Abstract
Though antibiotics have been used for decades to treat bacterial infections, there is a great need for new treatment methods. Bacteria are becoming resistant to conventional antibiotics, as is the case with Methicillin resistant Staphylococcus aureus (MRSA). Herein we report the design of a series of lipidated α/Sulfono-α-AA heterogeneous peptides as mimics for Host Defense Peptides (HDPs). Utilizing fluorescence microscopy and depolarization techniques, our compounds demonstrate the ability to kill Gram-positive bacteria through cell membrane disruption. This mechanism of action makes it difficult for bacteria to develop resistance. Further time kill studies and hemolytic assays have also proven these compounds to be efficient in their ability to eradicate bacteria cells while remaining non-toxic to human red blood cells. This new class of peptidomimetics shows promise for the future antibiotic treatment of MRSA.
Keywords: Host Defense Peptides, Peptidomimetics, Bacterial resistance, Lipidation, Antimicrobial
1. Introduction
For many years it has been the convention to use antibiotics in the treatment of bacterial infections. However, the overuse and misuse of these drugs has led to bacteria resistant to treatment.1,2 This occurrence has caused concern about the ability to tackle infections and is considered by the Center of Disease Control (CDC) to be a matter of global priority.3 The World Health Organization (WHO) also considers bacterial resistance to be a world-wide challenge, suggesting that negligence of this issue will lead to efforts like major surgery or chemotherapy becoming ineffective due to further illness or even death caused by these microbes.4
MRSA is a Gram-positive bacterium, while contagious in person to person contact it is also nosocomial.5–9 It leaves at risk patients who are in a hospital setting that are elderly, immunocompromised, have wounds from surgery or are using medically invasive devices.10 Throughout the years the rate of deaths caused by MRSA in the Intensive Care Unit has grown from 20% to 60%11; it is because of this threat that scientists continue to work on novel methods for the treatment.
When observing the study of bacterial eradiation, much attention has been drawn to Host Defense Peptides (HDPs).12,13 These are natural short and cationic peptides that are first responders to infection.14 They kill bacteria by means of immunomodulation and by disruption of the cell membrane.15,16 Their ability to disrupt the cell membrane is what leaves them less susceptible to bacterial resistance.17 HDPs can vary in their structure but they all share an amphiphilic nature, which consists of an overall positive charge and hydrophobic groups that allow for selectivity and penetration of the anionic, lipid based bacterial cell membrane.18–20 These compounds have become of much interest as they are believed to be the alternative strategy to combat antibiotic resistance.21,22
While peptidomimetics or peptide derivatives of HDPs allow for therapeutics that have a low propensity to develop resistance, there are some drawbacks in terms of selectivity for bacterial cells, stability, cost and efficiency of synthesis.23 Compound with small molecular weight but similar mechanism of action could be more preferred.24,25 Recently our group has designed a class of peptidomimetics – α-AApeptides based on the backbone of the α-chiral peptide nucleic acid (PNA)26–28 (Fig. 1). Our previous findings have shown that α-AApeptides are a new class of antimicrobial peptidomimetics by mimicking host-defense peptides.29,30 Among them, a subclass of low-molecular-weight peptide hybrids, containing a lipid tail, a canonical amino acid, and α-AApeptide building block, show enhanced broad spectrum antibacterial activity, the ability to eradicate bacterial cells efficiently, low toxicity and the ability to clear bacterial biofilm.31 Based on our previous studies, we speculated that a related class of antimicrobial peptidomimetics, lipidated α/sulfono-α-AA heterogeneous peptides could also possess the potential for the treatment of bacterial resistance.
Fig. 1.
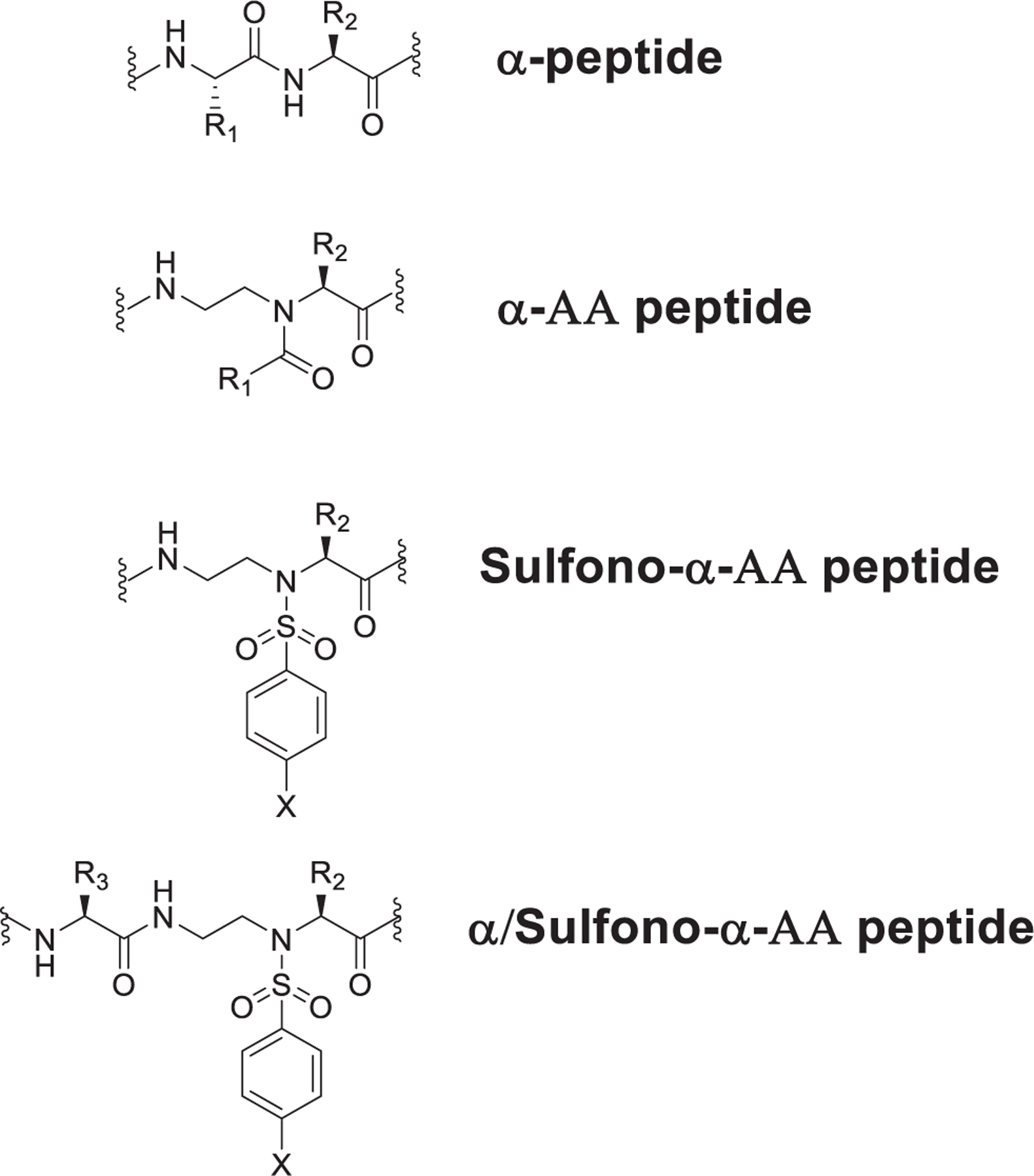
Structure of α-peptide, α-AA peptide, Sulfono-α-AA peptide, α /Sulfono-α-AA peptide, and lipidated α/sulfono-α-AA peptide.
2. Results and discussion
As such, we synthesized a series of sequences that contain a canonical lysine amino acid to introduce a positive charge for selective electrostatic interaction with bacterial cells which generally bear negatively charged membranes,31 a α-sulfono-AApeptide building block for hydrophobicity, and a lipid tail for added lipophilicity; which allows for binding and penetration of the bacterial cell membranes.32
All sequences were synthesized by the utilization of Solid Phase Peptide Synthesis (SPPS, Scheme 1; please also see supporting information for details), and their structures are shown in Fig. 2.
Scheme 1.
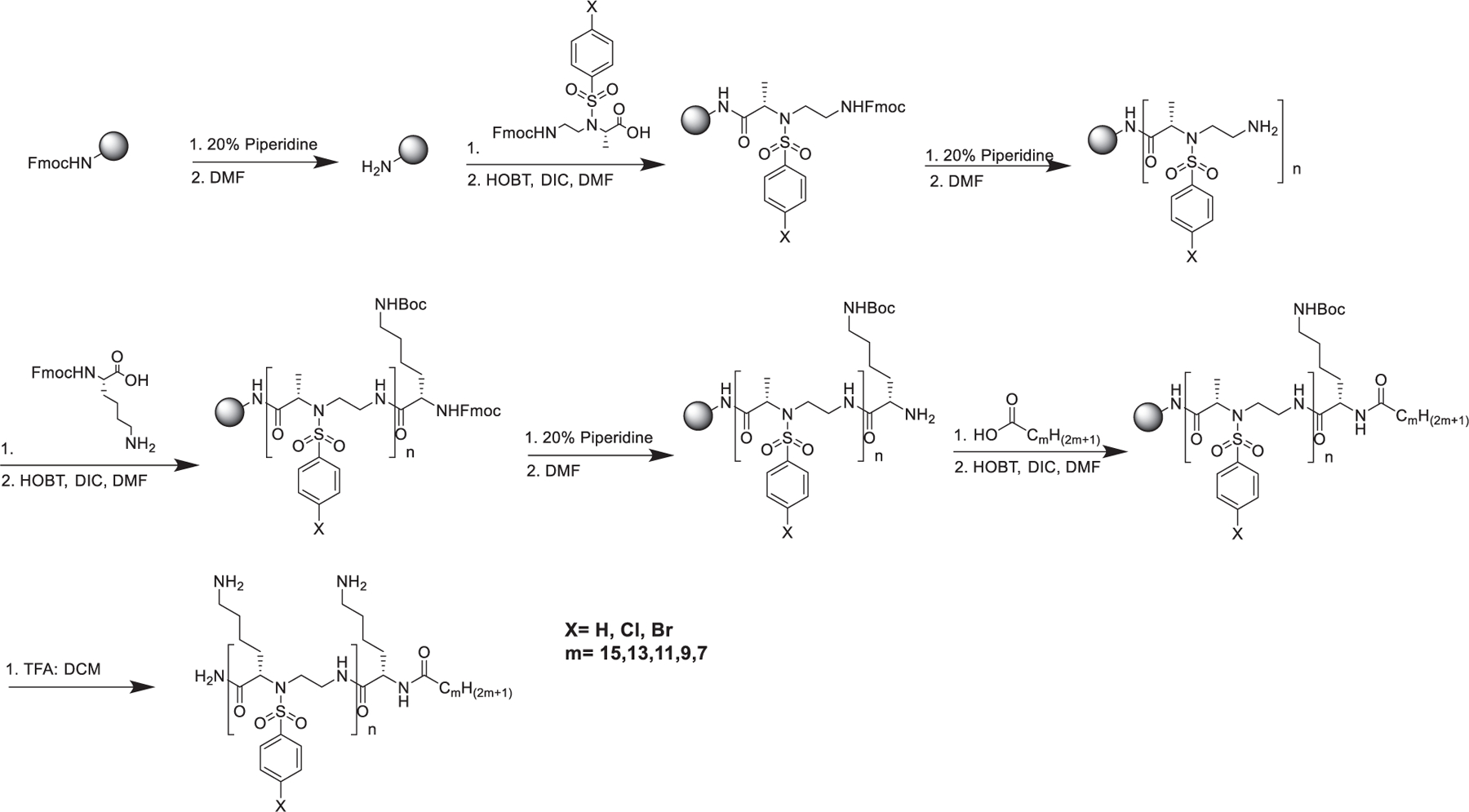
Synthetic scheme for the preparation of Lipidated α/Sulfono-α-AA heterogeneous peptides by SPPS.
Fig. 2.
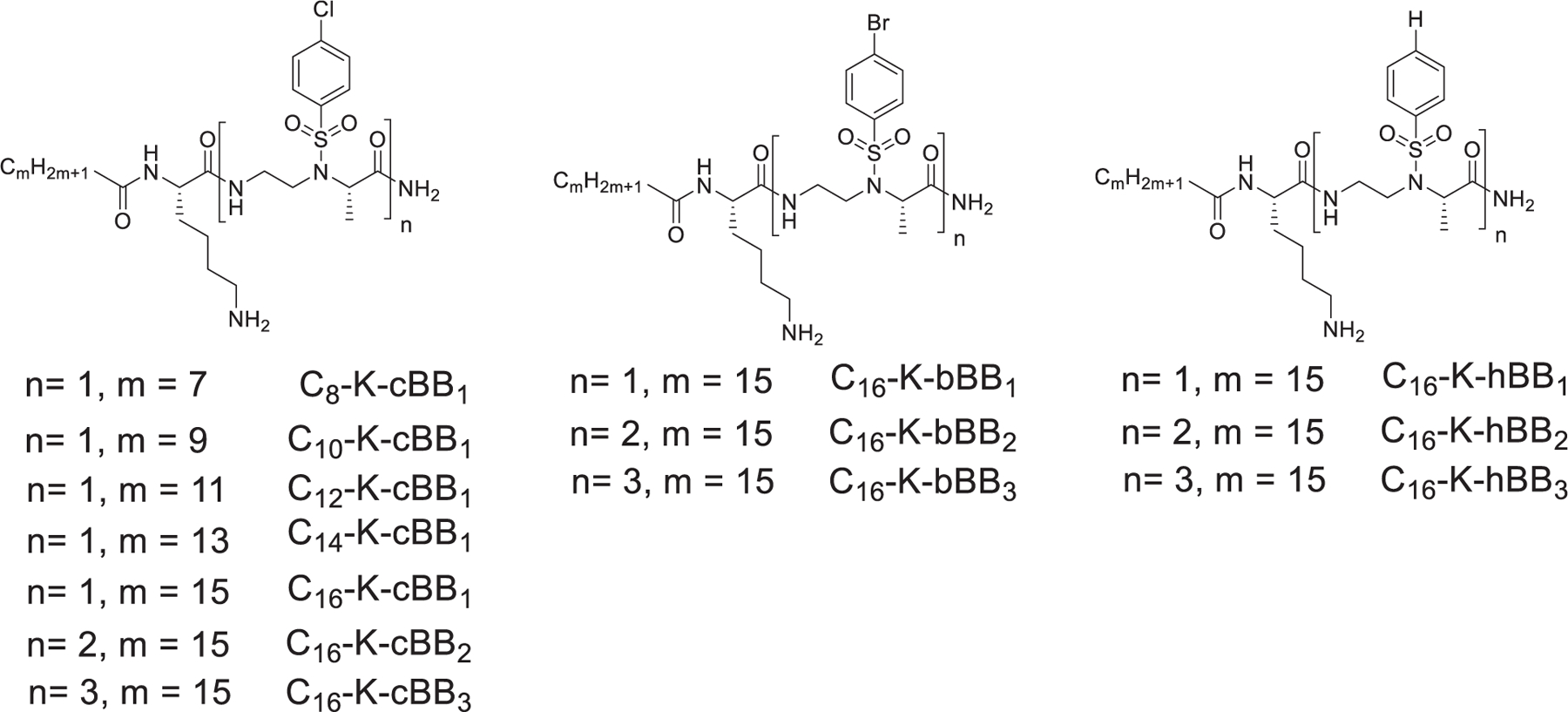
Final sequences synthesized by SPPS, indicating their varying number of building blocks and length of lipid tails.
After the completion of synthesis, the sequences were tested Gram-positive bacteria Methicillin resistant Staphylococcus aureus (MRSA).
As anticipated, C8-K-hBB1 and C10-K-bBB1 did not show any antibacterial activity, consistent to our previous findings that short lipid tails could not aid in penetrating bacterial membranes.33 However, to our delight, C12-K-cBB1, C14-K-cBB1, and C16-K-cBB1 all exhibited excellent activity against MRSA. In particular, C16-K-cBB1 is highly potent, with a MIC of 1 µg/mL. Meanwhile, this compound also has very good selectivity, as it has weak hemolytic activity. Interestingly, as the number of building blocks were increased, antimicrobial activity against MRSA decreased in the order of C16-K-bBB2, and C16-K-cBB3. Indeed, C16-K-hBB3, C16-K-bBB3, and C16-K-cBB3 showed little to no activity against MRSA. This phenomenon suggest the proper balance of hydrophobicity and hydrophilicity is desirable for antimicrobial activity.34 Furthermore, the substitution of different halogens on the side chain influences the hydrophobicity of the compound, hence changing the way the compounds interact with bacterial cells, as seen for C16-K-cBB1, C16-K-bBB1 and C16-K-hBB1. Interestingly, both Cl and Br substitution on the aromatic ring lead to better activity (Table 1.).
Table 1.
Antimicrobial activity of compounds shown in Fig. 2 against various bacterial strains. — indicates that activity was not tested.
| MIC (µg/mL) |
Hemolytic data HC50 (µg/mL) |
Selectivity of MRSA: HC50/ MICMRSA (µg/mL) |
|
|---|---|---|---|
| Compound | Gram + | ||
| C 8 -K-cBB 1 | > 25 | — | — |
| C 10 -K-cBB 1 | > 25 | — | — |
| C 12 -K-cBB 1 | 5 | — | — |
| C 14 -K-cBB 1 | 5 | — | — |
| C 16 -K-cBB 1 | 1 | 62.5 | 62.5 |
| C 16 -K-cBB 2 | 10 | — | — |
| C 16 -K-cBB 3 | > 25 | — | — |
| C 16 -K-bBB 1 | 2 | — | — |
| C 16 -K-bBB 2 | 5 | — | — |
| C 16 -K-bBB 3 | > 25 | — | — |
| C 16 -K-hBB 1 | 5 | — | — |
| C 16 -K-hBB 2 | 2 | — | — |
| C 16 -K-hBB 3 | 10 | — | — |
As the peptidomimetics for HDPs, they are expected to exert antimicrobial activity based on cell membrane disruption. As such, depolarization was carried out to probe the mechanism of action. The depolarization (Fig. 3) of MRSA cells was tested after the cells were treated with C16-K-cBB1. The results show an increase in fluorescence intensity with time, suggesting that the active compound was able to disrupt the cell membrane of the bacterial cells.
Fig. 3.
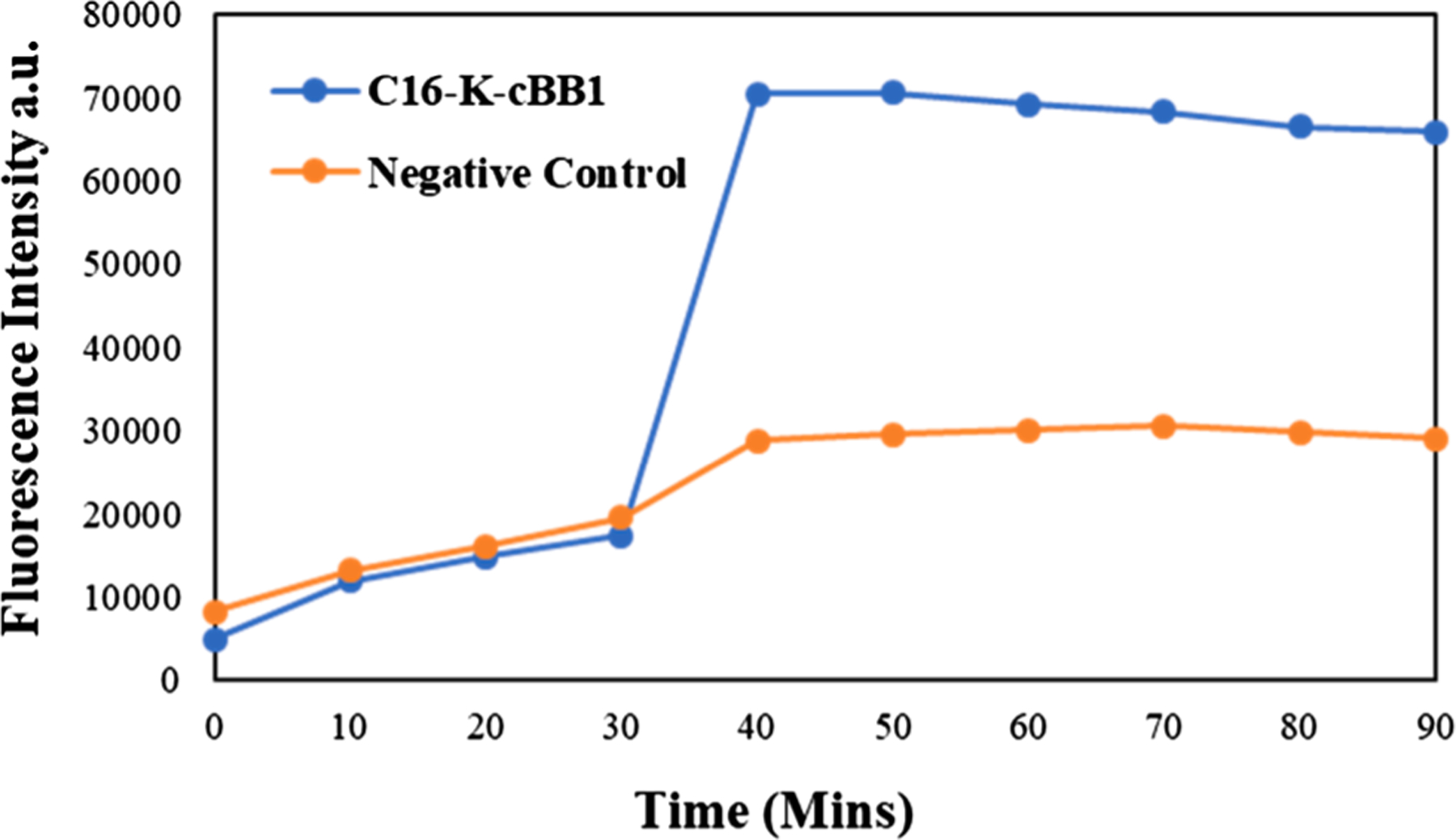
Bacterial cells treated with C16-K-cBB1 showed increased depolarization with time.
The action on the membrane was further investigated by fluorescence microscopy (Fig. 4). Cells were treated with active compound and analyzed to determine the presence of 4′,6-diamidino-2-phenylindole (DAPI) which stains both live and dead cells and Pyridium iodide (PI) which can only enter the cells through disrupted cells membrane. Fig. 4c shows the presence of PI in cells that were treated with C16-K-cBB1 after 2 h which is representative of dead MRSA cells.
Fig. 4.
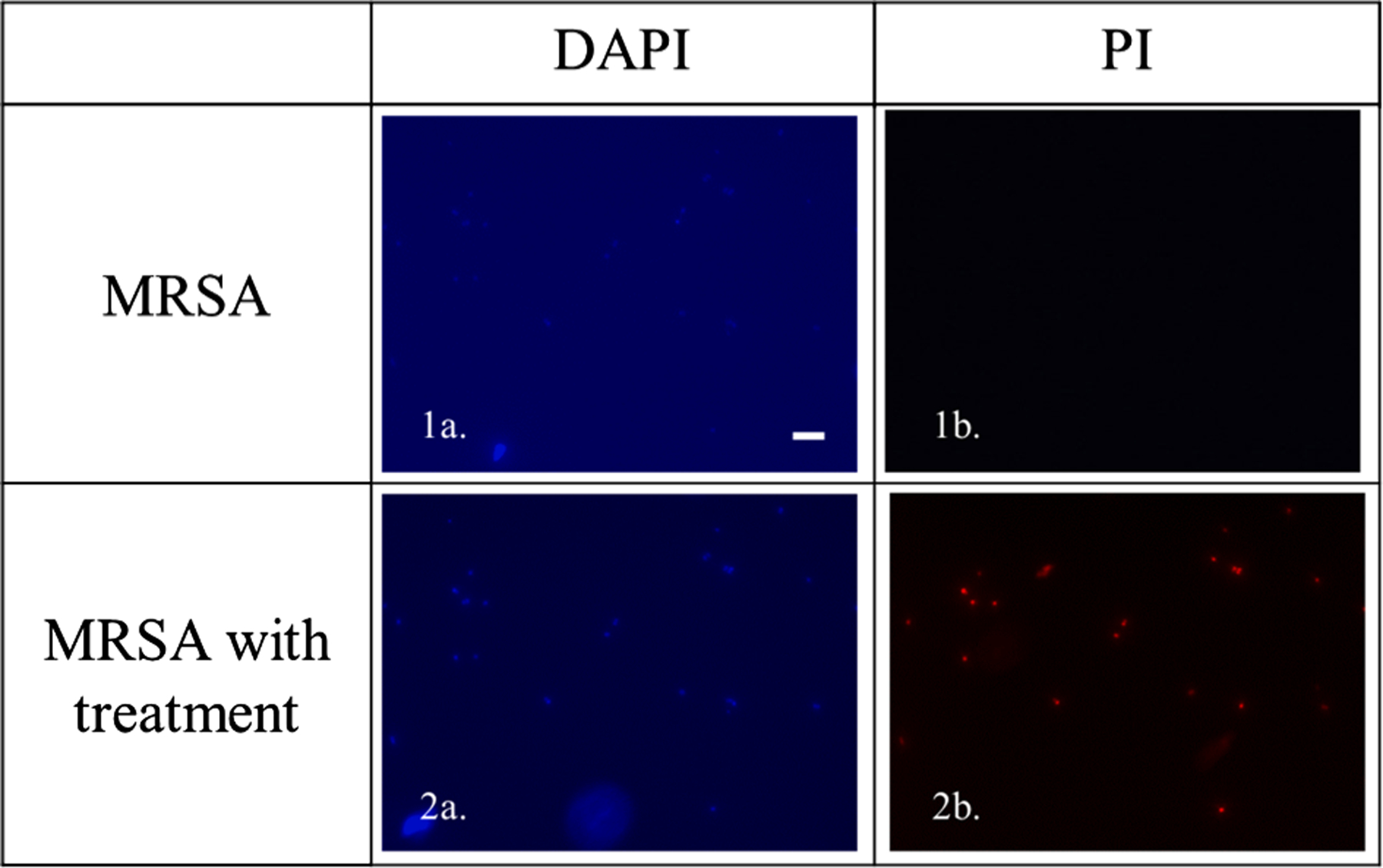
Fluorescence data of cells stained with DAPI and PI, untreated and treated with C16-K-cBB1 for 2 h (Scale bar 10 μM).
Apart from the mechanism of cell eradication by C16-K-cBB1 we also wanted to determine its efficiency of clearing MRSA infection through time-kill assay (Fig. 5).
Fig. 5.
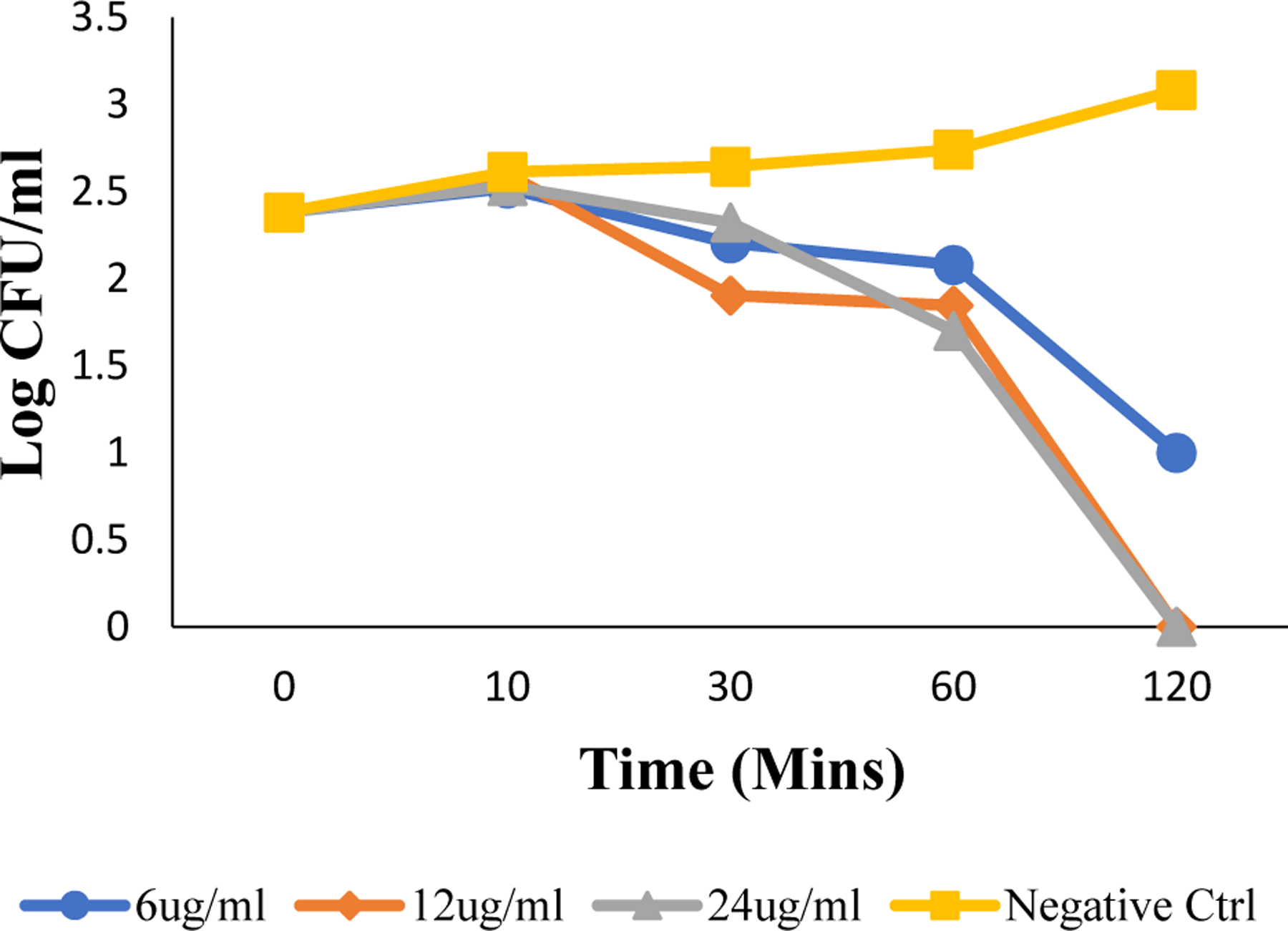
Time dependent killing efficiency assay as determined by the log of colony forming units per mL against time in minutes for Gram-positive MRSA.
Time kill assay indicated that C16-K-cBB1 was able to kill bacterial cells in a matter of 120 min at a concentration of 12.5 μg/mL suggesting that our compound is kinetically favorable as it relates to the clearing of bacteria.
3. Experimental procedure
3.1. General overview
All compounds were synthesized using solid phase peptide synthesis. To achieve this a peptide reaction vessel clamped on a Burrell Wrist-Action Shaker containing Rink amide with MBHA resin (100–200 mesh, 0.64 mmol/g) from Chem-Impex International, Inc was used. The compounds were then purified by High performance liquid chromatography (HPLC) utilizing a preparative C18 column (5 μm, 9 × 250 mm) and their molecular weight was confirmed using quadrupole time of flight mass spectrometer. The final products were then dried on a labcono lyophilizer. All other chemicals were purchased for either Sigma Aldrich or Fischer unless otherwise stated.
3.2. Solid phase peptide synthesis27:
This method was conducted by using Rink amide MBHA resin (100 mg) in a peptide reaction vessel on a shaker. 20% piperidine in dimethylformamide (DMF) was first added to the resin for 15 min to remove Fmoc group. This process was done twice and after each time the beads were washed with both 3 mL DMF and methylene chloride (DCM). Afterward, the building block (2 equiv.), hydroxybenzotriazole (HOBT) (6 equiv.) and N, N′-Diisopropylcarbodiimide (DIC) (6 equiv.) were shaken for 10 min in DMF, and then added to the vessel and left to shake overnight. The beads were washed with DMF and DCM. Fmoc group was removed, and either one more building block, or the lysine amino acid, or a lipid tail, was added to the beads, depending on the compound to be synthesized. The sequence of synthesis was continued until the desired compound was completed. A 1:1 ratio of trifluoroacetic acid (TFA): DCM was added for 2 h to cleave the final compound from the resin. The mixture was collected, dried using an air source, and the residue was dissolved in water. This was then purified by HPLC.
3.3. Minimum Inhibition Concentration (MIC) – antimicrobial assay35:
This assay is used to determine the smallest concentration of compound required to inhibit bacteria. This experiment utilized Gram-positive specimen Methicillin Resistant Staphylococcus aureus (MRSA, ATCC 33591). To initiate this protocol one colony of bacteria was taken from an agar plate and grown overnight at 37 °C in tryptic soy broth (TSB) media. Afterward, one more colony was taken from this batch and grown to mid-logarithm phase. 50 μL of 106 CFU/mL of bacteria was then obtained and added every well in 96 well plate followed by 50 μL of diluted lipidated α/Sulfono-α-AA peptides (100 to 0.5 μg/mL). These plated were incubated at 37 °C for 18 h and the MIC was determined at an optical density of 600 nm by using a Biotek microplate reader. All experiments were done in triplicates, each time being duplicates and the control was the bacteria not treated with any peptides. MIC was determined as the lowest concentration needed to inhibit bacterial growth.
3.4. Hemolytic assay34:
The percent hemolysis was determined by using the formula: % Hemolysis = (Abssample − AbsPBS)/(AbsTriton − AbsPBS) × 100. Controls were used to determine 0% hemolysis by incubation in phosphate buffered saline (PBS) and 100% hemolysis by treatment with 0.1% Triton-X-100 in PBS. To obtain these results human red blood cells were obtained and washed with PBS. After a few washed with PBS the cells were centrifuged at 1000g for 10 min. The red blood cells were then resuspended in PBS after the supernatant was removed. The resuspension of red blood cells was further diluted to obtain a 5% volume by volume concentration. C16-K-cBB1 was the further diluted two-fold into a 96 well plate to contain a total of 50 μL in each well and 50 μL of 5% volume by volume red blood cells were added to each well. The plate was then centrifuged for 10 min at 3500 rpm after being stored at 37 °C for 1 h. 100 μL of PBS was then used to dilute the supernatant and a Biotek microtiter plate reader (Synergy HT) was used to detect hemoglobin at 540 nm.
3.5. Fluorescence microscopy31:
This method utilized two dyes: 4′,6 diamidino-2-phenylindole dihydrochloride (DAPI, Sigma, > 98%) and propidium iodide (PI, Sigma). A combination of both these dyes were used to analyze the eradication of bacteria based on our active compound C16-K-cBB1. This is so because DAPI has a great ability to enter live cells and bind to the A-T abundant portions of double stranded DNA allowing for very intense fluorescence. PI on the other hand only breaches the wall of dead cells. To determine the viability of MRSA, bacterial cells were cultured to the mid-logarithmic phase, at 37 °C with C16-K-cBB1 (5 μg/mL) for 4 h. These cells were then centrifuged for 15 min at 3000g and the supernatant was discarded to obtain the pellets which were washed with PBS and incubated with DAPI (10 μg/mL) at 0 °C for 15 min in the dark. Afterwards DAPI was washed with 1 × PBS to remove any excess dye. This process was then repeated with PI (5 μg/mL) and the controls, however, for the controls the cells were not incubated with C16-K-cBB1. A Zeiss Axio Imager Z1 optical microscope (100×) was then used to analyze the cells.
3.6. Depolarization assay24:
After bacterial cells were grown to mid-log phase they were washed with 5 mM HEPES followed by 5 mM glucose, and re-suspended in a 1:1:1 ratio (108 CFU/mL) of 5 mM HEPES, 5 mM glucose and 100 nM of KCl. To a 96 well plate, 200 µM of suspension solution and 2 µM of 3,3′-Dipropylthiadicarbocyanine iodide (DiSC3) was added and monitored by fluorescence for 30 min at room temperature at the wavelengths of 622 nm for excitation and 670 nm for emission. Once the minimum value of fluorescence was obtained the cells in the 96 well plate was treated with the lead compound and a decrease in potential was observed with the increase in fluorescence. Three trials of the experiment were done with duplicates each time.
3.7. Time kill assay26:
This assay was done in to determine the efficiency of the peptide C16-K-cBB1 for the eradication of bacteria. To achieve this, the bacteria were incubated with different concentrations of the peptide at 0, 10, 20, 30, 60 and 120 min. From each time interval 100 μL of bacterial suspension was taken and further diluted. The diluted suspensions were incubated at for 20 h at 37 °C on agar plates and the colonies counted. All experiments were done in triplicates.
4. Conclusion
It can be concluded that we have established α/Sulfono-α-AA heterogeneous peptides as a new class of lipidated peptides. They show effective activity against clinically relevant Gram-positive bacterial stains as they are mimics for HDPs and can eradicate bacteria efficiently while expressing limited toxicity to mammalian cells. Our compound design indicates that lipidation, hydrophobicity, and positive charge, are crucial for the selectivity of specific bacterial cells and the disruption of bacterial cell membrane leaving them less susceptible to resistance. Therefore, lipidated α/Sulfono-α-AA heterogeneous peptides show potential as new generation of antibiotic agents with potential therapeutic applications for MRSA.
Supplementary Material
Acknowledgments
The work was supported by NSF 1708500 and NIH 1R01GM112652.
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary material
The supporting information consist of High-performance liquid chromatography (HPLC) information on purity of compounds and the molecular weight as determined by quadrupole time of flight mass spectrometer..Supplementary data to this article can be found online at https://doi.org/10.1016/j.bmc.2019.115241.
References
- 1.Ventola CL. The antibiotic resistance crisis. Pharm Ther 2015;40:277–283. [PMC free article] [PubMed] [Google Scholar]
- 2.Shallcross LJ, Davies DSC. Antibiotic overuse: a key driver of antimicrobial resistance. Br J Gen Pract 2014;64:604–605. 10.3399/bjgp14X682561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.CDC. Antibiotic resistance threatens everyone Centers for disease control and prevention; Published September 10, 2018. https://www.cdc.gov/drugresistance/index.html. Accessed 17 December 2018. [Google Scholar]
- 4.Prestinaci F, Pezzotti P, Pantosti A. Antimicrobial resistance: a global multifaceted phenomenon. Pathog Glob Health 2015;109:309–318. 10.1179/2047773215Y.0000000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alebachew Woldu M Klebsiella pneumoniae and Its growing concern in healthcare settings. Clin Exp Pharmacol 2016;6. 10.4172/2161-1459.1000199. [DOI] [Google Scholar]
- 6.Karchmer AW, Bayer AS. Methicillin-resistant Staphylococcus aureus: an evolving clinical challenge. Clin Infect Dis 2008;46(suppl 5):S342–S343. 10.1086/533589. [DOI] [PubMed] [Google Scholar]
- 7.Kampmeier S, Kossow A, Clausen LM, et al. Hospital acquired vancomycin resistant enterococci in surgical intensive care patients – a prospective longitudinal study. Antimicrob Resist Infect Control 2018;7. 10.1186/s13756-018-0394-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Joob B, Wiwanitkit V. Cancerous patients and outbreak of Escherichia coli: an important issue in oncology. Asian Pac J Trop Dis 2014;4:204–206. 10.1016/S2222-1808(14)60506-5. [DOI] [Google Scholar]
- 9.Rice LB. Antimicrobial resistance in gram-positive bacteria. Am J Infect Control 2006;34(5 suppl 1):S11–S19. 10.1016/j.ajic.2006.05.220 discussion S64–73. [DOI] [PubMed] [Google Scholar]
- 10.Raygada JL, Levine DP. Methicillin-resistant Staphylococcus aureus: a growing risk in the hospital and in the community. Am Health Drug Benefits 2009;2:86–95. [PMC free article] [PubMed] [Google Scholar]
- 11.Haddadin AS, Fappiano SA, Lipsett PA. Methicillin resistant Staphylococcus aureus (MRSA) in the intensive care unit. Postgrad Med J 2002;78:385–392. 10.1136/pmj.78.921.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lo JCY, Lange D. Current and potential applications of host-defense peptides and proteins in urology. BioMed Res Int 2015;2015. 10.1155/2015/189016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chellat MF, Raguž L, Riedl R. Targeting antibiotic resistance. Angew Chem Int Ed Engl 2016;55:6600–6626. 10.1002/anie.201506818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choi K-Y, Chow LNY, Mookherjee N. Cationic host defence peptides: multifaceted role in immune modulation and inflammation. J Innate Immun 2012;4:361–370. 10.1159/000336630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gupta S, Bhatia G, Sharma A, Saxena S. Host defense peptides: an insight into the antimicrobial world. J Oral Maxillofac Pathol JOMFP 2018;22:239–244. 10.4103/jomfp.JOMFP_113_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mansour SC, Pena OM, Hancock REW. Host defense peptides: front-line immunomodulators. Trends Immunol 2014;35:443–450. 10.1016/j.it.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 17.Ageitos JM, Sánchez-Pérez A, Calo-Mata P, Villa TG. Antimicrobial peptides (AMPs): ancient compounds that represent novel weapons in the fight against bacteria. Biochem Pharmacol 2017;133:117–138. 10.1016/j.bcp.2016.09.018. [DOI] [PubMed] [Google Scholar]
- 18.Zasloff M Antimicrobial peptides of multicellular organisms. Nature 2002;415:389–395. 10.1038/415389a. [DOI] [PubMed] [Google Scholar]
- 19.Pag U, Oedenkoven M, Papo N, Oren Z, Shai Y, Sahl H-G. In vitro activity and mode of action of diastereomeric antimicrobial peptides against bacterial clinical isolates. J Antimicrob Chemother 2004;53:230–239. 10.1093/jac/dkh083. [DOI] [PubMed] [Google Scholar]
- 20.Sani M-A, Separovic F How membrane-active peptides get into lipid membranes. Acc Chem Res 2016;49:1130–1138. 10.1021/acs.accounts.6b00074. [DOI] [PubMed] [Google Scholar]
- 21.Huang W, Seo J, Willingham SB, et al. Learning from host-defense peptides: cationic, amphipathic peptoids with potent anticancer activity. PLoS ONE 2014;9:e90397. 10.1371/journal.pone.0090397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Porter EA, Weisblum B, Gellman SH. Mimicry of host-defense peptides by unnatural oligomers: antimicrobial β-peptides. J Am Chem Soc 2002;124:7324–7330. 10.1021/ja0260871. [DOI] [PubMed] [Google Scholar]
- 23.Chongsiriwatana NP, Patch JA, Czyzewski AM, et al. Peptoids that mimic the structure, function, and mechanism of helical antimicrobial peptides. Proc Natl Acad Sci U S A 2008;105:2794–2799. 10.1073/pnas.0708254105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Niu Y, Wang M, Cao Y, et al. Rational design of dimeric lysine N-alkylamides as potent and broad-spectrum antibacterial agents. J Med Chem 2018;61:2865–2874. 10.1021/acs.jmedchem.7b01704. [DOI] [PubMed] [Google Scholar]
- 25.Teng P, Huo D, Nimmagadda A, et al. Small antimicrobial agents based on acylated reduced amide scaffold. J Med Chem 2016;59:7877–7887. 10.1021/acs.jmedchem.6b00640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.She F, Nimmagadda A, Teng P, Su M, Zuo X, Cai J. Helical 1:1 α/sulfono-γ-AA heterogeneous peptides with antibacterial activity. Biomacromolecules 2016;17:1854–1859. 10.1021/acs.biomac.6b00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Padhee S, Smith C, Wu H, et al. The development of antimicrobial α-AApeptides that suppress proinflammatory immune responses. ChemBioChem 2014;15:688–694. 10.1002/cbic.201300709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Niu Y, Wu H, Li Y, et al. AApeptides as a new class of antimicrobial agents. Org Biomol Chem 2013;11:4283. 10.1039/c3ob40444g. [DOI] [PubMed] [Google Scholar]
- 29.Li Y, Smith C, Wu H, et al. Short antimicrobial lipo-α/γ-AA hybrid peptides. ChemBioChem 2014;15:2275–2280. 10.1002/cbic.201402264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bolarinwa O, Cai J. Developments with investigating descriptors for antimicrobial AApeptides and their derivatives. Expert Opin Drug Discov 2018;13:727–739. 10.1080/17460441.2018.1487950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singh S, Nimmagadda A, Su M, Wang M, Teng P, Cai J. Lipidated α/α-AA heterogeneous peptides as antimicrobial agents. Eur J Med Chem 2018;155:398–405. 10.1016/j.ejmech.2018.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cavallo G, Metrangolo P, Milani R, et al. The halogen bond. Chem Rev 2016;116:2478–2601. 10.1021/acs.chemrev.5b00484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Niu Y, Padhee S, Wu H, et al. Lipo-γ-AApeptides as a new class of potent and broad-spectrum antimicrobial agents. J Med Chem 2012;55:4003–4009. 10.1021/jm300274p. [DOI] [PubMed] [Google Scholar]
- 34.Su M, Xia D, Teng P, et al. Membrane-active hydantoin derivatives as antibiotic agents. J Med Chem 2017;60:8456–8465. 10.1021/acs.jmedchem.7b00847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nimmagadda A, Liu X, Teng P, et al. Polycarbonates with potent and selective antimicrobial activity toward gram-positive bacteria. Biomacromolecules 2017;18:87–95. 10.1021/acs.biomac.6b01385. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


