Abstract
Introduction
Ukraine has a high prevalence of co-occurring disorders (COD), defined as having both substance use (SUD) and psychiatric disorders. Major depressive disorder (MDD) is the most prevalent psychiatric disorder among people with SUD. People with COD experience poor health outcomes, and international agencies propose integrated COD care. In Ukraine, treatment for SUD is delivered in specialized substance use clinics, without providing any other medical services for comorbidities, including MDD. Here we present the protocol, along the with the preliminary results of the MEDIUM project, including observations over the first 6 months.
Methods
A cluster-randomized type-2 hybrid trial was conducted to integrate MDD treatment into specialty clinics providing opioid agonist therapies (OAT) in Ukraine. Twelve clinics in four regions underwent randomization to control (N=1) vs experimental arms (N=2) in each region. Clinicians at experimental sites received tele-education through modified project ECHO using a facilitated screening, evaluation, and treatment algorithm of depression, with or without financial incentives. Service-, patient- and provider-level data were collected for the analysis every 6 months for 24 months.
Preliminary Results
For service delivery outcomes, 4421 patients enrolled on OAT across all sites were assessed for MDD for screening (76.7%), evaluation with diagnosis (43.5%) and treatment (30.7%) for MDD; 13.8% continued treatment at least for 6 months. For patient-level outcomes, 1345 patients and 54 providers participated in serial surveys every six months.
Conclusion
This study will be the first to explore integrated COD care in Ukraine and generate evidence on effective service integration and delivery strategies for people with COD receiving treatment at substance use clinics with broader implications for Eastern Europe and Central Asia region.
Keywords: Opioid Agonist Therapies (OAT), People who inject drugs (PWID), Opioids, Depression, Co-occurring disorders (COD), Implementation science, Ukraine
1. Introduction
Ukraine is a middle-income country in Eastern Europe that has a high prevalence of psychiatric (PD) and substance use disorders (SUD).1,2 PD like major depressive disorder (MDD) and SUD like opioid and alcohol use disorder are especially high. When they co-occur, termed co-occurring disorders (COD) or dual disorders,3 they include the combination of at least one PD and one SUD identified in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5).4,5 COD are common,6,7 and result in poorer psychosocial functioning, higher frequency of emergency room visits and hospitalization, a higher burden of disease, and worse health-related quality of life (HRQoL) for people with COD relative to those with either PD or SUD alone.6,8–10
Among the ~355,000 people who inject drugs (PWID) in Ukraine,11 over 80% have opioid use disorder (OUD); nearly all opioids are injected as oral prescription opioids are not readily available. The prevalence of PDs among PWID often exceeds 50%,6 with MDD being the most common PD among PWID.6,12,13 Independent of COD, PWID have poor health outcomes, including from high prevalence of medical comorbidities like HIV, viral hepatitis (HBV, HCV), tuberculosis, and other conditions.14–16 Furthermore, PWID and people with PDs experience stigma and discrimination,17,18 which may be heightened when PDs and OUD co-occur.
Maintenance with opioid agonist therapies (OAT) like methadone or buprenorphine are the most effective treatment for of OUD.19,20 Systematic reviews confirm that OAT consistently improves health outcomes by decreasing illicit opioid use, injection and injection-related risks, sex-related risk behavior, risk of HIV and HCV infections and overdose;21,22 OAT improves many social conditions like employment and interpersonal relationships while decreasing criminal activity.23 Similarly, the best evidence-based treatment for MDD is with antidepressants, including selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs).24
Organization of healthcare systems can greatly influence services delivery.25 Though there has been recent reform, Ukraine’s health system is built on the Soviet Semashko model, which prioritizes specialty care in separate settings.26 PDs are typically evaluated and treated in specialized psychiatric clinics while SUD treatment is provided independently in narcology (an addiction subspecialty of psychiatry) clinics.2,27 While narcologists can technically prescribe antidepressants, they are often reluctant to provide psychiatric care due to lack of experience, skills and motivation and, in some cases, unavailability of psychiatric medications.28 Consequently, Ukraine’s current standard of care (SOC) involves referring OAT patients to an off-site psychiatric clinic if MDD is suspected rather than provide onsite screening, diagnosis and treatment. Even though national guidelines recommend standardized screening tools, screening is either uncommon or not reported, and adherence to these guidelines is minimal. Consequently, the siloed healthcare delivery reduces access to care for patients who need it, with narcologists often reinforcing the existing status quo even though they have the training and the legal capacity treat COD patients.2
International agencies recommend integration of services, including for COD,29,30 as integration of COD care improves clinical outcomes.31–33 Effective tools, strategies and processes to integrate COD care, however, are variable and sometimes complex, and none have been tested in the Ukrainian context. To address this implementation gap and overcome the challenge of the siloed care in Ukraine, we implemented the integrated COD care model for OUD and MDD in Ukraine: Project MEDIUM. To guide implementation of integrating COD care, we used the integrated Promoting Action on Research Implementation in Health Services (i-PARiHS) framework,34 which includes key constructs of innovation of the evidence-base practice (EBP), facilitation style and fit, impact of the EPB on recipients, and context. Guided by i-PARIHS framework, implementation of integrated COD care was facilitated using expert coaching using Project ECHO, a collaborative learning tool effective for teaching and supporting non-specialists (i.e., narcologists) to provide specialty care (i.e., treatment for PDs) in OAT clinics. Here, we provide the rationale for MEDIUM, along with the trial protocol, research methods, study design, description of tools and EBPs, the evaluation plan, and preliminary results. Throughout this text, COD refers to the co-occurrence of OUD and MDD.
2. Methods
2.1. Local context
OAT were introduced in Ukraine in 2004, with 17,232 patients on OAT at 204 governmental clinics throughout the country just before Russia invaded Ukraine in February 2022, with scale-up continuing since the invasion.35,36 OAT coverage in Ukraine, however, remains low at 5.9%,37 well below the WHO recommended 40%.38 OAT scale-up has been thwarted by patient, provider and structural factors, including deep-seated myths and misconceptions, limited OAT convenience and accessibility, lack of integrated care, stigma and discrimination in specialty care settings, and police harassment at OAT sites.39,40
2.2. Implementation strategies and tools
2.2.1. SET, or Modified SBIRT (Screening, Brief Intervention and Referral to Treatment)
Guided by the i-PARiHS framework, we created a rapid, innovative implementation strategy, Screening, Evaluation and Treatment (SET) by modifying an existing tool, Screening, Brief Intervention and Referral to Treatment (SBIRT) for the delivery of evidence-based practice (i.e., antidepressants for MDD) by removing the component of offsite referral (Figure 1).
Figure 1. A Simplified Screening, Evaluation and Treatment (SET) strategy to guide implementation of integrating treatment for co-occurring disorders.
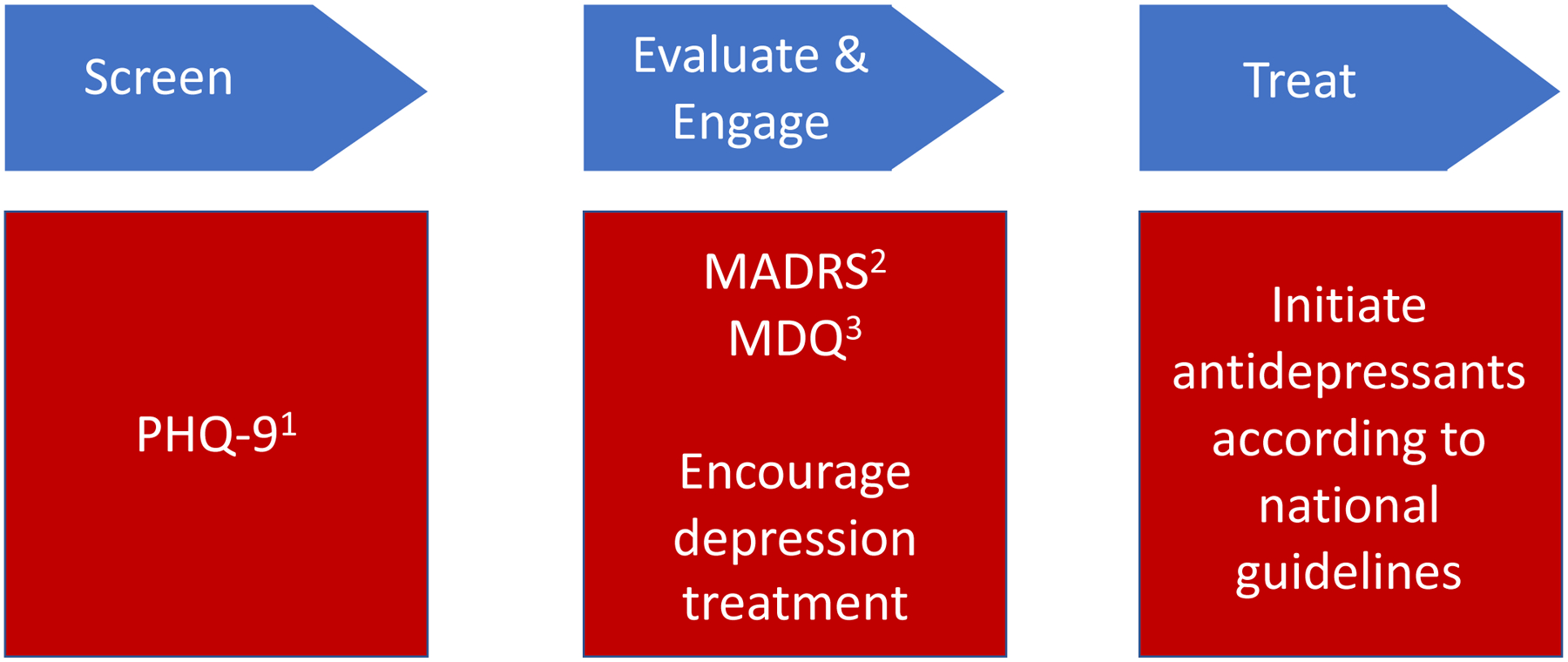
SET strategy was devised as a modification of SBIRT by removing the referral component.
Abbreviations: SBIRT Screening, Brief Intervention, Referral, Treatment; PHQ-9 Patient Health Questionnaire 9; MADRS Montgomery-Asberg Depression Rating Scale; MDQ Mood Disorder Questionnaire.
1. Levis B, Benedetti A, Thombs BD. Accuracy of Patient Health Questionnaire-9 (PHQ-9) for screening to detect major depression: individual participant data meta-analysis. BMJ. 2019;365:l1476. doi:10.1136/bmj.l1476
2. Hobden B, Schwandt ML, Carey M, et al. The Validity of the Montgomery-Asberg Depression Rating Scale in an Inpatient Sample with Alcohol Dependence. Alcohol Clin Exp Res. Jun 2017;41(6):1220–1227. doi:10.1111/acer.13400
3. Hirschfeld RM. The Mood Disorder Questionnaire: A Simple, Patient-Rated Screening Instrument for Bipolar Disorder. Prim Care Companion J Clin Psychiatry. Feb 2002;4(1):9–11. doi:10.4088/pcc.v04n0104
SBIRT is an implementation practice to guide treatment scale-up, including for SUD and PDs. SBIRT has been effectively deployed in primary care, emergency rooms, and other community settings,41–43 and it is recommended by Substance Abuse and Mental Health Services Administration in Treatment Improvement Protocol 42 for integrating COD treatment.29 SBIRT, however, is limited through its offsite “referral”, which has recently been successfully modified to SET to streamline the process.44 The SET toolkit eliminated the referral component by providing narcologists SET tools (i.e., brief screening and diagnostic instruments), expert facilitation to support evaluation and treatment of patients (i.e., ongoing tele-education). Free antidepressant medications were available to all study sites. An initial 2-day SET training was delivered to both intervention arm clinicians, along with a manual and an instruction sheet with the SET algorithm. Ongoing training and clinical support were provided continuously over 24 months using tele-education. According to SET, any patient screening positive for depression were immediately evaluated for diagnostic confirmation using standardized tools. For treatment, those with mild depression were monitored for stability and received supportive counselling. If diagnosed with moderate to severe depression, clinicians prescribed antidepressants in accordance with national guidelines. For patients that improved, antidepressants were maintained and monitored for at least one year. Patients non-responsive to antidepressants were referred to an offsite psychiatric hospital for expert consultation.
2.2.2. ECHO-COD, or modified Project ECHO (Extension for Community Healthcare Outcomes®) for COD
Facilitation is a key ingredient for effective implementation according to the i-PARiHS framework. As clinicians were geographically dispersed throughout Ukraine, we deployed a modified Project ECHO-like tele-education strategy to facilitate and support OAT clinicians to also provide psychiatric care. ECHO is based on established educational theories of social learning and behavior change45 where a group of non-specialists collaborate with specialists, resulting in an innovative healthcare delivery model that translates into high-quality care for patients in non-specialty settings.46,47 ECHO links experts with non-specialist clinicians through tele-education, in which the newly emerging experts co-manage cases and share expertise via mentoring, guidance, feedback, support and didactic education. ECHO learning for this study was modified to focus on co-management of COD, called ECHO-COD, and was provided bi-weekly for via zoom. ECHO-COD learning was guided by a U.S. expert and included case-based learning and mini-didactic clinical vignettes.
2.2.3. Pay-for-performance
Pay for performance (P4P) is a strategy used in healthcare that provides financial incentives to clinicians for adhering to clinical guidelines and achieving better health outcomes.48 Such practices are increasingly used globally, including in middle-income settings, with qualified support for P4P programs that deploy valid quality indicators, ensure patient and physician autonomy, adequately reward clinicians, and involve clinicians in the incentive process. In Ukraine, physicians, on average, earn $260 per month, a salary that is well below the income of other professionals.49,50 Given this, we introduced P4P to facilitate the successful implementation of SET among clinicians. With the focus on local context and on the evidence that successes from P4P strategies are optimized when adequate incentives target pre-specified indicators,51 we defined a set of measurable indicators as targets for clinicians that included (i) screening, (ii) diagnosis, (iii) treatment initiation and (iv) treatment retention. The indicators and payment structure were guided by a panel of OAT providers and international experts (Supplementary table 1). The incentives were transparent so that each provider at a P4P site could see the indicators achieved in their monthly status report and was paid monthly as an addition to clinicians’ monthly remuneration.
2.3. Implementation Framework
The implementation and evaluation of MEDIUM is guided by i-PARIHS framework34 that incorporates diffusion of innovation theory52 and implementation science. Central to i-PARiHS are its key constructs necessary for successful implementation: 1) Innovation in the EBP, which involves the SET delivery model for managing depression; 2) the Ukrainian context where substance use and PDs are managed separately and antidepressants are not prescribed in OAT clinics; 3) impact of the innovation on recipients, which is antidepressants for clients and P4P for clinicians; and 4) facilitation, which is guided by Project ECHO.
2.4. Overall Design
Based on the region-specific differences in COD prevalence and OAT practice in Ukraine,53,54 we selected three substance use centers as study sites in each geographic regions: Center, East, South, West. Characteristics of the regions, number of OAT patients and OAT coverage levels are provided in Table 1.11,55,56
Table 1.
Study context and implementation outcomes
| Context Information | Entire Reach of Implementation Outcomes: Preliminary Results over Six Months | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Region | Oblast | Number of PWlDs | Population in thousands | OAT Coverage by Feb 2022 | Study Site | Study Arm | Total N of OAT patients per site | Screened | Diagnosed | Treated with antidepressants | 6-month retention |
| South | Kirovohrad | 13,200 | 912 | 4.5% | Kropyvnitsky Regional Narcological Dispensary | SOC | 435 | 386 (88.7%) | 169 (38.9%) | 152 (34.9%) | 36 (8.3%) |
| Mykolaiv | 12,300 | 1,100 | 9.0% | Mykolaiv Regional Narcological Dispensary | ECHO-COD | 540 | 318 (58.9%) | 122 (22.6%) | 122 (22.6%) | 60 (11.1%) | |
| Mykolaiv City Hospital N5 | ECHO-COD+P4P | 175 | 169 (96.6%) | 137 (78.3%) | 85 (48.6%) | 32 (18.1%) | |||||
| East | Donetsk | 31,100 | 4,080 | 6.1% | Kramatorsk Narcological Dispensary | SOC | 254 | 212 (83.5%) | 229 (90.2%) | 135 (53.1%) | 22 (8.7%) |
| Dnipropetrovsk | 58,000 | 3,120 | 5.1% | Krivyi Rih Psycho-Neurological Dispensary | ECHO-COD | 698 | 430 (61.6%) | 179 (25.6%) | 114 (16.3%) | 67 (9.6%) | |
| Dnipropetrovsk Narcological Dispensary in Pavlograd | ECHO-COD+P4P | 334 | 203 (60.8%) | 175 (52.4%) | 117 (35.0%) | 57 (17.1%) | |||||
| Center | Poltava | 33,700 | 2,957 | 4.3% | Poltava Regional Narcological Dispensary | SOC | 479 | 323 (67.4%) | 174 (36.3%) | 135 (28.2%) | 49 (10.2%) |
| Kyiv City | 6,500 | 1,362 | 13.5% | Kyiv City Narcological Clinic “Sociotherapia” | ECHO-COD | 539 | 433 (80.3%) | 123 (22.8%) | 77 (14.3%) | 42 (7.8%) | |
| Vinnytsia | 9,400 | 1,519 | 8.5% | Vinnitsia Regional Narcological Dispensary “Sociotherapia” | ECHO-COD+P4P | 351 | 336 (95.7%) | 169 (38.5%) | 69 (19.7%) | 38 (10.8%) | |
| West | Lviv | 3,400 | 1,356 | 12.8% | Lviv Regional Center on Addiction Treatment and Prevention | SOC | 186 | 160 (86.0%) | 135 (72.6%) | 120 (64.5%) | 71 (38.2%) |
| Ternopil | 10,400 | 2,488 | 5.7% | Ternopil Regional Narcological Dispensary | ECHO-COD | 149 | 146 (98.0%) | 146 (98.0%) | 63 (42.3%) | 35 (23.5%) | |
| Ivano-Frankivsk | 4,400 | 1,026 | 3.1% | Ivano-Frankivsk Regional Narcological Dispensary | ECHO-COD+P4P | 281 | 277 (98.6%) | 201 (71.5%) | 168 (59.8%) | 99 (35.2%) | |
| Total | 4,421 | 3,393 (76.7%) | 1,925 (43.5%) | 1,357 (30.7%) | 608 (13.8%) | ||||||
Contextual information on the four regions of Ukraine where study clinics were situated are presented along with preliminary implementation outcomes.
Abbreviations: PWID People Who Inject Drugs, OAT Opioid Agonist Treatment, SOC Standard of Care, ECHO-COD Extension for Community Healthcare Outcomes® modified for Co-occurring Disorders, P4P Pay-for-Performance
We deployed a Type-2 hybrid, phase-in, cluster-randomized trial design.57 Consistent with a type-2 hybrid design, we measured both implementation process and intervention effectiveness. To measure implementation processes, we defined service-level outcomes as the COD cascade of care (screening, evaluation, treatment, retention). For effectiveness, we conducted patient surveys to measure changes in HRQoL and other health indicators. Participant enrollment was completed between August 2019 and January 2020, and the follow-up was completed by February 2022.
2.5. Randomization
As the intervention was clinic-based and the goal was to evaluate service-level outcomes, it was not appropriate to randomize participants within a specific clinic. Therefore, we chose a cluster-randomized trial design. Three sites each were selected within four regions and allocated randomly to receiving SOC (control arm) or integrated COD care (experimental arms) (Figure 2).
Figure 2. Project MEDIUM Study Design.
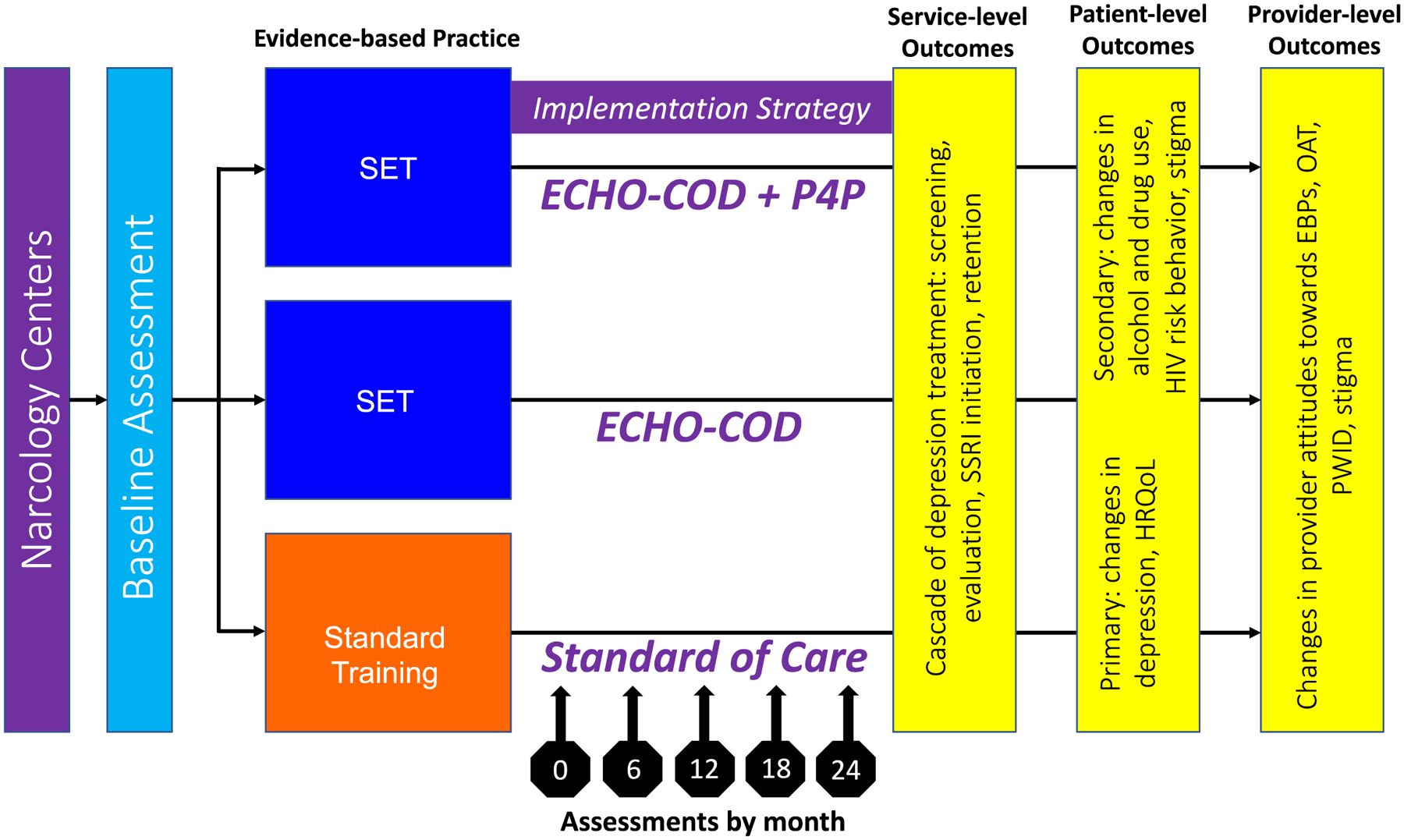
The MEDIUM study had three arms: Standard of Care, ECHO-COD, and ECHO-COD+P4P. Assessments were carried out on baseline and every 6 months for 24 months. Service-, patient- and provider-level outcomes are presented.
Abbreviations: SET: Screening Evaluation Treatment; ECHO-COD: Extension for Community Healthcare Outcomes® modified for Co-occurring Disorders; P4P: Pay for Performance; HRQoL Health-related Quality of Life, EBP Evidence based practice, OAT opioid agonist therapies, PWID people who inject drugs.
In the SOC arm, no additional training was provided. In the experimental arms, providers were trained to use SET procedures and supported through ECHO-COD, with or without P4P. As antidepressant treatment for MDD is an evidence-based practice,58 withholding it from the control arm would have been unethical, therefore all sites, including the ones providing SOC (control), were provided with a free supply of two antidepressant medications [sertraline (SSRI) and venlafaxine (SNRI), projected to be taken by a maximum of 20% and 5% of patients, respectively, at any given time point (with a possibility to request additional supply), and an adapted clinical guideline that described the SET algorithm for depressive disorder. Provision of antidepressants to all sites, including those providing SOC, ensured that outcomes would not be confounded by varying availability of antidepressants and ability of patients to pay for them. OAT with methadone and buprenorphine is free in Ukraine and were readily available at all study sites.
2.6. Study outcomes
2.6.1. Service-level implementation outcomes
We defined service-level outcomes according to the COD continuum of care as the following implementation measures: (i) depression screening, measured by the proportion of all OAT patients that were screened in the past 6 months; (ii) proportion evaluated by a physician, diagnosed and motivated to start treatment; (iii) proportion of patients initiated on antidepressant treatment; and (iv) proportion retained on antidepressants (SET cascade + retention). The denominator for the service-level outcome was the number of OAT patients. The numerator is different for each cascade element: number of patients screened, number of patients diagnosed with depression, number of patients started on antidepressants, and number of patients who received antidepressants for 6 months.
2.6.2. Patient-level outcomes
The primary patient-level efficacy outcomes were defined as the changes over time in depressive symptoms and health-related quality of life (QoL). Secondary outcomes were defined as changes in alcohol use, enacted, internalized, and anticipated stigma, HIV risk behavior and other health comorbidities.
2.6.3. Provider-level outcomes
Provider-level outcomes were defined as changes over time in self-reported provider stigma, attitudes towards PWID, OAT, and EBPs.
2.7. Study participants
2.7.1. Participants for service-level outcomes
Twelve OAT clinics from four regions of Ukraine (East, West, Center, South) were selected.59 OAT sites were selected if they had: (i) at least 75 patients receiving OAT; and (ii) regional administrator approval for the clinicians at the site to participate. All patients receiving OAT at each participating sites during the study period were included in the evaluation of the service-level outcomes. Dropout from the antidepressant treatment was not a reason for the follow-up discontinuation.
Transfer of patients between sites was uncommon in Ukraine before the war and did not occur during the study. Treatment of depression, once made available at the site, was available to every patient undergoing OAT at the given site. Therefore, the entire population of patients receiving OAT at the 12 sites between August 2019 and February 2022 were included in the assessment of the service-level outcomes.
2.7.2. Participants for patient-level effectiveness outcomes
To assess primary and secondary patient-level outcomes, a cohort of patients was randomly recruited to complete structured surveys every 6 months over 24 months. To enroll individual participants at each OAT site, selection criteria included: (a) age ≥18 years; (b) prescribed OAT; and (c) ability to provide informed consent. Recruitment was conducted in the following order: (i) each site submitted lists of identification numbers (IDs) of their OAT patients for random selection; (ii) for sites with over 115 patients, we randomly selected IDs and sent this list back to the sites; (iii) clinical staff at each site contacted patients using their ID from the list, and offered to refer them to research assistants to learn more about the study; (iv) if interested, the research assistants completed screening procedures, informed consent procedures, and conducted the baseline interview. Additional random selection was made to replace IDs of patients who refused to participate. Participants were followed up with the surveys even if they dropped out of OAT.
2.7.3. Participants for provider-level outcomes
To assess provider attitudes towards PWID, OAT, EBPs, organizational support and change, we selected a total sample of medical and administrative staff members from each site and conducted structured interviews after obtaining informed consent.
2.8. Study Assessments
2.8.1. Clinical Records Data
Clinical data for the service-level outcomes were collected at baseline and every 6 months for 24 months from all OAT patients at each site using clinical records, regardless of the arm of the trial or participation in the interviews. We collected data using the standard electronic instrument used by OAT providers for routine treatment monitoring.60 Charts were reviewed, and data were entered by authorized clinical personnel. Every quarter, after all up-to-date information was entered, the staff used the standard data export feature to export and encrypt a de-identified dataset containing information on OAT enrollment, medication prescription, mental health assessments, and other clinical assessments related to comorbidities.
2.8.2. Patient and provider surveys
Structured interviews were used to survey patients and providers at baseline and every 6 month for 24 months. A detailed list of survey instruments used in patient and provider interviews can be found in Table 2. Interviews were conducted in Ukrainian or Russian, based on participants’ preferences. As surveys for providers were sent out to providers at each clinic, linked individual responses could be measured over time for both the clinic and the individual. All survey data were collected using the REDCap data management system.
Table 2.
Patient and provider surveys
| Patients Surveys (N=1,345) | Provider Surveys (N=54) | ||
|---|---|---|---|
| Survey instrument | What it measures | Survey Instrument | What it measures |
| BASIS-2461 | Addiction severity | Feeling thermometers62 | Attitudes towards sociodemographic and health conditions: people with HIV, PWID, men who have sex with men, women who sell sex, and recently released prisoners |
| AUDIT-C63 | Alcohol use and alcohol use disorder | ||
| SOCRATES64 | Treatment readiness | ||
| Addiction treatment experience | Satisfaction, barriers and facilitators, adherence | ||
| HIV risk behaviors | Involvement in behaviors of heightened HIV transmission risk (e.g., needle-sharing) | Counselor Assessment Screen (CAS)65 | Attitudes towards PWID and OAT |
| Medical comorbidity and health assessment | HIV, HBV, HCV, TB and STIs statuses, and other comorbidities including NCDs | Multidimensional stigma scale66 | stigma towards PWID that includes five subscales on discrimination, prejudice, internal shame, fear, stereotypes towards PWID |
| PHQ-967 | Depressive symptoms | ||
| MADRS68 | Depressive symptoms and depression | ||
| Mood Disorder Questionnaire69 | Mood symptoms and mood disorders | Resistance to Change70 | individual resistance to organizational change |
| 12-Item Short Form Health Survey (SF-12)71 | Health-related quality of life using | EBPAS-3672 | Evidence-based practice attitudes, includes sub-scales measuring appeal, organizational support, feedback, etc. |
| Drug use and mental illness stigma73 | Substance use and mental health stigma | ||
| Sociodemographic characteristics | Age, gender, income, housing, education, marital status, etc. | Sociodemographic Characteristics | Age, gender, position, practice years, etc. |
The list includes patient and provider surveys that were conducted every 6 months for 24 months to evaluate patient- and provider-level outcomes.
Abbreviations: BASIS-24 Behavior and Symptom Identification Scale 24; AUDIT-C Alcohol Use Disorders Identification Test-Concise; MADRS Montgomery-Asberg Depression Rating Scale; PWID people who inject drugs; OAT opioid agonist therapies; EBPAS-36 Evidence-based Practices Attitudes Scale 36.
2.9. Statistical considerations
2.9.1. Sample Size
For the service-level outcomes, the total sample consisted of all patients that received OAT across all participating sites – there were no patient-level measurements aside from their participation in the cascade. For patient-level outcomes, however, we conservatively estimated a sample size of 405 per group (total sample size of 1215) using a two-sample t-test for a cluster randomized design to have at least 80% power at a 5% level of significance (Bonferroni corrected for 3 pairwise comparisons). We allowed for the within-site ICC of 0.01 to detect a small to medium standardized effect size (Cohens’ d) of 0.35 for a null hypothesis of no difference between the means of the three groups versus an alternative of at least one difference in means at the 24-month time point. The sample size calculation was performed using the PASS 2019 software.74 Given potential concerns about dropout, we inflated the sample size by 10% and recruited a total of 1350 patients for the patient-level outcome. For the provider-level outcomes, sample size was not pre-defined.
2.9.2. Statistical analysis plan
The preliminary descriptive analysis of the treatment cascade was performed. We calculated the percentage of the total study population at each step of the cascade (MDD screening, evaluation and diagnosis, antidepressant treatment initiation and retention). The service-, patient-, and provider-level data analyses described below are to follow.
To understand the effect of the interventions on the depression treatment implementation outcomes, we will test the following hypotheses: (i) facilities participating in ECHO-COD and their level of engagement will have better service-level and patient-level outcomes facilitated through SET training; (ii) clinics receiving P4P incentives have better service- and patient-level outcomes but are moderated by participation in ECHO-COD facilitation (ECHO-COD+P4P > ECHO-COD > SOC).
All analyses will be conducted using intention-to-treat, i.e., analysis of the site and individuals within the site as randomized. For the service-level outcomes, the treatment cascade will be calculated as the ratio of patients receiving OAT that has been screened, diagnosed, started, and retained on treatment. A repeated measures likelihood-based mixed model with missing at random assumptions will be used for the analysis of the service-level outcome to compare the three study arms, adjusted for site, age, gender, and OAT medication (methadone or buprenorphine). The results will be displayed as point estimates with corresponding 95% confidence limits. In addition, we will test for a linear trend among the intervention levels for the proposed outcomes, testing that ECHO-COD+P4P>ECHO-COD>SOC. We will test the treatment effects using similar methods for OAT dropout strata separately. Patient- and provider-level outcomes, including changes in depression symptoms, health-related QoL, stigma, alcohol use and HIV risk behavior, as well as provider attitudes, are continuous measures. Changes in these measures and their subscales over time, as well as differences in scores between study arms, will be evaluated using linear mixed-effects models with random intercepts to account for the intra-site clustering and within-subject variability by including a random intercept for each subject in the model. We will test for a treatment*time interaction. If the treatment*time interaction is significant at 0.10, we will use a linear contrast at 24-months to estimate the differences between treatment arms. If the treatment by time interaction is not significant, we will use the average of the measures over time to compare treatment arms using a linear contrast.
We do not anticipate missing data to be a concern since most of the data on service-level outcomes was derived from clinical source documentation and we were able to retain the participants in the study sample if they dropped out of the depression treatment as long as they continued receiving OAT at the study sites. For the patient-level data, we conducted follow-up interviews with the participants enrolled in the survey sample even if they dropped out from OAT. We plan to explore patterns of missing data and compare baseline characteristics of those with and without data (i.e., eliminate the missing completely at random assumption). We will conduct sensitivity analyses for missing not at random using an appropriate missing data method, such as pattern mixture models. SAS software version 9.4 or higher and R will be used for all analyses.75,76
2.10. Institutional review and ethical considerations
The research protocol was approved by Ukrainian Institute on Public Health Policy Institutional Review Board (IRB) for scientific content and compliance with applicable research and human subject regulations. The trial is registered at www.clinicaltrials.gov as NCT05646212.
Clinical record data were collected on all patients receiving OAT. As the data collected were identical to routine treatment quality monitoring performed by OAT providers and did not include any personally identifiable information, the IRB granted an informed consent wavier. To participate in the surveys, participants signed an informed consent document approved by the IRB.
3. Preliminary results
For the service-level implementation outcomes, all patients receiving OAT at all study sites were included (N=4421). For the patient-level outcomes, participant accrual happened according to the planned timeline, enrolling 1345 patients between August 2019 and January 2020. The completion of the 24-month follow-up was complicated by the COVID-19 pandemic, though the study team was able to adapt swiftly and provide remote interviews. Consequently, it was possible to complete the 24-month follow-up according to the protocol by January 2022.
Here we present the preliminary service-level results involving all patients receiving OAT at all sites. The COD cascade is presented in Figure 3, including the implementation gap, with 76.7% (N=3393) screened at least once, 43.5% diagnosed with MDD (N=1925), 30.7% initiated antidepressant treatment (N=1357), and 13.8% (N=608) were retained on antidepressants 6 months after initiation (Figure 3). Sites differed in terms of size (Table 1), ranging from 149 to 698 patients receiving OAT, with considerable variation in each level of the cascade by site. Specifically, screening ranged from 58.9% to 98.6%, while diagnosis ranged from 22.6% to 98.0%. Initiation of antidepressants ranged from 16.3% to 64.5% and 6-month retention ranged from 7.8% to 38.2%.
Figure 3. Screening, Evaluation and Treatment (SET) cascade.
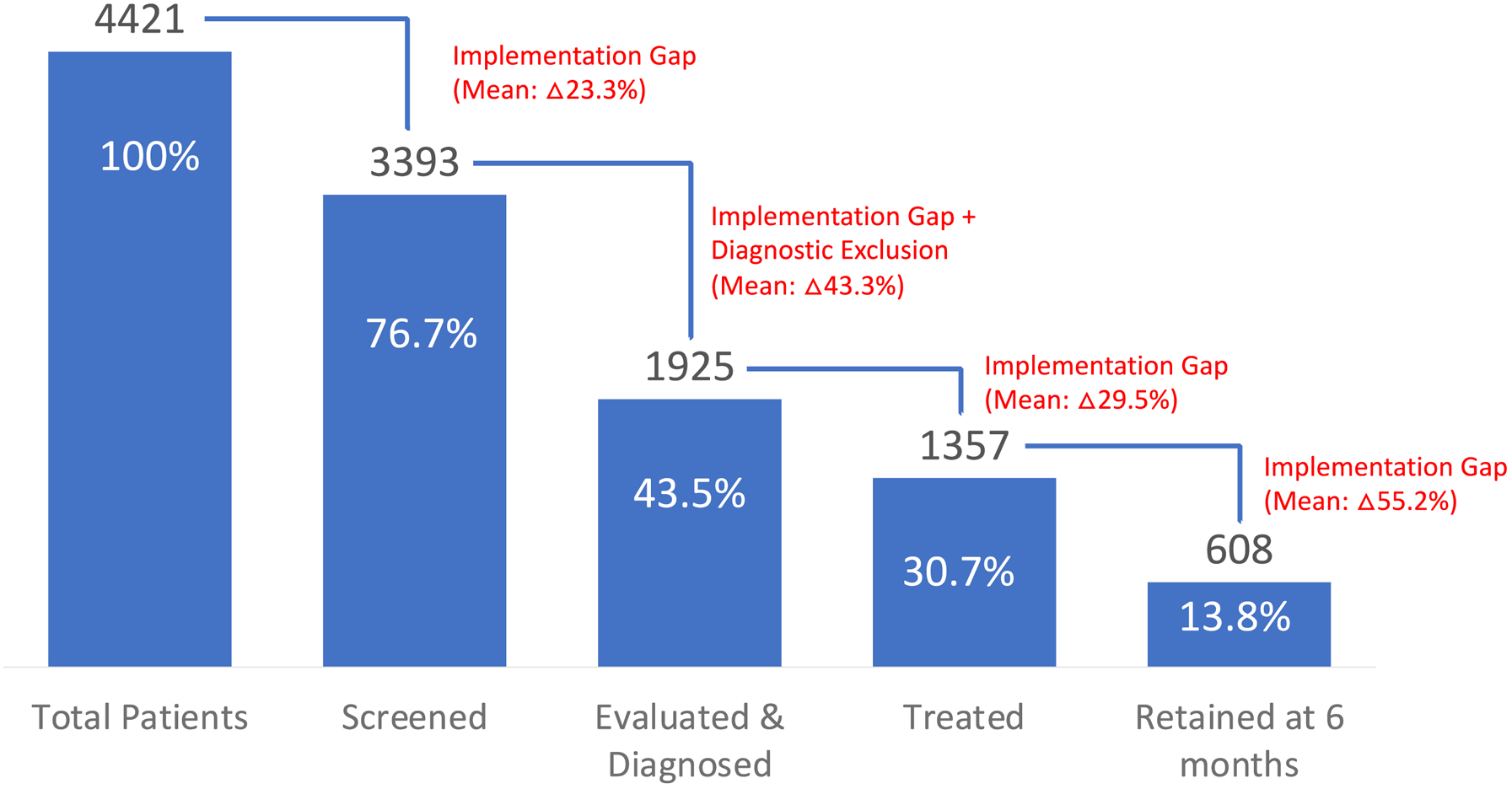
The denominator of each step in SET cascade is the total number of patients receiving opioid agonist treatment in all study clinics. The The numerator is different for each cascade element: number of patients screened, number of patients diagnosed with depression, number of patients started on antidepressants, and number of patients who were retained on antidepressants at 6 months.
In certain cases, number of patients treated or diagnosed was higher than number of patients screened, explained by clinicians administering the diagnostic tool without screening, or initiating treatment without administering the diagnostic tool.
For the patient-level effectiveness outcomes, 1350 randomly selected patients, 1345 had complete data for the baseline assessment. The baseline characteristics of the 1,345 participants, stratified by study arm, is presented in Table 3; where there are significant differences between the arms, those covariates will be controlled for the final analyses. For provider-level outcomes, 54 providers were enrolled at baseline, including 26 medical and 10 administrative staff members from experimental sites and 12 medical and 6 administrative staff members from SOC sites.
Table 3:
Baseline characteristics of randomly recruited participants for patient-level outcomes (N=1345)
| Characteristic | Total | Study Allocation | p-value | ||
|---|---|---|---|---|---|
| SOC (N=449) | ECHO-COD (N=448) | ECHO-COD + P4P (N=448) | |||
| Age in years, Mean (SD) | 40.7 (7.6) | 39.2 (7.1) | 42.5 (7.9) | 40.5 (7.3) | <0.001 |
| Male, N (%) | 1108 (82.4%) | 381 (84.9%) | 354 (79.0%) | 373 (83.3%) | 0.060 |
| Married, N (%) | 555 (41.3%) | 182 (40.5%) | 174 (38.3%) | 199 (44.4%) | 0.220 |
| Employed, N (%) | 874 (65.0%) | 331 (73.7%) | 261 (58.3%) | 282 (62.9%) | <0.001 |
| Below secondary education, N (%)a | 147 (10.9%) | 53 (11.8%) | 41 (9.2%) | 53 (11.8%) | 0.336 |
| Stably housed, N (%)b | 1178 (87.6%) | 377 (84.0%) | 400 (89.3%) | 401 (89.5%) | 0.017 |
| History of prior incarceration | 606 (45.1%) | 176 (39.2%) | 247 (55.1%) | 183 (40.8%) | <0.001 |
| Time on OAT < 3 months, N (%)c | 41 (3.0%) | 24 (5.3%) | 10 (2.2%) | 7 (1.6%) | 0.002 |
| HIV-positive | 483 (35.9%) | 131 (29.2%) | 208 (46.4%) | 144 (32.1%) | <0.001 |
| BASIS-24 Subscale scores | |||||
| Depression/Functioning, Mean (SD)d | 1.6 (0.6) | 1.5 (0.5) | 1.7 (0.6) | 1.5 (0.6) | <0.001 |
| Psychotic symptoms, Mean (SD)d | 0.6 (0.6) | 0.6 (0.6) | 0.7 (0.6) | 0.5 (0.6) | 0.002 |
| Interpersonal problems, Mean (SD)d | 2.4 (0.7) | 2.4 (0.7) | 2.3 (0.7) | 2.3 (0.7) | 0.052 |
| Emotional liability, Mean (SD)d | 1.5 (0.8) | 1.4 (0.8) | 1.6 (0.8) | 1.5 (0.9) | <0.001 |
| Self-harm, Mean (SD)d | 0.5 (0.7) | 0.4 (0.7) | 0.5 (0.8) | 0.5 (0.8) | 0.040 |
| Substance use, Mean (SD)d | 1.1 (1.0) | 1.1 (0.9) | 1.2 (1.0) | 1.1 (1.0) | 0.120 |
| Alcohol use disorder (AUDIT-C screening), N (%) | 179 (13.3%) | 38 (8.5%) | 77 (17.2%) | 64 (14.3%) | 0.001 |
| Any injection drug use in the last 30 days, N (%) | 272 (20.2%) | 73 (16.3%) | 96 (21.4%) | 103 (23.0%) | 0.032 |
Less than 12 years of secondary school
Stable housing: owned, rented or family-owned housing and high level of confidence of being able to stay in this housing next month
Time in OAT ever, not necessarily during the most recent treatment episode
Dimensions of the BASIS-24 questionnaire, each dimension is measured on a 0–4 scale, where 0 represents the absence of a problem and 4 represents the most severe problem.
Abbreviations: PWID People Who Inject Drugs, OAT Opioid Agonist Treatment, SOC Standard of Care, ECHO-COD Extension for Community Healthcare Outcomes® modified for Co-occurring Disorders, P4P Pay for Performance, SD standard deviation.
4. Adaptation during the COVID-19 pandemic
In response to the COVID-19 pandemic, Ukraine’s Ministry of Health issued emergency interim guidance to OAT clinics in March 2020 to encourage: (i) continued access for starting new patients on OAT; and (ii) transfer as many patients as safely possible from daily observed to take-home dispensing to mitigate harm to patients and providers. Though new enrollment decreased initially during the first three months, it went up thereafter with an immediate shift to take-home dosing from 54% to 82% of all OAT patients receiving take-home medications, with variable levels by region.77 In tandem, the research team shifted to telephone interviews as needed and there was no interruption in research activities, aside from occasional brief postponement in participant interviews and assessment windows extended. All research and clinical staff adhered to personal protection measures (wearing face masks, using sanitizers, and increasing physical distance).
5. Discussion
In this study, we explore innovative implementation strategies to facilitate integrated co-management of MDD in patients on OAT. The two new implementation strategies were designed and deployed to overcome known barriers: a) lack of experience and confidence by addiction treatment specialists to manage MDD; and b) low salaries for addiction treatment staff, which we postulated would increase co-management through P4P incentives. Moreover, this implementation study streamlined and adapted an EBP documented to increase treatment engagement, SBIRT, to its basic elements (screening-evaluation-treatment: SET) and incorporated efficient tools to achieve these goals. In Ukraine, the costs of psychiatric medications (except for emergency and acute care) must be borne by the patient. We overcame this impediment by making antidepressants available for prescription at all sites rather than make it a pragmatic trial. This was done to ensure the maximum potential benefit of what could be achieved through more effective implementation and allow assessment of patient-level outcomes in terms of response to treatment (i.e., antidepressants).
To our knowledge, MEDIUM represents the first integration of MDD services in OAT clinics in Ukraine, or even throughout the Eastern European and Central Asian (EECA) region. Potential opportunities as well as implementation challenges are highlighted. Preliminary results show that there is an important implementation gap for each step of the SET implementation cascade, which will allow identification of strategies that worked better in some but not in other settings (Figure 3). For example, settings like Lviv had the highest treatment retention (38.2%), with a big proportion (72.6%) of the participants diagnosed with MDD – with considerable variation between sites. Elsewhere in LMIC, screening rates in integrated settings are considerably lower,78,79 which allows further investigation of site-specific implementation strategies that may be more useful. Exploration of engagement in ECHO-COD, in which education, support and feedback was provided to clinicians may, in part, explain differences in treatment, but not screening outcomes, yet retention on antidepressants overall remained a challenge. Understanding a patient’s reason for antidepressant treatment discontinuation, providing frequent assessments and management of side effects as well as individually-tailored antidepressant choice and psychoeducation are among some of the strategies that can be used in the future projects to ensure not only the detection of MDD, but a higher rate of retention in treatment.80
A strength of this study is the deployment of a type-2 hybrid trial design, where both implementation process and patient-level effectiveness outcomes are assessed. From the implementation perspective, does engagement in ECHO-COD result in better knowledge and confidence in addiction treatment staff providing integrated COD services and, if so, does it result in better outcomes along the COD continuum of care? From the patient level, do these two additional implementation strategies reduce the levels of depression in a group already with high levels and, potentially improve their retention in care and health-related quality of life? These questions will be assessed in this trial, which if efficacious, can be further disseminated in Ukraine.
Already, there is a shift to creating a unified system of psychiatric care that is responsible for both psychiatric and SUD. Findings from this trial can serve as heuristic for how these services will evolve and provide tools for integrated COD care. Payments for care are being established as packages that follow the patient. These packages and their reimbursement are based on management of each condition but given the high prevalence of COD among patients on OAT, synergies that increase reimbursement may be later used to guide service delivery, including efficiencies created as part of this trial and allow for differentiation of reimbursement following the P4P approach, leading to better quality care. Moreover, while Ukraine has made bold steps to transform its healthcare system by the new creation of a National Health System, findings here will be viewed by other countries within the Easter Europe and Central Asia region that are evolving from the legacy pf the rigid Semashko healthcare system. Last, the recent war brought on by the Russian invasion creates a more urgent need to manage COD, as people with opioid use disorder are more susceptible to stressful situations, and their depressive symptoms are expected to be exacerbated.
Supplementary Material
Funding:
The trial was funded by the National Institutes on Drug Abuse (R01 DA045384), with additional support from F31 DA054861, R01 DA043125, and R01 DA033679.
Abbreviations:
- COD
Co-occurring disorders
- DSM-5
Diagnostic and statistical manual of mental disorders, fifth edition
- EBP
Evidence-based practice
- ECHO-COD
Extension for community healthcare outcomes for co-occurring disorders
- HBV
Hepatitis B virus
- HCV
Hepatitis C virus
- HRQoL
Health-related quality of life
- HIV
Human immunodeficiency virus
- i-PARiHS
Integrated promoting action on research implementation in health services
- IRB
Institutional review board
- MDD
Major depressive disorder
- OAT
Opioid agonist therapies
- OUD
Opioid use disorder
- P4P
Pay for performance
- PD
Psychiatric disorders
- PWID
People who inject drugs
- SBIRT
Screening, brief Intervention and referral to treatment
- SET
Screening, evaluation, treatment
- SOC
Standard of care
- SNRI
Serotonin-norepinephrine re-uptake inhibitor
- SSRI
Selective serotonin re-uptake inhibitor
- SUD
substance use disorders
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References:
- 1.Ukraine - WHO Special Initiative for Mental Health. Accessed October 10, 2022, https://cdn.who.int/media/docs/default-source/mental-health/who-special-initiative-country-report---ukraine---2020.pdf?sfvrsn=ad137e9_4
- 2.Bromet EJ, Gluzman SF, Paniotto VI, et al. Epidemiology of psychiatric and alcohol disorders in Ukraine: findings from the Ukraine World Mental Health survey. Soc Psychiatry Psychiatr Epidemiol. Sep 2005;40(9):681–90. doi: 10.1007/s00127-005-0927-9 [DOI] [PubMed] [Google Scholar]
- 3.Adan A, Torrens M. Special Issue: Diagnosis and Management of Addiction and Other Mental Disorders (Dual Disorders). Journal of Clinical Medicine. 2021;10(6):1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Co-Occurring Disorders and Other Health Conditions. SAMHSA. Accessed October 12, 2022, https://www.samhsa.gov/medications-substance-use-disorders/medications-counseling-related-conditions/co-occurring-disorders
- 5.Diagnostic and Statistical Manual of Mental Disorders. American Psychiatric Association https://dsm.psychiatryonline.org/doi/abs/10.1176/appi.books.9780890425596
- 6.Hunt GE, Malhi GS, Lai HMX, Cleary M. Prevalence of comorbid substance use in major depressive disorder in community and clinical settings, 1990–2019: Systematic review and meta-analysis. Journal of Affective Disorders. 2020/04/01/ 2020;266:288–304. doi: 10.1016/j.jad.2020.01.141 [DOI] [PubMed] [Google Scholar]
- 7.Jones CM, McCance-Katz EF. Co-occurring substance use and mental disorders among adults with opioid use disorder. Drug Alcohol Depend. Apr 1 2019;197:78–82. doi: 10.1016/j.drugalcdep.2018.12.030 [DOI] [PubMed] [Google Scholar]
- 8.Janssen B, Gaebel W, Haerter M, Komaharadi F, Lindel B, Weinmann S. Evaluation of factors influencing medication compliance in inpatient treatment of psychotic disorders. Psychopharmacology (Berl). Aug 2006;187(2):229–36. doi: 10.1007/s00213-006-0413-4 [DOI] [PubMed] [Google Scholar]
- 9.Marquez-Arrico JE, Navarro JF, Adan A. Health-Related Quality of Life in Male Patients under Treatment for Substance Use Disorders with and without Major Depressive Disorder: Influence in Clinical Course at One-Year Follow-Up. Journal of Clinical Medicine. 2020;9(10):3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hashemzadeh I, Navarro JF, Adan A. Circadian functioning and quality of life in substance use disorder patients with and without comorbid schizophrenia. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2023/01/10/ 2023;120:110623. doi: 10.1016/j.pnpbp.2022.110623 [DOI] [PubMed] [Google Scholar]
- 11.Sazonova Y, Duchenko G, Kovtun O, Kuzin I. Assessment of the number of key groups in Ukraine, Report. Accessed October 12, 2022. https://aph.org.ua/wp-content/uploads/2019/06/Otsinka-chiselnosti_32200.pdf
- 12.Davis L, Uezato A, Newell JM, Frazier E. Major depression and comorbid substance use disorders. Curr Opin Psychiatry. Jan 2008;21(1):14–8. doi: 10.1097/YCO.0b013e3282f32408 [DOI] [PubMed] [Google Scholar]
- 13.Reddon H, Pettes T, Wood E, et al. Incidence and predictors of mental health disorder diagnoses among people who inject drugs in a Canadian setting. Drug Alcohol Rev. Apr 2018;37 Suppl 1(Suppl 1):S285–s293. doi: 10.1111/dar.12631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Altice FL, Kamarulzaman A, Soriano VV, Schechter M, Friedland GH. Treatment of medical, psychiatric, and substance-use comorbidities in people infected with HIV who use drugs. Research Support, N.I.H., Extramural Review. Lancet. Jul 31 2010;376(9738):367–87. doi: 10.1016/S0140-6736(10)60829-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolfe D, Carrieri MP, Shepard D. Treatment and care for injecting drug users with HIV infection: a review of barriers and ways forward. Lancet. Jul 31 2010;376(9738):355–66. doi: 10.1016/s0140-6736(10)60832-x [DOI] [PubMed] [Google Scholar]
- 16.Degenhardt L, Mathers BM, Wirtz AL, et al. What has been achieved in HIV prevention, treatment and care for people who inject drugs, 2010–2012? A review of the six highest burden countries. Int J Drug Policy. Jan 2014;25(1):53–60. doi: 10.1016/j.drugpo.2013.08.004 [DOI] [PubMed] [Google Scholar]
- 17.van Boekel LC, Brouwers EP, van Weeghel J, Garretsen HF. Stigma among health professionals towards patients with substance use disorders and its consequences for healthcare delivery: systematic review. Drug Alcohol Depend. Jul 1 2013;131(1–2):23–35. doi: 10.1016/j.drugalcdep.2013.02.018 [DOI] [PubMed] [Google Scholar]
- 18.Corrigan PW, Watson AC. Understanding the impact of stigma on people with mental illness. World Psychiatry. Feb 2002;1(1):16–20. [PMC free article] [PubMed] [Google Scholar]
- 19.Strang J, Volkow ND, Degenhardt L, et al. Opioid use disorder. Nat Rev Dis Primers. Jan 9 2020;6(1):3. doi: 10.1038/s41572-019-0137-5 [DOI] [PubMed] [Google Scholar]
- 20.Santo T Jr., Clark B, Hickman M, et al. Association of Opioid Agonist Treatment With All-Cause Mortality and Specific Causes of Death Among People With Opioid Dependence: A Systematic Review and Meta-analysis. JAMA Psychiatry. Sep 1 2021;78(9):979–993. doi: 10.1001/jamapsychiatry.2021.0976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karki P, Shrestha R, Huedo-Medina TB, Copenhaver M. The Impact of Methadone Maintenance Treatment on HIV Risk Behaviors among High-Risk Injection Drug Users: A Systematic Review. Evid Based Med Public Health. 2016;2 [PMC free article] [PubMed] [Google Scholar]
- 22.Gowing L, Farrell MF, Bornemann R, Sullivan LE, Ali R. Oral substitution treatment of injecting opioid users for prevention of HIV infection. Cochrane Database Syst Rev. Aug 10 2011;(8):Cd004145. doi: 10.1002/14651858.CD004145.pub4 [DOI] [PubMed] [Google Scholar]
- 23.Degenhardt L, Grebely J, Stone J, et al. Global patterns of opioid use and dependence: harms to populations, interventions, and future action. Systematic Review. Lancet. Oct 26 2019;394(10208):1560–1579. doi: 10.1016/S0140-6736(19)32229-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cipriani A, Furukawa TA, Salanti G, et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet. Apr 7 2018;391(10128):1357–1366. doi: 10.1016/s0140-6736(17)32802-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mosadeghrad AM. Factors influencing healthcare service quality. Int J Health Policy Manag. Jul 2014;3(2):77–89. doi: 10.15171/ijhpm.2014.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.National Health Reform Strategy for Ukraine 2015–2020. Ministry of Health of Ukraine. Accessed December 10, 2022, https://en.moz.gov.ua/uploads/0/16-strategy_eng.pdf
- 27.Latypov AB. The Soviet doctor and the treatment of drug addiction: “A difficult and most ungracious task”. Harm Reduction Journal. 2011/12/30 2011;8(1):32. doi: 10.1186/1477-7517-8-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mental Health in Transition: Assessment and Guidance for Strengthening Integration of Mental Health into Primary Health Care and Community-Based Service Platforms in Ukraine. Accessed October 12, 2022, https://documents1.worldbank.org/curated/en/310711509516280173/pdf/120767-WP-Revised-WBGUkraineMentalHealthFINALwebvpdfnov.pdf
- 29.Substancce Use Disorder Treatment for People With Co-Occuring Disorders. Treatment Improvement Protocol (TIP) Series, No. 42. Substance Abuse and Mental Health Services Administration (SAMHSA). Accessed December 10, 2022. https://store.samhsa.gov/sites/default/files/SAMHSA_Digital_Download/PEP20-02-01-004_Final_508.pdf
- 30.World Health Organization. Mental Health Action Plan 2013–2020. Accessed December 10, 2022, http://apps.who.int/iris/bitstream/10665/89966/1/9789241506021_eng.pdf
- 31.Butler M, Kane RL, McAlpine D, et al. Integration of mental health/substance abuse and primary care. Evid Rep Technol Assess (Full Rep). Nov 2008;(173):1–362. [PMC free article] [PubMed] [Google Scholar]
- 32.Drake RE, Mercer-McFadden C, Mueser KT, McHugo GJ, Bond GR. Review of integrated mental health and substance abuse treatment for patients with dual disorders. Schizophr Bull. 1998;24(4):589–608. doi: 10.1093/oxfordjournals.schbul.a033351 [DOI] [PubMed] [Google Scholar]
- 33.Assefa MT, Ford JH, Osborne E, et al. Implementing integrated services in routine behavioral health care: primary outcomes from a cluster randomized controlled trial. BMC Health Services Research. 2019/10/24 2019;19(1):749. doi: 10.1186/s12913-019-4624-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harvey G, Kitson A. PARIHS revisited: from heuristic to integrated framework for the successful implementation of knowledge into practice. Implementation Science. 2016/03/10 2016;11(1):33. doi: 10.1186/s13012-016-0398-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Altice FL, Bromberg DJ, Dvoriak S, et al. Extending a lifeline to people with HIV and opioid use disorder during the war in Ukraine. Lancet Public Health. May 2022;7(5):e482–e484. doi: 10.1016/S2468-2667(22)00083-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Altice FL, Bromberg DJ, Klepikov A, et al. Collaborative learning and response to opioid misuse and HIV prevention in Ukraine during war. Lancet Psychiatry. Nov 2022;9(11):852–854. doi: 10.1016/S2215-0366(22)00318-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Public Health Center of the Ministry of Health of Ukraine. Accessed January 12, 2023, https://phc.org.ua/kontrol-zakhvoryuvan/zalezhnist-vid-psikhoaktivnikh-rechovin/zamisna-pidtrimuvalna-terapiya-zpt/statistika-zpt
- 38.WHO, UNODC, UNAIDS technical guide for countries to set targets for universal access to HIV prevention, treatment and care for injecting drug users Accessed November 23, 2022, https://www.who.int/publications/i/item/978924150437
- 39.Bojko MJ, Mazhnaya A, Makarenko I, et al. “Bureaucracy & Beliefs”: Assessing the Barriers to Accessing Opioid Substitution Therapy by People Who Inject Drugs in Ukraine. Drugs (Abingdon Engl). 2015;22(3):255–262. doi: 10.3109/09687637.2015.1016397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Madden L, Bojko MJ, Farnum S, et al. Using nominal group technique among clinical providers to identify barriers and prioritize solutions to scaling up opioid agonist therapies in Ukraine. Int J Drug Policy. Nov 2017;49:48–53. doi: 10.1016/j.drugpo.2017.07.025 [DOI] [PubMed] [Google Scholar]
- 41.Krupski A, Sears JM, Joesch JM, et al. Impact of brief interventions and brief treatment on admissions to chemical dependency treatment. Drug and Alcohol Dependence. 2010;110:126–136. doi: 10.1016/j.drugalcdep.2010.02.018 [DOI] [PubMed] [Google Scholar]
- 42.Madras BK, Compton WM, Avula D, Stegbauer T, Stein JB, Clark HW. Screening, brief interventions, referral to treatment (SBIRT) for illicit drug and alcohol use at multiple healthcare sites: comparison at intake and 6 months later. Drug Alcohol Depend. Jan 1 2009;99(1–3):280–95. doi: 10.1016/j.drugalcdep.2008.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hargraves D, White C, Frederick R, et al. Implementing SBIRT (Screening, Brief Intervention and Referral to Treatment) in primary care: lessons learned from a multi-practice evaluation portfolio. Public Health Rev. 2017;38:31. doi: 10.1186/s40985-017-0077-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sivakumar A, Madden L, DiDomizio E, Eller A, Villanueva M, Altice FL. Treatment of Hepatitis C virus among people who inject drugs at a syringe service program during the COVID-19 response: The potential role of telehealth, medications for opioid use disorder and minimal demands on patients. Int J Drug Policy. Mar 2022;101:103570. doi: 10.1016/j.drugpo.2021.103570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bandura A Social foundations of thought and action: A social cognitive theory.
- 46.Arora S, Kalishman S, Thornton K, et al. Expanding access to hepatitis C virus treatment--Extension for Community Healthcare Outcomes (ECHO) project: disruptive innovation in specialty care. Hepatology. Sep 2010;52(3):1124–33. doi: 10.1002/hep.23802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scott JD, Unruh KT, Catlin MC, et al. Project ECHO: a model for complex, chronic care in the Pacific Northwest region of the United States. J Telemed Telecare. Dec 2012;18(8):481–4. doi: 10.1258/jtt.2012.gth113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cromwell J, Trisolini MG, Pope GC, Mitchell JB, Greenwald LM. Pay for Performance in Health Care: Methods and Approaches. 2021. https://www.rti.org/rti-press-publication/pay-performance-health-care/fulltext.pdf
- 49.Colborne M In Ukraine, mistrust of doctors remains high. Cmaj. Jun 14 2016;188(9):E179. doi: 10.1503/cmaj.109-5247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Statistical Yearbook of Ukraine 2019, State Statistics Service of Ukraine. Accessed December 10, 2022, https://ukrstat.gov.ua/druk/publicat/kat_u/2020/zb/11/zb_yearbook_2019_e.pdf
- 51.Jha AK. Time to get serious about pay for performance. JAMA: Journal of the American Medical Association. 2013;309:347–348. doi: 10.1001/jama.2012.196646 [DOI] [PubMed] [Google Scholar]
- 52.Greenhalgh T, Robert G, Macfarlane F, Bate P, Kyriakidou O. Diffusion of innovations in service organizations: systematic review and recommendations. Milbank Q. 2004;82(4):581–629. doi: 10.1111/j.0887-378X.2004.00325.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Polonsky M, Azbel L, Wickersham JA, et al. Challenges to implementing opioid substitution therapy in Ukrainian prisons: Personnel attitudes toward addiction, treatment, and people with HIV/AIDS. Research Support, N.I.H., Extramural. Drug Alcohol Depend. Mar 1 2015;148:47–55. doi: 10.1016/j.drugalcdep.2014.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zaller N, Mazhnaya A, Larney S, et al. Geographic variability in HIV and injection drug use in Ukraine: implications for integration and expansion of drug treatment and HIV care. Int J Drug Policy. Jan 2015;26(1):37–42. doi: 10.1016/j.drugpo.2014.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Number of Present Population of Ukraine. Statistics Ukraine. Accessed November 23, 2022, https://ukrstat.gov.ua/druk/publicat/kat_u/2022/zb/05/zb_Сhuselnist.pdf
- 56.HIV Infection in Ukraine. Newsletter No. 52. Public Health Center of Ukraine 2021. Accessed 29 January, 2023, https://phc.org.ua/sites/default/files/users/user90/HIV_in_UA_52_2021.pdf
- 57.Curran GM, Bauer M, Mittman B, Pyne JM, Stetler C. Effectiveness-implementation hybrid designs: combining elements of clinical effectiveness and implementation research to enhance public health impact. Med Care. Mar 2012;50(3):217–26. doi: 10.1097/MLR.0b013e3182408812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.von Wolff A, Hölzel LP, Westphal A, Härter M, Kriston L. Selective serotonin reuptake inhibitors and tricyclic antidepressants in the acute treatment of chronic depression and dysthymia: a systematic review and meta-analysis. J Affect Disord. Jan 10 2013;144(1–2): 7–15. doi: 10.1016/j.jad.2012.06.007 [DOI] [PubMed] [Google Scholar]
- 59.Polonsky M, Azbel L, Wickersham JA, et al. Challenges to implementing opioid substitution therapy in Ukrainian prisons: Personnel attitudes toward addiction, treatment, and people with HIV/AIDS. Drug and Alcohol Dependence. 2015/03/01/ 2015;148:47–55. doi: 10.1016/j.drugalcdep.2014.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dumchev K, Dvoryak S, Chernova O, Morozova O, Altice FL. Retention in medication-assisted treatment programs in Ukraine-Identifying factors contributing to a continuing HIV epidemic. Int J Drug Policy. Oct 2017;48:44–53. doi: 10.1016/j.drugpo.2017.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Madden LM, Farnum SO, Bromberg DJ, et al. The development and initial validation of the Russian version of the BASIS-24. Addict Sci Clin Pract. Nov 26 2022;17(1):65. doi: 10.1186/s13722-022-00343-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Alwin DF. Feeling Thermometers Versus 7-Point Scales. Sociological Methods & Research. 1997;25(3):318–340. [Google Scholar]
- 63.Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA, Project ftACQI. The AUDIT Alcohol Consumption Questions (AUDIT-C): An Effective Brief Screening Test for Problem Drinking. Archives of Internal Medicine. 1998;158(16):1789–1795. doi: 10.1001/archinte.158.16.1789 [DOI] [PubMed] [Google Scholar]
- 64.Vijay A, Bazazi AR, Yee I, Kamarulzaman A, Altice FL. Treatment readiness, attitudes toward, and experiences with methadone and buprenorphine maintenance therapy among people who inject drugs in Malaysia. Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t. J Subst Abuse Treat. Jul 2015;54:29–36. doi: 10.1016/j.jsat.2015.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kang S-Y, Magura S, Nwakeze P, Demsky S. Counselor Attitudes in Methadone Maintenance. Journal of Maintenance in the Addictions. 1998/01/20 1998;1(2):41–58. doi: 10.1300/J126v01n02_04 [DOI] [Google Scholar]
- 66.Stein JA, Li L. Measuring HIV-related stigma among Chinese service providers: confirmatory factor analysis of a multidimensional scale. AIDS Behav. Sep 2008;12(5):789–95. doi: 10.1007/s10461-007-9339-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Levis B, Benedetti A, Thombs BD. Accuracy of Patient Health Questionnaire-9 (PHQ-9) for screening to detect major depression: individual participant data meta-analysis. BMJ. 2019;365:l1476. doi: 10.1136/bmj.l1476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hobden B, Schwandt ML, Carey M, et al. The Validity of the Montgomery-Asberg Depression Rating Scale in an Inpatient Sample with Alcohol Dependence. Alcohol Clin Exp Res. Jun 2017;41(6):1220–1227. doi: 10.1111/acer.13400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hirschfeld RM. The Mood Disorder Questionnaire: A Simple, Patient-Rated Screening Instrument for Bipolar Disorder. Prim Care Companion J Clin Psychiatry. Feb 2002;4(1):9–11. doi: 10.4088/pcc.v04n0104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Oreg S Resistance to change: Developing an individual differences measure. Journal of Applied Psychology. 2003;88:680–693. doi: 10.1037/0021-9010.88.4.680 [DOI] [PubMed] [Google Scholar]
- 71.Ware J Jr., Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. Mar 1996;34(3):220–33. doi: 10.1097/00005650-199603000-00003 [DOI] [PubMed] [Google Scholar]
- 72.Rye M, Torres EM, Friborg O, Skre I, Aarons GA. The Evidence-based Practice Attitude Scale-36 (EBPAS-36): a brief and pragmatic measure of attitudes to evidence-based practice validated in US and Norwegian samples. Implement Sci. Apr 4 2017;12(1):44. doi: 10.1186/s13012-017-0573-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Brown SA. Standardized measures for substance use stigma. Drug Alcohol Depend. Jul 1 2011;116(1–3):137–41. doi: 10.1016/j.drugalcdep.2010.12.005 [DOI] [PubMed] [Google Scholar]
- 74.PASS 2019 Power Analysis and Sample Size Software (2019). NCSS, LLC. Kaysville, Utah, USA, ncss.com/software/pass.; [Google Scholar]
- 75.SAS Institute Inc 2013. SAS/ACCESS® 9.4 Interface to ADABAS: Reference. Cary, NC: SAS Institute Inc.; [Google Scholar]
- 76.R Core Team (2022). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria; https://www.R-project.org/ [Google Scholar]
- 77.Meteliuk A, Galvez de Leon SJ, Madden LM, et al. Rapid transitional response to the COVID-19 pandemic by opioid agonist treatment programs in Ukraine. Research Support, N.I.H., Extramural. J Subst Abuse Treat. Feb 2021;121:108164. doi: 10.1016/j.jsat.2020.108164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fekadu A, Demissie M, Birhane R, et al. Under detection of depression in primary care settings in low and middle-income countries: a systematic review and meta-analysis. Syst Rev. Feb 5 2022;11(1):21. doi: 10.1186/s13643-022-01893-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wainberg ML, Scorza P, Shultz JM, et al. Challenges and Opportunities in Global Mental Health: a Research-to-Practice Perspective. Curr Psychiatry Rep. May 2017;19(5):28. doi: 10.1007/s11920-017-0780-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Solmi M, Miola A, Croatto G, et al. How can we improve antidepressant adherence in the management of depression? A targeted review and 10 clinical recommendations. Braz J Psychiatry. Mar-Apr 2021;43(2):189–202. doi: 10.1590/1516-4446-2020-0935 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


