felt that there was insufficient data to have histologic healing as a treatment goal because few clinical studies report on the use of histologic endpoints and in those that do, the histologic endpoints have been achieved in a small proportion of patients.
Achievement of endoscopic healing has emerged as an important treatment goal for patients with UC as several studies suggest that endoscopic healing is associated with long-term clinical remission, decreased colectomy rates, and decreased rates of dysplasia [3–8]. Multiple scoring systems are available to assess endoscopic activity, which has resulted in variable definitions of endoscopic remission in clinical trials [9]. The Mayo endoscopic score (MES) [10] and Ulcerative Colitis Endoscopic Index of Severity (UCEIS) [11] are commonly used to assess endoscopic healing, although the exact definition of endoscopic healing remains to be established [9]. Published studies suggest that more stringent endoscopic criteria may provide even better long-term benefits for patients [12–15]. Barreiro-de Acosta et al. [12] showed that patients who achieved MES = 0 (inactive disease) had a lower relapse rate at 12 months than those who achieved MES = 1 (mild disease). A recent meta-analysis [13] demonstrated that patients with MES = 0 had a 52% lower risk of clinical relapse at 12 months than patients with MES = 1. Persistent histologic inflammation is often observed in patients who achieve endoscopic healing [16–19] and is associated with an increased risk of relapse, colectomy, and colorectal neoplasia [8, 19–25]. More recently, histologic remission was shown to be associated with more favorable outcomes, including lower hospitalization and relapse rates, and lower cancer risk [14].
At present, there are few data available on the impact of a composite histologic and endoscopic endpoint on longer-term outcomes, particularly early in the treatment cycle. Two endpoints that combine endoscopic and histologic disease activity have been proposed and are currently being utilized in clinical trial settings [26–30]. The first composite endpoint is histologic endoscopic mucosal improvement (HEMI), defined as MES ≤ 1 and Geboes histologic score ≤ 3.1 [27, 28]. The second endpoint is a more stringent, novel composite endpoint, and histologic endoscopic mucosal remission (HEMR) or deep mucosal healing, defined as MES = 0 and Geboes histologic score < 2.0. We therefore aimed to investigate the effect of achieving a composite of endoscopic remission and histologic remission in UC early in the treatment course on long-term clinical outcomes in patients treated with upadacitinib in the U-ACHIEVE maintenance trial.
Methods
Study design and data source
The upadacitinib phase 3 program consists of 2 identical induction studies (U-ACHIEVE induction and U-ACCOMPLISH) and 1 maintenance study (U-ACHIEVE maintenance). In the induction studies, patients were randomized to receive upadacitinib 45 mg or placebo for 8 weeks. At Week 8, patients who did not achieve a clinical response were eligible to receive an additional 8 weeks of treatment with upadacitinib 45 mg. Patients who achieved a clinical response following upadacitinib 45 mg for 8 or 16 weeks were eligible to enroll in the U-ACHIEVE maintenance study and were randomized 1:1:1 to receive upadacitinib 15 mg, upadacitinib 30 mg, or placebo. Randomization was stratified by bio-IR status (bio-IR vs non-bio-IR), corticosteroid use (yes or no), and Adapted Mayo score (≤ 7 or > 7) at Baseline. Within bio-IR, the randomization was further stratified by number of prior biologic treatments (≤ 1 or > 1). Within non-bio-IR, the randomization was further stratified by previous biologic use (yes or no). We performed post hoc analyses of data from the Phase 3 U-ACHIEVE upadacitinib maintenance trial to evaluate the relationship between the HEMR/HEMI endpoints and achievement of long-term clinical and patient-reported outcomes at Week 52. Histologic, clinical, and patient-reported outcomes data from the Phase 3 U-ACHIEVE [NCT02819635] upadacitinib maintenance trial were evaluated in this analysis. The objective of the U-ACHIEVE maintenance trial was to evaluate the efficacy and safety of upadacitinib compared with placebo in achieving clinical remission in patients with moderately to severely active UC who had a clinical response per adapted Mayo score following induction with upadacitinib 45 mg once daily (QD). In the maintenance trial, patients were randomized 1:1:1 to upadacitinib 15 mg QD, upadacitinib 30 mg QD, or placebo QD. Details of the U-ACHIEVE induction and maintenance trials are reported elsewhere [28].
A full colonoscopy was performed for all patients at screening. At Weeks 8 and 52, endoscopies were either a colonoscopy or a flexible sigmoidoscopy, depending on the extent of disease at screening, and were performed up to the segment where a clear demarcation of inflammation was observed. In patients who required 16 weeks of upadacitinib induction therapy, an additional colonoscopy or a flexible sigmoidoscopy was performed at Week 16. During all endoscopies, biopsies for histologic evaluation were taken from the rectosigmoid colon (approximately 15–30 cm from the anal verge) and from the area of most inflammation. For follow-up endoscopies, in the absence of any visible lesions or areas of general inflammation characteristic of UC, biopsies were to be collected from normal mucosa in the same segments as noted above. Tissue samples were processed, mounted to slides, and digitized using a whole slides scanner. For each image, a central reader performed the reading and provided a histologic score. Both histologic and endoscopic scoring were performed by the central readers, who were properly trained regarding lesion definition and identification, proficient in scoring and blinded to other clinical or study data. The pathologists who participated as blinded central readers have extensive experience as pathologists (14, 17, 20, and 28 years) and specifically in the field of gastrointestinal pathology (8, 9, 12, and 16 years, respectively).
The Geboes histologic score is an index commonly used to measure histologic disease activity in UC, which has undergone content and construct validation and reliability testing [31–34]. Patients are assigned a Geboes histologic score between 0 and 5.4, based on the results from an endoscopy with biopsy. Higher scores indicate greater levels of inflammation, with the scores used to distinguish between inactive disease (grade 0 or 1), mildly active disease (grade 2 or 3), or moderate to severely active disease (grade 4 or 5).
The U-ACHIEVE maintenance trial was conducted in accordance with the protocol, International Conference on Harmonization (ICH) guidelines, applicable regulations and guidelines governing clinical study conduct and the ethical principles that have their origin in the Declaration of Helsinki. All participants provided written informed consent before any study-related procedures were performed. All authors had access to the study data and reviewed and approved the final manuscript.
Composite histologic endoscopic endpoints
HEMI
HEMI is a composite endpoint defined as a Mayo endoscopic sub-score of 0 or 1 and a Geboes histologic score ≤ 3.1 [27, 28]. Achieving the HEMI endpoint indicates improvement in the macroscopic appearance of the mucosal surface, as well as improvement in the microscopic and cellular features characteristic of mucosal inflammation. The Mayo endoscopic sub-score of 0 or 1 is defined by the lack of marked erythema, no friability, absence of vascular pattern and erosions, and no spontaneous bleeding or ulcerations that are frequently observed in patients with moderate to severe UC.
HEMR
HEMR is a novel, more stringent composite endpoint defined as a Mayo endoscopic sub-score of 0 and a Geboes histologic score < 2.0. Achieving the HEMR endpoint indicates that the mucosa appears normal upon endoscopic inspection and that there are no neutrophils in crypts or lamina propria, and no increase in eosinophils, no crypt destruction, and no erosions, ulcerations, or granulation tissue.
Outcomes assessed at Week 52
Clinical outcomes included corticosteroid-free remission, sustained clinical response defined as a decrease in the Adapted Mayo score ≥ 2 points and ≥ 30% from baseline, plus a decrease in rectal bleeding score (RBS) ≥ 1 or an absolute RBS ≤ 1 at Week 8/16 that was maintained at Week 52, clinical remission per full and adapted Mayo score, endoscopic improvement and remission (RBS = 0, SFS ≤ 1), and fecal calprotectin (FCP) levels ≤ 250 μg/g and ≤ 150 μg/g (Table 1). In addition to RBS and SFS, other patient-reported outcome measures included the Functional Assessment of Chronic Illness Therapy–Fatigue (FACIT-F) questionnaire, Ulcerative Colitis Symptoms Questionnaire (UC-SQ), Inflammatory Bowel Disease Questionnaire (IBDQ), Short Form Health Survey (SF-36) physical component summary (PCS) and mental component summary (MCS) scores, European Quality of Life Five Dimensions Five Levels (EQ-5D-5L) index, and Work Productivity and Activity Impairment (WPAI) questionnaire.
Table 1.
Baseline characteristics of patients in U-ACHIEVE maintenance trial (intent-to-treat)
| Characteristic | Placebo (n = 149) | Upadacitinib 15 mg OD (n = 148) | Upadacitinib 30 mg OD (n = 154) |
|---|---|---|---|
| Female, n (%) | 64 (43.0) | 53 (35.8) | 68 (44.2) |
| Race, n (%) | |||
| Caucasian | 93 (62.4) | 97 (65.5) | 101 (65.6) |
| Black or African American | 6 (4.0) | 7 (4.7) | 3 (1.9) |
| Asian | 42 (28.2) | 44 (29.7) | 48 (31.2) |
| American Indian or Alaska Native | 0 | 0 | 0 |
| Native Hawaiian or other Pacific Islander | 1 (0.7) | 0 | 1 (0.6) |
| Multiple | 7 (4.7) | 0 | 1 (0.6) |
| Age (years), median (IQR) | 40.0 (21.0) | 40.0 (22.0) | 41.0 (7.0) |
| Disease duration (years), median (IQR) | 6.2 (8.6) | 6.4 (10.6) | 6.0 (9.7) |
| Previous failure with biologic therapy, n (%) | 81 (54.4) | 71 (48.0) | 73 (47.4) |
| Endoscopic sub-score | |||
| = 3, n (%) | 98 (65.8) | 100 (65.6) | 108 (70.1) |
| Mean ± SD | 2.7 ± 0.48 | 2.7 ± 0.47 | 2.7 ± 0.48 |
Baseline demographics and disease characteristics were measured at baseline during the induction studies
IQR interquartile range, OD once daily, SD standard deviation
Data analysis
Data from patients who achieved a clinical response after 8 or 16 weeks of upadacitinib induction treatment and received upadacitinib maintenance treatment were analyzed in this study. Non-responder imputation was conducted in all Week 52 outcomes with no special data handling for missing data because of corona virus disease of 2019 (COVID-19). Missing data were not imputed for HEMI or HEMR at Week 8/16.
To assess the relative importance of HEMR compared with HEMI, cross-sectional and predictive analyses were conducted on the following patient groups: (1) patients who achieved HEMR, (2) patients who achieved HEMI without HEMR, and (3) patients who did not achieve HEMI. The cross-sectional analysis examined the likelihood of achieving long-term clinical and patient-reported outcomes at Week 52 among patients who achieved HEMR or HEMI without HEMR versus those who did not achieve HEMI. Sensitivity analyses compared outcomes in patients with vs without HEMR and with vs without HEMI. Predictive analysis assessed the relationship between achieving HEMR or HEMI without HEMR at the end of induction (Week 8/16) and outcomes at Week 52.
Clinically meaningful improvement in patient-reported outcomes was assessed as the likelihood of achieving a change from baseline (before starting treatment) in the patient-reported outcome score ≥ the corresponding meaningful within-patient change threshold (MWPC). The MWPC thresholds for UC-SQ (≥ 10) and FACIT-F (≥ 5) were estimated from anchor- and distribution-based analyses of upadacitinib phase 2 data. MWPC thresholds for IBDQ (≥ 16), [35–37] SF-36 PCS and MCS (≥ 4.1), [38] EQ-5D-5L index (≥ 0.076), [39] and WPAI (work time missed ≥ 6.5, impairment while working ≥ 6.1, overall work impairment ≥ 7.3, activity impairment ≥ 8.5) [40] were extracted from published literature.
Odds ratios (ORs) with 95% confidence intervals (CIs) were derived from logistic regression adjusting for the following characteristics: histologic score at baseline (continuous), maintenance treatment dosage (high or low), gender (male or female), disease extent (left-sided or extensive), disease duration, and baseline age and weight.
The percentages of patients who achieved HEMR or HEMI without HEMR at end of induction (Week 8/16) and attained long-term clinical and patient-reported outcomes at Week 52 were determined. The percentages of patients achieving these histological endpoints at end of maintenance (Week 52) and clinical and patient-reported outcomes at Week 52 were also calculated. Chi-square test was used to determine the significance test for both mucosal healing endpoints (HEMR and HEMI without HEMR) compared with no HEMI.
To further assess the benefits of the HEMI/HEMR end points, the number of UC-related hospitalizations and surgeries was tabulated and stratified by mutually exclusive HEMI and HEMR categories at Week 8/16 (end of induction) and Week 52 (end of maintenance). However, it was not feasible to perform logistic regression analyses because of the limited number of hospitalizations and surgeries observed in the upadacitinib trial data.
Results
Demographic data showed that nearly half of the patients were previously treated with biologics
Median age and median duration of disease of the study population who participated in the maintenance trial were approximately 40 years and 6 years, respectively (Table 1). At least 47% of the study population previously failed biologic therapy and at least 65% had an endoscopic sub-score of 3 at baseline of induction study. Of 125 patients who had a clinical response at end of induction (Week 16), 41.6% lost their clinical response at Week 52 (end of maintenance).
Patients who achieved HEMR had greater likelihood of improvement in clinical and patient-reported outcomes than those who achieved HEMI alone
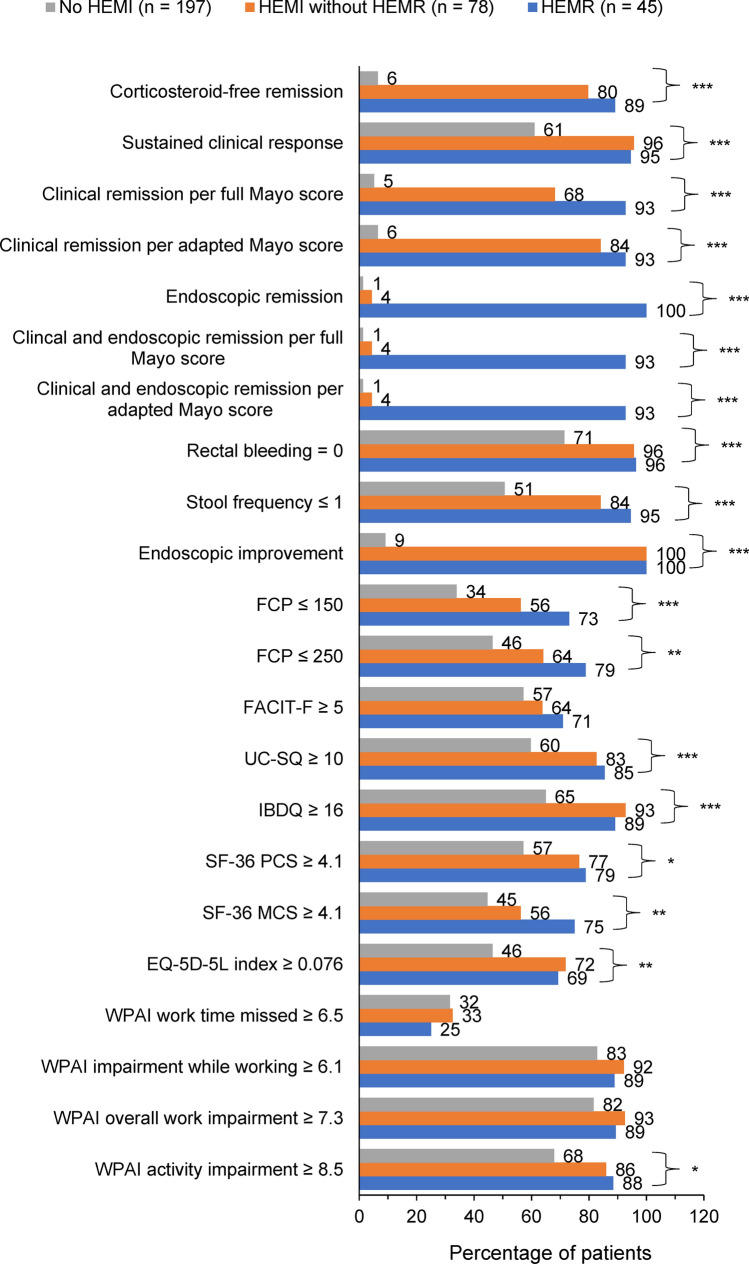
For the cross-sectional analysis, an equal or greater percentage of patients who achieved HEMR at Week 52 attained improved clinical outcomes and had clinically meaningful improvements from baseline in patient-reported outcomes at Week 52 compared with patients who achieved HEMI alone (Fig. 1). The cross-sectional regression analysis showed that patients who achieved HEMR (versus no HEMI) at Week 52 had significantly greater odds of attaining all clinical outcomes, including corticosteroid-free remission, sustained clinical response, and clinical and endoscopic remission per full Mayo and per adapted Mayo score at Week 52 (Table 2). These patients also had significantly greater odds of achieving clinically meaningful improvements in UC-SQ, IBDQ, SF-36 PCS, SF-36 MCS, EQ-5D-5L, and WPAI activity impairment at Week 52 (Table 2). Furthermore, the odds of improvement in all clinical outcomes and in FACIT-F, UC-SQ, SF-36 PCS, SF-36 MCS, and overall work impairment were numerically higher in patients who achieved HEMR vs no HEMI compared with those who achieved HEMI alone vs no HEMI.
Fig. 1.
Cross-sectional analysis: Percentage of patients who achieved HEMR or HEMI without HEMR at Week 52 (end of maintenance) and outcomes at Week 52. *p < 0.05, **p < 0.01, ***p ≤ 0.001 for joint significance of (HEMR and HEMI without HEMR) versus no HEMI. EQ-5D-5L European Quality of Life Five Dimensions Five Levels, FACIT-F Functional Assessment of Chronic Illness Therapy–Fatigue, FCP fecal calprotectin, HEMI histologic endoscopic mucosal improvement, HEMR histologic endoscopic mucosal remission, IBDQ Inflammatory Bowel Disease Questionnaire, SF-36 MCS Short Form Health Survey Mental Component Summary, SF-36 PCS Short Form Health Survey Physical Component Summary, UC-SQ Ulcerative Colitis Symptoms Questionnaire
Table 2.
Cross-sectional analyses: Adjusted odds ratios for HEMR or HEMI without HEMR at Week 52 (end of maintenance) and clinical outcomes at Week 52 in U-ACHIEVE
| Outcomes at Week 52a | Single regression per outcome for HEMR and HEMI without HEMR vs no HEMI | |||
|---|---|---|---|---|
| HEMR (n = 55b) vs no HEMI (n = 77b) | HEMI (n = 69b) without HEMR vs no HEMI (n = 77b) | |||
| Adjusted odds ratio (95% CI)c | p value | Adjusted odds ratio (95% CI)c | p value | |
| Clinical outcomes | ||||
| Corticosteroid-free remissionb,d | 476.3 (71.4, > 999.99) | < .001 | 119.2 (23.9, 594.9) | < .001 |
| Sustained clinical responseb,e | 25.0 (5.4, 115.5) | < .001 | 13.5 (3.3, 54.8) | < .001 |
| Clinical remission per full Mayo scoref | > 999.99 (180.2, > 999.99) | < .001 | 69.2 (16.1, 296.9) | < .001 |
| Clinical remission per adapted Mayo scoreb,g | > 999.99 (157.0, > 999.99) | < .001 | 253.4 (41.6, > 999.99) | < .001 |
| Endoscopic remissionb,h | N/A due to HEMR definition | 13.0 (0.6, 294.2) | .106 | |
| Clinical and endoscopic remission per full Mayo scoreb,f,h | > 999.99 (210.5, > 999.99) | < .001 | 5.2 (0.41, 65.4) | .204 |
| Clinical and endoscopic remission per adapted Mayo scoreb,g,h | > 999.99 (210.5, > 999.99) | < .001 | 5.2 (0.41, 65.4) | .204 |
| Rectal bleeding = 0b | 22.5 (4.0, 126.9) | < .001 | 7.3 (1.8, 29.2) | .005 |
| Stool frequency ≤ 1b | 34.2 (7.9, 147.4) | < .001 | 4.9 (2.0, 12.2) | < .001 |
| Endoscopic improvementb,i | N/A due to HEMI and HEMR definitions | |||
| FCP rangesj | ||||
| FCP ≤ 150 μg/g | 6.9 (2.7, 17.3) | < .001 | 3.7 (1.6, 8.6) | .003 |
| FCP ≤ 250 μg/g | 6.1 (2.4, 15.7) | < .001 | 2.8 (1.2, 6.4) | .015 |
| Clinically meaningful improvement from induction baseline in patient-reported outcomesk | ||||
| FACIT-F (≥ 5)b | 2.0 (0.9, 4.4) | .100 | 1.5 (0.7, 3.1) | .316 |
| UC-SQ (≥ 10)b | 4.5 (1.7, 11.9) | .002 | 2.9 (1.2, 6.9) | .016 |
| IBDQ (≥ 16)b | 4.2 (1.5, 11.7) | .007 | 7.0 (2.2, 22.6) | .001 |
| SF-36 PCS (≥ 4.1)j | 4.1 (1.6, 10.4) | .004 | 2.9 (1.2, 7.0) | .018 |
| SF-36 MCS (≥ 4.1)j | 4.9 (2.0, 12.2) | < .001 | 1.7 (0.8, 3.9) | .186 |
| EQ-5D-5L index (≥ 0.076)j | 4.5 (1.8, 11.1) | .001 | 4.8 (1.9, 12.0) | < .001 |
| WPAI | ||||
| Work time missed (≥ 6.5)l | 0.9 (0.3, 2.8) | .817 | 1.3 (0.4, 3.8) | .672 |
| Impairment while working (≥ 6.1)l | 2.7 (0.4, 16.5) | .281 | 3.0 (0.6, 15.0) | .191 |
| Overall work impairment (≥ 7.3)l | 3.4 (0.6, 19.6) | .173 | 2.9 (0.6, 14.0) | .197 |
| Activity impairment (≥ 8.5)j | 3.7 (1.3, 10.8) | .016 | 4.5 (1.6, 12.6) | .005 |
CI confidence interval, EQ-5D-5L European Quality of Life Five Dimensions Five Levels, FACIT-F Functional Assessment of Chronic Illness Therapy–Fatigue, FCP fecal calprotectin, HEMI histologic endoscopic mucosal improvement, HEMR histologic endoscopic mucosal remission, IBDQ Inflammatory Bowel Disease Questionnaire, N/A not applicable, NRI-NC non-responder imputation with no special data handling for missing due to COVID-19, SF-36 MCS Short Form Health Survey Mental Component Summary, SF-36 PCS Short Form Health Survey Physical Component Summary, UC ulcerative colitis, UC-SQ Ulcerative Colitis Symptoms Questionnaire, WPAI Work Productivity and Activity Impairment questionnaire
aNRI-NC was conducted in all Week 52 outcomes
bn = 201, includes patients who achieved a clinical response after 8 weeks or 16 weeks of upadacitinib induction treatment
cAdjusted for maintenance baseline Geboes histologic score, dosage, gender, age, weight, UC disease extent, UC disease duration, and use of extended therapy (16-week induction period). Due to the adjustment of covariates, patients with missing values on the covariates were dropped in the logistic regressions
dCorticosteroid-free remission was defined as achieving 90-day steroid-free clinical remission per adapted Mayo in patients who achieved clinical remission at the end of induction treatment
eSustained clinical response was defined as remained clinically responsive at the end of Week 52
fClinical remission per full Mayo was defined as total Mayo score ≤ 2 with no sub-score > 1
gClinical remission per adapted Mayo (full Mayo excluding physician’s global assessment) was defined as stool frequency sub-score ≤ 1 and not greater than baseline, rectal bleeding sub-score = 0, endoscopic sub-score ≤ 1 without friability
hEndoscopic remission defined as Mayo endoscopic sub-score = 0
iEndoscopic improvement defined as Mayo endoscopic sub-score ≤ 1
jn = 172, includes only patients who achieved a clinical response after 8 weeks of upadacitinib induction treatment
kClinically meaningful improvement in patient-reported outcomes was assessed as the likelihood of achieving a change from induction baseline in patient-reported outcome score ≥ the corresponding meaningful within-patient change threshold
lOnly includes patients who had baseline WPAI scores. n = 154 for work time missed, n = 142 for impairment while working, n = 154 for overall work impairment
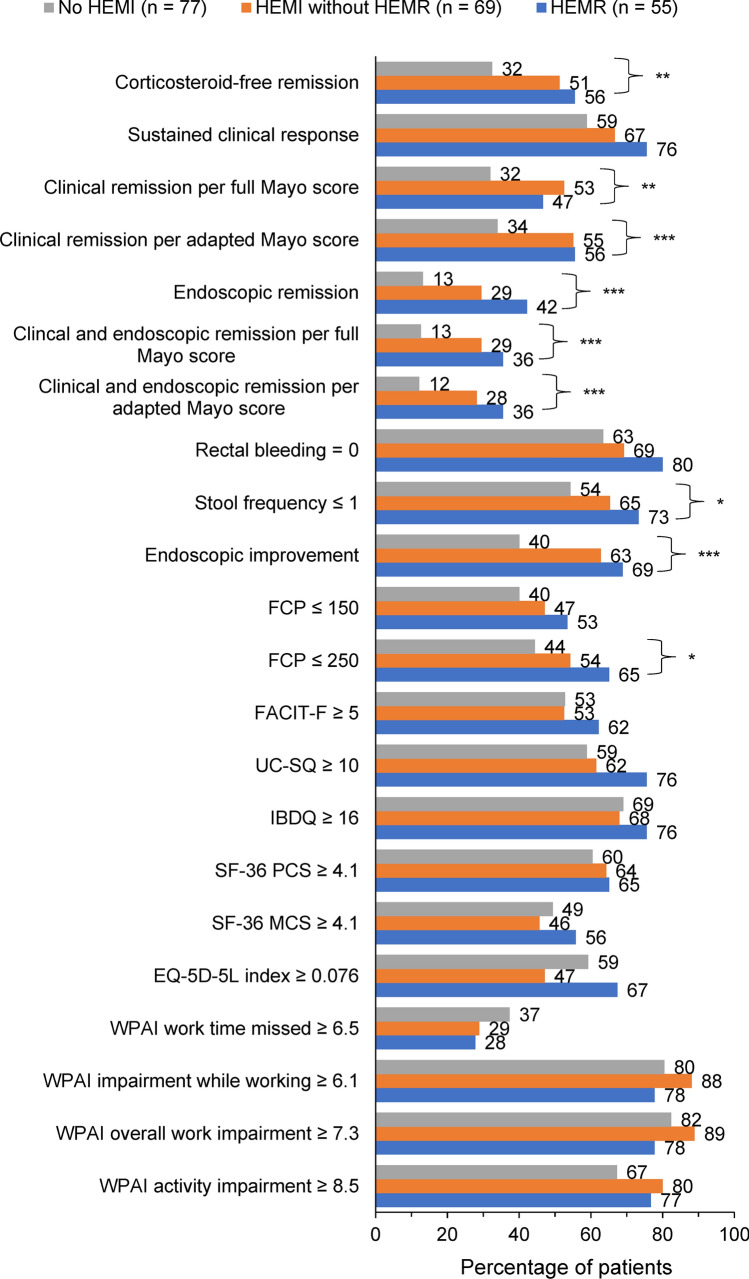
An equal or greater percentage of patients with HEMR at Week 8/16 attained clinical outcomes and had clinically meaningful improvements from baseline (before starting treatment) in patient-reported outcomes at Week 52 compared to patients who achieved HEMI without HEMR in the predictive analysis (Fig. 2). The predictive regression analyses showed that achievement of HEMR (vs no HEMI) at Week 8/16 was associated with independently and significantly greater odds of attaining all clinical outcomes (Table 3). Achievement of HEMR (vs no HEMI) at Week 8/16 was associated with greater odds of attaining clinically meaningful improvements from induction baseline in patient-reported outcomes at Week 52, although the improvements were not statistically significant (Table 3). Similar results were observed for patients who achieved HEMI versus no HEMI. However, it should be noted that the odds of attaining most clinical outcomes and improvements in most patient-reported outcomes were numerically higher in patients who achieved HEMR vs no HEMI compared with those who achieved HEMI alone vs no HEMI.
Fig. 2.
Predictive analysis: Percentage of patients who achieved HEMR or HEMI without HEMR at Week 8/16 (end of induction) and outcomes at Week 52 (end of maintenance). *p < 0.05, **p < 0.01, ***p ≤ 0.001 for joint significance of (HEMR and HEMI without HEMR) versus no HEMI. EQ-5D-5L European Quality of Life Five Dimensions Five Levels, FACIT-F Functional Assessment of Chronic Illness Therapy–Fatigue, FCP fecal calprotectin, HEMI histologic endoscopic mucosal improvement, HEMR histologic endoscopic mucosal remission, IBDQ Inflammatory Bowel Disease Questionnaire, SF-36 MCS Short Form Health Survey Mental Component Summary, SF-36 PCS Short Form Health Survey Physical Component Summary, UC-SQ Ulcerative Colitis Symptoms Questionnaire
Table 3.
Predictive analyses: adjusted odds ratios for HEMR or HEMI without HEMR at Week 8/16 (end of induction) and clinical outcomes at Week 52 in U-ACHIEVE
| Outcomes at Week 52a | Single regression per outcome for HEMR and HEMI without HEMR vs no HEMI | |||
|---|---|---|---|---|
| HEMR (n = 45b) vs no HEMI (n = 197b) | HEMI without HEMR (n = 78b) vs no HEMI (n = 197b) | |||
| Adjusted odds ratio (95% CI)ci | P value | Adjusted odds ratio (95% CI)c | P value | |
| Clinical outcomes | ||||
| Corticosteroid-free remissionb,d | 3.8 (1.8, 8.2) | < 0.001 | 2.8 (1.6, 5.1) | < 0.001 |
| Sustained clinical responseb,e | 2.8 (1.2, 6.5) | .014 | 1.9 (1.0, 3.4) | .044 |
| Clinical remission per full Mayo scoreb,f | 2.4 (1.2, 5.2) | .019 | 2.9 (1.6, 5.3) | < 0.001 |
| Clinical remission per adapted Mayo scoreb,g | 3.6 (1.7, 7.7) | .001 | 3.2 (1.7, 5.7) | < 0.001 |
| Endoscopic remissionb,h | 3.9 (1.7, 8.6) | < 0.001 | 2.4 (1.2, 4.7) | .010 |
| Clinical and endoscopic remission per full Mayo scoreb,f,h | 3.2 (1.4, 7.2) | .006 | 2.7 (1.4, 5.3) | .004 |
| Clinical and endoscopic remission per adapted Mayo scoreb,g,h | 3.5 (1.5, 7.9) | .003 | 2.7 (1.3, 5.3) | .006 |
| Rectal bleeding = 0b | 3.5 (1.4, 8.6) | .006 | 1.8 (1.0, 3.3) | .067 |
| Stool frequency ≤ 1b | 2.7 (1.2, 6.0) | .015 | 2.0 (1.1, 3.6) | .022 |
| Endoscopic improvementb,i | 5.0 (2.2, 11.3) | < 0.001 | 3.3 (1.8, 5.9) | < 0.001 |
| FCP rangesj | ||||
| FCP ≤ 150 μg/g | 2.3 (1.1, 5.0) | .032 | 1.6 (0.9, 2.9) | .122 |
| FCP ≤ 250 μg/g | 3.3 (1.5, 7.3) | .004 | 1.9 (1.1, 3.6) | .032 |
| Clinically meaningful improvement from induction baseline in patient-reported outcomesk | ||||
| FACIT-F (≥ 5)b | 1.5 (0.7, 3.0) | .314 | 1.1 (0.6, 1.9) | .753 |
| UC-SQ (≥ 10)b | 2.7 (1.2, 6.0) | .019 | 1.4 (0.8, 2.4) | .296 |
| IBDQ (≥ 16)b | 1.3 (0.6, 3.0) | .483 | 1.1 (0.6, 1.9) | .863 |
| SF-36 PCS (≥ 4.1)j | 1.2 (0.6, 2.6) | .603 | 1.4 (0.8, 2.6) | .277 |
| SF-36 MCS (≥ 4.1)j | 1.2 (0.6, 2.6) | .560 | 0.9 (0.5, 1.7) | .837 |
| EQ-5D-5L index (≥ 0.076)j | 1.4 (0.6, 3.0) | .413 | 0.8 (0.4, 1.4) | .348 |
| WPAI | ||||
| Work time missed (≥ 6.5)l | 0.8 (0.2, 2.8) | .758 | 0.7 (0.3, 1.6) | .414 |
| Impairment while working (≥ 6.1)l | 2.0 (0.4, 10.1) | .408 | 2.7 (0.8, 9.0) | .115 |
| Overall work impairment (≥ 7.3)l | 1.9 (0.4, 9.6) | .420 | 2.1 (0.7, 6.5) | .204 |
| Activity impairment (≥ 8.5)j | 1.6 (0.7, 3.6) | .298 | 2.4 (1.2, 5.0) | .016 |
CI confidence interval, EQ-5D-5L European Quality of Life Five Dimensions Five Levels, FACIT-F Functional Assessment of Chronic Illness Therapy–Fatigue, FCP fecal calprotectin, HEMI histologic endoscopic mucosal improvement, HEMR histologic endoscopic mucosal remission, IBDQ Inflammatory Bowel Disease Questionnaire, NRI-NC non-responder imputation with no special data handling for missing due to COVID-19, SF-36 MCS Short Form Health Survey Mental Component Summary, SF-36 PCS Short Form Health Survey Physical Component Summary, UC ulcerative colitis, UC-SQ Ulcerative Colitis Symptoms Questionnaire, WPAI Work Productivity and Activity Impairment questionnaire
aNRI-NC was conducted in all Week 52 outcomes. No missing data imputation was used for HEMI and HEMR at Week 8/16
bn = 320, includes patients who achieved a clinical response after 8 weeks or 16 weeks of upadacitinib induction treatment
cAdjusted for maintenance baseline Geboes histologic score, dosage, gender, age, weight, UC disease extent, UC disease duration, and use of extended therapy (16-week induction period). Due to the adjustment of covariates, patients with missing values on the covariates were dropped in the logistic regressions
dCorticosteroid-free remission was defined as achieving 90 days steroid-free clinical remission per adapted Mayo in patients who achieved clinical remission at the end of induction treatment
eSustained clinical response was defined as remained clinically responsive at the end of Week 52
fClinical remission per full Mayo was defined as total Mayo score ≤ 2 with no sub-score > 1
gClinical remission per adapted Mayo (full Mayo excluding physician’s global assessment) was defined as stool frequency sub-score ≤ 1 and not greater than baseline, rectal bleeding sub-score = 0, endoscopic sub-score ≤ 1 without friability
hEndoscopic remission defined as Mayo endoscopic sub-score = 0
iEndoscopic improvement defined as Mayo endoscopic sub-score ≤ 1
jn = 275, includes only patients who achieved a clinical response after 8 weeks of upadacitinib induction treatment
kClinically meaningful improvement in patient-reported outcomes was assessed as the likelihood of achieving a change from induction baseline in patient-reported outcome score ≥ the corresponding meaningful within-patient change threshold
lOnly includes patients who had baseline WPAI scores. n = 154 for work time missed, n = 142 for impairment while working, n = 154 for overall work impairment
Patients who achieved HEMR had improved outcomes compared with those who did not achieve HEMR
The cross-sectional regression analysis (Supplemental Table 1) demonstrated that achieving HEMR (versus not achieving HEMR) at Week 52 significantly increased the odds of achieving all clinical outcomes, as well as the increasing the odds of achieving clinically meaningful improvements from induction baseline in UC-SQ, SF-36 PCS, and SF-36 MCS. The predictive regression analysis (Supplemental Table 2) showed that achieving HEMR (versus not achieving HEMR) at Week 8/16 significantly increased the odds of attaining most clinical outcomes at Week 52 and numerically increased the odds of achieving clinically meaningful improvements from induction baseline in patient-reported outcomes at Week 52.
Patients who achieved HEMI had improved outcomes compared with those who did not achieve HEMI
The cross-sectional regression analysis demonstrated that achieving HEMI at Week 52 was associated with significantly greater odds of achieving all clinical outcomes at Week 52, as well as clinically meaningful improvements in UC-SQ, IBDQ, SF-36 PCS and MCS, EQ-5D-5L, and the WPAI activity impairment domain (Supplemental Table 1).
The predictive regression analysis showed that achieving HEMI (versus not achieving HEMI) at Week 8/16 was associated with significantly greater odds of achieving all clinical outcomes at Week 52 (Supplemental Table 2). The odds of achieving clinically meaningful improvements from induction baseline in patient-reported outcomes were increased, but not statistically significant for most outcomes.
Correlation of Geboes score with long-term clinical outcomes
The relationship between the Geboes score and long-term clinical outcomes was also evaluated. Patients were assigned to the following Geboes score intervals [0, 2.0), [2.0, 3.1], and (3.1, 5.4] based on the histologic evaluation of biopsy samples taken during the U-ACHIEVE maintenance trial. These intervals were selected to assess patients who achieved the histology threshold for mucosal healing (Geboes score < 2.0) and those who achieved the histologic mucosal improvement threshold without mucosal healing [2.0, 3.1]. Patients with GS < 2.0 at Week 52 had significantly greater odds of achieving all clinical endpoints at Week 52 compared to patients with GS > 3.1 (Supplemental Fig. 1). Patients with GS < 2.0 at end of induction had a significantly higher likelihood of achieving clinical remission per adapted Mayo score (OR = 1.8, P < 0.030) and endoscopic improvement (OR = 1.8, P < 0.015) at Week 52 than patients with GS > 3.1. Results were directionally similar, but not statistically significant, for all other outcomes (Supplemental Fig. 2).
In addition, we calculated Spearman correlations for the Geboes score and secondary measures at baseline and Week 8 (end of induction). At Week 8, moderate (r ≥ 0.3) to strong (r ≥ 0.5) positive Spearman correlations were found between the Geboes score and Mayo subscores as well as the Mayo Full and Partial score (Supplemental Table 3).
No hospitalizations or surgeries were observed during the 52-week maintenance phase in patients with HEMR at Week 8/16
None of the patients who achieved HEMR at Week 8/16 (n = 45) or Week 52 (n = 55) had an UC-related hospitalization or surgery during the 52-week maintenance phase (Supplemental Tables 4 and 5). In contrast, 4 patients who did not achieve HEMR (n = 275 by Week 8/16; n = 146 by Week 52) had an UC-related hospitalization or surgery during the 52-week maintenance phase. Of these 4 patients, 1 achieved HEMI only and the other 3 patients did not achieve HEMI.
Discussion
In this post hoc analysis of patients with UC treated with upadacitinib, we observed that both HEMR and HEMI were associated with better long-term clinical outcomes. Achievement of HEMR at Week 8/16 was associated with numerically greater odds of improved clinical and patient-reported outcomes at Week 52 compared with patients who achieved HEMI without HEMR. Also, no UC-related hospitalizations and surgeries were observed during the 52-week maintenance phase in patients who achieved HEMR at Week 8/16.
Results obtained in the present analysis support the long-term benefits to patients who achieve the stringent endpoint of HEMI. The HEMI endpoint combines both endoscopic and histologic improvement cutoffs (MES of 0 or 1 and Geboes histologic score ≤ 3.1, respectively). Patients who achieve the HEMI endpoint gained improvement in the macroscopic appearance of the mucosal surface as well as improvement in the microscopic and cellular features that are characteristic of mucosal inflammation [14, 31, 41]. The endoscopic improvement (MES = 0 or 1) portion of the HEMI score is defined by lack of marked erythema, no friability, absence of vascular pattern and erosions, and no spontaneous bleeding or ulcerations [31, 41]. Patients who achieve low MES scores (0 or 1) have been shown to have a lower risk of relapse than those who do not. Achieving the histologic threshold of Geboes histologic score ≤ 3.1 indicates that < 5% of the mucosal crypts have neutrophilic infiltrate and there is an absence of crypt destruction (Grade 4) or ulceration (Grade 5) [31]. Post hoc analysis of data from the VARSITY trial also supports the benefit of achieving histologic improvement [42]. Narula et al. demonstrated that a change in epithelial neutrophilic infiltrate (the defining feature of Geboes Grade 3) during induction was an accurate predictor of response (achievement of endoscopic and histologic improvement) to biologic therapy during maintenance treatment [42].
Results reported in this study also support previous evidence showing improved outcomes with an even stricter definition of mucosal healing. The HEMR endpoint (deep mucosal healing) combines both endoscopic and histologic remission cutoffs (MES = 0 and Geboes histologic score < 2.0, respectively) and requires that the mucosa appear normal upon endoscopic inspection, and that no acute inflammatory infiltrate (i.e., neutrophils and/or eosinophils) be present in either the crypts or lamina propria [31, 41]. The stricter definition of HEMR was associated with more favorable and durable long-term patient outcomes in our analysis. These results are consistent with previously published literature [12, 13, 42]. A longitudinal cohort study demonstrated that patients who achieved an MES of 0 were less likely to experience a relapse at 6 months than those with an MES of 1[12]. A meta-analysis confirmed that patients who achieved both of the more rigorous goals of endoscopic remission and histologic remission had a lower risk of clinical relapse at 1 year than patients who achieved only endoscopic remission [13]. Histologic remission (Geboes histologic score < 2.0) and its relation to patient outcomes, independent of endoscopic findings, was recently reviewed by Chateau et al., [14] and it was shown to be associated with more favorable prognoses and outcomes. The importance of early histologic remission is suggested by the findings of Choi et al. [43] who reported that the risk of colorectal neoplasia in UC is significantly correlated with the total amount of inflammatory damage accumulated over time. Histologic remission has also been associated with lower rates of high-grade dysplasia and colon cancer [44]. We also demonstrated that achievement of histologic remission (Geboes histologic < 2.0) at the end of induction or maintenance with upadacitinib was associated with the greatest likelihood of achieving desirable clinical and patient-reported outcomes after 1 year of maintenance treatment. Finally, our results show that HEMR has greater predictive power for clinical remission and endoscopic remission, as well as UC-SQ, compared to HEMI without HEMR, which further demonstrates the greater clinical relevance of this more stringent measure of deep mucosal healing. To our knowledge, no other clinical trials aside from U-ACHIEVE have evaluated HEMR as a primary or ranked secondary endpoint. Thus, the effects of other treatments on HEMR have not been evaluated. Until such studies are performed, it is difficult to predict whether findings obtained with other treatments would be similar to those obtained with upadacitinib.
Strengths of this study include patients’ receiving standard treatment and follow-up, as well as centralized histologic assessments as defined in the clinical trial protocol. Although previous studies have shown improved clinical outcomes with mucosal healing, these studies have not examined long-term outcomes in terms of histologic remission. Our study evaluated a unique composite endpoint of endoscopic histologic remission, which was defined as Mayo endoscopic score = 0 and Geboes histologic score < 2.0. To our knowledge, this is the first study that assesses the benefits of achieving mucosal healing based on this stringent endoscopic and histologic measure. We acknowledge a number of limitations in our study. One limitation of this study is that logistic regression analyses were not performed on hospitalizations and surgeries because of the limited number of patients who were hospitalized or had surgery in the upadacitinib data. While this study used data from a randomized clinical trial, residual confounding may still arise from factors not included as covariates in the logistic regression. In addition, although full colonoscopy was performed for all participants at screening, the biopsy specimen was taken only from the rectosigmoid region and it is possible that a more proximal segment may, in some cases, have a greater histologic disease burden than the rectosigmoid regions. Furthermore, our results are based on data from Phase 3 clinical trials, which may not correspond to outcomes observed in routine clinical practice. Finally, outcomes in the maintenance study were assessed after a relatively short period of time (at Week 52). However, additional data is currently being collected in an ongoing long-term extension study, which will allow analysis of outcomes for a longer period of time.
Conclusions
Based on our analysis of upadacitinib maintenance trial data, the stringent mucosal healing endpoints, HEMI, and HEMR were both clinically relevant with HEMR being associated with the greatest likelihood of improvement in long-term clinical and patient-reported outcomes. Early HEMI and HEMR are independent predictors of later remission and improved quality of life. Data on achieving the endoscopic remission and histologic remission endpoints and their benefit on long-term outcomes in upadacitinib-treated patients support HEMI and HEMR as desirable treatment goals in UC.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contribution
Conceptualization: FSL, YSG. Data curation: HD, FSL. Formal analysis: HD, EA, FSL. Funding acquisition: YSG. Investigation: all authors. Methodology: HD, EA, FSL, YSG. Project administration: YSG, FSL. Resources: YSG. Software: HD, EA. Supervision: YSG. Validation: HD, EA. Visualization: HD, EA. Writing—original draft: EA, YSG. Writing—review and editing: all authors.
Funding
Financial support for the study was provided by AbbVie. AbbVie participated in the interpretation of data, review, and approval of the manuscript. All authors contributed to the development of the article and maintained control over the final content. No honoraria or payments were made for authorship. RC Ungaro is supported by an NIH K23 Career Development Award K23KD111995-01A1.
Declarations
Conflict of interest
G Parkes received personal payments/honoraria/speaker fees and/or accepted travel grants or fellowships from AbbVie, Allergan, BMS, Celltrion, Ferring, Galapagos, Janssen, Napp, Takeda, and Tillotts; and is a Director and Shareholder in Ampersand Health. RC Ungaro served as an advisory board member or consultant for AbbVie, BMS, Janssen, Pfizer, and Takeda and has research grants from AbbVie, BI, and Pfizer. S Danese has received consultancy fees from AbbVie, Allergan, Amgen, AstraZeneca, Biogen, BI, Celgene, Celltrion, Ferring, Gilead, Hospira, Janssen, Johnson & Johnson, MSD, Mundipharma, Pfizer, Roche, Sandoz, Takeda, TiGenix, UCB, and Vifor Pharma. MT Abreu has been a consultant or served on an advisory board for, served as a teacher, lecturer, or speaker for, and/or has received grants/research support from AbbVie, Alimentiv, Arena, BMS, Gilead, Imedex, Intellisphere, LLC (HSCG) HCP Live Institutional Perspectives in GI, Janssen, Lilly, Microba, Pfizer, Prime, Prometheus, Takeda, and UCB. E Arenson is an employee of Adelphi Values and provides scientific support services within the pharmaceutical and device development industry. W Zhou, D Ilo, FS Laroux, and Y Sanchez Gonzalez are employees of AbbVie and may own stock or stock options. H Deng is a research fellow and student at University of Illinois at Chicago. L Peyrin-Biroulet received personal fees from Galapagos, AbbVie, Janssen, Genentech, Ferring, Tillots, Celltrion, Takeda, Pfizer, Index Pharmaceuticals, Sandoz, Celgene, Biogen, Samsung Bioepis, Inotrem, Allergan, MSD, Roche, Arena, Gilead, Amgen, BMS, Vifor, Norgine, Mylan, Lilly, Fresenius Kabi, OSE Immunotherapeutics, Enthera, Theravance, Pandion Therapeutics, Gossamer Bio, Viatris, Thermo Fisher; grants from Abbvie, MSD, Takeda, Fresenius Kabi; has stock options with CTMA.
Writing assistance
Medical writing assistance was provided by Joann Hettasch, Ph.D., of Fishawack Facilitate Ltd., part of Fishawack Health, and was funded by AbbVie Inc., North Chicago, IL.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
8/5/2023
Supplementary file was missing from this article and has now been uploaded.
References
- 1.Ungaro R, Mehandru S, Allen PB, et al. Ulcerative colitis. Lancet. 2017;389:1756–1770. doi: 10.1016/S0140-6736(16)32126-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Turner D, Ricciuto A, Lewis A, et al. STRIDE-II: an update on the selecting therapeutic targets in inflammatory bowel disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): determining therapeutic goals for treat-to-target strategies in IBD. Gastroenterology. 2021;160:1570–1583. doi: 10.1053/j.gastro.2020.12.031. [DOI] [PubMed] [Google Scholar]
- 3.Colombel JF, Rutgeerts P, Reinisch W, et al. Early mucosal healing with infliximab is associated with improved long-term clinical outcomes in ulcerative colitis. Gastroenterology. 2011;141:1194–1201. doi: 10.1053/j.gastro.2011.06.054. [DOI] [PubMed] [Google Scholar]
- 4.Feagins LA, Melton SD, Iqbal R, et al. Clinical implications of histologic abnormalities in colonic biopsy specimens from patients with ulcerative colitis in clinical remission. Inflamm Bowel Dis. 2013;19:1477–1482. doi: 10.1097/MIB.0b013e318281f4ae. [DOI] [PubMed] [Google Scholar]
- 5.Shah SC, Colombel JF, Sands BE, et al. Mucosal healing Is associated with improved long-term outcomes of patients with ulcerative colitis: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2016;14:1245–1255.e1248. doi: 10.1016/j.cgh.2016.01.015. [DOI] [PubMed] [Google Scholar]
- 6.Rutter M, Saunders B, Wilkinson K, et al. Severity of inflammation is a risk factor for colorectal neoplasia in ulcerative colitis. Gastroenterology. 2004;126:451–459. doi: 10.1053/j.gastro.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 7.Rutter MD, Saunders BP, Wilkinson KH, et al. Cancer surveillance in longstanding ulcerative colitis: endoscopic appearances help predict cancer risk. Gut. 2004;53:1813–1816. doi: 10.1136/gut.2003.038505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gupta RB, Harpaz N, Itzkowitz S, et al. Histologic inflammation is a risk factor for progression to colorectal neoplasia in ulcerative colitis: a cohort study. Gastroenterology. 2007;133:1099–105 (quiz 1340–1091). [DOI] [PMC free article] [PubMed]
- 9.Christensen B, Rubin DT. Understanding endoscopic disease activity in IBD: How to incorporate It into practice. Curr Gastroenterol Rep. 2016;18:5. doi: 10.1007/s11894-015-0477-6. [DOI] [PubMed] [Google Scholar]
- 10.D'Haens G, Sandborn WJ, Feagan BG, et al. A review of activity indices and efficacy end points for clinical trials of medical therapy in adults with ulcerative colitis. Gastroenterology. 2007;132:763–786. doi: 10.1053/j.gastro.2006.12.038. [DOI] [PubMed] [Google Scholar]
- 11.Ikeya K, Hanai H, Sugimoto K, et al. The ulcerative colitis endoscopic index of severity more accurately reflects clinical outcomes and long-term prognosis than the Mayo endoscopic score. J Crohns Colitis. 2016;10:286–295. doi: 10.1093/ecco-jcc/jjv210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barreiro-de Acosta M, Vallejo N, de la Iglesia D, et al. Evaluation of the risk of relapse in ulcerative colitis according to the degree of mucosal healing (Mayo 0 vs 1): a longitudinal cohort study. J Crohns Colitis. 2016;10:13–19. doi: 10.1093/ecco-jcc/jjv158. [DOI] [PubMed] [Google Scholar]
- 13.Yoon H, Jangi S, Dulai PS, et al. Incremental benefit of achieving endoscopic and histologic remission in patients with ulcerative colitis: a systematic review and meta-analysis. Gastroenterology. 2020;159:1262–1275.e1267. doi: 10.1053/j.gastro.2020.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chateau T, Feakins R, Marchal-Bressenot A, et al. Histological remission in ulcerative colitis: under the microscope Is the cure. Am J Gastroenterol. 2020;115:179–189. doi: 10.14309/ajg.0000000000000437. [DOI] [PubMed] [Google Scholar]
- 15.Parkes G, Ungaro R, Danese S, et al. Correlation of histological assessment of mucosal healing with long-term clinical and patientreported outcomes in patients with moderately to severely active ulcerative colitis treated with upadacitinib: results from the Phase 3 U-ACHIEVE maintenance trial. J Crohns Colitis. 2022;16(S1):i477–478. [Google Scholar]
- 16.Bryant RV, Winer S, Travis SP, et al. Systematic review: histological remission in inflammatory bowel disease. Is 'complete' remission the new treatment paradigm? An IOIBD initiative. J Crohns Colitis. 2014;8:1582–97. [DOI] [PubMed]
- 17.Korelitz BI. Mucosal healing as an index of colitis activity: back to histological healing for future indices. Inflamm Bowel Dis. 2010;16:1628–1630. doi: 10.1002/ibd.21268. [DOI] [PubMed] [Google Scholar]
- 18.Rosenberg L, Nanda KS, Zenlea T, et al. Histologic markers of inflammation in patients with ulcerative colitis in clinical remission. Clin Gastroenterol Hepatol. 2013;11:991–996. doi: 10.1016/j.cgh.2013.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Riley SA, Mani V, Goodman MJ, et al. Microscopic activity in ulcerative colitis: what does it mean? Gut. 1991;32:174–178. doi: 10.1136/gut.32.2.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Azad S, Sood N, Sood A. Biological and histological parameters as predictors of relapse in ulcerative colitis: a prospective study. Saudi J Gastroenterol. 2011;17:194–198. doi: 10.4103/1319-3767.80383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bessissow T, Lemmens B, Ferrante M, et al. Prognostic value of serologic and histologic markers on clinical relapse in ulcerative colitis patients with mucosal healing. Am J Gastroenterol. 2012;107:1684–1692. doi: 10.1038/ajg.2012.301. [DOI] [PubMed] [Google Scholar]
- 22.Bitton A, Peppercorn MA, Antonioli DA, et al. Clinical, biological, and histologic parameters as predictors of relapse in ulcerative colitis. Gastroenterology. 2001;120:13–20. doi: 10.1053/gast.2001.20912. [DOI] [PubMed] [Google Scholar]
- 23.Hefti MM, Chessin DB, Harpaz NH, et al. Severity of inflammation as a predictor of colectomy in patients with chronic ulcerative colitis. Dis Colon Rectum. 2009;52:193–197. doi: 10.1007/DCR.0b013e31819ad456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bryant RV, Burger DC, Delo J, et al. Beyond endoscopic mucosal healing in UC: histological remission better predicts corticosteroid use and hospitalisation over 6 years of follow-up. Gut. 2016;65:408–414. doi: 10.1136/gutjnl-2015-309598. [DOI] [PubMed] [Google Scholar]
- 25.Christensen B, Hanauer SB, Erlich J, et al. Histologic normalization occurs in ulcerative colitis and is associated with improved clinical outcomes. Clin Gastroenterol Hepatol. 2017;15:1557–1564.e1551. doi: 10.1016/j.cgh.2017.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jairath V, Peyrin-Biroulet L, Zou G, et al. Responsiveness of histological disease activity indices in ulcerative colitis: a post hoc analysis using data from the TOUCHSTONE randomised controlled trial. Gut. 2019;68:1162–1168. doi: 10.1136/gutjnl-2018-316702. [DOI] [PubMed] [Google Scholar]
- 27.Li K, Marano C, Zhang H, et al. Relationship between combined histologic and endoscopic endpoints and efficacy of ustekinumab treatment in patients with ulcerative colitis. Gastroenterology. 2020;159:2052–2064. doi: 10.1053/j.gastro.2020.08.037. [DOI] [PubMed] [Google Scholar]
- 28.Danese S, Vermeire S, Zhou W, et al. Upadacitinib as induction and maintenance therapy for moderately to severely active ulcerative colitis: results from three phase 3, multicentre, double-blind, randomised trials. Lancet. 2022;399:2113–2128. doi: 10.1016/S0140-6736(22)00581-5. [DOI] [PubMed] [Google Scholar]
- 29.Sandborn WJ, Feagan BG, Wolf DC, et al. Ozanimod induction and maintenance treatment for ulcerative colitis. N Engl J Med. 2016;374:1754–1762. doi: 10.1056/NEJMoa1513248. [DOI] [PubMed] [Google Scholar]
- 30.Peyrin-Biroulet L, Loftus EV, Jr, Colombel JF, et al. Histologic outcomes with vedolizumab versus adalimumab in ulcerative colitis: results from an efficacy and safety study of vedolizumab intravenous compared to adalimumab subcutaneous in participants with ulcerative colitis (VARSITY) Gastroenterology. 2021;161:1156–1167.e1153. doi: 10.1053/j.gastro.2021.06.015. [DOI] [PubMed] [Google Scholar]
- 31.Geboes K, Riddell R, Ost A, et al. A reproducible grading scale for histological assessment of inflammation in ulcerative colitis. Gut. 2000;47:404–409. doi: 10.1136/gut.47.3.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bressenot A, Salleron J, Bastien C, et al. Comparing histological activity indexes in UC. Gut. 2015;64:1412–1418. doi: 10.1136/gutjnl-2014-307477. [DOI] [PubMed] [Google Scholar]
- 33.Mosli MH, Parker CE, Nelson SA, et al. Histologic scoring indices for evaluation of disease activity in ulcerative colitis. Cochrane Database Syst Rev. 2017;5:Cd011256. [DOI] [PMC free article] [PubMed]
- 34.Mosli MH, Feagan BG, Zou G, et al. Development and validation of a histological index for UC. Gut. 2017;66:50–58. doi: 10.1136/gutjnl-2015-310393. [DOI] [PubMed] [Google Scholar]
- 35.Gregor JC, McDonald JW, Klar N, et al. An evaluation of utility measurement in Crohn's disease. Inflamm Bowel Dis. 1997;3:265–276. doi: 10.1097/00054725-199712000-00004. [DOI] [PubMed] [Google Scholar]
- 36.Irvine EJ. Development and subsequent refinement of the inflammatory bowel disease questionnaire: a quality-of-life instrument for adult patients with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 1999;28:S23–27. doi: 10.1097/00005176-199904001-00003. [DOI] [PubMed] [Google Scholar]
- 37.Irvine EJ. Quality of life of patients with ulcerative colitis: past, present, and future. Inflamm Bowel Dis. 2008;14:554–565. doi: 10.1002/ibd.20301. [DOI] [PubMed] [Google Scholar]
- 38.Coteur G, Feagan B, Keininger DL, et al. Evaluation of the meaningfulness of health-related quality of life improvements as assessed by the SF-36 and the EQ-5D VAS in patients with active Crohn's disease. Aliment Pharmacol Ther. 2009;29:1032–1041. doi: 10.1111/j.1365-2036.2009.03966.x. [DOI] [PubMed] [Google Scholar]
- 39.Stark RG, Reitmeir P, Leidl R, et al. Validity, reliability, and responsiveness of the EQ-5D in inflammatory bowel disease in Germany. Inflamm Bowel Dis. 2010;16:42–51. doi: 10.1002/ibd.20989. [DOI] [PubMed] [Google Scholar]
- 40.Sandborn WJ, Reilly MC, Brown MCJ, et al. Minimally important difference for WPAI:CD scores: defining relevant impact on work productivity in active Crohn's disease. Gastroenterology. 2007;102:S472. [Google Scholar]
- 41.Marchal Bressenot A, Riddell RH, Boulagnon-Rombi C, et al. Review article: the histological assessment of disease activity in ulcerative colitis. Aliment Pharmacol Ther. 2015;42:957–967. doi: 10.1111/apt.13375. [DOI] [PubMed] [Google Scholar]
- 42.Narula N, Wong ECL, Colombel JF, et al. Early change in epithelial neutrophilic infiltrate predicts long-term response to biologics in ulcerative colitis. Clin Gastroenterol Hepatol. 2022;20:1095–1104. [DOI] [PubMed]
- 43.Choi CR, Al Bakir I, Ding NJ, et al. Cumulative burden of inflammation predicts colorectal neoplasia risk in ulcerative colitis: a large single-centre study. Gut. 2019;68:414–422. doi: 10.1136/gutjnl-2017-314190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Korelitz BI, Sultan K, Kothari M, et al. Histological healing favors lower risk of colon carcinoma in extensive ulcerative colitis. World J Gastroenterol. 2014;20:4980–4986. doi: 10.3748/wjg.v20.i17.4980. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.